Abstract
Simple Summary
Sixteen G-protein-coupled receptors (GPCRs) have been involved in melanogenesis or melanomagenesis. Here, we review these GPCRs, their associated signaling, and therapies.
Abstract
G-protein-coupled receptors (GPCRs) serve prominent roles in melanocyte lineage physiology, with an impact at all stages of development, as well as on mature melanocyte functions. GPCR ligands are present in the skin and regulate melanocyte homeostasis, including pigmentation. The role of GPCRs in the regulation of pigmentation and, consequently, protection against external aggression, such as ultraviolet radiation, has long been established. However, evidence of new functions of GPCRs directly in melanomagenesis has been highlighted in recent years. GPCRs are coupled, through their intracellular domains, to heterotrimeric G-proteins, which induce cellular signaling through various pathways. Such signaling modulates numerous essential cellular processes that occur during melanomagenesis, including proliferation and migration. GPCR-associated signaling in melanoma can be activated by the binding of paracrine factors to their receptors or directly by activating mutations. In this review, we present melanoma-associated alterations of GPCRs and their downstream signaling and discuss the various preclinical models used to evaluate new therapeutic approaches against GPCR activity in melanoma. Recent striking advances in our understanding of the structure, function, and regulation of GPCRs will undoubtedly broaden melanoma treatment options in the future.
Keywords: G-protein-coupled receptor, mouse models, skin cancer, UVR, drug, network
1. Introduction
G-protein-coupled receptors (GPCRs) participate in intercellular communication by receiving extracellular stimuli from the microenvironment. They then amplify and transduce the signal, passing it on to the nucleus, where it triggers an appropriate cellular response. GPCRs can bind to a wide variety of ligands (hormones, proteins, peptides, amino acids, lipids, nucleotides, xenobiotics, etc.) and regulate numerous essential physiological processes during development and in adult life. GPCRs are the largest receptor family in the mammalian genome, with over 800 members [1]. The GPCR family is composed of receptors that share a common structure, consisting of seven transmembrane helices that are associated with a heterotrimeric G-protein. These receptors are known to regulate many essential physiological processes and their aberrant expression or activity can contribute to human diseases, including cancer. GPCRs are among the most common drug targets because they can be activated or blocked by low molecular weight molecules that have a very strong interaction with their receptors. Their importance in drug discovery is demonstrated by the fact that nearly 60% of drugs in the developmental stage and 36% of currently marketed drugs target human GPCRs, representing around 700 molecules [2]. However, only 10 of these molecules are used in cancer therapy and none have yet been approved for melanoma [3].
Melanoma is a skin cancer that arises from melanocytes, the cells responsible for pigmentation. It affects more than 320,000 people worldwide each year, resulting in the death of nearly 60,000 patients [4]. According to the WHO’s International Agency for Research on Cancer, the incidence is expected to continue to rise in the coming years, reaching half a million patients by 2040. Mortality estimates are also on the rise, with 97,000 deaths estimated for 2040, despite the development of new therapies in the second half of the 2010s [4]. These treatments are based on two approaches: (1) inhibition of the MAPK/ERK pathway in melanoma using a combination of activated BRAF and MEK inhibitors—targeted therapy (TT)—and (2) inhibition of immune cell exhaustion using the checkpoint inhibitors iCTL4 and iPD1—immunotherapy (IT)—[5]. Although these treatments have significantly increased patient survival, half of treated patients relapse [6]. Furthermore, despite significant recent progress in both targeted therapies and immunotherapies for treating advanced-stage disease, the long-term prognosis for patients with cutaneous melanoma is still poor. Therefore, an effective, reliable cure for melanoma undoubtedly requires further therapeutic innovation. Moreover, 40–60% patients are not responsive to current treatments.
GPCRs play crucial roles in various physiological processes, including neurotransmission, cardiac and sensory function, immune responses, and regulation of the pigmentary system. Pigmentation phenotypes have been observed with eight GPCRs (Table 1).
Table 1.
GPCR and pigmentation GPCRs involved in pigmentary phenotypes in mouse and human.
|
Gene
Cyto.loc |
Receptor Name | Signaling | Mouse Model | Human Disease | Function(s) | References |
|---|---|---|---|---|---|---|
|
DRD2
11q23.2 |
Dopamine D2 | Gαs | Hyperpigmentation eg. Drd2tm1Ebo/Drd2tm1Ebo |
αMSH synthesis | [7] | |
|
EDNRB
13q22.3 |
Endothelin B | Gαq | Hypopigmentation eg. Ednrbs-l/Ednrbs-l |
WS4A (#277580) ABCD syndrome (#600501) |
Proliferation Survival DNA repair |
[8] |
|
FZD4
11q14.2 |
Frizzled 4 | β-catenin | Hypopigmentation eg. Fzd4tm1Nat/Fzd4tm1Nat |
[9] | ||
|
GPR143
Xp22.2 |
Protein G143 | Gαq | Eye hypopigmentation eg. Gpr143tm1Inc/Y |
OA1 (#300808, #300500) |
Melanogenesis | [10] |
|
GPR161
1q24.2 |
Protein G141 | Gαs | White belly spot eg. Gpr161vl/Gpr161vl |
Shh negative regulator | [11] | |
|
GRM1
6q24.3 |
Glutamate receptor metabotropic 1 | Gαq | Hyperpigmentation eg. Tg(Dct-Grm1)ESzc |
Proliferation Survival |
[12] | |
|
MC1R
16q24.3 |
Melanocortin 1 receptor |
Gαs | Yellow coat Mc1re/Mc1re |
Light skin, red/blond hair | Switch eu/pheomelanin DNA repair |
[13] |
|
SMO
7q32.1 |
Smoothened | Gli | Striped depigmentation Curry-Jones syndrome (#601707) |
[14] |
The major signaling is indicated for each protein as well as the most characterized mouse mutant. For some of the proteins a human pathology is associated, the OMIM link is indicated (#). The reference is indicated in brackets in the last column. Cyto.loc. = cytogenic location, Ocular albinism = OA, Waardenburg syndrome = WS, Albinism, black lock, cell migration disorder of the neurocytes of the gut = ABCD.
Aside from its cosmetic role, pigmentation is a natural sunscreen that potently absorbs ultraviolet radiation (UVR) and is among the most important factors that determine UV sensitivity and melanoma risk. The melanin pigments responsible for the color of the skin and hair are synthesized within the melanosomes of melanocytes. In the epidermis, melanosomes generated by melanocytes are transferred to keratinocytes to allow homogenous pigmentation and protection of the entire skin against UVR. In the skin, melanocytes, keratinocytes, and dermal fibroblasts communicate with each other via secreted factors and cell-to-cell contact. The crosstalk of the various signaling pathways between these cells constitutes a complex network that controls pigmentation and melanocyte homeostasis. Genetics and in vitro studies have identified loci that regulate pigmentation, and among them, certain key regulators belong to the GPCR family. For example, the identification of the mouse extension locus (extension, recessive yellow, or Mc1r) associated with cloning of the melanocortin 1 receptor (MC1R) gene in human melanocytes identified this GPCR as the primary regulator of pigment synthesis. Apart from its effects on melanin production and consequently, UVR protection, the MC1R has functions extending beyond pigmentation, which explain how MC1R activity is directly involved in multiple aspects of melanomagenesis.
In contrast to epidermal melanocytes, which have a long-life span and low proliferative capacity during adult life, the precursor cells of melanocytes, called melanoblasts, proliferate and actively migrate during embryonic development to colonize the entire skin. Many signaling molecules/ligands are required during all stages of melanocyte development. These ligands educate neural crest cells (NCCs) to specify the melanocytic fate and instruct melanoblasts to proliferate, migrate, survive, and home (towards their final destination) prior to terminal differentiation into pigmented melanocytes. Approximately 100 genes have been shown to be specifically involved in melanocyte development. Among these genes, two GPCRs play a key role: the EDNRB (endothelin receptor type B, with its ligand endothelin 3) and FZL (frizzled receptor, with its ligands WNT1/3A and one of its mediators, β-catenin). Of note, the molecular and cellular mechanisms involved in the proliferation and migration of melanoblasts during development and those of melanoma cells during tumor progression are often closely related. Therefore, it is not surprising to find that key regulators of melanocyte development are also important players in melanomagenesis. The objective of this review is to provide an update on the GPCRs that have an important, well-identified role in melanomagenesis and to discuss the therapeutic strategies that have been used to target these GPCRs.
2. Impact of GPCRs on Melanoma Initiation and Progression
2.1. Melanomagenesis
During the multi-step process of melanomagenesis, skin melanocytes are transformed into melanoma. Briefly, the first steps consist of the benign proliferation of melanocytes to form a nevus, in which the melanocytes are grouped together and lose their characteristic contacts with keratinocytes. The melanocytes in the nevus eventually stop proliferating and become senescent. As melanomagenesis continues, nevus melanocytes are able to bypass senescence and enter the radial growth phase (RGP), where they typically superficially proliferate toward the basement membrane of the epidermis. These primary steps can be defined as “melanoma initiation”. Then, during the vertical growth phase (VGP), melanoma cells continue to actively proliferate and acquire migratory and invasive properties, allowing them to cross the basement membrane and invade the dermis. The cells eventually acquire metastatic characteristics as they enter the bloodstream and/or lymphatic vessels and eventually colonize various tissues and organs. These latter stages can be considered as the “progression” of the disease. Melanomagenesis is associated with changes in many cellular processes, such as proliferation, immortalization, pseudo-epithelial-mesenchymal transition, migration, and invasion. Cutaneous melanomas are molecularly classified into four groups based on their mutations: BRAF, RAS, NF1, and the “triple wildtype”. All four genomic subtypes of cutaneous melanomas are associated with aberrant activation of the MAPK and/or PI3K/AKT pathway that supports tumor cell growth, proliferation, survival, and anti-apoptotic signals.
The only effective way to demonstrate the causal role of a gene in tumor initiation is to use animal models, whose use allows a better understanding of tumor progression in a physiological context. Indeed, it is very difficult, if not impossible, to reproduce the in vivo cell-cell organization and microenvironment in vitro. However, simple or complex in vitro models are very useful for deciphering the involvement of key genetic elements in various cellular processes, but cannot be used to determine the causal role of a gene in tumorigenesis because (i) established melanoma cell lines are often derived from metastases in which the cells have already undergone a complete transformation process, (ii) the cells are grown on plastic, without their microenvironment, and (iii) colonization of distant organs is very difficult or impossible to assess. Various animal models allow evaluation of the proliferation and bypass of senescence (initiation) and invasion and metastasis (progression). Several animal models have been used to better understand melanomagenesis, including mouse, dog, pig, horse, chicken, and zebrafish. In this review, we focus on the currently best studied mouse models for melanomagenesis.
2.2. GPCRs in Melanoma
GPCRs regulate many key biological functions, such as cell differentiation, proliferation, migration, and metabolic activity. Thus, it is not surprising that they play a role in tumorigenesis, including melanomagenesis [15]. There are four main mechanisms by which GPCRs can drive tumorigenesis: (i) excess ligand availability, (ii) excess GPCR expression, (iii) activating mutations in GPCRs, and (iv) activating mutations in Gα proteins.
The role of certain GPCRs during melanomagenesis has been studied using natural (or chemically induced) mouse mutants of genes of interest or novel engineered gain- and loss-of-function mutants. A list of mouse mutants with pigmentation phenotypes is available and regularly updated (http://www.ifpcs.org/colorgenes/ accessed on 2 January 2022) [16]. In the case of genetically modified mutants, targeting of the melanocytic lineage is performed using the tyrosinase (Tyr), tyrosinase related protein 1 (Tyrp1), dopachrome tautomerase (Dct), or microphthalmia-associated transcription factor (Mitf) promoters/enhancers in the transgenic constructs [17]. Cre recombinase is used to generate conditional mutants, which are required when genes are essential in other lineages and/or during development: Tyr::Cre, Tyr::CreERT2-Lar, and Tyr::CreERT2-Bos [18,19,20].
The known GPCRs involved in melanomagenesis are presented in the following section. The role of certain GPCRs in melanocyte transformation is predictable, given their key function in melanocyte development and homeostasis (ET/EDNRB, MSH/MC1R, WNT/FZD), whereas the involvement of other GPCRs in melanomagenesis was less expected, such as GRM1, GRM3, GRM5, PAR1, CXCR4, CCR7, CCR10 and GPER1 (Table 2).
Table 2.
GPCR and melanoma.
|
Gene
Cyto.loc |
Receptor Name | Signaling | Animal Model | Melanomagenesis | Function(s) | References |
|---|---|---|---|---|---|---|
|
EDNRB
13q22.3 |
Endothelin B | Gαq | Mitf-cre/+; Rosa-fs-GNAQQ209L/+; EdnrbF/F | initiation progression |
Proliferation Invasion DNA repair |
[21] [22] |
|
MC1R
16q24.3 |
Melanocortin 1 | Gαs | Tyr::CreERT2/°; BrafV600E; MC1Re/e |
initiation | Proliferation DNA repair |
[23] [24] |
|
FZD7
2q33.1 |
Frizzled 7 | β-catenin | Xen., A375P, WM1361 shRNA-FZD7 |
progression | Proliferation | [25] [26] |
|
GPER1
7p22.3 |
G-protein-coupled estrogen receptor | Gαs | Xen., NHEM with BRAFV600E; p53R248W; CDK4R24C; hTERT | initiation | Differentiation | [27] |
|
GRM1
6q24.3 |
Glutamate receptor metabotropic 1 | Gαq | Dct::Grm1 | initiation progression |
Proliferation Invasion |
[12] [28] |
|
GRM3
7q21.11/12 |
Glutamate receptor metabotropic 3 | Gαq | Xen., A375 GRM3 S610L, E767K, or E870K; |
initiation progression |
Proliferation Migration |
[29] |
|
GRM5
11q14.2/3 |
Glutamate receptor metabotropic 5 | Gαq | Tyrp1::Grm5 | initiation progression |
Proliferation Invasion |
[30] |
|
PAR1/F2R
5q13.3 |
Coagulation factor II Thrombin |
Gαq | Xen. A375 shRNA-PAR1 |
progression: | Proliferation | [31] |
|
CXCR4
2q22.1 |
CXC motif chemokine 4 | Gαi/0 | Allograft B16 Ectopic expression Blocking peptide T22 |
progression | Attraction Growth |
[32] [33] |
|
CCR7
17q21.2 |
CC motif chemokine 7 | Gαi/0 | Allograft B16 Ectopic expression Neutral. anti-CCL21 ab |
progression | Attraction Growth | [34] |
|
CCR10
17q21.2 |
CC motif chemokine 10 | Gαi/0 | Allograft B16 Ectopic expression |
progression | Immune invasion Apop. resistance |
[35] |
GPCRs involved in melanoma from transgenic mice model or cell grafting or injection. The main associated signaling pathway is indicated as well as the cellular processes induced by the receptor activation. The reference is indicated in brackets in the last column. Cyto.loc. = cytogenic location, ab = Antibody, Xen. = Xenograft, Neutral. = Neutralizing, Apop. = Apoptosis.
2.2.1. Endothelin Receptor Type B (EDNRB)
The endothelin (ET) system consists of two class A G-protein-coupled receptors, endothelin receptors type A and B (EDNRA and EDNRB, respectively) and their three similar peptide ligands, endothelin-1, -2, and -3 (ET1, 2, 3). The EDNRB is the predominant receptor expressed by melanocytes/melanomas and binds all ETs with the same affinity. Edn3 and Ednrb were first found to play a major role during the development of melanocytes from NCCs using genetic knockout mouse models and then, by analogy, to the classic mouse mutants, piebald and lethal spotting [8,36]. Indeed, Ednrb and Edn3 genetically engineered mice (GEM) are both allelic to the spontaneous mouse mutations that occur at the piebald and lethal spotting loci. Recessive mutations at either of these loci give rise to similar phenotypes consisting of differing degrees of hypopigmentation and aganglionic megacolon, due to the absence of enteric ganglia, which have the same neural crest embryonic origin as melanocytes. Reciprocally, increased expression of Edn3 in the epidermis leads to increased numbers of melanocytes and hyperpigmentation [37]. Moreover, neonatal UV-irradiation of these mice overexpressing Edn3 in the epidermis leads to melanoma formation [38]. Germline Ednrb deletion does not lead to tumorigenesis but to the absence of melanocytes, mainly in the dermis. The role of Ednrb in melanomagenesis has been evaluated in the context of oncogenic GNAQQ209L signaling (see next paragraph). The expression of GNAQQ209L (encoding for Gαq) is not sufficient to replace EDNRB signaling during embryonic development, suggesting that Gαq may not be the only G-protein activated downstream of EDNRB (or other signaling pathways). Using a conditional knockout approach, GNAQQ209L–induced melanomagenesis is inhibited in the absence of Ednrb, including lung metastases (Mitf-cre/+; Rosa-fs-GNAQQ209L/+; EdnrbF/F background) [21]. Intriguingly, germline haploinsufficiency for Ednrb has the opposite effect in the RET mouse melanoma model (Metallothionein-1/RFP-RET; Ednrb+/− mice), in which it accelerates tumorigenesis, with an increase in lung metastases [39]. These two mouse models are of interest and clearly show that Ednrb expression affects melanomagenesis. However, they have the disadvantage of representing an uncommon oncogenic situation in human cutaneous melanoma, with haploinsufficiency or the lack of Ednrb combined with a GNAQQ209L driver mutation or increased RET signaling.
Interest in EDNRB in melanoma stems primarily from early observations in humans showing that EDNRB expression was positively associated with cutaneous melanoma progression; EDNRB mRNA and protein levels were found to increase from common nevi to dysplastic nevi and from primary to metastatic melanoma [40]. Consistent with this observation, in vitro experiments showed that ET promoted melanoma cell proliferation, migration, and invasion and that EDNRB inhibitors reduced melanoma cell growth and survival in culture and xenografts [41,42,43]. However, overexpression of EdnrB alone or combined with driver mutations was not performed in mice to genetically address its role in tumorigenesis in physiological situations.
Another aspect of ET signaling in melanoma is its activity in DNA repair, which has a role in reducing the genotoxic effect of UVR [22]. Indeed, ET signaling increases intracellular Ca++ mobilization and downstream activation of the stress-induced MAP kinases JNK and p38 in UV-irradiated human melanocytes. This activation in turn enhances the repair of cyclobutane pyrimidines (CPDs), the major form of DNA photoproducts [44]. Finally, a recent study has suggested that ET signaling has a multifunctional role in melanoma, acting on both tumorigenic and stromal cells, where it mediates immunosuppression by increasing Treg proliferation [45]. Thus, although the role of EDNRB signaling is relatively well understood during melanocyte development, its role in malignant transformation is much less clear, as it acts in multiple signaling pathways and is context dependent. As such, it is not surprising that therapies that target EDNRB have thus far not been very successful. Small-molecule inhibitors of EDNRB, A-192621 and BQ788, were shown to inhibit the growth and survival of melanoma cells in culture and in xenografts [42,43,46]. However, the dual EDNRA/EDNRB antagonist, Bosentan, was tested in phase II clinical trials and failed to produce a robust response in cutaneous melanoma patients, neither alone nor in combination with dacarbazine [47,48]. Similarly, A-192621 treatment of mice expressing Ednrb in the context of oncogenic GNAQQ209L showed no effect on tumorigenesis, whereas haploinsufficiency for Ednrb reduced it. Targeting EDNRB with an antibody-drug conjugate (DEDN6526A) is currently being tested in phase I [49]. It would be of great interest to generate a mouse model that reflects the human EdnRB situation in cutaneous melanoma: overexpression of EDNRB (human and mouse) in melanocytes/melanoma combined with oncogenic BRAFV600E or NRASQ61K/R, the major driver mutations in human cutaneous melanoma. Such models would allow a better understanding of the effect of Ednrb overexpression on melanomagenesis, the study of its downstream signaling, and the testing of its inhibitors in a human relevant preclinical mouse model before clinical trials.
2.2.2. The Melanocortin Receptor (MC1R)
Melanocytes express a receptor (MC1R) that controls melanogenesis. The MC1R belongs to a small subfamily of GPCRs, classified into five subtypes (MCR1-5) that contribute to important physiological processes. MC1R is the only melanocortin receptor expressed in melanocytes. MC1R is a class A receptor and is coupled to Gs protein. MC1R binds to the pro-opiomelanocortin-derived peptide α-melanocyte-stimulating hormone (α-MSH), resulting in the activation of downstream signaling cascades in a cAMP-PKA-dependent manner [50]. Upon UV exposure, α-MSH is released by keratinocytes, leading to stimulation of the MC1R at the melanocyte membrane, the activation of protein kinase A (PKA), and ultimately, to increased cAMP levels. Other pathways independent of UV exposure can lead to increased α-MSH production and hyperpigmentation, as observed in the Dopamine receptor D2 knockout (DRD2tm1Ebo) [7]. An important target of cAMP is the transcription factor CREB (CAMP-responsive element-binding protein), which becomes phosphorylated and then activates the promoter of MITF, which in turn up-regulates the transcription of the melanogenesis enzyme genes TYR, TYRP1, and DCT, as well as those regulating other cellular processes, including proliferation, invasion and metabolism [51,52]. In addition, binding of neurofibromin 1 (NF1) to MC1R regulates intracellular signaling pathways involved in pigmentation [53]. MC1R is the product of the gene located at the extension locus and stimulates the synthesis of the pigment, eumelanin (black, brown). The loss-of-function mutation in this locus, recessive yellow (e/e), results in the production of pheomelanin (yellow, red) instead of eumelanin [13,54]. An MC1R antagonist is the agouti signaling protein (ASP). Mutations in the mouse Agouti gene that cause increased and ectopic expression of ASIP (viable yellow, Avy) result in yellow coat color, similar to the phenotype of e/e mice, as well as obesity due to ASIP binding to the MC4R. In humans, more than 200 MC1R variants have been identified and high numbers of natural MC1R variants are strongly associated with pigmentary phenotypes, providing evidence that the MC1R is the main determinant of human pigmentation and central to eu- and pheo-melanin regulation [50]. Similar to mice, MC1R variants in humans can result in the reduction of receptor activity and a shift in melanin synthesis from eumelanin to pheomelanin. MC1R is inactivated in people with red hair, due to polymorphism(s) that make(s) them more susceptible to melanoma than dark-skinned individuals. For example, variants of the gene encoding MC1R, mainly R151C, R160W, and D294H, have been shown to be associated with light and poorly pigmented skin [55], whereas the WT form is associated with dark, highly pigmented skin [56,57]. Similarly, patients with mutations in proopiomelanocortin (POMC) genes—encoding the precursor of αMSH—have red hair [58] These variants decrease the sensitivity of the receptor and binding of the hormone α-MSH, produced by keratinocytes in response to UVR. Epidemiology studies have strongly established that the MC1R functions as a melanoma predisposition gene. However, it is still not clear whether this is due to the lack of eumelanin, to photoprotective and antioxidant activities, to the expression of pheomelanin, which is known to amplify UVA-induced reactive oxygen species (ROS), or to other functions not related to pigmentation. Indeed, pheomelanin was shown to promote melanomagenesis via the induction of oxidative DNA damage, without exposure to any carcinogens, such as UVR, in mice harboring the activating BrafV600E mutation combined with MC1Re/e [23]. Thus, loss of function of MC1R promotes initiation in a UV-independent manner, demonstrating its tumor suppressor activity and a key role in the initiation of melanoma.
Apart from its central role in pigment switching, it is now recognized that MC1R has non-pigmentary roles in antioxidant defenses and DNA-repair mechanisms [59,60,61]. The cAMP pathway enhances melanocyte nucleotide excision repair (NER) activity, which operates by a « cut and patch » mechanism, to remove UV lesions. Activation of the MC1R by α-MSH binding results in phosphorylation of the DNA damage sensors ataxia telangiectasia mutated (ATM) and Rad3 related (ATR), as well as in the recruitment of the xeroderma pigmentosum complementing proteins XPC (Group C) and XPA (Group A) [22]. Consistent with MC1R promoting DNA damage repair, impairment of the NER pathway in subjects carrying an MC1R loss-of function mutation has been observed. Additional non-pigmentation-related effects of MC1R can be attributed to the activation of MITF expression, which controls genes involved in DNA damage repair, chromosome stability, and centromere integrity [62].
Therapies involving defective MC1R signaling aim to restore its activities. Mouse mutants of MC1R have been characterized for years and can be used to evaluate therapies for better protection against UVR. Topical application of the cAMP permeable-inducer forskolin onto mice harboring loss-of-function mutations or haploinsufficiency of Mc1re/e stimulated eumelanogenesis and induced UV-resistance [24,63]. These studies confirmed epidemiological studies suggesting that MC1R haploinsufficiency increases mutagenic susceptibility to UVR and melanoma risk. Another therapeutic approach is to use MC1R agonists to increase pigmentation, antioxidant defense, and DNA repair. The best-known analogue is NDP-MSH, which is 100 times more potent than α-MSH and is currently used to treat photosensitivity diseases, such as erythropoietic protoporphyria (EPP). A very promising analog is the tripeptide (LK-514), which is >105 times more selective for MC1R than other melanocortin receptors [64]. The challenge of MC1R-based therapies is to use an analog that is highly specific to MC1R to prevent toxic effects due to the activation of other receptors and to avoid targeting expression of MC1R in non-melanocytes.
