Abstract
Objective: The purpose of this meta-analysis was to examine the effects of physical activity (PA) on cognition and activities of daily living in adults with Alzheimer’s Disease (AD). Methods: Six electronic databases (MEDLINE, CINAHL, PsycArticles, SPORTDiscus, EMBASE and CNKI) were used to search for potential studies from inception until October 2021. Randomized controlled trials (RCTs) investigating the effect of physical activity (PA) on cognition and activities of daily living in AD patients compared to a control condition were included. The effect sizes were synthesized using a random effects model with a 95% confidence interval (CI). Results: Sixteen articles including 945 participants (aged 70 to 88 years, 34.6% male) met the inclusion criteria. The pooled effect sizes demonstrated that PA intervention was associated with significant improvements in global cognition (Standard Mean Difference (SMD) = 0.41, 95% CI [0.24, 0.58], p < 0.01) and activities of daily living (SMD = 0.56, 95% CI [0.32, 0.79], p < 0.01) in AD patients. Subgroup analyses suggested that PA for 3–4 times per week for 30–45 min for more than 12 weeks had a relatively strong effect on improving global cognition in AD patients. The sensitivity analysis showed robust results. Conclusions: The findings from the current meta-analysis suggested that AD patients can improve their global cognition and Activities of Daily Living (ADL) through engaging in aerobic and mixed exercise (aerobic and anaerobic exercise).
Keywords: physical exercise, cognitive function, activities of daily living, Alzheimer’s disease
1. Introduction
Alzheimer’s disease (AD) is the most common type of dementia, accounting for 60–80% of the confirmed cases. It is a chronically non-communicable, irreversible, neurodegenerative disorder, primarily appearing in the population aged 65 years and older [1,2]. The causes of AD are multifactorial, including gene mutation, age, having less physical activity, lower education levels, and cardiovascular disease and complications [1]. Individuals diagnosed with AD have different symptoms, including memory impairment, apathy, disorientation, behavior changes, and even difficulty walking and speaking. Moreover, AD can result in a poor quality of life and a loss of independence [1]. According to the World Health Organization (WHO), approximately 50 million people have been diagnosed with dementia worldwide, and the recently estimated number will be increased to 82 million in 2030 and 152 million in 2050 [3].
Currently, faced with the above problem, it is necessary to adopt more effective methods to improve AD patients’ quality of life and health outcomes. Nevertheless, there are no specific and compelling drugs for treating AD, so researchers have a strong awareness of the shift from pharmacotherapy to physical activity (PA) and expect to find a cure approach for AD. A growing body of studies has suggested that PA has been considered a non-pharmacological therapy associated with improved mental health (e.g., depression and anxiety) and cardiovascular fitness [4,5,6]. Moreover, existing evidence has shown a positive relationship between more PA and cognitive performance in adults [7,8,9]. Therefore, as a complementary therapy, the PA plays a vital role in improving physical fitness, mental health, and even cognition in different populations. Specific to unhealthy aging, the existing reviews have not presented a comprehensive understanding of the effects of PA on cognition in AD patients. Previous studies have suggested that older adults should engage in PA to slow down cognitive impairment, especially dementia patients [10,11,12,13]. For instance, a review study with 26 longitudinal studies conducted by Blondell et al. found that a higher level of physical activity (i.e., aerobic and anaerobic exercise) was associated with a reduced risk of cognitive decline in dementia [14]. However, positive results are not always observed in systematic reviews. A recent systematic review conducted by Borges-Machado et al. shows uncertain effects of exercise on cognition in adults with dementia [15]. Similarly, Demurtas and colleagues could not be certain that PA improved cognition in dementia patients [16]. To support this point, a recent meta-analysis study including 11 studies showed no significant improvements in a Mini–Mental State Examination (MMSE) test [17].
In addition to cognitive decline, dementia patients also have a significant impairment in physical function, especially for Activities of Daily Living (ADL). The level of ADL capacity directly affects the quality of life and the burden cost of care in patients [18,19,20]. Many studies have documented that PA is a crucial way to improve muscle strength, muscle mass, balance, ADL dependence [2,21,22], and therefore their quality of life [23]. For example, exercise (e.g., aerobic and resistance exercise) helped improve the daily life capacity in individuals with dementia [24]. A recent study found that dementia patients showed improvements in ADL performance [15] and quality of life [25] after doing long-term PA. However, most systematic review studies have investigated ADL in adults with dementia, and little research has been done on the effects of exercise and ADL in people with AD. Meanwhile, the existing evidence from different studies is also inconsistent [10,26].
Although many systematic review studies have examined the effects of exercise on cognition and physical performance in adults with AD, they have commonly shown inconclusive results due to inconsistent inclusion criteria, considerable heterogeneity (I2 ≈ 80%) across studies, a small number of included studies, and different types of dementia populations (e.g., Lewy body dementia and vascular dementia) [16,17,27]. They have considered the limitations of previous studies and the fact that a growing body of new RCT studies published in Chinese and English language is investigating the effect of exercise on cognition and ADL in populations with AD. Promising and robust evidence is expected to confirm the effects of PA on promoting cognitive function and ADL in individuals with AD. Therefore, there is a need to conduct an updated meta-analysis (1) to investigate the effects of PA on global cognition and ADL in adults with sole AD; and (2) to evaluate the impacts of the attributes of PA on cognition using moderator analysis based on the exercise dose (frequency, type, time, and duration), the stage of AD, and the study design.
2. Methods
2.1. Search Strategy
Articles were retrieved from six electronic databases (MEDLINE, CINAHL, PsycArticles, SPORTDiscus, EMBASE and CNKI) from their inception until October 2021. In each database, we combined the following groups of terms for potential studies: (1) “physical activity” OR “physical training” OR “exercise” OR “aerobic exercise” OR “cycling” OR “walking” OR “resistance training” OR “strength exercise”; (2) “Alzheimer’s disease” OR “Alzheimer*” OR “AD”; (3) “randomized controlled trials”. Additional articles were manually found by studying the reference lists of the included studies. PRISMA (Preferred Repointing Items for Systematic Reviews and Meta-Analyses) was used in the current study [28].
2.2. Inclusion and Exclusion Criteria
The included articles met the following criteria: (i) participants had a clinical diagnosis of AD based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA), as well as the Diagnostic and Statistical Manual of Mental Health Disorders (DSM-IV); (ii) the interventions involved one or more similar PA therapies (e.g., walking, or walking and stretching) in the experimental group and either a placebo-control condition or a control condition, without using any other exercise interventions in the control group; (iii) the study design consisted of randomized controlled trials (RCTs); (iv) at least one outcome measurement (i.e., ADL and global cognition, e.g., (Mini-Mental State Examination (MMSE), the Cognitive section of the Alzheimer’s Disease Assessment Scale (ADAS-Cog), and the Rapid Evaluation of Cognitive Function (ERFC))) was reported; (v) studies were published in English or Chinese. Studies were excluded when (i) they were animal or in vitro studies, abstracts, case reports, reviews, or trial protocols; (ii) there were participants with other types of dementia (e.g., vascular dementia) or mild cognitive impairment; (iii) outcome measurements could not be used to calculate the effect size (ES).
