Abstract
Background
Post‐traumatic stress disorder (PTSD) is a severe and debilitating condition. Several pharmacological interventions have been proposed with the aim to prevent or mitigate it. These interventions should balance efficacy and tolerability, given that not all individuals exposed to a traumatic event will develop PTSD. There are different possible approaches to preventing PTSD; universal prevention is aimed at individuals at risk of developing PTSD on the basis of having been exposed to a traumatic event, irrespective of whether they are showing signs of psychological difficulties.
Objectives
To assess the efficacy and acceptability of pharmacological interventions for universal prevention of PTSD in adults exposed to a traumatic event.
Search methods
We searched the Cochrane Common Mental Disorders Controlled Trial Register (CCMDCTR), CENTRAL, MEDLINE, Embase, two other databases and two trials registers (November 2020). We checked the reference lists of all included studies and relevant systematic reviews. The search was last updated on 13 November 2020.
Selection criteria
We included randomised clinical trials on adults exposed to any kind of traumatic event. We considered comparisons of any medication with placebo or with another medication. We excluded trials that investigated medications as an augmentation to psychotherapy.
Data collection and analysis
We used standard Cochrane methodological procedures. In a random‐effects model, we analysed dichotomous data as risk ratios (RR) and number needed to treat for an additional beneficial/harmful outcome (NNTB/NNTH). We analysed continuous data as mean differences (MD) or standardised mean differences (SMD).
Main results
We included 13 studies which considered eight interventions (hydrocortisone, propranolol, dexamethasone, omega‐3 fatty acids, gabapentin, paroxetine, PulmoCare enteral formula, Oxepa enteral formula and 5‐hydroxytryptophan) and involved 2023 participants, with a single trial contributing 1244 participants. Eight studies enrolled participants from emergency departments or trauma centres or similar settings. Participants were exposed to a range of both intentional and unintentional traumatic events. Five studies considered participants in the context of intensive care units with traumatic events consisting of severe physical illness. Our concerns about risk of bias in the included studies were mostly due to high attrition and possible selective reporting. We could meta‐analyse data for two comparisons: hydrocortisone versus placebo, but limited to secondary outcomes; and propranolol versus placebo. No study compared hydrocortisone to placebo at the primary endpoint of three months after the traumatic event.
The evidence on whether propranolol was more effective in reducing the severity of PTSD symptoms compared to placebo at three months after the traumatic event is inconclusive, because of serious risk of bias amongst the included studies, serious inconsistency amongst the studies' results, and very serious imprecision of the estimate of effect (SMD ‐0.51, 95% confidence interval (CI) ‐1.61 to 0.59; I2 = 83%; 3 studies, 86 participants; very low‐certainty evidence). No study provided data on dropout rates due to side effects at three months post‐traumatic event. The evidence on whether propranolol was more effective than placebo in reducing the probability of experiencing PTSD at three months after the traumatic event is inconclusive, because of serious risk of bias amongst the included studies, and very serious imprecision of the estimate of effect (RR 0.77, 95% CI 0.31 to 1.92; 3 studies, 88 participants; very low‐certainty evidence). No study assessed functional disability or quality of life.
Only one study compared gabapentin to placebo at the primary endpoint of three months after the traumatic event, with inconclusive evidence in terms of both PTSD severity and probability of experiencing PTSD, because of imprecision of the effect estimate, serious risk of bias and serious imprecision (very low‐certainty evidence). We found no data on dropout rates due to side effects, functional disability or quality of life.
For the remaining comparisons, the available data are inconclusive or missing in terms of PTSD severity reduction and dropout rates due to adverse events. No study assessed functional disability.
Authors' conclusions
This review provides uncertain evidence only regarding the use of hydrocortisone, propranolol, dexamethasone, omega‐3 fatty acids, gabapentin, paroxetine, PulmoCare formula, Oxepa formula, or 5‐hydroxytryptophan as universal PTSD prevention strategies. Future research might benefit from larger samples, better reporting of side effects and inclusion of quality of life and functioning measures.
Keywords: Adult; Humans; Hydrocortisone; Hydrocortisone/therapeutic use; Paroxetine; Psychotherapy; Psychotherapy/methods; Quality of Life; Stress Disorders, Post-Traumatic; Stress Disorders, Post-Traumatic/psychology
Plain language summary
Medicines for preventing post‐traumatic stress disorder (PTSD)
Why is this review important?
Post‐traumatic stress disorder (PTSD) is a severe and disabling condition which may develop in people exposed to traumatic events. Such events can have long‐lasting negative repercussions on the lives of those who have experienced them, as well as on the lives of loved ones.
Research has shown that there are some alterations in how the brain works in people with PTSD. Some researchers have thus proposed using medicines to target these alterations soon after a traumatic event, as a way to prevent the development of PTSD. However, the majority of people who experience a traumatic event will not develop PTSD. Therefore, medicines that can be given soon after exposure to a traumatic event must be carefully evaluated for their effectiveness, including balancing the risk of side effects against the risk of developing PTSD.
Who will be interested?
‐ People exposed to traumatic events and their family, friends, and loved ones
‐ Professionals working in mental health
‐ Professionals working in traumatology and emergency medicine
‐ People caring for victims of traumatic experiences and veterans of the armed forces
What questions did this review try to answer?
For people exposed to a traumatic event, whether or not they have psychological symptoms, are some medicines more effective than other medicines or placebo (dummy pills) in:
‐ reducing the severity of symptoms of PTSD?
‐ reducing the number of people stopping the medication because of side effects?
‐ reducing the probability of developing PTSD?
Which studies were included?
We searched scientific databases for studies in which participants were randomly assigned to a medicine with the aim of preventing PTSD and its symptoms or reducing severity. We included studies published up until November 2020. We selected studies in adults who had experienced any kind of traumatic event, and which provided treatment, regardless of whether or not the participants had psychological symptoms.
We included 13 studies, with a total of 2023 participants. One study alone contributed 1244 participants. The studies took place in different settings and involved people exposed to a wide range of traumatic events. Some studies took place in emergency departments and considered people whose trauma resulted from intentional harm or unintentional harm. Other studies focused on life‐threatening illness as the source of trauma, including major surgeries or being admitted to intensive care units. The medicines most commonly given to participants in the studies included: hydrocortisone (which reduces the body's immune response), propranolol (used to treat heart problems and anxiety, amongst other conditions), and gabapentin (a medicine primarily used to treat seizures and nerve pain).
What did the evidence tell us?
We found four trials comparing hydrocortisone to placebo. These trials did not report how participants were doing at three months after a traumatic event, a time point that is usually useful to assess the evolution of PTSD symptoms.
We found very low‐certainty evidence about propranolol compared to placebo three months after a traumatic event. This evidence does not tell us whether or not propranolol is more effective than placebo in reducing the severity of PTSD symptoms and the probability of developing PTSD. We did not find evidence on the probability of people stopping the medication because of side effects, quality of life, or functional disability (a measure of how much one’s life is limited by symptoms).
We found very low‐certainty evidence about gabapentin compared to placebo three months after a traumatic event. This evidence does not tell us whether or not gabapentin is more effective than placebo in reducing the severity of PTSD symptoms and the probability of developing PTSD. We did not find evidence on the probability of people stopping the medication because of side effects, quality of life, or functional disability.
We found studies on additional medicines, for which information about the reduction of PTSD severity and the probability of people stopping the medication was either inconclusive or missing.
None of the included studies measured the functional disability of participants.
What should happen next?
The evidence we found does not support the use of any medicines for the prevention of PTSD in people exposed to a traumatic event, regardless of whether or not they have psychological symptoms. More higher quality studies involving more people are needed to draw conclusions about these treatments.
Summary of findings
Summary of findings 1. Hydrocortisone compared to placebo for preventing post‐traumatic stress disorder (PTSD).
| Hydrocortisone compared to placebo for preventing post‐traumatic stress disorder (PTSD) | |||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: N/A Intervention: hydrocortisone Comparison: placebo | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| PTSD severity at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| Dropout due to adverse events at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| PTSD rate at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| Functional disability at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| Quality of life at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| PTSD: post‐traumatic stress disorder | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
Summary of findings 2. Propranolol compared to placebo for preventing post‐traumatic stress disorder (PTSD).
| Propranolol compared to placebo for preventing post‐traumatic stress disorder (PTSD) | ||||||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: emergency departments and surgical trauma center Intervention: propranolol Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with propranolol | |||||
| PTSD severity at 3 months assessed with: CAPS (Hoge 2012; Pitman 2002), PCL‐C (Stein 2007) | See comment | SMD 0.51 lower (1.61 lower to 0.59 higher) | ‐ | 86 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | A general rule for interpreting SMDs is that 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect |
| Dropout due to adverse events at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ | ‐ | ||
| PTSD rate at 3 months assessed with: CAPS (Hoge 2012), DSM‐IV criteria (Pitman 2002), CIDI (Stein 2007) | Study population | RR 0.77 (0.31 to 1.92) | 88 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,d | ||
| 204 per 1000 | 157 per 1000 (63 to 392) | |||||
| Functional disability at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ | ‐ | ||
| Quality of life at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAPS: Clinician‐Administered PTSD Scale; CI: confidence interval; CIDI: Comprehensive International Diagnostic Interview; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders‐4th Edition; PCL‐C: Posttraumatic Stress Disorder Checklist ‐ Civilian version; PTSD: post‐traumatic stress disorder; RCTs: randomised controlled trials; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias. One study had a high attrition rate (Pitman 2002). Another study had imbalanced attrition rates between the intervention arms (Stein 2007). bDowngraded one level as the 95% confidence interval of one study overlaps minimally with those of the other two cDowngraded two levels for imprecision as the total number of participants is fewer than 400 and the confidence interval includes both appreciable harm and benefit dDowngraded two levels as the optimal information size is not met and the confidence interval includes both appreciable benefit and harm
Summary of findings 3. Gabapentin compared to placebo for preventing post‐traumatic stress disorder (PTSD).
| Gabapentin compared to placebo for preventing post‐traumatic stress disorder (PTSD) | ||||||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: surgical trauma center Intervention: gabapentin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with gabapentin | |||||
| PTSD severity at 3 months assessed with: PCL‐C | One study reports a GEE analysis of PCL‐C scores over the 4 months after the traumatic event of B = ‐0.48, SE= 0.85, ns | ‐ | (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| Dropout due to adverse events at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ | ‐ | ||
| PTSD rate at 3 months assessed with: CIDI | Study population | RR 0.80 (0.18 to 3.59) | 26 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | ||
| 250 per 1000 | 200 per 1000 (45 to 898) | |||||
| Functional disability at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ | ‐ | ||
| Quality of life at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CIDI: Comprehensive International Diagnostic Interview; GEE: generalised estimating equations; ns: non‐significant; PCL‐C: Posttraumatic Stress Disorder Checklist ‐ Civilian version; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; RR: risk ratio; SE: standard error | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias as the included study has imbalanced attrition rates between the intervention arms bDowngraded two levels for imprecision as the total number of participants is fewer than 400 and the confidence interval includes both appreciable harm and benefit cDowngraded two levels as the optimal information size is not met and the confidence interval includes both appreciable benefit and harm
Background
Description of the condition
Post‐traumatic stress disorder (PTSD) is a severe and disabling disorder which may develop in people exposed to traumatic events. Up to 80% of the adult population in the USA have been exposed to a traumatic event eligible for diagnosis of PTSD (Breslau 2012), and estimates are similar for Europe (De Vries 2009). Data from the World Health Organization World Mental Health Survey Initiative show that the 12‐month prevalence of PTSD is 1.1% and the lifetime prevalence is 3.9% (Karam 2014; Koenen 2017). Prevalence estimates rates are higher in displaced populations (Bogic 2015; Turrini 2017), and populations exposed to conflict (Steel 2009).
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5), traumatic events eligible for the diagnosis "include, but are not limited to, exposure to war as a combatant or civilian, threatened or actual physical assault, threatened or actual sexual violence, being kidnapped, being taken hostage, terrorist attack, torture, incarceration as a prisoner of war, natural or human‐made disasters, and severe motor vehicle accidents" (APA 2013). As stated by the DSM, this list is not exhaustive and many different traumatic events have proved capable of triggering PTSD. For instance, in recent years, there has been an increase in reports of PTSD in survivors of critical illness, with an estimated prevalence of 25% amongst this population (Wade 2013). With some limitations regarding the nature of the traumatic incident, witnessing a trauma, learning that a relative or close friend was exposed to trauma, or being exposed to aversive details about a trauma (as in the course of professional duties) may also precipitate PTSD (APA 2013).
The majority of individuals exposed to traumatic experiences do not develop PTSD. The likelihood of developing PTSD is associated with a number of pre‐, peri‐, and post‐traumatic factors (Bisson 2007; Qi 2016), such as: history of a psychiatric disorder; sex (females are more vulnerable than males); low socioeconomic status; belonging to a minority; history of previous trauma; genetic endowment and epigenetic regulation; impaired executive functioning and higher emotional reactivity (Aupperle 2012; Guthrie 2005); the severity of the trauma itself; the perceived threat to life; whether the event or the intent to harm was intentional or unintentional; peri‐traumatic emotions and dissociation (Ozer 2003); and the lack of social support and subsequent life stress (e.g. inability to work as a result of the event) (Brewin 2000).
Individuals who develop PTSD following a trauma may experience a wide range of symptoms, which are presented in four categories in the DSM‐5 (APA 2013).
Re‐experiencing; for example, recurrent unwanted intrusive memories, distressing dreams, flashbacks, distress at re‐exposure.
Avoidance of stimuli associated with the trauma behaviours; for example, the avoidance of distressing memories associated with the traumatic event or avoidance of external reminders.
Negative alteration in cognitions and mood associated with the traumatic event; for example, impairment in recalling important aspects of the trauma, negative thoughts and assumptions about oneself or the world, negative beliefs about the causes or consequences of the traumatic event, diminished interest or participation in significant activities, feeling of detachment from others, inability to experience positive emotions.
Arousal symptoms; for example, hypervigilance, insomnia, irritability, reckless or self‐destructive behaviour, problems concentrating.
The development and maintenance of PTSD is most likely the product of an interaction of different factors. Although current evidence alone cannot explain the complexity underlying PTSD, it is clear that multiple and interconnected systems are involved (Kelmendi 2016; Koch 2014; Lee 2016; Pitman 2012), with the contribution of biological and psychological mechanisms (Besnard 2012; Nickerson 2013).
Description of the intervention
Interventions for preventing the development of PTSD can be divided into two main categories: psychological and pharmacological. Although this review focuses on the latter, several other publications have examined and reviewed the former (Forneris 2013; Kearns 2012; Qi 2016; Roberts 2019; Rose 2002).
With respect to pharmacological interventions, drugs belonging to different classes have been examined by means of randomised clinical trials, and some reviews have already been published, including a previous Cochrane Review (Amos 2014; Astill Wright 2019; Sijbrandij 2015). It should be noted that the mechanisms underlying the onset of the disorder are likely to be different from the ones maintaining it, and therefore some of the interventions proposed to prevent the onset of the disorder differ from the interventions for treatment.
Glucocorticoids are synthetic analogues of hormones involved in immunity and stress response. They can be administered in several ways, including oral, intravenous, and intramuscular administration. Depending on the purpose, a treatment course can last from a single shot to several days. The trials testing steroids for PTSD prevention have used either single dose administration or a course of a few days in individuals with severe physical conditions (Delahanty 2013; Schelling 2001; Weis 2006). Hydrocortisone, along with some other steroids, is also included in the World Health Organization (WHO) Model List of Essential Medicines (WHO 2017), and is therefore expected to be commonly available in several global contexts. Propranolol is a beta blocker, primarily used for long‐term treatment in cardiology. Some trials have tested it on a three‐week time span for PTSD prevention (Hoge 2012; Pitman 2002; Stein 2007). Propranolol is also included in the WHO Model List of Essential Medicines (WHO 2017). A small trial has investigated a short course of temazepam, which belongs to the class of benzodiazepines (common anxiolytic drugs), but found an increase of PTSD onset rather than a decrease (Mellman 2002). Recently, there is growing interest in oxytocin, an endogenous hormone involved in sociability and stress regulation (Qi 2016), and an early trial investigated oxytocin administered in a single intranasal dose (Van Zuiden 2017). Escitalopram is a selective serotonin reuptake inhibitor (SSRI) antidepressant, and although this class has yielded good results in PTSD treatment, there is uncertainty about whether SSRIs are effective in reducing the incidence of PTSD (Shalev 2012; Zohar 2017a). Gabapentin, an anticonvulsant with anxiolytic properties and a benign side effect profile, has been included in trials of PTSD prevention (Stein 2007). Opioids have been proposed too; for example, a large retrospective study on American soldiers with combat injury found an association between morphine administration and lower PTSD incidence (Holbrook 2010).
How the intervention might work
The biological mechanisms underlying PTSD provide several possible targets for the pharmacological prevention of PTSD. Different rationales can potentially explain the efficacy of the investigated drugs.
Glucocorticoids
Glucocorticoids are involved in both hormonal stress response and memory formation. The hypothalamic‐pituitary‐adrenal (HPA) axis has long been a focus in PTSD investigations, and a role for hydrocortisone in facilitating extinction learning has been hypothesised (Hruska 2014). In a rodent model, a negative association has been found between a high dose of steroids and prevalence of PTSD‐like behaviour in rats exposed to predator scent stress (Cohen 2008), and consistent results were found in a human study (Zohar 2011). There is also epidemiologic evidence that lower urinary cortisol levels in the immediate aftermath of the trauma are associated with increased likelihood of future PTSD symptoms (Delahanty 2000; McFarlane 1997).
Beta blockers
A role for adrenaline in the formation of traumatic memories has long been postulated (Pitman 1989; Ressler 2020). It has been argued that a surge in adrenaline concentration, in conjunction with trauma, results in a strong emotional memory and fear conditioning that could prime PTSD. Later human studies supported a role for the beta adrenergic system in memory storing and in the enhanced memories associated with emotional arousal (Cahill 1994; Southwick 1999), and for propranolol to limit this process (Reist 2001).
Benzodiazepines
Benzodiazepines are known for reducing arousal and decreasing distress. They have amnesic properties as well, mostly inhibiting memory consolidation by impairing long‐term episodic storage (Barbee 1993). Despite this, no clinical research has found a positive effect for benzodiazepines in the management of traumatic stress symptoms (Howlett 2016).
Opioids
Studies on rodents have found retrograde amnesia properties for morphine, and a possible mechanism for that has been proposed via decreasing cyclic adenosine monophosphate or activating N‐methyl‐D‐aspartate (NMDA) receptors in the hippocampus (McNally 2003). Human observational studies support a protective effect for morphine (Bryant 2009; Mouthaan 2015).
Oxytocin
A possible role for oxytocin in the prevention of PTSD is quite a recent approach, which has been proposed on a dual assumption theory: a reduction in the amygdala activation and an increase in the activation of the social reward brain areas (Olff 2010). Behavioural data on rodents seem to confirm a plausible role for oxytocin in mitigating the behavioural response to stress (Cohen 2010).
SSRIs
SSRI antidepressants are generally considered the first‐line pharmacological treatment for PTSD (ISTSS 2018; NICE 2018; Stein 2006), and might thereby have a putative role in the prevention of the disorder. SSRIs enhance serotonergic neurotransmission by inhibiting the re‐uptake of serotonin from the synapsis as mediated by the SERT serotonin transporter (Leonard 2000). Further downstream mechanisms are likely responsible for the beneficial effects of SSRIs, as these effects develop only after a few weeks of treatment. An increased expression of the specific downstream genes is currently supposed to induce dendritic spine formation, synaptogenesis, and neurogenesis (Licznerski 2013; Santarelli 2003).
Mood stabilisers/anticonvulsants
As for SSRIs, mood stabilisers/anticonvulsants might have a putative role in PTSD prevention, considering their employment as adjuvant/second‐line treatment for anxiety disorders (Van Ameringen 2004). A trial of gabapentin has been reported in a previous meta‐analysis of PTSD prevention (Stein 2007). Gabapentin administration increases the release of the neurotransmitter GABA from brain glial cells (Lydiard 2003). Imbalances in the GABAergic system have been reported in people with PTSD and other anxiety disorders (Meyerhoff 2014).
Omega‐3 fatty acid compounds
Given their ability to promote neurogenesis in the hippocampus ‐ a key area in memory consolidation and fear maintenance ‐ a role has been proposed for omega‐3 fatty acids in PTSD prevention (Matsuoka 2011).
Why it is important to do this review
PTSD represents a heavy burden for the people affected, those around them, health systems, and society. Findings from the WHO World Mental Health Surveys showed a mean duration of symptoms of about six years. This time length was greatly influenced by the type of traumatic event: from about one year for natural disasters, up to 13 years for war combat‐related traumatic events (Kessler 2017). Moreover, PTSD is associated with poor general health status and unemployment (Zatzick 1997). Most of the evidence focuses on psychosocial intervention, amongst which trauma‐focused and exposure‐based therapies are the most promising ones. However, many of the studies are restricted by small sample sizes and methodological limitations (Birur 2017; Bisson 2021).
Despite knowledge of biological and clinical risk factors for PTSD and the various predictive strategies being researched (e.g. supervised machine learning (Galatzer‐Levy 2014; Karstoft 2015; Kessler 2014)), in clinical practice there is currently no effective way to predict who will develop PTSD after a traumatic experience. The biological features of PTSD provide several possible targets for the prevention of PTSD, and encouraging results were found in previous meta‐analyses of pharmacotherapy for PTSD prevention (Amos 2014; Sijbrandij 2015).
New trials on PTSD pharmacological prevention have now been published. Additionally, the Amos 2014 and Sijbrandij 2015 reviews considered together two different approaches: universal prevention (people exposed to a traumatic event) and indicated prevention (people exposed to a traumatic event and showing early symptoms). Although it would be valuable to have effective interventions for the prevention of PTSD, the risk‐to‐benefit ratio needs to be carefully assessed, as drugs will entail possible side effects for all of those receiving them, and not all of the individuals exposed to a traumatic event will develop PTSD.
Objectives
To assess the efficacy and acceptability of pharmacological interventions for universal prevention of post‐traumatic stress disorder (PTSD) in adults exposed to a traumatic event.
Methods
Criteria for considering studies for this review
Types of studies
We have included randomised controlled trials (RCTs) comparing one medication with placebo or one medication with another. We have considered trials for inclusion irrespective of language or publication status. We found no cross‐over trials, for which we had planned to consider the first randomised phase only.
Types of participants
Individuals
We have included trials in individuals with both of the following characteristics.
History of any traumatic event.
Aged 18 and older.
We have excluded studies targeting symptomatic patients at baseline, as these studies will be included in a second parallel review on early interventions (i.e. indicated prevention of PTSD) (Bertolini 2020), whilst the present review is on universal prevention.
Setting
We have considered trials performed in any type of setting.
Subset data
We planned to include trials in which only a portion of the sample met the above criteria, provided that the relevant data could be gained from the study report or by contacting the authors, and that the effect of randomisation was not affected by doing so. We did not find studies that required this treatment.
Types of interventions
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) regards the three months from the trauma as a relevant timeframe for symptom evolution (APA 2013). Thus, we included any pharmacological intervention administered with the intention to prevent the onset of PTSD or PTSD symptoms within such a timeframe. We set no restrictions regarding dose, duration, administration route of the intervention, nor on the presence of any co‐medication not related to PTSD prevention. We excluded trials that investigated medications as an augmentation to psychotherapy (e.g. cognitive enhancers), as those trials investigate a form of psychological prevention.
Based on our knowledge of the literature, we expected to find drugs from these pharmacological groups:
glucocorticoids;
beta blockers;
benzodiazepines;
opioids;
other hormones (oxytocin);
selective serotonin reuptake inhibitors (SSRIs);
mood stabilisers/anticonvulsants; and
omega‐3 fatty acid compounds.
Types of comparison
We have included studies using placebo or any active pharmacological agent as comparison. We have not considered studies comparing pharmacological interventions with only psychosocial interventions (i.e. with no other pharmacological or placebo arm). We have included studies meeting the above criteria, irrespective of whether they reported any of our outcomes of interest.
Types of outcome measures
Primary outcomes
PTSD severity (continuous): using the mean score on a validated rating scale such as the Clinician‐Administered PTSD Scale (CAPS) (Blake 1995), or the Posttraumatic Stress Disorder Checklist (PCL) (Weathers 2001), the Comprehensive International Diagnostic Interview (CIDI) (WHO 1997), or any other validated rating scale to assess symptom severity.
Dropouts due to adverse events (dichotomous): we considered the number of participants who left the assigned arms early due to side effects, out of the number of randomised individuals.
Secondary outcomes
PTSD rate (dichotomous): we considered PTSD rates, as measured by a DSM‐defined measure or International Classification of Diseases (ICD) (WHO 1992) diagnosis made with a clinician‐administered measure.
Depression severity (continuous): we considered the severity of depressive symptoms, using the score on validated scales such as the Montgomery‐Asberg Depression Rating Scale (MADRS; Montgomery 1979), the Hamilton Depression Rating Scale (Hamilton 1960), the Beck Depression Inventory (Beck 1961), or any other validated scale.
Anxiety severity (continuous): we considered the severity of the anxiety symptoms using the score on validated scales such as the Covi Anxiety Scale (CAS) (Covi 1984), the Beck Anxiety Inventory (Beck 1988), the Spielberger State‐Trait Anxiety Inventory (Spielberger 1970), the Hamilton Anxiety Rating Scale (Hamilton 1959), or any other validated scale.
Functional disability (continuous): we considered validated scales such as the Sheehan Disability Scale (Sheehan 1996), or any other validated scale.
Quality of life (continuous): we considered validated scales such as the Medical Outcomes Study (MOS) 36‐Item Short‐Form Health Survey (SF‐36) (Ware 1992), or any other validated scale to assess quality of life.
Dropout for any reason (dichotomous): we considered the number of participants who left the assigned arms early for any reason, out of the number of randomised individuals.
Hierarchy of outcome measures
The hierarchy of outcome measure scales has followed the order above. We expected that clinician‐administered scales would more frequently be employed. In the case of trials employing validated scales different from the ones mentioned above, for homogeneity reasons, we have given priority to clinician‐administered scales over self‐reported ones.
Timing of outcome measures
We have synthesised data at three months after exposure to the traumatic event, operationalised as the time point closest to three months of follow‐up (from two to four months of follow‐up). In addition, we have included data at study endpoint as a secondary time point.
Search methods for identification of studies
Cochrane Common Mental Disorders (CCMD) maintained a specialised register of randomised controlled trials (RCTs), the CCMDCTR, to June 2016. This register contains over 40,000 reference records (reports of RCTs) for anxiety and depressive disorders, bipolar disorder, eating disorders, self‐harm, and other mental disorders within the scope of CCMD. The CCMDCTR is a partially studies‐based register, with more than 50% of the reference records tagged to 12,600 study records, individually coded for participant, intervention, comparison, and outcome (PICO). Reports of trials for inclusion in the register were collated from (weekly) generic searches of MEDLINE (1950‐), Embase (1974‐) and PsycINFO (1967‐), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of trials were also sourced from international trial registries, drug companies, handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on CCMD's website, with an example of the MEDLINE search displayed in Appendix 1.
The CCMD trials register fell out of date with the relocation of the group from the University of Bristol to York University in June 2016.
Electronic searches
CCMDCTR studies and references register
We have cross‐searched the CCMDCTR studies and references register for condition alone, using the following terms: (PTSD or posttrauma* or post‐trauma* or "post trauma*" or "combat disorder*" or "stress disorder*") (all years to June 2016).
Biomedical database search
To account for the period after the CCMDCTR fell out of date, the CCMD's information specialists conducted additional searches on the following bibliographic databases, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource (see Appendix 2 for details of the search strategies).
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 11) in the Cochrane Library (June 2016 to 13 November 2020).
MEDLINE Ovid (June 2016 to 13 November 2020).
Embase Ovid (June 2016 to 13 November 2020).
PsycINFO Ovid (June 2016 to 13 November 2020).
Published International Literature On Traumatic Stress (PILOTS) EBSCO (June 2016 to 13 November 2020).
The search was for all reviews on PTSD within the scope of CCMD. After de‐duplication, at least two members of the CCMD editorial base staff screened the search results in Covidence, according to the following criteria.
Inclusion criteria
Any RCT for the treatment of PTSD (irrespective of intervention, age group or comorbidity)
Any RCT which might be seen as a PTSD prevention study
Any RCT for critical incident stress debriefing (CISD) (simulated crises not included)
Any RCT for debriefing after psychological trauma or any stress resilience studies
Any controlled clinical trial (CCT) where the treatment allocation was ambiguous
Corrigendums, errors, retractions, or substantial comments relating to the above
Exclusion criteria
All systematic reviews and meta‐analyses
Healthy populations
Simulated crises (e.g. for staff training in accident and emergency)
