Significance
The ability to identify individual objects or events as members of a kind (e.g., “knife,” “dog,” or “party”) is a fundamental aspect of human cognition. It allows us to quickly access a wealth of information pertaining to a newly encountered object or event and use it to guide our behavior. How is this information represented in the brain? We used functional MRI to analyze patterns of brain activity corresponding to hundreds of familiar concepts and quantitatively characterized the informational structure of these patterns. Our results indicate that conceptual knowledge is stored as patterns of neural activity that encode sensory-motor and affective information about each concept, contrary to the long-held idea that concept representations are independent of sensory-motor experience.
Keywords: semantic memory, concept representation, lexical semantics, embodied semantics, representational similarity analysis
Abstract
The nature of the representational code underlying conceptual knowledge remains a major unsolved problem in cognitive neuroscience. We assessed the extent to which different representational systems contribute to the instantiation of lexical concepts in high-level, heteromodal cortical areas previously associated with semantic cognition. We found that lexical semantic information can be reliably decoded from a wide range of heteromodal cortical areas in the frontal, parietal, and temporal cortex. In most of these areas, we found a striking advantage for experience-based representational structures (i.e., encoding information about sensory-motor, affective, and other features of phenomenal experience), with little evidence for independent taxonomic or distributional organization. These results were found independently for object and event concepts. Our findings indicate that concept representations in the heteromodal cortex are based, at least in part, on experiential information. They also reveal that, in most heteromodal areas, event concepts have more heterogeneous representations (i.e., they are more easily decodable) than object concepts and that other areas beyond the traditional “semantic hubs” contribute to semantic cognition, particularly the posterior cingulate gyrus and the precuneus.
The capacity for conceptual knowledge is arguably one of the most defining properties of human cognition, and yet it is still unclear how concepts are represented in the brain. Recent developments in functional neuroimaging and computational linguistics have sparked renewed interest in elucidating the information structures and neural circuits underlying concept representation (1–5). Attempts to characterize the representational code for concepts typically involve information structures based on three qualitatively distinct types of information, namely, taxonomic, experiential, and distributional information. As the term implies, a taxonomic information system relies on category membership and intercategory relations. Our tendency to organize objects, events, and experiences into discrete categories has led most authors—dating back at least to Plato (6)—to take taxonomic structure as the central property of conceptual knowledge (7). The taxonomy for concepts is traditionally seen as a hierarchically structured network, with basic-level categories (e.g., “apple,” “orange”) grouped into superordinate categories (e.g., “fruit,” “food”) and subdivided into subordinate categories (e.g., “Gala apple,” “tangerine”) (8). A prominent account in cognitive science maintains that such categories are represented in the mind/brain as purely symbolic entities, whose semantic content and usefulness derive primarily from how they relate to each other (9, 10). Such representations are seen as qualitatively distinct from the sensory-motor processes through which we interact with the world, much like the distinction between software and hardware in digital computers.
An experiential representational system, on the other hand, encodes information about the experiences that led to the formation of particular concepts. It is motivated by a view, often referred to as embodied, grounded, or situated semantics, in which concepts arise primarily from generalization over particular experiences, as information originating from the various modality-specific systems (e.g., visual, auditory, tactile, motor, affective) is combined and re-encoded into progressively more schematic representations that are stored in memory. Since, in this view, there is a degree of continuity between conceptual and modality-specific systems, concept representations are thought to reflect the structure of the perceptual, affective, and motor processes involved in those experiences (11–14).
Finally, distributional information pertains to statistical patterns of co-occurrence between lexical concepts (i.e., concepts that are widely shared within a population and denoted by a single word) in natural language usage. As is now widely appreciated, these co-occurrence patterns encode a substantial amount of information about word meaning (15–17). Although word co-occurrence patterns primarily encode contextual associations, such as those connecting the words “cow,” “barn,” and “farmer,” semantic similarity information is indirectly encoded since words with similar meanings tend to appear in similar contexts (e.g., “cow” and “horse,” “pencil” and “pen”). This has led some authors to propose that concepts may be represented in the brain, at least in part, in terms of distributional information (15, 18).
Whether, and to what extent, each of these types of information plays a role in the neural representation of conceptual knowledge is a topic of intense research and debate. A large body of evidence has emerged from behavioral studies, functional neuroimaging experiments, and neuropsychological assessments of patients with semantic deficits, with results typically interpreted in terms of taxonomic (19–24), experiential (13, 25–34), or distributional (2, 3, 5, 35, 36) accounts. However, the extent to which each of these representational systems plays a role in the neural representation of conceptual knowledge remains controversial (23, 37, 38), in part, because their representations of common lexical concepts are strongly intercorrelated. Patterns of word co-occurrence in natural language are driven in part by taxonomic and experiential similarities between the concepts to which they refer, and the taxonomy of natural categories is systematically related to the experiential attributes of the exemplars (39–41). Consequently, the empirical evidence currently available is unable to discriminate between these representational systems.
Several computational models of concept representation have been proposed based on these structures. While earlier models relied heavily on hierarchical taxonomic structure (42, 43), more recent proposals have emphasized the role of experiential and/or distributional information (34, 44–46). The model by Chen and colleagues (45), for example, showed that graded taxonomic structure can emerge from the statistical coherent covariation found across experiences and exemplars without explicitly coding such taxonomic information per se. Other models propose that concepts may be formed through the combination of experiential and distributional information (44, 46), suggesting a dual representational code akin to Paivio’s dual coding theory (47).
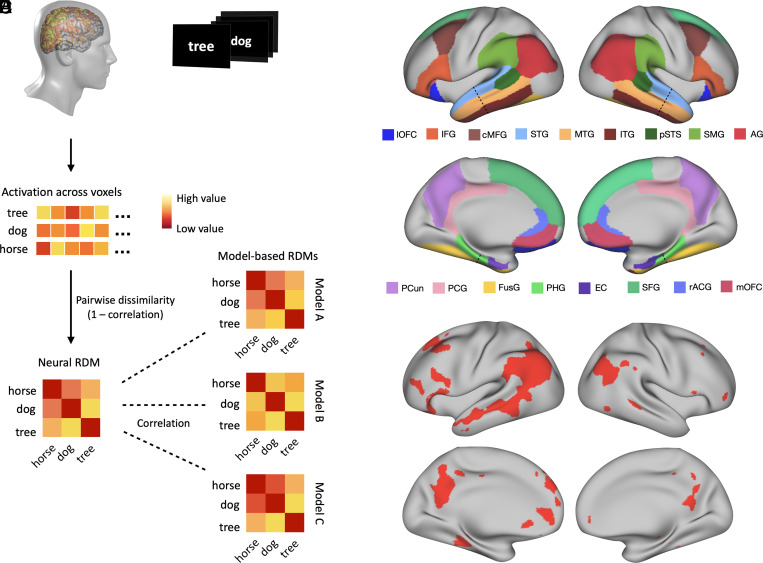
We investigated the relative contribution of each representational system by deriving quantitative predictions from each system for the similarity structure of a large set of concepts and then using representational similarity analysis (RSA) with high-resolution functional MRI (fMRI) to evaluate those predictions. Unlike the more typical cognitive subtraction technique, RSA focuses on the information structure of the pattern of neural responses to a set of stimuli (48). For a given stimulus set (e.g., words), RSA assesses how well the representational similarity structure predicted by a model matches the neural similarity structure observed from fMRI activation patterns (Fig. 1). This allowed us to directly compare, in quantitative terms, predictions derived from the three representational systems.
Fig. 1.
