Abstract
Background
Venous thromboembolism (VTE) often complicates the clinical course of cancer. The risk is further increased by chemotherapy, but the trade‐off between safety and efficacy of primary thromboprophylaxis in cancer patients treated with chemotherapy is uncertain. This is the third update of a review first published in February 2012.
Objectives
To assess the efficacy and safety of primary thromboprophylaxis for VTE in ambulatory cancer patients receiving chemotherapy compared with placebo or no thromboprophylaxis, or an active control intervention.
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Cochrane Vascular, CENTRAL, MEDLINE, Embase and CINAHL databases and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 3 August 2020. We also searched the reference lists of identified studies and contacted content experts and trialists for relevant references.
Selection criteria
Randomised controlled trials comparing any oral or parenteral anticoagulant or mechanical intervention to no thromboprophylaxis or placebo, or comparing two different anticoagulants.
Data collection and analysis
We extracted data on risk of bias, participant characteristics, interventions, and outcomes including symptomatic VTE and major bleeding as the primary effectiveness and safety outcomes, respectively. We applied GRADE to assess the certainty of evidence.
Main results
We identified six additional randomised controlled trials (3326 participants) for this update, bringing the included study total to 32 (15,678 participants), all evaluating pharmacological interventions and performed mainly in people with locally advanced or metastatic cancer. The certainty of the evidence ranged from high to very low across the different outcomes and comparisons. The main limiting factors were imprecision and risk of bias.
Thromboprophylaxis with direct oral anticoagulants (direct factor Xa inhibitors apixaban and rivaroxaban) may decrease the incidence of symptomatic VTE (risk ratio (RR) 0.43, 95% confidence interval (CI) 0.18 to 1.06; 3 studies, 1526 participants; low‐certainty evidence); and probably increases the risk of major bleeding compared with placebo (RR 1.74, 95% CI 0.82 to 3.68; 3 studies, 1494 participants; moderate‐certainty evidence).
When compared with no thromboprophylaxis, low‐molecular‐weight heparin (LMWH) reduced the incidence of symptomatic VTE (RR 0.62, 95% CI 0.46 to 0.83; 11 studies, 3931 participants; high‐certainty evidence); and probably increased the risk of major bleeding events (RR 1.63, 95% CI 1.12 to 2.35; 15 studies, 7282 participants; moderate‐certainty evidence).
In participants with multiple myeloma, LMWH resulted in lower symptomatic VTE compared with the vitamin K antagonist warfarin (RR 0.33, 95% CI 0.14 to 0.83; 1 study, 439 participants; high‐certainty evidence), while LMWH probably lowers symptomatic VTE more than aspirin (RR 0.51, 95% CI 0.22 to 1.17; 2 studies, 781 participants; moderate‐certainty evidence). Major bleeding was observed in none of the participants with multiple myeloma treated with LMWH or warfarin and in less than 1% of those treated with aspirin.
Only one study evaluated unfractionated heparin against no thromboprophylaxis, but did not report on VTE or major bleeding.
When compared with placebo or no thromboprophylaxis, warfarin may importantly reduce symptomatic VTE (RR 0.15, 95% CI 0.02 to 1.20; 1 study, 311 participants; low‐certainty evidence) and may result in a large increase in major bleeding (RR 3.82, 95% CI 0.97 to 15.04; 4 studies, 994 participants; low‐certainty evidence).
One study evaluated antithrombin versus no antithrombin in children. This study did not report on symptomatic VTE but did report any VTE (symptomatic and incidental VTE). The effect of antithrombin on any VTE and major bleeding is uncertain (any VTE: RR 0.84, 95% CI 0.41 to 1.73; major bleeding: RR 0.78, 95% CI 0.03 to 18.57; 1 study, 85 participants; very low‐certainty evidence).
Authors' conclusions
In ambulatory cancer patients, primary thromboprophylaxis with direct factor Xa inhibitors may reduce the incidence of symptomatic VTE (low‐certainty evidence) and probably increases the risk of major bleeding (moderate‐certainty evidence) when compared with placebo. LMWH decreases the incidence of symptomatic VTE (high‐certainty evidence), but increases the risk of major bleeding (moderate‐certainty evidence) when compared with placebo or no thromboprophylaxis. Evidence for the use of thromboprophylaxis with anticoagulants other than direct factor Xa inhibitors and LMWH is limited. More studies are warranted to evaluate the efficacy and safety of primary prophylaxis in specific types of chemotherapeutic agents and types of cancer, such as gastrointestinal or genitourinary cancer.
Keywords: Adult; Child; Humans; Ambulatory Care; Anticoagulants; Anticoagulants/adverse effects; Anticoagulants/therapeutic use; Antineoplastic Agents; Antineoplastic Agents/adverse effects; Antithrombins; Antithrombins/therapeutic use; Bias; Factor Xa Inhibitors; Factor Xa Inhibitors/therapeutic use; Hemorrhage; Hemorrhage/chemically induced; Heparin; Heparin/adverse effects; Heparin/therapeutic use; Heparin, Low-Molecular-Weight; Heparin, Low-Molecular-Weight/adverse effects; Heparin, Low-Molecular-Weight/therapeutic use; Neoplasms; Neoplasms/complications; Neoplasms/drug therapy; Primary Prevention; Primary Prevention/methods; Pulmonary Embolism; Pulmonary Embolism/etiology; Pulmonary Embolism/prevention & control; Randomized Controlled Trials as Topic; Venous Thromboembolism; Venous Thromboembolism/etiology; Venous Thromboembolism/prevention & control; Warfarin; Warfarin/adverse effects; Warfarin/therapeutic use
Plain language summary
Prevention of blood clots in non‐hospitalised cancer patients receiving chemotherapy
Background
Cancer patients are more likely than people without cancer to develop venous thromboembolism (blood clots in the veins). Chemotherapy may activate blood coagulation (clotting) and further increase this risk. Anticoagulants are medicines which are used to prevent and treat blood clots. They are sometimes known as blood thinners. This systematic review aimed to look at the effectiveness and safety of anticoagulants and mechanical interventions when used to prevent blood clots in cancer patients receiving chemotherapy.
Key results
We included 32 randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) involving 15,678 participants (current search to August 2020). All studies evaluated anticoagulants and were performed mainly in people with locally advanced (unlikely to be cured) or metastatic (where the cancer has spread from the part of the body where it started) cancer. Direct oral anticoagulants (anticoagulants that act by directly binding to and inhibiting specific coagulation factors – thrombin or activated factor X) may reduce the occurrence of blood clots and probably increase the risk of major bleeding in people with cancer. Low‐molecular‐weight heparins (anticoagulants that increase the activity of the natural anticoagulant antithrombin) were associated with a reduction in symptomatic blood clots, but increased the risk of major bleeding. In people with the blood‐related cancer, multiple myeloma, low‐molecular‐weight heparin reduced the number of symptomatic blood clots when compared with the vitamin K antagonist warfarin, while the difference with aspirin was not clear; there were no major bleeds with low‐molecular‐weight heparin or warfarin, and in participants treated with aspirin the rate was below 1%. One study evaluated unfractionated heparin and did not report on blood clots or major bleeding. Data for warfarin in comparison with placebo (pretend treatment) were too limited to support the use of warfarin in the prevention of symptomatic blood clots in cancer patients. One study in children evaluated antithrombin, which had no significant effect on any type of blood clots or major bleeding when compared with no antithrombin.
Quality of the evidence
The methodological quality of the included studies ranged from low to high, such that future studies may change our confidence in the results, in particular with regard to the safety of anticoagulants. The reliability of the findings ranged from high to very low across the different outcomes and comparisons. The main limiting factors, which were the reason for a decrease in reliability in some outcomes, were imprecision and risk of bias. The relatively low number of studies, participants, and clinical events prevented us from providing more definitive conclusions about the risk of bleeding in association with anticoagulants. None of the studies tested intermittent pneumatic compression (a mechanical device using an air pump and inflatable leggings to provide pulsing pressure that pushes blood through the veins) or graduated elastic stockings (special socks that improve blood flow in the leg veins and prevent blood from pooling in the legs) for the prevention of venous thromboembolism.
Summary of findings
Summary of findings 1. DOAC versus placebo.
| DOAC direct factor Xa inhibitors compared with placebo for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: DOAC direct factor Xa inhibitors (apixaban or rivaroxaban) Comparison: placebo | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With placebo Number of events per 1000 participants |
With DOAC (any dosage) Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: median 6 months |
RR 0.43 (0.18 to 1.06) | High‐risk populationc | 46 per 1000 fewer events (66 fewer to 5 more) | 1526 (3) | ⊕⊕⊝⊝ Lowd | DOACs may decrease the incidence of symptomatic VTE across different cancer types. | |
| 80 per 1000 | 34 per 1000 (14 to 85) | ||||||
|
Major bleeding Follow‐up: median 6 months |
RR 1.74 (0.82 to 3.68) | High‐risk populationc | 13 per 1000 more events (3 fewer to 49 more) | 1494 (3) | ⊕⊕⊕⊝ Moderatee | DOACs probably increase the incidence of major bleeding across different cancer types. | |
| 18 per 1000 | 32 per 1000 (15 to 67) | ||||||
|
Symptomatic PE Follow‐up: median 6 months |
RR 0.38 (0.10 to 1.47) | High‐risk populationc | 21 per 1000 fewer events (31 fewer to 16 more) | 1526 (3) | ⊕⊕⊝⊝ Lowd | DOACs may decrease the incidence of symptomatic PE across different cancer types. | |
| 34 per 1000 | 13 per 1000 (3 to 51) |
||||||
|
Symptomatic DVT Follow‐up: median 6 months |
RR 0.51 (0.21 to 1.22) | High‐risk populationc | 22 per 1000 fewer events (36 fewer to 10 more) | 1526 (3) | ⊕⊕⊝⊝ Lowd | DOACs may decrease the incidence of symptomatic DVT across different cancer types. | |
| 45 per 1000 | 23 per 1000 (9 to 55) | ||||||
|
Any VTE Follow‐up: median 6 months |
RR 0.55 (0.34 to 0.90) |
High‐risk populationc | 43 per 1000 fewer events (9 fewer to 63 fewer events) | 1404 (2) | ⊕⊕⊕⊝ Moderatee | DOACs probably decrease the incidence of any VTE across different cancer types. | |
| 95 per 1000 | 52 per 1000 (32 to 85) | ||||||
|
1‐year overall mortality Follow‐up: NA |
NAf | High‐risk populationc | NA | 0 (0) | NA | We do not know how DOAC affect overall mortality. | |
| NA | NA | ||||||
|
Clinically relevant bleeding Follow‐up: median 4.5 months |
RR 1.61 (0.82 to 3.15) | High‐risk populationc | 20 per 1000 more events (6 fewer to 69 more) | 931 (2) | ⊕⊕⊕⊝ Moderatee | DOACs probably increase the incidence of clinically relevant bleeding across different cancer types. | |
| 32 per 1000 | 52 per 1000 (26 to 101) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DOAC: direct oral anticoagulants; DVT: deep vein thrombosis; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the median control group risk across the studies. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cHigh‐risk population refers to the median observed risk to experience symptomatic VTE in the trials contributing to the analyses (71 per 1000). Rates from 7% and higher are considered high risk (Khorana 2008). dDowngraded two levels because of imprecision, inconsistency, and attrition bias, see Characteristics of included studies table. eDowngraded one level because of imprecision and risk of attrition bias, see Characteristics of included studies table. fNo trials contributed to this outcome.
Summary of findings 2. Low‐molecular‐weight heparin versus no thromboprophylaxis.
| LMWH compared with no thromboprophylaxis for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: LMWH Comparison: no thromboprophylaxis (placebo or no LMWH) | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risk (95% CI)* | Difference (95% CI)b | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With no thromboprophylaxis Number of events per 1000 participants |
With LMWH Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: median 10 months |
RR 0.62 (0.46 to 0.83) | High‐risk populationc | 27 per 1000 fewer events (12 fewer to 39 fewer) | 3931 (11) | ⊕⊕⊕⊕ Highd | LMWH decreases the incidence of symptomatic VTE across different cancer types. | |
| 71 per 1000 | 44 per 1000 (33 to 59) |
||||||
|
Major bleeding Follow‐up: median 10 months |
RR 1.63 (1.12 to 2.35) | High‐risk populationc | 7 per 1000 more major bleeds (1 more to 15 more) | 7282 (15) | ⊕⊕⊕⊝ Moderatee | LMWH probably increases major bleedings across different cancer types. | |
| 11 per 1000 | 18 per 1000 (12 to 26) |
||||||
|
Symptomatic PE Follow‐up: median 8 months |
RR 0.60 (0.42 to 0.88) | High‐risk populationc | 7 per 1000 fewer events (2 fewer to 11 fewer) | 5324 (8) | ⊕⊕⊕⊝ Moderatef | LMWH probably decreases the incidence of symptomatic PE across different cancer types. | |
| 18 per 1000 | 11 per 1000 (8 to 16) |
||||||
|
Symptomatic DVT Follow‐up: median 10 months |
RR 0.48 (0.35 to 0.67) | High‐risk populationc | 15 per 1000 fewer events (9 fewer to 18 fewer) | 5408 (9) | ⊕⊕⊕⊕ Highg |
LMWH decreases the incidence of symptomatic DVT across different cancer types. | |
| 28 per 1000 | 14 per 1000 (10 to 19) |
||||||
| Any VTE Follow‐up: median 8 months | RR 0.57 (0.46 to 0.71) | High‐risk populationc | 38 per 1000 fewer events (26 fewer to 48 fewer) | 5743 (10) | ⊕⊕⊕⊕ Highh |
LMWH decreases the incidence of any VTE across different cancer types. | |
| 90 per 1000 | 52 per 1000 (43 to 64) | ||||||
|
1‐year overall mortality Follow‐up: median 12 months |
RR 0.94 (0.83 to 1.07) | High‐risk populationc | 35 per 1000 fewer deaths (100 fewer to 41 more) | 2681 (9) | ⊕⊕⊝⊝ Lowi | LMWH may decrease the incidence of death across different cancer types. | |
| 586 per 1000 | 551 per 1000 (486 to 627) |
||||||
|
Clinically relevant bleeding Follow‐up: median 11 months |
RR 3.40 (1.20 to 9.63) | High‐risk populationc | 40 per 1000 more clinically relevant bleeds (3 more to 145 more) | 3105 (4) | ⊕⊕⊕⊝ Moderatej |
LMWH probably increases the incidence of clinically relevant bleeding across different cancer types. | |
| 17 per 1000 | 57 per 1000 (20 to 162) |
||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; LMWH: low‐molecular‐weight heparin; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the median control group risk across the studies. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cHigh‐risk population refers to the median observed risk to experience symptomatic VTE in the trials contributing to the analyses. It corresponds to 71 per 1000 for symptomatic VTE, which is consistent with previous literature, suggesting that rates of 7% or higher identify individuals at high risk of symptomatic VTE (Khorana 2008). The high‐risk label for other outcomes is based on the risk profile for symptomatic VTE. dAlthough 7/11 trials were not double‐blind, and 3/11 trials used dosages exceeding typical prophylactic dosages, results were consistent across trials, so we did not downgrade. eDowngraded one level because 10/15 trials contributing to the analyses were not double‐blind, and 4/15 trials did not use standard definitions to ascertain major bleeding. Overall, no relevant inconsistency was detected, so that the effects of non‐blinding, definitions, and other study characteristics were deemed to be small. One study reported zero events in both the intervention and control arm, and was not considered in the 'Summary of findings' table (Zwicker 2013). fDowngraded one level because risk of selective outcome reporting, with only 8/15 trials reporting symptomatic PE. gAlthough 5/9 trials were not double‐blind, and 2/9 trials used dosages exceeding typical prophylactic dosages, results were very consistent across trials, so we did not downgrade. hAlthough 7/10 trials were not double‐blind, and 4/10 trials used dosages exceeding typical prophylactic dosages, results were very consistent across trials, so we did not downgrade. iDowngraded two levels because the 95% CI included both small and appreciable benefit or harm; with some variability in estimates across trials due to heterogeneity other than sampling error (chance). jDowngraded one level due to unexplained between‐trial variation.
Summary of findings 3. Low‐molecular‐weight heparin versus with active control (1).
| LMWH: prophylactic dose compared with intermediate or therapeutic dosefor primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | ||||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: prophylactic dose LMWH Comparison: intermediate or therapeutic dose LMWH | ||||||||
| Outcomes | Control type | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | |||||||
|
With intermediate/therapeutic dose LMWH Number of events per 1000 participants |
With prophylactic dose LMWH Number of events per 1000 participants |
|||||||
| Intermediate‐risk populationc | ||||||||
|
Symptomatic VTE Follow‐up: median 3.5 months |
Intermediate | RR 2.89 (0.12 to 66.75) | 31 per 1000 | 90 per 1000 (4 to 2086) | 59 per 1000 more events (28 fewer events to 2055 more) | 51 (1) | ⊕⊕⊝⊝ Lowd |
Prophylactic‐dose LMWH may be associated with a higher risk of symptomatic VTE when compared to intermediate‐dose LMWH in ovarian cancer. |
| Therapeutic | RR 1.00 (0.07 to 15.15) | 53 per 1000 | 53 per 1000 (4 to 805) | 0 per 1000 fewer events (49 fewer events to 752 more) | 52 (1) | ⊕⊕⊝⊝ Lowd |
We do not know if prophylactic‐dose LMWH is associated with a higher risk of symptomatic VTE when compared to therapeutic‐dose LMWH in ovarian cancer. | |
| Intermediate‐risk populationc | ||||||||
|
Major bleeding Follow‐up: median 3.5 months |
Intermediate | Not estimablee | NA | NA | NA | NA | NA | As we have insufficient data to estimate the relative risk, we do not know how prophylactic‐dose LMWH affects major bleeding in ovarian cancer. |
| Therapeutic | Not estimablee | NA | NA | NA | NA | NA | ||
| Intermediate‐risk populationc | ||||||||
|
Symptomatic PE Follow‐up: median 3.5 months |
Intermediate | RR 2.89 (0.12 to 66.75) | NAf | NA | NA | NA | NA | As we have insufficient data to estimate the assumed risk, we do not know how prophylactic‐dose LMWH affects symptomatic PE in ovarian cancer. |
| Therapeutic | RR 3.00 (0.13 to 70.42) | NAf | NA | NA | NA | NA | ||
| Intermediate‐risk populationc | ||||||||
|
Symptomatic DVT Follow‐up: median 3.5 months |
Intermediate | Not estimablee | NA | NA | NA | NA | NA | We do not know how prophylactic‐dose LMWH affects symptomatic DVT across different cancer types. |
| Therapeutic | RR 0.33 (0.01 to 7.82) | 53 per 1000 | 18 per 1000 (1 to 415) | 36 per 1000 fewer DVT (53 fewer to 362 more) | 52 (1) | ⊕⊕⊝⊝ Lowd |
Prophylactic‐dose LMWH may reduce the risk of symptomatic DVT when compared to therapeutic‐dose LMWH in ovarian cancer, although this seems an implausible finding. | |
| Intermediate‐risk populationc | ||||||||
|
Any VTE Follow‐up: NA |
Intermediate |
RR 4.81 (0.24 to 95.58) |
NAf | NA | NA | NA | NA | As we have insufficient data to estimate the assumed risk, we do not know how prophylactic‐dose LMWH affects any VTE across different cancer types. |
| Therapeutic |
RR 5.00 (0.25 to 99.34) |
NAf | NA | NA | NA | NA | ||
| Intermediate‐risk populationc | ||||||||
|
1‐year overall mortality Follow‐up: NA |
Intermediate | NAg | NA | NA | NA | NA | NA | We do not know how prophylactic‐dose LMWH affects overall mortality when compared to intermediate or therapeutic‐dose LMWH across different cancer types. |
| Therapeutic | NAg | NA | NA | NA | NA | NA | ||
| Intermediate‐risk populationc | ||||||||
|
Clinically relevant bleeding Follow‐up: median 3.5 months |
Intermediate | NAe | NA | NA | NA | NA | NA | We do not know how prophylactic‐dose LMWH affects clinically relevant bleeding across different cancer types. |
| Therapeutic | RR 0.33 (0.01 to 7.82) | 38 per 1000h | 13 per 1000 (0 to 301) | 26 per 1000 fewer clinically relevant bleeding (38 fewer to 262 more) | 52 (1) | ⊕⊕⊝⊝ Lowd |
Prophylactic‐dose LMWH may reduce clinically relevant bleeding when compared to therapeutic‐dose LMWH in ovarian cancer. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; LMWH: low‐molecular‐weight heparin; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | ||||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||||
aThe assumed risk is calculated from the medium observed control group risk in Elit 2012 and Pelzer 2015 for the intermediate‐dose estimation, and from Elit 2012 and Maraveyas 2012 for therapeutic‐dose LMWH. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cIntermediate‐risk population refers to the median observed risk to experience symptomatic VTE in the trials contributing to the analyses (31 per 1000 and 53 per 1000). Rates between 2% and 7% are considered intermediate risk (Khorana 2008). dDowngraded two levels because of imprecision. eNot estimable due to zero event count in both trial arms. fWe have insufficient data to estimate the assumed risk due to the zero event rate in both the intermediate‐dose and therapeutic‐dose LMWH. gNo trials contributed to this outcome. hThe assumed risk was based on the small trial by Elit 2012 only (the observed event rate in the control group was 1 out of 26).
Summary of findings 4. Low‐molecular‐weight heparin versus active control (2).
| LMWH compared with aspirin for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: LMWH Comparison: aspirin | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With aspirin Number of events per 1000 participants |
With LMWH
(any dosage) Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: median 18.5 months |
RR 0.51 (0.22 to 1.17) | Intermediate‐risk populationc | 19 per 1000 fewer events (30 fewer to 7 more) | 781 (2) | ⊕⊕⊕⊝ Moderated | LMWH probably decreases the incidence of symptomatic VTE when compared with aspirin in multiple myeloma. | |
| 39 per 1000 | 20 per 1000 (9 to 45) | ||||||
|
Major bleeding Follow‐up: median 18.5 months |
RR 0.14 (0.01 to 2.76) | Intermediate‐risk populationc | 6 per 1000 fewer events (7 fewer to 12 more) | 781 (2) | ⊕⊕⊝⊝ Lowe | LMWH may reduce the incidence of major bleeding when compared with aspirin in multiple myeloma. | |
| 7 per 1000 | 1 per 1000 (0 to 19) | ||||||
|
Symptomatic PE Follow‐up: median 18.5 months |
RR 0.13 (0.02 to 1.03) | Intermediate‐risk populationc | 15 per 1000 fewer events (17 fewer to 1 more) | 781 (2) | ⊕⊕⊕⊝ Moderated | LMWH probably reduces the incidence of symptomatic PE when compared with aspirin in multiple myeloma. | |
| 18 per 1000 | 2 per 1000 (0 to 18) | ||||||
|
Symptomatic DVT Follow‐up: median 18.5 months |
RR 0.81 (0.32 to 2.04) | Intermediate‐risk populationc | 5 per 1000 fewer events (16 fewer to 25 more) | 781 (2) | ⊕⊕⊕⊝ Moderated | LMWH probably reduces the incidence of symptomatic DVT when compared with aspirin in multiple myeloma. | |
| 24 per 1000 | 19 per 1000 (8 to 49) | ||||||
|
Any VTE Follow‐up: NA |
NAf | Intermediate‐risk populationc | NA | NA | NA | We do not know how LMWH affects any VTE when compared with aspirin in multiple myeloma. | |
| NA | NA | ||||||
|
1‐year overall mortality Follow‐up: NA |
NAf | Intermediate‐risk populationc | NA | NA | NA | We do not know how LMWH affects 1‐year overall mortality when compared with aspirin in multiple myeloma. | |
| NA | NA | ||||||
|
Clinically relevant bleeding Follow‐up: NA |
NAf | Intermediate‐risk populationc | NA | NA | NA | We do not know how LMWH affects clinically relevant bleeding when compared with aspirin in multiple myeloma. | |
| NA | NA | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; LMWH: low‐molecular‐weight heparin; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the medium observed control group risk across the studies. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cIntermediate‐risk population refers to the median observed risk to experience symptomatic VTE in the trials contributing to the analyses (39 per 1000). Rates between 2% and 7% are considered intermediate risk (Khorana 2008). dDowngraded one level because of imprecision. eDowngraded two levels because of imprecision. fNo trials contributed to this outcome.
Summary of findings 5. Low‐molecular‐weight heparin versus active control (3).
| LMWH compared with VKA for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: LMWH Comparison: VKA | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With VKA Number of events per 1000 participants |
With LMWH
(any dosage) Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: median 25 months |
RR 0.33 (0.14 to 0.83) | High‐risk populationc | 55 per 1000 fewer events (14 fewer to 70 fewer) | 439 (1) | ⊕⊕⊕⊕ Highd | LMWH reduces the incidence of symptomatic VTE when compared to VKA in multiple myeloma. | |
| 82 per 1000 | 27 per 1000 (11 to 68) | ||||||
|
Major bleeding Follow‐up: median 25 months |
Not estimablee | High‐risk populationc | NA | NA | NA | We do not know how LMWH affects major bleeding when compared to VKA across different cancer types. | |
| NA | NA | ||||||
|
Symptomatic PE Follow‐up: median 25 months |
RR 0.11 (0.01 to 2.06) | High‐risk populationc | 16 per 1000 fewer events (18 fewer to 19 more) | 439 (1) | ⊕⊕⊝⊝ Lowf | LMWH may reduce the incidence of symptomatic PE when compared to VKA in multiple myeloma. | |
| 18 per 1000 | 2 per 1000 (0 to 37) | ||||||
|
Symptomatic DVT Follow‐up: median 25 months |
RR 0.43 (0.17 to 1.10) | High‐risk populationc | 36 per 1000 fewer events (53 fewer to 6 more) | 439 (1) | ⊕⊕⊕⊝ Moderateg | LMWH probably reduces the incidence of symptomatic DVT when compared to VKA in multiple myeloma. | |
| 64 per 1000 | 27 per 1000 (11 to 70) | ||||||
|
Any VTE Follow‐up: NA |
NAh | High‐risk populationc | NA | NA | NA | We do not know how LMWH affects any VTE when compared to VKA across different cancer types. | |
| NA | NA | ||||||
|
1‐year overall mortality Follow‐up: NA |
NAh | High‐risk populationc | NA | NA | NA | We do not know how LMWH affects 1‐year overall mortality when compared to VKA across different cancer types. | |
| NA | NA | ||||||
|
Clinically relevant bleeding Follow‐up: NA |
NAh | High‐risk populationc | NA | NA | NA | We do not know how LMWH affects clinically relevant bleeding when compared to VKA across different cancer types. | |
| NA | NA | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; LMWH: low‐molecular‐weight heparin; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; VKA: vitamin K antagonist; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the observed control group risk in Palumbo 2011. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cHigh‐risk population refers to the median observed risk to experience symptomatic VTE in the trials contributing to the analyses (82 per 1000). Rates from 7% and higher are considered high risk (Khorana 2008). dAlthough there was some risk of attrition bias, imputation of the missing data in various ways showed that estimates would not change in a clinically relevant manner (data not shown). eNot estimable due to zero event count in both trial arms. fDowngraded two levels because of imprecision. gDowngraded one level because of imprecision. hNo trials contributed to this outcome.
Summary of findings 6. Ultra‐low‐molecular‐weight heparin versus placebo.
| uLMWH (semuloparin) compared with placebo for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: semuloparin Comparison: placebo | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With placebo Number of events per 1000 participants |
With semuloparin Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: median 3.5 months |
RR 0.36 (0.22 to 0.60) | Intermediate‐risk populationc | 22 per 1000 fewer events (14 fewer to 27 fewer) | 3212 (1) | ⊕⊕⊕⊕ High |
Semuloparin decreases the incidence of symptomatic VTE across different cancer types. | |
| 34 per 1000 | 12 per 1000 (8 to 21) | ||||||
|
Major bleeding Follow‐up: median 3.5 months |
RR 1.05 (0.55 to 2.0) | Intermediate‐risk populationc | 1 per 1000 more events (5 fewer to 11 more) | 3172 (1) | ⊕⊕⊕⊝ Moderated |
Semuloparin probably has little effect on major bleedings across different cancer types. | |
| 11 per 1000 | 12 per 1000 (6 to 23) | ||||||
|
Symptomatic PE Follow‐up: median 3.5 months |
RR 0.48 (0.22 to 1.01) | Intermediate‐risk populationc | 7 per 1000 fewer events (0 fewer to 10 fewer) | 3212 (1) | ⊕⊕⊕⊝ Moderated |
Semuloparin probably decreases the incidence of symptomatic PE across different cancer types. | |
| 13 per 1000 | 6 per 1000 (3 to 13) | ||||||
|
Symptomatic DVT Follow‐up: median 3.5 months |
RR 0.32 (0.16 to 0.63) | Intermediate‐risk populationc | 14 per 1000 fewer events (8 fewer to 18 fewer) | 3212 (1) | ⊕⊕⊕⊕ High |
Semuloparin decreases the incidence of symptomatic DVT across different cancer types. | |
| 21 per 1000 | 7 per 1000 (3 to 13) | ||||||
|
Any VTE Follow‐up: median 3.5 months |
RR 0.36 (0.22 to 0.60) | Intermediate‐risk populationc | 22 per 1000 fewer (14 fewer to 27 fewer) | 3212 (1) | ⊕⊕⊕⊕ High |
Semuloparin decreases the incidence of any VTE across different cancer type. | |
| 34 per 1000 | 12 per 1000 (8 to 21) | ||||||
|
1‐year overall mortality Follow‐up: 1 year |
RR 1.02 (0.96 to 1.08) | Intermediate‐risk populationc | 11 per 1000 more events (22 fewer to 44 more) | 3212 (1) | ⊕⊕⊕⊝ Moderated |
Semuloparin probably has no effect on 1‐year overall mortality across different cancer types. | |
| 555 per 1000 | 566 per 1000 (533 to 599) | ||||||
|
Clinically relevant bleeding Follow‐up: median 3.5 months |
RR 1.40 (0.90 to 2.19) | Intermediate‐risk populationc | 8 per 1000 more events (2 fewer to 24 more) | 3172 (1) | ⊕⊕⊕⊝ Moderated |
Semuloparin probably increases the incidence of clinically relevant bleeding across different cancer types. | |
| 20 per 1000 | 28 per 1000 (18 to 44) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; uLMWH: ultra‐low‐molecular‐weight heparin; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the medium observed control group risk in the study. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cIntermediate risk population refers to the observed median risk to experience symptomatic VTE in the single trial contributing to the analyses (34 per 1000). Rates between 2% and 7% are considered intermediate risk (Khorana 2008). dDowngraded one level because of imprecision.
Summary of findings 7. Unfractionated heparin versus no thromboprophylaxis.
| UFH compared withno thromboprophylaxis for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: UFH Comparison: no thromboprophylaxis | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With no thromboprophylaxis Number of events per 1000 participants |
With UFH Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how UFH affects symptomatic VTE across different cancer types. | |
| NA | NA | ||||||
|
Major bleeding Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how UFH affects major bleeding across different cancer types. | |
| NA | NA | ||||||
|
Symptomatic PE Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how UFH affects symptomatic PE across different cancer types. | |
| NA | NA | ||||||
|
Symptomatic DVT Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how UFH affects symptomatic DVT across different cancer types. | |
| NA | NA | ||||||
|
Any VTE Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how UFH affects any VTE across different cancer types. | |
| NA | NA | ||||||
|
1‐year overall mortality Follow‐up: 1 year |
RR 0.86 (0.72 to 1.03) | Population at unclear riskd | 98 per 1000 fewer events (195 fewer to 21 more) | 277 (1) | ⊕⊕⊕⊝ Moderatee |
UFH probably decreases the incidence of 1‐year overall mortality in small‐cell lung cancer. | |
| 698 per 1000 | 600 per 1000 (502 to 719) | ||||||
|
Clinically relevant bleeding Follow‐up: median not reported, maximum of 4.9 years of follow‐up |
RR 2.01 (0.18 to 21.96) | Population at unclear riskd | 7 per 1000 more events (6 fewer to 151 more) | 277 (1) | ⊕⊕⊝⊝ Lowf |
UFH may increase the risk of clinically relevant bleeding in small‐cell lung cancer. | |
| 7 per 1000 | 14 per 1000 (1 to 158) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; UFH: unfractionated heparin; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the observed control group risk in Lebeau 1994. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cNo trials contributed to this outcome. dThe risk profile refers to the median observed risk to experience symptomatic VTEs. As Lebeau 1994 did not report this outcome, the risk profile remains unclear. eDowngraded one level because of imprecision. fDowngraded two levels because of imprecision.
Summary of findings 8. Vitamin K antagonists versus placebo or no thromboprophylaxis.
| VKA compared with placebo or no thromboprophylaxis for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: VKA Comparison: placebo or no thromboprophylaxis | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With placebo or no thromboprophylaxis Number of events per 1000 participants |
With VKA Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: mean 6 months |
RR 0.15 (0.02 to 1.2) | Intermediate‐risk populationc | 37 per 1000 fewer events (43 fewer to 9 more) | 311 (1) | ⊕⊕⊝⊝ Lowd | VKA may reduce the incidence of symptomatic VTE in breast cancer. | |
| 44 per 1000 | 7 per 1000 (1 to 53) | ||||||
|
Major bleeding Follow‐up: mean 6 months |
RR 3.82 (0.97 to 15.04) | Intermediate‐risk populationc | 18 per 1000 more events (0 fewer to 88 more) | 994 (4) | ⊕⊕⊝⊝ Lowe | VKA may increase the incidence of major bleeding in breast cancer and small‐cell lung cancer. | |
| 6 per 1000 | 24 per 1000 (6 to 95) | ||||||
|
Symptomatic PE Follow‐up: mean 6 months |
RR 1.05 (0.07 to 16.58) | Intermediate‐risk populationc | 0 per 1000 fewer events (6 fewer to 101 more) | 311 (1) | ⊕⊝⊝⊝ Verylowf | We have very little confidence in the estimated effect of VKA on symptomatic PE in breast cancer. | |
| 6 per 1000 | 7 per 1000 (0 to 108) | ||||||
|
Symptomatic DVT Follow‐up: mean 6 months |
RR 0.08 (0 to 1.42) | Intermediate‐risk populationc | 35 per 1000 fewer events (38 fewer to 16 more) | 311 (1) | ⊕⊕⊝⊝ Lowd | VKA may reduce the incidence of symptomatic DVT in breast cancer. | |
| 38 per 1000 | 3 per 1000 (0 to 54) | ||||||
|
Any VTE Follow‐up: NA |
NAg | Intermediate‐risk populationc | NA | NA | NA | We do not know how VKA affects any VTE across different cancer types. | |
| NA | NA | ||||||
|
1‐year overall mortality Follow‐up: NA |
NAg | Intermediate‐risk populationc | NA | NA | NA | We do not know how VKA affects 1‐year overall mortality across different cancer types. | |
| NA | NA | ||||||
|
Clinically relevant bleeding Follow‐up: NA |
NAg | Intermediate‐risk populationc | NA | NA | NA | We do not know how VKA affects clinically relevant bleeding across different cancer types. | |
| NA | NA | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; VKA: vitamin K antagonists; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the medium observed control group risk across the trials. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cIntermediate‐risk population refers to the median observed risk to experience symptomatic VTE in the trials contributing to the analyses (44 per 1000). Rates between 2% and 7% are considered intermediate risk (Khorana 2008). dDowngraded two levels because of imprecision, risk of publication bias (only 1/4 trials reported on this outcome), and potential risk of attrition bias, see Characteristics of included studies table. eDowngraded two levels because of imprecision and potential attrition bias in 2/4 trials. fDowngraded three levels because of imprecision (two levels), the risk for publication bias, as only 1/4 trials reported on this outcome, and potential attrition bias, see Characteristics of included studies table. gNo trials contributed to this outcome.
Summary of findings 9. Vitamin K antagonists versus active control.
| VKA compared with aspirin for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory cancer patients receiving chemotherapy Settings: outpatient clinics Intervention: VKA Comparison: aspirin | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With aspirin Number of events per 1000 participants |
With VKA Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: median 2.1 years |
RR 1.50 (0.74 to 3.04) | Intermediate‐risk populationc | 27 per 1000 more events (14 fewer to 211 more) | 440 (1) | ⊕⊕⊕⊝ Moderated | VKA probably increases the incidence of symptomatic VTE when compared to aspirin in multiple myeloma. | |
| 55 per 1000 | 82 per 1000 (40 to 166) | ||||||
|
Major bleeding Follow‐up: median 2.1 years |
RR 0.14 (0.01 to 2.75) | Intermediate‐risk populationc | 12 per 1000 fewer events (14 fewer to 24 more) | 440 (1) | ⊕⊕⊝⊝ Lowe | VKA may reduce the incidence of major bleeding when compared to aspirin in multiple myeloma. | |
| 14 per 1000 | 2 per 1000 (0 to 38) | ||||||
|
Symptomatic PE Follow‐up: median 2.1 years |
RR 1.00 (0.25 to 3.95) | Intermediate‐risk populationc | 0 per 1000 fewer events (14 fewer to 54 more) | 440 (1) | ⊕⊕⊕⊝ Moderated | VKA is probably as effective as aspirin in the prevention of symptomatic PE in multiple myeloma. | |
| 18 per 1000 | 18 per 1000 (5 to 72) | ||||||
|
Symptomatic DVT Follow‐up: median 2.1 years |
RR 1.75 (0.75 to 4.09) | Intermediate‐risk populationc | 27 per 1000 more events (9 fewer to 112 more) | 440 (1) | ⊕⊕⊕⊝ Moderated | VKA probably increases the incidence of symptomatic DVT when compared to aspirin in multiple myeloma. | |
| 36 per 1000 | 64 per 1000 (27 to 149) | ||||||
|
Any VTE Follow‐up: NA |
NAf | Intermediate‐risk populationc | NA | NA | NA | We do not know how VKA affects any VTE when compared to aspirin across different cancer types. | |
| NA | NA | ||||||
|
1‐year overall mortality Follow‐up: NA |
NAf | Intermediate‐risk populationc | NA | NA | NA | We do not know how VKA affects 1‐year overall mortality when compared to aspirin across different cancer types. | |
| NA | NA | ||||||
|
Clinically relevant bleeding Follow‐up: NA |
NAf | Intermediate‐risk populationc | NA | NA | NA | We do not know how VKA affects clinically relevant bleeding when compared to aspirin across different cancer types. | |
| NA | NA | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; VKA: vitamin K antagonists; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the observed control group risk in Palumbo 2011. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cIntermediate‐risk population refers to the median observed risk to experience symptomatic VTE in the trials contributing to the analyses (55 per 1000). Rates between 2% and 7% are considered intermediate risk (Khorana 2008). dDowngraded one level because of imprecision. Although attrition bias may have occurred, it is unlikely to have changed the results in a clinically relevant manner. eDowngraded two levels because of imprecision. Although attrition bias may have occurred, it is unlikely to have changed the results in a clinically relevant manner. fNo trials contributed to this outcome.
Summary of findings 10. Antithrombin versus no thromboprophylaxis.
| Antithrombin compared with placebo for primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy | |||||||
|
Patient or population: ambulatory paediatric cancer patients newly diagnosed with acute lymphoblastic leukaemia who received chemotherapy Settings: outpatient clinics Intervention: antithrombin Comparison: placebo | |||||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks* (95% CI) | Differenceb (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | What it means | |
| Assumed riska | Corresponding risk | ||||||
|
With placebo Number of events per 1000 participants |
With antithrombin
(any dosage) Number of events per 1000 participants |
||||||
|
Symptomatic VTE Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how antithrombin affects symptomatic VTE across different cancer types. | |
| NA | NA | ||||||
|
Major bleeding Follow‐up: median 4 months |
RR 0.78 (0.03 to 18.57) | Population at unclear riskd | 4 per 1000 fewer events (16 fewer to 293 more) | 85 (1) | ⊕⊝⊝⊝ Very lowe | We have very little confidence in the estimated effect of antithrombin on the incidence of major bleeding in acute lymphoblastic leukaemia. | |
| 17 per 1000 | 13 per 1000 (1 to 310) | ||||||
|
Symptomatic PE Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how antithrombin affects symptomatic PE across different cancer types. | |
| NA | NA | ||||||
|
Symptomatic DVT Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how antithrombin affects symptomatic DVT across different cancer types. | |
| NA | NA | ||||||
|
Any VTE Follow‐up: median 4 months |
RR 0.84 (0.41 to 1.73) |
Population at unclear riskd | 53 per 1000 fewer events (197 fewer to 243 more) | 85 (1) | ⊕⊝⊝⊝ Very lowe | We have very little confidence in the estimated effect of antithrombin on the incidence of any VTE in acute lymphoblastic leukaemia. | |
| 333 per 1000 | 280 per 1000 (137 to 577) | ||||||
|
1‐year overall mortality Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how antithrombin affects 1‐year overall mortality across different cancer types. | |
| NA | NA | ||||||
|
Clinically relevant bleeding Follow‐up: NA |
NAc | Population at unclear riskd | NA | NA | NA | We do not know how antithrombin affects clinically relevant bleeding across different cancer types. | |
| NA | NA | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; NA: not applicable; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | |||||||
|
GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||||
aThe assumed risk was calculated from the observed control group risk in Mitchell 2003. bDifference calculated as the absolute risk difference between the assumed risk and corresponding risk, expressed per 1000. cNo trials contributed to this outcome. dThe risk profile refers to the median observed risk to experience symptomatic VTEs. As Mitchell 2003 did not report this outcome, the risk profile remains unclear. eDowngraded three levels because of imprecision, indirectness and attrition bias, see Characteristics of included studies table.
