Abstract
The Adaptive Calibration Model of Stress Responsivity (ACM) suggests that developmental experiences predictably tune biological systems to meet the demands of the environment. Particularly important is the calibration of reward systems. Using a longitudinal sample (N = 184) followed since adolescence, this study models the dimensions of early life stress and their effects on epigenetic modification of the oxytocin receptor gene (OXTR) and individual differences in neural response to reward anticipation. We first created a latent variable model of developmental context using measures collected when participants were 13 years old. As adults, two subsets of participants completed a reward anticipation fMRI paradigm (N = 82) and agreed to have their blood assayed for (OXTR) DNA methylation (N = 112) at two CpG sites. Three latent constructs of developmental context emerged: Neighborhood Harshness, Family Harshness, and Abuse and Disorder. Greater OXTR DNA methylation at CpG sites −924 and −934 blunted the association between greater Neighborhood Harshness and increased neural activation in caudate in anticipation of rewards. Interaction effects were also found outside of reward-related areas for all three latent constructs. Results indicate an epigenetically derived differential susceptibility model whereby high methylation coincides with decreased association between developmental environment and neural reward anticipation.
Introduction
The Adaptive Calibration Model (ACM) of Stress Responsivity posits that adult phenotypes arise from individuals calibrating to meet the needs of their developmental context (Ellis, Giudice, Giudice, & Shirtcliff, 2017). Rather than early life stress (ELS) leading to just negative outcomes, gene by environment interactions produce biological and behavioral phenotypes that are functional if imperfect. It is from this perspective that we investigated a specific instance of gene by environment interaction, namely, the role of epigenetic modification of the oxytocinergic system in the developmental calibration of neural reward sensitivity. The oxytocin receptor gene is sensitive to developmental context and deeply involved in regulating neural reward systems (Love, 2014), which in turn are highly implicated in learning, motivation, mood, and psychopathology (Luking, Pagliaccio, Luby, & Barch, 2016). Understanding the unique interaction between dimensions of developmental adversity, OXTR epigenetic modification, and adult neural reward sensitivity can help us understand normative responses to developmental stressors. Importantly, the current study is translational, and taken from the prairie vole (Microtus ochrogaster) model system which supplies us with the necessary functional connections between ELS, DNA methylation, gene expression, and calibration of neural reward regions, to enact our study design.
Dimensions of Early Life Stress and Calibration of Reward Systems
Amassing research suggests ELS has a profound impact on individual differences from behavior to neurons (Fareri & Tottenham, 2016; Miller, Chen, & Parker, 2011; Wadsworth, Evans, Grant, Carter, & Duffy, 2016). However, the construct of ELS is not well defined. The dominant model of ELS is to characterize varied stressors from parental abandonment to resource scarcity as additive and create a cumulative risk factor which in turn compromises development and health. However, newer models, such as ACM, espouse a dimensional approach (Ellis, Figueredo, Brumbach, & Schlomer, 2009b; McLaughlin, Sheridan, & Lambert, 2014). Life History Theory, the foundation of ACM, separates developmental context into two dimensions: Harshness and Instability. Because bioenergetic resources are finite, the body in development takes its cues from the environment to determine what biological processes should be invested in and when. ACM predicts that children who develop in unreliable environments will calibrate towards less risk-adverse, more reward sensitive, and more impulsive adult phenotypes (Belsky, Steinberg, & Draper, 1991; Ellis, Figueredo, Brumbach, & Schlomer, 2009a). Accordingly higher Harshness and Instability should favor conditional adaptations that enhance neural systems supporting reward-motivation (Figueredo et al., 2006). Specifically, orbital frontal cortex (OFC) and striatum, especially the subregions of nucleus accumbens (NAcc) and caudate, should be implicated as these regions have all been implicated in the anticipation and receipt of reward, creating a putative motivational “reward system” (Liu, Hairston, Schrier, & Fan, 2011; Robbins & Everitt, 1996).
ACM is new, but support for its predictions can be found at the behavioral and neural levels. Behaviorally, the research indicates that children from lower SES families (high harshness) will discount bigger delayed rewards (Sturge-Apple et al., 2016) and even children from high SES will do this in the presence of an unreliable (high instability) adult (Michaelson, de la Vega, Chatham, & Munakata, 2013). Children with hawkish (faster life history trait) tendencies will also show enhanced problem solving for rewards (Suor, Sturge-Apple, Davies, & Cicchetti, 2017). At the neural level, research indicates that the striatum is sensitive to childhood experiences of harshness: abuse and neglect (Hanson, Hariri, & Williamson, 2015), low economic privilege (Cavanagh et al., 2013; Gonzalez, Allen, & Coan, 2016; Gonzalez, Puglia, Morris, & Connelly, 2017; Hanson et al., 2015), childhood social economic status (Cavanagh et al., 2013), and relative status (Ly, Haynes, Barter, Weinberger, & Zink, 2011). The impact of ELS on reward systems are not always in the expected ACM direction of increased reward-related response (perhaps due to the nebulous construct of ELS itself). However, in line with ACM, a recent meta-analysis of fMRI data indicates that lower SES is associated with structural and functional upregulation of reward-related regions including caudate and OFC (Yaple & Yu, 2020).
Developmental Calibration of OXTR and Reward Sensitivity
Many of the developmental stressors which coincide with changes to neural reward sensitivity are social in nature. This is not surprising given that humans have evolved to be highly social. Social perception (Shamay-Tsoory & Abu-Akel, 2016) and behaviors, including mate selection, copulation, and parenting strategies, are at least in part guided by variation in the oxytocinergic system (Carter, 2003; Feldman, Monakhov, Pratt, & Ebstein, 2016). In turn early life social experiences change the oxytocinergic system. Indeed, experiences of childhood abuse coincide with decreased oxytocin availability in the blood and cerebral spinal fluid (Heim et al., 2009; Opacka-Juffry & Mohiyeddini, 2012) and higher DNA methylation of OXTR (Gouin et al., 2017). At the neural level, the oxytocinergic system contributes to the response to social behaviors in putatively reward-related regions (Love, 2014) and its interaction with dopamine in these regions influences many important aspects of lifespan development: e.g, mother-infant attachment (Strathearn, 2011), sexual behavior (Melis et al., 2007), and pair bonding (Liu & Wang, 2003). Importantly, the effects of oxytocin on the brain likely depend on expression of its receptor, OXTR (Gimpl, Fahrenholz, & Gene, 2001; Yoshida et al., 2009).
If we synthesize the literature on ELS, oxytocin, and reward sensitivity we arrive at a reasonable ACM hypothesis: greater Harshness and Instability in the developmental context could calibrate the oxytocinergic system to promote a reward-sensitive phenotype through its impact on neural reward systems. We know that early life experiences are associated with variation of OXTR DNA methylation even in infancy (Krol, Puglia, Morris, Connelly, & Grossmann, 2019) and that variation in OXTR DNA methylation is associated with individual differences in the neural response to social cues in both adults (Jack, Connelly, & Morris, 2012; Puglia, Connelly, & Morris, 2018; Puglia, Lillard, Morris, & Connelly, 2015) and infants (Krol et al., 2019). DNA methylation is an epigenetic process by which the addition of a methyl group to cytosine-phosphate-guanine (CpG) sites in genes modifies transcription. This modification often reduces transcription, but other types of methylation can increase it (Zhang et al., 2018). A limitation of DNA methylation, however, is that it is often tissue specific (Lokk et al., 2014) and access to human brain tissue is severely limited. Guidance on using epigenetic imaging procedures suggest the importance of animal models in identification of appropriate genes, loci, functional relationship to neural tissue, and appropriate peripheral tissue as reporters of neural tissue (Lancaster, Morris, & Connelly, 2018). Luckily, the prairie vole model system provides us with the translational data necessary to pursue our hypothesis.
