Abstract
Objective
The aim of this exploratory study was to investigate the development of low‐grade inflammation during ageing and its relationship with frailty.
Methods
The trajectories of 18 inflammatory markers measured in blood samples, collected at 5‐year intervals over a period of 20 years from 144 individuals aged 65–75 years at the study endpoint, were related to the degree of frailty later in life.
Results
IFN‐γ‐related markers and platelet activation markers were found to change in synchrony. Chronically elevated levels of IL‐6 pathway markers, such as CRP and sIL‐6R, were associated with more frailty, poorer lung function and reduced physical strength. Being overweight was a possible driver of these associations. More and stronger associations were detected in women, such as a relation between increasing sCD14 levels and frailty, indicating a possible role for monocyte overactivation. Multivariate prediction of frailty confirmed the main results, but predictive accuracy was low.
Conclusion
In summary, we documented temporal changes in and between inflammatory markers in an ageing population over a period of 20 years, and related these to clinically relevant health outcomes.
Keywords: chemokines, chronic low‐grade inflammation, cytokines, frailty, healthy ageing, longitudinal study
This exploratory study gives unique insight into longitudinal changes in the immune profile of ageing men and women over a period of about 20 years, and revealed multiple associations of immune markers with clinically relevant outcomes at study endpoint, such as frailty, handgrip strength and lung function. More specifically, the findings implicate an important role for the IL‐6 pathway due to elevated markers such as CRP and, only in women, monocyte activation due to the involvement of sCD14.
![]()
Introduction
Understanding the ageing process is important for finding ways to prevent or delay age‐related morbidity and thus to ensure a good quality of life for an elderly population that is rapidly expanding worldwide. 1 One factor that is crucial for ‘successful’ ageing is an adequately functioning immune system necessary for combating pathogens and warding off internal threats such as malignant transformation. But immune processes can become out of balance in the elderly, leading to persistent low‐grade inflammation. 2 , 3 It is thought that those with long‐lasting low‐grade inflammation have reduced responses to pathogens and carcinogenesis, and are more prone to autoimmunity. This would render them more vulnerable to developing age‐related diseases and becoming frail. 2 , 3 In addition to ageing, a potential driver of chronic low‐grade inflammation could be the amount of body fat, since adipocytes can activate the immune system directly. 4
It is still largely unknown when and how low‐grade inflammation develops in the course of ageing, and how this is related to frailty. The few longitudinal studies on this subject showed that in frail people, often low‐grade inflammation was present over a long period of time. 5 , 6 , 7 , 8 In most studies, including our own, 7 the presence of chronic low‐grade inflammation was assessed by measuring the plasma concentrations of only one or two inflammatory markers, notably CRP and IL‐6. 6 However, inflammation is a complex process in which many proteins are involved. Some studies already suggested that looking at a larger panel of inflammatory biomarkers, including a broader range of (chemotactic) cytokines, would improve the understanding of the relationship between low‐grade inflammation and age‐related diseases. 9 Furthermore, taking sex differences into account seems relevant. Women generally have higher scores on frailty than men but nevertheless have a greater life expectancy. 10 In order to gain more insight into how long‐lasting low‐grade inflammation relates to frailty, and taking into account sex differences, we performed an exploratory study using data and blood samples from a selection of participants (n = 144) in the longitudinal Doetinchem Cohort Study. Blood samples and data were collected at 5‐year intervals covering a period of approximately 20 years.
We had several aims. First, we wanted to explore the development of low‐grade inflammation in an ageing population by investigating whether, and how, concentrations of multiple inflammatory markers change with age. Second, we wanted to know whether exposure to low‐grade inflammation over a prolonged period of time is related to frailty or the likelihood of becoming frail, and to specific ageing‐related clinical outcomes such as decreased physical (handgrip) strength and lung function (spirometry). Lastly, we investigated whether being overweight is an important factor in these relationships.
Results
Study population characteristics
The study group consisted of 144 participants, (73 men and 71 women; Figure 1), with an average age of 68.3 years (min 59.7, max 73.5 years) at the endpoint of follow‐up (Table 1). The average follow‐up time was 19.3 years (min 14.4, max 20.9 years). Since blood samples were taken every 5 years, a maximum of five samples per individual was available for analysis, which was the case for most participants (n = 107). Of the other study participants, either four samples (n = 34) or three samples (n = 3) were available. Highest concentrations were in the order of 106 pg mL−1, for example, of soluble CD14 (sCD14; average endpoint level: 2.19 × 106 pg mL−1) and C‐reactive protein (CRP; 1.28 × 106 pg mL−1; Supplementary table 1); lowest concentrations were around 1 pg mL−1, for example, of IL‐10 and IL‐6 (average endpoint level: 0.65 and 2.31 pg mL−1, respectively). Concentrations of sIL‐2R and IL‐1β were below the detection limit in > 40% of the cases; therefore, these were excluded from analysis, leaving a total of 18 inflammatory markers (Supplementary table 1).
Figure 1.
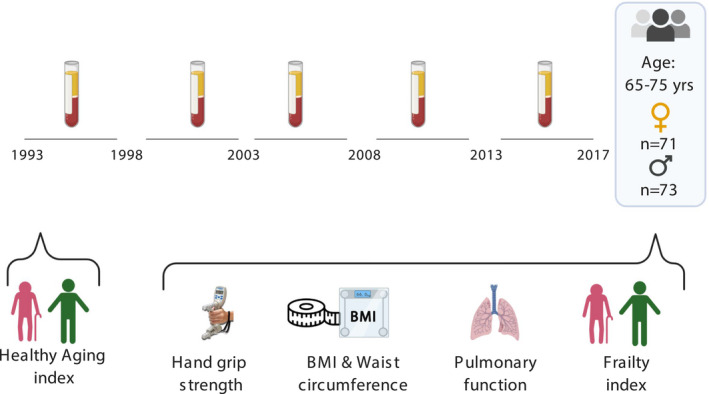
Timeline of the study. Participants aged 65–75 years at the study endpoint had been followed for more than 20 years, with plasma samples taken and stored at 5‐year intervals. Healthy ageing index scores at baseline were determined. At last assessment, a frailty index based on 35 health parameters, including lung function and handgrip strength, was calculated for each participant.
Table 1.
Baseline characteristics of the study population
| Value | |
|---|---|
| N | 144 |
| Women, % (n) | 49.3 (71) |
| Age at baseline (years) | 49 (SD 2.6, range 42.4–53.5) |
| Age at the endpoint (years) | 68.3 (SD 2.8, range 59.7–73.5) |
| Mean follow‐up timespan (years) | 19.3 (SD 1.8, range 14.4–20.9) |
| BMI | 26.5 (SD 4, range 20.5–46.8) |
Numbers are mean (SD) unless otherwise stated.
Sex‐specific changes in concentrations of inflammatory markers during ageing
We estimated the average inflammatory marker levels over the whole 20‐year follow‐up by calculating the area under the concentration versus time curve (AUC) for each individual and each inflammatory marker separately. The AUC values for CCL5 were consistently higher in women than in men (Supplementary figure 1). In both men and women, an increase during ageing was observed in the levels of CXCL10, CXCL11 and CCL27 (Figure 2). In women, but not in men, there were increases in CRP, sIL‐6R, CCL2, CCL11 and sCD14. These markers increased until age 60 approximately, after which the trajectories of inflammatory markers in men and women appeared to become more similar (Figure 2). Indeed, the sex differences that existed at study baseline, in particular higher concentrations in women of CCL5 and BDNF, and lower concentrations of CCL11 and CCL2, were no longer found at the study endpoint (data not shown).
Figure 2.
