Abstract
Glioblastoma (GBM) is a devastating type of brain tumor, and current therapeutic treatments, including surgery, chemotherapy, and radiation, are palliative at best. The design of effective and targeted chemotherapeutic strategies for the treatment of GBM require a thorough analysis of specific signaling pathways to identify those serving as drivers of GBM progression and invasion. The Wnt/β-catenin and PI3K/Akt/mTOR (PAM) signaling pathways are key regulators of important biological functions that include cell proliferation, epithelial–mesenchymal transition (EMT), metabolism, and angiogenesis. Targeting specific regulatory components of the Wnt/β-catenin and PAM pathways has the potential to disrupt critical brain tumor cell functions to achieve critical advancements in alternative GBM treatment strategies to enhance the survival rate of GBM patients. In this review, we emphasize the importance of the Wnt/β-catenin and PAM pathways for GBM invasion into brain tissue and explore their potential as therapeutic targets.
Keywords: glioblastoma, Wnt/β-catenin, PI3K/Akt/mTOR, autophagy, GBM survival
1. Introduction
Glioblastoma multiforme (GBM) is a type of fatal primary brain tumor in adults, with a median survival duration of about 16 months. Over the previous three decades, survival rates for patients diagnosed with GBM have improved by a mere three months. This is due to the locally aggressive and invasive nature of GBM, the ability of GBM cells to develop therapeutic resistance, and the limited number of drugs capable of reaching therapeutic concentrations in the brain [1,2]. Traditional interventions of surgery, radiation and temozolomide (TMZ) that attempt to reduce tumor mass and attenuate GBM proliferation have failed and have promoted the recurrence of therapy-resistant GBM cells [1,3,4,5]. Because a significant proportion of GBM tumors recur, there is an urgent need for more effective therapeutic strategies [2,6].
Various biochemical changes take place during the epithelium-to-mesenchymal transition (EMT), resulting in the loss of the epithelial structure (at the tissue level) and the development of a non-polar mesenchymal phenotype (at the cellular level). Acquiring a more mesenchymal-like phenotype is associated with greater cellular motility and reduced intercellular adhesion. The latter phenotype promotes migration, invasion, and metastasis [7]. In analogy to other solid tumors, interconnected signaling networks and their downstream genetic regulators have been shown to alter gene transcription and contribute to mesenchymal phenotypes in GBM [6,8]. The wingless/integrated (Wnt)/β-catenin pathway was found to be most active (as assessed by β-catenin nuclear staining) in 30 GBM patient specimens of the mesenchymal subtype [9]. The canonical Wnt pathway is activated when canonical Wnt ligands bind to frizzled (FZD) receptors. This inhibits GSK-3 and prevents β-catenin from being phosphorylated, which results in its ubiquitination and proeasomal degradation. In primary GBM cells, active Wnt/β-catenin signaling increases the expression of EMT activators such as zinc-finger E-box binding homeobox 1 (ZEB1), twist-related protein 1 (TWIST1), and snail-related zinc-finger transcription factor (SLUG1), and promotes the in vitro migration capacity [9,10,11,12]. Importantly, active Wnt/β-catenin signaling has been associated with decreased GBM patient survival. This emphasizes a need for further research to identify the exact roles of canonical Wnt signaling in GBM onset, progression, and recurrence [13]. The phosphoinositol 3-kinase (PI3K)/Akt signaling pathway is instrumental for cell proliferation, stem cell maintenance, and tumor formation [14,15]. Malignant glioma cells produce LIM and SH3 protein 1 (LASP1), LIM, and SH3 protein 1, all of which have been demonstrated to enhance cell proliferation and invasion by activating the PI3K/Akt/snail signaling cascade. The combination of PI3K/Akt inhibitors and TMZ was shown to disrupt the LASP1/PI3K/Akt/snail signaling pathway and significantly suppress tumor progression [16]. In IDH1 mutant glioma, PI3K/mammalian target of rapamycin (mTOR) suppression led to decreased production of the oncometabolite 2-hydroxyglutarate (2HG), and this is associated with improved survival [17].
GBM cells employ the Warburg effect by metabolizing glucose via glycolysis rather than oxidative phosphorylation, even under aerobic conditions, and this is further evidence for the functional involvement of PI3K signaling in GBM cells. The rate of glucose metabolism in human glioblastoma cells is linked to Akt activity, and cells expressing Akt are more vulnerable to mortality after glucose removal. As shown in glioma cells highly expressing Akt, glucose deprivation impairs fatty acid oxidation and causes cell death. Interestingly, stimulation of fatty acid oxidation with 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) is sufficient to sustain cell viability, indicating that activating Akt impairs the ability of GBM cells to metabolize non-glucose substrates, thus promoting glucose addiction. Akt also contributes to glycolysis regulation by controlling the localization of the glucose transporter 1 (GLUT1) at the plasma membrane and increasing the activity of the key glycolytic enzyme hexokinase (HK). Normally, when growth hormones are removed, cytochrome c is released from the mitochondria, and the caspase cascade is initiated. The pro-apoptotic protein Bax is glucose-sensitive and increasing glycolysis inhibits its activation. Thus, glucose-dependency enables cancer cells with active PI3K/Akt signaling to escape apoptosis in the absence of growth factors [18,19,20,21].
Despite the prevalence and apparent reliance on PI3K signaling in glioma, PI3K inhibitors exert cytostatic rather than cytotoxic effects in glioma cell lines and xenografts even when combined with inhibitors of the epidermal growth factor receptor (EGFR) and mTOR [22]. Thus, the inhibition of PI3K may activate other pathways and processes, such as Wnt/β-catenin and EMT, respectively, both of which enhance cell survival. Combining PI3K inhibitors with drugs blocking these pathways may promote GBM apoptosis.
2. Glioblastoma Invasion: Programmed Signaling Pathways
2.1. Wnt Pathway
Wnt signaling regulates the self-renewal, proliferation, and differentiation of neural progenitor cells (NPCs) in the brain during different stages of central nervous system (CNS) development. GBM and other cancers (e.g., of the digestive system) have been related to abnormal Wnt pathway activity. Wnt signaling can be subdivided into two categories: canonical and non-canonical (β-catenin independent). A function for the canonical pathway in the maintenance, expansion, and lineage determination of stem/progenitor cell pools has been demonstrated in embryonic and adult tissues. During gastrulation and development, the non-canonical Wnt pathway regulates cell motility and tissue polarity, as well as convergent extension motions of epithelial and neuronal cells [6].
LRP5/6 (low-density lipoprotein receptor-related protein 5, 6) is activated by the canonical Wnt components after binding to cell surface receptors such as FZD. Destabilization occurs when Wnt-binding receptors activate another key complex comprising the APC tumor suppressor gene, glycogen synthase kinase-3 (GSK3), and Axin. This results in the stabilization of β-catenin, which then moves to the nucleus, forms a compound with T cell factor/lymphocyte enhancer factor, and activates Wnt-target genes, including cyclin-D, c-Myc, and VEGF, all of which are essential for embryonic development [23].
The non-canonical pathway can be split into two parts. The disheveled (DVL)-c-Jun N terminal kinase (JNK) pathway/planar cell polarity pathway is involved in cellular polarity. When Wnt binds to FZD via DVL, the JNK kinase pathway is triggered, and this results in cytoskeletal changes. DVL and phospholipase C (PLC) activation by the interaction of Wnt proteins to FZD receptors leads to inositol 1,4,5-triphosphate (IP3) activation through the Ca2+-mediated pathway. Activation of protein kinase C, cell division cycle 42 (Cdc42), mitogen-activated protein kinase 7 (MAP3K7), Ca2+/calmodulin-dependent protein kinase II (CAMKII), Nemo-like kinase (NLK), and nuclear factor of activated T cell (NFAT) is triggered when IP3 binds to Ca2+ channels in the endoplasmic reticulum [23] (see Figure 1).
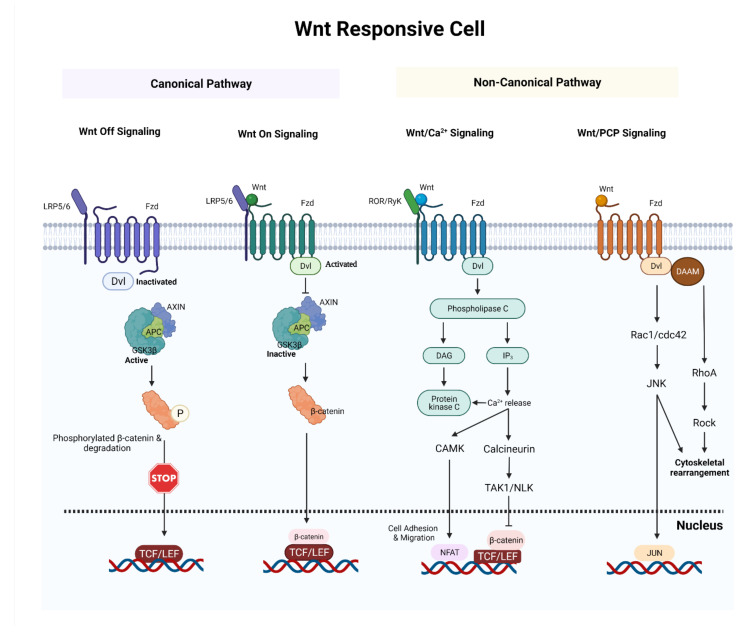
Figure 1.
A synopsis of the canonical and noncanonical Wnt signaling pathways. In canonical Wnt off signaling, a combination of AXIN and APC allows GSK3β to phosphorylate β-catenin and target it for proteolysis. In canonical Wnt on signaling, Wnt ligands bind and activate Fzd receptor and activated Fzd receptor recruits Dvl protein and AXIN. This blocks the formation of an AXIN–APC complex and inhibits GSK3β. As a result, β-catenin avoids destruction and accumulates in the nucleus. In non-canonical Wnt/Ca2+ signaling, the interaction of Wnt/Fzd with ROR/Ryk co-receptor leads to the formation of IP3 and DAG. DAG activates PKC. IP3 triggers ER Ca2+ release which stimulates calcineurin and CAMKⅡ. This activates TAK1/NLK and NFAT. Activated TAK1/NLK can inhibit TCF/β-catenin signaling and NFAT can regulate cell adhesion and migration. In non-canonical Wnt/PCP signaling, the interaction of Wnt/Fzd leads to the recruitment of Dvl, which utilizes its domains (PDZ and DIX) to produce a complex with DAAM. DAAM then stimulates RhoA. Activated RhoA can activate Rock. Dvl can also produce a complex with Rac1 to activate JNK. Abbreviations: APC, adenomatous polyposis coli; GSK3β, glycogen synthase kinase 3β; LRP5/6, low-density lipoprotein receptor-related protein 5/6; TCF/LEF, T-cell factor/lymphoid enhancer-binding factor; IP3, inositol trisphosphate; DAG, diacylglycerol; CAMKⅡ, calcium/calmodulin-dependent protein kinase II; TAK1, transforming growth factor beta-activated kinase 1; NLK, nemo like kinase; NFAT, nuclear factor of activated T-Cells; Dvl; disheveled; Fzd, frizzled; DAAM, disheveled-associated activator of morphogenesis, ROCK, Rho-associated kinase. (Created with https://biorender.com/, accessed on 18 December 2021).
2.1.1. Wnt Signaling in Glioblastoma
Wnt has a role in preserving the stemness of normal stem cells, enabling repair and regeneration. Dysregulation of Wnt signaling has been reported in several cancers [24]. Mutations in Wnt system components, including APC, β-catenin, AXIN, WTX, and TCF4, may be responsible for Wnt pathway activation in certain malignancies [24]. Colorectal cancers have the highest number of Wnt signaling mutations, and nearly 85% of colorectal cancers have a loss-of-function mutation in APC, while 50% of tumors without an APC mutation have an activating mutation in β-catenin. Mutations were also detected in APC, β-catenin, and AXIN1 in colon cancer and medulloblastoma. In GBM, ovarian, head and neck, breast, and stomach cancers, abnormalities of key components of the Wnt pathway are less common [24]. APC mutations were found in roughly 13% of GBM patients, with a mutation frequency of approximately 14.5%, based on recent studies from a small cohort [19]. Another investigation discovered a homozygous loss of atypical protocadherin FAT1, a tumor suppressor and negative regulator of the Wnt pathway [24]. A dysregulated Wnt pathway may lead to the emergence of cancer stem cells with enhanced aberrant proliferation [25,26,27,28].
2.1.2. Involvement of Wnt Signaling in Glioblastoma Invasion
β-catenin regulates cell–cell adhesion by binding to cadherin as part of the cell adhesion complex. Increased binding of β-catenin to cadherin as well as α-catenin to cadherin or β-catenin promotes glioma cell migration and EMT, and this is mediated by catenin phosphorylation of particular tyrosine residues induced by growth factor signaling. EGFR expression is increased in early GBM, and EGF ligand/EGFR signaling through extracellular signal-regulated kinases 1/2 (ERK1/2) and casein kinase2 (CK2) causes phosphorylation of β-catenin at serine 641 in glioma cells, which has been linked to glioma malignancy [26]. Glioma cell migration and transactivation are aided by β-catenin phosphorylation. Increased expression of the Fz antagonist sFRP2 was shown to prevent glioma invasion by reducing tyrosine phosphorylation of β-catenin and downregulating matrix metalloprotease-2 (MMP-2). Cancer invasion and metastasis have been linked to non-canonical Wnt-5a. For example, Wnt-5a enhances the migration of glioma cells by altering the synthesis of MMP-2 through β-catenin-independent signaling; this includes pathways regulating planar cell polarity and calcium dynamics. U251 astrocytoma cells are less invasive when Wnt-2, Wnt-5a, and Fz2 expression levels are suppressed. Thus, the β-catenin and Wnt signaling pathways seem to regulate selective metalloprotease regulation as an important downstream target to facilitate glioma invasion [26].
2.1.3. Emerging Links between Wnt Signaling and Autophagy in Glioma
An intricate relationship exists between Wnt signaling and autophagy. Canonical Wnt/β-catenin activity inhibits autophagy and the production of the autophagic adaptor p62. Nutrient deprivation promotes the destruction of β-catenin through the autophagic machinery [24,29,30]. Intriguingly, autophagy activation inhibits Wnt/β-catenin signaling in glioblastoma cells through β-catenin subcellular re-localization. The induction of autophagy increases the interaction of β-catenin and N-cadherin in glioma cells and is likely responsible for the formation of newly generated N-cadherin-mediated cell–cell junctions [31]. LC3 targets β-catenin and DVL for autophagic breakdown during nutritional restriction. Interestingly, β-catenin serves as a co-repressor of p62 when Wnt signaling is active. GSK3β is a critical inhibitory regulator of Wnt signaling and has been demonstrated to promote autophagy through phosphorylation of the tuberous sclerosis complex (TSC) [24,32] (see Figure 2).
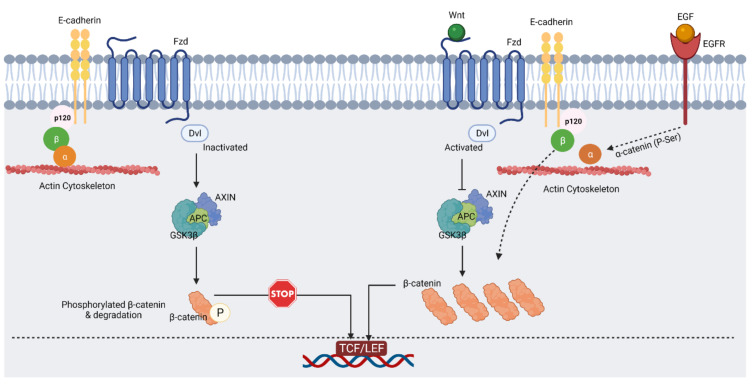
Figure 2.
In epithelial cell–cell adhesion and Wnt signaling, the E-cadherin/β-catenin complex plays a critical role. In the absence of Wnt ligand (on the left), activated GSK3β can phosphorylate β-catenin, which leads to its proteasomal degradation. As result of that, β-catenin remains in the cadherin/β-catenin/α-catenin complex that is attached to the cytoskeleton (created with https://biorender.com/, accessed on 12 September 2021). In the presence of Wnt ligand (on the right), inactivated GSK3β cannot phosphorylate β-catenin, and this results in free β-catenin and its nuclear accumulation. EGF signaling via EGFR, ERK1/2, and CK2 leads to phosphorylation of α-catenin and enhances β-catenin transactivation [26,33]. Abbreviations: EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ERK1/2, extracellular signal-regulated protein kinase; CK2, casein kinase 2. (Created with https://biorender.com/, accessed on 9 December 2021).
3. Different Forms of Epithelial-Mesenchymal Transition (EMT)
When polarized epithelial cells undergo EMT, they transform into mesenchymal-like cells with a greater capacity to migrate and resist genotoxic substances. Wound healing, embryonic development, and tissue remodeling all require EMT. The EMT process transforms epithelial tumor cells so that they detach from the basement membrane, becoming migratory and invasive without impairing viability. Glioma mesenchymal features may be triggered by similar factors that induce EMT and EMT and play a crucial role in the development of cancer stem cells. Typically, GBM with a mesenchymal subtype behaves aggressively and exhibits markers of neural stem cells. Glioma stem-like cells are extremely invasive and resistant to chemotherapy and radiation in both in vitro and clinical settings [34,35,36]. EMT can be classified into EMT types 1, 2, and 3. Type 1 is necessary for the transformation of primitive embryonic epithelial cells into motile mesenchymal cells, which are necessary for neural crest cell gastrulation and migration. Type 1 EMT plays a critical role throughout embryogenesis in generating morphologically and functionally diverse cell types [37]. EMT type 2 causes the development of excessive fibrosis during wound healing and tissue regeneration [38]. EMT type 3 can be described as a process in which subsets of cancer cells undergo phenotypic alterations that promote invasion, migration, and metastasis. Several studies have indicated that transforming growth factor (TGF) may promote EMT in epithelial cancer cells through the Smad or p38 mitogen-activated protein kinase/Ras homolog family member A pathways [39]. The activation of EMT processes by cues from the tumor microenvironment has also been hypothesized as a key pathway for cancer cells to acquire highly malignant phenotypes. Cancer cells with a transitional mesenchymal phenotype can undergo type 3 EMT and form metastatic tumor nodules at distant locales. While metastasis seldomly occurs in GBM, it is common in EMT [34,40]. In addition, autophagy modulates the transition from an epithelial to a mesenchymal phenotype in non-small cell lung cancer cells through TGFβ 1, and similar processes may be at play in glioma [7,41].
A Wnt Perspective of EMT in Glioblastoma Invasion
Twist is a transcriptionally active protein involved in cell differentiation and lineage determination. Mesenchymal progression of glioma cells results in the loss of adhesion molecules and cytoskeletal alterations. Twist operates independently of snail to repress E-cadherin and upregulate both N-cadherin and fibronectin through EMT during cancer invasion. Using orthotopic mouse models and brain slice cultures, twist was shown to increase in malignant gliomas and enhance glioma cell invasion via the mesenchymal target gene slug and fibroblast activation protein (FAP). Xenotransplantation experiments revealed that this occurred independently of the cadherin switch. Targeted suppression of twist expression results in a considerable decrease in GBM stem cell sphere formation [42,43,44,45].
