Key Points
Question
Is the recent use of non–vitamin K antagonist oral anticoagulants (NOACs) associated with increased risk of intracranial hemorrhage among patients with acute ischemic stroke treated with intravenous alteplase?
Findings
This US retrospective cohort study included 163 038 patients with acute ischemic stroke treated with alteplase. Among patients with use of NOACs within 7 days of hospital arrival vs patients with no use of anticoagulants, intracranial hemorrhage occurred in 3.7% vs 3.2%, respectively, a difference that was not statistically significant after multivariable adjustment.
Meaning
Recent use of NOACs was not significantly associated with increased risk of intracranial hemorrhage among patients with acute ischemic stroke treated with alteplase.
Abstract
Importance
Current guidelines recommend against use of intravenous alteplase in patients with acute ischemic stroke who are taking non–vitamin K antagonist oral anticoagulants (NOACs).
Objective
To evaluate the safety and functional outcomes of intravenous alteplase among patients who were taking NOACs prior to stroke and compare outcomes with patients who were not taking long-term anticoagulants.
Design, Setting, and Participants
A retrospective cohort study of 163 038 patients with acute ischemic stroke either taking NOACs or not taking anticoagulants prior to stroke and treated with intravenous alteplase within 4.5 hours of symptom onset at 1752 US hospitals participating in the Get With The Guidelines–Stroke program between April 2015 and March 2020, with complementary data from the Addressing Real-world Anticoagulant Management Issues in Stroke registry.
Exposures
Prestroke treatment with NOACs within 7 days prior to alteplase treatment.
Main Outcomes and Measures
The primary outcome was symptomatic intracranial hemorrhage occurring within 36 hours after intravenous alteplase administration. There were 4 secondary safety outcomes, including inpatient mortality, and 7 secondary functional outcomes assessed at hospital discharge, including the proportion of patients discharged home.
Results
Of 163 038 patients treated with intravenous alteplase (median age, 70 [IQR, 59 to 81] years; 49.1% women), 2207 (1.4%) were taking NOACs and 160 831 (98.6%) were not taking anticoagulants prior to their stroke. Patients taking NOACs were older (median age, 75 [IQR, 64 to 82] years vs 70 [IQR, 58 to 81] years for those not taking anticoagulants), had a higher prevalence of cardiovascular comorbidities, and experienced more severe strokes (median National Institutes of Health Stroke Scale score, 10 [IQR, 5 to 17] vs 7 [IQR, 4 to 14]) (all standardized differences >10). The unadjusted rate of symptomatic intracranial hemorrhage was 3.7% (95% CI, 2.9% to 4.5%) for patients taking NOACs vs 3.2% (95% CI, 3.1% to 3.3%) for patients not taking anticoagulants. After adjusting for baseline clinical factors, the risk of symptomatic intracranial hemorrhage was not significantly different between groups (adjusted odds ratio [OR], 0.88 [95% CI, 0.70 to 1.10]; adjusted risk difference [RD], −0.51% [95% CI, −1.36% to 0.34%]). There were no significant differences in the secondary safety outcomes, including inpatient mortality (6.3% for patients taking NOACs vs 4.9% for patients not taking anticoagulants; adjusted OR, 0.84 [95% CI, 0.69 to 1.01]; adjusted RD, −1.20% [95% CI, −2.39% to −0%]). Of the secondary functional outcomes, 4 of 7 showed significant differences in favor of the NOAC group after adjustment, including the proportion of patients discharged home (45.9% vs 53.6% for patients not taking anticoagulants; adjusted OR, 1.17 [95% CI, 1.06 to 1.29]; adjusted RD, 3.84% [95% CI, 1.46% to 6.22%]).
Conclusions and Relevance
Among patients with acute ischemic stroke treated with intravenous alteplase, use of NOACs within the preceding 7 days, compared with no use of anticoagulants, was not associated with a significantly increased risk of intracranial hemorrhage.
This retrospective cohort study uses data from hospitals participating in the Get With The Guidelines–Stroke registry on patients with acute ischemic stroke and compares the safety and functional outcomes of intravenous alteplase among patients who were taking non–vitamin K antagonist oral anticoagulants (NOACs) prior to stroke vs patients who were not taking long-term anticoagulants.
Introduction
Non–vitamin K antagonist oral anticoagulants (NOACs) have become the first-line therapy for the prevention of ischemic stroke associated with nonvalvular atrial fibrillation.1 Despite their efficacy in preventing thromboembolic events, approximately 1% to 2% of patients taking NOACs are anticipated to experience an ischemic stroke each year.2,3,4,5 Intravenous alteplase is the standard medical reperfusion therapy for acute ischemic stroke. It has been demonstrated to improve clinical outcomes when given to patients meeting appropriate criteria.6,7 However, with a perceived increased risk of symptomatic intracranial hemorrhage or other serious bleeding complications, current guidelines advise against the use of alteplase in patients who are taking NOACs unless test results from an appropriate coagulation measure (such as direct factor Xa activity) are normal or more than 48 hours have passed since the last NOAC dose.8 Nonetheless, there are limited robust clinical data that have assessed the safety of alteplase use in patients taking NOACs prior to their stroke.9,10,11 Treatment is often withheld in this situation and patients receiving NOAC therapy may be deprived of the potential beneficial effects of reperfusion therapy due to an unclear safety profile.
The use of alteplase in patients taking NOACs between October 2012 and March 2015 was preliminarily explored in the American Heart Association and American Stroke Association Get With The Guidelines–Stroke (GWTG-Stroke) registry, but data for only 251 patients were available.11 Because NOAC therapy is being rapidly adopted in clinical practice, the number of patients presenting with an acute ischemic stroke while taking NOACs has increased.12,13,14 Using updated data from the GWTG-Stroke registry and complementary data from the Addressing Real-world Anticoagulant Management Issues in Stroke (ARAMIS) registry, this study evaluated the use of intravenous alteplase in patients taking NOACs prior to their strokes, and compared treatment-related complications and in-hospital outcomes with patients who received alteplase and had not been taking long-term anticoagulants.
Methods
Study Design and Data Source
This study was a prespecified retrospective analysis of data from 2 interrelated registries of patients who had an acute ischemic stroke in the US. The primary data source was the GWTG-Stroke registry, which is an ongoing, voluntary, national registry and performance improvement initiative developed by the American Heart Association and the American Stroke Association. Details of the methods for data collection in the GWTG-Stroke registry and the validity and reliability of its data collection have been published.15,16 Briefly, trained hospital personnel use a patient management tool to collect data on consecutive patients with acute ischemic stroke admitted to the hospital. Standardized data available in the registry include patient demographics, medical history, medications prior to admission, diagnostic tests (including brain imaging), treatment, and in-hospital outcomes.
