Abstract
Background and aims:
Developmental motor speech impairment has been suspected, but rarely systematically examined, in low- and minimally verbal individuals with autism spectrum disorder. We aimed to investigate the extent of motor speech impairment in this population and its relation to number of different words produced during a semi-structured language sample.
Methods:
Videos of 54 low-verbal and minimally verbal individuals (ages 4;4–18;10) performing portions of a speech praxis test were coded for signs of motor speech impairment (e.g., childhood apraxia of speech). Age, autism spectrum disorder severity, nonspeech oral-motor ability, speech production ability, nonverbal IQ, and receptive vocabulary were compared between groups.
Results:
Four groups emerged: (1) speech within normal limits (n=12), (2) non-childhood apraxia of speech impairment (n=16), (3) suspected childhood apraxia of speech (n=13), and (4) insufficient speech to rate (n=13). Groups differed significantly in nonspeech oral-motor ability, speech production ability, nonverbal IQ, and receptive vocabulary. Overall, only speech production ability and receptive vocabulary accounted for significant variance in number of different words. Receptive vocabulary significantly predicted number of different words only in Groups 1 and 2, while speech production ability significantly predicted number of different words only in Groups 3 and 4.
Conclusions and implications:
If replicated, our findings have important implications for developing much-needed spoken language interventions in minimally verbal individuals with autism spectrum disorder.
Keywords: Autism spectrum disorder, speech, expressive language, motor speech disorder, childhood apraxia of speech
Introduction
Up to 25–30% of individuals with autism spectrum disorder (ASD) will remain minimally verbal (MV) by school age (Norrelgen et al., 2015; Tager-Flusberg & Kasari, 2013). Hence, in addition to experiencing impairments in social communication and the repetitive behaviors and restricted interests common to all children with ASD (American Psychiatric Association, 2013), these children also have extremely limited ability to communicate using spoken language. MV children with ASD typically use a small number of single words or fixed phrases to request items in familiar contexts (DiStefano & Kasari, 2016). Given that communication is both a basic need and the right of all human beings (Brady et al., 2016) and that better expressive communication is also associated with fewer maladaptive behaviors (Baghdadli, Pascal, Grisi, & Aussilloux, 2003; Dominick, Davis, Lainhart, Tager-Flusberg, & Folstein, 2007; Hartley, Sikora, & McCoy, 2008), there is a great need for further investigation and intervention options for MV children with ASD.
Speech production in MV ASD
The ability to use spoken language effectively requires the intent to communicate and skill in both language (the comprehension and use of a communication symbol system; American Speech-Language-Hearing Association (ASHA), 1993) and speech (the perception, motor production, or phonological representation of phonemes; ASHA, n.d.). We define language here as a learned code or rule system that enables us to communicate ideas and express wants and needs. Print, manual signing, and speaking are all forms of language. Language falls into two main divisions: expressive language (writing, signing, or speaking) and receptive language (understanding what is written, signed, or said). We use the term “spoken language” to include aspects of both expressive language and speech.
Though language development in MV children with ASD has received some attention by researchers (see, for example, Brignell et al., 2018; Luyster, Kadlec, Carter, & Tager-Flusberg, 2008, Tager-Flusberg, 2006, 2015, 2016; Tager-Flusberg & Caronna, 2007), speech development has received much less. In part, of course, this is because it is very challenging to assess the speech production of children who speak little, episodically, or sometimes not at all. Still, clinicians have long suspected the presence of motor speech disorders in at least some MV children with ASD. For example, Prizant (1996) proposed, based on clinical observation, that motor limitations specific to speech are significant factors limiting speech development in many individuals with ASD and that “information is needed into how specific motor and sensory limitations may impact on communicative … development” in MV children with ASD (p. 178). Further, Shriberg, Paul, Black, and van Santen (2011) articulated the hypothesis that “{childhood apraxia of speech} is a sufficient cause of lack of speech development in at least some children classified as nonverbal ASD” (p. 405), though these authors did not investigate that hypothesis.
Classification of developmental motor speech disorders
Developmental motor speech disorders typically include diagnoses either of dysarthria or of childhood apraxia of speech (CAS). Dysarthria is a disorder of neuromuscular execution that affects the accuracy, range, speed, strength, or steadiness of speech movement. Dysarthria occurs due to lesions in the corticobulbar tract (Liegeois & Morgan, 2012; Morgan et al., 2018) and hence is associated with weakness, spasticity, incoordination, involuntary movement, or altered muscle tone (Duffy, 2013). By contrast, CAS is a developmental neurological speech sound disorder in which the precision and consistency of the articulatory movements of speech are impaired in the absence of the neuromuscular deficits that give rise to dysarthria (ASHA, 2007). The core impairment in CAS is one of planning or programming the spatiotemporal aspects of speech movements, resulting in speech sound errors. CAS and dysarthria often co-occur in cases of severe disruption to neurodevelopment (Morgan et al., 2018; White et al., 2010). Further, it is common for children to experience speech impairments other than motor speech disorders (Morgan & Webster, 2018). Children with 16p11.2 deletion, for example, have been found to present with signs of CAS and dysarthria alongside other developmental speech sound disorders of phonological delay, phonological disorder, or articulation disorder (Fedorenko et al., 2015; Mei et al., 2018). Here, we use the term non-CAS speech impairment to denote individuals with speech impairment not meeting criteria for CAS. This could include dysarthria, articulation disorder, phonological disorders, or motor speech disorders not otherwise specified.
Though the works cited above are suggestive of motor speech comorbidities in some children with ASD, there is limited evidence that motor speech impairment commonly co-occurs in verbal or MV children with ASD. Adams (1998) found that children with ASD received significantly lower scores on the Kaufman Speech Praxis Test (KSPT; Kaufman, 1995) than age- and IQ-matched typical controls. Errors in the ASD group included prevocalic voicing of voiceless phonemes, phoneme substitutions, oral scanning/groping, syllable deletion, phoneme distortion, and cluster reduction. Velleman et al. (2010) found that 60% of a sample of 40 individuals with ASD between 1;10 and 22;0 showed speech signs consistent with dysarthria or CAS, 12.5% showed signs of CAS only, 10% showed signs of dysarthria only, and 37.5% showed signs that were ambiguous between diagnoses. These researchers also examined the speech of 10 children with ASD aged 4;0–6;5 using the Verbal Motor Production Assessment for Children (Hayden & Square, 1999). Only three of the children achieved scores within normal age limits. Six children scored in the “severe deficit” range, and the remaining child scored in the “moderate deficit” range. Five of the children showed deficits in sequencing of oromotor movements, one of the characteristics of CAS; and eight children had voice characteristics that aroused concern as being consistent with dysarthria.