E-cadherin expression is regulated by snail, a member of the snail family of transcriptional regulators. Snail also regulates the expression of epithelial (occludins, claudins, and cytokeratin) and mesenchymal markers during EMT (vitronectin and fibronectin). The transcriptional activity of snail is greatly influenced by its subcellular location. After phosphorylation, snail is exported from the nucleus to the cytoplasm and ceases to act as a transcription factor. In response to irradiation, mesenchymal cells release TGF-β, which stimulates snail nuclear localization through the Smad2/3 pathways [34,46,47]. Slug is another transcriptional activator of the snail family, and has been shown to play a key role in inhibiting the epithelial phenotype in a variety of cancer cells. Slug increases the migration and invasion of malignant gliomas. By profiling mRNA expression levels, The Cancer Genome Atlas (TCGA) identified slug and cluster of differentiation (CD)-44 as mesenchymal transition hallmarks in a wide range of cancers [34]. Modulation of autophagy can affect the migratory and invasion capacities of GBM cells by downregulating the EMT proteins snail and slug and, as a result, upregulate cadherin expression [48]. Hyperactivation of the mTORC1/2 complexes hampers autophagy and activates the Wnt pathway, enabling β-catenin to translocate to the nucleus and increase the transcription of pro-invasive factors. Cadherin is expressed and repressed in this situation by EMT players of the SNAI family. Genetic silencing of autophagy-related genes exacerbates the mesenchymal phenotype and increases the capacity of cells to migrate. When autophagy is induced, DVL is destroyed, and the Wnt pathway is inactivated. This results in the cytoplasmic accumulation of β-catenin. SNAI factors are downregulated in autophagic cells and, as a result, N-cadherin accumulates and binds β-catenin [49]. The ZEB proteins ZEB1 and ZEB2 are another notable family of transcription factors that mediate EMT in a variety of tumors, including gliomas [50]. ZEB proteins attach to the E-cadherin promoter region and inhibit the expression of this adhesion molecule, which causes diminished cell–cell interaction and increased tumor cell motility. Recurrences are significantly more common in GBM patients with high ZEB2 levels, and glioma cells become more invasive when activated nuclear factor-B induces ZEB1 expression [34,51,52,53].
RNA-based epigenetic regulation of EMT in GBM is an emerging area with potential therapeutic implications. The long noncoding RNA LINC-PINT inhibits GBM cell proliferation, invasion, and EMT by interfering with Wnt/β-catenin signaling [54]. The miR-101-3p suppresses EMT in GBM by targeting the EMT-promoting factor tripartite motif containing 44 (TRIM44). miR-101-3p suppresses the expression of TRIM44 by directly targeting its 3′UTR [55]. Another microRNA, miR 205, targets ZEB1 via the Akt/mTOR signaling pathway, and miR205 downregulation affects GBM cell motility, invasion, and EMT [56]. Small nucleolar RNA host gene 7 (SNHG7) increases GBM cell proliferation, motility, and invasion by suppressing miR-5095 and by concurrently stimulating the Wnt/β-catenin signaling pathway. miR-5095 and SNHG7 were shown to inversely regulate the Wnt/β-catenin signaling pathway in GBM [57]. Additionally, exosomal miR-133b generated from mesenchymal stem cells inhibits glioma development through the Wnt/β-catenin signaling pathway by targeting EZH2 [58]. Furthermore, miR-128-3p increases TMZ chemosensitivity in GBM by targeting c-Met and EMT [59].
4. Therapeutic Approaches to Decrease the Invasion and Survival of GBM Cells by Inhibiting Wnt/β-Catenin and Emt
Adaptive radio-resistance in GBM patients is mediated by the IGF1/N-cadherin/β-catenin/clusterin signaling axis. The accumulation of β-catenin at the cell surface caused by increased N-cadherin expression attenuates the signaling of Wnt/β-catenin and reduces neural cell growth. Transfection of wild type N-cadherin restored radio-resistance in N-cadherin knockout glioblastoma stem cells (GSCs) but not in mutant N-cadherin GSCs missing the β-catenin binding region, which demonstrates the functional significance of the interaction between N-cadherin and β-catenin. Additionally, N-cadherin enhances clusterin secretion to protect GSCs from death after radiotherapy, and N-cadherin deficiency and reduced clusterin release increase the sensitivity of cells to radiation treatment. Radiation was also shown to induce IGF1 secretion, which resulted in enhanced N-cadherin expression, causing an EMT-like phenotypic shift in GSCs [60].
The pharmacological inhibition of the Wnt pathway with the porcupine inhibitor LGK974 acts synergistically with TMZ to decrease glioma cell growth in vitro and is independent of the methylation status of the O6-alkylguanine DNA alkyltransferase (MGMT) promoter [61]. Further transcriptomic analyses revealed that cells treated with LGK974 and TMZ showed substantially decreased expression of aldehyde dehydrogenase 3A1 (ALDH3A1). Suppression of ALDH3A1 expression improved TMZ effectiveness and lowered glioma stem cell clonogenicity, as evidenced by decreased expression of the stem cell markers CD133, Nestin, and Sox2 [61].
Accumulating evidence suggests that TMZ therapy may promote autophagy, which could contribute to GBM therapeutic resistance. The absence of DOC-2/DAB2 interacting protein (DAB2IP) was shown to be responsible for TMZ resistance in GBM through ATG9B. By negatively regulating ATG9B expression, DAB2IP sensitized GBM to TMZ and inhibited TMZ-induced autophagy. ATG9B expression was much higher in GBM than in low-grade glioma. In GBM cells, silencing ATG9B expression inhibited both TMZ-induced autophagy and TMZ resistance. Additionally, DAB2IP decreased ATG9B expression by inhibiting the Wnt/β-catenin pathway. In an attempt to maximize the therapeutic efficacy of TMZ while avoiding therapeutic resistance in GBM, combinatorial therapeutic strategies of TMZ plus a small molecule inhibitor of the Wnt/β-catenin pathway have been proven to act synergistically in GBM cells [62].
Lumefantrine, an FDA-approved antimalarial medication, was recently demonstrated to reverse radiation and TMZ resistance in GBM. Lumefantrine may be a promising GBM treatment option by inhibiting the friend leukemia integration 1 (Fli-1)/HSPB1/EMT/ECM remodeling protein networks. Fli-1 signaling has been implicated in GBM oncogenesis and is overexpressed in radio-resistant and TMZ-resistant GBM, where Fli-1 regulates HSPB1 transcriptionally. Lumefantrine binds Fli-1, and this induces apoptosis in vitro in parental and radio/TMZ-resistant GBM. Moreover, it reduced tumor development in vivo in U87MG glioma cells and radio/TMZ-resistant GBM orthotopic tumor models without systemic side effects [63].
5. PI3K/Akt/mTOR (PAM) Signaling Cascade in Glioblastoma Invasion
The extracellular matrix and cytokines transduce their signals via the activation of tyrosine kinase receptors (RTK) and cytokine receptors, which leads to downstream activation of the PAM pathway. PAM is the focus of many cancer studies, since this signaling pathway is frequently overactive in several cancers and has critical roles in cancer cell survival, proliferation, invasion, and migration [64,65,66].
5.1. PAM in Gliomagenesis
5.1.1. Role of PAM in Glioma Growth and Invasion
The PAM signaling pathway promotes tumor growth and progression by regulating cell cycle activities. IDH-wildtype GBM has mutations in the growth factor receptor/PI3K/Akt/mTOR pathway [67]. The RTK/PI3K/Akt/mTOR cascade has long been known to boost glioma invasiveness. Upon phosphorylation by Akt, activated mTOR causes cyclin D1 to bind to cyclin dependent kinase (CDK) and initiate cell division. This G1 to S transition can promote carcinogenesis if cyclin D1 is expressed at high levels. Although inhibition of CDK activity by P27kip1 can stall the cell cycle, causing growth arrest, Akt-mediated phosphorylation of P27kip1 neutralizes this critical cell cycle barrier so that cell growth and differentiation are unimpeded. PAM’s powerful metabolic control on the formation of macromolecules, such as proteins, nucleotides and lipids, ensures the rapid growth of cancer cells [66,68,69].
Active PAM signaling networks are operational in the fast majority of GBM [70]. This pathway appears to aid the development of an invasive phenotype of GBM and pediatric brain tumor medulloblastoma (MB) by enhancing motility and stress resistance [71]. The most important PI3K isoform for cell growth and survival is class IA PI3K p110α. In GBM, the gene encoding this isoform, PIK3CA, is often altered [72]. Under anchorage-independent circumstances, the mutant version of PIK3CA plays a key role in cell proliferation in this malignancy. In addition, this PI3K isoform is generally overexpressed in MB where it supports cell proliferation through regulation of the leukemia inhibitory factor receptor α (LIFR α). In MB, inhibiting p110α reduces cancer cell proliferation, migration, and survival [73,74]. Other functionally relevant PI3K class IA isoforms have also been reported in brain tumors. For example, primary GBM overexpress mRNA for p110δ, a class IA isoform that regulates cell motility in these cancer cells [75,76,77]. Other components of the PI3K/Akt pathway are currently being evaluated as possible targets for inhibiting cell growth and migration in GBM and MB. Among these is Akt, which is frequently phosphorylated in malignant brain tumors. This has already led to some promising in vitro observations indicating that Akt inhibition with KP-372-1, KP-372-2, A-443654, or perifosine suppresses cell growth and radio-sensitizes GBM and MB [75,78,79,80].
Phosphatase and tensin homolog (PTEN) inhibits cell growth and interferes with cellular metabolism by downregulating the PAM pathway, whereas inhibiting PTEN activity stimulates Akt and its downstream signaling [81]. In recent work, alterations to the PI3K pathway were found in 44% of the 60,991 solid tumors studied, and PTEN (9.4%) was the second most commonly changed gene after PI3K (13.3%). Changes in PTEN, primarily mutations and profound deletions, are common in uterine, glioblastoma, prostate, lung, and melanoma cancers, according to a pan-cancer restricted analysis of various tumors [82]. Particularly in brain and breast cancer patients, loss of PTEN function has been related to metastasis and a lack of response to radiotherapy and chemotherapy, demonstrating that PTEN is a critical regulator of tumor susceptibility to several treatment options [83,84,85]. Frequently detected in GBM, the level of expression of an inactive form of the tumor suppressor PTEN inversely correlates with tumor aggressiveness. In MB, PTEN is rarely altered, but Akt activity is frequently downregulated [79,86]. PTEN, along with the MAPK signaling cascade, plays a key role in the control of G1/S cell cycle checkpoint-defective astrocytoma invasion, and PTEN loss in GBM cell lines enhances migration, invasion, and resistance to apoptosis [87,88,89]. PTEN regulates Src family kinase activity in a PI3K/Akt-independent manner, and this involves integrin-dependent migration [87,88,89]. Re-expression of functional PTEN in GBM cell lines enhances the cellular amount and activity of the P53 tumor suppressor protein. This causes cell cycle arrest and boosts tumor cell susceptibility to chemotherapeutic drugs, such as the topoisomerase inhibitor etoposide [87,88,89,90].
mTOR has emerged as a key cell growth checkpoint in the PI3K signaling pathway. In the brain, mTOR signaling is mediated by mTORC1 and mTORC2. The mTORC1 signaling route is well recognized due to a wealth of information on mTOR signaling. However, the mTORC2 signaling pathway is less well characterized [91,92,93]. mTORC1 controls cell size and growth in response to nutrition levels, whereas mTORC2 controls cytoskeletal dynamics and the activation of Akt [94,95]. Most GBM exhibit hyper-activation of mTOR signaling [96], and aberrant activation of these particular pathways has been linked to poor prognosis in GBM patients [97]. Tuberous sclerosis complex 1/2 (TSC1/2) is a tumor suppressor. The RAS homolog enriched in brain (RHEB) GTPase activates this complex, which subsequently binds directly to mTORC1 and activates kinase activity [98]. The transcription intermediary factor 1-alpha (TIF-1A) is activated by mTORC1. TIF-1A enables RNA polymerase to transcribe rRNA genes and blocks the MAF1 polymerase III repressor, thus permitting the transcription of 5sRNA and tRNA. mTORC1 also increases protein synthesis and promotes the rapid turnover of lipids and nucleotides typically observed in GBM. Additionally, mTORC1 promotes tumor cell proliferation by suppressing autophagy [99,100]. Unlike mTORC1, mTORC2 cannot sense changes in nutritional state but is activated by growth factors [98]. The activation of various AGC protein kinases by mTORC2 also increases cell proliferation, motility, and survival. In addition to Akt, mTORC2 phosphorylates protein kinase C (PKC)δ, PKCζ, PCKγ, and PKCε, all of which are involved in cytoskeletal construction and cell migration [101,102,103,104,105,106]. Furthermore, the induction of the Warburg effect is mediated by mTORC2 activity. Indeed, mTORC2 stimulates the expression of GLUT4 and the activation of the glycolytic enzymes HK2 and phosphofructokinase-1 (PFK-1) via increasing Akt phosphorylation on serine 473 [107]. Lastly, it was shown that mTORC1 and mTORC2 inhibition may be a promising new treatment for GBM. mTORC2 activity is linked to GBM cell growth and motility. Inhibition of mTOR or PI3K blocks the activation of P70S6K at residue Thr389, which prevents platelet-derived growth factor (PDGF)- or fibronectin (FN)-induced activation of STAT3Ser727 upon combined mTOR and PI3K inhibition. Silencing rictor, a co-protein of mTORC2, but not raptor, a co-protein of mTORC1, lowered pAKT expression, and dual mTOR and PI3K inhibition was more effective in reducing cell growth and motility. Pre-treatment with the mTOR inhibitor rapamycin (RAPA) prevented PDGF-induced nuclear localization of mTOR [108].
5.1.2. PAM in Angiogenesis of Brain Tumors
Angiogenesis is a process that involves the formation of new blood vessels and is required for tumors to grow larger than 1 mm in diameter. By providing nutrition and oxygen to tumors, angiogenesis allows tumors to progress and become invasive [109]. GBM tumors have a poor prognosis because they are frequently highly vascularized tumors [110]. Several regulators of angiogenesis promote vascularization during GBM progression, including VEGF, basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), TGF-β, MMPs, and angiopoietins (Angs) [111]. PAM signaling has been identified as a key contributor to GBM angiogenesis [95]. Fibroblast growth factor receptor (FGFR) controls several angiogenic activities, including FGF-induced glioma endothelial cell migration and proliferation, and has been shown to exert its effect on GBM cell survival and angiogenesis through the PAM pathway [112,113]. Like in other cancers, EGFR is active in many GBM and controls tumor formation and angiogenesis [113,114,115]. The RAS/MAPK and PAM signaling pathways have emerged as critical regulators of glioma cell proliferation, differentiation, tumor angiogenesis, and survival in GBM [113,114,115]. It has been demonstrated that rapamycin- and mTOR siRNA-mediated inhibition of the HIF1α and mTOR signaling pathways may decrease the ability of GBM cells to acquire genetic and/or phenotypic characteristics of endothelial cells and form microvascular channels as a way to enhance blood supply to the tumor, called vasculogenic mimicry or trans-differentiation. This supports the notion that mTOR may be a viable therapeutic target in GBM [107]. The triterpenoid celastrol suppresses vasculogenic mimicry formation and angiogenesis in glioma via modulating PAM signaling [116]. The cytotoxic impact of radiation and TMZ in GBM cells is enhanced when targeting the RTK/PI3K/Akt pathway [117]. Loss of PTEN leads to VEGFR2 expression in GBM tumors and may contribute to the failure of anti-angiogenic treatments in GBM [118]. In addition, the overexpression of VEGFR2 in tumor cells might cause early resistance to TMZ and anti-angiogenesis treatment with bevacizumab in GBM patients [119]. In both normal and cancer cells, EGFR stimulates the PI3K/Akt pathway, and EGFR gene amplification in glioma cells results in constitutive PI3K activation [120]. Inhibiting EGFR using EGFR inhibitors PD153035 or AG1478 resulted in the inactivation of this signaling arm [121]. EGFR/PI3K/mTOR inhibitory compounds as well as drugs targeting the Wnt/β-catenin signaling pathways utilized in preclinical and/or clinical investigations for glioma treatment were adapted from [122,123] review papers, respectively (see Figure 3).
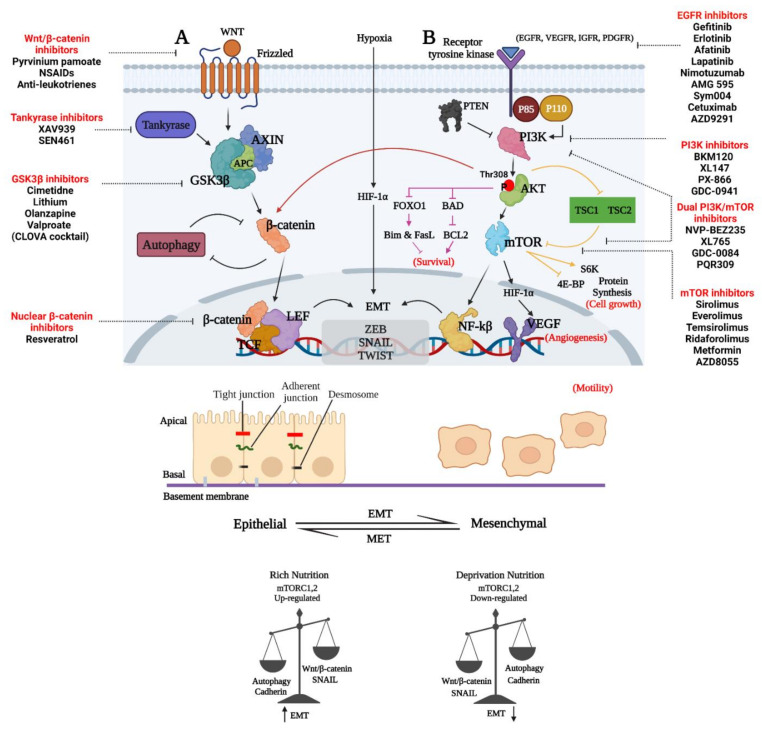
Figure 3.
Representative image of GBM invasion through the regulation of the interconnected Wnt/β-catenin, PAM, and EMT signaling pathways. (A) Disheveled protein (DVL) enhances the phosphorylation of LRP5/6 co-receptor and the recruitment of axin to the membrane. DVL is recruited in the presence of WNT ligands (WNT1, WINT3A and WNT8) bound to FZD. Non-phosphorylated β-catenin may enter the nucleus when the destruction complex is dissociated to form a complex with the TCF/LEF proteins [124]. (B) PI3K regulator (p85) and catalytic isoform (p110) are released from inhibition when growth factors interact with their cognate RTK, resulting in the activation of PI3K. An inhibitor of PTEN may prevent PIP2 synthesis from PIP3, a process catalyzed by PI3K. The PH domain of Akt is then recruited to the plasma membrane by PIP3 and forms an Akt-PIP3 complex. To alleviate the inhibitory effect of TSC1-TSC2 on mTORC1, Akt phosphorylates and inhibits TSC2. Prolonged tumor cell survival, proliferation, migration, invasion, and glucose metabolism are all promoted by activated Akt phosphorylation of downstream system components. The Akt–mTORC1 axis also influences protein synthesis and cell proliferation. An additional negative feedback loop is created when the mTORC1 pathway is activated [122]. Abbreviations: APC, adenomatous polyposis coli; CK1α, casein kinase 1α; GSK3β, glycogen synthase kinase 3β; LRP5/6, low-density lipoprotein receptor-related protein 5/6; β-TREP, beta-transducing repeat-containing protein; TCF/LEF, T-cell factor/lymphoid enhancer-binding factor; TSC1, TSC Complex Subunit 1; 4E-BP, 4E-binding protein 1; FOXO1, forkhead box O1; S6K, ribosomal protein S6 kinase; PDGFR, platelet-derived growth factor receptor; IGFR, insulin-like growth factor 1 receptor; VEGF, vascular endothelial growth factor. (Created with https://biorender.com/, accessed on 25 July 2021).
