Abstract
Introduction
Measurement of breath hydrogen (H2) and methane (CH4) excretion after ingestion of test‐carbohydrates is used for different diagnostic purposes. There is a lack of standardization among centers performing these tests and this, together with recent technical developments and evidence from clinical studies, highlight the need for a European guideline.
Methods
This consensus‐based clinical practice guideline defines the clinical indications, performance, and interpretation of H2‐CH4‐breath tests in adult and pediatric patients. A balance between scientific evidence and clinical experience was achieved by a Delphi consensus that involved 44 experts from 18 European countries. Eighty eight statements and recommendations were drafted based on a review of the literature. Consensus (≥80% agreement) was reached for 82. Quality of evidence was evaluated using validated criteria.
Results
The guideline incorporates new insights into the role of symptom assessment to diagnose carbohydrate (e.g., lactose) intolerances and recommends that breath tests for carbohydrate malabsorption require additional validated concurrent symptom evaluation to establish carbohydrate intolerance. Regarding the use of breath tests for the evaluation of oro‐cecal transit time and suspected small bowel bacterial overgrowth, this guideline highlights confounding factors associated with the interpretation of H2‐CH4‐breath tests in these indications and recommends approaches to mitigate these issues.
Conclusion
This clinical practice guideline should facilitate pan‐European harmonization of diagnostic approaches to symptoms and disorders, which are very common in specialist and primary care gastroenterology practice, both in adult and pediatric patients. In addition, it identifies areas of future research needs to clarify diagnostic and therapeutic approaches.
Keywords: fructose, intolerance, lactose, malabsorption, oro‐cecal transit time, small intestinal bacterial overgrowth
INTRODUCTION
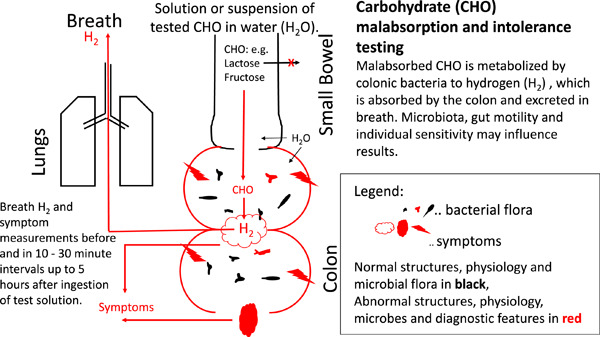
In the human body, hydrogen (H2) and methane (CH4) are derived exclusively through anaerobic fermentation of both endogenous and exogenous carbohydrates by enteric microflora. 1 , 2 Studies have shown that this process is rapid, and H2 can be measured in the breath less than 5 min after introduction of sugars and polysaccharides into the unprepared colon. 3 Increased concentrations of this gas in breath after oral ingestion of a fermentable carbohydrate therefore indicate that the substrate has not been fully absorbed by the small bowel and has come into contact with saccharolytic bacteria. This is the physiological basis for the detection of carbohydrate malabsorption by H2‐CH4 carbohydrate breath tests.
Measurement of H2 excretion in end‐expiratory breath for the assessment of carbohydrate malabsorption was introduced into clinical practice in the 1970s, 4 , 5 and has since been recommended and widely used for diagnostic purposes in adults and children. 6 , 7 , 8 , 9 More recently, additional measurement of CH4 concentrations has been proposed in order to improve test accuracy, 10 especially in patients who do not excrete measurable quantities of H2 in breath.
Hydrogen breath tests (H2BTs) have been used for (I) assessment of carbohydrate malabsorption of sugars, such as lactose and fructose, that are variably absorbed in the small bowel, (II) measurement of the time interval between ingestion of an unabsorbable carbohydrate, such as lactulose, and its contact with colonic bacteria in the cecum (oro‐cecal transit time, OCTT), and (III) contact of a test carbohydrate, such as glucose or lactulose, with abnormally high concentrations of bacteria in the small bowel (small intestinal bacterial overgrowth, SIBO). 6 , 7 , 10 Breath tests are non‐invasive, relatively simple to perform and safe diagnostic tools, which can be used both in adults and in children. The results are considered helpful in the evaluation of common abdominal symptoms such as bloating, flatulence, abdominal pain, and diarrhea, which can be caused by carbohydrate malabsorption and intolerance. 6 , 7 , 10
Although several national guidelines have provided guidance on indications and performance of H2 and CH4 breath tests, 6 , 7 , 10 there is a lack of standardization regarding performance and interpretation among expert centers in different countries. This is relevant because modifications of test procedures and of the evaluation of data may markedly influence test results, diagnosis, and, thus, clinical usefulness of the investigation. In addition, in recent years, clinical and scientific developments have considerably expanded the knowledge about how these tests should be performed and interpreted.
There is increased awareness that the clinical usefulness of breath tests for the detection of carbohydrate malabsorption in patients with abdominal symptoms is incompletely understood, and that an important discrepancy exists between the presence of malabsorption and intolerance. 11 The meaning and the use of the terms “lactose malabsorption” and “lactose intolerance” has not always been clearly defined 12 and the misuse of these terms (e.g., in patients with lactose malabsorption without a close temporal association with symptoms 13 ) may be the cause for conflicting results of clinical studies. Many other studies have recruited patients with malabsorption and symptoms following ingestion of high doses of sugars (e.g., 40–50 g lactose), which is of questionable clinical or therapeutic relevance. 14 , 15 , 16 , 17 Additionally, test‐specific symptom questionnaires for carbohydrate intolerance have rarely been applied in the past to document carbohydrate intolerance. 18 , 19 , 20 The current guideline will define terms and put a focus on the role of validated procedures and measurement for the detection of carbohydrate malabsorption and intolerance.
Recent studies have questioned the usefulness of H2BT in the detection of SIBO and in the measurement of rapid OCTT owing to difficulties in interpreting results because of potential overlap of test results in these two clinical entities. 21 , 22 This guideline will also address this issue.
The aim of this consensus‐based clinical practice guideline of H2‐CH4‐carbohydrate breath tests is to improve harmonization of diagnostic approaches in the assessment of functional gastrointestinal (GI) symptoms and disorders which are very common in specialist and primary care gastroenterology practice, both in adult and in pediatric patients. It should provide physicians with the information required to deliver high quality care and to communicate the best care options to patients. It is hoped that this will add to the quality of clinical care and, thus, the welfare of GI patients because it will allow a more rational, evidence‐based approach to diagnostic evaluation and treatment. The guideline will also help to minimize disparities between health care systems across Europe and facilitate cooperation between expert groups, and the performance of multi‐center clinical trials focused on the management of functional GI disease, carbohydrate intolerances, SIBO, and related conditions.
METHODS
The structured procedure, which was developed for the creation of this consensus‐based clinical practice guideline, has been published. 23 This procedure was initiated by three representatives of the contributing societies (heads of guideline) and started with formation of a representative core group of experts nominated from all participating societies and associations. This core group developed 88 statements and recommendations, which were then submitted to a wider group of reviewers in a three‐stage Delphi voting process. The participating societies, and the names of the heads of guideline, the core group leads, and core group members are listed as authors; the reviewers are listed as members of the European H2‐CH4‐breath test group.
Four core groups were established for the following topics: general methodology, assessment of carbohydrate malabsorption and intolerance, assessment of SIBO, and measurement of OCTT. Each core group developed recommendations and statements, which addressed indication, operating procedures, and interpretation of breath tests used in their assigned topics. A “recommendation” was developed if the core group felt that a suggestion or proposal as to the best course of action was adequate. A “statement” was drafted if the core group felt that a summary of current knowledge or procedures was adequate. Statements and recommendations were based on available research and consensus documents including those of participating societies, and systematic literature search in Medline/Pubmed and the Cochrane database using the PICO system as appropriate (i.e., patient population/problem, intervention, comparison/control, and outcome).
Key questions, which were addressed, were:
What are the general technical requirements and operating procedures for performance of H2‐CH4 breath tests, including preparation, dosage, breath sampling, and technical analysis?
What is the value of including measurements of CH4 in breath samples?
What is the role of H2‐CH4 breath tests in the evaluation of carbohydrate malabsorption, in suspected SIBO, and in the measurement of OCTT?
What is the role of including symptom measurements to diagnose carbohydrate intolerance?
What are the reporting requirements?
Scientific quality of evidence for statements and recommendations was assigned using a modified Oxford grading 24 with four levels of evidence: the highest level of evidence (systematic reviews, validating cohort studies) was designated “A” and the lowest level of evidence (expert opinion) was designated “D” (Table 1). Strength of recommendations was indicated by the wording used for the recommendation and graded with four grades with A (“has to be,” “is to be,” and “shall”) being the highest to D (“may”) being the lowest (Table 2).
TABLE 1.
Level of evidence for describing the quality of recommendations and statements (modified after reference 24)
| Level of Evidence | Diagnostic studies | |
|---|---|---|
| A: High | 1a | SR (with homogeneity) of level 1 diagnostic studies; CDR with 1b studies from different clinical centres |
| 1b | Validating cohort study with good reference standards; or CDR tested within one clinical centre | |
| 1c | Absolute SpPins and SnNouts | |
| B: Moderate | 2a | SR (with homogeneity) of level >2 diagnostic studies |
| 2b | Exploratory cohort study with good reference standards; CDR after derivation, or validated only on split‐sample or databases | |
| C: Weak | 3a | SR (with homogeneity) of 3b and better studies |
| 3b | Non‐consecutive study; or without consistently applied reference standards | |
| 4 | Case‐control study, poor or non‐independent reference standard | |
| D: Expert opinion | 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” |
Note: “Absolute SpPin”: a diagnostic finding whose specificity is so high that a positive result rules‐in the diagnosis. “Absolute SnNout”: a diagnostic finding whose Sensitivity is so high that a Negative result rules out the diagnosis. SNOUT: acronym for “Sensitive test when Negative rules OUT the disease,” SPIN: acronym for “Specific test when Positive rules IN the disease.”
Abbreviations: CDR, clinical decision rule; SR, Systematic review.
TABLE 2.
Descriptors of grading of strength of recommendations
| Descriptor | Meaning | Wording used for the recommendation |
|---|---|---|
| A–Strength high | Evidence or general accord that the recommendation is useful or effective. Further research is very unlikely to change our confidence in the estimate of effect. | ..has to be….. |
| …is to be….. | ||
| ….shall… | ||
| B–Strength moderate | Conflicting evidence or discordant opinions that the recommendation is useful or effective. The weight of evidence/opinion is in favour of utility. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | …should….. |
| …can….. | ||
| C–Strength low | Conflicting evidence or discordant opinions that the recommendation is useful or effective. Further research is VERY likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | …..could…. |
| D–Strength very low | Any estimate of effect is very uncertain. | ….may….. |
Statements and recommendations were distributed via email for a Delphi voting process (6‐point Likert scale) and commenting among reviewers for three Delphi voting rounds. Recommendations and statements were considered to be accepted if they had achieved ≥80% agreement (the highest two points of the 6‐point Likert scale) and <10% disagreement (the lowest two points of the 6‐point Likert scale). After each voting round recommendations and statements which were declined were modified according to comments, and modified statements and recommendations underwent further rounds of the Delphi process as previously described. 23 After three Delphi rounds, 82 statements and recommendations were accepted.
In the manuscript for each of the recommendations, quality “Q,” strength “S,” and the rates of agreement and disagreement (in %) are shown. For statements, only quality “Q” was assigned. Rates of agreement and disagreement do not necessarily add up to 100% if some of the votes were in the middle of the 6‐point Likert scale (indicating minor agreement or minor disagreement).
Most of the recommendations are valid for all ages from childhood to adult, except those in which specific circumstances of pediatric patients must be considered. These statements are marked with “Ped.”
METHODOLOGY OF BREATH TESTS
Any assessment of the diagnostic accuracy of H2BT and comparability between tests performed in different locations and laboratories requires defined test protocols. However, for research projects, test parameters such as dose or composition of the test substance or test meal, duration and interval of breath sampling, and cut‐off values defining normal versus abnormal can be varied to evaluate the impact of the variation on test results (agree 86%, disagree 2%).
Some of the carbohydrates used in the various H2BT are not completely soluble at doses recommended for their use in room temperature water at the suggested volumes of water. For practical purposes, a “suspension” of the sugar (by stirring the water and immediately drinking the suspension) is appropriate. Dissolution of test carbohydrates in warm tea or use of foods rich in the test carbohydrate (e.g., milk) shall be avoided because of potential interference of other components of these “test‐meals” with GI function. 25 , 26
How shall breath samples be collected?
Accurate results from breath testing rely on proper preparation of patients and instruction on how to perform the breath test maneuvre, on reliable sampling of end‐expiratory air, on stability of the stored sample if measurements are delayed, and on reproducibility and accuracy of the breath analyzer, including its algorithms for measuring or calculating H2 or CH4 concentration. 6 , 7 , 10 , 27 , 28 , 29 , 30 , 31 Correct collection of breath samples is a prerequisite for obtaining end‐expiratory breath samples which are less prone to dilution of H2 and CH4 by bronchial dead air volume. 6 Different sampling and measurement devices have been described or are commercially available. 12 , 29 , 30 , 32 , 33 , 34 For example, in young children a face mask, connected to a double bag by means of a T‐valve, is commonly used. 35 Adult breath collection techniques are used when the child can blow a balloon and thus can mentally and physically cooperate.
Some breath analyzers use algorithms based on the measurement of carbon dioxide (CO2) or oxygen (O2) in exhaled air to detect and correct for dead space air mixed into the breath sample. 36 Breath H2 samples are stable for 6 h at room temperature, and if measurements are delayed beyond this, storage at −20℃ is needed. 27 , 31
Recommendation 1.1
For collection and measurement of breath samples, certified medical products shall be used. Attention must be given to breath sampling, storage and stability of breath samples and the manufacturer's instructions on handling of the sampling devices and the breath analyzing instruments in order to guarantee accuracy of breath testing.
Q: C; S: A, 100% agree
Recommendation Ped 1.1
For collection and measurement of breath samples in young children who cannot use the technique used in adults validated alternative collection devices, like a face mask, nasal probe or others should be used.
Q: C, S: B; 100% agree
How shall patients be prepared for testing?
The majority of authors recommend performing the test in the morning, after mouth cleansing and to follow an overnight fasting condition. Smoking and exercise that may result in hyperventilation are not permitted before or during the test. 37 , 38 , 39
There is strong evidence that antibiotics 40 , 41 , 42 and colonic cleansing 40 alter the composition of, and metabolism by intestinal bacteria and thereby H2 production. There are no data on how long this effect lasts, but it has been suggested to delay H2BT for between 1 and 4 weeks after finishing antibiotic treatment or colonic cleansing. 6 , 7 , 10 Since H2BTs are generally performed to address chronic symptoms and are not emergency procedures, it is reasonable that the waiting period between antibiotic treatment or colonic cleansing and the breath test shall be long enough in order not to raise doubts about potential influence of these treatments or procedures.
A high fasting level of breath H2 might impair the detection of a rise in H2 concentration by fermentation of the test carbohydrate and therefore should be minimized. High fasting levels may be the result of a high fiber meal on the previous evening, 43 smoking before the test 44 , 45 or abnormal GI anatomy (e.g., small bowel diverticula) or function (e.g., constipation) resulting in a background activity of bacterial fermentation. A low fiber diet or a diet containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) 46 decreases breath H2 excretion. Previous recommendations have suggested different time intervals of up to 24 h of restricting diet before testing. 6 , 7 , 10 In constipated subjects, it may be preferable to avoid foods, which may result in gas production, for up to 48 h although there are no data supporting this. In pediatric patients, it may not always be possible to avoid certain foods, especially lactose containing foods. There are no strong data in the literature on how long smoking shall be avoided before the H2BT; however, a practical recommendation must consider how long a smoker might tolerate refraining from smoking, therefore a 2‐h period was considered to be reasonable by the guideline group.
Fast rises in H2 concentration, which may be due to oral bacteria, 47 , 48 can be prevented by mouthwash with 1% chlorhexidine solution. 49 Rinsing with chlorhexidine may be difficult to achieve in children. In the current guideline, a recommendation proposing that in this case a wet tissue may be used to clean the oral cavity did not reach agreement (agree: 59%, disagree 4%).
Hyperventilation reduces breath H2 concentrations 50 , 51 and previous guidelines have recommended limiting physical activity before and during the test. 6 , 7 , 10
Different drugs, 52 , 53 including probiotics, 6 , 54 may affect GI transit, 55 , 56 , 57 and bacterial metabolism of carbohydrates. 33 , 58 Interpretation of results of the breath test requires information on drugs taken by the patient at the time of H2BT. Whether drugs for symptomatic treatment, such as laxatives, antidiarrheals, and spasmolytics, can and should be stopped before the test, depends on the clinical situation and remains at the discretion of the clinician. Special consideration must be given to carbohydrate‐laxatives such as lactulose or fermentable dietary fibers (e.g., fig syrup and bran), which need to be stopped before the test and, if required, be replaced by non‐carbohydrate laxatives, such as polyethylene glycol. 53 Some medications that have a significant effect on GI physiology (e.g., opioids) may not be able to be stopped, but in these cases, the test results must be interpreted with caution.
Recommendation 1.2
Breath testing should be delayed until at least 4 weeks after finishing antibiotic therapy.
Q: D, S: B, 100% agree
Recommendation 1.3
Breath testing should be delayed until at least 2 weeks after colonic cleansing for endoscopic or surgical procedures.
Q: D, S: B, 83% agree, 0% disagree
Recommendation 1.4
For a minimum of one day before breath testing, foods containing poorly absorbed, fermentable carbohydrates and dietary fibers should be avoided.