2.3. Data Extraction and Quality Assessment
Eligible studies were evaluated by two independent authors, screening the titles, abstracts, and full texts. When disagreement appeared, a third author would be consulted. The following information was extracted from each included article: author, year of publication, characteristics of subjects, sample size, gender, age, intervention protocol (frequency, time, type, duration and intensity), outcome measurements (ADL and global cognition (e.g., MMSE and ADAS-Cog)), and raw data for effect size calculation.
The eligibility studies’ risk of bias was assessed by two independent reviewers, using the Physiotherapy Evidence Database (PEDro) scale [29], which is a systematic tool to assess the study quality. This scale encompasses 11 items, of which item 1 is not involved in calculating the total scores. The total valid scores range from 0 to 10. The methodological quality classifications are excellent (9–10 points), good (6–8 points), fair (4–5 points) and poor (<4 points) [29].
2.4. Statistical Analysis
Meta-analysis was performed using the Comprehensive Meta-Analysis software (v2 software, Biostat, Englewood, NJ, USA). The continuous data on cognition and ADL were extracted from the included studies. Standardized mean difference (SMD) was expressed as the effect size (ES) measure by calculating the mean change from pre- to post-intervention in the experimental and control groups, respectively. The standard deviation was calculated using the equation: SD = SE*. The ESs were calculated using a random effects model with a 95% confidence interval (CI) to avoid a high risk of false-positive results [30]. The pooled ESs were presented in a forest plot, and a positive ES value reflected a more effective PA. ES was commonly classified into three levels: small (0.2–0.39), moderate (0.4–0.60), and large (≥0.6) [31]. Heterogeneity was examined using the I2 statistic for each analysis: I2 < 25% (low heterogeneity), I2 < 50% (moderate heterogeneity), and I2 < 70% (high heterogeneity) [32]. Prediction intervals (PIs) were measured to reflect the variation in intervention effects when more than 10 studies were included in the meta-analysis according to the formula: 95% PI = MD ± 2 Tau2 [33]. Sensitivity analysis was performed to evaluate the stability of the results by excluding one study each time. Lastly, the funnel plot, Begg’s test, and Egger’s regression test were employed to examine the publication bias. Duvall and Tweedie’s trim and fill analysis was used to recalculate a new ES by adjusting the outlier values. The significance level was set at p < 0.05.
In addition, to investigate the confounding factors (study design, the stage of AD, location, and exercise principles), we performed subgroup analyses and meta-regression tests accordingly. In terms of the exercise principle, a prior study indicated that exercise modalities were likely to influence the findings [34]. Based on the included studies, we slightly modified the cut-off of the exercise duration (short-term: ≤12 weeks; medium-term: 13–23 weeks; long-term: ≥24 weeks) and session time (medium: 30–45 min; longer: >46 min) based on the American College of Sports Medicine [35]. With regard to gender, according to the proportion of males and females in original studies, we coded a high male percentage as ≥40% and a high female percentage as >60%.
3. Results
3.1. Search Results
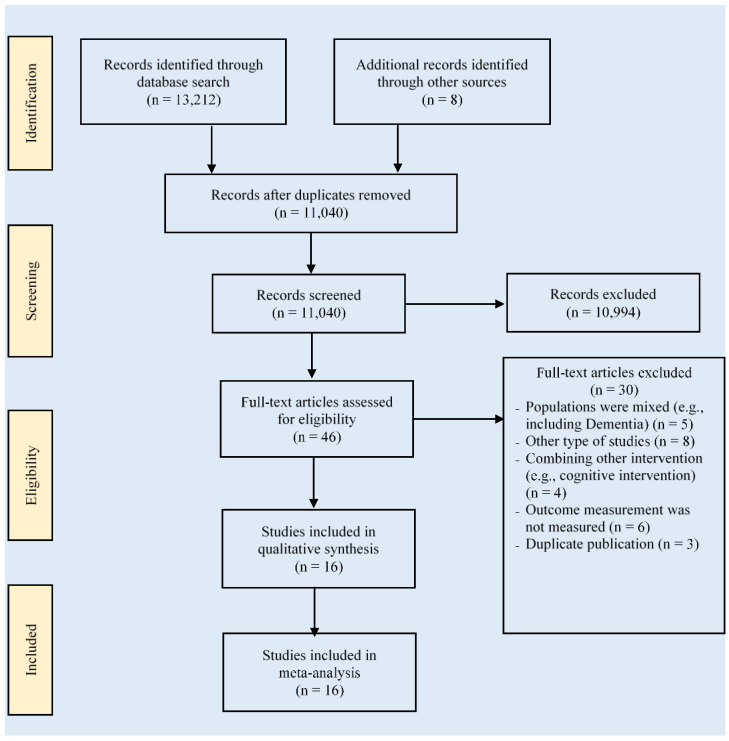
Figure 1 depicts the flowchart of the study selection for this meta-analysis. The initial search record of studies was 13,220. After removing duplicates (n = 2180), 11,040 studies were remaining. Furthermore, 10,994 studies were eliminated after reviewing the titles and abstracts. As a result of the screening process, 30 articles were excluded by assessing the full texts (n = 46), and 16 RCTs [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] met the inclusion criteria in this meta-analysis.
Figure 1.
The flowchart of study selection based on PRISMA.
3.2. Characteristics of Included Studies
Detailed information of the included studies is shown in Table 1. All studies were published after 2010. The included studies were from Asia, South America, and Europe. The sample size ranged from 20 to 200. A total of 945 participants were diagnosed with mild to severe AD (approximately 34.6% of males). The mean age of the participants ranged from 70 to 88 years. PA protocols were multiple interventions across the included studies. Eight studies used aerobic exercise (e.g., walking, cycling and gymnastics) [43,44,46,47,49,50,51,52], and eight studies used mixed exercises (aerobic exercise and resistance training) [37,38,39,40,41,42,45,48]. Moderate-intensity PA was used in all studies, except one where moderate to high intensity was used [40]. PA duration ranged from three to six months, and the training time for each session was 30 to 90 min, 2–7 times weekly. The participants in the control group of 11 studies [36,37,39,40,41,43,44,45,47,48,50] were mainly given the usual medical treatment (e.g., donepezil). Five studies used social activity [38,42,45,46,49], two studies used cognitive treatment [39,47], and one study used a health education intervention [51]. Regarding outcomes, global cognition (MMSE, ADAS-Cog and ERFC) and ADL (Barthel Index and Alzheimer’s Disease Cooperative Study—Activities of Daily Living Inventory) were usually considered in sixteen [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] and eight studies [36,38,39,41,44,48,49,50], respectively.
Table 1.
