Abstract
Fibroblast growth factor 23 (FGF23) is an important bone hormone that regulates phosphate homeostasis in the kidney along with active vitamin D (1,25(OH)2D3) and parathyroid hormone (PTH). Endocrine effects of FGF23 depend, at least in part, on αKlotho functioning as a co-receptor whereas further paracrine effects in other tissues are αKlotho-independent. Regulation of FGF23 production is complex under both, physiological and pathophysiological conditions. Physiological regulators of FGF23 include, but are not limited to, 1,25(OH)2D3, PTH, dietary phosphorus intake, and further intracellular and extracellular factors, kinases, cytokines, and hormones. Moreover, several acute and chronic diseases including chronic kidney disease (CKD) or further cardiovascular disorders are characterized by early rises in the plasma FGF23 level pointing to further mechanisms effective in the regulation of FGF23 under pathophysiological conditions. Therefore, FGF23 also serves as a prognostic marker in several diseases. Our review aims to comprehensively summarize the regulation of FGF23 in health and disease.
Keywords: Klotho, Vitamin D, PTH, Phosphate, CKD
Introduction
Fibroblast growth factor 23 (FGF23) was discovered as an endocrine factor produced in bone that may be considered as the missing link of the kidney-parathyroid gland-bone axis [9]. It helps maintain phosphate homeostasis not only by regulating parathyroid hormone (PTH) and 1,25(OH)2D3 (calcitriol), active vitamin D, secretion, but also by directly targeting renal phosphate transport [9]. Phosphate is essential for a bunch of cellular processes including nucleic acid production, energy metabolism, or signal transduction (phosphorylation/dephosphorylation of signaling molecules) [14]. Moreover, it is part of hydroxyapatite that makes up the essential inorganic compound of bone [14].
FGF family
FGF23 is a relatively new protein in evolution [82]. The mammalian family comprises two types of FGFs: intracellular FGFs and extracellular FGFs [82]. FGFs 11–14 function in the cell as signaling molecules and play a role in neuronal excitability [82]. Extracellular FGFs can be subdivided into endocrine and canonical (also named paracrine) members depending on heparin or heparan sulfate as a cofactor [82]. Endocrine FGF15/19, 21, and 23 exhibit low affinity for heparin cofactors and therefore require Klotho proteins as co-receptors [82].
FGF23
FGF23 (251 amino acids) displays the highest expression in bone (osteoblasts and osteocytes) but can also be detected in other organs including the liver, brain, heart, thyroid, intestine, and skeletal muscle [70, 87, 109]. As a prerequisite for its endocrine properties, it is devoid of the heparan-sulfate binding motif which would result in high extracellular matrix binding, allowing its secretion into blood [82]. FGF23 secretion is strongly influenced by posttranslational modification, i.e., O-glycosylation and phosphorylation [14]. The polypeptide N-acetylgalactosaminyltransferase 3 (GALNT3) O-glycosylates FGF23, resulting in its secretion and preventing its phosphorylation by family with sequence similarity 20 member C (FAM20C) which would lead to FGF23 breakdown [14]. Subtilisin-like proprotein convertases (SPC) cleave FGF23 at a certain motif leading to inactive C-terminal (25–179) and N-terminal (180–251) FGF23 residues [9]. Commercial ELISAs detecting C-terminal FGF23 (cFGF23) or uncleaved intact FGF23 (iFGF23) are commonly used for plasma samples. Possibly, cFGF23 is not only inactive, but may suppress FGF23 signaling [48]. FGF23 effects can be exerted in an αKlotho-independent or αKlotho-dependent fashion [48]. FGF23 receptors include fibroblast growth factor receptor (FGFR)1c, FGFR3c, and FGFR4 [82]. αKlotho binds to FGF23 thereby enhancing its receptor affinity [82].
αKlotho
The relevance of αKlotho was discovered in 1997: Mice with markedly reduced αKlotho expression exhibit accelerated aging with multiple aging-associated diseases and die early [66]. In its transmembrane form, αKlotho is a co-receptor for FGF23 while soluble αKlotho has FGF23-independent paracrine and endocrine effects [9]. Soluble αKlotho is generated by cleavage of its extracellular domain or alternative splicing [29]. It regulates membrane proteins including ion channels and controls intracellular pathways such as insulin-like growth factor I or Wnt signaling [65].
Effects of FGF23
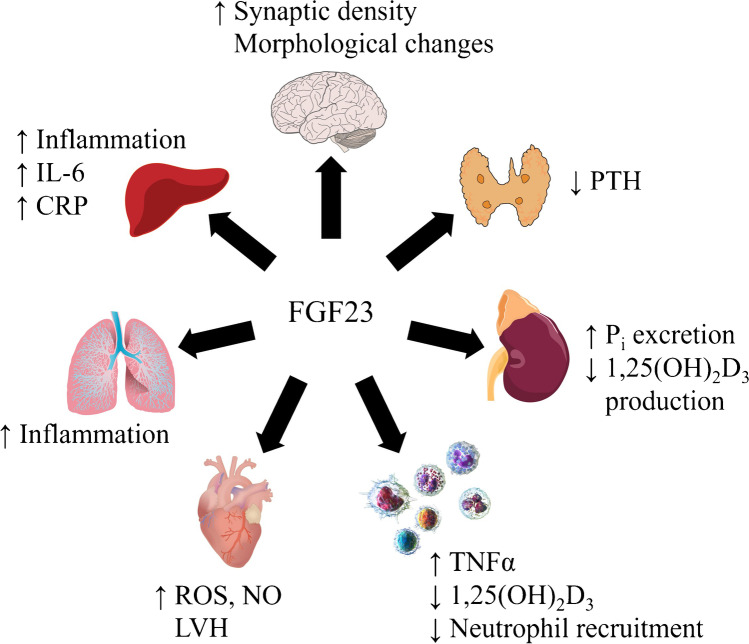
The effects of FGF23 in different organs, tissues, and cells are displayed in Fig. 1.
Fig. 1.
