Abstract
DNA damage plays a role in ultraviolet (UV)-induced melanoma. We previously showed that aspirin (ASA) can suppress prostaglandin-E2 (PGE2) and protect melanocytes from UV-induced DNA damage in mice, and suggested that taking ASA before acute sun exposure may reduce melanoma risk. We conducted a prospective randomized placebo-controlled trial to determine if orally administered ASA could suppress PGE2 in plasma and nevi and protect nevi from UV-induced DNA damage. After obtaining plasma and determining the minimal erythemal dose (MED) in 95 subjects at increased risk for melanoma, they were randomized to receive a daily dose of placebo, 81 mg ASA, or 325 mg ASA, in double-blind fashion for one month. After this intervention, one nevus was irradiated (dose = 1 or 2 MED) using a solar simulator. One day later, MED was re-determined, a second plasma sample was obtained, and the UV-irradiated nevus and an unirradiated nevus were removed. ASA metabolites were detected in the second plasma sample in subjects in the ASA arms. There were no significant differences in the pre- and post-intervention MED between those patients receiving ASA and placebo. Significantly reduced PGE2 levels were detected in plasma (second vs. first samples) and in nevi (both unirradiated and UV-treated) in subjects receiving ASA compared to placebo. Comparing UV-treated nevi from the ASA and placebo cohorts, however, did not reveal significant reductions in CD3-cell infiltration or 8-oxoguanine and cyclobutane pyrimidine dimers. Thus ASA did not effectively protect nevi from solar-simulated UV -induced inflammation and DNA damage under the conditions examined.
Keywords: aspirin, chemoprevention, nevi, UV, 8-OG, CPD
Prevention Relevance:
Despite promising rationale, ASA at conventional dosing was not able to protect nevi against UV-induced DNA damage under the conditions examined.
Introduction
The incidence of cutaneous melanoma has been rapidly rising over the past few decades (1) due to many factors including increased exposure to ultraviolet (UV) radiation (2). A randomized controlled trial in Australia demonstrated a 50% reduction in melanoma in sunscreen users (3) and use of sunscreen currently represents the most effective means of primary prevention. However, given that sunscreen is often inadequately applied (4), there would be great value in a systemic adjunct to sunscreen, particularly for high-risk subjects. Several systemic agents have been considered for melanoma chemoprevention, but none have been validated in prospective phase III trials in humans (5) and long-term administration of any drug may be associated with unexpected toxicities.
Although the relationship between UV exposure and melanoma development has long been established (6), the underlying mechanisms through which UV causes melanocyte transformation and tumor development have not been clearly defined. Acute UV exposure induces inflammation, reactive oxygen species, cytokine activation, and DNA damage which have been associated with melanoma development in mouse models (7–9). We suggested over a decade ago (10) that use of a systemic agent limited to periods immediately preceding or during sun exposure that targets UV-induced pathways could counter the cumulative deleterious effects of UV exposure while avoiding the pitfalls of chronic chemoprevention.
Aspirin (ASA) is a safe drug with both anti-inflammatory and antioxidant properties (11) that could potentially be suitable for this purpose. Although there are conflicting epidemiologic studies on the association of ASA use and melanoma risk in humans (12), the potential protective effects of ASA have not been evaluated prospectively in high-risk patients in the context of acute UV exposure. We recently reported that ASA can suppress acute UV-induced inflammation and DNA damage in mouse skin (13). In prior open-label studies in human subjects, we detected ASA metabolites and reduced levels of prostaglandin-E2 (PGE2) in plasma and melanocytic nevi following conventional daily ASA dosing (14). Here, we conducted a randomized placebo-controlled trial to determine if ASA could suppress solar-simulated UV (SSUV)-induced inflammation in nevi and protect against SSUV -induced DNA damage.
Materials and Methods
Human subjects
This study was approved by the Institutional Review Board (IRB #94424) of the University of Utah and conducted in accordance with recognized ethical guidelines (e.g., Declaration of Helsinki). All participants signed an IRB-approved consent form. They were recruited by D.G. from his pigmented lesion clinic at the Huntsman Cancer Institute, in which those with history of numerous or atypical nevi, and/or personal or family history of melanoma are regularly monitored. Subjects that were under age 18, critically ill or mentally handicapped, prisoners, pregnant or breast-feeding, non-English speaking, having history of severe asthma or allergic reaction to ASA, history of bleeding disorder or peptic ulcer disease, intense UV exposure (e.g. tanning bed use) in the preceding month, or those having taken ASA, any non-steroidal anti-inflammatory drug (NSAID), or blood thinner in the preceding two weeks were excluded (Fig. 1A). Females of child-bearing potential had a confirmed negative urine pregnancy test. Subjects were not charged for removal or histological examination of their nevi, and each subject was compensated $400 following their participation. The clinical trial was registered at ClinicalTrials.gov (identifier: NCT04066725) and was conducted in accordance with CONSORT guidelines.
Fig. 1.
