Abstract
Neurodegenerative disorders such as Alzheimer’s disease (AD) are distinguished by the irreversible degeneration of central nervous system function and structure. AD is characterized by several different neuropathologies—among others, it interferes with neuropsychiatrical controls and cognitive functions. This disease is the number one neurodegenerative disorder; however, its treatment options are few and, unfortunately, ineffective. In the new strategies devised for AD prevention and treatment, the application of plant-based natural products is especially popular due to lesser side effects associated with their taking. Moreover, their neuroprotective activities target different pathological mechanisms. The current review presents the anti-AD properties of several natural plant substances. The paper throws light on products under in vitro and in vivo trials and compiles information on their mechanism of actions. Knowledge of the properties of such plant compounds and their combinations will surely lead to discovering new potent medicines for the treatment of AD with lesser side effects than the currently available pharmacological proceedings.
Keywords: Alzheimer’s disease, neurodegeneration, dementia, polyphenols, terpenes, secondary plant metabolites
1. Introduction
Alzheimer’s disease (AD) is the most common cause of dementia globally. According to the World Alzheimer Report 2019, over 50 million patients suffer from AD. Long-lasting studies have revealed the multi-factorial character of the diseases that contribute to the complexity of the disorder [1].
Among the most intensively studied pro-neurodegenerative factors are the following: oxidative stress, amyloid-β accumulation leading to senile plaques, low level of neurotransmitters in the brain (i.e., acetyl- and butyrylcholine), high level of metal ions in the organism, and too high activity of monoamine oxidase (MAO) and neuroinflammation [2,3,4]. An effective substance should therefore be active against several possible pro-degeneration factors.
Similarly to other disorders, in the case of neurodegeneration, quick diagnosis of disease is crucial for effective treatment. Hence, an additional complication results from the non-specific character of the first AD symptoms. In the first stage of AD development, symptoms are similar to typical chronic tiredness, along with memory disturbances. Unfortunately, this stage is presented as crucial for treatment success. The middle stage and later symptoms are more characteristic for neurodegeneration. In this case, significant memory and learning disturbances are apparent, along with insomnia and problems with interpersonal contacts, which lead to complete dependency on third parties [1].
In the search for treatment for AD, besides synthetic drugs, recently, substances of natural origin have been very popular. It is known that plants are inexhaustible source of active compounds that are used as drugs in numerous disorders. Among the secondary plant metabolites, polyphenols and terpenes are the most popular due to their rich biological activities such as antioxidant, sedative, anti-inflammatory, antibacterial, enzyme inhibitory effects [5].
The presented review is focused on natural compounds that reveal positive activity (in vitro, in silico, in vivo) against various Alzheimer’s disease factors, along with their mechanisms of action.
2. Activity Targeting Cholinergic Neurotransmission
The cholinergic system is associated with a number of cognitive functions, e.g., learning and memory. Cholinergic neurons are major source of innervation in the cortex and hippocampus. They release choline O-acetyltransferase (ChAT), which takes part in the production of acetylcholine (ACh) by catalyzing the transfer of acetyl group from the coenzyme acetyl-CoA to choline [2]. Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), expressed at lower levels than AChE), hydrolyze ACh back to choline [6]. The cholinergic hypothesis proposes that the decline of cholinergic neurotransmission and loss of these neurons in AD patients cause cognitive deficits [2]. Researchers found that besides the decrease in the mRNA expression level of ChAT in the AD brain, its activity is also reduced, which is asynchronous with synaptic loss [7].
For many years, research on AChE inhibitors has been a major avenue for drug development for AD. In fact, three out of the four anti-AD drugs approved by the Food and Drug Administration are AChE inhibitors. Unfortunately, the results of the clinical studies demonstrate that their therapeutic effects are not as effective as expected. The brain of AD patients contains very high concentrations of BuChE [8]. Therefore, other anti-AD strategies targeting cholinergic neurons include BuChE inhibition and promotion of ChAT expression, as well as protection of cholinergic neurons by stimulating the expression of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and their receptors [6].
Both AChE and BuChE are associated with aggregation of Aβ plaques. AChE is able to increase Aβ peptide fibril aggregation to form Aβ–AChE complexes. In general, AChE activity is decreased in the AD brain, but its concentration could be enhanced while binding to Aβ plaques. However, the association between AChE and BuChE with other AD hallmarks remains largely unexplored [9].
An in vitro study on aqueous extracts from 80 traditional Chinese medicinal plants (from families drawn from Berberidaceae, Ranunculaceae, and Rutaceae) showed that extracts rich in isoquinoline alkaloids effectively inhibit AChE activity [10]. Moreover, extracts of Berberis bealei, Coptis chinensis, and Phellodendron chinense, which are characterized by a high content of isoquinoline alkaloids, were found to substantially constrain AChE. Furthermore, combinations of three of the alkaloids palmatine, berberine, and coptisine demonstrate a synergistic enhancement of ACh restriction. It is likely that the way of AChE inhibition by crude extracts of Coptis chinensis, Berberis bealei, and Phellodendron chinense is due to of this synergism of alkaloids. It should be emphasized that none of the active extracts are cytotoxic at the concentrations that limit AChE [11].
It was also observed many years ago that ‘Compound Danshen Tablet’, a traditional Chinese medicine, can improve spatial cognition. However, the in vivo neuroprotective mechanism of the Compound Danshen Tablet in models of spatial memory impairment in mice was not evaluated until 2014. The results of the research conducted by Teng et al. [12] have found that this medicine increased ChAT expression in the brain, induced BDNF production, and activated the protein kinase C (PKC) receptor to improve spatial recognition in an AD rat model.
Besides Compound Danshen Tablet, various other preparations and extracts used in Chinese medicine have demonstrated therapeutic effects on AD through their effects on the expression of NGF, BDNF, and their related receptors in vivo. The very popular Bushen-Yizhi formula was noted, for example, to be able to regulate NGF signal transduction and the anti-apoptotic cholinergic pathway to improve memory impairment in an AD rat model [13]. Moreover, bioactive components of ginger 6-shogaol have increased the levels of NGF and improved scopolamine-induced memory impairment in animal models of dementia [14]. In addition, Xanthoceras sorbifolium extracts, rich in transhinone II, were seen to save dendritic spines through the BDNF signal transduction pathway and improve cognition in an AD rat model. Moreover, tanshinone IIA was found to be helpful in promoting depolarization-induced BDNF synthesis [15], and Polygonum multiflorum Thunberg complex was recognized to increase BDNF level and synapse number in the hippocampus of an AD mice model [16]. Finally, extracts from Huperzia serrata (studies on AD mice) were found to inhibit AChE activity and ameliorate the cognitive impairment [17].
Beyond the aforementioned substances, extracts from Crocus sativus in in vitro study showed moderate inhibitory activity against AChE, while crocetin, dimethylcrocetin, and safranal extracted from C. sativus have all been found to possess moderate AChE inhibitory activities (IC50 values below or around 100 µM). Accordingly, results of kinetic analysis exhibited mixed-type inhibition. This was verified by in silico docking studies. Here, safranal was found to interact only with the binding site of the AChE, but crocetin and dimethylcrocetin bound simultaneously to the catalytic and peripheral anionic sites. The presented findings confirm previous results about the beneficial action of saffron against AD and may be of value for the development of novel therapeutic agents based on carotenoid-based dual binding inhibitors [18].
Other plant metabolites and extracts with anti-AD potential are listed in Table 1.
Table 1.
Plant products with anti-AD potential possess activity targeting cholinergic neurotransmission.
| Plant | Extract | Model and Assay | Target | Results | Ref. |
|---|---|---|---|---|---|
| Salvia triloba L. | aerial parts macerated in 70% methanol | AD rats, male (administration of AlCl3) | AChE, CRP, NF-κB, MCP-1 | ↓AChE activities in brain and serum, ↓CRP, ↓NF-κB, ↓MCP-1, ↑ACh |
[19] |
| Salvia triloba | samples extracted with 75% ethanol at r.t. | in vitro enzymatic assay (AChE), Swiss albino mice, male scopolamine-induced amnesia | AChE | AChE inhibition, IC50: 0.71 mg/mL, memory-enhancing effect: 57.1 and 71.4% at 200 and 400 mg/kg, respectively |
[20] |
| Melissa officinalis | samples extracted with 75% ethanol at r.t. | in vitro enzymatic assay (AChE), Swiss albino mice, male scopolamine-induced amnesia | AChE | ↓AChE activities in brain, memory-enhancing effect: no significance | [20] |
| Teucrium polium | samples extracted with 75% ethanol at r.t. | in vitro enzymatic assay (AChE), Swiss albino mice, male scopolamine-induced amnesia | AChE, | AChE inhibition, IC50: 0.55 mg/mL, memory-enhancing effect: 55.4 and 61.6% at 200 and 400 mg/kg, respectively | [20] |
| Piper nigrum | seeds extracted with 70% methanol at r.t. | SD rats, male (administration of AlCl3) | AChE, CRP, NF-κB, MCP-1 | ↓AChE activities in brain and serum, ↓CRP, ↓NF-κB, ↓MCP-1, ↑ACh |
[19] |
| Foeniculum vulgare | fruit extracted with 90% methanol at r.t. | Swiss mice, scopolamine- and aging-induced amnesia | AChE | amnesia behavioral improvement, ↓AChE activities↓ in brain |
[21] |
| Ocimum sanctum | water extract, refluxed at 75–80 °C | Wistar rats; male; maximal electroshock-, atropine-, and cyclosporine-induced dementia | AChE | cognitive behavioral performance improvement | [22] |
| Ocimum sanctum Linn | leaf extracted with 95% ethanol extract using Soxhlet | Wistar rats; male; maximal electroshock-, atropine-, and cyclosporine-induced dementia | AChE | cognitive behavioral performance improvement ↓AChE activities↓ in cortex, cerebellum, medulla oblongata, and midbrain region: 21%, 21%, 25%, and 30% at 500 mg/kg |
[22] |
| Lavandula angustifolia Mill. | essential oils obtained from steam distillation | C57BL/6J mice, male, scopolamine-induced amnesia; H2O2-induced PC12 (1.5–50 μg/mL LO for 24 h) | AChE, ROS, MMP | cognitive behavioral performance improvement, ↓AChE activities, ↓MDA, ↑SOD activities↑, ↑GPX activities, PC12 cells model: ↓LDH, ↓NO, ↓ROS, ↑MMP |
[23] |
| Olive oil (fruit oil of Olea europaea) | olive oil (rich in oleic acid) | ICR mice, male, intracerebroventricular injection of Aβ into the mice brain | MDA, NO, COX-2 | ↓MDA, ↓NO, ↓COX-2 | [24] |
| Corn oil (Zea mays) | corn oil (rich in linoleic acid) | ICR mice, male, intracerebroventricular injection of Aβ into the mouse brain | AChE, MDA, NO, iNOS, COX-2 | ↓AChE, ↓MDA, ↓NO, ↓COX-2, ↓iNOS | [24] |
| Perilla oil (Perilla frutescens) | perilla oil (rich in α-linolenic acid) | ICR mice; male, intracerebroventricular injection of Aβ into the mouse brain | AChE, MDA, NO, iNOS, COX-2, BDNF | ↓AChE, ↓MDA, ↓NO, ↓COX-2, ↓iNOS↓, ↑BDNF | [24] |
| Coffee | boiled water extraction | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 0.41 ± 0.004 mg/mL | [25] |
| Shaddock (Citrus maxima) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of 60.39% at 66.68 mL/L and of ≈ 28% at 16.67 mL/L | [26] |
| Grapefruit (Citrus paradisii) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of ≈ 52% at 66.68 mL/L; AChE inhibitory rate of ≈ 29% at 16.67 mL/L |
[26] |
| Lemon (Citrus limoni) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of ≈ 48% at 66.68 mL/L and of ≈ 22% at 16.67 mL/L | [26] |
| Orange (Citrus sinensis) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of ≈ 50% at 66.68 mL/L and of ≈ 30.89% at 16.67 mL/L | [26] |
| Tangerine (Citrus reticulata) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of ≈ 57% at 66.68 mL/L and of ≈ 20% at 16.67 mL/L | [26] |
| Extra-virgin olive oil (Olea europaea) | extra-virgin olive oil | TgSwDI model | Aβ, tau, ApoE, PPARγ, and LXRs | cognitive behavioral performance improvement, ↓Aβ, ↓tau, ↓phosphorylation of tau, ↑ApoE, ↑PPARγ, ↑LXRs ↑Aβ clearance pathways |
[27] |
| Green tea (Camellia sinensis) | water extract of green tea | in vitro enzymatic assay | AChE, BuChE, and BACE-1 | AChE inhibition, IC50: 7.2 μg/mL | [28] |
| White tea (Camellia sinensis, WTE) | water extract of white tea | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 8.06 μg/mL | [28] |
| Green tea (Camellia sinensis, GTE-PG) | water extract of green tea processed through simulated gastrointestinal digestion to obtain post-gastric digested extract | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 17.84 μg/mL | [28] |
| Green tea (Camellia sinensis, GTE-CA) | water extract of green tea processed through simulated gastrointestinal digestion to obtain colon-available digested extract | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 9.59 μg/mL | [28] |
| White tea (Camellia sinensis, WTE-PG) | water extract of white tea processed through simulated gastrointestinal digestion to obtain post-gastric digested extract | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 16.1 μg/mL | [28] |
| White tea (Camellia sinensis, WTE-CA) | water extract of white tea processed through simulated gastrointestinal digestion to obtain colon-available digested extract | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 4.22 μg/mL | [28] |
| Black tea (Camellia sinensis) | water extract of black tea | in vitro enzymatic assay | AChE and BuChE | AChE inhibition, IC50: 0.06 ± 0.005 mg/mL; BuChE inhibition, IC50: 0.05 ± 0.007 mg/mL |
[25] |
| Green tea (Camellia sinensis) | water extract of green tea | Wistar rats; male; injection with green tea extract, saline, or AlCl3 into the left-brain hemisphere cornu ammonis region 1 of the hippocampus | AChE | ↑COX and AChE activities with GTE injection, ↓AlCl3 neurotoxicity, 3-epigallocatechin gallate and epicatechin in extract improves cholinergic synaptic functions |
[29] |
| Black tea (Camellia sinensis) | brewed at 85 °C | Wistar rats, male, AlCl3 (100 mg/kg, i.p. 60 days) induced AD | AChE, APP, β and γ secretases, Aβ | memory-enhancing effect ↓TBARS, ↑GSH, ↑SOD, ↑catalase, ↑GPx |
[30] |
3. BACE-1 Inhibitory Activity
One of the most studied neurodegenerative factors is amyloid-β accumulation. This plays a key role in the aggregation of neurotic senile plaques. In young healthy organisms, the Aβ peptides are released outside of cells, removed, and excreted. With age, the process is disturbed, and the ability of the organism to get rid of amyloid compounds is reduced. This leads to accumulation of the forms in the brain. Many years of research have allowed for detailed analysis of the creation of Aβ structures, as well as their influence on brain functioning.