Translational Epigenetics of Early Life Stress and Neural Reward Sensitivity
A series of studies using the prairie vole by Perkeybile and colleagues (Perkeybile et al., 2019) suggest a functional relationship between developmental experience, OXTR DNA methylation in blood and brain, and OXTR mRNA expression in the brain. Specifically, the authors found that lower parental care was associated with increased OXTR DNA methylation at CpG sites −924 and −934 in the promoter region of OXTR. These CpG sites are homologous to sites in humans and are contained within a region of the gene that is important in regulating DNA methylation-dependent transcription of OXTR (Kusui et al., 2001). In voles, DNA methylation levels are reduced in both blood and brain tissue (NAcc) with low parental care, indicating the potential for the use of the blood as a marker of early experience. In fact, the authors showed that greater OXTR DNA methylation in the blood coincided with decreased mRNA expression in NAcc further validating the potential use of blood as marker of the transcription state of the brain. Interestingly, the environmentally responsive DNA methylation state of the blood was established in early life and persisted into adulthood. This study allows for translation of this model to humans because (a) prairie voles display human-like social behaviors including monogamous pair bonding and both maternal and paternal care of offspring (Gavish, Carter, & Getz, 1981; Getz, Carter, & Gavish, 1981; Tabbaa, Paedae, Liu, & Wang, 2017), (b) CpG sites −924 and −934 are conserved in humans (Perkeybile et al., 2019), giving us suitable loci, and (c) data suggest that DNA methylation in the blood reports on transcription in the NAcc, allowing us to non-invasively assay humans.
The Current Study
Our translational study explores the role of OXTR DNA methylation at CpG sites −924 and −934 in the developmental calibration of reward sensitivity. We hypothesize that like in prairie voles, human developmental context calibrates neural reward systems via modification of OXTR. Specifically, in line with ACM, greater ELS in the form of harshness and instability should coincide with increased DNA methylation and greater neural reward sensitivity. In turn, increased DNA methylation should also coincide with increased reward sensitivity. Using a longitudinal sample started when participants were 13 years of age, we first modeled developmental context and ELS using multi-reporter data during the first wave of data collection. Separate subsets of these participants later returned as adults (25–27 years of age) to complete the Monetary Incentive Delay (MID) task, an fMRI paradigm, and donated their blood for epigenetic analysis of OXTR. We then modeled the relationships between latent factors of developmental context, OXTR DNA methylation, and neural reward sensitivity. We predicted that greater Harshness and Instability would be associated with increased reward sensitivity and that this would be mediated or moderated by increased OXTR DNA methylation.
METHODS AND MATERIALS
Participants
The Virginia Institute of Development in Adulthood (VIDA) sample is comprised of 184 socioeconomically and racially diverse participants followed since they were 13 years of age (now aged ~33). Eighty-nine individuals subsequently underwent functional magnetic resonance imaging (fMRI) as adults (23–27) and 112 participants completed blood draws to assay OXTR DNA methylation (29–32). Table 1 displays sample demographics and subsamples included in each of the three study sub-analyses. All portions of this study were approved by the Institutional Review Board. Participants gave informed consent and were compensated for their time. All data were de-identified.
Table 1.
Demographics across study components, parental income and education beginning when participants were 13 years of age
| Demographics breakdown by analysis | ||||
|---|---|---|---|---|
| Exploratory factor analysis | Neuroimaging | Epigenetic | Neuroimaging & epigenetic | |
| Total | 184 | 82 | 112 | 59 |
| Gender | ||||
| Female | 98 | 44 | 66 | 33 |
| Ethnicity | ||||
| White/Caucasian | 58% | 52% | 57% | 53% |
| Black/African American | 29% | 33% | 29% | 32% |
| Other | 13% | 15% | 13% | 15% |
| Parent’s highest education | ||||
| Below high school | 4% | 6% | 4% | 7% |
| High school or GED | 18% | 17% | 17% | 14% |
| Some college or technical training | 27% | 29% | 28% | 29% |
| Associate’s degree | 6% | 4% | 5% | 3% |
| Bachelor’s degree | 15% | 11% | 13% | 8% |
| Some graduate work | 7% | 10% | 9% | 10% |
| Post college degree | 19% | 20% | 18% | 25% |
| Annual household income | ||||
| Under $5,000 | 3% | 4% | 2% | 2% |
| $5,000-$9,999 | 4% | 4% | 5% | 5% |
| $10,000-$14,999 | 5% | 5% | 4% | 5% |
| $15,000-$19,999 | 5% | 5% | 6% | 5% |
| $20,000-$29,999 | 17% | 18% | 19% | 22% |
| $30,000-$39,999 | 8% | 7% | 9% | 7% |
| $40,000-$59,999 | 20% | 23% | 19% | 22% |
| $60,000 or more | 30% | 27% | 29% | 27% |
Behavioral Data Procedures
In Wave 1, parents and adolescents (participants) completed several questionnaires. A high proportion of fathers (38%) did not complete data collection. We therefore used data from mothers unless only the father’s data were available (N = 2). Behavioral measures were chosen based on their relevance to economic and social adversity. Social adversity includes parental experiences which may negatively impact the parent–child relationship (e.g. mental health issues). All measures were previously validated and descriptions as well as who completed them (parent or child) and when (Wave 1 or retrospective) can be found in the Supplementary Materials. Measures used were: Adverse Childhood Experiences Questionnaire (ACE), Childhood Trauma Questionnaire (CTQ), Childhood Report of Parent Behavior (CRPB), Children’s Expectation of Social Behavior (CESB), Neighborhood Quality Questionnaire (NQQ), Beck Depression Inventory (BDI; parent’s depression at Wave 1), and household income and education.
Exploratory Factor Analyses
Our factor analysis methods are discussed in detail in the Supplementary Materials. Behavioral analyses were completed in RStudio (V 1.0.136). We used exploratory factor analysis on 123 individual items from the eight measures. Restricting the covariance matrix reduced these items to 84. Data missingness was at 3.8% and were imputed using multiple imputation estimation of missing data (K-fold = 1,000, missMDA package; Josse & Husson, 2016). An exploratory factor analysis was then conducted on the 84 identified items. Tests of sampling adequacy indicated that the sample was usable (Kaiser–Meyer–Oklin = 0.69; Bartlett’s test of sphericity: χ (184) = 19,242.97, p < .01, df = 3,486).