Background
Cancer is often complicated by venous thromboembolism (VTE), which can present as deep vein thrombosis (DVT) or pulmonary embolism (PE), or both (Ay 2017; Cohen 2017; Khorana 2009a; Timp 2013). Cancer patients with VTE have a two‐fold or greater increased mortality compared with cancer patients without thrombosis, which could be explained by the development of fatal PEs or by a more aggressive disease in patients who develop VTE (Sorensen 2000). VTE in cancer patients may be difficult to recognise due to aspecific symptoms, which may overlap and be confused with symptoms caused by the underlying cancer disease process or cancer treatments. VTE carries significant morbidity due to the need for hospitalisation and an increased risk of recurrent VTE or bleeding complications while on anticoagulation (Hutten 2000; Prandoni 2002). The occurrence of symptomatic or incidental VTE may delay the delivery of cancer treatments such as chemotherapy, with a negative impact on morbidity and potentially mortality. In addition, the occurrence of VTE brings further emotional strain for patients and their families, which negatively affects their quality of life. Finally, the costs related to the management of VTE may be considerable, resulting from the expenses related to the drugs and hospitalisation (Heit 2015).
Description of the condition
The incidence of VTE is higher in people with cancer compared with people without cancer, with similar rates of PE and proximal DVT (Heit 2015; Timp 2013). Compared with an incidence of about 0.1% in the general population, the absolute risk of VTE in people with cancer varies between 0.6% and about 8%, depending on patient and cancer characteristics, duration of follow‐up, and diagnostic tests used for VTE (Cohen 2017; Khorana 2009a; Timp 2013). In cancer patients with advanced disease, the incidence rate of VTE has been estimated to be as high as 68 per 1000 person‐years (Horsted 2012). About one‐half of all VTEs in cancer patients are incidentally detected on routine imaging without any clinical suspicion of VTE at the time of diagnosis (incidental VTE; Di Nisio 2017). The clinical relevance of incidental VTE seems to be comparable to that of symptomatic VTE with similar risk of recurrent thrombosis (Di Nisio 2017; Kraaijpoel 2019; van Es 2014). Chemotherapy has been recognised as an independent predictor for symptomatic VTE, with reported rates ranging from 11%, in Otten 2004, up to 75%, in Heit 2015 and Khorana 2009a, depending on the type of chemotherapeutic agent used. The risk of thrombosis in cancer patients receiving chemotherapy seems to vary based on the stage of the disease, ranging from 3% to 5% in patients with early‐stage cancer to 30% in those with metastatic or advanced malignancy (Khorana 2009a; Timp 2013). The benefit‐risk ratio of primary prophylaxis in ambulatory patients with cancer who are receiving chemotherapy is not well established. Current guidelines do not recommend routine thromboprophylaxis in such patients and suggest risk stratification to identify people with a higher risk of VTE who may have a greater benefit from thromboprophylaxis (Connors 2014; Key 2020).
Description of the intervention
Currently available drugs for the prevention of VTE include parenteral (e.g. unfractionated heparin (UFH), low‐molecular‐weight heparins (LMWH), and fondaparinux), and oral anticoagulants (e.g. vitamin K antagonists (VKAs), direct oral anticoagulants (DOACs) including the direct thrombin inhibitor dabigatran, and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban). In fact, each one of these agents may present disadvantages for long‐term prophylaxis in ambulatory patients with cancer. Heparins and fondaparinux, as well as the ultra‐low‐molecular‐weight heparin (uLMWH) semuloparin, require daily subcutaneous injections, which represent a considerable burden for the patient. Of note is that marketing applications for semuloparin have been withdrawn worldwide, and it is, therefore, unlikely to ever be commercially available (EMEA 2012). Treatment with VKAs requires laboratory monitoring with frequent dose‐adjustments and may be complicated by multiple drug and food interactions. Direct thrombin and factor Xa inhibitors offer the potential advantages of an oral route of administration, and in comparison with VKAs do not require routine laboratory monitoring and have fewer pharmacological interactions. VKAs and direct thrombin or factor Xa inhibitors can be difficult to administer in cancer patients with nausea or vomiting.
The use of pharmacological prophylaxis may be more challenging in people with cancer. The efficacy could be reduced by the intrinsic procoagulant state induced by the cancer itself, prothrombotic treatments for cancer (e.g. chemotherapy), as well as the decline in the patient's general condition leading to immobilisation. In contrast, the risk of bleeding events could be high even with prophylactic doses because of a number of predisposing factors such as the bleeding tendency at the site of the cancer, the relative decrease in the number of platelets in the blood (thrombocytopenia) secondary to chemotherapy, and the concomitant use of drugs (e.g. bevacizumab) that affect the vessel wall integrity (Kamphuisen 2014).
Currently available mechanical interventions for the prevention of VTE include intermittent pneumatic compression and graduated elastic stockings. These non‐pharmacological interventions may be a valid option in cancer patients who are at risk of bleeding; however, evidence supporting their benefit and assuring no harm is limited.
Why it is important to do this review
The overall burden of VTE in people with cancer is steadily increasing as a result of an ageing population, greater awareness, prothrombotic anticancer treatments, as well as the growing cancer population (Heit 2015). In addition, an increasing number of VTEs in cancer patients are diagnosed incidentally on imaging tests requested for baseline staging, treatment response evaluation, or routine surveillance while off anticancer treatment (Di Nisio 2017). Provision of widespread primary thromboprophylaxis for ambulatory cancer patients who receive chemotherapy may help in preventing VTE. However, the efficacy of thromboprophylaxis needs to be balanced with the associated risks of bleeding complications.
Objectives
To assess the efficacy and safety of primary thromboprophylaxis for VTE in ambulatory patients with cancer receiving chemotherapy compared with placebo, no thromboprophylaxis, or an active control intervention.
To compare the efficacy and safety of different types of primary thromboprophylaxis by stratifying the main results per type of drug or mechanical intervention, and by aggregating results from head‐to‐head comparisons.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐randomised trials (Higgins 2011).
Types of participants
We included participants who were ambulatory outpatients receiving chemotherapy at the time of randomisation or study entry. We included participants of any age (including children) with either a solid or haematological cancer, at any stage. We included any type of chemotherapy as described by the study authors.
We excluded studies of participants receiving anticoagulation for a previous VTE or an indication other than VTE if data could not be extracted separately for participants not receiving anticoagulants. We excluded studies evaluating prophylaxis for catheter‐related thrombosis, since this is already the subject of another Cochrane Review (Kahale 2018).
Types of interventions
We included studies that evaluated any oral or parenteral anticoagulant (e.g. UFH, LMWH, uLMWH, fondaparinux, direct thrombin or factor Xa inhibitors and VKAs) or mechanical intervention (intermittent pneumatic compression or graduated elastic stockings), or both, used to prevent VTE in ambulatory patients with cancer who were receiving chemotherapy. Comparison interventions included no thromboprophylaxis in the form of an inactive control intervention (placebo, no treatment, standard care) or an active control intervention (a different scheme or regimen of the same intervention, a different pharmacological type of prophylaxis, a different type of non‐pharmacological prophylaxis). We considered any frequency or duration of administration, dosage or intensity, and timing of delivery of pharmacological prophylaxis.
Types of outcome measures
We considered all outcomes as binary outcomes except for quality of life, which we considered a continuous outcome.
Primary outcomes
Symptomatic VTE: objectively verified by means of Doppler (compression) ultrasonography or venography for DVT, and spiral computed tomography, ventilation/perfusion lung scan, or pulmonary angiography for PE.
Major bleeding; typically defined as overt bleeding associated with a decrease in haemoglobin of 2 g/dL or more, or leading to a transfusion of two or more units of packed red blood cells or whole blood; bleeding that occurred at a critical site (intracranial, intraspinal, intraocular, pericardial, intra‐articular, intramuscular with compartment syndrome, retroperitoneal); or bleeding contributing to death (Schulman 2005).
Secondary outcomes
Symptomatic PE.
Symptomatic DVT.
Any VTE (symptomatic and incidental).
One‐year overall mortality.
Clinically relevant bleeding (major and clinically relevant non‐major bleeding); typically defined as overt bleeding that does not meet the criteria for major bleeding, but is associated with the need for medical intervention, contact with a physician, or interruption of the study drug or with discomfort or impairment of activities of daily life (Kaatz 2015).
Incidental VTE.
Minor bleeding; defined as a bleeding event not matching the criteria for major bleeding or clinically relevant non‐major bleeding.
Arterial thromboembolic events.
Superficial venous thrombosis.
Quality of life.
Any serious adverse event; defined as events resulting in patient hospitalisation, prolongation of hospitalisation, persistent or significant disability, congenital abnormality or birth defect of offspring, life‐threatening events. or death. For trials using LMWH as the intervention or control, we recorded heparin‐induced thrombocytopenia (HIT) and the incidence of osteoporosis, as defined by the trial authors.
For the 'Summary of findings' tables, we selected the following outcomes as the most patient‐relevant.
Symptomatic VTE.
Major bleeding.
Symptomatic PE.
Symptomatic DVT.
Any VTE.
One‐year overall mortality.
Clinically relevant bleeding.
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year, or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched from inception to 3 August 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2020, Issue 7);
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (searched from 1 January 2017 to 3 August 2020);
Embase Ovid (searched from 1 January 2017 to 3 August 2020);
CINAHL EBSCO (searched from 1 January 2017 to 3 August 2020);
AMED Ovid (searched from 1 January 2017 to 3 August 2020).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 3 August 2020:
the World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
The review authors searched the reference lists of identified studies and contacted content experts and trialists for relevant references. One review author (MC) screened the conference proceedings of the American Society of Clinical Oncology (from 2009 to 2018) and the International Society of Thrombosis and Haemostasis (from 2003 to 2019), combining the search terms of 'venous thrombosis', 'vein thrombosis', or 'pulmonary embolism' with 'cancer' or 'tumour'. We included studies if we could obtain adequate information from either the abstract or personal communication.
Data collection and analysis
Selection of studies
Two review authors (EV, MC) independently reviewed the titles and abstracts identified from the database searches to determine whether they met the inclusion criteria. Any disagreements were resolved through discussion between the review authors. The review authors were not blinded to the journal, institution, or results of the study. We applied no language restrictions. We reassessed studies with insufficient information if we were able to obtain additional details from the trial authors. We documented reasons for excluding studies in the Characteristics of excluded studies table. We considered all reports relating to the same trial if there were multiple reports. We collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram
Data extraction and management
Two review authors (EV, MC) independently extracted the data from the included studies onto standardised forms, resolving any disagreements by consensus or by involving a third review author (AWSR). We collected information on risk of bias, participant characteristics, characteristics of the intervention and control groups, and outcomes. Whenever possible, we extracted the results from an intention‐to‐treat analysis. If we could not calculate effect sizes, we contacted the trial authors to request additional data.
Assessment of risk of bias in included studies
Two review authors (EV, MC) independently assessed randomisation, blinding, and adequacy of analyses (Higgins 2011). We resolved disagreements by consensus or by involving a third review author (AWSR).
We assessed two components of randomisation: generation of allocation sequence and concealment of allocation. We considered generation of the allocation sequence to be adequate if it resulted in an unpredictable allocation schedule. Mechanisms considered to be adequate included random number tables, computer‐generated random numbers, minimisation, coin tossing, shuffling cards, and drawing lots. We considered trials using an unpredictable allocation sequence to be randomised. We considered trials using potentially predictable allocation mechanisms, such as alternation or allocation of participants according to date of birth, date of presentation, or case record number, to be quasi‐randomised (Higgins 2011).
We considered concealment of allocation to be adequate if participants and the investigators responsible for participant selection were unable to predict before allocation which treatment was next. Methods considered adequate included central randomisation; pharmacy‐controlled randomisation using identical, prenumbered containers; and sequentially numbered, sealed, opaque envelopes. We considered blinding of participants and therapists to be adequate if experimental and control preparations were explicitly described as indistinguishable, or if a study used a double‐dummy technique. We considered assessors to be blinded if this was explicitly mentioned by the investigators.
We considered the risk of attrition bias to be low if all randomised participants were included in the analyses according to the intention‐to‐treat principle. We classified the item 'selective reporting' as at low risk of bias if we had both the protocol and the full report of a given study, where the full report presented results for all outcomes listed in the protocol. We classified a study as at high risk of bias if a report did not present data on all outcomes reported in either the protocol or the methods section. We did not consider the item 'other bias' in this review. We assessed the reporting of primary outcomes and sample size calculations. Finally, we used GRADE to describe the certainty of the overall body of evidence, defined as the extent of our confidence in the estimates of treatment benefits and harms (Guyatt 2008; Higgins 2011).
Measures of treatment effect
We presented results as summary risk ratios (RRs) for dichotomous variables, determining a 95% confidence interval (CI) for each estimate. The unit of analysis was the participant throughout all outcomes. We planned to summarise results on quality of life with the standardised mean difference (SMD), but none of the studies provided quality of life data on the continuous scale. We used inverse‐variance random‐effects model meta‐analysis to combine the trials (DerSimonian 1986). For outcomes considered in the 'Summary of findings' tables, we also calculated clinical effect summary statistics such as the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) to express the final results of the review. NNTB and NNTH were only calculated in the case of statistically significant findings.
Assessment of heterogeneity
We identified between‐study variation visually by looking at the overlap of CIs of individual studies. In addition, we measured and described heterogeneity of the treatment effect between trials using the I2 statistic and the P value from the corresponding Chi2 test and the variance estimate Tau2.
Assessment of reporting biases
For the primary outcomes symptomatic VTE and major bleeding, we evaluated publication bias and other biases related to small‐study size using funnel plots, whenever 10 studies contributed. We plotted the RRs on the vertical axis against their standard errors on the horizontal axis (Sterne 2001). Funnel plot symmetry would be expected in the absence of any bias related to small‐study size. We used the Harbord–Egger's test to assess symmetry (Harbord 2006). We further explored any anomaly in stratified analyses, in which we investigated the effects of differences in types of LMWH, age, type of cancer, and suboptimal study design choices on the magnitude of the effects.
Data synthesis
In the main analyses, we analysed and presented data by stratifying for the type of thromboprophylaxis used and grouped comparisons according to whether control treatment included placebo/no thromboprophylaxis or active control treatment.
We planned to explore the between‐trial heterogeneity by stratifying the primary outcomes for the following trial characteristics: age (65 years or less versus above 65 years); type of cancer; stage of cancer (metastatic versus non‐metastatic); type of major bleeding (according to the definition provided by Schulman 2005 versus unclear or different definition); concealment of allocation (adequate versus inadequate or unclear); blinding (adequate versus inadequate or unclear); analysis in accordance with the intention‐to‐treat principle (yes versus no or unclear); selective outcome reporting (low versus high or unclear risk); and differences in the use of cointerventions in the trial groups. We planned to use univariate random‐effects model meta‐regression to determine whether treatment effects were affected by these factors and by three continuous variables at trial level: dosage of intervention, treatment duration, and length of follow‐up (Thompson 1999). Not all planned analyses could be performed, which is explained in the Differences between protocol and review section.
We performed the data analysis in Review Manager 5 (Review Manager 2014). We performed stratified analyses and funnel plot exploration in STATA release 15.1 (Stata 2019).
'Summary of findings' table
We presented the main findings of the review concerning the certainty of the evidence, magnitude of effect of the interventions examined, and sum of available data in 'Summary of findings' tables, according to the GRADE principles described by Higgins 2011 and Guyatt 2008. We created separate tables for different comparisons of thromboprophylaxis used and reported the findings of the outcomes symptomatic VTE, major bleeding, symptomatic PE, symptomatic DVT, any VTE, one‐year overall mortality, and clinically relevant bleeding. For the critical outcome symptomatic VTE, we applied cutoffs to define high‐ and intermediate‐risk groups. We used a cutoff of 7% to define high risk, in line with the cutoff proposed by Khorana 2008, which is between 6.7% and 7.1% over about three months, and with the results of a recent trial (Carrier 2019). We used event rates between 2% and 7% to define groups at intermediate risk for symptomatic VTEs (Khorana 2008).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies tables.
Results of the search
Following title and abstract screening, we considered 52 reports (31 trials) to be potentially eligible for this update. We included 26 reports related to six new trials (Campos‐Cabrera 2018; Carrier 2019; Ek 2018; Greiner 2019; Khorana 2019; Meyer 2018), and 15 reports related to previously included trials. We identified five new excluded studies (Groen 2019; NCT04106700; NCT04352439; Storrar 2019; Zwicker 2019). We added five reports to the Characteristics of ongoing studies table (ChiCTR‐TRC‐08000267; NCT01518465; NCT03090880; NCT03428373; O'Brien 2019). One study previously listed as awaiting classification has now been excluded (Salat 1990). See Figure 1 for the study flow diagram.
1.
Study flow diagram. RCT: randomised controlled trial; VTE: venous thromboembolism.
Included studies
For this update, we included six new studies (Campos‐Cabrera 2018; Carrier 2019; Ek 2018; Greiner 2019; Khorana 2019; Meyer 2018). Two of these were reported as ongoing studies in the previous version of the review (Carrier 2019; Ek 2018).
The review includes 32 RCTs randomising 15,678 participants. The treatments evaluated consisted of the uLMWH semuloparin (Agnelli 2012), LMWH (Agnelli 2009; Altinbas 2004; Ek 2018; Elit 2012; Greiner 2019; Haas 2012; Kakkar 2004; Khorana 2017; Klerk 2005; Larocca 2012; Lecumberri 2013; Macbeth 2016; Maraveyas 2012; Meyer 2018; Palumbo 2011; Pelzer 2015; Perry 2010; Sideras 2006; Vadhan‐Raj 2013; van Doormaal 2011; Zwicker 2013), UFH (Greiner 2019; Lebeau 1994), the VKA warfarin (Chahinian 1989; Levine 1994; Maurer 1997; Palumbo 2011; Zacharski 1981), antithrombin (Greiner 2019; Mitchell 2003), and the oral direct factor Xa inhibitors apixaban (Carrier 2019; Levine 2012) and rivaroxaban (Campos‐Cabrera 2018; Khorana 2019). None of the included RCTs used non‐pharmacological prophylaxis, or pharmacological thromboprophylaxis with fondaparinux, the direct thrombin inhibitor dabigatran, or the direct factor Xa inhibitor edoxaban. In 17/32 studies, inclusion was restricted to people with locally advanced or metastatic cancer, in three studies limited cancer was included, in six studies both early and advanced disease were included, while in the remaining studies the stage was not clear (see Characteristics of included studies table). Meyer 2018 recruited participants with completely resected stage I, II, or IIIA non‐small‐cell lung cancer. Greiner 2019 and Mitchell 2003 included children with acute lymphoblastic leukaemia.
Two studies assessed the use of the oral direct factor Xa inhibitors apixaban (Carrier 2019) and rivaroxaban (Khorana 2019) versus placebo in patients with cancer considered at intermediate‐to‐high risk of VTE (Khorana score 2 or greater).
Carrier 2019 recruited 574 participants with a Khorana score of 2 or greater and newly diagnosed cancer or progression of known cancer after complete or partial remission and who were initiating a new course of chemotherapy with a minimum treatment intent of three months. Participants were randomised to apixaban 2.5 mg twice daily or placebo for six months.
Khorana 2019 recruited 841 ambulatory adults with various cancers initiating a new systemic regimen and at increased risk for VTE (defined as Khorana score of 2 or greater) who had no DVT on screening ultrasonography. Participants were randomised 1:1 to rivaroxaban 10 mg once daily or placebo up to day 180.
In a pilot, phase II study, Levine 2012 recruited 125 participants receiving either first‐ or second‐line chemotherapy for advanced or metastatic lung, breast, gastrointestinal, bladder, ovarian, or prostate cancer; cancer of unknown origin; myeloma; or selected lymphomas. Participants were randomised to apixaban 5 mg (32 participants), 10 mg (30 participants), 20 mg (33 participants), and placebo (30 participants). The study treatment was given for 12 weeks, beginning within four weeks of starting chemotherapy.
Campos‐Cabrera 2018 recruited 23 participants with multiple myeloma who received thalidomide‐ and dexamethasone‐based triplet induction therapy and maintenance thalidomide. Participants were randomised 5:1 to receive aspirin 100 mg or rivaroxaban 10 mg until relapse and further treatment was needed.
One study assessed the uLMWH semuloparin versus placebo.
Agnelli 2012 recruited 3212 participants with metastatic or locally advanced solid cancer of the lung, pancreas, stomach, colon or rectum, bladder, or ovary and randomised them to the uLMWH semuloparin 20 mg once daily versus placebo starting on the first day of a first or new regimen of chemotherapy. The intervention was continued for three months unless chemotherapy was stopped earlier.
Twenty‐one studies assessed LMWH.
Seventeen studies evaluated LMWH either versus placebo or no thromboprophylaxis (Agnelli 2009; Altinbas 2004; Ek 2018; Haas 2012; Kakkar 2004; Khorana 2017; Klerk 2005; Lecumberri 2013; Macbeth 2016; Maraveyas 2012; Meyer 2018; Pelzer 2015; Perry 2010; Sideras 2006; Vadhan‐Raj 2013; van Doormaal 2011; Zwicker 2013). One study compared different doses from prophylactic to full therapeutic of LMWH with each other (Elit 2012). These 18 trials varied in the duration and type of LMWH, including eight weeks to 48 months of subcutaneous (SC) dalteparin, enoxaparin, certoparin, nadroparin, bemiparin, and tinzaparin. The dose of LMWH was prophylactic in most studies, intermediate in three (Ek 2018; Meyer 2018; Pelzer 2015), and therapeutic in one study (Maraveyas 2012). In two studies, initial therapeutic LMWH was followed by intermediate doses (Klerk 2005; van Doormaal 2011). Fifteen of these 18 studies reported a mean age at study entry of 65 years or younger, whereas Ek 2018 and Zwicker 2013 included participants with a mean age above 65 years.
Agnelli 2009 recruited 1150 participants with metastatic or locally advanced lung, gastrointestinal, pancreatic, breast, ovarian, or head and neck cancer and randomised them to nadroparin 3800 IU SC once daily versus placebo. Study treatment started on the same day as chemotherapy and was given for the duration of the chemotherapy or up to a maximum of 120 days (± 10 days).
Altinbas 2004 recruited 84 participants with histologically confirmed small‐cell lung carcinoma and randomised them to standard anticancer treatment with or without dalteparin 5000 IU SC once daily. Dalteparin was stopped with disease progression or at the end of the 18 weeks of chemotherapy.
Ek 2018 recruited 390 participants with newly diagnosed small‐cell lung cancer and randomised them to enoxaparin at a supraprophylactic dose (1 mg/kg) in addition to standard treatment versus standard treatment alone. Enoxaparin was started on the same day as chemotherapy and continued until the 21st day of the last chemotherapy cycle.
Elit 2012 recruited 77 women with newly diagnosed epithelial ovarian cancer and randomised them to receive standard chemotherapy and one of three SC doses of dalteparin (50 IU/kg, 100 IU/kg, or 150 IU/kg), once daily during the first three of six cycles of three‐weekly chemotherapy.
Haas 2012 recruited 353 participants with metastatic breast cancer or 547 participants with non‐small‐cell lung carcinoma and receiving first‐ or second‐line chemotherapy. Participants were randomised to six months of certoparin 3000 IU SC, once daily versus placebo.
Kakkar 2004 recruited 385 participants with histologically confirmed locally advanced or metastatic malignant disease of the breast, lung, gastrointestinal tract, pancreas, liver, genitourinary tract, ovary, or uterus and randomised them to dalteparin 5000 IU SC, once daily versus placebo. Study treatment was for one year or until the participant died, whichever occurred first.
Khorana 2017 recruited 98 participants with cancer at high risk for VTE (Khorana score 3 or greater) who initiated a new systemic chemotherapy regimen and randomised them to dalteparin 5000 IU SC once daily for 12 weeks versus no thromboprophylaxis.
Klerk 2005 recruited 302 participants with metastasised or locally advanced solid tumours and randomised them to nadroparin versus placebo. Study treatment was given using prefilled syringes containing a fixed volume of nadroparin (anti‐factor Xa 9500 IU/mL) or placebo according to the participant’s weight: 0.4 mL for those weighing less than 50 kg, 0.6 mL for those weighing between 50 kg and 70 kg, and 0.8 mL for those weighing more than 70 kg. Study treatment was to be administered SC twice daily during the initial 14 days of treatment and once daily thereafter for another four weeks.
Lecumberri 2013 recruited 39 participants with newly diagnosed, limited‐stage small‐cell lung cancer and randomised them to standard chemoradiotherapy alone or combined with bemiparin 3500 IU SC once daily for a maximum of 26 weeks.
Macbeth 2016 recruited 2202 participants with histopathological or cytological diagnosis of primary bronchial carcinoma of any stage and histology (small‐cell or non‐small‐cell) and randomised them to standard anticancer treatment (including active supportive or palliative care) with or without dalteparin 5000 IU SC once daily for a maximum of 24 weeks.
Maraveyas 2012 recruited 123 participants with advanced pancreatic cancer and randomised them to dalteparin (200 IU/kg SC, once daily for four weeks followed by 150 IU/kg for a further eight weeks) in combination with gemcitabine versus gemcitabine alone. Continuing dalteparin prophylaxis after 12 weeks was not recommended, but was left to the discretion of the investigator.
Meyer 2018 recruited 553 participants with completely resected stage I, II or IIIA non‐small‐cell lung cancer and randomised them to tinzaparin 100 IU/kg SC once daily for 12 weeks in addition to standard of care versus standard of care alone.
Pelzer 2015 recruited 312 participants with histologically or cytologically confirmed advanced pancreatic cancer and randomised them to standard anticancer treatment with or without enoxaparin 1 mg/kg SC once daily for three months, started simultaneously with palliative systemic chemotherapy; after 12 weeks of initial chemotherapy, all participants who had not progressed received the standard therapy with or without enoxaparin 40 mg SC once daily for an additional three months.
Perry 2010 recruited 186 participants with newly diagnosed, pathologically confirmed WHO grade 3 or grade 4 glioma and randomised them to six months of dalteparin 5000 IU SC once daily versus placebo starting within the first month after surgery. Participants were allowed to continue the study medication for 12 months.
Sideras 2006 recruited 138 participants with advanced breast cancer who did not respond to first‐line chemotherapy, advanced prostate cancer resistant to primary hormonal therapy, advanced lung cancer, or advanced colorectal cancer. In the first part of the study, participants were randomised to dalteparin 5000 IU SC once daily versus placebo, while in the second part participants were randomised to dalteparin 5000 IU SC once daily plus standard clinical care versus standard clinical care alone. Dalteparin (or placebo) was given for 18 weeks or until disease progression.
Vadhan‐Raj 2013 recruited 75 participants with advanced stage (unresectable or metastatic) adenocarcinoma of the pancreas planning to initiate systemic chemotherapy and randomised them to chemotherapy with or without dalteparin 5000 IU SC once daily for 16 weeks.
van Doormaal 2011 recruited 503 participants with non‐small‐cell lung cancer (stage IIIB), hormone‐refractory prostate cancer, or locally advanced pancreatic cancer and randomised them to standard anticancer treatment with or without nadroparin. Sc nadroparin was administered for six weeks (two weeks at therapeutic dose and four weeks at half therapeutic dose). The participants were eligible to receive additional cycles of nadroparin (two weeks at therapeutic dose and four weeks washout period) for a maximum of six cycles.
Zwicker 2013 recruited 34 participants with histologically confirmed advanced stage malignancy, which included adenocarcinoma of the pancreas (locally advanced or metastatic), colorectal (stage IV), non‐small‐cell lung cancer (stage III or IV), relapsed or stage IV ovarian, or surgically unresectable or metastatic gastric adenocarcinoma. Participants were randomised to enoxaparin 40 mg SC once daily for two months or observation.
Three additional studies compared LMWH against an active control.
Greiner 2019 recruited 949 participants aged one to 18 years with newly diagnosed acute lymphoblastic leukaemia and randomised them to low‐dose UFH (2 IU/kg body weight/hour), LMWH (enoxaparin 80 IU/kg to 100 IU/kg body weight once daily SC with a target anti‐Xa level not exceeding 0.4 U/L) or activity‐adapted antithrombin throughout induction therapy. Thromboprophylaxis was started on day eight and ended on day 33 of induction chemotherapy.
Larocca 2012 recruited 342 participants with newly diagnosed multiple myeloma treated with lenalidomide and low‐dose dexamethasone induction and melphalan‐prednisone‐lenalidomide consolidation. Participants were randomised to aspirin 100 mg per day or LMWH (enoxaparin 40 mg once daily). Prophylaxis was provided during the four (28‐day) cycles of induction and the six (28‐day) cycles of consolidation therapy.
Palumbo 2011 recruited 667 participants with previously untreated myeloma who received thalidomide‐containing regimens and randomised them to aspirin 100 mg once daily, low‐dose warfarin (1.25 mg once daily) or LMWH (enoxaparin 40 mg once daily). The prophylaxis was administered during the three cycles of induction therapy in participants aged 65 years or less and during the first six cycles of induction therapy in participants aged over 65 years.
Four studies compared the VKA warfarin against no thromboprophylaxis or placebo.
Chahinian 1989 recruited 328 participants with extensive carcinoma of the lung and randomised them to warfarin (dose to maintain a prothrombin time 1.5 to twice the control values) versus no warfarin. Warfarin was continued throughout the course of chemotherapy.
Levine 1994 recruited 311 participants with metastatic stage IV breast carcinoma who had been receiving first‐ or second‐line chemotherapy for four weeks or less and randomised them to warfarin (target of international normalised ratio (INR) 1.3 to 1.9) versus placebo. Study treatment began either at the start of chemotherapy or within the following four weeks and continued until one week after termination of chemotherapy.
Maurer 1997 recruited 347 participants with limited‐stage small‐cell lung cancer who were to receive chemotherapy and radiotherapy and randomised them to warfarin or no warfarin. Warfarin (dose of 10 mg once daily for the first three days and then at a dose to maintain the prothrombin time between 1.4 and 1.6 times the local institutional control standards) was continued through the complete course of chemotherapy and radiation therapy and was stopped three weeks after the last cycle of chemotherapy.
Zacharski 1981 recruited 50 participants with small‐cell lung cancer and randomised them to warfarin (dose to prolong the prothrombin time to approximately two times the control value) versus no warfarin.
One study each evaluated UFH and antithrombin against no thromboprophylaxis.
Lebeau 1994 recruited 277 participants with limited and extensive small‐cell lung cancer who had not been previously treated with chemotherapy or radiotherapy. The dose of UFH was initially adapted to weight (500 IU/kg/day), then adjusted by clotting times (different techniques used, and results had to be between two and three times the control value). UFH was administered in two or three daily injections for five weeks and stopped one week after the second course of chemotherapy.
Mitchell 2003 recruited 85 children newly diagnosed with acute lymphoblastic leukaemia and randomised them to receive, or not, weekly infusions of antithrombin.
Excluded studies
We excluded 30 studies for the following reasons: design other than an RCT (Baz 2005; Bocharov 2011; Kessler 2011; Meister 2008; Minnema 2004; NCT04106700; NCT04352439; Paydas 2008; Storrar 2019; Zangari 2003); studies on perioperative thromboprophylaxis (Bergqvist 1983; Heilmann 1995; Hills 1972; Macintyre 1974; Maxwell 2000; Sideras 2007; Welti 1981); inclusion of hospitalised cancer patients (Eichinger 2008; Haas 2011; Poniewierski 1988; Weber 2008); no relevant outcomes reported (Groen 2019; Rajan 1995; Salat 1990); no eligible intervention (Niesvizky 2007; Zwicker 2019); and prophylaxis was for catheter‐related thrombosis (NCT00004875). Three studies were terminated early: NCT00790452 because of a drug supply issue; NCT00662688 due to the lack of eligible patients; NCT00031837 with no reason for study termination reported.
Studies awaiting classification
There are two completed studies awaiting classification, one published in abstract form (Ciftci 2012), one published as trial registration (NCT00771563). Outcome data for these two trials are not yet published but may be available at the time of the next update.
Ongoing studies
Five new ongoing studies were identified for this update (ChiCTR‐TRC‐08000267; NCT01518465; NCT03090880; NCT03428373; O'Brien 2019), bringing the total to eight ongoing studies (ChiCTR‐TRC‐08000267; NCT00718354; NCT01518465; NCT02285738; NCT02555878; NCT03090880; NCT03428373; O'Brien 2019).
Risk of bias in included studies
The 'Risk of bias' summary is shown in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The random sequence was adequately generated in 24 studies (Agnelli 2009; Agnelli 2012; Carrier 2019; Chahinian 1989; Ek 2018; Elit 2012; Greiner 2019; Haas 2012; Kakkar 2004; Khorana 2019; Klerk 2005; Larocca 2012; Lecumberri 2013; Levine 1994; Levine 2012; Macbeth 2016; Maraveyas 2012; Meyer 2018; Mitchell 2003; Palumbo 2011; Pelzer 2015; Perry 2010; van Doormaal 2011; Zacharski 1981), but was unclear in the remaining eight studies due to poor reporting (Altinbas 2004; Campos‐Cabrera 2018; Khorana 2017; Lebeau 1994; Maurer 1997; Sideras 2006; Vadhan‐Raj 2013; Zwicker 2013).
Allocation was adequately concealed in 23 studies (Agnelli 2009; Agnelli 2012; Carrier 2019; Ek 2018; Elit 2012; Greiner 2019; Kakkar 2004; Khorana 2019; Klerk 2005; Larocca 2012; Lebeau 1994; Lecumberri 2013; Levine 2012; Macbeth 2016; Maraveyas 2012; Meyer 2018; Mitchell 2003; Palumbo 2011; Pelzer 2015; Perry 2010; Sideras 2006; van Doormaal 2011; Zwicker 2013), and was unclear in the remaining nine studies due to poor reporting (Altinbas 2004; Campos‐Cabrera 2018; Chahinian 1989; Haas 2012; Khorana 2017; Levine 1994; Maurer 1997; Vadhan‐Raj 2013; Zwicker 2013).
Blinding
Ten studies had a double‐blind design and were at low risk of performance and detection bias (Agnelli 2009; Agnelli 2012; Carrier 2019; Haas 2012; Kakkar 2004; Khorana 2019; Klerk 2005; Levine 1994; Levine 2012; Perry 2010), and 18 were open studies and at high risk of bias (Altinbas 2004; Ek 2018; Elit 2012; Greiner 2019; Khorana 2017; Larocca 2012; Lebeau 1994; Lecumberri 2013; Macbeth 2016; Maraveyas 2012; Meyer 2018; Mitchell 2003; Palumbo 2011; Pelzer 2015; Sideras 2006; Vadhan‐Raj 2013; van Doormaal 2011; Zwicker 2013). In four studies blinding was unclear due to poor reporting (Campos‐Cabrera 2018; Chahinian 1989; Maurer 1997; Zacharski 1981).
Incomplete outcome data
Fourteen studies performed the analysis according to the intention‐to‐treat principle and so were at low risk of attrition bias (Agnelli 2012; Elit 2012; Greiner 2019; Khorana 2017; Khorana 2019; Klerk 2005; Larocca 2012; Lebeau 1994; Macbeth 2016; Pelzer 2015; Perry 2010; Vadhan‐Raj 2013; Zacharski 1981; Zwicker 2013), while in 14 studies the percentages of participants randomised and subsequently excluded from the analyses ranged from 0.7% to 10%; we considered these at high risk of bias (Agnelli 2009; Carrier 2019; Chahinian 1989; Ek 2018; Haas 2012; Kakkar 2004; Lecumberri 2013; Levine 1994; Levine 2012; Maraveyas 2012; Meyer 2018; Palumbo 2011; Sideras 2006; van Doormaal 2011). The study involving children used a per‐protocol analysis and excluded 22% of the participants that were initially enrolled (Mitchell 2003); we considered this study at high risk of attrition bias. Attrition bias was unclear in three studies (Altinbas 2004; Campos‐Cabrera 2018; Maurer 1997).
Selective reporting
We judged 23 studies free of selective reporting and thus at low risk of reporting bias (Agnelli 2009; Agnelli 2012; Altinbas 2004; Carrier 2019; Ek 2018; Elit 2012; Greiner 2019; Haas 2012; Kakkar 2004; Khorana 2019; Khorana 2017; Klerk 2005; Larocca 2012; Lebeau 1994; Lecumberri 2013; Levine 1994; Levine 2012; Macbeth 2016; Maraveyas 2012; Meyer 2018; Mitchell 2003; Sideras 2006; van Doormaal 2011). In five studies one or more outcomes that were reported in the results were not anticipated in the methods sections of the publications; we considered these at unclear risk of reporting bias (Campos‐Cabrera 2018; Chahinian 1989; Maurer 1997; Vadhan‐Raj 2013; Zacharski 1981). In four studies not all outcomes were reported in the results; we considered these at high risk of reporting bias (Palumbo 2011; Pelzer 2015; Perry 2010; Zwicker 2013).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
The section Data and analyses depicts effects of interventions derived from studies conducted in adults. In this section, we describe outcome data from both paediatric and adult populations.
Direct oral anticoagulant versus placebo
We found no studies on the direct thrombin inhibitor dabigatran. Three RCTs evaluated the use of factor Xa inhibitors versus placebo (Carrier 2019; Khorana 2019; Levine 2012). We found low‐certainty evidence that factor Xa inhibitors may be associated with a reduction of symptomatic VTE (RR 0.43, 95% CI 0.18 to 1.06; 3 studies, 1526 participants; high heterogeneity, Tau2 = 0.35, Analysis 1.1). We downgraded the overall body of evidence because of imprecision, inconsistency, and risk of bias (Table 1). Levine 2012 was a pilot dose‐finding study that evaluated three regimens of apixaban prophylaxis that are currently not approved. Exclusion of Levine 2012 reduced between‐trial heterogeneity for symptomatic VTE and confirmed that factor Xa inhibitors may be associated with a lower symptomatic VTE (RR 0.57, 95% CI 0.29 to 1.14 for symptomatic VTE).
1.1. Analysis.