Belmonte et al. (2013) examined a group of 31 children with ASD aged 1;10–5;5 using a criterion-referenced developmental measure, the ComDEALL Developmental Checklist (Karanth, 2007), and an assessment of oral-motor skills, the ComDEALL OroMotor Assessment (Archana, 2008), distinguishing 11 children who experienced expressive language difficulty and impairment in oral motor functioning that was more severe than their impairments in other domains from the other 20 participants, whose expressive language and oral motor skills were commensurate with their abilities in other areas. More recently, Tierney et al. (2015) investigated a group of 30 children aged 2;0–4;7 who were assessed for ASD with the Childhood Autism Rating Scale-2 (Schopler, Van Bourgondien, Wellman, & Love, 2010) and for CAS with the KSPT. Across the sample four children met criteria for ASD only, 12 for CAS only, seven children met criteria for both disorders, and seven met criteria for neither disorder. However, the findings from this study must be interpreted cautiously given the potential for recruitment bias. Finally, signs of motor speech disorder have also been noted in recent work on spoken-language treatment in MV children with ASD (Chenausky, Kernbach, Norton, & Schlaug, 2017; Chenausky, Norton. Tager-Flusberg, & Schlaug, 2016).
Study aims
Past studies have shown that motor speech disorders can co-occur in children with ASD and suggested that these motor speech disorders may affect spoken language. To date, however, no study has investigated the potential presence or effect of motor speech impairment on expressive language specifically in low-verbal (LV) and MV individuals with ASD. Yet such a study would greatly inform not only the etiology of the MV phenotype(s), but also inform treatment options. Thus, we aimed to estimate the proportion of LV and MV individuals with ASD with motor speech impairment and to explore the contribution of age, ASD severity, nonspeech oral-motor ability, speech production ability, nonverbal IQ, and receptive vocabulary to variability in the number of different words (NDW) produced during a semi-structured language sample, both in the group as a whole and in subgroups defined on the basis of speech production ability. Our main question was whether participants would fall into more than one subgroup according to the presence and type of speech impairment. We also investigated the exploratory hypothesis that variance in NDW would be accounted for by different factors in different subgroups.
Methods
Participants
Participants were 54 LV and MV individuals with ASD (13 female), aged between 4;4 and 18;10, who were part of an ongoing phenotyping study. Families were recruited from the New England area of the US through online advertisements, presentations at schools for MV children with ASD, and parent support groups. Participants were classified as MV if parent interview indicated that they did not spontaneously use phrase speech (i.e., if they did not show evidence of productive syntax or word combinations, thus meeting criteria for Module 1 of the Autism Diagnostic Observation Schedule-2 (ADOS; Lord et al., 2012) or the Adapted ADOS (AADOS; Hus et al., 2011), as suggested in Hus Bal, Katz Bishop, & Krasileva, 2016). Participants who used phrase speech spontaneously and met criteria for Module 2 of the ADOS or AADOS were considered low verbal. Our aim was to deliberately cast a wide diagnostic net, in the interest of parsing heterogeneity. Participants were excluded if English was not the main language of their household or if the NDW they produced during the ADOS or AADOS was greater than 250. The research protocol was approved by the Institutional Review Board of Boston University, and parents of all participants gave written informed consent prior to enrolment.
Measures
ASD diagnosis.
Participants met criteria for ASD on the Autism Diagnostic Interview-Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003) and either the ADOS or the AADOS. All ASD diagnostic assessments were administered by research-reliable examiners. The ADI-R is a structured interview with the individual’s parent or main caregiver, while the ADOS and AADOS are semi-structured assessments with the individual him- or herself that include a variety of “communicative temptations”—opportunities to request or comment—such as free play with age-appropriate toys, taking turns on an activity like Connect-4 or blowing bubbles, or eating a snack. The ADOS was administered to participants younger than 12 years of age and the AADOS to participants 12 or older. The calibrated severity score (Shumway et al., 2012) was used to compare scores across versions.
Receptive vocabulary.
The Peabody Picture Vocabulary Test-4 (PPVT-4; Dunn & Dunn, 2007) was used to assess receptive vocabulary. Raw scores were used to identify the differences between individuals that would be obscured by percentile ranks.
Nonverbal intelligence.
An estimate of nonverbal intelligence (NVIQ) was obtained using standard scores from the Leiter International Performance Scale-Third Edition (Leiter-3; Roid & Miller, 2013).
Expressive language.
Expressive language ability was quantified using the NDW spoken by the individual during the ADOS or AADOS (Barokova & Tager-Flusberg, 2018). NDW was selected as a measure of spoken language because it combines aspects of both speech and language; is spontaneous, rather than imitated; and thus shows evidence of communicative intent. In addition, NDW captures lexical semantic ability and correlates highly with measures of syntax/ morphology in children who do use multi-word utterances (Tager-Flusberg et al., 2009). Words in chunked phrases were counted individually.
To derive this measure, transcripts of the assessments were prepared from video using Systematic Analysis of Language Transcripts (SALT; Miller, Andriacchi, & Knockerts, 2011) conventions to facilitate coding and analysis. We used SALT to automatically tally the NDW spoken by the participant. After undergoing training, one transcriber transcribed the video and a second transcriber reviewed the same file. If there were discrepancies, transcribers convened to reach a consensus. If the two were unable to reach a consensus, a third trained transcriber resolved the discrepancy.
Nonspeech oral-motor and speech production ability.
The first two sections of the KSPT were administered to all participants. Section 1 (KSPT1) includes 11 tasks assessing nonspeech oral-motor ability (e.g., “open your mouth”). Section 2 (KSPT2) includes 63 speech imitation tasks, ranging from single vowels or consonants to C1V1C2V2 words (e.g., “bunny”, [b^ni]).