2.2.3. The Wnt/Frizzled Receptor
The Wnt (fusion of the words wingless and integrated) pathway is one of the most important signaling pathways during embryonic development and adult homeostasis and its deregulation has often been linked to cancer. Wnt proteins activate at least three different intracellular signaling pathways: the Wnt/β-catenin (or canonical), Wnt/Ca2+, and Wnt/planar cell polarity pathways [65,66]. The type of Wnt protein secreted determines which of these three signaling cascades is activated. The Wnt family contains at least 19 secreted cysteine-rich glycoproteins in humans [67,68]. Wnt proteins bind to target cells via two families of receptors: the seven transmembrane receptors Frizzled (Fzd) and LDL-receptor-related proteins. The Frizzled (FZDs) receptors are comprised of ten members (FZD1–FZD10), most of which are coupled to the β-catenin (bcat) canonical signaling pathway.
The Wnt/β-catenin pathway is essential for melanocyte development from NCCs [69]. The ligands Wnt1 and Wnt3a are required for the specification, expansion, and differentiation of melanoblasts from NCCs [70,71]. β-catenin itself has been directly implicated in melanoblast determination in several models, with varying effects depending on the temporality of its activation [72,73]. In mice, loss of bcat from pre-migratory NCCs (Wnt1::Cre; Ctnnb1ex2-6F/F) or melanoblasts (Tyr::Cre; Ctnnb1ex2-6F/F) induces the disappearance of melanoblasts [73,74]. The expression of a stabilized form of bcat (Tyr::bcat-mut-nls-egfp) leads to mice with a ventral white coat area associated with a defect in melanoblast migration [75]. Various β-catenin targets have been shown to be involved in cell proliferation and include the ubiquitous genes Myc and CyclinD1 and the melanocyte-specific gene Mitf-M.
Only one member of the Frizzled family, FZD4, has been implicated in pigmentation. FZD4 knockout (Fzd4tm1Nat) induced a depigmentation of coat color in addition to multiple defect in the nervous system [9]. FZD receptors are frequently overexpressed in tumor tissues relative to normal tissues and are potentially associated with a poor prognosis. No FZD overexpression has been directly linked to melanoma. Nevertheless, the FZD7 receptor was found to be upregulated in metastatis-derivative melanoma cell lines compared to the parental A375P cell line, with its expression associated with amoeboid invasion [26]. FZD7 knock-down reduces tumor growth after subcutaneous injection of the WM1361 melanoma cell line as well as metastasis formation after tail-vein injection of several melanoma cells in NSG mice [25,26]. OMP-18R5, a monoclonal antibody targeting several FZD including FZD7, is able to block tumor growth in xenograft mouse models for multiple cancers, but was not evaluated for melanoma [76].
Although little evidence implicates FZD receptors in melanomagenesis, studies of its associated signaling with β-catenin clearly highlight a central role of this pathway in melanoma. Melanoma was one of the first cancers in which CTNNB1 mutations were identified. In mouse models, the activation of WNT/β-catenin signaling participates in the initiation of melanomagenesis but is not, alone, sufficient for initiation. Expression of a stabilized mutated bcat in melanocytes, along with a mutated human NRAS oncogene, constitutively activating the MAPK pathway (Tyr::NRASQ61K/°; Tyr::bcat-mut/°) led to accelerated onset and increased the number of melanomas in a mouse model [77]. This property has been linked to the increased immortalization of melanocytes in vitro by the repression of p16 expression. In a BrafV600E; Pten-/-; bcat-null-KO (Tyr::CreERT2-Bos; BrafCA; PtenF/F; Ctnnb1ex2-6F/F) mouse model, in which β-catenin is inactivated, the occurrence of melanoma is strongly delayed relative to that in a BrafV600E; Pten-/- model [78]. In a BrafV600E; Pten-/-; bcat (Tyr::CreERT2-Bos; BrafCA; PtenF/F; Ctnnb1ex3F/F) mouse model, in which bcat is activated, the occurrence of melanoma is accelerated [78]. In mouse models of BrafV600E, Pten-/-, and NRASQ61R melanoma, bcat activation increases the number of lung metastases, whereas bcat inactivation decreases the number of lymph-node and lung metastases [75,78]. In conclusion, the activation of β-catenin increases both the initiation and progression of melanoma in mouse models. Of note, in humans, several studies have linked the Wnt/β-catenin pathway to the antitumor immune response in melanoma [79,80,81].
2.2.4. Glutamate Receptors (GRM1, GRM3 and GRM5)
Glutamate is the most abundant excitatory neurotransmitter in the human central nervous system, where it plays a critical role in intercellular communication. Glutamate receptors are also expressed in tissues outside of the nervous system and are involved in the modulation of various normal and pathological processes. The glutamate receptor family is divided into two major groups: ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (Grms). The Grm1s belong to the class C family of GPCRs, characterized by a large, globular, extracellular ligand-binding domain. The Grm1 family consists of eight members (Grm1-8), which are organized by sequence homology, signaling effectors, and general localization. Group I Grms, consisting of Grm1 and Grm5, are multi-coupling receptors that can signal through both the Gαq and Gαi/o pathways. Group II Grms consist of Grm2 and Grm3 and couple to the Gαi/o pathway. Group III Grms consist of Grm4, Grm6, Grm7, and Grm8 and couple to Gαi/o signaling pathways. Three members of the metabotropic glutamate receptors (mGluR1, mGluR5, mGluR3) have been clearly identified as regulators of melanomagenesis (see [82] for review).
The involvement of metabotropic glutamate receptors in melanomagenesis was initially revealed by chance in a complex study using insertional mutagenesis, leading to aberrant expression of Grm1. Surprisingly, the mouse developed metastatic melanoma, whereas Grm1 is not detected in normal melanocytes in mice [83]. Confirming this initial observation, transgenic mice containing Grm1 under the control of the Dct promoter (Dct::Grm1) developed melanoma with 100% penetrance [12]. Initially, no distant organ metastases were observed but disseminated cells were later detected in distant organs, such as the lung and liver [28]. The importance of Grm1 on tumor growth in vivo was supported by the decrease in growth induced by Grm1 knock-down in xenografted cells [84]. A conditional transgenic model using the tetracycline-regulated system to express mGluR1 in adulthood demonstrated that Grm1 expression is required not only for the initiation of melanoma but also for its progression in vivo [85]. The gene encoding the human receptor (GRM1) is altered in melanoma by point mutations, amplification, and/or deletions. In humans, GRM1 expression is not detected in normal melanocytes but it is expressed in 80% of metastatic melanoma or cell lines. Works from the laboratory of S. Chen showed that GRM1 expression results from activation of the MAPK and PI3K/AKT pathways, the main pathways activated in melanoma [82,86,87]. Melanomas expressing GRM1 show elevated levels of glutamate in the tumor microenvironment, contributing to hyperactivation of the receptor and its downstream effectors. The identification of this autocrine loop between GRM1 expression and the secretion of glutamate led to clinical trials to test riluzole, known to reduce glutamate release and thus reduce activation of the receptor. Despite being efficient in mice, no objective responses were observed in humans [88,89]. Recently, the secretion of glutamate by melanoma cells has been associated with an increased glutaminolysis due to aberrant expression of glutaminase. Co-inhibition of glutaminolysis and GRM1 have proven to be efficient in decreasing melanoma xenograft growth in vivo [90]. This co-treatment remains to be tested in the clinic. Furthermore, riluzole monotherapy showed more immune cell infiltrates in stable disease patients than those with progressive disease, suggesting that combining riluzole with immune checkpoint blockade therapy could enhance the efficacy of either agent alone. However, this has not yet been tested.
In contrast to Grm1, Grm5 is normally expressed in both normal melanocytes and melanoma tumors. Transgenic mice overexpressing Grm5 (Tyrp1-Grm5) present with multiple melanoma located on the tail, with 100% penetrance and metastases, demonstrating that Grm5 drives melanoma initiation and progression [30]. Of note, mice with Grm1 or Grm5 melanoma both exhibit tumor formation on the hairless skin, including the pinnae and tails, rather than on trunk areas, as observed for BRAFV600E and NRASQ61K induced melanoma, suggesting that the origin of the transformed cell may not be identical (epidermal vs. hair follicle). No information concerning GRM5 in humans is available.
Exon capture sequencing of 734 GPCRs in malignant melanoma showed that a third glutamate receptor, GRM3, is frequently mutated in human melanoma. The identification of the same mutations (G18E/R, M518I) in multiple individuals suggests that these mutations may be “drivers” of the oncogenic process. GRM3 mutants selectively regulate the phosphorylation of MEK, leading to increased anchorage-independent growth and migration [29]. Mutated GRM3 cells are more sensitive to MEK inhibitors. To date, no transgenic models with GRM3 mutants to study its effect on cellular transformation and sensitivity to MEK inhibitors have been developed. GRM3 mutants may contribute to melanomagenesis through cross talk between the cAMP and MAPK signaling pathways [91]. The misexpression of two other glutamate receptors, GRM4 and GRM8, has been detected in melanoma, but their precise role has not yet been clearly demonstrated [82]. Over 60% of human melanomas express Grm, indicating the importance of glutamatergic signals in this type of tumor. It appears that glutamate receptors are not only involved in neuronal signaling and neuronal disorders but also in the transformation of the originating neural crest-derived cells, melanocytes, into melanoma. Note that other NCC derivatives express GRMs. As an example, GRM1 is expressed in chromaffin cells of the adrenal medulla and GRM5 is expressed in the Meckelian cartilage [92,93]. However, no other NCC derivatives transformed by glutamatergic signaling have been reported so far.
The GPCRs that are cited in the next paragraphs (PAR1, CXCR4, CCR7, CCR10 and GPER1) have been implicated in melanomagenesis. However, their functional role in melanoma initiation and in an immunocompetent environment have not been studied.
2.2.5. PAR1
The protease-activated receptors (PARs) are a family of GPCRs comprised of four members (PAR1–4) involved in the regulation of various cellular processes, including inflammation and coagulation. Cleavage of PAR1 (also known as the thrombin receptor) by thrombin activates the receptor and downstream signaling through multiple heterotrimeric G-proteins such as Gαq, Gαi/0, and Gα12/13. In turn, the MAPK and PI3-K signaling pathways are activated, along with phospholipase C-β (PLC-β). Elevated PAR-1 expression during melanoma progression has been suggested to promote key processes that contribute to melanoma metastasis. Targeting PAR-1 reduced tumor growth and the metastases of melanoma cells in xenograft experiments [31]. Overexpression of PAR-1, as well as the continuous activation of thrombin, promotes the upregulation of genes involved in adhesion, invasion, angiogenesis, and metastasis [94]. As PAR-1 signaling affects both melanoma cells and their microenvironment, it was considered to be an attractive therapeutic target for the treatment of melanoma patients. However therapeutic trials were not continued in melanoma due to the activity of PAR1 in coagulation.
2.2.6. Chemokine Receptors (CXCR4, CCR7, CCR10)
Chemokine receptors belong to the GPCR family and are classified into four groups, CXCR, CCR, XCR, and CX3CR. Each receptor can bind to several chemokines. A variety of chemokine receptors are expressed on the surface of both immune and tumor cells. Expression of CXCR4, CCR7 and CCR10 on the surface of melanoma cells is associated with a poor prognosis [95]. Aside from their critical role in the immune response, chemokines and their receptors have been studied for their capacity to guide cancer cells to specific organs. Chemokines have chemotactic properties and can attract melanoma cells expressing their corresponding receptors. High concentrations of CXCL12, the ligand of CXCR4, are produced in the lungs and injected CXCR4-expressing B16 melanoma cells are able to efficiently colonize the lung. Such colonization is reduced in the presence of T22, a specific inhibitor of CXCR4 [32]. In addition, a commercially available dermal filler, hyaluronic acid (HA)-based gel, loaded with CXCL12 was able to recruit and trap CXCR4-expressing B16 melanoma cells injected into mice, consequently leading to a reduction in lung metastases [33]. One hundred and thirty-eight (138) clinical trials with CXCR4 inhibitors are being/have been performed, none of which include(d) melanoma. In mice, the overexpression of CCR7 or CCR10 in B16 melanoma cells was shown to increase regional lymph node metastases, which was blocked by neutralizing its ligand, CCL21, using a specific antibody [34,35,95]. However, most of these experiments used mouse B16 melanoma cells, which are not necessarily the best representation of human melanoma. The effect of the various chemokines on the immune response is not discussed here.
2.2.7. G-Protein-Coupled Estrogen Receptor 1 (GPER1)
There are three estrogen receptors, two nuclear receptors, ERα and ERβ, that act mainly as transcription regulators, and the G-protein-coupled estrogen receptor 1 (GPER1 = GPER = GPR30), that can induce rapid, non-genomic estrogen signaling [96]. GPER1 coupled with Gαs protein induces the cAMP pathways. Using various mouse models, GPER1 has been shown to play pleiotropic functions particular in the endocrine, immune, cardiovascular and central nervous systems. In these mice, no pigmentary phenotype has been observed. The impact of estrogen in melanomagenesis is still controversial since multiple genetic and environmental factors can greatly influence the development of this cancer and its severity. Nevertheless, Natale at al., propose that repeated pregnancies inhibit the growth of BRAF-driven human melanocytic neoplasia in xenografts and that GPER1 signaling promotes cell differentiation instead of proliferation, inhibiting tumor development [27]. Furthermore, in this study, GPER1 signaling rendered melanoma cells more vulnerable to immunotherapy. These results led to the initiation of a clinical trial targeting GPER1 and anti-PD1 immunotherapies (Phase I/IIA trials: NCT04130516). It should be pointed out that there is an notable controversy concerning the role of GPER1 as the principal mediator of the estrogen response in vivo, because even though nuclear receptors are less expressed in melanoma than GPER1 (Median level for GPER1 = 1.86; Erα = 0.61; Erβ = 0.31Tpm according to TCGA data base), they have a higher affinity for 17β-estradiol (GPER1 = 3–6 nM and ERs = 0.1–1 nM) [97]. It is clear that the role of GPER1 in melanoma needs further investigation, particularly in physiologically relevant mouse melanoma models.
Other GPCRs, namely GPR143, GPR161, SMO, which couple to Gαq, Gαs and Gli, respectively, have produced pigmentation phenotypes in mice, but have not been implicated in melanomagenesis [10,11,14].
3. GPCR Associated Signaling in Melanoma
The activation of GPCRs leads to the modulation of the activity of cellular signaling pathways, marked by the production of second messengers. The first element of such signaling is the heterotrimeric G-proteins to which these receptors are coupled through their intracellular domain. These G-proteins consist of three subunits, Gα, Gβ, and Gγ [98]. The C-terminal subunit of the GPCR is responsible for the selectivity of receptor/G-protein binding [98,99]. The G subunit is also responsible for the selectivity of downstream signaling pathways [100]. There are a total of 17 Gα subunits grouped into four subfamilies: Gαs, Gαi/0, Gαq/11, and Gα12/13.
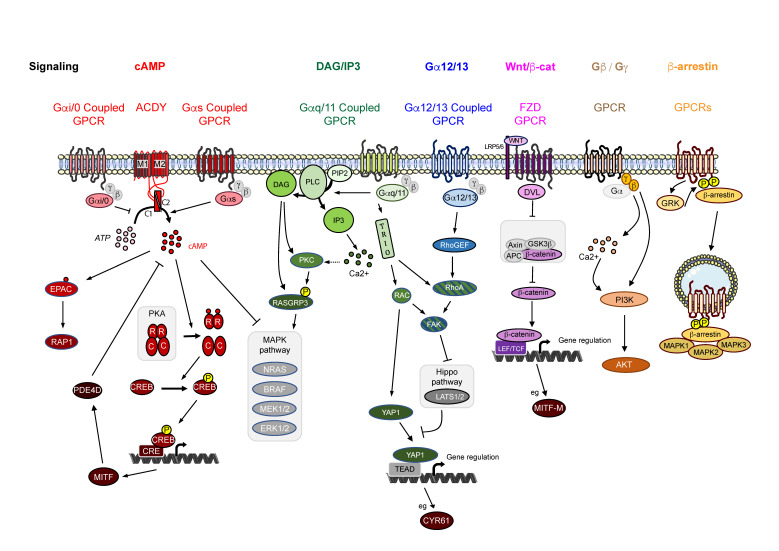
GPCRs generally couple to a specific G-protein but may interact with several different G-proteins [56,101]. Coupling appears to be cell-dependent. Thus, a careful analysis of the downstream signaling is required for each cell type. Binding of a ligand to its GPCR causes a conformational change in the GPCR that is transmitted to the Gα subunit, which exchanges its GDP to a GTP molecule. The binding of GTP induces the dissociation of Gα from the Gβ-Gγ subunits of the receptor. This dimer then modulates the activity of other intracellular proteins [98]. The GαGTP and Gβ/Gγ complexes then generate different intracellular signals that included cAMP, inositol-1,4,5 trisphosphate (IP3), diacylglycerol (DAG), and Rho proteins (Figure 1).
Figure 1.
GPCR signaling pathways. Note that the Gαq/11 et Gα12/13 G-proteins share a common downstream signaling intermediate—YAP1.
3.1. Signaling via cAMP
The induction of MC1R by its ligand activates the cAMP pathway, whereas the induction of GRM1, GRM3 and GRM5, CXCR4, CCR4, CCR7, and CCR10 represses this pathway. cAMP was the first second messenger to be discovered and regulates many downstream cellular processes [102,103]. Two classes of G subunits modulate intracellular cAMP levels, Gαs and Gαi/0, with diametrically opposite effects [100,104]. The enzyme responsible for cAMP production, adenylate cyclase (ADCY), is a membrane-associated enzyme that converts ATP to cAMP [104,105]. The difference between Gαs and Gαi/0 is due to the difference in the binding domain on the adenylyl cyclase: members of the Gαs family bind to the C2 intracellular domain of the adenylyl cyclase, which then activates its enzymatic activity, ultimately leading to an increase in intracellular cAMP levels [106]. Conversely, members of the Gαi/0 family bind to the C1 intracellular domain of the adenylyl cyclase, thereby inhibiting its activity.
The heterotrimeric G-protein subfamily Gαs is composed of three members: Gαs and Gαsxl, two splice variants of the GNAS gene, and Gαolf, encoded by the GNAL gene [100]. Only Gαs is expressed in melanocytes and melanoma [107] and is activated by an associated receptor, such as the MC1R [108]. Gain-of-function mutations of GNAS are clustered at amino acids R201 and Q227, located in the GTPase activity pocket. These mutations induce the loss of intrinsic GTPase activity and maintain the Gαs protein in an activated state. Mutations of GNAS are frequently found in various tumors of the pancreas, kidney, and stomach, but are more anecdotal in melanoma, affecting less than 1% of cases [109,110]. More strikingly, the GNAS T393C SNP polymorphism is associated with tumor progression in metastatic melanoma [111], as well as in other cancers, such as colorectal or bladder cancer [112,113]. How this polymorphism affects the activity of Gαs and the oncogenic process has not been evaluated. However, this observation suggests that Gαs inactivation favors melanoma progression.
The Gαi/0 heterotrimeric G-protein subfamily is composed of eight members: Gαi1, Gαi2, Gαi3, Gα0, Gαz, Gαt-r, Gαt-c, and Gαgust. However, only Gαi1, Gαi2, and Gαi3 are expressed in melanocytes and melanoma, and are encoded by GNAI1-3, respectively [100,110,114,115]. Gain-of-function mutations of GNAI2 are clustered at amino acids R179 and T182 and lead to constitutive activation of the Gαi2 subunit by increasing its GTP binding capacity [116,117]. GNAI2 is mostly involved in cell injury and inflammatory responses but these activating mutations can lead to tumors, depending on the cellular context, due to increased MAPK activity. Gαi2 R179 and T182 mutations are found in 1.4% of melanoma patients [110] but their impact on melanomagenesis has not yet been evaluated.
Gαs and Gαi/0 directly regulate adenylate cyclase. There are ten (10) enzymes encoded by ten (10) different ADCY1-10 genes [118,119]. All are membrane bound via their two series of six transmembrane helices (TM1 and TM2), followed by a cytoplasmic domain (C1 and C2, respectively). Only ADCY10, which is soluble, differs from the others, as its activation is GPCR independent, being activated by bicarbonate and calcium [120]. Most adenylate cyclases are expressed in melanoma, with the exception of ADCY5 and ADCY8 [110,115]. The expression of ADCY10 is unclear because, although teams have found the protein by IHC, databases suggest that the mRNA is absent [110,121]. It has been shown that metastatic melanomas express more ADCY1 mRNA than primary melanomas and that a high level of ADCY1 expression correlates with a poorer prognosis [122]. Consistent with this observation, the silencing of ADCY1 in vitro in mucosal melanoma cell lines decreases the ability of cells to form clones in a colony-formation assay, as well as their migratory and invasive capacity [123]. In xenograft experiments, decreased ADCY1 expression decreased subcutaneous cell growth as well as colonization of the lung after the injection of melanoma cells into the tail vein of NOD/SCID mice [123]. More generally, stimulation of adenylate cyclase activity by forskolin promotes tumor growth in the BrafCA/Pten−/− melanoma mouse model, whereas its pharmacological inhibition by SQ22536 leads to a decrease in tumor growth in a MAPK pathway-independent manner [124,125]. However, treatment of human primary and metastatic melanoma cell lines with SQ22536, even at high concentrations, does not alter cell survival. This implies that the targeting of transmembrane adenylate cyclase is not a feasible therapeutic strategy on its own. Adenylate cyclase activity is also involved in resistance to MAPK inhibitors. Indeed, treating BRAFV600E melanoma cells with forskolin increases ADCY9 expression and cAMP synthesis, leading to greater resistance to MAPK inhibition [126].
The intracellular concentration of cAMP is negatively regulated by phosphodiesterases (PDEs), which hydrolyze cAMP to AMP, thus controlling the amplitude and duration of the signal. There are 11 families of PDEs (PDE1-11) encoded by a total of 21 different genes [127]. Specific PDE isoforms are located in different subcellular compartments, where they regulate cAMP levels. Indeed, cAMP does not freely diffuse across the cell but is rather produced in subcellular compartments. This feature has important consequences, allowing only appropriate targets to be activated in microdomains [128].
In melanocytes, phosphodiesterase 4 (PD4E), more specifically its variant PD4ED3, is a direct target of MC1R-cAMP signaling, constituting a negative feedback mechanism [129]. Blocking PDE4D3 activity in conjunction with forskolin treatment can efficiently restore cAMP levels and pigmentation in MC1Re/e mice. In melanoma, the expression of numerous PDEs has been reported and their effect was initially shown on cell proliferation [130,131]. Their specific functions in BRAF or NRAS-mutated melanoma highlight the connection between the cAMP and MAPK pathways (see below). The overexpression of PDE4 and, therefore, the inhibition of cAMP signaling is critical for MAPK activation by oncogenic RAS in melanoma [131,132]. In BRAF-mutated melanoma, the inhibition of PDE4 activity by pharmacological inhibitors or RNA interference decreases melanoma cell invasion by interacting with the focal adhesion kinase FAK [133]. Overall, 3.5% of solid tumors (including melanoma) have homozygous microdeletions of PDE4D associated with increased expression and a tumor-promoting effect [130]. PDE4D expression is elevated in advanced melanoma and negatively associated with survival. More generally, inhibiting cAMP signaling through the expression of PDEs (PDE1, PDE2, PDE4, and PDE8) is associated with the oncogenic progression in melanoma [128]. Whether PDE inhibitors can prevent proliferation, invasion, and/or migration in melanoma needs to be evaluated in the future.
The first and main target of cAMP is protein kinase A (PKA) [100,134]. PKA is a serine/threonine kinase composed of four subunits: two regulatory and two catalytic. There are four isoforms for both the regulatory (RIα, RIβ, RIIα, and RIIβ) and catalytic subunits (Cα, Cβ, Cγ, and PRKX), with each isoform showing individual localization and specificity. In humans, all are expressed, but only Cγ is expressed in melanocytes and melanomas [135]. Theoretically, since RIs cannot interact with RIIs, a maximum of 36 combinations can be generated between regulatory and catalytic subunits. At this point, specific combinations have not been characterized in melanocytes or melanomas.
Binding of cAMP to the regulatory subunits induces their dissociation from the catalytic units, which become active and phosphorylate downstream targets [136]. More than 70% of patients with familial Carney complex, an autosomal dominant skin condition associated with spotty pigmentation [137], carry three mutations associated with pathogenic features (82C>T, 491_492 delTG, c.709-2_709-7 delATTTTT) in the PRKAR1A gene, which encodes the RIα subunit [138]. This mutation induces a dominant-negative action of the regulatory subunit and in consequence a constitutive activation of the catalytic subunit of PKA. Inactivating mutations of PRKAR1A lead to constitutive activation of the cAMP-PKA pathway through the loss of regulation of the catalytic subunits of PKA. In melanoma, mutations are found in 1.4% of cases, as well as loss of heterozygosity in 11.8% of patients [110]. Furthermore, loss of function of PRKAR1A is found in epithelioid pigmented melanocytomas, a rare intermediate/borderline form of melanoma [139]. By comparing PKA activity in primary and metastatic melanoma cells, Beebe and colleagues suggested that PKA activity is higher in melanoma metastases [140]. The pharmacological inhibition of PKA induces the growth and invasion of melanoma cells [141].