Comparison 1: Anticoagulants versus control: symptomatic venous thromboembolism, Outcome 1: Symptomatic VTE: DOAC vs placebo
We found moderate‐certainty evidence that factor Xa inhibitors probably increase major bleeding (RR 1.74, 95% CI 0.82 to 3.68; 3 studies, 1494 participants; no heterogeneity, Tau2 = 0.00; Analysis 2.1). We downgraded due to imprecision. After exclusion of Levine 2012, differences in effects compared to placebo remained similar (RR 1.95, 95% CI 0.88 to 4.30).
2.1. Analysis.

Comparison 2: Anticoagulants versus control: major bleeding, Outcome 1: Major bleeding: DOAC vs placebo
Factor Xa inhibitors may reduce symptomatic PE but between‐study variation was large and the estimate was imprecise (RR 0.38, 95% CI 0.10 to 1.47; 3 studies, 1526 participants; high heterogeneity, Tau2 = 0.65; low‐certainty evidence; Analysis 3.1). Similarly, there was low‐certainty evidence that factor Xa inhibitors may decrease symptomatic DVT when compared to placebo (RR 0.51, 95% CI 0.21 to 1.22; 3 studies, 1526 participants; high heterogeneity, Tau2 = 0.30; low‐certainty evidence; Analysis 5.1). We downgraded to low certainty because of imprecision, inconsistency, and risk of bias. Factor Xa inhibitors halved the risk of any VTE (RR 0.55, 95% CI 0.34 to 0.90; 2 studies, 1404 participants; moderate‐certainty evidence; Analysis 6.1). Assuming a background risk of 95 per 1000 participants, this corresponds to an NNTB of 24 (95% CI 16 to 106). Factor Xa inhibitors also halved incidental VTE (RR 0.50, 95% CI 0.25 to 0.98; 2 studies, 1404 participants; Analysis 9.1). Factor Xa inhibitors probably increase clinically relevant bleeding (RR 1.61, 95% CI 0.82 to 3.15; 2 studies, 931 participants; moderate‐certainty evidence; Analysis 8.1), probably decrease arterial thromboembolism (RR 0.57, 95% CI 0.17 to 1.94; Analysis 11.1), and probably have little effect on serious adverse events (RR 0.96, 95% CI 0.82 to 1.13; Analysis 13.1). We downgraded to moderate‐certainty evidence due to imprecision.
3.1. Analysis.

Comparison 3: Anticoagulants versus control: symptomatic pulmonary embolism, Outcome 1: Symptomatic PE: DOAC vs placebo
5.1. Analysis.

Comparison 5: Anticoagulants versus control: symptomatic deep vein thrombosis, Outcome 1: Symptomatic DVT: DOAC vs placebo
6.1. Analysis.

Comparison 6: Anticoagulants versus control: any venous thromboembolism, Outcome 1: Any VTE: DOAC vs placebo
9.1. Analysis.

Comparison 9: Anticoagulants versus control: incidental venous thromboembolism, Outcome 1: Incidental VTE: DOAC vs placebo
8.1. Analysis.

Comparison 8: Anticoagulants versus control: clinically relevant bleeding, Outcome 1: Clinically relevant bleeding: DOAC vs placebo
11.1. Analysis.

Comparison 11: Anticoagulants versus control: symptomatic arterial thromboembolism, Outcome 1: Symptomatic arterial thromboembolism: DOAC vs placebo
13.1. Analysis.

Comparison 13: Anticoagulants versus control: serious adverse events, Outcome 1: Serious adverse events: DOAC vs placebo
None of the studies reported the remaining outcomes of interest (one‐year overall mortality, superficial venous thrombosis, and quality of life).
Campos‐Cabrera 2018 randomised 23 patients with multiple myeloma 5:1 to receive aspirin or rivaroxaban. There was no VTE in participants who received rivaroxaban and one participant in the aspirin group. There were no cases of major bleeding in either group. The study did not report incidental VTE, clinically relevant bleeding, arterial thromboembolism, or serious adverse events.
Low‐molecular‐weight heparin versus placebo or no thromboprophylaxis
Seventeen studies evaluated LMWH versus placebo or no thromboprophylaxis (Agnelli 2009; Altinbas 2004; Ek 2018; Haas 2012; Kakkar 2004; Khorana 2017; Klerk 2005; Lecumberri 2013; Macbeth 2016; Maraveyas 2012; Meyer 2018; Pelzer 2015; Perry 2010; Sideras 2006; Vadhan‐Raj 2013; van Doormaal 2011; Zwicker 2013).
Based on high‐certainty evidence from 11 RCTs, there was a reduction in symptomatic VTE with LMWH compared with no thromboprophylaxis in the absence of heterogeneity (RR 0.62, 95% CI 0.46 to 0.83; 3931 participants; Tau2 = 0.00; Analysis 1.2). This corresponded to an NNTB of 37 (95% CI 26 to 83), assuming a background risk of 71 symptomatic VTE events per 1000 participants (Table 2 and Khorana 2008). Funnel plot exploration found no evidence of biases associated with small studies (Figure 3). Stratified analyses showed no effect of the type of LMWH, dosage, treatment duration, type or stage of cancer, or design characteristics on the relative risk of symptomatic VTE (Table 11). Similarly, we found no evidence for a linear association between treatment duration and the risk of symptomatic VTE using meta‐regression analysis (P = 0.643).
1.2. Analysis.

Comparison 1: Anticoagulants versus control: symptomatic venous thromboembolism, Outcome 2: Symptomatic VTE: LMWH vs no thromboprophylaxis
3.
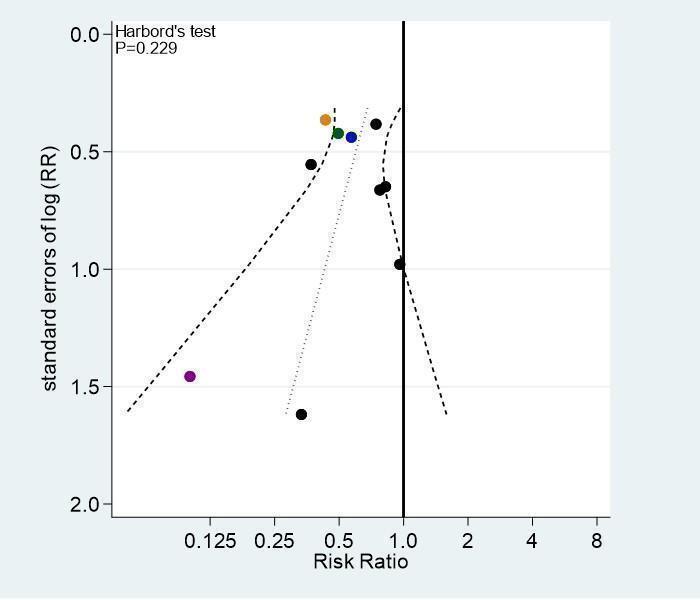
Funnel plot of comparison: 1 Anticoagulants versus control: symptomatic venous thromboembolism (VTE), outcome: 1.2 Symptomatic VTE: low‐molecular weight heparin versus no thromboprophylaxis.
1. Results of stratified analyses on symptomatic venous thromboembolism for LMWH versus no thromboprophylaxis.
| Variable | No of trials | No of participants (LMWH) | No of participants (control) | RR (95% CI) |
Heterogeneity I2 statistic/Tau2 |
P for interaction |
| All trials | 11 | 2168 | 1763 | 0.62 (0.46 to 0.83) | 0.0/0.00 | — |
| Type of LMWH | 0.530 | |||||
| Dalteparin | 6 | 508 | 491 | 0.66 (0.40 to 1.07) | 0.0/0.00 | — |
| Certoparin | 1 | 442 | 441 | 0.57 (0.24 to 1.35) | NA | |
| Nadroparin | 1 | 769 | 381 | 0.50 (0.22 to 1.13) | NA | |
| Enoxaparin | 1 | 160 | 152 | 0.43 (0.21 to 0.88) | NA | |
| Bemiparin | 1 | 20 | 18 | 0.10 (0.01 to 1.75) | NA | |
| Tinzaparin | 1 | 269 | 280 | 0.94 (0.51 to 1.73) | NA | |
| Type of dosage | 0.965 | |||||
| Prophylactic | 8 | 1680 | 1271 | 0.62 (0.42 to 0.93) | 0.0/0.00 | — |
| Higher than prophylactic | 3 | 488 | 492 | 0.58 (0.32 to 1.05) | 44.2/0.12 | |
| Treatment duration | 0.646 | |||||
| < 12 weeks | 3 | 378 | 388 | 0.74 (0.42 to 1.31) | 8.7/0.03 | — |
| 12–24 weeks | 3 | 879 | 493 | 0.56 (0.29 to 1.11) | 0.0/0.00 | |
| > 24 weeks | 5 | 911 | 882 | 0.56 (0.37 to 0.85) | 0.0/0.00 | |
| Type of cancera | 0.683 | |||||
| Mixed | 4 | 878 | 603 | 0.74 (0.36 to 1.49) | 0.0/0.00 | — |
| Lung | 5 | 798 | 684 | 0.62 (0.38 to 1.02) | 6.9/0.03 | |
| Pancreatic | 2 | 219 | 212 | 0.41 (0.23 to 0.75) | 0.0/0.00 | |
| Glioma | 1 | 99 | 87 | 0.74 (0.35 to 1.57) | NA | |
| Breast cancer | 1 | 174 | 177 | 0.76 (0.17 to 3.36) | NA | |
| Presence of metastatic diseaseb | 0.237 | |||||
| Yes, mixed population | 5 | 519 | 508 | 0.50 (0.30 to 0.82) | 0.0/0.00 | — |
| No | 2 | 289 | 298 | 0.48 (0.07 to 3.56) | 55.4/1.38 | |
| Allocation concealment | 0.935 | |||||
| Adequate | 8 | 1634 | 1232 | 0.62 (0.45 to 0.85) | 0.0/0.00 | — |
| Inadequate or unclear | 3 | 534 | 531 | 0.60 (0.28 to 1.28) | 0.0/0.00 | |
| Blinding of participants | 0.975 | |||||
| Double‐blind | 4 | 1500 | 1093 | 0.62 (0.40 to 0.96) | 0.0/0.00 | — |
| Inadequate or unclear blinding | 7 | 668 | 670 | 0.62 (0.42 to 0.91) | 0.0/0.00 | |
| Intention‐to‐treat analysis | 0.317 | |||||
| Yes | 5 | 388 | 365 | 0.51 (0.33 to 0.81) | 0.0/0.00 | — |
| No or unclear | 6 | 1780 | 1398 | 0.71 (0.48 to 1.04) | 0.0/0.00 | |
| Selective outcome reporting | 0.655 | |||||
| Adequate | 9 | 1909 | 1524 | 0.65 (0.46 to 0.92) | 0.0/0.00 | — |
| Incomplete or unclear | 2 | 259 | 239 | 0.56 (0.33 to 0.95) | 5.6/0.01 | |
CI: confidence interval; LMWH: low‐molecular‐weight heparin; NA: not applicable, only one trial contributing to this stratum; RR: risk ratio. Analyses performed in STATA. aHaas 2012 contributed to both the breast cancer and lung cancer strata; Agnelli 2009 contributed both to the lung cancer and mixed cancer strata bStudies that did not report the selection criteria for metastatic disease were omitted from this analyses (Agnelli 2009; Haas 2012; Khorana 2017; Perry 2010).
When compared with no thromboprophylaxis, we found moderate‐certainty evidence that LMWH was associated with an increase in major bleeding in the absence of heterogeneity (RR 1.63, 95% CI 1.12 to 2.35; 15 studies, 7282 participants; Tau2 = 0.00; Analysis 2.2). We downgraded by one level for risk of bias. Assuming a background risk of 11 major bleeding episodes per 1000 participants, this corresponds to an NNTH of 144 (95% CI 67 to 758). Visual examination of the funnel plot and Harbord–Egger's test (P = 0.350) found no asymmetry (Figure 4), so that we detected no publication bias or other biases related to small‐study size. The stratified analyses showed no effect of the type of LMWH, dosage, treatment duration, age, type or stage of cancer, definition of major bleeding, or other design characteristics on the relative risk of major bleeding (Table 12). We found no evidence for a linear association between treatment duration and the risk of major bleeding using meta‐regression analysis (P = 0.892). In Ek 2018, three (1.6%) participants in the enoxaparin group had a fatal bleeding compared to one (0.5%) in the control group. In Meyer 2018, three participants in both groups had a fatal bleeding and Khorana 2017 reported no fatal bleeds in either group.
2.2. Analysis.

Comparison 2: Anticoagulants versus control: major bleeding, Outcome 2: Major bleeding: LMWH vs no thromboprophylaxis
4.
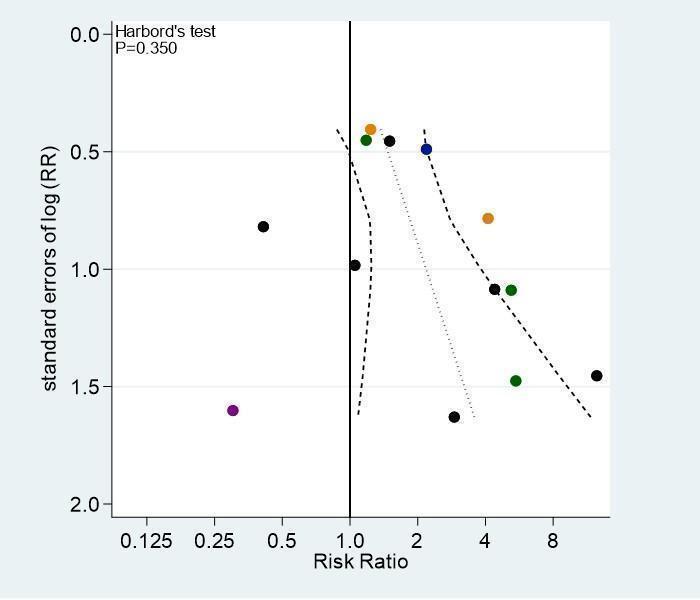
Funnel plot of comparison: 2 Anticoagulants versus control: major bleeding, outcome: 2.2 Major bleeding: low‐molecular weight heparin versus no thromboprophylaxis.
2. Results of stratified analyses on major bleeding for LMWH versus no thromboprophylaxis.
| Variable | No of trials | No of participants (LMWH) | No of participants (control) | RR (95% CI) |
Heterogeneity I2 statistic/Tau2 |
P for interaction |
| All trials | 14a | 3833 | 3449 | 1.63 (1.12 to 2.35) | 0.0/0.00 | — |
| Type of LMWH | 0.860 | |||||
| Dalteparin | 6 | 1567 | 1552 | 1.52 (0.70 to 3.28) | 15.1/0.15 | — |
| Certoparin | 1 | 447 | 451 | 2.19 (0.84 to 5.70) | NA | |
| Nadroparin | 3 | 1161 | 794 | 1.83 (0.69 to 4.85) | 13.8/0.15 | |
| Enoxaparin | 2a | 346 | 343 | 1.87 (0.61 to 5.72) | 46.1/0.33 | |
| Bemiparin | 1 | 20 | 18 | 0.30 (0.01 to 6.97) | NA | |
| Tinzaparin | 1 | 269 | 280 | 5.20 (0.25 to 107.89) | NA | |
| Type of dosage | 0.797 | |||||
| Prophylactic | 8 | 2744 | 2340 | 1.73 (0.94 to 3.21) | 11.2/0.09 | — |
| Higher than prophylactic | 6 | 1066 | 1098 | 1.55 (0.93 to 2.57) | 0.0/0.00 | |
| Treatment durationb | 0.348 | |||||
| Up to 12 weeks | 4 | 526 | 544 | 3.32 (1.02 to 10.80) | 0.0/0.00 | — |
| 12 to 24 weeks | 4 | 2182 | 1811 | 1.21 (0.68 to 2.15) | 0.0/0.00 | |
| more than 24 weeks | 5 | 916 | 892 | 1.62 (0.92 to 2.86) | 0.0/0.00 | |
| Age | 0.246 | |||||
| up to 65 years | 13 | 3624 | 3247 | 1.54 (1.05 to 2.25) | 0.0/0.00 | — |
| 66 years or older | 1 | 186 | 191 | 4.11 (0.88 to 19.09) | NA | |
| Type of cancer | 0.626 | |||||
| Mixed | 6 | 1293 | 1027 | 1.67 (0.68 to 4.12) | 25.4/0.32 | — |
| Lung | 6 | 2048 | 1943 | 1.79 (1.01 to 3.19) | 0.0/0.00 | |
| Pancreatic | 2 | 219 | 214 | 1.21 (0.58 to 2.51) | 0.0/0.00 | |
| Glioma | 1 | 99 | 87 | 4.39 (0.52 to 36.89) | NA | |
| Breast cancer | 1 | 174 | 178 | 7.16 (0.37 to 137.60) | NA | |
| Presence of metastatic diseasec | 0.967 | |||||
| Yes, mixed population | 8 | 2156 | 2173 | 1.38 (0.90 to 2.12) | 0.0/0.00 | — |
| No | 2 | 289 | 298 | 1.29 (0.08 to 21.04) | 38.9/1.58 | |
| Definition of major bleeding | 0.505 | |||||
| Standardd | 10 | 3127 | 2745 | 1.79 (1.13 to 2.82) | 0.0/0.00 | — |
| Alternative or unclear | 4 | 683 | 693 | 1.45 (0.56 to 3.77) | 39.1/0.37 | |
| Allocation concealment | 0.285 | |||||
| Adequate | 12 | 3313 | 2939 | 1.48 (0.99 to 2.22) | 0.0/0.00 | — |
| Inadequate or unclear | 2 | 497 | 499 | 3.05 (0.80 to 11.70) | 22.5/0.34 | |
| Blinding of participants | 0.403 | |||||
| Double‐blind | 6 | 1897 | 1516 | 1.97 (1.11 to 3.51) | 0.0/0.00 | — |
| Inadequate or unclear blinding | 8 | 1913 | 1922 | 1.44 (0.82 to 2.54) | 14.9/0.10 | |
| Intention‐to‐treat analysis | 0.895 | |||||
| Yes | 7 | 1637 | 1622 | 1.58 (0.95 to 2.65) | 0.0/0.00 | — |
| No or unclear | 7 | 2173 | 1816 | 1.69 (0.95 to 3.00) | 6.8/0.04 | |
| Selective outcome reporting | 0.726 | |||||
| Adequate | 12 | 3551 | 3199 | 1.69 (1.10 to 2.59) | 0.0/0.00 | — |
| Incomplete or unclear | 2 | 259 | 239 | 1.56 (0.59 to 4.11) | 16.7/0.13 | |
CI: confidence interval; LMWH: low‐molecular‐weight heparin; NA: not applicable, only one trial contributing to this stratum; RR: risk ratio. Analyses performed in STATA. aZwicker 2013, who reported zero events in both the LMWH and control group, was excluded from all analyses. bEk 2018 was excluded in the stratified analyses by treatment duration, as the duration of anticoagulation was unclear. cThe definition of major bleeding was considered 'standard' when it matched the definition of the International Society of Thrombosis and Haemostasis (Schulman 2005). dStudies that did not report the selection criteria for metastatic disease were omitted from this analyses (Agnelli 2009; Haas 2012; Khorana 2017; Perry 2010).
Pooled estimates of LMWH effects on symptomatic VTE and major bleeding were unchanged after excluding Meyer 2018, which enrolled participants with completely resected cancer (symptomatic VTE: RR 0.55, 95% CI 0.39 to 0.76: major bleeding: RR 1.60, 95% CI 1.10 to 2.32).
LMWH probably reduces symptomatic PE (RR 0.60, 95% CI 0.42 to 0.88; 8 studies, 5324 participants; Tau2 = 0.00; moderate‐certainty evidence; Analysis 3.2). We downgraded by one level due to selective outcome reporting. Assuming a background risk of 18 PE per 1000 participants, this corresponds to an NNTB of 138 (95% CI 95 to 458). LMWH may reduce fatal PE but four out of seven studies reporting this outcome did not contribute to the summary estimate as no fatal PE occurred in either trial arm, leading to a very imprecise pooled estimate (RR 0.37, 95% CI 0.11 to 1.21; 7 studies, 4286 participants; Tau2 = 0.00; low‐certainty evidence; Analysis 4.1).
3.2. Analysis.

Comparison 3: Anticoagulants versus control: symptomatic pulmonary embolism, Outcome 2: Symptomatic PE: LMWH vs no thromboprophylaxis
4.1. Analysis.

Comparison 4: Anticoagulants versus control: fatal PE, Outcome 1: Fatal PE: LMWH vs no thromboprophylaxis
The risk of symptomatic DVT was reduced by 52% (RR 0.48, 95% CI 0.35 to 0.67; 9 studies, 5408 participants; Tau2 = 0.00; high‐certainty evidence; Analysis 5.2). Assuming a background risk of 28 per 1000 participants, this corresponds to an NNTB of 69 (95% CI 55 to 108).
5.2. Analysis.

Comparison 5: Anticoagulants versus control: symptomatic deep vein thrombosis, Outcome 2: Symptomatic DVT: LMWH vs no thromboprophylaxis
The incidence of any VTE was reduced by 43% (RR 0.57, 95% CI 0.46 to 0.71; 10 studies, 5743 participants; Tau2 = 0.00; high‐certainty evidence; Analysis 6.2), which corresponds to an NNTB of 27 (95% CI 21 to 39), assuming a background risk of 90 per 1000 participants.
6.2. Analysis.

Comparison 6: Anticoagulants versus control: any venous thromboembolism, Outcome 2: Any VTE: LMWH vs no thromboprophylaxis
There was no clear difference detected for one‐year overall mortality (RR 0.94, 95% CI 0.83 to 1.07; 9 studies, 2681 participants; Tau2 = 0.02; low‐certainty evidence; Analysis 7.1). We downgraded by two levels because of imprecision and inconsistency. LMWH probably results in a large increase in clinically relevant bleeding (RR 3.40, 95% CI 1.20 to 9.63; 4 studies, 3105 participants; Tau2 = 0.73; moderate‐certainty evidence; Analysis 8.2). We downgraded by one level because of inconsistency. With a background risk of 17 per 1000 participants, the NNTH is 24 (95% CI 6 to 298). The incidence of incidental VTE is probably lowered by LMWH (RR 0.63, 95% CI 0.40 to 0.99; 5 studies, 4452 participants; Tau2 = 0.00; Analysis 9.2). LMWH may increase minor bleeding (Analysis 10.1; low‐certainty evidence) and may decrease symptomatic arterial thromboembolism (Analysis 11.2; low‐certainty evidence). The effects of LMWH on superficial venous thrombosis (Analysis 12.1; very low‐certainty evidence), or serious adverse events (Analysis 13.2; very low‐certainty evidence) were uncertain.
7.1. Analysis.

Comparison 7: Anticoagulants versus control: 1‐year overall mortality, Outcome 1: 1‐year overall mortality: LMWH vs no thromboprophylaxis
8.2. Analysis.

Comparison 8: Anticoagulants versus control: clinically relevant bleeding, Outcome 2: Clinically relevant bleeding: LMWH vs no thromboprophylaxis
9.2. Analysis.

Comparison 9: Anticoagulants versus control: incidental venous thromboembolism, Outcome 2: Incidental VTE: LMWH vs no thromboprophylaxis
10.1. Analysis.

Comparison 10: Anticoagulants versus control: minor bleeding, Outcome 1: Minor bleeding: LMWH vs no thromboprophylaxis
11.2. Analysis.

Comparison 11: Anticoagulants versus control: symptomatic arterial thromboembolism, Outcome 2: Symptomatic arterial thromboembolism: LMWH vs no thromboprophylaxis
12.1. Analysis.

Comparison 12: Anticoagulants versus control: superficial venous thrombosis, Outcome 1: Superficial venous thrombosis: LMWH vs no thromboprophylaxis
13.2. Analysis.

Comparison 13: Anticoagulants versus control: serious adverse events, Outcome 2: Serious adverse events: LMWH vs no thromboprophylaxis
Only two studies evaluated quality of life (Macbeth 2016; Sideras 2006). Macbeth 2016 used the Hospital Anxiety and Depression Score and the EuroQol 5 Dimensions (EQ‐5D) while Sideras 2006 used the single‐item visual analogue Uniscale and a 5‐item series of linear analogue self‐assessment measures supplemented by a 13‐item symptom distress scale. Sideras 2006 reported similar results across groups with respect to decreased quality of life of 10 or more points on the 0‐ to 100‐point visual analogue Uniscale (RR 1.06, 95% CI 0.77 to 1.45; 138 participants). Results on the symptom distress scale were incompletely reported in Sideras 2006, but they did describe that they found similar results in participants randomised to LMWH or no thromboprophylaxis, both at baseline and during the study period. Macbeth 2016 found no difference between LMWH and no thromboprophylaxis with respect to quality‐adjusted life years gained in the first year (mean difference (MD) not reported, 95% CI –0.02 to 0.03) and no difference in overall quality of life at six months (EQ‐5D: MD 0.11, 95% CI –3.18 to 3.40; 940 participants; P = 0.94) or 12 months (EQ‐5D: MD –0.34, 95% CI –5.25 to 4.57; 445 participants; P = 0.89).
Three studies reported no cases of HIT with LMWH use (Haas 2012; Klerk 2005; Pelzer 2015). Haas 2012 reported objectively verified skeletal events (including all fractures, spinal cord compressions, and requirements for surgery to treat fractures or for bone irradiation) in 16/442 participants in the LMWH group and 19/441 participants in the placebo group (RR 0.84, 95% CI 0.44 to 1.61).
Macbeth 2016 reported compliance with LMWH. Of the 977 (89%) participants in whom compliance was evaluated, 180 (18.4%) were considered as fully compliant, whereas 431 (39%) received half of the planned syringes or less. In Ek 2018, approximately 85% of the participants in the enoxaparin group reported full adherence.
Five studies reported symptomatic VTE and six studies on major bleeding in participants with non‐small‐cell lung cancer (Haas 2012; Meyer 2018), small‐cell lung cancer (Altinbas 2004; Lecumberri 2013; Ek 2018), or both (Agnelli 2009; Macbeth 2016). Pooled analysis of these trials showed a probable reduction in symptomatic VTE (RR 0.62, 95% CI 0.38 to 1.02), and possibly a higher risk of major bleeding with LMWH compared with the control treatment (RR 1.79, 95% CI 1.01 to 3.19; no evidence of statistical heterogeneity; Tau2 = 0.00; moderate‐certainty evidence; Table 11; Table 12).
Two studies reported symptomatic VTE and major bleeding in participants with advanced pancreatic cancer (Maraveyas 2012; Pelzer 2015). Pooled analysis of these trials showed that LMWH probably substantially reduce symptomatic VTE (RR 0.41, 95% CI 0.23 to 0.75) and may slightly increase major bleeding (RR 1.21, 95% CI 0.58 to 2.51; no evidence of statistical heterogeneity; Tau2 = 0.00) (Table 11; Table 12). Vadhan‐Raj 2013 also selectively included participants with advanced pancreatic cancer and reported two DVTs in the dalteparin group and eight VTEs (two PE and six DVT) in 37 participants receiving no thromboprophylaxis. The abstract did not report whether these events were symptomatic, incidental, or both. There were no clinically significant bleeding events with dalteparin, although the definition of bleeding was not provided, and it was not reported if any bleeding occurred in participants of the control group.
Low‐molecular‐weight heparin versus active control
Elit 2012 compared prophylactic, intermediate and therapeutic doses of dalteparin against each other. There were no symptomatic VTE or major bleeding events during dalteparin administration. Two participants developed symptomatic VTE and one was diagnosed with incidental PE after dalteparin discontinuation (see Analysis 1.3; Analysis 3.3; Analysis 5.3; Analysis 6.3; Analysis 9.3). The certainty of the evidence was low for symptomatic VTE and could not be evaluated for major bleeding as the RR was not estimable due to zero counts in all trial groups (see Table 3). There were no data on one‐year overall mortality, arterial thromboembolism, clinically relevant bleeding, and serious adverse events reported. Two participants had minor bleeding in the highest dose group (150 IU/kg). There were no cases of HIT. Compliance with injections was more than 80% in all three dose groups.
1.3. Analysis.

Comparison 1: Anticoagulants versus control: symptomatic venous thromboembolism, Outcome 3: Symptomatic VTE: prophylactic vs intermediate or therapeutic LMWH
3.3. Analysis.

Comparison 3: Anticoagulants versus control: symptomatic pulmonary embolism, Outcome 3: Symptomatic PE: prophylactic vs intermediate or therapeutic LMWH
5.3. Analysis.

Comparison 5: Anticoagulants versus control: symptomatic deep vein thrombosis, Outcome 3: Symptomatic DVT: prophylactic vs intermediate or therapeutic LMWH
6.3. Analysis.

Comparison 6: Anticoagulants versus control: any venous thromboembolism, Outcome 3: Any VTE: prophylactic vs intermediate vs therapeutic LMWH
9.3. Analysis.

Comparison 9: Anticoagulants versus control: incidental venous thromboembolism, Outcome 3: Incidental VTE: prophylactic vs intermediate or therapeutic LMWH
Two studies of participants with multiple myeloma receiving thalidomide‐ and lenalidomide‐based regimens compared LMWH against an active control, which in both studies was aspirin (Larocca 2012; Palumbo 2011), and in one of the studies was a VKA (warfarin) (Palumbo 2011). See Table 4. When compared with aspirin, pooled analysis showed a possible reduction (49%) in symptomatic VTE (RR 0.51, 95% CI 0.22 to 1.17; 2 studies, 781 participants; moderate‐certainty evidence; Analysis 1.4). There were 3/396 (0.75%) major bleeding events with aspirin and 0/385 with LMWH (RR 0.14, 95% CI 0.01 to 2.76; 2 studies, 781 participants; low‐certainty evidence; Analysis 2.4). The incidence of symptomatic PE was possibly reduced by 87% (RR 0.13, 95% CI 0.02 to 1.03; 2 studies, 781 participants; moderate‐certainty evidence). We downgraded due to imprecision. LMWH probably decreases the incidence of symptomatic DVT when compared to aspirin (RR 0.81, 95% CI 0.32 to 2.04; 2 studies, 781 participants; moderate‐certainty evidence; Analysis 5.4). Very low‐certainty evidence showed no clear differences between LMWH and aspirin regarding the incidence of minor bleeding (Analysis 10.3), and symptomatic arterial thromboembolism (Analysis 11.3). There were no data on one‐year overall mortality, clinically relevant bleeding, and serious adverse events.
1.4. Analysis.

Comparison 1: Anticoagulants versus control: symptomatic venous thromboembolism, Outcome 4: Symptomatic VTE: LMWH vs aspirin
2.4. Analysis.

Comparison 2: Anticoagulants versus control: major bleeding, Outcome 4: Major bleeding: LMWH vs aspirin
5.4. Analysis.

Comparison 5: Anticoagulants versus control: symptomatic deep vein thrombosis, Outcome 4: Symptomatic DVT: LMWH vs aspirin
10.3. Analysis.

Comparison 10: Anticoagulants versus control: minor bleeding, Outcome 3: Minor bleeding: LMWH vs aspirin
11.3. Analysis.

Comparison 11: Anticoagulants versus control: symptomatic arterial thromboembolism, Outcome 3: Symptomatic arterial thromboembolism: LMWH vs aspirin
In the study of Palumbo 2011, LMWH was associated with a 67% reduction in symptomatic VTE relative to warfarin (RR 0.33, 95% CI 0.14 to 0.83; 439 participants; high‐certainty evidence; Analysis 1.5), with no major bleeding events in either group. The pooled estimate for the reduction in symptomatic PE was very imprecise (RR 0.11, 95% CI 0.01 to 2.06; low‐certainty evidence; Analysis 3.5), whereas LMWH probably reduces symptomatic DVT more than active control (RR 0.43, 95% CI 0.17 to 1.10; moderate‐certainty evidence; Analysis 5.5). We downgraded by either one or two levels due to imprecision (see Table 5). There were no clear differences between LMWH and warfarin regarding the incidence of minor bleeding and symptomatic arterial thromboembolism. There were no data on one‐year overall mortality.
1.5. Analysis.

Comparison 1: Anticoagulants versus control: symptomatic venous thromboembolism, Outcome 5: Symptomatic VTE: LMWH vs warfarin
3.5. Analysis.

Comparison 3: Anticoagulants versus control: symptomatic pulmonary embolism, Outcome 5: Symptomatic PE: LMWH vs warfarin
5.5. Analysis.

Comparison 5: Anticoagulants versus control: symptomatic deep vein thrombosis, Outcome 5: Symptomatic DVT: LMWH vs warfarin
In the study of Greiner 2019, conducted in participants aged one to 18 years, the incidence of symptomatic VTE was reduced by both enoxaparin (3.5%) and antithrombin (1.9%) compared with UFH (8.0%; LMWH versus UFH: RR 0.41, 95% CI 0.20 to 0.85; antithrombin versus UFH: RR 0.22, 95% CI 0.09 to 0.54; 949 participants). Major bleeding occurred in four (1.1%) participants treated with UFU, three (0.9%) with antithrombin, and one (0.5%) with enoxaparin. The study did not report the remaining outcomes of interest.
Ultra‐low‐molecular‐weight heparin versus placebo
In one large trial of 3212 participants, semuloparin was associated with a reduction in symptomatic VTE (RR 0.36, 95% CI 0.22 to 0.60, high‐certainty evidence; Analysis 1.6), corresponding to an NNTB of 46 (95% CI 38 to 73) using a control group risk of 34 VTE per 1000 participants (Agnelli 2012). There were 19/1589 major bleeding events in the semuloparin group versus 18/1583 in the placebo group (RR 1.05, 95% CI 0.55 to 2.00; moderate‐certainty evidence; Analysis 2.6). We downgraded one level for imprecision (see Table 6). Semuloparin reduced symptomatic VTE by 64% in participants with lung cancer (9/591 with semuloparin versus 25/589 with placebo; RR 0.36, 95% CI 0.17 to 0.76) and by 78% in participants with pancreatic cancer (3/126 with semuloparin versus 14/128 with placebo; RR 0.22, 95% CI 0.06 to 0.74). The occurrence of major bleeding was not reported separately for these types of cancer.
1.6. Analysis.

Comparison 1: Anticoagulants versus control: symptomatic venous thromboembolism, Outcome 6: Symptomatic VTE: semuloparin vs placebo
2.6. Analysis.

Comparison 2: Anticoagulants versus control: major bleeding, Outcome 6: Major bleeding: semuloparin vs placebo
Semuloparin probably reduced the risk of symptomatic PE by 52% (RR 0.48, 95% CI 0.22 to 1.01; moderate‐certainty evidence; Analysis 3.6). We downgraded by one level for imprecision. Both symptomatic DVT (RR 0.32, 95% CI 0.16 to 0.63; high‐certainty evidence; Analysis 5.6) and any VTE (RR 0.36, 95% CI 0.22 to 0.60; high‐certainty evidence; Analysis 6.4) were reduced by about two‐thirds with semuloparin. Fatal PE occurred in 0.4% of participants on semuloparin and 0.6% of participants on placebo. Clinically relevant bleeding was reported in 2.8% of participants on semuloparin and 2.0% of participants on placebo (RR 1.40, 95% CI 0.90 to 2.19; moderate‐certainty evidence; Analysis 8.4). We downgraded by one level for imprecision. Semuloparin may reduce incidental VTE but the study was too small to estimate effects precisely (RR 0.14, 95% CI 0.01 to 2.76; Analysis 9.4). We found no evidence that semuloparin had an effect on one‐year overall mortality (RR 1.02, 95% CI 0.96 to 1.08; moderate‐certainty evidence; Analysis 7.2). The incidence of serious adverse events or thrombocytopenia was similar in the semuloparin and placebo groups (serious adverse effects: 26% with semuloparin versus 25% with placebo; thrombocytopenia: 7.1% with semuloparin versus 7.6% with placebo; Analysis 13.3), with no cases of HIT.
3.6. Analysis.

Comparison 3: Anticoagulants versus control: symptomatic pulmonary embolism, Outcome 6: Symptomatic PE: semuloparin vs placebo
5.6. Analysis.

Comparison 5: Anticoagulants versus control: symptomatic deep vein thrombosis, Outcome 6: Symptomatic DVT: semuloparin vs placebo
6.4. Analysis.

Comparison 6: Anticoagulants versus control: any venous thromboembolism, Outcome 4: Any VTE: semuloparin vs placebo
8.4. Analysis.

Comparison 8: Anticoagulants versus control: clinically relevant bleeding, Outcome 4: Clinically relevant bleeding: semuloparin vs placebo
9.4. Analysis.

Comparison 9: Anticoagulants versus control: incidental venous thromboembolism, Outcome 4: Incidental VTE: semuloparin vs placebo
7.2. Analysis.

Comparison 7: Anticoagulants versus control: 1‐year overall mortality, Outcome 2: 1‐year overall mortality: semuloparin vs placebo
13.3. Analysis.

Comparison 13: Anticoagulants versus control: serious adverse events, Outcome 3: Serious adverse events: semuloparin vs placebo
Unfractionated heparin versus no thromboprophylaxis
One study evaluated UFH against no thromboprophylaxis (Lebeau 1994), and did not report on VTE or major bleeding. UFH probably decreases the incidence of one‐year overall mortality in small‐cell lung cancer, although the CIs of the summary estimate did not conclusively rule out an increase in one‐year overall mortality (RR 0.86, 95% CI 0.72 to 1.03; moderate‐certainty evidence; Analysis 7.3). Clinically relevant bleeding occurred in 2/138 participants with UFH versus 1/139 participants with no thromboprophylaxis (RR 2.01, 95% CI 0.18 to 21.96; low‐certainty evidence; Analysis 8.5). We downgraded by one or two levels due to imprecision. See Table 7. The study by Lebeau and colleagues was too small to evaluate effects on minor bleeding (RR 3.02, 95% CI 0.12 to 73.54; Analysis 10.5), and they found no cases of HIT. The study did not report the remaining outcomes of interest.
7.3. Analysis.

Comparison 7: Anticoagulants versus control: 1‐year overall mortality, Outcome 3: 1‐year overall mortality: UFH vs no thromboprophylaxis
8.5. Analysis.

Comparison 8: Anticoagulants versus control: clinically relevant bleeding, Outcome 5: Clinically relevant bleeding: UFH vs no thromboprophylaxis
10.5. Analysis.

Comparison 10: Anticoagulants versus control: minor bleeding, Outcome 5: Minor bleeding: UFH vs no thromboprophylaxis
Vitamin K antagonist versus placebo or no thromboprophylaxis
Four studies compared the VKA warfarin against no thromboprophylaxis or placebo, but did not all report our primary outcomes (Chahinian 1989; Levine 1994; Maurer 1997; Zacharski 1981).
Levine 1994 found that warfarin may reduce symptomatic VTE substantially relative to placebo (RR 0.15, 95% CI 0.02 to 1.20; 311 participants; low‐certainty evidence; Analysis 1.7). We downgraded by two levels because of imprecision, potential risk of attrition bias, and risk of publication bias. No other study reported on VTE. There was no clear effect on major bleeding (RR 0.52, 95% CI 0.05 to 5.71), symptomatic PE (RR 1.05, 95% CI 0.07 to 16.58; 311 participants; very low‐certainty evidence; Analysis 3.7), whereas warfarin may decrease symptomatic DVT substantially (RR 0.08, 95% CI 0.00 to 1.42; 311 participants; low‐certainty evidence; Analysis 5.7), and may increase minor bleeding (RR 2.44, 95% CI 0.64 to 9.27; Analysis 10.6). There were no symptomatic arterial thromboembolic events in either group.
1.7. Analysis.

Comparison 1: Anticoagulants versus control: symptomatic venous thromboembolism, Outcome 7: Symptomatic VTE: vitamin K antagonists vs placebo
3.7. Analysis.