Q: D, S: B, 90% agree, 0% disagree
Recommendation Ped 1.4
In pediatric patients on the day before breath testing food containing fibers and poorly absorbable fermentable carbohydrates, like lactose, fructose, xylitol and other fermentable oligosaccharides, disaccharides, monosaccharides and polyols should be avoided, if possible.
Q: D, S: B, 100% agree
Recommendation 1.5
A minimum fasting period of 8 h should be observed before breath testing.
Q: D, S: B, 98% agree, 0% disagree
Recommendation Ped. 1.5
In children and adolescents, a minimum fasting period of 8 h should be observed before breath testing, whereas a fasting period of 4–6 h should be observed in infants (<1 year of age).
Q: D, S: B, 100% agree
Recommendation 1.6
To reduce the risk of H2 production from oral bacteria, the oral cavity should be rinsed with an antiseptic solution (e.g., chlorhexidine) immediately before the first (baseline) breath measurements are obtained.
Q: C, S: B, 83% agree, 7% disagree
Recommendation Ped 1.6
If the baseline H2 concentration before carbohydrate ingestion is ≥15 ppm, children should be asked to rinse their mouth with tap water and then provide a next breath sample. The breath test shall be continued only if the baseline H2 value is <15 ppm to exclude children with small intestinal bacterial overgrowth.
Q:D, S:B; 89% agree, 5% disagree
Recommendation 1.7
Smoking shall be avoided on the day of the test at least 2 h before and during the duration of the test.
Q: C, S: A, 98% agree, 0% disagree
Recommendation 1.8
Physical activity shall be limited for 2 h before and during the test to prevent the influence of respiration on breath H2 values.
Q: C, S: A, 95% agree, 0% disagree
Recommendation 1.9
Drugs that contain fermentable carbohydrates (e.g., lactulose or lactose in gram doses), prokinetics, laxatives and probiotics should be stopped at least 24 h prior to breath testing, if possible.
Q: D, S: B, 86% agree, 0% disagree
Recommendation 1.10
Information on drugs with pharmaceutical action in the gastrointestinal tract and probiotics taken by the patient within 24 h before the test shall be obtained.
Q: D, S: A, 93% agree, 0% disagree
Is there a need for additional gas measurements to improve diagnostic value?
Methanogenic flora, 2 dietary sulfate, 59 or acidic colonic pH 60 may contribute to low rates of colonic H2 accumulation, resulting in false negative tests due to “low H2 production” or “non‐excretion” in up to 20% of H2BT. 61 It has been suggested that additional measurement of breath concentrations of CH4 may help to improve sensitivity of the breath tests in H2 non‐excretors. 10 Some breath test analyzers use algorithms based on the measurement of CO2 or O2 in exhaled air to correct for dead space air in the breath samples. However, the detection rate of carbohydrate malabsorption has not been significantly affected by additional measurement of CH4 or of CO2 in children and adolescents. 62 The potential increase in test accuracy due to these additional measurements must be weighed up against higher costs of equipment and potentially more complicated breath collection. Independent from the use of CH4 for diagnosis of carbohydrate malabsorption the detection of CH4 in breath may be helpful for directing treatment of constipation. 63
Statement 1.11
Measuring breath CH4 excretion, if available, may be helpful in patients with low H2 excretion and/or lack of a clinically relevant increase in breath H2 after ingestion of a malabsorbed substrate like lactulose.
Q: D, 85% agree, 3% disagree
Statement 1.12
Measuring breath CO2 or O2 excretion, if available, may be helpful to confirm that end‐expiratory collection of breath is correct.
Q: D, 92% agree, 0% disagree
CARBOHYDRATE MALABSORPTION AND INTOLERANCE
Introduction
Incomplete absorption of carbohydrates in the small intestine may have different causes. For some monosaccharides such as fructose, or sugar alcohols such as sorbitol or xylitol, absorptive capacity in the small intestinal mucosa is limited, and high amounts of these carbohydrates may result in their incomplete absorption in normal, healthy individuals. 15 , 64 , 65 , 66 , 67 For other carbohydrates, such as the disaccharides lactose or trehalose, specific digestive enzymes, such as lactase or trehalase, are required to digest the disaccharide to absorbable monosaccharides. 66 , 68 Lack of these enzymes may result in malabsorption of the respective carbohydrate. 66 , 69 In fact, the list of poorly absorbable FODMAPs is long. The term FODMAP was introduced for a group of carbohydrates based on their chemical‐analytical criteria rather than biological effects 70 and does not include incompletely absorbable long chain carbohydrates, such as starches 71 , 72 , 73 and many dietary fibers that also pass unchanged into the large intestine. 74 , 75
The common end for all carbohydrates that are not fully absorbed in the small bowel is bacterial fermentation in the colon with production of short chain fatty acids and gases. 76 In addition to the effects of short‐chain carbohydrates themselves, the production of short chain fatty acids increases passive movement (osmosis) and active secretion of water and sodium into the lumen that can result in diarrhea. 77 , 78 At the same time production of gases, such as CO2, H2, or CH4, may contribute to the sensation of abdominal bloating, pain and flatulence, related to perception of colonic distension. 79 , 80 , 81 Whether these processes cause symptoms is related to many factors including the total dose of poorly absorbable carbohydrates ingested, the saccharolytic activity of bacteria in the colon, the structure and function of the GI tract, and patient factors which affect sensitivity to chemical and mechanical stimulation of the intestine. The complex interplay between these factors results in marked variation in the likelihood of symptom development after ingestion of poorly absorbable, fermentable carbohydrates between individuals and even in the same person over time. 12 , 17 , 58 , 68 , 82 , 83 , 84 , 85
The rationale for using the measurement of H2 excretion in breath to establish malabsorption of carbohydrates was established in the late 1970s, 4 based on three observations: first, H2 is not produced by human cells, and its production occurs almost entirely due to bacterial fermentation in the colon; second, this production markedly and rapidly increases when carbohydrates are delivered to colonic bacteria; and third, increased H2 production is readily detectable as an increase in H2 excretion in the breath.
Background and definitions
Age of onset helps to identify the different forms of lactose malabsorption occurring throughout pediatric ages and some of these also are relevant in adults.
Congenital lactose malabsorption is extremely rare, is genetically determined by absent lactase (alactasia), manifests with severe symptoms (intractable watery osmotic diarrhea associated with metabolic acidosis, dehydration and weight loss) in the first days of life, necessitates a complete lactose free infant formula, and has been reported mostly in Finland and Western Russia. 86
Developmental lactase deficiency refers to the relative lactase deficiency in preterm neonates of less than 34 weeks of gestation.
Primary lactose malabsorption (due to lactase non‐persistance with increasing age) usually presents after 3 years of life in more than half of the world population, depending on the geographical origin and ethnicity. 86 In many European populations, the persistence or decline of lactase activity is related to the point polymorphism C/T 13910, with the genotype CC in lactase deficiency, TT in lactase persistency, and C/T in the intermediate expression. 87 , 88 , 89 In other geographical regions, other point mutations have been described. 90 Northern European children and adults present with the lowest prevalence of primary lactase malabsorption, while lactase deficiency predominates in Asian and American adolescents and adults.
Secondary lactase deficiency, due to damage to the small intestinal mucosa, may occur at any age and may be caused by infectious enteritis (i.e., Rotavirus, particularly in infancy), enteropathy (i.e., celiac disease, Giardiasis, and Crohn's disease), or severe malnutrition and may, thus, be transient and related to the underlying condition. 86
Various methodologies are used which assess different parts of the process that leads from mucosal maldigestion to malabsorption and intolerance of carbohydrates, such as lactose and others. 13 The different parts of this process identified by these different methodologies have different diagnostic and potentially also therapeutic implications. The following definitions were agreed on during the Delphi process:
The terms “lactase deficiency,” “lactose malabsorption,” and “lactose intolerance” describe different aspects of a pathogenetic process, which are documented by different diagnostic procedures, have different clinical relevance and therapeutic consequences, and may be, but do not necessarily have to be, connected (97% agree, 0% disagree).
“Lactase deficiency” refers to a lack of the activity of the enzyme lactase, which is the disaccharidase responsible for digestion of the disaccharide lactose and is located in the mucosa of the small bowel. Lactase deficiency can be diagnosed directly by measurement of enzyme activity in mucosal biopsies, and indirectly either by genetic testing or by measurement of serum glucose concentration after ingestion of lactose. Lactase deficiency can be primary or secondary to another small bowel disease. Lactase deficient persons who are exposed to lactose may develop lactose malabsorption, depending on the amount of ingested lactose (94% agree, 3% disagree)
“Lactose malabsorption” refers to incomplete absorption of lactose in the small intestine with the consequence that ingested lactose reaches the colon. The ingestion of lactose containing food by people who have primary lactase deficiency is the most common cause of lactose malabsorption worldwide. Lactose malabsorption can be diagnosed by breath tests. Lactose malabsorption may result in symptoms (intolerance) that can be treated by diet and other means (94% agree, 3% disagree).
The term “Carbohydrate malabsorption” may be used for incomplete absorption of a poorly digestible carbohydrate in the small intestine. These carbohydrates include fermentable oligo‐, di‐, and monosaccharides and polyols and more complex poorly absorbable carbohydrates like polysaccharides, dietary fibers and starches (94% agree, 6% disagree)
“Lactose intolerance” refers to reports of GI symptoms after ingesting lactose or lactose containing food (88% agree, 3% disagree).
The term “Carbohydrate intolerance” may be used for symptoms after ingesting other non‐ or poorly absorbed, fermentable oligo‐, di‐, and monosaccharides (and polyols) and more complex carbohydrates, such as dietary fibers. This includes fructose, lactulose, inulin, xylitol, and sorbitol. (94% agree, 0% disagree)
Who benefits from investigation for carbohydrate malabsorption and intolerance?
In principle, documentation of lactose malabsorption and intolerance indicates the need for dietary treatment. To avoid unnecessary dietary restriction and possible negative outcomes, recommendation of an elimination diet or the use of enzyme supplements (e.g., containing lactase 91 or, in the case of fructose intolerance potentially xylose isomerase 52 ) should be limited to cases in which the relationship between ingestion of the carbohydrate and development of symptoms has been documented.
In addition to commonly tested simple carbohydrates, such as lactose and fructose, 92 many other incompletely absorbed, fermentable carbohydrates reach the large bowel and can be metabolized by the colonic microbiome. 65 , 93 , 94 , 95 Indeed, the mechanisms by which lactose or fructose malabsorption cause intolerance are shared by many other types of carbohydrate, including FODMAPs, 96 starch 71 , 73 , 97 and non‐starch polysaccharides. 98 However, statements with regard to the clinical utility of H2BT with symptom assessment after sorbitol or lactulose (a representative FODMAP) were not thought to be supported by enough evidence and did not reach the level of acceptance in the guideline process.
Statement 2.1
Established indications for carbohydrate breath tests and symptom assessment include intermittent diarrhea, abdominal pain, bloating, distension, nausea and flatulence in patients without evidence of organic disease on appropriate investigations and in whom carbohydrate intolerance is considered a possible or likely cause of symptoms.
Q: A, 91% agree, 0% disagree
Recommendation 2.2
Patients with alarm symptoms and/or signs should be investigated by biochemical, endoscopic and imaging investigations prior to performance of breath tests.
Q: B, S: B, 97% agree, 0% disagree
Statement 2.3
Results of H2 breath testing with symptom assessment for lactose malabsorption and intolerance have acceptable sensitivity and specificity for a clinically relevant condition and can direct effective therapy.
Q: B, 84% agree, 0% disagree
How shall the breath test and the intolerance test be performed?
H2BT was first introduced to identify carbohydrate malabsorption, in particular to detect lactose malabsorption due to primary lactase deficiency (lactase non‐persistence). In epidemiological studies, it is appropriate to use high doses of the test carbohydrate to ensure a high sensitivity for detection of malabsorption. High doses are also most likely to cause symptoms if malabsorption is present 6 , 7 , 10 , 29 , 99 ; however, the ingestion of 40–50 g lactose is the equivalent of approximately 1000 ml fresh milk and it is not representative of a normal dietary intake. Lower doses are considered to be more appropriate in investigations that aim to detect clinically relevant carbohydrate intolerance and to guide dietary management. 100 The dose of substrate used for H2BT must produce enough H2 from bacterial fermentation to be detected reliably in breath and to trigger symptoms in the large majority of patients with clinically relevant carbohydrate intolerance; however, it should not be so large as to exceed the absorptive capacity and cause symptoms in normal, healthy individuals. 68
The sensitivity and specificity of H2BT is related also to the increase in breath H2 concentrations used to define carbohydrate malabsorption. A diagnostic cut‐off of 20 parts per million (ppm) has a reported specificity of 100% at a sensitivity of 60% for this purpose, whereas a cut‐off of 10 ppm has a specificity of 92% and a sensitivity of 70%. 6 , 41 , 100 The interval between breath collections and the overall duration of the H2BT should achieve a balance between sensitivity for detecting H2 increases and appropriate use of resources in terms of the personnel required to collect breath samples. It is recommended that for the documentation of carbohydrate intolerance the increase in abdominal symptoms used to define intolerance should be assessed, preferably using test‐specific validated questionnaires for adult 20 , 101 and pediatric 18 patients.
It has been proposed that, to detect the amount of a given substrate that can be tolerated by an individual patient, testing could be repeated for a range of doses applied in randomized order and with the patient blinded to the protocol. 68 The cost‐utility ratio of the increased complexity to test procedures must be studied before this can be recommended for routine clinical practice.
For statements and recommendations specific for the pediatric patients, please see below.
Statement 2.4
A watery solution of a defined dose of a carbohydrate is appropriate for hydrogen breath testing and symptom assessment.
Q: A, 88% agree, 3% disagree
Recommendation 2.5
The dose of test substance in adults for diagnosis of lactose malabsorption should be 25–50 g of the disaccharide lactose.
Q: A, S: B, 93% agree, 3% disagree
Recommendation 2.6
The dose of test substance in adults for diagnosis of lactose intolerance should be 25 g lactose.
Q: A, S: B, 91% agree, 6% disagree
Recommendation 2.7
The dose of test substance in adults for diagnosis of fructose malabsorption and intolerance could be 20–25 g of the monosaccharide fructose.
Q: B, S: C, 94% agree, 0% disagree
Recommendation 2.8
The recommended test duration is 3–5 h, or shorter if positive diagnosis for malabsorption and intolerance is confirmed.
Q: A, S: A, 97% agree, 3% disagree
Recommendation 2.9
The standard measurement interval to assess malabsorption and intolerance is 30 min. Longer intervals up to 60 min may be adequate to assess malabsorption. Shorter intervals of 10–15 min may be required to provide evidence of intolerance (i.e., temporal relation between increase in H2 production and occurrence of symptoms).
Q: A, S: A, 83% agree, 5% disagree
Recommendation 2.10
A H2 cut‐off ≥20 parts per million increase above baseline at a single time point during the test shall indicate maldigestion or malabsorption.
Q: A, S: A, 94% agree, 0% disagree
Recommendation 2.11
A H2 cut‐off ≥10 parts per million increase above baseline at a single time point during the test may indicate maldigestion or malabsorption if concurrent with simultaneous imaging to confirm arrival of substrate in the large bowel.
Q: B, S: D 84% agree, 3% disagree
Statement 2.12
Blinded testing for intolerance is not required in routine clinical practice.
Q: B, 92% agree, 0% disagree
Recommendation 2.13
Minimum reporting criteria of a breath test used to detect carbohydrate maldigestion or malabsorption should include a statement whether there is evidence of (i) maldigestion or malabsorption and (ii) of intolerance.
Q: A, S: B, 100% agree, 0% disagree
What are the strengths and limitations of breath tests?
The key limitation of tests that detect the genetic predisposition to carbohydrate malabsorption (e.g., lactase non‐persistence), the deficiency of certain enzymes required for carbohydrate digestion (e.g., endoscopic biopsy for duodenal hypolactasia), or the presence of specific biomarkers (e.g., gaxilose test 102 ) is that carbohydrate malabsorption is not in itself pathological or even unusual in normal, healthy individuals. 68 In most cases, carbohydrate malabsorption is clinically relevant only if it causes abdominal symptoms (intolerance). A well‐performed H2BT addresses this limitation by detecting malabsorption (H2 increase above a set diagnostic threshold) and confirming the temporal relationship between this objective event and the occurrence of subjective symptoms. 13 , 52 , 83 , 91 , 92 , 103 , 104 , 105 However, there is little evidence on test‐retest reliability of H2BT, and large variations in breath H2 response to fructose have been observed with repeated testing. 106
It should be noted that neurological and other somatic symptoms (e.g., postprandial fatigue and dizziness) have also been linked to ingestion of certain carbohydrates. 11 However, studies have not confirmed a temporal association between malabsorption and the onset of these non‐specific symptoms and the biological mechanism for any such link remains speculative.
The accuracy of H2BT is limited by certain factors. A false‐positive H2BT, often characterized by a rapid increase in the concentration of H2 in the breath, can result from poor oral hygiene, SIBO, or rapid intestinal transit. 6 , 21 , 22 Conversely, a false‐negative H2BT result occurs in at least 10% of patients because the colonic microbiome does not produce sufficient H2 that can be detected by current technology. 6 , 61 If required, this can be confirmed by a lack of increase in breath H2 in a lactulose H2BT (lactulose is not digested by the small bowel). 61 False negatives may also occur if OCTT is prolonged and the substrate enters the large bowel after the test is completed, usually after 3 h. 61
Certain refinements to the H2BT protocol may improve test performance. Many of the above listed limitations can be mitigated if H2BT is combined with an independent measurement of oro‐cecal transit (e.g., scintigraphy). This methodology increases test sensitivity if low H2 cut‐off values are applied and ensures that potential false positive and false negative results related to SIBO and/or variation in OCTT are avoided. 21 , 107 Unfortunately, the addition of scintigraphy increases the cost of this test and this technology is not available in office‐based practice.
