Abstract
This document summarizes the work of the CPAP and bilevel PAP therapy for OSA Technical Expert Panel working group. For positive airway pressure (PAP) therapy, the most pressing current coverage barriers identified were: an insufficient symptom list describing all potential symptoms in patients with mild OSA; the 4 h per night of PAP usage requirement to keep the device; the additional sleep studies requirement to re-qualify for PAP or supplemental oxygen; and the inability to use telehealth visits for follow-up visits. Critical evidence supports changes to current policies and includes: symptom list inadequate to cover all scenarios based on updated clinical practice guidelines; published evidence that 2 h per night of PAP use can result in benefit to quality of life and other metrics; the costs of another sleep study not justified for all nonadherent patients or for supplemental oxygen due to other types of assessment currently available; and the remarkable success and acceptance of telehealth visits. To achieve optimal access for patients on PAP therapy, we make the following key suggestions: removing symptom criteria for mild OSA; reduce continued coverage criteria to > 2 h per night; eliminate the need for a sleep study to re-qualify if nonadherent or for new Centers for Medicare & Medicaid Services beneficiaries already on and adherent to PAP therapy; allow telehealth visits for documenting benefit and adherence; and allow PAP reports and domiciliary oximetry to qualify for supplemental oxygen with PAP if needed. This paper shares our best vision for bringing the right device to the right patient at the right time.
Key Words: access, Medicare, NIV, noninvasive ventilation, optimal, OSA, positive airway pressure, sleep apnea
Abbreviations: AASM, American Academy of Sleep Medicine; AHI, apnea-hypopnea index; BPAP, bilevel positive airway pressure; CMS, Centers for Medicare & Medicaid Services; DME, durable medical equipment; ESS, Epworth Sleepiness Scale; FOSQ, Functional Outcomes of Sleep Questionnaire; PAP, positive airway pressure; RDI, respiratory disturbance index; TEP, Technical Expert Panel
Note to the Reader: The current document is one of a series produced by a Technical Expert Panel (TEP), whose purpose was to propose changes to Centers for Medicare & Medicaid Services national coverage determinations for the use of noninvasive ventilation and home mechanical ventilation, which were formulated in 1998. Specifically, the TEP proposed changes to national coverage determinations for thoracic restrictive disorders (neuromuscular disease), COPD, hypoventilation syndromes, central sleep apnea, and OSA. The background, makeup of the TEP, and key recommendations are highlighted in an Executive Summary. CHEST, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society formed the “Optimal NIV Medicare Access Promotion (ONMAP)” to provide processes to obtain the “right device for the right patient at the right time.” More details and rationale for the proposed changes are available in the companion documents.
OSA is a highly prevalent disorder characterized by repetitive upper airway obstruction during sleep with intermittent hypoxemia and arousals.1 It is associated with adverse effects, including neurobehavioral impairments (eg, excessive sleepiness, impaired quality of life, fatigue, mood changes), cardiovascular events, metabolic dysregulation, and higher mortality. Positive airway pressure (PAP) therapy, including CPAP and bilevel PAP (BPAP), remains the most common treatment, regardless of OSA severity. Greater adherence to PAP therapy is associated with improved outcomes2 and is critical to satisfying current coverage determination policies.
Background
Current Coverage Guidelines
The Local Coverage Determination (LCD 33718) entitled “PAP Devices for the Treatment of Obstructive Sleep Apnea” was effective October 1, 2015, with its last revision on January 1, 2020.3 Our Technical Expert Panel (TEP) discussions with key stakeholders revealed aspects of the Local Coverage Determination that require updating or remain vague in light of newer research and guideline updates. Both providers and durable medical equipment (DME) companies have variable interpretations of the current coverage determination policies, which can lead to wasteful expenditure of CMS resources or needless denials. Furthermore, in light of the ongoing COVID-19 pandemic, the unique capability of PAP devices to transmit adherence and effectiveness data has led to the recognition that telehealth can complement or potentially replace in-person visits, while providing high-value health care. Herein, the TEP describes specific patient-provider situations that confound the ability of clinicians to deliver optimal noninvasive ventilation Medicare access with recommendations for improvement of current coverage determination policies.
