Abstract
Background
Elective cesarean section (ECS) is the most common reason for the increasing cesarean section rate worldwide, and it is reported to be related to adverse short-term and long-term outcomes in both mothers and infants. Findings on the association between ECS and overweight and obesity in children are controversial in recent studies. Therefore, we conducted a systematic review and meta-analysis to examine the effect of ECS on offspring's overweight and obesity.
Methods
PubMed, Science Direct, Web of Science, CNKI (China National Knowledge Infrastructure), Wanfang Database (in Chinese), and China Biology Medicine disc databases were searched using different combinations of three groups of keywords: “elective cesarean section,” “overweight/obesity,” and “children.” Nine cohort studies and 11 independent risk estimates were finally identified.
Results
We have observed significant association between ECS and children's obesity, the total pooled risk ratio (RR) being 1.10 (95% CI: 1.01–1.18; I2 = 32.4%). In subgroup analysis, ECS was found to be associated with the occurrence of obesity in preschoolers (RR = 1.12, 95% CI: 1.02–1.22; I2 = 16.8%). Furthermore, it revealed that ECS was related with the high risk of children's obesity where the rate of ECS exceeded 10%. No significant association was observed between ECS and children's overweight, and the RR was 1.12 (95% CI: 0.94–1.30; I2 = 55.6%).
Conclusions
Overall, it indicated that children born via ECS had an increased risk of later-life obesity. Given the global increase in childhood obesity, our findings would provide evidence-based reference for early life intervention on children's obesity.
Systematic Review Registration
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021267211, identifier: CRD42021267211.
Keywords: cesarean section, obesity, children, systematic review, meta-analysis
Introduction
With the development of perinatal medicine, cesarean section (CS) has become an effective means to save women's and infants' lives when encountering severe conditions. In recent years, the CS rate has been increasing rapidly around the world, and has been reported from 26.4% in the WHO Global Survey (2004–08) to 31.2% in the WHO Multi-Country Survey (2010–11) (1).
CS can be divided into elective CS (ECS) and emergency CS (EMCS). EMCS is performed under an acute situation that threatens the life of both mother and fetus and requires immediate termination of pregnancy. Unlike EMCS, ECS is not only based on medical indications, but also up to the request of mothers and their families. CS on maternal request is the most frequently cited reason for the increasing CS rates (2, 3). WHO has estimated that 6.2 million excess—i.e., not medically indicated—CS are being performed each year, 50% of which are in Brazil and China alone (4).
There is no evidence showing the benefits of CS for women or infants who do not require the surgery. In contrast, CS is reported to be associated with adverse short-term and long-term physical and psychological outcomes in both mothers and infants (5). For instance, CS is related to offspring's atopy and asthma, and may also cause reduced intestinal gut microbiome diversity (6), which may contribute to an individual's obesity in later life (7).
Overweight/obesity is widely recognized as a serious public health problem, as it relates to a range of health problems in children and adults, including cardiovascular disease, diabetes, orthopedic disease, low self-esteem, depression, and social marginalization (8). The prevalence of childhood and adult obesity has increased in the past several decades in both developed and developing countries (9). In 2016, the WHO estimated that 41 million children under five were affected by overweight or obesity worldwide, and half of them were in Asia (10). Early childhood overweight and obesity may persist into middle childhood and adolescence (11).
Previous studies have paid close attention to the relationship between CS and offspring's overweight and obesity, but the findings are contradictive. Mueller et al. found that compared to vaginal delivery (VD), CS was associated with a higher rate of offspring's weight gain over the first year (12). Other studies also showed that CS was associated with an increased risk of children's obesity (13, 14). However, a longitudinal cohort study in Ireland argued no association between modes of delivery and children's obesity at the ages of 2 and 5 (15). More than that, when investigating the association between CS and offspring's overweight/obesity, limited studies have distinguished ECS from non-ECS, and the findings are contradictory as well. Cai et al. found that ECS was significantly associated with the risk of infants' overweight or obesity. Masukume et al. revealed that there was a statistically significant association between ECS and obesity with children aged 24 months, but the relationship was not observed when they are 54 months old (16, 17). A Swedish population-based cohort study found no evidence of an association between non-elective or elective CS and obesity in young adulthood (18).
From what has been mentioned above, the association between ECS and offspring's overweight/obesity is controversial. It is regrettably that there is no systematic review on this topic with a matter of concern. The purpose of this study was thus to summarize the available evidence and systematically examine the association between ECS and offspring's weight development from birth to adolescence.
Methods
Literature Search and Inclusion Criteria
We searched for relevant publications in PubMed, Science Direct, Web of Science, CNKI (China National Knowledge Infrastructure), Wanfang Database (in Chinese), and China Biology Medicine disc databases from inception to the end of December 2020. We used different combinations on the three clusters of terms: (1) “cesarean/cesarean delivery/section,” “Planned cesarean/cesarean,” “elective cesarean/cesarean”; (2) “weight,” “fat*,” “adiposity,” “obesity,” “BMI,” “physical growth,” “physical development”; (3) “child*,” “adolescen*,” “infant,” “toddler,” “offspring” (Supplemental Table 1). After excluding duplicate records, we scanned titles and abstracts and then retrieved full texts of potentially relevant articles for detailed evaluation. All searches were conducted by Zhang SS and double-checked by Qin XY. The two reviewers reached a consensus at each stage before proceeding.
