Abstract
NAD+, as an emerging regulator of immune responses during viral infections, may be a promising therapeutic target for coronavirus disease 2019 (COVID-19). In this Opinion, we suggest that interventions that boost NAD+ levels might promote antiviral defense and suppress uncontrolled inflammation. We discuss the association between low NAD+ concentrations and risk factors for poor COVID-19 outcomes, including aging and common comorbidities. Mechanistically, we outline how viral infections can further deplete NAD+ and its roles in antiviral defense and inflammation. We also describe how coronaviruses can subvert NAD+-mediated actions via genes that remove NAD+ modifications and activate the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome. Finally, we explore ongoing approaches to boost NAD+ concentrations in the clinic to putatively increase antiviral responses while curtailing hyperinflammation.
Keywords: NAD+, COVID-19, inflammation, immune responses
NAD+ as a modulator of viral infection outcomes
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other viral infections render the body a battlefield, requiring mobilization of vast resources to mount an effective defense. These defenses can backfire when uncontrolled, resulting in deadly cytokine storms (see Glossary). A key to effective interventions is to trigger robust antiviral defenses but with checked inflammation. Therefore, molecules that suppress both viral replication and inflammation may be particularly important for fighting severe COVID-19 [1]. A growing body of evidence shows that the metabolite NAD+ is a mediator of both antiviral and anti-inflammatory mechanisms. Based on this evidence, we posit that therapies that boost NAD+ concentrations might play a role in preventing and treating severe COVID-19 and other viral infections.
First described as a yeast fermentation factor over a century ago, today, NAD+ has risen to prominence as a regulator of healthy aging. Low levels of NAD+ in tissues and organs are associated with aging, metabolic syndrome, and inflammation, while dietary interventions that slow age-related diseases increase NAD+ concentrations [2]. Here, we review the epidemiological and mechanistic data supporting a role for NAD+ in modulating the outcomes of viral infections, with a focus on SARS-CoV and SARS-CoV-2. We also explore ongoing approaches to boost NAD+ levels for therapeutic benefit in the clinic.
NAD+ and risk factors for severe COVID-19
Aging
A tragic and poorly understood aspect to COVID-19 is the increased susceptibility of the elderly to severe forms of the disease [3]. Rates of hospitalization, intensive care unit (ICU) admission, and death increase with age, while the young are often left relatively unscathed [4]. Most infectious diseases afflict both the very old and young, making this demographic pattern for COVID-19 susceptibility unusual [5]. A better understanding of the mechanisms that render older individuals more susceptible to developing severe disease could lead to new candidate strategies for therapeutic intervention.
We posit that one culprit may be a deficiency in NAD+, the concentration of which decreases during aging in nearly every tissue studied across species [6]. In rodents, this decrease has been observed in tissues and cells that are relevant to COVID-19 infection, such as the skeletal muscle, liver endothelial cells, and macrophages [7., 8., 9., 10.]. A growing body of human data corroborates an association between age and low NAD+ concentrations across multiple tissues including the skin, blood, liver, and muscle [10., 11., 12., 13.,117].
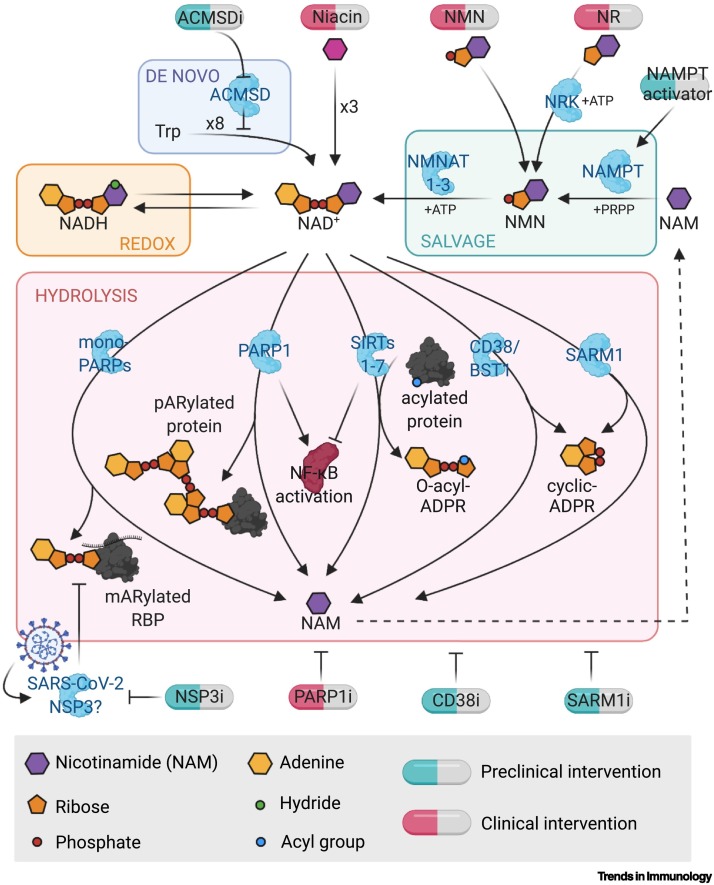
Why NAD+ levels fall during aging is an active area of research. Studies in mice have implicated an increase in the concentrations and activity of NAD+-consuming enzymes such as CD38, poly(ADP-ribose) polymerase 1 (PARP1)1, and sterile alpha and TIR motif containing 1 (SARM1) [9,14., 15., 16., 17.], as well as an insufficient flux from NAD+-producing enzymes such as nicotinamide phosphoribosyltransferase (NAMPT), indoleamine 2,3-dioxygenase (IDO), and quinolinate phosphoribosyltransferase (QPRT) [10,18., 19., 20.]. These enzymes all serve as potential pharmacological targets to raise NAD+ concentrations (Figure 1 ).
Figure 1.
NAD+ metabolism and points of pharmacological intervention.
Enzymes involved in NAD+ biosynthesis and hydrolysis play important roles in inflammation and immunity. Biosynthetic pathways include the NAD+ salvage pathway, which recycles nicotinamide to form NMN, then NAD+, and the de novo pathway that begins with tryptophan. Hydrolysis of NAD+ is largely carried out by PARPs, which tag target proteins with poly- or mono-(ADP ribose); sirtuins, which remove acyl groups and create O-acyl-ADP-ribose; and CD38, BST, and SARM1, which create (cyclic)-ADP-ribose [44]. There are multiple points of potential pharmacological intervention throughout NAD+ metabolic pathways. Created with BioRender.com. Abbreviations: i, inhibitor; NAMPT, nicotinamide phosphoribosyltransferase; NMN, nicotinamide mononucleotide; NR, nicotinamide ribose; NSP3, nonstructural protein 3; PARP, poly(ADP-ribose) polymerase; SARM1, sterile alpha and TIR motif containing 1; SIRT, sirtuin.