In an effort to monitor racial and ethnic inequities in stroke care, data on race and ethnicity were recorded by hospital staff from various sources, including patient self-designation, by administrative personnel during the registration process, or on nursing intake forms.17 The patient management tool supports a multiple selection option functionality that includes single racial, multiple racial, and ethnic categories, and a separate data element for Hispanic ethnicity (yes, no, or not documented). The ARAMIS registry, a multicenter cohort of patients who had an acute ischemic stroke while receiving long-term anticoagulation therapy, expands on the GWTG-Stroke registry with the collection of additional data on patients in common, including more information about the precise time of the last NOAC dose. Details of the design and conduct of the ARAMIS registry also have been published.18
Participating hospitals in the GWTG-Stroke registry received either human research approval to enroll patients without individual consent under the Common Rule,19 or a waiver of authorization and exemption from subsequent review by their institutional review boards. This study was approved by the Duke University Health System institutional review board. IQVIA served as the data collection and coordination center. The Duke Clinical Research Institute served as the data analysis center and has an agreement to analyze the aggregate deidentified data for research purposes.
Study Population
The study population included patients with acute ischemic stroke who were treated with intravenous alteplase within 4.5 hours of symptom onset at GWTG-Stroke hospitals between April 1, 2015, and March 31, 2020. Patients were either taking NOAC medications or not taking any anticoagulant medications prior to their admission. According to GWTG-Stroke coding instructions, use of NOACs was defined as documentation that the patient was taking dabigatran, rivaroxaban, apixaban, or edoxaban within the 7 days before hospital arrival.
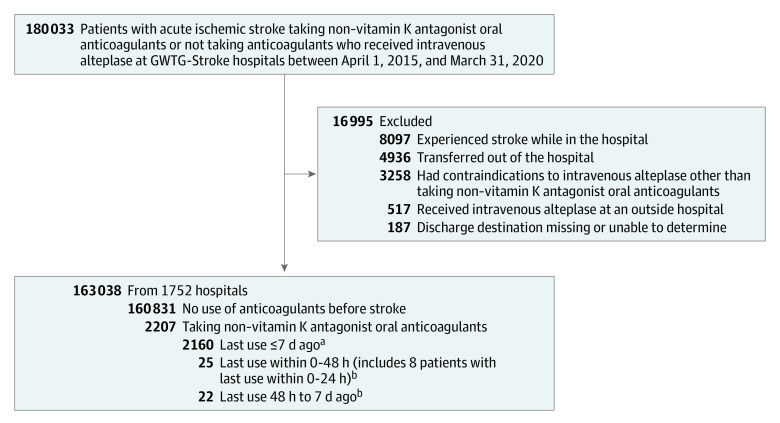
Patients were excluded if they were taking warfarin or any anticoagulant other than a NOAC, or taking multiple anticoagulants. Patients also were excluded if they received intravenous alteplase outside standard treatment guidelines (with the exception of NOAC use), had in-hospital strokes, received intravenous alteplase at an outside hospital, were transferred to another hospital, or had missing discharge information (Figure). The same inclusion and exclusion criteria were then applied to the ARAMIS cohort (eFigure in the Supplement).
Figure. Study Population in the Get With The Guidelines–Stroke (GWTG-Stroke) Registry.
aPatients had taken non–vitamin K antagonist oral anticoagulants within 7 days before hospital arrival but the exact timing of the last dose was not captured in the GWTG-Stroke registry.
bObtained from 47 patients in the Addressing Real-world Anticoagulant Management Issues in Stroke (ARAMIS) registry. The reported date and time that the patient last took their anticoagulant medication was used to generate the time epochs; however, if the exact date or time was not available, the response to the question “Did patient stop anticoagulant medication >2 days prior to stroke onset?” was alternatively used to define the time epochs of less than 48 hours and greater than 48 hours.
Outcome Measures
The primary outcome analysis focused on patients in the GWTG-Stroke registry who were taking NOACs vs those who were not taking any anticoagulants. The primary outcome was symptomatic intracranial hemorrhage occurring within 36 hours after intravenous alteplase administration. Symptomatic intracranial hemorrhage was defined as any intracranial hemorrhage documented by either computed tomography or magnetic resonance imaging, with a corresponding treating physician’s note indicating clinical deterioration from that hemorrhage.
There were 4 secondary safety outcomes: (1) inpatient mortality, (2) life-threatening or serious systemic hemorrhage within 36 hours, (3) any alteplase-related complication (symptomatic intracranial hemorrhage within 36 hours, life-threatening or serious systemic hemorrhage within 36 hours, or other serious complications [those that require additional medical interventions or prolonged length of stay]), and (4) combined in-hospital mortality or discharged to hospice.
There were 7 secondary functional outcomes assessed at hospital discharge, including independent ambulation, discharge location (home, hospice, inpatient rehabilitation facility, skilled nursing facility), free of disabilities (modified Rankin Scale score of 0-1), and functionally independent (modified Rankin Scale score of 0-2). The modified Rankin Scale is a measure of global disability ranging from a score of 0 (no symptoms) to 6 (death). A descriptive outcome was hospital length of stay. Patients with imaging evidence of an intracranial hemorrhage were classified by the type of hemorrhage (parenchymal hematoma type 2 [hematoma occupying ≥30% of the infarcted tissue with obvious mass effect], subarachnoid hemorrhage, intraventricular hemorrhage, hemorrhage outside area of infarction, or other positive finding).
Statistical Analysis
Baseline patient demographics and comorbidities, along with the characteristics of the treating hospitals, are described using proportions for categorical variables and medians with IQRs for continuous variables. The between-group comparisons of the characteristics are made using the absolute standardized difference, which was calculated as the difference in means or proportions divided by a pooled estimate of the SD and multiplied by 100. An absolute standardized difference value greater than 25 was considered a meaningful imbalance of the covariate between the 2 groups and a value between 10 and 25 was considered a potentially meaningful imbalance. In addition, the Pearson χ2 test or the Fisher exact test was used to compare binary outcomes with 95% CIs.
Multivariable logistic regression modeling was then performed to evaluate the relationship between anticoagulation therapy status (taking NOACs vs not taking anticoagulants) prior to stroke and in-hospital outcomes after thrombolytic therapy. To account for differences in baseline characteristics between the 2 groups, the analyses were conducted on a propensity-weighted population with propensity scores calculated using an overlap-weighting method20,21 (eMethods in the Supplement). The adjusted model controlled for baseline patient demographics, clinical variables, and hospital-level factors that are expected to be predictive of outcomes and have been used in prior GWTG-Stroke analyses.11,22,23,24,25 The patient-level variables included age, sex, race and ethnicity, history of atrial fibrillation or flutter, coronary artery disease or prior myocardial infarction, hypertension, dyslipidemia, diabetes, smoking, heart failure, prosthetic heart valve, previous stroke, previous transient ischemic attack, carotid stenosis, peripheral vascular disease, chronic kidney disease, medications prior to admission (including antiplatelets, antihypertensives, cholesterol reducers, and medications for diabetes), systolic blood pressure, blood glucose level, initial National Institutes of Health Stroke Scale (NIHSS) score (a measure of stroke severity with scores ranging from 0-42; a higher score indicates greater stroke severity), time from last known feeling well to alteplase initiation, receipt of endovascular therapy, insurance status, arrival by emergency medical services, and arrival time during regular working hours (7 am-6 pm Monday-Friday). The hospital-level variables included stroke center status, academic status, annual ischemic stroke volume, annual thrombolytic volume, number of hospital beds, geographic region, and rural or urban location.