It is known that amyloid-β is created in two stages. In the first, the amyloid precursor protein (APP) cleaves the enzyme BACE-1 (β-secretase), known also as Asp2. This stage leads to the generation of soluble versions of protein (sAPPβ) and a 99 amino acid fragment (C99), which, in turn, is treated with a second enzyme γ-secreatse to produce peptides composed of 38–43 amino acids (termed epsilon, ε; zeta, ζ; and gamma, γ) and AICD (APP intracellular domain) [31]. Among these, the greatest threat is Aβ40 and Aβ42, both being main products and simultaneously playing crucial roles in senile plaque creation. It is worth stating that Aβ42 is the most toxic form and has high predispositions towards aggregating. This is particularly evident in a familial form of AD [32,33].
The importance of BACE-1 activity results from its ability to determine total amyloid-β production and to induce overproduction of toxic Aβ42. In the luminal surface, it is a membrane anchored aspartyl protease responsible for APP cleaving [33]. From a structural point of view, the enzyme is characterized by a large and elongated active site (1000 Å and 20 Å, respectively), along with a large catalytic domain with centrally located catalytic aspartates Asp32 and Asp228 [34]. Molecular simulations revealed that the key interacting residue is Arg307 at the edge of the catalytic cleft [35]. Additionally, molecular simulations revealed four residues creating group-specification in the ligand binding side, namely, Pro70, Ile110, Ile126, and Asn233 [36]. It is known that the catalytic activity of β-secretase involves aspartic proteases hydrolyzing peptide bonds.
In accordance with Shimizu et al. [37], BACE-1 activity is directly affected by the behaviors of water (Wat) molecules, and the molecules participate in the creation of the following hydrogen-bonding network: Wat–Ser35–Asp32–Wat–Asp228. An equally important amino acid residue is Leu30, which is responsible for stabilizing the bound inhibitor conformation [37].
Several inhibitors that were tested in phase II or phase III clinical trials were the result of multiple years of research on the enzyme. Among them are the following: lanabecestat, verubecestat, elenbecestat, atabecestat, and umibecestat [31]. Selected doses of the substances led to reduction of Aβ in the cerebrospinal fluid by 90%. Of note, in the clinical trials, a high level of BACE inhibition to achieve Aβ lowering was the goal, and the effects of lower doses of active substances were not investigated [38].
Information obtained from the studies indicates that the optimal therapeutic window might be before the onset of appreciable Aβ plaque deposition; hence, the solution can be used in preventive therapy [39]. It is known that the effectiveness of preventive therapy depends on the length of drug administration, and thus side effects of the solution must be minimalized. In the case of BACE-1 inhibitors, adverse effects were observed both in non-clinical studies and clinical trials. The following negative changes were observed: off-target effects: retinal toxicity in an animal model and hepatotoxicity in humans [38,40], and mechanism-based effects: cognitive decline, anxiety, weight loss, sleep disturbances, and suicidal ideation [41,42,43].
When therapy based on BACE inhibitors is considered, a milestone is appropriate dosage. In accordance with available study results, there is a therapeutic window for the enzyme inhibitor concentration, namely, 3 nM concentration of BACE inhibitor caused 40% inhibition of APP processing, whereas concentration > 300 nM led to significant inhibition of BACE-dependent neuronal growth cone collapse [38,44].
In this subsection, attention will be focused on natural compounds derived from plants that were studied in vitro, in silico, and in vivo towards their BACE-1 inhibitory activity (Table 2). Taking into account an inexhaustible source of natural active compounds, it is highly probable to find substances that are highly active inhibitors that come without adverse side effects.
Table 2.
Selected natural compounds which activity towards BACE-1 inhibition was confirmed in in vitro, in silico, or in vivo studies.
| In Vitro and In Silico Studies towards BACE-1 Inhibition | ||||
|---|---|---|---|---|
| Compound | Type of Study/Methodology | Mechanism of Action | Studies Results/Comment | References |
| Two serratene-type triterpenoids: lycernuic acid A with a ρ-hydroxycinnamate group as an ester substituent and 21β-hydroxyserrat-14-en-3,16-dione extracted from Lycopodiella cernua L. |
|
Interactions with several pocket domains of the AChE, which were 5 Å from the inhibitors in the original complex. | IC50 = 0.23 μM and 0.98 μM, respectively. The compounds revealed higher inhibitory activity than quercetin, a positive control. | [31,45] |
| Embelin (3-undecyl-1,4-benzoquinone) from Embelia ribes |
|
Molecular docking revealed entering of embelin into the active site gorge and interacting with Tyr71 (via hydrogen bonding). | IC50 = 2.11 μM. Lower activity than donepezil, a positive control. | [31,46] |
| Five arylbenzofurans: sanggenofuran A, mulberrofuran D, mulberrofuran H, morusalfuran B, and mulberrofuran D2 from the root bark of Morus alba |
|
Molecular docking revealed the following interactions for mulberrofuran: D2 bound to the active allosteric site of BACE-1 through hydrogen bonds with Asn37, Ser36, and Tyr198, as well as hydrophobic interactions with Val69, Tyr71, Trp76, Phe108, Tyr198, and Ile126 | Sanggenofuran A revealed lower activity (IC50 = 5.64 μM) than mulberrofuran D (IC50 = 3.74 μM), and both compounds were less active than quercetin (IC50= 3.38 μM). The remaining compounds revealed higher activity in comparison to quercetin: mulberrofuran D2, mulberrofuran H, and morusalfuran B, for which IC50 was equal to: 0.73 μM, 1.04 μM, and 2.03 μM, respectively. | [31,47] |
| Fifteen ptesorin derivatives from Pteridium aquilinum |
|
(2R)-Pteroside D was able to bind (hydrogen bonds) with Asn37, Trp76, and Ile126; (2R,3R)-pteroside C was able to create hydrogen bonds with Ser36, Asn37, Asp228, and Thr231, as well as hydrophobic interactions with Ala39, Trp76, Val69, Ile118, and Arg129; (3S)-pteroside D was able to create hydrogen bonds with Ser36, Asn37, Ile126, and Asp228, as well as hydrophobic interactions with Val69, Tyr71, Trp76, and Arg128. | The most active compounds were the following: (2R)-pteroside D, (2S,3R)-pteroside C, (2R,3R)-pteroside C, and (3S)-pteroside D (IC50 = 2.55, 9.17, 3.77, and 27.4 μM, respectively). (2R)-Pteroside D, (2R,3R)-pteroside C, and (3S)-pteroside D revealed higher inhibitory activity than quercetin. The compounds revealed the ability to bind with crucial amino acid residues, creating BACE-1 binding sites. | [48] |
| Three phlorotannins: eckol, dieckol, and 8,8′-bieckol isolated from Ecklonia cava |
|
Dieckol revealed the ability to interact with Trp76, Thr232, and Lys321 through hydrogen bonds. 8,8′-Bieckol interacted with the BACE-1 active site by hydrogen bonding interactions with Lys107, Gly230, Thr231, and Ser325. | Dieckol and 8,8′-bieckol revealed higher inhibitory activity than reseveratrol (positive control) with IC50 = 2.34 and 1.62 μM, respectively. | [49] |
| Flavonoids and non-flavonoids: caffeic acid, hydroxytyrosol, oleuropein, verbascoside, quercetin, rutin, and luteloin isolated from Olea europaea L. |
|
The compound structure analysis suggests that the 3,4-dihydroxy group and double bond in olive biophenols can interfere with hydrogen bonds of the NH2 group and NH hydrogens in the core structure of the BACE-1 enzyme. The higher activity of flavonoid olive biophenols in comparison to non-flavonoid olive biophenols results from their chemistry-a 15-carbon skeleton consisting of two benzene rings linked via the heterocyclic pyrene ring-C. | Caffeic acid, hydroxytyrosol, oleuropein, verbascoside, quercetin, rutin, and luteloin revealed higher inhibitory activity than positive control epigallocatechin gallate, with the following IC50 values: 16.67, 0.035, 2.76, 0.0063, 0.55, 0.0038, and 0.52 μM, respectively. | [50] |
| Flavonoids: bavachin, bavachinin, bavachalcone, and iso-bavalchacone isolated from Psoralea fructus |
|
Structure analysis of studied compounds revealed that the chalcone backbone of bavachalcone and isobavachalcone was more flexible, which allowed them to fit more easily to the conformations of Aβ42 and enabled more hydrogen bonds than the flavanone of bavachin and bavachinin. Bavachalcone and isobavachalcone may stabilize Aβ42 monomers through their strong bindings, whereas bavachinin might induce intricate conformational changes of Aβ42 through binding, which leads to the off-pathway aggregation. | BACE-1 inhibition: 14% (bavachin at concentration 100 μM), 20% (bavachinin at concentration 100 μM), 68% (bavalchacone at concentration 100 μM), and 34% (iso-bavalchacone at concentration of 100 μM). | [51] |
| Linalool and 2,3,4,4-tetramethyl-5-methylene-cyclopent-2-enone isolated from Lavandula luisieri |
|
Lack of mechanisms analysis. | Inhibitory activity for linalool was equal to 4.7, whereas 2,3,4,4-tetramethyl-5-methylene-cyclopent-2-enone was 31.8% at a concentration of 45 μg/mL. | [52] |
| Ajmalicine and reserpine |
|
Strong binding of the compounds to the catalytic site of BACE-1. Reserpine interacted with Thr72, Asp32, and Asp217 by five hydrogen bonds, whereas ajmalicine was able to create hydrophobic interactions with Asp32 and Asp228. Thanks to the reserpine indole ring, the compound acted as a hydrogen bond donor capable of creating double hydrogen bonds with the catalytic site of the enzyme, whereas ajmalicine bound more strongly to the enzyme by hydrophobic interactions. | AJM showed the maximum inhibition of BACE-1 activity to be 69% at 50 μM concentration, whereas RES imparted 47% inhibition at the same concentration. |
[53] |
| (S)-5,7,3′,5′-Tetrahydroxy-flavanone-7-O-(6″-galloyl)-β-D-glucopyranose (1); flavanone: (S)-5,7,3′,5′- tetrahydroxy-flavanone-7-O-β-D-glucopyranose (2), and dihydrochalcones: 4,2′,6′-trihydroxy-dihydrochalcone-4′-O-(6″-galloyl)-β-D-glucopyranose (3); 3,4,2′,6′-tetrahydroxydihydroflavone- 4′-O-β-D-glucopyranose (4); 3,4,2′,6′-tetrahydroxy- dihydrochalcone-4′-O-(6″-galloyl)-β-D-glucopyranose (5); and phloretin 4′-O-[4′, 6′-O-(S)-HHDP]-β-D-glucoside (6) isolated from Balanophora involucrata Hook. |
|
Lack of mechanism analysis. | In vitro studies revealed the activity of the compounds to inhibit BACE-1; nevertheless, only compounds 1, 2, 4, and 5 turned out to be a little more active than the positive control. None of the substances achieved an inhibition capacity of 50% at 10 μM concentration. | [54] |
| The chemical components of W. fruticosa viz. botulin, betulinic acid, ursolic acid, ellagic acid, quercetin, kaempferol, oenothein C, and cyanidn-3,5-diglucoside. |
|
The high activity of ellagic acid resulted from hydrogen bonding with Thr231, Asp228, Gly34, and Trp76 amino acid residues. Additionally, hydrophobic interactions were observed between aromatic rings of the acid and Trp115 and Tyr71 residues. | Among the compounds, ellagic acid and quercetin revealed the highest activity (70% BACE-1 inhibition at 100 μM). The most active was ellagic acid (IC50 = 16.2 μM). | [55] |
| 3,4-di-o-Caffeylquinic acid, apigenin, and 7-o-methylwoonin isolated from A. paniculata |
|
The 3,4-di-o-caffeylquinic acid was able to bind with Trp71, Phe108, Gly34, Arg128 (first pose) and Ile126, Trp76, and Tyr198 (second pose) by hydrogen bonds. Hydrophobic interactions were also observed. | BACE-1 inhibition assay indicates that 3,4-di-o-caffeylquinic acid is the most promising inhibitor (activity slightly higher than quercetin), whereas the activity of 7-o-methylwogonin was similar to quercetin and the activity of apigenin was slightly weaker than quercetin. In accordance with molecular docking, 3,4-di-o-caffeylquinic acid showed the highest ability to bind with the BACE-1 active site. Hydrophobic interactions and hydrogen bonds allow achieving selective BACE-1 inhibition by the compound. | [56] |
| Proroberberine alkaloids: berberine, palmatine, jateorrhizine, epiberberine, coptisine, groenlandicine, and eporphine alkaloid-magnoflorine from Coptidis Rhizoma |
|
The activity of epiberberine and groenlandicine is closely related with the presence of the methylenedioxy group in the D ring that is responsible for the BACE-1 inhibitory activity of protoberberine alkaloids. | Among the compounds, only epiberberine and groenlandicine revealed good, non-competitive BACE-1 inhibitory activities, with IC50 = 8.55 and 19.68 μM, respectively. | [57] |
| In Vivo and Ex Vivo Studies towards BACE-1 Inhibition | ||||
| Compound | Animal Models/Type of Study/Methodology | Mechanism of Action | Studies Results/Comment | References |
| Berberine (isoquinoline alkaloid) | New Zealand white rabbits. Lesion (pro-Alzheimer’s disease) was induced by aluminum-maltol injection into intraventricular fissure. Berberine chloride (50 mg/kg) was administered intragastrically once daily for 14 days. Histopatological examinations (brain tissue) were performed. BACE-1 activity was detectable by RP-HPLC. | The mechanism of CNS cell damage prevention by berberine was based on BACE-1 inhibition, as well as its antioxidant, anti-inflammatory, and AChE inhibitory activities. | Results indicated that berberine chloride has a preventative effect on the degeneration of the hippocampus, along with the ability to decrease the activity of BACE-1. Berberine prevented the increase in enzyme activity in 40% of all cases, as compared with the control group. | [58] |