Comparison 3: Anticoagulants versus control: symptomatic pulmonary embolism, Outcome 7: Symptomatic PE: vitamin K antagonists vs placebo
5.7. Analysis.

Comparison 5: Anticoagulants versus control: symptomatic deep vein thrombosis, Outcome 7: Symptomatic DVT: vitamin K antagonists vs placebo
10.6. Analysis.

Comparison 10: Anticoagulants versus control: minor bleeding, Outcome 6: Minor bleeding: vitamin K antagonists vs placebo
The three remaining studies reported major bleeding events, but provided no data on the occurrence of symptomatic or incidental VTE (Chahinian 1989; Maurer 1997; Zacharski 1981). Pooled analysis of all four studies evaluating VKA versus placebo or no thromboprophylaxis showed that major bleeding may substantially increase with VKA, with evidence of a high degree of heterogeneity (RR 3.82, 95% CI 0.97 to 15.04; 4 studies, 994 participants; low‐certainty evidence; Tau2 = 0.71; Analysis 2.7).
2.7. Analysis.

Comparison 2: Anticoagulants versus control: major bleeding, Outcome 7: Major bleeding: vitamin K antagonists vs no thromboprophylaxis
The certainty of the evidence was low for symptomatic VTE, major bleeding, and symptomatic DVT and very low for symptomatic PE. We downgraded two or three levels due to imprecision and risk of bias concerns (see Table 8).
Vitamin K antagonist versus active control
Palumbo 2011 reported a possible increased risk of symptomatic VTE with VKA (warfarin) compared to aspirin in patients with multiple myeloma (RR 1.50, 95% CI 0.74 to 3.04; 440 participants; moderate‐certainty evidence; Analysis 1.8). There were 3/220 major bleeding events in the aspirin group and none (0/220) in the warfarin group (RR 0.14, 95% CI 0.01 to 2.75; 440 participants; low‐certainty evidence; Analysis 2.8). Evidence suggests that VKA and aspirin probably reduce the incidence of symptomatic PE to a similar extent (RR 1.00, 95% CI 0.25 to 3.95; 440 participants; moderate‐certainty evidence; Analysis 3.8). VKA is probably less effective than aspirin in reducing symptomatic DVT (RR 1.75, 95% CI 0.75 to 4.09; 440 participants; moderate‐certainty evidence; Analysis 5.8). The study by Palumbo and colleagues was too small to precisely estimate effects on other secondary outcomes minor bleeding (Analysis 10.7), and symptomatic arterial thromboembolism (Analysis 11.6). See Table 9.
1.8. Analysis.

Comparison 1: Anticoagulants versus control: symptomatic venous thromboembolism, Outcome 8: Symptomatic VTE: warfarin vs aspirin
2.8. Analysis.

Comparison 2: Anticoagulants versus control: major bleeding, Outcome 8: Major bleeding: warfarin vs aspirin
3.8. Analysis.

Comparison 3: Anticoagulants versus control: symptomatic pulmonary embolism, Outcome 8: Symptomatic PE: warfarin vs aspirin
5.8. Analysis.

Comparison 5: Anticoagulants versus control: symptomatic deep vein thrombosis, Outcome 8: Symptomatic DVT: warfarin vs aspirin
10.7. Analysis.

Comparison 10: Anticoagulants versus control: minor bleeding, Outcome 7: Minor bleeding: warfarin vs aspirin
11.6. Analysis.

Comparison 11: Anticoagulants versus control: symptomatic arterial thromboembolism, Outcome 6: Symptomatic arterial thromboembolism: warfarin vs aspirin
Results for the comparison of 'VKA versus LMWH' are presented in the previous section 'LMWH versus active control'.
Antithrombin versus no thromboprophylaxis
One study that recruited 85 children assessed antithrombin (Mitchell 2003). This study did not report on symptomatic VTE but did report any VTE. Effects of antithrombin compared to placebo were uncertain with regard to major bleeding (RR 0.78, 95% CI 0.03 to 18.57; 85 participants; very low‐certainty evidence), any VTE (RR 0.84, 95% CI 0.41 to 1.73; 85 participants; very low‐certainty evidence), and minor bleeding (RR 11.73, 95% CI 0.58 to 235.96; 85 participants; very low‐certainty evidence). We downgraded the certainty of the evidence due to imprecision and risk of bias. The study did not report the remaining outcomes. See Table 10.
Discussion
Summary of main results
Thromboprophylaxis with direct oral factor Xa inhibitors may decrease the incidence of symptomatic VTE (low‐certainty evidence) and probably increases the risk of major bleeding compared with placebo (moderate‐certainty evidence). See Table 1. Factor Xa inhibitors reduced the risk of any VTE by 45% and of incidental VTE by 50% There were no clear differences in symptomatic PE, symptomatic DVT, clinically relevant bleeding, arterial thromboembolism, or serious adverse events.
When compared with placebo or no thromboprophylaxis, LMWH reduced the incidence of symptomatic VTE by 38% (high‐certainty evidence; NNTB 37), but probably increased the risk of major bleeding by 63% (moderate‐certainty evidence; NNTH 144). LMWH probably reduced the incidence of symptomatic PE (moderate‐certainty evidence), reduced symptomatic DVT (high‐certainty evidence), any VTE (high‐certainty evidence), and incidental VTE and may decrease one‐year overall mortality (low‐certainty evidence). LMWH was associated with a probable three‐fold higher risk of clinically relevant bleeding compared with no thromboprophylaxis (moderate‐certainty evidence). See Table 2.
Evidence for the use of thromboprophylaxis with anticoagulants other than factor Xa inhibitors and LMWH appear to be preliminary.
Marketing applications for the uLMWH semuloparin have been withdrawn worldwide, and it is therefore unlikely to ever be commercially available (EMEA 2012).
In participants with multiple myeloma, LMWH probably reduces symptomatic VTE more than aspirin (moderate‐certainty evidence). There was major bleeding in none of the participants treated with LMWH and in less than 1% of those treated with aspirin (low‐certainty evidence). See Table 4. There is a possible increased risk of symptomatic VTE with VKA (warfarin) compared to aspirin (moderate‐certainty evidence) while VKA may be associated with a lower risk of major bleeding when compared to aspirin (low‐certainty evidence). See Table 9.
One study in participants with multiple myeloma receiving thalidomide‐ or lenalidomide‐based regimens showed that LMWH was associated with a 67% lower risk of symptomatic VTE compared with warfarin (high‐certainty evidence), but this study was underpowered to show differences for major bleeding (Palumbo 2011; Table 5). Similarly, the evidence was insufficient to precisely estimate the effects in people without myeloma. In the latter, warfarin may reduce symptomatic VTE (Analysis 1.7) and increase major bleeding (see Analysis 2.7), but the magnitude of effects remain uncertain.
The lack of an adequate control group receiving placebo or no thromboprophylaxis in the studies of participants with myeloma hampers definitive recommendations for one specific thromboprophylaxis over another. In addition, these trials focused on specific regimens (thalidomide‐ and lenalidomide‐based combinations), thus findings and conclusions may not apply to people with myeloma receiving other treatments. As renal insufficiency often complicates the course of multiple myeloma, the administration and dosing of drugs such as LMWH with a predominant renal clearance should be taken with great caution.
Only one study evaluated UFH against no thromboprophylaxis, but did not report on VTE or major bleeding. See Table 7.
When compared with placebo or no thromboprophylaxis, warfarin may reduce symptomatic VTE (low‐certainty evidence); and may increase major bleeding (low‐certainty evidence). See Table 8.
While additional studies could help clarify the efficacy and safety of VKAs, the bleeding concerns and the complexity of VKAs management remain significant barriers for VKAs use as primary prophylaxis in ambulatory cancer patients.
Antithrombin, evaluated in one study involving children, had no clear difference in effect on any VTE (very low‐certainty evidence) or major bleeding when compared with no antithrombin (very low‐certainty evidence). See Table 10.
Overall completeness and applicability of evidence
No RCTs evaluated fondaparinux, dabigatran, edoxaban, and mechanical interventions. The oral factor Xa inhibitors apixaban and rivaroxaban do not require routine laboratory monitoring and may be easy for patients to use. Results with these agents are encouraging although several issues remain. Levels of apixaban and rivaroxaban can be influenced by the concurrent administration of strong inhibitors and inducers of the P‐glycoprotein and CYP3A4. The clinical relevance of drug–drug interactions with chemotherapy and new target therapies interfering with P‐glycoprotein and CYP3A4 requires further investigation. In addition, prolonged nausea and vomiting, gastrointestinal toxicity from cancer treatment, or surgery involving the gastrointestinal tract may influence drug absorption and need careful consideration.
Comorbidities predisposing to bleeding, which often represent an exclusion criterion in RCTs on anticoagulants, might result in a greater number of major bleeding complications and limit the use of thromboprophylaxis in routine clinical practice. Additional concerns may be the use of thromboprophylaxis with apixaban or rivaroxaban in some types of cancers, such as those of the gastrointestinal or genitourinary tracts, which were more prone to bleed in the studies with DOAC (Carrier 2019; Khorana 2019).
We performed stratified analyses and there was no evidence to suggest that effects of LMWH versus placebo or no thromboprophylaxis on symptomatic VTE or major bleeding varied by type of cancer, presence of metastatic disease, treatment duration, or dosing. However, we acknowledge that there was an insufficient number of studies to make strong conclusions about the variation by type of cancer. Stratified analyses could not be performed for other comparisons as the number of identified studies was too low. Nevertheless, since this review mainly included participants with locally advanced or metastatic cancer, the results may not be generalisable to patients with earlier stages of cancer. Estimates may not apply to paediatric populations as the majority of evidence was derived from adult populations. Likewise, the very low‐certainty evidence of effects of antithrombin versus placebo on major bleeding and VTE was derived from a single study in a paediatric population, and the described effects may not apply to adult populations.
Quality of the evidence
The risk of bias of the individual studies, as assessed using Cochrane's risk of bias tool, ranged from low to high (Figure 2). Analytical exploration of the effects of design flaws was feasible only for the comparison of LMWH versus no thromboprophylaxis. We found no evidence of design‐related biases. An inspection of the funnel plot and formal analysis of asymmetry did not indicate asymmetry for the primary efficacy outcome symptomatic VTE and major bleeding (Figure 3; Figure 4), suggesting the absence of publication bias or other biases related to small‐study size.
Across comparisons, the certainty of the evidence for symptomatic VTE ranged from very low to high. While it is very unlikely that new evidence will change our confidence in the estimate of the effects on VTE of LMWH or semuloparin compared to placebo or no thromboprophylaxis or of LMWH compared to VKA (all high‐certainty evidence), we are less certain about the estimates of the other comparisons. The certainty of the evidence for major bleeding varied from very low to moderate, indicating that further research is likely to have an important effect on our confidence in the estimate of effect and may change the estimate (Guyatt 2008). Overall, the largest concern was imprecision due to the small‐study size of the majority of the trials. We could not judge the certainty of the evidence for several outcomes across comparisons due to incomplete reporting or the absence of events in both trial arms so these were downgraded for risk of bias concerns.
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10.
Potential biases in the review process
Our systematic approach to searching, study selection, and data extraction followed that described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). It is unlikely that we have missed relevant trials, but frequent updates of this review are warranted given that we identified several new trials since the previous version of this review, which covered published trials up to 2016 (Di Nisio 2016). We minimised data extraction errors by using two independent review authors (EV, MC). Judgements on the certainty of evidence were discussed with a third review author (AWSR). We acknowledge that risk of bias assessment leaves room for different interpretations, especially where the quality of reporting is poor. We applied strict rules regarding the risk of attrition bias, requiring that all randomised participants be analysed according to the intention‐to‐treat principle. We chose this rather strict approach, as the incidence of symptomatic VTE varies considerably between trials and may be rather low, so that even a small proportion of participants not analysed may impact on the study estimates if the fraction not analysed is associated with the outcome. Other reviews have also applied this approach (Juni 2001; Rutjes 2009; Rutjes 2012). Following Cochrane guidance, we included quotes and the arguments on which we based our risk of bias judgements, allowing the reader to reach their own conclusions. Our systematic approach and the consistency of the results (lack of significant heterogeneity) increase confidence in the internal validity of our findings.
One limitation in the interpretation of this review is the 'no evidence of a difference' findings. The lack of such evidence may be related to the small number of RCTs and small number of participants, events, or both, as well as the absence of a true effect. In this regard, the lack of a clear effect between the DOACs and symptomatic VTE or major bleeding could be the result of the relatively low number of events observed. The three studies comparing DOAC with placebo reported only 30 major bleeds in total, with a point estimate suggesting a 74% higher risk with DOAC and the upper value of the 95% CI not excluding a near four‐fold higher risk of major bleeding.
Another limitation related to the small number of RCTs, poor reporting, or both, was our inability to conduct some subgroup analyses (e.g. use of cointerventions) for the primary efficacy outcome symptomatic VTE, whereas other stratified analyses were hampered by the lack of contrast (e.g. age and presence of metastasis). We performed subgroup analysis by type of cancer for the lung and pancreatic cancers, albeit the data for the pooled analysis were derived from only seven (lung) and two (pancreatic) studies. The lack of reporting, as well as the heterogeneity of the cancers treated, prevented us from assessing the importance of background chemotherapy on the response to thromboprophylaxis. Finally, the lack of evidence precluded any inference on the use of mechanical prophylaxis.
Agreements and disagreements with other studies or reviews
The evidence on the use of thromboprophylaxis in ambulatory cancer patients receiving chemotherapy was summarised by the recently updated guidelines of the American Society of Clinical Oncology, the International Initiative on Thrombosis and Cancer (ITAC), and the National Comprehensive Cancer Network (Farge 2019; Key 2020; National Comprehensive Cancer Network 2020). One potential advantage of the current review is that we provided pooled estimates with 95% CIs for both efficacy and safety outcomes, allowing a better estimation of the risks and benefits of thromboprophylaxis in this setting. The use of a larger dataset allowed us to stratify multiple outcomes by type of treatment. Other narrative reviews summarised the evidence on the use of thromboprophylaxis for VTE in ambulatory cancer patients (Aikens 2013; Maxwell 2012). These reviews lacked a systematic search of the literature and, as for Farge 2019, Key 2020, and National Comprehensive Cancer Network 2020, there was no meta‐analysis or evaluation of study quality items and assessment of risk of bias performed.
The conclusions of our review are in agreement with those of the American Society of Clinical Oncology (Key 2020), and differ somewhat from the 2012 guidelines of the American College of Chest Physicians (Kahn 2012), which suggested primary thromboprophylaxis with LMWH or UFH in ambulatory patients with solid tumours who have additional risk factors for VTE (that is previous venous thrombosis, immobilisation, angiogenesis inhibitors, thalidomide and lenalidomide) and a low risk of bleeding.
Authors' conclusions
Implications for practice.
In ambulatory cancer patients, primary thromboprophylaxis with direct factor Xa inhibitors may reduce the incidence of symptomatic venous thromboembolism (VTE) (low‐certainty evidence) and probably increases the risk major bleeding (moderate‐certainty evidence) when compared with placebo. Low‐molecular‐weight heparin (LMWH) reduces symptomatic VTE with 37 participants requiring prophylaxis to prevent one event (high‐certainty evidence). This benefit comes at the cost of a higher incidence of major bleeding, where for each 144 participants treated, one event is expected to occur when compared against placebo or no thromboprophylaxis (moderate‐certainty evidence). When deciding whether to use primary antithrombotic prophylaxis in ambulatory cancer patients receiving chemotherapy, clinicians need to determine the patient's baseline risk of VTE with the help of risk‐stratification models and weigh the magnitude of benefit with antithrombotic prophylaxis, especially on major clinical endpoints, against the risk of major bleeding complications. Evidence for the use of thromboprophylaxis with anticoagulants other than direct factor Xa inhibitors and LMWH is limited.
Implications for research.
Further randomised studies are needed to establish the risk–benefit ratio of primary thromboprophylaxis in ambulatory cancer patients receiving chemotherapy. Additional studies may be useful to improve VTE risk stratification to identify subgroups of patients who may have larger benefits from thromboprophylaxis.
Although several tools have been proposed to stratify VTE risk in ambulatory cancer patients, the score developed by Khorana and colleagues remains one of the most extensively evaluated (Ay 2010; George 2011; Khorana 2008; Khorana 2009a; Khorana 2009b; Khorana 2018; Verso 2012; Zwicker 2013). A Khorana score of 2 or greater was recently used in Carrier 2019 and Khorana 2019 to identify and include patients with a high‐risk of VTE. In the control group, symptomatic VTE occurred at a similar rate as in previous studies which did not use any risk score (Analysis 1.2). According to the results of one recent large meta‐analysis of over 34,000 cancer patients, the incidence of thromboembolic complications in patients at low VTE risk according to the Khorana score may be not negligible (Mulder 2019). One potential limitation of current scoring systems is the overall low sensitivity, which may result in the exclusion of over half of patients who ultimately develop cancer‐associated VTE from the potential benefits of thromboprophylaxis. These observations suggest that further refinement of risk stratification tools could help to significantly reduce the burden of cancer‐associated VTE.
Several additional aspects related to thromboprophylaxis deserve further study, such as the development of bleeding‐risk models, optimal doses and duration of thromboprophylaxis, patient preferences, and quality of life.
Cost‐analysis data on the use of anticoagulation in people with cancer undergoing chemotherapy would be very valuable and supportive of a broader application of prophylaxis in the future.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2020 | New search has been performed | Searches rerun. Six new studies included, five new studies excluded, six new ongoing studies identified. |
| 17 December 2020 | New citation required and conclusions have changed | New author joined the review team. Searches rerun. Six new studies included, five new studies excluded, six new ongoing studies identified. Text updated to reflect current Cochrane standards. Conclusions changed. The authors' Declarations of interest have been updated to reflect the review's compliance with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. |
History
Protocol first published: Issue 5, 2010 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 11 December 2020 | Amended | Clarification message added to the Declarations of interest statement about the review's compliance with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. |
| 9 July 2016 | New search has been performed | Searches rerun. Five additional studies were added to the included studies. Two additional studies excluded on full‐text basis. |
| 9 July 2016 | New citation required and conclusions have changed | Searches rerun. Five additional studies were added to the included studies. Two additional studies excluded on full‐text basis. New authors joined the review team. 'Summary of findings' tables added. Conclusions not changed. |
| 24 July 2013 | New search has been performed | Searches rerun. Twelve additional studies were added to the included studies and nine additional studies to the excluded studies. |
| 24 July 2013 | New citation required but conclusions have not changed | Searches rerun. Twelve additional studies were added to the included studies and nine additional studies to the excluded studies. Risk of bias was reassessed in all included trials. Conclusions not changed. Change in author team. |
Acknowledgements
The review authors would like to thank the personnel from Cochrane Vascular for their invaluable assistance and advice. The review authors and the Cochrane Vascular editorial base are grateful to the following peer reviewers for their time and comments: Dr Hanny Al‐Samkari, Division of Hematology/Oncology, Harvard Medical School, USA; Professor John A Baron MD, MS, MSc, University of North Carolina, USA; Karla Duque Jacome MD, MPH, Ecuador; Tian Li, China.
Appendices
Appendix 1. Database searches
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Thrombosis 1690 #2 MESH DESCRIPTOR Thromboembolism 1159 #3 MESH DESCRIPTOR Venous Thromboembolism 500 #4 MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES 2453 #5 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*):TI,AB,KY 29547 #6 MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES 899 #7 (DVT or VTE):TI,AB,KY 3100 #8 (((vein* or ven*) near thromb*)):TI,AB,KY 10154 #9 (blood near3 clot*):TI,AB,KY 4945 #10 (pulmonary near3 clot*):TI,AB,KY 13 #11 (lung near3 clot*):TI,AB,KY 11 #12 MESH DESCRIPTOR Antineoplastic Protocols EXPLODE ALL TREES 12850 #13 MESH DESCRIPTOR Survival EXPLODE ALL TREES 129 #14 surviv*:TI,AB,KY 102184 #15 chemotherap*:TI,AB,KY 67936 #16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 165162 #17 MESH DESCRIPTOR Anticoagulants EXPLODE ALL TREES 10046 #18 (anticoagul* or anti‐coagu*):TI,AB,KY 12207 #19 MESH DESCRIPTOR Heparin EXPLODE ALL TREES 4448 #20 heparin*:TI,AB,KY 11287 #21 UFH:TI,AB,KY 667 #22 LMWH:TI,AB,KY 1267 #23 LMH:TI,AB,KY 9 #24 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren ):TI,AB,KY 14 #25 (Clexane or klexane or lovenox ):TI,AB,KY 157 #26 Fragmin:TI,AB,KY 215 #27 Innohep:TI,AB,KY 25 #28 clivarin*:TI,AB,KY 22 #29 (danaproid or danaparoid):TI,AB,KY 55 #30 antixarin:TI,AB,KY 2 #31 (Zibor or cy 222 or embolex or monoembolex):TI,AB,KY 38 #32 (rd 11885 or RD1185):TI,AB,KY 0 #33 (Kabi‐2165 or Kabi 2165):TI,AB,KY 39 #34 (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169):TI,AB,KY 8 #35 (fr‐860 or fr860 or cy‐216 or cy216):TI,AB,KY 53 #36 (kb101 or lomoparan or orgaran ):TI,AB,KY 32 #37 (fluxum or lohepa or lowhepa):TI,AB,KY 13 #38 (op 2123 or op2123):TI,AB,KY 1 #39 (ave 5026 or ave5026 ):TI,AB,KY 12 #40 (M118 or RO‐1):TI,AB,KY 10 #41 coumar*:TI,AB,KY 385 #42 (warfarin or (vitamin near/3 antagonist*)):TI,AB,KY 4427 #43 (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin):TI,AB,KY 692 #44 MESH DESCRIPTOR Antithrombins EXPLODE ALL TREES 1745 #45 MESH DESCRIPTOR Hirudin Therapy EXPLODE ALL TREES 75 #46 (thrombin near3 inhib*):TI,AB,KY 675 #47 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048):TI,AB,KY 48 #48 (ximelagatran or Exanta or Exarta or melagatran):TI,AB,KY 189 #49 (AZD0837 or AZD‐0837):TI,AB,KY 23 #50 (S35972 or S‐35972):TI,AB,KY 0 #51 MESH DESCRIPTOR Factor Xa Inhibitors 457 #52 (Factor X* near4 (antag* or inhib* or block*)):TI,AB,KY 914 #53 (FX* near4 (antag* or inhib* or block*)):TI,AB,KY 84 #54 (10* near4 (antag* or inhib* or block*) ):TI,AB,KY 1473 #55 (rivaroxaban or Xarelto):TI,AB,KY 1282 #56 (Bay‐597939 or Bay597939):TI,AB,KY 0 #57 (betrixaban or PRT054021):TI,AB,KY 79 #58 apixaban:TI,AB,KY 745 #59 (BMS‐562247 or BMS‐562247 or ELIQUIS):TI,AB,KY 36 #60 (DU‐176b or DU176b):TI,AB,KY 48 #61 (PRT‐054021 or PRT054021):TI,AB,KY 3 #62 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*):TI,AB,KY 101 #63 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893):TI,AB,KY 7 #64 (edoxaban or lixiana):TI,AB,KY 462 #65 etexilate:TI,AB,KY 273 #66 agatroban:TI,AB,KY 1 #67 MESH DESCRIPTOR Bandages EXPLODE ALL TREES 2603 #68 (stocking* or hosier* or tight* or sock* or bandag* ):TI,AB,KY 8039 #69 (jobst or surepress or activa or kendall or elbeo or levante or lloveras or cette or sigvaris or solidea or medilast or VenoTrain* or Ulcertec or ComfortPro or Comfort‐Pro or "Ulcer Kit"):TI,AB,KY 462 #70 MESH DESCRIPTOR Intermittent Pneumatic Compression Devices EXPLODE ALL TREES 125 #71 compres*:TI,AB,KY 9404 #72 (foot near3 impulse):TI,AB,KY 9 #73 MESH DESCRIPTOR Platelet Aggregation Inhibitors EXPLODE ALL TREES 10232 #74 MESH DESCRIPTOR Phosphodiesterase Inhibitors EXPLODE ALL TREES 6703 #75 MESH DESCRIPTOR Tetrazoles EXPLODE ALL TREES 3304 #76 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*):TI,AB,KY 5733 #77 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) near3 (antagonist or inhibitor)):TI,AB,KY 2854 #78 (((gp* or glycoprotein* or protease or P2Y12 or TXA2) near3 inhibit*)):TI,AB,KY 4468 #79 thienopyridine:TI,AB,KY 374 #80 (ticlopidine or Ticlid):TI,AB,KY 2311 #81 (clopidogrel or Plavix):TI,AB,KY 4952 #82 (Prasugrel or Effient or Efient or Prasita):TI,AB,KY 975 #83 (ticagrelor or AZD6140 or Brilinta):TI,AB,KY 1288 #84 (elinogrel or PRT060128 or PRT‐060128):TI,AB,KY 10 #85 (cangrelor or AR‐C6993* or ARC6993*):TI,AB,KY 119 #86 (SCH530348 or SCH‐530348):TI,AB,KY 25 #87 E5555:TI,AB,KY 12 #88 (terutroban or Triplion):TI,AB,KY 26 #89 (aspirin* or nitroaspirin ):TI,AB,KY 12416 #90 (acetylsalicylic acid):TI,AB,KY 5301 #91 (acetyl salicylic acid*):TI,AB,KY 150 #92 (triflusal or disgren):TI,AB,KY 111 #93 (Cilostazol or Pletal or Pletaal):TI,AB,KY 738 #94 (dipyridamol* or Persantine):TI,AB,KY 1359 #95 (OPC‐13013 or OPC13013):TI,AB,KY 6 #96 (picotamide or picotinamide):TI,AB,KY 46 #97 satigrel:TI,AB,KY 3 #98 vorapaxar:TI,AB,KY 120 #99 indobufen:TI,AB,KY 92 #100 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 73572 #101 #16 AND #100 18481 #102 MESH DESCRIPTOR Neoplasms EXPLODE ALL TREES 69579 #103 (malignan* or neoplas* or cancer*):TI,AB,KY 169102 #104 (carcinoma* or adenocarcinoma*):TI,AB,KY 43590 #105 (tumour* or tumor*):TI,AB,KY 64990 #106 (glio* or leukemia):TI,AB,KY 15433 #107 chemotherapy:TI,AB,KY 67159 #108 chemoanticoagul*:TI,AB,KY 0 #109 myeloma:TI,AB,KY 4924 #110 oncolog*:TI,AB,KY 22997 #111 metastas*:TI,AB,KY 22284 #112 MESH DESCRIPTOR Antineoplastic Agents EXPLODE ALL TREES 52113 #113 MESH DESCRIPTOR Neoplasm Metastasis EXPLODE ALL TREES 4722 #114 #102 OR #103 OR #104 OR #105 OR #106 OR #107 OR #108 OR #109 OR #110 OR #111 OR #112 OR #113 238167 #115 #101 AND #114 3106 #116 01/01/2019 TO 09/07/2019:CD 243198 #117 #115 AND #116 581 |
8 January 2019 – 3626 9 July 2019 – 581 14 October 19 – 43 3 August 2020 – 450 |
| Clinicaltrials.gov | chemotherapy OR malignancy OR neoplasm OR cancer OR tumour OR tumor OR carcinoma OR adenocarcinoma | Thrombosis OR Thromboembolism OR "venous thromboembolism" OR "venous thrombosi"s OR "pulmonary embolism" OR DVT OR VTE OR "deep vein thrombosis" | Anticoagulants OR Heparin OR Antithrombins OR Hirudin Therapy OR "Factor Xa Inhibitors" OR Bandages OR "Intermittent Pneumatic Compression Device"s OR "Platelet Aggregation Inhibitors" OR "Phosphodiesterase Inhibitors" OR Tetrazoles OR aspirin | 8 January 2019 – 35 9 July 2019 – 4 14 October 2019 – 2 3 August 2020 – 151 |
| ICTRP Search Portal | chemotherapy OR malignancy OR neoplasm OR cancer OR tumour OR tumor OR carcinoma OR adenocarcinoma | Thrombosis OR Thromboembolism OR "venous thromboembolism" OR "venous thrombosi"s OR "pulmonary embolism" OR DVT OR VTE OR "deep vein thrombosis" | Anticoagulants OR Heparin OR Antithrombins OR Hirudin Therapy OR "Factor Xa Inhibitors" OR Bandages OR "Intermittent Pneumatic Compression Device" OR "Platelet Aggregation Inhibitors" OR "Phosphodiesterase Inhibitors" OR Tetrazoles OR aspirin | 8 January 2019 – 6 9 July 2019 – 0 14 October 2019 – 0 3 August 2020 – portal not available |
| Medline (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present 2017, 2018, 2019 AND 2020 only |
1 THROMBOSIS/ 2 THROMBOEMBOLISM/ 3 exp Venous Thromboembolism/ 4 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*).ti,ab. 5 exp Pulmonary Embolism/ 6 (PE or DVT or VTE).ti,ab. 7 ((vein* or ven*) adj thromb*).ti,ab. 8 (blood adj3 clot*).ti,ab. 9 (pulmonary adj3 clot*).ti,ab. 10 (lung adj3 clot*).ti,ab. 11 or/1‐10 12 exp ANTICOAGULANTS/ 13 exp HEPARIN/ 14 exp ANTITHROMBINS/ 15 exp Hirudin Therapy/ 16 exp BANDAGES/ 17 exp Factor Xa Inhibitors/ 18 exp Intermittent Pneumatic Compression Devices/ 19 exp Platelet Aggregation Inhibitors/ 20 exp Phosphodiesterase Inhibitors/ 21 exp TETRAZOLES/ 22 (anticoagul* or anti‐coagu*).ti,ab. 23 heparin*.ti,ab. 24 UFH.ti,ab. 25 LMWH.ti,ab. 26 LMH.ti,ab. 27 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren).ti,ab. 28 (Clexane or klexane or lovenox).ti,ab. 29 Fragmin.ti,ab. 30 Innohep.ti,ab. 31 clivarin*.ti,ab. 32 (danaproid or danaparoid).ti,ab. 33 antixarin.ti,ab. 34 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 35 (Kabi‐2165 or Kabi 2165).ti,ab. 36 (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169).ti,ab. 37 (fr‐860 or fr860 or cy‐216 or cy216).ti,ab. 38 (kb101 or lomoparan or orgaran).ti,ab. 39 (fluxum or lohepa or lowhepa).ti,ab. 40 (ave 5026 or ave5026).ti,ab. 41 (M118 or RO‐1).ti,ab. 42 coumar*.ti,ab. 43 ((warfarin or vitamin) adj3 antagonist*).ti,ab. 44 (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).ti,ab. 45 (thrombin adj3 inhib*).ti,ab. 46 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048).ti,ab. 47 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 48 (AZD0837 or AZD‐0837).ti,ab. 49 (S35972 or S‐35972).ti,ab. 50 (Factor X* adj4 (antag* or inhib* or block*)).ti,ab. 51 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 52 (rivaroxaban or Xarelto).ti,ab. 53 (betrixaban or PRT054021).ti,ab. 54 apixaban.ti,ab. 55 (BMS‐562247 or BMS‐562247 or ELIQUIS).ti,ab. 56 (DU‐176b or DU176b).ti,ab. 57 (PRT‐054021 or PRT054021).ti,ab. 58 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*).ti,ab. 59 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893).ti,ab. 60 (edoxaban or lixiana).ti,ab. 61 etexilate.ti,ab. 62 agatroban.ti,ab. 63 (stocking* or hosier* or tight* or sock* or bandag*).ti,ab. 64 (jobst or surepress or activa or kendall or elbeo or levante or lloveras or cette or sigvaris or solidea or medilast or VenoTrain* or Ulcertec or ComfortPro or Comfort‐Pro or "Ulcer Kit").ti,ab. 65 (compres* or ICD).ti,ab. 66 (foot adj3 impulse).ti,ab. 67 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 68 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj2 inhibit*).ti,ab. 69 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj2 (antagonist or inhibitor)).ti,ab. 70 thienopyridine.ti,ab. 71 (ticlopidine or Ticlid).ti,ab. 72 (clopidogrel or Plavix).ti,ab. 73 (Prasugrel or Effient or Efient or Prasita).ti,ab. 74 (ticagrelor or AZD6140 or Brilinta).ti,ab. 75 (elinogrel or PRT060128 or PRT‐060128).ti,ab. 76 (cangrelor or AR‐C6993* or ARC6993*).ti,ab. 77 (SCH530348 or SCH‐530348).ti,ab. 78 E5555.ti,ab. 79 (terutroban or Triplion).ti,ab. 80 (aspirin* or nitroaspirin or ASA).ti,ab. 81 acetylsalicylic acid.ti,ab. 82 acetyl salicylic acid*.ti,ab. 83 (triflusal or disgren).ti,ab. 84 (Cilostazol or Pletal or Pletaal).ti,ab. 85 (dipyridamol* or Persantine).ti,ab. 86 (OPC‐13013 or OPC13013).ti,ab. 87 (picotamide or picotinamide).ti,ab. 88 satigrel.ti,ab. 89 vorapaxar.ti,ab. 90 indobufen.ti,ab. 91 or/12‐90 92 11 and 91 93 exp NEOPLASMS/ 94 exp Antineoplastic Agents/ 95 exp Neoplasm Metastasis/ 96 exp Antineoplastic Protocols/ 97 (malignan* or neoplas* or cancer*).ti,ab. 98 (carcinoma* or adenocarcinoma*).ti,ab. 99 (tumour* or tumor*).ti,ab. 100 (glio* or leukemia).ti,ab. 101 myeloma.ti,ab. 102 oncolog*.ti,ab. 103 metastas*.ti,ab. 104 chemotherap*.ti,ab. 105 or/93‐104 106 92 and 105 107 randomized controlled trial.pt. 108 controlled clinical trial.pt. 109 randomized.ab. 110 placebo.ab. 111 drug therapy.fs. 112 randomly.ab. 113 trial.ab. 114 groups.ab. 115 or/107‐114 116 exp animals/ not humans.sh. 117 115 not 116 118 106 and 117 119 (2017* or 2018* or 2019*).ed. 120 118 and 119 |
8 January 2019 – 644 9 July 2019 – 161 14 October 19 – 101 3 August 2020 – 539 |
| EMBASE 2017, 2018, 2019 AND 2020 only | 1 thrombosis/ 2 thromboembolism/ 3 exp venous thromboembolism/ 4 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*).ti,ab. 5 exp lung embolism/ 6 (PE or DVT or VTE).ti,ab. 7 ((vein* or ven*) adj thromb*).ti,ab. 8 (blood adj3 clot*).ti,ab. 9 (pulmonary adj3 clot*).ti,ab. 10 (lung adj3 clot*).ti,ab. 11 or/1‐10 12 exp anticoagulant agent/ 13 exp heparin/ 14 exp antithrombin/ 15 exp anticoagulant therapy/ 16 exp bandage/ 17 exp blood clotting factor 10a inhibitor/ 18 exp intermittent pneumatic compression device/ 19 exp antithrombocytic agent/ 20 exp phosphodiesterase inhibitor/ 21 exp tetrazole derivative/ 22 (anticoagul* or anti‐coagu*).ti,ab. 23 heparin*.ti,ab. 24 UFH.ti,ab. 25 LMWH.ti,ab. 26 LMH.ti,ab. 27 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren).ti,ab. 28 (Clexane or klexane or lovenox).ti,ab. 29 Fragmin.ti,ab. 30 Innohep.ti,ab. 31 clivarin*.ti,ab. 32 (danaproid or danaparoid).ti,ab. 33 antixarin.ti,ab. 34 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 35 (Kabi‐2165 or Kabi 2165).ti,ab. 36 (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169).ti,ab. 37 (fr‐860 or fr860 or cy‐216 or cy216).ti,ab. 38 (kb101 or lomoparan or orgaran).ti,ab. 39 (fluxum or lohepa or lowhepa).ti,ab. 40 (ave 5026 or ave5026).ti,ab. 41 (M118 or RO‐1).ti,ab. 42 coumar*.ti,ab. 43 ((warfarin or vitamin) adj3 antagonist*).ti,ab. 44 (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).ti,ab. 45 (thrombin adj3 inhib*).ti,ab. 46 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048).ti,ab. 47 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 48 (AZD0837 or AZD‐0837).ti,ab. 49 (S35972 or S‐35972).ti,ab. 50 (Factor X* adj4 (antag* or inhib* or block*)).ti,ab. 51 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 52 (rivaroxaban or Xarelto).ti,ab. 53 (betrixaban or PRT054021).ti,ab. 54 apixaban.ti,ab. 55 (BMS‐562247 or BMS‐562247 or ELIQUIS).ti,ab. 56 (DU‐176b or DU176b).ti,ab. 57 (PRT‐054021 or PRT054021).ti,ab. 58 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*).ti,ab. 59 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893).ti,ab. 60 (edoxaban or lixiana).ti,ab. 61 etexilate.ti,ab. 62 agatroban.ti,ab. 63 (stocking* or hosier* or tight* or sock* or bandag*).ti,ab. 64 (jobst or surepress or activa or kendall or elbeo or levante or lloveras or cette or sigvaris or solidea or medilast or VenoTrain* or Ulcertec or ComfortPro or Comfort‐Pro or "Ulcer Kit").ti,ab. 65 (compres* or ICD).ti,ab. 66 (foot adj3 impulse).ti,ab. 67 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 68 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj2 inhibit*).ti,ab. 69 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj2 (antagonist or inhibitor)).ti,ab. 70 thienopyridine.ti,ab. 71 (ticlopidine or Ticlid).ti,ab. 72 (clopidogrel or Plavix).ti,ab. 73 (Prasugrel or Effient or Efient or Prasita).ti,ab. 74 (ticagrelor or AZD6140 or Brilinta).ti,ab. 75 (elinogrel or PRT060128 or PRT‐060128).ti,ab. 76 (cangrelor or AR‐C6993* or ARC6993*).ti,ab. 77 (SCH530348 or SCH‐530348).ti,ab. 78 E5555.ti,ab. 79 (terutroban or Triplion).ti,ab. 80 (aspirin* or nitroaspirin or ASA).ti,ab. 81 acetylsalicylic acid.ti,ab. 82 acetyl salicylic acid*.ti,ab. 83 (triflusal or disgren).ti,ab. 84 (Cilostazol or Pletal or Pletaal).ti,ab. 85 (dipyridamol* or Persantine).ti,ab. 86 (OPC‐13013 or OPC13013).ti,ab. 87 (picotamide or picotinamide).ti,ab. 88 satigrel.ti,ab. 89 vorapaxar.ti,ab. 90 indobufen.ti,ab. 91 or/12‐90 92 11 and 91 93 exp neoplasm/ 94 exp antineoplastic agent/ 95 exp metastasis/ 96 (malignan* or neoplas* or cancer*).ti,ab. 97 (carcinoma* or adenocarcinoma*).ti,ab. 98 (tumour* or tumor*).ti,ab. 99 (glio* or leukemia).ti,ab. 100 myeloma.ti,ab. 101 oncolog*.ti,ab. 102 metastas*.ti,ab. 103 chemotherap*.ti,ab. 104 or/93‐103 105 92 and 104 106 randomized controlled trial/ 107 controlled clinical trial/ 108 random$.ti,ab. 109 randomization/ 110 intermethod comparison/ 111 placebo.ti,ab. 112 (compare or compared or comparison).ti. 113 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 114 (open adj label).ti,ab. 115 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 116 double blind procedure/ 117 parallel group$1.ti,ab. 118 (crossover or cross over).ti,ab. 119 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 120 (assigned or allocated).ti,ab. 121 (controlled adj7 (study or design or trial)).ti,ab. 122 (volunteer or volunteers).ti,ab. 123 trial.ti. 124 or/106‐123 125 105 and 124 126 (2017* or 2018* or 2019*).dc. 127 125 and 126 |
8 January 2019 – 1280 9 July 2019 – 547 14 October 2019 – 219 3 August 2020 – 717 |
| CINAHL 2017, 2018, 2019 AND 2020 only | S118 S116 AND S117 S117 EM 2017 OR EM 2018 OR EM 2019 S116 S100 AND S115 S115 S101 OR S102 OR S103 OR S104 OR S105 OR S106 OR S107 OR S108 OR S109 OR S110 OR S111 OR S112 OR S113 OR S114 S114 MH "Random Assignment" S113 MH "Triple‐Blind Studies" S112 MH "Double‐Blind Studies" S111 MH "Single‐Blind Studies" S110 MH "Crossover Design" S109 MH "Factorial Design" S108 MH "Placebos" S107 MH "Clinical Trials" S106 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S105 TX crossover OR "cross‐over" S104 AB placebo* S103 TX random* S102 TX trial* S101 TX "latin square" S100 S87 AND S99 S99 S88 OR S89 OR S90 OR S91 OR S92 OR S93 OR S94 OR S95 OR S96 OR S97 OR S98 S98 TX chemotherap* S97 TX metastas* S96 TX oncolog* S95 TX myeloma S94 TX glio* or leukemia S93 TX tumour* or tumor* S92 TX carcinoma* or adenocarcinoma* S91 TX malignan* or neoplas* or cancer* S90 (MH "Neoplasm Metastasis+") S89 (MH "Antineoplastic Agents+") S88 (MH "Neoplasms+") S87 S11 AND S86 S86 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 ... S85 TX indobufen S84 TX vorapaxar S83 TX satigrel S82 TX picotamide or picotinamide S81 TX OPC‐13013 or OPC13013 S80 TX dipyridamol* or Persantine S79 TX Cilostazol or Pletal or Pletaal S78 TX triflusal or disgren S77 TX acetyl salicylic acid* S76 TX acetylsalicylic acid S75 TX aspirin* or nitroaspirin or ASA S74 TX terutroban or Triplion S73 TX E5555 S72 TX SCH530348 or SCH‐530348 S71 TX cangrelor or AR‐C6993* or ARC6993* S70 TX elinogrel or PRT060128 or PRT‐060128 S69 TX ticagrelor or AZD6140 or Brilinta S68 TX Prasugrel or Effient or Efient or Prasita S67 TX clopidogrel or Plavix S66 TX ticlopidine or Ticlid S65 TX thienopyridine S64 TX (platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) N2 (antagonist or inhibitor) S63 TX (gp* or glycoprotein* or protease or P2Y12 or TXA2) N2 inhibit* S62 TX antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg* S61 TX foot N3 impulse S60 TX compres* or ICD S59 TX jobst or surepress or activa or kendall or elbeo or levante or lloveras or cette or sigvaris or solidea or medilast or VenoTrain* or Ulcertec or ComfortPro or Comfort‐Pro or "Ulcer Kit" S58 TX stocking* or hosier* or tight* or sock* or bandag* S57 TX agatroban S56 TX etexilate S55 TX edoxaban or lixiana S54 TX GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893 S53 TX YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176* S52 TX PRT‐054021 or PRT054021 S51 TX DU‐176b or DU176b S50 TX BMS‐562247 or BMS‐562247 or ELIQUIS S49 TX apixaban S48 TX betrixaban or PRT054021 S47 TX rivaroxaban or Xarelto S46 TX FX* N4 (antag* or inhib* or block*) S45 TX Factor X* N4 (antag* or inhib* or block*) S44 TX S35972 or S‐35972 S43 TX AZD0837 or AZD‐0837 S42 TX ximelagatran or Exanta or Exarta or melagatran S41 TX BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048 S40 TX thrombin N3 inhib* S39 TX VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin S38 TX (warfarin or vitamin) N3 antagonist* S37 TX coumar* S36 TX M118 or RO‐1 S35 TX ave 5026 or ave5026 S34 TX fluxum or lohepa or lowhepa S33 TX kb101 or lomoparan or orgaran S32 TX fr‐860 or fr860 or cy‐216 or cy216 S31 TX emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169 S30 TX Kabi‐2165 or Kabi 2165 S29 TX Zibor or cy 222 or embolex or monoembolex S28 TX antixarin S27 TX danaproid or danaparoid S26 TX clivarin* S25 TX Innohep S24 TX Fragmin S23 TX Clexane or klexane or lovenox S22 TX Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren S21 TX LMH S20 TX LMWH S19 TX UFH S18 TX heparin* S17 TX anticoagul* or anti‐coagu* S16 (MH "Phosphodiesterase Inhibitors+") S15 (MH "Platelet Aggregation Inhibitors+") S14 (MH "Elastic Bandages") S13 (MH "Heparin+") S12 (MH "Anticoagulants+") S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 S10 TX lung N3 clot* S9 TX pulmonary N3 clot* S8 TX blood N3 clot* S7 TX ((vein* or ven*) n thromb*) S6 TX PE or DVT or VTE S5 (MH "Pulmonary Embolism") S4 TX thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol* S3 (MH "Venous Thromboembolism") S2 (MH "Thromboembolism") S1 (MH "Thrombosis") |
8 January 2019 – 93 9 July 2019 – 22 14 October 2019 – 40 3 August 2020 – 100 |
| AMED 2017, 2018, 2019 AND 2020 only | 1 Thrombosis/ 2 Thromboembolism/ 3 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*).ti,ab. 4 exp Pulmonary embolism/ 5 (PE or DVT or VTE).ti,ab. 6 ((vein* or ven*) adj thromb*).ti,ab. 7 (blood adj3 clot*).ti,ab. 8 (pulmonary adj3 clot*).ti,ab. 9 (lung adj3 clot*).ti,ab. 10 or/1‐9 11 exp Anticoagulants/ 12 exp Heparin/ 13 exp Bandages/ 14 exp Platelet Aggregation Inhibitors/ 15 (anticoagul* or anti‐coagu*).ti,ab. 16 heparin*.ti,ab. 17 UFH.ti,ab. 18 LMWH.ti,ab. 19 LMH.ti,ab. 20 coumar*.ti,ab. 21 ((warfarin or vitamin) adj3 antagonist*).ti,ab. 22 (VKA or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).ti,ab. 23 (thrombin adj3 inhib*).ti,ab. 24 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 25 (Factor X* adj4 (antag* or inhib* or block*)).ti,ab. 26 (FX* adj4 (antag* or inhib* or block*)).ti,ab. 27 (rivaroxaban or Xarelto).ti,ab. 28 (stocking* or hosier* or tight* or sock* or bandag*).ti,ab. 29 (jobst or surepress or activa or kendall or elbeo or levante or lloveras or cette or sigvaris or solidea or medilast or VenoTrain* or Ulcertec or ComfortPro or Comfort‐Pro or "Ulcer Kit").ti,ab. 30 (compres* or ICD).ti,ab. 31 (foot adj3 impulse).ti,ab. 32 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 33 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj2 inhibit*).ti,ab. 34 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj2 (antagonist or inhibitor)).ti,ab. 35 (ticlopidine or Ticlid).ti,ab. 36 (clopidogrel or Plavix).ti,ab. 37 (aspirin* or nitroaspirin or ASA).ti,ab. 38 acetylsalicylic acid.ti,ab. 39 acetyl salicylic acid*.ti,ab. 40 (Cilostazol or Pletal or Pletaal).ti,ab. 41 (dipyridamol* or Persantine).ti,ab. 42 or/11‐41 43 10 and 42 44 exp Neoplasms/ 45 exp Antineoplastic agents/ 46 exp Neoplasm metastasis/ 47 (malignan* or neoplas* or cancer*).ti,ab. 48 (carcinoma* or adenocarcinoma*).ti,ab. 49 (tumour* or tumor*).ti,ab. 50 (glio* or leukemia).ti,ab. 51 myeloma.ti,ab. 52 oncolog*.ti,ab. 53 metastas*.ti,ab. 54 chemotherap*.ti,ab. 55 or/44‐54 56 43 and 55 57 exp CLINICAL TRIALS/ 58 RANDOM ALLOCATION/ 59 DOUBLE BLIND METHOD/ 60 Clinical trial.pt. 61 (clinic* adj trial*).tw. 62 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 63 PLACEBOS/ 64 placebo*.tw. 65 random*.tw. 66 PROSPECTIVE STUDIES/ 67 or/57‐66 68 56 and 67 69 ("2017" or "2018" or "2019").yr. 70 68 and 69 |
8 January 2019 – 0 9 July 2019 – 0 14 October 2019 – 0 3 August 2020 – 0 |
Appendix 2. Abbreviations and scientific terms
| Abbreviation | Scientific description | Lay description |
| Anticoagulation therapy | Blood‐thinning therapy | |
| GES | Graduated elastic stockings | Special socks that improve blood flow in the leg veins and prevent blood from pooling in the legs |
| Incidence | Number of newly diagnosed diseases, in this review cases of VTE | |
| IPC | Intermittent pneumatic compression | A mechanical intervention using an air pump and inflatable leggings to provide pulsing pressure that pushes blood through the veins |
| Primary prophylaxis | Primary protective treatment aiming at the prevention of disease development | |
| Thromboprophylaxis | Treatment to prevent the development of blood clots | |
| VTE | Venous thromboembolism | Blood clots |
Data and analyses
Comparison 1. Anticoagulants versus control: symptomatic venous thromboembolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Symptomatic VTE: DOAC vs placebo | 3 | 1526 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.18, 1.06] |
| 1.1.1 Apixaban | 2 | 685 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.06, 1.02] |
| 1.1.2 Rivaroxaban | 1 | 841 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.41, 1.54] |
| 1.2 Symptomatic VTE: LMWH vs no thromboprophylaxis | 11 | 3931 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.46, 0.83] |
| 1.2.1 Dalteparin | 6 | 999 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.40, 1.07] |
| 1.2.2 Certoparin | 1 | 883 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.24, 1.35] |
| 1.2.3 Nadroparin | 1 | 1150 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.22, 1.13] |
| 1.2.4 Enoxaparin | 1 | 312 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.21, 0.88] |
| 1.2.5 Bemiparin | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 1.75] |
| 1.2.6 Tinzaparin | 1 | 549 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.51, 1.73] |
| 1.3 Symptomatic VTE: prophylactic vs intermediate or therapeutic LMWH | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Prophylactic vs intermediate | 1 | 51 | Risk Ratio (IV, Fixed, 95% CI) | 2.89 [0.12, 67.75] |
| 1.3.2 Prophylactic vs therapeutic | 1 | 52 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.07, 15.15] |
| 1.4 Symptomatic VTE: LMWH vs aspirin | 2 | 781 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.22, 1.17] |
| 1.5 Symptomatic VTE: LMWH vs warfarin | 1 | 439 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.83] |
| 1.6 Symptomatic VTE: semuloparin vs placebo | 1 | 3212 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.22, 0.60] |
| 1.7 Symptomatic VTE: vitamin K antagonists vs placebo | 1 | 311 | Risk Ratio (IV, Fixed, 95% CI) | 0.15 [0.02, 1.20] |
| 1.8 Symptomatic VTE: warfarin vs aspirin | 1 | 440 | Risk Ratio (IV, Fixed, 95% CI) | 1.50 [0.74, 3.04] |
Comparison 2. Anticoagulants versus control: major bleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Major bleeding: DOAC vs placebo | 3 | 1494 | Risk Ratio (IV, Random, 95% CI) | 1.74 [0.82, 3.68] |
| 2.1.1 Apixaban | 2 | 685 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.60, 4.17] |
| 2.1.2 Rivaroxaban | 1 | 809 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.61, 6.57] |
| 2.2 Major bleeding: LMWH vs no thromboprophylaxis | 15 | 7282 | Risk Ratio (IV, Random, 95% CI) | 1.63 [1.12, 2.35] |
| 2.2.1 Dalteparin | 6 | 3119 | Risk Ratio (IV, Random, 95% CI) | 1.52 [0.70, 3.28] |
| 2.2.2 Certoparin | 1 | 898 | Risk Ratio (IV, Random, 95% CI) | 2.19 [0.84, 5.70] |
| 2.2.3 Nadroparin | 3 | 1955 | Risk Ratio (IV, Random, 95% CI) | 1.83 [0.69, 4.85] |
| 2.2.4 Enoxaparin | 3 | 723 | Risk Ratio (IV, Random, 95% CI) | 1.87 [0.61, 5.72] |
| 2.2.5 Bemiparin | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.01, 6.97] |
| 2.2.6 Tinzaparin | 1 | 549 | Risk Ratio (IV, Random, 95% CI) | 5.20 [0.25, 107.89] |
| 2.3 Major bleeding: prophylactic vs intermediate or therapeutic LMWH | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Prophylactic vs intermediate | 1 | 51 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 2.3.2 Prophylactic vs therapeutic | 1 | 52 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 2.4 Major bleeding: LMWH vs aspirin | 2 | 781 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.76] |
| 2.5 Major bleeding: LMWH vs warfarin | 1 | 439 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 2.6 Major bleeding: semuloparin vs placebo | 1 | 3172 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.55, 2.00] |
| 2.7 Major bleeding: vitamin K antagonists vs no thromboprophylaxis | 4 | 994 | Risk Ratio (IV, Random, 95% CI) | 3.82 [0.97, 15.04] |
| 2.8 Major bleeding: warfarin vs aspirin | 1 | 440 | Risk Ratio (IV, Fixed, 95% CI) | 0.14 [0.01, 2.75] |
2.3. Analysis.