In addition to adjusted odds ratios (ORs), adjusted risk differences (RDs) were calculated from the regression model by assuming an identity link function. Interaction analyses also were performed to determine whether associations between NOAC treatment and outcomes varied by receipt of endovascular therapy. A generalized estimating equations approach was used within the regression model to account for within-hospital clustering.
Patient-level data were either complete or had small missing rates (<3%). However, 6.5% of the data were missing for health insurance status. Hospital-level data were complete for stroke center status, academic status, annual ischemic stroke volume, annual thrombolytic volume, geographic region, and rural or urban location. Regarding the number of hospital beds, data were missing for less than 2% of the hospitals. For the patient-level variables with missing data, multiple imputations with 10 data sets were performed; imputed values were obtained using the fully conditional specification method.26 Estimates were combined using the rules of Rubin to appropriately reflect the added variability from imputing data. Imputed values were not obtained for patients with missing data on hospital-level variables, outcomes, or both, and these patients were excluded from the model.
Among the ARAMIS study population, exploratory analyses were conducted to evaluate the relationship between the time of last NOAC dose (0-24 hours, 0-48 hours, and >48 hours prior to admission) and thrombolytic therapy–related outcomes. The reported dates and times that patients last took their anticoagulant medications were used to generate the time epochs; however, if the exact date and time was not available, the response to the question “Did patient stop anticoagulant medication >2 days prior to stroke onset?” was alternatively used to define the time epochs of less than 48 hours (including 0-24 hours) and greater than 48 hours. The occurrence of the outcomes was reported as absolute counts and percentages for each time epoch. Adjusted analyses were not performed due to the small number of events for each outcome.
To determine whether potential selection bias existed regarding the use of thrombolytic therapy in patients taking NOACs, a separate group of patients with ischemic stroke in the GWTG-Stroke registry was identified who had arrived within 3.5 hours after symptom onset (ie, potentially eligible for a 4.5-hour treatment window), who did not have contraindications to thrombolytic therapy other than use of NOACs, and who did not receive alteplase. Their baseline characteristics were compared with those treated with alteplase.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). All P values are 2-sided, with values less than .05 considered statistically significant. Because of the potential for type I error due to multiple comparisons, the findings for the analyses of the secondary outcomes should be interpreted as exploratory.
Results
Baseline Characteristics of the Study Population
Of the 163 038 patients with ischemic stroke treated with alteplase (median age, 70 [IQR, 59-81] years; 49.1% women) across 1752 hospitals, 2207 (1.4%) were taking NOACs and 160 831 (98.6%) were not taking any anticoagulants prior to their stroke. Demographic and clinical characteristics appear in Table 1. Thrombolytic therapy metrics and hospital characteristics appear in Table 2.
Table 1. Patient Characteristics by Anticoagulant Therapy Status Prior to Stroke.
| Taking NOACs (n = 2207) | Not taking anticoagulants (n = 160 831) | Absolute standardized differencea | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, median (IQR), y | 75 (64-82) | 70 (58-81) | 26.8 |
| Sex, No. (%) | |||
| Male | 1186 (53.7) | 81 857 (50.9) | 5.7 |
| Female | 1021 (46.3) | 78 974 (49.1) | |
| Race and ethnicity, No. (%) | (n = 160 803) | ||
| Asian | 76 (3.4) | 4777 (3.0) | 2.7 |
| Hispanic | 196 (8.9) | 12 935 (8.0) | 3.0 |
| Non-Hispanic Black | 342 (15.5) | 25 980 (16.2) | 1.8 |
| Non-Hispanic White | 1488 (67.4) | 110 265 (68.6) | 2.5 |
| Otherb | 105 (4.8) | 6846 (4.3) | 2.4 |
| Health insurance status, No. (%) | (n = 2100) | (n = 150 348) | |
| Medicare | 1082 (51.5) | 65 907 (43.8) | 15.4 |
| Private | 745 (35.5) | 61 535 (40.9) | 11.2 |
| Medicaid | 222 (10.6) | 15 274 (10.2) | 1.4 |
| Self-pay or no insurance | 43 (2.0) | 6977 (4.6) | 14.5 |
| Not documented | 8 (0.4) | 655 (0.4) | 0.9 |
| Clinical characteristics | |||
| Year of stroke admission, No. (%) | |||
| 2015 | 201 (9.1) | 19 244 (12.0) | 9.3 |
| 2016 | 340 (15.4) | 29 574 (18.4) | 8.0 |
| 2017 | 419 (19.0) | 32 903 (20.5) | 3.7 |
| 2018 | 547 (24.8) | 35 642 (22.2) | 6.2 |
| 2019 | 617 (28.0) | 38 205 (23.8) | 9.6 |
| 2020 | 83 (3.8) | 5263 (3.3) | 2.7 |
| Medical history, No. (%) | |||
| Atrial fibrillation or flutter | 1614 (73.1) | 23 458 (14.6) | 146.1 |
| Coronary artery disease or prior MI | 624 (28.3) | 34 823 (21.7) | 15.4 |
| Heart failure | 421 (19.1) | 13 801 (8.6) | 30.8 |
| Prior stroke | 628 (28.5) | 32 825 (20.4) | 18.8 |
| Prior transient ischemic attack | 214 (9.7) | 14 525 (9.0) | 2.3 |
| Prosthetic heart valve | 21 (1.0) | 1231 (0.8) | 2.0 |
| Carotid stenosis | 58 (2.6) | 4385 (2.7) | 0.6 |
| Diabetes | 719 (32.6) | 46 926 (29.2) | 7.4 |
| Peripheral vascular disease | 75 (3.4) | 5128 (3.2) | 1.2 |
| Hypertension | 1753 (79.4) | 115 623 (71.9) | 17.6 |
| Smoker | 242 (11.0) | 29 045 (18.1) | 20.2 |
| Dyslipidemia | 1099 (49.8) | 72 112 (44.8) | 10.0 |
| Chronic kidney disease | 169 (7.7) | 11 830 (7.4) | 1.2 |
| Medication history, No. (%) | |||
| Any antiplatelets | 769 (34.8) | 73 319 (45.6) | 22.0 |
| Single antiplatelet | 707 (32.0) | 61 602 (38.3) | 13.2 |
