Abstract
In 2020 North Italy suffered the SARS-CoV-2-related pandemic with a high number of deaths and hospitalization. The effect of atmospheric parameters on the amount of hospital admissions (temperature, solar radiation, particulate matter, relative humidity and wind speed) is studied through about 8 months (May–December). Two periods are considered depending on different conditions: a) low incidence of COVID-19 and very few regulations concerning personal mobility and protection (“free/summer period”); b) increasing incidence of disease, social restrictions and use of personal protections (“confined/autumn period”). The “hospitalized people in medical area wards/100000 residents” was used as a reliable measure of COVID-19 spreading and load on the sanitary system. We developed a chemometric approach (multiple linear regression analysis) using the daily incidence of hospitalizations as a function of the single independent variables and of their products (interactions). Eight administrative domains were considered (altogether 26 million inhabitants) to account for relatively homogeneous territorial and social conditions.
The obtained models very significantly match the daily variation of hospitalizations, during the two periods. Under the confined/autumn period, the effect of non-pharmacologic measures (social distances, personal protection, etc.) possibly attenuates the virus diffusion despite environmental factors. On the contrary, in the free/summer conditions the effects of atmospheric parameters are very significant through all the areas. Particulate matter matches the growth of hospitalizations in areas with low chronic particulate pollution. Fewer hospitalizations strongly correspond to higher temperature and solar radiation. Relative humidity plays the same role, but with a lesser extent. The interaction between solar radiation and high temperature is also highly significant and represents surprising evidence. The solar radiation alone and combined with high temperature exert an anti-SARS-CoV-2 effect, via both the direct inactivation of virions and the stimulation of vitamin D synthesis, improving immune system function.
Keywords: SARS-CoV-2, COVID-19, Temperature, Solar radiation, Particulate matter, Relative humidity, Hospitalization, Chemometrics
1. Introduction
At the end of 2019 a novel coronavirus, now defined as SARS-CoV-2, has emerged in Wuhan (China) and it has rapidly spread worldwide, becoming a very serious health concern (Lu et al., 2020). Its related disease, known as COVID-19, has caused more than 5 million deaths across the globe (WHO, 2021). This pathogen is an enveloped non-segmented RNA virus with a spherical-shape (Xu et al., 2020). Other RNA viruses show some potential pathogenetic mechanisms and clinical manifestations similar to SARS-CoV-2, thus many studies have focused on researching possible similarities among these pathogens and have considered that the spreading of this new pandemic may be associated with seasonal and environmental factors (Fiorino et al., 2021b). After China, North Italy was one of the most impacted areas by the epidemic in the world since its beginning. However, its incidence has not resulted homogeneous across distinct regions and areas in this nation as well as depending on the different seasons and climatic conditions (Quaranta et al., 2020). These observations have led researchers to accurately consider physical/environmental/meteorological parameters (among others) as factors able to control the diffusion and the severity of the disease caused by SARS-CoV-2 infection (Lolli et al., 2020). This approach derives from the observation that other well-known respiratory viruses display seasonal fluctuations of their epidemic manifestations, with distinct peak incidence (Nichols et al., 2008; Moriyama et al., 2020). It is well-known that both outdoor and indoor distinct climate conditions and environmental factors have an important impact on the transmission efficiency of these pathogens as well as on the human susceptibility to them (Leung, 2021). Temperature, solar radiation, particulate matter, relative humidity, and wind speed have been identified as factors capable to influence the replicative fitness, transmissibility, and the pathogenicity of respiratory viruses as well as the severity of the infectious diseases caused by these pathogens. The above-mentioned climate- or ambient-conditions may act both by altering the survival and spread of viruses in the environment and by changing host's susceptibility against them (Moriyama et al., 2020; Audi et al., 2020). Only a few studies have examined the relationship between wind speed and the development of respiratory tract diseases, although no definitive conclusions have been reached, whereas no reports concerning the effects of wind speed on the immune system activities are currently available (Toczylowski et al., 2021). Furthermore, in a large social community, the meteorological conditions must be considered together with personal mobility and with the progressive restrictions adopted during the course of the pandemic, making more complex the interaction between climate, environment, and health. Moreover, the effect of atmospheric parameters could vary according to the mutual relationship among them.
The aim of our study was to better understand what factors may influence the transmissibility of SARS-CoV-2 in terms of hospital admission. Three main features distinguish our approach respect to many other works published on this subject: a) we considered the daily variation of factors within single administrative areas instead of comparing different zones; such a condition is more realistic, assuming that people doesn't move through different provinces to prevent the viral infection; moreover, other confounding factors (for instance the demographic variables) are not applicable within a single area; b) multivariate statistical models were used considering both single environmental parameters (single variables) as well as their interactions: this chemometric approach has never been used in this context up today and give rise to new findings; c) the effect of non-pharmacologic measures (social distances, personal protection, etc.) is considered by modelling two different time periods: one free of mobility and social restrictions (18 May – 6 November 2020), the other characterized by progressive and differentiated social limitations established during the local “second wave” (14 November – 31 December 2020). The study focuses on North Italy, because of its high incidence of the COVID-19 and as a representative area of the western countries for its socio-economic features (Postiglione et al., 2020; Rosano et al., 2020).
1.1. Effects of temperature, UV radiation, humidity, particulate matter, and wind speed
Atmospheric parameters can have a crucial impact on the pandemic crisis by several routes, at least three points can be considered: the direct effect on the virus survival, the interaction with the media necessary for the diffusion of the virions from person to person (for instance the droplets stability) and the effect on the human immune system. In open-air conditions all these features interact with each other, so it is possible that ecological studies highlight controversial roles of the atmospheric parameter in different areas or under different conditions; recent reviews show several of these unusual cases all over the world (Jayaweera et al., 2020; Paraskevis et al., 2021; Srivastava, 2021), but the subject is already well known for respiratory viruses (Pica and Bouvier, 2012).
1.1.1. Effect of atmospheric parameters on the human immune system
It has been shown that atmospheric factors may affect and also alter the normal function of several defense mechanisms in humans. In the course of viral infections, the first line of protection is provided by the respiratory mucosal barrier. It consists of three main elements, including: the mucus layer, the surface liquid layer and the cilia on the surface of the bronchus epithelia. All these elements cooperate in counteracting invading pathogens by promoting their mucociliary clearance (Kudo et al., 2019). Nevertheless, if the virus bypasses this defense mechanism, further protective responses are activated, and innate and adaptive arms of the host's immune system are triggered. Viral pathogen-associated molecular patterns (PAMPs) are recognized by Toll-Like Receptors on the membrane of antigen-presenting cells, leading to the activation of type-I interferons (IFNs) and to the transcription of a large spectrum of IFNs-regulated genes as well as to the stimulation of specific T- and B-cells. Some experimental studies have shown that low humidity conditions and dry air in the environment cause a higher susceptibility to some viruses, such as influenza viruses in humans, via an impairment in the function of the muco-ciliar barrier as well as in the activity of innate immunity (Shephard and Shek, 1998; Eccles, 2002; Kudo et al., 2019). Furthermore, some researchers have suggested that both environmental-as well as host's body temperature may affect both innate and adaptive immune-mediated antiviral responses in animals and humans, but, to date, the results of the available investigations are not univocal. In a model of guinea pigs, investigating the factors involved in influenza virus transmission, animals housed at 5 °C presented a higher viral transmission in comparison with ones housed at 20 °C and 30 °C, but innate responses were comparable between the three different groups of animals (Lowen et al., 2007). On the other hand, a study in mice has shown that elevated environmental temperature (36 °C) may impair adaptive immune responses to influenza A virus infection (Moriyama and Ichinohe, 2019). Furthermore, also the host's body temperature may influence innate and adaptive immune response. One research has suggested that warm temperature in mouse airway cells may improve innate defense against the common cold virus, decreasing its replication capability; however, the better antiviral effect may be detectable with a restricted range of temperature (35–37 °C) (Foxman et al., 2015). Exposure to particulate matter causes cell and mitochondrial damage in mammalian and human tissues. This event is associated with the generation of reactive oxygen species (ROS). The synthesis and release of these mediators mainly occurs during the phagocytosis of the pollutant particles by macrophages and by antigen-presenting cells. Furthermore, the usual production of ROS is up-regulated in mitochondria, following pollutants-mediated injury. The increase in the release of these free radicals triggers the onset/exacerbation of inflammatory events, leading to the release of cytokines, interleukins, and mediators and to the generation of a phlogistic microenvironment (Valacchi et al., 2020). Experimental “in vitro” studies have shown that the exposure of human peripheral blood mononuclear cells to metal-containing particulate matter shifts the balance of Th1/Th2 and Treg/Th17 lymphocyte, promoting the polarization of T cells towards Th1 and Th17 subsets. Th1 and Th17 cells induce the release of IFN-γ and IL-17. These mediators down-regulate the expression of FoxP3 protein, promoting the inhibition of T reg cells and stimulating pro-inflammatory activity of Th17 lymphocytes. These events lead to an exacerbation of tissue inflammation (Gałuszka et al., 2020; Matthews et al., 2016). It is well-known that sunlight prevents respiratory tract infection mediated by several viruses, such as the influenza virus (Walker and Ko, 2007; Tseng and Li, 2005). Exposure to solar radiation also affects the survival of several pathogens, exerting germicidal and antiviral activities. Current evidence suggests UV-B rays are effective in inactivating respiratory viruses via the induction of irreversible modifications in their nucleic acids or proteins (Ianevski et al., 2019) and in inducing the activation of vitamin D (VD) in the human skin. Some epidemiological and clinical studies suggest that this fat-soluble compound exerts important effects in counteracting respiratory tract viruses-mediated infections (Hughes and Norton, 2009). A relationship between vitamin D deficiency and a higher risk of intensive care admission (Remmelts et al., 2012) as well as more elevated mortality rates in subjects with more severe forms of pneumonia has been demonstrated since several years ago (Dancer et al., 2015). However, despite the protective role of VD has been reported, the prevalence of low vitamin D status is still a health problem worldwide, involving not only the oldest individuals but also all age classes and people living in geographical areas with sun exposure all year round (Holick and Chen, 2008; Hughes and Norton, 2009; Palacios and Gonzalez, 2014). VD, alone or in cooperation with other micronutrients, improves the function of the innate ad adaptive arm of the immune system. In particular, this fat-soluble compound prevents an excessive inflammatory response, by down-regulating the activity of some pro-inflammatory cytokines, such as IL-6, IL-8, IL-12, IFN-γ, and Th17 and Th1 lymphocytes, and promoting the up-regulation of T regulatory cells. This activity results in the modulation and control of immune response strength (Chang et al., 2010; Kim et al., 2020).
1.1.2. Laboratory studies and simulations
More firm indications arise from laboratory studies that have tested the effect of temperature on the survival of SARS-CoV-2 (Chin et al., 2020; Matson et al., 2020; Riddell et al., 2020) and also other DNA and RNA viruses (HBV, HCV, and HIV) under controlled conditions (Than et al., 2019; Doerrbecker et al., 2011; Paintsil et al., 2014; Song et al., 2010). A large series of reviews concerning in-vitro data about several coronavirus types (Aboubakr et al., 2020; Guillier et al., 2020), show that the increase of temperature reduces the viability of these pathogens (including SARS-CoV-2) on different materials in a similar way. A mathematical model has been developed, but the uncertainty in predicting the inactivation of any coronavirus strains, belonging to Alphacoronavirus and Betacoronavirus genera on different materials as a function of temperature, persists (Guillier et al., 2020).
Furthermore, it is now well-known that solar radiation, especially the UV-B component (315-280 nm), counteracts the survival of SARS-CoV-2 (Ratnesar-Shumate et al., 2020). On the other hand, the UV-C component (<280 nm) is unable to get the ground and is absorbed by the atmosphere, however, it is commonly used as a germicidal tool against viral particles (Walker and Ko, 2007). Theoretical computations based on laboratory results indicate that the dose of UV-B solar radiation reaching the Earth's surface is effective for the control of the SARS-CoV-2 diffusion (Sagripanti and Lytle, 2020; Nicastro et al., 2020). The UV-A (400-315 nm) channel has not a direct activity against SARS-CoV-2 (Darnell et al., 2004), but it may exert some benefits towards this pathogen, as the UV-A radiation induces cardiovascular and metabolic protective effects upon photo-release of nitric oxide from stores in the skin (Cherrie et al., 2021).
High values of air relative humidity are considered an effective inhibitory factor for SARS-CoV-2 (Chan et al., 2011), MERS-CoV (van Doremalen et al., 2013), SARS-CoV (Casanova et al., 2010), and Influenza A (Yang and Marr, 2011). Also, Campos et al. (2020) have reported that the SARS-CoV-2 is reduced by heating more efficiently when the relative humidity is higher, in agreement with other RNA viruses. Nevertheless, the increase of environmental relative humidity is less effective in promoting the inactivation of coronaviruses in both solid and liquid fomites in comparison with temperature. In particular, the data collected by Guillier et al. (2020) show that the plot describing relative humidity and the reduction of virus infectivity is extremely variable, and at >99% relative humidity the virus infectivity is unpredictable and any relationship between these parameters is lost. A specific study highlights that both high (>85%) and low (<60%) relative humidity determines a significant decrease of infectivity in models of influenza virus and SARS-CoV (Prussin et al., 2018), substantially in agreement with the review by Moriyama et al. (2020). The researchers have observed that in droplets the inactivation of influenza viruses occurs at intermediate relative humidity (40%–60%). The review by Ahlawat et al. (2020) has concluded that in indoor environment the chances of airborne transmission of SARS-CoV-2 are higher in dry conditions (<40% relative humidity) than those detectable in humid places (>90%).