Comparison 2: Anticoagulants versus control: major bleeding, Outcome 3: Major bleeding: prophylactic vs intermediate or therapeutic LMWH
2.5. Analysis.

Comparison 2: Anticoagulants versus control: major bleeding, Outcome 5: Major bleeding: LMWH vs warfarin
Comparison 3. Anticoagulants versus control: symptomatic pulmonary embolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Symptomatic PE: DOAC vs placebo | 3 | 1526 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.10, 1.47] |
| 3.1.1 Apixaban | 2 | 685 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.04, 0.67] |
| 3.1.2 Rivaroxaban | 1 | 841 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.29, 3.44] |
| 3.2 Symptomatic PE: LMWH vs no thromboprophylaxis | 8 | 5324 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.42, 0.88] |
| 3.2.1 Dalteparin | 5 | 2979 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.42, 0.94] |
| 3.2.2 Nadroparin | 1 | 1150 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.10, 2.44] |
| 3.2.3 Certoparin | 1 | 883 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.14, 2.49] |
| 3.2.4 Enoxaparin | 1 | 312 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.61] |
| 3.3 Symptomatic PE: prophylactic vs intermediate or therapeutic LMWH | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 Prophylactic vs intermediate | 1 | 51 | Risk Ratio (IV, Fixed, 95% CI) | 2.89 [0.12, 67.75] |
| 3.3.2 Prophylactic vs therapeutic | 1 | 52 | Risk Ratio (IV, Fixed, 95% CI) | 3.00 [0.13, 70.42] |
| 3.4 Symptomatic PE: LMWH vs aspirin | 2 | 781 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.02, 1.03] |
| 3.5 Symptomatic PE: LMWH vs warfarin | 1 | 439 | Risk Ratio (IV, Fixed, 95% CI) | 0.11 [0.01, 2.06] |
| 3.6 Symptomatic PE: semuloparin vs placebo | 1 | 3212 | Risk Ratio (IV, Fixed, 95% CI) | 0.48 [0.22, 1.01] |
| 3.7 Symptomatic PE: vitamin K antagonists vs placebo | 1 | 311 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.07, 16.58] |
| 3.8 Symptomatic PE: warfarin vs aspirin | 1 | 440 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.25, 3.95] |
3.4. Analysis.

Comparison 3: Anticoagulants versus control: symptomatic pulmonary embolism, Outcome 4: Symptomatic PE: LMWH vs aspirin
Comparison 4. Anticoagulants versus control: fatal PE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Fatal PE: LMWH vs no thromboprophylaxis | 7 | 4286 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.11, 1.21] |
| 4.1.1 Dalteparin | 3 | 2419 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.07, 1.84] |
| 4.1.2 Enoxaparin | 2 | 679 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.01, 4.25] |
| 4.1.3 Nadroparin | 1 | 1150 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 4.1.4 Bemiparin | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
Comparison 5. Anticoagulants versus control: symptomatic deep vein thrombosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Symptomatic DVT: DOAC vs placebo | 3 | 1526 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.21, 1.22] |
| 5.1.1 Apixaban | 2 | 685 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.04, 1.73] |
| 5.1.2 Rivaroxaban | 1 | 841 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.41, 1.54] |
| 5.2 Symptomatic DVT: LMWH vs no thromboprophylaxis | 9 | 5408 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.35, 0.67] |
| 5.2.1 Dalteparin | 6 | 3063 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.32, 0.77] |
| 5.2.2 Nadroparin | 1 | 1150 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.19, 1.31] |
| 5.2.3 Certoparin | 1 | 883 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.18, 1.20] |
| 5.2.4 Enoxaparin | 1 | 312 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.22, 0.93] |
| 5.3 Symptomatic DVT: prophylactic vs intermediate or therapeutic LMWH | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 Prophylactic vs intermediate | 1 | 51 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 5.3.2 Prophylactic vs therapeutic | 1 | 52 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.01, 7.82] |
| 5.4 Symptomatic DVT: LMWH vs aspirin | 2 | 781 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.32, 2.04] |
| 5.5 Symptomatic DVT: LMWH vs warfarin | 1 | 439 | Risk Ratio (IV, Fixed, 95% CI) | 0.43 [0.17, 1.10] |
| 5.6 Symptomatic DVT: semuloparin vs placebo | 1 | 3212 | Risk Ratio (IV, Fixed, 95% CI) | 0.32 [0.16, 0.63] |
| 5.7 Symptomatic DVT: vitamin K antagonists vs placebo | 1 | 311 | Risk Ratio (IV, Fixed, 95% CI) | 0.08 [0.00, 1.42] |
| 5.8 Symptomatic DVT: warfarin vs aspirin | 1 | 440 | Risk Ratio (IV, Fixed, 95% CI) | 1.75 [0.75, 4.09] |
Comparison 6. Anticoagulants versus control: any venous thromboembolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Any VTE: DOAC vs placebo | 2 | 1404 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.34, 0.90] |
| 6.1.1 Apixaban | 1 | 563 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.21, 0.79] |
| 6.1.2 Rivaroxaban | 1 | 841 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.42, 1.10] |
| 6.2 Any VTE: LMWH vs no thromboprophylaxis | 10 | 5743 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.46, 0.71] |
| 6.2.1 Dalteparin | 4 | 2494 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.41, 0.70] |
| 6.2.2 Nadroparin | 3 | 1955 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.48, 1.27] |
| 6.2.3 Certoparin | 1 | 883 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.37, 1.15] |
| 6.2.4 Enoxaparin | 2 | 411 | Risk Ratio (IV, Random, 95% CI) | 0.28 [0.12, 0.69] |
| 6.3 Any VTE: prophylactic vs intermediate vs therapeutic LMWH | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 6.3.1 Prophylactic vs intermediate | 1 | 51 | Risk Ratio (IV, Fixed, 95% CI) | 4.81 [0.24, 95.58] |
| 6.3.2 Prophylactic vs therapeutic | 1 | 52 | Risk Ratio (IV, Fixed, 95% CI) | 5.00 [0.25, 99.34] |
| 6.4 Any VTE: semuloparin vs placebo | 1 | 3212 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.22, 0.60] |
Comparison 7. Anticoagulants versus control: 1‐year overall mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 1‐year overall mortality: LMWH vs no thromboprophylaxis | 9 | 2681 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.83, 1.07] |
| 7.1.1 Dalteparin | 4 | 782 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.77, 1.21] |
| 7.1.2 Nadroparin | 2 | 1452 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.77, 1.18] |
| 7.1.3 Enoxaparin | 2 | 411 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.80, 1.16] |
| 7.1.4 Bemiparin | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.05, 0.70] |
| 7.2 1‐year overall mortality: semuloparin vs placebo | 1 | 3212 | Risk Ratio (IV, Fixed, 95% CI) | 1.02 [0.96, 1.08] |
| 7.3 1‐year overall mortality: UFH vs no thromboprophylaxis | 1 | 277 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.72, 1.03] |
Comparison 8. Anticoagulants versus control: clinically relevant bleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Clinically relevant bleeding: DOAC vs placebo | 2 | 931 | Risk Ratio (IV, Random, 95% CI) | 1.61 [0.82, 3.15] |
| 8.1.1 Apixaban | 1 | 122 | Risk Ratio (IV, Random, 95% CI) | 1.87 [0.23, 14.91] |
| 8.1.2 Rivaroxaban | 1 | 809 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.78, 3.21] |
| 8.2 Clinically relevant bleeding: LMWH vs no thromboprophylaxis | 4 | 3105 | Risk Ratio (IV, Random, 95% CI) | 3.40 [1.20, 9.63] |
| 8.3 Clinically relevant bleeding: prophylactic vs intermediate vs therapeutic LMWH | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 8.3.1 Prophylactic vs intermediate | 1 | 51 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 8.3.2 Prophylactic vs therapeutic | 1 | 52 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.01, 7.82] |
| 8.4 Clinically relevant bleeding: semuloparin vs placebo | 1 | 3172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.90, 2.19] |
| 8.5 Clinically relevant bleeding: UFH vs no thromboprophylaxis | 1 | 277 | Risk Ratio (IV, Fixed, 95% CI) | 2.01 [0.18, 21.96] |
8.3. Analysis.

Comparison 8: Anticoagulants versus control: clinically relevant bleeding, Outcome 3: Clinically relevant bleeding: prophylactic vs intermediate vs therapeutic LMWH
Comparison 9. Anticoagulants versus control: incidental venous thromboembolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Incidental VTE: DOAC vs placebo | 2 | 1404 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.25, 0.98] |
| 9.1.1 Apixaban | 1 | 563 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.12, 1.89] |
| 9.1.2 Rivaroxaban | 1 | 841 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.23, 1.10] |
| 9.2 Incidental VTE: LMWH vs no thromboprophylaxis | 5 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.40, 0.99] |
| 9.2.1 Dalteparin | 3 | 2419 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.34, 1.00] |
| 9.2.2 Nadroparin | 1 | 1150 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.21, 2.62] |
| 9.2.3 Certoparin | 1 | 883 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.26, 2.14] |
| 9.3 Incidental VTE: prophylactic vs intermediate or therapeutic LMWH | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 9.3.1 Prophylactic vs intermediate | 1 | 51 | Risk Ratio (IV, Fixed, 95% CI) | 2.89 [0.12, 67.75] |
| 9.3.2 Prophylactic vs therapeutic | 1 | 52 | Risk Ratio (IV, Fixed, 95% CI) | 3.00 [0.13, 70.42] |
| 9.4 Incidental VTE: semuloparin vs placebo | 1 | 3212 | Risk Ratio (IV, Fixed, 95% CI) | 0.14 [0.01, 2.76] |
Comparison 10. Anticoagulants versus control: minor bleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Minor bleeding: LMWH vs no thromboprophylaxis | 8 | 2901 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.92, 1.68] |
| 10.1.1 Dalteparin | 5 | 815 | Risk Ratio (IV, Random, 95% CI) | 1.32 [0.77, 2.24] |
| 10.1.2 Nadroparin | 1 | 1150 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.69, 1.45] |
| 10.1.3 Certoparin | 1 | 898 | Risk Ratio (IV, Random, 95% CI) | 1.96 [1.11, 3.46] |
| 10.1.4 Bemiparin | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.09, 2.17] |
| 10.2 Minor bleeding: prophylactic vs intermediate or therapeutic LMWH | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 10.2.1 Prophylactic vs intermediate | 1 | 51 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 10.2.2 Prophylactic vs therapeutic | 1 | 52 | Risk Ratio (IV, Fixed, 95% CI) | 0.20 [0.01, 3.97] |
| 10.3 Minor bleeding: LMWH vs aspirin | 2 | 781 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.17, 2.84] |
| 10.4 Minor bleeding: LMWH vs warfarin | 1 | 439 | Risk Ratio (IV, Fixed, 95% CI) | 3.01 [0.32, 28.75] |
| 10.5 Minor bleeding: UFH vs no thromboprophylaxis | 1 | 277 | Risk Ratio (IV, Fixed, 95% CI) | 3.02 [0.12, 73.54] |
| 10.6 Minor bleeding: vitamin K antagonists vs placebo | 1 | 311 | Risk Ratio (IV, Fixed, 95% CI) | 2.44 [0.64, 9.27] |
| 10.7 Minor bleeding: warfarin vs aspirin | 1 | 440 | Risk Ratio (IV, Fixed, 95% CI) | 0.17 [0.02, 1.37] |
10.2. Analysis.

Comparison 10: Anticoagulants versus control: minor bleeding, Outcome 2: Minor bleeding: prophylactic vs intermediate or therapeutic LMWH
10.4. Analysis.

Comparison 10: Anticoagulants versus control: minor bleeding, Outcome 4: Minor bleeding: LMWH vs warfarin
Comparison 11. Anticoagulants versus control: symptomatic arterial thromboembolism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Symptomatic arterial thromboembolism: DOAC vs placebo | 1 | 841 | Risk Ratio (IV, Fixed, 95% CI) | 0.57 [0.17, 1.94] |
| 11.2 Symptomatic arterial thromboembolism: LMWH vs no thromboprophylaxis | 5 | 4351 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.49, 1.22] |
| 11.2.1 Dalteparin | 2 | 2321 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.52, 1.53] |
| 11.2.2 Nadroparin | 2 | 1653 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.14, 1.03] |
| 11.2.3 Enoxaparin | 1 | 377 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.26, 9.11] |
| 11.3 Symptomatic arterial thromboembolism: LMWH vs aspirin | 2 | 781 | Risk Ratio (IV, Random, 95% CI) | 2.01 [0.37, 10.86] |
| 11.4 Symptomatic arterial thromboembolism: LMWH vs warfarin | 1 | 439 | Risk Ratio (IV, Fixed, 95% CI) | 9.04 [0.49, 166.92] |
| 11.5 Symptomatic arterial thromboembolism: vitamin K antagonists vs placebo | 1 | 311 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 11.6 Symptomatic arterial thromboembolism: warfarin vs aspirin | 1 | 440 | Risk Ratio (IV, Fixed, 95% CI) | 0.20 [0.01, 4.14] |
11.4. Analysis.

Comparison 11: Anticoagulants versus control: symptomatic arterial thromboembolism, Outcome 4: Symptomatic arterial thromboembolism: LMWH vs warfarin
11.5. Analysis.

Comparison 11: Anticoagulants versus control: symptomatic arterial thromboembolism, Outcome 5: Symptomatic arterial thromboembolism: vitamin K antagonists vs placebo
Comparison 12. Anticoagulants versus control: superficial venous thrombosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Superficial venous thrombosis: LMWH vs no thromboprophylaxis | 2 | 2033 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.30, 2.26] |
| 12.1.1 Certoparin | 1 | 883 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 12.1.2 Nadroparin | 1 | 1150 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.30, 2.26] |
| 12.2 Superficial venous thrombosis: LMWH vs aspirin | 1 | 342 | Risk Ratio (IV, Fixed, 95% CI) | 0.12 [0.01, 2.17] |
12.2. Analysis.

