Abstract
Background
Hip fractures are a major healthcare problem, presenting a challenge and burden to individuals and healthcare systems. The number of hip fractures globally is rising. The majority of extracapsular hip fractures are treated surgically.
Objectives
To assess the relative effects (benefits and harms) of all surgical treatments used in the management of extracapsular hip fractures in older adults, using a network meta‐analysis of randomised trials, and to generate a hierarchy of interventions according to their outcomes.
Search methods
We searched CENTRAL, MEDLINE, Embase, Web of Science and five other databases in July 2020.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing different treatments for fragility extracapsular hip fractures in older adults. We included internal and external fixation, arthroplasties and non‐operative treatment. We excluded studies of hip fractures with specific pathologies other than osteoporosis or resulting from high‐energy trauma.
Data collection and analysis
Two review authors independently assessed studies for inclusion. One review author completed data extraction which was checked by a second review author. We collected data for three outcomes at different time points: mortality and health‐related quality of life (HRQoL) ‐ both reported within 4 months, at 12 months and after 24 months of surgery, and unplanned return to theatre (at end of study follow‐up).
We performed a network meta‐analysis (NMA) with Stata software, using frequentist methods, and calculated the differences between treatments using risk ratios (RRs) and standardised mean differences (SMDs) and their corresponding 95% confidence intervals (CIs). We also performed direct comparisons using the same codes.
Main results
We included 184 studies (160 RCTs and 24 quasi‐RCTs) with 26,073 participants with 26,086 extracapsular hip fractures in the review. The mean age in most studies ranged from 60 to 93 years, and 69% were women.
After discussion with clinical experts, we selected nine nodes that represented the best balance between clinical plausibility and efficiency of the networks: fixed angle plate (dynamic and static), cephalomedullary nail (short and long), condylocephalic nail, external fixation, hemiarthroplasty, total hip arthroplasty (THA) and non‐operative treatment. Seventy‐three studies (with 11,126 participants) with data for at least two of these treatments contributed to the NMA.
We selected the dynamic fixed angle plate as a reference treatment against which other treatments were compared. This was a common treatment in the networks, providing a clinically appropriate comparison.
We downgraded the certainty of the evidence for serious and very serious risks of bias, and because some of the estimates included the possibility of transitivity owing to the proportion of stable and unstable fractures between treatment comparisons. We also downgraded if we noted evidence of inconsistency in direct or indirect estimates from which the network estimate was derived. Most estimates included the possibility of benefits and harms, and we downgraded the evidence for these treatments for imprecision.
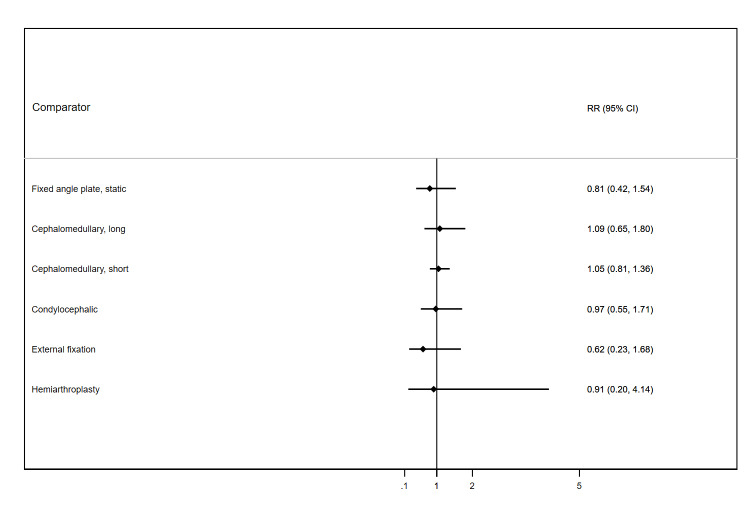
Overall, 20.2% of participants who received the reference treatment had died by 12 months after surgery. We noted no evidence of any differences in mortality at this time point between the treatments compared. Effect estimates of all treatments included plausible benefits as well as harms. Short cephalomedullary nails had the narrowest confidence interval (CI), with 7 fewer deaths (26 fewer to 15 more) per 1000 participants, compared to the reference treatment (risk ratio (RR) 0.97, 95% CI 0.87 to 1.07). THA had the widest CI, with 62 fewer deaths (177 fewer to 610 more) per 1000 participants, compared to the reference treatment (RR 0.69, 95% CI 0.12 to 4.03). The certainty of the evidence for all treatments was low to very low. Although we ranked the treatments, this ranking should be interpreted cautiously because of the imprecision in all the network estimates for these treatments.
Overall, 4.3% of participants who received the reference treatment had unplanned return to theatre. Compared to this treatment, we found very low‐certainty evidence that 58 more participants (14 to 137 more) per 1000 participants returned to theatre if they were treated with a static fixed angle plate (RR 2.48, 95% CI 1.36 to 4.50), and 91 more participants (37 to 182 more) per 1000 participants returned to theatre if treated with a condylocephalic nail (RR 3.33, 95% CI 1.95 to 5.68). We also found that these treatments were ranked as having the highest probability of unplanned return to theatre. In the remaining treatments, we noted no evidence of any differences in unplanned return to theatre, with effect estimates including benefits as well as harms. The certainty of the evidence for these other treatments ranged from low to very low.
We did not use GRADE to assess the certainty of the evidence for early mortality, but our findings were similar to those for 12‐month mortality, with no evidence of any differences in treatments when compared to dynamic fixed angle plate. Very few studies reported HRQoL and we were unable to build networks from these studies and perform network meta‐analysis.
Authors' conclusions
Across the networks, we found that there was considerable variability in the ranking of each treatment such that there was no one outstanding, or subset of outstanding, superior treatments. However, static implants such as condylocephalic nails and static fixed angle plates did yield a higher risk of unplanned return to theatre. We had insufficient evidence to determine the effects of any treatments on HRQoL, and this review includes data for only two outcomes. More detailed pairwise comparisons of some of the included treatments are reported in other Cochrane Reviews in this series. Short cephalomedullary nails versus dynamic fixed angle plates contributed the most evidence to each network, and our findings indicate that there may be no difference between these treatments. These data included people with both stable and unstable extracapsular fractures. At this time, there are too few studies to draw any conclusions regarding the benefits or harms of arthroplasty or external fixation for extracapsular fracture in older adults.
Future research could focus on the benefits and harms of arthroplasty interventions compared with internal fixation using a dynamic implant.
Plain language summary
Which are the best treatments for hip fractures in older adults?
Key messages
‐ There is no 'best treatment' for this type of broken hip.
‐ More people needed additional surgery on their broken hip after treatment with condylocephalic nails (where a nail is inserted upwards from the knee towards the hip joint) or static fixed angle plates (where pins or screws attach a plate to the broken bone).
‐ There may be no difference between a short cephalomedullary nail (where a nail is inserted downwards from the hip joint towards the knee) and a dynamic fixed angle plate (where the pins or screws attaching a plate to the broken bone are able to slide in a sleeve).
‐ We found too few studies to know whether any of these treatments were better at improving people's quality of life after surgery.
Hip fractures in older people
A hip fractures is a break at the top of the leg bone. There are two types of hip fractures; in this review, we included people with a break just outside the hip joint. The other type of hip fracture is a break just below the ball and socket joint ‐ we reviewed these fractures in another review. Both types of broken hip are common in older adults whose bones may be fragile because of a condition called osteoporosis.
What are the treatments?
‐ Using metal implants to fix the broken parts of the bone. A nail is inserted inside the thigh bone. These long or short nails may be inserted downwards from the hip joint towards the knee (cephalomedullary nails). Some nails may be inserted upwards from the knee towards the hip joint (condylocephalic nail). Alternatively, the surgeon may use a 'fixed angle plate' which sits on the outer edge of the broken bone and is attached to the bone with screws or pins. Often the screws for these plates slide in a sleeve and the plate is called a dynamic fixed angle plate. Without this, it is a static fixed angle plate.
‐ Replacing the broken hip with an artificial one. This can be done using a hemiarthroplasty (HA), which replaces only the ball part of the joint, or with a total hip arthroplasty (THA) which replaces all of the hip joint including the socket.
‐ Using external fixation. Pins or screws are placed into the bones around the fracture and a metal frame holds these nails in place. The frame sits outside the body, around the broken hip.
‐ Treatment without an operation, usually requiring a period of rest in bed whilst the leg is held in position using traction with weights.
What did we do?
We searched for studies that compared one or more of these treatments. We wanted to find out the benefits and harms of these different treatments. We combined the findings from studies, and created a 'network' (which is used when researchers compare all available treatments in a single analysis called a 'network meta‐analysis') to see if we could find out if some treatments were better than others.
What did we find?
We found 184 studies with 26,073 people who had this type of hip fracture. Most people were aged between 60 and 93 years, and 69% were women, which is usual for people with this type of broken bone. We included 73 of these studies in our 'network'.
We found little or no difference in how many people died with each treatment. We were not sure whether any of the treatments were better than another at reducing deaths within 12 months of surgery.
For most treatments, we also found little or no difference in whether people needed to have additional surgery on their broken hip. However, for the condylocephalic nail and the static fixed angle plate, more people needed additional surgery compared to people treated with a dynamic fixed angle plate. It seemed that these treatments increased the chance of needing additional surgery.
Very few studies reported whether or not people had better health‐related quality of life after their treatment.
Are we confident in what we found?
We are not very confident in these findings because:
‐ most of the studies were not well reported. It is possible that their study methods could introduce errors in their results;
‐ we found some differences between some of the study results which we could not explain;
‐ we found some differences in the types of fractures in some studies;
‐ most treatments included the possibility of a benefit (for example, fewer deaths) as well as the possibility of a harm (for example, more deaths). This made the result very uncertain.
The true effects of these treatments might be very different to what we have found in this review.
How up to date is this review?
The evidence is up to date to July 2020.
Summary of findings
Background
This review has been written in accordance with guidance for authors on preparing a protocol for a systematic review with multiple interventions (Chaimani 2017; CMIMG 2014).
Description of the condition
Epidemiology
A hip fracture, or proximal femoral fracture, is a break in the upper region of the femur (thigh bone) between the subcapital region (the area just under the femoral head) and 5 cm below the lesser trochanter (a bony projection of the upper femur). The incidence of hip fractures rises with age; they are most common in the older adult population (Court‐Brown 2017; Kanis 2001). Those seen in younger adults are usually associated with poor bone health (Karantana 2011; Rogmark 2018). A very small proportion of fractures in younger people are caused by high‐energy trauma such as road traffic collisions, industrial injuries and sports injuries. The overwhelming majority of hip fractures are fragility fractures associated with osteoporosis; such fractures are caused by mechanical forces that would not ordinarily result in fracture. The World Health Organization (WHO) has defined fragility fractures as those sustained from injuries equivalent to a fall from a standing height or less (Kanis 2001). In the UK, the mean age of a person with hip fracture is 83 years, and approximately two‐thirds occur in women (NHFD 2017).
Hip fractures are a major healthcare problem at the individual and population level. They present a huge challenge and burden to individuals, healthcare systems and society. The increased proportion of older adults in the world population means that the absolute number of hip fractures is rising rapidly across the globe. For example, in 2016 there were 65,645 new presentations of hip fracture to 177 trauma units in England, Wales and Northern Ireland (NHFD 2017). Based on population estimates for these regions for mid‐2016, this equates to an incidence rate of 109 cases per 100,000 population (ONS 2016). By 2050, it is estimated that the annual worldwide incidence of hip fracture will be 6 million (Cooper 2011; Johnell 2004). Incident hip fracture rates are higher in industrialised countries than in developing countries. Northern Europe and the USA have the highest rates of hip fracture, whereas Latin America and Africa have the lowest (Dhanwal 2011). European studies show that there are more hip fractures in the north of the region than in the south, and there is also a similar north‐south gradient in the USA (Dhanwal 2011). Factors thought to be responsible for this variation are population demographics (with older populations in countries with higher incidence rates) and the influence of ethnicity, latitude and environmental factors such as socioeconomic deprivation (Bardsley 2013; Cooper 2011; Dhanwal 2011; Kanis 2012).
Burden of disease
Hip fractures are associated with a high risk of death. For example, in England, Wales and Northern Ireland, the 30‐day mortality rate in 2016 remained high at 6.7%, despite a decline from 8.5% in 2011 and 7.1% in 2015 (NHFD 2017). The mortality rate one year after a hip fracture is approximately 30%; however, fewer than half of deaths are attributable to the fracture itself, which reflects the frailty of the patients and associated high prevalence of comorbidities and complications (Parker 1991; SIGN 2009). The impact of morbidity associated with hip fractures is similar to that of stroke, and entails a substantial loss of healthy life‐years in older people (Griffin 2015). Hip fractures commonly result in reduced mobility and greater dependency, with many people failing to return to their pre‐injury residence. In addition, the public health impact of hip fractures is significant: data from large prospective cohorts show the burden of disease due to hip fracture is 27 disability‐adjusted life years (DALYs) per 1000 individuals, which equates to an average loss of 2.7% of the healthy life expectancy in the population at risk of fragility hip fracture (Papadimitriou 2017). The direct economic burden of hip fractures is also substantial. Hip fractures are amongst the most expensive conditions seen in hospitals; the aggregated cost for 316,000 inpatient episodes in the USA in 2011 was nearly USD 4.9 billion (USD 4900 million; Torio 2011). In England, Wales and Northern Ireland, people with hip fractures occupy 1.5 million hospital bed days each year, and cost the National Health Service and social care GBP 1 billion (GBP 1000 million; NHFD 2017). Combined health and social care costs incurred during the first year following a hip fracture have been estimated at USD 43,669, which is greater than the cost for non‐communicable diseases such as acute coronary syndrome (USD 32,345) and ischaemic stroke (USD 34,772) (Williamson 2017). In established market economies, hip fractures represent 1.4% of the total healthcare burden (Johnell 2004).
Extracapsular hip fracture
Hip fractures either involve the region of the bone which is enveloped by the ligamentous hip joint capsule (intracapsular), or that outside the capsule (extracapsular). Extracapsular fractures traverse the femur within the area of bone bounded by the intertrochanteric line proximally, up to a distance of 5 cm from the distal part of the lesser trochanter. Several classification methods have been proposed to define different types of extracapsular fractures (AO Foundation 2018; Evans 1949; Jensen 1980). They are generally subdivided depending on their relationship to the greater and lesser trochanters (the two bony projections present at the upper end of the femur) and the complexity of the fracture configuration. It is increasingly clear that each of these classifications is limited in its generalisability since inter‐ and intra‐observer agreement is poor. Table 3 provides a description of the most recent classification of trochanteric fractures (AO Foundation 2018). For this review, we use a pragmatic simplification of these classifications, as follows.
1. Trochanteric region fractures: type and surgical management (Revised AO/OTA classification, January 2018).
| Type | Features | Stability | Description |
| Simple, pertrochanteric fractures (A1) |
|
Stable | The fracture line can begin anywhere on the greater trochanter and end either above or below the lesser trochanter. The medial cortex is interrupted in only one place. |
| Multifragmentary pertrochanteric fractures (A2) |
|
Unstable | The fracture line can start laterally anywhere on the greater trochanter and runs towards the medial cortex, which is typically broken in two places. This can result in the detachment of a third fragment which may include the lesser trochanter. |
| Intertrochanteric fractures (A3) |
|
Unstable | The fracture line passes between the two trochanters, above the lesser trochanter medially and below the crest of the vastus lateralis laterally. |
AO/OTA: Arbeitsgemeinschaft für Osteosynthesefragen (German for "Association for the Study of Internal Fixation")/Orthopaedic Trauma Association
Trochanteric fractures: those which lie mostly between the intertrochanteric line and a transverse line at the level of the lesser trochanter. These can be further divided into simple two‐part stable fractures, and comminuted or reverse obliquity unstable fractures.
Subtrochanteric fractures: those which mostly lie in the region bordered by the lesser trochanter and 5 cm distal to the lesser trochanter.
Approximately 40% of hip fractures are extracapsular, of which 90% are trochanteric and 10% are subtrochanteric (NHFD 2017).
Description of the intervention
Internationally, many guidelines exist concerning the management of hip fracture (e.g. AAOS 2014; Mak 2010; NICE 2011; SIGN 2009). Each recommend that early surgical management, generally within 24 to 48 hours, is the mainstay of care for the majority of hip fractures. The overall goal of surgery in the older population is to facilitate early rehabilitation, which enables early mobilisation and the return to premorbid function while minimising the complication risk. This approach has been associated with reductions in mortality in many worldwide registries (Neufeld 2016; Sayers 2017).
Osteosynthesis
The most common surgical treatment for extracapsular fractures is osteosynthesis. A variety of internal fixation implants exist, including both extramedullary and intramedullary types. External fixation, where external bars traverse the fracture and are attached to the bones by threaded pins, has also been applied. A description and proposed grouping of interventions is given in Table 4. Although less common, arthroplasty is an option in the management of these fractures. Descriptions and a proposed grouping of arthroplasty interventions is provided in Table 5.
2. Categorisation of internal and external fixation interventions for extracapsular hip fractures.
| Implant category | Grouping variable | Implant subcategory (entry point/static or dynamic) | Examplesa | Description |
| Extracapsular fractures | ||||
| External fixation | ||||
| External fixator | n/a | n/a |
|
Threaded pins are passed into the bone proximal and distal to the fracture. These pins are attached to external bars which may be arranged in numerous configurations to bridge the fracture. |
| Internal fixation | ||||
| Intramedullary nails | n/a | Cephalomedullary nails |
|
A nail is inserted antegrade into the intramedullary canal of the femur. Once the nail is in place, a pin, nail or screw is passed from the lateral cortex of the femur across the fracture and through the nail into the femoral head. The pin or screw can be fixed to the nail in various ways to allow or prevent sliding as well as provide rotational stability about the axis of the femoral neck. Küntscher Y‐nail: an early intramedullary nail based on the principles of stable fixation and closed nailing. Zickel nail: similar to the Küntscher Y‐nail. The Küntscher Y‐nail was considered to be more difficult to insert than the Zickel nail. The major complication of the Küntscher Y‐nail was distal migration of the intramedullary nail, although this may be prevented by the insertion of a bolt through the upper end of the nail. Zickel developed his device to address the difficulties with the former. Gamma nail (first generation, 1980s) or Standard Gamma Nail (SGN): a prototype intramedullary nailing system which became the most widely used for trochanteric fractures worldwide. Complications such as cut‐out, implant breakage, femoral shaft fractures, and reduction loss were reported with its use. Long Gamma Nail (LGN) (1992): used for subtrochanteric hip fractures, femoral shaft fractures and combined trochanter‐diaphyseal fractures of the femur. Trochanteric Gamma Nail (TGN) (1997): a modified SGN, replaced the SGN. Gamma3 Nailing System: third generation of intramedullary short and long Gamma fixation nails. Four locking grooves allow for quarter‐turn advancement of 0.8 mm to allow for precise lag screw positioning. PFN (Synthes, Solothurn, Switzerland) and ATN (DePuy, Warsaw, IN, USA): developed to address the mechanical complications of the standard gamma nail. These intramedullary implants provide sliding head‐neck screws. However, complications still occurred, such as lateral migration of head‐neck screw, cut‐out from head‐neck fragment and cut‐through of the antirotation screw into the joint. The expandable PFN was a development of this nail with a hydraulic expansion mechanism in the head reducing the need for reaming for the lag screw. PFNA (Synthes, Solothurn, Switzerland) was designed by the AO/ASIF group for improving the rotational stability. It used a single head‐neck fixation device called a 'helical blade'. The TFN and TFNA are the first‐ and second‐generation trochanteric entry point cephalomedullary nails made by Synthes (Switzerland) using lag screws or blades. ACE Trochanteric nail System (ATN) (DePuy): intended to treat stable and unstable proximal fractures of the femur including pertrochanteric fractures, intertrochanteric fractures, high subtrochanteric fractures and combinations of these fractures. Trochanteric Long Nail System: additionally indicated to treat pertrochanteric fractures associated with shaft fractures, pathologic fractures in osteoporotic bone of the trochanteric and diaphyseal areas, long subtrochanteric fractures, ipsilateral femoral fractures, proximal or distal non‐unions and malunions and revision procedures. Russel‐Taylor Recon nail: a second‐generation locking femoral nail. Intramedullary hip screw (IMHS): a short intramedullary nail with interlocking screws that can be used to treat subtrochanteric and intertrochanteric femur fractures. This nail, which has the biomechanical advantage of being an intramedullary appliance but can be placed percutaneously, is inserted under fluoroscopic control with the patient on a fracture table. Reaming is not usually necessary. Intramedullary Hip Screw Clinically Proven (IMHS™ CP): intramedullary hip screw device that provides a barrel through which a lag screw can slide. Introduced in 1991 with its design, the IMHS system provided a more minimally invasive technique than the traditional Compression Hip Screw. By featuring a centering sleeve to enhance lag screw sliding and medialising the implant to reduce the moment arm, this design improved implant biomechanics for the treatment of hip fractures. Targon® PFT nailing system: targeting device is suitable for all caput collum diaphyseal (CCD) angles and permits shorter and less invasive incisions thanks to an optimised geometry. Zimmer Natural Nail System: intramedullary nails, screws, instruments and other associated implants. Holland Nail™ System includes a short, universal nail in diameters of 9, 11 and 13 mm x 24 cm length and long, anatomic (L & R) nails in diameters of 9 mm and 12 mm in various lengths. A 7 mm cannulated, partially threaded screw is used for proximal reconstructive interlocking. A unique pilot thread screw is used for proximal and distal interlocking. Distal screw options offer shaft fracture compression while controlling rotation by a distal slot, or the option of static interlocking. Endovis cephalomedullary nail (Citieffe, Italy) is implanted without reaming and has two lag screws placed in the femoral head. The Trigen Intertan (Smith and Nephew) intramedullary nail was designed as a trochanteric entry nail especially shaped for fractures of the proximal femur. It offers an integrated interlocking screw option to increase stability and resistance to intraoperative and postoperative femoral head rotation, thus eliminating excessive sliding and the possibility of Z‐effect. Femoral intramedullary nail (Elos) is a trapezoidal section piriformis fossa entry point nail with cannulated cephalic lag screw. Affixus Hip Fracture Nail System (ZimmerBiomet) is a cephalomedullary nail with double cephalic lag screws. |
| n/a | Condylocephallic nails |
|
Condylocephalic nails are intramedullary nails which are inserted retrograde through the femoral canal across the fracture and into the femoral head. Ender nails are pre‐bent flexible rods. Three to five of these of appropriate length are inserted into the femoral canal. The femoral canal is thus 'stacked' with nails, whilst their tips should radiate out to produce a secure fixation within the femoral head. Harris nail is a larger nail used as a single nail. |
|
| Fixed angle plates | Sliding | Static |
|
Static device consisting of a nail, pin or screw which is passed across the fracture into the femoral head and connected to a plate on the lateral femur. These implants have no capacity for ‘sliding’ between the plate and pin or screw components and hence are termed static implants. Holt nail plate: a four‐flanged nail connected to a plate at the time of surgery. Jewett nail: the nail is fixed to the plate at manufacture. Thornton and McLaughlin nail plates: the nail is connected to the plate at the time of surgery with a locking bolt. RAB plate: similar to a Jewett fixed nail plate but has an additional oblique strut to connect the nail and the side plate. AO Angle Blade Plate (Synthes) is a well‐used fixed angle static implant where the cephalic placed blade is angled at various degrees from 90 to 150 with a plate attached to the lateral side of the femur. Less‐Invasive Stabilisation System (LISS) (Synthes) is a fixed angle plating system where screws fix into the plate but are statically locked. |
| Dynamic |
|
Dynamic device consisting of a nail, pin or screw which is passed across the fracture into the femoral head and connected to a plate on the lateral femur. These implants allow ‘sliding’ between the plate and pin or screw components and hence are termed dynamic implants. Weight bearing or translation during surgery causes the femoral head to become impacted on the femoral neck, leading to compression of the fracture. Precimed Hip Screw System: compression fixation system used for the treatment of femoral neck and distal femoral fractures. It consists of compression plates, lag screws, compression screws, bone screws and angled blade plates. The system functions to provide immediate stability and temporary fixation during the natural healing process following fractures of the femoral neck or distal femur. AMBI/Classic Hip Screw System: compression fixation system consisting of hip screw plates and nails. AMBI plates have a barrel design which is keyless but can be converted to keyed with the insertion of a small keying clip; Classic plates have a keyed barrel design only. AMBI/Classic Lag Screws: 18 lengths: 55 mm to 140 mm; nonself‐tapping for cancellous bone. Pugh nail: similar to SHS except instead of a lag screw being passed up the femoral neck, a nail with a trifin or three‐flanged terminus is inserted by a punching mechanism. This is then connected to the side plate in the same manner as for a SHS. Medoff plate: a modification of SHS, where a lag screw is passed up the femoral neck and attached to a plate on the side of the femur. The difference is that the plate has an inner and outer sleeve, which can slide between each other. This creates an additional capacity for sliding to occur at the level of the lesser trochanter as well as at the lag screw. An additional variant of the Medoff plate is the capability of compressing the fracture distally using the two interlocking plates and a compression screw. In addition, sliding at the lag screw can be prevented with a locking screw to create a 'one way' sliding Medoff instead of a 'two way' sliding Medoff. At a later date, the locking device on the lag screw can be removed to 'dynamise' the fracture. Gotfried PCCP has a side plate that is inserted via a small incision level to the lesser trochanter by use of a connecting jig. Using the latter, two sliding proximal screws are passed up the femoral neck and the plate is fixed to the femur shaft with three screws. DCS is an implant assembly that consists of a lag screw, angled barrel plate fixed to the bone (usually distal femur) by 4.5 cortical screws. DCS and DHS work on the same principle of the sliding nail that allows impaction of the fracture. This is due to insertion of wide diameter into the condyle (or femoral head). A side plate, which has a barrel at a fixed angle, is slid over the screw and fixed to the femoral shaft. DCS has all other components the same as DHS; only the side plate is different. The plate barrel angle is 95 degrees and the plate is shaped to accommodate the lateral aspect of lateral condyle. DCS was mainly developed for fractures of distal femoral condyles. It is also used in fixation of subtrochanteric fractures of femur. Sometimes, it can be used in revision surgeries of selected intertrochanteric fractures. Compression hip screw (Smith & Nephew) is similar to other types of sliding hip screw with an angled barrel, sliding lag screw and plate attached to the side of the femur. Richards sliding hip screw is similar to other types of sliding hip screw with an angled barrel, sliding lag screw and plate attached to the side of the femur. X‐BOLT®: an expanding bolt akin to a Chinese lantern with a central drive shaft. The opposing threads compress the expandable section from both ends to expand the wings perpendicularly to the shaft, without spinning, pushing or pulling the femoral head. |
||
| External fixation | ||||
| n/a | Pertrochanteric external fixator | Pertrochanteric external fixator: held outside the thigh by two pairs of pins. One pair is passed up the femoral neck under X‐ray control. The other pair is placed in the femur. The fixator is left in place until the fracture has healed, which usually takes about three months. It is then removed under local anaesthesia, generally as an outpatient procedure. |
||
aThis list is not exhaustive. n/a: not applicable
3. Categorisation of arthroplasty interventions for extracapsular hip fractures.
| Implant | Grouping variable | Implant subcategory | Examplesa | Description |
| Extracapsular fractures | ||||
| Arthroplasty | ||||
| Total hip arthroplasty | Articulation | Femoral head and acetabular bearing surface materials |
|
Bearing surfaces may be grouped into hard (ceramic and metal) and soft (polyethylene variants). Arthroplasties exist with many of the possible combinations of these bearing surfaces. |
| Femoral head size |
|
Over the development of hip arthroplasty, different sizes of femoral head have been used, from 22 mm to very large diameters approximating that of the native femoral head. The size of the head represents a compromise between stability and linear and volumetric wear at the articulation. The optimum size varies by indication and bearing materials. 36 mm is considered as a cut‐off between standard and large sizes. | ||
| Acetabular cup mobility |
|
A standard total hip arthroplasty has a single articulating surface between the femoral head and acetabulum bearing surface. Alternative designs incorporate two further articulations within the structure of the femoral head. | ||
| Fixation technique | Cemented |
|
Both components are cemented with poly(methyl methacrylate) bone cement that is inserted at the time of surgery. It sets hard and acts as grout between the prosthesis and the bone. | |
| Modern uncemented |
|
Neither component is cemented but rely on osseous integration forming a direct mechanical linkage between the bone and the implant. The femoral prosthesis may be coated with a substance such as hydroxyapatite which promotes bone growth into the prosthesis. Alternatively, the surface of the prosthesis may be macroscopically and microscopically roughened so that bone grows onto the surface of the implant. The acetabular component may be prepared similarly and may or may not be augmented with screws fixed into the pelvis. | ||
| Hybrid | Combinations | The femoral stem is cemented and the acetabular cup is uncemented. | ||
| Reverse hybrid | Combinations | The acetabular cup is cemented and the femoral stem is uncemented. | ||
| Hemiarthroplasty | Articulation | Unipolar |
|
A single articulation between the femoral head and the native acetabulum. The femoral component can be a single ‘monoblock’ of alloy or be modular, assembled from component parts during surgery. |
| Bipolar |
|
The object of the second joint is to reduce acetabular wear. This type of prosthesis has a spherical inner metal head with a size between 22 and 36 mm in diameter. This fits into a polyethylene shell, which in turn is enclosed by a metal cap. There are a number of different types of prostheses with different stem designs. | ||
| Fixation technique | First generation uncemented |
|
These prostheses were designed before the development of poly(methyl methacrylate) bone cement and were therefore originally inserted as a ’press fit’. Long‐term stability through osseus integration was not part of the design concept. | |
| Cemented |
|
The femoral stem is cemented with poly(methyl methacrylate) bone cement that is inserted at the time of surgery. It sets hard and acts as grout between the prosthesis and the bone. | ||
| Modern uncemented |
|
The femoral stem relies on osseous integration forming a direct mechanical linkage between the bone and the implant. A prosthesis may be coated with a substance such as hydroxyapatite which promotes bone growth into the prosthesis. Alternatively, the surface of the prosthesis may be macroscopically and microscopically roughened so that bone grows onto the surface of the implant. | ||
aThis list is not exhaustive.
In general, the majority of fractures must be reduced prior to fixation. Typically, fragility fractures are reduced closed, under X‐ray control using an image intensifier. However, if a fracture is irreducible using closed means, the fracture may be reduced open (exposed surgically to aid reduction). The reduced fracture is held by an implant passed across the fracture, or is bridged by an external fixator.
Extramedullary implants are those where a side plate is screwed to the lateral edge of the femur. They are grouped into static and dynamic designs. In static designs, the part of the implant that crosses the fracture is fixed in relation to the side plate; in dynamic designs, this can slide within the side plate, allowing collapse of the fracture along the axis of the femoral neck until the fracture is stable. There are also variable angles between the plate and the interfragmentary components of the system. In general, there are those which approximate a right angle, such as condylar screws and blade plates, and those which approximate the native angle of the femoral neck (approximately 130 degrees), such as the sliding hip screw.
Intramedullary implants are those which run along the internal course of the femur. They are grouped into cephalocondylic nails, which are advanced in an antegrade fashion into the femur, and condylocephalic nails, which are advanced retrograde. Retrograde nails, such as Ender nails, are passed from distal to proximal and a single implant traverses both the femoral canal and the fracture. Cephalomedullary nails, such as the Gamma or Proximal Femoral Nail (PFN), are passed from the tip of the greater trochanter or piriformis fossa into the medullary canal and subsequently an interfragmentary component is passed separately from the lateral femur through the centre of the nail and across the fracture. Cephalomedullary implants are grouped into short nails (where the tip of the nail ends in the region of the mid femur) and long nails (where the tip ends in the region of the distal metaphysis).
Arthroplasty
Arthroplasty entails replacing part or all of the hip joint with an endoprosthesis, an implant constructed of non‐biological materials such as metal, ceramic or polyethylene. Arthroplasties can be grouped into two main categories: hemiarthroplasty (where only the femoral head and neck are replaced) and total hip arthroplasty (THA, also known as 'total hip replacement' ) (where both the femoral head and the acetabulum or socket are replaced).
Hemiarthroplasty
Hemiarthroplasty involves replacing the femoral head with a prosthesis whilst retaining the natural acetabulum and acetabular cartilage. Hemiarthroplasties can be broadly divided into two groups: unipolar and bipolar. In unipolar hemiarthroplasties, the femoral head is a solid block of metal. Bipolar femoral heads include a single articulation which allows movement to occur, not only between the acetabulum and the prosthesis, but also at this joint within the prosthesis itself.
The best‐known of the early hemiarthroplasty designs are the Moore prosthesis (1952) and the FR Thompson Hip Prosthesis (1954). These are both monoblock implants and were designed before the development of poly(methyl methacrylate) bone cement; they were therefore originally inserted as a 'press fit'. The Moore prosthesis has a femoral stem, which is fenestrated (i.e. has holes or openings) and also has a square stem with a shoulder to enable stabilisation within the femur; this resists rotation within the femoral canal. This prosthesis is generally used without cement and, in the long term, bone in‐growth into the fenestrations can occur. The Thompson prosthesis has a smaller stem without fenestrations and is now often used in conjunction with cement. Numerous other designs of unipolar hemiarthroplasties exist, based on stems that have been used for THAs.
In bipolar prostheses, there is an articulation within the femoral head component itself. In this type of prosthesis, there is a spherical inner metal head which measures between 22 and 36 millimetres in diameter. This fits into a polyethylene shell, which in turn is enclosed by a metal cap. The objective of the second joint is to reduce acetabular wear by promoting movement at the interprosthetic articulation rather than with the native acetabulum. There are a number of different types of prostheses with different stem designs. Examples of bipolar prostheses are the Charnley‐Hastings, Bateman, Giliberty and the Monk prostheses, but many other types with different stem designs exist.
Total hip arthroplasty
THA involves the replacement of the acetabulum in addition to the femoral head. The first successful THA was developed by John Charnley, using metal alloy femoral heads articulating with polyethylene acetabular components. Subsequently, the articulating materials have diversified; designs using metal alloys, ceramics and various polyethylenes in various combinations have all been used.
Component fixation
Irrespective of the nature of the articulating surfaces, the components must be fixed to the bone to ensure longevity of the arthroplasty. The two approaches used to achieve this fixation are cemented and uncemented designs.
Cemented systems
In this approach, poly(methyl methacrylate) bone cement may be inserted at the time of surgery. It sets hard and acts as grout between the prosthesis and the implant at the time of surgery. Potential advantages of cement are a reduced risk of intraoperative fracture and later periprosthetic fracture, and that it does not rely on integration of the prosthesis with osteoporotic bone. Major side effects of cement are cardiac arrhythmias and cardiorespiratory collapse, which occasionally occur following its insertion. These complications may be fatal, and are caused by either embolism from marrow contents forced into the circulation (Christie 1994), or a direct toxic effect of the cement.
Uncemented systems
Uncemented systems rely on osseous integration forming a direct mechanical linkage between the bone and the implant. A prosthesis may be coated with a substance such as hydroxyapatite, which promotes bone growth into the prosthesis. Alternatively, the surface of the prosthesis may be macroscopically and microscopically roughened so that bone grows onto the surface of the implant.
The complications of arthroplasty are those general to surgical management of hip fracture ‐ for example, pneumonia, venous thromboembolism, infection, acute coronary syndrome and cerebrovascular accident ‐ and those specific to arthroplasty, including dislocation of the prosthesis, loosening of the components, acetabular wear and periprosthetic fracture.
Non‐operative management
Although the majority of extracapsular fractures are treated surgically, some people have non‐operative or conservative treatment. In 2016, 0.6% of people with an extracapsular fracture in England and Wales did not receive surgical management (NHFD 2017). Conservative or non‐operative management can consist of traction and may be of two types: skeletal traction (where traction is applied to the injured limb either via a pin inserted into the proximal tibia or distal femur) or skin traction (where adhesive tape or bandages are applied to the injured leg). Traction is then maintained, for a period of two to four months, by using 4 kg to 9 kg of weight. This ensures that the injured leg is immobilised whilst the fracture heals. Non‐operative treatment may be acceptable where modern surgical facilities are unavailable, where low income or different systems of care preclude an individual's access to surgery, or in medically unfit people with an unacceptably high risk of perioperative death.
Why it is important to do this review
Currently, there are six independent Cochrane Reviews that have focused on specific interventions for extracapsular fracture (Parker 2000; Parker 2006; Parker 2009; Lewis 2022a; Parker 2013; Queally 2014). The findings of the reviews varied. Although the sliding hip screw (SHS) is widely used in practice, there is uncertainty about the beneficial effects of intramedullary implants or the most appropriate implant for the specific type of extracapsular fracture (Mak 2010). Moreover, the implant design of the intramedullary nail is evolving substantially and a body of evidence supporting its use in certain situations is building.
It is difficult to determine the most effective treatment option for extracapsular fractures from the results of conventional pairwise meta‐analyses of direct evidence for three reasons:
some pairs of treatments have not been directly compared in a randomised controlled trial;
sometimes the direct evidence does not provide sufficient data and we need to support it with indirect evidence;
there are frequently multiple overlapping comparisons that potentially give inconsistent estimates of effect.
A network meta‐analysis (NMA) overcomes these problems by simultaneously synthesising direct and indirect evidence (comparisons of treatments that have not been tested in a randomised controlled trial). For each outcome, an NMA provides estimates of effect for all possible pairwise comparisons. This allows the ranking of the different interventions in order of effectiveness, and assessment of their relative effectiveness.
This Cochrane NMA has been developed in parallel with a sister NMA on surgical interventions for treating intracapsular hip fractures in older adults (Lewis 2022b).
Objectives
To assess the relative effects (benefits and harms) of all surgical treatments used in the management of extracapsular hip fractures in older adults, using a network meta‐analysis of randomised trials, and to generate a hierarchy of interventions according to their outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs assessing surgical interventions for the management of people with extracapsular hip fracture. Quasi‐RCTs are defined as trials in which the methods of allocating people to a trial are not random, but are intended to produce similar groups when used to allocate participants (Cochrane 2018). Studies published as conference abstracts were eligible for inclusion in the review, provided sufficient data relating to the methods and outcomes of interest were reported. We also considered unpublished data for inclusion.
Types of participants
Population
The fundamental assumption underpinning a network meta‐analysis is that of transitivity (Caldwell 2005; Cipriani 2013). This implies that the distribution of potential treatment effect modifiers is balanced across the available direct comparisons. Therefore, we assume that any individual who meets the inclusion criteria below is, in principle, equally able to have been randomised to any of the eligible interventions examined in this review; that is, they are 'jointly randomisable' (Salanti 2012).
We included older adults (at least 60 years of age) undergoing surgery in a hospital setting for a fragility extracapsular hip fracture. We included stable and unstable trochanteric fractures, and subtrochanteric fractures, which we expected to be caused by low‐energy trauma.
As a benchmark, representative of the general hip fracture population, we expected trial populations to have a mean age of between 80 and 85 years and include 70% women, 30% with chronic cognitive impairment, and 50% with an American Society of Anesthesiologists (ASA) score greater than two (NHFD 2017; NICE 2011).
We excluded studies that focused exclusively on the treatment of participants younger than 16 years of age, participants with fractures caused by specific pathologies other than osteoporosis, and participants with high‐energy fractures. However, we took a pragmatic approach to study inclusion criteria and included studies with mixed populations (fragility and other mechanisms, ages or pathologies). We expected that the proportion of participants with standard fragility fractures was most likely to outnumber those with high‐energy or local pathological fractures; therefore, the results will be generalisable to the fragility fracture population. If data were reported separately for standard fragility fractures, we planned to use this subgroup data in our main analysis. However, we excluded studies if we noted baseline characteristics indicated that participants were not representative of the general hip fracture population. We considered it unlikely that participants under 60 years of age would have experienced a fragility intracapsular hip fracture caused by low‐energy trauma.
Types of interventions
We included trials comparing at least two of the competing interventions in the synthesis set. All the eligible interventions are assumed to be legitimate treatment alternatives for people with extracapsular fractures and therefore 'jointly randomisable'. We expected randomised groups to be similar with respect to co‐interventions; for example, perioperative care, the use of intraoperative antibiotics or postoperative rehabilitation.
We included the following interventions.
Any implant used for fixation of an extracapsular hip fracture.
All hip endoprostheses: unipolar hemiarthroplasty (HA), bipolar HA, or total hip arthroplasty (THA) (small and large head) — applied with or without cement.
Non‐operative treatment, including treatment with or without traction.
Grouping interventions
We spoke to our clinical authors and the International Fragility Fracture Network in preparation for this review, to group possible interventions into homogenous therapeutic categories. We present these categories in Table 4 and Table 5. We updated these tables to include all interventions included within studies in this review. These interventions, or sufficiently similar variations of these interventions, are all potentially still in clinical use worldwide.
These categories formed the main nodes of the network. With our clinical authors, we explored differences within these nodes and made decisions on whether to group or split the nodes. This was guided by the data as well as considering the underlying assumptions (such as whether merging insufficiently similar interventions might violate transitivity).
We did not identify any unexpected interventions while searching for eligible studies. In this event, we had planned to consider these based on the context and whether they provided information to the network via a closed loop of treatment effects.
Types of outcome measures
We extracted data on the following critical outcomes.
Mortality.
Health‐related quality of life (HRQoL): measured using recognised scores such as the Short Form 36 questionnaire (SF‐36; Ware 1992) or EuroQol‐5D (EQ‐5D; Dolan 1997; EQ‐5D).
Unplanned return to theatre: secondary procedure required for a complication resulting directly or indirectly from the index operation/primary procedure.
We chose these outcomes by considering all relevant outcomes of benefit and harm, and also taking into account input from our stakeholder workshop (Sreekanta 2018).
Depending on the length of follow‐up reported, we categorised the endpoints for mortality and HRQoL into early (up to and including four months), 12 months (prioritising 12‐month data, but in its absence including data after four months and up to 24 months), and late (after 24 months) time points. We reported data at each of these time points for these two outcomes. For unplanned return to theatre, we extracted data at the end of study follow‐up.
Search methods for identification of studies
As well as developing a strategy for this review, we developed general search strategies for the large bibliographic databases to find records to feed into a number of Cochrane Reviews and review updates on hip fracture surgery (Lewis 2021; Lewis 2022a; Lewis 2022b; Lewis 2022c). We searched the main databases up to July 2020.
Electronic searches
We identified RCTs and quasi‐RCTs through literature searching with systematic and sensitive search strategies, as outlined in Chapter 4 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2019, hereafter referred to as the Cochrane Handbook). We applied no restrictions on language, date or publication status. We searched these databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL; CRS Web; 8 July 2020);
MEDLINE (Ovid; 1946 to 6 July 2020);
Embase (Ovid; 1980 to 7 July 2020);
Web of Science (SCI EXPANDED; 1900 to 8 July 2020);
Cochrane Database of Systematic Reviews (CDSR; Cochrane Library; 7 July 2020);
Database of Abstracts of Reviews of Effects (DARE; www.crd.york.ac.uk/CRDWeb/; 17 December 2018);
Health Technology Assessment (HTA) database (www.crd.york.ac.uk/CRDWeb/; 17 December 2018);
Epistemonikos (www.epistemonikos.org/; 9 July 2020);
ProQuest Dissertations and Theses (www.proquest.com/; 1743 to 8 July 2020);
National Technical Information Service (NTIS, for technical reports; www.ntis.gov/; 10 July 2020).
We developed a subject‐specific search strategy in MEDLINE and other listed databases. We adapted strategies with consideration of database interface differences as well as different indexing languages. In MEDLINE, we used the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2019). In Embase, we used the Cochrane Embase filter (www.cochranelibrary.com/central/central-creation) to focus on RCTs. The initial search was run in November 2018 and December 2018, and a top‐up search was run in July 2020 in all databases except for DARE and HTA, in which no new records have been added since the initial search. At the time of the search, CENTRAL was fully up‐to‐date with all records from the Cochrane Bone, Joint, and Muscle Trauma (BJMT) Group's Specialised Register, and so it was unnecessary to search this register separately. We developed the search strategy in consultation with Information Specialists (see Acknowledgements) and the Information Specialist for the BJMT Group. Search strategies can be found in Appendix 1.
We scanned ClinicalTrials.gov (www.clinicaltrials.gov/) for ongoing and unpublished trials on 10 July 2020.
Searching other resources
We handsearched abstracts from the following conferences from 2016 to November 2018.
Fragility Fractures Network Congress.
British Orthopaedic Association Congress.
Orthopaedic World Congress (SICOT).
Orthopaedic Trauma Association Annual Meeting.
Bone and Joint Journal Orthopaedic Proceedings.
American Academy of Orthopaedic Surgeons Annual Meeting.
Data collection and analysis
In order to reduce bias, we ensured that any review author who is also a co‐applicant on the Cochrane Programme Grant on the management of hip fracture, a study author, or who has or has had an advisory role on any potentially relevant study, remained independent of study selection decisions, risk of bias assessment and data extraction for their study.
Selection of studies
Two review authors screened titles and abstracts of all the retrieved bibliographic records in a web‐based systematic reviewing platform, Rayyan (Ouzzani 2016), and in the top‐up search using Covidence. We retrieved the full texts of all potentially eligible records passing the title and abstract screening. Two review authors independently examined the full texts, using the eligibility criteria mentioned in Criteria for considering studies for this review. Full‐text screening was conducted using Covidence. We resolved disagreements through discussion or adjudication by a third review author. We excluded duplicates and we collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We prepared a PRISMA flow‐diagram to outline the study selection process, numbers of records at each stage of selection, and reasons for exclusions of full‐text articles (Moher 2009). In the review, we reported details of key excluded studies, rather than all studies that were excluded from consideration of full‐text articles.
Data extraction and management
All review authors conferred on the essential data for extraction, and we structured a form to align with default headings in the Characteristics of included studies (see Appendix 2). Two review authors piloted the form on five studies and compared results. We then made changes to the template following additional discussion with the author team. For the remaining data extraction, one review author independently extracted data and a second review author checked all the data for accuracy. We extracted the following data.
Study methodology: publication type; sponsorship/funding/notable conflicts of interest of trial authors; study design; number of centres and locations; size and type of setting; study inclusion and exclusion criteria; randomisation method; number of randomised participants, losses (and reasons for losses), and number analysed for each outcome. (Collecting information relating to the participant flow helped the assessment of risk of attrition bias.)
Population: baseline characteristics of the participants, by group and overall (age, gender, smoking history, medication, body mass index (BMI), comorbidities, functional status such as previous mobility, place of residence before fracture, cognitive status, American Society of Anesthesiologists (ASA) status, fracture type and stability). This included data on the clinical and methodological variables that can act as effect modifiers across treatment comparisons. For intracapsular hip fractures, these have been identified as age, gender, baseline comorbidity, fracture displacement and cognitive status.
Interventions: details of each intervention (number and type, manufacturer details); general surgical details (number of clinicians and their skills and experience, perioperative care such as use of prophylactic antibiotics or antithromboembolics, mobilisation or weight‐bearing protocols).
Outcomes: all outcomes measured or reported by study authors; outcomes relevant to the review (to include measurement tools and time points of measure); extraction of outcome data into data and analysis tables in Review Manager 2014.
We extracted this data in accordance with recommendations in the DECiMAL (Data Extraction for Complex Meta‐Analysis) guide developed by Pedder and colleagues, which optimises data extraction for NMAs (Pedder 2016).
Assessment of risk of bias in included studies
One review author independently assessed risk of bias in the included studies using the Cochrane risk of bias tool (Higgins 2011a). A second author checked these decisions and a final judgement was made through discussion, if required. We assessed the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants, personnel (performance bias).
Blinding of outcome assessors (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other risks of bias.
In addition, we also considered performance bias related to the experience of the clinicians (whether clinicians were equally experienced with the implants used in the study). We considered risk of detection bias separately for: subjective outcomes measured by clinicians, objective outcomes measured by clinicians, and participant‐reported outcomes (e.g. pain and HRQoL). For each domain, two review authors judged whether study authors made sufficient attempts to minimise bias in their design. For each domain, we made judgements using three measures ‐ high, low or unclear risk of bias ‐ and we recorded these judgements in risk of bias tables.
Measures of treatment effect
Summary measures
At each data point, we extracted either:
mean or mean change from the baseline and standard deviations (SDs) per arm, or the information from which SDs could be derived, such as standard error or confidence interval (CI) for continuous outcomes;
number of events per arm.
If a trial presented outcomes at more than one time point, we extracted data for all relevant time points. We included three time points in the review for mortality and HRQoL: 'early' (up to and including four months), 12 months (prioritising 12‐month data, but in its absence including data after four months and up to 24 months), and 'late' (after 24 months).
Relative treatment effects
Studies reported HRQoL using different measurement tools and we therefore pooled data using standardised mean difference (SMD) (Hedges’ adjusted g). We entered data presented as a scale with a consistent direction of effect across studies. For interpretation, we re‐expressed the SMD in the measurement scale most commonly used in the estimate and used a minimal clinically important difference (MCID) for the selected measurement scale (Schünemann 2019).
For dichotomous outcomes, we reported the risk ratio (RR) and 95% CI. Results from NMA are presented as summary relative effect sizes ‐ SMDs and risk ratio (RR) ‐ for each possible pair of treatments.
Relative treatment ranking
We obtained a treatment hierarchy using the surface under the cumulative ranking curve (SUCRA), which is used to evaluate superiority of different treatments (Konig 2013; Mavridis 2015; Rucker 2015; Salanti 2008b; Salanti 2011; Salanti 2012). We prepared these for outcomes with sufficient data to form a network (early mortality, 12‐month mortality, and unplanned return to theatre). Generally, a larger SUCRA means a more effective intervention. We expressed SUCRA as a proportion (0 to 1.0). The higher the SUCRA value, the more likely the outcome of the respective treatment would be ranked first, or at least near the top of the rankings. Computations for SUCRA values were implemented in Stata (Stata) using the command 'sucra' (Chaimani 2013; Rucker 2015; Salanti 2011). We also calculated the estimated proportion of times each intervention would be ranked in each order position (from best to worst treatment) and from this, we presented an estimated mean rank for each intervention for each outcome in a network.
Unit of analysis issues
Alternative trial designs
We did not encounter any within‐person randomised trials or cluster‐randomised trials.
Reports of outcomes at different time points
When preparing the review, we found that outcomes were reported at a wider range of 'late' time points than we had anticipated. Following discussion with our clinical authors, we grouped these into three time points; we maintained an early time point (up to four months after surgery) and adopted two later time points ‐ one that prioritised data at 12 months (between four months and 24 months), and a final time point later than 24 months after surgery (which included final study follow‐up) (see Differences between protocol and review).
Studies with multiple treatment groups
We included multi‐armed trials and accounted for the correlation between the effect sizes in the network meta‐analysis. We followed guidance provided in the Cochrane Handbook on dealing with multiple groups from one study (Higgins 2011b), and NMA (Higgins 2011c).
We assumed that studies of different comparisons were similar in all ways apart from the interventions being compared.
Dealing with missing data
For each included study, we recorded the number of participant losses for each outcome. Unless reported otherwise, we assumed complete case data for mortality and unplanned return to theatre. For outcomes that required participant assessment at end of follow‐up (i.e. HRQoL), we prioritised intention‐to‐treat (ITT) data where these data were available. If ITT data were unavailable for these outcomes, and if study authors did not clearly report denominator figures for each group for the outcome, we reduced the denominator figure in each group to account for reported mortality. We did not impute missing data. We used the risk of bias tool to judge attrition bias. We judged studies to be at high risk of attrition bias if we noted large amounts of unexplained missing data, loss that could not be easily justified in the study population, or losses that were not sufficiently balanced between intervention groups.
Assessment of heterogeneity
Assessment of clinical and methodological heterogeneity within treatment comparisons
We assessed clinical and methodological diversity in terms of participants, interventions, outcomes and study characteristics for the included studies to determine whether a meta‐analysis was appropriate. We conducted this assessment by generating the descriptive statistics for trial and study population characteristics across all eligible trials that compared each pair of interventions, and observing these data from the data extraction tables.
Assumptions when estimating the heterogeneity
The network model allows for heterogeneity between studies within trial design by incorporating a study‐specific random effect. Standard pairwise meta‐analyses estimate different heterogeneity variances for each pairwise comparison. In NMAs, we assumed a common estimate for the heterogeneity variance across the different comparisons.
Measures and tests for heterogeneity
Pairwise comparisons
We assessed statistical heterogeneity within each pairwise comparison by visual inspection of the forest plots to detect any large differences of intervention effects across included studies. If the studies are estimating the same intervention effect, there should be overlap between the CIs for each effect estimate on the forest plot. However, if overlap is poor, or there are outliers, then statistical heterogeneity may be likely.
We used Stata to perform pairwise meta‐analysis (Stata). We produced the Chi2 statistic, which is the test for heterogeneity, and the I2 statistic, which is the test used to quantify heterogeneity and which calculates the proportion of variation due to heterogeneity rather than due to chance. A P value less than 0.10 was considered to be indicative of statistical heterogeneity; in the review, we refer to this as statistical inconsistency.
The I2 value ranges from 0% to 100%, with higher values indicating greater heterogeneity. As recommended in the Cochrane Handbook, an I2 value of 0% to 40% may be interpreted as "might not be important"; 30% to 60% as "may represent moderate heterogeneity"; 50% to 90% as "may represent substantial heterogeneity"; and 75% to 100% as "considerable heterogeneity" (Deeks 2019).
Entire network
The assessment of statistical heterogeneity in the entire network was based on the magnitude of the heterogeneity variance parameter (τ2) estimated from the NMA models (Jackson 2014). For dichotomous outcomes, the magnitude of the heterogeneity variance was compared with the empirical distribution, as derived by Turner (Turner 2012). For continuous outcomes where an SMD was used to summarise effect, we planned to use the same approach using the empirical distribution produced by Rhodes (Rhodes 2015). However, because no corresponding NMA models were carried out, this could not be done.
Assessment of statistical incoherence
We evaluated the statistical incoherence — which is the statistical disagreement between direct estimates (from direct comparisons of treatment) and indirect estimates (derived from the network comparisons) — by both local and global approaches, as follows (Chaimani 2017; Donegan 2013).
Global approaches for evaluating incoherence
To check the assumption of coherence in the entire network, we used the ‘design‐by‐treatment interaction’ model (Higgins 2012; White 2012). This method accounts for different sources of incoherence that can occur when studies with different designs (two‐armed trials versus three‐armed trials) give different results, as well as disagreement between direct and indirect evidence. Using this approach, we inferred the presence of incoherence from any source in the entire network based on a Chi2 test. The design‐by‐treatment model was performed in Stata, using the network command (Stata). We presented the results of this overall approach graphically in a forest plot using the network forest command in Stata (Stata).
Local approaches for evaluating incoherence
We evaluated the incoherence between direct and indirect comparisons using a statistical approach referred to as 'node splitting', conducted with the 'sidesplit' command in Stata, when a closed triangle or quadratic loop connecting no less than three arms existed (Dias 2010).
Investigation of heterogeneity
If we found important heterogeneity (inconsistency or incoherence, or both) across treatment comparisons, we planned to explore the possible sources. For extracapsular hip fractures, the effect modifiers have been identified as:
age;
gender;
baseline comorbidity index;
baseline functional status;
cognitive status;
fracture type; and
fracture stability.
However, there was insufficient variation between studies and a lack of reporting by subgroups for many of these effect modifiers within studies. When data were sufficient (such as for gender or fracture stability), we had insufficient studies within nodes to explore these effects practically.
Assessment of transitivity across treatment comparisons
We assessed the assumption of transitivity by comparing the distribution of the potential effect modifiers (such as stable and unstable fractures) across the different pairwise comparisons to ensure that they were, on average, balanced. We assessed control groups for their similarity across treatment comparisons.
Geometry of the network
Different eligibility criteria for interventions will result in different collections of evidence in the synthesis, and because of the inter‐relationships across direct and indirect evidence, this can lead to different effect estimates and relative rankings. We provided a qualitative description of network geometry accompanied by a network diagram of all competing interventions. The diagram gives a comprehensive definition of the nodes in the network and gives an indication of the volume of evidence within each comparison. It also gives a visual representation of the possible comparisons where any two modalities are compared.
We evaluated the quantitative metrics by assessing features of network geometry: the size of the nodes reflects the amount of evidence accumulated for each treatment (total number of participants) and the breadth of each edge is proportional to the inverse of the variance of the summary effect of each direct treatment comparison (Salanti 2008a). To understand which are the most influential comparisons in the network, and how direct and indirect evidence influences the final summary data, we used a contribution matrix that describes the percentage contribution of each direct meta‐analysis to the entire body of evidence (Chaimani 2015).
Presentation of results
We presented the following in our review, based on Salanti 2011.
A network diagram.
Direct pairwise results and assessment of between‐study heterogeneity.
Direct (the observed data), indirect and combined network estimates ‐ each reported in a single triangle table.
Treatment rankings.
Summary of findings tables for the primary networks accompanied by a forest plot of treatment effects.
Assessment of reporting biases
Standard systematic reviews consider the impact of possible reporting biases and small‐study effects (e.g. funnel plots and Egger’s test). These approaches have been extended for NMAs and we explored possible reporting biases when more than 10 relevant studies were available (early mortality, 12‐month mortality, and unplanned return to theatre). We produced comparison‐adjusted plots using the 'netfunnel' command in Stata to investigate any relationship between effect estimates and study size or precision (Chaimani 2012; Chaimani 2013). For the comparison‐adjusted funnel plot, we ordered interventions from the oldest to newest treatments in the entire evidence base, using date of publication as a proxy for old to new. We anticipated that published small trials may tend to be biased in the direction of new treatments.
Data synthesis
Methods for direct treatment comparisons
Initially, for every treatment comparison with at least two studies, we planned to perform standard pairwise meta‐analyses using a random‐effects model in Stata (Stata; White 2015); we considered this for all outcomes at each available time point. If any problems were evident with convergence, we planned to re‐analyse the data using a fixed‐effect model (White 2015). See Assessment of heterogeneity.
Methods for indirect and mixed comparisons
For each pairwise comparison, we synthesised data to obtain summary SMDs for continuous outcomes or risk ratios for dichotomous outcomes. Because the collected studies appeared to be sufficiently similar with respect to the distribution of effect modifiers, we conducted a random‐effects NMA to synthesise all evidence for each outcome and obtain a comprehensive ranking of all treatments. We performed our NMA with contrast‐level data by running the consistency and inconsistency (design‐by‐treatment interaction) models, using multivariate meta‐analysis approaches within the frequentist framework (White 2015). We used the network suite of Stata commands (Stata).
Subgroup analysis and investigation of heterogeneity
Although we planned to subgroup the data according to fracture type, we found limited variation in trochanteric and subtrochanteric fractures across most studies and therefore did not conduct subgroup analysis.
Sensitivity analysis
We did not conduct sensitivity analysis on the network estimates. See Differences between protocol and review.
Summary of findings and assessment of the certainty of the evidence
Credibility of the evidence
We used the GRADE approach to assess the certainty of the evidence for each outcome of interest in each paired comparison for which there is direct evidence (i.e. where two interventions have been compared in randomised trials). The GRADE system classifies evidence as 'high', 'moderate', 'low', or 'very low' certainty. The starting point for certainty in estimates for randomised trials is high, but for direct comparisons may be rated down based on limitations concerning risk of bias, imprecision, inconsistency, indirectness and publication bias (Guyatt 2008). We presented our GRADE assessments in a summary of findings table.
We also used the GRADE approach to assess the certainty in indirect and network (mixed) effect estimates (Brignardello‐Petersen 2018a; Puhan 2014). Using the 'node splitting' method, we calculated indirect effect estimates from the available 'loops' of evidence, including loops with a single common comparator (first order) or more than one intervening treatment (higher order) connecting the two interventions of the comparison of interest. To assess the certainty in evidence for each indirect comparison, we focused on the dominant first‐order loop (i.e. the first‐order loop that contributes most to the indirect estimate). The certainty‐of‐evidence rating for indirect comparisons was the lower of the ratings of certainty for the two direct estimates contributing to the dominant first‐order loop. For instance, if one of the direct comparisons was rated as low‐certainty evidence and the other was rated as moderate‐certainty evidence, we rated the certainty of indirect evidence as low.
For ratings of certainty for indirect comparisons, we also considered downgrading the certainty for intransitivity (Brignardello‐Petersen 2018a; Puhan 2014). The transitivity assumption implies similarity of the bodies of evidence (for instance, the trials assessing A versus C and B versus C informing a comparison of A versus B) informing indirect comparisons in terms of population, intervention, outcomes, settings and trial methodology (Salanti 2008b).
If both direct and indirect evidence are available and yield similar results, the NMA mixed‐estimate certainty rating comes from the higher certainty of the two that contribute substantially to the pooled estimate. If the direct and indirect estimates show important differences (incoherence) — addressed by the difference in point estimates, the extent of overlap of CIs, and a statistical test of incoherence — we considered further downgrading the certainty assessment of the mixed NMA effect (Brignardello‐Petersen 2018b).
Summary of findings tables
Typically, a summary of findings table presents the GRADE ratings, along with the intervention effects for the most important outcomes of the systematic review. In NMA, the comparison of multiple interventions is the main feature of the network and is likely to drive the structure of the tables. We followed the guidance for producing summary of findings tables for NMAs as outlined in Chapter 11 of the Cochrane Handbook (Chaimani 2018). Choosing the outcomes that yielded the most data, we produced a separate table for two outcomes in the review:
mortality at 12 months; and
unplanned return to the theatre at end of follow‐up.
All interventions were of direct interest to our main conclusions and were included in the summary of findings tables. We selected a reference comparator against which all other treatments were compared, and we reported relative effect estimates, baseline risk information, certainty of the evidence for the NMA, judgements for downgrading the body of the evidence, and text with definitions of NMA aspects (e.g. absolute effects) (Yepes‐Nuñez 2019).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Results of the search
After removal of duplicates from the search results, we screened 28,510 titles and abstracts, which included backward‐citation searches and searches of clinical trials registers. We reviewed the full texts of 1028 reports and selected 184 studies (with 269 records) for inclusion in this review. We excluded 725 records, and report the details of 21 key studies from these excluded records. Twelve studies are awaiting classification and we identified 20 ongoing studies. See Figure 1.
1.

Study flow diagram
Included studies
See Characteristics of included studies. Ten studies were reported only as abstracts with limited study characteristics (Acharya 2003; Benum 1994; Irvine 2014; Mehdi 2000; Merenyi 1995; Michos 2001; Mott 1993; Raimondo 2012; Trafton 1984; Weisbrot 2005).
Types of studies and settings
We included 184 studies (see Included studies). Twenty‐four studies allocated participants to interventions using methods that we have described as quasi‐randomised (Agrawal 2017; Bajpai 2015; Butt 1995; Dhamangaonkar 2013; Galanopoulos 2018; Guyer 1991; Hardy 1998; Kammerlander 2018; Lopez 2002; Lopez‐Vega 2015; Marques 2005; Park 1998; Park 2010; Schipper 2004; Sharma 2018; Verettas 2010; Watson 1998; Yamauchi 2014). Although we expected most other studies were randomised controlled trials, methods of randomisation were not always clearly reported.
Twenty‐two studies were conducted across multiple centres (Ahrengart 1994; Andalib 2020; Baumgaertner 1998; Benum 1994; Caiaffa 2016; Ciaffa 2018; Davis 1988; Ekstrom 2007; Fitzpatrick 2011; Griffin 2021; Kammerlander 2018; Lunsjo 2001; Matre 2013; McCormack 2013; Mott 1993; Okcu 2013; Rahme 2007; Reindl 2015; Sanders 2017; Schipper 2004; Vaquero 2012; Wu 2020), and the remainder were completed at a single centre.
Studies were conducted in:
China (Cao 2017; Chen 2018; Cheng 2014; Dong 2018; Gou 2013; Guo 2015; Han 2012; Huang 2006; Li 2010; Li 2015b; Li 2018; Pan 2009; Shi 2018; Song 2011 Su 2016; Tao 2013; Wang 2011; Wang 2019; Xu 2010b; Xu 2010a; Xu 2018; Yang 2011a; Yaozeng 2010; Yu 2015; Zhang 2013; Zhang 2018; Zhou 2012; Zou 2009);
UK (Adams 2001; Bannister 1990; Barton 2010; Bridle 1991; Butt 1995; Davis 1988; Esser 1986; Griffin 2016; Griffin 2021; Harrington 2002; Haynes 1996; Hornby 1989; Kosygan 2002; Little 2008; McLaren 1991; Mehdi 2000; Parker 2012; Parker 2017; Parker 2020; Radford 1993);
Sweden (Brostrom 1992; Dalen 1988; Ekstrom 2007; Lunsjo 2001; Mattsson 2004; Mattsson 2005; Miedel 2005; Nungu 1991; Olsson 2001; Sernbo 1988; Sernbo 1994; Stark 1992);
India (Agrawal 2017; Bajpai 2015; Barwar 2014; Dhamangaonkar 2013; Haq 2014; Jolly 2019; Kumar 2019; Singh 2017; Singh 2019; Vidyadhara 2007);
Italy (Andreani 2015; Caiaffa 2016; Carulli 2017; Catania 2019; Ciaffa 2018; Dall'Oca 2010; Moroni 2004; Moroni 2005; Pesce 2014; Raimondo 2012);
Greece (Aktselis 2014; Efstathopoulos 2007; Galanopoulos 2018; Kouvidis 2012; Makridis 2010; Michos 2001; Papasimos 2005; Verettas 2010; Vossinakis 2002);
Spain (Barrios 1993; Herrera 2002; Lopez 2002; Lopez‐Vega 2015; Marques 2005; Utrilla 1998; Utrilla 2005; Vaquero 2012; Varela‐Egocheaga 2009);
USA (Baumgaertner 1998; Chapman 1981; Fitzpatrick 2011; Goldhagen 1994; Shannon 2019; Trafton 1984; Watson 1998; Yang 2011b);
Turkey (Akinci 2010; Desteli 2015; Eceviz 2020; Okcu 2013; Ozkayin 2015; Seyhan 2015; Zehir 2015);
Germany (Berger‐Groch 2016; Fritz 1999; Hoffmann 1999; Hopp 2016; Stappaerts 1995; Wild 2010);
South Korea (Hong 2011; Kim 2005; Park 1998; Park 2010; Shin 2017);
Switzerland (Guyer 1991; Pelet 2001; Sadowski 2002; Saudan 2002; Stern 2011);
Belgium (De Grave 2012; Hardy 1998; Hardy 2003; Janzing 2002);
Canada (McCormack 2013; O'Brien 1995; Reindl 2015; Sanders 2017);
Norway (Benum 1994; Buciuto 1998; Haddon 2019; Matre 2013);
France (Dujardin 2001; Giraud 2005; Pitsaer 1993);
Iran (Andalib 2020; Kazemian 2014; Kazemian 2016);
Israel (Chechik 2014; Juhn 1988; Peyser 2007);
Mexico (Calderon 2013; Delgado 1990; Romero 2008);
Netherlands (Liem 1993; Pahlpatz 1993; Schipper 2004);
Two studies each in: Austria (Irvine 2014; Kukla 1997), Brazil (Guerra 2014; Sharma 2018), Denmark (Dalsgaard 1986; Hogh 1981), Finland (Pajarinen 2005; Teerenhovi 1984), Japan (Kuwabara 1998; Yamauchi 2014), and Pakistan (Adeel 2020; Akhtar 2016);
One study each in: Egypt (Selim 2020), Hong Kong (Leung 1992), Hungary (Merenyi 1995), Nepal (Karn 2006), and New Zealand (Hoffman 1996).
