Abstract
We evaluated the association between orthostatic hypertension and cardiovascular outcomes, and the effect of intensive blood pressure (BP) control on cardiovascular outcomes in patients with orthostatic hypertension. Post-hoc analyses of the SPRINT data were conducted; orthostatic hypertension was defined as increase in systolic BP ≥ 20 mm Hg or increase in diastolic BP ≥ 10 mm Hg with standing. Of 9329 participants, 1986 (21.2%) had orthostatic hypertension at baseline. Within the intensive treatment group, participants with orthostatic hypertension were at higher risk of developing the composite cardiovascular outcome (Hazard ratio (HR) 1.44 (95% confidence interval (CI) 1.1 to 1.87, p=0.007) compared to participants without orthostatic hypertension. Within the standard treatment group, there was no significant differences in cardiovascular outcome between participants with and without orthostatic hypertension. In participants with orthostatic hypertension, there was no statistically significant difference in risk of the composite cardiovascular outcome between the intensive and the standard BP treatment group (HR 1.07 (95% CI 0.78 to 1.47, p=0.68). In participants without orthostatic hypertension at baseline, the intensive treatment group was associated with a lower risk of the composite cardiovascular outcome (HR 0.67 (CI 0.56 to 0.79, p<0.0001). Orthostatic hypertension was associated with higher risk of cardiovascular outcomes in the intensive and not in the standard treatment group; intensive treatment of BP did not reduce the risk of cardiovascular outcomes compared to standard treatment in patients with orthostatic hypertension. These post-hoc analyses are hypothesis generating, and will need to be confirmed in future studies.
Keywords: Orthostatic hypertension, intensive blood pressure, cardiovascular outcomes, SPRINT, hypertension
Graphical Abstract
Introduction:
Orthostatic changes in blood pressure have been well described in the literature; most studies have focused on a decline in blood pressure after standing from a seated position (orthostatic hypotension). Orthostatic hypotension has been associated with lower cognitive function, higher risk of falls, and has been shown to be a marker of future cardiovascular risk.1,2 Less is known about patients who have a rise in blood pressure with standing, or “orthostatic hypertension”.3 The prevalence of orthostatic hypertension has ranged between 5% to 30% depending on the population studied, and also varied based on the definition used.4-6 Several studies have shown that orthostatic hypertension has been associated with higher risk of hypertension related target organ damage and cardiovascular events.5,7,8 However, there are very limited data about the optimal therapeutic approach to patients with orthostatic hypertension. Whether a strategy targeting intensive lowering of seated blood pressure improves cardiovascular outcomes in patients with orthostatic hypertension is not known. Given the paucity of data in this area, recent reviews have highlighted the need for additional research to understand the epidemiology, risk factors, outcomes and therapy of orthostatic hypertension.3,6
The Systolic Blood Pressure Intervention Trial (SPRINT) was a landmark clinical trial evaluating the efficacy of intensive treatment of blood pressure compared to a standard goal blood pressure in lowering risk of cardiovascular outcomes.9 It has been previously reported that though orthostatic hypotension was common in SPRINT participants, it was not worsened by intensive treatment of blood pressure and was not associated with a higher risk of cardiovascular outcomes.10,11 However, whether orthostatic hypertension influences outcomes in the context of intensive lowering of blood pressure is not known. The SPRINT trial offers a unique opportunity to evaluate this important question in a post –hoc analyses. The objectives of this paper are to evaluate the association between orthostatic hypertension and clinical outcomes, and to examine whether the effect of intensive blood pressure lowering on clinical outcomes is similar in patients with and without orthostatic hypertension.
Methods
Anonymized data and materials have been made publicly available at the NHLBI BioLINCC and can be accessed at https://biolincc.nhlbi.nih.gov/studies/sprint/
We conducted a post-hoc analyses of data from the SPRINT; this was a randomized, controlled, trial conducted at 102 clinical sites in the United States. The rationale, design and main results of SPRINT have been previously published.9,12 Briefly, SPRINT was designed to test whether a strategy targeted to lowering seated systolic blood pressure to <120 mm Hg reduces cardiovascular disease events compared to standard blood pressure control (<140 mm Hg). Inclusion criteria included age of at least 50 years, systolic blood pressure of 130 to 180 mm Hg, and high risk of cardiovascular disease defined as presence of one or more of the following: clinical or subclinical cardiovascular disease, chronic kidney disease, a 10-year risk of cardiovascular disease ≥15% estimated by the Framingham risk score, or age ≥75 years. Patients with type 2 diabetes mellitus, prior stroke and participants with standing systolic blood pressure <110 mm Hg at the screening visit were excluded from the study. The study was approved by the institutional review board at each participating site, and written informed consent was obtained from all participants.
Participants (n=9361) were enrolled between November 2010 and March 2013 and randomized to a systolic blood pressure target of <140 mm Hg (standard treatment arm) or <120 mm Hg (intensive treatment arm). The study was stopped early, after a median follow-up of 3.26 years due to a 25% reduction in the primary composite cardiovascular disease end point and a 27% reduction in all-cause mortality in the intensive treatment group. Demographic data were collected at baseline before randomization. Clinical and laboratory data were obtained at baseline and every 3 months thereafter.9
At each visit, trained clinical staff measured blood pressure with an automated device (Omron-HEM-907 XL, Omron Healthcare, Inc., Bannockburn, IL) using standardized procedures and three seated blood pressure readings were obtained. The details of the blood pressure measurement technique, strategy of adjustment of medication doses, and antihypertensive drug regimens during the trial in SPRINT have been previously published.9,13 After seated blood pressure measurement was obtained, participants were instructed to stand, and after 1 minute, standing blood pressure was measured. Standing measurements of blood pressure were obtained at screening, baseline, 1-month, 6-month, 12-month, and then yearly visits till the 60-month follow up visit.
