Abstract
Ineffective use of adaptive cognitive strategies (e.g., reappraisal) to regulate emotional states is often reported in a wide variety of psychiatric disorders, suggesting a common characteristic across different diagnostic categories. However, the extent of shared neurobiological impairments is incompletely understood. This study, therefore, aimed to identify the transdiagnostic neural signature of disturbed reappraisal using the coordinate‐based meta‐analysis (CBMA) approach. Following the best‐practice guidelines for conducting neuroimaging meta‐analyses, we systematically searched PubMed, ScienceDirect, and Web of Science databases and tracked the references. Out of 1,608 identified publications, 32 whole‐brain neuroimaging studies were retrieved that compared brain activation in patients with psychiatric disorders and healthy controls during a reappraisal task. Then, the reported peak coordinates of group comparisons were extracted and several activation likelihood estimation (ALE) analyses were performed at three hierarchical levels to identify the potential spatial convergence: the global level (i.e., the pooled analysis and the analyses of increased/decreased activations), the experimental‐contrast level (i.e., the analyses of grouped data based on the regulation goal, stimulus valence, and instruction rule) and the disorder‐group level (i.e., the analyses across the experimental‐contrast level focused on increasing homogeneity of disorders). Surprisingly, none of our analyses provided significant convergent findings. This CBMA indicates a lack of transdiagnostic convergent regional abnormality related to reappraisal task, probably due to the complex nature of cognitive emotion regulation, heterogeneity of clinical populations, and/or experimental and statistical flexibility of individual studies.
Keywords: activation likelihood estimation, coordinate‐based meta‐analysis, emotion regulation, reappraisal
The present study aimed to identify the transdiagnostic neural signature of disturbed reappraisal using the coordinate‐based meta‐analysis. Our findings indicate a lack of transdiagnostic convergent regional abnormality related to reappraisal task, probably due to the complex nature of cognitive emotion regulation, heterogeneity of clinical populations, and/or experimental and statistical flexibility of individual studies.
1. INTRODUCTION
Throughout our daily lives, we are constantly exposed to a wide range of emotionally arousing situations. Lacking the capacity to effectively use emotion regulation strategies to modify the occurrence, intensity, and duration of an emotional experience is referred to as emotion dysregulation, which can negatively affect our personal and social functioning and may cause serious mental health issues (Beauchaine & Cicchetti, 2019). Regulatory strategies are putatively considered “adaptive” or “maladaptive” based on their positive or negative associations with psychopathological symptoms (Aldao, Nolen‐Hoeksema, & Schweizer, 2010). Accordingly, healthy emotion regulation involves a balanced interplay between the use of adaptive and maladaptive strategies to reach a desired emotional state, whereas emotion dysregulation reflects unsuccessful handling of emotions caused by over‐reliance on maladaptive and/or failure in recruiting adaptive strategies (Aldao et al., 2010). It is estimated that emotion dysregulation occurs in about 40–70% of individuals diagnosed with psychiatric disorders (Jazaieri, Urry, & Gross, 2013), suggesting a transdiagnostic phenomenon (Aldao, 2016; Fernandez, Jazaieri, & Gross, 2016).
Emotion dysregulation has been extensively studied in the context of cognitive emotion regulation with a particular focus on reappraisal, an antecedent strategy that incorporates cognitive processes to alter the meaning or relevance of stimuli in order to change their emotional impact. (Aldao et al., 2010; Cludius, Mennin, & Ehring, 2020; D'Agostino, Covanti, Monti, & Starcevic, 2017; Werner & Gross, 2010). Reappraisal is a universal ability that can be used to maintain, decrease or increase negative and positive emotions (Nezlek & Kuppens, 2008). But, it is mainly required for reframing an emotionally aversive situation by creating a neutral or a more pleasant interpretation (Gross, 1998). Reappraisal has attracted attention as one of the most effective and adaptive strategies due to its immediate positive effects on emotional experience, as well as its long‐term beneficial outcomes for mental health (Hu et al., 2014; Webb, Miles, & Sheeran, 2012). Despite the health‐protective benefits of reappraisal, patients with mental illnesses generally report infrequent deployment of this regulation strategy, particularly in distressing or unpleasant situations (Aldao et al., 2010; Cludius et al., 2020; D'Agostino et al., 2017).