Speech group coding.
Videos of section 2 of the KSPT were coded for signs of CAS and other speech anomalies. Section 2 was used in order to have a consistent basis of comparison across participants. Videos were coded for the 11 signs of CAS from Iuzzini-Seigel, Hogan. Guarino, and Green (2015), as well as for three other types of anomaly: Inconsistent errors (i.e., the same target is produced differently on repeated attempts, such as [mamwi], [mabi], and [mambi] for “mommy”) is one of the core consensus-based ASHA criteria for CAS (ASHA, 2007). Abnormal pitch (too high or too low) and addition of phonemes (other than schwa) occurred commonly in the sample. In all cases, signs were marked as present if they occurred at least once in the video, in accordance with the procedures used in Iuzzini-Seigel et al. (2015). Note that this coding is distinct from the score on KSPT2; the latter is a simple count of the number of items correct and does not distinguish between CAS and other types of speech impairment.
Participants were grouped into one of four descriptive categories according to the clinical presentation of their speech: Group 1 (WNL) if their speech appeared within normal limits for their age, Group 2 (non-CAS) if their speech showed abnormalities that were not consistent with CAS, Group 3 (suspected CAS, or sCAS) if their speech showed at least five signs of CAS and was consistent with difficulties in motor planning, and Group 4 (Insufficient Speech) if the speaker did not produce enough speech on KSPT2 to rate.
A consensus method was used to establish reliability on assignment to one of the four categories. The first two authors (KC and AB) viewed a subset of 15 videos (26%). These were randomly selected using a random number generator (random.org) and included participants with high, medium, and low KSPT scores. The remaining videos were coded by the first author. Disagreements and questions were resolved through discussion with the third author (AM). Subsequently, a set of eight videos was independently coded by the two judges in order to establish intra- and inter-judge reliability. Both intra- and inter-judge reliability were 87.5% (7/8 videos were classified the same in each case). Cohen’s κ was 0.830 (very good) in both cases.
Analytic strategy
Analyses were conducted using SPSS v.25 (IBM Corp., 2017) and G*power (Faul, Erdfelder, Buchner, & Lang, 2009).
Group differences
Group differences in chronological age, ADOS severity score, raw scores on KSPT1 and KSPT2, Leiter standard score, PPVT raw score, and NDW were assessed using one-way ANOVAs with group as a between-subjects factor.
Regression analyses
Independent variables differing between groups (i.e., KSPT2 and PPVT raw score) were then entered into a hierarchical multiple regression analysis with NDW as the outcome variable in order to quantify the amount of variance in NDW that was accounted for by each of the independent variables.
Exploratory between-group analyses
To test whether the independent variables contributing to variance in NDW differed by group, two additional analyses were performed. First, a hierarchical multiple regression model was constructed that included the interaction terms (measure × group) for each variable that accounted for significant variance in NDW. A power analysis was performed to estimate the likelihood of Type II errors. Second, separate one-predictor regression models were constructed for each group and each independent variable. The results were then subjected to a false-discovery rate correction to estimate the likelihood of Type I errors (Benjamini & Hochberg, 1995).
Results
Speech coding and group differences
Participants were classified into one of the four categories noted earlier; their characteristics appear in Table 1. A brief qualitative description of the speech of each group appears below. Implications of these findings are considered in more detail in “Discussion“ section.
Table 1.
Participant characteristics.
| Group | N | Agea (yr;mo.) | ADOS | KSPT1 | KSPT2 | Leiter | PPVT | NDW |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 9;10±2;9 | 7.7±1.5 | 10.6±0.9 | 55.8±6.6 | 81.3±16.6 | 72.3±24.2 | 101.8±71.0 |
| (WNL) | [5;10–13;11] | [6–10] | [8–11] | [44–62] | [56–115] | [39–123] | [9–229] | |
| 2 | 16 | 9;6±4;6 | 7.8±1.2 | 8.5±2.6 | 44.0±12.6 | 71.9±20.9 | 47.7±26.9 | 62.3±51.3 |
| (non-CAS) | [4;4–18;10] | [6–10] | [3–11] | [23–58] | [39–112] | [4–106] | [4–211] | |
| 3 | 13 | 8;8±3;6 | 7.7±1.3 | 7.3±2.9 | 12.8±15.7 | 65.2–13.9 | 15.9±16.6 | 14.2±14.1 |
| (sCAS) | [5;5–15;8] | [6–10] | [3–11] | [0–61] | [39–83] | [0–47] | [0–45] | |
| 4 | 13 | 11;11±4;1 | 8.1±1.0 | 3.3±3.1 | 1.6±1.8 | 51.1±13.2 | 5.15±5.9 | 7.5±10.7 |
| (Insuff.) | [6;0–17;7] | [7–10] | [0–11] | [0–5] | [30–78] | [0–20] | [0–34] | |
| ANOVA | (0.181) | (0.744) | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 |
ADOS: Autism Diagnostic Observation Schedule or Adapted ADOS, calibrated severity score; KSPT1: Kaufman Speech Praxis Test, Section 1 (oral movement), max score 11; KSPT2: Kaufman Speech Praxis Test, Section 2 (simple speech), max score 63; Leiter: Leiter International Performance Scale-Third Edition (nonverbal IQ), standard score; PPVT: Peabody Picture Vocabulary Test, Fourth Edition, raw score; NDW: number of different words produced during a semi-structured language sample.
Figures are listed as mean±standard deviation [min–max].
We identified four qualitatively different subgroups within our sample. Group 1 (WNL, n=12) produced speech that was within normal limits on KSPT2. Mean score for this group on sections 1–3 of the KSPT was 114.2 (SD 38.6, range 55–150), out of a total possible score of 155. Six of the participants in this group were considered LV (Module 2 of the (A)ADOS) and six MV (Module 1 of the (A)ADOS).