| Aspirin | 691 (31.3) | 65 007 (40.4) | 19.1 |
| Clopidogrel | 132 (6.0) | 17 750 (11.0) | 18.2 |
| Ticagrelor | 2 (0.1) | 442 (0.3) | 4.3 |
| Prasugrel | 3 (0.1) | 170 (0.1) | 0.9 |
| Aspirin with dipyridamole | 1 (<0.1) | 835 (0.5) | 8.9 |
| Dual antiplatelet | 61 (2.8) | 11 590 (7.2) | 20.5 |
| Antihypertensives | 1560 (70.7) | 83 665 (52.0) | 39.1 |
| Cholesterol reducers | 1362 (61.7) | 68 844 (42.8) | 38.6 |
| Any diabetes medication | 471 (21.3) | 28 717 (17.9) | 8.8 |
| National Institutes of Health Stroke Scale score, median (IQR)c | 10 (5-17) | 7 (4-14) | 25.7 |
| Ambulatory status prior to hospital admission, No. (%) | (n = 1598) | (n = 115 953) | |
| Able to ambulate independently | 1490 (93.2) | 108 891 (93.9) | 2.7 |
| Requires assistance from person | 66 (4.1) | 4392 (3.8) | 1.8 |
| Unable to ambulate | 42 (2.6) | 2670 (2.3) | 2.1 |
| Ambulatory status at admission, No. (%) | (n = 1244) | (n = 83 025) | |
| Able to ambulate independently | 336 (27.0) | 26 740 (32.2) | 11.4 |
| Requires assistance from person | 335 (26.9) | 23 635 (28.5) | 3.4 |
| Unable to ambulate | 573 (46.1) | 32 650 (39.3) | 13.7 |
| Hospital arrival, No. (%) | |||
| By emergency medical services | 1746 (79.1) | 120 840 (75.1) | 9.5 |
| During off-hours | 1185 (53.7) | 85 406 (53.1) | 1.2 |
| Body mass index, median (IQR)d | 28.1 (24.3-33.3) | 28.0 (24.3-32.4) | 9.0 |
| Blood pressure, median (IQR), mm Hg | |||
| Systolic | 154 (136-173) | 156 (138-176) | 7.0 |
| Diastolic | 88 (75-100) | 85 (74-97) | 8.2 |
| Heart rate, median (IQR), /min | 82 (70-96) | 80 (70-92) | 11.7 |
| Blood glucose, median (IQR), mg/dL | 122 (103-153) | 119 (102-151) | 0.4 |
| International normalized ratio, median (IQR) | 1.1 (1.0-1.2) | 1.0 (1.0-1.1) | 47.6 |
| Serum creatinine, median (IQR), mg/dL | 1.0 (0.8-1.3) | 1.0 (0.8-1.2) | 0.7 |
Abbreviations: MI, myocardial infarction; NOACs, non–vitamin K antagonist oral anticoagulants.
SI conversion factors: To convert creatinine to μmol/L, multiply by 88.4; glucose to mmol/L, multiply by 0.0555.
Calculated as the difference in means or proportions divided by a pooled estimate of the SD and multiplied by 100.
Includes American Indian or Alaska Native, Native Hawaiian or Pacific Islander, multiracial, or any other non-Black or non-White racial categories.
A measure of stroke severity with scores ranging from 0-42; a higher score indicates greater stroke severity.
Calculated as weight in kilograms divided by height in meters squared.
Table 2. Thrombolytic Therapy Metrics and Hospital Characteristics by Anticoagulant Therapy Status Prior to Stroke.
| Taking NOACs (n = 2207) | Not taking anticoagulants (n = 160 831) | Absolute standardized differencea | |
|---|---|---|---|
| Thrombolytic therapy metrics | |||
| Time, median (IQR), min | |||
| Onset-to-needle | 122 (89-168) | 123 (91-168) | 1.9 |
| Door-to-needle | 51 (37-69) | 49 (36-66) | 7.7 |
| Onset-to-arrival | 62 (40-102) | 64 (42-106) | 6.5 |
| Received endovascular therapy, No. (%) | 415 (18.8) | 18 526 (11.5) | 20.4 |
| Presence of large vessel occlusion, No./total (%)b | 532/1214 (43.8) | 25 291/76 322 (33.1) | 22.1 |
| Site of occlusion, No. (%)b | (n = 532) | (n = 25 276) | |
| Middle cerebral artery | 442 (83.1) | 19 022 (75.3) | 19.4 |
| Internal carotid artery | 69 (13.0) | 4737 (18.7) | 15.9 |
| Other cerebral artery branch | 41 (7.7) | 2359 (9.3) | 5.8 |
| Basilar artery | 16 (3.0) | 904 (3.6) | 3.2 |
| Vertebral artery | 9 (1.7) | 1054 (4.2) | 14.7 |
| Hospital characteristics | |||
| No. of beds, median (IQR) | 411 (278-620) | 384 (255-585) | 6.8 |
| Annual ischemic stroke volume, median (IQR) | 368 (232-563) | 331 (211-530) | 8.8 |
| Annual intravenous thrombolytic therapy cases, median (IQR) | 51 (30-82) | 46 (26-73) | 13.5 |
| Academic hospital, No. (%) | 1663 (75.4) | 122 631 (76.2) | 2.1 |
| Stroke center status, No. (%) | |||
| Comprehensive | 278 (12.6) | 19 577 (12.2) | 1.3 |
| Primary | 1162 (52.7) | 88 717 (55.2) | 5.0 |
| Neither comprehensive nor primary | 767 (34.8) | 52 537 (32.7) | 4.4 |
| Geographic region, No. (%) | |||
| South | 1010 (45.8) | 66 393 (41.3) | 9.1 |
| West | 503 (22.8) | 34 935 (21.7) | 2.6 |
| Northeast | 373 (16.9) | 29 647 (18.4) | 4.0 |
| Midwest | 321 (14.5) | 29 856 (18.6) | 10.8 |
| Rural location, No. (%) | 41 (1.9) | 3800 (2.4) | 3.5 |
Abbreviation: NOACs, non–vitamin K antagonist oral anticoagulants.
Calculated as the difference in means or proportions divided by a pooled estimate of the SD and multiplied by 100.
Not available in the registry until 2019.
Patients taking NOACs were older (median age, 75 years [IQR, 64-82 years]) compared with those not taking anticoagulants (median age, 70 years [IQR, 58-81 years]) and had a higher prevalence of coexistent medical conditions, such as atrial fibrillation or flutter, coronary artery disease or prior myocardial infarction, hypertension, heart failure, and prior stroke (Table 1; all absolute standardized differences >10). Furthermore, patients taking NOACs experienced more severe strokes (median NIHSS score, 10 [IQR, 5-17] vs 7 [IQR, 4-14] for those not taking anticoagulants; absolute standardized difference, 25.7) and were more likely to undergo subsequent endovascular therapy (18.8% vs 11.5%, respectively; absolute standardized difference, 20.4). However, 2 measures of time to thrombolytic treatment initiation were not notably different between the 2 groups; there was a median onset-to-needle time of 122 minutes (IQR, 89-168 minutes) for patients taking NOACs vs 123 minutes (IQR, 91-168 minutes) for those not taking anticoagulants and a median door-to-needle time of 51 minutes (IQR, 37-69 minutes) vs 49 minutes (IQR, 36-66 minutes), respectively (Table 2).
