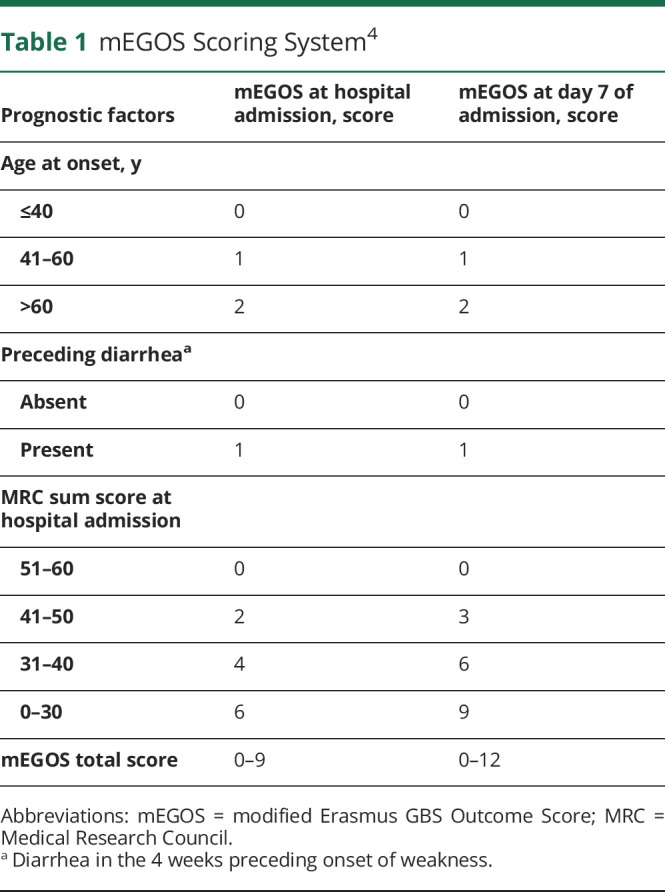
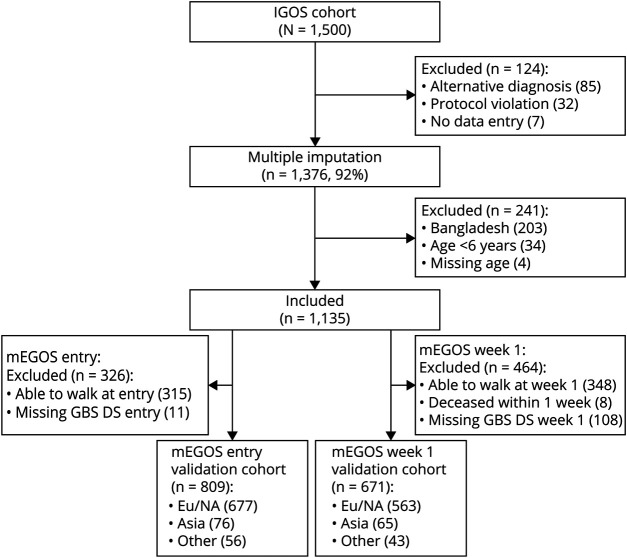
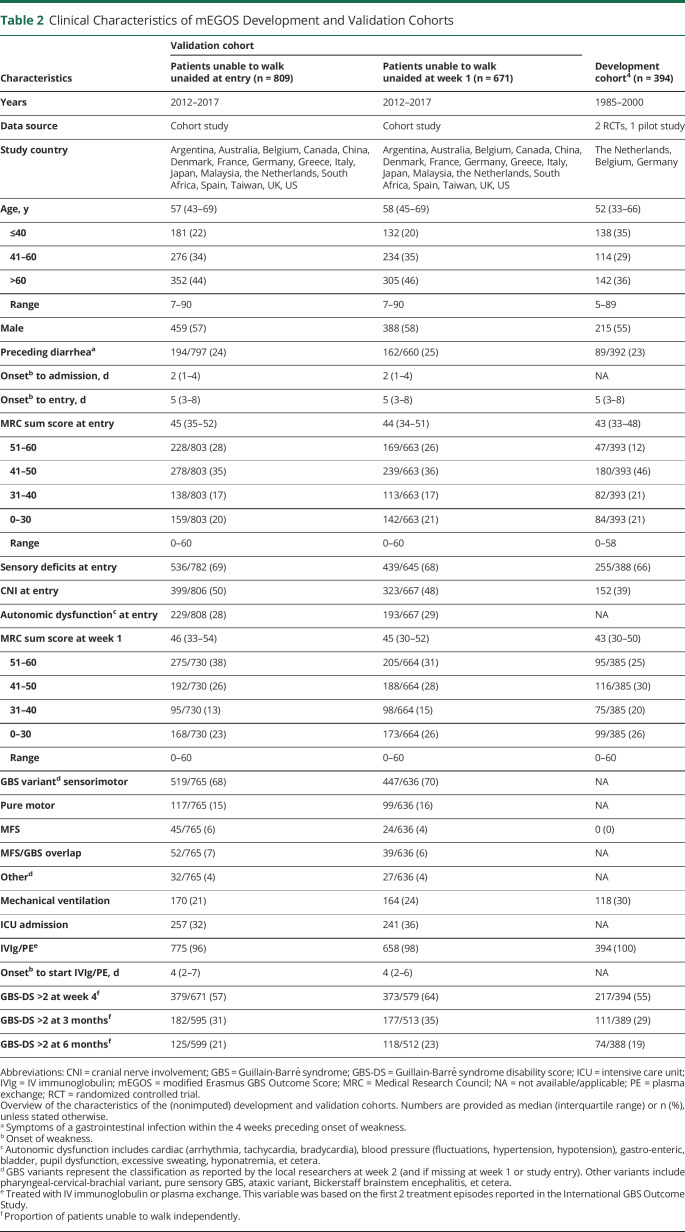
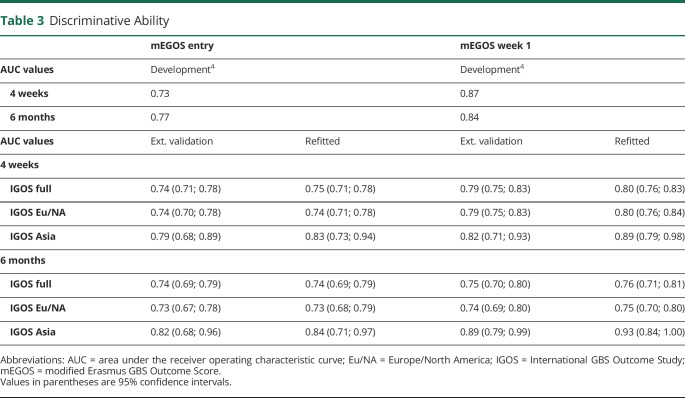
Alex Y Doets
Alex Y Doets, MD, PhD candidate
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Hester F Lingsma
Hester F Lingsma, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Christa Walgaard
Christa Walgaard, MD, PhD candidate
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Badrul Islam
Badrul Islam, MBBS, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Nowshin Papri
Nowshin Papri, MD, PhD candidate
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Amy Davidson
Amy Davidson, MD, PhD candidate
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Yuko Yamagishi
Yuko Yamagishi, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Susumu Kusunoki
Susumu Kusunoki, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Mazen M Dimachkie
Mazen M Dimachkie, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Waqar Waheed
Waqar Waheed, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Noah Kolb
Noah Kolb, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Zhahirul Islam
Zhahirul Islam, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Quazi Deen Mohammad
Quazi Deen Mohammad, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Thomas Harbo
Thomas Harbo, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Soren H Sindrup
Soren H Sindrup, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Govindsinh Chavada
Govindsinh Chavada, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Hugh J Willison
Hugh J Willison, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Carlos Casasnovas
Carlos Casasnovas, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Kathleen Bateman
Kathleen Bateman, MBChB
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
James AL Miller
James AL Miller, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Bianca van den Berg
Bianca van den Berg, MD, PhD candidate
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Christine Verboon
Christine Verboon, MD, PhD candidate
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Joyce Roodbol
Joyce Roodbol, MD, PhD candidate
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Sonja E Leonhard
Sonja E Leonhard, MD, PhD candidate
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Luana Benedetti
Luana Benedetti, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Satoshi Kuwabara
Satoshi Kuwabara, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Peter Van den Bergh
Peter Van den Bergh, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Soledad Monges
Soledad Monges, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Girolama A Marfia
Girolama A Marfia, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Nortina Shahrizaila
Nortina Shahrizaila, FRCP, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Giuliana Galassi
Giuliana Galassi, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Yann Péréon
Yann Péréon, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Jan Bürmann
Jan Bürmann, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Krista Kuitwaard
Krista Kuitwaard, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Ruud P Kleyweg
