Abstract
In recent years, some exogenous bioactive peptides have been shown to have promising anti-aging effects. These exogenous peptides may have a mechanism similar to endogenous peptides, and some can even regulate the release of endogenous active peptides and play a synergistic role with endogenous active peptides. Most aging studies use rodents that are easy to maintain in the laboratory and have relatively homogenous genotypes. Moreover, many of the anti-aging studies using bioactive peptides in rodent models only focus on the activity of single endogenous or exogenous active peptides, while the regulatory effects of exogenous active peptides on endogenous active peptides remain largely under-investigated. Furthermore, the anti-aging activity studies only focus on the effects of these bioactive peptides in individual organs or systems. However, the pathological changes of one organ can usually lead to multi-organ complications. Some anti-aging bioactive peptides could be used for rescuing the multi-organ damage associated with aging. In this paper, we review recent reports on the anti-aging effects of bioactive peptides in rodents and summarize the mechanism of action for these peptides, as well as discuss the regulation of exogenous active peptides on endogenous active peptides.
Keywords: bioactive peptide, anti-aging, rodents
1. Introduction
In modern society, the extension of average life expectancy and the decreased birth rate have led to aging-related burdens across many regions [1,2]. Aging is a dynamic process associated with accumulated cell damage, a decline in biological function, and susceptibility to disease occurring over time [3]. A common and widely recognized mechanism for aging is oxidative damage caused by the accumulation of reactive oxygen species (ROS) [4], resulting from decreased antioxidant capacity, mitochondrial dysfunction, inflammation, etc. [5]. Aging can lead to multiple age-related diseases (ARDs) [6], such as cancer, Alzheimer’s disease (AD), cardiovascular disease (CVD), metabolic syndrome, obesity, fatty liver, and many other chronic diseases. The aging process inevitably involves the aging of cells, which is usually caused by damage at the molecular and cellular level by long-term exposure to endogenous and exogenous stressors. These damaged cells eventually lose their proliferative capacity and promote aging at an organism level [7]. These senescent cells can release a variety of pro-inflammatory factors and chemokines to promote cellular dysfunction, causing senescence-related diseases. In the process of skin aging, oxidative stress and inflammation can increase the activity of matrix metalloproteinases (MMPs) and increase the degradation of collagen, resulting in skin sagging and wrinkle formation. In some neurodegenerative diseases, such as AD, oxidative stress and inflammation can increase the accumulation of amyloid plaques (Aβ) and promote lesions in the brain. Oxidative stress and inflammation also play an important role in the aging of several other organs, such as the heart, liver, and kidneys. Collectively, these pathological changes can cause a variety of complications that affect multiple systems in the body. Thus, ARDs seriously impact the quality of life, shorten the lifespan, and bring a heavy burden to families and society. Therefore, in-depth studies of aging are particularly important.
Bioactive peptides are short peptides consisting of 2–20 amino acid residues. They have positive effects on body functions and generally have antibacterial, antihypertensive, antioxidant, and anti-inflammatory effects [8]. Natural bioactive peptides can be generally divided into two categories: endogenous peptides, which are naturally released from precursor proteins and secreted from cells, and exogenous peptides, which are produced by enzymatic hydrolysis of proteins or by biosynthesis or organic synthesis [9,10]. Bioactive peptide resources have been found in plants (soybeans, walnuts, rice bran, etc.), animals (some fish, dairy products, etc.), and some fungi and bacteria (yeast, lactic acid bacteria, etc.). The bioactive peptides used in early research were mainly derived from milk, cheese, and other dairy products. As research has progressed, active peptides have also been derived from other foods, including animal products as well as plant products [11]. They have been widely used in animal research, especially in rodents, but with limited research in humans. This is because rodents are easy to breed in the laboratory setting, have a short life cycle, and can be rapidly bred. Rodents also share similar genes and physiological functions with humans, making them ideal experimental animal models [12,13]. In this paper, we review the recent progress in anti-aging research involving the use of bioactive peptides in animal models, especially in rodents. In addition, we also highlight that aromatic residue, such as Trp, in some of the reported active peptides, can confer their anti-inflammatory and antioxidant activity. Moreover, many studies on active peptides mainly focus on the direct effects of these exogenous active peptides but ignore their indirect effects through regulating endogenous antioxidants in vivo. For example, exogenous active peptides can enhance endogenous antioxidative activity by increasing the levels of glutathione (GSH), superoxide dismutase (SOD), and bone-derived neurotrophic factor (BDNF) [14,15,16].
We divide this review into several sections based on the anti-aging effects on different organs. In each section, we review the mechanism of aging and the mechanism of action for the anti-aging effect of these bioactive peptides in each organ. We also summarize their common mechanism of action in different organs and the synergistic regulatory effects between endogenous and exogenous active peptides.
2. Bioactive Peptides Delay Skin Aging
2.1. Skin Aging
Skin is the largest organ and the body’s first barrier of defense against external pathogens. The skin protects the body from environmental damage and invasion of pathogens, and it is responsible for managing body temperature, sensation, and secretion function. Aging can cause different degrees of skin damage and interfere with the normal physiological function of other organs in the body [17]. The etiology of skin aging includes many factors. Among them, internal aging and photoaging are most common. Aging can alter the structure, function, and appearance of the skin, eventually leading to the increase of wrinkles, loss of elasticity, sagging, and pigment precipitation [18]. The main mechanisms of skin aging are the decrease of antioxidants in the skin, inflammation, and the degradation of collagen by increased MMPs [19]. Anti-aging bioactive peptides often act on these aging mechanisms. For example, oral collagen hydrolysates (CHs) can inhibit the activity of MMPs to reduce the degradation of collagen fibers [20]. Active peptides can reduce skin photoaging by scavenging free radicals [21]. Some bioactive peptides can reduce inflammation. In general, both endogenous and exogenous active peptides can down-regulate the factors causing skin aging. We discuss these in detail below.
2.2. Antioxidant Peptides in Delaying Skin Aging
Bioactive peptides derived from some animal proteins have antioxidant activity. These bioactive peptides can delay skin aging by regulating oxidative stress (Figure 1). For example, the collagen peptide extracted from the swim bladder of Sturgeon can increase the activities of catalase (CAT), SOD, and GSH peroxidase (GSH-PX) and decrease the activity of MMPs in skin tissue from Sprague-Dawley rats, as well as reduce the degradation of collagen by MMPs [22]. In recent years, some insect proteins with biological activity have also been found. For example, Eupolyphaga sinensis walker polypeptides (EPs) is a polypeptide mixture with a molecular weight of less than 3.3 kDa obtained from enzymatic digestion that can significantly improve the activity of antioxidant enzymes and reduce the generation of harmful free radicals. Thus, EPs can reduce the UV-irradiation-induced increase in epidermal thickness and elastic fiber breakage and restore the content of collagen [23]. In both cases, the mechanism of action of these exogenous active peptides is mainly to improve the activity of antioxidant enzymes in the skin and reduce the activity of MMPs and the degradation of collagen.
Figure 1.
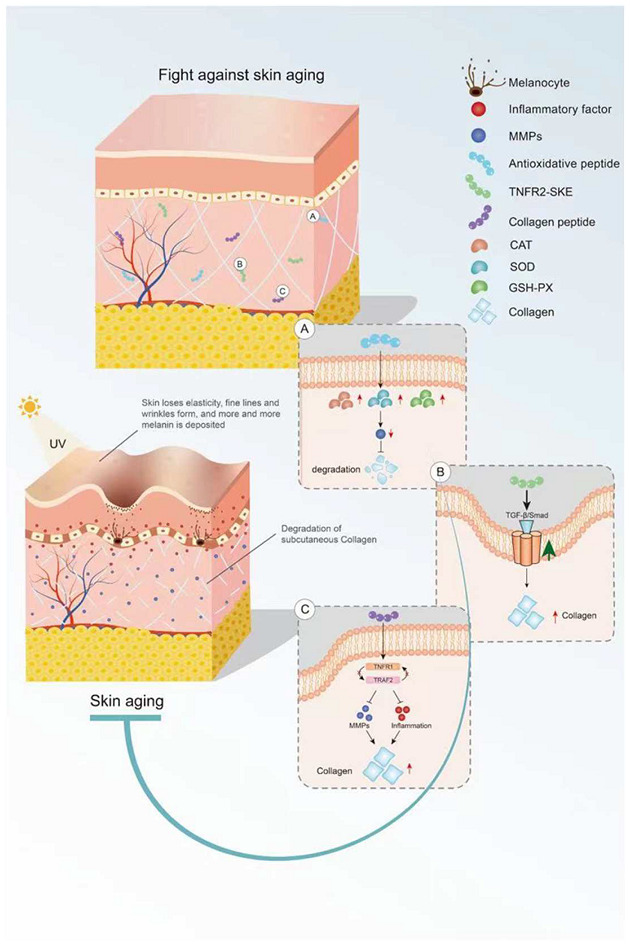
Mechanism of bioactive peptides in delaying skin aging. (A) Antioxidant peptides can increase the activity of antioxidant enzymes. (B) Bioactive peptides retard skin aging through the TGF-β/Smad pathway. (C) Active peptides inhibit inflammation and MMP activity. This figure cannot be reproduced without author permission.
2.3. Anti-Inflammatory Peptides in Delaying Skin Aging
Some endogenous peptides with anti-inflammatory effects have been used to delay skin aging. The tripeptide TNFR2-SKE (362.4 Da) derived from the tetrapeptide of TNF receptor-associated factor 2 (TNFR2) showed a good protective effect against skin photoaging. TNFR2-SKE can block the interaction between TNFR1 and TRAF2 and inhibit the inflammation induced by TNF-2 (Figure 1). Intraperitoneal administration of TNFR2-SKE to UVB-irradiated six-week-old male DBA/2 mice was shown to significantly improve epidermal thickness and pigment cell proliferation [24]. MOTS-C is a 16-peptide from the MDP family derived from mitochondria with a molecular weight of 2174.61 Da. This bioactive peptide can regulate cell metabolism and inflammation [25,26]. In a D galactose-induced aging mouse model, treatment with MOTS-c was shown to increase collagen fiber content in the dermis by increasing NRF2 and MFN2 and decreasing interleukin-6 (IL-6). The anti-aging activity of MOTS-c is likely achieved by reducing inflammation [27]. Thus, both TNFR2-SKE and MOTS-C active peptides showed good performance in significantly alleviating skin inflammation and increasing collagen fiber content in mice. These endogenous active peptides can delay skin aging through their anti-inflammatory effects. However, there are many endogenous anti-inflammatory polypeptides in the body, and their anti-aging effects on the skin remain to be explored.
