Abstract
Background
Diabetes is a risk factor for postoperative complications. Previous meta-analyses have shown that elevated glycated hemoglobin (HbA1c) levels are associated with postoperative complications in various surgical populations. However, this is the first meta-analysis to investigate the association between preoperative HbA1c levels and postoperative complications in patients undergoing elective major abdominal surgery.
Methods
PRISMA guidelines were adhered to for this study. Six databases were searched up to April 1, 2020. Primary studies investigating the effect of HbA1c levels on postoperative complications after elective major abdominal surgery were included. Risk of bias and quality of evidence assessments were performed. Data were pooled using a random effects model. Meta-regression was performed to evaluate different HbA1c cut-off values.
Results
Twelve observational studies (25,036 patients) were included. Most studies received a ‘good’ and ‘moderate quality’ score using the NOS and GRADE, respectively. Patients with a high HbA1c had a greater risk of anastomotic leaks (odds ratio [OR]: 2.80, 95% CI [1.63, 4.83], P < 0.001), wound infections (OR: 1.21, 95% CI [1.08, 1.36], P = 0.001), major complications defined as Clavien-Dindo [CD] 3–5 (OR: 2.16, 95% CI [1.54, 3.01], P < 0.001), and overall complications defined as CD 1–5 (OR: 2.12, 95% CI [1.48, 3.04], P < 0.001).
Conclusions
An HbA1c between 6% and 7% is associated with higher risks of anastomotic leaks, wound infections, major complications, and overall postoperative complications. Therefore, guidelines with an HbA1c threshold > 7% may be putting pre-optimized patients at risk. Future randomized controlled trials are needed to explore causation before policy changes are made.
Keywords: Diabetes mellitus, Elective surgical procedures, General surgery, Glycated hemoglobin A, Operative surgical procedures, Postoperative complications
Introduction
Diabetes mellitus is known to be a predisposing risk factor for postoperative complications, such as infections, poor wound healing, anastomotic leaks, and cardiac complications. Compared with non-diabetic patients, both in-hospital and long-term mortality rates are considerably higher in patients with diabetes [1]. Hence, glycemic control during the perioperative period could be a modifiable risk factor and a potential target for reducing postoperative complications.
The American Diabetes Association endorses the use of glycated hemoglobin (HbA1c) levels to monitor glycemic control in patients with diabetes [2]. This is a measure that reflects the average blood glucose level over a three-month period, providing an indirect measurement of how effectively blood glucose is controlled. Systematic reviews and meta-analyses have shown that increased levels of preoperative HbA1c are associated with higher rates of postoperative complications and poorer outcomes in surgical specialties, such as cardiothoracic [3], bariatric [4], and orthopedic surgery [5].
Major abdominal surgery, defined as a major operation involving the abdominal and/or retroperitoneal compartment, is associated with high postoperative morbidity due to the extensive nature of the surgery. Despite the clinical significance of this, no previous systematic review or meta-analysis has investigated the association between preoperative HbA1c levels and postoperative complications in this population. Furthermore, there is no consensus on the HbA1c threshold at which it would be advisable to postpone elective surgery. The Joint British Diabetes Societies for Inpatient Care and the Association of Anesthetists of Great Britain and Ireland recommend further optimization of glycemic control at an HbA1c threshold of 8.5% [6], while the US Society for Ambulatory Anesthesia recommends a threshold of 7.0% [7], and the Australian Diabetes Society recommends a threshold of 9.0% [8]. An HbA1c target set too low may be unrealistic and may delay a patient’s surgery unnecessarily, whereas an HbA1c target set too high may be inadequate in risk prognostication and in reducing postoperative complications.
Thus, there is a gap in the literature regarding the association between preoperative HbA1c levels and postoperative complications after elective major abdominal surgery despite the increasing incidence of both diabetes and abdominal surgery. The UK National Diabetes Inpatient Audit found that 21% of all surgical patients have diabetes, and general surgery (36%) and colorectal surgery (22%) are the surgical specialties with the highest prevalence [9]. Greater understanding of the association between preoperative HbA1c levels and postoperative complications after elective major abdominal surgery could therefore help with risk prognostication and perioperative management.
This is the first meta-analysis to evaluate all the available evidence regarding the association between preoperative HbA1c levels and postoperative complications in the unique population of patients undergoing elective major abdominal surgery. Furthermore, we investigated whether a threshold HbA1c level could be used to predict an increase in postoperative complications. The findings from this meta-analysis could have implications for policies in various countries, as different HbA1c cut-off thresholds are currently being used in clinical practice.
Materials and Methods
This meta-analysis has been reported in line with the PRISMA guidelines [10] and registered on PROSPERO (http://www.crd.york.ac.uk/PROSPERO, no. CRD 42020167347) [11]. A full description of the methodology has been described previously [12].
Search strategy
The following electronic databases were searched using the search strategy described in Supplementary Digital Content 1, from each database’s earliest record up to April 1, 2020: PubMed, Embase, MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Google Scholar, and China Knowledge Resource Integrated Database (CNKI).
Study selection
The study selection was performed by two independent reviewers (JKLW and YK). Discrepancies were resolved by a third reviewer (HRA). The eligibility criteria were as follows: randomized controlled trials (RCTs) and observational studies investigating the association between HbA1c levels and postoperative complications by reporting outcomes in at least two HbA1c groups in adult patients undergoing major abdominal surgery. Studies on patients undergoing bariatric, total pancreatectomy, pediatric, emergency, and transplant surgery were excluded [12].
Data collection
Data extraction was performed by two independent reviewers (JKLW and YK) and stored in proformas. The extracted data included study characteristics (author, year, country, study design, type of surgery), patient demographics (age, sex, sample size), intervention and comparator data (HbA1c cut-off value), and outcome data (postoperative complications including major, overall, gastrointestinal, infectious, cardiopulmonary, and renal complications), which were guided by the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) [13]. An exhaustive list of the extracted data items has been published previously [12]. The raw outcomes for each HbA1c level group were extracted and estimates of effects using the methods recommended by the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0.17) were calculated.
Risk of bias and quality of evidence assessment
The risk of bias for the non-randomized observational studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) [14] and converted to the Agency for Healthcare Research and Quality (AHRQ) standards (Supplementary Digital Content 2). The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach was used to grade the quality of evidence as recommended by Cochrane [15].
