Abstract
Classic taxonomies of memory distinguish explicit and implicit memory systems, placing motor skills squarely in the latter branch. This assertion is in part a consequence of foundational discoveries showing significant motor learning in amnesics. Those findings suggest that declarative memory processes in the medial temporal lobe (MTL) do not contribute to motor learning. Here, we revisit this issue, testing an individual (L. S. J.) with severe MTL damage on four motor learning tasks and comparing her performance to age-matched controls. Consistent with previous findings in amnesics, we observed that L. S. J. could improve motor performance despite having significantly impaired declarative memory. However, she tended to perform poorly relative to age-matched controls, with deficits apparently related to flexible action selection. Further supporting an action selection deficit, L. S. J. fully failed to learn a task that required the acquisition of arbitrary action–outcome associations. We thus propose a modest revision to the classic taxonomic model: Although MTL-dependent memory processes are not necessary for some motor learning to occur, they play a significant role in the acquisition, implementation, and retrieval of action selection strategies. These findings have implications for our understanding of the neural correlates of motor learning, the psychological mechanisms of skill, and the theory of multiple memory systems.
INTRODUCTION
Conventional taxonomies of human learning and memory place motor skill learning in the domain of implicit memory (Squire, 2004). This idea can be traced, in part, to a series of seminal studies on the hippocampal patient H. M., who suffered severe bilateral loss of his medial temporal lobe (MTL) from resection surgery for intractable epilepsy. Milner, Corkin, and colleagues revealed that, although H. M. was mostly unable to form new declarative memories (and lost much of his autobiographical memory), he retained an ability to improve his performance on novel motor tasks (Corkin, 1968, 2002; Shadmehr, Brandt, & Corkin, 1998; Milner, Corkin, & Teuber, 1968; Milner, 1962; Scoville & Milner, 1957). H. M. showed significant reductions of motor error during tasks including mirror tracing (Milner, 1962), rotary pursuit (Corkin, 1968), repetitive tapping (Corkin, 1968), bimanual tracking (Corkin, 1968), and force field adaptation (Shadmehr et al., 1998), even though he could not recall performing the tasks from session to session. A common interpretation of those findings is that H. M.'s spared neural circuits—such as the motor and premotor cortices, basal ganglia, and cerebellum—preserved his aptitude for learning (though it should be noted that his cerebellum was likely affected by antiepileptic drugs; Corkin, 2002).
A crucial and underappreciated fact of these previous studies is that H. M.'s motor performance was markedly inferior to matched controls on all tasks except simple repetitive finger tapping (Shadmehr et al., 1998; Corkin, 1968). His learning generally proceeded slowly, reached a lower asymptote, and was erratic (Corkin, 1968). Moreover, he typically required intensive bouts of coaching at the outset of each session to remind him of useful strategies for the tasks (Stanley & Krakauer, 2013). Beyond H. M., similar results have been observed in other studies of motor learning in amnesics (Brigard, 2019). Given these caveats, a more complete picture of the effects of MTL disruption on motor learning is needed.
One concept relevant to the neural correlates of motor learning is the protean nature of motor skills themselves—research in healthy adults demonstrates that even relatively simple motor learning tasks simultaneously leverage multiple qualitatively distinct memory systems (Krakauer, Hadjiosif, Xu, Wong, & Haith, 2019; McDougle, Ivry, & Taylor, 2016). A common dissociation in that literature distinguishes deliberative action selection from error-correcting implicit motor calibration. The latter has been linked to incremental learning processes, primarily involving the cerebellum (Shadmehr, Smith, & Krakauer, 2010; Taylor, Klemfuss, & Ivry, 2010). In terms of the former, recent studies have demonstrated that cognitive strategies play a prominent role in many human motor learning tasks and likely reflect the learning of action–outcome mappings (McDougle & Taylor, 2019). This dual (or hierarchical; Krakauer, 2019) model of motor learning implies that motor learning deficits in amnesics might be linked to more “explicit” aspects of learning, namely, the flexible use of deliberate, context-dependent action selection strategies.
The MTL is well suited to support flexible action selection given its role in associative binding (Davachi, 2006; Brasted, Bussey, Murray, & Wise, 2003; Murray & Wise, 1996), context sensitivity (Burgess, Maguire, & O'Keefe, 2002; Phillips & LeDoux, 1992), and episodic memory-guided decision-making (Bornstein, Khaw, Shohamy, & Daw, 2017; Davidow, Foerde, Galván, & Shohamy, 2016; Shohamy & Turk-Browne, 2013; Zeithamova, Dominick, & Preston, 2012). In particular, it has been linked to the formation and deployment of associations between visual cues and motor actions (Hindy, Ng, & Turk-Browne, 2016; Mattfeld & Stark, 2015; Brasted et al., 2003; Wirth et al., 2003; Murray & Wise, 1996; Petrides, 1985). With these established MTL functions in mind, we sought to revisit the role of the MTL in motor learning.
Here, we describe a case report of a patient (L. S. J.) with near-complete bilateral loss of her hippocampi (Figure 1). L. S. J. and age-matched controls (n = 40) performed behavioral experiments designed to engage different aspects of motor learning, including the following: (1) a 3-day “mirror reversal” experiment, where reaching movements were mirrored across an invisible vertical axis. This task is thought to require a form of difficult de novo motor learning (Wilterson & Taylor, 2021; Telgen, Parvin, & Diedrichsen, 2014); (2) a “savings” experiment, where a novel visuomotor mapping is learned, extinguished, and then relearned within a single session (Morehead, Qasim, Crossley, & Ivry, 2015). Savings in this context has been shown to primarily tax deliberate selection processes, where successful movement strategies must be retrieved from memory (Avraham, Morehead, Kim, & Ivry, 2021; Morehead et al., 2015); (3) a force field adaptation experiment designed to reveal separate “fast” and “slow” learning components that might reflect, respectively, explicit and implicit motor learning processes (McDougle, Bond, & Taylor, 2015; Smith, Ghazizadeh, & Shadmehr, 2006); and (4) an arbitrary visuomotor association learning task, where unintuitive action–outcome associations have to be acquired and remembered to navigate an object through a structured space (Fermin, Yoshida, Ito, Yoshimoto, & Doya, 2010). We expected to see action selection and retrieval-related deficits in L. S. J. in all tasks, with the most profound deficits predicted for the arbitrary visuomotor association learning task (Hindy et al., 2016; Mattfeld & Stark, 2015; Brasted et al., 2003; Wirth et al., 2003; Murray & Wise, 1996; Petrides, 1985).
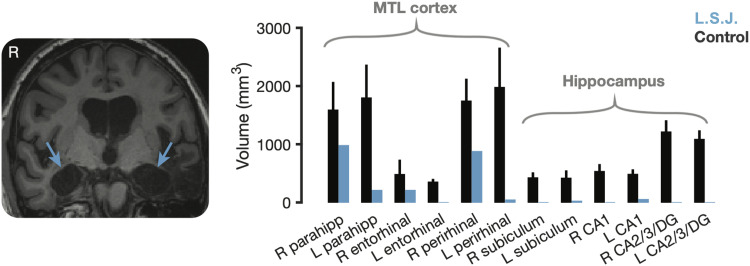
Figure 1. .
L. S. J.'s MTL anatomy. Left: Representative structural coronal slice (MP-RAGE) illustrating extensive bilateral MTL lesions in patient L. S. J. Right: MTL tissue volume in L. S. J. relative to controls, including parahippocampal cortex (parahipp), entorhinal cortex, perirhinal cortex, subiculum, CA1, and CA2/CA3/dentate gyrus (CA2/3/DG). Error bars denote ±1 SD. R = right; L = left. We note that the matched control cohort here (n = 4) is independent of the cohort tested in the main experiments. Data here are reproduced with permission from Schapiro et al. (2014).
METHODS
Participants
L. S. J. and 40 age-, education-, and handedness-matched controls (L. S. J. age: 65 years at time of testing for the reaching experiment, 67 years for the button-press experiments; reaching experiments mean control age = 61.3 years, range = 53–71 years, 7/10 female; button-press experiments mean control age = 63.7 years, range = 60–70 years, 17/30 female) participated in the experiments for monetary compensation. All participants, including L. S. J., were college-educated. The control participants were right-handed, had normal or corrected-to-normal vision and hearing, had no history of neurological disease, and were native English speakers. In accordance with the Princeton University institutional review board, control participants gave written, informed consent, and L. S. J. gave assent with consent given by her legal guardian. The Montreal Cognitive Assessment was used to screen control participants for cognitive deficits in all in-lab experiments (score cutoff: > 25). The Montreal Cognitive Assessment requires an in-person interview; thus, this cognitive screening was not performed on the online sample of control participants. To match L. S. J.'s repeated testing regime, 10 control participants performed all three reaching experiments in the same order as L. S. J.: three contiguous days of mirror reversal learning, followed by the savings experiment, and then the force field experiment. A separate sample of 30 control participants performed the arbitrary visuomotor map learning experiment (with two exclusions, resulting in an analyzed sample of 28; see below); these data were collected remotely through Amazon Mechanical Turk because of COVID-19 safety concerns, though we note that the methods were identical in the remote versus in-lab variants of this task. Control sample sizes were determined to be consistent with similar previous motor learning studies (McDougle et al., 2015; Keisler & Shadmehr, 2010), including those with amnesic individuals (Shadmehr et al., 1998).
Apparatus and Stimuli
All three reaching tasks were performed on a robotic manipulandum (Kinarm, BKIN Technologies). Participants were seated in front of the robotic arm, which had a central position 15 cm from the participant's body. The robot sampled hand position, velocity, and force at 1 kHz. Stimuli were displayed on a horizontally mounted mirror, which reflected images from an LCD flat screen.
Inspired by a previous motor learning study in amnesics (Shadmehr et al., 1998), we thought it would be important to first test L. S. J.'s ability to maintain and remember the abstract goal of typical reaching tasks with visual feedback—that is, getting a small circular “cursor” to land on a larger circular “target.” Our first attempt at measuring L. S. J.'s ability to learn simple motor tasks involved a pilot experiment designed to be similar in visual characteristics to other studies in the field. The general trial design, which was quickly aborted, matched the basic design used in our visuomotor rotation experiment. Critically, L. S. J. struggled to remember the goal of the task given the abstract nature of the visual feedback (i.e., floating, colored circles). To amend this, we pivoted to a variant of the task that required the participant to land a virtual airplane (cursor) on a virtual runway (target). We chose an aviation theme to leverage L. S. J.'s existing semantic knowledge, as she had been an avid amateur aviator before her injury. This adjustment—which gave the task a meaningful, intuitive goal—immediately solved L. S. J.'s problems with goal maintenance and was thus our approach in all further experiments.