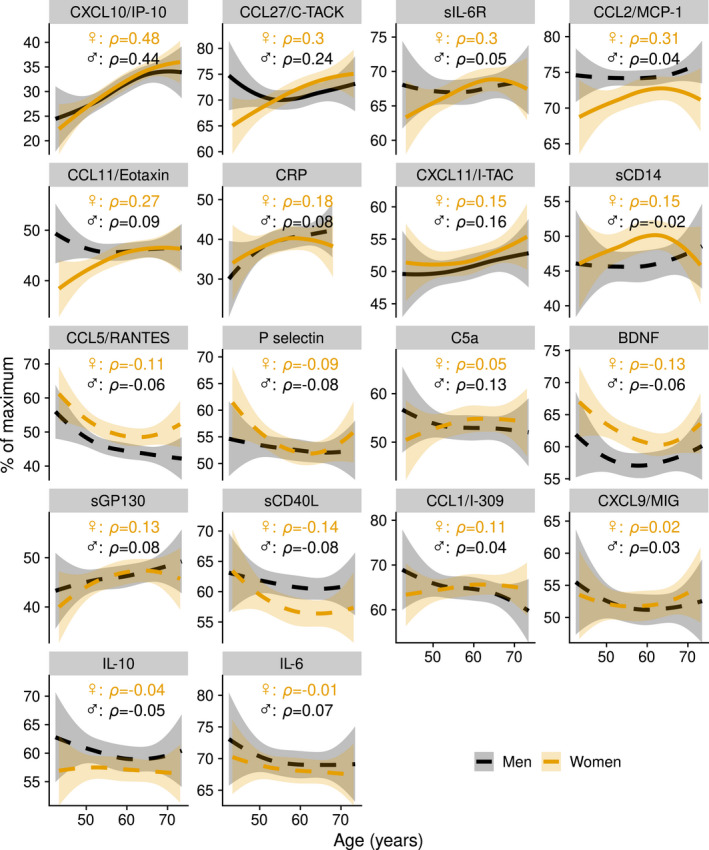
Trajectories of inflammatory markers as a function of age. Average trajectories are shown for men (n = 73) and women (n = 71) with 95% confidence intervals, estimated by local polynomial regression. A continuous line means that an association was found between inflammatory marker trajectory and age; a dashed line means that no association was found. The y‐axis shows the percentage of the maximum concentration per biomarker. Average concentrations per biomarker (pg mL−1) are given in Supplementary table 1.
The role of menopause
To study whether changes in inflammatory marker levels in women are affected by menopause, we compared the levels of inflammatory markers just before menopause with those shortly after menopause (enough information, including age at menopause, was available for 40 out of 70 women; Supplementary figure 2). The average self‐reported age at menopause was 50.3 years (95% CI 39.7–60.9), and the average time between measurement before and after menopause was 5.3 years (95% CI 2.6–8.1). After menopause, concentrations of CCL2, CCL11, sGP130, CCL27 and CXCL10 were higher. Thus, our data show that inflammatory marker trajectories differ between men and women although these become more similar with increasing age. This is possibly partly explained by the hormonal changes in menopause.
Correlations between inflammatory markers at the study endpoint
We studied relations between inflammatory markers by calculating their correlations at the study endpoint. This revealed multiple associations (Figure 3a). The strongest positive associations (ρ > 0.50) were as follows: IL‐6 with IL‐10 (ρ = 0.72); IL‐6 with sCD40L (ρ = 0.58); IL‐6 with CXCL9 (ρ = 0.53); sCD40L with CXCL9 (ρ = 0.50); and BDNF with CCL5 (ρ = 0.68). These associations were found both in men and in women, but were stronger in women (Supplementary figure 3a and b). Other positive associations were seen between the structurally related IFN‐γ‐inducible chemokines CXCL9, CXCL10 and CXCL11 (Figure 3a); again, these associations were stronger in women than in men (Supplementary figure 3 and b). BDNF correlated positively with most of the CXCL and CCL chemokines (CCL1, CCL2, CCL5, CCL11, CXCL9 and CXCL11) (Supplementary figure 3a and b).
Figure 3.
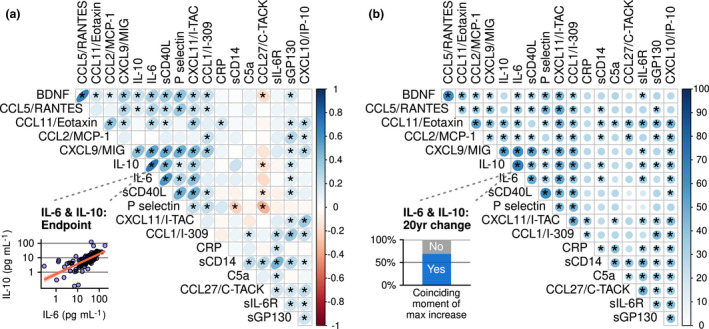
Relationships between inflammatory markers shown: (a) correlations between pairs of inflammatory markers at the study endpoint; and (b) similarities between pairs of inflammatory marker trajectories during the approximately 20 years of follow‐up. In (a), direction and strength of the association are visualised by ellipses of different eccentricities and a colour gradient. The inset shows the correlation between one pair of biomarkers (IL‐6 and IL‐10) as an example. In (b), the blue colour gradient and the size of the circles encode the percentage of participants in whom a pair of biomarkers peaked in concentration at the same time interval during follow‐up. For the pair of biomarkers IL‐6 and IL‐10, this was seen in 69% of the participants (see inset). * = existence of an association between two inflammatory markers is confirmed, based on a false discovery rate of 15%. n = 144.
Relations between inflammatory marker changes over time
In order to study how changes in concentrations of inflammatory markers during ageing relate to each other, we looked for synchronous changes in biomarkers. Our hypothesis was that when biomarkers change together, this probably reflects an underlying biological process inducing these changes. To identify synchronous changes, we determined in which of the four possible time intervals in the 20 years of follow‐up (based on five consecutive measurements once every 5 years), a marker had its greatest increase in concentration. Then, for every pair of biomarkers we tested in what proportion of participants this interval of greatest change was the same (e.g. the interval between the second and third blood withdrawal). In both men and women, the clearest evidence of synchronous change was found for IL‐6 and IL‐10 (Figure 3b), and for markers of the innate immune system, such as markers of platelet activation (sCD40L and P‐selectin) and of chemotaxis and granulocyte activation (CCL11 and CCL5; Figure 3b). Also, the structurally related chemokines CXCL9, CXCL11 and CXCL10 tended to change in lockstep. In addition, CXCL9 and CXCL11 were related to not only multiple other inflammatory markers, such as IL‐10 and IL‐6, but also CCL5, P‐selectin and sCD40L. This suggests the potential sensitivity of using a set of inflammatory markers for detecting IFN‐γ pathway activation in aged men and women.
In this longitudinal analysis, inflammatory marker profiles of men and women appear somewhat more similar than in the cross‐sectional analysis at the study endpoint. Thus, while in the latter analysis CCL5 correlated with more markers in women than in men (Supplementary figure 3a and b), this appeared to be less so in the longitudinal analysis (Supplementary figure 3c and d). In summary, multiple inflammatory marker levels were correlated with each other, with IL‐6 showing the strongest correlations with other markers, followed by markers related to platelet activation and activation of the IFN‐γ pathway. Correlations at the study endpoint were stronger and more numerous in women.
Levels of inflammatory markers over time related to frailty at the study endpoint
CRP concentrations over time (expressed as AUC) were most strongly associated with frailty at the study endpoint (Figure 4a and b; correlation in men, ρ = 0.44; in women, ρ = 0.50; frailty index based on 35 deficits, see Supplementary table 3). CRP levels appeared to be continuously elevated in those with higher frailty index scores at the endpoint (Figure 4b), as we reported previously. 7 In women only, associations with frailty were seen for sIL‐6R (ρ = 0.34), sCD14 (ρ = 0.33) and sCD40L (ρ = −0.29). Also, increases or decreases in biomarkers levels with advancing age (measured as fold change over the 20‐year study period) were associated with frailty at the study endpoint. This was seen in women for sCD14 and sIL‐6R (Figure 4c, ρ = 0.42 and ρ = 0.35, respectively).
Figure 4.