Three studies were conducted in more than one country: Australia and UK (Rahme 2007); Sweden and Finland (Ahrengart 1994); and Austria, Belgium, Germany, Israel, Norway and Switzerland (Kammerlander 2018). In two studies, there was insufficient information in the study report for us to determine the country (Acharya 2003; Weisbrot 2005).
Studies were published between 1980 and 2020. We noted that 25% of studies were published before 2000, and 52% were published since 2010.
Types of participants
In total, 26,073 participants with 26,086 extracapsular hip fractures were recruited across the 184 studies. Whilst most studies included a mix of stable and unstable fractures, almost a third of studies included only participants with unstable fractures and two studies included only participants with stable fractures (Eceviz 2020; Sharma 2018). Most studies included a mix of fracture instability. One study included only subtrochanteric fractures (Rahme 2007), and whilst some studies also included subtrochanteric fractures, these were generally a much smaller proportion of the overall fracture type in these studies.
Most participants were randomised within three days of injury, but 13 studies included participants at later time points after injury: up to four days (Pelet 2001), five days (Sernbo 1994), seven days (Akhtar 2016; Eceviz 2020; Karn 2006; O'Brien 1995; Vossinakis 2002 ), two weeks (Hong 2011; Reindl 2015), 15 days (Caiaffa 2016; Ciaffa 2018), or three weeks (Haq 2014; Zhou 2012).
Although some studies recruited participants from a younger starting age (e.g. at least 50 years or 55 years of age), we found that the mean age of participants in most studies (where reported) ranged from 60 to 93 years of age. In only five studies, we noted a mean age slightly younger than 60 years (Adeel 2020; Agrawal 2017; Akhtar 2016; Haq 2014; Singh 2017). Three studies recruited only female participants (Esser 1986; Moroni 2004; Moroni 2005). In the remaining studies that reported gender distribution, there were 15,944 females, which represents 69% of the participants included in these studies. We expected that female representation was higher than this in most studies because we noted that in 15 of these studies, there were more male participants than female participants, and we expected that these studies affected this percentage (Adeel 2020; Agrawal 2017; Akhtar 2016; Akinci 2010; Chapman 1981; Ciaffa 2018; Desteli 2015; Dhamangaonkar 2013; Dong 2018; Hong 2011; Huang 2006; Radford 1993; Romero 2008; Wang 2011; Zhang 2018).
Types of interventions
Only two studies included three comparison arms (Ciaffa 2018; Papasimos 2005). The remaining studies were two‐arm studies comparing only one type of intervention with another. Studies included the following interventions (see Table 4 and Table 5 for approaches to classification of interventions).
Cephalomedullary nails: Gamma nail, Gamma 3, Endovis nail, Holland nail, Trigen Intertan nail, Kűntscher‐Y nail, Zimmer nail, Uni‐nail, Elos Intramedullary nail, ACE Trochanteric nail, Gliding nail, Proximal Femoral Nail (PFN), expandable PFN, PFN‐antirotation (PFNA), Targon PFN, Fixion PFN, Intramedullary Hip Screw, Affixus Hip Fracture Nail System and unspecified cephalomedullary nails. Where reported, studies included nails that were short (or likely to be short) and long. Although less frequently reported, we noted other intervention characteristics such as whether the lag screw was dynamic or static, single or double, etc.
Condylocephalic nails: Ender nails and Harris nails.
Fixed angle plates: sliding hip screw, dynamic hip screw, compression hip screw, dynamic condylar screw, Medoff sliding plate, AMBI hip screw, percutaneous compression plate, X‐bolt Dynamic Hip Plating System, Contralateral reverse Distal Femoral Locking Compression Plate (DFLCP), Less Invasive Stabilisation System (LISS), locking compression plate, McLaughlin nail plate, Richards sliding hip screw, Pugh nail plate, Jewett nail plate, AO angle plate, proximal femoral locking plate, locking plate, blade plate, angle plate. Where reported, or when the implant was specified, we noted whether the capital screw or blade was dynamic or static within the barrel.
Arthroplasties, including all types of hemiarthroplasty and total hip arthroplasty.
External fixation.
Non‐operative treatment, including skin or skeletal traction.
Types of outcome measures
Forty nine studies did not report review outcomes (Acharya 2003; Adeel 2020; Akhtar 2016; Bajpai 2015; Bannister 1990; Barrios 1993; Barwar 2014; Calderon 2013; Cao 2017; Chen 2017; Chen 2018; Cheng 2014; Delgado 1990; Desteli 2015; Dhamangaonkar 2013; Dong 2018; Fitzpatrick 2011; Galanopoulos 2018; Guo 2015; Han 2012; Hong 2011; Juhn 1988; Karn 2006; Kuwabara 1998; Li 2010; Li 2018; Mehdi 2000; Moroni 2004; Moroni 2005; Pan 2009; Park 1998; Park 2010; Pesce 2014; Romero 2008; Selim 2020; Sernbo 1994; Shi 2018; Stappaerts 1995; Vidyadhara 2007; Wang 2011; Wang 2019; Watson 1998; Weisbrot 2005; Xin 2014; Xu 2010a; Xu 2018; Yamauchi 2014; Yu 2015; Zhang 2018). The remaining studies reported data for at least one of the review outcomes.
Sources of funding and declarations of interest
Approximately 40% of the studies declared that they had no conflicts of interest. However, we note that sources of funding were not always clearly reported, such that we could not determine how some of these studies were funded.
We judged that the funding was from independent sources (such as universities, hospitals or foundation trusts) in 11 studies (Cai 2016; Davis 1988; Esser 1986; Haddon 2019; Hornby 1989; Lunsjo 2001; Olsson 2001; Parker 2012; Parker 2017; Reindl 2015; Xu 2018). In 14 studies, all or part of the funding was received from a manufacturer of study implants (Ahrengart 1994; Griffin 2016; Griffin 2021; Hardy 1998; Haynes 1996; Kammerlander 2018; Matre 2013; Mattsson 2004; Mattsson 2005; Miedel 2005; Parker 2020; Sanders 2017; Schipper 2004; Vaquero 2012); this represented only 7.5% of all studies. However, we note that overall, two‐thirds of studies did not report whether they received funding for their research.
Excluded studies
Because the searches in this review were designed to feed into a series of related Cochrane Reviews about the surgical management of hip fracture, we have not included a bibliographic list of all excluded studies. We excluded most studies because they were study designs that were ineligible for inclusion in this review, or were not treating participants with the type of fracture or with the types of interventions that were eligible for this review. Some of the excluded studies were eligible for inclusion in the related Cochrane Reviews.
Here, we report the details of 21 key excluded studies (see Characteristics of excluded studies). We excluded three studies because studies recruited younger participants than the expected population for the type of fracture, or included mainly fractures from high‐energy trauma, or both (El‐Desouky 2016; Emami 2013; Lee 2007b). The decision to exclude younger participants was a change from our protocol (see Differences between protocol and review). Five studies were abstracts with insufficient detail on the number of participants in each group, meaning extraction of data was not feasible (Ahmad 2011; Gupta 2012; Kumar 2006; Savadhkoohi 2016; Sher 1985). We excluded six studies that investigated the surgical approach or a supplement to treatment rather than the interventions of interest for this review (Alobaid 2004; Bong 1981; Kumar 1996; Lee 2007a; Li 2015a; Wong 2009). We excluded seven clinical trials reports. Two of these were terminated early and have not published findings (ACTRN12608000162314; NCT03065101). Five of these were completed in 2011/2012, according to the clinical trials register; we excluded these because we expect publication of findings is now unlikely (NCT00323232; NCT00686023; NCT00736684; NCT01173744; NCT01238068). See Characteristics of excluded studies.
Ongoing studies
We identified 20 studies from clinical trials register searches. These studies evaluate: two different models of cephalomedullary nail (NCT01437176; NCT01509859; NCT01797237; NCT02627040; NTR1133); cephalomedullary nails that are locked or unlocked (ACTRN12618001431213), with or without a lateral plate (ChiCTR1900025588), with a static or dynamic lag screw (NCT04441723), and single lag screw nailing and helical blade nailing (CTRI/2019/02/017733; NCT04306198); cephalomedullary nail versus fixed angle plate (ACTRN12610000992000; IRCT20141209020258N80; NCT03906032); two different models of fixed angle plate (ChiCTR‐TRC‐11001642; CTRI/2019/11/022097); fixed angle plate with or without a static screw (IRCT20170621034689N2); fixed angle plate and external fixator (IRCT20181015041344N1); internal fixation versus hemiarthroplasty (JPRN‐UMIN000019523; NCT03407131); and hemiarthroplasty and total hip arthroplasty (ChiCTR‐INR‐17011841). The estimated enrolment is more than 2400 participants; this number does not include seven ongoing studies for which we did not have estimated numbers (ChiCTR1900025588; ChiCTR‐INR‐17011841; ChiCTR‐TRC‐11001642; CTRI/2019/02/017733; CTRI/2019/11/022097; IRCT20170621034689N2; IRCT20181015041344N1). See Characteristics of ongoing studies.
Awaiting classification
We found twelve studies that are awaiting classification. These studies are described as completed in a clinical trials register, or we expected they were completed, but do not yet have published results. The studies evaluate: two different models of cephalomedullary nails (ISRCTN48618754; JPRN‐UMIN000018307; NCT03635320); cephalomedullary nail with or without cement augmentation (NCT03000972); cephalomedullary nail and fixed angle plate (NCT01380444; NCT02788994; NCT03849014; NCT04240743; REGAIN 2008); two different models of fixed angle plate (ISRCTN32393360); fixed angle plate with or without trochanteric support plate (NCT02294747); and internal fixation and total hip arthroplasty (NCT02171897). Recruited participants are likely to number more than 1500; this number does not include two studies for which we did not have estimated participant numbers (ISRCTN32393360;ISRCTN48618754). See Studies awaiting classification.
Risk of bias in included studies
See Figure 2. We only conducted risk of bias assessment for studies with outcome data included in the networks, and we conducted risk of detection bias separately for each outcome. Blank spaces in the risk of bias figure indicate that assessments were not completed for these studies or domains.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Blank spaces indicate that risk of bias was not conducted because: (1) the study reported no review‐relevant outcomes; (2) no outcome data were included in analyses; (3) the study was not included in any networks; or (4) study authors did not report outcomes relevant to these domains.
Allocation
Twenty‐six studies described adequate methods to randomise participants to treatment groups, and we judged these studies to be at low risk of selection bias for sequence generation (Andalib 2020; Barton 2010; Eceviz 2020; Ekstrom 2007; Esser 1986; Giraud 2005; Guerra 2014; Haq 2014; Hoffman 1996; Kazemian 2014; Kazemian 2016; Kim 2005; Little 2008; Ovesen 2006; Pajarinen 2005; Parker 2012; Reindl 2015; Sadowski 2002; Saudan 2002; Sernbo 1988; Shannon 2019; Varela‐Egocheaga 2009; Vossinakis 2002; Xu 2010b; Zehir 2015; Zhou 2012). We judged 11 studies to be at high risk of selection bias for sequence generation because these were quasi‐randomised studies (Agrawal 2017; Butt 1995; Chapman 1981; Dalen 1988; Dalsgaard 1986; Goldhagen 1994; Guyer 1991; Hardy 2003; Haynes 1996; Leung 1992; Verettas 2010). The remaining studies reported insufficient information for us to judge risk of selection bias for sequence generation.
Thirteen studies described adequate methods to conceal allocation during the selection process and were at low risk of bias (Adams 2001; Ahrengart 1994; Aktselis 2014; Baumgaertner 1998; Eceviz 2020; Ekstrom 2007; Haynes 1996; Hoffman 1996; Ovesen 2006; Pajarinen 2005; Parker 2012; Parker 2017; Shannon 2019). We judged all the quasi‐randomised studies to be at high risk of bias for allocation concealment, and the remaining studies reported insufficient information for us to judge risk of bias for this domain.
Blinding
It is not possible to blind surgeons to the different study implants, but we did not expect that this lack of blinding would introduce bias and we judged all studies to be at low risk of performance bias for blinding of personnel. However, we believed that the experience with the interventions may affect performance. We judged 23 studies to be at low risk of other performance bias because surgeons were experienced, or likely to be experienced, with both types of implants (Adams 2001; Ahrengart 1994; Aktselis 2014; Barton 2010; Bridle 1991; Dalen 1988; Dujardin 2001; Eceviz 2020; Gou 2013; Haq 2014; Kouvidis 2012; Kukla 1997; Okcu 2013; Pajarinen 2005; Parker 2012; Parker 2017; Radford 1993; Sadowski 2002; Saudan 2002; Shannon 2019; Utrilla 1998; Utrilla 2005; Varela‐Egocheaga 2009). Thirteen studies were at high risk of bias because surgeons were more experienced using one of the study implants (Baumgaertner 1998; Benum 1994; Brostrom 1992; Goldhagen 1994; Guyer 1991; Hardy 1998; Harrington 2002; Haynes 1996; Hoffman 1996; Leung 1992; Little 2008; Tao 2013; Zhou 2012). There was insufficient detail about surgeon experience in the remaining studies.
We judged detection bias according to the type of outcome being measured. We expected that assessment of mortality was at low risk of detection bias in all studies. Although participants were not always blinded when providing assessment information for health‐related quality of life, we also expected that detection bias for this outcome was low in all relevant studies. However, we believed that decisions on return to theatre were subjective, were likely to be made by unblinded surgeons, and we judged all studies reporting this outcome to be at high risk of detection bias. Only one study reported methods used to conceal identity on radiographs to ensure blinded outcome assessment for unplanned return to theatre, and we judged risk of detection bias for this outcome to be low (Eceviz 2020).
Incomplete outcome data
We judged six studies to be at high risk of attrition bias because of unexplained losses affecting some or all of the outcomes or because we could not determine whether data were complete for all participants (Ahrengart 1994; Benum 1994; Brostrom 1992; Pajarinen 2005; Pitsaer 1993; Tao 2013). We judged the remaining studies to be at low risk of attrition bias.
Selective reporting
We judged only one study to be at low risk of selective reporting bias (Shannon 2019). This study was prospectively registered with a clinical trials register and reported outcomes in this study were consistent with those in the trials register documents. Four studies were retrospectively registered with a clinical trials register (Barton 2010; Eceviz 2020: Parker 2017; Reindl 2015). It was not feasible to use these registration documents to assess risk of selective reporting bias. The remaining studies did not report pre‐published protocols or clinical trials registration and we were unable to assess reporting bias.
Other potential sources of bias
We judged five studies to be at high risk of other bias because they were reported only in brief abstracts or reports which we expected were not peer‐reviewed (Benum 1994; Merenyi 1995; Pitsaer 1993; Trafton 1984). We noted a difference in clinical management between participant groups in three studies (Agrawal 2017; Akinci 2010;Tao 2013); we judged risk of other bias to be unclear because we could not be certain whether this could influence participant outcomes. We had only limited translation for Teerenhovi 1984, and risk of other bias was therefore unclear. We identified no other sources of bias in the remaining studies.
Effects of interventions
Summary of findings 1. Certainty of the effects and network estimates for treating extracapsular hip fractures in older adults: mortality at 12 months.
| Certainty of the effects and network estimates for treating extracapsular hip fractures in older adults: mortality at 12 months | |||||
|
Patient or population: older adults (> 60 years of age) with extracapsular hip fractures Intervention: static fixed angle plate, long cephalomedullary nail, short cephalomedullary nail, condylocephalic nail, external fixation, hemiarthroplasty, non‐operative treatment, total hip arthroplasty Comparison: dynamic fixed angle plate Outcome: mortality at 12 months; range of follow‐up time points from 5 months up to 24 months (in most studies, data were reported at 12 months) Setting: in hospital | |||||
|
Total studies: 56 Total participants: 8407 |
Relative effect (95% CI) | Anticipated absolute effect* (95% CI) | Certainty of the evidence | ||
| With comparison | With intervention | Difference | |||
|
Static fixed angle plate (5 studies; 847 participants)a |
RR 1.02 (0.77 to 1.36) |
201 per 1000 | 205 per 1000 | 4 per 1000 more (46 fewer to 72 more) |
Very low Downgraded for risk of bias,b,c and imprecisiond |
|
Long cephalomedullary nail (2 studies; 400 participants)a |
RR 1.27 (0.89 to 1.81) |
201 per 1000 | 255 per 1000 | 54 per 1000 more (22 fewer to 162 more) |
Very low Downgraded for risk of bias,b,c imprecisiond and intransitivitye |
|
Short cephalomedullary nail (33 studies; 5380 participants)a |
RR 0.97 (0.87 to 1.07) |
201 per 1000 | 194 per 1000 | 7 per 1000 fewer (26 fewer to 15 more) |
Very low Downgraded for risk of biasb,c and imprecisiond |
|
Condylocephalic nail (6 studies; 847 participants)a |
RR 0.93 (0.74 to 1.16) |
201 per 1000 | 186 per 1000 | 15 per 1000 fewer (52 fewer to 32 more) |
Very low Downgraded for risk of biasb,c and imprecisiond |
|
External fixation (1 study; 100 participants)a |
RR 0.75 (0.36 to 1.57) |
201 per 1000 | 150 per 1000 | 51 per 1000 fewer (130 fewer to 115 more) |
Low Downgraded for risk of bias,b and imprecisiond |
|
Hemiarthroplasty (No direct evidence, indirect evidence only) |
RR 1.36 (0.73 to 2.54) |
201 per 1000 | 274 per 1000 | 73 per 1000 more (54 fewer to 309 more) |
Very low Downgraded for risk of bias,b,c imprecisiond andintransitivitye |
|
Non‐operative treatment (1 study; 106 participants)a |
RR 1.00 (0.53 to 1.87) |
201 per 1000 | 201 per 1000 | 0 per 1000 fewer (94 fewer to 175 more) |
Low Downgraded for risk of biasb and imprecisione |
|
Total hip arthroplasty (1 study; 156 participants)a |
RR 0.69 (0.12 to 4.03) |
201 per 1000 | 139 per 1000 | 62 per 1000 fewer (177 fewer to 610 more) |
Low Downgraded for risk of biasb and imprecisione |
| Dynamic fixed angle plate | Reference comparator | ‐ | ‐ | ‐ | Reference comparator |
| *Anticipated absolute effects compare two risks by calculating the difference between the risk with the intervention group and the risk with the comparison/control group (reference comparator). CI: confidence interval; NMA: network meta‐analysis; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are confident that the true estimate lies close to the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of the effect. | |||||
aNetwork estimate derived from direct evidence and indirect evidence (number of studies and participants is for direct evidence contributing to the network estimate) bRisk of bias: all studies in direct and indirect estimates had high risks of detection bias, as well as unclear risks of bias in at least one other domain (downgraded by one level) cRisk of bias: studies in direct or indirect estimates (or both) had high risks of selection bias or 'other bias' (downgraded by one level) eImprecision: confidence interval in the network estimate included benefits as well as harms (downgraded by one level) eIntransitivity: indirect estimates included variation in proportion of stable/unstable fractures and intransitivity may be evident (downgraded by one level)
Summary of findings 2. Certainty of the effects and network estimates for treating extracapsular hip fractures in older adults: unplanned return to theatre.
| Certainty of the effects and network estimates for treating extracapsular hip fractures in older adults: unplanned return to theatre | |||||
|
Patient or population: older adults (> 60 years of age) with extracapsular hip fractures Intervention: static fixed angle plate, long cephalomedullary nail, short cephalomedullary nail, condylocephalic nail, external fixation, hemiarthroplasty Comparison: dynamic fixed angle plate Outcome: unplanned return to theatre: range of follow‐up time points from 6 weeks up to 5 years (in most studies, data were reported at 12 months; with only one study at 5 years) Setting: in hospital | |||||
|
Total studies: 55 Total participants: 9296 |
Relative effect (95% CI) | Anticipated absolute effect* (95% CI) | Certainty of the evidence | ||
| With comparison | With intervention | Difference | |||
|
Static fixed angle plate (4 studies; 622 participants)a |
RR 2.48 (1.36 to 4.50) |
39 per 1000 | 97 per 1000 | 58 per 1000 more (14 more to 137 more) |
Very low Downgraded for risk of biasb,c and inconsistencyd |
|
Long cephalomedullary nail (2 studies; 400 participants)a |
RR 1.58 (0.55 to 4.56) |
39 per 1000 | 62 per 1000 | 23 per 1000 more (18 fewer to 139 more) |
Very low Downgraded for risk of bias,b,c inconsistencyd and imprecisione |
|
Short cephalomedullary nail (34 studies; 6437 participants)a |
RR 1.12 (0.80 to 1.56) |
39 per 1000 | 43 per 1000 | 4 per 1000 more (8 fewer to 22 more) |
Very low Downgraded for risk of bias,b,c inconsistencyd and imprecisione |
|
Condylocephalic nail (7 studies; 996 participants)a |
RR 3.33 (1.95 to 5.68) |
39 per 1000 | 130 per 1000 | 91 per 1000 more (37 more to 182 more) |
Very low Downgraded for risk of biasb,c and inconsistencyd |
|
External fixation (1 studies; 100 participants)a |
RR 0.09 (0.00 to 1.87) |
39 per 1000 | 4 per 1000 | 35 per 1000 fewer (39 fewer to 34 more) |
Low Downgraded for risk of biasb and imprecisione |
|
Hemiarthroplasty (No direct evidence, indirect evidence only) |
RR 0.37 (0.01 to 10.26) |
39 per 1000 | 14 per 1000 | 25 per 1000 fewer (38 fewer to 361 more) |
Very low Downgraded for risk of bias,b,c inconsistency,d imprecisione and intransitivityf |
| Dynamic fixed angle plate | Reference comparator | ‐ | ‐ | ‐ | Reference comparator |
| *Anticipated absolute effects compare two risks by calculating the difference between the risk with the intervention group and the risk with the comparison/control group (reference comparator). CI: confidence interval; NMA: network meta‐analysis; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are confident that the true estimate lies close to the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of the effect. | |||||
aNetwork estimate derived from direct evidence and indirect evidence (number of studies and participants is for direct evidence contributing to the network estimate) bRisk of bias: all studies in direct and indirect estimates had high risks of detection bias, as well as unclear risks of bias in at least one other domain (downgraded by one level) cRisk of bias: studies in direct or indirect estimates (or both) had high risks of selection bias or 'other bias' (downgraded by one level) dInconsistency: evidence of statistical inconsistency in direct or indirect estimates, or both (downgraded by one level) eImprecision: confidence interval in the network estimate included benefits as well as harms (downgraded by one level) fIntransitivity: indirect estimates included variation in proportion of stable/unstable fractures and intransitivity may be evident (downgraded by one level)
Where data were available, we produced networks for each of our specified outcomes and time points, as described in Types of outcome measures, yielding three different networks. The overall approach to development of these networks was driven principally by consideration of the clinical appropriateness of lumping/splitting the nodes, carried out in a series of meetings between the author group and representatives from the Fragility Fracture Network. We refined the networks such that a balance was achieved between efficiency, where consideration was taken for how many studies could be included, and the best possible representation of the interventions and their component subtypes.
Although not all the networks included all nodes, we defined nine separate nodes across this review:
dynamic fixed angle plates;
static fixed angle plates;
long cephalomedullary nails;
short cephalomedullary nails;
condylocephalic nails;
external fixation;
hemiarthroplasty;
total hip arthroplasty; and
non‐operative treatment.
We had insufficient studies in this review to enable networks for late mortality and for HRQoL at any time point. Therefore, the networks of interest in this review are for:
early mortality (participant numbers in nodes ranged from 79 to 2050; studies ranged from 1 to 21 per comparison);
mortality at 12 months (participant numbers in nodes ranged from 76 to 2861; studies ranged from 1 to 33 per comparison);
unplanned return to theatre (participant numbers in nodes ranged from 29 to 4228; studies ranged from 1 to 34 per comparison).
The treatments in these networks were all connected (see Figure 3; Figure 4; Figure 5).
3.

Network geometry for early mortality. The size of the nodes is proportional to the number of trials investigating each treatment, whilst the thickness of the edges is proportional to the number of direct comparisons. Treatment nodes ‐ A: dynamic fixed angle plate;B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail;F: external fixation; G: hemiarthroplasty
4.

Network geometry for mortality at 12 months. The size of the nodes is proportional to the number of trials investigating each treatment, whilst the thickness of the edges is proportional to the number of direct comparisons. Treatment nodes ‐ A: dynamic fixed angle plate;B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail; F: external fixation; G: hemiarthroplasty; H: non‐operative treatment; I: total hip arthroplasty
5.

Network geometry for unplanned return to theatre. The size of the nodes is proportional to the number of trials investigating each treatment, whilst the thickness of the edges is proportional to the number of direct comparisons. Treatment nodes ‐ A: dynamic fixed angle plate; B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail; F: external fixation; G: hemiarthroplasty
We prepared summary of findings tables for two of these networks, and we selected dynamic fixed angle plates as our reference comparator against which we reported network effect estimates and assessed the certainty of the evidence. This treatment was included in all networks and was deemed clinically to be a reasonable candidate as a 'default' treatment that would likely be appropriate for the vast majority of people with an extracapsular fracture.
For each treatment in all of the networks, we calculated probabilities for each treatment for every possible rank between best and worst treatment, along with the mean rank and surface under the cumulative ranking (SUCRA) values. The magnitude of the estimated between‐study standard deviations for each of the networks conducted was in keeping with published estimates of non‐pharmacological interventions.
1. Early mortality
We included 36 studies (4822 randomised participants and 4791 analysed participants) in the network for mortality within four months of surgery (Barton 2010; Bridle 1991; Brostrom 1992; Chapman 1981; Dalen 1988; Dalsgaard 1986; Dujardin 2001; Giraud 2005; Guyer 1991; Hardy 1998; Harrington 2002; Hoffman 1996; Hoffmann 1999; Hogh 1981; Jolly 2019; Kazemian 2014; Kim 2005; Kukla 1997; Liem 1993; Little 2008; Miedel 2005; Ovesen 2006; Pajarinen 2005; Parker 2017; Radford 1993; Sadowski 2002; Saudan 2002; Shannon 2019; Utrilla 1998; Utrilla 2005; Varela‐Egocheaga 2009; Verettas 2010; Vossinakis 2002; Xu 2010b; Zehir 2015; Zhou 2012). The maximum number of randomised participants was 400 and the minimum number was 39.
Twenty additional studies reported early mortality which we did not include in the network. We dropped seven of these from the analysis because at least one of the treatments in the study did not correspond with our node definitions (e.g. mixed group of cephalomedullary nail lengths, or we could not determine the length of nail) (Chechik 2014; Matre 2013; Michos 2001; O'Brien 1995; Pahlpatz 1993; Sanders 2017; Wild 2010). We dropped the remaining 13 studies from the analysis because they compared treatments within a node (e.g. a short cephalomedullary nail with another short cephalomedullary nail) (Caiaffa 2016; Efstathopoulos 2007; Fritz 1999; Hardy 2003; Kosygan 2002; Makridis 2010; Olsson 2001; Papasimos 2005; Parker 2020; Peyser 2007; Schipper 2004; Vaquero 2012; Wu 2020). The network included no data for non‐operative treatment or total hip arthroplasty.
Direct comparisons
In the direct comparisons, we found evidence of a difference in early mortality between dynamic fixed angle plate versus long cephalomedullary nail (RR 1.80, 95% CI 1.02 to 3.18, favours fixed angle plate; 2 studies, 400 participants).
However, we found no evidence of a difference between any of the other treatments for this outcome (Table 6; Figure 6). On inspection of Table 6, we noted that CIs for each estimate overlapped and there was little evidence to suggest that any one of the treatments was either substantially better or worse than the other. However, the CIs were wide for most of these estimates, indicating substantial imprecision.
4. Early mortality: network estimates, direct estimates and indirect estimates.
| Dynamic fixed angle plate | D: 0.91 (0.36 to 2.32) I: 0.71 (0.28 to 1.79) | D: 1.80 (1.02 to 3.18) I: 0.53 (0.28 to 1.01) | D: 0.94 (0.75 to 1.19) I: 2.92 (1.34 to 6.39) |
D: 0.93 (0.48 to 1.80) I: 1.12 (0.36 to 3.53) |
D: 0.62 (0.23 to 1.68) I: too imprecise |
|
| 0.81 (0.42 to 1.54) | Static fixed angle plate | D: 1.56 (0.14 to 16.97) I: 1.28 (0.62 to 2.64) | D: 1.29 (0.61 to 2.70) I: 1.06 (0.35 to 3.16) | |||
| 1.09 (0.65 to 1.80) | 1.35 (0.59 to 3.06) | Long cephalomedullary nails | D: 1.79 (0.98 to 3.25) I: 0.53 (0.28 to 0.97) | |||
| 1.05 (0.81 to 1.36) | 1.30 (0.65 to 2.60) | 0.97 (0.58 to 1.61) | Short cephalomedullary nails | D: 0.87 (0.20 to 3.86) I: too imprecise | ||
| 0.97 (0.55 to 1.71) | 1.21 (0.66 to 2.21) | 0.90 (0.42 to 1.92) | 0.93 (0.50 to 1.72) | Condylocephalic nails | ||
| 0.62 (0.23 to 1.68) | 0.76 (0.23 to 2.52) | 0.57 (0.18 to 1.75) | 0.59 (0.21 to 1.66) | 0.63 (0.20 to 2.00) | External fixation | |
| 0.91 (0.20 to 4.14) | 1.14 (0.22 to 5.86) | 0.84 (0.18 to 4.06) | 0.87 (0.20 to 3.86) | 0.94 (0.19 to 4.71) | 1.49 (0.24 to 9.11) | Hemiarthroplasty |
Intervention effects, measured as risk ratios (with 95% confidence intervals), for early mortality (≤ 4 months). In the lower triangle (network estimates), the column‐defining intervention is the reference group; a risk ratio lower than 1 favours the row‐defining intervention for the network meta‐analysis results (network estimates). In the upper triangle (direct estimates (D) and indirect estimates (I)), the row‐defining intervention is the reference group; a risk ratio lower than 1 favours the column‐defining intervention for the pairwise meta‐analysis. Blank spaces indicate that no direct estimates were available and the network estimate was derived from indirect evidence only.
6.

Network forest plot for early mortality. Treatment nodes ‐ A: dynamic fixed angle plate;B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail;F: external fixation; G: hemiarthroplasty
Network meta‐analysis
The global test for incoherence was significant (P = 0.047); this appeared to be driven by comparisons of long versus short cephalomedullary nails, and dynamic fixed plates versus both long and short cephalomedullary nails. We could not determine any methodological or clinical reasons for this incoherence.
There was no evidence of any difference between any of the treatments in the network meta‐analysis (Table 6). We present the network estimates compared to dynamic fixed angle plates in Figure 7. There was no evidence of statistical inconsistency in any of the direct estimates, and no evidence of publication bias or small study effects. We noted variation between all treatments in the proportion of stable and unstable fractures, and other variables (age and gender) were reasonably balanced between treatment comparisons; we therefore did not consider intransitivity to be of concern in this network. However, we found evidence of incoherence in network estimates for long and short cephalomedullary nails, and all effect estimates included plausible benefits as well as harms. For this outcome, we could not rule out the possibility of selection bias because studies published only as abstracts did not adequately report data for this outcome.
7.
Network estimates of treatments compared to dynamic fixed angle plate for early mortality, as risk ratios with 95% confidence intervals (CI)
We found that external fixation and static fixed‐angle plate seemed to have the greatest likelihood of being ranked highly (mean ranks 2.4, 3.1; SUCRA values 0.8, 0.7 respectively) (Table 7; Figure 8). External fixation was ranked the highest, but we note that this was derived from two small studies (Kazemian 2014; Vossinakis 2002). Long and short cephalomedullary nails had the worst mean ranks (5 and 4.9) and the lowest SUCRA values (0.3 and 0.4) which would indicate that these treatments have the lowest probability of reducing early death. However, on inspection of the CIs in Table 6, we noted no evidence of a difference between the treatments in any of the network estimates for this outcome and we are cautious in drawing meaningful interpretations from the ranking of treatments in this network.
5. Early mortality: estimated probabilities of rankings.
| Rank | F | B | G | E | A | D | C |
| Best | 47.8 | 17.6 | 26.3 | 4 | 0.7 | 0.8 | 2.9 |
| 2nd | 21.6 | 29.9 | 15.7 | 14 | 6.3 | 4.3 | 8.3 |
| 3rd | 9.1 | 20.6 | 7.8 | 20.6 | 16.9 | 12.3 | 12.6 |
| 4th | 5.2 | 10.2 | 5.1 | 16.8 | 29.7 | 19.6 | 13.4 |
| 5th | 4.4 | 7.9 | 4.7 | 12.7 | 27.6 | 27.3 | 15.4 |
| 6th | 5.6 | 8.4 | 7.4 | 16.6 | 14.6 | 25 | 22.4 |
| Worst | 6.3 | 5.4 | 33 | 15.3 | 4.3 | 10.8 | 24.9 |
| MEAN RANK | 2.4 | 3.1 | 4 | 4.3 | 4.4 | 4.9 | 5 |
| SUCRA | 0.8 | 0.7 | 0.5 | 0.4 | 0.4 | 0.4 | 0.3 |
Estimated probabilities of rankings, mean rank and SUCRA (surface under the cumulative ranking curve) for each treatment in the early mortality network based on random‐effects consistency network meta‐analysis (sorted by mean rank from left to right). Treatment nodes ‐ A: dynamic fixed angle plate;B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail;F: external fixation; G: hemiarthroplasty
8.

Cumulative ranking probability curves for each treatment in the early mortality network. Each curve corresponds to the cumulative probability for all treatment ranks. From left to right along the x‐axis are the possible rankings for each treatment (12 in total; only best (1st) and worst (12th) shown in figure). Probabilities of each ranking are cumulatively summed from best to worst along the x‐axis for each treatment. Treatment nodes ‐ A: dynamic fixed angle plate;B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail;F: external fixation; G: hemiarthroplasty
The direct evidence from the dynamic fixed angle plate comparison with short cephalomedullary nails was the most informative for this network, contributing 22.7% to the network estimates. This was a notably higher contribution than the other direct comparisons, with the comparison between dynamic fixed angle plates and condylocephalic nails giving the next most informative contribution to the network estimates with 14.0%. This variation reflects the uneven nature of the available data when viewed as a network, with no direct evidence available for a number of the comparisons. Where direct evidence is present, the quantity of evidence varies substantially between comparisons. However, none of the direction comparisons appear to dominate the network estimates unduly.
2. Mortality at 12 months
We included 56 studies (8407 randomised participants; 8203 analysed participants) in the network for 12 months mortality (Adams 2001; Agrawal 2017; Ahrengart 1994; Akinci 2010; Aktselis 2014; Andalib 2020; Barton 2010; Baumgaertner 1998; Bridle 1991; Buciuto 1998; Butt 1995; Carulli 2017; Chapman 1981; Dalsgaard 1986; Dujardin 2001; Eceviz 2020; Ekstrom 2007; Esser 1986; Goldhagen 1994; Gou 2013; Guerra 2014; Hardy 1998; Harrington 2002; Haynes 1996; Hoffman 1996; Hogh 1981; Hornby 1989; Huang 2006; Jolly 2019; Kazemian 2016; Kim 2005; Kouvidis 2012; Kukla 1997; Leung 1992; Liem 1993; Little 2008; Miedel 2005; Nungu 1991; Okcu 2013; Ovesen 2006; Parker 2012; Parker 2017; Reindl 2015; Sadowski 2002; Saudan 2002; Sernbo 1988; Sharma 2018; Tao 2013; Trafton 1984; Utrilla 1998; Utrilla 2005; Varela‐Egocheaga 2009; Vossinakis 2002; Xu 2010b; Zehir 2015; Zhou 2012). The maximum number of randomised participants was 598 and the minimum number was 31.
Forty‐eight additional studies reported mortality at 12 months which we did not include in the network. We dropped thirteen studies from the analysis because at least one of the study treatments did not correspond with our node definitions (e.g. mixed group of cephalomedullary nail lengths, or we could not determine the length of nail) (Cai 2016; Chechik 2014; Davis 1988; Kammerlander 2018; Lopez 2002; Matre 2013; Pahlpatz 1993; Pelet 2001; Rahme 2007; Raimondo 2012; Sanders 2017; Singh 2019; Yang 2011a). We dropped the 34 other studies from the analysis because they compared treatments within a node (e.g. a short cephalomedullary nail with another short cephalomedullary nail) (Berger‐Groch 2016; Caiaffa 2016; Catania 2019; Ciaffa 2018; Dall'Oca 2010; De Grave 2012; Fritz 1999; Griffin 2016; Griffin 2021; Haddon 2019; Hardy 2003; Herrera 2002; Hopp 2016; Janzing 2002; Kosygan 2002; Kumar 2019; Li 2015b; Lopez‐Vega 2015; Lunsjo 2001; Makridis 2010; Marques 2005; Mattsson 2005; McCormack 2013; McLaren 1991; Parker 2020; Peyser 2007; Schipper 2004; Stern 2011; Su 2016; Vaquero 2012; Wu 2020; Yang 2011b; Yaozeng 2010; Zhang 2013).
Direct comparisons
In the direct comparisons, we found no evidence of a difference between any of the treatments in mortality at 12 months (Table 8; Figure 9). On inspection of Table 8, we noted that all CIs for each estimate overlapped and there was little evidence to suggest that any one of the treatments was either substantially better or worse than the other. However, the CIs were wide for most of these estimates, indicating substantial imprecision.
6. Mortality at 12 months: network estimates, direct estimates and indirect estimates.
| Dynamic fixed angle plate | D: 1.03 (0.74 to 1.42) I: 1.01 (0.57 to 1.79) | D: 1.28 (0.89 to 1.85) I: 1.05 (0.27 to 4.12) |
D: 0.97 (0.87 to 1.07) I: 0.98 (0.40 to 2.38) | D: 0.92 (0.73 to 1.16) I: 0.98 (0.49 to 1.97) | D: 0.88 (0.34 to 2.23) I: 0.57 (0.17 to 1.93) |
D: 0.91 (0.45 to 1.85) I: 1.40 (0.36 to 5.49) |
D: 0.69 (0.12 to 4.03) I: too imprecise | |
| 1.02 (0.77 to 1.36) | Static fixed angle plate | D: 0.86 (0.28 to 2.59) I: 0.95 (0.70 to 1.30) | D: 0.95 (0.51 to 1.78) I: 0.89 (0.60 to 1.32) |
|||||
| 1.27 (0.89 to 1.81) | 1.24 (0.79 to 1.95) | Long cephalomedullary nail | D: 0.92 (0.23 to 3.58) I: 0.75 (0.51 to 1.10) | |||||
| 0.97 (0.87 to 1.07) | 0.95 (0.70 to 1.28) | 0.76 (0.53 to 1.10) | Short cephalomedullary nail | D: 1.41 (0.77 to 2.61) I: too imprecise | ||||
| 0.93 (0.74 to 1.16) | 0.91 (0.65 to 1.26) | 0.73 (0.48 to 1.11) | 0.96 (0.75 to 1.23) | Condylocephalic nail | ||||
| 0.75 (0.36 to 1.57) | 0.73 (0.33 to 1.62) | 0.59 (0.26 to 1.34) | 0.77 (0.37 to 1.64) | 0.81 (0.37 to 1.75) | External fixation | 1.60 (0.59 to 4.33) I: 1.04 (0.32 to 3.37) | ||
| 1.36 (0.73 to 2.54) | 1.34 (0.68 to 2.64) | 1.08 (0.53 to 2.20) | 1.41 (0.77 to 2.61) | 1.47 (0.76 to 2.85) | 1.83 (0.69 to 4.81) | Hemiarthroplasty | ||
| 1.00 (0.53 to 1.87) | 0.98 (0.49 to 1.95) | 0.79 (0.38 to 1.62) | 1.03 (0.55 to 1.95) | 1.08 (0.55 to 2.10) | 1.34 (0.63 to 2.86) | 0.73 (0.30 to 1.77) | Non‐operative treatment | |
| 0.69 (0.12 to 4.03) | 0.68 (0.11 to 4.04) | 0.55 (0.09 to 3.30) | 0.72 (0.12 to 4.19) | 0.75 (0.13 to 4.41) | 0.93 (0.14 to 6.27) | 0.51 (0.08 to 3.29) | 0.69 (0.11 to 4.50) | Total hip arthroplasty |
Intervention effects, measured as risk ratios (with 95% confidence intervals), for unplanned return to theatre. In the lower triangle (network estimates), the column‐defining intervention is the reference group; a risk ratio lower than 1 favours the row‐defining intervention for the network meta‐analysis results (network estimates). In the upper triangle (direct estimates (D) and indirect estimates (I)), the row‐defining intervention is the reference group; a risk ratio lower than 1 favours the column‐defining intervention for the pairwise meta‐analysis. Blank spaces indicate that no direct estimates were available and the network estimate was derived from indirect evidence only.
9.

Network forest plot for mortality at 12 months. Treatment nodes ‐ A: dynamic fixed angle plate;B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail; F: external fixation; G: hemiarthroplasty; H: non‐operative treatment; I: total hip arthroplasty
Network meta‐analysis
The global test for incoherence was nonsignificant (P = 0.980). There was no evidence of any difference between any of the treatments in the network meta‐analysis (Table 8).
A summary of this outcome, compared to dynamic fixed angle plate, is in Table 1, and we present the network estimates against this reference comparator in Figure 10. The certainty of the evidence ranged from low to very low. We downgraded the evidence for all treatments by one level for risk of bias because all studies in the direct or indirect estimates (or both estimates) had unclear risks of bias in at least one domain. We also downgraded for a further level for risk of bias if estimates included studies at high risk of bias. We could not rule out the possibility of selection bias because studies published only as abstracts did not adequately report data for this outcome. There was no evidence of statistical inconsistency in any of the direct or indirect estimates and no evidence of publication bias or small study effects. We noted some variation in the proportion of stable and unstable fractures in some of the indirect estimates and we downgraded the evidence for treatments that included these indirect comparisons because of possible intransitivity. We also considered variation in age and gender, but judged these to be comparable between treatment comparisons. There was no evidence of incoherence in the network estimates. However, we downgraded all estimates for imprecision because network estimates included evidence of benefits as well as harms.
10.
Network estimates of treatments compared to fixed angle plate for mortality at 12 months, as risk ratios with 95% confidence intervals (CI).
We found that external fixation and total hip arthroplasty seemed to have the greatest likelihood of being ranked highly (mean ranks 2.9, 3.7; SUCRA values 0.8, 0.7 respectively) (Table 9; Figure 11). External fixation was ranked the highest, but we note that this was derived from two small studies (Kazemian 2014; Vossinakis 2002). Long cephalomedullary nails and hemiarthroplasty had the worst mean ranks (both 7.4) and the lowest SUCRA values (both 0.2) which would indicate that these treatments have the lowest probability of reducing late mortality. However, on inspection of the CIs in Figure 10, we noted no evidence of a difference between the treatments in any of the network estimates for this outcome and are cautious in drawing meaningful interpretations from the ranking of treatments in this network.
7. Mortality at 12 months: estimated probabilities of ranking.
| Rank | F | I | E | D | H | A | B | C | G |
| Best | 33.5 | 48.7 | 5.6 | 1.5 | 6.1 | 0.3 | 2.2 | 0.3 | 1.7 |
| 2nd | 31.4 | 9.6 | 18.1 | 9 | 16.8 | 1.9 | 8.1 | 1.3 | 3.7 |
| 3rd | 8.7 | 3.9 | 23.8 | 20 | 16.2 | 8.7 | 12.9 | 2.2 | 3.7 |
| 4th | 4 | 2 | 20.1 | 26.5 | 7.4 | 20.4 | 12.9 | 2.8 | 3.8 |
| 5th | 4.1 | 2.2 | 13.3 | 22.2 | 6.8 | 30.2 | 13.9 | 4.1 | 3.2 |
| 6th | 5.3 | 3.2 | 10.4 | 14.2 | 9.6 | 24.7 | 17.6 | 9.5 | 5.6 |
| 7th | 5.9 | 4.8 | 6.2 | 5.2 | 14 | 11.6 | 19 | 21.1 | 12.2 |
| 8th | 4.4 | 6.8 | 2.2 | 1.3 | 13.4 | 2 | 10.8 | 35.1 | 23.9 |
| Worst | 2.6 | 18.8 | 0.3 | 0.1 | 9.7 | 0.2 | 2.6 | 23.5 | 42.3 |
| MEAN RANK | 2.9 | 3.7 | 3.9 | 4.3 | 5 | 5.1 | 5.3 | 7.4 | 7.4 |
| SUCRA | 0.8 | 0.7 | 0.6 | 0.6 | 0.5 | 0.5 | 0.5 | 0.2 | 0.2 |
Estimated probabilities of rankings, mean rank and SUCRA for each treatment in the 12‐month mortality network based on random‐effects consistency network meta‐analysis (sorted by mean rank from left to right). Treatment nodes ‐ A: dynamic fixed angle plate;B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail; F: external fixation; G: hemiarthroplasty; H: non‐operative treatment; I: total hip arthroplasty
11.

Cumulative ranking probability curves for each treatment in the network for mortality at 12 months. Each curve corresponds to the cumulative probability for all treatment ranks. From left to right along the x‐axis are the possible rankings for each treatment (12 in total; only best (1st) and worst (12th) shown in figure). Probabilities of each ranking are cumulatively summed from best to worst along the x‐axis for each treatment. Treatment nodes ‐ A: dynamic fixed angle plate;B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail; F: external fixation; G: hemiarthroplasty; H: non‐operative treatment; I: total hip arthroplasty
The direct evidence from the dynamic fixed angle plate comparison with short cephalomedullary nails was the most informative for this network, contributing 18.8% to the network estimates. This was a notably higher contribution than the other direct comparisons, with the comparison between dynamic fixed angle plates and non‐operative treatment giving the next most informative contribution to the network estimates with 11.3%. This variation reflects the uneven nature of the available data when viewed as a network, with no direct evidence available for a number of the comparisons. Where direct evidence is present, the quantity of evidence varies substantially between comparisons. However, none of the direction comparisons appear to dominate the network estimates unduly.
3. Late mortality
Only two studies reported data for mortality later than 24 months after surgery (Akinci 2010; Berger‐Groch 2016). Akinci 2010 compared a dynamic fixed angle plate with a static plate, and we found no evidence of any differences in mortality between these treatments at five years (RR 0.83, 95% CI 0.57 to 1.22, favours dynamic plate; 157 participants).
Berger‐Groch 2016 compared treatments within the node for short cephalomedullary nails.
4. Early health‐related quality of life (HRQoL)
Only one study reported data for this outcome within four months of surgery (Griffin 2021). This study compared treatments within the dynamic fixed angle plate node.
5. HRQoL at 12 months
Three studies (260 randomised participants, 230 analysed participants) compared treatments for short cephalomedullary nails and dynamic fixed angle plates and reported HRQoL at 12 months (Aktselis 2014; Carulli 2017; Haq 2014). An additional four studies reported this outcome but compared treatments within a node (Andreani 2015; Griffin 2021; Vaquero 2012), or did not correspond to our node definitions because the study included cephalomedullary nails of mixed lengths (Singh 2019). We therefore could not conduct a network meta‐analysis.
In the direct comparison of these three studies, we found that HRQoL was improved when a dynamic fixed angle plate was used (SMD 0.35, 95% CI 0.09 to 0.61). After converting this estimate onto the Short Form 12‐item questionnaire (SF‐12) physical component summary score (PCS) scale (with which two studies measured this outcome), the difference between treatments was compatible with both no clinically important difference and plausible benefits (MD 3.68, 95% CI 0.94 to 6.42); this was based on a MCID for SF‐12 (PCS) of 4.6 (Berliner 2016).
6. Late HRQoL
No studies reported HRQoL later than 24 months after surgery.
5. Unplanned return to theatre
We included 55 studies (9296 randomised participants; 8739 analysed participants) in the network for unplanned return to theatre (Adams 2001; Ahrengart 1994; Akinci 2010; Aktselis 2014; Andalib 2020; Barton 2010; Benum 1994; Butt 1995; Carulli 2017; Chapman 1981; Dalen 1988; Dalsgaard 1986; Eceviz 2020; Ekstrom 2007; Giraud 2005; Goldhagen 1994; Guyer 1991; Haq 2014; Hardy 1998; Haynes 1996; Hoffman 1996; Hoffmann 1999; Hogh 1981; Kim 2005; Kouvidis 2012; Kukla 1997; Leung 1992; Liem 1993; Little 2008; Merenyi 1995; Miedel 2005; Nungu 1991; Okcu 2013; Ovesen 2006; Pajarinen 2005; Parker 2012; Parker 2017; Pitsaer 1993; Radford 1993; Reindl 2015; Sadowski 2002; Saudan 2002; Sernbo 1988; Shannon 2019; Sharma 2018; Stark 1992; Teerenhovi 1984; Trafton 1984; Utrilla 1998; Utrilla 2005; Vossinakis 2002; Xu 2010b; Zehir 2015; Zhou 2012; Zou 2009).
Forty‐one additional studies reported unplanned return to theatre which we did not include in the network. We dropped 15 of these studies from the analysis because at least one of the treatments in the study did not correspond with our node definitions (e.g. mixed group of cephalomedullary nail lengths, or we could not determine the length of nail) (Chechik 2014; Davis 1988; Irvine 2014; Kammerlander 2018; Lopez 2002; Matre 2013; Michos 2001; Mott 1993; O'Brien 1995; Ozkayin 2015; Pelet 2001; Rahme 2007; Sanders 2017; Singh 2017; Singh 2019). We dropped the remaining 26 studies from the analysis because they compared treatments within a node (e.g. a short cephalomedullary nail with another short cephalomedullary nail) (Catania 2019; De Grave 2012; Fritz 1999; Griffin 2021; Haddon 2019; Herrera 2002; Hopp 2016; Janzing 2002; Kumar 2019; Lopez‐Vega 2015; Lunsjo 2001; Marques 2005; Mattsson 2004; Mattsson 2005; McCormack 2013; Olsson 2001; Papasimos 2005; Parker 2020; Schipper 2004; Seyhan 2015; Shin 2017; Stern 2011; Su 2016; Wu 2020; Yaozeng 2010; Zhang 2013). The network included no data for non‐operative treatment or total hip arthroplasty.
Direct comparisons
In the direct comparisons, we found evidence of a difference in unplanned return to theatre between dynamic fixed angle plate versus condylocephalic nail (RR 3.57, 95% CI 1.91 to 6.66, favours fixed angle plate; 7 studies, 996 participants).
However, we found no evidence of a difference between any of the other treatments for this outcome (Table 10; Figure 12). On inspection of Table 9, we noted that all CIs for each estimate overlapped and there was little evidence to suggest that any one of the treatments was either substantially better or worse than the other. However, the CIs were wide for most of these estimates, indicating substantial imprecision.
8. Unplanned return to theatre: network estimates, direct estimates and indirect estimates.
| Dynamic fixed angle plate | D: 2.19 (0.97 to 4.92) I: 2.91 (1.17 to 7.22) | D: 1.09 (0.19 to 6.15) I: 1.98 (0.51 to 7.70) | D: 1.13 (0.81 to 1.59) I: 0.74 (0.14 to 3.95) | D: 3.57 (1.91 to 6.66) I: 2.74 (0.93 to 8.05) | D: 0.09 (0.00 to 1.87) I: too imprecise |
|
| 2.48 (1.36 to 4.50) | Static fixed angle plate | D: 0.39 (0.03 to 4.93) I: 0.45 (0.22 to 0.92) | D: 1.22 (0.58 to 2.59) I: 1.59 (0.59 to 4.30) | |||
| 1.58 (0.55 to 4.56) | 0.64 (0.19 to 2.15) | Long cephalomedullary nail | D: 0.57 (0.15 to 2.14) I: 1.03 (0.18 to 6.01) | |||
| 1.12 (0.80 to 1.56) | 0.45 (0.23 to 0.88) | 0.71 (0.25 to 2.01) | Short cephalomedullary nail | D: 0.33 (0.01 to 9.05) I: too imprecise |
||
| 3.33 (1.95 to 5.68) | 1.34 (0.75 to 2.42) | 2.11 (0.64 to 6.93) | 2.98 (1.59 to 5.60) | Condylocephalic nail | ||
| 0.09 (0.00 to 1.87) | 0.04 (0.00 to 0.80) | 0.06 (0.00 to 1.42) | 0.08 (0.00 to 1.71) | 0.03 (0.00 to 0.59) | External fixation | |
| 0.37 (0.01 to 10.26) | 0.15 (0.01 to 4.36) | 0.24 (0.01 to 7.53) | 0.33 (0.01 to 9.05) | 0.11 (0.00 to 3.22) | 3.98 (0.05 to 350.47) | Hemiarthroplasty |
Intervention effects, measured as risk ratios (with 95% confidence intervals), for unplanned return to theatre. In the lower triangle (network estimates), the column‐defining intervention is the reference group; a risk ratio lower than 1 favours the row‐defining intervention for the network meta‐analysis results (network estimates). In the upper triangle (direct estimates (D) and indirect estimates (I)), the row‐defining intervention is the reference group; a risk ratio lower than 1 favours the column‐defining intervention for the pairwise meta‐analysis. Blank spaces indicate that no direct estimates were available and the network estimate was derived from indirect evidence only.
12.

Network forest plot for unplanned return to theatre. Treatment nodes ‐ A: dynamic fixed angle plate; B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail; F: external fixation; G: hemiarthroplasty
Network meta‐analysis
The global test for incoherence was nonsignificant (P = 0.922). Based upon the network estimates, we noted a difference in unplanned return to theatre for the following comparisons.
Dynamic versus static fixed angle plate (RR 2.48, 95% CI 1.36 to 4.50, favours dynamic fixed angle plate); this effect was derived from direct evidence (RR 2.19, 95% CI 0.97 to 4.92; 4 studies, 622 participants), and indirect evidence (RR 2.91, 95% CI 1.17 to 7.22).
Dynamic fixed angle plate versus condylocephalic nail (RR 3.33, 95% CI 1.95 to 5.68, favours fixed angle plate); this effect was derived from direct evidence (RR 3.57, 95% CI 1.91 to 6.66; 7 studies, 996 participants), and indirect evidence (RR 2.74, 95% CI 0.93 to 8.05).
Static fixed angle plate versus short cephalomedullary nail (RR 0.45, 95% CI 0.23 to 0.88, favours cephalomedullary nail); this effect was derived from direct evidence (RR 0.39, 95% CI 0.03 to 4.93; 1 study, 68 participants), and indirect evidence (RR 0.45, 95% CI 0.22 to 0.92).
Static fixed angle plate versus external fixation (RR 0.04, 95% CI 0.00 to 0.80, favours external fixation); this effect was derived only from indirect evidence.
Short cephalomedullary nail versus condylocephalic nail (RR 2.98, 95% CI 1.59 to 5.60, favours cephalomedullary nail); this effect was derived only from indirect evidence.
Condylocephalic nail versus external fixation (RR 0.03, 95% CI 0.00 to 0.59, favours external fixation); this effect was derived only from indirect evidence).
A summary of this outcome, compared to dynamic fixed angle plate, is in Table 2, and we present the network estimates against this reference comparator in Figure 13. The certainty of the evidence ranged from low to very low. We downgraded the evidence for all treatments by one level for risk of bias because all studies in the direct or indirect estimates (or both estimates) had high risk of detection bias and unclear risks of bias in at least one domain. We also downgraded for a further level for risk of bias if estimates included studies at high risk of selection bias or 'other bias'. We noted statistical heterogeneity in some direct or indirect estimates and downgraded for inconsistency for treatments that may be affected by this, but found no evidence of publication bias or small study effects. We downgraded for intransitivity when we noted that indirect estimates included variation in the proportion of stable and unstable fractures; however, we judged variation in age and gender to be comparable between treatment comparisons. There was no evidence of incoherence in the network estimates. However, we downgraded estimates that included evidence of benefits as wells as harms for imprecision.
13.
Network estimates of treatments compared to fixed angle plate for unplanned return to theatre, as risk ratios with 95% confidence intervals (CI).
We found that external fixation and hemiarthroplasty seemed to have the greatest likelihood of being ranked highly (mean ranks 1.5, 2.7; SUCRA values 0.9, 0.7 respectively) (Table 11, Figure 14). External fixation was ranked the highest, but we note that this was derived from two small studies (Kazemian 2014; Vossinakis 2002). Condylocephalic nail and static fixed angle plate had the worst mean ranks (6.6 and 5.8) and the lowest SUCRA values (0.1 and 0.2) which would indicate that these treatments have the lowest probability of reducing unplanned return to theatre. Consistent with these low rankings, effect estimates in Figure 13 showed clinically important and statistically significant harms of both these treatments compared with the comparator intervention. The possibility of very substantial harms but also clinically important benefits was observed with long cephalomedullary nails, which ranked poorly too. It is likely that condylocephalic nails and static fixed angle plates yield worse unplanned returns to theatre and some evidence that long cephalomedullary nails may also be associated with very substantial harms.
9. Unplanned return to theatre: estimated probabilities of ranking .
| Rank | F | G | A | D | C | B | E |
| Best | 71.1 | 26.4 | 1.5 | 0.4 | 0.7 | 0 | 0 |
| 2nd | 22.1 | 43.9 | 20.3 | 6.8 | 6.8 | 0.1 | 0 |
| 3rd | 1.7 | 3.7 | 49.3 | 30.8 | 13.9 | 0.6 | 0 |
| 4th | 2 | 5.9 | 23.7 | 47.2 | 16 | 4.6 | 0.6 |
| 5th | 1.5 | 6.5 | 5.3 | 14.6 | 41 | 26 | 5.2 |
| 6th | 0.7 | 3.6 | 0 | 0.2 | 13 | 56.1 | 26.5 |
| Worst | 0.9 | 9.9 | 0 | 0 | 8.6 | 12.7 | 67.8 |
| MEAN RANK | 1.5 | 2.7 | 3.1 | 3.7 | 4.6 | 5.8 | 6.6 |
| SUCRA | 0.9 | 0.7 | 0.6 | 0.6 | 0.4 | 0.2 | 0.1 |
Estimated probabilities of rankings, mean rank and SUCRA for each treatment in the unplanned return to theatre network based on random‐effects consistency network meta‐analysis (sorted by mean rank from left to right). Treatment nodes ‐ A: dynamic fixed angle plate; B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail; F: external fixation; G: hemiarthroplasty
14.

Cumulative ranking probability curves for each treatment in the network for unplanned return to theatre. Each curve corresponds to the cumulative probability for all treatment ranks. From left to right along the x‐axis are the possible rankings for each treatment (12 in total; only best (1st) and worst (12th) shown in figure). Probabilities of each ranking are cumulatively summed from best to worst along the x‐axis for each treatment. Treatment nodes ‐ A: dynamic fixed angle plate; B: static fixed angle plate; C: long cephalomedullary nail; D: short cephalomedullary nail; E: condylocephalic nail; F: external fixation; G: hemiarthroplasty
The direct evidence from the dynamic fixed angle plate comparison with short cephalomedullary nails was the most informative for this network, contributing 24.9% to the network estimates. This was a notably higher contribution than the other direct comparisons, with the comparisons between dynamic fixed angle plates and external fixation and short cephalomedullary nails and hemiarthroplasty giving the next most informative contributions to the network estimates, each with 13.9%. This variation reflects the uneven nature of the available data when viewed as a network, with no direct evidence available for a number of the comparisons. Where direct evidence is present, the quantify of evidence varies substantially between comparisons. However, none of the direction comparisons appear to dominate the network estimates unduly.
Discussion
Summary of main results
In the review, we included 184 studies (160 RCTs, 24 quasi‐RCTs) with 26,073 participants with 26,086 fractures. Most of the studies included a mixed sample of participants with stable or unstable pertrochanteric fracture patterns. We selected nine interventions as network nodes that represented the most clinically relevant distinctions between treatments and which still yielded sufficient data with which to conduct network meta‐analysis. Overall, we included 73 studies with 11,126 participants in our network meta‐analyses. We selected mortality at 12 months and unplanned return to theatre as the primary analyses, balancing our prespecified critical outcomes with the availability of studies and data at each time point.
Mortality within 12 months of surgery was estimated, from the included studies, to be 20.2% for treatment with dynamic fixed angle plates. Comparing all other treatments to fixed angle plates, we found no evidence of any differences in mortality at this time point; the certainty of this evidence ranged from low to very low. Total hip arthroplasty and external fixation were the two treatments ranked highest in the network, with the greatest likelihood of reducing mortality. However, because of the imprecision in the network estimates and the lack of evidence of any between‐treatment differences in risk, we are cautious in drawing meaningful conclusions from this ranking.
We found very low‐certainty evidence that more participants had unplanned return to theatre when treated with a static fixed angle plate or with a condylocephalic nail compared with fixed angle plates. This finding was consistent with the treatment rankings from the network; both of these treatments had the lowest probability of reducing unplanned return to theatre. The network estimates for the other treatments provided low‐ and very low‐certainty evidence of no differences when compared with fixed angle plates.
We found insufficient data to allow us to construct a network for the health‐related quality of life (HRQoL) outcome at any time point.
We did not GRADE the certainty of the evidence for early mortality (within four months of surgery); this network was constructed from fewer studies than the later time point for mortality. However, we similarly found no evidence of any differences in early mortality in the network estimates for any of the treatments.
Overall completeness and applicability of evidence
Most of the studies reported a mixed sample of participants with stable or unstable pertrochanteric fracture patterns, one third reported an exclusively unstable group, two studies only stable patterns and only one exclusively subtrochanteric fractures. However, there were insufficient studies reporting this variation fully to be able to explore it effectively through subgroup analysis. We did not include the single study of subtrochanteric fractures in the NMAs because this study compared treatments that did not correspond with our node definitions. Where relevant baseline characteristics were reported, we noted that the majority of the studies included participants aged between 60 and 93 years and that most participants were female. Therefore, we consider that the included studies are largely representative of the general hip fracture population. However, we found that few studies reported American Society of Anesthesiologists (ASA) status or presence of cognitive impairment at baseline, such that we could not confidently state that the included studies were similarly representative for these characteristics.
We noted that studies included in the network meta‐analysis were published between 1981 and 2020, and 43% of these were published before 2000. Due to the limitations in the quality of the reporting in these older studies, we could not easily judge whether patient care protocols were equivalent to current standards of care. It is certainly possible that important developments have been made in co‐interventions ‐ such as the introduction of orthogeriatric care in some parts of the world ‐ that have yielded improved outcomes for patients. We are unable to comment on whether such co‐interventions may have changed the estimates of the relative benefits and harms between treatments reported here or rather changed the absolute risks following treatment for this injury.
Although we found that many of the included studies reported mortality and unplanned return to theatre, very few reported HRQoL. We were therefore unable to evaluate whether the benefits and harms of each treatment differed in terms of this crucial outcome for individuals. Few studies reported later outcomes, extending beyond 12 months, and so the late recovery profile for participants is therefore incomplete in this review.
Quality of the evidence
The overall certainty of the evidence for the outcomes in this review was low to very low. This was largely owing to risks of bias in the included studies. Many studies included in this review predate widespread uptake of current standards of reporting, such as preregistration of trial protocols and adherence to the CONSORT statement. It is therefore perhaps not surprising that this is reflected in the grade of the evidence. We judged that many studies were at unclear risk of selection bias because they did not provide information about the allocation methods. Some were at high risk of bias because they used quasi‐randomised methods to allocate participants to groups or were reported only in abstracts which we expected were not peer‐reviewed. We could not rule out the possibility of selection bias because outcomes were not always reported in abstract publications. We also assessed all studies to be at a high risk of detection bias for the outcome of unplanned return to theatre.
A small number of direct and indirect estimates included inconsistency and we downgraded the certainty of the evidence for this, where appropriate. We could not rule out that variation in fracture stability may have affected the transitivity assumption in the networks, and for some treatments, we downgraded the certainty of the evidence for the network estimate for intransitivity. We did not downgrade the evidence for indirectness (the studies included the relevant population, treatments and outcome measures) and we detected no evidence of publication bias from visual inspection of the comparison‐adjusted funnel plots.
Overall, our network for early mortality showed evidence for global incoherence. Through the side‐splitting approach, we identified that this was likely driven by studies including long cephalomedullary nails, short cephalomedullary nails and also dynamic fixed plates. However, we were unable to identify any apparent causes for the incoherences. There was no simplification of the network that would address these inconsistencies without removing most of the studies and treatments from the network. Accordingly, we reported the network estimates and note that many were imprecise, with confidence intervals that included clinical benefits as well as possible harms. This reduced our certainty in the estimates for most treatments. Findings from this network should be interpreted cautiously.
Potential biases in the review process
The review authors conducted a thorough search and independently assessed study eligibility, extracted data, and assessed risk of bias in the included studies before reaching consensus together or with one other review author.
Our decisions on lumping/splitting of nodes were necessarily subjective and meant that some studies were inevitably excluded from the networks. This often occurred either because interventions were insufficiently described in study reports, or because studies compared two treatments within a node (such as two different types of dynamic fixed angle plates). However, sometimes this was because investigators had used a pragmatic study design, which included interventions that belonged to more than one of our specified nodes.
We made a decision to include populations with mixed fracture types ‐ for example, stable and unstable trochanteric fractures and subtrochanteric fractures. Fracture type may be an important effect modifier and it may have been distributed unevenly across the comparisons within the networks. We were able to review the distribution of the potential effect modifiers of age, sex and fracture stability as these were sufficiently well reported. Fracture stability did seem to vary across comparisons within networks, but across the entire review there was limited evidence of inconsistency within the networks, excepting that for early mortality.
We recognise that important co‐interventions are likely to have been introduced into clinical practice which are not represented in the network. We did note some evidence of global inconsistency, but we did not undertake any exploratory analyses to explore how limiting the included studies may have impacted on our findings, since there seemed little clinical justification for removing specific studies.
In the conduct of this large review, we have chosen to alter or have been unable to deliver all of our planned methods. Given the complexity of the review, the sparsity of many of the networks and the often unclear and high risk of bias of included studies, we chose not to perform any sensitivity analyses. We chose to include only older participants, such that some potentially eligible studies were excluded, but this ensured less variation in age and that the evidence was representative of the target fragility fracture population. During data extraction, it became apparent that multiple time points were reported inconsistently between studies. We chose to create additional networks rather than group widely across time windows. Balancing this against the availability of data, we elected to move away from our prespecified preference for early time points to prioritise the more often reported 12 months' time point.
