ABSTRACT
Osteoarthritis (OA) is a degenerative disease of extremely high incidence in the elderly. Therefore, anti-aging may be an important prerequisite for treating OA. The senescence of chondrocytes and mesenchymal stem cells (MSCs) is one of the important factors that causes OA. Here, the effect of uridine (which is a functional food derived from plants or animals) on senescence of chondrocytes and MSCs was evaluated in in vivo and in vitro experiments. For this, we established the senescence model of chondrocyte and MSCs in vitro, and established the OA model in vivo, and a series of experiments (such as CLSM, ELISA, Western blot, etc.) were conducted to evaluate the effect of uridine on chondrocyte and MSCs senescence. The results showed that uridine could alleviate chondrocyte and MSCs senescence in vitro by evaluating a series of aging markers. Furthermore, uridine could also relieve OA in vivo. In summary, in the present work, we found that uridine can alleviate chondrocyte and MSCs senescence in in vitro and in vivo experiments. Uridine has shown great potential in the treatment of OA in vivo, suggesting that uridine could be used to treat and prevent OA induced by aging, and has potential clinical applications in future.
KEYWORDS: Uridine, chondrocyte cell line, MSC, aging, osteoarthritis (OA)
Introduction
Osteoarthritis (OA) is closely related to aging [1–3]. At present, population aging has become one of the important social problems in the twenty-first century [4,5]. OA is considered to be the inevitable result of aging. OA is closely associated with articular chondrocytes aging [3,6,7]. The increase in the positive rate of senescent cartilage cells reduces the repair ability of cartilage, aggravates cartilage degeneration, and promotes an increase in the incidence of OA [7]. Chondrocyte senescence plays an important role in the occurrence and development of OA. Therefore, relieving or inhibiting the aging of cartilage cells may reverse OA, protect the integrity of articular cartilage tissue, and alleviate OA patients’ pain Figure 1.
Figure 1.
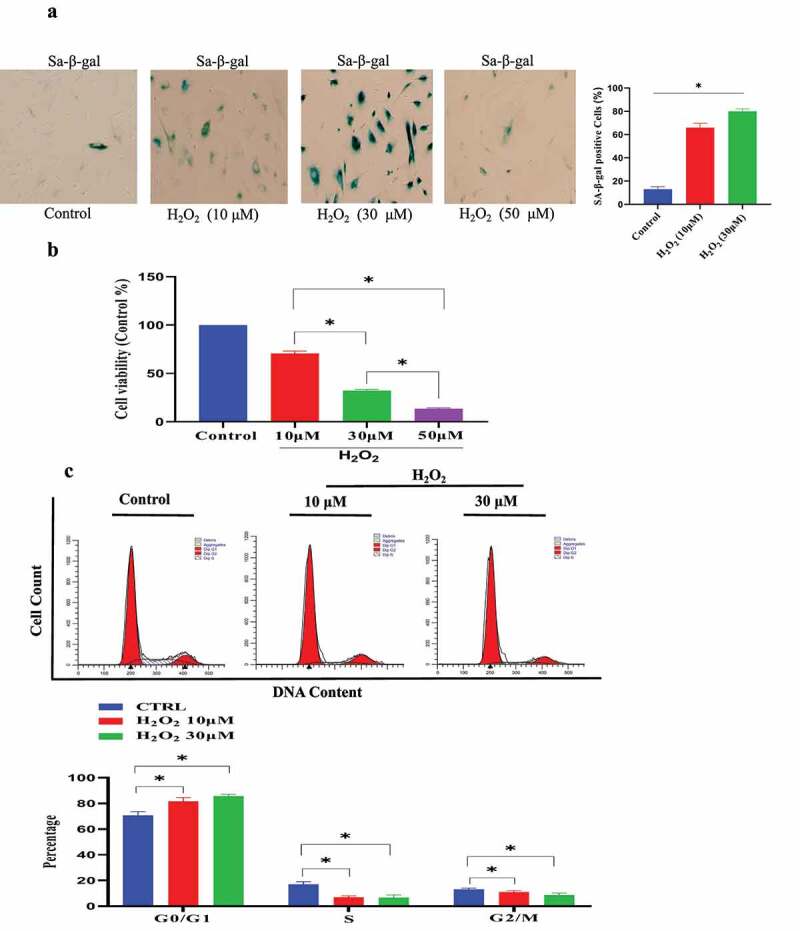
C-28/I2 chondrocyte cell senescence model was established by H2O2 treatment. (a) Sa-β-gal-positive cells were significantly up-regulated under H2O2 treatment. (b) H2O2 treatment reduced cell proliferation of C-28/I2. (c) The effect of H2O2 treatment on cell cycle phase by Flow cytometry analysis. (d) H2O2 (30 μmol/L) resulted in slight C-28/I2 chondrocyte apoptosis by Flow cytometry analysis. (e) The expression of P21 and P16 was increased under H2O2. (f). IL-1β and IL-6 expression was increased in the H2O2-induced C-28/I2 chondrocyte cell senescence model. Data are expressed as mean ± SD. The asterisk indicates a significant difference (p < 0.05).
Figure 1.
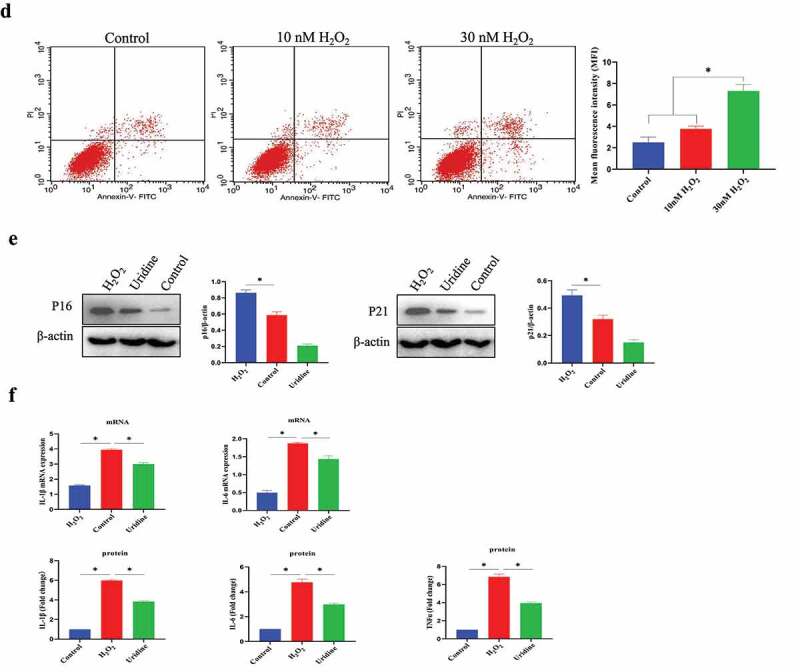
Continued.