Comorbidities
NAD+ concentrations are also low in certain comorbidities associated with COVID-19 severity. One such comorbidity is insulin resistance (IR) and diabetes mellitus [21]. Specifically, in mouse models, genetic (KK/HIJ), diet-induced (high-fat diet), streptozotocin-induced, and age-associated IR have been associated with lower NAD+ concentrations in liver and white adipose tissue, while restoration of NAD+ with NAD+ boosters such as nicotinamide ribose (NR) and nicotinamide mononucleotide (NMN) (oral, intraperitoneal, or infusion) reverses IR [22., 23., 24., 25., 26.]. In humans, metabolic syndrome and obesity have been associated with low NAD+ concentrations in adipose tissue [27]. NMN was recently shown to improve insulin sensitivity in prediabetic women, and further clinical studies are ongoing, discussed later in this review [28] (Table 1, Key table). In addition, a recently developed oral antidiabetic medication, imeglimin, can enhance glucose-stimulated ATP generation and induce the synthesis of NAD+ in pancreatic islets derived from diseased rodents with type 2 diabetes [29].
Table 1.
Key table. Selected clinical trials involving NAD+ boosters
| Clinicaltrials.gov ID | Phase | Interventions | Duration | Type | Enrollment (participants) | Inclusion criteria | Primary endpoint | Completion |
|---|---|---|---|---|---|---|---|---|
| NCT03151239 | N/A | NMN, 250 mg/d or placebo | 8 wk | Randomized, double-blind, placebo-controlled | 25 | Prediabetic postmenopausal women age 55–75 yr | Change in muscle insulin sensitivity | June 2021 |
| NCT05175768 | N/A | NMN, NMN+L-leucine, or placebo | Up to 28 d | Randomized, double-blind, placebo-controlled | 375 | Individuals age >40 yr hospitalized with COVID-19 requiring supplemental oxygen | COVID-19 associated fatigue | December 2022 |
| NCT04903210 | IV | NMN, 800 mg/d + lifestyle modification or lifestyle modification alone | 8 wk | Randomized, single blind | 20 | Individuals age 18–65 yr with mild essential hypertension (BP 130/80–159/99) | Hypertension (flow-mediated dilation and brachial–ankle pulse wave velocity) | July 2022 |
| NCT04664361 | N/A | NMN 250 mg/d, NMN 500 mg/d, or placebo | 38 d | Randomized, double-blind, placebo-controlled | 150 | Healthy men age 20–49 yr with regular moderate physical activity | Muscle recovery (post-endurance Wingate Anaerobic Test) | September 2022 |
| NCT02950441 | II | NR 1 g/d or placebo | 21 d, followed by washout and crossover | Randomized, double-blind, placebo-controlled, crossover | 12 | Men age 70–80 yr | Mitochondrial function (respirometry) and NAD+ concentrations in muscle biopsy | September 2019 |
| NCT02921659 | I/II | NR 1 g/d or placebo | 6 wk, followed by crossover | Randomized, double-blind, placebo-controlled, crossover | 30 | Individuals age 55–79 yr | Treatment-emergent adverse events | October 2016 |
| NCT04040959 | II | NR 1 g/d or placebo | 3 mo | Randomized, double-blind, placebo-controlled | 118 | Individuals age 35–80 yr with chronic kidney disease stage III or IV | Arterial stiffness (carotid–femoral pulse wave velocity) | September 2024 |
| NCT03821623 | II | NR 1 g/d or placebo | 3 mo | Randomized, double-blind, placebo-controlled | 118 | Individuals age ≥50 yr with systolic blood pressure between 120 and 139 mmHg | Resting systolic blood pressure | December 2023 |
| NCT04528004 | I | NR dose escalation to 1 g/d or placebo | 14 d | Randomized, double-blind, placebo-controlled | 40 | Adults with end-stage heart failure NYHA class IV | Whole blood NAD+ concentrations | August 2024 |
| NCT04407390 | II | NR 1 g/d or placebo | 14 d | Randomized, double-blind, placebo-controlled | 100 | Individuals age ≥70 yr with COVID-19 | Hypoxic respiratory failure | May 2022 |
| NCT04818216 | II | NR 1 g/d or placebo | 10 d | Randomized, double-blind, placebo-controlled | 100 | Adults hospitalized with COVID-19 and acute kidney injury | Whole blood NAD+ concentrations, adverse events, thrombocytopenia | June 2023 |
| NCT04573153 | II/III | NR + serine + L-carnitine tartrate + N-acetylcysteine + hydroxychloroquine vs. placebo + hydroxychloroquine | 14 d | Randomized, placebo-controlled | 400 | Adults with COVID-19, ambulatory and symptomatic | Hospitalization rate | March 2021 |
| NCT04809974 | IV | NR 2 g/d or placebo | 22 wk | Randomized, double-blind, placebo-controlled | 100 | Individuals age 18–65 yr, 2+ mo out from COVID-19 PCR diagnosis, currently PCR negative, with persistent cognitive and physical difficulties (long-COVID) | Cognitive functioning measured by executive functioning and memory composite scores | December 2022 |
Another risk factor for severe COVID-19 is cardiovascular disease (CVD) [30]. Several preclinical studies demonstrate that raising NAD+ concentrations in endothelial cells reverses vascular and endothelial dysfunction [8,31]. Further studies have shown a beneficial role for NAD+ in cardiovascular function in mouse models, including models of dyslipidemia, ischemia–reperfusion injury, and diastolic heart failure [32,33]. This link is supported in humans by epidemiological data demonstrating a correlation between dietary intake of the NAD+ precursor niacin (nicotinic acid) and vascular health, including brachial-artery flow-mediated dilation and serum low-density lipoprotein (LDL) concentrations [34]. In fact, niacin is an USA FDA-approved therapy for reducing LDL, ApoB, and triglycerides, and for raising high-density lipoprotein (HDL). Additionally, multiple ongoing clinical studies are testing the effects of other NAD+ boosters such as NMN and NR on hypertension and heart failure (discussed later) (Table 1).
Mechanisms of NAD+ in viral infections
Viral infections can deplete NAD+ concentrations
Not only are low NAD+ concentrations associated with risk factors for poor COVID-19 outcomes, but certain viral infections can further deplete NAD+ in infected cells. For example, lower NAD+ concentrations have been reported in human peripheral blood leukocytes infected with HIV-1 in vitro [35], human fibroblasts infected with herpes simplex virus 1 (HSV-1) [36], and in the skeletal muscle of individuals coinfected with HIV-1 and hepatitis C virus [37]. These effects are presumably due to the induction of NAD+-consuming enzymes such as CD38 and PARPs [17]. For instance, CD8+ T lymphocytes expressing CD38 have been proposed as a marker for HIV-1-mediated disease progression [38]. The decline of NAD+ concentrations in human fibroblasts induced by HSV-1 infection is associated with increased protein poly(ADP-ribosyl)ation, and can be blocked by pharmacological inhibition of PARP1/PARP2 [36].
