Abstract
James Alexander Logan, a second-year medical student at the Barts and The London School of Medicine and Dentistry, died in February 2001 after a distressing illness of three months duration. His family, friends and interested professionals subsequently set up the James Logan Trust to encourage doctors and others to have the confidence to recognise and treat cancer pain. The James Logan Trust has provided funds for an annual prize for the best essay on “The challenges of cancer pain assessment and management” to be submitted by a Queen’s University of Belfast undergraduate medical student after the completion of their fourth-year palliative medicine teaching.
Introduction
Pain is one of the most significant symptoms amongst cancer patients – nearly 40% of all patients in the oncological population experience moderate to severe pain.1 Responding to the urgent need for an international standard of care, the World Health Organisation published guidelines on cancer pain management in 1986.2 Despite this there is evidence to suggest that pain is under-managed. A 2008 review of studies reporting negative Pain Management Index (PMI) scores concluded that 43.4% of patients were being undertreated3 which decreased to 31.8% in a 2014 update.4 While the temporal trend is encouraging, approximately 30% of patients with cancer pain are still being under-treated. The causes are wide ranging, from inadequate pain assessment by healthcare professionals, to patients’ reluctance to report pain.5 The multitude of barriers in pain management reflect the complex nature of the symptom, which Cicely Saunders noted in her concept of “total pain.” Multiple dimensions beyond the physical contribute to pain6 and awareness of this is integral to the holistic approach. This essay will discuss oncological, pharmacological, psychological, social, and spiritual strategies to manage cancer pain.
Defining and assessing pain
Holistic management of pain must begin with a comprehensive assessment – this requires understanding the nature of the symptom. The International Association for the Study of Pain defines the symptom as ‘an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage’,7 with additional points expanding upon the definition noted in figure 1. The subjective nature of pain makes addressing it challenging, its presence cannot be verified with diagnostic tools such as imaging or blood tests, so patients’ self-reports of symptom improvement is the benchmark of treatment success.
Figure 1.

notes on pain definition [Figure]8
Cancer pain is primarily either nociceptive or neuropathic.9 The former is caused by injury to skin and organs which stimulates local nociceptors, the latter by damage to the central or peripheral nervous system. The quality of the pain is important to elicit in a pain history – sharp, localised pain is more often somatic while aching, dull pain may be visceral, neuropathic pain may be described as burning or shooting. This guides the clinician to the cause: somatic pain may be caused by tumour metastasis to bone, visceral pain by abdominal infiltration and neuropathic pain is due to compression or disruption of nerves, by tumour or surgical intervention.10
A key aspect of the pain management plan is setting expectations, which increases patients’ satisfaction regardless of the intensity of their pain.11 The WHO 1986 guidelines lay out the following goals: to decrease pain when a patient is carrying out activities as well as at rest, and to decrease pain at night to maximise pain-free sleep. To this end, a multi-pronged approach consisting of various modalities is recommended with multi-disciplinary input.
Oncological pain management
Oncological methods such as radiotherapy and chemotherapy reduce the source of pain and hence are ideal for symptom control.
Nearly 50% of cancer patients require some degree of radiotherapy, with around 40% of these patients receiving radiotherapy for symptom control.12 It is important to note that radiotherapy may take up to 6 weeks to relieve pain depending on the neoplastic cells’ stage in the cell cycle. The side effects of radiotherapy, however, are immediate and pharmacological management is necessary in the interim.13
Concomitant use of radiotherapy and bisphosphonates can markedly relieve pain amongst patients with bone metastases, associated with a reduction in the dose of opioid analgesia required by these patients.14 The utility of radiation treatments in reducing nociceptive signalling in this patient group is well corroborated in the literature.15-18 There is evidence to suggest that single fraction radiotherapy is as effective as multi-fraction radiotherapy in patients with bone metastasis uncomplicated by fractures,19 although patients treated with single fraction radiotherapy were more likely to require re-treatment.20 When it comes to brain metastases and tumours, treatment modality depends on prognostic factors such as the age of the patient, their performance status and the number of brain metastases. In suitable patients, whole brain radiotherapy (WBRT) reduces the severity of a number of symptoms including nausea and vomiting, seizures and headaches.21 In patients with poor prognoses there is less benefit from WBRT compared to corticosteroids and supportive care.22
Cytotoxic chemotherapeutic and targeted agents can function as anti-pain therapies through inhibiting tumour growth, hence reducing nociceptive pain by alleviating compression on viscera. In men with metastatic prostate cancer, a chemotherapeutic agent in combination with corticosteroids are superior to corticosteroids alone in reducing pain.23,24 Chemotherapy induced peripheral neuropathy is a concern with certain agents, including cisplatin, vincristine and ixabepilone. Use of this modality requires vigilance for neurological symptoms as well as alteration of the dosage and regimen of chemotherapeutic agents if necessary.25 Targeted therapies such as Tamoxifen26 and Abiraterone27 have been found to reduce cancer pain in breast and prostate cancer patients respectively; Imatinib, used to treat renal cancer, is particularly exciting for reducing abdominal pain in the majority of patients within weeks.24
Pharmacological pain management
The WHO 1986 guidelines included a simple three-step analgesic ladder ( figure 2) consisting of aspirin, codeine, and morphine.2 This was updated in 1997 to include more options at each rung of the ladder, although the aim was still to utilise readily available drugs to their full potential rather than introduce new expensive options that would not be equally accessible across the globe.28
Figure 2.
