Abstract
Objective:
To evaluate research retention of older minority women with urinary incontinence (UI) using a community-based participatory research (CBPR) versus a traditional research approach.
Methods:
An ancillary prospective study was conducted within an ongoing pilot randomized clinical trial (RCT) to treat UI. Participants were recruited using CBPR in collaboration with a local community versus a traditional research approach at an academic center. Inclusion criteria were women ≥65 years and symptomatic UI. The primary outcome was the randomization rate defined as the proportion of women randomized into the RCT out of screened participants. Screening and consent rates were also evaluated. Pearson Chi- square, Fisher’s Exact, and T-tests were utilized. The effect of CBPR on research retention rates was expressed as odds ratio (OR) with 95% Confidence Intervals (CI).
Results:
There were 10 and 88 women screened in CBPR and traditional research groups, respectively. CBPR participants were Hispanic (n=10, 100%) and older (78.4 ± 8.3 years, p<0.01). Majority of traditional research participants were non-Hispanic Black (n=55, 62.5%) and younger (71.0 ± 4.9 years). The CBPR group had higher rates of screening (76.9% versus 40.6%, p=0.01), consent (80% versus 44.3%, p=0.045), and randomization (50.0% versus 14.8%, p<0.01) compared to the traditional research group. CBPR increased the odds of research retention during screening (OR=4.9, 95% CI 1.3-18.2), consent (OR= 5.0, 95% CI=1.0-25.0), and randomization (OR=5.8, 95% CI 1.5-22.7).
Conclusion:
Compared to traditional research, CBPR yielded higher research retention among older minority women with UI in a clinical study.
Keywords: Urinary Incontinence, Community-Based Participatory Research, Racial and Ethnic Minority Disparity, Research Recruitment
Introduction
Research on racial and ethnic disparities in healthcare has grown significantly in the previous two decades.1 However, racial and ethnic minority participation in clinical research remains low. The pervasive under-representation of minorities in clinical research is concerning given the United States (U.S.) national mandate that requires all National Institutes of Health (NIH) funded clinical trials to include women and minority participants.2,3 Published reports reveal the most common barriers to clinical research participation among African Americans and Hispanics including issues concerning trust, experimentation, communication, and logistics.4,5 These barriers result in the paucity of scientific data, recommendations, and health policies specific to racial and ethnic minorities, thus, further perpetuating health disparities and inequities in these populations.
Community-based participatory research (CBPR) may provide an ideal recruitment strategy to enroll racial and ethnic minority women. 6-8 CBPR is defined as research focusing on active involvement of community members, organizational leaders, and researchers in all research aspects. CBPR increases research participation through its main principles such as building on the strengths and resources of the community, promoting co-learning among research partners, and achieving a balance that mutually benefits both science and the community. 8-9
Urinary incontinence (UI) is the most common pelvic floor disorder with some reports suggesting higher UI rates in Hispanic compared to White and Black women.10-14 Nonetheless, older racial and ethnic minority women are particularly underrepresented in UI research.15-18 The American College of Obstetrics and Gynecology recently emphasized the need to address racial and ethnic disparities in women’s health and urged physician-scientists to identify and evaluate factors that contribute to these disparities in gynecologic care.19 Therefore, the overarching aim of this study was to assess the effectiveness of a CBPR approach to increase research retention among older minority women in a UI clinical trial. Accordingly, it was hypothesized that a CBPR approach would yield higher rates of randomized participants compared to a traditional research approach at an academic medical center. A secondary objective was to examine barriers to research participation among racial and ethnic minority women in both groups.
Methods
This ancillary prospective pilot study was conducted within an ongoing pilot randomized clinical trial (RCT) to treat UI: Functional Assessment and Muscle Evaluation through Exercise (FAME) Trial (NCT03166150, clinicaltrials.gov).20 The protocol for the current study was approved by the Institutional Review Board (IRB) at Howard University Hospital (HUH) IRB (#18-MED-21). Eligible participants were enrolled from February 2019 through March 2020, when recruitment was suspended due to COVID-19. Inclusion criteria included women 65 years or older and reported symptomatic UI within 3 months with confirmed UI episodes on three-day bladder diary.