Data synthesis and statistical analysis
The primary and secondary aims of this meta-analysis were to investigate the associations between preoperative HbA1c levels and major and overall postoperative complications, respectively, where major complications were defined as those fulfilling the Clavien-Dindo (CD) classification grades 3–5, and overall complications were defined as those fulfilling the CD grades 1–5 [16]. Table 1 provides detailed information on the definitions of the CD classification grades 1 through 5. The corresponding primary and secondary outcomes are represented as the odds ratios (ORs) of postoperative complication events between the normal and elevated HbA1c groups.
Table 1.
Clavien-Dindo Classification Definitions
| Grades | Definition [16] |
|---|---|
| I | Any deviation from normal postoperative course without need for pharmacological treatment or surgical, endoscopic, or radiological interventions |
| Allowed therapeutic regimens: antiemetics, antipyretics, analgesics, diuretics, electrolytes, and physiotherapy | |
| This grade also includes wound infections opened at the bedside | |
| II | Requiring pharmacological treatment with drugs other than those included in the grade I complications |
| Also includes blood transfusions and total parenteral nutrition | |
| III | Requiring surgical, endoscopic, or radiological interventions |
| a: Not under general anesthesia | |
| b: Under general anesthesia | |
| IV | Life-threatening complication (including central nervous system complications)* requiring intensive care unit management |
| a: Single organ dysfunction (including dialysis) | |
| b: Multi-organ dysfunction | |
| V | Death |
Including brain hemorrhage, ischemic stroke, or subarachnoid bleeding but excluding transient ischemic attacks.
The postoperative complications extracted from the primary studies were initially graded according to the CD classification, and then grouped according to either the primary outcome (major postoperative complications) or secondary outcome (overall postoperative complications) analyses. Examples of postoperative complications that were included as major postoperative complications (primary outcome) include reoperation [17], anastomotic leak [18–23], 30-day mortality, [23] and major complications fulfilling the CD grades 3–5 [24–26]. Examples of secondary outcomes (overall complications) include anastomotic leak, postoperative ileus, overall infections, wound infections, pneumonia, sepsis, cardiopulmonary complications, and renal failure [13]. The primary and secondary outcomes were quantitatively analyzed. Qualitative analyses were conducted for outcomes reported by two or fewer studies.
Statistical analyses were performed using Stata (2019. Stata Statistical Software: Release 16. StataCorp LLC. StataCorp.). Funnel plots, Begg’s rank correlation tests, and Egger’s regression asymmetry tests were used to assess publication bias [27]. The Duval and Tweedie nonparametric trim and fill method to account for publication bias was performed to formalize the use of funnel plots and adjust the meta-analysis by incorporating theoretical missing trials [27]. The Q-statistic was used to investigate the heterogeneity between the studies. One limitation of Cochran’s Q-test is that it might be underpowered when studies in a meta-analysis have small sample sizes or low event rates. Therefore, Cochrane recommends that a higher standard be adopted to determine whether there is indeed no significant heterogeneity between the studies. Hence, a higher P value of 0.1 was used rather than the conventional 0.05 [28]. The I2 statistical test [29] was carried out to describe the proportion of total variation caused by heterogeneity [30]. An I2 < 30% was considered mild heterogeneity, > 50% as notable heterogeneity, and anything between 30% and 50% as moderate heterogeneity. However, these must be interpreted with caution, as inconsistency may not necessarily be important with low I2 values because the importance of the I2 value depends on the magnitude and direction of effects, strength of evidence for heterogeneity including the P value from the chi-squared test, and/or the confidence interval for I2 [28]. The random effects model (DerSimonian–Laird) was used to derive pool estimates to account for inter-study heterogeneity. A meta-regression was performed to evaluate the effect that different HbA1c cut-off values had on the following outcomes: major postoperative complications, overall postoperative complications, anastomotic leak, overall infections, and wound infections.
Ethics approval and consent to participate
No ethics approval or consent to participate was required, as only secondary data were used.
Results
Search results
The search yielded 2,539 records. One additional record was identified through a manual search of the bibliographies. Fifteen and twelve records met the criteria for qualitative and quantitative analyses, respectively (Fig. 1). The following three records were not included in the quantitative analysis: the study by Lee et al. [31] because it was the only study that used the outcome measures progression-free survival, cancer specific survival, and overall survival and hence could not be combined with other studies; the study by Goh et al. [25] because the authors used an HbA1c cut-off value of 8.0%, which was higher than the cut-off values used in other studies and would have confounded the quantitative analysis; and the study by Zhang [32] because the number of patients in each HbA1c level group was not reported in the study.
Fig. 1.
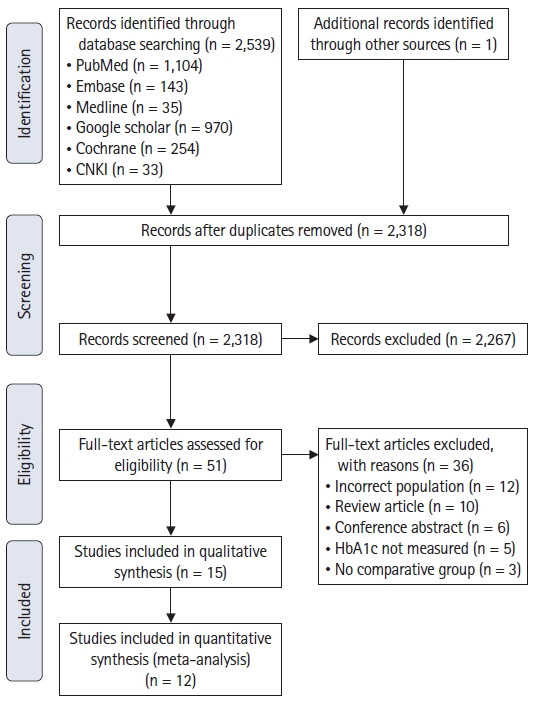
PRISMA flowchart.
Study characteristics
The study characteristics are summarized in Table 2. All the studies were conducted between 2008 and 2019. Eleven studies were conducted in Asia, two in the USA [24,33], and two in Europe [18,26]. There were no RCTs that met our inclusion criteria. Twelve studies were performed retrospectively and three employed a prospective design [18,24,26], where patients were followed up from two months [26] to four years [24]. Most studies included gastrointestinal (GI) tract surgeries [17,18,20–26,33–35], though one included esophagectomies [19], one included GI tract and hepato-pancreato-biliary surgeries [26], one included exclusively biliary surgeries [32], one included genitourinary surgeries [24], and one included exclusively genitourinary surgeries [31]. The HbA1c cut-off values used in the studies to dichotomize the case and control groups were variable. The most common cut-off values were 6.5%, which were used by five studies [19,24,26,33,34], and 7.0%, which was used in four studies [17,20,23,32]. Most studies used HbA1c levels taken within 3 months of the surgery, while one study used HbA1c levels taken within 6 months of the surgery [31]. Additionally, a few studies did not state the timeframe between the HbA1c measurements and the surgery [17,20–23,32,34]. A total of 25,036 patients were included in the quantitative analysis.