Specific Issues
Initial Coverage
Current coverage determination allows for PAP therapy to Medicare beneficiaries with mild OSA only if certain symptoms and comorbidities are present. Symptoms specifically listed include excessive daytime sleepiness and others such that auditors will strictly adhere only to these symptoms. However, many patients with mild OSA experience many other OSA-related symptoms not included in current policies that adversely impair their quality of life and can be excluded from coverage.2,3 As it is implicit that the patient presents to their caregiver because they are symptomatic, this “symptom” criteria might reasonably be excluded altogether.
BPAP for Patients Intolerant of CPAP
Existing policy allows BPAP if CPAP has been “tried and proven ineffective based on a therapeutic trial conducted in either a facility or in a home setting.”3 Policy-specific documentation later defines “tried and proven ineffective” to include documentation of issues related to “interface fit and comfort” and “pressure settings” that failed to adequately control the symptoms of OSA, improve sleep quality, or reduce the apnea-hypopnea index (AHI) or respiratory disturbance index (RDI) to acceptable levels. However, this approach may dictate unnecessary clinical efforts and has largely been ignored by commercial payers and DME providers because mask interface issues are unrelated to determination of BPAP as alternative treatment. In general, based on the TEP discussion, clinicians consider CPAP as “tried and proven ineffective” based on patient self-report or by technologist observation in the sleep laboratory of issues with pressure intolerance, disturbed sleep, failure to correct the AHI/RDI, sleep oximetry, or persistent hypercapnia while using CPAP.
Continued Coverage or Requalification of PAP Therapy Beyond the First 3 Months
The requirements for continued PAP coverage devices beyond 90 days in certain circumstances has created significant problems for Medicare beneficiaries and contributed to wasteful expenditures for additional sleep testing. Currently, the Centers for Medicare & Medicaid Services (CMS) requires beneficiaries with OSA to show adherence to PAP therapy, defined by ≥ 4 h per day, ≥ 70% of days in a consecutive 30-day period within the first 3 months, and an in-person visit with their treating provider showing that OSA symptoms are improved for continued coverage. The 4 h per day adherence threshold, which was based on clinical consensus in the early days of PAP adherence research, is too high for some patients to reach. In particular, those of lower socioeconomic status may be unfairly disadvantaged by this requirement.4 Evidence reveals that as few as 2 h of therapy per day benefits Medicare-aged patients,5 suggesting that adherence should be assessed in conjunction with clinical outcomes.6 Many patients struggle early on with therapy and require multiple mask adjustments, PAP-related desensitization, or other educational, troubleshooting, or behavioral interventions that may take > 90 days to optimize adherence. Furthermore, some patients are unreasonably “penalized” with the need for additional appointments with their provider even if they are highly adherent but inadvertently mis-scheduled for a follow-up visit prior to or following the official 31- to 90-day visit requirement.
Current coverage policies mandate that all beneficiaries with OSA who fail to satisfactorily meet the 90-day adherence guideline must undergo an in-person re-evaluation and a type 1 sleep study to secure another 90-day PAP trial. Mandating requalification of treatment-adherent beneficiaries who, due to extenuating circumstances (eg, travel or hospitalization), could not return within the 31- to 90-day window required for the in-person visit is burdensome, expensive, and of dubious benefit. Adherence and efficacy data captured by PAP devices combined with provider visits (in-person or telehealth) may allow for the requisite troubleshooting in patients beyond the 90-day period without requiring an attended, laboratory-based, or home-based sleep study if such patients continue to be engaged with their provider and express interest in continuing to optimize therapy. Those patients still engaged with the clinician managing their noninvasive ventilation therapy and who are attempting, although not yet meeting adherence criteria should be allowed another 90-day PAP trial prior to consideration of alternative therapy.