The relationship between the effect of water vapour on the stability of SARS-CoV-2 and on the stability of droplets is still to be defined: apparently, the pathogen suffers the complete dryness, in agreement with its reduced transmission in dry climates, whereas the same effect in wet environments contradicts this hypothesis (Corpet, 2021); also, it must be considered that the droplets enlarge and fall down if humidity is high enough (Jayaweera et al., 2020). The virus diffusion after sneezing calculated at 5 °C and 20 °C and at 50% and 90% relative humidity shows that the infection risk distance is less than 1.75 m at 5 °C and slightly more than 1.75 m at 20 °C. This effect is due to the balance between the stability of droplets which represent the most infectious media (liquid) and the dispersion of evaporation nuclei which may remain infectious for a considerable amount of time, travelling long distances (Wang et al., 2021).
Another atmospheric parameter that is considered very critical for the COVID-19 diffusion and severity is the particulate matter (PM). Genetic material from the SARS-CoV-2 virus was detected on particulate matter less than 10 μm (PM10) in the city of Bergamo (Italy), severely impacted by SARS-CoV-2 infection (Setti et al., 2020). Other data on Italian areas during the same COVID-19 crisis (Chirizzi et al., 2020) show that in crowded sites the PM10 results in a vector for SARS-CoV-2, whereas in different contexts the particulate resulted negative. In Madrid, no presence of SARS-CoV-2 was detected in particulate matter of various sizes (10, 2.5, and 0.1 μm) during a period with a relatively reduced circulation of the coronavirus, low PM concentration, and high temperature (Linillos-Pradillo et al., 2021). Very similar results have been reported from the Padova province (North Italy), in the earliest phases of the pandemic diffusion in that area (Pivato et al., 2021). Theoretically, the SARS-CoV-2 can persist on the particulate surfaces and this event may contribute to its transmission (Duval et al., 2021). Particulate materials may serve as a shuttle for the virions, but this aspect cannot be generalized, as the characteristics of particles surfaces are variable and several features of the virions-particle interaction can decrease the virus viability (Wathore et al., 2020; Nieto-Juarez and Kohn, 2013). The environmental and meteorological conditions may eventually exert additional effects (positive or negative) on the virus transmissibility (for instance humidity, UV irradiation, evaporation due to temperature or wind).
The effect of air movements on SARS-CoV-2 is difficult to measure, but simulations can help in deciphering the effect of wind alone. Li et al. (2020) have computed that the larger droplets (ranging from 100 to 1000 μm) increase their travel distance with the wind speed, taking into account also the evaporation rate at specific values of temperature and relative humidity. Similar conclusions are reported also for smaller droplets (10–100 μm) and, even if at high wind speed (3 m/s) the human thermal plume is destroyed, their deposition on a susceptible person downwind is ten times higher than at low speed (0.2 m/s; Yang et al., 2021). Under such a condition (downwind), the wind speed may be a major infection factor as it narrows the infectious plume and relatively-high virion concentrations can be found even at 9 m away from the infective person, at least under the studied conditions (wind speed 0.1–11.5 m/s; Rezaali and Fouladi-Fard, 2021).
1.1.3. Ecological studies - global considerations
In addition to the indications about the effects of a single parameter obtained by laboratory data or by simulations, a lot of ecological studies have investigated the association between meteorological and environmental conditions, the spread of the pandemic, and its severity. It is very difficult to compare these researches because they consider different areas, time periods, and parameters (for instance the temperature or the daily temperature excursion). Nevertheless, general reviews performed on the global scale or on continental or sub-continental areas tried to identify systematic relationships among the COVID-19 and environmental parameters. The results are often contradictory, but the efficacy of higher temperatures in preventing virus diffusion seems one of the few common features in all studies over the world (Kubota et al., 2020; Ma et al., 2020; Wu et al., 2020a; Asher et al., 2021; Grespan et al., 2021; Sanchez-Lorenzo et al., 2021; Srivastava, 2021; Notari, 2021), even if different results are reported for the first wave of the pandemic, up to spring 2020 (Sfîcă et al., 2020; Paraskevis et al., 2021).
Higher temperatures are commonly associated to higher solar radiation on a global scale, and the effect of the UV-B in slowing the diffusion of SARS-CoV-2 may be observed (Nicastro et al., 2020; Asher et al., 2021; Grespan et al., 2021; Srivastava, 2021; Tang et al., 2021). A possible confounding factor associated with the temperature and solar radiation anomalies is explicitly mentioned by Sfîcă et al. (2020) and by Byass (2020). The latter researcher has studied approximately 18000 cases of COVID-19 in China and has reached the following conclusion: although UV radiation is considered effective in counteracting virus survival, sunshine days induce a higher sociality and accordingly a higher probability of transmission.
The effect of air relative humidity on the SARS-CoV-2 diffusion has been considered by several authors and contradictory results have been described (Paraskevis et al., 2021). Some studies, enrolling large population cohorts, have detected no significant relationship with the pandemic (Asher et al., 2021; Grespan et al., 2021), whereas others have observed a protective effect (Gupta et al., 2020; Wu et al., 2020a; Ma et al., 2020; Srivastava, 2021), but also positive correlations with the COVID-19 diffusion have been observed (Gupta et al., 2020; Suhaimi et al., 2020). A preliminary evaluation of most impacted cities in Brazil during spring suggests that intermediate relative humidity (about 80%) favours the transmission rate (Auler et al., 2020). Further controversy is whether to consider humidity in relative or absolute terms. For instance, low absolute humidity (4–7 g/m3 humid air) and specific humidity (3–6 g/kg dry air) characterize the areas in the world, suffering a high spread of SARS-CoV-2. On the other hand, the relative humidity values of the same affected areas do not allow the identification of the poorly-affected territories by the pathogen in the northern hemisphere (Sajadi et al., 2020).
Further studies have shown that air pollution also is able to affect the transmission of SARS-CoV-2 (Wu et al., 2020b; Maleki et al., 2021; Pansini and Fornacca, 2021; Srivastava, 2021; Travaglio et al., 2021, among others). However, it remains unclear what is the pathogenetic role of particulate matter in humans, as it may act as a carrier for the virus, it may induce an acute inflammatory response in the respiratory system or it may cause a chronic injury in organs and tissues, persistently exposed to air pollutants (Comunian et al., 2020).
In general wind speed is not considered a major factor in SARS-CoV-2 spreading. Only a few studies have investigated the effects of this parameter in the transmission of the virus (Srivastava, 2021). In the review by Asher et al. (2021), this factor has been recognized as a protective agent (the higher the wind speed, the lower the deaths for COVID-19), in agreement with studies in Brazil (Rosario et al., 2020) and Iran (Ahmadi et al., 2020). However, no beneficial roles of this ambient parameter have been demonstrated in further studies (Gupta et al., 2020).
2. Materials and methods
2.1. Epidemiological and demographic data
In Italy, epidemiological data are available on daily basis since the 24th February 2020, from national authorities (https://github.com/pcm-dpc/COVID-19), in particular, the daily number of new cases should represent a possible measure of the pandemic spread, but unfortunately, it is not completely reliable (Sartor et al., 2020), as the number of daily tests changes strongly according to the administrative territory, day of the week (lower number of tests on Saturday and Sunday) and time since the beginning of the pandemic. So, the interpretation of the results available presents some problems and data are not comparable considering different administrative areas; moreover, it strongly depends on the efficacy of the contact-tracing survey, that during the strongest pandemic rate was not sufficient in identifying all the contagious persons (Pezzutto et al., 2021). It must also be considered that the contact-tracing cannot be done for asymptomatic individuals, who are not intercepted by screening tests. It is likely that a significant number of cases has been excluded, as about 43% of infected subjects were asymptomatic in February–March 2020 according to a whole-population screening performed in the Vo’ Euganeo village, in Regione Veneto (Lavezzo et al., 2020). For these reasons the prevalence of hospitalized people in medical area wards on 100000 residents (RCS) was used to account for the spread and incidence of the pandemic. This parameter was considered, as the criteria for patient hospitalization are more homogeneous, accurately counted, and ensure adequate numerosity; the Intensive Care Units cases were excluded because of lower numerosity and more severe clinical status of patients, non-reflecting the general health situation (Struyf et al., 2021; Lorenzoni et al., 2020). The number of hospitalized cases is available for regional administrations and autonomous provinces. In our study we have considered eight areas, that are contiguous and cover the physiographic area between the Alps (in the Northern border) and the Apennines (in the South): Val d’Aosta, Piemonte, Lombardia, Provincia autonoma di Trento (later on simply Trento), Provincia autonoma di Bolzano (later on simply Bolzano), Veneto, Friuli Venezia Giulia, Emilia-Romagna (Fig. 1 ). More than 26 million people live in these areas, confirmed positive cases were 1210752 at the 31st December 2020, hospital occupancies (excluding intensive care units) 2367162, 51024 deaths.
Fig. 1.
Location of the areas under study (Regions and Autonomous Provinces: bold line); the Provinces which compose some of the areas are marked by thinner border; urban centres in black.
Demographic data referred to the 2019 are taken from the national repository (http://dati.istat.it/Index.aspx?DataSetCode=DCIS_POPRES1).
2.2. Atmospheric parameters
In order to get homogeneous data on daily basis over the study area, the databases from Copernicus data sets (https://climate.copernicus.eu/) were used for collecting the following data:
-
•
temperature (labelled T, ERA5 hourly data on single levels from 1979 to present, average 2 m temperature at 12.00 and 24.00, recalculated as °C, https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-single-levels?tab=form),
-
•
UV solar radiation (labelled S, ERA5 hourly data on single levels from 1979 to present, sum of mean surface downward short-wave radiation flux at 8.00, 12.00 and 16.00, W/m2, https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-single-levels?tab=form),
-
•
particulate matter less than 2.5 μm (labelled P, CAMS daily air quality analyses and forecasts for Europe, concentration at 12.00, recalculated as μg/m3, https://ads.atmosphere.copernicus.eu/cdsapp#!/dataset/cams-europe-air-quality-forecasts?tab=form),
-
•
relative humidity (labelled H, ERA5 hourly data on pressure levels from 1979 to present, average at 8.00, 12.00 and 16.00, percentage, https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-pressure-levels?tab=form),
-
•
wind (labelled W, ERA5 hourly data on single levels from 1979 to present, module of the eastward component of the 10 m wind, maximum velocity at 08:00, 14:00 or 20:00, m/s, https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-single-levels?tab=form).
To compare regional sanitary data with atmospheric ones, the weighted average of T, S, P, H and W was calculated on regional basis by measuring the parameters of the main provincial localities and using the provincial inhabitants as weight. This calculation was adopted because in the study area most people live in urban zones (91%; according to the last Italian General Census, available for the 2011 at https://www.istat.it/it/archivio/104317).
2.3. Searching for delay from environmental stress to hospitalization
A multiple linear regression analysis has been performed to create multivariate models, considering the RCS as dependent variable (response) and the atmospheric parameters T, S, P, H, and W (rescaled from 0 to 1, as indicated in Brereton, 2007) as independent variables; moreover, not only the single independent variables were taken as factors, but also and products between independent variables were used as factors in the multivariate models: T∙S, T∙P, T∙H, T∙W, S∙P, S∙H, S∙W, P∙H, P∙W, and H∙W. This means that the general function relevant to the multivariate models has the following form:
| RCS = f (factor1, …, factor 15) = f (T, S, P, H, W, T, T∙S, T∙P, T∙H, T∙W, S∙P, S∙H, S∙W, P∙H, P∙W, H∙W) |
The mathematical form of the function f can be found using the well-known regression procedure.
The Solver routine of Microsoft Excel was used for the calculation; the standard deviation of regression (square root of the sum of square distances between RCS and the computed model, divided by the number of degrees of freedom), as the minimizing parameter; this is the Multiple Linear Regression approach (MLR) (Brereton, 2007). An initial analysis using the atmospheric parameters alone (T, S, P, H, and W) produced standard deviations systematically worse than including also the interactions (Fig. S1), so this simpler approach was no longer developed. We have used the concept of lag time, defined as the time period between atmospheric stress and hospitalization (Haghshenas et al., 2020). This variable represents an indication of how the computed model is realistic, as the lag interval should include both the incubation time and other possible causes of delay in the appearance of the symptoms and of the overt disease.
We checked for the best lag time ranging from 8 to 24 days (Fig. S1) and the lag time corresponding to the best fit was used for the multivariate analysis.
2.4. Multivariate analysis
Several atmospheric parameters (independent variables) have been considered: temperature (T), solar radiation (S), particulate matter (P), relative humidity (H), and wind speed (W). The chosen chemometric approach consists in writing equations (statistical models) in which the dependent variable (response) is a function of single independent variables and of their products two at a time (interactions):
| RCS = b0 + b1 T + b2 S + b3 P + b4 H + b5 W + |
| + b6 T∙S + b7 T∙P + b8 T∙H + b9 T∙H + b10 S∙P + b11 S∙H + b12 S∙W + b13 P∙H + b14 P∙W + b15 H∙W | (1) |
Single variables and interactions are called factors. In this case, the number of degrees of freedom is calculated as the difference between the number of observations and the numbers of regression coefficients in eq. (1), which are 16 (bi, i = 0, …, 15).
As a dependent variable (response) we used the hospitalized people in general medical wards on 100000 residents (labelled as RCS).
In fact, the classical statistical methods adopt a univariate approach considering one independent variable at a time; in this univariate approach, for each variable, the mean and standard deviation are used, and an equation putting the response as a function of the single variable is studied as a possible univariate statistical model. However, the univariate approach is very limited, because it cannot show correlations among all the variables capable of influencing the response; besides correlation, also interactions between independent variables do emerge: this means that one can understand whether the simultaneous changes of variables are capable of influencing the response. On the contrary, when the multivariate approach is adopted, it becomes possible to evaluate correlations, interactions and also to evaluate the relative influence of all the factors (single variables and products) on the response: this is performed by evaluating the significance of the regression coefficients (b0, …, b15 in eq. (1)) and their numerical values (opportunely scaled).