Studies were considered for inclusion if (1) the study was an original research published in English or in other languages with an English abstract; (2) the study used a cohort (historical or prospective) or case–control design and examined the association between ECS and birth weight or overweight/obesity in offspring; (3) the authors reported mean ± standard deviation of birth weight or RRs/ORs with 95% confidence intervals (CIs) for overweight/obesity in the study.
Data Extraction and Quality Assessment
Data extraction was independently extracted by two reviewers using a pretested structured form. Discrepancies were resolved on the basis of a consensus discussion between the two reviewers or with the third reviewer (Li PX).
Where possible, we extracted the adjusted effect estimates. If several multivariate-adjusted estimates were available, we only extracted the most adequately adjusted one. If a study longitudinally assessed offspring's overweight or obesity risk based on the same cohort, we extracted all estimates. If a study reported more than one estimate within the same group at different ages, we extracted only the estimate at the longest follow-up period. The extracted information mainly included author, study design, year of publication, country, study sample, children's age, definition of obesity, estimates of outcome, gestational age, and adjustment for confounding factors.
The quality of each included study was independently assessed by two reviewers according to the Newcastle-Ottawa Scale (NOS). The scale awarded a maximum score of 9 to each study: 4 for study group selection, 2 for the comparability between groups, and 3 for the ascertainment of outcome measurement for cohort studies. We defined a score of 7 as high quality, 4–6 as medium, and 3 as low quality. The quality assessment of all included studies is presented in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis.
| Author | Study design | Year of publication | Country | Study sample (n, ECSa /VDb) | Children's age | ECSa rate | Definition of obesity | Estimates of outcome | Gestational age (weeks, ECSa/VDb) | Adjustment for confounding factors | NOS. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sitarik et al. | Cohort Study | 2020 | USA | 82/358 | 10 years | 14.3% | BMI ≥ 95th percentile | Obesity: RR (95% CI):1.77 (1.16, 2.72) birth weight (g, ECS/VD): 3,446 ± 758/3,337 ± 529 |
38.3 ± 1.7/38.9 ± 1.6 | Marital status, maternal race, prenatal tobacco smoke exposure, maternal age, maternal BMI, any hypertensive disorders during pregnancy, gestational diabetes, prenatal antibiotic use, child sex, parity, birthweight z-score | 8 |
| Maharlouei et al. | Cohort Study | 2019 | Iran | 1,071/1,367 | Newborn | / | / | Birth weight (g, ECS/VD): 3,166 ± 442.4/3,213 ± 454.8 |
38.4 ± 1.2/39.2 ± 1.2 | / | 8 |
| Masukume et al. | Cohort Study | 2019 | UK | 1,669/12,567 | 3–5 years | 9.2% | IOTFc | Obesity: RR(95% CI):0.96 (0.67, 1.38) | / | Maternal age, ethnicity, education, marital status, couple income, infant sex, birth weight, smoking during pregnancy, gestational age, diabetes mellitus, parity, pre-pregnancy BMI | 8 |
| Masukume et al. | Cohort Study | 2019 | New Zealand | 618/5,067 | 4.5 years | 9.4% | IOTFc | Overweight: RR:(95%CI):1.27 (0.99, 1.63) Obesity: RR (95% CI): 0.85 (0.56, 1.29) |
/ | Maternal age, education, ethnicity, marital status, infant sex, birth weight, smoking, gestational age, gestational diabetes, parity, pre-pregnancy BMI. | 8 |
| Ahlqvist et al. | Cohort Study | 2019 | Sweden | 4,147/89,024 | 18 years | 4% | WHO BMI > 30 | Overweight: RR: (95%CI): 0.99 (0.90, 1.08) Obesity: RR (95% CI): 1.02 (0.88, 1.18) birth weight (g, ECS/VD) 3,444 ± 554.7/3,633.1 ± 499.4 |
38.2 ± 1.3/39.6 ± 1.5 | Pre-pregnancy maternal BMI, maternal diabetes at delivery, maternal hypertension at delivery, maternal smoking, parity, parental education, maternal age at delivery, birth weight standardized according to gestational age, preeclampsia, gestational age. | 8 |
| Zhou et al. | Cohort Study | 2019 | China | 737/730 | 4–7 years | 50.2% | Central obesity as waist circumference > 75th age- and sex-specific percentile of that for Chinese preschool children | Obesity: OR (95% CI): 1.33 (1.02, 1.72) | / | Maternal age, educational level, BMI in early pregnancy, gestational weight gain, micronutrient supplementation, sex, gestational age, sex-adjusted birthweight-for-gestational age z scores, and age at follow-up visit. | 8 |
| Cai et al. | Cohort Study | 2018 | Singapore | 74/505 | 1 year | 10.1% | Risk of overweight: 1sd < BAZd ≤ 2SD Overweight: BAZ > 2SD | Overweight: OR (95% CI): 2.05 (1.08, 3.90) | / | Ethnicity, maternal age at delivery, maternal educational level, parity, early pregnancy body mass index, antenatal active or passive smoking, hypertensive disorders of pregnancy, gestational diabetes, sex-adjusted birth weight–for–gestational agezscore. | 8 |
| Masukume et al. | Cohort Study | 2018 | Ireland | 1,402/6,579 | 5 years | 12.7% | IOTFc | Overweight: RR: (95% CI): 1.13 (0.94, 1.35) Obesity: RR (95% CI): 1.30 (0.98, 1.73) birth weight (g, ECS/VD) 3,431 ± 562/3,507 ± 502 |
38.7 ± 1.7/39.7 ± 1.9 | Maternal age, education, ethnicity, marital status, region, infant sex, gestational age, pre-eclampsia, gestational diabetes, parity | 8 |
| Black et al. | Cohort Study | 2015 | UK Scotland | 12,355/ 252,917 | 5 years | 3.8% | BMI > the 95th centile | Obesity (RR 95% CI): 1.12 (0.99, 1.26) birth weight (g, ECS/VD) 3,301 ± 494.1/3,379.4 ± 454.6 |
38.7 ± 1.0/39.8 ± 1.2 | Maternal age, gestation at birth, maternal Carstairs decile, maternal smoking status, birth weight, year of delivery, male infant, breastfeeding at 6 weeks, maternal BMI | 8 |
ECS, Elective Cesarean Section.