Characteristics of the included randomized controlled trials.
| Study/Country | Severity of AD Age (Years) Total Sample Size Male (%) |
Diagnostic Criteria | Time/Frequency/ Duration/Intensity |
Intervention Protocol | Outcomes (Instrument) | |
|---|---|---|---|---|---|---|
| Experiment (Details) | Control | |||||
| Chang, 2015 [36] China |
Mild to moderate 70.5 n = 57 38.6% |
DSM-IV MMSE |
60–90 min/3 times/week 16 weeks 50–70% HRmax moderate |
aerobic exercise (cycling, walking) | usual medical treatment | global cognition (MMSE) ADL |
| de Oliveira Silva, 2019 [37] Brazil |
Mild 79.4 n = 27 40.7% |
DSM-IV | 60 min/2 times/week 12 weeks 80% HRmax moderate |
mixed exercises (treadmill, weight-lifting, balance exercises) | usual medical treatment | global cognition (MMSE) |
| de Souto Barreto, 2017 [38] France |
Mod to severe 87.5 n = 91 15.4% |
DSM-IV MMSE |
60 min/2 times/week 24 weeks moderate |
mixed exercises (walking, weight-lifting, coordination, balance exercises) | social activity | global cognition (MMSE) ADL |
| Fonte, 2019 [39] Italy |
Mild 79 n = 60 35% |
NINCDS-ADRDA | 90 min/3 times/week 24 weeks 70% HRmax moderate |
mixed exercises (cycling, walking, weight-lifting) | C1: cognitive treatment C2: usual medical treatment |
global cognition (ADAS-Cog) ADL |
| Hoffmann, 2016 [40] Denmark |
Mild 70.5 n = 200 56.5% |
NINCDS-ADRDA | 60 min/3 times/week 16 weeks 70-80% HRmax moderate-high |
mixed exercise (ergometer bicycle, cross-trainer, treadmill, strength training) | usual medical treatment | global cognition (MMSE) |
| Holthoff, 2015 [41] Germany |
Mild or moderate 71.5 n = 30 50% |
NINCDS-ADRDA | 30 min/3 times/week 12 weeks moderate |
mixed exercises (movement trainer, resistance training) | usual medical treatment | global cognition (MMSE) ADL |
| Kemoun, 2010 [42] France |
Moderate to severe 82 n = 31 26% |
DSM-IV MMSE |
60 min/3 times/week 15 weeks moderate |
mixed exercises (walking, equilibrium, stamina, ergocycle with the arms and the legs) | social activity | global cognition (ERFC) |
| Liu, 2017 [43] China |
Mild 70.5 n = 48 43.8% |
MRI MMSE |
40 min/3 times/week 12 weeks moderate |
aerobic exercise (aerobic gymnastics: rowing movement, kicking movement) | usual medical treatment | global cognition (MMSE) |
| Mu, 2016 [44] China |
Mild to moderate 73 n = 78 37.2% |
NINCDS-ADRDA MMSE |
60 min/3 tims/week 16 weeks Moderate |
aerobic exercise (brisk walking) |
usual medical treatment | global cognition (MMSE) ADL (Barthel Index) |
| Pedroso, 2018 [45] Brazil |
Mild to moderate 78.3 n = 57 24.6% |
DSM-IV | 60 min/3 times/week 12 weeks 60–75% HRmax Moderate |
mixed exercises (walking, stretching, jogging, climbing, and descending stairs) | C1: social activity C2: usual medical treatment |
global cognition (MMSE) |
| Venturelli, 2011 [46] Italy |
Severe 84 n = 24 0% |
DSM-IV MMSE |
>30 min/4 times/week 24 weeks moderate |
aerobic exercise (walking) |
social activity | global cognition (MMSE) |
| Venturelli, 2016 [47] Italy |
Moderate to severe 84 n = 40 30% |
DSM-IV MMSE |
60 min/5 times/week 3 months moderate |
aerobic exercise (walking) |
cognitive treatment | global cognition (MMSE) |
| Vreugdenhil, 2012 [48] Australia |
Mild 74 n = 40 (16/24) 40% |
NINCDS-ADRDA | >30 min/7 times/week 4 months |
mixed exercises (brisk walking, upper and lower body strength, balance training) |
usual medical treatment | global cognition (MMSE) ADL |
| Wang, W 2014 [49] China |
Mild to moderate 70.5 n = 54 38.9% |
NINCDS-ADRDA | 40 min/3tims/week 6 months Moderate 70% HRmax |
aerobic exercise (cycling) |
social activity | global cognition (MMSE) ADL |
| Wang, Y 2014 [50] China |
Mild to moderate 70.7 n = 39 33.3% |
NINCDS-ADRDA | 30 min/3 times/week 12 weeks 50% HR reserve |
aerobic exercise (cycling) |
usual medical treatment | global cognition (MMSE) ADL |
| Yang, 2015 [51] China |
Mild to moderate 72 n = 50 34% |
NINCDS-ADRDA MMSE |
40 min/3 times/week 12 weeks 70% HRmax |
aerobic exercise (cycling) |
health education | global cognition (MMSE) |
Note: Total sample size means total number of samples in one experimental study; Male%: the Male percentage in the total sample size; ADAS-Cog: Cognitive section of the Alzheimer’s Disease Assessment Scale; ADL: Alzheimer’s Disease Cooperative Study–Activities of Daily Living Inventory; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition; ERFC: Rapid Evaluation of Cognitive Function; HR: Heart Rate; MMSE: Mini-Mental State Examination; MRI: Magnetic resonance imaging; (NINCDS-ADRDA: National Institute of Neurological and Communication Disorders and Stroke–Alzheimer Disease and Related Disorders Association.
According to the Physiotherapy Evidence Database (PEDro) scale, the methodological quality of each study is presented in Table 2. The study quality score fluctuated from six to eight, implying that all studies were considered to be of good methodological quality. Concealed allocation was used in eight studies [37,38,39,40,42,44,49,50]. Only two studies performed a blinding method to measure the outcomes [39,40].
Table 2.
Methodological quality of the included studies (PEDro analysis).
| Study | Score | Methodological Quality | PEDro Item Number | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Chang, 2015 [36] | 5 | Fair | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| de Oliveira Silva, 2019 [37] | 7 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| de Souto Barreto, 2017 [38] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Fonte, 2019 [39] | 8 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Hoffmann, 2016 [40] | 8 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Holthoff, 2015 [41] | 5 | Fair | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Kemoun, 2010 [42] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Liu, 2017 [43] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Mu, 2016 [44] | 7 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Pedroso, 2018 [45] | 5 | Fair | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Venturelli, 2011 [46] | 5 | Fair | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Venturelli, 2016 [47] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Vreugdenhil, 2012 [48] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Wang, W 2014 [49] | 7 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Wang, Y 2014 [50] | 7 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Yang, 2015 [51] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
Note: PEDro, Physiotherapy Evidence Database scale; Studies were classified as having excellent (9–10), good (6–8), fair (4–5), or poor (<4). Scale of item score: ✔, present. The PEDro scale involves (1) eligibility criteria; (2) random allocation; (3) concealed allocation; (4) similarity at baseline on key measures; (5) participant blinding; (6) instructor blinding; (7) assessor blinding; (8) more than 85% retention rate of at least one outcome; (9) intention-to-treat analysis; (10) between-group statistical comparison for at least one outcome; (11) point estimates and measures of variability provided for at least one outcome.