Representational similarity analysis. (A) An fMRI activation map was generated for each concept presented in the study, and the activation across voxels was reshaped as a vector. (B) The neural RDM for the stimulus set was generated by computing the dissimilarity between these vectors (1 − correlation) for every pair of concepts. (C) A model-based RDM was computed from each model, and the similarity between each model’s RDM and the neural RDM was evaluated via Spearman correlation. (D) Anatomically defined ROIs. The dashed line indicates the boundary where temporal lobe ROIs were split into anterior and posterior portions (see main text for acronyms). (E) Cortical areas included in the functionally defined semantic network ROI (49).
Results
In two experiments, participants made familiarity judgments on a large number of lexical concepts, which were selected from a broad range of taxonomic categories, while undergoing fMRI (details in Materials and Methods). Written nouns were presented, one at a time, on a computer screen, and participants rated each one according to how often they encountered the corresponding entity or event in their daily lives, on a scale from 1 (“rarely or never”) to 3 (“often”). RSAs were conducted for the following set of cortical areas previously associated with concept representation (49–51): angular gyrus (AG), supramarginal gyrus (SMG), temporal pole (TP), anterior superior temporal gyrus (aSTG), posterior superior temporal gyrus (pSTG), anterior middle temporal gyrus (aMTG), posterior middle temporal gyrus (pMTG), anterior inferior temporal gyrus (aITG), posterior inferior temporal gyrus (pITG), posterior superior temporal sulcus (pSTS), anterior parahippocampal gyrus (aPHG), posterior parahippocampal gyrus (pPHG), entorhinal cortex (EC), inferior frontal gyrus (IFG), caudal middle frontal gyrus (cMFG), superior frontal gyrus (SFG), precuneus (PCun), posterior cingulate gyrus (PCG), rostral anterior cingulate gyrus (rACG), medial orbitofrontal cortex (mOFC), lateral orbitofrontal cortex (lOFC), anterior fusiform gyrus (aFusG), and posterior fusiform gyrus (pFusG). These regions were anatomically defined following the Desikan-Killiany probabilistic parcellation map (52), with the border between anterior and posterior temporal lobe areas determined according to a plane perpendicular to the lobe’s main axis (53). We also conducted RSA for a distributed, functionally defined region-of-interest (ROI) based on the voxel-based meta-analysis by Binder and colleagues (49) (Fig. 1D; heretofore referred to as “semantic network ROI”). The semantic network ROI spanned a large swathe of heteromodal cortex, including most of the aforementioned cortical areas. The neural similarity structure of concept-related activation patterns in this ROI reflects not only information encoded within each cortical area but also information encoded in the pattern of activations across different areas, thus allowing us to examine the representational structure in the network as a whole.
From the fMRI data, we generated a whole-brain activation map for each concept, reflecting the unique spatial pattern of neural activity for that concept (Fig. 1). From these maps, a neural representational dissimilarity matrix (RDM) was generated for each ROI. RDMs consisted of all pairwise dissimilarities (1 − correlation) between the vectorized activation patterns across voxels. For each representational model investigated, a model-based RDM was computed, and its similarity to the neural RDM was evaluated via Spearman correlation. We evaluated six different representational models: two based on taxonomic information, two based on experiential information, and two based on distributional information (see details below). The resulting profile of relative model performances provided an assessment of the degree to which each type of information is reflected in the neural activation patterns corresponding to different concepts. Because the RDMs from different models were partly correlated with each other (SI Appendix, Table S1), we also conducted partial correlation RSAs to evaluate the unique contribution of each model to the neural activation patterns underlying lexical concepts. We stress that the representational models investigated here are models of information content (i.e., taxonomic, experiential, or distributional); they are not meant to model the neural architecture through which information is encoded in the brain (extended model descriptions in SI Appendix, Supplementary Text).
Taxonomic Models.
WordNet is the most influential representational model based on taxonomic information, having been used in several neuroimaging studies to successfully model semantic content (2, 22, 54, 55). It is organized as a knowledge graph in which words are grouped into sets of synonyms (“synsets”), with each expressing a distinct concept. Synsets are richly interconnected according to taxonomic relations, resulting in a hierarchically structured network encompassing 81,426 noun concepts (in the English version). Concept similarity is computed based on the shortest path connecting the two concepts in this network.
In contrast to WordNet, which provides a comprehensive taxonomy of lexical concepts, the Categorical model was customized to encode the particular taxonomic structure of the concept set in each study, based on a set of a priori categories. Therefore, the Categorical model ignores concept categories that were not included in the study, such as “furniture” or “clothing.” To reduce the level of subjectivity involved in the assignment of items to categories and in the evaluation of intercategory similarities, we tested 18 a priori versions of the Categorical model, with different numbers of categories and different levels of hierarchical structure, for the concepts in Study 1 (SI Appendix, Fig. S1). Each version was tested via RSA against the fMRI data, and the best-performing version was selected for comparisons against other types of models. The selected model for Study 1 (model N in SI Appendix, Fig. S1) consisted of 19 hierarchically structured categories, as follows: abstract (mental abstract, social abstract, social event, other abstract), event (social event, concrete event), animate (animal, human, body part), inanimate (artifact [musical instrument, vehicle, other artifact], food, other inanimate), and place.
In Study 2, the Categorical model consisted of two higher-level categories—object and event—with each consisting of four subcategories (animal, plant/food, tool, and vehicle; sound event, social event, communication event, and negative event).
Experiential Models.
The Exp48 model consists of 48 dimensions corresponding to distinct aspects of phenomenal experience, such as color, shape, manipulability, and pleasantness (SI Appendix, Table S2). This model is based on the experiential salience norms of Binder and colleagues (40), in which each dimension encodes the relative importance of an experiential attribute according to crowd-sourced ratings. Exp48 encompasses all perceptual, motor, spatial, temporal, causal, valuation, and valence dimensions present in those norms.
The SM8 model consists of a subset of the Exp48 dimensions, focusing exclusively on sensory-motor information. These dimensions represent the relevance of each sensory modality (vision, audition, touch, taste, and smell) and of action schemas performed with each motor effector (hand, foot, and mouth) to the concept. The concept “apple,” for instance, has high values for vision, touch, taste, mouth actions, and hand actions and low values for the other dimensions.
Distributional Models.
Distributional information was modeled with two of the most prominent distributional models available. Namely, word2vec (56) uses a deep neural network trained to predict a word based on a context window of a few words preceding and following the target word. In contrast, the model GloVe (57) is based on the ratio of cooccurrence probabilities between pairs of words across the entire corpus. In a comparative evaluation of distributional semantic models (17), word2vec and GloVe emerged as the two top-performing models in predicting human behavior across a variety of semantic tasks.
Study 1.