Orthostatic hypertension was defined as change in systolic blood pressure (standing systolic blood pressure –mean seated systolic blood pressure) ≥ 20 mm Hg OR change in diastolic blood pressure (standing diastolic blood pressure –mean seated diastolic blood pressure) ≥ 10 mm Hg. As a sensitivity analysis, we used an alternate definition of orthostatic hypertension; participants were defined as having orthostatic hypertension if they met the change in blood pressure criteria (defined above) at either baseline or 1 month visit. In addition, we conducted sensitivity analyses defining orthostatic hypertension based on change in systolic blood pressure, and diastolic blood pressure separately.
Participants were asked to self-report cardiovascular events in both treatment arms every 3 months. Medical records were obtained for documentation of events, and investigators blinded to the study group assignments adjudicated the clinical outcomes as specified in the protocol. The predefined primary cardiovascular outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, heart failure, and death from cardiovascular causes. As secondary outcomes, we evaluated each component of the composite outcome separately, kidney outcomes and all-cause mortality. The kidney outcome in participants with chronic kidney disease at baseline was a composite of a decrease in estimated glomerular filtration rate of 50% or more or the development of end stage renal disease requiring long-term dialysis or kidney transplantation. In participants without chronic kidney disease at baseline, the kidney outcome was defined by a decrease in the estimated glomerular filtration rate of 30% or more to a value of less than 60 ml per minute per 1.73 m2.
Statistical analyses
We compared demographic, clinical and laboratory characteristics of participants with and without orthostatic hypertension at baseline. Given adequate sample size and lack of need to assume equality of variances among the two groups, Welch two-sample t-tests were conducted for continuous variables. Chi-square tests were conducted for discrete variables, and sufficient cell counts were observed. For the primary outcome of composite cardiovascular disease as well as secondary outcomes, we applied the Weibull accelerated failure time model to estimate the hazard ratios of baseline orthostatic hypertension status, treatment and baseline orthostatic hypertension status by treatment interactions, and their 95% confidence intervals, adjusting for gender, race, smoking status, history of cardiovascular disease history, age, statin use, number of antihypertensive drugs, chronic kidney disease, body mass index, baseline seated systolic blood pressures, baseline total and HDL cholesterol, glomerular filtration rate and urine albumin/creatinine ratio. To model the hazard ratios of baseline orthostatic hypertension status, treatment and baseline orthostatic hypertension status by treatment interactions, for each model with interaction, we performed partitioned analysis of the least square-means for an interaction, to get the simple effects of each of subgroups. The Cox regression model was not used because the proportional hazard assumption appeared to be violated for models of all the outcomes. The accelerated failure time approach is an alternative strategy for the analysis of time‐to‐event data and can be suitable even when hazards are not proportional.14 Model goodness of fit statistics and residuals indicate that the Weibull accelerated failure time model fits the data well.
To understand factors that are associated with total incidence rates of orthostatic hypertension over the course of the study, we applied a negative binomial regression model. The outcome was total number of incidences of orthostatic hypertension, offset by the natural log of number of attended blood pressure measurement sessions, adjusted for baseline orthostatic hypertension status, treatment, gender, race, age, smoking status, cardiovascular disease history, statin use, number of antihypertensive drugs, chronic kidney disease, body mass index, baseline seated systolic blood pressures, baseline total and HDL cholesterol (above and below respective medians), glomerular filtration rate and urine albumin/creatinine ratio. The negative binomial distribution was adopted to account for over-dispersion.15 Estimated incidence rate ratios and their 95% confidence intervals were reported for model covariates.
All statistical analyses were performed using SAS 9.4. We defined significance based on two-sided P-values <0.05.
Results
Of 9361 randomized SPRINT participants, 32 had missing standing blood pressure at baseline and were excluded from the analyses; data from 9329 participants forms the basis of this report. The mean age of the study population was 67.86 ± 9.40 years, 35.6% were African-American, and 31.5% were female. Orthostatic hypertension was present in 1986 (21.3%) of the study population. In patients who met the definition of orthostatic hypertension, 22.2% had change in systolic blood pressure ≥ 20 mm Hg, 92.7% had change in diastolic blood pressure ≥ 10 mm Hg, and 14.9% had both change in systolic blood pressure ≥ 20 mm and change in diastolic blood pressure ≥ 10 mm Hg (percentages are not mutually exclusive). Participants with orthostatic hypertension were more likely to be female and African American, and less likely to have chronic kidney disease than participants without orthostatic hypertension. There were several other differences in clinical and lab characteristics between the two groups (Table 1).