Effect of FGF23 in different organs and cells. C-reactive protein (CRP), fibroblast growth factor 23 (FGF23), inorganic Phosphate (Pi), interleukin-6 (IL-6), left ventricular hypertrophy (LVH), parathyroid hormone (PTH), reactive oxygen species (ROS), tumor necrosis factor α (TNFα). Sources: Heart: Injurymap, CC BY 4.0, Leukocytes: Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014.” WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002–4436., CC BY 3.0
Kidney
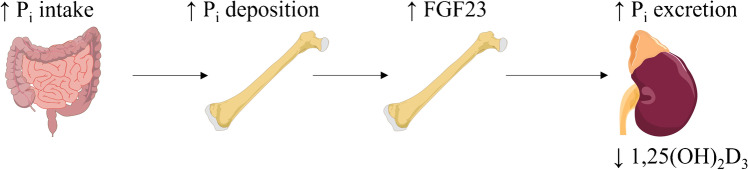
FGF23 is a major regulator of phosphate homeostasis that is dependent on the interplay of different organs: Alimentary phosphate is absorbed in the intestine; most extracellular phosphate is deposited in bone; and the kidney is responsible for urinary excretion of phosphate that is filtered in the glomeruli [57] (Fig. 2). Moreover, PTH and 1,25(OH)2D3 are further regulators of phosphate homeostasis and FGF23 [9]. FGF23 induces renal phosphate excretion by decreasing surface expression of NaPiIIa and NaPiIIc, the major Na+-dependent phosphate transporters of the proximal tubule [57]. FGF23 downregulates renal cytochrome P450 (Cyp)27b1 expression, the key enzyme for 1,25(OH)2D3 production, and enhances Cyp24a1 production catalyzing the inactivation of 1,25(OH)2D3 [57]. These effects of FGF23 are αKlotho-mediated [29].
Fig. 2.
FGF23 is upregulated upon alimentary phosphate intake and regulates renal phosphate and vitamin D handling
Parathyroid glands
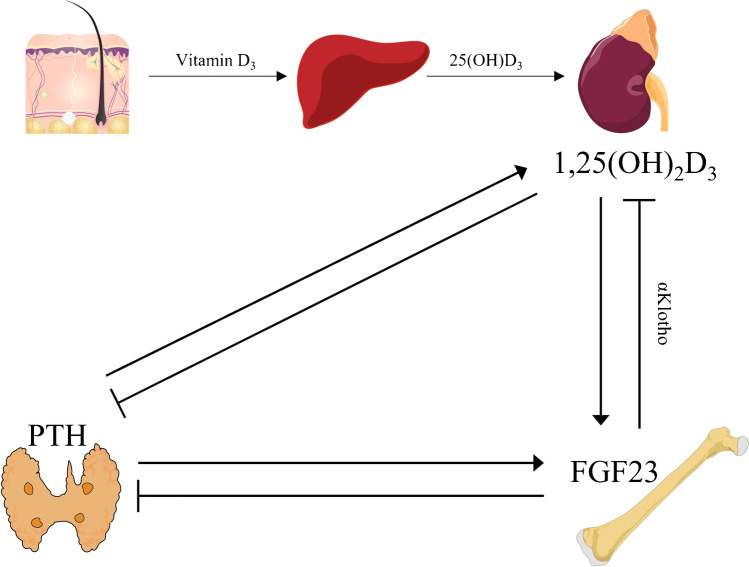
FGF23 inhibits Pth expression and lowers PTH plasma levels through mitogen-activated protein kinase (MAPK) signaling and, in an αKlotho-independent manner, through calcineurin/nuclear factor of activated T-cells (NFAT) signaling [14, 75]. The interdependence of FGF23, PTH, and 1,25(OH)2D3 is summarized in Fig. 3.
Fig. 3.
The interdependence of FGF23, PTH, and 1,25(OH)2D3. Source: Skin: DBCLS 統合TV, CC BY 4.0
Bone
FGF23 controls bone mineralization [78].
Brain
FGF23 increases synaptic density and changes morphology of hippocampal cells [53].
Heart
FGF23 induces left ventricular hypertrophy (LVH) through FGFR4 [49]. In isolated cardiac myocytes, FGF23 favors pro-fibrotic signaling [68]. FGF23 stimulates NO synthesis and reactive oxygen species (ROS) generation in human coronary endothelial cells [89].
Immune system
Lipopolysaccharide (LPS) and interferon γ (IFNγ) enhance Fgf23 expression while FGF23 stimulates tumor necrosis factor α (TNFα) production in pro-inflammatory macrophages [50]. FGF23 suppresses 1,25(OH)2D3 production in monocytes [3] and interferes with neutrophil recruitment [91].
Liver
FGF23 upregulates interleukin (IL)-6 and C-reactive protein (CRP) expression in the liver, thereby promoting inflammation in chronic kidney disease [96].
Lung
In bronchial epithelial cells, FGF23 also stimulates inflammation [63].
Muscle
Physical exercise enhances FGF23 production, and FGF23 increases mitochondrial function and helps cope with ROS production [70].
Regulation of FGF23
In the following, we in an alphabetical order summarize intracellular and extracellular factors regulating gene expression, production, and secretion of FGF23 (Table 1).
Table 1.
Regulators of FGF23
| Factor | Influence on FGF23 |
|---|---|
| 1,25(OH)2D3 | ↑ [9] |
| Acidosis | ↑ [64] |
| Actin cytoskeleton | ↑ [36] |
| Advanced glycation endproducts | ↑ [7] |
| Aldosterone | ↑ [84, 113] |
| AMPK | ↓ [47] |
| Cadmium | ↑ [62] |
| Calcineurin inhibitors | ↓ [5] |
| Calciprotein | ↑ [1] |
| Calcium | ↑ [19] |
| cFGF23 | Inhibits signaling [48] |
| DMP1 | ↓ [25, 73] |
| Endothelin-1 | ↓ [39] |
| ENPP1 | ↓ [54] |
| ERR-γ | ↑ [87] |
| Erythropoietin | ↑ [44] |
| FGFR1 signaling | ↑ [107] |
| Glucocorticoids | ↓ [40] |
| HIF1α | ↑ [104, 116] |
| High-fat diet | ↑ [46] |
| IL-1β | ↑ [59, 81, 110] |
| IL-6β | ↑ [24] |
| Insulin | ↓ [4] |
| Insulin-like growth factor | ↓ [4] |
| Iron | ↓ [52] |
| Lactic acid | ↑ [2] |
| Leptin | ↑ [102] |
| Lipocalin 2 | ↑ [17] |
| Lithium | ↑ [37, 114] |
| LPS | ↑ [81] |
| Lysophosphatidic acid | ↑ [95] |
| Myostatin | ↑ [32] |
| NF-κB | ↑ [2, 7, 33, 114, 115] |
| Nurr1 | ↑ [75] |
| p38MAPK | ↑ [33] |
| PHEX | ↓ [8, 111] |
| Phosphate | ↑ [9, 55] |
| PKC | ↑ [6] |
| Plasminogen activation | ↓ [30] |
| PPARα | ↓ [34] |
| Propranolol | ↓ [35] |
| PTH | ↑ [75, 81] |
| SOCE | ↑ [34, 41, 47, 114, 115] |
| Sympathetic activity | ↑ [35, 61] |
| TGF-β2 | ↑ [41] |
| TNFα | ↑ [46, 81] |
| Vitamin A | ↓ [88] |
Actin cytoskeleton
Reorganization of the actin cytoskeleton controlled by Rac1/PAK1 signaling is a prerequisite for Fgf23 expression in vitro [36].