Participants were screened during 30-45-minute telephone interviews, and once preliminary inclusion criteria were confirmed at an in-person study visit, participants signed the consent form with research staff. After consenting, eligible participants completed FAME questionnaires, a bladder diary, and a physical examination. Exclusion criteria to participate in FAME were inability to follow up or complete a bladder diary, cognitive impairment (mini-mental state evaluation score <25), uncontrolled medical comorbidities, and UI associated with hematuria, urinary tract infection, fistula, pelvic organ prolapse > stage 2, and fecal impaction.
Compared to traditional research approach, the experimental CBPR intended to partner with community members to overcome most common barriers to clinical research participation among African Americans and Hispanics by establishing trust, acceptance, and research engagement of community members.4,5 More specifically, the experimental approach consisted of working in partnership with a community-based organization, the National Hispanic Council on Aging (NHCOA), and its local community, Casa Iris, to increase research awareness and to culturally adapt research protocol and recruitment efforts so that they were tailored to the local population. NHCOA and Casa Iris staff underwent necessary clinical research training to participate in CBPR. NHCOA is the leading national organization working to improve the lives of older Hispanic adults and headquartered near Howard University.21 Casa Iris is a non-profit senior, housing, non-nursing home facility with 46 tenants (75% women) managed by NHCOA and located near Howard University.21 All CBPR participants were recruited at Casa Iris, mainly a Hispanic community, therefore, it was anticipated that CBPR group would predominately consist of older Hispanic women. Casa Iris and NHCOA staff were essential in the study design, recruitment, and study-related activities implementation, providing information directly to potential participants, and establishing trusting relationships with potential participants. Study documents (consents, instructions, bladder diary, and questionnaires) were translated into Spanish with the assistance of NHCOA staff and Department of World Languages and Cultures at Howard University. Moreover, participants had access to Spanish-speaking research staff. Adapted study advertisements were distributed by NHCOA staff at various health fairs, and personal referrals were made to potential participants. The principal investigator conducted several educational sessions about UI, its negative impact on quality of life, and treatment options including the ongoing UI RCT. Lastly, the RCT multimodal rehabilitation intervention was adapted to be performed at the Casa Iris community center under research staff supervision.
As opposed to CBPR, the traditional research approach implemented all study-related activities at an academic center, HUH, that primarily provided medical care for non-Hispanic black population. Therefore, it was anticipated that the traditional research group would predominately consist of older non-Hispanic Black women. The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) Community Engagement Core assisted with recruitment in this group. Traditional recruitment strategies were similar to recruitment and retention strategies advocated by the Urinary Incontinence Treatment Network (UITN) such as advertisements, the study website (https://wp4r.trialstoday.org/trial/NCT03166150),22 fliers, University announcements, letters to community physicians, payments for parking, some transportation assistance, and phone reminders.23 Research staff approached potential participants at community events, where women received information about UI and the FAME RCT. Traditional recruitment also included physician-based referrals at the HUH Urogynecology Clinic. Additionally, social media platforms (e.g. Facebook, Craigslist, etc.) were used to disseminate study advertisements. Potential participants were referred to the GHUCCTS website to enter their information or had the option to directly contact the study coordinator. The RCT multimodal rehabilitation intervention was administered at the HUH Clinical Research Unit. Pelvic floor physical therapy was performed at the HUH Urogynecology clinic by a pelvic floor physical therapist for both CBPR and traditional research groups.
Outcome variables included research retention rates among older minority women participating in the UI RCT. Participants who expressed interest in participating in UI clinical research were contacted, screened, assessed for eligibility criteria, and considered either eligible and randomized, or excluded if met exclusion criteria. The primary outcome was the randomization rate defined as the proportion of older racial and ethnic minority women randomized into the RCT out of screened participants using the CBPR versus traditional research approach. Recruitment rates were further compared at telephone screening assessments (proportion of screened participants out of those women who expressed an interest to participate in UI research) and in-person informed consents (proportion of consented participants out of those who were screened) between the groups. Reasons for exclusion from the enrollment process during the telephone screening, administration of informed consent, and randomization were recorded to assess barriers to research participation. Data were collected on demographic characteristics (age, body mass index (BMI), race and ethnicity) and medical history (hypertension, stroke, diabetes, hearing or vision impairments, and previous UI surgery).