Agreements and disagreements with other studies or reviews
Few network meta‐analyses of treatments for fragility extracapsular hip fracture have been reported. Cheng 2020 is the most comprehensive, including 36 studies and reporting blood loss, operative time and Harris Hip Score. These outcomes did not closely reflect the priorities of patients expressed in the core outcome set development (Haywood 2014). However, the data suggest that any difference in bleeding or operative time between treatments is very small, even if statistically significant, and unlikely to drive clinical decision‐making. Similarly, the between‐treatment differences in hip function measured using the Harris Hip Score, were very small. The Cheng 2020 review did not report mortality or HRQoL outcomes.
In the preparation of this network meta‐analysis, the author team has been involved in the production and updating of a suite of reviews that are relevant to the interpretation of the findings reported here (Lewis 2022a; Lewis 2022c). These reviews also conclude that there is no evidence of any important differences in mortality and unplanned return to theatre from pairwise comparisons of cephalomedullary implants with fixed angle plates or cemented versus uncemented hemiarthroplasty for extracapsular fractures.
An older review from 2006, comparing internal fixation with arthroplasty for extracapsular fractures, was unable to reach clear conclusions regarding the majority of outcomes (Parker 2006). This finding mirrors our network meta‐analysis but, importantly, we have been able to provide more precise estimates.
Authors' conclusions
Implications for practice.
The review includes older adults with both stable and unstable extracapsular fractures. Across the networks, we found that there was considerable variability in the ranking of each treatment such that there was no one outstanding, or subset of outstanding, superior treatments. However, static implants, such as condylocephalic and static fixed angle plates, did yield a higher risk of unplanned return to theatre. We had insufficient evidence to determine the effects of any treatments on health‐related quality of life (HRQoL), and this review only includes two outcomes. More detailed pairwise comparisons of some of the included treatments are reported in other Cochrane Reviews in this series. Cephalomedullary nails versus dynamic fixed angle plates contributed the most evidence to each network, and our findings indicate that there may be no difference between these treatments. We did not formally review cost effectiveness studies, but there was no evidence in this review to support the rising worldwide usage of the more expensive cephalomedullary nail over the dynamic fixed angle plate. At this time, there are too few studies to draw any conclusions regarding the benefits or harms of arthroplasty or external fixation for extracapsular fracture.
Implications for research.
Hip fracture continues to be a dynamic area within clinical research in trauma and orthopaedic surgery. We have identified 20 ongoing studies with an estimated enrolment of more than 2400 participants. Many of these studies are testing two interventions that we have lumped together within a single node; for example, two cephalomedullary nails from different manufacturers. We consider that such studies are unlikely to generate evidence that advances care for individuals. Three studies are comparing arthroplasty with internal fixation, however the likely impact of this additional evidence may be limited by the small sample size (approximately 140 participants) in these studies (ChiCTR‐INR‐17011841; JPRN‐UMIN000019523; NCT03407131).
However, despite this ongoing research interest, we note that many studies in this review ‐ even those reported more recently ‐ are at unclear or high risk of bias, and we urge authors to report studies in accordance with the CONSORT statement. We also encourage authors to review the core outcome set for hip fracture (Haywood 2014), and to ensure that, at a minimum, data are reported at four months ‐ the time point prioritised by people with hip fracture.
We have shown that static fixed angle implants cause harm compared with dynamic implants, which reflects worldwide practice of a fall in the use of such implants. Thirty‐four studies comparing dynamic fixed angle plates with short cephalomedullary nails were included in this review. It is unlikely that additional studies will yield fundamental changes in our knowledge of the outcomes from these major classes of treatments since the precision of the effect estimates in this review are approaching clinically not relevant differences. Therefore, we consider that additional studies of such comparisons are not the best use of scarce research resources.
However, future research should focus on the use of established technologies in this fracture group, particularly focusing on the benefits and harms of arthroplasty interventions compared with internal fixation with a dynamic implant, to properly assess their efficacy prior to any widespread adoption. Based upon our findings, we propose that such a study should be highly pragmatic, allowing surgeons to choose the type of dynamic implant. The evidence from this review suggests that any true difference between fixation implants is very likely to be small. We await the findings of the three such ongoing studies, which together will likely provide sufficient information to plan a definitive, multicentre randomised trial.
History
Protocol first published: Issue 8, 2019
Notes
Additional figures and data are available on request from the review authors of the Cochrane Bone, Joint and Muscle Trauma Group. These include the following.
Forest plots of direct comparisons of treatments (only studies in the networks).
Netfunnel plots.
Contribution matrix figures.
Bar charts showing distribution of key baseline characteristics (gender, age, fracture displacement).
Outcome data for all studies (included and not included in the networks).
Acknowledgements
We thank Joanne Elliott and Maria Clarke for editorial base support. We thank Liz Bickerdike and Kerry Dwan for their comments and help at editorial review. We thank Paul Baker, Harman Chaudhry and Ian Harris (external referees) for their helpful comments on the review. We thank Helen Handoll, Ashwini Sreekanta, Julie Glanville and Hannah Wood for their contributions to the development and publication of the protocol and for developing and running the searches in December 2018.
This project was funded and supported by the National Institute for Health Research (NIHR). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (CRS‐Web)
#1 MESH DESCRIPTOR Femoral Fractures EXPLODE ALL AND CENTRAL:TARGET #2 ((hip or hips or cervical) NEAR5 (fracture* or break* or broke*)) AND CENTRAL:TARGET #3 ((femoral* or femur* or acetabul*) NEAR5 (fracture* or break* or broke*)) AND CENTRAL:TARGET #4 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) NEAR5 (fracture* or break* or broke*)) AND CENTRAL:TARGET #5 ((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) NEAR5 (fracture* or break* or broke*)) AND CENTRAL:TARGET #6 ((head or neck or proximal) NEAR5 (fracture* or break* or broke*)) and (femoral* or femur*) AND CENTRAL:TARGET #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 AND CENTRAL:TARGET #8 MESH DESCRIPTOR Arthroplasty, Replacement, Hip AND CENTRAL:TARGET #9 MESH DESCRIPTOR Hip Prosthesis AND CENTRAL:TARGET #10 MESH DESCRIPTOR Arthroplasty, Replacement AND CENTRAL:TARGET #11 MESH DESCRIPTOR Hemiarthroplasty AND CENTRAL:TARGET #12 MESH DESCRIPTOR Joint Prosthesis AND CENTRAL:TARGET #13 ((arthroplast* or hemiarthroplast*) NEAR5 (hip or hips or femur* or femoral* or acetabul*)) AND CENTRAL:TARGET #14 ((hip or hips) NEAR5 (replac* or prosthes* or implant*)) AND CENTRAL:TARGET #15 ((joint* NEAR5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*)) AND CENTRAL:TARGET #16 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 AND CENTRAL:TARGET #17 MESH DESCRIPTOR Fractures, Bone AND CENTRAL:TARGET #18 MESH DESCRIPTOR Fracture Dislocation EXPLODE ALL AND CENTRAL:TARGET #19 MESH DESCRIPTOR Fractures, Closed AND CENTRAL:TARGET #20 MESH DESCRIPTOR Fractures, Comminuted AND CENTRAL:TARGET #21 MESH DESCRIPTOR Fractures, Compression AND CENTRAL:TARGET #22 MESH DESCRIPTOR Fractures, Malunited AND CENTRAL:TARGET #23 MESH DESCRIPTOR Fractures, Multiple AND CENTRAL:TARGET #24 MESH DESCRIPTOR Fractures, Open AND CENTRAL:TARGET #25 MESH DESCRIPTOR Fractures, Spontaneous AND CENTRAL:TARGET #26 MESH DESCRIPTOR Fractures, Stress AND CENTRAL:TARGET #27 MESH DESCRIPTOR Fractures, Ununited AND CENTRAL:TARGET #28 MESH DESCRIPTOR Intra‐Articular Fractures AND CENTRAL:TARGET #29 MESH DESCRIPTOR Osteoporotic Fractures AND CENTRAL:TARGET #30 MESH DESCRIPTOR Periprosthetic Fractures AND CENTRAL:TARGET #31 fracture* AND CENTRAL:TARGET #32 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 AND CENTRAL:TARGET #33 #32 AND #16 AND CENTRAL:TARGET #34 (pin or pins or nail or nails or screw or screws or plate or plates) AND CENTRAL:TARGET #35 MESH DESCRIPTOR Internal Fixators AND CENTRAL:TARGET #36 MESH DESCRIPTOR Bone Nails AND CENTRAL:TARGET #37 MESH DESCRIPTOR Bone Plates AND CENTRAL:TARGET #38 MESH DESCRIPTOR Bone Screws EXPLODE ALL AND CENTRAL:TARGET #39 (static NEXT (device* or implant*)) AND CENTRAL:TARGET #40 (dynamic NEXT (device* or implant*)) AND CENTRAL:TARGET #41 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 AND CENTRAL:TARGET #42 ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) AND CENTRAL:TARGET #43 (hip or hips or femur* or femoral* or acetabul*) AND CENTRAL:TARGET #44 #43 AND (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) AND CENTRAL:TARGET #45 #42 OR #44 AND CENTRAL:TARGET #46 #41 AND #45 AND CENTRAL:TARGET #47 #7 OR #33 OR #46 AND CENTRAL:TARGET #48 14/11/2018_TO_08/07/2020:CRSCREATED AND CENTRAL:TARGET #49 #47 AND #48
MEDLINE (Ovid)
1 exp Femoral Fractures/ 2 ((hip or hips or cervical) adj5 (fracture$ or break$ or broke$)).ti,ab,kf. 3 ((femoral$ or femur$ or acetabul$) adj5 (fracture$ or break$ or broke$)).ti,ab,kf. 4 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) adj5 (fracture$ or break$ or broke$)).ti,ab,kf. 5 ((extracapsular or extra‐capsular or trochant$ or subtrochant$ or pertrochant$ or intertrochant$) adj5 (fracture$ or break$ or broke$)).ti,ab,kf. 6 (((head or neck or proximal) adj5 (fracture$ or break$ or broke$)) and (femoral$ or femur$)).ti,ab,kf. 7 or/1‐6 8 randomized controlled trial.pt. 9 controlled clinical trial.pt. 10 randomized.ab. 11 placebo.ab. 12 clinical trials as topic.sh. 13 randomly.ab. 14 trial.ti. 15 or/8‐14 16 7 and 15 17 Arthroplasty, Replacement, Hip/ or Hip Prosthesis/ 18 Arthroplasty, Replacement/ or Hemiarthroplasty/ or Joint Prosthesis/ 19 ((arthroplast$ or hemiarthroplast$) adj5 (hip or hips or femur$ or femoral$ or acetabul$)).ti,ab,kf. 20 ((hip or hips) adj5 (replac$ or prosthes$ or implant$)).ti,ab,kf. 21 ((joint$1 adj5 (replac$ or prosthes$ or implant$)) and (hip or hips or femur$ or femoral$ or acetabul$)).ti,ab,kf. 22 or/17‐21 23 fractures, bone/ or exp fracture dislocation/ or fractures, closed/ or fractures, comminuted/ or fractures, compression/ or fractures, malunited/ or fractures, multiple/ or fractures, open/ or fractures, spontaneous/ or exp fractures, stress/ or fractures, ununited/ or intra‐articular fractures/ or osteoporotic fractures/ or periprosthetic fractures/ 24 fracture$.ti,ab,kf. 25 23 or 24 26 22 and 25 and 15 27 (pin or pins or nail or nails or screw or screws or plate or plates).ti,ab,kf. 28 internal fixators/ or bone nails/ or bone plates/ or exp bone screws/ 29 (static adj (device$1 or implant$1)).ti,ab,kf. 30 (dynamic adj (device$1 or implant$1)).ti,ab,kf. 31 or/27‐30 32 ((hip or hips or femur$ or femoral$ or acetabul$) and (fracture$ or break$ or broke$)).ti,ab,kf. 33 (hip or hips or femur$ or femoral$ or acetabul$).ti,ab,kf. and (fractures, bone/ or exp fracture dislocation/ or fractures, closed/ or fractures, comminuted/ or fractures, compression/ or fractures, malunited/ or fractures, multiple/ or fractures, open/ or fractures, spontaneous/ or exp fractures, stress/ or fractures, ununited/ or intra‐articular fractures/ or osteoporotic fractures/ or periprosthetic fractures/) 34 or/32‐33 35 31 and 34 and 15 36 16 or 26 or 35 37 exp animals/ not humans/ 38 36 not 37
Embase (Ovid)
1 exp Femur Fractures/ or exp hip fracture/ 2 ((hip or hips or cervical) adj5 (fracture$ or break$ or broke$)).ti,ab,kw. 3 ((femoral$ or femur$ or acetabul$) adj5 (fracture$ or break$ or broke$)).ti,ab,kw. 4 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) adj5 (fracture$ or break$ or broke$)).ti,ab,kw. 5 ((extracapsular or extra‐capsular or trochant$ or subtrochant$ or pertrochant$ or intertrochant$) adj5 (fracture$ or break$ or broke$)).ti,ab,kw. 6 (((head or neck or proximal) adj5 (fracture$ or break$ or broke$)) and (femoral$ or femur$)).ti,ab,kw. 7 or/1‐6 8 exp hip surgery/ or (joint surgery/ and exp hip/) 9 exp Hip Prosthesis/ 10 joint prosthesis/ and exp hip/ 11 Replacement Arthroplasty/ and exp hip/ 12 exp Hip arthroplasty/ 13 Arthroplasty/ and exp hip/ 14 Hemiarthroplasty/ and exp hip/ 15 Hip hemiarthroplasty/ 16 ((arthroplast$ or hemiarthroplast$) adj5 (hip or hips or femur$ or femoral$ or acetabul$)).ti,ab,kw. 17 ((hip or hips) adj5 (replac$ or prosthes$ or implant$)).ti,ab,kw. 18 ((joint$1 adj5 (replac$ or prosthes$ or implant$)) and (hip or hips or femur$ or femoral$ or acetabul$)).ti,ab,kw. 19 or/8‐18 20 fracture/ 21 Fracture dislocation/ 22 Comminuted fracture/ 23 Multiple fracture/ 24 Open fracture/ 25 Fragility fracture/ 26 exp Fracture healing/ 27 Stress fracture/ 28 intraarticular fracture/ 29 periprosthetic fracture/ 30 fracture$.ti,ab,kw. 31 or/20‐30 32 19 and 31 33 (pin or pins or nail or nails or screw or screws or plate or plates).ti,ab,kw. 34 internal fixator/ or exp bone nail/ or exp bone plate/ or exp bone pin/ or exp bone screw/ or exp femoral fixation device/ 35 (static adj (device$1 or implant$1)).ti,ab,kw. 36 (dynamic adj (device$1 or implant$1)).ti,ab,kw. 37or/33‐36 38 ((hip or hips or femur$ or femoral$ or acetabul$) and (fracture$ or break$ or broke$)).ti,ab,kw. 39 (hip or hips or femur$ or femoral$ or acetabul$).ti,ab,kw. 40 39 and 31 41 37 and (38 or 40) 42 7 or 32 or 41 43 Randomized controlled trial/ 44 Controlled clinical study/ 45 Random$.ti,ab. 46 randomization/ 47 intermethod comparison/ 48 placebo.ti,ab. 49 (compare or compared or comparison).ti. 50 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 51 (open adj label).ti,ab. 52 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 53 double blind procedure/ 54 parallel group$1.ti,ab. 55 (crossover or cross over).ti,ab. 56 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 57 (assigned or allocated).ti,ab. 58 (controlled adj7 (study or design or trial)).ti,ab. 59 (volunteer or volunteers).ti,ab. 60 human experiment/ 61 trial.ti. 62 or/43‐61 63 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 64 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 65 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 66 (Systematic review not (trial or study)).ti. 67 (nonrandom$ not random$).ti,ab. 68 "Random field$".ti,ab. 69 (random cluster adj3 sampl$).ti,ab. 70 (review.ab. and review.pt.) not trial.ti. 71 "we searched".ab. and (review.ti. or review.pt.) 72 "update review".ab. 73 (databases adj4 searched).ab. 74 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ 75 Animal experiment/ not (human experiment/ or human/) 76 or/63‐75 77 62 not 76 78 42 and 77
Web of Science
# 1 TOPIC: (((hip or hips or cervical) NEAR/5 (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 2 TOPIC: (((femoral* or femur* or acetabul*) NEAR/5 (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 3 TOPIC: (((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) NEAR/5 (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 4 TOPIC: (((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) NEAR/5 (fracture* or break* or broke*))) Indexes=SCIEXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 5 TOPIC: (((head or neck or proximal) NEAR/5 (fracture* or break* or broke*)) and (femoral* or femur*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 6 #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 7 TS=(((arthroplast* or hemiarthroplast*) NEAR/5 (hip or hips or femur* or femoral* or acetabul*)) and fracture*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 8 TS=( ((hip or hips) NEAR/5 (replac* or prosthes* or implant*)) and fracture*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 9 TS=(((joint* NEAR/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*)) and fracture*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 10 TS=( (pin or pins or nail or nails or screw or screws or plate or plates or fixator*) and ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 11 TS=((“static device*” OR “static implant*”) and ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 12 TS=((“dynamic device*” or “dynamic implant*”) and ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 13 #12 OR #11 OR #10 OR #9 OR #8 OR #7 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 14 #13 OR #6 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 15 TS=( random* or factorial* or crossover* or "cross‐over*" or placebo* or "doubl* blind*" or "singl* blind*" or assign* or allocat* or volunteer* or "trial" or "groups" or "controlled") Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 16 #15 AND #14 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 17 #16 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2018 # 18 TI=(RAT OR RATS OR MOUSE OR MOUSE OR DOG OR DOGS OR RABBIT OR RABBITS OR PIG OR PIGS OR SWINE OR PORCINE) Indexes=SCIEXPANDED, CPCI‐S Timespan=1900‐2020 # 19 #17 NOT #18 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2020
Cochrane Database of Systematic Reviews (CDSR)
#1 MeSH descriptor: [Femoral Fractures] explode all trees #2 ((hip or hips or cervical) NEAR/5 (fracture* or break* or broke*)) #3 ((femoral* or femur* or acetabul*) NEAR/5 (fracture* or break* or broke*)) #4 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) NEAR/5 (fracture* or break* or broke*)) #5 ((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) NEAR/5 (fracture* or break* or broke*)) #6 ((head or neck or proximal) NEAR/5 (fracture* or break* or broke*)) and (femoral* or femur*) #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 #8 MeSH descriptor: [Arthroplasty, Replacement, Hip] this term only #9 MeSH descriptor: [Hip Prosthesis] this term only #10 MeSH descriptor: [Arthroplasty, Replacement] this term only #11 MeSH descriptor: [Hemiarthroplasty] this term only #12 MeSH descriptor: [Joint Prosthesis] this term only #13 ((arthroplast* or hemiarthroplast*) NEAR/5 (hip or hips or femur* or femoral* or acetabul*)) #14 ((hip or hips) NEAR/5 (replac* or prosthes* or implant*)) #15 ((joint* NEAR/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*)) #16 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 #17 MeSH descriptor: [Fractures, Bone] this term only #18 MeSH descriptor: [Fracture Dislocation] explode all trees #19 MeSH descriptor: [Fractures, Closed] this term only #20 MeSH descriptor: [Fractures, Comminuted] this term only #21 MeSH descriptor: [Fractures, Compression] this term only #22 MeSH descriptor: [Fractures, Malunited] this term only #23 MeSH descriptor: [Fractures, Multiple] this term only #24 MeSH descriptor: [Fractures, Open] this term only #25 MeSH descriptor: [Fractures, Spontaneous] this term only #26 MeSH descriptor: [Fractures, Stress] explode all trees #27 MeSH descriptor: [Fractures, Ununited] this term only #28 MeSH descriptor: [Intra‐Articular Fractures] this term only #29 MeSH descriptor: [Osteoporotic Fractures] this term only #30 MeSH descriptor: [Periprosthetic Fractures] this term only #31 fracture* #32 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 #33 #16 AND #32 #34 (pin or pins or nail or nails or screw or screws or plate or plates) #35 MeSH descriptor: [Internal Fixators] this term only #36 MeSH descriptor: [Bone Nails] this term only #37 MeSH descriptor: [Bone Plates] this term only #38 MeSH descriptor: [Bone Screws] explode all trees #39 (static NEXT (device* or implant*)) #40 (dynamic NEXT (device* or implant*)) #41 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 #42 ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) #43 (hip or hips or femur* or femoral* or acetabul*) #44 #43 AND (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) #45 #42 OR #44 #46 #41 AND #45 #47 #7 OR #33 OR #46 in Cochrane Reviews
Database of Abstracts of Reviews of Effects (DARE)
1 (MeSH DESCRIPTOR Femoral Fractures EXPLODE ALL TREES) 2 ((hip or hips or cervical) near5 (fracture* or break* or broke*)) 3 ((fracture* or break* or broke*) near5 (hip or hips or cervical) ) 4 ((femoral* or femur* or acetabul*) near5 (fracture* or break* or broke*)) 5 ((fracture* or break* or broke*) near5 (femoral* or femur* or acetabul*)) 6 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) near5 (fracture* or break* or broke*)) 7 ((fracture* or break* or broke*) near5 (intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical)) 8 ((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near5 (fracture* or break* or broke*)) 9 ((fracture* or break* or broke*) near5 (extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*)) 10 ((head or neck or proximal) near5 (fracture* or break* or broke*) ) AND (femoral* or femur*) 11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 12 (MeSH DESCRIPTOR Arthroplasty, Replacement, Hip) OR (MeSH DESCRIPTOR Hip Prosthesis) 13 (MeSH DESCRIPTOR Arthroplasty, Replacement ) OR (MeSH DESCRIPTOR Hemiarthroplasty) OR (MeSH DESCRIPTOR Joint Prosthesis) 14 ((arthroplast* or hemiarthroplast*) near5 (hip or hips or femur* or femoral* or acetabul*)) 15 ((hip or hips or femur* or femoral* or acetabul*) near5 (arthroplast* or hemiarthroplast*)) 16 ((hip or hips) near5 (replac* or prosthes* or implant*)) 17 ((replac* or prosthes* or implant*) near5 (hip or hips)) 18 (joint* near5 (replac* or prosthes* or implant*) ) AND (hip or hips or femur* or femoral* or acetabul*) 19 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 20 (MeSH DESCRIPTOR fractures, bone) 21 (MeSH DESCRIPTOR fracture dislocation EXPLODE ALL TREES ) 22 (MeSH DESCRIPTOR fractures, closed ) 23 (MeSH DESCRIPTOR fractures, comminuted ) 24 (MeSH DESCRIPTOR fractures, compression ) 25 (MeSH DESCRIPTOR fractures, malunited ) 26 (MeSH DESCRIPTOR fractures, open ) 27 (MeSH DESCRIPTOR fractures, spontaneous) 28 (MeSH DESCRIPTOR fractures, stress EXPLODE ALL TREES ) 29 (MeSH DESCRIPTOR fractures, ununited ) 30 (MeSH DESCRIPTOR intra‐articular fractures ) 31 (MeSH DESCRIPTOR osteoporotic fractures ) 32 (MeSH DESCRIPTOR periprosthetic fractures ) 33 (MeSH DESCRIPTOR fractures, multiple ) 34 (fracture*) 35 #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 36 #19 AND #35 37 (pin or pins or nail or nails or screw or screws or plate or plates) 38 (MeSH DESCRIPTOR internal fixators ) 39 (MeSH DESCRIPTOR bone nails) 40 (MeSH DESCRIPTOR bone plates ) 41 (MeSH DESCRIPTOR bone screws EXPLODE ALL TREES ) 42 (static near (device* or implant*)) 43 ((device* or implant*) near static) 44 (dynamic near (device* or implant*)) 45 ((device* or implant*) near dynamic) 46 #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 47 ( (hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) 48 (hip or hips or femur* or femoral* or acetabul* ) 49 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33) 50 #48 AND #49 51 #47 OR #50 52 #46 AND #51 53 #11 OR #36 OR #52 54 * IN DARE 55 #53 AND #54
Health Technology Assessment (HTA)
1 (MeSH DESCRIPTOR Femoral Fractures EXPLODE ALL TREES) 2 ((hip or hips or cervical) near5 (fracture* or break* or broke*)) 3 ((fracture* or break* or broke*) near5 (hip or hips or cervical) ) 4 ((femoral* or femur* or acetabul*) near5 (fracture* or break* or broke*)) 5 ((fracture* or break* or broke*) near5 (femoral* or femur* or acetabul*)) 6 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) near5 (fracture* or break* or broke*)) 7 ((fracture* or break* or broke*) near5 (intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical)) 8 ((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near5 (fracture* or break* or broke*)) 9 ((fracture* or break* or broke*) near5 (extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*)) 10 ((head or neck or proximal) near5 (fracture* or break* or broke*) ) AND (femoral* or femur*) 11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 12 (MeSH DESCRIPTOR Arthroplasty, Replacement, Hip) OR (MeSH DESCRIPTOR Hip Prosthesis) 13 (MeSH DESCRIPTOR Arthroplasty, Replacement ) OR (MeSH DESCRIPTOR Hemiarthroplasty) OR (MeSH DESCRIPTOR Joint Prosthesis) 14 ((arthroplast* or hemiarthroplast*) near5 (hip or hips or femur* or femoral* or acetabul*)) 15 ((hip or hips or femur* or femoral* or acetabul*) near5 (arthroplast* or hemiarthroplast*)) 16 ((hip or hips) near5 (replac* or prosthes* or implant*)) 17 ((replac* or prosthes* or implant*) near5 (hip or hips)) 18 (joint* near5 (replac* or prosthes* or implant*) ) AND (hip or hips or femur* or femoral* or acetabul*) 19 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 20 (MeSH DESCRIPTOR fractures, bone) 21 (MeSH DESCRIPTOR fracture dislocation EXPLODE ALL TREES ) 22 (MeSH DESCRIPTOR fractures, closed ) 23 (MeSH DESCRIPTOR fractures, comminuted ) 24 (MeSH DESCRIPTOR fractures, compression ) 25 (MeSH DESCRIPTOR fractures, malunited ) 26 (MeSH DESCRIPTOR fractures, open ) 27 (MeSH DESCRIPTOR fractures, spontaneous) 28 (MeSH DESCRIPTOR fractures, stress EXPLODE ALL TREES ) 29 (MeSH DESCRIPTOR fractures, ununited ) 30 (MeSH DESCRIPTOR intra‐articular fractures ) 31 (MeSH DESCRIPTOR osteoporotic fractures ) 32 (MeSH DESCRIPTOR periprosthetic fractures ) 33 (MeSH DESCRIPTOR fractures, multiple ) 34 (fracture*) 35 #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 36 #19 AND #35 37 (pin or pins or nail or nails or screw or screws or plate or plates) 38 (MeSH DESCRIPTOR internal fixators ) 39 (MeSH DESCRIPTOR bone nails) 40 (MeSH DESCRIPTOR bone plates ) 41 (MeSH DESCRIPTOR bone screws EXPLODE ALL TREES ) 42 (static near (device* or implant*)) 43 ((device* or implant*) near static) 44 (dynamic near (device* or implant*)) 45 ((device* or implant*) near dynamic) 46 #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 47 ( (hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) 48 (hip or hips or femur* or femoral* or acetabul* ) 49 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33) 50 #48 AND #49 51 #47 OR #50 52 #46 AND #51 53 #11 OR #36 OR #52 54 * IN HTA 55 #53 AND #54
Epistemonikos
Search 1: Title/abstract (fracture* or break* or broke) AND Title/abstract (hip or hips or cervical or femoral* or femur* or acetabul* or intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical or extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*)
Search 2: Title/abstract (hip or hips or femur* or femoral* or acetabul*) and (replac* or prosthes* or implant*) and fracture* OR Title/abstract (arthroplast* or hemiarthroplast*) and (hip or hips or femur* or femoral* or acetabul*) and fracture*
Search 3: Title/abstract (pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators) AND Title/abstract (hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke)
Proquest DISSERTATIONS AND THESES
S1 ti(((hip or hips or cervical) near/5 (fracture* or break* or broke*))) OR ab(((hip or hips or cervical) near/5 (fracture* or break* or broke*))) S2 ti(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*))) OR ab(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*))) S3 ti(((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) near/5 (fracture* or break* or broke*))) OR ab(((intracapsular or intracapsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) near/5 (fracture* or break* or broke*))) S4 ti(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*))) OR ab(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*))) S5 ti((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))) OR ab((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))) S6 (ti(((hip or hips or cervical) near/5 (fracture* or break* or broke*))) OR ab(((hip or hips or cervical) near/5 (fracture* or break* or broke*)))) OR (ti(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*))) OR ab(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*)))) OR (ti(((intracapsular or intracapsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) near/5 (fracture* or break* or broke*))) OR ab(((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basicervical) near/5 (fracture* or break* or broke*)))) OR (ti(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*))) OR ab(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*)))) OR (ti((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))) OR ab((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*)))) S7 ti((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) OR ab((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) S8 ti( (hip or hips) near/5 (replac* or prosthes* or implant*)) OR ab( (hip or hips) near/5 (replac* or prosthes* or implant*)) S9 ti(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) OR ab(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) S10 (ti((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) OR ab((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*))) OR (ti( (hip or hips) near/5 (replac* or prosthes* or implant*)) OR ab( (hip or hips) near/5 (replac* or prosthes* or implant*))) OR (ti(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) OR ab(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*)))) S11 ti(fracture*) OR ab(fracture*) S12 ((ti((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) OR ab((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*))) OR (ti( (hip or hips) near/5 (replac* or prosthes* or implant*)) OR ab( (hip or hips) 183 near/5 (replac* or prosthes* or implant*))) OR (ti(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) OR ab(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))))) AND (ti(fracture*) OR ab(fracture*)) S13 ti((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) OR ab((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) S14 ti(static near (device* or implant*)) OR ab(static near (device* or implant*)) S15 ti(dynamic near (device* or implant*)) OR ab(dynamic near (device* or implant*)) S16 (ti((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) OR ab((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators))) OR (ti(static near (device* or implant*)) OR ab(static near (device* or implant*))) OR (ti(dynamic near (device* or implant*)) OR ab(dynamic near (device* or implant*))) S17 ti((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) OR ab((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) S18 ((ti((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) OR ab((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators))) OR (ti(static near (device* or implant*)) OR ab(static near (device* or implant*))) OR (ti(dynamic near (device* or implant*)) OR ab(dynamic near (device* or implant*)))) AND (ti((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) OR ab((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))) S19 ((ti(((hip or hips or cervical) near/5 (fracture* or break* or broke*))) OR ab(((hip or hips or cervical) near/5 (fracture* or break* or broke*)))) OR (ti(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*))) OR ab(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*)))) OR (ti(((intracapsular or intracapsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) near/5 (fracture* or break* or broke*))) OR ab(((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basicervical) near/5 (fracture* or break* or broke*)))) OR (ti(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*))) OR ab(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*)))) OR (ti((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))) OR ab((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))))) OR (((ti((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) OR ab((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*))) OR (ti( (hip or hips) near/5 (replac* or prosthes* or implant*)) OR ab( (hip or hips) near/5 (replac* or prosthes* or implant*))) OR (ti(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) OR ab(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))))) AND (ti(fracture*) OR ab(fracture*))) OR (((ti((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) OR ab((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators))) OR (ti(static near (device* or implant*)) OR ab(static near (device* or implant*))) OR (ti(dynamic near (device* or implant*)) OR ab(dynamic near (device* or implant*)))) AND (ti((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) OR ab((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))))
National Technical Information Service (NTIS)
Title: hip fractures OR Keyword: hip fractures
Keyword: Hip AND Keyword: Bone fractures
ClinicalTrials.gov
Advanced search limited to intervention studies in Condition or disease
Interventional Studies | (fracture OR fractures OR break OR broke OR broken ) AND ( hip OR hips OR femoral OR femur OR acetabular OR intracapsular OR intra‐capsular OR subcapital OR sub‐capital OR transcervical OR trans‐cervical OR basicervical OR basi‐cervical)
Interventional Studies | (fracture OR fractures OR break OR broke OR broken ) AND ( extracapsular OR extracapsular OR trochanter OR trochanteric OR subtrochanter OR subtrochanteric OR pertrochanter OR pertochanteric OR intertrochanter OR intertochanteric )
Interventional Studies | (hip OR hips OR femur OR femoral OR acetabular) AND (replace OR replacement OR prosthesis OR prostheses OR implant OR implants) AND (fracture OR fractures OR break OR broke OR broken)
Interventional Studies | (arthroplasty OR hemiarthroplasty) AND (hip OR hips OR femur OR femoral OR acetabular) AND (fracture OR fractures OR break OR broke OR broken)
Appendix 2. Template data extraction form
| Methods |
RCT or quasi‐randomised; parallel design Review comparison group: |
| Participants |
Total number of randomised participants: Inclusion criteria: Exclusion criteria: Setting:type of setting, how many sites & country Baseline characteristics Intervention group 1 (specify by name)
Intervention group 2 (specify by name)
Overall
Note:
|
| Interventions |
General details: to include number of clinicians (and their skills and experience), type of anaesthesia, pre‐ and postoperative care (e.g. use of prophylactic antibiotics or antithromboembolics), rehabilitation (e.g. time to mobilisation or weight bearing) Intervention group 1: type of implant (with manufacturer details), description of use; number randomised to group, number of losses (for relevant outcomes, and with reasons for losses), number analysed by review authors for each review outcome Intervention group 2: type of implant (with manufacturer details), description of use; number randomised to group, number of losses (for relevant outcomes, and with reasons for losses), number analysed by review authors for each review outcome Note:
|
| Outcomes | Outcomes measured/reported by study authors: include all outcomes reported by study authors. For outcomes relevant to the review, specify all time points measured by study authors Outcomes relevant to the review: include measurement tools and time point of measure used in review analysis Note:
|
| Notes |
Funding/sponsor/declarations of interest: Study dates: |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acharya 2003.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: fixed angle plate versus fixed angle plate: keyed (locked) or unkeyed (unlocked) |
|
| Participants |
Total number of randomised participants: 40 Inclusion criteria: people who have a proximal femoral fracture Exclusion criteria: not reported Setting: not reported Baseline characteristics Intervention group 1 (locked)
Intervention group 2 (unlocked)
Note:
|
|
| Interventions |
General details: participants assessed clinically and radiologically postoperatively at 3 months following discharge from hospital Intervention group 1
Intervention group 2
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: screw slide; fixation failure; VAS Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Notes:
|
|
Adams 2001.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus SHS |
|
| Participants |
Total number of randomised participants: 400 Inclusion criteria: diagnosis of intertrochanteric fractured femur Exclusion criteria: inability to give informed consent, too frail for any operative intervention, and residence outside the region of the hospital because of the difficulty of follow‐up Setting: single centre; orthopaedic hospital, UK Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: study authors report that surgeons were experienced with both implants; both groups received standard 3‐dose IV cefuroxime and routine antithrombotic prophylaxis; clinical follow‐up for 1 year or until death (3 months, 6 months, 12 months) Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; postoperative haemoglobin; tip‐apex distance; number of participants transfused; operative fracture of the femur; later fracture of the femur; cut‐out of implant; detachment of the plate from the femur; re‐operation; deep wound infection; superficial wound infection; DVT; mortality; use of walking aids; place of residence at follow‐up; HHS (available at 3, 6, 12 months) Outcomes relevant to the review: mortality (12 months); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: quote: "Although none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but are directed solely to a research fund, the Scottish Orthopaedic Research Trust into Trauma, a non‐profit organisation with which one or more of the authors is associated." Study dates: February 1994 to June 1995 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Quotes: "At admission, patients were randomized by a closed, opaque envelope method and were assigned to receive either..." Confirmed by Adams in 2001 that "the opaque envelopes were sequentially numbered" ‐ and that there was concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quote (from draft report): "The surgeons were experienced in the insertion of both implants". |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment: HRQoL (detection bias) | Low risk | Quote: "Observed‐blinded functional assessments were carried out by the unit research physiotherapist, by use of the Harris hip score." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses were explained by death, which is expected in this population, and losses were reasonably balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report details of pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Adeel 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus DHS |
|
| Participants |
Total number of randomised participants: 68 Inclusion criteria: 40 to 75 years of age; presenting with AO type A2 and A3 pertrochanteric fracture of femur diagnosed on history, clinical examination and radiograph Exclusion criteria: people with anaesthesia risk; pathological fracture; previous surgical intervention on the affected hip; metabolic bone disease diagnosed on history, clinical examination, baseline investigations, ECG, and radiograph Setting: single centre; hospital; Pakistan Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: single surgical team; ceftriaxone 1 g given half an hour before surgery, and continued 2 g per day for 3 postoperative days; general or spinal anaesthesia; encouraged to take up ankle and calf exercises from POD1, mobilised non‐weight bearing from POD2 depending on physical condition of participant Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: union, operation time, volume of blood loss, complications (infection, non‐union, mal‐union, and implant failure); functional outcome (using HHS with grades of excellent, good, fair, and poor; and mean scores) Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: no funding; study authors declare no conflicts of interest Study dates: September 2015 to September 2017 Note:
|
|
Agrawal 2017.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: fixed angle plate versus PFLCP |
|
| Participants |
Total number of randomised participants: 52 Inclusion criteria: people who gave consent for inclusion into the study; > 18 years of age; and closed intertrochanteric fractures Exclusion criteria: people refusing to give consent; AO type 31A3 fractures; neurovascular injury; open fractures; associated ipsilateral or contralateral major limb injury (including fractures) affecting the treatment or rehabilitation protocol; associated upper limb fractures requiring surgery; major systemic illness (malignancy, chronic kidney, liver disease, etc.) Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (PFLCP)
Note:
|
|
| Interventions |
General details: all surgeries performed on traction table; all surgeries performed by single surgeon; prophylactic antibiotics given preoperatively; mobilisation from POD1, toe‐touch weight bearing in DHS group at 7 days and in PFLCP group at least 6 weeks; follow‐up at 6, 12, 16, and 24 weeks, and at 3‐monthly intervals until radiological union Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of surgery; blood loss; need for postoperative blood transfusion; duration of hospital stay; time to radiological union; functional status; significant limb shortening; medialisation of shaft; mortality; complications (septicaemia, DVT, pulmonary embolism, stroke, MI); non‐specific hip pain; varus deformity; superficial wound infection; non‐union Outcomes relevant to the review: mortality Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors declare no external financial aid was provided with no conflict of interest Study dates: June 2011 to June 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants selected on odd/even numbers |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation because of the quasi‐randomised methods. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. All surgeries were performed by a single surgeon with no experience reported. We could not be certain whether the surgeon was equally experienced in using both implants. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Skills and experience of surgeon is not reported and we could not be certain whether experience comparable for both implants |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported that no participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It was not feasible to effectively assess risk of selective bias without these documents. |
| Other bias | Unclear risk | We noted differences in the time to weight bearing between the two groups. This could influence outcomes. |
Ahrengart 1994.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: intramedullary nail versus SHS |
|
| Participants |
Total number of randomised participants: 492 Inclusion criteria: intertrochanteric (stable and unstable) Exclusion criteria: subtrochanteric and pathologic fractures, earlier fractures or operations on the same hip, or if the surgeon was unfamiliar with the Gamma nail technique Setting: multicentre; 5 hospitals; Sweden and Finland Baseline characteristics: only reported for the 426 participants that completed the study (according to linked study report Ahrengart 2002) Intervention group 1 (Gamma nail) (n = 210)
Intervention group 2 (SHS) (n = 216)
Notes:
|
|
| Interventions |
General details: operations were carried out by surgeons of varying grades from junior resident to staff surgeons, and surgeons were excluded from the trial if not adequately experienced with the Gamma nail; 90% received spinal anaesthesia and 81% received antibiotic prophylaxis, 75% received anticoagulants, 56% received dextran and 18% received heparin or warfarin; compression stockings or other physical preventive measures occasionally used; open reduction in some cases; full weight bearing immediately in 88% of cases Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: LOS, residence at 6 months, lag screw position, length of skin incisions, operative time, blood loss, transfusion, superficial wound infection, deep wound infection, operative fracture of femur, fracture reduction, screw cut out, mortality, femoral medialisation (sliding of lag screw), lateral pain over the femoral head screw, pain at the top of the greater trochanter, thromboembolic complication (DVT, PE); clinical complications (pneumonia); shortening of leg; return to pre‐fracture residential status; use of walking aids; length of skin incision; all 6 months Outcomes relevant to the review: mortality (6 months); unplanned return to theatre (reported as revision, 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: supported by grants from the Karolinska Institute Foundation, Lund University, Skane County Council and Stryker‐Howmedica Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by consecutively opened sealed envelopes; no additional details |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was achieved using sealed envelopes in numerical order before the patient was taken to the operating room." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Surgery was done by various orthopaedic surgeons from junior residents to staff surgeons, and surgeons were excluded from trial participation if unfamiliar with the Gamma nail technique. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | Surgery was done by various orthopaedic surgeons from junior residents to staff surgeons, and surgeons were excluded from trial participation if unfamiliar with the Gamma nail technique. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | We could not adequately assess risk of attrition bias because findings were reported by different trial centres at different points in time, and we noted variation in numbers of lost participants which were not sufficiently explained. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trial register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Akhtar 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA vs DCS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: people with unstable proximal femur fracture of 31A2 and 31A3 within 7 days of fracture; 40 to 70 years of age; either gender Exclusion criteria: people with 31A1 type fracture, pathological fractures, presence of neurovascular injury, inability to walk before injury, significant medical comorbidity such as diabetes mellitus; not fit for anaesthesia Setting: hospital; single centre; Pakistan Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (DCS)
Note:
|
|
| Interventions |
General details: study authors reported no treatment details Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: union time Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: September 2015 to March 2016 Note:
|
|
Akinci 2010.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus AP |
|
| Participants |
Total number of randomised participants: 262 Inclusion criteria: people with trochanteric fractures of the femur and underwent surgical treatment Exclusion criteria: not reported Setting: single site; hospital; Turkey Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (AP)
Note:
|
|
| Interventions |
General details: traction applied to 53.5% participants. Participants in DHS group allowed to sit after 24 hours postoperatively, walk with crutches without weight bearing on POD 6 to 10, partial weight bearing weeks 4 to 6, full weight bearing weeks 8 to 12. Participants in AP group allowed to sit at 48 hours, walk on crutches without weight bearing on POD 10, partial weight bearing weeks 8 to 12, full weight bearing weeks 12 to 16 Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of hospital stay; mortality (available at 12 months and 5 years); infection necessitating removal of implant; infection responding to medical treatment; DVT; severe pulmonary embolism; additional fixation; other organ injuries (multiple rib fractures, ischium‐pubis fracture, ipsilateral femoral shaft fracture, colles fracture, radius and ulna forearm fracture, tibial condyle fracture and vertebral body fracture); complications (shortening, varus deformity, femoral head perforation, acetabular protrusion, device breakage, pseudoarthrosis, avascular necrosis, malunion, rotational deformity, re‐operation (follow‐up time unknown), partial prosthesis, total prothesis, hip movement restriction); position of screws; Foster's anatomical ranking (excellent, good, moderate and bad); Clawson functional classification (excellent, good, moderate and bad) Outcomes relevant to the review: mortality (reported at 12 months and 5 years); re‐operation (follow‐up time unknown) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: September 1988 to June 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although interventions are described as being randomly assigned, method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors did not report skills and experience of surgeons and we could not be certain that experience was comparable for both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We assumed that there were no additional losses other than for death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It was not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Unclear risk | We noted differences in criteria for postoperative mobilisation and weight bearing between the groups, and we could not be certain whether these differences could influence outcomes. |
Aktselis 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: short intramedullary Gamma nail versus SHS (AMBI) |
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: an unstable 31.A2.2 or 31.A2.3 (but not 31.A2.1) fracture type according to the AO/OTA classification, > 65 years old Exclusion criteria: bilateral fractures, pathologic fractures, previous chemotherapy or radiotherapy, or both, rheumatic diseases, polytrauma, a previous operation in the same hip/femur and an ASA score of IV or V Setting: single centre; hospital; Greece Baseline characteristics (only for analysed participants) Intervention group 1 (Gamma nail)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: fracture table; spinal anaesthesia; single dose antibiotics preoperatively continued 48 hours; no suction drain; mobilisation with a walker and weight bearing as tolerated and assessment of postoperative X‐rays; all operations supervised by consultant orthopaedic surgeons familiar with both procedures; clinical follow‐up at 1, 3, 6 and 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mobility (available at 1, 3, 6 and 12 months); daily function ‐ Barthel Index (available at 1, 3, 6 and 12 months); EQ‐5D ‐ HRQoL (available at 1, 3, 6 and 12 months); mortality; duration of surgery; radiation time; LOS; hip pain; mechanical failure; cut‐out; non‐union; fracture (intraoperative and late); fixation failure; infection; re‐operation Outcomes relevant to the review: HRQoL (EQ‐5D, 3 & 12 months); unplanned return to theatre (described as cut outs necessitating re‐operation; 12 months); mortality (12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: October 2008 until January 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Envelopes were picked from a box, however method of sequence generation is not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed, opaque envelopes picked from a box in the presence of 3 surgeons" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Operations supervised by 4 consultant surgeons with experience in both techniques |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment: HRQoL (detection bias) | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses, which were balanced between groups and explained by death, which is expected in this population |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trial register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Andalib 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: cephalomedullary nail vs DHS and DCS |
|
| Participants |
Total number of randomised participants: 113 Inclusion criteria: unstable intertrochanteric fractures, candidate for extramedullary or intramedullary surgery, ability to walk without any assistance before the fracture, signed informed consent Exclusion criteria: uncontrolled diabetes mellitis, using immunosuppressive drugs, any kind of malignancies as well as those who refused to continue the trial Setting: multi‐centre; 2 trauma centres; Iran Baseline characteristics (data only for those not lost to follow‐up/excluded) Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: no details Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: ADL (using LEM score; measured at baseline, 1, 3, 6 and 12 months after surgery); device failure (cut out, migration of screw, breakage of implant); need for re‐operation; fracture union; limb shortening; return to previous level of activity (before fracture); superficial and deep infections; mortality Outcomes relevant to the review: mortality (12 months), unplanned return to theatre (reported as re‐operation; at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: March 2016 to June 2018 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization into the groups was performed using stratification and blocking methods" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | We did not expect lack of blinding of surgeons to influence performance and outcome data for this review. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses were explained by death which is expected in this population. Other losses (due to being unable or unwilling to continue in the study) were few and were reasonably balanced between study groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trial register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Andreani 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: intramedullary nail versus intramedullary nail: Gamma 3 or ENDOVIS nail |
|
| Participants |
Total number of randomised participants: 81 Inclusion criteria: > 65 years of age; with a pertrochanteric fracture (31A1 and 31A2) after a low‐energy trauma Exclusion criteria: people who had pathological fractures; high‐energy trauma; 31A3 fractures; those who were not walking; < 65 years of age Setting: single centre; hospital; Italy Baseline characteristics Intervention group 1 (Gamma 3 nail)
Intervention group 2 (ENDOVIS nail)
Note:
|
|
| Interventions |
General details: prophylactic IV second‐generation cephalosporin administered preoperatively and continued until 24 hours postoperatively; heparin was received for 5 weeks postoperatively; all surgery performed by the same group of trauma surgeons; all operations done on traction table in supine position; mobilisation on POD1, with weight bearing as tolerated on POD2 Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: serum haemoglobin level; mobility status; hospitalisation; mortality (available at 12 months); functional status (ADL, mobility score and HRQoL); radiographical findings; fracture healing; EQ‐5D (available at 1, 3, 6 and 12 months); DVT Outcomes relevant to the review: mortality (reported at 12 months); EQ‐5D (reported at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report "no conflicts of interest of interest to disclose" Study dates: March 2012 to June 2013 Note:
|
|
Bajpai 2015.