Autonomic nerve system
The circadian rhythm governs sympathetic activity which enhances FGF23 production [61]. During the dark phase, Fgf23 expression goes up in bone [61]. This regulation is dependent on cryptochrome 1 [61]. In mice with a GSK3 mutation rendering it insensitive to PKB/Akt/SGK signaling, enhanced sympathetic activity is associated with elevated FGF23 serum levels [35]. The latter are lowered by β-adrenergic receptor blocker propranolol [35].
Calcineurin inhibitors
Ca2+-dependent phosphatase calcineurin inhibitors tacrolimus and ciclosporin A are widely used as immunosuppressants and inhibit Fgf23 gene expression in vitro [5].
Calcium
Hypocalcemia is associated with low FGF23 levels as a study of Gcm2−/− mice characterized by hypocalcemia, hyperphosphatemia, and low calcitriol and PTH levels and Cyp27b1−/− mice with hypocalcemia, hypophosphatemia, and low 1,25(OH)2D3 but high PTH levels has revealed [19]. Conversely, a high-calcium diet increases FGF23 serum concentration in the transgenic mice without affecting 1,25(OH)2D3 or PTH, pointing to an independent role of extracellular Ca2+ in regulating FGF23 [19]. Store-operated Ca2+ entry (SOCE) through Ca2+ release-activated calcium channel protein 1 (Orai1) in conjunction with Ca2+-sensing protein STIM1 is part of the cellular machinery enhancing Fgf23 transcription in vitro [115]. Calciprotein particles composed of calcium, phosphate, and fetuin-A also stimulate Fgf23 expression [1].
C-Term FGF23
C-terminal FGF23 inhibits FGF23 signaling by impeding formation of the αKlotho FGFR1c complex in vivo and in vitro [48].
Endothelins
Endothelin-1 (ET-1) reduces FGF23 production through endothelin B receptor (ETB) in vitro and in vivo [39].
Energy metabolism
Insulin and insulin-like growth factor 1 suppress FGF23 production in vitro and in vivo [4]. This effect is mediated by induction of PI3K/PKB/Akt activity inhibiting transcription factor FOXO1 [4]. Consequently, insulin-deficient mice are characterized by elevated FGF23 serum concentrations that is decreased by insulin administration [4]. In a human study, a negative correlation of plasma insulin and FGF23 was found [4]. Cellular energy sensor 5′-adenosine monophosphate (AMP)-activated kinase (AMPK) is activated in energy deficiency and inhibits FGF23 production in vivo and in vitro through suppression of Orai1-mediated SOCE [47]. Fibrates, agonists of lipid metabolism-associated transcription factor PPARα, downregulate FGF23 in vitro, an effect at least partly mediated by AMPK-dependent regulation of SOCE [34]. Adipokine leptin induces Fgf23 expression in vivo [102]. Acidosis is associated with enhanced FGF23 production [64]. Moreover, lactic acid concentrations encountered in severe lactic acidosis upregulate Fgf23 expression in vitro, an effect at least in part dependent on nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling [2]. Advanced glycation endproducts induce Fgf23 gene expression in an NF-κB-dependent manner [7].
ENPP1
In autosomal recessive hypophosphatemic rickets type 2 (ARHR2), ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (ENPP1) fails to keep FGF23 levels low due to inactivating mutations in the ENPP1 gene resulting in hypophosphatemia [54].
ERR-γ
Orphan nuclear estrogen-related receptor-γ (ERR-γ) increases hepatic FGF23 synthesis in acute kidney injury (AKI) [87].
DMP1
Dentin matrix acidic phosphoprotein 1 (DMP1) is a protein produced by osteoblasts and osteocytes and regulates the mineralization of extracellular matrix [25]. In vivo, DMP1 deficiency is associated with enhanced Fgf23 expression with hypophosphatemia [73], and in vitro DMP1 downregulates FGF23 through NFAT signaling [25].
G-3-P
Glycerol-3-phosphate (G-3-P) released in AKI is positively correlated with FGF23 levels in humans and enhances Fgf23 transcription in bone [95]. This effect is dependent on G-3-P acyltransferases converting G-3-P to lysophosphatidic acid that activates LPA receptor 1 in vitro [95].
Inflammation
As a mediator of inflammation-dependent upregulation of FGF23, pro-inflammatory IL-1β elevates FGF23 serum levels through bone resorption [110] and through enhanced gene expression in vitro [59]. Also, pro-inflammatory IL-6 directly stimulates Fgf23 expression through STAT3 signaling [24]. TNFα enhances FGF23 production in chronic inflammation [26] and in mice upon high-fat diet feeding [46]. An enhancer element 16 kb upstream of the start site of Fgf23 gene transcription accounts for LPS-, IL-1β-, TNF-α-, and PTH-induced Fgf23 expression [81]. NF-κB is a prominent transcription factor complex involved in pro-inflammatory responses [115]. In vitro, NF-κB induces Orai1 expression, facilitating SOCE which enables Fgf23 transcription [115]. Lipocalin 2 (LCN2) is an iron chelator and part of innate immune responses [17]. In CKD, it stimulates FGF23 production, at least in part through cAMP signaling [17].
Iron, EPO, and HIF1α
In mice, iron deficiency results in upregulated Fgf23 expression and iFGF23 as well as cFGF23 serum levels [52], an effect involving hypoxia inducible factor 1 α (HIF1α) [104] which is a transcriptional regulator of FGF23 [116]. HIF1α target erythropoietin (EPO) also stimulates FGF23 production [44].
Kinases
P38 mitogen-activated protein kinase (p38MAPK) is activated upon exposure of cells to stress and stimulates Fgf23 expression in vitro, an effect at least in part depending on NF-κB [33].
Metal ions
Cadmium impacts on post-translational modification of FGF23, stimulating its secretion in vitro and in vivo [62]. This effect requires p38MAPK-dependent activation of aryl hydrocarbon receptor leading to enhanced GALNT3 production [62]. Lithium stimulates FGF23 production in vitro and in vivo through NF-κB-dependent Orai1 and SOCE regulation [37, 114].
Nurr1
Nuclear receptor-associated protein1 (Nurr1) mediates PTH-dependent upregulation of Fgf23 expression in vitro and in vivo [75].
Paracrine/autocrine FGFR1 signaling
Regulation of FGFR1 signaling through autocrine and paracrine FGFs influences Fgf23 transcription, an effect involving PLCγ, MAPK, and PI3K/Akt signaling [107].