In all reaching tasks, the center of the displayed target was 10 cm from the center of the workspace. At the beginning of each trial, the robotic arm automatically guided the participant's hand to the center point. After a 500-msec delay, the runway appeared, and the participant could initiate their movement. If the center of the plane landed within the runway, a green square appeared around the runway and a higher-pitched tone (400 Hz) was played, signifying a “hit.” Otherwise, no square appeared, and a lower-pitched tone (200 Hz) was played, signifying a “miss.”
Mirror Reversal
Mirror reversal tasks are difficult and taxing motor skills, likely requiring de novo learning of a sensorimotor mapping (Telgen et al., 2014). Here, we tested the prediction that L. S. J. would learn in this task, as H. M. had learned a similar mirror drawing task (mirror-reversed star tracing; Milner, 1962). However, we also predicted that L. S. J. would show less flexible learning, perhaps reflecting an inability to maintain and recall goal-directed action selection strategies.
The mirror reversal task we implemented was modeled after a task used by Telgen et al. (2014), with several key differences. In brief, after a short baseline period with veridical feedback participants made reaching movements in a “mirrored” environment, where an invisible central vertical axis acted as a mirror on the x-coordinate of their movements (Figure 2A). Six targets were displayed, with two central “baseline” targets along the mirror axis and four off-axis targets, each 20°/−20° from either of the two central targets. Trial-specific target positions were pseudorandomized in blocks of six trials. Although previous work using a mirror reversal task focused on speed accuracy trade-offs (Telgen et al., 2014), we did not believe a strict response time constraint would be feasible with L. S. J. given her problems remembering unintuitive instructions. Thus, we measured how individuals learn a mirror reversal task given ample time to plan on each trial.
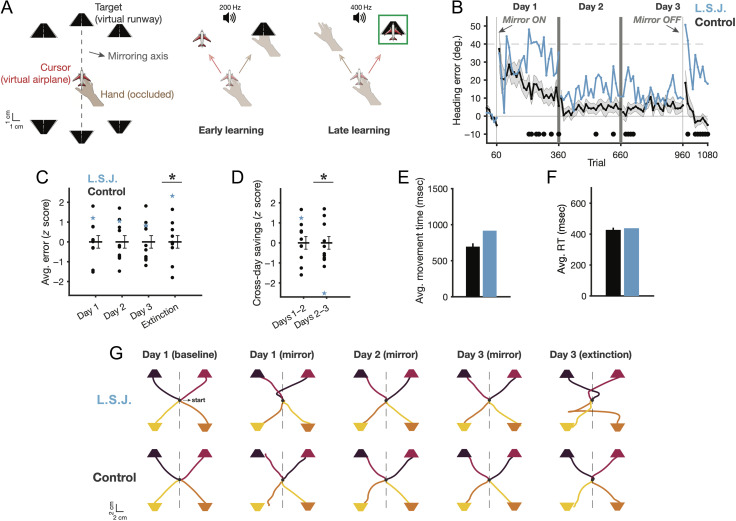
Figure 2. .
Mirror reversal learning. (A) Participants brought a visual cursor (virtual airplane) to a target (virtual runway) as a cover task for a motor learning experiment. Task design (left): Early in the task, the hand is directed toward the target resulting in large errors (center), whereas late in training, the hand should be directed toward the mirror solution direction (right). Target misses were signaled by a low-pitched tone; target hits were signaled by a high-pitched tone and a green border. (B) Learning curves, in eight trial bins. The line at 40° error represents the result of a reach straight to a target during the mirror trials. Black dots mark where L. S. J. has an error bin of z > 1.64 (p < .05) relative to controls. Error shading = 1 SEM. (C) Z score average error on each day and in the extinction block and (D) on the first two bins at the end of Day n and the beginning of Day n + 1. Error bars = 1 SEM. (E–F) Average (median) movement times and RTs during mirror blocks. (G) Average reach trajectories, aligned to peak speed. Target/trajectory colors are matched to depict trials associated with each specific target; center line is for mirroring axis illustration (i.e., not visible to the participant; central baseline targets not shown).
The task occurred over three consecutive days, with 1080 total trials. The first day began with 60 baseline trials where no perturbation was applied, followed by 300 trials with the mirror perturbation applied to the virtual plane feedback. Day 2 consisted of another 300 mirror trials. Day 3 consisted of 300 mirror trials, followed by 120 extinction trials, where the mirror perturbation was removed. A movement was considered complete when the hand passed an invisible ring around the center point, with a radius of 10 cm. If a movement took longer than 2000 msec, a “too slow” message was displayed. The virtual plane feedback was displayed continually throughout all movements.
Savings in Visuomotor Rotation
Savings refers to the phenomenon where learning is faster on the second exposure to a task, even after apparent extinction of a learned behavior. Various lines of evidence have recently shown that, in motor adaptation tasks, savings is driven by the explicit recollection of a goal-directed cognitive strategy (Avraham et al., 2021; Vandevoorde & Orban de Xivry, 2019; Morehead et al., 2015). Thus, we predicted that, although L. S. J. would show some learning in this task, she would have significantly impaired savings. This savings experiment used a common visuomotor rotation perturbation to elicit learning (Cunningham, 1989), where the virtual airplane was perturbed by a consistent −45° clockwise rotation on perturbation trials (Figure 3A). Four runway targets could appear at each of the cardinal directions (0°, 90°, 180°, and 270°), with trial-specific target positions pseudorandomized in blocks of four trials.
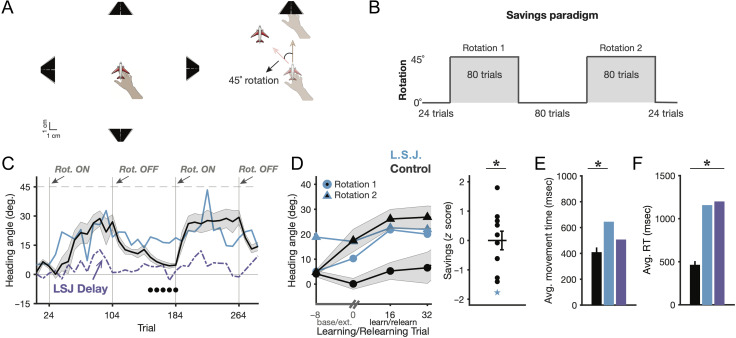
Figure 3. .
Savings in visuomotor rotation learning. (A) Task design (left) and example rotation trial and endpoint visual feedback. (B) Schematic of the “savings” paradigm. (C) Learning curves in eight trial bins. The line at +45° represents the ideal reach heading angle on rotation trials. Black dots mark where L. S. J.'s heading angle (bin) was |z| > 1.64 (p < .05) relative to controls. The purple line reflects L. S. J.'s learning curve in the delayed feedback task, where endpoint cursor (airplane) feedback was delayed by 1 sec. Error shading = 1 SEM. (D) Zoomed-in savings effect, showing early learning of the first and second rotations (left) and z score savings effects (right). (E–F) Average (median) movement times and RTs during rotation and extinction blocks (blue = L. S. J. typical task; purple = L. S. J. delayed feedback task).
The task took place over a single session of 288 trials (Figure 3B). The first 24 trials were baseline trials, where no perturbation was applied. The next 80 trials were the first rotation block, where the −45° clockwise rotation was applied on every trial. The following 80 trials were an extinction block, where the rotation was removed. The next 80 trials were the second rotation block, where the rotation was reapplied and savings was assessed. Finally, the last 24 trials consisted of a final extinction block, where the rotation was again removed. A movement was considered complete when the hand passed an invisible ring around the center point with a radius of 10 cm. If a movement took longer than 2000 msec, a “too slow” message was displayed. The virtual airplane feedback was only presented at the end of the movement, signifying where the plane had crossed the invisible ring.
In a brief follow-up experiment, L. S. J. performed the same task again on a separate day. Here, we delayed the visual endpoint feedback by 1 sec—a simple manipulation that has been shown to significantly attenuate implicit visuomotor adaptation but spare explicit learning processes (McDougle & Taylor, 2019; Schween, Langsdorf, Taylor, & Hegele, 2019; Brudner, Kethidi, Graeupner, Ivry, & Taylor, 2016; Kitazawa, Kohno, & Uka, 1995). Thus, in this experiment, we could infer if implicit learning played a major role in L. S. J.'s learning curve under the standard nondelayed feedback condition described above.
Fast and Slow Force Field Learning
Force field learning, a classic task where individuals adapt their reaching movements to counteract dynamic environmental force perturbations, is generally thought to be a pure assay of implicit motor adaptation (Figure 4A; Shadmehr & Mussa-Ivaldi, 1994). Recent evidence suggests that it may also involve a modest contribution from explicit goal-directed strategies, which may reflect a fast and flexible component of learning (Schween, McDougle, Hegele, & Taylor, 2020; McDougle et al., 2015). The “fast and slow” force field learning paradigm imposes a velocity-dependent force field perturbation on participants' movements in a manner thought to dissociate multiple motor learning processes (Smith et al., 2006): In this task, an initial force perturbation is applied for a substantial period, followed briefly by a second perturbation, and finally a “force channel” phase, which precludes visual errors and constricts the participant's movement along a straight trajectory (Figure 4B). Critically, in this force channel phase, people often show a bias toward echoing the movements they had made to counter the first perturbation, suggesting that, although a “fast” learning process drives rapid learning of the second perturbation, a second “slow” learning process remains stuck in the first perturbation state, which is revealed by the force channel (Smith et al., 2006).
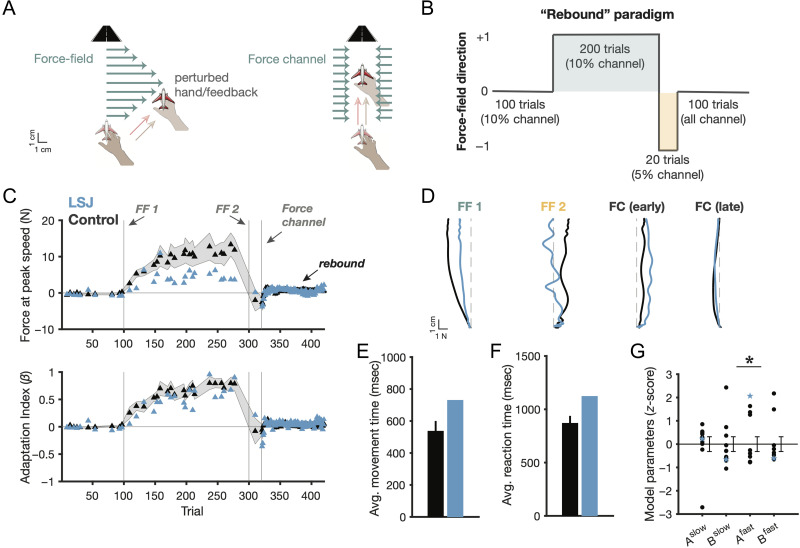
Figure 4. .