3.3. Effects of PA Intervention on Global Cognition
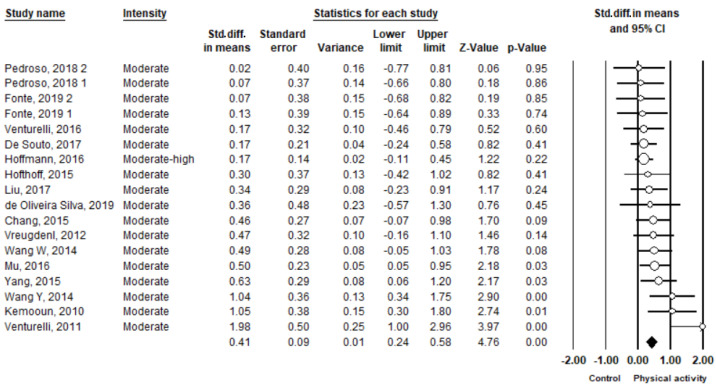
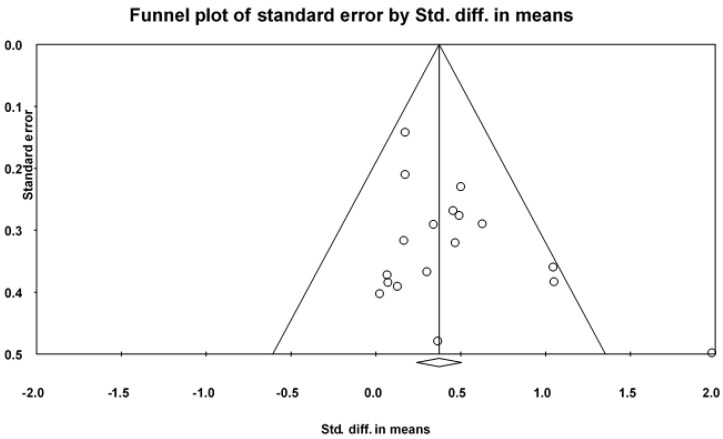
The pooled result from 18 studies showed a significant improvement in favor of PA (SMD = 0.41, 95% CI 0.24 to 0.58, I2 = 30%, p < 0.01; 95% PI = −0.04 to 0.85) (Figure 2, Supplement Figure S1). However, the PI revealed that PA may not elevate global cognition in more than 5% of trial settings. By removing one study at a time, the pooled result of global cognition was stable. A symmetrical funnel plot was shown in Figure 3, indicating no publication bias for global cognition. Begg’s test (Kendall’s tau = 0.07, p = 0.74) and Egger’s test (intercept = 1.48, p = 0.08) indicated no publication bias. Furthermore, the ES was recalculated using Duvall and Tweedie’s trim and fill procedure, adding two studies on the right sides of the funnel plot, and an adjusted ES was SMD = 0.40 (95% CI 0.28 to 0.60, p < 0.05).
Figure 2.
Forest plot showing the effects of physical activity vs. control on global cognition.
Figure 3.
The funnel plot of publication bias for global cognition.
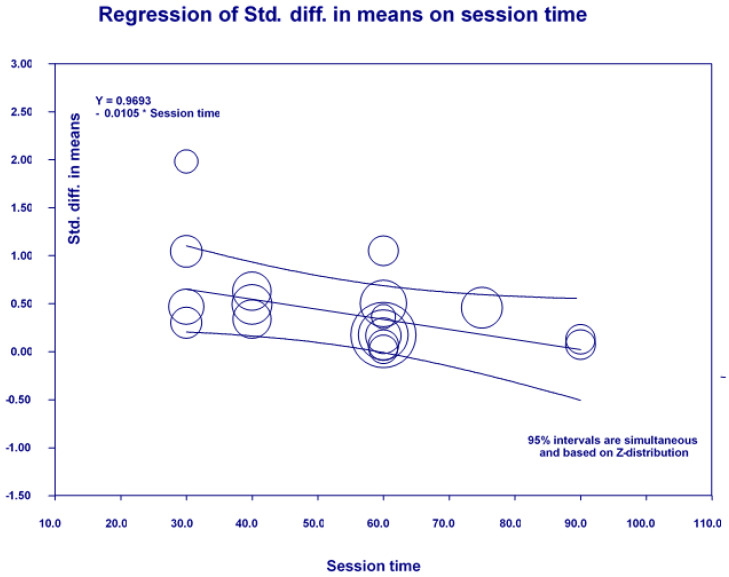
Additionally, we conducted subgroup analyses to examine potential factors affecting PA on cognition, shown in Table 3. There were significant differences in how exercise type (Q(1) = 4.69, p = 0.03), exercise session time (Q(1) = 4.41, p = 0.04), and AD stage (Q(1) = 6.26, p = 0.04) affected global cognition. This suggests that aerobic exercise (SMD = 0.60, 95% CI 0.32–0.88) had a greater effect than mixed exercises (SMD = 0.24, 95% CI 0.06–0.41) on global cognition. Moreover, an exercise session time of less than 45 min (SMD = 0.66, 95% CI 0.33–0.99) had a greater effect on global cognition than one of more than 45 min (SMD = 0.27, 95% CI 0.11–0.42). A moderate to severe AD stage (SMD = 0.75, 95% CI 0.03–1.47) had a greater effect on global cognition than a mild to moderate AD stage (SMD = 0.20, 95%CI −0.003–0.39). However, there were no significant differences in exercise duration (Q(1) = 0.22, p = 0.64), frequency per week (Q(2) = 0.79, p = 0.67), the stages of AD (Q(2) = 5.43, p = 0.07), control group type (Q(3) = 2.72, p = 0.44), or study quality (Q(1) = 0.24, p = 0.62). In addition, among six continuous predictors (the exercise session time, duration (weeks), frequency per week, total training time during the experiment, participant age, and male percentage) in meta-regression analyses, the session time generated a significant effect on global cognition (β = −0.0105, p = 0.03) (see Table 3 and Figure 4).
Table 3.
Moderator analysis for the effects of physical activity vs. control intervention on global cognition.