In addition, OA is also closely related to stem cell senescence [1]. Mesenchymal stem cells (MSCs) are special cells that have the potential to self-renew and differentiate into various types of cells. The MSCs can differentiate into chondrocytes, thereby supplementing the loss of chondrocytes [8], which provides a new way for OA treatment [9]. However, with aging, stem cells will also be aging, which will result in the ability of MSCs to differentiate into cartilage to gradually weaken (or even disappear) [10]. MSCs are currently being used in the various OA treatments [11].
Cells are the basic units of tissues and organs. Therefore, cellular senescence is a prerequisite for tissue and organ aging. In recent years, researchers have conducted in-depth research on cellular senescence and have proposed a variety of cellular senescence mechanisms [12,13]. However, so far, the nature of the mechanism of cellular senescence has not been fully revealed. At present, more and more research evidences indicate that aging is one of the biggest risk factors for OA [14,15].
In the current research, we have studied the potential anti-aging effect of uridine, which is a bioactive molecule. Uridine is a kind of nucleotide [16]. It is a synthetic material of RNA. In addition, it has been reported that uridine plays an important function in the synthesis of glycogen. Diet is an indirect source of uridine [17–19]. In the present work, we found that uridine can alleviate the senescence of chondrocytes and MSCs in vivo. Further work indicated that uridine could relieve OA in vivo.
Materials and methods
Materials
Anti-NFκB (ab32360, 1:200 dilution), anti-p16 (ab51243, 1:500 dilution), and anti-p21 (ab109520, 1:1000 dilution) were obtained from Abcam Ltd. (UK, Cambridge). Uridine was purchased from Sigma-Aldrich (CAS:58-96-8). BCA protein assay kit (#23,225) was purchased from Thermo Scientific (USA). RIPA lysate, glutathione peroxidase (GSH-PX) assay kit, and reactive oxygen assay species assay kit were purchased from Solarbio (Beijing, PR China). MDA assay kit and cell superoxide dismutase (SOD) assay kit were obtained from Shanghai Biyuntian Biotechnology Co., Ltd. (Shanghai, China). Fetal bovine serum (FBS, #1,616,964) and DMEM culture medium were obtained from Thermo Fisher (USA).
Cell culture
Mesenchymal stem cells (catalog number, A15652) were purchased from Thermo Fisher Scientific. Stem cells were cultured in an MSC medium supplemented with 10% FBS.
CCK8 detects cell proliferation ability
The cells were seeded into a 96-well plate (1 × 105 cells per well), and the cells were treated with uridine (0.4 mg/mL) as indicated in the figure legends and cultured for different time points. After incubation, the CCK8 solution was added and cultured for 4 h. The absorbance of each well was measured at 450 nm wavelength using a microplate reader (Thermo Fisher Scientific).
β-Galactosidase staining
The staining of β-Galactosidase (β-Gal) was carried out according to the β-galactosidase (SA-β-Gal) kit’s instruction (#RG0039, Beyotime). In brief, the cells were washed three times with PBS. The cells were then fixed at room temperature for 15 min. After the cells were washed three times with PBS, SA-β-Gal staining solution was added and incubated overnight at 37°C in the dark. The cell samples were observed under a microscope.
SOD level
Total Superoxide Dismutase Assay Kit was used to detect superoxide dismutase (SOD).
RT-PCR
Total RNA was extracted using an RNAzol isolation kit. The extracted RNA samples were then reverse-transcribed into complementary DNA using a TaKaRa RNA PCR Kit (TaKaRa, Shiga, Japan). RT-PCR was performed using the following primers: IL-1β: Fwd: CAACCAACAAGTGATATTCTCCATG, Rev: GATCCACACTCTCCAGCTGCA; IL-6: Fwd: GAGGATACCACTCCCAACAGACC, Rev: AAGTGCATCATCGTTGTTCATACA; TNFα: Fwd: CATCTTCTCAAAATTCGAGTGACAA, Rev: TGGGAGTAGACAAGGTACAACCC.
Western blot analysis
Proteins from cells or tissues were extracted. Protein samples were then centrifuged at 15,000 r/min at 4°C for 10 min, and the cell supernatant was collected. BCA kit was used to determine protein concentration. The protein samples were subjected to SDS-PAGE and transferred to PVDF membrane. After PVDF membranes were washed three times with TBST, the indicated primary antibodies were added and incubated at 4°C overnight. After washing PVDF membrane for three times, secondary antibodies were added and incubated for 1.5 h at RT. After the final three washes, the membranes were detected by ChemiDoc™ MP imager (Bio-Rad).
CLSM
The cells were rinsed and fixed with 4% PFA for 20 min at RT. After three washes with PBS, the cell samples were blocked with 5% BSA for 1.5 h at RT. The primary antibody diluted in the 1% BSA was added and incubated overnight at 4°C. The cell samples were rinsed with PBS and incubated with fluorescently labeled secondary antibody (diluted at 1:1000) for 1 h at 37°C. After three washes with PBS buffer, the cell samples were observed and photographed by a laser confocal scanning microscope (CLSM).
Cell cycle detection
Cell cycle was analyzed by cell cycle detection kit (Abcam, #ab112117) according to its manufacturer’s instructions. The C-28/I2 chondrocyte cells were harvested and transferred into a sterile centrifuge tube. After washing, the cells were fixed with 70% ethanol. After three washes, 500 μL of PI dye was added and the cells re-suspended. The cells were stained for 30 min at 4°C. After washing in PBS, the cell cycle was tested by flow cytometry (BD Accuri C6, Flow Cytometry).
Flow cytometry analysis
The C-28/I2 chondrocyte cells were collected. After washing, the cells were fixed with paraformaldehyde (PFA) and blocked with 5% BSA. After washing, the cells were incubated with the indicated primary antibodies at 4°C overnight. After washing, the fluorescently labeled secondary antibody was added and incubated for 1.5 h, followed by flow cytometry analysis (BD Accuri C6).
HE Staining
The joint tissue of the mice was fixed in 4% paraformaldehyde. The tissue was later dehydrated and embedded in paraffin. The pathological changes of joint tissue were observed by a microscope.
Establishment of OA model
OA model was established according to published protocol [20]. C57 mice were used in the present study. After the skin of the right-knee joint of the experimental mouse was cut, the knee joint cavity was exposed, and the tibial ligament was cut off.
Cell apoptosis analysis
Cell apoptosis was evaluated by flow cytometry. The cells were later treated with uridine, as indicated in the figure legends. The cells were then harvested and stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and PI solution according to manufacturer’s instructions. Cell samples were then detected by flow cytometry (BD Accuri C6).
