Abstract
Exosomes are small extracellular vesicles that are naturally produced and carry biomolecules such as proteins, microRNAs, and metabolites. Because of their small size and low level of biomolecule expression, the biological function of exosomes has only been identified recently. Despite the short history of investigation, exosomes seem to have remarkable potential as a delivery vehicle. With regards to cancer therapy, numerous antitumor agents demonstrate serious side effects (or toxicity), which has led to the unmet need for improving their selectivity and stability. Exosomes, either produced naturally or generated artificially, provide an attractive platform to load many types of molecules such as small molecules, biologics, and other therapeutic agents. Furthermore, the features of exosomes can be designed by selecting their source cells, or they can be engineered to incorporate affinity tags; thus, exosomes show promise as effective delivery vehicles for the complex tumor microenvironment. In this review, we focus on various exosomes produced from different cell types and their potential uses. Moreover, we summarize the current state of artificial exosomes as a drug carrier and provide an overview of the techniques used for their production.
Keywords: exosome, cell-derived vesicles, drug delivery, cancer therapy
1. Introduction
1.1. Drug Delivery Vehicles for Cancer Therapy
Cancer is the second leading cause of death globally, with a high mortality rate, causing 9 million deaths annually, and approximately 18.1 million new cases are identified every year [1]. Current cancer treatment options include surgical intervention, chemotherapy, and radiation therapy or a combination of these options [2]. Chemotherapy is one of the most widely employed clinical cancer treatments, which works by interfering with DNA synthesis and mitosis, leading to the death of rapidly growing and dividing cancer cells. These agents are nonselective and can damage normal tissues, causing severe undesired side effects such as nausea and vomiting. In fact, the severe adverse effects induced by chemotherapeutic drugs on normal tissues and organs are a major reason underlying the high mortality rate of patients with cancer [3]. Additionally, because of the poor tissue penetration of these drugs, higher doses are required, leading to elevated toxicity in normal cells. Therefore, it is desirable to develop chemotherapeutics that can effectively reach the target cancerous cells, thereby reducing adverse effects while improving therapeutic efficacy.
In the last few years, numerous attempts have been made to develop drug delivery systems (DDSs) with improved therapeutic efficacies. The use of nanotechnology has had a profound impact on clinical therapeutics. Compared with conventional chemotherapeutic agents, nanoscale drug carriers have several advantages; that is, they improve treatment efficacy while avoiding toxicity in normal cells due to features such as highly selective accumulation in tumors via the enhanced permeability and retention effect and active cellular uptake [4,5]. An active targeting approach can be achieved by binding nanocarriers, including chemotherapeutic agents, to molecules that bind to overexpressed antigens [6]. Most drug delivery vehicles are chemically synthesized using lipids or lipid-like molecules. Despite the remarkable advances and successes in the design and effectiveness of synthetic drug vehicles, some limitations to their practical application exist. The main disadvantages are their toxicity and low biocompatibility [7]. To overcome these limitations, there is increasing recognition of natural drug delivery vehicles due to their advantages of evasion of the host immune system and high efficacy of entering target cells. In the past, bacteria, viruses, red blood cells, and lymphocytes have been considered possible natural drug delivery vehicle candidates [8]. Recently, exosomes have attracted attention as novel DDSs. There has been a growing interest in exosome research in the last decade due to their emerging role as intercellular messengers and their potential in managing disease [9].
1.2. Basic Properties of Exosomes
Exosomes are a type of cell-derived vesicles characterized as extracellular vesicles (EVs). EVs are nanometer-sized small membrane vesicles secreted by most cells, containing proteins, lipids, and nucleic acids, which are specific to their cell origin [10]. EVs are categorized into three types—exosomes, apoptotic bodies, and microvesicles [11]. The difference between these EVs is thought to be due to biogenesis, which in turn determines the cargo contents and functions. Microvesicles are formed from the budding of the cell membrane, whereas exosomes are the result of endocytosis from multivesicular bodies (MVBs) that eventually fuse with the plasma membrane and are then released to the extracellular space [12]. Exosomes, with a diameter in the range of 40–100 nm, possess a lipid bilayer membrane with the same orientation as the plasma membrane and carry cargo that includes both proteins and genetic material [13]. Exosomes have an array of constituents such as surface proteins, heat shock proteins (HSPs), lysosomal proteins, tumor-derived genes, fusion proteins, and nucleic acids, each exhibiting certain functions. The lipid bilayer of the exosome is rich in cholesterol and diacylglycerol [14]. Lipids such as sphingomyelin and monosialotetrahexosylganglioside determine the rigidity of the exosomes. In addition, different types of phospholipid transportation enzymes in exosomes are expressed by phosphatidylserine [15]. Exosomes have similar components due to their endosomal origin, including HSPs, membrane transporters (annexins, Rab GTPases, and flotillin), and MVB proteins, including TSG101, Alix, integrins, and tetraspanins (CD9, CD63, CD81, and CD82), which mediate signaling, cell fusion, and migration [16].
Exosomes contain various nucleic acids. Messenger RNA (mRNA) is the mediator of horizontal transfer of genetic information in exosomes [17], whereas micro RNA (miRNA) serves the function of cell targeting and gene silencing [18]. Exosomes also have noncoding RNA, the shorter ones of which regulate gene expression [19], and long noncoding RNAs are involved in carcinogenesis and cancer progression [20]. Circulating DNA (cDNA), a heterogenous population of genomic and mitochondrial DNA, contains genetic alterations and reflects mutations, rearrangements, and amplifications in tumor tissues [21]. Exosomes have been reported to be involved in several processes such as cell–cell communication through the exchange of proteins and genetic materials, immunomodulatory functions, antigen presentation, tumor growth suppression, endothelial cell migration, and inflammation. The main function of exosomes is intercellular communication by transferring lipids, RNA, and cytosolic proteins. This finding indicates the possibility of using exosomes as DDSs to deliver therapeutic drugs.
Exosomes are produced by most cell types, including dendritic cells (DCs), neutrophils, epithelial cells, and tumor cells. They are also found in biological fluids [22]. Depending on the cell of origin, exosomes contain cell-specific proteins and lipid constituents that reflect their cellular source origin [23]. Furthermore, because of their stability, exosomes are widely distributed in biological fluids such as the blood, urine, bronchoalveolar lavage fluid, breast milk, amniotic fluid, synovial fluid, and ascites [23]. These properties suggest that exosomes are attractive vehicles for drug delivery. Mesenchymal stem/stromal cells (MSC)-derived exosomes do not contain class I and class II human major histocompatibility complex (MHC) proteins or co-stimulatory molecules such as CD80 and CD86, which helps them evade the human immune system. From the immunological perspective, MSC-derived exosomes are mostly used nowadays [24].