All women who expressed an interest to participate in UI clinical research were included in the analytic sample. The Research Electronic Data Capture (REDCap) software was used for data collection and management. Continuous variables were estimated as means with standard deviations (SD), and categorical variables were calculated as frequencies and percentages to summarize participants’ data. For inferential analyses, T-tests were used to examine mean differences of continuous variables between the CBPR and traditional research groups. Similarly, Chi-square or Fisher-exact tests were used to evaluate between-group differences in proportions for categorical variables. The effect of CBPR on recruitment rates was expressed as odds ratio (OR) with 95% Confidence Intervals (CI). Statistical significance was set to an a priori alpha value set of 0.05, and all analyses were performed using SPSS software (Version 26).
Results
Among 13 women in the CBPR group who expressed an interest, 10 successfully completed telephone screening and among the 217 in traditional research group, 88 successfully completed telephone screening (Fig. 1). The mean age among all participants were 72.0 ± 6.0 years and mean BMI was 31.0 ± 8.8 kg/m2 (Table 1). Participants recruited with the CBPR approach were significantly older than individuals enrolled in the traditional research group (78.4 ± 8.3 versus 71.0 ± 4.9 years, P<0.01). Among participants who successfully completed telephone screenings, most were non-Hispanic Black (n=55; 56.1%), followed by Hispanic (n=11; 11.2%), and non-Hispanic White (n=5; 5.1%). As expected, all women recruited using the CBPR approach were Hispanic (n=10; 100.0%), and most participants in the traditional recruitment group were non-Hispanic Black (n=55; 62.5%). There was no significant difference in the prevalence of medical comorbidities between groups, but prior UI surgery was more common among participants recruited using the CBPR approach.
Figure 1.
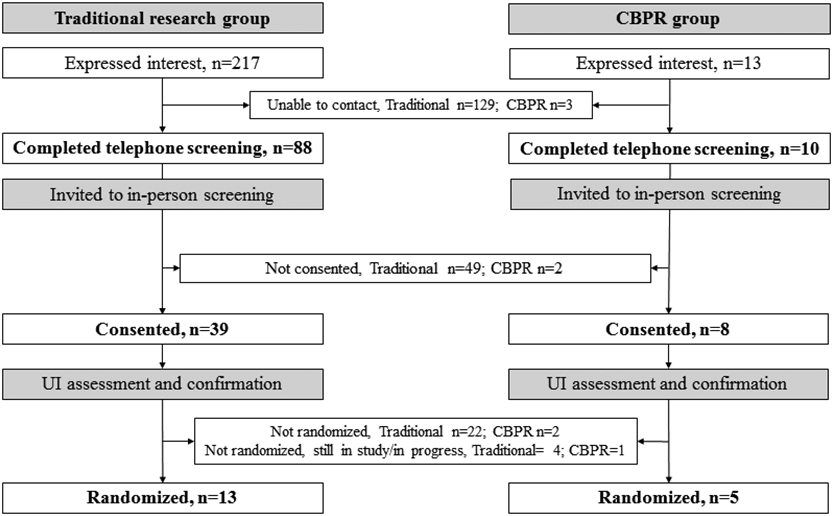
Study flowchart for the traditional and CBPR groups. Shows trajectory of recruitment as participants progress through each step of recruitment process. CBPR, Community-Based Participatory Research; UI, Urinary Incontinence.
Table 1.
Demographic Characteristics*
| Variables | Overall (n=98) | CBPR (n=10) | Traditional Research (n=88) | P† |
|---|---|---|---|---|
| Age, years (SD) | 72.0 ± 6.0 | 78.4 ± 8.3 | 71.0 ± 4.9 | <0.01 |
| BMI (SD) | 31.0 ± 8.8 | 28.3 ± 6.9 | 31.7 ± 9.1 | 0.28 |
| Race-Ethnicity | 0.02 | |||
| Hispanic | 11 (11.2) | 10.0 (100.0) | 1 (1.1) | |
| Non-Hispanic Black | 55 (56.1) | 0 | 55 (62.5) | |
| Non-Hispanic White | 5 (5.1) | 0 | 5 (5.7) | |
| Non-Hispanic Other | 1 (1.0) | 0 | 1 (1.1) | |
| Missing | 26 (26.5) | 0 | 26 (29.5) | |
| Medical History ‡ | ||||
| Hypertension | 37/57 (64.9) | 6/10 (60.0) | 31/47 (66.0) | 0.47 |
| Stroke | 6/54 (11.1) | 1/10 (10.0) | 5/44 (11.4) | 0.11 |
| High Cholesterol | 36/57 (63.2) | 8/10 (80.0) | 28/47 (59.6) | 0.45 |
| Diabetes | 21/57 (36.8) | 3/10 (30.0) | 18/47 (38.3) | 0.62 |
| Cancer | 4/57 (7.0) | 0 | 4/47 (8.5) | 0.34 |
| Hearing/Vision Impairment | 5/57 (8.8) | 0 | 5/47 (10.6) | 0.28 |
| UI Surgery | 2/55 (3.6) | 2/10 (20.0) | 0 | <0.01 |
Self-reported data from screened participants.