ELISA
The pro-inflammatory cytokine levels in the serum from mice (n = 5) that were treated with uridine or vehicle were evaluated using enzyme-linked immunosorbent assay (ELISA, eBioscience).
Micro-CT scanning analysis
In this experiment, micro-CT scanning of the specimens was performed using GE’s triph trimodality preclinical imaging system (GE Healthcare, USA). All reconstructed images were processed with Amira 5.2 image processing software (Visage Imaging, Inc., CA, USA).
Statistical analysis
Data were analyzed using GraphPad Prism version 8.0. All results are expressed as mean ± SD. SPSS23.0 software was used for single-factor analysis of variance (one-way ANOVA). A p-value of less than 0.05 was considered statistically significant.
Results
Establishment of C-28/I2 chondrocyte/MSCs senescence model by H2O2 treatment
The senescent C-28/I2 chondrocyte model was established by H2O2 treatment. In the preliminary experiment, the different concentrations of H2O2 were used to induce the senescence of C-28/I2 chondrocyte cell (0–50 μmol/L): (1) As shown in Figure s 1a, 30 μmol/L H2O2 successfully led to C-28/I2 chondrocyte senescence. Sa-β-gal-positive cells were significantly increased under H2O2 induction. (2) The results of CCK-8 tests showed that H2O2 treatment reduced cell growth of C-28/I2 cell (70.0 ± 8.1% in the 10 μM, 32.9 ± 10.4% in the 30 μM group, 13.5 ± 8.4% in the 50 μM group, compared with the control group) (Figure 1 b). (3) The cell cycle was analyzed by flow cytometry. As shown in Figure 1 c, H2O2 treatment resulted in a significant increase in the G0/G1 phase. Meanwhile, H2O2 treatment reduced the proportion of S-phase cell [17% (Control group) VS 7% (10 μM H2O2) and 6% (30 μM H2O2), P < 0.05]. Cells treated with 50 μM H2O2 could not be collected in sufficient amounts for flow cytometry analysis. (4) The results from flow cytometry indicated that H2O2 (30 μmol/L) only result in slight C-28/I2 chondrocyte apoptosis (Figure 1 d). Therefore, we chose 30 μmol/L H2O2 to induce C-28/I2 chondrocyte cell senescence in the current research. On this basis, further research showed that P16 and P21 (senescence marker) were significantly up-regulated (Figure 1 e and Figure S1). (4) Cellular senescence is closely related to inflammation. Therefore, we also tested the expression of the pro-inflammatory molecules in the protein and mRNA level, and found that interleukin-6 (IL)-6 and IL-1β were also unregulated by H2O2 treatment (Figure 1 f).
Next, we also used the same method to establish the cell senescence model of MSCs. The results showed that 100 μmol H2O2 treatment could successfully induce MSCs senescence. Sa-β-gal-positive cell ratio was significantly increased after H2O2 stimulation (Figure 2a). 2) CCK-8 assays indicated that H2O2 stimulation reduced MSC proliferation (73.0 ± 5.0% in the 50 μM group, 35.0 ± 8.9% in the 100 μM group, 15.0 ± 9.0% in the 200 μM group) (Figure 2b). (3) The cell cycle was evaluated by flow cytometry, and H2O2 stimulation increased the proportion of G0/G1 phase in the cell cycle, and the proportion of S phase was significantly reduced (Figure 2c). H2O2 treatment led to a significant increase in the G0/G1 phase. At same time, H2O2 stimulation reduced the proportion of S-phase cell [18% (Control group) VS 6% (50 μM H2O2)/5% (100 μM H2O2), P < 0.05]. Cells treated with 200 μM H2O2 could not be collected in sufficient amounts for flow cytometry analysis (data not shown). (4) Flow cytometry experiments indicated that H2O2 (100 μmol/L) did not induce significant MSCs apoptosis (Figure 2d). However, H2O2 (200 μM) induced a significant increase in apoptotic MSCs. Therefore, 100 μmol/L H2O2 was used to establish MSCs senescence model in the current work. Further study indicated that P16 and P21 (senescence marker) were significantly increased compared to control group (Figure 2e).
Figure 2.
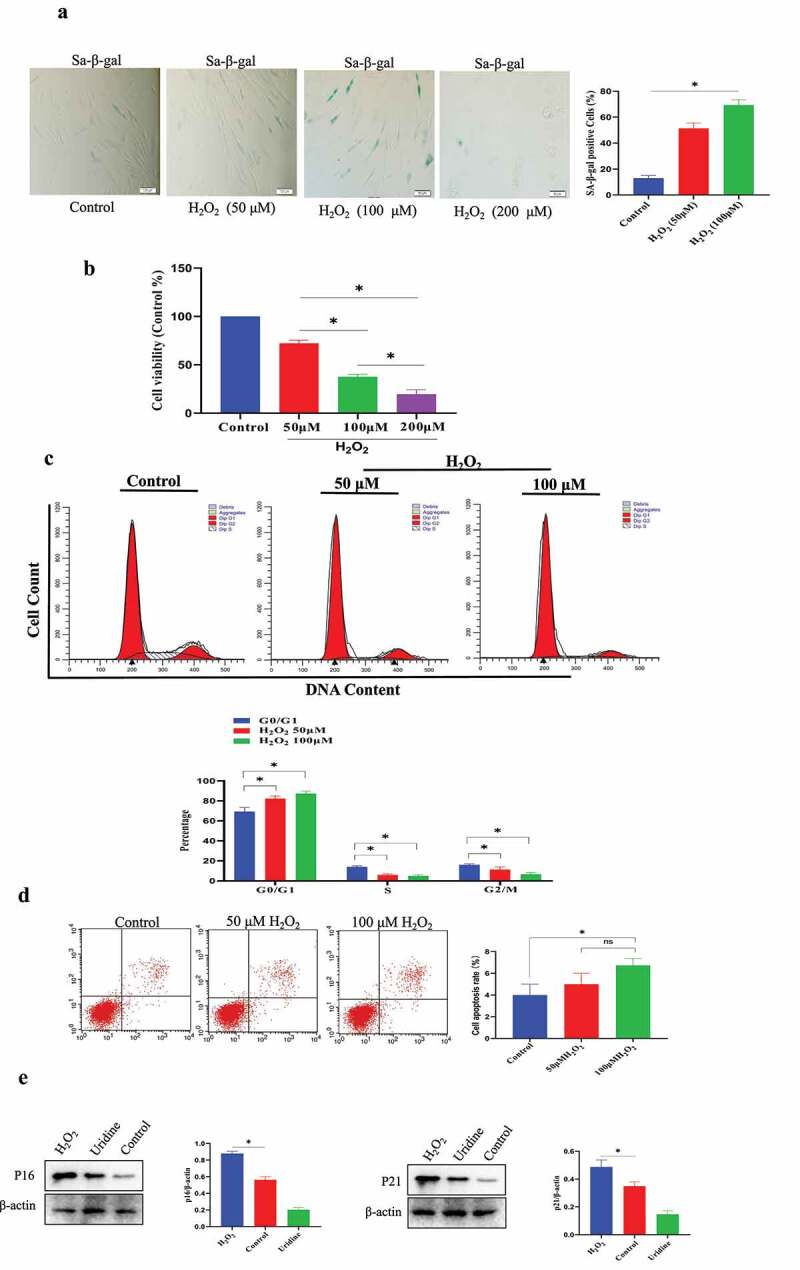
Establishment of MSC senescence model by H2O2 stimulation. (a) Sa-β-gal-positive cells were significantly increased under H2O2 treatment. (b) H2O2 treatment reduced cell proliferation of MSCs. (c) The effect of H2O2 treatment on cell cycle phase by Flow cytometry analysis. (d) H2O2 (100 μmol/L) only leaded slight MSCs apoptosis. (e) The expression of P21 and P16 was increased under H2O2 treatment. Data are expressed as mean ± SD (n = 5). The asterisk indicates a significant difference (p < 0.05).
