Abstract
Environmental fluctuations often select for adaptations such as diapause states, allowing species to outlive harsh conditions. The natural sugar trehalose which provides both cryo- and desiccation-protection, has been found in diapause stages of diverse taxa. Here, we hypothesize that trehalose deposition in resting stages is a locally adapted trait, with higher concentrations produced in harsher habitats. We used resting stages, produced under standardized conditions, by 37 genotypes of Daphnia magna collected from Western Palaearctic habitats varying in their propensity to dry in summer and freeze in winter. Resting eggs produced by D. magna from populations from summer-dry habitats showed significantly higher trehalose than those from summer-wet habitats, suggesting that trehalose has a protective function during desiccation. By contrast, winter-freezing did not explain variation in trehalose content. Adaptations to droughts are important, as summer dryness of water bodies is foreseen to increase with ongoing climate change.
Keywords: trehalose, desiccation, diapause, local adaptation, Daphnia magna
1. Introduction
Environmental factors determine the occurrence and geographical range of species, whose ability to persist in any given habitat depends on their capacity to develop behavioural, physiological or structural adaptations, especially to extreme environmental fluctuations [1,2]. These adaptive strategies, which have allowed most environments on the planet to be colonized [3], may involve moving to a different place (migration [4]) or suspending development and forming protective dormant stages [5].
Diapause, a programmed state of developmental arrest, is a form of dormancy often initiated in response to environmental triggers in anticipation of deteriorating environmental fluctuations [6–8]. It often goes hand-in-hand with seasonality [9]. During diapause, activities such as embryogenesis, growth, maturation, breeding and hatching may be postponed, resulting in dormant cysts, gemmules, eggs and larvae [8–10], capable of surviving conditions such as cold or heat stress [5]. Desiccation, a state of extreme dryness, is frequently observed as a stress factor that triggers diapause in many organisms. In diapause, some organisms can successfully survive desiccation, even when 99% of the water is removed from their cells [11,12]. This ability seems to have evolved early in evolutionary history and is observed in prokaryotes [13] and eukaryotes, including plants [14], fungi [15] and animals [7]. Specific mechanisms and adaptations, including the production of sugar molecules (e.g. sucrose and trehalose) [11,16,17] and small stress proteins (e.g. heat shock and late embryogenesis abundant proteins) [9,18,19] are required to survive the severe damage that desiccation would otherwise cause to cells [20]. The array of these mechanisms suggests the convergent evolution of those traits (reviewed in [20]).
The role of the natural sugar, trehalose, was first identified as being essential to diapause in Artemia salina [21,22], whose dormant eggs contained higher trehalose concentration than non-dormant eggs [22]. Trehalose not only helped organisms survive during diapause conditions, but also functioned as an energetic substrate, boosting their emergence from diapause and their further development [21]. Numerous studies have corroborated the role of trehalose as both a cryo- and desiccation-protectant, for instance in bacteria [23], fungi [24–26], nematodes [27], tardigrades [28], insects [29,30] and crustaceans [31]. Trehalose also aids organisms in other stressful conditions, such as when water salinity and temperature rise [19,20,32]. The main benefits of trehalose are its stability as a chemical with a low degradation rate; it is able to stabilize dry membranes, liposomes and proteins over the long-term by impeding their aggregation [27,33] and has a special ability to reach a vitrification state and fill cellular spaces left by water [11,22,25,34]. However, despite the fact that trehalose is a compatible solute in many organisms [35], its biosynthesis is energetically costly [26]. Furthermore, over-accumulations of trehalose have led to aberrations and seem to interfere with reactive oxygen species signalling and reducing programmed cell damage [36]. Thus, trehalose is a double-edged sword that should only be relied on when its benefits outweigh its costs. If these benefits depend on the environment, trehalose expression should show a signature of local adaptation, occurring in higher amounts in habitats with more severe diapause conditions.
Here, we test whether the concentration of trehalose in resting eggs is higher in genotypes of Daphnia magna from habitats with particularly harsh diapause conditions, namely water bodies that freeze in winter and desiccate during summer. Daphnia magna Straus 1820 is an ideal organism to study local adaptation in diapause. It inhabits brackish and freshwater bodies in a wide variety of habitats, from permanent to intermittent freshwater ponds [37] and it produces diapausing resting eggs which ensure survival during severe, otherwise unliveable conditions [38,39].
