Abstract
The potential of covert pulmonary arteriovenous malformations (PAVMs) to cause early onset, preventable ischemic strokes is not well known to neurologists. This is evident by their lack of mention in serial American Heart Association/American Stroke Association (AHA/ASA) Guidelines and the single case report biased literature of recent years. We performed PubMed and Cochrane database searches for major studies on ischemic stroke and PAVMs published from January 1, 1974, through April 3, 2021. This identified 24 major observational studies, 3 societal guidelines, 1 nationwide analysis, 3 systematic reviews, 21 other review/opinion articles, and 18 recent (2017–2021) case reports/series that were synthesized. Key points are that patients with PAVMs have ischemic stroke a decade earlier than routine stroke, losing 9 extra healthy life-years per patient in the recent US nationwide analysis (2005–2014). Large-scale thoracic CT screens of the general population in Japan estimate PAVM prevalence to be 38/100,000 (95% confidence interval 18–76), with ischemic stroke rates exceeding 10% across PAVM series dating back to the 1950s, with most PAVMs remaining undiagnosed until the time of clinical stroke. Notably, the rate of PAVM diagnoses doubled in US ischemic stroke hospitalizations between 2005 and 2014. The burden of silent cerebral infarction approximates to twice that of clinical stroke. More than 80% of patients have underlying hereditary hemorrhagic telangiectasia. The predominant stroke mechanism is paradoxical embolization of platelet-rich emboli, with iron deficiency emerging as a modifiable risk factor. PAVM-related ischemic strokes may be cortical or subcortical, but very rarely cause proximal large vessel occlusions. Single antiplatelet therapy may be effective for secondary stroke prophylaxis, with dual antiplatelet or anticoagulation therapy requiring nuanced risk–benefit analysis given their risk of aggravating iron deficiency. This review summarizes the ischemic stroke burden from PAVMs, the implicative pathophysiology, and relevant diagnostic and treatment overviews to facilitate future incorporation into AHA/ASA guidelines.
Introduction
Pulmonary arteriovenous malformations (PAVMs), also known as pulmonary arteriovenous fistulae, are pathologic low-resistance, high-flow conduits between a pulmonary artery and vein without intervening capillaries.1 The resulting “right-to-left” shunt directs a proportion of the venous return from the right (pulmonary) to the left (systemic) circulation.1 Fundamentally, PAVMs allow for a portion of the right ventricular stroke volume (deoxygenated blood) to bypass gas exchange and processing within the pulmonary capillary bed, resulting in hypoxemia. In addition, the failure to filter venous thrombi, bacteria, and other vasoactive materials may result in acute ischemic stroke (AIS), cerebral abscess, and migraines (Figure 1).2 Unlike right-to-left shunts across the cardiac atrial septum, such as patent foramen ovale (PFO), where right-to-left flow is only intermittent,1,2 PAVMs provide a continuous right-to-left shunt until abrogated by treatments, usually embolization.2 PAVMs can be sporadic, but most commonly occur in the setting of multisystemic vascular dysplasias such as hereditary hemorrhagic telangiectasia (HHT).2,5
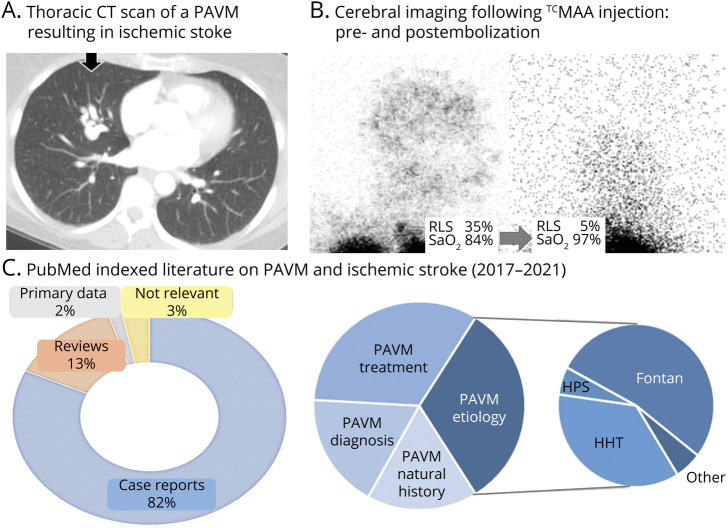
Figure 1. Pulmonary Arteriovenous Malformations and Right to Left Shunts.
(A) Thoracic CT scan of a pulmonary arteriovenous malformation (PAVM) in a patient admitted with acute ischemic stroke. (B) Diagnosis and quantification of pathologic right-to-left shunt (RLS) on right lateral head projections following injection of 99mTc-labeled albumin macroaggregates in a patient pre and post PAVM embolization, resulting in a visible reduction of shunt size and corresponding improvement in oxygen saturation (SaO2) levels.2,3 Note the intense activity in the lung apices is as expected, but that in the postembolization image, although the gain has been turned up, trivial cerebral activity is still visible. (C) PubMed-indexed English language literature (1 January 2017–April 3, 2021) on “pulmonary arteriovenous malformation” and “stroke” depicting a publication bias favoring case reports, especially those describing rare associations; for example, Fontan circulation and hepatopulmonary syndrome (HPS), rather than hereditary hemorrhagic telangiectasia (HHT), which is responsible for PAVMs in >70% of cases.4
Methods
We searched PubMed and the Cochrane databases for English-language studies published from January 1, 1974, through April 3, 2021, for randomized clinical trials (RCTs), meta-analyses, and observational studies (search terms are reported in the eAppendix in the Supplement). We also included studies referenced in review of the early PAVM literature performed previously by the senior author (C.L.S.)6 and manually searched the references of retrieved articles. Emphasis was given to selection of RCTs and to adult observational series with 50 or more patients with PAVM, as well as pediatric observational series with 20 or more patients (Table 1), for consideration of information of interest to a general neurology readership. Emphasis was also placed upon case reports and smaller series published in the past 3 years (Figure 1C) to capture a snapshot of current clinical practice.
Table 1.