PHEX
Loss of PHEX activity elevates plasma FGF23 levels, as typical of X-linked hypophosphatemia (XLH) [8]. This effect is dependent on PHEX enhancing FGF23 degradation through SPC or PHEX-DMP1-integrin complexes [111].
Phosphate
Phosphate induces Fgf23 transcription through ROS in vitro [55].
PKC
In vitro, protein kinase C (PKC) activation through phorbol ester enhances whereas PKC inhibition downregulates Fgf23 gene expression [6].
Plasminogen activation
Overexpression of plasminogen activator inhibitor-1 (PAI-1) elevates FGF23 levels in mice whereas tissue-type and urokinase-type plasminogen activators cleave FGF23 in vitro [30].
Steroid hormones
Anti-inflammatory glucocorticoids suppress Fgf23 expression in vitro and FGF23 serum levels in mice, at least in the short term [40]. Mineralocorticoid aldosterone upregulates Fgf23 transcription in vitro and in vivo [84, 113]. In Klotho deficiency, enhanced 1,25(OH)2D3 leads to extracellular volume depletion which further worsens outcome [43].
TGF-β
Transforming growth factor-β2 (TGF-β2) upregulates Fgf23 transcription and secretion through SOCE in vitro [41]. Myokine myostatin also stimulates Fgf23 expression and secretion in vitro [32].
Vitamin A
Retinoic acid receptor (RAR) signaling induced by vitamin A compounds inhibits Fgf23 expression and protein secretion in vitro [88].
Pathophysiological roles of FGF23
The pathophysiological role of FGF23 is not limited to diseases with hypophosphatemia or hyperphosphatemia. Also, further acute and chronic disorders not associated with altered phosphate metabolism are characterized by changes in the plasma FGF23 concentration.
Acute kidney injury
Acute kidney injury leads to increased FGF23 levels [87, 95].
Airway inflammation
In chronic obstructive pulmonary disease, FGF23 is elevated [63].
Autosomal dominant polycystic kidney disease
Patients with autosomal dominant polycystic kidney disease are mainly characterized by high cFGF23 and, in part also, high iFGF23 levels [85]. In rodent models of this disease, iFGF23 levels are elevated [97].
Cancer
Rare forms of colon adenocarcinoma are characterized by FGF23 secretion with hypophosphatemia [67] whereas in other forms, plasma FGF23 is increased [60]. In urothelial cancer, FGF23 is also elevated [71]. Further malignancies found to exhibit, at least in part, higher FGF23 levels are ovarian cancer [101], prostate cancer [42], and multiple myeloma [99]. For further review, see [31].
Cardiovascular disease
FGF23 induces LVH without αKlotho in mice [38]. However, Klotho deficiency also induces LVH without involvement of FGF23 [108]. Interestingly, cardiac Fgf23 overexpression in healthy mice does not cause LVH, supporting a role of αKlotho or phosphate status in the progression of LVH [69]. Due to these results, the exact role of FGF23 in heart disease remains somewhat controversial (Fig. 4) [98]. In human cohorts, FGF23 is positively associated with left ventricular heart mass in CKD patients [38]. In patients with coronary artery disease, higher FGF23 levels are associated with increased risk of death [83]. In CKD patients and in the elderly, increased levels of iFGF23 are positively correlated with aortic calcification [20, 76, 79]. Higher FGF23 levels are associated with atrial fibrillation in CKD [74]. High FGF23 is also a risk factor for myocardial infarction, hemorrhagic stroke [22], and heart failure [21].
Fig. 4.
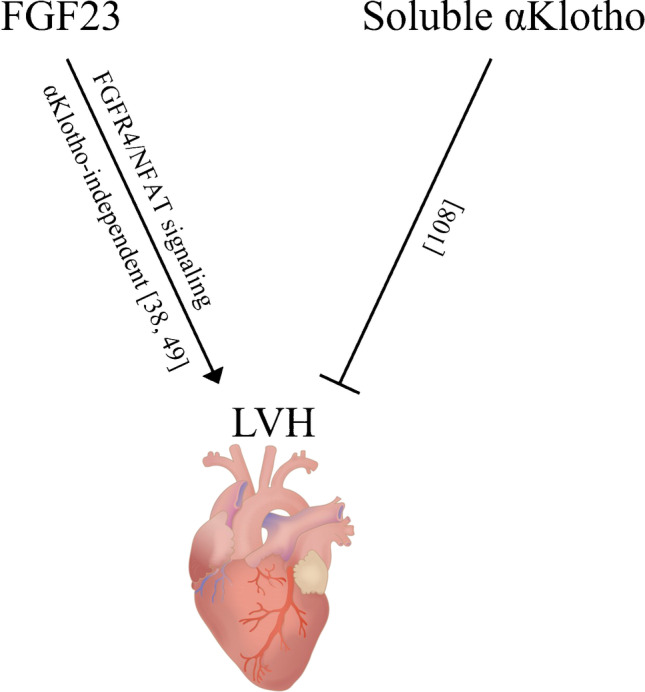
Effect of FGF23 and αKlotho in the heart. Heart: Injurymap, CC BY 4.0
CKD
CKD is often characterized by hyperphosphatemia due to failure of the kidney to excrete phosphate [56]. As hyperphosphatemia is a major trigger of enhanced FGF23 secretion, high FGF23 plasma levels are typical of CKD [103]. However, since FGF23 goes up early in CKD prior to the onset of hyperphosphatemia or hyperparathyroidism [58], other factors including inflammation are also effective [26]. FGF23 is a reliable prognostic marker in CKD correlating with outcome [45]. Upon kidney transplantation, cFGF23 is correlated with graft loss [16]. In CKD patients, higher abundance of oxidized PTH is observed [112]. In contrast to non-oxidized PTH, oxidized PTH is not correlated with plasma FGF23, and in vitro, oxidized PTH is less capable of inducing Fgf23 gene expression [112]. Moreover, in CKD, the positive association of plasma Klotho with GFR is absent in patients with high FGF23 levels [93].
Diabetes and obesity
FGF23 levels are positively associated with increased insulin resistance and obesity [51].
Hyperphosphatemic disorders
Hyperphosphatemic familial tumoral calcinosis type 1–3 (HFTC) is characterized by hyperphosphatemia, normal or high calcitriol levels, and phosphate retention [11]. It is due to loss of function mutation in the gene encoding GALNT3 (type I), FGF23 (type II), and αKlotho (type III) ultimately causing FGF23 deficiency or resistance to FGF23 [11].
PTH-dependent hyperphosphatemic disorders include pseudohypoparathyroidism, where PTH resistance causes a decrease of 1,25(OH)2D3 and an increase in serum FGF23 concentration [117].