Blood Collection and DNA Extraction
We performed venipuncture between Waves 16 and 17 (28–29 years of age). Eight and a half milliliters of whole blood were collected from each participant using a PAXgene Blood DNA Tube (PreAnalytiX, Hombrechtikon Switzerland). DNA was extracted and OXTR DNA methylation was assayed by bisulfite pyrosequencing using previously established methods(Jack et al., 2012; Puglia et al., 2015, 2018; see Supplementary Materials). Samples were run in triplicate, with methylation score averages used for all further analyses (average deviation of 1.86%). Methylation percentages for the two CpG sites were correlated at r = .65.
fMRI Data Acquisition and Preprocessing
Participants completed the MID Task to assay BOLD response related to the anticipation of monetary rewards. The paradigm is well described in the literature (Knutson, Westdorp, Kaiser, & Hommer, 2000) and in the Supporting Information. Briefly here, MID requires participants to press a button following a reward or punishment cue in order to earn money or prevent monetary loss. Participants briefly see a fixation cross in between pressing their button and receiving feedback and this allows us to look at the anticipation of monetary rewards.
Data were acquired using a Siemens 3.0 Tesla MAGNETOM Trio high-speed MRI device. Stimuli were presented through a CP transmit/receive head coil with an integrated mirror. Structural T1 echo-planar images (EPI) were first obtained (176, 1-mm slices). Functional T2-weighted EPIs were collected during MID (224 per MID run, volume = 28, 3.5-mm slices). Imaging data were preprocessed and analyzed using FMRIB Software Library (FSL) software (version 5.98; www.fmrib.ox.ac.uk/fsl). Standard pre-processing steps were completed before using FSL’s fMRI Expert Analysis Tool (FEAT) to model anticipation to monetary gain and loss using the standard lower-level contrasts (Knutson et al., 2000; Supplementary Materials).
fMRI Data Analysis
Associations between developmental context and OXTR DNA methylation on task-specific activity were assessed using FLAME. We corrected for multiple comparisons using estimated smoothness and Gaussian Random Field Theory to determine cluster size (Z = 2.3, p=.05). We conducted both region of interest analyses for a priori brain regions (NAcc, caudate, and OFC) and exploratory whole-brain analyses. Binarized region of interest (ROI) masks were created based on the Harvard-Oxford Subcortical Atlas probability map.
Developmental context and adult reward anticipation.
Each latent variable factor identified in the behavioral analysis (see Behavioral Results) was centered to the subsample (N = 82) and entered together into one general linear model. Negative and positive contrasts were computed to determine correlations between each latent construct and neural response to reward anticipation while statistically adjusting for the other constructs.
Interaction between OXTR DNA methylation and developmental context on reward anticipation.
Each latent factor and methylation percentage was Z-transformed on the overlapping sample (N = 59) and interaction terms computed. In accordance with best practices, mean anticipation reward, a developmental factor, DNA methylation at one CpG site, and the interaction term were entered into the model, but only the interaction was estimated. All models with DNA methylation as a predictor also included self-reported sex as a nuisance variable. Others have found sex-based methylation differences (Puglia et al., 2015), however we did not.
We conducted simple slopes analyses on linear regression models (Preacher, Curran, & Bauer, 2006) to understand interaction effects. We extracted the mean Z-statistic for the Reward Anticipation > Neutral contrast for each ROI from a model without covariates. Multiple linear models were used to probe the interaction effects between a single latent factor and a single CpG site on mean activation at each ROI. All models were adjusted for self-reported sex. Models were assessed for normality of residuals, linearity, heteroscedasticity, and influence.
RESULTS
Three factor solution for developmental context analysis.
Instead of a Harshness and an Instability latent construct, a three-factor solution emerged. Factors were extracted based on the following rules: (a) factors had to have an Eigenvalue greater than one and (b) each factor had to substantially increase the variance explained. A three-factor solution explained 28% of the data, and subsequent factors did not significantly increase this. The solution was examined using the ordinary least square minimum residuals measure bootstrapped with 1,000 iterations. Model fit was good (RMSEA = 0.02, 90% confidence interval = 0.02 to 0.148, Bayesian information criterion = −1075.92) though Chi Square testing rejected that three factors were sufficient (χ2 = 15,804.82, p < 0). Nevertheless, three factors struck the balance between simplicity and completeness. Factors were characterized as Neighborhood Harshness, Family Harshness, and Abuse & Disorder (see Figure 1; Supplementary Materials; Table 2 for item breakdowns). Factor scores were extracted using the ten Berge algorithm to preserve the correlations between factors (ten Berge, Krijnen, Wansbeek, & Shapiro, 1999).
Figure 1.
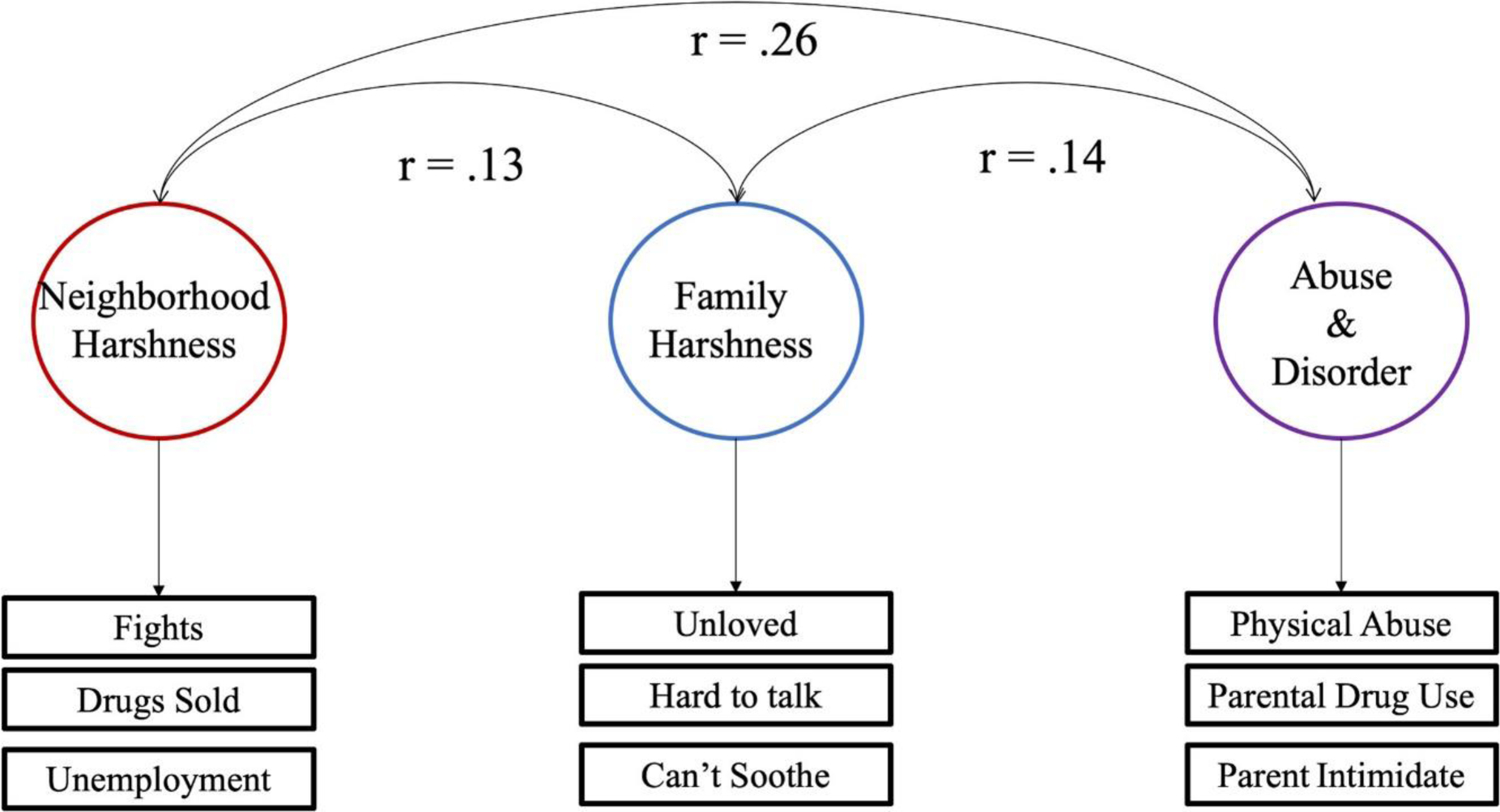
Three-factors that represent developmental context: Neighborhood Harshness, Family Harshness, Abuse and Neglect. Curved arrows indicate correlations between factors and the correlation coefficients are shown below each line. The boxes under each latent factor indicate a short description of the three most strongly loaded items for that factor.