Table 2.
Study Characteristics
| Study | Country | Study design | Type of surgery | Sample size, n | HbA1c cut-off (no. of patients, percentage) | Time window between HbA1c level result and surgery | Outcome measures |
|---|---|---|---|---|---|---|---|
| Lee et al. 2015 [31] | South Korea | Retrospective | Nephrectomy (radical and partial) for renal cell carcinoma | n = 3075 | ≥ 6.8% (n = 158, 50%) | Within 6 months of the surgery | · Progression-free survival |
| 316 (75.8%) patients had recorded HbA1c levels | < 6.8% (n = 158, 50%) | · Cancer specific survival | |||||
| *HbA1c 6.8% used as cut-off point as it was the median value | · Overall survival | ||||||
| Gustafsson et al. 2009 [18] | Sweden | Prospective | Elective colorectal resection (including cancer, inflammatory bowel disease, benign pathology) | n = 120 | > 6.0% (n = 31, 25.8%) | 1 day before surgery | · Postoperative glucose control |
| ≤ 6.0% (n = 89, 74.2%) | · Magnitude of inflammatory response | ||||||
| · Postoperative recovery | |||||||
| · 30-day overall morbidity | |||||||
| Goh et al. 2017 [25] | Singapore | Retrospective | Colorectal surgery | n = 149 | ≥ 8% (n = 31, 23.8%) | Within 3 months of the surgery | · Postoperative complications (CD grade 2 and above) |
| 130 (87.2%) patients had recorded HbA1c levels | < 8% (n = 99, 76.2%) | ||||||
| Goodenough et al. 2015 [24] | USA | Prospective | *Abdominal surgery | n = 1017 | ≥ 6.5% (n = 183, 41.8%) | Within 3 months of the surgery | · Primary: Major complication CD grade 3–5 within 30 days |
| 438 (43.1%) patients had recorded HbA1c levels | < 6.5% (n = 255, 52.8%) | · Secondary: Any complication, including CD grade 1–2 | |||||
| Kamarajah et al. 2018 [26] | UK | Prospective | Gastrointestinal and hepatobiliary surgery | n = 381 | ≥ 6.5% (n = 49, 27.1%) | Within 3 months of the surgery | · Primary: 30-day complications defined by CD |
| 181 (47.5%) patients had recorded HbA1c levels | < 6.5% (n = 132, 72.9%) | · Secondary: Major complications, 30-day readmission rates, postoperative care setting | |||||
| Huang et al. 2017 [23] | China | Retrospective | Surgical resection for gastrointestinal cancer | n = 209 | ≥ 7% (n = 67, 56.8%) | Not stated | · 30-day and 180-day mortality rates |
| 118 (56.4%) patients had recorded HbA1c levels | < 7% (n = 51, 43.2%) | · Postoperative complications | |||||
| · Length of hospital stay | |||||||
| Jones et al. 2017 [33] | USA | Retrospective | Gastrointestinal surgery | n = 21541 | > 6.5% (n = 8822, 41.0%) | Within 3 months of the surgery | · Any post-operative complication |
| 5.7–6. 5% (n = 8118, 37.7%) | · Infectious complications (wound infection, pneumonia, urinary tract infection, sepsis) | ||||||
| < 5.7% (n = 4601, 21.4%) | · Post-discharge outcomes (readmission within 14 d, readmission within 30 d) | ||||||
| Villamiel et al. 2019 [17] | Philippines | Retrospective | Elective colorectal surgery | n = 157 | > 7% (n = 15, 34.1%) | Not stated | · Primary: Length of hospital stay |
| 44 (28%) patients had recorded HbA1c levels | ≤ 7% (n = 29, 65.9%) | · Secondary: Discharge within 30 postoperative days, postoperative complications, reoperation, pneumonia, wound infection | |||||
| Okamura et al. 2017 [19] | Japan | Retrospective | Esophagectomy for esophageal cancer | n = 300 | ≥ 6.5% (n = 27, 9%) | Within 3 months of the surgery | · Anastomotic leak |
| 6.0–6.4% (n = 50, 16.7%) | |||||||
| < 6.0% (n = 223, 74.3%) | |||||||
| Oh et al. 2018 [35] | South Korea | Retrospective | Elective major laparoscopic abdominal surgery | n = 1885 | ≥ 6.0% (n = 628, 33.3%) | Within 1 month of the surgery | · Acute kidney injury (post-operative day 0–3, stage 1–3) |
| < 6.0% (n = 1257, 66.7%) | |||||||
| Chen et al. 2018 [21] | China | Retrospective | Colorectal surgery | n = 126 | > 6.3%, (n = 67, 53.2%) | Not stated | · Anastomotic leak |
| ≤ 6.3% (n = 59, 46.8%) | |||||||
| Zhou et al. 2019 [34] | China | Retrospective | Colorectal and upper gastrointestinal surgery | n = 118 | 7–8% (n = 27, 22.9%) | Not stated | · Postoperative delirium |
| 6.5 ≤ 7% (n = 27, 22.9%) | |||||||
| 5.7 ≤ 6.5% (n = 34, 28.8%) | |||||||
| < 5.7% (n = 30, 25.4%) | |||||||
| Dai et al. 2017 [20] | China | Retrospective | Colorectal surgery | n = 201 | > 7% (n = 112, 55.7%) | Not stated | · Anastomotic leak |
| ≤ 7% (n = 89, 44.3%) | · Length of stay | ||||||
| · Duration of surgery | |||||||
| · Major intra-operative bleeding | |||||||
| · Infections | |||||||
| · Acute myocardial infarction | |||||||
| Zhang et al. 2008 [32] | China | Retrospective | Cholecystectomy | n = 86 | > 7.0 | Not stated | · Anastomotic leak |
| < 7.0 | · Infections | ||||||
| Number of patients per group not reported | |||||||
| Wang et al. 2010 [22] | China | Retrospective | Gastrointestinal tumor surgery | n = 82 | < 6.2 (n = 47, 79.7%) | Not stated | · Bloatedness |
| ≥ 6.2 (n = 35, 42.7%) | · Nausea and vomiting | ||||||
| · Anastomotic leak | |||||||
| · Time to flatus | |||||||
| · Length of hospital stay |
Included four gynecological procedures that constituted only 0.7% of the total number of surgeries.