The measurement of CH4 in addition to H2 appears to improve test sensitivity in low H2 producers. 10 , 108 However, the methodology and the clinical utility of this approach remain controversial. Published research has not always required a temporal association between the appearance of CH4 in the breath and the occurrence of symptoms, instead the presence of CH4 at any stage of the investigation (even at baseline) may be reported as a “positive test.” The addition of CO2 monitoring has been proposed to ensure that an adequate breath sample has been collected. However in a recent pediatric study, the additional measurement of CH4 or CO2 did not significantly affect the detection rate of carbohydrate malabsorption. 62 Additionally, measurement of additional gases increases the cost and complexity of the test. 109
If the pre‐test probability of carbohydrate malabsorption is high, then the occurrence of typical symptoms 30–90 min after ingestion may be sufficient to establish the diagnosis, and H2BT may not need to be necessary. Conversely, if the pre‐test probability of carbohydrate malabsorption is intermediate or low, then the demonstration of malabsorption by H2BT can help to distinguish between symptoms caused by fermentation of carbohydrates, as compared to other GI process (e.g., small bowel distention, intestinal contractions), 13 or, importantly, a nocebo effect (i.e., a negative outcome due to a belief that the intervention will cause harm). 85
Statement 2.14
False negative results for carbohydrate malabsorption by breath testing may occur in patients with low H2 excretion, in those with slow oro‐cecal transit time in whom carbohydrate fermentation commences after conclusion of the breath test and in patients with elevated baseline H2 concentration.
Q: A, 94% agree, 3% disagree
Statement 2.15
False positive results for carbohydrate malabsorption by breath testing may occur in small intestinal bacterial overgrowth or in rapid oro‐cecal transit time.
Q: A, 94% agree, 3% disagree
Statement Ped. 2.15
Several factors affecting the intestinal microbiota, gut motility and the individual sensitivity may result in false positive and false negative results. H2 non‐excretion is reported in up to 10%–15% of pediatric patients.
Q: A, 94% agree, 0% disagree
Statement 2.16
The role of inclusion of CH4 measurement for improving clinical usefulness of the H2 breath testing for detection of clinically relevant carbohydrate malabsorption is unclear: increased sensitivity may be counteracted by decreased specificity.
Q: B, 84% agree, 5% disagree
Recommendation 2.17
A CH4 cut‐off ≥10 parts per million increase above baseline may indicate malabsorption.
Q: B, S: D, 87% agree, 0% disagree;
Why shall symptoms be recorded after a carbohydrate challenge and how shall they be recorded?
In symptomatic patients referred for the evaluation of the clinical suspicion of carbohydrate intolerance, an increase of breath H2 after ingestion of this carbohydrate does not confirm that the patient's symptoms are caused by malabsorption of the tested carbohydrate. 82 , 110 , 111 Documentation of the relation between carbohydrate ingestion and the occurrence of symptoms is of importance for correctly assigning symptoms to the ingestion of the test carbohydrate and should be the main indication for treatment aimed at improving abdominal symptoms. It has been demonstrated both in adults and in pediatric patients that it is intolerance after a carbohydrate challenge, and not malabsorption, which corresponds to a history of clinical symptoms, related to carbohydrate intake. 82 , 83 , 112 It has been shown that patients in whom lactose malabsorption was diagnosed with 25 g of lactose, had a greater frequency of symptom resolution on lactose withdrawal as compared to patients in whom lactose malabsorption was diagnosed after 50 g of lactose. 113
In the past, treatment studies for patients with abdominal symptoms thought to be caused by carbohydrates, have mainly included patients with documented carbohydrate malabsorption rather than documented intolerance, which may explain conflicting results of these treatment studies. 114 , 115 Whereas malabsorption is not a major determinant for the outcome of the diet, 46 the occurrence of symptoms during a lactose breath test may suggest a favorable response to diet. 116 It has been suggested that in documented carbohydrate intolerance, carbohydrate‐reduced products are advisable and effective, although the evidence is scarce. 68 , 110 This is most likely due to poor inclusion criteria in treatment studies.
The validity of symptom assessment is important for the diagnosis and the initiation of therapy but also for the evaluation of the treatment response. 68 , 117 In order to minimize diagnostic bias, to standardize symptom assessment and to achieve comparability between studies, test‐specific symptom questionnaires have been developed for the assessment of carbohydrate induced symptoms both for the pediatric 18 and the adult population. 20 The questionnaire for adults will be available as a smartphone App under the name “carboception.” 101 The pediatric questionnaire will have to be translated into child‐specific language, and the translation will have to be validated in the countries where it shall be used. Use of these symptom questionnaires has confirmed that only a proportion of carbohydrate malabsorbers develop symptoms, while on the other hand, carbohydrate‐induced symptoms can also arise without detectable malabsorption both in adults and in children. 80 , 82 , 118 The fact that patients with the irritable bowel syndrome (IBS) have lactose intolerance, but not malabsorption, more often than their non‐IBS counterparts and that IBS‐patients report more severe symptoms, 119 , 120 argues for visceral hypersensitivity to play a role in the realization of symptoms in carbohydrate malabsorption. Moreover, other pathogenetic mechanisms might induce symptoms that may be confused with intolerance symptoms such as in non‐celiac gluten or wheat sensitivity. 121
Recommendation 2.18
The recording of symptoms manifesting after carbohydrate ingestion is an integral part of a carbohydrate challenge test.
Q: C, S: A; 97% agree, 0% disagree
Statement Ped. 2.18
Apart from the determination of malabsorption, the recording of symptoms manifesting after carbohydrate ingestion is an integral part of a carbohydrate breath test in children and adolescents.
Q: C, 95% agree, 0% disagree
Recommendation 2.19
The use of a validated symptom assessment tool is recommended.
Q: C, S: A; 94% agree, 0% disagree
Recommendation Ped 2.19
Gastrointestinal symptoms that manifest after a carbohydrate challenge in children and adolescents should be assessed with a validated, pediatric‐specific questionnaire.
Q: B, S: B, 90% agree, 0% disagree
Statement 2.19.
The combination of carbohydrate breath tests with symptom assessment allows for the determination of four different entities after a carbohydrate load: (1) maldigestion or malabsorption plus symptoms, (2) maldigestion or malabsorption only, (3) symptoms only, and (4) none of the above.
Q: B, 93% agree, 0% disagree
Statement 2.20
A positive and long‐lasting response to dietary intervention may confirm the diagnosis of carbohydrate intolerance.
Q: D, 85% agree, 5% disagree
Statement Ped 2.21
The occurrence of symptoms during the breath test cannot distinguish primary from secondary carbohydrate malabsorption and intolerance.
Q: D, 93% agree, 0% disagree
Which factors are responsible for symptoms after carbohydrate ingestion?
Carbohydrate malabsorption results in unabsorbed carbohydrates reaching the lower parts of the small intestine and the colon, which may result in biological processes that can lead to symptoms of carbohydrate intolerance. 83 Dose dependency of carbohydrate induced diarrhea has been demonstrated with the use of lactulose, a non‐absorbable disaccharide. 77 , 78 The colon provides a large volume capacity and efficiency for bacterial metabolism of unabsorbed carbohydrates and for absorbing fermentation products. These colonic properties help to prevent diarrhea due to fecal excretion of osmotic loads. 122 , 123 , 124 This colonic salvage becomes saturated as the quantity of carbohydrates reaching the colon increases. 77 , 78 Short chain fatty acids, which are metabolic products of bacterial carbohydrate metabolism, considerably increase colonic transit time, especially in the left colon, and thereby allow for longer contact between the malabsorbed carbohydrate and bacterial flora. 55 The colon also has a high capacity to absorb gas, however with increasing accumulation of colonic gas the efficiency of colonic gas absorption decreases. 33 Colorectal distension by gas remaining in the colon results in symptoms such as bloating or pain. 81
Ingestion of as little as 3 g of lactose has been reported to induce symptoms in some individuals. 104 , 125 , 126 However, in controlled and blinded studies, most persons with lactose malabsorption can tolerate at least 12 g in the absence of a meal. A pooled analysis of studies has suggested that incremental doses of lactose increase the number of individuals who report abdominal symptoms. 110 In a double blind study performed in a Chinese population with primary lactase deficiency, there was a similar incremental increase in gas production with the dose of lactose ingested in both healthy controls and patients with diarrhea‐predominant IBS. The ingestion of 10 g lactose rarely induced abdominal symptoms in healthy controls, 22% reported symptoms after the ingestion of 20 g lactose, and this number increased to 73% after the ingestion of 40 g lactose. 120 The same dose‐dependent increase in symptoms was observed in IBS patients, although the percentage of patients reporting symptoms was always higher as compared to non‐IBS controls, especially at low to moderate doses. 120 Thus, the prevalence of lactose intolerance is higher in IBS than in a healthy control population, even though the prevalence of lactose malabsorption is comparable, 119 indicating that heightened visceral sensitivity or other factors unrelated to lactose malabsorption play a role in symptom development after carbohydrate ingestion.
The tolerance of lactose in milk may depend on whether milk is consumed alone or together with other food, and the lactose in milk may be better tolerated than in aqueous solutions. 127 When lactose malabsorbers ingest lactose with other nutrients, they usually tolerate the consumption of up to 18 g of lactose without notable symptoms. 128 , 129 Indeed, consumption of up to 70 g per day of lactose in divided doses may be tolerated without a change of clinical symptoms as compared to a low lactose control period. 110 , 130
A minimal dose of fructose that is tolerated by a majority of consumers has not been evaluated systematically, although doses below 25 g of fructose have not caused abdominal symptoms in healthy volunteers, even in verified fructose malabsorbers. 131
Statement 2.22
The likelihood of reporting symptoms and the severity of symptoms in individuals with lactose malabsorption depends on the dose of lactose.
Q: A, 85% agree, 0% disagree
Statement 2.23
Doses of lactose exceeding 10 g are required to induce appreciable symptoms (i.e., intolerance).
Q: C, 87% agree, 0% disagree
Statement 2.24
The likelihood of reporting symptoms and the severity of symptoms depends on the degree of visceral sensitivity.
Q: C, 81% agree, 0% disagree
Statement 2.25
The severity of symptoms depends on whether the carbohydrate is administered in a single or split dose or together with other nutrients.
Q: C, 81% agree, 0% disagree
Statement 2.26
Abdominal symptoms may arise after carbohydrate ingestion without objective evidence of malabsorption on breath test. This may be due to a false negative malabsorption test or other mechanisms not related to malabsorption such as a nocebo‐effect (i.e., patients expectation of symptoms) or visceral hypersensitivity to distension of the gastrointestinal tract by the test meal (e.g., functional dyspepsia, irritable bowel syndrome), food allergy (especially if symptoms occur after whole milk or other food) or other mechanisms that have not yet been described. This entity needs further studies as to its pathogenesis and therapeutic relevance.
Q: B, 85% agree, 0% disagree
Statement Ped. 2.27
In pediatric patients, there is no strong correlation between symptoms and the activity of lactase.
Q: C, 83% agree, 0% disagree
What is the time course of symptoms of carbohydrate intolerance?
Symptoms of lactose intolerance are diarrhea, abdominal pain, bloating, flatulence, vomiting, and nausea. 18 , 19 Various symptoms arise at different points in time after carbohydrate ingestion, and the duration of individual symptoms may differ as well. 11 , 82 In patients with intolerance, symptoms such as pain, bloating, and flatulence may precede onset of diarrhea by several hours after carbohydrate ingestion. The majority of intestinal symptoms occurs in the first 4 h after carbohydrate load. 132 However, symptoms may persist and diarrhea may occur after patients have left the outpatient clinic having resumed their normal daily activities.
The typical symptoms resulting from carbohydrate malabsorption are generally attributed to the consequences of the carbohydrate reaching the large intestine and its fermentation by colonic bacteria. However, there is a complex interplay between products of bacterial carbohydrate metabolism and different structures and functions of the human GI tract resulting in inter‐individual differences in symptom development. 68 While colonic events play a major role in symptom generation, some symptoms develop rapidly after a carbohydrate load before intestinal contents can reach the colon. This may suggest that distension of the small intestine by fluids 133 or a rapid increase in colonic luminal contents of gas 133 contribute to some symptoms after a carbohydrate load. 80
Patients who report symptoms within a few minutes (<10 min) after ingestion of the test carbohydrate are likely to have functional dyspepsia triggered by gastric distension rather than a specific food intolerance. 134 The possibility of SIBO should also be considered in subjects with early symptoms. 135
Statement 2.28
There is a different time course for each individual symptom after carbohydrate ingestion.
Q: A, 84% agree, 0% disagree
Recommendation 2.29
Duration of symptom assessment longer than 3–5 h may be useful, but in that case, the influence of food consumed during this period should be considered.
Q: C, S: C, 83% agree, 3% disagree
How can lactose malabsorption and intolerance manifest in children?
As in adults, the amount of lactose that causes symptoms has a high inter‐individual variability. It depends not only on the degree of enzyme deficiency, but also on the lactose amount and other components of lactose‐containing food, on sensitivity to chemical and mechanical gut stimulation, on gut motility and on differences in microbiota. 12 , 18 , 46 , 84 , 85 , 86 , 87 , 112 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 If symptoms develop in the first 60 min after ingestion of the test carbohydrate, functional dyspepsia related to visceral sensitivity to gastric distension and psychological factors should be considered.
The contribution of these factors is difficult to estimate but together they determine the presence of symptoms after ingestion of lactose and other carbohydrates. 82 , 114 , 115 Moreover, particularly in young infants, confusion may arise between symptoms caused by the lactose or the protein content of milk. Gastrointestinal symptoms after milk are mostly non‐IgE mediated (negative allergy tests), and infant milk formulas used for treatment (hydrolyzed cow's milk formulas or soy formulas) may be lactose free.
A diagnosis of lactose intolerance shall only be considered when symptoms are documented. In young children, symptom assessment may be challenging because of limited verbal communication and the caregiver's interpretation of symptoms of their children. Language of the questions should be adapted to be easily understandable for children and a Likert‐type face scale should be used, like in the recently published pediatric Carbohydrate Perception Questionnaire (pCPQ). 18 This questionnaire has been validated in German language in Austria in 215 children and adolescents who underwent a fructose or lactose H2BT for diagnostic workup of persistent non‐organic abdominal pain. 18 Patients completed the pCPQ with or without their caregivers' assistance at baseline and every 30 min up to 3 h during the breath tests. Noteworthy, in this study a larger proportion of children had symptoms after lactose ingestion than had malabsorption (46% vs. 32%), while the reverse was true after fructose ingestion (37% vs. 44%). Overall, 21% of this pediatric population reported symptoms despite the absence of malabsorption and, conversely, 18% of children with malabsorption did not report symptoms during the observation period of 3 h. 18 For its use in other languages, and in German language regions other than Austria, this questionnaire will have to be translated and validated using child‐specific terms, which may have regional differences even in the same language.
When clinical history reveals a relation between lactose intake and development of symptoms, preferably by the use of a validated questionnaire, lactose intolerance may be suspected, and a lactose free diet can be tried for a period of 2 weeks. If symptoms resolve on diet and recur at reintroduction of dairy foods, a H2BT may not be necessary to make the diagnosis. If clinical symptoms are uncertain or the correlation is unclear, H2BT with symptom measurement is the least invasive and most reliable test to diagnose lactose malabsorption and intolerance. 18 , 19 , 82 , 86
Statement Ped 2.30
In pediatric patients there is no strong correlation between symptoms and the activity of lactase.
Q: C, 83% agree, 0% disagree
Recommendation Ped 2.31
H2 breath testing with symptom assessment shall be performed in children with uncertain correlation between food containing lactose or fructose and gastrointestinal symptoms.
Q: C, S: A; 90% agree, 0% disagree
Recommendation Ped 2.32
H2 breath testing does not need to be performed in children with a clear correlation between ingestion of a specific carbohydrate and gastrointestinal symptoms, as documented by relief of symptoms when this carbohydrate is avoided and recurrence of symptoms when the carbohydrate is reintroduced in the diet.
Q: C, S: A; 100% agree
How shall the H2BT be performed and interpreted in children?