RCTs which fall outside the scope of CCMD, such as serious mental illness (schizophrenia), borderline personality disorder, alcohol use disorder (e.g. brief alcohol intervention in accident and emergency department), smoking cessation, traumatic brain injury, fibromyalgia (unless the comorbidity clearly fell within the scope of the search and was an outcome of the trial)
A first search was run in March 2018, with an update in March 2019, and a final update on 13 November 2020 (see Appendix 2).
Searching other resources
We have checked the reference lists of all included studies and relevant systematic reviews.
Data collection and analysis
Selection of studies
We imported all records obtained via the electronic search, plus the handsearch, into EndNote software in order to remove all duplicates. Two review authors (FB and LR) worked independently and in duplicate. We screened all potentially eligible papers' titles and abstracts and coded them as 'retrieve' or 'not retrieve'; obtained the full‐text publication of the records coded as 'retrieve'; and assessed inclusion and exclusion criteria. We resolved any disagreements through discussion or, if necessary, by involving a third review author (NM).
Data extraction and management
Two review authors (FB and LR), working independently and in duplicate, extracted data from the included trials. We used a data extraction sheet developed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (hereafter referred to as the Cochrane Handbook), section 7.5 (Higgins 2011a). We collected the following data.
First author, year of publication, journal, source of funding, notable conflict of interest of authors, total duration of study, number of centres and location.
Methodological characteristics of the trial: randomisation, blinding, allocation concealment, number of arms, follow‐up time points.
Sample characteristics: study setting, type of trauma, criteria for enrolling, age, gender, number of participants randomised to each arm, history of previous traumatic events.
Intervention details: time from the traumatic event to treatment, medication employed, period over which it was administered, dosage range, mean dosage prescribed.
Outcomes: time points of outcome assessment, instrument used to assess PTSD symptoms, instrument used to assess PTSD rate, instrument used to assess depression symptoms, instrument used to assess anxiety, instrument used to assess functional disability, outcome measure employed by original trial (primary and secondary), data for continuous (means and standard deviation or standard error if standard deviation was not provided) and dichotomous variables of interest, total number of dropouts, number of dropouts due to pharmacological side effects, whether the data reflected an intention‐to‐treat (ITT) model, methods of estimating the outcome for participants who dropped out (last observation carried forward (LOCF) or completer/observed case (OC) approach, or other).
Assessment of risk of bias in included studies
Two review authors (FB and LR), working independently and in duplicate, assessed the risk of bias for each study according to the criteria outlined in the Cochrane Handbook (Higgins 2011b). We resolved any disagreements through discussion, or if necessary, by involving a third review author (NM). We assessed the risk of bias according to the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personal (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We assessed performance, detection, and attrition bias on a per outcome basis rather than per study. We have rated each source of bias as high, low or unclear, with reasons to justify the rating.
Measures of treatment effect
Dichotomous data
For dichotomous data, we calculated risk ratio (RR) estimates and their 95% confidence interval (CI). RRs are more easily interpreted than odds ratios (ORs) (Boissel 1999), and as clinicians may risk interpreting ORs as RRs (Deeks 2002), this may lead to an overestimation of the effect. We also calculated the number needed to treat for an additional beneficial/harmful outcome (NNTB/NNTH).
Continuous data
For continuous data, we calculated the mean differences (MDs) and their 95% CI, where data were measured on the same scale. For studies that employed different scales, we have used standardised mean differences (SMDs). We gave preference to endpoint measures, considering the nature of the review (prevention) and that endpoint data are easier to interpret from a clinical point of view. In the case of reporting of change scores measures only, we had planned ‐ if sufficient data had been reported ‐ to convert change scores into endpoint data using standard formulas reported in the Cochrane Handbook (Deeks 2011), but this was unnecessary.
Unit of analysis issues
Cross‐over trials
We included no cross‐over trials in this review. For this design, we had planned to consider only the first phase from cross‐over trials, as the carryover effect cannot be excluded on a prevention measure, regardless of appropriate washout times.
Cluster‐randomised trials
We found no cluster‐randomised trial eligible for inclusion in this review. For eligible cluster‐RCTs which had not appropriately adjusted for the correlation between participants within clusters, we had planned to contact trial authors to obtain an estimate of the intracluster correlation (ICC), or to impute using estimates from the other included trials or from similar external trials. We planned to conduct a sensitivity analyses in the case of imputation of ICCs to examine the impact on estimates.
Multiple treatment group studies
We have compared each arm with placebo separately and included each pair‐wise comparison separately. In the case of pooling different interventions together, we had planned the following means to prevent 'double‐counting', in accordance with the Cochrane Handbook, section 16.5.4 (Higgins 2011c): in the case of dichotomous variables, to split the comparison group evenly amongst the intervention groups; in the case of continuous variables, to only divide the total number of participants and leave the mean and standard deviations (SDs) unchanged.
Dose‐ranging studies
We have not included studies with multiple arms with the same medication administered at different doses or for a different length of time. For these trials, we had planned to pool these intervention groups into a single one, as recommended by the Cochrane Handbook, section 16.5.4 (Higgins 2011c).
Dealing with missing data
As a first measure, we tried to contact study investigators to obtain missing data. When this was unsuccessful, we employed the following approaches.
Dichotomous data
We planned to use ITT data analysed on a 'once randomised, always analysed' basis, and for studies that did not perform an ITT analysis, to assume a negative outcome (i.e. onset of PTSD) for individuals lost to follow‐up. However, given the high attrition rates of some trials and that none used ITT analyses, we felt that this approach risked being further distant from the true value. Therefore, we decided to consider the number of participants with the event divided by the number of analysed participants (i.e. 'observed cases'), and added a sensitivity analysis with the number of participants with the event divided by the number of randomised participants.
Continuous data
We used ITT data when reported, favouring multiple imputations or mixed‐effects models where different imputational strategies had been used. In the context of prevention, last observation carried forward (LOCF) provides the least conservative option and therefore observed cases (OC) were preferred. For studies not reporting ITT analyses, we have not imputed missing data for continuous outcomes, as this usually requires access to individual participant data.
Missing statistics
In the case of missing statistics, we had planned to calculate SDs when only P values, CIs, standard errors, and so on were reported, but this was not possible. We also planned to calculate the arithmetic mean of SDs of studies using the same scale of the one with the missing SDs (as in Furukawa 2006), but again this was not possible.
Assessment of heterogeneity
We have assessed heterogeneity by means of:
visual inspection of the overlap of the CIs for individual studies in the forest plot;
Chi2 test, with a P value set at 0.10;
I2 statistic: in accordance with the suggestion in the Cochrane Handbook, section 9.5.2 (Deeks 2011), we have followed a rough guide for interpretation as follows: 0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity. We have also taken into account magnitude and direction of effects.
Assessment of reporting biases
We included fewer than 10 studies per outcome per comparison. If more than 10 studies had been included per primary outcome, we would have:
visually inspected the relative funnel plots, tested them for asymmetry, and investigated possible reasons for funnel plot asymmetry;
employed Egger's regression test (Egger 1997).
Data synthesis
Methods for pair‐wise meta‐analysis
We have performed standard pair‐wise meta‐analysis with a random‐effects model for every comparison with at least two studies, using Review Manager 5 (Review Manager 2014). We used a random‐effects model as we were expecting clinical heterogeneity. We have performed the pair‐wise comparison at individual medicine level (e.g. propranolol versus placebo) and planned a possible shift to drug class level if the number of studies was limited. We decided not to do so in the case of dexamethasone and hydrocortisone. Although both drugs are steroids, dexamethasone does not easily pass the blood‐brain barrier whilst hydrocortisone does.
Methods for network meta‐analysis
We had planned to perform a network meta‐analysis subject to feasibility. In consideration of the limited number of included studies and the lack of direct comparisons, we judged the network meta‐analysis infeasible.
Subgroup analysis and investigation of heterogeneity
We planned to assess the impact on effectiveness of subgrouping by intervention, starting within 12 hours from the traumatic event and after 12 hours from the traumatic event. However, this was feasible for only one comparison, as most of the comparisons we found included only one study, or the reported start time of the intervention was insufficiently specific. To limit the risk of false positive through multiple testing, we applied the subgroup analysis to primary outcomes only. An additional planned subgroup considering the setting of the intervention (e.g. acute and emergency departments, surgery or intensive care units) was not feasible, because for each comparison, all of the included studies took place in the same setting.
Sensitivity analysis
We could not carry out the following additional pre‐planned sensitivity analyses due to lack of data: excluding studies at high risk of bias defined by unclear allocation concealment or unblinded outcome assessment; impact using ITT data versus completers data; and impact of excluding cluster‐RCTs.
Summary of findings and assessment of the certainty of the evidence
We planned to present the results using a summary of findings table for each comparison. However, as we found nine comparisons, we considered this approach impractical. Instead, we prioritised those comparisons that are widely discussed in the literature and in previous guidelines (namely, hydrocortisone versus placebo, propranolol versus placebo, and gabapentin versus placebo) (ISTSS 2018; NICE 2018; Phoenix Australia 2020), as we expect these to be more relevant to decision makers. For comprehensiveness, we have reported summary of findings tables for the other comparisons in Additional tables.
The summary of findings tables considered the primary time point of three months after the traumatic event and these outcomes:
PTSD severity;
dropouts due to adverse events;
PTSD rate;
functional disability; and
quality of life.
We have used the five GRADE 'certainty assessment' domains (study design, risk of bias, inconsistency, indirectness, imprecision) to assess the certainty of the evidence of the studies that provided data for the specific outcome. We have used GRADEpro software (GRADEpro GDT), and applied the methods and recommendations from the Cochrane Handbook, section 11.5 (Schünemann 2011). Two review authors (FB and TW) independently graded the certainty of the evidence. We resolved any disagreements through discussion or, if required, by consulting a third review author (NM). We have used footnotes to justify the downgrading of the evidence. We have noted comments to aid the reader, when suitable. We have categorised the certainty of the evidence as high (further research is not likely to change our confidence in the estimate of effect), moderate (further research is likely to have an important impact on the estimate of effect and may change it), low (further research is very likely to have an important impact on the estimate of effect and is likely to change it), or very low (estimate of effect is very uncertain).
Results
Description of studies
Results of the search
The initial search identified 3339 titles and abstracts, and an updated search identified an additional 2208 titles and abstracts (see Figure 1 for study flow diagram). We screened each title (and abstract if available) for eligibility. We inspected a total of 100 full‐text publications, and identified 16 studies (32 reports) eligible for inclusion in the review. Of these, three are ongoing studies, leaving 13 studies (29 reports) for inclusion in the review. Thirty‐nine studies were ineligible for this review; of these, 18 have been marked for inclusion in the parallel review on early, indicated pharmacological intervention (Bertolini 2020).
1.
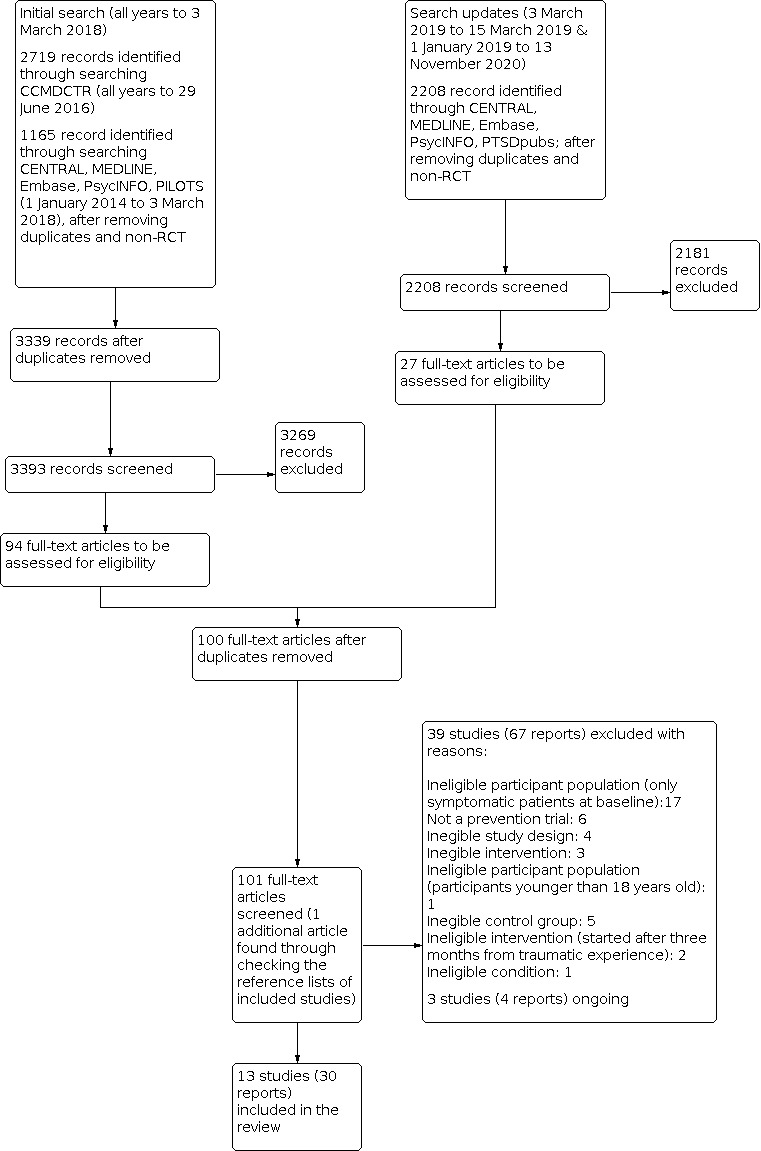
PRISMA study flow chart
Included studies
Design
All of the included studies are RCTs. All of them compared one active intervention against placebo, with the exception of Stein 2007, which compared two active interventions and placebo, and Kagan 2015, which compared two different enteral formulas.
Participants, traumatic events, and setting
A total of 2023 participants were included, with one single study contributing 1244 participants (Kok 2016).
The studies considered individuals exposed to a wide range of traumatic events, reflecting different recruitment settings. Eight studies recruited participants from emergency departments (Hoge 2012; Pitman 2002), trauma centres (Borrelli 2019; Shaked 2019; Stein 2007), intensive care units (ICUs) caring for trauma patients (Kagan 2015; Matsuoka 2015), or burn centres (Orrey 2015). Participants were exposed to both intentional and unintentional traumatic events, including assault, work injuries, motor vehicle accidents, and burns. Five studies recruited participants from ICUs, with septic shock (Denke 2008; Schelling 2001), or after cardiac surgery (Kok 2016; Weis 2006); or on the basis of ICU admission for non‐surgical reasons (Tincu 2016).
Two studies were multicentric (Kok 2016; Orrey 2015), nine were single centre (Borrelli 2019; Hoge 2012; Kagan 2015; Matsuoka 2015; Schelling 2001; Shaked 2019; Stein 2007; Tincu 2016; Weis 2006), and two provided insufficient detail to determine how many centres were involved (Denke 2008; Pitman 2002). Five studies were conducted in the USA (Borrelli 2019; Hoge 2012; Orrey 2015; Pitman 2002; Stein 2007), three in Germany (Denke 2008; Schelling 2001; Weis 2006), two in Israel (Kagan 2015; Shaked 2019), one in the Netherlands (Kok 2016), one in Japan (Matsuoka 2015), and one in Romania (Tincu 2016).
Interventions
The trials considered nine active interventions. Five trials investigated intravenous glucocorticoids, with one on dexamethasone (Kok 2016), and four on hydrocortisone (Denke 2008; Schelling 2001; Shaked 2019; Weis 2006). Four trials investigated propranolol (Hoge 2012; Orrey 2015; Pitman 2002; Stein 2007), with one of these having an additional gabapentin arm (Stein 2007). One trial was on omega‐3 fatty acids (docosahexaenoic acid and eicosapentaenoic acid) (Matsuoka 2015), one on paroxetine, an SSRI antidepressant (Borrelli 2019), one on 5‐hydroxytryptophan (Tincu 2016), and one on enteral nutrition formulas (Kagan 2015). In the included studies, dexamethasone was administered as a continuous infusion at 1 mg/kg body weight for 12 days (Kok 2016). Hydrocortisone was variably administered, with one study using a single bolus of 100 mg (Shaked 2019); one study using a 4‐day course, including 100 mg as initial loading dose followed by one day at 10 mg/hour and then tapering (Weis 2006); and two studies using courses of several days with either a fixed scheme of 50 mg every 6 hours for five days followed by progressive tapering in six days (Denke 2008), or a loading dose of 100 mg followed by continuous infusion of 0.18 mg/kg/hour for six days and later tapering after septic shock reversal (Schelling 2001). Propranolol administration schemes ranged from a 14‐day course with up to 120 mg daily (Stein 2007), to a 6‐week course with 240 mg daily for three weeks followed by a taper period (Orrey 2015). Gabapentin was administered to up to 1200 mg daily over a course of 14 days. The omega‐3 fatty acids dose consisted of 1470 mg of docosahexaenoic acid and 147 mg of eicosapentaenoic acid daily for 20 days (Matsuoka 2015). The dose of paroxetine was 20 mg with possible reduction to 10 mg for side effects or elderly participants (Borrelli 2019), and the 5‐hydroxytryptophan dose was 300 mg (Tincu 2016). The enteral formulas were administered so as to provide at least 80% of the energetic expenditure and were provided for the duration of participants' ICU stay, up to 28 days (Kagan 2015).
Outcome measures
Most of the studies assessed PTSD severity with either the CAPS (Hoge 2012; Matsuoka 2015; Pitman 2002), or PCL (Borrelli 2019; Kagan 2015; Stein 2007). Denke 2008 used the Post‐Traumatic Stress Symptom 10‐Question Inventory (PTSS‐10) questionnaire, whilst two other studies used a modified version of the PTSS‐10 validated for ICU patients (Schelling 2001; Weis 2006). Two studies used additional, different scales: the Post‐traumatic stress Diagnostic Scale (PDS) (Shaked 2019), and the Posttraumatic Symptom Scale‐Interview Version (PSS‐I) Orrey 2015).
Three studies used the CAPS to establish the presence of PTSD according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria (Hoge 2012; Matsuoka 2015; Pitman 2002), whilst two other studies used other validated DSM‐IV‐based interviews (Borrelli 2019; Schelling 2001). The remaining studies used several different tools to establish the presence of PTSD: the CIDI PTSD (Stein 2007), a validated modified version of the PTSS‐10 questionnaire (Weis 2006), the PSS‐I (Orrey 2015), the PTSS‐10 (Denke 2008), and the Self‐Rating Inventory for PTSD (SRIP, a Dutch questionnaire consistent with the DSM‐IV criteria for PTSD) (Kok 2016).
Depression was assessed with the Center for Epidemiologic Studies Depression Scale (CES‐D) (Stein 2007), the MADRS (Matsuoka 2015), the Quick Inventory of Depressive Symptomatology (QIDS‐SR) (Borrelli 2019), and an unspecified 'Depression scale' (Kagan 2015).
All the studies that assessed quality of life used the SF‐36 (Borrelli 2019; Denke 2008; Kok 2016; Matsuoka 2015; Weis 2006).
Timing of outcome assessment
Of the 13 included trials, only five assessed outcomes at three months (which is the review's primary time point, operationalised as between two and four months from the traumatic experience) (Borrelli 2019; Hoge 2012; Matsuoka 2015; Pitman 2002; Stein 2007). The timing of the studies' endpoints varied greatly, from two weeks (Tincu 2016), to 49 months (Schelling 2001), with three studies assessing the outcome only after one year (Denke 2008; Kok 2016; Schelling 2001).
Excluded studies
We excluded 39 studies from this review. About half were excluded because they restricted eligibility to participants experiencing psychological distress at baseline. These studies will be included in the parallel review (Bertolini 2020). We excluded five studies because the focus was not PTSD prevention (NCT00633685; Lijffijt 2019; Naylor 2013; Rabinak 2020; Takehiro 2014; Truppman Lattie 2020); five for lack of or inappropriate comparison arm (Matsumura 2011; Matsuoka 2010; Nishi 2012; Schelling 2004; Yang 2011); four for ineligible study design (Blaha 1999; Gelpin 1996; Mistraletti 2015; NCT02069366); five for ineligible intervention (including interventions started after three months from the traumatic event) (FDA 1999; Kaplan 2015; Nedergaard 2020; Treggiari 2009; Zoellner 2001); one for including participants under 18 years old (Stoddard 2011); and one study concerned an ineligible condition (NCT02505984).
Ongoing studies
We found three currently ongoing studies (McMullan 2020; NCT03997864; NCT04274361).
Risk of bias in included studies
See Figure 2 and Figure 3 for further details.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Random sequence generation
Ten studies described in sufficient detail a strategy for randomisation; we judged these as low risk of bias (Borrelli 2019; Denke 2008; Kagan 2015; Kok 2016; Matsuoka 2015; Orrey 2015; Schelling 2001; Shaked 2019; Stein 2007; Weis 2006). The remaining three studies reported a random assignment to intervention but with insufficient detail to assess the validity of the method (Hoge 2012; Pitman 2002; Tincu 2016). Therefore, we assessed these as having an unclear risk of bias.
Allocation
Seven studies described procedures that clearly resulted in or implied the concealment of the randomisation list (Denke 2008; Kagan 2015; Kok 2016; Matsuoka 2015; Orrey 2015; Stein 2007; Weis 2006); we judged these as low risk of bias. Six studies did not describe the allocation process with sufficient detail to ensure that allocation concealment was in place (Borrelli 2019; Hoge 2012; Pitman 2002; Schelling 2001; Shaked 2019; Tincu 2016); we judged these as having an unclear risk of bias.
Blinding
Ten studies reported blinding of participants (Borrelli 2019; Denke 2008; Hoge 2012; Kagan 2015; Kok 2016; Matsuoka 2015; Orrey 2015; Shaked 2019; Stein 2007; Weis 2006), and have been judged at low risk of bias. One study was designed as double‐blind, but trial authors raised a concern about the effectiveness of the blinding due to specific side effects that may have allowed the identification of the intervention (Pitman 2002). The reporting of another study was unclear about participants’ blinding, and if blinded, whether blinding was still in place at the time of assessment of PTSD outcomes (Schelling 2001). We judged these trials as having an unclear risk of bias. One study did not mention blinding in its reporting (Tincu 2016); we judged it to be at high risk of bias.
Eleven studies reported blinding of outcome assessors (Borrelli 2019; Denke 2008; Hoge 2012; Kok 2016; Matsuoka 2015; Orrey 2015; Pitman 2002; Schelling 2001; Shaked 2019; Stein 2007; Weis 2006). One study provided insufficient detail (Kagan 2015), and has been judged at unclear risk of bias. One study did not mention blinding in its reporting (Tincu 2016); we judged it to be at high risk of bias.
Incomplete outcome data
Only four studies reported low attrition rates for outcomes of interest (Hoge 2012; Matsuoka 2015; Orrey 2015; Weis 2006); we judged these as having a low risk of bias. Three studies provided insufficient detail to assess the attrition rate of intervention arms (Borrelli 2019; Shaked 2019; Tincu 2016); we assessed these as having an unclear risk of bias. We judged six studies to be at high risk of bias. For four of them, this was due to high (over 20%) or uneven attrition rates (Denke 2008; Kagan 2015; Pitman 2002; Stein 2007). Kok 2016 and Schelling 2001 assessed PTSD‐related outcomes in follow‐up studies of clinical trials in ICU patients. The trials had not originally planned for this, requiring additional informed consent and applying additional exclusion criteria post‐randomisation. Moreover, these assessments were performed some time after the original trials, and parts of the samples were lost due to death. We judged these studies to be at high risk of bias.
Selective reporting
Reporting bias could be excluded only for Matsuoka 2015, which we judged as having a low risk of bias. Eight studies lacked a published protocol or clinical trial registration entry finalised before the data were available (Borrelli 2019; Denke 2008; Hoge 2012; Pitman 2002; Shaked 2019; Stein 2007; Tincu 2016; Weis 2006); we judged these as having an unclear risk of bias. In the Orrey 2015 trial, some outcomes were changed whilst the trial was being carried out. Kok 2016 and Schelling 2001 did not plan PTSD‐related outcomes when the original trials were carried out, and the registration entry of Kagan 2015 did not report PTSD‐related outcomes. We judged these four trials as having a high risk of bias.
Other potential sources of bias
We did not find additional concerns for seven studies (Borrelli 2019; Hoge 2012; Matsuoka 2015; Orrey 2015; Pitman 2002; Shaked 2019; Stein 2007), and rated them at low risk of bias. The reporting of PTSD‐related outcomes for three trials relied on conference abstracts only (Denke 2008; Kagan 2015; Tincu 2016); for this reason, we judged these as having an unclear risk of bias. We judged the three remaining studies to be at high risk of bias. Kok 2016 required additional informed consent after randomisation at a time when part of the sample (critically‐ill participants) was lost due to death, and consenting participants were analysed at various time lengths after the traumatic event. We felt that a high risk of bias was therefore appropriate for this study. The authors of Schelling 2001 raised the concern that the investigated intervention might have been effective due to differences in the inotropic support (catecholamines have a role in PTSD development, see How the intervention might work). Moreover, additional inclusion criteria were applied after randomisation for the PTSD outcomes. A difference in the inotropic support was present in Weis 2006 too. Participants had different courses of the underlying condition between intervention arms and this could mediate an effect on PTSD development. We felt that a judgement of high risk of bias was therefore appropriate for these studies too.
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9 and Data and analyses.
1. Additional summary of findings table: dexamethasone compared to placebo for preventing post‐traumatic stress disorder (PTSD).
| Dexamethasone compared to placebo for preventing post‐traumatic stress disorder (PTSD) | |||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: N/A Intervention: dexamethasone Comparison: placebo | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| PTSD severity at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| Dropout due to adverse events at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| PTSD rate at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| Functional disability at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| Quality of life at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| PTSD: post‐traumatic stress disorder | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
2. Additional summary of findings table: omega‐3 fatty acids compared to placebo for preventing post‐traumatic stress disorder (PTSD).
| Omega‐3 fatty acids compared to placebo for preventing post‐traumatic stress disorder (PTSD) | ||||||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: intensive care unit of a disaster medical center Intervention: omega‐3 fatty acids Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with omega‐3 fatty acids | |||||
| PTSD severity at 3 months assessed with: CAPS (Matsuoka 2015) | The mean PTSD severity at 3 months was 9.22 | Mean 1.56 higher (4.06 lower to 7.18 higher) | ‐ | 100 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| Dropout due to adverse events at 3 months | Study population | Not estimable | 110 (1 RCT) | ⊕⊕⊝⊝ Lowb | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| PTSD rate at 3 months assessed with: DSM‐IV criteria | Study population | RR 2.44 (0.23 to 26.09) | 100 (1 RCT) | ⊕⊕⊝⊝ Lowc | ||
| 18 per 1000 | 44 per 1000 (4 to 474) | |||||
| Functional disability at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ | ‐ | ||
| Quality of life at 3 months assessed with: SF‐36, MSC | The mean quality of life at 3 months was 51.6 | Mean 3 lower (7.4 lower to 1.4 higher) | ‐ | 99 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAPS: Clinician‐Administered PTSD Scale; CI: confidence interval; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders‐4th Edition; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; RR: risk ratio; SF‐36, MSC: Medical Outcomes Study 36‐Item Short Form Health Survey, mental component summary | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision as far fewer than 400 participants were included and the confidence interval is wide bDowngraded two levels for imprecision as the number of participants is very far from the optimal information size (OIS) cDowngraded two levels as the OIS is not met and the confidence interval includes both appreciable benefit and harm
3. Additional summary of findings table: propranolol compared to gabapentin for preventing post‐traumatic stress disorder (PTSD).
| Propranolol compared to gabapentin for preventing post‐traumatic stress disorder (PTSD) | ||||||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: surgical trauma center Intervention: propranolol Comparison: gabapentin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with gabapentin | Risk with propranolol | |||||
| PTSD severity at 3 months ‐ not reported | No study reported this outcome at this timepoint | ‐ | ‐ | ‐ | ||
| Dropout due to adverse events at 3 months ‐ not reported | No study reported this outcome at this timepoint | ‐ | ‐ | ‐ | ||
| PTSD rate at 3 months assessed with: CIDI | Study population | RR 1.25 (0.26 to 6.07) | 22 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 200 per 1000 | 250 per 1000 (52 to 1000) | |||||
| Functional disability at 3 months ‐ not reported | No study reported this outcome | ‐ | ‐ | ‐ | ||
| Quality of life at 3 months ‐ not reported | No study reported this outcome | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CIDI: Comprehensive International Diagnostic Interview; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias as the included study has high attrition rates for these interventions bDowngraded two levels as the optimal information size is not met and the CI includes both appreciable benefit and harm
4. Additional summary of findings table: paroxetine compared to placebo for preventing post‐traumatic stress disorder (PTSD).
| Paroxetine compared to placebo for preventing post‐traumatic stress disorder (PTSD) | |||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: trauma center Intervention: paroxetine Comparison: placebo | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| PTSD severity at 3 months assessed with: PCL‐C | Change from baseline PCL‐C scores: paroxetine: ‐4.0, placebo +0.3, without statistical significance with alpha set at 0.05. No variance measure is reported nor the number of analysed participants. | (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
| Dropout for any reason at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| PTSD rate at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| Functional disability at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| Quality of life at 3 months assessed with: SF‐36 | Change from baseline SF‐36 scores: paroxetine: +4.1, placebo +4.4, without statistical significance with alpha set at 0.05. No variance measure is reported nor the number of analysed participants. | (1 RCT) | ⊕⊝⊝⊝ Very lowa,b |
| PCL‐C: Posttraumatic Stress Disorder Checklist ‐ Civilian version; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; SF‐36: Medical Outcomes Study 36‐Item Short‐Form Health Survey | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
aDowngraded one level for unclear risk of bias in allocation concealment, incomplete outcome data and selective reporting bDowngraded two levels for imprecision as much fewer than 400 participants were included
5. Additional summary of findings table: PulmoCare formula compared to Oxepa formula for preventing post‐traumatic stress disorder (PTSD).
| PulmoCare formula compared to Oxepa formula for preventing post‐traumatic stress disorder (PTSD) | |||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: N/A Intervention: PulmoCare formula Comparison: Oxepa formula | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| PTSD severity at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| Dropout due to adverse events at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| PTSD rate at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| Functional disability at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| Quality of life at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| PTSD: post‐traumatic stress disorder | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
6. Additional summary of findings table: 5‐hydroxytryptophan compared to placebo for preventing post‐traumatic stress disorder (PTSD).
| 5‐hydroxytryptophan compared to placebo for preventing post‐traumatic stress disorder (PTSD) | |||
| Patient or population: adults (aged 18 and older) exposed to a traumatic event Setting: N/A Intervention: 5‐hydroxytryptophan Comparison: placebo | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| PTSD severity at 3 months ‐ not measured | No study reported this outcome at this timepoint | ‐ | ‐ |
| Dropout due to adverse events at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| PTSD rate at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| Functional disability at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| Quality of life at 3 months ‐ not measured | No study reported this outcome | ‐ | ‐ |
| PTSD: post‐traumatic stress disorder | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
Comparison 1: hydrocortisone versus placebo
Four studies compared hydrocortisone to placebo (Denke 2008; Shaked 2019; Schelling 2001; Weis 2006).
Primary outcomes
1. PTSD severity
Three months after the traumatic event
No studies provided data on PTSD severity assessed three months after the traumatic event.
PTSD severity at study endpoint
One study provided data on PTSD severity at one month after the traumatic event (Shaked 2019), measured by the Post‐traumatic stress Diagnostic Scale (PDS). It is unclear whether hydrocortisone was less effective in reducing the severity of PTSD symptoms compared to placebo, as these data are based on only one small study, the confidence interval is rather wide and includes the null effect (MD 4.36, 95% CI ‐0.71 to 9.43; 1 study, 77 participants; Analysis 1.1). Two studies measured PTSD severity with a version of the PTSS‐10 questionnaire modified for ICU patients: one in a cohort of participants at various lengths of time after the traumatic event, ranging from 21 to 49 months (Schelling 2001); and one at 6 months after the traumatic event (Weis 2006). In both cases, we could not meta‐analyse the data due to non‐normal distribution. Schelling 2001 reported a reduction in PTSD symptoms for hydrocortisone compared with placebo, but there was substantial uncertainty due to the imprecision of the effect estimate and the very small sample size (hydrocortisone: median 27, 9 participants; placebo: median 36, 11 participants; P = 0.30). Weis 2006 also found a reduction in PTSD symptoms for hydrocortisone compared with placebo, but there was similar uncertainty due to the very small sample size (hydrocortisone: median 15.5, interquartile range (IQR) 14.8 to 21.8, 14 participants; placebo: median 25.5, IQR 16.8 to 33.0, 14 participants). One additional study measured PTSD severity with PTSS‐10 at one year after the traumatic event (Denke 2008). Outcome data were not reported.
1.1. Analysis.