Hypophosphatemic disorders
Autosomal dominant hypophosphatemic rickets (ADHR) is due to mutations rendering FGF23 resistant to cleavage [94]. In tumor-induced osteomalacia, tumor cells — often but not exclusively benign mesenchymal tumors — secrete FGF23 [15], resulting in hypophosphatemia as a hallmark. XLH is also caused by an abnormally high FGF23 plasma concentration that is due to loss-of-function mutations of the PHEX gene [100]. Inactivating mutations in the DMP1/ENPP1/FAM20C genes are responsible for ARHR1/2/3 with elevated FGF23 levels [57]. Fibrous dysplasia/McCune-Albright syndrome is caused by an activating mutation of GNAS resulting in high cAMP and FGF23 levels [10]. Activating mutations of PTH/PTHrP receptor gene account for Jansen’s metaphyseal chondrodysplasia characterized by high FGF23 plasma concentration [12]. Activating mutations of FGFR1 gene are the reason for osteoglophonic dysplasia characterized by high FGF23 levels and hypophosphatemia [105]. Increased αKlotho levels also result in hypophosphatemic rickets and increased iFGF23 plasma concentration [13].
Inflammatory diseases
In inflammatory diseases, a correlation of inflammatory activity and plasma FGF23 is observed (e.g., rheumatoid arthritis [92], inflammatory bowel disease [28], sepsis in CKD patients [23]). In CKD, a higher FGF23 plasma concentration is correlated with higher inflammatory activity [77]. Since inflammation also contributes to CKD, it may contribute to the rise in plasma FGF23 typical of this disease [18].
Iron deficiency
In the absence of CKD, iron deficiency is associated with an elevation of cFGF23 [106]. In general, treatment of iron deficiency with intravenous iron lowers cFGF23 on a transcriptional level while ferric carboxymaltose increases iFGF23 due to an inhibitory effect on its degradation [106]. In patients on dialysis, ferric carboxymaltose, however, decreases iFGF23 while elevating cFGF23 [90]. Upon renal transplantation, iron deficiency also drives an increase in cFGF23 and contributes to the poorer outcome of iron deficiency in CKD [27].
Liver disease
In patients with end stage liver disease, FGF23 is increased owing to hepatic FGF23 production [86].
Anti-FGF23 therapy
Burosumab is an antibody against FGF23 that is approved and therapeutically used in the treatment of X-linked hypophosphatemia [72]. Further FGF23-associated diseases for which anti-FGF23 therapy is tested include tumor-induced osteomalacia [80].
Conclusions
FGF23 is part of a complex network with a very high degree of interdependence of the constituting regulating factors. Better understanding of the regulation of FGF23 is of high interest in view of the many pathologies impacting on the plasma FGF23 concentration. The endocrine effects of FGF23 are nowadays well established. However, the multiple paracrine effects in different tissues are less well studied. Moreover, the regulation of FGF23 under both, physiological and pathophysiological conditions is ill-defined including transcriptional and post-transcriptional mechanisms. In particular, it is not yet clear in many cases whether the increase in plasma FGF23 concentration observed in many diseases only indicates disease or whether FGF23 actively contributes to disease progression as observed in the heart. Also the role of anti-FGF23 therapy needs to be investigated. Definitely, further research is warranted.
Author contribution
Steffen Rausch and Michael Föller wrote the paper.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors’ investigations into the regulation of FGF23 were funded by Deutsche Forschungsgemeinschaft (Fo695/2–1 and Fo695/6–1).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akiyama K-I, Miura Y, Hayashi H, et al. Calciprotein particles regulate fibroblast growth factor-23 expression in osteoblasts. Kidney Int. 2020;97:702–712. doi: 10.1016/j.kint.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Alber J, Föller M. Lactic acid induces fibroblast growth factor 23 (FGF23) production in UMR106 osteoblast-like cells. Mol Cell Biochem. 2021 doi: 10.1007/s11010-021-04287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacchetta J, Sea JL, Chun RF, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res. 2013;28:46–55. doi: 10.1002/jbmr.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bär L, Feger M, Fajol A, et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23) Proc Natl Acad Sci U S A. 2018;115:5804–5809. doi: 10.1073/pnas.1800160115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bär L, Großmann C, Gekle M, et al. Calcineurin inhibitors regulate fibroblast growth factor 23 (FGF23) synthesis. Naunyn-Schmiedeberg’s Arch Pharmacol. 2017;390:1117–1123. doi: 10.1007/s00210-017-1411-2. [DOI] [PubMed] [Google Scholar]
- 6.Bär L, Hase P, Föller M. PKC regulates the production of fibroblast growth factor 23 (FGF23) PLoS ONE. 2019;14:e0211309. doi: 10.1371/journal.pone.0211309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bär L, Wächter K, Wege N et al (2017) Advanced glycation end products stimulate gene expression of fibroblast growth factor 23. Mol Nutr Food Res 61. 10.1002/mnfr.201601019 [DOI] [PubMed]
- 8.Beck-Nielsen SS, Mughal Z, Haffner D, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14:58. doi: 10.1186/s13023-019-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharyya N, Wiench M, Dumitrescu C, et al. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27:1132–1141. doi: 10.1002/jbmr.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyce AM, Lee AE, Roszko KL, et al. Hyperphosphatemic tumoral calcinosis: pathogenesis, clinical presentation, and challenges in management. Front Endocrinol (Lausanne) 2020;11:293. doi: 10.3389/fendo.2020.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown WW, Jüppner H, Langman CB, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownstein CA, Adler F, Nelson-Williams C, et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. PNAS. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chande S, Bergwitz C. Role of phosphate sensing in bone and mineral metabolism. Nat Rev Endocrinol. 2018;14:637–655. doi: 10.1038/s41574-018-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong WH, Molinolo AA, Chen CC, et al. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53–77. doi: 10.1530/ERC-11-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu C, Elitok S, Zeng S, et al. C-terminal and intact FGF23 in kidney transplant recipients and their associations with overall graft survival. BMC Nephrol. 2021;22:125. doi: 10.1186/s12882-021-02329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courbon G, Francis C, Gerber C, et al. Lipocalin 2 stimulates bone fibroblast growth factor 23 production in chronic kidney disease. Bone Res. 2021;9:35. doi: 10.1038/s41413-021-00154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czaya B, Faul C (2019) The role of fibroblast growth factor 23 in inflammation and anemia. Int J Mol Sci 20. 10.3390/ijms20174195 [DOI] [PMC free article] [PubMed]