Comparison 12: Anticoagulants versus control: superficial venous thrombosis, Outcome 2: Superficial venous thrombosis: LMWH vs aspirin
Comparison 13. Anticoagulants versus control: serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Serious adverse events: DOAC vs placebo | 2 | 934 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.82, 1.13] |
| 13.2 Serious adverse events: LMWH vs no thromboprophylaxis | 5 | 1531 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.70, 1.07] |
| 13.2.1 Dalteparin | 3 | 343 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.45, 3.34] |
| 13.2.2 Nadroparin | 1 | 1150 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.68, 1.17] |
| 13.2.3 Bemiparin | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.43, 1.30] |
| 13.3 Serious adverse events: semuloparin vs placebo | 1 | 3172 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [0.92, 1.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agnelli 2009.
| Study characteristics | ||
| Methods | Trial acronym: PROTECHT Design: multicentre, double‐blind, placebo‐controlled trial with modified intention‐to‐treat analysis, including participants who received ≥ 1 dose of study treatment. Median duration of follow‐up: 111 days in nadroparin; 113 days in placebo |
|
| Participants | Ambulatory patients aged > 18 years who were receiving chemotherapy for metastatic or locally advanced lung, gastrointestinal, pancreatic, breast, ovarian, or head and neck cancer Mean age: 62.1 (SD 10.3) years in nadroparin group; 63.7 (SD 9.2) years in placebo group Gender, n (%) males: 372 (48.4%) in nadroparin group; 183 (48%) in placebo group Metastatic disease, n (%): not reported Previous VTE, n (%): 12 (1.6%) in nadroparin group; 6 (1.6%) in placebo group |
|
| Interventions | Intervention: LMWH, nadroparin 3800 IU SC, once daily Control: placebo Study treatment started on the same day as chemotherapy (the first cycle or a new course), and was given for the duration of chemotherapy or up to a maximum of 120 days (± 10 days). |
|
| Outcomes | Primary outcomes: composite of symptomatic venous or arterial thromboembolic events occurring during study treatment plus 10 days; major bleeding that occurred between randomisation and 48 hours after last injection of study drug Secondary efficacy outcomes: incidental thromboembolic events incidentally diagnosed; survival at end of study treatment and at 12 months; superficial venous thrombosis of lower limbs; response to chemotherapy; central venous catheter‐related complications of possible thrombotic origin Secondary safety outcome: minor bleeding |
|
| Notes | Antiplatelet agents, oral anticoagulants, fibrinolytic agents, UFH, or LMWH other than nadroparin not allowed during study Funding: Italfarmaco SpA, Milan, Italy Disclosure of potential conflicts of interest: the scientific director of Italfarmaco was involved as an author. Publication format: published as full text |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation list was generated by an independent statistician who used a standard permuted block of six without stratification. The list was generated with SAS version 8.2." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was available online to the investigators using the Hypernet web‐based system. At the time the investigator accessed the web‐based system with personal codes (user ID and password) and requested the treatment allocation for a new patient who fulfilled the eligibility criteria, the system assigned the next free number in accordance with the randomisation sequence" Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Patients and investigators did not know whether study drug or placebo was being given, since pre‐filled syringes were used which were identical in appearance. Treatment assignments were masked from all study personnel and participants for the duration of the study." "All study outcomes were assessed by a central independent adjudication committee whose members were unaware of patients’ study‐group allocation." Comment: double‐blind RCT and adequate methods of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All randomised patients who received at least one dose of the study treatment were included in the efficacy and safety analyses." Comment: 769/779 (98.7%) participants randomised were analysed in the LMWH group, 381/387 (98.4%) randomised were analysed in the placebo group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section were addressed in the results or discussion section. |
Agnelli 2012.
| Study characteristics | ||
| Methods | Trial acronym: SAVE‐ONCO study Design: multicentre, double‐blind RCT, with intention‐to‐treat for effectiveness and modified intention‐to‐treat analysis for safety outcomes, including participants who received ≥ 1 study dose Mean duration of follow‐up: not reported |
|
| Participants | Patients with metastatic or locally advanced solid cancer of the lung, pancreas, stomach, colon or rectum, bladder, or ovary who were beginning a course of chemotherapy Mean age: 59.8 years in semuloparin group; 59.4 years in placebo group Gender, n (%) males: 974 (60.6%) in semuloparin group; 956 (59.6%) in placebo group Metastatic disease: not reported Previous VTE: 2% in semuloparin group; 2.3% in placebo group |
|
| Interventions | Intervention: uLMWH semuloparin 20 mg SC, once daily Control: placebo The first dose of the study drug was administered on the first day of a course of chemotherapy (first regimen or a new regimen), continuing for the duration of chemotherapy (intended to be a minimum of 3 months). Median treatment duration was 3.5 months. |
|
| Outcomes | Primary efficacy outcome: composite of any symptomatic DVT, any non‐fatal PE, and death related to VTE Primary safety outcome: clinically relevant bleeding (major and non‐major) Secondary efficacy outcome: 1‐year overall survival or at study end date |
|
| Notes | Funding, quote: "Supported by Sanofi". "The study was designed by the steering committee members and sponsored by Sanofi. Data were collected through a clinical research organization and analyzed by Sanofi. No Sanofi employees were members of the steering committee or the data and safety monitoring board." Disclosure of potential conflicts of interest: in the section 'The Work Under Consideration for Publication,' some of the authors declared they were employed by Sanofi or had received consulting fee or honorarium and support for travel to meetings by Sanofi‐Aventis. Publication format: published as full text Marketing applications for semuloparin have been withdrawn worldwide, and it is, therefore, unlikely to ever be commercially available (EMEA 2012). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally by means of an interactive voice‐response system." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally by means of an interactive voice‐response system." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Efficacy and bleeding outcomes were assessed by a central independent adjudication committee, whose members were unaware of the study treatment" Comment: double‐blind RCT and blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients who underwent randomization were included in the primary efficacy population (intention‐to‐treat population), and those who underwent randomization and received at least one dose of the study treatment were included in the safety population" Comment: for safety, 1589/1608 (98.8%) participants randomised were analysed in uLMWH group, 1583/1604 (98.7%) participants randomised were analysed in placebo group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the protocol and in the methods section of the full report were addressed in the results or discussion section, except for 1 outcome mentioned in the protocol only: "Secondary efficacy variables include the initiation of curative treatment by the investigator after VTE," We did not consider this outcome to be relevant for the current review. |
Altinbas 2004.
| Study characteristics | ||
| Methods | Trial acronym: none reported Design: RCT with intention‐to‐treat analysis for survival outcomes Median duration of follow‐up: 10 (range 2–33) months |
|
| Participants | Patients aged 18–75 years with histologically confirmed small‐cell lung carcinoma with an ECOG performance status < 3 and normal haematological, renal, and hepatic function tests Median age: 58 (range 34–75) years Gender, n: 33 men and 9 women in dalteparin group; 35 men and 7 women in control group Metastatic disease: 19 in dalteparin group; 17 in control group Previous VTE: 0/84 |
|
| Interventions | Intervention: LMWH, dalteparin 5000 IU SC, once daily Control: no dalteparin Dalteparin was stopped with disease progression or at end of 18 weeks of chemotherapy Median duration of treatment: 18 weeks |
|
| Outcomes | Primary outcome: overall survival Secondary outcomes: progression‐free survival, adverse effects |
|
| Notes | Funding: not reported Disclosure of potential conflicts of interest: not disclosed, no COI forms available Publication format: published as full text |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to receive either CT [chemotherapy] or CT plus LMWH." Comment: method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized to receive either CT or CT plus LMWH." Comment: method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: trial is reported as a "Chemotherapy‐only" vs "Chemotherapy + LMWH" trial, without mentioning the use of placebo LMWH, or any attempt to blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: for effectiveness is not reported. For safety, survival was analysed according to the intention‐to‐treat principle. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. |
Campos‐Cabrera 2018.
| Study characteristics | ||
| Methods | Trial acronym: none reported Design: randomised study with active control Median duration of follow‐up: not reported |
|
| Participants | Patients with multiple myeloma who received thalidomide‐ and dexamethasone‐based triplet induction therapy, maintenance with thalidomide and creatinine clearance > 30 mL/minute and had an additional cardiovascular risk factor. Median age: 67.5 years in rivaroxaban group; 66.8 years in aspirin group Gender, n (%) males: 3 (60%) males in rivaroxaban group; 10 (55.6%) males in aspirin group Metastatic disease: not reported Previous VTE: not reported |
|
| Interventions | Intervention 1: rivaroxaban 10 mg once daily Intervention 2: aspirin 100 mg once daily Treatment was continued until relapse and need another treatment |
|
| Outcomes | VTE including symptomatic or incidental DVT and symptomatic PE; bleeding | |
| Notes | Funding: none reported Disclosure of potential conflicts of interest: "no relevant conflicts to declare." Publication format: published as conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear if all participants included were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all outcomes were reported. |
Carrier 2019.
| Study characteristics | ||
| Methods | Trial acronym: AVERT Design: double‐blind (participant, carer, investigator, outcomes assessor), parallel‐assignment RCT Median duration of follow‐up: 183 days in each group |
|
| Participants | Patients with a newly diagnosed cancer site or progression of the malignant disease after complete or partial remission who were initiating a new course of chemotherapy with a minimum intent of 3 months' therapy and who had a VTE risk stratification score of ≥ 2, according to the Khorana scoring method. Mean age: 61 years in whole study population; 61.2 (SD 12.4) years in apixaban group; 61.7 (SD 11.3) years in placebo group Gender, n (%) males: 121 (41.6%) in apixaban group; 119 (42%) in placebo group Metastatic disease, n (%): 73 (25.1%) in apixaban group; 67 (23.7%) in placebo group Previous VTE, n (%): 9 (3.1%) in apixaban group; 8 (2.8%) in placebo group |
|
| Interventions | Intervention: apixaban 2.5 mg twice daily for 6 months Control: placebo |
|
| Outcomes | Primary outcome: symptomatic or incidental VTE (DVT, PE, or both) at 6 months Secondary outcomes: rate of adverse events, clinical overt bleeding (major and minor bleeding), and death within the study period |
|
| Notes | Funded by the Canadian Institutes of Health Research and Bristol‐Myers Squibb–Pfizer Alliance; AVERT Disclosure of potential conflicts of interest (extracted for first, second, and last author):
Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients underwent randomization by means of a centralized, Web‐based randomization system to receive apixaban or placebo in a 1:1 ratio. Randomization was stratified according to age, sex, and participating center and occurred up to 5 days before the administration of the first chemotherapy." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible patients underwent randomization by means of a centralized, Web‐based randomization system to receive apixaban or placebo in a 1:1 ratio. Randomization was stratified according to age, sex, and participating center and occurred up to 5 days before the administration of the first chemotherapy." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All trial outcomes were adjudicated by an independent adjudication committee whose members were unaware of the treatment assignments." Comment: double‐blind RCT and blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 11/574 (1.9%) participants enrolled in the study were not considered for the analysis. Exclusions per trial arm were reported. 24 (4.2%) participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. |
Chahinian 1989.
| Study characteristics | ||
| Methods | Trial acronym: none reported, is a trial run by Cancer and Leukemia Group B (CALGB) institutions in USA Design: multicentre, 3‐arm RCT, type of analyses not reported Median duration of follow‐up: 36 months |
|
| Participants | Patients with extensive carcinoma of the lung Mean age: not reported. % patients aged ≥ 60 years: 55% in warfarin group; 60% in control group Gender, n (%) males: 70 (68%) in warfarin group; 129 (68%) in control group Metastatic or extensive disease, n (%): 294 (100%) Previous VTE: not reported |
|
| Interventions | Intervention: warfarin to maintain a prothrombin 1.5 to twice the control values Control 1: no warfarina Control 2: no warfarina aAll groups received chemotherapy with methotrexate, doxorubicin, cyclophosphamide, and lomustine (MACC), but control group 2 alternated mitomycin, etoposide, cisplatin, and hexamethylmelamin with MACC. Warfarin was continued throughout the course of chemotherapy, and it was withheld in participants with brain metastases during cranial irradiation and whenever platelet counts < 75,000/μL. The median time on warfarin was 162 (range 2–627) days. |
|
| Outcomes | Main outcomes: overall survival, failure‐free survival, and cancer response (complete response, partial response, and objective response rate) to therapy Secondary outcomes: toxicity |
|
| Notes | Funding: grants from the National Cancer Institute, Department of Health and Human Services, and the T.J. Martell Foundation for Leukemia and Cancer Research Disclosure of potential conflicts of interest: not disclosed, no COI forms available Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allocation was determined by a Latin square arrangement balancing the sequence within and across institutions." Comment: adequate method of sequence generation; stratified randomisation, use of Latin square design. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: blinding not reported, use of placebo warfarin not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 34/328 (10%) participants enrolled in the study were not considered for the analysis. Exclusions per trial group were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. Toxicity was addressed in the results, but not explicitly reported as an outcome in the methods section. |
Ek 2018.
| Study characteristics | ||
| Methods | Trial acronym: none reported Design: international, open‐label RCT Median follow‐up: 41 (IQR 21–81) months for participants still alive |
|
| Participants | Patients with histologically or cytologically verified newly diagnosed small‐cell lung cancer of all stages Mean age: 67 (SD 7.9) years in enoxaparin group; 68 (SD 8.5) years in control group Gender, n (%) males: 78 (42%) in enoxaparin group; 82 (43%) in control group Metastatic disease, n (%): extensive disease: 114 (61%) in enoxaparin group; 113 (59%) in control group Previous VTE: not reported |
|
| Interventions | Intervention: enoxaparin at a supraprophylactic dose (1 mg/kg) in addition to standard treatment. Enoxaparin was started on day 1 of chemotherapy and continued until the 21st day of the last chemotherapy cycle Control: standard treatment |
|
| Outcomes | Primary outcome: overall survival Secondary outcomes were progression‐free survival, incidence of VTE and haemorrhagic events |
|
| Notes | Funding: Swedish Research Councile (to MB, grant number: 2014‐3421); the Swedish Cancer Society (to MB, grant number: 2014/378); the Skane University Hospital donation funds (to MB, no grant number); the Medical Faculty, Lund University (to MB, no grant number); the Governmental funding of clinical research within the national health services (ALF) (to MB and EG, no grant number); the Gunnar Nilsson, Anna Lisa and Sven Eric Lundgren and Kamprad Foundations (to MB, no grant number); a restricted grant support from Sanofi Aventis, Sweden (to LE, no grant number); a donation by Viveca Jeppsson (to MB, no grant number); and received honoraria from Leo Pharma, AstraZeneca and Pfizer (to MB, no grant number) Disclosure of potential conflicts of interest: "the authors have declared no conflicts of interest." Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization procedure was conducted at the Clinical Research Unit at Lund University Hospital, using a computer algorithm." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization procedure was conducted at the Clinical Research Unit at Lund University Hospital, using a computer algorithm." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "international, open‐label trial." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 13/574 (3.3%) participants enrolled in the study were not considered for the analysis. Exclusions per trial group were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section |
Elit 2012.
| Study characteristics | ||
| Methods | Trial acronym: none reported, trial run by the Ontario Clinical Oncology Group Design: multicentre, open‐label, 4‐arm phase II randomised trial. The study was terminated early due to poor recruitment. Median duration of follow‐up: not reported, participants were followed until the end of chemotherapy |
|
| Participants | Women with newly diagnosed epithelial ovarian cancer stage IIB–IV Age, median: 61 (range 34–74) years Gender, n (%) females: 77 (100%) Metastatic disease: not reported Previous VTE, n (%): 4 (5%) |
|
| Interventions | Intervention 1: standard adjuvant chemotherapy (taxane and platinum‐based) and dalteparin 50 IU/kg SC once daily during the first 3 of 6 cycles of 3‐weekly chemotherapy Intervention 2: standard adjuvant chemotherapy (taxane and platinum‐based) and dalteparin 100 IU/kg SC once daily during the first 3 of 6 cycles of 3‐weekly chemotherapy Intervention 3: standard adjuvant chemotherapy (taxane and platinum‐based) and dalteparin 150 IU/kg SC once daily during the first 3 of 6 cycles of 3‐weekly chemotherapy Study medication was started within 7 days prior to the first 21‐day cycle of chemotherapy and continued until day 21 of cycle 3. Median duration of LMWH was 67 days. |
|
| Outcomes | Primary outcome: tumour response defined by ≥ 50% reduction in serum CA125 from baseline sustained for ≥ 28 days Secondary outcomes: major bleeding up to 24 hours after the last dose of dalteparin; any bleeding up to 24 hours after the last dose of dalteparin; symptomatic VTE up to 7 days after the last dose of dalteparin; death up to the last day of follow‐up; and compliance with dalteparin administration |
|
| Notes | Funding, quote: "The Steering Committee wishes to acknowledge the financial support from both the Juravinski Cancer Centre Foundation and Pfizer Canada Inc." Disclosure of potential conflicts of interest, quote: "There are no financial disclosures from any of the authors related to this work except for Dr. Lee who has provided educational lectures and received financial reimbursement from Pfizer Canada Inc." Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Concealed randomization was performed centrally … using a computer‐generated, permuted‐block randomization schedule." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed randomization was performed centrally … using a computer‐generated, permuted‐block randomization schedule." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Study outcomes were adjudicated by members of a Central Adjudication Committee masked to treatment assignment." Comment: open‐label study with blinded adjudication of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The primary analysis included all patients as randomized." Comment: all participants who were randomised were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes indicated in the methods were presented in the results. |
Greiner 2019.
| Study characteristics | ||
| Methods | Trial acronym: THROMBOTECT Design: open‐label, prospective, randomised, multicentre study Median duration of follow‐up: not reported |
|
| Participants | Patients aged 1–18 years with newly diagnosed acute lymphoblastic leukaemia Mean age: not reported. 54% of participants were aged 1–6 years, 19.8% were 6–10 years, and 26.2% were > 10 years Gender, n (%) males: 537 (56.6%) Metastatic disease: not applicable, haematological cancer Previous VTE: not reported |
|
| Interventions | Intervention 1: low‐dose UFH 2 IU/kg bodyweight/hour Intervention 2: prophylactic LMWH, enoxaparin (ClexaneTM) at a dose of 80–100 IU/kg bodyweight once daily SC with a target anti‐Xa level not exceeding 0.4 U/L, measured 4 hours after the third or fourth injection Intervention 3: activity‐adapted antithrombin throughout induction therapy Thromboprophylaxis started on day 8 and ended on day 33 of induction chemotherapy |
|
| Outcomes | Primary: symptomatic VTE Secondary: major and minor bleeding, event‐free survival, and overall survival |
|
| Notes | Funding: both interventional drugs were provided free of charge by the respective pharmaceutical companies: enoxaparin (Clexane) by Sanofi and antithrombin (Kybernin) by CSL Behring. Neither company was acting as a sponsor, they were not involved in the THROMBOTECT study design, neither in the collection and analysis of data nor in the content and wording of the manuscript. Neither of them had access to the THROMBOTECT data sets nor did they have information on unpublished results. Disclosures of potential conflicts of interest: 3/19 authors had disclosures unrelated to the work under consideration: Martin Schrappe: honoraria: prIMEOncology; research funding: Medac, Baxalta, SigmaTau; Speaker's Bureau: Baxalta; Wolfgang Korte: honoraria: Bayer, Boehringer Ingelheim, Pfizer, Daichii, Abbott, Siemens; consulting, medical advisor: Bayer, Boehringer Ingelheim, Pfizer, Daichii; research funding: CSL Behring; travel expenses: Bayer, Pfizer; Johannes Rischewski: honoraria, medical advisor, research funding: CSL Behring International and Switzerland Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally by the ALL‐BFM study coordination center using computer‐generated random number lists." Comment: adequate method of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally by the ALL‐BFM study coordination center using computer‐generated random number lists" and "The assigned arm was submitted to the center by fax." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "No systematic provision was made for blinding the attending physicians or radiologists to the randomization arm." Comment: open study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. |
Haas 2012.
| Study characteristics | ||
| Methods | Trial acronym: TOPIC‐1 and TOPIC‐2 Design: multicentre RCTs, intention‐to‐treat analysis for effectiveness and modified intention‐to‐treat analysis for safety outcomes. TOPIC‐1 was prematurely halted after an interim analysis. Median duration of follow‐up: not reported |
|
| Participants | Patients with metastatic breast cancer (n = 353) or non‐small‐cell lung carcinoma (n = 547) receiving first‐ or second‐line chemotherapy. Mean age in TOPIC‐1 study (participants with breast cancer): 54.6 (SD 10.3) years in certoparin group; 56.6 (SD 11.0) years in placebo group Mean age in TOPIC‐2 study (participants with lung cancer): 60.8 (SD 9.5) years in certoparin group; 60.3 (SD 10.0) years in placebo group Gender, n (%) males: TOPIC‐1: 0 (0%); TOPIC‐2: 227 (83.2%) overall Metastatic disease: not reported Previous VTE: 0/900 |
|
| Interventions | Intervention: LMWH, certoparin 3000 IU SC, once daily Control: placebo Study treatment given for 6 months. |
|
| Outcomes | Primary outcomes: symptomatic or incidental VTE, major bleeding Secondary outcomes: symptomatic VTE, overall thrombosis rate (to include arterial thrombotic events, superficial venous thrombosis, and central‐line thrombosis), minor bleeding, thrombocytopenia, heparin‐induced thrombocytopenia, osteoporotic fractures, survival Post hoc: mortality, symptomatic or incidental VTE according to tumour stage |
|
| Notes | Funding: grant from Novartis Pharma, Nuremberg, Germany. Quote: "The TOPIC studies were supported by an unrestricted grant from Novartis Pharma GmbH, Germany." Disclosure of potential conflicts of interest, quote: "The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article." Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer‐generated randomisation list" and "Randomization was block‐stratified according to treatment with hormone‐based chemotherapy." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization numbers were allocated sequentially as patients were enrolled at each center." Comment: concealment of allocation was poorly reported. It was not reported if sealed, opaque, and consecutively numbered envelopes, coded syringes, or other methods were used. In addition, it remains unclear what is meant by randomisation number in "Patients were allocated to the lowest available randomisation number available for each study center." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Efficacy outcomes were validated by a blinded, independent Central Thrombosis Evaluation Team; safety end points were validated by a Data Safety Monitoring Committee consisting of 2 clinicians (blinded to treatment) and an independent statistician with access to the treatment assignments." and "Only the external statistician from the Safety Committee had access to the randomization codes." Comment: double‐blind, placebo‐controlled RCT with blinding of participants, physicians, and outcome assessors#. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: for effectiveness, 442/447 (98.9%) in LMWH group and 441/453 (97.4%) in placebo group were analysed. For safety, 447/447 (100%) in LMWH group and 451/453 (99.6%) in placebo group were analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. However, the outcome osteoporotic fracture was incompletely reported; it remained unclear in which of the TOPIC‐2 trial arms the single event occurred. Post hoc analyses were reported transparently. |
Kakkar 2004.
| Study characteristics | ||
| Methods | Trial acronym: FAMOUS Design: double‐blind, placebo‐controlled, multicentre RCT; modified intention‐to‐treat analysis for both effectiveness and safety analyses, including participants with ≥ 1 study dose and 1 follow‐up visit Median duration of follow‐up: 10 months in dalteparin group; 9 months in placebo group |
|
| Participants | Patients aged 18–80 years with histologically confirmed advanced stage III or IV (locally advanced or metastatic) malignant disease of the breast, lung, gastrointestinal tract, pancreas, liver, genitourinary tract, ovary, or uterus. Mean age: 62 (IQR 54–68) years in dalteparin group; 60.9 (IQR 52–69) years in placebo group Gender, n (%) males: 77 (40.5%) in dalteparin group; 84 (45.7%) in placebo group Metastatic disease, n (%): 161 (85%) in dalteparin group; 161 (87.5%) in placebo group Previous VTE: 0/385 (0%) |
|
| Interventions | Intervention: LMWH, dalteparin 5000 IU SC, once daily Control: placebo (0.9% normal saline) Study treatment given for 1 year or until the participant died, whichever occurred sooner |
|
| Outcomes | Primary outcomes: mortality after 1 year of therapy Secondary outcomes: symptomatic, objectively confirmed VTE disease and bleeding complications |
|
| Notes | Funding: Pharmacia Corp, New York, NY Disclosure of potential conflicts of interest: the lead author declared having acted as a consultant for Pfizer. Quote: "The following authors or their immediate family members have indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. Acted as a consultant within the last 2 years: Ajay K. Kakkar, Pfizer. Received more than $2,000 a year from a company for either of the last 2 years: Ajay K. Kakkar, Pfizer." Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally by computer‐generated code." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally by computer‐generated code." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "placebo (0.9% normal saline), each supplied in 0.2‐mL prefilled syringes." Comment: trial reported as double‐blind, with active substance or placebo provided in prefilled syringes. It is not reported whether syringes were identical in appearance or if outcome assessor were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: both for effectiveness and safety, 190/196 (96.9%) participants were analysed in the LMWH group and 184/189 (97.4%) participants were analysed in the placebo group. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. |
Khorana 2017.
| Study characteristics | ||
| Methods | Trial acronym: PHACS Design: multicentre RCT Study terminated early due to poor accrual Median duration of follow‐up: not reported |
|
| Participants | Cancer patients at high risk for VTE (Khorana score ≥ 3) and initiating a new systemic chemotherapy regimen who had no VTE at initial baseline screening compression ultrasonography of the lower extremities and baseline computed tomography of the chest. Mean age: overall 59 years; 60 (SD 10) years in dalteparin group; 58 (SD 12) years in observation group Gender, n (%) males: 29 (58%) in dalteparin group; 24 (50%) in observation group Metastatic disease: not reported Previous VTE, n (%): 4 (8%) in dalteparin group; 2 (4%) in observation group |
|
| Interventions | Intervention: LMWH, dalteparin 5000 IU daily SC for 12 weeks Control: no dalteparin |
|
| Outcomes | Primary outcome: any VTE over 12 weeks. VTE included adjudicated symptomatic lower extremity DVT, PE and upper extremity thrombosis as well as all unsuspected DVT and PE diagnosed by screening ultrasonography and computed tomography tests, respectively, occurring during 12 weeks of the study treatment or observation. Participants in both arms were screened with lower extremity ultrasounds every 4 weeks of study. Primary safety endpoint: clinically relevant bleeding events over 13 weeks. Secondary outcomes: symptomatic VTE, all‐cause mortality Secondary safety outcomes: major bleeding and all bleeding including major, non‐major and minor bleeding events. |
|
| Notes | Funding: not reported. Dalteparin was provided free of charge by Eisai, Inc. Disclosure of potential conflicts of interest: all authors reported conflicts of interest. Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized to either dalteparin 5000 units daily subcutaneously or no prophylactic anticoagulation." Comment: method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomized to either dalteparin 5000 units daily subcutaneously or no prophylactic anticoagulation." Comment: method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study. Quote: "Thrombotic events were adjudicated by a thrombosis adjudication committee, comprising 2 radiologists who reviewed de‐identified imaging studies and were blinded to treatment assignment" and "Bleeding events were adjudicated by a bleeding committee comprising two hematologists who were blinded to treatment assignment." Comment: participants and personnel not blinded. Blinded adjudication of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of 117 enrolled patients, 19 were not randomized due to the presence of VTE on initial screening (N =10, 8.5%) or for other reasons (N = 9)." Comment: all randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes indicated in the methods of the abstract are reported in the results. |
Khorana 2019.
| Study characteristics | ||
| Methods | Trial acronym: CASSINI Design: double‐blind, randomised, placebo‐controlled, parallel‐group, multicentre Phase IIIB study Median follow‐up duration: not reported |
|
| Participants | High‐risk ambulatory patients with solid cancer or lymphoma who had a Khorana score of ≥ 2, had a plan to start a new systemic regimen within 1 week before or after initiating the trial regimen and had no DVT on screening ultrasonography. Enrolled patients underwent venous duplex compression ultrasonography of both legs to rule out pre‐existing proximal DVT. Median age: 63 (range 23–88) years overall; 63 (range 23–87) years in rivaroxaban group; 62 (range 28–88) years in placebo group Gender, n (%) males: 428 (50.9%) overall; 222 (52.9%) in rivaroxaban group; 206 (48.9%) in placebo group Metastatic disease: 54.5% overall in those with solid tumour Previous VTE, n (%): 15 (1.7%) overall; 13 (3.1%) in rivaroxaban group; 2 (0.5%) in placebo group |
|
| Interventions | Intervention: rivaroxaban 10 mg once daily up to day 180 Control: placebo up to day 180 Mean intervention period was 4.3 months |
|
| Outcomes | Primary efficacy endpoint: composite of objectively confirmed symptomatic or asymptomatic lower‐extremity proximal DVT, symptomatic upper extremity, symptomatic lower‐extremity distal DVT, symptomatic or incidental PE, and VTE‐related death Secondary efficacy endpoints: included components of the primary endpoint, symptomatic VTE, death from any cause, confirmed arterial thromboembolism, and confirmed visceral thromboembolism |
|
| Notes | Funding: by Janssen, Bayer, and the Sondra and Stephen Hardis Chair in Oncology Research (to Dr Khorana), by grants (U01HL143402 and R34 HL127156, to Dr Khorana) from the National Heart, Lung, and Blood Institute, and by the Cleveland Clinic Center of Excellence for Cancer‐Associated Thrombosis (to Dr Khorana) and the Porter Family Fund (to Dr Khorana). Disclosure of potential conflicts of interest: all authors reported conflicts of interest Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients without thrombosis were randomly assigned in a 1:1 ratio to receive rivaroxaban at a dose of 10 mg or placebo orally once daily for 180 days (with a window of ±3 days) according to a computer generated randomization schedule." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients without thrombosis were randomly assigned in a 1:1 ratio to receive rivaroxaban at a dose of 10 mg or placebo orally once daily for 180 days (with a window of ±3 days) according to a computer generated randomization schedule." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "… as adjudicated by an independent clinical end‐point committee whose members were unaware of the trial‐group assignments." and "Double‐blind, randomized, placebo‐controlled." Comment: double‐blind, randomised, placebo‐controlled and all endpoints adjudicated by blinded independent committees. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment; all participants enrolled were analysed as reported in the methods. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. |
Klerk 2005.
| Study characteristics | ||
| Methods | Trial acronym: MALT Design: multicentre, double‐blind, randomised, placebo‐controlled study with intention‐to‐treat analyses for both effectiveness and safety, including participants who received ≥ 1 study dose Mean duration of follow‐up: 12 months |
|
| Participants | Patients with metastasised or locally advanced solid tumours Median age: 63 (range 36–86) years in nadroparin group; 64 (range 28–83) years in placebo group Gender, n (%) males: 77 (52%) in nadroparin group; 81 (53%) in placebo group Metastatic disease, n (%): 137 (93%) in nadroparin group; 139 (90%) in placebo group Previous VTE: 0/302 (0%) overall |
|
| Interventions | Intervention: LMWH, nadroparin Control: placebo Prefilled syringes containing a fixed volume of nadroparin (9500 anti‐factor Xa U/mL) or placebo were provided according to participant’s weight: 0.4 mL for those weighing < 50 kg, 0.6 mL for those weighing 50–70 kg, and 0.8 mL for those weighing > 70 kg. Administered SC twice daily during the initial 14 days of treatment and once daily thereafter for another 4 weeks. |
|
| Outcomes | Primary efficacy outcome: death from any cause Primary safety outcome: major bleeding Secondary safety outcome: clinically relevant non‐major bleeding |
|
| Notes | Funding: treatment provided by Sanofi‐Synthelabo (Paris, France). The authors stated that "protocol design, data collection, and analysis were solely the responsibility of the authors." Disclosure of potential conflicts of interest: the senior author and statistician declared consultancy activities for various pharmaceutical companies, including Sanofi‐Synthelabo. Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sequentially numbered boxes of syringes with nadroparin or placebo were prepared using a central computer‐generated randomization schedule, stratified for body weight with blocks of four." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered boxes of syringes with nadroparin or placebo were prepared using a central computer‐generated randomization schedule, stratified for body weight with blocks of four." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Prefilled syringes containing a fixed volume of nadroparin (9,500 antifactor Xa U/mL) or placebo were provided according to patient’s weight." Comment: trial reported as double‐blind, with active substance or placebo provided in prefilled syringes. It was not reported whether syringes were identical in appearance or if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all enrolled participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. The authors reported reasons for the discontinuation of the study drug in the results section only, but this was for descriptive purposes, so unlikely to introduce bias. |
Larocca 2012.
| Study characteristics | ||
| Methods | Trial acronym: substudy of RV‐MM‐PI209 Design: prospective, multicentre, open‐label, randomised substudy of a phase III trial with modified intention‐to‐treat analyses of both effectiveness and safety outcomes, including participants who received ≥ 1 study dose Median follow‐up duration: 20 months |
|
| Participants | Patients with newly diagnosed multiple myeloma treated with lenalidomide and low‐dose dexamethasone induction and melphalan‐prednisone‐lenalidomide consolidation. Median age: 57 (IQR 51–61) years in aspirin group; 58 (IQR 52–62) years in LMWH group Gender, n (%) males: 87 (49%) in aspirin group; 99 (60%) in LMWH group Metastatic disease: not reported Previous VTE: 0/342 (0%) overall |
|
| Interventions | Intervention 1: LMWH, enoxaparin 40 mg/day SC Intervention 2: aspirin 100 mg/day Prophylaxis was provided during the 4 (28‐day) cycles of lenalidomide and low‐dose dexamethasone and the 6 (28‐day) cycles of melphalan‐prednisone‐lenalidomide consolidation. Median treatment duration: 3.6 months in aspirin group; 3.5 months in LMWH group |
|
| Outcomes | Primary endpoint: composite of symptomatic DVT, PE, arterial thrombosis, any acute cardiovascular event, or sudden otherwise‐unexplained death in the first 6 months after randomisation Secondary outcomes: major and minor bleeding, any complications related to thromboprophylaxis |
|
| Notes | Funding: main study (RV‐MM‐PI209) was supported by Fondazione Neoplasie Sangue Onlus, and Celgene supplied free lenalidomide. The authors declared that Celgene had no role in the study design, data analysis, data interpretation, or writing of the report. Disclosure of potential conflicts of interest: several authors declared having received honoraria or consultancy fees from various pharmaceutical companies, including Celgene. Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "simple randomization sequence run by a central computer, which generated an automated assignment procedure that was concealed from the investigators in each study center." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "simple randomization sequence run by a central computer, which generated an automated assignment procedure that was concealed from the investigators in each study center." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Open‐label" study Comment: open study with no blinding of participants, physicians, and outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. |
Lebeau 1994.
| Study characteristics | ||
| Methods | Trial acronym: 02 PC 85, run by the "Petites Cellules" group Design: multicentre, open‐label, randomised substudy, with intention‐to‐treat analyses Median duration of follow‐up: not reported |
|
| Participants | Patients with limited and extensive small‐cell lung cancer who had not been previously treated with chemotherapy or radiotherapy Mean age: not reported overall; 42 (15%) < 50 years; 104 (38%) 50–59 years; 88 (32%) 60–69 years, 44 (16%) 70–81 years Gender, n (%) males: 120 (87%) in heparin group; 132 (95%) in control group Metastatic disease, n (%): extensive disease: 74 (54%) in heparin group; 82 (59%) in control group Previous VTE: not reported |
|
| Interventions | Intervention: chemotherapy with SC UFH. The dose of UFH was initially adapted to weight (500 IU/kg/day) then adjusted by clotting times. UFH was administered in 2 or 3 daily injections for 5 weeks and stopped 1 week after the second course of chemotherapy. Control: chemotherapy without UFH |
|
| Outcomes | Primary outcome: overall survival, response to chemotherapy Secondary outcomes: bleeding, UFH‐related thrombocytopenia |
|
| Notes | Funding: none reported Disclosure of potential conflicts of interest: not disclosed, no COI forms available Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized through a centralized blind telephone assignment procedure." Comment: method of sequence generation not clearly reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "randomized through a centralized blind telephone assignment procedure." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "No bIinding procedure for patients and physicians was used." Comment: open‐label study with no blinding of participants or physicians. Not reported if there was blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient was lost to follow up." Comment: all participants enrolled in the randomised substudy were analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results section. |
Lecumberri 2013.
| Study characteristics | ||
| Methods | Trial acronym: Adjuvant Bemiparin in Small Cell Lung Cancer (ABEL) Design: a multicentre, investigator‐initiated, open‐label, randomised study Study terminated early due to slow recruitment Median duration of follow‐up: not reported |
|
| Participants | Patients with newly diagnosed, limited‐stage small‐cell lung cancer Mean age: 62.7 (SD 8.9) years overall Gender, n (%) males: 33 (87%) males overall Previous VTE: none Metastatic disease: none |
|
| Interventions | Intervention: standard chemoradiotherapy plus bemiparin 3500 IU daily for a maximum of 26 weeks Participants received a median of 26 weeks of LMWH. Bemiparin was started on the first day of the first cycle of chemotherapy and stopped at disease progression or at the end of the 26 weeks of treatment. Control: standard first‐line platinum‐based chemotherapy and radiotherapy |
|
| Outcomes | Primary efficacy outcome: progression‐free survival Primary safety outcome: major bleeding Secondary outcomes: overall survival, tumour response rate to chemoradiotherapy, incidence of objectively confirmed symptomatic VTE, minor bleeding, thrombocytopenia, death from any cause, and incidence of any other adverse event. |
|
| Notes | Funding, quote: "Bemiparin 3,500 IU syringes were provided without charge by Laboratorios Farmacéuticos ROVI. S.A. The company also gave economic support for the expenses of the CRO, but was not directly involved in the design of the study, collection or analysis of the data or in the preparation of the manuscript." Disclosure of potential conflicts of interest: "Drs. Lecumberri and Rocha report receiving investigational grant support and consulting and lecture fees from Rovi. No other potential conflict of interest relevant to this article was reported." Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed through an automatic central randomization system." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed through an automatic central randomization system." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "there was no central adjudication committee." Comment: open study with unblinded adjudication of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 1/39 (2.56%) included participants was excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes indicated in the methods were reported in the results. |
Levine 1994.
| Study characteristics | ||
| Methods | Trial acronym: none reported Design: multicentre, double‐blind, randomised, placebo‐controlled trial; intention‐to‐treat analysis Mean duration of follow‐up: 199 days (SD 126) in warfarin and 188 days (SD 137) in placebo |
|
| Participants | Patients with metastatic stage IV breast carcinoma who had been receiving first‐ or second‐line chemotherapy for 4 weeks or less. Mean age: 57.1 (SD 10.2) years in warfarin group; 56.1 (SD 10.9) years in placebo group Gender (%) females: 100% Metastatic disease: not reported Previous VTE: 0 in warfarin group; 2/159 in placebo group |
|
| Interventions | Intervention: warfarin 1 mg daily for 6 weeks and then adjusted to maintain the INR at 1.3–1.9 Control: placebo Study treatment began either at the start of chemotherapy or within the next 4 weeks and continued until 1 week after termination of chemotherapy. Median treatment duration: 181 (SD 123) days in warfarin group; 166 (SD 139) days in placebo group |
|
| Outcomes | Primary outcomes: VTE and arterial thrombosis; major and minor bleeding Secondary outcome: survival |
|
| Notes | Funding: study supported by a grant‐in‐aid from the National Cancer Institute of Canada Disclosure of potential conflicts of interest: none disclosed, no COI forms available Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a computer‐generated random arrangement." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "neither patients nor doctors were aware of treatment allocation" and "All outcome events were reviewed by a central adjudicating committee, unaware of treatment allocation" and "placebo patients took an identical inert tablet." Comment: adequate blinding of participants, physicians, and outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: for effectiveness and safety, 152/154 (98.7%) in warfarin group and 159/161 (98.8%) in placebo group were analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results. |
Levine 2012.
| Study characteristics | ||
| Methods | Trial acronym: none reported Design: randomised, double‐blind, phase II trial; intention‐to‐treat analyses not reported Trials closed prematurely due to slow accrual rate Median duration of follow‐up: not reported, maximum 114–121 days |
|
| Participants | Patients receiving either first‐ or second‐line chemotherapy for advanced or metastatic lung, breast, gastrointestinal, bladder, ovarian, or prostate cancer; cancer of unknown origin; myeloma; or selected lymphomas. Median age: 57 (range 41–67) years in apixaban 5 mg group, 60 (range 39–76) years in apixaban 10 mg group, 64 (range 25–86) years in apixaban 20 mg group, and 59 (range 20–82) years in placebo group Gender, n (%) males: 15 (46.9%) in apixaban 5 mg group; 13 (43.3%) in apixaban 10 mg group; 20 (60.6%) in apixaban 20 mg group; 15 (50%) in placebo group Metastatic disease (%): advanced or metastatic: 100% Previous VTE: 0/125 (0%) |
|
| Interventions | Intervention: factor Xa inhibitor, apixaban 5 mg, 10 mg, or 20 mg once daily orally Control: placebo Study treatment given for 12 weeks beginning within 4 weeks of starting chemotherapy. Median treatment duration: 79.2 (range 29–90) days in apixaban 5 mg group; 76.0 (range 16–90) days in apixaban 10 mg group; 73.6 (range 14–92) days in apixaban 20 mg group; 69.6 (range 7–91) days in placebo group |