The most studied outcomes were infections [17,18,20,22,23,32,33] and anastomotic leaks [18–23,32]. Some studies investigated the individual effects of different types of infections, such as pneumonia, urinary tract infections, wound infections, and sepsis [17,18,23,33], while others only investigated the collective effect of all infections [20,22,32]. A few studies only investigated total postoperative complications according to the CD classification [24–26].
Risk of bias and quality of evidence assessment
According to the risk of bias assessment, all studies scored at least a 7/9 on the NOS, which equates to a “good quality” score after conversion to the AHRQ standards. Only one was graded as “poor quality.” Most studies lost points in the selection and outcome parameters (Supplementary Digital Content 3). According to the quality of evidence assessment using the GRADE, two outcome parameters were “moderate” in quality and the remaining four were “low” in quality (Table 3).
Table 3.
GRADE Evidence Profile
| Quality assessment | No. of patients | Relative Effect (95% CI) | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Elevated HbA1c | Normal HbA1c | ||
| Major postoperative complications | ||||||||||
| 9 | Observational studies | Moderate | - | - | - | - | 586 | 1024 | OR 2.16 (1.54, 3.01) | ⊕⊕⊕⊖ |
| Moderate | ||||||||||
| Overall postoperative complications | ||||||||||
| 12 | Observational studies | Moderate | - | - | - | Large effect size | 10063 | 15030 | OR 2.12 (1.48, 3.04) | ⊕⊕⊕⊖ |
| Moderate | ||||||||||
| Anastomotic leak | ||||||||||
| 6 | Observational studies | Moderate | - | - | - | - | 339 | 608 | OR 2.80 (1.63, 4.83) | ⊕⊕⊖⊖ |
| Low | ||||||||||
| Overall infections | ||||||||||
| 6 | Observational studies | Moderate | - | - | Serious | 9082 | 13024 | OR 1.69 (1.05, 2.71) | ⊕⊕⊖⊖ | |
| Low | ||||||||||
| Wound infections | ||||||||||
| 3 | Observational studies | Moderate | - | - | Serious | 8920 | 12859 | OR 1.21 (1.08, 1.36) | ⊕⊕⊖⊖ | |
| Low | ||||||||||
| Pneumonia | ||||||||||
| 4 | Observational studies | Moderate | Serious | - | Serious | - | 8935 | 12888 | OR 0.77 (0.61, 0.97) | ⊕⊕⊖⊖ |
| Low | ||||||||||
OR: odds ratio.
Major postoperative complications
Nine studies were included in this analysis [17–24,26]. Neither Begg’s rank correlation test (P = 0.175) nor Egger’s regression asymmetry test (P = 0.565) showed significant publication bias in our meta-analysis, which was consistent with the funnel plots (Supplementary Digital Content 4). Neither the Q-statistic nor the I2-statistic showed heterogeneity among the included studies (P = 0.711, I2 = 0%). The pooled results showed that the patients with an elevated HbA1c level tended to have a higher risk of developing major complications after surgery (OR: 2.16, 95% CI [1.54, 3.01], P < 0.001) (Fig. 2).
Fig. 2.
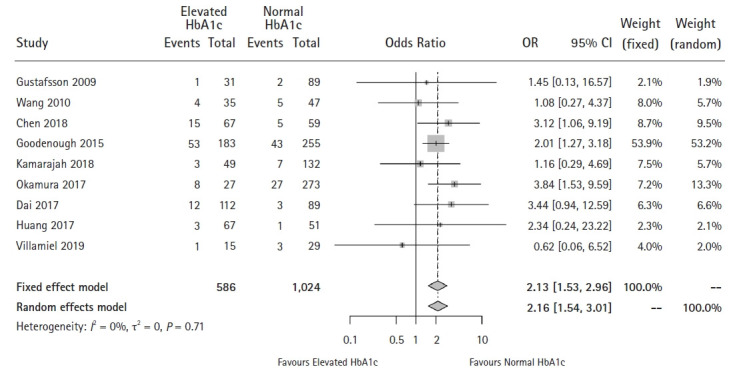
Forest plot of the effect of HbA1c level on major postoperative complications (P < 0.001).
Overall postoperative complications
Among the 12 studies reporting overall complications [17–24,26,33-35], Wang et al. [22] reported on anastomotic leak and postoperative infection data separately. We included this study in our primary analysis and conducted a sensitivity analysis with the excluded study to ensure that patients with complications were not counted twice, as we were unable to obtain the original patient-level data from the authors.
For the primary analysis, with Wang et al. ’s postoperative infection data included [22], Egger’s test for small-study effects found significant publication bias (P = 0.001), which was consistent with the funnel plots (Supplementary Digital Content 5). The Q-statistic and I2-statistic results showed heterogeneity among the studies (P < 0.001, I2 = 75.6%). Pooled results showed that patients with an elevated HbA1c level tended to have a higher risk of developing overall complications (CD grade ≥ 1) after surgery (OR: 2.12, 95% CI [1.48, 3.04], P < 0.001) (Fig. 3). The Duval and Tweedie nonparametric trim and fill method adopted to adjust for publication bias, and the meta-analysis using the trim and fill method resulted in similar conclusions. The sensitivity analysis also showed similar conclusions (OR: 2.00, 95% CI [1.41, 2.85]). The funnel and forest plots are shown in Supplementary Digital Content 6.
Fig. 3.
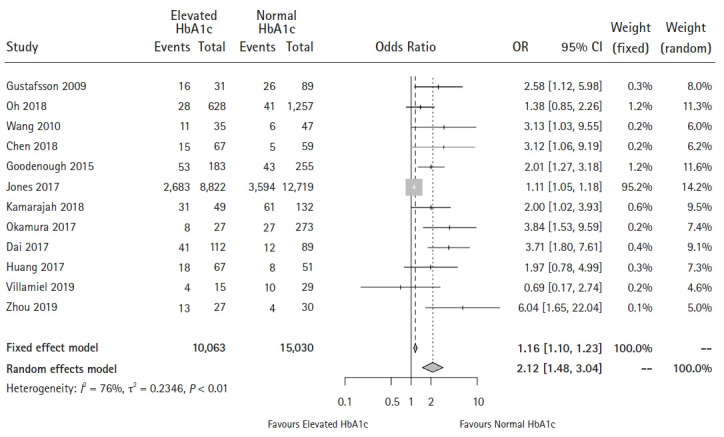
Forest plot of the effect of HbA1c level on overall complications (P < 0.001).