2.4. Peptides in Reducing Collagen Hydrolysis
Collagen is the main component of the dermis, and its content decreases with age. Skin sagging and wrinkles are caused by a decrease in collagen content. It is noteworthy that oral CHs can reduce skin laxity and wrinkles [28] and delay skin aging. Fish skin and fish scales are generally rich in collagen. Two collagen hydrolysates (ACH and CCH) prepared from fish skin can up-regulate the transforming growth factor β (TGF-β)/Smad signaling pathway related to collagen synthesis and increase the amount of collagen. CHs have a good protective effect on skin laxity, as shown in 13-month-old female KM mice [29]. Collagen hydrolysate CPNS (Gly-Pro and Pro-Hyp) [30] and CP [31] prepared from fish scales can significantly attenuate the increase in epidermal thickness and water loss and the decrease in dermal hyaluronic acid (HA) induced by UVB irradiation, as well as recover HA loss by regulating hyaluronan synthases 1 (HAS1), hyaluronan synthases 2 (HAS2), and hyaluronidase 2 (HYAL2). Another elastin hydrolysate (EH) prepared from the bovine artery is composed of four polypeptides: Gly-Leu-Pro-Tyr (GLPY), Pro-Tyr (PY), Gly-Leu-Gly-Pro-Gly-Val-Gly (GLGPGVG), and Gly-Pro-Gly-Gly-Val-Gly-Ala- Leu (GPGGVGAL). EH can inhibit UV-induced skin thickening and sebaceous gland hyperplasia in mice and promote moisturizing of the skin. GLPY and GPGGVGAL have better inhibitory effects on elastase and thus can reduce extracellular matrix (ECM) degradation and improve the activity of UV damaged fibroblasts [32]. Collagen hydrolysis is the main cause of skin sagging, and the supplement of some collagen hydrolytic peptides can reduce the hydrolysis of collagen by MMPs. However, the detailed underlying mechanism is still unclear and needs to be further explored.
3. Bioactive Peptides and Brain Aging
3.1. Brain Aging
In the process of aging, brain function will gradually decline, which is manifested by a decline of learning ability and memory, as well as attention, decision-making ability, sensory perception, and motor ability. The prevalence of some neurodegenerative diseases, such as AD, Parkinson’s disease (PD), and stroke, also increases with age. The development of these diseases is related to mitochondrial dysfunction, accumulation of oxidative damage, and increased inflammation [33]. AD is the most common neurodegenerative disease. Currently, abnormal folding of Aβ1-42 produced by the metabolism of amyloid precursor protein (APP) is considered to be the main cause of AD pathology [34]. Iron is involved in many biological processes in the brain and plays an important role in maintaining normal brain function. However, an iron imbalance can cause toxic effects on the brain. When the iron concentration is too high, it can increase the misfolding of Aβ and promote the development of AD [35]. The role of oxidative stress and inflammation in the development of AD is well known, and some new therapeutic targets have become research hotspots. Serotonin receptors (5-HT4R) have been found to reduce Aβ production. Many 5-HT4R agonists have been studied, but their potential therapeutic effect on AD has rarely been studied in vivo [36]. Glycosylation of proteins produces advanced glycation end products (AGEs) that can cause neurodegeneration. When glyoxalase activity is reduced, the ability of these toxic glycosylated proteins to be eliminated is significantly reduced, leading to neurological disease [37]. The relationship between the gut microbiome and aging and the development of AD has been confirmed, but no clear mechanism has been elucidated. In a recent report, we found that intestinal dysregulation of Firmicutes and Bacteroidetes promotes T helper 1 (Th1) cell infiltration and promotes microglia differentiation in a pro-inflammatory direction. This may be related to the development of AD [38]. Bioactive peptides can exert their anti-aging effect on the brain through various mechanisms. They can increase antioxidant enzyme activity, reduce inflammation, increase the removal ability of iron and AGEs, increase expression of 5-HT receptors, and regulate the gut microbiota.
3.2. Antioxidant Peptides in Delaying Brain Aging
Carnosine (CAR) is an endogenous dipeptide (β-Ala-L-His) existing in muscle, blood, and the brain. CAR has good antioxidant activity and can attenuate neurological diseases caused by aging; CAR supplementation reduces the accumulation of Aβ in the hypothalamus and prefrontal cortex of aging rats and has potential therapeutic effects on AD [39]. After CAR treatment, GSH levels and SOD and GSH-Px activity were increased, whereas acetylcholinesterase (AChE) activity was significantly decreased (Figure 2), and there was a significant reduction in neuronal apoptosis, brain edema, and inflammation in D-galactose treated rats [40]. With aging, iron gradually accumulates and induces the generation of free radicals, promoting the formation of Tau and Aβ oligomers, which are neurotoxic and the main cause of AD [41]. The amount of iron found in the brains of AD patients is much higher than that of normal brains, suggesting that excess iron may be one of the causes of AD [35,42]. To better understand the effects of iron, researchers have synthesized the peptides with the ability to remove iron ions. Pentapeptide YHEDA (Tyr-His-Glu-Asp-Ala) and polypeptide mixture HAYED (5) Five (His-Ala-Tyr-Glu-Asp) repeat sequences are two synthetic active peptides with good iron ion scavenging ability (Figure 2). They can prevent the decrease of blood oxygen metabolism, inhibit the generation of free radicals, and reduce the damage in brain tissue, effectively improving cognitive impairment in senescent (SN) mice (25 months old) [43,44]. However, many high-quality natural antioxidant peptides have yet to be discovered and utilized in anti-aging studies. For example, many plant-derived bioactive peptides have antioxidant activities, and the research and development of these active peptides in aging studies will be of great significance in delaying brain aging [45].
Figure 2.
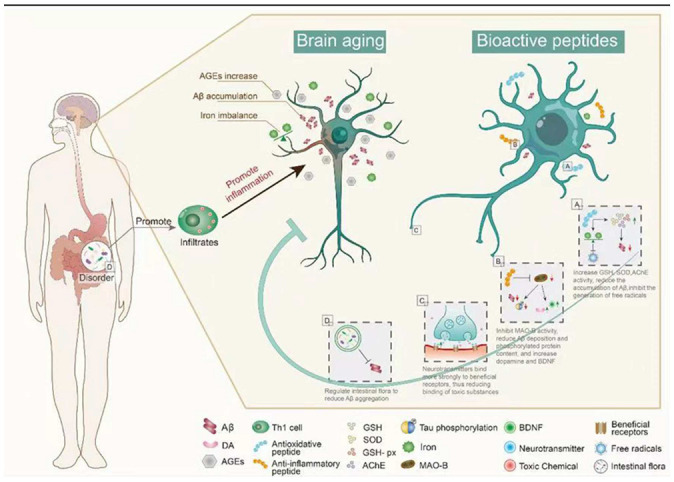
The main mechanism of bioactive peptides in delaying brain aging. (A) Bioactive peptides reduce Aβ accumulation by regulating oxidative stress. (B) The bioactive peptides inhibit the activity of MAO-B, up-regulate BDNF, and reduce the aggregation of Aβ. (C) Bioactive peptides reduce brain damage caused by toxic substances in the brain. (D) Bioactive peptides reduce Aβ aggregation by regulating intestinal microbiota. This figure cannot be reproduced without author permission.
3.3. Anti-Inflammatory Peptide in Delaying Brain Aging
Synthetic bioactive peptides are being increasingly produced for the treatment of different diseases. Liraglutide, a synthetic long-acting glucagon-like peptide 1 (GLP-1) analog, is widely used in the treatment of diabetes mellitus and CVDs. Recently, it has been speculated that liraglutide may have neuroprotective effects [46,47]. In senescence accelerated mouse P8 (SAMP8) mice (model of AD-like dementia), liraglutide treatment can improve spatial long-term memory and increase the number of hippocampal neurons [48,49]. The active peptides in dairy products have been long known, and there have been some reports that these active peptides can delay brain aging, mainly with the improvement of AD symptoms. The Whey protein hydrolysate tryptophan-methionine and tryptophan-tyrosine, extracted from fermented dairy products, can improve the cognitive impairment of AD mice. Inflammation and Aβ1-42 deposition in the cerebral cortex and hippocampus are also significantly reduced in 5 × FAD transgenic mice fed tryptophan-tyrosine [50]. Notably, Tryptophan-Tyrosine dipeptide and whey protein hydrolysate GTWY (Gly-Thr-Trp-Tyr) can increase dopamine (DA) content in the hippocampus and frontal cortex of AD mice by inhibiting the activity of monoamine oxidase B (MAO-B) [51,52,53]. β-lactolin, an active polypeptide extracted from whey protein hydrolysate, has been shown to improve cognitive impairment. Specifically, β-lactolin can reduce amyloid plaque deposition and phosphorylated Tau protein content in the cerebral cortex of 5 × FAD transgenic mice (AD mice), as well as increase DA and BDNF levels, thereby improving the cognitive impairment [54]. BDNF is one of the most widely distributed neurotrophic factors in the brain, and it plays an important role in regulating synaptic growth, neuroprotection, and affecting memory and cognition in vivo [55]. β-lactolin can increase the expression of BDNF in vivo (Figure 2). This is an example of how exogenous active peptides have a regulatory effect on endogenous active substances. Thus, exogenous active peptides not only play a therapeutic role in some antioxidant and anti-inflammatory pathways but also enhance the expression of endogenous active peptides to treat some diseases. The mechanism of action of exogenous active peptides may differ from endogenous ones, but they can supplement the body’s defense system.