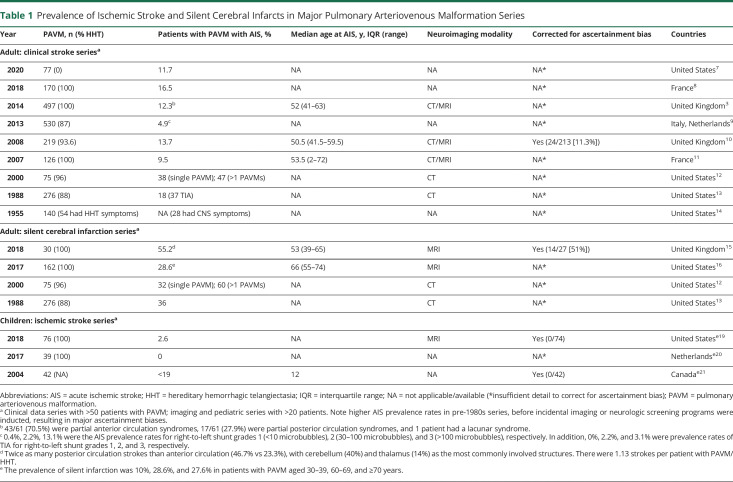
Prevalence of Ischemic Stroke and Silent Cerebral Infarcts in Major Pulmonary Arteriovenous Malformation Series
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Clinical Practice Guidelines for PAVMs and Ischemic Stroke
Pulmonary AVMs in patients with ischemic stroke pose a unique management challenge to clinicians as authoritative clinical practice guidelines are generally lacking. In the United States, there has been a historical absence of clinical practice guidelines addressing the acute management and secondary prevention of ischemic stroke in patients with PAVMs, including no recommendations from the American Thoracic Society or the American Heart Association/American Stroke Association (AHA/ASA). In fact, the condition of PAVMs has been mentioned in passing on only one occasion in the 1999–2021 AHA/ASA Guidelines,17 and have not been mentioned at all in the last 2 editions (2014 and 2021). Internationally, consensus position statements have addressed PAVM management, including from the British Thoracic Society (BTS),5 the Cardiovascular and Interventional Radiologic Society of Europe (CIRSE),18 and the Cochrane Database,4 all citing prevention of ischemic stroke as one of the major reasons to treat PAVMs, based on previously published primary literature (Table 1).
Epidemiology of Pulmonary AVMs and Ischemic Stroke
Advances in diagnostic imaging and clinical awareness have increased the frequency with which PAVMs are recognized.19 In an early US autopsy series, PAVMs were identified at a rate of ∼1/5,000 individuals.20 More recently, a Japanese population-wide cancer screening program using thoracic CT found a PAVM prevalence rate of 1/2,630 (95% confidence interval [CI] 1/1,315 to 1/5,555).21 On the other hand, recently published literature suggests that the combination of PAVMs and ischemic stroke is both rare and newly identified, with only one primary data report of more than 2 patients that mentioned both PAVMs and ischemic stroke (Figure 1C).8 To redress, in 2021, the first nationwide analysis of 4,271,910 patients with AIS admitted to US hospitals found that 822 patients had a PAVM diagnosis, with unique stroke risk markers and differential treatment patterns and outcomes.19
Ischemic Stroke as the First Presentation of a PAVM
PAVMs remain clinically silent in the overwhelming majority of patients.4,10 The usual temporal relationship between PAVM diagnosis and ischemic stroke is illustrated by the 18 case reports between 2017 and 2021: in 17 (94%), PAVMs had not been diagnosed until patients presented with an ischemic stroke.e1-e18 These case reportse1-e18 attest to older studies indicating that at the time of presentation with an ischemic stroke, two-thirds (20/30) of patients with PAVM were not diagnosed, until a mean delay of 2 years later.10 The limited diagnostic rates prior to a clinical stroke reflect the paucity of respiratory symptoms in patients with PAVMs. For instance, in one early series of 219 patients with PAVM (median age 50.5 years), only 21.5% (46/219) had PAVMs diagnosed because of respiratory symptoms.10
Silent Cerebral Infarction
The burden of silent cerebral infarction has been observed to be about twice more than clinical stroke (Table 1), with a recent study observing 1.13 silent infarcts per patient with PAVM/HHT.15 Furthermore, the silent infarct burden has been shown to increase with age, affecting 10%, 29%, and 28% of patients with PAVM aged 30–39, 60–69, and ≥70 years in one series.16 In the 18 international case reports on PAVM-related AIS published between 2017 and 2021, only 5 had described prior acute neurologic events,e2,e4,e11,e12,e16 with 11 (61%) having one or more additional silent ischemic lesions on diffusion-weighted imaging or fluid-attenuated inversion recovery sequences.e1-e3,e6,e10,e11,e16 The multiple lesions were found in 5,e11 4,e10 3,e1,e2 2,e3 or a singlee16 cortical/subcortical location. In addition, 4 patients had 2 lesions,e8,e9,e13,e18 with only 8 (44%) having a single infarct.e4,e5,e7,e8,e13-e15,e17
Symptomatic Ischemic Stroke
Although the 2021 Nationwide Inpatient Sample (NIS) database study confirmed that PAVMs constitute a minority of all AIS admissions (0.02% in their analysis), it also demonstrated an overall doubling of the PAVM diagnostic rate between 2005 and 2014 (Figure 2).19 MRI of the brain performed on patients with PAVM have found that nearly 50% have evidence of cerebral infarction by 50 years of age.15,16 Even when restricted to the subgroup of patients with a clinical stroke, nearly 25% of untreated patients are expected to become symptomatic by age 65 years.10 Assuming that the stroke risk from a PAVM is linearly distributed over time, then most patients with a PAVM would carry ∼0.4% annual risk for symptomatic ischemic stroke. Given PAVM prevalence estimates of ∼38 per 100,000 from population-wide screening studies along with the US population averaging 300,000,000 between 2005 and 2014, it can be projected that PAVMs should have been responsible for ∼4,560 PAVM-related AIS hospitalizations between 2005 and 2014; that is, ∼5-fold higher than that captured administratively.19 In other words, about 1 in 1,000 AIS hospitalizations may have been related to a PAVM, with only 1 in 5,000 captured between 2005 and 2014. Such projections likely underestimated the true stroke risk from PAVMs because they excluded events lasting less than 24 hours and those without supportive neuroimaging.10 In addition, some of the events diagnosed as migraines may have been ischemic strokes. Recent evidence has shown that patients with PAVM have migraine-like symptoms at the time of their stroke22 and that migraines can be precipitated by paradoxical emboli.23 On the other hand, patients hospitalized with stroke are more likely to have their PAVM identified, potentially introducing a selection bias that could inflate the observed stroke prevalence.