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | No baseline orthostatic hypertension (N=7343) |
Baseline orthostatic hypertension (N=1986) |
p – value |
|---|---|---|---|
| Age (yrs) mean (SD) | 67.86 (9.41) | 67.87 (9.35) | 0.97 |
| Gender (Female, n. (%) | 2490 (33.9) | 827 (41.6) | <0.001 |
| Race or ethnic group – no. (%) Black | 2150 (29.3) | 788 (39.7) | <0.001 |
| Chronic kidney disease – no. (%) ‡ | 2135 (29.1) | 503 (25.3) | 0.001 |
| Smoking status – no. (%) ** | 0.13 | ||
| Non smoker | 3219 (43.8) | 895 (45.1) | |
| Ever smoker | 4117 (56.1) | 1086 (54.7) | |
| Body-mass index, mean (SD) ∥ | 29.68 (5.48) | 30.22 (6.16) | <0.001 |
| Statin use – no. (%) | 3044 (41.4) | 802 (40.4) | 0.60 |
| Alpha blocker use – no. (%) | 497 (6.8) | 92 (4.6) | 0.0004 |
| Antihypertensive agents – no. (%) | 0.37 | ||
| Not using antihypertensive agents | 665 (9.1) | 202 (10.2) | |
| Using one antihypertensive agent | 2291 (31.2) | 643 (32.4) | |
| Using two antihypertensive agents | 2523 (34.4) | 661 (33.3) | |
| Using three antihypertensive agents | 1476 (20.1) | 382 (19.2) | |
| Using four antihypertensive agents | 388 (5.3) | 98 (4.9) | |
| Baseline blood pressure – mm Hg, mean (SD) | |||
| Seated systolic | 140.02 (15.41) | 138.28 (16.12) | <0.001 |
| Seated diastolic | 78.76 (11.90) | 75.78 (11.83) | <0.001 |
| Standing systolic | 137.91 (17.07) | 148.84 (18.28) | <0.001 |
| Standing diastolic | 79.76 (12.37) | 89.23 (12.23) | <0.001 |
| Seated heart rate mean (SD) | 66.4 (11.6) | 65.8(11.3) | 0.045 |
| Standing heart rate mean (SD) | 71.2 (13.0) | 72.5 (12.6) | 0.00015 |
| Estimated GFR ml/min/1.73m2 *, mean (SD) | 71.29 (20.52) | 73.43 (20.66) | <0.001 |
| Urinary albumin (mg) to creatinine (g) ratio, mean (SD) | 0.41 (1.60) | 0.40 (1.75) | 0.77 |
| Total cholesterol – mg/dl, mean (SD) | 189.40 (41.33) | 192.81 (40.46) | 0.001 |
| HDL cholesterol – mg/dl, mean (SD) | 52.51 (14.31) | 54.19 (14.94) | <0.001 |
Chronic kidney disease was defined as an estimated glomerular filtration rate of less than 60 ml per minute per 1.73 m2 of body-surface area,
39(0.4%) participants had missing GFR values,
12(0.1%) participants had missing smoking status,
Body-mass index is the weight in kilograms divided by the square of the height in meters
The proportion of participants with orthostatic hypertension over the course of the study is illustrated in figure 1. In negative binomial regression analyses evaluating the total incidence rates of orthostatic hypertension during the course of the trial, participants with orthostatic hypertension at baseline had a higher incidence rate of orthostatic hypertension than those without orthostatic hypertension (incidence rate ratio 3.18 (95% confidence interval 3.06-3.31) (Table S1). Older age, black race, higher HDL cholesterol and higher body mass index were statistically significantly associated with higher rates of orthostatic hypertension; male gender was associated with lower rates of orthostatic hypertension. There was no statistically significant association between randomization to intensive or standard group with rates of orthostatic hypertension during the course of the study. (Table S1)
Figure 1.
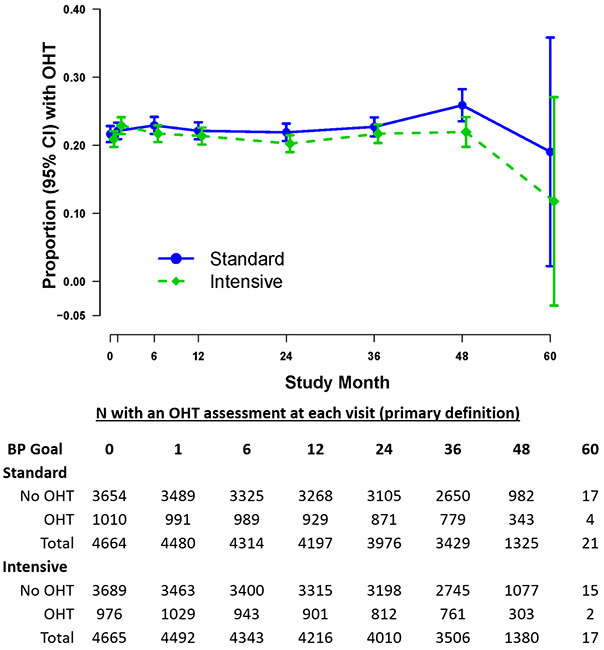
Proportion of participants with orthostatic hypertension (OHT) over the course of the study stratified by treatment arm.
Blood pressure over the course of trial was similar in participants with and without orthostatic hypertension (Table S2). Details regarding antihypertensive drug therapy at baseline and during the course of the trial are provided in table S3. At the baseline visit, in participants randomized to intensive treatment group, those with orthostatic hypertension were more likely to be prescribed thiazide diuretics and centrally acting agents than participants without orthostatic hypertension. In participants randomized to standard treatment group, those with orthostatic hypertension were less likely to be prescribed alpha-1 blockers than participants without orthostatic hypertension. Over the course of the trial, prescription of antihypertensive drug therapy was not different at most visits between participants with and without orthostatic hypertension.
Data comparing participants with orthostatic hypertension at baseline, to those without orthostatic hypertension at baseline
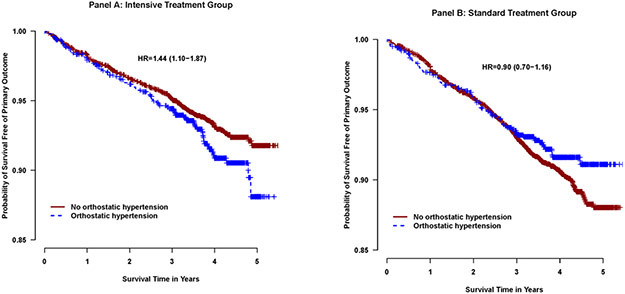
In analyses examining the association between orthostatic hypertension and clinical outcomes, there were significant interactions between orthostatic hypertension and treatment found for primary cardiovascular outcome (p-interaction=0.01) and heart failure (p interaction=0.03). Within the intensive treatment group, participants with orthostatic hypertension were at higher risk of developing the primary cardiovascular outcome (Hazard ratio 1.44 (95% confidence interval 1.10 to 1.87), p=0.007, table 3, Figure2, heart failure (HR 1.85 (95% CI 1.17 - 2.94), p=0.009) and cardiovascular death (HR 2.59 (95% CI 1.46 - 4.62), p=0.001) compared to participants without orthostatic hypertension (table 2). Within the standard treatment group, there were no statistically significant differences in any of the cardiovascular outcomes between participants with and without orthostatic hypertension. The association between orthostatic hypertension and all-cause mortality and kidney outcomes was consistent between the intensive and standard treatment groups (interaction p values >0.05, table 2). There was no statistically significant difference in risk of adverse events (serious adverse events, electrolyte abnormalities, syncopal episodes, injurious falls, acute kidney injury, hypotension, or bradycardia) between participants with vs without orthostatic hypertension for intensive treatment group or standard treatment group during the course of the trial (table S4).