Gastrointestinal complications
Anastomotic leak
Six studies were included in this analysis [18–23]. No significant publication bias was found using Egger’s test for small-study effects (P = 0.401) or Begg’s rank correlation test (P = 0.452) (Supplementary Digital Content 7). Neither the Q-statistic nor the I2-statistic showed significant heterogeneity between the studies (P = 0.600, I2 = 0%). Pooled results showed that patients with elevated HbA1c levels tended to have a higher risk of developing anastomotic leaks (OR: 2.80, 95% CI [1.63, 4.83], P < 0.001) (Fig. 4).
Fig. 4.
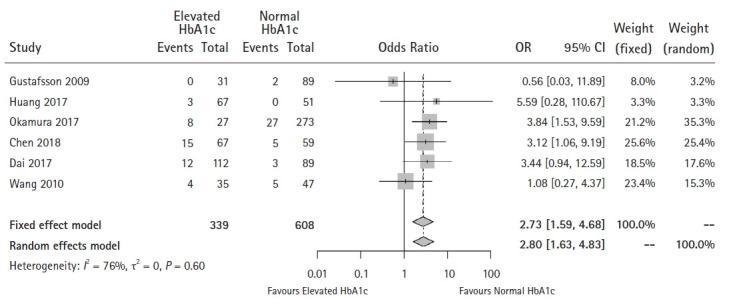
Forest plot of the effect of HbA1c level on anastomotic leak (P < 0.001).
Postoperative ileus
Only two studies investigated the impact of HbA1c levels on postoperative ileus [18,23]. Gustafsson et al. [18] found that the rate of events was 9.7% in the HbA1c level > 6% group and 1.1% in the HbA1c level ≤ 6% group. Although the rate of events in the HbA1c level > 6% group was higher, the significance was not reported. When an HbA1c cut-off of 7% was used, Huang et al. [23] found no difference in the rate of postoperative ileus between the HbA1c groups (P = 0.284).
Infectious complications
Overall infections
This analysis included six studies [17,18,20,22,23,33]. Egger’s test for small-study effects found significant publication bias (P = 0.038) (Supplementary Digital Content 8). Although the Q-statistic did not show significant heterogeneity among studies (P = 0.113), the I2-statistic found moderate heterogeneity (I2 = 43.8%). The pooled results showed that patients with an elevated HbA1c level tended to have a higher risk of developing infections (OR: 1.69, 95% CI [1.05, 2.71]) (Fig. 5). However, the meta-analysis using the trim and fill method showed this effect to be insignificant (OR: 1.18, 95% CI [0.77, 1.82]).
Fig. 5.
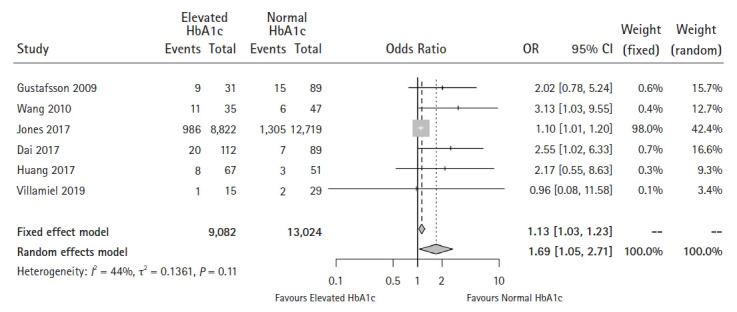
Forest plot of the effect of HbA1c level on overall infections (P = 0.031).
Wound infection
Three studies were included in this analysis [18,23,33]. No significant publication bias was found using either Egger’s test for small-study effects (P = 0.947) or Begg’s rank correlation test (P = 1.000). However, the funnel plot showed different results (Supplementary Digital Content 9). No significant heterogeneity was found among the studies using either the Q-statistic nor the I2-statistic (P = 0.757, I2 = 0%). Pooled results showed that patients with an elevated HbA1c level tended to have a higher risk of developing wound infections (OR 1.21, 95% CI 1.08–1.36, P = 0.001) (Fig. 6). The meta-analysis using the trim and fill method did not alter this conclusion.
Fig. 6.
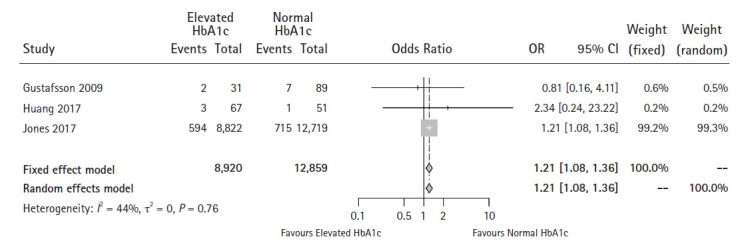
Forest plot of the effect of HbA1c level on wound infections (P = 0.001).
Pneumonia
Four studies were included in this analysis [17,18,23,33]. No significant publication bias was found using Egger’s test for small-study effects (P = 0.385) or Begg’s rank correlation test (P = 1.000) in our meta-analysis. However, the funnel plots showed different results. No significant heterogeneity between the studies was found using the Q-statistic or the I2-statistic (P = 0.424, I2 = 0%). The pooled results showed that patients with an elevated HbA1c level tended to have a lower risk of developing pneumonia after surgery (OR: 0.77, 95% CI [0.61, 0.97]). However, this effect became insignificant (OR: 0.74, 95% CI [0.44, 1.25], P = 0.026) when the trim and fill method was used to adjust for publication bias (Supplementary Digital Content 10).
Sepsis
Only two studies reported outcomes on postoperative sepsis [18,33]. Gustafsson et al. [18] found an event rate of 0% in the HbA1c level > 6% group and 1.1% in the HbA1c level ≤ 6% group. However, the significance was not reported. Jones et al. [33] used three HbA1c cut-off values: < 5.7%, 5.7–6.4% and ≥ 6.5%. There was no significant difference in the event rates between the three groups (P = 0.80). Using theHbA1c level < 5.7% group as the reference group, no differences in the adjusted OR were found between any of the groups in either study.