| 2,2′,4′-Trihydroxychalcone (TDC) from Glycyrrhiza glabra | APP-PS1 double transgenic mice model (B6C3-Tg (APPswe, PS1dE9)). The studied substance was administered i.p. by 100 days to two groups with different doses (9 mg/kg/day and 3 mg/kg/day). The mice were applied to the MWM spatial memory test. Additionally, Western blot analysis for BACE-1 was conducted. | This is a specific non-competitive BACE-1 inhibitor. Taking into account the low molecular weight of TDC, it is highly probable that the compound is able to cross the blood–brain barrier in vivo. | Administration of TDC (9 mg/kg/day) caused significant decreasing of Aβ production and senile plaque formation. The activity resulted in memory improvement, as observed in the Morris water maze test. It was also determined that the level of BACE-1 in TDC-treated Tg mice was almost kept unchanged, as compared with those in the vehicle-treated Tg mice. | [59] |
| Gallic acid | Male B6.Cg-Tg(APPswe, PSEN1dE9) 85Dbo/Mmjax mice (bearing ‘Swedish’ APPK595N/M596L and PS1 exon 9-deleted mutant human transgenes) on a congenic C57BL/6J background (designated APP/PS1 mice). GA was administered with 20 mg/kg/day for 6 months. Two behavioral tests were conducted: Y-maze and RAWM. | The activity of gallic acid towards BACE-1 inhibition led to nonamyloidogenic APP metabolic effects. GA is able to inhibit the enzyme activity post-translationally. | Gallic acid demonstrated the ability to mitigate impaired learning and memory and reduce cerebral amyloidosis. A 6 month oral therapy based on GA completely remediated behavioral deficits, ameliorated cerebral amyloidosis, and reduced amyloid abundance. | [60] |
| Anatabine | Measurement of BACE-1 expression by RT-qPCR according to SHSY-5Y cells. Pharmacokinetic studies of anatabine were performed using 43-week-old B6/SJL F1 mice. The studied substance was administered i.p. at dosages of 0.5 and 2.0 mg/kg/day over 4 days. |
Mechanism of Aβ reduction was based on the impact of anatabine on BACE-1 transcription. The compound was able to reduce BACE-1 protein levels in human neuronal-like SHSY-5Y cells. | Reduction was indicated of two forms of amyloid (soluble-40% reduction and insoluble-30% inhibition) after 4 days of drug administration at a dosage of 2 mg/kg. | [61] |
4. α-Synuclein Inhibition
Research results provide scientific evidence confirming the correlation of neuronal mitochondrial dysfunction with the pathogenesis of neurodegenerative diseases, including AD [62]. Abnormal accumulation of α-synuclein induces an alteration of normal mitochondrial function, leading to neuronal degeneration and strong oxidative stress [63]. This low molecular weight protein can activate microglia and release proinflammatory cytokines such as NO and ROS, resulting in microglial activation, neuronal death, and further inflammation [64].
Synucleinopathic disorders involve the accumulation of inclusions rich in α-sunuclein [65,66]. Different types of aggregates, e.g., fibrils, protofibrils, and oligomers, are produced during aggregation of these protein in synucleinopathies. Some aggregated species might be neurotoxic and lead to neurodegeneration [67]. For this reason, targeting neuronal accumulation of α-synuclein is appealing as a promising approach to delaying the progression of AD [68,69].
Limited amounts of research have been published thus far on inhibiting α-synuclein aggregation by natural compounds [70]. Ehrnhoefer et al. [71], however, demonstrated that epigallocatechin-3-gallate (EGCG) inhibits the fibrillogenesis of these protein by directly binding to natively unfolded polypeptides and preventing their conversion into toxic aggregation intermediates. Moreover, computational molecular docking analysis showed that this plant compound preferentially bound the C-terminus of α-synuclein. Other studies have revealed that epigallocatechin-3-gallate promotes the production of unstructured, nontoxic α-synuclein. These phenomena suggest its favorable effect on aggregation pathways in AD. In addition, the results of studies conducted by Hornedo-Ortega et al. [72] showed that protocatechuic acid (doses 10, 20, 50, and 100 μmol/L) inhibits Aβ and α-synuclein aggregation. What is more, protocatechuic acid disturbs the stability of prefabricated fibrils and inhibits Aβ- and α-synuclein-induced PC12 cell death.
5. MAO Inhibition
Monoamine oxidase (MAO) is an enzyme bound with the mitochondria that catalyzes the oxidative deamination of a range of neurotransmitters e.g., serotonin, tyramine, norepinephrine, and dopamine. This process produces (during the biochemical reaction) several harmful side compounds, including peroxides, ammonia, and aldehydes. This enzyme occurs in MAO-A and MAO-B isoforms. They show remarkable sequence similarity but differ in their substrate-inhibitor recognition sites and presence within the tissues. MAOs catalyze the oxidative deamination of several monoamines and play important roles in metabolism-released neurotransmitters [73]. Both isoforms MAO-A and MAO-B possess 73% sequence similarity; however, in the central nervous system, the MAO-A form is present mostly in catecholaminergic neurons, whereas the MAO-B form is mostly found in serotonergic neurons and in the glia [74].
In many neurodegenerative diseases, including AD, amended levels of neurotransmitters are observed [75]. Activated MAO causes amyloid beta aggregation by two successive cleft b-secretase and g-secretase effects upon the amyloid precursor protein. Moreover, this enzyme participates in cognitive damage through the destruction of cholinergic neurons, as well as through disturbance of the cholinergic system. MAOs also regulate mood control, motor function, and brain and motivational functions [76,77,78]. MAO enzyme inhibition causes an anti-AD effect as a result of oxidative stress decrease prompted by MAO. Inhibitors of MAO can block the catalytic activity of the enzyme and slow down the catabolism process of various monoamines. They also increase the production of the monoamine neurotransmitters that are accumulated in the nerve terminals. Inhibitors of this enzyme are applied as medicines in diseases where MAO is over-expressed. These drugs halt the production of neurotoxic side substances and thus prevent neuronal damage [79].
Radioenzymatic screening in brain autopsy has revealed that the alterations in MAO-A and MAO-B in the prefrontal cortex are present from the beginning of AD and remain constant in the later AD stages. In addition, levels of MAO-A and MAO-B and/or mRNA may rise in various brain areas, including in the frontal lobe of the neocortex and also in the parietal, temporal, occipital, and frontal cortices [73]. Studies using immunostaining demonstrate that in AD, the MAO-B level is significantly increased in the hippocampus and in the cortical areas, whereas MAO-A activity is enhanced in the frontal pole and hypothalamus [80]. This indicates that cell loss and substantial gliosis in these brain areas has occurred. The presence of MAO-A in the neurons is implicated in the pathology of AD as a predisposing factor, and activation of MAO occurs during AD cognitive dysfunction.
Monoamine neurotransmitter systems play significant roles in cognition at the biomolecular level, especially in memory, attention, paranoid thinking, behavior, and emotion, as well as orientation [73,81]. Oxidative stress related to MAO is a well-recognized cause of neurotransmitter dysfunction in AD [82]. Indeed, neuroinflammation participates significantly in cognitive loss and in oxidative stress, and in AD, MAO may have pro-inflammatory effects, as activated MAO increases levels of monoamine in the brain. Moreover, research indicates that MAO alters other neurotransmitter systems resulting in cogitative impairments [83]. In addition, changes in the concentration of dopamine and serotonin acid metabolites (homovanillic acid and 5-hydroxyindole-3-acetic acid), mediated by MAO and established via AD-related mouse models pathology, are known to be related to cognitive deficits [84].
Amyloid plaques are also produced through MAO activation. High oxidative stress in AD patients results in amyloid plaque formation, and an increased level (>3-fold) was notably found in sensitive astrocytes around plaques (amyloid-beta). In astrocytes, this increasing level of MAO-B is hypothesized to result in excessive deamination of monoamines and the release of large amounts of oxygen radicals; hence, it could contribute to the progress of AD. Research on AD mice demonstrates that MAO-B is firmly related with the formation of GABA (gamma-aminobutyric acid) in sensitive astrocytes, and this effect brings about memory deterioration [85].
Many natural substances are used in medicine, such as MAO-A and MAO-B inhibitors, in the treatment of neurodegenerative diseases, including AD. MAO-B inhibitors not only enhance dopaminergic neurotransmission, but they also reduce the radical production from toxins. Larit et al. [86] isolated quercetin and myricetin from Hypericum afrum, as well as genistein and chrysin from Cytisus villosus, and evaluated their effect upon recombinant hMAO-A and hMAO-B in in vitro studies. Therein, quercetin, myricetin, and chrysin induced MAO-A inhibition activity with IC50 values of 9.93, 1.52, and 0.25 µM, respectively, whereas genistein was found to be a most effective potent inhibitor of MAO-B, with an IC50 value of 0.65 µM. In addition, computational docking and dynamic simulation showed its ability to effectuate neuroprotection and MAO-A and MAO-B binding affinity at the molecular level [86].
Baek et al. [87] obtained bisdemethoxycurcumin and demethoxycurcumin from Curcuma longa. These compounds were tested for MAO-A and -B inhibitory activity. Both compounds were found to be potential inhibitors against the MAO-B enzyme, with high IC50 values [87]. This study suggests that the investigated curcumin derivatives could be potent inhibitors for the treatment of MAO related disorders. Other research [88] isolated alternariol monomethyl ether (AME) from Alternaria brassicae. In these experiments, AME exhibited high and selective hMAO-A inhibition. However, this compound was found to be less effective for MAO-B inhibition. Chaurasiya et al. [89], in turn, isolated acacetin 7-methyl ether from Turnera diffusa and analyzed its in vitro inhibitory activity against recombinant hMAO-A and hMAO-B. This compound was discovered to be a potent selective MAO-B inhibitor, with an IC50 value of 198 nM. Furthermore, the molecular docking and molecular dynamic experiments showed that the compound displayed selectivity stable and strong inhibition of the MAO-B enzyme.
Mohamed et al. [90] isolated 14 compounds from Zanthoxylum flavum stems and evaluated their recombinant human MAO inhibition. The results of the study revealed that compound 3-sesamin exhibited potent selective MAO-B impediment (IC50 value of 1.45 µM). The promising MAO-B inhibitory activity of sesamin inclined the authors to explore its kinetic properties, binding mode, and the mechanism of MAO-B restriction. Detailed investigation substantiated reversible binding and mixed MAO-B catalytic function constraint by sesamin. This study provided promising findings for further in vivo investigation to confirm the therapeutic potential of sesamin.
Rauhamäki et al. [91] designed and synthesized numerous 3-phenylcoumarin derivatives and subsequently screened them for MAO-B inhibitory activity. They found 24 coumarin derivatives that are promising inhibitors of selected MAO-B (IC50 in the range 100 nM–1 µM). This study also researched the best ligand lipophilicity efficiency. This work indicates that in the future, 3-phenylcoumarin derivative drug development can be developed into being pharmacologically more active inhibitors. Yang et al. [92], in turn, synthesized and studied the in vitro activity of 3-arylcoumarin derivatives. In the work, most of these substances exhibited good activity against MAO, AChE, and BuChE enzymes; thus, potentially, they could become anti-AD drugs. The optimal compound was particularly noted to be a very effective inhibitor of MAO (IC50 = 27.03 µM).
Other studies have synthesized new derivatives of hydroxypyridinone-coumarin and developed their potential towards AD. These compounds display potential MAO-B inhibitory activity as demonstrated under in vitro assay. Herein, 1-((7-((3-fluorobenzyl)oxy)-2-oxo-2H-chromen-3-yl) methyl)-3-hydroxy-2-methylpyridin-4(1H)-one hydrochloride exhibited the highest active anti-MAO-B (IC50 = 14.7 nM). Molecular docking shows that this compound is a potential drug for AD treating because it can bind both substrate and entrance cavity of MAO-B [93].
To discover different inhibitors, Xie et al. developed novel coumarin-dithiocarbamate derivatives. They explored the options for treating AD by restriction of selective MAO-B isoforms. Compound 3-((3-chloro-4-methyl-2-oxo-2H-chromen-7-yl)oxy)propyl2,6-dimethyl-piperidine-1 carbodithioate evidenced the strongest MAO-B (IC50 = 0.101 µM). It also did not show any critical toxicity in mice in therapeutic doses, and, according to the researchers, prevented cognitive dysfunction in the AD-infected mice [94]. Repsold et al. [95] produced multitargeted directed ligands based on a coumarin scaffold that demonstrated inhibitory activities at two main enzymes (MAO-B and AChE) for the treatment of AD. Biological assay indicated that one of the coumarin-morpholine ether conjugates was a most promising hMAO-B inhibitor, while one of the coumarin-piperidine conjugates was an effective AChE inhibitor.