6. Therapeutic Approaches Targeting PAM in GBM
Several studies have associated over-active mTOR signaling with GBM, and blocking downstream effects mTOR has emerged as a popular treatment strategy [125,126]. The repurposing of several drugs originally intended for other diseases may have promise for treating GBM. The biguanidine drug metformin is typically used to treat type 2 diabetes and acts by activating AMP-activated protein kinase (AMPK) to reduce hepatic glucose synthesis. Activated AMPK is a well-recognized regulator of mTOR activity by phosphorylating and activating TSC2 [127]. There is evidence that metformin has the ability to sensitize GBM and GBM stem cells to TMZ, and this has served as a rationale in an early clinical trial for the treatment of GBM [128,129]. Pimozide is an FDA-approved diphenylbutylpiperidine-class antipsychotic drug that can target STAT5. Pimozide was shown to inhibit Fn14 expression in a STAT5-dependent manner by promoting the TNFR family member FGF inducible 14 (Fn14). Fn14 is known to facilitate cancer cell invasion and survival. One application for pimozide may be as a classical GBM subtype. Excessive STAT5 signaling downstream of constitutively active EGFR variant III (EGFRvIII) was capable of inhibiting the migration and survival of these GBM cells in a STAT5-dependent manner [130]. In addition, the combination of TMZ and pimozide has also been demonstrated to be more effective than TMZ alone.
Chlorpromazine is an antipsychotic medication used for the treatment of schizophrenia and bipolar disorder. Recently, chlorpromazine treatment of C6 glioma cells was shown to arrest the cell cycle in the G2/M phase through transcriptional activation of p21 (Waf1/Cip1) [131]. This transcriptional activation was independent of p53, but involved activation of early growth response-1 (EGR-1). Additionally, chlorpromazine inhibited PAM signaling, resulting in caspase-independent cell death [131]. In other work, TMZ and the combination of endothelial-monocyte-activating polypeptide-ii and miR-590-3p/MACC1 was demonstrated to block PAM signaling in human GBM stem cells [132].
The activation of RTK leads to downstream stimulation of the PI3K/Akt pathway, but RTK inhibitors have shown poor clinical efficacy. It has been proposed that PI3K signaling inhibitors may be helpful in GBM. Buparlisib, a well-tolerated and blood–brain barrier (BBB)-permeable panPI3K inhibitor, attenuates GBM cell growth in vitro and GBM tumor formation in vivo [133,134]. Buparlisib is the most frequently utilized panPI3K inhibitor in clinical studies for GBM therapy, but this drug showed limited efficacy as a single agent in recurrent glioblastoma phase II studies [135]. Sonolisib is an irreversible wortmannin analogue that inhibits PI3K for longer time periods than wortmannin. Sonolisib reduced invasion and angiogenesis in GBM cell lines and improved survival in vivo in orthotopic xenograft models [136,137]. Despite these encouraging preclinical findings, a phase II trial in patients with recurrent GBM had low response rates for sonolisib and no increase in the survival of recurrent GBM patients [138].
The Akt, RAF/ERK, and STAT3 pathways are mainly regulated by EGFR in GBM [139,140]. EGFR mutations increase the invasion and proliferation of GBM cells by activating ERK1/2 and matrix metallopeptidase 1 (MMP1) signaling [139]. Paradoxically, first- and second-generation EGFR inhibitors had limited to no effect on GBM. AZD9291 is the first novel and irreversible third-generation anti-EGFR agent that shows higher efficacy than other EGFR inhibitors in suppressing GBM cell activity and prolonging the life of mice with orthotopic tumor grafts of GBM [141]. While AZD9291 decreases RTK function permanently through covalent EGFR binding, adenosine triphosphate (ATP) analogs and the EGFR inhibitors gefitinib and erlotinib compete reversibly with ATP at the EGFR and require high concentrations to show appreciable drug effects [141,142,143,144,145].
PI3K inhibitors are typically categorized based on their isoform selectivity into pan-PI3K, isoform-selective, and dual PI3K/mTOR inhibitors. At present, approximately 50 PI3K inhibitors are being developed and manufactured for cancer therapy. Table 1 summarizes the characteristics and structural formulae of the most widely used and new PI3K inhibitors. Of note, only a few of these candidates, including BKM120, XL147, and XL765, have thus far entered clinical trials for the treatment of GBM [146,147] (Table 1).
Table 1.
PI3K inhibitors and their potential therapeutic use in GBM [146].
| Classification | Drug Name | IC50 (nM) | References | |||||
|---|---|---|---|---|---|---|---|---|
| p110α | p110β | p110δ | p110γ | mTORC1/2 | ||||
| Pan-PI3K inhibitors | Pictilisib | 3 | 33 | 3 | 75 | 580 | Inhibits tumor proliferation | [148,149] |
| Taselisib | 0.29 | 9.1 | 0.12 | 0.97 | 1200 | Inhibits tumor proliferation | [150] | |
| Buparlisib | 52 | 166 | 116 | H | 4600 | Apoptotic cell death and p53 deleted cells | [134,151] | |
| Pilaralisib | 39 | 383 | 36 | 23 | >15,000 | Inhibits tumor proliferation | [152] | |
| Copanlisib | 0.5 | 3.7 | 0.7 | 6.4 | 45 | Induces apoptosis | [153,154] | |
| Sonolisib | 0.1 | >300 | 2.9 | N/A | N/A | Complete tumor growth control in the early stages of treatment | [155] | |
| ZSTK474 | 16 | 44 | 4.6 | 49 | >10,000 | Cell apoptosis | [156,157] | |
| AMG511 | 4 | 6 | 2 | 1 | N/A | Inhibits tumor growth | [158] | |
| Isoform-selective inhibitors | Alpelisib | 5 | 1200 | 290 | 250 | >9100 | Inhibits tumor growth | [159] |
| Idelalisib | 820 | 565 | 2.5 | 89 | >1000 | Suppresses GBM cell proliferation and migration | [160,161] | |
| AMG319 | 3300 | 2700 | 18 | 850 | N/A | [162] | ||
| AZD6482 | 870 | 10 | 80 | 1090 | N/A | PI3K inhibition as anti-platelet therapy; mild generalized anti-platelet effect | [163] | |
| CH5132799 | 14 | 120 | 500 | 36 | 1600 | Potent antiproliferative activity | [164] | |
| AS-605240 | 60 | 270 | 300 | 8 | N/A | Leukocyte activation and migration | [165] | |
| MLN1117 | 15 | 4500 | 1900 | 13,390 | 1670 | p110α inhibition; attenuated B cell receptor (BCR)-dependent AKT activation, proliferation, and survival when supported by cytokines BAFF and IL-4 | [166] | |
| Dual PI3K/mTOR inhibitors | Dactolisib | 4 | 75 | 7 | 5 | 6 | Effective and specific blocking of dysfunctional PI3K activation in human tumor cell models; G1 arrest | [167,168] |
| NVP-BGT226 | 4 | 63 | N/A | 38 | N/A | p110α inhibition; attenuated B cell receptor (BCR)-dependent AKT activation, proliferation, and survival when supported by cytokines BAFF and IL-4 | [166] | |
| Omipalisib | 0.019 | 0.13 | 0.024 | 0.06 | 0.18/0.3 | mTOR inhibitor augmenting anti-proliferative efficacy of PI3K/AKT pathway inhibition | [169] | |
| Voxtalisib | 39 | 113 | 41 | 9 | 160/910 | Anti-proliferative, anti-angiogenic, pro-apoptotic | [170] | |
| Apitolisib | 5 | 27 | 7 | 14 | 17 | mTOR inhibition | [171] | |
| GDC-0084 | 2 | 46 | 3 | 10 | 70 | PI3K inhibition | [172] | |
| VS-5584 | 16 | 68 | 42 | 25 | 37 | anti-proliferative | [173] | |
| PF-04691502 | 1.8 | 2.1 | 1.6 | 1.9 | 16 | Inhibits cell proliferation | [174] | |
| Gedatolisib | 0.4 | N/A | N/A | 5.4 | 1.6 | Inhibits PI3K/Akt signaling; attenuates proliferation, survival, protein synthesis, and glucose metabolism | [175] | |
Bevacizumab was approved by the FDA in 2009 for use as a monotherapy or in combination with chemotherapy in the second line treatment of recurrent GBM [176,177]. Bevacizumab inhibits the neovascularization of tumors by neutralizing the impact of VEGF-A but cannot prevent vasculogenic mimicry or the supply of blood from pre-existing vasculature [178,179]. Indeed, histological examination revealed that bevacizumab treatment normalized the vascular structure, reduced microvessel density, and enhanced tumor oxygenation [180]. When used as a first-line therapy, bevacizumab caused an increase in symptom severity, worsening quality of life, and a rapid loss in neurological function [181]. In light of these results, bevacizumab should only be used in individuals who are unresponsive to other treatments. GBM patients likely to relapse during treatment also commonly relapse while treated with bevacizumab [182,183]. GBM recurrence in the absence of VEGF blockade often involves mesenchymal transition in tumor cells, and this is likely mediated by c-Met (a receptor tyrosine kinase of hepatocyte growth factor) activation. In normoxic GBM tissue regions, but not hypoxic regions where VEGF functions are impaired, a functional Met/VEGFR2 complex recovers and accelerates tumor cell invasion and mesenchymal transition [184].
Despite anti-angiogenic therapy increasing tumor oxygenation, hypoxia persists overall in treatment-resistant tumors [180]. The hypoxic microenvironment reactivates HIF-1α, which results in the activation of stromal cell-derived factor 1 (SDF-1) and VEGF and the migration of bone marrow-derived pro-angiogenic monocytic cells, endothelial cells, and pericyte progenitors [185]. Placental growth factor (PGF) is another protein known to promote angiogenesis in the tumor microenvironment by stimulating the VEGF pathways [186,187]. However, when an anti-PGF monoclonal antibody was evaluated in phase I for patients with recurrent GBM, no substantial clinical benefit was observed [188]. The alkylating cytotoxic drug lomustine may have an adjuvant therapeutic effect in recurrent GBM when given in combination with other drugs, as nitrosoureas readily penetrate the BBB [189]. Nonetheless, the impact of chemotherapy is temporary and dependent on treatment dosage and duration until the vasculature returns to normal after anti-angiogenic treatment [190]. An additional treatment option for recurrent GBM is to provide angiotensin system inhibitors (ASI) (given as antihypertensive medications) along with cytotoxic bevacizumab combination therapy [191]. These inhibitors affect both the signaling of the angiotensin II receptor type I (AT1) and angiotensin converting enzyme (ACE). Improved clinical outcomes have been reported in GBM patients receiving standard-of-care (radiotherapy + TMZ) therapy in combination with ASI, but further prospective trials are warranted to validate these observations [192]. Supporting the clinical benefit of ASI and providing mechanistic insight, it was previously demonstrated that inhibiting AT1 with ASI decreased tumor VEGF levels in preclinical glioma models [193].
7. GBM Cell Metabolic Functions as a Target of Wnt and PAM Therapeutics
7.1. Glucose and Lactate Are Main Fuel Sources for GBM Cells
Non-oxidative glucose metabolism occurs even at normal oxygen levels in cancer cells, while normal cells use the oxidative phosphorylation (OxPhos) pathway to convert glucose into energy [194]. Cancer cells use several different substrates to create raw materials that are needed for cell maintenance and energy production. The importance of these substrates in cancer cell metabolism is becoming increasingly apparent in GBM [195].
Energy metabolization of glucose occurs by means of oxygen-dependent aerobic respiration and oxygen-independent anaerobic fermentation. The most effective form of energy extraction is a chemical process known as OxPhos. The Krebs (tricarboxylic acid, or TCA) cycle is able to use the pyruvate produced during OxPhos as a source of energy for respiring cells. NADH (reduced nicotinamide adenine dinucleotide) fed into the electron transport chain generates ATP at a rate of 36 molecules per glucose molecule. As a result of the Warburg effect, reduction from pyruvate to lactate occurs, and lactate is released into the extracellular space as a byproduct of glycolysis. Only two ATP molecules are produced per glucose molecule, making this procedure a far less efficient way of producing cellular energy equivalents [195]. Even in the presence of sufficient oxygen, cancer cells nearly always adopt this less efficient glycolytic route. It is entirely clear why cancer cells choose to utilize the Warburg effect, but it has been proposed that the amount of ATP is not a limiting factor in cancer cells, considering that this metabolic phenotype manifests prior to the onset of hypoxia [196,197]. Because glycolysis is an inefficient energy-generating metabolic pathway, cancer cells need to consume considerable amounts of glucose and generate large quantities of lactate that decrease the extracellular pH in the tumor microenvironment to acidic (pH ranging from 6.0 to 6.5) conditions. This acidity promotes metastasis, angiogenesis, and, most significantly, immunosuppression, which has been linked to a poor clinical outcome. Thus, lactate might be seen as a critical oncometabolite in metabolic reprogramming during cancer [198]. Cancer cell populations adapt to this process of chronic (several weeks) lactate-induced acidity of the microenvironment by promoting beta-oxidation. This metabolic strategy, known as the Corbet–Feron effect, includes the activation of acetylated proteins and DNA in mitochondria and histone deacetylation in the nucleus. While active in SiHa cervical cancer cells, the FaDu pharynx squamous cell carcinoma line, HCT-116 cells and HT-29 colon cancer cells, GBM cells do not adopt the Corbet–Feron effect. Hence, it is uncertain as to whether this metabolic strategy is functionally relevant in malignant brain tumors [195,199]. However, it has been demonstrated that lactate is a significant alternative energy and biosynthetic substrate that sustains the development of glioma stem cells in the absence of glucose [200].
7.2. Role of Fatty Acids in GBM Metabolism
Despite advancements in the genetic characterization of GBM subtypes, metabolic abnormalities that contribute to its aggressive behavior are only now becoming apparent. Some cancer cells, including gliomas, have been demonstrated to have other sources of oxidation in addition to the Warburg effect, suggesting that various substrates are oxidized [196]. Fatty acids may function as important bioenergetic substrates for glioma cells. In particular, primary cultured human glioma cells grown in serum-free medium were shown to oxidize fatty acids to sustain respiratory and proliferative activity. According to results from 13C carbon radiolabeling studies in animal models of malignant glioma, more than half of the brain tumor oxidative activity comes from acetate, whereas less than a third comes from glucose [201,202,203]. A recent study suggested that fatty acid β-oxidation (FAO) is the main metabolic node in GBM, providing metabolic flexibility that enables these cells to adapt to their dynamic microenvironment [204]. While glioma cells depend on fatty acids for energy generation, it is currently unclear as to whether these carbon chains derive from the circulation or as a metabolic product from glioma cells themselves. Glucose may be delivered into cells and metabolized into fatty acids via the enzyme fatty acid synthase (FASN); these fatty acids can then be imported into the mitochondria for β-oxidation (futile cycle). In support of this notion, FASN has been demonstrated in glioma cells, and its expression increases with tumor aggressiveness [205].
7.3. Amino Acid Metabolism in GBM
Amino acids are a viable nutritional option for the maintenance of glioma. Increased glutathione production by utilizing glutamine may alleviate oxidative and radiation stress and increase chemotherapy tolerance. Glycogen depletion in the liver may be replenished with the help of glutamine, which aids the increased glycolytic rate in cancer cells by increasing blood glucose levels [206,207]. Flushing molecules from the TCA cycle into other metabolic pathways to facilitate tumor growth is possible with a high abundance of glutamine in GBM. Due to a shortage of NADH2/FADH2 for extended OxPhos, glutamine removal may reverse the Warburg effect and lead to cell death in GBM [206,207,208,209]. Deprivation from glycolysis, carbohydrates, or glutamine may be a potential therapeutic option. However, this strategy is not applicable to all GBM. Glutamate generated from glutamine was unexpectedly found to be secreted rather than metabolized. GBM cell development was found to be unaffected by glutamine deprivation because glutamine synthase activity prevented glutamine from entering the TCA cycle. Only a small subset of GBM, mesenchymal in origin and lacking the stem cell marker CD133, might be susceptible to targeting glutamine metabolism. More research is required in this field, especially considering the fact that glutamine is connected to many aspects of cancer metabolism, which may necessitate the deployment of different combinatorial therapeutic strategies for proper metabolic targeting of selected glioma cases [206,207,208,209].
7.4. Research Progress on the Changes in Glycolysis and HIF in GBM Invasion
GBM recurrences are frequent and the result of tumor cell infiltration into normal brain tissue which precludes the complete surgical resection of GBM. Bevacizumab is known to enhance the invasion of GBM cells, and bevacizumab-resistant GBM cells are more glycolytic. HIF1 levels were reduced and mitochondrial complex-I levels elevated in invasive versus angiogenic GBM models. Additionally, glycolysis was increased in invasive GBM cells after long-term but not short-term hypoxia. This suggests dynamic metabolic adaptations of GBM cells based on cues from microenvironmental conditions. Emerging evidence suggests an impact of enhanced glycolysis on the invasion and recurrence of GBM [210,211,212]. In vitro, increased glucose-6-phosphatase (G6PC) conferred resistance to glycolytic inhibition by 2-DG on GBM cells, while G6PC knockdown reduced migration, invasion, and proliferation in vitro and in vivo [213]. Corroborating this result, the expression and activity of glycolytic enzymes were increased in both bulk tumor cells and brain tumor initiating cells from invasive GBM models [214]. A decrease in intracellular ATP results in a rise in the AMP:ATP ratio and activation of the energy-sensing AMPK, which is known to regulate mTORC1 [215]. AMPK functions as an activator of TSC2 and an inhibitor of the mTORC1 component adaptor protein raptor [127,216,217]. In GBM, activation of mTOR signaling results in increased production of transcription factors (such as c-Myc) [218], which in turn increase glycolytic gene expression [219]. Akt also contributes to the glycolytic GBM phenotype by boosting the expression and membrane translocation of GLUT1 and 3 overexpressed in GBM [220,221].
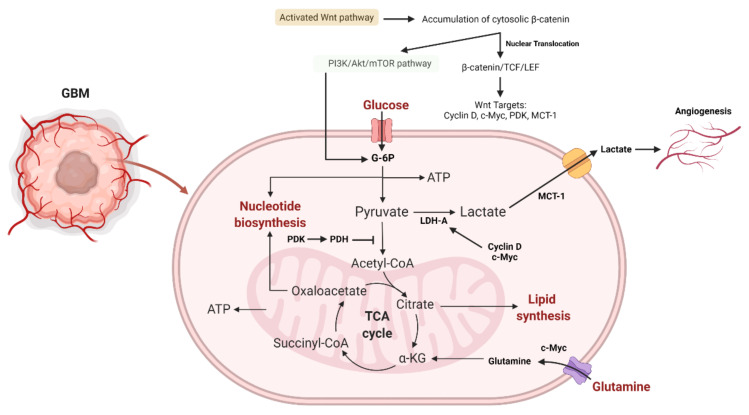
HIF-1 has been implicated in the onset and progression of GBM. Hypoxia increases HIF-1 production, resulting in the transition from aerobic to anaerobic glycolysis, angiogenesis, enhanced cell migratory potential, and genetic changes that prevent hypoxia-induced death [222,223,224]. As a result of the activation of oncogenic signaling pathways, such as PI3K/Akt, MAPK/ERK, and STAT3, HIF-1 transcriptional expression and glucose consumption in GBM are increased, even when oxygen is plentiful [225,226]. HIF-1 activation may promote the production of anti-apoptotic proteins, such as Bcl-2, and thereby inhibit GBM cell death [227,228]. In order to meet the high-energy requirements, HIF-1 acts as a master regulator of aerobic glycolysis in GBM cells while also protecting them from hypoxia-induced damage (see Figure 4 and Figure 5).