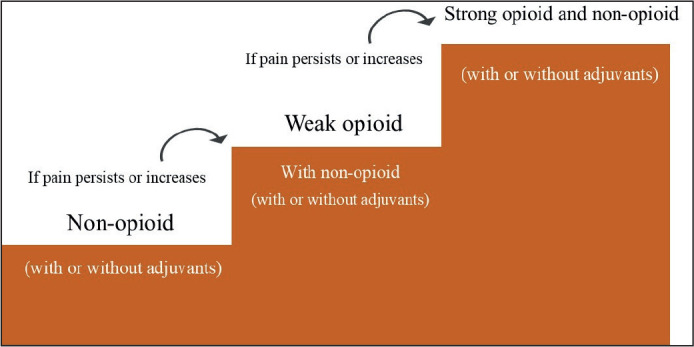
The WHO analgesic ladder for cancer pain management [Figure]29
Although the ladder has been subject to criticism and numerous adaptations have been suggested, the WHO approach has been found to be effective for 70-80% of patients.30 In practice, non-opioid analgesics are rarely enough as sole agents and opioids are often added. Different opioids vary in their affinity for receptors at the various binding sites, as well as varying in pharmacokinetics.31 This leads to variation in adverse effects and tolerance within the drug class.
Six key points are noted relating to the ladder in the 1986 guidelines:
Individual dosing is required. It is important to let the patient’s perception of the pain guide the dose rather than healthcare professionals’ perceptions. When patients say they are in pain, we must believe them. Clinicians must be mindful of racial32 and gendered33 bias in pain assessment.
Oral therapy should be used in the first instance unless parenteral therapy is absolutely required.
Nocturnal pain control is emphasised to maximise pain-free hours of sleep – this may require increased doses of morphine.
Patients should be monitored for pain and adverse effects of pharmacological therapy. They should be reviewed regularly and re-assessed if there is an onset of new pain.
Adverse effects of strong opioids must be treated. Constipation, nausea, vomiting and respiratory depression can be as significant to the patient’s quality of life as pain, and the majority will require co-prescriptions of laxatives and/or anti-emetics.
For patients with intractable anxiety and depression, adjuvants may be useful.
Complications of long-term opioid therapy include tolerance and addiction,34 opioid-induced inhibition of adrenal androgen production,35 and osteoporosis.36
Adjuvants such as tricyclic antidepressants, local anaesthetics, carbamazepine, bisphosphonates and NSAIDs can be effective in the management of cancer pain syndromes.37 The advantage of this approach is reducing dosages and hence toxicity; there may also be a synergistic effect from multiple therapies acting on many cellular targets simultaneously.
Neuropathic pain in cancer patients requires a different approach than in other patient populations, as typical treatments such as amitriptyline may not be as effective when given as sole agents. Mishra et al. found that a combination of opioids, gabapentin, amitriptyline and dexamethasone were effective for neuropathic pain.38
Psychological, social, and spiritual pain management
Pain manifested physically is perhaps simpler to understand than the other components of total pain (psychological, emotional, social, and spiritual), but the latter are no less important. Psychological distress can both be caused by pain and exacerbate it. Cancer is frequently comorbid with depression39 which is itself associated with pain and a lower quality of life. In an interesting analysis, Wang et al. found that treatment for depression resulted in a greater decrease in pain than the reverse.40 The psychological effects of cancer and treatment depend on a host of factors including the diagnosis, prognosis, underlying psychological vulnerabilities and internal locus of control. Interventions that may improve psychological wellbeing during treatment include Cognitive Behavioural Therapy (CBT), hypnosis and patient education about the condition and management.