3.4. Regulation of Peptide Receptors in Delaying Brain Aging
Serotonin (5-HT) is an important neurotransmitter that is involved in a variety of brain activities and functions. 5-HT receptors decrease gradually in the aging process. Serotonergic neurons are widely distributed in the brain. Reduction of 5-HT receptors can cause functional impairment of these neurons and lead to cognitive impairment. CAR is a dipeptide extracted from the meat. It can enhance 5-HT binding to its receptor and restore the regional senage-induced decrease in serotonin to normal levels [56,57]. Pituitary adenylate cyclase activated polypeptide (PACAP) is an endogenous active polypeptide with 38 amino acid residues and has a neuroprotective effect. It is widely distributed in the brain, pancreas, gonad, and respiratory tract. PACAP38 can be cleaved to form a 27 amino acid polypeptide, PACAP27 [58]. The level of PACAP gradually decreases in the normal aging process, and decreased PACAP levels have been found in the brain tissues of AD patients [59]. PACAP27 and PACAP38 can reduce the accumulation of Aβ in the brain by activating pituitary adenylate cyclase-activating polypeptide (PAC1), which causes the shedding of the receptor for advanced glycation end products (RAGE) of late glycation end products on the cell surface [60]. In summary, these peptides act on receptors, promoting the binding of beneficial receptors in neurons but reducing the binding of toxic substances.
3.5. Intestinal Microbiota Regulation by Peptides in Delaying Brain Aging
The link between the gut microbiota and AD is widely recognized, and many substances, including bioactive peptides, have been reported to regulate the gut microbiota. Some active peptides can regulate the intestinal microbiota in a beneficial direction by reducing Aβ aggregation, which has a potential role in the treatment of AD by regulating the intestinal microbiota [61]. The walnut protein hydrolysate PW5 (Pro-Pro-Lys-Asn-Trp) identified from walnut protein can reduce Aβ aggregation and improve cognitive impairment in mice by regulating intestinal microbiota (Figure 2). PW5 fed to APP/PS mice (AD mice) can increase firmicutes in the intestinal microbiota, which may be associated with reduced Aβ aggregation in mice [62]. The association between intestinal microbiota and AD has been widely recognized, and many bioactive peptides have been used to regulate intestinal microbiota to improve AD symptoms, but the mechanism is still not deeply studied, and further exploration is needed.
4. Bioactive Peptides and Aging in Other Organs
Aging is an irreversible biological process. Organs in the body cannot avoid aging. This leads to a variety of chronic diseases, including CVD, chronic obstructive pulmonary disease (COPD), intermittent lung disease, and asthma [63,64]. The aging processes of these important organs are correlated, and complications of one organ often lead to multi-organ disease. For example, lung aging causes COPD, which causes systemic inflammation and increases the risk of non-alcoholic liver disease. Moreover, people with non-alcoholic liver disease are more likely to have chronic kidney disease (CKD) and CVD. Oxidative stress and inflammation play an important role in the pathogenesis of these diseases. Many bioactive peptides with antioxidant and anti-inflammatory activities have been used in the prevention and treatment of these diseases. However, the role of a peptide in a disease is often limited, and there is still a lack of research on the complications of these diseases.
4.1. Lung Aging
COPD is a major form of lung disease characterized by chronic inflammation of the windpipe. Aging and smoking are the main causes of COPD. People over the age of 65 are five times more likely to develop the disease than younger people [65,66]. COPD is often associated with metabolic abnormalities, CVD, skeletal muscle atrophy, and other chronic diseases. In the later stages of COPD, arteriosclerosis, oxidative stress, and inflammation are the main mechanisms of its progression. Persistent inflammation disrupts the normal function of the lungs and is one of the causes of other complications [67]. Other researchers point to systemic inflammation from COPD as a major cause of non-alcoholic fatty liver disease (NAFLD) [68].
4.1.1. Antioxidant Peptides in Delaying Lung Aging
The human body is rich in peptides that play various biological activities in the body to adapt to different needs. The tripeptide GHK (glycyl-L-histidyl-L-lysine) is an active peptide existing in the human body, which has a high affinity for copper and can form a GHK-Cu complex. GHK-Cu has anti-inflammatory and antioxidant functions and can promote blood vessel growth and increase neural nutrition [69]. GHK-Cu has been shown to improve the symptoms of acute lung injury (ALI), which is usually accompanied by severe oxidative stress and inflammation. In ALI mice treated with GHK-Cu, SOD activity and GSH levels were significantly increased, and the NF-κB signaling pathway was blocked to reduce the release of inflammatory factors [70]. GHK-Cu is also a potential drug candidate for treating some chronic lung diseases such as COPD, asthma, and lung cancer [71].
4.1.2. Anti-Inflammatory Peptide in Delaying Lung Aging
Since many plants are rich in active substances, they are widely studied for use in drug development. The cyclic peptide CPE extracted from hydrolysates of Pseudostellariae can effectively relieve the symptoms of COPD. CPE treatment can significantly reduce the degree of alveolar destruction and lung inflammation, increase alveolar space, and regulate various cytokines. CPE treatment also reduces several mRNAs for TLR4, the adaptor protein MyD88 and activator protein-1 (AP-1), and active phosphorylated forms of proteins (P-JNK, P-P38, and P-TAK1) in alveolar macrophages in a COPD rat model [72]. These results suggest that CPE can act on the TLR4-MyD88-JNK/P38 signaling pathway and inhibit the release of important inflammatory factors to reduce lung inflammation. Thus, CPE has therapeutic potential for treating COPD. As an exogenous active peptide, CPE has a similar mechanism of action as GHK-Cu; both can block inflammatory pathways and reduce the release of inflammatory factors to attenuate lung inflammation. However, it would be interesting to explore whether CPE plays a synergistic role with GHK-Cu in vivo.
4.2. Liver Aging
A high-fat diet can cause NAFLD and non-alcoholic hepatitis (NASH), which is one of the major causes of cirrhosis and hepatocellular carcinoma (HCC). According to research, older people are more likely to develop NAFLD [73]. The liver is an important organ in the body. Dysfunction of antioxidant enzymes can reduce the ability of liver cells to remove peroxides, leading to the damage of mitochondrial DNA and mitochondrial dysfunction, resulting in liver aging [74,75]. Changes in the gut microbiome can also cause liver disease. For example, chronic inflammation, known as “metabolic inflammation”, caused by changes in the microbial metabolites of the gut microbiome, can lead to NAFLD. Analysis of these altered gut microbes has revealed a significant increase in Proteobacteria, a group of microbes that may be responsible for NAFLD [76]. NAFLD, in turn, can increase the risk of atherosclerosis and accelerate the development of atherosclerosis symptoms. This is supported by a correlation in lesions of several organs [77]. Internal organs also interfere with each other as the body ages. This is exemplified by the fact that the severity of NAFLD increases the risk and severity of CKD [78].
4.2.1. Antioxidant and Anti-Inflammatory Peptides in Delaying Liver Aging
The body secretes some active polypeptides when maintaining normal physiological functions. Adropin is a peptide hormone that is expressed in the liver and can regulate blood glucose and lipid homeostasis. Studies have shown that adropin knockout can increase liver inflammation, liver steatosis, and fibrosis in mice, promoting the development of NASH. The underlying changes due to the knockout, such as reduction of Nrf2 transcriptional activity, GSH level, and mitochondrial membrane potential, can be normalized by adropin supplementation. These results indicate that adropin can delay the development of NASH by maintaining mitochondrial homeostasis and increasing antioxidant enzyme activity [79]. This is an example of how the liver can secrete beneficial active peptides to maintain normal physiological function. However, in the aging process, when the liver has reduced ability to produce such peptides, there is subsequent liver damage. Thus, the supplementation of these active peptides in an aging individual can maintain the normal function of the liver and prevent liver damage. It is worth mentioning that after long-term research on the anti-aging of active peptides, our research group has found that the mice treated with the active peptide derived from rice bran have reduced aging characteristics caused by galactose. This peptide is extracted from rice bran protein hydrolysate, named KF-8 (Lys-His-Asn-Arg-Gly-Asp-Glu-Phe), can reduce oxidative stress in D-gal-treated mouse livers by inhibiting the NF-κB/p38 signal transduction pathway and delaying liver aging [80]. This suggests that some exogenous active peptides with antioxidant activity could be added to the diet as anti-aging supplements.
4.2.2. Intestinal Microbiota Regulation by Peptides in Delaying Liver Aging
Liraglutide (discussed above) can improve the symptoms of NAFLD by regulating the gut microbiome. Specifically, liraglutide treatment can reduce Proteobacteria, a common factor in many diseases, and increase Verucommicrobia, which contributes to intestinal health and glucose homeostasis in the intestines of obese mice. Changes in the abundance of gut microbiota by liraglutide are associated with improvement of NAFLD symptoms and a reduction in inflammatory cell infiltration in the cecum and liver [81]. Mechanistically, liraglutide regulates the gut microbiome to reduce liver inflammation. There are many other bioactive peptides that can regulate the intestinal microbiome; however, the detailed mechanisms of their effects have not yet been elucidated.
4.3. Kidney Aging
The physiological function of the kidneys gradually deteriorates with aging, causing some kidney diseases [82]. With the increase of age, kidneys are also more vulnerable to oxidative damage, especially in the mitochondria of the kidney cells. Impaired mitochondrial function and cellular metabolism eventually lead to chronic renal failure [83]. CKD is an important cause of CVD because it can lead to high blood pressure and a decrease in the capillary density of the cardiac tissue. In addition, CKD reduces nitric oxide synthase expression in the vascular endothelium and increases renin–angiotensin system activity, resulting in increased release of superoxide and inflammatory cytokines and subsequent CVD [84].