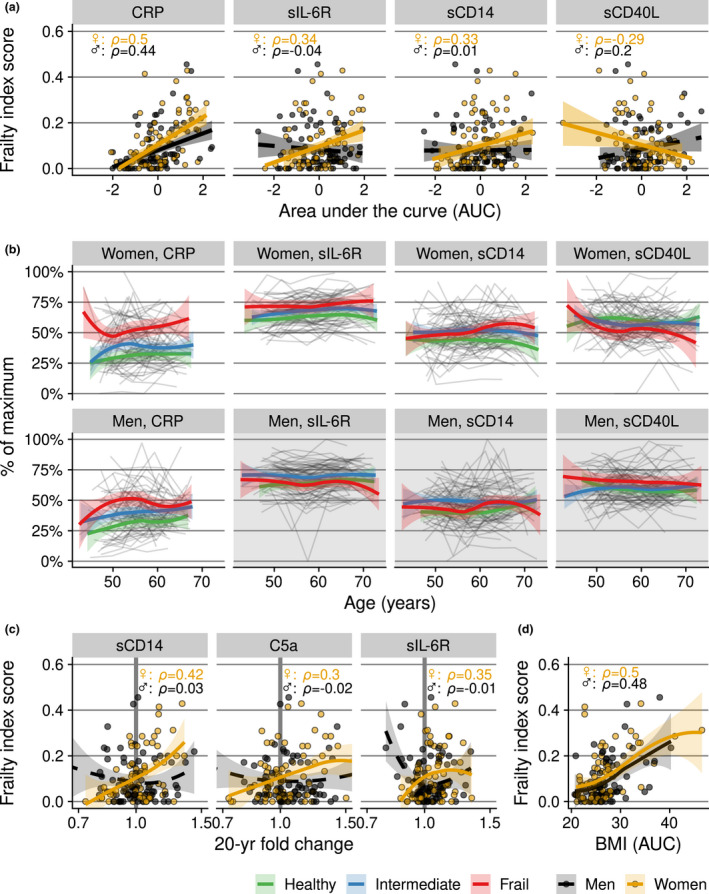
Relationship between frailty at the study endpoint and inflammatory marker levels over time in men (n = 73) and women (n = 71). (a) The cumulative ‘exposure’ to inflammatory marker levels over time, expressed as the area under the inflammatory marker concentration curve versus time (AUC), standardised to take into account differences in follow‐up periods and transformed into z‐scores for visualisation and better comparison between inflammatory markers. (b) Trajectories of individuals (grey lines) and the local polynomial regression lines with 95% confidence intervals per frailty category (bold coloured lines), based on frailty index score at the study endpoint (concentrations are scaled to percentage of maximum concentration per marker). This complements the analysis in (a) and visualises marker levels evolving over time. The frailty categories in (b) were used for illustration purposes only; in the statistical analyses, the continuous frailty index score was used. Inflammatory markers are displayed when their AUCs showed an association with the frailty index score at the endpoint in at least one of the sexes. Plot area backgrounds in (b) are rendered in grey if no association was found. (c) Frailty index score (y‐axis) versus fold change in inflammatory markers over the 20‐year period (x‐axis). A vertical reference line of no increase (fold change of 1.0) is shown in bold grey. (d) Frailty index score (y‐axis) versus average body mass index (BMI) over time, expressed as AUC values (x‐axis).
Inflammatory markers, BMI and waist circumference, and frailty
We investigated the relationship between the inflammatory marker profile and two measures of body fat, namely body mass index (BMI) and waist circumference. Average BMI increased with age in both sexes over the 20 years of follow‐up (men, ρ = 0.24; women, ρ = 0.37; Supplementary figure 4a). In both men and women, greater BMI corresponded to higher frailty scores (Figure 4d). In women, average BMI and waist circumference during the follow‐up were positively associated with the AUCs of CRP (ρ = 0.55) and of sIL‐6R (ρ = 0.28; Supplementary figure 4b and c). In men, a larger waist circumference was positively associated with CRP.
We further analysed how adjusting for BMI influenced these associations. Apart from the association between CRP and frailty, this revealed an association of BDNF with frailty in men that was not found without adjusting for BMI (ρ = 0.35 and ρ = 0.21, respectively).
Prediction of frailty with multiple inflammatory marker trajectories
Since multiple associations were found between inflammatory marker trajectories and frailty, we further investigated how well the AUCs of all the biomarkers combined would predict the frailty index score using a random forest algorithm with BMI and age at the last measurement also included in the model. The predictive accuracy was 12% in men and 10% in women. With predictive accuracy not being greater, only signification can be given to the top results of the algorithm (Figure 5). These top results confirmed findings of the association study: BMI and CRP were the most important predictors (in men and women), followed closely by sCD14 (only in women).
Figure 5.
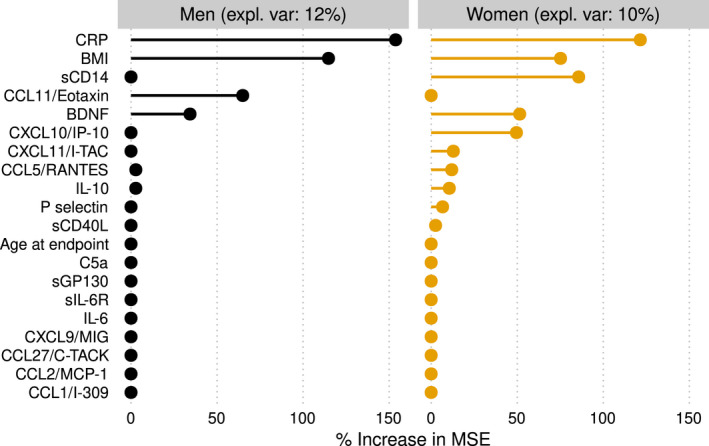
Variable importance of the inflammatory marker concentrations over the previous 20 years (area under the curve) per individual in predicting frailty at the study endpoint using a random forest algorithm. Age at the study endpoint and BMI were included as variables in the model. Expl. Var: explained variance. % increase in mean‐squared error: percentage increase in mean‐squared error of the prediction of frailty after the variable is replaced by ‘random noise’. A higher value thus means that the variable is more important in predicting frailty.
Trajectories of inflammatory markers related to an increase in frailty
Next, we studied whether the development of low‐grade inflammation is related to the onset of frailty. This was done in two different ways. First, we investigated whether chronic low‐grade inflammation was related to an increase in frailty over a period of 5 years, that is between measurement rounds 5 and 6, for which we had enough data to calculate the frailty index. We found no evidence for this over such a relatively brief period of time (Supplementary table 2). Second, to prospectively investigate the risk of becoming frail over a longer period of time, we selected a subgroup of individuals who were ‘healthy’ at study baseline. Being 'healthy' was defined using an alternative health index with fewer items, which could be assessed at study baseline (score of 9 or 10 out of 10 on the healthy ageing index score; 31 women and 27 men; Figure 6a). 11 We then related the frailty score at the study endpoint to the levels of inflammatory markers over time. In women, frailty development was associated with higher AUCs of CRP and sIL‐6R (ρ = 0.44 and 0.56, respectively; Figure 6b and c). As participants who became frail tended to have higher BMIs (Figure 6d), we also adjusted for BMI, which resulted in weaker associations (correlation coefficient of frailty and sIL‐6R: ρ = 0.49; of frailty and CRP: ρ = 0.22). Thus, it is likely that BMI plays an important role in shaping the relation between chronic inflammation and frailty.
Figure 6.