Force field adaptation. (A) Schematic of force field trials (left) and force channel trials (right), where the latter is designed to measure learning and suppress errors. (B) Schematic of the “rebound” force field learning paradigm. (C) Learning curves, reflected in lateral forces (against the perturbation) at peak speed (top), and measured as an “adaptation index,” which involved regressing produced forces on a given force channel trial against the ideal force profile for that trial (i.e., an adaptation index of 1 = perfect learning). Error shading = 1 SEM. (D) Force profiles on outbound phase of reaches, with the abscissa representing lateral force (in Newtons) and the ordinate representing position (in cm). (E–F) Average (median) movement times and RTs during force field blocks. (G) Z score model parameters after fitting the “two-state” model of motor adaptation. Error bars = 1 SEM.
The first 100 trials consisted of baseline trials where no forces were applied. During force trials (Trials 101–320), the motors of the robot applied a force (f) to the hand. The force applied was proportional to the velocity (V) of the hand, and the direction of the force was perpendicular to the hand motion as follows:
where M is the matrix of forces applied to the hand velocity vector V. In the initial force block (F1; Trials 101–300), the force field pushed the hand in a clockwise direction. In the second force block (F2; Trials 301–320), M was multiplied by −1, yielding a counterclockwise force.
Probe force channel trials occurred in 10% of the baseline trials and F1 block trials, in a single trial of the F2 block, and in all 100 trials of the force channel block (Trials 321–420). The force channel was implemented using a simulated spring (6 kN/m) and damper (20 N/sec/m), which keeps the hand perpendicular to the target during movement and limits any visual deviations from a straight path to that target. All movements were made to a single straight-ahead target (90° position). A movement was considered complete when the hand stopped on the runway (velocity < 2.5 m/sec). If the full movement took longer than 2000 msec, a “too slow” message was displayed. The visual airplane feedback was presented continually throughout all movements.
Arbitrary Visuomotor Map Learning
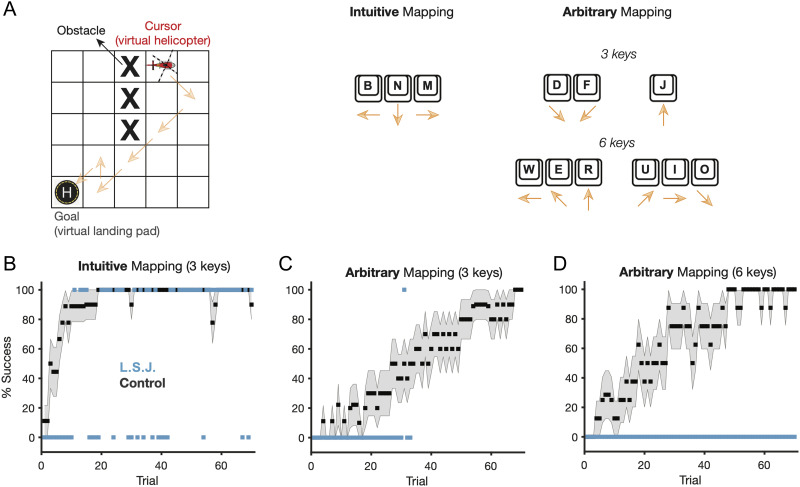
L. S. J. performed this study on an in-lab PC computer; control participants performed this study remotely because of COVID-19 restrictions, using their personal computers and peripherals. Participants used the letter keys “D-F-J,” “B-N-M,” or “W-E-R-U-I-O” on their keyboard to manipulate a virtual helicopter cursor around a 5 × 5 grid. The experiment was controlled by custom software coded in HTML, CSS, and JavaScript and was hosted on Amazon Mechanical Turk for the remote experiment. After agreeing to participate in the experiments, participants were presented with the following instruction: “The goal of the game is to land your helicopter safely on the landing pad in as few moves as possible. To play, use the ([KEYS]; e.g., B, N, and M) keys to move the helicopter across the board. Be careful, those keys might not do what you think they should. Don't take too long thinking! You have a short amount of time to make it to the landing pad before you run out of fuel.”
Each trial began with the helicopter and target visible on a 5 × 5 grid. Participants were able to begin moving across the board immediately and had 6 sec to reach the target, with unlimited moves per trial (Fermin et al., 2010). If the time expired and the participant had not reached the target, the feedback “Out of Fuel. Try Again! 0 points” was displayed. If the participant reached the target, the helicopter remained on the target location for the duration of the 6-sec window. If the target was hit with the minimal number of moves (six presses), the feedback read “Perfect! 100 points.” Within each trial, for every move over that minimum, 5 points were deducted from the perfect 100. That is, feedback for a trial in which one additional move was used to reach the target would read “Nicely done! 95 points.” A running total of points appeared under the trial feedback. All feedback was displayed for 2 sec.
Movement of the helicopter was determined by one of three key mappings: The “intuitive” mapping was made to follow preconceived notions about how the relative position of keys relate to movement (e.g., the right-most key moved the helicopter to the right). The three- and six-key “arbitrary” mappings were chosen to be unintuitive and required use of both diagonals and cardinal directions (Figure 6A).
Figure 6. .
Arbitrary visuomotor map learning. (A) Task design and display (left), intuitive visuomotor mappings (center), and arbitrary visuomotor mappings (right). (B) Learning curves (left) in the intuitive condition, shown as percent success (i.e., successful navigation to the target within 6 sec). Note that for L. S. J., 100% reflects a single successful trial. Error shading = 1 SEM. (C) Learning curves (left) and average presses per trial (right) in the three-key arbitrary mapping condition and (D) six-key arbitrary mapping conditions.
The experiment consisted of 70 training trials, each requiring the participant to move between a single start–end pair using the assigned mapping, avoiding several visual obstacles used to limit positional degrees of freedom. Each start–end pair forced the participant to use every key at least once to reach the target in the minimum number of moves, and the minimum number of moves was always 6. Crucially, each condition had a single start–end pair (i.e., initial helicopter position, final goal position, and obstacle placement); thus, if the correct mapping was learned, the task in theory became a simple repeated sequence execution task. Each control participant only trained on one of each mapping, though L. S. J. performed all three mappings (starting with intuitive, then three-key, then six-key mapping), with ∼30-min breaks in between. Finally, we note that during piloting we found that L. S. J. occasionally forgot which keys moved the helicopter. To address this issue, we removed all but the relevant keys across three separate computer keyboards (one for each condition). In this way, the only keys available to her were the affordances of the task.
Kinematic Analysis
In all three reaching tasks, participants, including L. S. J., occasionally made slow movements, contrary to the instructions. Thus, any movements that took over 2000 msec in all three reaching experiments were excluded from analysis (the percentage of excluded trials are delineated below).
In the mirror reversal task, error was measured as the difference between the participant's movement angle and the ideal movement angle for that trial (e.g., if the mirror perturbation was ignored in a trial with one of the peripheral targets, a 40° error would result). Because we sought to measure feedforward learning, we only analyzed the early portion of movements before feedback corrections and online control would dominate. Movement angles were computed as the angle of the hand 2.5 cm into the full 10-cm reach. As planned, we did not include movements to the two central baseline targets in our main analyses to isolate learning at the targets positioned off the mirroring axis. Outlier movements were excluded from the mirror reversal blocks if the movement error exceeded 3 SD from the mean movement error (mean ± 1 SD: 0.81 ± 0.48% for controls; 0.37% for L. S. J.) or the 2000 msec movement time cutoff (3.34 ± 2.14% for controls; 9.54% for L. S. J.).
For the visuomotor rotation savings experiment, the heading angle represented the participant's reach angle relative to the target (e.g., a correct heading angle on a rotation trial would be +45°). Heading angle was again computed as the angle of the hand 2.5 cm into the full 10-cm reach. The savings metric was computed in a manner similar to a prior study (Morehead et al., 2015): Savings was calculated by subtracting the mean heading angle for the first 16 trials in the R1 block from the first 16 trials of the R2 block. Importantly, those values were first corrected by subtracting the mean heading angle on the 16 trials preceding the corresponding block. We note here that cross-day savings was calculated in a similar fashion in the mirror reversal experiment, but without the correction as the learning blocks were contiguous. Outliers were excluded from each block if the movement error exceeded 3 SD from the mean movement error (0.90 ± 0.66% for controls, 1.39% for L. S. J.) or the 2000 msec movement time cutoff (0.56 ± 0.50% for controls; 4.51% for L. S. J.).
In the force field learning experiment, learning was quantified with two metrics: First, we computed the force produced in channel trials in the direction orthogonal to the movement direction (i.e., against the force field) while the hand was moving at peak velocity. Second, because the force field perturbation was velocity dependent, we also computed a “normalized” measure of learning. To do this, we used the typical “adaptation index” metric, which involves regressing the participant's full-reach trajectory (once the hand was moving >2.5 cm/sec) against the ideal trajectory needed to fully counteract the imposed force field (Smith et al., 2006). Thus, an adaptation index of 1 connotes perfect learning and 0 connotes no learning. We note that for the second reversed-sign force field perturbation (F2), we set the sign of the ideal force profile in the positive direction such that perfect learning of F2 would be reflected by an adaptation index of −1. Finally, no outliers (exceeding 3 SD from the mean) in the adaptation index were observed; trials were excluded that exceeded the 2000 msec movement time cutoff (0.29 ± 0.27% for controls; 0.95% for L. S. J.).
Lastly, in the arbitrary visuomotor map learning experiment, learning was quantified as the number of successful trials the participant completed throughout the task (i.e., wherein the target was reached within 6 sec). We also computed the median number of button presses per trial across the three conditions. Trials were excluded if no presses were made (1.94 ± 5.00% for controls, 1.43% for L. S. J.). One of the Mechanical Turk control participants was excluded (six-key condition) for not performing the task (pressing only a single button) on over >50% of the trials. Another was excluded from the same condition for holding down a single button (e.g., over 20 repeated presses) on over >50% of the trials.