Cardiopulmonary complications
Only two studies reported cardiopulmonary complications [18,20]. Although the complication rates for respiratory failure, pleural fluid, cardiac failure, and cardiac arrhythmia were reported, no P values were reported by Gustafsson et al. [18] For acute myocardial infarctions, Dai et al. [20] reported an event rate of 8% in the HbA1c level > 7% group and 2.2% in the HbA1c level < 7% group (P < 0.05).
Renal complications
Only one study reported acute kidney injury (AKI) events postoperatively. Oh et al. [35] measured the association between an HbA1c cut-off value of 6% and the AKI stage (The Kidney Disease: Improving Global Outcomes or KDIGO staging) and the total number of AKI events. There was no difference for any of the AKI stages between the groups with an HbA1c level < 6% or ≥ 6% (P > 0.05). Similarly, for the total number of AKI events, there was no difference between the groups (OR:1.38, 95% CI [0.85, 2.26], P = 0.194).
Meta-regression
For the range of HbA1c cut-off values between 5.7% and 7.0%, there were no statistically significant effects on the development of major postoperative complications, overall postoperative complications, anastomotic leaks, overall infections, or wound infections (all P > 0.05). Bubble plots of the meta-regressions are presented in Supplementary Digital Content 11.
Discussion
Results from our meta-analysis showed that elevated HbA1c (> 6–7%) was associated with a higher risk of anastomotic leaks, wound infections, major postoperative complications (CD grades 3–5) and overall postoperative complications (CD grades 1–5), but not with overall infections and pneumonia.
The most important finding from this meta-analysis was that elevated HbA1c levels are associated with a higher risk of anastomotic leaks. This is an important observation as anastomotic leaks are one of the most serious complications associated with gastrointestinal surgery, resulting in a mortality rate as high as 16.4% and long hospital and intensive care unit admissions [36]. Another important finding was that wound infections were the only type of infection associated with elevated HbA1c levels. Taken together, these results indicate that elevated HbA1c levels may be an indicator of impairment in wound healing physiology. Impaired glucose tolerance causes both macrovascular and microvascular complications, which may result in inadequate angiogenesis and decreased perfusion to the wound site [37] as well as poorer immune function [38]. These results are consistent with previous findings regarding different types of surgeries with various levels of evidence [3,39,40]. If a target HbA1c level was set preoperatively for patients undergoing elective surgery, the risk of anastomotic leaks and wound infections could be markedly reduced.
Our meta-analysis also found that lower HbA1c levels are not only associated with a lower risk of major postoperative complications (CD grade 3–5), but also with a lower risk of overall postoperative complications (CD grade 1–5). This has significant implications as it suggests that postponing elective surgery until an optimal HbA1c level is achieved may reduce the risk of both major and overall postoperative complications that negatively affect patients’ quality of life after surgery. These findings may also facilitate counseling during preoperative assessments to motivate patients to make lifestyle modifications and improve medication adherence.
It should be noted that a significant association between preoperative HbA1c levels and the risk of overall infections and pneumonia was not found in our pooled results. These findings were not consistent with a well-cited study by Dronge et al. [41], who showed that a HbA1c cut-off value of 7% was significantly associated with lower postoperative infection risks in the major non-cardiac surgical population (which also included non-abdominal surgeries). This inconsistency could be explained by the different HbA1c cut-off values used, as Dronge et al. used a cut-off of 7%, while our study accepted a range between 6% and 7%. This may suggest that an HbA1c level of 6% may be too low for making prognoses regarding postoperative infections.
Regarding the rationales for excluding certain populations, patients undergoing pancreatic and bariatric surgery were excluded from this meta-analysis because the postoperative glucose metabolism in these patients is different from that in patients undergoing other types of abdominal surgeries [42,43]. As perioperative glucose control has been demonstrated to be an independent predictor of postoperative complications [44], we determined it would be unfair to group pancreatic and bariatric surgery patients with other non-pancreatic and non-bariatric patients undergoing surgery. Patients undergoing emergency surgery were also excluded because this patient population is different from that undergoing elective surgery, as these patients are by default subject to higher postoperative complications due to the nature of the surgery (e.g., unprepared bowel, fecal contamination, hemodynamic instability, sepsis). Additionally, preoperative HbA1c optimization is impossible in patients undergoing emergency surgery due to the lack of a preoperative period. Finally, transplant patients were excluded because the nature of transplant surgery is unique to that of major abdominal surgery, as defined in our Methods sections.
The main strength of this study is that this is the first meta-analysis investigating the association between preoperative HbA1c levels and postoperative complications exclusively in the elective major abdominal surgery population, as the majority of previous meta-analyses have been conducted on cardiac, bariatric, and orthopedic populations [3-5]. Another strength is our inclusion of the Chinese database CNKI, which helped to ensure an extensive search of the available literature, as the database has grown significantly in the past decade. Furthermore, the inclusion of the CNKI also ensures ethnic diversity and representation.
This meta-analysis has some limitations. Some studies that met the inclusion criteria of abdominal surgery had to be excluded since they also included non-abdominal surgeries, and we were unable to attain the data on abdominal surgeries separately. To overcome this limitation, we applied the Duval and Tweedie nonparametric trim and fill method to adjust the meta-analysis by incorporating theoretical missing trials. Some studies categorized patients according to their diabetes diagnosis status instead of their HbA1c status, and not everyone who had a diabetes diagnosis had an elevated HbA1c level. To adjust for this, we only included patients with HbA1c levels available and categorized them according to their HbA1c status. Another limitation was the inclusion of studies that used different HbA1c cut-off points. For this reason, we have provided a conservative conclusion that an HbA1c level > 6–7% is associated with higher risk of postoperative complications. Additionally, it was not possible to perform subgroup analyses, although these are crucial, accounting for the fact that some of the included patients had comorbidities such as cancer and patient-level data for these factors were unavailable. While this is a possible limitation, for diabetes optimization, HbA1c levels also allow for an attempt to optimize the preoperative phase similar to how we optimize pre-operative patients at high risk of malnutrition (for example, patients with gastrointestinal cancers).