Overall, a significant number of structurally distinct synthetic and natural compounds can inhibit both MAO-A and MAO-B isoforms with different degree of potency and selectivity. However, it is not easy to categorize the chemical structures for their affinity towards the MAO-A and MAO-B isoform.
6. Anti NFTs Accumulation
The tau proteins play an important role in cell integrity. They are predominantly found in neurons, but small amounts of tau are also located in the astrocytes and oligodendrocytes. The different tau isoforms are encoded by the microtubule-associated gene on human chromosome 17q21 [6]. The microtubule-associated protein tau is disordered and shows high flexibility and lack of a stable conformation. The major hallmark of the tau hypothesis of AD pathogenesis is the formation of NFTs, which are aggregates of abnormal tau proteins. In AD patients, the density of NFTs is related to the degree of cognitive deficit [96], and therefore, tau needs to be detached from microtubules and then transferred into abnormal aggregates before a patient develops AD. This modification is probably caused by a series of post-translational processes, e.g., phosphorylation, glycosylation, nitration, acetylation, ubiquitination, and methylation. Abnormal phosphorylation is the most important modification. In AD, both the total and phosphorylated tau levels increase, along with the disease progression. It has been revealed that tau is 3–4 times more phosphorylated in the brains of AD patients compared to healthy brains [97].
Tau protein hyperphosphorylation causes the dissociation of tau from microtubules and induces abnormal tau aggregation. Approaches to blocking tau-mediated neurotoxicity includes primarily restricting tau post-translational modifications and directly inhibiting tau aggregation. Tau protein dephosphorylation is mainly brought about by protein phosphatase 2A (PP2A). This phosphatase has reduced activity in the AD brain and is a difficult target for drugs [98]. Moreover, research suggests that inhibition of PP2A could induce tau hyperphosphorylation [6]. This fact indicates that PP2A might regulate normal tau protein phosphorylation by preventing excessive activation of tau kinases. It is believed that tau phosphorylation could be the result of equilibrium between tau protein phosphatases and kinases. Therefore, protein kinase inhibitors are usually targeted towards other kinases (rather than directly on PP2A) to impede tau hyperphosphorylation or reduce tau aggregation [99]. When this enzymatic equilibrium is disturbed, tau hyperphosphorylation occurs. This process will lead to NFT formation and cognitive deficits.
In AD animal models, some plant compounds and products have been shown to constrain tau hyperphosphorylation through modulating the activity of glycogen synthase kinase-3 (GSK3) or cyclin-dependent kinase-5 (CDK5), or directly through PP2A control [100]. ‘Tongmai Yizhi’, a decoction derived from Chinese medicine, has been demonstrated to significantly decrease CDK5 and CDK5 expression in the hippocampus of model rats [101]. This decoction contains plants such as Daemonorops draco (Willd.), Panax ginseng C.A. Meyer, Rehmannia glutinosa Libosch, Alpinia oxyphylla Miq., Gastrodia elata Blume, and Whitmania pigra Whitman. Multidrug compatibility is regarded as the essence of Tongmai Yizhi decoction activity. However, due to the complex components and numerous targets involved, fully elucidating its mechanism is challenging [102].
Safflower yellow is one of the traditional Chinese medicines extracted from safflower (Carthamus tinctorius), which is suggested to have therapeutic potential for neurodegenerative disorders. Data obtained by Ma et al. [103] indicate that this extract can serve as a therapeutic candidate for AD. They have found that safflower yellow inhibits the GSK-3 activation and GSK-5 signaling pathways so as to protect against tau hyperphosphorylation by Aβ1–42, and in this way improves learning and memory functions in AD model rats.
It is known that ginsenoside Rd (Rd), one of the main active ingredients in Panax ginseng, increases PP2A activity and decreases okadaic acid-induced neurotoxicity, as well as tau hyperphosphorylation in vitro and in vivo [104]. Zhang et al. [105] investigated whether Rd could reduce tau phosphorylation and sequential cognition impairment after ischemic stroke. The results of the study demonstrated that Rd treatment reduces ischemia-induced enhancement of tau phosphorylation and ameliorated behavior impairment. Moreover, Rd inhibits the activity of GSK-3β but enhances the activity of protein kinase B (PKB/AKT), an important kinase suppressing GSK-3β activity. The authors also concluded that LY294002, an antagonist for the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, significantly decreases the inhibitory effect of Rd on GSK-3β activity. These findings provide evidence that Rd may reduce cerebral ischemia-induced tau phosphorylation via the PI3K/AKT/GSK-3β pathway.
Epigallocatechin-3-gallate is an active plant metabolite that has therapeutic potential against various disorders, including inhibition of tau aggregation. EGCG interacts with full-length tau protein at several residues with unstable interactions. This compound restricts aggregation of tau and dissolves tau fibrils and oligomers. It is likely that EGCG forms higher-order structures and degrades them without allowing the formation of mature aggregates [106].
Many of the tau aggregation inhibitors are natural plant compounds with antioxidant activity. Crocin from Crocus sativus can interfere with tau protein nucleation and inhibit tau protein filament formation in vitro [100]. In vitro, the aqueous extract of Glycyrrhiza inflata can improve the growth of the repeat domain and axons in mutant tau protein to prevent tau aggregation. This extract is also able to upregulate unfolded protein response-mediated chaperones to reduce tau misfolding. Cornel iridoid glycoside is the main compound extracted from Cornus officinalis. The findings obtained by Yang et al. [107] suggest that it may be used as a promising anti-AD drug. The results presented by the authors provide novel insights into how cornel iridoid glycoside constrains tau hyperphosphorylation. Among other approaches, this compound impedes GSK-3β activity through promoting the phosphatidylinositol 3-kinase/AKT signaling pathway. Moreover, it can elevate PP2A activity via constraining PME-1-induced PP2A catalytic C subunit demethylation, and, subsequently, limiting GSK-3β activity. In this way, cornel iridoid glycoside regulates the crosstalk between GSK-3β and PP2A signaling and, consequently, inhibits tau hyperphosphorylation.
Sonawane et al. [108] screened the potency of baicalein, a polyphenol from the Scutellaria baicalensis Georgi, against in vitro tau aggregation and tau filaments dissolution. Their study suggests the potency of baicalein against two pathological tau activities, namely, this plant metabolite efficiently inhibits tau formation by promoting off pathway oligomers, as well as by dissolving tau filaments. This research highlights the potential of baicalein in ameliorating multifactorial neurodegenerative pathologies.
Curcumin works in a similar way as baicalein. Studies indicate that curcumin inhibits the oligomerization of tau and could disaggregate tau filaments [109]. Another plant compound able to constrain tau protein aggregation is resveratrol, which inhibits the aggregation of the repeat domain of tau (and shows several other neuroprotective mechanisms) [110]. Two more are folic acid, which slows down tau aggregation via stabilizing its native state [111], and purpurin, which counteracts tau fibrillization and breaks down the pre-formed fibrils [112].
7. Neuroinflammation
A significant factor attributable to neurodegeneration is neuroinflammation. This is a cellular and biochemical response that increases inflammatory mediators (cytokines, chemokines) and activates glia cells and leukocyte invasion of brain tissue [113]. A significant side effect of the process is increased permeability of the BBB (blood–brain barrier). It is known that this neuroinflammation is strictly connected with innate (the first line of defense) and adaptive immune responses. In the case of Alzheimer’s disease, neuroinflammatory contribution to pathogenesis equals that of senile plaques and NFTs [114]. The following neuroinflammatory landscapes that are associated with AD are the most intensive studied:
Microglia: the resident phagocytes of central nervous system. In the case of AD, the structure binds to soluble Aβ oligomers and Aβ fibrils via the following receptors: SCARA1, CD36, CD14, α6β1 integrin, CD47, and Toll-like receptors. Binding of Aβ with CD36, TLR4, and TLR6 leads to activation of microglia and the production of proinflammatory cytokines and chemokines [114,115].
Astroglia: accumulates around senile plaques. The structures release cytokines, interleukins, nitric oxide, and other potentially cytotoxic molecules. ApoE is needed for astrocyte-mediated clearance of Aβ, and astrocyte-dependent lipidation of ApoE increases the capability of microglia to clear Aβ [114].
There are many factors that contribute to neuroinflammation. Besides typical pro-neuroinflammatory factors such as senile plaques, there are numerous linkage with phenomena such as (1) systematic inflammation, for which studies revealed explicit correlation with inflammatory changes in the brain [116]; (2) obesity, which is characterized by white fat having a high level of activated macrophages that constantly secrete proinflammatory cytokines [117]; (3) traumatic brain injury leading to microglia activation, which can persist for months or years after traumatic brain injury [118]; and (4) locus coeruleus degeneration being strictly connected with loss of noradrenaline, which is due to compromised microglial migration and Aβ phagocytosis [119].
One of the most often administered tests towards determining anti-inflammatory activity is the carrageenan-induced rat paw edema test. This was applied for assessing the activity of extracts of Acalypha hispida (Euphorbiaceae) leaves, which are rich in ellagic acid, gallic acid, and rutin [120]. The basis of the test is a marked edema formation that is mediated by histamine, serotonin, and bradykinins (first phase), as well as the release of prostaglandins and nitric oxide (second phase). The study results revealed that extracts of Acalypha hispida significantly decreased edema formation and histamine-induced rat paw edema. The most probable mechanism is based on antihistaminic activity, but inhibition of carrageenan-induced inflammatory responses was also noted; hence, the main components of the studied extract might follow several inflammatory pathways. The compound that is likely to be responsible for the activity is ellagic acid, which is known to constrain COX-2 and NO synthase expression [121].
A well-known and commonly administered secondary plant metabolite is lycopene, a hydrocarbon carotenoid. The compound has demonstrated a variety of biological activities, including high antioxidant activity. In rat model studies, lycopene and human amniotic epithelial cells (HAECs) were used as therapeutic agents for assessing immunomodulatory effects at the choroid plexus. Here, the results revealed that lycopene administration has a significant impact on the level of proinflammatory mediators such as TNF-α and IL-1β. In addition, the metabolite was found to increase the anti-inflammatory mediators IL-10 and TGF-β1 in the cerebro-spinal fluid and hippocampal tissues. Additional analysis revealed that lycopene can positively affect upregulation of Toll-like receptor 4, leading to reversion of Aβ [122].
Terpenes are another group of secondary plant metabolites revealing rich biological activity. The group consists of over 55,000 compounds that are well diversified in terms of structure and effect. Among them are triterpenoids that demonstrate anti-neuroinflammatory activity. For example, interesting results were obtained for novel triterpenoids derived from seeds of Quercus serrata Thunb (acorns). Here, studies based on NO production restriction induced by LPS in microglia cells revealed the potent inhibitory impact of triterpenoids on the mRNA expression of iNOS and COX-2 in LPS-induced BV-2 cells [123].
As mentioned previously, nitric oxide is one of the most studied promoters of neuroinflammation. The phenomenon results from the fact that localization of NOSs (NO synthases) allow for the synthesis of nitric oxide in macrophages microglia, neurons, and endothelial cells, leading to immunomodulation and neuroinflammation [124]. Lignans and neolignans are among the compounds revealing NO-inhibiting activity. These can counteract neuroinflammation and NO production by reducing the expression of PGE2, TNF-α, IL-1β, and COX2, as well as by downregulating the MAPK, ERK, and JNK pathways. Among them, the most intensively studied are balanophonin [125], chaenomiside A [126], sambucuside [127], melongenamide C, and cannabisin F [128].
The polyphenols revealed similarly high anti-neuroinflammatory and NO generation inhibitory activity. Gingerol, a compound isolated from Zingiber officinale, is commonly recognized as an anti-neurodegeneration agent. It is known that the polyphenol is able to inhibit NO production and pro-inflammatory cytokines via the NF-κB pathway. Additionally, other ingredients of Zingiber officinale (i.e., zingerone, 6-gingerol) inhibit NO production, IL-6, IL-1β, TNF-α, and mRNA levels in BV2 microglial cells activated by LPS [124]. Related activity was also observed for coumarins. Here, omphalocarpin obtained from Toddaliae asiaticae (Rutaceae) revealed an impact on the expression of proinflammatory mediators, such as NO, TNF-α, and IL-1β, and fostered the downregulation of COX-2 and NOS expression in LPS-stimulated BV2 cells [124,129].
8. The Influence of Iron Ions on Neurodegeneration Process
What are deemed ‘essential metals’ play crucial roles in the maintaining good health. Among the metals, the most important are iron, zinc, copper, chromium, and manganese. These are responsible for a number of crucial processes in our body, including enzymatic reactions (catalase, hydrogenase) and cellular activities [4]. Nevertheless, there is a thin line between the beneficial and harmful role of the elements. Numerous studies reveal the effects of excessive levels of trace metal ions with regard to mitochondrial dysfunctions, endoplasmic reticulum stress, oxidative stress, and autophagy dysregulation. One of the most important metals in this regard is iron and its ions. These have been intensively studied towards their influence on neurodegeneration processes, including AD [130].
A critical factor leading to various cellular changes is oxidative stress. The basis of free radical creation is the Haber–Weiss reaction, which is the source of the most dangerous hydroxyl radical:
Here, acting as catalysts, Fe2+/Fe3+ drive the reaction via the Fenton reaction, in which Fe2+ reacts with H2O2, leading to the generation of Fe3+, which is subsequently reduced with O2•− [131].
Similarly to the free redox active form of iron, heme-iron plays an important role in oxidative stress generation. An overload of free heme is toxic due to their pro-oxidant activity resulting from their being part of the prosthetic group in proteins [132].