Similar depletion of NAD+ has been reported with coronavirus infections. Specifically, blood from severe COVID-19 patients contains lower amounts of the NAD+ precursor NMN compared with blood from healthy individuals [39]. Moreover, expression changes in genes involved in NAD+ synthesis and utilization such as Nampt, Parp9, Parp12, and Parp14 have been observed in epithelial cell lines and enterocyte organoids infected with SARS-CoV-2 compared with mock infection [40]. Similar NAD+-related gene expression changes were also reported in the lung tissue of a deceased COVID-19 patient and in the bronchoalveolar lavage fluid of SARS-CoV-2-infected individuals relative to healthy controls [40]. Furthermore, infection of a coronavirus, mouse hepatitis virus (MHV), in mouse bone-marrow-derived macrophages (BMDMs) induced NAD+ depletion [40] as well as increased gene expression of many PARPs including Parp7, Parp9, Parp10, Parp11, Parp12, Parp13, and Parp14 relative to mock infection [41]. These observations suggest that NAD+ metabolic pathways are under increased demand during SARS-CoV-2 infection, and highlights the potential relevance of NAD+ in modulating COVID-19 disease outcomes.
NAD+-consuming enzymes in antiviral mechanisms
NAD+ harbors an important role in fueling the activity of enzymes that regulate mammalian immune responses [42,43]. Classically, NAD+ participates in redox processes, but it also participates in non‐redox reactions in which it is hydrolyzed, and it non‐enzymatically regulates protein–protein interactions [16] (Figure 1). In these latter functions, NAD+ acts as a signaling molecule, serving as a marker of energy availability and directing a cell to respond to metabolic changes via the action of NAD+-utilizing enzymes [44].
PARPs and sirtuins are two NAD+-dependent enzyme families that participate in immune responses [42,43]. By adding or removing post-translational modifications on key proteins such as nuclear factor kappa B (NF-κB), they can coordinate the intensity of inflammatory and immune responses [42]. This places NAD+ in an important position for both promoting strong immune responses to pathogens, and for keeping those responses in check.
PARP1, the pre-eminent member of the PARP family, is a potent coactivator of the proinflammatory transcription factor NF-κB, and therefore participates in initiating specific immune responses [42]. However, this is a double-edged sword, as PARP1 may also increase the severity of cytokine storms as it regulates the expression of many NF-κB-dependent cytokines and chemokines [42]. Indeed, inhibiting or deleting PARP1 has ameliorated the severity of symptoms in several inflammatory disease rodent models including asthma and colitis [45., 46., 47., 48., 49., 50., 51.]. For example, PARP1 inhibition (pharmacological or genetic) has prevented ovalbumin-induced lung inflammation in mice [45] and in a guinea pig model of asthma [46]. Treatment with PARP inhibitors has attenuated inflammation associated with colitis seen in interleukin-10 (IL-10) deficient (Il10 -/-) mice [47], as well as in trinitrobenzene sulfonic acid-treated rats [49]. Furthermore, PARP1 knockout (KO) (Parp1 -/-) mice are protected from dextran sulfate sodium-induced colitis compared with wild-type mice [48].
Several PARPs harbor potent antiviral functions [42,52., 53., 54., 55., 56., 57., 58., 59.]. Indeed, PARP13 is a powerful antiviral factor that recognizes various viruses from several families, including Retroviridae, Filoviridae, Alphaviridae, and Hepadnaviridae [53., 54., 55., 56.]. It binds to specific sequences of viral RNAs during infection and mediates their degradation via the cellular mRNA decay machinery; however, these functions are not dependent on PARP-mediated ADP-ribosylation [54., 55., 56.]. Expression of PARP7, PARP10, and the long isoform of PARP12 (PARP12L) efficiently inhibits cellular translation and the replication of Venezuelan equine encephalitis virus and other alphaviruses in vertebrate cells [52., 59.]. These effects of PARP12L are dependent on its catalytic activity. Moreover, PARP9 and PARP14 are also upregulated in macrophages stimulated by interferon (IFN)-γ and have opposing roles in macrophage activation [58]. PARP9 activates IFNγ–STAT1 signaling and induces proinflammatory activation while PARP14 ADP-ribosylation reduces STAT1 phosphorylation in IFNγ-treated human macrophages [58]. , Additionally, the nucleocapsid proteins of several coronaviruses, including SARS-CoV, MERS-CoV, and MHV, are ADP-ribosylated in infected cells, presumably by PARPs, which may indicate a common use of this pathway among viruses. However, the functional consequences of such ADP‐ribosylation remain to be investigated [57]. We argue that since PARP enzymatic activity requires NAD+[44], maintaining a sufficient NAD+ concentration may be crucial for achieving PARP-related antiviral mechanisms.
Sirtuins also play a role in antiviral defenses [43,60., 61., 62., 63., 64.]. Indeed, sirtuin 1 (SIRT1) KO or inhibition promotes the lifecycle and replication of vesicular stomatitis virus in mouse embryonic fibroblasts (MEFs) and Kaposi’s sarcoma-associated herpesvirus in human lymphoma cell lines [61,62]. Disruption of SIRT1 also increases HPV16 E1–E2 replication [60]. Moreover, knockdown via siRNA of each of the seven sirtuins in human fibroblast cells promoted the growth of a diverse set of human viruses after infection, including human cytomegalovirus (CMV), HSV1, adenovirus type 5, and influenza virus (H1N1) [63]. Furthermore, SIRT1-activating drugs such as resveratrol and CAY10602 have inhibited the replication of these viruses [63]. SIRT6 promotes tumor necrosis factor (TNF)α secretion, as evidenced from the suppression of TNFα release from SIRT6 KO MEFs, whereby TNFα secretion would be expected to promote the eradication of pathogens [64]. Indeed, pharmacological inhibition and siRNA knockdown of SIRT6 and NAMPT, an NAD+-synthetic enzyme (Figure 1), in mouse fibroblasts promoted CMV replication [65].
Other NAD+-utilizing enzymes that also play roles during immune responses include CD38 [66], BST1 [2], and SARM1 [43]. Indeed, CD38 and BST1 are highly expressed on the surface of macrophages and lymphocytes and produce extracellular cyclic(ADP-ribose), a calcium-mobilizing second messenger that is important for immune cell activation [2,66,67]. SARM1, another potent NADase, contains a Toll/IL-1 receptor domain, which might elicit neuroprotective innate immune responses, as suggested from mouse models of neurodegeneration [68].
Viruses fighting back
One antiviral mechanism that is associated with PARP activity is the formation of cytosolic, membraneless organelles called stress granules (SGs) to sequester viral RNAs and arrest proviral translation [69,70]. Indeed, ADP-ribosylation of SG components by PARPs promotes the formation of antiviral SGs [71., 72., 73.]. Several antiviral PARPs including PARP5a, 12, 13, 14, and 15 localize to SGs in human cells, presumably to execute this modification [71].