A large number of cytosolic and nuclear proteins have been identified as substrates for PKA [102]. Importantly, PKA is located at the crossroads between cAMP and MAPK/ERK. Constitutive activation of cAMP leads to the phosphorylation and inactivation of CRAF by PKA in melanoma [131]. CRAF is important in maintaining activation of the MAPK pathway in RAS-mutated cancers because ERK1/2 has a negative feedback action through the phosphorylation of BRAF, which causes its inhibition [131,132,142]. As a consequence, activation of MAPK pathways through CRAF requires that the cAMP pathway in melanoma cells be inactivated to release cAMP-mediated inhibition of CRAF. PKA can also directly phosphorylate BRAF on serine 365, dissociating the RAS/BRAF/KSR complex and thus activating BRAF [143]. The catalytic Cα subunit renders BRAFV600E melanoma cells resistant to MAPK inhibitors [126]. The CRTC3 protein, a co-activator of CREB that is phosphorylated and activated by PKA and ERK [144], lies at the interface between signaling through the cAMP and MAPK pathways. A knockout mouse model for Crct3 showed graying of the coat due to defects in melanocyte maturation [144]. Mutations in CRCT3 have been identified in 23% of human melanomas, most leading to an increase in its expression and activity and reduced patient survival [144]. Thus, CRT3 inhibition could be beneficial for such patients.
The regulation of transcription by PKA is mainly achieved by the phosphorylation of CREB. CREB phosphorylation leads to dimerization of this transcription factor and its subsequent binding to cAMP response elements (CRE) in target genes and its interaction with transcription co-activators, such as CREB-binding protein (CBP) and p300. CRE binding sites are located in the promoter regions of many genes, including the master melanocyte regulator MITF [51,145]. MITF regulates numerous major cellular processes essential for melanogenesis and melanomagenesis, including pigmentation, growth, survival, migration, and invasion [52]. In melanoma, CREB overexpression is associated with transition from the radial to vertical growth phase [146].
The inhibition of CREB in melanoma cell lines was shown to decrease metastasis formation after injection into the tail veins of mice [147,148]. This loss of metastatic potential can be explained, at least in part, by the loss of the expression of the metalloproteinase MMP2 and the adhesion molecule MCAM/MUC18. Surprisingly, CREB, which generally acts as a transactivator, negatively regulates the transcription factor AP2α and the gene encoding cellular communication network factor 1 (CCN1/CYR61). In early publications, these two genes were considered to be tumor suppressor genes in melanoma but more recent studies have proposed that AP2α and CCN1 facilitate melanoma progression [149,150,151]. The RNA-editing enzyme adenosine deaminase acting on RNA1 (ADAR1) has been recently identified as a new target of CREB. Silencing ADAR1 enhances the invasiveness of melanoma cells [152]. CREB has been associated with resistance to MAPK inhibitors. Phospho-CREB is restored in relapsing melanomas previously treated by MAPK inhibitors, possibly by the up-regulation of adipocyte enhancer-binding protein 1, AEBP1 [126,153].
The second major target of cAMP is the cAMP-activated exchange protein (EPAC) [154]. EPAC is a guanine nucleotide exchange factor (GEF) for small GTPases, e.g., RAP1 (Ras-related protein 1). Activation of RAP1 occurs through the exchange of GDP for GTP [102,154]. The consequences of EPAC activation on the growth of melanoma are still unclear and reports are conflicting. Indeed, pharmacological activation of EPAC using an EPAC-specific cAMP analog increases the growth of HMG cells [155] but has no effect on PMP melanoma [141]. The use of shRNA targeting Rap1 increases the growth and survival of cells derived from primary but not metastatic melanomas [124]. The current hypothesis is that the EPAC-Rap1 pathway is anti-proliferative in metastatic melanoma and pro-proliferative in primary melanoma [156].
The last major effectors of cAMP are the cAMP-dependent ion channels: the cyclic nucleotide-gated ion channel (CNG) and the hyperpolarization-activated cyclic nucleotide-gated channel (HCN) [154,157]. These channels are relatively nonselective cation channels and have not yet been studied in melanoma.
3.2. Signaling via Inositol Triphosphate and Diacylglycerol
The induction of EDNRB and PAR1 by its ligand activates the IP3/DAG pathway. It has to be noted that GRM1 and GRM5 induce the IP3/DAG pathway, but repress the cAMP pathway. Cellular levels of IP3 and DAG are highly regulated by the stimulation of GPCRs of the Gαq/11 class. This class contains four members: Gαq, Gα11, Gα14, and Gα16 [100]. All can be expressed in melanocytes and melanoma but only Gαq and Gα11 are highly expressed in these cells [110,115]. Activation of the receptor induces the exchange of GDP bound to the alpha subunit for GTP, resulting in activation of this subunit. Mutations affecting G-proteins of the Gαq/11 class are almost systematically found in uveal melanoma. Approximately 90% of metastatic uveal melanomas are mutated for Gαq or Gα11, affecting mainly glutamine 209 in both proteins, but also, to a lesser extent, arginine 183 [158,159]. GNAQ, which encodes Gαq, is mutated in 50 to 85% of non-epithelial melanocytic lesions, including blue nevi and leptomeningeal melanocytic neoplasms. Mutations in GNA11, which encodes Gα11, are more frequent in uveal melanomas [160]. These mutations are found at lower frequencies (~1–6%) in other types of cutaneous melanoma [110,161]. Q209L/P and R183C/Q mutations in GNAQ or GNA11 affect the GTPase domain [162] but only Q209L/P mutations have actually been characterized. These mutations reduce the GTPase activity of the Gαq subunit and cause hyperactive signaling [162]. Regardless of the tumor context, the mutations are mutually exclusive [158] and are also mutually exclusive with BRAF and NRAS mutations. Of note, in uveal melanoma, mutations of CYSLTR2 (L129G), encoding a Gαq/11-coupled GPCR, are found in a mutually exclusive manner with Gαq and Gα11 mutations in approximately 3% of patients. This mutation is also present in blue nevi [158,163]. This CYSLTR2L129G mutation constitutively activates the receptor and thus downstream signaling. Depending on the murine models used and the cells targeted (neural crest cells or melanoblasts), embryonic GαqQ209L expression is able to induce a range of lesions from dermal hyperpigmentation to leptomeningeal melanocytoma, nevi, and dermal melanoma to malignant uveal melanomas with lung invasion [164,165]. Similarly, postnatal expression of Gα11Q209L in melanocytes induces hyperpigmented melanocytic lesions in the uveal tract, skin, and leptomeninges that progress to melanoma with lung invasion [166]. Mice transplanted under the skin with Gαq mutated melanoma cells show inhibition of MAPK signaling and tumor growth following treatment with FR900359 [167]. To date, the inhibition of uveal melanoma with FR900359 appears to be more potent than inhibition with YM254890 [168]. The activation of Gαq/11 is a mechanism of resistance to MAPK pathway inhibition through the overexpression of c-Jun [169]. These data are consistent with the gain in resistance to MAPK inhibitors shown by activation of EDNRB [170]. Conversely, inhibition of Gαq by YM-254890 resulted in inhibition of MAPK signaling, with evidence of a rebound after 24 h in xenograft experiments of uveal melanoma. Combined treatment with YM-254890 and a MEK inhibitor led to sustained MAPK inhibition and tumor shrinkage [171]. A combination of PKC and MEK1 inhibitors is currently under clinical evaluation for solid tumors harboring GNAQ/11 mutations or PRKS fusions (Phase I/II trials: NCT03947385).
The primary target of Gαq/11 subunits is phospholipase C beta (PLCβ) [172,173,174]. This subfamily is encoded by four genes (PLCB1-4) encoding seven proteins, all of which have two isoforms, except PLCβ3 [175]. All PLCβs are likely expressed in cutaneous melanoma [110], whereas, only the PLCB2-4 genes are expressed in uveal melanoma [158]. PLCβs function by hydrolyzing membrane phosphatidylinositol-4,5-biphosphates (PIP2) into IP3 and DAG [176]. PLCB4 mutations occur in approximately 5% of uveal melanoma and are mutually exclusive with GNAQ, GNA11, and CYSLTR2 mutations [177]. PLCβ4D630Y mutations affect the Y domain of the catalytic core of PLC4 [177]. Their effect has not been precisely studied, but their exclusivity with the GNAQ, GNA11, and CYSLTR2 mutations and PLCβ4 being downstream of these proteins suggest that PLCβ4 mutations have a similar effect on the oncogenicity of uveal melanomas [177]. Outside the context of melanoma, the other PLCs have often been shown to induce migration and/or to reduce the immune response [178,179].
One of the two secondary messengers produced by PLCs is DAG, which remains anchored in the membrane. Phorbol esters, such as 12-O-tetradeconoyl phorbol-13-acetate (TPA) and phorbol 12-myristate 13-acetate (PMA), are synthetic analogues of DAG. TPA is essential for melanocyte growth in vitro [180,181,182]. Interestingly, the effect of TPA on the proliferation of melanoma cell lines appears to be cell dependent [118,181,183,184,185]. PMA increases cell survival, invasion, and resistance to anoikis [184,186,187]. TPA and PMA activate the PKC and MAPK pathways [184]. The other secondary messenger produced by PLCs is IP3. The binding of IP3 to the IP3 receptor (IP3R) increases intracellular Ca2+ levels [188]. Calcium release induced by IP3 supports melanoma cell migration and invasion [189,190,191].
DAG and calcium activate protein kinase C by binding to the C1 and C2 domains of PKC, respectively [192]. There are nine genes that encode PKC: PKCα, PKCβ, PKCγ, PKCδ, PKCθ, PKCε, PKCη, PKCι, and PKCζ [192]. They are divided into three classes according to their activation mechanism. The classical PKCs (cPKCs) consist of PKCα, PKCβ, and PKCγ and are activated by calcium and DAGs. The novel PKCs (nPKCs) (PKCδ, PKCθ, PKCε, PKCη) are activated by DAGs alone. Atypical PKCs (aPKCs) (PKCι and PKCζ) are not activated by calcium or DAGs [192]. At least one PKC from each class is expressed in melanoma cells, except PKCγ [110,192]. PKC activity is regulated by the presence of their substrates and cofactors and their recruitment by scaffolding proteins, such as receptor for activated kinases C (RACK) or protein kinase A scaffolding protein 5 (AKAP5) [193,194,195,196]. PKC regulates invasion of melanoma cells but the various members have different effects: PKCα and PKCδ induce melanoma migration, invasion, and lung colonization [197]. Conversely, PKCβ decreases invasion and promotes cell differentiation and pigmentation [198,199]. PRKCB is frequently mutated and its expression is often lost in melanoma, but the impact of these mutations on PKCβ activity has not been evaluated [110,196]. Similarly, the effect of PKC on cell growth is dependent on the isotype [200,201,202].
Among the targets of PKC is Ras guanine-releasing protein 3 (RASGRP3), a guanine nucleotide exchange factor for RAS family proteins [166,200]. PKC phosphorylates RASGRP3 on Thr 133, which contributes to its activation in conjunction with DAG binding [203,204,205]. The inhibition of RASGRP3 induces the loss of GTP binding to RAS and thus its activity [166,200]. This decrease in activity is accompanied by a decrease in MAPK pathway activity and is associated with a decrease in cell proliferation [166]. This molecular mechanism may explain the activation of the MAPK pathway seen after PKC activation [86,206,207,208]. PKCε and PKCη are able to shunt the pharmacological inhibition of BRAFV600E, rendering melanoma cells resistant to these drugs [209]. Consistent with this finding, the use of PKC inhibitors inhibits the survival and migration of melanoma cells resistant to vemurafenib [210].
The activity of Yes-associated protein 1 (YAP1) is positively regulated by many GPCRs but negatively regulated by the Hippo pathway [211,212]. In uveal melanoma, YAP1 is activated by Gαq, inducing cell growth and survival [164,213,214]. Inhibition of YAP activity by verteporfin reduces tumor growth [213,214]. Gαq activates the guanine nucleotide exchange factor TRIO, which in turn activates the small GTPases RhoA and Rac1 [213,215]. This likely has a dual action: (i) the activation of FAK, which in turn inhibits the LATS1/2 kinase of the Hippo pathway, inactivating the action of YAP1, and (ii) the direct activation of YAP1 by releasing the angiomotin transcription factor (AMOT) [213,216]. In uveal melanoma, the activation of Rho and Rac is linked to the activation of the MAP kinases JNK & p38 [215]. JNK and p38 phosphorylate c-Jun and induce the expression of AP-1 targets [217]. Phosphorylation of c-Jun induces cell proliferation via AP1 targets that regulate the cell cycle, such as cyclin D1, p53, p21cip1/Waf1, p19ARF, and p16 [217,218,219,220]. Activation of p38 and JNK are likely to be involved in mechanisms of resistance to MAPK inhibitors [218,219]. In cutaneous melanoma, the activation of YAP1 is required for cell invasion [221,222,223,224], as well as viability and resistance to anoikis [221,222,224]. Although the effect of YAP1 on invasion is clearly documented, its effect on melanoma growth is still debated [221,223]. YAP1 activity has also been shown to be associated with cell migration via regulation of the arp2/3 complex 3 [221,225]. The invasive phenotype of melanoma cells has been correlated with the activation signature of YAP [223]. In vivo, YAP1 activation induces the formation of very large numbers of metastases in the lungs after the injection of cells carrying the activating mutation of YAP1-5SA under the skin of mice [223]. Lung colonization after the injection of cells into the tails of mice also decreases when YAP1 levels are genetically decreased [222,224]. Most interestingly, inhibition of the Hippo pathway replicates this effect, favoring lung colonization [226]. The pro-invasive action of YAP1 is mediated through the transcription of a number of its targets, such as CCN1, AXL, and THBS1 [223]. These targets are well known in melanoma. AXL expression is associated with tumor growth and cell invasion and migration and has also been shown to be associated with resistance to MAPK inhibitors [227,228,229]. CCN1 has been shown to be associated with increased metastatic potential and angiogenesis [150,230]. Genetic inhibition of THBS1 is associated with decreased cell invasion [231,232]. YAP1 requires the transcriptional cofactors TEAD1-4 to bind to its targets. Genetic inhibition of TEAD1-4 recapitulates the in vitro effects of YAP1 on invasion, with a clear decrease in the invasive capacity of melanoma cells [233]. TEAD1-4 are also involved in resistance to MAPK inhibitors and inhibition of all four TEADs sensitizes cells to these inhibitors [233].
3.3. Signaling via Gα12/13
The induction of PAR1 by its ligand activates Gα12/13. It has to be noted that PAR1 induces both the IP3/DAG and Gα12/13 pathways. The third major pathway of GPCR signaling involved in melanoma is the pathway involving Gα12/13 [100]. This class of G subunits has two members, Gα12 and Gα13, encoded by the GNA12 and GNA13 genes, respectively. The expression of these genes is ubiquitous [100,110,115]. Activation of these subunits activates Rho-GEFs, such as leukemia-associated Rho-GEF (LARG) or p115 Rho-GEF [234,235]. Activation is achieved by the attachment of the G12/13 subunit to the RH domain of Rho-GEFs [236]. Once activated, Rho-GEFs induce RhoA activation [237,238,239,240]. Rho is a converging point for Gα12/13 and Gαq/11 signaling [239]. Signaling induced downstream occurs through the activation of YAP1, as described above [212]. Gα12/13 signaling has been poorly analyzed in the context of melanoma. PAR1 and 2 receptors, coupled to Gα12/13 proteins, are expressed in melanoma [241]. Activation of PAR1 receptors by its ligand TRAP6 induces the activation of YAP1 in a HEK293A cell model [242]. YAP1 activation subsequently leads to activation of RhoA and inhibition of LATS1 [242]. Similar to Gαq/11 signaling, Gα12/13 signaling appears to increase invasion and migration while not altering cell growth [243,244]. However, these results were obtained in breast and prostate cancer and need to be confirmed in melanoma. Of note, activation of LPA receptors (LPA1-LPA6), coupled to G12/13 receptors, is still poorly documented in melanoma, although it has been reported to enhance chemoresistance and increase the survival of melanoma cells in vitro [245].
3.4. Signaling via WNT/β-Catenin
The induction of FZD7 by its ligand activates the WNT/β-catenin signaling pathway. WNT ligands activate three intracellular pathways: the canonical WNT/β-catenin, WNT/Ca++ and WNT/PCP. Only the canonical WNT/β-catenin pathway will be described in this chapter. WNT/Ca++ corresponds to the Gαq/11 pathway already described in 3.2. and the WNT/PCP pathway has not been studied in melanoma.
The WNT/β-catenin pathway has been widely studied and reviewed. Here, we provide the current knowledge of this pathway in melanoma, especially through the activity of β-catenin, which is encoded by CTNNB1 [68,246].
Mutations in the CTNNB1 gene are rare: around 3% in melanoma except for the deep penetrating nevus (DPN) that harbors 90% of activating mutations in CTNNB1. As such, activated β-catenin is a marker caracterizing this specific melanocytoma/melanoma type from other cutaneous melanocytic tumors [247,248]. However, cytoplasmic or nuclear localization of β-catenin, indicating activation of the pathway by other mechanisms other than mutation in its gene, was found in 20–30% of human melanoma [249,250]. The canonical WNT pathway is activated only in response to the formation of a complex containing WNT, FZD, and LRP. WNT proteins are difficult to purify in an active form and only a few antibodies are available for their detection. The WNT proteins most studied in the context of β-catenin activation in melanocytes/melanoma are WNT1 and WNT3a. WNT5a has different roles and acts as an antagonist or agonist of the canonical WNT/β-catenin pathway, depending on the cellular context. WNT proteins are subject to post-translational modifications, including glycosylation and lipid modifications. Acylation on conserved serine and cysteine residues is required for WNT secretion and efficient binding to the Frizzled receptor [251,252]. In the basal state, Axin protein interacts through distinct domains with GSK-3, CK1α, APC, and β-catenin and is considered to be the limiting component of the β-catenin destruction complex [253,254]. Modulation of its levels would therefore be an effective way to regulate β-catenin destruction. APC is a large protein that interacts with both β-catenin and Axin. It contains three Axin-binding domains, interspaced between armadillo repeat domains (ARMs), which bind to β-catenin. β-catenin is sequentially phosphorylated by CK1α and GSK-3 on serines (S) and a threonine (T) (S45, T41, S37, and S33) in the N-terminal region of the protein, resulting in its interaction with and ubiquitination by β-TRCP1 before being degraded by the proteasome.
Binding of the WNT ligand leads to the dimerization of Frizzled with the coreceptor LRP5/6. This dimerization results in a conformational change of the receptors, leading to relocalization of the degradation complex to the cell membrane under the double interaction of Axin with DVL (itself associated with Frizzled) and with the cytoplasmic end of LRP. Such membrane relocalization decreases the activity of the destruction complex, such that the amount of unphosphorylated cytoplasmic β-catenin rapidly increases. The stabilization of cytoplasmic β-catenin results in an increase in nuclear β-catenin. The balance between the amount of cytoplasmic and nuclear β-catenin is dynamic, resulting from multiple mechanisms of transport and retention between the two compartments. In the nucleus, β-catenin binds to the T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) family of transcription factors, which themselves are already associated with DNA. In the absence of β-catenin, TCF factors interact with transcriptional co-repressors of the Groucho/transducin-like enhancer of split (TLE) family and repress the expression of their target genes. Nuclear accumulation of β-catenin leads to the association of TCF with β-catenin, resulting in dissociation from Groucho/TLE1 and allowing the recruitment of other coactivators for transcriptional activation through its C-terminal transcriptional activation domain. Many transcription factors outside the TCF/LEF family of transcription factors have been reported to be capable of associating with β-catenin to activate or repress transcription [255]. In addition, it has to be noted that alternative pathways activate β-catenin independently of WNTs [256].
In melanocytes/melanoma, MITF interacts with β-catenin and redirects β-catenin-mediated transcriptional activity from canonical Wnt/β-target genes to specific MITF target genes to activate their transcription [257]. For instance, in mutated β-catenin melanoblasts, stabilised form of β-catenin increases Mitf-M levels, which may interfere with β-catenin transactivation, inhibiting the activation of Myc and CyclinD1, therefore reducing proliferation [74]. During melanocyte establishment, β-catenin and MITF-M levels are likely maintained within a very narrow range, with any reduction or increase, such as those observed in the bcat mutants, altering melanoblast proliferation. Other levels of cross-signaling between the WNT/β-catenin pathway and MITF have been described. For example, it has been shown that β-catenin/TCF transcriptionally upregulates MITF-M expression [72]. It has also been shown that MITF-M binds to and upregulates its own promoter through a direct interaction with LEF1 [258]. It would appear to be difficult to target β-catenin in melanoma without affecting MITF expression and/or activity. MITF could potentially be an attractive target for melanoma therapy but the drug-targeting of MITF is highly challenging. As mentioned already, MITF is considered to be the “master gene” of melanocyte differentiation and has an essential role in the proliferation, survival, senescence, migration, invasion, DNA repair, and metabolism of melanoma cells [52,259]. Mitf expression is regulated by multiple signaling pathways outside of the canonical Wnt/β-catenin pathway, such as the cAMP/CREB, YAP1/PAX3, TGFβ/GLI2, and TNF/NFκB pathways, and by transcription factors, such as SOX10 and BRN2, themselves regulated by multiple pathways in melanoma. The basic concept in melanoma is that the proliferative and invasive states are defined, in part, by the high level/activity of MITF and low level/activity MITF, respectively. High and low MITF level/activity co-exist in melanoma tumors and the switch in MITF expression (high and low) is reversible and responsible for melanoma heterogeneity and plasticity. MITF is also involved in the resistance to BRAF inhibitors. One current view for the therapeutic strategy is to increase MITF levels and therefore those of melanoma antigens, such as MART-1 and GP-100, to increase the recognition of melanoma cells by T cells and improve the immune response [260]. In any case, therapeutic strategies have to address the versatility and heterogeneity of melanoma cells.
3.5. Signaling via Gβ/Gγ Subunits
All GPCRs activate the Gβ/Gγ signaling pathways, but this pathway remains poorly studied. The activation of GPCRs mainly induces the activation of Gα subunits, but also that of the Gβ/Gγ complex. There are five Gβ subunits (Gβ1-5) in humans encoded by the GNB1-5 genes. All except GNB5 are expressed in melanoma [100]. There are 16 Gγ subunits encoded by the GNG1-16 genes, of which only Gγ2, Gγ4, Gγ5, Gγ6, Gγ7, Gγ10, Gγ11, and Gγ12 are expressed in melanoma [100,110,115]. The significance of Gβ/Gγ signaling in melanoma has not yet been fully assessed. Gβ/Gγ subunits activate the PI3K signaling pathway. This activation is either direct or indirect via calcium release [261,262,263,264]. Classically, PI3K activation induces AKT activation and increases cell survival [265]. Also, Gβ/Gγ were shown to inhibit melanoma migration in vitro through EPAC inhibition [261]. Given the known importance of the PI3K/AKT pathways, as well as the role of migration in melanoma, its regulation by Gβ/Gγ subunits needs to be further analyzed.
3.6. β-Arrestin Signaling
All GPCRs activate β-arrestin biased signaling, but this remains poorly studied in melanoma. At the end of the 1990s, it was observed that Src family tyrosine kinases are recruited by β-arrestins (encoded by ARRB1-2 genes) to the adrenergic receptor 2a, a member of the GPCR family [266]. Surprisingly, the binding of protein kinases induced activation of the MAPK/ERK pathway only if the receptor was internalized [266,267]. Other observations in the mid-2000s showed that such signaling was non-canonical and independent of G-proteins. Indeed, the activation of GPCRs activates three types of proteins: G-proteins, GPCR protein kinases (GRKs), and arrestins. GRKs and arrestins are the most important elements involved in the termination of GPCR activation. GRKs phosphorylate the receptor on its C-terminal residues, which prevents the activation of G-proteins [268]. Such phosphorylation recruits the non-visual arrestins, β-arrestin 1 & 2, which in turn recruit clathrin, resulting in receptor internalization by clathrin coated-pits [268,269,270]. Depending on the affinity of the GPCR/β-arrestin complex, receptors can be recycled or degraded in the proteasome [271]. In early endosomes, the GPCR/β-arrestin complex is able to form a signalosome by recruiting signaling proteins, such as members of the MAPK pathway [270,272,273]. As a result, several pathways can be activated, such as the MAPK/ERK and Src pathways [266,274], AKT [275], MAPK/JNK [276], MAPK/p38 [277], or PDEs [278].
GRK-arrestin signaling has been poorly studied in melanoma. β-arrestin2 is able to bias MC1R signaling by promoting activation of the MAPK pathway towards that of cAMP-dependent signaling [279]. A transcript of the MC1R (MC1R-203) naturally promotes such biased signaling toward the MAPK pathway [280]. Mutants of metabolic glutamate receptors (mGluR3G848E) can promote such biased signaling, characterized by prolonged internalization of the receptor [281]. This mutation is found in rare cases of cutaneous melanoma (<1%) [110]. Conversely, in uveal melanoma, the CYSLTR2L129Q mutation forces signaling via Gαq/11 and disfavors β-arrestin-biased signaling [282]. GPCR signaling is certainly much more complex than what is presented here: their interactions with GRKs and arrestins and the dynamics of their desensitization add another level of complexity that needs to be investigated in the future.