Lower reappraisal tendency in psychiatric patients might be related to their inability to implement this strategy in an effective way (Silvers & Moreira, 2019). Reappraisal is a top‐down and effortful process that depends on intact cognitive control and executive functioning (McRae, Jacobs, Ray, John, & Gross, 2012; Schmeichel & Tang, 2015). Thus, having trouble recruiting such higher‐order processes might lead to a decreased desire for using this strategy over time, which in turn could diminish its efficient health outcomes (Ford, Karnilowicz, & Mauss, 2017). Neuroimaging studies on reappraisal in healthy (Etkin, Büchel, & Gross, 2015; Ochsner & Gross, 2005; Ochsner, Silvers, & Buhle, 2012; Öner, 2018) and clinical populations (Green & Malhi, 2006; Silvers, Buhle, & Ochsner, 2014; Taylor & Liberzon, 2007; Zilverstand, Parvaz, & Goldstein, 2017) appear to support altered mechanisms of reappraisal across different patient groups. Accordingly, inefficient reappraisal performance is thought to be associated with a transdiagnostic pattern of aberrant brain activation in frontal cognitive control regions which are necessary to modulate the activation in regions subserving emotion generation (Zilverstand et al., 2017). Supporting this hypothesis, several transdiagnostic studies have reported the common pathways of disturbances in the neural mechanisms underlying cognitive control and executive functioning (Malloy‐Diniz, Miranda, & Grassi‐Oliveira, 2017; McTeague, Goodkind, & Etkin, 2016), emotion processing system as the regulation target (McTeague et al., 2020; Schulze, Schulze, Renneberg, Schmahl, & Niedtfeld, 2019), and their network interactions (Kebets et al., 2020).
This literature, therefore, could be construed to claim that there may exist a consistent pattern of regional abnormality underlying ineffective reappraisal performance across various diagnostic representations. However, the available meta‐analytic evidence at this point is not sufficient to draw such a conclusion due to the inconsistent results (McTeague et al., 2020; Picó‐Pérez, Radua, Steward, Menchón, & Soriano‐Mas, 2017; Wang et al., 2018). For example, although McTeague et al. (2020) found the right VLPFC as the only convergent region related to emotion dysregulation spanning different psychiatric diagnoses, this region was not identified in other meta‐analyses on a combination of mood and anxiety (Picó‐Pérez et al., 2017) and on a range of anxiety disorders (Wang et al., 2018). Divergent findings across these meta‐analyses encouraged us to address the dispute of transdiagnostic disruptions underlying reappraisal by performing a more comprehensive meta‐analysis on the largest available number of clinical neuroimaging studies concerning brain activation during a reappraisal task. In order to do so, we used the activation likelihood estimation (ALE) technique (Eickhoff et al., 2016; Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012) to integrate the neuroimaging results. In particular, we performed the analyses in a hierarchical order (global level, experimental‐contrast level, and disorder‐group level) to map the neural correlates of reappraisal disruptions based on the increasing homogeneity of data. In this regard, we first pooled all the available data to provide an overview of the neural alterations in patients compared with healthy controls. Then, we clustered data by the factors with a potential to contribute to heterogeneity (i.e., regulation direction, stimulus valence, and instruction rule). Finally, we used a narrowing down approach to make clustering of disorders (from the most heterogeneous to the most homogenous ones) across the data representing downregulation of negative emotions.