In addition to oxidative stress involving iron ions, the metal is engaged in Aβ creation (Figure 1). Intensive studies have revealed the explicit correlation between senile plaque deposition and the level of iron ions. Detailed molecular docking simulations indicate that His6, His13, and His14 amino acid residues of amyloid β are able to interact with iron ions. An additional factor promoting the reaction of iron–Aβ interactions is the reductive brain environment, as well as the high level of metal ions in this organ [133]. The mechanism of the Aβ formation with iron ion participation can be controlled by intracellular iron via the iron regulatory element RNA stem loop in the 5ʹ unsaturated region of the APP transcript. The element was found to physiologically bind with iron response protein 1 and not with iron response protein 2 in human neuronal cells [134].
Figure 1.
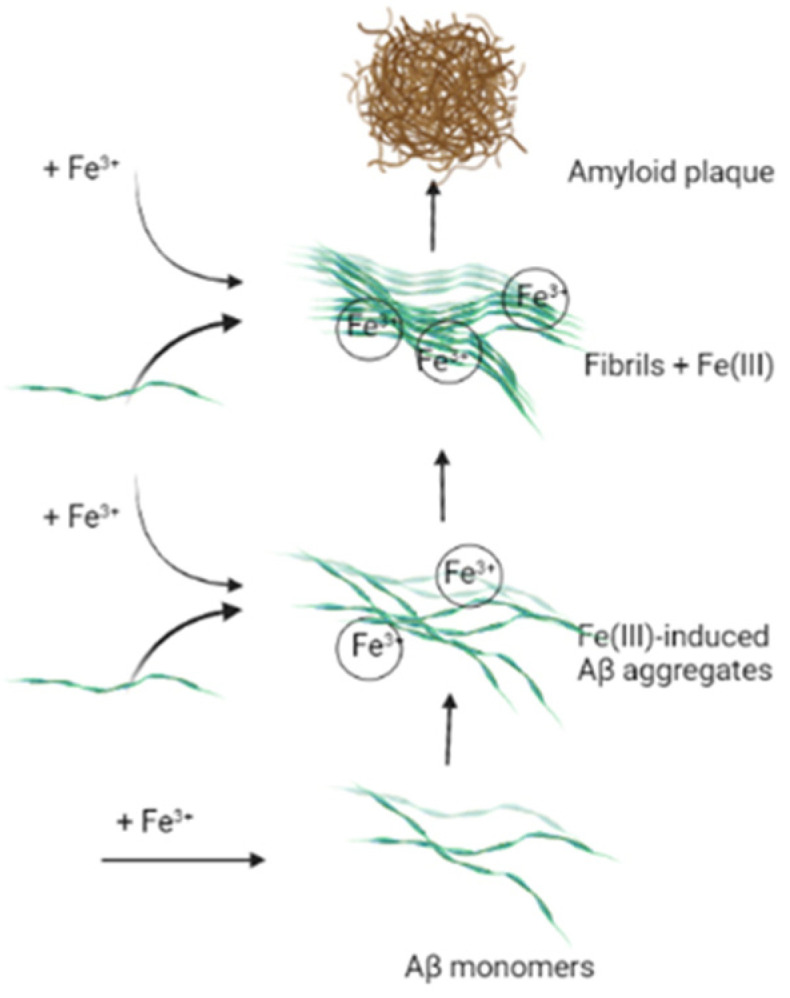
Amyloid plaques formation in Fe(III) participation.
An equally significant mechanism of Aβ generation to which iron contributes is explained by furin and secretase activity, both of which are engaged in nonamyloidogenic and amyloidogenic changes in APP. Silvestri et al. revealed the explicit dependency between level of cellular iron and furin level, namely, that the protein level decreased along with an excess of the former, and simultaneously, iron content supported enhanced β-secretase activity and the development of the amyloidogenic pathway [135].
The presence of intraneuronal neurofibrillary tangles (NFTs) is an equally important factor contributing to neurodegeneration and Alzheimer’s disease. Herein, detailed and long-lasting studies have revealed a clear correlation between Fe3+ and the aggregation of hyperphosphorylated tau. The mechanism of the process is explained by iron metal participation in tau-tau interactions and dimerization [136]. Additionally, scientists indicated phosphorylation as an agent in metal interactions. NMR analysis confirmed strong interactions between Fe(III) and His residue of tau [137].
Proposed solutions to the negative influence of excessive levels of iron ions upon the organism include metal ion reduction (Fe3+) and metal ion chelation (Fe2+/3+). Both approaches are thought to allow for the keeping of an appropriate level of the metal ions in the body, while counteracting neurodegeneration development. Intense studies, both in vitro and in vivo, have revealed the ability of natural compounds to reduce and/or chelate iron ions. The most interesting and promising results are presented below.
Phenols, a rich group of secondary plant metabolites, have been intensive studied towards iron ion reduction and chelation activity. The phenomenon results from the rich biological activities of the compounds, as well as their structures. The catechol (1,2-dihydroxybenzene) nucleus, for example, has an affinity for metal ion. Moreover, the keto groups and their nearby hydroxyl groups in the flavonoids, also contribute towards iron ion reduction [138].
Studies performed for 3-hydroxyflavone, 5,7-dihydroxyflavone, and 4′-dihydroxyflavone demonstrate the positive influence of the moieties on phenol metal binding. Here, stability constant analysis reveals that 3′,4′-hydroxy substitutions at the catecholic site are most significant for ferric complexation [139].
As mentioned above, iron ions take part in ROS generation via Fenton reaction. The impact of polyphenols on the process was analyzed in terms of the participation in an inhibitory way (via formation of inert metal complexes) and in a stimulatory/pro-oxidant manner. Interesting study results have been obtained, for example, for quercetin, a well-known phenolic present in numerous fruits and vegetables. In this study, the iron-binding ability of the compound was analyzed by means of NMR and EPR spectroscopies. The resulting binding constant analysis explicitly indicated that quercetin can bind Fe(II) stronger that ferrozine, a well-known Fe(II) chelator. The researchers concluded that the high ability of quercetin to chelate Fe(II) can completely inhibit the Fenton reaction, leading to significantly improvement of oxidative stress [140].
Besides catechol moiety, combinations of hydroxyl and carbonyl groups play pivotal roles. In this case, the metal binding site is defined by their assemblage. Polyphenols can be divided into the following groups: (1) ‘one-metal binding site’, having one potential chelator site—these include the curcuminoids, lignans, stilbenes, isoflavonoids, flavanols, and anthocyanins; (2) ‘two-metal binding site’, having two potential chelator sites, among others, the flavones and flavonones; (3) ‘three-metal binding site’, having three potential chelator sites—these embrace the flavonols, flavanols, and tannins; and (4) compounds having four or more metal chelator sites [141] (Figure 2).
Figure 2.
Examples of polyphenols with various numbers of metal chelator sites.
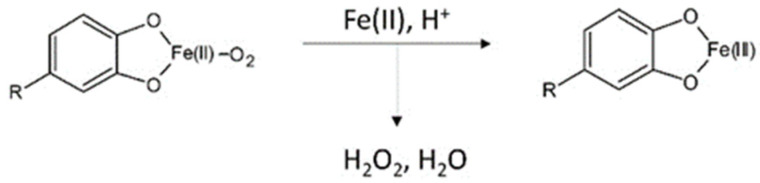
A further aspect of iron ions binding by phenols is the difference in kinetic reaction in Fe(II) and Fe(III) binding. In order to expand knowledge in this direction, researchers performed studies based on gallic acid, caffeic acid, catechin, and rutin. Accordingly, affinity of the common phenols for iron ions turned out to be different. However, assessment of metal-polyphenol interactions and the redox process is difficult due to sensitivity of metal autoxidation processes and redox potentials to pH. This was overcome by performing the experiments in neutral phosphate buffer. The outcome of the work established that (1) spectral changes following Fe(II) addition are much faster and more intense than with Fe(III); (2) Fe(II)-polyphenol binding does not provide protection to Fe(II) against autoxidation; and (3) Fe(II)-polyphenol binding is faster than autoxidation of free Fe(II) [138]. Conclusions drawn on the basis of the conducted research indicate that the common polyphenols can bind Fe(II) and Fe(III), but the second is captured more slowly. The mechanism of the reaction is explained by electrons transfer from Fe(II) with the concomitant formation of Fe(III)-phenol complexes (Figure 3). The reaction could also take place starting from Fe(III)-phenol complexes; however, the slow preliminary step of Fe(III) reduction by phenols hampers it.
Figure 3.
Possible mechanism reaction of Fe(II)-phenol binding reaction. The figure was prepared on the basis of [138].
Another important and well-known phenol is chlorogenic acid. Similarly to other secondary plant metabolites, the compound reveals a variety of biological activities. Studies were performed to ascertain the ability of the phenol to chelate Fe(II), to determine hydroxyl radical generation as influenced by the compound during iron release, and to understand the impact of chlorogenic acid on iron-involved polymerization. The outcome of such work revealed that chlorogenic acid can interact with Fe(III) to form complexes that can interact with ferritin via hydrogen bonds. Additionally, the generation of hydroxyl radicals is significantly reduced by the phenol during ion release. Moreover, chlorogenic acid can also promote the rates of ion oxidative deposition and ion release from ferritin [142].
Reduction or chelation of iron ions by natural compounds such as phenols was confirmed repeatedly in studies based on essential oils rich in these compounds. In most of these studies, correlation between high ability of essential oils to bind iron ions and content of phenols, terpenes, or other secondary plant metabolites indicate that the high biological activity can be related to the main components of the essential oils [143].
Activity of secondary plant metabolites towards regulating organism levels of iron ions by their reduction and chelation was confirmed in in vivo studies using rat models. Both an acute and a long-term study using rat models have indicated the significant influence of the polyphenol on iron level. In the acute study, duodenal mucosa to quercetin was found to increase apical iron intake and to decrease subsequent basolateral iron efflux in the circulation. The probable mechanism of action is thought to be based on the chelation ability of quercetin in binding iron between 3-hydroxyl and 4-carbonyl groups and by methylation of the 3-hydroxyl group. In the case of the long-term study, assessment of the positive influence of quercetin was based on recapitulation in Caco-2 cells exposed to quercetin. Here, reporter assays in Caco-2 cell suggested that the repression of FPN by quercetin was not a transcriptional event but might be mediated by miRNA interaction with the FPN 3′UTR [144].
Another natural compound revealing excellent biological activity is tannic acid. Besides reduction and chelation activity in vitro, the compound has revealed an ability to counteract iron overload by its chelator activity. The obtained research results were similar to that of the control substance (desirox-a standard iron chelator). Additionally, it is probable that tannic acid might modulate DMT-1, block L-type calcium channels, and reverse iron overload in the organism [145].
Epigallocatechin-3-gallate (EGCG) is a similarly important and well-known natural compound. Detailed analysis has demonstrated the ability of EGCG to decrease cellular assimilation of heme iron, along with the ability to limit its basolateral efflux. The data confirmed that the polyphenol constrains heme iron absorption by reducing basolateral iron exit, rather than by decreasing apical heme iron uptake in intestinal cells [146].
9. Conclusions
Although not fully understood, the pathological processes associated with AD are influenced by many factors. Many in vitro and in vivo studies have demonstrated that natural plant products and phytochemicals exhibit neuroprotective effects. Among the many mechanisms are activity targeting cholinergic neurotransmission and neuroinflammation; generating an imbalance of iron in the organism; inhibiting α-synuclein, as well as BACE and MAO; affecting Aβ accumulation and aggregation; and reducing tau phosphorylation, as well as inducing anti-inflammatory and antioxidant effects.
The plant metabolites and their combinations are a valuable collection of natural products that should be tested to prevent and effective treat AD. These naturally based drugs will surely have fewer side effects than the currently available pharmacological treatments.