Model Fitting
The force field experiment was directly inspired by a previous study revealing dissociable fast and slow processes of motor adaptation (Smith et al., 2006). We thus fit this same “two-state” state–space model to participants' behavior data. The model tracks changes in the learning state (x) on each trial:
where e is the error on the current trial n, determined by the difference between the current state and the current force field perturbation p (p takes values of +1, −1, or 0, depending on the trial). and are respectively the slow and fast learning processes, with respective retention factors A and learning rates B. Models were fit to each participant's adaptation index data by optimizing the four free parameters to minimize the root-mean-square error between the model and the data using MATLAB optimization routines (fmincon). To delineate the two processes, the model is subject to an additional constraint such that Afast < Aslowand Bfast > Bslow. Fits were iterated 100 times with randomized initial parameter values to avoid local minima in the error surface.
PCAs
We performed an exploratory PCA on learning data from the three reaching tasks, using metrics for initial learning, unlearning (extinction), and savings (visuomotor rotation experiment). Initial learning rates in the mirror reversal and visuomotor rotation experiment were computed using the (baseline-corrected) first 16 trials of initial exposure to the perturbations. In the force field experiment, we used the first force channel trial after the onset of the perturbation to compute an initial learning rate. Unlearning rates were computed analogously, but during the initial trials of the respective extinction phases (the single reverse force field force channel data were used for the force field extinction rate). We also included the visuomotor rotation savings metric in our PCA, which is described above. The seven variables were z-scored and entered into a PCA using the pca function in MATLAB.
Statistics
We took a two-pronged approach for statistical comparisons. First, we computed z scores for point metric comparisons of a priori interest and compared L. S. J. to controls via a p value derived from a z table. Second, we also used a well-known, more conservative adjusted t test (reported as “ATT”) designed for clinical case studies (Crawford & Howell, 1998). We note that reported z and t tests were one tailed when we had specific a priori directional predictions concerning L. S. J.'s performance versus controls (i.e., attenuated performance) and two tailed, otherwise (noted in the latter case). Our significance threshold alpha value was set to .05 for all statistical tests; thus, for a predicted result, |z| > 1.644 for L. S. J. signals statistical significance.
RESULTS
In 2007, L. S. J. suffered wide-scale loss of hippocampal and neighboring MTL cortex tissue because of viral encephalitis. L. S. J. exhibits profound retrograde and anterograde memory impairments (Gregory, McCloskey, Ovans, & Landau, 2016; Gregory, McCloskey, & Landau, 2014; Schapiro, Gregory, Landau, McCloskey, & Turk-Browne, 2014). A previously published (Schapiro et al., 2014) structural analysis of her lesions in subregions of MTL cortex and subfields of the hippocampus is reproduced in Figure 1. L. S. J. has little remaining tissue in the left parahippocampal cortex, the left entorhinal cortex, and the left perirhinal cortex, and virtually none in the hippocampus bilaterally.
Lesions in the right hemisphere were localized to the MTL but in the left hemisphere extended to other temporal regions, including the temporal pole and mid-fusiform gyrus, as well as the left insula and OFC (Gregory et al., 2016). This broader damage may confound an MTL-focused interpretation of L. S. J.'s deficits, though previous studies have revealed strikingly precise behavioral deficits in L. S. J.'s retrograde and anterograde declarative memory, with preserved reasoning skills, perceptual acuity, working memory capacity, item recognition, language ability, an above average vocabulary, and a spared capacity to perform overtrained motor skills (e.g., writing and violin playing; Gregory et al., 2014, 2016; Schapiro et al., 2014). Thus, although L. S. J.'s lesions are not circumscribed within the MTL, MTL deficits are predominant in both her neurological and neuropsychological profiles.
Mirror Reversal Learning
Inspired by previous work, we began our investigation with a mirror reversal task that echoed the one originally used on H. M. to delineate putative explicit and implicit forms of memory (Milner, 1962). Here, we implemented a more recent iteration of the mirror reversal task (Telgen et al., 2014) that does not require continuous tracing, so as to have better experimental control over feedforward learning.
Mirror reversal learning has proven to be a more complex task than initially thought: Implicit motor calibration appears to operate in opposition to task performance (Hadjiosif, Krakauer, & Haith, 2021), and explicit strategies appear to be required to fully overcome reversals, at least during early training (Wilterson & Taylor, 2021). These revelations make this a worthwhile task to revisit in the context of MTL contributions to motor learning. We predicted that aspects of L. S. J. performance—such as her ability to flexibly adjust to changing task demands—might be impaired, even though significant learning should still be seen. If confirmed, this would provide one piece of evidence for a role for the MTL in motor learning.
L. S. J. and age-matched controls (n = 10) performed a reaching experiment that required learning a novel mirror-reversed mapping. In this task, the x-dimension of participants' reaching movements are mirrored about an invisible vertical axis (Telgen et al., 2014), requiring consistent adjustment of movements to achieve task success (Figure 2A). The task was completed over a 3-day span, allowing us to measure long-term retention and cross-day savings (Ebbinghaus, 2013).
Both L. S. J. and controls showed comparable learning, reducing their reaching errors across the 3 days of exposure to the mirror reversal (Figure 2B; mean error in last 16 trials of Day 3 minus first 16 trials of Day 1; controls: μ = −26.87 ± 9.75° SEM, t(9) = 2.76, p = .02 two-tailed; L. S. J.: μ = −22.96°; comparisons: z score, L. S. J. versus controls = 0.13, ns; adjusted t test [Crawford & Howell, 1998] ATT: p = .45). However, L. S. J. displayed several difficulties during learning, seen most clearly in an apparent collapse in performance during the later phase of the first day of training (last half of Day 1 trials; z = 1.93, p < .05; ATT: p < .05). On the whole, although her average total error on each of the 3 days was numerically higher than controls, these were not significant differences (Figure 2C; z = 1.20 Day 1, z = 1.05 Day 2, z = 0.82 Day 3, all ns; ATT: all ps > 0.13). In the broadest sense, we thus replicated previous findings with patient H. M.—some aspects of learning to invert one's movements across a mirroring axis do not appear to require an intact MTL (Milner, 1962).
Aside from generally weaker performance, however, L. S. J. was significantly impaired in key phases of the task. First, when the mirror reversal perturbation was removed on Day 3 (“Extinction”), L. S. J. showed significantly impaired performance, perseverating on her previously learned mirror-reversed movement policy, whereas controls easily reverted to the baseline unperturbed mapping (Figure 2C; z = 2.32, p < .01; ATT: p < .05). This impairment suggests that, although L. S. J. could learn the mirror reversal to some degree, her learning was inflexible and unresponsive to context shifts. This result provides preliminary support for the idea that retrieving previous strategies, perhaps generated by a deliberative action selection system, invokes the MTL.
In further analyses, we asked whether L. S. J. showed significant retention (“savings”) in her across-day performance. H. M.'s ability to perform mirror tracing was not only preserved from day to day but was subtly enhanced across days (Milner, 1962). Replicating those findings, L. S. J. had an unimpaired savings effect (computed as the difference between early error on Day n and late error on Day n − 1; see Methods) between Days 1 and 2, quantified as decreased error across those days (Figure 2D; z = 1.24, ns; ATT: p = .13). In contrast, her savings from Day 2 to Day 3 were severely impaired relative to controls (z = −2.52, p < .01; ATT: p < .05). This again suggests that her ability to retrieve a previously successful strategy was unreliable.
Several control analyses ruled out alternative explanations for L. S. J.'s performance deficits: First, both her average movement time (Figure 2E) and average RT (Figure 2F) on mirror-reversed trials were numerically slower but not significantly slower than controls (zs = 1.42 and 0.22, respectively, ns; ATT; ps = .11 and .21). This supports the observation that she attended to the task and performed it as instructed. Similarly, the shape of her reaching trajectories was comparable to controls (Figure 2G), demonstrating that L. S. J. was likely not adopting a qualitatively different movement strategy in the task.
Taken together, the results of our mirror reversal experiment not only replicate previous work with severe amnesic cases (i.e., showing somewhat preserved motor learning) but also complicate the strict claim that the MTL does not contribute to motor learning—L. S. J. showed globally weaker learning, a less stable learning function, and a significantly reduced ability to arbitrate between conflicting motor control policies. Our next experiment targeted this latter point more directly using a sensorimotor savings paradigm.
Savings in Visuomotor Learning
One foundational result in the psychology of learning is Ebbinghaus' “savings” effect (Ebbinghaus, 2013). Savings is defined as faster learning, relative to initial exposure, when information is presented again after being putatively forgotten. This effect has typically been attributed to a persisting latent memory trace that is unmasked upon reexposure to previously seen information. In the case of motor learning tasks like visuomotor rotation learning, the savings effect has been attributed to the explicit retrieval of a previous (successful) action selection strategy; that is, if a human participant learns to redirect their movements in response to a novel visuomotor rotation of their visual feedback (Exposure 1) and then unlearns that perturbed visuomotor mapping (extinction), upon reexposure, they will rapidly recall their original action selection policy and reexpress the learned behavior (Avraham et al., 2021; Vandevoorde & Orban de Xivry, 2019; Morehead et al., 2015). We reasoned that this important component of motor learning—deliberate retrieval of an action selection strategy—would recruit the MTL and thus be impaired in L. S. J.
L. S. J. and age- and education-matched controls (n = 10) performed a reaching experiment that required learning a novel rotated visuomotor mapping. During exposure, visual feedback was rotated by 45° relative to the direction of movement (Figure 3A; see Methods for further details). This simple perturbation has been shown to involve both implicit sensorimotor recalibration as well as explicit, goal-directed strategizing (McDougle et al., 2016). In the savings variant of this task, initial rotation learning is reversed via an extinction phase where the rotation is removed, and savings is then measured in a third phase (re-exposure) where the rotation is reapplied (Figure 3B).
Both controls and L. S. J. showed significant learning (Figure 3C; late learning for Rotation 1: controls: μ = 24.41 ± 5.08°, t(9) = 4.80, two-tailed p < .001; L. S. J.: μ = 25.99°; comparison: z = 0.10, ns; ATT: p = .46). Echoing her mirror reversal impairments, L. S. J. perservated when the perturbation was removed: Although controls adapted back to baseline reaching angles, L. S. J. showed only subtle unlearning in the extinction phase (Figure 3C; hand heading angle comparison: z = 3.76, p < .005; ATT: p < .005). Crucially, this inflexibility extended into the reexposure phase, where controls showed faster relearning of the second versus the first rotation (“savings”; t(9) = 3.88, two-tailed p < .005), but L. S. J.'s savings were impaired relative to controls (Figure 3D; significant z test, z = −1.77, p < .05; ATT (marginal): p = .06). This again suggests that the ability to flexibly retrieve action selection strategies might recruit the MTL.