Table 3.
Clinical outcomes in participants randomized to the intensive compared to the standard blood pressure group, stratified by baseline orthostatic hypertension
| Outcomes | Orthostatic hypertension | No orthostatic hypertension | Interaction p-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard treatment group |
Intensive treatment group |
HR2 (95% CI3) |
p-value | Standard treatment group |
Intensive treatment group |
HR2 (95% CI3) |
p-value | ||||||
| n | Event rate1 |
n | Event rate1 |
n | Event rate1 |
n | Event rate1 |
||||||
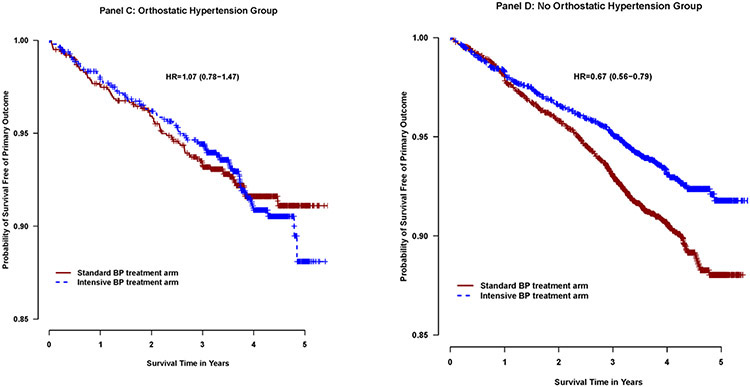
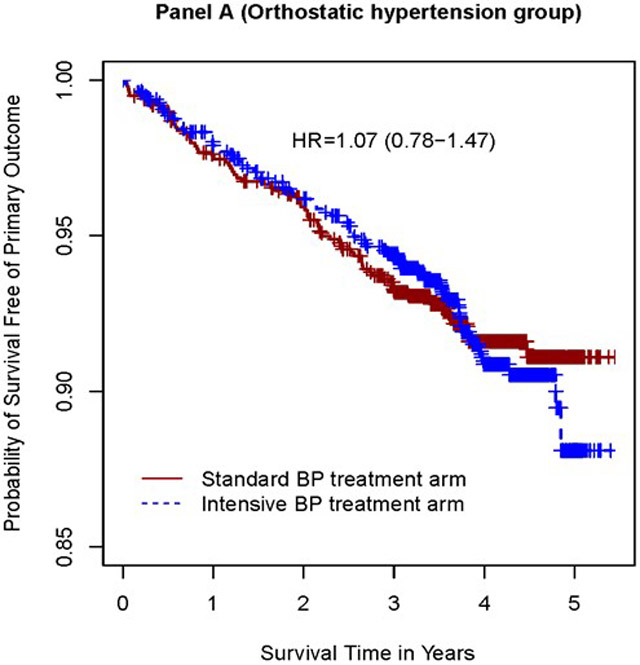
| Primary outcome | 77 | 2.11 | 77 | 2.17 | 1.07 (0.78-1.47) | 0.68 | 333 | 2.50 | 236 | 1.74 | 0.67 (0.56-0.79) | <0.0001 | 0.01 |
| Components of primary outcome | |||||||||||||
| Myocardial infarction | 32 | 0.86 | 20 | 0.55 | 0.69 (0.39-1.21) | 0.19 | 128 | 0.95 | 94 | 0.68 | 0.71 (0.54-0.93) | 0.012 | 0.94 |
| Acute coronary syndrome | 9 | 0.24 | 8 | 0.22 | 0.97 (0.37-2.50) | 0.94 | 38 | 0.28 | 37 | 0.27 | 0.90 (0.57-1.42) | 0.65 | 0.89 |
| Stroke | 19 | 0.51 | 19 | 0.53 | 1.05 (0.55-2.00) | 0.88 | 74 | 0.54 | 58 | 0.42 | 0.75 (0.53-1.06) | 0.11 | 0.37 |
| Heart failure | 20 | 0.53 | 27 | 0.75 | 1.44 (0.80-2.59) | 0.22 | 93 | 0.68 | 70 | 0.51 | 0.68 (0.49-0.93) | 0.02 | 0.03 |
| Cardiovascular disease death | 15 | 0.40 | 19 | 0.52 | 1.53 (0.76-3.06) | 0.23 | 72 | 0.52 | 36 | 0.26 | 0.49 (0.33-0.75) | 0.001 | 0.07 |
| All Cause Mortality | 56 | 1.48 | 55 | 1.51 | 1.11 (0.76-1.61) | 0.60 | 218 | 1.58 | 170 | 1.22 | 0.75 (0.622-0.93) | 0.007 | 0.08 |
| Kidney outcome (In participants without CKD at baseline) | 10 | 0.36 | 31 | 1.17 | 3.22 (1.54 -6.71) | 0.001 | 37 | 0.38 | 127 | 1.33 | 3.30 (2.21-4.93) | <0.001 | 0.95 |
| Kidney outcome (In participants with CKD at baseline) | 1 | 0.1 | 4 | 0.45 | 3.53 (0.35 -35.46) | 0.28 | 18 | 0.46 | 13 | 0.32 | 0.81 (0.38-1.74) | 0.59 | 0.24 |
Events rates are measured as % per year
HR: Hazards ratio Intensive treatment compared to standard group CI: Confidence interval
Adjusting gender, race, smoking status, history of cardiovascular disease history, age, statin use, number of antihypertensive drugs, chronic kidney disease, body mass index, baseline seated systolic blood pressures, baseline total and HDL cholesterol, glomerular filtration rate and urine albumin/creatinine ratio
Figure 2.
Kaplan Meier curves comparing the SPRINT primary composite cardiovascular outcome in the patients with and without orthostatic hypertension, stratified by intensive treatment group (Panel A) and standard treatment group (Panel B). Hazard ratio for participants with orthostatic hypertension vs those without orthostatic hypertension.
Table 2.