Ruud P Kleyweg, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Cintia Marchesoni
Cintia Marchesoni, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
María J Sedano Tous
María J Sedano Tous, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Luis Querol
Luis Querol, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Isabel Illa
Isabel Illa, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Yuzhong Wang
Yuzhong Wang, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Eduardo Nobile-Orazio
Eduardo Nobile-Orazio, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Simon Rinaldi
Simon Rinaldi, MBChB, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Angelo Schenone
Angelo Schenone, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Julio Pardo
Julio Pardo, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Frederique H Vermeij
Frederique H Vermeij, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Helmar C Lehmann
Helmar C Lehmann, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Volkan Granit
Volkan Granit, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Guido Cavaletti
Guido Cavaletti, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Gerardo Gutiérrez-Gutiérrez
Gerardo Gutiérrez-Gutiérrez, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Fabio A Barroso
Fabio A Barroso, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Leo H Visser
Leo H Visser, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Hans D Katzberg
Hans D Katzberg, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Efthimios Dardiotis
Efthimios Dardiotis, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Shahram Attarian
Shahram Attarian, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Anneke J van der Kooi
Anneke J van der Kooi, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Filip Eftimov
Filip Eftimov, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Paul W Wirtz
Paul W Wirtz, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Johnny PA Samijn
Johnny PA Samijn, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
H Jacobus Gilhuis
H Jacobus Gilhuis, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Robert DM Hadden
Robert DM Hadden, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
James KL Holt
James KL Holt, FRCP, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Kazim A Sheikh
Kazim A Sheikh, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Summer Karafiath
Summer Karafiath, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Michal Vytopil
Michal Vytopil, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Giovanni Antonini
Giovanni Antonini, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Thomas E Feasby
Thomas E Feasby, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Catharina G Faber
Catharina G Faber, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Cees J Gijsbers
Cees J Gijsbers, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Mark Busby
Mark Busby, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Rhys C Roberts
Rhys C Roberts, MB BChir PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Nicholas J Silvestri
Nicholas J Silvestri, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Raffaella Fazio
Raffaella Fazio, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Gert W van Dijk