As noted above, L. S. J.'s learning did not fully wash out during the extinction phase, complicating the savings results. That is, her lack of savings could be due to erratic action selection strategies—as we hypothesized—or due to overactive implicit learning processes such as use-dependent learning (Verstynen & Sabes, 2011), where movement directions that are repeatedly revisited bias future movements toward the repeated direction. Alternatively, L. S. J.'s perseveration may have been the result of a combination of multiple processes. One approach to this issue is to minimize the role of implicit adaptation processes to allow for a more direct assay of L. S. J.'s action selection system.
To that end, we performed a short follow-up experiment on L. S. J. several weeks after the initial task. It has been demonstrated that when visual feedback is slightly delayed during motor adaptation, implicit learning processes are attenuated or even abolished while explicit strategies are unaffected (McDougle & Taylor, 2019; Schween et al., 2019; Brudner et al., 2016; Kitazawa et al., 1995). We reasoned that L. S. J.'s ability to perform visuomotor rotation learning mainly relied on implicit learning, and thus, successful performance would be virtually abolished or be made more erratic if visual feedback was briefly delayed (feedback delay = 1 sec each trial). Indeed, this is what we observed (Figure 3C): Under delayed visual feedback, L. S. J. showed sporadic, short-lived signatures of learning in the task (every delayed learning trial bin was attenuated relative to the corresponding nondelayed learning trial bin). This suggests that her behavior in the standard nondelayed task reflected a combination of erratic action selection and implicit learning processes. We note that, in similar savings paradigms, older controls show both reliable explicit learning and savings (Vandevoorde & Orban de Xivry, 2019). This further supports the idea that her impaired savings (Figure 3D) was likely the result of a deficit in deliberate action selection and retrieval.
Additional analyses did reveal that L. S. J. had significantly increased learning phase movement times (Figure 3E) and RTs (Figure 3F; zs = 1.95 and 4.83, respectively, ps < .05; ATT: ps < .05). The movement time difference was modest (235.80 msec) and only significant in the nondelayed condition (z = 0.81 for delay, ns; ATT: p = .23). Because our learning metrics measured feedforward adjustments to reaches (see Methods), it is unlikely that differences in movement time significantly influenced learning. However, the RT difference between L. S. J. and controls was rather large (693.15 msec). We did not have a priori predictions concerning RT, and this increase was inconsistent with the results of the mirror reversal task (Figure 2F) and subsequent results (see below). We return to this unexpected finding later (see Discussion).
The results of this experiment demonstrate that, although L. S. J. showed some learning during a visuomotor adaptation task, her learning was less flexible than controls (i.e., minimal extinction), her ability to retrieve recently learned action selection strategies was apparently impaired (i.e., reduced savings), and—as revealed by the delayed feedback task—her ability to learn a motor adaptation task was likely reliant on implicit, putatively subcortical processes (e.g., cerebellar error-based motor learning). These results corroborate and extend our interpretation of the mirror reversal findings, demonstrating again that although the MTL is clearly not necessary for some motor learning to occur, it may contribute to flexible action selection.
Force Field Adaptation
Adaptation to dynamic sensorimotor perturbations is typically investigated by perturbing movements via externally generated velocity-dependent force fields (Shadmehr & Mussa-Ivaldi, 1994). This type of learning has been interpreted as reflecting implicit adaptation of a mapping between movement goals and applied forces (Shadmehr & Mussa-Ivaldi, 1994), though several studies have hinted at a small role for deliberate action selection processes in these tasks as well (McDougle et al., 2015; Keisler & Shadmehr, 2010; Hwang, Smith, & Shadmehr, 2006). Indeed, we have recently provided direct evidence for a modest—though reliable—contribution of deliberative strategizing when people adapt to dynamic force fields (Schween et al., 2020). This deliberative process appears to adjust quickly if demanded so by a change in context (i.e., via instructions or abrupt removal of the perturbations) and is fast decaying. Thus, we reasoned that this process might correspond to one aspect of a popular model of motor adaptation—the “two-state” state–space model—where distinct fast and slow processes simultaneously contribute to the learning curve (Smith et al., 2006). Indeed, there is evidence in both visuomotor rotation learning and force field adaptation that the putative fast process shares resources with explicit memory systems (Vandevoorde & Orban de Xivry, 2019; McDougle et al., 2015; Keisler & Shadmehr, 2010) and may track changes in context (Heald, Lengyel, & Wolpert, 2021).
L. S. J. and age- and education-matched controls (n = 10) performed a reaching experiment that required first counteracting a novel dynamic force field (FF 1), quickly adapting to an opposite force field (FF 2), and subsequently reaching in an errorless “force channel” (FC) that measures adaptation aftereffects (Smith et al., 2006; Figure 4A, B). As predicted, L. S. J. showed robust learning in the force field adaptation task (Figure 4C; adaptation index final channel trial of FF 1: controls: μ = 0.79 ± 0.13, t(9) = 6.21, two-tailed p < .001; L. S. J.: 0.90; comparison: z = 0.28, ns; ATT: p = .25). Learning is depicted in Figure 4C as both the lateral forces produced against the force field at peak reaching speed and a regression metric that relates produced versus ideal force profiles for each probe channel trial (an “adaptation index”; Smith et al., 2006). Because applied forces were velocity dependent, our analyses focus on this normalized adaptation index metric—indeed, L. S. J. tended to move slower in the task relative to controls (Figure 4E; see below). We note the fact that she generally moved slower in this velocity-dependent perturbation task somewhat complicates our comparisons between L. S. J. and controls, and interpretation of these results should be made with caution.
Inspection of participants' force profiles (Figure 4D) echoed the results of the learning curves—L. S. J. generally responded in the correct manner in each phase of the task, pushing against the initial force field (FF 1), deadapting during the presentation of the second force field (FF 2), perseverating on the second force field in the early phase of the force channel block (FC “early,” first five trials only), and then showing “rebound” back to her initial compensatory responses in the remainder of the FC trials. L. S. J.'s movement times (Figure 4E) and RTs (Figure 4F) were numerically, but not significantly, higher than the controls' (zs = 0.96 and 1.16, respectively, both ns; ATT: ps > .14). L. S. J. thus showed mostly normal force field adaptation, replicating previous findings in amnesics (Shadmehr et al., 1998).
Although L. S. J.'s learning was robust, she did show numerically weaker learning during the second force field (FF 2) and then appeared to perseverate more when the perturbations were removed as she entered the FC block. To synthesize multiple learning effects and test our a priori hypothesis, we turned to computational modeling. We fit the aforementioned two-state model to participants' adaptation index data. Resulting (normalized) model parameter results are shown in Figure 4G.
L. S. J. showed a significant difference relative to controls in the operation of the fast motor learning process—namely, her so-called “retention” parameter (Afast) was significantly elevated (z = 2.06, p < .05; ATT: p < .05), suggesting that her fast learning process was more rigid than controls. No other model parameters significantly differed between L. S. J. and controls (all |z|s < 0.66, ns; ATT: ps > .27). Fitted model parameter values (mean ± 1 SD) were as follows: Afast = 0.25 ± 0.30 for controls, 0.87 for L. S. J.; Bfast = 0.17 ± 0.23 for controls, 0.03 for L. S. J.; Aslow = 0.99 ± 0.02 for controls, 0.99 for L. S. J.; and Bslow = 0.03 ± 0.02 for controls, 0.02 for L. S. J.
At first glance, it may seem counterintuitive that L. S. J.'s “retention” parameter (Afast) was elevated given her severe amnesia. To illustrate how the A parameter of the fast learning process is inversely related to behavioral flexibility, we correlated, across participants, the fitted Afast parameters with two behavioral analogues of strategy shifting—the rate of unlearning that occurred at the onset of the second force field (i.e., FF 2 adaptation minus the last force channel trial of FF 1 adaptation) and the amount of perseveration seen in the early phase of the force channel block (i.e., counteracting FF 2, with more perseveration signaling less flexibility). Both metrics were significantly correlated with Afast in the predicted directions (Afast and rate of unlearning: ρ = −0.62, p = .04; Afast and degree of perseveration: ρ = .71, p = .01), demonstrating that Afast (or more precisely, its inverse) is a proxy for flexibility during learning. Thus, L. S. J.'s elevated Afast parameter reflected reduced flexibility.
Together these results suggest that, like other amnesic individuals, L. S. J.'s ability to learn novel movement dynamics is mostly intact (Shadmehr et al., 1998). This lends support to the notion that force field learning, for the most part, may not require an intact MTL. However, echoing the previous experiments, L. S. J. was impaired in a manner that again implied a role for the MTL in flexible action selection.
Exploratory PCA
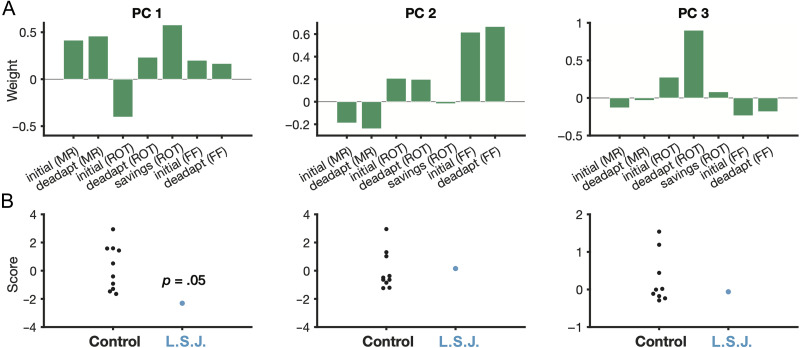
Given the similarity of our first three experiments and the fact that all participants completed each task, we performed a post hoc PCA. We reasoned that if L. S. J. was impaired in a specific manner, a PCA could reveal a single summary metric that captured her deficits across the three studies. We entered the seven key behavioral measures from the above experiments into the PCA (i.e., initial learning rates, deadaptation/extinction rates, and savings in the rotation experiment; see Methods). The resulting first three principal components (PCs) explained a combined 78% of the variance, with PC1 explaining 41% alone. As shown in Figure 5A, PC1 appeared to reflect a performance metric that captured rates of learning, extinction, and savings (with the exception of initial learning in the rotation experiment). L. S. J. had the lowest score on this PC (z = −1.59, p [marginal] = .05; ATT [marginal]: p = .08; Figure 5B) but was unimpaired on the others (all other |z|s < 0.12; ATT: ps > .46). Speculatively, although PC1 appeared to reflect general learning efficiency across the tasks, PC2 appeared to track implicit motor adaptation, which has been shown to be more involved in force field learning versus rotation learning (Schween et al., 2020), to be uninvolved in savings (Avraham et al., 2021; Morehead et al., 2015), and to work against performance in mirror reversal learning (Hadjiosif et al., 2021; Wilterson & Taylor, 2021). These incidental findings support the use of PCA to synthesize behavioral results across these disparate tasks.