Concurrent Use of Oxygen With PAP Therapy
Although PAP therapy is adequate for most patients with OSA, some will experience persistent nocturnal hypoxemia due to underlying cardiopulmonary conditions. Current policies on concurrent oxygen use with PAP therapy have resulted in disparate practices in qualifying patients for oxygen and have represented regulatory barriers to optimal patient care. One of the interpretive challenges has been whether a patient can qualify for supplemental oxygen and PAP therapy during an initial split-night sleep study, in which diagnoses of OSA and sleep-related hypoxemia despite PAP therapy are established. Another ambiguity is whether the patient must subsequently show PAP adherence as an outpatient to be considered in a “chronic stable state” and then undergo retesting to observe if sleep-related hypoxemia persists despite PAP therapy. An affirmative interpretation leaves some patients vulnerable to adverse health outcomes during the PAP-only period. Another challenge has been that some interpret the policy to require that the AHI must be reduced to < 10 events/h (or below the baseline AHI if it was 5.0-9.9 events/h) on CPAP for a minimum of 2 h during polysomnography vs just a portion of the 2-h minimum PAP titration duration. Furthermore, the location and timing of “nocturnal oximetry” documenting hypoxemia on optimal PAP settings are ambiguous.
Beneficiaries Entering Medicare
Many Medicare beneficiaries will be on PAP therapy prior to Medicare enrollment. Current coverage determination policies require that patients who have already received PAP therapy prior to enrollment must provide a diagnostic sleep study showing the presence of OSA and have an in-person evaluation with their treating provider that documents the diagnosis of OSA and continued PAP use by the beneficiary. However, this policy may present a regulatory barrier to appropriate patient care if long-term adherent patients or their treating providers cannot produce a qualifying sleep study from the distant past for a variety of reasons. New beneficiaries who have long been established on PAP therapy are then required to undergo repeat diagnostic sleep testing, which results in an unnecessary expense for CMS and undesirable re-testing burdens (eg, sleeping without their PAP device for the night or having to stop PAP therapy for 2 nights prior to the sleep study to ensure that OSA severity returns to baseline).
Telehealth
Early in the COVID-19 pandemic, seniors were recognized as representing a vulnerable group for COVID-19 infection, and these individuals disproportionately experience the most severe of the COVID-19 outcomes.7 As a result, most providers have pivoted to providing care via telehealth visits, which was supported under the CMS medical emergency waiver and has been well received by patients and health care providers alike. The experiences of many providers have shown that both new and follow-up telehealth visits can be successfully used but require time and resources similar to those of in-person visits. Given recent experiences, current policies should be expanded. The American Medical Association (AMA) has strongly supported inclusion of virtual visits by proposing resolution 203 on November 17, 2020, stating: “…AMA advocates for equitable access to telehealth services, especially for at-risk and under-resourced patient populations and communities, …and support the use of telehealth to reduce health disparities and promote access to health care.”8
Current Evidence/Clinical Consensus/Practice Guidelines
BPAP for Patients Intolerant of CPAP
Clinical guidelines2 continue to recommend CPAP or autoadjustable PAP over BPAP when initiating treatment for OSA in adults. Nevertheless, current guidelines recognize situations in which patients with OSA may benefit from BPAP therapy. For example, one study examined the potential role of BPAP as rescue therapy following at least 2 weeks of suboptimal adherence while using CPAP.9 Those randomized to use BPAP vs continuing CPAP at 2 months reported greater nightly adherence to PAP, with 49% of the BPAP group compared with 28% of the CPAP group subsequently meeting the CMS definition of adherence. A retrospective study of US veterans, with many of similar age to Medicare beneficiaries, suggested that those initially prescribed BPAP were more likely to be adherent at 30 months.10 In another study examining BPAP as rescue therapy, patients nonadherent to CPAP at 90 days who were transitioned to auto-BPAP reported improvements in adherence, sleepiness, and sleep-related quality of life at 10 weeks compared with the period of time on CPAP.11 The available evidence supporting the use of BPAP in OSA indicates that BPAP is an appropriate treatment option for patients with OSA when CPAP has been tried and proven intolerable or ineffective.