2.4.1. MLR
The multivariate models corresponding to eq. (1) are created by the MLR method.
MLR is the multivariate extension of the univariate Ordinary Least Squares (OLS) method: in OLS the independent variable is a vector (n x 1, meaning n lines and 1 column), while in MLR the m factors are m columns in the n x m matrix (X) representing the starting dataset. The response y is a vector (n x 1) both for OLS and MLR. The equation of the model is:
| (2) |
where b is the vector of the regression coefficients. When also the constant value b0 (intercept) is computed in eq. (1), the number of regression coefficients is m’ = m+1 and the dimension of the vector b is m’ x 1, while the matrix X becomes n x m’ and its first column is made of unitary values.
The advantages of MLR with respect to OLS are great. By MLR it is possible to evaluate: all the possible correlations and the interactions among all the variables; the relevance of variables in influencing the response; the relative importance of variables; the predictive ability of the model. The capability of a MLR model to predict unknown samples can be evaluated by a simple chemometric tool: Cross Validation (CV). The power of CV lays in the fact that it does not need standard samples: this is perfect in a situation where standards do not exist, like the case of the present work. The CV method simply works by calculating the distance of each sample from the model created by all the other samples, and then calculating the statistical mean of all the obtained distances: square root of the ratio between the summation of the distances and the degrees of freedom of the model (n-m’), called Root Mean Square Error in CV (RMSECV). In order to have models highly performing in prediction, RMSECV must tend towards zero.
Once a MLR model is created, the validity of the equation must be verified by an ANOVA test (Analysis Of VAriance): the variability due to the model (MSS, Model Sum of Squares, m degrees of freedom) must be much higher than the variability due to uncertainty (RSS; Residual Sum of Squares, n-m’ degrees of freedom). Consequently, the Fisher coefficient F obtained as the ratio between MSS/m and RSS/(n-m’) must be much higher than 1: the corresponding p-value (p) of F-test should be very low.
After passing the ANOVA test, some figures of merit must be evaluated: the correlation coefficient R, RMSE, and the corresponding values in prediction mode (RCV and RMSECV). The coefficient R quantifies the correlation between the response and the independent variables, while RMSE quantifies the mean distance between the experimental y values and the relevant values re-calculated by the model. These figures of merit can be calculated by creating the response plot, in which the recalculated values of y are plotted versus the experimental values of y; in this plot, the target line has null intercept and unitary slope.
As for the relative relevance of variables, it is estimated through the numerical values of the relevant regression coefficients. In order to make the coefficients directly comparable, independently of the order of magnitude of the variables, the independent variables were range-scaled between 0 and 1: each value was subtracted of the minimum value, and this difference was divided by the difference between the maximum and the minimum value of the considered variable.
As for the significance of the variables, it was evaluated by comparing the absolute numerical value of each regression coefficient with the null value, by a t-test. If the corresponding p-value is low this means that the coefficient is very far from zero, hence it is significant and the relevant variable significantly influences the response; otherwise, the coefficient is not significantly different from zero, meaning that the corresponding variable does not influence the response. The significance of a variable increases with decreasing the p-value of the corresponding regression coefficient, in the t-test comparing it with the null value.
2.4.2. PCA
Principal Components Analysis (PCA) is an explorative chemometric tool, allowing to plot the objects (rows of the dataset) as points in a strongly informative plot, called scores plot, whose axes are called Principal Components (PCs). To create a scores plot, the m-dimensional space formed by the original variables is rotated towards a new mathematical space, where the first axis (PC1) is the one containing the maximum information (explained variance, EV); the remaining m-1 axes are orthogonal to each other and sorted in order of decreasing EV. Plotting the scores relevant to a PC (for instance PC1) vs. the scores relevant to another PC (for instance PC2), one can project the objects onto a highly informative plot and view the object as points in this plot. Once calculated the PCs, one can choose to further modelling data using only the first M scores which give an adequate cumulative EV. For instance, distances between objects will be calculated using the first M scores. Distances allow exploring eventual differences between the objects. Two distances are particularly useful: the distance between a point on the scores plot and the origin of the plot is called Hotelling distance (T2) and is characterized by a threshold depending on the chosen significance; the distance between an object and the scores plot, calculated as perpendicular distance out of the plane, is indicated as Q; also, for Q a threshold can be calculated depending on significance. The plot reporting T2 vs. Q is called influence plot; it allows to find out objects that far exceed the thresholds, and also the variables responsible for this diversity.
2.5. Time periods considered
In Italy, the so-called first wave of the pandemic involved many people and very severe health effects occurred leading the national authorities to establish a lockdown strategy from the 8 March 2020 to the 18 May 2020 (DPCM, 2020c; DPCM, 2020d); the sequence of restrictions during the first wave impacted on personal mobility which, in turn, was found to account for the spreading of the pandemic in the first wave (Gatto et al., 2020; Cartenì et al., 2020).
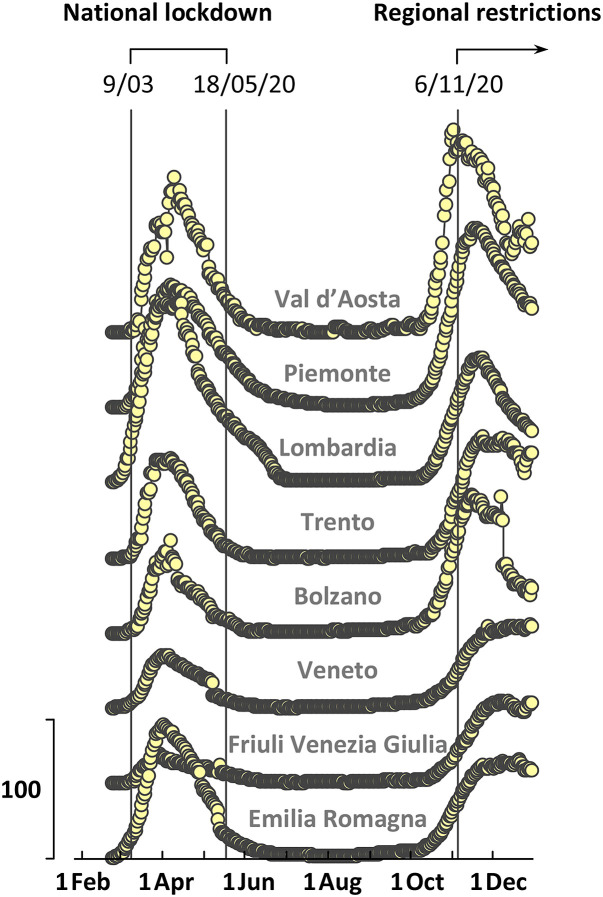
After the 18 May the number of infections continued to slow down, all public shops, bars, restaurants, sporting centres, theatres, and cinemas were open, people were free to move within regional territories (except various needs) and the use of personal surgery mask was mandatory only in closed places (DPCM, 2020a). This is the beginning of a period with “quite” pandemic diffusion (Fig. 2 ) not mitigated by severe restrictions, so it was chosen as a reference time to test possible effects of the atmospheric parameters under relatively large freedom of personal mobility, possibly also dealing with psychological feeling of relaxation and trust. We have indicated this time period in a concise way adopting the informal label “free/summer period”. This term is used also thereafter. The free/summer period ended on the 6 November, when new drastic restrictions were established after a sudden increase of cases observed during October (night curfew, halved occupancy of public transport, closure of high schools, partial or total closure of shops depending on the severity of the pandemic diffusion) (DPCM, 2020b). The fast increase of infections during October in the whole nation was the beginning of the so-called “second wave” during which a weekly adjustment of the restrictions was taken for the different regions according to the number of infections and availability of hospital care. Several limitations to the normal human activities were introduced or removed in the different Italian Regions; moreover, during the Christmas period (and the pre-Christmas shopping time) the changes of rules were even more frequent. The second wave developed in a diachronous way through Northern Italy, more severe and early in western regions (Fig. 2). In order to test possible effects of the atmospheric parameters under a strong limitation of personal mobility and possibly psychological feeling of stress, a reference time period was chosen from the 14 November up to the end of the year, beginning a few days after the activation of the new rules, to make our system stable for our analysis. We have indicated this time period in a concise way adopting the informal label “confined/autumn period”. This term is also used thereafter.
Fig. 2.
Number of hospitalized people in general medical wards on 100000 residents (RCS). The dates of the main restriction rules are reported.
3. Results
3.1. Delay between environmental stress and hospitalization
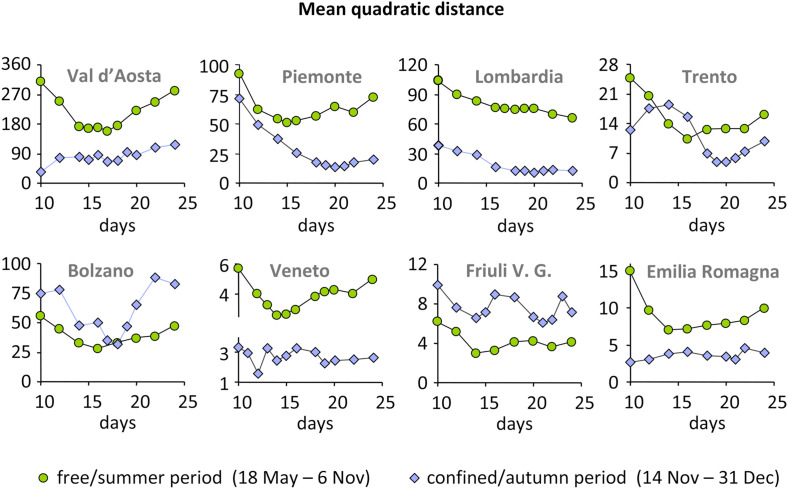
The scanning of progressive lag time for the free/summer period shows that the best fit between the model and the observed data (RCS) occurs for a delay between 14 and 16 days for most areas. The standard deviation against the lag time (days) reported in Fig. 3 shows that the errors decrease approaching the lag time corresponding to the best fit and then increase again. The trend is regular and it is very easy to identify the lag time for the lowest standard deviation. For Val d’Aosta, the lag time is represented by a flat interval of minimum values between 14 and 18 days (17 days represent the lower standard deviation because of a very little difference in comparison with the other days). Only for Lombardia, there is a continuous decreasing trend, never seen in the other areas; in this case, a relative minimum at 18 days was identified and chosen as lag time.
Fig. 3.
Mean quadratic distance between RCS and the model obtained by multiple linear regression of atmospheric parameters shifted by 10–24 days.
For the confined/autumn period the lag time is a bit higher (18–21 days) but the shape shown by standard deviation values is more irregular respect to the previous period (Fig. 3). In particular, for three areas (Val d’Aosta, Veneto and Emilia Romagna) there is an early minimum at 10–12 days, not reliable as defined by only one isolated point (Veneto) or too much early (Aguilar et al., 2020; Argenziano et al., 2020); in Val d’Aosta, Veneto and Emilia Romagna the shape of the standard deviation points is rather flat, even if a minimum value can be identified.
3.2. The multivariate model
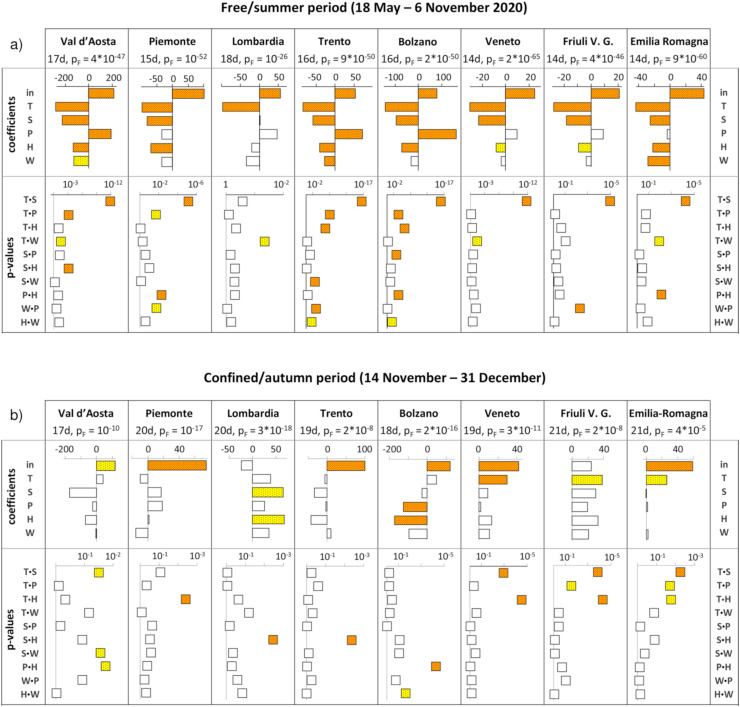
The results of the multivariate analysis developed after the identification of the lag time are reported in Fig. 4 in terms of significance F of the model (ANOVA p-value of the model, briefly here indicated as pF), the significance of the statistical coefficients (p-value of t-test) and coefficient values. The coefficients represent also the load of the factors as they were rescaled from 0 to 1 (Brereton, 2007). Figures of merit are also reported in Table 1 . Detailed regression coefficients are reported in Table S1, together with relevant standard deviations (Std.e.) and p-values of t-test for the comparison with the null value.
Fig. 4.