The test is commonly performed over a period of 2–3 h in the morning after overnight fasting, although for the assessment of diarrhea, a more prolonged recording time of up to 4–6 h may be needed. If the baseline value is ≥15 ppm, children are commonly asked to rinse their mouth with tap water and then repeat the breath sample collection. It has been suggested, that the H2BT shall only be started if the H2 baseline value is <15 ppm in order to exclude children with SIBO. 35
In pediatric subjects, there is no consensus on the dose of the carbohydrate used for the test. For the lactose H2BT, the administered dose in children varies from 0.5 to 2.0 g/kg lactose dissolved or suspended in water to obtain a 10%–20% concentration, up to a maximum of 25–50 g of lactose (corresponding to the lactose content in 500–1000 ml of cow's milk). 18 , 35 , 86 , 136 Fructose and sorbitol H2BT have been introduced in the last decades but with less clear indications and standardized methodology. For fructose, the dose used has ranged between 0.5 and 1.0 g/kg in a 10% water solution or suspension, up to a maximum of 25–50 g; for sorbitol, the dose used has ranged between 0.2 g/kg in a 10% watery solution with a volume of 6 ml/kg (maximum volume 300 ml), and a total dose of 5–10 g. 35 , 82 , 137 It is recommended not to use fluids other than water for dissolving or suspending the carbohydrate because this may lead to symptoms already in the first 30 min. 82 Correlation between a history of symptoms and malabsorption of fructose in children is poor 82 , 136 ; hence, the clinical utility of fructose H2BT is still debated. The identification of fructose intolerance with a validated symptom questionnaire 18 , 101 may in the future help to identify children who would benefit from a fructose reduced diet. Another potential therapeutic option, which needs to be studied in more detail in the future, is the use of a dietary supplement containing D‐xylose isomerase, which catalyzes the conversion of fructose to glucose 145 and has been shown to decrease breath H2 excretion, nausea, and abdominal pain in adult patients with malabsorption of fructose during a fructose H2 breath test. 52 Fructose malabsorption or fructose intolerance needs to be distinguished from hereditary fructosemia, which is a genetic metabolic disease resulting from an enzyme defect leading to hypoglycaemia and symptoms starting in the first months of life as soon as fructose is introduced into diet. 146
Breath samples are commonly collected and analyzed every 15–30 min after ingestion of the test‐carbohydrate. Usually an increase of H2 concentration of ≥20 ppm over baseline after 60 min is diagnostic for lactose malabsorption. It has been suggested, that if the baseline H2 is ≥10 ppm, a two‐fold increase of H2 in three consecutive breath samples can also be interpreted as a positive lactose H2BT. 136 An early peak of expired H2 in the first 30 min is suggestive of SIBO. H2 non‐excretion has been reported in 10%–15% of pediatric patients, 86 and for these patients, assessment of CH4 levels has been proposed, with an increase of ≥10 ppm being considered as a positive test result. However, CH4 measurements require more expensive technical equipment and collection of breath samples may be more complicated and therefore not applicable to all pediatric patients, 109 , 147 , 148 , 149 and the diagnostic gain of additional CH4‐measurement over measurement of H2 alone in children and adolescents is disputed. 62
Statement Ped. 2.33
There is no consensus on the amount of carbohydrate intake to be tested in pediatric patients.
Q: C, 89% agree, 5% disagree
Statement Ped. 2.34
For lactose, the dose used for the test in pediatric patients varies from 0.5 to 2.0 g/kg dissolved in a 10%–20% water solution, up to a maximum of 25–50 g.
Q: B, 95% agree, 5% disagree
Statement Ped. 2.35
For fructose, the dose used for the test in pediatric patients ranges between 0.5 and 1.0 g/kg in a 10% water solution, up to a maximum of 25–50 g.
Q: B, 89% agree, 5% disagree
Recommendation Ped. 2.36
Breath samples should be collected every 30 min after the carbohydrate ingestion over a period of 3 h.
Q: C, S: B, 96% agree, 0% disagree
Recommendation Ped. 2.37
The methodology, interpretation and clinical utility of testing for fructose and sorbitol in pediatric patients have to be clarified and further standardized in future studies.
Q: C, S. A, 94% agree, 0% disagree
SMALL INTESTINAL BACTERIAL OVERGROWTH
Introduction
Small intestinal bacterial overgrowth is a condition in which the small bowel is colonized by excessive numbers of aerobic and anaerobic microbes that are normally found in the large intestine. 150 , 151 The normal balance between bacterial flora and host is maintained by many factors. The most important control mechanisms are gastric acid secretion, anatomical integrity of the digestive tract, propulsive peristaltic activity, IgA secretive immunoglobulins and, to a lesser extent, other secretions such as saliva, bile and pancreatic juice. Failure of these mechanisms can be responsible for the development of intestinal microbial imbalance such as SIBO. 6 , 10 , 150 , 151
In many studies, SIBO is defined as the microbiological presence of at least >105 colony‐forming units per ml of colonic bacteria in jejunal aspirate. The qualitative microbiological composition of contaminating flora is important. 10 Most reports indicate a predominant role of colonic Gram‐negative anaerobe bacteria in this condition, with the presence of other organisms considered not to have the same impact on health. The clinical presentation of SIBO is highly variable. It may be asymptomatic, cause abdominal pain and bloating indistinguishable from IBS or result in severe symptoms with effects not only on carbohydrate digestion but also the metabolism of amino acids and bile acids with impaired uptake of vitamin B12 and other nutrients. 107 , 150 , 152 , 153 , 154 In such cases, the use of antibiotics can improve the digestion and absorption of nutrients and improve symptoms; however, if the underlying condition that led to colonization of the small bowel with colonic bacteria is not addressed, then the problem can recur. 21 , 150 , 155 , 156 , 157 , 158 , 159
There is no strong consensus regarding the appropriate performance and interpretation of diagnostic tests for SIBO. With regard to H2BT, the impact of confounding factors such as variability in OCTT on test results, and uncertainty regarding diagnostic cut‐offs has led to the absence of a universally accepted definition of SIBO based on this technology. 160
Statement 3.1
Small intestinal bacterial overgrowth is the abnormal presence of excessive numbers of bacteria in the small intestine.
Q: A, 94% agree, 0% disagree
Statement 3.2
Small intestinal bacterial overgrowth may be more likely to be clinically relevant if the bacteria in the small bowel are anaerobes.
Q: B, 81% agree, 3% disagree
Which patients shall be tested for SIBO?
SIBO is characterized by a wide spectrum of clinical manifestations, ranging from unspecific, “functional” abdominal symptoms (e.g., bloating, abdominal discomfort, and flatulence) to less frequent severe generalized malabsorption and nutrient deficiency (diarrhea, anemia, deficiency of vitamins, and iron, steatorrhea, weight loss). 150
Multiple independent risk factors have been identified for SIBO including: anatomical abnormalities such as small intestinal diverticulosis; postsurgical structural changes such as ileocecal valve resection, gastric bypass, and Roux‐en‐Y; medications that slow gut motility such as narcotics, anticholinergics, and anti‐diarrheals; hypo‐ or achlorhydria due to surgery, autoimmune gastritis, or proton pump inhibitors; and small bowel dysmotility irrespective of the cause (e.g., inflammatory bowel disease, celiac disease, radiation enteritis, small bowel adhesions, and systemic diseases associated with dysmotility such as scleroderma, diabetes, and amyloidosis). In addition, SIBO has been associated with multiple conditions including IBS, rosacea, hepatic encephalopathy, obesity, gastroparesis, Parkinson's disease, fibromyalgia, chronic pancreatitis, end‐stage renal disease, and inflammatory bowel diseases. 150 , 161
The culture of jejunal aspirate has been considered for long time the gold standard diagnostic test for SIBO. 6 However this approach is invasive, difficult to perform, 162 , 163 and aspirates from the proximal jejunum lack sensitivity in all but the most severe cases of bacterial overgrowth which extend towards the upper parts of the small bowel. 164 H2BT have long been used to detect SIBO based on the principle that an increase in H2 indicates the contact between carbohydrates and bacteria in the GI tract. H2BT has become the most used test for SIBO in clinical practice 10 ; however, this is largely due to its ease of use, non‐invasive character and low cost and not based on evidence from clinical trials.
Statement 3.3
Small intestinal bacterial overgrowth is characterized by a wide clinical spectrum ranging from mild and unspecific intestinal symptoms to a severe malabsorption syndrome.
Q: A, 94% agree, 3% disagree
Recommendation 3.4
Until a true gold standard is established, H2 breath testing can be used for the diagnostic evaluation of small intestinal bacterial overgrowth.
Q: A, S: B, 80% agree, 3% disagree
Recommendation 3.5
Evaluation of small intestinal bacterial overgrowth with an H2BT can be considered in the presence of bloating, abdominal discomfort and flatulence and/or signs of malabsorption, in the absence of another diagnosis on endoscopy or imaging, especially if there are underlying conditions which increase the risk of small intestinal bacterial overgrowth (i.e., moderate to high pre‐test probability).
Q: A, S: B, 85% agree, 3% disagree
Recommendation 3.6
After the basal fasting breath sampling, a standard 15‐min sampling rate is recommended in clinical studies.
Q: A, S: B, 88% agree, 3% disagree
Recommendation 3.7
A standard 120‐min test duration is recommended in the investigation of small intestinal bacterial overgrowth by glucose H2 breath testing.
Q: A S: B 90% agree, 0% disagree
Which test substrate should be used for the diagnosis of SIBO?
Substrates, which have been used for breath testing for SIBO, include glucose and lactulose. The dose and the rate of absorption of the substrate, the rate of gastric and intestinal transit, as well as the extent of SIBO (if present), will determine where and when contact between the test carbohydrate and enteric bacteria will take place. Glucose is rapidly absorbed in the duodenum and jejunum and this may restrict the sensitivity of the test, with false negative breath tests occurring with this substrate if the bacteria occupy only the lower parts of the small intestine which are not reached by the ingested glucose. 164 , 166 Conversely, false positive results occur in patients with relatively rapid OCTT in whom glucose may reach the colon. 165 , 166 Lactulose is not absorbed by the small intestine 77 and a breath test using this substrate will identify contact with bacteria both in the small bowel and in the colon. 33 , 37 , 58 Therefore, used in isolation, only early increases in breath H2 concentration observed during the lactulose H2BT may be interpreted as being due to bacteria in the small bowel, although early increases may also be due to rapid OCTT. 22 , 167 Overall, specificity is similar with both substrates (80%–85%), but the glucose H2BT is claimed to have a higher sensitivity (62% vs. 52%) and diagnostic accuracy (72% vs. 55%) than lactulose H2BT, especially in non‐surgical patients 163 , 167 , 168 and if the conventional double‐peak criterion on lactulose H2BT is used to diagnose SIBO. For both substrates, the diagnostic accuracy of breath testing for SIBO can be greatly improved by combining this technique with an independent assessment of OCTT (e.g., scintigraphy). 21 , 22 , 107 , 165
Different protocols exist for H2BT in the diagnosis of SIBO, with doses as high as 100 g. 169 , 170 In the case of glucose, 50 g diluted in 250 ml of water has been commonly used. 168 The North American Consensus has recommended the use of 75 g of glucose, 10 but validation studies have indicated that this large dose is commonly associated with false positive diagnoses. 165
In the case of lactulose, the use of 10–25 g of lactulose has been suggested without causing an excessive acceleration of OCTT. The advantage of a higher lactulose dose is an increased sensitivity due to increased H2 production. Moreover, higher doses provide the opportunity to assess a potential carbohydrate intolerance (lactulose is a representative FODMAP). 21 , 171 , 172 , 173 Concurrent assessment of breath H2 and symptoms may provide direct evidence that carbohydrate fermentation is associated with patient reports of abdominal bloating, pain, or diarrhea in IBS patients. 172 , 173 , 174
Recommendation 3.8
For the assessment of small intestinal bacterial overgrowth by H2 breath testing, glucose or lactulose can be used.
Q: B, S: B, 82% agree, 5% disagree
Statement 3.9
H2 breath testing with lactulose or glucose may result in false‐positive diagnoses of small intestinal bacterial overgrowth caused by rapid transit with early colonic fermentation of the substrate.
Q: A, 94% agree, 0% disagree
Statement 3.10
In the absence of concomitant scintigraphy to assess small bowel transit time, glucose should be preferred in non‐surgical patients because the false positive rate for detection of small intestinal bacterial overgrowth is lower with glucose than with lactulose.
Q: B, 83% agree, 0% disagree
Recommendation 3.11
The standard dose of glucose for small intestinal bacterial overgrowth testing by H2 breath testing shall be 50 g diluted in 250 ml water.
Q: A, S: B, 83% agree, 0% disagree
Recommendation Ped 3.11
In children the dose of glucose for small intestinal bacterial overgrowth testing by H2 breath testing shall be 2 g/kg (maximum 50 g) diluted in 200–250 ml water.
Q: A, 100% agree
Statement 3.12
A commonly used dose of lactulose for small intestinal bacterial overgrowth testing by H2 breath testing is 10–20 g diluted in 250 ml water.
Q: A, 90% agree 5% disagree
Recommendation Ped 3.12
In children the dose of lactulose for small intestinal bacterial overgrowth testing by H2 breath testing shall be 10–20 g diluted in 100–200 ml water.
Q: A, 95% agree, 5% disagree
How shall results of H2BT for SIBO be interpreted?
For both glucose and lactulose H2BTs, SIBO is confirmed if there is a relevant increase in breath H2 before the test substrate enters the cecum. In SIBO, glucose usually results in a single “early” peak of H2 excretion. The most used cut‐off value for test positivity is 10–12 ppm. Conversely, lactulose may result in an early peak with progressive increase in H2 excretion as the substrate enters the colon or two distinct H2 peaks: the first “early” peak being linked to the small intestinal microflora activity and the second “late” peak indicating colonic bacterial metabolism. 175 , 176
The key limitations of H2BT for diagnosis of SIBO are the high variability in OCTT in health and disease 21 , 22 , 177 resulting in a high rate of false‐positive diagnoses of SIBO by lactulose breath test 21 , 22 and glucose breath test, 165 and the variations in breath H2 response to lactulose with repeated testing. 106 In particular, rapid small bowel transit is common in IBS and this confounds SIBO diagnosis when based on an early rise in breath H2 during lactulose breath testing. Combination of H2BT with an independent measurement of OCTT (usually scintigraphy) has been proposed to increase the specificity of SIBO diagnosis. 22 , 178 , 179 , 180 , 181 A low H2 cut‐off value of 5 ppm may increase sensitivity without sacrificing specificity when performed using the combined lactulose breath test/scintigraphy technique. 21
The guideline refrains from requesting the routine use of scintigraphy in combination with breath tests, because scintigraphy has a limited availability. If a breath test with concurrent scintigraphy is not available, then two options remain: (1) Interpretation of results considering pre‐test probability of SIBO and (2) Serial tests, with the H2BT being followed by a transit test with scintigraphy to distinguish SIBO from rapid transit. The use of serial tests may be less ideal because of relatively high day‐to‐day variation in OCTT.
The measurement of CH4 excretion may improve test performance in H2BTs performed to detect SIBO. Patients with CH4‐predominant bacterial overgrowth may have increased prevalence of abdominal bloating and abdominal distension with constipation. Further, the severity of constipation may correlate with CH4 excretion. 6 , 10 , 150 , 151 , 161 , 163 , 182
Statement 3.13
The diagnostic criteria for diagnosis of small intestinal bacterial overgrowth using breath testing have not been confirmed and uniformly accepted, and the clinical relevance of a positive result needs to be considered in the light of the pre‐test probability of small intestinal bacterial overgrowth in the individual patient.
Q: A, 91% agree; 0% disagree
Statement 3.14
The use of H2 breath testing for diagnosis of small intestinal bacterial overgrowth is non‐invasive, safe and inexpensive; however, the interpretation of results is limited by important confounding factors, in particular the variability of oro‐cecal transit time.
Q: A, 100% agree, 0% disagree
Statement 3.15
The risk of a false positive or false negative breath test for small intestinal bacterial overgrowth can be reduced by combining the breath test with an independent measurement of oro‐cecal transit time, for example scintigraphy.
Q: A, 91% agree, 3% disagree
ORO‐CECAL TRANSIT TIME
Introduction
The OCTT reflects the sum of esophageal, gastric, and small bowel transit. 183 The time between the ingestion of an unabsorbable fermentable carbohydrate and the start of a quantifiable rise of H2 concentration in end‐expiratory breath, can be used to estimate OCTT, assuming that the bacterial metabolism of the ingested test carbohydrate happens in the colon and that there are no relevant numbers of bacteria in the small bowel (SIBO). Several technical factors, such as the consistency and type of test food, or type and dose of the test substrate, may affect the expected normal range and the reproducibility of H2BT for OCTT. The H2BT does not generate a measure of OCTT in “low H2 producers” or “H2 non‐excretors.” 1 Measurement of OCTT with unabsorbable carbohydrates, such as lactulose or inulin, is well tolerated and safe. Colonic fermentation of carbohydrates can induce the perception of bloating and abdominal distension, which is dose‐dependent and usually of mild intensity. 184 A range of methodologies have been applied for OCTT measurement by H2BT. Since there are no comparative studies of these different approaches, the guideline team decided to refrain from making specific recommendations for this section of the guideline and, instead, to summarize the current approaches in statements.
What is the background of using H2BT for measuring OCTT?
The measurement of OCTT with H2BT is supported by evidence of significant correlation between the transit times measured with this technique, commonly using lactulose as the non‐absorbable fermentable carbohydrate, with transit times measured by barium meal, 185 scintigraphy 21 , 22 , 179 or magnetic resonance imaging. 186 Results of H2BT for measuring OCTT of a caloric meal with added inulin as a fermentable carbohydrate, or of a mixed meal containing baked beans coincide with the transit time measured with lactose 13C‐ureide breath test 187 , 188 or with the arrival of radioactive marker in the cecum. 189
Fast OCTT has been reported in patients with IBS with diarrhea, 22 , 190 partial gastrectomy, 191 postvagotomy diarrhea, 192 and hyperthyroidism. 193 , 194 , 195 Delayed OCTT has been reported in patients with depression, 196 constipation, 197 IBS with constipation, 22 acromegalia, 198 diabetes, 199 pregnancy, 200 cholecystectomy, 201 obesity, 202 cirrhosis, 203 scleroderma, 204 and gallstones before and after cholecystectomy. 205 Accelerated OCTT has been shown with various medications including misoprostol, 206 erythromycin, 207 metoclopramide, 208 paroxetine, 209 cisapride, 210 and prucalopride, 211 and OCTT may be delayed by loperamide, 212 , 213 ritodrine, 214 dopamine, 215 peppermint oil, 210 imipramine, 209 and n‐butylscopolamine. 210
Although alterations in OCTT have been demonstrated in a wide variety of conditions, no routine clinical indications for measuring OCTT have been identified, 216 and therefore this method is applied only to address specific clinical issues such as exclusion of generalized motility disorders in patients under consideration for subtotal colectomy in the management of treatment refractory constipation. Otherwise, this technique is generally restricted in use to addressing scientific questions.