VD, Vaginal Delivery.
IOTF, International Obesity Task Force.
BAZ, BMI-for-age z scores.
Statistical Analysis
All analyses were conducted by using stata11. Main outcomes in the current study included offspring's birth weight and later overweight/obesity. When estimating the overall effect of ECS on offspring's overweight/obesity, since the OR values do not define the actual risk of the association of interest, we converted all ORs into RRs by the following formula: (RR: risk ratio; OR: odds ratio; Pref: Prevalence of the outcome in the reference group) (19).
We also performed two subgroup analyses to examine the effect of ECS on children's obesity. First, we re-run the analysis stratified by children's age. In age stratification, “preschool-age period” referred to 3–7 years and “adolescence” referred to 10–18 years. Second, we examined the effect of ECS on children's obesity, respectively, in the studies with “high” ECS rate (>10%) and “low” ECS rate (10%).
Forest plot was used to present the RR value of overweight/obesity. We used I2 to evaluate the statistical heterogeneity between studies. If I2 < 50%, and the heterogeneity was low and acceptable, then the fixed effect model was used for meta-analysis; if I2 > 50% and heterogeneity between studies was high, the random effect model was adopted. Publication bias was detected by the funnel plot and Egger's/Begg's test. Sensitivity analysis was introduced to assess the effect of each individual study on the overall estimate. We also used sensitivity analysis to investigate the stability of the outcome. The pooled risk ratios were observed after removing each study. If the pooled risk ratios exceeded the original confidence interval, the finding was regarded as unstable; otherwise, the finding was considered to be stable.
Protocol Registration
The protocol for this systematic review and meta-analysis has been registered in the PROSPERO (Protocol No. CRD42021267211).
Results
Characteristics of the Selected Studies
A total of 2,023 studies were obtained through literature search. After deleting the repeated literature, there were 1,552 articles left. After reviewing the titles and abstracts, 43 relevant studies were obtained. After reading the full text, a total of 9 studies met the inclusion criteria and were used for final meta-analysis (16–18, 20–25) (Figure 1).
Figure 1.
Flowchart on literature search and study selection.
The characteristics of the nine eligible studies are listed in Table 1. These nine studies were conducted in eight countries and were all cohort studies. With the exception of one study that only covered boys, the others included both boys and girls in their samples. The total sample size was 391,269, ranging between 74 and 252,917 in individual studies. The participants were followed up from birth to median 18 years of age.
The Association Between ECS and Infants' Birth Weight
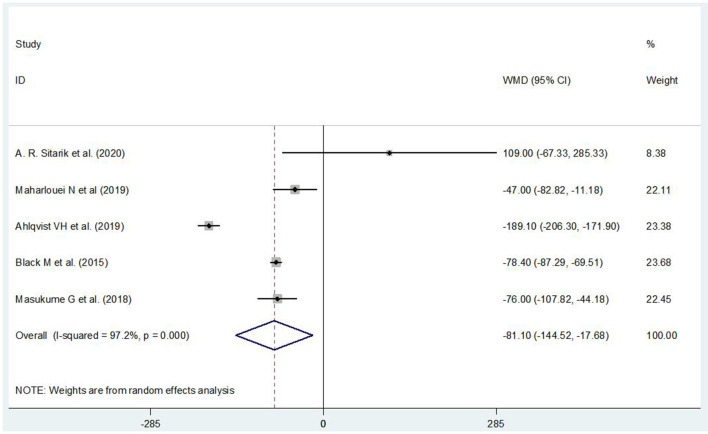
Five of the 9 cohort studies reported children's birth weight (n = 369,282) born via ECS and VD. Birth weight in children born via ECS was found to be lower than that in children born via VD (WMD = −81.10, 95% CI: −144.52, −17.68) (Figure 2). Publication bias was detected by the funnel plot and Egger's test (P > |t| = 0.986), and it indicated that there was no publication bias. The sensitivity analysis showed that the pooled RRs did not exceed the original confidence interval after removing each study in turn, and indicated that the result was stable.