Figure 3.
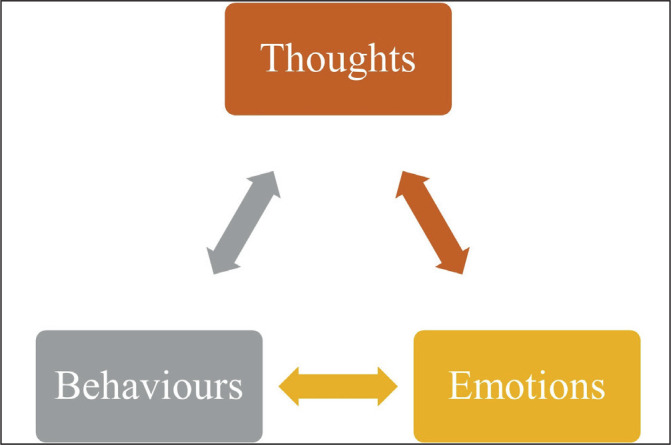
the ‘cognitive triangle’ [figure]
CBT is the first line treatment for mild depression and is highly recommended as an adjunct in moderate to severe depression.41 The principles of CBT rely on the connection between thoughts, emotions and behaviour. A patient may understandably have a negative reaction to their situation but ruminating on thoughts such as “This pain is going to kill me, I can’t bear this,” will negatively affect their mood. “Catastrophising” has a deleterious effect on emotions, which in turn can cause behavioural changes such as social withdrawal. CBT aims to break the cycle by training patients to identify and question catastrophic thinking. Behaviours may also influence emotions and thoughts. Hence behavioural changes such as engaging in exercise, establishing sleep hygiene, and trying relaxation techniques are encouraged. There is some evidence for the benefits of this approach in breast cancer patients.42
There appears to be a relationship between the severity of cancer pain and hours spent on social activity with friends and family. Patients with mild pain spend more time on social activity while the converse is true for those suffering more severe pain.43 This raises the intriguing question of whether there is a similar temporal relationship between the two as with depression. Koopman et al. carried out a study investigating pain in a sample of women with metastatic breast carcinoma, finding that pain was greater in those with more “total life stress” regardless of levels of social support. However, more social support reduced levels of mood disturbance.44
The patient-carer relationship is a key aspect of social pain. Caregivers often struggle with depression and feelings of hopelessness.45 There is some evidence to suggest that carers’ emotional distress is associated with the patients’ pain, however, it is difficult to ascertain whether the pain is caused by the carers’ distress or vice versa.46 Interventions to socially support both patients and carers vary in scope and method, but meta-analyses have shown significant improvements to depression, caregiver burden, and symptom control.47 Where more social support is needed, the valuable input of social work and occupational therapy is should be considered.
Spiritual pain can be defined as a loss of feelings of connection to a higher power or a lack of meaning. Cancer patients may experience spiritual suffering at the unpredictable nature of their condition, wondering why they were the ones to be afflicted. Being too incapacitated to carry out religious rituals, such as prayer, may also contribute to spiritual pain. Providing spiritual resources such as access to faith-based therapy reduced spiritual pain and symptoms of depression in a population of Muslim breast cancer patients.48 Similar results have been found in Christian patient populations.49 Clinicians must work with chaplains and spiritual authorities to support patients in their journeys.
A final aspect of the holistic approach may involve complementary and alternative medicine (CAM). This includes practices such as acupuncture, massage, reflexology, and aromatherapy. There is some evidence that CAMs in combination with conventional medicine improve quality of life,50 however, there are concerns regarding lack of regulation.
To summarise, pain management is integral to high-quality care for cancer patients. Guiding principles were standardised by the WHO in 1986, these are: to assess patients comprehensively; to take a careful approach to pharmacological therapies; to consider all aspects of an individual’s pain from physical pain to spiritual suffering; and to involve the multidisciplinary team in managing these aspects. The most important principle of all is to practice empathy and understand that while pain may not be measurable, it is very real. Patients are the experts of their bodies – we must trust them.
Footnotes
UMJ is an open access publication of the Ulster Medical Society (http://www.ums.ac.uk).