4. Perspectives of Targeting GPCRs in Melanoma
The molecular and cellular consequences of the activation of GPCRs in melanoma clearly indicate that GPCRs could be interesting targets for therapy. Targeting GPCRs has the advantage of seeking readily available membrane molecules instead of signaling proteins with molecules that need to cross the plasma membrane without deteriorating the intracellular environment and/or endocytotic vesicles and lysosomes [283,284,285].
4.1. Limitation of Available Tools
Large-scale sequencing conducted by the cancer genome atlas consortium (TCGA) has shown that approximately 92% of the melanoma patients tested had at least one nonsense or missense mutation of at least one non-olfactory GPCR [110,286]. Each melanoma patient has an average of 10 mutated GPCRs [110,286]. The functions of the vast majority of the receptors and associated mutations remain uncharacterized. The most frequent GPCR recurrent mutation in melanoma is GPR139R217C, but this mutation does not exceed 1% of the patients and may have a driver function. A large number of mutations have been found in various GPCRs, but can be considered as passengers since none of these mutations are recurrent in melanoma. Indeed, the Adhesion G Protein-Coupled Receptor V1 (ADGRV1) is mutated in approximately 30% of patients, but only a few recurrent mutations are found. The high number of mutations in ADGRV1 is most likely associated with the size of its cDNA (19,557 nucleotides) [110,286]. These data suggest that if we cannot offer patients a personalized and targeted therapy (one patient = one mutation = one drug), patients could be grouped by activated signaling for therapy that is still targeted but less personalized. This less direct choice of therapy would consider the positioning of the GPCR within the signaling pathways and target the downstream node(s) [287]. Such a therapeutic option would also be attractive in the setting of patients who do not have mutated GPCRs but rather mutations in downstream signaling elements.
Receptor activation may result by a mechanism independent of the presence of a mutations in the gene. This could be related to overexpression or de novo expression of a receptor, which would induce a higher basal level of receptor activity, or activation of the receptor by the production of its ligand in the environment [288,289,290]. Binding of the ligand to the receptor may occur in the primary melanoma in an autocrine/paracrine mode, with ligand production by the melanoma cell or by cells in the microenvironment [291]. For example, activation of EDNRB by ET-1 secreted by surrounding melanoma cells induces reactivation of the MAPK pathway after BRAFi treatment [292]. In this model, ET is secreted by melanoma cells and activates EDNRB in an autocrine and/or paracrine manner [292]. Alternatively, ligand production may only occur at the metastatic site and thus only affect metastasis formation and not melanoma initiation [293,294]. Ligand production may attract tumor cells into the target tissue by chemoattraction, promote cell survival and/or proliferation, or induce resistance to drugs. In this perspective, ET-1 is highly expressed in the lungs and can promote the colonization of melanoma cells that express ENDRB in this organ. The reactivation of MAPK pathways by expression of EDNRB may occur at primary sites, as well as in distant organs. Ligand-dependent tissue expression can be observed, in particular, in the lungs, where only 50 highly expressed ligands are found [114,295,296,297,298]. An important limitation is our lack of knowledge about the level of gene expression in melanoma cells that colonize distant organs. Transcriptomic analyses would be extremely useful in determining which GPCRs are expressed in melanoma in distant metastases. The correlation of GPCR expression data in melanoma at sites of metastasis with expression in the target tissue of ligands for these GPCRs would allow the selection of potential receptors of interest.
An alternative to transcriptional analysis would be the identification of GPCRs at metastatic sites on the basis of protein expression. However, the identification of GPCRs by immunolabeling is difficult, due to the small exposed area of extracellular epitopes and very high conformational variability. Conventional mass spectrometry is also challenging, as receptors are not readily isolatable from membranes [299,300,301].
The improvement of antibody-isolation technologies associated with our knowledge of GPCRs will lead to the generation of new, more selective antibodies. The development of nanobodies appears to be promising for the detection and targeting of GPCRs [302]. The major limitation in the high-throughput identification of GPCRs via mass spectrometry is the depletion of GPCRs from mass spectrometry samples. This bias can be avoided by performing surfaceomes, which will significantly enrich samples for membrane glycoproteins and thus potentially reveal the expression of GPCRs [301,303,304]. Finally, GPCRs can also be indirectly identified by analyzing cell-binding ligands rather than the receptors directly [305].
Data mining of the literature generates databases, such as TCGA. To date, current databases have been extremely useful for the identification of driver mutations and prognostic biomarkers in melanoma. However, they are composed of 80% primary tumors or skin or lymphatic metastases [110,286], whereas visceral, bone, and nervous system melanoma metastases are poorly represented. The presence of characteristic and highly aggressive mutations in distant metastasis may be hidden by the small sample size. Furthermore, current databases were generated before the generalization of current treatments and the samples constituting the large databases came from patients who were naive to any treatment with MAPK or checkpoint inhibitors. The changes in genetic/epigenetic expression due to such treatments could involve the activation of GPCR expression or the selection of subclones in which GPCR signaling is activated. The generation of databases enriched with samples from distant metastases and patients treated with MAPK and immune checkpoint inhibitors would be of great interest to the scientific community.
Large-scale high-throughput screening using RNA interference or CRISPR-Cas9 could be performed to identify the GPCR-dependence of melanoma cells for growth or invasion. Such screens can also be performed in vivo. To evaluate the role of specific GPCRs, transplantation of genetically modified cells into animals would reveal their roles in internal organ colonization and in melanoma progression [306]. Such screening could also be performed by generating transgenic animal models, but the experiments would become extremely complex, time consuming and expensive. Zebrafish may be a suitable model for these types of studies. Indeed, it is relatively easy to generate and maintain large groups of transgenic animals [307,308,309,310]. Furthermore, zebrafish express many of the GPCRs expressed in humans, as well as their signaling machinery [311,312]. In addition, there are already many melanoma models that could be used to test genetic modifications in melanoma-producing animals, for example, to assess the pro-metastatic effect [313,314]. Particular attention should be paid to studies based on in vivo cell-injections. Indeed, the type of injection will determine the preferential location of metastasis formation. For example, in mice, tail-vein injections are more likely to result in lung metastasis formation, whereas intrasplenic injections are more likely to result in liver metastases [315]. Thus, the use of a particular type of injection may mask the action of a GPCR on metastasis formation in a tissue not targeted by the injection method. The study of the tissue expression profile of GPCR ligands and the use of an appropriate method can address this concern.
4.2. Novel Structures and Drug Design Approaches
To efficiently target GPCRs, two parallel and complementary approaches are used: binding of compounds (including small molecules, antibodies and radiotherapies) to GRPRs, and structure determination of GRPRs. The structure of GPCR can be elucidated using X-ray crystallography, Cryo-EM, NMR and artificial intelligence.
A better knowledge of GPCR structure and molecular ligand-receptor interactions is critical for structure-based molecule design and the design of new receptor-activating agonist or antagonist molecules [316]. Such structural knowledge needs to be as accurate as possible and is currently acquired by two main experimental methods and one predictive method [316,317].
The first three-dimensional structure of a GPCR, rhodopsin, was solved by X-ray crystallization in 2000 [318]. This method requires obtaining stable crystals of the receptor extracted from its membrane in solution, which has been a major obstacle to structure determination [319]. This problem has been solved by modifying GPCRs to make them more stable while retaining their activity [319,320,321,322]. This can be achieved, for example, by fusing the T4 lysozyme protein as a replacement for intracellular loop 3 [321] or by mutating the receptor to thermostabilize it [320]. A new technique for crystal structure resolution has recently been developed: X-ray-free electron laser (XFEL) crystallography. The use of lasers reduces the size of the crystals required and, therefore, increases their stability [323,324].
Crystallization has been completed by cryo-electronmicroscopy (Cryo-EM). Significant improvements in detectors and structure determination algorithms have enabled the resolution of structures at the particle scale [325,326,327]. Cryo-EM does not require the formation of crystals, as the receptors are directly vitrified after purification, but requires more computational time to solve the structure, resulting in lower resolution [317,328,329,330]. These various constraints and the relative novelty of these techniques mean that, currently, the structure of only 20% of non-olfactory GPCRs has been solved [328]. Nuclear magnetic resonance (NMR) allows the acquisition of dynamic data and thus complements the data from the fixed structures of crystallography and Cryo-EM [331].
More recently, advances in artificial intelligence have made it possible to approach structures using machine learning. Protein structure prediction was revolutionized in 2021 by the publication of the algorithms AlphaFold2 and RoseTTAfold, which generated highly accurate three-dimensional structures of any protein of interest [332,333,334]. These algorithms are completed by GPCR-specific algorithms, such as the already developed arsenal, which is specific to the GPCR field [335,336,337,338]. Nonetheless, although these algorithms all regularly perform well, they can still be improved. GPCRs differ, particularly in their loops, which results in a deficit for machine-learning algorithms and thus low confidence in the models, often producing false predictions [335,339]. Generating and publishing more receptor structures will increase both direct knowledge about receptors and knowledge about other receptors via increased substrates for algorithms and in silico prediction. Predictions of molecular anchors in receptors can also be made by site-directed mutagenesis (SDM) studies to find amino acids that interact with known ligands of the receptor [340,341]. Crystallization and SDM techniques can be used synergistically to better understand the binding of the molecule to the receptor and produce much more refined and/or efficient molecules [340,341,342].
For the development of an effective therapy, stable, receptor-specific agonists or antagonists must be found. The screening of drug libraries is performed to find molecular scaffolds capable of binding to receptors and modulating their activity [343]. Screening can be performed using affinity assays by assessing the ability of molecules to bind to the target as purified proteins, on whole cells expressing the target, or on isolated membranes [344,345,346]. Purified proteins are free of binding to secondary targets, but the ability of the molecules to cross a membrane is not analyzed. Moreover, the presence of the membrane is often necessary for receptor stability [345].
Affinity tests are based on the ability of molecules to displace—and replace on the receptor—a reference ligand known to bind the receptor. Detection is either by radio-labeling of the reference ligand, regularly labeled with iodine-125 or tritium [347,348,349] or by fluorescence resonance energy transfer (FRET), in which both the reference ligand and the receptor are bound to a fluorophore that allows resonance from one molecule to the other [350,351]. Such screening is only able to identify ligands that bind to the same site as the competitor. To overcome this bias, the analysis of the activity of various elements of GPCR signaling, such as IP1 concentration for the Gαq pathway or cAMP for the Gαs pathway, can be jointly performed on a large scale [352,353]. The activity of molecules on downstream G-protein signaling pathways can also be assessed using kinase assays from protein extracts or purified protein or by FRET [354,355,356]. These screens can be virtually performed with high efficiency if the receptor structure is known [357,358,359]. The hits that are found are generally of low affinity for their targets and require optimization to be usable [343]. They will then need to undergo pharmaco-modulation that will be directed through structural data and docking algorithms to result in lead generation [360,361,362]. These leads will have to be tested in vivo to verify their pharmacokinetic parameters (absorption, distribution, metabolism, elimination) at the risk of obtaining only molecules incapable of producing an effect in vivo and condemned to be used only as an in vitro tool [363,364]. Molecules identified as leads can be used to verify the efficacy of these molecules on the cellular and molecular processes of melanoma progression, as well as on the formation of metastases and resistance to treatment.
In addition to small chemical molecules, GPCRs can be targeted by monoclonal antibodies. Several have been generated and approved as therapeutic targets, such as mogamulizumab, a humanized antibody against chemokine receptor type 4. The advantage of using monoclonal antibodies in treating diseases is notable because they have a long half-life.
Finally, targeted radiotherapy is currently being developed and constitutes a promising strategy to target GPCRs in cancer. The radioactivity is delivered to a specific tumor by means of a systemic injection. For melanoma treatment, targeted treatment can be achieved by labeling small molecules, such as melanin ligands, peptides that recognize a specific receptor (MC1R), or antibodies (anti-melanin, anti-GD3) [365,366]. The efficacy of targeted radiotherapy depends on the dose delivered, which in turn depends on the radionuclide used and the time that the labeled compound remains associated with the target. Targeted radiotherapy is highly relevant for the treatment of disseminated lesions and overcoming tumor heterogeneity through cross-fire irradiation with β-radionuclides characterized by a decay spectrum of between a few nanometers and 2 mm. The reception of adequate doses of radiation from neighboring receptor-expressing cells can kill tumor cells lacking the targeted receptor in the tumor. This property is particularly important for melanoma, in which gene expression is often heterogeneous. Targeted radiotherapy of the somatostatin receptor is used for the clinical treatment of neuroendocrine tumors. The same strategy could be adapted for targeting GPCRs in melanoma.
5. Conclusions
Of the eight GPCRs shown to be involved in pigmentation, only three have been studied in the context of melanoma (EDNRB, MC1R, and GRM1). The remaining five receptors (DRD2, FZD4, GPR143, GPR161, and SMO) may be of interest in the context of melanoma, but they have not been studied yet. Conversely, it would be relevant to evaluate the importance of the eight receptors described only in a melanoma context (FZD7, GPER1, GRM3, GRM5, PAR1/F2R, CXCR4, CCR7, and CCR10), for their implication in the melanocyte lineage as a whole, whether during embryonic development, melanocyte homeostasis/renewal and/or melanogenesis. The identification of completely novel GPCRs, important in melanomagenesis, will require detailed studies of primary and/or metastatic tumors through the development of new and more powerful analysis tools as their detection may escape current RNAseq and proteomic techniques.
The low number of GPCR mutations and their modification of expression/activity often dependent on temporal, tissue and molecular contexts render their studies in vivo essential to precisely define their roles. The creation of novel Omics databases generated from distant melanoma metastases treated with immune checkpoint or/and MAPK inhibitors would reveal novel GPCR players involved in resistance and metastasis. Once such GPCR target(s) would be identified and characterized, small molecules will have to be developed to directly target it/them. These molecules can be screened from chemical libraries or designed according to their structure, based on their crystal or artificial intelligence using Alphafold for instance.
Prior to 2010, surgery remained the primary treatment option for resectable melanoma, with eventual chemotherapies, although these treatments did not provide a survival benefit for patients when metastasis arose. Over the past decade, effective therapeutic approaches such as immunotherapies and targeted therapies, alone or in combination, have significantly changed the prognosis of patients with advanced melanoma. However, despite the success of these treatments, their long-term efficacy remains limited by mechanisms of acquired resistance. In this review, we have highlighted the involvement of GRPRs in the initiation and progression of melanoma and mentioned their therapeutic evaluation in preclinical or clinical trials. Most of these studies are in the early stages of clinical development, being studied in phase I or phase I/II. The success of these trials will depend on understanding the activity of GPCRs and the quality/stability of the drug used in clinical development. GPCRs signal through one or more G-proteins and thus can regulate multiple signaling pathways simultaneously. Therefore, targeting GPCR activities and downstream signaling nodes may simultaneously inhibit several key signaling pathways in melanomagenesis. This strategy should be more effective (multi-pathway inhibition) in a greater proportion of patients (BRAF or NRAS mutated) than existing targeted therapies, and should minimize the development of cancer cell resistance. A better understanding of the specific function of GPCRs in melanoma will certainly lead to innovative therapy in the future and will open new avenues for the treatment of melanoma.
Acknowledgments
We are grateful to the members of Larue laboratories for discussions and comments.
Author Contributions
Conceptualization, J.H.R., Z.A., L.L., V.D.; writing—original draft preparation, J.H.R., Z.A., L.L., V.D.; writing—review and editing, J.H.R., Z.A., L.L., V.D.; funding acquisition, V.D., L.L. All authors have read and agreed to the published version of the manuscript.
Funding
JHR had a fellowship from PSL and Ligue nationale contre le cancer. This work was supported by FRM, Institut Carnot, Cancer, CNRS, INSERM, Gefluc and is under the program «Investissements d’Avenir» launched by the French Government and implemented by ANR Labex CelTisPhyBio (ANR-11-LABX-0038 and ANR-10-IDEX-0001-02 PSL).
Conflicts of Interest
The authors declare no competing interest. The funders had no role in the writing of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Insel P.A., Sriram K., Gorr M.W., Wiley S.Z., Michkov A., Salmerón C., Chinn A.M. GPCRomics: An Approach to Discover GPCR Drug Targets. Trends Pharmacol. Sci. 2019;40:378–387. doi: 10.1016/j.tips.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser A.S., Attwood M.M., Rask-Andersen M., Schiöth H.B., Gloriam D.E. Trends in GPCR Drug Discovery: New Agents, Targets and Indications. Nat. Rev. Drug Discov. 2017;16:829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Usman S., Khawer M., Rafique S., Naz Z., Saleem K. The Current Status of Anti-GPCR Drugs against Different Cancers. J. Pharm. Anal. 2020;10:517–521. doi: 10.1016/j.jpha.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Luke J.J., Flaherty K.T., Ribas A., Long G.V. Targeted Agents and Immunotherapies: Optimizing Outcomes in Melanoma. Nat. Rev. Clin. Oncol. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 6.Carlino M.S., Larkin J., Long G.V. Immune Checkpoint Inhibitors in Melanoma. Lancet. 2021;398:1002–1014. doi: 10.1016/S0140-6736(21)01206-X. [DOI] [PubMed] [Google Scholar]
- 7.Baik J.H., Picetti R., Saiardi A., Thiriet G., Dierich A., Depaulis A., Le Meur M., Borrelli E. Parkinsonian-like Locomotor Impairment in Mice Lacking Dopamine D2 Receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 8.Hosoda K., Hammer R.E., Richardson J.A., Baynash A.G., Cheung J.C., Giaid A., Yanagisawa M. Targeted and Natural (Piebald-Lethal) Mutations of Endothelin-B Receptor Gene Produce Megacolon Associated with Spotted Coat Color in Mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Huso D., Cahill H., Ryugo D., Nathans J. Progressive Cerebellar, Auditory, and Esophageal Dysfunction Caused by Targeted Disruption of Thefrizzled-4 Gene. J. Neurosci. 2001;21:4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Incerti B., Cortese K., Pizzigoni A., Surace E.M., Varani S., Coppola M., Jeffery G., Seeliger M., Jaissle G., Bennett D.C., et al. Oa1 Knock-out: New Insights on the Pathogenesis of Ocular Albinism Type 1. Hum. Mol. Genet. 2000;9:2781–2788. doi: 10.1093/hmg/9.19.2781. [DOI] [PubMed] [Google Scholar]
- 11.Matteson P.G., Desai J., Korstanje R., Lazar G., Borsuk T.E., Rollins J., Kadambi S., Joseph J., Rahman T., Wink J., et al. The Orphan G Protein-Coupled Receptor, Gpr161, Encodes the Vacuolated Lens Locus and Controls Neurulation and Lens Development. Proc. Natl. Acad. Sci. USA. 2008;105:2088–2093. doi: 10.1073/pnas.0705657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollock P.M., Cohen-Solal K., Sood R., Namkoong J., Martino J.J., Koganti A., Zhu H., Robbins C., Makalowska I., Shin S.-S., et al. Melanoma Mouse Model Implicates Metabotropic Glutamate Signaling in Melanocytic Neoplasia. Nat. Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 13.Robbins L.S., Nadeau J.H., Johnson K.R., Kelly M.A., Roselli-Rehfuss L., Baack E., Mountjoy K.G., Cone R.D. Pigmentation Phenotypes of Variant Extension Locus Alleles Result from Point Mutations That Alter MSH Receptor Function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- 14.Twigg S.R.F., Hufnagel R.B., Miller K.A., Zhou Y., McGowan S.J., Taylor J., Craft J., Taylor J.C., Santoro S.L., Huang T., et al. A Recurrent Mosaic Mutation in SMO, Encoding the Hedgehog Signal Transducer Smoothened, Is the Major Cause of Curry-Jones Syndrome. Am. J. Hum. Genet. 2016;98:1256–1265. doi: 10.1016/j.ajhg.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.J., Wall B., Chen S. G-Protein-Coupled Receptors and Melanoma. Pigment Cell Melanoma Res. 2008;21:415–428. doi: 10.1111/j.1755-148X.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxter L.L., Watkins-Chow D.E., Pavan W.J., Loftus S.K. A Curated Gene List for Expanding the Horizons of Pigmentation Biology. Pigment Cell Melanoma Res. 2019;32:348–358. doi: 10.1111/pcmr.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aktary Z., McMahon M., Larue L. Animal Models of Melanoma. In: Fisher D.E., Bastian B.C., editors. Melanoma. Springer; New York, NY, USA: 2017. pp. 1–31. [Google Scholar]