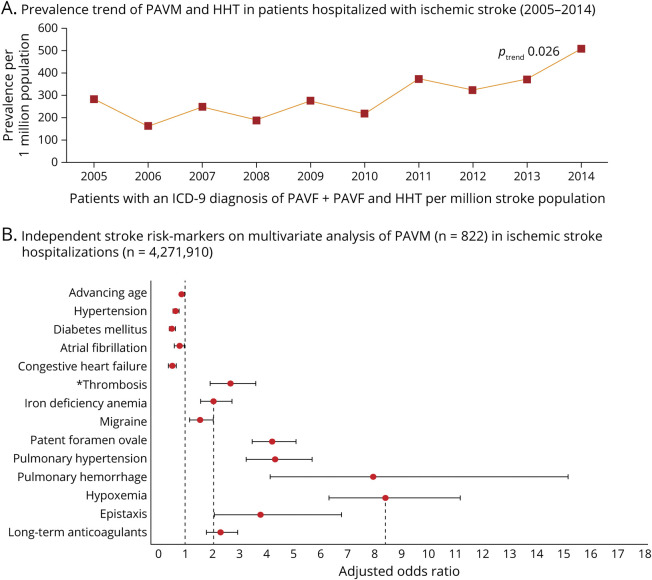
Figure 2. Prevalence and Risk Markers of Pulmonary Arteriovenous Malformation in Patients Hospitalized With Ischemic Stroke.
(A) 2021 nationwide analysis of pulmonary arteriovenous malformations (PAVMs) across a non-selected US population of 4,271,910 patients hospitalized with acute ischemic stroke (2005–2014), observing a significant increase in the prevalence of PAVMs in a combined cohort of patients with and without a concomitant ICD-9-CM diagnosis of hereditary hemorrhagic telangiectasia (HHT).19 (B) Clinical variables found to be independently associated with ischemic stroke on multivariable logistic regression analysis included hypoxemia as the strongest independent stroke risk marker (odds ratio [OR] 8.4, 95% confidence interval [CI] 6.3–11.2); iron deficiency anemia (OR 2.03, 95% CI 1.5–2.7) and epistaxis (OR 3.7, 95% CI 2.0–6.7), a common cause of iron deficiency anemia in patients with HHT, were the major modifiable risk markers. *Patients with PAVMs were 2.3-fold more likely to be on long-term anticoagulation even after adjusting for age, atrial fibrillation, primary hypercoagulable states, venous thrombosis, and pulmonary embolism.
Clinical Features of AIS in Patients With PAVMs
Younger age at stroke admission and a significantly higher loss of disability-adjusted life-years were prominent features of PAVM-related AIS in both the 2005–2014 NIS study and the 18 case reports published between 2017 and 2021.19,e1-e18 In the NIS data, when compared to ischemic stroke due to other causes, patients with AIS with PAVMs were younger at admission (57.5 years vs 72.5 years) and death (67 years vs 79.9 years), with higher median years of life lost (18.5 years vs 9.7 years) and disability-adjusted life-years (19.2 years vs 10.3 years).19 In the 2017–2021 international case report series, the median age at presentation with PAVM-induced ischemic stroke was 47 years (range 19–84 years).e1-e18
Several studies have shown that in patients with PAVM-related ischemic stroke, conventional stroke risk factors are less frequent (Figure 2). In the 2021 NIS study, the patients with PAVM-AIS were less likely than the main AIS cohort to have hypertension (55.3% vs 79.4%, age-adjusted univariate p < 0.001) or diabetes (15.6% vs 34%, age-adjusted univariate p < 0.001).19 Similarly, no conventional neurovascular risk markers were identified in 10/15 (67%) reported single cases,e1-2,e10,e12-18 nor in 29/61 (47.5%) of AIS-PAVM cases in an earlier series.2 Instead, stroke risk was associated with several PAVM and non-PAVM factors, as detailed below.
PAVM Etiology and Complications
More than 80% of PAVMs are part of a multisystemic vascular dysplasia, bringing additional considerations for the patient (Table 2) and often concurrently diagnosing a family at risk of further preventable neurologic complications.
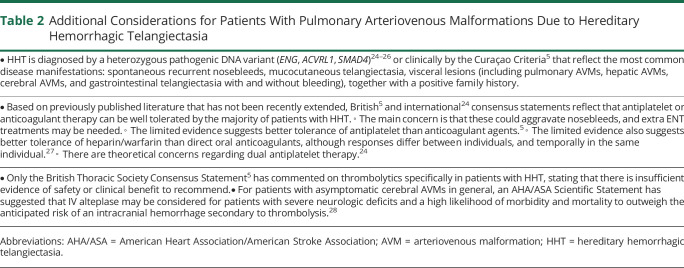
Table 2.
Additional Considerations for Patients With Pulmonary Arteriovenous Malformations Due to Hereditary Hemorrhagic Telangiectasia
The most common of these is HHT,24 which is inherited as an autosomal dominant trait, due to a single disease-causing variant that is usually identified in ENG, ACVRL1, or SMAD4,24,25,29 leading to familial nosebleeds, mucocutaneous telangiectasia, iron deficiency anemia (usually secondary to epistaxis), and arteriovenous malformations (AVMs). Visceral AVMs include PAVMs that affect approximately 50% of people with HHT.24,25 Symptoms and signs of HHT may be subtle, with one study of 205 patients with PAVM with HHT (median age 50.5 years) finding that 60% were unaware that they had HHT at the time of their PAVM diagnosis.7 In more recent data of 124 consecutive patients with PAVMs who were the first in their family to be tested for HHT, 67% had a positive HHT gene test, but almost 24% lacked suggestive clinical features other than nosebleeds in adult life, none of whom had a family history of HHT, and only 15% had classic HHT telangiectasia.30
Recent observational studies highlight that PAVMs also occur outside of the setting of HHT. Case series from the United States7 and the Netherlands31 of consecutive patients with PAVM diagnosed during 13-year31 to 18-year7 intervals found that non-HHT PAVMs account for up to 22% of cases. Non-HHT PAVMs are mostly single (83%–90%), unlike in HHT, where they tend to be multiple. Uncommon causes of acquired PAVMs include hepatopulmonary syndrome, choriocarcinoma, and surgery for cyanotic congenital heart disease diverting vena-caval blood into a single lung where PAVMs develop in the lung not receiving hepatic venous effluent. Although these are all very rare causes of PAVMs, they are overrepresented in recent literature (Figure 1C). Traumatic origin is reported but notably, in our experience, these are almost always misdiagnosed systemic-to-pulmonary artery communications.32
Most PAVMs are small with minimal effect on gas exchange. For larger PAVMs, even when significant hypoxemia and exuberant ventilation is present, dyspnea and exercise limitation is uncommon. Recent data33 add to previously published evidence2,5 and indicate that most patients successfully compensate for PAVM-induced hypoxemia, unless iron deficiency develops.33 Hemorrhage resulting in hemoptysis and hemothorax is also rare in PAVMs, reflecting the normally low pulmonary artery pressure.5 Higher rates are seen in pregnancy (with ∼1% risk emphasized in a 2020 review article),34 or if perfusion pressure rises to systemic arterial pressures, for example after the development of systemic collaterals to previously treated PAVMs.35 Chest pain has also been emphasized to be due usually to alternate pathologies that triggered the imaging that identified coexisting PAVMs.5 The most common and severe complications result from paradoxical emboli. These include cerebral abscess and migraine in addition to embolic AIS.5,10,18,19,36 While there appears to be little overlap between ischemic stroke and cerebral abscess,10,36 there are strong links between migraine and ischemic stroke,22,23 with one of the recent case reports further highlighting the association.e7 In persistently demonstrated data5,8 that surprise many clinicians and patients, for people with HHT, the neurologic effect from pulmonary AVMs far exceeds the risks from cerebral AVMs (Table 1).