REFERENCES
- 1.Van den Beuken-van Everdingen MH Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–90.e9. doi: 10.1016/j.jpainsymman.2015.12.340. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Cancer pain relief. Geneva: World Health Organisation; 1986. [Google Scholar]
- 3.Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol Off J Eur Soc Med Oncol. 2008;19(12):1985–91. doi: 10.1093/annonc/mdn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149–54. doi: 10.1200/JCO.2014.56.0383. [DOI] [PubMed] [Google Scholar]
- 5.Kwon J. Overcoming barriers in cancer pain management. J Clin Oncol. 2014;32(16):1727–33. doi: 10.1200/JCO.2013.52.4827. [DOI] [PubMed] [Google Scholar]
- 6.Lewis M. Cicely Saunders: founder of the hospice movement. Selected letters 1959 – 1999. Mortality. 2007;12(4):385–6. [Google Scholar]
- 7.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–82. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. Four decades later: revision of the IASP definition of pain and notes. Pain. 2020 [Google Scholar]
- 9.Hewitt DJ. The management of pain in the oncology patient. Obstet Gynecol Clin North Am. 2001;28(4):819–46. doi: 10.1016/s0889-8545(05)70238-2. [DOI] [PubMed] [Google Scholar]
- 10.Swarm RA, Abernethy AP, Anghelescu DL, Benedetti C, Buga S, Cleeland C, et al. Adult cancer pain: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;11(8):992–1022. doi: 10.6004/jnccn.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck SL, Towsley GL, Berry PH, Lindau K, Field RB, Jensen S. Core aspects of satisfaction with pain management: cancer patients’ perspectives. J Pain Symptom Manage. 2010;39(1):100–15. doi: 10.1016/j.jpainsymman.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Sejpal SV, Bhate A, Small WJ. Palliative radiation therapy in the management of brain metastases, spinal cord compression, and bone metastases. Semin Intervent Radiol. 2007;24(4):363–74. doi: 10.1055/s-2007-992324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gough N, Miah A, Linch M. Nonsurgical oncological management of cancer pain. Curr Opin Support Palliat Care. 2014;8(2):102–11. doi: 10.1097/SPC.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 14.Vassiliou V, Kardamakis D. The management of metastatic bone disease with the combination of bisphosphonates and radiotherapy: from theory to clinical practice. Anticancer Agents Med Chem. 2009;9(3):326–35. doi: 10.2174/1871520610909030326. [DOI] [PubMed] [Google Scholar]
- 15.Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer. 1997;21(3):129–83. doi: 10.1016/s0147-0272(97)80004-9. [DOI] [PubMed] [Google Scholar]
- 16.Reale C, Turkiewicz AM, Reale CA. Antalgic treatment of pain associated with bone metastases. Crit Rev Oncol Hematol. 2001;37(1):1–11. doi: 10.1016/s1040-8428(99)00066-9. [DOI] [PubMed] [Google Scholar]
- 17.Ciezki JP, Komurcu S, Macklis RM. Palliative radiotherapy. Semin Oncol. 2000;27(1):90–3. [PubMed] [Google Scholar]
- 18.Van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443–9. doi: 10.1053/j.seminoncol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24(2):112–24. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–36. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 21.Suteu P, Fekete Z, Todor N, Nagy V. Survival and quality of life after whole brain radiotherapy with 3D conformal boost in the treatment of brain metastases. Med Pharm Reports. 2019;92(1):43–47. doi: 10.15386/cjmed-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–14. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 24.Osoba D, Tannock IF, Scott Ernst D, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17(6):1654–63. doi: 10.1200/JCO.1999.17.6.1654. [DOI] [PubMed] [Google Scholar]
- 25.Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci. 2019;20(6):1451. doi: 10.3390/ijms20061451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston S, Stebbing J. Breast cancer (metastatic) Clin Evid (Online) 2003;(10):1975–2002. [PubMed] [Google Scholar]
- 27.Logothetis C, Basch E, Molina A, Fizazi K, North S, Chi K, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13(12):1210–7. doi: 10.1016/S1470-2045(12)70473-4. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira KA, Kimura M, Teixeira MJ. The WHO analgesic ladder for cancer pain control, twenty years of use. How much pain relief does one get from using it? Support Care Cancer. 2006;14(11):1086–93. doi: 10.1007/s00520-006-0086-x. [DOI] [PubMed] [Google Scholar]