Value of the coefficients for the atmospheric parameters and p-values for the interactions obtained by MLR, computed for the lag time corresponding to the best fit between RCS and the model; the ANOVA p-value of the model is reported (pF). The parameters with very significant p-values (<0.01) are marked by dense pattern and orange background; a lighter pattern and yellow background is used for significant p-values (<0.05); d = days corresponding to the lag time considered; a): free/summer period (18 May - 6 November 2020); b) confined/autumn period (4 November - 31 December 2020). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Figures of merit for all the MLR models created. Cross-validation: full.
| free/summer period |
confined/autumn period |
|||||||
|---|---|---|---|---|---|---|---|---|
| R | RCV | RMSE | RMSECV | R | RCV | RMSE | RMSECV | |
| Val d'Aosta | 0.895 | 0.861 | 13.1 | 14.3 | 0.937 | 0.803 | 9.87 | 13.9 |
| Piemonte | 0.911 | 0.887 | 7.48 | 8.02 | 0.979 | 0.921 | 4.45 | 2.64 |
| Lombardia | 0.794 | 0.729 | 9.05 | 9.74 | 0.981 | 0.955 | 4.05 | 1.80 |
| Trento | 0.903 | 0.868 | 3.35 | 3.70 | 0.911 | 0.746 | 2.71 | 3.61 |
| Bolzano | 0.905 | 0.870 | 5.53 | 6.13 | 0.981 | 0.955 | 4.07 | 5.07 |
| Veneto | 0.940 | 0.917 | 1.66 | 1.86 | 0.944 | 0.875 | 1.87 | 2.26 |
| Friuli Venezia Giulia | 0.893 | 0.843 | 1.83 | 2.10 | 0.910 | 0.767 | 3.02 | 3.84 |
| Emilia-Romagna | 0.929 | 0.904 | 2.78 | 3.07 | 0.847 | 0.427 | 2.13 | 2.97 |
For the free/summer period the significance of the model is always very high, corresponding to very low p-values of F-test on the model (10−26 ─ 2∙10−65). The R values are always very high: there is strong correlation between the response and the factors; the RCV show very high values for all regions: the capability of the models to predict is surprisingly high. Several atmospheric parameters and also many interactions are significant or very significant. An additional feature deals with the sign of the coefficients of T, S and P: these parameters have a known effect on the viability or infectivity of the SARS-CoV-2 coronavirus, according to controlled laboratory conditions or considering the deleterious effect of particulate matter on human health (see section 1). For most of the areas the multivariate analysis gives rise to very significant coefficients of T, S and P and their signs agree with the results of the mentioned literature. The coefficients of relative humidity are always negative and for all the areas (but Lombardia) are also significant or very significant. The wind speed results at least significant in three areas, with a negative sign.
As for the interactions, the p-values relevant to their regression coefficients quantify their importance: for low p-values a strong synergy of the two multiplied variables is unveiled. A remarkable and novel result is that in several cases the interactions are significant or very significant; T∙S is very significant in 7 areas over 8 (Lombardia is the exception) and with high load: this means that the simultaneous variation of T and S results in a strong effect on RCS; as for the sign of this effect, it is accordingly to the signs of the regression coefficients of the single variables, as long as these coefficients are significant. In fact, when a regression coefficient is not significant, it means that it is not different from zero, hence its sign has no experimental meaning. All the interactions turn out to be at least significant in one area, even if S∙P, S∙H, and S∙W are significant only for one of the considered areas. There is no interaction which never gets significant levels.
Considering the confined/autumn period, the R values are always very high, indicating a strong correlation between the response and the factors; the RCV show very high values, with the only exception on Emilia-Romagna in which the predictive ability is significant but not high: but in all the other regions the capability of the models to predict is again surprisingly high. The RMSE and RMSECV are in all cases very low, considering that the maximum value for y is in the order of hundreds. As for the regression coefficients, the results are less homogeneous and the coefficients which are significant or very significant are fairly less frequent. A peculiar feature regards the sign of the coefficients for T, S and P, which only in five cases are at least significant, but with a sign opposite with respect to the results of the free/summer period. The coefficients of the three factors are never significant (W, S∙P and W∙P).
3.3. PCA: influence plots
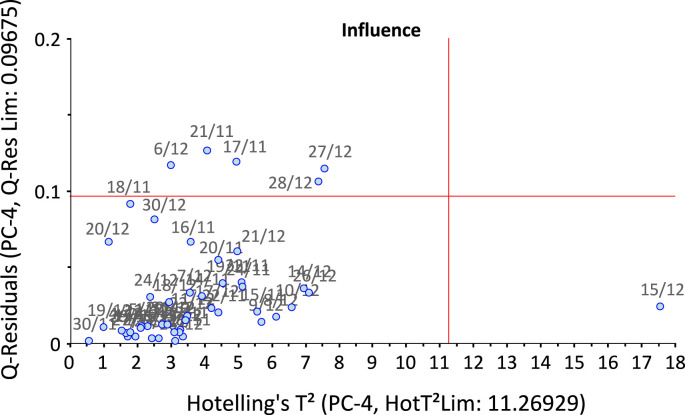
As an example, the Influence Plot relevant to Val d'Aosta in the confined/autumn period is reported (Fig. 5 ). The reported plot has been computed using the first four PC, containing 93.4% of the full EV. It is evident that the object 15/12 (15 December) is very far from the T2 threshold. The variables responsible for this diversity are W and W∙P. The complete analogous analysis of the influence plots for the free/summer period and for the confined/autumn period is reported in Table 2 . For the free/summer period, three regions (Val d'Aosta, Trento, Friuli Venezia Giulia) show outliers in June, all related to the interaction S∙H; all regions except Veneto and Friuli Venezia Giulia show outliers in October, with no particular regularity as regards responsible variables: several interactions are involved, while the effect of single variables on outliers is observed for Val d'Aosta (P), Piemonte (W), Emilia-Romagna (S); all regions except Val d'Aosta and Emilia-Romagna show outliers in early November, and in this case P is always involved, as a single variable or in interaction with others.
Fig. 5.
Influence plot for Val d'Aosta in the confined/autumn period. Number of PCs: 4. Explained variance: 93.4%.
Table 2.
Analysis of the influence plot (Principal Components: 4, significance for threshold: 0.05).
| free/summer period |
confined/autumn period |
|||||
|---|---|---|---|---|---|---|
| EV | objects far outside threshold | responsible variables | EV | objects far outside threshold | responsible variables | |
| Val d'Aosta | 90.9% | 4/6; 5, 12/10 | S∙H; P, S∙P, T∙P | 93.4% | 15/12 | W, W∙P |
| Piemonte | 91.6% | 10, 12/10; 4/11 | W; W∙P | 91.8% | 18/12 | W, W∙P |
| Lombardia | 92.5% | 23/9; 13/10; 6/11 | S∙P, T∙P; W∙S; S∙P, T∙P, P∙H | 92.6% | 18/12 | P, W, W∙P |
| Trento | 94.0% | 27/6; 31/10; 5/11 | S∙H; S∙T, W∙S; P, P∙U | 93.2% | 24/12, 25/12 | S∙P, T∙H |
| Bolzano | 93.9% | 18/10; 5/11 | S∙H, P∙H; P, P∙H | 92.6% | 18/12, 26/12 | P, W, W∙P |
| Veneto | 93.1% | 22/8; 4/11 | S∙H; P, P∙H | 94.0% | 25/12 | W, W∙H |
| Friuli Venezia Giulia | 91.0% | 24/6; 4/11 | S∙H; P, P∙H | 92.9% | 20/12 | W |
| Emilia-Romagna | 92.9% | 10/10 | S, W∙U | 92.9% | 12/12 | W∙S |
As for the confined/autumn interval, few outliers are present between 12 and 26 December, and the main factors involved are the single variables P and W, and several interactions (with W∙P particularly recurring, in the regions Val d'Aosta, Piemonte, Lombardia, Bolzano).
4. Discussion
4.1. The lag time
The blind search for an appropriate lag time developed in this study is consistent with other studies that take into account the hospitalized cases as a measure of the pandemic spread (Lolli et al., 2020). The time period between the atmospheric stress and hospitalization agrees with clinical observations (Aguilar et al., 2020; Argenziano et al., 2020) According to these researchers a brief period of 3–5 days occurs between the infection and the insurgence of symptoms, then during the successive 5–7 days the first stage of the clinical course is concluded with mild symptoms. In the following 10–14 days a possible progressive worsening of individuals' clinical status may arise, leading to the need of hospitalization (Aguilar et al., 2020). The hospital load is a significant sign of the stress suffered by the sanitary system and of the socio-economic impact due to working days lost. The agreement of our results with the biological criteria adopted to compute incubation-latency period validates the approach used for this study (and in other cases, e.g. Pirouz et al., 2020; Adhikari and Yin, 2021) and can be helpful for predictive purposes of SARS-CoV-2 recurrence. A significant finding is the longer lag time observed under confined conditions (17–21 days) with respect to the free period (15–17 days), suggesting that the preventive measures are effective in diluting the load of hospitalization over a wider time span (Pana et al., 2021).
4.2. The effect of atmospheric variables emerging from Italian studies
In Italy the pandemic developed earlier in comparison with other countries (Lolli et al., 2020; Wu et al., 2020a), therefore a lot of studies have been published on this topic. These investigations have examined the effect of atmospheric parameters on the COVID-19 diffusion in the most impacted zone (especially Lombardia, and its main city: Milano), and during the most dramatic period, up to March 2020, i.e. the first month (Pivato et al., 2021; Pirouz et al., 2020; Collivignarelli et al., 2021; Haghshenas et al., 2020; Bontempi, 2020; Passerini et al., 2020; Ye et al., 2021; Sfîcă et al., 2020; Khursheed et al., 2021; Coker et al., 2020; Bianconi et al., 2020; Accarino et al., 2021; Perone, 2021; Filippini et al., 2021; Kotsiou et al., 2021), or up to April 2020 (Coccia, 2020; Delnevo et al., 2020; Fazzini et al., 2020; Filippini et al., 2020; Fattorini and Regoli, 2020; Zoran et al., 2020; De Angelis et al., 2021; Benedetti et al., 2020; Agnoletti et al., 2020). Only a few works have considered a wider time span, up to the end of spring (Ho et al., 2021; Pansini and Fornacca, 2021; Lolli et al., 2020; Cascetta et al., 2021). The paper by Fiasca et al. (2020) has studied the effect of air pollution until the end of November. Our analysis covers a bit different time periods and conditions because we considered what happened after the effective diffusion of the SARS-CoV-2 virus over the study area (as shown by the occurrence of cases and recoveries in all the regions), a circumstance resembling the actual conditions and possibly our near future.
4.2.1. The effect of atmospheric variables during the free/summer period
The more evident role of atmospheric variables can be seen during the free/summer period, when the significant parameters are more abundant and with similar effects in different areas (Fig. 4a) with respect to the confined/autumn period (Fig. 4b). The most important meteorological factors are represented by T and S, which show a protective role in 7 areas over 8 (only T in Lombardia). The protective effect of T has been widely recognized in North Italy (Benedetti et al., 2020; Lolli et al., 2020; De Angelis et al., 2021; Perone, 2021; Coker et al., 2020; Khursheed et al., 2021). However, this relationship was not significant during the first month from the starting of the Sars-CoV-2 pandemic. (Pirouz et al., 2020; Haghshenas et al., 2020). An adverse effect of the variable T during the first month since the beginning of outbreak has been reported just in Lombardia, where a positive correlation with new cases has been observed (Passerini et al., 2020).
The effect of S on the viral spreading capability has been rarely investigated in Lombardia (Fazzini et al., 2020) and in Italy (Sfîcă et al., 2020), but the results are not univocal. Furthermore, an interesting new finding emerges from our analysis: the interaction between the T and S variables generates a protective effect against SARS-CoV-2 diffusion in the population. This action is highly significant in 7 areas over 8 (Fig. 4a), and it has been never noticed previously. The idea that T and S can play synergic effects against respiratory viruses agrees also with the studies based on machine learning and laboratory investigations, concerning the spread of the influenza virus in Northern Europe (Ianevski et al., 2019). Sunlight may provide protection to humans against SARS-CoV-2, by means of a double action. It has been suggested that UV-B radiation may inactivate or damage the virions and may improve the immune system activity of individuals. Some reports have suggested that VD exerts protective effects, by inhibiting virus transmission, via direct inhibition of the interaction between viral spike protein and ACE-2 receptor on cell membrane surface (Shoemark et al., 2021), or by improving immune system responses. Therefore, its use has been proposed in the initial phase of SARS-CoV-2 associated pandemic, since March 2020, with preventive or therapeutic purpose in patients with COVID-19 at higher risk of developing severe forms of diseases, such as aged people or immunocompromised individuals (Fiorino et al., 2020, 2021a). Several meta-analyses have confirmed these preliminary suggestions and have reported that individuals with vitamin D deficiency are at higher risk of SARS-CoV-2 infections and of a poorer clinical outcome in comparison with subjects with normal levels of this micronutrient (Kaya et al., 2021; Ghasemian et al., 2021; Szarpak et al., 2021; Oscanoa et al., 2021), whereas only a few studies have examined the effects of vitamin D supplementation in patients suffering from COVID-19. Although these investigations have shown interesting results, the number of enrolled individuals is small. Therefore, no definitive conclusions may be drawn and further trials are needed to clarify this topic (Entrenas Castillo et al., 2020; Elamir et al., 2021).