2. Material and methods
(a) . Daphnia samples
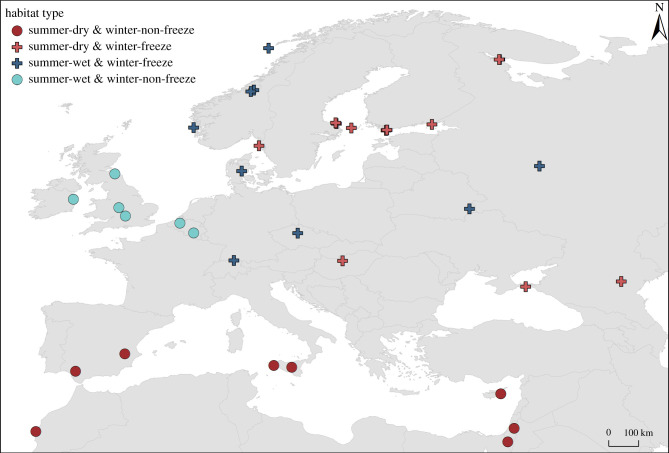
Thirty-seven genotypes, each from a distinct population across the Western Palaearctic were selected from the D. magna Diversity Panel (e.g. [40]; figure 1 and electronic supplementary material, table S1). Water bodies were characterized by their tendency to dry out during summer or not (based on observation and reports, see [40]), and to freeze regularly during winter or not (indicated as average temperature of the coldest month below zero). This resulted in four distinct habitat categories ([41,42]; figure 1, electronic supplementary material, table S1).
Figure 1.
Geographical distribution of sampling sites and their habitat types.
(b) . Resting stage production
Resting eggs were produced by selfing from genotypes kept as clonal lines for five months under standardized laboratory conditions. The number of resting eggs produced depends on the genotype [42], but can be triggered by short photoperiod or crowding [38,43]. We kept crowded monoclonal populations at 16 and 20°C and 8 : 16 dark : light cycle in 400-ml medium jars with artificial medium [44], feeding three times per week with the green algae Scenedesmus sp. Medium was changed once a month. Resting eggs were collected weekly and kept in closed jars with the same medium conditions for five months maximum.
(c) . Trehalose extraction and concentration measure
For each genotype, eggs (actually embryos in developmental arrest) were removed from the resting egg-case. In total eight biological replicates per genotype were used, each containing five eggs. For each replicate, we calculated the total egg volume (assuming the eggs were ellipsoids) by measuring length and width per egg using an eyepiece graticule (2 mm ± 0.01) in a stereomicroscope. Eggs were cleaned with deionized water, placed in a 0.5-ml Eppendorf tube filled with 25 µl of ultrapure water and disintegrated using a sonicator (Biorupter Next Generation System—UCD300, Diagenode), with up to three runs of three cycles of 90 s each, until achieve a homogeneous solution.
For trehalose extraction, samples were incubated at 95°C for 60 min and centrifuged for 15 min at 4°C at 13 200 r.p.m. and 16 100g. We used 20 µl of the supernatant to determine trehalose concentration following the manufacturer protocol of the Megazyme trehalose kit (Megazyme, Bray, Ireland). This method relies on the difference in NADPH+, before and after trehalose degradation by trehalase. Falcon Microtest 96 microplates were used for absorbance reads in an Infinite M200 Tecan spectrophotometer at 340 nm. For each 96-well plate (including two biological replicates each), we added eight blanks and two trehalose standards solution to calibrate and validate the reaction. After shaking 3 s and 5 min pause, 16 measurements were taken per sample (4 × 4 matrix per well). Absorbance values were retrieved by i-control Microplate Reader Software by Tecan before and after trehalase addition. Calculation of trehalose concentration followed the manufacturer's instructions, accounting for egg volume and standard calibrations. According to the manufacturer's instructions, absorbance estimates below 0.1 are unreliable, which was the case for 13 of our 296 individual measures (see electronic supplementary material, tables S1 and S2 for details). We, therefore, excluded those replicates from statistical analysis, even though including them (setting estimates below 0.1 to an absorbance of 0.1) did not affect the outcome of the analysis (see electronic supplementary material, table S3). To compare our estimates with other studies, we estimated dry weight to volume ratio per egg by using four replicates of 100 eggs each. We compared our trehalose estimates with another commonly used estimation method (high-performance liquid anion exchange chromatography) and were able to show that both methods reach the same results (electronic supplementary material, section S5), providing us with confidence in the spectrophotometric method used here.