2. Natural Cell-Type Specific Exosomes
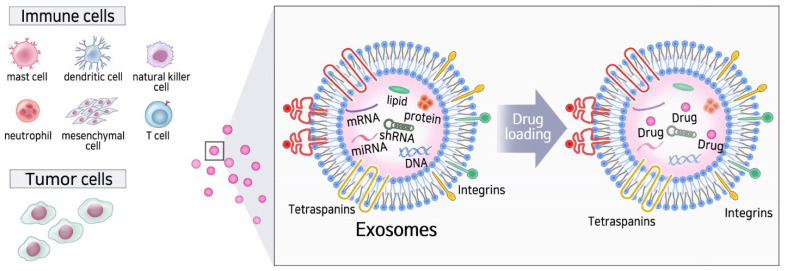
Numerous different cell types such as DCs, mast cells (MCs), B cells, T cells, platelets, and tumor cells are known to secrete exosomes (Figure 1) [25]. Exosomes released from tumors have been widely studied in various cancer types, such as renal cancer, breast cancer, and melanoma. Tumor cells continuously secrete membrane vesicles into the extracellular environment. Exosomes released by malignant tumor cells contain specific proteins, lipids, DNA molecules, miRNAs, mRNAs, and noncoding RNAs, which are important for cancer cell communication with the environment [26]. Tumor-derived exosomes or tumor-related exosomes are considered to be closely associated with the pathogenesis and microenvironmental formation of cancer because the number of exosomes in cancer cells is higher than that in normal cells [27].
Figure 1.
Diverse sources of exosomes and the effect on the immune system.
By contrast, DCs play a central role in initiating antigen-specific immunity and tolerance [28]. In cancer, DCs act as the initial link between oncogenesis and the host immune system, which is the first step of the immunity cycle that aims to eliminate cancer cells through the activation of T cells. DC-derived exosomes are nanometer-sized membrane vesicles that are secreted by the antigen-presenting cells of the immune system. DCs secrete a large number of exosomes to induce effective anti-cancer effects. DC-derived exosomes containing MHC I, MHC II, CD86, and HSP70/HSP90 chaperones can trigger CD4+ and CD8+ T cell activation. Under costimulation of secreted IL-2 and exosomal peptides, MHC I is passed to CD8+ T cells and induces more effective antitumor immunity in vivo [29,30,31].
As the source of immune cell-derived exosomes, NK cells contribute to immunosurveillance and function as the body’s first line of defense against several human disorders, including pathogen infections and cancers. NK cells can directly recognize and effectively kill oncogenic transformed cells that are normally devoid of class I MHC antigen expression, thus participating in anti-cancer immunity [32]. NK cell-derived exosomes also harbor prototype NK markers and killer proteins [33]. Additionally, NK exosomes can exert their cytolytic activity by directly diffusing into tumor tissues and subsequently overcoming the homing deficiency of NK cells to tumor sites [34]. In addition to exosome-specific markers (e.g., tsg 101, CD81, CD63, and CD9), NK cell markers (NKG2D, CD94, perforin, granzymes, and CD40L) are also expressed in NK-derived exosomes, which are both involved in cytotoxicity and immune responses. These exosomes can induce target cell death by multiple killing mechanisms [35,36].
MC is an important component of the innate immune system and plays a crucial role in Th2 responses [37]. MCs can secrete exosomes that display biological functions in RNA and protein transfer, intercellular communication, and immune regulation [38]. MC-derived exosomes can affect the biological functions of DCs, T cells, and B cells [38,39]. For example, CD63+ and OX40L+ exosomes from MCs promote the proliferation and differentiation of CD4+ Th2 cells via the OX40L–OX40 interaction [40]. MC-derived exosomes also induce immature DCs to upregulate MHC II, CD40, CD80, and CD86 expression and to confer the antigen-presenting capacity to T cells, thereby leading to the initiation of antigen-specific immune responses [41]. Similarly, neutrophil-derived exosomes also contain proteins, mRNA, and miRNAs, which are associated with inflammatory reactions, immune response, and cell communication [42,43,44]. They can affect the activity of other immune cells, such as macrophages, by transferring several proinflammatory factors [45]. These exosomes have been reported to bind and degrade extracellular matrix (ECM) via integrin Mac-1 and neutrophil elastase, consequently leading to inflammatory disease progression [46].
As another exosome source, MSCs are multipotent nonhematopoietic adult cells, discovered by Alexander Friedenstein [47]. MSCs, possibly originating from the mesoderm, were reported to express CD73, CD90, and CD105 plasma membrane markers, and not CD14, CD34, and CD45 [48]. Relative to other cell types, MSCs possess distinct advantages as an exosome source. They release higher numbers of exosomes than other cells. MSC-derived EVs are relatively well tolerated in different animal models and show more stability and sustainability in human plasma [49].
3. Artificial Exosomes as a Drug Delivery Vehicle
Exosomes have been suggested to be ideal DDSs with potential for application in a broad range of pathologies, including cancer, because of their organotrophic properties [26]. However, the low yield, high cost, and laborious methods of production of cell-derived exosomes are limitations, together with the lack of standardization for relevant processes [50]. Recently, artificial exosomes have been developed to overcome the drawbacks of natural exosomes as new theragnostic biomaterials for potential clinical applications [51]. A recent study reported the incorporation of CRISPR/gRNA into exosome [52]. In addition, siRNA, aptamer, and antisense oligonucleotide can be delivered via exosomes [53]. Despite promising results of exosome-mediated drug delivery, the translation of exosomes is challenged by massive production, purification, modification, drug loading, and storage. Because of the shortcomings of natural exosomes, a growing number of studies are aiming to develop artificial exosomes using the top-down, bottom-up, or biohybrid approach. The development of artificial exosomes, which have the advantages of both natural and synthetic nanoparticles, through nanobiotechnology holds great promise for advanced drug delivery.
3.1. Limitations of Artificial Lipid Bilayer Nanoparticles
When drug-loaded synthetic nanoparticles enter the bloodstream, there are two main issues with drug nanoformulations: toxicity and rapid clearance by the mononuclear phagocyte systems. Macrophages in the reticuloendothelial system (RES), located in the liver and spleen, take up particles bound with serum proteins [54]. Several efforts have been made to overcome this clearance of particles and improve distribution in vivo. The most widely used method is the steric stabilization of the liposomal surface by using polyethyleneglycol (PEG) [55]. It is hypothesized that PEG on the surface of liposomes attracts a water shell, resulting in reduced adsorption of opsonins and recognition of the liposomes by the mononuclear phagocytic systems [56]. This, in turn, leads to extended circulation time and improvement in tumor delivery. However, although PEGylation decreases clearance by the MPS, it reduces the interaction of the nanoformulation with target and barrier cells, thus decreasing the drug biodistribution in diseased tissues. Furthermore, PEG induces antibody-related immune reactions and accelerates blood clearance [57,58,59]. Moreover, surface modification of nanoparticles using CD47 or peptide derivatives from this marker, termed the “don’t eat me” signal, has proven effective for enhancing drug delivery [60].