Defining Symptomatic Patients With OSA
The current coverage policies allow for CMS beneficiaries with mild OSA to obtain PAP, whether CPAP or BPAP, in the setting of a number of common symptoms associated with OSA; however, the list does not fully encompass the range of symptoms associated with OSA that impair sleep-related quality of life and can be improved with treatment. For example, the 2019 American Academy of Sleep Medicine (AASM) guidelines2 made a conditional recommendation that CPAP should be used to treat OSA in adults with many symptoms that impair sleep-related quality of life. The AASM recommendation was supported by a meta-analysis of common sleep-related quality of life questionnaires, including the Function Outcomes of Sleep Questionnaire (FOSQ) and the Sleep Apnea Quality of Life Index. One of these studies specifically examined improvements in sleep-related quality of life by using the Sleep Apnea Quality of Life Index in adults aged ≥ 65 years.5 A subsequent randomized controlled trial from the United Kingdom found moderate to large improvements in vitality and fatigue following 3 months of CPAP for mild OSA, with 81% of participants wishing to continue with CPAP.12 Thus, the available studies indicate that the current symptom list which qualifies Medicare beneficiaries for PAP in the setting of mild OSA is too limited.
Definitions for Continued Coverage
A critical element of continued coverage for PAP for CMS beneficiaries with OSA is meeting the CMS definition of adherence: “use of PAP ≥ 4 hours per night on 70% of nights during a consecutive thirty (30) day period anytime during the first three (3) months of initial usage.” The basis of the original adherence definition was from a paper examining CPAP use via an objective monitor and was based on expert clinical opinion.13 However, subsequent studies, including randomized controlled studies,14, 15, 16, 17, 18, 19 indicate that improvement in OSA-related symptoms and outcomes can occur below the CMS threshold and have also shown a dose-response relationship. For example, in the PREDICT (Continuous Positive Airway Pressure in Older People With Obstructive Sleep Apnoea Syndrome) trial of older adults (age ≥ 65 years), a clinically significant improvement in the Epworth Sleepiness Scale (ESS) score was observed at 3 months, despite a median CPAP adherence of 1 h and 52 min per night (interquartile range: 19 min to 5 h 12 min).14 In another study of 150 adults with severe OSA,6 approximately 20% to 50% of participants using PAP between 2 and 4 h per night reported normalization of the ESS, FOSQ, or multiple sleep latency test, with greater use associated with a greater proportion of the study sample with normalized values. A similar dose-response relationship with measures of the ESS and FOSQ was seen in a subsequent study of adults with moderate to severe OSA treated with CPAP.20
Furthermore, the CMS requirement to meet the adherence threshold within the first 90 days may disadvantage some Medicare beneficiaries. For instance, some patients may take > 90 days to reach conventional adherence definitions, as highlighted in one study which showed that 30.4% became adherent for the remainder of the first year after only becoming adherent beyond the initial 90-day period.21 Other studies suggest that the adherence requirement may, in particular, disadvantage Medicare beneficiaries of lower socioeconomic status5 and older adults who are eligible for Medicaid. Thus, the current definitions of adherence necessary for continued coverage run counter to the aim of the CMS to eliminate disparities in health care quality and access, and should be revised.
Requalification for PAP
Beneficiaries who fail criteria to qualify for PAP therapy in the initial 90-day period have a pathway to requalification. There was consensus within the TEP panel that patients “fail” PAP therapy for a wide variety of reasons. Factors for nonadherence or nonresolution of OSA-related symptoms can, in most instances, be determined through careful evaluation by the treating provider to address issues such as underlying sinus or nasal condition, changing masks, adjusting pressure settings, switching PAP modes, or even considering alternative diagnoses. In most instances, a facility-based type 1 sleep study should be optional but not mandated for requalification of PAP therapy coverage. Furthermore, if further sleep testing is deemed necessary, the type of test (facility or home based) should be at the discretion of the treating provider.