T-tests (Continuous Variables), Fisher exact test or Pearson χ2 (Categorical Variables).
Column totals do not equal 100 percent because information was missing for some participants.
Data are n (%) or mean (± SD) unless otherwise specified.
Abbreviations: CBPR, Community Based Participatory Research; BMI, Body Mass Index; UI, Urinary Incontinence.
Table 2 reveals that CBPR group had higher rates of screening (76.9% versus 40.6%, p=0.01), consent (80% versus 44.3%, p=0.045), and randomization (50.0% versus 14.8%, p<0.01) compared to the traditional research group. CBPR increased the odds of research retention among older minority women with UI, including during screening (OR=4.9, 95% CI 1.3-18.2), consent (OR= 5.0, 95% CI 1.0-25.0), and randomization (OR=5.8, 95% CI 1.5-22.7).
Table 2.
Research Retention Rates Among Older Minority Women with Urinary Incontinence
| Retention Rate | Overall | CBPR | Traditional Research | P* | OR (95% CI) |
|---|---|---|---|---|---|
| Expressed Interest/Contacted | 230 (100) | 13 (100.0) | 217 (100) | ||
| Screened† | 98/230 (42.6) | 10/13 (76.9) | 88/217 (40.6) | 0.01 | 4.9 (1.3-18.2) |
| Consented‡ | 47/98 (48.0) | 8/10 (80.0) | 39/88 (44.3) | 0.045 | 5.0 (1.0-25.0) |
| Randomized§ | 18/98 (18.4) | 5/10 (50.0) | 13/88 (14.8) | <0.01 | 5.8 (1.5-22.7) |
Fisher exact test or Pearson χ2 (Categorical Variables).
Proportion of screened participants out of women who expressed an interest to participate in UI research.
Proportion of consented participants out of screened women.
Proportion of randomized participants into a clinical randomized trial out of screened participants.
Data are n (%) unless otherwise specified.
Abbreviations: CBPR, Community Based Participatory Research; OR, Odds Ratio; CI, Confidence Interval.
The inability to contact women who expressed an interest to participate in UI research was a major barrier during the early stages of recruitment. For example, 129 (59.4%) participants in the traditional research group could not be contacted for screening compared to only 3 (23.1%) women in the CBPR group (P=0.01) (Fig. 1). Figure 2 depicts the reasons that prevented participants from consenting after completing the telephone screens. More specifically, in the CBPR group, 2 participants did not consent due to failure to follow up. In the traditional research group, 49 participants did not consent due declining to participate during screening (n=13), not meeting RCT eligibility criteria (n=15), failing to follow up after screening (n=14), and refusing to participate in RCT after screening (n=7). Figure 3 reveals that among 24 women who were not randomized, the majority (n=13, 54.1%) met RCT exclusion criteria, including uncontrolled medical conditions (n=5), no UI episodes on bladder diary (n=4), prolapse > stage 2 (n=3), and functional disability (n=1). Other barriers to randomization were participants changing their mind regarding participation or not being able to follow up (n=8), lacking transportation (n=2), and being advised against enrolling by a primary care physician (n=1).
Figure 2.