Risk Factors for PAVM Ischemic Stroke
PAVM-Related Factors
Intuitively and traditionally, anatomically larger PAVMs were thought to have a higher likelihood of ischemic stroke. Early attention focused on the diameter of the feeding artery: in one study of 170 patients with HHT with PAVM, a positive correlation was observed with feeding artery diameter (FAD) (stroke vs nonstroke [mean ± SD] 4.87 ± 2.96 mm vs 3.19 ± 2.07 mm, assessed using CT scan).8 However, 2 other studies of 219 and 75 patients with PAVM found no association with FAD.10,12 Instead, one noted near doubling of ischemic stroke prevalence in patients with multiple PAVMs (28% vs 14%).12
Pathophysiologic reasoning would suggest the likelihood of ischemic stroke would be higher when there is physiologic evidence of a greater proportion of the cardiac output passing through PAVMs, that is, a larger shunt fraction. This expectation receives support from several lines of evidence, with multiple surrogates of right-to-left shunt demonstrating some relationship with ischemic stroke risk: the shunt fraction is inversely proportional to SaO2,2,3 which, in addition to higher right-to-left shunt grade, and lower pulmonary artery pressure, has been associated with greater stroke risk (Figure 2). Reinforcing earlier data,4 the recent NIS study also found hypoxemia as the strongest independent stroke risk marker (odds ratio [OR] 8.4, 95% CI 6.3–11.2) in a study of 822 patients with PAVM/AIS.19
The potential relevance of a coexisting PFO has been examined by several studies. Because PFO prevalence in the general population exceeds 25% and PAVMs are not thought to influence that risk, the continuous right-to-left shunt in a patient with PAVM may be augmented by intermittent right-to-left shunting through a coexisting PFO. In the 2021 NIS study,19 patients with PAVM/AIS had 4.2 times higher odds (95% CI 3.5–5.1) of also having a diagnosis of PFO. The authors suspected that a proportion of that positive correlation was a result of ascertainment biases.19 Although both forms of right-to-left shunting (PFO and PAVM) individually add to the risk of paradoxical embolism, the far greater stroke risk proportionally is from PAVMs, in which more than 50% of patients have evidence of cerebral infarction by age 50 years.15,16 This is understandable from their pathophysiology: PAVMs provide a continuous right-to-left shunt (as pulmonary artery pressure typically exceeds pulmonary venous pressure), whereas PFOs result in a left-to-right shunt (because left atrial pressures are normally higher than right atrial pressures), and only subject to intermittent reversal, for example, with Valsalva-like provocative maneuvers.37
Non-PAVM Factors Potentiating Stroke Risk
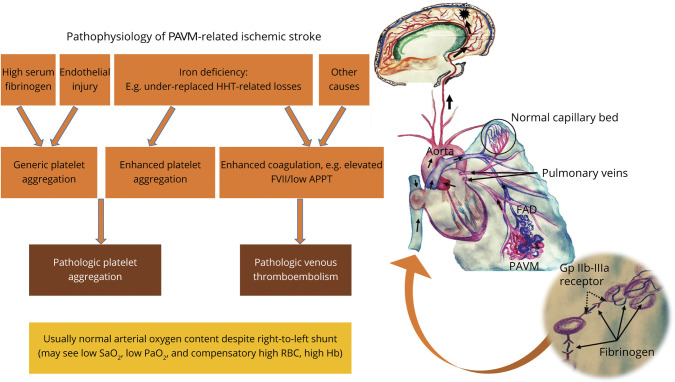
As noted above and illustrated in Figure 2, the prevalence of conventional neurovascular risk markers is low in patients with AIS associated with PAVMs, but few studies have examined other potential risk-heightening factors. The 2021 NIS study found a positive association with iron deficiency anemia (adjusted OR 2.0, 95% CI 1.5–2.7). One explanation could be that iron deficiency anemia was acting as a surrogate marker for patients with HHT in the cohort, and that patients with HHT had a higher ischemic stroke risk. However, in an earlier study restricting a PAVM cohort to 497 cases of HHT, low serum iron had a linear, inverse relationship with ischemic stroke (age/sex-adjusted OR 0.96 [95% CI 0.9–1.0] per μmol/L rise in serum iron, Figure 3).3 In addition, and replicating 4-decades-old studies in the general population, iron-deficient patients with PAVM/HHT were found to have an exuberant secondary wave of platelet aggregation in response to serotonin (5HT) that was not seen in non-iron-deficient controls, and was partially reduced by iron repletion.3 There was no association between ischemic stroke and venous thromboembolism, polycythemia, or coagulation screen parameters, but this study identified significantly elevated serum fibrinogen (which normally crosslinks glycoprotein IIb-IIIa platelet receptors) in patients with PAVM with ischemic stroke, supporting a role for platelets as a critical thromboembolic constituent in this population.3
Figure 3. Schematic Model of the Implicative Pathophysiology of Pulmonary Arteriovenous Malformation–Related Ischemic Stroke.