3.2. Advantages of Artificial Exosomes Compared to Artificial Lipid Bilayer Nanoparticles
Compared to artificial, human-engineered nanoparticles, as natural nanovesicles, exosomes are good candidates for drug delivery due to their low immunogenicity and ability to enter tissues. Exosomes have advantages of both synthetic nanocarriers and cell-mediated drug delivery, avoiding the rapid clearance and toxicity associated with synthetic vehicles, as well as the complexity in utilizing cell-mediated DDSs in the clinic. These unique features make exosomes an attractive option for use as a drug delivery vehicle for cancer treatment. While artificial nanoparticles cannot pass the blood–brain barrier, endothelium, cell, and tissue barriers, exosomes have the natural ability to cross the normal blood–brain vascular barrier by transcytosis [61,62]. Thus, they are available for systemic treatment of CNS-inflammatory disorders and possibly cancers. Furthermore, exosomes have great resistance to various noxious environments. Exosomes resist the stomach acid and can likely also survive in phagolysosomes after cellular uptake and can resist the harsh tissue conditions of hypoxia [63,64]. These characteristics enable exosomes to function in the combined acidic and hypoxic environments of cancers and other types of tissue necrosis. Exosomes can naturally and easily evade the RES and avoid immune detection. Thus, they have a long in vivo duration of action. Furthermore, artificial nanoparticles demonstrate poor penetration of solid tumors and tissue-inflammatory infiltrates. However, exosomes can naturally penetrate tissues that have dense inflammation to target particular cells without any alterations for subsequent specific affinity targeting of target cells [62].
3.3. Challenges Associated with Artificial Exosomes Compared to Lipid Bilayer Nanoparticles
Despite the several advantages of exosomes as drug delivery vehicles, the application of artificial exosomes is still challenging in terms of massive production, standard purification protocols, cargo loading, storage stability, and modification cost. Because physical and biological stability is typically limited to a shorter time period, the International Society of EVs recommends storage at −80 °C in phosphate-buffered saline [65]. However, this storage condition is unfavorable in terms of energy consumption, transportation, and, most importantly, clinical application. Generally, freezing–thawing is considered to destabilize EVs, for example, by changing the EV morphology, function, particle size, and concentration [66]. Freezing–thawing studies have revealed improved colloidal EV stability in the presence of sucrose or potassium phosphate buffer instead of sodium phosphate buffer or phosphate-buffered saline [67]. Less aggregation and/or vesicle fusion occur at neutral pH than at slightly acidic or alkaline pH. In addition, the purification method is time-consuming. Some EVs are similar to exosomes in their physical properties, such as size and density, which makes the isolation of exosomes considerably challenging. Therefore, it is hard to produce and purify exosomes on a large scale [51], making it one of the active areas of research as described below.
4. Purification and Drug Loading of Exosomes
4.1. Approaches for the Isolation of Exosomes
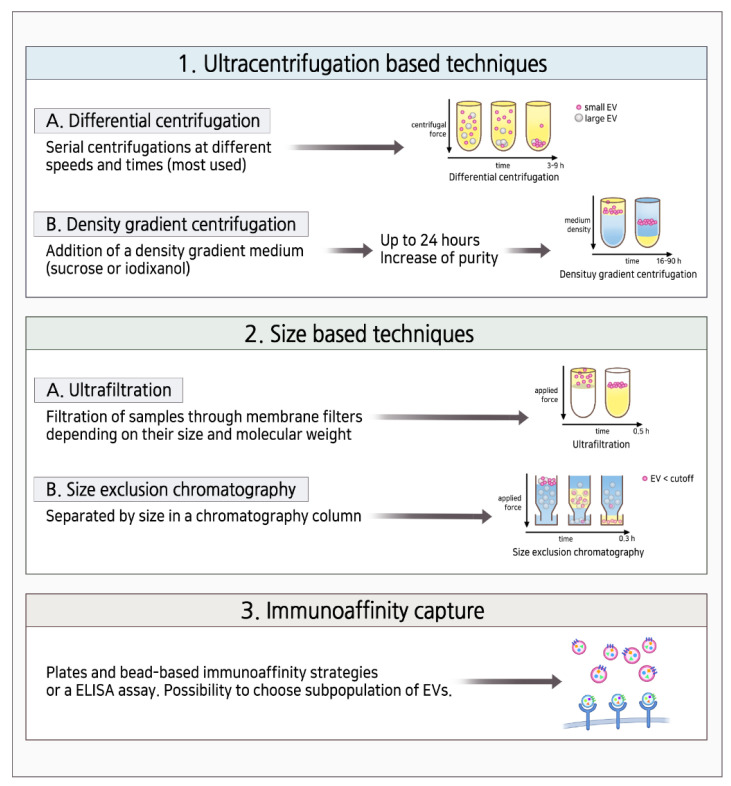
To use exosomes as biomarkers and DDSs, their isolation, purification, and characterization are important and can be improved by innovative technologies. Numerous methods have been developed to facilitate the isolation of exosomes from biological resources. Ultracentrifugation is the gold standard of exosome isolation (Figure 2). Ultracentrifugation is based on the sedimentation coefficient difference between exosomes and other extracellular content. Under certain centrifugal forces, different extracellular components of fluidic samples can be sequentially separated based on the density, size, and shape. Among them, recently, density gradient ultracentrifugation has achieved the purest exosome samples. However, this method is time-consuming since it takes a while to attain the equilibrium of solutions [68,69]. Ultrafiltration is a membrane separation technique based on the size and molecular weight of exosomes and other contents. Exosomes can be separated from macromolecules using membranes containing pores equivalent to exosomes with a size of 100 nm so that they pass through, and other contents are retained on the membrane. Multiple steps of membrane washing increase the processing time. However, compared with the ultrafiltration method, ultrafiltration-based exosome isolation dramatically shortens the processing time and does not require special equipment, presenting an ideal substitute to the classical ultracentrifugation strategy [70]. The principle of immunological separation is based on the antigen–antibody reaction to capture exosomes (Figure 2). This method exploits the presence of various proteins on exosome membranes to capture them. Recent studies have focused on antibody-coated plates, chromatography matrices, and beads for immunological separation with high purity and less time consumption. It is an expensive method, as it involves special reagents and cell-free samples and limits the use of large-scale samples [68].
Figure 2.
Schematic representation of the various methods used for exosome isolation.