Table 2.
Local maxima for Reward > Neutral × Developmental Context Analyses. Tables shows clusters significantly related to Neighborhood Harshness. For whole-brain analyses, brain regions are approximate and based on Harvard-Oxford Cortical and Subcortical Atlases
| Local maxima | ||||||
|---|---|---|---|---|---|---|
| Max Z Stat | X | Y | Z | k | p | |
| ROI analysis: Nucleus accumbens | ||||||
| Right | 3.82 | 14 | 22 | −2 | 140 | .00677 |
| Left | 3.14 | −10 | 20 | 2 | 55 | .0466 |
| ROI analysis: Caudate | ||||||
| Right | 3.82 | 14 | 22 | −2 | 357 | .000699 |
| Left | 3.3 | 12 | −4 | 18 | 155 | .0197 |
| Whole-brain analysis | ||||||
| Brain region | ||||||
| Right caudate | 3.82 | 14 | 22 | −2 | 1,021 | 1.24E-05 |
| Right parietal operculum | 3.66 | 30 | −28 | 24 | 656 | .000702 |
| Left occipital pole | 3.84 | −22 | −104 | 2 | 493 | .0054 |
| Left temporal gyrus | 4.24 | −48 | −44 | −6 | 450 | .00956 |
| Right occipital pole | 3.72 | 24 | −92 | 10 | 377 | .0263 |
Greater Neighborhood Harshness coincides with increased neural reward sensitivity in a priori ROIs.
Neighborhood Harshness was associated with increased activation in NAcc, caudate, and OFC in response to reward anticipation while controlling for Family Harshness and Abuse and Disorder. Associations between Family Harshness or Abuse and Disorder and reward anticipation at these ROIs were not significant when controlling for the other two factors.
OXTR DNA methylation does not mediate the association between Neighborhood Harshness and neural reward sensitivity.
Step two of creating a mediation model involved investigating the simple associations between Neighborhood Harshness and OXTR DNA methylation. We first determined that DNA methylation did not vary by race (−924: F(1,110) = 0.54, p = .46; −934: F(1,110) = 1.5, p = .22) or self-reported sex (−924: F(1,110) = 0.13, p = .72; −934: F(1,110) = 0.0, p = .98) in our current sample. We next created a general linear model predicting DNA methylation from Neighborhood Harshness, but this was found to be non-significant (−924: F(3:108) = 0.34, p = .79; −934: N = 112, F(3:108) = 0.57, p = .63. Without this direct relationship, we could not further pursue a mediation model.
OXTR DNA methylation blunts the association between Neighborhood Harshness and neural reward sensitivity.
There was a two-way interaction in caudate, and OFC between Neighborhood Harshness and methylation at CpG site −924 (Table 3; Figure 2a). Interactions between Neighborhood Harshness and CpG site −934 reached significance in caudate (Table 3; Figure 2b) and trended in NAcc, but not OFC. Simple slopes analysis (Figure 3) indicated that OXTR methylation appears to blunt the effect of adolescent Neighborhood Harshness in caudate. However, higher methylation at CpG −924 was associated with increased OFC activation to reward anticipation, but only with low Neighborhood Harshness (Figure 4).
Table 3.
Results of regions of interest analyses modeling Reward > Neutral × Neighborhood Harshness × OXTR methylation. Local maxima reflect voxels in a priori regions maximally associated with the interaction of Neighborhood Harshness and methylation at either CpG site −924 or −934
| Local maxima | ||||||
|---|---|---|---|---|---|---|
| Max Z Stat | X | Y | Z | k | p | |
| CpG 924 | ||||||
| Caudate | ||||||
| Right | 3.64 | 12 | 16 | 8 | 137 | .0247 |
| Left | 3.3 | −14 | 18 | −2 | 119 | .0362 |
| Orbital frontal cortex | ||||||
| Left | 3.08 | −48 | 22 | −2 | 155 | .0357 |
| CpG 934 | ||||||
| Caudate | ||||||
| Right | 3.7 | 16 | 16 | 6 | 274 | .00191 |
Figure 2.
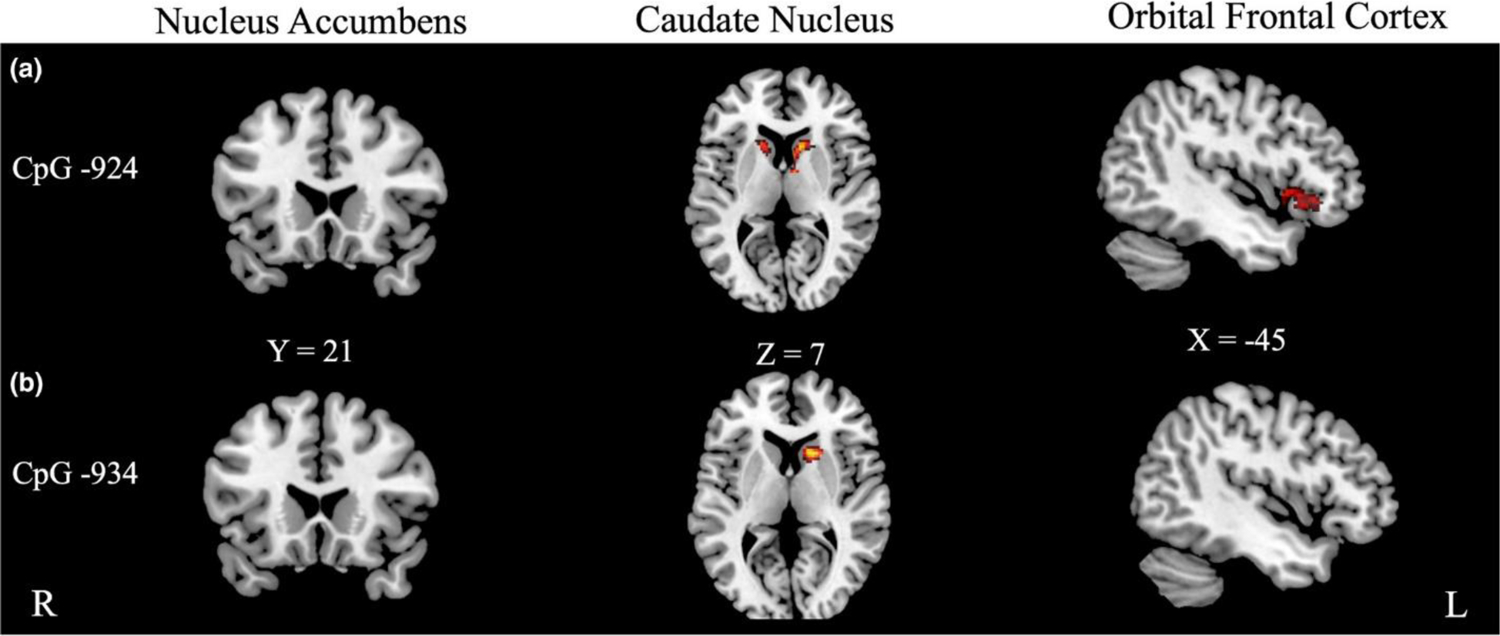
Significant results for regions of interest analyses modeling Reward > Neutral × Neighborhood Quality × OXTR methylation. (a) Methylation at OXTR CpG site −924 moderated the relationships between Neighborhood Harshness and Adult Reward Sensitivity in caudate and left orbital frontal cortex, but not nucleus accumbens. (b) Methylation OXTR CpG site −934 moderated the relationship between Neighborhood Harshness and Adult Reward Sensitivity in left caudate, but neither nucleus accumbens nor orbital frontal cortex.