Uridine alleviated the senescence of C-28/I2 and MSCs
Here, the effect of uridine on C-28/I2 chondrocyte senescence was studied, and the results showed that uridine could reduce C-28/I2 chondrocyte senescence. Uridine treatment significantly reduced the number of SA-β-gal-positive cells compared to the control group, and the expression of P16 and P21 was also significantly decreased (Figure 3 (a,b)). In addition, cell proliferation assay showed that uridine treatment increased the C-28/I2 cell proliferation (Figure 3c), and cell cycle experiments also showed that the S-phase ratio of uridine-treated C-28/I2 chondrocyte cells was significantly increased (Figure 3d). In addition, the percentage of Ki67-positive C-28/I2 chondrocyte cell is significantly up-regulated (Figure 3e).
Figure 3.
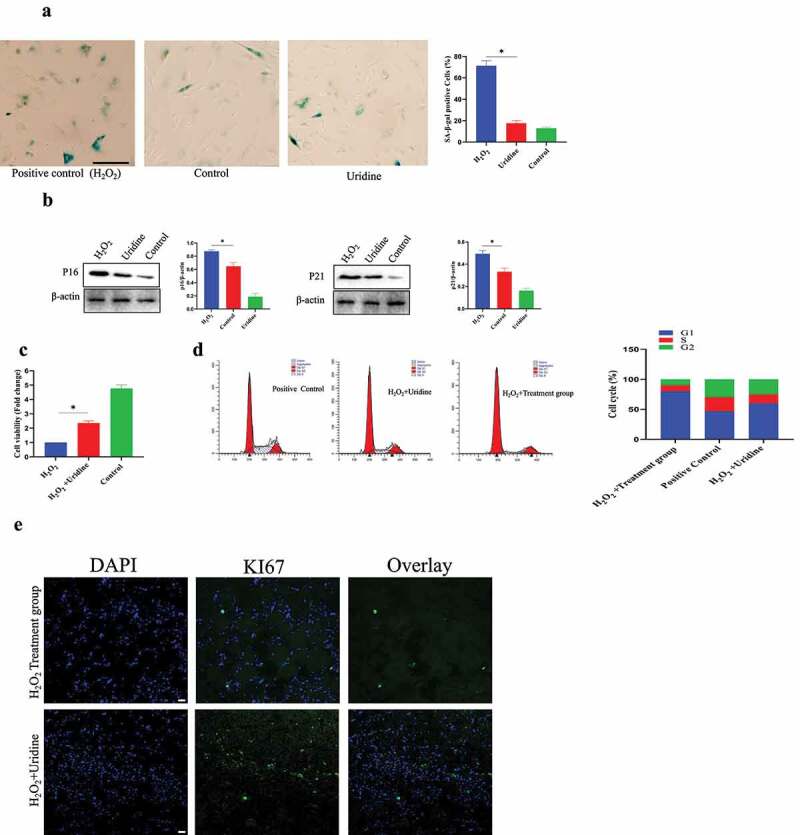
(a) Uridine treatment reduced the ratio of β-gal positive cells. (b) The expression level of P16 and P21 was reduced by uridine treatment. (c) Uridine treatment increased the C-28/I2 chondrocyte cell proliferation. (d) Cell cycle was changed by uridine treatment (n = 5). E. Ki67 was up-regulated by uridine treatment. Data are expressed as mean ± SD. The asterisk indicates a significant difference (p < 0.05).
Next, we also studied the effect of uridine on MSCs senescence and found that uridine could relieve the MSC senescence. Uridine stimulation significantly reduced the number of SA-β-gal-positive cells compared to the control group. In addition, the expression of P16 and P21 was also significantly down-regulated (Figure 4 A and B). Furthermore, uridine treatment promoted the MSC proliferation (Figure 4c), and cell cycle analyses also indicated that the S-phase ratio of MSC in the uridine treatment group was significantly increased (Figure 4d). Additionally, the percentage of Ki67-positive MSC was significantly increased by uridine treatment (Figure 4e).
Figure 4.