Among various isolation methods for EV, size exclusion chromatography (SEC) is considered an effective way to obtain homogeneous EVs [71]. SEC is also reported to remove soluble protein contaminants and is relatively easy to scale up for manufacturing clinical-grade products [72]. For clinical trials of exosomes, a frequently applied method is tangential flow fractionation combined with ultracentrifugation, as indicated in a recent report [73]. This method can maximize the purity, uniformity, and integrity of the exosomes.
Table 1 summarizes the pros and cons of the routinely used three exosome isolation methods [74].
Table 1.
Pros and cons of the three methods for exosome isolation [74].
| Method | Pros | Cons |
|---|---|---|
| Ultracentrifugation | Low cost, high purity, massive production | Time-consuming, mechanical damage, specialized equipment requirement |
| Ultrafiltration | Low cost, less time consuming, good portability | Moderate purity, mechanical damage, high cost |
| Immunological separation | High purity, no chemical contamination, simple | Small volume production, high cost |
4.2. Approaches for Drug Loading on Exosomes
Methods for encapsulating cargo into exosomes can be divided into two types: cell-based loading methods and non-cell-based loading methods. In the cell-based loading approach, cargo is usually delivered into the donor cells first. After being packaged into EVs, the cargo can be secreted and collected in an EV-carrying manner for therapeutic use [75]. The non-cell-based loading approach involves directly loading drugs into the isolated EVs through electroporation, sonication, incubation, and/or transfection [76]. Table 2 summarizes various exosome drug loading methods. Considering previous results of measured efficiency, sonication seems to work well in macrophage-derived exosomes, whereas electroporation seems better for primary DC-derived exosomes [77].
Table 2.
Various drug loading methods on exosomes and their efficiency [77].
| Loading Method |
Extracellular Vesicle (EV) Source | Loading Content |
Loading Measurement | Efficiency (Type, %) | |
|---|---|---|---|---|---|
| Sonication | Raw 264.7 macrophages (mouse) | Paclitaxel (PTX) | High-performance liquid chromatography (HPLC) | Loading capacity | 28.29 (SEM ± 1.38%) |
| Raw 264.7 macrophages (mouse) | Dox | Fluorescence of Dox | Encapsulation efficiency | 8.0–11.0% | |
| Raw 264.7 macrophages (mouse) | Catalase | Catalase enzymatic activity | Loading capacity | 26.1 (SEM ± 1.2%) | |
| Saponin permeabilization | Raw 264.7 macrophages (mouse) | Catalase | Catalase enzymatic activity | Loading capacity | 18.5 (SEM ± 1.3%) |
| Mixing | Raw 264.7 macrophages (mouse) | Paclitaxel (PTX) | High-performance liquid chromatography (HPLC) | Loading capacity | 1.4 (SEM ± 0.38%) |
| LNCaP and PC-3 (human) | PTX | Ultra-performance liquid chromatography (UPLC) | Encapsulation efficiency | 9.2% (SD ± 4.5%) | |
| Milk (bovine) | PTX | UPLC | Encapsulation efficiency | 7.9 ± 1.0% | |
| Raw 264.7 macrophages (mouse) | Catalase | Catalase enzymatic activity | Loading capacity | 4.9 (SEM ± 0.5%) | |
| Electroporation | Raw 264.7 macrophages (mouse) | Paclitaxel (PTX) | High-performance liquid chromatography (HPLC) | Loading capacity | 5.3 (SEM ± 0.48%) |
| Immature dendritic cells (mouse) | Doxorubicin (Dox) | Fluorescence of Dox | Encapsulation efficiency | <20% | |
| Primary immature dendritic cells (mouse) | Glyceraldehyde 3-phosphate dehydrogenase | qPCR analysis, fluorescence microscopy | Encapsulation efficiency | 10–38% | |
| Primary dendritic cells (mouse) | Vascular endothelial growth factor (VEGF) siRNA | qPCR analysis | Encapsulation efficiency | 3% | |
5. Therapeutic Aspects of Exosomes as a DDS
5.1. Exosomes: The Natural Drug Delivery Vehicle
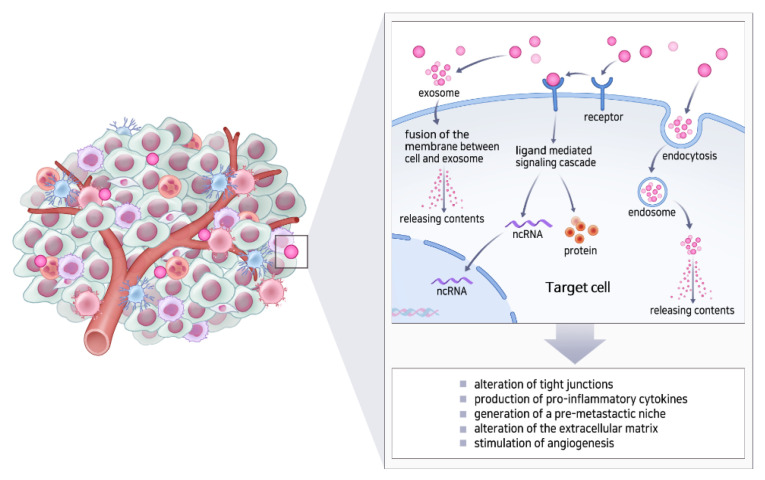
Exosomes have benefits as drug delivery vehicles, such as tissue specificity, safety, and stability. They can deliver their cargo across the plasma membranes of target cells into the correct cellular compartment to exert a functional response. For example, exosomes derived from DCs can modulate the immune cell response by transferring peptide-loaded MHC class I and II cells complexed to DCs [78]. Another highly attractive feature as a drug delivery vehicle is the ability to home to target tissues. For example, melanoma exosomes home to sentinel nodes, demonstrating that exosomes do have intrinsic homing capability [79]. Exosomes loaded with anti-cancer drugs have already shown promise as a new therapeutic approach in animal models. The released exosomes loaded with cargo affect the target cells through the following mechanisms [80]. First, they activate certain signaling pathways of the target cells by interacting with specific ligand receptors. Next, the exosomes transfer surface receptors from one cell to another target cell by budding, followed by fusion with the plasma membrane. Then, they enter the cells using endocytic mechanisms such as receptor-mediated endocytosis, phagocytosis, and micropinocytosis and release their content into the cytoplasm. However, to use exosomes as biomarkers and DDSs, their isolation, purification, and characterization are extremely important and can be improved by using novel technologies.