Primary Outcome
Overall, 5210 patients (3.2%; 95% CI, 3.1% to 3.3%) developed symptomatic intracranial hemorrhage within 36 hours after intravenous alteplase administration. The unadjusted rate of symptomatic intracranial hemorrhage was 3.7% (95% CI, 2.9% to 4.5%) in patients taking NOACs vs 3.2% (95% CI, 3.1% to 3.3%) in patients not taking anticoagulants (Table 3). After adjustment for NIHSS score and other baseline clinical factors, the risk of symptomatic intracranial hemorrhage was not significantly different between the 2 groups (adjusted OR, 0.88 [95% CI, 0.70 to 1.10]; adjusted RD, −0.51% [95% CI, −1.36% to 0.34%]).
Table 3. Outcomes of Patients With Acute Ischemic Stroke Treated With Intravenous Alteplase by Anticoagulant Therapy Status Prior to Stroke.
| Taking NOACs (n = 2207) |
Not taking anticoagulants (n = 160 831) |
Odds ratio (95% CI) | Adjusted absolute risk difference, % (95% CI)a | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Primary outcome | |||||
| Symptomatic intracranial hemorrhage within 36 h, No. (%) | 81 (3.7) | 5129 (3.2) | 1.18 (0.94 to 1.47) | 0.88 (0.70 to 1.10) | −0.51 (−1.36 to 0.34) |
| Secondary outcomes | |||||
| Life-threatening or serious systemic hemorrhage within 36 h, No. (%) | 16 (0.7) | 898 (0.6) | 1.28 (0.79 to 2.07) | 0.95 (0.57 to 1.60) | −0.03 (−0.39 to 0.32) |
| Any alteplase complication, No. (%)b | 152 (6.9) | 9728 (6.0) | 1.15 (0.97 to 1.37) | 0.87 (0.73 to 1.04) | −0.94 (−2.09 to 0.21) |
| Inpatient mortality, No. (%) | 139 (6.3) | 7925 (4.9) | 1.28 (1.05 to 1.56) | 0.84 (0.69 to 1.01) | −1.20 (−2.39 to −0) |
| In-hospital mortality or discharged to hospice, No. (%) | 273 (12.4) | 15 109 (9.4) | 1.35 (1.16 to 1.56) | 0.87 (0.76 to 1.00) | −1.63 (−3.20 to −0.06) |
| Able to ambulate independently at hospital discharge, No./total (%) | 1008/1951 (51.7) | 83 807/144 751 (57.9) | 0.81 (0.74 to 0.88) | 1.25 (1.12 to 1.40) | 5.65 (2.91 to 8.40) |
| Free of disabilities (modified Rankin Scale score of 0-1 at hospital discharge), No./total (%)c | 372/1382 (26.9) | 34 548/101 554 (34.0) | 0.74 (0.65 to 0.83) | 1.22 (1.06 to 1.42) | 3.71 (0.91 to 6.52) |
| Functionally independent (modified Rankin Scale score of 0-2 at hospital discharge), No./total (%)c | 513/1382 (37.1) | 45 212/101 554 (44.5) | 0.76 (0.68 to 0.85) | 1.27 (1.11 to 1.45) | 5.28 (2.15 to 8.41) |
| Discharge location, No./total (%) | |||||
| Home | 1014/2207 (45.9) | 86 171/160 831 (53.6) | 0.75 (0.69 to 0.82) | 1.17 (1.06 to 1.29) | 3.84 (1.46 to 6.22) |
| Hospice | 135/2207 (6.1) | 7288/160 831 (4.5) | 1.36 (1.12 to 1.65) | 0.91 (0.76 to 1.10) | −0.57 (−1.71 to 0.56) |
| Inpatient rehabilitation facility | 490/2179 (22.5) | 34 110/158 499 (21.5) | 1.06 (0.95 to 1.17) | 1.01 (0.90 to 1.14) | 0.20 (−1.82 to 2.22) |
| Skilled nursing facility | 403/2179 (18.5) | 23 228/158 499 (14.7) | 1.31 (1.17 to 1.47) | 0.90 (0.80 to 1.02) | −1.59 (−3.51 to 0.33) |
| Hospital length of stay >4 d, No./total (%) | 1032/2194 (47.0) | 62103/160 183 (38.8) | 1.30 (1.19 to 1.43) | 0.93 (0.84 to 1.03) | −1.73 (−4.22 to 0.76) |
Abbreviation: NOACs, non–vitamin K antagonist oral anticoagulants.
Adjusted for potential confounders including age, sex, race and ethnicity, medical history of atrial fibrillation or flutter, coronary artery disease or prior myocardial infarction, hypertension, dyslipidemia, diabetes, smoking, heart failure, prosthetic heart valve, previous stroke, previous transient ischemic attack, carotid stenosis, peripheral vascular disease, chronic kidney disease, medications prior to hospital admission (including antiplatelets, antihypertensives, cholesterol reducers, and any diabetes), systolic blood pressure, blood glucose level, initial National Institutes of Health Stroke Scale score, last known time of being well to alteplase initiation, receipt of endovascular therapy, insurance status, arrival by emergency medical services, arrival time during regular working hours (7 am-6 pm Monday-Friday), and hospital-level variables (including stroke center status, academic status, annual ischemic stroke volume, annual thrombolytic volume, number of beds, geographic region, and rural or urban location). Additional details appear in the eMethods in the Supplement.
Includes symptomatic intracranial hemorrhage within 36 hours, life-threatening or serious systemic hemorrhage within 36 hours, or other serious complications (those that required additional medical interventions or prolonged length of stay).
The modified Rankin Scale has a score range of 0 to 6 with 0 being no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death.
Secondary Outcomes
The unadjusted rate for life-threatening or serious systemic hemorrhage was 0.7% (95% CI, 0.4% to 1.2%) in patients taking NOACs vs 0.6% (95% CI, 0.5% to 0.6%) in patients not taking anticoagulants and the rate for any alteplase-related complication was 6.9% (95% CI, 5.9% to 8.0%) vs 6.0% (95% CI, 5.9% to 6.2%), respectively. There were no significant differences noted after risk adjustment for these outcomes (Table 3). Among patients with imaging evidence of an intracranial hemorrhage, there were no significant differences in the type of intracranial hemorrhage seen on imaging between patients taking NOACs and those not taking anticoagulants (eTable 1 in the Supplement).