Iron deficiency, which in patients with hereditary hemorrhagic telangiectasia (HHT) is most likely to be a result of underreplacement of hemorrhagic losses, has been shown to mediate a complex serotonergic proaggregatory platelet response facilitated by a dysfunctional platelet monoamine oxidase.3 Patients with ischemic stroke with pulmonary arteriovenous malformations (PAVMs) have also been found to have elevated plasma fibrinogen levels, a protein that normally serves to crosslink the glycoprotein (Gp) IIb-IIIa platelet receptors (inset), further accentuating the role of platelet aggregation.3 While iron deficiency has also been associated with elevated serum factor VIII levels and venous thromboembolism (VTE), clinical3 and epidemiologic19 studies have not found conventional VTE to be commonly associated with PAVM-related ischemic stroke. Recurrent endothelial injury (e.g., from dysplastic vasculature) or other molecular mechanisms of hypercoagulability implicated in different right-to-left shunts (e.g., elevated homocysteine levels in patent foramen ovale)38 may further constitute additional thromboembolic or clot-independent mechanisms of cerebrovascular injury. APPT = activated partial thromboplastin time; FAD = feeding artery diameter; FVII = factor VIII; Hb = hemoglobin; RBC = red blood cell.
Diagnosis of PAVMs
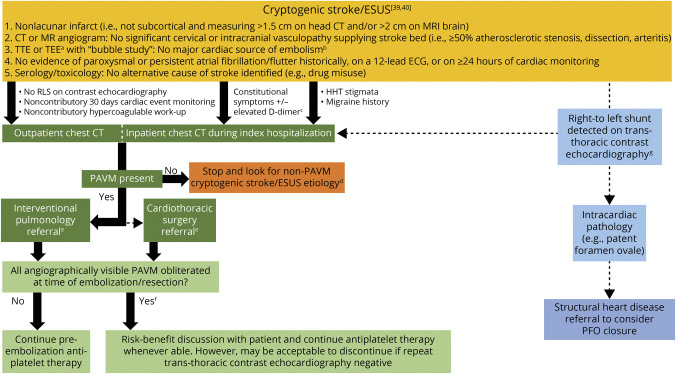
The construct of cryptogenic stroke or embolic stroke of undetermined source (ESUS) has evolved over the past decade,39,40 with diagnostic algorithms subject to institutional practice. Our proposed diagnostic algorithm for PAVM-related embolic stroke (Figure 4) incorporates recommendations from the BTS Consensus Statement, which underwent revisions following a formal public/professional consultation period, and emphasized thoracic CT imaging (with or without contrast) as the better test of choice for PAVM screening than transthoracic contrast echocardiography, where low-grade intrapulmonary shunting may be missed by less experienced observers.5 In addition, CT scans are ∼100% specific at distinguishing pulmonary AVM from mimics,37 identifying all PAVMs that are technically feasible to embolize.5,37
Figure 4. Diagnostic and Management Algorithm for Embolic Stroke Related to Pulmonary Arteriovenous Malformations.
Proposed diagnostic algorithm incorporating thoracic CT scans in the workup of embolic stroke of undetermined source (ESUS).39 aTransesophageal echocardiography (TEE) is considered when the transthoracic echocardiography (TTE) is technically limited or patient age is ≤60 years and there is high suspicion for atrial cardiopathy or aortic pathology.40 bPermanent or paroxysmal atrial fibrillation, sustained atrial flutter, intracardiac thrombus, prosthetic cardiac valve, atrial myxoma or other cardiac tumors, mitral valvulopathy, recent (<4 weeks) myocardial infarction, left ventricular ejection fraction less than 30%, or infective/other endocarditis.39 cCT chest may be performed as a part of a screening CT chest/abdomen/pelvis on a case-by-case basis, which may help identify a potentially occult solid organ malignancy.40 dPAVMs do not grow by any measurable extent in adult life (exceptions may include postpregnancy),5 and thus there is little value is repeating PAVM screening, even if the patient presents with subsequent ischemic stroke. eIf the risk-benefit analysis after considering surgical risk stratification, baseline functional status, concomitant vascular risk factor profile, and age (>60 years with a life expectancy <5 years considered a relative contraindication) is favorable, then endovascular embolization is performed. Rarely is surgical resection indicated when PAVMs are too diffuse to realistically achieve endovascular cure. fThe majority of treated patients will have residual right-to-left shunt (RLS), as demonstrated in Figure 1B. gLeft heart contrast opacification from an intrapulmonary RLS occurs within seconds, is not affected by cardiac cycles or respirations, and becomes certain if the bubble density is greater within left-heart than right (requires >30 seconds of recording).37 HHT = hereditary hemorrhagic telangiectasia; PFO = patent foramen ovale.
Contrast-enhanced transthoracic echocardiography (“bubble study”) has become a routine test in the workup for ESUS,39 and consistently detects more intrapulmonary right-to-left shunting in patients with HHT than any other screening modality.37 However, approximately 8% of the general population demonstrates opacification of the left heart on contrast echocardiography (up to 90% with exercise),41 and as a result, positive predictive value for a treatable PAVM has remained low and variable, even when performed at highly experienced centers.37 Therefore, while acknowledging what is already conducted in poststroke workup, we emphasize that a positive bubble study is not a prerequisite for a CT scan to seek potentially causative PAVMs in patients with ESUS.5 It is not clear whether “CT-negative” right-to-left shunting enhances stroke risk, although data from the HHT literature indicate that any enhanced risk would be very small.9
In the general population, pulmonary angiography is usually reserved for interventional care,4,5,18 as confirmed by recent consensus statements from respiratory medicine and radiology.4,7 Given the high sensitivity of thoracic CT scans for identifying PAVMs amenable to intervention, a neurologist generally need not push for a formal pulmonary angiogram to rule out a PAVM (Figure 4).