Figure 5. .
PCA results. (A) Weights from the first three components yielded by a PCA, which was conducted on initial learning rates for each task, early deadaptation (extinction) rates for each task, and the savings metric in the visuomotor rotation task. (B) Individual scores for each PC for controls (black) and L. S. J. (cyan). MR = mirror reversal; ROT = visuomotor rotation; FF = force field adaptation.
The results for PC1, though on the statistical cusp, suggest that a single factor (a damaged MTL) might explain L. S. J.'s impairments on the three tasks. That is, L. S. J. appeared to show a general impairment in flexibly deploying action selection strategies during visuomotor learning.
How are such strategies formed in the first place, and does that process require an intact MTL? Arguably, the strategies required for the previous three motor learning tasks require simply implementing a single rule on a well-learned movement—that is, to reach in a direction that negates the perturbation (the second and third experiments) or that is flipped across a single axis (the first experiment). However, in many real-world motor learning tasks, implementing a simple rule does not apply, and new associations must be formed from scratch (e.g., learning to operate a novel tool). This key issue motivated our fourth and final study, where we tested the more targeted hypothesis that the acquisition of a complex, novel action selection policy requires the MTL.
Arbitrary Visuomotor Map Learning
The previous motor learning tasks (Figures 2–5) require the modification of overlearned, intuitive visuomotor mappings (Krakauer et al., 2019). In our final study, we implemented a task designed to directly tax a putatively more cognitive form of motor learning—arbitrary visuomotor map learning (Fermin et al., 2010). This task departs from both typical motor sequence learning (SRT tasks; Nissen & Bullemer, 1987) and simpler stimulus–response learning tasks (McDougle & Collins, 2021; Hardwick, Forrence, Krakauer, & Haith, 2019; Collins & Frank, 2012), as it requires the acquisition of a novel structured visuomotor mapping.
L. S. J. and age-matched controls (n = 30; n = 10/condition) performed a computerized experiment that required pressing buttons on a keyboard to navigate a virtual helicopter toward a goal on a grid (Figure 6A). We implemented three conditions: In the “intuitive” condition, the keys available to the participant were mapped onto movements of the helicopter in an easily understood, direct manner (e.g., key B = left, key M = right). We assumed this condition would reveal minimal (if any) deficits in L. S. J. In the two “arbitrary” conditions, the mapping was unintuitive (e.g., D = down/right, F = down/left), requiring exploratory action selection and the integration and retrieval of an action–outcome mapping across time. Given our previous results, we predicted that successful arbitrary visuomotor map learning might rely on an intact MTL.
L. S. J. and controls were able to learn in the intuitive condition (Figure 6B), significantly improving their performance throughout training (last five trials minus first five trials; controls: μ = 65.67 ± 11.07%; L. S. J.: 60.00%; comparison: z = −0.13, ns; ATT: p = .45). Moreover, the average (median) number of presses L. S. J. performed to get to the goal matched that of controls, and both were equal to the minimum number needed to succeed on each trial (six). These results imply that L. S. J. understood the premise of the task and could implement a straightforward action selection strategy to navigate to the goal when the mapping between actions and sensory outcomes was intuitive.
In the arbitrary mapping conditions, L. S. J. simply could not learn—in the three-key condition, she gave up halfway through the task having performed only one successful trial out of 33 (Figure 6C). L. S. J. completed the six-key version (Figure 6D), performing exactly zero successful trials (learning comparison: z = −6.22, p < .001; ATT: p < .001). Neither result could be easily explained by her implementing a different global strategy, as her average number of presses per trial did not significantly differ from controls (with control values in the three-key conditions matched for the number of trials L. S. J. completed; zs = 0.63 and 0.90 for the three- and six-key conditions, respectively, both ns; ATT: ps > 0.20). These results support the idea that the acquisition of complex, novel action–outcome mappings depends on the MTL.
DISCUSSION
The finding that H. M. could improve at motor learning tasks was a watershed moment in neuropsychology (Brigard, 2019; Corkin, 2002; Milner, 1962). A notable aspect of these studies was H. M.'s ability to retain a motor memory over time in spite of his inability to remember performing the tasks from session to session. This aspect of his behavior arguably led to the conventional taxonomy of explicit versus implicit memory (Brigard, 2019; Stanley & Krakauer, 2013). Critically, it was also clear in these studies that H. M. performed worse than neurologically intact individuals on virtually all of the motor learning tasks that he attempted (Corkin, 1968). Thus, there is likely a more complex story regarding the role of the MTL in motor learning. We revisited this issue in a patient (L. S. J.) with dramatic MTL loss similar in magnitude to H. M.'s. We chose a variety of motor learning tasks and analysis approaches to better understand the role of the MTL in motor learning.
L. S. J. was tested on four motor learning tasks: mirror reversal learning, visuomotor rotation learning, dynamic force field adaptation, and arbitrary visuomotor map learning. Consistent with previous results, L. S. J. showed significant learning in most (three of four) tasks she attempted. She also showed several consistent impairments, which appeared to reflect deficits in flexibly retrieving deliberative action selection strategies. This capacity was directly taxed during arbitrary visuomotor map learning, where the learner has to link movements to abstract outcomes and remember those associations across time to achieve a goal. L. S. J. completely failed at this de novo learning task (Figure 6). As a whole, our results both replicate and expand upon influential earlier findings, suggesting that although a functioning MTL is not strictly necessary for (some) motor learning to occur, it has a role to play.
As mentioned above, previous studies on this topic have similarly shown that amnesics (including H. M.) are typically worse at motor tasks versus matched controls, even though they still show signatures of learning (Brigard, 2019; Corkin, 1968). This raises several general questions that are critical to issues of motor skill and memory taxonomies. First, what exactly is the computational role of the MTL system in motor skill? Second, how should we define a motor skill, and do typical laboratory assays capture our folk notions? We believe that our results speak to these questions.
In terms of the computational role of MTL, it has been suggested that the type of knowledge acquired by the MTL system is fundamental to many real-life motor skills (Stanley & Krakauer, 2013). This is supported by the current study, as well as by a large body of research showing that cognitive processes are ubiquitous during motor learning (Krakauer et al., 2019; McDougle et al., 2016). Indeed, even at the level of reflexive behaviors such as the long-latency stretch reflex, explicit contextual cues (e.g., the size and shape of a visual target) significantly impact how that reflex is expressed (Krakauer, 2019; Nashed, Crevecoeur, & Scott, 2012). The mechanisms by which these types of cognitive variables shape motor learning is poorly understood. However, an older account has experienced a renaissance in recent years, in which motor skills progress from a highly deliberative phase to a qualitatively distinct automatic phase (Fitts & Posner, 1967). This qualitative transition could reflect a shift from algorithmic strategies (i.e., simulating the consequences of potential actions) to flexible retrieval of “cached” action policies (McDougle & Taylor, 2019; Haith & Krakauer, 2018; Logan, 1988). We speculate that one role for the MTL in this process might be to link different contexts to cached motor memories (Julian & Doeller, 2021; Gulli et al., 2020; Goldfarb, Chun, & Phelps, 2016; Chun & Phelps, 1999), contributing to the maintenance of context-dependent sensorimotor repertoires (Collins & McDougle, 2021; Heald et al., 2021). This notion is supported here by L. S. J.'s consistent tendency to perseverate on inappropriate movement strategies even after the context had changed.
In terms of how to capture motor skill in the laboratory, the present tasks move beyond a simplistic definition centered on overly constrained basic motor functions (e.g., repetitive finger tapping). It is likely counterproductive to define motor skills circularly, such that the ability of an amnesic to perform a given task defines that task as a motor skill. Motor adaptation tasks, like force field adaptation (Figure 4) and visuomotor rotation learning (Figure 3), are thought to reflect the restoration of performance of an overlearned skill (e.g., short, straight reaches), rather than true de novo learning (Krakauer et al., 2019; Telgen et al., 2014). In our view, the arbitrary visuomotor mapping task (Figure 6) gets closer to the concept of a de novo skill—although it does not require relearning how to use one's fingers, it does require acquisition of a structured, goal-directed mapping from movements to sensory outcomes, arguably a key component of motor skill. Indeed, these results echo previous work by Drs. Milner and Corkin, showing that individuals with bilateral hippocampal damage, including H. M., struggle to acquire and retain the solutions to visually and tactually guided mazes (Corkin, 1965; Milner, 1965). Our visuomotor map learning task also departs from typical motor sequence learning tasks (Nissen & Bullemer, 1987) where people simply learn to chain actions together; in such tasks, there is no underlying novel mapping to be learned. Our mirror reversal task (Figure 2) may lie somewhere between adaptation and de novo learning; here, a new control policy must be learned, but it is constrained to a single salient spatial rule (Telgen et al., 2014).
Echoing others (Stanley & Krakauer, 2013), we propose that one would be hard-pressed to cite a motor skill that does not require, at some point, the application of deliberative strategies. Even at the expert level, where conventional wisdom implies that skills should be fully “proceduralized,” it is still critical to flexibly adjust movements in response to contextual cues (e.g., consider a tennis pro deliberately adjusting her serve to account for wind conditions). With these issues in mind, our results may speak more to the nature of motor skill than to the function of the MTL—well-established capacities of the declarative memory system may be fundamental for skilled motor behavior (Hindy et al., 2016; Mattfeld & Stark, 2015; Brasted et al., 2003; Wirth et al., 2003; Murray & Wise, 1996; Petrides, 1985).
We note that this case report has several limitations beyond those already intrinsic to single-patient studies. First, we did not directly measure L. S. J.'s explicit, deliberate learning strategies, even though there are techniques available for doing so (Maresch, Werner, & Donchin, 2021; Taylor, Krakauer, & Ivry, 2014). This choice was a practical reality of working with a deeply amnesic patient: In an aborted “aim report” pilot study, where participants were tasked with reporting their intended direction of movement before every trial, we struggled to get L. S. J. to adhere to the instructions. Importantly, recent work has shown that such interventions can bias performance (Maresch et al., 2021) and thus may have confounded our results anyway. We also note that we here operate under the assumption that, during motor adaptation, explicit strategies and implicit adaptation are not interacting; however, recent work suggests that they may indeed interact (Albert et al., 2020; Leow, Marinovic, de Rugy, & Carroll, 2018, 2020; Miyamoto, Wang, & Smith, 2020). These potential interactions have been difficult to parse out and have tended to be quite subtle. Future work will be needed to address potential system interactions and a role for the MTL in that process.