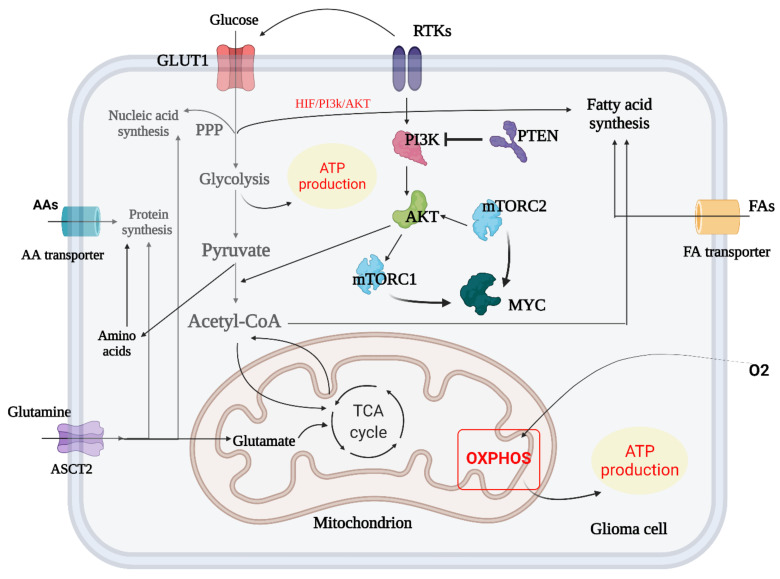
Figure 4.
Metabolic reprogramming in glioma. The metabolic switch between glycolysis and TCA cycle in GBM is summarized and amino acid biosynthesis and transport in crosstalk with mTORC1 and mTORC2 are shown. In addition, the crosstalk of fatty acid biosynthesis and TCA/glycolysis cycle is briefly outlined (created with https://biorender.com/, accessed on 14 July 2021).
Figure 5.
The role of the Wnt and PI3K/Akt/mTOR signaling pathways in glioma aerobic glycolysis. The association of β-catenin to TCF-LEF results in the transcription of Wnt-sensitive genes (PDK, c-Myc, cyclin D, MCT-1). MCT-1 promotes lactate extrusion from the cytosol, hence promoting angiogenesis. Activating PI3K/Akt results in an increase in glucose metabolism. By activating HIF-1α, which inhibits glucose entry into the TCA cycle, Akt-transformed cells defend against ROS damage. HIF-1α-induced PDK1 phosphorylates PDH, culminating in the conversion of cytosolic pyruvate to lactate through the activation of LDH-A. Because PDK inhibits the PDH complex in the mitochondria, pyruvate cannot be converted completely to acetyl-CoA and enter the TCA cycle. Additionally, c-Myc and cyclin D activate LDH-A, which catalyzes the conversion of cytosolic pyruvate to lactate. c-Myc facilitates the entrance of glutamine into the cytosol and mitochondria. Glutamate generated by c-Myc promotes aspartate and nucleotide synthesis [229]. Abbreviations: PDK, pyruvate dehydrogenase kinase; MCT-1, monocarboxylate transporter 1, ROS, reactive oxygen species; PDH, pyruvate dehydrogenase; LDH-A, lactate dehydrogenase A (created with https://biorender.com/, accessed on 28 December 2021).
8. Therapeutic Targeting Wnt and PAM Metabolic Functions to Decrease Invasion and Survival of GBM
Oncogenesis in GBM is thwarted by mTOR inhibitors, which block the activity of mTORC1/2 [230,231]. Metformin and other oral anti-diabetic drugs, as well as chlorpromazine, have beneficial anti-cancer actions by interfering with AMPK/mTORC1 signaling [232]. However, GBM cells can counteract mTOR inhibition by increasing glutamine metabolism via glutaminase (GLS). The combination of mTOR and GLS inhibitors was shown to synergistically reduce GBM tumor formation in a preclinical setting [232]. Hexokinase 2 (HK2), the rate-limiting enzyme that catalyzes the initial step of glycolysis, provides a direct connection between energy metabolism and the autophagy suppressor mTORC1. When glucose shortage occurs, HK2 physically binds to mTORC1 to inhibit its activity and promote protective autophagy [233]. AMPK is required for this process and considered a critical target for reducing autophagy-dependent cancer cell survival in GBM and other gliomas [234,235]. Additionally, the combination of metformin with non-metabolizable 2-deoxy-d-glucose (2-DG) exhibits a powerful inhibitory effect on both glycolysis and oxidative phosphorylation. This resulted in the death of GBM tumor spheres and increased survival in an orthotopic xenograft mouse model [236]. Through acetylation of FOXO and overexpression of c-Myc, mTORC2 regulates glycolytic metabolism in GBM [218]. Metabolic reprogramming of glucose is a significant characteristic of malignant tumors, including GBM, and miRNAs may play a critical role in regulating this process in GBM cells. miR-199a-3p, which specifically targets mTORC2, is significantly downregulated in GBM relative to healthy brain [237,238]. Additionally, miR-34a targets the mTORC2 binding partner rictor in GBM [239]. The tumor suppressor miR-181b may inhibit the growth of glioma cells by blocking SP1-mediated glucose metabolism [240]. Table 2 summarizes repurposed drugs that have shown potential efficacy in the treatment of GBM, along with their hypothesized mechanism of action in tumor cells and associated results.
Table 2.
Licensed drugs targeting Wnt or PAM pathways that have shown potential as repurposed anticancer agents in GBM therapy determined by in vitro and in vivo research and clinical trials [241].
| Drug | Suggested Mechanism of Action | IC50 | Outcome | References |
|---|---|---|---|---|
| Chloroquine | Autophagy induction, inhibition of MMP-2 activity, TGF-β secretion and signaling | 30 μΜ (U251, LN229, U87MG) 40 μΜ (U251-TMZR, LN229-TMZR, U87MG-TMZR) |
Primary cultures of GBM cell lines and specimens showed a 50% reduction in proliferation. | [242,243] |
| Hydroxy-chloroquine | Autophagy induction | - | Hydroxy-chloroquine eliminated TMZ-resistant glioma cells. | [242] |
| Mefloquine | Autophagy induction | 10 μΜ (U251, LN229, U87) 15 μΜ (U251-TMZR, LN229-TMZR, U87-TMZR) |
Mefloquine was capable to killing U251 and U251-TMZ resistant cells at far lower doses than chloroquine. | [242,244] |
| Quinacrine | Autophagy induction | 5 μΜ (U251, LN229, U87) 8 μΜ (U251-TMZR, LN229-TMZR, U87-TMZR) |
50 mg/kg of quinacrine substantially slowed the growth of tumors in a subcutaneous human xenograft U87MG model. | [242,245] |
| Pyrvinium pamoate | Inhibition of Wnt/β-catenin signaling | 239.8 nmol/L (BT241), 122.5 nmol/L (BT486) | For 48 h, 200 nmol/L of pyrvinium decreased CD133POS cell fractions in primary (BT428) and recurrent (BT 566) GBM cells. | [246] |
| Itraconazole | Inhibition of cell proliferation | - | Itraconazole inhibited the proliferation of GBM cells in vitro (2–80 μM, U87MG and rat C6 glioma cells) and in vivo (75 mg/kg, nude mice with U87MG subcutaneous xenografts). | [247] |
| Salinomycin | OxPhos inhibition in mitochondria | - | Salinomycin decreased the cell viability of GL261 neurospheres and GL261 adherent cells. Salinomycin depleted neurosphere-forming GL261 stem cells from tumorspheres. | [248] |
| Minocycline | Growth inhibition, autophagy induction, caspase-3 mediated apoptosis | 30 μM (C6) | 50 μM of minocycline decreased the cell viability of U87MG, U251 and C6 glioma cells; 20 or 100 mg/kg of minocycline (IP) showed slower tumor growth compared controls in Mice injected with C6 cells. | [249] |
| Chlorpromazine | Inhibition of PAM signaling, autophagy induced cell death | 18.8–27.7 μM (C6) 15 μM (SH-SY5Y) |
Cell viability was significantly lowered in cells treated with chlorpromazine (≥20 μM) for 24 h. Overall survival greatly improved for U251-TMZR orthotopic mouse xenograft models. | [250] |
| Quetiapine | Inhibition of Wnt/β-catenin signaling | - | Relatively high doses of quetiapine (>25 μM) may inhibit cell proliferation by retarding cell cycle in the G2-M phase; 20 mg/kg of quetiapine (IP) alone or combined with TMZ slowed tumor development in orthotopic xenograft mouse model. | [251] |
| Lithium | Inhibition of GSK-3 activation | - | 20 mM lithium for 48 h reduced in viability of 20% of U87MG cells. Through GSK-3 inhibition, lithium concentrations above 5 mM can affect the proliferation, apoptosis and migration of glioma cells. Combination of 1.2 mM lithium and TMZ increased cell death in TP53wt glioma cells and prevented tumor growth in vivo with increased median survival times of mice. | [252,253] |
| Fluvoxamine | Inhibition of FAK and Akt/mTOR | 30 μM | 20–30 μM of fluvoxamine inhibited lamellipodia formation, migration and invasion of U87MG and U251 cells in vitro; 50 mg/kg of fluvoxamine inhibited GBM cell invasion and prolonged survival in mice bearing glioma tumors. | [254] |
| Imipramine | Autophagy induction; inhibition of PI3K/Akt/mTOR | - | 60 μM of imipramine was cytotoxic and strongly reduced colony formation of U87MG and C6 cells, but not primary cultured rat astrocytes; 10 μM of imipramine inhibited mitochondrial activity relative to oxygen content in the atmosphere (from 6% in hypoxia, 11% in mild hypoxia, to 19% in medium re-oxygenated at 26% oxygen). | [255,256,257] |
| Dimethyl fumarate | Autophagy induction | - | - | [258] |
| Simvastatin | Inhibition of cell growth and migration; inhibition of Ras/ERK and Ras/Akt pathways to induce caspase-3 mediated apoptosis; downregulation of PI3K/Akt | - | 10 μM of simvastatin was cytotoxic to U251 and U87MG by inducing aopotosis. | [259,260,261] |
| Mevastatin, fluvastatin | Inhibition of cell growth; inhibition of Ras/ERK and Ras/Akt; induction of apoptosis | 0.922 μM (A 172) | 5 and 10 μM of mevastatin and fluvastatin are cytotoxic. | [259,261] |
| Mibefradil | Inhibition of tumor growth; cell cycle inhibition; activation of pro-apoptotic survivin and BAX pathways; inhibition of Akt/mTOR | - | 2.5–5 μmol/L greatly inhibited cell growth and enhanced the inhibition of GSC growth by TMZ; 24 mg/kg (bodyweight) of mibefradil (oral gavage) significantly inhibited growth of tumor. | [262,263] |
| Losartan | Reducing tumor and cell growth; reduced number of capillary blood vessels; decreased levels of VEGF, PDGF and FGF; apoptosis induction | [193,264] | ||
| Metformin | Autophagy and induction of apoptosis; activated AMPK and down-regulation of Akt/mTOR pathway; inhibition of CLIC1 activity with G1 cell arrest | - | 10 mM significantly decreased GBM cell proliferation (U87MG, U251, LN18 and SF767). | [265,266] |
| Pioglitazone | Inhibition of β-catenin expression; reduced cell viability; apoptosis induction | 85 μM (U87MG) | 100–200 μM significantly reduced the viability of glioma cells (U251, T98G, and U87MG) in a concentration- and time-dependent manner; 100 μM pioglitazone via reducing MMP-2 expression can inhibit U251 cell migration; 50 μM significantly reduced the metabolic activity of G144 cells; 10 μM promoted a minor decrease in metabolic activity in GliNS2 cells. | [267,268] |
| Aprepitant | Enhanced blockage in Akt phosphorylation due to NK-1 signaling | 32 µM (GAMG) | Maximum inhibition at 70 μM after 48 h, with no surviving cells (GAMG glioma cell line). | [269] |
| Cimetidine | GSK-3β inhibition; reduction in endogenous receptors required for cell adhesion and migration | - | Decreased growth rates of U373 GBM and 9L gliosarcoma cells at concentrations equal to or higher than 100 mM; 100 and 1000 mM cimetidine significantly decreased migration of both cell lines; doses <100 mM did not affect cell cycle kinetics or apoptosis. | [270,271] |
| Ivermectin | Deactivation of the Akt/mTOR pathways | - | 1, 5, and 10 μM inhibited proliferation of U87MG and T98G cells in a dose-dependent manner with ED50 of ∼5 μM. | [272] |
| Rapamycin | Interfering with the AMPK/mTORC1 axis | [108] | ||
| Gefitinib and Erlotinib | ATP-competitive and reversible EGFR inhibitors | [141] | ||
| Sonolisib | Reduced invasion and angiogenesis in GBM cell lines in vitro; improved survival in orthotopic xenograft models | [136,137] | ||
| Buparlisib | Well-tolerated, BBB permeable PI3K inhibitor (most often utilized PI3K inhibitor in clinical studies for GBM therapy) | [135] | ||
| Pimozide | Inhibition of migration and survival of GBM cells in a STAT5- dependent manner | [130] |
9. Conclusions and Perspective
GBM is known for its invasive nature, frequent recurrence, and poor response to therapy. The inter- and intra-tumoral genetic variants and diverse histological appearance of GBM render this tumor one of the most problematic and challenging cancers. Current treatment of GBM includes surgical excision followed by adjuvant radio-chemotherapy. Despite the improvements in neurosurgical procedures and radiation treatment, the limited efficacy of chemotherapy remains a major challenge. It is important to identify efficient and well-tolerated pharmacological strategies capable of generating lasting responses in specific patient populations, especially with GBM, where frequent recurrence coincide with the emergence of therapeutic resistance. As outlined, the PAM signaling pathways are vital for glioma growth. Preliminary preclinical investigations focusing on the potential effectiveness of PAM inhibitors revealed positive findings. However, thus far, clinical trials have shown little to no benefit from single or combination PAM treatment regimens. Possible explanations for the therapeutic failure of these trials include the limited BBB permeability of particular compounds or the development of GBM resistance or tolerance mechanisms in response to continuous treatment [122].
A significant body of research now supports the claim that Wnt signaling is vital to the pathophysiology of malignant glioma and linked to malignant glioma stem cell proliferation, invasiveness, and treatment resistance. In addition to some promising early clinical findings, there is convincing preclinical evidence of Wnt’s participation in the development of glioma, emphasizing the need for randomized clinical studies on the efficacy of Wnt inhibition in GBM. Advantages of inhibiting Wnt signaling include a reduction in tumor invasiveness, treatment resistance, and tumor cell stemness. Moreover, Wnt inhibition impairs angiogenesis and improves BBB permeability for better delivery of chemotherapy [273,274]. Considering the fact that PAM and Wnt/β-catenin are two of the most important pathways governing cell proliferation and survival in GBMs, targeting PAM and Wnt/β-catenin represents a compelling avenue for developing treatments against gliomas. In this review, we have outlined how the networks composed of PAM, Wnt/β-catenin, and EMT signaling pathways convey information that critically impacts on GBM development. Furthermore, we provided a paradigm for dual-targeted treatments of GBM and suggest potentially promising treatment strategies. More preclinical and clinical studies are required to provide further insights into glioma maintenance, treatment resistance, and recurrence and elucidate how the interactions between PAM, Wnt/β-catenin and EMT affect clinical outcomes.
Acknowledgments
Authors acknowledge the Silesian University of Technology for language editing of the paper.