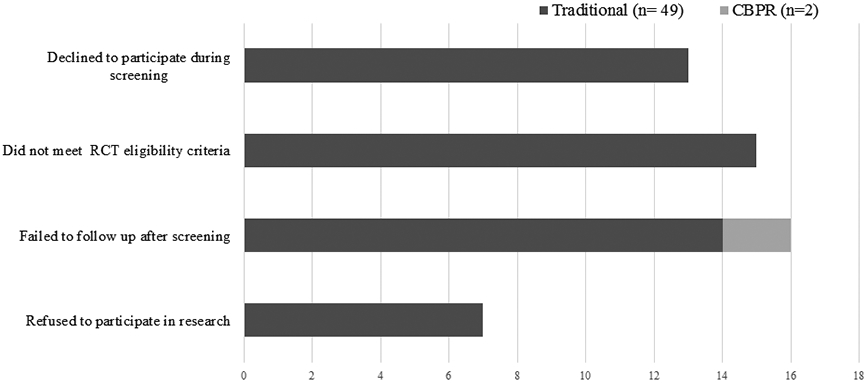
Barriers to consent for CBPR and Traditional Research groups. CBPR, Community-Based Participatory Research; RCT, Randomized Control Trial.
Figure 3.
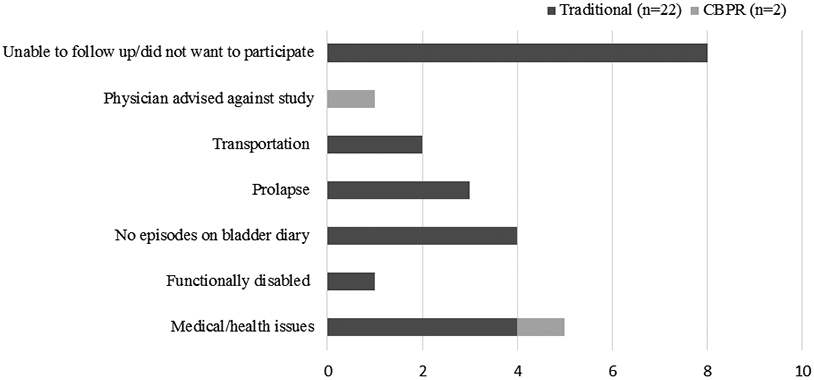
Barriers to randomization for CBPR and Traditional Research groups. CBPR, Community-Based Participatory Research.
Discussion
This ancillary prospective pilot study indicates that compared to traditional research, CBPR yielded higher research retention among older racial and ethnic minority women with UI. A major barrier to enrollment during the initial stage of recruitment was the inability to contact interested participants, particularly when using a traditional research approach not tailored to the local communities. Other barriers to screening and randomization included declining to participate, not meeting study criteria, failing to follow up, changing their mind regarding participation, being advised against participation by a primary care physician, and lacking transportation.
The results from the present study are congruent with recent systematic review conclusions that CBPR is effective in recruiting minority participants and could improve the under-representation of disadvantaged populations.24 The current study reveals high consent (80%) and randomization (50%) rates among screened older Hispanic women with UI in CBPR group that is comparable to reported high recruitment rates in other CBPR studies.25, 26 Although promising, the CBPR group only included 13 Hispanic women participants; and therefore, further confirmatory research using larger and more diverse samples is necessary.
This pilot study contributes scientific data on the challenges facing clinical research highlighted by the UITN including difficulties in recruitment, community involvement, and diversity of participants.23 Prior research has estimated that less than 25% of patients who respond to research recruitment advertisements showed up for initial screening, of which 5% subsequently completed clinical trials.27 In this study, traditional research group had similar trends, while CBPR approach resulted in significantly higher randomization rates. These results suggest that using a CBPR approach may be the optimal strategy to recruit and retain older racial and ethnic minority women with UI while traditional research recruitment models may be poorly suited to achieve their inclusion in UI clinical trials.