Abbreviations
| Ach | acetylcholine |
| AChE | acetylcholinesterase |
| AME | alternariol monomethyl ether |
| Apo E | apolipoprotein E |
| APP | amyloid precursor protein |
| BACE | β-secretase |
| BDNF | brain-derived neurotrophic factor |
| BuChE | butyrylcholinesterase |
| ChAT | choline O-acetyltransferase |
| CDK5 | cyclin-dependent kinase 5 |
| COX | cyclooxygenase |
| CRP | C-reactive protein |
| EGCG | epigallocatechin-3-gallate |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GSK | glycogen synthase kinase |
| iNOS | inducible nitric oxide synthase |
| LDH | lactate dehydrogenase |
| LXR | liver X receptor |
| MAO | monoamine oxidase |
| MDA | malondialdehyde |
| MCP-1 | monocyte chemoattractant protein-1 |
| MMP | mitochondrial membrane potential |
| NF-κB | nuclear factor kappa-B |
| NGF | nerve growth factor |
| NO | nitric oxide |
| PI3K | phosphatidylinositol 3-kinase |
| PKB | protein kinase B |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| PP2A | protein phosphatase 2A |
| ROS | reactive oxygen species |
| r.t. | room temperature |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid reactive substances |
Author Contributions
Conceptualization, K.W.-K. and A.O.; methodology, T.O.; software, T.O. and J.M.; validation; J.M., I.K. and J.S.; formal analysis, K.W.-K.; investigation, A.O.; resources, K.W.-K.; data curation, T.O. and I.K.; writing—original draft preparation, K.W.-K., A.O., T.O. and J.M.; writing—review and editing, A.O., I.K. and J.S.; visualization, T.O.; supervision, K.W.-K.; project administration, I.K.; funding acquisition, K.W.-K. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Medical University of Lublin.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar A., Singh A., Ekavali A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampel H., Hardy J., Blennow K., Chen C., Perry G., Kim S.H., Villemagne V.L., Aisen P., Vendruscolo M., Iwatsubo T., et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry. 2021:1–23. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojtunik-Kulesza K., Oniszczuk A., Waksmundzka-Hajnos M. An attempt to elucidate the role of iron and zinc ions in development of Alzheimer’s and Parkinson’s diseases. Biomed. Pharmacother. 2019;111:1277–1289. doi: 10.1016/j.biopha.2018.12.140. [DOI] [PubMed] [Google Scholar]
- 5.Wojtunik-Kulesza K.A., Kasprzak K., Oniszczuk T., Oniszczuk A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019;16:e1900434. doi: 10.1002/cbdv.201900434. [DOI] [PubMed] [Google Scholar]
- 6.Peng Y., Tao H., Wang S., Xiao J., Wang Y., Su H. Dietary intervention with edible medicinal plants and derived products for prevention of Alzheimer’s disease: A compendium of time-tested strategy. J. Funct. Foods. 2021;81:104463. doi: 10.1016/j.jff.2021.104463. [DOI] [Google Scholar]
- 7.Tiraboschi P., Hansen L.A., Alford M., Masliah E., Thal L.J., Corey-Bloom J. The decline in synapses and cholinergic activity is asynchronous in Alzheimer’s disease. Neurology. 2000;55:1278–1283. doi: 10.1212/WNL.55.9.1278. [DOI] [PubMed] [Google Scholar]
- 8.Mesulam M.-M. Butyrylcholinesterase in Alzheimer’s Disease. In: Giacobini E., Becker R.E., editors. Alzheimer Disease: Therapeutic Strategies. Birkhäuser; Boston, MA, USA: 1994. pp. 79–83. [Google Scholar]
- 9.Inestrosa N.C., Sagal J.P., Colombres M. Acetylcholinesterase Interaction with Alzheimer Amyloid β. In: Harris J.R., Fahrenholz F., editors. Alzheimer’s Disease. Subcellular Biochemistry. Vol. 38. Springer; Boston, MA, USA: 2005. pp. 299–317. [DOI] [PubMed] [Google Scholar]
- 10.Chen X., Drew J., Berney W., Lei W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells. 2021;10:1309. doi: 10.3390/cells10061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann D., Kaur Dogra A., Tahrani A., Herrmann F., Wink M. Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target. Molecules. 2016;21:1161. doi: 10.3390/molecules21091161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng Y., Zhang M.-Q., Wang W., Liu L.-T., Zhou L.-M., Miao S.-K., Wan L.-H. Compound danshen tablet ameliorated aβ25-35-induced spatial memory impairment in mice via rescuing imbalance between cytokines and neurotrophins. BMC Complement. Altern. Med. 2014;14:23. doi: 10.1186/1472-6882-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou X., Song H., Chen Y., Cheng S., Fang S., Zhang J., Wang Q. Effects of Bushen-Yizhi formula on age-related inflammation and oxidative stress in senescence-accelerated mice. Mol. Med. Rep. 2018;17:6947–6960. doi: 10.3892/mmr.2018.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon M., Kim H.G., Choi J.G., Oh H., Lee P., Ha S.K., Kim S.Y., Park Y., Huh Y., Oh M.S. Corrigendum to “6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia” [BBRC 449 (2014) 8-13] Biochem. Biophys. Res. Commun. 2019;521:545. doi: 10.1016/j.bbrc.2019.10.150. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Xu J., Xu P., Song S., Liu P., Chi T., Ji X., Jin G., Qiu S., Hou Y., et al. Xanthoceras sorbifolia extracts ameliorate dendritic spine deficiency and cognitive decline via upregulation of BDNF expression in a rat model of Alzheimer’s disease. Neurosci. Lett. 2016;629:208–214. doi: 10.1016/j.neulet.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Park H.R., Kim J.Y., Lee Y., Chun H.J., Choi Y.W., Shin H.K., Choi B.T., Kim C.M., Lee J. PMC-12, a traditional herbal medicine, enhances learning memory and hippocampal neurogenesis in mice. Neurosci. Lett. 2016;617:254–263. doi: 10.1016/j.neulet.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Ohba T., Yoshino Y., Ishisaka M., Abe N., Tsuruma K., Shimazawa M., Oyama M., Tabira T., Hara H. Japanese Huperzia serrata extract and the constituent, huperzine A, ameliorate the scopolamine-induced cognitive impairment in mice. Biosci. Biotechnol. Biochem. 2015;79:1838–1844. doi: 10.1080/09168451.2015.1052773. [DOI] [PubMed] [Google Scholar]
- 18.Geromichalos G.D., Lamari F., Papandreou M.A., Trafalis D.T., Margarity M., Papageorgiou A., Sinakos Z. Saffron as a Source of Novel Acetylcholinesterase Inhibitors: Molecular Docking and in Vitro Enzymatic Studies. J. Agric. Food Chem. 2012;60:6131–6138. doi: 10.1021/jf300589c. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed H.H., Salem A.M., Sabry G.M., Husein A.A., Kotob S.E. Possible Therapeutic Uses of Salvia triloba and Piper nigrum in Alzheimer’s Disease–Induced Rats. J. Med. Food. 2013;16:437–446. doi: 10.1089/jmf.2012.0165. [DOI] [PubMed] [Google Scholar]
- 20.Orhan I., Aslan M. Appraisal of scopolamine-induced antiamnesic effect in mice and in vitro antiacetylcholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. J. Ethnopharmacol. 2009;122:327–332. doi: 10.1016/j.jep.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Joshi H., Parle M. Cholinergic Basis of Memory-Strengthening Effect of Foeniculum vulgare Linn. J. Med. Food. 2006;9:413–417. doi: 10.1089/jmf.2006.9.413. [DOI] [PubMed] [Google Scholar]
- 22.Giridharan V.V., Thandavarayan R.A., Mani V., Dundapa T.A., Watanabe K., Konishi T. Ocimum sanctum Linn. Leaf Extracts Inhibit Acetylcholinesterase and Improve Cognition in Rats with Experimentally Induced Dementia. J. Med. Food. 2011;14:912–919. doi: 10.1089/jmf.2010.1516. [DOI] [PubMed] [Google Scholar]
- 23.Xu P., Wang K., Lu C., Dong L., Gao L., Yan M., Aibai S., Liu X. Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and H2O2 induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 2016;193:408–415. doi: 10.1016/j.jep.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Lee A.Y., Choi J.M., Lee J., Lee M.H., Lee S., Cho E.J. Effects of Vegetable Oils with Different Fatty Acid Compositions on Cognition and Memory Ability in Aβ25–35-Induced Alzheimer’s Disease Mouse Model. J. Med. Food. 2016;19:912–921. doi: 10.1089/jmf.2016.3737. [DOI] [PubMed] [Google Scholar]
- 25.Okello E.J., Savelev S.U., Perry E.K. In vitro anti-β-secretase and dual anti-cholinesterase activities of Camellia sinensis L. (tea) relevant to treatment of dementia. Phytother. Res. 2004;18:624–627. doi: 10.1002/ptr.1519. [DOI] [PubMed] [Google Scholar]
- 26.Ademosun A.O., Oboh G. Inhibition of Acetylcholinesterase Activity and Fe2+-Induced Lipid Peroxidation in Rat Brain In Vitro by Some Citrus Fruit Juices. J. Med. Food. 2012;15:428–434. doi: 10.1089/jmf.2011.0226. [DOI] [PubMed] [Google Scholar]
- 27.Qosa H., Mohamed L.A., Batarseh Y.S., Alqahtani S., Ibrahim B., LeVine H., Keller J., Kaddoumi A. Extra-virgin olive oil attenuates amyloid-β and tau pathologies in the brains of TgSwDI mice. J. Nutr. Biochem. 2015;26:1479–1490. doi: 10.1016/j.jnutbio.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okello E.J., Leylabi R., McDougall G.J. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct. 2012;3:651–661. doi: 10.1039/c2fo10174b. [DOI] [PubMed] [Google Scholar]
- 29.Jelenković A., Jovanović M.D., Stevanović I., Petronijević N., Bokonjić D., Živković J., Igić R. Influence of the Green Tea Leaf Extract on Neurotoxicity of Aluminium Chloride in Rats. Phytother. Res. 2013;28:82–87. doi: 10.1002/ptr.4962. [DOI] [PubMed] [Google Scholar]
- 30.Mathiyazahan D.B., Thenmozhi A.J., Manivasagam T. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. J. Funct. Foods. 2015;16:423–435. doi: 10.1016/j.jff.2015.05.001. [DOI] [Google Scholar]
- 31.Ferreira J.P., Albuquerque H.M., Cardoso S.M., Silva A.M., Silva V.L. Dual-target compounds for Alzheimer’s disease: Natural and synthetic AChE and BACE-1 dual-inhibitors and their structure-activity relationship (SAR) Eur. J. Med. Chem. 2021;221:113492. doi: 10.1016/j.ejmech.2021.113492. [DOI] [PubMed] [Google Scholar]
- 32.Bursavich M.G., Harrison B.A., Blain J.-F. Gamma Secretase Modulators: New Alzheimer’s Drugs on the Horizon? J. Med. Chem. 2016;59:7389–7409. doi: 10.1021/acs.jmedchem.5b01960. [DOI] [PubMed] [Google Scholar]
- 33.Maia M.A., Sousa M.E. BACE-1 and γ-Secretase as Therapeutic Targets for Alzheimer’s Disease. Pharmaceuticals. 2019;12:41. doi: 10.3390/ph12010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pont C., Ginex T., Griñán-Ferré C., Scheiner M., Mattellone A., Martínez N., Arce E.M., Soriano-Fernández Y., Naldi M., De Simone A., et al. From virtual screening hits targeting a cryptic pocket in BACE-1 to a nontoxic brain permeable multitarget anti-Alzheimer lead with disease-modifying and cognition-enhancing effects. Eur. J. Med. Chem. 2021;225:113779. doi: 10.1016/j.ejmech.2021.113779. [DOI] [PubMed] [Google Scholar]
- 35.Di Pietro O., Juárez-Jiménez J., Muñoz-Torrero D., Laughton C.A., Luque F.J. Unveiling a novel transient druggable pocket in BACE-1 through molecular simulations: Conformational analysis and binding mode of multisite inhibitors. PLoS ONE. 2017;12:e0177683. doi: 10.1371/journal.pone.0177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirsafian H., Ripen A.M., Merican A., Bin Mohamad S. Amino Acid Sequence and Structural Comparison of BACE1 and BACE2 Using Evolutionary Trace Method. Sci. World J. 2014;2014:1–6. doi: 10.1155/2014/482463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu H., Tosaki A., Kaneko K., Hisano T., Sakurai T., Nukina N. Crystal Structure of an Active Form of BACE1, an Enzyme Responsible for Amyloid β Protein Production. Mol. Cell. Biol. 2008;28:3663–3671. doi: 10.1128/MCB.02185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDade E., Voytyuk I., Aisen P., Bateman R.J., Carrillo M.C., De Strooper B., Haass C., Reiman E.M., Sperling R., Tariot P.N., et al. The case for low-level BACE1 inhibition for the prevention of Alzheimer disease. Nat. Rev. Neurol. 2021;17:703–714. doi: 10.1038/s41582-021-00545-1. [DOI] [PubMed] [Google Scholar]
- 39.Mintun M.A., Lo A.C., Evans C.D., Wessels A.M., Ardayfio P.A., Andersen S.W., Shcherbinin S., Sparks J., Sims J.R., Brys M., et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021;384:1691–1704. doi: 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
- 40.Cai J., Qi X., Kociok N., Skosyrski S., Emilio A., Ruan Q., Grant M.B., Saftig P., Serneels L., Golde T., et al. β-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol. Med. 2012;4:980–991. doi: 10.1002/emmm.201101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egan M.F., Mukai Y., Voss T., Kost J., Stone J., Furtek C., Mahoney E., Cummings J.L., Tariot P.N., Aisen P.S., et al. Further analyses of the safety of verubecestat in the phase 3 EPOCH trial of mild-to-moderate Alzheimer’s disease. Alzheimer’s Res. Ther. 2019;11:1–12. doi: 10.1186/s13195-019-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez C.L., Tariot P.N., Caputo A., Langbaum J.B., Liu F., Riviere M., Langlois C., Rouzade-Dominguez M., Zalesak M., Hendrix S., et al. The Alzheimer’s Prevention Initiative Generation Program: Study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer’s disease. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2019;5:216–227. doi: 10.1016/j.trci.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tüshaus J., Müller A.S., Kataka E.S., Zaucha J., Monasor L.S., Su M., Güner G., Jocher G., Tahirovic S., Frishman D., et al. An optimized quantitative proteomics method establishes the cell type-resolved mouse brain secretome. EMBO J. 2020;39:e105693. doi: 10.15252/embj.2020105693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barão S., Gärtner A., Leyva-Díaz E., Demyanenko G., Munck S., Vanhoutvin T., Zhou L., Schachner M., López-Bendito G., Maness P.F., et al. Antagonistic Effects of BACE1 and APH1B-γ-Secretase Control Axonal Guidance by Regulating Growth Cone Collapse. Cell Rep. 2015;12:1367–1376. doi: 10.1016/j.celrep.2015.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen V.T., To D.C., Tran M.H., Oh S.H., Kim J.A., Ali Y., Woo M.-H., Choi J.S., Min B.S., Nguyen V.T., et al. Isolation of cholinesterase and β-secretase 1 inhibiting compounds from Lycopodiella cernua. Bioorg. Med. Chem. 2015;23:3126–3134. doi: 10.1016/j.bmc.2015.04.080. [DOI] [PubMed] [Google Scholar]