(d) . Data analysis
Data analysis was performed using the eight biological replicates, distributed evenly across four microtitre plates. We did not detect an effect of the microtitre plate (block-effect). The lme4 package was used to estimate the genotype variance component for trehalose content (lmer(conc_trehalose∼(1|genotype)). Analysis of variance used summer-dry (Y/N) and winter-freeze (Y/N) as independent explanatory variables, and genotype variable as error, to test for differences in trehalose content (table 1). Our data followed normality and homoscedasticity assumptions. All analyses were performed with R (v. 3.5) in R studio (v. 1.2.5033). All material used is available in electronic supplementary material, table S2 and section S4.
Table 1.
Analysis of variance for the effect of habitat type and host genotype on trehalose concentration of Daphnia magna resting eggs. Significant p-values (p ≤ 0.001) are shown in italics.
| factor | d.f. | mean of squares | F-value | p-value |
|---|---|---|---|---|
| summer-dry | 1 | 327.3 | 12.0 | 0.001 |
| winter-freeze | 1 | 47.0 | 1.73 | 0.198 |
| summer-dry: winter-freeze | 1 | 16.5 | 0.61 | 0.442 |
| residuals | 33 | 27.3 | ||
| error: genotype | 246 | 15.69 |
3. Results
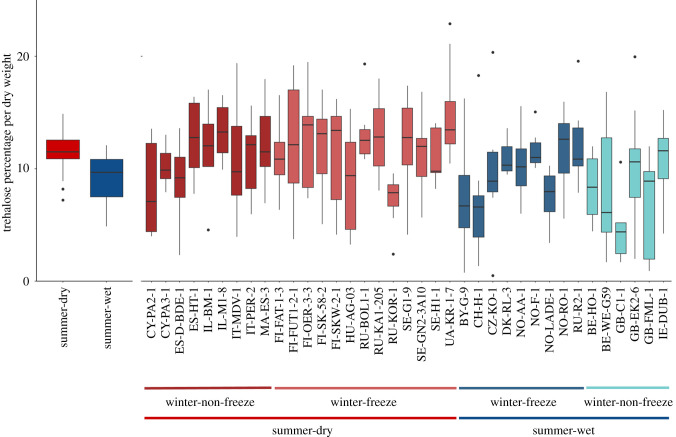
Mean percentage of trehalose in resting eggs was 10.55% of dry weight (s.e. = 4.45). The among genotype variance component for these estimates was 14%. The mean percentage is similar to reports for some invertebrates with dry resting stages (nematodes [45], insects [46] and the lower crustacean Artemia (15% of dry weight) [22]). There was strong variation among and within genotypes (figure 2). Analysis of variance revealed a higher trehalose concentration (p-value = 0.001) in resting eggs from summer-dry habitat populations (table 1 and figure 2). Factoring for winter-freezing did not reveal a significant difference, nor did the interaction between both factors (table 1 and figure 2).
Figure 2.
Average trehalose concentration for summer-dry (red) and summer-wet (blue) separated for habitat types (on the left) and for genotypes (on the right). Trehalose concentration is given as a percentage of dry weight per resting egg. Box plots show median, first and third quartile. Whiskers extend to 1.5 times from the interquartile range upper and lower limits. The dots show data points beyond whiskers.
4. Discussion
In this study, we show that trehalose concentration in resting stages varies among D. magna genotypes and that this variation is partially influenced by the local habitat type. While previous studies focused on quantifying trehalose between species or between directly developing eggs and dormant stages [22,24,47], our study is the first to determine genetic variation within the same egg type of a species. Since all our genotypes were acclimated under similar laboratory conditions and produced resting eggs using eight independent replicates under the same conditions unrelated to their environment of origin, our results reflect genetic differences among the 37 genotypes studied here. This allows us to examine the evolution of trehalose concentration and test for its adaptive role across different environments. Our hypothesis—that trehalose concentration would be higher for genotypes from habitats with more severe conditions during diapause—was corroborated here, as genotypes from habitats with a high propensity for summer desiccation produced resting eggs containing about 20% more trehalose. This difference might be even greater when considering natural environmental triggers. The high (sometimes extreme) temperatures of the dry pond sediment (greater than 50°C [40]) constitute a severe stressful condition, requiring an efficient protection mechanism that trehalose is able to provide, as it fills the spaces left by water in the resting embryos' tissue with a glass-like structure and maintains the stability of cells and their contents [22,33].