2. METHODS AND MATERIALS
The current study was pre‐registered at the International Prospective Register of Systematic Reviews (PROSPERO, CRD42019119121) and the search strategy was based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement (Moher, Liberati, Tetzlaff, & Altman, 2010). Following the recent best‐practice guidelines for neuroimaging meta‐analyses (Müller et al., 2018; Tahmasian et al., 2019), we performed several ALE analyses on the existing reappraisal neuroimaging studies that assessed the regulating disturbances in psychiatric patients compared with healthy controls. In a typical reappraisal experiment, participants are presented with a series of evocative stimuli and are instructed to either naturally respond to them or apply the reappraisal strategy by implementing a given tactic or choosing the tactic themselves. Generally, two specific tactics are used in reappraisal experiments: reinterpretation or changing one's reinterpretation of the emotional stimulus; and distancing or changing the one's psychological distance from the emotional stimulus (Webb et al., 2012). Reappraisal performance is assessed by contrasting the brain activations during the two conditions of reappraisal implementation and natural responding across individual studies.
2.1. Search strategies and selection criteria
The literature search was conducted in February 2020 through PubMed, ScienceDirect, and Web of Science databases with the following search terms: (cognitive OR volitional OR voluntary OR effortful) AND (emotion OR affect) AND (regulat* OR reappraisal OR reinterpretation OR distancing) AND (fMRI OR “functional MRI” OR “functional magnetic resonance imaging” OR PET OR “positron emission tomography”) AND (patient OR disorder). Additional publications were identified by reference tracking from reviews/meta‐analyses. The resulting pool consisted of 1,608 records, assessed by two authors independently (T.K. and Z.S.). Eligible studies were selected in two steps: Firstly, non‐English language publications, case reports, letters to editors, reviews/meta‐analyses, and structural or task‐free imaging studies were excluded by screening the abstracts. Secondly, full‐texts of all remaining studies were screened carefully and studies that met the following criteria were included:
functional neuroimaging studies (i.e., fMRI/PET) that compared the reappraisal task between patients suffering from any kind of psychiatric disorders and healthy subjects,
if significant brain activation results were reported for the contrast of interest (reappraisal vs. natural responding),
if “whole‐brain” analyses were performed and coordinates from the peak of task‐based activations were reported in Montreal Neurological Institute (MNI) or Talairach spaces,
if standardized diagnostic criteria (DSM‐IV‐TR, or DSM‐5, or ICD‐10) for patient recruitment was used,
if adult subjects were recruited (range age between 18 and 60),
if at least seven subjects were in each group.
2.2. Data extraction
For all the included studies, sample size, demographic data of participants (age, gender), clinical characteristics of patients (diagnosis, diagnostic tool, symptom severity, medication status, and comorbidities), experimental setup (stimulus arousal, stimulus valence, regulation strategy and regulation direction, imaging modality, scanner type, analysis software package, and statistical analysis criteria), and the peak coordinates of between‐group experiments for the reappraisal versus natural responding contrast were extracted (Table 1). In the present meta‐analysis, “study” reflects an individual publication and “experiment” indicates a set of coordinates belonging to a particular analysis or contrast of interest (i.e., patients vs. controls group comparison) in a given study (Müller et al., 2018). Subsequently, the experiments were coded as “increased” when the brain activation during the reappraisal condition was higher in patients than healthy controls (patients > controls) or “decreased” when it was lower in patients than controls (patients < controls). The peak coordinates reported in Talairach space were converted into MNI space to set all the coordinates in the same reference space (Lancaster et al., 2007). To avoid convergence over the analyses performed on the same/overlapping samples (reported within or between studies), we merged the coordinates from multiple experiments (e.g., increased/decreased) pertaining to the studies with the same/overlapping samples, making sure each study contributes once per analysis (Turkeltaub et al., 2012).
TABLE 1.