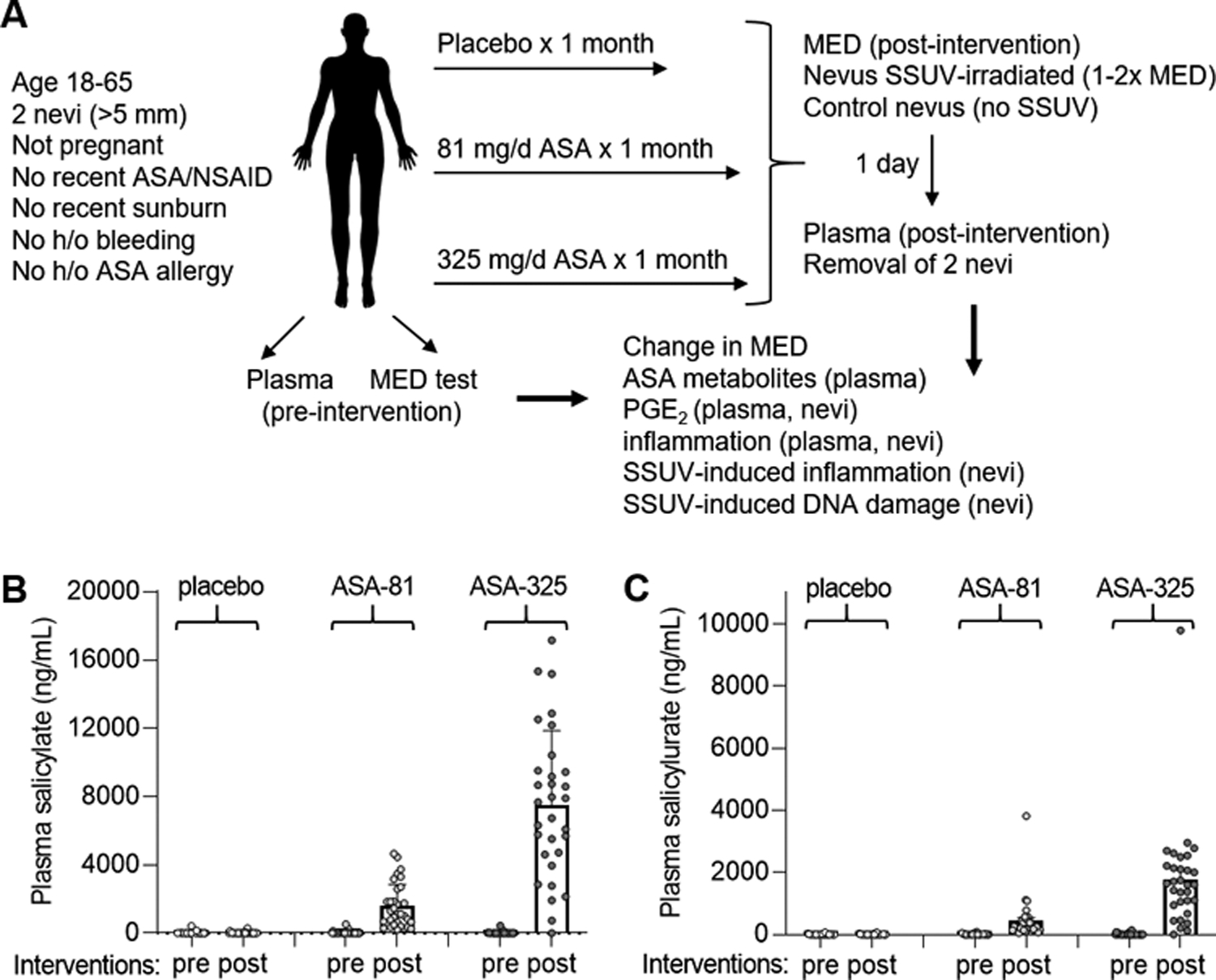
Trial design and measurement of ASA metabolites. A, Summary of randomized controlled trial, procedures and analyses performed. B, Mean plasma salicylate levels in pre- and post-trial samples from subjects in the placebo and two ASA cohorts. Error bars indicate SEM. C, Mean plasma salicylurate levels in pre- and post-trial samples. Error bars indicate SEM.
MED
The minimal erythemal dose (MED) needed to produce skin erythema at 24 hours was determined for each subject both prior to and at the end of the trial using a 16S-300–003 solar simulator calibrated with a PMA2100 radiometer (SolarLight, Glenside, PA) as described previously (15). The emission spectrum of the device encompasses wavelength from 295 to 400 nm, and is weighted to mimic the spectrum of sunlight.
Study visits
There were four study visits for each subject. At the first visit (day 1), after obtaining informed consent, MED testing was initiated and the first blood sample was obtained. At the second visit (day 2), the MED was determined and unlabeled capsules were provided. At the third visit (approximately day 28), repeat MED testing was initiated and one nevus was SSUV -treated. At the fourth visit (day after 3rd visit), repeat MED was determined, a second blood sample was obtained, and both the UV-treated and an unirradiated nevus were removed.
ASA
Placebo- and ASA-containing capsules were prepared by the Huntsman Cancer Institute investigational pharmacy. Avicel PH105 microcrystalline cellulose NF powder (Letco, NDC 6299120072) was used to fill “00” capsule shells (DRK GRN, NDC 6299141215) for the placebo. Single ASA tablets containing either 81 mg (Rugby, NDC 00536123441) or 325 mg (Bayer, NDC 00284211010) were added along with enough Avicel to fill the remaining capsules. The tops of the capsules were locked into place and added to plastic bottles labeled by subject number according to a randomization scheme provided by the statistician (K.M.B.). The bottles were stored at room temperature. The investigators and subjects were blinded as to the contents of each numbered bottle. Subjects were asked to take a capsule each evening at bedtime, and provided a log sheet to document date and time of each dose. They were contacted each week to confirm adherence and inquire about potential side effects. The last study visit occurred approximately 7–14 hours after the last dose.
Plasma
Blood was collected from each subject at the start and end of the trial in EDTA-containing vacutainers, and plasma was isolated and stored at −80 °C as described previously (14).
Nevi
Two nevi (>5 mm in diameter) were selected on each subject. These were lesions not clinically suspicious for melanoma by visual inspection and dermoscopy and/or confirmed to be stable by comparison to the subject’s baseline photographs used for monitoring during regular clinic visits. At the end of the trial, one nevus was SSUV -irradiated at a dose equivalent to 1 (pink nevi) or 2 (brown nevi) MED (based on initial MED determination for each subject). Approximately 24 hours later, both the unirradiated and SSUV -treated nevus were removed as described previously (16). A representative 1 mm slice from each nevus was placed in 10% formalin for paraffin embedding and sectioning. The remaining nevus fragments were stored at −80 °C. A hematoxylin and eosin (H&E)-stained section was later reviewed by a dermatopathologist (S.R.F.) to confirm the lesion was a nevus and not melanoma. The presence of dysplasia was also noted. Additional unstained sections were prepared for immunohistochemistry.
ASA metabolites
Metabolites of ASA were detected and quantitated in plasma samples by liquid chromatography-mass spectrometry as previously described (14).
PGE2
PGE2 was quantitated in plasma and nevus specimens by ELISA using a PGE2 assay kit as described previously (14).
SSUV -induced inflammation and DNA damage in nevi
Sunburn cells were quantitated in H&E-stained sections by light microscopy as previously described (17). Inflammatory cell infiltrates and expression of markers of DNA damage was determined by immunohistochemistry on nevus sections using mouse anti-myeloperoxidase (clone MPO-7, DAKO), rabbit anti-CD3 (Abcam ab5690), mouse anti-CD4 (clone 4B12, Leica Biosystems), mouse anti-CD8 (clone 4B11, Leica), mouse anti-CD163 (clone 10D6, Leica), mouse anti-CD20 (clone L26, Leica), mouse anti-8-oxoguanine (8-OG, Genox Corp.), and mouse anti-cyclobutane pyrimidine dimer (CPD, Cosmo Bio) antibodies as described previously (13).