- 46.Nuthakki V., Sharma A., Kumar A., Bharate S.B. Identification of embelin, a 3-undecyl-1,4-benzoquinone from Embelia ribes as a multitargeted anti-Alzheimer agent. Drug Dev. Res. 2019;80:655–665. doi: 10.1002/ddr.21544. [DOI] [PubMed] [Google Scholar]
- 47.Paudel P., Seong S.H., Zhou Y., Ha M.T., Min B.S., Jung H.A., Choi J.S. Arylbenzofurans from the Root Bark of Morus alba as Triple Inhibitors of Cholinesterase, β-Site Amyloid Precursor Protein Cleaving Enzyme 1, and Glycogen Synthase Kinase-3β: Relevance to Alzheimer’s Disease. ACS Omega. 2019;4:6283–6294. doi: 10.1021/acsomega.9b00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jannat S., Balupuri A., Ali Y., Hong S.S., Choi C.W., Choi Y.-H., Ku J.-M., Kim W.J., Leem J.Y., Kim J.E., et al. Inhibition of β-site amyloid precursor protein cleaving enzyme 1 and cholinesterases by pterosins via a specific structure−activity relationship with a strong BBB permeability. Exp. Mol. Med. 2019;51:1–18. doi: 10.1038/s12276-019-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J., Jun M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study. Mar. Drugs. 2019;17:91. doi: 10.3390/md17020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omar S.H., Scott C.J., Hamlin A.S., Obied H.K. Biophenols: Enzymes (β-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.) Fitoterapia. 2018;128:118–129. doi: 10.1016/j.fitote.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Xu Q.-X., Hu Y., Li G.-Y., Xu W., Zhang Y.-T., Yang X.-W. Multi-Target Anti-Alzheimer Activities of Four Prenylated Compounds from Psoralea fructus. Molecules. 2018;23:614. doi: 10.3390/molecules23030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Videira R., Castanheira P., Grãos M., Salgueiro L., Faro C., Cavaleiro C. A necrodane monoterpenoid fromLavandula luisieriessential oil as a cell-permeable inhibitor of BACE-1, theβ-secretase in Alzheimer’s disease. Flavour Fragr. J. 2013;28:380–388. doi: 10.1002/ffj.3156. [DOI] [Google Scholar]
- 53.Kashyap P., Kalaiselvan V., Kumar R., Kumar S. Ajmalicine and Reserpine: Indole Alkaloids as Multi-Target Directed Ligands Towards Factors Implicated in Alzheimer’s Disease. Molecules. 2020;25:1609. doi: 10.3390/molecules25071609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tao J., Zhao J., Zhao Y., Cui Y., Fang W. BACE inhibitory flavanones from Balanophora involucrata Hook. Fitoterapia. 2012;83:1386–1390. doi: 10.1016/j.fitote.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Raghuvanshi R., Nuthakki V.K., Singh L., Singh B., Bharate S.S., Bhatti R., Bharate S.B. Identification of plant-based multitargeted leads for Alzheimer’s disease: In-vitro and in-vivo validation of Woodfordia fruticosa (L.) Kurz. Phytomedicine. 2021;91:153659. doi: 10.1016/j.phymed.2021.153659. [DOI] [PubMed] [Google Scholar]
- 56.Panche A., Chandra S., Diwan A.D. Multi-Target β-Protease Inhibitors from Andrographis paniculata: In Silico and In Vitro Studies. Plants. 2019;8:231. doi: 10.3390/plants8070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung H.A., Min B.-S., Yokozawa T., Lee J.-H., Kim Y.S., Choi J.S. Anti-Alzheimer and Antioxidant Activities of Coptidis Rhizoma Alkaloids. Biol. Pharm. Bull. 2009;32:1433–1438. doi: 10.1248/bpb.32.1433. [DOI] [PubMed] [Google Scholar]
- 58.Panahi N., Mahmoudian M., Mortazavi P., Hashjin G.S. Experimental research Effects of berberine on β-secretase activity in a rabbit model of Alzheimer’s disease. Arch. Med. Sci. 2013;1:146–150. doi: 10.5114/aoms.2013.33354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Z., Li C., Wang X., Yang Z., Chen J., Hu L., Jiang H., Shen X. 2,2′,4′-Trihydroxychalcone from Glycyrrhiza glabra as a new specific BACE1 inhibitor efficiently ameliorates memory impairment in mice. J. Neurochem. 2010;114:374–385. doi: 10.1111/j.1471-4159.2010.06751.x. [DOI] [PubMed] [Google Scholar]
- 60.Mori T., Koyama N., Yokoo T., Segawa T., Maeda M., Sawmiller D., Tan J., Town T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020;295:16251–16266. doi: 10.1074/jbc.RA119.012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paris D., Beaulieu-Abdelahad D., Bachmeier C., Reed J., Ait-Ghezala G., Bishop A., Chao J., Mathura V., Crawford F., Mullan M. Anatabine lowers Alzheimer’s Aβ production in vitro and in vivo. Eur. J. Pharmacol. 2011;670:384–391. doi: 10.1016/j.ejphar.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 62.Rahman H., Bajgai J., Fadriquela A., Sharma S., Trinh T.T., Akter R., Jeong Y.J., Goh S.H., Kim C.-S., Lee K.-J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules. 2021;26:5327. doi: 10.3390/molecules26175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushik S., Cuervo A.M. Proteostasis and aging. Nat. Med. 2015;21:1406–1415. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 64.Wales P., Pinho R., Lázaro D.F., Outeiro T.F. Limelight on Alpha-Synuclein: Pathological and Mechanistic Implications in Neurodegeneration. J. Parkinsons Dis. 2013;3:415–459. doi: 10.3233/JPD-130216. [DOI] [PubMed] [Google Scholar]
- 65.Brás I.C., Dominguez-Meijide A., Gerhardt E., Koss D., Lázaro D.F., Santos P.I., Vasili E., Xylaki M., Outeiro T.F. Synucleinopathies: Where we are and where we need to go. J. Neurochem. 2020;153:433–454. doi: 10.1111/jnc.14965. [DOI] [PubMed] [Google Scholar]
- 66.Goedert M., Jakes R., Spillantini M.G. The Synucleinopathies: Twenty Years On. J. Parkinsons Dis. 2017;7:S51–S69. doi: 10.3233/JPD-179005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Outeiro T.F. Emerging concepts in synucleinopathies. Acta Neuropathol. 2021;141:469–470. doi: 10.1007/s00401-021-02290-7. [DOI] [PubMed] [Google Scholar]
- 68.Gonçalves P., Sodero A., Cordeiro Y. Green Tea Epigallocatechin-3-gallate (EGCG) Targeting Protein Misfolding in Drug Discovery for Neurodegenerative Diseases. Biomolecules. 2021;11:767. doi: 10.3390/biom11050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teil M., Arotcarena M.-L., Faggiani E., Laferriere F., Bezard E., Dehay B. Targeting alpha-Synuclein for PD Therapeutics: A Pursuit on All Fronts. Biomolecules. 2020;10:391. doi: 10.3390/biom10030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pervin M., Unno K., Ohishi T., Tanabe H., Miyoshi N., Nakamura Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules. 2018;23:1297. doi: 10.3390/molecules23061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ehrnhoefer E.D., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 72.Hornedo-Ortega R., Álvarez-Fernández M.A., Cerezo A.B., Richard T., Troncoso A.M., Garcia-Parrilla M.C. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-β and α-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016;64:7722–7732. doi: 10.1021/acs.jafc.6b03217. [DOI] [PubMed] [Google Scholar]
- 73.Manzoor S., Hoda N. A comprehensive review of monoamine oxidase inhibitors as Anti-Alzheimer’s disease agents: A review. Eur. J. Med. Chem. 2020;206:112787. doi: 10.1016/j.ejmech.2020.112787. [DOI] [PubMed] [Google Scholar]
- 74.Behl T., Kaur D., Sehgal A., Singh S., Sharma N., Zengin G., Andronie-Cioara F., Toma M., Bungau S., Bumbu A. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules. 2021;26:3724. doi: 10.3390/molecules26123724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Youdim M.B.H., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 76.Ramsay R.R. Monoamine Oxidases: The Biochemistry of the Proteins as Targets in Medicinal Chemistry and Drug Discovery. Curr. Top. Med. Chem. 2012;12:2189–2209. doi: 10.2174/156802612805219978. [DOI] [PubMed] [Google Scholar]
- 77.Tripathi A.C., Upadhyay S., Paliwal S., Saraf S.K. Privileged scaffolds as MAO inhibitors: Retrospect and prospects. Eur. J. Med. Chem. 2018;145:445–497. doi: 10.1016/j.ejmech.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Yoshimoto M., Hirata M., Kagawa S., Magata Y., Ohmomo Y., Temma T. Synthesis and characterization of novel radiofluorinated probes for positron emission tomography imaging of monoamine oxidase B. J. Label. Compd. Radiopharm. 2019;62:580–587. doi: 10.1002/jlcr.3779. [DOI] [PubMed] [Google Scholar]
- 79.Kristal B.S. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic. Biol. Med. 2001;30:924–931. doi: 10.1016/S0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 80.Sparks D.L., Woeltz V.M., Markesbery W.R. Alterations in Brain Monoamine Oxidase Activity in Aging, Alzheimer’s Disease, and Pick’s Disease. Arch. Neurol. 1991;48:718–721. doi: 10.1001/archneur.1991.00530190064017. [DOI] [PubMed] [Google Scholar]
- 81.Barnett J.H., Xu K., Heron J., Goldman D., Jones P. Cognitive effects of genetic variation in monoamine neurotransmitter systems: A population-based study of COMT, MAOA, and 5HTTLPR. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2010;156:158–167. doi: 10.1002/ajmg.b.31150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schneier F.R. Pharmacotherapy of social anxiety disorder. Expert Opin. Pharmacother. 2011;12:615–625. doi: 10.1517/14656566.2011.534983. [DOI] [PubMed] [Google Scholar]
- 83.Dhull D.K., Jindal A., Dhull R.K., Aggarwal S., Bhateja D., Padi S.S.V. Neuroprotective Effect of Cyclooxygenase Inhibitors in ICV-STZ Induced Sporadic Alzheimer’s Disease in Rats. J. Mol. Neurosci. 2011;46:223–235. doi: 10.1007/s12031-011-9583-6. [DOI] [PubMed] [Google Scholar]
- 84.Vermeiren Y., Van Dam D., Aerts T., Engelborghs S., Martin J.-J., De Deyn P.P. The monoaminergic footprint of depression and psychosis in dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimer’s Res. Ther. 2015;7:7–18. doi: 10.1186/s13195-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carter S.F., Schöll M., Almkvist O., Wall A., Engler H., Långström B., Nordberg A. Evidence for Astrocytosis in Prodromal Alzheimer Disease Provided by 11C-Deuterium-L-Deprenyl: A Multitracer PET Paradigm Combining 11C-Pittsburgh Compound B and 18F-FDG. J. Nucl. Med. 2012;53:37–46. doi: 10.2967/jnumed.110.087031. [DOI] [PubMed] [Google Scholar]
- 86.Larit F., Elokely K.M., Chaurasiya N.D., Benyahia S., Nael M.A., Leon F., Abu-Darwish M.S., Efferth T., Wang Y.-H., Belouahem-Abed D., et al. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine. 2017;40:27–36. doi: 10.1016/j.phymed.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baek S.C., Choi B., Nam S.-J., Kim H. Inhibition of monoamine oxidase A and B by demethoxycurcumin and bisdemethoxycurcumin. J. Appl. Biol. Chem. 2018;61:187–190. doi: 10.3839/jabc.2018.027. [DOI] [Google Scholar]
- 88.Lee H.W., Kim Y.J., Nam S.-J., Kim H. Potent Selective Inhibition of Monoamine Oxidase A by Alternariol Monomethyl Ether Isolated from Alternaria brassicae. J. Microbiol. Biotechnol. 2017;27:316–320. doi: 10.4014/jmb.1610.10053. [DOI] [PubMed] [Google Scholar]
- 89.Chaurasiya N.D., Zhao J., Pandey P., Doerksen R.J., Muhammad I., Tekwani B.L. Selective Inhibition of Human Monoamine Oxidase B by Acacetin 7-Methyl Ether Isolated from Turnera diffusa (Damiana) Molecules. 2019;24:810. doi: 10.3390/molecules24040810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohamed S.M., Chaurasiya N.D., Mohamed N.M., Bayoumi S.A.L., Tekwani B.L., Ross S.A. Promising selective MAO-B inhibition by sesamin, a lignan from Zanthoxylum flavum stems. Saudi Pharm. J. 2020;28:409–413. doi: 10.1016/j.jsps.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rauhamäki S., Postila P.A., Niinivehmas S., Kortet S., Schildt E., Pasanen M., Manivannan E., Ahinko M., Koskimies P., Nyberg N., et al. Structure-Activity Relationship Analysis of 3-Phenylcoumarin-Based Monoamine Oxidase B Inhibitors. Front. Chem. 2018;6:41. doi: 10.3389/fchem.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang J., Zhang P., Hu Y., Liu T., Sun J., Wang X. Synthesis and biological evaluation of 3-arylcoumarins as potential anti-Alzheimer’s disease agents. J. Enzym. Inhib. Med. Chem. 2019;34:651–656. doi: 10.1080/14756366.2019.1574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang C., Yang K., Yu S., Su J., Yuan S., Han J., Chen Y., Gu J., Zhou T., Bai R., et al. Design, synthesis and biological evaluation of hydroxypyridinone-coumarin hybrids as multimodal monoamine oxidase B inhibitors and iron chelates against Alzheimer’s disease. Eur. J. Med. Chem. 2019;180:367–382. doi: 10.1016/j.ejmech.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 94.He Q., Liu J., Lan J.-S., Ding J., Sun Y., Fang Y., Jiang N., Yang Z., Sun L., Jin Y., et al. Coumarin-dithiocarbamate hybrids as novel multitarget AChE and MAO-B inhibitors against Alzheimer’s disease: Design, synthesis and biological evaluation. Bioorg. Chem. 2018;81:512–528. doi: 10.1016/j.bioorg.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 95.Repsold B.P., Malan S.F., Joubert J., Oliver D.W. Multi-targeted directed ligands for Alzheimer’s disease: Design of novel lead coumarin conjugates. SAR QSAR Environ. Res. 2018;29:231–255. doi: 10.1080/1062936X.2018.1423641. [DOI] [PubMed] [Google Scholar]