Management
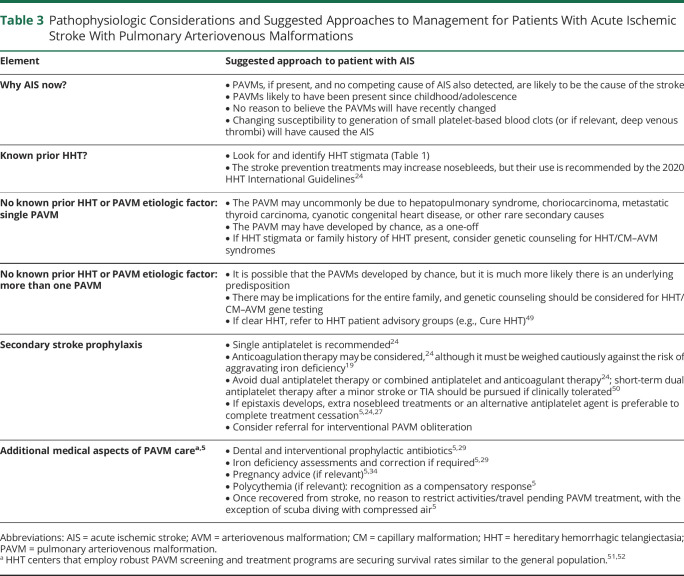
In our experience, patients already anxious regarding their potential recovery from a first AIS event are relieved when a cause is found for which there are potential treatments to avert recurrent stroke. But there can be unrealistic expectations of treatment effectiveness when PAVMs are multiple and residual shunting inevitable after maximal treatment. A cautious description of treatment potential is suggested in the acute setting (Table 3).
Table 3.
Pathophysiologic Considerations and Suggested Approaches to Management for Patients With Acute Ischemic Stroke With Pulmonary Arteriovenous Malformations
Acute Stroke Reperfusion Therapy
No cohort studies or case series to date have described use of IV thrombolytics or mechanical thrombectomy in patients with PAVM with ischemic stroke. Even with a third of PAVM literature since 2010 constituting single case reports (eFigure 1, links.lww.com/WNL/B696),42 only a handful have described the use of thrombolytics.e6-e9 None of the case reports described a proximal (internal carotid artery, M1–middle cerebral artery [MCA], basilar) large vessel occlusion, with only one report of a right M2 MCA occlusion.43 In the 2005–2014 NIS study, patients with and without PAVM received IV thrombolytics at similar rates (5.9% vs 5.8%), but patients with PAVM had lower rates of mechanical thrombectomy (0% [n = 0] vs 0.7% [n = 29,262]), which the authors suggested was consistent with a propensity to medium and small vessel occlusions rather than large vessel occlusions arising from PAVM-related emboli.19 In support, an older observational cohort of 61 patients with PAVM with ischemic stroke demonstrated that none had large vessel occlusion strokes.3
Secondary Stroke Prevention
The AHA/ASA guidelines recommend PFO closure devices in younger patients with ischemic stroke with right-to-left shunts caused by a PFO, without a statement for PAVMs.17 In older patients with PFO-associated stroke, antithrombotic therapy with either antiplatelet agents or anticoagulants is recommended. Concurrently, no randomized trials or large cohort studies have evaluated secondary stroke prophylactic measures such as long-term antiplatelet therapy or anticoagulation therapy in the PAVM population, with wide variations in recent case reports.e1-e18 Identifying that NIS-captured patients with AIS with PAVMs (United States, 2005–2014) were ∼2-fold more likely to be on long-term anticoagulation in crude and confounder-adjusted analyses, and recognizing the potential for greater adverse event rates in patients with HHT on anticoagulants, the authors suggested a cautious review of their continued use vs switching to antiplatelet therapy.19 Data from recent PAVM-AIS case reportse1-e18 found that only 2 patients had deep venous thrombosis on lower extremity ultrasounds,e2,e7 with 6 patients receiving anticoagulants and 3 patients receiving antiplatelet agents,e2,e5,e7,e9,e10,e13 indicating continued practitioner uncertainty regarding the best antithrombotic regimen. Although venous sources for paradoxical embolism also include sites not identified on lower extremity ultrasound (e.g., pelvic veins), these findings indicate a limited appreciation that ischemic strokes in patients with PAVM are only rarely associated with conventional venous thromboemboli that warrant the additional risks of anticoagulation. The most recent international guidelines for HHT management recommend that, when indicated, preventive antithrombotic regimens consist of either single antiplatelet or anticoagulant therapy, avoiding double antiplatelet therapy or combined antiplatelet and anticoagulant therapy.24 These comments are expected to evolve in the near future as more evidence for individual variability in stroke risk (from concurrent genetic changes)44 or pharmacogenomic variations in sensitivity/resistance to antiplatelets such as clopidogrel45 emerge.
Pulmonary Arteriovenous Malformations
As emphasized by the BTS, CIRSE, and Cochrane Database Consensus Statements/Reviews,4,5,18 most patients will have PAVMs treated by embolization, with parenchymal sparing surgery also an option in highly selected cases. It is worth noting that the previous “3-mm rule” suggesting PAVMs with feeding artery diameters below 3 mm rarely caused cerebral events5 is based on 20-year-old literature and does not hold up well to recent evidence.3–5,10 Potential limitations to embolotherapy include early pregnancy (radiation exposure), renal impairment (contrast exposure), and pulmonary hypertension (theoretical risk of increase in pulmonary artery pressure),5 with a major complication rate of ∼1%.5,18 For details and current areas of technical advances, the reader is directed to the CIRSE statement.18
Concern over MRI safety of embolization devices has caused delays in emergent neuroimaging46; however, the safety of 1.5T scans is now well established, with a 2019 BJR Advances in Knowledge statement indicating that “MRI of patients who have had PAVMs treated by embolization can be implemented without contacting specialist pulmonary arteriovenous malformation treatment centres for approval.”46
PAVMs can be cured, and their treatment has been shown to reduce the risk of ischemic stroke,10 migraine,47 and cerebral abscess.10 One study that examined stroke rates before and after obliteration of all angiographically visible PAVM examined 219 patients with PAVMs and found a significant reduction of recurrent stroke (13.6% vs 0%) at a mean of 3.7 years follow-up.10 Two other studies (199747 and 200448) provided retrospective follow-up data after PAVM closure, reporting recurrent stroke/TIA rates of 2.7% and 4.5% over a 5-year follow-up. There are no current guidelines from AHA/ASA regarding PAVM repair. The consensus from the BTS,5 CIRSE,18 and the latest Cochrane Database review4 is that every CT-visible PAVM should be considered for endovascular treatment, even when asymptomatic, and regardless of feeding artery diameter.