Statistics
A biostatistician (K.M.B.) constructed the subject randomization scheme, and performed all statistical analyses using “R” software (Vienna, Austria). Analyses of MED, PGE2, and sunburn cells was performed on the log scale. Because some sunburn cell counts were equal to zero, 0.5 was added to all sunburn cell counts before taking the logarithm. The results were converted back to the original scale for plotting and interpretation. Paired t-tests were used for differences in PGE2 within a single treatment group, and Welch’s two-sample t tests were used for differences in PGE2 between treatment groups. Additionally, a combined analysis adjusted for nevus color using linear regression on the log-transformed values with color as an adjustment variable was employed. Multiple regression was used to analyze differences in sunburn cells between treatment groups, adjusting for nevus color (and SSUV dosage). Analysis of covariance with treatment group as fixed effect and pre-treatment value as a continuous adjustment variable was used to analyze differences in post treatment MED and serum PGE2. P-values from Wald tests are reported for the analysis of covariance and regression models. T tests were used to analyze 8-OG, and the results are presented on the original scale. Analysis was also done on the log scale and with an analysis of covariance model for the difference between the SSUV -irradiated and unirradiated nevi with similar results. For the CPD data, we used non-parametric Wilcoxon tests for pairwise comparisons because the zero values seen in many of the unirradiated nevi violates normality assumptions required for a t-test. For paired analyses of CPD data, zero values were replaced by 0.5 (half of limit of detection). P values ≤ 0.05 were considered statistically significant.
Results
Randomization of subjects to one month of placebo or ASA
A total of 95 eligible subjects were randomized to receive placebo (n=32), 81 mg ASA (ASA-81, n=32), or 325 mg ASA (ASA-325, n=31) daily for one month (Fig. 1A). There were no significant differences in age, sex, personal of family history of melanoma, body surface area, or hair and eye color among the cohorts (Table I). Four subjects assigned to the placebo group withdrew due to unrelated events or exposure to ASA or NSAIDs in violation of protocol (Fig 2). The remaining 91 subjects completed the study. The rate of reported side effects was 3.6%, 6.3%, and 9.7% in the placebo, ASA-81 and ASA-325 cohorts, respectively. One subject developed transient vision loss and withdrew from the study. The other side effects reported were transient auditory symptoms, sleep disturbance, stomach upset, heartburn and bruising, and their incidence is noted in Fig. 2). No significant levels of ASA metabolites were detected in plasma samples from subjects in the placebo cohort, or in pre-trial samples from subjects in either ASA cohort (Fig. 1B,C), confirming subject compliance with protocol. Post-trial plasma samples from the ASA cohorts revealed dose-dependent levels of salicylate (Fig. 1B) and salicylurate (Fig. 1C). Gentesate and salicylacyl glucoronide were not detected.
Table I.
Demographics of study participants
| Placebo (n=28) | 81 mg ASA (n=32) | 325 mg ASA (n=31) | P-value | |
|---|---|---|---|---|
| Age (mean, range) | 47, 18–61 | 44, 24–63 | 44, 29–64 | 0.39 |
| Sex (M, F) | 11, 17 | 14, 18 | 12, 19 | 0.68 |
| Personal history melanoma (No., %) | 12, 43% | 13, 41% | 21, 68% | 0.067 |
| Family history melanoma (No., %) | 15, 54% | 12, 39% | 18, 58% | 0.24 |
| Body surface area (m2, mean, range) | 1.9, 1.5–3.0 | 1.9, 1.5–2.5 | 1.9, 1.5–2.4 | 0.77 |
| Hair color (No., %) | ||||
| Brown | 14, 59% | 21, 66% | 19, 59% | 0.54 |
| Red | 1, 4% | 2, 7% | 3, 11% | |
| Blonde | 13, 42% | 9, 29% | 9, 29% | |
| Eye color (No., %) | 0.19 | |||
| Brown | 4, 14% | 6, 19% | 5, 16% | |
| Green | 0, 0% | 4, 13% | 6, 19% | |
| Hazel | 7, 25% | 6, 19% | 9, 29% | |
| Blue | 17, 61% | 16, 50% | 11, 35% |
Fig. 2.
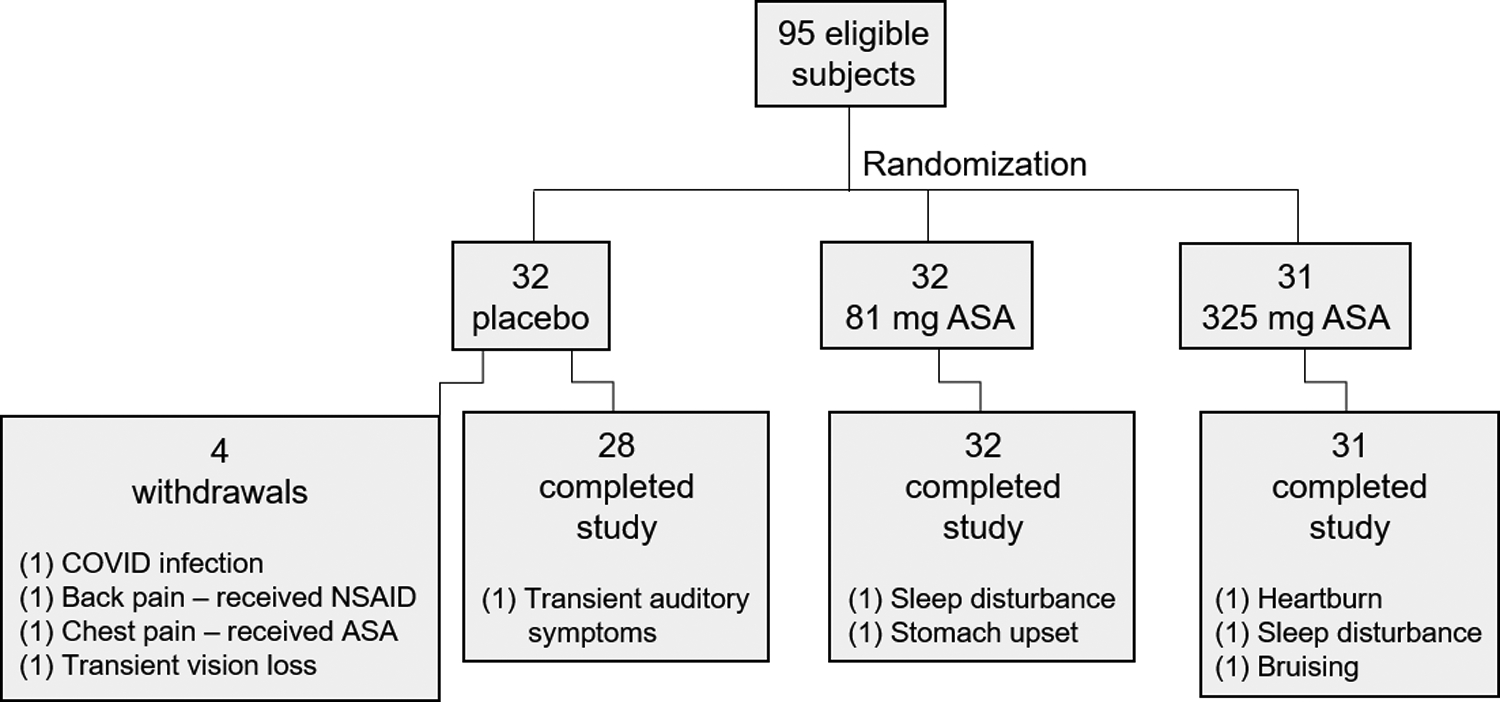
Subject randomization, withdrawals, and reported side effects. A total of 95 subjects were randomized to either daily placebo (n=28), 81 mg ASA (n=32), or 325 mg ASA (n=31) as indicated. There were 4 withdrawals from the placebo group. Shown are the numbers of subjects in each group who completed the study and the number (in parentheses) reporting various side effects.