The unadjusted rates for inpatient mortality were higher in patients taking NOACs (6.3% [95% CI, 5.3% to 7.4%]) than in those not taking anticoagulants (4.9% [95% CI, 4.8% to 5.0%]); however, there was no significant difference after risk adjustment (adjusted OR, 0.84 [95% CI, 0.69 to 1.01]; adjusted RD, −1.20% [95% CI, −2.39% to −0%]). The unadjusted rates were higher for the combined outcome of in-hospital mortality or discharge to hospice (12.4% [95% CI, 11.0% to 13.8%] for patients taking NOACs vs 9.4% [95% CI, 9.3% to 9.5%] for those not taking anticoagulants). However, the adjusted combined outcome of in-hospital mortality or discharge to hospice reversed directionality, with an adjusted OR of 0.87 (95% CI, 0.76 to 1.00) and an adjusted RD of −1.63% (95% CI, −3.20% to −0.06%), suggesting that the higher unadjusted rates seen in the NOAC group may be due to the greater risk profile of those patients such as older age, greater stroke severity (higher NIHSS score), and presence of existing comorbidities (Table 3).
After risk adjustment, patients taking NOACs were significantly more likely to ambulate independently at hospital discharge (51.7%) compared with patients not taking anticoagulants (57.9%) (adjusted OR, 1.25 [95% CI, 1.12 to 1.40]; adjusted RD, 5.65% [95% CI, 2.91% to 8.40%]), be discharged home (45.9% vs 53.6%, respectively; adjusted OR, 1.17 [95% CI, 1.06 to 1.29]; adjusted RD, 3.84% [95% CI, 1.46% to 6.22%]), be free of disabilities (modified Rankin scale score of 0-1) at hospital discharge (26.9% vs 34.0%; adjusted OR, 1.22 [95% CI, 1.06 to 1.42]; adjusted RD, 3.71% [95% CI, 0.91% to 6.52%]), and be functionally independent (modified Rankin scale score of 0-2) at hospital discharge (37.1% vs 44.5%; adjusted OR, 1.27 [95% CI, 1.11 to 1.45]; adjusted RD, 5.28% [95% CI, 2.15% to 8.41%]).
There were no significant differences in the proportion of patients who were discharged to hospice, an inpatient rehabilitation facility, or a skilled nursing facility between the 2 groups (Table 3). For hospital length of stay longer than 4 days, the proportion of patients was 47.0% for patients taking NOACs vs 38.8% for patients not taking anticoagulants (adjusted OR, 0.93 [95% CI, 0.84 to 1.03]; adjusted RD, −1.73% [95% CI, −4.22% to 0.76%]). The associations between NOAC treatment and outcomes after thrombolytic therapy did not significantly differ by receipt of endovascular therapy (eTable 2 in the Supplement).
Exploratory Analysis
Baseline characteristics of patients taking NOACs who were treated with alteplase and included in the ARAMIS registry were similar to those in the GWTG-Stroke registry (eTable 3 in the Supplement). Of the 47 patients prescribed NOACs who had a time of last NOAC dose reported or had a recorded response to the question of whether they had stopped their anticoagulant medication more than 2 days prior to stroke onset, 8 (17.0%) took their last dose between 0 and 24 hours ago, 25 (53.2%) took their last dose between 0 and 48 hours ago, and 22 (46.8%) took their last dose more than 48 hours prior to hospital admission. Of the 25 patients who took their last NOAC dose between 0 and 48 hours ago, 2 (8.0% [95% CI, 1.0% to 26.0%]) developed symptomatic intracranial hemorrhage after receiving thrombolytic therapy (Table 4). There were no reported alteplase-related complications among patients who had their last NOAC dose between 0 and 24 hours (0/8) or more than 48 hours (0/22) prior to hospital admission.
Table 4. Outcomes of Patients Taking a NOAC Who Had an Acute Ischemic Stroke and Received Intravenous Alteplase in the ARAMIS Registry.
| No. (%) of patients who had an acute ischemic stroke by timing of last NOAC dose prior to strokea | ||||
|---|---|---|---|---|
| Overall (n = 47) | 0-24 h ago (n = 8) | 0-48 h ago (n = 25) | >48 h ago (n = 22) | |
| Primary outcome | ||||
| Symptomatic intracranial hemorrhage within 36 h | 2 (4.3) | 0 | 2 (8.0) | 0 |
| Secondary outcomes | ||||
| Life-threatening or serious systemic hemorrhage within 36 h | 0 | 0 | 0 | 0 |
| Any alteplase complicationb | 3 (6.4) | 0 | 3 (12.0) | 0 |
| Inpatient mortality | 2 (4.3) | 0 | 1 (4.0) | 1 (4.6) |
| In-hospital mortality or discharged to hospice | 4 (8.5) | 0 | 2 (8.0) | 2 (9.1) |
| Able to ambulate independently at hospital dischargec | 28/45 (62.2) | 5/8 (62.5) | 12/24 (50.0) | 16/21 (76.2) |
| Free of disabilities (modified Rankin Scale score of 0-1 at hospital discharge)d | 14/40 (35.0) | 1/7 (14.3) | 4/21 (19.0) | 10/19 (52.6) |
| Functionally independent (modified Rankin Scale score of 0-2 at hospital discharge)d | 17/40 (42.5) | 2/7 (28.6) | 6/21 (28.6) | 11/19 (57.9) |
| Discharge location | ||||
| Home | 26 (55.3) | 5 (62.5) | 10 (40.0) | 16 (72.7) |
| Hospice | 2 (4.3) | 0 | 1 (4.0) | 1 (4.5) |
| Inpatient rehabilitation facility | 11 (23.4) | 2 (25.0) | 7 (28.0) | 4 (18.2) |
| Skilled nursing facility | 6 (12.8) | 1 (12.5) | 6 (24.0) | 0 |
| Hospital length of stay >4 d | 19 (40.4) | 3 (37.5) | 14 (56.0) | 5 (22.7) |
Abbreviations: ARAMIS, Addressing Real-world Anticoagulant Management Issues in Stroke; NOAC, non–vitamin K antagonist oral anticoagulant.
These data are from a subset of patients participating in the ARAMIS registry. The reported date and time that the patient last took their anticoagulant medication was used to generate the time epochs; however, if the exact date and time was not available, the response to the question “Did patient stop anticoagulant medication >2 days prior to stroke onset?” was alternatively used to define the time epochs of less than 48 hours and greater than 48 hours.
Includes symptomatic intracranial hemorrhage within 36 hours, life-threatening or serious systemic hemorrhage within 36 hours, or other serious complications (those that required additional medical interventions or prolonged length of stay).
Data are expressed as No./total (%).
Data are expressed as No./total (%). The modified Rankin Scale has a score range of 0 to 6 with 0 being no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death.
Potential Treatment Selection
From the same period in the GWTG-Stroke registry, there were 22 977 patients taking NOACs who arrived within 3.5 hours after stroke symptom onset, who did not have any other contraindications to thrombolytic therapy, and who did not receive alteplase (eTable 4 in the Supplement). The patients who received treatment with alteplase were younger (median age, 75 years vs 77 years for eligible patients not treated with alteplase), had fewer comorbidities, and presented with more severe strokes (median NIHSS score of 10 vs 5, respectively).