The speech of individuals in this group occasionally showed one or two signs classified as abnormalities. For example, three participants showed at least one consonant distortion (i.e., a manner or place of articulation error or a subphonemic error) and two made a voicing error (this includes productions of ambiguous voicing status). Finally, scores also reflect the varied motivation of MV individuals with ASD to participate in structured assessments—one participant, for example, responded to the examiner’s prompts in a sing-song voice until he was told to stop. However, the fact that he was easily redirected from this behavior suggests that he was in control of his speech and that his altered prosody was not a sign of a speech disorder.
The speech of Group 2 (non-CAS, n=16) was characterized by the presence of more abnormal signs than in Group 1, but was not judged consistent with a diagnosis of CAS. Mean score for this group on sections 1–3 of the KSPT was 84.5 (SD 37.8, range 28–137). Two participants in this group were considered LV and the remaining 14 were MV. Often, speakers in this group produced speech that was significantly under-articulated (“mumbled”) unless specifically prompted to “say it nice and loud!”. For this reason, vowel errors (16/16) and nasality errors (13/16) were common in this group, due to vowel reduction/centralization and hypernasality, respectively. Sixteen participants also showed minor abnormalities such as labiodental production of /m/ or /b/ (i.e., producing labial consonants by touching the upper teeth to the lower lip instead of touching upper and lower lips) or residual errors on /r/. Thus, this group may comprise a mixed bag of individuals whose speech is still developing (the youngest child in this group was 4;4) and individuals who may show mild or subclinical signs of a recognized speech disorder (CAS, phonological disorder, mild dysarthria due to low muscle tone).
The speech of participants in Group 3 (sCAS, n=13) was severely disordered, was judged consistent with a disorder in planning and programming the spatiotemporal parameters of speech movement, and showed at least five of the signs of CAS detailed in Iuzzini-Seigel et al. (2015). All participants in this group were MV. Mean score for this group on sections 1–3 of the KSPT was 25.4 (SD 35.3, range 4–140). The most common signs were consonant distortions, nasality errors, and vowel errors (in 13/13 participants). Voicing errors were present in 12 and stress errors in eight participants. Intrusive schwa, slow rate, and difficulty with initial configuration were less common (six participants); as were syllable segmentation and increased difficulty with longer words (four participants). Only one participant showed obvious silent groping. Table 2 shows the signs of motor speech disorder that were coded for and how many participants in each group showed each sign.
Table 2.
Signs of motor speech disorder that were coded, and number of participants per group showing each sign.
| Group |
||||
|---|---|---|---|---|
| Signs | 1=WNL | 2=nCAS | 3=sCAS | 4=Insuff. speech |
| Iuzzini-Seigel et al. (2015) | ||||
| Vowel error | 9 | 14 | 12 | 4 |
| Consonant distortion | 3 | 14 | 12 | 4 |
| Stress error | 0 | 7 | 8 | 2 |
| Syllable segregation | 0 | 3 | 4 | 2 |
| Groping | 0 | 1 | 1 | 0 |
| Intrusive schwa | 1 | 4 | 6 | 0 |
| Voicing error | 2 | 11 | 11 | 2 |
| Slow rate | 0 | 4 | 5 | 0 |
| Increased difficulty with multisyllabic words | 1 | 5 | 4 | 0 |
| Nasality disturbance | 3 | 12 | 12 | 3 |
| Difficulty with initial configuration/transitions | 1 | 1 | 6 | 0 |
| Other | ||||
| Inconsistent errors | 0 | 1 | 3 | 0 |
| Abnormal pitch | 3 | 2 | 4 | 0 |
| Addition of phonemes (except schwa) | 2 | 4 | 8 | 3 |
nCAS: non-childhood apraxia of speech; sCAS: suspected childhood apraxia of speech; WNL: speech appeared within normal limits; Insuff. speech: insufficient speech to diagnose.
Finally, Group 4 (insufficient speech to rate, n=13) consisted of participants who produced too little speech during KSPT2 to be analyzable. Mean score for this group on sections 1–2 of the KSPT was 4.8 (SD 4.1, range 0–16). All participants in this group were MV. Three participants produced no vocalizations at all during the KSPT, while five others produced mainly or exclusively nonspeech vocalizations such as vocal stims, moans, or squeals. The remaining five could phonate on request, though often with breathy or rough, non-modal phonation, or imitate simple syllables. Yet here, too, participants showed considerable variation in NDW during the spontaneous language sample, ranging from five or fewer words (nine participants) to more than 10 words (four participants).
A post-hoc ANOVA performed on the mean number of speech abnormalities identified per group for Groups 1 (WNL), 2 (non-CAS), and 3 (sCAS) to verify that they differed according to this measure was significant, F(2,39)=32.850, p<0.0005. The mean number of abnormalities was 1.8 (SD 1.7) for Group 1, 5.2 (SD 2.0) for Group 2, and 7.7 (SD 1.8) for Group 3.
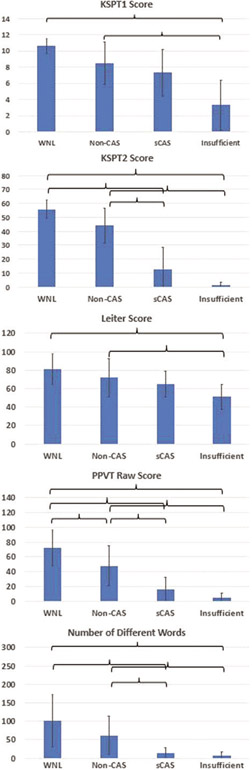
One-way ANOVAs were also performed to determine whether the groups differed on chronological age, ADOS severity score, KSPT1 and KSPT2 raw scores, Leiter standard score, PPVT raw score, and NDW. Groups did not differ significantly on age (F(3,53)=1.688, p=0.181) or ADOS severity score (F(3,53)=0.414, p=0.744). Groups did differ significantly on KSPT1 (F(3,53)=19.491), KSPT2 (F(3,53)=76.795), Leiter (F(3,53)=7.809), PPVT raw score (F(3,53)=28.506), and NDW (F(3,53)=7.334; all p<0.0005. Note that scores of 0 on KSPT1 and KSPT2 could mean either that the participant attempted at least some items but did not produce them accurately, or that the participant did not attempt any items (i.e., was essentially mute and/or unable to imitate nonspeech oral movements). Figure 1 plots group means for these five variables, with significant between-group differences indicated.