ASA did not affect MED
There was considerable variation in MED values among the subjects, and between the pre-trial and post-trial measurements for each subject (Fig. 3A). The MED increased by approximately 15–17% in all the subjects, but paired analyses of pre-trial and post-trial values did not reveal significant changes in MED within any cohort or between either ASA cohort and placebo (P=0.7 for all comparisons).
Fig. 3.
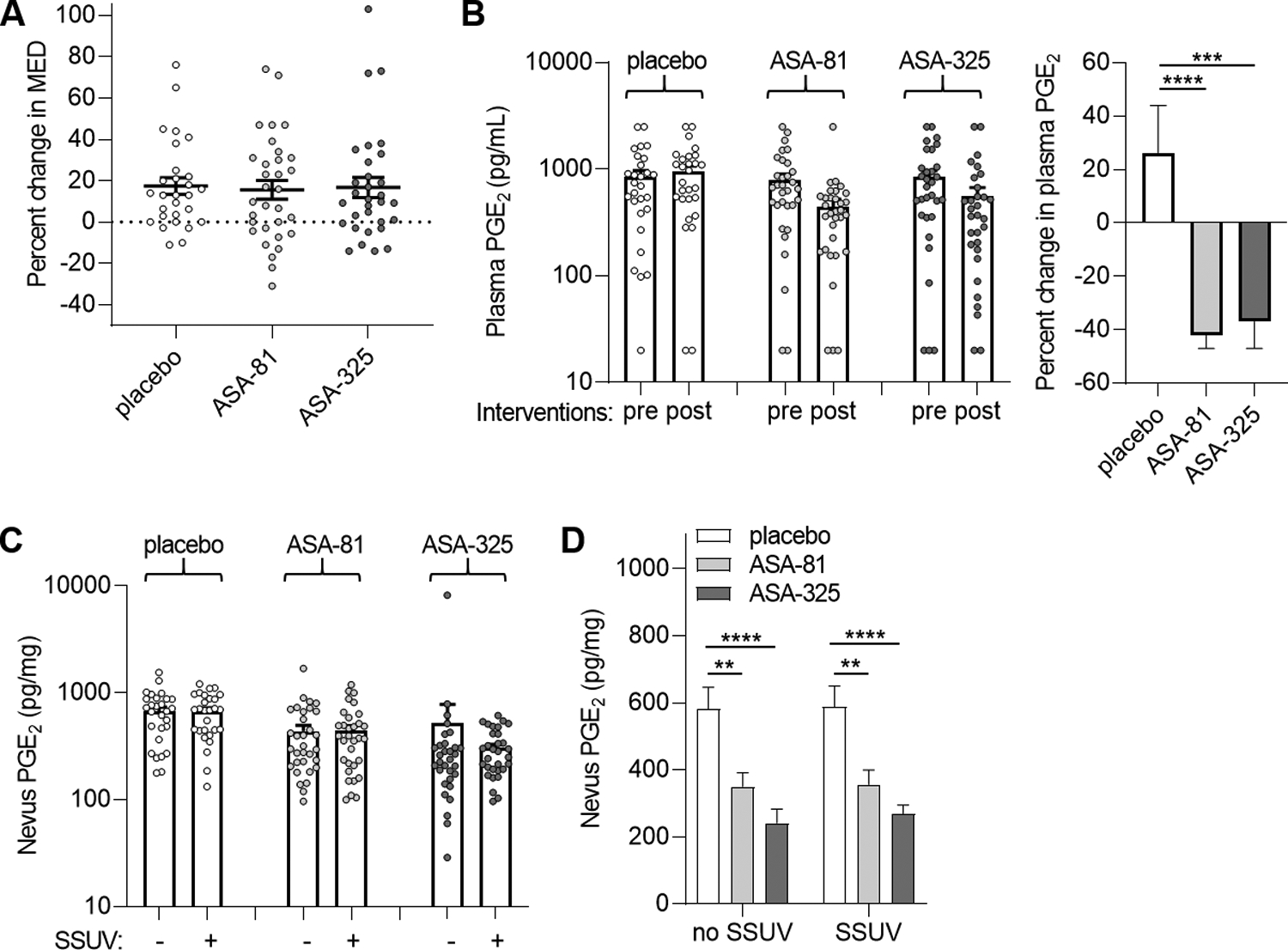
MED and modulation of PGE2 in plasma and nevi. A, Mean percent change in MED (post-intervention compared to pre-intervention measurements) in the cohorts receiving placebo (n=28), 81 mg daily ASA (ASA-81, n=32), and 325 mg daily ASA (ASA-325, n=31). Error bars indicate SEM. Paired analyses did not reveal significant changes in MED in any cohort or between either ASA cohort and placebo (P=0.7). B, Mean PGE2 levels in plasma obtained before (pre) and after (post) intervention (left panel). Percent changes in plasma PGE2 for each group (right panel). Error bars represent SEM. ***P=0.001, ****P<0.0001, paired tests. C, Mean PGE2 levels in unirradiated and solar-simulated UV (SSUV) -treated nevi. Error bars represent SEM. Paired analyses did not reveal significant differences in PGE2 between unirradiated and SSUV-treated nevi within the placebo (P=0.93), ASA-81 (P=0.85) or ASA-325 (P=0.48) cohorts. D, Comparison of nevus PGE2 levels among the groups for unirradiated (no SSUV) and SSUV-treated nevi. **P<0.01, ****P<0.0001, paired t tests. Values were lower in the ASA-325 compared to the ASA-81 cohorts which approached statistical significance for unirradiated (P=0.08) and SSUV-treated nevi (P=0.07).