Author Contributions
A.B.B., Z.T. and F.J. prepared the draft of the manuscript, reviewed the literature, and prepared the graphical abstract as well as figures. M.J.Ł. and S.G. supervised the team. M.J.Ł., S.G. and T.K. edited the draft and finalized the paper. S.G. drafted the sections related to autophagy. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hombach-Klonisch S., Mehrpour M., Shojaei S., Harlos C., Pitz M., Hamai A., Siemianowicz K., Likus W., Wiechec E., Toyota B.D., et al. Glioblastoma and chemoresistance to alkylating agents: Involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol. Ther. 2018;184:13–41. doi: 10.1016/j.pharmthera.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Wu W., Klockow J.L., Zhang M., Lafortune F., Chang E., Jin L., Wu Y., Daldrup-Link H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021;171:105780. doi: 10.1016/j.phrs.2021.105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shojaei S., Alizadeh J., Thliveris J., Koleini N., Kardami E., Hatch G.M., Xu F., Hombach-Klonisch S., Klonisch T., Ghavami S. Statins: A new approach to combat temozolomide chemoresistance in glioblastoma. J. Investig. Med. 2018;66:1083–1087. doi: 10.1136/jim-2018-000874. [DOI] [PubMed] [Google Scholar]
- 4.Shojaei S., Koleini N., Samiei E., Aghaei M., Cole L.K., Alizadeh J., Islam I., Vosoughi A., Albokashy M., Butterfield Y., et al. Simvastatin increases temozolomide-induced cell death by targeting the fusion of autophagosomes and lysosomes. FEBS J. 2020;287:1005–1034. doi: 10.1111/febs.15069. [DOI] [PubMed] [Google Scholar]
- 5.Siebzehnrubl F.A., Silver D.J., Tugertimur B., Deleyrolle L.P., Siebzehnrubl D., Sarkisian M.R., Devers K.G., Yachnis A.T., Kupper M.D., Neal D., et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol. Med. 2013;5:1196–1212. doi: 10.1002/emmm.201302827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta S., Cascio C.L. Developmentally regulated signaling pathways in glioma invasion. Cell. Mol. Life Sci. 2018;75:385–402. doi: 10.1007/s00018-017-2608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alizadeh J., Shojaei S., Sepanjnia A., Hashemi M., Eftekharpour E., Ghavami S. Simultaneous Detection of Autophagy and Epithelial to Mesenchymal Transition in the Non-small Cell Lung Cancer Cells. Methods Mol. Biol. 2019;1854:87–103. doi: 10.1007/7651_2017_84. [DOI] [PubMed] [Google Scholar]
- 8.Madan B., Virshup D.M. Targeting Wnts at the Source—New Mechanisms, New Biomarkers, New Drugs. Mol. Cancer Ther. 2015;14:1087–1094. doi: 10.1158/1535-7163.MCT-14-1038. [DOI] [PubMed] [Google Scholar]
- 9.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahlert U., Maciaczyk D., Doostkam S., Orr B.A., Simons B., Bogiel T., Reithmeier T., Prinz M., Schubert J., Niedermann G., et al. Activation of canonical WNT/β-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett. 2012;325:42–53. doi: 10.1016/j.canlet.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Foltz G., Yoon J.-G., Lee H., Ma L., Tian Q., Hood L., Madan A. Epigenetic Regulation of Wnt Pathway Antagonists in Human Glioblastoma Multiforme. Genes Cancer. 2010;1:81–90. doi: 10.1177/1947601909356103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson J.M., Rank K.B., Zeng Y., Capen A., Yadav V., Manro J.R., Engler T.A., Chedid M. Activating the Wnt/β-catenin pathway for the treatment of melanoma–application of LY2090314, a novel selective inhibitor of glycogen synthase kinase. PLoS ONE. 2015;10:e0125028. doi: 10.1371/journal.pone.0125028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C., Tu Y., Sun X., Jiang J., Jin X., Bo X., Li Z., Bian A., Wang X., Liu D., et al. Wnt/beta-Catenin pathway in human glioma: Expression pattern and clinical/prognostic correlations. Clin. Exp. Med. 2010;11:105–112. doi: 10.1007/s10238-010-0110-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Ng P.K.-S., Kucherlapati M., Chen F., Liu Y., Tsang Y.H., De Velasco G., Jeong K.J., Akbani R., Hadjipanayis A., et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell. 2017;31:820–832.e3. doi: 10.1016/j.ccell.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhavan D., Cloughesy T.F., Mischel P.S. mTOR signaling in glioblastoma: Lessons learned from bench to bedside. Neuro-Oncology. 2010;12:882–889. doi: 10.1093/neuonc/noq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong C., Li X., Tao B., Peng L., Peng T., Yang X., Xia X., Chen L. LIM and SH3 protein 1 induces glioma growth and invasion through PI3K/AKT signaling and epithelial-mesenchymal transition. Biomed. Pharmacother. 2019;116:109013. doi: 10.1016/j.biopha.2019.109013. [DOI] [PubMed] [Google Scholar]
- 17.Batsios G., Viswanath P., Subramani E., Najac C., Gillespie A.M., Santos R.D., Molloy A.R., Pieper R.O., Ronen S.M. PI3K/mTOR inhibition of IDH1 mutant glioma leads to reduced 2HG production that is associated with increased survival. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-47021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzzai M., Bauer E.D., Jones R.G., DeBerardinis R.J., Hatzivassiliou G., Elstrom R.L., Thompson C.B. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid β-oxidation. Oncogene. 2005;24:4165–4173. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- 19.Elstrom R.L., Bauer D.E., Buzzai M., Karnauskas R., Harris M.H., Plas D.R., Zhuang H., Cinalli R.M., Alavi A., Rudin C., et al. Akt Stimulates Aerobic Glycolysis in Cancer Cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 20.Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 21.Rathmell J.C., Fox C.J., Plas D.R., Hammerman P.S., Cinalli R.M., Thompson B.C. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng C.K., Fan Q.-W., Weiss W.A. PI3K Signaling in Glioma-Animal Models and Therapeutic Challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan R., Zhang X., Guo M. Glioblastoma stem cells and Wnt signaling pathway: Molecular mechanisms and therapeutic targets. Chin. Neurosurg. J. 2020;6:1–6. doi: 10.1186/s41016-020-00207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorzadeh S., Kohan L., Ghavami S., Azarpira N. Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:118926. doi: 10.1016/j.bbamcr.2020.118926. [DOI] [PubMed] [Google Scholar]
- 25.Morris L.G.T., Kaufman A.M., Gong Y., Ramaswami D., Walsh L., Turcan S., Eng S., Kannan K., Zou Y., Peng L., et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat. Genet. 2013;45:253–261. doi: 10.1038/ng.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nager M., Bhardwaj D., Cantí C., Medina L., Nogués P., Herreros J. β-Catenin Signalling in Glioblastoma Multiforme and Glioma-Initiating Cells. Chemother. Res. Pract. 2012;2012:192362. doi: 10.1155/2012/192362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang C., Guo J., Chen H., Yao C.-J., Zhuang D.-X., Wang Y., Tang W.-J., Ren G., Yao Y., Wu J.-S., et al. Gene mutation profiling of primary glioblastoma through multiple tumor biopsy guided by 1H-magnetic resonance spectroscopy. Int. J. Clin. Exp. Pathol. 2015;8:5327–5335. [PMC free article] [PubMed] [Google Scholar]
- 28.Takebe N., Harris P.J., Warren R.Q., Ivy S.P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 2010;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 29.Petherick K.J., Williams A.C., Lane J.D., Ordóñez-Morán P., Huelsken J., Collard T.J., Smartt H.J.M., Batson J., Malik K., Paraskeva C., et al. Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J. 2013;32:1903–1916. doi: 10.1038/emboj.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eshraghi M., Adlimoghaddam A., Mahmoodzadeh A., Sharifzad F., Yasavoli-Sharahi H., Lorzadeh S., Albensi B., Ghavami S. Alzheimer’s Disease Pathogenesis: Role of Autophagy and Mitophagy Focusing in Microglia. Int. J. Mol. Sci. 2021;22:3330. doi: 10.3390/ijms22073330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colella B., Faienza F., Carinci M., D’Alessandro G., Catalano M., Santoro A., Cecconi F., Limatola C., Di Bartolomeo S. Autophagy induction impairs Wnt/β-catenin signalling through β-catenin relocalisation in glioblastoma cells. Cell Signal. 2019;53:357–364. doi: 10.1016/j.cellsig.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Alizadeh J., Lorzadeh S., Ghavami S. Autophagy and cancer metastasis: A Trojan horse. J. Investig. Med. 2021;69:1145–1147. doi: 10.1136/jim-2021-002016. [DOI] [PubMed] [Google Scholar]
- 33.Ji H., Wang J., Nika H., Hawke D., Keezer S., Ge Q., Fang B., Fang X., Litchfield D.W., Aldape K., et al. EGF-induced ERK activation promotes CK2-mediated disassociation of α-catenin from β-catenin and transactivation of β-catenin. Mol. Cell. 2009;36:547–559. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwadate Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol. Lett. 2016;11:1615–1620. doi: 10.3892/ol.2016.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahlert U., Nikkhah G., Maciaczyk J. Epithelial-to-mesenchymal(-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett. 2013;331:131–138. doi: 10.1016/j.canlet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Koen E.J., Collier A.B., Maharaj S.K. Particle-in-cell simulations of beam-driven electrostatic waves in a plasma. Phys. Plasmas. 2012;19:042101. doi: 10.1063/1.3695402. [DOI] [Google Scholar]
- 37.Imran S.A.M., Yazid M.D., Idrus R.B.H., Maarof M., Nordin A., Razali R.A., Lokanathan Y. Is There an Interconnection between Epithelial-Mesenchymal Transition (EMT) and Telomere Shortening in Aging? Int. J. Mol. Sci. 2021;22:3888. doi: 10.3390/ijms22083888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan W., Wang Y., Chen Y., Chen C. Cell tracing reveals the transdifferentiation fate of mouse lung epithelial cells during pulmonary fibrosis in vivo. Exp. Ther. Med. 2021;22:1–9. doi: 10.3892/etm.2021.10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abolhassani A., Riazi G.H., Azizi E., Amanpour S., Muhammadnejad S., Haddadi M., Zekri A., Shirkoohi R. FGF10: Type III Epithelial Mesenchymal Transition and Invasion in Breast Cancer Cell Lines. J. Cancer. 2014;5:537–547. doi: 10.7150/jca.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bierie B., Moses H.L. TGFβ: The molecular Jekyll and Hyde of cancer. Nat. Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 41.Alizadeh J., Glogowska A., Thliveris J., Kalantari F., Shojaei S., Hombach-Klonisch S., Klonisch T., Ghavami S. Autophagy modulates transforming growth factor beta 1 induced epithelial to mesenchymal transition in non-small cell lung cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:749–768. doi: 10.1016/j.bbamcr.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Castanon I., Baylies M.K. A Twist in fate: Evolutionary comparison of Twist structure and function. Gene. 2002;287:11–22. doi: 10.1016/S0378-1119(01)00893-9. [DOI] [PubMed] [Google Scholar]
- 43.Elias M.C., Tozer K.R., Silber J.R., Mikheeva S., Deng M., Morrison R.S., Manning T.C., Silbergeld D.L., Glackin C.A., Reh T.A., et al. TWIST is Expressed in Human Gliomas, Promotes Invasion. Neoplasia. 2005;7:824–837. doi: 10.1593/neo.04352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikheeva S.A., Mikheev A.M., Petit A., Beyer R., Oxford R.G., Khorasani L., Maxwell J.-P., Glackin C.A., Wakimoto H., González-Herrero I., et al. TWIST1 promotes invasion through mesenchymal change in human glioblastoma. Mol. Cancer. 2010;9:194. doi: 10.1186/1476-4598-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang M.-H., Wu M.-Z., Chiou S.-H., Chen P.-M., Chang S.-Y., Liu C.-J., Teng S.-C., Wu K.-J. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat. Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 46.Mahabir R., Tanino M., Elmansuri A., Wang L., Kimura T., Itoh T., Ohba Y., Nishihara H., Shirato H., Tsuda M., et al. Sustained elevation of Snail promotes glial-mesenchymal transition after irradiation in malignant glioma. Neuro Oncol. 2014;16:671–685. doi: 10.1093/neuonc/not239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng W.-Y., Kandel J.J., Yamashiro D.J., Canoll P., Anastassiou D. A Multi-Cancer Mesenchymal Transition Gene Expression Signature Is Associated with Prolonged Time to Recurrence in Glioblastoma. PLoS ONE. 2012;7:e34705. doi: 10.1371/journal.pone.0034705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catalano M., D’Alessandro G., Lepore F., Corazzari M., Caldarola S., Valacca C., Faienza F., Esposito V., Limatola C., Cecconi F., et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol. Oncol. 2015;9:1612–1625. doi: 10.1016/j.molonc.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colella B., Faienza F., Di Bartolomeo S. EMT Regulation by Autophagy: A New Perspective in Glioblastoma Biology. Cancers. 2019;11:312. doi: 10.3390/cancers11030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang H., Zheng Y., Zhao Y., Xiu X., Wang J. miR-590-3p suppresses cancer cell migration, invasion and epithelial-mesenchymal transition in glioblas-toma multiforme by targeting ZEB1 and ZEB. Biochem. Biophys. Res. Commun. 2015;468:739–745. doi: 10.1016/j.bbrc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 51.Qi S., Song Y., Peng Y., Wang H., Long H., Yu X., Li Z., Fang L., Wu A., Luo W., et al. ZEB2 Mediates Multiple Pathways Regulating Cell Proliferation, Migration, Invasion, and Apoptosis in Glioma. PLoS ONE. 2012;7:e38842. doi: 10.1371/journal.pone.0038842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sánchez-Tilló E., Liu Y., de Barrios O., Siles L., Fanlo L., Cuatrecasas M., Darling D.S., Dean D.C., Castells A., Postigo A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell. Mol. Life Sci. 2012;69:3429–3456. doi: 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q., Li X., Zhu Y., Yang P. MicroRNA-16 suppresses epithelial-mesenchymal transition-related gene expression in human glioma. Mol. Med. Rep. 2014;10:3310–3314. doi: 10.3892/mmr.2014.2583. [DOI] [PubMed] [Google Scholar]
- 54.Zhu H., Chen Z., Shen L., Tang T., Yang M., Zheng X. Long Noncoding RNA LINC-PINT Suppresses Cell Proliferation, Invasion, and EMT by Blocking Wnt/β-Catenin Signaling in Glioblastoma. Front. Pharmacol. 2021;11:11. doi: 10.3389/fphar.2020.586653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L., Shao M.-Y., Zou S.-C., Xiao Z.-F., Chen Z.-C. MiR-101-3p inhibits EMT to attenuate proliferation and metastasis in glioblastoma by targeting TRIM. J. Neuro-Oncol. 2019;141:19–30. doi: 10.1007/s11060-018-2973-7. [DOI] [PubMed] [Google Scholar]
- 56.Chen W., Kong K., Xu X., Chen C., Li H., Wang F., Peng X., Zhang Z., Li P., Li J., et al. Downregulation of miR-205 is associated with glioblastoma cell migration, invasion, and the epithelial-mesenchymal transition, by targeting ZEB1 via the Akt/mTOR signaling pathway. Int. J. Oncol. 2018;52:485–495. doi: 10.3892/ijo.2017.4217. [DOI] [PubMed] [Google Scholar]
- 57.Ren J., Yang Y., Xue J., Xi Z., Hu L., Pan S.-J., Sun Q. Long noncoding RNA SNHG7 promotes the progression and growth of glioblastoma via inhibition of miR-5095. Biochem. Biophys. Res. Commun. 2018;496:712–718. doi: 10.1016/j.bbrc.2018.01.109. [DOI] [PubMed] [Google Scholar]
- 58.Xu H., Zhao G., Zhang Y., Jiang H., Wang W., Zhao D., Hong J., Yu H., Qi L. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res. Ther. 2019;10:1–14. doi: 10.1186/s13287-019-1446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao C., Guo R., Guan F., Ma S., Li M., Wu J., Liu X., Li H., Yang B. MicroRNA-128-3p Enhances the Chemosensitivity of Temozolomide in Glioblastoma by Targeting c-Met and EMT. Sci. Rep. 2020;10:9471. doi: 10.1038/s41598-020-65331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osuka S., Zhu D., Zhang Z., Li C., Stackhouse C.T., Sampetrean O., Olson J.J., Gillespie G.Y., Saya H., Willey C.D., et al. IGF1/N-cadherin/b-catenin/Clusterin signaling axis can mediates adaptive radioresistance in glioblastoma; Proceedings of the AACR Annual Meeting 2021; Philadelphia, PA, USA. 10–15 April 2021. [Google Scholar]
- 61.Suwala A.K., Koch K., Rios D.H., Aretz P., Uhlmann C., Ogorek I., Felsberg J., Reifenberger G., Köhrer K., Deenen R., et al. Inhibition of Wnt/beta-catenin signaling downregulates expression of aldehyde dehydrogenase isoform 3A1 (ALDH3A1) to reduce resistance against temozolomide in glioblastoma in vitro. Oncotarget. 2018;9:22703–22716. doi: 10.18632/oncotarget.25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yun E.-J., Kim S., Hsieh J.-T., Baek S.T. Wnt/β-catenin signaling pathway induces autophagy-mediated temozolomide-resistance in human glioblastoma. Cell Death Dis. 2020;11:771. doi: 10.1038/s41419-020-02988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajesh Y., Biswas A., Kumar U., Banerjee I., Das S., Maji S., Das S.K., Emdad L., Cavenee W.K., Mandal M., et al. Lumefantrine, an antimalarial drug, reverses radiation and temozolomide resistance in glioblastoma. Proc. Natl. Acad. Sci. USA. 2020;117:12324–12331. doi: 10.1073/pnas.1921531117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deprez J., Vertommen D., Alessi D., Hue L., Rider M.H. Phosphorylation and Activation of Heart 6-Phosphofructo-2-kinase by Protein Kinase B and Other Protein Kinases of the Insulin Signaling Cascades. J. Biol. Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 65.Gottlob K., Majewski N., Kennedy S., Kandel E., Robey R.B., Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu F., Na L., Li Y., Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10:1–12. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Brennan C.W., Verhaak R.G.W., McKenna A., Campos B., Noushmehr H., Salama S.R., Zheng S., Chakravarty D., Sanborn J.Z., Berman S.H., et al. Erratum: The somatic genomic landscape of glioblastoma (Cell (2013) 155 (462-477)) Cell. 2014;157:753. doi: 10.1016/j.cell.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vadlakonda L., Pasupuleti M., Reddanna P. Role of PI3K-AKT-mTOR and Wnt signaling pathways in transition of G1-S phase of cell cycle in cancer cells. Front. Oncol. 2013;3:85. doi: 10.3389/fonc.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Von Achenbach C., Weller M., Kaulich K., Gramatzki D., Zacher A., Fabbro D., Reifenberger G., Szabó E. Synergistic growth inhibition mediated by dual PI3K/mTOR pathway targeting and genetic or direct pharmacological AKT inhibition in human glioblastoma models. J. Neurochem. 2020;153:510–524. doi: 10.1111/jnc.14899. [DOI] [PubMed] [Google Scholar]
- 70.Langhans J., Schneele L., Trenkler N., Von Bandemer H., Nonnenmacher L., Karpel-Massler G., Siegelin M.D., Zhou S., Halatsch M.-E., Debatin K.-M., et al. The effects of PI3K-mediated signalling on glioblastoma cell behaviour. Oncogenesis. 2017;6:1–9. doi: 10.1038/s41389-017-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holand K., Salm F., Arcaro A. The phosphoinositide 3-kinase signaling pathway as a therapeutic target in grade IV brain tumors. Curr. Cancer Drug Targets. 2011;11:894–918. doi: 10.2174/156800911797264743. [DOI] [PubMed] [Google Scholar]