- 19.David V, Dai B, Martin A, et al. Calcium regulates FGF-23 expression in bone. Endocrinology. 2013;154:4469–4482. doi: 10.1210/en.2013-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desjardins L, Liabeuf S, Renard C, et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2012;23:2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 21.Di Giuseppe R, Buijsse B, Hirche F, et al. Plasma fibroblast growth factor 23, parathyroid hormone, 25-hydroxyvitamin D3, and risk of heart failure: a prospective, case-cohort study. J Clin Endocrinol Metab. 2014;99:947–955. doi: 10.1210/jc.2013-2963. [DOI] [PubMed] [Google Scholar]
- 22.Di Giuseppe R, Kühn T, Hirche F, et al. Plasma fibroblast growth factor 23 and risk of cardiovascular disease: results from the EPIC-Germany case-cohort study. Eur J Epidemiol. 2015;30:131–141. doi: 10.1007/s10654-014-9982-4. [DOI] [PubMed] [Google Scholar]
- 23.Dounousi E, Torino C, Pizzini P, et al. Intact FGF23 and α-Klotho during acute inflammation/sepsis in CKD patients. Eur J Clin Invest. 2016;46:234–241. doi: 10.1111/eci.12588. [DOI] [PubMed] [Google Scholar]
- 24.Durlacher-Betzer K, Hassan A, Levi R, et al. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94:315–325. doi: 10.1016/j.kint.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Dussold C, Gerber C, White S, et al. DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res. 2019;7:12. doi: 10.1038/s41413-019-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egli-Spichtig D, Imenez Silva PH, Glaudemans B, et al. Tumor necrosis factor stimulates fibroblast growth factor 23 levels in chronic kidney disease and non-renal inflammation. Kidney Int. 2019;96:890–905. doi: 10.1016/j.kint.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Eisenga MF, van Londen M, Leaf DE, et al. C-terminal fibroblast growth factor 23, iron deficiency, and mortality in renal transplant recipients. JASN. 2017;28:3639–3646. doi: 10.1681/ASN.2016121350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Hodhod MA-A, Hamdy AM, Abbas AA, et al. Fibroblast growth factor 23 contributes to diminished bone mineral density in childhood inflammatory bowel disease. BMC Gastroenterol. 2012;12:44. doi: 10.1186/1471-230X-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erben RG, Andrukhova O. FGF23-Klotho signaling axis in the kidney. Bone. 2017;100:62–68. doi: 10.1016/j.bone.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Eren M, Place AT, Thomas PM, et al. PAI-1 is a critical regulator of FGF23 homeostasis. Sci Adv. 2017;3:e1603259. doi: 10.1126/sciadv.1603259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewendt F, Feger M, Föller M. Role of fibroblast growth factor 23 (FGF23) and αKlotho in cancer. Front Cell Dev Biol. 2020;8:601006. doi: 10.3389/fcell.2020.601006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewendt F, Feger M, Föller M. Myostatin regulates the production of fibroblast growth factor 23 (FGF23) in UMR106 osteoblast-like cells. Pflugers Arch. 2021;473:969–976. doi: 10.1007/s00424-021-02561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ewendt F, Föller M. p38MAPK controls fibroblast growth factor 23 (FGF23) synthesis in UMR106-osteoblast-like cells and in IDG-SW3 osteocytes. J Endocrinol Invest. 2019;42:1477–1483. doi: 10.1007/s40618-019-01073-y. [DOI] [PubMed] [Google Scholar]
- 34.Ewendt F, Hirche F, Feger M, et al. Peroxisome proliferator-activated receptor α (PPARα)-dependent regulation of fibroblast growth factor 23 (FGF23) Pflugers Arch. 2020;472:503–511. doi: 10.1007/s00424-020-02363-8. [DOI] [PubMed] [Google Scholar]
- 35.Fajol A, Chen H, Umbach AT, et al. Enhanced FGF23 production in mice expressing PI3K-insensitive GSK3 is normalized by β-blocker treatment. FASEB J. 2016;30:994–1001. doi: 10.1096/fj.15-279943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fajol A, Honisch S, Zhang B, et al. Fibroblast growth factor (Fgf) 23 gene transcription depends on actin cytoskeleton reorganization. FEBS Lett. 2016;590:705–715. doi: 10.1002/1873-3468.12096. [DOI] [PubMed] [Google Scholar]
- 37.Fakhri H, Ricken R, Adli M, et al. Impact of lithium treatment on FGF-23 serum concentrations in depressive patients. J Clin Psychopharmacol. 2014;34:745–747. doi: 10.1097/JCP.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feger M, Ewendt F, Menzel M, et al. Endothelin receptor B controls the production of fibroblast growth factor 23. FASEB J. 2020;34:6262–6270. doi: 10.1096/fj.201903109R. [DOI] [PubMed] [Google Scholar]
- 40.Feger M, Ewendt F, Strotmann J, et al. Glucocorticoids dexamethasone and prednisolone suppress fibroblast growth factor 23 (FGF23) J Mol Med (Berl) 2021;99:699–711. doi: 10.1007/s00109-021-02036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feger M, Hase P, Zhang B, et al. The production of fibroblast growth factor 23 is controlled by TGF-β2. Sci Rep. 2017;7:4982. doi: 10.1038/s41598-017-05226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng S, Wang J, Zhang Y, et al. FGF23 promotes prostate cancer progression. Oncotarget. 2015;6:17291–17301. doi: 10.18632/oncotarget.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer SS, Kempe DS, Leibrock CB, et al. Hyperaldosteronism in Klotho-deficient mice. Am J Physiol Renal Physiol. 2010;299:F1171–F1177. doi: 10.1152/ajprenal.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flamme I, Ellinghaus P, Urrego D, et al. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS ONE. 2017;12:e0186979. doi: 10.1371/journal.pone.0186979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 46.Glosse P, Fajol A, Hirche F, et al. A high-fat diet stimulates fibroblast growth factor 23 formation in mice through TNFα upregulation. Nutr & Diabetes. 2018;8:36. doi: 10.1038/s41387-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glosse P, Feger M, Mutig K, et al. AMP-activated kinase is a regulator of fibroblast growth factor 23 production. Kidney Int. 2018;94:491–501. doi: 10.1016/j.kint.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Goetz R, Nakada Y, Hu MC, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. PNAS. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grabner A, Schramm K, Silswal N, et al. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci Rep. 2017;7:1993. doi: 10.1038/s41598-017-02068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han X, Li L, Yang J, et al. Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2 D in macrophages. FEBS Lett. 2016;590:53–67. doi: 10.1002/1873-3468.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanks LJ, Casazza K, Judd SE, et al. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS ONE. 2015;10:e0122885. doi: 10.1371/journal.pone.0122885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanudel MR, Chua K, Rappaport M, et al. Effects of dietary iron intake and chronic kidney disease on fibroblast growth factor 23 metabolism in wild-type and hepcidin knockout mice. Am J Physiol Renal Physiol. 2016;311:F1369–F1377. doi: 10.1152/ajprenal.00281.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hensel N, Schön A, Konen T, et al. Fibroblast growth factor 23 signaling in hippocampal cells: impact on neuronal morphology and synaptic density. J Neurochem. 2016;137:756–769. doi: 10.1111/jnc.13585. [DOI] [PubMed] [Google Scholar]
