Abstract
Fibroblast growth factors (FGFs) play important roles in various growth signaling processes, including proliferation, development, and differentiation. Endocrine FGFs, i.e., atypical FGFs, including FGF15/19, FGF21, and FGF23, function as endocrine hormones that regulate energy metabolism. Nutritional status is known to regulate the expression of endocrine FGFs through nuclear hormone receptors. The increased expression of endocrine FGFs regulates energy metabolism processes, such as fatty acid metabolism and glucose metabolism. Recently, a relationship was found between the FGF19 subfamily and stress signaling during stresses such as endoplasmic reticulum stress and oxidative stress. This review focuses on endocrine FGFs and the recent progress in FGF studies in relation to stress signaling. In addition, the relevance of the stress–FGF pathway to disease and human health is discussed.
Keywords: FGF15/19, FGF21, FGF23, endocrine FGFs, stress signaling, ER stress, oxidative stress
1. Introduction
Fibroblast growth factors (FGFs) are polypeptides that regulate various physiological functions, including growth, differentiation, development, wound healing, and energy metabolism [1,2]. FGFs activate intracellular signaling pathways by binding to cell-surface FGF receptors (FGFRs). FGFs contain a homologous core region of 120–130 amino acids arranged into 12 antiparallel β-strands, flanked by divergent amino- and carboxyl-termini. Sequence variation of the amino- and carboxyl-terminal tails usually accounts for the different biological functions of FGFs [3,4]. To date, twenty-two FGFs and four FGFRs have been identified in mammals. In invertebrates, two (egl-17 and let-756) and three genes (branchless, pyramus, and thisbe) for FGFs have been reported in Caenorhabditis elegans and Drosophila melanogaster, respectively. These genes encode polypeptides with a core region similar to mammalian FGFs. FGFRs are also found in Caenorhabditis elegans (egl-15) and Drosophila melanogaster (breathless and heartless), suggesting that FGF/FGFR signaling is evolutionarily conserved [5,6,7,8,9]. In mammals, FGFs can be divided into seven subfamilies (FGF1, 4, 7, 8, 9, 11, and 19 subfamilies) based on their biological functions, sequence homology, and evolutionary relationships. Five subfamilies (FGF1, 4, 7, 8, and 9) are the autocrine/paracrine FGFs, which are mainly important for multiple developmental processes. The FGF11 subfamily is the intracellular FGFs. Unlike other FGF subfamilies, these FGFs are not secreted, but interact with cytosolic proteins to regulate intracellular signaling, such as ion channels. The FGF19 subfamily (FGF15/19, FGF21, and FGF23) is the endocrine FGF. In mammals, human FGF15 and mouse FGF19 are lacking, as human FGF19 and mouse FGF15 are orthologs based on comparative genomics (in this review, we describe them as “FGF15/19”). Most FGF members have a high affinity for heparan sulfate glycosaminoglycans in the extracellular matrix, which enables them to act in an autocrine and a paracrine manner. Unlike conventional FGFs, endocrine FGFs (i.e., FGF15/19, FGF21, and FGF23) have a weak affinity for heparan sulfate glycosaminoglycans; therefore, they require a single-pass transmembrane protein, Klotho (αKlotho, βKlotho, and lactase-like), as a coreceptor to allow binding to FGFRs. Upon binding to FGFs, FGFRs are activated by dimerization and autophosphorylation; subsequently, cytosolic substrates of FGFRs, such as the FGFR substrate 2α and mitogen-activated protein kinases, are activated [1]. αKlotho functions as a coreceptor for FGF23, whereas βKlotho serves as a coreceptor for both FGF15/19 and FGF21 [10,11,12,13,14,15]. Although FGFRs are broadly expressed, the expression of Klotho occurs only in specific tissues [16]. This allows endocrine FGFs to enter circulation and function as hormones. The biological functions of FGFs are classically considered to be related to development and differentiation. Recent studies, however, have found that FGF15/19 and FGF21 are important regulators of nutrient and energy metabolism, whereas FGF23 is vital for phosphate and vitamin D homeostasis [17,18]. Furthermore, endocrine FGFs may play certain roles in metabolic disorders; hence, these polypeptides have become attractive target molecules for the development of therapies [19].
The number of patients with metabolic abnormalities, such as obesity, diabetes mellitus, and hypertension, continues to increase worldwide. These disorders, which are caused by an imbalance in energy intake and expenditure, as well as an unhealthy lifestyle (e.g., a western-style diet and physical inactivity), are major risk factors for serious disease, including cardiovascular and cerebrovascular diseases. Therefore, lifestyle modification is important to prevent metabolic abnormalities [20,21]. In addition, the intake of functional food factors, such as bioactive food-derived molecules, is an attractive approach to preventing metabolic disorders [22,23].
Adaptation to a multitude of environmental stresses is essential for the survival of multicellular organisms. When cells are exposed to stress, intracellular stress signaling is activated to prevent stress-induced damage and to maintain cellular homeostasis. Physical and chemical stresses can include endoplasmic reticulum (ER) stress and oxidative stress. It is now known that endocrine FGFs play an important role in stress signaling as well as energy metabolism. In this review, we summarize the role of endocrine FGFs in the regulation of energy metabolism, and we detail their contribution to various stress signaling processes. In addition, we discuss how the stress–FGF pathway is relevant to disease and human health.
2. Endocrine FGFs
2.1. FGF15/19
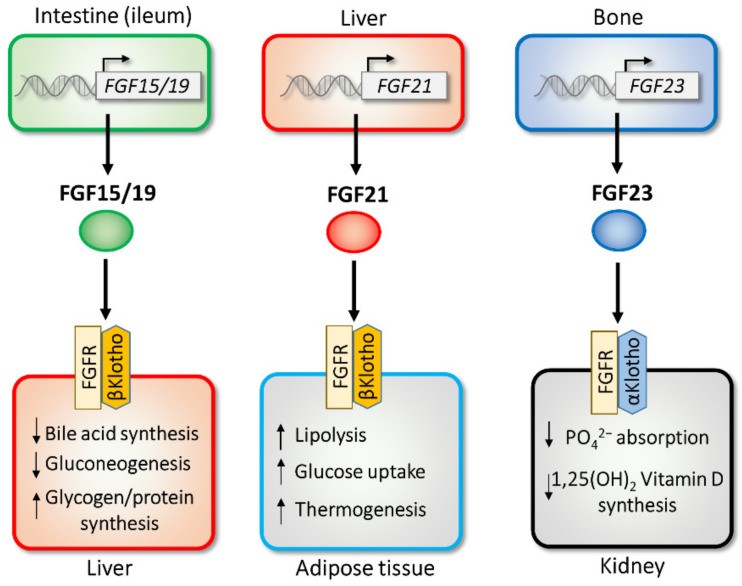
Mouse FGF15 was initially found to be expressed in the developing nervous system [24], and human FGF19 was discovered following a homology-based search [25]. Although the amino acid sequences of mouse FGF15 and human FGF19 are dissimilar, comparative genomics has shown that their genes are orthologs [26]; thus, the two are referred to as “FGF15/19”. The primary source of FGF15/19 is the ileum, a distal part of the small intestine that absorbs bile acids [16]. Once released from the ileum, FGF15/19 travels to target tissues, including the liver (Figure 1). For binding to FGF receptors, FGF15/19 requires the presence of another transmembrane protein, βKlotho [11,27]. In the liver, FGF15/19 strongly represses the expression of Cyp7a1, a rate-limiting enzyme involved in bile acid biosynthesis, which helps maintain postprandial bile acid homeostasis [28,29]. This negative feedback regulation is important, as bile acid has strong and toxic detergent properties. FGF15/19 also regulates other postprandial responses, including the inhibition of gluconeogenesis, activation of glycogen and protein synthesis [30,31], and gallbladder filling [32]. The regulation of bile acid by FGF15/19 is dependent on βKlotho [33,34]. Interestingly, FGF15/19 and insulin (a representative postprandial hormone) share several postprandial effects, e.g., the induction of protein and glycogen synthesis [30]. Although plasma insulin levels are quickly elevated by feeding (within 1 h), the peak level of FGF15/19 in serum is achieved around 3 h after a meal [35], suggesting that FGF15/19 has a late-phase postprandial effect.
Figure 1.
Endocrine functions of FGF15/19, FGF21, and FGF23. FGF15/19, FGF21, and FGF23 are expressed in intestine, liver, and bone, respectively. Secreted FGFs selectively act on the target tissues through FGF receptor (FGFR)/Klotho receptor complexes.
Feeding predominantly regulates the transcription of FGF15/19. Upon feeding, bile (containing bile acids) is released into the small intestine, which leads to the induction of FGF15/19 expression via the nuclear bile acid receptor, farnesoid X receptor (FXR) [28,29,36]; this in turn regulates the transcription of target genes through interactions with the retinoid X receptor as a heterodimer. Other nuclear hormone receptors that are activated by bile acids, e.g., a vitamin D receptor, VDR [37] and a xenobiotic receptor pregnane X receptor [38], also regulate FGF15/19, indicating that they play an important role in bile acid metabolism. Bile acid is synthesized from cholesterol in the liver; sterol regulatory response element-binding protein 2, a master regulator of cholesterol synthesis, negatively regulates FGF15/19 expression through interactions with FXR [39]. Although studies have reported various regulation processes for FGF15/19 transcription, little is known about the mechanism of FGF15/19 secretion. A recent study found that a natural genetic variant of the human DIET1 gene increases the secretion of FGF15/19 in vitro [40,41].
2.2. FGF21
FGF21 was identified as a homolog of FGF15/19 [42], which is also a metabolic regulator in adipocytes [43]. FGF21 activates glucose uptake by upregulating the transcription of the glucose transporter GLUT1. FGF21 is predominantly expressed in the liver and weakly expressed in white and brown adipose tissue [16,44]. Almost all of the circulating FGF21 in plasma is considered to be derived from the liver [45,46,47]. FGF21 produced in adipose tissue can function in an autocrine or a paracrine manner [48,49,50]. Released FGF21 works on target tissues, including adipose tissues, where the coreceptor βKlotho is expressed [11,51,52,53,54] (Figure 1). In adipose tissue, FGF21 stimulates a thermogenic response through the regulation of the uncoupling protein 1 and deiodinase-2 genes [52,55], and it activates peroxisome proliferator-activated receptor γ (PPARγ), a nuclear hormone receptor, by preventing its sumoylation. Such posttranslational regulation by FGF21 is important for the insulin-sensitizing effects of the drug thiazolidinedione, which is a chemical ligand for PPARγ [56]. Recent studies have also revealed that FGF21 can stimulate sympathetic nerve activity [57,58]. Interestingly, this nervous system activity is essential for the effects of FGF21 on energy metabolism [53,59]. FGF21 improves energy metabolism through multiple mechanisms. In adipose tissue, FGF21 activates fatty acid oxidation by upregulating lipolytic enzymes, hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) [52,53,59,60]. FGF21 also activates nuclear fatty acid receptor PPARγ, a target of insulin sensitizer thiazolidinediones (TZDs), in adipose tissue. FGF21 prevents the sumoylation of PPARγ, resulting in an improvement in insulin sensitivity [56,61]. FGF21 reduces fatty liver by activating fatty acid β oxidation and reducing the expression of lipogenic genes [52,54,62,63]. FGF21 also regulates important fasting responses, including gluconeogenesis and ketone body synthesis [60,64,65,66]. During fasting, FGF21 induces the hepatic expression of peroxisome proliferator-activated receptor coactivator protein-1α (PGC-1α) to activate gluconeogenesis, and it increase ketogenesis by the induction of ketogenic enzymes hydroxymethylglutaryl-CoA synthase 2 (HMGCS2) and carnitine palmitoyl transferase 1a (CPT1a). The other important roles of FGF21 include the regulation of growth [67], longevity [68], and pancreatic proteostasis [69]. The transgenic expression of FGF21 reduces size and growth by reducing growth hormone (GH) and the insulin-like growth factor-1 (IGF-1) signaling pathway through the decrease in the phosphorylation of signal transducer and activator of transcription 5 (STAT5) [67]. The other phenotype of FGF21 transgenic mice is an extension of lifespan through GH and IGF-1 signaling [68]. In an exocrine pancreas, where FGF21 is highly expressed, FGF21 functions as a digestive enzyme secretagogue and maintains proteostasis during the postprandial state [69].