Optimal NIV Medicare Access Proposal (ONMAP): Revisions to the Coverage Decision Policies
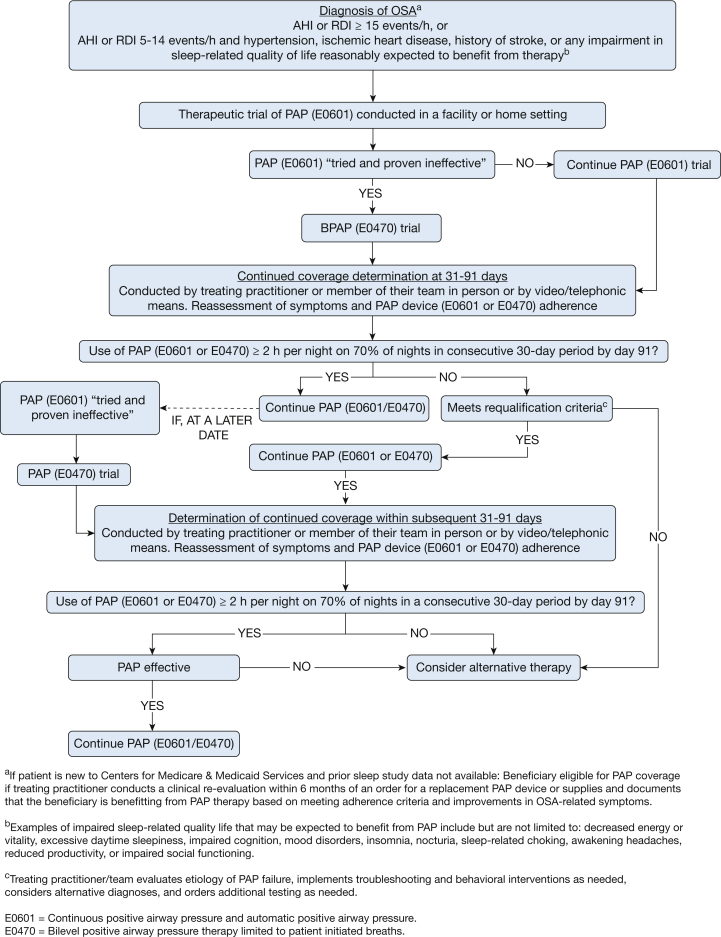
Based on the data reviewed in the preceding sections, the TEP proposes the following changes to policies. Figure 1 presents a suggested pathway.
Figure 1.
Proposed treatment with CPAP and BPAP for patients with OSA. AHI = apnea-hypopnea index; BPAP = bilevel positive airway pressure; PAP = positive airway pressure; RDI = respiratory disturbance index.
Policy for Initial Coverage
The coverage policy should continue to provide initial coverage for patients diagnosed with moderate to severe OSA. However, initial coverage of a PAP device (CPAP or E0601 or E0470 BPAP without a backup rate) for mild OSA should be revised to allow coverage for:
-
1.
Any impairment in sleep-related quality of life that in the judgment of the treating physician may be expected to benefit from therapy.
-
2.
Comorbid cardiovascular conditions (eg, hypertension, diabetes, ischemic heart disease, history of stroke).
Bilevel PAP for Patients Intolerant of CPAP
The current criteria should continue as they provide appropriate coverage to beneficiaries for an E0470 (BPAP without a backup rate capability), when “an E0601 [CPAP] device has been tried and proven ineffective based on a therapeutic trial conducted in either a facility or in a home setting.”3 The details of the intolerant and/or ineffective CPAP response should be documented in the patient record, but the specifics should be determined by the treating practitioner.
Policy for Continued Coverage
The coverage determination policy regarding continued coverage for either a PAP device (CPAP or BPAP without a backup rate E0601 or E0470) as outlined in Figure 1 should be revised as follows:
-
•
The treating practitioner (or designee) conducts a clinical re-evaluation and documents that the beneficiary is using and benefiting from PAP therapy.
-
•
The clinical re-evaluation can be performed either by the treating practitioner or members of their health care team within the scope of their clinical practice.
-
•
Objective adherence to the use of the PAP device based on utilization data should be reviewed and documented by the treating practitioner.
-
•
Adherence to therapy should be considered as acceptable with use of PAP ≥ 2 h per night on 70% of nights during a consecutive 30-day period anytime during the first 3 months of initial usage. If use and/or benefits have not been reached by day 91, the beneficiary must requalify.
Requalification for PAP
The revised coverage determination policy should include the following to requalify for PAP therapy:
-
•
The treating practitioner will re-evaluate and document the reason the beneficiary has suboptimal PAP therapy adherence and reason for reconsidering the treatment of OSA with PAP.