|
| Outcomes | Primary outcome: major bleeding or clinically relevant non‐major bleeding Secondary outcomes: VTE, grade III or higher adverse events related to study drug |
|
| Notes | Funding, quote: "The study was sponsored by Bristol‐Myers Squibb and Pfizer Inc." Disclosure of potential conflicts of interest: no other COI reported, no COI forms available, but 2 of the authors were employees of the sponsor. Publication format: full‐text publication Pilot dose‐finding study of 3 apixaban regimens (5 mg, 10 mg, or 20 mg once daily orally) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed centrally by contacting a computerised telephone voice response system provided by Bristol Myers Squibb." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed centrally by contacting a computerised telephone voice response system provided by Bristol Myers Squibb" and "BMS generated and kept the randomization schedules." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind" study, "treatment groups or all placebo tablets for the placebo treatment group such that the study supplies for subjects in all treatment groups were identical in appearance", and "All bleeding and VTE events were adjudicated by a committee unaware of treatment allocation." Comment: participants, physicians, and outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: for effectiveness and safety, 32/32 (100%) participants analysed in the 5 mg group; 29/30 (96.7%) analysed in the 10 mg group; 32/33 (97%) analysed in the 20 mg group; and 29/30 (96.7%) analysed in the placebo group. None of these excluded participants received study treatment, and we could not rule out that their exclusion was associated with the outcome. In addition to these 3 excluded participants, it also remains unclear why the 5 participants (4%) enrolled after the protocol amendment were not considered in the analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results section. |
Macbeth 2016.
| Study characteristics | ||
| Methods | Trial acronym: FRAGMATIC Design: an open‐label, multicentre, parallel‐group, superiority, randomised phase III trial. Median follow‐up of 23.1 (IQR 3.6–31.2) months |
|
| Participants | Patients with histopathological or cytological diagnosis of primary bronchial carcinoma of any stage and histology (small‐cell or non‐small‐cell) within 6 weeks Median age: 65 (IQR 59–71) years in LMWH group; 64 (IQR 58–71) years in control group Gender, n (%) males: 61 (60.0%) in LMWH group; 656 (59.6%) in control group Metastatic disease, n (%): 670 (60.9%) in LMWH group; 666 (60.5%) in control group Previous VTE: not reported |
|
| Interventions | Intervention: standard anticancer treatment (including active supportive or palliative care) plus dalteparin 5000 IU SC once daily for a maximum of 24 weeks Dalteparin was started as soon as possible and before first definitive anticancer treatment Control: standard anticancer treatment (including active supportive or palliative care) Use of prophylactic anticoagulant outside of trial (short‐term use, e.g. inpatient thromboprophylaxis, and therapeutic anticoagulation were allowed if clinically indicated according to local guidelines), n (%): 106 (9.7%) in LMWH group; 88 (8.0%) in control group |
|
| Outcomes | Primary outcome: overall survival Secondary outcomes: VTE‐free survival, bleeding (major and clinically relevant non‐major), metastasis‐free survival, toxic effects, quality of life, dyspnoea, cost‐effectiveness, and cost utility Compliance with dalteparin was assessed by counting empty syringes at follow‐up visits and from the local pharmacy logs. |
|
| Notes | Funding, quote: "Supported by Cancer Research UK Grant No. CR UK/06/007, an educational grant from Pfizer, and the National Institute for Health Research Cancer Network; sponsored by Velindre National Health Service Trust, Cardiff; and coordinated by the Cancer Research UK core‐funded Wales Cancer Trials Unit at Cardiff University." Disclosure of potential conflicts of interest: some of the authors reported COI. Publication format: full‐text publication Quote: "The trial did not reach its intended number of events for the primary analysis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned by the Wales Cancer Trials Unit in a 1:1 ratio to receive either LMWH or no LMWH, by use of a computer algorithm using the method of minimization and a random element." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was by research nurses (who recruited patients) telephoning the Wales Cancer Trials Unit, where randomization and treatment allocation was done by a trial/data manager using a computerized system." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The study had an open‐label design." Comment: not reported if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were performed using intention to treat." Comment: for the analysis of the primary outcomes and most of the secondary outcomes, all randomised participants were apparently included in the analysis. For the evaluation of compliance with LMWH, 977/1101 participants were assessed. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes indicated in the methods were reported in the results of the main or related papers. |
Maraveyas 2012.
| Study characteristics | ||
| Methods | Trial acronym: FRAGEM Design: phase IIb RCT; intention‐to‐treat analyses not reported Median duration of follow‐up: 19.3 months |
|
| Participants | Patients with non‐resectable, recurrent, or metastatic pancreatic adenocarcinoma Median age: 63 (range 40–82) years overall Gender, n (%) males: 72 (59%) overall Metastatic disease (%): 54% overall Previous VTE: 0/123 (0%) overall |
|
| Interventions | Intervention: LMWH, dalteparin 200 IU/kg once daily, SC for 4 weeks followed by a stepdown to 150 IU/kg for a further 8 weeks and gemcitabine Continuing dalteparin prophylaxis beyond 12 weeks was not recommended, but was left to the discretion of the investigator. Control: gemcitabine with no dalteparin |
|
| Outcomes | Primary outcome: reduction of all‐type vascular thromboembolism during the study period. All‐type vascular thromboembolism included DVT, PE, all arterial events (e.g. cerebrovascular accident/myocardial infarction), and all visceral thromboembolic events diagnosed on the basis of clinical symptomatology, postmortem, or incidentally. Outcome data kindly provided by the authors: VTE |
|
| Notes | Central venous access devices and inferior vena cava filters were not allowed. Funding: the Hull and East Yorkshire Hospitals National Health Service Trust; Pfizer provided a grant covering the cost of dalteparin; Lilly provided a grant covering the cost of biostatistics. Disclosure of potential conflicts of interest: the lead author has received honoraria and participated on advisory boards for Pfizer. Another author received travel expenses from Pfizer. None of the other authors had any conflicting interests. Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in the facilities of the Postgraduate Medical Institute in Hull with software developed by York University". Allocation and stratification were done through remote telephone "block" randomisation (personal communication). Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Comment: performed centrally at the Medical Institute in Hull for all of the 7 recruiting sites. Allocation and stratification were done through remote telephone "block" randomisation (personal communication). |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: open study (personal communication). Not reported if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: for effectiveness and safety, 59/60 (98.3%) were analysed in the LMWH group, and 62/63 (98.4%) were analysed in the control group. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results section. |
Maurer 1997.
| Study characteristics | ||
| Methods | Trial acronym: CALGB Protocol 8534, run by the Cancer and Leukemia Group B study Design: multicentre RCT; intention‐to‐treat analyses not reported Median duration of follow‐up: 69 months in those still alive |
|
| Participants | Patients with limited‐stage small‐cell lung cancer receiving chemotherapy and radiotherapy Participants aged ≥ 60 years: 57.6% Gender, n (%) males: 225 (64.8%) Metastatic disease: none Previous VTE: not reported |
|
| Interventions | Intervention: warfarin 10 mg/day for the first 3 days and then at a dose to maintain the prothrombin time between 1.4 and 1.6 times the local institutional control standards Control: no warfarin Warfarin was continued through the complete course of chemotherapy and radiotherapy and stopped 3 weeks after the last cycle of chemotherapy. Warfarin was administered for a median of 112.5 days. |
|
| Outcomes | Primary outcomes: overall survival and cancer response to therapy Secondary outcomes: failure‐free survival, disease‐free survival, patterns of relapse, toxicity |
|
| Notes | Funding: grants from the National Cancer Institute, Bethesda, MD Disclosure of potential conflicts of interest: not reported, no COI forms available Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to receive warfarin or no warfarin." Comment: method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized to receive warfarin or no warfarin." Comment: method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: it is not reported whether participants, physicians, and outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Table 6 of the study full‐text indicated that not all randomised participants were analysed, but the exact numbers were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: only the outcomes of overall survival and complete tumour response were specified in the methods section. All other outcomes were addressed in the results section only, including the survival analyses at 8 months, and 2, 3, and 4 years. Only the 8 months' analyses were reported to be exploratory. |
Meyer 2018.
| Study characteristics | ||
| Methods | Trial acronym: TILT Design: randomised, multicentre, open, controlled trial with blinded adjudication of outcome Median follow‐up: 5.7 years |
|
| Participants | Patients with completely resected stage I, II, or IIIA non‐small‐cell lung cancer Mean age: 61.6 (SD 9.0) years in tinzaparin group; 61.6 (SD 8.8) years in control group Gender, n (%) males: 167 (62.1%) in tinzaparin group; 189 (67.5%) in control group Metastatic disease n (%): 0; 190 (34.6%) participants had stage II–III disease, and 220 (40.1%) participants received adjuvant chemotherapy Previous VTE: not reported |
|
| Interventions | Intervention: tinzaparin (Innohep, Leo Pharma France) 100 IU/kg SC once a day for 12 weeks in addition to standard of care Control: standard of care |
|
| Outcomes | Primary outcome: overall survival Secondary outcomes: serious bleeding recorded during the 12‐week treatment period in tinzaparin group or corresponding period in control group, recurrence‐free survival, cancer‐related mortality, and symptomatic VTE recorded during the whole follow‐up period |
|
| Notes | The study was supported by 2 grants issued by the French Ministry of Health (PHRC AOM05185 and PHRC AOM12612). Leo Pharma provided the study drug and a complementary grant. Disclosure of potential conflicts of interest: the first author received research funding and paid travel expenses from Leo Pharma. The last author received honoraria for consultancy and paid travel expenses from Leo Pharma. All other authors declared no relevant COI. Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned in a 1:1 ratio to receive either tinzaparin or no anticoagulants according to a list of randomisation numbers with treatment assignments. This list was computer‐generated, used alternate blocks of small size (2,4,6) to make it unpredictable and was stratified according to centre and tumour stage (I versus II‐III). An Internet application (CleanWeb) allowed central randomisation." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly assigned in a 1:1 ratio to receive either tinzaparin or no anticoagulants according to a list of randomisation numbers with treatment assignments. This list was computer‐generated, used alternate blocks of small size (2,4,6) to make it unpredictable and was stratified according to centre and tumour stage (I versus II‐III). An Internet application (CleanWeb) allowed central randomisation." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The trial was open‐label with blinded central adjudication of study outcomes." and "All suspected outcome events and deaths were adjudicated by an independent clinical events committee whose members were unaware of treatment assignment." Comment: open‐label study with blinded adjudication of outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 4/553 (0.7%) participants enrolled in the study were not considered for the analysis. Exclusions per trial arm were reported. 2 (0.4%) participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. |
Mitchell 2003.
| Study characteristics | ||
| Methods | Trial acronym: PARKAA Design: multicentre, open, phase II RCT; per‐protocol analysis Median duration of follow‐up: not reported |
|
| Participants | Children newly diagnosed with acute lymphoblastic leukaemia treated with L‐asparaginase and a functioning central venous line placed within 2 weeks of initiating induction chemotherapy. Median age: 3.8 (range 1.6–17.2) years in antithrombin group; 5.9 (range 1.9–16.7) years in control group Gender, n (%) males: 15 (60%) in antithrombin group; 37 (61.7%) in control group Metastatic disease: not applicable, haematological cancer Previous VTE: not reported |
|
| Interventions | Intervention: Thrombate III, a sterile, lyophilised preparation of purified human antithrombin manufactured and supplied by Bayer Corporation, USA. Antithrombin infused once weekly for 4 weeks to increase plasma concentrations of antithrombin to approximately 3.0 U/mL but no more than 4.0 units/mL Control: standard care |
|
| Outcomes | Primary outcomes: clinically symptomatic or incidental thrombotic event in any location; major and minor bleeding Secondary outcome: surrogate outcome for thrombotic events by measuring markers of thrombin generation |
|
| Notes | Participants received small amounts of UFH for prophylaxis of central venous line blockage either by continuous infusion (1–3 U/mL) or intermittent flushes (50–100 U/mL up to 4 times per day) according to local standard of care. Funding: study was supported by a grant from the Canadian Institutes of Health Research and Bayer Inc. Disclosure of potential conflicts of interest: not reported, no COI forms available Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by the pharmacist‐on‐call using a computer generated random number list." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators at participating centres were blinded to the randomisation code and unaware of patient treatment allocation until after patients had been randomised." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The PARKAA study was an open, randomised, multi‐centre extended phase II clinical study" and "The thrombotic events outcomes were adjudicated centrally by committees consisting of physicians with appropriate expertise, who were not involved with study patients’ care and were blinded to treatment groups." Comment: participants and physicians were not blinded, whereas outcome assessors were. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: for effectiveness and safety, 25/37 (67.6%) participants were analysed in the antithrombin group, and 60/72 (83.3%) participants were analysed in the control group. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results section. |
Palumbo 2011.
| Study characteristics | ||
| Methods | Trial acronym: substudy of GIMEMA MM‐BO2005 and GIMEMA‐MM‐03‐05 Design: randomised, open‐label, multicentre study; modified intention‐to‐treat analysis, including participant receiving ≥ 1 study dose The trial sampled participants from 2 distinct RCTs, of which participants who received thalidomide‐based regimens were eligible to the substudy randomising antithrombotic prophylaxis treatments Median follow‐up time: 24.9 months |
|
| Participants | Patients with previously untreated myeloma who received thalidomide‐containing regimens and had no clinical indication or contraindication for a specific antiplatelet or anticoagulant therapy Median age: 61 (range 55–66) years in aspirin group; 60 (range 54–66) years in warfarin group; 62 (range 55–66) years in heparin group Gender, n (%) males: 117 (53%) in aspirin group; 115 (52%) in warfarin group; 130 (59%) in heparin group Metastatic disease: not reported Previous VTE: none |
|
| Interventions | Intervention 1: aspirin 100 mg/day Intervention 2: low‐dose warfarin (1.25 mg/day) Intervention 3: LMWH (enoxaparin 40 mg/day) Prophylaxis was administered during the 3 cycles of induction therapy in participants aged ≤ 65 years and during the first 6 cycles of induction therapy in participants aged > 65 years. Median treatment duration: 2.6 months in aspirin group; 2.4 months in low‐dose warfarin group; 2.6 months in LMWH group |
|
| Outcomes | Primary outcomes: a composite measure of a first episode of objectively confirmed symptomatic DVT, PE, arterial thrombosis, acute myocardial infarction or stroke, or sudden, otherwise‐unexplained death during the first 6 months from random assignment Secondary outcomes: each component of the composite primary endpoint; long‐term cumulative incidence of the primary endpoint; major and minor bleeding events; any toxicity that required interruption of study prophylaxis |
|
| Notes | Funding: none reported Disclosure of potential conflicts of interest: several authors reported paid consultant or advisory roles, honoraria, and research funds that were relevant to the subject matter under consideration in their trial report. Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A simple random assignment sequence was generated by a centralized computer." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "After registration in a centralized database through the Internet and validation of eligibility, patients were randomly allocated to treatments using an automated assignment procedure concealed to the investigators." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "open‐label." Comment: this was an open‐label study. It is not reported whether outcomes were assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: for effectiveness and safety, 220/224 (98.2%) participants in aspirin group, 220/222 (99.1%) participants in warfarin group, and 219/221 (99.1%) participants in LMWH group were analysed. In addition, 1 participant was not randomised by "clinician mistake." |
| Selective reporting (reporting bias) | High risk | Comment: the outcome "any toxicity that required interruption of study prophylaxis" was not reported in the final report. |
Pelzer 2015.
| Study characteristics | ||
| Methods | Trial acronym: CONKO 004 Design: open‐label, multicentre RCT; intention‐to‐treat and per‐protocol analyses Median follow‐up: 30.4 weeks |
|
| Participants | Outpatients with histologically confirmed advanced pancreatic cancer treated with first‐line chemotherapy Median age: 62 (range 32–81) years in enoxaparin group; 63 (range 27–83) years in control group Gender, n (%) males: 91 (57%) in enoxaparin group; 94 (62%) in control group Metastatic disease, n (%): 119 (74%) in enoxaparin group; 118 (78%) in control group Previous VTE: not reported |
|
| Interventions | Intervention: LMWH, enoxaparin (1 mg/kg once daily) for 3 months started simultaneously to palliative systemic chemotherapy Control: no enoxaparin Quote: "After 3 months of initial enoxaparin use at half the therapeutic dosage (time point of primary end point), treatment was continued with a fixed dose of 40 mg daily until disease progression." |
|
| Outcomes | Primary outcome: symptomatic VTE within 3 months after random assignment Secondary outcomes: progression‐free survival; overall survival; overall symptomatic VTE after 6, 9, and 12 months; major bleeding Additional outcomes reported in related references: incidental DVT during months 6, 9, and 12; toxicity of the therapeutic regimen; time to cancer progression; remission at 3, 6, 9, and 12 months; quality of life |
|
| Notes | Funding, quote: "Supported by Charité–Forschungsförderung, Arbeitsgemeinschaft Internistische Onkologie, Deutsche Krebsgesellschaft, Amgen, Eli Lilly, and sanofi‐aventis, which provided enoxaparin free of charge." Disclosure of potential conflicts of interest: quote: "Employment or Leadership Position: None Consultant or Advisory Role: Helmut Oettle, Celgene (C), Eli Lilly (C), Fresenius (C); Hanno Riess, sanofi‐aventis (C) Stock Ownership: None Honoraria: Helmut Oettle, Celgene; Hanno Riess, sanofi‐aventis, Roche, Amgen, Bayer, Novartis, Eli Lilly Research Funding: Helmut Oettle, Celgene, Eli Lilly Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: Uwe Pelzer, sanofi‐aventis, Roche, Eli Lilly, Amgen; Jens M. Stieler, sanofi‐aventis, Roche, Eli Lilly, Amgen." Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random numbers generated at the study coordination center at the Charité–Universitätsmedizin Berlin." Comment: adequate method of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "computer‐generated random numbers generated at the study coordination center at the Charité–Universitätsmedizin Berlin." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "All symptomatic VTEs and major hemorrhages were documented using the serious adverse event form, centrally reviewed and evaluated by an independent, blinded event review board." Comment: open‐label study, with blinded outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | High risk | Comment: some of the outcomes indicated in the related reports or in the main article (quality of life) are not reported. |
Perry 2010.
| Study characteristics | ||
| Methods | Trial acronym: PRODIGE Design: phase III, randomised, placebo‐controlled trial; intention‐to‐treat analysis Median duration of follow‐up: not reported, planned follow‐up up to 12 months |
|
| Participants | Patients aged > 18 years with newly diagnosed, pathologically confirmed WHO grade 3 or grade 4 glioma Mean age: 57 (range 30–81) years in dalteparin group; 55 (26–77) years in placebo group Gender, n (%) males: 61 (62%) in dalteparin group; 50 (57%) in placebo group Metastatic disease: not reported Previous VTE: none |
|
| Interventions | Intervention: LMWH, dalteparin (5000 IU SC, once daily) Control: placebo Study treatment given for 6 months starting within the first month after surgery. Participants were allowed to continue study medication for 12 months. Median treatment duration: 183 days in LMWH group; 157 days in placebo group |
|
| Outcomes | Primary outcomes: objectively documented symptomatic DVT or PE occurring during the 6 months postrandomisation Secondary outcomes: major and all bleeding, quality of life, cognition assessments, and death |
|
| Notes | Funding: Pfizer Inc, Ontario Clinical Oncology Group, Crolla Chair in Brain Tumour Research Disclosure of potential conflicts of interest: the lead author disclosed research support (and funding) by Pfizer Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer‐generated randomization list." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Consenting patients were randomized by contacting the Ontario Clinical Oncology Group (OCOG) Coordinating and Methods Centre at the Henderson Research Centre." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "In our study, investigators, patients and outcome assessors were blinded to treatment allocation. In addition, VTE and bleeding outcomes were adjudicated by a central committee unaware of treatment assignment." Comment: participants, physicians, and outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | High risk | Comment: the outcomes quality of life and cognition assessment were mentioned in the methods but not reported in the results. |
Sideras 2006.
| Study characteristics | ||
| Methods | Trial acronym: none reported, trial of the North Central Cancer Treatment Group and Mayo Clinic Design: multicentre, placebo‐controlled 2‐arm randomised study; type of analyses not reported After 52 accrued participants, the study was modified because of concerns that the low accrual rate was related to the requirements for placebo injections. The saline placebo injections were eliminated, then, unblinded LMWH was compared with standard clinical care (with 89 more participants accrued after that point). Median duration of follow‐up: not reported, planned minimum follow‐up of 18 months |
|
| Participants | Patients with advanced breast cancer who had failed first‐line chemotherapy; advanced prostate cancer who had failed primary hormonal therapy; advanced lung cancer; or advanced colorectal cancer. Median age: 64.5 years in for blinded LMWH group; 63.5 years in placebo group; 68.5 years in unblinded LMWH group; 70.5 years in standard care group. SDs not reported Gender, n (%) males: 12 (50%) in blinded LMWH group; 11 (42%) in placebo group; 28 (64%) in unblinded LMWH group; 31 (70%) in standard care group Metastatic disease, n (%): not reported, but all had advanced incurable cancer Previous VTE, n (%): 1 (4%) in blinded LMWH group; 1 (4%) in placebo group; 2 (5%) in unblinded LMWH group; 0 (0%) in standard care group |
|
| Interventions |
First part of the study, double‐blind (52 participants): LMWH, dalteparin (5000 IU SC, once daily) plus standard care Control: placebo (saline injections) plus standard care Second part of the study, open (86 participants): LMWH, dalteparin (5000 IU SC, once daily) plus standard care Control: standard care alone Duration: 18 weeks or until disease progression |
|
| Outcomes | Primary outcome: overall survival Secondary outcomes: toxic effects, incidence of thromboembolic events, changes in quality of life |
|
| Notes | Funding: Public Health Services grants from the National Cancer Institute, Department of Health and Human Services Disclosure of potential conflicts of interest: not reported and no COI forms available Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization processes applied were handled through the North Central Cancer Treatment Group (NCCTG) Randomization Office." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: the study used a double‐blind design in the first part of the trial, and an open‐label design in the second part. It is not reported if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: for effectiveness and safety, 68/69 (98.6%) participants were analysed in the LMWH group, and 70/72 (97.2%) were analysed in the placebo group. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion sections. |
Vadhan‐Raj 2013.
| Study characteristics | ||
| Methods | Trial acronym: none reported, registry Identifier of NCI CTRP: NCI‐2011‐01773 Design: randomised, open‐label, parallel‐group trial Median duration of follow‐up: not reported |
|
| Participants | Patients aged ≥ 18 years with a diagnosis of advanced stage (unresectable or metastatic) adenocarcinoma of the pancreas planning to initiate systemic chemotherapy within 2 weeks, ECOG performance status 0–2, adequate renal function (creatinine clearance > 50 mL/minute). Mean age: 52 (range 36–77) years overall; 59 (range 36–75) years in dalteparin group; 64 (range 38–77) years in control group Gender, n (%) males: 41 (54.7%) males overall; 20 (52.6%) in dalteparin group; 21 (56.8%) in control group Metastatic disease: not reported Previous VTE: not reported |
|
| Interventions | Intervention: LMWH, dalteparin (5000 IU SC, once daily) for 16 weeks during chemotherapy Control: chemotherapy alone |
|
| Outcomes | Primary outcome: venous thromboembolic events during 16 weeks of treatment Other outcomes mentioned in the abstract: adverse events, clinically significant bleeding, overall survival |
|
| Notes | Funding: not reported; however, Eisai Inc. is listed as collaborator at ClinicalTrials.gov Disclosure of potential conflicts of interest: not reported Publication format: published conference abstract Baseline characteristics and overall VTE outcome data available at clinicaltrials.gov/ct2/show/results/NCT00966277. The trial database was used as source for data extraction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomized 1:1 to dalteparin vs control arms." Comment: method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "were randomized 1:1 to dalteparin vs control arms." Comment: method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: open‐label study. It is not reported in the abstract if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 75 patients were evaluable for response in an intent‐to‐treat analysis." Comment: all randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study not published as full report yet. The conference abstract did not address all planned outcomes in sufficient detail. |
van Doormaal 2011.
| Study characteristics | ||
| Methods | Trial acronym: INPACT Design: multicentre, open‐label RCT; intention‐to‐treat analyses for mortality Median duration of follow‐up: 10.4 months |
|
| Participants | Patients with non‐small‐cell lung cancer (stage IIIB), hormone‐refractory prostate cancer, or locally advanced pancreatic cancer Age, mean: 65 (SD 10) years in nadroparin group; 65 (SD 9.8) years in no‐nadroparin group Gender, n (%) males: 197 (81%) in nadroparin group; 206 (80%) in no‐nadroparin group Metastatic disease in prostate cancer, n (%): 73 (73.7%) in nadroparin group; 85 (87.6%) in no‐nadroparin group Previous VTE: none |
|
| Interventions | Intervention: LMWH, nadroparin in addition to standard anticancer treatment SC nadroparin was administered for 6 weeks (2 weeks at therapeutic dose and 4 weeks at half therapeutic dose). Participants were eligible to receive additional cycles of nadroparin (2 weeks at therapeutic dose and 4 weeks of washout period) for a maximum of 6 cycles. Mean duration of treatment: 12.6 weeks Control: standard anticancer treatment (no nadroparin) |
|
| Outcomes | Primary efficacy outcome: all‐cause mortality Primary safety outcome: major bleeding Secondary efficacy outcomes: time to disease progression, clinically relevant non‐major bleeding, VTE, arterial thromboembolic events |
|
| Notes | Funding: the study was supported by a grant from GlaxoSmithKline (Paris, France). Disclosure of potential conflicts of interest: 2 authors reported consultant or advisory roles, honoraria, and research funds that were relevant to the subject matter under consideration in their trial report. Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation of treatment proceeded centrally by using an interactive‐voice response system." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation of treatment proceeded centrally by using an interactive‐voice response system." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "all study outcomes were adjudicated by an independent, blinded committee." Comment: open study with blinded outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: for effectiveness and safety, the overall percentage of participants enrolled and subsequently excluded from the analysis was 2.2% (11/503). |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were addressed in the results or discussion section. |
Zacharski 1981.
| Study characteristics | ||
| Methods | Trial acronym: Veterans Administration Study No. 75 Design: multicentre RCT, type of analyses not reported Median duration of follow‐up: not reported, maximum follow‐up was approximately 95 weeks in warfarin group and 94 weeks in control group (approximated from figure) |
|
| Participants | Patients with small‐cell lung carcinoma treated with chemotherapy and radiotherapy Mean age: 58.9 (SD not reported) in warfarin group: 59.8 (SD not reported) in control group Gender, n (%) males: 50 (100%) Metastatic disease: extensive cancer in 13 (52%) in warfarin group; 12 (48%) in control group Previous VTE: not reported |
|
| Interventions | Intervention: warfarin at doses to prolong the prothrombin time to approximately 2 times the control value Control: no warfarin Median duration of warfarin administration: 27 weeks |
|
| Outcomes | Primary efficacy outcomes: survival and cancer response to treatment | |
| Notes | Funding: VA Cooperative Studies Program Disclosure of potential conflicts of interest: not reported, no COI forms available Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjected to computer randomization." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "subjected to computer randomization." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: it is not reported whether participants, physicians, and outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient has been lost to follow‐up." Comment: all enrolled participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: bleeding was addressed in the results section, but not mentioned in the methods section. |
Zwicker 2013.
| Study characteristics | ||
| Methods | Trial acronym: MicroTEC Design: 3‐arma randomised, multicentre phase II study; use of intention‐to‐treat analyses reported Median duration of follow‐up: 2 months for the primary efficacy endpoint |
|
| Participants | Patients with histologically confirmed advanced‐stage malignancy for which standard curative therapies did not exist. Eligible malignancies included: adenocarcinoma of the pancreas (locally advanced or metastatic), colorectal (stage IV), non‐small‐cell lung cancer (stage III or IV), relapsed or stage IV ovarian cancer, or surgically unresectable or metastatic gastric adenocarcinoma. Median age: 68.1 (range 46.6–80.1) years in LMWH group; 67.5 (range 28.8–78.7) years in observation group Gender, n (%) males: 14 (61%) in LMWH group; 5 (46%) in observation group Metastatic disease: 52 (78.8%) overall across 3 trial arms Previous VTE: none |
|
| Interventions | Intervention: LMWH, enoxaparin (40 mg SC, once daily) Control: observation Treatment was given for 2 months |
|
| Outcomes | Primary efficacy outcome: cumulative incidence of VTE (i.e. any symptomatic proximal or distal lower extremity DVT, incidental proximal DVT, symptomatic PE, or fatal PE) at 2 months Primary safety outcome: major bleeding Secondary: toxicity and survival |
|
| Notes |
a2/3 trial arms with high tissue factor‐bearing microparticles (TFMP) were considered in this review. The trial arm with low TFMP without enoxaparin was excluded. Funding, quote: "the study was supported by grants from the National Institutes of Health, K23 HL84052 (JIZ) and R01 HL095084 (BF), as well as a research grant from Sanofi." Disclosure of potential conflicts of interest: 1 author had served on steering committees for Sanofi, and another had received research funds and served on advisory boards for Sanofi and Eisai Publication format: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomized (2:1) to enoxaparin 40 mg subcutaneously once daily or observation." Comment: method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Study coordination, randomization, and monitoring were performed by the Quality Assurance Office for Clinical Trials (QACT) at Dana Farber/Harvard Cancer Center." Comment: method of allocation concealment not clearly specified. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: both the treating physicians and participants in the observation arms were blinded to microparticle status. However, participants in the control group were only observed; the use of placebo, blinding method, or an independent and blinded adjudication committee was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants were included in the analysis. 4/70 participants initially enrolled were excluded prior to randomisation. |
| Selective reporting (reporting bias) | High risk | Comment: the outcome toxicity was not reported in the results section. |
COI: conflict of interest; DVT: deep vein thrombosis; ECOG: Eastern Cooperative Oncology Group; INR: international normalised ratio; IQR: interquartile range; LMWH: low‐molecular‐weight heparin; n: number of participants; PE: pulmonary embolism; RCT: randomised controlled trial; SC: subcutaneous; SD: standard deviation; UFH: unfractionated heparin; uLMWH: ultra‐low‐molecular‐weight heparin; VTE: venous thromboembolism; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baz 2005 | Not an RCT. |
| Bergqvist 1983 | Perioperative thromboprophylaxis. |
| Bocharov 2011 | Not an RCT and study included surgical patients. |
| Eichinger 2008 | Inadequate population: hospitalised cancer patients. |
| Groen 2019 | Other: none of the primary outcomes of this review were reported. |
| Haas 2011 | Inadequate population: hospitalised cancer patients. |
| Heilmann 1995 | Perioperative thromboprophylaxis. |
| Hills 1972 | Perioperative thromboprophylaxis. |
| Kessler 2011 | Not an RCT. |
| Macintyre 1974 | Perioperative thromboprophylaxis. |
| Maxwell 2000 | Perioperative thromboprophylaxis. |
| Meister 2008 | Not an RCT. |
| Minnema 2004 | Not an RCT. |
| NCT00004875 | Prophylaxis for catheter‐related thrombosis. |
| NCT00031837 | Study was terminated early, and no results were posted on ClinicalTrials.gov (accessed at clinicaltrials.gov/ct2/show/NCT00031837 on 13 June 2013). |
| NCT00662688 | Study terminated. No published data available and results not reported in ClinicalTrials.gov. |
| NCT00790452 | Study was terminated early because of a drug supply issue. Results of a single participant were posted (accessed at clinicaltrials.gov/ct2/show/results/NCT00790452 on 11 December 2012). |
| NCT04106700 | Not an RCT. |
| NCT04352439 | Not an RCT. |
| Niesvizky 2007 | Inadequate type of intervention: antiplatelet agent vs placebo. |
| Paydas 2008 | Not an RCT. |
| Poniewierski 1988 | Inadequate population: hospitalised cancer patients. |
| Rajan 1995 | Inadequate outcomes. |
| Salat 1990 | No outcome data extractable and unlikely that trial will be published as full report in future. |
| Sideras 2007 | Perioperative thromboprophylaxis. |
| Storrar 2019 | Not an RCT. |
| Weber 2008 | Inadequate population: hospitalised cancer patients. |
| Welti 1981 | Perioperative thromboprophylaxis. |
| Zangari 2003 | Not an RCT. |
| Zwicker 2019 | Inadequate type of intervention: flavonoid. |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Ciftci 2012.
| Methods | Single‐centre, randomised study |
| Participants | Patients with lung cancer |
| Interventions | Intervention: warfarin in addition to standard anticancer treatment. Warfarin orally for 6 months starting on day 1 of chemotherapy at a dose of 5 mg/day to achieve a target international normalised ratio of 1.5–2.5 Control: standard anticancer treatment |
| Outcomes | No clear distinction between primary and secondary outcomes. Outcomes reported in the abstract: overall median survival, response rates (complete and partial), bleeding |
| Notes | Reason to be listed as awaiting classification: no outcome data extractable, trial not yet published as full article. |
NCT00771563.
| Methods | Open‐label RCT |
| Participants | Patients with locally advanced or metastatic non‐small‐cell lung cancer (stage IIIB or IV) who were not candidates for radical combined‐modality treatments or high‐dose radiotherapy |
| Interventions | Intervention: chemotherapy (cisplatin + docetaxel) and enoxaparin 1 mg/kg/day SC Control: chemotherapy (cisplatin + docetaxel) |
| Outcomes | Primary outcome: progression‐free survival Secondary outcomes: symptom control evaluated with the Lung Cancer Symptoms Scale, overall survival, best overall response, incidence of total documented thromboembolic and haemorrhagic events, overall safety, and tolerability |
| Notes | ClinicalTrials.gov identifier: NCT00771563 Reason to be listed as awaiting classification: no outcome data extractable, trial not yet published as full article. |
RCT: randomised controlled trial; SC: subcutaneous.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐08000267.
| Study name | The role of LMWH combined with TACE in hepatocellular carcinoma |
| Methods | Randomised parallel controlled trial Phase: postmarket |
| Participants | Adults with hepatocellular carcinoma that is not amenable to surgical resection, liver transplantation, or local ablative therapy; without metastasis out of liver |
| Interventions | Intervention: hypodermic injection LMWH 4100 IU each 12 hours in 6 weeks Control: no intervention |
| Outcomes | Primary outcomes: survival time; survival time with no tumour progression Secondary outcome: response rate |
| Starting date | 1 December 2008 |
| Contact information | Jiamei Yang, yang‐jia‐mei@163.com |
| Notes | Recruitment status: completed Primary sponsor: Shanghai Eastern Hepatobiliary Surgery Hospital |
NCT00718354.
| Study name | Randomized, phase III‐b, multi‐centre, open‐label, parallel study of enoxaparin (low molecular weight heparin) given concomitantly with chemotherapy vs chemotherapy alone in patients with inoperable gastric and gastro‐oesophageal cancer |
| Methods | Randomised, open‐label, multicentre study. Methods of randomisation and allocation concealment unclear |
| Participants | Patients with inoperable (locally advanced) or metastatic newly diagnosed gastric or gastro‐oesophageal cancer |
| Interventions | Intervention: LMWH, enoxaparin (1 mg/kg SC once daily) in addition to standard chemotherapy up to 6 months Control: standard chemotherapy (up to 6 cycles) |
| Outcomes | Primary outcome: event‐free survival (composite endpoint of overall survival plus free of symptomatic VTE) Secondary outcomes: incidence of symptomatic VTE, overall survival, major and minor bleeding during chemotherapy or up to 30 days after last dose is provided, serious adverse events, all reported adverse events, HIT |
| Starting date | July 2008 |
| Contact information | Maganji JM, mmaganji@tri‐london.ac.uk |
| Notes |
NCT00718354 Study status in ClinicalTrials.gov is "complete." |
NCT01518465.
| Study name | Dalteparin, lenalidomide, and low‐dose dexamethasone in treating patients with previously untreated multiple myeloma |
| Methods | Randomised, open‐label, pilot phase II trial |
| Participants | Patients with a diagnosis of active multiple myeloma requiring treatment |
| Interventions | Intervention: dalteparin 5000 IU SC once daily on days 1–28; lenalidomide on days 1–21; and low‐dose dexamethasone on days 1, 8, 15, and 22 Control: dalteparin 200 IU/kg SC on days 1–21 |
| Outcomes | Primary outcome: number of participants who experienced grade 4 haemorrhage regardless of attribution, or grade 3 haemorrhage that is possibly, probably, or definitely attributable to dalteparin Secondary outcome: toxicities observed at each dose level |
| Starting date | January 2012 |
| Contact information | Ann Mohrbacher |
| Notes |
NCT01518465 The study status in ClinicalTrials.gov is "terminated" due to insufficient accrual. |
NCT02285738.
| Study name | Anti‐platelet and statin therapy to prevent cancer‐associated thrombosis: a pilot study |
| Methods | Open‐label, parallel‐assignment RCT |
| Participants | Patients with a histological diagnosis of malignancy of a solid organ or lymphoma who have a VTE risk score of ≥ 1 and will be initiating a new systemic chemotherapy regimen |
| Interventions | Intervention 1: aspirin Intervention 2: simvastatin Control: observation |
| Outcomes | Primary outcome: change in average soluble P‐selectin levels Secondary outcomes: major bleeding complications or clinically significant non‐bleeding complications; change in circulating biomarkers; thrombotic events including venous thrombosis, PE, visceral vein thrombosis; arterial thromboembolic events including stroke, myocardial infarction, or arterial embolism |
| Starting date | December 2014 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT02285738 |
NCT02555878.
| Study name | Efficacy and safety of rivaroxaban prophylaxis compared with placebo in ambulatory cancer patients initiating systemic cancer therapy and at high risk for venous thromboembolism |
| Methods | Multicentre, randomised, double‐blind (participant, carer, investigator), placebo‐controlled, parallel‐group superiority study |
| Participants | Patients with histologically confirmed solid malignancy including but not limited to: pancreas, lung, stomach, colon, rectum, bladder, breast, ovary, renal, or lymphoma (haematological), with locally advanced or metastatic disease who have a Khorana thromboembolic risk score ≥ 2 |
| Interventions | Intervention: rivaroxaban 10 mg tablet orally once daily for 180 days Control: placebo |
| Outcomes | Primary efficacy outcomes: symptomatic and incidental lower extremity proximal DVT, symptomatic upper extremity DVT, symptomatic non‐fatal PE, incidental PE, VTE‐related death Primary safety outcome: major bleeding Secondary outcomes: symptomatic VTE and VTE‐related deaths, all‐cause mortality, clinically relevant non‐major bleeding, minor bleeding, any bleeding adverse events, and serious adverse events |
| Starting date | September 2015 |
| Contact information | Janssen Research & Development, LLC Clinical Trial |
| Notes | ClinicalTrials.gov identifier: NCT02555878 |
NCT03090880.
| Study name | Prophylaxis of venous thromboembolism in advanced lung cancer (PROVE) |
| Methods | Randomised, phase III, open, multicentre trial with blinded adjudication of endpoints |
| Participants | Adults aged > 18 years with stage IV non‐small‐cell lung cancer and elevated D‐dimer > 1500 µg/L |
| Interventions | Intervention: tinzaparin 4500 IU SC once daily for 6 months Control: usual care |
| Outcomes | Primary outcome: VTE including symptomatic or incidental PE, symptomatic or incidental proximal DVT of the lower extremity, symptomatic DVT of the upper extremity, VTE‐related death during the six‐month treatment period Secondary outcomes: symptomatic VTE, any VTE, major bleeding, death at 6 and 12 months |
| Starting date | March 2017 |
| Contact information | Guy Meyer |
| Notes | ClinicalTrials.gov identifier: NCT03090880 |
NCT03428373.
| Study name | ASA vs. rivaroxaban in newly diagnosed or relapsed and refractory multiple myeloma patients treated with Len‐Dex combination therapy (RithMM) |
| Methods | Multicentre, open‐label, pilot, RCT. A web‐based randomisation system will ensure allocation concealment |
| Participants | Patients with newly diagnosed or relapsed and refractory multiple myeloma treated with lenalidomide dexamethasone combination therapy |
| Interventions | Intervention 1: aspirin 81 mg daily Intervention 2: rivaroxaban 10 mg daily for 6 months |
| Outcomes | Venous or arterial thromboembolism, treatment‐related adverse events |
| Starting date | 1 January 2019 |
| Contact information | Martha Louzada, mailto:Martha.Louzada%40lhsc.on.ca?subject=NCT03428373, 10014356, ASA vs. Rivaroxaban in Newly Diagnosed or Relapsed and Refractory Multiple Myeloma Patients Treated With Len‐Dex Combination Therapy. |
| Notes |
O'Brien 2019.
| Study name | PREVAPIX‐ALL: apixaban compared to standard of care for prevention of venous thrombosis in paediatric acute lymphoblastic leukaemia (ALL) – rationale and design |
| Methods | Multinational, multicentre, randomised, open‐label trial |
| Participants | Children and adolescents with acute lymphoblastic leukaemia and T/B cell lymphoblastic lymphoma receiving standard induction chemotherapy with asparaginase and the presence of a central venous access device |
| Interventions | Intervention: apixaban. Children 5 years or older randomised to the apixaban arm and weighing ≥ 35 kg may be administered either 2.5 mg tablets, 0.5 mg tablets, or oral solution apixaban twice daily for approximately 28 days, while children < 5 years and < 35 kg may be administered 0.5 mg tablets only. Children weighing ≥ 35 kg will be administered the adult dose of apixaban 2.5 mg twice daily Control: standard of care |
| Outcomes | Primary efficacy endpoint: composite of symptomatic and asymptomatic VTE that includes DVT, PE, cerebral sinovenous thrombosis, or VTE‐related death Primary safety outcome: major bleeding Secondary outcomes: central line‐associated infections, patency and line replacement, superficial thrombosis, arterial events, and death |
| Starting date | Not reported |
| Contact information | Sarah H O'Brien, sarah.obrien@nationwidechildrens.org |
| Notes |
DVT: deep vein thrombosis; HIT: heparin‐induced thrombocytopenia; IV: intravenous; LMWH: low‐molecular‐weight heparin; PE: pulmonary embolism; RCT: randomised controlled trial; SC: subcutaneous; TACE: transarterial chemoembolisation; VTE: venous thromboembolism.
Differences between protocol and review
In this update, we added fatal PE to the secondary outcomes.