As a counter-mechanism to the host, many viruses, including coronaviruses and alphaviruses, deploy ADP-ribosylhydrolases to remove SG-promoting ADP-ribose modifications [74., 75., 76., 77.]. SARS-CoV-2 has such a domain with mono-ADP-ribosylhydrolase activity in its largest encoded protein, nonstructural protein 3 (NSP3) [79,80]. Ectopic expression of the SARS-CoV-2 NSP3 macrodomain reverses PARP9-dependent ADP-ribosylation of target proteins [81]. In live viruses, this domain is important for both viral replication and virulence. Mutations of this domain in MHV and SARS-CoV led to attenuated viral replication, and thus, the mutant viruses were unable to cause lung disease in infected mice, while treatment with pan-PARP inhibitors enhanced SARS-CoV replication and inhibited IFN production in BMDMs infected with macrodomain-deficient mutant coronavirus [41,82]. These findings suggest that the SARS-CoV-2 NSP3 protein might also interfere with SG formation and thereby allow for evasion of cellular antiviral responses (Figure 2 ). We argue that providing more NAD+ to fuel the activity of PARPs might shift the antiviral balance back in favor of host cells.
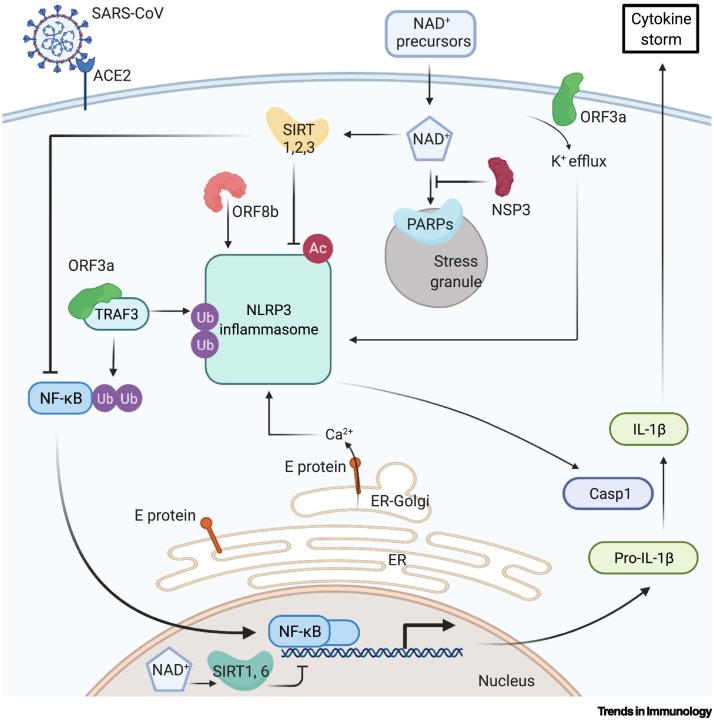
Figure 2.
Regulation of the NLRP3 inflammasome by SARS-CoV infection and NAD+.
Several proteins encoded by SARS-CoV promote NLRP3 inflammasome activity and the release of proinflammatory cytokines. ORF3a activates NF-κB through TRAF3-dependent ubiquitination, which facilitates ASC speck formation and the assembly of NLRP3 inflammasome [86]. ORF3a also has transmembrane domains and ion channel activity that drives a K+ efflux [85]. E protein is located at the ER–Golgi compartment and promotes Ca2+efflux [87,88]. ORF8bdirectly interacts with NLRP3. These mechanisms all activate the inflammasome [89]. Host cell SIRT1, SIRT2, and SIRT3 all suppress the NLRP3 inflammsome [94., 95., 96., 97., 98.]. SIRT1 deacetylates NF-κB, suppressing its activity, and reduces oxidative stress, decreasing inflammasome activation. SIRT2 deacetylates NLRP3. SIRT3 suppresses mitochondrial ROS production, decreasing inflammasome activation. SIRT6 reduces inflammation via H3K9 deacetylation in the promoters of NF-κB target genes [99]. PARPs promote antiviral SG formation through ADP-ribosylation of SG components [71., 72., 73.]. Created with BioRender.com. Abbreviations: Ac, acetylation; ACE-2, angiotensin-converting enzyme 2; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain Casp1, caspase-1; ER, endoplasmic reticulum; NF-κB, nuclear factor κB; NLRP3, NOD-, LRR-, and pyrin domain-containing protein 3; ORF, open reading frame; SARS-CoV, severe acute respiratory syndrome coronavirus 1; SIRT, sirtuin; TRAF3, TNF receptor-associated factor 3; Ub, ubiquitination.
Viruses can drive uncontrolled inflammation
One of the potential catastrophic effects of SARS-CoV-2 infections is cytokine storms. Indeed, the concentrations of circulating proinflammatory factors such as IL-1, IL-6, and TNFα are strongly associated with ICU admission and mortality in COVID-19 patients [83,84]. Studies of the first SARS pandemic coronavirus, SARS-CoV, have shown that viral proteins play an active role in the processing and release of the two specific proinflammatory cytokines, IL-1β and IL-18, by enhancing NF-κB transcriptional activity and promoting the formation of the NLRP3 inflammasome (Figure 2) [85., 86., 87., 88., 89., 90.].
The SARS-CoV proteins that facilitate these processes include open reading frame (ORF)3a, envelope protein (E), and ORF8b [85., 86., 87., 88., 89., 90.]. ORF3a mediates NF-κB activation through TRAF3-dependent ubiquitination of an NF-κB inhibitory subunit, and mediates speck formation of the inflammasome subunit ASC, which accompanies assembly of the NLRP3 inflammasome in human cells [86]. Additionally, ORF3a has transmembrane domains and ion channel (IC) activity that drives K+ efflux, which further promotes activation of the NLRP3 inflammasome. Indeed, IL-1β secretion was completely blocked when BMDMs, stimulated with lentiviruses expressing the SARS-CoV ORF3a, were treated with K+-rich medium [78,85]. The SARS-CoV E protein also promotes inflammasome activation through its intracellular activity; it forms lipid–protein channels at the endoplasmic reticulum (ER)–Golgi intermediate compartment, and the resulting Ca2+ stimulates the activation of the NLRP3 inflammasome. The E protein also promotes IL-1β release in mammalian cells expressing the NLRP3 inflammasome, while E protein mutants lacking ion conductance cannot boost IL-1β secretion [87,88]. ORF8b causes inflammasome activation in human macrophages and interacts directly with NLRP3 in vitro; moreover, it can form protein aggregates and trigger ER stress and lysosomal damage that activate the inflammasome in HeLa cells [89]. These processes not only promote virulence but may also facilitate viral replication [87,90]. For example, a comparative study of the functional motifs included within the SARS-CoV viroporins showed that full-length E and ORF3a proteins were required for maximal SARS-CoV replication and virulence in infected mice [87].