- 29.Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Vol. 56, Canadian Family Physician. College of Family Physicians of Canada. 2010;56(6):514. Figure 1. [PMC free article] [PubMed] [Google Scholar]
- 30.Vargas-Schaffer G. Is the WHO analgesic ladder still valid?: twenty-four years of experience. Can Fam Physician. 2010;56(6):514–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Meert T, Vermeirsch H. A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacol Biochem Behav. 2005;80(2):309–26. doi: 10.1016/j.pbb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296. doi: 10.1073/pnas.1516047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samulowitz A, Gremyr I, Eriksson E, Hensing G. “Brave men” and “emotional women”: a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res Manag. 2018;2018 doi: 10.1155/2018/6358624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballantyne J, LaForge S. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129(3):235–55. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Daniell H. DHEAS deficiency during consumption of sustained-action prescribed opioids: evidence for opioid-induced inhibition of adrenal androgen production. J pain. 2006;7(12):901–7. doi: 10.1016/j.jpain.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Daniell H. Opioid osteoporosis. Arch Intern Med. 2004;164(3):338. doi: 10.1001/archinte.164.3.338-a. [DOI] [PubMed] [Google Scholar]
- 37.Mitra R, Jones S. Adjuvant analgesics in cancer pain: a review. Am J Hosp Palliat Care. 2012;29(1):70–9. doi: 10.1177/1049909111413256. [DOI] [PubMed] [Google Scholar]
- 38.Mishra S, Bhatnagar S, Gupta D, Nirwani Goyal G, Jain R, Chauhan H. Management of neuropathic cancer pain following WHO analgesic ladder: a prospective study. Am J Hosp Palliat Care. 2008;25(6):447–51. doi: 10.1177/1049909108322288. [DOI] [PubMed] [Google Scholar]
- 39.Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32(16):1703. doi: 10.1200/JCO.2013.54.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Kroenke K, Wu J, Tu W, Theobald D, Rawl S. Predictors of cancer-related pain improvement over time. Psychosom Med. 2012;74(6):642–7. doi: 10.1097/PSY.0b013e3182590904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark DM. Implementing NICE guidelines for the psychological treatment of depression and anxiety disorders: the IAPT experience. Int Rev Psychiatry. 2011;23(4):318. doi: 10.3109/09540261.2011.606803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatrow K, Montgomery G. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med. 2006;29(1):17–27. doi: 10.1007/s10865-005-9036-1. [DOI] [PubMed] [Google Scholar]
- 43.Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. J Pain Symptom Manage. 2002;24(5):526–42. doi: 10.1016/s0885-3924(02)00497-9. [DOI] [PubMed] [Google Scholar]
- 44.Koopman C, Hermanson K, Diamond S, Angell K, Spiegel D. Social support, life stress, pain and emotional adjustment to advanced breast cancer. Psychooncology. 1998;7:101–11. doi: 10.1002/(SICI)1099-1611(199803/04)7:2<101::AID-PON299>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Mystakidou K, Tsilika E, Parpa E, Galanos A, Vlahos L. Caregivers of advanced cancer patients: feelings of hopelessness and depression. Cancer Nurs. 2007;30(5):412–8. doi: 10.1097/01.NCC.0000290807.84076.73. [DOI] [PubMed] [Google Scholar]
- 46.De Laurentis M, Rossana B, Andrea B, Riccardo T, Valentina I. The impact of social-emotional context in chronic cancer pain: patient-caregiver reverberations. Support Care Cancer. 2019;27(2):705–13. doi: 10.1007/s00520-018-4530-5. [DOI] [PubMed] [Google Scholar]
- 47.Kent EE, Rowland JH, Northouse L, Litzelman K, Chou W-YS, Shelburne N, et al. Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer. 2016;122(13):1987–95. doi: 10.1002/cncr.29939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khait AA, Lazenby M. Psychosocial-spiritual interventions among Muslims undergoing treatment for cancer: an integrative review. BMC Palliat Care 2021 201. 2021;20(1):1–22. doi: 10.1186/s12904-021-00746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee Y-H. Spiritual care for cancer patients. Asia-Pacific J Oncol Nurs. 2019;6(2):101. doi: 10.4103/apjon.apjon_65_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh P, Chaturvedi A. Complementary and alternative medicine in cancer pain management: a systematic review. Indian J Palliat Care. 2015;21(1):105. doi: 10.4103/0973-1075.150202. [DOI] [PMC free article] [PubMed] [Google Scholar]