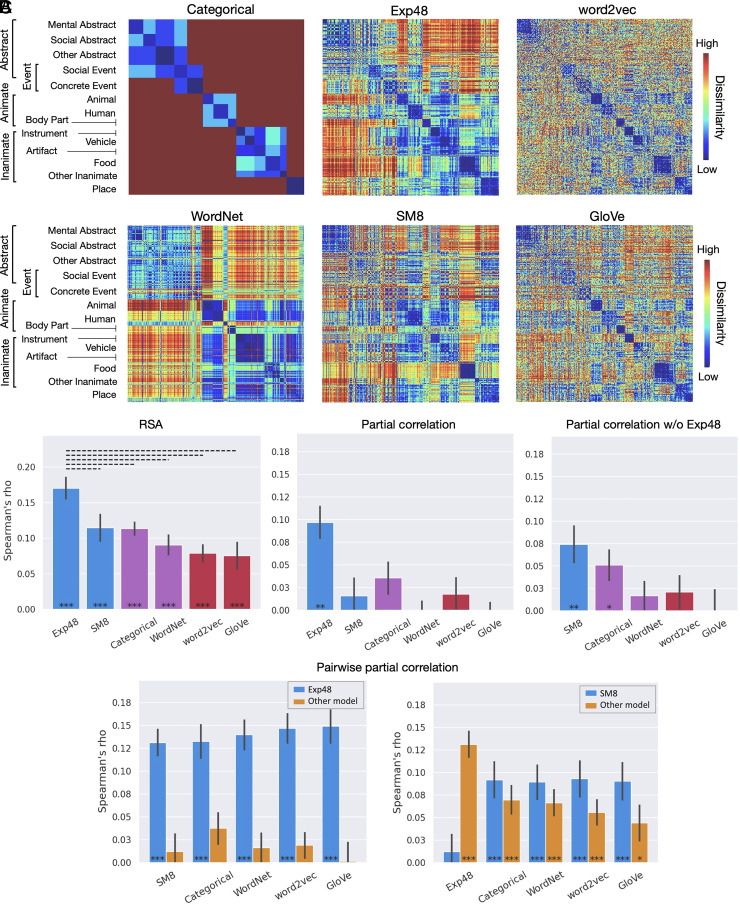
Study 1 evaluated these 6 models with a set of 300 concepts spanning a wide variety of semantic categories, including animate objects (e.g., “elephant,” “student”), places (e.g., “kitchen,” “beach”), artifacts (e.g., “comb,” “rocket”) and other inanimate objects (e.g., “cloud,” “ice”), events (e.g., “hurricane,” “election”), and highly abstract concepts (e.g., “fate,” “hygiene”) (Fig. 2A and SI Appendix, Tables S3 and S4).
Fig. 2.
Study 1. (A) Dissimilarity matrices for the representational spaces tested. Rows and columns represent each of the 300 concepts used in the study, grouped according to the categorical model to reveal taxonomic structure. (B) RSA results for the semantic network ROI. Experiential (blue), taxonomic (purple), and distributional (red) models. Left: Correlations between the group-averaged neural RDM and each model-based RDM. Center: Partial correlation results for each model while controlling for its similarity with all other models. Right: Partial correlation results when Exp48 was excluded from the analysis. (C) Pairwise partial correlations for the semantic network ROI, with blue bars representing Exp48 (Left) or SM8 (Right) while controlling for its similarity to each of the other model-based RDMs; yellow bars correspond to each of the other model-based RDMs while controlling for their similarity to the model represented in blue. ***P < 0.0005, **P < 0.005, *P < 0.05, Mantel test; solid bar: P < 0.001; dashed bar: P < 0.05, permutation test. All P values are FDR corrected for multiple comparisons (q = 0.05). Error bars represent the SE.
In the functionally defined semantic network ROI, the experiential models achieved the highest performance, followed by the taxonomic models (Fig. 2 B and C). Exp48 performed significantly better than any other model, with no other differences between models reaching significance. To investigate the unique contribution of each type of information to the similarity structure of activation patterns, we conducted partial correlation RSAs. These analyses assessed how well each model explained the neural data after controlling for its similarity to other models. We conducted RSAs with the RDM of each model after regressing out the RDMs of all other models. As shown in Fig. 2B (Center), only Exp48 explained significant variance in the neural data after controlling for the other models. To verify whether Exp48 individually explains all of the variance accounted for by each of the other models, we performed pairwise partial correlation RSAs (Fig. 2C, Left). These analyses showed that no other model accounted for any measurable additional variance after controlling for their similarity to Exp48. In other words, Exp48 was the only model that made unique contributions to explaining the representational structure of lexical concepts in the semantic network ROI, while the other models only predicted the data to the extent that their similarity structure correlated with that of Exp48.
We then investigated whether information about the relative importance of eight sensory-motor modalities, by itself, successfully predicted the neural similarity structure of concepts after controlling for taxonomic and distributional information. The results showed that, when Exp48 was not included in the partial correlation analysis, SM8 remained highly significantly correlated with the neural data after controlling for all other models, while WordNet, word2vec, and GloVe did not (Fig. 2B, Right). Categorical was the only other model displaying a significant partial correlation, although it was numerically lower and less significant than that of SM8. Pairwise partial correlation RSAs showed that SM8 did not explain all of the variance accounted for by WordNet, word2vec, or GloVe; however, there was a trend toward stronger partial correlations for SM8 than for any of the nonexperiential models (Fig. 2C, Right). Together, these results suggest that experiential information—including but not restricted to sensory-motor information—plays a fundamental role in the neural representation of conceptual knowledge in heteromodal cortical areas, while taxonomic and distributional representational systems appear to contribute relatively little independent information.
All six models predicted the similarity structure of concept-related neural activation patterns in left AG, PCun, SFG, and IFG (SI Appendix, Figs. S2 and S3). In all of these areas, as well as in left and right PCG, left PHG, right IFG, and right SFG, the Exp48 model achieved the strongest correlation. SM8 did not perform as well as Exp48, and this difference reached significance in several ROIs, particularly in the left IFG. The same areas also showed a significant advantage for Exp48 over WordNet. The advantage of Exp48 over the Categorical model reached significance in left AG, PCun, PC, and PHG, and in right IFG and SFG. There was a trend toward higher correlations for the Categorical model than for WordNet in most areas, but this difference only reached significance in the left pSTS, which was the only area in which the Categorical (or any other) model significantly outperformed both experiential models. Besides the pSTS, the Categorical model also achieved the highest correlation of any model in the left pFusG, pMTG, pSTG, and SMG, although this advantage only reached significance relative to word2vec in pSTG and pMTG and relative to SM8 in pMTG. Several areas showed a trend toward lower performance for word2vec and GloVe relative to the other models, and in most areas, these two models performed similarly, with the following two exceptions: in left IFG, GloVe significantly outperformed word2vec, while the inverse effect was found in right PCun. The relatively low correlations for all models found in the aITG, aFusG, TP, EC, aPHG, and mOFC may be due to lower signal-to-noise ratio in those areas (SI Appendix, Table S7).
Study 2.
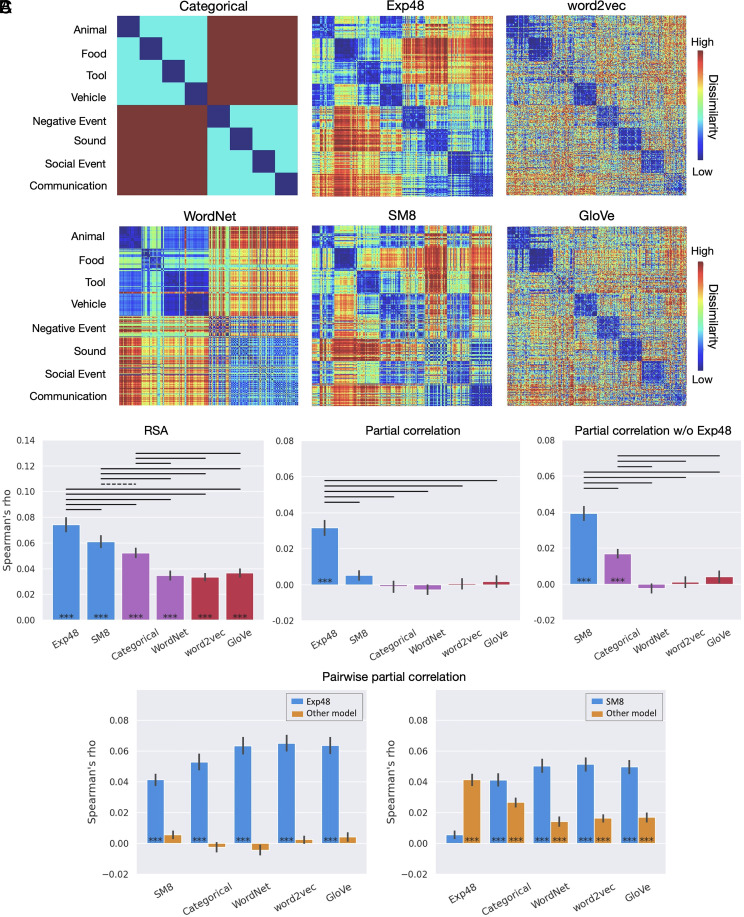
Study 2 was designed to further investigate the information content of concept-related activation patterns with a different set of concepts, a larger participant sample size, and matched subsets of object and event concepts. This study examined whether the pattern of model performances found in Study 1 would be observed independently for objects and events or—given their markedly distinct ontological status—whether these two types of concepts would differ in the degree to which they encode experiential, taxonomic, and distributional information. The 320 concepts included in the study were selected to be typical exemplars of four categories of objects (animals, tools, plants/foods, and vehicles) and four categories of events (sounds, negative events, social events, and communication events). The larger sample size (36 participants) allowed for statistical testing across participants as well as across stimuli (i.e., words).