4.3.1. Antioxidant Peptides in Delaying Renal Aging
In the aging process, increased oxidative stress and chronic inflammation can lead to some kidney diseases. Most of the endogenous active peptides in the endocrine system can reduce oxidative stress and inflammation, and thus, these peptides have potential therapeutic for alleviating kidney diseases. Mitochondrial targeted peptide SBT-20 (also known as SS-20) is a synthetic tetrapeptide that can reduce ROS and maintain the normal production of the electron transport chain and ATP. SBT-20 can reduce the expression of mitochondrial mitotic protein Drp1 and increase the expression of mitochondrial fusion protein 2 (Mfn2) to maintain the normal structure and function of mitochondria. SBT-20 treatment can decrease the expression of inflammatory cytokines IL-1β, IL-6, NF-κB1, and NF-κB2 in the kidney and alleviate the symptoms of chronic renal failure (CRF) in CRF mice [85]. Compared with endogenous active peptides, SBT-20 also has regulatory effects on inflammation and oxidative stress. With the technological development of peptide synthesis in vitro, the synthesis of these active peptides to meet the therapeutic needs of different diseases would be a powerful and desirable approach in the future.
4.3.2. Anti-Inflammatory Peptides in Delaying Renal Aging
Compared with endogenous active peptides produced in animals, exogenous active peptides from plants can also reduce the symptoms of CRF through similar mechanisms. Soy is rich in proteins that can be hydrolyzed into some bioactive peptides. The soybean protein hydrolysate (SPH) can lower blood pressure and maintain normal renal function. We showed that feeding rats (5/6 nephrectomized model) SPH can reduce ACE activity and TNF-α levels. These results suggest that SPH can reduce renal inflammation in CRF by down-regulating TNF-α activity [86]. Thus, exogenous active peptides are similar to endogenous active peptides in slowing CRF progression. Since exogenous active peptides are more widely derived, they hold great promise for providing therapeutic effects for many diseases.
4.4. Aging of the Heart and Blood Vessels
Complications of many diseases can increase the risk of CVD, and CVD can also promote lesions in other organs. The impaired endothelial cell function that is associated with aging can lead to vascular dilation and decreased anti-thrombotic ability, and eventually CVD. The main causes of CVD are activation of inflammatory signals induced by NF-κB, increases in MMP-9, and changes in TGF-β [87,88]. In addition, increased inflammation with aging can induce the expression of vascular endothelial growth factor (VEGF) family proteins. Although VEGF plays a beneficial role in some CVDs, over-expression of VEGF promotes the formation of new blood vessels, which in turn contributes to the development of atherosclerotic pathology [89].
4.4.1. Antioxidant Peptides in Delaying Cardiovascular Aging
Some active peptides extracted from animals and plants have potential therapeutic effects on CVD. For example, some exogenous active peptides extracted from grains may improve CVD [90]. Rice α-globulin hydrolysate Try-Try-Gly-Gly-Glu-Ser-Ser-Ser-Glu-Gln-Gly (YYGGESSSEQG) and Ser-Glu-Ser-Glu-Met (SESEM) extracted from rice can improve the symptoms of atherosclerosis in mice. They can down-regulate the TNF-α pathway and NF-κB in the aorta and aortic root tissues and reduce oxidative stress levels and inflammatory factors in apolipoprotein E-deficient mice [91]. Another exogenous active peptide, zebra blenny protein hydrolysates (ZBPHs), extracted from zebrafish protein, has a good antioxidant effect and can reduce the lipid deposition and apoptosis of cardiac cells induced by a high cholesterol diet in hypercholesterolemic rats. This is a potential therapeutic agent for CVD [92]. Since these two exogenous active peptides are isolated from rice and zebrafish proteins, respectively, they are widely available and can easily be incorporated into the diet.
4.4.2. Anti-Inflammatory Peptide in Delaying Cardiovascular Aging
In normal conditions, the heart can secrete some active peptides to maintain the physiological functions of the cardiovascular system. Natriuretic peptide (NP) is a kind of polypeptide that can maintain normal function of the heart, blood vessels, and kidney, and is associated with some CVDs [93]. NPs mainly exist in two forms: atrial na-triuretic peptide (ANP) and cerebral natriuretic peptide (BNP). BNP has been reported to treat myocardial infarction in mice. BNP can promote endothelial cell proliferation and myocardial vascularization in the infarcted and non-infarcted areas in the hearts of mice following myocardial infarction. The action of BNP is mediated by P38 MAP kinase [94]. Adropin, a peptide used to treat the non-alcoholic liver disease, can also be used to treat CVDs. It can reduce inflammation and migration of vascular smooth muscle cells, improve symptoms, and reduce intravascular plaque significantly [95]. Since adropin can be used to treat liver aging and atherosclerosis, it would have an added therapeutic potential to treat the cardiovascular complications of NASH.
5. Conclusions
Continuous improvement of biomedical research and healthcare has resulted in a significant increase in life span and the aging population. However, this has created a subsequent problem because aging is associated with many diseases. Therefore, great efforts have been made in anti-aging research, and many bioactive peptides have been discovered to have anti-aging activity. Bioactive peptides can be endogenously produced in the body, but more and more bioactive peptides are being exogenously produced from natural products or biosynthesis. The mechanisms of action of these bioactive peptides mainly involve their antioxidant and anti-inflammatory activities. Interestingly, some exogenous and endogenous active peptides have synergistic effects. Several organs in the body share similar aging mechanisms, and one organ disease can affect multiple organs. Thus, bioactive peptides can have anti-aging effects on multiple organs.
Although bioactive peptides have been widely used in anti-aging studies of rodents, it is not clear whether these active peptides exert their anti-aging effects in humans through similar mechanisms. Therefore, anti-aging research using genetically similar animal models, such as primates, is needed before many bioactive peptides can be tested in human clinical trials.
Here, we have reviewed the anti-aging activity of bioactive peptides, most of them from the hydrolysate of some food, as well as some synthetic active peptides and endogenous active peptides. These peptides were ingested by mouth, gavage, and injection in rodent models, and behavioral and physiological changes in these animals demonstrated the protective benefits of these peptides (reduced disease symptoms). It is worth mentioning that these active peptides have no adverse side effects and toxicity to experimental animals within the range of experimental concentrations, which also indicates that bioactive peptides are non-toxic and hypoallergenic active substances.
In this paper, we have introduced some of the anti-aging activities of active peptides, but there is additional research in the literature on other peptides. In view of this, Table 1 provides more information on other active peptides that are not discussed in detail in the text, as well as summarizing the animal models used in the studies of these active peptides and methods and dosages of peptide administration for reference of interested scholars. In addition, the main abbreviations that appear in this article are given in Table A1 of the Appendix A.
Table 1.
Bioactive peptides with anti-aging activity.
| Classification | Name and Delivery Way | Source | Rodent Model | Target Organ | Mechanism |
|---|---|---|---|---|---|
| Food-derived active peptide | Walnut protein hydrolysates(WPH) Oral gavage for 21 days Low: 333 mg/kg High: 666 mg/kg |
Walnut | Alzheimer’s disease model mice aged 6–8 weeks scopolamine solution (1.0 mg/kg) |
Brain | SOD↑ GSH-Px↑ CAT↑ Nrf2↑ BDNF↑ CREB↑ MDA↓ TNFα↓ AchE↓ Trp-, Tyr-, or Phe-containing peptide has high affinity to Keap1 and Ache, so it can increase the activity of NRF2 and reduce the activity of Ache, which ultimately increases antioxidant capacity and anti-inflammatory ability and leads to increased BDNF, CREB transcription [15] |
| Walnut protein hydrolysate and its low-molecular-weight fraction (WPH/WPHL) Oral gavage for 21 daysWPH: 666 mg/kgWPHL: 666 mg/kg |
Walnut | Alzheimer’s disease model mice aged 6–8 weeks LPS (300 μg/kg bw) |
Brain | SOD↑ GSH-Px↑ CAT↑ MDA↓ TNFα↓ TNFα↓ IL-6↓ IL-1β↓ Trp, Gly, Leu residues, hydrophobic amino acids, and aromatic amino acids in polypeptides can inhibit the expression of pro-inflammatory factors TNF-α, IL-1β, and IL-6 and reduce inflammation [16] |
|
| Tyr-Val-Leu-Leu-Pro-Ser-Pro-Ly (walnut protein hydrolysates) Continuous injection for 4 weeks 60 mg/kg bw |
Walnut | Alzheimer’s disease model mice (C57BL/6) 5–6 week oldscopolamine solution (1 mg/kg bw) |