ASA suppresses PGE2 in plasma and nevi
Plasma PGE2 levels also varied considerably among all the subjects, but paired analyses comparing post- and pre-trial samples revealed significant reductions of 35–40% for plasma PGE2 in both ASA cohorts but not in the placebo cohort (Fig. 3B). There was not a significant difference in percent change between the two ASA cohorts (p = 0.69). For nevi, while PGE2 levels were increased by 1.3%, 8.8%, and 1.6% between paired unirradiated and UV-treated nevi in the placebo, ASA-81 and ASA-325 cohorts, respectively (Fig. 3C), none of these UV-induced increases were significant (P=0.3–0.9). Similarly, no differences between paired unirradiated and UV-treated nevi were seen among the placebo, ASA-81 and ASA-325 groups when the analysis was adjusted for nevus color with adjustment variables brown and pink (P=0.19, 0.97 and 0.86, respectively). On the other hand, PGE2 was significantly reduced among both the unirradiated nevi and UV-treated nevi in both of the ASA cohorts compared to the placebo cohort (Fig. 3D). Reductions in PGE2 were greater among unirradiated nevi (P=0.07) and UV-treated nevi (P=0.04) in the ASA-325 cohort compared to the ASA-81 cohort. Reductions in PGE2 levels in both SSUV-treated (ASA-81 and ASA-325 vs. placebo, P<0.0001) and unirradiated (ASA-81 vs. placebo, P=0.001; ASA-325 vs. placebo, P<0.0001) nevi remained significant when adjusted for nevus color. Thus, while PGE2 levels in nevi were not significantly affected by UV treatment, PGE2 was suppressed in both plasma and all the nevi in subjects receiving ASA.
ASA does not affect SSUV -induced apoptosis, inflammation, or DNA damage in nevi
Sunburn cells undergoing SSUV -induced apoptosis (18) were generally not seen in unirradiated nevi and consistently observed in SSUV -treated nevus specimens. However, there were no significant differences between SSUV -treated nevi in comparing the ASA-81 (P= 0.8) or ASA-325 (P=0.55) cohorts with the placebo cohort (Fig. 4A). Since brown nevi were treated with twice the MED-equivalent SSUV dose than pink nevi, we performed a stratified analysis for this covariate and again found no significant differences in brown or pink UV-treated nevi between the ASA-81 (P= 0.42 and 0.19, respectively) or ASA-325 (P=0.98 and 0.37, respectively) cohorts with the placebo cohort.
Fig. 4.
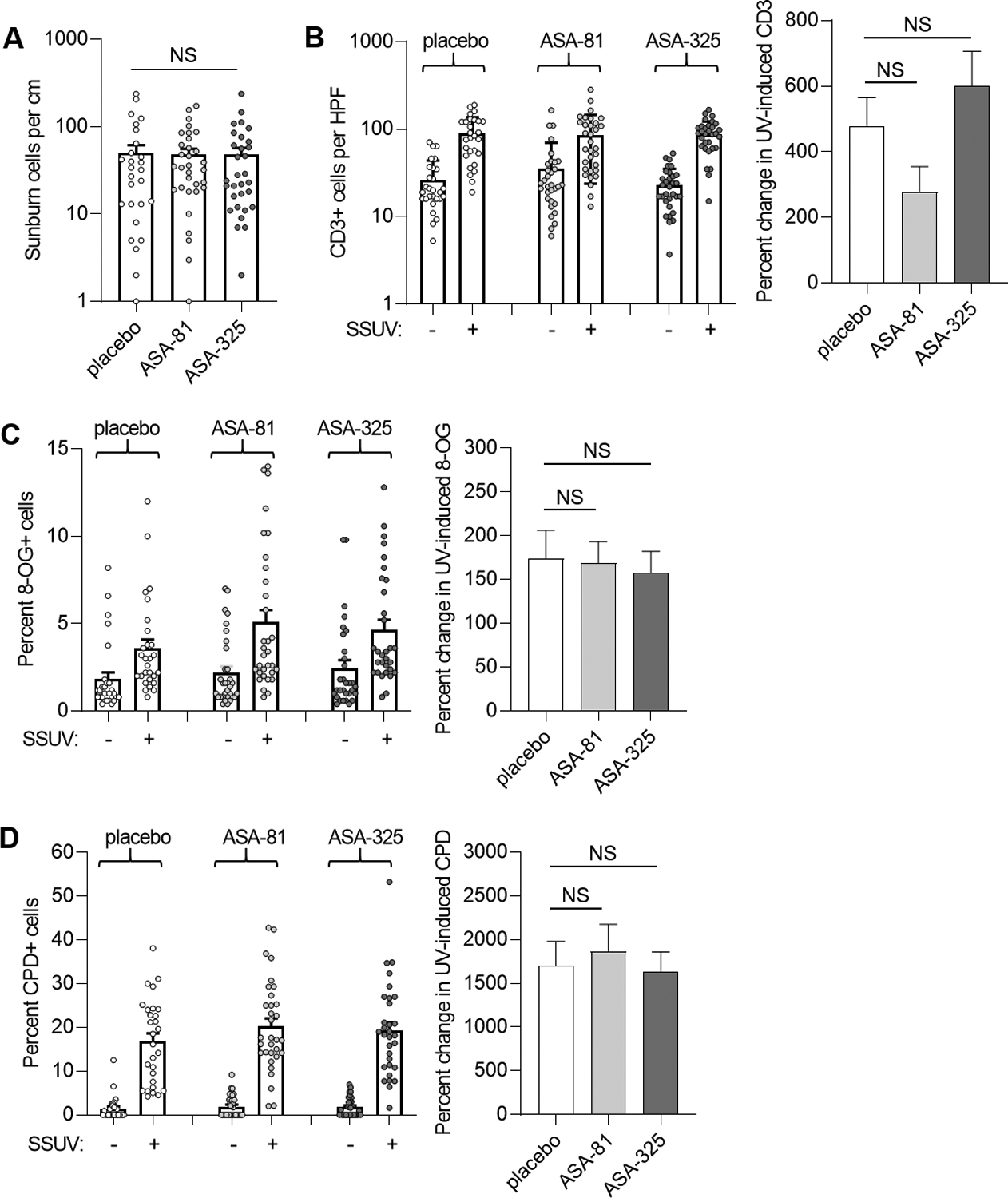
SSUV responses in nevi. A, Sunburn cells were quantitated from H&E sections of UV-treated nevi from the cohorts receiving placebo (n=28), 81 mg daily ASA (ASA-81, n=32), and 325 mg daily ASA (ASA-325, n=31). Few or no sunburn cells were observed in unirradiated nevi. Bars represent mean and error bars represent SEM. Analyses did not reveal significant differences between SSUV-treated nevi in the ASA-81 (P= 0.8) or ASA-325 (P=0.55) and placebo cohorts. NS, not significant. B, CD3 expression in unirradiated and SSUV-treated nevi (left panel). Percent changes in SSUV-induced CD3 count for each group (right panel). Bars represent mean and error bars represent SEM. There were no significant differences in CD3 between unirradiated or SSUV-treated nevi in either the ASA-81 (P=0.37 and 0.45, respectively) or ASA-325 (P=0.60 and 0.75, respectively) cohort compared to placebo. Similarly, paired analyses comparing unirradiated and SSUV-treated nevi did not demonstrate significant differences in SSUV-induced CD3 for either the ASA-81 (P=0.13) or ASA-325 (P=0.53) cohort compared to placebo. NS, not significant. C, 8-OG. Percent cells staining for 8-OG in unirradiated and SSUV-treated nevi within each of the indicated cohorts. There were no significant differences in 8-OG between unirradiated or SSUV -treated nevi in either the ASA-81 (P=0.48 and 0.10, respectively) or ASA-325 (P=0.29 and 0.17, respectively) cohort compared to placebo (left panel). Paired analyses comparing unirradiated and SSUV-treated nevi did not demonstrate significant differences in SSUV -induced 8-OG for either the ASA-81 (P=0.07) or ASA-325 (P=0.37) cohort compared to placebo (right panel). D, CPD. Percent cells staining for CPD in unirradiated and SSUV-treated nevi within each of the indicated cohorts. There were no significant differences in CPD between unirradiated or SSUV-treated nevi in either the ASA-81 (P=0.46 and 0.25, respectively) or ASA-325 (P=0.45 and 0.47, respectively) cohort compared to placebo (left panel). Paired analyses comparing unirradiated and SSUV-treated nevi did not demonstrate significant differences in SSUV-induced CPD for either the ASA-81 (P=0.25) or ASA-325 (P=0.39) cohort compared to placebo (right panel).