As with SARS-CoV, SARS-CoV-2 can also activate the inflammasome [91]. Active NLRP3 inflammasomes have been found in peripheral blood mononuclear cells (PBMCs) and tissues from deceased COVID-19 patients upon autopsy, along with higher concentrations of serum IL-18 which correlated with COVID-19 severity. SARS-CoV-2 may activate the inflammasome through mechanisms similar to those of SARS-CoV [91]. Overexpression of SARS-CoV-2 ORF3a in human cells can induce K+ efflux, NLRP3 activation, and IL-1β release. Restricting K+ efflux with K+-rich media impairs the ability of ORF3a to trigger NLRP3 inflammasome assembly, as evidenced from coimmunoprecipitation (co-IP) assays [92]. SARS-CoV-2 N protein can interact directly with NLRP3, supported by results of reciprocal co-IP and confocal microscopy, thus promoting inflammasome activation and IL-1β release in cells. In Nlrp3 +/+ mice infected with adeno-associated virus (AAV)-Lung-N, serum IL-1β concentrations were increased (detected via ELISA), whereas IL-1β was not induced by AAV-N in the sera of Nlrp3 −/− mice [93]. The higher cytokine concentrations stemming from NLRP3 activation suggest an increased likelihood of uncontrolled inflammation in response to SARS-CoV-2 infection.
NAD+-consuming enzymes in anti-inflammatory mechanisms
NAD+ may contribute to the resolution of inflammation, and to limiting or preventing the effects of cytokine storms; it might do so by increasing the activity of sirtuins (Figure 2) [44]. SIRT1, SIRT2, and SIRT3 all suppress the activity of NF-κB and the NLRP3 inflammasome via multiple mechanisms [94., 95., 96., 97., 98.]. From a biochemical standpoint, SIRT1 physically interacts with and deacetylates NF-κB and thereby suppresses its transcriptional activity in human epithelial cells [95]. SIRT1 also inhibits lipopolysaccharide-induced NLRP3 inflammasome activation by reducing oxidative stress, as demonstrated in human trophoblasts with an shRNA knockdown of SIRT1 [94]. SIRT2 directly deacetylates NLRP3 and inactivates the NLRP3 inflammasome, as shown in SIRT2 KO (Sirt2 -−) mouse macrophages [97]. SIRT3 mediates inflammasome activation by suppressing mitochondrial reactive oxygen species (ROS) production, which can activate the NLRP3 inflammasome. SiRNA depletion of SIRT3 in human macrophages results in increased ROS production and inflammasome activation compared with controls [98]. SIRT6 also promotes the resolution of inflammation via histone H3 lysine 9 (H3K9) deacetylation in the promoters of NF-κB target genes [99]. However, the effects of sirtuins on the NLRP3 inflammasome have yet to be extensively studied in the context of viral infection.
We posit that NAD+ boosters that suppress NF-κB and NLRP3 inflammasome activity might also represent potential treatments to ameliorate the inflammatory symptoms of COVID-19. Indeed, treatment with NR, an NAD+ precursor, for 24 hours has been reported to decrease the release of IL-1β and blunt inflammasome activation in PBMCs from healthy and fasted individuals [98]. NR also promotes SIRT1 expression, suppresses NLRP3 expression, and reduces secretion of proinflammatory factors TNFα and IL-6 in mouse hepatocytes [100]. Another NAD+ precursor, nicotinic acid, also reduced inflammation in a rodent model of type 2 diabetes (KK/HIJ mice) by modulating NLRP3 activity [26]. However, consideration should be given to the fact that some studies show that extracellular nucleotides such as NAD+ can also promote inflammation via the activation of P2X7 receptors in mouse macrophages and T cells [101].
The promise of NAD+ boosters in the clinic
Adding more fuel
Given that low NAD+ concentrations might exacerbate COVID-19 severity, boosting the levels of this metabolite in at-risk populations is one potential therapeutic strategy for mitigating severe disease. The most straightforward way to raise NAD+ concentrations is to provide additional precursors to boost NAD+ synthesis [2]. Canonically, NAD+ cannot be taken up directly by the cell, but its precursors can [102,115]. The NAD+ precursors nicotinic acid, NR, and NMN, all have putative transporters, high safety thresholds, and are orally bioavailable [2]. They do, however, vary in their pharmacokinetic profiles and seem to raise NAD+ concentrations to different extents in different tissues [103], although this remains an area of active investigation.
Structurally, the closest molecule to NAD+ is NMN, requiring only one enzymatic step to be converted to NAD+. In a double blind, placebo-controlled trial, NMN was shown to increase insulin sensitivity in prediabetic women [28] (NCT03151239; Table 1). It is currently being studied in hospitalized COVID-19 patients with hypertension and metabolic syndrome for its effects on fatigue, duration of hospitalization, and viral load (NCT05175768; Table 1). Ongoing clinical trials with NMN are also investigating its effect on hypertension and postexercise muscle recovery (NCT04903210 and NCT04664361; Table 1).
NR, which is two enzymatic steps removed from NAD+, is also being studied clinically. In one placebo-controlled trial, oral NR reduced circulating concentrations of inflammatory cytokines IL-2, IL-5, and IL-6 [104] (NCT02950441; Table 1). In another trial, oral NR reduced blood pressure and possibly aortic stiffness in healthy middle-aged and older adults [105] (NCT02921659; Table 1). At the time this review was written, over 30 active studies testing NR had been registered on clinicaltrials.gov, including many for COVID-19 risk factors such as vascular disease, hypertension, and heart failure (NCT04040959, NCT03821623, and NCT04528004; Table 1). At least four ongoing studies are directly testing NR in COVID-19 patients, some in patients with particular risk factors such as advanced age or acute kidney injury, with readouts such as rates of hospitalization, respiratory failure, and long-COVID symptoms (NCT04407390, NCT04818216, NCT04573153, and NCT04809974; Table 1).
Other approaches to raising NAD+ concentrations
Another approach to raising NAD+ concentrations in humans is to inhibit the activity of NAD+-consuming enzymes. For instance, as PARP1 is a major consumer of NAD+ in cells [42], PARP1 inhibitors such as olaparib have been extensively studied for the treatment of breast and ovarian cancer, given that PARP1 is synthetically lethal with BRCA mutations [106]. Regarding COVID-19, several PARP1 inhibitors, CVL218 and stenoparib, were recently shown to limit SARS-CoV-2 replication and proinflammatory cytokine production in human PBMCs and lung cells. One hypothesized mechanism of action is via inhibition of PARP1-dependent NAD+ depletion [107,116].
Several inhibitors of other NAD+-consuming enzymes are in preclinical development, including against CD38 [108,109], SARM1 [110], and ACMSD (which consumes a precursor of NAD+ in the de novo pathway) [20]. In humans, treatment with luteolin, a naturally occurring CD38 inhibitor, reduced serum TNF and IL-6 concentrations [111].
The activation of NAD+ biosynthetic enzymes is another strategy to raise NAD+ concentrations; a small molecule NAMPT activator, SBI-797812, is currently in preclinical development for cardiovascular and metabolic disease [112]. Finally, a virally‐encoded protein is a potential target that interferes with NAD+ metabolism: the macrodomain of NSP3 can antagonize the antiviral activities of host PARPs, although the implications of this activity entail further investigation for SARS-CoV-2 [41,80., 81., 82.]. A few inhibitors of NSP3 were recently identified in a chemical screen and were shown to exhibit antiviral properties against Chikungunya virus in human epithelial cells [113].