In the semantic network ROI, Exp48 outperformed all other models when tested across participants and across stimuli (Fig. 3B, Left, and SI Appendix, Fig. S6, Left), and in both analyses, it was the only model that retained explanatory power after controlling for the predictions of all other models (Fig. 3B, Center, and SI Appendix, Fig. S6, Center). SM8 also performed significantly better than all nonexperiential models, and the Categorical model outperformed WordNet, word2vec, and GloVe.
Fig. 3.
Study 2. (A) Dissimilarity matrices for the representational spaces tested. Rows and columns have been grouped according to the Categorical model to reveal taxonomic structure. (B) RSA results across participants for the semantic network ROI. Group mean values of the correlations between each participant’s neural RDM and the model-based RDMs. Full RSA correlations (Left) and partial correlations with Exp48 included (Center) and excluded (Right). (C) Pairwise partial correlations, with blue bars representing Exp48 (Left) or SM8 (Right) while controlling for its similarity to each of the other models; yellow bars correspond to each of the other models while controlling for their similarity to the model represented in blue. ***P < 0.0005 ; solid bar: P < 0.001; dashed bar: P < 0.05; Wilcoxon signed-rank tests. All P values are FDR corrected for multiple comparisons (q = 0.05). Error bars represent the SEM.
Partial correlation RSAs revealed that Exp48 accounted for all the variance explained by the other models (Fig. 3 B, Center, and C, Left). When Exp48 was left out of the analysis, the SM8 and Categorical models together accounted for all the variance explained (Fig. 3B, Right), with SM8 performing significantly better than Categorical. Confirming the main findings of Study 1, these results indicate that experiential information plays a central role in the representation of lexical concepts in high-level heteromodal cortical areas.
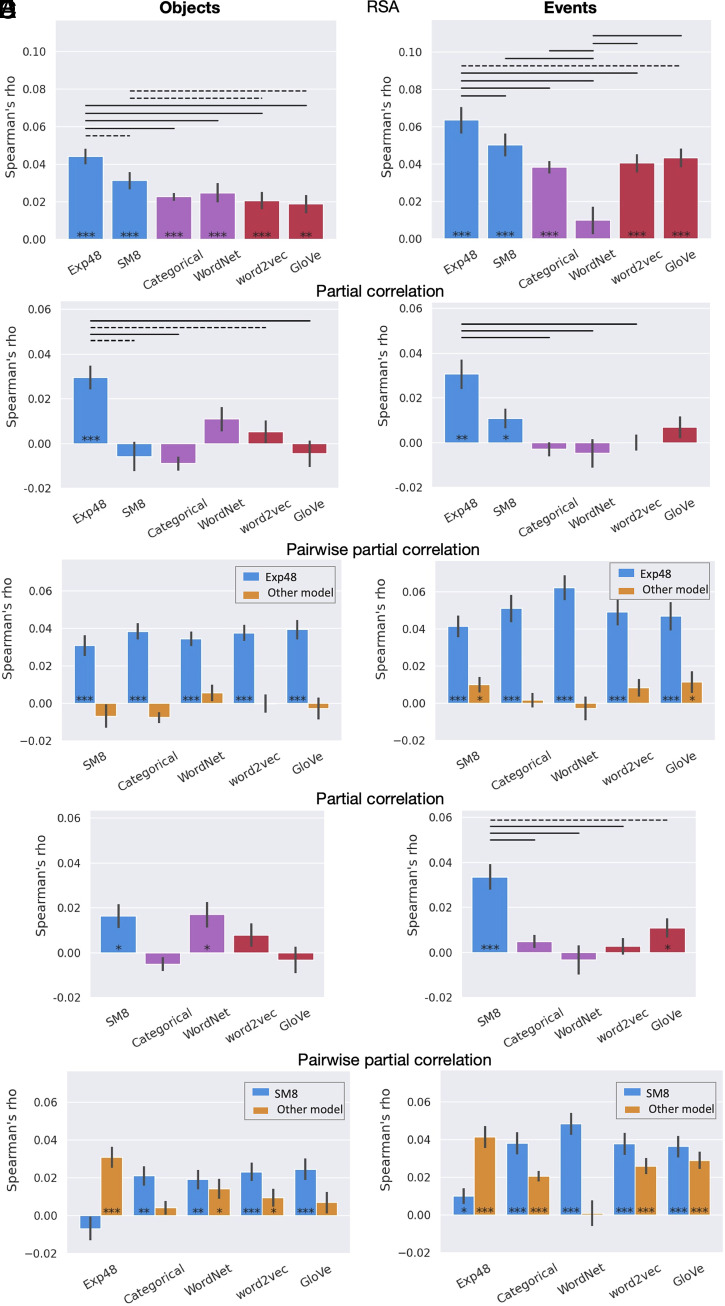
Separate analyses for objects and events revealed two main differences between these two types of concepts (Fig. 4 and SI Appendix, Figs. S7–S10). First, RSA correlations were substantially higher for events than for objects, across all three types of representational models, in almost all ROIs. This result reflects the higher variability of pairwise similarities for the neural representations of event concepts, as evidenced by the higher noise ceiling in this condition (mean noise ceiling across anatomical ROIs, lower bound = 0.17) relative to object concepts (0.14). Inspection of the neural RDM (SI Appendix, Fig. S11) reveals a slightly more pronounced categorical structure for events than for objects, with particularly high pairwise similarities for communication events and social events.
Fig. 4.
Results for object concepts (Left) and event concepts (Right) for the semantic network ROI in Study 2 (across participants). (A) RSA results. (B) Partial correlations. (C) Pairwise partial correlations for Exp48. (D) Partial correlations with Exp48 excluded from the analysis. (E) Pairwise partial correlations for SM8. In the pairwise partial correlation charts, blue bars represent an experiential model (Exp48 in C and SM8 in E) while controlling for its similarity to each of the other models; yellow bars correspond to each of the other models while controlling for their similarity to the experiential model represented in blue. ***P < .0005, **P < .005, *P < .05; solid bar, P < .001; dashed bar, P < .05; Wilcoxon signed-rank tests. All P values are FDR-corrected for multiple comparisons (q = .05). Error bars represent the standard error of the mean.