In our prior studies in mouse skin, acute UV exposure resulted in a mixed inflammatory cell infiltrate composed of macrophages and lymphocytes (13). Most of the SSUV -treated nevi also demonstrated robust inflammatory cell infiltration of the dermis. We selected 6 different SSUV-treated nevi from the placebo group, and performed immunostains for myeloperoxidase and CD-163 to assess macrophage/monocyte and CD3, CD4, CD8, and CD20 to assess lymphocytes in the SSUV-induced inflammatory infiltrates. We found that the predominant inflammatory cell type was CD3+ lymphocytes (Supplemental Fig. S1), with a CD4:CD8 ratio of approximately 2:1 as in normal skin (19). Few or no cells expressed the B-cell marker CD20. Finally, there was very minimal staining for myeloperoxidase or CD-163, which was similar to that seen in unirradiated nevi. Thus, we focused our analysis of SSUV -induced inflammation to all nevus sections stained for CD3. As shown in Fig. 4B, both unirradiated and SSUV -treated nevi from each cohort expressed variable expression of CD3 cells and the average value was higher in SSUV -treated compared to unirradiated nevi within each cohort. SSUV -induced CD3 expression was 2–5 fold higher in SSUV -treated compared to unirradiated nevi in all cohorts, however, there was not significantly reduced UV-induced CD3 expression in either ASA cohort compared to placebo (Fig. 4B). In addition, no significant differences were observed in analyses restricted to brown or pink SSUV -treated nevi between the ASA-81 (P= 0.24 and 0.34, respectively) or ASA-325 (P=0.24 and 0.81, respectively) cohorts and the placebo cohort.
Next, we examined expression of 8-OG and CPD in nevi as a measure of SSUV -induced DNA damage. Both unirradiated and SSUV -treated nevi from each cohort demonstrated variable expression of 8-OG and the average percent positive-staining cells was higher in SSUV -treated compared to unirradiated nevi within each cohort. Notably, there were not significant differences in 8-OG between unirradiated or SSUV -treated nevi in either the ASA-81 (P=0.48 and 0.10, respectively) or ASA-325 (P=0.29 and 0.17, respectively) cohort compared to placebo (Fig. 4C). UV-induced 8-OG was approximately 1.5-fold higher in SSUV -treated compared to unirradiated nevi in all cohorts, however paired analyses comparing unirradiated and SSUV -treated nevi did not demonstrate significant differences in SSUV -induced 8-OG in either the ASA-81 (P=0.07) or ASA-325 (P=0.37) cohort compared to placebo. For CPD, expression in both unirradiated and SSUV -treated nevi from each cohort was variable and the average percent positive-staining cells was higher in SSUV -treated compared to unirradiated nevi within each cohort. However, there were not significant differences in CPD between unirradiated or SSUV -treated nevi in either the ASA-81 (P=0.46 and 0.25, respectively) or ASA-325 (P=0.45 and 0.47, respectively) cohort compared to placebo (Fig. 4D). SSUV -induced CPD was approximately 10–15 fold higher in SSUV -treated compared to unirradiated nevi in all cohorts, however paired analyses comparing unirradiated and SSUV -treated nevi did not demonstrate significant differences in SSUV -induced CPD in either the ASA-81 (P=0.25) or ASA-325 (P=0.39) cohort compared to placebo. Thus, ASA administration did not appear to reduce SSUV -induced apoptosis, inflammation, or DNA damage in nevi under the conditions examined.
Finally, we reviewed all the SSUV—treated nevi for dysplasia and found the following proportions with dysplasia in the placebo 11/29 (38%), ASA-81 11/32 (34%), and ASA-325 11/31 (35%) groups. There was no significant difference between the groups (p = 0.96, Fisher’s Exact test).