Statement 4.1
The time between the ingestion of an unabsorbable fermentable organic compound and the start of colonic fermentation, revealed by a quantifiable rise of H2 concentration in the expiratory breath, can be used to estimate oro‐cecal transit time.
Q: A, 92% agree, 0% disagree.
Statement 4.2
Measurement of oro‐cecal transit time with H2 breath testing is a widely available, non‐invasive, and safe technique, not associated with radiation exposure.
Q: A, 100% agree
Recommendation 4.3
H2 breath testing may be used to demonstrate the effect of a pharmacological intervention on gastrointestinal transit.
Q: A, S: B, 85% agree, 0% disagree
Statement 4.4
Measurement of oro‐cecal transit time by H2 breath testing has not yet found a clear indication in clinical practice.
Q.A, 92% agree, 0% disagree
Which test meals and fermentable substrates shall be used for OCTT?
Solid and liquid meals with added fermentable unabsorbable carbohydrates have been used to assess OCTT by H2BT. Expected normal ranges of OCTT are influenced by composition and consistency of the test meal 217 and the composition and dose of the unabsorbable carbohydrate. Solid meals have a longer OCTT and a better reproducibility as compared to liquid meals. 189 , 218 , 219 , 220
The most frequently used fermentable unabsorbable carbohydrate is lactulose. Commonly, a small dose (10 g) is used in adults, although also larger doses of up to 30 g have been used. 218 , 221 Higher doses of lactulose result in more pronounced increases of H2 concentration in breath 58 and therefore may increase sensitivity of the H2BT 179 , 218 ; however, lactulose dose‐dependently accelerates small bowel transit time 187 and increases variability of OCTT. There may be less variability of OCTT with liquid meals. 218 As an alternative to osmotically active lactulose 77 and its effects on transit, 55 , 179 inulin has been used as a fermentable substrate for OCTT. In a study in which 5 g of inulin was added to a 330 Kcal solid food pancakes meal 187 or to a 400 ml liquid enteral standard nutrition 188 inulin had no influence on transit, whereas lactulose significantly shortened OCTT. 187
The start and the interval of breath collections has to take into account that very short OCTT (<15 min) has been demonstrated by scintigraphy in IBS, 22 , 165 suggesting that short time intervals between breath samples (<15 min) are needed in selected patients (i.e., IBS with diarrhea), especially at the beginning of the study. Scintigraphic studies have also demonstrated, that liquid meals containing lactulose or glucose can reach the colon in considerably less than 90 min in patients with rapid OCTT. 22 , 165 , 166
Statement 4.5
There is no uniformly accepted protocol for using H2 breath testing for measurement of oro‐cecal transit time.
Q: B, 95% agree, 3% disagree
Statement 4.6
Several liquid and solid substrates have been used in the assessment of oro‐cecal transit time. The substrates often used are (1) the non‐absorbable sugar lactulose diluted in water or added to a liquid meal and (2) the polysaccharide inulin added to a solid meal.
Q: A, 98% agree, 0% disagree
Statement 4.7
A dose of 10–20 g of lactulose in 100 ml of water has been used to measure oro‐cecal transit time of a liquid meal. Transit time shortens with increasing doses of lactulose.
Q: A, 95% agree; 0% disagree
Statement Ped. 4.7
In pediatric patients 10 g lactulose has been used to measure oro‐cecal transit time in volumes of intake ranging from 20 ml of a 50% solution to 100 ml of a 10% solution.
Q: C, 94% agree, 0% disagree
Statement 4.8
A dose of 5 g of inulin in a defined caloric meal has been used to measure oro‐cecal transit time of a solid meal.
Q: A, 86% agree, 3% disagree
Recommendation 4.9
End‐expiratory breath samples should be collected at baseline and thereafter at every 10–15 min at least for 240 min after ingestion of the unabsorbable fermentable organic compound, or until the increase in breath hydrogen has occurred.
Q: A, S: B; 92% agree, 0% disagree
How shall results of OCTT be interpreted?
Increments of 3, 5, or 10 ppm of H2 above baseline have been used to indicate arrival of the substrate in the cecum. The OCTT lengthens as cut‐off values of H2 concentration above baseline are increased. 185 Early H2‐peaks, which may be wrongly interpreted as rapid OCTT, 222 may be due to the fermentation by bacteria in the mouth, 48 emptying of ileal residues from a previous meal into the colon 222 or by SIBO. 10 , 223 Care has to be observed in interpreting results of H2BT because of large variations in breath H2 response to lactulose with repeated testing. 106
The simultaneous assessment of OCTT with a scintigraphic technique is useful to distinguish early peaks of H2 due to fast OCTT from peaks due to SIBO. 21 , 22 However, the guideline refrains from requesting the routine use of scintigraphy in combination with breath tests, because scintigraphy has a limited availability.
Statement 4.10
Increments of at least 3, 5 or 10 ppm of hydrogen above baseline (mean of two pre‐meal samples) maintained or increased in the two following determinations have been used to define the oro‐cecal transit time. The oro‐cecal transit time lengthens as the chosen cut‐off value of H2 increases.
Q: A, 85% agree, 0% disagree
Statement 4.11
The oro‐cecal transit time in adult healthy subjects depends on the test substance, its dose and the type of the meal. Transit times between 40 and 170 min have been described for a lactulose liquid non‐caloric meal and between 420 and 570 min for inulin added to a caloric meal.
Q: B, 95% agree, 3% disagree
Statement Ped. 4.11
In healthy children the oro‐cecal transit time ranges between 30 and 120 min for a lactulose breath test.
Q: C, 94% agree,0% disagree
CONCLUSIONS AND FUTURE PERSPECTIVES
This consensus‐based clinical practice guideline aims to assist physicians and provide them with the information required to deliver high quality care for patients with suspected carbohydrate malabsorption, carbohydrate intolerance, SIBO, or suspicion of altered OCTT. In a consensus process in which representatives from all regions of Europe and specialists representing European scientific societies participated, the available evidence was evaluated, taking account of local facilities, diverse clinical practice, and health care environments. Patient involvement was not covered in our guideline because of its focus on diagnostic recommendations.
We have previously described the structured procedure which we have developed to organize this multinational guideline. 23 A balance between the weight of evidence, the expertise of leading scientists, and the results of the Delphi voting process representing the opinions of experienced physicians working in different European regions and in a wide range of clinical circumstances was found. Consensus was achieved, by acknowledging majority positions, which were not always evidence‐based, and by formulating statements and recommendations that acknowledged the absence of evidence but provided clear guidance based on clinical experience. Regarding some applications of breath testing, it can be argued that the evidence base is scarce and contradictory so that this specific test should not be performed at all. However, some methods and some tests are so well established in clinical practice that these techniques should not be excluded from a consensus clinical guideline. We consider that this approach is justified if it helps to ensure that the guideline will be widely adopted and that tests will be performed, analyzed, and interpreted to consistent standards.
The guideline identifies areas in which further research is needed and sets the agenda for research in diagnosis and treatment of carbohydrate intolerances, in SIBO, and in OCTT. The clear definition of terms and the new focus on validated symptom assessment to detect carbohydrate intolerances 18 , 20 , 101 should in the future allow more focused clinical research in the detection and treatment of specific intolerances in diseases such as IBS, in which many of the documented trigger foods contain large quantities of simple or complex carbohydrates. 224 Therapeutic trials focusing on patients with documented carbohydrate intolerance, rather than carbohydrate malabsorption, may help to improve knowledge about the rational use of treatment options like diets or drugs. 52 , 91 , 96 , 173 , 225 , 226 , 227 , 228 , 229 Future studies should also address whether in diseases such as IBS the detection and treatment of specific carbohydrate intolerances (“precision knife approach”) will improve the patients' quality of life, costs of living, long‐term safety, and health outcomes as compared to empirical and less specific dietary advice (“shot gun approach”). 96 , 115 , 230 In the face of criticism of the clinical relevance of H2BT in the past, 100 future studies will have to show to which extent validated symptom measurement for the diagnosis and clinical follow‐up of carbohydrate intolerances 18 , 20 , 101 will find a place as an adjunct or may even become a replacement of H2BT.
For the use of H2BT in the diagnosis of SIBO, future studies may focus on better defining the role of different substrates used for the test, on diagnostic criteria, on a combination of breath tests with independent measurement of OCTT and on the comparison of H2BT with the new and developing methods of microbiological analysis of intestinal contents. Given the limitations of H2BT, it is hoped that new technology may improve the diagnosis of SIBO. Recently, a wireless gas sensing capsule has been described that greatly increases sensitivity and signal‐to‐noise ratio in measuring luminal H2 concentrations and may provide concurrent information on the site of intestinal gas production. If confirmed, this device has potential for improving diagnostic precision for disorders such as SIBO. 231 , 232 At the same time, the introduction of advanced molecular techniques has opened new clinical scenarios for gut microbiota involvement that go beyond the confine of a classical malabsorption syndrome and may allow clinicians to dissect the contribution of intestinal bacteria to different clinical manifestations. 150 For the use of breath tests in OCTT, agreement on more uniformly accepted test protocols, including agreement on test carbohydrates, test meals and test protocol, is needed.
After publication of the guideline, a qualitative and quantitative assessment of guideline adoption into clinical practice is planned, including patient and public involvement. Moreover, new recommendations in the guideline, such as the use of validated symptom assessment to diagnose carbohydrate intolerance, and introduction of new methodology to diagnose SIBO, should be evaluated not only with regard to their acceptance by patients and medical practitioners but also to assess the cost‐benefit and utility of these approaches in clinical practice.
MEMBERS OF EUROPEAN H2‐CH4‐BREATH TEST GROUP
Altorjay, Istvan; Barbara, Giovanni; Basilisco, Guido; Baumann‐Durchschein, Franziska; Belei, Oana; Benninga, Marc; Borrelli, Osvaldo; Churchev, Stanislav; Dominguez‐Munoz, Enrique; Dumitrascu, Dan; Effenberger, Maria; Fox, Mark; Fürst, Stefan; Gasbarrini, Antonio; Götze, Oliver; Haas, Stephan; Hammer, Heinz; Hammer, Johann; Hammer, Karin; Hauser, Bruno; Herszenyi, Laszlo; Keller, Jutta; Krznaric, Zeljko; Leja, Marcis; Lopetuso, Loris; Mion, Francois; Mulak, Agata; Nakov, Radislav; Nakov, Ventsislav; Pohl, Daniel; Reinisch, Sieglinde; Salvatore, Silvia; Shvets, Oleg; Simren, Magnus; Sonyi, Marc; Surdea‐Blaga, Teodora; Tepes, Bojan; Thapar, Nikhil; Törnblom, Hans; Tutuian Radu; Verbeke, Kristin; Vogelsang, Harald; Vranesic‐Bender, Darija; Wilder‐Smith, Clive.
CONFLICT OF INTEREST
Heinz F. Hammer and Johann Hammer are shareholders of Carboception GmbH. The other authors do not declare any conflict of interest.
ETHICS STATEMENT
The guideline was supported by a guideline development grant from UEG. Heinz Hammer and Johann Hammer are shareholders of Carboception GmbH
Hammer HF, Fox MR, Keller J, Salvatore S, Basilisco G, Hammer J, et al. European guideline on indications, performance, and clinical impact of hydrogen and methane breath tests in adult and pediatric patients: European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Neurogastroenterology and Motility, and European Society for Paediatric Gastroenterology Hepatology and Nutrition consensus. United European Gastroenterol J. 2022;10(1):15–40. 10.1002/ueg2.12133
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281:122–7. 10.1056/NEJM196907172810303 [DOI] [PubMed] [Google Scholar]
- 2. Vernia P, Di Camillo M, Marinaro V, Caprilli R. Effect of predominant methanogenic flora on the outcome of lactose breath test in irritable bowel syndrome patients. Eur J Clin Nutr. 2003;57:1116–9. [DOI] [PubMed] [Google Scholar]
- 3. Read NW, Al‐Janabi MN, Bates TE, Holgate AM, Cann PA, Kinsman RI, et al. Interpretation of the breath hydrogen profile obtained after ingesting a solid meal containing unabsorbable carbohydrate. Gut. 1985;26:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levitt MD, Donaldson RM. Use of respiratory hydrogen (H2) excretion to detect carbohydrate malabsorption. J Lab Clin Med. 1970;75:937–45. [PubMed] [Google Scholar]
- 5. Schoeller DA, Schneider JF, Solomons NW, Watkins JB, Klein PD. Clinical diagnosis with the stable isotope 13C in CO2 breath tests: methodology and fundamental considerations. J Lab Clin Med. 1977;90:412–21. [PubMed] [Google Scholar]
- 6. Gasbarrini A, Corazza GR, Gasbarrini G, Montalto M, Di Stefano M, Basilisco G, et al. Methodology and indications of H2‐breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29(Suppl 1):1–49. 10.1111/j.1365-2036.2009.03951.x [DOI] [PubMed] [Google Scholar]
- 7. Keller J, Franke A, Storr M, Wiedbrauck F, Schirra J. [Clinically relevant breath tests in gastroenterological diagnostics–recommendations of the German Society for Neurogastroenterology and Motility as well as the German Society for Digestive and Metabolic Diseases]. Z Gastroenterol. 2005;43:1071–90. 10.1055/s-2005-858479 [DOI] [PubMed] [Google Scholar]
- 8. Maffei HV, Metz GL, Jenkins DJ. Hydrogen breath test: adaptation of a simple technique to infants and children. Lancet. 1976;1:1110–1. 10.1016/s0140-6736(76)90069-6 [DOI] [PubMed] [Google Scholar]
- 9. Maffei HV, Metz G, Bampoe V, Shiner M, Herman S, Brook CGD. Lactose intolerance, detected by the hydrogen breath test, in infants and children with chronic diarrhoea. Arch Dis Child. 1977;52:766–71. 10.1136/adc.52.10.766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, et al. Hydrogen and methane‐based breath testing in gastrointestinal disorders: the North American Consensus. Am J Gastroenterol. 2017;112:775–84. 10.1038/ajg.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilder‐Smith CH, Olesen SS, Materna A, Drewes AM. Fermentable sugar ingestion, gas production, and gastrointestinal and central nervous system symptoms in patients with functional disorders. Gastroenterology. 2018;155:1034–44. 10.1053/j.gastro.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 12. Hammer HF, Petritsch W, Pristautz H, Krejs GJ. Evaluation of the pathogenesis of flatulence and abdominal cramps in patients with lactose malabsorption. Wien Klin Wochenschr. 1996;108:175–9. [PubMed] [Google Scholar]
- 13. Hammer HF, Hammer J, Fox M. Mistakes in the management of carbohydrate intolerance and how to avoid them. UEG Education. 2019;19:9–14. [Google Scholar]
- 14. Abaturov AY, Stepanov YM, Nikulina AA. Treatment of lactase deficiency in children's obesity with genotype c/c 13910 of lactase gene. Wiad Lek. 2019;72:17–21. [PubMed] [Google Scholar]
- 15. Fernandez‐Banares F, Rosinach M, Esteve M, Forné M, Espinós JC, Maria Viver J. Sugar malabsorption in functional abdominal bloating: a pilot study on the long‐term effect of dietary treatment. Clin Nutr. 2006;25:824–31. 10.1016/j.clnu.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 16. Medow MS, Thek KD, Newman LJ, Berezin S, Glassman MS, Schwarz SM. Beta‐galactosidase tablets in the treatment of lactose intolerance in pediatrics. Am J Dis Child. 1990;144:1261–4. 10.1001/archpedi.1990.02150350093034 [DOI] [PubMed] [Google Scholar]
- 17. Misselwitz B, Pohl D, Fruhauf H, Fried M, Vavricka SR, Fox M. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J. 2013;1:151–9. 10.1177/2050640613484463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammer J, Memaran N, Huber WD, Hammer K. Development and validation of the paediatric Carbohydrate Perception Questionnaire (pCPQ), an instrument for the assessment of carbohydrate‐induced gastrointestinal symptoms in the paediatric population. Neuro Gastroenterol Motil. 2020;32:e13934. 10.1111/nmo.13934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammer K, Hammer J. Valid assessment of carbohydrate intolerance and the need for a distinction to carbohydrate malabsorption. Comment on “roles of lactose and fructose malabsorption and dietary outcomes in children presenting with chronic abdominal pain.”, nutrients, 2019, 11, 3063. Nutrients. 2020;12(6):1546. 10.3390/nu12061546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hammer J, Sonyi M, Engesser KM, Riedl G, Luong S, Hammer HF. Carbohydrate‐induced gastrointestinal symptoms: development and validation of a test‐specific symptom questionnaire for an adult population, the adult Carbohydrate Perception Questionnaire. Eur J Gastroenterol Hepatol. 2020;32:171–7. 10.1097/MEG.0000000000001880 [DOI] [PubMed] [Google Scholar]
- 21. Zhao J, Zheng X, Chu H, Zhao J, Cong Y, Fried M, et al. A study of the methodological and clinical validity of the combined lactulose hydrogen breath test with scintigraphic oro‐cecal transit test for diagnosing small intestinal bacterial overgrowth in IBS patients. Neuro Gastroenterol Motil. 2014;26:794–802. 10.1111/nmo.12331 [DOI] [PubMed] [Google Scholar]
- 22. Yu D, Cheeseman F, Vanner S. Combined oro‐caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro‐caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–40. 10.1136/gut.2009.205476 [DOI] [PubMed] [Google Scholar]
- 23. Sonyi M, Keller J, Fox M, Hammer HF. Development of a multinational clinical practice guideline: a practical structured procedure. Dig Dis. 2020. Epub ahead of print. 10.1159/000511007 [DOI] [PubMed] [Google Scholar]
- 24. Medicine OCfEB. https://www.cebm.ox.ac.uk/resources/levels‐of‐evidence/oxford‐centre‐for‐evidence‐based‐medicine‐levels‐of‐evidence‐march‐2009 (2009). Accessed 28 Sept 2020.