Comparison 1: Hydrocortisone versus placebo, Outcome 1: PTSD severity at studies' endpoint
2. Dropouts due to adverse events
No studies provided data on dropouts due to adverse events
Secondary outcomes
1. PTSD rate
Three months after the traumatic event
No studies provided data on PTSD rate at three months after the traumatic event.
Study endpoint
Two studies provided data on PTSD rate, as measured at various lengths of time after the traumatic event, ranging from 21 to 49 months (Schelling 2001), and at 6 months after the traumatic event (Weis 2006). These studies suggested that hydrocortisone may reduce the probability of experiencing PTSD compared to placebo (RR 0.23, 95% CI 0.06 to 0.96; NNTB 3, 95% CI NNTB 2 to NNTH 56; I2 = 0%; 2 studies, 48 participants; Analysis 1.2). However, this is based on two very small studies and the effect estimate is not clearly determined as the confidence interval is very wide. We conducted a sensitivity analysis exploring the effect of considering cases divided by the number of randomised participants instead of divided by the number of analysed participants. This sensitivity analysis showed similar results (RR 0.22, 95% CI 0.05 to 0.92; NNTB 3, 95% CI NNTB 3 to NNTH 33; I2 = 0%; 2 studies, 60 participants; Analysis 1.4). We could not perform a planned sensitivity analysis excluding studies at high risk of bias as the analysis would have included one study only (Weis 2006). One study assessed PTSD rate at one year after the traumatic event (Denke 2008); however, data were not reported in sufficient detail for meta‐analysis. The study authors report that the incidence of PTSD did not differ between intervention groups (18 participants).
1.2. Analysis.