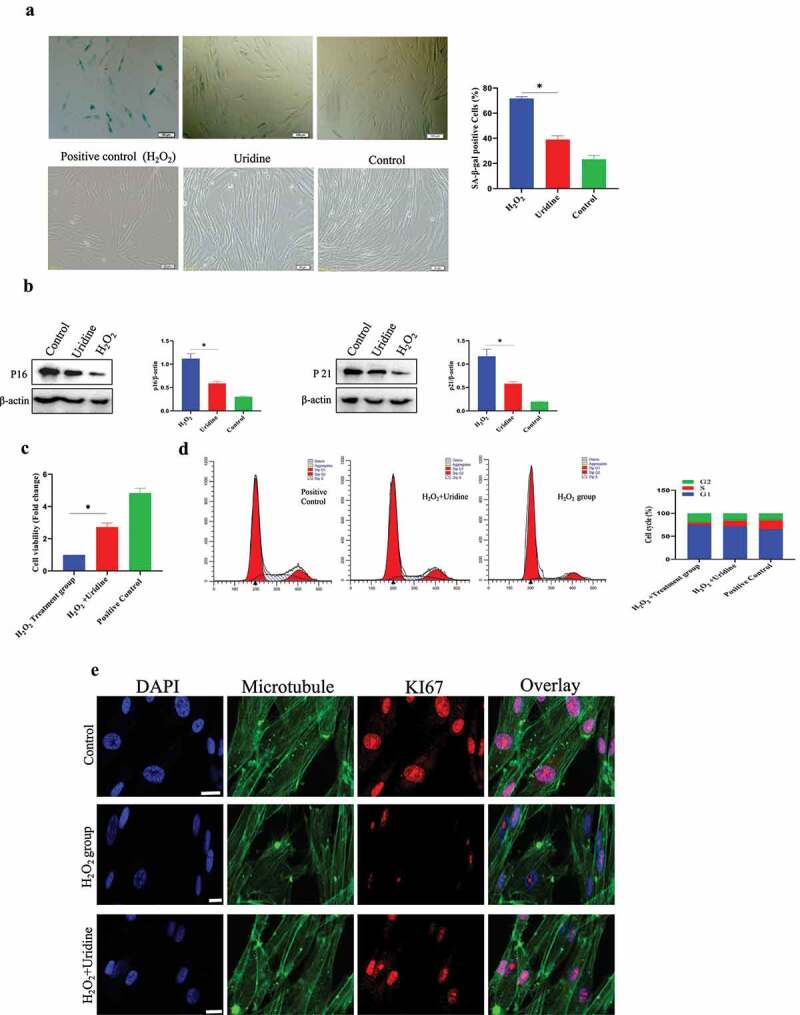
(a) Uridine treatment reduced the ratio of β-gal positive cells. (b) The expression levels of P16 and P21 were reduced by uridine treatment. (c) Uridine treatment increased the C-28/I2 chondrocyte cell proliferation (n = 5). (d) Cell cycle was changed by uridine treatment. E. Ki67 was up-regulated by uridine treatment. Data are expressed as mean ± SD. The asterisk indicates a significant difference (p < 0.05).
The effect of uridine on inflammation in the senescent C-28/I2 and MSCs
The effect of uridine on inflammation was studied, and results showed that the TNF-α, interleukin-1β (IL-1β), and IL-6 were significantly reduced by uridine treatment (Figure 5a). We further explore the molecular mechanism by which uridine could relieve inflammation. For this, we analyzed the inflammatory-related signaling pathway and found that inflammatory-related signaling pathway was inhibited by uridine in C-28/I2 and MSC (Figure 5b). This may be one of the potential molecular mechanisms by which uridine relieves inflammation.
Figure 5.
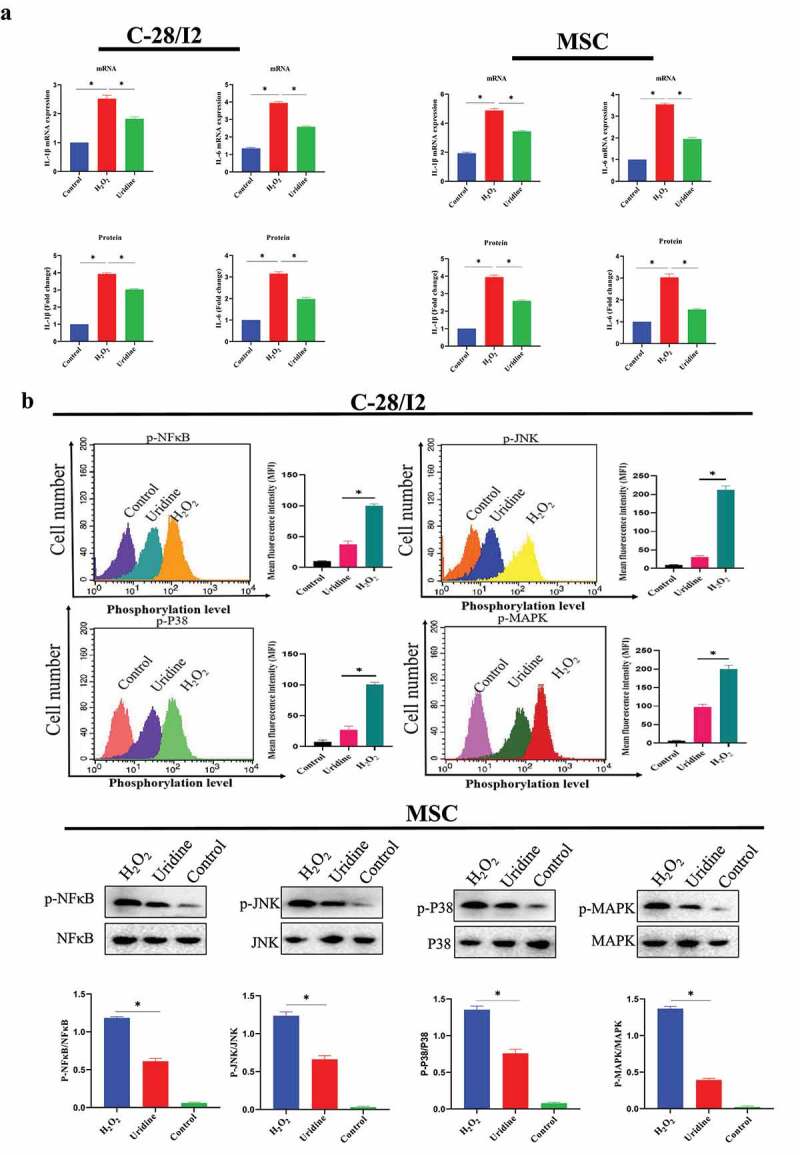
(a) Uridine relieves inflammation caused by aging in C-28/I2 (left penal) and MSC (right penal). (b) Inflammatory-related signaling pathway was inhibited by uridine in C-28/I2 and MSC (n = 5). Data are expressed as mean ± SD. The asterisk indicates a significant difference (p < 0.05).
Effect of uridine on oxidative stress in vitro
Aging is closely linked with oxidative stress [21]. The oxidative stress theory of aging has been the most widely accepted [22]. Aging results from the accumulation of oxidative damage, which is related to the release of reactive oxygen species. It is well known that H2O2 could lead to oxidative stress. As expected,the level of oxidative stress was markedly increased after H2O2 treatment,Figure 6,Figure 7 shown that the expression level of SOD, CAT, GSH and GPx were obviously down-regulated.However, uridine treatment significantly increased the levels of SOD, CAT, GSH, and GPx, and reduced the levels of ROS and MDA in C-28/I2 and MSC. These findings suggest that uridine has an anti-oxidative stress effect for C-28/I2 and MSCs.
Figure 6.