While UITN recognized the challenges of recruitment, there is a lack of data on specific barriers that prevent older minority women with UI from participating in clinical research.23 Most common facilitators to increase participants’ retention are desire to help others, perceived need and benefits of research, positive healthcare experiences with a researcher/provider, personal suffering from a condition, familiarity with staff, compensation, and a provider encouragement.28 Most helpful in retaining participants in the CBPR group in this study was establishing the positive, trusting relationships between potential participants, community-based organization, and researchers. Given secondary outcomes indicated that a major barrier to participation was the inability to follow-up after a participant expressed interest, especially in the traditional recruitment group, this study demonstrates that a tailored recruitment is needed to increase research retention among older ethnic and minority women. Finally, we advocate to work closely with primary care providers and educate them about UI and clinical research benefits, as one potential participant in the CBPR group was advised against enrolling and primary care providers’ perception towards UI has been cited as a barrier to receiving UI care.29-31
There are several limitations to this pilot study. Foremost, the majority (62.5%) of women in the traditional recruitment group were non-Hispanic Black while the CBPR participants were all Hispanic. Therefore, it was not possible to determine whether research methods performed differently by race and/or ethnicity. CBPR group had fewer participants compared to traditional research group, as CBPR was conducted within a small community of 46 residents. The Hispanic women in the CBPR group were older and more likely to have prior UI surgery. A higher rate of previous bladder surgery for UI among these participants might be indicative of greater severity and more bothersome UI symptoms, and resultantly, led to better retention because of greater perceived benefit. We did not evaluate whether traditional research approach in a living community would perform similarly to CBPR. Lastly, in-depth qualitative interviews and/or focus group discussions were not conducted to evaluate research barriers or factors promoting research retention in CBPR. The noted limitations are mitigated by the strengths of the study, which include a large sample of screened older minority women suffering from UI with detailed data collected prospectively. Enrollment procedures were culturally adapted through collaborative efforts while maintaining a rigorous scientific approach with strict inclusion/exclusion criteria and validated UI evaluation.
To summarize, findings from this study imply that utilizing CBPR rather than a traditional research approach may be a useful modality to increase research retention among racially and ethnically diverse communities, including older minority women with UI. While CBPR requires additional efforts, training, and resources, it is effective in research retention of underrepresented populations. CBPR could be one of the strategies to decrease the gap in UI care health disparities and improve outcomes among these patients.
Acknowledgements:
We would like to acknowledge the National Hispanic Council on Aging (NHCOA) and Casa Iris staff, including Dr. Yanira Cruz, the President of NHCOA, Ms. Christine Perez, and Ms. Nataly Moran for their contributions to this study
Funding:
This study was supported by Georgetown-Howard Universities Center for Clinical and Translational Science (UL1TR001409), and a grant from the National Institute on Aging (R03 AG053281)
Footnotes
Clinical Trial Registration: clinicaltrials.gov (NCT03166150)
References
- 1.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. (Smedley BD, Stith AY, Nelson AR, eds.). National Academies Press (US); 2003. Accessed July 18, 2020. http://www.ncbi.nlm.nih.gov/books/NBK220358/ [PubMed] [Google Scholar]
- 2.Studies I of M (US) C on E and LIR to the I of W in C, Mastroianni AC, Faden R, Federman D. NIH Revitalization Act of 1993 Public Law 103-43. National Academies Press (US); 1994. Accessed August 18, 2020. https://www.ncbi.nlm.nih.gov/books/NBK236531/ [Google Scholar]
- 3.NHLBI Policy for the Inclusion of Women, Minorities, and Participants Across the Lifespan in Clinical Research ∣ NHLBI, NIH. Accessed November 6, 2020. https://www.nhlbi.nih.gov/grants-and-training/policies-procedures-and-guidelines/nhlbi-policy-inclusion-women-and-minorities-clinical-research [Google Scholar]
- 4.Luebbert R, Perez A. Barriers to Clinical Research Participation Among African Americans. J Transcult Nurs. 2016;27(5):456–463. [DOI] [PubMed] [Google Scholar]
- 5.Diaz Rios LK, Chapman-Novakofski K. Latino/Hispanic Participation in Community Nutrition Research: An Interplay of Decisional Balance, Cultural Competency, and Formative Work. Journal of the Academy of Nutrition and Dietetics. 2018;118(9):1687–1699. [DOI] [PubMed] [Google Scholar]