The main implication of this study is to guide future RCTs. Our findings suggest that an elevated HbA1c level of 6–7% may be associated with a higher risk of postoperative complications. Currently, only the US guidelines recommend a target HbA1c of 7% [7], while the Great Britain and Australian guidelines recommend a target HbA1c of 8.5% and 9%, respectively [6,8]. Our findings may suggest that under the current guidelines, patients are undergoing elective surgery pre-optimized and would thus not have the best chance of being complication-free postoperatively. These implications should be considered with caution, however, as an association should not be mistaken for causation. Conducting an RCT to determine causation in the relationship between HbA1c levels and postoperative complications is necessary to determine if changes to the current guidelines are warranted. However, we accept that there are challenges in conducting RCTs in this field. Many elective major abdominal surgery operations are undertaken for cancer resection and are therefore urgent cases that do not allow sufficient time for the pre-optimization of HbA1c levels. Future studies should also investigate the specific HbA1c cut-off value that is associated with an increase in complications for different types of surgeries using a receiver-operating characteristic (ROC) analysis design.
In conclusion, the findings from our meta-analysis show that elevated HbA1c levels are associated with a higher risk of developing anastomotic leaks, wound infections, and major and overall postoperative complications, but not overall infections and pneumonia. This implies that patients fare better postoperatively if a target HbA1c level ≤ 7% is set before undergoing elective major abdominal surgery. Our findings can help to guide future RCTs to determine if current guidelines on the recommended cut-off values for HbA1c levels should be reviewed, as the HbA1c thresholds currently used in clinical practice are all above 7%. Further studies using ROC analyses to investigate the exact HbA1c cut-off value associated with an increase in postoperative complications should also be performed.
Footnotes
Funding
This work was supported by the funding department of the Department of Anesthesiology, Singapore General Hospital, Singapore. H.R.A. is a recipient of the SingHealth Duke-NUS Nurturing Clinician Scientists Scheme Award (project number 12/FY2017/P1/15-A29) and the National Medical Research Council (NMRC), Singapore, Clinician Investigator Salary Support scheme 2018–2020. The funding sources played no role in the design of this study or the analysis and interpretation of the results.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Joanna K. L. Wong (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft)
Yuhe Ke (Data curation; Formal analysis; Investigation; Methodology; Writing – original draft)
Yi Jing Ong (Data curation; Formal analysis; Investigation; Methodology; Writing – original draft)
HuiHua Li (Formal analysis; Investigation; Methodology; Writing – original draft)
Ting Hway Wong (Investigation; Methodology; Writing – review & editing)
Hairil Rizal Abdullah (Conceptualization; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing)
Supplementary Materials
Search strategy.
Thresholds for converting the Newcastle-Ottawa scales to AHRQ standards.
Newcastle-Ottawa Scale for Risk of Bias Assessment of Studies
Funnel plot of major postoperative complications.
Funnel plot of overall complications (CD1 and above, using Wang et al’s data on postoperative infections).
(A) Funnel plot and (B) forest plot of overall complications (CD1 and above, using Wang et al’s data on anastomotic leak) (P < 0.001).
Funnel plot of all anastomotic leaks.
Funnel plot of all infectious complications.
Funnel plot of all wound infections.
(A) Funnel plot and (B) forest plot of all pneumonia (P = 0.026).
Bubble plots displaying meta-regression for (A) Major postoperative complications (CD3-5), (B) Overall postoperative complications (CD1-5), (C) Anastomotic leaks, (D) Overall infections, and (E) Wound infections.
References
- 1.Krolikowska M, Kataja M, Poyhia R, Drzewoski J, Hynynen M. Mortality in diabetic patients undergoing non-cardiac surgery: a 7-year follow-up study. Acta Anaesthesiol Scand. 2009;53:749–58. doi: 10.1111/j.1399-6576.2009.01963.x. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes--2011. Diabetes Care. 2011;34 Suppl 1(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biancari F, Giordano S. Glycated hemoglobin and the risk of sternal wound infection after adult cardiac surgery: a systematic review and meta-analysis. Semin Thorac Cardiovasc Surg. 2019;31:465–7. doi: 10.1053/j.semtcvs.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Chow A, Switzer NJ, Dang J, Shi X, de Gara C, Birch DW, et al. A systematic review and meta-analysis of outcomes for type 1 diabetes after bariatric surgery. J Obes. 2016;2016:6170719. doi: 10.1155/2016/6170719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shohat N, Muhsen K, Gilat R, Rondon AJ, Chen AF, Parvizi J. Inadequate glycemic control is associated with increased surgical site infection in total joint arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2018;33:2312–21. doi: 10.1016/j.arth.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Dhatariya K, Levy N, Kilvert A, Watson B, Cousins D, Flanagan D, et al. NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med. 2012;29:420–33. doi: 10.1111/j.1464-5491.2012.03582.x. [DOI] [PubMed] [Google Scholar]
- 7.Joshi GP, Chung F, Vann MA, Ahmad S, Gan TJ, Goulson DT, et al. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111:1378–87. doi: 10.1213/ANE.0b013e3181f9c288. [DOI] [PubMed] [Google Scholar]
- 8.Australian Diabetes Society Peri-operative Diabetes Management Guidelines [Internet] Sydney: ADS; 2012 Jul [cited 2021 Jul 2]. Available from https://diabetessociety.com.au/documents/PerioperativeDiabetesManagementGuidelinesFINALCleanJuly2012.pdf.
- 9.National Health Service National Diabetes Inpatient Audit (NaDIA), Open data-2013 [Internet] Wales: NHS; 2014 Jun 26 [cited 2021 Jul 2]. Available from http://www.hscic.gov.uk/catalogue/PUB14358.
- 10.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong JK, Ke Y, Ong Y, Abdullah HR. The impact of preoperative Hba1c on postoperative complications in major abdominal surgery PROSPERO 2020 [Internet] London: National Institute for Health Research; 2020 Apr 23 [cited 2021 Jul 2]. Available from https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=167347.
- 12.Wong JK, Ke Y, Ong YJ, Li HH, Abdullah HR. Impact of preoperative HbA1c on postoperative complications after elective major abdominal surgery: a systematic review protocol. BMJ Open. 2020;10:e039422. doi: 10.1136/bmjopen-2020-039422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Surgeons ACS National Surgical Quality Improvement Program [Internet] Chicago: ACS; 2020 [cited 2021 Jul 2]. Available from https://www.facs.org/quality-programs/acs-nsqip.
- 14.Wells G, Sheah B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] 2000 [cited 2021 Jul 2]. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook [Internet] London: Cochrane; 2013 Oct [cited 2021 Jul 2]. Available from https://training.cochrane.org/resource/grade-handbook.