The expression of hepatic FGF21 is strongly induced by fasting, which is the most well-known regulator of its transcription [60,65,70]. During fasting, free fatty acids travel from white adipose tissue (WAT) to the liver, where the nuclear fatty acid receptor PPARα activates the promoter activity of the FGF21 gene by binding with peroxisome proliferator response elements comprising a direct repeat-1 element [60,65,70]. Other transcription factors that are activated during fasting, such as the glucocorticoid receptor (GR) [71,72] and cAMP responsive element-binding protein H (CREBH) [73,74], also regulate FGF21 expression.
In addition to transcriptional regulation, it has been reported that circulating FGF21 protein has a short half-life [51,75,76]. Fibroblast activation protein (FAP), a serine protease, cleaves proteolytically and inactivates FGF21 [77,78,79,80]. As FAP protein is detected in human plasma, the pharmacological inhibition of FAP could be a therapeutic drug target for metabolic diseases, such as obesity and diabetes.
2.3. FGF23
The FGF23 gene was first identified in a mutated form in patients with autosomal dominant hypophosphatemic rickets (ADHR) [81]. FGF23 is highly expressed in osteocytes and osteoblasts; however, it is weakly expressed in the brain and thymus [16,82]. FGF23 is proteolytically processed to generate inactive fragments; however, some patients express FGF23 polypeptides which are resistant to proteolysis [81]. Moreover, excess circulating FGF23 can lead to hypophosphatemia and tumor-induced osteomalacia [83]. FGF23 expression is regulated by vitamin D and dietary phosphate [84,85,86,87]. Transgenic mice overexpressing FGF23 exhibit hypophosphatemia, a decrease in circulating 1,25-dihydroxy vitamin D, and an increase in the renal release of phosphate [88,89,90]. FGF23-null mice and αKlotho-knockout mice share a similar phenotype, which includes increased levels of 1α-hydroxylase, an enzyme that functions in the production of active vitamin D [91,92,93]. This shared phenotype led to the association of the FGF23–αKlotho pathway. αKlotho is a type I transmembrane protein, and αKlotho-knockout mice exhibit an aging phenotype [93]. In contrast to FGF15/19 and FGF21, FGF23 requires αKlotho, which is predominantly expressed in the kidney, to bind to FGFR [10,12,94] (Figure 1). The FGF23–αKlotho pathway regulates phosphate excretion in the kidney and reduces the synthesis of vitamin D and parathyroid hormone (PTH). As an excess amount of circulating FGF23 is associated with ADHR/osteomalacia, several drug discovery studies have targeted FGF23 [19]. Currently, an antibody against FGF23 (burosumab) is used for patients with FGF23-related ADHR/osteomalacia, such as those with X-linked hypophosphatemia [95,96].
Vitamin D is a major regulator of FGF23 transcription [87]. Indeed, 1α,25-dihydroxyvitamin D, an active form of vitamin D that is synthesized by 25-hydroxyvitamin D-1α-hydroxylase and expressed in the kidney, induces FGF23 expression [97,98]. FGF23 induction by 1α,25-dihydroxyvitamin D is mediated by the nuclear vitamin D receptor VDR [99,100]. PTH, a target molecule of the FGF23–αKlotho pathway, also regulates FGF23 transcription through cAMP-dependent protein kinase A (PKA) and the Wnt pathway [101,102]. The PKA signal increases the mRNA expression of orphan nuclear receptor Nurr1, and then Nurr1 activates FGF23 transcription [103]. In rickets model mice (Hyp, DMP1 knockout, and PHEX mutant), circulating FGF23 levels are substantially increased [104,105,106,107,108]. In these models, the activation of the nuclear factor of activated T-cells (NFAT) contributes to FGF23 induction [109,110].
3. Stress Signaling and Endocrine FGFs
3.1. ER Stress
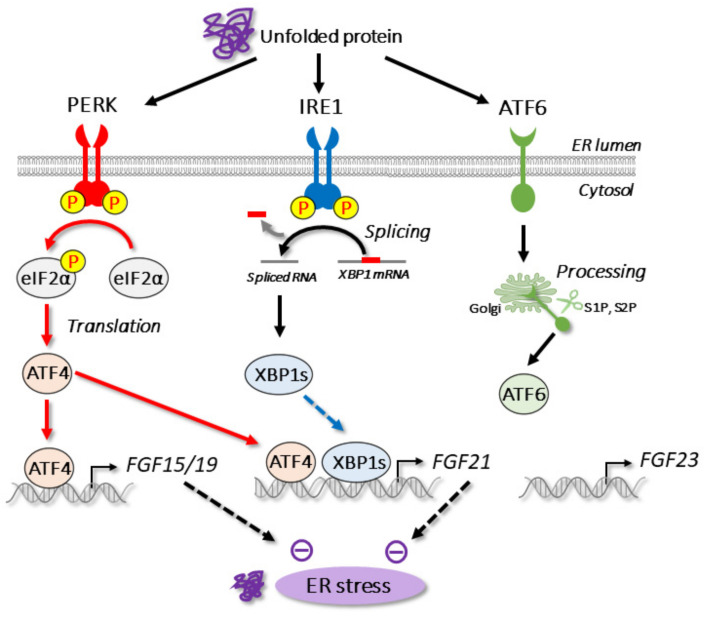
The ER is the subcellular organelle responsible for many cellular functions, including the folding and maturation of proteins, the synthesis of lipids, and the regulation of calcium storage in eukaryotic cells. To maintain proper protein folding in the ER, various ER-resident molecular chaperones assist with ER quality control. BiP/GRP78 (immunoglobulin heavy chain binding protein) is the major molecular chaperone in the ER; it is an important regulator of the unfolded protein response pathway. When ER homeostasis is dysregulated by pathological and pharmacological conditions, unfolded or misfolded proteins accumulate in the ER lumen; this is referred to as ER stress. Upon ER stress, three branches of unfolded protein response (UPR) are activated to restore ER homeostasis. In mammals, the UPR pathway adjusts the protein folding capacity in the ER by activating three ER-resident transmembrane sensors, namely, inositol requiring enzyme 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6) [111] (Figure 2). IRE1, a type I transmembrane protein, was first identified as a UPR transducer in yeast [112,113]. Mammals express two IRE1 homologs: IRE1α and IRE1β [114,115]. IRE1α is ubiquitously expressed and functions as an ER stress transducer, whereas the expression of IRE1β is restricted to the gut. Upon ER stress, IRE1 is oligomerized to activate its RNase domain. IRE1 cleaves a substrate mRNA from Hac1 in yeast and XBP1 in mammals, respectively. After unconventional splicing by IRE1, the mature mRNA of Hac1 and XBP1 is translated to produce a basic leucine zipper-type transcriptional factor. PERK, another UPR transducer, is also a type I transmembrane protein that resembles IRE1 [116,117,118]. In the presence of ER stress, PERK is oligomerized for the activation of autophosphorylation. In contrast to IRE1, active PERK phosphorylates the α subunit of eukaryotic translation initiation factor 2 (eIF2α), which leads to the inhibition of global translation and protein loading to the ER. In contrast, PERK selectively activates the translation of mRNA that encodes ATF4, a basic leucine zipper-type transcriptional factor. ATF4 regulates the expression of genes related to amino acid metabolism and antioxidative responses [119]. ATF6, a type II transmembrane protein, is another UPR transducer. In mammals, ATF6 has two subtypes (ATF6α and ATF6β) that are ubiquitously expressed. Upon ER stress, ATF6 transits from the ER to the Golgi, where it receives two-step proteolysis by site-1 and site-2 proteases [120]. This cleavage results in the release of the N-terminal transcription factor domain (ATF6-N). In the nucleus, ATF6-N regulates transcription related to ER chaperones and ER-associated degradation [121].
Figure 2.
ER stress and endocrine FGFs. Upon ER stress, three branches (PERK, IRE1, and ATF6) are activated to maintain ER homeostasis. Expression of FGF15/19 and FGF21 genes, but not FGF23 gene, is regulated by ER stress.
Several studies have revealed that the transcriptional regulation of endocrine FGFs occurs with ER stress (Figure 2, Table 1). In addition to bile acids, ER stress is also a regulator of FGF15/19 transcription [122]. FGF15/19 is a direct target gene of ATF4, which is stimulated by ER stress. ATF4 is also known to bind to the FGF15/19 promoter through an amino acid response element (AARE) upon ER stress. As ER stress is triggered by high concentrations of bile acids [123,124], the ATF4–FGF15/19 pathway may have a function in preventing the toxicity that can be induced by excess bile acids. In addition to ER stress, ATF4 activation is regulated by various stress signaling pathways, such as oxidative stress and amino acid deprivation [111]. We previously found that FGF15/19 is selectively regulated by ER stress; however, it is not regulated by other ATF4 stimuli [125].
Table 1.
Regulation of endocrine FGFs by stress signaling.
| FGFs | Regulator | Stimuli | Refs |
|---|---|---|---|
| FGF15/19 | ATF4 | ER stress | [122] |
| ATF4 1 | Oxidative stress | [125] | |
| ATF4 1 | Amino acid deprivation | [125] | |
| FGF21 | ATF4 | ER stress | [125,126,127] |
| XBP-1 | ER stress | [128] | |
| ATF4 | Oxidative stress | [125,126] | |
| ATF4 | Amino acid deprivation | [125,129,130,131,132,133] | |
| NRF2 2 | Oxidative stress | [134,135,136,137,138] | |
| ATF4 | Mitochondrial stress | [130,139] | |
| ATF2 | Cold stress | [55,140] | |
| PPARα | Fasting | [60,65,70] | |
| GR | Fasting | [71,72] | |
| CREBH | Fasting | [73,74] | |
| ChREBP | High carbohydrate | [141,142,143,144,145,146,147] | |
| PPARγ | Obesity/feeding | [56,148,149] | |
| FGF23 | - | - | - |
1 Only in vitro. 2 Both positive and negative regulations have been reported.
In addition to FGF15/19, ATF4 is reportedly an important regulator of FGF21 transcription [125,126,127,129,130]. Interestingly, the FGF21 promoter contains three AAREs, which provide potent induction by ER stress [150]. We found that FGF21 expression is induced by ER stress, oxidative stress, and amino acid deprivation, whereas FGF15/19 expression is ER stress selective [125]. Another ER stress-activated transcription factor, X box–binding protein-1 (XBP-1), has also been reported to regulate FGF21 expression; however, further research is required in this area as the binding element of XBP-1 is not conserved among species and XBP-1 failed to activate the human FGF21 promoter [150].