| Moderator | Level | Number of Trials | Sub-Analysis | Between-Group Homogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| SMD | 95% CI | I2 % | Q-Value | df(Q) | p-Value | |||
| Type | Aerobic exercise | 8 | 0.60 | 0.32 to 0.88 | 43.6% | 4.69 | 1 | 0.03 |
| Mixed exercises | 10 | 0.24 | 0.06 to 0.41 | 0% | ||||
| Duration | ≤12 weeks | 8 | 0.39 | 0.15 to 0.63 | 0% | 0.12 | 1 | 0.73 |
| ≥13 weeks | 10 | 0.45 | 0.20 to 0.69 | 50.3% | ||||
| Low (≤2) | 2 | 0.20 | −0.17 to 0.58 | 0% | 1.48 | 2 | 0.48 | |
| Frequency | Medium (3–4) | 14 | 0.46 | 0.25 to 0.67 | 42.7% | |||
| High (5–7) | 2 | 0.31 | −0.13 to 0.76 | 0% | ||||
| Session time | ≤45 min | 7 | 0.66 | 0.33 to 0.99 | 45.4% | 4.41 | 1 | 0.04 |
| >45 min | 11 | 0.27 | 0.11 to 0.42 | 0% | ||||
| AD stage | Mild AD | 7 | 0.20 | −0.003 to 0.39 | 0% | 6.26 | 2 | 0.04 |
| Mild to moderate AD | 7 | 0.54 | 0.32 to 0.76 | 0% | ||||
| Moderate to severe AD | 4 | 0.75 | 0.03 to 1.47 | 79% | ||||
| Control type | Health education | 1 | 0.63 | 0.06 to 1.20 | 0% | 2.72 | 3 | 0.44 |
| Cognitive treatment | 2 | 0.15 | −0.33 to 0.63 | 0% | ||||
| Social activity | 5 | 0.66 | 0.11 to 1.21 | 72.7% | ||||
| Usual medical treatment | 11 | 0.35 | 0.19 to 0.52 | 0% | ||||
| Study quality | Good | 13 | 0.36 | 0.21 to 0.51 | 66% | 0.24 | 1 | 0.62 |
| Fair | 5 | 0.51 | 0.05 to 1.06 | 2% | ||||
| Moderator | Number of Trials | β | 95% CI | Q-Value | df(Q) | p-Value | ||
| Session time | 18 | −0.0105 | −0.0197 to −0.0013 | 4.98 | 1 | 0.03 | ||
| Duration (weeks) | 18 | −0.0163 | −0.0523 to 0.0196 | 0.79 | 1 | 0.37 | ||
| Frequency (per week) | 18 | 0.0430 | −0.1166 to 0.2026 | 0.28 | 1 | 0.60 | ||
| Total training time (during experiment) | 18 | −0.0001 | −0.0002 to 0.0001 | 1.38 | 1 | 0.24 | ||
| Age | 18 | −0.0031 | −0.03333 to 0.0271 | 0.042 | 1 | 0.84 | ||
| Male percentage | 18 | −0.0022 | −0.0145 to 0.0101 | 0.12 | 1 | 0.73 | ||
AD = Alzheimer’s disease; CI = Confidence interval; df = degree of freedom; SMD = Standardized mean difference. ≤45 min = 30–45 min.
Figure 4.
Effect size by exercise session time in meta-regression for global cognition.
3.4. Effects of PA Intervention on Activities of Daily Living
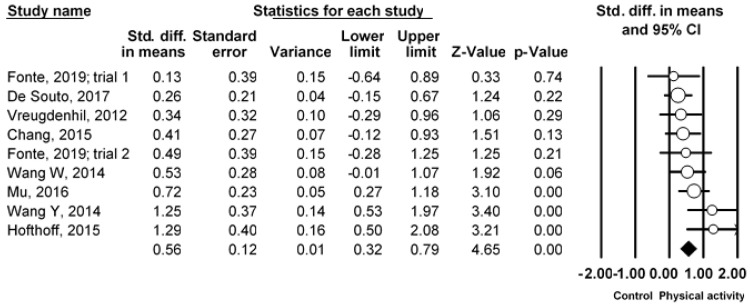
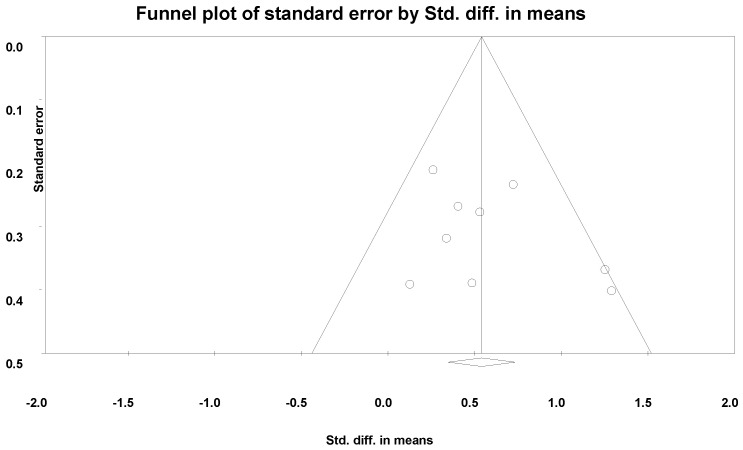
Figure 5 shows a forest plot of the meta-analysis. The pooled result from nine studies revealed that the PA intervention had a significant benefit on ADL (SMD = 0.56, 95% CI 0.32 to 0.79, I2 = 29.86%, p < 0.001). By removing one study at a time, the pooled result of ADL was stable. A symmetrical funnel plot was shown in Figure 6, indicating no publication bias for ADL. Begg’s test (p = 0.60) and Egger’s test (p = 0.29) indicated no publication bias. However, Duvall and Tweedie’s trim and fill procedure revealed that no study was missing after adjusting for outlier values. A new ES was SMD = 0.54 (95% CI 0.32 to 0.79, p < 0.05).
Figure 5.
Forest plot showing the effects of physical activity vs. the control on activities of daily living.
Figure 6.
The funnel plot of publication bias for activities of daily living.
4. Discussion
This systematic review with meta-analysis examined the effects of PA on adults with AD, as well as the estimated ESs of PA on cognition and ADL. Compared to previous meta-analyses, there were six studies [36,39,43,44,49,50] newly included in this review. Thus, a total of 945 participants (34.6% male) were involved in 16 RCTs. Overall, the methodological quality of the included studies was high. The findings showed that PA significantly benefited global cognition and ADL in adults with AD.
Although many meta-analyses have indicated the effects of exercise on adults with AD [17,27,53], there are differences in selection criteria between our study and previous studies. Due to the different mechanisms of dementia, abnormal protein deposits may coexist with neuro-vasculature at different stages of dementia and influence brain function [54]. For example, Lewy body dementia is associated with the α-synuclein protein; AD is associated with the β–amyloid and tau-proteins; vascular dementia is attributed to a lack of blood flow [55]. Therefore, we included participants with only Alzheimer’s type of dementia in this current study. Some meta-analyses intended to target people with AD, but the actual analysis included people with different types of dementia [17,27,53]. Zhu et al. conducted the meta-analysis by including both RCTs and non-RCTs, which directly increased the risk of bias [53]. Moreover, only exercise intervention was considered to meet the inclusion criteria. Some meta-analyses have combined the exercise with other therapies (e.g., psychotherapy) in the experimental group, which influenced the effectiveness of sole exercise [53,56]. Our study, compared to other systematic reviews, avoided the flaws of the above aspects and excluded some original studies. In this context, the findings from previous studies may not necessarily generalize the specific individuals with AD. On the contrary, our meta-analysis presented a low level of heterogeneity (approximately I2 = 28%, p > 0.1) by implementing strict inclusion criteria, showing reliable evidence of the effectiveness of PA on global cognition and ADL in adults with AD.