Brain | ATP↑ PINK1↑ Parkin↑ NRF2↑ LC3 II/LC3 I↑ Beclin↑ KEAP1↓ p62↓ It can increase antioxidant capacity through Nrf2 signaling pathway and increase the expression of Beclin-1, Parkin, and PINK1 to enhance mitochondrial autophagy capacity [96] |
|
| Alcalase potato-protein hydrolysates (IF) Oral administration 3 weeks 1 mg/kg bw |
Potato | Senescence-Accelerated mice (SAMP8) 6 months high-fat diet |
Liver/heart | pAKT↑ Sirt1↑ pAMPK↑ PGC1α↑ pFOXO3a↑ Bax↓ GOT↓ GPT↓ LDL↓ ANP↓ BNP↓ pGATA4↓ It can down-regulate cardiac hypertrophy markers ANP and BNP, reduce inflammation in the heart and liver, and reduce apoptosis by stimulating the activity of Sirt1 [97] |
|
| Alcalase potato protein hydrolysate (APPH) Oral administration 4 weeks Low: 15 mg/kg/day Middle: 45 mg/kg/day High: 75 mg/kg/day |
Potato | Sprague-Dawley (SD) rat 23 months old high-fat diet |
Heart | p-p38/p38↓ GSN↓ p-Gata4↓ TGFβ↓ APPH has good lipid solubility and can reduce myocardial hypertrophy and fibrosis in aging rats through TGF-β/GSN pathway [98] |
|
| Casein hydrolysates Continuous injection 10 weeks 200 mg/kg |
Casein | Diabetic rat high-fat diet |
Liver | NRF2↑ HO-1↑ SOD↑ GSH↑ MDA↓ By enhancing Nrf2 translation, the activity of antioxidant enzymes was enhanced, and the activities of DPP-IV and ACE were inhibited, among which dipeptide WM could inhibit Keap1/Nrf2 interaction [99] |
|
| Wheat germ albumin hydrolysates ((Ala-Asp-Trp-Gly-Gly-Pro-Leu-Pro-His)) Continuous injection 1 week 4 mg/kg |
Wheat | Diabetic mice 6 weeks old |
Vascular | pAMPK/AMP↑ pPKCζ/PKCζ↓ NOX4↓ ROS↓ pAKT/AKT↓ Inhibition of NOX4 expression through the PKCζ/AMPK signaling pathway reduced oxidative stress levels and the release of inflammatory factors [100] |
|
| Collagen hydrolysate Pro-Hyp Oral administration 4 weeks 210 mg/kg |
Porcine skin | Chronic kidney disease mice 6 weeks old |
Kidney | Liver iron content↑ EPO↑ HIF-2α↑ Hepcidin↓ TNF-α↓ IL-1β↓ IL-6↓ NF-κB↓ COX2↓ It reduces inflammation by regulating inflammatory pathways and plays a protective role in regulating HIF-2α, EPO, and Hepcidin [101] |
|
| Anchovy hydrolysates Pro-Ala-Tyr-Cys-Ser (PAYCS) 20 days 0.2 mM/kg/day |
Anchovy | Alzheimer’s disease model mice 6 weeks old Scopolamine solution (1 mg/kg bw) |
Brain | Ach↑ AchR↑ Nrf2↑ BDNF↑ SOD↑ The antioxidative effects of PAYCS and PAY may be related to the Try active phenolic structure in the sequence and the hydrogen donor of the sulfhydryl group in Cys. Both active peptides have the ability to promote the binding of Ach and AchR [102] |
|
| Soy protein isolate (SPI) Oral administration 8 weeks |
Soy | Obese rat 6 weeks old |
Liver | NPTX2↑ GPT↑ INMT↑ HAL↑ The increased expression of NPTX2 reduced the inflammation of the rat liver, the increased expression of GPT may be related to mitochondrial energy metabolism, the increased expression of INMT may be related to the relief of NAFLD symptoms, and the increased expression of HLT can consume excess protein in the liver [103] |
|
| Walnut protein hydrolysate Oral administration Low: 0.32 g/L Middle: 0.96 g/L High: 2.88 g/L |
Walnut | Skin-aging model rat Exposed to UV-R |
Skin | Elastin↑ Fibrillin-1↑ MMP-1↓ Increasing the expression of Col I, Col III, HYP, and HA and significantly attenuated the activity of MMP-1 [104] |
|
| Eucheuma hydrolysate (EZY-1) 28 day 0.25 mg/kg 0.5 mg/kg 1 mg/kg 50 mg/kg |
Eucheuma | Pulmonary fibrosis mice (C57BL/6J) 8 weeks old injected with 3.5 mg/kg of bleomycin |
Lung | T-SOD↑ GSH-Px↑ HYP↓ MDA↓ pSmad3↓ EZY- 1 is easily absorbed in the intestinal tract, and its hydrophobic point facilitates the entry of EZY-1 into cells, while EZY-1 can reduce pulmonary fibrosis through TGF-β/Samd signaling pathway. [105] |
|
| Egg white protein hydrolysate (EWPs) Gavage 14 days Low: 50 mg/kg Middle: 100 mg/kg High: 200 mg/kg |
Egg | Colitis model mice (BALB/c) administered 3% (w/v) DSS |
Gut | Candidatus_Sacchar-imonas↑ norank_f_Ruminococcaceae↓ Ruminiclostridium↓ TNF-α↓ IL-6↓ IL-8↓ EWPs contain Trp, Try, His, and Met, which make it have good antioxidant activity and can reduce the release of inflammatory factors by increasing the content of Lactobacillus and Candidatus-Saccharimonas in the gut [106] |
|
| Whey protein hydrolysate (WHP) Gavage 30 days Low: 0.3 g/kg Middle: 1.5 g/kg High: 3.0 g/kg |
Egg | D-galactose-treated mice (C57BL/6N) 6 months 100 mg/kg |
Brain | SOD↑ GSH-Px↑ AChE↑ p-CaMKII↑ MDA↓ TNF-α↓ IL-1β↓ TNF-α↓ WHP can reduce the release of inflammatory factors, increase the activity of antioxidant enzymes, and enhance the activities of AchE and P-CamKII, which play an important role in maintaining synaptic plasticity [107] |
|
| A peptide encrypted from the venom of Tityus serrulatus scorpion | Lys-Pro-Pro (KPP) | Scorpion | Mice 10 weeks old |
Heart | pPLN/PLN↓ pERK/ERK↓ KPP regulates cellular stress-related proteins and exerts cardioprotective effects through PLN dephosphorylation [108] |
| Secretory bioactive peptide | Humanin (HNG) Injections 14 months 4 mg/kg |
Mitochondria | Aging mice (C57BL/6N) 18 months |
Heart | pAKT↑ pGSK3β↓ 4-HNE↓ TGF-β1↓ FGF-2↓ MMP-2↓ HNG down-regulated the expression of GSK-3β through Akt pathway, reduced myocardial apoptosis, down-regulated FGF-2 and MMP-2 expression, and inhibited cardiac fibrosis [109] |
| Peptide hormone | Melatonin Injections 30 days 10 mg/kg |
Pineal gland | Aging mice 8 weeks old D-galactose 100 mg/kg |
Brain | SNAP-25↑ PSD95↑ GluR1↑ p-CREB↑ ROS↓ GFAP↓ p-IKKβ↓ NF-κB↓ COX-2↓ NOS2↓ IL-1β↓ TNFα↓ p-JNK↓ Melatonin can reduce synaptic damage caused by oxidative stress and neuroinflammation through RAGE/NFκB/JNK pathway and has a good therapeutic effect on neurodegeneration [110] |
Appendix A
Table A1.
The full name of abbreviations.
| Abbreviations | Full Name | Abbreviations | Full Name |
|---|---|---|---|
| SOD | Superoxide dismutase | Samd | Drosophila mothers against decapentaplegic protein |
| GSH-Px | Glutathione peroxidase | pAMPK/AMP | AMP-activated protein kinase/AMP |
| GSH | Glutathione | pPKCζ/PKCζ | Anti-phospho-protein kinase ζ |
| CAT | Catalase | NOX4 | Antibodies against NADPH oxidase4 |
| Nrf2 | Transcription factor nuclear factor erythroid 2-related factor 2 | EPO | Erythropoietin |
| BDNF | Brain-derived neurotrophic factor | HIF-2α | Hypoxia-inducible factor |
| CREB | cAMP-response element-binding protein | NF-κB | Nuclear factor-kappa beta |
| AchE | Acetylcholinesterase | COX2 | Cyclooxygenase |
| MDA | Malondialdehyde | Ach | Acetylcholine |
| TNFα | Tumour necrosis factor-α | AchR | Cetylcholine receptor |
| IL-8 | Interleukin-8 | NPTX2 | Neuronal pentraxin 2 |
| IL-6 | Interleukin-6 | INMT | Indolethylamine N-methyltransferas |
| IL-1β | Interleukin-1β | HAL | Histamine ammonia-lyase |
| ATP | Adenosine triphosphate | MMP | Atrix metalloproteinase |
| PINK1 | Mutations in the PTEN-induced kinase 1 | ERK | Extracellular signal-regulated kinase |
| Parkin | Parkin RBR E3 ubiquitin protein ligase | HYP | Hydroxyproline |
| LC3Ⅱ/LC3Ⅰ | Microtubule-associated protein light chain 3 | p-CaMKII | Phosphorylated Ca2+/calmodulin-dependent protein kinase II |
| KEAP1 | Kelch-like ECH-associated protein 1 | PLN | Dephosphorylation of phospholamban |
| p62 | Protein sequestosome 1/p62 | ERK | Extracellular regulated protein kinases |
| pAKT | Phosphorylated protein kinase B | pGSK3β | Phosphorylated glycogen synthase kinase-3beta |
| Sirt1 | Silencing information regulator 2 related enzyme | 4-HNE | 4-hydroxynonenal |
| pAMPK | Phosphorylated AMP-activated protein kinase | FGF-2 | Fibroblast growth factor 2 |
| PGC1α | Peroxisome proliferator-activated receptor-γ co-activator-1α | SNAP-25 | Synaptosomal associated protein 25 |
| pFOXO3a | Phospho forkhead box O3a | PSD95 | Postsynaptic density proteins |
| GOT | Glutamic oxaloacetic transaminase | GluR1 | Anti-phospho-AMPARs |
| GPT | Glutamic-pyruvic transaminase | p-CREB | Phosphorylated cAMP-response element-binding protein |
| LDL | Low-density lipoprotein | GFAP | Astrocytosis |
| ANP | Atrial natriuretic peptide | p-IKKβ | Phosphorylated IKKbeta |
| BNP | Cerebral natriuretic peptide | NOS2 | Nitric oxide synthase-2 |
| pGATA4 | Phosphorylated GATA binding protein 4 | p-JNK | Hospho-c-JunN-terminal Kinase |
| p-p38/p38 | Phosphorylated p38 kinase/p38 kinase | TGFβ | Transforming growth factor-beta |
| GSN | Gelsolin |
Author Contributions
J.W. and Y.W.: writing—original draft preparation and data curation; Z.C. and Y.C.: software and investigation; Q.L.: supervision and funding acquisition; Y.L.: conceptualization, writing—reviewing and editing, and visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Natural Science Foundation for Distinguished Young Scholars of Hunan Province (No.2021JJ10078), the Natural Science Foundation of Hunan Province (No. 2020JJ4138), Hunan Furong Scholars Program, Huxiang Youth Talents Supporting Program (No. 2016RS3033), Scientific Research Foundation of Hunan Provincial Education Department (No. 18A160), and Grain-Oil Process and Quality Control 2011 Collaborative and Innovative Grant from Hunan Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository (https://pubmed.ncbi.nlm.nih.gov, accessed on 21 January 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Partridge L., Fuentealba M., Kennedy B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020;19:513–532. doi: 10.1038/s41573-020-0067-7. [DOI] [PubMed] [Google Scholar]