- 18.Delmas V., Martinozzi S., Bourgeois Y., Holzenberger M., Larue L. Cre-Mediated Recombination in the Skin Melanocyte Lineage. Genesis. 2003;36:73–80. doi: 10.1002/gene.10197. [DOI] [PubMed] [Google Scholar]
- 19.Yajima I., Belloir E., Bourgeois Y., Kumasaka M., Delmas V., Larue L. Spatiotemporal Gene Control by the Cre-ERT2 System in Melanocytes. Genesis. 2006;44:34–43. doi: 10.1002/gene.20182. [DOI] [PubMed] [Google Scholar]
- 20.Bosenberg M., Muthusamy V., Curley D.P., Wang Z., Hobbs C., Nelson B., Nogueira C., Horner II J.W., DePinho R., Chin L. Characterization of Melanocyte-Specific Inducible Cre Recombinase Transgenic Mice. Genesis. 2006;44:262–267. doi: 10.1002/dvg.20205. [DOI] [PubMed] [Google Scholar]
- 21.Jain F., Longakit A., Huang J.L.-Y., Raamsdonk C.D.V. Endothelin Signaling Promotes Melanoma Tumorigenesis Driven by Constitutively Active GNAQ. Pigment Cell Melanoma Res. 2020;33:834–849. doi: 10.1111/pcmr.12900. [DOI] [PubMed] [Google Scholar]
- 22.Swope V.B., Starner R.J., Rauck C., Abdel-Malek Z.A. Endothelin-1 and α-Melanocortin Have Redundant Effects on Global Genome Repair in UV-Irradiated Human Melanocytes despite Distinct Signaling Pathways. Pigment Cell Melanoma Res. 2020;33:293–304. doi: 10.1111/pcmr.12823. [DOI] [PubMed] [Google Scholar]
- 23.Mitra D., Luo X., Morgan A., Wang J., Hoang M.P., Lo J., Guerrero C.R., Lennerz J.K., Mihm M.C., Wargo J.A., et al. An Ultraviolet-Radiation-Independent Pathway to Melanoma Carcinogenesis in the Red Hair/Fair Skin Background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Orazio J.A., Nobuhisa T., Cui R., Arya M., Spry M., Wakamatsu K., Igras V., Kunisada T., Granter S.R., Nishimura E.K., et al. Topical Drug Rescue Strategy and Skin Protection Based on the Role of Mc1r in UV-Induced Tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 25.Tiwary S., Xu L. FRIZZLED7 Is Required for Tumor Initiation and Metastatic Growth of Melanoma Cells. PLoS ONE. 2016;11:e0147638. doi: 10.1371/journal.pone.0147638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Hernandez I., Maiques O., Kohlhammer L., Cantelli G., Perdrix-Rosell A., Monger J., Fanshawe B., Bridgeman V.L., Karagiannis S.N., Penin R.M., et al. WNT11-FZD7-DAAM1 Signalling Supports Tumour Initiating Abilities and Melanoma Amoeboid Invasion. Nat. Commun. 2020;11:5315. doi: 10.1038/s41467-020-18951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natale C.A., Li J., Zhang J., Dahal A., Dentchev T., Stanger B.Z., Ridky T.W. Activation of G Protein-Coupled Estrogen Receptor Signaling Inhibits Melanoma and Improves Response to Immune Checkpoint Blockade. Elife. 2018;7:e31770. doi: 10.7554/eLife.31770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiffner S., Chen S., Becker J.C., Bosserhoff A.-K. Highly Pigmented Tg(Grm1) Mouse Melanoma Develops Non-Pigmented Melanoma Cells in Distant Metastases. Exp. Derm. 2012;21:786–788. doi: 10.1111/j.1600-0625.2012.01560.x. [DOI] [PubMed] [Google Scholar]
- 29.Prickett T.D., Wei X., Cardenas-Navia I., Teer J.K., Lin J.C., Walia V., Gartner J., Jiang J., Cherukuri P.F., Molinolo A., et al. Exon Capture Analysis of G Protein-Coupled Receptors Identifies Activating Mutations in GRM3 in Melanoma. Nat. Genet. 2011;43:1119–1126. doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi K.Y., Chang K., Pickel J.M., Badger J.D., Roche K.W. Expression of the Metabotropic Glutamate Receptor 5 (MGluR5) Induces Melanoma in Transgenic Mice. Proc. Natl. Acad. Sci. USA. 2011;108:15219–15224. doi: 10.1073/pnas.1107304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villares G.J., Zigler M., Wang H., Melnikova V.O., Wu H., Friedman R., Leslie M.C., Vivas-Mejia P.E., Lopez-Berestein G., Sood A.K., et al. Targeting Melanoma Growth and Metastasis with Systemic Delivery of Liposome-Incorporated Protease-Activated Receptor-1 Small Interfering RNA. Cancer Res. 2008;68:9078–9086. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami T., Maki W., Cardones A.R., Fang H., Tun Kyi A., Nestle F.O., Hwang S.T. Expression of CXC Chemokine Receptor-4 Enhances the Pulmonary Metastatic Potential of Murine B16 Melanoma Cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 33.Ieranò C., D’Alterio C., Giarra S., Napolitano M., Rea G., Portella L., Santagata A., Trotta A.M., Barbieri A., Campani V., et al. CXCL12 Loaded-Dermal Filler Captures CXCR4 Expressing Melanoma Circulating Tumor Cells. Cell Death Dis. 2019;10:562. doi: 10.1038/s41419-019-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiley H.E., Gonzalez E.B., Maki W., Wu M.T., Hwang S.T. Expression of CC Chemokine Receptor-7 and Regional Lymph Node Metastasis of B16 Murine Melanoma. J. Natl. Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 35.Murakami T., Cardones A.R., Finkelstein S.E., Restifo N.P., Klaunberg B.A., Nestle F.O., Castillo S.S., Dennis P.A., Hwang S.T. Immune Evasion by Murine Melanoma Mediated through CC Chemokine Receptor-10. J. Exp. Med. 2003;198:1337. doi: 10.1084/jem.20030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baynash A.G., Hosoda K., Giaid A., Richardson J.A., Emoto N., Hammer R.E., Yanagisawa M. Interaction of Endothelin-3 with Endothelin-B Receptor Is Essential for Development of Epidermal Melanocytes and Enteric Neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 37.Garcia R.J., Ittah A., Mirabal S., Figueroa J., Lopez L., Glick A.B., Kos L. Endothelin 3 Induces Skin Pigmentation in a Keratin-Driven Inducible Mouse Model. J. Invest. Derm. 2008;128:131–142. doi: 10.1038/sj.jid.5700948. [DOI] [PubMed] [Google Scholar]
- 38.Benaduce A.P., Batista D., Grilo G., Jorge K., Cardero D., Milikowski C., Kos L. Novel UV-Induced Melanoma Mouse Model Dependent on Endothelin3 Signaling. Pigment Cell Melanoma Res. 2014;27:839–842. doi: 10.1111/pcmr.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumasaka M.Y., Yajima I., Hossain K., Iida M., Tsuzuki T., Ohno T., Takahashi M., Yanagisawa M., Kato M. A Novel Mouse Model for De Novo Melanoma. Cancer Res. 2010;70:24–29. doi: 10.1158/0008-5472.CAN-09-2838. [DOI] [PubMed] [Google Scholar]
- 40.Demunter A., De Wolf-Peeters C., Degreef H., Stas M., van den Oord J.J. Expression of the Endothelin-B Receptor in Pigment Cell Lesions of the Skin. Evidence for Its Role as Tumor Progression Marker in Malignant Melanoma. Virchows Arch. 2001;438:485–491. doi: 10.1007/s004280000362. [DOI] [PubMed] [Google Scholar]
- 41.Asundi J., Reed C., Arca J., McCutcheon K., Ferrando R., Clark S., Luis E., Tien J., Firestein R., Polakis P. An Antibody-Drug Conjugate Targeting the Endothelin B Receptor for the Treatment of Melanoma. Clin. Cancer Res. 2011;17:965–975. doi: 10.1158/1078-0432.CCR-10-2340. [DOI] [PubMed] [Google Scholar]
- 42.Lahav R., Heffner G., Patterson P.H. An Endothelin Receptor B Antagonist Inhibits Growth and Induces Cell Death in Human Melanoma Cells in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA. 1999;96:11496–11500. doi: 10.1073/pnas.96.20.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lahav R., Suvà M.-L., Rimoldi D., Patterson P.H., Stamenkovic I. Endothelin Receptor B Inhibition Triggers Apoptosis and Enhances Angiogenesis in Melanomas. Cancer Res. 2004;64:8945–8953. doi: 10.1158/0008-5472.CAN-04-1510. [DOI] [PubMed] [Google Scholar]
- 44.von Koschembahr A.M., Swope V.B., Starner R.J., Abdel-Malek Z.A. Endothelin-1 Protects Human Melanocytes from UV-Induced DNA Damage by Activating JNK and P38 Signalling Pathways. Exp. Derm. 2015;24:269–274. doi: 10.1111/exd.12638. [DOI] [PubMed] [Google Scholar]
- 45.Freitas J.T., Lopez J., Llorian C., Boroni M., Kos L. The Immunosuppressive Role of Edn3 Overexpression in the Melanoma Microenvironment. Pigment Cell Melanoma Res. 2021;34:1084–1093. doi: 10.1111/pcmr.13002. [DOI] [PubMed] [Google Scholar]
- 46.Bagnato A., Natali P.G. Endothelin Receptors as Novel Targets in Tumor Therapy. J. Transl. Med. 2004;2:16. doi: 10.1186/1479-5876-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kefford R., Beith J.M., Van Hazel G.A., Millward M., Trotter J.M., Wyld D.K., Kusic R., Shreeniwas R., Morganti A., Ballmer A., et al. A Phase II Study of Bosentan, a Dual Endothelin Receptor Antagonist, as Monotherapy in Patients with Stage IV Metastatic Melanoma. Invest. New Drugs. 2007;25:247–252. doi: 10.1007/s10637-006-9014-7. [DOI] [PubMed] [Google Scholar]
- 48.Kefford R.F., Clingan P.R., Brady B., Ballmer A., Morganti A., Hersey P. A Randomized, Double-Blind, Placebo-Controlled Study of High-Dose Bosentan in Patients with Stage IV Metastatic Melanoma Receiving First-Line Dacarbazine Chemotherapy. Mol. Cancer. 2010;9:69. doi: 10.1186/1476-4598-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandhu S., McNeil C.M., LoRusso P., Patel M.R., Kabbarah O., Li C., Sanabria S., Flanagan W.M., Yeh R.-F., Brunstein F., et al. Phase I Study of the Anti-Endothelin B Receptor Antibody-Drug Conjugate DEDN6526A in Patients with Metastatic or Unresectable Cutaneous, Mucosal, or Uveal Melanoma. Invest. New Drugs. 2020;38:844–854. doi: 10.1007/s10637-019-00832-1. [DOI] [PubMed] [Google Scholar]
- 50.García-Borrón J.C., Abdel-Malek Z., Jiménez-Cervantes C. MC1R, the CAMP Pathway and the Response to Solar UV: Extending the Horizon beyond Pigmentation. Pigment Cell Melanoma Res. 2014;27:699–720. doi: 10.1111/pcmr.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertolotto C., Abbe P., Hemesath T.J., Bille K., Fisher D.E., Ortonne J.P., Ballotti R. Microphthalmia Gene Product as a Signal Transducer in CAMP-Induced Differentiation of Melanocytes. J. Cell Biol. 1998;142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goding C.R., Arnheiter H. MITF—the First 25 Years. Genes Dev. 2019;33:983–1007. doi: 10.1101/gad.324657.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deraredj Nadim W., Hassanaly S., Bénédetti H., Kieda C., Grillon C., Morisset-Lopez S. The GTPase-Activating Protein-Related Domain of Neurofibromin Interacts with MC1R and Regulates Pigmentation-Mediated Signaling in Human Melanocytes. Biochem. Biophys. Res. Commun. 2021;534:758–764. doi: 10.1016/j.bbrc.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Tamate H.B., Takeuchi T. Action of the e Locus of Mice in the Response of Phaeomelanic Hair Follicles to α-Melanocyte-Stimulating Hormone in Vitro. Science. 1984;224:1241–1242. doi: 10.1126/science.6328651. [DOI] [PubMed] [Google Scholar]
- 55.Smith R., Healy E., Siddiqui S., Flanagan N., Steijlen P.M., Rosdahl I., Jacques J.P., Rogers S., Turner R., Jackson I.J., et al. Melanocortin 1 Receptor Variants in an Irish Population. J. Invest. Derm. 1998;111:119–122. doi: 10.1046/j.1523-1747.1998.00252.x. [DOI] [PubMed] [Google Scholar]
- 56.Harding R.M., Healy E., Ray A.J., Ellis N.S., Flanagan N., Todd C., Dixon C., Sajantila A., Jackson I.J., Birch-Machin M.A., et al. Evidence for Variable Selective Pressures at MC1R. Am. J. Hum. Genet. 2000;66:1351–1361. doi: 10.1086/302863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scott M.C., Wakamatsu K., Ito S., Kadekaro A.L., Kobayashi N., Groden J., Kavanagh R., Takakuwa T., Virador V., Hearing V.J., et al. Human Melanocortin 1 Receptor Variants, Receptor Function and Melanocyte Response to UV Radiation. J. Cell Sci. 2002;115:2349–2355. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- 58.Krude H., Biebermann H., Luck W., Horn R., Brabant G., Grüters A. Severe Early-Onset Obesity, Adrenal Insufficiency and Red Hair Pigmentation Caused by POMC Mutations in Humans. Nat. Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 59.Herraiz C., Martínez-Vicente I., Maresca V. The α-Melanocyte-Stimulating Hormone/Melanocortin-1 Receptor Interaction: A Driver of Pleiotropic Effects beyond Pigmentation. Pigment Cell Melanoma Res. 2021;34:748–761. doi: 10.1111/pcmr.12980. [DOI] [PubMed] [Google Scholar]
- 60.Swope V.B., Abdel-Malek Z.A. Significance of the Melanocortin 1 and Endothelin B Receptors in Melanocyte Homeostasis and Prevention of Sun-Induced Genotoxicity. Front. Genet. 2016;7:146. doi: 10.3389/fgene.2016.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castejón-Griñán M., Herraiz C., Olivares C., Jiménez-Cervantes C., García-Borrón J.C. CAMP-Independent Non-Pigmentary Actions of Variant Melanocortin 1 Receptor: AKT-Mediated Activation of Protective Responses to Oxidative DNA Damage. Oncogene. 2018;37:3631–3646. doi: 10.1038/s41388-018-0216-1. [DOI] [PubMed] [Google Scholar]
- 62.Guida S., Guida G., Goding C.R. MC1R Functions, Expression, and Implications for Targeted Therapy. J. Investig. Dermatol. 2022;142:293–302.e1. doi: 10.1016/j.jid.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Bautista R.-M.F., Carter K.M., Jarrett S.G., Napier D., Wakamatsu K., Ito S., D’Orazio J.A. Cutaneous Pharmacologic CAMP Induction Induces Melanization of the Skin and Improves Recovery from Ultraviolet Injury in Melanocortin 1 Receptor-Intact or Heterozygous Skin. Pigment Cell Melanoma Res. 2020;33:30–40. doi: 10.1111/pcmr.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koikov L., Starner R.J., Swope V.B., Upadhyay P., Hashimoto Y., Freeman K.T., Knittel J.J., Haskell-Luevano C., Abdel-Malek Z.A. Development of HMC1R Selective Small Agonists for Sunless Tanning and Prevention of Genotoxicity of UV in Melanocytes. J. Invest. Derm. 2021;141:1819–1829. doi: 10.1016/j.jid.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veeman M.T., Axelrod J.D., Moon R.T. A Second Canon. Functions and Mechanisms of Beta-Catenin-Independent Wnt Signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/S1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 66.Abou Azar F., Lim G.E. Metabolic Contributions of Wnt Signaling: More Than Controlling Flight. Front. Cell Dev. Biol. 2021;9:709823. doi: 10.3389/fcell.2021.709823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nusse R., Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 68.Larue L., Delmas V. The WNT/Beta-Catenin Pathway in Melanoma. Front. Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- 69.Dorsky R.I., Moon R.T., Raible D.W. Control of Neural Crest Cell Fate by the Wnt Signalling Pathway. Nature. 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- 70.Dunn K.J., Williams B.O., Li Y., Pavan W.J. Neural Crest-Directed Gene Transfer Demonstrates Wnt1 Role in Melanocyte Expansion and Differentiation during Mouse Development. Proc. Natl. Acad. Sci. USA. 2000;97:10050–10055. doi: 10.1073/pnas.97.18.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikeya M., Lee S.M.K., Johnson J.E., McMahon A.P., Takada S. Wnt Signalling Required for Expansion of Neural Crest and CNS Progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 72.Dorsky R.I., Raible D.W., Moon R.T. Direct Regulation of Nacre, a Zebrafish MITF Homolog Required for Pigment Cell Formation, by the Wnt Pathway. Genes Dev. 2000;14:158–162. doi: 10.1101/gad.14.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hari L., Brault V., Kléber M., Lee H.-Y., Ille F., Leimeroth R., Paratore C., Suter U., Kemler R., Sommer L. Lineage-Specific Requirements of β-Catenin in Neural Crest Development. J. Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luciani F., Champeval D., Herbette A., Denat L., Aylaj B., Martinozzi S., Ballotti R., Kemler R., Goding C.R., De Vuyst F., et al. Biological and Mathematical Modeling of Melanocyte Development. Development. 2011;138:3943–3954. doi: 10.1242/dev.067447. [DOI] [PubMed] [Google Scholar]
- 75.Gallagher S.J., Rambow F., Kumasaka M., Champeval D., Bellacosa A., Delmas V., Larue L. Beta-Catenin Inhibits Melanocyte Migration but Induces Melanoma Metastasis. Oncogene. 2013;32:2230–2238. doi: 10.1038/onc.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y., Wang X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delmas V., Beermann F., Martinozzi S., Carreira S., Ackermann J., Kumasaka M., Denat L., Goodall J., Luciani F., Viros A., et al. Beta-Catenin Induces Immortalization of Melanocytes by Suppressing P16INK4a Expression and Cooperates with N-Ras in Melanoma Development. Genes Dev. 2007;21:2923–2935. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Damsky W.E., Curley D.P., Santhanakrishnan M., Rosenbaum L.E., Platt J.T., Gould Rothberg B.E., Taketo M.M., Dankort D., Rimm D.L., McMahon M., et al. β-Catenin Signaling Controls Metastasis in Braf-Activated Pten-Deficient Melanomas. Cancer Cell. 2011;20:741–754. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nsengimana J., Laye J., Filia A., O’Shea S., Muralidhar S., Poźniak J., Droop A., Chan M., Walker C., Parkinson L., et al. β-Catenin-Mediated Immune Evasion Pathway Frequently Operates in Primary Cutaneous Melanomas. J. Clin. Investig. 2018;128:2048–2063. doi: 10.1172/JCI95351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah K.V., Chien A.J., Yee C., Moon R.T. CTLA-4 Is a Direct Target of Wnt/β-Catenin Signaling and Is Expressed in Human Melanoma Tumors. J. Investig. Dermatol. 2008;128:2870–2879. doi: 10.1038/jid.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spranger S., Bao R., Gajewski T.F. Melanoma-Intrinsic β-Catenin Signalling Prevents Anti-Tumour Immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 82.Eddy K., Chen S. Glutamatergic Signaling a Therapeutic Vulnerability in Melanoma. Cancers. 2021;13:3874. doi: 10.3390/cancers13153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen S., Zhu H., Wetzel W.J., Philbert M.A. Spontaneous Melanocytosis in Transgenic Mice. J. Investig. Derm. 1996;106:1145–1151. doi: 10.1111/1523-1747.ep12340194. [DOI] [PubMed] [Google Scholar]
- 84.Shin S.-S., Namkoong J., Wall B.A., Gleason R., Lee H.J., Chen S. Oncogenic Activities of Metabotropic Glutamate Receptor 1 (Grm1) in Melanocyte Transformation. Pigment Cell Melanoma Res. 2008;21:368–378. doi: 10.1111/j.1755-148X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohtani Y., Harada T., Funasaka Y., Nakao K., Takahara C., Abdel-Daim M., Sakai N., Saito N., Nishigori C., Aiba A. Metabotropic Glutamate Receptor Subtype-1 Is Essential for in Vivo Growth of Melanoma. Oncogene. 2008;27:7162–7170. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- 86.Marín Y.E., Namkoong J., Cohen-Solal K., Shin S.-S., Martino J.J., Oka M., Chen S. Stimulation of Oncogenic Metabotropic Glutamate Receptor 1 in Melanoma Cells Activates ERK1/2 via PKCepsilon. Cell Signal. 2006;18:1279–1286. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 87.Shin S.-S., Wall B.A., Goydos J.S., Chen S. AKT2 Is a Downstream Target of Metabotropic Glutamate Receptor 1 (Grm1) Pigment Cell Melanoma Res. 2010;23:103–111. doi: 10.1111/j.1755-148X.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Namkoong J., Shin S.-S., Lee H.J., Marín Y.E., Wall B.A., Goydos J.S., Chen S. Metabotropic Glutamate Receptor 1 and Glutamate Signaling in Human Melanoma. Cancer Res. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 89.Mehnert J.M., Silk A.W., Lee J.H., Dudek L., Jeong B.-S., Li J., Schenkel J.M., Sadimin E., Kane M., Lin H., et al. A Phase II Trial of Riluzole, an Antagonist of Metabotropic Glutamate Receptor 1 (GRM1) Signaling, in Patients with Advanced Melanoma. Pigment Cell Melanoma Res. 2018;31:534–540. doi: 10.1111/pcmr.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah R., Singh S.J., Eddy K., Filipp F.V., Chen S. Concurrent Targeting of Glutaminolysis and Metabotropic Glutamate Receptor 1 (GRM1) Reduces Glutamate Bioavailability in GRM1+ Melanoma. Cancer Res. 2019;79:1799–1809. doi: 10.1158/0008-5472.CAN-18-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neto A., Ceol C.J. Melanoma-Associated GRM3 Variants Dysregulate Melanosome Trafficking and CAMP Signaling. Pigment Cell Melanoma Res. 2018;31:115–119. doi: 10.1111/pcmr.12610. [DOI] [PubMed] [Google Scholar]
- 92.Fan Y.-N., Li C., Huang L., Chen L., Tang Z., Han G., Liu Y. Characterization of Group I Metabotropic Glutamate Receptors in Rat and Human Adrenal Glands. Front. Physiol. 2020;11:401. doi: 10.3389/fphys.2020.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tucker B., Richards R.I., Lardelli M. Contribution of MGluR and Fmr1 Functional Pathways to Neurite Morphogenesis, Craniofacial Development and Fragile X Syndrome. Hum. Mol. Genet. 2006;15:3446–3458. doi: 10.1093/hmg/ddl422. [DOI] [PubMed] [Google Scholar]
- 94.Zigler M., Kamiya T., Brantley E.C., Villares G.J., Bar-Eli M. PAR-1 and Thrombin: The Ties That Bind the Microenvironment to Melanoma Metastasis. Cancer Res. 2011;71:6561–6566. doi: 10.1158/0008-5472.CAN-11-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacquelot N., Duong C.P.M., Belz G.T., Zitvogel L. Targeting Chemokines and Chemokine Receptors in Melanoma and Other Cancers. Front. Immunol. 2018;9:2480. doi: 10.3389/fimmu.2018.02480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Revankar C.M., Cimino D.F., Sklar L.A., Arterburn J.B., Prossnitz E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 97.Luo J., Liu D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in Vivo? Front. Endocrinol (Lausanne) 2020;11:148. doi: 10.3389/fendo.2020.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamm H.E. The Many Faces of G Protein Signaling. J. Biol. Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 99.Martin E.L., Rens-Domiano S., Schatz P.J., Hamm H.E. Potent Peptide Analogues of a G Protein Receptor-Binding Region Obtained with a Combinatorial Library. J. Biol. Chem. 1996;271:361–366. doi: 10.1074/jbc.271.1.361. [DOI] [PubMed] [Google Scholar]
- 100.Wettschureck N., Offermanns S. Mammalian G Proteins and Their Cell Type Specific Functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 101.Inoue A., Raimondi F., Kadji F.M.N., Singh G., Kishi T., Uwamizu A., Ono Y., Shinjo Y., Ishida S., Arang N., et al. Illuminating G-Protein-Coupling Selectivity of GPCRs. Cell. 2019;177:1933–1947.e25. doi: 10.1016/j.cell.2019.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sassone-Corsi P. The Cyclic AMP Pathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011148. doi: 10.1101/cshperspect.a011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sutherland E.W., Rall T.W. Fractionation and Characterization of a Cyclic Adenine Ribonucleotide Formed by Tissue Particles. J. Biol. Chem. 1958;232:1077–1091. doi: 10.1016/S0021-9258(19)77423-7. [DOI] [PubMed] [Google Scholar]