In a hepatocellular carcinoma cell line, FGF15/19 reduces ER stress through the activation of the antioxidative transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) [151]. The overexpression of FGF15/19 increases the phosphorylation of glycogen synthase kinase-3β (GSK3β), leading to the inhibition of the proteasomal degradation of NRF2.
In contrast to FGF15/19, several studies have described that secreted FGF21 inhibits ER stress [128,152,153,154]. FGF21 alleviates drug-induced ER stress through MAP kinase [128,154]. FGF21 also reduced ER stress-induced steatosis [128]. ER stress is also triggered by physiological conditions, such as the postprandial state and a secretagogue response in the pancreas [155,156,157]. We previously reported that FGF21 overexpression by adenovirus is effective in reducing refeeding-induced ER stress [152]. In skeletal muscle, although the basal expression of FGF21 is low [16], FGF21 expression is strongly induced by the forced activation of PERK; this can help prevent obesity [158].
Unlike FGF15/19 and FGF21, the regulation of FGF23 by ER stress has not been reported. We observed that two ER stress inducers, tunicamycin and thapsigargin, fail to increase the gene expression of FGF23 in osteoblasts (Shimizu et al., unpublished observation). Thus, ER stress seems to selectively regulate FGF15/19 and FGF21 but not FGF23 (Figure 2).
3.2. Oxidative Stress
Oxidative stress is triggered by the disruption of the balance between the production of reactive oxygen species and antioxidants. Excess oxidative stress causes oxidative damage to cellular components, including proteins, DNA, and lipids.
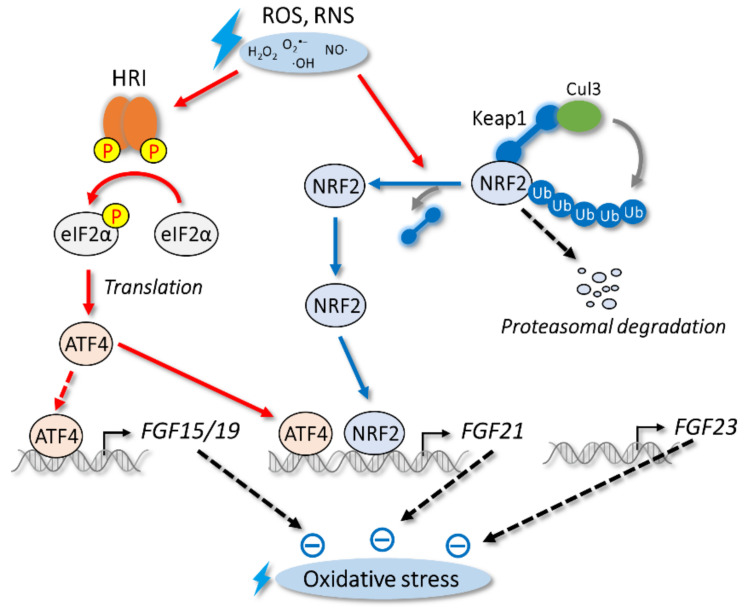
NRF2 is a key transcriptional regulator of antioxidant responses; it regulates the expression of the phase II detoxifying enzyme and antioxidant-responsive genes by binding to antioxidant-responsive elements that are present in the promoter regions of target genes [159,160]. Under unstressed conditions, NRF2 interacts with Kelch-like ECH-associated protein 1 (Keap1), an actin-binding cytoplasmic protein, to repress NRF2 activity through degradation by cullin 3 (Cul3) ubiquitin E3 ligase [161]. However, oxidative stress triggers the oxidation of the cysteine residues of Keap1, which leads to its conformational change and the liberation of NRF2 to the nucleus for the induction of its target genes (Figure 3).
Figure 3.
Oxidative stress and endocrine FGFs. HRI–ATF4 and Keap1–NRF2 pathways are activated in response to oxidative stress. Expression of FGF15/19 and FGF21 genes, but not FGF23 gene, is regulated by oxidative stress.
ATF4 is also a transcriptional regulator of oxidative stress. Unlike ER stress, oxidative stress increases ATF4 translation through the activation of heme-regulated inhibitor (HRI), another eIF2α kinase. HRI is also stimulated by various stresses, including heme deprivation and iron deficiency [162]. Similar to PERK, HRI is activated by its autophosphorylation, which leads to the phosphorylation of eIF2α and an increase in ATF4 translation (Figure 3).
FGF15/19 expression is increased by oxidative stress in intestinal cell lines; however, oxidative stress fails to increase FGF15/19 expression in vivo, whereas other ATF4 target genes are induced [125] (Figure 3, Table 1). As HRI and ATF4 are expressed in the intestine, the mechanism of FGF15/19 selective regulation is currently unclear. In human hepatocytes that express FGF15/19, oxidative stress increases the expression of FGF15/19, suggesting a conserved regulation by oxidative stress at least in vitro [151]. During the postprandial state, secreted FGF15/19 increases the phosphorylation of GSK3β, an inactive form of GSK3β, which stimulates glycogen synthesis [30]. Interestingly, the stability of the NRF2 protein is regulated by GSK3β [163,164]. Consistent with this phenomenon, FGF15/19 activates the NRF2 pathway by inactivating GSK3β in hepatocytes and cardiomyocytes, which helps protect cells and tissues [151,165,166] (Figure 3).
In contrast to FGF15/19, oxidative stress increases the expression of FGF21 both in vitro and in vivo [125,126] (Figure 3, Table 1). Although ATF4 induces FGF21 expression, several studies have reported negative regulation by NRF2 [134,136,137,138]. NRF2 is also reported to activate FGF21 expression in diabetes [135]. Several studies have found that oxidative stress is reduced by FGF21 [167,168,169,170] (Figure 3). In human umbilical vein endothelial cells, FGF21 prevents hydrogen peroxide-induced oxidative damage and cytotoxicity by affecting stress-responsive kinases, including p38 MAP kinase and JNK [170]. In the liver, FGF21 reduces acetaminophen-induced oxidative stress through an increase in NRF2 expression. A transcriptional coactivator, PGC-1α [167], mediates NRF2 induction by FGF21. The reduction in oxidative stress due to this pathway is lost in FGF21-knockout mice, which indicates the physiological importance of this pathway. In cardiomyocytes, FGF21 prevents cardiac hypertrophy by reducing oxidative stress [168]. FGF21 also induces the expression of antioxidant proteins, including superoxide dismutase (SOD) 2 and uncoupling protein 3, but such effects are not observed in FGF21-knockout mice or following treatment with FGF21 antibody. Circulating FGF21 levels are known to be increased in patients with rheumatoid arthritis [171]. When FGF21 is administrated to rheumatoid arthritis model mice, the levels of some antioxidant proteins, including SODs, increase, which in turn reduces oxidative stress and inflammation [169].
Although the regulation of FGF23 expression by oxidative stress has not been reported, FGF23 is known to activate NRF2 signaling in osteoblasts [172]. Dexamethasone (DEX), a synthetic glucocorticoid, is used for patients with a chronic inflammatory disease. DEX is known to induce reactive oxygen species in osteoblasts [173,174], and DEX-induced osteoporosis is a major side effect [175]. The treatment of osteoblasts with FGF23 increases NRF2 protein levels through the FGFR1–Akt pathway and reduces oxidative stress, which in turn protects against DEX-induced cytotoxicity [172] (Figure 3). Interestingly, the FGFR1–Akt pathway can be activated in the absence of αKlotho [156]. As the medical use of NRF2 activators at high concentrations is limited due to side effects, FGF23 is an attractive target for therapies.
3.3. Mitochondrial Stress
The mitochondria, an organelle with a double membrane and unique circular DNA, has many important functions, including ATP synthesis. Mitochondrial dysfunction caused by metabolic changes within mitochondria and the disruption of mitochondrial quality control results in mitochondrial stress. Unlike that of ER stress, the precise mechanism of the mitochondrial stress response is not well characterized. However, recent findings indicate that mitochondrial stress affects several metabolic pathways and diseases [176].
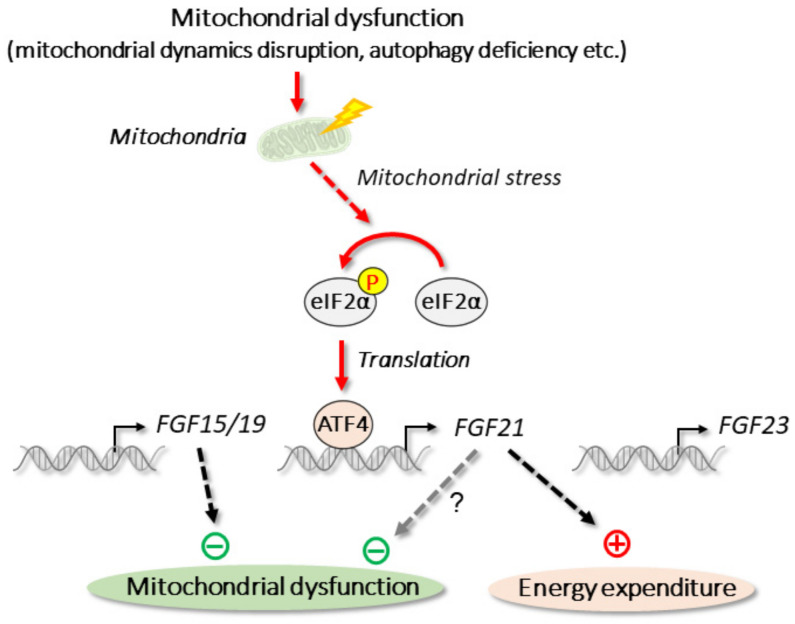
The basal expression of FGF21 is low in skeletal muscle [16], but FGF21 is now recognized as a myokine, i.e., a protein produced and released from muscle fibers [177,178]. Mitochondrial dynamics (mitochondrial fusion and fission) are important for maintaining mitochondrial function. The deficiency of optic atrophy 1 (OPA1), an essential protein for mitochondrial fusion, causes the potent induction of FGF21 in skeletal muscle [139]. The ablation of OPA1 in skeletal muscle causes mitochondrial stress response, which increases the expression of ATF4 and FGF21 (Figure 4). In OPA1/FGF21 double-knockout mice, muscle atrophy caused by OPA1 deficiency is partially recovered. Thus, skeletal muscle-derived FGF21 apparently functions in an autocrine manner. Although βKlotho can be detected in skeletal muscle [139], it is expressed at low levels [16]. Recombinant FGF21 treatment fails to increase the phosphorylation of ERK1/2, a target of FGFR substrate 2 (FRS2), which is activated by FGF21 signaling, in skeletal muscle [13]. Thus, further studies may be required to confirm the precise functional mechanism of FGF21 in skeletal muscle. The disruption of autophagy-related gene 7 (ATG7), an important factor for autophagosome expansion and completion, in skeletal muscle results in autophagy deficiency. The resultant mitochondrial stress, as well as the inhibition of the mitochondrial respiratory chain, leads to the induction of FGF21 through the eIF2α–ATF4 pathway [130]. This skeletal muscle-specific deletion of ATG7 increases energy expenditure and prevents diet-induced obesity and the amelioration of insulin resistance by the activation of lipolysis and the browning of white adipose tissue. Both ATF7 and FGF21 deficiency diminish these metabolic changes, indicating their physiological importance. FGF21 induction by mitochondrial stress is also observed in other mitochondrial dysfunctions, including mitochondrial myopathy and mutations of mitochondrial DNA [179,180]. Therefore, mitochondrial stress appears to induce FGF21 expression to protect against metabolic abnormalities. Although FGF21 induction contributes to muscle atrophy and production of inflammatory cytokines [139], FGF21 activates mitophagy to degrade dysfunctional mitochondria [181]. Thus, further studies are required to fully understand the function of FGF21 on mitochondrial dysfunctions (Figure 4).