Comparison 1: Hydrocortisone versus placebo, Outcome 2: PTSD rate at studies' endpoint
1.4. Analysis.

Comparison 1: Hydrocortisone versus placebo, Outcome 4: Sensitivity analysis: PTSD rate at studies' endpoint (cases out of randomised)
2. Depression severity
No studies provided data on depression severity.
3. Anxiety severity
No studies provided data on anxiety severity.
4. Functional disability
No studies provided data on functional disability.
5. Quality of life
Three months after the traumatic event
No studies provided data on quality of life at three months after the traumatic event.
Study endpoint
One study measured quality of life at one year after the traumatic event with the 36‐Item Short‐Form Health Survey (SF‐36) score, but outcome data were not reported (Denke 2008). Another study measured quality of life at six months after the traumatic event with the SF‐36 score (Weis 2006). Data were not reported in sufficient detail for meta‐analysis. However, authors reported that hydrocortisone group participants (N = 14) compared to placebo group participants (N = 14) had higher scores on seven of eight scales, comprising both physical and mental aspects, with higher scores on SF‐36 physical and mental component summaries.
6. Dropout for any reason
Three months after the traumatic event
No studies provided data on dropout for any reason at three months after the traumatic event.
Study endpoint
Two studies provided data on dropout for any reason at various lengths of time after the traumatic event, ranging from 21 to 49 months (Schelling 2001), and at 6 months after the traumatic event (Weis 2006). It was unclear whether hydrocortisone increased the risk of dropout for any reason compared to placebo, as the confidence interval is rather wide and these data are based only on two small studies (RR 1.38, 95% CI 0.49 to 3.89; NNTH 16, 95% CI NNTB 12 to NNTH 2; I2 = 0%; 2 studies, 60 participants; Analysis 1.3). We could not perform a planned sensitivity analysis excluding studies at high risk of bias as the analysis would have included one study only (Weis 2006).
1.3. Analysis.

Comparison 1: Hydrocortisone versus placebo, Outcome 3: Dropout for any reason at studies' endpoint
Comparison 2: propranolol versus placebo
Four studies compared propranolol versus placebo (Hoge 2012; Orrey 2015; Pitman 2002; Stein 2007).
Primary outcomes
1. PTSD severity
Three months after the traumatic event
Three studies provided data on PTSD severity at three months after the traumatic event (Hoge 2012; Pitman 2002; Stein 2007). The evidence is inconclusive: it is not possible to determine if there is a clinically important difference between propranolol and placebo in reducing the severity of PTSD symptoms (SMD ‐0.51, 95% CI ‐1.61 to 0.59; I2 = 83%; 3 studies, 86 participants; very low‐certainty evidence; Analysis 2.1). We downgraded this outcome from high‐ to very low‐certainty evidence due to serious risk of bias amongst the included studies, serious inconsistency amongst the studies' results, and very serious imprecision of the effect estimate. In a subgroup analysis by timing of the intervention, there was a statistically significant difference (Chi2 = 11.47, degrees of freedom (df) = 1, P < 0.001) between two studies providing propranolol within 12 hours after the traumatic event (Hoge 2012; Pitman 2002) (SMD 0.06, 95% CI ‐0.46 to 0.58; I2 = 0%; 2 studies, 58 participants) and one study providing propranolol more than 12 hours after the traumatic event (Stein 2007) (SMD ‐1.73, 95% CI ‐2.62 to ‐0.83; 28 participants). We could not perform a planned sensitivity analysis excluding studies at high risk of bias as the analysis would have included one study only (Hoge 2012).
2.1. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 1: PTSD severity at three months
Study endpoint
Four studies provided data on PTSD severity at various time points: 6 weeks (Orrey 2015), 12 weeks (Hoge 2012), and 3 months (Pitman 2002; Stein 2007). It is unclear whether propranolol was more effective in reducing the severity of PTSD symptoms compared to placebo, as the confidence interval is very wide and includes the null effect (SMD ‐0.42, 95% CI ‐1.16 to 0.32; I2 = 74%; 4 studies, 125 participants; Analysis 2.4). In a subgroup analysis by timing of the intervention, there was no statistically significant difference (Chi2 = 1.52, df = 1, P = 0.22) between the two studies providing the interventions within 12 hours after the traumatic event (Hoge 2012; Pitman 2002) (SMD 0.06, 95% CI ‐0.46 to 0.58, I2 = 0%; 2 studies, 58 participants) and the two studies providing the interventions more than 12 hours after the traumatic event (Orrey 2015; Stein 2007) (SMD ‐0.93, 95% CI ‐2.42 to 0.56; I2 = 86%; 2 studies, 67 participants). We could not perform a planned sensitivity analysis excluding studies at high risk of bias as the analysis would have included one study only (Hoge 2012).
2.4. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 4: PTSD severity at studies' endpoint
2. Dropouts due to adverse events
Three months after the traumatic event
No study provided data on dropout due to adverse events at three months after the traumatic event.
Study endpoint
One study provided data for dropout due to adverse events at six weeks after the traumatic event (Orrey 2015). It is unclear whether propranolol increased the risk of dropout rate due to adverse events compared to placebo as these data are based on only one small study, the confidence interval is very wide and includes the null effect (RR 5.21, 95% CI 0.26 to 102.98; NNTH 11, 95% CI NNTB 20 to NNTH 5; 47 participants; Analysis 2.5).
2.5. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 5: Dropout due to adverse events at studies' endpoint
Secondary outcomes
1. PTSD rate
Three months after the traumatic event
Three studies provided data for PTSD rate at three months after the traumatic event (Hoge 2012; Pitman 2002; Stein 2007). The evidence is inconclusive: it is not possible to determine if there is a clinically important difference between propranolol and placebo in reducing the probability of experiencing PTSD (RR 0.77, 95% CI 0.31 to 1.92; NNTB 20, 95% CI NNTB 7 to NNTH 5; I2 = 0%; 3 studies, 88 participants; very low‐certainty evidence; Analysis 2.2). We downgraded this outcome from high‐ to very low‐certainty evidence because of serious risk of bias amongst the included studies and very serious imprecision of the effect estimate. We conducted a sensitivity analysis exploring the effect of considering cases divided by the number of randomised participants instead of dividing by the number of analysed participants. This sensitivity analysis showed similar results (RR 0.62, 95% CI 0.24 to 1.59; NNTB 13, 95% CI NNTB 13 to NNTH 9; I2 = 0%; 3 studies, 118 participants; Analysis 2.8). We could not perform a planned sensitivity analysis excluding studies at high risk of bias as the analysis would have included one study only (Hoge 2012).
2.2. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 2: PTSD rate at three months
2.8. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 8: Sensitivity analysis: PTSD rate at three months (cases out of randomised)
Study endpoint
Four studies provided data on PTSD rate at various time points: six weeks (Orrey 2015), 12 weeks (Hoge 2012), and three months (Pitman 2002; Stein 2007). It is unclear whether propranolol reduced the risk of experiencing PTSD compared with placebo, as the confidence interval is wide and includes the null effect (RR 0.73, 95% CI 0.35 to 1.51; NNTB 15, 95% CI NNTB 6 to NNTH 8; I2 = 0%; 4 studies, 127 participants; Analysis 2.6). We conducted a sensitivity analysis exploring the effect of considering cases divided by the number of randomised participants instead of dividing by the number of analysed participants. This sensitivity analysis showed similar results (RR 0.59, 95% CI 0.28 to 1.24; NNTB 11, 95% CI NNTB 6 to NNTH 19; I2 = 0%; 4 studies, 165; Analysis 2.9). We could not perform a planned sensitivity analysis excluding studies at high risk of bias as the analysis would have included one study only (Hoge 2012).
2.6. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 6: PTSD rate at studies' endpoint
2.9. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 9: Sensitivity analysis: PTSD rate at studies' endpoint (cases out of randomised)
2. Depression severity
One study provided data on depression severity (Stein 2007). Data were not reported in sufficient detail for meta‐analysis. The study authors report that, over the course of the study (maximum follow‐up: four months from the traumatic event), Center for Epidemiologic Studies Depression Scale (CES‐D) scores compared by a generalised estimating equation (GEE) analysis did not differ significantly between propranolol (17 randomised) and placebo (17 randomised) groups. However, this is based on one small trial; therefore, data were insufficient to conclude if there are any differences between groups.
3. Anxiety severity
No study provided data on anxiety severity.
4. Functional disability
No study provided data on functional disability.
5. Quality of life
No study provided data on quality of life.
6. Dropout for any reason
Three months after the traumatic event
Three studies provided data on dropout for any reason at three months after the traumatic event (Hoge 2012; Pitman 2002; Stein 2007). It is unclear whether propranolol increased the risk of dropout for any reason compared with placebo, as the confidence interval is very wide and includes the null effect (RR 1.53, 95% CI 0.77 to 3.01; NNTH 7, 95% CI NNTB 17 to NNTH 2; I2 = 6%; 3 studies, 118 participants; Analysis 2.3). We could not carry out a planned sensitivity analysis excluding studies at high risk of bias as the analysis would have included one study only (Hoge 2012).
2.3. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 3: Dropout for any reason at three months
Study endpoint
Four studies provided data on dropout for any reason at study endpoint (Hoge 2012; Orrey 2015; Pitman 2002; Stein 2007). It is unclear whether propranolol increased the risk of dropout for any reason compared with placebo, as the confidence interval is very wide and includes the null effect (RR 1.81, 95% CI 0.95 to 3.48; NNTH 6, 95% CI NNTB 91 to NNTH 2; I2 = 12%; 4 studies, 165 participants; Analysis 2.7). We could not carry out a planned sensitivity analysis excluding studies at high risk of bias as the analysis would have included one study only (Hoge 2012).
2.7. Analysis.

Comparison 2: Propranolol versus placebo, Outcome 7: Dropout for any reason at studies' endpoint
Comparison 3: dexamethasone versus placebo
One study compared dexamethasone to placebo (Kok 2016).
Primary outcomes
1. PTSD severity
Kok 2016 did not provide data on PTSD severity.
2. Dropouts due to adverse events
Kok 2016 did not provide data on dropouts due to adverse events.
Secondary outcomes
1. PTSD rate
Three months after the traumatic event
Kok 2016 did not provide data on PTSD rate at three months after the traumatic event.
Study endpoint
Kok 2016 provided data on PTSD rate in a cohort of participants at various lengths of time after the traumatic event, ranging from 1.5 to 4 years. It is unclear whether dexamethasone reduced the risk of experiencing PTSD compared to placebo, as the confidence interval is very wide and includes the null effect (RR 0.79, 95% CI 0.56 to 1.12; NNTB 50, 95% CI NNTB 17 to NNTH 100; 1125 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3: Dexamethasone versus placebo, Outcome 1: PTSD rate at studies' endpoint
2. Depression severity
Kok 2016 did not provide data on depression severity.
3. Anxiety severity
Kok 2016 did not provide data on anxiety severity.
4. Functional disability
Kok 2016 did not provide data on functional disability.
5. Quality of life
Three months after the traumatic event
Kok 2016 did not provide data on quality of life at three months after the traumatic event.
Study endpoint
Kok 2016 provided data on quality of life assessed with the SF‐36 Mental Component Summary score in a cohort of participants at various lengths of time after the traumatic event, ranging from 1.5 to 4 years. Authors report that data were not normally distributed and could therefore not be meta‐analysed. Authors report that there was no significant difference between the dexamethasone group (median 55.99, IQR 50.85 to 58.91; n = 561) and placebo group (median 55.68, IQR 50.76 to 58.81; n = 564). Information about statistical significance should be interpreted with caution due to skewed data.
6. Dropout for any reason
Three months after the traumatic event
Kok 2016 did not provide data on dropout for any reason at three months after the traumatic event.
Study endpoint
Kok 2016 provided data on dropout for any reason in a cohort of participants at various lengths of time after the traumatic event, ranging from 1.5 to 4 years. It is unclear whether dexamethasone reduced the risk of dropout for any reason compared to placebo, as the confidence interval is wide and includes the null effect (RR 0.93, 95% CI 0.66 to 1.31; NNTB 100, 95% CI NNTB 25 to NNTH 33; 1244 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3: Dexamethasone versus placebo, Outcome 2: Dropout for any reason at studies' endpoint
Comparison 4: omega‐3 fatty acids versus placebo
One study compared omega‐3 fatty acids (docosahexaenoic acid and eicosapentaenoic acid) to placebo (Matsuoka 2015). In the Matsuoka 2015 trial, the study endpoint corresponds to the review's primary time point of three months.
Primary outcomes
1. PTSD severity
Matsuoka 2015 provided data on PTSD severity at three months after the traumatic event, as assessed with CAPS. The evidence is inconclusive and so it is not possible to determine if there is a clinically important difference between omega‐3 fatty acids and placebo in reducing the PTSD severity (MD 1.56, 95% CI ‐4.06 to 7.18; 100 participants; low‐certainty evidence; Analysis 4.1). We downgraded this outcome from high‐ to low‐certainty evidence because of very serious imprecision due to limited sample size and wide confidence interval.
4.1. Analysis.

Comparison 4: Omega‐3 fatty acids versus placebo, Outcome 1: PTSD severity at three months
2. Dropouts due to adverse events
Matsuoka 2015 provided data on dropouts due to adverse events at three months after the traumatic event. In both intervention groups, no participants dropped out due to adverse events (100 participants; low‐certainty evidence; see Analysis 4.2). We downgraded this outcome from high‐ to low‐certainty evidence because of very serious imprecision due to the sample being very far from the optimal information size (OIS).
4.2. Analysis.

Comparison 4: Omega‐3 fatty acids versus placebo, Outcome 2: Dropout due to adverse events at three months
Secondary outcomes
1. PTSD rate
Matsuoka 2015 provided data on PTSD rate at three months after the traumatic event, as assessed with DSM‐IV criteria for PTSD. The evidence is inconclusive: it is not possible to determine if there is a clinically important difference between omega‐3 fatty acids and placebo in reducing the risk of experiencing PTSD (RR 2.44, 95% CI 0.23 to 26.09; NNTH 33, 95% CI NNTB 25 to NNTH 10; 100 participants; low‐certainty evidence; Analysis 4.3). We downgraded this outcome from high‐ to low‐certainty evidence because of very serious imprecision due to the sample being very far from the OIS.
4.3. Analysis.

Comparison 4: Omega‐3 fatty acids versus placebo, Outcome 3: PTSD rate at three months
2. Depression severity
Matsuoka 2015 provided data on depression severity at three months after the traumatic event, as assessed with the Montgomery‐Asberg Depression Rating Scale (MADRS). It is unclear whether omega‐3 fatty acids were less effective compared to placebo in reducing the severity of depression symptoms as these data are based on a single study, and the confidence interval includes the null effect (MD 1.82, 95% CI ‐1.65 to 5.29; 106 participants; Analysis 4.4).
4.4. Analysis.

Comparison 4: Omega‐3 fatty acids versus placebo, Outcome 4: Depression severity at three months
3. Anxiety severity
Matsuoka 2015 did not provide data on anxiety severity.
4. Functional disability
Matsuoka 2015 did not provide data on functional disability.
5. Quality of life
Matsuoka 2015 provided data on quality of life at three months after the traumatic event, as assessed with the SF‐36 Mental Component Summary. The evidence is inconclusive: it is not possible to determine if there is a clinically important difference between omega‐3 fatty acids and placebo in terms of quality of life (MD ‐3.00, 95% CI‐7.40 to 1.40; 99 participants; low‐certainty evidence; Analysis 4.5). We downgraded this outcome from high‐ to low‐certainty evidence because of very serious imprecision due to small sample size and wide confidence interval.
4.5. Analysis.

Comparison 4: Omega‐3 fatty acids versus placebo, Outcome 5: Quality of life at three months
6. Dropout for any reason
Matsuoka 2015 provided data on dropout for any reason at three months after the traumatic event (Matsuoka 2015). It is unclear whether omega‐3 fatty acids increased the risk of dropout for any reason compared to placebo as these data are based on a single study, the confidence interval is very wide and includes the null effect (RR 4.30, 95% CI 0.96 to 19.35; NNTH 8, 95% CI NNTB 100 to NNTH 5; 110 participants; Analysis 4.6).
4.6. Analysis.

Comparison 4: Omega‐3 fatty acids versus placebo, Outcome 6: Dropout for any reason at three months
Comparison 5: propranolol versus gabapentin
One study compared propranolol to gabapentin (Stein 2007). In this trial, the available study endpoint corresponds to the review's primary time point of three months.
Primary outcomes
1. PTSD severity
Stein 2007 provided data on PTSD severity at three months after the traumatic event, as assessed with the Post‐traumatic Stress Disorder Checklist ‐ Civilian version (PCL‐C). Data were not reported in sufficient detail for meta‐analysis. The study authors report that propranolol did not differ significantly compared to gabapentin in terms of reduction of PTSD symptoms over time. However, this is based on one small trial, so there are insufficient data to conclude whether there are any differences between groups.
2. Dropouts due to adverse events
Stein 2007 provided data on dropouts due to adverse events. The study authors report that no participant discontinued the assigned medication due to adverse events during the first treatment week.
Secondary outcomes
1. PTSD rate
Stein 2007 provided data on PTSD rate at three months after the traumatic event, as assessed with the Comprehensive International Diagnostic Interview (CIDI) for PTSD. The evidence is inconclusive: it is not possible to determine if there is a clinically important difference between propranolol and gabapentin in reducing the severity of PTSD symptoms (RR 1.25, 95% CI 0.26 to 6.07; NNTH 20, 95% CI NNTB 3 to NNTH 3; 22 participants; very low‐certainty evidence; Analysis 5.1). We downgraded this outcome from high‐ to very low‐certainty evidence because of serious risk of bias and very serious imprecision due to small sample size and wide confidence interval.
5.1. Analysis.

Comparison 5: Propranolol versus gabapentin, Outcome 1: PTSD rate at three months
Three months after the traumatic event
Stein 2007 assessed depression severity with the Center for Epidemiologic Studies Depression Scale (CES‐D) at three months after the traumatic event. Data were not reported. The study had three arms (propranolol, gabapentin, and placebo) and authors report that GEE analyses for both propranolol versus placebo and gabapentin versus placebo did not find statistically significant differences. However, this is based on one small trial; therefore, there are insufficient data to conclude whether there are any differences between groups.
3. Anxiety severity
Stein 2007 did not provide data on anxiety severity.
4. Functional disability
Stein 2007 did not provide data on functional disability.
5. Quality of life
Stein 2007 did not provide data on quality of life.
6. Dropout for any reason
Stein 2007 provided data on dropout for any reason at three months after the traumatic event. It is unclear whether propranolol was less effective in reducing the risk of dropout for any reason compared to gabapentin as these data are based on only one small study, the confidence interval is very wide and includes the null effect (RR 1.03, 95% CI 0.34 to 3.12; NNTH 100, 95% CI NNTB 3 to NNTH 3; 31 participants; Analysis 5.2).
5.2. Analysis.

Comparison 5: Propranolol versus gabapentin, Outcome 2: Dropout for any reason at three months
Comparison 6: gabapentin versus placebo
One study compared gabapentin to placebo (Stein 2007). In this trial, the available study endpoint corresponds to the review's primary time point of three months.
Primary outcomes
1. PTSD severity
Stein 2007 provided data on PTSD severity at three months after the traumatic event, as assessed with PCL‐C. Data were not reported in sufficient detail for meta‐analysis. The study authors report that gabapentin did not differ significantly on the reduction of PTSD symptoms over time compared to placebo. However, this is based on one small trial; therefore, there are insufficient data to conclude whether there are any differences between groups. A GEE analysis found no significant difference (B = ‐0.48, B standard error = 0.85, very low‐certainty evidence). We downgraded this outcome from high‐ to very low‐certainty evidence because of serious risk of bias and very serious imprecision due to small sample size and the confidence interval including both appreciable harm and appreciable benefit.
2. Dropouts due to adverse events
Stein 2007 did not provide data on dropouts due to adverse events.
Secondary outcomes
1. PTSD rate
Stein 2007 provided data on PTSD rate at three months after the traumatic event, as assessed with CIDI for PTSD. The evidence is inconclusive: it is not possible to determine if there is a clinically important difference between gabapentin and placebo in reducing the risk of experiencing PTSD (RR 0.80, 95% CI 0.18 to 3.59; NNTB 20, 95% CI NNTB 3 to NNTH 4; 26 participants; very low‐certainty evidence; Analysis 6.1). We downgraded this outcome from high‐ to very low‐certainty evidence because of serious risk of bias and very serious imprecision due to small sample size and the confidence interval including both appreciable harm and appreciable benefit.
6.1. Analysis.

Comparison 6: Gabapentin versus placebo, Outcome 1: PTSD rate at three months
2. Depression severity
Stein 2007 assessed depression severity with CES‐D at three months after the traumatic event. Data were not reported in sufficient detail for meta‐analysis. The study authors report that a GEE analysis for gabapentin (14 randomised) versus placebo (17 randomised) did not find a statistically significant difference. However, this is based on one small trial; therefore, there are insufficient data to conclude whether there are any differences between groups.
3. Anxiety severity
Stein 2007 did not provide data on anxiety severity.
4. Functional disability
Stein 2007 did not provide data on functional disability.
5. Quality of life
Stein 2007 did not provide data on quality of life.
6. Dropout for any reason
Stein 2007 provided data on dropout for any reason at three months after the traumatic event. It is unclear whether gabapentin increased the risk of dropout for any reason compared to placebo as these data are based on only one small study, the confidence interval is very wide and includes the null effect (RR 4.86, 95% CI 0.61 to 38.65; NNTH 4, 95% CI NNTB 33 to NNTH 2; 31 participants; Analysis 6.2).
6.2. Analysis.