Figure 2.
The association between ECS and infants' birth weight.
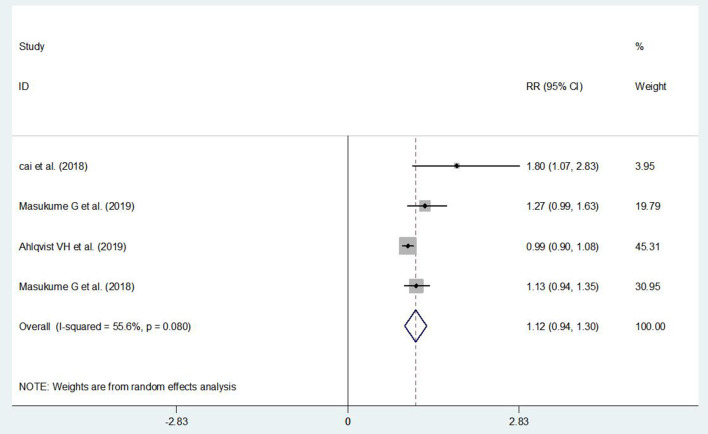
The Association Between ECS and Children's Overweight
It did not show a significant association between ECS and children's overweight (RR = 1.12, 95% CI: 0.94–1.30) (Figure 3). Publication bias was detected by the funnel plot and Egger's test (P > |t| = 0.001), and it indicated that there was publication bias. Sensitivity analysis had shown that the finding was stable.
Figure 3.
The association between ECS and children's overweight.
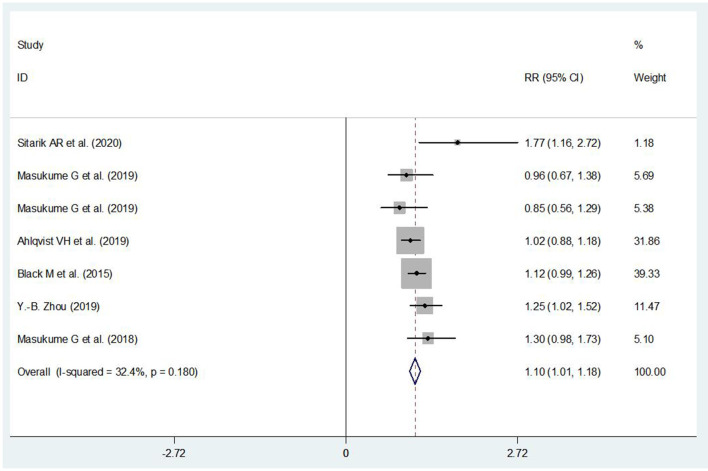
The Association Between ECS and Children's Obesity
Compared with those being born via VD, it showed an increased risk of being obesity in children born via ECS (RR = 1.10, 95% CI: 1.01, 1.18) (Figure 4). Publication bias was detected by the funnel plot and Egger' s test (P > |t| = 0.638), and it indicated that there was no statistically significant publication bias, as it was not likely to substantially affect the results.
Figure 4.
The association between ECS and children's obesity.
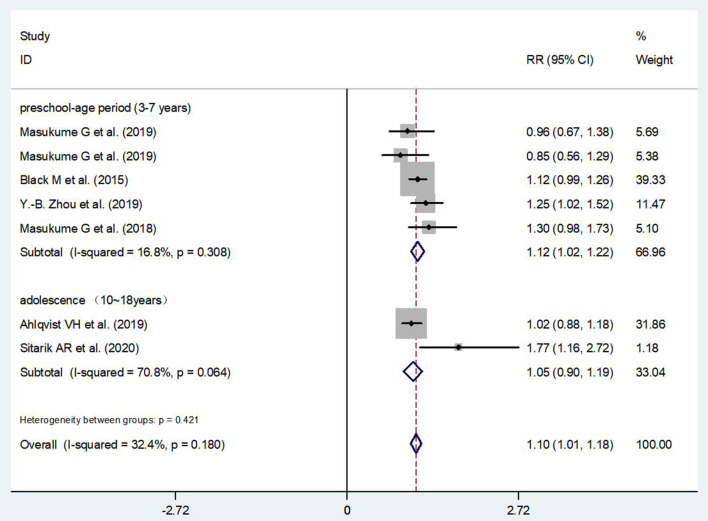
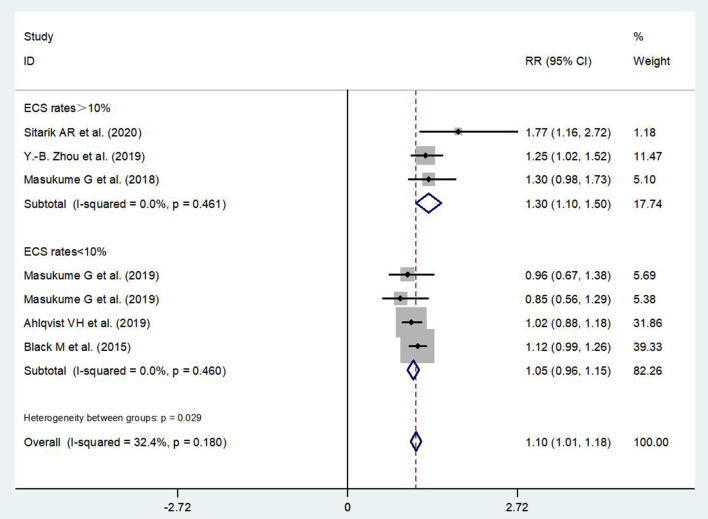
According to the available data, we have performed a subgroup analysis on the association between ECS and children's obesity stratified by age. It showed that ECS was associated with a high risk of obesity in children at preschool-age period (RR = 1.12, 95% CI: 1.02–1.22) (Figure 5). In other subgroup analysis, the effect of ECS on children's obesity was found in places where the ECS rate exceeded 10% (RR = 1.30, 95% CI: 1.10–1.50) (Figure 6).