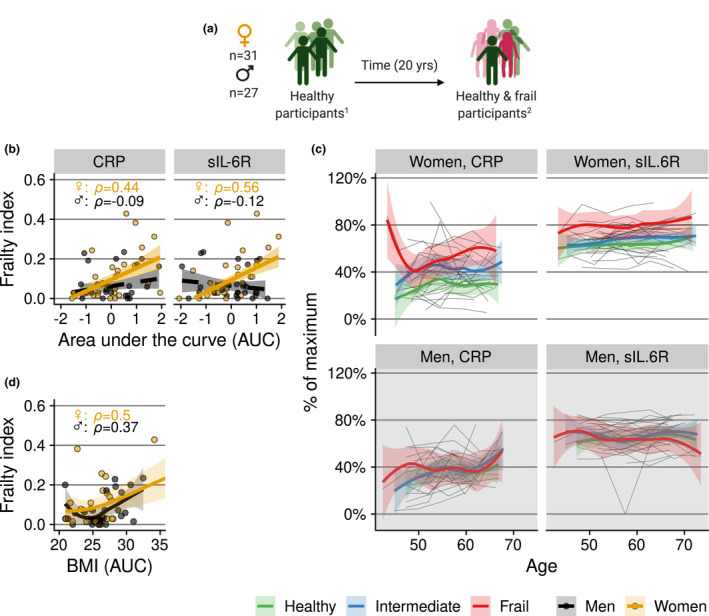
(a) Subgroup of selected participants who were ‘healthy’ at baseline. 1Health status at baseline was defined by a healthy ageing index (‘healthy’ taken to be a score of 9 or 10 out of 10). 2 Health status at the endpoint was defined by a frailty index score. (b, c) Relation of frailty at the study endpoint with trajectories of CRP and sIL‐6R in the subgroup of people who were ‘healthy’ at study baseline. To capture the cumulative ‘exposure’, trajectories of CRP and sIL‐6R in (b) and of BMI in (d) are expressed as the area under the concentration/BMI versus time curve per individual. Grey lines in (c) are individual trajectories. Bold coloured lines in (b) are (robust) linear regression lines, and in (c) and (d), local polynomial regression lines with 95% confidence intervals. In (c), the colour denotes the ‘frailty category’ at the study endpoint. Frailty categories are used for visualisation of the longitudinal trajectory; for the statistical analyses, the continuous frailty index score was used. Concentrations in (c) are scaled to percentage of maximum concentration per marker.
Trajectories of inflammatory markers related to lung function
Lung function is an important determinant of health that is known to decline with age, and for which clinically accepted quantitative measures have been defined. To investigate whether chronic inflammation is associated with a sharper decline in lung function with age, we related inflammatory marker trajectories over the previous 20 years to spirometric measurements at the endpoint, in particular the forced expiratory volume in one second (FEV1) and the forced vital capacity (FVC). In women, higher CRP levels were associated with both smaller FEV1 and FVC (correlation of the AUC of CRP with ρ = −0.48 for both). In contrast, higher levels of CXCL11 and of sCD40L were associated with greater FEV1 (ρ = 0.33 for both; Figure 7a). In men, no associations were found between the inflammatory marker trajectories and the FEV1. In conclusion, stronger lung function decline is associated with higher CRP levels and with lower concentrations of CXCL11 and of sCD40L in women only.
Figure 7.
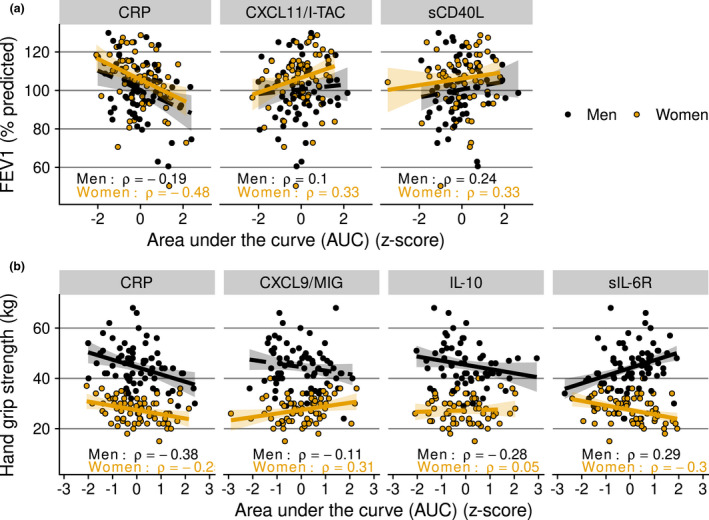
Inflammatory marker trajectories in men and women of those that showed an association with physiological clinical parameters of ageing at the study endpoint. (a) Lung function assessed by forced expiratory volume in one second (FEV1); and (b) handgrip strength measured as a proxy for physical strength. As a measure of cumulative ‘exposure’, inflammatory marker levels were assessed as the area under the inflammatory marker concentration versus time curve per individual (AUC) during the 20‐year follow‐up. AUC values were transformed to z‐scores for visualisation. % predicted: FEV1 value compared with the reference values from the Global Lung Initiative, 60 specific for age, length and sex. An association is indicated by a continuous trendline; otherwise, a dashed trendline is shown.
Trajectories of inflammatory markers related to handgrip strength
Another important determinant of health that declines with advancing age is muscle strength. We related handgrip strength, as measured with a dynamometer, to inflammatory marker trajectories (Figure 7b). In both sexes, we found a negative association between the AUCs of CRP trajectories and handgrip strength. But only in men did this association hold out after adjusting for BMI, a potential confounder. Also only in men, a negative association was found between IL‐10 trajectories and handgrip strength (ρ = −0.28), also after adjusting for BMI.
Discussion
In this unique longitudinal study with 20 years of follow‐up data, we quantified the presence of chronic low‐grade inflammation in ageing men and women using multiple inflammatory markers. Our main findings are that low‐grade chronic inflammation is associated with frailty and could be an important mediator of the relationship between BMI and frailty. CRP, sIL‐6R, sCD14 and sCD40L were identified as the most important biomarkers. The associations of biomarkers with frailty were stronger and more numerous in women than in men, although with advancing age, inflammatory marker trajectories became more similar between the sexes. Levels of several immune markers were found to change simultaneously during ageing in our study population, indicating possible relationships between these markers, but not all age‐related biomarkers were related to frailty.
This study revealed that changes in markers of the IL‐6 pathway, of platelet activation and of monocyte activation are associated with clinically relevant health outcomes at older age, in particular frailty. Body fat turned out to be an important factor in this process, since BMI and waist circumference were shown to be related to both frailty and markers of the IL‐6 pathway. However, the power to predict frailty with the inflammatory marker trajectories was weak, probably because of the heterogeneity in the study population and the small numbers of individuals.
Age‐related increase in inflammatory marker levels
We detected an increase in multiple biomarker levels with advancing age, which is in line with studies reporting chronic low‐grade inflammation at older age. 2 , 12 Both in men and in women, IFN‐γ‐related chemokines clearly changed with age, resulting in higher concentrations of CXCL10 and CXCL11 at older ages. Elevated concentrations of these IFN‐γ‐induced and structurally related chemokines could be a sign of activation of both the innate and the adaptive immune systems. This could be clinically important, since these chemokines have been proposed as possible biomarkers for heart failure. 13 CXCL10 has previously been described to increase with age in studies with shorter follow‐up times. 14 , 15 Also, CCL27 levels increased with age. This is possibly related to an accumulation of skin damage, since CCL27 is a cytokine involved in T‐cell‐mediated homing to the skin. 16 , 17
In women, other inflammatory markers were also found to increase with advancing age. This included the IL‐6 pathway‐related markers CRP and sIL‐6R, and two chemokines involved in innate immune cell activation, namely CCL2 and CCL11. These sex‐specific increases all seemed to reach a plateau at around the age of 60 years. Differences in the immune profile between men and women could be because of hormonal differences, as stated previously. 18 , 19 This is in line with our data, which suggest that the hormonal shifts of menopause can partially explain why concentrations of multiple inflammatory markers increase over time in women before the age of 60, and then level off. Of note is that while sIL‐6R and CRP were found to increase with age in women, IL‐6 levels were not. This may be explained by the molar excess of sIL‐6R in the circulation, which naturally binds IL‐6 and thereby may limit its detection. Thus, IL‐6 concentrations as measured in plasma are likely an underestimation of the biological activity of IL‐6 via both the classical and trans‐signalling pathways. 20 Still, we expected to find an association between IL‐6 levels and age. IL‐6 levels were found to be higher at older age in several previous studies, 6 , 21 , 22 which is why it is thought to be a key player in chronic low‐grade inflammation in the elderly. This discrepancy may further be because of the relatively small sample size in our longitudinal study and the relatively narrow age range of the participants at study endpoint, when frailty was assessed, in comparison with these prior studies. However, other studies also did not find a relation between IL‐6 and age. 15 , 23 , 24 One of these also claimed this was because of the limited age range of its participants. 24 Interestingly, another study on this topic found similar elevations of sIL‐6R and CRP, but not IL‐6, with frailty. 25 Taken together, all studies point to associations of IL‐6 pathway markers with frailty.