- 104.Zaccolo M., Zerio A., Lobo M.J. Subcellular Organization of the CAMP Signaling Pathway. Pharm. Rev. 2021;73:278–309. doi: 10.1124/pharmrev.120.000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanoune J., Defer N. Regulation and Role of Adenylyl Cyclase Isoforms. Annu. Rev. Pharm. Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 106.Dessauer C.W., Scully T.T., Gilman A.G. Interactions of Forskolin and ATP with the Cytosolic Domains of Mammalian Adenylyl Cyclase. J. Biol. Chem. 1997;272:22272–22277. doi: 10.1074/jbc.272.35.22272. [DOI] [PubMed] [Google Scholar]
- 107.Plagge A., Kelsey G., Germain-Lee E.L. Physiological Functions of the Imprinted Gnas Locus and Its Protein Variants Gαs and XLαs in Human and Mouse. J. Endocrinol. 2008;196:193–214. doi: 10.1677/JOE-07-0544. [DOI] [PubMed] [Google Scholar]
- 108.Sánchez-Más J., Guillo L.A., Zanna P., Jiménez-Cervantes C., García-Borrón J.C. Role of G Protein-Coupled Receptor Kinases in the Homologous Desensitization of the Human and Mouse Melanocortin 1 Receptors. Mol. Endocrinol. 2005;19:1035–1048. doi: 10.1210/me.2004-0227. [DOI] [PubMed] [Google Scholar]
- 109.Innamorati G., Wilkie T.M., Kantheti H.S., Valenti M.T., Dalle Carbonare L., Giacomello L., Parenti M., Melisi D., Bassi C. The Curious Case of Gαs Gain-of-Function in Neoplasia. BMC Cancer. 2018;18:293. doi: 10.1186/s12885-018-4133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.The Cancer Genome Atlas Network Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frey U., Fritz A., Rotterdam S., Schmid K., Potthoff A., Altmeyer P., Siffert W., Brockmeyer N. GNAS1 T393C Polymorphism and Disease Progression in Patients with Malignant Melanoma. Eur J. Med. Res. 2010;15:422–427. doi: 10.1186/2047-783X-15-10-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frey U.H., Alakus H., Wohlschlaeger J., Schmitz K.J., Winde G., van Calker H.G., Jöckel K.-H., Siffert W., Schmid K.W. GNAS1 T393C Polymorphism and Survival in Patients with Sporadic Colorectal Cancer. Clin. Cancer Res. 2005;11:5071–5077. doi: 10.1158/1078-0432.CCR-05-0472. [DOI] [PubMed] [Google Scholar]
- 113.Frey U.H., Eisenhardt A., Lümmen G., Rübben H., Jöckel K.-H., Schmid K.W., Siffert W. The T393C Polymorphism of the G Alpha s Gene (GNAS1) Is a Novel Prognostic Marker in Bladder Cancer. Cancer Epidemiol. Biomark. Prev. 2005;14:871–877. doi: 10.1158/1055-9965.EPI-04-0720. [DOI] [PubMed] [Google Scholar]
- 114.Thul P.J., Åkesson L., Wiking M., Mahdessian D., Geladaki A., Ait Blal H., Alm T., Asplund A., Björk L., Breckels L.M., et al. A Subcellular Map of the Human Proteome. Science. 2017;356:eaal3321. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 115.Uhlen M., Zhang C., Lee S., Sjöstedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z., Edfors F., et al. A Pathology Atlas of the Human Cancer Transcriptome. Science. 2017;357:eaan2507. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 116.Nishina H., Nimota K., Kukimoto I., Maehama T., Takahashi K., Hoshino S., Kanaho Y., Katada T. Significance of Thr182 in the Nucleotide-Exchange and GTP-Hydrolysis Reactions of the Alpha Subunit of GTP-Binding Protein Gi2. J. Biochem. 1995;118:1083–1089. doi: 10.1093/jb/118.5.1083. [DOI] [PubMed] [Google Scholar]
- 117.Pace A.M., Wong Y.H., Bourne H.R. A Mutant Alpha Subunit of Gi2 Induces Neoplastic Transformation of Rat-1 Cells. Proc. Natl. Acad. Sci. USA. 1991;88:7031–7035. doi: 10.1073/pnas.88.16.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cooper D.M.F. Regulation and Organization of Adenylyl Cyclases and CAMP. Biochem. J. 2003;375:517–529. doi: 10.1042/bj20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Halls M.L., Cooper D.M.F. Adenylyl Cyclase Signalling Complexes—Pharmacological Challenges and Opportunities. Pharmacol. Ther. 2017;172:171–180. doi: 10.1016/j.pharmthera.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Kleinboelting S., Diaz A., Moniot S., van den Heuvel J., Weyand M., Levin L.R., Buck J., Steegborn C. Crystal Structures of Human Soluble Adenylyl Cyclase Reveal Mechanisms of Catalysis and of Its Activation through Bicarbonate. Proc. Natl. Acad. Sci. USA. 2014;111:3727–3732. doi: 10.1073/pnas.1322778111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Magro C.M., Neil Crowson A., Desman G., Zippin J.H. Soluble Adenylyl Cyclase Antibody Profile as a Diagnostic Adjunct in the Assessment of Pigmented Lesions. Arch. Derm. 2012;148:335–344. doi: 10.1001/archdermatol.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen J., Wu F., Shi Y., Yang D., Xu M., Lai Y., Liu Y. Identification of Key Candidate Genes Involved in Melanoma Metastasis. Mol. Med. Rep. 2019;20:903–914. doi: 10.3892/mmr.2019.10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma M., Dai J., Tang H., Xu T., Yu S., Si L., Cui C., Sheng X., Chi Z., Mao L., et al. MicroRNA-23a-3p Inhibits Mucosal Melanoma Growth and Progression through Targeting Adenylate Cyclase 1 and Attenuating CAMP and MAPK Pathways. Theranostics. 2019;9:945–960. doi: 10.7150/thno.30516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rodríguez C.I., Castro-Pérez E., Prabhakar K., Block L., Longley B.J., Wisinski J.A., Kimple M.E., Setaluri V. EPAC–RAP1 Axis-Mediated Switch in the Response of Primary and Metastatic Melanoma to Cyclic AMP. Mol. Cancer Res. 2017;15:1792–1802. doi: 10.1158/1541-7786.MCR-17-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rodríguez C.I., Castro-Pérez E., Longley B.J., Setaluri V. Elevated Cyclic AMP Levels Promote BRAFCA/Pten-/- Mouse Melanoma Growth but PCREB Is Negatively Correlated with Human Melanoma Progression. Cancer Lett. 2018;414:268–277. doi: 10.1016/j.canlet.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johannessen C.M., Johnson L.A., Piccioni F., Townes A., Frederick D.T., Donahue M.K., Narayan R., Flaherty K.T., Wargo J.A., Root D.E., et al. A Melanocyte Lineage Program Confers Resistance to MAP Kinase Pathway Inhibition. Nature. 2013;504:138–142. doi: 10.1038/nature12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Omori K., Kotera J. Overview of PDEs and Their Regulation. Circ. Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 128.Bang J., Zippin J.H. Cyclic Adenosine Monophosphate (CAMP) Signaling in Melanocyte Pigmentation and Melanomagenesis. Pigment Cell Melanoma Res. 2021;34:28–43. doi: 10.1111/pcmr.12920. [DOI] [PubMed] [Google Scholar]
- 129.Khaled M., Levy C., Fisher D.E. Control of Melanocyte Differentiation by a MITF–PDE4D3 Homeostatic Circuit. Genes Dev. 2010;24:2276–2281. doi: 10.1101/gad.1937710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin D.-C., Xu L., Ding L.-W., Sharma A., Liu L.-Z., Yang H., Tan P., Vadgama J., Karlan B.Y., Lester J., et al. Genomic and Functional Characterizations of Phosphodiesterase Subtype 4D in Human Cancers. Proc. Natl. Acad. Sci. USA. 2013;110:6109–6114. doi: 10.1073/pnas.1218206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marquette A., André J., Bagot M., Bensussan A., Dumaz N. ERK and PDE4 Cooperate to Induce RAF Isoform Switching in Melanoma. Nat. Struct. Mol. Biol. 2011;18:584–591. doi: 10.1038/nsmb.2022. [DOI] [PubMed] [Google Scholar]
- 132.Dumaz N., Hayward R., Martin J., Ogilvie L., Hedley D., Curtin J.A., Bastian B.C., Springer C., Marais R. In Melanoma, RAS Mutations Are Accompanied by Switching Signaling from BRAF to CRAF and Disrupted Cyclic AMP Signaling. Cancer Res. 2006;66:9483–9491. doi: 10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 133.Delyon J., Servy A., Laugier F., André J., Ortonne N., Battistella M., Mourah S., Bensussan A., Lebbé C., Dumaz N. PDE4D Promotes FAK-Mediated Cell Invasion in BRAF-Mutated Melanoma. Oncogene. 2017;36:3252–3262. doi: 10.1038/onc.2016.469. [DOI] [PubMed] [Google Scholar]
- 134.Walsh D.A., Perkins J.P., Krebs E.G. An Adenosine 3′,5′-Monophosphate-Dependant Protein Kinase from Rabbit Skeletal Muscle. J. Biol. Chem. 1968;243:3763–3765. doi: 10.1016/S0021-9258(19)34204-8. [DOI] [PubMed] [Google Scholar]
- 135.Skalhegg B.S., Tasken K. Specificity in the CAMP/PKA Signaling Pathway. Differential Expression, Regulation, and Subcellular Localization of Subunits of PKA. Front. Biosci. 2000;5:D678–D693. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- 136.Taskén K., Skålhegg B.S., Taskén K.A., Solberg R., Knutsen H.K., Levy F.O., Sandberg M., Orstavik S., Larsen T., Johansen A.K., et al. Structure, Function, and Regulation of Human CAMP-Dependent Protein Kinases. Adv. Second Messenger Phosphoprot. Res. 1997;31:191–204. doi: 10.1016/s1040-7952(97)80019-5. [DOI] [PubMed] [Google Scholar]
- 137.Kirschner L.S., Carney J.A., Pack S.D., Taymans S.E., Giatzakis C., Cho Y.S., Cho-Chung Y.S., Stratakis C.A. Mutations of the Gene Encoding the Protein Kinase A Type I-Alpha Regulatory Subunit in Patients with the Carney Complex. Nat. Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 138.Liu Q., Tong D., Liu G., Yi Y., Zhang D., Zhang J., Zhang Y., Huang Z., Li Y., Chen R., et al. Carney Complex with PRKAR1A Gene Mutation. Medicine. 2017;96:e8999. doi: 10.1097/MD.0000000000008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cohen J.N., Yeh I., Mully T.W., LeBoit P.E., McCalmont T.H. Genomic and Clinicopathologic Characteristics of PRKAR1A-Inactivated Melanomas: Toward Genetic Distinctions of Animal-Type Melanoma/Pigment Synthesizing Melanoma. Am. J. Surg. Pathol. 2020;44:805–816. doi: 10.1097/PAS.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 140.Beebe S.J., Salomonsky P., Holroyd C., Becker D. Differential Expression of Cyclic AMP-Dependent Protein Kinase Isozymes in Normal Human Melanocytes and Malignant Melanomas. Cell Growth Differ. 1993;4:1005. [PubMed] [Google Scholar]
- 141.Hiramoto K., Murata T., Shimizu K., Morita H., Inui M., Manganiello V.C., Tagawa T., Arai N. Role of Phosphodiesterase 2 in Growth and Invasion of Human Malignant Melanoma Cells. Cell Signal. 2014;26:1807–1817. doi: 10.1016/j.cellsig.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lyons J., Bastian B.C., McCormick F. MC1R and CAMP Signaling Inhibit Cdc25B Activity and Delay Cell Cycle Progression in Melanoma Cells. Proc. Natl. Acad. Sci. USA. 2013;110:13845–13850. doi: 10.1073/pnas.1201917110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takahashi M., Li Y., Dillon T.J., Stork P.J.S. Phosphorylation of Rap1 by CAMP-Dependent Protein Kinase (PKA) Creates a Binding Site for KSR to Sustain ERK Activation by CAMP. J. Biol. Chem. 2017;292:1449–1461. doi: 10.1074/jbc.M116.768986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ostojić J., Yoon Y.-S., Sonntag T., Nguyen B., Vaughan J.M., Shokhirev M., Montminy M. Transcriptional Co-Activator Regulates Melanocyte Differentiation and Oncogenesis by Integrating CAMP and MAPK/ERK Pathways. Cell Rep. 2021;35:109136. doi: 10.1016/j.celrep.2021.109136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Buscà R., Ballotti R. Cyclic AMP a Key Messenger in the Regulation of Skin Pigmentation. Pigment. Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 146.Mobley A.K., Braeuer R.R., Kamiya T., Shoshan E., Bar-Eli M. Driving Transcriptional Regulators in Melanoma Metastasis. Cancer Metastasis Rev. 2012;31:621–632. doi: 10.1007/s10555-012-9358-8. [DOI] [PubMed] [Google Scholar]
- 147.Dobroff A.S., Wang H., Melnikova V.O., Villares G.J., Zigler M., Huang L., Bar-Eli M. Silencing CAMP-Response Element-Binding Protein (CREB) Identifies CYR61 as a Tumor Suppressor Gene in Melanoma. J. Biol. Chem. 2009;284:26194–26206. doi: 10.1074/jbc.M109.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie S., Price J.E., Luca M., Jean D., Ronai Z., Bar-Eli M. Dominant-Negative CREB Inhibits Tumor Growth and Metastasis of Human Melanoma Cells. Oncogene. 1997;15:2069–2075. doi: 10.1038/sj.onc.1201358. [DOI] [PubMed] [Google Scholar]
- 149.Braeuer R.R., Zigler M., Villares G.J., Dobroff A.S., Bar-Eli M. Transcriptional Control of Melanoma Metastasis: The Importance of the Tumor Microenvironment. Semin. Cancer Biol. 2011;21:83–88. doi: 10.1016/j.semcancer.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen J., Zhou X., Yang J., Sun Q., Liu Y., Li N., Zhang Z., Xu H. Circ-GLI1 Promotes Metastasis in Melanoma through Interacting with P70S6K2 to Activate Hedgehog/GLI1 and Wnt/β-Catenin Pathways and Upregulate Cyr61. Cell Death Dis. 2020;11:596. doi: 10.1038/s41419-020-02799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.White J.R., Thompson D.T., Koch K.E., Kiriazov B.S., Beck A.C., van der Heide D.M., Grimm B.G., Kulak M.V., Weigel R.J. AP-2α-Mediated Activation of E2F and EZH2 Drives Melanoma Metastasis. Cancer Res. 2021;81:4455–4470. doi: 10.1158/0008-5472.CAN-21-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nemlich Y., Baruch E.N., Besser M.J., Shoshan E., Bar-Eli M., Anafi L., Barshack I., Schachter J., Ortenberg R., Markel G. ADAR1-Mediated Regulation of Melanoma Invasion. Nat. Commun. 2018;9:2154. doi: 10.1038/s41467-018-04600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu W., Jin L., Jiang C.C., Long G.V., Scolyer R.A., Wu Q., Zhang X.D., Mei Y., Wu M. AEBP1 Upregulation Confers Acquired Resistance to BRAF (V600E) Inhibition in Melanoma. Cell Death Dis. 2013;4:e914. doi: 10.1038/cddis.2013.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang H., Kong Q., Wang J., Jiang Y., Hua H. Complex Roles of CAMP–PKA–CREB Signaling in Cancer. Exp. Hematol. Oncol. 2020;9:32. doi: 10.1186/s40164-020-00191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Narita M., Murata T., Shimizu K., Nakagawa T., Sugiyama T., Inui M., Hiramoto K., Tagawa T. A Role for Cyclic Nucleotide Phosphodiesterase 4 in Regulation of the Growth of Human Malignant Melanoma Cells. Oncol. Rep. 2007;17:1133–1139. doi: 10.3892/or.17.5.1133. [DOI] [PubMed] [Google Scholar]
- 156.Rodriguez C.I., Setaluri V. EPAC Mediates the Dual Role of CAMP Signaling in Melanoma. Oncoscience. 2018;6:283–284. doi: 10.18632/oncoscience.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fajardo A.M., Piazza G.A., Tinsley H.N. The Role of Cyclic Nucleotide Signaling Pathways in Cancer: Targets for Prevention and Treatment. Cancers. 2014;6:436–458. doi: 10.3390/cancers6010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Robertson A.G., Shih J., Yau C., Gibb E.A., Oba J., Mungall K.L., Hess J.M., Uzunangelov V., Walter V., Danilova L., et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell. 2017;32:204–220.e15. doi: 10.1016/j.ccell.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Van Raamsdonk C.D., Griewank K.G., Crosby M.B., Garrido M.C., Vemula S., Wiesner T., Obenauf A.C., Wackernagel W., Green G., Bouvier N., et al. Mutations in GNA11 in Uveal Melanoma. N. Engl. J. Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Urtatiz O., Van Raamsdonk C.D. Gnaq and Gna11 in the Endothelin Signaling Pathway and Melanoma. Front. Genet. 2016;7:59. doi: 10.3389/fgene.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.O’Hayre M., Vázquez-Prado J., Kufareva I., Stawiski E.W., Handel T.M., Seshagiri S., Gutkind J.S. The Emerging Mutational Landscape of G Proteins and G-Protein-Coupled Receptors in Cancer. Nat. Rev. Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maziarz M., Leyme A., Marivin A., Luebbers A., Patel P.P., Chen Z., Sprang S.R., Garcia-Marcos M. Atypical Activation of the G Protein Gαq by the Oncogenic Mutation Q209P. J. Biol. Chem. 2018;293:19586–19599. doi: 10.1074/jbc.RA118.005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Möller I., Murali R., Müller H., Wiesner T., Jackett L.A., Scholz S.L., Cosgarea I., van de Nes J.A., Sucker A., Hillen U., et al. Activating Cysteinyl Leukotriene Receptor 2 (CYSLTR2) Mutations in Blue Nevi. Mod. Pathol. 2017;30:350–356. doi: 10.1038/modpathol.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang J.L.-Y., Urtatiz O., Van Raamsdonk C.D. Oncogenic G Protein GNAQ Induces Uveal Melanoma and Intravasation in Mice. Cancer Res. 2015;75:3384–3397. doi: 10.1158/0008-5472.CAN-14-3229. [DOI] [PubMed] [Google Scholar]
- 165.Urtatiz O., Cook C., Huang J.L.-Y., Yeh I., Van Raamsdonk C.D. GNAQQ209L Expression Initiated in Multipotent Neural Crest Cells Drives Aggressive Melanoma of the Central Nervous System. Pigment Cell Melanoma Res. 2020;33:96–111. doi: 10.1111/pcmr.12843. [DOI] [PubMed] [Google Scholar]
- 166.Moore A.R., Ran L., Guan Y., Sher J.J., Hitchman T.D., Zhang J.Q., Hwang C., Walzak E.G., Shoushtari A.N., Monette S., et al. GNA11 Q209L Mouse Model Reveals RasGRP3 as an Essential Signaling Node in Uveal Melanoma. Cell Rep. 2018;22:2455–2468. doi: 10.1016/j.celrep.2018.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Annala S., Feng X., Shridhar N., Eryilmaz F., Patt J., Yang J., Pfeil E.M., Cervantes-Villagrana R.D., Inoue A., Häberlein F., et al. Direct Targeting of Gαq and Gα11 Oncoproteins in Cancer Cells. Sci. Signal. 2019;12:eaau5948. doi: 10.1126/scisignal.aau5948. [DOI] [PubMed] [Google Scholar]
- 168.Kostenis E., Pfeil E.M., Annala S. Heterotrimeric Gq Proteins as Therapeutic Targets? J. Biol. Chem. 2020;295:5206–5215. doi: 10.1074/jbc.REV119.007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ambrosini G., Pratilas C.A., Qin L.-X., Tadi M., Surriga O., Carvajal R.D., Schwartz G.K. Identification of Unique MEK-Dependent Genes in GNAQ Mutant Uveal Melanoma Involved in Cell Growth, Tumor Cell Invasion and MEK-Resistance. Clin. Cancer Res. 2012;18:3552–3561. doi: 10.1158/1078-0432.CCR-11-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Asundi J., Lacap J.A., Clark S., Nannini M., Roth L., Polakis P. MAPK Pathway Inhibition Enhances the Efficacy of an Anti-Endothelin B Receptor Drug Conjugate by Inducing Target Expression in Melanoma. Mol. Cancer. 2014;13:1599–1610. doi: 10.1158/1535-7163.MCT-13-0446. [DOI] [PubMed] [Google Scholar]
- 171.Hitchman T.D., Bayshtok G., Ceraudo E., Moore A.R., Lee C., Jia R., Wang N., Pachai M.R., Shoushtari A.N., Francis J.H., et al. Combined Inhibition of Gαq and MEK Enhances Therapeutic Efficacy in Uveal Melanoma. Clin. Cancer Res. 2021;27:1476–1490. doi: 10.1158/1078-0432.CCR-20-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Smrcka A.V., Hepler J.R., Brown K.O., Sternweis P.C. Regulation of Polyphosphoinositide-Specific Phospholipase C Activity by Purified Gq. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- 173.Taylor S.J., Chae H.Z., Rhee S.G., Exton J.H. Activation of the Beta 1 Isozyme of Phospholipase C by Alpha Subunits of the Gq Class of G Proteins. Nature. 1991;350:516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- 174.Waldo G.L., Boyer J.L., Morris A.J., Harden T.K. Purification of an AlF4- and G-Protein Beta Gamma-Subunit-Regulated Phospholipase C-Activating Protein. J. Biol. Chem. 1991;266:14217–14225. doi: 10.1016/S0021-9258(18)98670-9. [DOI] [PubMed] [Google Scholar]
- 175.Lyon A.M., Tesmer J.J.G. Structural Insights into Phospholipase C-β Function. Mol. Pharm. 2013;84:488–500. doi: 10.1124/mol.113.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chua V., Lapadula D., Randolph C., Benovic J.L., Wedegaertner P.B., Aplin A.E. Dysregulated GPCR Signaling and Therapeutic Options in Uveal Melanoma. Mol. Cancer Res. 2017;15:501–506. doi: 10.1158/1541-7786.MCR-17-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johansson P., Aoude L.G., Wadt K., Glasson W.J., Warrier S.K., Hewitt A.W., Kiilgaard J.F., Heegaard S., Isaacs T., Franchina M., et al. Deep Sequencing of Uveal Melanoma Identifies a Recurrent Mutation in PLCB4. Oncotarget. 2016;7:4624–4631. doi: 10.18632/oncotarget.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li J., Zhao X., Wang D., He W., Zhang S., Cao W., Huang Y., Wang L., Zhou S., Luo K. Up-Regulated Expression of Phospholipase C, Β1 Is Associated with Tumor Cell Proliferation and Poor Prognosis in Hepatocellular Carcinoma. Onco Targets. 2016;9:1697–1706. doi: 10.2147/OTT.S97189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sengelaub C.A., Navrazhina K., Ross J.B., Halberg N., Tavazoie S.F. PTPRN2 and PLCβ1 Promote Metastatic Breast Cancer Cell Migration through PI(4,5)P2-dependent Actin Remodeling. EMBO J. 2016;35:62–76. doi: 10.15252/embj.201591973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arita Y., O’Driscoll K.R., Weinstein I.B. Growth of Human Melanocyte Cultures Supported by 12-O-Tetradecanoylphorbol-13-Acetate Is Mediated through Protein Kinase C Activation. Cancer Res. 1992;52:4514–4521. [PubMed] [Google Scholar]
- 181.Petit V., Raymond J., Alberti C., Pouteaux M., Gallagher S.J., Nguyen M.Q., Aplin A.E., Delmas V., Larue L. C57BL/6 Congenic Mouse NRASQ61K Melanoma Cell Lines Are Highly Sensitive to the Combination of Mek and Akt Inhibitors in Vitro and in Vivo. Pigment Cell Melanoma Res. 2019;32:829–841. doi: 10.1111/pcmr.12807. [DOI] [PubMed] [Google Scholar]
- 182.Tamura A., Halaban R., Moellmann G., Cowan J.M., Lerner M.R., Lerner A.B. Normal Murine Melanocytes in Culture. In Vitro Cell Dev. Biol. 1987;23:519–522. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]
- 183.Iwasaki T., Yamauchi M., Liang Z., Itai A., Sakaguchi M., Nagano T., Kamada S., Oka M. TPA Inhibits Melanoma Growth through Inactivation of STAT3 through Protein Tyrosine Phosphatases. J. Dermatol. Sci. 2017;86:e94. doi: 10.1016/j.jdermsci.2017.02.275. [DOI] [Google Scholar]
- 184.Jørgensen K., Skrede M., Cruciani V., Mikalsen S.-O., Slipicevic A., Flørenes V.A. Phorbol Ester Phorbol-12-Myristate-13-Acetate Promotes Anchorage-Independent Growth and Survival of Melanomas through MEK-Independent Activation of ERK1/2. Biochem. Biophys. Res. Commun. 2005;329:266–274. doi: 10.1016/j.bbrc.2005.01.143. [DOI] [PubMed] [Google Scholar]
- 185.La Porta C.A., Porro D., Comolli R. Opposite Effects of TPA on G1/S Transition and on Cell Size in the Low Metastatic B16F1 with Respect to High Metastatic BL6 Murine Melanoma Cells. Cancer Lett. 1998;132:159–164. doi: 10.1016/S0304-3835(98)00177-3. [DOI] [PubMed] [Google Scholar]
- 186.Dissanayake S.K., Wade M., Johnson C.E., O’Connell M.P., Leotlela P.D., French A.D., Shah K.V., Hewitt K.J., Rosenthal D.T., Indig F.E., et al. The Wnt5A/Protein Kinase C Pathway Mediates Motility in Melanoma Cells via the Inhibition of Metastasis Suppressors and Initiation of an Epithelial to Mesenchymal Transition. J. Biol. Chem. 2007;282:17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhao X., Geltinger C., Kishikawa S., Ohshima K., Murata T., Nomura N., Nakahara T., Yokoyama K.K. Treatment of Mouse Melanoma Cells with Phorbol 12-Myristate 13-Acetate Counteracts Mannosylerythritol Lipid-Induced Growth Arrest and Apoptosis. Cytotechnology. 2000;33:123–130. doi: 10.1023/A:1008129616127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Foskett J.K., White C., Cheung K.-H., Mak D.-O.D. Inositol Trisphosphate Receptor Ca2+ Release Channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cox J.L., Lancaster T., Carlson C.G. Changes in the Motility of B16F10 Melanoma Cells Induced by Alterations in Resting Calcium Influx. Melanoma Res. 2002;12:211–219. doi: 10.1097/00008390-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 190.Sun J., Lu F., He H., Shen J., Messina J., Mathew R., Wang D., Sarnaik A.A., Chang W.-C., Kim M., et al. STIM1- and Orai1-Mediated Ca2+ Oscillation Orchestrates Invadopodium Formation and Melanoma Invasion. J. Cell Biol. 2014;207:535–548. doi: 10.1083/jcb.201407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Umemura M., Baljinnyam E., Feske S., De Lorenzo M.S., Xie L.-H., Feng X., Oda K., Makino A., Fujita T., Yokoyama U., et al. Store-Operated Ca2+ Entry (SOCE) Regulates Melanoma Proliferation and Cell Migration. PLoS ONE. 2014;9:e89292. doi: 10.1371/journal.pone.0089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Denning M.F. Specifying Protein Kinase C Functions in Melanoma. Pigment Cell Melanoma Res. 2012;25:466–476. doi: 10.1111/j.1755-148X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 193.Hoshi N., Langeberg L.K., Gould C.M., Newton A.C., Scott J.D. Interaction with AKAP79 Modifies the Cellular Pharmacology of PKC. Mol. Cell. 2010;37:541–550. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Park H.-Y., Wu H., Killoran C.E., Gilchrest B.A. The Receptor for Activated C-Kinase-I (RACK-I) Anchors Activated PKC-Beta on Melanosomes. J. Cell Sci. 2004;117:3659–3668. doi: 10.1242/jcs.01219. [DOI] [PubMed] [Google Scholar]