Figure 4.
Mitochondrial stress and endocrine FGFs. Mitochondrial stress is triggered by mitochondrial dysfunctions or by deficiency of mitochondrial dynamics-related gene OPA1 or autophagy-related gene ATG7. In skeletal muscle, mitochondrial stress induces FGF21 through eIF2α–ATF4 pathway.
Unlike FGF21, the regulation of FGF15/19 and FGF23 by mitochondrial stress has not been reported. FGF15/19 is, however, known to alleviate mitochondrial dysfunction through the AMPK–PGC-1α pathway [182] (Figure 4).
3.4. Thermal (Cold) Stress
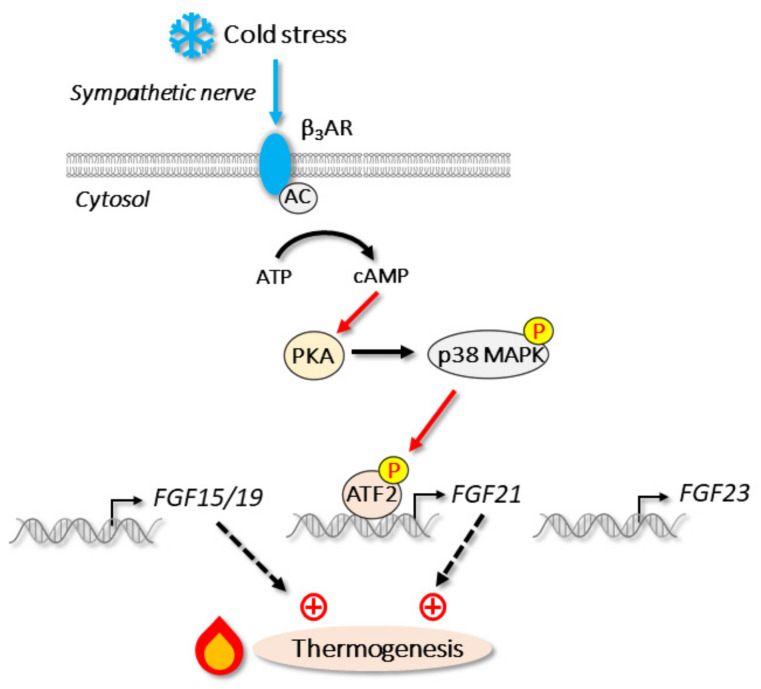
In humans, the thermoregulation system maintains the core body temperature at around 37 °C. Whereas white adipose tissue stores chemical energy as triglycerides, brown adipose tissue is a specialized tissue that dissipates chemical energy to produce heat in a process known as nonshivering thermogenesis [183,184]. In addition to brown adipose tissue, other types of thermogenic adipocytes, termed beige or brite adipocytes, are known to exist. In response to acute cold stress, uncoupling protein 1 (UCP1), the mitochondrial uncoupling protein, is potently induced in both brown adipose tissue and beige adipocytes by a set of transcription factors, including PGC-1α and ATF2 [183]. UCP1 uncouples electron transport from ATP synthesis by dissipating the mitochondrial proton motive force (Δp) and thereby increases thermogenesis. As brown adipose tissue and beige adipocytes consume triglycerides, these tissues may be attractive targets for the treatment of obesity and type 2 diabetes [184].
FGF21, but not FGF15/19 or FGF23, is expressed in white adipose tissue and brown adipose tissue [16]. Upon cold stress, FGF21 is strongly induced in these tissues independent of PPARα, a key regulator of hepatic FGF21 [55,140]. Instead, p38 MAPK-mediated ATF2 activation is important for FGF21 induction during cold stress [55] (Figure 5). Interestingly, G protein-coupled receptor 120, which is activated by long chain fatty acids, stimulates the release of FGF21 from adipocytes [185]. The levels of circulating FGF21 are almost abolished by the liver-specific deletion of FGF21, whereas they are unchanged by adipose-specific deficiency. Thus, FGF21 produced in brown adipose tissue and white adipose tissue is thought to function in an autocrine and a paracrine fashion but not an endocrine fashion, unlike that produced in the liver [49,56]. Chronic FGF21 treatment activates the thermogenic response of adipocytes [49,186] at least partly through the increase in thermogenic coactivator PGC-1α protein levels and the subsequent increase in UCP1 expression [49]. This thermogenic activation by FGF21 is also observed in human neck-derived primary adipocytes [187]. Thus, FGF21 is a cold stress-induced adipokine, and it activates the thermogenic response to protect against further cold stress (Figure 5). Although the regulation of FGF15/19 by cold stress has not been reported, circulating FGF15/19 levels positively correlate with UCP1 expression [188]. The overexpression of FGF15/19 induces the expression of thermogenic genes, including UCP1 and PGC-1α in subcutaneous WAT, whereas FGF15/19 deficiency prevents this induction. Thus, FGF15/19 activates the thermogenic response through a browning of WAT (Figure 5).
Figure 5.
Cold stress and endocrine FGFs: upon cold stress, β3-adrenergic receptor (β3AR) stimulates the production of second messenger cAMP through adenylate cyclase (AC). Subsequent pathway actives p38 MAPK and transcription factor ATF2, which induces FGF21 expression during cold stress.
3.5. Nutrient Stress
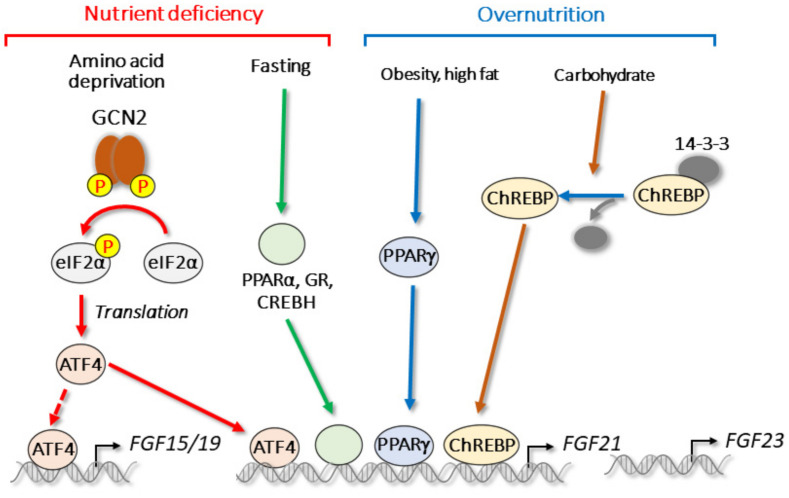
The expression of FGF15/19 and FGF21 is observed in tissues that are important for nutrient sensing, including the intestine, liver, and adipose tissue [16]. In these tissues, signaling pathways for nutrient stress are activated in response to severe nutritional states, including nutrient deficiency or overnutrition. Nutrient deficiency, fasting, or over nutrition elicit nutritional stress signals and compensatory survival mechanisms. FGF15/19 and FGF21 are important responsive genes for feeding or fasting [29,60,65]. During fasting, the expression of FGF21 is regulated by several transcription factors, including PPARα, GR, and CREBH [60,65,70,71,72,73,74]. In addition, the overconsumption or deficiency of each major macronutrient (e.g., amino acids, lipids, and carbohydrates) triggers nutrient stress signaling (Figure 6).
Figure 6.
Nutrient stress and endocrine FGFs. Both nutrient deficiency and overnutrition regulate endocrine FGFs.
Amino acid deprivation or protein restriction are known to activate transcription factor ATF4. Unlike during ER stress and oxidative stress, general control non-derepressible 2 (GCN2) phosphorylates eIF2α, leading to an increase in ATF4 translation [189]. Although both ATF4 and GCN2 are expressed in the intestine, we did not observe a significant change in FGF15/19 expression under a leucine-deficient diet [125]. In contrast, hepatic FGF21 is reportedly induced upon amino acid deprivation and protein restriction both in vitro and in vivo [125,129,132,133]. During amino acid or protein restriction, induced FGF21 reduces the size of adipocytes through the activation of lipolysis, and it activates thermogenesis through the induction of UCP1 in brown adipose tissue [132,190]. We previously reported that FGF21 expression is induced by β-conglycinin, a soy protein [191]; the administration of β-conglycinin to mice results in a methionine imbalance in the portal vein, which in turn activates the ATF4–FGF21 pathway. FGF21 deficiency prevents β-conglycinin-induced improvements in energy metabolism, including the reduction in body weight gain and adipose tissue weight.
In addition to the effects of feeding and bile acids, FGF15/19 expression is increased by saturated fatty acids, which cause lipotoxicity and ER stress [122,192,193]. In obese patients, circulating FGF15/19 is known to be decreased [192,194]. Furthermore, FGF15/19 prevents hepatic steatosis and reduces hepatic ER stress in high-fat-diet fed mice [192,195,196].
In contrast to the effect of nutrient deficiency [60,65,70,71,72,73,74], FGF21 expression is increased by overnutrition. For example, under a high-carbohydrate diet, hepatic FGF21 is strongly induced through the activation of carbohydrate response element-binding protein (ChREBP) [141,142,143,144,145,146,147]. The overexpression of ChREBP induces FGF21 expression and improves glucose tolerance and plasma triglyceride, despite the occurrence of fatty liver [197,198]. FGF21 can also help decrease sugar intake and preference [199,200,201], suggesting that a negative feedback loop regulates sugar consumption via the ChREBP–FGF21 pathway. The expression of hepatic FGF21 is also increased in obese mice, such as ob/ob mice, and under high-fat diet and fatty liver conditions [44,70,202,203,204,205,206,207,208,209], in which FGF21 improves energy metabolism. In contrast to the liver, FGF21 in WAT is induced by feeding, which is likely mediated by PPARγ [56,148,149]. The expression of FGF21 in WAT is also increased during obesity [148] when adipose PPARγ is activated to promote adipogenesis and lipid accumulation [210]. Thiazolidinediones (TZD), an antidiabetic PPARγ ligand, have been reported to improve insulin sensitivity through FGF21 induction [56]. FGF21 increases PPARγ activity through the prevention of its sumoylation. The effects of FGF21 on WAT are likely mediated in an autocrine or paracrine fashion, but not via endocrine action [45,56].