Chronic low‐grade inflammation related to (an increase in) frailty
As expected, in line with our previous study, 7 the association of higher CRP levels in frailer men and women was detected in this subcohort again. In fact, CRP turned out to be one of the inflammatory markers with the strongest association with frailty and with the chance of becoming frail over time. This was followed by sIL‐6R in women, indicative of involvement of the IL‐6 pathway. Previous literature about immune marker alterations preceding the risk of becoming frail is limited, with one study showing associations between higher CRP and the risk of becoming frail. 5 In another study, associations were reported between IL‐6 and ‘incident frailty’ over a period of 5 years. sIL‐6R was not measured in that study. 15 Probably an important driver in this process is being (over)weight, since the associations with frailty in our study disappeared after adjustments for BMI. A plausible reason for this is that a higher BMI both increases the risk of becoming frail and raises inflammatory biomarker levels. 4 Excessive adipose tissue, especially visceral adipose tissue, is known to contribute to chronic inflammation by increased secretion of adipokines and inflammatory cytokines, and by inducing accumulation of macrophages. 4 , 26 , 27 It has been suggested that waist circumference is a better estimate of visceral pro‐inflammatory body fat than BMI in the elderly. 28 Indeed, we saw stronger associations of IL‐6 pathway markers with waist circumference than with BMI in men. Of note is that even after adjusting for BMI, frailer women also showed higher levels of sCD14, mostly because of an increase in this marker's level in the preceding 20 years. sCD14 is a marker of monocyte activation since it is released from monocytes after stimulation with Toll‐like receptor ligands, 29 in particular lipopolysaccharide (LPS). sCD14 also binds to LPS, and it has been suggested that sCD14 is necessary for platelets and endothelial cells to respond adequately to LPS. 30 , 31 Thus, sCD14 might be important in the defence against bacterial infections. Its increase in concentration over time in frailer women is in line with previous reports showing higher monocyte numbers in frailer women. 32 , 33
As low‐grade inflammation occurs more frequently in frail participants, most associations between inflammatory marker levels and frailty were positive, as expected. Yet in women, we also found a negative association of sCD40L levels with frailty (not after adjustment for BMI). sCD40L, which is released mainly by activated platelets, 34 , 35 is a soluble membrane glycoprotein involved in B‐cell responses, 36 and in macrophage and monocyte signalling. 37 It thus plays an important role in the communication between innate and adaptive immune responses. sCD40L is commonly seen as a pro‐inflammatory molecule, 38 but it might also have an immunosuppressive role, as has been suggested in studies on HIV infection and cancer, by inducing the expansion of regulatory T‐cell populations, 36 , 39 and suppressing T‐cell proliferation. 39 This could imply that lower levels of sCD40L indicate a failing regulation of immune responses, with higher low‐grade inflammation as a result.
Lung function and physical strength
Elevated CRP levels have often been found to be associated with unfavorable health outcomes such as impaired lung function 40 and reduced handgrip strength. 41 , 42 , 43 We largely confirmed these results in our study, showing that inflammatory marker levels had persistently been higher over an age range of 20 years in people who were found to have reduced lung function and handgrip strength at the end of this period. This confirms that low‐grade inflammation in a proportion of the older population is indeed ‘chronic’. In addition, only in women, higher levels of sCD40L and CXCL11 were found to be associated with better lung function measures. We are not aware that these markers have been previously reported to be related to lung function in a community‐dwelling population. While as discussed above, sCD40L was shown in previous studies to possibly have an anti‐inflammatory role, this is not the case with CXCL11. In fact, we would rather expect a negative correlation of this marker with lung function because of its pro‐inflammatory effect in the IFN‐γ pathway. Such a negative association was seen in cross‐sectionally performed studies on sarcoidosis 44 and other diseases affecting the lung. 45 These showed higher levels of CXCL11 to be associated with worse lung function, which is in contrast with our findings. Further studies are needed to confirm our findings in a community‐dwelling population, and to explain why in our study this relationship was found only in women.
Interestingly, in men we also found a negative correlation of handgrip strength with concentrations of IL‐10, a cytokine commonly seen as anti‐inflammatory. A similar relationship of greater muscle strength (knee extension strength) and lower IL‐10 values was also described in a previous study, 46 although in most studies no association between IL‐10 and grip strength was detected. 47 , 48 Our results also show that IL‐10 correlates strongly with several pro‐inflammatory markers such as IL‐6, which is in line with previous studies. 15 , 46 Further research is needed to confirm the results and to investigate whether these elevated IL‐10 levels could be a compensatory response to low‐grade inflammation.
Strengths and limitations
Our longitudinal analysis of a well‐described cohort is a major strength of this study. To the best of our knowledge, our study is unique in that an extensive panel of inflammatory markers was measured repeatedly at 5‐year intervals in the same individuals over a period of 20 years. Since variation within individuals was much smaller than the variation between individuals, we had more power to detect changes in protein levels with advancing age than in cross‐sectional studies, even when these have larger sample sizes. Furthermore, while the sample size for cross‐sectional analyses of a single analyte was limited compared with large cohort studies, our study made use of a spectrum of inflammatory markers, thus giving a more comprehensive insight into inflammation than studies that measured only a few inflammatory markers. Also, since inflammatory marker levels can vary considerably between studies because of methodological differences, an advantage of our study was that all inflammatory markers were measured in the same assay and that all samples of the same individual were measured on the same plate, giving the opportunity to relate them to each other. A limitation is that we did not have a frailty index measurement at baseline, which restricted our ability to analyse the relationship between levels of inflammatory markers and the risk of becoming frail. Thus, while we assumed that the participants who we selected to be healthy at baseline had a low frailty index score, this selection was based on a relatively simple health score rather than the frailty index score that we used at the study endpoint. Other limitations are that the study population might not be fully representative of the general population, given the relative small number of participants in this study and possibly selective dropout of participants, which is commonly seen in longitudinal cohorts. However, the response rate in the Doetinchem Cohort Study is good, generally above 70%. 49 A further potential limitation is that the prolonged storage of the plasma samples could have affected the sample quality. However, the samples were consistently stored at low temperature and the trajectories were remarkably stable within individuals, indicating that protein degradation was probably of minor influence.
Concluding remarks
Our exploratory study gives a unique insight into longitudinal changes in the immune profile of ageing men and women over a period of about 20 years, and revealed multiple associations of immune markers with clinically relevant outcomes at the study endpoint, such as frailty, handgrip strength and lung function. More specifically, the findings implicate an important role for the IL‐6 pathway as indicated by elevated markers such as CRP and, in women, monocyte activation as indicated by sCD14. As BMI and waist circumference are related to elevations of immune markers in the IL‐6 pathway, chronic inflammation might be an important mediator of the relationship between BMI and frailty.