Concluding remarks
NAD+ metabolism appears to be linked to infections of SARS-CoV-2 and other viruses via multiple lines of evidence, epidemiological and mechanistic. Further research is warranted to better understand its mechanistic role and utility as a target of intervention (see Outstanding questions). Clinical translation of basic scientific discoveries is difficult, and there are many examples of antiviral and anti-inflammatory molecules that have shown early promise but have not materialized. However, while human studies are still at an early stage, we are excited about the promise of interventions that modulate the concentrations of NAD+ or the activity of NAD+-consuming enzymes. Such interventions may soon have a place in our arsenal to fight current and future viral infections.
Outstanding questions.
Why are NAD+ tissue levels reduced during aging? Many hypotheses exist to date, but there is currently no consensus, especially as multiple mechanisms may be involved.
Are low NAD+ concentrations associated with severe COVID-19? There is little data to date showing a direct association.
Can raising NAD+ tissue concentrations reduce COVID-19 symptoms, severity, and mortality in the elderly and in the young?
Can NAD+ boosters reduce COVID-19 severity after the onset of symptoms, or only prophylactically?
What might be the best approach for raising NAD+ concentrations in the clinic: would this be NMN, NR, activators of NAD+ biosynthesis, or inhibitors of enzymes that consume NAD+?
What role does NAD+ play in promoting SG-dependent antiviral mechanisms?
How does SARS-CoV-2 modulate NAD+ and NSP3 to increase virulence?
Alt-text: Outstanding questions
Declaration of interests
D.A.S. is a founder, equity owner, advisor to, director of, board member of, consultant to, investor in and/or inventor on patents licensed to GlaxoSmithKline, Segterra, Animal Biosciences, AFAR, Cohbar, Galilei, Zymo Research, Immetas, EdenRoc Sciences and affiliates (Arc-Bio, Dovetail Genomics, Claret Bioscience, MetroBiotech, Astrea, Liberty Biosecurity and Delavie), Life Biosciences, and Levels Health. D.A.S. is an inventor on a patent application licensed to Elysium Health. More at https://sinclair.hms.harvard.edu/david-sinclairs-affiliations.
Glossary
- Apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC)
inflammasome adaptor that plays a key role in the assembly and activation of the inflammasome. The NLRP3 inflammasome consists of a sensor (NLRP3), an adaptor (ASC), and an effector (caspase-1). Upon sensing-specific triggers, NLRP3 undergoes conformational changes which catalyze ASC oligomerization to form a macromolecular signaling complex known as the ASC speck. ASC then recruits caspase-1. Active caspase-1 cleaves pro-IL-1β and pro-IL-18, which are vital cytokines during infection and inflammation.
- Cytokine storm
life-threatening event triggered by uncontrolled inflammatory responses with the release of a large amounts of proinflammatory cytokines, including IL-6, IL-1, TNFα, and IFN. This leads to the recruitment and expansion of various immune cells into injury sites which can result in tissue and organ damage.
- Histone H3 lysine 9 (H3K9) deacetylation
epigenetic modification; H3K9 is generally acetylated when a gene is transcriptionally activated and is deacetylated (and/or methylated) when a gene is silenced.
- Imeglimin
novel oral agent for the treatment of type 2 diabetes. Its mechanism of action involves amplification of glucose-stimulated insulin secretion and enhanced insulin action. Approved for use in Japan in 2021.
- P2X purinoceptor 7
expressed in an increasing number of cell types; primarily mediates inflammation and cell death; ligand-gated cation channel activated by high concentrations of extracellular ATP, triggering the assembly and activation of the NLRP3 inflammasome and subsequent release of IL-1β and IL-18.
- Poly(ADP-ribose) polymerases (PARPs)
with coenzyme NAD+, PARP enzymes catalyze ADP-ribosylation or the transfer of ADP-ribosyl groups from NAD+ to nucleophilic side chains of proteins. They can also ADP-ribosylate DNA and RNA. In humans, there are 17 identified PARPs. PARPs participate in diverse cellular functions such as DNA repair, apoptosis, unfolded protein response, pathogen response, and inflammation.
- Sirtuins
enzymes that couple NAD+ degradation to deacylation (e.g., deacetylation, desuccinylation, and demyristoylation). With NAD+, sirtuins remove acyl groups from lysine residues on proteins, sense intracellular NAD+ concentrations, and transduce a signal via protein deacylation. In humans, there are seven sirtuins at different cellular locations that regulate diverse functions, including transcription, genome stability, metabolism, and cell signaling.
- Stress granules (SGs)
dense, membraneless, organelles in the cytosol that appear in response to cellular stress to promote cell survival. Ribonucleoprotein assemblies that store mRNAs stalled at translation initiation. The introduction of viral RNA into the cytoplasm triggers the formation of stress granules.
- TNF receptor-associated factor 3 (TRAF3)
member of the TRAF family; contains a RING domain with E3 ubiquitin ligase activity, and a TRAF domain mediating protein–protein interactions; functions in inflammation, antiviral defense, and apoptosis. There are seven TRAF proteins identified in mammals.
- Toll/interleukin-1 receptor domain
intracellular signaling domain in proteins that mediates protein–protein interactions between Toll-like receptors and signal transduction proteins. When activated, the TIR domain recruits cellular adaptor proteins and induces downstream activation of kinases.