Comparison 6: Gabapentin versus placebo, Outcome 2: Dropout for any reason at three months
Comparison 7: paroxetine versus placebo
One study compared paroxetine to placebo (Borrelli 2019).
Primary outcomes
1. PTSD severity
Three months after the traumatic event
Borrelli 2019 provided data on PTSD severity at three months after the traumatic event, as assessed with PCL‐C. Data were not reported in sufficient detail for meta‐analysis. The study authors report that mean change in scores from baseline was ‐4.0 for paroxetine (60 randomised) and +0.3 for placebo (60 randomised) (very low‐certainty evidence). We downgraded this outcome from high‐ to very low‐certainty evidence because of serious risk of bias and very serious imprecision due to a very small sample size.
Study endpoint
Borrelli 2019 provided data on PTSD severity at study endpoint, as assessed with PCL‐C at six months after the traumatic event. Data were not reported in sufficient detail for meta‐analysis. The study authors report that mean change in scores after baseline were ‐4.4 for paroxetine (60 randomised) and ‐0.5 for placebo (60 randomised) without statistical significance with alpha set at 0.05. The lack of statistical significance is likely due to a small sample size and therefore imprecision. Additionally, a generalised linear mixed model (GLMM) analysis found no significant difference between intervention groups (F = 0.23; denominator degree of freedom = 119, P = 0.873).
2. Dropouts due to adverse events
Borrelli 2019 did not provide data on dropouts due to adverse events.
Secondary outcomes
1. PTSD rate
Three months after the traumatic event
Borrelli 2019 did not provide data on PTSD rate at three months after the traumatic event.
Study endpoint
Borrelli 2019 provided data on PTSD rate at twelve months after the traumatic event, as assessed with the Diagnostic Interview Schedule for DSM‐IV. Data were not reported in sufficient detail for meta‐analysis. The study authors report that the percentage of participants with PTSD did not differ significantly between intervention groups (paroxetine 17.7%, placebo 16.7%; Chi2 = 0.006, df = 1, P = 0.939). Although event rates were very similar in both groups, there is insufficient precision to conclude there was no difference between treatments.
2. Depression severity
Three months after the traumatic event
Borrelli 2019 did not provide data on depression severity at three months after the traumatic event.
Study endpoint
Borrelli 2019 assessed depression severity with the Quick Inventory of Depressive Symptomatology (QIDS‐SR) at eight weeks after the traumatic event. Data were not reported in sufficient detail for meta‐analysis. The study authors report that a Wilcoxon test for difference between baseline and follow‐up scores did not find a significant difference (Wilcoxon S = 1.00, P = 0.219). However, information about statistical significance should be interpreted with caution due to a small sample size.
3. Anxiety severity
Borrelli 2019 did not provide data on anxiety severity.
4. Functional disability
Borrelli 2019 did not provide data on functional disability.
5. Quality of life
Three months after the traumatic event
Borrelli 2019 assessed quality of life at three months after the traumatic event with SF‐36. Data were not reported in sufficient detail for meta‐analysis. The study authors report that mean change in scores from baseline were 11.3 for paroxetine (60 randomised) and 7.6 for placebo (60 randomised). This was judged to be very low‐certainty evidence due to imprecision and risk of bias.
Study endpoint
Borrelli 2019 assessed quality of life at 12 months after the traumatic event with SF‐36. Data were not reported in sufficient detail for meta‐analysis. The study authors report that mean change in scores from baseline were 18.4 for paroxetine and 5.0 for placebo with statistical significance (P ≤ 0.05). However, a GLMM analysis for scores from baseline to 12 months after the traumatic event did not find a significant difference between the paroxetine and placebo. Information about P values should be interpreted with caution as 95% CIs were not reported, there was only one trial with a small sample size.
6. Dropout for any reason
In the Borrelli 2019 trial, data on dropout and number of analysed participants were not reported in sufficient detail to assess dropout rate at three months after the traumatic event or at the study endpoint.
Comparison 8: PulmoCare formula versus Oxepa formula
One study compared PulmoCare formula to Oxepa formula (Kagan 2015).
Primary outcomes
1. PTSD severity
Three months after the traumatic event
Kagan 2015 did not provide data on PTSD severity at three months after the traumatic event.
Study endpoint
Kagan 2015 assessed PTSD severity with the Post‐traumatic Checklist Scale at six months after ICU discharge. Data were not reported in sufficient detail for meta‐analysis. The study authors report that mean scores at follow‐up did not differ significantly between intervention groups (47.03 versus 43.08, P = 0.46). Information about statistical significance should be interpreted with caution as the evidence is based on one relatively small trial and therefore may have reflected a lack of precision.
2. Dropouts due to adverse events
Kagan 2015 did not provide data on dropouts due to adverse events.
Secondary outcomes
1. PTSD rate
Kagan 2015 did not provide data on PTSD rate.
2. Depression severity
Three months after the traumatic event
Kagan 2015 did not provide data on depression severity at three months after the traumatic event.
Study endpoint
Kagan 2015 assessed depression severity with an unspecified 'Depression Scale'. Data were not reported in sufficient detail for meta‐analysis. The study authors report that mean scores at follow‐up did not differ significantly between intervention groups (9.11 versus 7.986, P = 0.33). Information about statistical significance should be interpreted with caution as the evidence is based on one relatively small trial and therefore may have reflected a lack of precision.
3. Anxiety severity
Kagan 2015 did not provide data on anxiety severity.
4. Functional disability
Kagan 2015 did not provide data on functional disability.
5. Quality of life
Kagan 2015 did not provide data on quality of life.
6. Dropout for any reason
Kagan 2015 did not report data on dropout or number of analysed participants in sufficient detail to assess dropout rate at three months after the traumatic event or at the study endpoint.
Comparison 9: 5‐hydroxytryptophan versus placebo
One study compared 5‐hydroxytryptophan to placebo (Tincu 2016)
Primary outcomes
1. PTSD severity
Three months after the traumatic event
Tincu 2016 did not provide data on PTSD severity at three months after the traumatic event.
Study endpoint
Tincu 2016 assessed PTSD severity with CAPS at 14 days. Data were not reported in sufficient detail for meta‐analysis. The study authors report that mean score at follow‐up was significantly higher in the placebo group compared to the 5‐hydroxytryptophan group (P = 0.006). Although the authors report information about P values, this should be interpreted with caution as 95% CIs were not reported and the trial had a very small sample size.
2. Dropouts due to adverse events
Tincu 2016 did not provide data on dropouts due to adverse events.
Secondary outcomes
1. PTSD rate
Tincu 2016 did not provide data on PTSD rate.
2. Depression severity
Tincu 2016 did not provide data on depression severity.
3. Anxiety severity
Tincu 2016 did not provide data on anxiety severity.
4. Functional disability
Tincu 2016 did not provide data on functional disability.
5. Quality of life
Tincu 2016 did not provide data on quality of life.
6. Dropout for any reason
Tincu 2016 did not provide data on dropout for any reason.
Discussion
Summary of main results
During the review process, we identified 13 studies comparing one medication to placebo or one medication to another for the prevention of PTSD. We identified nine comparisons, the majority of which included only one study. As we were expecting a multitude of interventions, we planned a network meta‐analysis. However, all but one of the comparisons were against placebo and the resulting network plot was deemed unfit for an informative network meta‐analysis. We had sufficient data to perform meta‐analysis for two comparisons: hydrocortisone versus placebo and propranolol versus placebo, and found very limited evidence of effectiveness for hydrocortisone only.
In terms of PTSD severity, there is inconsistent evidence that hydrocortisone may be more effective than placebo in decreasing PTSD severity, with one small study reporting a favouring result (Weis 2006), and three not finding a significant difference (Denke 2008; Schelling 2001; Shaked 2019). In the pooled analysis at study endpoint, hydrocortisone performed marginally better than placebo in preventing the development of PTSD, but the 95% CI was remarkably wide and next to the no‐significance point, due to a pooled sample size of 48 participants. We could assess negative outcomes in terms of dropouts for any reason only, and found no difference between active and inactive groups.
There were also very wide 95% CIs in all comparisons of propanolol with placebo for PTSD severity, PTSD prevention, or dropouts due to adverse events or for any reason.
All of the remaining comparisons included only one study. For dexamethasone versus placebo, omega‐3 fatty acids versus placebo, propranolol versus gabapentin, gabapentin versus placebo, paroxetine versus placebo, and PulmoCare formula versus Oxepa formula, we found no significant difference in terms of PTSD prevention or dropout rates. A small trial on 5‐hydroxytryptophan versus placebo reported efficacy at 14 days after ICU admission on PTSD severity, a time frame too short to draw any conclusions on the long‐term effectiveness of the intervention.
Overall completeness and applicability of evidence
The body of evidence from RCTs for universal PTSD prevention remains overall limited and sparse. We included 13 studies spread across nine comparisons. We could pool data for two comparisons only, and the trials informing the other comparisons generally had limited sample sizes. Lack of direct comparisons prevented the performance of a network meta‐analysis. Additionally, the quality of trial conduct and reporting varied greatly and was often low.
Many authors have reported difficulty in performing trials in this population, in particular in recruiting possible participants in emergency department settings. This difficulty could relate to practical aspects; for instance, the embedding of research personnel in the busy emergency department setting. In addition, there is a high likelihood of reluctance on the part of potential participants. Stein 2007 reports that many people declined participation out of the desire to leave the emergency department setting as soon as possible, denial of possible mental health repercussions, or concerns for possible side effects.
Most of the trials addressed the efficacy of the interventions in terms of PTSD severity, PTSD rate or both. Conversely, we found little data on tolerability assessed as dropout rates due to side effects. The rates of dropout due to any reason are less reliable in informing intervention‐associated adverse events, considering that high dropout rates are frequent in prevention trials and for psychiatric trials in general. Limited data were available to assess depressive symptoms and quality of life. Notably, no study reported assessing anxiety severity or functional disability.
We aimed at reviewing outcomes three months after the traumatic event, a time point at which a pharmacological treatment could be initiated if PTSD had developed. However, many trials did not assess outcomes at that time. Study endpoint assessments varied greatly in terms of length from the traumatic event, ranging from a few weeks after the traumatic event to several years after. Data summarised at study endpoints are therefore limited in their clinical applicability (see heterogeneity below).
The included trials considered various settings. A body of trials explored interventions in the context of intensive care units (ICUs). Results from these trials are limited in their applicability to other contexts: the traumatic event consisted of the ICU stay itself or the surgery that participants were undergoing; and the interventions often were primarily intended to address the underlying physical condition and results in the PTSD outcome might reflect a different course of this underlying condition, rather than a direct effect on PTSD development. In the other trials, the traumatic events that led to trial enrolment were variable, covering a rather wide spectrum of possible traumatic events, both intentional and unintentional, with a prevalence of motor vehicle accidents. We did not find any eligible trials that considered large‐scale events, such as acts of war, terrorist attacks, or natural disasters.
Almost all of the trials took place in high‐income countries. However, most of the evidence relates to hydrocortisone and propranolol, two drugs currently listed in the World Health Organization (WHO) essential drug list. As such, they can also be expected to be available in low‐ and middle‐income countries. With the exception of dexamethasone, none of the other interventions are on the WHO essential drug list.
The last search update for this review was undertaken on 13 November 2020. It is therefore possible that additional eligible trials have been published between that date and the publication of this review.
Quality of the evidence
Heterogeneity
Hydrocortisone versus placebo
Clinical and statistical heterogeneity overall were limited. The two studies contributing to the meta‐analysis were from the same research group (Schelling 2001; Weis 2006): both took place in an ICU setting, the traumatic event was a life‐threatening condition in addition to the ICU stay, and the overall designs were similar. Statistical heterogeneity was coherently low.
Propranolol versus placebo
Propranolol‐investigating trials considered participants from emergency departments, except for one study which considered burn centres. Dosing regimes varied in lengths and maximum daily dose. In this context, statistical heterogeneity was low in most outcomes, but in the PTSD severity outcome, a considerable level of heterogeneity was introduced by a single trial (Stein 2007). Considering the small number of included participants, this might be due to chance, as the same heterogeneity was not found for the related PTSD rate outcome.
Other comparisons
As all of the other comparisons rely on single trials, heterogeneity could not be assessed.
Methodological certainty
We used the GRADE domains to assess the quality of the evidence for the review's primary time point of three months after the traumatic event. The two main reasons for downgrading the level of quality were the risk of bias and not meeting the optimal information size. The risk of bias of the body of evidence concerns mainly the attrition and reporting domains (detailed assessments are found in the Characteristics of included studies; Figure 2 and Figure 3 provide a graphic summary). Attrition is a frequent phenomenon in RCTs concerning mental health and prevention. A large number of included participants might counterbalance this issue. In this review, however, most of the trials have small sample sizes, adding to this limitation, and downgrading our confidence in the estimates. Some trials had additional concerns of bias, related to their specific designs: possible mediating effects on PTSD not directly related to the intervention per se (but rather on the course of the ICU stays), or the inclusion of additional informed consent and inclusion criteria after the study randomisation because PTSD outcomes were not originally planned. Overall, the certainty of evidence ranged from very low (further research is very likely to have an important impact on the estimate of effect and is likely to change it), to low (estimate of effect is very uncertain).
Potential biases in the review process
This review followed Cochrane guidelines (Higgins 2011). Two review authors independently screened search results, checked the full texts of studies marked for possible inclusion against inclusion criteria, extracted relevant data, and assessed the risk of bias. We resolved disagreements through discussion or by involving a third review author. We followed Cochrane guidelines in performing the statistical analyses. Two review authors applied the GRADE tool to assess the certainty of the evidence in line with what is suggested by both Cochrane and GRADE. These methods should have minimised the risk of bias in the review process, although some possible issues remain. We could not properly assess the risk of publication bias through funnel plots due to the low numbers of studies per comparison. As some trials on PTSD pharmacological prevention are from specific fields of medicine (e.g. ICU treatments, burn treatments), despite our efforts to systematically search for potentially eligible trials, there is a possibility we overlooked some other RCTs, especially if their primary focus was not on PTSD. We have found limited data concerning adverse events. However, knowledge about the possible adverse effects of the interventions we have reviewed here already exists, and this could mitigate this limitation of the review.
Agreements and disagreements with other studies or reviews
This Cochrane Review, and its parallel review (Bertolini 2020), are the first to consider separately universal and indicated pharmacological prevention for PTSD. Previously published reviews, including Amos 2014, Astill Wright 2019 and Bisson 2021 as the most recent ones, have considered jointly the two types of prevention, and thus the included trials differ on the basis of symptom presentation at baseline. Despite this, and some minor methodological differences, overall, the main results of this review are consistent with those previously reported. Notwithstanding promising theoretical premises, propranolol has not shown efficacy in either PTSD severity or PTSD rate. There is some modest evidence for hydrocortisone in reducing PTSD severity and PTSD rate. However, this evidence comes from specific settings (ICUs) and severely physically ill participants, and its generalisability to other contexts is currently uncertain. Compared to previous reviews, we found three additional trials investigating new comparisons for PTSD prevention (paroxetine, 5‐hydroxytryptophan, enteric formulas), without conclusive evidence of efficacy.
Current guidelines do not recommend routine use of pharmacological intervention for universal prevention of PTSD. The UK's National Institute for Health and Care Excellent (NICE) advises that drugs should not be offered to prevent PTSD (NICE 2018). The USA's Department of Veteran Affairs and Department of Defense guidelines on PTSD do not address universal prevention, but found insufficient evidence to recommend pharmacotherapy for the indicated prevention of people with acute stress disorder (ASD) (Veterans Affairs/Department of Defense 2017). Phoenix Australia guidelines do not list drugs as a possible preventive intervention but recognise a role for hydrocortisone in the research context (Phoenix Australia 2020). The International Society for Traumatic Stress Studies (ISTSS) guidelines list hydrocortisone as a universal intervention, with emerging evidence that it could be considered in people with severe physical illness or injury (ISTSS 2018). The results of this review are consistent with current guidance, as the evidence on the effectiveness of hydrocortisone is limited to severely ill people and is low quality.
Authors' conclusions
Implications for practice.
This review provides uncertain evidence only regarding the use of propranolol, dexamethasone, omega‐3 fatty acids, gabapentin, paroxetine, PulmoCare and Oxepa formula, and 5‐hydroxytryptophan as universal post‐traumatic stress disorder (PTSD) prevention strategies. Whilst we found limited evidence that hydrocortisone may be effective in the prevention of PTSD, the evidence base is limited in quality and there are generalisability concerns. Additionally, although we did not find acceptability differences compared to placebo, tolerability could not be properly assessed, which is a major limitation of current evidence. Hydrocortisone is a well‐known drug, widely employed in other medical fields, for which several side effects are known, including psychiatric ones such as agitation and abnormally elevated mood. Preventive measures, especially universal ones, would require a careful assessment for expected benefit against side effects, particularly given that not all of the possible candidates will develop PTSD.
Implications for research.
Future research might benefit from addressing the current limitations of the evidence base. There is a need for better study reporting, including trial registration on online repositories before study completion, accurate and complete outcome reporting, and assurance of allocation concealment. Although participant enrolment represents a challenge for these trials, larger sample sizes are needed to yield stronger conclusions. They would also allow investigation of whether specific subgroups or trauma events might benefit more from the intervention (e.g. women rather than men, interpersonal trauma rather than non‐interpersonal trauma). As far as possible, assessment and reporting of dropout reasons would better inform the relevance of tolerability, a key aspect for preventions trials. Specific high‐risk populations, such as displaced populations or people exposed to conflict, are not currently represented in the evidence base.
Most of the investigated interventions have been thoroughly investigated in terms of side effects in other research fields. Still, future trials might consider a better reporting of side effects in this specific context. We believe that both potential recipients and clinicians need a robust assessment of possible benefits and side effects to be confident in a pharmacological intervention for universal PTSD prevention.
Rarely has quality of life been assessed in this context, and no study assessed functional disability. Even if these outcomes are somehow related to PTSD severity, their assessment would still provide a more comprehensive picture of the effects of the interventions.
History
Protocol first published: Issue 10, 2019
Acknowledgements
We thank Giovanni Mistraletti for providing additional clarifications on the Mistraletti 2015 trial.
Cochrane Common Mental Disorders (CCMD) supported the authors in the development of this review. We are grateful to the CCMD editorial team for guidance provided during review production.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Robert Boyle, Senior Editor, Cochrane Mental Health and Neuroscience Network, and Imperial College London; Nuala Livingstone, Associate Editor, Cochrane Mental Health and Neuroscience Network
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Jessica Hendon, CCMD, Centre for Reviews and Dissemination, University of York
Information specialist (developed the search strategies, provided editorial guidance to authors, edited the article): Sarah Dawson, CCMD & University of Bristol
Peer‐reviewers (provided comments and recommended an editorial decision): Natalie Simon, Cardiff and Vale University Health Board, Cardiff (clinical/content review); Myfanwy J Williams (consumer review); Hector Pardo‐Hernandez, Iberoamerican Cochrane Centre, World Health Organization (methods review); Gillian Worthy, Freelance (methods review).
Copy Editor (copy‐editing and production): Faith Armitage
The authors and the CCMD Editorial Team are grateful to the peer reviewers for their time and comments. They would also like to thank Cochrane Copy Edit Support for the team's help.
Cochrane Group funding acknowledgement: the UK National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: the views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health and Social Care.
Appendices
Appendix 1. CCMDCTR (core MEDLINE search)
Core search strategy used to inform specialised register: Ovid MEDLINE (1946 to June 2016)
A weekly search alert based on condition + RCT filter only
1. [MeSH Headings]:
eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/2. [Title/ Author Keywords]:
(eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.3. [RCT filter]:
(controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)4. (1 and 2 and 3)Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record.
Similar weekly search alerts are also conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. CCMD Editorial Base search strategy
In March 2018, CCMD's Information Specialist (Chris Cooper) ran a search for all PTSD studies (treatment or prevention, RCTs, condition only) on the main biomedical databases listed below. This was to account for the period when the CCMDCTR was out of date and to cover all PTSD reviews within the scope of CCMD.
Search results were deduplicated and screened in Covidence. Each record was screened by at least two members of the CCMD editorial base staff.
Inclusion criteria
Any RCT for the treatment of PTSD (irrespective of intervention, age group or comorbidity)
Any RCT which might be seen as a PTSD prevention study
Any RCT for critical incident stress debriefing (CISD) (simulated crises not included)
Any RCT for debriefing after psychological trauma or any stress resilience studies
Any CCT where the treatment allocation is ambiguous
Corrigendums, errors, retractions or substantial comments relating to the above
Exclusion criteria
All systematic reviews and meta‐analyses
Healthy populations
Simulated crises (e.g. for staff training in accident and emergency)
RCTs which fall outside the scope of CCMD, e.g. serious mental illness (schizophrenia), borderline personality disorder, alcohol use disorder (e.g. brief alcohol intervention in accident and emergency department), smoking cessation, traumatic brain injury, fibromyalgia (unless the comorbidity clearly fell within the scope of the search and was an outcome of the trial).
| Databases | Hits |
| MEDLINE | 1742 |
| Embase | 3319 |
| CENTRAL | 2028 |
| PsycINFO | 1449 |
| PILOTS | 879 |
| Total | 9417 |
| ‐duplicates | ‐4635 |
| Studies to screen | 4782 |
| Search date | 3 Mar 18 |
1. Cochrane Central Register of Controlled Trials (CENTRAL) Host: Wiley interface Data Parameters: Cochrane Central Register of Controlled Trials: Issue 2 of 12, February 2018 Date Searched: Monday, 3 March 2018 Searched by: Chris Cooper Hits: 2028 ID Search Hits #1 MeSH descriptor: [Stress Disorders, Post‐Traumatic] this term only 1492 #2 (PTSD or ((posttrauma* or post‐trauma* or post trauma*) near/3 (stress* or disorder* or psych* or symptom*)) or acute stress disorder* or combat disorder* or war neuros*) 5065 #3 (((acute or traumatic) near/1 stress*) and (expos* or psyc*)) 1525 #4 (traumatised near/1 (victim* or survivor*)) 2 #5 (traumatized near/1 (victim* or survivor*)) 4 #6 (trauma* near/2 (event* or memor* or flashback* or nightmare*)) 553 #7 ((trauma* or posttrauma* or post‐trauma* or victim* or survivor*) and (exposure near/3 (therap* or psychotherap* or training or counsel*))) 417 #8 MeSH descriptor: [Crisis Intervention] this term only 166 #9 (critical incident near/1 (stress or debrief* or de‐brief*)) 24 #10 (debriefing or de‐briefing) 328 #11 (crisis intervention* or CISD) 1003 #12 ((stress or group* or psychological or crisis) near/3 (debrief* or de‐brief*)) 107 #13 (trauma* near/2 (event* or memor* or flashback* or nightmare*)) 553 #14 (EMDR or (eye movement desensitization and reprocessing)) 225 #15 (EMDR or (eye movement desensitisation and reprocessing)) 197 #16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 Publication Year from 2014 to 2018 2893 Notes: N/A File: VO1 CENTRAL n2028.txt
2. Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present Host: OVID Data Parameters: 1946‐Current (date limits applied, 2014 onwards) Date Searched: Monday, 3 March 2018 Searched by: Chris Cooper Hits: 1742
| # | Searches | Results |
| 1 | Stress Disorders, Post‐Traumatic/ | 27503 |
| 2 | (PTSD or ((posttrauma* or post‐trauma* or post trauma*) adj3 (stress* or disorder* or psych* or symptom?)) or acute stress disorder* or combat disorder* or war neuros*).ti,ab,kf,kw,id. | 31111 |
| 3 | (((acute or traumatic) adj stress*) and (expos* or psyc*)).ti,ab,kf,kw,id. | 10567 |
| 4 | (traumati#ed adj (victim? or survivor?)).ti,ab,kf,kw,id. | 34 |
| 5 | (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab,kf,kw,id. | 8174 |
| 6 | ((trauma* or posttrauma* or post‐trauma* or victim* or survivor?) and (exposure adj3 (therap* or psychotherap* or training or counsel*))).ti,ab,kf,kw,id,hw. | 901 |
| 7 | Crisis Intervention/ | 5457 |
| 8 | (critical incident adj (stress or debrief* or de‐brief*)).ti,ab,kf,kw,id. | 223 |
| 9 | (debriefing or de‐briefing).ti,kf,kw,id. | 577 |
| 10 | (crisis intervention? or CISD).ti,ab,kf,kw,id. | 1744 |
| 11 | ((stress or group? or psychological or crisis) adj3 (debrief* or de‐brief*)).ti,ab,kf,kw,id. | 406 |
| 12 | (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,kf,kw,id. | 1150 |
| 13 | (EMDR or (eye movement desensiti#ation and reprocessing)).ti,ab,kf,kw,id,sh. | 510 |
| 14 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 52168 |
| 15 | randomized controlled trial.pt. | 454849 |
| 16 | controlled clinical trial.pt. | 92204 |
| 17 | randomized.ab. | 404382 |
| 18 | placebo.ab. | 186843 |
| 19 | clinical trials as topic.sh. | 182777 |
| 20 | randomly.ab. | 285994 |
| 21 | trial.ti. | 178689 |
| 22 | 15 or 16 or 17 or 18 or 19 or 20 or 21 | 1136215 |
| 23 | 14 and 22 | 4000 |
| 24 | (2014* or 2015* or 2016* or 2017* or 2018*).yr,dt,ed,ep. | 5444042 |
| 25 | 23 and 24 | 1742 |
Notes: N/A File: VO1 MEDLINE n1742.txt
3. Embase Host: OVID Data Parameters: 1974 to 2 March 2018 (date limits applied, 2014 onwards) Date Searched: Monday, 3 March 2018 Searched by: Chris Hits: 3319 Search Strategy:
| # | Searches | Results |
| 1 | posttraumatic stress disorder/ | 48854 |
| 2 | "trauma and stressor related disorders"/ | 34962 |
| 3 | combat disorders/ | 26663 |
| 4 | psychological trauma/ | 5351 |
| 5 | stress disorders, post‐traumatic/ | 16743 |
| 6 | stress disorders, traumatic, acute/ | 751 |
| 7 | (PTSD or ((posttrauma* or post‐trauma* or post trauma*) adj3 (stress* or disorder* or psych* or symptom?)) or acute stress disorder* or combat disorder* or war neuros*).ti,ab,kw. | 39945 |
| 8 | (((acute or traumatic) adj stress*) and (expos* or psyc*)).ti,ab,kw. | 15122 |
| 9 | (traumati#ed adj (victim? or survivor?)).ti,ab,kw. | 51 |
| 10 | (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab,kw. | 10514 |
| 11 | (EMDR or (eye movement desensiti#ation and reprocessing)).ti,kw. | 527 |
| 12 | ((trauma* or posttrauma* or post‐trauma* or victim* or survivor?) and (exposure adj3 (therap* or psychotherap* or training or counsel*))).ti,ab,kw. | 1096 |
| 13 | (critical incident adj (stress or debrief* or de‐brief*)).ti,ab,kw. | 275 |
| 14 | (debriefing or de‐briefing).ti,ab,kw. | 4133 |
| 15 | (crisis intervention? or CISD).ti,ab,kw. | 2273 |
| 16 | ((stress or group? or psychological or crisis) adj3 (debrief* or de‐brief*)).ti,ab,kw. | 602 |
| 17 | (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab,kw. | 10514 |
| 18 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 | 74063 |
| 19 | crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ or (random* or factorial* or crossover* or cross over* or placebo* or (doubl* adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer*).tw. | 1970074 |
| 20 | 18 and 19 | 7601 |
| 21 | (2014* or 2015* or 2016* or 2017* or 2018*).yr,dc. | 7084132 |
| 22 | 20 and 21 | 3319 |
Notes: N/A File: VO1 Embase n3319.txt
4. PsycINFO Host: OVID Data Parameters: 1806 to February Week 4 2018 (date limits applied, 2014 onwards) Date Searched: Monday, 3 March 2018 Searched by: Chris Cooper Hits: 1449 Search Strategy:
| # | Searches | Results |
| 1 | posttraumatic stress disorder/ or complex ptsd/ or desnos/ or acute stress disorder/ or combat experience/ or "debriefing (psychological)"/ or emotional trauma/ or post‐traumatic stress/ or exp stress reactions/ or traumatic neurosis/ | 50806 |
| 2 | exp disasters/ | 8186 |
| 3 | (PTSD or ((posttrauma* or post‐trauma* or post trauma*) adj3 (stress* or disorder* or psych* or symptom?)) or acute stress disorder* or combat disorder* or war neuros*).ti,ab. | 38985 |
| 4 | (((acute or traumatic) adj stress*) and (expos* or psyc*)).ti,ab. | 16755 |
| 5 | (traumati#ed adj (victim? or survivor?)).ti,ab. | 68 |
| 6 | (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab. | 11819 |
| 7 | (EMDR or (eye movement desensiti#ation and reprocessing)).ti,ab. | 1640 |
| 8 | ((trauma* or posttrauma* or post‐trauma* or victim* or survivor?) and (exposure adj3 (therap* or psychotherap* or training or counsel*))).ti,ab. | 1086 |
| 9 | crisis intervention/ | 3314 |
| 10 | (critical incident adj (stress or debrief* or de‐brief*)).ti,ab. | 443 |
| 11 | (debriefing or de‐briefing).ti,ab. | 2186 |
| 12 | (crisis intervention? or CISD).ti,ab. | 3505 |
| 13 | ((stress or group? or psychological or crisis) adj3 (debrief* or de‐brief*)).ti,ab. | 596 |
| 14 | (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab. | 11819 |
| 15 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 | 80813 |
| 16 | clinical trials.sh. | 10820 |
| 17 | (randomi#ed or randomi#ation or randomi#ing).ti,ab,id. | 72509 |
| 18 | (RCT or at random or (random* adj3 (assign* or allocat* or control* or crossover or cross‐over or design* or divide* or division or number))).ti,ab,id. | 82020 |
| 19 | (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,id,hw. | 25590 |
| 20 | ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,id. | 24054 |
| 21 | trial.ti. | 25583 |
| 22 | placebo.ti,ab,id,hw. | 37267 |
| 23 | treatment outcome.md. | 18762 |
| 24 | treatment effectiveness evaluation.sh. | 21858 |
| 25 | mental health program evaluation.sh. | 2028 |
| 26 | 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 | 169119 |
| 27 | 15 and 26 | 4124 |
| 28 | (2014* or 2015* or 2016* or 2017* or 2018*).yr,dc,mo. | 782907 |
| 29 | 27 and 28 | 1449 |
Notes: N/A File: VO1 PsycINFO n1449.txt
5.PILOTS: Published International Literature On Traumatic Stress Host: Pro Quest Data Parameters: 1871‐Current (date limits applied, 2014 onwards) Date Searched: Monday, 3 March 2018 Searched by: Chris Cooper Hits: 879 Search Strategy
Set#: S1 Searched for: ti((posttrauma* near/4 (stress* or disorder* or psych* or symptom*))) OR ab((posttrauma* near/4 (stress* or disorder* or psych* or symptom*))) Results: 16999* Set#: S2 Searched for: ti((post‐trauma* near/4 (stress* or disorder* or psych* or symptom*))) OR ab((post‐trauma* near/4 (stress* or disorder* or psych* or symptom*))) Results: 6647° Set#: S3 Searched for: ti((post trauma* near/4 (stress* or disorder* or psych* or symptom*))) OR ab((post trauma* near/4 (stress* or disorder* or psych* or symptom*))) Results: 7214° Set#: S4 Searched for: ti((PTSD or acute stress disorder* or combat disorder* or war neuros*) ) OR ab((PTSD or acute stress disorder* or combat disorder* or war neuros*) ) Results: 30435* Set#: S5 Searched for: ti((((acute or traumatic) near/2 stress*) and (expos* or psyc*)) ) OR ab((((acute or traumatic) near/2 stress*) and (expos* or psyc*)) ) Results: 2341° Set#: S6 Searched for: ti((traumatised near/2 (victim* or survivor*)) ) OR ab((traumatised near/2 (victim* or survivor*)) ) Results: 84° Set#: S7 Searched for: ti((trauma* near/3 (event* or memor* or flashback* or nightmare*)) ) OR ab((trauma* near/3 (event* or memor* or flashback* or nightmare*)) ) Results: 6974° Set#: S8 Searched for: ti(((trauma* or posttrauma* or post‐trauma* or victim* or survivor*) and (exposure near/4 (therap* or psychotherap* or training or counsel*))) ) OR ab(((trauma* or posttrauma* or post‐trauma* or victim* or survivor*) and (exposure near/4 (therap* or psychotherap* or training or counsel*))) ) Results: 787° Set#: S9 Searched for: ti((critical incident near/2 (stress or debrief* or de‐brief*)) ) OR ab((critical incident near/2 (stress or debrief* or de‐brief*)) ) Results: 385° Set#: S10 Searched for: ti((debriefing or de‐briefing)) OR ab((debriefing or de‐briefing)) Results: 685° Set#: S11 Searched for: ti((crisis intervention* or CISD)) OR ab((crisis intervention* or CISD)) Results: 784° Set#: S12 Searched for: ti(((stress or group* or psychological or crisis) near/4 (debrief* or de‐brief*)) ) OR ab(((stress or group* or psychological or crisis) near/4 (debrief* or de‐brief*)) ) Results: 464° Set#: S13 Searched for: ti((trauma* near/3 (event* or memor* or flashback* or nightmare*)) ) OR ab((trauma* near/3 (event* or memor* or flashback* or nightmare*)) ) Results: 6974° Set#: S14 Searched for: ti((EMDR or (eye movement desensitisation and reprocessing))) OR ab((EMDR or (eye movement desensitisation and reprocessing))) Results: 888° Set#: S15 Searched for: ti((EMDR or (eye movement desensitiZation and reprocessing))) OR ab((EMDR or (eye movement desensitiZation and reprocessing))) Results: 888° Set#: S16 Searched for: (s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15) Results: 36840* Set#: S17 Searched for: MAINSUBJECT.EXACT("Randomized Clinical Trial") Results: 1210° Set#: S18 Searched for: ab((randomized or randomised or placebo or randomly)) Results: 2931° Set#: S19 Searched for: ti(trial) Results: 784° Set#: S20 Searched for: (S17 or S18 or S19) Results: 3226° Set#: S21 Searched for: S16 and s20 Results: 2654° Set#: S22 Searched for: (S16 and s20) AND pd(20140101‐20180301) Results: 879°
* Duplicates are removed from your search, but included in your result count. ° Duplicates are removed from your search and from your result count.
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
PTSD update search (15 March 2019):
• CLib:CENTRAL (Issue 3 of 12, March 2019, date limited 2018 onwards), n=514 (116 of these are from ClinicalTrials.gov) • Ovid MEDLINE (2018 to 15‐Mar‐2019), n=599 • Ovid Embase (2018 to 15‐Mar‐2019), n=1035 • Ovid PsycINFO (2018 to 15‐Mar‐2019), n=445 • Proquest PTSDpubs, (2018‐03‐01 to 2019‐03‐15) n=197 Total=2790 Duplicates removed, n=1178 Records screened by CCMD editorial base staff n=1612
RCTs, n=781
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
PTSD update search (13 November 2020)
PTSD ‐ 2020 update summary (results retrieved per database) • CLib:CENTRAL (Issue 11 of 12, November 2020, date limited 2019 onwards), n=2748 [1288 of these are from ClinicalTrials.gov] • Ovid MEDLINE (2019 to 13‐Nov‐2020), n=1019 • Ovid Embase (2019 to 2020 Week 46), n=1984 • Ovid PsycINFO (2019 to 2020 November Week 2), n=849 • Proquest PTSDpubs, (2019 to 13‐Nov‐2020) n=194 Total=6794 Duplicates removed within this batch, n=2434 Duplicates removed from previous update search (March 2019), n=607 Records screened by CCMD editorial base staff, n=3753 [Covidence identified 6 further duplicates]
RCTs, n=1427
Data and analyses
Comparison 1. Hydrocortisone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 PTSD severity at studies' endpoint | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 4.36 [‐0.71, 9.43] |
| 1.2 PTSD rate at studies' endpoint | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.96] |
| 1.3 Dropout for any reason at studies' endpoint | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.49, 3.89] |
| 1.4 Sensitivity analysis: PTSD rate at studies' endpoint (cases out of randomised) | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.92] |
Comparison 2. Propranolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 PTSD severity at three months | 3 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.61, 0.59] |
| 2.1.1 Intervention started within 12 hours from the traumatic event | 2 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.46, 0.58] |
| 2.1.2 Intervention started after 12 hours from the traumatic event | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐2.62, ‐0.83] |
| 2.2 PTSD rate at three months | 3 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.31, 1.92] |
| 2.3 Dropout for any reason at three months | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.77, 3.01] |
| 2.4 PTSD severity at studies' endpoint | 4 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.16, 0.32] |
| 2.4.1 Intervention started within 12 hours from the traumatic event | 2 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.46, 0.58] |
| 2.4.2 Intervention started after 12 hours from the traumatic event | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐2.42, 0.56] |
| 2.5 Dropout due to adverse events at studies' endpoint | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 5.21 [0.26, 102.98] |