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Festejo Villamiel KM, Yao C, Sioson M. Enhanced recovery after surgery (ERAS) outcomes in patients with prior diagnosis of diabetes. J ASEAN Fed Endocr Soc. 2019;34:73–9. doi: 10.15605/jafes.034.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafsson UO, Thorell A, Soop M, Ljungqvist O, Nygren J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg. 2009;96:1358–64. doi: 10.1002/bjs.6724. [DOI] [PubMed] [Google Scholar]
- 19.Okamura A, Watanabe M, Imamura Y, Kamiya S, Yamashita K, Kurogochi T, et al. Preoperative glycosylated hemoglobin levels predict anastomotic leak after esophagectomy with cervical esophagogastric anastomosis. World J Surg. 2017;41:200–7. doi: 10.1007/s00268-016-3763-z. [DOI] [PubMed] [Google Scholar]
- 20.Dai Z, Yang Y, Liu Y. Preoperative HbA1C and its effect on post operative complications in colorectal surgery patients. People’s Mil Surg. 2017;60:875–8. [Google Scholar]
- 21.Chen S, Cha IR, Tu S, Wan Z, Chen B. Association of postoperative anastomotic leak with preoperative level of glycosylated hemoglobin in elderly patients with colorectal cancer and type 2 diabetes mellitus. Chin J Heal Lab Tec. 2018;28:2632–5. [Google Scholar]
- 22.Wang Q, Cao W, Zhao Y. The correlations between intensive glucose lowering and the post-operative complications in the diabetic patients underwent curative surgery for gastrointestinal tumor. Pract J Cancer. 2010;25:381–3. [Google Scholar]
- 23.Huang Y, Zheng H, Chen P, Yang J, Lin S, Liu T, et al. An elevated HbA1c level is associated with short-term adverse outcomes in patients with gastrointestinal cancer and type 2 diabetes mellitus. J Clin Med Res. 2017;9:303–9. doi: 10.14740/jocmr2607w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodenough CJ, Liang MK, Nguyen MT, Nguyen DH, Holihan JL, Alawadi ZM, et al. Preoperative glycosylated hemoglobin and postoperative glucose together predict major complications after abdominal surgery. J Am Coll Surg. 2015;221:854–61. doi: 10.1016/j.jamcollsurg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Goh SN, Yeoh E, Tan KY. Impact of perioperative hypoglycaemia in subjects with diabetes undergoing colorectal surgery. Int J Colorectal Dis. 2017;32:209–14. doi: 10.1007/s00384-016-2680-9. [DOI] [PubMed] [Google Scholar]
- 26.Kamarajah SK, Adlan A, Barmayehvar B, Sowida M, Reihill C, Ellahee P. Preoperative glycosylated haemoglobin (HbA1c) does impact on postoperative complications in patients undergoing gastrointestinal and hepatobiliary surgery. Asian J Anesthesiol. 2018;56:83–91. doi: 10.6859/aja.201809_56(3).0003. [DOI] [PubMed] [Google Scholar]
- 27.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: John Wiley & Sons Ltd; 2000. [Google Scholar]
- 28.Cochrane Identifying and measuring heterogeneity [Internet] London: Cochrane; 2020 [cited 2021 Jul 2]. Available from https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm.
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17:841–56. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Lee H, Kwak C, Kim HH, Byun SS, Lee SE, Hong SK. Diabetes mellitus as an independent predictor of survival of patients surgically treated for renal cell carcinoma: a propensity score matching study. J Urol. 2015;194:1554–60. doi: 10.1016/j.juro.2015.05.097. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M. The effects of HbA1C in post operative complications of cholecystectomy. J Chang Med Coll. 2008;22:427–8. [Google Scholar]
- 33.Jones CE, Graham LA, Morris MS, Richman JS, Hollis RH, Wahl TS, et al. Association between preoperative hemoglobin A1c levels, postoperative hyperglycemia, and readmissions following gastrointestinal surgery. JAMA Surg. 2017;152:1031–8. doi: 10.1001/jamasurg.2017.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Zhang Z, Zhou B. Relationship between postoperative delirium and preoperative HbA1c level in elderly surgical patients. Jiangsu Med J. 2019;45:1125. [Google Scholar]
- 35.Oh TK, Han S, Oh AY, Kim S, Ryu JH. Chronic hyperglycemia with elevated glycated hemoglobin level and its association with postoperative acute kidney injury after a major laparoscopic abdominal surgery in diabetes patients. J Anesth. 2018;32:740–7. doi: 10.1007/s00540-018-2551-3. [DOI] [PubMed] [Google Scholar]
- 36.Li YW, Lian P, Huang B, Zheng HT, Wang MH, Gu WL, et al. Very early colorectal anastomotic leakage within 5 post-operative days: a more severe subtype needs relaparatomy. Sci Rep. 2017;7:39936. doi: 10.1038/srep39936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–22. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin ET, Kaye KS, Knott C, Nguyen H, Santarossa M, Evans R, et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37:88–99. doi: 10.1017/ice.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang S, Han H, Yoon KS. Preoperative HbA1c is a good marker for predicting wound complications after TKA in diabetics. Orthop Proc. 2018;92-B:135. [Google Scholar]
- 41.Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141:375–80. doi: 10.1001/archsurg.141.4.375. [DOI] [PubMed] [Google Scholar]
- 42.Wu JM, Ho TW, Yang CY, Lee PH, Tien YW. Changes in glucose metabolism after distal pancreatectomy: a nationwide database study. Oncotarget. 2018;9:11100–8. doi: 10.18632/oncotarget.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith EP, Polanco G, Yaqub A, Salehi M. Altered glucose metabolism after bariatric surgery: What’s GLP-1 got to do with it? Metabolism. 2018;83:159–66. doi: 10.1016/j.metabol.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Pomposelli JJ, Baxter JK 3rd, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy.
Thresholds for converting the Newcastle-Ottawa scales to AHRQ standards.
Newcastle-Ottawa Scale for Risk of Bias Assessment of Studies
Funnel plot of major postoperative complications.
Funnel plot of overall complications (CD1 and above, using Wang et al’s data on postoperative infections).
(A) Funnel plot and (B) forest plot of overall complications (CD1 and above, using Wang et al’s data on anastomotic leak) (P < 0.001).
Funnel plot of all anastomotic leaks.
Funnel plot of all infectious complications.
Funnel plot of all wound infections.
(A) Funnel plot and (B) forest plot of all pneumonia (P = 0.026).
Bubble plots displaying meta-regression for (A) Major postoperative complications (CD3-5), (B) Overall postoperative complications (CD1-5), (C) Anastomotic leaks, (D) Overall infections, and (E) Wound infections.