Figure 5.
Subgroup analysis on the association between ECS and children's obesity stratified by age.
Figure 6.
Subgroup analysis on the association between ECS and children's obesity stratified by ECS rate.
Discussion
The current study indicated that, compared with children born via VD, children delivered by ECS had lower birth weight and an increased risk of obesity from infancy to adolescence. As far as we know, this is the first systematic review and meta-analysis that focuses on the association between ECS and children's long-term weight development.
Our findings on lower birth weight in children born via ECS were contrary to the study of Arikan et al. (26), which indicated that the weight of newborns delivered by ECS (ECS on maternal request due to fear of childbirth) is heavier than those born via VD. There may exist a bidirectional relationship between ECS and infants' birth weight. On one hand, birth weight might be a result of ECS. If there are serious pregnancy complications that require early termination of pregnancy, the gestational age might be small and thus the birth weight might be accordingly low (2). Although the American Congress of Obstetricians and Gynecologists recommend that that ECS should not be performed before a gestational age of 39 weeks (27), still there are many ECSs conducted before 39 gestational weeks, even for ECS on maternal request (28). On the other hand, birth weight might be the cause of ECS. For instance, if fetal ultrasound indicates the possibility of macrosomia and causes difficulties to have VD, ECS would be advised or requested (2). In the current study, we found that the gestational age of newborns born via ECS was about 1 week younger than that of newborns born via VD (WMD: −0.93, 95% CI: −1.13, −0.73), but we could not tell the exact causality between the two variables. However, whether birth weight is regarded as a confounder or a mediator, the studies we included had all controlled birth weight in data analysis.
Still, lower birth weight and younger gestational age of newborns delivered by ECS were not enough to indicate children's exact intrauterine development, but the possibility of potential IUGR in children delivered by ECS could not be fully ruled out. It is speculated that the risk of obesity in children born of ECS might be due to the catch-up growth of IUGR infants via this operative delivery mode.
The 10% increased risk of obesity identified following ECS compared with VD is consistent with the other studies. Li et al. (29) showed that the overall pooled odds ratio (OR) of overweight/obesity in offspring delivered by CS was 1.33 (95% CI: 1.19, 1.48) compared with those born via VD. Although Kuhle et al. (30) did not distinguish CS from ECS and non-ECS, they had performed a meta-analysis in 2015 and found that children born via CS had a 34% higher risk of obesity than those born via VD.
Recent studies have suggested that the link between delivery modes and offspring's overweight/obesity may be caused by different mother-to-child microbiome at birth. Different exposures to the vaginal, perineal, and fecal microflora in CS (especially ECS) and VD infants are thought to determine the different initial composition intestinal microflora (31). A Norwegian Birth Cohort study examined the relationship between early gut microbiota and children's BMI at age 12 and found that composition of the gut microbiota at days 10 and 730 was significantly associated with childhood BMI (32). Studies have shown that Escherichia-Shigella and Bacteroides species were underrepresented in infants born by CS (33, 34). A lack of exposure to these microbes at birth may increase the risk of obesity later in life, as biological dysregulation or depletion of the microbes may impede normal energy acquisition and alter metabolic procedures (33–36), whereas in emergency CS, membranes usually have ruptured, with the consequent infants' exposure to vaginal microbiota, as this may partially reduce the risk of later life obesity compared with infants born via ECS with intact membranes (16).
Stress hormone triggered by different delivery modes may also play a role. Normal increase in the stress hormone, cortisol, during labor is beneficial for the newborn's adaptation to the extra-uterine environment (37). Research suggests that the lowest stress response is found in infants born via ECS, a lower response is found in those born via emergency CS, and a high response is found in those born via normal VD (38, 39). Studies propose that lower levels of fetal cortisol in ECS may cause metabolic perturbations at birth and future obesity (40). Kiriakopoulos' study found a higher level of growth hormone (GH) in the umbilical cord blood in infants with ECS compared to those with VD. Experimental studies concur that increased GH level is associated with dysfunction of the adipose tissue and thus leads to increased body weight (41).