| 2.6 PTSD rate at studies' endpoint | 4 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.51] |
| 2.7 Dropout for any reason at studies' endpoint | 4 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.95, 3.48] |
| 2.8 Sensitivity analysis: PTSD rate at three months (cases out of randomised) | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.24, 1.59] |
| 2.9 Sensitivity analysis: PTSD rate at studies' endpoint (cases out of randomised) | 4 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.28, 1.24] |
Comparison 3. Dexamethasone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 PTSD rate at studies' endpoint | 1 | 1125 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.56, 1.12] |
| 3.2 Dropout for any reason at studies' endpoint | 1 | 1244 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.66, 1.31] |
Comparison 4. Omega‐3 fatty acids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 PTSD severity at three months | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 1.56 [‐4.06, 7.18] |
| 4.2 Dropout due to adverse events at three months | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.3 PTSD rate at three months | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.23, 26.09] |
| 4.4 Depression severity at three months | 1 | 106 | Mean Difference (IV, Random, 95% CI) | 1.82 [‐1.65, 5.29] |
| 4.5 Quality of life at three months | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐7.40, 1.40] |
| 4.6 Dropout for any reason at three months | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [0.96, 19.35] |
Comparison 5. Propranolol versus gabapentin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 PTSD rate at three months | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.26, 6.07] |
| 5.2 Dropout for any reason at three months | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.34, 3.12] |
Comparison 6. Gabapentin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 PTSD rate at three months | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.18, 3.59] |
| 6.2 Dropout for any reason at three months | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 4.86 [0.61, 38.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Borrelli 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, double‐blind Number of centres: 1 Primary location: Dallas, Texas, USA Locations other than primary: none Number of arms: 2 (paroxetine and placebo) Follow‐up time point(s): post‐injury week 6, months 3, 6 and 12 Imputational strategy: generalised linear mixed models Original study outcomes (name, measure, time points): PTSD and major depression rates (Diagnostic Interview Schedule for DMS‐IV) at 6 and 12 months; PTSD symptoms level (PTSD Checklist for DSM‐IV (PCL)); general health (SF‐36) and musculoskeletal function (Short Musculoskeletal Functional Assessment (SMFA)) at week 6, months 3, 6 and 12; level of depression symptoms (Quick Inventory of Depressive Symptomatology (QIDS‐SR)) at 3 months Total duration of study: three years, beginning in March 2010 |
|
| Participants |
Sample size: 120 Baseline characteristics Overall
Baseline group differences: no baseline group differences for PCL scores, PCL avoidance and numbing scores, SF‐36 scores or SMFA scores Inclusion criteria: admitted to level I trauma centre with an orthopaedic injury and an Injury Severity Score (ISS) of more than 8 Exclusion criteria: (1) cognitive problems precluding informed consent; (2) closed head injury with a Glasgow Coma Scale score of less than 13; (3) pre‐existing psychiatric disorder at the time of injury; (4) current pregnancy; and (5) current use of triptans, tricyclic antidepressants, monoamine oxidase inhibitors, fentanyl, lithium, tramadol, tryptophan, buspirone, thioridazine, St. John's wort, or warfarin; and (6) current incarceration |
|
| Interventions |
Setting: urban level I trauma centre ‐ Parkland Memorial Hospital Trauma Service Intervention characteristics Paroxetine
Placebo
|
|
| Outcomes |
PTSD severity
PTSD rate
Depression severity
Quality of life
|
|
| Identification |
Sponsorship source: funded by the Orthopedic Trauma Association Country: USA Author's name: Joseph Borrelli Institution: BayCare Medical Group, Lutz, FL Email: jborthodoc58@gmail.com Address: 4211 Van Dyke Rd, Suite 200 Lutz, FL 33558, USA |
|
| Declarations of interest among primary researchers | Study authors report no conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computerized randomization program, study participants were selected to receive paroxetine (n = 60) or treatment as usual (n = 60) starting at the 2‐week postinjury appointment. Patients received either paroxetine or a placebo according to a double‐blinded randomization schedule" (p. e59). |
| Allocation concealment (selection bias) | Unclear risk | See quote above: no details are provided regarding the allocation concealment strategy. Baseline characteristics are not reported in sufficient detail to assess a possible problem with randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients received either paroxetine or a placebo according to a double‐blind randomization schedule" (p. e59). Comment: likely done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See quote and comment above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Only 18.3% of the sample was completely lost to follow‐up, with 19.2% (23/120) of the sample providing full follow‐up data for all assessment points" (p. e60). Comment: dropouts are not reported in sufficient detail to asses attrition on a per‐outcome basis. |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration entry was not available for this trial. |
| Other bias | Low risk | No conflicts of interest reported. No other sources of bias found |
Denke 2008.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, double‐blind Number of centres: unclear Primary location: unclear (Germany) Locations other than primary: unclear Number of arms: 2 (hydrocortisone and placebo) Follow‐up time point(s): 1 year after discharge from ICU Imputational strategy: no Original study outcomes (name, measure, time points): mental disorders (SKID 1, BDI); PTSD (PTSS‐10, KPS); HRQoL (SF‐36); assessed 1 year after discharge from ICU Total duration of study: not reported |
|
| Participants |
Sample size: 18/84 participants from original study interviewed (44 dead, 20 declined participation, 2 dropouts) Baseline characteristics: no baseline characteristics provided Type of traumatic event: ICU stay for septic shock Baseline group differences: no information on baseline group differences provided Inclusion criteria: all 4 required: (1) Clinical evidence of infection within the previous 72 hours with either presence of polymorphonuclear cells in a normally sterile body fluid (excluding blood); culture or Gram stain of blood, sputum, urine or normally sterile body fluid positive for a pathogenic micro‐organism; focus of infection identified by visual inspection (e.g. ruptured bowel with the presence of free air or bowel contents in the abdomen found at the time of surgery, wound with purulent drainage); or other clinical evidence of infection – treated community‐acquired pneumonia, purpura fulminans, necrotising fascitis, etc. (2) Evidence of a systemic response to infection defined by the presence of two or more of the following signs within the previous 24 hours: fever (temperature > 38.3°C) or hypothermia (rectal temperature < 35.6°C); tachycardia (heart rate of > 90 beats/min); tachypnoea (respiratory rate > 20 breaths/min, PaCO2 < 32 mmHg) or need for invasive mechanical ventilation; alteration of the white cell count > 12,000 cells/mm, < 4000 cells/mm or > 10% immature neutrophils (bands). (3) Evidence of shock within the previous 72 hours (originally within the previous 24 hours) defined by (both A + B required): 1A. a systolic blood pressure (SBP) < 90 mmHg or a decrease in SBP of more than 50 mmHg from baseline in previous hypertensive patients (for at least one hour) despite adequate fluid replacement OR need for vasopressors for at least one hour (infusion of dopamine ≥ 5 mcg/kg/min or any dose of epinephrine, norepinephrine, phenylephrine or vasopressin) to maintain a SBP ≥ 90 mmHg; and B. Hypoperfusion or organ dysfunction attributable to sepsis, including one of: 1. Sustained oliguria (urine output < 0.5 ml/kg/hr for a minimum of 1 hour); 2. Metabolic acidosis [pH of < 7.3, or arterial base deficit of ≥ 5.0 mmol/L, or an increased lactic acid concentration (> 2 mmol/L)]; 3. Arterial hypoxaemia (PaO2/FIO2 < 280 in the absence of pneumonia) (PaO2/FIO2 < 200 in the presence of pneumonia); 4. Thrombocytopenia – platelet count ≤ 100,000 cells/mm3; 5. Acute altered mental status (Glasgow Coma Scale < 14 or acute change from baseline). (4) Informed consent according to local regulations.* Patients had to be hypotensive or receiving vasopressors at the time of enrolment. Exclusion criteria: (1) Pregnancy. (2) Age < 18 years. (3) Underlying disease with a prognosis for survival < 3 months. (4) Cardiopulmonary resuscitation within 72 hours before enrolment. (5) Drug‐induced immunosuppression, including chemotherapy or radiation therapy within 4 weeks before the study. (6) Administration of chronic corticosteroids in the last 6 months or acute steroid therapy (any dose) within 4 weeks (including inhaled steroids). (7) Human immunodeficiency virus (HIV) positivity. (8) Presence of an advanced directive to withhold or withdraw life‐sustaining treatment (i.e. do not resuscitate (DNR)). (9) Advanced cancer with a life expectancy < 3 months. (10) Acute myocardial infarction or pulmonary embolus. (11) Another experimental drug study within the last 30 days. (12) Moribund people likely to die within 24 hours. (13) People in the ICU > 2 months. |
|
| Interventions |
Setting: intensive care units Intervention characteristics Hydrocortisone
Placebo
|
|
| Outcomes |
PTSD rate
PTSD severity
Quality of life
|
|
| Identification |
Sponsorship source: supported by a contract (QLK2‐CT‐2000‐00589) from the European Commission, the European Society of Intensive Care Medicine, the European Critical Care Research Network, the International Sepsis Forum, and the Gorham Foundation. Roche Diagnostics provided the Elecsys cortisol immunoassay. Country: Germany Comments: this is a sub‐study of the CORTICUS study, a trial on hydrocortisone for septic shock. Author's name: Claudia Denke Institution: Charité Universitaetsmedizin Berlin Address: Berlin, Germany |
|
| Declarations of interest among primary researchers | Not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization (in a 1:1 ratio) was stratified according to study center in blocks of four with the use of a computerized random‐number generator list provided by a statistician who was not involved in the determination of eligibility, administration of a study drug, or an assessment of outcomes” (Sprung 2008, p. 112). |
| Allocation concealment (selection bias) | Low risk | Quote: “In each center, the study drug (hydrocortisone or placebo) was sealed in sequentially numbered, identical boxes that contained the entire treatment for each patient to be administered sequentially. The sequence was concealed from the investigators” (Sprung 2008, p. 112). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “All patients, medical and nursing staff members, pharmacists, investigators, and members of the monitoring board remained unaware of study‐group assignments throughout the study period” (Sprung 2008, p. 112). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See quote above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 21.4% (14/84) of the participants completed the study. |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration entry was not available for this trial. |
| Other bias | Unclear risk | PTSD‐related outcomes are reported only in a conference abstract. |
Hoge 2012.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group Number of centres: 1 Primary location: Boston, MA, USA Locations other than primary: none Number of arms: 2 (propranolol and placebo) Follow‐up time point(s): 4, 5, 12 and 13 weeks Imputational strategy: no Original study outcomes (name, measure, time points): PTSD severity and rate as assessed by the Clinician‐Administered PTSD Scale (CAPS) Total Score, 4 and 12 weeks after trauma; physiological posterior probability of PTSD as assessed by the Psychophysiologic Responses During Script‐Driven Mental Imagery at 5 and 13 weeks after trauma Total duration of study: about four years (recruitment took place from September 2004 to May 2008) |
|
| Participants |
Sample size: 43 Baseline characteristics Propranolol
Placebo
Baseline group differences: there were no significant differences in age, gender, peri‐traumatic emotional distress level, hours elapsed from traumatic event to first dose of study medication, heart rate prior to or 90 minutes after first dose of study medication, or medication adherence between the two treatment groups. Inclusion criteria: traumatic event that met DSM‐IV PTSD Criteria A.1 and A.2; both genders, aged 18 to 65. Occurence of the traumatic event no more than 12 hours before first dose of medication (originally the permissible time from event to first medication dose was 4 hours; this criterion was amended in order to obtain more candidates, and an additional criterion of heart rate above 80 bpm was dropped for the same purpose). Exclusion criteria: physical injury that would complicate participation, hospital stay longer than overnight, head injury with loss of consciousness, a medical condition that contraindicated the administration of propranolol, use of medications with potentially dangerous interactions with propranolol, previous adverse reaction to a beta blocker, blood alcohol concentration above 0.02% or presence of substances of abuse on saliva testing, pregnancy, traumatic event reflecting ongoing victimisation, contraindicating psychiatric condition such as psychotic, bipolar, major depressive, or post‐traumatic stress disorder from another event, suicidality or homicidality, unwillingness or inability to come to Boston for the research visits, or treating physician did not concur with enrolment in the study |
|
| Interventions |
Setting: Emergency Department at the Massachusetts General Hospital in Boston Intervention characteristics Propranolol
Placebo
|
|
| Outcomes |
PTSD severity
PTSD rate
Dropout for any reason
|
|
| Identification |
Sponsorship source: NIMH grant #MH068603 to Roger K Pitman Country: USA Comments: 2 participants (1 from each arm) dropped out after randomisation and before starting medication: trial authors have excluded them from all of the analysis Author's name: Elizabeth A. Hoge Institution: Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA Email: ehoge@partners.org Address: Elizabeth A. Hoge, MD, Massachusetts General Hospital, One Bowdoin Square, 6th Flr, Boston |
|
| Declarations of interest among primary researchers | Study authors report no conflict of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Following screening, each participant was randomized to receive an initial oral dose of either 40 mg short‐acting propranolol or placebo" (p. 22). Comment: no details provided regarding the generation of the random sequence. Baseline characteristics are balanced between groups and do not suggest a possible problem with randomisation |
| Allocation concealment (selection bias) | Unclear risk | See quote above: no details provided regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No details on blinding strategy and its effectiveness are provided within the paper. However, a previous meta‐analysis reports a personal correspondence with the lead author (Amos 2014), confirming blinding of participants, personnel and assessors through use of blinded medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates for the 3‐month outcomes: propranolol arm: 6/22, 27.7%; placebo arm: 3/21 14.3%. Although there is some imbalance, considering the small sample sizes, these figures have likely not resulted in biased outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available for this trial. Original outcomes listed on clinicaltrials.gov (NCT00158262), were submitted on September 2005 whilst the trial was already being carried out. Outcomes specified within the methods section of the paper are reported. |
| Other bias | Low risk | No other source of bias was identified for this trial. |
Kagan 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, double‐blind Number of centres: 1 Primary location: Israel Locations other than primary: none Number of arms: 2 Follow‐up time point(s): 6 months after ICU discharge Imputational strategy: no Original study outcomes (name, measure, time points): Post Traumatic Check List Scale, Hospital Anxiety (unspecified 'established questionnaire') and depression scale (unspecified 'established questionnaire'), Rivermead Post Concussion symptoms questionnaire for head trauma patients, Rivermead Post Concussion symptoms questionnaire for head trauma patients and Revised Illness Perceptions Questionnaire at 6 months Total duration of study: 44 months |
|
| Participants |
Sample size: 150 participants originally included, 52 interviewed (98 excluded due to death within 6 months, inability to communicate, declined to participate, unreachable or organisational reason) Baseline characteristics Overall
Baseline group differences: no information on baseline group differences provided Inclusion criteria: people between the ages of 18 and 90 years with a diagnosis of multiple trauma, defined as physical insults or injuries occurring simultaneously in more than one part of the body, or of isolated head trauma, which required mechanical ventilation and had an anticipated ICU stay of ≥ 2 days Exclusion criteria: presence of any contraindication for commencing enteral nutrition (EN) within the first 36 hours of ICU admission, such as mechanical or functional small bowel obstruction, high‐output fistula, gastrointestinal tract discontinuity and/or surgeon reluctance to commence EN immediately following abdominal surgery; treatment with immunosuppressive drugs; second‐/third‐degree burns covering > 66% of body surface area; pregnancy |
|
| Interventions |
Setting: general ICU of the Rabin Medical Center, Petah Tikva, Israel Intervention characteristics Enteral nutrition enriched with eicosapentaenoic acid, gamma‐linolenic acid and antioxidants (PulmoCare formula)
High‐fat, low‐carbohydrate enteral formula (Oxepa formula)
|
|
| Outcomes |
PTSD severity
Depression severity
Dropout for any reason
|
|
| Identification |
Sponsorship source: Israeli Ministry of Security Country: Israel Comments: PTSD outcomes are reported in a conference abstract but not within the main publication or registration entry at ClinicalTrials.gov. Original trial paper reports 120 randomised, abstract on PTSD reports 150 and does not specify how many to each arm. Author's name: Ilya Kagan Institution: Department of General Intensive Care, Institute for Nutrition Research, Rabin Medical Center, Petah Tikva, Israel Email: psinger@clalit.org.il Address: Department of General Intensive Care, Institute for Nutrition Research, Rabin Medical Center, 49100 Petah Tikva, Israel |
|
| Declarations of interest among primary researchers | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was achieved using a computer‐based block randomization generated by a statistical software program which was concealed to all investigators apart from the principal investigator (PS)” (Kagan 2015b, p. 462). |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The two feeds were decanted from their commercial packaging and presented at the bedside in a blinded manner. All healthcare workers involved in the daily care of the patients were blinded to the type of EN administered” (Kagan 2015b, p. 462). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether outcome assessment was conducted by blinded investigators as this is not explicitly stated in the paper and the principal investigator is instead described as unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 52 out of 150 randomised participants were interviewed. |
| Selective reporting (reporting bias) | High risk | The PTSD, depression and anxiety outcomes were not predefined within the original trial or trial registration at clinicaltrials.gov (NCT01099501). |
| Other bias | Unclear risk | There is some inconsistency in the number of randomised participants: 120 randomised in original study but conference abstract reports that 150 participants were randomised. |
Kok 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, double‐blind, placebo‐controlled Number of centres: 5: University Medical Center Utrecht, Isala Clinics, Amphia Hospital, University Medical Center Groningen, and Erasmus Medical Center Primary location: University Medical Center Utrecht, Utrech Locations other than primary: all in the Netherlands: Isala Clinics, Amphia Hospital, University Medical Center Groningen, Erasmus Medical Center Number of arms: 2 (dexamethasone, placebo) Follow‐up time point(s): unique follow‐up with participants at various lengths of time after intervention: from 1.5 to 4 years Imputational strategy: missing values occurred in both baseline characteristics and outcome variables in 0% to 9.9% of the cases and were accounted for using multiple imputation. The imputation model contained independent and dependent variables and 10 imputed data sets were created; analyses were conducted in each of these data sets, and results were pooled according to Rubin’s rule. Original study outcomes (name, measure, time points): Self‐Rating Inventory for PTSD (SRIP) (Dutch questionnaire consistent with the DSM‐IV criteria for PTSD ‐ score of 39 as a cutoff), Beck Depression Inventory (BDI) II (cutoff score of 13.5) and QoL Short Form‐36 (SF‐36) (mental and physical component considered); between 1.5 and 4 years Total duration of study: original study: April 2006 to November 2011; follow‐up study: 8 months ‐ December 2012 to July 2013 |
|
| Participants |
Sample size: 1244 (618 + 626 participants who provided additional informed consent) Baseline characteristics Dexamethasone
Placebo
Type of traumatic event: cardiac surgery and consequent ICU stay Baseline group differences: no baseline differences in demographic, preoperative, surgical and ICU characteristics, SRIP, BDI, or psychopathology Inclusion criteria: participants of 18 years or older scheduled to undergo cardiac surgery and requiring cardiopulmonary bypass. For the follow‐up study, only participants within 4 years from enrolment were considered. Exclusion criteria: emergency procedures, off‐pump interventions, or a life expectancy of 6 months or less. |
|
| Interventions |
Setting: post‐cardiac surgery ICUs Intervention characteristics Dexamethasone
Placebo
|
|
| Outcomes |
PTSD rate
Dropout for any reason
Quality of life
|
|
| Identification |
Sponsorship source: the Dexamethasone for Cardiac Surgery trial itself was supported by grants 80‐82310‐98‐08607 from the Netherlands Organization for Health Research and Development (ZonMw) and 2007B125 from the Dutch Heart Foundation Country: the Netherlands Comments: this is an additional follow‐up on PTSD, depression and QoL that was not originally planned. An additional informed consent from participants was required. Continuous outcomes are not normally distributed and reported as median and inter‐quartile ranges. Dropouts have been counted starting from randomised participants that provided the additional informed consent. Author's name: Lotte Kok Institution: Department of Anesthesiology and Intensive Care, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, the Netherlands Email: L.Kok‐3@umcutrecht.nl Address: Department of Anesthesiology and Intensive Care, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, the Netherlands |
|
| Declarations of interest among primary researchers | Dr Hillegers received support for article research from the Netherlands Foundation for Mental Health (Fonds Psychische Gezondheid [personal grant], project 201126672, Postoperative psychopathology after cardiac surgery: Effects of dexamethasone and relation with corticosteroid receptor single nucleotide polymorphisms) and served as a board member for Benecke advisory board. He lectured for Benecke, Astra Zeneca, Shire, and Lundbeck (payment for lectures). Dr van der Maaten’s institution received support for participation in review activities from the University Medical Center Utrecht (UMC Utrecht, which paid the University Medical Center). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician created a computer‐generated 1:1 randomization scheme, which was stratified to participating center and in blocks of 40" (Dieleman 2012, p. 1762). |
| Allocation concealment (selection bias) | Low risk | Quote: "The research pharmacist [...] prepared and delivered batches of 40 ampoules to each center. When a consenting patient arrived [...and] the ampoule had been opened and the study drug was administered, the patient was considered randomized and the corresponding study number was assigned to that patient." "The study drug was supplied in packaged ampoules, each assigned to a unique study number" (Dieleman 2012, p. 1762). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients and observers of the present follow‐up study were blinded to treatment allocation" (Kok 2016, p. 513). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See quote above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates were high overall (although mostly due to death/exceeding time from intervention to follow‐up): dexamethasone 658/1219, 54%; placebo: 675/1239, 54.5%. Most of the participants that gave additional informed consent provided data (dexamethasone: 561/618 = 90.8%; placebo: 564/626 = 90.1%). |
| Selective reporting (reporting bias) | High risk | The follow‐up on PTSD, depression and quality of life has been an additional investigation after the main study, requiring collection of additional informed consent; therefore, the PTSD outcomes were not predefined. |
| Other bias | High risk | Although the two interventions groups considered in this follow‐up do not differ in baseline characteristics, additional informed consent was required after randomisation, and some participants were already dead by that point. It's unclear how this might have affected randomisation. Additionally, not all participants were analysed at the same time length from intervention administration. |
Matsuoka 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, double‐blind Number of centres: 1 Primary location: National Disaster Medical Center, Japan Locations other than primary: none Number of arms: 2 (omega‐3 fatty acids (docosahexaenoic acid (DHA) and eicosapentaenoic acid) and placebo) Follow‐up time point(s): 4 and 12 weeks Imputational strategy: multiple imputation using PROC MI and MIANALYZE in SAS software (applied as sensitivity analysis, only P values reported) Original study outcomes (name, measure, time points): at 1 and 3 months: PTSD severity measured by CAPS total score (3‐month time point is trial primary outcome); PTSD incidence measured by ‘structured interviews’ to asses DSM‐IV criteria; partial PTSD incidence (DSM‐IV criteria: 2 of 3 symptom criteria (B [re‐experiencing], C [avoidance], or D [hyperarousal]) and also at least 1 of the criteria A‐1 (stressor), E (duration), or F (impairment)); additional measure of PTSD severity with Impact of Event Scale–Revised (IES‐R ); major depression disorder incidence evaluated by Mini‐International Neuropsychiatric Interview (MINI); depression severity as measured by Montgomery‐Asberg Depression Rating Scale (MADRS) total score; score on Hospital Anxiety and Depression scale (HADS); health‐related quality of life scale (SF‐36); resilience as measured by Conner‐Davidson Resilience Scale (CD‐RISC) score; Buss‐Perry Aggression Questionnaire (BAQ); brain‐derived neurotrophic factor (BDNF); dehydroepiandrosterone (DHEA); neuropeptide Y (NPY), IL‐1 beta, IL‐6, TNF alpha, D‐serine, L‐serine, DL‐serine and activin levels. At 3 months only: number of days of leave taken from the time of injury, autonomic response measured before, during and after script driven imagery and acoustic stimulation Total duration of study: participant recruitment started on 16 December 2008 and ended on 6 June 2013. Follow‐up assessment was completed on 29 August 2013. |
|
| Participants |
Sample size: 110 Baseline characteristics Omega‐3 fatty acids
Placebo
Type of traumatic event: traffic accident ‐ intervention group: 40 (75.5%); placebo group: 43 (75.4%); falling from a high place ‐ intervention group: 9 (17.0%); placebo group: 10 (17.5%); workplace accident and other ‐ intervention group: 4 (7.5%); placebo group: 4 (7.0%) Baseline group differences: at baseline, the two groups did not differ in demographic variables, clinical characteristics, body mass index, or erythrocyte DHA composition Inclusion criteria: accidental injury; aged 18 years or older; native Japanese speaker; contact with research team within 240 hours after injury; physical and psychological ability to understand the scope of the trial and to provide written consent for study participation Exclusion criteria: (1) acute brain parenchymal damage that is obviously irretrievable or subdural or subarachnoidal bleeding detected by computed tomography and/or magnetic resonance imaging; (2) cognitive impairment, defined as a score of < 24 on the Mini‐Mental State Examination; (3) a serious drinking problem or high γ‐GTP blood level of ≥ 100 IU/L on admission; (4) a smoking habit of ≥ 40 cigarettes per day; (5) history and current suspicion of psychosis or bipolar I disorder; (6) suspicion of alcohol‐ or capsule‐related disorder or eating disorder; (7) serious psychiatric symptoms such as suicidal ideation, self‐harm behaviour or severe dissociation, or in need of rapid psychiatric treatment; (8) regular treatment with anti‐epilepsy medication, lithium, ethylicosapentate, aspirin, or warfarin within the last 3 months; (9) regular consumption of polyunsaturated fatty acid supplements within the last 3 months; (10) a habit of eating fish ≥ 4 times per week, to ensure comparability to people studied in Western countries |
|
| Interventions |
Setting: ICU of the National Disaster Medical Center, Tokyo, Japan Intervention characteristics Omega‐3 fatty acids
Placebo
|
|
| Outcomes |
PTSD severity
Dropouts due to adverse events
PTSD rate
Depression severity
Quality of life
Dropout for any reason
|
|
| Identification |
Sponsorship source: this study was supported by CREST, the Japan Science and Technology Agency (primary investigator: Dr Matsuoka). All supplements used in the study were supplied by Kentech Co, Ltd, Toyama, Japan. Country: Japan Author's name: Yutaka Matsuoka Institution: Department of Psychiatry, National Disaster Medical Center, Tachikawa, Tokyo, Japan; CREST, Japan Science and Technology Agency, Tokyo, Japan Email: matsuoka‐psy@umin.ac.jp Address: Yutaka Matsuoka, MD, PhD, Department of Psychiatry, National Disaster Medical Center, 3256 Midoricho, Tachikawa, Tokyo 190‐0014, Japan |
|
| Declarations of interest among primary researchers | Dr Matsuoka has received research grants from the Japan Science and Technology Agency; the National Center of Neurology and Psychiatry, Japan; and the Ministry of Health, Labour and Welfare of Japan. He has been a paid speaker for Ono, Mochida, Takeda, Suntory Wellness, Otsuka, and the DHA & EPA Association. Dr Nishi has received research grants from the Japan Society for the Promotion of Science and lecture fees from Qol, the DHA & EPA Association, NTT DoCoMo, and Emotional Quotient Academy. Dr K. Hamazaki has received research support from the National Center of Neurology and Psychiatry, Japan; the Japan Society for the Promotion of Science; the Tamura Foundation for Promotion of Science and Technology; and the Ichiro Kanehara Foundation for Promotion of Medical Sciences and Medical Care. He has received consultant fees from Polyene Project and scholarship donations from Otsuka. He has been a paid speaker for the DHA & EPA Association. Mr Yonemoto has received research grants from the Japan Science and Technology Agency; the National Center of Neurology and Psychiatry, Japan; and Ministry of Health, Labour and Welfare of Japan. Dr Matsumura has received research grants from the Japan Society for the Promotion of Science and developed the iPhysioMeter app, distributed for free at the iTunes App Store (Apple, Inc). There are no further patents, products in development, or marketed products to declare. Dr Hashimoto has served as a scientific consultant to Astellas and Taisho. He has also received research support from Abbvie, Dainippon Sumitomo, Otsuka, and Taisho. Dr T. Hamazaki has received research support from the Japan Society for the Promotion of Science, the Open Research Center for Lipid Nutrition (Kinjo Gakuin University), and Nippon Suisan Kaisha, Ltd; consultancy fees from Polyene Project and Otsuka; lecture fees from Otsuka; and travel expenses from Aker BioMarine. Dr Noguchi declares that she has no competing interests in relation to this work. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician (Dr. Akiko Kada) composed tables of block randomization with three stratification factors using a computer‐generated random allocation sequence. Stratification factors included sex, age (< 40 or ≥ 40 years), and sense of life threat. This resulted in eight (2×2×2) different stratified tables of supplement numbers" (Matsuoka 2013, p. 6). |
| Allocation concealment (selection bias) | Low risk | Quote: "Those [randomization] tables were sent to an independent pharmacist (Professor Satoru Kobayashi); he securely kept the tables and prepared numbered supplements bottles according to the tables" (Matsuoka 2013, p. 6). Quote: "An allocation Excel sheet file was masked and securely kept under passcode by the pharmacist. Both the research team and participants will be blinded to randomization until the last participant has completed the protocol and the spreadsheets of all results are finalized" (Matsuoka 2015, p. e1016). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We added a small amount of not fully deodorized fish oil to the base of the control oil to give it a fishy odor and make it indistinguishable from the active oil" (Matsuoka 2015, p. e1016). Quote: "All members of the research team, including all authors, physicians, nurses, research assistants, and study participants, remained blinded to the actual intervention assignments until data collection was completed and confirmed and erythrocyte fatty acids were analyzed" (Matsuoka 2015, p. e1017). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See quote above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates are below 20% for both interventions: omega‐3 fatty acids: 8/53, 15.1%; placebo: 2/57, 3.5%. There is some imbalance between the two attrition rates but this could be due to the relatively small number of randomised participants. |
| Selective reporting (reporting bias) | Low risk | According to the protocol, many outcomes should have been measured at one month post‐randomisation but the data for this time point are not usually provided by the authors. Results of the more clinically relevant time point of three months are provided and the hierarchy of primary/secondary outcomes respected. The published protocol was submitted for publication on July 2012, while recruitment was started in December 2008. Still, the registration entry at clinicaltrials.gov (NCT00671099 ‐ outcomes) was originally submitted on May 2008. |
| Other bias | Low risk | Quote: "The Japan Science and Technology Agency and Kentech Co, Ltd, had no role in the design and conduct of the study, data collection, data management, analysis, interpretation of the data, review or approval of the manuscript, and decision to submit the manuscript for publication" (Matsuoka 2015, p. e1021). No other source of bias found |
Orrey 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, pilot genotype‐based, multisite, double‐blind Number of centres: 4 Primary location: University of North Carolina, Chapel Hill, NC Locations other than primary: MedStar Health Research Institute, Washington, DC; Wake Forest University Health System, Wake Forest, NC; Crozer‐Keystone Health System, Upland, PA Number of arms: 2 (propranolol and placebo) Follow‐up time point(s): 6 weeks Imputational strategy: no imputational strategy employed for mental health outcomes Original study outcomes (name, measure, time points): PTSD symptoms by the Posttraumatic Symptom Scale‐Interview Version (PSS‐I) at 1 and 6 weeks; pain by Numeric Rating Scale 0‐10, assessed daily from study day 5 to 19 Total duration of study: 6 weeks |
|
| Participants |
Sample size: 47 Baseline characteristics Propranolol
Placebo
Type of traumatic event: major burn injury Baseline group differences: no apparent major imbalances at baseline Inclusion criteria: individuals admitted to participating burn centres within 72 hours of sustaining a thermal burn injury involving ≤ 20% total body surface area Exclusion criteria: "Patients with the CC genotype at rs4818 (high activity COMT haplotype). Estimated hospital stay at the time of admission of <5 days or >40 days, intentional injury, substantial concomitant non burn injury, and greater than first‐degree cardiac conduction blockade, already taking a b‐adrenergic antagonist medication, non‐English speaking, clinically unstable, prisoners, history of asthma diabetes coronary artery disease psychotic disorder or hepatic renal or congestive heart failure, patients whose highest pain score between admission and recruitment was <4 (0 to 10 numeric rating scale [NRS]) or who were on opioid medications for chronic pain before their burn injury." |
|
| Interventions |
Setting: four network burn centres Intervention characteristics Propranolol
Placebo
|
|
| Outcomes |
PTSD severity
Dropouts due to adverse events
PTSD rate
Dropout for any reason
|
|
| Identification |
Sponsorship source: Award Number UL1RR025747 from the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the National Institutes of Health (Bethesda, MD), the NC Jaycee Burn Center Fund (Chapel Hill, NC), the Firefighters Research Fund, the DC Firefighters Burn Foundation (Washington, DC), and UNC Institutional Resources (Chapel Hill, NC) Country: USA Author's name: Samuel McLean Institution: UNC Chapel Hill Email: smclean@aims.unc.edu Address: Department of Surgery, University of North Carolina at Chapel Hill, 101 Manning Drive, Medical School Wing C, Room B45, Chapel Hill, NC 27599‐7010 |
|
| Declarations of interest among primary researchers | None reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated protocol created a numbered sequence of treatment assignments (allocation list) at each study site using permuted blocks (block sizes of 2 and 4) stratified by race (European‐American, African‐American, or other) and sex" (Orrey 2015, p. 22). |
| Allocation concealment (selection bias) | Low risk | Quote: “Other than the study biostatistician, all burn unit staff, research data collectors, and investigators were blinded to randomization schedule. Investigational drug pharmacy personnel at each study site maintained the unblinded study site allocation list and assigned participants to treatment arm” (Orrey 2015, p. 22). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Other than the study biostatistician, all burn unit staff, research data collectors, and investigators were blinded to randomization schedule. Capsules (sight, taste, smell) and medication bottles were identical across treatment arm. Bottles were marked by unique study ID numbers only" (Orrey 2015, p. 22). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Other than the study biostatistician, all burn unit staff, research data collectors, and investigators were blinded to randomization schedule" (Orrey 2015, p. 22). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates overall were low: propranolol 3/23, 13.0%; placebo 1/24, 4.2%. |
| Selective reporting (reporting bias) | High risk | A protocol was not available for this study. Some outcomes reported in the conference abstract are not in the main 2015 publication. Pain outcome was changed during the course of the study: "However, soon after starting the study it was observed that patients sometimes provided an “average” pain rating greater than their worst reported pain or less than their least reported pain. These observations, together with the high degree of educational disadvantage in this population, led us to appreciate that our interview question regarding “average pain” was poorly designed for the study population and did not reliably yield valid data. We therefore used linear mixed modelling to combine the pain measurements assessed from all participants on primary outcome days (waking, worst, and least pain) into an “overall pain” score for each of these days. This alternative primary acute pain outcome was defined after the study start and before study analyses (secondary analyses also evaluated our original primary outcome measure, average pain severity)" (Orrey 2015, p. 23). |