Figure 1.
Group means for examined variables. Brackets indicate significant post-hoc between-group comparisons (p<0.05, Bonferroni-corrected). Error bars:±1 SD.
Regression analyses
Next, KSPT1, KSPT2, Leiter, and PPVT scores (the independent variables differing significantly between groups) were entered into a hierarchical multiple regression model to determine which variables accounted for significant variance in NDW over the entire group. The overall regression model including all four independent variables was significant, F(4,52)=17.971, p<0.0005, and accounted for 60.0% of the variance (R2) in NDW. However, KSPT1 and Leiter accounted for insignificant amounts of variance (ΔR2=0.0.026, p=0.122 for KSPT1; ΔR2=0.008, p=0.379 for Leiter) and were removed.
A reduced model including just KSPT2 and PPVT was also significant, F(2,51)=35.657, p<0.0005, and accounted for 58.3% of the variance in NDW. KSPT2 contributed a ΔR2 of 0.464 (p<0.0005); PPVT contributed a ΔR2 of 0.120 (p<0.0005). Post-hoc analysis showed that the two independent variables were significantly correlated (Pearson’s r=0.852, p<0.005); however, the variance inflation factor associated with them was acceptably low (VIF=3.5), indicating no collinearity. Table 3 shows the regression parameters for this model.
Table 3.
Parameters for regression of number of different words on KSPT2 and PPVT scores.
| β | SE | p | |
|---|---|---|---|
| Constant | −2.546 | 8.008 | (0.752) |
| KSPT2 | 0.313 | 0.397 | (0.434) |
| PPVT | 1.129 | 0.295 | <0.0005 |
KSPT2: Kaufman Speech Praxis Test, Section 2 (simple speech); PPVT: Peabody Picture Vocabulary Test, Fourth Edition.
Exploratory between-group analyses
To investigate the exploratory hypothesis that NDW differed according to subgroup, we performed one analysis examining the whole group and a second to examine subgroups. In view of the small sample size in this study, we also performed analyses to assess the likelihood of Type I and Type II errors.
The first analysis tested whether the interaction terms (measure×group) were significant when added to the regression model. The overall model including KSPT2, KSPT2×Group, PPVT, and PPVT×Group was significant, F(4,53)=21.102, p<0.0005. The KSPT2×Group interaction term was not associated with a significant increase in R2 ΔR2=0.036, p=0.061); however, the PPVT×Group interaction term was associated with a significant increase in R2 ΔR2=0.042, p=0.021). Because the variance of an interaction term is approximately four times the variance of a main effect (Leon & Heo, 2009), large sample sizes are typically needed to detect significant interaction effects. Hence we calculated both the a priori sample size required to detect a significant interaction with the effect size from our regression model and the post-hoc power for our sample of 54. The ΔR2 of 0.042 associated with the larger of the two interaction terms, PPVT×Group, corresponds to an f2 effect size of 0.04 (Cohen, 1992). With α=0.05 and at 80% power, a sample size of 365 is needed to detect a significant ΔR2 of 4.2%. A sample size of 54 provides only 25% power to detect a significant ΔR2 of this size.
Next, we tested single-predictor regression models for KSPT2 and PPVT (dependent variable NDW) for each subgroup, a total of eight models. KSPT2 significantly predicted NDW in Groups 3 and 4; but not in Group 1. PPVT significantly predicted NDW in Groups 1 and 2, but not Groups 3 and 4. Details appear in Table 4.
Table 4.
Single-predictor regression results by group.
| PPVT → NDW |
KSPT2 → NDW |
|||||
|---|---|---|---|---|---|---|
| Group | F | p | R 2 | F | p | R 2 |
| 1 | F(1,11)=8.262 | 0.017 | 0.452 | F(1,11)=1.118 | (0.315) | 0.101 |
| 2 | F(1,15)=5.686 | 0.032 | 0.289 | F(1,15)=3.757 | (0.073) | 0.212 |
| 3 | F(1,11)=0.008 | (0.932) | 0.001 | F(1,11)=5.474 | 0.039 | 0.332 |
| 4 | F(1,11)=0.349 | (0.566) | 0.031 | F(1,11)=4.930 | 0.032 | 0.355 |
KSPT2: Kaufman Speech Praxis Test, Section 2 (simple speech); NDW: number of different words; PPVT: Peabody Picture Vocabulary Test, Fourth Edition.
To control for multiple comparisons, we applied a false-discovery rate correction (Benjamini & Hochberg, 1995). All significant comparisons survived a false-discovery rate correction of 0.1.
Discussion
In this paper, we aimed to address an under-studied aspect of spoken language in low and MV individuals with ASD, namely motor speech production ability, and its relationship to the NDW produced during a semi-structured language sample. Among our 54 participants with ASD, there was considerable heterogeneity with respect to both language and speech production ability. Though all participants produced fewer than 250 different words during language sampling, eight used occasional phrase speech and the remaining 36 did not. Some participants were essentially mute (0 different words, unable to vocalize on request). One had significantly disordered speech, yet used phrases and produced over 200 different words during language sampling; and one showed speech that appeared within normal limits on our assessments while at the same time producing only a small NDW (<10) and no phrase speech. By including participants with a wide range of performance and employing the assessment techniques described in Tager-Flusberg et al. (2017) for use with MV individuals, we were able to determine that predictors of expressive language ability do differ according to different factors in different individuals.
When looking at the sample as a whole, only KSPT2 score and raw PPVT score significantly predicted NDW. As interesting as what did predict expressive language is what did not: age and NVIQ were unrelated to expressive language ability. This finding is consistent with previous work (Chenausky, Norton, Tager-Flusberg, & Schlaug, 2018), and has important clinical implications that will be discussed below. Note, however, that it differs from other research (e.g., Ellis Weismer & Kover, 2015; Thurm, Lord, Lee, & Newschaffer, 2007; Venter, Lord, & Schopler, 1992), where NVIQ has been shown to be related to expressive language development. A factor that may explain these differences is that these previous studies included children with ASD with higher levels of language proficiency and used standardized measures of expressive language rather than NDW from language samples.