-
•
The need for additional sleep testing should be at the discretion of the treating practitioner, rather than mandatory, and may be either home- or facility-based.
-
•
If use and/or benefits have not been reached during a subsequent 90-day period, alternative treatments should be considered.
Beneficiaries Entering Medicare
-
•
Beneficiaries newly enrolling in Medicare with OSA currently using PAP therapy should not be denied coverage on the basis of absence of documentation of a prior diagnostic sleep study demonstrating OSA or the in-person assessment for OSA by the provider required within 6 months of the initial diagnostic sleep study. Coverage should be continued if the treating practitioner (or designee) has conducted a clinical re-evaluation within 6 months of an order for a replacement PAP device or supplies and documented that the beneficiary was benefiting from PAP therapy based on meeting the previously defined adherence criteria and improvement in OSA-related symptoms.
Concurrent Oxygen With PAP
Although the TEP recognizes that the concurrent use of oxygen with PAP is outside of the coverage policy for BPAP, it is important to comment on potential revisions needed for optimal care given the wide regional variation in implementation of current policies by the DME Medicare administrative contractors and DME providers. The TEP suggests the following revisions to coverage determination policies:
-
•For beneficiaries to be considered in a chronic, stable state, adequate treatment of OSA should be defined as an AHI ≤ 10 events/h or a 75% reduction from the baseline AHI either:
-
○During a PAP titration polysomnography with at least 2 h of the titration portion OR
-
○Based on a concurrent domiciliary oximetry AND PAP report
-
○
-
•Coverage criteria for supplemental oxygen is achieved if during the PAP titration polysomnography OR concurrent domiciliary oximetry and PAP reports:
-
○The oxygen saturation remains ≤ 88% for a total of 5 min (noncontinuous) AND
-
○Adequate PAP treatment (as discussed earlier) is achieved
-
○
Telehealth Provision
To enhance access to the highest quality care and build on the experiences gained from the COVID-19 pandemic, the TEP suggests that all required face-to-face re-evaluations for CMS beneficiaries with OSA following initiation of PAP therapy may also be accomplished by video/telephonic means.
Recommendations for Other Areas CMS Should Consider Addressing
The TEP also identified additional areas of the coverage determination policy that have overlap with other policies that should be considered for updates and revisions. A brief summary and suggestions follow:
-
•
Hypopnea definition: The policy for PAP therapy coverage for OSA defines hypopneas as a ≥ 10-s event with a minimum 30% decrease in thoracoabdominal effort or airflow and a ≥ 4% decrease in oxygen saturation. The CMS definition stands in contrast to the definition used by most clinicians, which defines hypopneas based on either a 3% oxygen desaturation or an arousal from sleep.22 Research suggests similar associations of this consensus definition as the CMS hypopnea definition with OSA-related outcome, including cardiovascular and cerebrovascular disease, quality of life, daytime sleepiness, occupational and motor vehicle accidents, metabolic disease, and mortality.23 The TEP strongly suggests that CMS re-evaluate the hypopnea definition to identify those who will benefit from treatment.
-
•
Definitions of RDI and AHI: Current CMS use of the terms AHI and RDI do not conform to definitions used by clinicians. The RDI is used by the CMS to refer to sleep apnea severity based on home-based testing, whereas standards set by national organizations use this term to include apneas, hypopneas, and respiratory-effort related arousals.22 Currently, the term respiratory event index has been recommended to reflect sleep apnea severity determined based on monitoring time because some home-based tests, which assess sleep time, can determine an AHI or RDI.
-
•
Orphan diagnoses in which PAP is beneficial: The TEP also identified patients with disorders other than sleep apnea who may benefit from PAP therapy due to its ability to act as an airway stent. These include disorders such as tracheobronchomalacia with excessive dynamic airway collapse, multisystem atrophy in which patients may develop nocturnal stridor, and other similar disorders. CMS beneficiaries with these disorders are denied PAP therapy unless they meet coverage determination policies for OSA, although many clearly benefit from PAP therapy and need an appropriate pathway to obtain symptomatic relief.
Summary of New Recommendations
-
•
Documentation by the clinician that CPAP was “tried and proven ineffective” and the patient-specific issues with CPAP treatment should be considered sufficient for BPAP.