By contrast to summer-dryness, we found no relation between trehalose concentration and winter-freezing. This finding is not very surprising because the resting stages in the pools we classified as winter-freezing pools may often not freeze solid; they generally only acquire ice at the surface. Except for very shallow pools such as Nordic rock pool habitats [40]. Thus, resting eggs can often overwinter on the surface of the pond sediment without freezing stress. Also, the pools included in our study do not dry out in winter, so the severe combination of drying and freezing does not occur [5]. A more detailed study with better data about local winter conditions may reveal an effect of winter harshness on trehalose concentration.
If trehalose is beneficial for the survival of resting eggs, one may expect it to be found equally in the resting eggs of all genotypes. Since this is not verified, trehalose production may be costly. A trade-off was found between storing and using energy metabolites for desiccation versus starvation stress in Drosophila melanogaster [48]. Trehalose might also be involved in biotic interactions, as suggested in symbioses between higher plants and microorganisms [36,49], and in pathogenic interactions [50]. An unexpected link may also exist between host trehalose concentrations and infection susceptibility, based on observations that only D. magna populations from summer-dry habitats are susceptible to the persistence of a virulent microsporidian parasite [41,51]; however, it is unclear if elevated trehalose in resting eggs plays a causal role here. In interactions between Plasmodium falciparum and Anopheles gambiae mosquitoes, trehalose is likely a source of energy that enhances infection success [52]. Further investigations in our study system might examine the relationship between host–parasite interaction and trehalose production.
Hengherr et al. [31] presented an estimate of trehalose in one genotype of D. magna resting eggs (0.5% of trehalose per dry weight), which is much lower than our estimates (about 10%). Since the quantification method differed between the two studies, we contacted the laboratory and conducted an experiment to quantify trehalose using duplicated samples of the same biological material, that were analysed by each laboratory following the methods previously applied to each study (this study and [31]). The two methods resulted in very similar trehalose estimates and were in accordance to values presented in our study (see electronic supplementary material, section S5). The lower values presented in Hengherr et al. [31] might be explained by an extreme case of low trehalose concentration in resting eggs of a D. magna genotype from a summer-wet population.
Our study indicates that Daphnia resting eggs are locally adapted to the desiccation of their habitat in summer, allowing the species to inhabit a wider range of habitats and geographical areas, including very small water bodies that frequently dry up [53] and desert pools, where water is only available for a limited period after rainfall [54]. With ongoing climate change, an increased incidence of droughts across large geographical regions is predicted and can already be seen in the greater incidence of pools drying up in summer [55]. Daphnia magna as an important component of many fresh- and brackish-water ecosystems will be strongly affected by such changes. Survival of local populations may critically depend on its ability to produce resting stages that can survive summer dryness. Understanding local adaptation to summer dryness is a first step to predict how species may evolve to cope with this aspect of climate change and provide insights on the future of abiotic and biotic interactions.
Acknowledgements
We thank to Jürgen Hottinger, Urs Stiefel, Michelle Krebs, Nicolas Boileau and Heidi Schiffer for their technical support. We greatly appreciated the contributions of Ralph O. Schill and Arnd G. Heyer in later experiments and discussion. Suzanne Zweizig improved the manuscript's language.
Data accessibility
Data, additional analysis and R scripts for this study are available in the electronic supplementary material [56].
Authors' contributions
J.L.S.: conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing; D.E.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by Swiss National Science Foundation and by the Universität Basel, Switzerland.