- 195.Schechtman D., Mochly-Rosen D. Adaptor Proteins in Protein Kinase C-Mediated Signal Transduction. Oncogene. 2001;20:6339–6347. doi: 10.1038/sj.onc.1204778. [DOI] [PubMed] [Google Scholar]
- 196.Voris J.P., Sitailo L.A., Rahn H.R., Defnet A., Gerds A.T., Sprague R., Yadav V., Caroline Le Poole I., Denning M.F. Functional Alterations in Protein Kinase C Beta II Expression in Melanoma. Pigment Cell Melanoma Res. 2010;23:216–224. doi: 10.1111/j.1755-148X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 197.Matsuoka H., Tsubaki M., Yamazoe Y., Ogaki M., Satou T., Itoh T., Kusunoki T., Nishida S. Tamoxifen Inhibits Tumor Cell Invasion and Metastasis in Mouse Melanoma through Suppression of PKC/MEK/ERK and PKC/PI3K/Akt Pathways. Exp. Cell Res. 2009;315:2022–2032. doi: 10.1016/j.yexcr.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 198.Oka M., Kikkawa U., Nishigori C. Protein Kinase C-BetaII Represses Hepatocyte Growth Factor-Induced Invasion by Preventing the Association of Adapter Protein Gab1 and Phosphatidylinositol 3-Kinase in Melanoma Cells. J. Investig. Derm. 2008;128:188–195. doi: 10.1038/sj.jid.5700961. [DOI] [PubMed] [Google Scholar]
- 199.Park H.Y., Russakovsky V., Ohno S., Gilchrest B.A. The Beta Isoform of Protein Kinase C Stimulates Human Melanogenesis by Activating Tyrosinase in Pigment Cells. J. Biol. Chem. 1993;268:11742–11749. doi: 10.1016/S0021-9258(19)50262-9. [DOI] [PubMed] [Google Scholar]
- 200.Chen X., Wu Q., Depeille P., Chen P., Thornton S., Kalirai H., Coupland S.E., Roose J.P., Bastian B.C. RasGRP3 Mediates MAPK Pathway Activation in GNAQ Mutant Uveal Melanoma. Cancer Cell. 2017;31:685–696.e6. doi: 10.1016/j.ccell.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lau E., Kluger H., Varsano T., Lee K., Scheffler I., Rimm D.L., Ideker T., Ronai Z.A. PKCε Promotes Oncogenic Functions of ATF2 in the Nucleus While Blocking Its Apoptotic Function at Mitochondria. Cell. 2012;148:543–555. doi: 10.1016/j.cell.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mhaidat N.M., Thorne R.F., Zhang X.D., Hersey P. Regulation of Docetaxel-Induced Apoptosis of Human Melanoma Cells by Different Isoforms of Protein Kinase C. Mol. Cancer Res. 2007;5:1073–1081. doi: 10.1158/1541-7786.MCR-07-0059. [DOI] [PubMed] [Google Scholar]
- 203.Aiba Y., Oh-hora M., Kiyonaka S., Kimura Y., Hijikata A., Mori Y., Kurosaki T. Activation of RasGRP3 by Phosphorylation of Thr-133 Is Required for B Cell Receptor-Mediated Ras Activation. Proc. Natl. Acad. Sci. USA. 2004;101:16612–16617. doi: 10.1073/pnas.0407468101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Johnson J.E., Goulding R.E., Ding Z., Partovi A., Anthony K.V., Beaulieu N., Tazmini G., Cornell R.B., Kay R.J. Differential Membrane Binding and Diacylglycerol Recognition by C1 Domains of RasGRPs. Biochem. J. 2007;406:223–236. doi: 10.1042/BJ20070294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Teixeira C., Stang S.L., Zheng Y., Beswick N.S., Stone J.C. Integration of DAG Signaling Systems Mediated by PKC-Dependent Phosphorylation of RasGRP3. Blood. 2003;102:1414–1420. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- 206.Cozzi S.-J., Parsons P.G., Ogbourne S.M., Pedley J., Boyle G.M. Induction of Senescence in Diterpene Ester-Treated Melanoma Cells via Protein Kinase C-Dependent Hyperactivation of the Mitogen-Activated Protein Kinase Pathway. Cancer Res. 2006;66:10083–10091. doi: 10.1158/0008-5472.CAN-06-0348. [DOI] [PubMed] [Google Scholar]
- 207.Schönwasser D.C., Marais R.M., Marshall C.J., Parker P.J. Activation of the Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Pathway by Conventional, Novel, and Atypical Protein Kinase C Isotypes. Mol. Cell Biol. 1998;18:790–798. doi: 10.1128/MCB.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tsubaki M., Matsuoka H., Yamamoto C., Kato C., Ogaki M., Satou T., Itoh T., Kusunoki T., Tanimori Y., Nishida S. The Protein Kinase C Inhibitor, H7, Inhibits Tumor Cell Invasion and Metastasis in Mouse Melanoma via Suppression of ERK1/2. Clin. Exp. Metastasis. 2007;24:431–438. doi: 10.1007/s10585-007-9080-z. [DOI] [PubMed] [Google Scholar]
- 209.Johannessen C.M., Boehm J.S., Kim S.Y., Thomas S.R., Wardwell L., Johnson L.A., Emery C.M., Stransky N., Cogdill A.P., Barretina J., et al. COT Drives Resistance to RAF Inhibition through MAP Kinase Pathway Reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fu Y., Rathod D., Patel K. Protein Kinase C Inhibitor Anchored BRD4 PROTAC PEGylated Nanoliposomes for the Treatment of Vemurafenib-Resistant Melanoma. Exp. Cell Res. 2020;396:112275. doi: 10.1016/j.yexcr.2020.112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kwon H., Kim J., Jho E.-H. Role of the Hippo Pathway and Mechanisms for Controlling Cellular Localization of YAP/TAZ. FEBS J. 2021 doi: 10.1111/febs.16091. in press . [DOI] [PubMed] [Google Scholar]
- 212.Yu F.-X., Zhao B., Panupinthu N., Jewell J.L., Lian I., Wang L.H., Zhao J., Yuan H., Tumaneng K., Li H., et al. Regulation of the Hippo-YAP Pathway by G-Protein Coupled Receptor Signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Feng X., Degese M.S., Iglesias-Bartolome R., Vaque J.P., Molinolo A.A., Rodrigues M., Zaidi M.R., Ksander B.R., Merlino G., Sodhi A., et al. Hippo-Independent Activation of YAP by the GNAQ Uveal Melanoma Oncogene through a Trio-Regulated Rho GTPase Signaling Circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yu F.-X., Luo J., Mo J.-S., Liu G., Kim Y.C., Meng Z., Zhao L., Peyman G., Ouyang H., Jiang W., et al. Mutant Gq/11 Promote Uveal Melanoma Tumorigenesis by Activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vaqué J.P., Dorsam R.T., Feng X., Iglesias-Bartolome R., Forsthoefel D.J., Chen Q., Debant A., Seeger M.A., Ksander B.R., Teramoto H., et al. A Genome-Wide RNAi Screen Reveals a Trio-Regulated Rho GTPase Circuitry Transducing Mitogenic Signals Initiated by G Protein-Coupled Receptors. Mol. Cell. 2013;49:94–108. doi: 10.1016/j.molcel.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Paradis J.S., Acosta M., Saddawi-Konefka R., Kishore A., Lubrano S., Gomes F., Arang N., Tiago M., Coma S., Wu X., et al. Synthetic Lethal Screens Reveal Cotargeting FAK and MEK as a Multimodal Precision Therapy for GNAQ-Driven Uveal Melanoma. Clin. Cancer Res. 2021;27:3190–3200. doi: 10.1158/1078-0432.CCR-20-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shaulian E., Karin M. AP-1 in Cell Proliferation and Survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 218.Du L., Anderson A., Nguyen K., Ojeda S.S., Ortiz-Rivera I., Nguyen T.N., Zhang T., Kaoud T.S., Gray N.S., Dalby K.N., et al. JNK2 Is Required for the Tumorigenic Properties of Melanoma Cells. ACS Chem. Biol. 2019;14:1426–1435. doi: 10.1021/acschembio.9b00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Estrada Y., Dong J., Ossowski L. Positive Crosstalk between ERK and P38 in Melanoma Stimulates Migration and in Vivo Proliferation. Pigment Cell Melanoma Res. 2009;22:66–76. doi: 10.1111/j.1755-148X.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 220.Pathria G., Garg B., Garg K., Wagner C., Wagner S.N. Dual C-Jun N-Terminal Kinase-Cyclin D1 and Extracellular Signal-Related Kinase-c-Jun Disjunction in Human Melanoma. Br. J. Derm. 2016;175:1221–1231. doi: 10.1111/bjd.14713. [DOI] [PubMed] [Google Scholar]
- 221.Ma Y., Wang L., He F., Yang J., Ding Y., Ge S., Fan X., Zhou Y., Xu X., Jia R. LACTB Suppresses Melanoma Progression by Attenuating PP1A and YAP Interaction. Cancer Lett. 2021;506:67–82. doi: 10.1016/j.canlet.2021.02.022. [DOI] [PubMed] [Google Scholar]
- 222.Nallet-Staub F., Marsaud V., Li L., Gilbert C., Dodier S., Bataille V., Sudol M., Herlyn M., Mauviel A. Pro-Invasive Activity of the Hippo Pathway Effectors YAP and TAZ in Cutaneous Melanoma. J. Investig. Derm. 2014;134:123–132. doi: 10.1038/jid.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang X., Yang L., Szeto P., Abali G.K., Zhang Y., Kulkarni A., Amarasinghe K., Li J., Vergara I.A., Molania R., et al. The Hippo Pathway Oncoprotein YAP Promotes Melanoma Cell Invasion and Spontaneous Metastasis. Oncogene. 2020;39:5267–5281. doi: 10.1038/s41388-020-1362-9. [DOI] [PubMed] [Google Scholar]
- 224.Zhao B., Xie J., Zhou X., Zhang L., Cheng X., Liang C. YAP Activation in Melanoma Contributes to Anoikis Resistance and Metastasis. Exp. Biol. Med. 2021;246:888–896. doi: 10.1177/1535370220977101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lui J.W., Moore S.P.G., Huang L., Ogomori K., Li Y., Lang D. YAP Facilitates Melanoma Migration through Regulation of Actin-Related Protein 2/3 Complex Subunit 5 (ARPC5) Pigment Cell Melanoma Res. 2021;32:52–65. doi: 10.1111/pcmr.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tan S., Zhao Z., Qiao Y., Zhang B., Zhang T., Zhang M., Qi J., Wang X., Meng M., Zhou Q. Activation of the Tumor Suppressive Hippo Pathway by Triptonide as a New Strategy to Potently Inhibit Aggressive Melanoma Cell Metastasis. Biochem. Pharmacol. 2021;185:114423. doi: 10.1016/j.bcp.2021.114423. [DOI] [PubMed] [Google Scholar]
- 227.Flem-Karlsen K., McFadden E., Omar N., Haugen M.H., Øy G.F., Ryder T., Gullestad H.P., Hermann R., Mælandsmo G.M., Flørenes V.A. Targeting AXL and the DNA Damage Response Pathway as a Novel Therapeutic Strategy in Melanoma. Mol. Cancer. 2020;19:895–905. doi: 10.1158/1535-7163.MCT-19-0290. [DOI] [PubMed] [Google Scholar]
- 228.Müller J., Krijgsman O., Tsoi J., Robert L., Hugo W., Song C., Kong X., Possik P.A., Cornelissen-Steijger P.D.M., Foppen M.H.G., et al. Low MITF/AXL Ratio Predicts Early Resistance to Multiple Targeted Drugs in Melanoma. Nat. Commun. 2014;5:5712. doi: 10.1038/ncomms6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tizpa E., Young H.J., Bonjoc K.-J.C., Chang C.-W., Liu Y., Foulks J.M., Chaudhry A. Role of AXL in Metastatic Melanoma and Impact of TP-0903 as a Novel Therapeutic Option for Melanoma Brain Metastasis. J. Clin. Oncol. 2020;38:e22021. doi: 10.1200/JCO.2020.38.15_suppl.e22021. [DOI] [Google Scholar]
- 230.Kunz M., Moeller S., Koczan D., Lorenz P., Wenger R.H., Glocker M.O., Thiesen H.-J., Gross G., Ibrahim S.M. Mechanisms of Hypoxic Gene Regulation of Angiogenesis Factor Cyr61 in Melanoma Cells. J. Biol. Chem. 2003;278:45651–45660. doi: 10.1074/jbc.M301373200. [DOI] [PubMed] [Google Scholar]
- 231.Borsotti P., Ghilardi C., Ostano P., Silini A., Dossi R., Pinessi D., Foglieni C., Scatolini M., Lacal P.M., Ferrari R., et al. Thrombospondin-1 Is Part of a Slug-Independent Motility and Metastatic Program in Cutaneous Melanoma, in Association with VEGFR-1 and FGF-2. Pigment Cell Melanoma Res. 2015;28:73–81. doi: 10.1111/pcmr.12319. [DOI] [PubMed] [Google Scholar]
- 232.Jayachandran A., Anaka M., Prithviraj P., Hudson C., McKeown S.J., Lo P.-H., Vella L.J., Goding C.R., Cebon J., Behren A. Thrombospondin 1 Promotes an Aggressive Phenotype through Epithelial-to-Mesenchymal Transition in Human Melanoma. Oncotarget. 2014;5:5782–5797. doi: 10.18632/oncotarget.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Verfaillie A., Imrichova H., Atak Z.K., Dewaele M., Rambow F., Hulselmans G., Christiaens V., Svetlichnyy D., Luciani F., Van den Mooter L., et al. Decoding the Regulatory Landscape of Melanoma Reveals TEADS as Regulators of the Invasive Cell State. Nat. Commun. 2015;6:6683. doi: 10.1038/ncomms7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hart M.J., Jiang X., Kozasa T., Roscoe W., Singer W.D., Gilman A.G., Sternweis P.C., Bollag G. Direct Stimulation of the Guanine Nucleotide Exchange Activity of P115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 235.Suzuki N., Nakamura S., Mano H., Kozasa T. Galpha 12 Activates Rho GTPase through Tyrosine-Phosphorylated Leukemia-Associated RhoGEF. Proc. Natl. Acad. Sci. USA. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen Z., Singer W.D., Sternweis P.C., Sprang S.R. Structure of the P115RhoGEF RgRGS Domain-Galpha13/I1 Chimera Complex Suggests Convergent Evolution of a GTPase Activator. Nat. Struct. Mol. Biol. 2005;12:191–197. doi: 10.1038/nsmb888. [DOI] [PubMed] [Google Scholar]
- 237.Fukuhara S., Murga C., Zohar M., Igishi T., Gutkind J.S. A Novel PDZ Domain Containing Guanine Nucleotide Exchange Factor Links Heterotrimeric G Proteins to Rho. J. Biol. Chem. 1999;274:5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- 238.Suzuki I., Cone R.D., Im S., Nordlund J., Abdel-Malek Z.A. Binding of Melanotropic Hormones to the Melanocortin Receptor MC1R on Human Melanocytes Stimulates Proliferation and Melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- 239.Vogt S., Grosse R., Schultz G., Offermanns S. Receptor-Dependent RhoA Activation in G12/G13-Deficient Cells: Genetic Evidence for an Involvement of Gq/G11. J. Biol. Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- 240.Wells C.D., Liu M.-Y., Jackson M., Gutowski S., Sternweis P.M., Rothstein J.D., Kozasa T., Sternweis P.C. Mechanisms for Reversible Regulation between G13 and Rho Exchange Factors. J. Biol. Chem. 2002;277:1174–1181. doi: 10.1074/jbc.M105274200. [DOI] [PubMed] [Google Scholar]
- 241.Elste A.P., Petersen I. Expression of Proteinase-Activated Receptor 1-4 (PAR 1-4) in Human Cancer. J. Mol. Histol. 2010;41:89–99. doi: 10.1007/s10735-010-9274-6. [DOI] [PubMed] [Google Scholar]
- 242.Mo J.-S., Yu F.-X., Gong R., Brown J.H., Guan K.-L. Regulation of the Hippo–YAP Pathway by Protease-Activated Receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kelly P., Stemmle L.N., Madden J.F., Fields T.A., Daaka Y., Casey P.J. A Role for the G12 Family of Heterotrimeric G Proteins in Prostate Cancer Invasion. J. Biol. Chem. 2006;281:26483–26490. doi: 10.1074/jbc.M604376200. [DOI] [PubMed] [Google Scholar]
- 244.Kelly P., Moeller B.J., Juneja J., Booden M.A., Der C.J., Daaka Y., Dewhirst M.W., Fields T.A., Casey P.J. The G12 Family of Heterotrimeric G Proteins Promotes Breast Cancer Invasion and Metastasis. Proc. Natl. Acad. Sci. USA. 2006;103:8173–8178. doi: 10.1073/pnas.0510254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Minami K., Ueda N., Ishimoto K., Tsujiuchi T. Lysophosphatidic Acid Receptor-2 (LPA2)-Mediated Signaling Enhances Chemoresistance in Melanoma Cells Treated with Anticancer Drugs. Mol. Cell Biochem. 2020;469:89–95. doi: 10.1007/s11010-020-03730-w. [DOI] [PubMed] [Google Scholar]
- 246.Zhan T., Rindtorff N., Boutros M. Wnt Signaling in Cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.de la Fouchardière A., Caillot C., Jacquemus J., Durieux E., Houlier A., Haddad V., Pissaloux D. β-Catenin Nuclear Expression Discriminates Deep Penetrating Nevi from Other Cutaneous Melanocytic Tumors. Virchows Arch. 2019;474:539–550. doi: 10.1007/s00428-019-02533-9. [DOI] [PubMed] [Google Scholar]
- 248.Yeh I., Lang U.E., Durieux E., Tee M.K., Jorapur A., Shain A.H., Haddad V., Pissaloux D., Chen X., Cerroni L., et al. Combined Activation of MAP Kinase Pathway and β-Catenin Signaling Cause Deep Penetrating Nevi. Nat. Commun. 2017;8:644. doi: 10.1038/s41467-017-00758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rimm D.L., Caca K., Hu G., Harrison F.B., Fearon E.R. Frequent Nuclear/Cytoplasmic Localization of Beta-Catenin without Exon 3 Mutations in Malignant Melanoma. Am. J. Pathol. 1999;154:325–329. doi: 10.1016/S0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Demunter A., Libbrecht L., Degreef H., De Wolf-Peeters C., van den Oord J.J. Loss of Membranous Expression of β-Catenin Is Associated with Tumor Progression in Cutaneous Melanoma and Rarely Caused by Exon 3 Mutations. Mod. Pathol. 2002;15:454–461. doi: 10.1038/modpathol.3880546. [DOI] [PubMed] [Google Scholar]
- 251.Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. Monounsaturated Fatty Acid Modification of Wnt Protein: Its Role in Wnt Secretion. Dev. Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 252.Willert K., Brown J.D., Danenberg E., Duncan A.W., Weissman I.L., Reya T., Yates J.R., Nusse R. Wnt Proteins Are Lipid-Modified and Can Act as Stem Cell Growth Factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 253.Lee E., Salic A., Krüger R., Heinrich R., Kirschner M.W. The Roles of APC and Axin Derived from Experimental and Theoretical Analysis of the Wnt Pathway. PLoS Biol. 2003;1:e10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Salic A., Lee E., Mayer L., Kirschner M.W. Control of Beta-Catenin Stability: Reconstitution of the Cytoplasmic Steps of the Wnt Pathway in Xenopus Egg Extracts. Mol. Cell. 2000;5:523–532. doi: 10.1016/S1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 255.Valenta T., Hausmann G., Basler K. The Many Faces and Functions of β-Catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Aktary Z., Bertrand J.U., Larue L. The WNT-Less Wonder: WNT-Independent β-Catenin Signaling. Pigment Cell Melanoma Res. 2016;29:524–540. doi: 10.1111/pcmr.12501. [DOI] [PubMed] [Google Scholar]
- 257.Schepsky A., Bruser K., Gunnarsson G.J., Goodall J., Hallsson J.H., Goding C.R., Steingrimsson E., Hecht A. The Microphthalmia-Associated Transcription Factor Mitf Interacts with Beta-Catenin to Determine Target Gene Expression. Mol. Cell. Biol. 2006;26:8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Saito H., Yasumoto K.-I., Takeda K., Takahashi K., Fukuzaki A., Orikasa S., Shibahara S. Melanocyte-Specific Microphthalmia-Associated Transcription Factor Isoform Activates Its Own Gene Promoter through Physical Interaction with Lymphoid-Enhancing Factor 1. J. Biol. Chem. 2002;277:28787–28794. doi: 10.1074/jbc.M203719200. [DOI] [PubMed] [Google Scholar]
- 259.Kawakami A., Fisher D.E. The Master Role of Microphthalmia-Associated Transcription Factor in Melanocyte and Melanoma Biology. Lab. Investig. 2017;97:649–656. doi: 10.1038/labinvest.2017.9. [DOI] [PubMed] [Google Scholar]
- 260.Ballotti R., Cheli Y., Bertolotto C. The Complex Relationship between MITF and the Immune System: A Melanoma ImmunoTherapy (Response) Factor? Mol. Cancer. 2020;19:170. doi: 10.1186/s12943-020-01290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Baljinnyam E., Umemura M., De Lorenzo M.S., Xie L.-H., Nowycky M., Iwatsubo M., Chen S., Goydos J.S., Iwatsubo K. Gβγ Subunits Inhibit Epac-Induced Melanoma Cell Migration. BMC Cancer. 2011;11:256. doi: 10.1186/1471-2407-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bonacci T.M., Ghosh M., Malik S., Smrcka A.V. Regulatory Interactions between the Amino Terminus of G-Protein Betagamma Subunits and the Catalytic Domain of Phospholipase Cbeta2. J. Biol. Chem. 2005;280:10174–10181. doi: 10.1074/jbc.M412514200. [DOI] [PubMed] [Google Scholar]
- 263.Leopoldt D., Hanck T., Exner T., Maier U., Wetzker R., Nürnberg B. Gβγ Stimulates Phosphoinositide 3-Kinase-γ by Direct Interaction with Two Domains of the Catalytic P110 Subunit. J. Biol. Chem. 1998;273:7024–7029. doi: 10.1074/jbc.273.12.7024. [DOI] [PubMed] [Google Scholar]
- 264.Pfeil E.M., Brands J., Merten N., Vögtle T., Vescovo M., Rick U., Albrecht I.-M., Heycke N., Kawakami K., Ono Y., et al. Heterotrimeric G Protein Subunit Gαq Is a Master Switch for Gβγ-Mediated Calcium Mobilization by Gi-Coupled GPCRs. Mol. Cell. 2020;80:940–954.e6. doi: 10.1016/j.molcel.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 265.Sellers L.A., Alderton F., Carruthers A.M., Schindler M., Humphrey P.P. Receptor Isoforms Mediate Opposing Proliferative Effects through Gbetagamma-Activated P38 or Akt Pathways. Mol. Cell Biol. 2000;20:5974–5985. doi: 10.1128/MCB.20.16.5974-5985.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Luttrell L.M., Ferguson S.S., Daaka Y., Miller W.E., Maudsley S., Della Rocca G.J., Lin F., Kawakatsu H., Owada K., Luttrell D.K., et al. Beta-Arrestin-Dependent Formation of Beta2 Adrenergic Receptor-Src Protein Kinase Complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 267.Miller W.E., Maudsley S., Ahn S., Khan K.D., Luttrell L.M., Lefkowitz R.J. Beta-Arrestin1 Interacts with the Catalytic Domain of the Tyrosine Kinase c-SRC. Role of Beta-Arrestin1-Dependent Targeting of c-SRC in Receptor Endocytosis. J. Biol. Chem. 2000;275:11312–11319. doi: 10.1074/jbc.275.15.11312. [DOI] [PubMed] [Google Scholar]
- 268.Krupnick J.G., Goodman O.B., Keen J.H., Benovic J.L. Arrestin/Clathrin Interaction. Localization of the Clathrin Binding Domain of Nonvisual Arrestins to the Carboxy Terminus. J. Biol. Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- 269.Gurevich V.V., Gurevich E.V. Arrestins: Critical Players in Trafficking of Many GPCRs. Prog Mol. Biol. Transl. Sci. 2015;132:1–14. doi: 10.1016/bs.pmbts.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shenoy S.K., Lefkowitz R.J. Multifaceted Roles of Beta-Arrestins in the Regulation of Seven-Membrane-Spanning Receptor Trafficking and Signalling. Biochem. J. 2003;375:503–515. doi: 10.1042/bj20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Oakley R.H., Laporte S.A., Holt J.A., Caron M.G., Barak L.S. Differential Affinities of Visual Arrestin, ΒArrestin1, and ΒArrestin2 for G Protein-Coupled Receptors Delineate Two Major Classes of Receptors. J. Biol. Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 272.Miller W.E., Lefkowitz R.J. Expanding Roles for Beta-Arrestins as Scaffolds and Adapters in GPCR Signaling and Trafficking. Curr. Opin. Cell Biol. 2001;13:139–145. doi: 10.1016/S0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 273.Shenoy S.K., Lefkowitz R.J. Seven-Transmembrane Receptor Signaling through Beta-Arrestin. Sci. STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 274.Zhai P., Yamamoto M., Galeotti J., Liu J., Masurekar M., Thaisz J., Irie K., Holle E., Yu X., Kupershmidt S., et al. Cardiac-Specific Overexpression of AT1 Receptor Mutant Lacking G Aq/Gαi Causes Hypertrophy and Bradycardia in Transgenic Mice. J. Clin. Investig. 2005;115:3045–3056. doi: 10.1172/JCI25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Beaulieu J.-M., Sotnikova T.D., Marion S., Lefkowitz R.J., Gainetdinov R.R., Caron M.G. An Akt/Beta-Arrestin 2/PP2A Signaling Complex Mediates Dopaminergic Neurotransmission and Behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 276.McDonald P.H., Chow C.-W., Miller W.E., Laporte S.A., Field M.E., Lin F.-T., Davis R.J., Lefkowitz R.J. β-Arrestin 2: A Receptor-Regulated MAPK Scaffold for the Activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 277.Luttrell L.M., Roudabush F.L., Choy E.W., Miller W.E., Field M.E., Pierce K.L., Lefkowitz R.J. Activation and Targeting of Extracellular Signal-Regulated Kinases by β-Arrestin Scaffolds. Proc. Natl. Acad. Sci. USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Perry S.J., Baillie G.S., Kohout T.A., McPhee I., Magiera M.M., Ang K.L., Miller W.E., McLean A.J., Conti M., Houslay M.D., et al. Targeting of Cyclic AMP Degradation to Β2-Ad.drenergic Receptors by β-Arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 279.Abrisqueta M., Herraiz C., Pérez Oliva A.B., Sanchez-Laorden B.L., Olivares C., Jiménez-Cervantes C., García-Borrón J.C. Differential and Competitive Regulation of Human Melanocortin 1 Receptor Signaling by β-Arrestin Isoforms. J. Cell Sci. 2013;126:3724–3737. doi: 10.1242/jcs.128322. [DOI] [PubMed] [Google Scholar]