References
- 1.Stebbing J., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajman L., et al. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 2018;27:529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller A.L., et al. Why does COVID-19 disproportionately affect the elderly? Aging. 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luk J., et al. Observations on mortality during the 1918 influenza pandemic. Clin. Infect. Dis. 2001;33:1375–1378. doi: 10.1086/322662. [DOI] [PubMed] [Google Scholar]
- 6.Fang E.F., et al. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol. Med. 2017;23:899–916. doi: 10.1016/j.molmed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes A.P., et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das A., et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89.e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camacho-Pereira J., et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minhas P.S., et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 2019;20:50–63. doi: 10.1038/s41590-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement J., et al. The plasma NAD+ metabolome is dysregulated in “normal” aging. Rejuvenation Res. 2019;22:121–130. doi: 10.1089/rej.2018.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massudi H., et al. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C.-C., et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br. J. Pharmacol. 2016;173:2352–2368. doi: 10.1111/bph.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essuman K., et al. The SARM1 Toll/interleukin-1 receptor domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron. 2017;93:1334–1343.e5. doi: 10.1016/j.neuron.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai P., et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., et al. A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science. 2017;355:1312–1317. doi: 10.1126/science.aad8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covarrubias A.J., et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2020;2:1265–1283. doi: 10.1038/s42255-020-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braidy N., et al. Effects of kynurenine pathway inhibition on NAD metabolism and cell viability in human primary astrocytes and neurons. Int. J. Tryptophan Res. 2011;4:29–37. doi: 10.4137/IJTR.S7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frederick D.W., et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 2016;24:269–282. doi: 10.1016/j.cmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsyuba E., et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature. 2018;563:354–359. doi: 10.1038/s41586-018-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu L., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantó C., et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshino J., et al. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills K.F., et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trammell S.A.J., et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci. Rep. 2016;6:26933. doi: 10.1038/srep26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H.J., et al. Nicotinamide riboside ameliorates hepatic metaflammation by modulating NLRP3 inflammasome in a rodent model of type 2 diabetes. J. Med. Food. 2015;18:1207–1213. doi: 10.1089/jmf.2015.3439. [DOI] [PubMed] [Google Scholar]
- 27.Jukarainen S., et al. Obesity is associated with low NAD(+)/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. J. Clin. Endocrinol. Metab. 2016;101:275–283. doi: 10.1210/jc.2015-3095. [DOI] [PubMed] [Google Scholar]
- 28.Yoshino M., et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372:1224–1229. doi: 10.1126/science.abe9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallakou-Bozec S., et al. Mechanism of action of Imeglimin: a novel therapeutic agent for type 2 diabetes. Diabetes Obes. Metab. 2021;23:664–673. doi: 10.1111/dom.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., et al. A meta-analysis on the risk factors adjusted association between cardiovascular disease and COVID-19 severity. BMC Public Health. 2021;21:1533. doi: 10.1186/s12889-021-11051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Picciotto N.E., et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong D., et al. NAD+ repletion reverses heart failure with preserved ejection fraction. Circ. Res. 2021;128:1629–1641. doi: 10.1161/CIRCRESAHA.120.317046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kane A.E., Sinclair D.A. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ. Res. 2018;123:868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplon R.E., et al. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J. Appl. Physiol. 2014;116:156–163. doi: 10.1152/japplphysiol.00969.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray M.F., et al. HIV infection decreases intracellular nicotinamide adenine dinucleotide [NAD] Biochem. Biophys. Res. Commun. 1995;212:126–131. doi: 10.1006/bbrc.1995.1945. [DOI] [PubMed] [Google Scholar]
- 36.Grady S.L., et al. Herpes simplex virus 1 infection activates poly(ADP-ribose) polymerase and triggers the degradation of poly(ADP-ribose) glycohydrolase. J. Virol. 2012;86:8259–8268. doi: 10.1128/JVI.00495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran T., et al. Reduced levels of NAD in skeletal muscle and increased physiologic frailty are associated with viral coinfection in asymptomatic middle-aged adults. J. Acquir. Immune Defic. Syndr. 2022;89:S15–S22. doi: 10.1097/QAI.0000000000002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurya S.P., et al. Effect of Withania somnifer on CD38 expression on CD8+ T lymphocytes among patients of HIV infection. Clin. Immunol. 2019;203:122–124. doi: 10.1016/j.clim.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Xiao N., et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021;12:1618. doi: 10.1038/s41467-021-21907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heer C.D., et al. Coronavirus infection and PARP expression dysregulate the NAD metabolome: an actionable component of innate immunity. J. Biol. Chem. 2020;295:17986–17996. doi: 10.1074/jbc.RA120.015138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grunewald M.E., et al. The coronavirus macrodomain is required to prevent PARP-mediated inhibition of virus replication and enhancement of IFN expression. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brady P.N., et al. Poly(ADP-Ribose) polymerases in host-pathogen interactions, inflammation, and immunity. Microbiol. Mol. Biol. Rev. 2019;83 doi: 10.1128/MMBR.00038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shang J., et al. NAD+-consuming enzymes in immune defense against viral infection. Biochem. J. 2021;478:4071–4092. doi: 10.1042/BCJ20210181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 45.Boulares A.H., et al. Gene knockout or pharmacological inhibition of poly(ADP-ribose) polymerase-1 prevents lung inflammation in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. 2003;28:322–329. doi: 10.1165/rcmb.2001-0015OC. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki Y., et al. Inhibition of poly(ADP-ribose) polymerase prevents allergen-induced asthma-like reaction in sensitized Guinea pigs. J. Pharmacol. Exp. Ther. 2004;311:1241–1248. doi: 10.1124/jpet.104.072546. [DOI] [PubMed] [Google Scholar]
- 47.Jijon H.B., et al. Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G641–G651. doi: 10.1152/ajpgi.2000.279.3.G641. [DOI] [PubMed] [Google Scholar]
- 48.Larmonier C.B., et al. Transcriptional reprogramming and resistance to colonic mucosal injury in poly(ADP-ribose) polymerase 1 (PARP1)-deficient mice. J. Biol. Chem. 2016;291:8918–8930. doi: 10.1074/jbc.M116.714386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zingarelli B., et al. Inhibitors of poly (ADP-ribose) polymerase modulate signal transduction pathways in colitis. Eur. J. Pharmacol. 2003;469:183–194. doi: 10.1016/s0014-2999(03)01726-6. [DOI] [PubMed] [Google Scholar]
- 50.Altmeyer M., et al. Absence of poly(ADP-ribose) polymerase 1 delays the onset of Salmonella enterica serovar Typhimurium-induced gut inflammation. Infect. Immun. 2010;78:3420–3431. doi: 10.1128/IAI.00211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czapski G.A., et al. Poly(ADP-ribose) polymerase-1 inhibition protects the brain against systemic inflammation. Neurochem. Int. 2006;49:751–755. doi: 10.1016/j.neuint.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Atasheva S., et al. New PARP gene with an anti-alphavirus function. J. Virol. 2012;86:8147–8160. doi: 10.1128/JVI.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodier J.L., et al. The broad-spectrum antiviral protein ZAP restricts human retrotransposition. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Y., et al. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15834–15839. doi: 10.1073/pnas.1101676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Müller S., et al. Inhibition of filovirus replication by the zinc finger antiviral protein. J. Virol. 2007;81:2391–2400. doi: 10.1128/JVI.01601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao R., et al. Inhibition of hepatitis B virus replication by the host zinc finger antiviral protein. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grunewald M.E., et al. The coronavirus nucleocapsid protein is ADP-ribosylated. Virology. 2018;517:62–68. doi: 10.1016/j.virol.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwata H., et al. PARP9 and PARP14 cross-regulate macrophage activation via STAT1 ADP-ribosylation. Nat. Commun. 2016;7:12849. doi: 10.1038/ncomms12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atasheva S., et al. Interferon-stimulated poly(ADP-Ribose) polymerases are potent inhibitors of cellular translation and virus replication. J. Virol. 2014;88:2116–2130. doi: 10.1128/JVI.03443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das D., et al. The deacetylase SIRT1 regulates the replication properties of human papillomavirus 16 E1 and E2. J. Virol. 