References
- 1.Brown JH, Stevens GC, Kaufman DM. 1996. The geographic range: size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 27, 597-623. ( 10.1146/annurev.ecolsys.27.1.597) [DOI] [Google Scholar]
- 2.Louthan AM, Doak DF, Angert AL. 2015. Where and when do species interactions set range limits? Trends Ecol. Evol. 30, 780-792. ( 10.1016/j.tree.2015.09.011) [DOI] [PubMed] [Google Scholar]
- 3.Lubzens E. 2015. Frére Jacques/Dormez vous? Dormancy, an intriguing phenomenon shared by many forms of life. Mol. Reprod. Dev. 82, 3. ( 10.1002/mrd.22480) [DOI] [PubMed] [Google Scholar]
- 4.Bauer S, Hoye BJ. 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344, 1242552. ( 10.1126/science.1242552) [DOI] [PubMed] [Google Scholar]
- 5.Iwaya-Inoue MI, Sakurai M, Uemura M. 2018. Survival strategies in extreme cold and desiccation: adaptation mechanisms and their applications, vol. 1081. Advances in Experimental Medicine and Biology. Singapore: Springer Nature. [Google Scholar]
- 6.Hanks HV. 1987. Insect dormancy: an ecological perspective. Ottawa, Canada: Biological Survey of Canada. [Google Scholar]
- 7.Hand SC, Denlinger DL, Podrabsky JE, Roy R. 2016. Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R1193-R1211. ( 10.1152/ajpregu.00250.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alekseev VR, Pinel-Alloul B. 2019. Dormancy in aquatic organisms. In Theory, human use and modeling, vol. 92. Monographiae Biologicae. Berlin, Germany: Springer International Publishing. [Google Scholar]
- 9.Denlinger DL. 2002. Regulation of diapause. Annu. Rev. Entomol. 47, 93-122. ( 10.1146/annurev.ento.47.091201.145137) [DOI] [PubMed] [Google Scholar]
- 10.Renfree MB, Shaw G. 2000. Diapause. Annu. Rev. Physiol. 62, 353-375. ( 10.1146/annurev.physiol.62.1.353) [DOI] [PubMed] [Google Scholar]
- 11.Crowe JH, Hoekstra FA, Crowe LM. 1992. Anhydrobiosis. Annu. Rev. Physiol. 54, 579-599. ( 10.1146/annurev.ph.54.030192.003051) [DOI] [PubMed] [Google Scholar]
- 12.Alpert P. 2005. The limits and frontiers of desiccation-tolerant life. Integr. Comp. Biol. 45, 685-695. ( 10.1093/icb/45.5.685) [DOI] [PubMed] [Google Scholar]
- 13.Potts M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58, 755-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bewley JD. 1979. Physiological aspects of desiccation tolerance. Annu. Rev. Plant. Physiol. 30, 195-238. ( 10.1146/annurev.pp.30.060179.001211) [DOI] [Google Scholar]
- 15.Gadd GM, Chalmers K, Reed RH. 1987. The role of trehalose in dehydration resistance of Saccharomyces cerevisiae. FEMS Microbiol. Lett. 48, 249-254. ( 10.1111/j.1574-6968.1987.tb02551.x) [DOI] [Google Scholar]
- 16.Koster KL, Leopold AC. 1988. Sugars and desiccation tolerance in seeds. Plant Physiol. 88, 829-832. ( 10.1104/pp.88.3.829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoekstra FA, Golovina EA, Buitink J. 2001. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 6, 431-438. ( 10.1016/S1360-1385(01)02052-0) [DOI] [PubMed] [Google Scholar]
- 18.Battista JR, Park M-J, McLemore AE. 2001. Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology 43, 133-139. ( 10.1006/cryo.2001.2357) [DOI] [PubMed] [Google Scholar]
- 19.Crowe JH, Crowe LM, Oliver AE, Tsvetkova N, Wolkers W, Tablin F. 2001. The trehalose myth revisited: introduction to a symposium on stabilization of cells in the dry state. Cryobiology 43, 89-105. ( 10.1006/cryo.2001.2353) [DOI] [PubMed] [Google Scholar]
- 20.Somero GN, Lockwood BL, Tomanek L. 2017. Biochemical adaptation: response to environmental challenges from life's origins to the Anthropocene. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 21.Clegg JS. 1964. The control of emergence and metabolism by external osmotic pressure and the role of free glycerol in developing cysts of Artemia salina. J. Exp. Biol. 41, 879-892. ( 10.1242/jeb.41.4.879) [DOI] [PubMed] [Google Scholar]