Second, L. S. J.'s elevated movement and RT data in the savings experiment (Figure 3) diverged from the other experiments. This could simply reflect increased distraction, an interpretation that we cannot rule out. On the other hand, increased RT has actually been associated with better learning and performance in visuomotor adaptation tasks (McDougle & Taylor, 2019; Haith, Huberdeau, & Krakauer, 2015; Fernandez-Ruiz, Wong, Armstrong, & Flanagan, 2011), reflecting time-consuming planning processes (Haith et al., 2015); here, L. S. J. showed the opposite effect (weaker performance). We note that increased RTs were also observed in H. M. when he performed motor learning tasks (Corkin, 1968), and further work is needed to interpret these effects.
Third, the severity of L. S. J.'s deficits clearly varied across experiments (e.g., compare Figures 4 and 6). This variation could reflect a different balancing of deliberative strategies versus implicit motor learning between the tasks themselves, differences in L. S. J.'s vigilance across days, or some combination of these (and perhaps other) factors. A PCA (Figure 5) did imply shared processing between the first three tasks (e.g., PC 1), but this was an exploratory measure. In addition, because of COVID-19-related issues, control data for the arbitrary visuomotor map learning experiment were collected online, whereas L. S. J.'s behavior was measured in-lab. Although we do not think this affected our results, we cannot rule out an effect of these different contexts.
Finally, although the extent of L. S. J.'s neural damage goes beyond the MTL (e.g., left insula and OFC; Gregory et al., 2016), her impairments appear to be primarily limited to memory function. Other aspects of executive function appear to be relatively preserved, notably her reasoning and working memory skills (Gregory et al., 2014, 2016; Schapiro et al., 2014). Nonetheless, it is possible that subtle, subclinical impairments in these processes could interact with her severe memory dysfunction, leading to a complex pattern of deficits across a myriad of tasks.
Taken together, our results support a relaxation of conventional assumptions about skill learning and strict memory taxonomies. Motor skills in particular are a crowning achievement of human evolution and intelligence—the range and complexity of the average human's motor skill repertoire arguably outstrips that of any other living species. It should not be surprising then that such abilities draw on multiple neural systems.
Acknowledgments
We thank Adrian Haith and John Krakauer for input early on in the study and thoughtful discussions, Anna Schapiro for generously sharing the anatomical data, and Aline Johnson for invaluable support and guidance.
Reprint requests should be sent to Samuel D. McDougle, Department of Psychology, Yale University, 2 Hillhouse Avenue, New Haven, CT 06520, or via e-mail: samuel.mcdougle@yale.edu.
Author Contributions
Samuel D. McDougle: Conceptualization; Formal analysis; Investigation; Methodology; Writing—Original draft; Writing—Review & editing. Sarah A. Wilterson: Investigation; Methodology; Writing—Review & editing. Nicholas B. Turk-Browne: Conceptualization; Methodology; Project administration; Supervision; Writing—Review & editing. Jordan A. Taylor: Conceptualization; Funding acquisition; Methodology; Project administration; Supervision; Writing—Review & editing.
Funding Information
This work was supported by the National Institute of Health (https://dx.doi.org/10.13039/100000025), grant number: F32MH119797 awarded to S. D. M., and National Institute of Neurological Disorders and Stroke (https://dx.doi.org/10.13039/100000065), grant number: R01 NS-084948 awarded to J. A. T.
Diversity in Citation Practices
Retrospective analysis of the citations in every article published in this journal from 2010 to 2021 reveals a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .407, W(oman)/M = .32, M/W = .115, and W/W = .159, the comparable proportions for the articles that these authorship teams cited were M/M = .549, W/M = .257, M/W = .109, and W/W = .085 (Postle and Fulvio, JoCN, 34:1, pp. 1–3). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article's gender citation balance. The authors of this article report its proportions of citations by gender category to be as follows: M/M = .611; W/M = .167; M/W = .056; W/W = .167.
REFERENCES
- Albert, S. T., Jang, J., Haith, A. M., Lerner, G., Della-Maggiore, V., Krakauer, J. W., et al. (2020). Competition between parallel sensorimotor learning systems. bioRxiv 2020.12.01.406777. 10.1101/2020.12.01.406777 [DOI] [PMC free article] [PubMed]
- Avraham, G., Morehead, J. R., Kim, H. E., & Ivry, R. B. (2021). Reexposure to a sensorimotor perturbation produces opposite effects on explicit and implicit learning processes. PLoS Biology, 19, e3001147. 10.1371/journal.pbio.3001147, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein, A. M., Khaw, M. W., Shohamy, D., & Daw, N. D. (2017). Reminders of past choices bias decisions for reward in humans. Nature Communications, 8, 15958. 10.1038/ncomms15958, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasted, P. J., Bussey, T. J., Murray, E. A., & Wise, S. P. (2003). Role of the hippocampal system in associative learning beyond the spatial domain. Brain, 126, 1202–1223. 10.1093/brain/awg103, [DOI] [PubMed] [Google Scholar]
- Brigard, F. D. (2019). Know-how, intellectualism, and memory systems. Philosophical Psychology, 32, 719–758. 10.1080/09515089.2019.1607280 [DOI] [Google Scholar]
- Brudner, S. N., Kethidi, N., Graeupner, D., Ivry, R. B., & Taylor, J. A. (2016). Delayed feedback during sensorimotor learning selectively disrupts adaptation but not strategy use. Journal of Neurophysiology, 115, 1499–1511. 10.1152/jn.00066.2015, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, N., Maguire, E. A., & O'Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron, 35, 625–641. 10.1016/S0896-6273(02)00830-9 [DOI] [PubMed] [Google Scholar]
- Chun, M. M., & Phelps, E. A. (1999). Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience, 2, 844–847. 10.1038/12222, [DOI] [PubMed] [Google Scholar]
- Collins, A. G., & Frank, M. J. (2012). How much of reinforcement learning is working memory, not reinforcement learning? A behavioral, computational, and neurogenetic analysis. European Journal of Neuroscience, 35, 1024–1035. 10.1111/j.1460-9568.2011.07980.x, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, A. G. E., & McDougle, S. D. (2021). Context is key for learning motor skills. Nature, 600, 387–388. 10.1038/d41586-021-03028-x, [DOI] [PubMed] [Google Scholar]
- Corkin, B. (1965). Tactually-guided maze learning in man: Effects of unilateral cortical excisions and bilateral hippocampal lesions. Neuropsychologia, 3, 339–351. 10.1016/0028-3932(65)90006-0 [DOI] [Google Scholar]
- Corkin, S. (1968). Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia, 6, 255–265. 10.1016/0028-3932(68)90024-9 [DOI] [Google Scholar]
- Corkin, S. (2002). What's new with the amnesic patient H. M.? Nature Reviews Neuroscience, 3, 153–160. 10.1038/nrn726, [DOI] [PubMed] [Google Scholar]
- Crawford, J. R., & Howell, D. C. (1998). Comparing an Individual's test score against norms derived from small samples. Clinical Neuropsychologist, 12, 482–486. 10.1076/clin.12.4.482.7241 [DOI] [Google Scholar]
- Cunningham, H. A. (1989). Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. Journal of Experimental Psychology: Human Perception and Performance, 15, 493–506. 10.1037/0096-1523.15.3.493, [DOI] [PubMed] [Google Scholar]
- Davachi, L. (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16, 693–700. 10.1016/j.conb.2006.10.012, [DOI] [PubMed] [Google Scholar]
- Davidow, J. Y., Foerde, K., Galván, A., & Shohamy, D. (2016). An upside to reward sensitivity: The hippocampus supports enhanced reinforcement learning in adolescence. Neuron, 92, 93–99. 10.1016/j.neuron.2016.08.031, [DOI] [PubMed] [Google Scholar]
- Ebbinghaus, H. (2013). Memory: A contribution to experimental psychology. Annals of Neurosciences, 20. 10.5214/ans.0972.7531.200408, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermin, A., Yoshida, T., Ito, M., Yoshimoto, J., & Doya, K. (2010). Evidence for model-based action planning in a sequential finger movement task. Journal of Motor Behavior, 42, 371–379. 10.1080/00222895.2010.526467, [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz, J., Wong, W., Armstrong, I. T., & Flanagan, J. R. (2011). Relation between reaction time and reach errors during visuomotor adaptation. Behavioural Brain Research, 219, 8–14. 10.1016/j.bbr.2010.11.060, [DOI] [PubMed] [Google Scholar]
- Fitts, P. M., & Posner, M. I. (1967). Human performance. Belmont, CA: Brooks/Cole. [Google Scholar]
- Goldfarb, E. V., Chun, M. M., & Phelps, E. A. (2016). Memory-guided attention: Independent contributions of the hippocampus and striatum. Neuron, 89, 317–324. 10.1016/j.neuron.2015.12.014, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory, E., McCloskey, M., & Landau, B. (2014). Profound loss of general knowledge in retrograde amnesia: Evidence from an amnesic artist. Frontiers in Human Neuroscience, 8, 287. 10.3389/fnhum.2014.00287, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory, E., McCloskey, M., Ovans, Z., & Landau, B. (2016). Declarative memory and skill-related knowledge: Evidence from a case study of amnesia and implications for theories of memory. Cognitive Neuropsychology, 33, 220–240. 10.1080/02643294.2016.1172478, [DOI] [PubMed] [Google Scholar]
- Gulli, R. A., Duong, L. R., Corrigan, B. W., Doucet, G., Williams, S., Fusi, S., et al. (2020). Context-dependent representations of objects and space in the primate hippocampus during virtual navigation. Nature Neuroscience, 23, 103–112. 10.1038/s41593-019-0548-3, [DOI] [PubMed] [Google Scholar]
- Hadjiosif, A. M., Krakauer, J. W., & Haith, A. M. (2021). Did we get sensorimotor adaptation wrong? Implicit adaptation as direct policy updating rather than forward-model-based learning. Journal of Neuroscience, 41, 2747–2761. 10.1523/JNEUROSCI.2125-20.2021, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith, A. M., Huberdeau, D. M., & Krakauer, J. W. (2015). The influence of movement preparation time on the expression of visuomotor learning and savings. Journal of Neuroscience, 35, 5109–5117. 10.1523/JNEUROSCI.3869-14.2015, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith, A. M., & Krakauer, J. W. (2018). The multiple effects of practice: Skill, habit and reduced cognitive load. Current Opinion in Behavioral Sciences, 20, 196–201. 10.1016/j.cobeha.2018.01.015, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, R. M., Forrence, A. D., Krakauer, J. W., & Haith, A. M. (2019). Time-dependent competition between goal-directed and habitual response preparation. Nature Human Behaviour, 3, 1252–1262. 10.1038/s41562-019-0725-0, [DOI] [PubMed] [Google Scholar]