Figure 3.
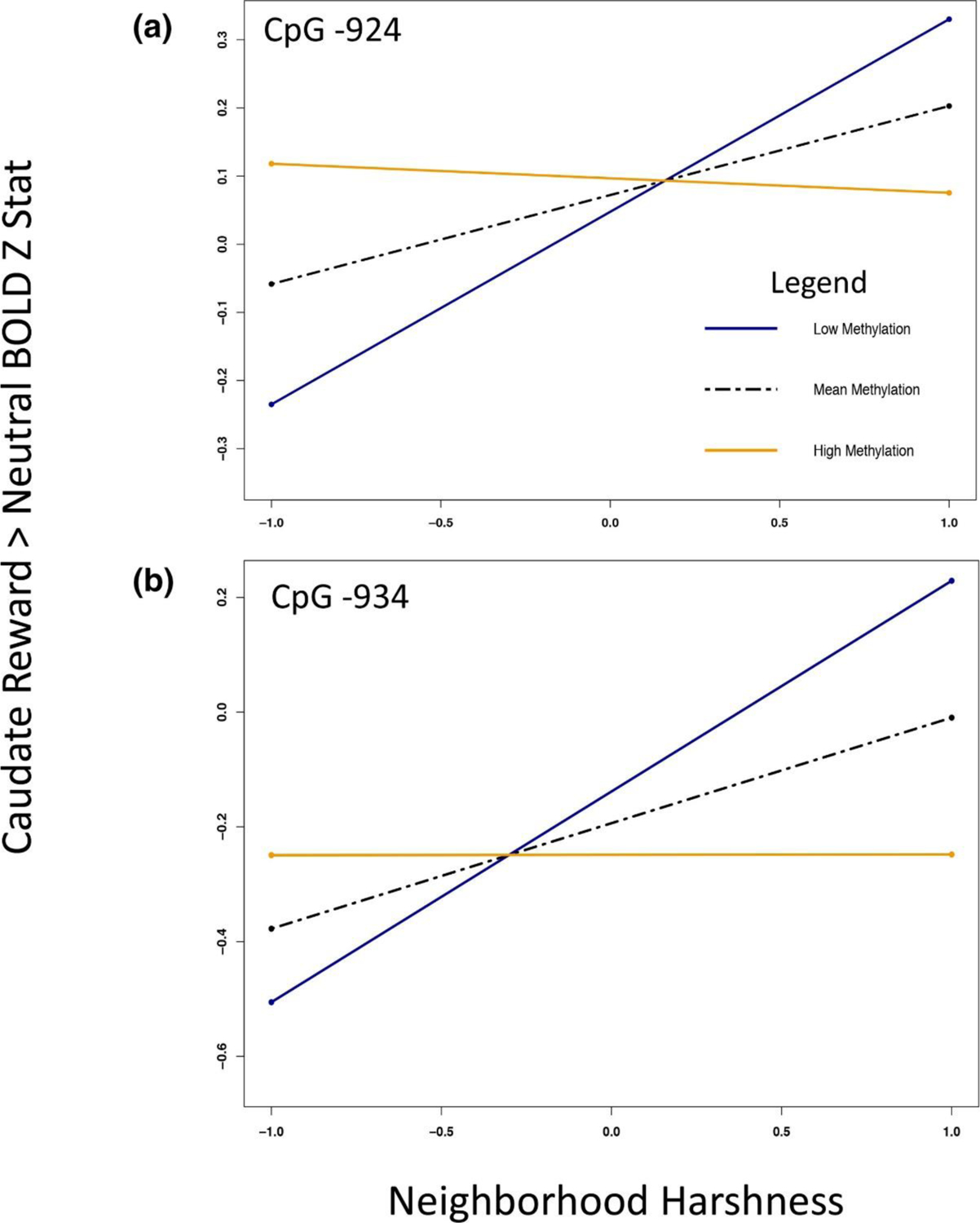
OXTR DNA methylation blunts association between Neighborhood Harshness and reward sensitivity in Caudate. Maximum likelihood estimation of simple slopes for OXTR × Neighborhood Harshness interactions on neural activation in caudate for both CpG sites. (a) High methylation (orange line), defined as one standard deviation away from the Z-transformed methylation percentage, blunts the positive association between Neighborhood Harshness and reward activation relative to individuals with mean (black dashed line) or low levels of methylation (1 standard deviation below the mean, blue line). (b) The same association is found in CpG site −934.
Figure 4.