4. Conclusions
Several studies have indicated that endocrine FGFs, especially FGF15/19 and FGF21, are attractive therapeutic target molecules for the treatment of metabolic disorders. Both FGF15/19 and FGF21 activate energy expenditure and reduce body weight gain despite their transcriptional regulator and tissue expression patterns being different. Cellular stress and energy metabolism are closely related. For example, ER stress not only disrupts ER homeostasis due to protein folding, but also has effects on obesity and type 2 diabetes [211]; moreover, reducing ER stress improves energy metabolism [212]. An imbalance between oxidants and antioxidant systems can lead to a variety of diseases, including type 2 diabetes and atherosclerosis. Among the endocrine FGFs, FGF15/19 and FGF21 are selectively responsive to stress signaling. In particular, FGF21 is described as a “stress hormone” as it is strongly induced by various stress signals [213]. ATF4 is likely a key regulator of FGF15/19 and FGF21 during stress signaling. Endocrine FGFs have protective effects against cellular stresses in addition to improving energy metabolism. As both FGF15/19 and FGF21 are target genes of ATF4, it would be interesting to develop an ATF4 activator as an inducer of FGF15/19 and FGF21 expression. The soy protein β-conglycinin is a good example of an ATF4 activator that can prevent metabolic disorders through the induction of FGF21 without ER stress [191]. In conclusion, endocrine FGFs, especially FGF15/19 and FGF21, play important roles in stress signaling. However, further studies on the role of stress–endocrine FGF pathways are required to evaluate potential therapeutic targets for stress toxicity and metabolic disorders.
Author Contributions
Writing—original draft preparation: M.S. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by JSPS KAKENHI (JP25504003 and JP16H06195 to M.S., JP15H05781 to R.S.), research grants from Fuji Foundation for Protein Research (R.S.), and Japanese Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP Project ID 14533567 to R.S.), and Japanese Agency for Medical Research and Development Grant (16gm0910008h0001 to R.S.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beenken A., Mohammadi M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh N., Ornitz D.M. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadi M., Olsen S.K., Ibrahimi O.A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Phan P., Saikia B.B., Sonnaila S., Agrawal S., Alraawi Z., Kumar T.K.S., Iyer S. The Saga of Endocrine FGFs. Cells. 2021;10:2418. doi: 10.3390/cells10092418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdine R.D., Chen E.B., Kwok S.F., Stern M.J. egl-17 encodes an invertebrate fibroblast growth factor family member required specifically for sex myoblast migration in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1997;94:2433–2437. doi: 10.1073/pnas.94.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roubin R., Naert K., Popovici C., Vatcher G., Coulier F., Thierry-Mieg J., Pontarotti P., Birnbaum D., Baillie D., Thierry-Mieg D. let-756, a C. elegans fgf essential for worm development. Oncogene. 1999;18:6741–6747. doi: 10.1038/sj.onc.1203074. [DOI] [PubMed] [Google Scholar]
- 7.Muha V., Müller H.A. Functions and Mechanisms of Fibroblast Growth Factor (FGF) Signalling in Drosophila melanogaster. Int. J. Mol. Sci. 2013;14:5920–5937. doi: 10.3390/ijms14035920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stathopoulos A., Tam B., Ronshaugen M., Frasch M., Levine M. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 2004;18:687–699. doi: 10.1101/gad.1166404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland D., Samakovlis C., Krasnow M.A. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/S0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- 10.Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K.P., Baum M.G., Schiavi S., Hu M.C., Moe O.W., et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurosu H., Choi M., Ogawa Y., Dickson A.S., Goetz R., Eliseenkova A.V., Mohammadi M., Rosenblatt K.P., Kliewer S.A., Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., Fujita T., Fukumoto S., Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa Y., Kurosu H., Yamamoto M., Nandi A., Rosenblatt K.P., Goetz R., Eliseenkova A.V., Mohammadi M., Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. USA. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharitonenkov A., Dunbar J.D., Bina H.A., Bright S., Moyers J.S., Zhang C., Ding L., Micanovic R., Mehrbod S.F., Knierman M.D., et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J. Cell. Physiol. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 15.Wu X., Ge H., Gupte J., Weiszmann J., Shimamoto G., Stevens J., Hawkins N., Lemon B., Shen W., Xu J., et al. Co-receptor requirements for fibroblast growth factor-19 signaling. J. Biol. Chem. 2007;282:29069–29072. doi: 10.1074/jbc.C700130200. [DOI] [PubMed] [Google Scholar]
- 16.Fon Tacer K., Bookout A.L., Ding X., Kurosu H., John G.B., Wang L., Goetz R., Mohammadi M., Kuro-o M., Mangelsdorf D.J., et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurosu H., Kuro-O M. Endocrine fibroblast growth factors as regulators of metabolic homeostasis. Biofactors. 2009;35:52–60. doi: 10.1002/biof.12. [DOI] [PubMed] [Google Scholar]
- 18.Potthoff M.J., Kliewer S.A., Mangelsdorf D.J. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degirolamo C., Sabbà C., Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat. Rev. Drug Discov. 2016;15:51–69. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- 20.Takahara M., Shimomura I. Metabolic syndrome and lifestyle modification. Rev. Endocr. Metab. Disord. 2014;15:317–327. doi: 10.1007/s11154-014-9294-8. [DOI] [PubMed] [Google Scholar]
- 21.Yamaoka K., Tango T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med. 2012;10:138. doi: 10.1186/1741-7015-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu M., Cao Y., Xiao J., Song M., Ho C.T. Molecular mechanisms of the anti-obesity effect of bioactive ingredients in common spices: A review. Food Funct. 2018;9:4569–4581. doi: 10.1039/C8FO01349G. [DOI] [PubMed] [Google Scholar]
- 23.Goto T. A review of the studies on food-derived factors which regulate energy metabolism via the modulation of lipid-sensing nuclear receptors. Biosci. Biotechnol. Biochem. 2019;83:579–588. doi: 10.1080/09168451.2018.1559025. [DOI] [PubMed] [Google Scholar]
- 24.McWhirter J.R., Goulding M., Weiner J.A., Chun J., Murre C. A novel fibroblast growth factor gene expressed in the developing nervous system is a downstream target of the chimeric homeodomain oncoprotein E2A-Pbx1. Development. 1997;124:3221–3232. doi: 10.1242/dev.124.17.3221. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura T., Utsunomiya Y., Hoshikawa M., Ohuchi H., Itoh N. Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim. Biophys. Acta. 1999;1444:148–151. doi: 10.1016/S0167-4781(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 26.Katoh M. Evolutionary conservation of CCND1-ORAOV1-FGF19-FGF4 locus from zebrafish to human. Int. J. Mol. Med. 2003;12:45–50. doi: 10.3892/ijmm.12.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Lin B.C., Wang M., Blackmore C., Desnoyers L.R. Liver-specific activities of FGF19 require Klotho beta. J. Biol. Chem. 2007;282:27277–27284. doi: 10.1074/jbc.M704244200. [DOI] [PubMed] [Google Scholar]
- 28.Holt J.A., Luo G., Billin A.N., Bisi J., McNeill Y.Y., Kozarsky K.F., Donahee M., Wang D.Y., Mansfield T.A., Kliewer S.A., et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G., Luo G., Jones S.A., Goodwin B., Richardson J.A., et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Kir S., Beddow S.A., Samuel V.T., Miller P., Previs S.F., Suino-Powell K., Xu H.E., Shulman G.I., Kliewer S.A., Mangelsdorf D.J. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potthoff M.J., Boney-Montoya J., Choi M., He T., Sunny N.E., Satapati S., Suino-Powell K., Xu H.E., Gerard R.D., Finck B.N., et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi M., Moschetta A., Bookout A.L., Peng L., Umetani M., Holmstrom S.R., Suino-Powell K., Xu H.E., Richardson J.A., Gerard R.D., et al. Identification of a hormonal basis for gallbladder filling. Nat. Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 33.Tomiyama K., Maeda R., Urakawa I., Yamazaki Y., Tanaka T., Ito S., Nabeshima Y., Tomita T., Odori S., Hosoda K., et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc. Natl. Acad. Sci. USA. 2010;107:1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito S., Fujimori T., Furuya A., Satoh J., Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J. Clin. Investig. 2005;115:2202–2208. doi: 10.1172/JCI23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundåsen T., Gälman C., Angelin B., Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim I., Ahn S.H., Inagaki T., Choi M., Ito S., Guo G.L., Kliewer S.A., Gonzalez F.J. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt D.R., Holmstrom S.R., Fon Tacer K., Bookout A.L., Kliewer S.A., Mangelsdorf D.J. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J. Biol. Chem. 2010;285:14486–14494. doi: 10.1074/jbc.M110.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wistuba W., Gnewuch C., Liebisch G., Schmitz G., Langmann T. Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by PXR. World J. Gastroenterol. 2007;13:4230–4235. doi: 10.3748/wjg.v13.i31.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyata M., Hata T., Yamazoe Y., Yoshinari K. SREBP-2 negatively regulates FXR-dependent transcription of FGF19 in human intestinal cells. Biochem. Biophys. Res. Commun. 2014;443:477–482. doi: 10.1016/j.bbrc.2013.11.126. [DOI] [PubMed] [Google Scholar]
- 40.Lee J.M., Ong J.R., Vergnes L., de Aguiar Vallim T.Q., Nolan J., Cantor R.M., Walters J.R.F., Reue K. Diet1, bile acid diarrhea, and FGF15/19: Mouse model and human genetic variants. J. Lipid Res. 2018;59:429–438. doi: 10.1194/jlr.M078279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vergnes L., Lee J.M., Chin R.G., Auwerx J., Reue K. Diet1 functions in the FGF15/19 enterohepatic signaling axis to modulate bile acid and lipid levels. Cell Metab. 2013;17:916–928. doi: 10.1016/j.cmet.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura T., Nakatake Y., Konishi M., Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta. 2000;1492:203–206. doi: 10.1016/S0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 43.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J., Sandusky G.E., Hammond L.J., Moyers J.S., Owens R.A., et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mraz M., Bartlova M., Lacinova Z., Michalsky D., Kasalicky M., Haluzikova D., Matoulek M., Dostalova I., Humenanska V., Haluzik M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. 2009;71:369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 45.Markan K.R., Naber M.C., Ameka M.K., Anderegg M.D., Mangelsdorf D.J., Kliewer S.A., Mohammadi M., Potthoff M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ameka M., Markan K.R., Morgan D.A., BonDurant L.D., Idiga S.O., Naber M.C., Zhu Z., Zingman L.V., Grobe J.L., Rahmouni K., et al. Liver Derived FGF21 Maintains Core Body Temperature during Acute Cold Exposure. Sci. Rep. 2019;9:630. doi: 10.1038/s41598-018-37198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang Q., Zhong L., Zhang J., Wang Y., Bornstein S.R., Triggle C.R., Ding H., Lam K.S., Xu A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63:4064–4075. doi: 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- 48.Abu-Odeh M., Zhang Y., Reilly S.M., Ebadat N., Keinan O., Valentine J.M., Hafezi-Bakhtiari M., Ashayer H., Mamoun L., Zhou X., et al. FGF21 promotes thermogenic gene expression as an autocrine factor in adipocytes. Cell Rep. 2021;35:109331. doi: 10.1016/j.celrep.2021.109331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F., Wu J., Kharitonenkov A., Flier J.S., Maratos-Flier E., et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Justesen S., Haugegaard K.V., Hansen J.B., Hansen H.S., Andersen B. The autocrine role of FGF21 in cultured adipocytes. Biochem. J. 2020;477:2477–2487. doi: 10.1042/BCJ20200220. [DOI] [PubMed] [Google Scholar]