The effect of H on the pandemic diffusion has been debated and it still results unclear at a global scale (see 1.1.1) also in North Italy, where an increase of the pandemic effects has been associated with higher humidity (Pirouz et al., 2020; Haghshenas et al., 2020; Perone, 2021; De Angelis et al., 2021, for absolute humidity), although opposite conclusions have been also reported (Passerini et al., 2020; Khursheed et al., 2021; Zoran et al., 2020; Lolli et al., 2020; Zhu et al., 2020, for absolute humidity). Fazzini et al. (2020) has found no significant effects. In the free/summer period the protective effect of H has been observed in the study area (Fig. 4a). Lombardia also has shown the same trend but without reaching a statistical significance. A pivotal role of H, poorly noticed before, arises also by the occurrence of significant interaction with S (Val d'Aosta), P (Piemonte and Bolzano), T (Trento and Bolzano) and W (Trento and Bolzano).
Among the parameters considered, only W is rarely very significant, with some exceptions. In these cases, a lower number of hospital admissions is systematically observed during stronger windy days. A possible weakness of W, as a significant factor, agrees with other studies in the considered area (Haghshenas et al., 2020; Passerini et al., 2020; Fazzini et al., 2020; Lolli et al., 2020), only in Veneto a protective effect has been reported but it has been described only during the first month of pandemic (Pirouz et al., 2020). It could be mentioned that the wind speed and direction is severely conditioned by the position and extension of buildings in urban centres, so, the wind experienced by urbanized citizens could differs with respect to the meteorological data.
A more difficult task is to evaluate the role of P, because air pollution is highly significant only in the mountain areas (Val d'Aosta, Trento and Bolzano; Fig. 4a), with a negative connotation for the health, according to the well-known deleterious effect of particulate matter on the human health. However, in Lombardia and Veneto, the most polluted regions among those considered (for instance Stafoggia et al., 2020), only the tendency of P to increase the RCS is confirmed, but without significant p-values. The positive relationship between particulate matter pollution measured each day and the spreading of COVID-19 was already observed in the Trento area (Lolli et al., 2020), whereas in Lombardia a more delimited area around the metropolis of Milano was studied, confirming the same effects up to April (Zoran et al., 2020) and June 2020 (Lolli et al., 2020). Other studies about the role of particulate matter on the COVID-19 were performed for the areas studied also in the present work and following similar approach (comparison between day by day parameters of particulate matter pollution during the pandemic and health effects); despite the previous studies consider an earlier time period, their conclusions are in agreement with our results: the daily air pollution does not seems a crucial factor in modulating the pandemic diffusion in North Italy (Bontempi, 2020; Collivignarelli et al., 2021). A similar daily-based study was carried out in Emilia-Romagna, considering several air pollutants (PM10, PM2.5 and NO2: Mirri et al., 2020) or following a Granger causality statistical hypothesis (Delnevo et al., 2020), that was designed to handle pairs of variables and could suffer when additional variables take part in the relation. Under such Granger causality perspective, a positive correlation between particulate matter and COVID-19 diffusion is observed up to the first 8 weeks of local pandemic, but this result still refers to an earlier time period respect to our study. Additionally, the results by Mirri et al. (2020) are very interesting and show that within the same region (Emilia-Romagna) each of the 9 provinces should be analyzed separately to account for the COVID-19 occurrence; unfortunately, such operation is not possible as the number of individuals hospitalized in general medical wards is provided in aggregate form. The need for detailed local research, focusing on epidemiologic-environmental relationships, emerges also from the spreading of COVID-19 in Catalonia (Tobías et al., 2021), and also from studies on the effect of particulate matter on other pathologies (for instance Scartezzini et al., 2021).
The evaluation of several polluting factors (O3, NO2, SO2 in addition to PM10 and PM2.5) by Ho et al. (2021) has led to finding that only the chronic pollution by particulate matter is significant for COVID-19 effects in Lombardia and Veneto, whereas on a daily basis the PM10 and PM2.5, are not, up to May 2020. A different conclusion was proposed by Ye et al. (2021) in the same time period (up to May 2020). The researchers have observed a significant impact of PM on all-cause mortality during the pandemic period. The latter study differs from our approach, because these authors have considered a very short lag time (0–3 days), and all the Italian territory, which includes areas with different socio-economic status. This aspect represents a known confounding effect in epidemiological studies (Rosano et al., 2020). The association between COVID-19 incidence rates and PM2.5 pollution was observed during the COVID-19 period in Italy over a more extended period (up to 3 November 2020, Fiasca et al., 2020) and during the first wave (1 January - 31 March 2020, Accarino et al., 2021) but these studies have compared different individual provinces, whereas our approach is to check for a possible day by day association between the RCS and atmospheric parameters within each single administrative area.
Despite the daily variations of PM does not seem to represent an essential factor in highly populated plain areas from our data, a different role emerges when the effect of long-term pollution of particulate matter (years-months) is taken into account, according to the evidence available in the literature. Several studies have considered this effect in Italy and have reported higher pandemic diffusion in more historically polluted areas (Bianconi et al., 2020; Coker et al., 2020; Fattorini and Regoli, 2020; Cascetta et al., 2021; De Angelis et al., 2021; Fiasca et al., 2020; Ho et al., 2021; Kotsiou et al., 2021; Pansini and Fornacca, 2021; Perone, 2021; Ye et al., 2021). The wide plain area of the Northern Italy (Fig. 1) is one of the most impacted areas for pollution by PM all over Europe (EEA, 2020). The comparison of literature data with our results shows a twofold conclusion: 1) the mountain areas studied (Val d'Aosta, Trento and Bolzano) are poorly affected by long-term PM 2.5 pollution (Stafoggia et al., 2020 and Table S2), but the day by day variation of P shows a highly significant correlation with RCS (Fig.4a); 2) in the other areas, which are severely affected by long-term PM 2.5 pollution, our data detect no significant correlation with P variations. We have no data explaining such an apparent paradox, but possible speculation is that exceeding a threshold limit, the day-by-day variation of P is not effective in changing the chronic exposure to a specific pollutant. In agreement with our findings, a recent meta-analysis has shown that an association exists between COVID-19 mortality and long-term exposure to PM2.5 but not to short-term one (Zang et al., 2022).
A final consideration on the free/summer period refers to the number of interactions that have turned out to be significant in influencing human health (Fig. 4a). Some couples of parameters (for instance temperature and particulate matter, Stafoggia et al., 2008) had been already recognized as important factors involved in this action. However, our paper reinforces this preliminary conclusion: a large spectrum of atmospheric parameters may generate cooperative interactions and produce important effects on human health.
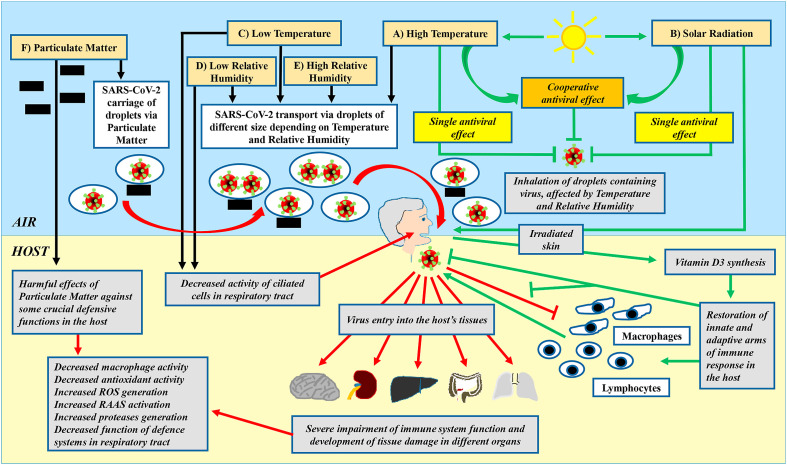
A possible scenario showing the relationships among environmental factors and the host's defense mechanisms on the fitness and infectious capability of SARS-CoV-2 is sketched in Fig. 6 .
Fig. 6.
The figure summarizes the current knowledge on the possible role of atmospheric conditions (High and Low Temperature, High and Low Relative Humidity and Solar Radiation) and of air pollutants (Particulate Matter) as well as of host's defense mechanisms on the fitness and infectious capability of SARS-CoV-2. Our analysis refers to the period, ranging from 18 May to 6 November 2020, in several regions of North Italy. Each of the above-mentioned factors may exert an its own individual activity or it may establish reciprocal interactions with the other climate-, environmental-parameters and with host's defense functions and produce a wide spectrum of effects. Experimental evidence suggests that: A) High temperature, as a single factor or together with Solar Radiation, may reduce viability and survival of SARS-CoV-2 through a direct anti-viral effect probably by: a) decreasing the stability of virions, b) preserving the normal function of ciliate cells, preventing their damage by airborne pathogens and particulate matter, c) affecting desiccation or hydration of viral droplets, modulating the size of droplets in cooperation with Relative Humidity (see also D and E); B) Solar Radiation, alone or in cooperation with environmental High Temperature, displays an antiviral function. Solar UV-B rays may impair the stability of viral capsids by: a) directly reducing the stability of SARS-CoV-2 virions and decreasing their fitness and survival b) indirectly promoting an increase in vitamin D synthesis. This fat-soluble micronutrient regulates the normal activity of different components and elements, belonging to immune system. An adequate antiviral response by host requires a cooperative interplay among these elements, such as cells (lymphocytes/macrophages) and mediators (chemokines, interleukins and oxygen species). Vitamin D3 may contribute to restore a proper function of both innate and adaptive arms of immune system. In particular, SARS-CoV-2 and particulate matter may impair the functionality of both lymphocytes and macrophages, via different mechanisms. Vitamin D3 may counteract the harmful effects caused by virus and by air pollutants and it may promote the reactivation of an adequate antiviral response by lymphocytes and macrophages in the host; C) Low Temperature as a single factor may promote SARS-CoV-2 infectious capability and its survival by: a) impairing host's defences in respiratory tract, via the damage of barrier system (the mucus layer, the surface liquid layer and the cilia on the surface of the bronchus epithelia) as, in normal conditions, these factors are able to counteract virus entry, or via the alteration of innate and adaptive immune response (the network of interferons, macrophages, lymphocytes and interleukins/cytokines) as these elements may block the virus which has by-passed the host's barrier system (b) affecting the physical characteristics of droplets, which carry the virus. In particular, Low Temperature alone or in cooperation with Low or High Relative Humidity may modulate the size of droplets, carrying SARS-CoV-2 and therefore it may influence the capability of this pathogen to infect the host (see also D and E); D) Low and E) High Relative Humidity as single factors may promote the desiccation or hydration of droplets, carrying SARS-CoV-2, and therefore they induce a reduction or an increase in their sizes. These events may influence the infectious capability of the virus, but the data available in literature are not univocal. In particular, data emerging from our analysis indicate that High Relative Humidity is associated with low rate of hospital admission. The role of Relative Humidity is debated and represents a proper example of how the mutual interplay among atmospheric, environmental and host's factors impacts on the virus spreading and on human health. In particular, it is possible that the size of droplets may exert a critical role in influencing the capability of SARS-CoV-2 to spread in the environment and to infect the host. It is probable that Low Humidity itself decreases viral viability and alone or with the concomitant presence of elevated temperature induces the formation of droplets containing viral particles with a reduced fitness and having suboptimal sizes for the spreading in the environment and for the entry in host's respiratory tract. On the other hand, High Relative Humidity may increase SARS-CoV-2 fitness and infectious capability, but, even in presence of low temperature, it may promote the generation of bigger droplets, but having sizes not adequate for a proper diffusion and infectivity of the virus. All these considerations may contribute to explain the not univocal data detectable in literature, concerning this topic. It is possible that the final effects depend on the overall balance among all these interactions. F) Particulate matter may affect SARS-CoV-2 infectivity and spreading, by: a) impairing several host's functions. The most important alterations induced by air pollution include a decrease in macrophage and lymphocyte activity, a reduction of antioxidant cell systems as well as an increase of protease generation. Furthermore, particulate matter may increase RAAS activation. A further possible effect of particulate matter is to act as carrier of droplets. RAAS: Renin-Angiotensin-Aldosterone-System; ROS: Reactive Oxygen Species generation; SARS-CoV-2: Severe-Acute-Respiratory-Syndrome associated with Coronavirus 2. Green arrows indicate protective antiviral actions, mediated by environmental- and atmospheric-factors as well as elicited by host's defensive mechanisms. Red and black arrows indicate harmful viral activities, induced by environmental- and atmospheric-factors as well as elicited by host's responses. Green lines with flat termination indicate inhibitory actions of environmental- and atmospheric-factors as well as of host's responses against SARS-CoV-2 with protective antiviral effects. Red lines with flat termination indicate harmful actions of SARS-CoV-2, inhibiting host's protective antiviral functions. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.2.2. The effect of atmospheric variables during the confined/autumn period
During the confined/autumn period the significant parameters have resulted to be in lower number and with variable role (sign of coefficients); also, the similarities among different areas, observed in the free/summer period, are no more detectable. A possible explanation relies on the establishment of new social restrictions after the 6 November 2020. Moreover, the restrictions adopted in the different areas were not synchronous and identical (DPCM, 2020b), so that the effect of atmospheric factors in different areas could be different. The high efficacy of public health interventions in Italy (social distances, restrictions in public and private places, individual protective devices, etc.) had already been observed in comparison with other countries during this time period (the so-called second wave; Bontempi, 2021). A modification of the relationships between atmospheric parameters and the COVID-19 diffusion had been already observed earlier in Italy and it was due to the lockdown during the spring season (Benedetti et al., 2020; Ho et al., 2021). Also, on a global scale the public health interventions are considered to induce a major impact on the prevention of COVID-19 diffusion in comparison with seasonal factors (Jüni et al., 2020; Paraskevis et al., 2021).