- 54.Höppner J, Kornak U, Sinningen K, et al. Autosomal recessive hypophosphatemic rickets type 2 (ARHR2) due to ENPP1-deficiency. Bone. 2021;153:116111. doi: 10.1016/j.bone.2021.116111. [DOI] [PubMed] [Google Scholar]
- 55.Hori M, Kinoshita Y, Taguchi M, et al. Phosphate enhances Fgf23 expression through reactive oxygen species in UMR-106 cells. J Bone Miner Metab. 2016;34:132–139. doi: 10.1007/s00774-015-0651-9. [DOI] [PubMed] [Google Scholar]
- 56.Hruska KA, Mathew S, Lund R, et al. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–157. doi: 10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang X, Jiang Y, Xia W. FGF23 and phosphate wasting disorders. Bone Res. 2013;1:120–132. doi: 10.4248/BR201302002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito N, Wijenayaka AR, Prideaux M, et al. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol. 2015;399:208–218. doi: 10.1016/j.mce.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Jacobs E, Martinez ME, Buckmeier J, et al. Circulating fibroblast growth factor-23 is associated with increased risk for metachronous colorectal adenoma. J Carcinog. 2011;10:3. doi: 10.4103/1477-3163.76723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawai M, Kinoshita S, Shimba S, et al. Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm-dependent manner. J Biol Chem. 2014;289:1457–1466. doi: 10.1074/jbc.M113.500850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kido S, Fujihara M, Nomura K, et al. Molecular mechanisms of cadmium-induced fibroblast growth factor 23 upregulation in osteoblast-like cells. Toxicol Sci. 2014;139:301–316. doi: 10.1093/toxsci/kfu043. [DOI] [PubMed] [Google Scholar]
- 63.Krick S, Grabner A, Baumlin N et al (2018) Fibroblast growth factor 23 and Klotho contribute to airway inflammation. Eur Respir J 52. 10.1183/13993003.00236-2018 [DOI] [PMC free article] [PubMed]
- 64.Krieger NS, Culbertson CD, Kyker-Snowman K, et al. Metabolic acidosis increases fibroblast growth factor 23 in neonatal mouse bone. Am J Physiol Renal Physiol. 2012;303:F431–F436. doi: 10.1152/ajprenal.00199.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuro-o M. The FGF23 and Klotho system beyond mineral metabolism. Clin Exp Nephrol. 2017;21:64–69. doi: 10.1007/s10157-016-1357-6. [DOI] [PubMed] [Google Scholar]
- 66.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 67.Leaf DE, Pereira RC, Bazari H, et al. Oncogenic osteomalacia due to FGF23-expressing colon adenocarcinoma. J Clin Endocrinol Metab. 2013;98:887–891. doi: 10.1210/jc.2012-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leifheit-Nestler M, Kirchhoff F, Nespor J, et al. Fibroblast growth factor 23 is induced by an activated renin-angiotensin-aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dial Transplant. 2018;33:1722–1734. doi: 10.1093/ndt/gfy006. [DOI] [PubMed] [Google Scholar]
- 69.Leifheit-Nestler M, Wagner MA, Richter B, et al. Cardiac fibroblast growth factor 23 excess does not induce left ventricular hypertrophy in healthy mice. Front Cell Dev Biol. 2021;9:745892. doi: 10.3389/fcell.2021.745892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li D-J, Fu H, Zhao T, et al. Exercise-stimulated FGF23 promotes exercise performance via controlling the excess reactive oxygen species production and enhancing mitochondrial function in skeletal muscle. Metabolism. 2016;65:747–756. doi: 10.1016/j.metabol.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Li J-R, Chiu K-Y, Ou Y-C, et al. Alteration in serum concentrations of FGF19, FGF21, and FGF23 in patients with urothelial carcinoma. BioFactors. 2019;45:62–68. doi: 10.1002/biof.1460. [DOI] [PubMed] [Google Scholar]
- 72.Linglart A, Imel EA, Whyte MP, et al. Sustained efficacy and safety of burosumab, a monoclonal antibody to FGF23, in children with X-linked hypophosphatemia. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgab729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehta R, Cai X, Lee J, et al. Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the Chronic Renal Insufficiency Cohort Study. JAMA Cardiol. 2016;1:548–556. doi: 10.1001/jamacardio.2016.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meir T, Durlacher K, Pan Z, et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 2014;86:1106–1115. doi: 10.1038/ki.2014.215. [DOI] [PubMed] [Google Scholar]
- 76.Mirza MAI, Hansen T, Johansson L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–3131. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 77.Munoz Mendoza J, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and Inflammation in CKD. CJASN. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murali SK, Roschger P, Zeitz U, et al. FGF23 regulates bone mineralization in a 1,25(OH)2 D3 and Klotho-independent manner. J Bone Miner Res. 2016;31:129–142. doi: 10.1002/jbmr.2606. [DOI] [PubMed] [Google Scholar]
- 79.Nasrallah MM, El-Shehaby AR, Salem MM, et al. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:2679–2685. doi: 10.1093/ndt/gfq089. [DOI] [PubMed] [Google Scholar]
- 80.Oe Y, Kameda H, Nomoto H, et al. Favorable effects of burosumab on tumor-induced osteomalacia caused by an undetectable tumor: a case report. Medicine (Baltimore) 2021;100:e27895. doi: 10.1097/MD.0000000000027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Onal M, Carlson AH, Thostenson JD, et al. A novel distal enhancer mediates inflammation-, PTH-, and early onset murine kidney disease-induced expression of the mouse Fgf23 gene. JBMR Plus. 2018;2:32–47. doi: 10.1002/jbm4.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–648. doi: 10.7326/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pathare G, Anderegg M, Albano G, et al. Elevated FGF23 levels in mice lacking the thiazide-sensitive NaCl cotransporter (NCC) Sci Rep. 2018;8:3590. doi: 10.1038/s41598-018-22041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pavik I, Jaeger P, Kistler AD, et al. Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 2011;79:234–240. doi: 10.1038/ki.2010.375. [DOI] [PubMed] [Google Scholar]