| Other bias | Low risk | The institutional sponsors do not seem to carry a bias towards a specific result. No other source of bias was identified for this trial. |
Pitman 2002.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, pilot, double‐blind, placebo controlled Number of centres: one Primary location: Massachusetts General Hospital, Boston, USA Locations other than primary: Number of arms: 2 (propranolol and placebo) Follow‐up time point(s): 1 and 3 months Imputational strategy: no Original study outcomes (name, measure, time points): PTDS severity measured by CAPS; PTSD incidence by DSM‐IV criteria, script‐driven imagery procedure, all measured at 1 and 3 months Total duration of study: not reported |
|
| Participants |
Sample size: 41 Baseline characteristics Propranolol
Placebo
Baseline group differences: no group differences approached statistical significance Inclusion criteria: emergency department (ED) patients who (a) had just experienced a traumatic event that met the DSM‐IV PTSD A.1 (stressor) and A.2 (response) criteria; had a heart rate (HR) of 80 beats per minute (BPM) or greater at the time of ED presentation; upon mental status examination were found competent to understand the purpose of the study and the nature of the procedures; gave written informed consent after the procedures had been fully explained Exclusion criteria: serious physical injury, systolic blood pressure under 100 mmHg, substance intoxication, pregnancy or lifetime history of congestive heart failure, heart block or bronchial asthma |
|
| Interventions |
Setting: emergency department Intervention characteristics Propranolol
Placebo
|
|
| Outcomes |
PTSD severity
PTSD rate
Dropout for any reason
|
|
| Identification |
Sponsorship source: supported by US Public Health Service Grant MH58671 Country: USA Author's name: Roger K. Pitman Institution: Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts Address: Roger K. Pitman, PTSD Research Laboratory, Massachusetts General Hospital‐East, Bldg. 149, 13th Street, Charlestown Massachusetts 02129. |
|
| Declarations of interest among primary researchers | Not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on random sequence generation provided. The trial is described as randomised. Baseline characteristics are balanced between groups and do not suggest a possible problem with randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details are provided regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial is described as double‐blind; however, assessing the efficacy of blindness is difficult. Authors acknowledge that nurses might have paid more attention to hydrocortisone patients because they were more likely to experience adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although blinding of assessors is only implied and not specifically stated, it seems likely that this was the case given study design and description of the personnel assessing the participants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates were uneven between the two intervention groups but within the context of a small sample size. However, in both groups, attrition rates were high: propranolol: 39%, placebo: 35%. |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration entry was not available for this trial. Predefined outcomes are reported. |
| Other bias | Low risk | No other source of bias was identified for this trial. |
Schelling 2001.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, double‐blind Number of centres: 1 Primary location: Munich, Germany Locations other than primary: none Number of arms: 2 (hydrocortisone and placebo) Follow‐up time point(s): unique follow‐up of participants at various lengths of time between 21 and 49 months, median 31 months Imputational strategy: no Original study outcomes (name, measure, time points): incidence of PTSD (Structured Clinical Interview for DSM‐IV (SCID‐IV)); severity of PTSD (modified German version of the Post‐Traumatic Stress Syndrome 10‐Question Inventory); traumatic memories from the ICU ("structured questionnaire" evaluating different categories of traumatic memory [from ICU]). All outcomes assessed at unique follow‐up that took place between 21 and 49 months (median 31 months) after ICU discharge Total duration of study: 49 months |
|
| Participants |
Sample size: original trial had 20 + 20 = 40 participants. By the time the additional criteria for PTSD were applied, the sample was reduced to 15 + 14 = 29 due to deaths; of these, 11 + 13 = 26 were eligible for the PTSD sub‐study Baseline characteristics Hydrocortisone
Placebo
Type of traumatic event: septic shock and ICU stay Baseline group differences: no significant differences between groups at baseline Inclusion criteria: ICU‐admitted individuals fulfilling criteria for hyperdynamic septic shock as proposed by the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) Exclusion criteria: original trial criteria: aged < 18 or > 75, pregnancy, irreversible underlying diseases, organ transplant recipients, patients with burns, hemorrhagic shock, or those who had suffered myocardial infarction in the 6 months preceding the study, treatment with vasopressors or glucocorticoids for > 72 hours. Additional follow‐up study criteria: pre‐existing neurologic or psychiatric diseases (including alcohol and drug abuse) or those who could not complete a questionnaire in German language |
|
| Interventions |
Setting: multidisciplinary ICU of a tertiary care university hospital Intervention characteristics Hydrocortisone
Placebo
|
|
| Outcomes |
PTSD rate
Dropout for any reason
PTSD Severity
|
|
| Identification |
Sponsorship source: this study was supported by grants from Hoffman‐La Roche, Grenzach–Wyhlen and the Eli‐Lilly International Foundation, Bad Homburg, all in Germany Country: Germany Comments: this is an additional follow‐up of a study originally on septic shock; the PTSD outcomes were not originally planned. PTSD severity on modified PTSS‐10 is not normally distributed. Dropouts have been calculated starting from the number of randomised participants included under the criteria for being considered for the PTSD outcomes Author's name: Gustav Schelling Institution: Departments of Anesthesiology Ludwig‐Maximilians‐University, Munich Email: not reported Address: Dr. G. Schelling, Department of Anesthesiology, Klinikum Grossfrunden, 81377 Muenchen, Germany |
|
| Declarations of interest among primary researchers | Not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were assigned to random permuted blocks” (Briegel 1999, p. 724). |
| Allocation concealment (selection bias) | Unclear risk | No details are provided regarding allocation concealment. Baseline characteristics are balanced between groups and do not suggest a possible problem with randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "During the study, the attending physicians, the investigators, and the nursing staff were blinded with regard to the results of these measurements" (Schelling 2001, p. 979). Not clear if participants were blinded to treatment. Moreover, it's unclear if blinding has been maintained after the original trial end. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The psychiatrists were blinded with regard to treatment characteristics (group assignment, principal diagnosis, traumatic experiences, duration of treatment, etc.)”. “The patients were blinded regarding the facts that their interviewers were psychiatrists and that the aim of the interviews was the diagnosis of PTSD” (Schelling 2001, p. 980). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A low attrition risk of bias is difficult to establish. This is a follow‐up study which applied additional exclusion criteria to an already‐randomised sample. Moreover, part of the randomised sample died before the time of this follow‐up study. |
| Selective reporting (reporting bias) | High risk | This is a follow‐up study which was not planned at the time of the original investigation. |
| Other bias | High risk | Hoffman‐La Roche and Eli‐Lilly founded the trial. Their role in designing, conducting, and writing the publications is not addressed within the papers. The authors recognise that participants receiving placebo received more inotropic support (although this difference did not reach statistical significance), and that this might have a role in a higher PTSD incidence within the placebo group. Additional inclusion criteria for considering participants for this sub‐study were applied post randomisation. |
Shaked 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, double‐blind, placebo controlled Number of centres: 1 Primary location: Beer Sheva, Israel Locations other than primary: none Number of arms: 2 (hydrocortisone and placebo) Follow‐up time point(s): 1 month post injury Imputational strategy: no Original study outcomes (name, measure, time points): Neck Disability Index (NDI), Numeric Pain Rating Scale (NPRS), Tampa Scale for Kinesiophobia (TSK‐11) and Post‐traumatic stress Diagnostic Scale (PDS), all at 1 month Total duration of study: the study was conducted between 2014 and 2017 |
|
| Participants |
Sample size: 77 Baseline characteristics (overall sample)
Baseline group differences: no group differences reported on socio‐demographic parameters, medical background, previous history of neck pain, pain level and cortisol concentration upon arrival at the emergency department, characteristics of motor vehicle collision (MVC), comparison of vital signs but for mean pulse Inclusion criteria: involvement in a motor vehicle accident, whiplash‐type mechanism of injury, neck pain, age between 18 and 70 years old, signed informed consent. Screening was limited to morning hours to prevent bias due to circadian fluctuations of cortisol. Exclusion criteria: head injury; psychiatric disorder; adrenal gland disease; chronic medication treatment including steroids, antidepressants, chronic analgesics, amphetamines; drug abuse; pregnancy; time interval > 6 hours from accident; polytrauma requiring hospitalisation |
|
| Interventions |
Setting: level I trauma centre in Beer Sheva, Israel Intervention characteristics Hydrocortisone
Placebo
|
|
| Outcomes |
PTSD severity
|
|
| Identification |
Sponsorship source: no sponsorship source reported Country: Israel Author's name: Gad Shaked Institution: Department of General Surgery and Trauma Unit, Soroka University Medical Center and Ben‐Gurion University Email: shakedg@bgu.ac.il Address: Department of General Surgery and Trauma Unit, Soroka University Medical Center and Ben‐Gurion University, Wingate St. 64, 84101 Beer Sheva, Israel |
|
| Declarations of interest among primary researchers | Study authors report no conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were randomly assigned to either a test or a control group based on a computer generated randomization table" (Shaked 2019, p. 1117). |
| Allocation concealment (selection bias) | Unclear risk | No information provided regarding allocation concealment strategy. Baseline characteristics do not suggest an imbalance between randomisation groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the patient and the researcher who injected the study drug were blinded to the nature of the given drug" (Shaked 2019, p. 1117). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patient and the researcher who conducted the interview were both blinded to whether the participant received the study drug or placebo" (Shaked 2019, p. 1117) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Seventy‐seven patients were enrolled in the study and completed the 1 month follow‐up" (Shaked 2019, p. 1117). Comment: it's unclear if this implies that all of the randomised participants completed the 1 month follow‐up, or if the paper reports data for follow‐up completers only. |
| Selective reporting (reporting bias) | Unclear risk | For the PTSD outcome, the registration entry at clinicaltrials.gov (NCT02090309) reports as measurement instrument "PTSD questionnaire (PDA)", while the paper reports "Post‐Traumatic Stress Diagnostic Scale (PDS). Possible spelling error? |
| Other bias | Low risk | Authors report no conflicts of interest; no other source of bias found. |
Stein 2007.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, proof‐of‐concept pilot, double‐blind, placebo controlled Number of centres: 1 Primary location: level 1 surgical trauma centre (University of California San Diego (UCSD)), California Locations other than primary: none Number of arms: 3 (propranolol, gabapentin, placebo) Follow‐up time point(s): 1, 4 and 8 months post injury Imputational strategy: no Original study outcomes (name, measure, time points): acute stress disorder scale at 1 month, Comprehensive International Diagnostic Interview (CIDI) for PTSD; major depressive disorder and panic disorder at 4 and 8 months, Posttraumatic Stress Disorder Checklist–Civilian Version modified to reflect symptoms from the time of injury (PCL‐C) at 1, 4, and 8 months; Center for Epidemiologic Studies Depression Scale (CES‐D), 1, 4, 8 months Total duration of study: 39 months |
|
| Participants |
Sample size: eligible N = 905; randomised N = 48 (336 discharged before trialist could make contact, 521 declined to participate) Baseline characteristics (data provided for overall sample)
Baseline group differences: no significant differences between randomised groups Inclusion criteria: aged 18 to 65; admitted to UCSD Surgical Trauma Centre for a severe, physical injury requiring specialised, emergent trauma care Exclusion criteria: main exclusion reasons: lived too far away for monitoring; too medically unstable; did not speak English; over 65 or under 18; suicidal or taking psychotropics; homeless, in jail or police hold; cardiac or seizure medications; active military |
|
| Interventions |
Setting: University of California San Diego (UCSD) Level 1 Surgical Trauma Center (admission to this service reflected a severe physical injury requiring specialised, emergent trauma care) Intervention characteristics Propranolol
Gabapentin
Placebo
|
|
| Outcomes |
PTSD severity
PTSD rate
Depression severity
Dropout for any reason
|
|
| Identification |
Sponsorship source: supported by NIMH grants MH62037 (R21) and MH64122 (K24) to MBS Country: USA Author's name: Murray B Stein Institution: Departments of Psychiatry, and Family & Preventive Medicine, University of California San Diego, and the VA San Diego Healthcare System, San Diego, CA Email: mstein@ucsd.edu Address: Murray B. Stein, University of California San Diego; Department of Psychiatry and Department of Family & Preventive Medicine; 8950 Villa La Jolla Drive; Suite B‐218; La Jolla, CA 92037 |
|
| Declarations of interest among primary researchers | Not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization schedule was set up and maintained by the UCSD Research Pharmacy" (Stein 2007, p. 926). |
| Allocation concealment (selection bias) | Low risk | Quote: "When a subject was enrolled, the study nurse notified one of the attending physicians on the Trauma Service, who authorized the Research Pharmacy to provide the medication supplies (according to the randomization schedule) to the subject" (Stein 2007, p. 926). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study medications were supplied in identical capsules to avoid breaking the blind study. When a subject was enrolled, the study nurse notified one of the attending physicians on the Trauma Service, who authorized the Research Pharmacy to provide the medication supplies (according to the randomization schedule) to the subject" (Stein 2007, p. 926). The study is described as double‐blind, the administration schema was identical among the different interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study nurse, who was also blind to treatment allocation, conducted assessments" (Stein 2007, p. 926). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates are not clearly stated and are inferred from the rates of PTSD at 4‐month follow‐up time. Attrition rates are over 20% for both propranolol (29.4%) and gabapentin (28.6%), and low for placebo (6.0%). For continuous outcomes, an imputational strategy (generalised estimating equations) has been employed, but authors recognised that “the possibility of differential drop‐out across groups creates a missing data problem that even the use of GEE analyses may not solve” (Stein 2007). |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration entry was not available for this trial. |
| Other bias | Low risk | No other source of bias was identified for this trial. |
Tincu 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group Number of centres: 1 Primary location: Bucharest, Romania Locations other than primary: none Number of arms: 2 (placebo and 5‐hydroxytryptophan) Follow‐up time point(s): 7 and 14 days post admission Imputational strategy: no Original study outcomes (name, measure, time points): serotonin plasma levels; CAPS scores Total duration of study: 14 days |
|
| Participants |
Sample size: 60 Baseline characteristics Placebo
5‐hydroxytryptophan
Type of traumatic event: ICU admission for non‐surgery causes Baseline group differences: at baseline, no significant differences regarding serotonin plasma levels (P > 0.05) or age (P > 0.05) Inclusion criteria: admission to ICU for non‐surgery causes Exclusion criteria: psychiatric disorders; malignancies; treated with drugs that interfere with the metabolism of serotonin |
|
| Interventions |
Setting: intensive care unit Intervention characteristics Placebo
5‐hydroxytryptophan
|
|
| Outcomes |
PTSD severity
PTSD rate
|
|
| Identification |
Sponsorship source: not reported Country: Romania Comments: the only source of information on this study is a conference abstract. Author's name: Radu Ciprian Tincu Institution: Bucharest Clinical Emergency Hospital, Bucharest, Romania Email: r_tincu@yahoo.com |
|
| Declarations of interest among primary researchers | Not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is provided regarding randomisation strategy. Baseline characteristics are not reported in sufficient detail to assess a possible problem with randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided regarding allocation concealment. Baseline characteristics are not reported in sufficient detail to assess a possible problem with randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not mentioned: likely not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of dropouts is not provided and it is not possible to assess attrition rates. |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration entry was not available for this trial. |
| Other bias | Unclear risk | A conference abstract is the only source of information on this study. |
Weis 2006.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel group, double‐blind Number of centres: 1 Primary location: Munich, Germany Locations other than primary: none Number of arms: 2 (hydrocortisone and placebo) Follow‐up time point(s): 6 months after cardiac surgery/discharge from ICU Imputational strategy: no Original study outcomes (name, measure, time points): health‐related quality of life measured by Medical Outcomes Study Short‐Form Survey (SF‐36) (self‐administered); chronic stress symptoms measured by a modified version of the Post‐Traumatic Stress Symptom 10‐Question Inventory (PTSS‐10) questionnaire (validated in patients after ICU treatment); PTSD rate assessed with same scale ("A summary score of more than 35 is associated with a high probability of patients fulfilling the diagnostic criteria for PTSD"); evaluation of traumatic memories by a narrative questionnaire. All outcomes at 6 months. Total duration of study: 1 year (September 2002 to September 2003) |
|
| Participants |
Sample size: randomised N = 36; completed 6 month questionnaires N = 30; excluded from final analyses (missing data) N = 2; final sample N = 28 Baseline characteristics Hydrocortisone
Placebo
Overall
Type of traumatic event: high‐risk cardiac surgery and ICU stay Baseline group differences: participants from the hydrocortisone and placebo groups did not differ with regard to the type of surgical procedures, age and sex distribution, or the duration of cardiopulmonary bypass or aortic cross clamping. Compared with participants from the placebo group, however, participants who received hydrocortisone had a significantly shorter postoperative stay in the ICU, had significantly lower Therapeutic Intervention Scoring System (TISS) scores, showed a strong trend toward lower values of the pro inflammatory cytokine IL‐6 (P = .05), and required significantly less norepinephrine (both with regard to maximal dosage and duration of administration). Inclusion criteria: high‐risk individuals undergoing cardiac surgery with cardiopulmonary bypass ("preoperative left ventricular ejection fraction of less than 35% or an expected duration of CPB of greater than 97 minutes") Exclusion criteria: pregnancy; emergency operation; hepatic dysfunction (bilirubin > 3 mg/dL); renal dysfunction (plasma creatinine > 2 mg/dL); a positive serologic test result for HIV; manifest insulin‐dependent diabetes mellitus; an extracardial septic focus; chronic or acute inflammatory disease; and inability to provide informed consent. In addition, people who required glucocorticoids other than hydrocortisone were excluded. |
|
| Interventions |
Setting: ICU Intervention characteristics Hydrocortisone
Placebo
|
|
| Outcomes |
PTSD severity
PTSD rate
Dropout for any reason
Quality of life
|
|
| Identification |
Sponsorship source: not reported Country: Germany Comments: baseline characteristics, and QoL and PTSD severity scores are described as non normally distributed. Participants receiving hydrocortisone had better short‐term physical outcomes (ICU stay length, TISS scores, norepinephrine requirements) Author's name: Gustav Schelling Institution: Departments of Anesthesiology and Cardiac Surgery and the Institute for Medical Informatics, Biometry and Epidemiology, e Ludwig‐Maximilians‐University, Munich, Germany Email: gustav.schelling@med.uni‐muenchen.de Address: Gustav Schelling, MD, PhD, Ludwig‐Maximilians University, Klinikum Grosshadern, Department of Anaesthesiology, 81377 Muenchen, Germany |
|
| Declarations of interest among primary researchers | Not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomisation list" (Weis 2006, p. 278). |
| Allocation concealment (selection bias) | Low risk | Quote: "The vials were prepared by a study nurse who was not involved in the care of patients participating in the trial" (Weis 2006, p. 278). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "One group of patients received stress doses of hydrocortisone […], and patients from the other group (the placebo group) received normal saline in identical vials in a double‐blind fashion. The vials were prepared by a study nurse who was not involved in the care of patients participating in the trial" (Weis 2006, p. 278). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were self‐reported through questionnaires ‐ see quote above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no significant differences with regard to patient or treatment characteristics between included or excluded patients" (Weis 2006, p. 280). The attrition rates are similar given the small sample size (hydrocortisone: 26.3%; placebo: 17.6%). |
| Selective reporting (reporting bias) | Unclear risk | A protocol or trial registration entry was not available for this trial. All prespecified outcomes in the paper are reported. |
| Other bias | High risk | Participants in the hydrocortisone arm had better physical results, including shorter ICU stays, compared to participants receiving placebo. This factor by itself could have mediated the difference in traumatic stress symptoms. Moreover, placebo participants required more noradrenergic support and higher norepinephrine urinary levels have been associated with increased PTSD incidence: this could be an alternative mediating factor on the difference in PTSD symptoms. |
BDI: Beck Depression Inventory; bpm: beats per minute; CAPS: Clinician‐Administered PTSD Scale; DSM‐IV/5: Diagnostic and Statistical Manual of Mental Disorders ‐ Fourth/Fifth Edition; ED: emergency department EN: enteral nutrition; ER: extended release; HRQoL: health‐related quality of life; ICU: intensive care unit; IQR: interquartile range; ISS: Injury Severity Score; IU: international units; IV: intravenous(ly); KPS: Karnofsky performance status; MADRS: Montgomery‐Asberg Depression Rating Scale; MVA: motor vehicle accident; MVC: motor vehicle collision; PCL: Post‐traumatic Stress Disorder Checklist; PSS‐I: Posttraumatic Symptom Scale‐Interview Version; PTSD: post‐traumatic stress disorder; PTSS‐10: Post‐Traumatic Stress Symptom 10‐Question Inventory; QIDS‐SR: Quick Inventory of Depressive Symptomatology; QoL: quality of life; SCID‐IV: Structured Clinical Interview for DSM‐IV; SF‐36 (MCS): Short Form 36‐item questionnaire (Mental Component Summary); SKID‐1: Strukturierte Klinische Interview für DSM‐IV, Achse‐I (German version of SCID‐IV for Axis I Disorders); SMFA: Short Musculoskeletal Functional Assessment; SRIP: Self‐Rating Inventory for PTSD
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blaha 1999 | Ineligible study design |
| Delahanty 2013 | Study targeting symptomatic participants at baseline |
| EUCTR‐000088‐12‐DE | Study targeting symptomatic participants at baseline |
| EUCTR‐004177‐83‐NL | Study targeting symptomatic participants at baseline |
| FDA 1999 | Intervention started after three months from traumatic experience |
| Frankova 2017 | Study targeting symptomatic participants at baseline |
| Frijling 2016 | Study targeting symptomatic participants at baseline |
| Gelpin 1996 | Ineligible study design |
| Kaplan 2015 | Intervention started after three months from traumatic experience |
| Lijffijt 2019 | Not a prevention trial |
| Matsumura 2011 | No control arm |
| Matsuoka 2010 | No control arm |
| Mellman 2002 | Study targeting symptomatic participants at baseline |
| Mistraletti 2015 | Ineligible study design. Secondary outcomes related to mental health were not systematically assessed despite what was originally planned. |
| Naylor 2013 | Not a prevention trial |
| NCT00633685 | Not a prevention trial |
| NCT00674570 | Study targeting symptomatic participants at baseline |
| NCT01039766 | Study targeting symptomatic participants at baseline |
| NCT02069366 | Ineligible study design |
| NCT02505984 | Ineligible condition |
| NCT03724448 | Study targeting symptomatic participants at baseline |
| NCT04071600 | Study targeting symptomatic participants at baseline |
| Nedergaard 2020 | Ineligible interventions: participants were randomised to being sedated or not during mechanical ventilation (not a drug versus another drug or placebo) |
| Nishi 2012 | No placebo or medication control group |
| Rabinak 2020 | Not a prevention trial |
| Rucklidge 2012 | Study targeting symptomatic participants at baseline |
| Schelling 2004 | No placebo or medication control group (control group was "standard treatment", which was also administered to the hydrocortisone group) |
| Shalev 2012 | Study targeting symptomatic participants at baseline |
| Stoddard 2011 | Ineligible participants (< 18 years old) |
| Suliman 2015 | Study targeting symptomatic participants at baseline |
| Takehiro 2014 | Not a prevention trial |
| Treggiari 2009 | Ineligible intervention (“deep” versus “light” sedation, apparently both accomplished with midazolam) |
| Truppman Lattie 2020 | Not a prevention trial |
| Van Zuiden 2017 | Study targeting symptomatic participants at baseline |
| Yang 2011 | No placebo or medication control group |
| Zoellner 2001 | Ineligible intervention |
| Zohar 2011 | Study targeting symptomatic participants at baseline |
| Zohar 2017a | Study targeting symptomatic participants at baseline |
| Zohar 2017b | Study targeting symptomatic participants at baseline |
Characteristics of ongoing studies [ordered by study ID]
McMullan 2020.
| Study name | Intranasal ketamine as an adjunct to fentanyl for the prehospital treatment of acute traumatic pain |
| Methods | Randomised, triple‐blind (participant, care provider, investigator) |
| Participants | People experiencing pain due to acute trauma (i.e. extremity deformity, tourniquet placement, or severe burns) |
| Interventions | ketamine IN, placebo IN |
| Outcomes | Primary: pain reduction on the Verbal numeric Rating Scale after 30 minutes from administration. Secondary outcomes: pain at emergency department (ED) arrival; adverse event incidence; opiate requiments prior to ED arrival and in the first three hours of ED care; chronic pain (Brief pain inventory) at 90 days after injury; PTSD (PTSD checklist for DSM‐5) at 90 days after injury; overall satisfaction with life (Satisfaction With Life Scale) at 90 days after injury |
| Starting date | 3 October 2017 |
| Contact information | Jason McMullan, University of Cincinnati |
| Notes |
NCT03997864.
| Study name | Administration of prazosin to prevent PTSD in adult women after sexual assault |
| Methods | Randomised, parallel group, quadruple‐blind (participant, care provider, investigator, outcomes assessor) |
| Participants | 40 adult females, ages 18 to 50 years, who are evaluated and treated at the University of Colorado Hospital after an alleged sexual assault on their person |
| Interventions | Prazosin, placebo |
| Outcomes | Primary: change in PTSD symptoms and severity measured with Clinician‐Administered PTSD Scale (CAPS‐5) at one month and three months after the traumatic event. Secondary: Pittsburgh Sleep Quality Index (PSQI) assessed weekly until study completion (expected 3 months); Pittsburgh Sleep Quality Index ‐ Trauma Addendum (PSQI‐A) assessed weekly until study completion (expected 3 months); Patient Health Questionnaire (PHQ‐9) assessed weekly for seven weeks and at study completion (expected 3 months). |
| Starting date | 23 February 2020 |
| Contact information | Steven J Berkowitz, MD, steven.berkowitz@cuanschutz.edu |
| Notes |
NCT04274361.
| Study name | Ketamine for pain control after severe traumatic injury |
| Methods | RCT, open label, parallel groups |
| Participants | Acutely injured adult trauma hospital inpatients with an Injury Severity Score (ISS) > 15 |
| Interventions | Ketamine, placebo |
| Outcomes | Cumulative opioid morphine equivalent dose after 24 hours |
| Starting date | |
| Contact information | |
| Notes | The study is currently ongoing. Effect of pain on future risk of PTSD development is mentioned in the 'Detailed description' but the only outcome currently listed is "Cumulative opioid morphine equivalent dose [Time Frame: The first 24 hours]" (NCT04274361). It is unclear if the study will consider PTSD or focus on pain only. |
Differences between protocol and review
As we were expecting a multitude of interventions, we made plans for a network meta‐analysis. Lack of direct comparisons between interventions prevented its execution. The title of the review changed to reflect this: from 'Early pharmacological interventions for preventing post‐traumatic stress disorder (PTSD): a network meta‐analysis' to 'Early pharmacological interventions for universal prevention of post‐traumatic stress disorder (PTSD)'.
At the protocol stage, we stated that we intended to search the World Health Organization's trials portal (ICTRP), and the National Institute of Health's trials website (clinicaltrials.gov). This has not been done as these resources are already covered by the CENTRAL database.
At the protocol stage, for the outcome PTSD rate, we planned to consider missing participants as participants who had a negative event (PTSD). In consideration of the high attrition rate, we felt that this approach made an unrealistically strong assumption, particularly in the context of a preventive intervention. Therefore, our main analyses used observed case data (i.e. the number of participants with the event divided by those who completed). However, we performed sensitivity analyses where the number of randomised participants was used as the denominator.
Contributions of authors
All review authors other than DS and TW contributed to the protocol. DS and TW joined the author team at the review stage.
FB: writing of protocol and review, development of the selection criteria and methodology, screening search results, data extraction, risk of bias and certainty of evidence (GRADE) assessment, interpretation of results (clinical perspective)
LR: screening search results, data extraction, risk of bias assessment, interpretation of results (methodological perspective)
JIB: development of the selection criteria, interpretation of results (clinical perspective)
NM: assisted in writing the protocol. Development of the methodology, assisted with risk of bias ratings and GRADE assessment, contributed to the writing of the results. Interpretation of results (methodological perspective)
RC: interpretation of results
GO: interpretation of results (clinical perspective)
DS: interpretation of results (clinical perspective)
TW: certainty of evidence (GRADE) assessment, interpretation of results (clinical perspective)
CB: contributed to the write‐up of the review, interpretation of results (clinical perspective)
Sources of support
Internal sources
University of York, UK
University of Verona, Italy
External sources
-
National Institute for Health Research (NIHR), UK
LR, NM & RC contributions to the protocol were funded from Cochrane Infrastructure funding to the Common Mental Disorders Cochrane Review Group
Declarations of interest
FB: no conflicts of interest
LR: works as part of the Cochrane Common Mental Disorders Review Group Editorial Base. LR was not involved in the editorial process for this review.
JIB: has been involved in the development of a guided self‐help programme for post‐traumatic stress disorder (PTSD), which has been tested in a Phase II randomised controlled trial (RCT) in partnership with the Healthcare Learning Company. JIB is leading an application for grant funding for a Phase III RCT of the programme. Cardiff University and JIB stand to benefit from royalties if the product is commercialised.
NM: is Deputy Co‐ordinating Editor of the Cochrane Common Mental Disorders Review Group. NM was not involved in the editorial process for this review.
RC: is Joint Co‐ordinating Editor of the Cochrane Common Mental Disorders Review Group. RC was not involved in the editorial process for this review. Cochrane Common Mental Disorders, which has supported parts of the review process, is largely funded by a grant from the National Institute for Health Research (NIHR) in the UK.
GO: no conflicts of interest
DS: has received research grants and/or consultancy honoraria from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier, and Sun.
TW: no conflicts of interest
CB: no conflicts of interest
New
References
References to studies included in this review
Borrelli 2019 {published data only}
Denke 2008 {published data only}
- Denke C, Deja M, Carstens S, Sprung CL, Annane D, Briegel J, et al.Effects of hydrocortisone on posttraumatic stress disorder after septic shock: results from the CORTICUS Berlin Study Group. Critical Care 2008;12(2):P421. [DOI: 10.1186/cc6642] [DOI] [Google Scholar]
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al.Hydrocortisone therapy for patients with septic shock. New England Journal of Medicine 2008;358(2):111-24. [DOI: 10.1056/NEJMoa071366] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoge 2012 {published data only}
- Hoge EA, Worthington JJ, Nagurney JT, Chang Y, Kay EB, Feterowski CM, et al.Effect of acute posttrauma propranolol on PTSD outcome and physiological responses during script-driven imagery. CNS Neuroscience & Therapeutics 2012;18(1):21-7. [DOI: 10.1111/j.1755-5949.2010.00227.x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kagan 2015 {published data only}
- Kagan I, Ben David I, Cohen J, Baniel I, Silva V, Grinev M, et al.SUN-PP054: Long term effects of EPA, GLA and antioxidant administration on post traumatic stress disorder (PTSD) following multiple trauma; a prospective randomized double blind study. Clinical Nutrition 2015;34(Suppl 1):S43. [DOI: 10.1016/S0261-5614(15)30205-3] [DOI] [Google Scholar]
- Kagan I, Cohen J, Stein M, Bendavid I, Pinsker D, Silva V, et al.Preemptive enteral nutrition enriched with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in severe multiple trauma: a prospective, randomized, double-blind study. Intensive Care Medicine 2015;41(3):460-9. [DOI: 10.1007/s00134-015-3646-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kok 2016 {published data only}
- Dieleman JM, Nierich AP, Rosseel PM, Van der Maaten JM, Hofland J, Diephuis JC, et al.Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA 2012;308(17):1761-7. [DOI: 10.1001/jama.2012.14144] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kok L, Hillegers M, Veldhuijzen D, Joels M, Van Dijk D.Individual susceptibility to stress-related psychopathology after cardiac surgery. Psychoneuroendocrinology 2015;61:24. [Google Scholar]
- Kok L, Hillegers MH, Veldhuijzen DS, Boks MP, Dieleman JM, Van Dijk D, et al.Genetic variation in the glucocorticoid receptor and psychopathology after dexamethasone administration in cardiac surgery patients. Journal of Psychiatric Research 2018;103:167-72. [DOI: 10.1016/j.jpsychires.2018.05.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kok L, Hillegers MH, Veldhuijzen DS, Cornelisse S, Nierich AP, Van der Maaten JM, et al.The effect of dexamethasone on symptoms of posttraumatic stress disorder and depression after cardiac surgery and intensive care admission: longitudinal follow-up of a randomized controlled trial. Critical Care Medicine 2016;44(3):512-20. [DOI: 10.1097/CCM.0000000000001419] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kok L, Hillegers MH, Veldhuijzen DS, Cornelisse S, Sep MS, Peelen LM, et al.The effect of dexamethasone on symptoms of post-traumatic stress disorder and depression after cardiac surgery. European Neuropsychopharmacology 2015;44:S74-5. [Google Scholar]
Matsuoka 2015 {published data only}
- Matsumura K, Noguchi H, Nishi D, Hamazaki K, Hamazaki T, Matsuoka YJ.Effects of omega-3 polyunsaturated fatty acids on psychophysiological symptoms of post-traumatic stress disorder in accident survivors: a randomized, double-blind, placebo-controlled trial. Journal of Affective Disorders 2017;224:27-31. [DOI: 10.1016/j.jad.2016.05.054] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Nishi D, Hamazaki K, Yonemoto N, Matsumura K, Noguchi H, et al.Docosahexaenoic acid for selective prevention of posttraumatic stress disorder among severely injured patients: a randomized, placebo-controlled trial. Journal of Clinical Psychiatry 2015;76(8):e1015-22. [DOI: 10.4088/JCP.14m09260] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Nishi D, Tanima Y, Itakura M, Kojima M, Hamazaki K, et al.Serum BDNF as a treatment biomarker for response to docosahexaenoic acid in traumatized people vulnerable to developing psychological distress: a randomized controlled trial. Psychotherapy and Psychosomatics 2015;84 (Suppl 1):47. [DOI: 10.1159/000438780] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Nishi D, Tanima Y, Itakura M, Kojima M, Hamazaki K, et al.Serum pro-BDNF/BDNF as a treatment biomarker for response to docosahexaenoic acid in traumatized people vulnerable to developing psychological distress: a randomized controlled trial. Translational Psychiatry 2015;5:e596. [DOI: 10.1038/tp.2015.89] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Nishi D, Yonemoto N, Hamazaki K, Matsumura K, Noguchi H, et al.Tachikawa project for prevention of posttraumatic stress disorder with polyunsaturated fatty acid (TPOP): study protocol for a randomized controlled trial. BMC Psychiatry 2013;13:8. [DOI: 10.1186/1471-244X-13-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Nishi D.Possible use of omega 3 polyunsaturated fatty acids for prevention of PTSD. Seishin Shinkeigaku Zasshi (Psychiatria et Neurologia Japonica) 2009;111(12):1527-30. [PubMed]
- Noguchi H, Nishi D, Matsumura K, Hamazaki K, Hamazaki T, Matsuoka YJ.Limited effect of omega-3 fatty acids on the quality of life in survivors of traumatic injury: a randomized, placebo-controlled trial. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2017;127:1-5. [10.1016/j.plefa.2017.09.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Noguchi H, Nishi Y, Matsumura K, Hamazaki K, Hamazaki T, Matsuoka Y.The effect of docosahexaenoic acid on quality of life among traumatized people: a randomized, placebo-controlled trial. Psychotherapy and Psychosomatics 2015;84 (Suppl 1):52. [DOI: 10.1159/000438780] [DOI] [Google Scholar]