Discussion
We previously demonstrated that orally-delivered ASA could reduce UV-induced sunburn cell formation, inflammation and DNA damage in mouse skin (13), and showed that a 1-week course of daily ASA could suppress PGE2 in the plasma and nevi of human subjects (14). Despite achieving similar reductions in plasma and nevus PGE2 however, we did not observe significant protection against these SSUV -induced sequelae in human nevi of subjects following ASA administration under the conditions examined. It is important to consider, however, a number of differences between the prior mouse study and this randomized controlled trial in human subjects.
First, there were differences in ASA dosing and the time intervals between ASA delivery, UV treatment, and harvest of tissue for analysis in the two model systems. In the prior mouse study, adult mice received 0.4 mg ASA daily by gavage (~20 mg/kg) approximately 1–2 hours prior to UV exposure and 3–4 hours prior to skin preservation (13). In the present study, subjects in the highest dose group (ASA-325 cohort) took 325 mg ASA daily (~3–6 mg/kg, dose not adjusted for weight) approximately 7–14 hours prior to SSUV treatment and removal of the nevi. It is possible that subjects in the present study were under-dosed (3–6 vs. 20 mg/kg) and too much time had expired between their last ASA dose and SSUV treatment (7–14 vs. 1–2 hours) and removal of nevi (7–14 vs. 3–4 hours). ASA is rapidly metabolized and not detectable in plasma (20), but examination of its immediate and most stable metabolite salicylate revealed mean levels of approximately 8000 (+/− 2000) ng/mL in the ASA-325 cohort which was comparable to that measured in our prior open-label human study (14) and in adult mice 4 hours after ASA gavage in an earlier study (21). We have previously shown that plasma salicylate levels peak in humans about 4–8 hours after a single 325 mg ASA dose (14). The longer time intervals in the present trial between last ASA dose, SSUV treatment, and nevus harvest compared to the prior mouse studies were necessitated by when it was convenient for most subjects to return for clinic visits. While we could have provided subjects higher daily doses of ASA to achieve greater salicylate levels over a longer period of time, given the roughly 10% incidence of side effects reported in the ASA-325 cohort it is likely that higher doses would not have been tolerable in many subjects.
Despite these limitations, ASA dosing in this trial was associated with 30–50% reductions in both plasma and nevus PGE2 levels in subjects randomized to ASA. This level of PGE2 suppression was comparable to that seen in plasma and nevi in the prior open-label human study (14) and in plasma and skin of adult mice in the prior mouse study (13). The mechanism(s) by which ASA protects against UV-induced DNA damage may be multi-factorial and has not been clearly defined. We previously showed that ASA-mediated inhibition of melanoma cell motility and melanin production in vitro was mediated by inhibition of PGE2 synthesis as addition of PGE2 to the culture medium was sufficient to reverse these effects of ASA (21). While it is possible that the level of PGE2 suppression achieved here was insufficient for protection of nevi, it is also conceivable that ASA-mediated protection of melanocytes and nevi involves PGE2-independent mechanisms which is a subject that merits further investigation.
Additional considerations are the differences between mouse skin and human nevi, the sources of UV employed, and the time point for tissue examination following UV exposure. The epidermis is considerably thicker in human compared to mouse skin and nevi often extend deeply into the dermis (22). UVB-emitting bulbs were used in the prior mouse study, while in the present study we employed SSUV consisting of both UVB and UVA. UVA consists of lower energy but longer wavelengths that may cause less DNA damage but penetrate deeper into the dermis than UVB (23). In our prior mouse studies showing ASA-mediated reduction in UV-induced inflammation and DNA damage (13), the protective effect of ASA was seen at multiple time points (1, 6, 24, and 48 hours) following UV exposure. Since the current trial design involved a second measurement of the MED, which was read 24 hours after SSUV exposure, it was most practical to have the MED initiated on the same day as the SSUV treatment of nevi so both could be evaluated on the same next day (24 hours after SSUV). Thus the spectrum and time course for UV damage and the ability of ASA to protect against it may vary in the context of these different UV sources and tissues.
A prior chemoprevention metabolomics study of ASA in healthy subjects reported that a dosing regimen of 325 mg daily for two months was associated with approximately 20% reduction in plasma levels of the oncometabolite 2-hydroxyglutarate (24). We similarly performed metabolomics in our prior open-label study but did not recapitulate this finding in subjects given 325 mg daily for 1 week (14). We considered that although dosing for one week was sufficient for PGE2 suppression, longer treatment regimens may be required for a wider spectrum of ASA effects. Thus, we conducted the present trial for a course of one month in balancing these considerations with the efforts associated with extended dosing regimens. It was not feasible to test multiple variables such as higher doses of ASA and duration and timing of drug administration relative to SSUV (before vs. after) exposure in a randomized controlled setting.
ASA remains a safe drug with demonstrated chemopreventive activity in subjects at increased risk for colon cancer (25), although its daily use is not recommended by the U.S. Preventive Services Task Force (26) in healthy subjects over age 60 given the small but increased risk of bleeding (27) and lack of overall survival benefit (28). Our initially proposed paradigm for melanoma chemoprevention, in which an anti-oxidant could be taken in anticipation of UV exposure to prevent UV-induced pro-carcinogenic oxidative damage (10), may be suitable for ASA but further studies are required to determine if alternate dosing regimens or evaluation of nevi at different time points following SSUV treatment could demonstrate protection against UV-induced inflammation and DNA damage.
Supplementary Material
Acknowledgements
D. Grossman was supported by The Department of Dermatology at the University of Utah and the Huntsman Cancer Foundation.
Abbreviations:
- 8-OG
8-oxoguanine
- CPD
cyclobutane pyrimidine dimers
- MED
minimal erythemal dose
- ROS
reactive oxygen species
- SSUV
solar-simulated ultraviolet
- UV
ultraviolet
Footnotes
Conflicts of interest: None declared.