During the confined/autumn period some significant atmospheric parameters have acted in a surprising way, for instance T has increased the RCS in Veneto, Friuli Venezia Giulia and Emilia-Romagna, P in Bolzano has been associated with a decrease of RCS, an opposite sign has characterized H in Lombardia and Bolzano. Significant interactions occurred also in this period, the more recurrent have been T∙S (Val d'Aosta, Veneto, Friuli Venezia Giulia and Emilia-Romagna) and T∙H (Piemonte, Veneto, Friuli Venezia Giulia and Emilia-Romagna). The atmospheric parameters under these climatic and sociality conditions have possibly acted both directly on biological targets (human immune system and the virus) and also on social behaviors, increasing or decreasing the interactions between individuals according to meteorological conditions. An opposite effect was already stressed, suggesting that warm days during the cold season stimulate outdoor people mobility/sociality in winter (Byass, 2020; Sfîcă et al., 2020).
5. Conclusions
This work accomplishes several meteorological parameters (temperature, solar radiation, relative humidity, wind speed) and a well-known pollution index (PM 2.5), which is especially dangerous to human health. The multivariate statistical models here developed are characterized by a very high significance, enabling a detailed evaluation of the effects of the studied environmental factors on the pandemic. All the atmospheric parameters in turn play a significant role in determining the hospital admission for COVID-19 during a time period subsequent to the first wave of the pandemic, along about 6 months. This effect is appreciable following the daily incidence of hospitalization within all the 8 territorial areas considered. The admission of people in general medical wards is not only an effect of the SARS-CoV-2 spreading but also represents a social and economic load for territories. Another finding is that some of the environmental parameters considered exert major rule respect to others. In particular, T is highly significant in all the 8 areas, confirming the results of other studies, but our new findings stress the pivotal role of S and H, both of which have an appreciable effect in 7 areas over the 8 considered; only for Lombardia, S and P are not significant, which point out a particular effect of the relationship between pandemic and environment in such region.
The obtained results also show that the role of P deserves special attention, because in the areas with more severe chronic pollution by PM the day-by-day variation of P does not load on the hospital admissions, whereas in mountain territories (which are fairly less impacted by PM pollution) P is highly significant in increasing the hospitalization. The reason for such different effects is beyond the aim of the present work, but considering the deleterious effect that PM has on human health in general, our findings encourage more studies to be done.
The chemometric approach adopted allows to highlight, on a solid statistical basis, not only the role of single atmospheric factors, but also of their interactions. This new approach is particularly interesting as several interactions are significant or very significant in all the areas, in particular T∙S plays an important role everywhere, with the exception of Lombardia which is confirmed as a peculiar area also under this point of view.
During the beginning of the local second wave, when social restrictions occurred, the atmospheric parameters that significantly influence the multivariate models are much less numerous, suggesting that people's movements and sociality play a substantial effect.
The present work shows that several environmental parameters and their interactions should be considered in fighting the pandemic. The present work shows that several environmental parameters and their interactions should be considered in fighting the pandemic. Common environmental factors considered in this study can act on the viability of the virus and on the efficiency of the host's immune system, as sketched in Fig. 6. Despite the scheme cannot be considered exhaustive lacking other considerations (for instance about mobility and sociality), it emphasizes direct and cooperative effects that emerge from our study as significant in conditioning the pandemic evolution. Besides the comparison of different territories (which inevitably bring their own social and environmental features), also the day-by-day variations of atmospheric parameters inside a defined area can lead to reliable models of pandemic diffusion, and this is especially important under the threat of increasing intensity and frequency of extreme novel epidemics (Marani et al., 2021).
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge Simonetta Righi (Biblioteca Centralizzata, PS Orsola-Malpighi, Università di Bologna, Bologna, Italy) for her support in the search of scientific bibliography.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.112921.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound. Emerg. Dis. 2020:1–17. doi: 10.1111/tbed.13707. 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accarino G., Lorenzetti S., Aloisio G. Assessing correlations between short-term exposure to atmospheric pollutants and COVID-19 spread in all Italian territorial areas. Environ. Pollut. 2021;268:115714. doi: 10.1016/j.envpol.2020.115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A., Yin J. Lag effects of ozone, PM2.5, and meteorological factors on COVID-19 new cases at the disease epicenter in queens, New York. Atmosphere. 2021;12(3):357. doi: 10.3390/atmos12030357. [DOI] [Google Scholar]
- Agnoletti M., Manganelli S., Piras F. Landscape and urban planning covid-19 and rural landscape: the case of Italy trend of COVID-19 cases in Italy. Landsc. Urban Plann. 2020;204:103955. doi: 10.1016/j.landurbplan.2020.103955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar R.B., Hardigan P., Mayi B., Sider D., Piotrkowski J., Mehta J.P., Dev J., Seijo Y., Camargo A.L., Andux L., Hagen K., Hernandez M.B. Current understanding of COVID-19 clinical course and investigational treatments. Front. Med. 2020;7:555301. doi: 10.3389/fmed.2020.555301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlawat A., Wiedensohler A., Mishra S.K. An overview on the role of relative humidity in airborne transmission of SARS-CoV-2 in indoor environments. Aerosol Air Qual. Res. 2020;2:1856–1861. doi: 10.4209/aaqr.2020.06.0302. [DOI] [Google Scholar]
- Ahmadi M., Sharifi A., Dorosti S., Ghoushchi S.J., Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci. Total Environ. 2020;729:138705. doi: 10.1016/j.scitotenv.2020.138705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenziano M.G., Bruce S.L., Slater C.L., Jonathan R.T., Baldwin M.R., Barr R.G., Chang B.P., Chau K.H., Choi J.J., Gavin N., Goyal P., Mills A.M., Patel A.A., Romney M.L.S., Safford M.M., Schluger N.W., Sengupta S., Sobieszczyk M.E., Zucker J.E., Asadourian P.A., Bell F.M., Boyd R., Cohen M.F., Colquhoun M.A.I., Colville L.A., de Jonge J.H., Dershowitz L.B., Dey S.A., Eiseman K.A., Girvin Z.P., Goni D.T., Harb A.A., Herzik N., Householder S., Karaaslan L.E., Lee H., Lieberman E., Ling A., Lu R., Shou A.Y., Sisti A.C., Snow Z.E., Sperring C.P., Xiong Y., Zhou H.W., Natarajan K., Hripcsak G., Chen R. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher E.E., Ashkenazy Y., Havlin S., Sela A. Optimal COVID-19 infection spread under low temperature, dry air, and low UV radiation. New J. Phys. 2021;23:33044. doi: 10.1088/1367-2630/abed0d. [DOI] [Google Scholar]
- Audi A., AlIbrahim M., Kaddoura M., Hijazi G., Yassine H.M., Zaraket H. Seasonality of respiratory viral infections: will COVID-19 follow suit? Front. Public Health. 2020;8:567184. doi: 10.3389/fpubh.2020.567184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auler A.C., Cassaro F.A.M., da Silva V.O., Pires L.F. Evidence that high temperatures and intermediate relative humidity might favor the spread of COVID-19 in tropical climate: a case study for the most affected Brazilian cities. Sci. Total Environ. 2020;729:139090. doi: 10.1016/j.scitotenv.2020.139090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Pachetti M., Marini B., Ippodrino R., Gallo R.C., Ciccozzi M., Zella D. Inverse correlation between average monthly high temperatures and COVID 19 related death rates in different geographical areas. J. Transl. Med. 2020:1–7. doi: 10.1186/s12967-020-02418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi V., Bronzo P., Banach M., Sahebkar A., Mannarino M.R., Pirro M. Clinical research Particulate matter pollution and the COVID-19 outbreak: results from Italian regions and provinces. Arch. Med. Sci. 2020;16:985–992. doi: 10.5114/aoms.2020.95336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020;186:109639. doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. The europe second wave of COVID-19 infection and the Italy “strange” situation. Environ. Res. 2021;193:110476. doi: 10.1016/j.envres.2020.110476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton R.G. John Wiley & Sons; 2007. Applied Chemometrics for Scientists. [Google Scholar]
- Byass P. Eco-epidemiological assessment of the COVID-19 epidemic in China, January-February 2020. Glob. Health Action. 2020;13:1760490. doi: 10.1080/16549716.2020.1760490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos R.K., Jin J., Rafael G.H., Zhao M., Liao L., Simmons G., Chu S., Weaver S.C., Chiu W., Cui Y. Decontamination of SARS-CoV 2 and other RNA viruses from N95 level meltblown polypropylene fabric using heat under different humidities. ACS Nano. 2020;14:14017–14025. doi: 10.1021/acsnano.0c06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartenì A., Di Francesco L., Martino M. How mobility habits influenced the spread of the COVID-19 pandemic: results from the Italian case study. Sci. Total Environ. 2020;741:140489. doi: 10.1016/j.scitotenv.2020.140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascetta E., Henke I., Di Francesco L. The effects of air pollution, sea exposure and altitude on COVID-19 hospitalization rates in Italy. Int. J. Environ. Res. Publ. Health. 2021;18:452. doi: 10.3390/ijerph18020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., MalikPeiris J.S., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011:1–7. doi: 10.1155/2011/734690. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.H., Chung Y., Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J. Biol. Chem. 2010;285(50):38751–38755. doi: 10.1074/jbc.C110.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrie M., Clemens T., Colandrea C., Feng Z., Webb D.J., Weller R.B.D., Dibben C. Ultraviolet A radiation and COVID-19 deaths in the USA with replication studies in England and Italy. Br. J. Dermatol. 2021;185:363–370. doi: 10.1111/bjd.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microb. 2020;1(1):e237. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirizzi D., Conte M., Feltracco M., Dinoi A., Gregoris E., Barbaro E., La Bella G., Ciccarese G., La Salandra G., Gambaro A., Contini D. SARS-CoV-2 concentrations and virus-laden aerosol size distributions in outdoor air in north and south of Italy. Environ. Int. 2020;146:106255. doi: 10.1016/j.envint.2020.106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729:138474. doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker E.S., Cavalli L., Fabrizi E., Guastella G., Lippo E., Laura M., Nicola P., Massimiliano P., Varacca A., Vergalli S. The effects of air pollution on COVID 19 related mortality in northern Italy. Environ. Resour. Econ. 2020;76:611–634. doi: 10.1007/s10640-020-00486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collivignarelli M.C., Abbà A., Caccamo F.M., Pedrazzani R., Baldi M., Ricciardi P., Miino M.C. Can particulate matter be identified as the primary cause of the rapid spread of CoViD-19 in some areas of Northern Italy? Environ. Sci. Pollut. Res. 2021;28:33120–33132. doi: 10.1007/s11356-021-12735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunian S., Dongo D., Milani C., Palestini P. Air pollution and covid-19: the role of particulate matter in the spread and increase of covid-19's morbidity and mortality. Int. J. Environ. Res. Publ. Health. 2020;17:1–22. doi: 10.3390/ijerph17124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet D.E. Why does SARS-CoV-2 survive longer on plastic than on paper. Med. Hypotheses. 2021;146:2–4. doi: 10.1101/2020.05.07.20094805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer R.C., Parekh D., Lax S., D'Souza V., Zheng S., Bassford C.R., Park D., Bartis D.G., Mahida R., Turner A.M., Sapey E., Wei W., Naidu B., Stewart P.M., Fraser W.D., Christopher K.B., Cooper M.S., Gao F., Sansom D.M., Martineau A.R., Perkins G.D., Thickett D.R. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E.R., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome. SARS-CoV. J. Virol. Method. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis E., Renzetti S., Volta M., Donato F., Calza S., Placidi D., Lucchini R.G., Rota M. COVID-19 incidence and mortality in Lombardy, Italy: an ecological study on the role of air pollution, meteorological factors, demographic and socioeconomic variables. Environ. Res. 2021;195:110777. doi: 10.1016/j.envres.2021.110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnevo G., Mirri S., Roccetti M. Particulate matter and COVID-19 disease diffusion. Computation. 2020;8:1–16. doi: 10.3390/computation8020059. [DOI] [Google Scholar]
- Doerrbecker J., Friesland M., Ciesek S., Erichsen T.J., Mateu-Gelabert P., Steinmann Joerg, Steinmann Jochen, Pietschmann T., Steinmann E. Inactivation and survival of hepatitis C virus on inanimate surfaces. J. Infect. Dis. 2011;204:1830–1838. doi: 10.1093/infdis/jir535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DPCM 17 May. 2020. https://www.gazzettaufficiale.it/eli/id/2020/05/17/20A02717/sg [Google Scholar]
- DPCM 3 November. 2020. https://www.gazzettaufficiale.it/eli/id/2020/11/04/20A06109/sg [Google Scholar]
- DPCM 8 March. 2020. https://www.gazzettaufficiale.it/eli/id/2020/03/08/20A01522/sg [Google Scholar]
- DPCM 9 March. 2020. https://www.gazzettaufficiale.it/eli/id/2020/03/09/20A01558/sg [Google Scholar]