Orrey 2015 {published data only}
- Orrey D, Bortsov A, Liberzon I, Shupp JW, Jones S, Hwang J, et al.Failure of propranolol to decrease anxiety and PTSD symptoms in a genotype-based RCT of patients with major thermal burn injury [abstract]. Biological Psychiatry 2012;71(8):56S. [DOI: 10.1016/j.biopsych.2012.02.012] [DOI] [Google Scholar]
- Orrey D, Bortsov A, Shupp J, Jones S, Hwang J, Jordan M, et al.Individuals with elevated PTSD symptoms experience increased itch symptom morbidity after major burn injury [abstract]. Biological Psychiatry 2012;71(8):242S. [DOI: 10.1016/j.biopsych.2012.02.014] [DOI] [Google Scholar]
- Orrey DC, Halawa OI, Bortsov AV, Shupp JW, Jones SW, Haith LR, et al.Results of a pilot multicenter genotype-based randomized placebo-controlled trial of propranolol to reduce pain after major thermal burn injury. Clinical Journal of Pain 2015;31(1):21-9. [DOI: 10.1097/Ajp.0000000000000086] [PMID: ] [DOI] [PMC free article] [PubMed]
Pitman 2002 {published data only}
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al.Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry 2002;51(2):189-92. [DOI] [PubMed] [Google Scholar]
Schelling 2001 {published data only}
- Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al.Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Critical care medicine 1999;27(4):723-32. [DOI: 10.1097/00003246-199904000-00025] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhausler HB, Kapfhammer HP.The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biological Psychiatry 2001;50(12):978-85. [DOI: 10.1016/s0006-3223(01)01270-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schelling G, Stoll C, Wichmann A, Haller M, Schuffel W, Briegel J.The effect of hydrocortisone (HC) during septic shock on incidence and intensity of post-traumatic stress disorder (PTSD) in long term survivors. Critical Care Medicine 1998;26:131A. [Google Scholar]
Shaked 2019 {published data only}
- Shaked G, Shaked D, Sebbag G, Czeiger D.The effect of steroid treatment on whiplash associated syndrome: a controlled randomized prospective trial. European Journal of Trauma and Emergency Surgery 2019;6:1115-22. [DOI: 10.1007/s00068-019-01282-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stein 2007 {published data only}
- Stein MB, Kerridge C, Dimsdale JE, Hoyt DB.Pharmacotherapy to prevent PTSD: results from a randomized controlled proof-of-concept trial in physically injured patients. Journal of Traumatic Stress 2007;20(6):923-32. [DOI: 10.1002/jts.20270] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tincu 2016 {published data only}
- Tincu R, Cobilinschi C, Tomescu D, Ghiorghiu Z, Macovei R.Can 5-hydroxytriptophan prevent post-traumatic stress disorder in critically ill patients? [abstract]. Critical Care 2016;20(Suppl 2):119. [Google Scholar]
Weis 2006 {published data only}
- Weis F, Kilger E, Roozendaal B, De Quervain D, Lamm P, Schmidt M, et al.Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. Journal of Thoracic and Cardiovascular Surgery 2006;131(2):277-82. [DOI: 10.1016/j.jtcvs.2005.07.063] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blaha 1999 {published data only}
- Blaha J, Svobodova K, Kapounkova Z.Therapeutical aspects of using citalopram in burns. Acta Chirurgiae Plasticae 1999;41(1):25-32. [PubMed] [Google Scholar]
Delahanty 2013 {published data only}
- Delahanty DL, Gabert-Quillen C, Ostrowski SA, Nugent NR, Fischer B, Morris A, et al.The efficacy of initial hydrocortisone administration at preventing posttraumatic distress in adult trauma patients: a randomized trial. CNS Spectrums 2013;18(2):103-11. [DOI: 10.1017/S1092852913000096)] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty DL, Ostrowski S, Nugent N, Gabert C, Fallon W.Hydrocortisone as a secondary intervention to prevent subsequent PTSD symptoms [abstract]. Psychosomatic Medicine 2011;73(3):A107. [Google Scholar]
EUCTR‐000088‐12‐DE {published data only}
- EUCTR-000088-12-DE.A prospective, single-blinded (rater-blinded), randomized, parallel group study of the efficacy of Quetiapine XR in the treatment of patients with Acute Stress Disorder (DSM-VI 308.3) - QSR-ASD. www.clinicaltrialsregister.eu/ctr-search/trial/2007-000088-12/DE (first received 6 August 2009).
EUCTR‐004177‐83‐NL {published data only}
- EUCTR-004177-83-NL.Oxytocin and social support as early interventions in acute trauma. www.clinicaltrialsregister.eu/ctr-search/trial/2011-004177-83/NL (first received 7 September 2011).
FDA 1999 {published data only}
- FDA.Review and evaluation of clinical data; October 1998. Available at www.accessdata.fda.gov/drugsatfda_docs/nda/99/19-839S026_Zoloft_Clinr_P1.pdf 1999.
Frankova 2017 {published data only}
- Frankova I.Will early intervention with SSRI versus benzodiazepines reduce the risk of developing posttraumatic stress disorder? European Neuropsychopharmacology 2017;27:S998-9. [Google Scholar]
Frijling 2016 {published data only}
- Frijling Jl, Van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M.Effects of intranasal oxytocin on amygdala reactivity to emotional faces in recently trauma-exposed individuals. Social Cognitive and Affective Neuroscience 2016;11(2):327-36. [DOI: 10.1093/scan/nsv116] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling JL, Van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M.Intranasal oxytocin modulates neural processing of emotional faces in recently traumatised individuals at increased risk for PTSD. European Neuropsychopharmacology 2014;27:S602. [DOI: 10.1016/S0924-977X(14)70965-4] [DOI] [Google Scholar]
Gelpin 1996 {published data only}
- Gelpin E, Bonne O, Peri T, Brandes D, Shalev AY.Treatment of recent trauma survivors with benzodiazepines: a prospective study. Journal of Clinical Psychiatry 1996;57(9):390-4. [PubMed] [Google Scholar]
Kaplan 2015 {published data only}
- Kaplan BJ, Rucklidge JJ, Romijn AR, Dolph M.A randomised trial of nutrient supplements to minimise psychological stress after a natural disaster. Psychiatry Research 2015;228(3):373-9. [DOI] [PubMed] [Google Scholar]
Lijffijt 2019 {published data only}
- Lijffijt M, Green CE, Balderston N, Iqbal T, Atkinson M, Vo-Le B, et al.A proof-of-mechanism study to test effects of the NMDA receptor antagonist lanicemine on behavioral sensitization in individuals with symptoms of PTSD. Frontiers in Psychiatry 2019;10:846. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed]
Matsumura 2011 {published data only}
- Matsumura K, Noguchi H, Nishi D, Matsuoka Y.The effect of omega-3 fatty acids on psychophysiological assessment for the secondary prevention of posttraumatic stress disorder: an open-label pilot study. Global Journal of Health Science 2012;4(1):3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuoka 2010 {published data only}
- Matsuoka Y, Nishi D, Yonemoto N, Hamazaki K, Hamazaki T, Hashimoto K.Potential role of brain-derived neurotrophic factor in omega-3 fatty acid supplementation to prevent posttraumatic distress after accidental injury: an open-label pilot study. Psychotherapy and Psychosomatics 2011;80(5):310-2. [DOI] [PubMed] [Google Scholar]
Mellman 2002 {unpublished data only}
Mistraletti 2015 {published data only}
- Mistraletti G, Umbrello M, Sabbatini G, Miori S, Taverna M, Cerri B, et al.Melatonin reduces the need for sedation in ICU patients: a randomized controlled trial. Minerva Anestesiologica 2015;81(12):1298-310. [PMID: ] [PubMed] [Google Scholar]
Naylor 2013 {published data only}
- Naylor JC, Dolber TR, Strauss JL, Kilts JD, Strauman TJ, Bradford DW, et al.A pilot randomized controlled trial with paroxetine for subthreshold PTSD in Operation Enduring Freedom/Operation Iraqi Freedom era veterans. Psychiatry Research 2013;206(2-3):318-20. [DOI: 10.1016/j.psychres.2012.11.008] [PMID: ] [DOI] [PMC free article] [PubMed]
NCT00633685 {published data only}
- NCT00633685.Predictors of treatment response to fluoxetine in PTSD following a recent history of war zone stress exposure. clinicaltrials.gov/ct2/show/NCT00633685 (first received March 4 2008).
NCT00674570 {published data only}
- NCT00674570.Effects of hydrocortisone and D-cycloserine on fear extinction in veterans with posttraumatic stress disorder. clinicaltrials.gov/show/nct00674570 (first received 8 May 2008).
NCT01039766 {published data only}
- NCT01039766.The efficacy of a single dose of intranasal oxytocin in the prevention of post traumatic stress disorder (PTSD). clinicaltrials.gov/ct2/show/record/NCT01039766 (first received 25 December 2009).
NCT02069366 {published data only}
- NCT02069366.Cannabinoid control of fear extinction neural circuits in post-traumatic stress disorder. clinicaltrials.gov/show/NCT02069366 (first received 24 February 2014). [clinicaltrials.gov/show/NCT02069366]
NCT02505984 {unpublished data only}
- NCT02505984.Preventing postpartum depression with intranasal oxytocin (IN-OXT). clinicaltrials.gov/ct2/show/NCT02505984 (first received 22 July 2015).
NCT03724448 {published data only}
- NCT03724448.The efficacy of a herbal supplement in the prevention of PTSD. clinicaltrials.gov/show/NCT03724448 (first received 30 October 2018).
NCT04071600 {published data only}
- NCT04071600.Intranasal neuropeptide Y in clinical trial in level two trauma patients for PTSD and acute stress disorder. clinicaltrials.gov/show/NCT04071600 (first received 28 August 2019).
Nedergaard 2020 {published data only}
- Nedergaard HK, Jensen HI, Stylsvig M, Olsen HT, Strom T, Toft P.Effect of non-sedation on post-traumatic stress and psychological health in survivors of critical illness: a substudy of the NONSEDA randomized trial. Acta Anaesthesiologica Scandinavica 2020;64(8):1136-43. [DOI: 10.1111/aas.13648] [PMID: ] [DOI] [PubMed]
Nishi 2012 {published data only}
- JPRN-UMIN000005367.Omega-3 polyunsaturated fatty acids for secondary prevention of critical incident stress after Japan earthquake March 11 in disaster medical assistance team (DMAT) workers: a randomized controlled trial. rctportal.niph.go.jp/en/detail?trial_id=UMIN000005367 (first received 1 April 2011).
- Matsuoka Y, Nishi D, Koido YI, Nakaya N, Sone T, Noguchi H, et al.Fish oil for attenuating posttraumatic stress symptoms among rescue workers after the Great East Japan earthquake: a randomized controlled trial. Psychosomatic Medicine 2013;75(3):A-131. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Nishi D, Nakaya N, Sone T, Hamazaki K, Hamazaki T, et al.Attenuating posttraumatic distress with omega-3 polyunsaturated fatty acids among disaster medical assistance team members after the Great East Japan Earthquake: the APOP randomized controlled trial. BMC Psychiatry 2011;11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi D, Koido Y, Nakaya N, Sone T, Noguchi H, Hamazaki K, et al.Fish oil for attenuating posttraumatic stress symptoms among rescue workers after the great east Japan earthquake: a randomized controlled trial. Psychotherapy and Psychosomatics 2012;81(5):315-7. [DOI] [PubMed] [Google Scholar]
- Nishi D, Matsuoka Y.Omega-3 fatty acids for attenuating posttraumatic stress symptoms after the earthquake: a randomized controlled trial. Psychotherapy and Psychosomatics 2013;82 Suppl 1:75. [DOI] [PubMed] [Google Scholar]
- Susukida R, Nishi D, Kawashima Y, Koido Y, Mojtabai R, Matsuoka Y J.Generalizability of findings from a randomized controlled trial of fish oil supplementation for attenuating posttraumatic stress symptoms among rescue workers in Japan. Psychotherapy and Psychosomatics 2018;21:21. [DOI] [PubMed] [Google Scholar]
Rabinak 2020 {published data only}
- Rabinak CA, Blanchette A, Zabik NL, Peters C, Marusak HA, Iadipaolo A, et al.Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: a preliminary study. Psychopharmacology 2020;237(6):1813-26. [DOI: 10.1007/s00213-020-05499-8] [PMID: ] [DOI] [PMC free article] [PubMed]
Rucklidge 2012 {published data only}
- Rucklidge JJ, Andridge R, Gorman B, Blampied N, Gordon H, Boggis A.Shaken but unstirred? Effects of micronutrients on stress and trauma after an earthquake: RCT evidence comparing formulas and doses. Human Psychopharmacology 2012;27(5):440-54. [DOI: 10.1002/hup.2246] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Blampied N, Gorman B, Gordon HA, Sole E.Psychological functioning 1 year after a brief intervention using micronutrients to treat stress and anxiety related to the 2011 Christchurch earthquakes: a naturalistic follow-up. Human Psychopharmacology 2014;29(3):230-43. [DOI: 10.1002/hup.2392] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schelling 2004 {published data only}
- Schelling G, Kilger E, Roozendaal B, De Quervain DJ, Briegel J, Dagge A, et al.Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biological Psychiatry 2004;55(6):627-33. [DOI] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, De Quervain DJ.Can posttraumatic stress disorder be prevented with glucocorticoids? Annals of the New York Academy of Sciences 2004;1032:158-66. [DOI] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, De Quervain D, Briegel J.Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Annals of the New York Academy of Sciences 2006;1071:46-53. [DOI] [PubMed] [Google Scholar]
Shalev 2012 {published data only}
- Shalev AY, Ankri Y, Gilad M, Israeli-Shalev Y, Adessky R, Qian M, et al.Long-term outcome of early interventions to prevent posttraumatic stress disorder. Journal of Clinical Psychiatry 2016;77(5):e580-7. [DOI: 10.4088/JCP.15m09932] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shalev AY, Ankri Y, Gilad M, Israeli-Shalev Y, Qian M, Galatzer-Levy I, et al.The differential effect of early and efficient interventions for acute PTSD declines with time. Neuropsychopharmacology 2013;38(Suppl):S365-6. [Google Scholar]
- Shalev AY, Ankri Y, Israeli-Shalev Y, Peleg T, Adessky R, Freedman S.Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem Trauma Outreach And Prevention study. Archives of General Psychiatry 2012;69(2):166-76. [DOI: 10.1001/archgenpsychiatry.2011.127] [PMID: ] [DOI] [PubMed]
- Shalev AY, Ankri YL, Peleg T, Israeli-Shalev Y, Freedman S.Barriers to receiving early care for PTSD: results from the Jerusalem trauma outreach and prevention study. Psychiatric Services 2011;62(7):765-73. [DOI: 10.1176/ps.62.7.pss6207_0765] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoddard 2011 {published data only}
- NCT00182078.A study of sertraline to prevent PTSD. clinicaltrials.gov/ct2/show/NCT00182078 (first received 16 September 2005).
- Stoddard FJ Jr, Luthra R, Sorrentino EA, Saxe GN, Drake J, et al.A randomized controlled trial of sertraline to prevent posttraumatic stress disorder in burned children. Journal of Child and Adolescent Psychopharmacology 2011;21(5):469-77. [DOI] [PubMed] [Google Scholar]
Suliman 2015 {published data only}
- Suliman S, Seedat S, Pingo J, Sutherland T, Zohar J, Stein DJ.Escitalopram in the prevention of posttraumatic stress disorder: a pilot randomized controlled trial. BMC Psychiatry 2015;15:24. [DOI: 10.1186/s12888-015-0391-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takehiro 2014 {published data only}
- Takehiro N, Shen G, Shin T, Satomi T, Yasutake M, Soichiro K, et al.Treatment of posttraumatic stress disorder using the traditional Japanese herbal medicine saikokeishikankyoto: a randomized, observer-blinded, controlled trial in survivors of the great east Japan earthquake and tsunami. Evidence-based Complementary and Alternative Medicine : ECAM 2014;2014:683293. [DOI: 10.1155/2014/683293] [PMID: ] [DOI] [PMC free article] [PubMed]
Treggiari 2009 {published data only}
- Treggiari MM, Romand J-A, Yanez ND, Deem SA, Goldberg J, Hudson L, et al.Randomized trial of light versus deep sedation on mental health after critical illness. Critical Care Medicine 2009;37(9):2527-34. [DOI] [PubMed] [Google Scholar]
Truppman Lattie 2020 {published data only}
- Truppman Lattie D, Nehoff H, Neehoff S, Gray A, Glue P.Anxiolytic effects of acute and maintenance ketamine, as assessed by the Fear Questionnaire subscales and the Spielberger State Anxiety Rating Scale. Journal of Psychopharmacology 2020 Sept 9 [Epub ahead of print]. [DOI: 10.1177/0269881120953991] [PMID: ] [DOI] [PubMed]
Van Zuiden 2017 {published data only}
- Engel S, Van Zuiden M, Frijling JL, Koch SB, Nawijn L, Schumacher S, et al.Patterns of recovery from early posttraumatic stress symptoms after a preventive intervention with oxytocin: hormonal contraception use is a prognostic factor. Biological Psychiatry 2019;85(12):e71-3. [DOI: 10.1016/j.biopsych.2019.01.014] [PMID: ] [DOI] [PubMed]
- Engel S, Van Zuiden M, Frijling JL, Koch SB, Nawijn L, Yildiz RL, et al.Early posttraumatic autonomic and endocrine markers to predict posttraumatic stress symptoms after a preventive intervention with oxytocin. European Journal of Psychotraumatology 2020;11(1):1761622. [DOI: 10.1080/20008198.2020.1761622] [PMID: ] [DOI] [PMC free article] [PubMed]
- Frijling J, Van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M.Intranasal oxytocin attenuates amygdala functional connectivity after a trauma reminder in recently trauma-exposed individuals. European Neuropsychopharmacology 2015;25(Suppl 2):S560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling J, Van Zuiden M, Nawijn L, Koch SB, Veltman DJ, Olff M.Repeated intranasal oxytocin administration as early preventive intervention for PTSD: a randomized controlled trial. European Neuropsychopharmacology 2016;26:S129-30. [Google Scholar]
- Frijling JL, Van Zuiden M, Koch SB, Nawijn L, Goslings JC, Luitse JS, et al.Efficacy of oxytocin administration early after psychotrauma in preventing the development of PTSD: study protocol of a randomized controlled trial. BMC Psychiatry 2014;14:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling JL, Van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M.Intranasal oxytocin affects amygdala functional connectivity after trauma script-driven imagery in distressed recently trauma-exposed individuals. Neuropsychopharmacology 2016;41(5):1286-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling JL, Van Zuiden M, Nawijn L, Koch SB, Veltman DJ, Olff M.Repeated intranasal oxytocin administration as early preventive intervention for PTSD: a randomized controlled trial. European Neuropsychopharmacology 2016;26(Suppl 1):S65-6. [Google Scholar]
- Frijling JL.Preventing PTSD with oxytocin: effects of oxytocin administration on fear neurocircuitry and PTSD symptom development in recently trauma-exposed individuals. European Journal of Psychotraumatology 2017;8(1):1302652. [DOI: 10.1080/20008198.2017.1302652] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, et al.Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PLoS One 2013;8(8):e70084. [DOI: 10.1371/journal.pone.0070084] [PMID: ] [DOI] [PMC free article] [PubMed]
- Olff M, Van Zuiden M, Nawijn L, Koch SB, Frijling JL, Veltman DJ.The effects of intranasal oxytocin after trauma. European Neuropsychopharmacology 2017;27:S527. [DOI] [PubMed] [Google Scholar]
- Van Zuiden M, Frijling JL, Nawijn L, Koch SB, Goslings J, Luitse JS, et al.Intranasal oxytocin to prevent posttraumatic stress disorder symptoms: a randomized controlled trial in emergency department patients. Biological Psychiatry 2017;81(12):1030-40. [DOI] [PubMed] [Google Scholar]
- Van Zuiden M, Frijling JL, Nawijn L, Koch SB, Veltman DJ, Olff M.Prevention of PTSD by intranasal oxytocin administration and moderating effects of glucocorticoids. Biological Psychiatry 2016;1:68S. [Google Scholar]
Yang 2011 {published data only}
- Yang HT, Xiong Y, Zhou F.Immunoprotection of dexmedetomidine from acute psychological stress. Journal of Dalian Medical University 2011;33(5):479-82. [Google Scholar]
Zoellner 2001 {published data only}
- Zoellner LA, Foa EB, Feeny NC, Meadows EA, Hembree EA.Prevention of chronic PTSD in recent assault victims. In: 17th Annual Meeting, International Society for Traumatic Stress Studies; 2001 December 6 - 9; New Orleans (LA). 2001.
Zohar 2011 {published data only}
- Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, et al.High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. European Neuropsychopharmacology 2011;21(11):796–809. [DOI] [PubMed] [Google Scholar]
- Zohar J.High dose hydrocortisone immediately after trauma may alter the trajectory of posttraumatic stress disorder: translational interplay between clinical and animal studies [abstract]. Neuropsychopharmacology 2011;36:S24. [DOI] [PubMed] [Google Scholar]
- Zohar J.Hydrocortisone in the golden hours and PTSD trajectory-clinical and animal studies. International Journal of Neuropsychopharmacology 2012;15 (Supp 1):18. [Google Scholar]
- Zohar J.New insights into secondary prevention in PTSD. International Journal of Neuropsychopharmacology 2014;17(Suppl 1):20. [DOI: 10.1017/S1461145714000741] [DOI]
Zohar 2017a {published data only}
- Zohar J.Long-term effects of high-dose hydrocortisone immediately after trauma: a double blind, placebo-controlled prospective study. Neuropsychopharmacology 2017;43:S89-90. [Google Scholar]
Zohar 2017b {published data only}
- Zohar J, Fostick L, Juven-Wetzler A, Kaplan Z, Shalev H, Schreiber G, et al.Secondary prevention of chronic PTSD by early and short-term administration of escitalopram: a prospective randomized, placebo-controlled, double-blind trial. Journal of Clinical Psychiatry 2017;11:11. [DOI] [PubMed] [Google Scholar]
- Zohar J.Golden hours in psychiatric disorders: focus on PTSD [abstract]. Indian Journal of Psychiatry 2012;54:S30-1. [Google Scholar]
References to ongoing studies
McMullan 2020 {published data only}
- McMullan J, Droege C, Strilka R, Hart K, Lindsell C.Intranasal ketamine as an adjunct to fentanyl for the prehospital treatment of acute traumatic pain: design and rationale of a randomized controlled trial. Prehospital Emergency Care 2020:1-11. [DOI: 10.1080/10903127.2020.1808746] [PMID: ] [DOI] [PubMed]
NCT03997864 {unpublished data only}
- NCT03997864.Administration of prazosin to prevent PTSD in adult women after sexual assault. clinicaltrials.gov/show/NCT03997864 (first received 25 June 2019).
NCT04274361 {published data only}
- NCT04274361.Ketamine for pain control after severe traumatic injury. clinicaltrials.gov/show/NCT04274361 (first received 18 February 2020).
Additional references
Amos 2014
- Amos T, Stein DJ, Ipser JC.Pharmacological interventions for preventing post-traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD006239. [DOI: 10.1002/14651858.CD006239.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Fifth edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Astill Wright 2019
- Astill Wright L, Sijbrandij M, Sinnerton R, Lewis C, Roberts NP, Bisson JI.Pharmacological prevention and early treatment of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Translational Psychiatry 2019;9(1):334. [DOI: 10.1038/s41398-019-0673-5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aupperle 2012
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP.Executive function and PTSD: disengaging from trauma. Neuropharmacology 2012;62(2):686-94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbee 1993
- Barbee JG.Memory, benzodiazepines, and anxiety: integration of theoretical and clinical perspectives. Journal of Clinical Psychiatry 1993;54 Suppl:86-101. [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J.An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA.An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertolini 2020
- Bertolini F, Robertson L, Ostuzzi G, Meader N, Bisson JI, Churchill R, et al.Early pharmacological interventions for acute traumatic stress symptoms: a network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013613. [DOI: 10.1002/14651858.CD013613] [CD013613] [DOI] [PMC free article] [PubMed] [Google Scholar]
Besnard 2012
- Besnard A, Caboche J, Laroche S.Reconsolidation of memory: a decade of debate. Progress in Neurobiology 2012;99(1):61-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Birur 2017
- Birur B, Moore NC, Davis LL.An evidence-based review of early intervention and prevention of posttraumatic stress disorder. Community Mental Health Journal 2017;53(2):183-201. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bisson 2007
- Bisson JI.Post-traumatic stress disorder. BMJ (Clinical Research Ed.) 2007;334(7597):789-93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisson 2021
- Bisson JI, Wright LA, Jones KA, Lewis C, Phelps AJ, Sijbrandij M, et al.Preventing the onset of post traumatic stress disorder. Clinical Psychology Review 2021;86:102004. [DOI: 10.1016/j.cpr.2021.102004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Blake 1995
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al.The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress 1995;8(1):75-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bogic 2015
- Bogic M, Njoku A, Priebe S.Long-term mental health of war-refugees: a systematic literature review. BMC International Health and Human Rights 2015;15:29. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al, Groupe d'Etude des Indices D'efficacite.The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique. 3: Comparaison des indices et utilisation]. Therapie 1999;54(4):405-11. [PMID: ] [PubMed] [Google Scholar]
Breslau 2012
- Breslau N.Epidemiology of posttraumatic stress disorder in adults. In: Beck JG, Sloan DM, editors(s). The Oxford Handbook of Traumatic Stress Disorders. Oxford, UK: Oxford University Press, 2012:84-97. [Google Scholar]
Brewin 2000
- Brewin CR, Andrews B, Valentine JD.Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology 2000;68(5):748-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bryant 2009
- Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC.A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biological Psychiatry 2009;65(5):438-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cahill 1994
- Cahill L, Prins B, Weber M, McGaugh JL.Beta-adrenergic activation and memory for emotional events. Nature 1994;371(6499):702-4. [DOI] [PubMed] [Google Scholar]
Cohen 2008
- Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J.Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biological Psychiatry 2008;64(8):708-17. [DOI] [PubMed] [Google Scholar]
Cohen 2010
- Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J.Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. Journal of Neuroendocrinology 2010;22(8):889-904. [DOI] [PubMed] [Google Scholar]
Covi 1984
- Covi L, Lipman RS.Primary depression or primary anxiety. A possible psychometric approach to a diagnostic dilemma. Clinical Neuropharmacology 1984;7:S502–3. [Google Scholar]
Deeks 2002
- Deeks JJ.Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575-600. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors).Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook-5-1.cochrane.org.
Delahanty 2000
- Delahanty DL, Raimonde AJ, Spoonster E.Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biological Psychiatry 2000;48(9):940-7. [DOI] [PubMed] [Google Scholar]
De Vries 2009
- De Vries GJ, Olff M.The lifetime prevalence of traumatic events and posttraumatic stress disorder in the Netherlands. Journal of Traumatic Stress 2009;22(4):259-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C.Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote [Computer program]
- EndNote.Philadelphia (PA): Clarvariate Analytics, 2019.
Forneris 2013
- Forneris CA, Gartlehner G, Brownley KA, Gaynes BN, Sonis J, Coker-Schwimmer E, et al.Interventions to prevent post-traumatic stress disorder: a systematic review. American Journal of Preventitive Medicine 2013;44(6):635-50. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N.Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [DOI] [PubMed] [Google Scholar]
Galatzer‐Levy 2014
- Galatzer-Levy IR, Karstoft KI, Statnikov A, Shalev AY.Quantitative forecasting of PTSD from early trauma responses: a Machine Learning application. Journal of Psychiatric Research 2014;59:68-76. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Version accessed prior to 17 September 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guthrie 2005
- Guthrie RM, Bryant RA.Auditory startle response in firefighters before and after trauma exposure. American Journal of Psychiatry 2005;162(2):283-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M.The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32(1):50-5. [DOI: 10.1111/j.2044-8341.1959.tb00467.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M.A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JP, Deeks JJ.Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook-5-1.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors).Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook-5-1.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors).Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook-5-1.cochrane.org.
Holbrook 2010
- Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL.Morphine use after combat injury in Iraq and post-traumatic stress disorder. New England Journal of Medicine 2010;362(2):110-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Howlett 2016
- Howlett JR, Stein MB.Prevention of trauma and stressor-related disorders: a review. Neuropsychopharmacology 2016;41(1):357-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hruska 2014
- Hruska B, Cullen PK, Delahanty DL.Pharmacological modulation of acute trauma memories to prevent PTSD: considerations from a developmental perspective. Neurobiology of Learning and Memory 2014;112:122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISTSS 2018
- International Society for Traumatic Stress Studies.Posttraumatic Stress Disorder Prevention and Treatment Guidelines. Available at istss.org/getattachment/Treating-Trauma/New-ISTSS-Prevention-and-Treatment-Guidelines/ISTSS_PreventionTreatmentGuidelines_FNL.pdf.aspx 2018.
Karam 2014
- Karam EG, Friedman MJ, Hill ED, Kessler RC, McLaughlin KA, Petukhova M, et al.Cumulative traumas and risk thresholds: 12-month PTSD in the World Mental Health (WMH) surveys. Depression and Anxiety 2014;31(2):130-42. [DOI: 10.1002/da.22169] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karstoft 2015
- Karstoft KI, Galatzer-Levy IR, Statnikov A, Li Z, Shalev AY.Bridging a translational gap: using machine learning to improve the prediction of PTSD. BMC Psychiatry 2015;15:30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearns 2012
- Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO.Early interventions for PTSD: a review. Depression and Anxiety 2012;29(10):833-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelmendi 2016
- Kelmendi B, Adams TG, Yarnell S, Southwick S, Abdallah CG, Krystal JH.PTSD: from neurobiology to pharmacological treatments. European Journal of Psychotraumatology 2016;7:31858. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2014
- Kessler RC, Rose S, Koenen KC, Karam EG, Stang PE, Stein DJ, et al.How well can post-traumatic stress disorder be predicted from pre-trauma risk factors? An exploratory study in the WHO World Mental Health Surveys. World Psychiatry Oct 2014;13(3):265-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2017
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet EJ, Cardoso G, et al.Trauma and PTSD in the WHO World Mental Health Surveys. European Journal of Psychotraumatology 2017;8(Suppl 5):1353383. [DOI: 10.1080/20008198.2017.1353383] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koch 2014
- Koch SB, Van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M.Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinology 2014;40:242-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Koenen 2017
- Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, et al.Posttraumatic stress disorder in the World Mental Health Surveys. Psychological Medicine 2017;47(3):2260–74. [DOI: 10.1017/S0033291717000708] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2016
- Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW.Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systematic review and meta-analyses to determine first-line treatments. Depression and Anxiety 2016;33(9):792-806. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leonard 2000
- Leonard BE, Richelson E.Synaptic effects of antidepressants. In: Buckley PF, Waddington JL, editors(s). Schizophrenia and Mood Disorders: The New Drug Therapies in Clinical Practice. Boston (MA): Butterworth-Heinemann, 2000:67–84. [Google Scholar]
Licznerski 2013
- Licznerski P, Duman RS.Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience 2013;251:33-50. [DOI: 10.1016/j.neuroscience.2012.09.057] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lydiard 2003
- Lydiard RB.The role of GABA in anxiety disorders. Journal of Clinical Psychiatry 2003;64(Suppl 3):21-7. [PMID: ] [PubMed] [Google Scholar]
Matsuoka 2011
- Matsuoka Y.Clearance of fear memory from the hippocampus through neurogenesis by omega-3 fatty acids: a novel preventive strategy for posttraumatic stress disorder? BioPsychoSocial Medicine 2011;5:3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McFarlane 1997
- McFarlane AC, Atchison M, Yehuda R.The acute stress response following motor vehicle accidents and its relation to PTSD. Annals of the New York Academy of Sciences 1997;821:437-41. [DOI] [PubMed] [Google Scholar]
McNally 2003
- McNally GP, Westbrook RF.Temporally graded, context-specific retrograde amnesia and its alleviation by context preexposure: effects of postconditioning exposures to morphine in the rat. Journal of Experimental Psychology 2003;29(2):130-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Meyerhoff 2014
- Meyerhoff DJ, Mon A, Metzler T, Neylan TC.Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep 2014;37(5):893-900. [DOI: 10.5665/sleep.3654] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M.A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [DOI: 10.1192/bjp.134.4.382] [DOI] [PubMed] [Google Scholar]
Mouthaan 2015
- Mouthaan J, Sijbrandij M, Reitsma JB, Luitse JS, Goslings JC, Gersons BP, et al.The role of early pharmacotherapy in the development of posttraumatic stress disorder symptoms after traumatic injury: an observational cohort study in consecutive patients. General Hospital Psychiatry 2015;37(3):230-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence.Post-traumatic stress disorder: management (NG116); December 2018. Available at www.nice.org.uk/guidance/ng116. [PubMed]
Nickerson 2013
- Nickerson A, Aderka IM, Bryant RA, Hofmann SG.The role of attribution of trauma responsibility in posttraumatic stress disorder following motor vehicle accidents. Depression and Anxiety 2013;30(5):483-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Olff 2010
- Olff M, Langeland W, Witteveen A, Denys D.A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectrums 2010;15(8):522-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ozer 2003
- Ozer EJ, Best SR, Lipsey TL, Weiss DS.Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological Bulletin 2003;129(1):52-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Phoenix Australia 2020
- Phoenix Australia.The Australian Guidelines for the Prevention and Treatment of Acute Stress Disorder (ASD), Posttraumatic Stress Disorder (PTSD) and Complex PTSD. www.phoenixaustralia.org/australian-guidelines-for-ptsd/ (accessed prior to 19 January 2021).
Pitman 1989
Pitman 2012
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al.Biological studies of post-traumatic stress disorder. Nature Reviews. Neuroscience 2012;13(11):769-87. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plot Digitizer 2015 [Computer program]
- Plot Digitizer.Huwaldt JA, Version 2.6.8. SourceForge, 24 October 2015. Available at plotdigitizer.sourceforge.net/.
Qi 2016
- Qi W, Gevonden M, Shalev A.Prevention of post-traumatic stress disorder after trauma: current evidence and future directions. Current Psychiatry Reports 2016;18(2):20. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reist 2001
- Reist C, Duffy JG, Fujimoto K, Cahill L.beta-Adrenergic blockade and emotional memory in PTSD. International Journal of Neuropsychopharmacology 2001;4(4):377-83. [DOI] [PubMed] [Google Scholar]
Ressler 2020
- Ressler KJ.Translating across circuits and genetics toward progress in fear- and anxiety-related disorders. American Journal of Psychiatry 2020;177(3):214-22. [DOI: 10.1176/appi.ajp.2020.20010055] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2019
- Roberts NP, Kitchiner NJ, Kenardy J, Robertson L, Lewis C, Bisson JI.Multiple session early psychological interventions for the prevention of post-traumatic stress disorder. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD006869. [DOI: 10.1002/14651858.CD006869.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2002
- Rose S, Bisson J, Churchill R, Wessely S.Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD000560. [DOI: 10.1002/14651858.CD000560] [DOI] [PubMed] [Google Scholar]
Santarelli 2003
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al.Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301(5634):805-9. [DOI: 10.1126/science.1083328] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH.Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook-5-1.cochrane.org.
Sheehan 1996
- Sheehan DV, Harnett-Sheehan K, Raj BA.The measurement of disability. International Clinical Psychopharmacology 1996;11 Suppl 3:89-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sijbrandij 2015
- Sijbrandij M, Kleiboer A, Bisson JI, Barbui C, Cuijpers P.Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Lancet: Psychiatry 2015;2(5):413-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Southwick 1999
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Arnsten A, Charney DS.Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry 1999;46(9):1192-204. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch RL, Lushene RE.Manual for the Stait-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press, 1970. [Google Scholar]
Steel 2009
- Steel Z, Chey T, Silove D, Marnane C, Bryant RA, Van Ommeren M.Association of torture and other potentially traumatic events with mental health outcomes among populations exposed to mass conflict and displacement: a systematic review and meta-analysis. JAMA 2009;302(5):537-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stein 2006
- Stein DJ, Ipser JC, Seedat S.Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD002795. [DOI: 10.1002/14651858.CD002795.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turrini 2017
- Turrini G, Purgato M, Ballette F, Nose M, Ostuzzi G, Barbui C.Common mental disorders in asylum seekers and refugees: umbrella review of prevalence and intervention studies. International Journal of Mental Health Systems 2017;11:51. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Ameringen 2004
- Van Ameringen M, Mancini C, Pipe B, Bennett M.Antiepileptic drugs in the treatment of anxiety disorders: role in therapy. Drugs 2004;64(19):2199-220. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Zuiden 2017
- Van Zuiden M, Frijling JL, Nawijn L, Koch SB, Goslings JC, Luitse JS, et al.Intranasal oxytocin to prevent posttraumatic stress disorder symptoms: a randomized controlled trial in emergency department patients. Biological Psychiatry 2017;81(12):1030-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Veterans Affairs/Department of Defense 2017
- Veterans Affairs/Department of Defense.VA/DoD Clinical Practice Guidelines: Management of Posttraumatic Stress Disorder and Acute Stress Disorder; 2017. Available at www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf.
Wade 2013
- Wade D, Hardy R, Howell D, Mythen M.Identifying clinical and acute psychological risk factors for PTSD after critical care: a systematic review. Minerva Anestesiologica 2013;79(8):944-63. [PMID: ] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD.The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Medical Care 1992;30:473-81. [PubMed] [Google Scholar]
Weathers 2001
WHO 1992
- World Health Organization.The ICD-10 Classification of Mental and Behavioural Disorders. Geneva, Switzerland: World Health Organization, 1992. [Google Scholar]
WHO 1997
- World Health Organization.Composite International Diagnostic Interview (CIDI) 2.1. Geneva, Switzerland: World Health Organization, 1997. [Google Scholar]
WHO 2017
- World Health Organization.The selection and use of essential medicines: report of the WHO Expert Committee, 2017 (including the 20th WHO Model List of Essential Medicines and the 6th WHO Model List of Essential Medicines for Children). Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
Zatzick 1997
- Zatzick DF, Marmar CR, Weiss DS, Browner WS, Metzler TJ, Golding JM, et al.Posttraumatic stress disorder and functioning and quality of life outcomes in a nationally representative sample of male Vietnam veterans. American Journal of Psychiatry 1997;154(12):1690-5. [PMID: ] [DOI] [PubMed] [Google Scholar]