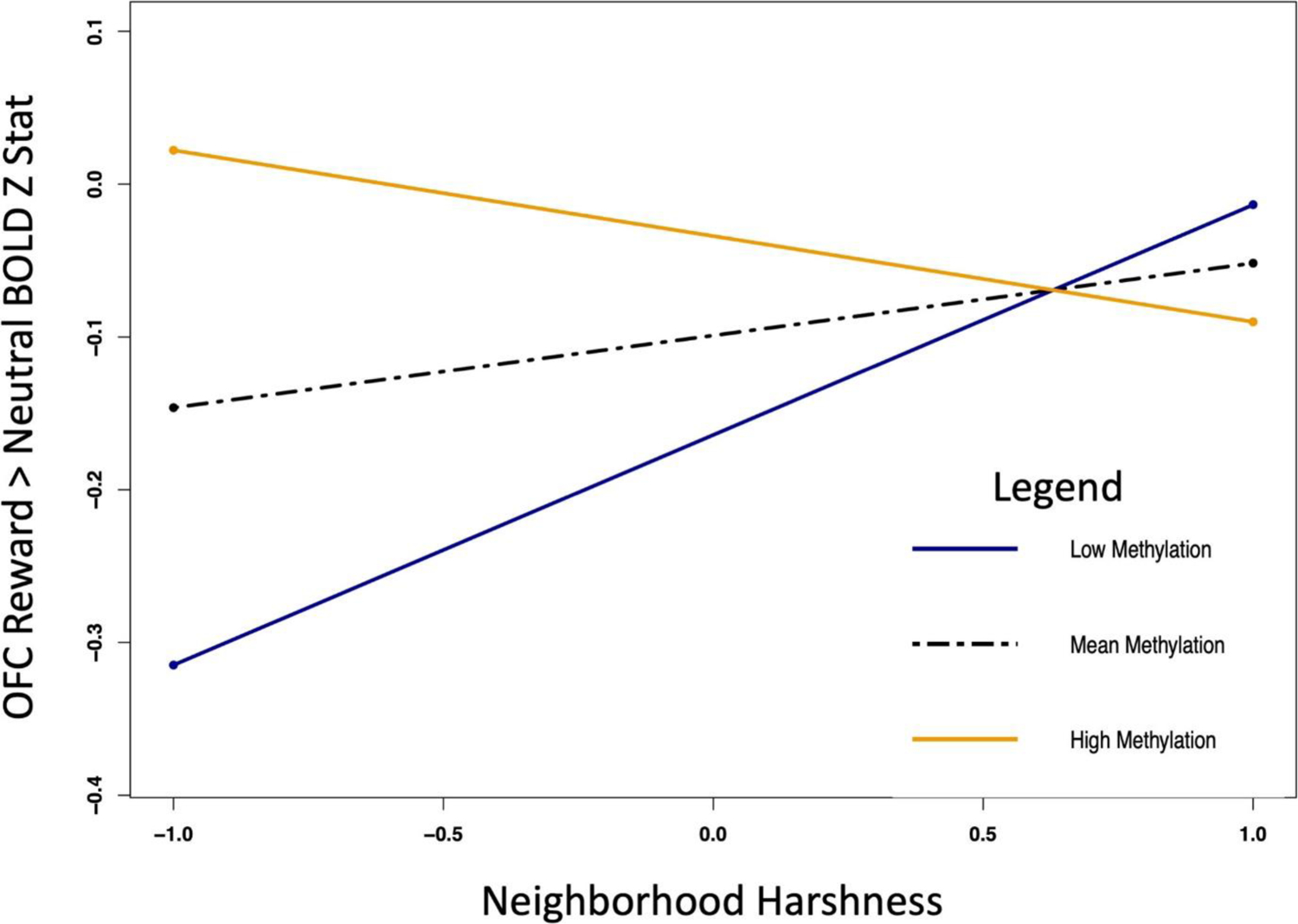
OXTR DNA Methylation reverses association between Neighborhood Harshness and reward sensitivity in OFC. Maximum likelihood estimation of simple slopes for OXTR × Neighborhood Harshness interactions on neural activation in OFC. Individuals with High Methylation at CpG site −924 (orange line) show increased anticipatory activation in OFC if they experienced low Neighborhood Harshness during adolescence. In contrast, those with low levels of methylation (blue line) show low activation in the OFC if they experienced low Neighborhood Harshness, but higher activation if they experienced high Neighborhood Harshness. This indicates an epigenetic by environment interaction on OFC response to the anticipation of rewards.
We did not find interaction effects between OXTR DNA methylation and either the Family Harshness or Abuse and Disorder constructs on neural activation at our a priori ROIs. However, whole-brain exploratory analysis across all three developmental factor-types revealed interaction effects with both CpG sites in regions of the default mode network (Supplementary Figures 5 and 7).
DISCUSSION
Our study tested a translational model derived from the prairie vole model system which links ELS and OXTR DNA methylation to adult neural reward systems. Based on ACM, we expected to model developmental context as Harshness and Instability, to associate higher levels of these dimension to increased OXTR DNA methylation, and to associate DNA methylation with increased neural activation in putatively reward-related regions of interest. We found partial support for our original hypotheses. Findings suggest that (a) our developmental context was best modeled by proximal and distal measures of ELS, (b) reward and motivational neural systems calibrated more to neighborhood harshness rather than immediate family dynamics, (c) greater neighborhood harshness coincided with a more reward-sensitive endophenotype and (d) greater OXTR DNA methylation in the promoter region was associated with the diminished importance of adolescent neighborhood harshness in the calibration of putative neural reward regions.
Neighborhood Characteristics as the Calibrating Developmental Environment
We first established a three latent factor model of adolescent developmental context. These factors—Neighborhood Harshness, Family Harshness, and Abuse and Disorder—included different types and intensities of Harshness, but not Instability. Though research on ELS and psychobiology has grown remarkably, we have little understanding of how different types of adversity may differentially impact the calibration of stress systems (Chen & Paterson, 2006; McLaughlin et al., 2014). We found that the distal Neighborhood Harshness construct, and not the proximal Family Harshness, and Abuse and Disorder constructs, was related to neural response to reward anticipation. Above individual SES, neighborhood SES and neighborhood dynamics influence risk-taking (Furr-Holden, Milam, Reynolds, MacPherson, & Lejuez, 2012). Mortality cues in neighborhoods have been related to life history behaviors such as early sexual debut (Carlson, McNulty, Bellair, & Watts, 2014; Wilson & Daly, 1997). Notably, a recent study found that neighborhood poverty, above individual SES, was associated with decreased activation in striatum during a response inhibition task in children and adolescents (Tomlinson et al., 2020). Adding to these previous findings, our data suggest that neighborhood factors, above individual family factors, calibrate neural reward systems towards a more appetitive endophenotype.
An Epigenetically Derived Differential Susceptibility Model
Greater DNA methylation in CpG sites −924 and −934 blunted the association between Neighborhood Harshness and reward-related activation in caudate. Those with low methylation had strong associations between Neighborhood Harshness and reward sensitivity in the expected life history directions (greater reward sensitivity). However, those with high methylation had no association at all. In other words, our effect looked like an epigenetically derived differential susceptibility model (Belsky & Pluess, 2009), where low methylation instead of a particular genotype rendered participants susceptible to the adolescent context. Importantly, this model would not extend to OFC findings, where we saw a full cross-over effect, perhaps owing to its regulatory rather than appetitive role in reward and motivational processes (Haber & Knutson, 2009). Future animal studies are particularly poised to disentangle differential effects across neural regions.
Another way to look at the data is to suppose that earlier experiences led to DNA methylation and this in turn led to decreases in adolescent neural plasticity to the immediate context. This sequence would align well with ACM’s position that more extreme stressors during earlier and more sensitive periods will lead to physiological systems (in this case, regions of the brain) being less open to iterative input from the environment. In the face of high mortality cues, the need for experiential canalization increases. In such a case, ACM predicts that we would trade plasticity and flexibility in favor of fixed action patterns useful for survival (Ellis, Giudice, et al., 2017; Figueredo et al., 2006). Epigenetic tuning at early stages of development (Krol et al., 2019) and even in utero (Unternaehrer et al., 2016) may then better explain the diminished receptivity to environmental inputs in adolescence.
Risk or Resilience?