- Duval J.F.L., Leeuwen H.P. Van, Norde W., Town R.M. Chemodynamic features of nanoparticles: application to understanding the dynamic life cycle of SARS-CoV-2 in aerosols and aqueous biointerfacial zones. Adv. Colloid Interface Sci. 2021;290:102400. doi: 10.1016/j.cis.2021.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol. 2002;122:183–191. doi: 10.1080/00016480252814207. [DOI] [PubMed] [Google Scholar]
- EEA (European Environment Agency) 2020. Air Quality in Europe - 2020 Report, EEA Report. [DOI] [Google Scholar]
- Elamir Y.M., Amir H., Lim S., Rana Y.P., Lopez C.G., Feliciano N.V., Omar A., Grist W.P., Via M.A. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone. 2021;154:116175. doi: 10.1016/j.bone.2021.116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcalá Díaz J.F., López Miranda J., Bouillon R., Quesada Gomez J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in. Environ. Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzini M., Baresi C., Bisci C., Bna C., Cecili A., Giuliacci A., Illuminati S., Pregliasco F., Miccadei E. Preliminary analysis of relationships between COVID19 and climate, morphology, and urbanization in the Lombardy region. Int. J. Environ. Res. Publ. Health. 2020;17:6955. doi: 10.3390/ijerph17196955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiasca F., Minelli M., Maio D., Minelli M., Vergallo I., Necozione S., Mattei A. Associations between COVID-19 incidence rates and the exposure to PM2.5 and NO2: a nationwide observational study in Italy. Int. J. Environ. Res. Publ. Health. 2020;17:9318. doi: 10.3390/ijerph17249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T., Rothman K.J., Gof A., Ferrari F., Maffeis G., Orsini N., Vinceti M. Satellite-detected tropospheric nitrogen dioxide and spread of SARS-CoV-2 infection in Northern Italy. Sci. Total Environ. 2020;739:140278. doi: 10.1016/j.scitotenv.2020.140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T., Rothman K.J., Cocchio S., Narne E., Mantoan D., Saia M., Gof A., Ferrari F., Maffeis G., Orsini N., Baldo V., Vinceti M. Associations between mortality from COVID-19 in two Italian regions and outdoor air pollution as assessed through tropospheric nitrogen dioxide. Sci. Total Environ. 2021;760:143355. doi: 10.1016/j.scitotenv.2020.143355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino S., Gallo C., Zippi M., Sabbatani S., Manfredi R., Moretti R., Fogacci E., Maggioli C., Travasoni Loffredo F., Giampieri E., Corazza I., Dickmans C., Denitto C., Cammarosano M., Battilana M., Orlandi P.E., Del Forno F., Miceli F., Visani M., Acquaviva G., De Leo A., Leandri P., Hong W., Brand T., Tallini G., Jovine E., Jovine R., de Biase D. Cytokine storm in aged people with CoV-2: possible role of vitamins as therapy or preventive strategy. Aging Clin. Exp. Res. 2020;32:2115–2131. doi: 10.1007/s40520-020-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino S., Zippi M., Gallo C., Sifo D., Sabbatani S., Manfredi R., Rasciti E., Rasciti L., Giampieri E., Corazza I., Leandri P., De Biase D. The rationale for a multi-step therapeutic approach based on antivirals, drugs and nutrients with immunomodulatory activity in patients with coronavirus-SARS2-induced disease of different severities. Br. J. Nutr. 2021;125:275–293. doi: 10.1017/S0007114520002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman E.F., Storer J.A., Fitzgerald M.E., Wasik B.R., Hou L., Zhao H., Turner P.E., Pyle A.M., Iwasaki A. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc. Natl. Acad. Sci. U.S.A. 2015;112:827–832. doi: 10.1073/pnas.1411030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałuszka A., Stec M., Węglarczyk K., Kluczewska A., Siedlar M., Baran J. Transition metal containing particulate matter promotes Th1 and Th17 inflammatory response by monocyte activation in organic and inorganic compounds dependent manner. Int. J. Environ. Res. Publ. Health. 2020;17(4):1227. doi: 10.3390/ijerph1704122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto M., Bertuzzo E., Mari L., Miccoli S., Carraro L., Casagrandi R., Rinaldo A. Spread and dynamics of the COVID-19 epidemic in Italy: effects of emergency containment measures. Proc. Natl. Acad. Sci. U.S.A. 2020;17:2004978117. doi: 10.1073/pnas.2004978117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemian R., Shamshirian A., Heydari K., Malekan M., Alizadeh-Navaei R., Ebrahimzadeh M.A., Ebrahimi Warkiani M., Jafarpour H., Razavi Bazaz S., Rezaei Shahmirzadi A., Khodabandeh M., Seyfari B., Motamedzadeh A., Dadgostar E., Aalinezhad M., Sedaghat M., Razzaghi N., Zarandi B., Asadi A., Yaghoubi Naei V., Beheshti R., Hessami A., Azizi S., Mohseni A.R., Shamshirian D. The role of vitamin D in the age of COVID-19: a systematic review and meta-analysis. Int. J. Clin. Pract. 2021;75 doi: 10.1111/ijcp.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grespan B., Faria L. De, Lopes F., Alexandre L. How the global health security index and environment factor influence the spread of COVID-19: a country level analysis. One Health. 2021;12:100235. doi: 10.1016/j.onehlt.2021.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillier L., Martin-Latil S., Chaix E., Thebault A., Pavio N., Le Poder S., on behalf of Covid-19 Emergency Collective Expert Appraisal Group, Batejat C., Biot F., Koch L., Schaffner D.W., Sanaa M. Modeling the inactivation of viruses from the coronaviridae family in response to temperature and relative humidity in suspensions or on surfaces. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.01244-20. e01244-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Banerjee S., Das S. Significance of geographical factors to the COVID-19 outbreak in India. Model. Earth Syst. Environ. 2020;6:2645–2653. doi: 10.1007/s40808-020-00838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghshenas Sina Shaffiee, Pirouz, Behrouz, Haghshenas, Shaffiee Sami, Pirouz Behzad, Piro P., Na K.S., Cho S.E., Geem Z.W. Prioritizing and analyzing the role of climate and urban parameters in the confirmed cases of COVID-19 based on artificial intelligence applications. Int. J. Environ. Res. Publ. Health. 2020;17:3730. doi: 10.3390/ijerph17103730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C., Hung S., Ho W. Effects of short- and long-term exposure to atmospheric pollution on COVID-19 risk and fatality: analysis of the first epidemic wave in northern Italy. 2021. 199111293. [DOI] [PMC free article] [PubMed]
- Holick M.F., Chen T.C. Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- Hughes D.A., Norton R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009;158:20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianevski A., Zusinaite E., Shtaida N., Kallio-Kokko H., Valkonen M., Kantele A., Telling K., Lutsar I., Letjuka P., Metelitsa N., Oksenych V., Dumpis U., Vitkauskiene A., Stašaitis K., Öhrmalm C., Bondeson K., Bergqvist A., Cox R.J., Tenson T., Merits A., Kainov D.E. Low temperature and low UV indexes correlated with peaks of influenza virus activity in Northern Europe during 2010–2018. Viruses. 2019;11(3):207. doi: 10.3390/v11030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüni P., Rothenbühler M., Bobos P., Thorpe K.E., Da Costa B.R., Fisman D.N., Slutsky A.S., Gesink D. Impact of climate and public health interventions on the COVID-19 pandemic: a prospective cohort study. CMAJ (Can. Med. Assoc. J.) 2020;192:E566–E573. doi: 10.1503/cmaj.200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya M.O., Pamukçu E., Yakar B. The role of vitamin D deficiency on the Covid-19: a systematic review and meta-analysis of observational studies. Epidemiol. Health. 2021 doi: 10.4178/epih.e2021074. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursheed A., Faisal M., Akhtar A. Investigating the roles of meteorological factors in COVID-19 transmission in Northern Italy. Environ. Sci. Pollut. Res. 2021;28:48459–48470. doi: 10.1007/s11356-021-14038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Baek S., Hong S.M., Lee J., Jung S.M., Lee J., Cho M.L., Kwok S.K., Park S.H. 11,25-dihydroxy vitamin D3 and interleukin-6 blockade synergistically regulate rheumatoid arthritis by suppressing interleukin-17 production and osteoclastogenesis. J. Kor. Med. Sci. 2020;35(6):e40. doi: 10.3346/jkms.2020.35.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsiou O.S., Kotsios V.S., Lampropoulos I., Zidros T., Zarogiannis S.G., Gourgoulianis K.I. PM 2.5 pollution strongly predicted COVID-19 incidence in four high-polluted urbanized Italian cities during the pre-lockdown and lockdown periods. Int. J. Environ. Res. Publ. Health. 2021;18:5088. doi: 10.3390/ijerph18105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Shiono T., Kusumoto B., Fujinuma J. Multiple drivers of the COVID-19 spread: the roles of climate, international mobility, and region-specific conditions. PLoS One. 2020;15:1–15. doi: 10.1371/journal.pone.0239385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo E., Song E., Yockey L.J., Rakib T., Wong P.W., Homer R.J., Iwasaki A. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. U.S.A. 2019;116(22):10905–10910. doi: 10.1073/pnas.1902840116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C., Rossi L., Manganelli R., Loregian A., Navarin N., Abate D., Sciro M., Merigliano S., De Canale E., Vanuzzo M.C., Besutti V., Saluzzo F., Onelia F., Pacenti M., Parisi S.G., Carretta G., Donato D., Flor L., Cocchio S., Masi G., Sperduti A., Cattarino L., Salvador R., Nicoletti M., Caldart F., Castelli G., Nieddu E., Labella B., Fava L., Drigo M., Gaythorpe K.A.M., Ainslie K.E.C., Baguelin M., Bhatt S., Boonyasiri A., Boyd O., Cattarino L., Ciavarella C., Coupland H.L., Cucunubá Z., Cuomo-Dannenburg G., Djafaara B.A., Donnelly C.A., Dorigatti I., van Elsland S.L., FitzJohn R., Flaxman S., Gaythorpe K.A.M., Green W.D., Hallett T., Hamlet A., Haw D., Imai N., Jeffrey B., Knock E., Laydon D.J., Mellan T., Mishra S., Nedjati-Gilani G., Nouvellet P., Okell L.C., Parag K.V., Riley S., Thompson H.A., Unwin H.J.T., Verity R., Vollmer M.A.C., Walker P.G.T., Walters C.E., Wang H., Wang Y., Watson O.J., Whittaker C., Whittles L.K., Xi X., Ferguson N.M., Brazzale A.R., Toppo S., Trevisan M., Baldo V., Donnelly C.A., Ferguson N.M., Dorigatti I., Crisanti A. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021;19:528–545. doi: 10.1038/s41579-021-00535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Leong F.Y., Xu G., Ge Z., Kang C.W., Lim K.H. Dispersion of evaporating cough droplets in tropical outdoor environment. Phys. Fluids. 2020;32:113301. doi: 10.1063/5.0026360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linillos-Pradillo B., Rancan L., Vara E., Arias J. Determination of SARS-CoV-2 RNA in different particulate matter size fractions of outdoor air samples in Madrid during the lockdown. Environ. Res. 2021;195:110863. doi: 10.1016/j.envres.2021.110863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolli S., Chen Y.C., Wang S.H., Vivone G. Impact of meteorological conditions and air pollution on COVID 19 pandemic transmission in Italy. Sci. Rep. 2020;10:16213. doi: 10.1038/s41598-020-73197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzoni G., Lanera C., Azzolina D., Berchialla P., Gregori D., Covid19ita Working Group Is a more aggressive COVID-19 case detection approach mitigating the burden on ICUs? Some reflections from Italy. Crit. Care. 2020;24(1):175. doi: 10.1186/s13054-020-02881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724:138226. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki M., Anvari E., Hopke P.K., Noorimotlagh Z. An updated systematic review on the association between atmospheric particulate matter pollution and prevalence of SARS-CoV-2. Environ. Res. 2021;195:110898. doi: 10.1016/j.envres.2021.110898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marani M., Katulx G.G., Pan W.K., Parolari A.J. Intensity and frequency of extreme novel epidemics. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118(35) doi: 10.1073/pnas.2105482118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson M.J., Yinda C.K., Seifert S.N., Bushmaker T., Fischer R.J., Doremalen N. Van, Lloyd-smith J.O., Munster V.J. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg. Infect. Dis. 2020;26(9):2276–2278. doi: 10.3201/eid2609.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N.C., Pfeffer P.E., Mann E.H., Kelly F.J., Corrigan C.J., Hawrylowicz C.M., Lee T.H. Urban particulate matter-activated human dendritic cells induce the expansion of potent inflammatory Th1, Th2, and Th17 effector cells. Am. J. Respir. Cell Mol. Biol. 2016;54(2):250–262. doi: 10.1165/rcmb.2015-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirri S., Delnevo G., Roccetti M. Is a COVID-19 second wave possible in Emilia-Romagna (Italy)? Forecasting a future outbreak with particulate pollution and machine learning. Computation. 2020;8:74. doi: 10.3390/computation8030074. [DOI] [Google Scholar]
- Moriyama M., Ichinohe T. High ambient temperature dampens adaptive immune responses to influenza A virus infection. Proc. Natl. Acad. Sci. U.S.A. 2019;116(8):3118–3125. doi: 10.1073/pnas.1815029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections: will COVID-19 follow suit? Annu. Rev. Virol. 2020;7:83–101. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- Nicastro F., Sironi G., Antonello E., Bianco A., Biasin M., Brucato J.R., Ermolli I., Pareschi G., Salvati M., Tozzi P., Trabattoni D., Clerici M. Forcing seasonality of influenza-like epidemics with daily solar resonance of influenza-like epidemics with daily solar resonance. iScience. 2020;23:101605. doi: 10.1016/j.isci.2020.101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W.G., Peck Campbell A.J., Boeckh M. Respiratory viruses other than influenza virus: impact and therapeutic advances. Clin. Microbiol. Rev. 2008;21:274–290. doi: 10.1128/CMR.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Juarez J.I., Kohn T. Virus removal and inactivation by iron (hydr)oxide-mediated Fenton-like processes under sunlight and in the dark. Photochem. Photobiol. Sci. 2013;12:1596–1605. doi: 10.1039/c3pp25314g. [DOI] [PubMed] [Google Scholar]