Association between baseline orthostatic hypertension and clinical outcomes, stratified by blood pressure treatment group.
| Outcomes | Intensive treatment group | Standard treatment group | Interaction p-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No orthostatic hypertension |
Orthostatic hypertension |
HR2 (95% CI3) |
p- value |
No orthostatic hypertension |
Orthostatic hypertension |
HR2 (95% CI3) |
p- value |
||||||
| n | Event rate1 |
n | Event rate1 |
n | Event rate1 |
n | Event rate1 |
||||||
| Primary outcome | 236 | 1.74 | 77 | 2.17 | 1.44 (1.10-1.87) | 0.007 | 333 | 2.50 | 77 | 2.11 | 0.90 (0.70-1.16) | 0.41 | 0.01 |
| Components of primary outcome | |||||||||||||
| Myocardial infarction | 94 | 0.68 | 20 | 0.55 | 0.97 (0.60-1.57) | 0.90 | 128 | 0.95 | 32 | 0.86 | 0.99 (0.67-1.47) | 0.98 | 0.94 |
| Acute coronary syndrome | 37 | 0.27 | 8 | 0.22 | 1.03 (0.48-2.22) | 0.95 | 38 | 0.28 | 9 | 0.24 | 0.96 (0.46-1.99) | 0.90 | 0.89 |
| Stroke | 58 | 0.42 | 19 | 0.53 | 1.37 (0.80-2.33) | 0.25 | 74 | 0.54 | 19 | 0.51 | 0.98 (0.59-1.62) | 0.93 | 0.37 |
| Heart failure | 70 | 0.51 | 27 | 0.75 | 1.85 (1.17-2.94) | 0.009 | 93 | 0.68 | 20 | 0.53 | 0.87 (0.53-1.42) | 0.58 | 0.03 |
| Cardiovascular disease death | 36 | 0.26 | 19 | 0.52 | 2.59 (1.46-4.62) | 0.001 | 72 | 0.52 | 15 | 0.40 | 0.84 (0.47-1.50) | 0.56 | 0.07 |
| All Cause Mortality | 170 | 1.22 | 55 | 1.51 | 1.48 (1.08-2.01) | 0.01 | 218 | 1.58 | 56 | 1.48 | 1.01 (0.75-1.36) | 0.96 | 0.08 |
| Kidney outcome (In participants without CKD at baseline) | 127 | 1.33 | 31 | 1.17 | 0.91 (0.60-1.37) | 0.64 | 37 | 0.38 | 10 | 0.36 | 0.93 (0.46-1.88) | 0.84 | 0.95 |
| Kidney outcome (In participants with CKD at baseline) | 13 | 0.32 | 4 | 0.45 | 1.80 (0.57-5.72) | 0.32 | 18 | 0.46 | 1 | 0.10 | 0.41 (0.05-3.63) | 0.43 | 0.24 |
Events rates are measured as % per year
HR: Hazards ratio for orthostatic hypertension, compared to no orthostatic hypertension, CI: Confidence interval
Adjusting gender, race, smoking status, history of cardiovascular disease history, age, statin use, number of antihypertensive drugs, chronic kidney disease, body mass index, baseline seated systolic blood pressures, baseline total and HDL cholesterol, glomerular filtration rate and urine albumin/creatinine ratio
Data comparing participants randomized to the intensive or standard group
In examining the association between randomization to the intensive and standard group and the primary cardiovascular composite outcome and components of primary endpoints, there were significant interactions based on presence of orthostatic hypertension at baseline (p = 0.01 for primary outcome and p=0.03 for heart failure,) (Table 3). In participants with orthostatic hypertension at baseline, there was no statistically significant difference in risk of the primary outcome and heart failure between the intensive and the standard treatment group (HR 1.07 (95% CI 0.78 to 1.47), p=0.68 (Table 5, Figure 3); HR 1.44 (95% CI 0.80 to 2.59), p=0.22 (Table5) ). In participants without orthostatic hypertension at baseline, the intensive treatment group was associated with a lower risk of the primary outcome (HR 0.67 (95%CI 0.56 to 0.79), p<0.0001), a lower risk of heart failure (HR 0.68 (95%CI 0.49 - 0.93), p=0.02), p<0.016).
Figure 3.
Kaplan Meier curves comparing the SPRINT primary composite cardiovascular outcome in the patients randomized to the intensive and standard treatment arms, stratified by presence (Panel C) or absence (Panel D) of orthostatic hypertension. Hazard Ratio (HR) Intensive treatment compared to standard treatment arm
The association between intensive treatment and other components of the primary endpoint (myocardial infarction, acute coronary syndrome, stroke, and cardiovascular death), all-cause mortality, and kidney outcome in participants with chronic kidney disease at baseline was consistent in patients with and without orthostatic hypertension at baseline (interaction p values >0.05) (Table 3). Intensive treatment was associated with a higher risk of the kidney outcome in participants without chronic kidney disease; this was consistent in participants with and without orthostatic hypertension at baseline. Participants in the intensive treatment group were more likely to have acute kidney injury and hypotension than those in the standard treatment group; results were consistent in participants with and without orthostatic hypertension (table S4). There was no statistically significant difference in risk of adverse events (serious adverse events, electrolyte abnormalities, syncopal episodes, injurious falls, acute kidney injury, hypotension, or bradycardia) participants intensive vs. standard treatment groups for participants with or without orthostatic hypertension during the course of the trial (table S5).
We conducted sensitivity analysis defining orthostatic hypertension based on meeting blood pressure change criteria as defined above, either at the baseline or the one month follow up visit; associations with outcomes were qualitatively similar to those seen with the primary definition (Table S6). In additional sensitivity analyses, we defined orthostatic hypertension based on change in systolic blood pressure alone (n=441); perhaps due to the lower counts of orthostatic hypertension at baseline , the interaction effect between intensive treatment and orthostatic hypertension was not significant for all outcomes, but the results were qualitatively similar to the original analysis (Table S6 and S8). We also conducted analyses defining orthostatic hypertension based on change in diastolic blood pressure alone (n=1842); the results (tables S9 and S10) were qualitatively similar to the primary analysis. We also evaluated the interaction effect between orthostatic hypertension at baseline and race; interaction terms by race were not statistically significant for any of the outcomes, suggesting that results are consistent in both racial groups (data not presented).