Given the association between ELS and dysregulated motivational systems to poorer mental health outcomes, it is natural to wonder which differential susceptibility pattern might constitute risk or resilience. From an allostatic load perspective, short-term gains could lead to long-term risk factors (McEwen & Gianaros, 2011). The ACM perspective, goes further to say that the ability of any physiological calibration to act as a risk or resilience factor is dependent on the match between the phenotype and the environment (Ellis, Bianchi, Bianchi, Griskevicius, & Frankenhuis, 2017). Like in other differential susceptibility models, any mechanistic effects are contextually bound. In other words, there is nothing inherently pathophysiological about having more or less neural plasticity, methylation, or neural reward sensitivity.
However, there are still clinical implications. If the model is true, adolescents with low OXTR methylation will have neural reward systems susceptible to the influence of their environments. Accordingly, interventions will be maximally effective for individuals with emerging mental health issues related to reward endophenotypes. It also means that highly methylated and mentally healthy adolescents may be resilient to aspects of the adolescent context that might otherwise compromise their more susceptible peers. Effects are contextually bound. These are conjectures, but they do imagine exciting new opportunities from which to maximize clinical outcomes based on molecular profiles, as others have called for (Bakermans-Kranenburg & van Ijzedoorn, 2014).
Limitations and Future Directions
While results of these analyses are bolstered by the mechanistic and functional animal data, our conclusions are still preliminary and necessitate replication and expansion through future studies. A clear limitation of our study is the limited number of people with complete developmental, neuroimaging, and epigenetic data (N = 59). Recent multisite longitudinal data collection efforts may shed light on some of the developmental sequalae suggested here (Casey et al., 2018), but targeted data collection is also warranted. Future data collection should focus on translating animal models as these provide the best mechanistic support. Importantly, our analysis failed to show a significant association between OXTR DNA methylation and any of our latent factors of developmental context despite others having found increased DNA methylation with experiences of childhood abuse (Smearman et al., 2016). Because DNA methylation occurs in utero and can change with very early life experience (Krol et al., 2019; Perkeybile et al., 2018; Unternaehrer et al., 2016), obtaining measures of DNA methylation at birth and through development along with developmental context may be necessary. Elucidating how different aspects of the developmental environment tune oxytocinergic and motivational systems can bring us closer to mechanisms of normative development and contextually bound understandings on putative endophenotypes of psychopathology.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institute of Mental Health (R01MH080725) awarded to James A. Coan, grants from the National Institute of Child Health and Human Development and the National Institute of Mental Health (9R01 HD058305-11A1 & R01-MH58066) awarded to Joseph P. Allen. Special thanks to Meghan Puglia who provided consultation and an extra set of eyes.
Footnotes
CONFLICT OF INTEREST
The authors, Marlen Z. Gonzalez, Kelly L. Wroblewski, James A. Coan, Joseph P. Allen, and Jessica J. Connelly, declare no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Bakermans-Kranenburg MJ, & van Ijzedoorn MH (2014). The hidden efficacy of interventions: Gene × environment experiments from a differential susceptibility perspective. Annual Review of Psychology, 1–29. 10.1146/annurev-psych-010814-015407 [DOI] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135(6), 885–908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, & Draper P (1991). Childhood experience, interpersonal development, and reproductive strategy: And evolutionary theory of socialization. Child Development, 62(4), 647–670. 10.1111/1467-8624.ep9109162242 [DOI] [PubMed] [Google Scholar]
- Carlson DL, McNulty TL, Bellair PE, & Watts S (2014). Neighborhoods and racial/ethnic disparities in adolescent sexual risk behavior. Journal of Youth and Adolescence, 43(9), 1536–1549. 10.1007/s10964-013-0052-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2003). Developmental consequences of oxytocin. Physiology and Behavior, 79(3), 383–397. 10.1016/S0031-9384(03)00151-3 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, … Dale AM (2018). Developmental cognitive neuroscience the adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32(January), 43–54. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J, Krishnadas R, Batty GD, Burns H, Deans KA, Ford I, … McLean J (2013). Socioeconomic status and the cerebellar grey matter volume. Data from a well-characterised population sample. The Cerebellum, 12(6), 882–891. 10.1007/s12311-013-0497-4 [DOI] [PubMed] [Google Scholar]
- Chen E, & Paterson LQ (2006). Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 25(6), 704–714. 10.1037/0278-6133.25.6.704 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Bianchi JM, Griskevicius V, & Frankenhuis WE (2017). Beyond risk and protective factors: An adaptation-based approach to resilience. Perspectives on Psychological Science, 12(4), 561–587. 10.1177/1745691617693054 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, & Schlomer GL (2009a). Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature, 20(2), 204–268. 10.1007/s12110-009-9063-7 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, & Schlomer GL (2009b). Fundamental dimensions of environmental risk. Human Nature, 20(2), 204–268. 10.1007/s12110-009-9063-7 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Giudice MD, & Shirtcliff EA (2017). The adaptive calibration model of stress responsitivity. Child and Adolescent Psychopathology, 29(3), 237–276. [Google Scholar]
- Fareri DS, & Tottenham N (2016). Effects of early life stress on amygdala and striatal development. Developmental Cognitive Neuroscience, 19, 233–247. 10.1016/j.dcn.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, & Ebstein RP (2016). Oxytocin pathway genes: Evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biological Psychiatry, 79(3), 174–184. 10.1016/j.biopsych.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Figueredo A, Vasquez G, Brumbach B, Schneider S, Sefcek J, Tal I, … Jacobs W (2006). Consilience and life history theory: From genes to brain to reproductive strategy. Developmental Review, 26(2), 243–275. 10.1016/j.dr.2006.02.002 [DOI] [Google Scholar]
- Furr-Holden CDM, Milam AJ, Reynolds EK, MacPherson L, & Lejuez CW (2012). Disordered neighborhood environments and risk-taking propensity in late childhood through adolescence. Journal of Adolescent Health, 50(1), 100–102. 10.1016/j.jadohealth.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish L, Carter CS, & Getz LL (1981). Further evidences for monogamy in the prairie vole. Animal Behaviour, 29(3), 955–957. 10.1016/S0003-3472(81)80035-8 [DOI] [Google Scholar]
- Getz LL, Carter CS, & Gavish L (1981). The mating system of the prairie vole, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behavioral Ecology and Sociobiology, 8(3), 189–194. 10.1007/BF00299829 [DOI] [Google Scholar]
- Gimpl G, Fahrenholz F, & Gene C (2001). The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews, 81(2), 629–683. [DOI] [PubMed] [Google Scholar]
- Gonzalez MZ, Allen JP, & Coan JA (2016). Lower neighborhood quality in adolescence predicts higher mesolimbic sensitivity to reward anticipation in adulthood. Developmental Cognitive Neuroscience, 22, 48–57. 10.1016/j.dcn.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MZ, Puglia MH, Morris JP, & Connelly JJ (2017). Oxytocin receptor genotype and low economic privilege reverses ventral striatum-social anxiety association. Social Neuroscience, 14(1), 67–79. 10.1080/17470919.2017.1403954 [DOI] [PubMed] [Google Scholar]