ClinicalTrials.gov identifier: NCT04066725
References
- 1.Welch HG, Mazer BL, Adamson AS. The Rapid Rise in Cutaneous Melanoma Diagnoses. N Engl J Med 2021;384(1),72–79. doi: 10.1056/NEJMsb2019760 [DOI] [PubMed] [Google Scholar]
- 2.Muzumdar S, Lin G, Kerr P, Grant-Kels JM. Is Melanoma Overdiagnosed? A Review of the Evidence. J Am Acad Dermatol 2021;doi: 10.1016/j.jaad.2021.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011;29(3),257–263. doi: 10.1200/JCO.2010.28.7078 [DOI] [PubMed] [Google Scholar]
- 4.Petersen B, Datta P, Philipsen PA, Wulf HC. Sunscreen use and failures--on site observations on a sun-holiday. Photochem Photobiol Sci 2013;12(1),190–196. doi: 10.1039/c2pp25127b [DOI] [PubMed] [Google Scholar]
- 5.Jeter JM, Bowles TL, Curiel-Lewandrowski C, Swetter SM, Filipp FV, Abdel-Malek ZA, et al. Chemoprevention agents for melanoma: A path forward into phase 3 clinical trials. Cancer 2019;125(1),18–44. doi: 10.1002/cncr.31719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med 1999;340(17),1341–1348. doi: 10.1056/NEJM199904293401707 [DOI] [PubMed] [Google Scholar]
- 7.Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature 2011;469(7331),548–553. doi: 10.1038/nature09666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan AM, Lo J, Fisher DE. How does pheomelanin synthesis contribute to melanomagenesis?: Two distinct mechanisms could explain the carcinogenicity of pheomelanin synthesis. Bioessays 2013;35(8),672–676. doi: 10.1002/bies.201300020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day CP, Marchalik R, Merlino G, Michael H. Mouse models of UV-induced melanoma: genetics, pathology, and clinical relevance. Lab Invest 2017;97(6),698–705. doi: 10.1038/labinvest.2016.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodson AG, Cotter MA, Cassidy P, Wade M, Florell SR, Liu T, et al. Use of oral N-acetylcysteine for protection of melanocytic nevi against UV-induced oxidative stress: towards a novel paradigm for melanoma chemoprevention. Clin Cancer Res 2009;15(23),7434–7440. doi: 10.1158/1078-0432.CCR-09-1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dovizio M, Tacconelli S, Sostres C, Ricciotti E, Patrignani P. Mechanistic and pharmacological issues of aspirin as an anticancer agent. Pharmaceuticals (Basel) 2012;5(12),1346–1371. doi: 10.3390/ph5121346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman JR, Grossman D. Aspirin and other NSAIDs as chemoprevention agents in melanoma. Cancer Prev Res (Phila) 2014;7(6),557–564. doi: 10.1158/1940-6207.CAPR-14-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman H, Kumar D, Liu T, Okwundu N, Lum D, Florell SR, et al. Aspirin Protects Melanocytes and Keratinocytes against UVB-Induced DNA Damage In Vivo. J Invest Dermatol 2021;141(1),132–141 e133. doi: 10.1016/j.jid.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 14.Varedi A, Rahman H, Kumar D, Catrow JL, Cox JE, Liu T, et al. ASA Suppresses PGE2 in Plasma and Melanocytic Nevi of Human Subjects at Increased Risk for Melanoma. Pharmaceuticals (Basel) 2020;13(1),doi: 10.3390/ph13010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodson AG, Varedi A, Hull C, Grossman D. A safe and efficient model for ultraviolet radiation-induced herpes simplex labialis. Photodermatol Photoimmunol Photomed 2015;31(3),170–172. doi: 10.1111/phpp.12168 [DOI] [PubMed] [Google Scholar]
- 16.Cassidy PB, Liu T, Florell SR, Honeggar M, Leachman SA, Boucher KM, et al. A Phase II Randomized Placebo-Controlled Trial of Oral N-acetylcysteine for Protection of Melanocytic Nevi against UV-Induced Oxidative Stress In Vivo. Cancer Prev Res (Phila) 2017;10(1),36–44. doi: 10.1158/1940-6207.CAPR-16-0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman D, Kim PJ, Blanc-Brude OP, Brash DE, Tognin S, Marchisio PC, et al. Transgenic expression of survivin in keratinocytes counteracts UVB-induced apoptosis and cooperates with loss of p53. J Clin Invest 2001;108(7),991–999. doi: 10.1172/JCI13345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Hanks AN, Boucher K, Florell SR, Allen SM, Alexander A, et al. UVB-induced apoptosis drives clonal expansion during skin tumor development. Carcinogenesis 2005;26(1),249–257. doi: 10.1093/carcin/bgh300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuckols JD, Shea CR, Horenstein MG, Burchette JL, Prieto VG. Quantitation of intraepidermal T-cell subsets in formalin-fixed, paraffin-embedded tissue helps in the diagnosis of mycosis fungoides. J Cutan Pathol 1999;26(4),169–175. doi: 10.1111/j.1600-0560.1999.tb01824.x [DOI] [PubMed] [Google Scholar]
- 20.Mullangi R, Sharma K, Srinivas NR. Review of HPLC methods and HPLC methods with mass spectrometric detection for direct determination of aspirin with its metabolite(s) in various biological matrices. Biomed Chromatogr 2012;26(8),906–941. doi: 10.1002/bmc.2694 [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Rahman H, Tyagi E, Liu T, Li C, Lu R, et al. Aspirin Suppresses PGE2 and Activates AMP Kinase to Inhibit Melanoma Cell Motility, Pigmentation, and Selective Tumor Growth In Vivo. Cancer Prev Res (Phila) 2018;11(10),629–642. doi: 10.1158/1940-6207.CAPR-18-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lever WF, Schaumburg-Lever G, Histopathology of the Skin. 7th Edition ed. 1990, Philadelphia: J.B. Lippincott. [Google Scholar]
- 23.Romanhole RC, Ataide JA, Moriel P, Mazzola PG. Update on ultraviolet A and B radiation generated by the sun and artificial lamps and their effects on skin. Int J Cosmet Sci 2015;37(4),366–370. doi: 10.1111/ics.12219 [DOI] [PubMed] [Google Scholar]
- 24.Liesenfeld DB, Botma A, Habermann N, Toth R, Weigel C, Popanda O, et al. Aspirin Reduces Plasma Concentrations of the Oncometabolite 2-Hydroxyglutarate: Results of a Randomized, Double-Blind, Crossover Trial. Cancer Epidemiol Biomarkers Prev 2016;25(1),180–187. doi: 10.1158/1055-9965.EPI-15-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 2009;101(4),256–266. doi: 10.1093/jnci/djn485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Preventive Services Task Force [Internet]. Draft Recommendation Statement. Aspirin Use to Prevent Cardiovascular Disease: Preventive Medication. [cited 2021 Oct 26. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication.
- 27.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med 2018;379(16),1509–1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med 2018;379(16),1519–1528. doi: 10.1056/NEJMoa1803955 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