- 2.Kane A.E., Sinclair D.A. Sirtuins and NAD+in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018;123:868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo J., Mills K., Le Cessie S., Noordam R., Van Heemst D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020;57:100982. doi: 10.1016/j.arr.2019.100982. [DOI] [PubMed] [Google Scholar]
- 4.Da Costa J.P., Vitorino R., Silva G.M., Vogel C., Duarte A.C., Rocha-Santos T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 2016;29:90–112. doi: 10.1016/j.arr.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner K.H., Cameron-Smith D., Wessner B., Franzke B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients. 2016;8:338. doi: 10.3390/nu8060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurau F., Baldoni S., Prattichizzo F., Espinosa E., Amenta F., Procopio A.D., Albertini M.C., Bonafe M., Olivieri F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018;46:14–31. doi: 10.1016/j.arr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Kim D.H., Bang E., Jung H.J., Noh S.G., Yu B.P., Choi Y.J., Chung H.Y. Anti-aging Effects of Calorie Restriction (CR) and CR Mimetics based on the Senoinflammation Concept. Nutrients. 2020;12:422. doi: 10.3390/nu12020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez A., Vázquez A. Bioactive peptides: A review. Food Qual. Saf. 2017;1:29–46. doi: 10.1093/fqs/fyx006. [DOI] [Google Scholar]
- 9.Gorguc A., Gencdag E., Yilmaz F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020;136:109504. doi: 10.1016/j.foodres.2020.109504. [DOI] [PubMed] [Google Scholar]
- 10.Bechaux J., Gatellier P., Le Page J.F., Drillet Y., Sante-Lhoutellier V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019;10:6244–6266. doi: 10.1039/C9FO01546A. [DOI] [PubMed] [Google Scholar]
- 11.Rutherfurd-Markwick K.J. Food proteins as a source of bioactive peptides with diverse functions. Br. J. Nutr. 2012;108((Suppl. 2)):S149–S157. doi: 10.1017/S000711451200253X. [DOI] [PubMed] [Google Scholar]
- 12.Azzu V., Valencak T.G. Energy Metabolism and Ageing in the Mouse: A Mini-Review. Gerontology. 2017;63:327–336. doi: 10.1159/000454924. [DOI] [PubMed] [Google Scholar]
- 13.Brunet A. Old and new models for the study of human ageing. Nat. Rev. Mol. Cell. Biol. 2020;21:491–493. doi: 10.1038/s41580-020-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes M.J.C., Lima S.L.S., Alves N.E.G., Assis A., Moreira M.E.C., Toledo R.C.L., Rosa C.O.B., Teixeira O.R., Bassinello P.Z., De Mejia E.G., et al. Common bean protein hydrolysate modulates lipid metabolism and prevents endothelial dysfunction in BALB/c mice fed an atherogenic diet. Nutr. Metab. Cardiovasc. Dis. 2020;30:141–150. doi: 10.1016/j.numecd.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Wang S., Su G., Zhang X., Song G., Zhang L., Zheng L., Zhao M. Characterization and Exploration of Potential Neuroprotective Peptides in Walnut (Juglans regia) Protein Hydrolysate against Cholinergic System Damage and Oxidative Stress in Scopolamine-Induced Cognitive and Memory Impairment Mice and Zebrafish. J. Agric. Food Chem. 2021;69:2773–2783. doi: 10.1021/acs.jafc.0c07798. [DOI] [PubMed] [Google Scholar]
- 16.Wang S., Zheng L., Zhao T., Zhang Q., Liu Y., Sun B., Su G., Zhao M. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food Chem. 2020;68:2381–2392. doi: 10.1021/acs.jafc.9b07670. [DOI] [PubMed] [Google Scholar]
- 17.Chambers E.S., Vukmanovic-Stejic M. Skin barrier immunity and ageing. Immunology. 2020;160:116–125. doi: 10.1111/imm.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu Y., Han J., Jiang C., Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020;59:101036. doi: 10.1016/j.arr.2020.101036. [DOI] [PubMed] [Google Scholar]
- 19.Lephart E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016;31:36–54. doi: 10.1016/j.arr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z., Wang Q., Wang L., Xu W., He Y., Li Y., He S., Ma H. Improvement of skin condition by oral administration of collagen hydrolysates in chronologically aged mice. J. Sci. Food Agric. 2017;97:2721–2726. doi: 10.1002/jsfa.8098. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre-Cruz G., León-López A., Cruz-Gómez V., Jiménez-Alvarado R., Aguirre-Álvarez G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants. 2020;9:181. doi: 10.3390/antiox9020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L., Wang X., Bai F., Fang Y., Wang J., Gao R. The anti-skin-aging effect of oral administration of gelatin from the swim bladder of Amur sturgeon (Acipenser schrenckii) Food Funct. 2019;10:3890–3897. doi: 10.1039/C9FO00661C. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N., Zhao Y., Shi Y., Chen R., Fu X., Zhao Y. Polypeptides extracted from Eupolyphaga sinensis walker via enzymic digestion alleviate UV radiation-induced skin photoaging. Biomed. Pharmacother. 2019;112:108636. doi: 10.1016/j.biopha.2019.108636. [DOI] [PubMed] [Google Scholar]
- 24.Lee K.J., Park K.H., Hahn J.H. Alleviation of Ultraviolet-B Radiation-Induced Photoaging by a TNFR Antagonistic Peptide, TNFR2-SKE. Mol. Cells. 2019;42:151–160. doi: 10.14348/molcells.2018.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K.H., Son J.M., Benayoun B.A., Lee C. The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell Metab. 2018;28:516–524.e7. doi: 10.1016/j.cmet.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C., Zeng J., Drew B.G., Sallam T., Martin-Montalvo A., Wan J., Kim S.J., Mehta H., Hevener A.L., de Cabo R., et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–454. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Lu H., Hu G., Ye Z., Zhai D., Yan Z., Wang L., Xiang A., Lu Z. Earlier changes in mice after D-galactose treatment were improved by mitochondria derived small peptide MOTS-c. Biochem. Biophys. Res. Commun. 2019;513:439–445. doi: 10.1016/j.bbrc.2019.03.194. [DOI] [PubMed] [Google Scholar]
- 28.Song H., Zhang S., Zhang L., Li B. Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice. Nutrients. 2017;9:1209. doi: 10.3390/nu9111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L., Zhang S., Song H., Li B. Ingestion of collagen hydrolysates alleviates skin chronological aging in an aged mouse model by increasing collagen synthesis. Food Funct. 2020;11:5573–5580. doi: 10.1039/D0FO00153H. [DOI] [PubMed] [Google Scholar]
- 30.Lee H.J., Jang H.L., Ahn D.K., Kim H.J., Jeon H.Y., Seo D.B., Lee J.H., Choi J.K., Kang S.S. Orally administered collagen peptide protects against UVB-induced skin aging through the absorption of dipeptide forms, Gly-Pro and Pro-Hyp. Biosci. Biotechnol. Biochem. 2019;83:1146–1156. doi: 10.1080/09168451.2019.1580559. [DOI] [PubMed] [Google Scholar]
- 31.Kang M.C., Yumnam S., Kim S.Y. Oral Intake of Collagen Peptide Attenuates Ultraviolet B Irradiation-Induced Skin Dehydration In Vivo by Regulating Hyaluronic Acid Synthesis. Int. J. Mol. Sci. 2018;19:3551. doi: 10.3390/ijms19113551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Su G., Zhou F., Zhang J., Zheng L., Zhao M. Protective Effect of Bovine Elastin Peptides against Photoaging in Mice and Identification of Novel Antiphotoaging Peptides. J. Agric. Food Chem. 2018;66:10760–10768. doi: 10.1021/acs.jafc.8b04676. [DOI] [PubMed] [Google Scholar]
- 33.Mattson M.P., Arumugam T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018;27:1176–1199. doi: 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 35.Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalut J., Karila D., Dallemagne P., Rochais C. Modulating 5-HT and 5-HT receptors in Alzheimer’s disease treatment. Future Med. Chem. 2017;9:781–795. doi: 10.4155/fmc-2017-0031. [DOI] [PubMed] [Google Scholar]
- 37.Hipkiss A.R. Glycotoxins: Dietary and Metabolic Origins; Possible Amelioration of Neurotoxicity by Carnosine, with Special Reference to Parkinson’s Disease. Neurotox. Res. 2018;34:164–172. doi: 10.1007/s12640-018-9867-5. [DOI] [PubMed] [Google Scholar]
- 38.Wang X., Sun G., Feng T., Zhang J., Huang X., Wang T., Xie Z., Chu X., Yang J., Wang H., et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019;29:787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banerjee S., Mukherjee B., Poddar M.K., Dunbar G.L. Carnosine improves aging-induced cognitive impairment and brain regional neurodegeneration in relation to the neuropathological alterations in the secondary structure of amyloid beta (Abeta) J. Neurochem. 2021;158:710–723. doi: 10.1111/jnc.15357. [DOI] [PubMed] [Google Scholar]
- 40.Aydin A.F., Coban J., Dogan-Ekici I., Betul-Kalaz E., Dogru-Abbasoglu S., Uysal M. Carnosine and taurine treatments diminished brain oxidative stress and apoptosis in D-galactose aging model. Metab. Brain Dis. 2016;31:337–345. doi: 10.1007/s11011-015-9755-0. [DOI] [PubMed] [Google Scholar]