2017;91:00102–00117. doi: 10.1128/JVI.00102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campagna M., et al. SIRT1 stabilizes PML promoting its sumoylation. Cell Death Differ. 2011;18:72–79. doi: 10.1038/cdd.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Q., et al. Activation of Kaposi’s sarcoma-associated herpesvirus (KSHV) by inhibitors of class III histone deacetylases: identification of sirtuin 1 as a regulator of the KSHV life cycle. J. Virol. 2014;88:6355–6367. doi: 10.1128/JVI.00219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koyuncu E., et al. Sirtuins are evolutionarily conserved viral restriction factors. MBio. 2014;5 doi: 10.1128/mBio.02249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang H., et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496:110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dantoft W., et al. Metabolic regulators nampt and sirt6 serially participate in the macrophage interferon antiviral cascade. Front. Microbiol. 2019;10:355. doi: 10.3389/fmicb.2019.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horenstein A.L., et al. CD38 in the age of COVID-19: a medical perspective. Physiol. Rev. 2021;101:1457–1486. doi: 10.1152/physrev.00046.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernández-Campo P.M., et al. Normal patterns of expression of glycosylphosphatidylinositol-anchored proteins on different subsets of peripheral blood cells: a frame of reference for the diagnosis of paroxysmal nocturnal hemoglobinuria. Cytometry B Clin. Cytom. 2006;70:71–81. doi: 10.1002/cyto.b.20087. [DOI] [PubMed] [Google Scholar]
- 68.Figley M.D., DiAntonio A. The SARM1 axon degeneration pathway: control of the NAD+ metabolome regulates axon survival in health and disease. Curr. Opin. Neurobiol. 2020;63:59–66. doi: 10.1016/j.conb.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCormick C., Khaperskyy D.A. Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 2017;17:647–660. doi: 10.1038/nri.2017.63. [DOI] [PubMed] [Google Scholar]
- 70.Raaben M., et al. Mouse hepatitis coronavirus replication induces host translational shutoff and mRNA decay, with concomitant formation of stress granules and processing bodies. Cell. Microbiol. 2007;9:2218–2229. doi: 10.1111/j.1462-5822.2007.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leung A.K.L., et al. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol. Cell. 2011;42:489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel A., et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 73.Altmeyer M., et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose) Nat. Commun. 2015;6:8088. doi: 10.1038/ncomms9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saikatendu K.S., et al. Structural basis of severe acute respiratory syndrome coronavirus ADP-ribose-1″-phosphate dephosphorylation by a conserved domain of nsP3. Structure. 2005;13:1665–1675. doi: 10.1016/j.str.2005.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jayabalan A.K., et al. Stress granule formation, disassembly, and composition are regulated by alphavirus ADP-ribosylhydrolase activity. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2021719118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abraham R., et al. ADP-ribosyl-binding and hydrolase activities of the alphavirus nsP3 macrodomain are critical for initiation of virus replication. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E10457–E10466. doi: 10.1073/pnas.1812130115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alhammad Y.M.O., Fehr A.R. The viral macrodomain counters host antiviral ADP-ribosylation. Viruses. 2020;12:384. doi: 10.3390/v12040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minakshi R., Padhan K. The YXXΦ motif within the severe acute respiratory syndrome coronavirus (SARS-CoV) 3a protein is crucial for its intracellular transport. Virol. J. 2014;11:75. doi: 10.1186/1743-422X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frick D.N., et al. Molecular basis for ADP-ribose binding to the Mac1 domain of SARS-CoV-2 nsp3. Biochemistry. 2020;59:2608–2615. doi: 10.1021/acs.biochem.0c00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alhammad Y.M.O., et al. The SARS-CoV-2 conserved macrodomain is a mono-ADP-ribosylhydrolase. J. Virol. 2021;95 doi: 10.1128/JVI.01969-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Russo L.C., et al. The SARS-CoV-2 Nsp3 macrodomain reverses PARP9/DTX3L-dependent ADP-ribosylation induced by interferon signaling. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fehr A.R., et al. The conserved coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome coronavirus infection. MBio. 2016;7 doi: 10.1128/mBio.01721-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herold T., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen I.-Y., et al. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siu K.-L., et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castaño-Rodriguez C., et al. Role of severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis. MBio. 2018;9 doi: 10.1128/mBio.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nieto-Torres J.L., et al. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi C.-S., et al. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nieto-Torres J.L., et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodrigues T.S., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021;218 doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu H., et al. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology. 2022;568:13–22. doi: 10.1016/j.virol.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pan P., et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021;12:4664. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park S., et al. SIRT1 Alleviates LPS-induced IL-1β production by suppressing NLRP3 inflammasome activation and ROS production in trophoblasts. Cells. 2020;9:3. doi: 10.3390/cells9030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeung F., et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y., et al. Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology. 2017;222:552–561. doi: 10.1016/j.imbio.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 97.He M., et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab. 2020;31:580–591.e5. doi: 10.1016/j.cmet.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Traba J., et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J. Clin. Invest. 2015;125:4592–4600. doi: 10.1172/JCI83260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawahara T.L.A., et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee H.J., Yang S.J. Nicotinamide riboside regulates inflammation and mitochondrial markers in AML12 hepatocytes. Nutr. Res. Pract. 2019;13:3–10. doi: 10.4162/nrp.2019.13.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hong S., et al. Differential regulation of P2X7 receptor activation by extracellular nicotinamide adenine dinucleotide and ecto-ADP-ribosyltransferases in murine macrophages and T cells. J. Immunol. 2009;183:578–592. doi: 10.4049/jimmunol.0900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grozio A., et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 2019;1:47–57. doi: 10.1038/s42255-018-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu L., et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018;27:1067–1080.e5. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elhassan Y.S., et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28:1717–1728.e6. doi: 10.1016/j.celrep.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martens C.R., et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 2018;9:1286. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farmer H., et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 107.Ge Y., et al. An integrative drug repositioning framework discovered a potential therapeutic agent targeting COVID-19. Signal Transduct. Target. Ther. 2021;6:165. doi: 10.1038/s41392-021-00568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haffner C.D., et al. Discovery, synthesis, and biological evaluation of thiazoloquin(az)olin(on)es as potent CD38 inhibitors. J. Med. Chem. 2015;58:3548–3571. doi: 10.1021/jm502009h. [DOI] [PubMed] [Google Scholar]
- 109.Tarragó M.G., et al. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab. 2018;27:1081–1095.e10. doi: 10.1016/j.cmet.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Loring H.S., et al. Identification of the first noncompetitive SARM1 inhibitors. Bioorg. Med. Chem. 2020;28 doi: 10.1016/j.bmc.2020.115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsilioni I., et al. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gardell S.J., et al. Boosting NAD+ with a small molecule that activates NAMPT. Nat. Commun. 2019;10:3241. doi: 10.1038/s41467-019-11078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shimizu J.F., et al. Is the ADP ribose site of the Chikungunya virus NSP3 Macro domain a target for antiviral approaches? Acta Trop. 2020;207 doi: 10.1016/j.actatropica.2020.105490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kropotov A., et al. Equilibrative nucleoside transporters mediate the import of nicotinamide riboside and nicotinic acid riboside into human cells. Int. J. Mol. Sci. 2021;22:1391. doi: 10.3390/ijms22031391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stone N.E., et al. Stenoparib, an inhibitor of cellular poly(ADP-ribose) polymerase, blocks replication of the SARS-CoV-2 and HCoV-NL63 human coronaviruses in vitro. mBio. 2021;12 doi: 10.1128/mBio.03495-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Janssens G.E., et al. Healthy aging and muscle function are positively associated with NAD+ abundance in humans. Nat. Aging. 2022 doi: 10.1038/s43587-022-00174-3. Published online February 17, 2022. [DOI] [PubMed] [Google Scholar]