- 72.Hartmann C., Bartels G., Gehlhaar C., Holtkamp N., von Deimling A. PIK3CA mutations in glioblastoma multiforme. Acta Neuropathol. 2005;109:639–642. doi: 10.1007/s00401-005-1000-1. [DOI] [PubMed] [Google Scholar]
- 73.Guerreiro A.S., Fattet S., Fischer B., Shalaby T., Jackson S.P., Schoenwaelder S.M., Grotzer M.A., Delattre O., Arcaro A. Targeting the PI3K p110α isoform inhibits medulloblastoma proliferation, chemoresistance, and migration. Clin. Cancer Res. 2008;14:6761–6769. doi: 10.1158/1078-0432.CCR-08-0385. [DOI] [PubMed] [Google Scholar]
- 74.Salm F., Dimitrova V., Von Bueren A.O., Ćwiek P., Rehrauer H., Djonov V., Anderle P., Arcaro A. The Phosphoinositide 3-Kinase p110α Isoform Regulates Leukemia Inhibitory Factor Receptor Expression via c-Myc and miR-125b to Promote Cell Proliferation in Medulloblastoma. PLoS ONE. 2015;10:e0123958. doi: 10.1371/journal.pone.0123958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koul D., Shen R., Bergh S., Sheng X., Shishodia S., LaFortune T.A., Lu Y., De Groot J.F., Mills G.B., Yung W.A. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol. Cancer Ther. 2006;5:637–644. doi: 10.1158/1535-7163.MCT-05-0453. [DOI] [PubMed] [Google Scholar]
- 76.Luk S.K., Piekorz R.P., Nürnberg B., To S.-S.T. The catalytic phosphoinositol 3-kinase isoform p110δ is required for glioma cell migration and invasion. Eur. J. Cancer. 2012;48:149–157. doi: 10.1016/j.ejca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Mueller W., Mizoguchi M., Silen E., D’Amore K., Nutt C.L., Louis D.N. Mutations of the PIK3CA gene are rare in human glioblastoma. Acta Neuropathol. 2005;109:654–655. doi: 10.1007/s00401-005-1001-0. [DOI] [PubMed] [Google Scholar]
- 78.Gallia G.L., Tyler B.M., Hann C.L., Siu I.-M., Giranda V.L., Vescovi A.L., Brem H., Riggins G.J. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol. Cancer Ther. 2009;8:386–393. doi: 10.1158/1535-7163.MCT-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartmann W., Digon-Söntgerath B., Koch A., Waha A., Endl E., Dani I., Denkhaus D., Goodyer C.G., Sörensen N., Wiestler O.D., et al. Phosphatidylinositol 3′-Kinase/AKT Signaling Is Activated in Medulloblastoma Cell Proliferation and Is Associated with Reduced Expression ofPTEN. Clin. Cancer Res. 2006;12:3019–3027. doi: 10.1158/1078-0432.CCR-05-2187. [DOI] [PubMed] [Google Scholar]
- 80.Kumar A., Fillmore H.L., Kadian R., Broaddus W.C., Tye G.W., van Meter T.E. The alkylphospholipid perifosine induces apoptosis and p21-mediated cell cycle arrest in medulloblastoma. Mol. Cancer Res. 2009;7:1813–1821. doi: 10.1158/1541-7786.MCR-09-0069. [DOI] [PubMed] [Google Scholar]
- 81.Naderali E., Khaki A.A., Rad J.S., Ali-Hemmati A., Rahmati M., Charoudeh H.N. Regulation and modulation of PTEN activity. Mol. Biol. Rep. 2018;45:2869–2881. doi: 10.1007/s11033-018-4321-6. [DOI] [PubMed] [Google Scholar]
- 82.Millis S.Z., Jardim D.L., Albacker L., Ross J.S., Miller V.A., Ali S.M., Kurzrock R. Phosphatidylinositol 3-kinase pathway genomic alterations in 60,991 diverse solid tumors informs targeted therapy opportunities. Cancer. 2019;125:1185–1199. doi: 10.1002/cncr.31921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang Z., Pore N., Cerniglia G.J., Mick R., Georgescu M.-M., Bernhard E.J., Hahn S.M., Gupta A.K., Maity A. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res. 2007;67:4467–4473. doi: 10.1158/0008-5472.CAN-06-3398. [DOI] [PubMed] [Google Scholar]
- 84.Juric D., Castel P., Griffith M., Griffith O., Won H.H., Ellis H., Ebbesen S.H., Ainscough B., Ramu A., Iyer G., et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature. 2015;518:240–244. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L., Zhang S., Yao J., Lowery F.J., Zhang Q., Huang W.-C., Li P., Li M., Wang X., Zhang C., et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knobbe C.B., Reifenberger G. Genetic Alterations and Aberrant Expression of Genes Related to the Phosphatidyl-lnositol-3′-Kinase/Protein Kinase B (Akt) Signal Transduction Pathway in Glioblastomas. Brain Pathol. 2006;13:507–518. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dey N., Crosswell H.E., De P., Parsons R., Peng Q., Su J.D., Durden D.L. The Protein Phosphatase Activity of PTEN Regulates Src Family Kinases and Controls Glioma Migration. Cancer Res. 2008;68:1862–1871. doi: 10.1158/0008-5472.CAN-07-1182. [DOI] [PubMed] [Google Scholar]
- 88.Mayo L.D., Dixon J.E., Durden D.L., Tonks N.K., Donner D.B. PTEN Protects p53 from Mdm2 and Sensitizes Cancer Cells to Chemotherapy. J. Biol. Chem. 2002;277:5484–5489. doi: 10.1074/jbc.M108302200. [DOI] [PubMed] [Google Scholar]
- 89.Vitucci M., Karpinich N.O., Bash R.E., Werneke A.M., Schmid R.S., White K.K., McNeill R.S., Huff B., Wang S., Van Dyke T., et al. Cooperativity between MAPK and PI3K signaling activation is required for glioblastoma pathogenesis. Neuro-Oncology. 2013;15:1317–1329. doi: 10.1093/neuonc/not084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daniele S., Costa B., Zappelli E., Da Pozzo E., Sestito S., Nesi G., Campiglia P., Marinelli L., Novellino E., Rapposelli S., et al. Combined inhibition of AKT/mTOR and MDM2 enhances Glioblastoma Multiforme cell apoptosis and differentiation of cancer stem cells. Sci. Rep. 2015;5:9956. doi: 10.1038/srep09956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Conciatori F., Ciuffreda L., Bazzichetto C., Falcone I., Pilotto S., Bria E., Cognetti F., Milella M. mTOR Cross-Talk in Cancer and Potential for Combination Therapy. Cancers. 2018;10:23. doi: 10.3390/cancers10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D.-H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/S0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 93.Sarbassov D.D., Ali S.M., Kim D.-H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. Rictor, a Novel Binding Partner of mTOR, Defines a Rapamycin-Insensitive and Raptor-Independent Pathway that Regulates the Cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 94.Duzgun Z., Eroglu Z., Avci C.B. Role of mTOR in glioblastoma. Gene. 2016;575:187–190. doi: 10.1016/j.gene.2015.08.060. [DOI] [PubMed] [Google Scholar]
- 95.Wu S.-H., Bi J.-F., Cloughesy T., Cavenee W.K., Mischel P.S. Emerging function of mTORC2 as a core regulator in glioblastoma: Metabolic reprogramming and drug resistance. Cancer Biol. Med. 2014;11:255–263. doi: 10.7497/j.issn.2095-3941.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gini B., Zanca C., Guo D., Matsutani T., Masui K., Ikegami S., Yang H., Nathanson D., Villa G.R., Shackelford D., et al. The mTOR kinase inhibitors, CC214-1 and CC214-2, preferentially block the growth of EGFRvIII-activated glioblastomas. Clin. Cancer Res. 2013;19:5722–5732. doi: 10.1158/1078-0432.CCR-13-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rich J.N., Hans C., Jones B., Iversen E.S., McLendon R.E., Rasheed B.K.A., Dobra A., Dressman H.K., Bigner D.D., Nevins J.R., et al. Gene Expression Profiling and Genetic Markers in Glioblastoma Survival. Cancer Res. 2005;65:4051–4058. doi: 10.1158/0008-5472.CAN-04-3936. [DOI] [PubMed] [Google Scholar]
- 98.Laplante M., Sabatini D.M. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen S., Han Q., Wang X., Yang M., Zhang Z., Li P., Chen A., Hu C., Li S. IBP-mediated suppression of autophagy promotes growth and metastasis of breast cancer cells via activating mTORC2/Akt/FOXO3a signaling pathway. Cell Death Dis. 2013;4:e842. doi: 10.1038/cddis.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mecca C., Giambanco I., Donato R., Arcuri C. Targeting mTOR in Glioblastoma: Rationale and Preclinical/Clinical Evidence. Dis. Markers. 2018;2018:1–10. doi: 10.1155/2018/9230479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Altman J.K., Szilard A., Goussetis D.J., Sassano A., Colamonici M., Gounaris E., Frankfurt O., Giles F.J., Eklund E.A., Beauchamp E., et al. Autophagy is a survival mechanism of acute myelogenous leukemia precursors during dual mTORC2/mTORC1 targeting. Clin. Cancer Res. 2014;20:2400–2409. doi: 10.1158/1078-0432.CCR-13-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gan X., Wang J., Wang C., Sommer E.M., Kozasa T., Srinivasula S.M., Alessi D., Offermanns S., Simon M.I., Wu D. PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Gα 12. Nat. Cell Biol. 2012;14:686–696. doi: 10.1038/ncb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hütt-Cabezas M., Karajannis M.A., Zagzag D., Shah S., Horkayne-Szakaly I., Rushing E.J., Cameron J.D., Jain D., Eberhart C.G., Raabe E.H., et al. Activation of mTORC1/mTORC2 signaling in pediatric low-grade glioma and pilocytic astrocytoma reveals mTOR as a therapeutic target. Neuro-Oncology. 2013;15:1604–1614. doi: 10.1093/neuonc/not132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li X., Gao T. mTORC 2 phosphorylates protein kinase Cζ to regulate its stability and activity. EMBO Rep. 2014;15:191–198. doi: 10.1002/embr.201338119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 106.Thomanetz V., Angliker N., Cloëtta D., Lustenberger R.M., Schweighauser M., Oliveri F., Suzuki N., Rüegg M.A. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J. Cell Biol. 2013;201:293–308. doi: 10.1083/jcb.201205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang M., Ke Y., Sun X., Yu L., Yang Z., Zhang Y., Du M., Wang J., Liu X., Huang S. Mammalian target of rapamycin signaling is involved in the vasculogenic mimicry of glioma via hypoxia-inducible factor-1α. Oncol. Rep. 2014;32:1973–1980. doi: 10.3892/or.2014.3454. [DOI] [PubMed] [Google Scholar]
- 108.Jhanwar-Uniyal M., Gulati N., Karsy M., Albert L., Murali R. Involvement of mTORC1 and mTORC2 in regulation of glioblastoma multiforme growth and motility. Int. J. Oncol. 2009;35:731–740. doi: 10.3892/ijo_00000386. [DOI] [PubMed] [Google Scholar]
- 109.Nagy J.A., Dvorak H.F. Heterogeneity of the tumor vasculature: The need for new tumor blood vessel type-specific targets. Clin. Exp. Metastasis. 2012;29:657–662. doi: 10.1007/s10585-012-9500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahir B.K., Engelhard H.H., Lakka S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020;57:2461–2478. doi: 10.1007/s12035-020-01892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gacche R.N., Meshram R.J. Angiogenic factors as potential drug target: Efficacy and limitations of anti-angiogenic therapy. Biochim. Biophys. Acta. 2014;1846:161–179. doi: 10.1016/j.bbcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 112.Batchelor T.T., Reardon D.A., De Groot J.F., Wick W., Weller M. Antiangiogenic Therapy for Glioblastoma: Current Status and Future Prospects. Clin. Cancer Res. 2014;20:5612–5619. doi: 10.1158/1078-0432.CCR-14-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Franklin R.A., Montalto G., Cervello M., Libra M., Candido S., Malaponte G., et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: How mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 2012;3:1068–1111. doi: 10.18632/oncotarget.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nicholas M.K., Lukas R.V., Jafri N.F., Faoro L., Salgia R. Epidermal Growth Factor Receptor–Mediated Signal Transduction in the Development and Therapy of Gliomas. Clin. Cancer Res. 2006;12:7261–7270. doi: 10.1158/1078-0432.CCR-06-0874. [DOI] [PubMed] [Google Scholar]
- 115.Vogt P.K., Hart J. PI3K and STAT3: A New Alliance. Cancer Discov. 2011;1:481–486. doi: 10.1158/2159-8290.CD-11-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu Y., Liu X., Zhao P., Zhao H., Gao W., Wang L. Celastrol Suppresses Glioma Vasculogenic Mimicry Formation and Angiogenesis by Blocking the PI3K/Akt/mTOR Signaling Pathway. Front. Pharmacol. 2020;11:25. doi: 10.3389/fphar.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choi E.J., Cho B.J., Lee D.J., Hwang Y.H., Chun S.H., Kim H.H., Kim I.A. Enhanced cytotoxic effect of radiation and temozolomide in malignant glioma cells: Targeting PI3K-AKT-mTOR signaling, HSP90 and histone deacetylases. BMC Cancer. 2014;14:17. doi: 10.1186/1471-2407-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Cristofano A., Pandolfi P.P. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/S0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 119.Kessler T., Sahm F., Blaes J., Osswald M., Rübmann P., Milford D., Urban S., Jestaedt L., Heiland S., Bendszus M., et al. Glioma cell VEGFR-2 confers resistance to chemotherapeutic and antiangiogenic treatments in PTEN-deficient glioblastoma. Oncotarget. 2015;6:31050–31068. doi: 10.18632/oncotarget.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Datta S., Brunet A., Greenberg M.E. Cellular survival: A play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 121.Ghosh M.K., Sharma P., Harbor P.C., Rahaman S.O., Haque S.J. PI3K-AKT pathway negatively controls EGFR-dependent DNA-binding activity of Stat3 in glioblastoma multiforme cells. Oncogene. 2005;24:7290–7300. doi: 10.1038/sj.onc.1208894. [DOI] [PubMed] [Google Scholar]
- 122.Colardo M., Segatto M., Di Bartolomeo S. Targeting RTK-PI3K-mTOR Axis in Gliomas: An Update. Int. J. Mol. Sci. 2021;22:4899. doi: 10.3390/ijms22094899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zuccarini M., Giuliani P., Ziberi S., Carluccio M., Di Iorio P., Caciagli F., Ciccarelli R. The Role of Wnt Signal in Glioblastoma Development and Progression: A Possible New Pharmacological Target for the Therapy of This Tumor. Genes. 2018;9:105. doi: 10.3390/genes9020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Robert J. Textbook of Cell Signalling in Cancer. Springer; Singapore: 2015. [Google Scholar]
- 125.Johnson B.E., Mazor T., Hong C., Barnes M., Aihara K., McLean C.Y., Fouse S.D., Yamamoto S., Ueda H., Tatsuno K., et al. Mutational Analysis Reveals the Origin and Therapy-Driven Evolution of Recurrent Glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamada D., Hoshii T., Tanaka S., Hegazy A.M., Kobayashi M., Tadokoro Y., Ohta K., Ueno M., Ali M.A., Hirao A. Loss of Tsc1 accelerates malignant gliomagenesis when combined with oncogenic signals. J. Biochem. 2013;155:227–233. doi: 10.1093/jb/mvt112. [DOI] [PubMed] [Google Scholar]
- 127.Inoki K., Zhu T., Guan K.-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell. 2003;115:577–590. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 128.Byron S.A., Tran N.L., Halperin R.F., Phillips J.J., Kuhn J.G., De Groot J.F., Colman H., Ligon K.L., Wen P.Y., Cloughesy T.F., et al. Prospective Feasibility Trial for Genomics-Informed Treatment in Recurrent and Progressive Glioblastoma. Clin. Cancer Res. 2018;24:295–305. doi: 10.1158/1078-0432.CCR-17-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu Z., Zhao G., Xie G., Zhao L., Chen Y., Yu H., Zhang Z., Li C., Li Y. Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells in vitro and in vivo. Oncotarget. 2015;6:32930–32943. doi: 10.18632/oncotarget.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roos A., Dhruv H.D., Peng S., Inge L.J., Tuncali S., Pineda M., Millard N., Mayo Z., Eschbacher J.M., Loftus J.C., et al. EGFRvIII–Stat5 Signaling Enhances Glioblastoma Cell Migration and Survival. Mol. Cancer Res. 2018;16:1185–1195. doi: 10.1158/1541-7786.MCR-18-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shin S.Y., Kim C.G., Kim S.H., Kim Y.S., Lim Y., Lee Y.H. Chlorpromazine activates p21 Waf1/Cip1 gene transcription via early growth response-1 (Egr-1) in C6 glioma cells. Exp. Mol. Med. 2010;42:395–405. doi: 10.3858/emm.2010.42.5.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou W., Liu L., Xue Y., Zheng J., Liu X., Ma J., Li Z., Liu Y. Combination of endothelial-monocyte-activating polypeptide-II with temozolomide suppress malignant biological behaviors of human glioblastoma stem cells via miR-590-3p/MACC1 Inhibiting PI3K/AKT/mTOR signal path-way. Front. Mol. Neurosci. 2017;10:68. doi: 10.3389/fnmol.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burger M.T., Pecchi S., Wagman A., Ni Z.-J., Knapp M., Hendrickson T., Atallah G., Pfister K., Zhang Y., Bartulis S., et al. Identification of NVP-BKM120 as a Potent, Selective, Orally Bioavailable Class I PI3 Kinase Inhibitor for Treating Cancer. ACS Med. Chem. Lett. 2011;2:774–779. doi: 10.1021/ml200156t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koul D., Fu J., Shen R., LaFortune T.A., Wang S., Tiao N., Kim Y.-W., Liu J.-L., Ramnarian D., Yuan Y., et al. Antitumor Activity of NVP-BKM120—A Selective Pan Class I PI3 Kinase Inhibitor Showed Differential Forms of Cell Death Based on p53 Status of Glioma Cells. Clin. Cancer Res. 2012;18:184–195. doi: 10.1158/1078-0432.CCR-11-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wen P.Y., Touat M., Alexander B.M., Mellinghoff I.K., Ramkissoon S., McCluskey C.S., Pelton K., Haidar S., Basu S.S., Gaffey S.C., et al. Buparlisib in patients with recurrent glioblastoma harboring phosphatidylinositol 3-kinase pathway activation: An open-label, multicenter, multi-arm, phase II trial. J. Clin. Oncol. 2019;37:741–750. doi: 10.1200/JCO.18.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gwak H.S., Shingu T., Chumbalkar V., Hwang Y.-H., DeJournett R., Latha K., Koul D., Yung W.K.A., Powis G., Farrell N.P., et al. Combined action of the dinuclear platinum compound BBR3610 with the PI3-K inhibitor PX-866 in glioblastoma. Int. J. Cancer. 2011;128:787–796. doi: 10.1002/ijc.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koul D., Shen R., Kim Y.-W., Kondo Y., Lu Y., Bankson J., Ronen S.M., Kirkpatrick D.L., Powis G., Yung W.K.A. Cellular and in vivo activity of a novel PI3K inhibitor, PX-866, against human glioblastoma. Neuro-Oncology. 2010;12:559–569. doi: 10.1093/neuonc/nop058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pitz M.W., Eisenhauer E.A., MacNeil M.V., Thiessen B., Easaw J.C., Macdonald D.R., Eisenstat D.D., Kakumanu A.S., Salim M., Chalchal H., et al. Phase II study of PX-866 in recurrent glioblastoma. Neuro-Oncology. 2015;17:1270–1274. doi: 10.1093/neuonc/nou365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu F., Hon G.C., Villa G.R., Turner K.M., Ikegami S., Yang H., Ye Z., Li B., Kuan S., Lee A.Y., et al. EGFR Mutation Promotes Glioblastoma through Epigenome and Transcription Factor Network Remodeling. Mol. Cell. 2015;60:307–318. doi: 10.1016/j.molcel.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tanaka K., Babic I., Nathanson D., Akhavan D., Guo D., Gini B., Dang J., Zhu S., Yang H., de Jesus J., et al. Oncogenic EGFR signaling activates an mTORC2–NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1:524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pao W., Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat. Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang C.-Y., Kuan Y.-H., Ou Y.-C., Li J.-R., Wu C.-C., Pan P.-H., Chen W.-Y., Huang H.-Y., Chen C.-J. Autophagy contributes to gefitinib-induced glioma cell growth inhibition. Exp. Cell Res. 2014;327:102–112. doi: 10.1016/j.yexcr.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 143.Pédeboscq S., L’Azou B., Passagne I., De Giorgi F., Ichas F., Pometan J.-P., Cambar J. Cytotoxic and apoptotic effects of bortezomib and gefitinib compared to alkylating agents on human glioblastoma cells. J. Exp. Ther. Oncol. 2008;7:99–111. [PubMed] [Google Scholar]