- 22.Clegg JS. 1965. The origin of trehalose and its significance during the formation of encysted dormant embryos of Artemia salina. Comp. Biochem. Physiol. 14, 135-143. ( 10.1016/0010-406X(65)90014-9) [DOI] [PubMed] [Google Scholar]
- 23.Welsh DT, Herbert RA. 1999. Osmotically induced intracellular trehalose, but not glycine betaine accumulation promotes desiccation tolerance in Escherichia coli. FEMS Microbiol. Lett. 174, 57-63. ( 10.1111/j.1574-6968.1999.tb13549.x) [DOI] [PubMed] [Google Scholar]
- 24.Thevelein JM, den Hollander JA, Shulman RG. 1984. Trehalase and the control of dormancy and induction of germination in fungal spores. Trends Biochem. Sci. 9, 495-497. ( 10.1016/0968-0004(84)90321-9) [DOI] [Google Scholar]
- 25.Tapia H, Koshland DE. 2014. Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr. Biol. 24, 2758-2766. ( 10.1016/j.cub.2014.10.005) [DOI] [PubMed] [Google Scholar]
- 26.Wiemken A. 1990. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek 58, 209-217. ( 10.1007/BF00548935) [DOI] [PubMed] [Google Scholar]
- 27.Koshland D, Tapia H. 2019. Desiccation tolerance: an unusual window into stress biology. Mol. Biol. Cell 30, 737-741. ( 10.1091/mbc.E17-04-0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hengherr S, Heyer AG, Köhler H, Schill RO. 2008. Trehalose and anhydrobiosis in tardigrades—evidence for divergence in responses to dehydration. FEBS J. 275, 281-288. ( 10.1111/j.1742-4658.2007.06198.x) [DOI] [PubMed] [Google Scholar]
- 29.Kimura MT, Awasaki T, Ohtsu T, Shimada K. 1992. Seasonal changes in glycogen and trehalose content in relation to winter survival of four temperate species of Drosophila. J. Insect Physiol. 38, 871-875. ( 10.1016/0022-1910(92)90098-X) [DOI] [Google Scholar]
- 30.Tamang AM, Kalra B, Parkash R. 2017. Cold and desiccation stress induced changes in the accumulation and utilization of proline and trehalose in seasonal populations of Drosophila immigrans. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 203, 304-313. ( 10.1016/j.cbpa.2016.10.011) [DOI] [PubMed] [Google Scholar]
- 31.Hengherr S, Heyer AG, Brümmer F, Schill RO. 2011. Trehalose and vitreous states: desiccation tolerance of dormant stages of the crustaceans Triops and Daphnia. Physiol. Biochem. Zool. 84, 147-153. ( 10.1086/658499) [DOI] [PubMed] [Google Scholar]
- 32.Kosar F, Akram NA, Sadiq M, Al-Qurainy F, Ashraf M. 2019. Trehalose: a key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant. Growth. Regul. 38, 606-618. ( 10.1007/s00344-018-9876-x) [DOI] [Google Scholar]
- 33.Crowe LM. 2002. Lessons from nature: the role of sugars in anhydrobiosis. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 131, 505-513. ( 10.1016/S1095-6433(01)00503-7) [DOI] [PubMed] [Google Scholar]
- 34.Crowe JH, Carpenter JF, Crowe LM. 1998. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 60, 73-103. ( 10.1146/annurev.physiol.60.1.73) [DOI] [PubMed] [Google Scholar]
- 35.Avonce N, Mendoza-Vargas A, Morett E, Iturriaga G. 2006. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 6, 109. ( 10.1186/1471-2148-6-109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez O, Béthencourt L, Quero A, Sangwan RS, Clément C. 2010. Trehalose and plant stress responses: friend or foe? Trends Plant Sci. 15, 409-417. ( 10.1016/j.tplants.2010.04.004) [DOI] [PubMed] [Google Scholar]
- 37.Hebert PDN. 1974. Enzyme variability in natural populations of Daphnia magna II: genotypic frequencies in permanent populations. Genetics 77, 323-334. ( 10.1093/genetics/77.2.323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleiven OT, Larsson P, Hobæk A, Hobaek A. 1992. Sexual reproduction in Daphnia magna requires three stimuli. Oikos 65, 197. ( 10.2307/3545010) [DOI] [Google Scholar]
- 39.Brendonck L, De Meester L. 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491, 65-84. ( 10.1023/A:1024454905119) [DOI] [Google Scholar]