- Notari A. Temperature dependence of COVID-19 transmission. Sci. Total Environ. 2021;763:144390. doi: 10.1016/j.scitotenv.2020.144390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscanoa T.J., Amado J., Vidal X., Laird E., Ghashut R.A., Romero-Ortuno R. The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyvitamin D concentration - a metaanalysis. Adv. Respir. Med. 2021;89(2):145–157. doi: 10.5603/ARM.a2021.0037. [DOI] [PubMed] [Google Scholar]
- Paintsil E., Binka M., Patel A., Lindenbach B.D., Heimer R. Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J. Infect. Dis. 2014;209:1205–1211. doi: 10.1093/infdis/jit648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios C., Gonzalez L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014;144:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pana T.A., Bhattacharya S., Gamble D.T., Pasdar Z., Szlachetka W.A., Perdomo-Lampignano J.A., Ewers K.D., McLernon D.J., Myint P.K. Country-level determinants of the severity of the first global wave of the COVID-19 pandemic: an ecological study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansini R., Fornacca D. Early spread of COVID-19 in the air-polluted regions of eight severely affected countries. Atmosphere. 2021;12:795. doi: 10.3390/atmos12060795. [DOI] [Google Scholar]
- Paraskevis D., Georgia E., Alygizakis N., Thomaidis N.S., Cartalis C., Tsiodras S., Athanasios M. A review of the impact of weather and climate variables to COVID-19: in the absence of public health measures high temperatures cannot probably mitigate outbreaks. Sci. Total Environ. 2021;768:144578. doi: 10.1016/j.scitotenv.2020.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini G., Mancinelli E., Morichetti M., Virgili S., Rizza U. A preliminary investigation on the statistical correlations between SARS-CoV-2 spread and local meteorology. Int. J. Environ. Res. Publ. Health. 2020;17:4051. doi: 10.3390/ijerph17114051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perone G. The determinants of COVID-19 case fatality rate (CFR) in the Italian regions and provinces: an analysis of environmental, demographic, and healthcare factors. Sci. Total Environ. 2021;755:142523. doi: 10.1016/j.scitotenv.2020.142523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzutto M., Bono Rosselló N., Schenato L., Garone E. Smart testing and selective quarantine for the control of epidemics. Annu. Rev. Control. 2021;51:540–550. doi: 10.1016/j.arcontrol.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012;2:90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirouz Behrouz, Sha S., Pirouz Behzad. Development of an assessment method for investigating the impact of climate and urban parameters in confirmed cases of COVID-19: a new challenge in sustainable development. Int. J. Environ. Res. Publ. Health. 2020;17:2801. doi: 10.3390/ijerph17082801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivato A., Amoruso I., Formenton G., Maria F. Di, Bonato T., Vanin S., Marion A., Baldovin T. Evaluating the presence of SARS-CoV-2 RNA in the particulate matters during the peak of COVID-19 in Padua, northern Italy. Sci. Total Environ. 2021;784:147129. doi: 10.1016/j.scitotenv.2021.147129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postiglione P., Cartone A., Panzera D. Economic convergence in EU NUTS 3 regions: a spatial econometric perspective. Sustainability. 2020;12:6717. doi: 10.3390/SU12176717. [DOI] [Google Scholar]
- Prussin A.J., Schwake D.O., Lin K., Gallagher D.L., Buttling L., Marr L.C. Survival of the enveloped virus Phi6 in droplets as a function of relative humidity, absolute humidity, and temperature. Appl. Environ. Microbiol. 2018;84:1–10. doi: 10.1128/AEM.00551-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta G., Formica G., Tenreiro Machado J., Lacarbonara W., Masri S.F. Understanding COVID-19 nonlinear multi-scale dynamic spreading in Italy. Nonlinear Dynam. 2020;101:1583–1619. doi: 10.1007/s11071-020-05902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I., Beck K., Yeager J., Altamura L.A., Biryukov J., Yolitz J., Schuit M., Wahl V., Hevey M., Dabisch P. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmelts H.H.F., van de Garde E.M.W., Meijvis S.C.A., Peelen E.L.G.C.A., Damoiseaux J.G.M.C., Grutters J.C., Biesma D.H., Bos W.J.W., Rijkers G.T. Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin. Infect. Dis. 2012;55:1488–1494. doi: 10.1093/cid/cis751. [DOI] [PubMed] [Google Scholar]
- Rezaali M., Fouladi-Fard R. Aerosolized SARS-CoV-2 exposure assessment: dispersion modeling with AERMOD. J. Environ. Health Sci. Eng. 2021;19:285–293. doi: 10.1007/s40201-020-00602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS CoV 2 on common surfaces. Virol. J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano A., Pacellic B., Zengarini N., Costa G., Cislaghi C., Caranci N. Aggiornamento e revisione dell’indice di deprivazione italiano 2011 a livello di sezione di censimento. Epidemiol. Prev. 2020;44(2–3):162–170. doi: 10.19191/EP20.2-3.P162.039. [DOI] [PubMed] [Google Scholar]
- Rosario D.K.A., Mutz Y.S., Bernardes P.C., Conte-Junior C.A. Relationship between COVID-19 and weather: case study in a tropical country. Int. J. Hyg Environ. Health. 2020;229:113587. doi: 10.1016/j.ijheh.2020.113587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagripanti J.L., Lytle C.D. Estimated inactivation of coronaviruses by solar radiation with special reference to COVID-19. Photochem. Photobiol. 2020;96:731–737. doi: 10.1111/php.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of coronavirus disease 2019 (COVID-19) JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lorenzo A., Vaquero-Martínez J., Calbó J., Wild M., Santurtún A., Lopez-Bustins J.A., Vaquero J.M., Folini D., Antón M. Did anomalous atmospheric circulation favor the spread of COVID-19 in Europe? Environ. Res. 2021;194:110626. doi: 10.1016/j.envres.2020.110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor G., Del Riccio M., Dal Poz I., Bonanni P., Bonaccorsi G. COVID-19 in Italy: considerations on official data. Int. J. Infect. Dis. 2020;98:188–190. doi: 10.1016/j.ijid.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scartezzini A., Tateo F., Perini P., Benacchio L., Ermani M., Ferro A., Cadaldini M., Grazia M., Colledan L., Freddi N., Gallo P., Puthenparampil M. Association of Multiple Sclerosis with PM 2.5 levels. Further evidence from the highly polluted area of Padua Province. Italy. Mult. Scler. Relat. Disord. 2021;48:102677. doi: 10.1016/j.msard.2020.102677. [DOI] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., Clemente L., Pallavicini A., Ruscio M., Piscitelli P., Miani A. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: first evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfîcă L., Bulai M., Amihăesei V.-A., Ion C., Ștefan M. Weather conditions (with focus on UV radiation) associated with COVID-19 outbreak and worldwide climate-based prediction for future prevention. Aerosol Air Qual. Res. 2020;20:1862–1873. doi: 10.4209/aaqr.2020.05.0206. [DOI] [Google Scholar]
- Shephard R.J., Shek P.N. Cold exposure and immune function. Can. J. Physiol. Pharmacol. 1998;76:828–836. doi: 10.1139/cjpp-76-9-828. [DOI] [PubMed] [Google Scholar]
- Shoemark D.K., Colenso C.K., Toelzer C., Gupta K., Sessions R.B., Davidson A.D., Berger I., Schaffitzel C., Spencer J., Mulholland A.J. Molecular simulations suggest vitamins, retinoids and steroids as ligands of the free fatty acid pocket of the SARS-CoV-2 spike protein. Angew. Chem. Int. Ed. 2021;60:7098–7110. doi: 10.1002/anie.202015639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino Sirio, Tateo Fabio, De Biase Dario, Gallo Claudio G., Orlandi Paolo E., Corazza Ivan, Budriesi Roberta, Micucci Matteo, Visani Michela, Loggi Elisabetta, Hong Wandong, Pica Roberta, Lari1 Federico, Zippi Maddalena. SARS-CoV-2: lessons from both the history of medicine and from the biological behavior of other well-known viruses. Future Microbiol. 2021;16(14):1105–1133. doi: 10.2217/fmb-2021-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Li J., Shi S., Yan L., Zhuang H., Li K. Thermal stability and inactivation of hepatitis C virus grown in cell culture. Virol. J. 2010;7:40. doi: 10.1186/1743-422X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. COVID-19 and air pollution and meteorology-an intricate relationship: a review. Chemosphere. 2021;263:128297. doi: 10.1016/j.chemosphere.2020.128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafoggia M., Cattani G., Ancona C., Ranzi A. Exposure assessment of air pollution in Italy 2016-2019 for future studies on air pollution and COVID-19. Epidemiol. Prev. 2020;44:161–168. doi: 10.19191/EP20.5-6.S2.115. [DOI] [PubMed] [Google Scholar]
- Stafoggia M., Schwartz J., Forastiere F., Perucci C.A., SISTI Group Does Temperature Modify the Association between Air Pollution and Mortality? A Multicity Case-Crossover Analysis in Italy. Am. J. Epidemiol. 2008;167(12):1476–1485. doi: 10.1093/aje/kwn074. [DOI] [PubMed] [Google Scholar]
- Struyf T., Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Leeflang M.M.G., Spijker R., Hooft L., Emperador D., Domen J., Horn S.R.A., Van den Bruel A., Cochrane Covid-19 Diagnostic Test Accuracy Group Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst. Rev. 2021;2(2):CD013665. doi: 10.1002/14651858.CD013665.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhaimi N.F., Jalaludin J., Latif M.T. Demystifying a possible relationship between COVID-19, air quality and meteorological factors: evidence from Kuala Lumpur, Malaysia. Aerosol Air Qual. Res. 2020;20:1520–1529. doi: 10.4209/aaqr.2020.05.0218. [DOI] [Google Scholar]
- Szarpak L., Rafique Z., Gasecka A., Chirico F., Gawel W., Hernik J., Kaminska H., Filipiak K.J., Jaguszewski M.J., Szarpak L. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol. J. 2021;28(5):647–654. doi: 10.5603/CJ.a2021.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Liu M., Ren B., Wu Z., Yu X., Peng C., Tian J. Sunlight ultraviolet radiation dose is negatively correlated with the percent positive of SARS-CoV-2 and four other common human coronaviruses in the. U.S. Sci. Total Environ. 2021;751:141816. doi: 10.1016/j.scitotenv.2020.141816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than T.T., Jo E., Todt D., Nguyen P.H., Steinmann J., Steinmann E., Windisch M.P. High environmental stability of hepatitis B virus and inactivation requirements for chemical biocides. J. Infect. Dis. 2019;219:1044–1048. doi: 10.1093/infdis/jiy620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobías A., Molina T., Rodrigo M., Saez M. Meteorological factors and incidence of COVID-19 during the first wave of the pandemic in Catalonia (Spain): a multi-county study. One Health. 2021;12:100239. doi: 10.1016/j.onehlt.2021.100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczylowski K., Wietlicka-Piszcz M., Grabowska M., Sulik A. Cumulative effects of particulate matter pollution and meteorological variables on the risk of influenza-like illness. Viruses. 2021;13(4):556. doi: 10.3390/v13040556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Selley L., Leal N.S., Martins L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021;268:115859. doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.C., Li C.S. Inactivation of virus-containing aerosols by ultraviolet germicidal irradiation. Aerosol Sci. Technol. 2005;39:1136–1142. doi: 10.1080/02786820500428575. [DOI] [Google Scholar]
- Valacchi G., Magnani N., Woodby B., Ferreira S.M., Evelson P. Particulate matter induces tissue OxInflammation: from mechanism to damage. Antioxidants Redox Signal. 2020;33(4):308–326. doi: 10.1089/ars.2019.8015. [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Munster V.J. Stability of middle east respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18(38):pii=20590. doi: 10.2807/1560-7917.ES2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- Walker C.M., Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ. Sci. Technol. 2007;41:5460–5465. doi: 10.1021/es070056u. [DOI] [PubMed] [Google Scholar]
- Wang J., Alipour M., Soligo G., Roccon A., Paoli M., De Picano F. Short-range exposure to airborne virus transmission and current guidelines. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118(37) doi: 10.1073/pnas.2105279118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathore R., Gupta A., Bherwani H., Labhasetwar N. Understanding air and water borne transmission and survival of coronavirus: insights and way forward for SARS-CoV-2. Sci. Total Environ. 2020;749:141486. doi: 10.1016/j.scitotenv.2020.141486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2021. WHO Coronavirus (COVID-19) Dashboard.https://covid19.who.int/ accessed 28 October 2021. [Google Scholar]
- Wu Y., Jing W., Liu J., Ma Q., Yuan J., Wang Y., Du M., Liu M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total Environ. 2020;729:139051. doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Marr L.C. Dynamics of Airborne influenza A viruses indoors and dependence on humidity. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yang H., Ou C., Luo Z., Hang J. Airborne transmission of pathogen-laden expiratory droplets in open outdoor space. Sci. Total Environ. 2021;773:145537. doi: 10.1016/j.scitotenv.2021.145537. [DOI] [PubMed] [Google Scholar]
- Ye T., Xu R., Yu W., Chen Z., Guo Y. Vulnerability and burden of all-cause mortality associated with particulate air pollution during COVID-19 pandemic: a nationwide observed study in Italy. Toxics. 2021;9(3):56. doi: 10.3390/toxics9030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang S., Luan J., Li L., Yu H., Wu Q., Chang Q., Zhao Y. Ambient air pollution and COVID-19 risk: evidence from 35 observational studies. Environ. Res. 2022;204:112065. doi: 10.1016/j.envres.2021.112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Liu X., Huang H., Avellán-Llaguno R.D., Lazo M.M.L., Gaggero A., Soto-Rifo R., Patiño L., Valencia-Avellan M., Diringer B., Huang Q., Zhu Y. Meteorological impact on the COVID-19 pandemic: A study across eight severely affected regions in South America. Sci. Total Environ. 2020;744:140881. doi: 10.1016/j.scitotenv.2020.140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan. Italy. Sci. Total Environ. 2020;738:139825. doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.