The protocol specified that we would evaluate heterogeneity in results between trials with the I2 statistic (Higgins 2003; Rücker 2008). However, we added the variance estimate Tau2 to indicate and interpret heterogeneity, as added to forest plots by default.
For the comparison of LMWH versus no thromboprophylaxis, we could not perform stratified analyses of the main outcomes by differences in the use of cointerventions in the trial groups due to poor reporting. Although we were unable to analyse dosage as a continuous variable, we could stratify the analyses according to trials using prophylactic dosage versus those using other (higher than prophylactic) dosages. We could not use the univariable random‐effects meta‐regression model by dosage of intervention.
Compared to earlier versions of this review, we added a stratified analysis by the risk of selective outcome reporting (low versus high or unclear risk), which is one of the standard risk of bias items in Cochrane Reviews.
We planned to perform meta‐regression on both treatment duration and follow‐up duration. The treatment duration equalled the follow‐up duration in all studies except in Pelzer 2015 and Meyer 2018. Therefore, we only analysed the effect of treatment duration on major bleeding and symptomatic VTE for the comparison LMWH versus no thromboprophylaxis. In all other comparisons, there was no exploration of the effects of participant or trial characteristics on symptomatic VTE or major bleeding due to the low number of studies identified.
Contributions of authors
Contribution to previous versions of this review are found in Di Nisio 2010; Di Nisio 2012; Di Nisio 2014; and Di Nisio 2016.
Contributions to the current version.
AWSR: acquisition of data, risk of bias assessments, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, grading of the evidence.
EP: analysis and interpretation of data, critical revision of the manuscript for important intellectual content.
MC: acquisition of data, risk of bias assessments, analysis and interpretation of data, critical revision of the manuscript for important intellectual content.
EV: acquisition of data, risk of bias assessments, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, grading of the evidence.
MDN: oversight, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content.
Sources of support
Internal sources
-
None, Italy
No funding source available
-
None, Netherlands
No funding source available
External sources
-
None, Italy
No funding source available
-
None, Netherlands
No funding source available
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
AWSR: none. EP: none. MC: none. EV: none. MDN: has declared that he has received consultancy fees from Bayer, Daiichi Sankyo, LEO Pharma, BMS‐Pfizer and Aspen, outside of the submitted work.
When the previous version of this review was published(Di Nisio 2016), the authors declared the below conflicts of interest. From 29 October 2020, the above conflicts of interest were declared. These conflicts applied during the period that the review update was in preparation.
MDN: I have received consultancy fees from Bayer, Grifols, and Daiichi Sankyo not related to the present review. EP: none known MC: none known MDT: none known IR: none known AWSR: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Agnelli 2009 {published data only}
- *.Agnelli G, Gussoni G, Bianchini C, Verso M, Mandala M, Cavanna L, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncology 2009;10(10):943-9. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Tonato M. Nadroparin for prevention of thromboembolic events in cancer patients receiving chemotherapy: a randomized placebo-controlled double-blind study. Supportive Care in Cancer 2009;17:857-1039. [Google Scholar]
- Barni S, Bonizzoni E, Verso M, Gussoni G, Petrelli F, Perrone T, et al. The effect of low-molecular-weight heparin in cancer patients: the mirror image of survival? Blood 2014;124:155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barni S, Labianca R, Agnelli G, Bonizzoni E, Mandalà M, Verso M, et al. Thromboembolic risk related to type of chemotherapy and efficacy of nadroparin in cancer outpatients with metastatic or locally advanced cancer. Supportive Care in Cancer 2011;19 (Suppl 2):S206. [Google Scholar]
- Barni S, Labianca R, Agnelli G, Bonizzoni E, Verso M, Mandala M, et al. Chemotherapy-associated thromboembolic risk in cancer outpatients and effect of nadroparin thromboprophylaxis: results of a retrospective analysis of the PROTECHT study. Journal of Translational Medicine 2011;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barni S, Petrelli F, Bonizzoni E, Verso M, Gussoni G, Perrone T, et al. Survival benefit with low-molecular-weight heparin in patients with advanced solid tumors: a post hoc analysis of PROTECHT Trial. Journal of Clinical Oncology 2014;32 (Suppl):Abstract 9640. [Google Scholar]
Agnelli 2012 {published data only}
- Agnelli G, George D, Fisher WD, Kakkar AK, Lassen MR, Mismetti P, et al. Ultra-low-molecular-weight heparin (ULMWH) semuloparin for prevention of venous thromboembolism (VTE) in cancer patients receiving chemotherapy: consistent beneficial effect across cancer stage and location subgroups. European Journal of Cancer 2011;47:S222. [Google Scholar]
- *.Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, et al, for the SAVE-ONCO Investigators. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. New England Journal of Medicine 2012;366(7):601-9. [DOI] [PubMed] [Google Scholar]
- Hull RD. Semuloparin reduced venous thromboembolism in patients receiving chemotherapy for cancer. Annals of Internal Medicine 2012;156:JC6-5. [DOI] [PubMed] [Google Scholar]
- NCT00694382. A multinational, randomized, double blind, placebo-controlled study to evaluate the efficacy and safety of AVE5026 in the prevention of venous thromboembolism (VTE) in cancer patients at high risk for VTE and who are undergoing chemotherapy. clinicaltrials.gov/show/NCT00694382 (first received 7 May 2008).
Altinbas 2004 {published data only}
- Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. Journal of Thrombosis and Haemostasis 2004;2(8):1266-71. [DOI] [PubMed] [Google Scholar]
Campos‐Cabrera 2018 {published data only}
- Campos-Cabrera G, Mendez-Garcia E, Campos-Cabrera S, Campos-Villagomez JL, Campos-Cabrera V. Rivaroxaban or aspirin as thromboprophylaxis in multiple myeloma. Blood 2018;132(Suppl 1):5068. [DOI: 10.1182/blood-2018-99-111579] [DOI] [Google Scholar]
Carrier 2019 {published data only}
- *.Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. New England Journal of Medicine 2019;380:711-9. [DOI] [PubMed] [Google Scholar]
- Castellucci L, Carrier M, Mallick R, Wells P. Bleeding in the prevention of cancer-associated venous thromboembolism: secondary analysis of the AVERT study. Research and Practice in Thrombosis and Haemostasis 2019;3 (Suppl 1):719. [Google Scholar]
- Kimpton M, Wells PS, Carrier M. Apixaban for the prevention of venous thromboembolism in high-risk ambulatory cancer patients receiving chemotherapy: rational and design of the AVERT trial. Thrombosis Research 2018;164:S124-9. [DOI] [PubMed] [Google Scholar]
- Knoll W, Mallick R, Wells P, Carrier M. Safety and efficacy of apixaban thromboprophylaxis in cancer patients with metastatic disease: a subgroup analysis of the avert trial. Blood 2019;134 (Suppl 1):1140. [DOI] [PubMed] [Google Scholar]
- Miranda S, Benhamou Y, Wells P, Carrier M. Safety of primary thromboprophylaxis using apixaban in ambulatory cancer patients with intracranial metastatic disease or primary brain tumors. Thrombosis and Haemostasis 2019;119(11):1886-7. [DOI] [PubMed] [Google Scholar]
- NCT02048865. Apixaban for the prevention of venous thromboembolism in cancer patients (AVERT). clinicaltrials.gov/ct2/show/NCT02048865 (first received 27 January 2014).
Chahinian 1989 {published data only}
- Chahinian AP, Propert KJ, Ware JH, Zimmer B, Perry MC, Hirsh V, et al. A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small-cell lung cancer by the Cancer and Leukemia Group B. Journal of Clinical Oncology 1989;7(8):993-1002. [DOI] [PubMed] [Google Scholar]
Ek 2018 {published data only}
- *.Ek L, Gezelius E, Bergman B, Bendahl PO, Anderson H, Sundberg J, et al. Randomized phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: the RASTEN trial. Annals of Oncology 2018;29(2):398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezelius E, Bendahl P, Goncalves de Oliveira K, Ek L, Bergman B, Sundberg J, et al. Low-molecular-weight heparin adherence and effects on survival within a randomised phase III lung cancer trial (RASTEN). European Journal of Cancer 2019;118:82-90. [DOI] [PubMed] [Google Scholar]
- Gezelius E, Ek L, Bergman B, Bendahl P, Anderson H, Falkmer U, et al. Randomized phase III trial of enoxaparin in addition to standard treatment in small cell lung cancer: the RASTEN trial. Journal of Thoracic Oncology 2017;12(11):S2045-6. [DOI] [PMC free article] [PubMed]
- Gezelius E, Flou Kristensen A, Bendahl PO, Hisada Y, Risom Kristensen S, Ek L, et al. Coagulation biomarkers and prediction of venous thromboembolism and survival in small cell lung cancer: a sub-study of RASTEN – a randomized trial with low molecular weight heparin. PloS One 2018;13(11):e0207387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00717938. A study of standard treatment +/- enoxaparin in small cell lung cancer (RASTEN). clinicaltrials.gov/ct2/show/NCT00717938 (first received 16 July 2008).
Elit 2012 {published data only}
- *.Elit LM, Lee AY, Parpia S, Swystun LL, Liaw PC, Hoskins P, et al. Dalteparin low molecular weight heparin (LMWH) in ovarian cancer: a phase II randomized study. Thrombosis Research 2012;130:894-900. [DOI] [PubMed] [Google Scholar]
- NCT00239980. A phase II randomized study of Fragmin in ovarian cancer: utility on survival (FOCUS). clinicaltrials.gov/show/NCT00239980 (first received 13 October 2005).
Greiner 2019 {published data only}
- Greiner J, Schrappe M, Claviez A, Zimmermann M, Niemeyer C, Kolb R, et al. THROMBOTECT – a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica 2019;104(4):756-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2012 {published data only}
- Freund M, Kakkar AK, Haas S, Heilmann L, von-Tempelhoff GF, Brom J, et al. A randomized trial of the low molecular weight of heparin certoparin against placebo in the long-term prevention of venous thromboembolism in patients with metastatic breast cancer. Blood 2003;102(11 (Pt1)):210a. [Google Scholar]
- Gatzemeier U, Freund M, Haas S, Kakkar A, Zatloukai P, Kelbel C, et al. Prevention of thromboembolic complications with the low-molecular-weight heparin certoparin in non-small-cell lung carcinoma (TOPIC-2). Lung Cancer (Amsterdam, Netherlands) 2005;49:S56.
- *.Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, Tempelhoff GF, TOPIC Investigators. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clinical and Applied Thrombosis Hemostasis 2012;18(2):159-65. [DOI] [PubMed] [Google Scholar]
- Haas SK, Kakkar AK, Kemkes-Matthes B, Freund M, Gatzemeier U, Heilmann L, et al. Prevention of venous thromboembolism with low-molecular-weight heparin in patients with metastatic breast or lung cancer – results of the TOPIC studies. Journal of Thrombosis and Haemostasis 2005;3(1):Abstract OR059. [Google Scholar]
- Verso M, Gussoni G, Agnelli G. Prevention of venous thromboembolism in patients with advanced lung cancer receiving chemotherapy: a combined analysis of the PROTECHT and TOPIC-2 studies. Journal of Thrombosis and Haemostasis 2010;8(7):1649-51. [DOI] [PubMed] [Google Scholar]
Kakkar 2004 {published data only}
- Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). Journal of Clinical Oncology 2004;22(10):1944-8. [DOI] [PubMed] [Google Scholar]
Khorana 2017 {published data only}
- Khorana AA, Francis CW, Kuderer N, Carrier M, Ortel TL, Wun T, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Blood 2015;126:427. [DOI] [PubMed] [Google Scholar]
- *.Khorana AA, Francis CW, Kuderer NM, Carrier M, Ortel TL, Wun T, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thrombosis Research 2017;151:89-95. [DOI] [PubMed] [Google Scholar]
- NCT00876915. A prospective randomized multicenter study of dalteparin prophylaxis in high-risk ambulatory cancer patients. clinicaltrials.gov/show/NCT00876915 (first received 31 March 2009).
Khorana 2019 {published data only}
- Anonymous. CASSINI trial data on preventing blood clots in cancer patients. Oncology Times 2019;41:19. [Google Scholar]
- Khorana A, McNamara M, Kakkar A, Streiff M, Riess H, Vijapurkar U, et al. Assessing full benefit of rivaroxaban prophylaxis in high-risk ambulatory patients with cancer: thromboembolic events in the randomized CASSINI trial. TH Open 2020;4(2):E107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana A, Soff G, Kakkar A, Vadhan-Raj S, Riess H, Wun T, et al. Rivaroxaban thromboprophylaxis in high-risk ambulatory cancer patients receiving systemic therapy: results of a randomized clinical trial (CASSINI). Blood 2018;132 (Suppl 1):LBA-1. [Google Scholar]
- *.Khorana AA, Soff GA, Kakkar AK, Vadhan‑Raj S, Riess H, Wun T, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. New England Journal of Medicine 2019;380:720-8. [DOI] [PubMed] [Google Scholar]
- Khorana AA, Vadhan-Raj S, Kuderer NM, Wun T, Liebman H, Soff G, et al. Rivaroxaban for preventing venous thromboembolism in high-risk ambulatory patients with cancer: rationale and design of the CASSINI trial. Thrombosis and Haemostasis 2017;117:2135-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VadhanRaj S, McNamara M, Venerito M, Riess H, O'Reilly E, Overman M, et al. Rivaroxaban thromboprophylaxis in ambulatory patients with pancreatic cancer: results from a prespecified subgroup analysis of the CASSINI study. Journal of Clinical Oncology. Conference 2019;37 (Suppl 15):4016. [Google Scholar]
Klerk 2005 {published data only}
- Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. Journal of Clinical Oncology 2005;23(10):2130-5. [DOI] [PubMed] [Google Scholar]
Larocca 2012 {published data only}
- Cavallo F, Caravita T, Di Raimondo F, Ciccone G, Lupo B, Marcatti M, et al. A phase III study of enoxaparin vs aspirin as thromboprophylaxis for patients with newly diagnosed of multiple myeloma treated with lenalidomide-based regimens. Haematologica 2011;96:S30. [Google Scholar]
- Cavallo F, Di Raimondo F, Harda I, Lupo B, Romano A, Catalano L, et al. A phase III study of enoxaparin vs aspirin as thromboprophylaxis for newly diagnosed myeloma patients treated with lenalidomide-based regimen. Blood 2010;116:Abstract 1092. [Google Scholar]
- Larocca A, Caravita TT, Di Raimondo F, Cavallo F, Cascavilla N, Galli M, et al. Thromboprophylaxis for newly diagnosed myeloma patients treated with lenalidomide-based regimens: an interim analysis of a prospective, randomized study of enoxaparin vs aspirin. Haematologica 2013;95:S40. [Google Scholar]
- *.Larocca A, Cavallo F, Bringhen S, Di Raimondo F, Falanga A, Evangelista A, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012;119(4):933-9. [DOI] [PubMed] [Google Scholar]
Lebeau 1994 {published data only}
- Lebeau B, Chastang C, Brechot J-M, Capron F, Dautzenberg B, Delaisements C, et al. Subcutaneous heparin treatment increases survival in small cell lung cancer. "Petites Cellules" Group. Cancer 1994;74(1):38-45. [DOI] [PubMed] [Google Scholar]
Lecumberri 2013 {published data only}
- *.Lecumberri R, Lopez VG, Font A, Gonzalez BE, Gurpide A, Gomez CJ, et al. Adjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: results from the ABEL study. Thrombosis Research 2013;132:666-70. [DOI] [PubMed] [Google Scholar]
- NCT00324558. Multicenter, randomized, open and sequential study to evaluate the efficacy and safety of bemiparin administration on the response to treatment in patients diagnosed with limited small cell lung cancer. clinicaltrials.gov/show/NCT00324558 (first received 9 May 2006).
Levine 1994 {published data only}
- Dickson L, Levine M, Gent M. Efficacy of double-blinding in a randomized controlled trial (RCT) of low dose warfarin to prevent thromboembolic disease in patients with metastatic breast cancer. Controlled Clinical Trials 1993;14(5):462. [Google Scholar]
- *.Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, et al. Double-blind randomised trial of very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet 1994;343(8902):886-9. [DOI] [PubMed] [Google Scholar]
Levine 2012 {published data only}
- Levine MN, Deitchman D, Julian J, Liebman H, Escalante C, O'Brien MC, et al. A randomized phase II trial of a new anticoagulant, apixaban, in metastatic cancer. Journal of Clinical Oncology 2009;27(15):e20514. [Google Scholar]
- *.Levine MN, Gu C, Liebman HA, Escalante CP, Solymoss S, Deitchman D, et al. A randomized phase II trial of Apixaban for the prevention of thromboembolism in patients with metastatic cancer. Journal of Thrombosis and Haemostasis 2012;10(5):807-14. [DOI] [PubMed] [Google Scholar]
- Levine MN, Liebman HA, Esclanate CP, Julian JA, Deitchman D, O'Brien MC, et al. Randomized phase II trial of an oral factor Xa inhibitor in patients with metastatic cancer on chemotherapy. Thrombosis Research 2010;125 (Suppl 2):S161-2. [Google Scholar]
- Liebman H, Levine MN, Deitchman D, Julian J, Escalante CP, O'Brien MC, et al. Apixaban in patients with metastatic cancer: a randomized phase II feasibility study. Journal of Thrombosis and Haemostasis 2009;7 Suppl 2:Abstract PP-WE-489. [DOI] [PubMed] [Google Scholar]
- NCT00320255. A randomized, double-blind, placebo-controlled study of apixaban for the prevention of thromboembolic events in patients undergoing treatment for advanced cancer: a phase 2 pilot study. clinicaltrials.gov/show/NCT00320255 (first received 28 April 2006).
Macbeth 2016 {published data only}
- Griffiths GO, Burns S, Noble SI, Macbeth FR, Cohen D, Maughan TS. FRAGMATIC: a randomised phase III clinical trial investigating the effect of fragmin added to standard therapy in patients with lung cancer. BMC Cancer 2009;9:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth F, Carter B, Noble S, Hood K. Further results of the FRAGMATIC trial of thromboprophylaxis in lung cancer. Translational Lung Cancer Research 2016;5:347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Macbeth F, Noble S, Evans J, Ahmed S, Cohen D, Hood K, et al. Randomized phase III trial of standard therapy plus low molecular weight heparin in patients with lung cancer: FRAGMATIC trial. Journal of Clinical Oncology 2016;34:488-94. [DOI] [PubMed] [Google Scholar]
- Macbeth F, Noble S, Griffiths G, Chowdhury R, Rolfe C, Hood K, et al. Preliminary results from the fragmatic trial: a randomised phase iii clinical trial investigating the effect of fragmin added to standard therapy in patients with lung cancer. Journal of Thoracic Oncology 2013;8(Suppl 2):O27.02. [Google Scholar]
- NCT00519805. Dalteparin in preventing blood clots in patients with lung cancer. clinicaltrials.gov/show/NCT00519805 (first received 23 August 2007).
- Noble S, Harrop E, Edwards M, Sivell S, Hood K, Griffiths G, et al. Applying the results of the Fragmatic trial to real life: longitudinal qualitative study exploring the experiences of patients participating in a randomised phase III clinical trial investigating the effect of Fragmin added to standard therapy in lung cancer. Journal of Thoracic Oncology 2013;8 (Suppl 2):S1011-2. [Google Scholar]
Maraveyas 2012 {published data only}
- Maraveyas A, Ettelaie C, Echrish H, Li C, Gardiner E, Greenman J, et al. Weight-adjusted dalteparin for prevention of vascular thromboembolism in advanced pancreatic cancer patients decreases serum tissue factor and serum-mediated induction of cancer cell invasion. Blood Coagulation and Fibrinolysis 2010;21(5):452-8. [DOI] [PubMed] [Google Scholar]
- *.Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. European Journal of Cancer 2012;48(9):1283-92. [DOI] [PubMed] [Google Scholar]
- Maraveyas A, Waters J, Roy R, Propper D, Fyfe D, Lofts F, et al. Gemcitabine with or without prophylactic weight-adjusted dalteparin in patients with advanced or metastatic pancreatic cancer (APC): a multicentre, randomised phase IIB trial (the UK FRAGEM study). European Journal of Cancer Supplements 2009;7(2):362. [Google Scholar]
- NCT00462852. A phase II randomised study of chemo-anticoagulation (gemcitabine-dalteparin) vs chemotherapy alone (Gemcitabine) for locally advanced and metastatic pancreatic adenocarcinoma. clinicaltrials.gov/show/NCT00462852 (first received 18 April 2007).
Maurer 1997 {published data only}
- Maurer LH, Herndon JE II, Hollis DR, Aisner J, Carey RW, Skarin AT, et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: a Cancer and Leukemia Group B study. Journal of Clinical Oncology 1997;15(11):3378-87. [DOI] [PubMed] [Google Scholar]
Meyer 2018 {published data only}
- *.Meyer G, Besse B, Doubre H, Charles-Nelson A, Aquilanti S, Izadifar A, et al. Anti-tumour effect of low molecular weight heparin in localised lung cancer: a phase III clinical trial. European Respiratory Journal 2018;52:1801220. [DOI] [PubMed] [Google Scholar]
- Meyer G, Besse B, Doubre H, Charles-Nelson A, Aquilanti S, Izadifar A, et al. Effect of low molecular weight heparin on survival of patients with resected non-small cell lung cancer: the Tinzaparin in Lung Tumors (TILT) randomized phase III trial. Research and Practice in Thrombosis and Haemostasis 2017;1(Suppl 1):S212. [Google Scholar]
- NCT00475098. Effect of low molecular weight heparin: Tinzaparin in Lung Tumours (TILT). clinicaltrials.gov/ct2/show/NCT00475098 (first posted 17 May 2007).
Mitchell 2003 {published data only}
- Mitchell L, Andrew M, Hanna K, Abshire T, Halton J, Wu J, et al. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l-asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thrombosis and Haemostasis 2003;90(2):235-44. [DOI] [PubMed] [Google Scholar]
Palumbo 2011 {published data only}
- Cavo M, Palumbo A, Bringhen S, Di RF, Patriarca F, Rossi D, et al. Phase III study of enoxaparin versus aspirin versus low dose warfarin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated up front with thalidomide-containing regimens. Haematologica 2010;95 Suppl 2:391, Abstract 0941. [Google Scholar]
- Cavo M, Palumbo A, Bringhen S, Falcone A, Musto P, Ciceri F, et al. A phase III study of enoxaparin versus low-dose warfarin versus aspirin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated up-front with thalidomide-containing regimens. Blood 2008;110:3017. [Google Scholar]
- Cavo M, Palumbo A, Bringhen S, Falcone A, Musto P, Ciceri F, et al. A phase III study of enoxaparin versus low-dose warfarin versus aspirin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated up-front with thalidomide-containing regimens. Haematologica 2009;94:s4. [Google Scholar]
- Magarotto V, Brioli A, Patriarca F, Rossi D, Petrucci MT, Nozzoli C, et al. Enoxaparin, aspirin, or warfarin for the thromboprophylaxis in newly diagnosed myeloma patients receiving thalidomide: a randomized controlled trial. XI Congress of the Italian Society of Experimental Hematology; 2010 Oct 6-8; Turin, Italy.
- Palumbo A, Cavo M, Bringhen S, Patriarca F, Rossi D, Petrucci MT, et al. A phase III study of enoxaparin versus aspirin versus low-dose warfarin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated up-front with thalidomide based-regimens. Haematologica 2009;94 Suppl 2:213.
- *.Palumbo A, Cavo M, Bringhen S, Zamagni E, Romano A, Patriarca F, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. Journal of Clinical Oncology 2011;29:986-93. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Rus C, Cavallo F, Bertola A, Petrucci MT, Liberati AM, et al. Enoxaparin or aspirin for the prevention of recurrent thromboembolism in newly diagnosed myeloma patients treated with melphalan and prednisone plus thalidomide or lenalidomide. Haematologica 2006;91 Suppl 1:Abstract 0234. [DOI] [PubMed]
Pelzer 2015 {published data only}
- NCT00785421. Chemotherapy with or without enoxaparin in pancreatic cancer (PROSPECT). clinicaltrials.gov/ct2/show/NCT00785421 (first received 5 November 2008).
- Pelzer U, Deutschinoff G, Opitz B, Stauch M, Reitzig P, Hahnfeld S, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy. First results of the CONKO-004 trial. Onkologie-DGHO meeting; 2009 Oct 2-6; Mannheim, Germany. Abstract 580.
- Pelzer U, Deutschinoff G, Opitz B, Stauch M, Reitzig P, Hahnfeld S, et al. The impact of low molecular weight heparin in first-line pancreatic cancer treatment – final results of the CONKO-004 trial. Onkologie 2010;33(6):200. [Google Scholar]
- Pelzer U, Hilbig A, Stieler J, Roll L, Riess H, Dörken B, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy (PROSPECT – CONKO 004). Onkologie 2005;28 Suppl 3:Abstract 151. [DOI] [PMC free article] [PubMed]
- Pelzer U, Hilbig A, Stieler JM, Bahra M, Sinn M, Gebauer B, et al. Intensified chemotherapy and simultaneous treatment with heparin in outpatients with pancreatic cancer – the CONKO 004 pilot trial. BMC Cancer 2014;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer U, Oettle H, Stauch M, Opitz B, Stieler J, Scholten T, et al. Prospective, randomized open trial of enoxaparin in patients with advanced pancreatic cancer undergoing first-line chemotherapy. XXIst Congress of the International Society on Thrombosis and Haemostasis; 2007 Jul 6-12; Geneva, Switzerland. Abstract P-T-488.
- *.Pelzer U, Opitz B, Deutschinoff G, Stauch M, Reitzig PC, Hahnfeld S, et al. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 Trial. Journal of Clinical Oncology 2015;33:2028-34. [DOI] [PubMed] [Google Scholar]
- Pelzer U, Sinn M, Stieler J, Reiss H. Primary pharmacological prevention of thromboembolic events in ambulatory patients with advanced pancreatic cancer treated with chemotherapy? Deutsche Medizinische Wochenschrift 2013;138:2084-8. [DOI] [PubMed] [Google Scholar]
- Riess H, Pelzer U, Hilbig A, Stieler J, Opitz B, Scholten T, et al. Rationale and design of PROSPECT-CONKO 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy. BMC Cancer 2008;8:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess HB, Pelzer U, Opitz B, Hilbig A, Strauch M, Hahnfeld S, et al. Late breaking clinical trial: a prospective, randomized trial of chemotherapy with and without the low molecular weight heparin (LMWH) enoxaparin in advanced pancreatic cancer patients. International Society on Thrombosis and Haemostasis 2009;7 (Suppl 2):1204. [Google Scholar]
Perry 2010 {published data only}
- NCT00135876. Dalteparin low molecular weight heparin for primary prophylaxis of venous thromboembolism in brain tumour patients. clinicaltrials.gov/ct2/show/NCT00135876 (first received 26 August 2005).
- *.Perry JR, Julian JA, Laperriere NJ, Geerts W, Agnelli G, Rogers LR, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. Journal of Thrombosis and Haemostasis 2010;8(9):1959-65. [DOI] [PubMed] [Google Scholar]
- Perry JR, Rogers L, Laperriere N, Julian J, Geerts W, Agnelli G, et al. PRODIGE: a phase III randomized placebo-controlled trial of thromboprophylaxis using dalteparin low molecular weight heparin (LMWH) in patients with newly diagnosed malignant glioma. Journal of Clinical Oncology 2007;25(18 Suppl):2011. [Google Scholar]
Sideras 2006 {published data only}
- Sideras K, Schaefer PL, Okuno SH, Sloan JA, Kutteh L, Fitch TR, et al. Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clinic Proceedings 2006;81(6):758-67. [DOI] [PubMed] [Google Scholar]
Vadhan‐Raj 2013 {published data only}
- NCT00966277. Randomized clinical trial of dalteparin for primary venous thromboembolism (VTE) prophylaxis in pancreatic cancer patients undergoing chemotherapy treatment. clinicaltrials.gov/show/NCT00966277 (first received 25 August 2009).
- *.Vadhan-Raj S, Zhou X, Varadhachary GR, Milind J, Fogelman D, Shroff R, et al. Randomized controlled trial of dalteparin for primary thromboprophylaxis for venous thromboembolism (VTE) in patients with advanced pancreatic cancer (APC): risk factors predictive of VTE. 55th Annual Meeting of the American Society of Hematology; 2013 Dec 7-10; New Orleans, LA.
- Vadhan-Raj S, Zhou X, Varadhachary GR, Milind J, Fogelman D, Shroff R, et al. Randomized controlled trial of dalteparin for primary thromboprophylaxis for venous thromboembolism (VTE) in patients with advanced pancreatic cancer (APC): risk factors predictive of VTE. Blood 2013;122(21):580. [Google Scholar]
van Doormaal 2011 {published data only}
- Doormaal FF, Di Nisio M, Otten H-M, Richel DJ, Prins M, Buller HR. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. Journal of Clinical Oncology 2011;29(15):2071-6. [DOI] [PubMed] [Google Scholar]
Zacharski 1981 {published data only}
- Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell CJ Jr, Forcier RJ, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75. Cancer 1984;53(10):2046-52. [DOI] [PubMed] [Google Scholar]
- *.Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell CJ Jr, Forcier RJ, et al. Effect of warfarin on survival in small cell carcinoma of the lung. Veterans Administration Study No. 75. JAMA 1981;245(8):831-5. [PubMed] [Google Scholar]
Zwicker 2013 {published data only}
- NCT00908960. Enoxaparin thromboprophylaxis in cancer patients with elevated tissue factor bearing microparticles. clinicaltrials.gov/ct2/show/NCT00908960 (first received 12 December 2013).
- Zwicker J, Liebman HA, Bauer KA, Caughey T, Rosovsky R, Mantha S, et al. A randomized-controlled phase II trial of primary thromboprophylaxis with enoxaparin in cancer patients with elevated tissue factor bearing microparticles (The Microtec Study). Journal of Thrombosis and Haemostasis 2013;11 (Suppl s3):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Zwicker JI, Liebman HA, Bauer KA, Caughey T, Campigotto F, Rosovsky R, et al. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study). British Journal of Haematology 2013;160(4):530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Baz 2005 {published data only}
- Baz R, Li L, Kottke-Marchant K, Srkalovic G, McGowan B, Yiannaki E, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clinic Proceedings 2005;80(12):1568-74. [DOI] [PubMed] [Google Scholar]
Bergqvist 1983 {published data only}
- Bergqvist D, Lindblad B. Is it possible to potentiate the thromboprophylactic effect of dextran with elastic compression stockings? Thrombosis and Haemostasis 1983;50(1):247 Abstract 0777.
Bocharov 2011 {published data only}
- Bocharov AV, Cherenkov VG, Ukhanov AP, Chentsov VI. Potential endovascular prophylaxis for pulmonary thromboembolism in the combined treatment of cancer patients. Voprosy Onkologii 2011;57:513-6. [PubMed] [Google Scholar]
Eichinger 2008 {published data only}
- Eichinger S, Traby L, Kaider A, Quehenberger P, Kyrle PA. Prevention of venous thrombosis in cancer patients: a randomized, double-blind study comparing two different dosages of low-molecular weight heparin. Blood 2008;112(11):690 Abstract 1977. [Google Scholar]
Groen 2019 {published data only}
- Groen HJ, Heijden EH, Klinkenberg TJ, Biesma B, Aerts J, Verhagen A, et al. Randomised phase 3 study of adjuvant chemotherapy with or without nadroparin in patients with completely resected non-small-cell lung cancer: the NVALT-8 study. British Journal of Cancer 2019;121(5):372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2011 {published data only}
- Haas S, Schellong SM, Tebbe U, Gerlach H-E, Bauersachs R, Melzer N, et al. Heparin based prophylaxis to prevent venous thromboembolic events and death in patients with cancer – a subgroup analysis of CERTIFY. BMC Cancer 2011;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heilmann 1995 {published data only}
- Heilmann L, Schneider D, Herrle B, von Tempelhoff G-F, Manstein J, Wolf H. A prospective randomized trial of low molecular weight heparin (LMWH) versus unfractionated heparin (UFH) in patients with gynecologic cancer. Thrombosis and Haemostasis 1995;73(6):974 Abstract No 286.
Hills 1972 {published data only}
- Hills NH, Pflug JJ, Jeyasingh K, Boardman L, Calnan JS. Prevention of deep vein thrombosis by intermittent pneumatic compression of calf. British Medical Journal 1972;1(5793):131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2011 {published data only}
- Kessler P, Pour L, Gregora E, Zemanova M, Penka M, Brejcha M, et al, Czech Myeloma Group. Low molecular weight heparins for thromboprophylaxis during induction chemotherapy in patients with multiple myeloma. Klinicka Onkologie 2011;24(4):281-6. [PubMed] [Google Scholar]
- Kessler P. Prophylaxis and treatment of venous thromboembolism in patients with multiple myeloma. Onkologie 2011;5(3):160-2. [Google Scholar]
Macintyre 1974 {published data only}
- Macintyre MC, Vasilescu C, Jones DR. Heparin versus dextran in the prevention of deep-vein thrombosis. A multi-unit controlled trial. Lancet 1974;2(7873):118-20. [PubMed] [Google Scholar]
Maxwell 2000 {published data only}
- Maxwell GL. A prospective randomized trial comparing external pneumatic compression stockings (EPC) to the low molecular weight heparin (LMWH) dalteparin in the prevention of thromboembolic events (TE) among gynecologic oncology patients. Proceedings of the American Society of Clinical Oncology 2000;19:388a, Abstract 1535.
Meister 2008 {published data only}
- Meister B, Kropshofer G, Klein Franke A, Strasak AM, Hager J, Streif W. Comparison of low-molecular-weight heparin and antithrombin versus antithrombin alone for the prevention of symptomatic venous thromboembolism in children with acute lymphoblastic leukemia. Pediatric Blood and Cancer 2008;50(2):298-303. [DOI] [PubMed] [Google Scholar]
Minnema 2004 {published data only}
- Minnema MC, Breitkreutz I, Auwerda JJ, van-der Holt B, Cremer FW, van-Marion AM, et al. Prevention of venous thromboembolism with low molecular-weight heparin in patients with multiple myeloma treated with thalidomide and chemotherapy. Leukemia 2004;18(12):2044-6. [DOI] [PubMed] [Google Scholar]
NCT00004875 {unpublished data only}
- NCT00004875. Heparin or enoxaparin in patients with cancer. clinicaltrials.gov/show/NCT00004875 (first received 7 March 2000).
NCT00031837 {unpublished data only}
- NCT00031837. A prospective randomized controlled multicenter study of the effect of dalteparin on quality of life in unresectable pancreatic cancer. clinicaltrials.gov/show/NCT00031837 (first received 8 March 2002).
NCT00662688 {unpublished data only}
- NCT00662688. Chemotherapy with or without dalteparin in treating patients with metastatic pancreatic cancer (PAM07). clinicaltrials.gov/show/NCT00662688 (first received 18 April 2008).
NCT00790452 {unpublished data only}
- NCT00790452. A randomized phase II trial of aspirin for primary prophylaxis of venous thromboembolism in glioblastoma. clinicaltrials.gov/show/NCT00790452 (first received 7 November 2008).
NCT04106700 {published data only}
- NCT04106700. Prophylaxis with apixaban in transplant eligible patients with multiple myeloma receiving induction therapy with IMiDs. clinicaltrials.gov/ct2/show/NCT04106700 (first received 27 September 2019).
NCT04352439 {published data only}
- NCT04352439. Aspirin for prevention of venous thromboembolism among ovarian cancer patients receiving neoadjuvant chemotherapy. clinicaltrials.gov/ct2/show/NCT04352439 (first received 20 April 2020).
Niesvizky 2007 {published data only}
- Niesvizky R, Martinez-Banos D, Jalbrzikowski J, Christos P, Furst J, De Sancho M, et al. Prophylactic low-dose aspirin is effective antithrombotic therapy for combination treatments of thalidomide or lenalidomide in myeloma. Leukemia and Lymphoma 2007;48(12):2330-7. [DOI] [PubMed] [Google Scholar]
Paydas 2008 {published data only}
- Paydas S. Tailored thromboprophylaxis for patients with multiple myeloma treated by IMIDs. Leukemia and Lymphoma 2008;49(8):1644-5. [DOI] [PubMed] [Google Scholar]
Poniewierski 1988 {published data only}
- *.Poniewierski M, Barthels M, Kuhn M, Poliwoda H. Efficacy of low molecular weight heparin (Fragmin) for thromboprophylaxis in medical patients: a randomized double blind trial. Medizinische Klinik 1988;83(7):241-5. [PubMed] [Google Scholar]
- Poniewierski M, Barthels M, Poliwoda H. The safety and efficacy of a low molecular weight heparin (fragmin) in the prevention of deep vein thrombosis in medical patients: a randomized double-blind trial. Thrombosis and Haemostasis 1987;58(1):119 Abstract 425.
Rajan 1995 {published data only}
- Rajan R, Gafni A, Levine M, Hirsh J, Gent M. Very low-dose warfarin prophylaxis to prevent thromboembolism in women with metastatic breast cancer receiving chemotherapy: an economic evaluation. Journal of Clinical Oncology 1995;13(1):42-6. [DOI] [PubMed] [Google Scholar]
Salat 1990 {published data only}
- Salat C, Breitruck H, Reinhardt B, Hiller E. Thromboprophylaxis with low molecular weight heparin (LMWH) and conventional low dose heparin in patients with malignant diseases. Blut 1990;61(2-3):142. [Google Scholar]
Sideras 2007 {published data only}
Storrar 2019 {published data only}
- Storrar NP, Mathur A, Johnson PR, Roddie PH. Safety and efficacy of apixaban for routine thromboprophylaxis in myeloma patients treated with thalidomide- and lenalidomide-containing regimens. British Journal of Haematology 2019;185:142-4. [DOI] [PubMed] [Google Scholar]
Weber 2008 {published data only}
- Weber C, Merminod T, Herrmann FR, Zulian GB. Prophylactic anti-coagulation in cancer palliative care: a prospective randomised study. Support Care in Cancer 2008;16(7):847-52. [DOI] [PubMed] [Google Scholar]
Welti 1981 {published data only}
- Welti H. Thrombo-embolytic prophylaxis using physiotherapy with and without low doses of heparin in gynecology and obstetrics. Results of a controlled and randomized multi-cancer study. Revue Medicale de la Suisse Romande 1981;101(11):925-34. [PubMed] [Google Scholar]
Zangari 2003 {published data only}
- Zangari M, Barlogie B, Saghafifar F, Eddlemon P, Jacobson J, Lee CK, et al. Effect of anticoagulation on development and recurrence of deep vein thrombosis (DVT) in multiple myeloma patients treated with chemotherapy and thalidomide (total therapy II). Hematology Journal 2003;4 Suppl 1:S248. [Google Scholar]
Zwicker 2019 {published data only}
- Zwicker JI, Schlechter BL, Stopa JD, Liebman HA, Aggarwal A, Puligandla M, et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019;4:e125851. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ciftci 2012 {published data only}
- Ciftci A, Altiay G. The effect of warfarin on survival in patients with lung cancer. Journal of Thoracic Oncology 2012;7:S122. [Google Scholar]
NCT00771563 {published data only}
- NCT00771563. Enoxaparin low molecular weight heparin (LMWH) in advanced non small cell lung cancer: effect on survival and symptom control in patients undergoing first line chemotherapy (SYRINGES). clinicaltrials.gov/ct2/show/NCT00771563 (first received 10 October 2008).
References to ongoing studies
ChiCTR‐TRC‐08000267 {unpublished data only}
- ChiCTR-TRC-08000267. The role of LMWH combined with TACE in hepatocellular carcinoma. chictr.org.cn/com/25/showproj.aspx?proj=9261 (first received 29 December 2008).
NCT00718354 {unpublished data only}
- NCT00718354. Overall survival of inoperable gastric/gastrooesophageal cancer subjects on treating with LMWH + chemotherapy (CT) vs standard CT (GASTRANOX). clinicaltrials.gov/ct2/show/NCT00718354 (first received 18 July 2008).
NCT01518465 {unpublished data only}
- NCT01518465. Dalteparin, lenalidomide, and low-dose dexamethasone in treating patients with previously untreated multiple myeloma. clinicaltrials.gov/ct2/show/NCT01518465 (first received 26 January 2012).
NCT02285738 {published data only}
- NCT02285738. Anti-platelet and statin therapy to prevent cancer-associated thrombosis. clinicaltrials.gov/ct2/show/NCT02285738 (first received 5 November 2014).
NCT02555878 {published data only}
- NCT02555878. Efficacy and safety of rivaroxaban prophylaxis compared with placebo in ambulatory cancer patients initiating systemic cancer therapy and at high risk for venous thromboembolism. clinicaltrials.gov/ct2/show/NCT02555878 (first received 18 September 2015).
NCT03090880 {unpublished data only}
- NCT03090880. Prophylaxis of venous thromboembolism in advanced lung cancer (PROVE). clinicaltrials.gov/ct2/show/NCT03090880 (first received 27 March 2017).
NCT03428373 {published data only}
- NCT03428373. ASA vs. rivaroxaban in newly diagnosed or relapsed and refractory multiple myeloma patients treated with Len-Dex combination therapy. clinicaltrials.gov/ct2/show/NCT03428373 (first received 9 February 2018).
O'Brien 2019 {unpublished data only}
- O'Brien SH, Li D, Mitchell LG, Hess T, Zee P, Yee DL, et al. PREVAPIX-ALL: apixaban compared to standard of care for prevention of venous thrombosis in paediatric acute lymphoblastic leukaemia (ALL) – rationale and design. Thrombosis and Haemostasis 2019;119:844-53. [DOI] [PubMed] [Google Scholar]
Additional references
Aikens 2013
- Aikens GB, Rivey MP, Hansen CJ. Primary venous thromboembolism prophylaxis in ambulatory cancer patients. Annals of Pharmacotherapy 2013;47(2):198-209. [DOI] [PubMed] [Google Scholar]
Ay 2010
- Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116(24):5377-82. [DOI] [PubMed] [Google Scholar]
Ay 2017
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thrombosis and Haemostasis 2017;117:219-30. [DOI] [PubMed] [Google Scholar]
Cohen 2017
- Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thrombosis and Haemostasis 2017;117:57-65. [DOI] [PubMed] [Google Scholar]
Connors 2014
- Connors JM. Prophylaxis against venous thromboembolism in ambulatory patients with cancer. New England Journal of Medicine 2014;370:2515-9. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Di Nisio 2017
- Di Nisio M, Carrier M. Incidental venous thromboembolism: is anticoagulation indicated? Hematology 2017;1:121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMEA 2012
- European Medicines Agency (EMEA). Withdrawal of the marketing authorisation application for Mulsevo (semuloparin sodium). www.emea.europa.eu/docs/en_GB/document_library/Medicine_QA/2012/07/WC500130168.pdf (accessed 11 December 2012).
Farge 2019
- Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncology 2019;20(10):e566-81. [DOI] [PubMed] [Google Scholar]
George 2011
- George D, Agnelli G, Fisher W, Kakkar A, Lassen MR, Mismetti P, et al. Venous thromboembolism (VTE) prevention with semuloparin in cancer patients initiating chemotherapy: benefit-risk assessment by VTE risk in SAVE-ONCO. Blood 2011;118:Abstract 206. [Google Scholar]
Guyatt 2008
- Guyatt G, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7656):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI] [PubMed] [Google Scholar]
Heit 2015
- Heit JA. Epidemiology of venous thromboembolism. Nature Reviews. Cardiology 2015;12:464-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horsted 2012
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Medicine 2012;9(7):e1001275.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutten 2000
- Hutten B, Prins M, Gent M, Ginsberg J, Tijsen JG, Büller HR. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. Journal of Clinical Oncology 2000;18(17):3078-83. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaatz 2015
- Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. Journal of Thrombosis and Haemostasis 2015;13:2119-26. [DOI] [PubMed] [Google Scholar]
Kahale 2018
- Kahale LA, Tsolakian IG, Hakoum MB, Matar CF, Barba M, Yosuico VE, et al. Anticoagulation for people with cancer and central venous catheters. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD006468. [DOI: 10.1002/14651858.CD006468.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahn 2012
- Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th edition: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):e195S-226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamphuisen 2014
- Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thrombosis Research 2014;133(S2):S49-55. [DOI] [PubMed] [Google Scholar]
Key 2020
- Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. Journal of Clinical Oncology 2020;38(5):496-520. [DOI] [PubMed] [Google Scholar]
Khorana 2008
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111(10):4902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khorana 2009a
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. Journal of Clinical Oncology 2009;27(29):4839-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khorana 2009b
- Khorana AA, Streiff MB, Farge D, Mandala M, Debourdeau P, Cajfinger F, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. Journal of Clinical Oncology 2009;27(29):4919-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khorana 2018
- Khorana AA, Francis CW. Risk prediction of cancer-associated thrombosis: appraising the first decade and developing the future. Thrombosis Research 2018;Suppl 1:S70-6. [DOI] [PubMed] [Google Scholar]
Kraaijpoel 2019
- Kraaijpoel N, Bleker SM, Meyer G, Mahé I, Muñoz A, Bertoletti L, et al. Treatment and long-term clinical outcomes of incidental pulmonary embolism in patients with cancer: an international prospective cohort study. Journal of Clinical Oncology 2019;37(20):1713-20. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
Maxwell 2012
- Maxwell WD, Bennett CL. Thromboprophylaxis guidelines in cancer with a primary focus on ambulatory patients receiving chemotherapy: a review from the southern network on adverse reactions (SONAR). Seminars in Thrombosis and Hemostasis 2012;38(8):759-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulder 2019
- Mulder FI, Candeloro M, Kamphuisen PW, Di Nisio M, Bossuyt PM, Guman N, et al. The Khorana score for prediction of venous thromboembolism in cancer patients: a systematic review and meta-analysis. Haematologica 2019;104(6):1277-87. [DOI: 10.3324/haematol.2018.209114] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Comprehensive Cancer Network 2020
- National Comprehensive Cancer Network (NCCN). Clinical practice guideline in oncology: cancer-associated venous thromboembolic disease. www.nccn.org/professionals/physician_gls/default.aspx#detection (accessed 16 April 2020).
Otten 2004
- Otten H-M, Mathijssen J, ten Cate H, Soesan M, Inghels M, Richel DJ, et al. Symptomatic venous thromboembolism in cancer patients treated with chemotherapy. An underestimated phenomenon. Archives of Internal Medicine 2004;164(2):190-4. [DOI] [PubMed] [Google Scholar]
Prandoni 2002
- Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100(10):3484-8. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rücker 2008
- Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Medical Research Methodology 2008;8(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2009
- Rutjes AW, Nüesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD002823. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2012
- Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Annals of Internal Medicine 2012;157(3):180-91. [DOI] [PubMed] [Google Scholar]
Schulman 2005
- Schulman S, Kearon C on behalf of the subcommittee on control of anticoagulation of the Scientific and Standardization committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. Scientific and Standardization Committee Communication. Journal of Thrombosis and Haemostasis 2005;3:692-4. [DOI] [PubMed] [Google Scholar]
Sorensen 2000
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. New England Journal of Medicine 2000;343(25):1846-50. [DOI] [PubMed] [Google Scholar]
Stata 2019 [Computer program]
- Stata Statistical Software. Version 15.1. College Station, TX: StataCorp LP, 2019.
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046-55. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693-708. [DOI] [PubMed] [Google Scholar]
Timp 2013
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712-23. [DOI] [PubMed] [Google Scholar]
van Es 2014
- Es N, Bleker SM, Di Nisio M. Cancer-associated unsuspected pulmonary embolism. Thrombosis Research 2014;133(Suppl 2):S172-8. [DOI] [PubMed] [Google Scholar]
Verso 2012
- Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Internal and Emergency Medicine 2012;7(3):291-2. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Di Nisio 2010
- Di Nisio M, Rutjes AW, Ferrante N, Otten HM, Porreca E, Cuccurullo F. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD008500. [DOI: 10.1002/14651858.CD008500] [DOI] [PubMed] [Google Scholar]
Di Nisio 2012
- Di Nisio M, Porreca E, Ferrante N, Otten HM, Cuccurullo F, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD008500. [DOI: 10.1002/14651858.CD008500.pub2] [DOI] [PubMed] [Google Scholar]
Di Nisio 2014
- Di Nisio M, Porreca E, Otten HM, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews 2014, Issue 8. Art. No: CD008500. [DOI: 10.1002/14651858.CD008500.pub3] [DOI] [PubMed] [Google Scholar]
Di Nisio 2016
- Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD008500. [DOI: 10.1002/14651858.CD008500.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