- 6.Clark LT, Watkins L, Piña IL, et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Current Problems in Cardiology. 2019;44(5):148–172. [DOI] [PubMed] [Google Scholar]
- 7.Simonds VW. Community-Based Participatory Research: Its Role in Future Cancer Research and Public Health Practice. Prev Chronic Dis. 2013;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holkup PA, Tripp-Reimer T, Salois EM, Weinert C. Community-based participatory research: an approach to intervention research with a Native American community. ANS Adv Nurs Sci. 2004;27(3):162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israel B, Schulz A, Parker E, Becker A. Community-based participatory research: policy recommendations for promoting a partnership approach in health research. Education for health. 2001;14(2):182–197. [DOI] [PubMed] [Google Scholar]
- 10.Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstetrics & Gynecology. 2014;123(1):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend MK, Curhan GC, Resnick NM, Grodstein F. Remission and progression of urinary incontinence among Asian, black, and white women in the United States. Am J Nurs. 2011;111(4):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daneshgari F, Imrey PB, Risendal B, Dwyer A, Barber MD, Byers T. Differences in urinary incontinence between Hispanic and non-Hispanic white women: a population-based study. BJU International. 2008;101(5):575–579. [DOI] [PubMed] [Google Scholar]
- 14.Thom DH, Van Den Eeden SK, Brown JS. Evaluation of Parturition and Other Reproductive Variables as Risk Factors for Urinary Incontinence in Later Life. Obstetrics & Gynecology. 1997;90(6):983–989. [DOI] [PubMed] [Google Scholar]
- 15.Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356(21):2143–2155. [DOI] [PubMed] [Google Scholar]
- 16.Richter HE, Albo ME, Zyczynski HM, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morse AN, Labin LC, Young SB, Aronson MP, Gurwitz JH. Exclusion of Elderly Women from Published Randomized Trials of Stress Incontinence Surgery. Obstetrics & Gynecology. 2004;104(3):498–503. [DOI] [PubMed] [Google Scholar]
- 18.Goode PS, FitzGerald MP, Richter HE, et al. Enhancing Participation of Older Women in Surgical Trials. J Am Coll Surg. 2008;207(3):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racial and Ethnic Disparities in Obstetrics and Gynecology. Accessed July 10, 2020. https://www.acog.org/en/Clinical/ClinicalGuidance/CommitteeOpinion/Articles/2015/12/RacialandEthnicDisparitiesinObstetricsandGynecology
- 20.Urinary Incontinence, Mobility & Muscle Function in Older Women: Functional Assessment and Muscle Evaluation Through Exercise (FAME) Trial. clinicaltrials.gov; 2020. Accessed December 16, 2020. https://clinicaltrials.gov/ct2/show/NCT03166150 [Google Scholar]
- 21.NHCOA. Accessed November 27, 2020. http://www.nhcoa.org/
- 22.We Partner 4 Research. Accessed December 18, 2020. https://wp4r.trialstoday.org/trial/NCT03166150
- 23.Steers W, Richter H, Nyberg L, et al. Challenges of Conducting Multi-Center, Multi-Disciplinary Urinary Incontinence Clinical Trials: Experience of the Urinary Incontinence Treatment Network. Neurourol Urodyn. 2009;28(3):170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Las Nueces D, Hacker K, DiGirolamo A, Hicks LS. A Systematic Review of Community-Based Participatory Research to Enhance Clinical Trials in Racial and Ethnic Minority Groups. Health Serv Res. 2012;47(3 Pt 2):1363–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greiner KA, Friedman DB, Adams SA, et al. Effective recruitment strategies and community-based participatory research: Community Networks Program Centers’ recruitment in cancer prevention studies. Cancer Epidemiol Biomarkers Prev. 2014;23(3):416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams SA, Heiney SP, Brandt HM, et al. A Comparison of a Centralized Versus De-centralized Recruitment Schema in Two Community-Based Participatory Research Studies for Cancer Prevention. J Community Health. 2015;40(2):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung NS, Crowley WF, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–1287. [DOI] [PubMed] [Google Scholar]
- 28.Hughes TB, Varma VR, Pettigrew C, Albert MS. African Americans and Clinical Research: Evidence Concerning Barriers and Facilitators to Participation and Recruitment Recommendations. Gerontologist. 2017;57(2):348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiqui NY, Levin PJ, Phadtare A, Pietrobon R, Ammarell N. Perceptions about female urinary incontinence: a systematic review. Int Urogynecol J. 2014;25(7):863–871. [DOI] [PubMed] [Google Scholar]
- 30.Mazloomdoost D, Westermann LB, Crisp CC, Oakley SH, Kleeman SD, Pauls RN. Primary care providers’ attitudes, knowledge, and practice patterns regarding pelvic floor disorders. Int Urogynecol J. 2017;28(3):447–453. [DOI] [PubMed] [Google Scholar]
- 31.Willis-Gray MG, Sandoval JS, Maynor J, Bosworth HB, Siddiqui NY. Barriers to urinary incontinence care seeking in White, Black, and Latina women. Female Pelvic Med Reconstr Surg. 2015;21(2):83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