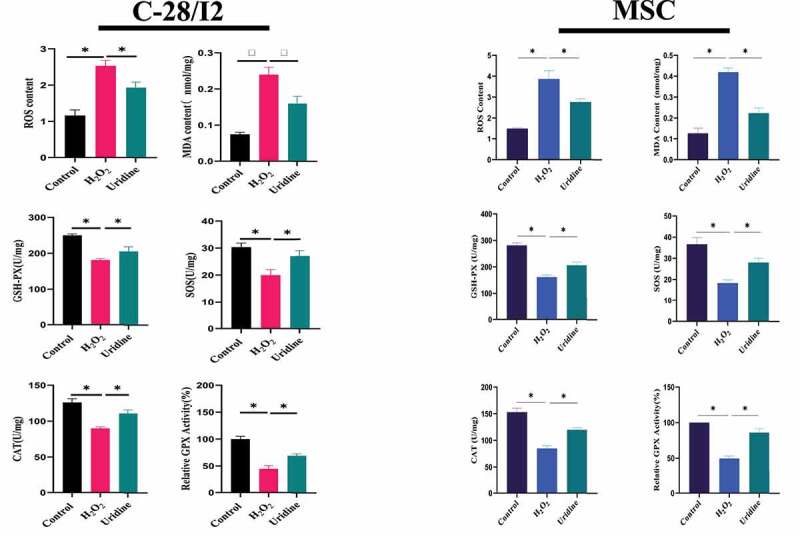
Effect of uridine on oxidative stress in C-28/I2 and MSC. The detailed process of the experiment has been described in detail in Materials and Methods section. Data are expressed as mean ± SD (n = 5). The asterisk indicates a significant difference (p < 0.05).
Figure 7.
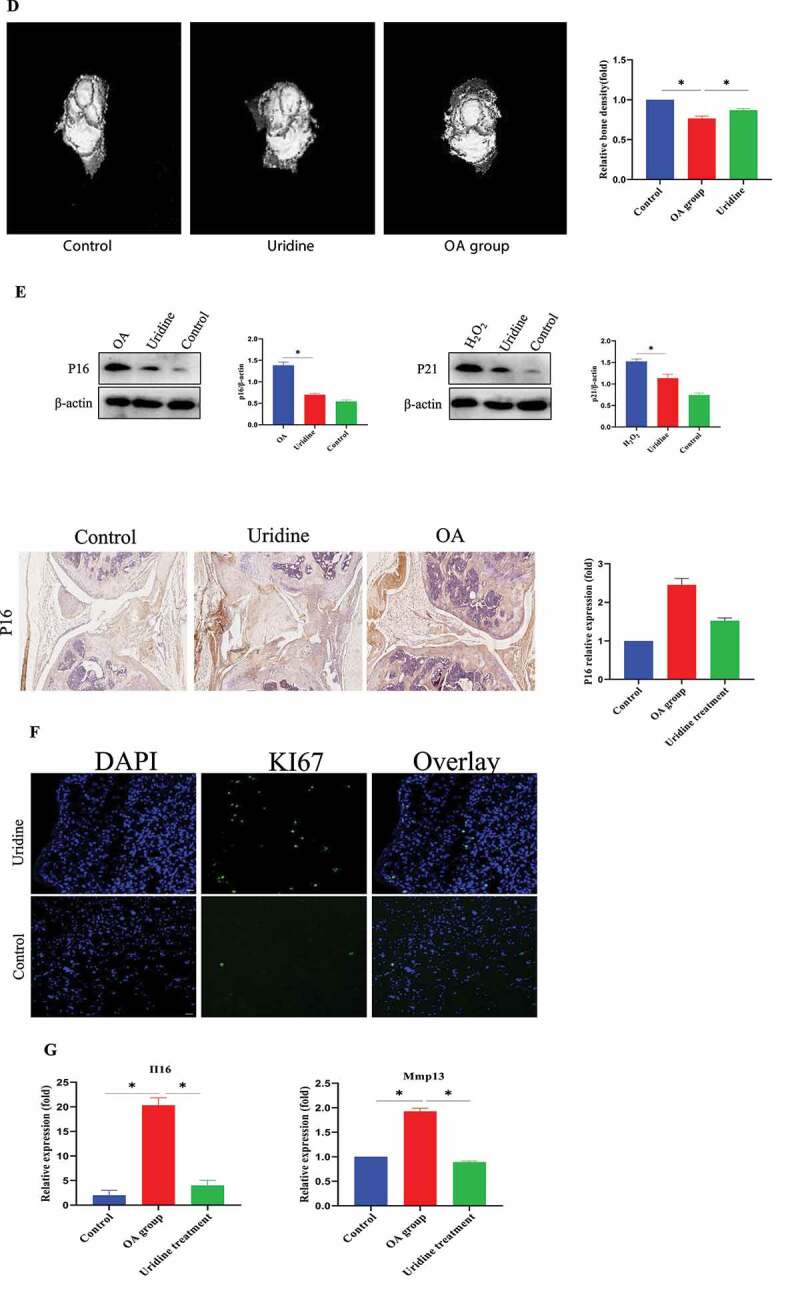
(a-c) Hematoxylin-eosin staining (HE) Safranin O-Fast Green staining showing that uridine can relieve OA. The experimental process has been described in detail in the Materials and Methods section. (d) The effect of uridine treatment on bone density by micro-CT analysis (n = 3). (e) p16 and p21 were down-regulated by uridine treatment. (f) Ki67 expression in uridine treatment group was increased compared to OA group. (g) Inflammation factors were down-regulated by uridine treatment. Data are expressed as mean ± SD (n = 3). The asterisk indicates a significant difference (p < 0.05).
Figure 7.
Continued.