It is important to remember that simply meeting criteria for a list of signs of CAS on one assessment, or failing to do so, is not the equivalent of receiving a comprehensive and detailed examination that carefully rules CAS in or out. Many of the tests in a complete battery to differentially diagnose motor speech disorders from phonological disorder, such as the Goldman–Fristoe Test of Articulation (Goldman & Fristoe, 2015) and diadochokinetic tasks (repetition of syllables such as “pa” or syllable sequences such as “pataka”), are infeasible for individuals with extremely limited speech output. Even in verbal individuals, accurate diagnosis of CAS is challenging, owing to the lack of a validated measure with high specificity and sensitivity (Strand, McCauley, Weigand, Stoeckel, & Baas, 2013). Also, signs such as consonant distortions are common across speech disorders (ASHA, 2007) and, in fact, occurred in each of the four groups we describe here. Therefore, in addition to showing the requisite number of signs of CAS, we also relied on clinical judgment that the presentation must be consistent with a motor planning disorder. Still, we cannot rule out the possibility that the children in this group experience a motor speech disorder similar to CAS but unique to MV children with ASD, or that their speech may fit the Motor Speech Disorder-Not Otherwise Specified category of Shriberg et al. (2017). Furthermore, to the extent that speech development may have been delayed in this group, their speech production may also reflect a lack of maturity (or practice). Neither can an investigation of this nature address the issue of whether the motor speech impairment identified in some of our participants is a comorbidity or an inextricable part of the subphenotype, with the same biological factors giving rise to ASD and the speech profile together. These are certainly areas for further research.
Consistent with the recommendations in the ASHA Technical Report on CAS (ASHA, 2007), we therefore consider participants in Group 3 to be “suspected to have CAS”. That said, given the recommendation (ASHA, 2007) that assessment for suspected CAS include measures of nonspeech oromotor skill, speech production and perception, prosody, voice, and language; and include both spontaneous and imitated speech, the fact that our Group 3 participants still met criteria for CAS using just the imitation tasks on the KSPT2 underscores their severity and lends credence to their status.
Regarding Group 4 (insufficient speech), our finding that one participant in this group produced 34 different words during language sampling but not enough responses on the KSPT to be able to rate for signs of speech disorder also underscores the necessity of employing a variety of tasks and speaking contexts—including dynamic assessment (Strand et al., 2013)—to make an accurate diagnosis. Caveats about the challenges of diagnosing CAS, or any motor speech disorder, in individuals whose speech is minimal or absent apply here as in our discussion of Group 3. We might hypothesize that a speech disorder such as CAS can be so severe as to render an individual functionally mute; however, that too is a topic for further research and discussion in the field.
Finally, it is important to see the current subgroup results in context. Since the sample size has low power to detect significant interaction effects, requiring more than six times the participants we have, it is a reasonable possibility that the finding of a nonsignificant KSPT2×group interaction is actually a Type II error (missing a significant finding that actually exists in the population as a whole) and suggests that the significant PPVT×Group interaction can generalize beyond our sample. The subgroup analysis is consistent with this, since all significant comparisons survive correction for false-discovery rate. We conclude provisionally that speech production and receptive vocabulary both contribute to expressive language in LV and MV individuals with ASD, but that the contribution differs from person to person, depends on the severity of speech impairment, and is related to language skill. By definition, in individuals with no speech impairment, speech production ability would not be expected to limit expressive language.
Clinical implications
The current results support the need for careful and detailed clinical assessments, conducted by speech pathologists experienced in pediatric speech disorders and addressing all the factors that may affect expressive language. They also support the use of CAS-specific therapy for some MV children with ASD (e.g., Rogers et al., 2006), but with the caveat that it be reserved for those who meet criteria for CAS. Furthermore, the fact that age and NVIQ were unrelated to concurrent expressive language skills or to response to spoken-language treatment (Chenausky et al., 2018) should remind clinicians to periodically revisit treatment goals for older, MV individuals with ASD. The American Speech-Language-Hearing Association’s guidelines on admission and discharge criteria caution clinicians that, even when treatment is discontinued because it no longer results in measurable benefits, “re-evaluation should be considered at a later date to determine whether the patient/client’s status has changed or whether new treatment options have become available.” (ASHA, 2004). Because an individual’s profile of challenges and skills can change with treatment or maturation, the focus of treatment can and should change accordingly. In particular, determining whether the primary factor limiting spoken language for a child is impaired speech motor control may be important for identifying those children who might benefit from augmentative and alternative communication interventions in addition to other forms of speech-language therapy.
Limitations and future work
As with many other studies of autism, a limitation of the current work is sample size, and replication in larger groups will be an important next step. In addition, and related to the clinical comments above, longitudinal studies should be carried out to determine whether and how children’s speech profiles evolve with time. Based on our findings, these studies should include detailed, prospective assessments for CAS and other developmental motor speech disorders using appropriate assessment tools such as the Dynamic Evaluation of Motor Speech Skill (DEMSS; Strand & McCauley, 2019).
More in-depth investigations of the nature of the impairments in Groups 2, 3, and 4 are also warranted. Regarding the variety of findings in Group 2, questions that should be addressed include the potential origin of their speech impairment and whether some children in this group show a form of childhood dysarthria due to identifiable neurological abnormalities, other types of speech disorder (e.g., articulation disorder, consistent speech sound disorder, inconsistent speech sound disorder), or combinations of these. For Group 3, it is yet to be determined whether they have CAS or a motor speech disorder unique to MV ASD. Finally, the question to be addressed in Group 4 is whether a motor speech disorder can be so profound that it renders an individual nonvocal (which may have been the case with the three nonvocal individuals in the present study). Here, too, assessment must take into account other factors, such as motivation to communicate and joint attention ability, that may affect an individual’s ability to imitate speech sounds on request. Any light we can shed on these issues will serve our ultimate clinical goals for individuals with ASD: treatment that is personalized to each person’s specific profile of strengths and challenges.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author has received funding from National Institute on Deafness and Other Communication Disorders.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Karen Chenausky, Sargent College, Boston University, Boston, MA, USA.
Amanda Brignell, Speech and Language, Murdoch Children’s Research Institute, Melbourne, Australia; University of Melbourne, Melbourne, Australia.