-
•
For patients with mild OSA, any impairment in sleep-related quality of life that in the judgment of the treating physician may be expected to benefit from therapy should be afforded initial coverage.
-
•
Patients with OSA can have improvements in quality of life symptoms with as little as 2 h of PAP use during sleep daily. In addition, many patients have initial challenges meeting a 4-h per night goal; therefore, adherence criteria should be redefined as use of PAP ≥ 2 h per night on 70% of nights during a consecutive 30-day period anytime during the first 3 months of initial usage. Patients who do not meet these criteria within the first 3 months may only requalify if they meet requalification criteria.
-
•
Patients who have not met continuing coverage criteria should be allowed to requalify under certain circumstances. Specifically, these patients may qualify after a reevaluation with the treating practitioner that documents the etiology of the failure to respond to PAP therapy and consideration of alternative treatments for OSA. The treating practitioner should document the reasons for an additional trial of PAP, and the need for additional sleep testing should be at the practitioner’s discretion.
-
•
Telehealth should be continued as an alternative for patient assessment and monitoring response to therapy.
-
•
Supplemental oxygen with PAP criteria should be simplified to showing adequate treatment of OSA (AHI < 10 events/h or a 75% reduction from the baseline AHI either during a PAP titration polysomnography, in which the entirety of the titration portion is at least 2 h, OR based on a concurrent domiciliary oximetry AND a PAP report. Coverage would be provided if during the PAP titration polysomnography OR concurrent domiciliary oximetry and PAP reports the oxygen saturation remains ≤ 88% for a total of 5 min (need not be continuous) and adequate PAP treatment is achieved.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
∗ONMAP Technical Expert Panel members: Overall Chairs: Peter C. Gay, MD, FCCP, and Robert L. Owens, MD. Thoracic Restrictive Disorders: Chair/Co-Chair—Lisa F. Wolfe, MD, FCCP, and Joshua O. Benditt, MD, FCCP. Thoracic Restrictive Disorders Committee Members—Loutfi S. Aboussouan, MD, FCCP, John M. Coleman III, MD, and Dean R. Hess, PhD, RRT. COPD: Chair/Co-chair—Nicholas S. Hill, MD, FCCP, and Gerard J. Criner, MD, FCCP. COPD Committee Members—Richard D. Branson, MSc, RRT, Bartolome R. Celli, MD, FCCP, Neil R. MacIntyre, MD, FCCP, and Amen Sergew, MD. Central Sleep Apnea: Chair/Co-Chair—Timothy I. Morgenthaler, MD, and Atul Malhotra, MD, FCCP. Central Sleep Apnea Committee Members—Richard B. Berry, MD, Karin G. Johnson, MD, and Marc I. Raphaelson, MD. Hypoventilation: Chair/Co-chair—Babak Mokhlesi, MD, FCCP, and Christine H. Won, MD. Hypoventilation Committee Members—Bernardo J. Selim, MD, FCCP, Barry J. Make, MD, FCCP, and Bernie Y. Sunwoo, MBBS. OSA: Chair/Co-chair—Nancy A. Collop, MD, Master FCCP, and Susheel P. Patil, MD, PhD. ATSF. OSA Committee Members—Alejandro D. Chediak, MD, FCCP, Eric J. Olson, MD, and Kunwar Praveen Vohra, MD, FCCP.
Footnotes
Dr Patil is currently at Case Western Reserve University (Cleveland, OH).
Contributor Information
Susheel P. Patil, Email: susheel.patil@uhhospitals.org.