Gert W van Dijk, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Marcel PJ Garssen
Marcel PJ Garssen, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Chiara SM Straathof
Chiara SM Straathof, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Kenneth C Gorson
Kenneth C Gorson, MD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,
Bart C Jacobs
Bart C Jacobs, MD, PhD
1From the Departments of Neurology (A.Y.D., C.W., B.v.d.B., C.V., J.R., S.E.L., K.K., B.C.J.), Public Health (H.F.L.), and Immunology (B.C.J.), Erasmus MC, University Medical Centre Rotterdam; Department of Neurology (C.W., J.P.A.S.), Maasstad Hospital, Rotterdam, the Netherlands; Laboratory of Gut-Brain Signaling (B.I., N.P., Z.I.), Laboratory Sciences and Services Division, Dhaka, Bangladesh; Department of Neurology (A.D., G. Chavada, H.J.W.), College of Medical, Veterinary and Life Sciences, University of Glasgow, UK; Department of Neurology (Y.Y., S. Kusunoki), Kindai University Faculty of Medicine, Osaka-Sayama City, Osaka, Japan; Department of Neurology (M.M.D.), University of Kansas Medical Center, Kansas City; Department of Neurology (W.W., N.K.), University of Vermont Medical Centre, Burlington; National Institute of Neurosciences and Hospital (Q.D.M.), Agargoan, Bangladesh; Department of Neurology (T.H.), Aarhus University Hospital; Department of Neurology (S.H.S.), Odense University Hospital and University of Southern Denmark, Odense, Denmark; Department of Neurology, Neuromuscular Unit (C.C.), Bellvitge University Hospital–IDIBELL, CIBERER, Barcelona, Spain; Department of Neurology (K.B.), Groote Schuur Hospital, University of Cape Town, South Africa; Department of Neurology (J.A.L.M.), Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK; Department of Neurology (B.v.d.B., C.J.G.), Franciscus Vlietland Hospital, Schiedam, the Netherlands; Department of Neurology (L.B.), IRCCS Ospedale Policlinico San Martino, Genova, Italy; Department of Neurology (S. Kuwabara), Chiba University, Japan; Department of Neurology (P.V.d.B.), Neuromuscular Reference Centre, University Hospital Saint-Luc, University of Louvain, Brussels, Belgium; Department of Neurology (S.M.), Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; Dysimmune Neuropathies Unit, Department of Systems Medicine (G.A.M.), Tor Vergata University Hospital, Rome, Italy; Department of Medicine (N.S.), University of Malaya, Kuala Lumpur; Department of Neurology (G.G.), University Hospital of Modena, Italy; Department of Clinical Neurophysiology, Reference Centre for NMD (Y.P.), CHU Nantes, France; Department of Neurology (J.B.), Saarland University Medical School, Homburg-Saarland (previous affiliation); MVZ Pfalzklinikum (J.B.), Kusel, Germany (current affiliation); Department of Neurology (K.K., R.P.K.), Albert Schweitzer Hospital, Dordrecht, the Netherlands; Department of Neurology (C.M.), Hospital Británico, Buenos Aires, Argentina; Department of Neurology (M.J.S.T.), Hospital Marques de Valdecilla, Santander; Department of Neurology (L.Q., I.I.), Hospital de la Santa Creu i Santa Pau, U.A.B. CIBERER and ERN-NMD, Barcelona, Spain; Department of Neurology (Y.W.), Affiliated Hospital of Jining Medical University, Shandong Province, China; Neuromuscular and Neuroimmunology Service (E.N.