- Gouin JP, Zhou QQ, Booij L, Boivin M, Côté SM, Hébert M, … Vitaro F (2017). Associations among oxytocin receptor gene (OXTR) DNA methylation in adulthood, exposure to early life adversity, and childhood trajectories of anxiousness. Scientific Reports, 7(1), 1–14. 10.1038/s41598-017-07950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2009). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 1–23. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, & Williamson DE (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78(9), 598–605. 10.1016/j.biopsych.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, & Nemeroff CB (2009). Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry, 14(10), 954–958. 10.1038/mp.2008.112 [DOI] [PubMed] [Google Scholar]
- Jack A, Connelly JJ, & Morris JP (2012). DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Frontiers in Human Neuroscience, 6(October), 280. 10.3389/fnhum.2012.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse J, & Husson F (2016). missMDA: A package for handling missing values in multivariate data analysis. Journal of Statistical Software, 70. 10.18637/jss.v70.i01 [DOI] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, & Hommer D (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. 10.1006/nimg.2000.0593 [DOI] [PubMed] [Google Scholar]
- Krol KM, Puglia MH, Morris JP, Connelly JJ, & Grossmann T (2019). Epigenetic modification of the oxytocin receptor gene is associated with emotion processing in the infant brain. Developmental Cognitive Neuroscience, 37(August 2018), 100648. 10.1016/j.dcn.2019.100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusui C, Kimura T, Ogita K, Nakamura H, Matsumura Y, Koyama M, … Murata Y (2001). DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochemical and Biophysical Research Communications, 289(3), 681–686. 10.1006/bbrc.2001.6024 [DOI] [PubMed] [Google Scholar]
- Lancaster K, Morris JP, & Connelly JJ (2018). Neuroimaging epigenetics: Challenges and recommendations for best practices. Neuroscience, 370, 88–100. 10.1016/j.neuroscience.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, & Fan J (2011). Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219–1236. 10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, & Wang ZX (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience, 121(3), 537–544. 10.1016/S0306-4522(03)00555-4 [DOI] [PubMed] [Google Scholar]
- Lokk K, Modhukur V, Rajashekar B, Märtens K, Mägi R, Kolde R, … Tõnisson N (2014). DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biology, 15(4), 10.1186/gb-2014-15-4-r54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM (2014). Oxytocin, motivation and the role of dopamine. Pharmacology Biochemistry and Behavior, 119, 49–60. 10.1016/j.pbb.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; CrossrefCASPubMedWeb of Science®Google ScholarGo here for SFX
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2016). Reward processing and risk for depression across development. Trends in Cognitive Sciences, 20(6), 456–468. 10.1016/j.tics.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M, Haynes MR, Barter JW, Weinberger DR, & Zink CF (2011). Subjective socioeconomic status predicts human ventral striatal responses to social status information. Current Biology, 21(9), 794–797. 10.1016/j.cub.2011.03.050 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews, 47, 578–591. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, … Argiolas A (2007). Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. European Journal of Neuroscience, 26(4), 1026–1035. 10.1111/j.1460-9568.2007.05721.x [DOI] [PubMed] [Google Scholar]
- Michaelson L, de la Vega A, Chatham CH, & Munakata Y (2013). Delaying gratification depends on social trust. Frontiers in Psychology, 4(June), 355. 10.3389/fpsyg.2013.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opacka-Juffry J, & Mohiyeddini C (2012). Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress, 15(1), 1–10. 10.3109/10253890.2011.560309 [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Carter CS, Wroblewski KL, Puglia MH, Kenkel WM, Lillard TS, … Connelly JJ (2019). Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology, 99, 128–136. 10.1016/j.psyneuen.2018.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJKJ, Curran PJPJ, & Bauer DJJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31(4), 437–448. 10.3102/10769986031004437 [DOI] [Google Scholar]
- Puglia MH, Connelly JJ, & Morris JP (2018). Epigenetic regulation of the oxytocin receptor is associated with neural response during selective social attention. Translational Psychiatry, 8(1). 10.1038/s41398-018-0159-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MH, Lillard TS, Morris JP, & Connelly JJ (2015). Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proceedings of the National Academy of Sciences, 112(11), 3308–3313. 10.1073/pnas.1422096112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, & Everitt BJ (1996). Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology, 6(2), 228–236. 10.1016/S0959-4388(96)80077-8 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79(3), 194–202. 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Smearman EL, Almli LM, Conneely KN, Brody GH, Sales JM, Bradley B, … Smith AK (2016). Oxytocin receptor genetic and epigenetic variations: Association with child abuse and adult psychiatric symptoms. Child Development, 87(1), 122–134. 10.1111/cdev.12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L (2011). Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. Journal of Neuroendocrinology, 23(11), 1054–1065. 10.1111/j.1365-2826.2011.02228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturge-Apple ML, Suor JH, Davies PT, Cicchetti D, Skibo MA, & Rogosch FA (2016). Vagal tone and childrens delay of gratification: Differential sensitivity in resource-poor and resource-rich environments. Psychological Science, 27(6), 885–893. 10.1177/0956797616640269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suor JH, Sturge-Apple ML, Davies PT, & Cicchetti D (2017). A life history approach to delineating how harsh environments and hawk temperament traits differentially shape children’s problem-solving skills. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58(8), 902–909. 10.1111/jcpp.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbaa M, Paedae B, Liu Y, & Wang Z (2017). Neuropeptide regulation of social attachment: The prairie vole model. Comprehensive Physiology, 7(1), 81–104. 10.1002/cphy.c150055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge JMF, Krijnen WP, Wansbeek T, & Shapiro A (1999). Some new results on correlation-preserving factor scores prediction methods. Linear Algebra and Its Applications, 289(1–3), 311–318. 10.1016/S0024-3795(97)10007-6 [DOI] [Google Scholar]
- Tomlinson RC, Burt SA, Waller R, Jonides J, Miller AL, Gearhardt AN, … Hyde LW (2020). Neighborhood poverty predicts altered neural and behavioral response inhibition. NeuroImage, 209, 116536. 10.1016/j.neuroimage.2020.116536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer E, Bolten M, Nast I, Staehli S, Meyer AH, Dempster E, … Meinlschmidt G (2016). Maternal adversities during pregnancy and cord blood oxytocin receptor (OXTR) DNA methylation. Social Cognitive and Affective Neuroscience, 11(9), 1460–1470. 10.1093/scan/nsw051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME, Evans GW, Grant K, Carter JS, & Duffy S (2016). Poverty and the development of psychopathology. Developmental Psychopathology, IV, 1–44. 10.1002/9781119125556.devpsy404 [DOI] [Google Scholar]
- Wilson M, & Daly M (1997). Life expectancy, economic inequality, homicide, and reproductive timing in Chicago neighbourhoods. BMJ (Clinical Research Ed.), 314(7089), 1271–1274. 10.1136/bmj.314.7089.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaple ZA, & Yu R (2020). Functional and structural brain correlates of socioeconomic status. Cerebral Cortex (New York, NY: 1991), 30(1), 181–196. 10.1093/cercor/bhz080 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, & Nishimori K (2009). Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(7), 2259–2271. 10.1523/JNEUROSCI.5593-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T-Y, Keown CL, Wen X, Li J, Vousden DA, Anacker C, … Meaney MJ (2018). Environmental enrichment increases transcriptional and epigenetic differentiation between mouse dorsal and ventral dentate gyrus. Nature Communications, 9(1), 1–11. 10.1038/s41467-017-02748-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