At a time when the global burden of stroke in people under 65 years of age is rising, PAVMs constitute a treatable cause of stroke in the young. Recent studies have identified patients with PAVM-related stroke as a unique cohort—one that is significantly younger and lacks traditional stroke risk factors, while simultaneously harboring modifiable risk factors of distinctive relevance to PAVM-associated stroke, and having a high silent infarct burden. There is a lack of systematic research with the overwhelming majority of current PAVM literature comprising case reports, particularly for PAVM-related ischemic stroke. Future research avenues should include epidemiologic evaluation of the silent infarct burden, including resultant vascular cognitive impairment and dementia in this cohort; systematic large patient cohort evaluations of the role of reperfusion therapy in the acute setting; and safety/efficacy of secondary prophylaxis with antiplatelet and anticoagulant therapies, as well as iron repletion on ischemic stroke rates in these patients. Finally, formal consensus statements from the American Thoracic Society/AHA/ASA are desirable to provide authoritative guidance to practitioners on diagnostic, acute treatment, and secondary stroke prophylactic management approaches for PAVM-induced ischemic stroke.
Acknowledgment
The authors thank Dr. Jethwani for guidance and support throughout the writing and editing of this manuscript.
Glossary
- AHA/ASA
American Heart Association/American Stroke Association
- AIS
acute ischemic stroke
- AVM
arteriovenous malformation
- BTS
British Thoracic Society
- CI
confidence interval
- CIRSE
Cardiovascular and Interventional Radiologic Society of Europe
- ESUS
embolic stroke of undetermined source
- FAD
feeding artery diameter
- HHT
hereditary hemorrhagic telangiectasia
- MCA
middle cerebral artery
- NIS
Nationwide Inpatient Sample
- OR
odds ratio
- PAVM
pulmonary arteriovenous malformation
- PFO
patent foramen ovale
- RCT
randomized clinical trial
Appendix. Authors

Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Majumdar S, McWilliams JP. Approach to pulmonary arteriovenous malformations: a comprehensive update. J Clin Med. 2020;9(6):1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shovlin CL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med. 2014;190(11):1217-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shovlin CL, Chamali B, Santhirapala V, et al. Ischaemic strokes in patients with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia: associations with iron deficiency and platelets. PLoS One. 2014;9(2):e88812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CC, Kwan GN, Evans-Barns H, van Driel ML. Embolisation for pulmonary arteriovenous malformation. Cochrane Database Syst Rev. 2018;1(1):CD008017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shovlin CL, Condliffe R, Donaldson JW, Kiely DG, Wort SJ, British Thoracic Society. British Thoracic Society clinical statement on pulmonary arteriovenous malformations. Thorax. 2017;72(12):1154-1163. [DOI] [PubMed] [Google Scholar]
- 6.Shovlin CL, Letarte M. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax. 1999;54(8):714-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albitar HAH, Segraves JM, Almodallal Y, Pinto CA, De Moraes AG, Iyer VN. Pulmonary arteriovenous malformations in non-hereditary hemorrhagic telangiectasia patients: an 18-year retrospective study. Lung. 2020;198(4):679-686. [DOI] [PubMed] [Google Scholar]
- 8.Etievant J, Si-Mohamed S, Vinurel N, et al. Pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia: correlations between computed tomography findings and cerebral complications. Eur Radiol. 2018;28(3):1338-1344. [DOI] [PubMed] [Google Scholar]
- 9.Velthuis S, Buscarini E, Van Gent MWF, et al. Grade of pulmonary right-to-left shunt on contrast echocardiography and cerebral complications: a striking association. Chest. 2013;144(2):542-548. [DOI] [PubMed] [Google Scholar]
- 10.Shovlin CL, Jackson JE, Bamford KB, et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax. 2008;63(3):259-266. [DOI] [PubMed] [Google Scholar]
- 11.Cottin V, Chinet T, Lavolé A, et al. ; Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P). Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine. 2007;86(1):1-17. [DOI] [PubMed] [Google Scholar]
- 12.Moussouttas M, Fayad P, Rosenblatt M, et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology. 2000;55(7):959-964. [DOI] [PubMed] [Google Scholar]
- 13.White RI Jr, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988;169(3):663-669. [DOI] [PubMed] [Google Scholar]
- 14.Stringer CJ, Stanley AL, Bates RC, Summers JE. Pulmonary arteriovenous fistula. Am J Surg. 1955;89(5):1054-1080. [DOI] [PubMed] [Google Scholar]
- 15.Fatania G, Gilson C, Glover A, et al. Uptake and radiological findings of screening cerebral magnetic resonance scans in patients with hereditary haemorrhagic telangiectasia. Intractable Rare Dis Res. 2018;7(4):236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinjikji W, Nasr DM, Wood CP, Iyer VN. Pulmonary arteriovenous malformations are associated with silent brain infarcts in hereditary hemorrhagic telangiectasia patients. Cerebrovasc Dis. 2017;44(3-4):179-185. [DOI] [PubMed] [Google Scholar]
- 17.Furie KL, Kasner SE, Adams RJ, et al. ; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. [DOI] [PubMed] [Google Scholar]
- 18.Müller-Hülsbeck S, Marques L, Maleux G, et al. CIRSE standards of practice on diagnosis and treatment of pulmonary arteriovenous malformations. Cardiovasc Intervent Radiol. 2020;43(3):353-361. [DOI] [PubMed] [Google Scholar]
- 19.Topiwala KK, Patel SD, Pervez M, Shovlin CL, Alberts MJ. Ischemic stroke in patients with pulmonary arteriovenous fistulas. Stroke. 2021;52(7):e311–e315. [DOI] [PubMed] [Google Scholar]
- 20.Sloan RD, Cooley RN. Congenital pulmonary arteriovenous aneurysm. Am J Roentgenol Radium Ther Nucl Med. 1953;70(2):183-210. [PubMed] [Google Scholar]
- 21.Nakayama M, Nawa T, Chonan T, et al. Prevalence of pulmonary arteriovenous malformations as estimated by low-dose thoracic CT screening. Intern Med. 2012;51(13):1677-1681. [DOI] [PubMed] [Google Scholar]
- 22.Elphick A, Shovlin CL. Relationships between epistaxis, migraines, and triggers in hereditary hemorrhagic telangiectasia. Laryngoscope. 2014;124(7):1521-1528. [DOI] [PubMed] [Google Scholar]