-O.), IRCCS Humanitas Clinical and Research Institute, Milan University, Rozzano, Italy; Nuffield Department of Clinical Neurosciences (S.R.), University of Oxford and Oxford University Hospitals NHS Foundation Trust, UK; Department of Neurosciences, Ophthalmology, Rehabilitation, Genetics and Maternal Sciences (A.S.), University of Genova; IRCCS San Martino Hospital (A.S.), Genova, Italy; Department of Neurology (J.P.), Hospital Clínico de Santiago, Santiago de Compostela (A Coruña), Spain; Department of Neurology (F.H.V.), Franciscus Vlietland Hospital (location: Franciscus Gasthuis), Rotterdam, the Netherlands; Department of Neurology (H.C.L.), University Hospital of Cologne, Germany; Department of Neurology (V.G.), Montefiore Medical Centre, Bronx, NY; Department of Neurology (G. Cavaletti), University Milano-Bicocca, Monza, Italy; Department of Neurology (G.G.-G.), Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Spain; Department of Neurology (F.A.B.), Instituto de Investigaciones Neurológicas Raúl Carrea, Buenos Aires, Argentina; Department of Neurology (L.H.V.), St. Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands; Department of Neurology (H.D.K.), University Health Network, University of Toronto, Canada; Department of Neurology (E.D.), University Hospital of Larissa, Greece; Department of Neurology, Reference Centre for NMD (S.A.), CHU Timone, Marseille, France; Department of Neurology (A.J.v.d.K., F.E.), Amsterdam University Medical Centre, University of Amsterdam, Neuroscience Institute, Netherlands Neuromuscular Centre, Euro-NMD; Department of Neurology (P.W.W.), Haga Hospital, Den Haag; Department of Neurology (H.J.G.), Reinier de Graaf Hospital, Delft, the Netherlands; Department of Neurology (R.D.M.H.), King's College Hospital, London; Department of Neurology (J.K.L.H.), The Walton Centre, Liverpool, UK; Department of Neurology (K.A.S.), University of Texas Health Science Centre at Houston; Department of Neurology (S. Karafiath), University of Utah School of Medicine, Salt Lake City; Department of Neurology (M.V.), Lahey Hospital and Medical Center, Tufts University School of Medicine, Burlington, MA; Department of Neurology (G.A.), Mental Health and Sensory Organs (NESMOS), University of Rome “Sapienza,” Sant’Andrea Hospital, Rome, Italy; Department of Clinical Neurosciences (T.E.F.), University of Calgary, Canada; Department of Neurology (C.G.F.), Maastricht University Medical Centre, the Netherlands; Department of Neurology (M.B.), Leeds Teaching Hospitals; Department of Neurology (R.C.R.), Addenbrooke's Hospital, Cambridge, UK; Department of Neurology (N.J.S.), University at Buffalo Jacobs School of Medicine and Biomedical Sciences, NY; Department of Neurology (R.F.), Scientific Institute San Raffaele, Milan, Italy; Department of Neurology (G.W.v.D.), Canisius Wilhelmina Hospital, Nijmegen; Department of Neurology (M.P.J.G.), Jeroen Bosch Hospital, ‘s-Hertogenbosch; Department of Neurology (C.S.M.S.), Leiden University Medical Centre, the Netherlands; and Department of Neurology (K.C.G.), St. Elizabeth's Medical Centre, Tufts University, School of Medicine, Boston, MA.
1,✉;
on behalf of the IGOS Consortium1