| Study characteristics | ||
| Methods | Quasi‐randomised trial; parallel design Review comparison group: screw PFN versus helical PFN |
|
| Participants |
Total number of randomised participants: 77 Inclusion criteria: skeletally‐mature participants > 50 years of age; new mobility score of 9 (Palmer and Parker 1993); closed unstable trochanteric fracture classified as AO 31A2 and A3 Exclusion criteria: immature skeleton; pathological fracture of any cause other than osteoporosis, open fractures, inability to walk independently prior to injury event Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (screw PFN)
Intervention group 2 (helical PFN)
Note:
|
|
| Interventions |
General details: all operations were performed on fracture table in supine position under general anaesthesia; crutch walking with partial weight bearing was allowed after 48‐hour drain removal; suture was removed on 12th day Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss; duration of hospitalisation; time before operation; HHS; time to union Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: June 2008 to August 2011 Note:
|
|
Bannister 1990.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus Jewett nail plate |
|
| Participants |
Total number of randomised participants: 155 Inclusion criteria: > 64 years of age; trochanteric fractures Exclusion criteria: not reported Setting: single centre, hospital, UK Baseline characteristics Overall:
Note:
|
|
| Interventions |
General details: 95% of procedures were carried out by orthopaedic residents; neither antibiotic nor antithromboembolic prophylaxis was used routinely; the postoperative management was identical in both participating institutions; weight bearing after 2 days; participants were reviewed 2, 4 and 12 months after fracture Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain; mobility; manner of walking; social independence; deep infection; superficial infection; failed union; mortality (available at 12 months); mechanical failures; unplanned return to theatre (available at 12 months) Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 1981 Note:
|
|
Barrios 1993.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Ender Nails versus SSP |
|
| Participants |
Total number of randomised participants: 149 recruited; 92 reported Inclusion criteria: > 50 years of age, trochanteric hip fractures Exclusion criteria: not reported Setting: single centre; hospital; Spain Baseline characteristics (only participants completing follow‐up are reported) Intervention group 1 (Ender)
Intervention group 2 (SSP)
Overall:
Note:
|
|
| Interventions |
General details: not reported Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: quality of reduction; healing status; implant failures; pain; mobility Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: no details on funding or conflict of interest were reported Study dates: June 1982 to May 1985 Note:
|
|
Barton 2010.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: long Gamma intramedullary nail versus SHS |
|
| Participants |
Total number of randomised participants: 210 Inclusion criteria: > 18 years of age; AO/OTA 31‐A2 fracture of the proximal part of the femur Exclusion criteria: pathological fractures; previous proximal femoral fractures; reverse oblique fractures (AO/OTA 31‐A3), decision by surgeon not to include individual in the study Setting: single centre; orthopaedic hospital; UK Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (SHS)
Overall:
Note:
|
|
| Interventions |
General details: traction table; aspirin and thromboembolism‐deterrent stockings for thromboprophylaxis; mobilisation with weight bearing; clinical follow‐up at 3, 6 and 12 months; 32 consultant orthopaedic surgeons all had experience with both techniques Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: number of participants transfused; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union; deep wound infection; re‐operation; LOS; mortality; change in mobility score (measured on a 5‐point ordinal scale); change in residential status (measured on a 5‐point ordinal scale); quality‐adjusted life‐years (EQ‐5D scores); length of follow‐up: 12 months Outcomes relevant to the review: mortality; unplanned return to theatre (12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: no external funding Study dates: April 2003 to April 2006 (from trial registration documents) Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was prepared by a medical statistician and we assumed that this was done adequately and independently. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was carried out with use of sealed envelopes generated by a medical statistician. Once a patient was considered to be appropriate for inclusion, consent was obtained. An envelope was then selected and opened at a daily trauma meeting." Comment: study authors do not report if envelopes were opaque or sequentially‐numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | All 32 surgeons were experienced with both implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses were explained by death, which is expected in this population, and were reasonably balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Retrospective trial registration document (ISRCTN79362886; received in March 2009). It is not feasible to use these retrospective documents to effectively assess risk of selective reporting bias. |
| Other bias | Low risk | We identified no other sources of bias. |
Barwar 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus DHS: conventional side plate or locking side plate |
|
| Participants |
Total number of randomised participants: 50 Inclusion criteria: people with intertrochanteric fracture; people who gave written and informed consent for the surgery Exclusion criteria: not reported Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (conventional DHS)
Intervention group 2 (DHS with locking side plate)
Note:
|
|
| Interventions |
General details: a single injection of cephazolin was given 30 minutes before incision as prophylaxis; all participants were placed in supine position on a traction table under spinal or general anaesthesia; postoperatively, weight bearing on tolerance was allowed in both groups; full weight bearing was not allowed in both groups till good amount of fracture consolidation; participants were followed up at 6 weeks, 12 weeks, 6 months and 1 year Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mobility; rate of restoration; TAD; angle of anteversion restoration; fracture union duration; deep infection; avascular necrosis; DVT; unplanned return to theatre Outcomes relevant to the review: unplanned return to theatre (reported at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Baumgaertner 1998.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: IMHS versus SHS |
|
| Participants |
Total number of randomised participants: 131; 135 trochanteric femoral fractures (4 of these were fractures which occurred several months later in the same participants) Inclusion criteria: intertrochanteric fracture Exclusion criteria: pathological fractures Setting: 2 orthopaedic hospitals; USA Baseline characteristics (overall)
Baseline characteristics Intervention group 1 (hip screw)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: antibiotic and DVT prophylactic; weight bearing according to individual participant characteristics (17 allowed weight bearing as tolerated, 107 restricted to partial weight bearing); surgical experience: Gamma nail: familiar with Intramedullary nailing but not the Gamma nail; SHS routine; surgery by residents under supervision, 30 participating surgeons (all had been using SHS, but had not used the IMHS); clinical follow‐up at 6 weeks, 3, 6, 12 and 24 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; transfusion; radiographic screening time; operative fracture of the femur; later fracture of the femur; cut‐out of implant; wound haematoma; major medical complication; LOS; hospital charges; mortality; hip pain at follow‐up; return to pre‐fracture residence; patient mobility; length of follow‐up: mean 28 months (range 4 to 54 months) Outcomes relevant to the review: mortality (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: March 1992 to March 1994 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of sealed envelopes, but method of sequence generation is not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "two hundred sealed opaque envelopes were randomly (cards were shuffled) assigned to either the IMHS or CHS, and numbered in sequential order, after enrolment in the study the next envelope was opened to reveal the device selected for the patient, no one was aware of the next upcoming device." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | All participating attending surgeons had been using SHS before the start of the study, and although they were familiar with nailing, they previously had not used the IMHS. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were balanced between groups and were mostly explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trial register or reference for a pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Benum 1994.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus CHS Note:
|
|
| Participants |
Total number of randomised participants: 912 Inclusion criteria: trochanteric and subtrochanteric proximal femoral fractures. One study publication referred to the Jensen and Zickel classifications and tabulated stable, unstable and subtrochanteric fractures (Aune 1994). Exclusion criteria: not reported Setting: orthopaedic hospitals, Norway Baseline characteristics (overall)
Note:
|
|
| Interventions |
General details: surgical experience is unknown for all centres but for a subgroup in 1 centre (Aune 1993) Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes measured/reported by study authors: length of surgery; blood loss; operative fracture of the femur; later fracture of the femur; cut‐out of implant (fracture dislocation); non‐union (fracture healing); re‐operation; wound infection; DVT; PE; length of hospital stay; mortality; institutional stay; walking function Outcomes relevant to the review: mortality; unplanned return to theatre (reported as re‐operation; 6 months) | |
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 1990 to 1992 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization was done by drawing on among mixed envelopes containing information allocating the patient to either treatment." Comment: study authors do not report if envelopes are sealed, sequentially‐numbered or opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Report from one centre (Aune 1994) refers to treatment by "younger surgeons" and in consequence that "the learning curve becomes important". We have assumed from this information that surgeons may not be equally experienced using both implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Because studies are reported in numerous abstracts with interim publications and later publications for only subsets of participants, we were concerned that attrition was not well‐explained or justified. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | High risk | For most data, we have used information reported in an abstract, which we expected was not peer‐reviewed and likely at high risk of bias. |
Berger‐Groch 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: 2‐screw cephalomedullary nail versus single‐screw cephalomedullary nail: InterTan or Gamma 3 |
|
| Participants |
Total number of randomised participants: 104 Inclusion criteria: skeletally‐mature participants with a displaced intertrochanteric fracture Exclusion criteria: pathological fractures; previous ipsilateral fracture treatment; acute neurologic or psychiatric disorders Setting: single centre; hospital; Germany Baseline characteristics Intervention group 1 InterTan
Intervention group 2 Gamma 3 nail
Overall: IT + G3
Note:
|
|
| Interventions |
General details: full weight bearing was achieved earlier in the IT group collective (IT group: 3.0 ± 1.6 days, G3 group: 12.9 ± 0.7 days; P = 0.03) Intervention group 1: 2‐screw
Intervention group 2: single screw
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: time to surgery; hospital stay; surgery time; x‐ray; ICU stay; mortality (available in hospital, 6 months and at 5 years); HRQoL (using SF‐36; available at hospital discharge, 6 weeks, 3 months and 6 months); HHS Outcomes relevant to the review: mortality (reported at 6 months and 5 years); SF‐36 (reported at 3 months and 6 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. Study authors report no conflicts of interest Study dates: 2009 to 2010 Note:
|
|
Bridle 1991.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus DHS |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: intertrochanteric proximal femoral fractures Exclusion criteria: < 60 years of age Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: 4 senior surgeons experienced with closed nailing techniques; general anaesthesia (n = 87), spinal anaesthesia (n = 13); clinical follow‐up 6 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union; re‐operation (incomplete data); wound infection; wound haematoma; bronchopneumonia; pressure sore; PE; any medical complication; LOS; shortening of femur (leg) (no information); mortality; pain (no information); eventual discharge residence; patient mobility; length of follow‐up: 6 months Outcomes relevant to the review: mortality (during hospital stay, 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: quote: "no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quote: "All the operations were performed by one of four senior surgeons, all experienced in closed nailing techniques." |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were balanced and explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Brostrom 1992.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus Ender nail |
|
| Participants |
Total number of randomised participants: 149 Inclusion criteria: > 50 years of age; with trochanteric fractures Exclusion criteria: people with pathological fractures (tumours) Setting: single site; hospital; Sweden Baseline characteristics Intervention group 1 (DHS; baseline data reported for 76 participants)
Intervention group 2 Ender flexible nails (baseline data reported for 44 participants)
Overall:
Note:
|
|
| Interventions |
General details: operations performed by 12 different surgeons (7 were regarded as experienced and 5 were regarded as inexperienced); if no contraindications were present, all operations were performed the first day after admission to the hospital under spinal or epidural anaesthesia; all participants received dextran antithrombotic therapy preoperatively and 3 days postoperatively but prophylactic antibiotics were not used routinely; exercises were started on POD1 and all participants encouraged to walk with full weight bearing and all had the same postoperative regimen Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: DVT; mortality (available at six week); mean hospital stay time; walking aid; blood loss; mean operation time; leg shortening; general complications; pain level; use of walking aids Outcomes relevant to the review: mortality (reported at 6 weeks) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors have not reported any study information Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although participants were randomly selected, method of randomisation is not specified. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by 7 experienced surgeons and 5 surgeons regarded as inexperienced but we could not be certain whether experienced surgeons were equally experienced in using the study implants. |
| Other performance bias: surgeon experience of both implants | High risk | Some surgeons were inexperienced and study authors do not specify whether inexperienced surgeons were equally balanced in use of both implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is not specified how many participants were lost from each group except for death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Buciuto 1998.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: RAB plate versus CHS plate |
|
| Participants |
Total number of randomised participants: 233 Inclusion criteria: unstable trochanteric hip fractures Exclusion criteria: not reported Setting: single centre, hospital, Norway Baseline characteristics Intervention group 1 (RAB)
Intervention group 2 (CHS)
|
|
| Interventions |
General details: all fractures were operated on within 24 hours of admission; the mean follow‐up time for remaining participants was 2 years; all participants were encouraged to bear full weight from the first postoperative day; serial radiographs were taken until union was complete Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: penetration of femoral head; cutting‐out; implant failure; varus dislocation > 10°; malunion; non‐union; femoral head necrosis; deep infection; median perioperative blood loss; median operation time; fractures healed without complication; mortality (available at 12 months) Outcomes relevant to the review: mortality (reported at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors do not provide details on funding or conflicts of interest Study dates: August 1991 to January 1994 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Experience of surgeons is not reported, and it is therefore uncertain whether they were equally experienced both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost, these losses were balanced between groups, and reasons for loss were clearly explained. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Butt 1995.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: Gamma nail versus DHS |
|
| Participants |
Total number of randomised participants: 95 Inclusion criteria: trochanteric and subtrochanteric proximal femoral fractures Exclusion criteria: not reported Setting: single centre; orthopaedic hospital, UK Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: standard surgical procedures; surgical experience is unknown; same surgeons did both operations Intervention group 1
Intervention group 2
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; later fracture of the femur; cut‐out of implant (incomplete data); non‐union (time to union); re‐operation (total inferred); wound infection; pneumonia; pressure sore; DVT; any medical complication; LOS; mortality; length of follow‐up: 'to fracture union' (generally < 6 months) Outcomes relevant to the review: mortality (5 months); unplanned return to theatre Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: quote: "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients admitted on even‐numbered weeks were treated with a DHS and patients admitted on odd‐numbered weeks were treated with a gamma nail." |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation because of methods used to randomise participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Same surgeons did both operations, but no mention of experience and interim modification of surgical technique by the manufacturers |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were balanced and explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Cai 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: intramedullary versus extramedullary implants |
|
| Participants |
Total number of randomised participants: 222 Inclusion criteria: stable, comminuted, intertrochanteric femoral fracture; > 65 years of age; the ability to walk independently (with or without an aid) prior to fracture; and the sustaining of a low‐energy injury within 24 hours prior to admission Exclusion criteria: a compound femoral fracture; < 65 years of age; a history of previous fracture; any contraindication to surgery; nonambulatory status prior to the presenting injury, or any other traumatic fracture Setting: single site; hospital; China Baseline characteristics (only for analysed participants) Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Overall:
Note:
|
|
| Interventions |
General details: all surgeries were carried out by 3 surgeons all of whom had more than 15 years of clinical experience (all were familiar with both techniques) Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss; functional recovery; postoperative complications (superficial wound infection, deep wound infection, pneumonia, UTI, delayed union, nonunion, cutting of lag screw, implant failure, electrolyte imbalance, hypoproteinaemia); mortality (available at 12 months) Outcomes relevant to the review: mortality (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: supported by the National Natural Science Foundation. Study authors declare no conflicts of interest Study dates: 2011 to 2014 Note:
|
|
Caiaffa 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: short intramedullary nails with no distal locking versus static distal locking |
|
| Participants |
Total number of randomised participants: 357 Inclusion criteria: the presence of an acute (treatment within 15 days from trauma) pertrochanteric stable fracture type 31A1/31A2; people > 65 years of age; able to walk before the injury with or without crutches Exclusion criteria: medical contraindications; medical illness; cognitive disorders precluding participation in follow‐up examination; unwillingness to participate; open fracture; bilateral fractures; pathological fractures and previous ipsilateral hip or femur surgery Setting: hospital; 6 sites; Italy Baseline characteristics (only for analysed participants) Intervention group 1 (locking)
Intervention group 2 (unlocking)
Overall:
Note:
|
|
| Interventions |
General details: 13 different surgeons with more than 10 years of experience performed the operations; depending on their general condition, participants in both groups were allowed to sit upright on the second postoperative day, and partial weight bearing with a walker was allowed on fourth postoperative day; follow‐up at 1 month, 3 months, 6 months and 1 year Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; fluoroscopy time; blood loss; total length of incision; time of fracture union; mortality (available at 30 days, 90 days, 180 days and 12 months); participants infused; length of hospital stay; complications; cutting out; diaphyseal fracture; avascular necrosis of the femoral head; deep infection; haematomas; superficial infections; DVT; mobility; SF‐36 (available at 12 months); VAS; HHS; thigh pain Outcomes relevant to the review: mortality (reported at 3 months and 12 months) HRQoL using SF‐36 (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report that they have no conflicts of interest Study dates: April 2012 to October 2014 Note:
|
|
Calderon 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus DHS |
|
| Participants |
Total number of randomised participants: 32 Inclusion criteria: 60 to 90 years of age; type II intertrochanteric fracture of Boyd and Griffin classification, < 48 hours from injury Exclusion criteria: previous fractures on limb or contralateral side which affected rehabilitation; pathological fractures; dementia; non‐consent to participate. Also excluded were participants who failed to attend follow‐up, participants with incomplete medical records and participants who withdrew from the trial. Setting: single centre; university hospital, Mexico Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (DHS)
Overall:
Note:
|
|
| Interventions |
General details: experience of surgeons not reported; clinical follow‐up at 2, 4 and 8 weeks and 6 months after surgery; active and passive mobility from first postoperative day, then full weight bearing as indicated by daily VAS assessment Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain (VAS ‐ range of scores not reported); incision size; intraoperative bleeding; length of surgery; HHS; time to start partial or total weight bearing; time to union; complications: reported on later fracture, 'varus collapse' (without clinical implication); length of follow‐up: 6 months (or 16 weeks ‐ inconsistently reported in article) Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Cao 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA versus HA; distal fixation augmented with or without bone cement |
|
| Participants |
Total number of randomised participants: 250 Inclusion criteria: intertrochanteric fracture; age of no less than 66 years old; indication of artificial hip joint replacement; normal walking without or with single stick before intertrochanteric fracture; Evans‐Jensen type III‐V fracture complicated with many underlying diseases, inappropriate for long‐term bedridden after the surgery or complicated with serious osteoporosis; or Evans‐Jensen type II fracture with the contraindication of fracture reduction and internal fixation Exclusion criteria: new or old cerebral thrombosis or Evans‐Jensen type I fracture; cardiac insufficiency; pathological fracture; complicated with coagulation disorders; mental diseases Setting: single centre; hospital; China Baseline characteristics (overall)
Note:
|
|
| Interventions |
General details: subarachnoid block combined epidural anaesthesia; postlateral approach; prophylactic anticoagulation and antibiotics; DVT prevention; mobilisation within 3 days to 1 week postoperatively; partial weight bearing after 3 weeks post‐surgery; full weight bearing after 3 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; total blood loss; ambulation time; HHS; loosening; neurovascular injury; infection; fracture; dislocation; pain in non‐femoral region; mortality Outcomes relevant to the review: mortality Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: declared no conflicts of interest Study dates: January 2012 to January 2016 Note:
|
|
Carulli 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN vs DHS |
|
| Participants |
Total number of randomised participants: 140 Inclusion criteria: adults with trochanteric fracture (31A1 or 31A2), able to give full consent Exclusion criteria: people with 31A3 fracture; psychiatric diseases; any form of neurologic deficit to lower limbs; any contraindication to surgery Setting: single centre; university hospital; Italy Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: all participants were studied by conventional radiology in emergency room, received antibiotic and antithromboembolic prophylaxis. For postoperative care: all participants given 2 bags of heterologous blood. For rehabilitation, POD1 ‐ passive motion in bed. POD2 ‐ allowed to sit in bed with active knee and ankle exercises. POD3 ‐ assisted standing and gait exercises. Participants sent to rehabilitation facilities to complete functional recovery. Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (at 12 months); blood loss; complications (pulmonary infection, DVT, UTI, superficial wound infection; mechanical complications ‐ spiral blade migration, lateral blade protrusion, migration of plate screws, failure); LOS; walking with partial or full weight bearing at discharge; independent walking at 3 months; restore walking activity and health status to pre‐fracture level; HRQoL Outcomes relevant to the review: HRQoL (SF‐12, PCS and MCS at 12 months); mortality (at 12 months); unplanned return to theatre (at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. "All authors disclose any financial and personal relationships with other people or organizations that could have inappropriately influenced or biased their work" ‐ these disclosures are not detailed in the study report Study dates: January 2007 to December 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes. Study authors do not report if envelopes were opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment: HRQoL (detection bias) | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were balanced and mostly explained by death, which is expected in this population |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Catania 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Elos‐Intrauma nail versus Gamma 3‐Stryker nail |
|
| Participants |
Total number of randomised participants: 323 Inclusion criteria: pertrochanteric fractures; between 65 and 90 years of age; fracture pattern AO/OTA 31.A1 or 31.A2; being able to walk without aids before fracture; good cognitive and motor abilities Exclusion criteria: pathological fractures or presence of an ongoing metastatic disease; polytrauma; presence of a high grade of hip osteoarthrosis; haematological disease or therapy with anticoagulants and antiplatelet drugs; ASA score of V Setting: single centre; hospital; Italy Baseline characteristics Intervention group 1 (Elos)
Intervention group 2 (Gamma)
Note:
|
|
| Interventions |
General details: surgeries were performed by experienced surgeons; minimum follow‐up was 12 months; cefazolin 2 g was administered 30 minutes before surgery; participants received spinal anaesthesia and were operated on in a supine position with the use of a traction table; participants underwent a standard postoperative rehabilitation protocol (when allowed by their clinical status and osteosynthesis stability). It consisted of 1 day bed rest with passive mobilisation of the lower limb, followed by assisted walking with tolerance weight bearing; follow‐up was done every 5 days for the first 15 days and at 1, 6 and 12 months Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of surgery; intraoperative blood loss; VAS; HHS; WOMAC; lag screw cut‐outs; local infections; malunion; proximal screw dislocation; nail breakage; unplanned return to theatre (available at 12 months); mortality (available at 12 months) Outcomes relevant to the review: unplanned return to theatre (reported at 12 months), mortality (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: funding is described as "not applicable" but study authors declare no conflict of interest Study dates: 1 July 2015 to 31 October 2017 Note:
|
|
Chapman 1981.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: compression sliding hip screw versus Ender pins |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: people with extracapsular hip fractures (all fractures at the base of the femoral neck, intertrochanteric fractures, subtrochanteric fractures not more than 2.5 cm distal to lesser trochanter, and any combination thereof) Exclusion criteria: pathological fractures Setting: single centre; hospital; USA Baseline characteristics Intervention group 1 (compression sliding hip screw)
Intervention group 2 (Ender pins)
Overall
Note:
|
|
| Interventions |
General details: no prophylaxis against DVT used; prophylactic sodium cefazolin routinely used; all participants were initially placed on skeletal traction with a pin in the proximal part of the tibia; all operations were performed by resident surgeons with an attending surgeon; unless contraindicated, all participants were placed in a chair at bedside on the first postoperative day; walking with immediate partial weight bearing and rapid progression to full weight bearing, using appropriate assistive devices, was begun as soon as possible; participants were discharged to their homes or an extended‐care facility as their condition warranted; minimum follow‐up of 6 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: time under anaesthesia; time from incision to closure; blood loss; mortality (available at 6 weeks, 6 months and later than 6 months ‐ study authors report average end of follow‐up at 13.5 months); unplanned return to theatre (available at end of follow up); deep wound infections; complications (UTI; pneumonia or pulmonary atelectasis, or both; cardiac arrhythmia; acute congestive heart failure; subphrenic abscess; upper gastrointestinal haemorrhage; renal lithiasis; decubitus ulcers); thrombophlebitis; pulmonary embolisms; total period of hospitalisation Outcomes relevant to the review: mortality (reported at 6 weeks and an average of 13.5 months); unplanned return to theatre (reported at end of follow‐up) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: October 1976 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants assigned based on even/odd number basis |
| Allocation concealment (selection bias) | High risk | We could not be certain of the criteria used to allocate participants and assume that this meant no concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. All surgeries were attended by a consultant but it is not specified whether consultant was equally experienced in both methods. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Performed by resident surgeons alongside the attending surgeon. It is uncertain whether resident surgeons were equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost, these losses were balanced between groups, and reasons for loss were clearly explained. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Chechik 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: EPFN vs DHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: unilateral extracapsular (31A1 and 31A2) hip fracture following low‐energy trauma Exclusion criteria: < 60 years of age, pathologic fractures, life‐threatening disease (ASA ≥ 4), subtrochanteric or reverse oblique fracture patterns (31A3), inability to give informed consent due to dementia or confusional state, previous fracture or previous surgery of the affected leg Setting: single centre; hospital; Israel Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: IV antibiotics given immediately before surgery; spinal anaesthesia (15) and general anaesthesia (45); low‐molecular‐weight heparin for 6 weeks after surgery. After surgery, participants allowed weight bearing as tolerated, all encouraged to begin walking with a frame on POD 1 Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (30 days); CVA (time point described as 'early postoperative'); LOS (days); re‐operation (1 year); discharge location (1 year); mobility score (Parker & Palmer; 1 year); functional outcome at 1 year (HHS; total mean score ‐ also reported as pain, support, distance, and limp); periprosthetic fracture; ADL (used Jensen's independence score; at 1 yr); pain (measured as a separate category in HHS); loosening of prosthesis (plate/screw failure; 1 yr); wound infection (defined as wound discharge); acute coronary syndrome; cut out; plate screw failure; independence (Jensen's score); change of independence; femur shortening; reduced offset; shaft medialisation; heterotopic ossification; blood transfusion (reported as mean units); radiation time, scar length, quality of reduction, intraoperative fracture, acute coronary syndrome, CVA, wound discharge, hospitalisation Outcomes relevant to the review: mortality (30 days); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported; study authors declare no conflicts of interest Study dates: June 2008 to February 2010 Note:
|
|
Chen 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Proximal femoral nail versus hemiarthroplasty |
|
| Participants |
Total number of randomised participants: 67 Inclusion criteria: unstable intertrochanteric fractures and osteoporosis Exclusion criteria: not reported Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (hemiarthroplasty)
Note:
|
|
| Interventions |
General details: unknown Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; intraoperative blood loss; length of incision; postoperative bed time; HHS Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: unknown Study dates: unknown Note:
|
|
Chen 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus SHS |
|
| Participants |
Total number of randomised participants: 36 Inclusion criteria: meeting the international definition of the elderly and diagnostic criteria for intertrochanteric fracture of femur Exclusion criteria: people with the following conditions: HIV; coagulation disorders; hepatic and renal insufficiency; severe circulatory system diseases; mental disorder Setting: single centre; orthopaedic hospital; China Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: combined spinal‐epidural anaesthesia; conventional anti‐inflammatory treatment after operation, and antithrombotic drugs were given on day one; no details reported regarding experience of surgeons or familiarity with interventions Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative bleeding; length of surgery; LOS; short‐term complications; time to weight bearing (partial/full); fracture healing time; functions (Sanders: 55 to 60, excellent; 45 to 54, good; 35 to 44, poor; < 34, fail) Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported; study authors declare no conflicts of interest Study dates: June 2016 to June 2017 Note:
|
|
Cheng 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PCCP versus DHS |
|
| Participants |
Total number of randomised participants: 133 Inclusion criteria: > 65 years of age; an intertrochanteric fracture amenable to satisfactory reduction (type AO/OTA 31.A1 to A2, Evans type 2); ability to ambulate independently prior to the fracture with or without assistive devices; people who were mentally competent and gave their consent to participate Exclusion criteria: reversed oblique fractures (type AO/OTA 31.A3 or Evans type 2); nonunion, pathological fractures, or the presence of metastatic disease; bilateral hip fractures, previous ipsilateral lower‐limb surgery, or contralateral hip fracture within the last year; people who required intensive care or treatment in other departments; people with diabetes difficult to control Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (PCCP)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: prophylactic antibiotics, and prophylactic low‐molecular‐weight heparin given subcutaneously for 6 weeks postoperatively; all participating surgeons were experienced in both techniques; short‐term antibiotic regimens were used to clear infections; ambulation with partial weight‐bearing form POD2 or POD3 for stable fractures, and delayed for at least 2 weeks for unstable fractures; each patient was reviewed monthly for the initial 6 months, then 9, 12, 18 and 24 months after surgery Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss and transfusion necessity; pain evaluation; length of hospital stay; HHS; time until fracture union; postoperative complications (up to 6 months); implant‐related failure; mortality Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: October 2007 to February 2011 Note:
|
|
Ciaffa 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: unlocked femoral nail versus dynamic femoral nail versus static femoral nail |
|
| Participants |
Total number of randomised participants: 240 Inclusion criteria: presence of an acute (treatment within 15 days from trauma) stable (31‐A1) or unstable (31‐A2) intertrochanteric fracture; > 65 years of age; ability to walk prior to injury, with or without crutches Exclusion criteria: people with medical contraindications; medical illness or cognitive disorders precluding participation in the follow‐up examination; unwillingness to participate; open fracture; bilateral fractures; pathological fracture; previous ipsilateral hip or femur surgery Setting: multi‐centre (9 centres): level II trauma centres; Italy Baseline characteristics (for analysed participants at the end of follow‐up, excluding those who died) Intervention group 1 (unlocked)
Intervention group 2 (dynamic)
Intervention group 3 (static)
Overall:
Note:
|
|
| Interventions |
General details: 10 different surgeons with more than 10 years of experience performed surgeries; clinical and radiological follow‐up was at 1, 3, 6 and 12 months; depending on their general condition, participants of all groups were allowed to sit upright on POD2; partial weight bearing with a walker was allowed on POD4; all cases of wound infections were successfully treated with debridement and antibiotic therapy Intervention group 1
Intervention group 2
Intervention group 3
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: blood loss; fluoroscopy time; blood transfusion; length of incision; surgery time; length of hospital stay, mortality (available at 12 months); HHS; HRQoL (available at 12 months); time of fracture union; length of lag screws back out; weight bearing; Barthel Index; walking ability; level of satisfaction; hip pain (VAS); complications (haematoma, DVT, cut out, loss of reduction > 5, diaphyseal fracture, non‐union, lag screws breakage, AVN, wound infection, deep infection); patient satisfaction Outcomes relevant to the review: mortality (reported at 12 months); HRQoL (using SF‐12, reported at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report 'no funding was received in support of this study. None of the other authors have any conflicts of interest to declare.' Study dates: January 2015 to March 2016 Note:
|
|
Dalen 1988.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: Ender nail versus McLauglin nail‐plate fixation |
|
| Participants |
Total number of randomised participants: 130 Inclusion criteria: trochanteric fractures Exclusion criteria: pathological fractures; outside of catchment area; default allocation (not specified in the study report); different medical reasons (not specified in the study report) Setting: single centre; hospital; Sweden Baseline characteristics Intervention group 1 (Ender)
Intervention group 2 (nail‐plate fixation)
Note:
|
|
| Interventions |
General details: participants with displaced fractures had pin traction preoperatively; general or epidural anaesthesia, extension fracture table and biplane fluoroscopy were used; operations were performed by all staff surgeons; antibiotics as prophylactics were not used; after operation, full weight bearing was allowed, and rehabilitation programme was identical for the 2 methods of operation; follow‐up time was about 6 years Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (available at 2 weeks, 6 years); unplanned return to theatre (available at 6 years); days in hospital; deep infections; blood loss; operation time; leg lengths; range of hip movement; hip pain; knee pain; walking capability Outcomes relevant to the review: mortality (reported at two weeks); unplanned return to theatre (reported at 6 years) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 1978 to December 1980 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation based on birth year: even years in one group; odd years in the other |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation because method is based on year of birth. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report all surgeries were performed by 'staff surgeons' but it is not reported whether surgeons were equally experienced in both techniques. |
| Other performance bias: surgeon experience of both implants | Low risk | All operations by staff surgeons. We assumed that they were equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Dall'Oca 2010.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: Gamma nail (cephalic screw cement augmentation technique) versus Gamma nail (conventional technique) |
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: unstable trochanteric fracture, defined as fracture with 3 parts or more; > 80 years of age; Singh score of 1 or 2 Exclusion criteria: dementia, neuromuscular or musculoskeletal deficiency which could limit the ability to perform objective functional tests; malignancy; massive corticosteroid treatment; concurrent other fractures or operations which could affect postoperative functional outcome Setting: single centre; hospital; Italy Baseline characteristics Intervention group 1 (cement augmentation)
Intervention group 2 (conventional)
Overall:
Note:
|
|
| Interventions |
General details: participants were followed up at 6 months and 12 months; the day after surgical operation, participants, according to their clinical conditions, started passive and active physiotherapy allowing free weight bearing of the operated limb Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional status (HHS); mortality (available at 12 months); blood loss; operation time; mean hospital stay; TAD; mean sliding distance of screw; screw displacement; fracture; non‐unions; infections Outcomes relevant to the review: mortality (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: 'the author did not receive any outside funding or grants in support of their research for or preparation of this work. Neither they nor any immediate member of their families received payments or other benefits or commitment or agreement to provide such benefits from commercial entity' Study dates: January 2006 to March 2010 Note:
|
|
Dalsgaard 1986.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: Ender versus SSP |
|
| Participants |
Total number of randomised participants: 101 Inclusion criteria: pertrochanteric fractures Exclusion criteria: not reported Setting: single centre, hospital, Denmark Baseline characteristics Intervention group 1 (Ender)
Intervention group 2 (SSP)
Note:
|
|
| Interventions |
General details: prophylactic antibiotics were not used. 30% of participants were operated on by senior house officers, and remaining were operated on by consultants and senior registrars; regular clinical follow‐up for minimum of 6 months Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: unplanned return to theatre (available at six months); operations time; distal slipping; deep infection; shortening of the leg; walking ability Outcomes relevant to the review: unplanned return to theatre (reported at 6 months); mortality (6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: no details on funding or conflict of interest are provided Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised using date of birth |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors do not report the experience and skills of the surgeons and we could not be certain whether surgeons were equally experienced with both types of implants. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses are reported. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Davis 1988.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Küntscher‐Y nail versus SHS |
|
| Participants |
Total number of randomised participants: 230 Inclusion criteria: intertrochanteric proximal femoral fractures; fit for surgery Exclusion criteria: < 50 years of age; pathological and Paget's fractures Setting: 2 orthopaedic hospitals, UK Baseline characteristics Intervention group 1 (nail)
Intervention group 2 (SHS)
Overall:
Note:
|
|
| Interventions |
General details: general or spinal anaesthetic; prophylactic antibiotics image intensification; weight bearing encouraged after 48 hours; clinical follow‐up at 6 weeks, 3, 6 and 12 months; operations performed by consultants or trainees Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: LOS; LOS and convalescence; mortality (1 month and 6 months); radiographic healing time; time to weight bearing; Salvati and Wilson score; functional deficit; power and motion at hip; knee mobility; time till painless mobilisation and failure to regain pre‐fracture mobility; complications: infection, UTI, chest infection, venous thromboembolic phenomena; implant failure; cut out; LOS (not reported by group); Mental Test Score Outcomes relevant to the review: mortality (12 months); unplanned return to theatre Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: funded by the Northern Regional Health Authority Study dates: June 1983 to May 1985 Notes:
|
|
De Grave 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma 3 nail versus ACE trochanteric nail |
|
| Participants |
Total number of randomised participants: 112 Inclusion criteria: pertrochanteric femoral fracture resulting from a low‐energy fall Exclusion criteria: pathological fractures; multiple injuries; high likelihood of loss to follow‐up Setting: single centre; hospital; Belgium Baseline characteristics Intervention group 1 (Gamma 3)
Intervention group 2 (ACE)
Overall:
Note:
|
|
| Interventions |
General details: a senior orthopaedic resident performed the operations; all participants were given one dose of cefuroxime before the operation and low‐molecular‐weight heparin for 4 weeks post surgery; they were mobilised with full weight bearing as tolerated; regular radiographic and clinical examinations were performed in all participants between 6 weeks and 1 year postoperatively Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: non‐union; DVT; peripheral nerve injury; failure of internal fixation; wound infection; implant failure; mortality (available at 12 months); Merle d'Aubigné hip score Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no benefits or funds were received in support of this study Study dates: August 2006 to July 2009 Note:
|
|
Delgado 1990.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Richards hip compression plate with and without Universal Angle Guide |
|
| Participants |
Total number of randomised participants: 20 Inclusion criteria: intertrochanteric fractures Exclusion criteria: not reported Setting: single centre; hospital; Mexico Baseline characteristics Intervention group 1 (using Universal Angle Guide)
Intervention group 2 (conventional method)
Note:
|
|
| Interventions |
General details: all participants given prophylactic antibiotics Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: fracture angle, complications, duration of surgery, mortality Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: December 1988 to May 1990 Note:
|
|
Desteli 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus cementless bipolar hemiarthroplasty |
|
| Participants |
Total number of randomised participants: 92 Inclusion criteria: isolated, unstable intertrochanteric fractures; > 60 years of age Exclusion criteria: pathological fractures due to primary and metastatic tumours; fractures associated with polytrauma; people with psychiatric or neurologic disorders; accompanying major systemic diseases; fracture type 31A1.1 and 31A1.2 Setting: single centre; hospital; Turkey Baseline characteristics (only for participants that did not die) Intervention group 1 (PFN)
Intervention group 2 (HA)
Overall:
Note:
|
|
| Interventions |
General details: all of the participants in each treatment arm received similar operative treatment and postoperative care from the same medical staff; follow‐up visits were performed at the 3rd, 12th and 24th months postoperatively; participants were treated with either regional anaesthesia or general anaesthesia Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: social function score, mobility score, HRQoL (available at 3 months, 12 months and 24 months); fracture union; operation time; time in hospital Outcomes relevant to the review: HRQoL (using EQ‐5D, at ≤ 4 months and 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 2009 to 2013 Note:
|
|
Dhamangaonkar 2013.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: proximal femoral locking plate versus DHS |
|
| Participants |
Total number of randomised participants: 44 Inclusion criteria: unstable intertrochanteric femoral fractures Exclusion criteria: children and people with compound fractures and associated head, chest or abdominal injuries were excluded Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (proximal femoral locking plate)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: antibiotics and analgesics were given according to hospital protocol; participants were allowed to perform quadriceps‐strengthening exercises the next day; mobilisation with no weight bearing was allowed, followed by partial weight bearing on crutches or with walking frame after 6 to 8 weeks; follow‐up at 2 weeks, 6 weeks and thereafter monthly for 18 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: blood loss; mean hospital stay; mean time to union; mean neck shaft angle; mean limb shortening; mean normal‐side neck shaft angles; medialisation of the shaft; varus collapse; functional hip score; deep wound infection; implant cut‐out Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: study authors declared no conflicts of interest. No details of funding Study dates: not reported Note:
|
|
Dong 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus Gamma nail |
|
| Participants |
Total number of randomised participants: 178 Inclusion criteria: ≥ 60 years of age; have self‐care ability prior to injury; femoral intertrochanteric fracture; eligible for surgery Exclusion criteria: pathological fractures; multiple injuries; those who had received another operation on affected side Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (Gamma)
Note:
|
|
| Interventions |
General details: participants underwent continuous epidural anaesthesia, in supine position, in traction bed before operation Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss; length of stay; postoperative haemoglobin decline values; rotation of caput femoris; amount of fixing needle slippage; TAD; collodiaphyseal angle; time to discharge; healing time; infections; femoral ruptures; hip varus; nonunion; dysfunction; pain; functional status (HHS) Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2015 to April 2016 Note:
|
|
Dujardin 2001.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: mini‐invasive static intramedullary nail versus SHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: intertrochanteric proximal femoral fracture (stable and unstable fractures), informed consent, ≥ 60 years of age; surgery within first 2 days after fracture Exclusion criteria: pathological; lower limb arteriopathy; fractures extending to the diaphysis; previous lesions of the hip; cutaneous lesions; abnormal calcium or phosphorus metabolism and no consent Setting: single centre; orthopaedic hospital; France Baseline characteristics Intervention group 1 (intramedullary nail)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: traction table; 6 week thromboembolic prophylaxis with low‐molecular‐weight heparin; postoperative care identical in both groups; weight bearing authorised when no pain existed; all operations were undertaken by 2 surgeons with experience of the surgical technique; 1 surgeon did all the SHS operations and the other did all the nail operations; both described as a senior surgeon; type of anaesthesia is at the discretion of attending anaesthetist Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; mean units blood transfused; radiographic screening time; non‐union; time to union; early postoperative complications (infection, thromboembolism, further operation); pneumonia; pressure sores; all medical complications; LOS; varus deformity (reported for the nail group); angular restoration; mortality; various aspects of hip function, including pain, power and mobility, were measured using the Salvati and Wilson score; pain; time to effective weight bearing; hip function; knee mobility; length of follow‐up: 6 months Outcomes relevant to the review: mortality (1 and 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: no external funding Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | All operations were undertaken by two surgeons with experience of the surgical technique; one surgeon did all the SHS operations and the other did all the nail operations. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Eceviz 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: cephalomedullary nail vs SHS |
|
| Participants |
Total number of randomised participants: 64 Inclusion criteria: basicervical fracture, ≥ 65 years of age, isolated fracture, ability to walk independently (with or without an aid) before fracture, fracture that had occurred < 1 week prior to admission Exclusion criteria: history of ipsilateral femoral fracture, fracture due to malignancy, limited life expectancy due to medical comorbidities, any contraindication to surgery, diagnosed dementia, any other traumatic fracture Setting: tertiary hospital; single centre; Turkey Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: 2 senior surgeons (> 10 years of surgical experience in treating basicervical fractures and familiar with both surgical techniques); closed reduction under fluoroscopic guidance on a traction table; postoperatively, all participants were allowed immediate weight bearing as tolerated, regardless of the method of fixation; clinical follow‐up at 6 weeks, 3 months, 6 months, and 12 months Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mobility score (0 to 9), HHS; ADL (using modified Barthel Index, range 0 to 100); tip apex distance and fracture settling, quality of reduction; mortality; revision surgery; wound infections Outcomes relevant to the review: mortality (12 months); unplanned return to theatre (revision surgery; at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: no funding; study authors declare no conflicts of interest Study dates: January 2016 to January 2018 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly allocated to a study group by permuted blocks of randomly mixed sizes and stratification according to the type of surgery (CMN or SHS)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was applied using pre‐prepared randomisation cards, which were placed in opaque, sealed envelopes and given to the surgeons to open just prior to surgery, and the designated procedure was then performed". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Surgeons were experienced with both implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | Low risk | Quote: "The clinical follow‐up evaluations were performed by two independent orthopaedic surgeons who had access to all the patients’ files and documents. They were also blinded to the preceded treatment." Comment: participants were assigned a four‐digit number to conceal their identity and the radiographs were kept in digital folders. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were balanced and mostly explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors reported registration with a clinical trials register (NCT04240743); however, this registration was made after completion of the study (in January 2020) and it was not feasible to effectively assess risk of selective reporting bias from these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Efstathopoulos 2007.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma trochanteric nail versus ACE trochanteric nail |
|
| Participants |
Total number of randomised participants: 112 Inclusion criteria: intertrochanteric hip fracture Exclusion criteria: < 65 years of age; pathological fractures; non‐ambulatory people; ASA score V; previous ipsilateral or contralateral hip fractures Setting: single centre; hospital; Greece Baseline characteristics Intervention group 1 (Gamma)
Intervention group 2 (ACE)
Overall:
Note:
|
|
| Interventions |
General details: operations were performed by 2 experienced surgeons; type of anaesthesia was spinal for most participants; postoperatively, all participants were given prophylactic antibiotics for 24 to 48 hours and DVT prophylactics (low‐molecular‐weight heparin) for 6 weeks; participants were mobilised on the first postoperative day, regardless of fracture pattern, and full weight bearing was permitted as tolerated; regular radiological and clinical examinations were performed in all participants 1,3 and 6 months postoperatively Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood transfusion; fluoroscopy time; complications (implant failures, DVT, superficial wound infection, general complications); mobility status; mortality (available at first week) Outcomes relevant to the review: mortality (reported in first week) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Ekstrom 2007.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus the Medoff sliding plate |
|
| Participants |
Total number of randomised participants: 210 Inclusion criteria: unstable trochanteric fracture classified 3‐5 (Jensen 1981); AO/OTA: 31 A2.1‐3 and A3.1‐3; subtrochanteric fracture classified as AO/OTA: 32 A1.1 and B1.1 (Seinsheimer 1978); adults with a closed growth plate and an unstable trochanteric fracture or a subtrochanteric fracture with the most distal fracture ending < 5 cm distal to the lesser trochanter Exclusion criteria: people with stable trochanteric fractures, high‐energy trauma, pathological fractures, previous surgery to the proximal femur, daily steroids of more than 10 mg of prednisolone, ongoing chemotherapy, irradiation treatment, presence of degenerative osteoarthrosis of the injured hip Setting: two orthopaedic hospitals, Sweden Baseline characteristics (only for 203 participants) Intervention group 1 (PFN)
Intervention group 1 (Medoff sliding plate)
Note:
|
|
| Interventions |
General details: preoperative antibiotics; subcutaneous low‐molecular‐weight heparin (thromboembolic prophylaxis) for 7 days; spinal anaesthesia was used, although 13 participants had general anaesthesia and 1 participant had a combination of both; participants were mobilised according to the treatment protocol at the 2 hospitals; weight bearing as tolerated or restricted weight bearing; clinical follow‐up at 6 weeks, 4 and 12 months; operations performed by 43 different surgeons, consultants or trainees; 2 senior consultants with extensive experience with both implants gave theoretical and practical instructions before start of study Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; radiographic screening time; cut‐out of implant; non‐union; operative fracture of the femur; later fracture of the femur; other fracture healing complications; re‐operation; wound infection; wound haematoma; LOS; mortality; failure to return to pre‐fracture residential status; pain; inability to walk 15 metres; inability to rise from the chair; inability to climb a curb; need to use walking aids; abductor strength Outcomes relevant to the review: mortality (12 months); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "based on a computer generated list. Randomization was stratified according to trochanteric or subtrochanteric fractures." |
| Allocation concealment (selection bias) | Low risk | Randomised "using consecutive numbered and sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Quotes: "Surgery was undertaken by 43 different surgeons employed as regular staff at the two hospital", "two senior consultations ... with extensive experience and familiar with both surgical methods, gave theoretical and practical instructions before the start of the study". Comment: we did not expect that this provided sufficient protection against performance bias. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Large loss to follow‐up at 12 months for some outcomes (and reasons are not reported by group, and explained by "general health problems and death"). However, for outcomes relevant to the review, we included most participants. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Esser 1986.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus Jewett nail plate |
|
| Participants |
Total number of randomised participants: 98 Inclusion criteria: elderly women, > 60 years of age, with trochanteric fractures; residents of North Wales Exclusion criteria: not reported Setting: single centre; hospital; Wales Baseline characteristics (overall):
Note:
|
|
| Interventions |
General details: clinical follow‐up at 6 weeks, 3 months, and 6 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; mobility; pain; complications; mortality Outcomes relevant to the review: mortality (during study period; 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: supported by a grant from Gwynedd Research Committee Study dates: November 1981 to February 1983 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to treatment groups. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors do not report the experience and skills of the surgeons and we could not be certain whether they were equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are reported only for participants that were treated with the interventions. However, the number of losses are few. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Fitzpatrick 2011.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus Dynamic Helical Hip System |
|
| Participants |
Total number of randomised participants: 51 Inclusion criteria: > 50 years of age; intertrochanteric hip fractures (AO/OTA classification 31A1, 31A2); admitted to study hospitals Exclusion criteria: reverse obliquity fractures; basicervical fractures; fractures extending into the subtrochanteric region; pathological fractures; previous ipsilateral hip surgery; multiple injuries; non‐ambulators prior to injury; dementia severe enough to limit ability to comply with postoperative rehabilitation Setting: multicentre (2 sites: level 2 trauma centre, and level 3 trauma centre); hospital; USA Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (Dynamic Helical Hip System)
Note:
|
|
| Interventions |
General details: sessions were held to make sure all surgeons were experienced with both techniques, and personnel were encouraged to use the Dynamic Helical Hip System exclusively for 3 months during the run‐up to the study; antibiotic prophylaxis was provided for 24 hours; spinal or general anaesthesia at the discretion of attending anaesthetist; in absence of bleeding concerns, participants were placed on low‐molecular‐weight heparin beginning postoperative day one, which continued 2 weeks postoperatively, at which time participants were switched to aspirin; compression hose and compression devices were used routinely while participants were in the hospital; all participants were allowed weight bearing as tolerated with physical therapy beginning on first postoperative day; participants were discharged to a skilled nursing facility or home when medically stable; clinical follow‐up at 2 weeks, 6 weeks, and then at 6‐weekly intervals until healing occurred Intervention group 1:
Intervention group 2:
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mean amount of sliding; TAD; screw‐tip location; quality of fracture reduction; cut‐out Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Fritz 1999.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: gliding nail versus Gamma nail Note:
|
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: unstable trochanteric fractures (A2, A3) Exclusion criteria: intracapsular femoral neck fractures; pathological fractures as well as a concomitant severe coxarthrosis Setting: single centre; hospital; Germany Baseline characteristics Intervention group 1 (gliding)
Intervention group 2 (Gamma)
Note:
|
|
| Interventions |
General details: 6 surgeons with sufficient traumatological experience with the fixation systems did the operation; if one of the 7 surgeons‐in‐training carried out the operation, this was done only under the supervision of a specialist; all participants had either spinal or general anaesthesia used; follow‐up was done at 6 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss; hospital stay; discharge destination; dislocation; distraction of fraction; CCD angle difference; score; intraoperative complications; postoperative complications (local and general); mortality (available before discharge and 6 months); general complications (cerebrovascular accident, bronchopneumonia, apoplexy, UTI, decubitus, fracture of forearm); mobility; pain; lengthening of length; internal malrotation; external malrotation Outcomes relevant to the review: mortality (reported before discharge and 6 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: December 1995 to December 1996 Note:
|
|
Galanopoulos 2018.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: short intramedullary nail versus long intramedullary nail |
|
| Participants |
Total number of randomised participants: 50 Inclusion criteria: low‐energy unstable (AO/OTA 31A2 and 31A3) pertrochanteric hip fractures Exclusion criteria: unstable pertrochanteric pathological fractures; previous pertrochanteric fractures of the contralateral hip Setting: single centre; hospital; Greece Baseline characteristics Intervention group 1 (short)
Intervention group 2 (long)
Overall:
Note:
|
|
| Interventions |
General details: all participants were operated under spinal anaesthesia in a supine position on a fracture table; all participants were mobilised in the sitting position on POD2, and allowed protective, partial (approx. 30% of body weight) weight bearing with a walker for 1 month, allowing weight bearing with the walker or canes as tolerated, thereafter; routine postoperative clinical and radiographic examination was done at 1, 3, 6 and 12 months and then annually until the last follow‐up Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: time for weight bearing with the use of a single crutch or cane; leg length discrepancy; Trendelenburg gait; fracture healing; varus/valgus loss of reduction on anteroposterior radiographs; distance between the distal line of the fracture and the distal locking screw of the nail; operation time; complications (periprosthetic fracture, z‐effect phenomenon) Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: authors declare that they have no conflicts of interest in relation to this article Study dates: 2012 to 2016 Note:
|
|
Giraud 2005.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Targon PFN versus DHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: intertrochanteric proximal femoral fracture (stable and unstable fractures: AO 31‐A1, A2 and A3) Exclusion criteria: not reported Setting: single centre; orthopaedic hospital; France Baseline characteristics Intervention group 1 (Targon PFN)
Intervention group 2 (DHS)
Overall:
Note:
|
|
| Interventions |
General details: experience of surgeons is unknown Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; cut‐out of implant; later fracture of the femur; re‐operation; wound infection (none); pneumonia (pulmonary congestion: "Pulmonaire"); DVT; LOS; mortality; time to walking; HHS; length of follow‐up: 3 months Outcomes relevant to the review: mortality (at 3 months); unplanned return to theatre (due to cut‐out) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: December 2003 and June 2004 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses explained by death, which is expected in this population |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Goldhagen 1994.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: Gamma intramedullary nail versus CHS |
|
| Participants |
Total number of randomised participants: 75 Inclusion criteria: adults; trochanteric and subtrochanteric proximal femoral fractures; fracture amenable to being treated with Gamma nail or CHS Exclusion criteria: previous ipsilateral hip fracture, hip surgery or congenital abnormality Setting: single centre; orthopaedic hospital, USA Baseline characteristics (overall)
Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (CHS)
Notes:
|
|
| Interventions |
General details: prophylactic antibiotics and DVT; physical therapy commenced on the first or second POD; weight bearing as tolerated; clinical follow‐up minimum of 6 months; experience of surgeons is not reported Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; radiographic screening time; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union; re‐operation; LOS; mortality; pain at follow‐up; non‐return to previous residence; impaired walking Outcomes relevant to the review: mortality (6 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 1990 to January 1991 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: " ..fractures ..were prospectively randomized into two groups according to their medical record number." |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation because of methods used to allocate participants to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Study authors refer to "a significant learning curve for the GN [Gamma nail]", and a "multiplicity of operating surgeons". We expected that surgeons were not all equally experienced with each implant. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were due to death, which is expected in this population. Although we noted some small discrepancies in denominators in some outcomes, we did not expect these to influence data. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Gou 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus PCCP |
|
| Participants |
Total number of randomised participants: 90 Inclusion criteria: > 60 years old; intertrochanteric fractures (type 31‐A1 and 31‐A2 based on OTA classification); Evans stable and unstable; ASA status score of I to IV Exclusion criteria: subtrochanteric fractures (type 31‐A3 in OTA classification); ASA V; existing or previous fractures in the same or contralateral hip; injuries that could affect the outcome measures; abnormalities that could affect the outcome measures Setting: single centre; orthopaedic hospital, China Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (PCCP)
Note:
|
|
| Interventions |
General details: performed according to the standard protocols provided by the manufacturer; inserted using a percutaneous technique; regional anaesthesia; preoperative antibiotics; traction table; prophylactic antibiotics for 3 days; exercise from first POD; walking with weight bearing as soon as possible; clinical follow‐up at 3, 6, 9, and 12 months; surgical experience: "all operations were performed by expert surgeons who had equal levels of experience with both the PCCP and PFNA" Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; intraoperative blood loss; perioperative blood loss; LOS; mortality; hip pain; OHS; HHS; mobility; cardiac failure; pneumonia; UTI; DVT; postoperative fracture; superficial infection; cerebral infarction; urosepsis; haematoma; fat embolism syndrome Outcomes relevant to the review: mortality |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported; authors state that no conflicts exist Study dates: January 2008 and October 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "using a sealed‐envelope system” Comment: study authors do not report if envelopes are opaque and sequentially‐numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | “All operations were performed by expert surgeons who had equal levels of experience with both the PCCP and PFNA” |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Griffin 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: X‐bolt dynamic hip plating system versus SHS |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: ≥ 60 years of age, with an AO/OTA type A2 and A3 fracture of the proximal femur; including those with cognitive impairment Exclusion criteria: treated non‐operatively Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (X‐bolt)
Intervention group 2 (SHS)
|
|
| Interventions |
General details: all participants received perioperative antibiotics; surgery on fracture table, closed reduction when possible; performed or supervised by an appropriately trained surgeon; length of plate and supplementary fixation at the discretion of the attending surgeon; clinical assessment at 4, 16 and 52 weeks Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: EQ‐5D‐3L; OHS; ICECAP‐O; mortality; revision; length of hospital stay Outcomes relevant to the review: HRQOL (EQ‐5D‐3L); mortality; unplanned return to theatre. All at 12 months |
|
| Notes |
Funding/sponsor/declarations of interest: funded by X‐Bolt Orthopaedics; study authors report that "benefits have been or will be directed to a research fund, foundation, educational institution, or other nonprofit organisation with which one or more of the authors are associated" Study dates: February 2013 to June 2014 Note:
|
|
Griffin 2021.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: X‐Bolt Dynamic Plating System versus SHS |
|
| Participants |
Total number of randomised participants: 1128 Inclusion criteria: trochanteric fracture of the hip (determined with AO/OTA classification) Exclusion criteria: < 60 years of age; subtrochanteric fractures; people who were managed non‐operatively Setting: 10 hospitals; multicentre; UK Baseline characteristics Intervention group 1 (X‐Bolt)
Intervention group 2 (SHS)
Overall:
Note:
|
|
| Interventions |
General details: routine investigations, anaesthetic management, antibiotic and venous thromboprophylaxis used according to local policy; internal fixation followed manufacturer guidelines; positioning of participant, surgical approach and wound closure at discretion of attending surgeon; mobilisation of participants through early, active, full weight bearing; follow‐up was at 4 months and 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HRQoL (available at 4 months and 12 months); mortality (available at 12 months); functional status; residential status; re‐operations (available at 12 months) Outcomes relevant to the review: HRQoL (using EQ‐5D‐5L, reported at 4 months and 12 months); mortality (available at 12 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report that "although none of the authors has received or will receive benefits for personal or professional use from commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non‐profit organisation with which one or more of the authors are associated. The study was sponsored by the University of Oxford. The study was funded by X‐Bolt Ltd and was supported by the National Institute for Health Research Oxford Biomedical Research Centre. All decisions relating to the design, conduct, analysis, write‐up, and publication of research are independent of each of these funders". Study dates: June 2016 to April 2018 Note:
|
|
Guerra 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus DHS |
|
| Participants |
Total number of randomised participants: 31 Inclusion criteria: > 65 years old; intertrochanteric fracture of the femur (AO classification 31 A1 or 31 A2) Exclusion criteria: compound femoral fracture; contraindications to surgery; non ambulatory before the presenting injury or presence of any other fractures Setting: single centre; orthopaedic hospital, Brazil Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: clinical follow‐up at 3, 6 and 12 months; experience of surgeon is not reported Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: FRS questionnaire (available at 3 months, 6 months, and 12 months); ASA status; mortality Outcomes relevant to the review: mortality (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors declare that no funding was received and that they have no conflicts of interest Study dates: from October 2007; no end date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Random selection from a box containing 20 envelopes. (10 DHS and 10 PFN)” Comment: envelopes replaced following selection |
| Allocation concealment (selection bias) | Unclear risk | Study authors do not report if envelopes are sealed, opaque and sequentially‐numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses are explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Guo 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS (conventional) versus DHS with lesser trochanteric reduction fixation system |
|
| Participants |
Total number of randomised participants: 66 Inclusion criteria: intertrochanteric fractures of Evans type III Exclusion criteria: unknown Setting: single centre; hospital; China Baseline characteristics unknown |
|
| Interventions |
General details: unknown Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; intraoperative blood loss; neck‐shaft angle; bone healing time; ratio of successful fixations; functional evaluation of hip joint after operation Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: NR Study dates: January 2010 to July 2012 Note:
|
|
Guyer 1991.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: Gamma intramedullary nail versus DHS |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: trochanteric and subtrochanteric proximal femoral fractures. Classification Evans modified by Jensen (stable and unstable) Exclusion criteria: not reported Setting: single centre; orthopaedic hospital, Switzerland Baseline characteristics (overall)
Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: surgeons inexperienced with both devices; surgery within 24 hours; prophylactic antibiotics and low‐dose heparin; mobilisation as tolerated within 3 days of surgery Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes measured/reported by study authors: length of surgery; blood loss; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union; re‐operation; deep wound infection; wound haematoma; LOS; shortening of leg (> 1 cm); mortality (available at 3 days, 30 days, and 3 months); pain at follow‐up (pain on walking); place of residence at 3 months; mobility (impaired walking and categorical data according to walking aids) Outcomes relevant to the review: mortality (3 months); unplanned return to theatre (3 months) | |
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: September 1989 to June 1990 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote (translation from German): "AO DHS and gamma nails were implanted alternatively." |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation using this method of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. Surgeons, care personnel and participants not blinded |
| Other performance bias: surgeon experience of both implants | High risk | Study authors describe surgeons as inexperienced with the implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses are explained by death which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Haddon 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: SHS with trochanteric support plates (TSP) versus SHS without trochanteric support plates |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: low‐energy, unstable, trochanteric fractures (Evans Jensen type 3 to 5); in later stages of the study (from 2011 onwards), also included people with cognitive impairment (previously excluded) Exclusion criteria: reverse oblique fractures; subtrochanteric fractures Setting: single centre; hospital; Norway Baseline characteristics Intervention group 1 (with TSP)
Intervention group 2 (no TSP)
Note:
|
|
| Interventions |
General details: front and lateral standard radiographs were obtained pre‐ and immediately postoperatively and new radiographs were obtained at 12 month follow‐up Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: place of residence; modified Merle d'Aubigné‐Postel score; postoperative radiograph; healed fracture; fixation failure (including those needing re‐operation); mortality (available at 12 months); pain; deep infections; DVT; stroke; pulmonary embolism; ileus; amputation Outcomes relevant to the review: mortality reported at 12 months; unplanned return to theatre because of fixation failure Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report "this study was funded solely by our employer, St.Olavs University Hospital" and declare no conflicts of interest. Study dates: 2008 to 2013 Note:
|
|
Han 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail vs Proximal femoral locking plate |
|
| Participants |
Total number of randomised participants: 83 Inclusion criteria: > 60 years of age; Jenson type II and above classification of fracture Exclusion criteria: ASA grade IV and V; unable to tolerate anaesthesia Setting: single centre; orthopaedic hospital; China Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: no mention of surgical experience Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of operation; intraoperative bleeding; haemoglobin reduction on POD 2; fracture healing ‐ local pain and percussion pain as a marker of healing; fracture healing ‐ radiographic parameters; functional recovery ‐ Parker and Palmer mobility score Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: unknown Study dates: June 2008 to June 2010 Note:
|
|
Haq 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus reverse distal femoral locking plate |
|
| Participants |
Total number of randomised participants: 40 Inclusion criteria: unstable intertrochanteric fracture with compromised lateral wall (AO 31A 2.2 to 3.3); surgery within 3 weeks Exclusion criteria: aged < 18 years; pathological fracture; multiple injuries; fractures with significant subtrochanteric extension (> 3 cm); unable or unwilling to give informed consent; unfit for surgical intervention Setting: single centre; university hospital; India Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (distal femoral locking plate)
Overall:
Note:
|
|
| Interventions |
General details: weight bearing as soon as possible; clinical follow‐up at 2 and 6 weeks, 3, 6 and 12 months; surgical experience: "the surgeons doing the procedure were adequately trained in both the procedures and had been doing it regularly before the start of the trial" Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of surgery; blood loss during surgery; fluoroscopy time; type of reduction; difficulty in reduction; surgeon's perception of surgery; position of implant; Parker Palmer mobility score; HHS (mean scores and categorical data); ADL: SF‐12 (physical and mental component scores); revision surgery; non‐union; malunion; shortening; length of follow‐up: 1 year Outcomes relevant to the review: HRQoL (SF‐12, 12 months); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: November 2011 and October 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Quotes: “opaque envelope technique”. "The envelope was opened 24 hours before surgical intervention by the treating surgeon." Comment: study authors do not report if envelopes are sealed and sequentially‐numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | “The surgeons doing the procedure were adequately trained in both the procedures and had been doing it regularly before the start of the trial". |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment: HRQoL (detection bias) | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were few and balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Hardy 1998.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: IMHS versus SHS |
|
| Participants |
Total number of randomised participants: 100 (see notes) Inclusion criteria: trochanteric proximal femoral fractures; classification according to Jensen: stable (types I & II) and unstable (types II, IV & V) Exclusion criteria: < 60 years of age; pathological fractures; incorrect anatomy; history of fracture or operation involving same limb; Paget's disease Setting: single centre; hospital; Belgium Baseline characteristics Intervention group 1 (IMHS)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: spinal or general anaesthesia; weight bearing on POD 4; clinical assessment at 1, 6 and 12 months; surgeon experience ‐ for IMHS, study report refers to prolonged learning curve required for insertion and SHS is routine; 2 senior operating surgeons, 3 junior attending surgeons Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; transfusion; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union; re‐operation; wound infection; wound haematoma; pneumonia; thromboembolic complications (DVT, PE); UTI; leg shortening; mortality; mid‐thigh pain; hip pain at follow‐up; mobility (available at 1, 3, 6, and 12 months); social function; length of follow‐up: 1 year (see notes) Outcomes relevant to the review: mortality (during hospital stay; 12 months); unplanned return to theatre (all at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: funding received from Smith and Nephew Richards Study dates: December 1993 to January 1995 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "prospectively randomised into two treatment groups according to the medical record number" |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation with this method of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Quote: "The different levels of experience of the ...operating surgeons and ... attending surgeons ..and the prolonged learning curve for insertion of intramedullary hip‐screws may have also affected the operative time." |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses were explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Hardy 2003.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: IHS statically locked versus IHS dynamically locked |
|
| Participants |
Total number of randomised participants: 81 Inclusion criteria: fracture featuring loss of the medial buttress (type IV and V of the classification of Jensen and Michaelsen) or reversed oblique fracture Exclusion criteria: not reported Setting: single centre; hospital; Belgium Baseline characteristics Intervention group 1 (IHS ‐ statically locked)
Intervention group 2 (IHS ‐ dynamically locked)
Note:
|
|
| Interventions |
General details: antibiotics (2 g cefazolin given at induction of anaesthesia) were administered to all participants, and prophylactic doses of low‐molecular‐weight heparin (calcium nadroparin, 7500 IU Anti‐Xa, Sanofi‐Winthrop, France) were given once a day for 15 days to participants who did not have a history of myocardial infarction or stroke in the previous 6 months, haemorrhage (genitourinary tract, gastrointestinal), tendency to bleed (thrombocytes < 150 X 10⋀6/mL or prothrombin time < 65%), or known allergies to low‐molecular‐weight heparin; all participants allowed to weight bear as tolerated; followed up after 1 month, 3 months, 6 months, 1 year, 2 years and 3 years; in IHS static group, 23 doctors were junior and 16 were senior and in the IHS dynamic group, 28 doctors were junior and 13 were senior Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; intraoperative blood loss; postoperative drainage; level of haemoglobin; transfused packed blood cells; mode of reduction; tip‐apex distance; discharge destination; mortality (available at 3 months, 6 months, 1 year and 2 years) Outcomes relevant to the review: mortality (3 months, 1 year) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Harrington 2002.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: IMHS versus SHS |
|
| Participants |
Total number of randomised participants: 102 Inclusion criteria: unstable trochanteric proximal femoral fractures; > 65 years of age; Evans classification III, IV and V Exclusion criteria: pathological fractures; previous fracture; other fracture; dementia meaning inability to consent Setting: single centre; orthopaedic hospital, UK Baseline characteristics Intervention group 1 (IMHS)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: no details on prophylaxis or rehabilitation program; clinical follow‐up at 3, 6 and 12 months by observers blind to procedure; surgeons familiarised themselves with the IMHS prior to the study, but experience was not balanced between both implants Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; radiographic screening time; transfusion requirements; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union of fracture; other fracture healing complications; LOS; mortality; patient mobility; regain of pre‐fracture living status; length of follow‐up: 12 months Outcomes relevant to the review: mortality (during hospital stay and at 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised on admission using a sealed envelope method". Comment: study authors do not report if envelopes are opaque and sequentially‐numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Quote: "Participating surgeons were required to familiarise themselves with the intramedullary implant and its insertion in supervised bone model sessions prior to using it in the clinical setting". Comment: we considered this insufficient for the purposes of the trial. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Haynes 1996.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus DHS |
|
| Participants |
Total number of randomised participants: 50 Inclusion criteria: trochanteric or 'high' subtrochanteric proximal femoral fractures Exclusion criteria: previous non‐consolidated femur fracture Setting: single centre; orthopaedic hospital, UK Baseline characteristics (overall)
Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: manufacturer's recommended procedures; mobilised as quickly as possible. For experience of surgeon: DHS commonly used but a minimum of 5 Gamma nails were used by each surgeon before any cases were included in the trial (also see note about unfamiliarity of the surgeons as a reason for exclusion) Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes | Outcomes measured/reported by study authors: length of surgery; blood loss; operative fracture of femur; cut‐out; non‐union; re‐operation; wound infection; pneumonia; pressure sore; wound haematoma; DVT; PE; LOS; shortening of leg; mortality; pain at follow‐up; place of residence (6 months after surgery); impaired walking Outcomes relevant to the review: mortality (6 months); unplanned return to theatre | |
| Notes |
Funding/sponsor/declarations of interest: sponsored and part administered by Howmedica Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Use of "randomisation cards". However, the imbalance in numbers was explained by the unfamiliarity of surgeons with Gamma nail treatment. Quote: "This resulted in a temptation to omit the patient from the trial if a Gamma nail was drawn as treatment, from the randomisation cards". |
| Allocation concealment (selection bias) | Low risk | We presumed from the information regarding selection of participants, that allocation on the randomisation cards was adequately concealed with decisions made by surgeons after selection of a card. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Surgical procedures were as recommended by the implant manufacturers, and a "minimum of 5 Gamma nails were then inserted by each surgeon before any cases were included in the trial". Comment: SHS was used routinely. However, mention of unfamiliarity of the surgeons (various) with the treatment was a putative reason for post‐randomisation exclusion and we therefore assumed that not all surgeons were sufficiently experienced with the Gamma nails. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses were explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Herrera 2002.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus PFN |
|
| Participants |
Total number of randomised participants: 250 Inclusion criteria: trochanteric fractures Exclusion criteria: not reported Setting: single centre; hospital; Spain Baseline characteristics Intervention group 1 (Gamma)
Intervention group 2 (PFN)
Overall:
Note:
|
|
| Interventions |
General details: follow‐up was done at 1, 3, 6 and 12 months; general anaesthesia was administered to 191 participants and intradural in the remainder; attempts were systematically made to have all participants adopt a sitting position within the first 48 hours after surgery; participants were encouraged to try weight bearing with the aid of crutches or a frame during the first postoperative week Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of operation; average haematocrit; transfusion; average healing time; fracture reduction; complications; re‐operations (available at 12 months); average hospitalisation time; mortality (available at 12 months) Outcomes relevant to the review: unplanned return to theatre (reported at 12 months); mortality (available at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 1997 to 2000 Note:
|
|
Hoffman 1996.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus SHS (AMBI hip screw) |
|
| Participants |
Total number of randomised participants: 69 (2 died prior to surgery and were not reported in the numbers randomised to each group) Inclusion criteria: trochanteric proximal femoral fractures. Jensen types 1 to 5; stable and unstable based on Evans; > 50 years of age Exclusion criteria: pathological fractures Setting: single centre; orthopaedic hospital; New Zealand Baseline characteristics Intervention group 1 (Gamma nails)
Intervention group 2 (SHS)
Overall
Note:
|
|
| Interventions |
General details: prophylactic antibiotics; general anaesthesia (50 participants), spinal anaesthesia (17 participants); closed reduction; image intensifier; manufacturer's guidelines followed for each device; mobilised with weight bearing as soon as possible; clinical assessment at 6 and 12 weeks and 6 months; surgeons did not have comparable experience with implants (longer learning curve with Gamma nail than with SHS; 4 orthopaedic trainees, normal supervision) Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; radiographic screening time; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union (time to union); re‐operation; wound infection; pneumonia; pressure sores; DVT; any medical complication; LOS; shortening of leg; mortality; pain at follow‐up (unresolved pain in patients with intertrochanteric fractures); non return to previous residence; participant mobility; length of follow‐up: 6 months Outcomes relevant to the review: mortality (in hospital and during follow‐up); unplanned return to theatre (6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated blocked randomization" |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment selections ... were sealed into opaque numbered envelopes that also contained a stiff card to further prevent disclosure of allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Quotes: most operations carried out by "one of four orthopaedic trainees ... supervised as appropriate.." and "longer learning curve for the Gamma nail may be the reason for the differences noted." |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Hoffmann 1999.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: IMHS versus SHS |
|
| Participants |
Total number of randomised participants: 110 Inclusion criteria: pertrochanteric proximal femoral fractures. Classification based on Evans‐Jensen: all 5 categories: stable and unstable fractures. Also AO 31 A1, A2 and A3 (just 2 fractures) Exclusion criteria: pathological fractures, old fractures, bedridden patients, polytrauma Setting: single centre; orthopaedic hospital, Germany Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: surgeons were not experienced (operations by junior and senior staff); surgery within 24 hours of admission; prophylactic antibiotics; postoperative thromboembolics with heparin Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of anaesthesia; length of surgery; operative blood loss; difference in haemoglobin; radiographic screening time; operative fracture of the femur; later fracture of the femur; loss of fracture reduction requiring re‐operation; re‐operation; wound infection; deep wound infection; wound haematoma; thromboembolic complication; clinical complications; LOS (acute); shortening of leg (> 1 cm); rotational deformity ('relevant'); mortality; pain (on walking); return to pre‐fracture residential status; impaired walking; Merle d'Aubigné hip score; length of follow‐up: mean 3.7 months Outcomes relevant to the review: mortality (3 to 4 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 1994 to 1996 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes, but no additional details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Involved both senior and junior surgeons ‐ tendency for more senior surgeons for the nail operations, and we could not be certain whether experience in both devices was equivalent |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Hogh 1981.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Ender versus McLaughlin |
|
| Participants |
Total number of randomised participants: 145 Inclusion criteria: trochanteric fractures Exclusion criteria: not reported Setting: single centre, hospital, Denmark Baseline characteristics Intervention group 1 (Ender)
Intervention group 2 (McLaughlin)
Overall
Note:
|
|
| Interventions |
General details: heparin given twice a day, until participant was mobilised. Use of traction table. Programme of exercises on POD 1. Walk with weight bearing and crutches on POD7. Clinical follow‐up at 1, 3, 6 and 12 months Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: anaesthesia time; operation time; duration of hospitalisation; mortality (available at 1 month, 6 months and 12 months); unplanned return to theatre; complications; deep infection Outcomes relevant to the review: mortality (reported at 1 month; 12 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: April 1976 to September 1978 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "on the principle of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Surgeon experience is not reported. It is unclear if surgeons were equally experienced with both implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Hong 2011.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus DHS |
|
| Participants |
Total number of randomised participants: 20 Inclusion criteria: > 60 years of age; intertrochanteric fracture; AO classification A1 or A2; surgery within 2 weeks of fracture; no prior disease that could affect serum markers Exclusion criteria: pathologic fracture; multi trauma or open fractures; drug or alcohol abuse; non‐ambulatory status; surgery beyond 2 weeks after trauma Setting: single centre; hospital; South Korea Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (DHS)
Overall:
Note:
|
|
| Interventions |
General details: single surgeon; fracture reduction; fluoroscopic guidance; clinical follow‐up at 6 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: pre‐ and postoperative bone healing status; data related to complications; incision length; operation time (skin to skin); estimated blood loss; blood samples at screening and on the morning before surgery for creatinine kinase, c‐reactive protein and serum myoglobin; blood samples taken postoperatively in the recovery room and at 8, 16, 24, 36, 48 and 72 hours postoperatively; haemoglobin and haematocrit measured preoperatively and at 16, 36 and 72 hours postoperatively; cardiac troponin I levels taken on the morning before surgery and 16 hours postoperatively Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: authors state that no funding was received and no conflicts exist Study dates: May 2009 to October 2009 Note:
|
|
Hopp 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma 3 nail or InterTAN nail |
|
| Participants |
Total number of randomised participants: 78 Inclusion criteria: > 60 years of age; unstable intertrochanteric femur fractures as a result of low‐impact trauma Exclusion criteria: inability to walk prior to the fracture; pathological fractures; associated severe osteoarthritis of the affected hip; comorbidities that preclude surgical intervention; refusal to give informed consent for the study Setting: single centre; hospital; Germany Baseline characteristics Intervention group 1 (Gamma)
Intervention group 2 (InterTAN)
Overall:
Note:
|
|
| Interventions |
General details: all surgeries were performed by 5 consultants with experience of at least 5 procedures with both Gamma and InterTAN nails, or the resident doctors under supervision inclusive; the procedures were done under general anaesthesia and both groups received a standard dose of single‐shot cefuroxime as routine prophylaxis; all procedures were performed using a traction table and by insertion of the nail via a percutaneous approach; follow‐ups were done at 6 weeks, 12 weeks, 6 months or until union or failure of the fracture Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative complications; fracture reduction time; intraoperative blood loss; image intensifier time during surgery; pre‐/postoperative haemoglobin value; requirement for blood transfusion; postoperative time on ICU; initial postoperative pain; time to discharge; difficulty of surgery; adequacy of osteosynthesis; rating of the implant; mortality (available at 6 months); quality of reduction; lateral sliding of the lag screw; CCD angle; reduction loss; heterotopic ossification; secondary dislocation; re‐operations (available at 6 months); HHS; mobility; leg‐length discrepancy Outcomes relevant to the review: mortality (reported at 6 months); unplanned return to theatre (reported at 6 months, for secondary dislocation with or without cut‐out) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report 'no benefits or funds were received in support of this study. The authors report no conflict of interest' Study dates: October 2007 to May 2009 Note:
|
|
Hornby 1989.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus conservative (traction) treatment |
|
| Participants |
Total number of randomised participants: 106 Inclusion criteria: > 60 years of age; extracapsular fractures Exclusion criteria: lack of fitness for operation; refusal; unavailability of informed consent Setting: Single centre; hospital; UK Baseline characteristics Intervention group 1 (operative)
Intervention group 2 (traction)
Note:
|
|
| Interventions |
General details: general or spinal anaesthesia was used for the operative technique and local anaesthesia was used for the conservative technique; all but 6 operations were performed by the same surgeon Intervention group 1
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (available at 6 months); average operation time; length of hospital stay; re‐operations (available at 6 months); complications (pin track infection, pin loosening, nerve palsy, traction sores, delayed union, other complications); varus/valgus deformity; rotational misalignment; fixed flexation deformity; leg shortening; pain, leg swelling; unhealed sore Outcomes relevant to the review: mortality (reported at 6 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report 'this study was supported by a grant from the Newcastle District Research Committee. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article'. Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Study authors report 'using a system of sealed envelopes, patients within each stratum were randomly allocated for treatment by operation or traction' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to treatment groups. However, we did not expect that this would influence performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors do not report the experience and skills of the surgeons, and we could not be certain whether experience was equivalent for both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect lack of blinding to influence detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported that no participants were lost to follow‐up. Only participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Huang 2006.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: hip joint replacement versus DHS |
|
| Participants |
Total number of randomised participants: 156 Inclusion criteria: intertrochanteric fractures Exclusion criteria: no details Setting: single centre; hospital; China Baseline characteristics unknown |
|
| Interventions |
General details: unknown Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of operation; intraoperative blood loss; total volume of blood transfusion; complications; walking time; function of affected limb; HHS Outcomes relevant to the review: mortality (end of follow‐up) |
|
| Notes |
Funding/sponsor/declarations of interest: unknown Study dates: September 1999 to June 2004 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to treatment groups. However, we did not expect that this would influence performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Skills and experience of surgeons are unknown |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect lack of blinding to influence detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Unclear risk | We were unable to ascertain other risks of bias because we were only able to extract data from an English abstract. |
Irvine 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail 3 versus PFNA |
|
| Participants |
Total number of randomised participants: 151 Inclusion criteria: AO/OTA 31‐A2 fractures Exclusion criteria: not reported Setting: single centre; hospital; Austria Baseline characteristics not reported |
|
| Interventions |
General details: no details Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: Parker's ratio values; re‐operations Outcomes relevant to the review: unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Notes:
|
|
Janzing 2002.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PCCP versus DHS |
|
| Participants |
Total number of randomised participants: 115 Inclusion criteria: 31A1 or 31A2 pertrochanteric hip fractures; > 60 years of age; Exclusion criteria: severe coxarthrosis of the ipsilateral hip; multiple injuries; reverse or bifocal fractures Setting: single centre, hospital, Belgium Baseline characteristics Intervention group 1 (PCCP)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: enoxaparin postoperatively; low‐molecular‐weight heparin as prophylaxis; cefazolin was given in a single dose at induction; mobilised immediately with full weight‐bearing status Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (available at 12 months); unplanned return to theatre (available at 12 months); time in theatre; postoperative transfusion; decrease in haemoglobin level; postoperative pain; postoperative fracture; fracture impaction Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: June 1998 to May 1999 Note:
|
|
Jolly 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus cemented hemiarthroplasty |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: written informed consent; unstable intertrochanteric fractures of femur; > 75 years of age and < 85 years of age Exclusion criteria: stable intertrochanteric fractures of femur; unfit or unwilling for surgery; polytrauma; pathological fractures; compound fractures; those who dropped out later in the study Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (hemiarthroplasty)
Note:
|
|
| Interventions |
General details: study authors do not provide any of the general details; followed‐up at 3 and 6 weeks, and 3 months intervals Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operating time; blood loss; HHS; mobility score; superficial infections; deep infections; bed sores; UTI; venous thromboembolism; time to full weight bearing; lower respiratory tract infection; mortality (available at 1 month, 3 months, 6 months and 12 months); functional status (HHS); mobility (MMS) Outcomes relevant to the review: mortality (reported at 1 months and 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflicts of interest to be declared but no details on funding are reported Study dates: January 2012 to January 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation reported as being from a table of random numbers, no further details on randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation is not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Skills and experience of surgeons are not specified, and we were uncertain if surgeons were equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect lack of blinding to influence detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost, these losses were balanced between groups, and reasons for loss were clearly explained. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Juhn 1988.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: nail‐plate fixation versus Ender's nailing |
|
| Participants |
Total number of randomised participants: 201 Inclusion criteria: intertrochanteric fractures of the hip Exclusion criteria: not reported Setting: single centre; hospital; Israel Baseline characteristics Intervention group 1 (nail‐plate)
Intervention group 2 (Ender)
Overall:
Note:
|
|
| Interventions |
General details: 'the operations were performed with patients under general or regional anaesthesia (except for 5 cases in which Ender's nailing was done with the patient initially under local anaesthesia; in our opinion this is not to be recommended)'; all participants received antibiotics perioperatively; none were treated with coagulants; participants were out of their bed the day after surgery, weight bearing was allowed according to individual capacity Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: anaesthesia time; operative time; length of hospital stay; complications (backing‐out of nails; proximal penetration of nails; fracture, femoral cortex; supracondylar fracture ‐ femur; superficial wound infection; deep wound infection) Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: April 1979 to August 1983 Note:
|
|
Kammerlander 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus PFNA: with or without cement augmentation |
|
| Participants |
Total number of randomised participants: 253 (30 were later excluded due to post‐enrolment eligibility failures, 16 with augmentation, 14 without) Inclusion criteria: ≥ 75 years of age; closed trochanteric fractures (AO type 31 A2‐A3) due to low‐energy trauma; indication for fixation with a PFNA within 72 hours after admission; ability to walk independently (customary walking aids were allowed); basic knowledge of the national language; informed consent Exclusion criteria: fractures due to malignancy; additional or open fractures; polytrauma; any implant at the same hip or hemiplegia; recent history of substance abuse; active malignancy; classified according to the ASA classification as class V and VI; participation in any other clinical trial of a drug or device possibly affecting the result of the present study within the previous months; those with a legal guardian; anyone for whom surgeons decided to use a different operative technique Setting: 9 sites; hospitals; Germany, Austria, Norway, Belgium, Switzerland, Israel Baseline characteristics Intervention group 1 (with cement augmentation)
Intervention group 2 (without cement augmentation)
Overall:
Note:
|
|
| Interventions |
General details: follow‐up data were taken immediately after surgery, and at 3 months, 6 months and 12 months; full weight bearing as tolerated was allowed right after surgery; all participants were mobilised under physiotherapeutic supervision within the first 2 days after surgery, starting as soon as the participants' condition allowed it Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: TUG; mortality (available at 12 months); blade migration, joint space, blade position, tip‐apex distance, complications; Parker Mobility Score; Barthel Index; re‐operations related to the implant Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report 'the present clinical investigation was performed with the support of the AO foundation via the AOTK Trauma and the AOTrauma networks' and 'C Kammerlander declares grants and personnel fees from AOTrauma and DePuy Synthes during the conduct of the study, and personal fees from DePuy Synthes and Eli Lilly outside the submitted work. T Klopfer, A Sermon and O Bach report research support from the AO foundation via the AOTK Trauma and the AOTrauma networks during the conduct of the study. M Blauth declares grants and personal fees from DePuy Synthes and personal fees from the AO foundation outside the submitted work. R Babst, M Dietrich, F Gebhard, ES Hem, and Y Weil have nothing to disclose'. Study dates: March 2012 to July 2015 Note:
|
|
Karn 2006.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: external fixation versus SHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: trochanteric fractures Exclusion criteria: presenting more than a week after injury; pathological fractures; subtrochanteric extensions; reverse obliquity; multiple fractures; bone and joint disease that could interfere with rehabilitation Setting: hospital, single centre, Nepal Baseline characteristics Intervention group 1 (External fixation)
Intervention group 2 (SHS)
Overall:
Note:
|
|
| Interventions |
General details: gradually progressive programme of weight bearing using a walking frame, from the first day of surgery. All the participants were reviewed at 6, 12, 18 and 24 weeks. No details reported of surgeons' experience with each technique. Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional status (WOMAC, HHS); surgery time; blood loss; LOS; shortening; malrotation; knee movement; varus angulation; superficial infection; deep infection; cost of treatment Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: authors declared no conflicts of interest Study dates: not reported Note:
|
|
Kazemian 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus external fixation |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: intertrochanteric fracture from a minor trauma Exclusion criteria: reverse oblique fractures; previous hip fracture; pathological fractures; an open fracture; hard‐ or soft‐tissue infection at the fracture site; multiple fractures Setting: hospital; number of sites not reported; Iran Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (external fixation)
Overall:
Note:
|
|
| Interventions |
General details: Cephalosporin (1 g intravenously) preoperatively and every 8 hours during the first postoperative day; we noted some differences in mobilisation strategy in each group; in DHS group, 22 had spinal anaesthesia and 8 had general anaesthesia and in external fixation group, all had spinal anaesthesia; follow‐up at 14, 45, 90, 180 and 360 days
Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: cut out; radiographic union; infection; operative time; blood transfusions; LOS; walking ability; functional status (HHS, at 12 months); pain (VAS); bedsores; pneumonia; UTI; DVT; mortality Outcomes relevant to the review: mortality (postoperative, described as death unrelated to causes) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors state they received no external funding and state that they have no conflicts of interest Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly placed in two groups, by a computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. We could not be certain whether surgeons were equally experienced in using the study implants. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Skills and experience of surgeons are not reported and it is uncertain whether surgeons have comparable experience with both types of implants |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of assessors of objective measures would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report a pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Kazemian 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: external fixation versus non‐operative |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: age above 60, an AO/OTA A1 or A2 type fracture from low‐energy trauma Exclusion criteria: reverse obliquity fractures, previous hip fracture, pathological fractures, infection at the fracture site and open or multiple fractures Setting: hospital, number of sites not reported, Iran Baseline characteristics Intervention group 1 (external)
Intervention group 2 (non‐operative)
Overall:
Note:
|
|
| Interventions |
General details: Cephalosporin (1 g intravenously) preoperatively and then every eight hours for the first postoperative day; low‐molecular‐weight heparin (enoxaparin) administered to both groups for 28 days; follow‐up at 14, 45, 90, 180 and 360 days after surgery
Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mobility (independent walking); blood transfusions; pain (VAS); functional status (HHS, 12 months); range of motion; infection; bed sores; pneumonia; UTI; DVT; LOS Outcomes relevant to the review: mortality (described as unrelated reasons, at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: June 2011 and August 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized by a computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. We could not be certain whether surgeons were equally experienced in using the study implants. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Skills and experience of surgeons are not reported and it is uncertain whether surgeons have comparable experience with both types of implants |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of assessors of objective measures would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report a pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Kim 2005.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: cementless calcar‐replacement hemiarthroplasty versus PFN |
|
| Participants |
Total number of randomised participants: 58 Inclusion criteria: unstable comminuted intertrochanteric femoral fracture (AO/OTA type 31‐A2 and Evans type III or IV); ≥ 75 years of age; informed consent Exclusion criteria: AO/OTA type 31‐A1 or A3 fracture Setting: single centre; hospital; South Korea Baseline characteristics Intervention group 1 (hemiarthroplasty)
Intervention group 2 (intramedullary nail)
Note:
|
|
| Interventions |
General details: the same surgeon performed the surgery for both arms of the trial; the mean duration of follow‐up was 35 months, ranging from 24 to 58 months in hemiarthroplasty group and 24 to 57 months in intramedullary nail group; the use of prophylactic antibiotics was the same in the two groups; prophylaxis against DVT was not administered in either group, as it is customary with patients with hip fractures in Korea as a result of low incidence of this complication Intervention group 1
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS; operative time; blood loss; transfusion data; complications (respiratory complication, cardiovascular complication, UTI, DVT, neurological complication, wound complication, superficial infection, deep infection) mortality (available at hospital stay, 12 months and between 1 and 3 years); mean fluoroscopy time; non‐unions; mobility; mean hospital stay; reduction; re‐operations (available at 3 years); cutting‐out of lag screw; delayed infection; breakage of antirotatory pin; breakage of distal locking screw; dislocation Outcomes relevant to the review: mortality (hospital stay, 12 months) and unplanned return to theatre (reported at 3 years) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report 'the authors did not receive grants or outside funding in support of their research or preparation of this manuscript. They did not receive payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, educational institution, or other charitable or nonprofit organisation with which the authors are affiliated or associated' Study dates: November 1998 to May 2001 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Skills and experience of surgeons are not reported and it is uncertain whether surgeons have comparable experience with both types of implants |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported that no participants were lost to follow‐up. The only participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Kosygan 2002.