Furthermore, a greater proportion of patients taking NOACs and treated with alteplase arrived at the hospital by emergency medical services and were more likely to arrive earlier (median time from symptom onset to arrival of 62 minutes vs 78 minutes for patients who were not treated with alteplase). Patients treated with alteplase were also more likely to have a large vessel occlusion (43.8% vs 33.6% for patients who were not treated with alteplase) and undergo subsequent endovascular therapy treatment (18.8% vs 12.7%, respectively). In addition, patients who received thrombolytic therapy were more likely to have received their care at centers with more thrombolytic therapy experience than patients who did not receive thrombolytic therapy (median of 51 annual cases vs 40 cases). For the abovementioned variables, all absolute standardized differences were greater than 10.
Discussion
In this retrospective, registry-based study of patients with acute ischemic stroke treated with intravenous alteplase at hospitals in the US, recent use of NOACs before stroke was not independently associated with increased rates of symptomatic intracranial hemorrhage, life-threatening or serious systemic hemorrhage, any alteplase-related complication, or inpatient mortality. Compared with patients not taking anticoagulants, prior use of NOACs was significantly associated with better adjusted outcomes in terms of patients being discharged home, their ambulatory status, their freedom from disability, and their functional independence at hospital discharge.
To our knowledge, this study represents the largest report on the safety and outcomes of thrombolytic therapy in the setting of recent NOAC use. Compared with a previous report using the GWTG-Stroke registry and a multicenter observational pilot study in Europe,10,11 the current study population is an order of magnitude larger. Accordingly, the confirmation of the absence of increased symptomatic intracranial hemorrhage, life-threatening or serious systemic hemorrhage, and inpatient mortality in the present study provides evidence that may potentially support the safety of intravenous alteplase in patients with acute ischemic stroke who have recently used NOACs within the past 7 days. In addition, even though the prior GWTG-Stroke study also found that NOAC use was associated with higher rates of discharge home and independent ambulation at discharge, it showed only nominally, not statistically significant, higher rates of freedom from disability and functional independence at discharge.
It is possible that some of the patients in the GWTG-Stroke registry experienced a stroke because of nonadherence or interruption of their NOAC intake, whereas the ischemic stroke for others may have been a breakthrough event that occurred despite not missing an anticoagulant dose. By including data from the more detailed, affiliated ARAMIS cohort, the current investigation provided additional insight into the timing of most recent NOAC use that was unavailable in earlier studies. Among the ARAMIS study patients, 25 of 47 patients (53.2%) reported taking their last dose of NOAC less than 48 hours prior to alteplase therapy. Among these patients, 8 of 25 (32.0%) reported taking their last dose within the prior 24 hours.
Because patients in the ARAMIS cohort were similar in patient and hospital characteristics to patients in the overall GWTG-Stroke cohort, it may be that similar frequencies are present in the broader GWTG-Stroke population in terms of timing of the last NOAC dose. Of the 25 patients who took their last dose of NOAC within 48 hours prior to hospital admission, 2 (8.0%) developed symptomatic intracranial hemorrhage after receiving thrombolytic therapy. Of the 8 patients who took their last NOAC dose within 24 hours prior to hospital admission, no cases of symptomatic intracranial hemorrhage were reported. The rates of symptomatic intracranial hemorrhage in these subsets seem notably different from the rate for the overall GTWG-Stroke population (3.7%). However, due to the small number of patients in the ARAMIS cohort, the differences should be interpreted with caution.
Compared with patients not taking any anticoagulants, patients taking NOACs were significantly more likely to be discharged home, ambulate independently, and have less global disability at discharge. There are several potential mechanisms that may help explain the better functional outcomes seen among patients taking NOACs. One possibility is that patients who had recently taken NOACs may have low, rather than absent, serum NOAC concentrations at the time of alteplase treatment either because of slow metabolism in some of the patients who took their last dose more than 48 hours before hospital admission or ineffective dosing in other patients who took their last dose within 48 hours and had breakthrough ischemic strokes. As such, low concentrations of NOACs could potentiate beneficial effects of alteplase in recanalizing the target occlusion without unduly increasing bleeding adverse effects.
Another possibility may stem from differences in the composition of the target occlusions between patient groups. In the NOAC population in which atrial fibrillation predominated as a stroke cause, the target occlusion typically would be an embolism from a detached thrombus originating from the heart. In the non-NOAC population, the composition of the target occlusion may be more variable, and sometimes include admixed atherosclerosis and supervening thrombosis. Recanalization after alteplase may be more enduring in relatively normal recipient vessels from which thrombi have been cleared than in the vessels with residual atherosclerosis prone to reocclusion. Furthermore, it is also possible that these results may reflect residual or unmeasured confounding.
Patients who present with an ischemic stroke and are subsequently found to have a large vessel occlusion pose another clinical dilemma. The decision to proceed with a mechanical thrombectomy (ie, catheter-based removal of a proximal intracranial arterial clot) is typically made independent of whether the patient received intravenous alteplase or not. Previous studies have not examined the outcomes of patients taking NOACs who were treated with intravenous alteplase prior to undergoing mechanical thrombectomy.11 The complication of greatest concern is risk of symptomatic intracranial hemorrhage from reperfusion. In this study, interaction analyses were performed to determine whether adjusted associations between NOAC treatment and outcomes after thrombolytic therapy varied by receipt of endovascular therapy. In these analyses, none of the outcomes, including rate of symptomatic intracranial hemorrhage, was influenced by receipt of endovascular therapy, suggesting endovascular therapy is probably safe for select patients with acute ischemic stroke with recent exposure to NOACs within the preceding 7 days and treated with alteplase.
Limitations
This study has several limitations. First, this was an observational cohort analysis and not a randomized clinical trial. There were several important differences in baseline characteristics between patients taking NOACs and those who were not taking any anticoagulants. Although a propensity-weighted analysis was performed to adjust for these confounding variables, the presence of any residual or unmeasured confounding may bias comparisons and outcomes.
Second, there is strong potential for selection bias related to which patients taking NOACs received alteplase. By comparing patients prescribed NOACs who were treated vs not treated with alteplase, it appears that patients who were treated with alteplase had more severe strokes, arrived at the hospital earlier, and received care at centers with more experience with thrombolytic administration. Despite not meeting the standard eligibility criteria, these patients may have been given alteplase for other reasons not fully captured in the GWTG-Stroke registry. In addition, nearly 5000 patients who were transferred to another hospital were excluded because outcomes after transfer were not captured in the registry, which could have biased the study population.
Third, more precise information on the time of last NOAC dose was available in only a small number of patients from the ARAMIS cohort, which makes the delineation of a clear relationship between bleeding risk and the timing of the last NOAC dose challenging. Although 53.2% of patients in the ARAMIS registry had their last NOAC dose within 48 hours, it is possible that a substantial portion of patients taking NOACs within the GWTG-Stroke cohort may have had an interruption in their NOAC intake for more than 48 hours. Therefore, the generalizability of the findings to all patients taking NOACs who experience acute ischemic stroke is not possible. Further research is needed to determine whether intravenous alteplase is safe in an unselected population of patients taking NOACs and especially in the subset of the population with more recent exposure to NOACs within 24 hours or within 24 to 48 hours of hospital admission.