ONMAP Technical Expert Panel:
Peter C. Gay, Robert L. Owens, Lisa F. Wolfe, Joshua O. Benditt, Loutfi S. Aboussouan, John M. Coleman, III, Dean R. Hess, Nicholas S. Hill, Gerard J. Criner, Richard D. Branson, Bartolome R. Celli, Neil R. MacIntyre, Amen Sergew, Timothy I. Morgenthaler, Atul Malhotra, Richard B. Berry, Karin G. Johnson, Marc I. Raphaelson, Babak Mokhlesi, Christine H. Won, Bernardo J. Selim, Barry J. Make, Bernie Y. Sunwoo, Nancy A. Collop, Susheel P. Patil, Alejandro D. Chediak, Eric J. Olson, and Kunwar Praveen Vohra
References
- 1.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patil S.P., Ayappa I.A., Caples S.M., Kimoff R.J., Patel S.R., Harrod C.G. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15(2):301–334. doi: 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Medicare & Medicaid Services Local coverage determination: positive pressure airway devices for the treatment of obstructive sleep apnea. https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=33718&ver=20&TAId=99&NCDId=330&ncdver=1&SearchType=Advanced&CoverageSelection=Both&NCSelection=NCA|CAL|NCD|MEDCAC|TA|MCD&ArticleType=Ed|Key|SAD|FAQ&PolicyType=Final&s=5|6|66|67|44&KeyWord=obstructive+sleep+apnea&KeyWordLookUp=Doc&KeyWordSearchType=Exact&kq=true&bc=IAAAADgAAAAA&
- 4.Platt A.B., Field S.H., Asch D.A., et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32(6):799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMillan A., Bratton D.J., Faria R., et al. PREDICT Investigators. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2(10):804–812. doi: 10.1016/S2213-2600(14)70172-9. [DOI] [PubMed] [Google Scholar]
- 6.Weaver T.E., Maislin G., Dinges D.F., et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahid Z., Kalayanamitra R., McClafferty B., et al. Covid-19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Medical Association COVID-19 emergency and expanded telemedicine regulations. https://www.ama-assn.org/system/files/2020-10/nov20-203.pdf Resolution 203. Introduced November 2020.
- 9.Ballard R.D., Gay P.C., Strollo P.J. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007;3(7):706–712. [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz S.W., Rosas J., Iannacone M.R., Foulis P.R., Anderson W.M. Correlates of a prescription for bilevel positive airway pressure for treatment of obstructive sleep apnea among veterans. J Clin Sleep Med. 2013;9(4):327–335. doi: 10.5664/jcsm.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentina T., Fortin F., Douay B., et al. Auto bi-level with pressure relief during exhalation as a rescue therapy for optimally treated obstructive sleep apnoea patients with poor compliance to continuous positive airways pressure therapy—a pilot study. Sleep Breath. 2011;15(1):21–27. doi: 10.1007/s11325-009-0322-y. [DOI] [PubMed] [Google Scholar]
- 12.Wimms A.J., Kelly J.L., Turnbull C.D., et al. MERGE Trial Investigators Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(4):349–358. doi: 10.1016/S2213-2600(19)30402-3. [DOI] [PubMed] [Google Scholar]
- 13.Kribbs N.B., Pack A.I., Kline L.R., et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 14.McMillan A., Morrell M.J. Sleep disordered breathing at the extremes of age: the elderly. Breathe (Sheff) 2016;12(1):50–60. doi: 10.1183/20734735.003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engleman H.M., Martin S.E., Deary I.J., Douglas N.J. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572–575. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 16.Engleman H.M., Martin S.E., Deary I.J., Douglas N.J. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. 1997;52(2):114–119. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engleman H.M., Kingshott R.N., Wraith P.K., Mackay T.W., Deary I.J., Douglas N.J. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 18.Redline S., Adams N., Strauss M.E., Roebuck T., Winters M., Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3 pt 1):858–865. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 19.McEvoy R.D., Antic N.A., Heeley E., et al. SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 20.Antic N.A., Catcheside P., Buchan C., et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naik S., Al-Halawani M., Kreinin I., Kryger M. Centers for Medicare and Medicaid Services positive airway pressure adherence criteria may limit treatment to many Medicare beneficiaries. J Clin Sleep Med. 2019;15(2):245–251. doi: 10.5664/jcsm.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry R.B., Quan S.F., Abreu A.S., et al. for the American Academy of Sleep Medicine . American Academy of Sleep Medicine; Darien, IL: 2020. The AASM Manual for the Scoring of Sleep and Associated Event: Rules, Terminology and Technical Specifications. [Google Scholar]
- 23.Berry R.B., Budhiraja R., Gottlieb D.J., et al. American Academy of Sleep Medicine Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]