| Study characteristics | ||
| Methods | RCT, parallel groups Review comparison group: CHS versus PCCP |
|
| Participants |
Total number of randomised participants: 111 Inclusion criteria: extracapsular fractures Exclusion criteria: pathological fractures; subtrochanteric fractures Setting: hospital, single centre, UK Baseline characteristics Intervention group 1 (CHS)
Intervention group 2 (PCCP)
Note:
|
|
| Interventions |
General details: perioperative antibiotics were given and antithromboembolic stockings were worn; "three experienced surgeons who had been instructed in the use of the PCCP by its inventor before embarking on this project"; postoperative rehabilitative regime was identical for the two groups; weight bearing on first or second postoperative day; follow‐up at 6 weeks and 3 and 6 months after surgery Intervention group 1
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; blood transfusion; LOS; DVT; PE; chest infection; cardiac complication; CA; gastrointestinal bleeding; superficial and deep infection; pressure sore; cut‐out; heel raise required; mortality (available within 48 hours, 2 to 30 days, and 30 days to 6 months) Outcomes relevant to the review: mortality (with 30 days, 6 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors state no external funding was received Study dates: not reported Note:
|
|
Kouvidis 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Endovis nail ("dual lag screw cephalomedullary nail") versus DHS |
|
| Participants |
Total number of randomised participants: 165 Inclusion criteria: low‐energy intertrochanteric fractures (AO type 31‐A) Exclusion criteria: < 65 years of age; multi‐trauma patients; people with previous ipsilateral hip or femur surgery possibly affecting functional outcome; people with pathological fractures Setting: single setting; orthopaedic ward in hospital, Greece Baseline characteristics Intervention group 1 (cephalomedullary nail)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: fracture table; spinal anaesthesia; closed reduction; use of an image intensifier; small lateral approach; standard postoperative protocol; immediate passive exercises; weight bearing encourage on second day; clinical examinations at 3 weeks and 4 months. Surgical experience: most operations were carried out by orthopaedic residents under a senior surgeon's assistance. Residents had almost equal experience with both implants. Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: FRS; mortality; length of surgery; LOS; duration of fluoroscopy; number receiving blood transfusion; later fracture of the femur; cut‐out of implant; implant breakage; non‐union; re‐operation; wound infection; implant‐related complications (non‐union, cut‐out); LOS; tip‐apex distance to assess position of implants; participant mobility (90% recovery or bed‐bound or wheelchair dependent); length of follow‐up: 12 months Outcomes relevant to the review: mortality (12 months); unplanned return to theatre (12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2005 to December 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes, but study authors do not reported if envelopes are opaque and sequentially‐numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quote: “vast majority of operations in our study were performed by orthopaedic residents under a senior surgeon’s experience. The participating residents had almost equal experience in both implants. The senior surgeons had already performed more than fifteen Endovis procedures each prior to this study”. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses explained by death, which is expected in this population |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Kukla 1997.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus DHS |
|
| Participants |
Total number of randomised participants: 120 Inclusion criteria: > 60 years old; unilateral fracture (AO/ASIF 31‐A1.1 to A3.3); ambulatory prior to trauma Exclusion criteria: pathological fractures; people with multiple injuries Setting: single setting; orthopaedic hospital, Austria Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Overall
Note:
|
|
| Interventions |
General details: spinal or general anaesthesia; clinical follow‐up at 6 months. Senior surgeons experienced in both operations Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union; re‐operation; wound infection; deep wound infection; wound haematoma; pneumonia; DVT; PE; any medical complication; LOS; shortening of leg (> 2 cm); mortality; non‐return to previous residence; impaired walking; length of follow‐up: 6 months Outcomes relevant to the review: mortality (in hospital, and 6 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: authors do not report funding or conflicts of interest Study dates: August 1993 to March 1994
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from direct communication with study authors): "random permutation" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation to the 2 groups was achieved by randomized, sealed envelopes" Comment: study authors do not report whether envelopes are opaque and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Comment: "Senior surgeons who, having operated on at least 80 cases each, were experienced in the use of both devices." |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses which we did not expect to influence data |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Kumar 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus PFNA |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: independently ambulant before injury; closed unstable trochanteric fracture classified as AO A2.2‐A2.3 and A3.1‐A3.3; written and informed consent Exclusion criteria: < 50 years of age; pathological fractures of any cause other than osteoporosis; open fractures; inability to walk independently prior to injury event; neurological and psychiatric disorders that would preclude assessment (e.g. Parkinsonism, multiple sclerosis, severe depression) Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (PFNA)
Note:
|
|
| Interventions |
General details: minimum follow‐up was at 12 months and maximum follow‐up was 2 years; surgery was performed on a fracture table in supine position under regional anaesthesia (combined spinal and epidural anaesthesia) by the on‐call surgeon or under their supervision by a resident; a 3rd generation cephalosporin was used for antibiotic prophylaxis for first 24 hours only, with first dose being administered at induction of anaesthesia; participants were mobilised early as per institutional protocol with protected weight bearing (partial weight bearing) on day one after operation; soluble aspirin along with anti‐embolism stockings were given for 6 weeks as thromboprophylaxis in most cases who could be mobilised early within first 24 hours postoperatively whereas low‐molecular‐weight heparin was used if there was any delay in mobilising the participant; ambulation with weight bearing as tolerated was continued till radiological evidence of union; each participant was followed up postoperatively clinically, radiologically and functionally at 6 weeks, 3 months, 6 months and 1 year Intervention group 1
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of surgery; blood loss; intraoperative imaging requirements; postoperative complications; time in hospital; implant‐related complications; mortality (available at 12 months); HHS; use of walking aid; persistent pain; return to pre‐fracture walking status Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflicts of interest. No details of funding are reported Study dates: not reported Note:
|
|
Kuwabara 1998.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus CHS |
|
| Participants |
Total number of randomised participants: 43 Inclusion criteria: trochanteric proximal femoral fractures. Evans classification: stable, unstable and 'type 2' (1 fracture) Exclusion criteria: < 65 years of age Setting: single centre; orthopaedic hospital; Japan Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (CHS)
Note:
|
|
| Interventions |
General details: level of surgical experience is unknown Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; operative fracture of the femur; later fracture of the femur; cut‐out of implant; wound infection; inversion deformity; inversion deformity; loss in mobility and use of walking aids; length of follow‐up: mean 6 months (5.7 and 6.5 months, respectively, for the two groups) Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: no details of funding or conflicts being reported Study dates: not reported Note:
|
|
Leung 1992.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: Gamma intramedullary nail versus DHS |
|
| Participants |
Total number of randomised participants: 225 participants; 226 fractures Inclusion criteria: peritrochanteric proximal femoral fractures; classified as "pertrochanteric or intertrochanteric with or without subtrochanteric extension" Exclusion criteria: < 65 years of age; purely subtrochanteric fractures Setting: single‐centre; orthopaedic hospitals; Hong Kong Baseline characteristics (only for survivors) Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: prophylactic antibiotics; general or spinal anaesthetic; traction table for closed reduction under fluoroscopic control; immediate mobilisation with full weight bearing; clinical follow‐up at 6 weeks, 3 and 6 months. Most of the Gamma nail operations were performed by 1 senior surgeon with a special interest in intramedullary nailing, whilst the SHS operations were performed by a number of less experienced surgeons (from email communication with study authors). Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes measured/reported by study authors: length of surgery; blood loss; radiographic screening time; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union (fracture healing); re‐operation; deep wound infection; chest infection/pneumonia; any medical complication; LOS (mixed location); external rotational deformity; shortening of leg (> 2 cm); varus displacement (> 10 degrees); mortality; pain at follow‐up (pain in hip and pain in thigh); impaired walking; length of follow‐up: mean 7 months Outcomes relevant to the review: mortality (6 months); unplanned return to theatre (at 6 months) | |
| Notes |
Funding/sponsor/declarations of interest: quote: "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | We identified no other sources of bias. |
| Allocation concealment (selection bias) | High risk | We identified no other sources of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Most of the Gamma nail operations were performed by one senior surgeon with a special interest in intramedullary nailing, whilst the SHD operations were performed by a number of less experienced surgeons. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Li 2010.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: anatomical plate versus DHS |
|
| Participants |
Total number of randomised participants: 48 Inclusion criteria: intertrochanteric fractures Exclusion criteria: unknown Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (anatomical plate group)
Intervention group 2 (DHS)
|
|
| Interventions |
General details: all participants were followed up for 12.6 months (range 5 to 18 months) Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operative time; blood loss; walking time; HHS Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: May 2007 to June 2009 Note:
|
|
Li 2015b.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: distally locked PFNA II versus unlocked nailing PFNA II |
|
| Participants |
Total number of randomised participants: 70 Inclusion criteria: > 65 years of age; pertrochanteric fractures classified as AO/OTA 31‐A1 and 31‐A2 according to the orthopaedic Trauma Association classification system Exclusion criteria: pathological fractures; open fractures; bilateral fractures; fractures associated with a neurovascular injury as well as previous ipsilateral hip or femur surgery Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (locking)
Intervention group 2 (unlocking)
Overall:
Note:
|
|
| Interventions |
General details: all operations were performed by the same group of experienced surgeons; the rehabilitation protocol was identical in each group; all participants were encouraged to move their hip, knee and ankle joints on the first postoperative day under guidance of the surgeon; on the following day, partial weight bearing, or restricted weight bearing as tolerated or walking with full weight bearing assisted by a walking frame was allowed in accordance with surgeons' advice; clinical and radiographic re‐examinations were performed at 3 months, 6 months and 12 months postoperatively Intervention group 1
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; volume of intraoperative blood loss; blood transfusion; total fluoroscopy time; length of incision; mortality (available at 12 months); postoperative complications; hospital stay length; HHS; mobility Outcomes relevant to the review: mortality (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: the authors declare no conflict of interest but no details on funding are reported Study dates: March 2013 to July 2014 Note:
|
|
Li 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA vs DHS |
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: elderly people ≥ 60 years of age, with osteoporosis, with femoral intertrochanteric fractures Exclusion criteria: people with bone or joint motor system diseases, diabetes mellitus, severe cardiorespiratory, hepatic, or renal dysfunctions, mental disorders, coagulation disorders, systemic malignant tumours, malignant tumour cachexia, or contraindications after intra‐spinal anaesthesia puncture; using analgesia devices or drugs after the operation; declined to consent to enrolment Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: spinal epidural anaesthesia; wound drain for all cases Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation duration, blood loss, postoperative drainage volume, HHS, pain, bone mineral density and calcitonin level, 10‐metre walking speed, 5‐times sit‐to‐stand test time, fracture healing and weight bearing time, complications (coxa vara, loose nail, bone non‐union, delayed union of fracture, femoral head necrosis and DVT) Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. Study authors declared no conflicts of interest Study dates: January 2013 to December 2014 Note:
|
|
Liem 1993.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus Ender |
|
| Participants |
Total number of randomised participants: 136 Inclusion criteria: pertrochanteric fractures of the femur Exclusion criteria: not reported Setting: single centre; hospital; Netherlands Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (Ender)
Note:
|
|
| Interventions |
General details: preoperative antibiotic prophylaxis was started with 1 g Keforal IV which was repeated 3 times during the first 24 hours; unless contraindicated, all participants were permitted immediate weight bearing; follow‐up for 6 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operating time; operative blood loss; mean hospital stay; superficial wound infections; mortality (available at 14 days and 6 months); intercurrent disease; re‐operations (available at 6 months); other complications Outcomes relevant to the review: mortality (reported at 14 days and 6 months); unplanned return to theatre (reported at 6 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: October 1982 to October 1985 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Skills and experience of surgeons are not reported and it is uncertain whether surgeons were equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported that no participants were lost to follow‐up. Only participant loss was because of death, which is expected in this population |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Little 2008.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: long Holland intramedullary nail versus CHS |
|
| Participants |
Total number of randomised participants: 190 Inclusion criteria: low‐energy extracapsular intertrochanteric fracture; classification AO/ASIF A1, A2 and A3 (stable and unstable fractures) Exclusion criteria: people with subtrochanteric fractures Setting: single centre; orthopaedic hospital; United Kingdom Baseline characteristics Intervention group 1 (Holland nail)
Intervention group 2 (DHS)
Overall:
Note:
|
|
| Interventions |
General details: pre‐ and postoperative care was the same for both groups; single‐dose antibiotic teicoplanin and gentamicin at induction; anaesthesia was either regional, regional and general, or general; traction table for closed reduction; standard operative technique either recommended by the manufacturer or by previous studies; antibiotic and thromboembolism prophylaxis were routinely given; aspirin once daily for 6 weeks; standardised pain relief; mobilised (fully weight bearing) on POD1; rehabilitation was standardised; clinical follow‐up at six weeks, 6 and 12 months; specialist registrar under supervision or by a consultant who was familiar with both procedures is claimed, but there is also a reference to the possible influence of a learning curve on some outcomes Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; radiographic screening time; number of participants transfused; cut‐out of the implant; re‐fracture around the implant; re‐operation; superficial wound infection; deep wound infection; pneumonia; DVT; PE; TIA; mortality; failure to regain mobility; mobility score; days until mobilisation; length of follow‐up: mean 12 months Outcomes relevant to the review: mortality (30 days and 12 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: study authors clearly state that no funding was received and no conflicts existed Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated a sequential study number and were randomised by computer to be treated with a DHS or a Holland nail." |
| Allocation concealment (selection bias) | Unclear risk | No additional details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Quote: "Each procedure was carried out by a specialist registrar under supervision or by a consultant who was familiar with both procedures." Comment: the report suggested that the longer operating and radiation times in the Holland nail group "may be a function of the learning curve in its use". |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses are explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Lopez 2002.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: Gamma nail versus DHS |
|
| Participants |
Total number of randomised participants: 103 Inclusion criteria: trochanteric proximal femoral fractures (no prominent subtrochanteric extension) Exclusion criteria: not reported Setting: single centre; orthopaedic hospital; Spain Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: experience of surgeons is not reported Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; postoperative transfusion; change in haematocrit; radiographic screening time; operative fracture of the femur; later fracture of the femur; cut‐out of implant; re‐operation; wound infection; wound haematoma; DVT; pneumonia; pressure sores; mortality; mobility score; mean time to fracture consolidation; length of follow‐up: 12 months Outcomes relevant to the review: mortality (12 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: February 1998 to April 1999 Note:
|
|
Lopez‐Vega 2015.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: Gamma 3 nail with distal lock versus Gamma 3 nail without distal lock |
|
| Participants |
Total number of randomised participants: 177 Inclusion criteria: intertrochanteric femoral fractures (31‐A1 or 31‐A2 in the AO classification) Exclusion criteria: high‐energy trauma (falls and traffic accidents); people who were incorrectly randomised according to their date of birth; unstable fractures requiring the use of a blocked nail or longer nails (particularly in AO 31‐A2.3 and 31‐A3 fractures); pathological fractures; implants from other commercial brands; people who did not wish to be included in the study Setting: single centre; hospital; Spain Baseline characteristics Intervention group 1 (with)
Intervention group 2 (without)
Overall:
Note:
|
|
| Interventions |
General details: all participants were allowed immediate load depending on their tolerance; antibiotic prophylaxis was prescribed for the first 24 hours after the intervention, along with antithrombotic prophylaxis with low‐molecular‐weight heparins for 30 days after discharge Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: surgical time; blocking screw; angulation of the screw; cephalic screw; distance from tip to apex; level of reduction; radiation absorbed; fluroscopy time; blood loss; re‐interventions; medical complications; biomechanical complications; mortality (available at 12 months) Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflict of interest; however, no information on funding is reported Study dates: June 2011 to January 2013 Note:
|
|
Lunsjo 2001.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: SHS versus MSP |
|
| Participants |
Total number of randomised participants: 569 Inclusion criteria: unstable intertrochanteric fracture, low‐energy trauma Exclusion criteria: pathological fractures; previous fractures; fractures > 5 cm distal to lesser trochanter; two‐part fractures Setting: 8 hospitals, Sweden Baseline characteristics Intervention group 1 (SHS)
Intervention group 2 (MSP)
Note:
|
|
| Interventions |
General details: MSP recently introduced; low‐molecular‐weight heparin; spinal anaesthesia; immediate weight bearing; follow up at 1 and 12 months Intervention group 1
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: blood loss, operating time, deep and superficial infection; PE; DVT; fixation failures; lag screw penetration; cut‐out; mobility; postoperative fractures; mobility; place of residence at 12 months; LOS Outcomes relevant to the review: unplanned return to theatre; mortality (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: supported by grants from the Thelma Zoéga Foundation and the Stig and Ragna Gorthon Foundation, Helsingborg, and the Clinical Research Foundation of Malmöhus County Council, Lund, and the Swedish Medical Research Council Study dates: March 1993 to June 1995 Note:
|
|
Makridis 2010.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: intramedullary nail versus intramedullary nail: IMHS or Endovis |
|
| Participants |
Total number of randomised participants: 215 Inclusion criteria: pertrochanteric fractures after low‐energy injury; > 60 years of age Exclusion criteria: pathological fractures; high‐energy injury; < 60 years of age Setting: single centre; hospital; Greece Baseline characteristics Intervention group 1 (IMHS)
Intervention group 2 (Endovis)
Note:
|
|
| Interventions |
General details: prophylactic intravenous second‐generation cephalosporin was administered before operation and discontinued 48 hours postoperatively; participants were mobilised on second postoperative day, allowing them to bear weight as much as they could tolerate; all cases received anticoagulant prophylactic therapy with low‐molecular‐weight heparin, starting on admission and for 4 weeks postoperatively; operations were performed on a fracture table under spinal anaesthesia and image intensifier control; clinical follow‐up at 1, 3, 6, and 12 months after surgery Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: surgical time; blood loss; any intraoperative complication; level of haemoglobin; mobility status at time of discharge; duration of hospital stay; mortality (available in hospital and at 12 months); functional status; fracture healing; tip to apex distance; transfusions; re‐operations (available at 12 months) Outcomes relevant to the review: mortality (reported in hospital and at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors declare they have no conflicts of interest. No detail on funding is provided. Study dates: July 2005 and June 2007 Note:
|
|
Marques 2005.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: intertrochanteric Gamma nail versus AO PFN |
|
| Participants |
Total number of randomised participants: 156 Inclusion criteria: > 65 years of age; unstable trochanteric fracture with any subtrochanteric fracture extension less than 2 cm Exclusion criteria: pathological fractures, types A2 3.1 and A3 Setting: single centre; hospital; Spain Baseline characteristics Intervention group 1 (Gamma)
Intervention group 2 (PFN)
Overall:
Note:
|
|
| Interventions |
General details: in the postoperative period, participants were allowed to weight bear as tolerated; surgeries were performed by a combination of junior residents, senior residents and staff Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operative time; transfusion requirements; mechanical complications; pain; intertrochanteric bursitis; re‐operations (available at 12 months); length of stay; mortality (available at hospital admission, 12 months); final independent mobility Outcomes relevant to the review: unplanned return to theatre (reported at 12 months); mortality (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: November 1999 to May 2002 Notes:
|
|
Matre 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Trigen Intertan versus SHS |
|
| Participants |
Total number of randomised participants: 684 (697 were initially randomised but 13 were excluded because of preoperative deaths, withdrawal from study before surgery, and due to not meeting inclusion criteria) Inclusion criteria: > 60 years of age; trochanteric or subtrochanteric fracture Exclusion criteria: pathological fractures Setting: 5 centres, hospitals, Norway Baseline characteristics Intervention group 1 (intramedullary nail)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: surgeons participated in at least 5 operations involving use of the Intertan nail before they could participate; clinical examinations at 5 days, 3 and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of the surgery; participants' haemoglobin level; number of blood transfusions; LOS; radiographs (quality of fracture reduction + tip‐apex distance); EQ‐5D questionnaire; postoperative pain ‐ VAS; TUG; LOS; complication and re‐operation rates; participants' residence; walking ability; HHS; mortality; major complications (failure of osteosynthesis; deep infection or postoperative haematoma requiring surgical intervention; cut‐out; femoral fracture; removal of whole implants); minor complications (locking screws missing the nail or removal of a single locking or lag screw; surgical removal of a drain) Outcomes relevant to the review: HRQoL (EQ‐5D at 3 and 12 months); unplanned return to theatre (assumed to be 12 months); mortality (at 4 & 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: quote: "Smith & Nephew supported the study, but otherwise the company had no influence on the study." Quote: "One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution, has had a financial relationship, in the thirty‐six months prior to submission of this work, with an entity in the biomedical arena that could be received to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work". Study dates: February 2008 to February 2009 Note:
|
|
Mattsson 2004.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus DHS augmented with calcium‐phosphate cement |
|
| Participants |
Total number of randomised participants: 26 Inclusion criteria: unstable trochanteric fracture (Jensen types IV to V), AO 31 A2, caused by a low‐energy trauma; signed informed consent; ambulatory without walking aid or with 1 cane prior to injury; a normal contralateral hip Exclusion criteria: senility; pathological fracture; concurrent fracture that would affect postoperative weight bearing Setting: single centre; hospital; Sweden Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (augmentation)
Overall:
Note:
|
|
| Interventions |
General details: all operations were performed under spinal anaesthesia by the authors; the first RSA examination was done within 24 hours after surgery, before weight bearing, after which all participants were allowed unrestricted weight bearing; the normal postoperative rehabilitation regime at the department Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: re‐operations (available at 12 months); degrees of rotation; amount of translation; mortality (available at 12 months) Outcomes relevant to the review: re‐operations (reported at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: cement supplied by manufacturer Study dates: not reported Note:
|
|
Mattsson 2005.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus DHS augmented with calcium‐phosphate cement |
|
| Participants |
Total number of randomised participants: 112 Inclusion criteria: unstable trochanteric fractures (Jensen types 4 to 5 or AO 31 to A2); > 65 years of age; walking with or without support; less than 72 hours between the fracture and surgery; signed informed consent Exclusion criteria: dementia; serious concomitant illness or mental instability; neurosensory, neuromuscular or musculoskeletal deficiency which limited the ability to perform objective functional tests; soft‐tissue infection at the operation site; ongoing radiotherapy or chemotherapy for malignancy; pathological fracture; a clotting disorder; corticosteroid treatment exceeding 5 mg/day; a concurrent fracture which would affect the postoperative functional outcome or an earlier operation on the contralateral hip Setting: single centre; hospital; Sweden Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (augmentation)
Note:
|
|
| Interventions |
General details: all surgeries were performed by the study authors; 98 surgeries were under spinal anaesthesia with sedation and 14 under general anaesthesia; all participants were allowed immediate weight bearing after surgery and were followed by clinical and radiological examination immediately after surgery and at 1 week, 6 weeks and 6 months after the procedure Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain; HRQoL (available at 1 week, 6 weeks and 6 months); isometric hip abductor muscle strength; reduction of fracture; adequacy of fixation; mortality (available at 6 months); failure of the implant; sliding of lag screw; healing of the fracture; non‐union; blood loss; mean hospital stay; re‐operations (available at 6 months) Outcomes relevant to the review: HRQoL (in SF‐36, reported at 6 months); mortality (reported at 6 months); unplanned return to theatre (reported at 6 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: implants supplied by manufacturer. Funding from Trygg Hansa Study dates: 1997 to 2000 Note:
|
|
McCormack 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus MSP |
|
| Participants |
Total number of randomised participants: 163 Inclusion criteria: > 60 years of age; unstable intertrochanteric hip fracture (determined by the treating surgeon) as defined by Type IV and Type V in the Jensen and Michaelsen (modified Evans) classification or OTA 31‐A2 (fractures involving the lesser trochanter ‐ loss of posteromedial support) Exclusion criteria: stable intertrochanteric fracture (determined by treating surgeon) as defined by types I, II and III in the Jensen and Michaelsen (modified Evans) classification or OTA 31‐A1; a previous hip fracture on the affected side; confinement to bed (non‐ambulatory people); a pathological fracture; a fracture with significant medical comorbidities (Detsky score > 30 points and ASA Grades IV and V); reverse obliquity fracture Setting: 3 centres; hospitals; Canada Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (MSP)
Overall:
Note:
|
|
| Interventions |
General details: mobilisation on POD1, unrestricted weight bearing and physiotherapy whilst in hospital; follow‐up assessments at 4 to 6 weeks, 3 or 4 months and 6 months after surgery Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: re‐operations (available at 6 months); participant function; morbidity; mortality (reported at 6 months); hospital stay; quality of reduction; radiographs, fixation, ROM, leg length; incidence of malunion and non‐union; ADL Outcomes relevant to the review: unplanned return to theatre (reported at 6 months); mortality (reported at 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflicts of interest but no information on funding is provided Study dates: October 1998 to March 2006 Note:
|
|
McLaren 1991.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: Pugh nail versus DHS |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: intertrochanteric fractures of the femur Exclusion criteria: not reported Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (Pugh)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: participants were seen at 6 weeks, 3 months and 6 months postoperatively Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain; operation time; mortality (available at 6 months); length of hospital stay; walking ability; complications Outcomes relevant to the review: mortality (reported at 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: September 1986 to December 1987 Note:
|
|
Mehdi 2000.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: IMHS versus SHS |
|
| Participants |
Total number of randomised participants: 180 Inclusion criteria: extracapsular proximal femoral fractures; AO 31 A1, A2, A3; stable and unstable fractures Exclusion criteria: not reported Setting: single centre; orthopaedic hospital; UK Baseline characteristics Intervention group 1 (IMHS)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: no surgical details described Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; operative fracture of the femur; later fracture of femur (none); cut‐out of implant; perioperative complication; fracture reduction; wound infection (superficial and deep); mortality; mobility; HHS Outcomes relevant to the review: none Note: because of the large range of final follow‐up times and high and unequal losses to follow‐up, we decided against presenting final follow‐up results (mortality) in the review. |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Merenyi 1995.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Ender nail versus angle‐plate Note:
|
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: unstable inter‐ or subtrochanteric fracture (AO type A2.2 ‐ A3.3) Exclusion criteria: NR Setting: single centre; hospital; Hungary Baseline characteristics Intervention group 1 (Ender)
Intervention group 2 (angle‐plate)
Note:
|
|
| Interventions |
General details: not reported Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: surgical trauma; blood loss; time till weight bearing; fracture healing; resumption of level of activity previous to trauma; local and general complications; re‐operations (time point not reported) Outcomes relevant to the review: unplanned return to theatre (time point not reported) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: August 1992 to March 1994 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Skills and experience of surgeons are not reported. It is uncertain whether surgeons were equally experienced both types of implants. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | High risk | Study report was available only as an abstract which we expected was not peer‐reviewed. In addition, because of limited detail in the abstract, we could not be certain of other risks of bias. |
Michos 2001.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus SHS |
|
| Participants |
Total number of randomised participants: 52 Inclusion criteria: trochanteric proximal femoral fractures. Some may have had subtrochanteric extension Exclusion criteria: not reported Setting: single site; orthopaedic hospital; Greece Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: experience of surgeon is not reported Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operative blood loss; later fracture of the femur; cut‐out of implant; non‐union; plate detachment; mortality (perioperative); length of follow‐up: 3 to 6 months Outcomes relevant to the review: mortality (during perioperative period); unplanned return to theatre (up to 6 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Miedel 2005.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus Medoff sliding plate |
|
| Participants |
Total number of randomised participants: 217 Inclusion criteria: unstable trochanteric (Jensen & Michaelsen type 3 to 5) fractures; subtrochanteric (Seinsheimer) proximal femoral fractures; fractures occurred due to a simple fall Exclusion criteria: pathological fractures; rheumatoid arthritis; osteoarthritis; fractures extending more than 5 cm distal to the lesser trochanter Setting: single centre; orthopaedic hospital; Sweden Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (sliding plate)
Note:
|
|
| Interventions |
General details: fracture table; low‐molecular‐weight heparin before and for approximately 10 to 14 days after operation; single dose of antibiotic preoperatively; mobilised with full weight bearing as tolerated; identical care programmes; 50% of operations performed by consultant orthopaedic surgeons Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; postoperative transfusion; operative fracture of the femur; technical failure; later fracture of the femur; cut‐out of implant; displacement (medialisation of the femur requiring surgery); re‐operation; wound infection (superficial and deep); severe medical complications (cardiac, pulmonary, thromboembolic or cerebrovascular); LOS; discharge location; mortality (available in hospital, at 4 months and at 12 months); mobility; pain; hip function; ADL; HRQoL Outcomes relevant to the review: unplanned return to theatre (12 months); mortality (4 & 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: supported in part from grants from the Trygg‐Hansa Insurance Company, the Swedish Orthopaedic Association, and from Stryker Howmedica (Gamma nail) and Swemac (Medoff sliding plate) Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised (sealed‐envelope system)" Comment: no additional details |
| Allocation concealment (selection bias) | Unclear risk | Study authors do not report whether envelopes are opaque and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Quote: only half of the operations in each group "were performed by consultant orthopaedic surgeons". Comment: study authors did not describe whether all surgeons were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses are balanced between groups and mostly explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Moroni 2004.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus DHS with HA coating |
|
| Participants |
Total number of randomised participants: 120 Inclusion criteria: women with osteoporosis and trochanteric fractures Exclusion criteria: previous hip fracture, open fracture, fracture secondary to malignancy, hard or soft tissue infection at the fracture site, chemotherapy, multiple fractures Setting: single centre, hospital, Italy Baseline characteristics Intervention group 1 (standard DHS)
Intervention group 2 (Hydroxyapatite‐coated DHS)
Note:
|
|
| Interventions |
General details: follow up at 1, 3 and 6 months; all operated on with 48 hours; cephalosporin was administered preoperatively (1 g) and continued every 8 hours for 72 hours; spinal anaesthesia; completed by senior surgeons; mobilised with 24 hours of surgery; full weight bearing was encouraged Intervention group 1:
Intervention group 2:
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional status (HHS); HRQoL (SF‐36); cut‐out; femoral neck shaft angle; tip‐apex distance; revision Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Notes:
|
|
Moroni 2005.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus Orthofix Pertrochanteric Fixator (OPF) |
|
| Participants |
Total number of randomised participants: 40 Inclusion criteria: female; ≥ 65 years of age; AO fracture type A1 or A2; pertrochanteric fracture resulting from minor trauma; ability to respond comprehensively; ability to walk unassisted prior to injury; BMD at the contralateral hip less than ‐2.5 T‐score Exclusion criteria: Setting: single centre; hospital; Italy Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (OPF)
Note:
|
|
| Interventions |
General details: not reported Intervention group 1
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS; operative time; postoperative complications; number of blood transfusions; level of pain; length of hospital stay; femoral neck‐shaft angle Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Mott 1993.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus SHS |
|
| Participants |
Total number of randomised participants: 69 Inclusion criteria: trochanteric proximal femoral fractures. Defined as 2‐, 3‐ or 4‐part with additional classifications for basilar neck/high intertrochanteric (7 fractures) and high subtrochanteric/low intertrochanteric (3 fractures). Reference made to classification according to Jensen's modification of Evans but types not reported Exclusion criteria: judged inoperable for medical reasons Setting: multi‐centre; three orthopaedic hospitals; USA. Baseline characteristics (overall)
Note:
|
|
| Interventions |
General details: not reported Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; blood transfusion; operative fracture of the femur; later fracture of the femur; cut‐out of implant; re‐operation; deep wound infection; superficial wound infection; wound haematoma; DVT; MI; pneumonia; UTI; mortality (1 week); length of follow‐up: not stated Outcomes relevant to the review: unplanned return to theatre; mortality (1 week) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
Nungu 1991.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Ender nails versus SSP |
|
| Participants |
Total number of randomised participants: 220 Inclusion criteria: intertrochanteric fractures Exclusion criteria: fractures due to malignant metastasis; fractures older than 1 week Setting: single centre; hospital; Sweden Baseline characteristics Intervention group 1 (Ender)
Intervention group 2 (SSP)
Overall:
Note:
|
|
| Interventions |
General details: surgical procedure was carried out under spinal anaesthesia; operations performed by 30 different surgeons with varying range of experience; until they were mobile, participants received either high‐molecular‐weight dextran or heparin as thromboembolic prophylaxis; clinical follow‐up at 4 months and 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operating time; blood loss; reduction; positioning of osteosynthesis materials; deep‐wound infections; re‐operations (available at 12 months); distal migration; mortality (available at 12 months); mobility; pain; rotation Outcomes relevant to the review: unplanned return to theatre (reported at 12 months); mortality (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 1987 to February 1989 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation described as 'closed envelope method' |
| Allocation concealment (selection bias) | Unclear risk | Randomisation described as 'closed envelope method' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Surgeons with varying degrees of experience. It is uncertain whether experience was equally balanced for both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect lack of blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost, these losses were balanced between groups, and reasons for loss were clearly explained |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
O'Brien 1995.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus DHS |
|
| Participants |
Total number of randomised participants: 101 participants with 102 fractures Inclusion criteria: trochanteric proximal femoral fractures; stable and unstable (Evans) Exclusion criteria: fractures > 1 week old; pathological fractures; subtrochanteric fractures Setting: single centre; orthopaedic hospital; Canada Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: all but 4 participants received prophylactic antibiotics; fracture table; image intensifier; no details of surgeons' experience Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; radiographic screening time; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union (time to union); re‐operation; wound infection; deep wound infection; wound haematoma; pneumonia; pressure sores; PE; any medical complication; LOS; mortality; pain at follow‐up; loss of independence; loss in mobility (dropped ≥ 1 level in walking‐aid dependence) Outcomes relevant to the review: mortality (early postoperative period); unplanned return to theatre (12 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: November 1989 to April 1991 Note:
|
|
Okcu 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus PFNA: standard nail or long nail |
|
| Participants |
Total number of randomised participants: 40 Inclusion criteria: reverse oblique type trochanteric fractures Exclusion criteria: AO/OTA 31‐A1 to A2 fractures; pathological fractures; previous proximal femoral fractures; open fractures; polytrauma with Injury Severity Score of greater than 16; severe concomitant medical conditions Setting: 2 centres; hospitals; Turkey Baseline characteristics (excludes participants who were lost to follow‐up/died) Intervention group 1 (standard)
Intervention group 2 (long)
Overall:
Note:
|
|
| Interventions |
General details: 3 experienced orthopaedic surgeons performed all operations; all participants were followed up until fracture union or a revision operation was performed; the choice of anaesthetic method was made by the anaesthesiologist; postoperatively, all participants had antibiotic prophylaxis for 48 hours and DVT prophylaxis for 4 weeks; postoperative rehabilitation protocol was identical for both groups; participants were mobilised as soon as possible, usually on second postoperative day with unrestricted weight bearing; formal therapy was instituted, working on muscle strengthening, conditioning and hip and knee ROM exercises; participants were evaluated at 6 weeks, 12 weeks, 6 months and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (available at 12 months); operation time; fluoroscopy time; hospital stay; walking ability; HHS; complications; change in neck‐shaft angle; migration of the blade; TAD; re‐operations (available at 12 months); malunion Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report that each author certifies that he or she, or a member or his or her immediate family, has no funding or commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article Study dates: January 2009 to December 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using random allocation software |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons |
| Other performance bias: surgeon experience of both implants | Low risk | Experienced surgeons for all operations |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported that no participants were lost to follow‐up. Only participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Olsson 2001.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: CHS versus Medoff sliding plate |
|
| Participants |
Total number of randomised participants: 114 Inclusion criteria: intertrochanteric fractures Exclusion criteria: surgery of the ipsilateral femur, pathological fractures Setting: single centre, hospital, Sweden Baseline characteristics Intervention group 1 (CHS)
Intervention group 2 (Medoff)
Note:
|
|
| Interventions |
General details: performed by 22 surgeons; subcutaneous enoxaparin (40 mg daily) was begun before the operation and continued until the participant was mobilised; antibiotic therapy (cefuroxime 1500 mg) was given intraoperatively with one subsequent dose after 24 hours; immediate weight bearing as tolerated Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operative time; bleeding; LOS; mortality, residential status, mobility, femoral shortening, lag screw migration, sliding of the lag screw, fixation failure, blood transfusion, wound infection, thromboembolism Outcomes relevant to the review: mortality (4 months), unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: no conflicts of interest stated; supported by grants from the Stig and Ragna Gorthon Foundation and the Thelma Zoega Foundation, Helsingborg, and from the clinical research foundation of Malmöhus County Council, Lund Study dates: November 1998 and October 1999 Note:
|
|
Ovesen 2006.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: trochanteric Gamma intramedullary nail (TGN) versus DHS |
|
| Participants |
Total number of randomised participants: 150 participants with 151 fractures (see Notes) Inclusion criteria: intertrochanteric fractures; AO 31 A11, A2 & A3 Exclusion criteria: subtrochanteric or a pathological fracture Setting: single centre; orthopaedic hospital; Denmark Baseline characteristics Intervention group 1 (TGN)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: prophylaxis for DVT and PE once daily starting from admission until mobilisation; antibiotic prophylaxis; fracture table; fluoroscopy; clinical follow‐up at 4 and 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes | Outcomes measured/reported by study authors: length of surgery; blood loss; transfusion; operative fracture of the femur (none); later fracture of the femur; cut‐out of implant; non‐union (none); re‐operation; wound infection; medical complications (none); LOS; mortality at 12 months; use of walking aids at discharge and 4 months; length of follow‐up: 12 months Outcomes relevant to the review: mortality (at 4 and 12 months); unplanned return to theatre (12 months) | |
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: April 2001 and October 2003 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from direct communication with study authors): "computer‐generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomized by consecutive drawing of opaque envelopes". Comment: envelopes were confirmed as sealed in direct communication with the study author |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Over two thirds of operations done by residents: 49 surgeons participated in trial. Study authors did not describe whether surgeons were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses which were balanced between groups and explained by study authors |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Ozkayin 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus hemiarthroplasty |
|
| Participants |
Total number of randomised participants: 54 Inclusion criteria: > 75 years of age; intertrochanteric femur fractures Exclusion criteria: people who did not want to be included in the study; pathological fractures Setting: single centre; hospital; Turkey Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (hemiarthroplasty)
Overall:
Note:
|
|
| Interventions |
General details: operations were performed by only 2 surgeons; participants were given either general or regional block anaesthesia; all participants were administered prophylactic antibiotics and low‐molecular‐weight heparin for 4 weeks as a means of thromboprophylaxis; all participants were mobilised full weight bearing within 48 hours after surgery; after hospital discharge, participants were followed up in the outpatient clinics for clinical and radiological evaluation at 1.5, 3, 6, 12 and 18 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; hospital stay; superficial infection; late deep infection; non‐union; shortening; re‐operations (available at 18 months); mobility; HHS; functional score Outcomes relevant to the review: unplanned return to theatre (reported at 18 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report 'the authors of this manuscript do not have financial or proprietary interest in the subject matter or materials discussed in the manuscript, including (but not limited to) employment, consultancies, stock ownership, honoraria, and paid expert testimony. No funds were received in the support of this study' and no conflict of interest Study dates: October 2006 to December 2012 Note:
|
|
Pahlpatz 1993.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus DHS |
|
| Participants |
Total number of randomised participants: 153 Inclusion criteria: trochanteric and subtrochanteric proximal femoral fractures; stable, unstable and subtrochanteric (Evans classification) Exclusion criteria: multiple fractures; open epiphyseal lines Setting: single centre; orthopaedic hospital; the Netherlands Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Baseline characteristics (overall)
Note:
|
|
| Interventions |
General details: mostly performed ≤ 24 hours; fracture table with image intensifier; operations by surgical residents with assistance of staff member as required; closed reductions; full weight bearing day after operation Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality; failure to regain residential status; length of follow‐up: 6 months minimum Outcomes relevant to the review: mortality (3 months and 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: July 1989 to January 1991 Note:
|
|
Pajarinen 2005.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus DHS |
|
| Participants |
Total number of randomised participants: 108 Inclusion criteria: low‐energy extracapsular pertrochanteric femoral fractures (AO category 31‐A) Exclusion criteria: pathological fractures; multiple injuries Setting: single centre; orthopaedic hospital; Finland Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (DHS)
Overall:
Note:
|
|
| Interventions |
General details: operations usually performed within 2 days of admission; in most cases by a senior orthopaedic resident (study authors confirmed all surgeons were experienced in both procedures); closed reduction; prophylactic antibiotics; low‐molecular‐weight heparin during hospital stay; weight bearing on POD1 or POD2; clinical examinations at 6 weeks and 4 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; units of blood transfused; later fracture of femur; cut‐out; failure of fixation (re‐displacement); re‐operation; superficial wound infection; deep wound infection; DVT; femoral neck and shaft shortening on X‐ray; LOS; mortality; failure to regain pre‐fracture residential status; non‐recovery of previous mobility; length of follow‐up: 4 months Outcomes relevant to the review: unplanned return to theatre; mortality (at 4 months) |
|
| Notes |
Funding/sponsor/declarations of interest: quote: "no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" Study dates: October 1999 and February 2001 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "strict randomisation" Comment: method used to generate random sequence is not described |
| Allocation concealment (selection bias) | Low risk | Quote: "The mode of treatment was determined by strict randomisation, using sealed envelopes." Comment: study author confirmed during direct communication that "it was impossible to see the number through the envelope". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quotes (from direct communication with study authors): "both procedures are standard procedures at our clinic" and "our surgeons are very experienced" |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25 participants were not included in final analysis and most of these losses were because participants were too ill to attend final follow‐up. Study authors did not report attrition by group. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Pan 2009.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus PFN |
|
| Participants |
Total number of randomised participants: 131 Inclusion criteria: femoral intertrochanteric fractures Exclusion criteria: Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (PFN)
Overall:
|
|
| Interventions |
General details: unknown Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: pre‐/postoperative blood loss; blood transfusion volume; operating time; length of hospital stay; infection; non‐union; delayed union of fracture; shortening of injured limb Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2005 to January 2008 Note:
|
|
Papasimos 2005.
| Study characteristics | ||
| Methods | RCT; parallel design; 3 study arms Review comparison group: PFN versus TGN versus DHS |
|
| Participants |
Total number of randomised participants: 141 Inclusion criteria: unstable trochanteric proximal femoral fracture (see Notes); AO 31‐A2 and A3; > 60 years of age Exclusion criteria: unable to walk before injury; pathologic fractures; previous ipsilateral hip or femur surgery; any fracture with extension 5 cm distal to the inferior border of the lesser trochanter; stable trochanteric fractures classified as AO Type 31‐A1 Setting: single centre; orthopaedic hospital; Greece Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (TGN)
Intervention group 3 (DHS)
Note:
|
|
| Interventions |
General details: 4 surgeons (extensive experience of TGN and DHS but limited with PFN); prophylactic antibiotics intraoperatively and 2 doses postoperatively; subcutaneous low‐molecular‐weight heparin for 6 weeks; rehabilitation was identical in all groups; mobilisation on the second postoperative day and subsequent ambulation with weight bearing as tolerated Intervention group 1
Intervention group 2
Intervention group 3
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; radiographic screening time; operative fracture (some of greater trochanter); cut‐out of implant; later fracture of the femur; non‐union; re‐operation; superficial wound infection; haematoma; medical complications; chest infection; pneumonia; mental disturbances; DVT; PE; urinary infection; LOS; time to fracture consolidation; function: scores using Salvati 1973; length of follow‐up: mean 12 months Outcomes relevant to the review: mortality (during hospital stay); unplanned return to theatre (at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2000 to December 2003 Note:
|
|
Park 1998.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: Gamma AP (Asia‐Pacific) intramedullary nail versus CHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: intertrochanteric femoral fracture. Tronzo classification: stable (II) and unstable (III & IV) Exclusion criteria: not reported Setting: single centre; university hospital; South Korea Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (CHS)
Overall
Note:
|
|
| Interventions |
General details: only limited details of clinical management reported. Mobilisation in Gamma nail group started using crutches 2 weeks after operation. In CHS group, people with unstable fractures were allowed to bear weight after minimal callus was evident on radiographs Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; operative fracture of femur (none); later fracture of femur (greater trochanter); cut‐out of implant; non‐union (time to union); wound infection; varus deformity; mobility Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 1993 and June 1995 Note:
|
|
Park 2010.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: PFN versus PFN: screw or helical |
|
| Participants |
Total number of randomised participants: 40 Inclusion criteria: intertrochanteric fractures Exclusion criteria: not reported Setting: single centre; hospital; Korea Baseline characteristics Intervention group 1 (screw)
Intervention group 2 (helical)
Note:
|
|
| Interventions |
General details: clinical follow‐up was during the immediate postoperative period, at discharge, and at 1, 3, 6, 12, 18, 24 and 48 months postoperatively; operations were performed under general anaesthesia; all participants had suction drains for 48 hours and received antibiotic and thromboembolic prophylaxis; the rehabilitation protocol was identical for both groups: participants remained in bed for 2 days but were allowed to sit up, and then, if able, ambulation with partial weight bearing with a parallel bar or walker was allowed; full weight bearing was allowed as tolerated and when fixation stability was adequate Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss; mobility score; BMD; duration of hospital stay; social function score; time to ambulation; neck‐shaft angle Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report having no relevant financial relationships to disclose Study dates: January 2005 to January 2007 Note:
|
|
Parker 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Targon PFN versus SHS |
|
| Participants |
Total number of randomised participants: 598 participants with 600 fractures Inclusion criteria: trochanteric hip fractures Exclusion criteria: subtrochanteric fractures, subtrochanteric extension that required a plate longer than 5 holes, pathological fractures, previously‐treated fractures, conservative treatments, people with senile dementia, people with significant arthritis to be treated with THA Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: all undertaken or supervised by a single specialised hip fracture surgeon; early mobilisation with full weight bearing, early discharge to previous residence when possible Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (available at 6 weeks, 3, 6, 9 and 12 months); acute ward stay; blood transfusion and volume of transfused blood; non‐union; avascular necrosis; re‐operation (arthroplasty or revision fixation); superficial and deep wound infection; confusion/delirium; pneumonia; pressure sores; urine retention; DVT; PE; fat embolism; CVA; MI; clostridia diarrhoea; gastrointestinal bleed; peritonitis; septicaemia; acute renal failure; pain (Charnley scale at 2, 3, 6, 9 and 12 months; VAS; using a 6‐point scale at 6 weeks, lower scores indicates no pain); available at 6 weeks, 3, 6, 9, 12 months); mobility score (9‐point scale ‐ 1 = no need for mobility aids; available at 8 weeks, 3, 6, 9, 12 months); penetration of lag screw, plate detachment from femur, fracture below implant Outcomes relevant to the review: mortality (at 3 and 12 months); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: funded internally from the Peterborough Hospitals Hip Fracture Research Fund to cover research expenses and those of the research nurse. Study author received benefits for personal or professional use from a commercial party related directly or indirectly to the study Study dates: April 2002 to November 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes prepared by person who was independent to the study |
| Allocation concealment (selection bias) | Low risk | Use of sealed, opaque, numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | All surgeries undertaken by a single surgeon experienced with both implants |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes (such as decision to re‐operate). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses are explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration or pre‐published protocol. It is not possible to effectively assess risk of reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Parker 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Targon PFN versus SHS |
|
| Participants |
Total number of randomised participants: 400 Inclusion criteria: surgically‐treated trochanteric fractures (stable A1, unstable A2, and transtrochanteric A3); participants with dementia were included with consent of next of kin Exclusion criteria: subtrochanteric fractures; subtrochanteric extension that required a plate longer than 5 holes; pathological fractures; previously treated fractures; conservative treatments; participants with senile dementia for whom permission of their next of kin was not obtained; arthritis of the hip Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (SHS)
Note:
|
|
| Interventions |
General details: all undertaken or supervised by a single specialised hip fracture surgeon; early mobilisation with full weight bearing; early discharge to previous residence when possible Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (available at 30 days, 8 weeks, 3, 6, 9 and 12 months); acute ward stay; blood transfusion and volume of transfused blood; confusion/delirium; non‐union; avascular necrosis; re‐operation (arthroplasty or revision fixation); superficial and deep wound infection; pneumonia; DVT; CVA; MI; acute renal failure; pain (using a 6‐point scale in the first 600 participants (in Parker 2012), and a 9‐point scale in the later 400 participants ‐ in both scales, lower scores indicate lower pain; available at 8 weeks, 3 months, 6 months, 9 months, 12 months); mobility score (9‐point scale ‐ 1 = no need for mobility aids; available at 8 weeks, 3, 6, 9, 12 months); pressure sores, urine retention, PE, congestive cardiac failure, cardiac arrhythmia, gastrointestinal bleed, peritonitis, intestinal obstruction, clostridia diarrhoea, septicaemia, fat embolism; cut‐out, plate off the femur or fracture below implant Outcomes relevant to the review: mortality (at 3 and 12 months); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: funded internally from the Peterborough Hospitals Hip Fracture Research Fund to cover research expenses and those of the research nurse. Study author received benefits for personal or professional use from a commercial party related directly or indirectly to the study Study dates: December 2010 to September 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised by the opening of numbered, opaque, sealed envelopes. No further info in the paper or the 2012 or 2017 publications |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by the opening of numbered, opaque, sealed envelopes to fixation of the fracture with either the SHS or an intramedullary nail |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | All surgeries undertaken by a single surgeon experienced with both implants |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect lack of blinding of participants to influence reporting of these outcomes. Data were collected from participants by a research nurse who was unaware of treatment allocation. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes (such as decision to re‐operate). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses are explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | The study was retrospectively registered on a clinical trials register (NCT02680028; first posted February 2016 and NCT03172923; June 2017); it is not feasible to effectively assess risk of selective reporting bias from this document. |
| Other bias | Low risk | We identified no other sources of bias. |
Parker 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: short versus standard length intramedullary nail |
|
| Participants |
Total number of randomised participants: 229 Inclusion criteria: stable trochanteric fracture (A1) or unstable trochanteric fracture (A2); consent Exclusion criteria: pathological fractures from tumours Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (short)
Intervention group 2 (standard)
Note:
|
|
| Interventions |
General details: all participants were encouraged to mobilise fully and bear weight as able with no restriction on hip movements; postoperative care protocols were identical for both groups; all participants without contraindications received chemical thrombopathy with low‐molecular‐weight heparin for 28 days; all surgeries performed (or supervised) by lead surgeon Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss; pain; mobility; social dependency; length of hospital stay; mortality (available at 12 months); amount of blood transfused; implant cut‐out; non‐union; femoral aneurysm; re‐operations (available at 30 days, 120 days and 12 months); reaming of femur required; pneumonia; urinary retention; DVT; pressure sores; delirium; gastrointestinal bleed; congestive cardiac failure; acute renal failure; myocardial infarction; atrial fibrillation Outcomes relevant to the review: mortality (reported at 30 and 120 days, and 12 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report "the author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article" and "M Parker has received royalties from B. Braun Inc. related to the design and development of an implant used for the internal fixation of intracapsular hip fractures. Travel expenses have been paid to M Parker to attend and speak at a number of meetings by implant manufacturers" Study dates: November 2015 to May 2018 Note:
|
|
Pelet 2001.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus angled plate |
|
| Participants |
Total number of randomised participants: 26 Inclusion criteria: trochanteric proximal femoral fractures, classified by the Kyle system as type IV. These are equivalent to type A3 (AO classification): reversed and transverse fracture lines at the level of the lesser trochanter Exclusion criteria: Kyle types I to III; < 16 years of age; refusing to consent; not operated within 4 days Setting: single centre; orthopaedic hospital; Switzerland Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (angled plate)
Note:
|
|
| Interventions |
General details: all operated on within 48 hours; preoperative prophylactic antibiotics; general or epidural anaesthesia; mobilised after 24 hours with weight bearing according to radiographs; clinical follow‐up at 10 days, 1, 2, 3,6 and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: quality of the reduction; length of surgery; operative blood loss; operative fracture of the femur; cut‐out; non‐union (and time to consolidation); avascular necrosis; implant failure; re‐operation; wound infection; PE; cardiac failure; all medical complications; LOS; discharge destination, external rotation deformity; hip flexion; mortality; pain at follow‐up; use of walking aids; time to start of weight bearing; time to full weight bearing; length of follow‐up: 12 months Outcomes relevant to the review: mortality (at 12 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: November 1993 to January 1995 Note:
|
|
Pesce 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: hydroxyapatite‐coated screw versus nail and head standard screws |
|
| Participants |
Total number of randomised participants: 54 Inclusion criteria: femoral lateral fractures; 60 to 94 years of age: AO classification 31‐A3; co‐operative people; osteoporosis disease if T‐score < ‐2.5 DS; BMI < 30.0 kg/m2 Exclusion criteria: people with heart, kidney, neurological diseases, diabetes and systemic diseases who were unable to follow the rehabilitation programme; previous surgery or severe osteoarthritis of lower limbs; specific drugs treatments such as anticoagulants or psychiatric drugs Setting: single centre; hospital; Italy Baseline characteristics Intervention group 1 (hydroxyapatite‐coated screw)
Intervention group 2 (standard)
Overall:
Note:
|
|
| Interventions |
General details: all participants were under spinal anaesthesia and placed on a traction bed; weight bearing was delayed until 10 days then participants were permitted progressive weight bearing with crutches Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: TAD; bone density; HHS Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2010 to July 2011 Note:
|
|
Peyser 2007.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PCCP versus CHS |
|
| Participants |
Total number of randomised participants: 104 Inclusion criteria: ≥ 60 years of age; intertrochanteric fracture of the hip, type AO/OTA 31.A1 to A2, which was amenable to closed reduction; informed consent Exclusion criteria: reverse obliquity fractures (type AO/OTA 31.A3); pathological fractures; presence of metastatic disease; ipsilateral lower‐limb surgery; contralateral hip fracture within the past 12 months Setting: single centre; hospital; Israel Baseline characteristics Intervention group 1 (PCCP)
Intervention group 2 (CHS)
Note:
|
|
| Interventions |
General details: prophylactic antibiotics (1 g of cefazolin) were administered intravenously 3 times a day for the first 24 hours and low‐molecular‐weight heparin (enoxaparin 40 mg/d) was given prophylactically for 1 month postoperatively; participants in both groups were allowed immediate weight bearing as tolerated on the affected lower limb with the use of walking aids; the participants were reviewed at 6, 12 and 24 weeks and 12 months postoperatively Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mean time of reduction; operating time; length of hospital stay; intensive‐care unit admissions; re‐admission for non‐orthopaedic reasons; blood loss; DVT; UTI; pulmonary embolism; cardiovascular events (myocardial infarction; arrhythmia); cerebrovascular accident; gastrointestinal, renal and pulmonary (respiratory failure; pneumonia) complications; wound infection; mortality (available at 3, 6 and 12 months); cutting‐out of the hip screw; secondary fractures; failure of the implant; axial compression; alteration of the neck‐shaft angle; collapse Outcomes relevant to the review: mortality (reported at 3, and 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no benefits were received. No details on conflict of interest are reported. Study dates: March 2002 to June 2003 Note:
|
|
Pitsaer 1993.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus McLaughlin nail plate |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: > 60 years of age; intertrochanteric fracture of the femur Exclusion criteria: pathological fractures Setting: single centre; hospital; France Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (McLaughlin nail plate)
Note:
|
|
| Interventions |
General details: not reported Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: walking ability; pain; re‐operations (available at 6 months); mortality (available at 6 months) Outcomes relevant to the review: unplanned return to theatre (reported at 6 months); mortality (reported at 6 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Number of clinicians and their skills and experience is not reported. It is uncertain whether surgeons were equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes (such as decision to re‐operate). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data are not complete, and do not include re‐operation rates amongst those who died within 6 months |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trial registration or pre‐published protocol. It is not possible to effectively assess risk of reporting bias without these documents. |
| Other bias | High risk | Short report. We could not be certain whether information in this report is complete. |
Radford 1993.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma intramedullary nail versus DHS |
|
| Participants |
Total number of randomised participants: 200 Inclusion criteria: > 60 years of age, pertrochanteric proximal femoral fractures. Stable and unstable fractures (Evans) Exclusion criteria: not reported Setting: single centre; orthopaedic hospital; UK Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: surgeons at registrar level or higher, experienced in both techniques and supervised by the study authors; image intensifier; closed reduction where possible; traction table; aimed for central screw position, 5 mm to 10 mm from subchondral bone; suction drains; perioperative antibiotic prophylaxis; mobilised on POD2; clinical review at 3 and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; operative fracture of the femur; later fracture of the femur; cut‐out of implant; non‐union; re‐operation; wound infection; deep wound infection; DVT; LOS; mortality; transfer to long term care; mobility level; length of follow‐up: 12 months Outcomes relevant to the review: mortality (3 months); unplanned return to theatre (time point unclear unless stated, assumed to be 12 month as end of follow‐up period) |
|
| Notes |
Funding/sponsor/declarations of interest: quote: "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to groups. No additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quote: "only surgeons of registrar grade and above .. took part in trial. They were already experienced in the use of the DHS and intramedullary nailing, and were personally instructed in the operative technique for the Gamma nail. ...The first two Gamma nail operations performed by each surgeon were not included in the trial." |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported losses were explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Rahme 2007.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus blade plate |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: subtrochanteric proximal femoral fractures, all types (Seinsheimer classification) Exclusion criteria: ipsilateral femoral shaft or femoral neck fractures Setting: multicentre; 2 orthopaedic hospitals; Australia Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (blade plate)
|
|
| Interventions |
General details: bone grafting was at the discretion of the surgeon. Non‐weight bearing mobilisation was allowed postoperatively for 12 weeks, or until callus was seen on radiographs Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; mean units of blood transfused; non‐union and delayed union; re‐operation; wound infection; LOS; mortality; general health (SF‐36); length of follow‐up: 12 months Outcomes relevant to the review: unplanned return to theatre (at end of follow‐up); mortality (unclear but assumed to be 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: August 2001 and August 2003 Note:
|
|
Raimondo 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: ITST nail versus PCCP plate |
|
| Participants |
Total number of randomised participants: 70 Inclusion criteria: not reported; described as elderly participants Exclusion criteria: not reported Setting: single centre; trauma unit; Italy Baseline characteristics (overall)
Note:
|
|
| Interventions |
General details: type of anaesthesia (general or locoregional) was consistent between groups Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood transfusion; LOS; complications; functional status (HHS, 40 days, 6 and 12 months) Outcomes relevant to the review: mortality (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 2006 to 2010 Note:
|
|
Reindl 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: intramedullary devices versus DHS |
|
| Participants |
Total number of randomised participants: 204 Inclusion criteria: unstable intertrochanteric hip fracture; ≥ 55 years of age; type 2 (AO/OTA 31 ‐ A2); isolated fracture; occurred < 2 weeks prior to the time of enrolment Exclusion criteria: fracture due to malignancy; inability to walk before the fracture; severe dementia; limited life expectancy due to substantial medical comorbidities; medical contraindication; inability to comply with rehab or complete the forms Setting: multicentre; 9 sites; Canada Baseline characteristics Intervention group 1 (intramedullary device)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: fracture table; attempted closed reduction; use of fluoroscopic guidance; clinical evaluations at 6 weeks, 3, 6 and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: available at 6 weeks, 3, 6 and 12 months: LEM; FIM; TUG; 2MWT; radiographic findings; implant position ‐ tip‐apex distance; femoral neck shortening; heterotopic ossification ‐ Brooker stage; complications; length of follow‐up: 12 months Outcomes relevant to the review: mortality and unplanned return to theatre (both 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: the study was directed by the Canadian Orthopaedic Trauma Society (COTS) with no other conflicts reported Study dates: February 2007 to November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: “Permuted block randomisation”,“randomly generated modality” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “sealed envelopes” Comment: study authors do not report whether envelopes are opaque and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Trial appears pragmatic in design. Multicentre trial with no information available on surgeon expertise |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were balanced between groups and were mostly explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Registration with clinical trials register NCT00597779: first registered in January 2008 although study commenced in February 2007. It was not feasible to effectively assess risk of reporting bias using retrospectively‐prepared documents. We noted that SF‐36 was listed as an outcome, but was later dropped from the outcome list on the clinical trials register and was not reported in the published study report. |
| Other bias | Low risk | We identified no other sources of bias. |
Romero 2008.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: PCCP versus DHS |
|
| Participants |
Total number of randomised participants: 26 Inclusion criteria: AO 31A1 and A2 fractures; > 60 years of age Exclusion criteria: A3 fractures, osteoarthritis, other diseases, previous hip surgery Setting: single centre; hospital; Mexico Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (PCCP)
Note:
|
|
| Interventions |
General details: prophylactic antibiotics and antithromboembolics Intervention group 1
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: hospital stay; wound length; postoperative complications; consolidation time; surgery time; pain Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: December 2004 to February 2005 Note:
|
|
Sadowski 2002.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus DCS |
|
| Participants |
Total number of randomised participants: 39 Inclusion criteria: 31‐A3 low‐energy fractures; ≥ 55 years of age Exclusion criteria: pathological fractures; fractures associated with polytrauma; a pre‐existing femoral deformity preventing hip screw osteosynthesis or intramedullary nailing; previous surgery on the ipsilateral hip or femur; fractures extending 5 cm distal to the inferior border of the lesser trochanter Setting: single centre; orthopaedic hospital; Switzerland Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (DCS)
Note:
|
|
| Interventions |
General details: single dose of prophylactic antibiotics preoperatively; low‐molecular‐weight heparin from the day of surgery; prophylactic anticoagulation on the fifth postoperative day; performed by staff surgeons on a fracture table; mobilised out of bed on the second postoperative day; walking with weight bearing as tolerated on the third or fourth day; rehabilitation protocol identical for both groups; surgeons were experienced with both devices (had performed at least eight of each operation before the study) Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; mean units transfused; number of participants transfused; radiographic screening time; cut‐out; non‐union (and time to consolidation); implant failure; re‐operation; wound infection; pneumonia; pressure sores; DVT; PE; urinary infection; cardiac failure/infarction; all medical complications; mortality; pain at follow‐up; social function; transfer to long‐term care; mobility level; length of follow‐up: 12 months Outcomes relevant to the review: mortality (in hospital and 12 months); unplanned return to theatre (reported as major re‐operation) (at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors clearly report that no grants or outside funding was received Study dates: March 1998 and June 1999 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "No patient refused randomization, which was accomplished with use of computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quote (from direct communication with study authors): "All the surgeons involved in this study had performed an average of eight procedures with the PFN prior to the initiation of the randomized clinical trial." |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were relatively balanced between groups and were mostly explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Sanders 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Intertan (short and long nails) vs SHS |
|
| Participants |
Total number of randomised participants: 250 Inclusion criteria: people with intertrochanteric fractures; ≥ 55 years of age; ambulatory; able to participate in follow‐up activities; provided informed consent Exclusion criteria: polytrauma; pathological fractures; no fixed address Setting: multicentre (5 level‐1 trauma centres); Canada Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: use of general or spinal anaesthesia; perioperative antibiotics; treated with indirect reduction and percutaneous techniques. Surgeon's preference determined reduction technique, plate length, number of screws, use of a compression screw, use of ancillary fixation, nail length (long or short), and number of distal interlocking screws Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional measures (FIM and TUG; 2MWT, LEM; data available at discharge, 6 weeks, 3 months, 6 months, 1 year); union and non‐union, complications (screw, plate, and rod breakage; loss of mechanical instability; alignment), place of residence at discharge, LOS, Self‐Administered Comorbidities Questionnaire, Geriatric Depression Scale, transfusion rates and haemoglobin level; infection, medical complications, implant failure, or periprosthetic fracture; mortality Outcomes relevant to the review: mortality (3 and 12 months); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: unrestricted educational grant from Smith and Nephew Richards Study dates: 2008 to 2013 Note:
|
|
Saudan 2002.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus DHS |
|
| Participants |
Total number of randomised participants: 206 Inclusion criteria: low‐energy trochanteric fractures; > 55 years of age Exclusion criteria: pathologic fractures; polytrauma; previous ipsilateral hip or femur surgery; any fracture with extension 5 cm distal to the inferior border of the lesser trochanter; AO/OTA Type 31‐A3 Setting: single setting; orthopaedic hospital; Switzerland Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: preoperative prophylactic antibiotics; low‐molecular‐weight heparin followed by Coumadin (warfarin) as prophylactic anticoagulation for 6 weeks; identical rehabilitation protocol, mobilised out of bed on the second day, ambulation with weight bearing on the third or fourth day; clinical follow‐up at 3, 6 and 12 months; all surgeons had performed ≥ 8 of each operation before the study Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; operative blood loss; mean units transfused; number of participants transfused; radiographic screening time; cut‐out; non‐union (and time to consolidation); implant failure; re‐operation; wound infection; pneumonia; pressure sores; DVT; PE; urinary infection; cardiac failure/infarction; all medical complications; mortality; pain at follow‐up; social function; transfer to long‐term care; length of follow‐up: 12 months Outcomes relevant to the review: mortality (during hospital stay and 12 months); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: quote: "No benefits in any form have been received or will be received from a commercial party directly or indirectly to the subject of this article. No funds were received in support of this study" Study dates: March 1998 to July 2000 Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "No patient refused randomization, which was accomplished with use of computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quote (from direct communication with study authors): "All the surgeons involved in this study had performed an average of eight procedures with the PFN prior to the initiation of the randomized clinical trial." |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most participant loss was because of death, which is expected in this population. Additional loss to follow‐up due to participants leaving the country, all clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Schipper 2004.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus PFN |
|