The subgroup analysis indicated an increased risk of obesity in preschoolers born via ECS. This is consistent with the trajectory of body fat development, which continues to grow in childhood and tends to be stable in adolescence (42, 43). Meanwhile, low gut microbiome diversity and richness in infant caused by ECS has widely been reported and lead to the subsequent expansion of adiposity tissue in infancy (33–36). A large amount of evidence supports that obesity during birth and infancy has a great influence on obesity in later life stage, including preschool-age and adolescence (44). Zhou's study had focused on the dynamic changes in children's growth and development and confirmed that CS significantly increased the risk of “progressive obesity” trajectory (OR = 2.50, 95% CI: 1.42, 4.41) at the ages of 10–15 years (45). Due to the limited data, we could not provide powerful findings on the effect of ECS on the occurrence of obesity in adolescents in the current study. More research data are needed to identify the association between ECS and adolescents' obesity.
In our study, the effect of ECS on children's obesity was observed in places where the ECS rate exceeded 10%. Previous studies showed that CS rates at the population level exceeding 10% were not associated with reductions in maternal and newborn mortality any more (46, 47). Our findings toward children's obesity here also provide new evidence on the adverse health outcome caused by cesarean section from the perspective of offspring's growth and development.
There are some strengths in our study. First of all, it is the first systematic analysis on the association between ECS and offspring's long-term weight development. ECS and non-ECS have been clearly distinguished in the current study. The finding is of high public health value without doubt as ECS (especially ECS on maternal request) is a potentially modifiable risk factor for children's obesity. Then, all the included studies are cohort designed, with high quality, and the sample size is large. It allows more opportunities to clarify causal relationship between ECS and children's overweight and obesity. Furthermore, heterogeneity was low in all primary analyses given all studies were observational.
Meanwhile, some limitations must be acknowledged. Because a limited number of studies distinguished ECS from non-ECS when examining the effect of cesarean section on children's obesity, there were a small number of studies included in the current meta-analyses. Thus, the results should be viewed with caution. However, the original studies included were all cohort studies with large sample size covering a wide range of areas; our findings, therefore, would provide credible evidence to a certain extent. Secondly, due to limited information in recruited articles, we were not able to distinguish ECS with medical indications from that without any indications. The former is a necessary surgical procedure to save mothers' and infants' lives, while the latter may violate the eugenics principles and adversely affect the offspring's development (2, 6). The public health significance between the two kinds of ECS, therefore, is absolutely different. Thirdly, the effects of early life exposure on child development are usually sex-specific (48). Few studies had distinguished the children's sex when examining the association between ECS and children's overweight and obesity, which hinders us in exploring the sex-specific effect of ECS on children's weight development. Although all the included studies in the current study had adjusted for children' sex, it was not possible to examine the specific effect of ECS on obesity, respectively, in boys and girls. Moreover, genetic determinants, as well as paternal characteristics, were not fully considered or adjusted in the recruited studies. These are also proven to be potential factors affecting children's overweight/obesity occurrence (49).
In summary, ECS is observed to be related with an increased risk of children's obesity. Under the condition of global high ECS rate, our findings will provide evidence for both obstetricians and women in their informed choice of cesarean section.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SZ collated and analyzed the data and was the main author of the first draft. XQ participated in literature search and data analysis. PL participated in literature retrieval and review. KH reviewed and revised the first draft and supervised and led this study. All authors contributed to the article and approved the submitted version.