- 96.Farber N.B., Rubin E.H., Newcomer J.W., Kinscherf D.A., Miller J.P., Morris J.C., Olney J.W., McKeel D.W. Increased Neocortical Neurofibrillary Tangle Density in Subjects With Alzheimer Disease and Psychosis. Arch. Gen. Psychiatry. 2000;57:1165–1173. doi: 10.1001/archpsyc.57.12.1165. [DOI] [PubMed] [Google Scholar]
- 97.Tapia-Rojas C., Cabezas-Opazo F., Deaton C.A., Vergara E.H., Johnson G.V.W., Quintanilla R.A. It’s all about tau. Prog. Neurobiol. 2019;175:54–76. doi: 10.1016/j.pneurobio.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lauretti E., Praticò D. Alzheimer’s disease: Phenotypic approaches using disease models and the targeting of tau protein. Expert Opin. Ther. Targets. 2020;24:319–330. doi: 10.1080/14728222.2020.1737012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hanger D.P., Anderton B.H., Noble W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Karakani A.M., Riazi G., Ghaffari S.M., Ahmadian S., Mokhtari F., Firuzi M.J., Bathaie S.Z. Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro. Iran. J. Basic Med. Sci. 2015;18:485–492. doi: 10.22038/IJBMS.2015.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feng J.-H., Cai B.-C., Guo W.-F., Wang M.-Y., Ma Y., Lu Q.-X. Neuroprotective effects of Tongmai Yizhi Decoction (通脉益智汤) against Alzheimer’s disease through attenuating cyclin-dependent kinase-5 expression. Chin. J. Integr. Med. 2016;23:132–137. doi: 10.1007/s11655-016-2507-0. [DOI] [PubMed] [Google Scholar]
- 102.Shi H., Dong C., Wang M., Liu R., Wang Y., Kan Z., Wang L., Si G. Exploring the mechanism of Yizhi Tongmai decoction in the treatment of vascular dementia through network pharmacology and molecular docking. Ann. Transl. Med. 2021;9:164. doi: 10.21037/atm-20-8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma Q., Ruan Y.-Y., Xu H., Shi X.-M., Wang Z.-X., Hu Y.-L. Safflower yellow reduces lipid peroxidation, neuropathology, tau phosphorylation and ameliorates amyloid β-induced impairment of learning and memory in rats. Biomed. Pharmacother. 2015;76:153–164. doi: 10.1016/j.biopha.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Li L., Liu J., Yan X., Qin K., Shi M., Lin T., Zhu Y., Kang T., Zhao G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011;138:135–141. doi: 10.1016/j.jep.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 105.Zhang X., Shi M., Ye R., Wang W., Liu X., Zhang G., Han J., Zhang Y., Wang B., Zhao J., et al. Ginsenoside Rd Attenuates Tau Protein Phosphorylation Via the PI3K/AKT/GSK-3β Pathway After Transient Forebrain Ischemia. Neurochem. Res. 2014;39:1363–1373. doi: 10.1007/s11064-014-1321-3. [DOI] [PubMed] [Google Scholar]
- 106.Sonawane S.K., Chidambaram H., Boral D., Gorantla N.V., Balmik A.A., Dangi A., Ramasamy S., Marelli U.K., Chinnathambi S. EGCG impedes human Tau aggregation and interacts with Tau. Sci. Rep. 2020;10:12579. doi: 10.1038/s41598-020-69429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang C., Li X., Gao W., Wang Q., Zhang L., Li Y., Li L., Zhang L. Cornel Iridoid Glycoside Inhibits Tau Hyperphosphorylation via Regulating Cross-Talk Between GSK-3β and PP2A Signaling. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sonawane S.K., Uversky V.N., Chinnathambi S. Baicalein inhibits heparin-induced Tau aggregation by initializing non-toxic Tau oligomer formation. Cell Commun. Signal. 2021;19:1–16. doi: 10.1186/s12964-021-00704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rane J.S., Bhaumik P., Panda D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimer’s Dis. 2017;60:999–1014. doi: 10.3233/JAD-170351. [DOI] [PubMed] [Google Scholar]
- 110.Yu K.C., Kwan P., Cheung S.K., Ho A., Baum L. Effects of resveratrol and morin on insoluble tau in tau transgenic mice. Transl. Neurosci. 2018;9:54–60. doi: 10.1515/tnsci-2018-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghasemzadeh S., Riazi G.H. Inhibition of Tau amyloid fibril formation by folic acid: In-vitro and theoretical studies. Int. J. Biol. Macromol. 2019;154:1505–1516. doi: 10.1016/j.ijbiomac.2019.11.032. [DOI] [PubMed] [Google Scholar]
- 112.Viswanathan G.K., Shwartz D., Losev Y., Arad E., Shemesh C., Pichinuk E., Engel H., Raveh A., Jelinek R., Cooper I., et al. Purpurin modulates Tau-derived VQIVYK fibrillization and ameliorates Alzheimer’s disease-like symptoms in animal model. Cell. Mol. Life Sci. 2019;77:2795–2813. doi: 10.1007/s00018-019-03312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alberti G., Paladino L., Vitale A., Caruso Bavisotto C., Conway de Macario E., Campanella C., Macario A., Marino Gammazza A. Functions and Therapeutic Potential of Extracellular Hsp60, Hsp70, and Hsp90 in Neuroinflammatory Disorders. Appl. Sci. 2021;11:736. doi: 10.3390/app11020736. [DOI] [Google Scholar]
- 114.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kummer M.P., Heneka M.T. Truncated and modified amyloid-beta species. Alzheimer’s Res. Ther. 2014;6:28–29. doi: 10.1186/alzrt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang H., Ochani M., Li J., Qiang X., Tanovic M., Harris H.E., Susarla S., Ulloa L., Wang H., DiRaimo R., et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA. 2003;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bastard J.-P., Maachi M., Lagathu C., Kim M.J., Caron M., Vidal H., Capeau J., Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine. Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 118.Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 119.Kalinin S., Gavrilyuk V., Polak P.E., Vasser R., Zhao J., Heneka M.T., Feinstein D.L. Noradrenaline deficiency in brain increases β-amyloid plaque burden in an animal model of Alzheimer’s disease. Neurobiol. Aging. 2007;28:1206–1214. doi: 10.1016/j.neurobiolaging.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Siraj A., Shilpi J.A., Hossain G., Uddin S.J., Islam K., Jahan I.A., Hossain H. Anti-Inflammatory and Antioxidant Activity of Acalypha hispida Leaf and Analysis of its Major Bioactive Polyphenols by HPLC. Adv. Pharm. Bull. 2016;6:275–283. doi: 10.15171/apb.2016.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gainok C.J., Daniels C.R., Golembiowski C.D., Strickland C.R., Garrett N. Investigation of the Anti-Inflammatory, Antinociceptive Effect of Ellagic Acid as Measured by Digital Paw Pressure via the Randall-Selitto Meter in Male Sprague- Dawley Rats. AANA J. 2011;79:S28–S34. [PubMed] [Google Scholar]
- 122.Xu Z., Liu C., Wang R., Gao X., Hao C., Liu C. A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. J. Nutr. Biochem. 2020;88:108558. doi: 10.1016/j.jnutbio.2020.108558. [DOI] [PubMed] [Google Scholar]
- 123.Mai Y., Wang Z., Wang Y., Xu J., He X. Anti-neuroinflammatory triterpenoids from the seeds of Quercus serrata Thunb. Fitoterapia. 2020;142:104523. doi: 10.1016/j.fitote.2020.104523. [DOI] [PubMed] [Google Scholar]
- 124.Förstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lim S.Y., Subedi L., Shin D., Kim C.S., Lee K.R., Kim S.Y. A New Neolignan Derivative, Balanophonin Isolated from Firmiana simplex Delays the Progress of Neuronal Cell Death by Inhibiting Microglial Activation. Biomol. Ther. 2017;25:519–527. doi: 10.4062/biomolther.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim C.S., Subedi L., Kim S.Y., Choi S.U., Kim K.H., Lee K.R. Lignan Glycosides from the Twigs of Chaenomeles sinensis and Their Biological Activities. J. Nat. Prod. 2015;78:1174–1178. doi: 10.1021/acs.jnatprod.5b00090. [DOI] [PubMed] [Google Scholar]
- 127.Suh W.S., Kim C.S., Subedi L., Kim S.Y., Choi S.U., Lee K.R. Iridoid Glycosides from the Twigs of Sambucus williamsii var. coreana and Their Biological Activities. J. Nat. Prod. 2017;80:2502–2508. doi: 10.1021/acs.jnatprod.7b00410. [DOI] [PubMed] [Google Scholar]
- 128.Sun J., Gu Y.-F., Su X.-Q., Li M.-M., Huo H.-X., Zhang J., Zeng K.-W., Zhang Q., Zhao Y.-F., Li J., et al. Anti-inflammatory lignanamides from the roots of Solanum melongena L. Fitoterapia. 2014;98:110–116. doi: 10.1016/j.fitote.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 129.Zeng Z., Tian R., Feng J., Yang N.-A., Yuan L. A systematic review on traditional medicine Toddalia asiatica (L.) Lam.: Chemistry and medicinal potential. Saudi Pharm. J. 2021;29:781–798. doi: 10.1016/j.jsps.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jan A.T., Azam M., Siddiqui K., Ali A., Choi I., Haq Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015;16:29592–29630. doi: 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kehrer J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 132.Kumar S., Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 133.Uranga R.M., Salvador G.A. Unraveling the Burden of Iron in Neurodegeneration: Intersections with Amyloid Beta Peptide Pathology. Oxidative Med. Cell. Longev. 2018;2018:1–12. doi: 10.1155/2018/2850341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ha C., Ryu J., Park C.B. Metal Ions Differentially Influence the Aggregation and Deposition of Alzheimer’s β-Amyloid on a Solid Template. Biochemistry. 2007;46:6118–6125. doi: 10.1021/bi7000032. [DOI] [PubMed] [Google Scholar]
- 135.Silvestri L., Pagani A., Camaschella C. Furin-mediated release of soluble hemojuvelin: A new link between hypoxia and iron homeostasis. Blood. 2008;111:924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 136.Ahmadi S., Ebralidze I.I., She Z., Kraatz H.-B. Electrochemical studies of tau protein-iron interactions—Potential implications for Alzheimer’s Disease. Electrochim. Acta. 2017;236:384–393. doi: 10.1016/j.electacta.2017.03.175. [DOI] [Google Scholar]
- 137.Nair N.G., Perry G., Smith M.A., Reddy V.P. NMR Studies of Zinc, Copper, and Iron Binding to Histidine, the Principal Metal Ion Complexing Site of Amyloid-β Peptide. J. Alzheimer’s Dis. 2010;20:57–66. doi: 10.3233/JAD-2010-1346. [DOI] [PubMed] [Google Scholar]
- 138.Nkhili E., Loonis M., Mihai S., El Hajji H., Dangles O. Reactivity of food phenols with iron and copper ions: Binding, dioxygen activation and oxidation mechanisms. Food Funct. 2014;5:1186–1202. doi: 10.1039/C4FO00007B. [DOI] [PubMed] [Google Scholar]
- 139.Engelmann M.D., Hutcheson A.R., Cheng I.F. Stability of Ferric Complexes with 3-Hydroxyflavone (Flavonol), 5,7-Dihydroxyflavone (Chrysin), and 3‘,4‘-Dihydroxyflavone. J. Agric. Food Chem. 2005;53:2953–2960. doi: 10.1021/jf048298q. [DOI] [PubMed] [Google Scholar]
- 140.Guo M., Perez C., Wei Y., Rapoza E., Su G., Bou-Abdallah F., Chasteen N.D. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007:4951–4961. doi: 10.1039/b705136k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lakey-Beitia J., Burillo A.M., La Penna G., Hegde M.L., Rao K. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021;82:S335–S357. doi: 10.3233/JAD-200185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang R., Tian J., Liu Y., Zhu L., Sun J., Meng D., Wang Z., Wang C., Zhou Z., Chen L. Interaction mechanism of ferritin protein with chlorogenic acid and iron ion: The structure, iron redox, and polymerization evaluation. Food Chem. 2021;349:129144. doi: 10.1016/j.foodchem.2021.129144. [DOI] [PubMed] [Google Scholar]
- 143.Chatoui K., Harhar H., El Kamli T., Tabyaoui M. Chemical Composition and Antioxidant Capacity of Lepidium sativum Seeds from Four Regions of Morocco. Evidence-Based Complement. Altern. Med. 2020;2020:1–7. doi: 10.1155/2020/7302727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lesjak M., Hoque R., Balesaria S., Skinner V., Debnam E.S., Srai S.K.S., Sharp P.A. Quercetin Inhibits Intestinal Iron Absorption and Ferroportin Transporter Expression In Vivo and In Vitro. PLoS ONE. 2014;9:e102900. doi: 10.1371/journal.pone.0102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Basu T., Panja S., Shendge A., Das A., Mandal N. A natural antioxidant, tannic acid mitigates iron-overload induced hepatotoxicity in Swiss albino mice through ROS regulation. Environ. Toxicol. 2018;33:603–618. doi: 10.1002/tox.22549. [DOI] [PubMed] [Google Scholar]
- 146.Ma Q., Kim E.-Y., Han O. Bioactive Dietary Polyphenols Decrease Heme Iron Absorption by Decreasing Basolateral Iron Release in Human Intestinal Caco-2 Cells. J. Nutr. 2010;140:1117–1121. doi: 10.3945/jn.109.117499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.