| Participants |
Total number of randomised participants: 424 Inclusion criteria: radiological diagnosis of an unstable trochanteric femoral fracture, classified as 31A2.1 to 3 and 31A3.1 to 3; > 60 years of age; signed informed consent Exclusion criteria: inability to walk before the fracture; other fracture interfering with rehabilitation; (suspicion of) pathological fracture Setting: multicentre; 9 hospitals; the Netherlands Baseline characteristics Intervention group 1 (Gamma)
Intervention group 2 (PFN)
Note:
|
|
| Interventions |
General details: follow‐up was at 4 weeks, 4 months and 12 months; the anaesthesia was either general or regional but did not differ between groups nor did the level of experience of the surgeons; surgeons had experience with at least 5 procedures with both implants; routine prophylaxis for thrombosis given preoperatively and during postoperative hospital stay; prophylactic antibiotics given; all participants encouraged to walk fully weight bearing, starting as soon as possible after operation with support of a physiotherapist Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: amount of fracture healing; intraoperative complications; re‐operations (available at 4 weeks, 4 months and 12 months); mortality (available at 4 weeks, 4 months and 12 months); blood loss; complications; mobility; operating time; fluoroscopy time; reduction of fracture Outcomes relevant to the review: mortality (reported at 4 months and 12 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report "this research was equally supported by Stryker Howmedica and Mathys Medical Nederland. The authors have received benefits for professional use from both commercial parties related directly to the subject of this article. In addition, benefits have been directed to a research fund, foundation, educational institution, or other non‐profit organisations with which one or more of the authors is associated". Study dates: September 1998 to January 2002 Note:
|
|
Selim 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS with trochanteric stabilising plate versus PFLP |
|
| Participants |
Total number of randomised participants: 40 Inclusion criteria: unstable pertrochanteric fractures AO 31A2.2/AO 31A2.3; informed consent Exclusion criteria: open fractures; preoperative neurological deficits Setting: single centre; hospital; Egypt Baseline characteristics not reported Note:
|
|
| Interventions |
General details: all surgeries were done by 3 senior surgeons; immediate weight bearing, as tolerated, was allowed for all participants under physiotherapeutic guidance utilising a frame; participants were followed‐up clinically and radiologically for a minimum of 1 year at intervals of 2 weeks, 6 weeks, 3 months, 6 months and at 1 year Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: TAD; time to union; operation time; blood loss; length of hospital stay; functional status (HHS); superficial infections; deep infections; DVT; non‐union; implant failure; cut‐out Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: study authors declare that "there are no potential conflicts of interest or financial relationships relevant to this review" Study dates: January 2016 to December 2018 Note:
|
|
Sernbo 1988.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Ender pins versus CHS |
|
| Participants |
Total number of randomised participants: 206 Inclusion criteria: unstable intertrochanteric fracture of the hip Exclusion criteria: fractures sustained more than a week preoperatively; people in very poor health; pathological or subtrochanteric fractures Setting: single centre; hospital; Sweden Baseline characteristics Intervention group 1 (Ender)
Intervention group 2 (CHS)
Note:
|
|
| Interventions |
General details: spinal anaesthesia was used in all participants except 6 in Ender group and 8 in CHS group; the postoperative regimens of mobilisation and weight bearing were ordered by the surgeon, with no set protocol; all participants in CHS group received prophylactic antibiotics Intervention group 1
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of operation; duration of hospitalisation; mortality (available at 6 months and 12 months); malrotation; place of residence; re‐operations (available at 12 months); delayed and non‐unions; technical failure of stabilisation; loss of fixation; varus deformity; superficial infection; deep infection; distal migration of pins; pain, use of walking aids Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report 'no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. No funds were received in support of this study' Study dates: July 1983 to December 1985 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: used a random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment only described as using "sealed envelopes" with no other detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Number of surgeons and their skills and experience is not reported. It is uncertain whether surgeons were equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported that no participants were lost to follow‐up. Only participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Sernbo 1994.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: CHS with key and compressing screw versus CHS without compressing screw |
|
| Participants |
Total number of randomised participants: 153 Inclusion criteria: trochanteric hip fractures Exclusion criteria: fracture over 5 days old, pathological fractures, subtrochanteric fractures Setting: single centre, hospital, Sweden Baseline characteristics Intervention group 1 (CHS with key and compressing screws)
Intervention group 2 (CHS without key and compressing screws)
Note:
|
|
| Interventions |
General details: early weight bearing permitted in 75% of group 1 and 73% of group 2 participants Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery, bleeding, varsification, non‐union, sliding of lag screw Outcomes relevant to the review: none Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 1987 to 1988 Note:
|
|
Seyhan 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus InterTAN |
|
| Participants |
Total number of randomised participants: 75 Inclusion criteria: trochanteric fractures Exclusion criteria: pathological fractures; multiple traumas; people who died in the early postoperative period; people who were lost to follow‐up Setting: single centre; hospital; Turkey Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (InterTAN)
Overall:
Note:
|
|
| Interventions |
General details: same surgeon performed all surgeries on a fracture table using a closed approach; flexion exercises immediately after surgery; other than exceptional cases, 3 doses of cefazolin sodium (1 g IV) were used for antibiotic prophylaxis; subcutaneous enoxaparin sodium (1 x 40 mg (0.4 mL)) injection and antiembolic stockings were used to prevent DVT for 35 days; each participant was mobilised with a walker ≤ 48 hours postoperatively; participants were advised to use walkers with touch‐down weight bearing, but those who failed to achieve this task proceeded with weight bearing as tolerated; clinical follow‐up at 1, 2, 3, 6, 12 and 24 months Intervention group 1
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of surgery; fluoroscopy use; tip‐apex distance; tip‐cortex distance; time to mobilisation; time to full weight bearing; time to union; HHS; fracture gap before and after compression; lateral extension of nail; length of proximal femur; femur neck‐shaft angle; revision surgery Outcomes relevant to the review: unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflicts of interest and no detail of funding is reported Study dates: not reported Note:
|
|
Shannon 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: cephalomedullary nail versus cephalomedullary nail: short or long |
|
| Participants |
Total number of randomised participants: 220 Inclusion criteria: isolated OTA/AO 31A1‐3 pertrochanteric femur fractures; ≥ 18 years of age Exclusion criteria: < 18 years of age; dementia limiting the ability to engage in patient‐reported outcomes; pathological fractures Setting: single centre; level‐1 trauma centre; USA Baseline characteristics Intervention group 1 (short)
Intervention group 2 (long)
Note:
|
|
| Interventions |
General details: the supervising surgeon was 1 of 4 traumatologists for 70% of short nails and 76% of the long nails with the remaining participants being covered by general orthopaedic surgeons; follow‐up occurred at 2 weeks, 6 weeks, 3 months, 6 months and 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HRQoL (available at 3 months); HHS; TAD subtrochanteric fracture line extension; operative time; estimated blood loss; re‐operations (available at 12 months); mortality (available at 3 months) Outcomes relevant to the review: HRQoL (in SF‐36, reported at 3 months); unplanned return to theatre (reported at 12 months); mortality (reported at 3 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: December 2014 to December 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Done using computer‐generated randomisation by block randomisation strategy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were all performed by senior surgeons but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Other performance bias: surgeon experience of both implants | Low risk | Most operations were conducted by experienced surgeons. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Blinding of outcome assessment: HRQoL (detection bias) | Low risk | Study authors report participants were not blinded to the intervention. We did not expect lack of blinding to influence detection bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost, these losses were balanced between groups, and reasons for loss were clearly explained. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered with a clinical trials register (NCT02285127). Outcomes in the published report are consistent with those in the trial registration document. |
| Other bias | Low risk | We identified no other sources of bias. |
Sharma 2018.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: ultra‐short PFN vs DHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: cases of stable intertrochanteric fractures in adults > 18 years of age Exclusion criteria: cases with marrow cavity blocked by another implant, deformed femur, narrow marrow cavity, pathological fracture or old, complicated fracture Setting: single centre; government secondary‐level hospital; Brazil Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: 1 surgeon operated on all cases; exercises from POD1, early mobilisation with walker as soon as possible with non‐weight bearing, later partial weight bearing started depending on compliance of participant Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative observations (length of incision, radiation exposure, duration of surgery, average blood loss, need for blood transfusion, failure to achieve closed reduction, hospital LOS, duration of full weight bearing); early complications (iatrogenic fracture, technical error, superficial infection, DVT); late complications (loss of reduction, implant failure, second surgery, mean shortening, non‐union, malunion, deaths); functional outcome (HHS; measured at 1 month, 3 months, 6 months, and 2 years) Outcomes relevant to the review: mortality (reported as after 3 months, we have assumed final time point of 24 months); unplanned return to theatre (assumed to be up to 24 months) |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: 2011 to 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quasi‐randomised trial. Participants allocated alternately to each intervention |
| Allocation concealment (selection bias) | High risk | Allocation is alternate, with same surgeon performing all operations. It is not possible to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors do not describe whether the surgeon was equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Shi 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: hemiarthroplasty versus internal fixation with PFLP |
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: intertrochanteric fractures; signed informed consent for enrolment, operation and anaesthesia Exclusion criteria: severe cardiopulmonary insufficiency; severe liver and kidney function deficiency; coagulation disorder; spinal deformity; malignant tumours; fractures in other parts of lower limbs; mental illness; used analgesia device after operation; refused enrolment Setting: single centre, hospital, China Baseline characteristics Intervention group 1 (observation)
Intervention group 2 (PFLP)
Note:
|
|
| Interventions |
General details: all participants were treated with spinal‐epidural anaesthesia in a lateral position; followed up at 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; intraoperative blood loss; postoperative drainage time; walking speed; 5‐times sit‐to‐stand test time; HHS; pain; hospital stay; time walking on crutches and walking without; complications; HRQoL (available at 12 months) Outcomes relevant to the review: HRQoL (in SF‐12, reported at 12 months, physical and psychological scores are available) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no funding was received with no conflicts of interest Study dates: May 2014 to December 2016 Note:
|
|
Shin 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: cephalomedullary nail versus cephalomedullary nail: Zimmer natural nail or PFNA II |
|
| Participants |
Total number of randomised participants: 380 Inclusion criteria: intertrochanteric hip fractures; written and informed consent Exclusion criteria: < 65 years of age; ASA score of V; poor pre‐fracture walking ability; pathological intertrochanteric hip fractures; multiple fractures Setting: single centre; hospital; South Korea Baseline characteristics (only for analysed participants) Intervention group 1 (Zimmer)
Intervention group 2 (PFNA)
Note:
|
|
| Interventions |
General details: the same postoperative rehabilitation regimen was used in both groups; isometric quadriceps exercises were started on the first day after surgery and weight bearing as tolerated with a walker was resumed 2 days after surgery Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS; operation time; fluoroscopy time; lateral hip pain; Parker and Palmer walking ability score; TAD; re‐operations (available at 12 months); fracture reduction; screw sliding distance; cut‐out distance; avascular necrosis; cleveland zone; mortality (available at 12 months) Outcomes relevant to the review: unplanned return to theatre (reported at 12 months), mortality (reported at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflict of interest and no details on funding Study dates: January 2011 to August 2014 Note:
|
|
Singh 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus locking compression plate (LCP) |
|
| Participants |
Total number of randomised participants: 48 Inclusion criteria: people with unilateral, closed, unstable trochanteric fractures (31.A2 & 31.A3), > 18 years of age Exclusion criteria: bilateral fractures, polytrauma, pathologic fractures, open fractures (ASA status IV or V), associated hip osteoarthritic (Kellgren‐Lawrence grade 3 or 4) Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: before surgery, each participant’s standard plain radiographs (1 anteroposterior, 1 lateral) were evaluated. Participants underwent surgery as soon as their general medical condition allowed. Knee and ankle exercises on POD1. Non‐weight bearing walking with bilateral axillary crutches usually on POD3 to 5. Progressive weight bearing started after 6 weeks Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: perioperative measures: operative time, incision length, radiologic exposure, LOS, blood loss, union rate, time to union, reduction quality. Complications: deep and superficial infections; local site pain; non‐union; implant‐related breakage, cut‐out, or Z‐effect; unrelated to fracture (bed sore, chest infection and DVT; revision surgery, shortening). Functional outcome (HHS; at final 2‐year follow‐up); mobility (Palmer and Parker Mobility score; at final 2‐year follow‐up) Outcomes relevant to the review: unplanned return to theatre (at 2 years) |
|
| Notes |
Funding/sponsor/declarations of interest: funding sources not reported. Study authors report no actual or potential conflicts of interest Study dates: April 2009 to June 2011 Note:
|
|
Singh 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus DHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: elderly participants (> 60 years of age); with stable intertrochanteric fractures (31 A.1 to A2.1); willing to give informed consent Exclusion criteria: younger people (< 60 years of age), with pathological fractures; unstable intertrochanteric fractures (31 A2.2 to A3.3); unfit for surgery; polytrauma; previous hip surgery; refusal to participate Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: under supervision of 2 consultant surgeons with adequate skill in using both implants; encouraged to perform exercises on POD1. Weight bearing with a walker, and physiotherapy support, on POD2 Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative variables (blood loss, fluoroscopy time, duration of surgery); neck shaft angle, TAD; functional outcomes (modified HHS; SF‐12 PCS and MCS); complications (varus collapse; lateral migration of blade/screw; cut‐out; non‐union; implant failure; infection; fracture shaft of femur; re‐operation; symptomatic DVT; decubitus ulcer, hyponatraemia; AF, pneumonia); mortality Outcomes relevant to the review: mortality (6 months); HRQoL (SF‐12 PCS; at 1 year); unplanned return to theatre (at 1 year) |
|
| Notes |
Funding/sponsor/declarations of interest: funding sources, or declarations of interest not reported Study dates: September 2014 to October 2016 Note:
|
|
Song 2011.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus DHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: elderly people (> 60 years of age); with stable intertrochanteric fractures (31 A.1 to A2.1); willing to give informed consent Exclusion criteria: clinical signs of infection; neoplasia; other operative procedures within the previous 3 months; pathological fractures; unstable fractures; perioperative myocardial infarction; inflammatory myopathy Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: standard traction table; supine position; performed under an X‐ray amplifier; low‐molecular‐weight heparin calcium injection Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: C‐reactive protein levels; creatine kinase level Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: study authors declare no funding sources and that there were no conflicts of interest Study dates: January 2008 and December 2009 Note:
|
|
Stappaerts 1995.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus VDP endoprosthesis |
|
| Participants |
Total number of randomised participants: 90 Inclusion criteria: ≥ 70 years of age; fresh unstable peritrochanteric fractures; non‐arthritic hip Exclusion criteria: reversed fractures Setting: single centre; hospital; Germany Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (VDP)
Overall:
Note:
|
|
| Interventions |
General details: not reported Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss, transfusion need; mortality (available at 30 days and 3 months); severe hypotension; cardiac arrhythmia; functional capacity/mobility Outcomes relevant to the review: mortality (reported at 30 days and 3 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: May 1989 to May 1992 Note:
|
|
Stark 1992.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: SHS versus Ender nails |
|
| Participants |
Total number of randomised participants: 149 Inclusion criteria: > 50 years of age; trochanteric fractures Exclusion criteria: 18 who died; 3 with early re‐operations; 2 who refused; 4 changed residence; 15 serious medical problems; 15 with weakness Setting: single centre; hospital; Sweden Baseline characteristics Intervention group 1 (SHS)
Intervention group 2 (Ender)
Note:
|
|
| Interventions |
General details: spinal anaesthesia was used in 86 participants and general anaesthesia in 6 participants; all participants received dextran or low‐dose heparin; participants were examined 3 and 6 months after operation Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; perioperative bleeding; postoperative bleeding; deep infections; re‐operations (available at 6 months); fracture reduction; position of the implant; Ender nail migration; screw sliding; redislocation; bone healing; use of walking aids; union rate; pain evaluation; range of motion; walking distance; walkway function Outcomes relevant to the review: unplanned return to theatre (reported at 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: June 1982 to May 1985 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Number of surgeons and their skills and experience are not reported. It is uncertain whether surgeons were equally experienced with both types of implants. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes (such as decision to re‐operate). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trial registration or pre‐published protocol. It is not possible to effectively assess risk of reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Stern 2011.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: screws versus helical blades Note:
|
|
| Participants |
Total number of randomised participants: 335 Inclusion criteria: extracapsular hip fractures classified according to AO/OTA as 31‐A1 and A2 (pertrochanteric fractures), and 31‐A3 (intertrochanteric fractures); > 60 years of age; fracture caused by low‐energy injury Exclusion criteria: pathological fractures; previous ipsilateral hip or femur surgery; refusal to participate in the study Setting: single centre; hospital; Switzerland Baseline characteristics Intervention group 1 (screw)
Intervention group 2 (blade)
Note:
|
|
| Interventions |
General details: operations were performed by a large number of surgeons with varying levels of experience; all participants were managed in the same fashion postoperatively, regardless of implant; they were mobilised out of bed and started on a physical programme of weight bearing as tolerated within the first few days; implant was at the discretion of the operating surgeon Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: position of cephalic implant in the femoral head; re‐operations (available at 12 months); correlation of the implant position with cut‐out of the screw or helical blade; TAD; degree of ambulation; pain; mortality (available at 12 months) Outcomes relevant to the review: unplanned return to theatre (reported at 12 months); mortality (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no funding was received for this work, any conflicts of interest are not reported Study dates: October 2006 to July 2009 Note:
|
|
Su 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma 3 versus InterTAN |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: uniquely unstable intertrochanteric fractures (AO/OTA classification, 31‐A2.2 to 31‐A3.3); fracture caused by low‐energy trauma; consent Exclusion criteria: polytrauma; high‐energy trauma; open fractures; pathological fractures; ASA score of V; inability to work before injury; presence of arthritis in injured hip Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (Gamma 3)
Intervention group 2 (InterTAN)
Note:
|
|
| Interventions |
General details: all participants received prophylactic antibiotics (cefuroxime 1.5 g) intravenously 30 minutes before skin incision and for 48 hours after surgery; all participants were treated with rivaroxaban for 35 days from the first day after operation as prophylactic anticoagulation; all operations were performed by two experienced orthopaedic surgeons; all participants were placed supine on a fracture table after general anaesthesia and closed reduction; participants were instructed to use walkers with touch‐down weight bearing postoperatively; 6 weeks after surgery, partial weight bearing with walker assistance was routine for all participants and full weight bearing was not permitted until sufficient bone consolidation; participants were followed at 1, 2, 3, 4, 6 and 12 months after surgery Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: time required for the closed reduction, X‐rayed times, operation time, estimated intraoperative blood loss; number of transfusions; TAD; decrease in length of the femoral neck; time to mobilisation; fracture healing; blood loss; pre‐ and postoperative haemoglobin; reduction results; superior position of lag screw; pulmonary embolism; DVT; lag screw cut‐out; hip pain; mortality (available at 12 months); re‐operations (available at 1 and 3 months); union time; HHS Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre (because of cut‐out) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflict of interest but no details on funding Study dates: January 2011 to August 2014 Note:
|
|
Tao 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus reverse less invasive stabilisation plate (LISS) |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: people with intertrochanteric femoral fractures; > 65 years of age Exclusion criteria: pathological fractures, osteoarthritis of the hips, ASA status IV or V Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: 3 orthopaedic consultants (surgeons are familiar with PFNA but not with LISS); prophylactic IV first‐generation cephalosporin started before surgery and continued up to 48 to 72 hours postoperatively; partial and full weight bearing allowed on 3rd and 6th postoperative week for PFNA group; partial and full weight bearing on 6th and 12th postoperative week for LISS group Intervention group 1:
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of surgery, fluoroscopy time, blood loss, quality of reduction (open reduction cases), LOS, bone‐healing time, postoperative walking ability, HHS, postoperative complications (pressure sore, urinary infection, pulmonary infection, DVT), mortality Outcomes relevant to the review: mortality |
|
| Notes |
Funding/sponsor/declarations of interest: funding or declarations of interest not reported Study dates: September 2010 to August 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Study authors state that surgeons were familiar with PFNA but not with reverse LISS. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number randomised to each group and the numbers of losses in each group was not reported and we therefore could not ascertain amount of attrition in the study. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Unclear risk | We noted a difference in postoperative/rehabilitation management regarding time at which weight bearing was allowed in each group. |
Teerenhovi 1984.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: AO angled plate versus DHS |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: proximal fracture of femur Exclusion criteria: not reported Setting: single centre; hospital; Finland Baseline characteristics not available (see notes) |
|
| Interventions |
General details: no details Intervention group 1
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: re‐operations (available at 12 months); varus deformity; ability to move; range of hip flexion Outcomes relevant to the review: unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: October 1981 to March 1983 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | We could not be certain whether surgeons were equally experienced with both implants. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Unclear risk | Because we had only a limited translation, we could not be certain of other risks of bias. |
Trafton 1984.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: CHS versus Harris condylocephalic nail |
|
| Participants |
Total number of randomised participants: 84 Inclusion criteria: intertrochanteric fractures Exclusion criteria: not reported Setting: single centre, hospital, USA Baseline characteristics
|
|
| Interventions |
General details: follow‐up was done after 6 weeks and up to 6 months Intervention group 1
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; fraction reduction; nail placement; fixation failure; osteoporosis; unstable fractures; varus reduction; re‐operations (available at 6 weeks); blood replacement; infection rate; mortality (available at 6 months) Outcomes relevant to the review: unplanned return to theatre (reported at 6 weeks); mortality (reported at 6 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report the experience and skills of the surgeons |
| Other performance bias: surgeon experience of both implants | Unclear risk | It is uncertain whether surgeons are equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect lack of blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Because of limited information in the abstract, study flow is unclear. For the purpose of reporting the review outcomes, we assumed that there were no losses. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | High risk | Study report was available only as an abstract which we expected was not peer‐reviewed. In addition, because of limited detail in the abstract, we could not be certain of other risks of bias. |
Utrilla 1998.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: CSP versus fixed nail plate (RAB monoblock nail plate) |
|
| Participants |
Total number of randomised participants: 200 Inclusion criteria: > 60 years of age; trochanteric fracture of the femur Exclusion criteria: not reported Setting: single centre; hospital; Spain Baseline characteristics Intervention group 1 (CSP)
Intervention group 2 (RAB)
Note:
|
|
| Interventions |
General details: all participants were treated by the same group of 4 surgeons, who had the same degree of experience; postoperative activities were programmed as follows: sitting down at 24 hours, and a trial deambulation at 48 hours, if tolerable; the participants were reviewed, clinically and radiologically, at 6 weeks, 3 months, 6 months and 12 months postoperatively Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of procedure; surgical difficulty; complications; blood loss; mortality (available at 1 month, 3 months and 6 months); re‐operations (available at 12 months); shortening of limb; able to sit up; hospital stay; ambulation; hip tenderness; hip flexion; external rotation; shortening of limb; complications Outcomes relevant to the review: mortality (reported at 3 months and 6 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to treatment groups. Study authors report that interventions were performed by surgeons who were equally experienced in using both types of study implants. |
| Other performance bias: surgeon experience of both implants | Low risk | Experienced surgeons and we expected that they experienced with both types of implants |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect lack of blinding to influence detection bias for this outcome. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of intervention could influence judgments made by surgeons when assessing this subjective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost, these losses were balanced between groups, and reasons for loss were clearly explained. |
| Selective reporting (reporting bias) | Unclear risk | Study authors did not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Utrilla 2005.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: TGN versus CHS |
|
| Participants |
Total number of randomised participants: 210 Inclusion criteria: trochanteric proximal femoral fractures; ≥ 65 years of age Exclusion criteria: subtrochanteric fractures; pathologic fractures; history of a previous lower limb injury; severe concomitant medical condition (ASA score of V) Setting: single centre; orthopaedic hospital; Spain Baseline characteristics Intervention group 1 (TGN)
Intervention group 2 (CHS)
Overall
Note:
|
|
| Interventions |
General details: fracture fixation was performed within 4 days; 4 surgeons experienced with Gamma nails; first 3 TGN operations performed by each surgeon were not included in the study and served as the learning curve; spinal anaesthesia (all but 3 participants); traction table with fluoroscopic control; suction drains for 48 hours; antibiotic and thromboembolic prophylaxis; clinical examination at 1, 3, 6 and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood transfusion; radiographic screening time; operative fracture of the femur; later fracture of the femur; cut‐out of implant; re‐operation; deep wound sepsis; local wound healing complications; DVT; shortening; hip flexion; mobility; pain (hip and thigh pain); mortality (available at 1, 3, 6 and 12 months); length of follow‐up: 12 months Outcomes relevant to the review: mortality (at 3 months and at 12 months); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: quote: "No financial support of this project occurred. None of the authors received anything of value" Study dates: October 1998 to December 2000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized for treatment into 2 groups based on sequence of admission, sealed envelopes were opened before the surgeon attempted a closed reduction of the fracture." Comment: no additional details |
| Allocation concealment (selection bias) | Unclear risk | Study authors do not report whether envelopes are opaque and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quote: "Four surgeons experienced in the standard Gamma nail did all the operations; however, the first 3 TGN operations performed by the surgeons were not included in the study and served as the learning curve for the new instrumentation." |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were balanced between groups and were mostly explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Vaquero 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus Gamma 3 |
|
| Participants |
Total number of randomised participants: 64 Inclusion criteria: isolated, unstable, closed or type I open trochanteric fractures treated with intramedullary fixation; ≥ 55 years of age; written and informed consent; agreed to attend all planned follow‐up evaluations Exclusion criteria: pathological fractures of any origin other than osteoporosis; type II and type II open fractures; rheumatoid arthritis; existing neurological and psychiatric disorder (e.g. Parkinson's disease, multiple sclerosis, severe depression or dementia) that would preclude reliable assessment; active malignancy; wound and/or bone healing disorders of any cause other than diabetes mellitus or smoking; polytrauma; people with a life expectancy of < 3 months; refusal to sign the informed consent form; those unable to walk independently (excluding use of one stick) prior to injury Setting: multicentre; 6 hospitals; Spain Baseline characteristics (for analysed participants only) Intervention group 1 (PFNA)
Intervention group 2 (Gamma)
Note:
|
|
| Interventions |
General details: all participants received similar preoperative treatment including a combination of skin traction, prophylactic medication for DVT and surgical infection and adequate pain relief; surgery was performed on a traction table under spinal anaesthesia, except for 1 Gamma 3 participant who received general anaesthesia; participants were encouraged to partially weight‐bear, starting as soon as possible; participants were discharged to their previous residence or a rehabilitation facility as soon as their health status allowed Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HRQoL (available at 6 months and 12 months); HHS; baseline pain; mortality (available at 3 months, 6 months and 12 months); fracture fixation; fracture healing; fluoroscopy time; surgery duration; implant/surgery complications (breakage; loosening, and/or migration of hardware components, loss of reduction, fracture or difficulty with nail insertion, difficulty with distal screw locking, blade/screw measurement; guide wires); bone/fracture complications (loss of reduction; delayed healing; malunion; non‐union; fracture impaction; refracture; avascular head necrosis); soft tissue/wound complications (superficial or deep infection; wound haematoma or seroma; trochanteric bursitis; tendinitis of the gluteus medius or minimus; local pain; neurological injury); general complications (pressure sores; thromboembolic complications; pneumonia; sepsis); time of hospital stay; TAD Outcomes relevant to the review: HRQoL (in SF‐36 with PCS, and EQ‐5D; reported at 12 months); mortality (reported at 3 months, and 12 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report "data collection and data analysis was supported by the AO Foundation and a financial grant from Synthes, GmbH, Switzerland." The authors also state no conflicts of interest that could inappropriately influence their work Study dates: January 2008 to October 2009 Note:
|
|
Varela‐Egocheaga 2009.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail versus PCCP |
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: > 60 years; stable intertrochanteric fracture (AO/OTA 31.A1–31.A2.1) Exclusion criteria: open reduction; reverse obliquity fractures (AO/OTA 31.A3); unstable intertrochanteric fractures; pathological fracture; presence of metastatic malignant disease; ipsilateral lower limb surgery; contralateral hip fracture within the past 12 months Setting: single centre; orthopaedic hospital; Spain Baseline characteristics Intervention group 1 (Gamma 3)
Intervention group 2 (PCCP)
Note:
|
|
| Interventions |
General details: fracture table; immediate postoperative full weight bearing; prophylactic antibiotics and prophylactic low‐molecular‐weight heparin (6 weeks postoperatively); 1‐year period prior to study as 'learning curve' period for surgeons Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood transfusion; fall in haemoglobin; cut‐out of implant; confusion; stroke; congestive cardiac failure; pneumonia; genitourinary infection; LOS; mortality; discharge to intermediate care; postoperative analgesia (duration and dose of metamizole); failure to regain mobility; length of follow‐up: 12 months Outcomes relevant to the review: mortality (during hospital stay and 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: June 2006 and March 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized using a table of randomized numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Study authors described prior 'learning curve' period before start of the trial, and we judged that surgeons were therefore likely to have experience with both implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses, which were balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Verettas 2010.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: intramedullary nail versus DHS |
|
| Participants |
Total number of randomised participants: 120 Inclusion criteria: unstable trochanteric proximal femoral fractures (AO/OTA type 31‐A2 and Evans type III or IV); > 70 years of age; walk independently with or without aid; low‐energy injury within 24 hours of admission Exclusion criteria: rheumatoid arthritis; pathological fracture Setting: single centre; orthopaedic hospital; Greece Baseline characteristics Intervention group 1 (Intramedullary nail)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: operated as soon as possible after their admission and in no case later than 24 hours; general parenteral opiate or spinal analgesia dependent on the anaesthetist’s assessment; reduced by closed methods; prophylactic antibiotics (cephalosporin) for 48 hours; prophylactic low‐molecular‐weight heparin for a total of 3 weeks; postoperative analgesia included a non‐steroid anti‐inflammatory medication; surgeons had previous experience of the use of these implants Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; blood loss; radiographic screening time; number of participants transfused; superficial wound infection; DVT ("immediate post‐operative"); cardiovascular complication ("immediate post‐operative"); neurologic complication/ delirium ("immediate post‐operative"); respiratory complication ("immediate post‐operative"); haematocrit; oxygen saturation and pressure; mental test score; LOS; days to being able to walk with a walker; mortality (in hospital); pain score; length of follow‐up: duration of hospital stay (mean 10 days) Outcomes relevant to the review: mortality (during hospital stay) |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported; study authors declare no conflicts of interests Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The patients were allocated to each group alternatively on their admission." |
| Allocation concealment (selection bias) | High risk | It was not possible to conceal allocation with this method of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Quote: "In our study the operating time was similar in both groups, possibly because the surgeons had previous experience of the use of these implants." However, there was a change in the type of nail used during the study period, and we could not be certain whether all surgeons were equally experienced with the newer implant. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses which are explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Vidyadhara 2007.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma nail (one femoral neck screw) versus Ace nail (two femoral neck screws) |
|
| Participants |
Total number of randomised participants: 73 Inclusion criteria: > 60 years of age; unstable trochanteric fractures Exclusion criteria: not reported Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (Gamma nail)
Intervention group 2 (Ace nail)
Overall:
Note:
|
|
| Interventions |
General details: surgery was performed in all cases by the same senior surgeon; participants received a prophylactic dose of an intravenous antibiotic; weight bearing within the limits of pain was allowed with walking frame or crutches as soon as possible in all participants; participants were discharged when comfortably walking; all participants were strictly advised to start full weight bearing without walking aids after the 4th week postoperatively; a standardised uniform rehabilitation protocol was followed in all participants regardless of the method of fixation used or the progress of fracture union; participants were reviewed with clinico‐radiological assessment at 4 months, 12 months and 24 months after operation; 7 participants had general anaesthesia and the other 66 had spinal anaesthesia Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operating time; blood loss; bent guide wires; broken drill bit; mean fracture reduction time; difficult lag screw insertions; ideal implant position; fracture reduction; TAD; sliding of the lag screw; HHS; shortening; delay in walking; hip pain at 1 month; thigh pain at one month; limp; difficulty in squatting and sitting crossed legged; time to weight bearing Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2001 to May 2003 Note:
|
|
Vossinakis 2002.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: SHS versus external fixation |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: pertrochanteric fractures Exclusion criteria: participants with dementia, basicervical fractures, pathological fractures, presenting more than one week after injury, subtrochanteric extension or reverse obliquity, people with associated fractures which could interfere with rehabilitation, people at high surgical risk (ASA > 4) Setting: hospital, single centre, Greece Baseline characteristics Intervention group 1 (SHS)
Intervention group 2 (external fixation)
Notes:
|
|
| Interventions |
General details: 4 surgeons; perioperative chemoprophylaxis (cefuroxime, 750 mg), thromboprophylaxis with low‐molecular‐weight heparin (enoxaparin), wearing of graduated compression stockings for 2 weeks; mobilisation using a walking frame, allowing full weight bearing, attempted from the second postoperative day; followed up for 6 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operating time; intraoperative complications; blood loss; blood transfusions; pain; mobility; LOS; place of residence; mortality, shortening; deep infection; re‐operations Outcomes relevant to the review: mortality (in hospital, and at 6 months); unplanned return to theatre Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors report "no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article" Study dates: 1997 to 1998 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "sealed envelopes containing cards, indicating the treatment for each patient" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors do not report surgeons' experience levels |
| Other performance bias: surgeon experience of both implants | Unclear risk | It is uncertain whether surgeons were equally experienced with both types of implants. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of assessors of objective measures would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We expected surgeons were likely to assess this outcome, and decisions to re‐operate could be subjective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trial registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Wang 2011.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: expandable intramedullary nail versus PFN |
|
| Participants |
Total number of randomised participants: 46 Inclusion criteria: intertrochanteric fractures Exclusion criteria: unknown Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (expandable intramedullary nail)
Intervention group 2 (PFN)
Note:
|
|
| Interventions |
General details: not reported Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; intraoperative blood loss; length of incision; X‐ray exposure; duration of inpatient stay; time to bone union; HHS Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: study authors state that they received no financial or other benefits and have no conflicts of interest Study dates: August 2005 to June 2009 Notes:
|
|
Wang 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA vs DHS |
|
| Participants |
Total number of randomised participants: 114 Inclusion criteria: diagnosed with intertrochanteric fractures on X‐ray; no cardiac accidents before admission; no cognitive disorder; preoperative BP and blood sugar (and other common diseases) controlled in a normal state Exclusion criteria: severe cardiovascular and cerebrovascular disease; combined with other fractures; pathological fracture; mental illness before fracture; surgical contraindications Setting: hospital; single centre; China Baseline characteristics (overall)
Note:
|
|
| Interventions |
General details: all given antibiotics; epidural anaesthesia; on POD2, allowed to sit, half‐squat, sit up, turn over, and perform contractile functional exercises of active and passive muscle, as well as knee flexion and extension exercises Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; volume of intraoperative blood loss; postoperative drainage volume; time to weight bearing; serum inflammatory markers; serum levels of MI markers and heart failure markers Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2016 to February 2018 Note:
|
|
Watson 1998.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: DHS versus Medoff |
|
| Participants |
Total number of randomised participants: 178 participants, 182 hips Inclusion criteria: intertrochanteric hip fracture Exclusion criteria: previous hip fracture or surgery; pathological fractures Setting: hospital, single centre, USA Baseline characteristics (data only reported for participants followed up at 6 months) Intervention group 1 (DHS)
Intervention group 1 (Medoff)
Overall:
Note:
|
|
| Interventions |
General details: surgery within 48 hours; performed by resident staff supervised by 1 of 4 attending staff; prophylactic antibiotics for 48 hours; closed suction drainage for 36 hours; compression stockings; subcutaneous heparin; mobilised on second day; weight bearing where appropriate; follow‐up at 2 and 6 weeks, 3, 6 and 12 months Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: blood loss, ambulatory function; living status; pain; time to union; LOS; fixation failure; mortality Outcomes relevant to the review: mortality (in hospital) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: November 1994 to December 1996 Note:
|
|
Weisbrot 2005.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PCCP versus DHS |
|
| Participants |
Total number of randomised participants: 110 Inclusion criteria: trochanteric fractures Exclusion criteria: not reported Setting: single centre; hospital; country is not specified Baseline characteristics not reported |
|
| Interventions |
General details: no details Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: DVT; cardiac complication; chest infection; pressure sores; implant failure; deep wound infection Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 2001 to 2003 Notes:
|
|
Wild 2010.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus Targon PF |
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: pertrochanteric femoral fractures (AO type 31.A2) Exclusion criteria: not reported Setting: single centre; hospital; Germany Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (Targon PF)
Overall:
|
|
| Interventions |
General details: surgery completed on average 1.2 days post‐injury; implantation was performed by the same two experienced surgeons who were familiar with the implants and had already been using them for many years; participants were examined clinically and radiologically at 2 weeks, 6 weeks, 3 months, 6 months and 12 months postoperatively Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional status (HHS); operative time; fluoroscopy time; mortality; hip motion; superficial infection; postoperative fractures; non‐union; cut‐out Outcomes relevant to the review: mortality (perioperative) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2006 to December 2007 Note:
|
|
Wu 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: Gamma 3 versus PFNA‐II |
|
| Participants |
Total number of randomised participants: 415 Inclusion criteria: informed consent; trochanteric fractures Exclusion criteria: < 60 years of age; not available for examination in outpatient clinic because of residing in other provinces or countries; previous hip fracture on the affected site; multiple fractures; confinement to a bed; pathological fractures; subtrochanteric fracture; limited life expectancy because of significant medical comorbidities (ASA grads IV and V); reverse obliquity fracture Setting: multicentre; 3 hospitals; China Baseline characteristics Intervention group 1 (Gamma 3)
Intervention group 2 (PFNA‐II)
Note:
|
|
| Interventions |
General details: all surgeries were performed by 32 orthopaedic trauma fellows who ‐ although familiar with both implants ‐ were defined as beginners because they had previously not performed the operations independently; general and spinal anaesthesia were used in both groups; all participants were operated on in the supine position on traction table; participants received prophylactic intravenous antibiotics starting 1 hour before operation; postoperatively, chemical venous thromboembolism prophylaxis (weight‐adjusted doses of Fraxiparine) was adopted; regardless of any fracture type and different internal fixation, all participants complied with the same rehabilitation protocol; the active part or full weight bearing should be performed gradually as tolerated thereafter; follow‐up occurred at 1, 3, 6 and 12 months postoperatively (mean 27.2 months (range 14 to 42 months)) Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: quality of reduction; fracture gap; lateral wall fragment; closed/open reduction; nail length; TAD; operation time; fluoroscopy time; time to mobilisation; blood units transfused; hospital stay; functional status (HHS); hip and thigh pain; non‐union; re‐operations (available at 12 months); mortality (in hospital and at 12 months); complications related to fixation (cut‐out, implant breakage, implant loosening, periprosthetic fracture, redisplacement, tractus irritation) Outcomes relevant to the review: unplanned return to theatre (reported at 12 months); mortality (reported during hospital stay and at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflicts of interest to disclose and that the research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors. Study dates: January 2011 to February 2017 Note:
|
|
Xin 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: DHS versus DHS with antirotation |
|
| Participants |
Total number of randomised participants: 96 Inclusion criteria: unstable intertrochanteric fractures Exclusion criteria: Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (DHS)
Intervention group 2 (DHS with antirotation screw)
Note:
|
|
| Interventions |
General details: unknown Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: surgical time; intraoperative blood loss; exposure time of X‐ray; fracture healing time; postoperative HHS; postoperative complications Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: study authors declare no conflicts of interest Study dates: June 2005 to June 2012 Note:
|
|
Xu 2010a.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus Gamma 3 |
|
| Participants |
Total number of randomised participants: 136 Inclusion criteria: unstable proximal femoral fracture; ASA score of I to IV; signed informed consent; > 60 years of age Exclusion criteria: Pathological fractures; inability to walk prior to injury; severe concomitant medical condition Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (Gamma 3)
Note:
|
|
| Interventions |
General details: operations were performed by surgeons who had experience with at least 5 procedures using the PFNA and the Gamma 3; each participant was given antibiotics with ceftriaxone preoperatively; either spinal or general anaesthesia was used; participants underwent surgery on a traction table Intervention group 1
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; fluoroscopy time; blood loss; intraoperative complications; result of fracture reduction; time till hospital discharge; number of transfusions; number of open reductions; time to fracture healing; complications (femoral shaft fracture, superficial wound infection, haematoma, lateral migration, decubitus ulcer, chest infection, UTI); pain; mobility Outcomes relevant to the review: none
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 2006 to August 2008 Note:
|
|
Xu 2010b.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus DHS |
|
| Participants |
Total number of randomised participants: 106 Inclusion criteria: unstable proximal femoral fracture (AO category 31‐A2) Exclusion criteria: < 65 years of age; pathological fractures; fractures associated with polytrauma; previous surgery on the ipsilateral hip or femur; inability to work before injury; severe concomitant medical condition (ASA status V) Setting: single centre; orthopaedic hospital; China Intervention group 1 (PFNA)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: performed through an open approach with direct exposure of the fracture; all operations were performed by surgeons who had performed ≥ 3 procedures with both interventions; preoperative ceftriaxone (2 g); general or spinal anaesthesia; prophylactic antibiotics for 3 to 5 days; movement of hip, knee and ankle joints on POD1; continuous passive motion rehabilitation devices used twice daily; clinical examination at 1, 3, 6 and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (at 3 and 12 months); operation time; fluoroscopy time; blood loss; blood transfusion; cut‐out; union; fixation failure; wound infection; lower respiratory tract infection; decubital ulcer; UTI; cerebral infarction; LOS; mobility score (Parker scale at 3 and 12 months); time to mobilise with frame; tIme to achieve preoperative mobility; return to preoperative mobility at 12 months; shortening of the femur on radiograph at 12 months Outcomes relevant to the review: unplanned return to theatre (12 months); mortality (at 3 and 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported, authors declare no conflicts of interest Study dates: August 2006 and June 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “consecutive numbered and sealed envelopes based on a computer generated list” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “sealed envelopes were opened before the surgeon performed the operation” Comment: study authors do not report whether envelopes are opaque or sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Low risk | Quote: “All operations were performed by surgeons who had performed at least three procedures with both the PFNA and the DHS”. |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type of implant could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were balanced between groups, and mostly explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Xu 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus DHS |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: ≥ 65 years of age; femoral neck bone density score ≤ 2.5 standard deviations; primary femoral intertrochanteric fracture Exclusion criteria: femoral head necrosis; Evans type II fracture or other serious complications detected in imaging examinations; surgical indications Setting: hospital; single centre; China Baseline characteristics Intervention group 1 (intramedullary)
Intervention group 2 (extramedullary)
Note:
|
|
| Interventions |
General details: prophylactic preoperative antibiotics; prophylactic anti‐inflammatory therapy, subcutaneous injection of low‐molecular‐weight heparin calcium, and anti‐osteoporosis drugs. Lower limb muscle contraction exercises on POD1. Time to weight bearing was determined according to X‐ray examinations (1, 4, 6, and 12 weeks after surgery) Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time, LOS, volume of blood loss, time to postoperative weight bearing, callusing time, swelling reduction time, transforming growth factor (TGF)‐beta2 expression; hip function scores; complications (hip varus, femoral shaft fracture, cut‐out of femoral head, fracture site infection, internal fixation breakage) Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: supported by the Research Project of the Jiangsu Health and Family Planning Commission; study authors declare no competing interests Study dates: January 2016 to January 2017 Note:
|
|
Yamauchi 2014.
| Study characteristics | ||
| Methods | Quasi‐randomised; parallel design Review comparison group: extra small PFN vs DHS |
|
| Participants |
Total number of randomised participants: 19 Inclusion criteria: simple intertrochanteric fractures, 31‐A1.1 and A1.2. All participants selected for this study reported walking independently without the use of walking aids such as walking frames or canes before sustaining their initial fracture (i.e. they had equivalent ADL) Exclusion criteria: simple fractures such as femoral basal neck fracture, minor trochanter as fracture fragment, comminuted greater trochanteric fractures; pathological fractures, high‐energy injuries or other multiple injuries. Participants with apparent dementia or other psychological problems and severe perioperative or postoperative complications that would result in delayed postoperative rehabilitation Setting: single centre; hospital; Japan Baseline characteristics Intervention group 1 (intramedullary; baseline data for only 10 participants)
Intervention group 2 (extramedullary; baseline data for only 8 participants)
Note:
|
|
| Interventions |
General details: a physiotherapist supervised full weight bearing and walking exercises that were performed on POD1. Plain anteroposterior and lateral radiographs were also obtained for each participant to confirm complete union of the bone. Intervention group 1:
Intervention group 2:
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: surgical variables (duration of surgery, intraoperative blood loss, haemoglobin). Pain and ADL scores (measured at 1, 2, 3 and 4 weeks after surgery), active ROM, angle of hip flexion, and abduction, time to achieve straight leg raise, time to achieve independent standing on the surgical leg Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: no funding. Study authors declared no conflicts of interest Study dates: 2009 to 2012 Note:
|
|
Yang 2011a.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus IHS |
|
| Participants |
Total number of randomised participants: 215 Inclusion criteria: intertrochanteric fractures from low‐energy trauma, 60 to 90 years of age, ASA status ≤ III; informed consent Exclusion criteria: high‐energy trauma; < 60 years of age; hip joint tumours; tuberculosis, arthritis in hip, conditions not conducive to surgery Setting: single centre, hospital, China Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (IHS)
|
|
| Interventions |
General details: preoperative antibiotics, prophylactic anticoagulation therapy, active within 24 hours of surgery; clinical follow‐up at 1, 3, 6 and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: blood loss and transfusion; mortality; postoperative complications (dislocation, fixation failure, postoperative fracture, cut‐out, infection); walking ability Outcomes relevant to the review: mortality (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: July 2008 to June 2010 Note:
|
|
Yang 2011b.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PCCP compared with SHS |
|
| Participants |
Total number of randomised participants: 66 Inclusion criteria: ≥ 18 years of age; A1 or A2 AO/OTA intertrochanteric proximal femoral fracture Exclusion criteria: existing or previous fractures of the same or contralateral hip; other fractures or injuries that could confound the outcome measure of interest; an inability to appear for follow‐up for any reason one year after surgery; inability to understand the purpose of the study and/or an inability to give informed consent for inclusion Setting: single centre; hospital; USA Baseline characteristics Note:
|
|
| Interventions |
General details: all participants began weight bearing as tolerated after surgery and were out of bed on the first or second postoperative day; physical therapy was initiated within 4 days of the procedure, depending on a variety of factors; mean follow‐up time was 36 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional status; VAS; HRQoL (available at 2 weeks, 4 weeks and 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 months); mortality (available at 12 months); surgical time; incision length; blood loss; transfusions Outcomes relevant to the review: HRQoL (in SF‐36, at 4 months and 12 months); mortality (reported at 12 months) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors declare that there was no external funding for this study. Study authors declare that some authors received fees or benefits of < $10,000 from commercial entities (Synthes, Orhofix, Smeith & Nephew, and Stryker) Study dates: July 2004 to September 2007 Note:
|
|
Yaozeng 2010.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus Gamma 3 |
|
| Participants |
Total number of randomised participants: 107 (143 initially recruited) Inclusion criteria: trochanteric fracture of the femur; informed consent Exclusion criteria: < 60 years of age; ASA score of V; unable to walk before injury; refusal to participate Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (Gamma 3)
Note:
|
|
| Interventions |
General details: all operations were performed by surgeons who had experience with at least 5 procedures with both implants; preoperative intravenous antibiotics with 2 g of ceftriaxone; general and spinal anaesthesia were used in both groups; operated on a traction table in a supine position; suction drains for 2 days; prophylactic antibiotics for 3 days; movement of hip, knee and ankle joints from the first postoperative day; encouraged full weight bearing as soon as possible; follow‐up was undertaken at 3, 6 and 12 postoperative months, and yearly thereafter Intervention group 1:
Intervention group 2:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operative time; fluoroscopy time; blood loss; intraoperative complications (femoral shaft fracture, locking difficulties, inappropriate length of screws); motion range of hip; hip pain; thigh pain; walking ability score; open reductions; hospital stay; mortality (available at 12 months); complications (lateral blade migration, delayed union, superficial wound infection, haematoma, decubital ulcer, pneumonia, UTI, cerebral infarction), re‐operations (available at 12 months) Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: April 2007 to May 2008 Note:
|
|
Yu 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFN versus PFNA |
|
| Participants |
Total number of randomised participants: 70 Inclusion criteria: osteoporotic intertrochanteric fracture Exclusion criteria: not specified in English abstract Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (PFN)
Intervention group 2 (PFNA)
Note:
|
|
| Interventions |
General details: unknown Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; intraoperative blood loss; fracture healing time; postoperative complications; HHS; mortality Outcomes relevant to the review: mortality |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported; study authors declare no conflicts of interest Study dates: June 2012 to June 2014 Note:
|
|
Zehir 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA vs DHS |
|
| Participants |
Total number of randomised participants: 198 Inclusion criteria: unstable trochanteric fractures (31 A2), > 65 years of age Exclusion criteria: multiple ipsilateral or contralateral fragmented or pathological fractures, intracapsular fractures, stable fractures; unable to walk or bedridden or wheelchair‐bound; history of previous hip surgery at either side Setting: single centre; tertiary university hospital; Turkey Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: 1 of 2 surgeons experienced in hip surgery; prophylactic antibiotics; under spinal, epidural, general anaesthesia, or regional; all participants mobilised out of bed and allowed weight bearing on POD1 or POD2 Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery; fluoroscopy times, volume of blood loss, mortality (in hospital, and at end of follow‐up); LOS; superficial infection; deep infection; haematoma; cut‐out; screw migration; pain (hip and thigh); re‐operation; DVT; PE; decompensated heart failure; UTI; pneumonia; pressure ulcer; time to healing; recovery of walking ability and independent mobility; discharged to home; mean tip‐apex distance Outcomes relevant to the review: mortality (in hospital and at end of follow‐up, median follow‐up is 15.95); unplanned return to theatre (12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. Study authors declared no conflicts of interest Study dates: January 2010 and March 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer‐based random number generator, patients were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Although sealed envelopes were used, study authors do not report if envelopes were opaque or sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Experienced hip surgeons, but study authors do not report if surgeons are experienced with using both types of implants in this study |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | Clinically‐reported subjective measures were assessed by independent radiographers. However, we assume that no attempts were made to conceal types of interventions, in which case there is a lack of blinding for these subjective measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses explained by death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Zhang 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: InterTAN nail versus PFNA‐II |
|
| Participants |
Total number of randomised participants: 113 Inclusion criteria: unstable trochanteric fractures of the femur; informed consent; low‐energy trauma Exclusion criteria: high‐energy trauma; pathological fractures; open fractures; multiple fractures; ASA score of V; inability to walk before injury; presence of degenerative osteoarthritis/arthritis in the injured hip Setting: single centre, hospital, China Baseline characteristics Intervention group 1 (PFNA II)
Intervention group 2 (InterTAN)
Note:
|
|
| Interventions |
General details: all surgeries were performed by surgeons who had performed at least 5 procedures independently with each implant; general or spinal anaesthesia were used; participants underwent prophylaxis for thrombosis perioperatively and received prophylactic antibiotics 30 minutes preoperatively; participants were operated on while in a supine position on a traction table; postoperatively, all participants had suction drains placed for 48 hours and were given prophylactic antibiotics for 24 hours; all participants were encouraged to walk with full weight bearing, while assisted by a physiotherapist, as soon as possible postoperatively; follow‐up occurred at 1, 3, 6 and 12 months postoperatively and yearly thereafter Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative time; fluoroscopy time; intraoperative blood loss; length of hospital stay; complications (superficial wound infection, deep infection, haematoma, cut‐out, lateral hip migration screw, femoral shaft fracture, DVT, pulmonary embolism, pressure sores, UTI); fixation failures; hip range of motion; pain in the hip and thigh; walking ability score; postoperative complications (wound infection and pulmonary, cardiovascular, thromboembolic, renal and gastrointestinal disorders); fracture complications (cut‐out, implant breakage, revision surgery); HHS; TAD; mortality (reported at 12 months); mean time to bone healing; re‐operations (available at 12 months); walking Outcomes relevant to the review: mortality (reported at 12 months); unplanned return to theatre (reported at 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: the study authors have no relevant financial relationships to disclose Study dates: July 2009 to September 2010 Note:
|
|
Zhang 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus hip arthroplasty |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: > 18 years of age; no history of bone fractures; clinically diagnosed unstable osteoporotic femoral intertrochanteric fracture; decision to receive surgery Exclusion criteria: severe dysfunction of liver and kidney; open fracture; pathological fracture; complicated with coagulation disorders, manifested surgical contraindication, inability to co‐operate Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (hip arthroplasty)
Note:
|
|
| Interventions |
General details: general anaesthesia was used for all surgeries Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of surgery, intraoperative blood loss, length of hospital stay; DVT; pulmonary infection; joint dislocation; prosthetic loosening; HHS Outcomes relevant to the review: none |
|
| Notes |
Funding/sponsor/declarations of interest: study authors report no conflict of interest with no detail of funding reported Study dates: January 2015 to December 2017 Note:
|
|
Zhou 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PNFA versus LISS (less‐invasive stabilisation system) |
|
| Participants |
Total number of randomised participants: 68 Inclusion criteria: OTA Type 31A proximal femoral fracture; closed fractures; treated within 3 weeks of injury Exclusion criteria: open fractures; pathologic fractures; delayed fractures; multiple fractures; periprosthetic fractures Setting: single centre; orthopaedic hospital; China Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (LISS)
Note:
|
|
| Interventions |
General details: fracture table and image intensifier were used; performed by 3 senior surgeons; preoperative intravenous antibiotics with 1.5 g cefuroxime; spinal or general anaesthesia; low‐molecular‐weight heparin was used as thromboembolic prophylaxis for 5 days; postoperative prophylactic antibiotics (1.5 g cefuroxime, 3 doses); weight bearing dependent on radiographs and partial healing; clinical examination at 1, 3, 6 and 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality; postoperative complications; unplanned return to theatre; intraoperative time; intraoperative blood loss; LOS; hip function (HHS); radiograph evaluation; length of follow‐up: mean 26.8 months (range 21 to 36 months) Outcomes relevant to the review: mortality (at 1 months and 6 months); unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: quote: "no financial support was received for the work on this project" Study dates: December 2006 to March 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the patients were randomised by a computer generated list” |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | High risk | Quotes: “Surgery was performed by three senior surgeons” and “The longer operative time in the LISS group compared with the PFNA group in the study may be the result of the learning curve" Comment: we judged that surgeons were not equally experienced with both implants |
| Blinding of outcome assessment: mortality (detection bias) | Low risk | We did not expect that lack of blinding of outcome assessors would influence objective outcome data. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the intervention could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported for all outcomes. We noted some discrepancies between text and tables in the study report, but these were for a small number of participants and we did not expect that they would influence the data. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Zou 2009.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: PFNA versus DHS |
|
| Participants |
Total number of randomised participants: 121 Inclusion criteria: low‐energy trochanteric proximal femoral fractures. Exclusion criteria: pathological fracture or multiple injuries Setting: single centre; orthopaedic hospital; China Baseline characteristics Intervention group 1 (PFNA)
Intervention group 2 (DHS)
Note:
|
|
| Interventions |
General details: supine position on a fracture table; participants were mobilised and given standard rehabilitation instructions; prophylactic intravenous antibiotic; clinical examinations at 6 weeks, 3, 6 and 9 months, and then annually; no details on surgeons' experience Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional outcome at 12 months using the Salvati and Wilson (Salvati 1973) scoring system: categorised as excellent (≥ 32), good (24 to 31), fair (16 to 23) or poor (≤ 15); length of surgery; operative blood loss; radiographic screening time; cut‐out of the implant; fracture; non‐union; implant breakage; re‐operation; superficial and deep wound infection; DVT; LOS; time point unclear, assumed to be 12 months unless reporting operative data Outcomes relevant to the review: unplanned return to theatre |
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported; study authors declared no conflicts of interest Study dates: January 2006 and December 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not possible to blind surgeons to the type of intervention. We did not, however, expect that this would influence surgeon performance. |
| Other performance bias: surgeon experience of both implants | Unclear risk | Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment: unplanned return to theatre (detection bias) | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the intervention could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report registration with a clinical trials register or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
ADL: activities of daily living; AF: atrial fibrillation; AO/OTA: Arbeitsgemeinschaft für Osteosynthesefragen (German for "Association for the Study of Internal Fixation")/Orthopaedic Trauma Association; AP: angled blade plate;AMTS: abbreviated mental test score; ASA: American Society of Anesthesiologists; AVN: avascular necrosis; BMD: bone mineral density; BMI: body mass index; CAD: coronary artery disease; CaP: calcium phosphate; CCD: caput‐collum‐diaphyseal; CHF: chronic heart failure; CHS: compression hip screw; COPD: chronic obstructive pulmonary disease; CRF: chronic renal failure; CSP: compression screw‐plate; CVA: cerebrovascular accident; DCS: dynamic compression screw; DHS: dynamic hip screw; DHHS: dynamic helical hip system; DHPS: dynamic hip plating system; DVT: deep vein thrombosis; EQ‐5D (‐3L/‐5L): EuroQol 5 dimensions (3 levels/5 levels); FIM: Functional Independence Measure; FRS: functional recovery score; G3: Gamma 3; HA: hemiarthroplasty; HHS: Harris hip score; HIV: human immunodeficiency virus; HRQoL: health related quality of life; ICECAP‐O: ICEpop capability measure for older people; ICU: intensive care unit; IHS: intramedullary hip screw; IM: intramedullary; IMHS: intramedullary hip screw; IQR: interquartile range; IT: InterTan; ITST: type of nail (not defined in study report); ITT: intention‐to‐treat; IU: international units; IV: intravenous(ly); LEM: Lower Extremity Measure; LISS: less‐invasive stabilisation system; LOS: length of stay; MCL: McLaughlin nail‐plate; MI: myocardial infarction; MIDHS: minimally invasive dynamic hip screw; MMS: mean mobility score; MMSE: mini‐mental state examination; MSP: Medoff sliding plate; NR: not reported; OPF: Orthofix pertrochanteric fixator; PC(C)P: percutaneous compression plate; PE: pulmonary embolism; PFLP: proximal femoral locking plate; PFLCP: proximal femur locking compression plate; PFN: proximal femoral nail; PFNA: proximal femoral nail antirotation; POD: postoperative day; RAB: Rigidity Augmentation Baixauli; RCT: randomised controlled trial; ROM: range of motion; RSA: radiostereometry; TSD: standard deviation; SEM: standard error of the mean; SF‐12 (PCS; MCS): Short Form 12‐item questionnaire (Physical component score; Mental component score);SF‐36: Short Form 36‐item questionnaire; SHS: sliding hip screw; SPMSQ: short portable mental status questionnaire; SSP: sliding screw plate; TAD: tip‐apex distance; TGN: trochanteric Gamma nail; THA: total hip arthroplasty; TIA: transient ischaemic attack; TSP: trochanteric support plate; TUG: timed up‐and‐go test; 2MWT: Two‐Minute Walk Test; WOMAC: Western Ontario and McMaster Universities Arthritis Index; UTI: urinary tract infection; VAS: visual analogue scale; VDP: Vandeputte; yr: year
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12608000162314 | Study comparing Gamma nail versus DHS. We received communication from the study contact (Rob Molnar; on 3 August 2015) to explain that the study was abandoned early because of poor recruitment. We excluded this study because no outcome data are available. |
| Ahmad 2011 | RCT comparing intramedullary hip screw versus compression hip screw. Published only as abstracts which contain insufficient information to justify inclusion in the review. |
| Alobaid 2004 | Quasi‐RCT comparing minimally‐invasive surgical technique with a conventional surgical technique using a DHS in both groups. We excluded this study because it did not compare interventions from our review criteria. |
| Bong 1981 | RCT comparing skeletal traction with tibial pin versus medial displacement osteotomy versus valgus osteotomy. We excluded this study because the comparisons were not consistent with our review criteria. |
| El‐Desouky 2016 | RCT comparing proximal femoral locking plate versus anatomical reduction. We excluded this study because it included mostly younger participants with high‐energy trauma. |
| Emami 2013 | Quasi‐RCT comparing DHS with bipolar HA. We excluded this study because the participants were aged between 45 and 65 years of age, and therefore did not meet our review criterion of older participants. |
| Gupta 2012 | RCT comparing condylocephalic nail versus compression hip screw. We excluded this study because it was reported as an abstract with insufficient information. |
| Kumar 1996 | RCT comparing anatomical reduction with medialisation osteotomy using a DHS in both groups. We excluded this study because it did not compare interventions from our review criteria. |
| Kumar 2006 | RCT comparing DHS versus DHS with modular TSP fixation in unstable intertrochanteric fractures. We excluded this study because it was reported as an abstract with insufficient information. |
| Lee 2007a | RCT comparing minimally‐invasive surgical technique with a conventional surgical technique using a DHS in both groups. We excluded this study because it did not compare interventions from our review criteria. |
| Lee 2007b | Quasi‐RCT comparing Russell‐Taylor reconstruction intramedullary nail versus dynamic condylar screw. We excluded this study which evaluates hip fractures in younger adults < 55 years of age. |
| Li 2015a | RCT comparing traditional Chinese medicine with conventional treatment using a DHS in both groups. We excluded this study because it did not compare interventions from our review criteria. |
| NCT00323232 | RCT comparing DHS as either a 2‐hole or 4‐hole device. Study is registered as completed in the clinical trials register in 2011. We have not sourced a full‐text report for this study and we do not expect the results of this study will be published. |
| NCT00686023 | RCT comparing inflatable PFN versus DHS. Clinical trials register states that expected study completion was in 2012. The trial register has not been updated. We excluded this study because we presume that this study has not been, or is unlikely to be, completed. |
| NCT00736684 | RCT comparing Gamma nail versus PFNA. Clinical trials register states that study completion was in 2009. The trial register has not been updated. We excluded this study because we presume that this study has not been, or is unlikely to be, completed. |
| NCT01173744 | RCT comparing Gamma nails with DHS. Clinical trials register states that expected study completion was in 2012. The trial register has not been updated. We excluded this study because we presume that this study has not been, or is unlikely to be, completed. |
| NCT01238068 | RCT comparing Gamma nail with DHS. Clinical trials register states that expected study completion was in 2011. The trial register has not been updated. We excluded this study because we presume that this study has not been, or is unlikely to be, completed. |
| NCT03065101 | RCT comparing Trigen Intertan nail with SHS. Clinical trials register states that the study was terminated because of low recruitment. We excluded this study because we did not have contact details for the principal investigator to confirm study status/recruitment, and we presume that the results of this study are unavailable. |
| Savadhkoohi 2016 | RCT comparing intramedullary nail with dynamic compression screw in subtrochanteric fractures. We excluded this study because it was reported as an abstract with insufficient information. |
| Sher 1985 | Oral presentation of RCT comparing SHS with Küntscher Y nail. We excluded this study because the short report/abstract contained insufficient information. |
| Wong 2009 | RCT comparing minimally‐invasive surgical technique with a conventional surgical technique using a DHS in both groups. We excluded this study because it did not compare interventions from our review criteria. |
DHS: dynamic hip screw; HA: hemiarthroplasty; PFN(A): proximal femoral nail (antirotation); RCT: randomised controlled trial; TSP: trochanteric support plate
Characteristics of studies awaiting classification [ordered by study ID]
ISRCTN32393360.
| Methods | RCT; parallel design |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: people with extracapsular fractures |
| Interventions | Compression hip screw versus intramedullary hip screw |
| Outcomes | Stroke volume, cardiac output, mean arterial pressure; oxygen saturation; length of hospital stay; pulmonary embolism; mortality |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was unavailable. We have used limited information available in the Cochrane Central Register of Controlled Trials. The year of publication is reported as 2005, and we therefore expect that this study is completed. We do not have contact details and have not been able to determine if this study is already published. |
ISRCTN48618754.
| Methods | RCT; parallel design |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: people presenting with an extracapsular hip fracture at Huddersfield Royal Infirmary |
| Interventions | Hansson Twin Hook versus lag screw |
| Outcomes | General and local complications, length of hospital stay, pain, mobility, hip characteristics, hip function, surgical/radiological evaluations |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was unavailable. We have used limited information available in the Cochrane Central Register of Controlled Trials. The year of publication is reported as 2004, and we therefore expect that this study is completed. We do not have contact details and have not been able to determine if this study is already published. |
JPRN‐UMIN000018307.
| Methods | RCT; parallel design |
| Participants |
Estimated participant enrolment: 195 Inclusion criteria: femoral trochanteric fracture (candidate for short femoral nail fixation); AO classification A1 and A2; written consent was obtained to participate in the study Exclusion criteria: adolescent who needed informed consent; individual who may present difficulties for CT analysis due to metal artefacts by metal implantation at proximal femur or other factors; ruled unfit for the study by a surgeon |
| Interventions | Short femoral nail with an integrated lag screw versus short femoral nail with a single lag screw |
| Outcomes | Rotational angle of proximal fragment; cut out; position of implant |
| Notes | Study described in clinical trials register as completed in March 2019. Some brief data reported in the clinical trials register. We await publication of the full study report. |
NCT01380444.
| Methods | RCT; parallel design |
| Participants |
Estimated number of participants: 736 participants Inclusion criteria
Exclusion criteria
|
| Interventions | Gamma 3 nail (Stryker) versus the Sliding Hip Screw |
| Outcomes | Length of follow‐up: 2 years
|
| Notes | Expected completion date: March 2017 Sponsor: Stryker Trauma GmbH This trial is being conducted at 26 centres in 12 countries. It is likely that the REGAIN 2008 study has acted as a pilot for this trial. Study authors contacted in April 2021. They confirmed that a publication was imminent but it was not possible to obtain preprint data. |
NCT02171897.
| Methods | RCT; parallel design |
| Participants |
Expected number of participants: 70 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Fixation with nail, plate, or screw versus THA |
| Outcomes | HHS; functional score; time to weight bearing; subjective satisfaction score (EQ‐5D, and VAS score for pain at rest); Parker score; complications (loosening, fractures, infections, phlebitis pulmonary embolism), mortality (12 months) |
| Notes | Study listed as completed in the clinical trials register. We await publication of the full study results. |
NCT02294747.
| Methods | RCT; parallel design |
| Participants |
Expected number of participants: 31 Inclusion criteria:
Exclusion criteria:
|
| Interventions | SHS with and without trochanteric support plate |
| Outcomes | Fracture displacement healing; perioperative blood loss; time of surgery; EQ‐5D; time to union; HHS; pain; TUG; satisfaction with operated hip motion during healing |
| Notes | Study listed as complete in the clinical trials register. We await publication of full study results. |
NCT02788994.