Funding
Funding was provided by the National Natural Science Foundation of China (81872630), the University Synergy Innovation Program of Anhui Province (GXXT-2020-067), the Sci-tech Basic Resources Research Program of China (2017FY101107), the Special Project Reproductive Health, Prevention and Control of Major Birth Defects of National Key Research and Development Program (2016YFC1000204-2), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT310002), and the Research Fund of Anhui Institute of Translational Medicine (ZHYX2020A001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.793400/full#supplementary-material
References
- 1.Roberts CL, Nippita TA. International caesarean section rates: the rising tide. Lancet Glob Health. (2015) 3:e241–2. 10.1016/S2214-109X(15)70111-7 [DOI] [PubMed] [Google Scholar]
- 2.Mylonas I, Friese K. Indications for and risks of elective cesarean section. Dtsch Arztebl Int. (2015) 112:489–95. 10.3238/arztebl.2015.0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar J, Carroli G, Zavaleta N, Donner A, Wojdyla D, Faundes A, et al. Maternal and neonatal individual risks and benefits associated with caesarean delivery: multicentre prospective study. BMJ. (2007) 335:1025. 10.1136/bmj.39363.706956.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbons L, Belizán JM, Lauer JA, Betrán AP, Merialdi M, Althabe F. The Global Numbers Costs of Additionally Needed Unnecessary Caesarean Sections Performed per Year: Overuse as a Barrier to Universal Coverage. WHO; (2010). Available online at: https://www.who.int/publications/m/item/the-global-numbers-and-costs-of-additionally-needed-and-unnecessary-caesarean-sections-performed-per-year-overuse-as-a-barrier-to-universal-coverage (accessed June 11, 2021). [Google Scholar]
- 5.World Health Organization . WHO Statement on Caesarean Section Rates. WHO reference number: WHO / RHR / 15.02.2015 (2015). [Google Scholar]
- 6.Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet. (2018) 392:1349–57. 10.1016/S0140-6736(18)31930-5 [DOI] [PubMed] [Google Scholar]
- 7.Martinez KA, 2nd, Devlin JC, Lacher CR, Yin Y, Cai Y, Wang J, et al. Increased weight gain by C-section: functional significance of the primordial microbiome. Sci Adv. (2017) 3:eaao1874. 10.1126/sciadv.aao1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YS, Biddle S, Chan MF, Cheng A, Cheong M, Chong YS, et al. Health Promotion Board-Ministry of Health clinical practice guidelines: obesity. Singapore Med J. (2016) 57:292–300. 10.11622/smedj.2016141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2014) 384:766–81. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Final Report of the Commission on Ending Childhood Obesity. Geneva: World Health Organization; (2016). [Google Scholar]
- 11.Reilly JJ, Bonataki M, Leary SD, Wells JC, Davey-Smith G, Emmett P, et al. Progression from childhood overweight to adolescent obesity in a large contemporary cohort. Int J Pediatr Obes. (2011) 6:e138–43 10.3109/17477166.2010.497538 [DOI] [PubMed] [Google Scholar]
- 12.Mueller NT, Zhang M, Hoyo C, Østbye T, Benjamin-Neelon SE. Does cesarean delivery impact infant weight gain and adiposity over the first year of life? Int J Obes. (2019) 43:1549–55. 10.1038/s41366-018-0239-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan C, Gaskins AJ, Blaine AI, Zhang C, Gillman MW, Missmer SA, et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. (2016) 170:e162385. 10.1001/jamapediatrics.2016.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu S, Zhang Y, Jiang Y, Sun W, Zhu Q, Liu S, et al. Cesarean section and risks of overweight and obesity in school-aged children: a population-based study. QJM. (2018) 111:859–65. 10.1093/qjmed/hcy195 [DOI] [PubMed] [Google Scholar]
- 15.Masukume G, McCarthy FP, Baker PN, Kenny LC, Morton SM, Murray DM, et al. Association between caesarean section delivery and obesity in childhood: a longitudinal cohort study in Ireland. BMJ Open. (2019) 9:e025051. 10.1136/bmjopen-2018-025051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai M, Loy SL, Tan KH, Godfrey KM, Gluckman PD, Chong YS, et al. Association of elective and emergency cesarean delivery with early childhood overweight at 12 months of age. JAMA Netw Open. (2018) 1:e185025. 10.1001/jamanetworkopen.2018.5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masukume G, McCarthy FP, Russell J, Baker PN, Kenny LC, Morton SM, et al. Caesarean section delivery and childhood obesity: evidence from the growing up in New Zealand cohort. J Epidemiol Community Health. (2019) 73:1063–70. 10.1136/jech-2019-212591 [DOI] [PubMed] [Google Scholar]
- 18.Ahlqvist VH, Persson M, Magnusson C, Berglind D. Elective and nonelective cesarean section and obesity among young adult male offspring: a Swedish population-based cohort study. PLoS Med. (2019) 16:e1002996. 10.1371/journal.pmed.1002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane SP. Odds Ratio to Risk Ratio Conversion. ClinCalc. Available online at: https://clincalc.com/Stats/ConvertOR.aspx (accessed July 1, 2017).