- 23.Patel T, Elphick A, Jackson JE, Shovlin CL. Injections of intravenous contrast for computerized tomography scans precipitate migraines in hereditary hemorrhagic telangiectasia subjects at risk of paradoxical emboli: implications for right-to-left shunt risks. Headache. 2016;56(10):1659-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faughnan ME, Mager JJ, Hetts SW, et al. Second international guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia. Ann Intern Med. 2020;173(12):989-1001. [DOI] [PubMed] [Google Scholar]
- 25.Topiwala KK, Patel SD, Nouh AM, Alberts MJ. Novel GDF2 gene mutation associated with pulmonary arteriovenous malformation. J Stroke Cerebrovasc Dis. 2020;29(12):105301. [DOI] [PubMed] [Google Scholar]
- 26.Shovlin CL, Simeoni I, Downes K, et al. Mutational and phenotypic characterisation of hereditary hemorrhagic telangiectasia. Blood. 2020;136(17):1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shovlin CL, Millar CM, Droege F, et al. Safety of direct oral anticoagulants in patients with hereditary hemorrhagic telangiectasia. Orphanet J Rare Dis 2019;14(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. ; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(2):581-641. [DOI] [PubMed] [Google Scholar]
- 29.Shovlin CL, Buscarini E, Kjeldsen AD, et al. European reference network for rare vascular diseases (VASCERN) outcome measures for hereditary haemorrhagic telangiectasia (HHT). Orphanet J Rare Dis. 2018;13(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma L, Alsafi A, Ferguson T, Redhead J, West London Genomic Medicine Centre Consortium, Shovlin CL. Pulmonary arteriovenous malformations: genetic versus clinical evidence of underlying hereditary haemorrhagic telangiectasia. Thorax. 2021;76(suppl 1). [Google Scholar]
- 31.Kroon S, van den Heuvel DAF, Vos JA, et al. Idiopathic and hereditary haemorrhagic telangiectasia associated pulmonary arteriovenous malformations: comparison of clinical and radiographic characteristics. Clin Radiol. 2021;76(5):394.e1-394.e8. [DOI] [PubMed] [Google Scholar]
- 32.Alsafi A, Shovlin CL, Jackson JE. Transpleural systemic artery-pulmonary artery communications in the absence of chronic inflammatory lung disease: a case series and review of the literature. Clin Radiol 2021;76(9):711.e9-711.e15. [DOI] [PubMed] [Google Scholar]
- 33.Gawecki F, Strangeways T, Amin A, et al. Exercise capacity reflects airflow limitation rather than hypoxaemia in patients with pulmonary arteriovenous malformations. QJM. 2019;112(5):335-342. [DOI] [PubMed] [Google Scholar]
- 34.Dupuis O, Delagrange L, Dupuis-Girod S. Hereditary haemorrhagic telangiectasia and pregnancy: a review of the literature. Orphanet J Rare Dis. 2020;15(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorsi U, Bansal A, Jugpal TS, Chaluvashetty SB, Sandhu MS. Endovascular management of massive hemoptysis secondary to systemic collaterals in previously treated pulmonary arteriovenous malformation. Vasc Endovascular Surg. 2019;53(8):674-678. [DOI] [PubMed] [Google Scholar]
- 36.Boother EJ, Brownlow S, Tighe HC, Bamford KB, Jackson JE, Shovlin CL. Cerebral abscess associated with odontogenic bacteremias, hypoxemia, and iron loading in immunocompetent patients with right-to-left shunting through pulmonary arteriovenous malformations. Clin Infect Dis. 2017;65(4):595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill SS, Roddie ME, Shovlin CL, Jackson JE. Pulmonary arteriovenous malformations and their mimics. Clin Radiol. 2015;70(1):96-110. [DOI] [PubMed] [Google Scholar]
- 38.Deng W, McMullin D, Inglessis-Azuaje I, et al. Effect of patent foramen ovale (PFO) closure after stroke on circulatory biomarkers. Neurology. 2021;97(2):e203-e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart RG, Diener HC, Coutts SB, et al. ; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429-438. [DOI] [PubMed] [Google Scholar]
- 40.Fuentes B, Gutiérrez-Zúñiga R, Díez-Tejedor E. It's time to say goodbye to the ESUS construct. Front Neurol. 2020;11:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duke JW, Elliott JE, Laurie SS, et al. Bubble and macroaggregate methods differ in detection of blood flow through intrapulmonary arteriovenous anastomoses in upright and supine hypoxia in humans. J Appl Physiol. 2017;123(6):1592-1598. [DOI] [PubMed] [Google Scholar]
- 42.Shovlin CL, Gossage JR. Pulmonary arteriovenous malformations: evidence of physician under-education. ERJ Open Res. 2017;3(2):00104-02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yassi N, Yan B, Dowling R, Mitchell PJ. A rare cause of embolic stroke in hereditary hemorrhagic telangiectasia. J Stroke Cerebrovasc Dis. 2014;23(5):1245-1246. [DOI] [PubMed] [Google Scholar]
- 44.Joyce KE, Onabanjo E, Brownlow S, et al. ; Genomics England Research Consortium. High definition analyses of single cohort, whole genome sequencing data provides a direct route to defining subphenotypes and personalizing medicine. medRxiv 2021. doi: 10.08.28.21262560 [Google Scholar]
- 45.Duarte JD, Cavallari LH. Pharmacogenetics to guide cardiovascular drug therapy. Nat Rev Cardiol. 2021;18(9):649-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsafi A, Jackson JE, Fatania G, Patel MC, Glover A, Shovlin CL. Patients with in-situ metallic coils and Amplatzer vascular plugs used to treat pulmonary arteriovenous malformations since 1984 can safely undergo magnetic resonance imaging. Br J Radiol. 2019;92(1098):20180752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee DW, White RI Jr, Egglin TK, et al. Embolotherapy of large pulmonary arteriovenous malformations: long-term results. Ann Thorac Surg. 1997;64(4):930-940. [DOI] [PubMed] [Google Scholar]
- 48.Mager JJ, Overtoom TT, Blauw H, Lammers JW, Westermann CJ. Embolotherapy of pulmonary arteriovenous malformations: long-term results in 112 patients. J Vasc Interv Radiol. 2004;15(5):451-456. [DOI] [PubMed] [Google Scholar]
- 49.Cure HHT. curehht.org. Published 2021.
- 50.Johnston SC, Easton JD, Farrant M, et al. ; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kjeldsen A, Aagaard KS, Tørring PM, Möller S, Green A. 20-year follow-up study of Danish HHT patients-survival and causes of death. Orphanet J Rare Dis 2016;11(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeGussem EM, Kroon S, Hosman AE, et al. Hereditary hemorrhagic telangiectasia (HHT) and survival: the importance of systematic screening and treatment in HHT centers of excellence. J Clin Med. 2020;9(11):3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Data available (references e1-e21): links.lww.com/WNL/B696
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.