5.2. Exosomes in the Tumor Microenvironment (TME)
The TME plays an important role in the proliferation and metastasis of tumor cells [81]. The TME comprises fibroblasts, stromal cells, and the ECM. Cancer-associated fibroblasts (CAF) and tumor-associated macrophages (TAM) are major cell populations in the stroma of all solid tumors and often exert protumorigenic functions [82,83]. Because CAF and TAM are known to modulate disease progression, we can expect that targeting cytokine and chemokine (e.g., CXCL, IL-6, and TGF-β) secretion by CAF could improve anti-cancer efficiency [84]. Several IL-6 inhibitors are already approved for immune disorders and are being investigated for their role in anti-cancer therapy. Exosomes can promote the formation of TME and also help in cell-to-cell communication in the TME by delivering proteins, nucleic acids, lipids, and signaling molecules (Figure 3). Moreover, exosomes are critical for tumor development due to their ten-fold higher secretory efficiency in cancer cells than in normal cells [85]. Thus, exosomes can release mRNAs and oncogenic proteins into target cells, which can fuse with the membrane and regulate tumor cell proliferation, invasion, and metastasis. Furthermore, exosomes from tumor cells induce adaptive changes in distant organs to create a “pre-metastatic” environment that is conducive to their growth and the formation of secondary metastatic foci [86].
Figure 3.
Strategies of exosomes to promote the formation of oncogenic microenvironment.
Costa-Silva et al. found that exosomes derived from pancreatic cancer cells induce transforming growth factor β signaling, leading to the activation of hepatic stellate cells and ECM remodeling. In turn, fibronectin accumulation promotes an influx of bone marrow-derived macrophages (and potentially neutrophils) to the liver, providing a favorable niche for liver metastasis [87]. Breast cancer cell-derived exosomes play an important role in promoting breast cancer bone metastasis, which is associated with the formation of a pre-metastatic niche via transferring miR-21 to osteoclasts [88]. Because exosomes closely interact with the TME, by attaching CAF-targeting molecules or receptors, they can effectively reach cancer cells. Targeting CAFs or TAMs with exosomes could be of high impact for improving future targeted treatment strategies [89]. By contrast, HSPs mainly function as molecular chaperones. However, in cancer, they can suppress apoptosis, evade immune responses, and enhance angiogenesis and metastasis. Moreover, HSP also plays a role as a mediator of the resistance-associated secretory phenotype [90]. Hence, if possible, HSPs need not be incorporated in the production of exosomes to minimize such protumorigenic effects [91].
5.3. Engineering of Exosomes for Drug Delivery
Exosomes used as drug delivery vehicles have multiple advantages over existing synthetic systems. They have phospholipid bilayers, which can directly fuse with the plasma membrane of the target cell, thus improving the cellular internalization of the encapsulated drug. Targeted delivery of compounds to tumor vessels and tumor cells can enhance tumor detection and therapy. Docking-based (synaphic) targeting strategies use peptides, antibodies, and other molecules that bind to tumor vessels and tumor cells to deliver more drugs to tumors than to normal tissues [92]. A strategy to deliver drug-loaded exosomes to the tumor parenchyma is to use tumor-homing peptides such as iRGD, a novel cyclic peptide composed of 9-amino acids comprising an Arg-Gly-Asp (RGD) motif, on the surface. iRGD has a high binding affinity to αvβ3 and αvβ5 integrins abundant in tumor vasculatures [93]. Tian et al. found that combining DC-derived exosomes with specific iRGD peptides endows the exosomes with the ability to target breast cancer more efficiently than the chemical drug used alone [94]. Conversely, certain proteins or biomolecules with high affinity to normal cells (such as immune cells or other organ-specific cells) should be avoided during EV formation. One of the main issues with EV-based DDS is rapid clearance by mononuclear phagocyte systems. The most widely used “don’t eat me” signal is to bind PEG on the vesicle surface [95]. A recent report showed that surface modification using CD47 reduced uptake by RES [96]. In the same report, a cationized mannan-modified EV derived from DC2.4 cells was administered to saturate the MPS (eat me strategy) [95]. Alternatively, metalloproteinases that are naturally found in exosomes are another important component [97]. They can regulate the proteolytic activity in exosomes, thereby altering their contents. Moreover, they can degrade the ECM, which can enhance the efficiency of exosome-mediated drug delivery.
5.4. Clinical Applications of Artificial Exosomes
The role of exosomes in cancer initiation and progression is becoming increasingly apparent from preclinical and clinical investigations (summarized in the Table 3), and therefore, they are in the spotlight for potential use as cancer therapeutics [98]. With these characteristics, there are in vitro and clinical studies which show that anti-cancer drugs can be delivered more effectively when the drug is loaded into the exosome than when only the drug is administered.
Table 3.
Studies that investigated the use of exosomes for cancer therapy [77].
| Source of Exosomes | Disease Type | Drugs | Isolation Methods |
|---|---|---|---|
| Raw 264.7 macrophages (mouse) | Multi-drug resistant cancers (in vitro and mouse models) | Doxorubicin and paclitaxel | Low-speed centrifugation with precipitating reagents and purifying column |
| Primary dendritic cells (mouse) | Breast cancer (in vitro and mouse models) | VEGF siRNA | Differential centrifugation and UC |
| Neutrophils | Malignant glioma | Doxorubicin | Ultracentrifugation |
| MSC | Colorectal cancer | Doxorubicin | Ultracentrifugation |
| Milk (bovine) | Lung cancer (in vitro and mouse models) | Paclitaxel | Differential gradient centrifugation and UC |
| MCF-7 breast carcinoma cells (human) | Breast carcinoma (in vitro) | Doxorubicin | Differential gradient centrifugation |
| LNCaP and PC-3 prostate cancer cells (human) | Prostate cancer (in vitro) | Paclitaxel | Differential centrifugation |
| Lewis lung carcinoma cells (mouse) | Lung cancer (in vitro) | Methotrexate | Differential gradient centrifugation |
| Immature dendritic cells (mouse) | Breast cancer (in vitro and mouse models) | Doxorubicin | Ultrafiltration, UC, and gradient centrifugation |
| HeLa cervical cancer cells (human) | Cervical cancer (in vitro) | Dextran | Precipitating reagents (total exosome isolation kit, Invitrogen) |
| H22 hepatocarcinoma cells (mouse) | Hepatocarcinoma (in vitro and mouse models) | Cisplatin | Differential gradient centrifugation |
| Gastric cancer (SKBR-3) | Gastric cancer | Trastuzumab | Ultracentrifugation |
| EL-4 lymphoma cells (mouse) | Tumor-induced inflammation (in vitro and mouse models) | Curcumin | Sucrose gradient centrifugation |
| Bone-marrow-derived MSCs (human) | Lung cancer (in vitro) | TRAIL | Filtration |
| Pleural mesothelioma (in vitro) | TRAIL | Filtration | |
| Renal cancer (in vitro) | TRAIL | Filtration | |
| Breast adenocarcinoma (in vitro) | TRAIL | Filtration | |
| Neuroblastoma (in vitro) | TRAIL | Filtration | |
| B16-F10 melanoma cells (mouse) | Melanoma (in vitro) | Superparamagnetic iron oxide nanoparticles | Ultracentrifugation (UC) |
| B16BL6 melanoma cells (mouse) | Melanoma (in vitro and mouse models) | CpG DNA | Filtration and differential UC |
| ADR/MCF-7 breast carcinoma cells (human) | Breast carcinoma (in vitro) | Cisplatin | Differential gradient centrifugation |
| A549 lung carcinoma cells (human) | Lung carcinoma (in vitro, mouse models, and stage IV human patients) | Doxorubicin | Differential gradient centrifugation |
| M1 macrophage | Pancreatic cancer | Gemcitabine/Deferasirox | Ultracentrifugation |
| Human breast cancer cell line (EFM-192A) | Breast cancer | Trastuzumab | Ultracentrifugation |
Like other drugs, exosomes can be administered through various routes [99]. For in vivo analysis of exosome distribution, intravenous (IV) injection of exosomes was the dominant (78%) administration route, followed by intraperitoneal injection. The administration of exosomes through intranasal, hock, subcutaneous, and retro-orbital venous sinus routes was rare. The tissues with the most frequent accumulation of exosomes after IV injection were the liver, lung, spleen, and kidney.