- 86.Prié D, Forand A, Francoz C, et al. Plasma fibroblast growth factor 23 concentration is increased and predicts mortality in patients on the liver-transplant waiting list. PLoS ONE. 2013;8:e66182. doi: 10.1371/journal.pone.0066182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radhakrishnan K, Kim Y-H, Jung YS et al (2021) Orphan nuclear receptor ERR-γ regulates hepatic FGF23 production in acute kidney injury. PNAS 118. 10.1073/pnas.2022841118 [DOI] [PMC free article] [PubMed]
- 88.Rausch S, Barholz M, Föller M, et al. Vitamin A regulates fibroblast growth factor 23 (FGF23) Nutrition. 2020;79–80:110988. doi: 10.1016/j.nut.2020.110988. [DOI] [PubMed] [Google Scholar]
- 89.Richter B, Haller J, Haffner D, et al. Klotho modulates FGF23-mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflugers Arch. 2016;468:1621–1635. doi: 10.1007/s00424-016-1858-x. [DOI] [PubMed] [Google Scholar]
- 90.Roberts MA, Huang L, Lee D, et al. Effects of intravenous iron on fibroblast growth factor 23 (FGF23) in haemodialysis patients: a randomized controlled trial. BMC Nephrol. 2016;17:177. doi: 10.1186/s12882-016-0391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossaint J, Oehmichen J, van Aken H, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126:962–974. doi: 10.1172/JCI83470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sato H, Kazama JJ, Murasawa A, et al. Serum fibroblast growth factor 23 (FGF23) in patients with rheumatoid arthritis. Intern Med. 2016;55:121–126. doi: 10.2169/internalmedicine.55.5507. [DOI] [PubMed] [Google Scholar]
- 93.Scholze A, Liu Y, Pedersen L, et al. Soluble α-klotho and its relation to kidney function and fibroblast growth factor-23. J Clin Endocrinol Metab. 2014;99:E855–E861. doi: 10.1210/jc.2013-4171. [DOI] [PubMed] [Google Scholar]
- 94.Shimada T, Muto T, Urakawa I, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 95.Simic P, Kim W, Zhou W, et al. Glycerol-3-phosphate is an FGF23 regulator derived from the injured kidney. J Clin Invest. 2020;130:1513–1526. doi: 10.1172/JCI131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh S, Grabner A, Yanucil C, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90:985–996. doi: 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spichtig D, Zhang H, Mohebbi N, et al. Renal expression of FGF23 and peripheral resistance to elevated FGF23 in rodent models of polycystic kidney disease. Kidney Int. 2014;85:1340–1350. doi: 10.1038/ki.2013.526. [DOI] [PubMed] [Google Scholar]
- 98.Stöhr R, Schuh A, Heine GH, et al. FGF23 in cardiovascular disease: innocent bystander or active mediator? Front Endocrinol (Lausanne) 2018;9:351. doi: 10.3389/fendo.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suvannasankha A, Tompkins DR, Edwards DF, et al. FGF23 is elevated in multiple myeloma and increases heparanase expression by tumor cells. Oncotarget. 2015;6:19647–19660. doi: 10.18632/oncotarget.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takashi Y, Kawanami D, Fukumoto S. FGF23 and hypophosphatemic rickets/osteomalacia. Curr Osteoporos Rep. 2021 doi: 10.1007/s11914-021-00709-4. [DOI] [PubMed] [Google Scholar]
- 101.Tebben PJ, Kalli KR, Cliby WA, et al. Elevated fibroblast growth factor 23 in women with malignant ovarian tumors. Mayo Clin Proc. 2005;80:745–751. doi: 10.4065/80.6.745. [DOI] [PubMed] [Google Scholar]
- 102.Tsuji K, Maeda T, Kawane T, et al. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res. 2010;25:1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 103.Wahl P, Wolf M. FGF23 in chronic kidney disease. Adv Exp Med Biol. 2012;728:107–125. doi: 10.1007/978-1-4614-0887-1_8. [DOI] [PubMed] [Google Scholar]
- 104.Wheeler JA, Clinkenbeard EL. Regulation of fibroblast growth factor 23 by iron, EPO, and HIF. Curr Mol Biol Rep. 2019;5:8–17. doi: 10.1007/s40610-019-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.White KE, Cabral JM, Davis SI, et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–1803. doi: 10.1002/jbmr.1923. [DOI] [PubMed] [Google Scholar]
- 107.Xiao Z, Huang J, Cao L, et al. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS ONE. 2014;9:e104154. doi: 10.1371/journal.pone.0104154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie J, Yoon J, An S-W, et al. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. JASN. 2015;26:1150–1160. doi: 10.1681/ASN.2014040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 110.Yamazaki M, Kawai M, Miyagawa K, et al. Interleukin-1-induced acute bone resorption facilitates the secretion of fibroblast growth factor 23 into the circulation. J Bone Miner Metab. 2015;33:342–354. doi: 10.1007/s00774-014-0598-2. [DOI] [PubMed] [Google Scholar]
- 111.Yuan B, Feng JQ, Bowman S, et al. Hexa-D-arginine treatment increases 7B2•PC2 activity in hyp-mouse osteoblasts and rescues the HYP phenotype. J Bone Miner Res. 2013;28:56–72. doi: 10.1002/jbmr.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeng S, Querfeld U, Feger M, et al. Relationship between GFR, intact PTH, oxidized PTH, non-oxidized PTH as well as FGF23 in patients with CKD. FASEB J. 2020;34:15269–15281. doi: 10.1096/fj.202000596R. [DOI] [PubMed] [Google Scholar]
- 113.Zhang B, Umbach AT, Chen H, et al. Up-regulation of FGF23 release by aldosterone. Biochem Biophys Res Commun. 2016;470:384–390. doi: 10.1016/j.bbrc.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 114.Zhang B, Yan J, Schmidt S, et al. Lithium-sensitive store-operated Ca2+ entry in the regulation of FGF23 release. NSG. 2015;23:34–48. doi: 10.1159/000442602. [DOI] [PubMed] [Google Scholar]
- 115.Zhang B, Yan J, Umbach AT, et al. NFκB-sensitive Orai1 expression in the regulation of FGF23 release. J Mol Med (Berl) 2016;94:557–566. doi: 10.1007/s00109-015-1370-3. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Q, Doucet M, Tomlinson RE, et al. The hypoxia-inducible factor-1α activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 2016;4:16011. doi: 10.1038/boneres.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu Y, He Q, Aydin C, et al. Ablation of the stimulatory G protein α-subunit in renal proximal tubules leads to parathyroid hormone-resistance with increased renal Cyp24a1 mRNA abundance and reduced serum 1,25-dihydroxyvitamin D. Endocrinology. 2016;157:497–507. doi: 10.1210/en.2015-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]