Angela Morgan, Speech and Language, Murdoch Children’s Research Institute, Melbourne, Australia; University of Melbourne, Melbourne, Australia.
Helen Tager-Flusberg, Sargent College, Boston University, Boston, MA, USA.
References
- Adams L (1998). Oral-motor and motor-speech characteristics of children with autism. Focus on Autism and Other Developmental Disabilities, 13(2), 108–112. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (edition 5.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- American Speech-Language-Hearing Association (ASHA). (n.d.). Speech sound disorders-articulation and phonology (practice portal). Retrieved 16 April 2019 from https://www.asha.org/Practice-Portal/Clinical-Topics/Articulation-and-Phonology/ [Google Scholar]
- American Speech-Language-Hearing Association (ASHA). (1993). Definitions of communication disorders and variations [Relevant Paper]. Retrieved from www.asha.org/policy
- American Speech-Language-Hearing Association (ASHA). (2004). Admission/discharge criteria in speech-language pathology [Guidelines]. Retrieved from www.asha.org/policy
- American Speech-Language-Hearing Association (ASHA). (2007). Childhood apraxia of speech [Technical Report]. Retrieved from www.asha.org/policy
- Archana G (2008). A manual from communicaid: assessment of oromotor skills in toddlers. Bangalore, India: TheComDEALLTrust. [Google Scholar]
- Baghdadli A, Pascal C, Grisi S, & Aussilloux C (2003). Risk factors for self-injurious behaviors among 222 young children with autistic disorders. Journal of Intellectual Disability Research, 47(8), 622–627. [DOI] [PubMed] [Google Scholar]
- Barokova M, & Tager-Flusberg H (2018). Commentary: Measuring language change through natural language samples. Journal of Autism and Developmental Disorders. Retrieved from 10.1007/s10803-018-3628-4 [DOI] [PubMed] [Google Scholar]
- Belmonte M, Saxena-Chandhok T, Cherian R, Muneer R, George L, & Karanth P (2013). Oral motor deficits in speech-impaired children with autism. Frontiers in Integrative Neuroscience, 7, Article 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Brady N, Bruce S, Goldman A, Erickson K, Mineo B, Ogletree B, Wilkonson K (2016) Communication services and supports for individuals with severe disabilities: Guidance for assessment and intervention. American Journal on Intellectual and Developmental Disabilities, 121(2), 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignell A, Morgan A, Woolfenden S, Klopper F, May T, Sarkozy V, & Williams K (2018). A systematic review and meta-analysis of the prognosis of language outcomes for individuals with autism spectrum disorder. Autism and Developmental Language Impairments, 3, 1–19. [Google Scholar]
- Chenausky K, Kernbach J, Norton A, & Schlaug G (2017). White matter integrity and treatment-based change in speech performance in minimally verbal children with autismspectrum disorder. Frontiers in Human Neuroscience, 11, Article 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenausky K, Norton A, Tager-Flusberg H, & Schlaug G (2016). Auditory-motor mapping training: Comparing the effects of a novel speech treatment to a control treatment for minimally verbal children with autism. PLOS One, 11(11), e0164930. doi: 10.1371/journal.pone.0164930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenausky K, Norton A, Tager-Flusberg H, & Schlaug G (2018). Behavioral predictors of improved speech output in minimally verbal children with autism. Autism Research, 11, 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 155–159. [DOI] [PubMed] [Google Scholar]
- DiStefano C, & Kasari C (2016). The window to language is still open: Distinguishing between preverbal and minimally verbal children with ASD. Perspectives of the ASHA Special Interest Groups SIG 1, Vol. 1(Part 1). [Google Scholar]
- Dominick K, Davis N, Lainhart J, Tager-Flusberg H, & Folstein S (2007). Atypical behaviors in children with autism and children with a history of language impairment. Research in Developmental Disabilities, 28, 145–162. [DOI] [PubMed] [Google Scholar]
- Duffy J (2013). Motor speech disorders: Substrates, differential diagnosis, and management. St. Louis, MO: Elsevier. [Google Scholar]
- Dunn L, & Dunn D (2007). PPVT-4: Peabody picture vocabulary test. Minneapolis, MN: Pearson Assessments. [Google Scholar]
- Ellis Weismer S, & Kover S (2015). Preschool language variation, growth, and predictors in children on the autism spectrum. Journal of Child Psychology and Psychiatry, 56(12), 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang A-G (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Morgan A, Murray E, Cardinaux A, Mei C, Tager-Flusberg H, … Kanwisher N (2015). A highly penetrant form of childhood apraxia of speech due to deletion of 16p11.2. European Journal of Human Genetics, 24(2), 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R, & Fristoe M (2015). The Goldman-Fristoe test of articulation – 3rd edition. Minneapolis, MN: Pearson Assessments. [Google Scholar]
- Hartley S, Sikora D, & McCoy R (2008). Prevalence and risk factors of maladaptive behaviour in young children with autistic disorder. Journal of Intellectual Disability Research, 52(10), 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden D, & Square P (1999). Verbal motor production assessment for children. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Hus V, Maye M, Harvey L, Guthrie W, Liang J, & Lord C (2011). The adapted ADOS – Preliminary findings using a modified version of the ADOS for adults who are nonverbal or have limited language. In: Poster presented at the international meeting for autism research. San Diego, CA, 13 May. [Google Scholar]
- Hus Bal V, Katz T, Bishop S, & Krasileva K (2016). Understanding definitions of minimally verbal across instruments: Evidence for subgroups within minimally verbal children and adolescents with autism spectrum disorder. Journal of Child Psychology and Psychiatry, 57(12), 1424–1433. [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2017). IBM SPSS statistics for Windows, Version 25.0 Armonk, NY: IBM Corp. [Google Scholar]
- Iuzzini-Seigel J, Hogan T, Guarino A, & Green JR (2015). Reliance on auditory feedback in children with childhood apraxia of speech. Journal of Communication Disorders, 54, 32–42. [DOI] [PubMed] [Google Scholar]
- Karanth P (2007). Communication DEALL developmental checklists. Bangalore, India: TheComDEALLTrust. [Google Scholar]
- Kaufman NR (1995). Kaufman speech praxis test. Detroit, MI: Wayne State University Press. [Google Scholar]