In all anatomically defined ROIs, RSAs based on the entire concept set (i.e., all categories included) revealed an advantage for experiential models relative to taxonomic and distributional models (SI Appendix, Figs. S4 and S5). As in Study 1, correlations were particularly strong in the left IFG, AG, and PCun, with the IFG showing substantially stronger correlations than other ROIs. For all models, correlations were stronger in left hemisphere ROIs than in their right hemisphere homologs, which is consistent with left lateralization of lexical semantic representations. The relatively low correlations found in the aITG, aFusG, TP, EC, aPHG, and mOFC may be due to lower signal-to-noise ratio in those areas (SI Appendix, Table S7). In almost all ROIs examined (45 out of 46), correlations were significantly stronger for the experiential models than those for taxonomic or distributional models. Both experiential models significantly outperformed all other models in 17 ROIs, and nowhere did a taxonomic or distributional model perform significantly better than SM8 or Exp48 (the superiority of the categorical model in the pSTS observed in Study 1 was not replicated here). Exp48 significantly outperformed SM8 in left aSTG, pSTG, AG, pMTG, IFG, pFusG, cMFG, and SFG and in right AG, SMG, pSTS, cMFG, and SFG. No significant differences between the two experiential models were found in the other ROIs.
Except for the left AG, WordNet appears to perform worse for events than for objects in all other ROIs, both in absolute terms and in relation to other models. This difference, which was strongest in the PCG, is likely due to taxonomic trees for events being relatively shallower in this model (mean tree depth for objects and events is 10.6 and 8.1, respectively), resulting in a narrower range of possible pairwise similarities. Apart from WordNet, the overall profile of relative model performances was similar across objects and events. For both types of concepts, experiential models achieved the strongest RSA correlations in most anatomical regions, with no ROIs showing significantly stronger correlations for any taxonomic or distributional model. Partial correlation RSAs in the semantic network ROI showed that, for object concepts, Exp48 accounted for all the variance explained by any other model (Fig. 4 B and C). For events, SM8 and GloVe maintained relatively weak but statistically significant correlations after controlling for Exp48. SM8 was second to Exp48 in explaining the most variance for both objects and events (Fig. 4 D and E).
Validation Analyses.
While the experiential feature ratings included in Exp48 and SM8 were meant to capture particular qualities of phenomenal experience, it is possible that the ratings themselves were influenced by other types of information as well, including more abstract knowledge about the target concept or about contextually associated concepts. Therefore, it is conceivable that a model based on semantic features that do not focus on experiential information could also outperform the taxonomic and distributional models. To assess this possibility, we conducted another set of analyses in which we included a model based on semantic features that are not explicitly focused on experiential information. This model was based on the Semantic Feature Production Norms (SFPN), which are the largest and best-known effort to characterize word meanings in terms of descriptive semantic features (58–60). These features are derived from descriptive properties generated by human participants in a property listing task, resulting in concept depictions based on thousands of features. They encode various types of information, including taxonomic (e.g., “is a mammal”), functional (e.g., “used for cooking”), and contextual association, as well as more elaborate features (e.g., “lays eggs,” “lives in the water”).
These analyses were based on the concepts included in our studies for which SFPN features are available (220 concepts in Study 1 and 196 concepts in Study 2). The results show that SFPN was the worst-performing model of the seven models tested and that both Exp48 and SM8 significantly outperformed it in both studies (SI Appendix, Fig. S12). These results indicate that the high performance of Exp48 and SM8 relative to the other models is indeed due to their focus on experiential information.
Discussion
In two studies, we conducted a quantitative assessment of the degree to which the three main information structures proposed for concept representation are encoded in fMRI activation patterns corresponding to individual lexical concepts. We found that Exp48—a representational model based on 48 distinct experiential dimensions grounded in known neurocognitive systems—consistently outperformed taxonomic and distributional models in predicting the neural similarity structure of a large number of lexical concepts (524 across both studies), spanning a wide variety of semantic categories. Additionally, SM8, a relatively impoverished experiential model based on only eight sensory-motor dimensions reflecting the relative importance of neural systems dedicated to vision, hearing, touch, taste, and smell, as well as motor control of the hands, mouth, and feet, exceeded the performance of the best-performing taxonomic and distributional models in the semantic network ROI in study 2. SM8 significantly outperformed those models in 17 anatomical ROIs, while matching their performance in the remaining ones. These results provide compelling evidence that experiential information grounded in sensory, motor, spatial, temporal, affective, and reward neural systems is a fundamental aspect of the representational code underlying conceptual knowledge—not only in sensory-motor cortical areas, as indicated in previous studies, but also in high-level association areas.
In both experiments, Exp48 was the only model whose predictions correlated with the neural similarity structure of the entire concept set when controlling for the predictions of all other models, and in Study 2, this was also true for object concepts in particular. For event concepts, SM8 was the only other model that independently accounted for variance in the neural data. The fact that taxonomic and distributional models did not predict the neural RDM when controlling for the predictions of Exp48 alone (Figs. 2C and 3C, Left) indicates that those models provide no measurable additional information about concept similarity structure. This suggests that taxonomic information, while seemingly central to the organization of conceptual knowledge, may be represented only indirectly, via its interdependency with experiential information (40).
Another important finding was that RSA correlations in the PCG and in the PCun were among the strongest across all ROIs examined, rivaling or even surpassing those found in the AG and in the temporal lobe. PCG and PCun are not typically included in discussions of cortical semantic hubs (3, 4, 28), although they have been consistently associated with semantic processing in functional neuroimaging studies (30, 49, 61, 62). This finding, replicated across studies 1 and 2, indicates that these regions play an important and yet unrecognized role in semantic cognition.
One limitation of the present study is that the representational spaces used to model experiential information content are relatively coarse. Neural concept representations must encode detailed information about aspects of the phenomenal experience associated with different lexical concepts, such as particular colors, shapes, or motor schemas, but Exp48 and SM8 only encode the relative importance of each kind of experience. In other words, these models represent information about how much the neural system underlying a particular process (e.g., color perception or motor control of the hand) contributes to a given lexical concept, but it contains no information about the particular experiences contributed by each system (e.g., different hues or different motor schemas). This coarseness, which results from practical limitations in obtaining participant ratings about fine-grained experiential attributes, imposes limitations on our ability to detect experiential information in the neural data. Nevertheless, it is still surprising that these models performed so well compared to some of the most sophisticated taxonomic and distributional models available, pointing to the development of more detailed experiential models as a promising direction for future work.
Although most researchers agree that the representation of conceptual information per se should be independent of the modality of the stimulus, concept-related neural activation patterns elicited by nonverbal stimuli may differ to some extent from those elicited by words. We used words as stimuli primarily for methodological reasons. First, unlike pictures, word forms are arbitrarily associated with conceptual content. The visual, motor, or auditory representations of a word form carry no information about the semantic properties of the lexical concept associated with it. Pictorial stimuli, on the other hand, carry information about visual properties of the concept, which can bias participants to focus on those properties at the expense of properties that are not explicitly conveyed by the stimulus. Second, word stimuli allow for the inclusion of relatively abstract concepts (e.g., “belief” and “year”) that are not easily conveyed via other stimulus modalities. Finally, in most experiential, taxonomic, and distributional model implementations, concepts are labeled exclusively using word forms.
Task requirements can also have an influence on activation patterns. For example, asking participants to make decisions about the stimulus based on a particular semantic criterion (e.g., living vs. nonliving, large vs. small, or natural vs. man-made) emphasizes those aspects of meaning to the detriment of all others. Our approach was to use a task that would be as neutral as possible regarding the conceptual content of the items. We see no reason to believe that our task favors some particular aspect of meaning over another, although that possibility has not been experimentally ruled out.