Characteristics of 32 included studies in the present meta‐analysis
| Study | Number (M:F) | Mean age (SD) | Diagnosis | Medication (n) | Comorbidity (n) | Symptom severity | ER tactic/direction | Stimulus | Arousal/valence | Statistical threshold | Software | MRI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | |||||||||||
| Major depressive disorder (MDD) | ||||||||||||||
| Stephanou, Davey, Kerestes, Whittle, and Harrison (2017) | 53 (22:31) | 64 (24:40) | 19.72 (2.68) | 19.03 (2.45) | SCID‐IV | No (within last 4 weeks) | No (lifetime) | MADRS [MDD = 32.80 (4.80); controls = 2.14 (2.99)] | Reinterpretation/downregulation | IAPS (negative social scenes), EPS, online sources | NA | FDR | SPM 8 | 3 T |
| Wang, Zhou, Dai, Ji, and Feng (2017) | 12 (5:7) | 15 (7: 8) | 29.50 (8.46) | 25.80 (5.89) | SCID‐IV | No (within last 2 weeks) | No (current psychiatric and lifetime neurologic) | BDI [MDD = 26.17 (12.65); controls = 4.27 (4.23)] | Not‐specified/downregulation and upregulation | IAPS (positive and negative images), other sources | NA | FWE (REST, AlphaSim) | SPM 8 | 3 T |
| Radke et al. (2018) | 22 (13:9) | 22 (13:9) | 32.6 (10.9) | 34.5 (9.9) | SCID‐IV | 15 | 3 | BDI [MDD = 13.8 (9.5); controls = 2.7 (3.4)] | Distancing/upregulation | FACES (angry face) | NA | FWE | SPM 8 | 3 T |
| Greening, Osuch, Williamson, and Mitchell (2014) | 19 (6:13) | 19 (6:13) | 26.79 (11.4) | 27.63 (11.0) | SCID‐IV | 9 | 7 | BDI [MDD = 25.53 (10.4); controls = 1.6 (2.3)] | Reinterpretation/downregulation | IAPS (sad and positive scenes) | Normative mean arousal sad = 5.08 (.62); mean arousal positive = 5.03 (.55) | FWE (AlphaSim) | AFNI 2012 | 3 T |
| Smoski, Keng, Schiller, Minkel, and Dichter (2013) | 18 (4:15) | 19 (7:12) | 24.5 (5.4) | 27.9 (6.3) | SCID‐IV | 5 | No (current) | BDI [MDD = 2.9 (5.0); controls = 1.4 (2.4)] | Not‐specified/downregulation | IAPS (sad pictures), other normed images | NA | FWE (AFNI, 3dClustSim) | FSL | 3 T |
| Heller et al. (2009) | 27 (12:15) | 19 (9:10) | 31.48 (11.58) | 31.84 (14.65) | DSM‐IV | No (lifetime) | No (current psychiatric and lifetime bipolar/anxiety) | HAMD [MDD = 20.6 (2.39); controls = 1.2 (1.6)] | Not‐specified/downregulation and upregulation | IAPS (positive and negative pictures) | Normative mean valence negative = 2.95 (.87); mean arousal negative = 5.44 (.8); mean valence positive = 7.13 (.62); mean arousal positive = 5.28 (.58) | FWE (AlphaSim) | AFNI | 3 T |
| Sheline et al. (2009) | 24 (12:12) | 21 (6:15) | 34 (9.4) | 35 (7.3) | DSM‐IV | No (within last 4 weeks) | No (lifetime) | HAMD [MDD = 21 (3.5); controls = 0 (.04)] | Not‐specified/downregulation | IAPS (positive and negative pictures) | NA | NA | NA | 3 T |
| Johnstone, van Reekum, Urry, Kalin, and Davidson (2007) | 21 (8:13) | 28 (7:21) | 33 (12) | 28 (12) | DSM‐IV | No (current) | No (current psychiatric and lifetime bipolar) | HAMD [MDD = 21 (2.5); controls = .5 (.6)] | Not‐specified/downregulation and upregulation | IAPS (positive and negative pictures) | Normative mean valence negative = 2.95 (.87); mean arousal negative = 5.44 (.8); mean valence positive = 7.13 (.62); mean arousal positive = 5.28 (.58) | FWE (AlphaSim) | AFNI | 3 T |