- 51.Kharitonenkov A., Wroblewski V.J., Koester A., Chen Y.F., Clutinger C.K., Tigno X.T., Hansen B.C., Shanafelt A.B., Etgen G.J. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 52.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y., Moller D.E., Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 53.Owen B.M., Ding X., Morgan D.A., Coate K.C., Bookout A.L., Rahmouni K., Kliewer S.A., Mangelsdorf D.J. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y.S., Lindberg R.A., et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hondares E., Iglesias R., Giralt A., Gonzalez F.J., Giralt M., Mampel T., Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutchak P.A., Katafuchi T., Bookout A.L., Choi J.H., Yu R.T., Mangelsdorf D.J., Kliewer S.A. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owen B.M., Bookout A.L., Ding X., Lin V.Y., Atkin S.D., Gautron L., Kliewer S.A., Mangelsdorf D.J. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 2013;19:1153–1156. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bookout A.L., de Groot M.H., Owen B.M., Lee S., Gautron L., Lawrence H.L., Ding X., Elmquist J.K., Takahashi J.S., Mangelsdorf D.J., et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douris N., Stevanovic D.M., Fisher F.M., Cisu T.I., Chee M.J., Nguyen N.L., Zarebidaki E., Adams A.C., Kharitonenkov A., Flier J.S., et al. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology. 2015;156:2470–2481. doi: 10.1210/en.2014-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Katafuchi T., Holland W.L., Kollipara R.K., Kittler R., Mangelsdorf D.J., Kliewer S.A. PPARγ-K107 SUMOylation regulates insulin sensitivity but not adiposity in mice. Proc. Natl. Acad. Sci. USA. 2018;115:12102–12111. doi: 10.1073/pnas.1814522115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fisher F.M., Chui P.C., Nasser I.A., Popov Y., Cunniff J.C., Lundasen T., Kharitonenkov A., Schuppan D., Flier J.S., Maratos-Flier E. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology. 2014;147:1073–1083.e1076. doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka N., Takahashi S., Zhang Y., Krausz K.W., Smith P.B., Patterson A.D., Gonzalez F.J. Role of fibroblast growth factor 21 in the early stage of NASH induced by methionine- and choline-deficient diet. Biochim. Biophys. Acta. 2015;1852:1242–1252. doi: 10.1016/j.bbadis.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R., Mohammadi M., Finck B.N., Mangelsdorf D.J., Kliewer S.A., et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Badman M.K., Koester A., Flier J.S., Kharitonenkov A., Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inagaki T., Lin V.Y., Goetz R., Mohammadi M., Mangelsdorf D.J., Kliewer S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., Xie Y., Berglund E.D., Coate K.C., He T.T., Katafuchi T., Xiao G., Potthoff M.J., Wei W., Wan Y., et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coate K.C., Hernandez G., Thorne C.A., Sun S., Le T.D.V., Vale K., Kliewer S.A., Mangelsdorf D.J. FGF21 Is an Exocrine Pancreas Secretagogue. Cell Metab. 2017;25:472–480. doi: 10.1016/j.cmet.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lundåsen T., Hunt M.C., Nilsson L.M., Sanyal S., Angelin B., Alexson S.E., Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem. Biophys. Res. Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 71.Patel R., Bookout A.L., Magomedova L., Owen B.M., Consiglio G.P., Shimizu M., Zhang Y., Mangelsdorf D.J., Kliewer S.A., Cummins C.L. Glucocorticoids regulate the metabolic hormone FGF21 in a feed-forward loop. Mol. Endocrinol. 2015;29:213–223. doi: 10.1210/me.2014-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vispute S.G., Bu P., Le Y., Cheng X. Activation of GR but not PXR by dexamethasone attenuated acetaminophen hepatotoxicities via Fgf21 induction. Toxicology. 2017;378:95–106. doi: 10.1016/j.tox.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Kim H., Mendez R., Zheng Z., Chang L., Cai J., Zhang R., Zhang K. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor α to regulate metabolic hormone FGF21. Endocrinology. 2014;155:769–782. doi: 10.1210/en.2013-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakagawa Y., Satoh A., Yabe S., Furusawa M., Tokushige N., Tezuka H., Mikami M., Iwata W., Shingyouchi A., Matsuzaka T., et al. Hepatic CREB3L3 controls whole-body energy homeostasis and improves obesity and diabetes. Endocrinology. 2014;155:4706–4719. doi: 10.1210/en.2014-1113. [DOI] [PubMed] [Google Scholar]
- 75.Xu J., Stanislaus S., Chinookoswong N., Lau Y.Y., Hager T., Patel J., Ge H., Weiszmann J., Lu S.C., Graham M., et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 2009;297:E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 76.Hecht R., Li Y.S., Sun J., Belouski E., Hall M., Hager T., Yie J., Wang W., Winters D., Smith S., et al. Rationale-Based Engineering of a Potent Long-Acting FGF21 Analog for the Treatment of Type 2 Diabetes. PLoS ONE. 2012;7:e49345. doi: 10.1371/journal.pone.0049345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dunshee D.R., Bainbridge T.W., Kljavin N.M., Zavala-Solorio J., Schroeder A.C., Chan R., Corpuz R., Wong M., Zhou W., Deshmukh G., et al. Fibroblast Activation Protein Cleaves and Inactivates Fibroblast Growth Factor 21. J. Biol. Chem. 2016;291:5986–5996. doi: 10.1074/jbc.M115.710582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhen E.Y., Jin Z., Ackermann B.L., Thomas M.K., Gutierrez J.A. Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem. J. 2016;473:605–614. doi: 10.1042/BJ20151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coppage A.L., Heard K.R., DiMare M.T., Liu Y., Wu W., Lai J.H., Bachovchin W.W. Human FGF-21 Is a Substrate of Fibroblast Activation Protein. PLoS ONE. 2016;11:e0151269. doi: 10.1371/journal.pone.0151269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sánchez-Garrido M.A., Habegger K.M., Clemmensen C., Holleman C., Müller T.D., Perez-Tilve D., Li P., Agrawal A.S., Finan B., Drucker D.J., et al. Fibroblast activation protein (FAP) as a novel metabolic target. Mol. Metab. 2016;5:1015–1024. doi: 10.1016/j.molmet.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Consortium A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 82.Yamashita T., Yoshioka M., Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 83.Shimada T., Mizutani S., Muto T., Yoneya T., Hino R., Takeda S., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagano N., Miyata S., Abe M., Kobayashi N., Wakita S., Yamashita T., Wada M. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006;69:531–537. doi: 10.1038/sj.ki.5000020. [DOI] [PubMed] [Google Scholar]
- 85.Burnett S.M., Gunawardene S.C., Bringhurst F.R., Jüppner H., Lee H., Finkelstein J.S. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J. Bone Miner. Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 86.Nishi H., Nii-Kono T., Nakanishi S., Yamazaki Y., Yamashita T., Fukumoto S., Ikeda K., Fujimori A., Fukagawa M. Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin. Pract. 2005;101:c94–c99. doi: 10.1159/000086347. [DOI] [PubMed] [Google Scholar]
- 87.Kolek O.I., Hines E.R., Jones M.D., LeSueur L.K., Lipko M.A., Kiela P.R., Collins J.F., Haussler M.R., Ghishan F.K. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: The final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 88.Larsson T., Marsell R., Schipani E., Ohlsson C., Ljunggren O., Tenenhouse H.S., Jüppner H., Jonsson K.B. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 89.Bai X., Miao D., Li J., Goltzman D., Karaplis A.C. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 90.Shimada T., Urakawa I., Yamazaki Y., Hasegawa H., Hino R., Yoneya T., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem. Biophys. Res. Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 91.Tsujikawa H., Kurotaki Y., Fujimori T., Fukuda K., Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 92.Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T., Fukumoto S., Tomizuka K., Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004;113:561–568. doi: 10.1172/JCI200419081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 94.Goetz R., Beenken A., Ibrahimi O.A., Kalinina J., Olsen S.K., Eliseenkova A.V., Xu C., Neubert T.A., Zhang F., Linhardt R.J., et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamazaki Y., Tamada T., Kasai N., Urakawa I., Aono Y., Hasegawa H., Fujita T., Kuroki R., Yamashita T., Fukumoto S., et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J. Bone Miner. Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 96.Lamb Y.N. Burosumab: First Global Approval. Drugs. 2018;78:707–714. doi: 10.1007/s40265-018-0905-7. [DOI] [PubMed] [Google Scholar]
- 97.Liu S., Tang W., Zhou J., Stubbs J.R., Luo Q., Pi M., Quarles L.D. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 98.Saini R.K., Kaneko I., Jurutka P.W., Forster R., Hsieh A., Hsieh J.C., Haussler M.R., Whitfield G.K. 1,25-dihydroxyvitamin D(3) regulation of fibroblast growth factor-23 expression in bone cells: Evidence for primary and secondary mechanisms modulated by leptin and interleukin-6. Calcif. Tissue Int. 2013;92:339–353. doi: 10.1007/s00223-012-9683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu X., Sabbagh Y., Davis S.I., Demay M.B., White K.E. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005;36:971–977. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 100.Inoue Y., Segawa H., Kaneko I., Yamanaka S., Kusano K., Kawakami E., Furutani J., Ito M., Kuwahata M., Saito H., et al. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem. J. 2005;390:325–331. doi: 10.1042/BJ20041799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lavi-Moshayoff V., Wasserman G., Meir T., Silver J., Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Ren. Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 102.Kulkarni N.H., Halladay D.L., Miles R.R., Gilbert L.M., Frolik C.A., Galvin R.J., Martin T.J., Gillespie M.T., Onyia J.E. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell Biochem. 2005;95:1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- 103.Meir T., Durlacher K., Pan Z., Amir G., Richards W.G., Silver J., Naveh-Many T. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 2014;86:1106–1115. doi: 10.1038/ki.2014.215. [DOI] [PubMed] [Google Scholar]
- 104.Martin A., Liu S., David V., Li H., Karydis A., Feng J.Q., Quarles L.D. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiao L., Naganawa T., Lorenzo J., Carpenter T.O., Coffin J.D., Hurley M.M. Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J. Biol. Chem. 2010;285:2834–2846. doi: 10.1074/jbc.M109.030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu S., Tang W., Fang J., Ren J., Li H., Xiao Z., Quarles L.D. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23:1505–1518. doi: 10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin A., David V., Li H., Dai B., Feng J.Q., Quarles L.D. Overexpression of the DMP1 C-terminal fragment stimulates FGF23 and exacerbates the hypophosphatemic rickets phenotype in Hyp mice. Mol. Endocrinol. 2012;26:1883–1895. doi: 10.1210/me.2012-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakatani T., Ohnishi M., Razzaque M.S. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 2009;23:3702–3711. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dussold C., Gerber C., White S., Wang X., Qi L., Francis C., Capella M., Courbon G., Wang J., Li C., et al. DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res. 2019;7:12. doi: 10.1038/s41413-019-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Han X., Xiao Z., Quarles L.D. Membrane and integrative nuclear fibroblastic growth factor receptor (FGFR) regulation of FGF-23. J. Biol. Chem. 2015;290:10447–10459. doi: 10.1074/jbc.M114.609230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schröder M., Kaufman R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 112.Cox J.S., Shamu C.E., Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-A. [DOI] [PubMed] [Google Scholar]