Together, the present results indicate that concept-related fMRI activation patterns in heteromodal cortical areas encode information about features of phenomenal experience grounded in sensory-motor, spatiotemporal, affective, and reward systems. While other studies have shown that areas involved in sensory perception and motor control are activated during concept retrieval (13, 28, 30), our results imply that information pertaining to these systems is directly encoded in high-level association areas during concept retrieval. The idea that concepts are neurally represented as amodal symbols whose representational code is independent of modality-specific systems has a long history (23, 37); however, the finding that taxonomic and distributional models performed significantly worse than both SM8 and Exp48 in Study 2 challenges that view. While our results do not rule out the notion that concepts are organized in hierarchical taxonomic networks, they are more aligned with a view in which concept representations emerge from the multimodal integration of signals originating in modality-specific systems during concept acquisition, and in which taxonomic organization emerges from correlations between experiential features (14, 30). In this framework, concept retrieval consists of the transient activation of a neuronal cell assembly distributed across the heteromodal cortical hubs that make up the semantic network, with possible downstream activation of the relevant modality-specific assemblies depending on contextual demands. Further research is required to determine the extent to which different experiential features contribute to concept representation in different parts of the semantic network and how their relative importance varies across ontological categories.
The present study focused on the overall nature of the informational code underlying conceptual knowledge; therefore, in both experiments, the analyses included a wide variety of semantic categories. Our results, however, do not imply that all portions of the semantic network contribute equally to different categories. In fact, an experiential view of concept representation would predict otherwise, and this issue should be explored in future studies.
It is worth noting that the detection of concept-related similarity structure in a given brain region does not necessarily implicate that region in the long-term storage of concept representations. In principle, the activation patterns detected in a region during concept retrieval may reflect information received from other regions. In the present study, this is likely to be the case for prefrontal cortical areas, which are typically implicated in executive control functions. If prefrontal areas are involved in the controlled retrieval or short-term maintenance of multimodal semantic representations, we should expect them to be tuned to particular sensory-motor and affective features at a relatively fine spatial scale and to transiently reflect representational structures stored elsewhere. Further studies are required to characterize the precise computational role played by the various cortical areas that constitute the general semantic network.
In addition to their implications for the nature of the representations underlying conceptual knowledge, the present results are also relevant to computational approaches to natural language processing in the field of artificial intelligence. They suggest that incorporating experiential information into computational models of word meaning could lead to more human-like performance. These findings can also inform the development of brain-machine interface systems by demonstrating that information about the experiential content of conceptual thought can be decoded from the spatial distribution of neural activity throughout the cortex.
Materials and Methods
Study 1.
This study was designed for RSA across stimuli rather than across participants, with a large number of trials (1,800 trials) and long scanning times, which allowed sufficient power to be achieved with a relatively small sample size (n = 8).
Participants.
Eight native speakers of English (four women), ages 19 to 37 (mean = 28.5), took part in study 1. Participants were all right-handed according to the Edinburgh Handedness Scale (63), had at least a high school education, and had no history of neurological or psychiatric conditions. All participants provided written informed consent. This study was approved by the Medical College of Wisconsin Institutional Review Board.
Stimuli.
Stimuli included 300 English nouns of various categories, including concrete and abstract concepts. Nouns were relatively familiar, with mean log-transformed Hyperspace Analogue to Language (HAL) frequency of 8.7 (range, 4.0 to 12.7) and mean age of acquisition of 6.7 y (range, 2.7 to 13.8; English Lexicon Project [https://elexicon.wustl.edu]) (64) (SI Appendix, Tables S3 and S4).
Task.
Participants rated each noun according to how often they encountered the corresponding entity or event in their daily lives, on a scale from 1 (“rarely or never”) to 3 (“often”). The task was designed to encourage semantic processing of the word stimuli without emphasizing any particular semantic features or dimensions. Participants indicated their response by pressing one of three buttons on a response pad with their right hand. On each trial, a noun was displayed in written form on the center of the screen for 500 ms, followed by a 3.5-s blank screen. Each trial was followed by a central fixation cross with variable duration between 1 and 4 s (mean = 2 s). The entire stimulus set was presented six times over the course of the study. In each presentation, the order of the stimuli was randomized. Each presentation was split into 3 runs of 100 trials each. The task was performed over the course of three scanning sessions on separate days, with two presentations (six runs) per session. Each run started and ended with an 8-s fixation cross.
Equipment.
Scanning was performed on a GE Healthcare Discovery MR750 3T MRI scanner at the Medical College of Wisconsin’s Center for Imaging Research. Stimulus presentation and response recording were performed via E-prime 2.0 software running on a Windows desktop computer and a Psychology Software Tools Serial Response Box. Stimuli were back-projected on a screen positioned behind the scanner bore and viewed through a mirror attached to the head coil.
Scanning protocol.
MRI scanning was conducted over three separate sessions on different days. Each session consisted of a structural T1-weighted magnetization-prepared radio-frequency pulses and rapid gradient-echo (MPRAGE) scan, a structural T2-weighted Cube scan, 3 pairs of T2-weighted spin echo echo-planar scans (5 volumes each) acquired with opposing phase-encoding directions (for correction of geometrical distortions in the functional images), and 6 gradient echo echo-planar runs using simultaneous multislice acquisition (8× multiband, repetition time = 1,200 ms, echo time = 33.5 ms, 512 volumes, flip angle = 65, matrix = 104 × 104, slice thickness = 2.0 mm, axial acquisition, 72 slices, field-of-view = 208 mm, voxel size = 2 × 2 × 2 mm).
Data analysis.
The fMRI images were preprocessed (slice timing correction, motion correction, distortion correction, volume alignment, and scaling) using the software package Analysis of Functional NeuroImages (AFNI) (65). Statistical analyses were conducted in each participant’s original coordinate space. Activation (beta) maps were generated for each noun relative to the mean signal across all other nouns using AFNI’s 3dDeconvolve. Response time z scores, and head motion parameters were included as regressors of no interest. Anatomically defined ROIs were created based on the probabilistic Desikan-Killiany parcellation atlas included in AFNI (TT_desai_dk_mpm; SI Appendix, Fig. S12). The functionally defined semantic network ROI corresponded to the 1% most consistently activated voxels in an activation likelihood estimate meta-analysis of 120 neuroimaging studies of semantic language processing (49) (Fig. 1D). All ROI masks were created in standard coordinate space (MNI152 2009c) and nonlinearly aligned to each participant’s anatomical scan using AFNI’s 3dQwarp. Neural RDMs were computed for each ROI as the Spearman correlation distance (1 − rho) for all pairs of concepts. A group-averaged neural RDM was computed by averaging the neural RDMs of all participants. A model-based RDM was computed from each model (except WordNet) as the cosine distance (1 − cosine) for all pairs of concept vectors. The WordNet RDM was based on the Wu & Palmer similarity metric (WPsim), which relies on the depth of the two synsets in the taxonomic tree and that of their Least Common Subsumer (LCS; i.e., their most specific common hypernym):
We used the package Natural Language Toolkit (NLTK 3.4.5; https://www.nltk.org) to compute WPsim; WordNet dissimilarity was computed as 1 − WPsim.
The RSAs computed the Spearman correlation between the group-averaged neural RDMs and each model-based RDM. Statistical significance was tested using the Mantel test with 10,000 permutations. Differences in RSA correlations between models were assessed for significance via permutation tests. Significance was controlled for multiple comparisons via false discovery rate (FDR) with q = 0.05. Since the model-based RDMs can be strongly correlated between models (SI Appendix, Table S1), these analyses were supplemented with partial correlation analyses to reveal the unique contribution of each model after controlling for other models.