| Methods | RCT, parallel design |
| Participants |
Expected number of participants: 60 Inclusion criteria
|
| Interventions | Endovis BA2 nail versus DHS |
| Outcomes | Mobility (TUG); length of hospital stay |
| Notes | We contacted trialists (Peter Giannoudis) in April 2021. Trialists confirmed that publication is in process but were unable to share data at this point. |
NCT03000972.
| Methods | RCT; parallel design |
| Participants |
Expected number of participants: 20 Inclusion criteria:
Exclusion criteria:
|
| Interventions | PFNA with and without cement augmentation |
| Outcomes | Fluoride uptake; HHS |
| Notes | Study listed as completed in clinical trials register. We await publication of the full study results. |
NCT03635320.
| Methods | RCT; parallel design |
| Participants |
Expected number of participants: 188 Inclusion criteria:
According to AO fracture classification, subjects with following fracture type:
Exclusion criteria:
|
| Interventions | TFNA vs PFNA‐II |
| Outcomes | Fracture union; adverse events; revision; re‐operation; HRQoL (SF‐12; EQ‐5D); HHS |
| Notes | Study listed as completed in clinical trials register. We await publication of the full study report. |
NCT03849014.
| Methods | RCT; parallel design |
| Participants |
Expected number of participants: 52 Inclusion criteria:
Exclusion criteria:
|
| Interventions | PFN versus DHS |
| Outcomes | Mortality and complications |
| Notes | Study listed as completed in clinical trials register. We await publication of the full study report. |
NCT04240743.
| Methods | RCT; parallel design |
| Participants |
Expected number of participants: 64 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Cephalomedullary nail vs SHS |
| Outcomes | Mobility; HHS; BI: TAP; fracture settling |
| Notes | Study listed as completed in clinical trials register. We await publication of the full study report. |
REGAIN 2008.
| Methods | RCT; parallel design |
| Participants |
Estimated number of participants: 90 participants; 85 participants reported in a conference abstract (see Notes) Inclusion criteria
Exclusion criteria
|
| Interventions | Gamma 3 intramedullary nail (Stryker) versus the sliding hip screw |
| Outcomes | Length of follow‐up: 2 years
|
| Notes | On WHO International Clinical Trials Registry (ICTR) platform, the trial (NCT00555945) is documented as recruitment complete with no results posted. No response was received from Dr Sprague who was emailed on 1 August 2015 requesting a further update on the trial regarding publication. This trial, which was conducted at three centres in Canada, Denmark and Sweden, was reported in a conference abstract (Bhandari 2011). It is termed a pilot study and thus it is very likely to be the pilot for NCT01380444. |
ASA: American Society of Anesthesiologists; BI: Barthel Index; DHS: dynamic hip screw; EQ‐5D: EuroQol 5‐dimension quality of life questionnaire; HHS: Harris Hip Score; HRQoL: health‐related quality of life; PFN: proximal femoral nail; PFNA: proximal femoral nail antirotation; RCT: randomised controlled trial; SF‐12: Short Form 12‐item questionnaire; SHS: sliding hip screw; TAP: tip‐apex distance; TFNA: trochanteric fixation nail advanced; THA: total hip arthroplasty; TUG: timed up and go test;VAS: visual analogue scale; WHO: World Health Organization; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000992000.
| Study name | Locking plate for treatment of intertrochanteric hip fractures |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 100 Inclusion criteria: primary intertrochanteric hip fracture; BMI < 30 kg/m2; no other diseases likely to affect outcome of fracture healing; no other previous surgical treatments Exclusion criteria: BMI > 30 kg/m2; other surgical treatment; health condition is not stable; other diseases affecting bone healing (such as cancer, kidney disease, needing corticosteroids) |
| Interventions | Locking plate versus Gamma nail |
| Outcomes | Incision length; transfusion volume; healing of fracture; functional scores (HHS); range of movement |
| Starting date | November 2010; register describes study as still recruiting |
| Contact information | Zhao Xiang; dr.zhaoxiang@gmail.com |
| Notes |
ACTRN12618001431213.
| Study name | Evaluating the treatment methods of proximal femur fractures in elderly trauma patients |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 900 Inclusion criteria: traumatic extracapsular hip fracture; closed injury; aged over 60 years; ability to be followed for up to 6 months; presentation to hospital within 14 days of injury Exclusion criteria: concomitant injuries affecting treatment and rehabilitation of the affected limb; associated neurovascular injuries requiring immediate surgery; consent is refused; limited English proficiency including family members |
| Interventions | InterTAN (locked and unlocked) versus Gamma nail (unlocked) |
| Outcomes | Device failure; injury‐specific complications (perioperative technical complications, surgical site infection, unplanned surgery, mortality); functional independence measure (FIM); TUG; re‐operation; general medical complications; return to mobility circumstances; gross motor activity; duration of operation; fluoroscopy time; TAD; range of motion; hip join contact forces and moments; hip muscle function; hip muscle asymmetry; nail and screw sizes used by operating team; HHS; pain (VAS) |
| Starting date | August 2018 |
| Contact information | A/Prof Mark Rickman; mark.rickman@sa.gov.au |
| Notes |
ChiCTR1900025588.
| Study name | A randomized control trial for combined proximal femur intramedullary nail and lateral wall plate for the treatment of pantrochanteric fracture |
| Methods | Unknown |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: at least 60 years of age; male and female; diagnosis of internal and external unstable femoral intertrochanteric fractures (Babhulkar modified type B and C fractures); undergoing surgery in the hospital; informed consent |
| Interventions | Intramedullary nail versus intramedullary nail with lateral plate |
| Outcomes | Failure rate; fracture‐associated complications; operative time; blood loss during surgery; length of hospital stay; medical complications; VAS; HHS |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was not available. We have used limited information available in the Cochrane Central Register of Controlled Trials. |
ChiCTR‐INR‐17011841.
| Study name | Intertrochanteric fracture in the elderly: a randomized comparison of reduction and fixation and total hip arthroplasty |
| Methods | Unknown |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: > 65 years of age; intertrochanteric fracture; informed consent |
| Interventions | Femoral head replacement or THA |
| Outcomes | Radiological evaluation; routine blood text; HHS; blood transfusion rate; DVT |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was not available. We have used limited information available in the Cochrane Central Register of Controlled Trials. |
ChiCTR‐TRC‐11001642.
| Study name | Minimally invasive percutaneous compression plating versus dynamic hip screw for intertrochanteric fractures |
| Methods | Unknown |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: > 60 years of age; intertrochanteric fracture amenable to satisfactory reduction (type AO/OTA 31.A1‐A2, Evans type1); ability to ambulate independently prior to the fracture with or without assistive devices |
| Interventions | PCCP versus DHS |
| Outcomes | Blood loss; postoperative complications; VAS; HHS |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was not available. We have used limited information available in the Cochrane Central Register of Controlled Trials. |
CTRI/2019/02/017733.
| Study name | A clinical study to evaluate the outcomes of singe lag screw and helical blade nailing in treatment of intertrochanteric femur fractures |
| Methods | Unknown |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: people with intertrochanteric fracture presenting to the institution during this period; low‐energy trauma; fracture < 3 weeks old |
| Interventions | Single lag screw nailing versus helical blade nailing |
| Outcomes | Functional outcomes (modified HHS); comorbidities; pullout rate; neck shaft angle; implant position and cut‐out rate; union rate; neck length |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was not available. We have used limited information available in the Cochrane Central Register of Controlled Trials. |
CTRI/2019/11/022097.
| Study name | A study to compare the results using dynamic hip screw and modified proximal femoral locking compression plate in fractures around the hip |
| Methods | Unknown |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: people with post‐traumatic intertrochanteric fractures; adults > 18 years of either sex; intertrochanteric fractures with subtrochanteric extension; fractures included in 31A1 and 31A2 AO classification |
| Interventions | Modified proximal femoral locking compression plate versus DHS |
| Outcomes | Functional and radiological outcomes; complications |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was not available. We have used limited information available in the Cochrane Central Register of Controlled Trials. |
IRCT20141209020258N80.
| Study name | Comparison proximal femoral nailing (PFN) versus dynamic hip screw (DHS) in intertrochanteric fracture |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 36 Inclusion criteria: intertrochanteric fracture, ≥ 18 years of age, either gender, fracture < 2 weeks old, lack of multiple fractures, absence of pathologic fracture, lack of background bone disease |
| Interventions | PFN versus DHS |
| Outcomes | Wound healing; clinical improvement of fracture by radiological examination |
| Starting date | March 2018 |
| Contact information | Fariba Farokhi; f.farokhi@arakmu.ac.ir; Arak University of Medical Sciences; Iran |
| Notes |
IRCT20170621034689N2.
| Study name | Results of intertrochanteric fractures treated with dynamic hip screw (DHS) with or without use of static screw |
| Methods | Unknown |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: intertrochanteric fractures |
| Interventions | DHS (conventional use) versus DHS with an additional static screw superior to the DHS |
| Outcomes | Failure of treatment; HHS; VAS; fracture union |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was not available. We have used limited information available in the Cochrane Central Register of Controlled Trials. |
IRCT20181015041344N1.
| Study name | Comparison of dynamic hip screw and dynamic external fixator in intertochanteric fractures |
| Methods | RCT |
| Participants |
Estimated participant enrolment: unknown Inclusion criteria: intertrochanteric fracture of type A1 or A2; > 65 years of age; bone density less than 2.5 in T score; able to walk without help before the fracture; participant should be at high anaesthetic risk |
| Interventions | DHS versus dynamic external fixator |
| Outcomes | Pain; length of hospital stay; haematocrit |
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | At the time of adding this study to the Review Manager file, the WHO search trial portal was unavailable. We have used limited information available in the Cochrane Central Register of Controlled Trial. |
JPRN‐UMIN000019523.
| Study name | Prospective study for treatment of femoral intertrochanteric fracture ‐ ORIF vs bipolar prosthesis |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 40 Inclusion criteria: < 80 years of age; with femoral intertrochanteric fracture Exclusion criteria: bilateral cases; severe dementia; neurological impairment |
| Interventions | Open reduction and internal fixation (ORIF) versus bipolar hip prosthesis |
| Outcomes | Muscle strength; functional evaluation using Barthel Index for walking or stair climbing |
| Starting date | August 2015 |
| Contact information | Mitsuaki Noda; m‐noda@muf.biglobe.ne.jp |
| Notes |
NCT01437176.
| Study name | Treatment of intertrochanteric fracture with new type of intramedullary nail |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 36 Inclusion criteria
Exclusion criteria
|
| Interventions | PFNA versus new type of intramedullary nail (Weigao Orthopaedic Device Co. Ltd) |
| Outcomes | Bone healing; rates of revision surgery; quality of life (SF‐36, ADL, FIM); complications (mortality, non‐union, implant breakage/failure, infection, DVT) |
| Starting date | September 2011; clinical trials register states that study is still recruiting |
| Contact information | Tang Peifu; Chinese PLA General Hospital |
| Notes |
NCT01509859.
| Study name | Comparing weight bearing after intramedullary fixation devices for the proximal femur fracture |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 100 Inclusion criteria
Exclusion criteria
|
| Interventions | PFNA versus InterTan nail |
| Outcomes | Not reported in clinical trials register |
| Starting date | September 2012; status in clinical trials register states that study is recruiting |
| Contact information | Yona Kosashvili, MD; Rabin Medical Center |
| Notes |
NCT01797237.
| Study name | Outcome comparison between PFNA and InterTAN |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 60 Inclusion criteria
Exclusion criteria
|
| Interventions | PFNA vs InterTan |
| Outcomes | Quality of life (SF‐36, ADL, FIM); bone healing |
| Starting date | October 2012; status in clinical trials register states that study is recruiting |
| Contact information | Peifu Tang; Chinese PLA General Hospital |
| Notes |
NCT02627040.
| Study name | Intertrochanteric femoral fracture fixation trial |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 95 Inclusion criteria
Exclusion criteria
|
| Interventions | Gamma 3 nail versus InterTan |
| Outcomes | Hip function (HHS); functional outcome; walking ability; hip range of motion; pain |
| Starting date | November 2015 |
| Contact information | Hassan R Mir, MD, MBA; Florida Orthopaedic Institute |
| Notes |
NCT03407131.
| Study name | Internal fixation or joint replacement therapy for aged hip fracture patients |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 100 Inclusion criteria
Exclusion criteria
|
| Interventions | Joint replacement versus intramedullary nail fixation |
| Outcomes | Postoperative drainage volume and bleeding volume during surgery; joint rehabilitation index, complication rate, functional rehabilitation index, surgical related index |
| Starting date | February 2018 |
| Contact information | Baoguo Jiang; Peking University People's Hospital |
| Notes |
NCT03906032.
| Study name | Comparison of sliding hip screw to intramedullary nailing in the treatment of intertrochanteric hip fracture |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 352 Inclusion criteria
Exclusion criteria
|
| Interventions | TFNA IM Nail versus SHS |
| Outcomes | Blood loss; mortality, analgesia use; mobility (TUG), function (HHS), kinematic gait parameters at hip; length of hospital stay |
| Starting date | April 2019 |
| Contact information | May Cleary; may.cleary@hse.ie |
| Notes | Estimated completion date April 2023 |
NCT04306198.
| Study name | Helical blade vs lag screw fixation for cephalomedullary nailing of low energy intertrochanteric hip fractures |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 200 Inclusion criteria
Exclusion criteria
|
| Interventions | Trochanteric fixation nail, using a lag screw versus a helical blade as proximal fixation |
| Outcomes | Cut‐out; complications (infection and non‐union); Parker Mobility Score |
| Starting date | March 2020 |
| Contact information | Tomas Amenabar MD; Instituto Traumatologico |
| Notes |
NCT04441723.
| Study name | Comparison of dynamic versus static lag screw modes for cephalomedullary nails used to fix intertrochanteric fragility fractures |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 150 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Gamma 3 nail using static versus dynamic lage screw as a proximal fixation |
| Outcomes | Cut‐out; complications (infection and non‐union); offset |
| Starting date | January 2020 |
| Contact information | Dr Teodoro Gebauer Weisser; Instituto Traumatologico |
| Notes |
NTR1133.
| Study name | Intramedullary nailing of proximal femur fractures: Gamma 3 Nail versus Fixion Proximal Femur Nailing System |
| Methods | RCT, parallel design |
| Participants |
Estimated participant enrolment: 244 Inclusion criteria: proximal femur fractures with AO‐classification 31 A1.1 ‐ A3.3; > 18 years; admitted to hospital Exclusion criteria: primary bone disease; life expectancy < 1 year; unable to speak Dutch |
| Interventions | Gamma 3 nail versus Fixion Proximal Femur Nailing System |
| Outcomes | Major and minor implant‐related complications; procedure time; per‐operative fluoroscopic time; number of infections; resumption of full activities; mortality |
| Starting date | January 2008 |
| Contact information | AM Bek; A.bek@student.unimaas.nl |
| Notes |
ADL: activities of daily living; BMI: body mass index; DHS: dynamic hip screw; DVT: deep vein thrombosis; FIM: functional independence measure; HHS: Harris Hip Score:MRI: magnetic resonance imaging; ORIF: open reduction and internal fixation; OTA: Orthopaedic Trauma Association; PCCP: percutaneous compression plate; PFN: proximal femoral nail; PLA: People's Liberation Army; RCT: randomised controlled trial; SF‐36: Short Form 36‐item questionnaire; SHS: sliding hip screw; TFNA IM: TFN‐Advanced® proximal femoral nailing system ‐ intramedullary nail; TAD: tip apex distance; THA: total hip arthroplasty; TKA: total knee arthroplasty; TUG: timed up and go test; VAS: visual analogue scale; WHO: World Health Organization
Differences between protocol and review
Review information
Title: we edited the title to better reflect the older adult population included in the review.
Review authors: five new review authors joined the review team (SL, RM, JL, JG, JS) and four authors left the review author team (AS, AJ, HW, JMG).
Objectives
We edited the objectives to reflect the restriction to older adult populations.
Methods
Criteria for considering studies for this review
Types of participants: we edited the criteria to state the inclusion of older adults (at least 60 years of age). We excluded studies in which all study participants were not representative of the general hip fracture population, and in which we expected that most hip fractures were not caused by low‐energy trauma. We reported these exclusions in Excluded studies.
Types of outcomes: we edited the time points in the review to reflect the wider variation in data in the included studies. In addition to the early data at 4 months or earlier, we added collection of data at 12 months (prioritising 12‐month data, but in its absence including data after 4 months and up to 24 months) and late (after 24 months) time points. We did not prioritise early time points when reporting results. Data for all three time points were reported, and in the summary of findings tables, abstract and PLS, we selected the time point which yielded the most data (i.e. 12 months after surgery).
Types of interventions: we did not number the intervention groups in order to specify the direction of the comparisons. We ensured that the direction of effect was always reported within any cited effect estimate.
Search methods for identification of studies
Electronic searches: we did not search the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/) because, at the time of searching, the platform was not available because of the COVID‐19 pandemic. We believed that clinical trials register searches remained comprehensive because CENTRAL also includes studies from international trials registers.
Data collection and analysis
Data extraction and management: we planned that data extraction would be completed independently by two reviewers. In practice, one author extracted data which was checked for accuracy by a second review author. We edited the data collected to describe the flow of study participants. Rather than collecting "study disposition (number randomised, number by protocol, number available for analysis)", we collected "number of randomised participants, losses (and reasons for losses), and number analysed for each outcome".
Summary measures: we were able to extract dichotomous data from all studies as number of events per arm. We did not need to use other data such as P values. We did not use 'count data' in which studies reported more than one observation during the course of follow‐up. We explained the method used to interpret any estimates reported with SMD.
Relative treatment ranking: we presented SUCRA as a proportion rather than a percentage, and have edited the methods to reflect this. In addition, we also provided an estimation of mean rank for each treatment, and described this in the methods.
Unit of analysis issues: we did not include any cluster‐randomised trials in the review.
Reports of outcomes at different time points: as described above ('types of outcomes'), we added an additional time point for collecting data. As we believed this approach best fit the data within the studies, as well as being most clinically appropriate, we did not consider alternative methods of grouping these time points.
Dealing with missing data: we attempted to contact study authors of recently published studies (since 2012) when we noted data were missing or not clearly reported for critical review outcomes. Most studies in the review were published more than 20 years ago and we did not expect study authors of older studies to have access to study data. We specified that we used the Characteristics of included studies to note when study authors reported data that we were unable to use because of an unknown number of losses or because data were reported unclearly.
Geometry of the network: we did not present network diagrams that were coloured according to the risk of bias.
Local approaches for evaluating inconsistency/incoherence: we did not use 'loop‐specific' approaches to evaluate incoherence. Instead, we only used the node‐splitting (sidesplit) approach.
Investigation of heterogeneity: we did not explore possible effect modifiers through network meta‐regression as we found that there was insufficient variation between studies for these effect modifiers, and individual studies did not report subgroup data by these effect modifiers. Similarly, we did not attempt to run network meta‐regression models to detect associations between study size and effect size as originally planned.
Investigation of heterogeneity: in order to make the distinction between different types of statistical heterogeneity, we changed the term for statistical heterogeneity within pairwise comparisons to 'statistical inconsistency' and the term for statistical heterogeneity within the entire network (locally or globally) to 'statistical incoherence'. This terminology is used in the Cochrane Handbook for Systematic Reviews of Interventions (Chaimani 2018).
Sensitivity analysis: we planned to explore the effect of excluding studies based on particular criteria. However, we did not conduct sensitivity analyses in this review. For studies at high risk of bias, we found that we had very few studies in most of the individual treatment arms such that sensitivity analysis would produce less meaningful results. Very few studies had substantial amounts of missing data, and we found insufficient variation in fracture classifications to warrant sensitivity analysis. We no longer believed that sensitivity analysis was necessary for the time points ('early' and 'late' time points) as we had addressed this by re‐defining the time points. Finally, we judged that all interventions, or sufficiently similar variations of these interventions, were in clinical use in a worldwide setting.
Credibility of the evidence: we presented tables of direct, indirect and network estimates for all outcomes but, given the number of possible direct and indirect estimates and the expected similarity in the GRADE judgements (low to very low), we did not also present GRADE judgements of certainty for each pairwise comparison. We removed this from this section of the methods.
Summary of findings tables: we specified the outcomes (and time points) for which we prepared summary of findings tables, the inclusion of all available interventions (from our nodes), and the decision to choose a reference comparator to present network estimates against in the tables. We did not include ranking values in the summary of findings tables; we were advised to drop this information from the table by a Methodological Editor in the Cochrane Methods Support Unit.
Contributions of authors
SL (systematic reviewer): sifted and identified included studies, extracted study data, interpreted the findings and drafted the review. RM (systematic reviewer): sifted and identified included studies, extracted study data, interpreted the findings and drafted the review. JL (systematic reviewer): extracted study data and drafted the review. JS (statistician): prepared estimates for the networks and conducted statistical analyses according to the protocol, interpreted the findings and approved the final draft of the review. JG (content expert, Trauma and Orthopaedics): agreed network nodes, extracted data and approved the final draft of the review. JC (statistician): prepared estimates for the networks and conducted statistical analyses according to the protocol, interpreted the findings and approved the final draft of the review. WE (content expert, Trauma and Orthopaedics): agreed network nodes, and reviewed and approved the final review. MP (content expert, Trauma and Orthopaedics): agreed network nodes, and reviewed and approved the final review. XG (content expert, Trauma and Orthopaedics): interpreted the findings, drafted the review, approved the final review and is the guarantor of the content.
Editorial contributions
Faith Armitage (Copy Editor): copy‐edited the review. Liz Bickerdike (Acute and Emergency Care Network Associate Editor): advised on methodology and review content. Mike Brown (Acute and Emergency Care Network Senior Editor): approved the final version for publication. Maria Clarke (Information Specialist): ran literature searches and edited the search methods section. Kerry Dwan (Statistical Editor): advised on methodology and review content. Joanne Elliott (Managing Editor): co‐ordinated the editorial process and edited the review.
Xavier Griffin and Sharon Lewis are members of the editorial base but were not involved in the editorial process or decision‐making for this review.
Sources of support
Internal sources
National Institute for Health Research Oxford Biomedical Research Centre, UK
External sources
-
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group, UK
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
SL: none known RM: none known JL: none known JS: none known JG: none known JC: none known WE has an advisory role on infection control with Orthofix, Bone Support and Stryker, but this is unrelated to this review. He has no known conflicts of interest. MP has received and may continue to receive financial payment from manufacturing companies of orthopaedic implants for attending meetings organised by these companies and for advising on the design and use of hip fracture implants. He remained independent of study selection decisions, risk of bias assessment and any data extraction of any of the studies on which he is an author, co‐applicant or has had an advisory role. XG is funded by a National Institute for Health Research Clinician Scientist Grant. Further funding from industry and charitable grants are and have been made available to his institution. He has ongoing expert consultancy with several companies; none involve the development of any implant for use in hip fracture care. All decisions relating to the design, conduct, analysis, write‐up and publication of research are independent of these funders. He remained independent of study selection decisions, risk of bias assessment and any data extraction of any of the studies on which he is an author, co‐applicant or has had an advisory role.
New
References
References to studies included in this review
Acharya 2003 {published data only}
- Acharya MR, Eastwood G, Bing A, Harper WH.Fixation of hip fractures with a sliding hip screw: a randomised controlled trial comparing fixation of the plate in the keyed and unkeyed position [abstract]. Injury 2003;34(629):no pagination. [Google Scholar]
Adams 2001 {published data only}
- Adams CI, Robinson CM, Court-Brown C, McQueen MM.Prospective randomised controlled trial of an intramedullary nail versus dynamic hip screw and plate for intertrochanteric fractured femur. Journal of Orthopaedic Trauma 2001;15(6):394-400. [2886707] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bartonicek I, Dousa P.Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate of intertrochanteric fractures of the femur [letter]. Journal of Orthopaedic Trauma 2002;16(5):363-4. [2886708] [DOI] [PubMed] [Google Scholar]
Adeel 2020 {published data only}
- Adeel K, Nadeem RD, Akhtar M, Sah RK, Mohy-Ud-Din I.Comparison of proximal femoral nail (PFN) and dynamic hip screw (DHS) for the treatment of AO type A2 and A3 pertrochanteric fractures of femur. Journal of the Pakistan Medical Association 2020;70(5):815-9. [16964099] [PMID: ] [DOI] [PubMed] [Google Scholar]
Agrawal 2017 {published data only}
- Agrawal P, Gaba S, Das S, Singh R, Kumar A, Yadav G.Dynamic hip screw versus proximal femur locking compression plate in intertrochanteric femur fractures (AO 31A1 and 31A2): a prospective randomized study. Journal of Natural Science, Biology & Medicine 2017;8(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahrengart 1994 {published data only}
- Ahrengart L, Thornkvist H, Lindgren U, Fornander P, Thorngren KG, Wahlstrom P, et al.Gamma nail vs. compression hip screw for trochanteric fractures - complications and patient outcome [abstract]. Acta Orthopaedica Scandinavica. Supplementum 1995;265:23. [2886710] [Google Scholar]
- Ahrengart L, Tornkvist H, Fornander P, Thorngren KG, Pasanen L, Wahlstrom P, et al.A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clinical Orthopaedics and Related Research 2002;401:209-22. [2886711] [DOI] [PubMed] [Google Scholar]
- Ahrengart L, Tornkvist H, Lindgren U, Fornander P, Thorngren KG, Wahlstrom P, et al.Gamma nail vs. compression hip screw for trochanteric and subtrochanteric fractures. Complications and patient outcome [abstract]. Orthopaedic Transactions 1995;19(1):154. [2886712] [Google Scholar]
- Fornander P, Thorngren K-G, Tornqvist H, Ahrengart L, Lindgren U.Swedish experience of the first 209 randomized patients with Gamma nail vs. screw-plate [abstract]. Acta Orthopaedica Scandinavica. Supplementum 1992;248:90. [2886713] [Google Scholar]
- Fornander P, Thorngren K-G, Tornqvist H, Ahrengart L, Lingren U.Swedish experience with the Gamma nail vs. sliding hip screw in 209 randomised cases. International Journal of Orthopaedic Trauma 1994;4(3):118-22. [2886714] [Google Scholar]
Akhtar 2016 {published data only}
- Akhtar MS, Gillani HU, Khan KR.Comparison between proximal femoral nail antirotation (PFNA) and dynamic condylar screw (DCS) in the management of unstable proximal femur fractures in terms of mean union time. Pakistan Journal of Medical and Health Sciences 2016;10(2):555-8. [16964101] [Google Scholar]
Akinci 2010 {published data only}
- Akinci O, Akalin Y, Reisoglu A, Kayali C.Comparison of long-term results of dynamic hip screw and AO 130 degrees blade plate in adult trochanteric region fractures. Acta Orthopaedica et Traumatologica Turcica 2010;44(6):443-51. [DOI] [PubMed] [Google Scholar]
Aktselis 2014 {published data only}
- Aktselis I, Kokoroghiannis C, Fragkomichalos E, Koundis G, Deligeorgis A, Daskalakis E, et al.Prospective randomised controlled trial of an intramedullary nail versus a sliding hip screw for intertrochanteric fractures of the femur. International Orthopaedics 2014;38(1):155-61. [2886716] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andalib 2020 {published data only}
- Andalib A, Etemadifar M, Yavari P.Clinical outcomes of intramedullary and extramedullary fixation in unstable intertrochanteric fractures: a randomized clinical trial. Archives of Bone & Joint Surgery 2020;8(2):190-7. [16964103] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreani 2015 {published data only}
- Andreani L, Bonicoli E, Piolanti N, Parchi P, Niccolai F, Carmignani A, et al.Prospective randomized controlled trial of two different intramedullary nails for pertrochanteric fractures of the femur. Surgical Technology International 2015;27:210-4. [PubMed] [Google Scholar]
Bajpai 2015 {published data only}
- Bajpai J, Maheshwari R, Bajpai A, Saini S.Treatment options for unstable trochanteric fractures: screw or helical proximal femoral nail. Chinese Journal of Traumatology English Edition 2015;18(6):342-46. [DOI] [PubMed] [Google Scholar]
Bannister 1990 {published data only}
- Bannister GC, Gibson AG, Ackroyd CE, Newman JH.The fixation and prognosis of trocantheric fractures. A randomized prospective controlled trial. Clinical Orthopaedics and Related Research 1990;254:242-6. [PubMed] [Google Scholar]
- Bannister GC, Gibson AG.Jewett nail plate or AO dynamic hip screw for trochanteric fractures? A randomised prospective controlled trail [Abstract]. Journal of Bone and Joint Surgery. British Volume 1983;65:218. [Google Scholar]
Barrios 1993 {published data only}
- Barrios C, Brostrom LA, Stark A, Walheim G.Factors predicting failures of internal fixation in trochanteric hip fractures - a multivariate analysis comparing Ender pins and a dynamic hip screw [abstract]. Acta Orthopaedica Scandinavica 1992;63(Suppl 248):87. [Google Scholar]
- Barrios C, Walheim G, Brostrom LA, Olsson E, Stark A.Walking ability after internal fixation of trochanteric hip fractures with Ender nails or sliding screw plate. A comparative study of gait. Clinical Orthopaedics and Related Research 1993;294:187-92. [PubMed] [Google Scholar]
Barton 2010 {published data only}
- Barton T, Chesser T, Harries W, Gleeson R, Topliss C, Greenwood R.A prospective randomised control trial comparing the long gamma nail with the sliding hip screw for the treatment of AO/OTA 31 A2 fractures of the proximal femur [abstract]. Orthopaedic Proceedings 2012;94-B(Suppl XLI):116. [2886718] [Google Scholar]
- Barton T.Personal communication 5 May 2010. [2886719]
- Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ.A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur; a prospective randomized trial. Journal of Bone and Joint Surgery. American Volume 2010;92(4):792-8. [2886720] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Barton TM, Gleeson R, Topliss C, Harries WJ, Chesser T.A prospective trial comparing the long gamma nail with the sliding hip screw for the treatment of unstable pertrochanteric hip fractures. Orthopaedic Proceedings January 2011;93-B(Suppl I):33. [2886721] [Google Scholar]
- ISRCTN79362886.A prospective randomised controlled trial comparing the long gamma nail with the sliding hip screw for the treatment of unstable pertrochanteric hip fractures. www.controlled-trials.com/ISRCTN79362886 (first received 26 March 2009). [2886722]
Barwar 2014 {published data only}
- Barwar N, Meena S, Aggarwal SK, Garhwal P.Dynamic hip screw with locking side plate: a viable treatment option for intertrochanteric fracture. Chinese Journal of Traumatology 2014;17(2):88-92. [PubMed] [Google Scholar]
Baumgaertner 1998 {published data only}
- Baumgaertner MR, Curtin SL, Lindskog D.A randomized, prospective comparison of the intramedullary hip screw (IMHS) to the compression hip screw and sideplate [abstract]. Orthopaedic Transactions 1995;19(1):153-4. [2886724] [Google Scholar]
- Baumgaertner MR, Curtin SL, Lindskog DM.Intramedullary versus extramedullary fixation for the treatment of intertrochanteric hip fractures. Clinical Orthopaedics and Related Research 1998;348:87-94. [2886725] [PMID: ] [PubMed] [Google Scholar]
- Baumgaertner MR, Curtin SL, Lindskog DM.The Intramedullary Hip Screw (IMHS) and the Compression Hip Screw (CHS): a prospective clinical trial [poster]. In: Final programme of the 20th World Congress SICOT; 1996 Aug 18-23. Vol. 313. Amsterdam, 1996. [2886726]
- Curtin S, Baumgaertner M.The IMHS: a better way to fix hip fractures? [abstract]. Orthopaedic Transactions 1994;18(1):198. [2886727] [Google Scholar]
Benum 1994 {published data only}
- Aune AK, Ekeland A, Odegaard B, Grogaard B, Alho A.Gamma nail vs compression screw for trochanteric femoral fractures. 15 reoperations in a prospective, randomized study of 378 patients. Acta Orthopaedica Scandinavica 1994;65:127-30. [2886729] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Benum P, Grontvedt T, Braten M, Rossvoll I, Walloe A, Ekeland A, et al.Gamma nailing versus CHS in intertrochanteric and subtrochanteric femoral fractures: a prospective randomized multicentre study [abstract]. Acta Orthopaedica Scandinavica. Supplementum 1996;260:33-4. [2886730] [Google Scholar]
- Benum P, Grontvedt T, Braten M, Walloe A, Ekeland A, Raugstad S, et al.Gamma nail versus CHS in intertrochanteric and subtrochanteric femoral fractures - a preliminary report of a prospective randomized study [abstract]. Acta Orthopaedica Scandinavica. Supplementum 1992;247:7-8. [2886731] [Google Scholar]
- Ekeland A, Aune AK, Odegaard B, Grogaard B, Alho A.Complications after Gamma nailing of proximal femoral fractures [abstract]. Orthopaedic Transactions 1993;17:1049. [2886732] [Google Scholar]
- Ekeland A, Aune AK, Odegaard B, Grogaard B, Alho A.Reoperations after use of gamma nail or hip compression screw for proximal femoral fractures [abstract]. Journal of Bone and Joint Surgery. British Volume 1993;75(Suppl 2):199. [2886733] [Google Scholar]
- Madsen JE, Naess L, Aune AK, Alho A, Ekeland A, Stromsoe K.Dynamic hip screw with trochanteric stabilizing plate in the treatment of unstable proximal femoral fractures: a comparative study with the Gamma nail and compression hip screw. Journal of Orthopaedic Trauma 1998;12(4):241-8. [2886734] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Madsen JE, Noess L, Aune AK, Alho A, Ekeland A, Stromsoe K.Unstable per- and subtrochanteric femoral fractures - a comparison of treatment with the Gamma nail, compression hip screw, or dynamic hip screw with a trochanter stabilizing plate [abstract]. Acta Orthopaedica Scandinavica. Supplementum 1996;270:35-6. [2886735] [Google Scholar]
Berger‐Groch 2016 {published data only}
- Berger-Groch J, Rupprecht M, Schoepper S, Schroeder M, Rueger JM, Hoffmann M.Five-year outcome analysis of intertrochanteric femur fractures: a prospective randomized trial comparing a 2-screw and a single-screw cephalomedullary nail. Journal of Orthopaedic Trauma 2016;30(9):483-8. [DOI] [PubMed] [Google Scholar]
Bridle 1991 {published data only}
- Bridle SH, Bircher M, Patel AD, Calvert PT.The gamma nail for pertrochanteric fractures of the femur: a prospective comparison with the dynamic hip screw [abstract]. Journal of Bone and Joint Surgery. British Volume 1990;72(6):1085. [2886737] [DOI] [PubMed] [Google Scholar]
- Bridle SH, Patel AD, Bircher M, Calvert PT.Fixation of intertrochanteric fractures of the femur: a randomised prospective comparison of the gamma nail and the dynamic hip screw. Journal of Bone and Joint Surgery. British Volume 1991;73:330-4. [2886738] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brostrom 1992 {published data only}
- Brostrom LA, Barrios C, Kronberg M, Stark A, Walheim G.Clinical features and walking ability in the early postoperative period after treatment of trochanteric hip fractures. Results with special reference to fracture type and surgical treatment. Annales Chirurgiae et Gynaecologiae 1992;81(1):66-71. [PubMed] [Google Scholar]
Buciuto 1998 {published data only}
- Buciuto R, Hammer R, Herder A.Spontaneous subcapital femoral neck fracture after healed trochanteric fracture. Clinical Orthopaedics & Related Research 1997;342:156-63. [PubMed] [Google Scholar]
- Buciuto R, Uhlin B, Hammerby S, Hammer R.RAB-plate vs Richards CHS plate for unstable trochanteric hip fractures. A randomized study of 233 patients with 1-year follow-up. Acta Orthopaedica Scandinavica 1998;69(1):25. [DOI] [PubMed] [Google Scholar]
- Hammer R.RAB-plate vs Richards CHS plate for unstable trochanteric hip fractures. A randomized study of 233 patients with 1-year follow-up [1]. Acta Orthopaedica Scandinavica 1999;70(1):101-2. [DOI] [PubMed] [Google Scholar]
Butt 1995 {published data only}
- Butt MS, Krikler SJ, Nafie S, Ali MS.Comparison of dynamic hip screw and gamma nail: a prospective, randomized, controlled trial. Injury 1995;26(9):615-8. [2886740] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cai 2016 {published data only}
- Cai L, Wang T, Di L, Hu W, Wang J.Comparison of intramedullary and extramedullary fixation of stable intertrochanteric fractures in the elderly: a prospective randomised controlled trial exploring hidden perioperative blood loss. BMC Musculoskeletal Disorders 2016;17(1):475. [17673592] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caiaffa 2016 {published data only}
- Caiaffa V, Vicenti G, Mori C, Panella A, Conserva V, Corina G, et al.Is distal locking with short intramedullary nails necessary in stable pertrochanteric fractures? A prospective, multicentre, randomised study. Injury 2016;47(Suppl 4):S98-S106. [DOI] [PubMed] [Google Scholar]
Calderon 2013 {published data only}
- Claderon A, Ramos T, Vilchez F, Mendoza-Lemus O, Pena V, Cardenas-Estrada E, et al.Comparison of proximal femoral intramedullary nail (PFN) versus plate (DHS) to treat intertrochanteric fractures, prospective analysis [Comparación del clavo intramedular femoral proximal (PFN) versus placa DHS para el tratamiento de fracturas intertrocantéricas. Análisis prospectivo]. Acta Ortopedica Mexicana 2013;27(4):236-9. [2886742] [PubMed] [Google Scholar]
Cao 2017 {published data only}
- Cao X, Kong X, Li A.Auxiliary biological cemented femoral stem was effective in treating elderly patients with intertrochanteric fracture. Biomedical Research (India) 28;9:4071-5. [Google Scholar]
Carulli 2017 {published data only}
- Carulli C, Piacentini F, Paoli T, Civinini R, Innocenti M.A comparison of two fixation methods for femoral trochanteric fractures: a new generation intramedullary system vs sliding hip screw. Clinical Cases in Mineral and Bone Metabolism 2017;14(1):40-7. [16964105] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Catania 2019 {published data only}
- Catania P, Passaretti D, Montemurro G, Ripanti S, Carbone S, Candela V, et al.Intramedullary nailing for pertrochanteric fractures of proximal femur: a consecutive series of 323 patients treated with two devices. Journal of Orthopaedic Surgery 2019;14(1):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 1981 {published data only}
- Chapman MW, Bowman WE, Csongradi JJ, Day LJ, Trafton PG, Bovill EG Jr.The use of Ender's pins in extracapsular fractures of the hip. Journal of Bone and Joint Surgery. American Volume 1981;63(1):14-28. [PubMed] [Google Scholar]
Chechik 2014 {published data only}
- Chechik O, Amar E, Khashan M, Pritsch T, Drexler M, Goldstein Y, et al.Favorable radiographic outcomes using the expandable proximal femoral nail in the treatment of hip fractures - a randomized controlled trial. Journal of Orthopaedics 2014;11(2):103-9. [16964107] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen LP, Liu FS, Yang YS.Proximal femoral nail versus hemiarthroplasty for unstable intertrochanteric fractures in osteoporotic patients. Chinese Journal of Tissue Engineering Research 2017;21(27):4277-82. [Google Scholar]
Chen 2018 {published data only}
- Chen K, Chen S, Yi J.Efficacy of proximal femoral nail anti-rotation and dynamic hip screw internal fixation in the treatment of hip fracture in the elderly patients. International Journal of Clinical and Experimental Medicine 2018;11(4):4188-92. [17673594] [1940-5901/IJCEM0071998] [Google Scholar]
Cheng 2014 {published data only}
- Cheng Q, Huang W, Gong X, Wang C, Liang X, Hu N.Minimally invasive percutaneous compression plating versus dynamic hip screw for intertrochanteric fractures: a randomized control trial. Chinese Journal of Traumatology 2014;17(5):249-55. [PubMed] [Google Scholar]
Ciaffa 2018 {published data only}
- Ciaffa V, Vicenti G, Mori CM, Panella A, Conserva V, Corina G, et al.Unlocked versus dynamic and static distal locked femoral nails in stable and unstable intertrochanteric fractures. A prospective study. Injury 2018;49:S19-S25. [DOI] [PubMed] [Google Scholar]
Dalen 1988 {published data only}
- Dalen N, Jacobsson B, Eriksson PA.A comparison of nail-plate fixation and Ender's nailing in pertrochanteric fractures. Journal of Trauma: Injury, Infection and Critical Care 1988;28(3):405-6. [DOI] [PubMed] [Google Scholar]
Dall'Oca 2010 {published data only}
- Dall'Oca C, Maluta T, Moscolo A, Lavini F, Bartolozzi P.Cement augmentation of intertrochanteric fractures stabilised with intramedullary nailing. Injury 2010;41(11):1150-5. [DOI] [PubMed] [Google Scholar]
Dalsgaard 1986 {published data only}
- Dalsgaard J, Yde J, Olsen A.Trochanteric fractures - a prospective and comparative study between Ender nailing and sliding screw plate [Abstract]. Acta Orthopaedica Scandinavica 1986;57:181. [Google Scholar]
- Dalsgard J, Yde JR, Olsen AD.Pertrochanteric fractures. Comparison between 2 surgical methods of internal fixation. Ugeskrift for Laeger 1987;149(14):900-4. [PubMed] [Google Scholar]
Davis 1988 {published data only}
- Davis TR, Sher JL, Checketts RG, Porter BB.Intertrochanteric fractures of the femur: a prospective study comparing the use of the Küntscher-Y nail and a sliding hip screw. Injury 1988;19(6):421-6. [2886744] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Grave 2012 {published data only}
- Winnock de Grave P, Tampere T, Byn P, Van Overschelde J, Pattyn C, Verdonk R.Intramedullary fixation of intertrochanteric hip fractures: a comparison of two implant designs. A prospective randomised clinical trial. Acta Orthopaedica Belgica 2012;78(2):192-8. [PubMed] [Google Scholar]
Delgado 1990 {published data only}
- Delgado B, Humberto A, Prez C, Jorge F.Stable intertrochanteric hip fractures Universal Guide Angle modification to the surgical technique of the Richards screw-plate. A new device developed at the Military Central Hospital of Mexico. Revista Mexicana de Ortopedia y Traumatologia 1990;4(3):91-5. [Google Scholar]
Desteli 2015 {published data only}
- Desteli EE, Imren Y, Erdogan M, Aydagun O.Quality of life following treatment of trochanteric fractures with proximal femoral nail versus cementless bipolar hemiarthroplasty in elderly. Clinical & Investigative Medicine - Medecine Clinique et Experimentale 2015;38(2):E63-72. [DOI] [PubMed] [Google Scholar]
Dhamangaonkar 2013 {published data only}
- Dhamangaonkar AC, Joshi D, Goregaonkar AB, Tawari AA.Proximal femoral locking plate versus dynamic hip screw for unstable intertrochanteric femoral fractures. Journal of Orthopaedic Surgery 2013;21(3):317-22. [DOI] [PubMed] [Google Scholar]
Dong 2018 {published data only}
- Dong Q, Zhang YG, Tian W.The effect of PFNA minimally invasive internal fixation on the postoperative slippage of fixing needle in elderly patients with femoral intertrochanteric fracture and the change of reset image. Acta Medica Mediterranea 2018;34(3):831-7. [Google Scholar]
Dujardin 2001 {published data only}
- Dujardin FH, Benez C, Polle G, Alain J, Biga N, Thomine JM.Prospective randomized comparison between a dynamic hip screw and a mini-invasive statis nail in fractures of the trochanateric area: preliminary results. Journal of Orthopaedic Trauma 2001;15(6):401-6. [2886746] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eceviz 2020 {published data only}
- Eceviz E, Cevik HB, Bulut G.Comparison of intramedullary and extramedullary fixation of basicervical fractures of the femur in the elderly: a prospective randomized study. Haseki Tip Bulteni 2020;58(2):169-75. [16964109] [Google Scholar]
Efstathopoulos 2007 {published data only}
- Efstathopoulos NE, Nikolaou VS, Lazarettos JT.Intramedullary fixation of intertrochanteric hip fractures: a comparison of two implant designs. International Orthopaedics 2007;31(1):71-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekstrom 2007 {published data only}
- Ekstrom W, Karlsson-Thur C, Larsson S, Ragnarsson B, Alberts K-A.Functional outcome in treatment of unstable trochanteric and subtrochanteric fractures with the proximal femoral nail and the Medoff sliding plate. Journal of Orthopaedic Trauma 2007;21(1):18-25. [2886748] [PMID: ] [DOI] [PubMed] [Google Scholar]
Esser 1986 {published data only}
- Esser MP, Kassab JY, Jones DH.Trochanteric fractures of the femur. a randomised prospective trial comparing the Jewett nail-plate with the dynamic hip screw. Journal of Bone and Joint Surgery. British Volume 1986;68(4):557-60. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2011 {published data only}
- Fitzpatrick DC, Sheerin DV, Wolf BR, Wuest TK.A randomized, prospective study comparing intertrochanteric hip fracture fixation with the dynamic hip screw and the dynamic helical hip system in a community practice. Iowa Orthopaedic Journal 2011;31:166-72. [PMC free article] [PubMed] [Google Scholar]
Fritz 1999 {published data only}
- Fritz T, Hiersemann K, Krieglstein C, Friedl W.Prospective randomized comparison of gliding nail and gamma nail in the therapy of trochanteric fractures. Archives of Orthopaedic & Trauma Surgery 1999;119(1):1. [DOI] [PubMed] [Google Scholar]
Galanopoulos 2018 {published data only}
- Galanopoulos IP, Mavrogenis AF, Megaloikonomos PD, Vottis CT, Mitsiokapa E, Koulouvaris P, et al.Similar function and complications for patients with short versus long hip nailing for unstable pertrochanteric fractures. SICOT-J 2018;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giraud 2005 {published data only}
- Giraud B, Dehoux E, Jovenin N, Madi K, Harisboure A, Usandizaga G.Pertrochanteric fractures: a randomized prospective study comparing dynamic screw plate and intramedullary fixation [Comparaison vis-plaque dynamique et ostéosynthèse intra-médullaire antérograde dans les fractures pertrochantériennes: une etude prospective randomisée]. Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur 2005;91(8):732-6. [2886750] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Giraud B, Dehoux E, Madi K, Harisboure A, Segal P.Intratrochanteric fractures; randomized prospective comparison of treatment with a dynamic hip screw (DHS) and anterograde intramedullary nailing (Targon PF). Journal of Bone and Joint Surgery. British Volume 2008;90(Suppl II):291. [2886751] [Google Scholar]
- Giraud B.Personal communication 4 July 2007. [2886752]
Goldhagen 1994 {published data only}
- Goldhagen P, O'Connor DR, Schwarze D, Schwartz EA.A prospective comparative study of compression hip screw and the Gamma nail [abstract]. Orthopaedic Transactions 1993;17(4):1048-9. [2886754] [DOI] [PubMed] [Google Scholar]
- Goldhagen PR, O'Connor DR, Schwarze D, Schwartz E.A prospective comparative study of the compression hip screw and the gamma nail. Journal of Orthopaedic Trauma 1994;8(5):367-72. [2886755] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gou 2013 {published data only}
- Guo Q, Shen Y, Zong Z, Zhao Y, Liu H, Hua X, et al.Percutaneous compression plate versus proximal femoral nail anti-rotation in treating elderly patients with intertrochanteric fractures: a prospective randomized study. Journal Orthopaedic Science 2013;18(6):977-86. [2886757] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2016 {published data only}
- Griffin XL, McArthur J, Achten J, Parsons N, Costa ML.The Warwick Hip Trauma Evaluation One - an abridged protocol for the WHiTE One Study: an embedded randomised trial comparing the X-bolt with sliding hip screw fixation in extracapsular hip fractures. Bone & Joint Research 2013;2(10):206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin XL, Parsons N, McArthur J, Achten J, Costa ML.The Warwick Hip Trauma Evaluation One: a randomised pilot trial comparing the X-Bolt Dynamic Hip Plating System with sliding hip screw fixation in complex extracapsular hip fractures: WHiTE (One). Bone & Joint Journal 2016;98(5):686-9. [DOI] [PubMed] [Google Scholar]
Griffin 2021 {published data only}
- Griffin XL, Achten J, O'Connor HM, Cook J, Costa ML.Effect on health-related quality of life of the X-Bolt dynamic plating system versus the sliding hip screw for the fixation of trochanteric fractures of the hip in adults: the WHiTE Four randomized clinical trial. Bone & Joint Journal 2021;103-B(2):256-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin XL, Achten J, Sones W, Cook J, Costa ML.Randomised controlled trial of the sliding hip screw versus x-bolt dynamic hip plating system for the fixation of trochanteric fractures of the hip in adults: a protocol study for WHiTE 4 (WHiTE4). BMJ Open 2018;8(1):e019944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guerra 2014 {published data only}
- Guerra M, Pasqualin S, Souza M, Lenz R.Functional recovery of elderly patients with surgically-treated intertrochanteric fractures: preliminary results of a randomised trial comparing the dynamic hip screw and proximal femoral nail techniques. Injury 2014;45(Suppl 5):S26-31. [2886759] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guo 2015 {published data only}
- Guo X, Zhang Y, Xiao J, Xie H, Yu J.Design and clinical application of lesser trochanteric reduction fixation system. Chinese Journal of Reparative and Reconstructive Surgery 2015;29(2):133-7. [PubMed] [Google Scholar]
Guyer 1991 {published data only}
- Guyer P, Landolt M, Eberle C, Keller H.The gamma-nail as a resilient alternative to the dynamic hip screw in unstable proximal femoral fractures in the elderly [Der Gamma-nagel als belastungsstabile alternative zur DHS bei der instabilen proximalen femurfraktur des alten menschen]. Helvetica Chirurgica Acta 1991;58(5):697-703. [2886761] [PMID: ] [PubMed] [Google Scholar]
- Guyer P, Landolt M, Keller H, Eberle C.The Gamma nail in per- and intertrochanteric femoral fractures - alternative or complementary to the DHS? A prospective randomised study. In: Marti RK, Dunki Jacobs PB, editors(s). Proximal Femoral Fractures. Operative Technique and Complications. Vol. 2. London (UK): Medical Press Limited, 1993:481-98. [2886763] [PMID: 1685055]1685055 [Google Scholar]
- Guyer P, Landolt M, Keller H, Eberle C.The Gamma Nail in per- and intertrochanteric femoral fractures - alternative or supplement to the dynamic hip screw? A prospective randomized study of 100 patients with per- and intertrochanteric femoral fractures in the surgical clinic of the City Hospital of Triemli, Zurich, September 1989 - June 1990 [Der Gamma-Nagel bei per- und intertrochantaren Femurfrakturen--Alternative oder Erganzung zur DHS? Eine prospektive randomisierte Studie anhand von 100 Patienten mit per- und intertrochantaren Femurfrakturen an der Chirurgischen Klinik des Stadtspitals Triemli, Zurich, September 1989-Juni 1990]. Aktuelle Traumatolologie 1991;21(6):242-9. [2886762] [PMID: ] [PubMed] [Google Scholar]
- Guyer P, Landolt M, Kelter H, Eberle C.Gamma-nails verses DHS be per- and intertrochateral femur fractures. Hefte zur der Unfallchirurg 1993;230:854-6. [2886764] [Google Scholar]
Haddon 2019 {published data only}
- Haddon J, Buciato R, Johnsen L.A prospective randomized trial of 100 patients using trochanteric support plates: worth their mettle? Injury 2019;50:733-37. [DOI] [PubMed] [Google Scholar]
Han 2012 {published data only}
- Han G, Wei W, Gu J.Comparison of proximal femoral locking plate and Gamma nail in the treatment of the femoral intertrochanteric fractures in the elder. China Journal of Orthopaedic Trauma 2012;25(10):796-9. [2886766] [PMID: ] [PubMed] [Google Scholar]
Haq 2014 {published data only}
- Haq RU, Manhas V, Pankaj A, Srivastava A, Dhammi IK, Jain AK.Proximal femoral nails compared with reverse distal femoral locking plates in intertrochanteric fractures with a compromised lateral wall; a randomised controlled trial. International Orthopaedics 2014;38(7):1443-9. [2886768] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardy 1998 {published data only}
- De Ridder VA, De Lange S.Use of an intramedullary hip-screw compared with a compression hip-screw with a plate for intertrochanteric femoral fractures. A prospective, randomized study of one hundred patients [letter; comment]. Journal of Bone and Joint Surgery. American Volume 1999;81(10):1502-3. [2886772] [PMID: ] [PubMed] [Google Scholar]
- Hardy DC, Delince P.Intramedullary hip screw (IMHS) versus compression hip screw plate (CHSP) for intertrochanteric hip fractures. A prospective, randomised trial of 160 patients [Abstract]. Journal of Bone and Joint Surgery. British Volume 1999;81(Suppl 2):163-4. [2886771] [Google Scholar]
- Hardy DC, Descamps P, Krallis P, Fabeck L, Smets P, Bertens CL, et al.Use of an intramedullary hip-screw compared with a compression hip-screw with a plate for intertrochanteric femoral fractures. A prospective, randomised study of one hundred patients. Journal of Bone and Joint Surgery. American Volume 1998;80(5):618-30. [2886770] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hardy 2003 {published data only}
- Hardy DC, Drossos K.Slotted intramedullary hip screw nails reduce proximal mechanical unloading. Clinical Orthopaedics & Related Research 2003;406:176-84. [DOI] [PubMed] [Google Scholar]
Harrington 2002 {published data only}
- Harrington P, Nihal A, Singania A, Howell F.Compression hip syndrome or intramedullary hip screw for unstable peri-trochanteric fractures? A prospective randomised study [abstract]. Journal of Bone and Joint Surgery. British Volume 1999;81(Suppl 3):296. [2886774] [Google Scholar]
- Harrington P, Nihal A, Singhania AK, Howell FR.Intramedullary hip screw versus sliding hip screw for unstable intertrochanteric femoral fractures in the elderly. Injury 2002;33(1):23-8. [2886775] [PMID: ] [DOI] [PubMed] [Google Scholar]
Haynes 1996 {published data only}
- Haynes RC.Internal hip fracture fixation systems [PhD thesis]. Bath, UK: University of Bath, 1996. [2886777] [Google Scholar]
Herrera 2002 {published data only}
- Herrera A, Domingo LJ, Calvo A, Martinez A, Cuenca J.A comparative study of trochanteric fractures treated with the gamma nail or the proximal femoral nail. International Orthopaedics 2002;26(6):365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 1996 {published data only}
- Hoffman CW, Lyndskey TG.Intertrochanteric fractures of the femur; a randomised prospective comparison of the gamma nail and the Ambi hip screw [abstract]. Journal of Bone and Joint Surgery. British Volume 1993;75(Suppl 1):50. [2886779] [PMID: 8639131]8639131 [Google Scholar]
- Hoffman CW, Lynskey TG.Intertrochanteric fractures of the femur: a randomized prospective comparison of the Gamma nail and the Ambi hip screw. New Zealand Journal of Surgery 1996;66(3):151-5. [2886780] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoffmann 1999 {published data only}
- Hoffmann R, Schmidmaier G, Schulz R, Schutz M, Sudkamp NP.Classic nail versus DHS. A prospective randomised study of fixation of trochanteric femur fractures [Classic-Nagel vs. dynamische Huftschraube (DHS). Eine prospektiv-randomisierte Studie zur Behandlung pertrochantarer Femurfrakturen]. Unfallchirurg 1999;102:182-90. [2886782] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hogh 1981 {published data only}
- Hogh J, Lund B, Lauritzen J.Ender or McLaughlin in trochanteric fractures? [Abstract]. Acta Orthopaedica Scandinavica 1980;51(359):20. [Google Scholar]
- Hogh J, Lund B, Lucht U.Trochanteric and subtrochanteric fractures. the operative results in a prospective and comparative study of Ender nailing and McLaughlin osteosynthesis. Acta Orthopaedica Scandinavica 1981;52:639-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hong 2011 {published data only}
- Hong JY, Suh SW, Park JH, Shin YS, Yoon JR, Yang JH.Comparison of soft-tissue serum markers in stable intertrochanteric fracture: dynamic hip screw versus proximal femoral nail—a preliminary study. Injury 2011;42(2):204-8. [2886784] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hopp 2016 {published data only}
- Hopp S, Wirbel R, Ojodu I, Pizanis A, Pohlemann T, Fleischer J.Does the implant make the difference? Prospective comparison of two different proximal femur nails. Acta Orthopaedica Belgica 2016;82(2):319-31. [PubMed] [Google Scholar]
Hornby 1989 {published data only}
- Hornby R, Evans JG, Vardon V.Operative or conservative treatment for trochanteric fractures of the femur. A randomised epidemiological trial in elderly patients. Journal of Bone and Joint Surgery. British Volume 1989;71(4):619-23. [DOI] [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang XQ, Liao YH, Wang XD, Wang XM, Gon SH.[Recovery of lower limb function in elder patients with intertrochanteric fractures treated with hip joint replacement versus dynamic hip screw]. Chinese Journal of Clinical Rehabilitation 2006;10(4):36-8. [Google Scholar]
Irvine 2014 {published data only}
- Irvine JN Jr, Widhalm H.A comparison of Parker's ratios for the treatment of AO/OTA 31-A2 fractures with third generation gamma nail or proximal femoral nail antirotation: is central positioning always the best? Journal of the American College of Surgeons 2014;1:S70. [Google Scholar]
Janzing 2002 {published data only}
- Brandt SE, Lefever S, Janzing HM, Broos PL, Pilot P, Houben BJ.Percutaneous compression plating (PCCP) versus the dynamic hip screw for pertrochanteric hip fractures: preliminary results. Injury 2002;33(5):413-8. [DOI] [PubMed] [Google Scholar]
- Janzing HM, Houben BJ, Brandt SE, Chhoeurn V, Lefever S, Broos P, et al.The Gotfried PerCutaneous Compression Plate versus the Dynamic Hip Screw in the treatment of pertrochanteric hip fractures: minimal invasive treatment reduces operative time and postoperative pain. Journal of Trauma: Injury, Infection and Critical Care 2002;52(2):293-8. [DOI] [PubMed] [Google Scholar]
Jolly 2019 {published data only}
- Jolly A, Bansal R, More AR, Pagadala MB.Comparison of complications and functional results of unstable intertrochanteric fractures of femur treated with proximal femur nails and cemented hemiarthroplasty. Journal of Clinical Orthopaedics and Trauma 2019;10(2):296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juhn 1988 {published data only}
- Juhn A, Krimerman J, Mendes DG.Intertrochanteric fracture of the hip. Comparison of nail-plate fixation and Ender's nailing. Archives of Orthopaedic & Traumatic Surgery 1988;107(3):136-9. [DOI] [PubMed] [Google Scholar]
Kammerlander 2018 {published data only}
- Kammerlander C, Hem ES, Klopfer T, Gebhard F, Sermon A, Dietrich M, et al.Cement augmentation of the proximal femoral nail antirotation (PFNA) - a multicentre randomized controlled trial. Injury 2018;49(8):1436-44. [DOI] [PubMed] [Google Scholar]
Karn 2006 {published data only}
- Karn NK, Singh GK, Kumar P, Shrestha B, Singh MP, Gowda MJ.Comparison between external fixation and sliding hip screw in the management of trochanteric fracture of the femur in Nepal. Journal of Bone and Joint Surgery. British Volume 2006;88-B(10):1347-50. Erratum in: Journal of Bone and Joint Surgery. Series B, 2007; 89(3)421. [DOI] [PubMed] [Google Scholar]
Kazemian 2014 {published data only}
- Kazemian GH, Manafi AR, Najafi F, Najafi MA.Treatment of intertrochanteric fractures in elderly high risk patients: dynamic hip screw vs. external fixation. Injury 2014;45(3):568-72. [DOI] [PubMed] [Google Scholar]
Kazemian 2016 {published data only}
- Kazemian GH, Emami M, Manafi A, Najafi F, Najafi MA.External fixation vs. skeletal traction for treatment of intertrochanteric fractures in the elderly. Trauma Monthly 2016;21(1):e15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim SY, Kim YG, Hwang JK.Cementless calcar-replacement hemiarthroplasty compared with intramedullary fixation of unstable intertrochanteric fractures. A prospective, randomized study. Journal of Bone and Joint Surgery. American Volume 2005;87(10):2186-92. [DOI] [PubMed] [Google Scholar]
Kosygan 2002 {published data only}
- Gotfried Y, Newman RJ, Kosygan KP, Mohan R.The Gotfried percutaneous compression plate compared with the conventional classic hip screw for the fixation of intertrochanteric fractures of the hip (multiple letters). Journal of Bone and Joint Surgery. Series B 2003;85(1):148-9. [DOI] [PubMed] [Google Scholar]
- Kosygan KP, Mohan R, Newman RJ.The Gotfried percutaneous compression plate compared with the conventional classic hip screw for the fixation of intertrochanteric fractures of the hip. Journal of Bone and Joint Surgery. British Volume 2002;84(1):19-22. [DOI] [PubMed] [Google Scholar]
- Kosygan KP, Newman RJ.A trial of the percutaneous compression plate and the classic hip screw for the fixation of intertrochanteric fractures of the hip [abstract]. Journal of Bone and Joint Surgery. British Volume 2005;85(1):no pagination. [DOI] [PubMed] [Google Scholar]
Kouvidis 2012 {published data only}
- Kouvidis G, Sakellariou, Mavrogenis AF, Stavrakakis J, Kampas D, Galanakis J, et al.Dual lag screw cephalomedullary nail versus the classic sliding hip screw for the stabilisation of intertrochanteric fractures. A prospective randomized study. Strategies in Trauma and Limb Reconstruction 2012;7:155-62. [2886786] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kukla 1997 {published data only}
- Kukla C, Berger G.Randomised comparison of the Gamma nail and the dynamic hip screw in 120 patients over 60 years of age. In: Gahr RH, Leung WS, Rosenwasser MO, Roth W, editors(s). The Gamma Locking Nail - Ten Years Results and Surgical Experience. Reinbek (Germany): Einhorn-Presse Verlag, 1999:294-302. [Google Scholar]
- Kukla C, Heinz T, Berger G, Kwasny O, Rosenberger A, Vecsei V.Gamma nail vs. dynamic hip screw in 120 patients over 60 years - a randomized trial. Acta Chirurgica Austriaca 1997;29(5):290-3. [DOI: 10.1007/BF02621324] [DOI] [Google Scholar]
- Kukla C, Heinz T, Berger G, Kwasny O, Wien A.Prospective randomised comparison of the Gamma nail and DHS in 120 patients [Prospektiv randomisierter vergleich zwischen Gammanagel und DHS bei 120 Patienten]. In: Rommens PM, Vecsei V, editors(s). Osteosynthese International. Leuven (Belgium): Leuven University Press, 1994:265-8. [Google Scholar]
- Vecsei V, Kukla C, Heinz T.Gamma-Nail versus DHS: prospective randomised comparison in 120 cases [abstract]. In: 5th International Orthopaedic and Trauma Meeting. Combined meeting of Austrian Trauma Association and Malaysian Orthopaedic Association; 1995 Oct 26-31; Kuala Lumpur (Malaysia). 1995:1-2. [2886792]
- Vecsei V, Kukla C, Heinz T.Gamma nail versus DHS - a prospective randomised trial of 120 patients [abstract]. Journal of Bone and Joint Surgery. British Volume 1995;77(Suppl 2):136. [2886791] [Google Scholar]
Kumar 2019 {published data only}
- Kumar CN, Srivastava MP.Screw versus helical proximal femoral nail in the treatment of unstable trochanteric fractures in the elderly. Journal of Clinical Orthopaedics and Trauma 2019;10(4):779-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuwabara 1998 {published data only}
- Kuwabara H, Wada T, Minagi Y, Iwasaki T, Tsuji H.Compression hip screw and gamma nail for intertrochanteric fractures - randomized prospective study. Hokkaido Journal of Orthopaedics & Traumatology 1998;40(2):29-33. [2886794] [Google Scholar]
Leung 1992 {published data only}
- Leung KS, So WS, Shen WY, Hui PW.Gamma nails and dynamic hip screws for peritrochanteric fractures. A randomised prospective study in elderly patients. Journal of Bone and Joint Surgery. British Volume 1992;74(3):345-51. [2886798] [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li J, Zhang ZS, Zhao HP, Zhang P, Pan YQ, Wu YF.Anatomical plate versus dynamic hip screw internal fixation for the treatment of femoral intertrochanteric fractures. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14(35):6627-31. [Google Scholar]
Li 2015b {published data only}
- Li X, Zhang L, Hou Z, Meng Z, Chen W, Wang P, et al.Distal locked and unlocked nailing for perthrochanteric fractures - a prospective comparative randomized study. International Orthopaedics (SICOT) 2015;39(8):1645-52. [DOI] [PubMed] [Google Scholar]
Li 2018 {published data only}
- Li H, Wang Q, Dai GG, Peng H.PFNA vs. DHS helical blade for elderly patients with osteoporotic femoral intertrochanteric fractures. European Review for Medical and Pharmacological Sciences 2018;22:1-7. [16964111] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liem 1993 {published data only}
- Liem FT.The use of Ender nails as opposed to the DHS. A randomised prospective study. Proximal Femoral Fractures. Operative Technique and Complications 1993;2(16):381-8. [Google Scholar]
Little 2008 {published data only}
- Fernando JC, Khaleel A, Elliot D.Holland nail vs DHS in intertrochanteric femoral fractures [abstract]. Journal of Bone and Joint Surgery. British Volume 2006;88(Suppl 1):71. [2886800] [Google Scholar]