- 41.Derry P.J., Hegde M.L., Jackson G.R., Kayed R., Tour J.M., Tsai A.L., Kent T.A. Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog. Neurobiol. 2020;184:101716. doi: 10.1016/j.pneurobio.2019.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts B.R., Ryan T.M., Bush A.I., Masters C.L., Duce J.A. The role of metallobiology and amyloid-beta peptides in Alzheimer’s disease. J. Neurochem. 2012;120((Suppl. 1)):149–166. doi: 10.1111/j.1471-4159.2011.07500.x. [DOI] [PubMed] [Google Scholar]
- 43.Zou Z., Cai J., Zhong A., Zhou Y., Wang Z., Wu Z., Yang Y., Li X., Cheng X., Tan J., et al. Using the synthesized peptide HAYED (5) to protect the brain against iron catalyzed radical attack in a naturally senescence Kunming mouse model. Free Radic. Biol. Med. 2019;130:458–470. doi: 10.1016/j.freeradbiomed.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Zou Z., Shao S., Zou R., Qi J., Chen L., Zhang H., Shen Q., Yang Y., Ma L., Guo R., et al. Linking the low-density lipoprotein receptor-binding segment enables the therapeutic 5-YHEDA peptide to cross the blood-brain barrier and scavenge excess iron and radicals in the brain of senescent mice. Alzheimers Dement. 2019;5:717–731. doi: 10.1016/j.trci.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui X., Lin Q., Liang Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020;12:209. doi: 10.3389/fnagi.2020.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly A.S., Auerbach P., Barrientos-Perez M., Gies I., Hale P.M., Marcus C., Mastrandrea L.D., Prabhu N., Arslanian S., Investigators N.N.T. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020;382:2117–2128. doi: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 47.Wicinski M., Socha M., Malinowski B., Wodkiewicz E., Walczak M., Gorski K., Slupski M., Pawlak-Osinska K. Liraglutide and its Neuroprotective Properties-Focus on Possible Biochemical Mechanisms in Alzheimer’s Disease and Cerebral Ischemic Events. Int. J. Mol. Sci. 2019;20:1050. doi: 10.3390/ijms20051050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen H.H., Fabricius K., Barkholt P., Niehoff M.L., Morley J.E., Jelsing J., Pyke C., Knudsen L.B., Farr S.A., Vrang N. The GLP-1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2015;46:877–888. doi: 10.3233/JAD-143090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan W., Pang M., Yu Y., Gou X., Si P., Zhawatibai A., Zhang Y., Zhang M., Guo T., Yi X., et al. The neuroprotection of liraglutide on diabetic cognitive deficits is associated with improved hippocampal synapses and inhibited neuronal apoptosis. Life Sci. 2019;231:116566. doi: 10.1016/j.lfs.2019.116566. [DOI] [PubMed] [Google Scholar]
- 50.Ano Y., Yoshino Y., Uchida K., Nakayama H. Preventive Effects of Tryptophan-Methionine Dipeptide on Neural Inflammation and Alzheimer’s Pathology. Int. J. Mol. Sci. 2019;20:3206. doi: 10.3390/ijms20133206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ano Y., Ayabe T., Ohya R., Kondo K., Kitaoka S., Furuyashiki T. Tryptophan-Tyrosine Dipeptide, the Core Sequence of beta-Lactolin, Improves Memory by Modulating the Dopamine System. Nutrients. 2019;11:348. doi: 10.3390/nu11020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ano Y., Yoshino Y., Kutsukake T., Ohya R., Fukuda T., Uchida K., Takashima A., Nakayama H. Tryptophan-related dipeptides in fermented dairy products suppress microglial activation and prevent cognitive decline. Aging. 2019;11:2949–2967. doi: 10.18632/aging.101909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ano Y., Ayabe T., Kutsukake T., Ohya R., Takaichi Y., Uchida S., Yamada K., Uchida K., Takashima A., Nakayama H. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging. 2018;72:23–31. doi: 10.1016/j.neurobiolaging.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Ano Y., Ohya R., Takaichi Y., Washinuma T., Uchida K., Takashima A., Nakayama H. beta-Lactolin, a Whey-Derived Lacto-Tetrapeptide, Prevents Alzheimer’s Disease Pathologies and Cognitive Decline. J. Alzheimers Dis. 2020;73:1331–1342. doi: 10.3233/JAD-190997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kowianski P., Lietzau G., Czuba E., Waskow M., Steliga A., Morys J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banerjee S., Ghosh T.K., Poddar M.K. Carnosine reverses the aging-induced down regulation of brain regional serotonergic system. Mech. Ageing Dev. 2015;152:5–14. doi: 10.1016/j.mad.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee S., Poddar M.K. Aging-induced changes in brain regional serotonin receptor binding: Effect of Carnosine. Neuroscience. 2016;319:79–91. doi: 10.1016/j.neuroscience.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Vaudry D., Falluel-Morel A., Bourgault S., Basille M., Burel D., Wurtz O., Fournier A., Chow B.K., Hashimoto H., Galas L., et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 59.Reglodi D., Atlasz T., Szabo E., Jungling A., Tamas A., Juhasz T., Fulop B.D., Bardosi A. PACAP deficiency as a model of aging. Geroscience. 2018;40:437–452. doi: 10.1007/s11357-018-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metz V.V., Kojro E., Rat D., Postina R. Induction of RAGE shedding by activation of G protein-coupled receptors. PLoS ONE. 2012;7:e41823. doi: 10.1371/journal.pone.0041823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu S., Bekhit A.E.-D.A., Wu Q., Chen M., Liao X., Wang J., Ding Y. Bioactive peptides and gut microbiota: Candidates for a novel strategy for reduction and control of neurodegenerative diseases. Trends Food Sci. Technol. 2021;108:164–176. doi: 10.1016/j.tifs.2020.12.019. [DOI] [Google Scholar]
- 62.Wang M., Amakye W.K., Guo L., Gong C., Zhao Y., Yao M., Ren J. Walnut-Derived Peptide PW5 Ameliorates Cognitive Impairments and Alters Gut Microbiota in APP/PS1 Transgenic Mice. Mol. Nutr. Food Res. 2019;63:e1900326. doi: 10.1002/mnfr.201900326. [DOI] [PubMed] [Google Scholar]
- 63.Cho S.J., Stout-Delgado H.W. Aging and Lung Disease. Annu. Rev. Physiol. 2020;82:433–459. doi: 10.1146/annurev-physiol-021119-034610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren J., Zhang Y. Targeting Autophagy in Aging and Aging-Related Cardiovascular Diseases. Trends Pharmacol. Sci. 2018;39:1064–1076. doi: 10.1016/j.tips.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Easter M., Bollenbecker S., Barnes J.W., Krick S. Targeting Aging Pathways in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020;21:6924. doi: 10.3390/ijms21186924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnes P.J., Baker J., Donnelly L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019;200:556–564. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 67.Chan S.M.H., Selemidis S., Bozinovski S., Vlahos R. Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): Clinical significance and therapeutic strategies. Pharmacol. Ther. 2019;198:160–188. doi: 10.1016/j.pharmthera.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lonardo A., Nascimbeni F., Ponz de Leon M. Nonalcoholic fatty liver disease and COPD: Is it time to cross the diaphragm? Eur. Respir J. 2017;49:1700546. doi: 10.1183/13993003.00546-2017. [DOI] [PubMed] [Google Scholar]
- 69.Pickart L., Vasquez-Soltero J.M., Margolina A. The human tripeptide GHK-Cu in prevention of oxidative stress and degenerative conditions of aging: Implications for cognitive health. Oxid. Med. Cell Longev. 2012;2012:324832. doi: 10.1155/2012/324832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park J.-R., Lee H., Kim S.-I., Yang S.-R. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget. 2016;7:58405–58417. doi: 10.18632/oncotarget.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meiners S., Eickelberg O. Next-generation personalized drug discovery: The tripeptide GHK hits center stage in chronic obstructive pulmonary disease. Genome Med. 2012;4:70. doi: 10.1186/gm371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu F., Yang H., Lin S.D., Zhao L., Jiang C., Chen Z.B., Liu Y.Y., Kan Y.J., Hu J., Pang W.S. Cyclic Peptide Extracts Derived From Pseudostellaria heterophylla Ameliorates COPD via Regulation of the TLR4/MyD88 Pathway Proteins. Front. Pharmacol. 2020;11:850. doi: 10.3389/fphar.2020.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niemann J., Johne C., Schroder S., Koch F., Ibrahim S.M., Schultz J., Tiedge M., Baltrusch S. An mtDNA mutation accelerates liver aging by interfering with the ROS response and mitochondrial life cycle. Free Radic. Biol. Med. 2017;102:174–187. doi: 10.1016/j.freeradbiomed.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 75.Chen Z., Tian R., She Z., Cai J., Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 76.Tilg H., Zmora N., Adolph T.E., Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020;20:40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 77.Lonardo A., Nascimbeni F., Mantovani A., Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 78.Musso G., Gambino R., Tabibian J.H., Ekstedt M., Kechagias S., Hamaguchi M., Hultcrantz R., Hagstrom H., Yoon S.K., Charatcharoenwitthaya P., et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen X., Xue H., Fang W., Chen K., Chen S., Yang W., Shen T., Chen X., Zhang P., Ling W. Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox. Biol. 2019;21:101068. doi: 10.1016/j.redox.2018.101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Cui X., Lin Q., Cai J., Tang L., Liang Y. Active Peptide KF-8 from Rice Bran Attenuates Oxidative Stress in a Mouse Model of Aging Induced by d-Galactose. J. Agric. Food Chem. 2020;68:12271–12283. doi: 10.1021/acs.jafc.0c04358. [DOI] [PubMed] [Google Scholar]