There was no statistically significant independent association between standing blood pressure at baseline and the SPRINT primary outcome (table S11). Standing blood pressure was lower in participants in the intensive treated arm than those in the standard arm (table S12).
Discussion:
In this post-hoc analyses of the SPRINT, presence of orthostatic hypertension at baseline was associated with higher risk of cardiovascular outcomes in participants randomized to intensive blood pressure treatment goal and not the standard treatment goal. In participants with orthostatic hypertension at baseline, intensive treatment of blood pressure was not associated with reduction in risk of cardiovascular outcomes compared to standard treatment. There were no differences in safety related outcomes between participants with and without orthostatic hypertension.
The prevalence rates of orthostatic hypertension have varied widely in the literature ranging between 5% to 30%; the variability likely reflects the fact that definition of orthostatic hypertension, and the patient populations differed between published studies. The definition of orthostatic hypertension used in this study (increase in systolic BP of ≥20 mm Hg or of diastolic BP of ≥ 10 mm on standing) has been used in other large studies, and has been recommended to be the standard definition in a recent review.6,16,17 We noted that change in diastolic blood pressure was more common than change in systolic blood pressure in meeting the definition of orthostatic hypertension.7 Older age, female gender, black race and high body mass index were associated with higher rates of orthostatic hypertension. This is consistent with findings in previous studies. 18-20
Several epidemiologic studies have evaluated the association between orthostatic hypertension and clinical outcomes. Orthostatic hypertension in young adults may predict the onset of sustained hypertension in adulthood.21 In cross-sectional studies, presence of orthostatic hypertension has been associated with silent cerebrovascular disease, left ventricular hypertrophy, peripheral arterial disease and stroke.4,18,22 In large prospective studies in Italy and France, and in a post hoc analyses of the Systolic Hypertension in the Elderly Program, orthostatic hypertension was associated with high risk of cardiovascular disease and mortality.5,7,8 However, one study in a geriatric patient population showed no association between orthostatic hypertension and survival.23 To our knowledge, this is the first study evaluating the relationship between intensive treatment of blood pressure and orthostatic hypertension. We report several novel findings; intensive treatment of blood pressure did not change the rate of orthostatic hypertension over the course of the study. Orthostatic hypertension was associated with higher risk of cardiovascular events compared to patients without orthostatic hypertension only in patients randomized to the intensive treatment arm. The higher risk of developing the primary cardiovascular outcome was driven by higher risk of heart failure and cardiovascular disease death. Seated office blood pressure and antihypertensive drug therapy was similar between the two groups during the course of the trial making that unlikely to explain the difference in outcomes between the groups. In complementary analyses, we note that the beneficial effect of intensive blood pressure lowering on clinical outcomes was not seen in patients with orthostatic hypertension at baseline. However, this finding should be interpreted with caution since orthostatic hypertension was not a pre-specified sub-group in SPRINT and unknown confounders may be influencing these results. Since orthostatic changes in blood pressure are relatively easy to measure in practice, our findings identify a subset of hypertensive patients who may not benefit from efforts to intensively lower blood pressure.
The mechanisms underlying the lack of benefit of intensive blood pressure control in patients with orthostatic hypertension are not clear in our current study and will require future research. We can speculate about several mechanisms that may explain these findings; differences in ambulatory blood pressure profile have been seen in patients with and without orthostatic hypertension.24 Since ambulatory readings are typically obtained with participants doing their usual day to day activities, many readings may be in the standing (rather than seated) position. The tendency to have higher standing blood pressure in patients with orthostatic hypertension may translate into a higher daytime blood pressure. Therefore, achievement of lower target office blood pressure during clinic visits may not fully reflect ambulatory blood pressure, and that difference may be magnified in patients with orthostatic hypertension. Our previous work using ambulatory blood pressure monitoring in SPRINT demonstrated the presence of a “masked” effect; some participants in both the intensive and standard treatment arms had higher twenty four hour daytime blood pressure than office blood pressure.25 However, the association between orthostatic hypertension and masked hypertension in SPRINT participants has not been studied. A few other studies have evaluated the association between orthostatic hypertension and masked hypertension; in a cohort of 304 patients with a mean age of 66 years, the presence of orthostatic hypertension was associated with more than a threefold higher prevalence of masked hypertension. 26 Similarly, in normotensive subjects, the prevalence of masked hypertension was higher in those with orthostatic hypertension compared with controls (odds ratio=3.01, P=0.001).27 Thus, it’s possible that orthostatic hypertension may be a clue to presence of underlying masked hypertension which is associated with worse cardiovascular outcomes.28 In addition, orthostatic hypertension has been associated with other abnormalities on ambulatory blood pressure monitoring such as morning blood pressure surge and extreme dipping at night which may contribute to higher risk of cardiovascular outcomes and stroke.18,24,29 The mechanisms of how orthostatic hypertension increases cardiovascular risk, and why patients with orthostatic hypertension do not seem to benefit from intensive blood pressure control will require further study.
The pathophysiology of orthostatic hypertension is not well understood. It is thought that excessive sympathetic response to standing results in vasoconstriction which raises blood pressure higher than the seated position.6,31,32 Several other metabolic and homeostatic derangements are associated with orthostatic hypertension; these include autonomic dysregulation, activation of the renin angiotensin axis and increased arterial stiffness.18,33-38 All of these may contribute to an increased risk of adverse cardiovascular events which may not be fully mitigated by intensive blood pressure control. Most studies have evaluated orthostatic hypertension based on a blood pressure readings obtained at one point in time.3 We conducted exploratory analyses to evaluate whether orthostatic hypertension is a reproducible profile over time. Though SPRINT is not the ideal setting to assess this (intensive blood pressure control was targeted to lower seated blood pressure), our findings indicate that participants who had orthostatic hypertension at baseline were much more likely to have orthostatic hypertension during follow up supporting the reproducibility of this profile. In addition, our sensitivity analyses suggests that using both systolic and diastolic blood pressure changes in the definition of orthostatic hypertension identifies a high risk population who may not benefit from intensive treatment of blood pressure. These findings are relevant from a methodologic perspective in refining the phenotype of orthostatic hypertension.