- 113.Mori K., Ma W., Gething M.J., Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 114.Tirasophon W., Welihinda A.A., Kaufman R.J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X.Z., Harding H.P., Zhang Y., Jolicoeur E.M., Kuroda M., Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shen X., Ellis R.E., Lee K., Liu C.Y., Yang K., Solomon A., Yoshida H., Morimoto R., Kurnit D.M., Mori K., et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/S0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 117.Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 118.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 119.Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 120.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoshida H., Haze K., Yanagi H., Yura T., Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- 122.Shimizu M., Li J., Maruyama R., Inoue J., Sato R. FGF19 (fibroblast growth factor 19) as a novel target gene for activating transcription factor 4 in response to endoplasmic reticulum stress. Biochem. J. 2013;450:221–229. doi: 10.1042/BJ20121393. [DOI] [PubMed] [Google Scholar]
- 123.Adachi T., Kaminaga T., Yasuda H., Kamiya T., Hara H. The involvement of endoplasmic reticulum stress in bile acid-induced hepatocellular injury. J. Clin. Biochem. Nutr. 2014;54:129–135. doi: 10.3164/jcbn.13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gafar A.A., Draz H.M., Goldberg A.A., Bashandy M.A., Bakry S., Khalifa M.A., AbuShair W., Titorenko V.I., Sanderson J.T. Lithocholic acid induces endoplasmic reticulum stress, autophagy and mitochondrial dysfunction in human prostate cancer cells. PeerJ. 2016;4:e2445. doi: 10.7717/peerj.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shimizu M., Morimoto H., Maruyama R., Inoue J., Sato R. Selective Regulation of FGF19 and FGF21 Expression by Cellular and Nutritional Stress. J. Nutr. Sci. Vitaminol. 2015;61:154–160. doi: 10.3177/jnsv.61.154. [DOI] [PubMed] [Google Scholar]
- 126.Schaap F.G., Kremer A.E., Lamers W.H., Jansen P.L., Gaemers I.C. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95:692–699. doi: 10.1016/j.biochi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 127.Wan X.S., Lu X.H., Xiao Y.C., Lin Y., Zhu H., Ding T., Yang Y., Huang Y., Zhang Y., Liu Y.L., et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. Biomed Res. Int. 2014;2014:807874. doi: 10.1155/2014/807874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang S., Yan C., Fang Q.C., Shao M.L., Zhang Y.L., Liu Y., Deng Y.P., Shan B., Liu J.Q., Li H.T., et al. Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. J. Biol. Chem. 2014;289:29751–29765. doi: 10.1074/jbc.M114.565960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Sousa-Coelho A.L., Marrero P.F., Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem. J. 2012;443:165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- 130.Kim K.H., Jeong Y.T., Oh H., Kim S.H., Cho J.M., Kim Y.N., Kim S.S., Kim d.H., Hur K.Y., Kim H.K., et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 131.Ozaki Y., Saito K., Nakazawa K., Konishi M., Itoh N., Hakuno F., Takahashi S., Kato H., Takenaka A. Rapid increase in fibroblast growth factor 21 in protein malnutrition and its impact on growth and lipid metabolism. Br. J. Nutr. 2015;114:1410–1418. doi: 10.1017/S0007114515002846. [DOI] [PubMed] [Google Scholar]
- 132.Pérez-Martí A., Garcia-Guasch M., Tresserra-Rimbau A., Carrilho-Do-Rosário A., Estruch R., Salas-Salvadó J., Martínez-González M., Lamuela-Raventós R., Marrero P.F., Haro D., et al. A low-protein diet induces body weight loss and browning of subcutaneous white adipose tissue through enhanced expression of hepatic fibroblast growth factor 21 (FGF21) Mol. Nutr. Food Res. 2017:61. doi: 10.1002/mnfr.201600725. [DOI] [PubMed] [Google Scholar]
- 133.Solon-Biet S.M., Cogger V.C., Pulpitel T., Heblinski M., Wahl D., McMahon A.C., Warren A., Durrant-Whyte J., Walters K.A., Krycer J.R., et al. Defining the Nutritional and Metabolic Context of FGF21 Using the Geometric Framework. Cell Metab. 2016;24:555–565. doi: 10.1016/j.cmet.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 134.Chartoumpekis D.V., Ziros P.G., Psyrogiannis A.I., Papavassiliou A.G., Kyriazopoulou V.E., Sykiotis G.P., Habeos I.G. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes. 2011;60:2465–2473. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Furusawa Y., Uruno A., Yagishita Y., Higashi C., Yamamoto M. Nrf2 induces fibroblast growth factor 21 in diabetic mice. Genes Cells. 2014;19:864–878. doi: 10.1111/gtc.12186. [DOI] [PubMed] [Google Scholar]
- 136.Lu H., Cui W., Klaassen C.D. Nrf2 protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced oxidative injury and steatohepatitis. Toxicol. Appl. Pharmacol. 2011;256:122–135. doi: 10.1016/j.taap.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y.K., Yeager R.L., Tanaka Y., Klaassen C.D. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol. Appl. Pharmacol. 2010;245:326–334. doi: 10.1016/j.taap.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Y.K., Wu K.C., Liu J., Klaassen C.D. Nrf2 deficiency improves glucose tolerance in mice fed a high-fat diet. Toxicol. Appl. Pharmacol. 2012;264:305–314. doi: 10.1016/j.taap.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tezze C., Romanello V., Desbats M.A., Fadini G.P., Albiero M., Favaro G., Ciciliot S., Soriano M.E., Morbidoni V., Cerqua C., et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017;25:1374–1389.e1376. doi: 10.1016/j.cmet.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chartoumpekis D.V., Habeos I.G., Ziros P.G., Psyrogiannis A.I., Kyriazopoulou V.E., Papavassiliou A.G. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol. Med. 2011;17:736–740. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Uebanso T., Taketani Y., Yamamoto H., Amo K., Ominami H., Arai H., Takei Y., Masuda M., Tanimura A., Harada N., et al. Paradoxical regulation of human FGF21 by both fasting and feeding signals: Is FGF21 a nutritional adaptation factor? PLoS ONE. 2011;6:e22976. doi: 10.1371/journal.pone.0022976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fisher F.M., Kim M., Doridot L., Cunniff J.C., Parker T.S., Levine D.M., Hellerstein M.K., Hudgins L.C., Maratos-Flier E., Herman M.A. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol. Metab. 2017;6:14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iizuka K., Takeda J., Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009;583:2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 144.Iroz A., Montagner A., Benhamed F., Levavasseur F., Polizzi A., Anthony E., Régnier M., Fouché E., Lukowicz C., Cauzac M., et al. A Specific ChREBP and PPARα Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. Cell Rep. 2017;21:403–416. doi: 10.1016/j.celrep.2017.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lundsgaard A.M., Fritzen A.M., Sjøberg K.A., Myrmel L.S., Madsen L., Wojtaszewski J.F.P., Richter E.A., Kiens B. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol. Metab. 2017;6:22–29. doi: 10.1016/j.molmet.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maekawa R., Seino Y., Ogata H., Murase M., Iida A., Hosokawa K., Joo E., Harada N., Tsunekawa S., Hamada Y., et al. Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice. J. Nutr. Biochem. 2017;49:71–79. doi: 10.1016/j.jnutbio.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 147.Sánchez J., Palou A., Picó C. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: Striking effects on fibroblast growth factor-21 induction. Endocrinology. 2009;150:5341–5350. doi: 10.1210/en.2009-0466. [DOI] [PubMed] [Google Scholar]
- 148.Muise E.S., Azzolina B., Kuo D.W., El-Sherbeini M., Tan Y., Yuan X., Mu J., Thompson J.R., Berger J.P., Wong K.K. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol. Pharm. 2008;74:403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 149.Oishi K., Konishi M., Murata Y., Itoh N. Time-imposed daily restricted feeding induces rhythmic expression of Fgf21 in white adipose tissue of mice. Biochem. Biophys. Res. Commun. 2011;412:396–400. doi: 10.1016/j.bbrc.2011.07.125. [DOI] [PubMed] [Google Scholar]
- 150.Maruyama R., Shimizu M., Li J., Inoue J., Sato R. Fibroblast growth factor 21 induction by activating transcription factor 4 is regulated through three amino acid response elements in its promoter region. Biosci. Biotechnol. Biochem. 2016;80:929–934. doi: 10.1080/09168451.2015.1135045. [DOI] [PubMed] [Google Scholar]
- 151.Teng Y., Zhao H., Gao L., Zhang W., Shull A.Y., Shay C. FGF19 Protects Hepatocellular Carcinoma Cells against Endoplasmic Reticulum Stress via Activation of FGFR4-GSK3β-Nrf2 Signaling. Cancer Res. 2017;77:6215–6225. doi: 10.1158/0008-5472.CAN-17-2039. [DOI] [PubMed] [Google Scholar]
- 152.Maruyama R., Shimizu M., Hashidume T., Inoue J., Itoh N., Sato R. FGF21 Alleviates Hepatic Endoplasmic Reticulum Stress under Physiological Conditions. J. Nutr. Sci. Vitam. 2018;64:200–208. doi: 10.3177/jnsv.64.200. [DOI] [PubMed] [Google Scholar]
- 153.Ouyang R., Zhao X., Zhang R., Yang J., Li S., Deng D. FGF21 attenuates high uric acid-induced endoplasmic reticulum stress, inflammation and vascular endothelial cell dysfunction by activating Sirt1. Mol. Med. Rep. 2022:25. doi: 10.3892/mmr.2021.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liang P., Zhong L., Gong L., Wang J., Zhu Y., Liu W., Yang J. Fibroblast growth factor 21 protects rat cardiomyocytes from endoplasmic reticulum stress by promoting the fibroblast growth factor receptor 1-extracellular signal-regulated kinase 1/2 signaling pathway. Int. J. Mol. Med. 2017;40:1477–1485. doi: 10.3892/ijmm.2017.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deng Y., Wang Z.V., Tao C., Gao N., Holland W.L., Ferdous A., Repa J.J., Liang G., Ye J., Lehrman M.A., et al. The Xbp1s/GalE axis links ER stress to postprandial hepatic metabolism. J. Clin. Investig. 2013;123:455–468. doi: 10.1172/JCI62819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kubisch C.H., Logsdon C.D. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1804–G1812. doi: 10.1152/ajpgi.00078.2007. [DOI] [PubMed] [Google Scholar]
- 157.Oyadomari S., Harding H.P., Zhang Y., Oyadomari M., Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miyake M., Nomura A., Ogura A., Takehana K., Kitahara Y., Takahara K., Tsugawa K., Miyamoto C., Miura N., Sato R., et al. Skeletal muscle-specific eukaryotic translation initiation factor 2α phosphorylation controls amino acid metabolism and fibroblast growth factor 21-mediated non-cell-autonomous energy metabolism. FASEB J. 2016;30:798–812. doi: 10.1096/fj.15-275990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 161.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Girardin S.E., Cuziol C., Philpott D.J., Arnoult D. The eIF2α kinase HRI in innate immunity, proteostasis, and mitochondrial stress. FEBS J. 2021;288:3094–3107. doi: 10.1111/febs.15553. [DOI] [PubMed] [Google Scholar]