6. Summary and Future Perspective
Exosomes as drug delivery vehicles possess huge advantages with low immunogenicity, long-term safety, and lack of cytotoxicity [62,100]. Conventional methods of delivering miRNAs, proteins, and chemical drugs show some limitations. For example, miRNAs are easily degraded in vivo, and chemical drugs are highly toxic to healthy cells. These obstacles can be solved by using exosomes as drug carriers. Currently, natural exosomes are used in preliminary clinical trials. Their translation, massive production, stabilized preparation, storage protocols, and quality control are challenges that must be overcome. As mentioned in a previous report, EV-based drug delivery remains challenging due to a lack of standardized isolation and purification methods, limited drug loading efficiency, and insufficient clinical-grade production [101]. Further development of cell-derived artificial exosomes and their engineering for isolation, purification, and drug loading will overcome these shortcomings. Artificial exosomes have commercial advantages for their up-scale productivity. Furthermore, by anchoring specific surface molecules on exosomes, we can increase the local concentration of exosomes at target cells or target disease sites, thereby reducing the toxicity and undesirable effects and maximizing therapeutic effects. The combination of artificial exosomes with anti-cancer drugs can lead to pivotal development in the treatment of cancer. In the future, novel and multifunctional artificial exosomes will be developed to improve healthcare. Therefore, further studies are needed to explore novel strategies of exosome-mediated therapies, particularly for cancer.
Author Contributions
Conceptualization, S.C. and B.M.; investigation, B.M.; writing—original draft preparation, B.M.; writing—review and editing, S.C.; supervision, S.C.; funding acquisition, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant of the MD-Ph.D./Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare (Korea), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant number: 2021R1A2C2005472), and a grant from the Asan Institute for Life Sciences (Grant No. 2020IL0036-2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., Jemal A., Kramer J.L., Siegel R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 3.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release. 2000;65:271–284. doi: 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 5.Koo H., Huh M.S., Sun I.C., Yuk S.H., Choi K., Kim K., Kwon I.C. In vivo targeted delivery of nanoparticles for theranosis. Acc. Chem. Res. 2011;44:1018–1028. doi: 10.1021/ar2000138. [DOI] [PubMed] [Google Scholar]
- 6.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 7.Sun W., Gu Z. Engineering DNA scaffolds for delivery of anticancer therapeutics. Biomater. Sci. 2015;3:1018–1024. doi: 10.1039/C4BM00459K. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha D., Roy S., Saha P., Chatterjee N., Bishayee A. Trends in Research on Exosomes in Cancer Progression and Anticancer Therapy. Cancers. 2021;13:326. doi: 10.3390/cancers13020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thery C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 12.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng W., Hao Y., He C., Li L., Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol. Cancer. 2019;18:57. doi: 10.1186/s12943-019-0982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antimisiaris S.G., Mourtas S., Marazioti A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics. 2018;10:218. doi: 10.3390/pharmaceutics10040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana S., Saieva L., Taverna S., Alessandro R. Contribution of proteomics to understanding the role of tumor-derived exosomes in cancer progression: State of the art and new perspectives. Proteomics. 2013;13:1581–1594. doi: 10.1002/pmic.201200398. [DOI] [PubMed] [Google Scholar]
- 17.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 19.Jacob F., Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 1961;3:318–356. doi: 10.1016/S0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- 20.Frank S., Aguirre A., Hescheler J., Kurian L. A lncRNA Perspective into (Re)Building the Heart. Front. Cell Dev. Biol. 2016;4:128. doi: 10.3389/fcell.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin Z., Ljubimov V.A., Zhou C., Tong Y., Liang J. Cell-free circulating tumor DNA in cancer. Chin. J. Cancer. 2016;35:36. doi: 10.1186/s40880-016-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai R.C., Yeo R.W., Padmanabhan J., Choo A., de Kleijn D.P., Lim S.K. Isolation and Characterization of Exosome from Human Embryonic Stem Cell-Derived C-Myc-Immortalized Mesenchymal Stem Cells. Methods Mol. Biol. 2016;1416:477–494. doi: 10.1007/978-1-4939-3584-0_29. [DOI] [PubMed] [Google Scholar]
- 23.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi Y.W., Lee J.H., Kim S.Y., Pack C.G., Ha D.H., Park S.R., Youn J., Cho B.S. Advances in Analysis of Biodistribution of Exosomes by Molecular Imaging. Int. J. Mol. Sci. 2020;21:665. doi: 10.3390/ijms21020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Justiz Vaillant A.A., Sabir S., Jan A. Physiology, Immune Response. StatPearls; Treasure Island, FL, USA: 2021. [Google Scholar]
- 26.Dai J., Su Y., Zhong S., Cong L., Liu B., Yang J., Tao Y., He Z., Chen C., Jiang Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020;5:145. doi: 10.1038/s41392-020-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenjaroenpun P., Kremenska Y., Nair V.M., Kremenskoy M., Joseph B., Kurochkin I.V. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ. 2013;1:e201. doi: 10.7717/peerj.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman R.M. Decisions about dendritic cells: Past, present, and future. Annu. Rev. Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 29.Chaput N., Taieb J., Schartz N.E., Andre F., Angevin E., Zitvogel L. Exosome-based immunotherapy. Cancer Immunol. Immunother. 2004;53:234–239. doi: 10.1007/s00262-003-0472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viaud S., Thery C., Ploix S., Tursz T., Lapierre V., Lantz O., Zitvogel L., Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: What’s next? Cancer Res. 2010;70:1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 31.Hao S., Liu Y., Yuan J., Zhang X., He T., Wu X., Wei Y., Sun D., Xiang J. Novel exosome-targeted CD4+ T cell vaccine counteracting CD4+25+ regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8+ CTL responses. J. Immunol. 2007;179:2731–2740. doi: 10.4049/jimmunol.179.5.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J., Gao F.X., Wang C., Qin M., Han F., Xu T., Hu Z., Long Y., He X.M., Deng X., et al. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019;38:321. doi: 10.1186/s13046-019-1310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fais S. NK cell-released exosomes: Natural nanobullets against tumors. Oncoimmunology. 