With regard to global cognition, all included studies, except for two studies using ADAS-Cog [39] and ERFC [42], examined the effect of PA on global cognition using the MMSE instrument. Both assessment tools have similar components (i.e., memory, attention, orientation, etc.) to evaluate global cognition. Our findings indicated that PA led to a significantly moderate improvement in global cognition (SMD = 0.42) for AD patients compared with the control group. This result was in line with previous meta-analyses, which demonstrated that exercise intervention contributed to an increase in global cognition amongst AD and dementia patients [54,57]. Although our result (SMD = 0.42, I2 = 30%) is slightly smaller than those of the previous study (SMD = 0.48, I2 = 85%) [54], the robustness of our result with lower heterogeneity is assured by including new RCT studies published in recent years. However, the PI for global cognition ranged from –0.04 to 0.85, implying that future research projects are likely to report a larger effect in the PA group, although all trials may not show a positive effect. Therefore, the results of this study are useful for researchers or therapists to better target intervention goals and methods, and for patients to know what to expect.
Additionally, subgroup analysis showed that exercise session time showed a significantly positive correlation with global cognition (p = 0.03), and exercise for 30–45 min had a more significant effect than exercise for more than 45 min. However, due to the moderate heterogeneity and a limited number of studies in interventions lasting 30–45 min, the evidence from the subgroup analysis of exercise session time does not very strongly determine whether an exercise session time of 30–45 min is better than one of more than 45 min. Jia et al. found that, in dementia patients, the positive effect on cognition was greater with an exercise session time of up to 30 min compared with one of more than 30 min [58]. Indeed, the included studies show that performing 30–45 min of exercise, compared with more than 45 min, produces better effects on global cognition. According to the current study, we moderately suggested that practicing moderate-intensity PA 3–4 times per week for 30–45 min for more than 12 weeks positively affected global cognition in adults with AD. However, future studies are warranted to verify our recommendation.
In terms of ADL, the ADCS-ADL is usually used to evaluate primary and operational activities of daily living for individuals with AD. In this current meta-analysis study, six studies directly reported ADL scores using the ADCS-ADL scale [36,38,39,41,49,50], and one study examined the ADL with the Barthel Index [44]. In older adults, impaired cognitive function is associated with difficulties in goal-directed activities (e.g., dressing and household duties) [59]. Reversely, improved cognitive performance leads to an improvement in physical function [59]. After participating in PA, participants showed a significant improvement in their ADL. The results from this current meta-analysis revealed that PA might have a considerable benefit in improving ADL in adults with AD. The calculated ES for ADL was 0.56, which is considered moderate. Because of the limited number of studies, the PI analysis for PA and ADL was not performed. Our finding is in line with previous review studies [53,57], which suggested that PA improved executive function and enhanced the ability of ADL in older adults. Meanwhile, exercise can adequately protect against a decline in ADL by improving skeletal muscle quality [60]. Given the limited number of included studies, subgroup analyses were not conducted to examine the impact of exercise dose on ADL. Further research investigating PA’s effects on improving ADL is needed. It is possible to make firm recommendations on an optimal PA strategy for AD patients.
This meta-analysis was an updated and comprehensive investigation of the beneficial effects of PA on global cognition and ADL. RCTs and sole AD patients were included, which avoids the influence of the bias of non-RCTs or an inconsistency in population. In particular, sensitivity analysis indicated that the findings maintained robust. These findings are vital and timely to help clinicians or caregivers provide exercise guidance to AD patients during physical rehabilitation since PA is a cost-effective intervention for all age groups. It is possible to develop a public health policy to help AD patients prevent cognitive decline. In addition, PA can lead to other health benefits, such as reduced depression and improved quality of life; depression and reduced quality of life are common in AD patients [61,62]. In contrast, the most effective PA program for AD patients is yet to be developed. Long-term participation in PA is more difficult for this population because older adults initiate PA programs and commonly discontinue exercise within half a year [63]. Thus, establishing methods and strategies to encourage AD patients to maintain long-term exercise is needed in future studies.
Several limitations in this meta-analysis should be considered. First, only articles published in Chinese and English were included, likely resulting in language bias. Second, we only examined global cognition and ADL without analyzing other indicators, such as the subdomains of cognition (attention, processing speed, etc.). Further, the accuracy of these outcomes needs to be examined using other sophisticated instruments (i.e., magnetic resonance imaging) due to the possible bias of self-reported results.
5. Conclusions
To examine the pooled effects of PA on global cognition and ADL in adults with AD, our meta-analysis study included articles with a relatively high methodological quality. The findings from this current study support that both aerobic exercise and mixed exercises benefited global cognition and ADL in patients with AD. Further research with large sample sizes and a high methodological quality is needed to acknowledge these findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19031216/s1, Figure S1: Distribution of true effects with prediction interval.
Author Contributions
Conceptualization, S.Z., Y.Z. and W.L.; methodology, S.Z., M.Z., S.C. and X.L.; software, Y.Z., S.C. and X.L.; validation, S.Z., X.L. and Y.Z.; formal analysis, Y.Z., M.Z. and S.C.; investigation, S.Z., M.Z., S.C. and Y.Z.; resources, S.Z., X.L. and Y.Z.; data curation, S.C., Y.Z. and X.L.; writing—original draft preparation, S.Z., Y.Z. and W.L.; writing—review and editing, S.C. and Y.Z.; visualization, Y.Z., S.C. and W.L.; supervision, W.L.; project administration, Y.Z. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original research presented in the study is included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Association Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018;14:367–429. [Google Scholar]
- 2.Ahn N., Kim K. Effects of an elastic band resistance exercise program on lower extremity muscle strength and gait ability in patients with Alzheimer’s disease. J. Phys. Ther. Sci. 2015;27:1953–1955. doi: 10.1589/jpts.27.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace L., Walsh S., Brayne C. The legacy of the 2013 G8 Dementia Summit: Successes, challenges, and potential ways forward. Lancet Health Longev. 2021;2:e455–e457. doi: 10.1016/S2666-7568(21)00145-8. [DOI] [PubMed] [Google Scholar]