- Heald, J. B., Lengyel, M., & Wolpert, D. M. (2021). Contextual inference underlies the learning of sensorimotor repertoires. Nature. 10.1038/s41586-021-04129-3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindy, N. C., Ng, F. Y., & Turk-Browne, N. B. (2016). Linking pattern completion in the hippocampus to predictive coding in visual cortex. Nature Neuroscience, 19, 665–667. 10.1038/nn.4284, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, E. J., Smith, M. A., & Shadmehr, R. (2006). Dissociable effects of the implicit and explicit memory systems on learning control of reaching. Experimental Brain Research, 173, 425–437. 10.1007/s00221-006-0391-0, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian, J. B., & Doeller, C. F. (2021). Remapping and realignment in the human hippocampal formation predict context-dependent spatial behavior. Nature Neuroscience, 1–10. 10.1038/s41593-021-00835-3, [DOI] [PubMed] [Google Scholar]
- Keisler, A., & Shadmehr, R. (2010). A shared resource between declarative memory and motor memory. Journal of Neuroscience, 30, 14817–14823. 10.1523/JNEUROSCI.4160-10.2010, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa, S., Kohno, T., & Uka, T. (1995). Effects of delayed visual information on the rate and amount of prism adaptation in the human. Journal of Neuroscience, 15, 7644–7652. 10.1523/JNEUROSCI.15-11-07644.1995, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer, J. W. (2019). The intelligent reflex. Philosophical Psychology, 32, 822–830. 10.1080/09515089.2019.1607281 [DOI] [Google Scholar]
- Krakauer, J. W., Hadjiosif, A. M., Xu, J., Wong, A. L., & Haith, A. M. (2019). Motor learning. Comprehensive Physiology, 9, 613–663. 10.1002/cphy.c170043, [DOI] [PubMed] [Google Scholar]
- Leow, L.-A., Marinovic, W., de Rugy, A., & Carroll, T. J. (2018). Task errors contribute to implicit aftereffects in sensorimotor adaptation. European Journal of Neuroscience, 48, 3397–3409. 10.1111/ejn.14213, [DOI] [PubMed] [Google Scholar]
- Leow, L.-A., Marinovic, W., de Rugy, A., & Carroll, T. J. (2020). Task errors drive memories that improve sensorimotor adaptation. Journal of Neuroscience, 40, 3075–3088. 10.1523/JNEUROSCI.1506-19.2020, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, G. D. (1988). Toward an instance theory of automatization. Psychological Review, 95, 492–527. 10.1037/0033-295X.95.4.492 [DOI] [Google Scholar]
- Maresch, J., Werner, S., & Donchin, O. (2021). Methods matter: Your measures of explicit and implicit processes in visuomotor adaptation affect your results. European Journal of Neuroscience, 53, 504–518. 10.1111/ejn.14945, [DOI] [PubMed] [Google Scholar]
- Mattfeld, A. T., & Stark, C. E. L. (2015). Functional contributions and interactions between the human hippocampus and subregions of the striatum during arbitrary associative learning and memory. Hippocampus, 25, 900–911. 10.1002/hipo.22411, [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle, S. D., Bond, K. M., & Taylor, J. A. (2015). Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. Journal of Neuroscience, 35, 9568–9579. 10.1523/JNEUROSCI.5061-14.2015, [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle, S. D., & Collins, A. G. E. (2021). Modeling the influence of working memory, reinforcement, and action uncertainty on reaction time and choice during instrumental learning. Psychonomic Bulletin & Review, 28, 20–39. 10.3758/s13423-020-01774-z, [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle, S. D., Ivry, R. B., & Taylor, J. A. (2016). Taking aim at the cognitive side of learning in sensorimotor adaptation tasks. Trends in Cognitive Sciences, 20, 535–544. 10.1016/j.tics.2016.05.002, [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle, S. D., & Taylor, J. A. (2019). Dissociable cognitive strategies for sensorimotor learning. Nature Communications, 10, 40. 10.1038/s41467-018-07941-0, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, B. (1962). Les troubles de la memoire accompagnant des lesions hippocampiques bilaterales. In Passouant P. (Ed.), Physiologie de l'Hippocampe (pp. 257–272). Paris: Centre National de la Recherche Scientifique. [Google Scholar]
- Milner, B. (1965). Visually-guided maze learning in man: Effects of bilateral hippocampal, bilateral frontal, and unilateral cerebral lesions. Neuropsychologia, 3, 317–338. 10.1016/0028-3932(65)90005-9 [DOI] [Google Scholar]
- Milner, B., Corkin, S., & Teuber, H.-L. (1968). Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of HM. Neuropsychologia, 6, 215–234. 10.1016/0028-3932(68)90021-3 [DOI] [Google Scholar]
- Miyamoto, Y. R., Wang, S., & Smith, M. A. (2020). Implicit adaptation compensates for erratic explicit strategy in human motor learning. Nature Neuroscience, 23, 443–455. 10.1038/s41593-020-0600-3, [DOI] [PubMed] [Google Scholar]
- Morehead, J. R., Qasim, S. E., Crossley, M. J., & Ivry, R. (2015). Savings upon re-aiming in visuomotor adaptation. Journal of Neuroscience, 35, 14386–14396. 10.1523/JNEUROSCI.1046-15.2015, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, E. A., & Wise, S. P. (1996). Role of the hippocampus plus subjacent cortex but not amygdala in visuomotor conditional learning in Rhesus monkeys. Behavioral Neuroscience, 110, 1261–1270. 10.1037/0735-7044.110.6.1261, [DOI] [PubMed] [Google Scholar]
- Nashed, J. Y., Crevecoeur, F., & Scott, S. H. (2012). Influence of the behavioral goal and environmental obstacles on rapid feedback responses. Journal of Neurophysiology, 108, 999–1009. 10.1152/jn.01089.2011, [DOI] [PubMed] [Google Scholar]
- Nissen, M. J., & Bullemer, P. (1987). Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology, 19, 1–32. 10.1016/0010-0285(87)90002-8 [DOI] [Google Scholar]
- Petrides, M. (1985). Deficits on conditional associative-learning tasks after frontal-and temporal-lobe lesions in man. Neuropsychologia, 23, 601–614. 10.1016/0028-3932(85)90062-4, [DOI] [PubMed] [Google Scholar]
- Phillips, R. G., & LeDoux, J. E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106, 274–285. 10.1037/0735-7044.106.2.274, [DOI] [PubMed] [Google Scholar]
- Schapiro, A. C., Gregory, E., Landau, B., McCloskey, M., & Turk-Browne, N. B. (2014). The necessity of the medial temporal lobe for statistical learning. Journal of Cognitive Neuroscience, 26, 1736–1747. 10.1162/jocn_a_00578, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schween, R., Langsdorf, L., Taylor, J. A., & Hegele, M. (2019). How different effectors and action effects modulate the formation of separate motor memories. Scientific Reports, 9, 17040. 10.1038/s41598-019-53543-1, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schween, R., McDougle, S. D., Hegele, M., & Taylor, J. A. (2020). Assessing explicit strategies in force field adaptation. Journal of Neurophysiology, 123, 1552–1565. 10.1152/jn.00427.2019, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville, W. B., & Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20, 11–21. 10.1136/jnnp.20.1.11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr, R., Brandt, J., & Corkin, S. (1998). Time-dependent motor memory processes in amnesic subjects. Journal of Neurophysiology, 80, 1590–1597. 10.1152/jn.1998.80.3.1590, [DOI] [PubMed] [Google Scholar]
- Shadmehr, R., & Mussa-Ivaldi, F. A. (1994). Adaptive representation of dynamics during learning of a motor task. Journal of Neuroscience, 14, 3208–3224. 10.1523/JNEUROSCI.14-05-03208.1994, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr, R., Smith, M. A., & Krakauer, J. W. (2010). Error correction, sensory prediction, and adaptation in motor control. Annual Review of Neuroscience, 33, 89–108. 10.1146/annurev-neuro-060909-153135, [DOI] [PubMed] [Google Scholar]
- Shohamy, D., & Turk-Browne, N. B. (2013). Mechanisms for widespread hippocampal involvement in cognition. Journal of Experimental Psychology: General, 142, 1159–1170. 10.1037/a0034461, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. A., Ghazizadeh, A., & Shadmehr, R. (2006). Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biology, 4, e179. 10.1371/journal.pbio.0040179, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire, L. R. (2004). Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory, 82, 171–177. 10.1016/j.nlm.2004.06.005, [DOI] [PubMed] [Google Scholar]
- Stanley, J., & Krakauer, J. W. (2013). Motor skill depends on knowledge of facts. Frontiers in Human Neuroscience, 7, 503. 10.3389/fnhum.2013.00503, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. A., Klemfuss, N. M., & Ivry, R. B. (2010). An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum, 9, 580–586. 10.1007/s12311-010-0201-x, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. A., Krakauer, J. W., & Ivry, R. B. (2014). Explicit and implicit contributions to learning in a sensorimotor adaptation task. Journal of Neuroscience, 34, 3023–3032. 10.1523/JNEUROSCI.3619-13.2014, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgen, S., Parvin, D., & Diedrichsen, J. (2014). Mirror reversal and visual rotation are learned and consolidated via separate mechanisms: Recalibrating or learning de novo? Journal of Neuroscience, 34, 13768–13779. 10.1523/JNEUROSCI.5306-13.2014, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoorde, K., & Orban de Xivry, J.-J. (2019). Internal model recalibration does not deteriorate with age while motor adaptation does. Neurobiology of Aging, 80, 138–153. 10.1016/j.neurobiolaging.2019.03.020, [DOI] [PubMed] [Google Scholar]
- Verstynen, T., & Sabes, P. N. (2011). How each movement changes the next: An experimental and theoretical study of fast adaptive priors in reaching. Journal of Neuroscience, 31, 10050–10059. 10.1523/JNEUROSCI.6525-10.2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilterson, S. A., & Taylor, J. A. (2021). Implicit visuomotor adaptation remains limited after several days of training. eNeuro, 8. 10.1523/ENEURO.0312-20.2021, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth, S., Yanike, M., Frank, L. M., Smith, A. C., Brown, E. N., & Suzuki, W. A. (2003). Single neurons in the monkey hippocampus and learning of new associations. Science, 300, 1578–1581. 10.1126/science.1084324, [DOI] [PubMed] [Google Scholar]
- Zeithamova, D., Dominick, A. L., & Preston, A. R. (2012). Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron, 75, 168–179. 10.1016/j.neuron.2012.05.010, [DOI] [PMC free article] [PubMed] [Google Scholar]