2013;2:e22337. doi: 10.4161/onci.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Pace A.L., Tumino N., Besi F., Alicata C., Conti L.A., Munari E., Maggi E., Vacca P., Moretta L. Characterization of Human NK Cell-Derived Exosomes: Role of DNAM1 Receptor in Exosome-Mediated Cytotoxicity Against Tumor. Cancers. 2020;12:661. doi: 10.3390/cancers12030661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Federici C., Shahaj E., Cecchetti S., Camerini S., Casella M., Iessi E., Camisaschi C., Paolino G., Calvieri S., Ferro S., et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Front. Immunol. 2020;11:262. doi: 10.3389/fimmu.2020.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu L., Kalimuthu S., Gangadaran P., Oh J.M., Lee H.W., Baek S.H., Jeong S.Y., Lee S.W., Lee J., Ahn B.C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics. 2017;7:2732–2745. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone K.D., Prussin C., Metcalfe D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekstrom K., Valadi H., Sjostrand M., Malmhall C., Bossios A., Eldh M., Lotvall J. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J. Extracell. Vesicles. 2012;1:1–12. doi: 10.3402/jev.v1i0.18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun B., Peng J., Wang S., Liu X., Zhang K., Zhang Z., Wang C., Jing X., Zhou C., Wang Y. Applications of stem cell-derived exosomes in tissue engineering and neurological diseases. Rev. Neurosci. 2018;29:531–546. doi: 10.1515/revneuro-2017-0059. [DOI] [PubMed] [Google Scholar]
- 40.Li F., Wang Y., Lin L., Wang J., Xiao H., Li J., Peng X., Dai H., Li L. Mast Cell-Derived Exosomes Promote Th2 Cell Differentiation via OX40L-OX40 Ligation. J. Immunol. Res. 2016;2016:3623898. doi: 10.1155/2016/3623898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skokos D., Botros H.G., Demeure C., Morin J., Peronet R., Birkenmeier G., Boudaly S., Mecheri S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J. Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 42.Vargas A., Roux-Dalvai F., Droit A., Lavoie J.P. Neutrophil-Derived Exosomes: A New Mechanism Contributing to Airway Smooth Muscle Remodeling. Am. J. Respir. Cell Mol. Biol. 2016;55:450–461. doi: 10.1165/rcmb.2016-0033OC. [DOI] [PubMed] [Google Scholar]
- 43.Shao S., Fang H., Zhang J., Jiang M., Xue K., Ma J., Zhang J., Lei J., Zhang Y., Li B., et al. Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis via activating keratinocytes. FASEB J. 2019;33:6813–6828. doi: 10.1096/fj.201802090RR. [DOI] [PubMed] [Google Scholar]
- 44.Brook A.C., Jenkins R.H., Clayton A., Kift-Morgan A., Raby A.C., Shephard A.P., Mariotti B., Cuff S.M., Bazzoni F., Bowen T., et al. Neutrophil-derived miR-223 as local biomarker of bacterial peritonitis. Sci. Rep. 2019;9:10136. doi: 10.1038/s41598-019-46585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen E.R., Lempke S.L., Miller M.M., Bush D.M., Braswell B.G., Estes C.L., Benedict E.L., Mahon A.R., Sabo S.L., Greenlee-Wacker M.C. Effect of extracellular vesicles from S. aureus-challenged human neutrophils on macrophages. J. Leukoc. Biol. 2020;108:1841–1850. doi: 10.1002/JLB.3AB0320-156R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genschmer K.R., Russell D.W., Lal C., Szul T., Bratcher P.E., Noerager B.D., Abdul Roda M., Xu X., Rezonzew G., Viera L., et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell. 2019;176:113–126.e15. doi: 10.1016/j.cell.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianco P., Robey P.G., Simmons P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teixeira F.G., Carvalho M.M., Sousa N., Salgado A.J. Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell. Mol. Life Sci. 2013;70:3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomari H., Forouzandeh Moghadam M., Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. OncoTargets Ther. 2018;11:5753–5762. doi: 10.2147/OTT.S173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., Corbett A.L., Taatizadeh E., Tasnim N., Little J.P., Garnis C., Daugaard M., Guns E., Hoorfar M., Li I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3:011503. doi: 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y.J., Wu J.Y., Liu J., Xu W., Qiu X., Huang S., Hu X.B., Xiang D.X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnol. 2021;19:242. doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gee P., Lung M.S.Y., Okuzaki Y., Sasakawa N., Iguchi T., Makita Y., Hozumi H., Miura Y., Yang L.F., Iwasaki M., et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat. Commun. 2020;11:1334. doi: 10.1038/s41467-020-14957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts T.C., Langer R., Wood M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020;19:673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S.D., Huang L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Biophys. Acta. 2009;1788:2259–2266. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohamed M., Abu Lila A.S., Shimizu T., Alaaeldin E., Hussein A., Sarhan H.A., Szebeni J., Ishida T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019;20:710–724. doi: 10.1080/14686996.2019.1627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishida T., Ichihara M., Wang X., Yamamoto K., Kimura J., Majima E., Kiwada H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dams E.T., Laverman P., Oyen W.J., Storm G., Scherphof G.L., van Der Meer J.W., Corstens F.H., Boerman O.C. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 59.Semple S.C., Harasym T.O., Clow K.A., Ansell S.M., Klimuk S.K., Hope M.J. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic Acid. J. Pharmacol. Exp. Ther. 2005;312:1020–1026. doi: 10.1124/jpet.104.078113. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez P.L., Harada T., Christian D.A., Pantano D.A., Tsai R.K., Discher D.E. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen C.C., Liu L., Ma F., Wong C.W., Guo X.E., Chacko J.V., Farhoodi H.P., Zhang S.X., Zimak J., Segaliny A., et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Andaloussi S., Lakhal S., Mager I., Wood M.J. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug. Deliv. Rev. 2013;65:391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Rahman M.M., Shimizu K., Yamauchi M., Takase H., Ugawa S., Okada A., Inoshima Y. Acidification effects on isolation of extracellular vesicles from bovine milk. PLoS ONE. 2019;14:e0222613. doi: 10.1371/journal.pone.0222613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wasik M., Nazimek K., Nowak B., Askenase P.W., Bryniarski K. Delayed-Type Hypersensitivity Underlying Casein Allergy Is Suppressed by Extracellular Vesicles Carrying miRNA-150. Nutrients. 