- 81.Moreira G.V., Azevedo F.F., Ribeiro L.M., Santos A., Guadagnini D., Gama P., Liberti E.A., Saad M., Carvalho C. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J. Nutr. Biochem. 2018;62:143–154. doi: 10.1016/j.jnutbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 82.Choudhury D., Levi M. Kidney aging—Inevitable or preventable? Nat. Rev. Nephrol. 2011;7:706–717. doi: 10.1038/nrneph.2011.104. [DOI] [PubMed] [Google Scholar]
- 83.Kimura T., Isaka Y., Yoshimori T. Autophagy and kidney inflammation. Autophagy. 2017;13:997–1003. doi: 10.1080/15548627.2017.1309485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., Jafar T.H., Heerspink H.J.L., Mann J.F., Matsushita K., Wen C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 85.Sun L., Xu H., Wang Y., Ma X., Xu Y., Sun F. The mitochondrial-targeted peptide SBT-20 ameliorates inflammation and oxidative stress in chronic renal failure. Aging. 2020;12:18238–18250. doi: 10.18632/aging.103681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang H.-Y., Chen J.-R., Chang L.-S. Effects of soy protein hydrolysate on blood pressure and angiotensin-converting enzyme activity in rats with chronic renal failure. Hypertens. Res. 2008;31:957–963. doi: 10.1291/hypres.31.957. [DOI] [PubMed] [Google Scholar]
- 87.Paneni F., Diaz Canestro C., Libby P., Luscher T.F., Camici G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017;69:1952–1967. doi: 10.1016/j.jacc.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 88.Donato A.J., Machin D.R., Lesniewski L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Camare C., Pucelle M., Negre-Salvayre A., Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox. Biol. 2017;12:18–34. doi: 10.1016/j.redox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gong X., An Q., Le L., Geng F., Jiang L., Yan J., Xiang D., Peng L., Zou L., Zhao G., et al. Prospects of cereal protein-derived bioactive peptides: Sources, bioactivities diversity, and production. Crit. Rev. Food Sci. Nutr. 2020:1–17. doi: 10.1080/10408398.2020.1860897. [DOI] [PubMed] [Google Scholar]
- 91.Tong L.-T., Ju Z., Wang L., Qiu J., Liu L., Zhou X., Liang T., Geng D., Zhou S. Peptides derived from rice α-globulin reduce atherosclerosis in apolipoprotein E-deficient mice by inhibiting TNF-α-induced vascular endothelial cells injury. J. Funct. Foods. 2019;63:103582. doi: 10.1016/j.jff.2019.103582. [DOI] [Google Scholar]
- 92.Ktari N., Bkhairia I., Nasri R., Ben Abdallah Kolsi R., Ben Slama-Ben Salem R., Ben Amara I., Zeghal N., Ben Salah B., Ben Salah R., Nasri M. Zebra blenny protein hydrolysates as a source of bioactive peptides with prevention effect against oxidative dysfunctions and DNA damage in heart tissues of rats fed a cholesterol-rich diet. Food Res. Int. 2017;100:423–432. doi: 10.1016/j.foodres.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 93.Rubattu S., Volpe M. Natriuretic Peptides in the Cardiovascular System: Multifaceted Roles in Physiology, Pathology and Therapeutics. Int. J. Mol. Sci. 2019;20:3991. doi: 10.3390/ijms20163991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li N., Rignault-Clerc S., Bielmann C., Bon-Mathier A.C., Deglise T., Carboni A., Ducrest M., Rosenblatt-Velin N. Increasing heart vascularisation after myocardial infarction using brain natriuretic peptide stimulation of endothelial and WT1(+) epicardial cells. Elife. 2020;9:e61050. doi: 10.7554/eLife.61050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sato K., Yamashita T., Shirai R., Shibata K., Okano T., Yamaguchi M., Mori Y., Hirano T., Watanabe T. Adropin Contributes to Anti-Atherosclerosis by Suppressing Monocyte-Endothelial Cell Adhesion and Smooth Muscle Cell Proliferation. Int. J. Mol. Sci. 2018;19:1293. doi: 10.3390/ijms19051293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao F., Liu C., Fang L., Lu H., Wang J., Gao Y., Gabbianelli R., Min W. Walnut-Derived Peptide Activates PINK1 via the NRF2/KEAP1/HO-1 Pathway, Promotes Mitophagy, and Alleviates Learning and Memory Impairments in a Mice Model. J. Agric. Food Chem. 2021;69:2758–2772. doi: 10.1021/acs.jafc.0c07546. [DOI] [PubMed] [Google Scholar]
- 97.Asokan S.M., Wang T., Wang M.F., Lin W.T. A novel dipeptide from potato protein hydrolysate augments the effects of exercise training against high-fat diet-induced damages in senescence-accelerated mouse-prone 8 by boosting pAMPK/SIRT1/PGC-1α/pFOXO3 pathway. Aging. 2020;12:7334–7349. doi: 10.18632/aging.103081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu W.S., Ting W.J., Tamilselvi S., Day C.H., Wang T., Chiang W.D., Viswanadha V.P., Yeh Y.L., Lin W.T., Huang C.Y. Oral administration of alcalase potato protein hydrolysate-APPH attenuates high fat diet-induced cardiac complications via TGF-beta/GSN axis in aging rats. Environ. Toxicol. 2019;34:5–12. doi: 10.1002/tox.22651. [DOI] [PubMed] [Google Scholar]
- 99.Wang C., Zheng L., Su G., Zeng X.A., Sun B., Zhao M. Evaluation and Exploration of Potentially Bioactive Peptides in Casein Hydrolysates against Liver Oxidative Damage in STZ/HFD-Induced Diabetic Rats. J. Agric. Food Chem. 2020;68:2393–2405. doi: 10.1021/acs.jafc.9b07687. [DOI] [PubMed] [Google Scholar]
- 100.Wang F., Weng Z., Lyu Y., Bao Y., Liu J., Zhang Y., Sui X., Fang Y., Tang X., Shen X. Wheat germ-derived peptide ADWGGPLPH abolishes high glucose-induced oxidative stress via modulation of the PKCzeta/AMPK/NOX4 pathway. Food Funct. 2020;11:6843–6854. doi: 10.1039/D0FO01229G. [DOI] [PubMed] [Google Scholar]
- 101.Zhu S., Wu L., Zhang J., Miao Y., Zhao Y., Zeng M., Li D., Wu H. Collagen Hydrolysate Corrects Anemia in Chronic Kidney Disease via Anti-Inflammatory Renoprotection and HIF-2alpha-Dependent Erythropoietin and Hepcidin Regulation. J. Agric. Food Chem. 2020;68:11726–11734. doi: 10.1021/acs.jafc.0c04459. [DOI] [PubMed] [Google Scholar]
- 102.Zhao T., Zheng L., Zhang Q., Wang S., Zhao Q., Su G., Zhao M. Stability towards the gastrointestinal simulated digestion and bioactivity of PAYCS and its digestive product PAY with cognitive improving properties. Food Funct. 2019;10:2439–2449. doi: 10.1039/C8FO02314J. [DOI] [PubMed] [Google Scholar]
- 103.Kozaczek M., Bottje W., Greene E., Lassiter K., Kong B., Dridi S., Korourian S., Hakkak R. Comparison of liver gene expression by RNAseq and PCR analysis after 8 weeks of feeding soy protein isolate- or casein-based diets in an obese liver steatosis rat model. Food Funct. 2019;10:8218–8229. doi: 10.1039/C9FO01387C. [DOI] [PubMed] [Google Scholar]
- 104.Xu D., Li D., Zhao Z., Wu J., Zhao M. Regulation by walnut protein hydrolysate on the components and structural degradation of photoaged skin in SD rats. Food Funct. 2019;2019:6792–6802. doi: 10.1039/C8FO01833B. [DOI] [PubMed] [Google Scholar]
- 105.Yu H., Zhang Z., Huang H., Wang Y., Lin B., Wu S., Ma J., Chen B., He Z., Wu J., et al. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the novel peptide EZY-1 purified from Eucheuma. Food Funct. 2019;10:3198–3208. doi: 10.1039/C9FO00308H. [DOI] [PubMed] [Google Scholar]
- 106.Ge H., Cai Z., Chai J., Liu J., Liu B., Yu Y., Liu J., Zhang T. Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 2021;360:129981. doi: 10.1016/j.foodchem.2021.129981. [DOI] [PubMed] [Google Scholar]
- 107.Yu X.C., Li Z., Liu X.R., Hu J.N., Liu R., Zhu N., Li Y. The Antioxidant Effects of Whey Protein Peptide on Learning and Memory Improvement in Aging Mice Models. Nutrients. 2021;13:2100. doi: 10.3390/nu13062100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gomez-Mendoza D.P., Lemos R.P., Jesus I.C.G., Gorshkov V., McKinnie S.M.K., Vederas J.C., Kjeldsen F., Guatimosim S., Santos R.A., Pimenta A.M.C., et al. Moving Pieces in a Cellular Puzzle: A Cryptic Peptide from the Scorpion Toxin Ts14 Activates AKT and ERK Signaling and Decreases Cardiac Myocyte Contractility via Dephosphorylation of Phospholamban. J. Proteome Res. 2020;19:3467–3477. doi: 10.1021/acs.jproteome.0c00290. [DOI] [PubMed] [Google Scholar]
- 109.Qin Q., Mehta H., Yen K., Navarrete G., Brandhorst S., Wan J., Delrio S., Zhang X., Lerman L.O., Cohen P., et al. Chronic treatment with the mitochondrial peptide humanin prevents age-related myocardial fibrosis in mice. Am. J. Physiol. Heart Circ. Physiol. 2018;315:H1127–H1136. doi: 10.1152/ajpheart.00685.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ali T., Badshah H., Kim T.H., Kim M.O. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J. Pineal Res. 2015;58:71–85. doi: 10.1111/jpi.12194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available in a publicly accessible repository (https://pubmed.ncbi.nlm.nih.gov, accessed on 21 January 2022).