- 25. Wirts CW, Rehfuss ME, Snape WJ, Swenson PC. Effect of tea on gastric secretions and motility. J Am Med Assoc. 1954;155:724–9. [PubMed] [Google Scholar]
- 26. Chaudhuri L, Basu S, Seth P, Chaudhuri T, Besra SE, Vedasiromoni JR, et al. Prokinetic effect of black tea on gastrointestinal motility. Life Sci. 2000;66:847–54. 10.1016/s0024-3205(99)00657-8 [DOI] [PubMed] [Google Scholar]
- 27. Ellis CJ, Kneip JM, Levitt MD. Storage of breath samples for H2 analyses. Gastroenterology. 1988;94:822–4. 10.1016/0016-5085(88)90260-0 [DOI] [PubMed] [Google Scholar]
- 28. Levitt MD, Ellis C, Furne J. Influence of method of alveolar air collection on results of breath tests. Dig Dis Sci. 1998;43:1938–45. 10.1023/a:1018874223418 [DOI] [PubMed] [Google Scholar]
- 29. Metz G, Jenkins DJ, Peters TJ, Newman A, Blendis L. Breath hydrogen as a diagnostic method for hypolactasia. Lancet. 1975;1:1155–7. 10.1016/s0140-6736(75)93135-9 [DOI] [PubMed] [Google Scholar]
- 30. Brummer RJ, Karibe M, Stockbrugger RW. Lactose malabsorption. Optimalization of investigational methods. Scand J Gastroenterol Suppl. 1993;200:65–9. [PubMed] [Google Scholar]
- 31. Corazza GR, Sorge M, Maurino E, Strocchi A, Lattanzi MC, Gasbarrini G. Methodology of the H2 breath test. I. Collection and storage for gas measurement. Ital J Gastroenterol. 1990;22:200–4. [PubMed] [Google Scholar]
- 32. Corazza GR, Sorge M, Strocchi A, Lattanzi MC, Benati G, Gasbarrini G. Methodology of the H2 breath test. II. Importance of the test duration in the diagnosis of carbohydrate malabsorption. Ital J Gastroenterol. 1990;22:303–5. [PubMed] [Google Scholar]
- 33. Hammer HF. Colonic hydrogen absorption: quantification of its effect on hydrogen accumulation caused by bacterial fermentation of carbohydrates. Gut. 1993;34:818–22. 10.1136/gut.34.6.818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shrestha A, Prodhan UK, Mitchell SM, Sharma P, Barnett MPG, Milan AM, et al. Validity of a portable breath analyser (AIRE) for the assessment of lactose malabsorption. Nutrients. 2019;11:1636. 10.3390/nu11071636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anania C, Pacifico L, Olivero G, Osborn JF, Bonaiuto E, Chiesa C. Breath tests in pediatrics. Clin Chim Acta. 2008;397:1–12. 10.1016/j.cca.2008.07.023 [DOI] [PubMed] [Google Scholar]
- 36. Niu HC, Schoeller DA, Klein PD. Improved gas chromatographic quantitation of breath hydrogen by normalization to respiratory carbon dioxide. J Lab Clin Med. 1979;94:755–63. [PubMed] [Google Scholar]
- 37. Saad RJ, Chey WD. Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol 2014;12:1964–72;quiz e1119‐1920. 10.1016/j.cgh.2013.09.055 [DOI] [PubMed] [Google Scholar]
- 38. D'Angelo G, Di Rienzo TA, Scaldaferri F, Del Zompo F, Pizzoferrato M, Lopetuso LR, et al. Tricks for interpreting and making a good report on hydrogen and 13C breath tests. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 2):90–8. [PubMed] [Google Scholar]
- 39. Fox MR, Kahrilas PJ, Roman S, Roman S, Gyawali CP, Scott SM, et al. Clinical measurement of gastrointestinal motility and function: who, when and which test? Nat Rev Gastroenterol Hepatol. 2018;15:568–79. 10.1038/s41575-018-0030-9 [DOI] [PubMed] [Google Scholar]
- 40. Gilat T, Ben Hur H, Gelman‐Malachi E, Terdiman R, Peled Y. Alterations of the colonic flora and their effect on the hydrogen breath test. Gut. 1978;19:602–5. 10.1136/gut.19.7.602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strocchi A, Corazza G, Ellis CJ, Gasbarrini G, Levitt MD. Detection of malabsorption of low doses of carbohydrate: accuracy of various breath H2 criteria. Gastroenterology. 1993;105:1404–10. 10.1016/0016-5085(93)90145-3 [DOI] [PubMed] [Google Scholar]
- 42. Hogenauer C, Hammer HF, Krejs GJ, Reisinger EC. Mechanisms and management of antibiotic‐associated diarrhea. Clin Infect Dis. 1998;27:702–10. 10.1086/514958 [DOI] [PubMed] [Google Scholar]
- 43. Levitt MD, Hirsh P, Fetzer C, Sheahan M, Levine AS. H2 excretion after ingestion of complex carbohydrates. Gastroenterology. 1987;92:383–9. [DOI] [PubMed] [Google Scholar]
- 44. Thompson D, Binfield P, De Belder A, O'Brien J, Warren S, Wilson M. Extra intestinal influences on exhaled breath hydrogen measurements during the investigation of gastrointestinal disease. Gut. 1985;26:1349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosenthal A, Solomons N. Time‐course of cigarette smoke contamination of clinical hydrogen breath‐analysis tests. Clin Chem. 1983;29:1980–1. [PubMed] [Google Scholar]
- 46. Wilder‐Smith C, Olesen SS, Materna A, Drewes AM. Predictors of response to a low‐FODMAP diet in patients with functional gastrointestinal disorders and lactose or fructose intolerance. Aliment Pharmacol Ther. 2017;45:1094–106. 10.1111/apt.13978 [DOI] [PubMed] [Google Scholar]
- 47. Brummer RJ, Armbrecht U, Bosaeus I, Dotevall G, Stockbruegger RW. The hydrogen (H2) breath test. Sampling methods and the influence of dietary fibre on fasting level. Scand J Gastroenterol. 1985;20:1007–13. 10.3109/00365528509088863 [DOI] [PubMed] [Google Scholar]
- 48. Thompson DG, O'Brien JD, Hardie JM. Influence of the oropharyngeal microflora on the measurement of exhaled breath hydrogen. Gastroenterology. 1986;91:853–60. 10.1016/0016-5085(86)90686-4 [DOI] [PubMed] [Google Scholar]
- 49. Mastropaolo G, Rees WD. Evaluation of the hydrogen breath test in man: definition and elimination of the early hydrogen peak. Gut. 1987;28:721–5. 10.1136/gut.28.6.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perman JA, Modler S, Engel RR, Heldt G. Effect of ventilation on breath hydrogen measurements. J Lab Clin Med. 1985;105:436–9. [PubMed] [Google Scholar]
- 51. Payne DL, Welsh JD, Claypool PL. Breath hydrogen (H2) response to carbohydrate malabsorption after exercise. J Lab Clin Med. 1983;102:147–50. [PubMed] [Google Scholar]
- 52. Komericki P, Akkilic‐Materna M, Strimitzer T, Weyermair K, Hammer HF, Aberer W. Oral xylose isomerase decreases breath hydrogen excretion and improves gastrointestinal symptoms in fructose malabsorption–a double‐blind, placebo‐controlled study. Aliment Pharmacol Ther. 2012;36:980–7. 10.1111/apt.12057 [DOI] [PubMed] [Google Scholar]
- 53. Hammer HF, Hammer J, Gasche C. [Polyethylene glycol (Macrogol)–an overview of its use in diagnosis and therapy of gastrointestinal diseases]. Wien Klin Wochenschr. 2000;112:53–60. [PubMed] [Google Scholar]
- 54. Barrett JS, Canale KE, Gearry RB, Irving PM, Gibson PR. Probiotic effects on intestinal fermentation patterns in patients with irritable bowel syndrome. World J Gastroenterol. 2008;14:5020–4. 10.3748/wjg.14.5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fritz E, Hammer HF, Lipp RW, Hogenauer C, Stauber R, Hammer J. Effects of lactulose and polyethylene glycol on colonic transit. Aliment Pharmacol Ther. 2005;21:259–68. 10.1111/j.1365-2036.2005.02244.x [DOI] [PubMed] [Google Scholar]
- 56. Fruhwald S, Herk E, Hammer HF, Holzer P, Metzler H. Differential reversal of drug‐induced small bowel paralysis by cerulein and neostigmine. Intensive Care Med. 2004;30:1414–20. 10.1007/s00134-004-2317-2 [DOI] [PubMed] [Google Scholar]
- 57. Pimentel M, Morales W, Lezcano S, Sun‐Chuan D, Low K, Yang J. Low‐dose nocturnal tegaserod or erythromycin delays symptom recurrence after treatment of irritable bowel syndrome based on presumed bacterial overgrowth. Gastroenterol Hepatol. 2009;5:435. [PMC free article] [PubMed] [Google Scholar]
- 58. Hammer HF, Sheikh MS. Colonic gas excretion in induced carbohydrate malabsorption–effect of simethicone. Eur J Gastroenterol Hepatol. 1992;4:141–5. [Google Scholar]
- 59. Christl S, Gibson G, Cummings J. Role of dietary sulphate in the regulation of methanogenesis in the human large intestine. Gut. 1992;33:1234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vogelsang H, Ferenci P, Frotz S, Meryn S, Gangl A. Acidic colonic microclimate–possible reason for false negative hydrogen breath tests. Gut. 1988;29:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hammer HF, Petritsch W, Pristautz H, Krejs GJ. Assessment of the influence of hydrogen nonexcretion on the usefulness of the hydrogen breath test and lactose tolerance test. Wien Klin Wochenschr. 1996;108:137–41. [PubMed] [Google Scholar]
- 62. Hammer K, Hasanagic H, Memaran N, Huber WD, Hammer J. Relevance of methane and carbon dioxide evaluation in breath tests for carbohydrate malabsorption in a paediatric cohort. J Pediatr Gastroenterol Nutr. 2021. [DOI] [PubMed] [Google Scholar]
- 63. Ghoshal UC, Srivastava D, Misra A. A randomized double‐blind placebo‐controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: a pilot study. Indian J Gastroenterol. 2018;37:416–23. 10.1007/s12664-018-0901-6 [DOI] [PubMed] [Google Scholar]
- 64. Nobigrot T, Chasalow FI, Lifshitz F. Carbohydrate absorption from one serving of fruit juice in young children: age and carbohydrate composition effects. J Am Coll Nutr. 1997;16:152–8. 10.1080/07315724.1997.10718666 [DOI] [PubMed] [Google Scholar]
- 65. Coyne MJ, Rodriguez H. Carbohydrate malabsorption in black and Hispanic dialysis patients. Am J Gastroenterol. 1986;81:662–5. [PubMed] [Google Scholar]
- 66. Montalto M, Gallo A, Ojetti V, Gasbarrini A. Fructose, trehalose and sorbitol malabsorption. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 2):26–9. [PubMed] [Google Scholar]
- 67. Born P, Zech J, Stark M, Classen M, Lorenz R. [Carbohydrate substitutes: comparative study of intestinal absorption of fructose, sorbitol and xylitol]. Med Klin. 1994;89:575–8. [PubMed] [Google Scholar]
- 68. Misselwitz B, Butter M, Verbeke K, Fox MR. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut. 2019;68:2080–91. 10.1136/gutjnl-2019-318404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Auricchio S, Rubino A, Landolt M, Semenza G, Prader A. Isolated intestinal lactase deficiency in the adult. Lancet. 1963;2:324–6. 10.1016/s0140-6736(63)92991-x [DOI] [PubMed] [Google Scholar]
- 70. Meance S, Giordano J, Chuang E, Schneider H. Food regulations: low FODMAP labeling and communication goals. J Gastroenterol Hepatol. 2017;32(Suppl 1):62–3. 10.1111/jgh.13699 [DOI] [PubMed] [Google Scholar]
- 71. Flourie B, Leblond A, Florent C, Rautureau M, Bisalli A, Rambaud J‐C. Starch malabsorption and breath gas excretion in healthy humans consuming low‐ and high‐starch diets. Gastroenterology. 1988;95:356–63. 10.1016/0016-5085(88)90491-x [DOI] [PubMed] [Google Scholar]
- 72. Scheppach W, Fabian C, Ahrens F, Spengler M, Kasper H. Effect of starch malabsorption on colonic function and metabolism in humans. Gastroenterology. 1988;95:1549–55. 10.1016/s0016-5085(88)80076-3 [DOI] [PubMed] [Google Scholar]
- 73. Shulman RJ. Starch malabsorption in infants. J Pediatr Gastroenterol Nutr. 2018;66(Suppl 3):S65–S67. 10.1097/MPG.0000000000001856 [DOI] [PubMed] [Google Scholar]
- 74. Bortolotti M, Levorato M, Lugli A, Mazzero G. Effect of a balanced mixture of dietary fibers on gastric emptying, intestinal transit and body weight. Ann Nutr Metab. 2008;52:221–6. 10.1159/000138127 [DOI] [PubMed] [Google Scholar]
- 75. Kaur A, Rose DJ, Rumpagaporn P, Patterson JA, Hamaker BR. In vitro batch fecal fermentation comparison of gas and short‐chain fatty acid production using “slowly fermentable” dietary fibers. J Food Sci. 2011;76:H137–H142. 10.1111/j.1750-3841.2011.02172.x [DOI] [PubMed] [Google Scholar]
- 76. Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50–60. 10.5740/jaoacint.sge_macfarlane [DOI] [PubMed] [Google Scholar]
- 77. Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest. 1989;84:1056–62. 10.1172/JCI114267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hammer HF, Fine KD, Santa Ana CA, Porter JL, Schiller LR, Fordtran JS. Carbohydrate malabsorption. Its measurement and its contribution to diarrhea. J Clin Invest. 1990;86:1936–44. 10.1172/JCI114927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Linlawan S, Patcharatrakul T, Somlaw N, Gonlachanvit S. Effect of rice, wheat, and mung bean ingestion on intestinal gas production and postprandial gastrointestinal symptoms in non‐constipation irritable bowel syndrome patients. Nutrients. 2019;11:2061. 10.3390/nu11092061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate‐related symptoms in individuals with irritable bowel syndrome. Gastroenterology. 2017;152:124–33. 10.1053/j.gastro.2016.09.062 [DOI] [PubMed] [Google Scholar]
- 81. Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol. 1998;274:G584–G590. 10.1152/ajpgi.1998.274.3.G584 [DOI] [PubMed] [Google Scholar]
- 82. Hammer V, Hammer K, Memaran N, Huber W‐D, Hammer K, Hammer J. Relationship between abdominal symptoms and fructose ingestion in children with chronic abdominal pain. Dig Dis Sci. 2018;63:1270–9. 10.1007/s10620-018-4997-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Deng Y, Misselwitz B, Dai N, Fox M. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients. 2015;7:8020–35. 10.3390/nu7095380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang J, Fox M, Cong Y, Chu H, Zheng X, Long Y, et al. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment Pharmacol Ther. 2014;39:302–11. 10.1111/apt.12582 [DOI] [PubMed] [Google Scholar]
- 85. Zheng X, Chu H, Cong Y, Deng Y, Long Y, Zhu Y, et al. Self‐reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: prevalence, risk factors, and impact on food choices. Neuro Gastroenterol Motil. 2015;27:1138–46. 10.1111/nmo.12602 [DOI] [PubMed] [Google Scholar]
- 86. Heyman MB, Committee on N. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118:1279–86. 10.1542/peds.2006-1721 [DOI] [PubMed] [Google Scholar]
- 87. Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice–myths and realities. Aliment Pharmacol Ther. 2008;27:93–103. 10.1111/j.1365-2036.2007.03557.x [DOI] [PubMed] [Google Scholar]
- 88. Hogenauer C, Hammer HF, Mellitzer K, Renner W, Krejs GJ, Toplak H. Evaluation of a new DNA test compared with the lactose hydrogen breath test for the diagnosis of lactase non‐persistence. Eur J Gastroenterol Hepatol. 2005;17:371–6. 10.1097/00042737-200503000-00018 [DOI] [PubMed] [Google Scholar]
- 89. Marton A, Xue X, Szilagyi A. Meta‐analysis: the diagnostic accuracy of lactose breath hydrogen or lactose tolerance tests for predicting the North European lactase polymorphism C/T‐13910. Aliment Pharmacol Ther. 2012;35:429–40. 10.1111/j.1365-2036.2011.04962.x [DOI] [PubMed] [Google Scholar]
- 90. Wanes D, Husein DM, Naim HY. Congenital lactase deficiency: mutations, functional and biochemical implications, and future perspectives. Nutrients. 2019;11:461. 10.3390/nu11020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Francesconi CF, Machado MB, Steinwurz F, Nones RB, Quilici FA, Catapani WR, et al. Oral administration of exogenous lactase in tablets for patients diagnosed with lactose intolerance due to primary hypolactasia. Arq Gastroenterol. 2016;53:228–34. 10.1590/S0004-28032016000400004 [DOI] [PubMed] [Google Scholar]
- 92. Escobar MA, Jr. , Lustig D, Pflugeisen BM, Amoroso PJ, Sherif D, Saeed R, et al. Fructose intolerance/malabsorption and recurrent abdominal pain in children. J Pediatr Gastroenterol Nutr. 2014;58:498–501. 10.1097/MPG.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 93. Bolin TD, Myo K, Soe A, Genge JR, Duncombe VM. Correlation of hydrogen and methane production to rice carbohydrate malabsorption in Burmese (Myanmar) children. J Pediatr Gastroenterol Nutr. 1996;22:144–7. 10.1097/00005176-199602000-00003 [DOI] [PubMed] [Google Scholar]
- 94. Caballero B, Solomons NW, Torun B. Fecal reducing substances and breath hydrogen excretion as indicators of carbohydrate malabsorption. J Pediatr Gastroenterol Nutr. 1983;2:487–90. 10.1097/00005176-198302030-00016 [DOI] [PubMed] [Google Scholar]
- 95. Maffei HV, Daher SR, Moreira FL. Carbohydrate malabsorption in infants with diarrhea: diagnostic and evolutive aspects. Arq Gastroenterol. 1984;21:136–42. [PubMed] [Google Scholar]
- 96. Bellini M, Tonarelli S, Nagy AG, Pancetti A, Costa F, Ricchiuti A, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:12. 10.3390/nu12010148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Levitt MD. Malabsorption of starch: a normal phenomenon. Gastroenterology. 1983;85:769–70. [PubMed] [Google Scholar]
- 98. Wolever TM, Robb PA. Effect of guar, pectin, psyllium, soy polysaccharide, and cellulose on breath hydrogen and methane in healthy subjects. Am J Gastroenterol. 1992;87:305–10. [PubMed] [Google Scholar]
- 99. Newcomer AD, McGill DB, Thomas PJ, Hofmann AF. Prospective comparison of indirect methods for detecting lactase deficiency. N Engl J Med. 1975;293:1232–6. 10.1056/NEJM197512112932405 [DOI] [PubMed] [Google Scholar]
- 100. Suarez F, Levitt MD. Should we test for lactose malabsorption? Ital J Gastroenterol Hepatol. 1997;29:113–6. [PubMed] [Google Scholar]
- 101. www.carboception.com. Accessed 11 Aug 2021.