- 144.Thress K.S., Paweletz C.P., Felip E., Cho B.C., Stetson D., Dougherty B., Lai Z., Markovets A., Vivancos A., Kuang Y., et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat. Med. 2015;21:560–562. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yosaatmadja Y., Silva S., Dickson J.M., Patterson A.V., Smaill J.B., Flanagan J.U., Mckeage M., Squire C.J. Binding mode of the breakthrough inhibitor AZD9291 to epidermal growth factor receptor revealed. J. Struct. Biol. 2015;192:539–544. doi: 10.1016/j.jsb.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 146.Zhao H.-F., Wang J., Shao W., Wu C.-P., Chen Z.-P., To S.S.T., Li W.-P. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: Current preclinical and clinical development. Mol. Cancer. 2017;16:100. doi: 10.1186/s12943-017-0670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tzeng H.-E., Yang L., Chen K., Wang Y., Liu Y.-R., Pan S.-L., Gaur S., Hu S., Yen Y. The pan-PI3K inhibitor GDC-0941 activates canonical WNT signaling to confer resistance in TNBC cells: Resistance reversal with WNT inhibitor. Oncotarget. 2015;6:11061–11073. doi: 10.18632/oncotarget.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raynaud F.I., Eccles S.A., Patel S., Alix S., Box G., Chuckowree I., Folkes A., Gowan S., Brandon A.D.H., Di Stefano F., et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: From PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol. Cancer Ther. 2009;8:1725–1738. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Folkes A.J., Ahmadi K., Alderton W.K., Alix S., Baker S.J., Box G., Chuckowree I.S., Clarke P.A., Depledge P., Eccles S.A., et al. The Identification of 2-(1H-Indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a Potent, Selective, Orally Bioavailable Inhibitor of Class I PI3 Kinase for the Treatment of Cancer. J. Med. Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 150.Ndubaku C.O., Heffron T.P., Staben S.T., Baumgardner M., Blaquiere N., Bradley E., Bull R., Do S., Dotson J., Dudley D., et al. Discovery of 2-{3-[2-(1-Isopropyl-3-methyl-1 H-1, 2–4-triazol-5-yl)-5, 6-dihydrobenzo [f] imidazo [1, 2-d][1, 4] oxazepin-9-yl]-1 H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): A β-sparing phosphoinositide 3-kinase inhib-itor with high unbound exposure and robust in vivo antitumor activity. J. Med. Chem. 2013;56:4597–4610. doi: 10.1021/jm4003632. [DOI] [PubMed] [Google Scholar]
- 151.Maira S.-M., Pecchi S., Huang A., Burger M., Knapp M., Sterker D., Schnell C., Guthy D., Nagel T., Wiesmann M., et al. Identification and Characterization of NVP-BKM120, an Orally Available Pan-Class I PI3-Kinase Inhibitor. Mol. Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 152.Foster P., Yamaguchi K., Hsu P.P., Qian F., Du X., Wu J., Won K.-A., Yu P., Jaeger C.T., Zhang W., et al. The Selective PI3K Inhibitor XL147 (SAR245408) Inhibits Tumor Growth and Survival and Potentiates the Activity of Chemotherapeutic Agents in Preclinical Tumor Models. Mol. Cancer Ther. 2015;14:931–940. doi: 10.1158/1535-7163.MCT-14-0833. [DOI] [PubMed] [Google Scholar]
- 153.Liu N., Rowley B.R., Bull C.O., Schneider C., Haegebarth A., Schatz C.A., Fracasso P.R., Wilkie D.P., Hentemann M., Wilhelm S.M., et al. BAY 80-6946 Is a Highly Selective Intravenous PI3K Inhibitor with Potent p110α and p110δ Activities in Tumor Cell Lines and Xenograft Models. Mol. Cancer Ther. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 154.Scott W.J., Hentemann M.F., Rowley R.B., Bull C.O., Jenkins S., Bullion A.M., Johnson J., Redman A., Robbins A.H., Esler W., et al. Discovery and SAR of novel 2, 3-dihydroimidazo [1, 2-c] quinazoline PI3K inhibitors: Identification of copanlisib (BAY 80-6946) ChemMedChem. 2016;11:1517–1530. doi: 10.1002/cmdc.201600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ihle N.T., Paine-Murrieta G., Berggren M.I., Baker A., Tate W.R., Wipf P., Abraham R.T., Kirkpatrick D.L., Powis G. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non–small cell lung cancer xenografts. Mol. Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lin L., Gaut D., Hu K., Yan H., Yin D., Koeffler H.P. Dual targeting of glioblastoma multiforme with a proteasome inhibitor (Velcade) and a phosphatidylinositol 3-kinase inhibitor (ZSTK474) Int. J. Oncol. 2013;44:557–562. doi: 10.3892/ijo.2013.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kong D., Yamori T. ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms. Cancer Sci. 2007;98:1638–1642. doi: 10.1111/j.1349-7006.2007.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Norman M.H., Andrews K.L., Bo Y.Y., Booker S.K., Caenepeel S., Cee V.J., D’angelo N.D., Freeman D.J., Herberich B.J., Hong F.-T., et al. Selective Class I Phosphoinositide 3-Kinase Inhibitors: Optimization of a Series of Pyridyltriazines Leading to the Identification of a Clinical Candidate, AMG 511. J. Med. Chem. 2012;55:7796–7816. doi: 10.1021/jm300846z. [DOI] [PubMed] [Google Scholar]
- 159.Furet P., Guagnano V., Fairhurst R.A., Imbach-Weese P., Bruce I., Knapp M., Fritsch C., Blasco F., Blanz J., Aichholz R., et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 2013;23:3741–3748. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 160.Lannutti B.J., Meadows S.A., Herman S.E.M., Kashishian A., Steiner B., Johnson A.J., Byrd J.C., Tyner J.W., Loriaux M.M., Deininger M., et al. CAL-101, a p110δ selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao H.-F., Wang J., Jiang H.-R., Chen Z.-P., To S.S.T. PI3K p110β isoform synergizes with JNK in the regulation of glioblastoma cell proliferation and migra-tion through Akt and FAK inhibition. J. Exp. Clin. Cancer Res. 2016;35:78. doi: 10.1186/s13046-016-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cushing T.D., Hao X., Shin Y., Andrews K., Brown M., Cardozo M., Chen Y., Duquette J., Fisher B., de Turiso F.G.-L., et al. Discovery and in Vivo Evaluation of (S)-N-(1-(7-Fluoro-2-(pyridin-2-yl)quinolin-3-yl)ethyl)-9H-purin-6-amine (AMG319) and Related PI3Kδ Inhibitors for Inflammation and Autoimmune Disease. J. Med. Chem. 2015;58:480–511. doi: 10.1021/jm501624r. [DOI] [PubMed] [Google Scholar]
- 163.Nylander S., Kull B., Björkman J.A., Ulvinge J.C., Oakes N., Emanuelsson B.M., Andersson M., Skärby T., Inghardt T., Fjellström O., et al. Human target validation of phosphoinositide 3-kinase (PI3K) β: Effects on platelets and insulin sensitivity, using AZD6482 a novel PI3Kβ inhibitor. J. Thromb. Haemost. 2012;10:2127–2136. doi: 10.1111/j.1538-7836.2012.04898.x. [DOI] [PubMed] [Google Scholar]
- 164.Ohwada J., Ebiike H., Kawada H., Tsukazaki M., Nakamura M., Miyazaki T., Morikami K., Yoshinari K., Yoshida M., Kondoh O., et al. Discovery and biological activity of a novel class I PI3K inhibitor, CH5132799. Bioorg. Med. Chem. Lett. 2011;21:1767–1772. doi: 10.1016/j.bmcl.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 165.Camps M., Rückle T., Ji H., Ardissone V., Rintelen F., Shaw J., Ferrandi C., Chabert C., Gillieron C., Françon B., et al. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 166.So L., Yea S.S., Oak J.S., Lu M., Manmadhan A., Ke Q.H., Janes M.R., Kessler L.V., Kucharski J.M., Li L.-S., et al. Selective Inhibition of Phosphoinositide 3-Kinase p110α Preserves Lymphocyte Function. J. Biol. Chem. 2013;288:5718–5731. doi: 10.1074/jbc.M112.379446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maira S.-M., Stauffer F., Brueggen J., Furet P., Schnell C., Fritsch C., Brachmann S., Chène P., de Pover A., Schoemaker K., et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 168.Yu Z., Xie G., Zhou G., Cheng Y., Zhang G., Yao G., Chen Y., Li Y., Zhao G. NVP-BEZ235, a novel dual PI3K–mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells. Cancer Lett. 2015;367:58–68. doi: 10.1016/j.canlet.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 169.Knight S.D., Adams N.D., Burgess J.L., Chaudhari A.M., Darcy M.G., Donatelli C.A., Luengo J.I., Newlander K.A., Parrish C.A., Ridgers L.H., et al. Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. ACS Med. Chem. Lett. 2010;1:39–43. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yu P., Laird A.D., Du X., Wu J., Won K.-A., Yamaguchi K., Hsu P.P., Qian F., Jaeger C.T., Zhang W., et al. Characterization of the Activity of the PI3K/mTOR Inhibitor XL765 (SAR245409) in Tumor Models with Diverse Genetic Alterations Affecting the PI3K Pathway. Mol. Cancer Ther. 2014;13:1078–1091. doi: 10.1158/1535-7163.MCT-13-0709. [DOI] [PubMed] [Google Scholar]
- 171.Sutherlin D.P., Bao L., Berry M., Castanedo G., Chuckowree I., Dotson J., Folks A., Friedman L., Goldsmith R., Gunzner J., et al. Discovery of a potent, selective, and orally available class I phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) kinase inhibitor (GDC-0980) for the treatment of cancer. J. Med. Chem. 2011;54:7579–7587. doi: 10.1021/jm2009327. [DOI] [PubMed] [Google Scholar]
- 172.Heffron T.P., Ndubaku C.O., Salphati L., Alicke B., Cheong J., Drobnick J., Edgar K., Gould S.E., Lee L.B., Lesnick J.D., et al. Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of PI3K and mTOR. ACS Med. Chem. Lett. 2016;7:351–356. doi: 10.1021/acsmedchemlett.6b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hart S., Novotny-Diermayr V., Goh K.C., Williams M., Tan Y.C., Ong L.C., Cheong A., Ng B.K., Amalini C., Madan B., et al. VS-5584, a Novel and Highly Selective PI3K/mTOR Kinase Inhibitor for the Treatment of Cancer. Mol. Cancer Ther. 2013;12:151–161. doi: 10.1158/1535-7163.MCT-12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yuan J., Mehta P.P., Yin M.-J., Sun S., Zou A., Chen J., Rafidi K., Feng Z., Nickel J., Engebretsen J., et al. PF-04691502, a Potent and Selective Oral Inhibitor of PI3K and mTOR Kinases with Antitumor Activity. Mol. Cancer Ther. 2011;10:2189–2199. doi: 10.1158/1535-7163.MCT-11-0185. [DOI] [PubMed] [Google Scholar]
- 175.Venkatesan A.M., Dehnhardt C.M., Santos E.D., Chen Z., Santos O.D., Ayral-Kaloustian S., Khafizova G., Brooijmans N., Mallon R., Hollander I., et al. Bis (morpholino-1, 3, 5-triazine) derivatives: Potent adenosine 5′-triphosphate competitive phos-phatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: Discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J. Med. Chem. 2010;53:2636–2645. doi: 10.1021/jm901830p. [DOI] [PubMed] [Google Scholar]
- 176.Friedman H.S., Prados M.D., Wen P.Y., Mikkelsen T., Schiff D., Abrey L.E., Yung W.A., Paleologos N., Nicholas M.K., Jensen R., et al. Bevacizumab Alone and in Combination with Irinotecan in Recurrent Glioblastoma. J. Clin. Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 177.Kreisl T.N., Kim L., Moore K., Duic P., Royce C., Stroud I., Garren N., Mackey M., Butman J.A., Camphausen K., et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jain R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 179.Takano S., Ishikawa E., Nakai K., Masumoto T., Yamamoto T., Matsumura A., Matsuda M. Bevacizumab in Japanese patients with malignant glioma: From basic research to clinical trial. OncoTargets Ther. 2014;7:1551–1562. doi: 10.2147/OTT.S67621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tamura R., Tanaka T., Miyake K., Tabei Y., Ohara K., Sampetrean O., Kono M., Mizutani K., Yamamoto Y., Murayama Y., et al. Histopathological investigation of glioblastomas resected under bevacizumab treatment. Oncotarget. 2016;7:52423–52435. doi: 10.18632/oncotarget.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gilbert M.R., Dignam J.J., Armstrong T.S., Wefel J.S., Blumenthal D.T., Vogelbaum M.A., Colman H., Chakravarti A., Pugh S., Won M., et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gil-Gil M.J., Mesia C., Rey M., Bruna J. Bevacizumab for the Treatment of Glioblastoma. Clin. Med. Insights Oncol. 2013;7:123–135. doi: 10.4137/CMO.S8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tamura R., Tanaka T., Miyake K., Yoshida K., Sasaki H. Bevacizumab for malignant gliomas: Current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 2017;34:62–77. doi: 10.1007/s10014-017-0284-x. [DOI] [PubMed] [Google Scholar]
- 184.Lu K.V., Chang J.P., Parachoniak C.A., Pandika M.M., Aghi M.K., Meyronet D., Isachenko N., Fouse S.D., Phillips J.J., Cheresh D.A., et al. VEGF Inhibits Tumor Cell Invasion and Mesenchymal Transition through a MET/VEGFR2 Complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Labussière M., Cheneau C., Prahst C., Pérez-Larraya J.G., Farina P., Lombardi G., Mokhtari K., Rahimian A., Delattre J., Eichmann A., et al. Angiopoietin-2 may be involved in the resistance to bevacizumab in recurrent glioblastoma. Cancer Investig. 2016;34:39–44. doi: 10.3109/07357907.2015.1088948. [DOI] [PubMed] [Google Scholar]
- 186.Carmeliet P., Moons L., Luttun A., Vincenti V., Compernolle V., de Mol M., Wu Y., Bono F., Devy L., Becket H., et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 187.Tabouret E., Denicolaï E., Delfino C., Graillon T., Boucard C., Nanni I., Padovani L., Figarella-Branger D., Chinot O. Changes in PlGF and MET-HGF expressions in paired initial and recurrent glioblastoma. J. Neuro-Oncol. 2016;130:431–437. doi: 10.1007/s11060-016-2251-5. [DOI] [PubMed] [Google Scholar]
- 188.Lassen U., Chinot O.L., McBain C., Mau-Sørensen M., Larsen V.A., Barrie M., Roth P., Krieter O., Wang K., Habben K., et al. Phase 1 dose-escalation study of the antiplacental growth factor monoclonal antibody RO5323441 combined with bevacizumab in patients with recurrent glioblastoma. Neuro-Oncology. 2015;17:1007–1015. doi: 10.1093/neuonc/nov019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wick W., Puduvalli V.K., Chamberlain M.C., van den Bent M.J., Carpentier A.F., Cher L.M., Mason W., Weller M., Hong S., Musib L., et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J. Clin. Oncol. 2010;28:1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Levin V.A., Chan J., Datta M., Yee J.L., Jain R.K. Effect of angiotensin system inhibitors on survival in newly diagnosed glioma patients and recurrent glioblastoma patients receiving chemotherapy and/or bevacizumab. J. Neuro-Oncol. 2017;134:325–330. doi: 10.1007/s11060-017-2528-3. [DOI] [PubMed] [Google Scholar]
- 191.Carpentier A.F., Ferrari D., Bailon O., Ursu R., Banissi C., Dubessy A.-L., Belin C., Levy C. Steroid-sparing effects of angiotensin-II inhibitors in glioblastoma patients. Eur. J. Neurol. 2012;19:1337–1342. doi: 10.1111/j.1468-1331.2012.03766.x. [DOI] [PubMed] [Google Scholar]
- 192.Januel E., Ursu R., Alkhafaji A., Marantidou A., Doridam J., Belin C., Levy-Piedbois C., Carpentier A.F. Impact of renin-angiotensin system blockade on clinical outcome in glioblastoma. Eur. J. Neurol. 2015;22:1304–1309. doi: 10.1111/ene.12746. [DOI] [PubMed] [Google Scholar]
- 193.Arrieta O., Guevara P., Escobar E., García-Navarrete R., Pineda B., Sotelo J. Blockage of angiotensin II type I receptor decreases the synthesis of growth factors and induces apoptosis in C6 cultured cells and C6 rat glioma. Br. J. Cancer. 2005;92:1247–1252. doi: 10.1038/sj.bjc.6602483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Warburg O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925;9:148–163. doi: 10.1158/jcr.1925.148. [DOI] [Google Scholar]
- 195.Strickland M., Stoll E.A. Metabolic Reprogramming in Glioma. Front. Cell Dev. Biol. 2017;5:43. doi: 10.3389/fcell.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Heiden M.G.V., Cantley L.C., Thompson C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Woolf E.C., Curley K.L., Liu Q., Turner G.H., Charlton J.A., Preul M.C., Scheck A.C. The ketogenic diet alters the hypoxic response and affects expression of proteins associated with angio-genesis, invasive potential and vascular permeability in a mouse glioma model. PLoS ONE. 2015;10:e0130357. doi: 10.1371/journal.pone.0130357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.De la Cruz-López K.G., Castro-Muñoz L.J., Reyes-Hernández D.O., García-Carrancá A., Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol. 2019;9:1143. doi: 10.3389/fonc.2019.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Corbet C., Pinto A., Martherus R., de Jesus J.P.S., Polet F., Feron O. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 2016;24:311–323. doi: 10.1016/j.cmet.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 200.Minami N., Tanaka K., Sasayama T., Kohmura E., Saya H., Sampetrean O. Lactate Reprograms Energy and Lipid Metabolism in Glucose-Deprived Oxidative Glioma Stem Cells. Metabolites. 2021;11:325. doi: 10.3390/metabo11050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lin H., Patel S., Affleck V.S., Wilson I., Turnbull D., Joshi A.R., Maxwell R., Stoll E.A. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro-Oncology. 2017;19:43–54. doi: 10.1093/neuonc/now128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maher E.A., Marin-Valencia I., Bachoo R.M., Mashimo T., Raisanen J., Hatanpaa K.J., Jindal A., Jeffrey F.M., Choi C., Madden C., et al. Metabolism of [U-13C] glucose in human brain tumors in vivo. NMR Biomed. 2012;25:1234–1244. doi: 10.1002/nbm.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mashimo T., Pichumani K., Vemireddy V., Hatanpaa K.J., Singh D., Sirasanagandla S., Nannepaga S., Piccirillo S.G.M., Kovacs Z., Foong C., et al. Acetate Is a Bioenergetic Substrate for Human Glioblastoma and Brain Metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kant S., Kesarwani P., Prabhu A., Graham S.F., Buelow K.L., Nakano I., Chinnaiyan P. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dy-namic nutrient microenvironment. Cell Death Dis. 2020;11:253. doi: 10.1038/s41419-020-2449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tao B.-B., He H., Shi X.-H., Wang C.-L., Li W.-Q., Li B., Dong Y., Hu G.-H., Hou L.-J., Luo C., et al. Up-regulation of USP2a and FASN in gliomas correlates strongly with glioma grade. J. Clin. Neurosci. 2013;20:717–720. doi: 10.1016/j.jocn.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 206.Michalak K.P., Maćkowska-Kędziora A., Sobolewski B., Woźniak P. Key Roles of Glutamine Pathways in Reprogramming the Cancer Metabolism. Oxidative Med. Cell. Longev. 2015;2015:1–14. doi: 10.1155/2015/964321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tardito S., Oudin A., Ahmed S.U., Fack F., Keunen O., Zheng L., Miletic H., Sakariassen P.Ø., Weinstock A., Wagner A., et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 2015;17:1556–1568. doi: 10.1038/ncb3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oizel K., Chauvin-Fleurence C., Oliver L., Gratas C., Geraldo F., Jarry U., Scotet E., Rabe M., Alves-Guerra M.-C., Teusan R., et al. Efficient Mitochondrial Glutamine Targeting Prevails Over Glioblastoma Metabolic Plasticity. Clin. Cancer Res. 2017;23:6292–6304. doi: 10.1158/1078-0432.CCR-16-3102. [DOI] [PubMed] [Google Scholar]