- 4.Gordon B.R., McDowell C.P., Lyons M., Herring M.P. The Effects of Resistance Exercise Training on Anxiety: A Meta-Analysis and Meta-Regression Analysis of Randomized Controlled Trials. Sports Med. 2017;47:2521–2532. doi: 10.1007/s40279-017-0769-0. [DOI] [PubMed] [Google Scholar]
- 5.Awick E.A., Ehlers D.K., Aguiñaga S., Daugherty A.M., Kramer A.F., McAuley E. Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. Gen. Hosp. Psychiatry. 2017;49:44–50. doi: 10.1016/j.genhosppsych.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sousa N., Mendes R., Abrantes C., Sampaio J., Oliveira J. Effectiveness of combined exercise training to improve functional fitness in older adults: A randomized controlled trial. Geriatr. Gerontol. Int. 2014;14:892–898. doi: 10.1111/ggi.12188. [DOI] [PubMed] [Google Scholar]
- 7.Stillman C.M., Esteban-Cornejo I., Brown B., Bender C.M., Erickson K.I. Effects of Exercise on Brain and Cognition Across Age Groups and Health States. Trends Neurosci. 2020;43:533–543. doi: 10.1016/j.tins.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y.Y., Lee Y.H., Chan J.C., Chiou P.Y., Chou X.Y., Chiu W.T., Hung C.T. Effects of exercise interventions on social and cognitive functioning of men with prostate cancer: A meta-analysis. Support. Care Cancer. 2020;28:2043–2057. doi: 10.1007/s00520-019-05278-y. [DOI] [PubMed] [Google Scholar]
- 9.Falck R.S., Davis J.C., Best J.R., Crockett R.A., Liu-Ambrose T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging. 2019;79:119–130. doi: 10.1016/j.neurobiolaging.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Meng Q., Lin M.S., Tzeng I.S. Relationship Between Exercise and Alzheimer’s Disease: A Narrative Literature Review. Front. Neurosci. 2020;14:131. doi: 10.3389/fnins.2020.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toots A., Littbrand H., Lindelöf N., Wiklund R., Holmberg H., Nordström P., Lundin-Olsson L., Gustafson Y., Rosendahl E. Effects of a High-Intensity Functional Exercise Program on Dependence in Activities of Daily Living and Balance in Older Adults with Dementia. J. Am. Geriatr. Soc. 2016;64:55–64. doi: 10.1111/jgs.13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groot C., Hooghiemstra A.M., Raijmakers P.G.H.M., van Berckel B.N.M., Scheltens P., Scherder E.J.A., van der Flier W.M., Ossenkoppele R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Du Z., Li Y., Li J., Zhou C., Li F., Yang X. Physical activity can improve cognition in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Interv. Aging. 2018;13:1593–1603. doi: 10.2147/CIA.S169565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blondell S.J., Hammersley-Mather R., Veerman J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borges-Machado F., Silva N., Farinatti P., Poton R., Ribeiro Ó., Carvalho J. Effectiveness of Multicomponent Exercise Interventions in Older Adults With Dementia: A Meta-Analysis. Gerontologist. 2020;61:e449–e462. doi: 10.1093/geront/gnaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demurtas J., Schoene D., Torbahn G., Marengoni A., Grande G., Zou L., Petrovic M., Maggi S., Cesari M., Lamb S., et al. Physical Activity and Exercise in Mild Cognitive Impairment and Dementia: An Umbrella Review of Intervention and Observational Studies. J. Am. Med. Dir. Assoc. 2020;21:1415–1422. doi: 10.1016/j.jamda.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 17.López-Ortiz S., Valenzuela P.L., Seisdedos M.M., Morales J.S., Vega T., Castillo-García A., Nisticò R., Mercuri N.B., Lista S., Lucia A., et al. Exercise interventions in Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021;72:101479. doi: 10.1016/j.arr.2021.101479. [DOI] [PubMed] [Google Scholar]
- 18.Chang W.-T., Kuo Y.-J., Huang Y.-Y., Tsai M.-J., Chen Y.-P. Poor Activities of Daily Living Function Reflect Poor Quality of Life after Hip Fracture Surgery for Geriatric Patients. Soc. Health Behav. 2019;2:41. [Google Scholar]
- 19.Medhi G., Sarma J., Pala S., Bhattacharya H., Bora P., Visi V. Association between health related quality of life (HRQOL) and activity of daily living (ADL) among elderly in an urban setting of Assam, India. J. Fam. Med. Prim. Care. 2019;8:1760. doi: 10.4103/jfmpc.jfmpc_270_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehghani A., Khoramkish M., Isfahani S.S. Challenges in the daily living activities of patients with multiple sclerosis: A qualitative content analysis. Int. J. Community Based Nurs. Midwifery. 2019;7:201–210. doi: 10.30476/IJCBNM.2019.44995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwak Y.-S., Um S.-Y., Son T.-G., Kim D.-J. Effect of Regular Exercise on Senile Dementia Patients. Int. J. Sports Med. 2008;29:471–474. doi: 10.1055/s-2007-964853. [DOI] [PubMed] [Google Scholar]
- 22.Sobol N.A., Hoffmann K., Frederiksen K.S., Vogel A., Vestergaard K., Brændgaard H., Gottrup H., Lolk A., Wermuth L., Jakobsen S., et al. Effect of aerobic exercise on physical performance in patients with Alzheimer’s disease. Alzheimer’s Dement. 2016;12:1207–1215. doi: 10.1016/j.jalz.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Abd El-Kader S.M., Al-Jiffri O.H. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with alzheimer’s disease. Afr. Health Sci. 2016;16:1045–1055. doi: 10.4314/ahs.v16i4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam F.M., Huang M.Z., Liao L.R., Chung R.C., Kwok T.C., Pang M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018;64:4–15. doi: 10.1016/j.jphys.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Ojagbemi A., Akin-Ojagbemi N. Exercise and Quality of Life in Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Appl. Gerontol. 2019;38:27–48. doi: 10.1177/0733464817693374. [DOI] [PubMed] [Google Scholar]
- 26.Laver K., Dyer S., Whitehead C., Clemson L., Crotty M. Interventions to delay functional decline in people with dementia: A systematic review of systematic reviews. BMJ Open. 2016;6:e010767. doi: 10.1136/bmjopen-2015-010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisani S., Mueller C., Huntley J., Aarsland D., Kempton M.J. A meta-analysis of randomised controlled trials of physical activity in people with Alzheimer’s disease and mild cognitive impairment with a comparison to donepezil. Int. J. Geriatr. Psychiatry. 2021;36:1471–1487. doi: 10.1002/gps.5581. [DOI] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maher C., Sherrington C., Herbert R., Moseley A., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J.P.T., Li T., Deeks J.J. Choosing effect measures and computing estimates of effect. Cochrane Handb. Syst. Rev. Interv. 2019:143–176. doi: 10.1002/9781119536604.ch6. [DOI] [Google Scholar]
- 31.Wu Y., Wang Y., Burgess E.O., Wu J. The effects of Tai Chi exercise on cognitive function in older adults: A meta-analysis. J. Sport Health Sci. 2013;2:193–203. doi: 10.1016/j.jshs.2013.09.001. [DOI] [Google Scholar]
- 32.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons Ltd.; Chichester, UK: 2011. [Google Scholar]
- 33.Borenstein M., Higgins J.P.T., Hedges L.V., Rothstein H.R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res. Synth. Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Loprinzi P.D., Yang L., Liu J., Liu S., Zou L. The Beneficial Effects of Traditional Chinese Exercises for Adults with Low Back Pain: A Meta-Analysis of Randomized Controlled Trials. Medicina. 2019;55:118. doi: 10.3390/medicina55050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Zou L., Chen S.-T., Bae J.H., Kim D.Y., Liu X., Song W. Effects and Moderators of Exercise on Sarcopenic Components in Sarcopenic Elderly: A Systematic Review and Meta-Analysis. Front. Med. 2021;8:649748. doi: 10.3389/fmed.2021.649748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang C., Wang W., Zhu Y. Clinical effects of aerobic training in the treatment of Alzheimer’s disease. Chin. J. Rehabil. Med. 2015;30:1131–1134. [Google Scholar]