Methods
The Doetinchem Cohort Study (DCS) is a population‐based longitudinal study of 7769 participants at study entry that have been followed since 1987. 49 , 50 Every 5 years, plasma samples were taken and data regarding the participants' lifestyle and health were collected. For the present study, a subgroup of 144 people aged 65–75 years were selected from the DCS, stratified by sex and with equal numbers of participants classified as relatively healthy, frail or of intermediate health status, as described elsewhere. 7 In brief, the healthy ageing/frailty were defined as those with the 15% lowest/highest frailty index score (see below) among their age‐ and sex‐matched peers. The individuals selected were still actively participating in the study at least until 2016 and had taken part in at least four out of the previous five assessment rounds of the DCS. The measurements of the present study were performed as an extension of the regular DCS assessments, with the sample size being restricted by budgetary and logistic constraints.
Frailty index
Details on the frailty index score used in this study can be found elsewhere. 7 Its definition was based on previous studies. 51 , 52 In our implementation, it consists of 35 potential age‐ and health‐related ‘deficits’, covering a broad range of domains, including cognitive, physical and psychological functioning. The score used in this study differed from the one previously used and validated in the DCS 7 in two respects. First, it was updated including the latest available data. Second, being overweight was left out of the score so that we could better investigate the influence of being overweight on our results. Details and cut‐off values for all of the 35 deficits are shown in Supplementary table 3. All deficits are bound between zero (best possible score) and one (worst possible), and the frailty index is the average score per individual. The frailty index was calculated in the two latest available DCS assessment rounds (rounds 5 and 6). Frailty categories (‘healthy’, ‘intermediate’ and ‘frail’; see DCS subgroup selection above) were only used for visualisation in some figures; in all statistical analyses, the continuous frailty index score was used.
Healthy ageing index
An alternative health status score, the healthy ageing index, was measured at study baseline and was used to select a subgroup of participants that were ‘healthy’ at baseline. The score is based on health deficits defined by systolic blood pressure, plasma glucose concentration, plasma creatinine concentration, lung function (forced vital capacity) and global cognitive function, 53 all treated as categorical variables, as described elsewhere. 11 Except for plasma glucose levels, the deficits were comparable to some of those included in the frailty index score. Every item has a similar weight in the score, and the score ranges from 0 to 10, with 10 being the best and 0 the worst possible outcome.
Measurements of inflammatory markers
Plasma samples were collected repeatedly from the same individuals over a period of approximately 20 years, from 1991 to 2017, and stored at −80°C. 49 , 50 For this study, the samples were thawed at room temperature, and shortly after thawing, a large panel of inflammatory markers was measured, consisting of the following cytokines: chemokines and soluble receptors – C‐C motif chemokine ligand (CCL)1 (alternative name I‐309), CCL2 (MCP‐1), CCL5 (RANTES), CCL11 (Eotaxin), CCL27 (C‐TACK), C‐X‐C motif chemokine ligand (CXCL)9 (MIG), CXCL10 (IP‐10), CXCL11 (I‐TAC), interleukin (IL)‐1β, IL‐10, IL‐6, soluble CD40 ligand (sCD40L), soluble CD14 (sCD14), soluble IL‐2 receptor (sIL‐2R), soluble IL‐6 receptor (sIL‐6R), soluble glycoprotein 130 (sGP130), complement factor 5a (C5a), brain‐derived neurotrophic factor (BDNF) and P‐selectin. Concentrations of the markers were measured using a validated bead‐based multiplex immunoassay (Flexmap 3D®; Luminex, Austin, TX, USA) at the Multiplex Core Facility Lab, University Medical Center Utrecht, The Netherlands. That laboratory has expertise in measuring combinations of cytokines and chemokines by Luminex assays in various patient cohorts and validated each bead region per cytokine. 54 , 55 All detection antibodies were coupled to magnetic beads and were tested for specificity. To minimise assay variation within individuals, all samples from the same participant (n = 6) were measured on the same plate. As an additional quality control anti‐bead antibodies were detected in the plasma samples, these antibodies may give false‐positive results in a bead‐based assay. Measurement of samples took place in 2018, in three independent batches. Levels of C‐reactive protein (CRP) in plasma had been measured previously in DCS rounds 2, 3, 4 and 5. 56 Apart from sIL‐2R and IL‐1β, the proportion of samples in which concentrations were below the limit of quantification (LOQ) was less than 20% for all inflammatory markers. Concentrations below the LOQ were imputed with a random value below the LOQ based on the maximum‐likelihood estimation. 57 sIL‐2R and IL‐1β were excluded from analysis since more than 65% of the samples were below the LOQ. For IL‐6 and IL‐10, 85 samples out of a total of 689 (from 15 of the 144 participants) were found to contain bead binding antibodies and were therefore excluded. This reduced the sample size for these cytokines to n = 129 out of the total n = 144 individuals (n = 65 men and n = 64 women). A sensitivity analysis including these samples showed that their exclusion did not affect the outcomes of our statistical analyses. The sample size for sGP130 was also not complete (n = 67 men and n = 66 women) since sGP130 was not measured in the first batch of cytokine measurements.
Anthropometric measurements
Body mass index was calculated as body mass in kilograms divided by the square of the height in metres. A tape measure was used to measure waist circumference, which was done with the participant in an upright standing position and wrapping the tape measure horizontally around the waist, at the midpoint between the lower rib and the iliac crest.
Handgrip strength
Participants applied as much force as possible with their dominant hand to a hydraulic dynamometer (Jamar, Patterson Medical, Warrenville, IL, USA), with their elbow at a 90° angle. The greatest applied force out of three separate attempts was used.
Lung function
At the study endpoint, a heated pneumotachometer (E Jaeger, Wurzburg, Germany) was used to measure forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) as previously explained in detail. 58 Spirometric measurements were done and evaluated according to the American Thoracic Society and European Respiratory Society guidelines. 59 FEV1 and FVC values were transformed to percentage of predicted values, with predictions based on the Global Lung Initiative's (2012) reference values 60 specific for age, length, sex and ethnicity, using the R macro provided on https://www.ers‐education.org/guidelines/global‐lung‐function‐initiative/spirometry‐tools.aspx.
Statistical analysis
Longitudinal biomarker trajectory estimators
To estimate the ‘inflammatory burden over time’ for each individual and each inflammatory marker, the area under the concentration versus time curve (AUC) was calculated and was divided by the total time of follow‐up to adjust for possible differences in follow‐up time. The AUC values of every inflammatory marker were subsequently log‐transformed and mean‐centred per measurement batch to adjust for possible confounding batch effects. To estimate the changes in inflammation over time, we also calculated the within‐individual proportional increase or decrease per year. To do this, we used a slope of log‐transformed concentration over time per individual, calculated in a median‐based linear model, 61 as an estimator for rate of change over time. A median‐based linear model is less sensitive to outliers or to deviations from a normal distribution than ordinary linear regression models. 62 , 63
Association studies
All the results were adjusted for multiple testing by controlling the false discovery rate 64 separately for each analysis. A nominal false discovery rate of at most 15% (meaning, roughly speaking, an average of 1.5 false discoveries per 10 discoveries presented as such) was tolerated in view of the number of tests carried out. For testing associations, we used the permutation versions of the Spearman and Wilcoxon–Mann–Whitney tests blocked for certain background or confounding variables (e.g. age) as implemented in the R package coin 65 with P‐values estimated by simulation. The concept of blocking in experimental designs is explained elsewhere. 66 Strengths of associations were thus expressed as Spearman's ρ coefficient of correlation, ranging between 0 (no correlation) and 1 (perfect correlation).
To investigate whether changes in plasma concentrations of inflammatory markers change with age, we carried out Spearman's tests between each marker's concentrations and age blocking by a participant. Thus, we focused on within‐individual increases in the marker's concentration during the 20 years of follow‐up. Should a marker tend to increase or decrease with age, the Spearman correlation between the marker's concentration and a participant's age should tend to exhibit consistently large or consistently small values across the cohort.
Single associations between an inflammatory marker's trajectory (the AUC) and sex were tested with the Wilcoxon–Mann–Whitney test, blocking for age at last measurement (in two categories, 65–70 and 70–75 years) and BMI (three categories) to adjust for these variables.