The strengths of our study include the large and diverse population sample size and the methodologic rigor of the SPRINT study, with close follow up, and careful adjudication of clinical outcomes. However, important limitations need to be considered; this was not a pre specified subgroup during the trial design and was a post-hoc analysis. Therefore, our findings are hypothesis generating, and not conclusive. Our data may prompt future studies to better understand the relationship between orthostatic hypertension and intensive treatment of blood pressure. Since diabetic patients were excluded in SPRINT, the applicability of our findings to diabetics, who often have diabetic autonomic neuropathy, is unclear. Also, orthostatic hypertension was defined on the basis of a single measurement of blood pressure obtained only one minute after standing, unlike other studies which used tilt-table testing to define orthostatic hypertension.42 Finally, orthostatic testing is usually conducted from the supine to the standing position; in SPRINT, blood pressure was measured in the seated and standing position.
Supplementary Material
Perspectives.
In this post-hoc analyses of SPRINT, orthostatic hypertension was present in approximately 22% of study participants, mostly driven by change in diastolic blood pressure. Intensive treatment of blood pressure did not affect the rate of orthostatic hypertension. Orthostatic hypertension was associated with higher risk of cardiovascular outcomes in participants in the intensive treatment group and not the standard treatment group. In participants with orthostatic hypertension at baseline, intensive treatment of blood pressure was not associated with reduction in risk of cardiovascular outcomes. While these data identify a subgroup of patients who may not benefit from intensive blood pressure lowering, further studies that confirm these findings, and evaluate the mechanisms of the relationship between orthostatic hypertension and intensive blood pressure control are needed prior to implementation in practice.
Novelty and Significance.
1. What Is New?
Blood pressure can change when patients stand up after being seated for some time. We studied whether a rise in blood pressure with standing is associated with any long term consequences.
2. What Is Relevant?
We showed that patients whose blood pressure rises when they stand (by more than 20 mm Hg) are less likely to benefit from intensive treatment of blood pressure.
3. Summary
More research is needed before these findings can be translated into practice, but our findings suggest that measuring blood pressure in both seated and standing blood pressure may be important, and may help a provider choose a blood pressure target best suited to an each individual patient.
Acknowledgments
We acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420.
Sources of Funding
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm
Footnotes
Disclosures
Dr. Rahman has research funding (clinical trials) from Bayer and Duke Clinical Research Institute, and honoraria from Relypsa and Reata pharmaceuticals. None of this is related to the work in the manuscript.
References
- 1.Joseph A, Wanono R, Flamant M, Vidal-Petiot E. Orthostatic hypotension: A review. Nephrol Ther. 2017;13 Suppl 1:S55–S67. [DOI] [PubMed] [Google Scholar]
- 2.Jones CD, Loehr L, Franceschini N, et al. Orthostatic hypotension as a risk factor for incident heart failure: the atherosclerosis risk in communities study. Hypertension (Dallas, Tex : 1979). 2012;59(5):913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan J, Ricci F, Hoffmann F, Hamrefors V, Fedorowski A. Orthostatic Hypertension: Critical Appraisal of an Overlooked Condition. Hypertension (Dallas, Tex : 1979). 2020;75(5):1151–1158. [DOI] [PubMed] [Google Scholar]
- 4.Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM. Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension (Dallas, Tex : 1979). 2011;57(2):167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnoletti D, Valbusa F, Labat C, Gautier S, Mourad JJ, Benetos A. Evidence for a Prognostic Role of Orthostatic Hypertension on Survival in a Very Old Institutionalized Population. Hypertension (Dallas, Tex : 1979). 2016;67(1):191–196. [DOI] [PubMed] [Google Scholar]
- 6.Magkas N, Tsioufis C, Thomopoulos C, et al. Orthostatic hypertension: From pathophysiology to clinical applications and therapeutic considerations. Journal of clinical hypertension (Greenwich, Conn). 2019;21(3):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostis WJ, Sargsyan D, Mekkaoui C, et al. Association of orthostatic hypertension with mortality in the Systolic Hypertension in the Elderly Program. Journal of human hypertension. 2019;33(10):735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veronese N, De Rui M, Bolzetta F, et al. Orthostatic Changes in Blood Pressure and Mortality in the Elderly: The Pro.V.A Study. American journal of hypertension. 2015;28(10):1248–1256. [DOI] [PubMed] [Google Scholar]
- 9.Wright JT Jr., Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juraschek SP, Taylor AA, Wright JT Jr., et al. Orthostatic Hypotension, Cardiovascular Outcomes, and Adverse Events: Results From SPRINT. Hypertension (Dallas, Tex : 1979). 2020;75(3):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend RR, Chang TI, Cohen DL, et al. Orthostatic changes in systolic blood pressure among SPRINT participants at baseline. Journal of the American Society of Hypertension : JASH. 2016;10(11):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clinical trials (London, England). 2014;11(5):532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KC, Whelton PK, Cushman WC, et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension (Dallas, Tex : 1979). 2018;71(5):848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collet D Modelling Survival Data in Medical Research. Boca Raton: CRC Press, Taylor & Francis; 2015. [Google Scholar]
- 15.Hilbe JM. Negative binomial regression New York,: Cambridge University Press; 2011. [Google Scholar]