- Leon A, & Heo M (2009). Sample sizes required to detect interactions between two binary fixed-effects in a mixed-effects linear regression model. Computational Statistics & Data Analysis, 53(3), 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois F, & Morgan AT (2012). Neural bases of childhood speech disorders: Lateralization and plasticity for speech functions during development. Neuroscience and Biobehavioral Reviews, 36, 439–458. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule (modules 1–4) (2nd ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- Luyster R, Kadlec M, Carter A, & Tager-Flusberg H (2008). Language assessment and development in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 38, 1426–1438. [DOI] [PubMed] [Google Scholar]
- Mei C, Fedorenko E, Amor DJ, Boys A, Hoeflin C, Carew P, … Morgan A (2018). Deep phenotyping of speech and language skills in individuals with 16p11.2 deletion. European Journal of Human Genetics, 26, 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Andriacchi K, & Knockerts A (2011). Assessing language production using SALT software: A clinician’s guide to language sample analysis. Middleton, WI: SALT Software, LLC. [Google Scholar]
- Morgan AT, van Haaften L, van Hulst K, Edley C, Mei C, Yang Tan T, … Koolen DA (2018). Early speech development in Koolen de Vries Syndrome limited by oral praxis and hypotonia. European Journal of Human Genetics, 26(1), 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AT, & Webster R (2018). Aetiology of childhood apraxia of speech: A clinical practice update for paediatricians. Journal of Paediatrics and Child Health, 54, 1090–1095. [DOI] [PubMed] [Google Scholar]
- Norrelgen F, Fernell E, Eriksson M, Hedvall A, Persson C, Sjölin M, … Kjellmer L (2015). Children with autism spectrum disorders who do not develop phrase speech in the preschool years. Autism, 19, 934–943. [DOI] [PubMed] [Google Scholar]
- Prizant B (1996). Brief report: Communication, language, social, and emotional development. Journal of Autism and Developmental Disorders, 26(2), 173–178. [DOI] [PubMed] [Google Scholar]
- Rogers S, Hayden D, Hepburn S, Charlifue-Smith R, Hall T, & Hayes A (2006). Teaching young nonverbal children with autism useful speech: A pilot study o the Deonver Model and PROMPT interventions. Journal of Autism and Developmental Disorders, 36, 1007–1024. [DOI] [PubMed] [Google Scholar]
- Roid G, & Miller L (2013). Leiter-3: Leiter International Performance Scale, third edition. Torrance, CA: Western Psychological Services. [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2003). Autism diagnostic interview – Revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Schopler E, Van Bourgondien M, Wellman J, & Love S (2010). Childhood Autism Rating Scale-Second Edition (CARS2). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Shriberg L, Paul R, Black L, & van Santen J (2011). The hypothesis of apraxia of speech in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 41, 405–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg L, Strand E, Fourakis M, Jakielski KJ, Hall SD, Karlsson HB, … Wilson DL (2017). A diagnostic marker to discriminate childhood apraxia of speech from speech delay: I. Development and description of the pause marker. Journal of Speech, Language, and Hearing Research, (Suppl.), 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway S, Farmer C, Thurm A, Joseph L, Black D, & Golden C (2012). The ADOS calibrated severity score: Relationship to phenotypic variables and stability over time. Autism Research, 5, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand E, & McCauley R (2019). Dynamic evaluation of motor speech skill (DEMSS). Baltimore, MD: Brookes Publishing. [Google Scholar]
- Strand E, McCauley R, Weigand S, Stoeckel RE, & Baas BS (2013). A motor speech assessment for children with severe speech disorders: Reliability and validity evidence. Journal of Speech, Language, and Hearing Research, 56, 505–520. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H (2006). Defining language phenotypes in autism. Clinical Neuroscience Research, 6, 219–224. [Google Scholar]
- Tager-Flusberg H (2015). Defining language impairments in a subgroup of children with autism spectrum disorder. Science China. Life Sciences, 58(10). [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H (2016). Risk factors associated with language in autism spectrum disorder: Clues to underlying mechanisms. Journal of Speech, Language, and Hearing Research, 59, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, & Caronna E (2007). Language disorders: Autism and other pervasive developmental disorders. Pediatric Clinics of North America, 54(3), 439–481. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, & Kasari C (2013). Minimally verbal school-aged children with autism spectrum disorder: The neglected end of the spectrum. Autism Research, 6(6), 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Plesa Skwerer D, Joseph R, Brukilacchio B, Decker J, Eggleston B, … Yoder A (2017). Conducting research with minimally verbal participants with autism spectrum disorder. Autism, 21(7), 852–861. DOI: 10.1177/1362361316654605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R, … Yoder P (2009). Defining spoken langauge benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research, 52, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm A, Lord C, Lee L-C, & Newschaffer C (2007). Predictors of language acquisition in preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37, 1721–1734. 10.1007/s10803-006-0300-1 [DOI] [PubMed] [Google Scholar]
- Tierney C, Mayes S, Lohs S, Black A, Gisin E, & Veglia M (2015). How valid is the checklist for autism spectrum disorder when a child has apraxia of speech? Journal of Developmental Behavioral Pediatrics, 0, 1–6. [DOI] [PubMed] [Google Scholar]
- Velleman S, Andrianopoulos M, Boucher M, Perkins JJ, Averback KE, Currier AR, … Van Emmerik R (2010). Motor speech disorders in children with autism. In Paul R & Flipsen P (Eds.), Speech sound disorders in children (pp. 141–180). San Diego, CA: Plural Publishing. [Google Scholar]
- Venter A, Lord C, & Schopler E (1992). A follow-up study of high-functioning autistic children. Journal of Child Psychology and Psychiatry, 33(3), 489–507. [DOI] [PubMed] [Google Scholar]
- White SM, Morgan A, Da Costa A, Lacombe D, Knight SJL, Houlston R, … Hurst JA (2010). The phenotype of Floating-Harbor syndrome in 10 patients. American Journal of Medical Genetics Part A, 152A, 821–829. [DOI] [PubMed] [Google Scholar]