Fourth, some patients may have received reduced dosing of alteplase either because of their history of NOAC use or because of a plan to subsequently receive mechanical thrombectomy. However, alteplase dosing information was not captured in the registry during this study period. The current guidelines continue to recommend a standard dose of alteplase after publication of the Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED), which did not find noninferiority for the lower dose.8,27 Therefore, use of reduced dose alteplase is probably uncommon in US clinical practice because it goes against US Food and Drug Administration labeling and against guidelines from the American Stroke Association.
Fifth, levels of drug-specific coagulation assays were neither available in the data set nor widely available in a timely manner in general US clinical practice during the study period. Wider availability and use of these assays in the future may help inform thrombolytic treatment decision-making among patients taking NOAC therapy.
Sixth, GWTG-Stroke and ARAMIS are both voluntary registries of enrolled hospitals that have the capacity to meet certain program requirements. As such, these results may not be generalizable to patients treated at nonregistry hospitals or in other countries.
Conclusions
Among patients with acute ischemic stroke treated with intravenous alteplase, use of NOACs within the preceding 7 days, compared with no use of anticoagulants, was not associated with a significantly increased risk of intracranial hemorrhage.
eFigure. Study Population in the ARAMIS Registry
eMethods. Propensity Score Overlap Weighting
eTable 1. Brain Imaging Results
eTable 2. Associations between NOAC Treatment and Post-Thrombolytic Outcomes by Receipt of Endovascular Therapy (interaction P values)
eTable 3. Baseline Patient and Hospital Characteristics of Patients Taking NOACs and Treated with Alteplase by ARAMIS and GWTG-Stroke Registries
eTable 4. Baseline Patient and Hospital Characteristics of Patients Taking NOACs Who Are Eligible for Intravenous Alteplase, Stratified by Treatment Status
References
- 1.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Published correction appears in Circulation. 2019;140(6):e285. Circulation. 2019;140(2):e125-e151. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. Published correction appears in N Engl J Med. 2010;363(19):1877. N Engl J Med. 2009;361(12):1139-1151. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. Published correction appears in Stroke. 2019;50(12):e440-e441. Stroke. 2019;50(12):e344-e418. [DOI] [PubMed] [Google Scholar]
- 9.Jin C, Huang RJ, Peterson ED, et al. Intravenous tPA (tissue-type plasminogen activator) in patients with acute ischemic stroke taking non-vitamin K antagonist oral anticoagulants preceding stroke. Stroke. 2018;49(9):2237-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiffge DJ, Hooff RJ, Nolte CH, et al. Recanalization therapies in acute ischemic stroke patients. Circulation. 2015;132(13):1261-1269. [DOI] [PubMed] [Google Scholar]
- 11.Xian Y, Federspiel JJ, Hernandez AF, et al. Use of intravenous recombinant tissue plasminogen activator in patients with acute ischemic stroke who take non–vitamin K antagonist oral anticoagulants before stroke. Circulation. 2017;135(11):1024-1035. [DOI] [PubMed] [Google Scholar]
- 12.Sindet-Pedersen C, Pallisgaard JL, Staerk L, et al. Temporal trends in initiation of VKA, rivaroxaban, apixaban and dabigatran for the treatment of venous thromboembolism. Sci Rep. 2017;7(1):3347. doi: 10.1038/s41598-017-03596-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu AYX, Malo S, Svenson LW, Wilton SB, Hill MD. Temporal trends in the use and comparative effectiveness of direct oral anticoagulant agents versus warfarin for nonvalvular atrial fibrillation. J Am Heart Assoc. 2017;6(11):e007129. doi: 10.1161/JAHA.117.007129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. Pharmacotherapy. 2018;38(9):907-920. doi: 10.1002/phar.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonarow GC, Reeves MJ, Smith EE, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get With The Guidelines–Stroke. Circ Cardiovasc Qual Outcomes. 2010;3(3):291-302. [DOI] [PubMed] [Google Scholar]
- 16.Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get With The Guidelines–Stroke (GWTG-Stroke). Am Heart J. 2012;163(3):392-398, 398.e1. [DOI] [PubMed] [Google Scholar]
- 17.Schwamm LH, Reeves MJ, Pan W, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121(13):1492-1501. [DOI] [PubMed] [Google Scholar]
- 18.Xian Y, Hernandez AF, Harding T, et al. Acute management of stroke patients taking non–vitamin K antagonist oral anticoagulants. Am Heart J. 2016;182:28-35. [DOI] [PubMed] [Google Scholar]
- 19.Office for Human Research Protections (OHRP) . Pre-2018 requirements. Accessed December 9, 2021. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/regulatory-text/index.html
- 20.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113(521):390-400. doi: 10.1080/01621459.2016.126046629930437 [DOI] [Google Scholar]
- 21.Thomas LE, Li F, Pencina MJ. Overlap weighting. JAMA. 2020;323(23):2417-2418. [DOI] [PubMed] [Google Scholar]
- 22.Xian Y, Liang L, Smith EE, et al. Risks of intracranial hemorrhage among patients with acute ischemic stroke receiving warfarin and treated with intravenous tissue plasminogen activator. JAMA. 2012;307(24):2600-2608. [DOI] [PubMed] [Google Scholar]
- 23.Xian Y, Federspiel JJ, Grau-Sepulveda M, et al. Risks and benefits associated with prestroke antiplatelet therapy among patients with acute ischemic stroke treated with intravenous tissue plasminogen activator. JAMA Neurol. 2016;73(1):50-59. [DOI] [PubMed] [Google Scholar]
- 24.Smith EE, Shobha N, Dai D, et al. Risk score for in-hospital ischemic stroke mortality derived and validated within the Get With The Guidelines–Stroke program. Circulation. 2010;122(15):1496-1504. [DOI] [PubMed] [Google Scholar]
- 25.Menon BK, Saver JL, Prabhakaran S, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke. 2012;43(9):2293-2299. [DOI] [PubMed] [Google Scholar]
- 26.Enders CK. Applied Missing Data Analysis. Guilford Press; 2010. [Google Scholar]
- 27.Anderson CS, Robinson T, Lindley RI, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374(24):2313-2323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Study Population in the ARAMIS Registry
eMethods. Propensity Score Overlap Weighting
eTable 1. Brain Imaging Results
eTable 2. Associations between NOAC Treatment and Post-Thrombolytic Outcomes by Receipt of Endovascular Therapy (interaction P values)
eTable 3. Baseline Patient and Hospital Characteristics of Patients Taking NOACs and Treated with Alteplase by ARAMIS and GWTG-Stroke Registries
eTable 4. Baseline Patient and Hospital Characteristics of Patients Taking NOACs Who Are Eligible for Intravenous Alteplase, Stratified by Treatment Status