- 163.Kay H.Y., Kim Y.W., Ryu D.H., Sung S.H., Hwang S.J., Kim S.G. Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKCδ-GSK3β pathway. Br. J. Pharmacol. 2011;163:1653–1665. doi: 10.1111/j.1476-5381.2010.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jain A.K., Jaiswal A.K. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 165.Fang Y., Zhao Y., He S., Guo T., Song Q., Guo N., Yuan Z. Overexpression of FGF19 alleviates hypoxia/reoxygenation-induced injury of cardiomyocytes by regulating GSK-3β/Nrf2/ARE signaling. Biochem. Biophys. Res. Commun. 2018;503:2355–2362. doi: 10.1016/j.bbrc.2018.06.161. [DOI] [PubMed] [Google Scholar]
- 166.Li X., Wu D., Tian Y. Fibroblast growth factor 19 protects the heart from oxidative stress-induced diabetic cardiomyopathy via activation of AMPK/Nrf2/HO-1 pathway. Biochem. Biophys. Res. Commun. 2018;502:62–68. doi: 10.1016/j.bbrc.2018.05.121. [DOI] [PubMed] [Google Scholar]
- 167.Ye D., Wang Y., Li H., Jia W., Man K., Lo C.M., Lam K.S., Xu A. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology. 2014;60:977–989. doi: 10.1002/hep.27060. [DOI] [PubMed] [Google Scholar]
- 168.Planavila A., Redondo-Angulo I., Ribas F., Garrabou G., Casademont J., Giralt M., Villarroya F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc. Res. 2015;106:19–31. doi: 10.1093/cvr/cvu263. [DOI] [PubMed] [Google Scholar]
- 169.Yu Y., Li S., Liu Y., Tian G., Yuan Q., Bai F., Wang W., Zhang Z., Ren G., Zhang Y., et al. Fibroblast growth factor 21 (FGF21) ameliorates collagen-induced arthritis through modulating oxidative stress and suppressing nuclear factor-kappa B pathway. Int. Immunopharmacol. 2015;25:74–82. doi: 10.1016/j.intimp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 170.Zhu W., Wang C., Liu L., Li Y., Li X., Cai J., Wang H. Effects of fibroblast growth factor 21 on cell damage in vitro and atherosclerosis in vivo. Can. J. Physiol. Pharmacol. 2014;92:927–935. doi: 10.1139/cjpp-2014-0227. [DOI] [PubMed] [Google Scholar]
- 171.Hulejová H., Andrés Cerezo L., Kuklová M., Pecha O., Vondráček T., Pavelka K., Vencovský J., Haluzík M., Senolt L. Novel adipokine fibroblast growth factor 21 is increased in rheumatoid arthritis. Physiol. Res. 2012;61:489–494. doi: 10.33549/physiolres.932324. [DOI] [PubMed] [Google Scholar]
- 172.Ji F., Hu X., Hu W., Hao Y.D. FGF23 protects osteoblasts from dexamethasone-induced oxidative injury. Aging. 2020;12:19045–19059. doi: 10.18632/aging.103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guo S., Mao L., Ji F., Wang S., Xie Y., Fei H., Wang X.D. Activating AMP-activated protein kinase by an α1 selective activator compound 13 attenuates dexamethasone-induced osteoblast cell death. Biochem. Biophys. Res. Commun. 2016;471:545–552. doi: 10.1016/j.bbrc.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 174.Almeida M., Han L., Ambrogini E., Weinstein R.S., Manolagas S.C. Glucocorticoids and tumor necrosis factor α increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J. Biol. Chem. 2011;286:44326–44335. doi: 10.1074/jbc.M111.283481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.den Uyl D., Bultink I.E., Lems W.F. Glucocorticoid-induced osteoporosis. Clin. Exp. Rheumatol. 2011;29:S93–S98. [PubMed] [Google Scholar]
- 176.D’Amico D., Sorrentino V., Auwerx J. Cytosolic Proteostasis Networks of the Mitochondrial Stress Response. Trends Biochem. Sci. 2017;42:712–725. doi: 10.1016/j.tibs.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 177.Itoh N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front. Endocrinol. 2014;5:107. doi: 10.3389/fendo.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tezze C., Romanello V., Sandri M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019;10:419. doi: 10.3389/fphys.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Suomalainen A., Elo J.M., Pietiläinen K.H., Hakonen A.H., Sevastianova K., Korpela M., Isohanni P., Marjavaara S.K., Tyni T., Kiuru-Enari S., et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: A diagnostic study. Lancet Neurol. 2011;10:806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tyynismaa H., Carroll C.J., Raimundo N., Ahola-Erkkilä S., Wenz T., Ruhanen H., Guse K., Hemminki A., Peltola-Mjøsund K.E., Tulkki V., et al. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 2010;19:3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]
- 181.Oost L.J., Kustermann M., Armani A., Blaauw B., Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J. Cachexia. Sarcopenia Muscle. 2019;10:630–642. doi: 10.1002/jcsm.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guo A., Li K., Xiao Q. Fibroblast growth factor 19 alleviates palmitic acid-induced mitochondrial dysfunction and oxidative stress via the AMPK/PGC-1α pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 2020;526:1069–1076. doi: 10.1016/j.bbrc.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 183.Inagaki T., Sakai J., Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 2016;17:480–495. doi: 10.1038/nrm.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sidossis L., Kajimura S. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Investig. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Quesada-López T., Cereijo R., Turatsinze J.V., Planavila A., Cairó M., Gavaldà-Navarro A., Peyrou M., Moure R., Iglesias R., Giralt M., et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat. Commun. 2016;7:13479. doi: 10.1038/ncomms13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Emanuelli B., Vienberg S.G., Smyth G., Cheng C., Stanford K.I., Arumugam M., Michael M.D., Adams A.C., Kharitonenkov A., Kahn C.R. Interplay between FGF21 and insulin action in the liver regulates metabolism. J. Clin. Investig. 2014;124:515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee P., Linderman J.D., Smith S., Brychta R.J., Wang J., Idelson C., Perron R.M., Werner C.D., Phan G.Q., Kammula U.S., et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Morón-Ros S., Uriarte I., Berasain C., Avila M.A., Sabater-Masdeu M., Moreno-Navarrete J.M., Fernández-Real J.M., Giralt M., Villarroya F., Gavaldà-Navarro A. FGF15/19 is required for adipose tissue plasticity in response to thermogenic adaptations. Mol. Metab. 2021;43:101113. doi: 10.1016/j.molmet.2020.101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sonenberg N., Hinnebusch A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De Sousa-Coelho A.L., Relat J., Hondares E., Pérez-Martí A., Ribas F., Villarroya F., Marrero P.F., Haro D. FGF21 mediates the lipid metabolism response to amino acid starvation. J. Lipid Res. 2013;54:1786–1797. doi: 10.1194/jlr.M033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hashidume T., Kato A., Tanaka T., Miyoshi S., Itoh N., Nakata R., Inoue H., Oikawa A., Nakai Y., Shimizu M., et al. Single ingestion of soy β-conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects. Sci. Rep. 2016;6:28183. doi: 10.1038/srep28183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Alvarez-Sola G., Uriarte I., Latasa M.U., Fernandez-Barrena M.G., Urtasun R., Elizalde M., Barcena-Varela M., Jiménez M., Chang H.C., Barbero R., et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: Development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut. 2017;66:1818–1828. doi: 10.1136/gutjnl-2016-312975. [DOI] [PubMed] [Google Scholar]
- 193.Wang D., Wei Y., Pagliassotti M.J. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 194.Gallego-Escuredo J.M., Gómez-Ambrosi J., Catalan V., Domingo P., Giralt M., Frühbeck G., Villarroya F. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int. J. Obes. 2015;39:121–129. doi: 10.1038/ijo.2014.76. [DOI] [PubMed] [Google Scholar]
- 195.Tomlinson E., Fu L., John L., Hultgren B., Huang X., Renz M., Stephan J.P., Tsai S.P., Powell-Braxton L., French D., et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 196.Fu L., John L.M., Adams S.H., Yu X.X., Tomlinson E., Renz M., Williams P.M., Soriano R., Corpuz R., Moffat B., et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 197.Iizuka K., Takao K., Kato T., Horikawa Y., Takeda J. ChREBP Reciprocally Regulates Liver and Plasma Triacylglycerol Levels in Different Manners. Nutrients. 2018;10:1699. doi: 10.3390/nu10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Benhamed F., Denechaud P.D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J., et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Investig. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Søberg S., Sandholt C.H., Jespersen N.Z., Toft U., Madsen A.L., von Holstein-Rathlou S., Grevengoed T.J., Christensen K.B., Bredie W.L.P., Potthoff M.J., et al. FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab. 2017;25:1045–1053.e1046. doi: 10.1016/j.cmet.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 200.von Holstein-Rathlou S., BonDurant L.D., Peltekian L., Naber M.C., Yin T.C., Claflin K.E., Urizar A.I., Madsen A.N., Ratner C., Holst B., et al. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016;23:335–343. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Talukdar S., Owen B.M., Song P., Hernandez G., Zhang Y., Zhou Y., Scott W.T., Paratala B., Turner T., Smith A., et al. FGF21 Regulates Sweet and Alcohol Preference. Cell Metab. 2016;23:344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barb D., Bril F., Kalavalapalli S., Cusi K. Plasma Fibroblast Growth Factor 21 Is Associated With Severity of Nonalcoholic Steatohepatitis in Patients With Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019;104:3327–3336. doi: 10.1210/jc.2018-02414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li H., Fang Q., Gao F., Fan J., Zhou J., Wang X., Zhang H., Pan X., Bao Y., Xiang K., et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J. Hepatol. 2010;53:934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 204.Nygaard E.B., Møller C.L., Kievit P., Grove K.L., Andersen B. Increased fibroblast growth factor 21 expression in high-fat diet-sensitive non-human primates (Macaca mulatta) Int. J. Obes. 2014;38:183–191. doi: 10.1038/ijo.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Strączkowski M., Karczewska-Kupczewska M., Adamska A., Otziomek E., Kowalska I., Nikołajuk A. Serum fibroblast growth factor 21 in human obesity: Regulation by insulin infusion and relationship with glucose and lipid oxidation. Int. J. Obes. 2013;37:1386–1390. doi: 10.1038/ijo.2013.10. [DOI] [PubMed] [Google Scholar]
- 206.Yang W., Chen X., Liu Y., Chen M., Jiang X., Shen T., Li Q., Yang Y., Ling W. N-3 polyunsaturated fatty acids increase hepatic fibroblast growth factor 21 sensitivity via a PPAR-γ-β-klotho pathway. Mol. Nutr. Food Res. 2017:61. doi: 10.1002/mnfr.201601075. [DOI] [PubMed] [Google Scholar]
- 207.Zhang X., Yeung D.C., Karpisek M., Stejskal D., Zhou Z.G., Liu F., Wong R.L., Chow W.S., Tso A.W., Lam K.S., et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 208.Giannini C., Feldstein A.E., Santoro N., Kim G., Kursawe R., Pierpont B., Caprio S. Circulating levels of FGF-21 in obese youth: Associations with liver fat content and markers of liver damage. J. Clin. Endocrinol. Metab. 2013;98:2993–3000. doi: 10.1210/jc.2013-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela-Rey M., Crawley M., Fisher F.M., Badman M.K., Martinez-Chantar M.L., Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tontonoz P., Spiegelman B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 211.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Görgün C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 212.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R.O., Görgün C.Z., Hotamisligil G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim K.H., Lee M.S. FGF21 as a Stress Hormone: The Roles of FGF21 in Stress Adaptation and the Treatment of Metabolic Diseases. Diabetes Metab. J. 2014;38:245–251. doi: 10.4093/dmj.2014.38.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.