- 280.Martínez-Vicente I., Abrisqueta M., Herraiz C., Jiménez-Cervantes C., García-Borrón J.C., Olivares C. Functional Characterization of a C-Terminal Splice Variant of the Human Melanocortin 1 Receptor. Exp. Derm. 2020;29:610–615. doi: 10.1111/exd.14118. [DOI] [PubMed] [Google Scholar]
- 281.Abreu N., Acosta-Ruiz A., Xiang G., Levitz J. Mechanisms of Differential Desensitization of Metabotropic Glutamate Receptors. Cell Rep. 2021;35:109050. doi: 10.1016/j.celrep.2021.109050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ceraudo E., Horioka M., Mattheisen J.M., Hitchman T.D., Moore A.R., Kazmi M.A., Chi P., Chen Y., Sakmar T.P., Huber T. Direct Evidence That the GPCR CysLTR2 Mutant Causative of Uveal Melanoma Is Constitutively Active with Highly Biased Signaling. J. Biol. Chem. 2021;296:100163. doi: 10.1074/jbc.RA120.015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bhullar K.S., Lagarón N.O., McGowan E.M., Parmar I., Jha A., Hubbard B.P., Rupasinghe H.P.V. Kinase-Targeted Cancer Therapies: Progress, Challenges and Future Directions. Mol. Cancer. 2018;17:48. doi: 10.1186/s12943-018-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Menichetti R., Kanekal K.H., Bereau T. Drug–Membrane Permeability across Chemical Space. ACS Cent. Sci. 2019;5:290–298. doi: 10.1021/acscentsci.8b00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sharma A., Vaghasiya K., Ray E., Verma R.K. Lysosomal Targeting Strategies for Design and Delivery of Bioactive for Therapeutic Interventions. J. Drug Target. 2018;26:208–221. doi: 10.1080/1061186X.2017.1374390. [DOI] [PubMed] [Google Scholar]
- 286.Liu D., Schilling B., Liu D., Sucker A., Livingstone E., Jerby-Arnon L., Zimmer L., Gutzmer R., Satzger I., Loquai C., et al. Integrative Molecular and Clinical Modeling of Clinical Ou.utcomes to PD1 Blockade in Patients with Metastatic Melanoma. Nat. Med. 2019;25:1916–1927. doi: 10.1038/s41591-019-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Campbell A.P., Smrcka A.V. Targeting G Protein-Coupled Receptor Signalling by Blocking G Proteins. Nat. Rev. Drug Discov. 2018;17:789–803. doi: 10.1038/nrd.2018.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chidiac P., Hebert T.E., Valiquette M., Dennis M., Bouvier M. Inverse Agonist Activity of Beta-Adrenergic Antagonists. Mol. Pharm. 1994;45:490–499. [PubMed] [Google Scholar]
- 289.de Ligt R.A.F., Kourounakis A.P., IJzerman A.P. Inverse Agonism at G Protein-Coupled Receptors: (Patho)Physiological Relevance and Implications for Drug Discovery. Br. J. Pharm. 2000;130:1–12. doi: 10.1038/sj.bjp.0703311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Pozvek G., Hilton J.M., Quiza M., Houssami S., Sexton P.M. Structure/Function Relationships of Calcitonin Analogues as Agonists, Antagonists, or Inverse Agonists in a Constitutively Activated Receptor Cell System. Mol. Pharm. 1997;51:658–665. doi: 10.1124/mol.51.4.658. [DOI] [PubMed] [Google Scholar]
- 291.Wu V., Yeerna H., Nohata N., Chiou J., Harismendy O., Raimondi F., Inoue A., Russell R.B., Tamayo P., Gutkind J.S. Illuminating the Onco-GPCRome: Novel G Protein–Coupled Receptor-Driven Oncocrine Networks and Targets for Cancer Immunotherapy. J. Biol. Chem. 2019;294:11062–11086. doi: 10.1074/jbc.REV119.005601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Smith M.P., Rowling E.J., Miskolczi Z., Ferguson J., Spoerri L., Haass N.K., Sloss O., McEntegart S., Arozarena I., von Kriegsheim A., et al. Targeting Endothelin Receptor Signalling Overcomes Heterogeneity Driven Therapy Failure. EMBO Mol. Med. 2017;9:1011–1029. doi: 10.15252/emmm.201607156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ben-Baruch A. Site-Specific Metastasis Formation. Cell Adh. Migr. 2009;3:328–333. doi: 10.4161/cam.3.4.9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chen W., Hoffmann A.D., Liu H., Liu X. Organotropism: New Insights into Molecular Mechanisms of Breast Cancer Metastasis. NPJ Precis. Onc. 2018;2:4. doi: 10.1038/s41698-018-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteom. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Forrest A.R.R., Kawaji H., Rehli M., Kenneth Baillie J., de Hoon M.J.L., Haberle V., Lassmann T., Kulakovskiy I.V., Lizio M., Itoh M., et al. A Promoter-Level Mammalian Expression Atlas. Nature. 2014;507:462–470. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Melé M., Ferreira P.G., Reverter F., DeLuca D.S., Monlong J., Sammeth M., Young T.R., Goldmann J.M., Pervouchine D.D., Sullivan T.J., et al. Human Genomics. The Human Transcriptome across Tissues and Individuals. Science. 2015;348:660–665. doi: 10.1126/science.aaa0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pierson E., the GTEx Consortium. Koller D., Battle A., Mostafavi S. Sharing and Specificity of Co-Expression Networks across 35 Human Tissues. PLoS Comput. Biol. 2015;11:e1004220. doi: 10.1371/journal.pcbi.1004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jo M., Jung S.T. Engineering Therapeutic Antibodies Targeting G-Protein–Coupled Receptors. Exp. Mol. Med. 2016;48:e207. doi: 10.1038/emm.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Latorraca N.R., Venkatakrishnan A.J., Dror R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2017;117:139–155. doi: 10.1021/acs.chemrev.6b00177. [DOI] [PubMed] [Google Scholar]
- 301.Pauwels J., Fijałkowska D., Eyckerman S., Gevaert K. Mass Spectrometry and the Cellular Surfaceome. Mass Spectrom. Rev. 2021 doi: 10.1002/mas.21690. in press . [DOI] [PubMed] [Google Scholar]
- 302.Alhosaini K., Azhar A., Alonazi A., Al-Zoghaibi F. GPCRs: The Most Promiscuous Druggable Receptor of the Mankind. Saudi Pharm J. 2021;29:539–551. doi: 10.1016/j.jsps.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kuhlmann L., Cummins E., Samudio I., Kislinger T. Cell-Surface Proteomics for the Identification of Novel Therapeutic Targets in Cancer. Expert Rev. Proteom. 2018;15:259–275. doi: 10.1080/14789450.2018.1429924. [DOI] [PubMed] [Google Scholar]
- 304.Sun F., Suttapitugsakul S., Wu R. Unraveling the Surface Glycoprotein Interaction Network by Integrating Chemical Crosslinking with MS-Based Proteomics. Chem. Sci. 2021;12:2146–2155. doi: 10.1039/D0SC06327D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Qin S., Meng M., Yang D., Bai W., Lu Y., Peng Y., Song G., Wu Y., Zhou Q., Zhao S., et al. High-Throughput Identification of G Protein-Coupled Receptor Modulators through Affinity Mass Spectrometry Screening. Chem. Sci. 2018;9:3192–3199. doi: 10.1039/C7SC04698G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Crotty S., Pipkin M.E. In Vivo RNAi Screens: Concepts and Applications. Trends Immunol. 2015;36:315–322. doi: 10.1016/j.it.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Driever W., Solnica-Krezel L., Schier A.F., Neuhauss S.C., Malicki J., Stemple D.L., Stainier D.Y., Zwartkruis F., Abdelilah S., Rangini Z., et al. A Genetic Screen for Mutations Affecting Embryogenesis in Zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- 308.Haffter P., Granato M., Brand M., Mullins M.C., Hammerschmidt M., Kane D.A., Odenthal J., van Eeden F.J., Jiang Y.J., Heisenberg C.P., et al. The Identification of Genes with Unique and Essential Functions in the Development of the Zebrafish, Danio Rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- 309.Keatinge M., Tsarouchas T.M., Munir T., Porter N.J., Larraz J., Gianni D., Tsai H.-H., Becker C.G., Lyons D.A., Becker T. CRISPR GRNA Phenotypic Screening in Zebrafish Reveals Pro-Regenerative Genes in Spinal Cord Injury. PLoS Genet. 2021;17:e1009515. doi: 10.1371/journal.pgen.1009515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Trubiroha A., Gillotay P., Giusti N., Gacquer D., Libert F., Lefort A., Haerlingen B., De Deken X., Opitz R., Costagliola S. A Rapid CRISPR/Cas-Based Mutagenesis Assay in Zebrafish for Identification of Genes Involved in Thyroid Morphogenesis and Function. Sci Rep. 2018;8:5647. doi: 10.1038/s41598-018-24036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., Collins J.E., Humphray S., McLaren K., Matthews L., et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Langenhan T., Barr M.M., Bruchas M.R., Ewer J., Griffith L.C., Maiellaro I., Taghert P.H., White B.H., Monk K.R. Model Organisms in G Protein–Coupled Receptor Research. Mol. Pharm. 2015;88:596–603. doi: 10.1124/mol.115.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Frantz W.T., Ceol C.J. From Tank to Treatment: Modeling Melanoma in Zebrafish. Cells. 2020;9:1289. doi: 10.3390/cells9051289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Patton E.E., Mueller K.L., Adams D.J., Anandasabapathy N., Aplin A.E., Bertolotto C., Bosenberg M., Ceol C.J., Burd C.E., Chi P., et al. Melanoma Models for the next Generation of Therapies. Cancer Cell. 2021;39:610–631. doi: 10.1016/j.ccell.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Manzotti C., Audisio R.A., Pratesi G. Importance of Orthotopic Implantation for Human Tumors as Model Systems: Relevance to Metastasis and Invasion. Clin. Exp. Metastasis. 1993;11:5–14. doi: 10.1007/BF00880061. [DOI] [PubMed] [Google Scholar]
- 316.Lee Y., Basith S., Choi S. Recent Advances in Structure-Based Drug Design Targeting Class A G Protein-Coupled Receptors Utilizing Crystal Structures and Computational Simulations. J. Med. Chem. 2018;61:1–46. doi: 10.1021/acs.jmedchem.6b01453. [DOI] [PubMed] [Google Scholar]
- 317.García-Nafría J., Tate C.G. Cryo-Electron Microscopy: Moving Beyond X-Ray Crystal Structures for Drug Receptors and Drug Development. Annu. Rev. Pharm. Toxicol. 2020;60:51–71. doi: 10.1146/annurev-pharmtox-010919-023545. [DOI] [PubMed] [Google Scholar]
- 318.Okada T., Le Trong I., Fox B.A., Behnke C.A., Stenkamp R.E., Palczewski K. X-ray Diffraction Analysis of Three-Dimensional Crystals of Bovine Rhodopsin Obtained from Mixed Micelles. J. Struct. Biol. 2000;130:73–80. doi: 10.1006/jsbi.1999.4209. [DOI] [PubMed] [Google Scholar]
- 319.Tate C.G., Schertler G.F.X. Engineering G Protein-Coupled Receptors to Facilitate Their Structure Determination. Curr. Opin. Struct. Biol. 2009;19:386–395. doi: 10.1016/j.sbi.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 320.Lebon G., Bennett K., Jazayeri A., Tate C.G. Thermostabilisation of an Agonist-Bound Conformation of the Human Adenosine A(2A) Receptor. J. Mol. Biol. 2011;409:298–310. doi: 10.1016/j.jmb.2011.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rosenbaum D.M., Cherezov V., Hanson M.A., Rasmussen S.G.F., Thian F.S., Kobilka T.S., Choi H.-J., Yao X.-J., Weis W.I., Stevens R.C., et al. GPCR Engineering Yields High-Resolution Structural Insights into Beta2-Adrenergic Receptor Function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 322.Tate C.G. Practical Considerations of Membrane Protein Instability during Purification and Crystallisation. Methods Mol. Biol. 2010;601:187–203. doi: 10.1007/978-1-60761-344-2_12. [DOI] [PubMed] [Google Scholar]
- 323.Ishchenko A., Stauch B., Han G.W., Batyuk A., Shiriaeva A., Li C., Zatsepin N., Weierstall U., Liu W., Nango E., et al. Toward G Protein-Coupled Receptor Structure-Based Drug Design Using X-Ray Lasers. IUCrJ. 2019;6:1106–1119. doi: 10.1107/S2052252519013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Liu H., Lee W. The XFEL Protein Crystallography: Developments and Perspectives. Int. J. Mol. Sci. 2019;20:3421. doi: 10.3390/ijms20143421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.McMullan G., Faruqi A.R., Henderson R. Direct Electron Detectors. Methods Enzym. 2016;579:1–17. doi: 10.1016/bs.mie.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 326.Vinothkumar K.R., Henderson R. Single Particle Electron Cryomicroscopy: Trends, Issues and Future Perspective. Q. Rev. Biophys. 2016;49:e13. doi: 10.1017/S0033583516000068. [DOI] [PubMed] [Google Scholar]
- 327.Zivanov J., Nakane T., Forsberg B.O., Kimanius D., Hagen W.J., Lindahl E., Scheres S.H. New Tools for Automated High-Resolution Cryo-EM Structure Determination in RELION-3. Elife. 2018;7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Congreve M., de Graaf C., Swain N.A., Tate C.G. Impact of GPCR Structures on Drug Discovery. Cell. 2020;181:81–91. doi: 10.1016/j.cell.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 329.Danev R., Belousoff M., Liang Y.-L., Zhang X., Eisenstein F., Wootten D., Sexton P.M. Routine Sub-2.5 Å Cryo-EM Structure Determination of GPCRs. Nat. Commun. 2021;12:4333. doi: 10.1038/s41467-021-24650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang M., Gui M., Wang Z.-F., Gorgulla C., Yu J.J., Wu H., Sun Z.J., Klenk C., Merklinger L., Morstein L., et al. Cryo-EM Structure of an Activated GPCR-G Protein Complex in Lipid Nanodiscs. Nat. Struct. Mol. Biol. 2021;28:258–267. doi: 10.1038/s41594-020-00554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Shimada I., Ueda T., Kofuku Y., Eddy M.T., Wüthrich K. GPCR Drug Discovery: Integrating Solution NMR Data with Crystal and Cryo-EM Structures. Nat. Rev. Drug Discov. 2019;18:59–82. doi: 10.1038/nrd.2018.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Baek M., DiMaio F., Anishchenko I., Dauparas J., Ovchinnikov S., Lee G.R., Wang J., Cong Q., Kinch L.N., Schaeffer R.D., et al. Accurate Prediction of Protein Structures and Interactions Using a Three-Track Neural Network. Science. 2021;373:871–876. doi: 10.1126/science.abj8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tunyasuvunakool K., Adler J., Wu Z., Green T., Zielinski M., Žídek A., Bridgland A., Cowie A., Meyer C., Laydon A., et al. Highly Accurate Protein Structure Prediction for the Human Proteome. Nature. 2021;596:590–596. doi: 10.1038/s41586-021-03828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Bender B.J., Marlow B., Meiler J. Improving Homology Modeling from Low-Sequence Identity Templates in Rosetta: A Case Study in GPCRs. PLoS Comput. Biol. 2020;16:e1007597. doi: 10.1371/journal.pcbi.1007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Esguerra M., Siretskiy A., Bello X., Sallander J., Gutiérrez-de-Terán H. GPCR-ModSim: A Comprehensive Web Based Solution for Modeling G-Protein Coupled Receptors. Nucleic Acids Res. 2016;44:W455–W462. doi: 10.1093/nar/gkw403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Worth C.L., Kreuchwig F., Tiemann J.K.S., Kreuchwig A., Ritschel M., Kleinau G., Hildebrand P.W., Krause G. GPCR-SSFE 2.0-a Fragment-Based Molecular Modeling Web Tool for Class A G-Protein Coupled Receptors. Nucleic Acids Res. 2017;45:W408–W415. doi: 10.1093/nar/gkx399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zhang J., Yang J., Jang R., Zhang Y. GPCR-I-TASSER: A Hybrid Approach to G Protein-Coupled Receptor Structure Modeling and the Application to the Human Genome. Structure. 2015;23:1538–1549. doi: 10.1016/j.str.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mullard A. What Does AlphaFold Mean for Drug Discovery? Nat. Rev. Drug Discov. 2021;20:725–727. doi: 10.1038/d41573-021-00161-0. [DOI] [PubMed] [Google Scholar]
- 340.Potterton A., Heifetz A., Townsend-Nicholson A. Synergistic Use of GPCR Modeling and SDM Experiments to Understand Ligand Binding. In: Heifetz A., editor. Computational Methods for GPCR Drug Discovery. Springer; New York, NY, USA: 2018. pp. 335–343. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 341.Zhou Q., Yang D., Wu M., Guo Y., Guo W., Zhong L., Cai X., Dai A., Jang W., Shakhnovich E.I., et al. Common Activation Mechanism of Class A GPCRs. eLife. 2019;8:e50279. doi: 10.7554/eLife.50279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Di Roberto R.B., Chang B., Peisajovich S.G. The Directed Evolution of Ligand Specificity in a GPCR and the Unequal Contributions of Efficacy and Affinity. Sci Rep. 2017;7:16012. doi: 10.1038/s41598-017-16332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hughes J., Rees S., Kalindjian S., Philpott K. Principles of Early Drug Discovery. Br. J. Pharm. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.von Ahsen O., Bömer U. High-Throughput Screening for Kinase Inhibitors. ChemBioChem. 2005;6:481–490. doi: 10.1002/cbic.200400211. [DOI] [PubMed] [Google Scholar]
- 345.Jones A.J.Y., Gabriel F., Tandale A., Nietlispach D. Structure and Dynamics of GPCRs in Lipid Membranes: Physical Principles and Experimental Approaches. Molecules. 2020;25:4729. doi: 10.3390/molecules25204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kitaeva K.V., Rutland C.S., Rizvanov A.A., Solovyeva V.V. Cell Culture Based in Vitro Test Systems for Anticancer Drug Screening. Front. Bioeng. Biotechnol. 2020;8:322. doi: 10.3389/fbioe.2020.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.González N., Mantey S.A., Pradhan T.K., Sancho V., Moody T.W., Coy D.H., Jensen R.T. Characterization of Putative GRP- and NMB-Receptor Antagonist’s Interaction with Human Receptors. Peptides. 2009;30:1473–1486. doi: 10.1016/j.peptides.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Uehara H., González N., Sancho V., Mantey S.A., Nuche-Berenguer B., Pradhan T., Coy D.H., Jensen R.T. Pharmacology and Selectivity of Various Natural and Synthetic Bombesin Related Peptide Agonists for Human and Rat Bombesin Receptors Differs. Peptides. 2011;32:1685–1699. doi: 10.1016/j.peptides.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zhang R., Xie X. Tools for GPCR Drug Discovery. Acta Pharm. Sin. 2012;33:372–384. doi: 10.1038/aps.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Maurel D., Comps-Agrar L., Brock C., Rives M.-L., Bourrier E., Ayoub M.A., Bazin H., Tinel N., Durroux T., Prézeau L., et al. Cell-Surface Protein-Protein Interaction Analysis with Time-Resolved FRET and Snap-Tag Technologies: Application to GPCR Oligomerization. Nat. Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Valencia C., Dujet C., Margathe J.-F., Iturrioz X., Roux T., Trinquet E., Villa P., Hibert M., Dupuis E., Llorens-Cortes C., et al. A Time-Resolved FRET Cell-Based Binding Assay for the Apelin Receptor. ChemMedChem. 2017;12:925–931. doi: 10.1002/cmdc.201700106. [DOI] [PubMed] [Google Scholar]
- 352.Titus S., Neumann S., Zheng W., Southall N., Michael S., Klumpp C., Yasgar A., Shinn P., Thomas C.J., Inglese J., et al. Quantitative High-Throughput Screening Using a Live-Cell CAMP Assay Identifies Small-Molecule Agonists of the TSH Receptor. J. Biomol. Screen. 2008;13:120–127. doi: 10.1177/1087057107313786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Trinquet E., Bouhelal R., Dietz M. Monitoring Gq-Coupled Receptor Response through Inositol Phosphate Quantification with the IP-One Assay. Expert Opin. Drug Discov. 2011;6:981–994. doi: 10.1517/17460441.2011.608658. [DOI] [PubMed] [Google Scholar]
- 354.Katsuya K., Hori Y., Oikawa D., Yamamoto T., Umetani K., Urashima T., Kinoshita T., Ayukawa K., Tokunaga F., Tamaru M. High-Throughput Screening for Linear Ubiquitin Chain Assembly Complex (LUBAC) Selective Inhibitors Using Homogenous Time-Resolved Fluorescence (HTRF)-Based Assay System. SLAS Discov. 2018;23:1018–1029. doi: 10.1177/2472555218793066. [DOI] [PubMed] [Google Scholar]
- 355.Lotta L.A., Mokrosiński J., Mendes de Oliveira E., Li C., Sharp S.J., Luan J., Brouwers B., Ayinampudi V., Bowker N., Kerrison N., et al. Human Gain-of-Function MC4R Variants Show Signaling Bias and Protect against Obesity. Cell. 2019;177:597–607.e9. doi: 10.1016/j.cell.2019.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zindel D., Vol C., Lecha O., Bequignon I., Bilgic M., Vereecke M., Charrier-Savournin F., Romier M., Trinquet E., Pin J.-P., et al. HTRF® Total and Phospho-YAP (Ser127) Cellular Assays. In: Hergovich A., editor. The Hippo Pathway: Methods and Protocols. Springer; New York, NY, USA: 2019. pp. 153–166. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 357.Liu L., Jockers R. Structure-Based Virtual Screening Accelerates GPCR Drug Discovery. Trends Pharmacol. Sci. 2020;41:382–384. doi: 10.1016/j.tips.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 358.Shoichet B.K., Kobilka B.K. Structure-Based Drug Screening for G Protein-Coupled Receptors. Trends Pharm. Sci. 2012;33:268–272. doi: 10.1016/j.tips.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Stein R.M., Kang H.J., McCorvy J.D., Glatfelter G.C., Jones A.J., Che T., Slocum S., Huang X.-P., Savych O., Moroz Y.S., et al. Virtual Discovery of Melatonin Receptor Ligands to Modulate Circadian Rhythms. Nature. 2020;579:609–614. doi: 10.1038/s41586-020-2027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ebalunode J.O., Zheng W., Tropsha A. Application of QSAR and Shape Pharmacophore Modeling Approaches for Targeted Chemical Library Design. In: Zhou J.Z., editor. Chemical Library Design. Humana Press; Totowa, NJ, USA: 2011. pp. 111–133. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 361.Green H., Koes D.R., Durrant J.D. DeepFrag: A Deep Convolutional Neural Network for Fragment-Based Lead Optimization. Chem. Sci. 2021;12:8036–8047. doi: 10.1039/D1SC00163A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Neves B.J., Braga R.C., Melo-Filho C.C., Moreira-Filho J.T., Muratov E.N., Andrade C.H. QSAR-Based Virtual Screening: Advances and Applications in Drug Discovery. Front. Pharm. 2018;9:1275. doi: 10.3389/fphar.2018.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Chung T.D.Y., Terry D.B., Smith L.H. In Vitro and In Vivo Assessment of ADME and PK Properties During Lead Selection and Lead Optimization—Guidelines, Benchmarks and Rules of Thumb. In: Markossian S., Grossman A., Brimacombe K., Arkin M., Auld D., Austin C.P., Baell J., Chung T.D.Y., Coussens N.P., Dahlin J.L., et al., editors. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences; Bethesda, MD, USA: 2004. [PubMed] [Google Scholar]
- 364.Guan L., Yang H., Cai Y., Sun L., Di P., Li W., Liu G., Tang Y. ADMET-Score—A Comprehensive Scoring Function for Evaluation of Chemical Drug-Likeness. Med. Chem. Commun. 2018;10:148–157. doi: 10.1039/C8MD00472B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Akil H., Quintana M., Raymond J.H., Billoux T., Benboubker V., Besse S., Auzeloux P., Delmas V., Petit V., Larue L., et al. Efficacy of Targeted Radionuclide Therapy Using [131I]ICF01012 in 3D Pigmented BRAF- and NRAS-Mutant Melanoma Models and In Vivo NRAS-Mutant Melanoma. Cancers. 2021;13:1421. doi: 10.3390/cancers13061421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Norain A., Dadachova E. Targeted Radionuclide Therapy of Melanoma. Semin. Nucl. Med. 2016;46:250–259. doi: 10.1053/j.semnuclmed.2015.12.005. [DOI] [PubMed] [Google Scholar]