- 16.Bhuachalla BN, McGarrigle CA, O’Leary N, et al. Orthostatic hypertension as a risk factor for age-related macular degeneration: Evidence from the Irish longitudinal study on ageing. Experimental gerontology. 2018;106:80–87. [DOI] [PubMed] [Google Scholar]
- 17.Wijkman M, Lanne T, Ostgren CJ, Nystrom FH. Diastolic orthostatic hypertension and cardiovascular prognosis in type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. 2016;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kario K, Eguchi K, Hoshide S, et al. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. Journal of the American College of Cardiology. 2002;40(1):133–141. [DOI] [PubMed] [Google Scholar]
- 19.Bursztyn M, Jacobs JM, Hammerman-Rozenberg A, Stessman J. Prevalence of orthostatic hypertension in the very elderly and its relationship to all-cause mortality. Journal of hypertension. 2016;34(10):2053–2058. [DOI] [PubMed] [Google Scholar]
- 20.Nardo CJ, Chambless LE, Light KC, et al. Descriptive epidemiology of blood pressure response to change in body position. The ARIC study. Hypertension (Dallas, Tex : 1979). 1999;33(5):1123–1129. [DOI] [PubMed] [Google Scholar]
- 21.Thomas RJ, Liu K, Jacobs DR Jr., Bild DE, Kiefe CI, Hulley SB. Positional change in blood pressure and 8-year risk of hypertension: the CARDIA Study. Mayo Clin Proc. 2003;78(8):951–958. [DOI] [PubMed] [Google Scholar]
- 22.Fan XH, Wang Y, Sun K, et al. Disorders of orthostatic blood pressure response are associated with cardiovascular disease and target organ damage in hypertensive patients. American journal of hypertension. 2010;23(8):829–837. [DOI] [PubMed] [Google Scholar]
- 23.Weiss A, Beloosesky Y, Grossman A, Shlesinger A, Koren-Morag N, Grossman E. The association between orthostatic hypertension and all-cause mortality in hospitalized elderly persons. J Geriatr Cardiol. 2016;13(3):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kario K, Eguchi K, Nakagawa Y, Motai K, Shimada K. Relationship between extreme dippers and orthostatic hypertension in elderly hypertensive patients. Hypertension (Dallas, Tex : 1979). 1998;31(1):77–82. [DOI] [PubMed] [Google Scholar]
- 25.Drawz PE, Pajewski NM, Bates JT, et al. Effect of Intensive Versus Standard Clinic-Based Hypertension Management on Ambulatory Blood Pressure: Results From the SPRINT (Systolic Blood Pressure Intervention Trial) Ambulatory Blood Pressure Study. Hypertension (Dallas, Tex : 1979). 2017;69(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barochiner J, Cuffaro PE, Aparicio LS, et al. Predictors of masked hypertension among treated hypertensive patients: an interesting association with orthostatic hypertension. American journal of hypertension. 2013;26(7):872–878. [DOI] [PubMed] [Google Scholar]
- 27.Tabara Y, Igase M, Miki T, Ohyagi Y, Matsuda F, Kohara K. Orthostatic hypertension as a predisposing factor for masked hypertension: the J-SHIPP study. Hypertens Res. 2016;39(9):664–669. [DOI] [PubMed] [Google Scholar]
- 28.Komori T, Eguchi K, Kario K. The measurement of orthostatic blood pressure as a screening tool for masked hypertension with abnormal circadian blood pressure rhythm. Hypertens Res. 2016;39(9):631–632. [DOI] [PubMed] [Google Scholar]
- 29.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension (Dallas, Tex : 1979). 2001;38(4):852–857. [DOI] [PubMed] [Google Scholar]
- 30.Upadhya B, Rocco M, Lewis CE, et al. Effect of Intensive Blood Pressure Treatment on Heart Failure Events in the Systolic Blood Pressure Reduction Intervention Trial. Circulation Heart failure. 2017;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Kim HA. Orthostatic hypertension: An underestimated cause of orthostatic intolerance. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2016;127(4):2102–2107. [DOI] [PubMed] [Google Scholar]
- 32.Vriz O, Soon G, Lu H, Weder AB, Canali C, Palatini P. Does orthostatic testing have any role in the evaluation of the young subject with mild hypertension?: an insight from the HARVEST study. American journal of hypertension. 1997;10(5 Pt 1):546–551. [DOI] [PubMed] [Google Scholar]
- 33.Streeten DH, Auchincloss JH Jr., Anderson GH Jr., Richardson RL, Thomas FD, Miller JW. Orthostatic hypertension. Pathogenetic studies. Hypertension (Dallas, Tex : 1979). 1985;7(2):196–203. [DOI] [PubMed] [Google Scholar]
- 34.Jordan J, Tank J, Shannon JR, et al. Baroreflex buffering and susceptibility to vasoactive drugs. Circulation. 2002;105(12):1459–1464. [DOI] [PubMed] [Google Scholar]
- 35.Jordan J, Shannon JR, Black BK, et al. Malignant vagotonia due to selective baroreflex failure. Hypertension (Dallas, Tex : 1979). 1997;30(5):1072–1077. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Wang Y, He B, et al. Sympathetic Overactivation From Supine to Upright Is Associated With Orthostatic Hypertension in Children and Adolescents. Frontiers in pediatrics. 2020;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson D Orthostatic hypertension: the last hemodynamic frontier. Hypertension (Dallas, Tex : 1979). 2011;57(2):158–159. [DOI] [PubMed] [Google Scholar]
- 38.Hoshide S, Kario K, Eguchi K, Ishikawa J, Morinari M, Shimada K. Altered aortic properties in elderly orthostatic hypertension. Hypertens Res. 2005;28(1):15–19. [DOI] [PubMed] [Google Scholar]
- 39.Kario K, Matsui Y, Shibasaki S, et al. An alpha-adrenergic blocker titrated by self-measured blood pressure recordings lowered blood pressure and microalbuminuria in patients with morning hypertension: the Japan Morning Surge-1 Study. Journal of hypertension. 2008;26(6):1257–1265. [DOI] [PubMed] [Google Scholar]
- 40.Hoshide S, Parati G, Matsui Y, Shibazaki S, Eguchi K, Kario K. Orthostatic hypertension: home blood pressure monitoring for detection and assessment of treatment with doxazosin. Hypertens Res. 2012;35(1):100–106. [DOI] [PubMed] [Google Scholar]
- 41.Chhabra L, Spodick DH. Orthostatic hypertension: recognizing an underappreciated clinical condition. Indian Heart J. 2013;65(4):454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finucane C, van Wijnen VK, Fan CW, et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res. 2019;29(4):427–441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.