2019;11:907. doi: 10.3390/nu11040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witwer K.W., Buzas E.I., Bemis L.T., Bora A., Lasser C., Lotvall J., Nolte-’t Hoen E.N., Piper M.G., Sivaraman S., Skog J., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maroto R., Zhao Y., Jamaluddin M., Popov V.L., Wang H., Kalubowilage M., Zhang Y., Luisi J., Sun H., Culbertson C.T., et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles. 2017;6:1359478. doi: 10.1080/20013078.2017.1359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trenkenschuh E., Richter M., Heinrich E., Koch M., Fuhrmann G., Friess W. Enhancing the Stabilization Potential of Lyophilization for Extracellular Vesicles. Adv. Healthc. Mater. 2021:e2100538. doi: 10.1002/adhm.202100538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tauro B.J., Greening D.W., Mathias R.A., Ji H., Mathivanan S., Scott A.M., Simpson R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheruvanky A., Zhou H., Pisitkun T., Kopp J.B., Knepper M.A., Yuen P.S., Star R.A. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Ren. Physiol. 2007;292:F1657–F1661. doi: 10.1152/ajprenal.00434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J.W., Wieckowski E., Taylor D.D., Reichert T.E., Watkins S., Whiteside T.L. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin. Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 72.Sidhom K., Obi P.O., Saleem A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020;21:6466. doi: 10.3390/ijms21186466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corso G., Mager I., Lee Y., Gorgens A., Bultema J., Giebel B., Wood M.J.A., Nordin J.Z., Andaloussi S.E. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017;7:11561. doi: 10.1038/s41598-017-10646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel G.K., Khan M.A., Zubair H., Srivastava S.K., Khushman M., Singh S., Singh A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019;9:5335. doi: 10.1038/s41598-019-41800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanada M., Bachmann M.H., Hardy J.W., Frimannson D.O., Bronsart L., Wang A., Sylvester M.D., Schmidt T.L., Kaspar R.L., Butte M.J., et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA. 2015;112:E1433–E1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Familtseva A., Jeremic N., Tyagi S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019;459:1–6. doi: 10.1007/s11010-019-03545-4. [DOI] [PubMed] [Google Scholar]
- 77.Walker S., Busatto S., Pham A., Tian M., Suh A., Carson K., Quintero A., Lafrence M., Malik H., Santana M.X., et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–8017. doi: 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu H., Chen L., Peng Y., Yu S., Liu J., Wu L., Zhang L., Wu Q., Chang X., Yu X., et al. Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget. 2018;9:2887–2894. doi: 10.18632/oncotarget.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hood J.L., San R.S., Wickline S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y., Liu Y., Liu H., Tang W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ilkhani K., Bastami M., Delgir S., Safi A., Talebian S., Alivand M.R. The Engaged Role of Tumor Microenvironment in Cancer Metabolism: Focusing on Cancer-Associated Fibroblast and Exosome Mediators. Anticancer Agents Med. Chem. 2021;21:254–266. doi: 10.2174/1871520620666200910123428. [DOI] [PubMed] [Google Scholar]
- 82.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 83.Ohlund D., Elyada E., Tuveson D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lambrechts D., Wauters E., Boeckx B., Aibar S., Nittner D., Burton O., Bassez A., Decaluwe H., Pircher A., Van den Eynde K., et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 85.Roma-Rodrigues C., Fernandes A.R., Baptista P.V. Exosome in tumour microenvironment: Overview of the crosstalk between normal and cancer cells. Biomed. Res. Int. 2014;2014:179486. doi: 10.1155/2014/179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan X., Qian N., Ling S., Li Y., Sun W., Li J., Du R., Zhong G., Liu C., Yu G., et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics. 2021;11:1429–1445. doi: 10.7150/thno.45351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou W., Fong M.Y., Min Y., Somlo G., Liu L., Palomares M.R., Yu Y., Chow A., O’Connor S.T., Chin A.R., et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raskov H., Orhan A., Gaggar S., Gogenur I. Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in Cancer and Cancer Immunotherapy. Front. Oncol. 2021;11:668731. doi: 10.3389/fonc.2021.668731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eguchi T., Taha E.A., Calderwood S.K., Ono K. A Novel Model of Cancer Drug Resistance: Oncosomal Release of Cytotoxic and Antibody-Based Drugs. Biology. 2020;9:47. doi: 10.3390/biology9030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wyciszkiewicz A., Kalinowska-Lyszczarz A., Nowakowski B., Kazmierczak K., Osztynowicz K., Michalak S. Expression of small heat shock proteins in exosomes from patients with gynecologic cancers. Sci. Rep. 2019;9:9817. doi: 10.1038/s41598-019-46221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kulkarni P., Haldar M.K., Karandish F., Confeld M., Hossain R., Borowicz P., Gange K., Xia L., Sarkar K., Mallik S. Tissue-Penetrating, Hypoxia-Responsive Echogenic Polymersomes for Drug Delivery to Solid Tumors. Chemistry. 2018;24:12490–12494. doi: 10.1002/chem.201802229. [DOI] [PubMed] [Google Scholar]
- 93.Zuo H.D., Yao W.W., Chen T.W., Zhu J., Zhang J.J., Pu Y., Liu G., Zhang X.M. The effect of superparamagnetic iron oxide with iRGD peptide on the labeling of pancreatic cancer cells in vitro: A preliminary study. Biomed. Res. Int. 2014;2014:852352. doi: 10.1155/2014/852352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 95.Belhadj Z., He B., Deng H., Song S., Zhang H., Wang X., Dai W., Zhang Q. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J. Extracell. Vesicles. 2020;9:1806444. doi: 10.1080/20013078.2020.1806444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fam S.Y., Chee C.F., Yong C.Y., Ho K.L., Mariatulqabtiah A.R., Tan W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials. 2020;10:787. doi: 10.3390/nano10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimoda M., Khokha R. Metalloproteinases in extracellular vesicles. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1989–2000. doi: 10.1016/j.bbamcr.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 98.Zhou X., Xie F., Wang L., Zhang L., Zhang S., Fang M., Zhou F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell. Mol. Immunol. 2020;17:323–334. doi: 10.1038/s41423-020-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marcus M.E., Leonard J.N. FedExosomes: Engineering Therapeutic Biological Nanoparticles that Truly Deliver. Pharmaceuticals. 2013;6:659–680. doi: 10.3390/ph6050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meng W., He C., Hao Y., Wang L., Li L., Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug. Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.