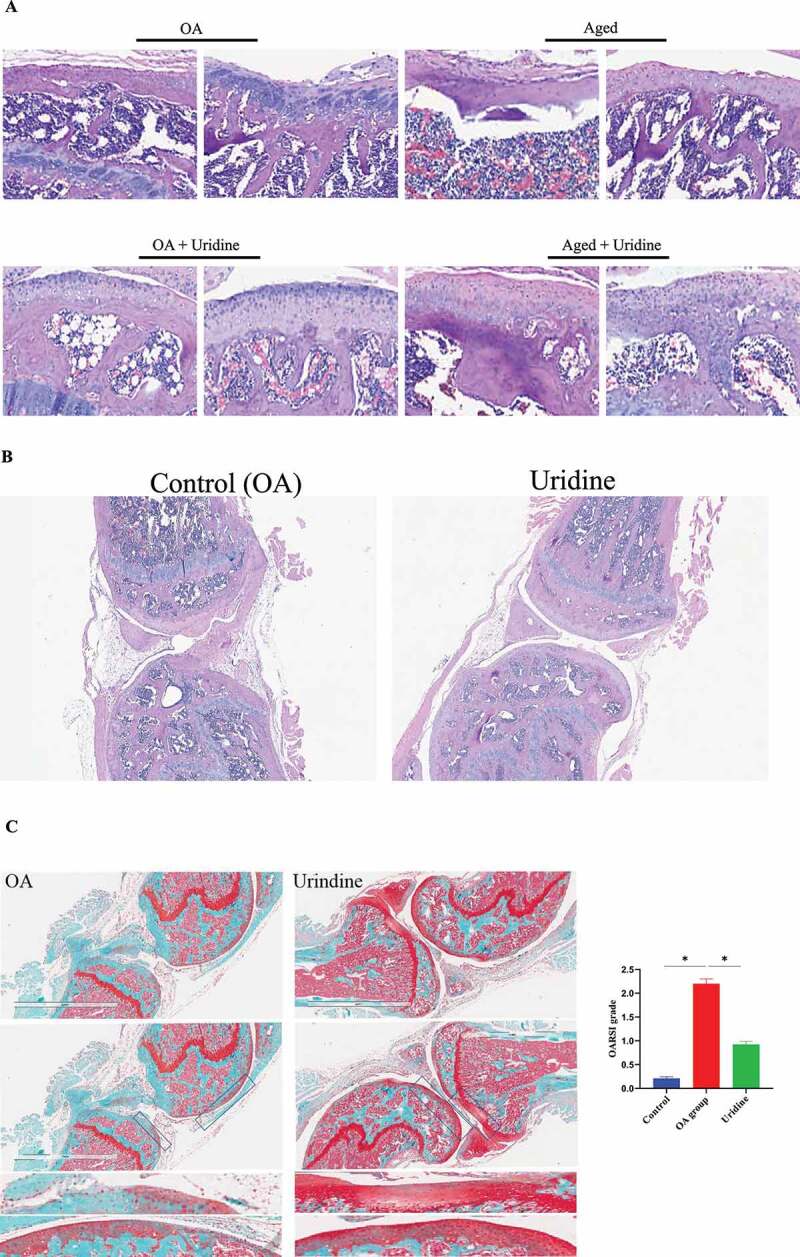
The effect of uridine on OA in vivo
OA is one of the most common chronic diseases among aged population. Therefore, it is closely to aging. Here, we established a model of OA and then studied the effect of uridine on OA. Hematoxylin–eosin staining (HE) (Figure 7 (a,b)) and Safranin-O/Fast Green staining (Figure 7 c) showed that the knee joint was severely injured in the OA model group and the aged mice group, and the cartilage became thinner. Uridine treatment induced cartilage regeneration in the articular cavities; these findings indicated that uridine can relieve joint damage in vivo.
In addition, microcomputed tomography (micro-CT) analyses showed that in contrast to the OA group, the bone quality of the OA mice treated with uridine was well maintained as those of the mice without OA (Figure 7d). Uridine could down-regulate p16 and p21 expression by Western blot and immunohistochemistry (Figure 7 e). In addition, Ki67 expression in the treatment group was increased compared to the OA group (figure 7 f). Additionally, the mRNA levels of typical markers of inflammation factors were up-regulated in OA, and uridine treatment reduced the expression of inflammation factors (Figure 7 g).
Effects of uridine on serum SOD, MDA, CAT, and GSH
As indicated in Figure 8a, aged mice treated with uridine significantly increased the levels of SOD, CAT, and GSH compared to control aged mice, and MDA level was down-regulated, suggesting that uridine has an anti-oxidative stress effect.
Figure 8.
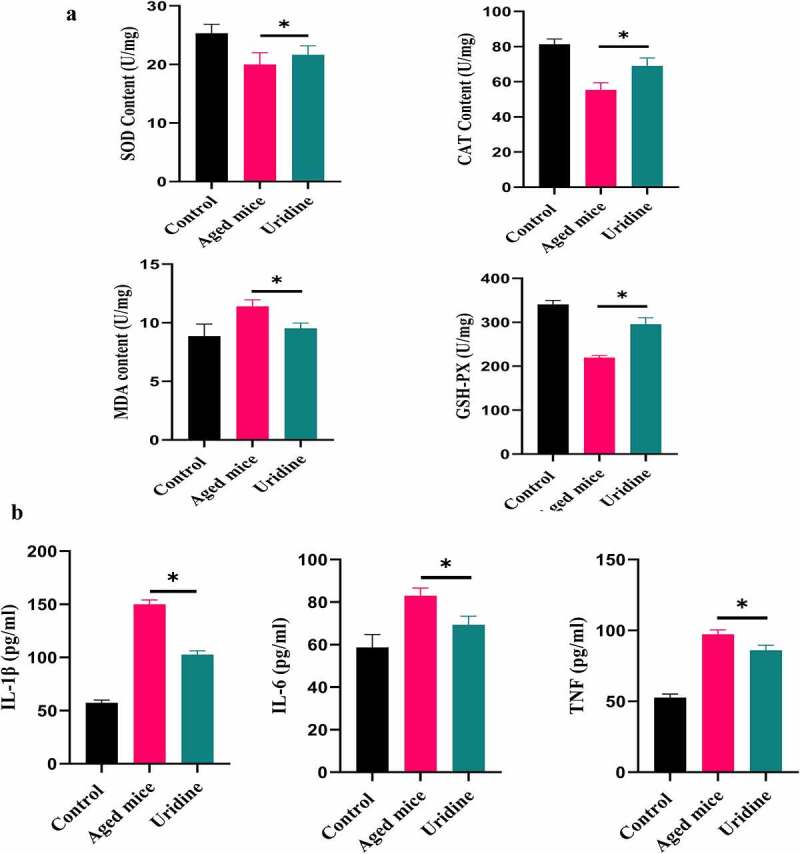
(a) Effects of uridine on serum SOD, MDA, CAT, and GSH. Effects of uridine on cytokine levels. The level of IL-6 and IL-1β in serum was determined by ELISA Kit according to the manufacturer’s instruction. Data are expressed as mean ± SD (n = 5). The asterisk indicates a significant difference (p < 0.05).
In addition, we also analyzed the effects of uridine on cytokine levels in serum. As illustrated in Figure 8b, IL-6 and IL-1β (pro-inflammatory cytokines) in serum from uridine-treated mice showed different degrees of decrease, which shows that uridine has a potential anti-inflammatory effect in vivo.
Discussion
As the global aging population increases, the incidence of OA is also gradually increasing [2]. Until now, scientists have been looking for effective methods to treat OA, including the following methods [23,24]: 1) Autologous chondrocyte implantation (ACI); 2) Stem cell therapy; 3) Growth factor therapy: in normal articular cartilage, a variety of growth factors (such as G-CSF, TGF-β, IGF-1, FGF-2) work together to regulate tissue development and internal environment balance, and promote cell growth and differentiation; and 4) Gene therapy: gene therapy is an emerging therapeutic strategy to treat OA. However, aging may be one of the most important risk factors for OA. Therefore, how to alleviate aging may be the most promising treatment strategy for preventing and treating OA. To address this, in the present study, we explore the effect of uridine on the senescence of C-28/I2 chondrocyte MSC. Further study indicated that uridine could relieve OA in vivo. These findings indicated that uridine can be used to treat and prevent aging and OA.