- 40.Seefeldt L, Ebert D. 2019. Temperature- versus precipitation-limitation shape local temperature tolerance in a Holarctic freshwater crustacean. Proc. R. Soc. B 286, 20190929. ( 10.1098/rspb.2019.0929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lange B, Kaufmann AP, Ebert D. 2015. Genetic, ecological and geographic covariables explaining host range and specificity of a microsporidian parasite. J. Anim. Ecol. 84, 1711-1719. ( 10.1111/1365-2656.12421) [DOI] [PubMed] [Google Scholar]
- 42.Roulin AC, Routtu J, Hall MD, Janicke T, Colson I, Haag CR, Ebert D. 2013. Local adaptation of sex induction in a facultative sexual crustacean: insights from QTL mapping and natural populations of Daphnia magna. Mol. Ecol. 22, 3567-3579. ( 10.1111/mec.12308) [DOI] [PubMed] [Google Scholar]
- 43.Carvalho GR, Hughes RN. 1983. The effect of food availability, female culture-density and photoperiod on ephippia production in Daphnia magna Straus (Crustacea: Cladocera). Freshw. Biol. 13, 37-46. ( 10.1111/j.1365-2427.1983.tb00655.x) [DOI] [Google Scholar]
- 44.Klüttgen B, Dülmer U, Engels M, Ratte HT. 1994. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 28, 743-746. ( 10.1016/0043-1354(94)90157-0) [DOI] [Google Scholar]
- 45.Madin KAC, Crowe JH. 1975. Anhydrobiosis in nematodes: carbohydrate and lipid metabolism during dehydration. J. Exp. Zool. 193, 335-342. ( 10.1002/jez.1401930309) [DOI] [Google Scholar]
- 46.Watanabe M, Kikawada T, Minagawa N, Yukuhiro F, Okuda T. 2002. Insect survival in extreme conditions. J. Exp. Biol. 205, 2799-2802. ( 10.1242/jeb.205.18.2799) [DOI] [PubMed] [Google Scholar]
- 47.Watanabe M. 2006. Anhydrobiosis in invertebrates. Appl. Entomol. Zool. 41, 15-31. ( 10.1303/aez.2006.15) [DOI] [Google Scholar]
- 48.Parkash R, Aggarwal DD. 2012. Trade-off of energy metabolites as well as body color phenotypes for starvation and desiccation resistance in montane populations of Drosophila melanogaster. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 161, 102-113. ( 10.1016/j.cbpa.2011.09.010) [DOI] [PubMed] [Google Scholar]
- 49.Mellor RB. 1992. Is trehalose a symbiotic determinant in symbioses between higher plants and microorganisms? Symbiosis 12, 113-129. [Google Scholar]
- 50.Keen NT, Williams PH. 1969. Translocation of sugars into infected cabbage tissues during clubroot development. Plant Physiol. 44, 748-754. ( 10.1104/pp.44.5.748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krebs M, Routtu J, Ebert D. 2017. QTL mapping of a natural genetic polymorphism for long-term parasite persistence in Daphnia populations. Parasitology 144, 1686-1694. ( 10.1017/S0031182017001032) [DOI] [PubMed] [Google Scholar]
- 52.Liu K, Dong Y, Huang Y, Rasgon JL, Agre P. 2013. Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proc. Natl Acad. Sci. USA 110, 17 504-17 509. ( 10.1073/pnas.1316709110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altermatt F, Ebert D. 2010. Populations in small, ephemeral habitat patches may drive dynamics in a Daphnia magna metapopulation. Ecology 91, 2975-2982. ( 10.1890/09-2016.1) [DOI] [PubMed] [Google Scholar]
- 54.Goren L, Ben-Ami F. 2013. Ecological correlates between cladocerans and their endoparasites from permanent and rain pools: patterns in community composition and diversity. Hydrobiologia 701, 13-23. ( 10.1007/S10750-012-1243-5) [DOI] [Google Scholar]
- 55.Altermatt F, Pajunen VI, Ebert D. 2008. Climate change affects colonization dynamics in a metacommunity of three Daphnia species. Glob. Chang. Biol. 14, 1209-1220. ( 10.1111/j.1365-2486.2008.01588.x) [DOI] [Google Scholar]
- 56.Santos JL, Ebert D. 2022. Trehalose provisioning in Daphnia resting stages reflects local adaptation to the harshness of diapause conditions. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Santos JL, Ebert D. 2022. Trehalose provisioning in Daphnia resting stages reflects local adaptation to the harshness of diapause conditions. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data, additional analysis and R scripts for this study are available in the electronic supplementary material [56].