We also conducted a temporal signal-to-noise ratio (tSNR) analysis to assess data quality across the cortical mantle. A general linear model (GLM) was conducted with parametric regressors for word concreteness, word length, response time, and head motion parameters and a binary regressor for trial onset. Each regressor was convolved with a canonical hemodynamic response function (HRF to generate the design matrix. A GLM was conducted on the scaled, smoothed (6 mm) imaging data for each participant, and a tSNR map was computed by dividing the mean signal by the SD of the residuals. The individual maps were warped into the MNI152 2009c template via affine transformation and averaged together to generate a group-averaged tSNR map (SI Appendix, Fig. S13).
Study 2.
Study 2 was designed to assess the contribution of each representational system separately for object and event concepts in the semantic network ROI and to achieve sufficient power for model comparisons within anatomically defined ROIs with RSA across participants as well as across stimuli, which required a substantially larger sample size (n = 36).
Participants.
A total of 36 native speakers of English (21 women), ages 20 to 41 (mean = 28.7) took part in study 2. Participants were all right-handed according to the Edinburgh Handedness Scale (63), had at least a high school education, and had no history of neurological or psychiatric conditions. None of the participants took part in study 1. All participants provided written informed consent. This study was approved by the Medical College of Wisconsin Institutional Review Board.
Stimuli.
Stimuli included 160 object nouns (animals, foods, tools, and vehicles—40 of each) and 160 event nouns (social events, verbal communication events, nonverbal sound events, and negative events—40 of each) (SI Appendix, Tables S5 and S6). Of the 320 concepts included in study 2, 62 objects and 34 events were also used in study 1.
Task.
Task instructions were identical to study 1. On each trial, a noun was displayed in written form on the center of the screen for 500 ms, followed by a 2.5-s blank screen. Each trial was followed by a central fixation cross with variable duration between 1 and 3 s (mean = 1.5 s). The entire stimulus set was presented six times over the course of the study in randomized order. Each presentation was split into 4 runs of 80 trials each. The task was performed over the course of three scanning sessions on separate days, with two presentations (eight runs) per session. Each run started and ended with an 8-s fixation cross.
Equipment.
Scanning was performed on a GE Healthcare Premier 3T MRI scanner with a 32-channel Nova head coil at the Medical College of Wisconsin’s Center for Imaging Research. Stimulus presentation and response recording were performed with Psychopy 3 software (66) running on a Windows desktop computer and a Celeritas fiber optic response system (Psychology Software Tools, Inc.). Stimuli were displayed on an MRI-compatible LCD screen positioned behind the scanner bore and viewed through a mirror attached to the head coil.
Scanning protocol.
MRI scanning was conducted on three separate visits. Each session consisted of a structural T1-weighted MPRAGE scan, a structural T2-weighted CUBE scan, 3 pairs of T2-weighted spin echo echo-planar scans (5 volumes each) acquired with opposing phase-encoding directions, and 8 gradient echo echo-planar functional scans (4× multiband, TR = 1,500 ms, TE = 33 ms, 251 volumes, flip angle = 50, in-plane matrix = 104 × 104, slice thickness = 2.0 mm, axial acquisition, 68 slices, field-of-view = 208 mm, voxel size = 2 × 2 × 2 mm).
Data analysis.
Data preprocessing and statistical analysis were performed as described in study 1. RDMs for the models tested in study 2 are depicted in Fig. 3 (main text), and intermodel correlations are displayed in SI Appendix, Table S1.
RSAs were conducted across stimuli (i.e., words) and across participants. In the analysis across stimuli, voxel-based RDMs from all participants were combined into a group-averaged neural RDM, and for each model, a single correlation was computed between this RDM and the model-based RDM. These correlations were tested for significance via the Mantel test with 10,000 permutations. In the analysis across participants, a correlation was computed, for each model, between each participant’s neural RDM and the model-based RDM. The Fisher Z-transformed correlation coefficients were then averaged across participants to compute the group mean rho, and significance was tested via Wilcoxon’s signed-rank test.
Differences in RSA correlations between models were tested across words via the permutation test (Mantel) and across participants via Wilcoxon’s signed-rank test. Significance was controlled for multiple comparisons via FDR with q = 0.05.
A group-averaged tSNR map was computed as in study 1.
Supplementary Material
Acknowledgments
We wish to thank Elizabeth Awe and Jed Mathis for assistance with data collection and management and two anonymous reviewers for valuable comments and suggestions on a previous version of the article. This project was supported by National Institute on Deafness and Other Communication Disorders Grant R01-DC016622 to J.R.B. and Advancing a Healthier Wisconsin Project #5520462 to Brian-Fred Fitzsimmons.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108091119/-/DCSupplemental.
Data Availability
Anonymized blood oxygen level–dependent MRI data have been deposited in the Open Science Framework (67) (http://osf.io/HB6DE). Previously published data were used for this work (https://code.google.com/archive/p/word2vec; https://github.com/doomlab/Word-Norms-2).
References
- 1.Mitchell T. M., et al. , Predicting human brain activity associated with the meanings of nouns. Science 320, 1191–1195 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Huth A. G., de Heer W. A., Griffiths T. L., Theunissen F. E., Gallant J. L., Natural speech reveals the semantic maps that tile human cerebral cortex. Nature 532, 453–458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carota F., Kriegeskorte N., Nili H., Pulvermüller F., Representational similarity mapping of distributional semantics in left inferior frontal, middle temporal, and motor cortex. Cereb. Cortex 27, 294–309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ralph M. A., Jefferies E., Patterson K., Rogers T. T., The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 18, 42–55 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Pereira F., et al. , Toward a universal decoder of linguistic meaning from brain activation. Nat. Commun. 9, 963 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross W. D., Plato’s Theory of Ideas (Clarendon Press, 1951). [Google Scholar]
- 7.Smith E. E., Medin D. L., Categories and Concepts (Harvard University Press, 2013). [Google Scholar]
- 8.Mervis C. B., Rosch E., Categorization of natural objects. Annu. Rev. Psychol. 32, 89–115 (1981). [Google Scholar]
- 9.Collins A. M., Loftus E. F., A spreading-activation theory of semantic processing. Psychol. Rev. 82, 407–428 (1975). [Google Scholar]
- 10.Anderson J. R., A spreading activation theory of memory. J. Verbal Learn. Verbal Behav. 22, 261–295 (1983). [Google Scholar]
- 11.Damasio A. R., Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition 33, 25–62 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Glenberg A. M., What memory is for. Behav. Brain Sci. 20, 1–19, discussion 19–55 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Barsalou L. W., Kyle Simmons W., Barbey A. K., Wilson C. D., Grounding conceptual knowledge in modality-specific systems. Trends Cogn. Sci. 7, 84–91 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Binder J. R., Desai R. H., The neurobiology of semantic memory. Trends Cogn. Sci. 15, 527–536 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landauer T. K., Dumais S. T., A solution to Plato’s problem: The latent semantic analysis theory of acquisition, induction, and representation of knowledge. Psychol. Rev. 104, 211–240 (1997). [Google Scholar]
- 16.Jones M. N., Kintsch W., Mewhort D. J. K., High-dimensional semantic space accounts of priming. J. Mem. Lang. 55, 534–552 (2006). [Google Scholar]
- 17.Pereira F., Gershman S., Ritter S., Botvinick M., A comparative evaluation of off-the-shelf distributed semantic representations for modelling behavioural data. Cogn. Neuropsychol. 33, 175–190 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Andrews M., Frank S., Vigliocco G., Reconciling embodied and distributional accounts of meaning in language. Top. Cogn. Sci. 6, 359–370 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Markman E. M., Constraints children place on word meanings. Cogn. Sci. 14, 57–77 (1990). [Google Scholar]