- 37.De Oliveira Silva F., Ferreira J.V., Plácido J., Sant’Anna P., Araújo J., Marinho V., Laks J., Camaz Deslandes A. Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: A randomized controlled trial. Maturitas. 2019;126:28–33. doi: 10.1016/j.maturitas.2019.04.217. [DOI] [PubMed] [Google Scholar]
- 38.De Souto Barreto P., Cesari M., Denormandie P., Armaingaud D., Vellas B., Rolland Y. Exercise or Social Intervention for Nursing Home Residents with Dementia: A Pilot Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2017;65:E123–E129. doi: 10.1111/jgs.14947. [DOI] [PubMed] [Google Scholar]
- 39.Fonte C., Smania N., Pedrinolla A., Munari D., Gandolfi M., Picelli A., Varalta V., Benetti M.V., Brugnera A., Federico A., et al. Comparison between physical and cognitive treatment in patients with MCI and Alzheimer’s disease. Aging. 2019;11:3138–3155. doi: 10.18632/aging.101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann K., Sobol N.A., Frederiksen K.S., Beyer N., Vogel A., Vestergaard K., Brændgaard H., Gottrup H., Lolk A., Wermuth L., et al. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: A randomized controlled trial. J. Alzheimer’s Dis. 2016;50:443–453. doi: 10.3233/JAD-150817. [DOI] [PubMed] [Google Scholar]
- 41.Holthoff V.A., Marschner K., Scharf M., Steding J., Meyer S., Koch R., Donix M. Effects of physical activity training in patients with alzheimer’s dementia: Results of a pilot RCT study. PLoS ONE. 2015;10:e0121478. doi: 10.1371/journal.pone.0121478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemoun G., Thibaud M., Roumagne N., Carette P., Albinet C., Toussaint L., Paccalin M., Dugué B. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement. Geriatr. Cogn. Disord. 2010;29:109–114. doi: 10.1159/000272435. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Wang T., Zhu Y., Wang W., Yin M., Zhang W., Zhang T., Wu T. Aerobic exercise improves cognitive function in patients with Alzheimer’s disease. Chin. J. Rehabil. 2017;32:386–389. [Google Scholar]
- 44.Mu H., Lv J., Hao Z., Li W., Li M. Effect of aerobic exercise on abilities in daily life, cognitive function and psychological symptoms in patients with mild to moderate Alzheimer’s disease. Chin. J. Mult. Organ Dis. Elder. 2016;15:451–545. [Google Scholar]
- 45.Pedroso R.V., Ayán C., Fraga F.J., Da Silva T.M.V., Cancela J.M., Santos-Galduròz R.F. Effects of functional-task training on older adults with Alzheimer’s disease. J. Aging Phys. Act. 2018;26:97–105. doi: 10.1123/japa.2016-0147. [DOI] [PubMed] [Google Scholar]
- 46.Venturelli M., Scarsini R., Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am. J. Alzheimers Dis. Other Demen. 2011;26:381–388. doi: 10.1177/1533317511418956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venturelli M., Sollima A., Cè E., Limonta E., Bisconti A.V., Brasioli A., Muti E., Esposito F. Effectiveness of Exercise- and Cognitive-Based Treatments on Salivary Cortisol Levels and Sundowning Syndrome Symptoms in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2016;53:1631–1640. doi: 10.3233/JAD-160392. [DOI] [PubMed] [Google Scholar]
- 48.Vreugdenhil A., Cannell J., Davies A., Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: A randomized controlled trial. Scand. J. Caring Sci. 2012;26:12–19. doi: 10.1111/j.1471-6712.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang W., Zhu Y., Yang S., Chang C., Wang Y., Li H., Wang Q., Wang W., Wang T., Wu T. The effect of aerobic exercise on cognitive function and activity of daily living in patients with Alzheimer’s disease. Chin. J. Rehabil. Med. 2014;29:1151–1155. [Google Scholar]
- 50.Wang Y., Shen F., Zhu Y., Yang S., Li H., Wang Q., Wang W., Wu T. Clinical Effects of Aerobic Exercises Training with Moderate and High Intensity in Alzheimer’s Disease Treatment. China J. Clin. Neurosci. 2014;22:504–509. [Google Scholar]
- 51.Yang S.-Y., Shan C.-L., Qing H., Wang W., Zhu Y., Yin M.-M., Machado S., Yuan T.-F., Wu T. The Effects of Aerobic Exercise on Cognitive Function of Alzheimer’s Disease Patients. CNS Neurol. Disord. Drug Targets. 2015;14:1292–1297. doi: 10.2174/1871527315666151111123319. [DOI] [PubMed] [Google Scholar]
- 52.Qin X.Y., Zhang S.P., Cao C., Loh Y.P., Cheng Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2016;73:1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 53.Zhu L., Li L., Wang L., Jin X., Zhang H. Physical Activity for Executive Function and Activities of Daily Living in AD Patients: A Systematic Review and Meta-Analysis. Front. Psychol. 2020;11:3227. doi: 10.3389/fpsyg.2020.560461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardona M.I., Afi A., Lakicevic N., Thyrian J.R. Physical activity interventions and their effects on cognitive function in people with dementia: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2021;18:8753. doi: 10.3390/ijerph18168753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raz L., Knoefel J., Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016;36:172–186. doi: 10.1038/jcbfm.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao A.K., Chou A., Bursley B., Smulofsky J., Jezequel J. Systematic review of the effects of exercise on activities of daily living in people with alzheimers disease. Am. J. Occup. Ther. 2014;68:50–56. doi: 10.5014/ajot.2014.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L.Y., Pei J., Zhan Y.J., Cai Y.W. Overview of Meta-Analyses of Five Non-pharmacological Interventions for Alzheimer’s Disease. Front. Aging Neurosci. 2020;12:594432. doi: 10.3389/fnagi.2020.594432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia R.X., Liang J.H., Xu Y., Wang Y.Q. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 2019;19:181. doi: 10.1186/s12877-019-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brigola A.G., Rossetti E.S., dos Santos B.R., Neri A.L., Zazzetta M.S., Inouye K., Pavarini S.C.I. Relationship between cognition and frailty in elderly: A systematic review. Dement. Neuropsychol. 2015;9:110–119. doi: 10.1590/1980-57642015DN92000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimada H., Makizako H., Doi T., Park H., Tsutsumimoto K., Verghese J., Suzuki T. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients With Mild Cognitive Impairment: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2018;19:584–591. doi: 10.1016/j.jamda.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 61.Herold F., Törpel A., Schega L., Müller N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—A systematic review. Eur. Rev. Aging Phys. Act. 2019;16:10. doi: 10.1186/s11556-019-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potter R., Ellard D., Rees K., Thorogood M. A systematic review of the effects of physical activity on physical functioning, quality of life and depression in older people with dementia. Int. J. Geriatr. Psychiatry. 2011;26:1000–1011. doi: 10.1002/gps.2641. [DOI] [PubMed] [Google Scholar]
- 63.Timmons J.F., Griffin C., Cogan K.E., Matthews J., Egan B. Exercise Maintenance in Older Adults 1 Year After Completion of a Supervised Training Intervention. J. Am. Geriatr. Soc. 2020;68:163–169. doi: 10.1111/jgs.16209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original research presented in the study is included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.