Associations between inflammatory markers at the study's endpoint were adjusted for age at last measurement and the immunoassay batch number (three batches). Longitudinal relationships between pairs of biomarkers were tested by investigating synchronous changes in biomarker levels. For every pair of markers, we investigated whether the interval of maximum increase in one marker coincided with that in another marker more often than what might be expected by chance, by carrying out one‐tailed binomial tests postulating the ‘probability of success’ of ¼ under the null hypothesis of no association.
To address our research question as to whether chronic low‐grade inflammation is related to frailty, we carried out several association studies, all separately for men and women. First, we tested associations, with Spearman's tests, between the AUC of each marker's inflammatory trajectory and the frailty index score, adjusted for age at last measurement. Analogous associations were tested using BMI as an additional blocking variable, to get an idea about the importance of BMI in these relationships. Then, we investigated the association between the individual's slope of the inflammatory protein trajectories and frailty at the endpoint, using Spearman's tests blocked by the baseline level of the marker (in tertiles) and BMI. Next, the relation was investigated between every immune marker trajectory and change in frailty index score from round 5 to round 6, adjusting the associations for age at last measurement and for the initial frailty index score at round 5 (using tertiles for blocking). The latter was done to adjust for a possible regression to the mean effect and to give more weight to smaller increases in frailty index of people that already had a high index in the beginning.
The tested relationships of the inflammatory markers with lung function parameters were also adjusted for smoking with two blocking categories: current smokers versus non‐smokers or former smokers. The relationships with handgrip strength were adjusted for age and were repeated with adjustment for BMI.
Prediction analysis
We used a random forest prediction algorithm 67 to investigate whether frailty could be predicted using several dependent variables, namely the AUCs of all the inflammatory markers, age at the endpoint and BMI. The overall performance of the prediction analysis was assessed in terms of the percentage explained variance. The relative weights of the independent variables in predicting frailty were assessed by ranking their ‘importance’. This variable importance was quantified in terms of the percentage increase in mean‐squared error (MSE) when the effect of that variable was removed.
All statistical analyses were performed with R version 3.6.2, 68 with several general packages for data processing 69 , 70 and for visualisation. 71 , 72 , 73 Correlations between inflammatory markers were visualised using the corrplot package. 74
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Leonard Daniël Samson: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Validation; Visualization; Writing – original draft; Writing – review & editing. Anne‐Marie Buisman: Conceptualization; Funding acquisition; Supervision; Writing – review & editing. José A Ferreira: Formal analysis; Methodology; Writing – review & editing. H Susan J Picavet: Resources; Writing – review & editing. W M Monique Verschuren: Resources; Supervision; Writing – review & editing. Annemieke M H Boots: Conceptualization; Project administration; Supervision; Writing – review & editing. Peter Engelfriet: Conceptualization; Funding acquisition; Investigation; Methodology; Supervision; Writing – review & editing.
Supporting information
Supplementary Material
Acknowledgments
We thank the DCS respondents for their participation in the study and the epidemiologists and fieldworkers of the Municipal Health Service in Doetinchem for their contribution to the data collection. In addition, we thank Petra Vissink for her help with the blood sample collection. Figure 1, Figure 7a and the graphical abstract in the online version were created using BioRender.com.
References
- 1. United Nations . World Population Ageing 2015. UN; 2017. https://www.un‐ilibrary.org/population‐and‐demography/world‐population‐ageing‐2015_88fa44e7‐en (accessed 3 July 2020). [Google Scholar]
- 2. Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Healthspan 2013; 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘Garb‐aging’. Trends in Endocr Metab 2017; 28: 199–212. [DOI] [PubMed] [Google Scholar]
- 4. Ghigliotti G, Barisione C, Garibaldi S et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation 2014; 37: 1337–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gale CR, Baylis D, Cooper C, Sayer AA. Inflammatory markers and incident frailty in men and women: the English Longitudinal Study of Ageing. Age 2013; 35: 2493–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Puzianowska‐Kuźnicka M, Owczarz M, Wieczorowska‐Tobis K et al. Interleukin‐6 and C‐reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing 2016; 13: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samson LD, Boots AMH, Verschuren WMM, Picavet HSJ, Engelfriet P, Buisman A‐M. Frailty is associated with elevated CRP trajectories and higher numbers of neutrophils and monocytes. Exp Gerontol 2019; 125: 110674. [DOI] [PubMed] [Google Scholar]
- 8. Walker KA, Walston J, Gottesman RF, Kucharska‐Newton A, Palta P, Windham BG. Midlife systemic inflammation is associated with frailty in later life: the ARIC study. J Gerontol A Biol Sci Med Sci 2019; 74: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morrisette‐Thomas V, Cohen AA, Fülöp T et al. Inflamm‐aging does not simply reflect increases in pro‐inflammatory markers. Mech Ageing Dev 2014; 139: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta‐analysis. Exp Gerontol 2017; 89: 30–40. [DOI] [PubMed] [Google Scholar]
- 11. Dieteren CM, Samson LD, Schipper M et al. The healthy aging index analyzed over 15 years in the general population: the Doetinchem Cohort study. Prev Med 2020; 139: 106193. [DOI] [PubMed] [Google Scholar]
- 12. Franceschi C, Bonafè M, Valensin S et al. Inflamm‐aging: an evolutionary perspective on immunosenescence. Ann NY Acad Sci 2006; 908: 244–254. [DOI] [PubMed] [Google Scholar]
- 13. Altara R, Gu Y‐M, Struijker‐Boudier HAJ, Thijs L, Staessen JA, Blankesteijn WM. Left ventricular dysfunction and CXCR3 ligands in hypertension: from animal experiments to a population‐based pilot study. PLoS One 2015; 10: e0141394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hearps AC, Martin GE, Angelovich TA et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012; 11: 867–875. [DOI] [PubMed] [Google Scholar]
- 15. Hsu B, Hirani V, Cumming RG et al. Cross‐sectional and longitudinal relationships between inflammatory biomarkers and frailty in community‐dwelling older men: the concord health and ageing in men project. J Gerontol A Biol Sci Med Sci 2019; 74: 835–841. [DOI] [PubMed] [Google Scholar]
- 16. Homey B, Alenius H, Müller A et al. CCL27–CCR10 interactions regulate T cell–mediated skin inflammation. Nat Med 2002; 8: 157–165. [DOI] [PubMed] [Google Scholar]
- 17. Richmond JM, Strassner JP, Essien KI, Harris JE. T‐cell positioning by chemokines in autoimmune skin diseases. Immunol Rev 2019; 289: 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bupp MRG. Sex, the aging immune system, and chronic disease. Cell Immunol 2015; 294: 102–110. [DOI] [PubMed] [Google Scholar]
- 19. Furman D, Hejblum BP, Simon N et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA 2014; 111: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rose‐John S. The soluble interleukin 6 receptor: advanced therapeutic options in inflammation. Clin Pharmacol Ther 2017; 102: 591–598. [DOI] [PubMed] [Google Scholar]
- 21. Ferrucci L, Corsi A, Lauretani F et al. The origins of age‐related proinflammatory state. Blood 2005; 105: 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giuliani N, Sansoni P, Girasole G et al. Serum interleukin‐6, soluble interleukin‐6 receptor and soluble gp130 exhibit different patterns of age‐ and menopause‐related changes. Exp Gerontol 2001; 36: 547–557. [DOI] [PubMed] [Google Scholar]
- 23. Beharka AA, Meydani M, Wu D, Leka LS, Meydani A, Meydani SN. Interleukin‐6 production does not increase with age. J Gerontol A Biol Sci Med Sci 2001; 56: B81–B88. [DOI] [PubMed] [Google Scholar]