- 102. Monsalve‐Hernando C, Crespo L, Ferreiro B, Martín V, Aldeguer X, Opio V, et al. Phase IV noninferiority controlled randomized trial to evaluate the impact on diagnostic thinking and patient management and the test‐retest reproducibility of the gaxilose test for hypolactasia diagnosis. Medicine (Baltim). 2018;97:e13136. 10.1097/MD.0000000000013136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Berni Canani R, Pezzella V, Amoroso A, Cozzolino T, Di Scala C, Passariello A. Diagnosing and treating intolerance to carbohydrates in children. Nutrients. 2016;8:157. 10.3390/nu8030157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shaukat A, Levitt MD, Taylor BC, MacDonald R, Shamliyan TA, Kane RL, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med. 2010;152:797–803. 10.7326/0003-4819-152-12-201006150-00241 [DOI] [PubMed] [Google Scholar]
- 105. Usai‐Satta P, Scarpa M, Oppia F, Cabras F. Lactose malabsorption and intolerance: what should be the best clinical management? World J Gastrointest Pharmacol Therapeut. 2012;3:29–33. 10.4292/wjgpt.v3.i3.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yao CK, Tuck CJ, Barrett JS, Canale KE, Philpott HL, Gibson PR. Poor reproducibility of breath hydrogen testing: implications for its application in functional bowel disorders. United European Gastroenterol J. 2017;5:284–92. 10.1177/2050640616657978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhao J, Fox M, Cong Y, Chu H, Shang Y, Fried M, et al. Lactose intolerance in patients with chronic functional diarrhoea: the role of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2010;31:892–900. 10.1111/j.1365-2036.2010.04252.x [DOI] [PubMed] [Google Scholar]
- 108. Houben E, De Preter V, Billen J, Van Ranst M, Verbeke K. Additional value of CH(4) measurement in a combined (13)C/H(2) lactose malabsorption breath test: a retrospective analysis. Nutrients. 2015;7:7469–85. 10.3390/nu7095348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. De Lacy Costello BP, Ledochowski M, Ratcliffe NM. The importance of methane breath testing: a review. J Breath Res. 2013;7:024001. 10.1088/1752-7155/7/2/024001 [DOI] [PubMed] [Google Scholar]
- 110. Levitt M, Wilt T, Shaukat A. Clinical implications of lactose malabsorption versus lactose intolerance. J Clin Gastroenterol. 2013;47:471–80. 10.1097/MCG.0b013e3182889f0f [DOI] [PubMed] [Google Scholar]
- 111. Di Costanzo M, Berni Canani R. Lactose intolerance: common misunderstandings. Ann Nutr Metab. 2018;73(Suppl 4):30–7. 10.1159/000493669 [DOI] [PubMed] [Google Scholar]
- 112. Chumpitazi BP, Shulman RJ. Dietary carbohydrates and childhood functional abdominal pain. Ann Nutr Metab. 2016;68(Suppl 1):8–17. 10.1159/000445390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ghoshal UC, Kumar S, Misra A, Mittal B. Lactose malabsorption diagnosed by 50‐g dose is inferior to assess clinical intolerance and to predict response to milk withdrawal than 25‐g dose in an endemic area. J Gastroenterol Hepatol. 2013;28:1462–8. 10.1111/jgh.12273 [DOI] [PubMed] [Google Scholar]
- 114. Turco R, Salvatore S, Miele E, Romano C, Marseglia GL, Staiano A. Does a low FODMAPs diet reduce symptoms of functional abdominal pain disorders? A systematic review in adult and paediatric population, on behalf of Italian Society of Pediatrics. Ital J Pediatr. 2018;44:53. 10.1186/s13052-018-0495-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pensabene L, Salvatore S, Turco R, Tarsitano F, Concolino D, Baldassarre ME, et al. Low FODMAPs diet for functional abdominal pain disorders in children: critical review of current knowledge. J Pediatr. 2019;95:642–56. 10.1016/j.jped.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 116. Vernia P, Ricciardi MR, Frandina C, Bilotta T, Frieri G. Lactose malabsorption and irritable bowel syndrome. Effect of a long‐term lactose‐free diet. Ital J Gastroenterol. 1995;27:117–21. [PubMed] [Google Scholar]
- 117. Choi BC, Pak AW. A catalog of biases in questionnaires. Prev Chronic Dis. 2005;2:A13. [PMC free article] [PubMed] [Google Scholar]
- 118. Wilder‐Smith CH, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013;37:1074–83. 10.1111/apt.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Varju P, Gede N, Szakacs Z, Hegyi P, Cazacu IM, Pécsi D, et al. Lactose intolerance but not lactose maldigestion is more frequent in patients with irritable bowel syndrome than in healthy controls: a meta‐analysis. Neuro Gastroenterol Motil. 2019;31:e13527. 10.1111/nmo.13527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yang J, Deng Y, Chu H, Cong Y, Zhao J, Pohl D, et al. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11:262–8 e261. 10.1016/j.cgh.2012.11.034 [DOI] [PubMed] [Google Scholar]
- 121. Catassi C, Elli L, Bonaz B, Bouma G, Carroccio A, Castillejo G, et al. Diagnosis of non‐celiac gluten sensitivity (NCGS): the Salerno experts' criteria. Nutrients. 2015;7:4966–77. 10.3390/nu7064966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hammer J, Pruckmayer M, Bergmann H, Kletter K, Gangl A. The distal colon provides reserve storage capacity during colonic fluid overload. Gut. 1997;41:658–63. 10.1136/gut.41.5.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hammer J, Phillips SF. Fluid loading of the human colon: effects on segmental transit and stool composition. Gastroenterology. 1993;105:988–98. 10.1016/0016-5085(93)90941-5 [DOI] [PubMed] [Google Scholar]
- 124. Hammer HF, Hammer J. Diarrhea caused by carbohydrate malabsorption. Gastroenterol Clin N Am. 2012;41:611–27. 10.1016/j.gtc.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 125. Gudmand‐Hoyer E, Simony K. Individual sensitivity to lactose in lactose malabsorption. Am J Dig Dis. 1977;22:177–81. 10.1007/BF01072273 [DOI] [PubMed] [Google Scholar]
- 126. Bedine MS, Bayless TM. Intolerance of small amounts of lactose by individuals with low lactase levels. Gastroenterology. 1973;65:735–43. [PubMed] [Google Scholar]
- 127. Leichter J. Comparison of whole milk and skim milk with aqueous lactose solution in lactose tolerance testing. Am J Clin Nutr. 1973;26:393–6. 10.1093/ajcn/26.4.393 [DOI] [PubMed] [Google Scholar]
- 128. Suarez FL, Adshead J, Furne JK, Levitt MD. Lactose maldigestion is not an impediment to the intake of 1500 mg calcium daily as dairy products. Am J Clin Nutr. 1998;68:1118–22. 10.1093/ajcn/68.5.1118 [DOI] [PubMed] [Google Scholar]
- 129. Wilt TJ, Shaukat A, Shamliyan T, Taylor BC, MacDonald R, Tacklind J, et al. Lactose intolerance and health. Evid Rep Technol Assess. 2010:1–410. [PMC free article] [PubMed] [Google Scholar]
- 130. Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr. 1996;64:232–6. 10.1093/ajcn/64.2.232 [DOI] [PubMed] [Google Scholar]
- 131. Rumessen JJ, Gudmand‐Hoyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27:1161–8. 10.1136/gut.27.10.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pohl D, Savarino E, Hersberger M, Behlis Z, Stutz B, Goetze O, et al. Excellent agreement between genetic and hydrogen breath tests for lactase deficiency and the role of extended symptom assessment. Br J Nutr. 2010;104:900–7. 10.1017/S0007114510001297 [DOI] [PubMed] [Google Scholar]
- 133. Murray K, Wilkinson‐Smith V, Hoad C, Costigan C, Cox E, Lam C, et al. Differential effects of FODMAPs (fermentable oligo‐, di‐, mono‐saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109:110–9. 10.1038/ajg.2013.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fuhrer M, Hammer J. Lack of an effect of gastric capsaicin on the rectal component of the gastrocolonic response. Dig Dis Sci. 2017;62:3542–9. 10.1007/s10620-017-4822-5 [DOI] [PubMed] [Google Scholar]
- 135. Takakura W, Pimentel M. Small intestinal bacterial overgrowth and irritable bowel syndrome–an update. Front Psychiatr. 2020;11:664. 10.3389/fpsyt.2020.00664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dabritz J, Muhlbauer M, Domagk D, Voos N, Henneböhl G, Siemer ML, et al. Significance of hydrogen breath tests in children with suspected carbohydrate malabsorption. BMC Pediatr. 2014;14:59. 10.1186/1471-2431-14-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Glatstein M, Reif S, Scolnik D, Rom L, Yerushalmy‐Feler A, Dali‐Levy M, et al. Lactose breath test in children: relationship between symptoms during the test and test results. Am J Therapeut. 2018;25:e189–e193. 10.1097/MJT.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 138. Pawlowska K, Seredynski R, Umlawska W, Iwańczak B. Hydrogen excretion in pediatric lactose malabsorbers: relation to symptoms and the dose of lactose. Arch Med Sci. 2018;14:88–93. 10.5114/aoms.2016.57884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Amieva‐Balmori M, Coss‐Adame E, Rao NS, Dávalos‐Pantoja BM, Rao SSC. Diagnostic utility of carbohydrate breath tests for SIBO, fructose, and lactose intolerance. Dig Dis Sci. 2020;65:1405–13. 10.1007/s10620-019-05889-9 [DOI] [PubMed] [Google Scholar]
- 140. Borghini R, Donato G, Alvaro D, Picarelli A. New insights in IBS‐like disorders: Pandora's box has been opened; a review. Gastroenterol Hepatol Bed Bench. 2017;10:79–89. [PMC free article] [PubMed] [Google Scholar]
- 141. Carroccio A, Montalto G, Cavera G, Notarbatolo A. Lactose intolerance and self‐reported milk intolerance: relationship with lactose maldigestion and nutrient intake. Lactase Deficiency Study Group. J Am Coll Nutr. 1998;17:631–6. 10.1080/07315724.1998.10718813 [DOI] [PubMed] [Google Scholar]
- 142. Catanzaro R, Sciuto M, Singh B, Pathak S, Marotta F. Irritable bowel syndrome and lactose intolerance: the importance of differential diagnosis. A monocentric study. Minerva Gastroenterol Dietol. 2020. Epub 2020 Jul 3. 10.23736/S1121-421X.20.02734-8 [DOI] [PubMed] [Google Scholar]
- 143. Choi YK, Johlin FC, Jr. , Summers RW, Jackson M, Rao SSC. Fructose intolerance: an under‐recognized problem. Am J Gastroenterol. 2003;98:1348–53. 10.1111/j.1572-0241.2003.07476.x [DOI] [PubMed] [Google Scholar]
- 144. Vernia P, Di Camillo M, Foglietta T, Avallone VE, De Carolis A. Diagnosis of lactose intolerance and the “nocebo” effect: the role of negative expectations. Dig Liver Dis. 2010;42:616–9. 10.1016/j.dld.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 145. Bhosale SH, Rao MB, Deshpande VV. Molecular and industrial aspects of glucose isomerase. Microbiol Rev. 1996;60:280–300. 10.1128/mr.60.2.280-300.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rennert OM, Greer M. Hereditary fructosemia. Neurology. 1970;20:421. 10.1212/wnl.20.5.421 [DOI] [PubMed] [Google Scholar]
- 147. Corazza GR, Benati G, Strocchi A, Malservisi S, Gasbarrini G. The possible role of breath methane measurement in detecting carbohydrate malabsorption. J Lab Clin Med. 1994;124:695–700. [PubMed] [Google Scholar]
- 148. Knudsen CD, Di Palma JA. Carbohydrate challenge tests: do you need to measure methane? South Med J. 2012;105:251–3. 10.1097/SMJ.0b013e318252d428 [DOI] [PubMed] [Google Scholar]
- 149. Myo K, Bolin TD, Khin Mar O, Tin‐Oo, Kyaw‐Hla S, Thein‐Myint T. Ineffectiveness of breath methane excretion as a diagnostic test for lactose malabsorption. J Pediatr Gastroenterol Nutr. 1999;28:474–9. 10.1097/00005176-199905000-00006 [DOI] [PubMed] [Google Scholar]
- 150. Quigley EMM, Murray JA, Pimentel M. AGA clinical practice update on small intestinal bacterial overgrowth: expert review. Gastroenterology. 2020;159:1526–32. 10.1053/j.gastro.2020.06.090 [DOI] [PubMed] [Google Scholar]
- 151. Rezaie A, Pimentel M, Rao SS. How to test and treat small intestinal bacterial overgrowth: an evidence‐based approach. Curr Gastroenterol Rep. 2016;18:8. 10.1007/s11894-015-0482-9 [DOI] [PubMed] [Google Scholar]
- 152. Donaldson RM, Jr. Studies on the pathogenesis of steatorrhea in the blind loop syndrome. J Clin Invest. 1965;44:1815–25. 10.1172/JCI105289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Drasar BS, Shiner M, McLeod GM. Studies on the intestinal flora. I. The bacterial flora of the gastrointestinal tract in healthy and achlorhydric persons. Gastroenterology. 1969;56:71–9. [PubMed] [Google Scholar]
- 154. Drasar BS, Shiner M. Studies on the intestinal flora. II. Bacterial flora of the small intestine in patients with gastrointestinal disorders. Gut. 1969;10:812–9. 10.1136/gut.10.10.812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Achufusi TGO, Sharma A, Zamora EA, Manocha D. Small intestinal bacterial overgrowth: comprehensive review of diagnosis, prevention, and treatment methods. Cureus. 2020;12:e8860. 10.7759/cureus.8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Avelar Rodriguez D, Ryan PM, Toro Monjaraz EM, Ramirez Mayans JA, Quigley EM. Small intestinal bacterial overgrowth in children: a state‐of‐the‐art review. Front Pediatr. 2019;7:363. 10.3389/fped.2019.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bohm M, Siwiec RM, Wo JM. Diagnosis and management of small intestinal bacterial overgrowth. Nutr Clin Pract. 2013;28:289–99. 10.1177/0884533613485882 [DOI] [PubMed] [Google Scholar]
- 158. Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, et al. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25:237–40. 10.1159/000103892 [DOI] [PubMed] [Google Scholar]
- 159. Ginnebaugh B, Chey WD, Saad R. Small intestinal bacterial overgrowth: how to diagnose and treat (and then treat again). Gastroenterol Clin N Am. 2020;49:571–87. 10.1016/j.gtc.2020.04.010 [DOI] [PubMed] [Google Scholar]
- 160. Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2009;7:1279–86. 10.1016/j.cgh.2009.06.031 [DOI] [PubMed] [Google Scholar]
- 161. Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis. 2013;4:223–31. 10.1177/2040622313496126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Abu‐Shanab A, Quigley EM. Diagnosis of small intestinal bacterial overgrowth: the challenges persist! Expet Rev Gastroenterol Hepatol. 2009;3:77–87. 10.1586/17474124.3.1.77 [DOI] [PubMed] [Google Scholar]
- 163. Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci. 2008;53:1443–54. 10.1007/s10620-007-0065-1 [DOI] [PubMed] [Google Scholar]
- 164. Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. J Am Med Assoc. 2004;292:852–8. 10.1001/jama.292.7.852 [DOI] [PubMed] [Google Scholar]
- 165. Lin EC, Massey BT. Scintigraphy demonstrates high rate of false‐positive results from glucose breath tests for small bowel bacterial overgrowth. Clin Gastroenterol Hepatol. 2016;14:203–8. 10.1016/j.cgh.2015.07.032 [DOI] [PubMed] [Google Scholar]
- 166. Sellin JH, Hart R. Glucose malabsorption associated with rapid intestinal transit. Am J Gastroenterol. 1992;87:584–9. [PubMed] [Google Scholar]
- 167. Ghoshal UC, Shukla R, Ghoshal U. Small intestinal bacterial overgrowth and irritable bowel syndrome: a bridge between functional organic dichotomy. Gut Liver. 2017;11:196–208. 10.5009/gnl16126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Parodi A, Dulbecco P, Savarino E, Giannini EG, Bodini G, Corbo M, et al. Positive glucose breath testing is more prevalent in patients with IBS‐like symptoms compared with controls of similar age and gender distribution. J Clin Gastroenterol. 2009;43:962–6. 10.1097/MCG.0b013e3181a099a5 [DOI] [PubMed] [Google Scholar]