Aging is a complex biological process involving molecular, cellular, and organic changes [25]. In order to evaluate the effect of uridine on C-28/I2 chondrocyte cell senescence, we first established an aging model of chondrocyte. H2O2 was used to establish the C-28/I2 chondrocyte senescence model. In the pre-experiment, different concentrations of H2O2 were used to induce the C-28/I2 chondrocyte senescence. However, we found that when high concentrations of H2O2 were used, the apoptosis rate of C-28/I2 cell was significantly increased, but low concentrations of H2O2 only slightly induced C-28/I2 cell apoptosis, which is consistent with previous reports [25]. Further experiments showed that uridine could significantly reduce the senescence level of C-28/I2 chondrocyte. We also found that uridine could also reduce the level of inflammation in senescent cells, and mechanism studies have found that uridine treatment could down-regulate inflammation-related signaling pathways.
MSCs are special cells that have the potential to self-renew and differentiate into multiple types of cells. MSCs can differentiate into chondrocyte, which provides a new way for OA treatment. Initially, the treatment of OA using MSCs was thought to be because MSCs could differentiate into chondrocytes and induce cartilage regeneration. However, recent studies have shown that MSCs differentiating into chondrocytes is not the only mechanism by which MSCs can treat OA. At present, the mechanism using MSCs to treat OA can be divided into three aspects [26] 1) MSCs differentiate into chondrocytes; 2) MSCs have immunomodulatory activity; and 3) MSCs could prevent chondrocyte apoptosis because MSCs can secrete a variety of growth factors, such as IGF-1 and TGF-β. OA is also closely related to stem cell senescence. In the present study, we have also studied the potential effect of uridine on MSCs, and the results show that uridine can significantly alleviate MSCs senescence, which may be a potential mechanism by which uridine could alleviate OA in vivo.
It is well known that aging is closely linked with oxidative stress [25]. In the present study, the level of oxidative stress was markedly increased by H2O2 treatment. However, uridine treatment significantly increased the levels of SOD, CAT, GSH, and GPx, and reduced the levels of ROS and MDA. These findings suggest that uridine has anti-oxidative stress effects. Taken together, we found that uridine can alleviate the senescence of C-28/I2 chondrocyte MSCs in vitro. Further study showed that uridine could relieve OA in vivo.
In the present work, we found that uridine can alleviate chondrocytes and MSCs in vivo. Further work indicated that uridine could relieve OA in vivo (Figure S2). These findings suggest that uridine (also as a functional food) can be used to treat and prevent aging and OA. Uridine has several advantages: First, it is a metabolite of the body, which is mainly used to synthesize nucleic acids; therefore, it is a safe bio-molecule; second, it can be taken for a long time (low dose); and third, at present, uridine is commercialized and can be purchased from pharmacies.
Supplementary Material
Acknowledgments
This work was supported by People’s Hospital of Wuhan University.
Funding Statement
This work was supported by People’s Hospital of Wuhan University;People’s Hospital of Wuhan University.
Disclosure statement
The authors declare that they have no financial conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Ke Z, Zhu J.. Stem-cell derived exosomes for the treatment of osteoarthritis. Curr Stem Cell Res Ther. 2020;15(7):597–601. [DOI] [PubMed] [Google Scholar]
- [2].Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. 2011;23(5):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;25(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhan H, Xizhe P. Strategic changes and policy choices in the governance of China’s aging societ y. Soc Sci China. 2020;41(4):185–208. [Google Scholar]
- [5].Clark N, Albris K. In the interest(s) of many: governing data in crises. Politics Gov. 2020;8:421–431. [Google Scholar]
- [6].Stolz M, Gottardi R, Raiteri R, et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat Nanotechnol. 2009;4(3):186–192. [DOI] [PubMed] [Google Scholar]
- [7].Gao SG, Zeng C, Li LJ. Correlation between senescence-associated beta-galactosidase expression in articular cartilage and disease severity of patients with knee osteoarthritis [J]. Int J Rheum Dis. 2016;19(3):226–232. [DOI] [PubMed] [Google Scholar]
- [8].Bai X, Xiao Z, Pan Y, et al. Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun. 2004;325(2):453–460. [DOI] [PubMed] [Google Scholar]
- [9].Tsuchiya K, Chen G, Ushida T, et al. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mat Sci Eng. 2004;24(3):391–396. [Google Scholar]
- [10].Szychlinska MA, Stoddart MJ, D’Amora U, et al. Mesenchymal stem cell-based cartilage regeneration approach and cell senescence: can we manipulate cell aging and function? Tissue Eng Part B Rev. 2017;23(6):529–539. [DOI] [PubMed] [Google Scholar]
- [11].Shariatzadeh M, Song J, Wilson SL. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019;378(3):399–410. [DOI] [PubMed] [Google Scholar]
- [12].Sikora E, Bielak-Zmijewska A, Mosieniak G. Cellular senescence in ageing, age-related disease and longevity. Curr Vasc Pharmacol. 2013;12:5. [DOI] [PubMed] [Google Scholar]
- [13].Thoppil H, Riabowol K. Senolytics: a translational bridge between cellular senescence and organismal aging. Front Cell Dev Biol. 2020;7:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17(8):971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3(5):257–264. [DOI] [PubMed] [Google Scholar]
- [16].Yamamoto T, Koyama H, Kurajoh M, et al. Biochemistry of uridine in plasma. Clin Chim Acta. 2011;412(19–20):1712–1724. 18. [DOI] [PubMed] [Google Scholar]
- [17].Connolly GP, Duley JA. Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol Sci. 1999;20(5):218–225. [DOI] [PubMed] [Google Scholar]
- [18].Connolly GP. Uridine and pyrimidine nucleotides in cell function. In: Purinergic and Pyrimidinergic Signalling I. Berlin: Springer; 2011. p. 403–421. [Google Scholar]
- [19].Dobolyi A, Juhász G, Kovács Z, et al. Uridine function in the central nervous system. Curr Top Med Chem. 2011;11(8):1058–1067. [DOI] [PubMed] [Google Scholar]
- [20].Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–1069. [DOI] [PubMed] [Google Scholar]
- [21].Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. [DOI] [PubMed] [Google Scholar]
- [23].Rahmati M, Nalesso G, Mobasheri A, et al. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30. [DOI] [PubMed] [Google Scholar]
- [24].Grässel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Res. 2020;9:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73–82. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kong L, Zheng LZ, Qin L, et al. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017;9:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