- 20.Sitarik AR, Havstad SL, Johnson CC, Jones K, Levin AM, Lynch SV, et al. Association between cesarean delivery types and obesity in preadolescence. Int J Obes. (2020) 44:2023–34. 10.1038/s41366-020-00663-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maharlouei N, Mansouri P, Zahmatkeshan M, Lankarani KB. Low-risk planned caesarean versus planned vaginal delivery at term: early and late infantile outcomes. East Mediterr Health J. (2019) 25:503–13. 10.26719/emhj.18.066 [DOI] [PubMed] [Google Scholar]
- 22.Masukume G, Khashan AS, Morton SMB, Baker PN, Kenny LC, McCarthy FP. Caesarean section delivery and childhood obesity in a British longitudinal cohort study. PLoS ONE. (2019) 14:e0223856. 10.1371/journal.pone.0223856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA. (2015) 314:2271–9. 10.1001/jama.2015.16176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou YB, Li HT, Si KY, Zhang YL, Wang LL, Liu JM. Association of elective cesarean delivery with metabolic measures in childhood: a prospective cohort study in China. Nutr Metab Cardiovasc Dis. (2019) 29:775–82. 10.1016/j.numecd.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Masukume G, O'Neill SM, Baker PN, Kenny LC, Morton SMB, Khashan AS. The impact of caesarean section on the risk of childhood overweight and obesity: new evidence from a contemporary cohort study. Sci Rep. (2018) 8:15113. 10.1038/s41598-018-33482-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arikan I, Barut A, Harma M, Harma IM, Gezer S, Ulubasoglu H. Cesarean section with relative indications versus spontaneous vaginal delivery: short-term outcomes of maternofetal health. Clin Exp Obstet Gynecol. (2012) 39:288–92. [PubMed] [Google Scholar]
- 27.Ecker J. Elective cesarean delivery on maternal request. JAMA. (2013) 309:1930–6. 10.1001/jama.2013.3982 [DOI] [PubMed] [Google Scholar]
- 28.Huang K, Yan S, Wu X, Zhu P, Tao F. Elective caesarean section on maternal request prior to 39 gestational weeks and childhood psychopathology: a birth cohort study in China. BMC Psychiatry. (2019) 19:22. 10.1186/s12888-019-2012-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes. (2013) 37:893–9. 10.1038/ijo.2012.195 [DOI] [PubMed] [Google Scholar]
- 30.Kuhle S, Tong OS, Woolcott CG. Association between caesarean section and childhood obesity: a systematic review and meta-analysis. Obes Rev. (2015) 16:295–303. 10.1111/obr.12267 [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. (2010) 107:11971–5. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanislawski MA, Dabelea D, Wagner BD, Iszatt N, Dahl C, Sontag MK, et al. Gut microbiota in the first 2 years of life and the association with body mass index at age 12 in a norwegian birth cohort. mBio. (2018) 9:e01751–18. 10.1128/mBio.01751-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yassour M, Vatanen T, Siljander H, Hämäläinen AM, Härkönen T, Ryhänen SJ, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. (2016) 8:343ra81. 10.1126/scitranslmed.aad0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller NT, Shin H, Pizoni A, Werlang IC, Matte U, Goldani MZ, et al. Delivery mode and the transition of pioneering gut-microbiota structure, composition and predicted metabolic function. Genes. (2017) 8:364. 10.3390/genes8120364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. (2013) 185:385–94. 10.1503/cmaj.121189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. (2016) 8:343ra82. 10.1126/scitranslmed.aad7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tribe RM, Taylor PD, Kelly NM, Rees D, Sandall J, Kennedy HP. Parturition and the perinatal period: can mode of delivery impact on the future health of the neonate? J Physiol. (2018) 596:5709–22. 10.1113/JP275429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller NM, Fisk NM, Modi N. Stress responses at birth: determinants of cord arterial cortisol and links with cortisol response in infancy. BJOG. (2005) 112:921–6. 10.1111/j.1471-0528.2005.00620.x [DOI] [PubMed] [Google Scholar]
- 39.Schuller C, Känel N, Müller O, Kind AB, Tinner EM, Hösli I, et al. Stress and pain response of neonates after spontaneous birth and vacuum-assisted and cesarean delivery. Am J Obstet Gynecol. (2012) 207:416.e1–6. 10.1016/j.ajog.2012.08.024 [DOI] [PubMed] [Google Scholar]
- 40.Shokry E, Marchioro L, Uhl O, Bermúdez MG, García-Santos JA, Segura MT, et al. Investigation of the impact of birth by cesarean section on fetal and maternal metabolism. Arch Gynecol Obstet. (2019) 300:589–600. 10.1007/s00404-019-05213-w [DOI] [PubMed] [Google Scholar]
- 41.Berryman DE, List EO. Growth hormone's effect on adipose tissue: quality versus quantity. Int J Mol Sci. (2017) 18:1621. 10.3390/ijms18081621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. (1982) 35:1169–75. 10.1093/ajcn/35.5.1169 [DOI] [PubMed] [Google Scholar]
- 43.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. (2000) 80:649–80. 10.1152/physrev.2000.80.2.649 [DOI] [PubMed] [Google Scholar]
- 44.Orsso CE, Colin-Ramirez E, Field CJ, Madsen KL, Prado CM, Haqq AM. Adipose tissue development and expansion from the womb to adolescence: an overview. Nutrients. (2020) 12:2735. 10.3390/nu12092735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Zhang Y, Sun Y, Zhang D. Association of cesarean birth with body mass index trajectories in adolescence. Int J Environ Res Public Health. (2020) 17:2003. 10.3390/ijerph17062003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betrán AP, Torloni MR, Zhang J, Yu J, Deneux-Tharaux C, Oladapo OT, et al. What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reprod Health. (2015) 12:57. 10.1186/s12978-015-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye J, Zhang J, Mikolajczyk R, Torloni MR, Gülmezoglu AM, Betran AP. Association between rates of caesarean section and maternal and neonatal mortality in the 21st century: a worldwide population-based ecological study with longitudinal data. BJOG. (2016) 123:745–53. 10.1111/1471-0528.13592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. (2017) 13:161–73 10.1038/nrendo.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aris IM, Bernard JY, Chen LW, Tint MT, Pang WW, Soh SE, et al. Modifiable risk factors in the first 1000 days for subsequent risk of childhood overweight in an Asian cohort: significance of parental overweight status. Int J Obes. (2018) 42:44–51. 10.1038/ijo.2017.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.