- 209.Rieger J., Bähr O., Maurer G.D., Hattingen E., Franz K., Brucker D., Walenta S., Kämmerer U., Coy J.F., Weller M., et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int. J. Oncol. 2014;44:1843–1852. doi: 10.3892/ijo.2014.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Keunen O., Johansson M., Oudin A., Sanzey M., Rahim S.A.A., Fack F., Thorsen F., Taxt T., Bartoš M., Jirik R., et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl. Acad. Sci. USA. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kuang R., Jahangiri A., Mascharak S., Nguyen A., Chandra A., Flanigan P.M., Yagnik G., Wagner J.R., de Lay M., Carrera D., et al. GLUT3 upregulation promotes metabolic reprogramming associated with antiangiogenic therapy resistance. JCI Insight. 2017;2:e88815. doi: 10.1172/jci.insight.88815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ruiz-Ontañon P., Orgaz J.L., Aldaz B., Elosegui-Artola A., Martino J., Berciano M.T., Montero J.A., Grande L., Nogueira L., Diaz-Moralli S., et al. Cellular Plasticity Confers Migratory and Invasive Advantages to a Population of Glioblastoma-Initiating Cells that Infiltrate Peritumoral Tissue. Stem Cells. 2013;31:1075–1085. doi: 10.1002/stem.1349. [DOI] [PubMed] [Google Scholar]
- 213.Abbadi S., Rodarte J.J., Abutaleb A., Lavell E., Smith C.L., Ruff W., Schiller J., Olivi A., Levchenko A., Guerrero-Cazares H., et al. Glucose-6–phosphatase Is a Key Metabolic Regulator of Glioblastoma Invasion. Mol. Cancer Res. 2014;12:1547–1559. doi: 10.1158/1541-7786.MCR-14-0106-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Libby C., Tran A., Scott S.E., Griguer C., Hjelmeland A.B. The pro-tumorigenic effects of metabolic alterations in glioblastoma including brain tumor initiating cells. Biochim. Biophys. Acta. 2018;1869:175–188. doi: 10.1016/j.bbcan.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hardie D.G., Hawley S.A. AMP-activated protein kinase: The energy charge hypothesis revisited. BioEssays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 216.Choo A.Y., Roux P.P., Blenis J. Mind the GAP: Wnt Steps onto the mTORC1 Train. Cell. 2006;126:834–836. doi: 10.1016/j.cell.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 217.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Masui K., Tanaka K., Akhavan D., Babic I., Gini B., Matsutani T., Iwanami A., Liu F., Villa G.R., Gu Y., et al. mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell Metab. 2013;18:726–739. doi: 10.1016/j.cmet.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Miller D.M., Thomas S.D., Islam A., Muench D., Sedoris K. c-Myc and Cancer Metabolism. Clin. Cancer Res. 2012;18:5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Boado R.J., Black K.L., Pardridge W. Gene expression of GLUT3 and GLUT1 glucose transporters in human brain tumors. Mol. Brain Res. 1994;27:51–57. doi: 10.1016/0169-328X(94)90183-X. [DOI] [PubMed] [Google Scholar]
- 221.Wieman H.L., Wofford J.A., Rathmell J.C. Cytokine Stimulation Promotes Glucose Uptake via Phosphatidylinositol-3 Kinase/Akt Regulation of Glut1 Activity and Trafficking. Mol. Biol. Cell. 2007;18:1437–1446. doi: 10.1091/mbc.e06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Birner P., Piribauer M., Fischer I., Gatterbauer B., Marosi C., Ambros P.F., Ambros I.M., Bredel M., Oberhuber G., Rössler K., et al. Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: Evidence for distinct angiogenic subtypes. Brain Pathol. 2003;13:133–143. doi: 10.1111/j.1750-3639.2003.tb00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Flynn J.R., Wang L., Gillespie D., Mph G.J.S., Reid J.K., Owens J., Bs G.B.E., Salzman K.L., Rn A.Y.K., Jensen R.L. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113:1032–1042. doi: 10.1002/cncr.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mashiko R., Takano S., Ishikawa E., Yamamoto T., Nakai K., Matsumura A. Hypoxia-inducible factor 1α expression is a prognostic biomarker in patients with astrocytic tumors associated with necrosis on MR image. J. Neuro-Oncol. 2011;102:43–50. doi: 10.1007/s11060-010-0292-8. [DOI] [PubMed] [Google Scholar]
- 225.Yang Y., Ju T., Yang D.-I. Induction of hypoxia inducible factor-1 attenuates metabolic insults induced by 3-nitropropionic acid in rat C6 glioma cells. J. Neurochem. 2005;93:513–525. doi: 10.1111/j.1471-4159.2005.03032.x. [DOI] [PubMed] [Google Scholar]
- 226.Yuan G., Yan S.-F., Xue H., Zhang P., Sun J.-T., Li G. Cucurbitacin I Induces Protective Autophagy in Glioblastoma in Vitro and in Vivo. J. Biol. Chem. 2014;289:10607–10619. doi: 10.1074/jbc.M113.528760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gammoh N., Fraser J., Puente C., Syred H.M., Kang H., Ozawa T., Lam D., Acosta J.C., Finch A.J., Holland E., et al. Suppression of autophagy impedes glioblastoma development and induces senescence. Autophagy. 2016;12:1431–1439. doi: 10.1080/15548627.2016.1190053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Maugeri G., D’Amico A.G., Reitano R., Magro G., Cavallaro S., Salomone S., D’Agata V. PACAP and VIP inhibit the invasiveness of glioblastoma cells exposed to hypoxia through the regula-tion of HIFs and EGFR expression. Front. Pharmacol. 2016;7:139. doi: 10.3389/fphar.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vallée A., LeCarpentier Y., Guillevin R., Vallée J.-N. Thermodynamics in Gliomas: Interactions between the Canonical WNT/Beta-Catenin Pathway and PPAR Gamma. Front. Physiol. 2017;8:352. doi: 10.3389/fphys.2017.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fan Q., Aksoy O., Wong R.A., Ilkhanizadeh S., Novotny C.J., Gustafson W.C., Truong A., Cayanan G., Simonds E., Haas-Kogan D., et al. A Kinase Inhibitor Targeted to mTORC1 Drives Regression in Glioblastoma. Cancer Cell. 2017;31:424–435. doi: 10.1016/j.ccell.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kahn J., Hayman T.J., Jamal M., Rath B.H., Kramp T., Camphausen K., Tofilon P.J. The mTORC1/mTORC2 inhibitor AZD2014 enhances the radiosensitivity of glioblastoma stem-like cells. Neuro-Oncology. 2014;16:29–37. doi: 10.1093/neuonc/not139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tanaka K., Sasayama T., Irino Y., Takata K., Nagashima H., Satoh N., Kyotani K., Mizowaki T., Imahori T., Ejima Y., et al. Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. J. Clin. Investig. 2015;125:1591–1602. doi: 10.1172/JCI78239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Roberts D.J., Tan-Sah V.P., Ding E., Smith J.M., Miyamoto S. Hexokinase-II Positively Regulates Glucose Starvation-Induced Autophagy through TORC1 Inhibition. Mol. Cell. 2014;53:521–533. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hegazy A.M., Yamada D., Kobayashi M., Kohno S., Ueno M., Ali M.A.E., Ohta K., Tadokoro Y., Ino Y., Todo T., et al. Therapeutic Strategy for Targeting Aggressive Malignant Gliomas by Disrupting Their Energy Balance. J. Biol. Chem. 2016;291:21496–21509. doi: 10.1074/jbc.M116.734756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sanduja S., Feng Y., Mathis R.A., Sokol E.S., Reinhardt F., Halaban R., Gupta P.B. AMPK promotes tolerance to Ras pathway inhibition by activating autophagy. Oncogene. 2016;35:5295–5303. doi: 10.1038/onc.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kim E.H., Lee J.-H., Oh Y., Koh I., Shim J.-K., Park J., Choi J., Yun M., Jeon J.Y., Huh Y.M., et al. Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin. Neuro-Oncology. 2016;19:197–207. doi: 10.1093/neuonc/now174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Masui K., Cavenee W.K., Mischel P.S. mTORC2 in the center of cancer metabolic reprogramming. Trends Endocrinol. Metab. 2014;25:364–373. doi: 10.1016/j.tem.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shen L., Sun C., Li Y., Li X., Sun T., Liu C., Zhou Y., Du Z. MicroRNA-199a-3p suppresses glioma cell proliferation by regulating the AKT/mTOR signaling pathway. Tumor Biol. 2015;36:6929–6938. doi: 10.1007/s13277-015-3409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rathod S.S., Rani S.B., Khan M., Muzumdar D., Shiras A. Tumor suppressive miRNA-34a suppresses cell proliferation and tumor growth of glioma stem cells by targeting Akt and Wnt signaling pathways. FEBS Open Bio. 2014;4:485–495. doi: 10.1016/j.fob.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yin J., Shi Z., Wei W., Lu C., Wei Y., Yan W., Li R., Zhang J., You Y., Wang X. MiR-181b suppress glioblastoma multiforme growth through inhibition of SP1-mediated glucose metabolism. Cancer Cell Int. 2020;20:1–12. doi: 10.1186/s12935-020-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Basso J., Miranda A., Sousa J., Pais A., Vitorino C. Repurposing drugs for glioblastoma: From bench to bedside. Cancer Lett. 2018;428:173–183. doi: 10.1016/j.canlet.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 242.Golden E.B., Cho H.-Y., Hofman F.M., Louie S.G., Schönthal A.H., Chen T.C. Quinoline-based antimalarial drugs: A novel class of autophagy inhibitors. Neurosurg. Focus. 2015;38:E12. doi: 10.3171/2014.12.FOCUS14748. [DOI] [PubMed] [Google Scholar]
- 243.Roy L.-O., Poirier M.-B., Fortin D. Chloroquine inhibits the malignant phenotype of glioblastoma partially by suppressing TGF-beta. Investig. New Drugs. 2015;33:1020–1031. doi: 10.1007/s10637-015-0275-x. [DOI] [PubMed] [Google Scholar]
- 244.Li Q., Chen Z.-G., Xia Q., Lin J.-P., Yan Z.-Q., Yao Z.-J., Dong J. Mefloquine inhibits chondrocytic proliferation by arresting cell cycle in G2/M phase. Int. J. Clin. Exp. Pathol. 2015;8:12583–12588. [PMC free article] [PubMed] [Google Scholar]
- 245.Erkoc P., Cingoz A., Onder T.B., Kizilel S. Quinacrine Mediated Sensitization of Glioblastoma (GBM) Cells to TRAIL through MMP-Sensitive PEG Hydrogel Carriers. Macromol. Biosci. 2016;17:1600267. doi: 10.1002/mabi.201600267. [DOI] [PubMed] [Google Scholar]
- 246.Venugopal C., Hallett R.M., Vora P., Manoranjan B., Mahendram S., Qazi M.A., McFarlane N., Subapanditha M.K., Nolte S.M., Singh M., et al. Pyrvinium Targets CD133 in Human Glioblastoma Brain Tumor–Initiating Cells. Clin. Cancer Res. 2015;21:5324–5337. doi: 10.1158/1078-0432.CCR-14-3147. [DOI] [PubMed] [Google Scholar]
- 247.Liu R., Li J., Zhang T., Zou L., Chen Y., Wang K., Lei Y., Yuan K., Li Y., Lan J., et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: Involvement of abnormal cholesterol trafficking. Autophagy. 2014;10:1241–1255. doi: 10.4161/auto.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Naujokat C., Steinhart R. Salinomycin as a Drug for Targeting Human Cancer Stem Cells. J. Biomed. Biotechnol. 2012;2012:1–17. doi: 10.1155/2012/950658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu W.-T., Lin C.-H., Hsiao M., Gean P.-W. Minocycline inhibits the growth of glioma by inducing autophagy. Autophagy. 2011;7:166–175. doi: 10.4161/auto.7.2.14043. [DOI] [PubMed] [Google Scholar]
- 250.Oliva C.R., Zhang W., Langford C., Suto M.J., Griguer C.E. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit. Oncotarget. 2017;8:37568–37583. doi: 10.18632/oncotarget.17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang Y., Huang N., Li H., Liu S., Chen X., Yu S., Wu N., Bian X.W., Shen H.Y., Li C., et al. Promoting oligodendroglial-oriented differentiation of glioma stem cell: A repurposing of quetiapine for the treatment of malignant glioma. Oncotarget. 2017;8:37511–37524. doi: 10.18632/oncotarget.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Han S., Meng L., Jiang Y., Cheng W., Tie X., Xia J., Wu A. Lithium enhances the antitumour effect of temozolomide against TP53 wild-type glioblastoma cells via NFAT1/FasL signalling. Br. J. Cancer. 2017;116:1302–1311. doi: 10.1038/bjc.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nowicki M.O., Dmitrieva N., Stein A.M., Cutter J.L., Godlewski J., Saeki Y., Nita M., Berens M.E., Sander L.M., Newton H.B., et al. Lithium inhibits invasion of glioma cells; possible involvement of glycogen synthase kinase-3. Neuro-Oncology. 2008;10:690–699. doi: 10.1215/15228517-2008-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hayashi K., Michiue H., Yamada H., Takata K., Nakayama H., Wei F., Fujimura A., Tazawa H., Asai A., Ogo N., et al. Fluvoxamine, an anti-depressant, inhibits human glioblastoma invasion by disrupting actin polymerization. Sci. Rep. 2016;6:23372. doi: 10.1038/srep23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bielecka-Wajdman A.M., Lesiak M., Ludyga T., Sieron A.L., Obuchowicz E. Reversing glioma malignancy: A new look at the role of antidepressant drugs as adjuvant therapy for glioblastoma multiforme. Cancer Chemother. Pharmacol. 2017;79:1249–1256. doi: 10.1007/s00280-017-3329-2. [DOI] [PubMed] [Google Scholar]
- 256.Jeon S., Kim S.H., Kim Y., Kim Y.S., Lim Y., Lee Y.H., Shin S.Y. The tricyclic antidepressant imipramine induces autophagic cell death in U-87MG glioma cells. Biochem. Biophys. Res. Commun. 2011;413:311–317. doi: 10.1016/j.bbrc.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 257.Shchors K., Massaras A., Hanahan D. Dual Targeting of the Autophagic Regulatory Circuitry in Gliomas with Repurposed Drugs Elicits Cell-Lethal Autophagy and Therapeutic Benefit. Cancer Cell. 2015;28:456–471. doi: 10.1016/j.ccell.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 258.Booth L., Cruickshanks N., Tavallai S., Roberts J.L., Peery M., Poklepovic A., Dent P. Regulation of dimethyl-fumarate toxicity by proteasome inhibitors. Cancer Biol. Ther. 2014;15:1646–1657. doi: 10.4161/15384047.2014.967992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jiang P., Mukthavaram R., Chao Y., Nomura N., Bharati I.S., Fogal V., Pastorino S., Teng D., Cong X., Pingle S.C., et al. In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. Br. J. Cancer. 2014;111:1562–1571. doi: 10.1038/bjc.2014.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wu H., Jiang H., Lu D., Xiong Y., Qu C., Zhou D., Mahmood A., Chopp M. Effect of Simvastatin on Glioma Cell Proliferation, Migration, and Apoptosis. Neurosurgery. 2009;65:1087–1097. doi: 10.1227/01.NEU.0000360130.52812.1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yanae M., Tsubaki M., Satou T., Itoh T., Imano M., Yamazoe Y., Nishida S. Statin-induced apoptosis via the suppression of ERK1/2 and Akt activation by inhibition of the geranyl-geranyl-pyrophosphate biosynthesis in glioblastoma. J. Exp. Clin. Cancer Res. 2011;30:74. doi: 10.1186/1756-9966-30-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Keir S.T., Friedman H.S., Reardon D.A., Bigner D.D., Gray L.A. Mibefradil, a novel therapy for glioblastoma multiforme: Cell cycle synchronization and interlaced therapy in a murine model. J. Neuro-Oncol. 2013;111:97–102. doi: 10.1007/s11060-012-0995-0. [DOI] [PubMed] [Google Scholar]
- 263.Zhang Y., Cruickshanks N., Yuan F., Wang B., Pahuski M., Wulfkuhle J., Gallagher I., Koeppel A.F., Hatef S., Papanicolas C., et al. Targetable T-type Calcium Channels Drive Glioblastoma. Cancer Res. 2017;77:3479–3490. doi: 10.1158/0008-5472.CAN-16-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rivera E., Arrieta O., Guevara P., Duarte-Rojo A., Sotelo J. AT1 receptor is present in glioma cells; its blockage reduces the growth of rat glioma. Br. J. Cancer. 2001;85:1396–1399. doi: 10.1054/bjoc.2001.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gritti M., Würth R., Angelini M., Barbieri F., Peretti M., Pizzi E., Pattarozzi A., Carra E., Sirito R., Daga A., et al. Metformin repositioning as antitumoral agent: Selective antiproliferative effects in human glioblastoma stem cells, via inhibition of CLIC1-mediated ion current. Oncotarget. 2014;5:11252–11268. doi: 10.18632/oncotarget.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sesen J., Dahan P., Scotland S.J., Saland E., Dang V.-T., Lemarié A., Tyler B.M., Brem H., Toulas C., Moyal E.C.-J., et al. Metformin Inhibits Growth of Human Glioblastoma Cells and Enhances Therapeutic Response. PLoS ONE. 2015;10:e0123721. doi: 10.1371/journal.pone.0123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cilibrasi C., Butta V., Riva G., Bentivegna A. Pioglitazone Effect on Glioma Stem Cell Lines: Really a Promising Drug Therapy for Glioblastoma? PPAR Res. 2016;2016:1–8. doi: 10.1155/2016/7175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wan Z., Shi W., Shao B., Shi J., Shen A., Ma Y., Chen J., Lan Q. Peroxisome proliferator-activated receptor γ agonist pioglitazone inhibits β-catenin-mediated glioma cell growth and invasion. Mol. Cell. Biochem. 2011;349:1–10. doi: 10.1007/s11010-010-0637-9. [DOI] [PubMed] [Google Scholar]
- 269.Kast R.E., Ramiro S., Lladó S., Toro S., Coveñas R., Muñoz M. Antitumor action of temozolomide, ritonavir and aprepitant against human glioma cells. J. Neuro-Oncol. 2015;126:425–431. doi: 10.1007/s11060-015-1996-6. [DOI] [PubMed] [Google Scholar]
- 270.Furuta T., Sabit H., Dong Y., Miyashita K., Kinoshita M., Uchiyama N., Hayashi Y., Hayashi Y., Minamoto T., Nakada M. Biological basis and clinical study of glycogen synthase kinase- 3β-targeted therapy by drug repositioning for glioblastoma. Oncotarget. 2017;8:22811–22824. doi: 10.18632/oncotarget.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lefranc F., James S., Camby I., Gaussin J.-F., Darro F., Brotchi J., Gabius H.-J., Kiss R. Combined cimetidine and temozolomide, compared with temozolomide alone: Significant increases in survival in nude mice bearing U373 human glioblastoma multiforme orthotopic xenografts. J. Neurosurg. 2005;102:706–714. doi: 10.3171/jns.2005.102.4.0706. [DOI] [PubMed] [Google Scholar]
- 272.Liu Y., Fang S., Sun Q., Liu B. Anthelmintic drug ivermectin inhibits angiogenesis, growth and survival of glioblastoma through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2016;480:415–421. doi: 10.1016/j.bbrc.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 273.Liu J., Pan S., Hsieh M.H., Ng N., Sun F., Wang T., Kasibhatla S., Schuller A.G., Li A.G., Cheng D., et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mccord M., Mukouyama Y.-S., Gilbert M.R., Jackson S. Targeting WNT Signaling for Multifaceted Glioblastoma Therapy. Front. Cell. Neurosci. 2017;11:318. doi: 10.3389/fncel.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.