- 169. Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non‐specific diarrhea. J Neurogastroenterol Motil. 2010;16:40–6. 10.5056/jnm.2010.16.1.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sachdeva S, Rawat AK, Reddy RS, Puri AS. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. J Gastroenterol Hepatol. 2011;26(Suppl 3):135–8. 10.1111/j.1440-1746.2011.06654.x [DOI] [PubMed] [Google Scholar]
- 171. Le Neve B, Brazeilles R, Derrien M, Tap J, Guyonnet D, Ohman L, et al. Lactulose challenge determines visceral sensitivity and severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2016;14:226–33 e221‐223. 10.1016/j.cgh.2015.09.039 [DOI] [PubMed] [Google Scholar]
- 172. Pohl D, Van Oudenhove L, Tornblom H, Le Nevé B, Tack J, Simrén M. Functional dyspepsia and severity of psychologic symptoms associate with postprandial symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2018;16:1745–53 e1741. 10.1016/j.cgh.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 173. Schindler V, Giezendanner S, Butikofer S, Murray F, Runggaldier D, Schnurre L, et al. Differentiation of functional gastrointestinal disorders from healthy volunteers by lactulose hydrogen breath test and test meal. J Gastroenterol Hepatol. 2019;34:843–51. 10.1111/jgh.14551 [DOI] [PubMed] [Google Scholar]
- 174. Le Neve B, Posserud I, Bohn L, Guyonnet D, Rondeau P, Tillisch K, et al. A combined nutrient and lactulose challenge test allows symptom‐based clustering of patients with irritable bowel syndrome. Am J Gastroenterol. 2013;108:786–95. 10.1038/ajg.2013.75 [DOI] [PubMed] [Google Scholar]
- 175. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–6. [DOI] [PubMed] [Google Scholar]
- 176. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double‐blind, randomized, placebo‐controlled study. Am J Gastroenterol. 2003;98:412–9. 10.1111/j.1572-0241.2003.07234.x [DOI] [PubMed] [Google Scholar]
- 177. Connolly L, Chang L. Combined orocecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects orocecal transit, not small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gastroenterology. 2011;141:1118–21. 10.1053/j.gastro.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 178. Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD, Thomas MC. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795–803. [PubMed] [Google Scholar]
- 179. Miller MA, Parkman HP, Urbain JL, Brown KL, Donahue DJ, Knight LC, et al. Comparison of scintigraphy and lactulose breath hydrogen test for assessment of orocecal transit: lactulose accelerates small bowel transit. Dig Dis Sci. 1997;42:10–8. 10.1023/a:1018864400566 [DOI] [PubMed] [Google Scholar]
- 180. Bratten JR, Spanier J, Jones MP. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103:958–63. 10.1111/j.1572-0241.2008.01785.x [DOI] [PubMed] [Google Scholar]
- 181. Rao S, Lele V. Scintigraphy of the small intestine: a simplified standard for study of transit with reference to normal values. Eur J Nucl Med Mol Imag. 2002;29:39–45. [DOI] [PubMed] [Google Scholar]
- 182. Low K, Hwang L, Hua J, Zhu A, Morales W, Pimentel M. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol. 2010;44:547–50. 10.1097/MCG.0b013e3181c64c90 [DOI] [PubMed] [Google Scholar]
- 183. Bertram F, Andresen V, Layer P, Keller J. Simultaneous non‐invasive measurement of liquid gastric emptying and small bowel transit by combined 13C‐acetate and H2‐lactulose breath test. J Breath Res. 2014;8:046007. 10.1088/1752-7155/8/4/046007 [DOI] [PubMed] [Google Scholar]
- 184. Basilisco G, Marino B, Passerini L, Ogliari C. Abdominal distension after colonic lactulose fermentation recorded by a new extensometer. Neuro Gastroenterol Motil. 2003;15:427–33. 10.1046/j.1365-2982.2003.00426.x [DOI] [PubMed] [Google Scholar]
- 185. Hirakawa M, Iida M, Kohrogi N, Fujishima M. Hydrogen breath test assessment of orocecal transit time: comparison with barium meal study. Am J Gastroenterol. 1988;83:1361–3. [PubMed] [Google Scholar]
- 186. Savarino E, Savarino V, Fox M, Di Leo G, Furnari M, Marabotto E, et al. Measurement of oro‐caecal transit time by magnetic resonance imaging. Eur Radiol. 2015;25:1579–87. 10.1007/s00330-014-3575-1 [DOI] [PubMed] [Google Scholar]
- 187. Geboes KP, Luypaerts A, Rutgeerts P, Verbeke K. Inulin is an ideal substrate for a hydrogen breath test to measure the orocaecal transit time. Aliment Pharmacol Ther. 2003;18:721–9. 10.1046/j.1365-2036.2003.01750.x [DOI] [PubMed] [Google Scholar]
- 188. Schneider AR, Jepp K, Murczynski L, Biniek U, Stein J. The inulin hydrogen breath test accurately reflects orocaecal transit time. Eur J Clin Invest. 2007;37:802–7. 10.1111/j.1365-2362.2007.01862.x [DOI] [PubMed] [Google Scholar]
- 189. Read NW, Miles CA, Fisher D, Holgate AM, Kime ND, Mitchell MA, et al. Transit of a meal through the stomach, small intestine, and colon in normal subjects and its role in the pathogenesis of diarrhea. Gastroenterology. 1980;79:1276–82. [PubMed] [Google Scholar]
- 190. Corbett CL, Thomas S, Read NW, Hobson N, Bergman I, Holdsworth CD. Electrochemical detector for breath hydrogen determination: measurement of small bowel transit time in normal subjects and patients with the irritable bowel syndrome. Gut. 1981;22:836–40. 10.1136/gut.22.10.836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bond JH, Levitt MD. Use of breath hydrogen (H2) to quantitate small bowel transit time following partial gastrectomy. J Lab Clin Med. 1977;90:30–6. [PubMed] [Google Scholar]
- 192. O'Brien JD, Thompson DG, McIntyre A, Burnham WR, Walker E. Effect of codeine and loperamide on upper intestinal transit and absorption in normal subjects and patients with postvagotomy diarrhoea. Gut. 1988;29:312–8. 10.1136/gut.29.3.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Shafer RB, Prentiss RA, Bond JH. Gastrointestinal transit in thyroid disease. Gastroenterology. 1984;86:852–5. [PubMed] [Google Scholar]
- 194. Bozzani A, Camboni MG, Tidone L, Cesari P, Della Mussia F, Quatrini M, et al. Gastrointestinal transit in hyperthyroid patients before and after propranolol treatment. Am J Gastroenterol. 1985;80:550–2. [PubMed] [Google Scholar]
- 195. Tobin MV, Fisken RA, Diggory RT, Morris AI, Gilmore IT. Orocaecal transit time in health and in thyroid disease. Gut. 1989;30:26–9. 10.1136/gut.30.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Gorard DA, Gomborone JE, Libby GW, Farthing MJ. Intestinal transit in anxiety and depression. Gut. 1996;39:551–5. 10.1136/gut.39.4.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Altomare DF, Portincasa P, Rinaldi M, Di Ciaula A, Martinelli E, Amoruso A, et al. Slow‐transit constipation: solitary symptom of a systemic gastrointestinal disease. Dis Colon Rectum. 1999;42:231–40. 10.1007/BF02237134 [DOI] [PubMed] [Google Scholar]
- 198. Resmini E, Parodi A, Savarino V, Greco A, Rebora A, Minuto F, et al. Evidence of prolonged orocecal transit time and small intestinal bacterial overgrowth in acromegalic patients. J Clin Endocrinol Metab. 2007;92:2119–24. 10.1210/jc.2006-2509 [DOI] [PubMed] [Google Scholar]
- 199. Scarpello JH, Greaves M, Sladen GE. Small intestinal transit in diabetics. Br Med J. 1976;2:1225–6. 10.1136/bmj.2.6046.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lawson M, Kern F, Jr. , Everson GT. Gastrointestinal transit time in human pregnancy: prolongation in the second and third trimesters followed by postpartum normalization. Gastroenterology. 1985;89:996–9. 10.1016/0016-5085(85)90199-4 [DOI] [PubMed] [Google Scholar]
- 201. Fort JM, Azpiroz F, Casellas F, Andreu J, Malagelada J. Bowel habit after cholecystectomy: physiological changes and clinical implications. Gastroenterology. 1996;111:617–22. 10.1053/gast.1996.v111.pm8780565 [DOI] [PubMed] [Google Scholar]
- 202. Basilisco G, Camboni G, Bozzani A, Vita P, Doldi S, Bianchi PA. Orocecal transit delay in obese patients. Dig Dis Sci. 1989;34:509–12. 10.1007/BF01536325 [DOI] [PubMed] [Google Scholar]
- 203. Maheshwari A, Thuluvath PJ. Autonomic neuropathy may be associated with delayed orocaecal transit time in patients with cirrhosis. Auton Neurosci. 2005;118:135–9. 10.1016/j.autneu.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 204. Di Ciaula A, Covelli M, Berardino M, Wang DQ, Lapadula G, Palasciano G, et al. Gastrointestinal symptoms and motility disorders in patients with systemic scleroderma. BMC Gastroenterol. 2008;8:7. 10.1186/1471-230X-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Di Ciaula A, Molina‐Molina E, Bonfrate L, Wang DQ‐H, Dumitrascu DL, Portincasa P. Gastrointestinal defects in gallstone and cholecystectomized patients. Eur J Clin Invest. 2019;49:e13066. 10.1111/eci.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Rutgeerts P, Vantrappen G, Hiele M, Ghoos Y, Onkelinx C. Effects on bowel motility of misoprostol administered before and after meals. Aliment Pharmacol Ther. 1991;5:533–42. 10.1111/j.1365-2036.1991.tb00522.x [DOI] [PubMed] [Google Scholar]
- 207. Minocha A, Katragadda R, Rahal PS, Ries A. Erythromycin shortens orocaecal transit time in diabetic male subjects: a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 1995;9:529–33. 10.1111/j.1365-2036.1995.tb00416.x [DOI] [PubMed] [Google Scholar]
- 208. Staniforth DH. Effect of drugs on oro‐caecal transit time assessed by the lactulose/breath hydrogen method. Eur J Clin Pharmacol. 1987;33:55–8. 10.1007/BF00610380 [DOI] [PubMed] [Google Scholar]
- 209. Gorard DA, Libby GW, Farthing MJ. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:159–66. 10.1111/j.1365-2036.1994.tb00273.x [DOI] [PubMed] [Google Scholar]
- 210. Goerg KJ, Spilker T. Effect of peppermint oil and caraway oil on gastrointestinal motility in healthy volunteers: a pharmacodynamic study using simultaneous determination of gastric and gall‐bladder emptying and orocaecal transit time. Aliment Pharmacol Ther. 2003;17:445–51. 10.1046/j.1365-2036.2003.01421.x [DOI] [PubMed] [Google Scholar]
- 211. Emmanuel AV, Roy AJ, Nicholls TJ, Kamm MA. Prucalopride, a systemic enterokinetic, for the treatment of constipation. Aliment Pharmacol Ther. 2002;16:1347–56. 10.1046/j.1365-2036.2002.01272.x [DOI] [PubMed] [Google Scholar]
- 212. Basilisco G, Bozzani A, Camboni G, Recchia M, Quatrini M, Conte D, et al. Effect of loperamide and naloxone on mouth‐to‐caecum transit time evaluated by lactulose hydrogen breath test. Gut. 1985;26:700–3. 10.1136/gut.26.7.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Basilisco G, Camboni G, Bozzani A, Paravicini M, Bianchi PA. Oral naloxone antagonizes loperamide‐induced delay of orocecal transit. Dig Dis Sci. 1987;32:829–32. 10.1007/BF01296704 [DOI] [PubMed] [Google Scholar]
- 214. Basilisco G, Camboni MG, Bozzani A, Molgora M, Bianchi P. Single doses of ritodrine delay orocaecal transit in patients with irritable bowel syndrome. Br J Clin Pharmacol. 1990;29:355–8. 10.1111/j.1365-2125.1990.tb03647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Levein NG, Thorn SE, Wattwil M. Dopamine delays gastric emptying and prolongs orocaecal transit time in volunteers. Eur J Anaesthesiol. 1999;16:246–50. 10.1046/j.1365-2346.1999.00471.x [DOI] [PubMed] [Google Scholar]
- 216. Keller J, Bassotti G, Clarke J, Clarke J, Dinning P, Fox M, et al. Expert consensus document: advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15:291–308. 10.1038/nrgastro.2018.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Diggory RT, Cuschieri A. The effect of dose and osmolality of lactulose on the oral‐caecal transit time determined by the hydrogen breath test and the reproducibility of the test in normal subjects. Ann Clin Res. 1985;17:331–3. [PubMed] [Google Scholar]
- 218. La Brooy SJ, Male PJ, Beavis AK, Misiewicz JJ. Assessment of the reproducibility of the lactulose H2 breath test as a measure of mouth to caecum transit time. Gut. 1983;24:893–6. 10.1136/gut.24.10.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Camboni G, Basilisco G, Bozzani A, Bianchi PA. Repeatability of lactulose hydrogen breath test in subjects with normal or prolonged orocecal transit. Dig Dis Sci. 1988;33:1525–7. 10.1007/BF01535941 [DOI] [PubMed] [Google Scholar]
- 220. Di Lorenzo C, Dooley CP, Valenzuela JE. Role of fasting gastrointestinal motility in the variability of gastrointestinal transit time assessed by hydrogen breath test. Gut. 1991;32:1127–30. 10.1136/gut.32.10.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Bond JH, Jr. , Levitt MD, Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975;85:546–55. [PubMed] [Google Scholar]
- 222. Kerlin P, Phillips S. Differential transit of liquids and solid residue through the human ileum. Am J Physiol. 1983;245:G38–G43. 10.1152/ajpgi.1983.245.1.G38 [DOI] [PubMed] [Google Scholar]
- 223. Rhodes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small‐bowel bacterial overgrowth. Scand J Gastroenterol. 1979;14:333–6. 10.3109/00365527909179892 [DOI] [PubMed] [Google Scholar]
- 224. McKenzie YA, Alder A, Anderson W, Wills A, Goddard L, Gulia P, et al. British Dietetic Association evidence‐based guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet. 2012;25:260–74. 10.1111/j.1365-277X.2012.01242.x [DOI] [PubMed] [Google Scholar]
- 225. Cappello G, Marzio L. Rifaximin in patients with lactose intolerance. Dig Liver Dis. 2005;37:316–9. 10.1016/j.dld.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 226. Yerushalmy‐Feler A, Soback H, Lubetzky R, Ben‐Tov A, Dali‐Levy M, Galai T, et al. One‐third of children with lactose intolerance managed to achieve a regular diet at the three‐year follow‐up point. Acta Paediatr. 2018;107:1389–94. 10.1111/apa.14305 [DOI] [PubMed] [Google Scholar]
- 227. Catassi G, Lionetti E, Gatti S, Catassi C. The low FODMAP diet: many question marks for a catchy acronym. Nutrients. 2017;9:292. 10.3390/nu9030292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Halmos EP. When the low FODMAP diet does not work. J Gastroenterol Hepatol. 2017;32(Suppl 1):69–72. 10.1111/jgh.13701 [DOI] [PubMed] [Google Scholar]
- 229. Krogsgaard LR, Lyngesen M, Bytzer P. Systematic review: quality of trials on the symptomatic effects of the low FODMAP diet for irritable bowel syndrome. Aliment Pharmacol Ther. 2017;45:1506–13. 10.1111/apt.14065 [DOI] [PubMed] [Google Scholar]
- 230. O'Keeffe M, Jansen C, Martin L, Williams M, Seamark L, Staudacher HM, et al. Long‐term impact of the low‐FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neuro Gastroenterol Motil 2018; 30. Epub 2017 Jul 15. 10.1111/nmo.13154 [DOI] [PubMed] [Google Scholar]
- 231. Kalantar‐Zadeh K, Yao CK, Berean KJ, Ha N, Ou JZ, Ward SA, et al. Intestinal gas capsules: a proof‐of‐concept demonstration. Gastroenterology. 2016;150:37–9. 10.1053/j.gastro.2015.07.072 [DOI] [PubMed] [Google Scholar]
- 232. Berean KJ, Ha N, Ou JZ, Chrimes AF, Grando D, Yao CK, et al. The safety and sensitivity of a telemetric capsule to monitor gastrointestinal hydrogen production in vivo in healthy subjects: a pilot trial comparison to concurrent breath analysis. Aliment Pharmacol Ther. 2018;48:646–54. 10.1111/apt.14923 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