- 24. Van Epps P, Oswald D, Higgins PA et al. Frailty has a stronger association with inflammation than age in older veterans. Immun Ageing 2016; 13: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu Y, Tan CTY, Nyunt MSZ et al. Inflammatory and immune markers associated with physical frailty syndrome: findings from Singapore longitudinal aging studies. Oncotarget 2016; 7: 28783–28795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 2017; 4: 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crow RS, Lohman MC, Titus AJ et al. Association of obesity and frailty in older adults: NHANES 1999–2004. J Nutr Health Aging 2019; 23: 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29: 1263–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Damien P, Cognasse F, Eyraud M‐A et al. LPS stimulation of purified human platelets is partly dependent on plasma soluble CD14 to secrete their main secreted product, soluble‐CD40‐Ligand. BMC Immunol 2015; 16: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lloyd‐Jones KL, Kelly MM, Kubes P. Varying importance of soluble and membrane CD14 in endothelial detection of lipopolysaccharide. J Immunol 2008; 181: 1446–1453. [DOI] [PubMed] [Google Scholar]
- 32. Leng SX, Xue QL, Tian J, Huang Y, Yeh SH, Fried LP. Associations of neutrophil and monocyte counts with frailty in community‐dwelling disabled older women: results from the Women’s Health and Aging Studies I. Exp Gerontol 2009; 44: 511–516. [DOI] [PubMed] [Google Scholar]
- 33. Samson LD, Boots AMH, Ferreira JA et al. In‐depth immune cellular profiling reveals sex‐specific associations with frailty. Immun Ageing 2020; 17: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Danese S. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut 2003; 52: 1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagasawa M, Zhu Y, Isoda T et al. Analysis of serum soluble CD40 ligand (sCD40L) in the patients undergoing allogeneic stem cell transplantation: platelet is a major source of serum sCD40L. Eur J Haematol 2005; 74: 54–60. [DOI] [PubMed] [Google Scholar]
- 36. Jenabian MA, Patel M, Kema I et al. Soluble CD40‐ligand (sCD40L, sCD154) plays an immunosuppressive role via regulatory T cell expansion in HIV infection. Clin Exp Immunol 2014; 178: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suttles J, Stout RD. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin Immunol 2009; 21: 257–264. [DOI] [PubMed] [Google Scholar]
- 38. Aloui C, Prigent A, Tariket S et al. Levels of human platelet‐derived soluble CD40 ligand depend on haplotypes of CD40LG‐CD40 ‐ ITGA2. Sci Rep 2016; 6: 24715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang J, Jochems C, Talaie T et al. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood 2012; 120: 3030–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahmadi‐Abhari S, Kaptoge S, Luben RN, Wareham NJ, Khaw K‐T. Longitudinal association of C‐reactive protein and lung function over 13 years: the EPIC‐Norfolk Study. Am J Epidemiol 2014; 179: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing 2014; 43: 10–12. [DOI] [PubMed] [Google Scholar]
- 42. Smith L, Yang L, Hamer M. Handgrip strength, inflammatory markers, and mortality. Scand J Med Sci Sports 2019; 29: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 43. Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta‐analysis. Ageing Res Rev 2020; 64: 101185. [DOI] [PubMed] [Google Scholar]
- 44. Arger NK, Ho M, Woodruff PG, Koth LL. Serum CXCL11 correlates with pulmonary outcomes and disease burden in sarcoidosis. Respir Med 2019; 152: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kameda M, Otsuka M, Chiba H et al. CXCL9, CXCL10, and CXCL11; biomarkers of pulmonary inflammation associated with autoimmunity in patients with collagen vascular diseases–associated interstitial lung disease and interstitial pneumonia with autoimmune features. PLoS One 2020; 15: e0241719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Calvani R, Marini F, Cesari M et al. Systemic inflammation, body composition, and physical performance in old community‐dwellers. J Cachexia Sarcopenia Muscle 2017; 8: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cesari M, Penninx BWJH, Pahor M et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2004; 59: M242–M248. [DOI] [PubMed] [Google Scholar]
- 48. Goldeck D, Pawelec G, Norman K et al. No strong correlations between serum cytokine levels, CMV serostatus and hand‐grip strength in older subjects in the Berlin BASE‐II cohort. Biogerontology 2016; 17: 189–198. [DOI] [PubMed] [Google Scholar]
- 49. Picavet HSJ, Blokstra A, Spijkerman AMW, Verschuren WMM. Cohort profile update: the Doetinchem Cohort Study 1987–2017: lifestyle, health and chronic diseases in a life course and ageing perspective. Int J Epidemiol 2017; 46: 1751–1751g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verschuren WM, Blokstra A, Picavet HS, Smit HA. Cohort profile: the Doetinchem Cohort Study. Int J Epidemiol 2008; 37: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 51. Collerton J, Martin‐Ruiz C, Davies K et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross‐sectional findings from the Newcastle 85+ Study. Mech Ageing Dev 2012; 133: 456–466. 10.1016/j.mad.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 52. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J 2001; 1: 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nooyens ACJ, Bueno‐de‐Mesquita HB, van Boxtel MPJ, van Gelder BM, Verhagen H, Verschuren WMM. Fruit and vegetable intake and cognitive decline in middle‐aged men and women: the Doetinchem Cohort Study. Br J Nutr 2011; 106: 752–761. [DOI] [PubMed] [Google Scholar]
- 54. Scholman RC, Giovannone B, Hiddingh S et al. Effect of anticoagulants on 162 circulating immune related proteins in healthy subjects. Cytokine 2018; 106: 114–124. [DOI] [PubMed] [Google Scholar]
- 55. de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Vaccine Immunol 2003; 10: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hulsegge G, Herber‐Gast GC, Spijkerman AM et al. Obesity and age‐related changes in markers of oxidative stress and inflammation across four generations. Obesity (Silver Spring) 2016; 24: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 57. Lubin JH, Colt JS, Camann D et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 2004; 112: 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Oostrom SH, Engelfriet PM, Verschuren WMM et al. Aging‐related trajectories of lung function in the general population—the Doetinchem Cohort Study. PLoS One 2018; 13: e0197250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Enright PL, Johnson LR, Connett JE, Voelker H, Buist AS. Spirometry in the lung health study: 1. Methods and quality control. Am Rev Respir Dis 1991; 143: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 60. Quanjer PH, Stanojevic S, Cole TJ et al. Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Komsta L. mblm: Median‐based Linear Models; 2019. https://CRAN.R‐project.org/package=mblm [Google Scholar]
- 62. Theil H. A rank‐invariant method of linear and polynomial regression analysis. Indag Math 1950; 12: 85–91. [Google Scholar]
- 63. Wilcox R. A Note on the Theil‐Sen regression estimator when the regressor is random and the error term is heteroscedastic. Biom J 1998; 40: 261–268. [Google Scholar]
- 64. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57: 289–300. [Google Scholar]
- 65. Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a class of permutation tests: the coin package. J Stat Soft 2008; 28: 1–27. [Google Scholar]
- 66. Krzywinski M, Altman N. Analysis of variance and blocking. Nat Methods 2014; 11: 699–700. [DOI] [PubMed] [Google Scholar]
- 67. Liaw A, Wiener M. Classification and regression by randomForest. R News 2002; 2: 18–22. [Google Scholar]
- 68. R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2019. https://www.R‐project.org/ [Google Scholar]
- 69. Wickham H, François R, Henry L, Müller K. dplyr: A grammar of data manipulation; 2020. https://CRAN.R‐project.org/package=dplyr
- 70. Wickham H, Henry L. tidyr: Tidy messy data; 2020. https://CRAN.R‐project.org/package=tidyr
- 71. Pedersen TL. Patchwork: The composer of plots; 2019. https://CRAN.R‐project.org/package=patchwork
- 72. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer‐Verlag New York, 2016. https://ggplot2.tidyverse.org [Google Scholar]
- 73. Wilke CO. cowplot: Streamlined plot theme and plot annotations for ‘ggplot2’; 2019. https://CRAN.R‐project.org/package=cowplot
- 74. Wei T, Simko V. R package ‘corrplot’: Visualization of a correlation matrix; 2017. https://github.com/taiyun/corrplot
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