- 20.Lucas M., Semantic priming without association: A meta-analytic review. Psychon. Bull. Rev. 7, 618–630 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Caramazza A., Shelton J. R., Domain-specific knowledge systems in the brain the animate-inanimate distinction. J. Cogn. Neurosci. 10, 1–34 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Crangle C. E., Perreau-Guimaraes M., Suppes P., Structural similarities between brain and linguistic data provide evidence of semantic relations in the brain. PLoS One 8, e65366-16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handjaras G., et al. , How concepts are encoded in the human brain: A modality independent, category-based cortical organization of semantic knowledge. Neuroimage 135, 232–242 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Xu Y., et al. , Doctor, teacher, and stethoscope: Neural representation of different types of semantic relations. J. Neurosci. 38, 3303–3317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer M. H., Zwaan R. A., Embodied language: A review of the role of the motor system in language comprehension. Q J Exp Psychol (Hove) 61, 825–850 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Rogers T. T., Graham K. S., Patterson K., Semantic impairment disrupts perception, memory, and naming of secondary but not primary colours. Neuropsychologia 70, 296–308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warrington E. K., McCarthy R. A., Categories of knowledge. Further fractionations and an attempted integration. Brain 110, 1273–1296 (1987). [DOI] [PubMed] [Google Scholar]
- 28.Kiefer M., Pulvermüller F., Conceptual representations in mind and brain: Theoretical developments, current evidence and future directions. Cortex 48, 805–825 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Fernandino L., et al. , Parkinson’s disease disrupts both automatic and controlled processing of action verbs. Brain Lang. 127, 65–74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandino L., et al. , Concept representation reflects multimodal abstraction: A framework for embodied semantics. Cereb. Cortex 26, 2018–2034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandino L., Humphries C. J., Conant L. L., Seidenberg M. S., Binder J. R., Heteromodal cortical areas encode sensory-motor features of word meaning. J. Neurosci. 36, 9763–9769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson A. J., et al. , Predicting neural activity patterns associated with sentences using a neurobiologically motivated model of semantic representation. Cereb. Cortex 27, 4379–4395 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Patterson K., Nestor P. J., Rogers T. T., Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8, 976–987 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Rogers T. T., et al. , Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychol. Rev. 111, 205–235 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Jones M. N., Mewhort D. J. K., Representing word meaning and order information in a composite holographic lexicon. Psychol. Rev. 114, 1–37 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Günther F., Dudschig C., Kaup B., Predicting lexical priming effects from distributional semantic similarities: A replication with extension. Front. Psychol. 7, 1646 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahon B. Z., Caramazza A., A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J. Physiol. Paris 102, 59–70 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Zwaan R. A., Embodiment and language comprehension: Reframing the discussion. Trends Cogn. Sci. 18, 229–234 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Malt B. C., Smith E. E., Correlated properties in natural categories. J. Verbal Learn. Verbal Behav. 23, 250–269 (1984). [Google Scholar]
- 40.Binder J. R., et al. , Toward a brain-based componential semantic representation. Cogn. Neuropsychol. 33, 130–174 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Riordan B., Jones M. N., Redundancy in perceptual and linguistic experience: Comparing feature-based and distributional models of semantic representation. Top. Cogn. Sci. 3, 303–345 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Rumelhart D. E., Todd P. M., “Learning and connectionist representations” in Attention and Performance 14: Synergies in Experimental Psychology, Artificial Intelligence, and Cognitive Neuroscience, Meyer D. E., Kornblum S., Eds. (MIT Press, 1993), pp. 3–30. [Google Scholar]
- 43.Rogers T. T., McClelland J. L., Précis of semantic cognition: A parallel distributed processing approach. Behav. Brain Sci. 31, 689–714 (2008). [Google Scholar]
- 44.Andrews M., Vigliocco G., Vinson D., Integrating experiential and distributional data to learn semantic representations. Psychol. Rev. 116, 463–498 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Chen L., Lambon Ralph M. A., Rogers T. T., A unified model of human semantic knowledge and its disorders. Nat. Hum. Behav. 1, 39 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman P., McClelland J. L., Lambon Ralph M. A., Concepts, control, and context: A connectionist account of normal and disordered semantic cognition. Psychol. Rev. 125, 293–328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paivio A., Dual coding theory: Retrospect and current status. Can. J. Psychol. Can. Psychol. 45, 255–287 (1991). [Google Scholar]
- 48.Kriegeskorte N., Mur M., Bandettini P., Representational similarity analysis—Connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binder J. R., Desai R. H., Graves W. W., Conant L. L., Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 19, 2767–2796 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser M., Jefferies E., Lambon Ralph M. A., Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. J. Cogn. Neurosci. 22, 1083–1094 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Wang J., Conder J. A., Blitzer D. N., Shinkareva S. V., Neural representation of abstract and concrete concepts: A meta-analysis of neuroimaging studies. Hum. Brain Mapp. 31, 1459–1468 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desikan R. S., et al. , An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Rice G. E., Lambon Ralph M. A., Hoffman P., The roles of left versus right anterior temporal lobes in conceptual knowledge: An ALE meta-analysis of 97 functional neuroimaging studies. Cereb. Cortex 25, 4374–4391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller G. A., Beckwith R., Fellbaum C., Gross D., Miller K. J., Introduction to WordNet: An on-line lexical database. Int. J. Lexicogr. 3, 235–244 (1990). [Google Scholar]
- 55.Anderson A. J., Murphy B., Poesio M., Discriminating taxonomic categories and domains in mental simulations of concepts of varying concreteness. J. Cogn. Neurosci. 26, 658–681 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Mikolov T., Chen K., Corrado G., Dean J., “Efficient estimation of word representations in vector space” in 1st International Conference on Learning Representations, ICLR 2013 - Workshop Track Proceedings (ICLR, Scottsdale, AZ, 2013), pp. 1–12.
- 57.Pennington J., Socher R., Manning C. D., GloVe: Global Vectors for Word Representation in Empirical Methods in Natural Language Processing (EMNLP, 2014), pp. 1532–1543. [Google Scholar]
- 58.McRae K., Cree G. S., Seidenberg M. S., McNorgan C., Semantic feature production norms for a large set of living and nonliving things. Behav. Res. Methods 37, 547–559 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Cree G. S., Armstrong B. C., “Computational models of semantic memory” in The Cambridge Handbook of Psycholinguistics, Spivey M. J., McRae K., Joanisse M., Eds. (Cambridge University Press, 2012), pp. 259–282. [Google Scholar]
- 60.Buchanan E. M., Valentine K. D., Maxwell N. P., English semantic feature production norms: An extended database of 4436 concepts. Behav. Res. Methods 51, 1849–1863 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Fairhall S. L., Caramazza A., Brain regions that represent amodal conceptual knowledge. J. Neurosci. 33, 10552–10558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liuzzi A. G., Aglinskas A., Fairhall S. L., General and feature-based semantic representations in the semantic network. Sci. Rep. 10, 8931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oldfield R. C., The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113 (1971). [DOI] [PubMed] [Google Scholar]
- 64.Balota D. A., et al. , The English Lexicon Project. Behav. Res. Methods 39, 445–459 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Cox R. W., AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996). [DOI] [PubMed] [Google Scholar]
- 66.Peirce J. W., PsychoPy—Psychophysics software in Python. J. Neurosci. Methods 162, 8–13 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.L. Fernandino, J.-Q. Tong, L. Conant, C. Humphries, J. Binder, Dataset: Decoding the Information Structure Underlying the Neural Representation of Concepts. Open Science Framework. http://osf.io/HB6DE. Deposited 29 April 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized blood oxygen level–dependent MRI data have been deposited in the Open Science Framework (67) (http://osf.io/HB6DE). Previously published data were used for this work (https://code.google.com/archive/p/word2vec; https://github.com/doomlab/Word-Norms-2).