- Little NJ, Verma V, Fernando C, Elliott DS, Khaleel A.A prospective trial comparing the Holland nail with the dynamic hip screw in the treatment of intertrochanteric fractures of the hip. Journal of Bone and Joint Surgery. British Volume 2008;90(8):1073-8. [2886801] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lopez 2002 {published data only}
- Lopez FM, Lopez XP, Casas OG, Valencia MR, Vao AL, Soleda JB.Prospective, comparative, randomized study of the sliding screw and Gamma nail in the treatment of pertrochanteric fractures [Estudio prospectivo aleatorio comparativo del tornillo deslizante y el clavo gamma en el tratamiento de las fracturas pertrocantereas]. Revista de Ortopedia y Traumatologia 2002;46(6):505-9. [17673596] [Google Scholar]
Lopez‐Vega 2015 {published data only}
- Lopez-Vega M, Gil-Monzo ER, Rodrigo-Perez JL, Lopez-Valenciano J, Salanova-Paris RH, Peralta-Nieto J, et al.Randomized prospective study on the influence distal block and Gamma 3 nail on the treatment of intertrochanteric fractures of femur. Revista Espanola de Cirugia Ortopedica y Traumatologia 2015;59(1):26-35. [DOI] [PubMed] [Google Scholar]
Lunsjo 2001 {published data only}
- Lunsjo K, Ceder L, Hamberg P, Tidermark J, Larsson BE, Allvin I, et al.The Medoff sliding plate versus standard dynamic screw systems in unstable inter-trochanteric and subtrochanteric hip fractures - a preliminary report of a prospective randomized multicenter trial [Abstract]. Acta Orthopaedica Scandinavica. Supplementum 1997;68(Suppl 274):97. [Google Scholar]
- Lunsjo K, Ceder L, Skytting B, Tidermark J, Larsson BE, Lindahl LE, et al.A randomized multicenter trial of the Medoff sliding plate versus standard screw-plate systems in subtrochanteric fractures. Acta Orthopaedica Scandinavica. Supplementum 1999;284:70. [DOI] [PubMed] [Google Scholar]
- Lunsjo K, Ceder L, Thorngren KG, Skytting B, Tidermark J, Berntson PO, et al.Extramedullary fixation of 569 unstable intertrochanteric fractures: a randomized multicenter trial of the Medoff sliding plate versus three other screw-plate systems. Acta Orthopaedica Scandinavica 2001;72(3):133-40. [DOI] [PubMed] [Google Scholar]
- Lunsjo K, Ceder L, Tidermark J, Hamberg P, Larsson BE, Ragnarsson B, et al.Extramedullary fixation of 107 subtrochanteric fractures: a randomized multicenter trial of the Medoff sliding plate versus 3 other screw-plate systems. Acta Orthopaedica Scandinavica 1999;70(5):459-66. [DOI] [PubMed] [Google Scholar]
- Lunsjo K.Dynamic fixation of unstable trochanteric hip fractures - a clinical and radiographic evaluation of the Medoff sliding plate [Abstract]. Acta Orhopaedica Scandinavica 1998;69(Suppl 282):68. [Google Scholar]
Makridis 2010 {published data only}
- Makridis KG, Georgaklis V, Georgoussis M, Mandalos V, Kontogeorgakos V, Badras L.Comparing two intramedullary devices for treating trochanteric fractures: a prospective study. Journal of Orthopaedic Surgery 2010;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marques 2005 {published data only}
- Marques F, Leon A, Mestre C, Ballester J, Caceres E.Prospective comparative study of the intertrochanteric Gamma nail and AO proximal femoral nail in the treatment of unstable intertrochanteric fractures. Revista de Ortopedia y Traumatologia 2005;49(1):11. [Google Scholar]
Matre 2013 {published data only}
- Matre K, Vinje T, Havelin LI, Gjertsen J, Furnes O, Espehaug B, et al.Trigen intertan intramedulllary nail versus sliding hip screw. A prospective, randomised multicenter study on pain, function and complications in 684 patients with an intertrochanteric or subtrochanteric fracture and one year follow-up. Journal of Bone and Joint Surgery. American Volume 2013;95:200-8. [DOI: 10.2106/JBJS.K.01497] [2886805] [DOI] [PubMed] [Google Scholar]
- Matre K, Vinje T, Havelin LI, Gjertsen J-E, Furnes O, Espehaug B, et al.Pain, function, and complications after operations with a sliding hip screw or an intertan nail for trochanteric and subtrochanteric fractures. A prospective randomized multicentre study with one year follow-up. Orthopaedic Proceedings 2012;Vol. 94-B:230. [2886806] [Google Scholar]
Mattsson 2004 {published data only}
- Mattsson P, Larsson S.Unstable trochanteric fractures augmented with calcium phosphate cement. A prospective randomized study using radiostereometry to measure fracture stability. Scandinavian Journal of Surgery: SJS 2004;93(3):223-8. [DOI] [PubMed] [Google Scholar]
Mattsson 2005 {published data only}
- Mattsson P, Alberts A, Dahlberg G, Sohlman M, Hyldahl HC, Larsson S.Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures. A prospective, randomised multicentre study. Journal of Bone and Joint Surgery. British Volume 2005;87(9):1203-9. [DOI] [PubMed] [Google Scholar]
McCormack 2013 {published data only}
- McCormack R, Panagiotopolous K, Buckley R, Penner M, Perey B, Pate G, et al.A multicentre, prospective, randomised comparison of the sliding hip screw with the Medoff sliding screw and side plate for unstable intertrochanteric hip fractures. Injury 2013;44(12):1904-9. [DOI] [PubMed] [Google Scholar]
McLaren 1991 {published data only}
- McLaren CA, Buckley JR, Rowley DI.Intertrochanteric fractures of the femur: a randomized prospective trial comparing the Pugh nail with the dynamic hip screw. Injury 1991;22(3):193-6. [DOI] [PubMed] [Google Scholar]
Mehdi 2000 {published data only}
- Kinninmonth A.Comparison of the intramedullary hip screw with Richard's classic hip screw in the management of pertrochanteric hip fractures. In: National Research Register, Issue 2, 2001. Oxford (UK): Update Software. [2886808]
- Mehdi SA, Kinninmonth AW, MacLeod C, McKenzie E, James PJ.Extracapsular hip fracture fixation: a prospective randomised comparison of the intramedullary hip screw with the sliding hip screw [abstract]. Injury 2000;31:287. [2886809] [Google Scholar]
- Mehdi SA.Personal communication 24 April 2006. [2886810]
Merenyi 1995 {published data only}
- Merenyi G, Zagh I, Kovacs A.Gamma nail versus Ender nails and angle-plate in the proximal fractures of the femur - a randomized prospective study. Journal of Bone and Joint Surgery. British volume 1995;77:215. [Google Scholar]
Michos 2001 {published data only}
- Michos I, Brakoulakis E, Pastroudis A, Loutriotis A, Adamopoulos G.The Gamma nail system compared to sliding nail and plate for peritrochanteric fractures [abstract]. Journal of Bone and Joint Surgery. British Volume 2001;83(Suppl 2):193. [2886812] [Google Scholar]
Miedel 2005 {published data only}
- Miedel R, Ponzer S, Tornkvist H, Soderqvist A, Tidermark J.The standard Gamma nail or the Medoff sliding plate for unstable trochanteric and sub-trochanteric fractures: a randomised, controlled trial. Journal of Bone and Joint Surgery. British Volume 2005;87(1):68-75. [DOI: 10.1302/0301-620X.87B1.15295] [2886814] [DOI] [PubMed] [Google Scholar]
- Tidermark J, Miedel R, Ponzer S, Tornkvist H.The standard Gamma nail or the Medoff sliding plate for unstable trochanteric and sub-trochanteric fractures: a randomized controlled trial [abstract]. Orthopaedic Trauma Association Annual Meeting; 2004 Oct 8-10; Hollywood (FL). Available at www.hwbf.org/ota/am/ota04/otapa/OTA041059.htm (accessed 24 January 2004). [2886815]
Moroni 2004 {published data only}
- Moroni A, Faldini C, Pegreffi F, Giannini S, BenNissan B, Sher D, et al.Hydroxyapatite-coated screws prevent lag screw cut-out in trochanteric fractures fixed with DHS: a prospective randomized study. Key Engineering Materials 2003;240:859-61. [Google Scholar]
- Moroni A, Faldini C, Pegreffi F, Giannini S.Better clinical outcomes for osteoporotic trochanteric fracture patients: DHS fixed with hydroxyapatite-coated AO/ASIF screws [abstact]. Journal of Bone and Joint Surgery. British Volume 2004;86:243. [Google Scholar]
- Moroni A, Faldini C, Pegreffi F, Giannini S.HA-coated screws decrease the incidence of fixation failure in osteoporotic trochanteric fractures. Clinical Orthopaedics and Related Research 2004;425:87-92. [DOI] [PubMed] [Google Scholar]
- Moroni A.Surgical techniques for osteoporotic bone. Orthopaedic Proceedings 2006;88-B(Suppl 1):11. [Google Scholar]
Moroni 2005 {published data only}
- Moroni A, Faldini C, Pegreffi F, Giannini S.External fixation for osteoporotic trochanteric fracture patients: a minimally invasive treatment option [abstract]. Journal of Bone and Joint Surgery. British Volume 2004;86:226. [Google Scholar]
- Moroni A, Faldini C, Pegreffi F, Hoang-Kim A, Vannini F, Giannini S, et al.Comparing dynamic hip screw to external fixation for treatment of osteoporotic pertrochanteric fractures: a prospective randomized study. Key Engineering Materials 2005;284:1037-40. [DOI] [PubMed] [Google Scholar]
Mott 1993 {published data only}
- Mott MP, Kronik JL, Fitzgerald RH, Morawa LG, Georgiadis GM, Salot WH.Gamma nail versus the sliding hip screw: a prospective randomized comparison [abstract]. Orthopaedic Transactions 1993;17:1049. [2886817] [Google Scholar]
Nungu 1991 {published data only}
- Nungu S, Olerud C, Rehnberg L.Treatment of intertrochanteric fractures: comparison of Ender nails and sliding screw plates. Journal of Orthopaedic Trauma 1991;5(4):452-7. [PubMed] [Google Scholar]
O'Brien 1995 {published data only}
- O'Brien PJ, Meek RN, Blachut PA, Broekhuyse HM, Sabharwal S.Fixation of intertrochanteric hip fractures: Gamma nail versus dynamic hip screw. A randomised, prospective study. Canadian Journal of Surgery 1995;38(6):516-20. [2886819] [PMID: ] [PubMed] [Google Scholar]
- O'Brien PJ, Meek RN, Blachut PA, Broekhuyse HM, Sabharwal S.Intertrochanteric hip fracture fixation - Gamma nail vs dynamic hip screw. A randomized, prospective study [abstract]. Orthopaedic Transactions 1994;18(1):19. [2886820] [Google Scholar]
- Sabharwal S, O'Brien PJ, Meek RN, Blackut PA, Broekhuyse HM.Intertrochanteric hip fracture fixation - Gamma nail versus dynamic hip screw. A randomized prospective study [abstract]. Journal of Bone and Joint Surgery. British Volume 1992;74(Suppl 3):281. [2886821] [Google Scholar]
Okcu 2013 {published data only}
- Okcu G, Ozkayin N, Okta C, Topcu I, Aktuglu K.Which implant is better for treating reverse obliquity fractures of the proximal femur: a standard or long nail? Clinical Orthopaedics and Related Research 2013;471(9):2768-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsson 2001 {published data only}
- Olsson O, Ceder L, Hauggaard A.Femoral shortening in intertrochanteric fractures. A comparison between the Medoff sliding plate and the compression hip screw. Journal of Bone and Joint Surgery. British Volume 2001;83(4):572-8. [DOI] [PubMed] [Google Scholar]
- Olsson O.Alternative techniques in trochanteric hip fracture surgery. Clinical and biomechanical studies on the Medoff sliding plate and the Twin hook. Acta Orthopaedica Scandinavica 2000;71(Suppl 295):1-31. [PubMed] [Google Scholar]
Ovesen 2006 {published data only}
- Ovesen O, Andersen M, Poulsen T, Nymark T, Overgaard S, Rock ND.The trochanteric gamma nail versus the dynamic hip screw: a prospective randomised study. One-year follow-up of 146 intertrochanteric fractures. Hip International 2006;16(4):293-8. [2886823] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ovesen O.Personal communication 25 June 2007. [2886824]
- Svenson O, Andersen M, Poulsen T, Nymark T, Overgaard S, Rock ND.A prospective randomised study comparing the trochanteric gamma nail (TGN) and the dynamic hip screw (DHS) in 146 intertrochanteric fractures [abstract]. Journal of Bone and Joint Surgery. British Volume 2006;88(Suppl 1):70. [2886825] [Google Scholar]
Ozkayin 2015 {published data only}
- Ozkayin N, Okcu G, Aktuglu K.Intertrochanteric femur fractures in the elderly treated with either proximal femur nailing or hemiarthroplasty: a prospective randomised clinical study. Injury 2015;46:S3-8. [DOI] [PubMed] [Google Scholar]
Pahlpatz 1993 {published data only}
- Pahlplatz PV, Langius FB.Comparing the Gamma nail and the dynamic hip screw in the treatment of pertrochanteric fractures. Preliminary results of a prospective randomised study. In: Marti RK, Dunki Jacobs PB, editors(s). Proximal Femoral Fractures. Operative Technique and Complications. Vol. 2. London (UK): Medical Press Limited, 1993:475-80. [2886827] [Google Scholar]
Pajarinen 2005 {published data only}
- Pajarinen J, Lindahl J, Michelsson O, Savolainen V, Hirvensalo E.Pertrochanteric femoral fractures treated with a dynamic hip screw or a proximal femoral nail: a randomised study comparing post-operative rehabilitation. Journal of Bone and Joint Surgery. British Volume 2005;87(1):76-81. [2886829] [PMID: ] [PubMed] [Google Scholar]
- Pajarinen J, Lindahl J, Savolainen V, Michelsson O, Hirvensalo E.Femoral shaft medialisation and neck-shaft angle in unstable pertrochanteric femoral fractures. International Orthopaedics 2004;28(6):347-53. [2886830] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarinen J.Personal communication 24 February 2005. [2886831]
Pan 2009 {published data only}
- Pan SH, Liu XW, Zhang CC, Xu SG, Fu QG.Implantation of Gamma nail and proximal femoral nail for the treatment of femoral intertrochanteric fractures in the elderly: a randomized follow-up for 131 cases. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13(39):7647-50. [Google Scholar]
Papasimos 2005 {published data only}
- Papasimos S, Koutsojannis CM, Panagopoulos A, Megas P, Lambiris E.A randomised comparison of AMBI, TGN and PFN for treatment of unstable trochanteric fractures. Archives of Orthopaedic and Trauma Surgery 2005;125(7):462-8. [2886833] [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 1998 {published data only}
- Kang JS, Park SR, Lee WH, Kim YH.Treatment of intertrochanteric fracture with the Gamma AP nail [poster]. In: Final programme of the 20th World Congress SICOT; 1996 Aug 18-23; Amsterdam. 1996:315. [2886835]
- Park SR, Kang JS, Kim HS, Lee WH.Treatment of intertrochanteric fracture with the Gamma AP locking nail or by a compression hip screw - a randomised prospective trial. International Orthopaedics 1998;3:157-60. [2886836] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2010 {published data only}
- Park JH, Lee YS, Park JW, Wang JH, Kim JG.A comparative study of screw and helical proximal femoral nails for the treatment of intertrochanteric fractures. Orthopedics 2010;33(2):81-5. [DOI] [PubMed] [Google Scholar]
Parker 2012 {published data only}
- Parker MJ, Bowers TR, Pryor GA.Sliding hip screw versus the Targon PF nail in the treatment of trochanteric fractures of the hip; a randomised trial of 600 fractures. Journal of Bone & Joint Surgery 2012;94(3):391-7. [17673598] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 2017 {published data only}
- Parker M.Intramedullary fixation with a third generation nail versus the sliding hip screw for trochanteric hip fractures; a randomised trial of 400 patients. Orthopaedic Proceedings 2011;Vol. 93-B:22. [17673599] [Google Scholar]
- Parker M.Intramedullary fixation with a third generation nail versus the sliding hip screw for trochanteric hip fractures: a randomised trial of 400 patients. Orthopaedic Proceedings 2010;Vol. 92-B:556. [17673600] [Google Scholar]
- Parker MJ, Cawley S.Sliding hip screw versus the Targon PFT nail for trochanteric hip fractures. Bone and Joint Journal 2017;99(9):1210-5. [16964113] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Parker MJ.Sliding hip screw versus intramedullary nail for trochanteric hip fractures; a randomised trial of 1000 patients with presentation of results related to fracture stability. Injury 2017;48(12):2762-7. [16964114] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 2020 {published data only}
- Parker MJ, Cawley S.Short (175 mm) versus standard (220 mm) length intramedullary nail for trochanteric hip fractures: a randomized trial of 229 patients. Bone and Joint Journal 2020;102(B(3)):394-9. [DOI] [PubMed] [Google Scholar]
Pelet 2001 {published data only}
- Pelet S, Arlettaz Y, Chevalley F.Osteosynthesis of per- and subtrochanteric fractures by blade plate versus gamma nail. A randomized prospective study. Swiss Surgery 2001;7(3):126-33. [2886842] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pesce 2014 {published data only}
- Pesce V, Maccagnano G, Vicenti G, Notarnicola A, Moretti L, Tafuri S, et al.The effect of hydroxyapatite coated screw in the lateral fragility fractures of the femur. A prospective randomized clinical study. Journal of Biological Regulators & Homeostatic Agents 2014;28(1):125-32. [PubMed] [Google Scholar]
Peyser 2007 {published data only}
- Peyser A, Weil Y, Brocke L, Sela Y, Mosheiff R, Mattan Y, et al.Randomized, prospective study comparing the efficacy of PCCP and CHS devices for the fixation of intertrochanteric hip fractures [abstract]. Journal of Bone and Joint Surgery. British Volume 2005;87:247. [DOI] [PubMed] [Google Scholar]
- Peyser A, Weil YA, Brocke L, Sela Y, Mosheiff R, Mattan Y, et al.A prospective, randomised study comparing the percutaneous compression plate and the compression hip screw for the treatment of intertrochanteric fractures of the hip (Abstract). Journal of Bone and Joint Surgery. British Volume 2007;89(9):1210-17. [DOI] [PubMed] [Google Scholar]
- Peyser A, Weil YA, Brocke L, Sela Y, Mosheiff R, Mattan Y, et al.A prospective randomised study comparing the percutaneous compression plate and the compression hip screw for the treatment of intertrochanteric fractures of the hip (correction to J Bone Joint Surg [Br] 2007; 89-B:1210–7). Journal of Bone and Joint Surgery. British Volume 2008;90(11):1533. [DOI] [PubMed] [Google Scholar]
Pitsaer 1993 {published data only}
- Pitsaer E, Samuel AW.Functional outcome after intertrochanteric fractures of the femur: does the implant matter? A prospective study of 100 consecutive cases. Injury 1993;24(1):35-6. [DOI] [PubMed] [Google Scholar]
Radford 1993 {published data only}
- Radford PJ, Needoff M, Webb JK.A prospective randomised comparison of the dynamic hip screw and the gamma locking nail. Journal of Bone and Joint Surgery. British Volume 1993;75(5):789-93. [2886844] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Radford PJ, Needoff M, Webb JK.The Gamma nail compared to the dynamic hip screw for pertrochanteric fractures of the femur [abstract]. Journal of Bone and Joint Surgery. British Volume 1992;74(Suppl 2):133-4. [2886845] [Google Scholar]
- Radford PJ, Needoff M.Intramedullary or extramedullary fixation for pertrochanteric fractures of the femur? [abstract]. Journal of Bone and Joint Surgery. British Volume 1992;74(Suppl 3):281. [2886846] [Google Scholar]
Rahme 2007 {published data only}
- Harris I, Rahme D.A prospective randomised controlled trial of subtrochanteric femur fractures treated with a proximal femoral nail compared to a 95-degree blade plate [abstract]. Journal of Bone and Joint Surgery. British Volume 2005;87(Suppl 3):310-1. [2886848] [Google Scholar]
- Rahme DM, Harris IA.Intramedullary nailing versus fixed angle blade plating for subtrochanteric femoral fractures: a prospective randomised controlled trial. Journal of Orthopaedic Surgery 2007;15(3):278-81. [2886849] [PMID: ] [DOI] [PubMed] [Google Scholar]
Raimondo 2012 {published data only}
- Raimondo E, Marchesi L, Pelis A, Grisone B, Giubilato A, Pietrogrande L.Minimally invasive surgical techniques in the treatment of proximal femoral fractures: PCCP plate versus ITST nail. A prospective randomized matched study. Journal of Orthopaedics and Traumatology 2013;13(Suppl 1):S57-89. [DOI: 10.1007/s10195-012-0210-2] [17673602] [DOI] [Google Scholar]
Reindl 2015 {published data only}
- NCT00597779.Randomised comparison of 2 fixation techniques for unstable intertrochanteric hip fractures (EMvsIM). clinicaltrials.gov/show/NCT00597779 (first received 18 January 2008). [2886852]
- Reindl R, Harvey EJ, Berry GK, Rahme E.Intramedullary versus extramedullary fixation for unstable intertrochanteric fractures: a prospective randomized controlled trial. Journal of Bone and Joint Surgery. American Volume 2015;97:1905-12. [2886851] [PMID: ] [DOI] [PubMed] [Google Scholar]
Romero 2008 {published data only}
- Romero JM, Diaz RA, Oropeza YM.Dynamic hip screw vs. percutaneous compression plate for trochanteric fractures. Acta Ortopedica Mexicana 2008;22(2):115-9. [PubMed] [Google Scholar]
Sadowski 2002 {published data only}
- Sadowski C, Lubbeke A, Saudan M, Riand N, Stern R, Hoffmeyer P.Treatment of reverse oblique and transverse intertrochanteric fractures with use of an intramedullary nail or a 95 degree screw-plate. Journal of Bone and Joint Surgery. American Volume 2002;84(3):372-81. [2886854] [PMID: ] [PubMed] [Google Scholar]
- Saudan M, Lubecke A, Sadowski C, Riand N, Stern R, Hoffmeyer P.Is there an indication for intramedullary fixation of intertrochanteric fractures? [abstract]. In: European Federation of National Associations of Orthopaedics and Traumatology; 2001 Jun 1-7; Rhodes (Greece). 2001. [2886855]
Sanders 2017 {published data only}
- Sanders D, Bryant D, Tieszer C, Lawendy AR, MacLeod M, Papp S, et al.Multicenter randomized control trial comparing a novel intramedullary device (InterTAN) versus conventional treatment (Sliding Hip Screw) of geriatric hip fractures. Journal of Orthopaedic Trauma 2017;31(1):1-8. [16964116] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saudan 2002 {published data only}
- Saudan M, Lubbeke A, Sadowski C, Riand N, Stern R, Hoffmeyer P.Pertrochanteric fractures: is there an advantage to an intramedullary nail? A randomized, prospective study of 206 patients comparing the dynamic hip screw and proximal femoral nail. Journal of Orthopaedic Trauma 2002;16(6):386-93. [2886857] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Saudan M, Lubecke A, Sadowski C, Riand N, Stern R, Hoffmeyer P.Is there an indication for intramedullary fixation of intertrochanteric fractures? [abstract]. In: European Federation of National Associations of Orthopaedics and Traumatology; 2001 Jun 1-7; Rhodes (Greece). 2001. [2886858]
- Saudan M, Lubecke-Wolff A, Sadowski C, Riand N, Hoffmeyer P.The proximal femoral nail (PFN) and the dynamic hip screw (DHS): a prospective clinical trial [abstract]. Journal of Bone and Joint Surgery. British Volume 1999;81(Suppl 2):163. [2886859] [Google Scholar]
- Stern RE, Sadowski C, Lubbeke A, Saudan M, Riand N, Hoffmeyer P.Pertrochanteric fractures: is there an advantage to an intramedullary nail? [abstract]. In: Annual Meeting of the Orthopaedic Trauma Association; 2001 Oct 18-20; San Diego (California). Available at www.hwbf.org/ota/am/ota01/otapo/OTP01065.html. Orthopaedic Trauma Association, 2001, (accessed 14 November 2001). [2886860]
Schipper 2004 {published data only}
- Schipper IB, Steyerberg EW, Castelein RM, Van der Heijden FH, Den Hoed PT, Kerver AJ, et al.Treatment of unstable trochanteric fractures. Randomised comparison of the gamma nail and the proximal femoral nail. Journal of Bone and Joint Surgery. British Volume 2004;86(1):86-94. [PubMed] [Google Scholar]
Selim 2020 {published data only}
- Selim AA, Beder FK, Algeaidy IT, Farhat AS, Diab NM, Barakat AS.Management of unstable pertrochanteric fractures, evaluation of forgotten treatment options. SICOT-J 2020;6(21):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sernbo 1988 {published data only}
- Sernbo I, Johnell O, Gentz CF, Nilsson JA.Unstable intertrochanteric fractures of the hip. Treatment with Ender pins compared with a compression hip-screw. Journal of Bone and Joint Surgery. American Volume 1988;70(9):1297-303. [PubMed] [Google Scholar]
Sernbo 1994 {published data only}
- Sernbo I, Johnell O, Gardsell A.A prospective randomized trial in trochanteric hip fractures treated with compression hip screw with and without locking and compression of the lag screw [Abstract]. Acta Orthopaedica Scandinavica 1990;61(237):55. [DOI] [PubMed] [Google Scholar]
- Sernbo I, Johnell O, Gardsell A.Locking and compression of the lag screw in trochanteric fractures is not beneficial. A prospective, randomized study of 153 cases. Acta Orthopaedica Scandinavica 1994;65(1):24-6. [DOI] [PubMed] [Google Scholar]
Seyhan 2015 {published data only}
- Seyhan M, Turkmen I, Unay K, Ozkut AT.Do PFNA devices and Intertan nails both have the same effects in the treatment of trochanteric fractures? A prospective clinical study. Journal of Orthopaedic Science 2015;20(6):1053-61. [DOI] [PubMed] [Google Scholar]
Shannon 2019 {published data only}
- Shannon SF, Yuan BJ, Cross WW, Barlow JD, Torchia ME, Holte PK, et al.Short versus long cephalomedullary nails for pertrochanteric hip fractures: a randomized prospective study. Journal of Orthopaedic Trauma 2019;33(10):480-6. [DOI] [PubMed] [Google Scholar]
Sharma 2018 {published data only}
- Sharma A, Sethi A, Sharma S.Treatment of stable intertrochanteric fractures of the femur with proximal femoral nail versus dynamic hip screw: a comparative study. Revista Brasileira de Ortopedia 2018;53(4):477-81. [16964118] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2018 {published data only}
- Shi SH.Curative effect of artificial femoral head replacement and its effect on hip joint function and complications of senile patients with femoral intertrochanteric fracture. Experimental and Therapeutic Medicine 2018;16(2):623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2017 {published data only}
- Shin YS, Chae JE, Kang TW, Han SB.Prospective randomized study comparing two cephalomedullary nails for elderly intertrochanteric fractures: Zimmer natural nail versus proximal femoral nail antirotation II. Injury 2017;48(7):1550-7. [DOI] [PubMed] [Google Scholar]
Singh 2017 {published data only}
- Singh AK, Narsaria N, Arun GR, Srivastava V.Treatment of unstable trochanteric femur fractures: Proximal Femur Nail versus Proximal Femur Locking Compression Plate. American Journal of Orthopedics 2017;46(2):E116-23. [16964120] [PMID: ] [PubMed] [Google Scholar]
Singh 2019 {published data only}
- Singh NK, Sharma V, Trikha V, Gamanagatti S, Roy A, Balawat AS, et al.Is PFNA-II a better implant for stable intertrochanteric fractures in elderly population? A prospective randomized study. Journal of Clinical Orthopaedics and Trauma 2019;10:S71-6. [16964122] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NK.A prospective randomized control study comparing functional and radiological outcome in elderly patients with type 31 A1 and A2 intertrochateric fractures treated by proximal femoral nail anti-rotation (PFNA) vs dynamic hip screw (DHS). Ann Arbor All India Institute of Medical Sciences, New Delhi (India) 2016;11004638:117. [16964123] [Google Scholar]
Song 2011 {published data only}
- Song W, Chen Y, Shen H, Yuan T, Zhang C, Zeng B.Biochemical markers comparison of dynamic hip screw and gamma nail implants in the treatment of stable intertrochanteric fracture: a prospective study of 60 patients. Journal of International Medical Research 2011;39(2):822-9. [2886935] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stappaerts 1995 {published data only}
- Stappaerts KH, Deldycke J, Broos PL, Staes FF, Rommens PM, Claes P.Treatment of unstable peritrochanteric fractures in elderly patients with a compression hip screw or with the Vandeputte (VDP) endoprosthesis: a prospective randomized study. Journal of Orthopaedic Trauma 1995;9(4):292-7. [DOI] [PubMed] [Google Scholar]
Stark 1992 {published data only}
- Stark A, Brostrom LA, Barrios C, Walheim G, Olsson E.A prospective randomized study of the use of sliding hip screws and Ender nails for trochanteric fractures of the femur. International Orthopaedics 1992;16(4):359-62. [DOI] [PubMed] [Google Scholar]
Stern 2011 {published data only}
- Stern R, Lubbeke A, Suva D, Miozzari H, Hoffmeyer P.Prospective randomised study comparing screw versus helical blade in the treatment of low-energy trochanteric fractures. International Orthopaedics 2011;35(12):1855-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2016 {published data only}
- Su H, Sun K, Wang X.A randomized prospective comparison of intertan and gamma3 for treating unstable intertrochanteric fractures. International Journal of Clinical and Experimental Medicine 2016;9(5):8640-7. [Google Scholar]
Tao 2013 {published data only}
- Tao R, Lu Y, Xu H, Zhou ZY, Wang YH, Liu F.Internal fixation of intertrochanteric hip fractures: a clinical comparison of two implant designs. Scientific World Journal 2013;2013:834825. [16964125] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teerenhovi 1984 {published data only}
- Teerenhovi O, Tunturi T, Suoniemi H.AO angled plate versus dynamic hip screw in the treatment of proximal fractures of the femur. Suomen Ortopedia ja Traumatologia 1984;7(3):16-21. [Google Scholar]
Trafton 1984 {published data only}
- Trafton PG, Day LJ, Cohen HA, Kaye RA, Bovill EG.A comparative study of compression hip screw and Condylocephalic nail for intertrochanteric fractures of the femur [Abstract]. Orthopaedic Transactions 1984;8(3):391. [Google Scholar]
Utrilla 1998 {published data only}
- Utrilla LA, Gomez CR, Forcada SE.Compression screw plate or fixed nail plate in trochanteric femoral fractures in the elderly [Tornillo-placa a compresión o clavo-placa monobloque en las fracturas trocantéreas del fémur del anciano]. Revista de Ortopedia y Traumatologia 1998;42(5):368-73. [Google Scholar]
Utrilla 2005 {published data only}
- Utrilla AL, Reig JS, Munoz FM, Tufanisco CB.Trochanteric Gamma nail and compression hip screw for trochanteric fractures: a randomized, prospective, comparative study in 210 elderly patients with a new design of the Gamma nail. Journal of Orthopaedic Trauma 2005;19(4):229-33. [2886862] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vaquero 2012 {published data only}
- Vaquero J, Munoz J, Prat S, Ramirez C, Aguado HJ, Moreno E, et al.Proximal Femoral Nail Antirotation versus Gamma3 nail for intramedullary nailing of unstable trochanteric fractures. A randomised comparative study. Injury 2012;43:S47-54. [DOI] [PubMed] [Google Scholar]
Varela‐Egocheaga 2009 {published data only}
- Varela-Egocheaga JR, Iglesias-Colao R, Suarez-Suarez MA, Fernandez-Villan M, Gonzalez-Sastre V, Murcia-Mazon A.Minimally invasive osteosynthesis in stable trochanteric fractures: a comparative study between Gotfried percutaneous compression plate and Gamma 3 intramedullary nail. Archives of Orthopaedic and Trauma Surgery 2009;129(10):1401-7. [2886864] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verettas 2010 {published data only}
- Verettas D-A, Ifantidis P, Chatzipapas CN, Drosos GI, Xarchas KC, Chloropoulou P, et al.Systematic effects of surgical treatment on hip fractures: gliding screw-plating vs intramedullary nailing. Injury 2010;41(3):279-84. [2886866] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Verettas D-A.Personal communication 25 May 2010. [2886867]
Vidyadhara 2007 {published data only}
- Vidyadhara S, Roa SK.One and two femoral neck screws with intramedullary nails for unstable trochanteric fractures of femur in the elderly - randomised clinical trial. Injury 2007;38(7):806-14. [DOI] [PubMed] [Google Scholar]
Vossinakis 2002 {published data only}
- Baumgaertner MR.The pertrochanteric external fixator reduced pain, hospital stay, and mechanical complications in comparison with the sliding hip screw. Journal of Bone and Joint Surgery. Series A 2002;84(8):1488. [DOI] [PubMed] [Google Scholar]
- Vossinakis IC, Badras LS.The external fixator compared with the sliding hip screw for pertrochanteric fractures of the femur. Journal of Bone and Joint Surgery. British Volume 2002;84(1):23. [DOI] [PubMed] [Google Scholar]
- Vossinakis IC, Skretas E, Bitounis E, Badras LS.Comparison between external fixation and the sliding hip screw in pertrochanteric fractures - a prospective randomised study. Journal of Bone and Joint Surgery. British Volume 2001;83:168. [Google Scholar]
Wang 2011 {published data only}
- Wang W, Hao H, Wang Jf, Yu L, Liang Hw.Comparison of expandable intradullary nail and proximal femoral nail in the treatment of intertrochanteric fractures. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15(39):7319-22. [Google Scholar]
Wang 2019 {published data only}
- Wang B, Liu Q, Liu Y, Jiang R.Comparison of proximal femoral nail antirotation and dynamic hip screw internal fixation on serum markers in elderly patients with intertrochanteric fractures. Journal of the College of Physicians and Surgeons Pakistan 2019;29(7):644-8. [16964127] [PMID: ] [DOI] [PubMed] [Google Scholar]
Watson 1998 {published data only}
- Watson JT, Moed BR, Cramer KE, Karges DE.Comparison of the compression hip screw with the Medoff sliding plate for intertrochanteric fractures. Clinical Orthopaedics and Related Research 1998;348:79-86. [PubMed] [Google Scholar]
- Watson JT, Moed BR, Karges DE, Cramer KE.DHS versus Medoff plate for the treatment of unstable intertrochanteric fractures: a randomized prospective study [Abstract]. Orthopaedic Transactions 1997;21(4):no pagination. [Google Scholar]
Weisbrot 2005 {published data only}
- Weisbrot M, Garti A, Pirotzki A, Yassin M, Hendel D, Robinson D.Percutaneous compression plate vs. DHS in trochanteric fractures. A randomized prospective study [abstract]. Journal of Bone and Joint Surgery. British Volume 2005;87:379. [Google Scholar]
Wild 2010 {published data only}
- Wild M, Jungbluth P, Thelen S, Laffree Q, Gehrmann S, Betsch M, et al.The dynamics of proximal femoral nails: a clinical comparison between PFNA and Targon PF. Orthopedics 2010;33:8. [DOI] [PubMed] [Google Scholar]
Wu 2020 {published data only}
- Wu K, Xu Y, Zhang L, Zhang Y, Xu W, Chu J, et al.Which implant is better for beginners to learn to treat geriatric intertrochanteric femur fractures: a randomised controlled trial of surgeons, metalwork, and patients. Journal of Orthopaedic Translation 2020;21:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xin 2014 {published data only}
- Xin F, Zhu GX, Gu YL.Dynamic hip screw combined with antirotation screw in repair of unstable femoral intertrochanteric fractures. Chinese Journal of Tissue Engineering Research 2014;18(48):7786-91. [Google Scholar]
Xu 2010a {published data only}
- Xu Y, Geng D, Yang H, Wang X, Zhu G.Treatment of unstable proximal femoral fractures: comparison of the proximal femoral nail antirotation and gamma nail 3. Orthopedics 2010;33(7):473. [DOI] [PubMed] [Google Scholar]
Xu 2010b {published data only}
- Xu YZ, Geng DC, Mao HQ, Zhu XS, Yang HL.A comparison of the proximal femoral nail antirotation device and dynamic hip screw in the treatment of unstable pertrochanteric fracture. Journal of International Medical Research 2010;38:1266-75. [2886869] [PMID: ] [DOI] [PubMed] [Google Scholar]
Xu 2018 {published data only}
- Xu R, Ru J, Ji F, Liu J, Ji Y, Wu Z, et al.Comparison of efficacy, complications and TGF-beta2 expression between DHS and PFNA in elderly patients with osteoporotic femoral intertrochanteric fracture. Experimental and Therapeutic Medicine 2018;16(1):394-9. [16964129] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamauchi 2014 {published data only}
- Yamauchi K, Fushimi K, Shirai G, Fukuta M.Comparison of functional recovery in the very early period after surgery between plate and nail fixation for correction of stable femoral intertrochanteric fractures: a controlled clinical trial of 18 patients. Geriatric Orthopaedic Surgery & Rehabilitation 2014;5(2):63-8. [16964131] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2011a {published data only}
- Yang N, Sun TS, Guo YZ, Zhang ZC, Wang XW.Intramedullary hip screw versus proximal femoral nail for intertrochanteric fracture: a randomized controlled study. Journal of Clinical Rehabilitave Tissue Engineering Research 2011;15(17):3093-7. [Google Scholar]
Yang 2011b {published data only}
- Yang E, Qureshi S, Trokhan S, Joseph D.Gotfried percutaneous compression plating compared with sliding hip screw fixation of intertrochanteric hip fractures: a prospective randomized study. Journal of Bone and Joint Surgery. American Volume 2011;93(10):942-7. [DOI] [PubMed] [Google Scholar]
Yaozeng 2010 {published data only}
- Yaozeng X, Dechun G, Huilin Y, Guangming Z, Xianbin W.Comparative study of trochanteric fracture treated with the proximal femoral nail anti-rotation and the third generation of gamma nail. Injury 2010;41(12):1234-8. [DOI] [PubMed] [Google Scholar]
Yu 2015 {published data only}
- Yu YY.Complication of two intramedullary nail treatments of osteoporotic intertrochanteric fractures. Chinese Journal of Tissue Engineering Research 2015;19(12):2103-7. [Google Scholar]
Zehir 2015 {published data only}
- Zehir S, Zehir R, Azboy I, Haykir N.Proximal femoral nail antirotation against dynamic hip screw for unstable trochanteric fractures: a prospective randomized comparison. European Journal of Trauma & Emergency Surgery 2015;41(4):393-400. [16964133] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang S, Zhang K, Jia Y, Yu B, Feng W.InterTan nail versus Proximal Femoral Nail Antirotation-Asia in the treatment of unstable trochanteric fractures. Orthopedics 2013;36(3):e288-94. [DOI] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang J, Liu J, Wang W, Fu Q, Zheng Y, Yuan X.Proximal femoral nail anti-rotation versus hip arthroplasty for osteoporotic intertrochanteric fracture: surgical effects and indications. International Journal of Clinical and Experimental Medicine 2018;11(7):7146-51. [Google Scholar]
Zhou 2012 {published data only}
- Zhou F, Zhang Z, Yang H, Tian Y, Ji HQ, Guo Y, et al.Less invasive stabilization system (LISS) versus Proximal Femoral Nail Anti-rotation (PFNA) in treating proximal femoral fractures: a prospective randomized study. Journal of Orthopaedic Trauma 2012;26(3):155-62. [2886871] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zou 2009 {published data only}
- Zou J, Xu Y, Yang H.A comparison of proximal femoral nail antirotation and dynamic hip screw devices in trochanteric fractures. Journal of International Medical Research 2009;37(4):1057-64. [2886873] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12608000162314 {published data only}
- ACTRN12608000162314.Prospective randomised pilot study comparing the dynamic hip screw and intramedullary gamma nail regarding the treatment of intertrochanteric hip fracture [Prospective randomised pilot study comparing the dynamic hip screw and intramedullary gamma nail regarding functional recovery following the treatment of intertrochanteric hip fractures]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ACTRN12608000162314 (first received 1 August 2008). [17673604]
- Molnar R.Trial status [personal communication]. Email 3 August 2015. [17673605]
Ahmad 2011 {published data only}
- Ahmad M, Bajwa A, Patil S, Bhattacharya R, Nanda R, Danjoux G, et al.Haemodynamic changes during fixation of extracapsular proximal femoral fractures: RCT comparing compression hip screw versus intramedullary hip screw [abstract]. Journal of Bone and Joint Surgery. British Volume 2011;93(Suppl III):313-4. [2886951] [Google Scholar]
- Ahmad M, Bhattacharya R, Nanda R, Bajwa A, Danjoux G, Hui AC.An RCT comparing haemodynamic changes during the fixation of extracapsular proximal femoral fractures using the compression hip screw versus the intramedullary hip screw [abstract]. Surgeon: Journal of the Royal Colleges of Surgeons of Edinburgh and Ireland 2005;3(Suppl 3):S10. [2886952] [Google Scholar]
- Ahmad M.Randomised controlled trial to compare the magnitude and incidence of haemodynamic changes during fixation of extracapsular fractures of the neck of femur using the compression hip screw versus the intramedullary hip screw. National Research Register Archive. www.nihr.ac.uk/Profiles/NRR.aspx?Publication_ID=N0227149002 (accessed 7 June 2010). [2886953]
Alobaid 2004 {published data only}
- Alobaid A, Harvey EJ, Elder GM, Lander P, Guy P, Reindl R.Minimally invasive dynamic hip screw: prospective randomized trial of two techniques of insertion of a standard dynamic fixation device. Journal of Orthopaedic Trauma 2004;18(4):207-12. [DOI] [PubMed] [Google Scholar]
Bong 1981 {published data only}
- Bong SC, Lau HK, Leong JC, Fang D, Lau MT.The treatment of unstable intertrochanteric fractures of the hip: a prospective trial of 150 cases. Injury 1981;13(2):139-46. [DOI] [PubMed] [Google Scholar]
El‐Desouky 2016 {published data only}
- El-Desouky II, Mohamed MM, Kandil AE.Clinical outcome of conventional versus biological fixation of subtrochanteric fractures by proximal femoral locked plate. Injury 2016;47(6):1309-17. [DOI] [PubMed] [Google Scholar]
Emami 2013 {published data only}
- Emami M, Manafi A, Hashemi B, Nemati A, Safari S.Comparison of intertrochanteric fracture fixation with dynamic hip screw and bipolar hemiarthroplasty techniques. Archives of Bone & Joint Surgery 2013;1(1):14. [PMC free article] [PubMed] [Google Scholar]
Gupta 2012 {published data only}
- Gupta A, Cooke C, Wilkinson M, Grazette A.Randomised control trial comparing long cephlocondylic nail vs compression hip screw for treatment of intertrochanteric hip fractures (abstract). Journal of Bone and Joint Surgery. British Volume 2012;94(Suppl XLI):117. [2886955] [Google Scholar]
Kumar 1996 {published data only}
- Kumar MM, Sudhakar GM, Shah DD, Pathak RH.A study of the role of osteotomy in unstable intertrochanteric fractures. Journal of Postgraduate Medicine 1996;42(1):4. [PubMed] [Google Scholar]
Kumar 2006 {published data only}
- Kumar S, Sen RK, Nagi ON.Hip abductor strength in unstable trochanteric fractures stabilised with DHS-TSP. European Journal of Trauma 2006;32:24. [Google Scholar]
Lee 2007a {published data only}
- Lee P-C, Hsieh P-H, Yu S-W, ShIao C-W, Kao H-K, Wu C-C.Biologic plating versus intramedullary nailing for comminuted subtrochanteric fractures in young adults: a prospective, randomised study of 66 cases. Journal of Trauma: Injury, Infection and Critical Care 2007;63(6):1283-91. [2886796] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2007b {published data only}
- Lee YS, Huang HL, Lo TY, Huang CR.Dynamic hip screw in the treatment of intertrochanteric fractures: a comparison of two fixation methods. International Orthopaedics 2007;31(5):683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2015a {published data only}
- Li P.Clinical analysis of syndrome differentiation treatment combined with semi-open open reduction and DHS fixation treatment of intertrochanteric fractures for 25 cases. Chinese Medicine Modern Distance Education of China 2015;13(3):57-8. [Google Scholar]
NCT00323232 {published data only}
- NCT00323232.Comparison of treatment outcomes in hip fractures surgically fixed with either a two or four hole device. clinicaltrials.gov/ct2/show/NCT00323232 (first received 9 May 2006).
NCT00686023 {published data only}
- NCT00686023.Comparing Surgical Techniques for CRIF of Pertrochanteric Fractures. clinicaltrials.gov/ct2/show/NCT00686023 (first received 29 May 2008). [17673607]
NCT00736684 {published data only}
- NCT00736684.Proximal Femoral Nail Antirotation™ (PFNA) versus Gamma Nail 3™ (Gamma3) for intramedullary nailing of unstable trochanteric fractures (PROGAINT-ES). clinicaltrials.gov/ct2/show/NCT00736684 (first received 18 August 2008). [17673609]
NCT01173744 {published data only}
- NCT01173744.Comparison of Gamma nail versus Dynamic Hip Screw for the treatment of unstable intertrochanteric fractures. clinicaltrials.gov/ct2/show/NCT01173744 (first received 2 August 2010). [17673611]
NCT01238068 {published data only}
- NCT01238068.Comparison of the results of treatment by Gamma nail versus dynamic hip screw for unstable intertrochanteric hip fractures. clinicaltrials.gov/show/NCT01238068 (first received 10 November 2010). [2886957]
NCT03065101 {published data only}
- NCT03065101.Trigen InterTAN vs Sliding Hip Screw RCT. clinicaltrials.gov/ct2/show/NCT03065101 (first received 27 February 2017). [17673613]
Savadhkoohi 2016 {published data only}
- Savadkoohi D, Siavashi B, Pendar E, Aalirezaei A.Subtrochanteric fractures: comparison of dynamic compression screw (DCS) and intramedullary nail fixation outcomes. HIP International 2016;26:S86-S87. [Google Scholar]
Sher 1985 {published data only}
- Sher JL, Stevens J, Porter BB, Checketts RG.A comparison of operative and conservative treatment for unstable trochanteric fractures [abstract]. Journal of Bone and Joint Surgery 1985;3:495. [Google Scholar]
Wong 2009 {published data only}
- Wong TC, Chiu Y, Tsang WL, Leung WY, Yeung SH.A double-blind, prospective, randomised, controlled clinical trial of minimally invasive dynamic hip screw fixation of intertrochanteric fractures. Injury 2009;40(4):422-7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ISRCTN32393360 {published data only}
- ISRCTN32393360.Randomised controlled trial to compare the magnitude and incidence of haemodynamic changes during fixation of extracapsular fractures of the neck of femur using the compression hip screw versus the intramedullary hip screw. who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN32393360 (first received 30 September 2005).
ISRCTN48618754 {published data only}
- ISRCTN48618754.Stabilisation of extracapsular fractures of the hip with a Hansson twin hook or a lag screw - a prospective randomised controlled trial. who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN48618754 (first received 30 September 2004).
JPRN‐UMIN000018307 {published data only}
- JPRN-UMIN000018307.Multicenter study for evaluation of rotational instability of femoral trochanteric fractures. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000021145 (first received 29 July 2015).
NCT01380444 {published data only}
- NCT01380444.Intramedullary nail versus sliding hip screw inter-trochanteric evaluation (INSITE). clinicaltrials.gov/show/NCT01380444 (first received 27 June 2011). [17673617]
NCT02171897 {published data only}
- NCT02171897.Pertrochanter arthroplasty versus osteosynthesis [Comparison of functional recovery after pertrochanter fracture treated with either total hip replacement or centromedullary nailing]. clinicaltrials.gov/ct2/show/record/NCT02171897 (first received 24 June 2014).
NCT02294747 {published data only}
- NCT02294747.Trochanteric hip fractures (AO A2) SHS with or without trochanteric stabilizing plate - RCT using RSA [Trochanteric hip fractures (AO A2) treated with sliding hip screw with or without trochanteric stabilizing plate - a randomized controlled trial using radiostereometry]. clinicaltrials.gov/ct2/show/record/NCT02294747 (first received 19 November 2014).
NCT02788994 {published data only}
- NCT02788994.Stabilization of fresh unilateral unstable pertrochanteric hip fracture (PET). clinicaltrials.gov/show/NCT02788994 (first received 2 June 2016). [17673619]
NCT03000972 {published data only}
- NCT03000972.PFN-A augmentation for intertrochanteric femoral fractures [PFN-A intramedullary nail and intertrochanteric hip Fracture. Augmentation of the femoral head assessed by PET-CT (Positron Emission Tomography - Computed Tomography)- a randomized trial]. clinicaltrials.gov/ct2/show/NCT03000972 (first received 22 December 2016).
NCT03635320 {published data only}
- NCT03635320.The CHINA TFNA study [A prospective study evaluating trochanteric Fixation Nail Advanced (TFNA) in a Chinese patient population]. clinicaltrials.gov/ct2/show/record/NCT03635320 (first received 17 August 2018).
NCT03849014 {published data only}
- NCT03849014.Comparison of biochemical changes in patients with trochanteric region fracture fixation with DHS versus PFN. clinicaltrials.gov/show/NCT03849014 (first received 21 February 2019). [17673621]
NCT04240743 {published data only}
- NCT04240743.Fixation methods of basicervical fractures [Comparison of intramedullary and extramedullary fixation of basicervical fractures of the femur in the elderly: a prospective, randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT04240743 (first received 27 Jaunuary 2020).
REGAIN 2008 {published data only}
- Bhandari M, Bojan A, Eckholm C, Brink O, Adili A, Sprague S, et al.Functional outcomes following intramedullary nailing of trochanteric hip fractures: a pilot multicentre, randomised controlled trial (abstract). Journal of Bone and Joint Surgery. British Volume 2011;93(Suppl IV):574. [17673623] [Google Scholar]
- NCT00555945.Re-evaluation of Gamma3 intramedullary nails in hip fracture (REGAIN). clinicaltrials.gov/ct2/show/NCT00555945 (first received 9 November 2007). [17673624]
References to ongoing studies
ACTRN12610000992000 {published data only}
- ACTRN12610000992000.Locking plate for treatment of intertrochanteric hip fractures [A prospective,randomised, controlled trial to evaluate the effect of locking plates compared with gamma nails on individuals with intertrochanteric hip fractures]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000992000 (first received 14 November 2010).
ACTRN12618001431213 {published data only}
- ACTRN12618001431213.Evaluating the treatment methods of proximal femur fractures in elderly trauma patients [A multi-centre, single blinded prospective randomised controlled trial of the Gamma 3 intramedullary nail to the unlocked and locked Intertan intramedullary nail for the treatment of proximal femur fractures]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375338 (first received 27 August 2018).
- Sivakumar A, Thewlis D, Ladurner A, Edwards S, Rickman M.Proximal Femoral Nail Unlocked versus Locked (ProFNUL): a protocol for a multicentre, parallel-armed randomised controlled trial for the effect of femoral nail mode of lag screw locking and screw configuration in the treatment of intertrochanteric femur fractures. BMJ Open 2020;10(2):e032640. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR1900025588 {published data only}
- ChiCTR1900025588.A randomized control trial for combined proximal femur intramedullary nail and lateral wall plate for the treatment of pantrochanteric fracture. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900025588 (first received 2 September 2019).
ChiCTR‐INR‐17011841 {published data only}
- ChiCTR‐INR‐17011841.Intertrochanteric fracture in the elderly: a randomized comparison of reduction and fixation and total hip arthroplasty. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-INR-17011841 (first received 03 July 2017).
ChiCTR‐TRC‐11001642 {published data only}
- ChiCTR-TRC-11001642.Minimally invasive percutaneous compression plating versus dynamic hip screw for intertrochanteric fractures. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-11001642 (first received 25 October 2011).
CTRI/2019/02/017733 {published data only}
- CTRI/2019/02/017733.A clinical study to evaluate the outcomes of single lag screw and helical blade nailing in treatment of intertrochanteric femur fractures. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/02/017733 (first received 20 February 2019).
CTRI/2019/11/022097 {published data only}
- CTRI/2019/11/022097.A study to compare the results using Dynamic Hip Screw and modified Proximal Femoral Locking Compression Plate in fractures around the hip. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/11/022097 (first received 21 November 2019).
IRCT20141209020258N80 {published data only}
- IRCT20141209020258N80.Comparison proximal femoral nailing (PFN) versus dynamic hip screw(DHS) in intertrochanteric fracture. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=IRCT20141209020258N80 (first received 11 June 2018). [17673626]
IRCT20170621034689N2 {published data only}
- IRCT20170621034689N2.Results of intertrochanteric fractures treated with Dynamic Hip Screw (DHS) with or without use of static screw. who.int/trialsearch/Trial2.aspx?TrialID=IRCT20170621034689N2 (first received 10 March 2018).
IRCT20181015041344N1 {published data only}
- IRCT20181015041344N1.Comparison of Dynamic hip screw and Dynamic external fixator in intertrochantric fractures. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20181015041344N1 (first received 24 May 2019).
JPRN‐UMIN000019523 {published data only}
- JPRN-UMIN000019523.Prospective study for treatment of femoral intertrochanteric fracture - ORIF VS bipolar prosthesis. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000022579 (first received 27 October 2015).
NCT01437176 {published data only}
- NCT01437176.Treatment of intertrochanteric fracture with new type of intramedullary nail. clinicaltrials.gov/ct2/show/record/NCT01437176 (first received 20 September 2011).
NCT01509859 {published data only}
- NCT01509859.Comparing weight bearing after intramedullary fixation devices for the proximal femur fracture. clinicaltrials.gov/ct2/show/record/NCT01509859 (first received 13 January 2012).
NCT01797237 {published data only}
- NCT01797237.Outcome comparison between PFNA and InterTAN. clinicaltrials.gov/ct2/show/NCT01797237 (first received 22 February 2013).
NCT02627040 {published data only}
- NCT02627040.Intertrochanteric femoral fracture fixation trial. clinicaltrials.gov/ct2/show/NCT02627040 (first received 18 November 2019).
NCT03407131 {published data only}
- NCT03407131.Internal fixation or joint replacement therapy for aged hip fracture patients. clinicaltrials.gov/ct2/show/NCT03407131 (first received 23 January 2018).
NCT03906032 {published data only}
- NCT03906032.Comparison of sliding hip screw to intramedullary nailing in the treatment of intertrochanteric hip fracture. clinicaltrials.gov/ct2/show/NCT03906032 (first received 8 April 2019). [17673628]
NCT04306198 {published data only}
- NCT04306198.Helical blade vs lag screw fixation for cephalomedullary nailing of low energy intertrochanteric hip fractures. clinicaltrials.gov/ct2/show/results/NCT04306198 (first received 12 March 2020).
NCT04441723 {published data only}
- NCT04441723.Comparison of dynamic versus static lag screw modes for cephalomedullary nails used to fix intertrochanteric fragility fractures. clinicaltrials.gov/ct2/show/NCT04441723 (first received 24 June 2020).
NTR1133 {published data only}
- NTR1133.Intramedullary nailing of proximal femur fractures: Gamma 3 Nail versus Fixion Proximal Femur Nailing System. trialregister.nl/trial/1098 (first received 11 December 2007).
Additional references
AAOS 2014
- American Academy of Orthopaedic Surgeons (AAOS).Management of hip fractures in the elderly. Evidence-based clinical practice guideline. www.aaos.org/research/guidelines/HipFxGuideline.pdf (accessed 22 March 2019).
AO Foundation 2018
- AO Foundation.Femur. Journal of Orthopaedic Trauma 2018;32 Suppl 1:S33-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bardsley 2013
- Smith P, Ariti C, Bardsley M.Nuffield Trust & Health Foundation: Focus on hip fracture. Trends in emergency admissions for fractured neck of femur, 2001 to 2011. www.nuffieldtrust.org.uk/research/focus-on-hip-fracture (accessed 9 January 2019).
Berliner 2016
- Berliner JL, Brodke DJ, Chan V, SooHoo NF, Bozic KJ.John Charnley Award: preoperative patient-reported outcome measures predict clinically meaningful improvement in function after THA. Clinical Orthopaedics and Related Research 2016;474(2):321-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brignardello‐Petersen 2018a
- Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al, GRADE Working Group.Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical Epidemiology 2018;93:36-44. [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2018b
- Brignardello-Petersen R, Mustafa RA, Siemieniuk RA, Murad MH, Agoritsas T, et al.GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. Journal of Clinical Epidemiology 2018;108:77-85. [DOI] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JP.Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331(7521):897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G.Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G.Graphical tools for network meta-analysis in STATA. PloS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2015
- Chaimani A, Salanti G.Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 2015;15(4):905-50. [Google Scholar]
Chaimani 2017
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G.Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. [DOI] [PubMed] [Google Scholar]
Chaimani 2018
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G.Chapter 11: Undertaking network meta-analyses. Draft version (16 September 2018) for inclusion in: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2018. Available from www.training.cochrane.org/handbook.
Cheng 2020
- Cheng Y-X, Sheng X.Optimal surgical methods to treat intertrochanteric fracture: a Bayesian network meta-analysis based on 36 randomized controlled trials. Journal of Orthopaedic Surgery and Research 2020;15:402. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christie 1994
- Christie J, Burnett R, Potts HR, Pell AC.Echocardiography of transatrial embolism during cemented and uncemented hemiarthroplasty of the hip. Journal of Bone and Joint Surgery. British Volume 1994;76(3):409-12. [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G.Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [DOI] [PubMed] [Google Scholar]
CMIMG 2014
- Comparing Multiple Interventions Methods Group.Protocol template for a Cochrane intervention review that compares multiple interventions. methods.cochrane.org/cmi/comparing-multiple-interventions-cochrane-reviews (accessed 31 October 2018).
Cochrane 2018
- Cochrane.Glossary. community.cochrane.org/glossary (accessed 2 November 2018).
Cooper 2011
- Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, et al.Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporosis International 2011;22(5):1277-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Court‐Brown 2017
- Court-Brown CM, Clement ND, Duckworth AD, Biant LC, McQueen MM.The changing epidemiology of fall-related fractures in adults. Injury 2017;48(4):819-24. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence.Melbourne, Australia: Veritas Health Innovation, accessed prior to 16 December 2021. Available at covidence.org.
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors).Chapter 10: Analysing data and undertaking meta-analyses. Draft version (29 January 2019) for inclusion in: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Dhanwal 2011
- Dhanwal DK, Dennison EM, Harvey NC, Cooper C.Epidemiology of hip fracture: worldwide geographic variation. Indian Journal of Orthopaedics 2011;45(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE.Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. [DOI] [PubMed] [Google Scholar]
Dolan 1997
- Dolan P.Modeling valuations for EuroQol health states. Medical Care 1997;35(11):1095-108. [DOI] [PubMed] [Google Scholar]
Donegan 2013
- Donegan S, Williamson P, D'Alessandro U, Tudur Smith C.Assessing key assumptions of network meta-analysis: a review of methods. Research Synthesis Methods 2013;4(4):291-323. [PMID: ] [DOI] [PubMed] [Google Scholar]
EQ‐5D
- EQ-5D. euroqol.org/ (accessed 14 August 2019).
Evans 1949
- Evans EM.The treatment of trochanteric fractures of the femur. Journal of Bone and Joint Surgery. British Volume 1949;31(2):190-203. [PMID: ] [PubMed] [Google Scholar]
Griffin 2015
- Griffin XL, Parsons N, Achten J, Fernandez M, Costa ML.Recovery of health-related quality of life in a United Kingdom hip fracture population: the Warwick Hip Trauma Evaluation — a prospective cohort study. Bone and Joint Journal 2015;97(3):372-82. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al.GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haywood 2014
- Haywood KL, Griffin XL, Achten J, Costa ML.Developing a core outcome set for hip fracture trials. Bone and Joint Journal 2014;96-B(8):1016-23. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG (editors).Chapter 16.5: Studies with more than two intervention groups. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG (editors).Chapter 16.6: Indirect comparisons and multiple treatments meta-analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Higgins 2012
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR.Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Research Synthesis Methods 2012;3(2):98-110. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2014
- Jackson D, Barrett JK, Rice S, White IR, Higgins JP.A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Statistics In Medicine 2014;33(21):3639-54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 1980
- Jensen JS.Classification of trochanteric fractures. Acta Orthopaedica Scandinavica 1980;51(5):803-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnell 2004
- Johnell O, Kanis JA.An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporosis International 2004;15(11):897-902. [DOI] [PubMed] [Google Scholar]
Kanis 2001
- Kanis JA, Oden A, Johnell O, Jonsson B, Laet C, Dawson A.The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporosis International 2001;12(5):417-27. [DOI] [PubMed] [Google Scholar]
Kanis 2012
- Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C.A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporosis International 2012;23(9):2239-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karantana 2011
- Karantana A, Boulton C, Bouliotis G, Shu KS, Scammell BE, Moran CG.Epidemiology and outcome of fracture of the hip in women aged 65 years and under: a cohort study. Journal of Bone and Joint Surgery. British Volume 2011;93(5):658-64. [DOI] [PubMed] [Google Scholar]
Konig 2013
- Konig J, Krahn U, Binder H.Visualizing the flow of evidence in network meta-analysis and characterizing mixed treatment comparisons. Statistics in Medicine 2013;32(30):5414-29. [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Lewis 2021
- Lewis SR, Macey R, Eardley WG, Dixon JR, Cook J, Griffin XL.Internal fixation implants for intracapsular hip fractures in older adults. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013409. [DOI: 10.1002/14651858.CD013409.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2022a
- Lewis SR, Macey R, Gill JR, Parker MJ, Griffin XL.Cephalomedullary nails versus extramedullary for extracapsular hip fractures in older adults. Cochrane Database of Systematic Reviews 2022, Issue 1. Art. No: CD000093. [DOI: 10.1002/14651858.CD000093.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2022b
- Lewis SR, Macey R, Stokes J, Cook JA, Eardley WGP, Griffin XL.Surgical interventions for treating intracapsular hip fractures in older adults: a network meta-analysis. Cochrane Database of Systematic Reviews. Art. No: CD013404. [DOI: 10.1002/14651858.CD013404.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2022c
- Lewis SR, Macey R, Parker MJ, Cook J, Griffin XL.Arthroplasties for hip fracture in adults. Cochrane Database of Systematic Reviews. Art. No: CD013410. [DOI: 10.1002/14651858.CD013410.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mak 2010
- Mak JC, Cameron ID, March LM.Evidence-based guidelines for the management of hip fractures in older persons: an update. Medical Journal of Australia 2010;192(1):37-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mavridis 2015
- Mavridis D, Giannatsi M, Cipriani A, Salanti G.A primer on network meta-analysis with emphasis on mental health. Evidence-Based Mental Health 2015;18(2):40-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neufeld 2016
- Neufeld ME, O'Hara NN, Zhan M, Zhai Y, Broekhuyse HM, Lefaivre KA, et al.Timing of hip fracture surgery and 30-day outcomes. Orthopedics 2016;39(6):361-8. [DOI] [PubMed] [Google Scholar]
NHFD 2017
- NHFD.The National Hip Fracture Database (NHFD) annual report 2017. www.nhfd.co.uk (accessed 30 October 2018).
NICE 2011
- National Clinical Guideline Centre (UK).The management of hip fracture in adults. NICE clinical guidelines, no. 124. London: Royal College of Physicians (UK); 2011. www.nice.org.uk/guidance/cg124/evidence/full-guideline-pdf-183081997 (accessed 30 October 2018). [PubMed]
ONS 2016
- Office for National Statistics.Mid-2016 population estimates for the UK, England and Wales, Scotland and Northern Ireland. bit.ly/28IITPF (accessed 30 October 2018).
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.Rayyan - a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papadimitriou 2017
- Papadimitriou N, Tsilidis KK, Orfanos P, Benetou V, Ntzani EE, Soerjomataram I, et al.Burden of hip fracture using disability-adjusted life-years: a pooled analysis of prospective cohorts in the CHANCES consortium. Lancet Public Health 2017;2(5):e239-e46. [DOI] [PubMed] [Google Scholar]
Parker 1991
- Parker MJ, Anand JK.What is the true mortality of hip fractures? Public Health 1991;105(6):443-6. [DOI] [PubMed] [Google Scholar]
Parker 2000
- Parker MJ, Handoll HH, Bhonsle S, Gillespie WJ.Condylocephalic nails versus extramedullary implants for extracapsular hip fractures. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD000338. [DOI: 10.1002/14651858.CD000338] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2006
- Parker MJ, Handoll HH.Replacement arthroplasty versus internal fixation for extracapsular hip fractures in adults. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD000086. [DOI: 10.1002/14651858.CD000086.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2009
- Parker MJ, Handoll HH.Osteotomy, compression and other modifications of surgical techniques for internal fixation of extracapsular hip fractures. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD000522. [DOI: 10.1002/14651858.CD000522.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2013
- Parker MJ, Das A.Extramedullary fixation implants and external fixators for extracapsular hip fractures in adults. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD000339. [DOI: 10.1002/14651858.CD000339.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedder 2016
- Pedder H, Sarri G, Keeney E, Nunes V, Dias S.Data extraction for complex meta-analysis (DECiMAL) guide. Systematic Reviews 2016;5:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al.A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [PMID: ] [DOI] [PubMed] [Google Scholar]
Queally 2014
- Queally JM, Harris E, Handoll HH, Parker MJ.Intramedullary nails for extracapsular hip fractures in adults. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD004961. [DOI: 10.1002/14651858.CD004961.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 2015
- Rhodes KM, Turner RM, Higgins JP.Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. Journal of Clinical Epidemiology 2015;68(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogmark 2018
- Rogmark C, Kristensen MT, Viberg B, Ronnquist SS, Overgaard S, Palm H.Hip fractures in the non-elderly: who, why and whither? Injury 2018;49(8):1445-50. [DOI] [PubMed] [Google Scholar]
Rucker 2015
- Rucker G, Schwarzer G.Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Medical Research Methodology 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2008a
- Salanti G, Kavvoura FK, Ioannidis JP.Exploring the geometry of treatment networks. Annals of Internal Medicine 2008;148(7):544-53. [DOI] [PubMed] [Google Scholar]
Salanti 2008b
- Salanti G, Higgins JP, Ades AE, Ioannidis JP.Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279-301. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP.Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G.Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. [DOI] [PubMed] [Google Scholar]
Sayers 2017
- Sayers A, Whitehouse MR, Berstock JR, Harding KA, Kelly MB, Chesser TJ.The association between the day of the week of milestones in the care pathway of patients with hip fracture and 30-day mortality: findings from a prospective national registry – The National Hip Fracture Database of England and Wales. BMC Medicine 2017;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al.Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:403-32. [Google Scholar]
SIGN 2009
- Scottish Intercollegiate Guidelines Network (SIGN).Management of hip fracture in older people. A national clinical guideline. www.sign.ac.uk/assets/sign111.pdf (accessed 30 October 2018).
Sreekanta 2018
- Sreekanta A, Felix L, Grant R, Handoll H, Elliott J, Cook JA, et al.Optimising stakeholder engagement for informing direction and scope of a programme of Cochrane Reviews on hip fracture management. In: Abstracts of the 25th Cochrane Colloquium, Edinburgh, UK. Cochrane Database of Systematic Reviews 2018;(9 Suppl 1):204. [DOI: 10.1002/14651858.CD201801] [DOI]
Stata [Computer program]
- Stata.College Station, TX, USA: StataCorp, 2015. Available at www.stata.com.
Torio 2011
- Torio CM, Andrews RM.National inpatient hospital costs: the most expensive conditions by Payer, 2011. Healthcare Cost and Utilization Project Statistical Brief #160. www.hcup-us.ahrq.gov/reports/statbriefs/sb160.pdf (accessed 9 January 2019).
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP.Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818-27. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD.The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JP.Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012;3(2):111-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2015
- White IR.Network meta-analysis. Stata Journal 2015;15(4).
Williamson 2017
- Williamson S, Landeiro F, McConnell T, Fulford-Smith L, Javaid MK, Judge A, et al.Costs of fragility hip fractures globally: a systematic review and meta-regression analysis. Osteoporosis International 2017;28(10):2791-800. [DOI] [PubMed] [Google Scholar]
Yepes‐Nuñez 2019
- Yepes-Nuñez JJ, Li S, Guyatt G, Jack SM, Brożek JL, Beyene J, et al.Development of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Summary of Findings (SoF) table for network meta-analysis. Journal of Clinical Epidemiology 2019 May 02 [Epub ahead of print]. [DOI: 10.1016/j.jclinepi.2019.04.018] [DOI] [PubMed]