| Schizophrenia | ||||||||||||||
| Zhang et al. (2020) | 16 (12:4) | 15 (10:5) | 31.75 (8.7) | 33.60 (11.1) | DSM‐IV and ICD‐10 | NA | No (current) | PANSS [schizophrenia = 26.69 (7.1)] | Reinterpretation/downregulation | IAPS (negative pictures) | NA | FWE (AFNI 2018, 3dClustSim) | SPM 12 | 3 T |
| Larabi, van der Meer, Pijnenborg, Ćurčić‐Blake, and Aleman (2018) | 30 (22:8) | 15 (10:5) | 35 (10.16) | 33.6 (11.11) | DSM‐IV and ICD‐1O | 28 | No (current) | PANSS [schizophrenia = 57.9 (14.71)] | Reinterpretation/downregulation | IAPS (negative images) | Normative mean valence negative = 2.6; mean arousal negative = 5.7; mean valence neutral = 1.3; mean arousal neutral = 1.9 | FWE | SPM 12 | 3 T |
| van der Meer et al. (2014) | 20 (14:6) | 20 (16:4) | 35.5 (11.7) | 35.2 (10.8) | DSM‐IV and ICD‐10 | 20 | NA | PANSS [schizophrenia = 29.9 (7.7)] | Reinterpretation/downregulation | IAPS (negative images) | Normative mean valence negative = 2.6; mean arousal negative = 5.7; mean valence neutral = 1.3; mean arousal neutral = 1.9 | FWE | SPM 5 | 3 T |
| Morris, Sparks, Mitchell, Weickert, and Green (2012) | 12 (8:4) | 15 (6:9) | 44 (3) | 35 (2) | DSM‐IV | 12 | NA | PANSS [schizophrenia = 32 (2)] | Distancing/downregulation and upregulation | IAPS (negative threat and suffering images) | NA | FWE | SPM 8 | 3 T |
| Social anxiety disorder (SAD) | ||||||||||||||
| Ziv, Goldin, Jazaieri, Hahn, and Gross (2013) | 27 (15:12) | 27 (14:13) | 31.1 (7.6) | 32.6 (9.5) | DSM‐IV | No (current) | 8 | LSAS‐SR [SAD = 99.3 (11.8); controls = 15.3 (9.1)] | Reinterpretation/downregulation | Anger and contempt facial expressions | NA | FWE (AlphaSim) | AFNI | 3 T |
| Goldin, Manber, Hakimi, Canli, and Gross (2009) | 15 (6:9) | 17 (8:9) | 31.6 (9.7) | 32.1 (9.3) | DSM‐IV | No (current) | No (current psychiatric and lifetime neurologic) | LSAS‐SR [SAD = 67.6 (21.1); controls = 29.3 (20.9)] | Distancing/downregulation | Facial action coding system (angry and contempt facial expression), and physical threat scenes | NA | FWE (AlphaSim) | AFNI | 3 T |
| Goldin, Manber‐Ball, Werner, Heimberg, and Gross (2009) | 27 (15:12) | 27 (15:12) | 32.1 (9.2) | 32.2 (9.5) | DSM‐IV | No (current) | 6 | LSAS‐SR [SAD = 80.1 (16.8); controls = 15.7 (8.7)] | Reinterpretation/downregulation | Written social situations | NA | FWE (AlphaSim) | AFNI | 3 T |
| Posttraumatic stress disorder (PTSD) | ||||||||||||||
| Butler et al. (2018) | 18 (18:0) | 27 (27:0) | 28.3 (6.4) | 32.7 (5.9) | ICD‐10 | No (current) | No (lifetime axis‐II) | PDS [PTSD = 36.28 (10.65); controls = 15.7 (8.7)] | Reinterpretation/downregulation | Combat images | NA | FWE (AFNI 2016, 3dClustSim) | SPM 8 | 3 T |
| Xiong et al. (2013) | 20 (13:7) | 20 (14:6) | 32.92 (8.48) | 31.53 (7.43) | SCID‐IV | No (lifetime) | No (lifetime, except of past depression) | CAPS [PTSD = 52.33 (9.44); controls = 8.26 (9.31)] | Reinterpretation/downregulation and upregulation | IAPS (negative images) | Normative mean valence negative = 2.17 (.34); mean arousal negative = 6.23 (.26); mean valence neutral = 5.12 (1.04); mean arousal neutral = 4.18 (.72) | FWE (REST, AlphaSim) | SPM 8 | 3 T |
| New et al. (2009) | 14 (0:14) | 14 (0:14) | 38.7 (11.2) | 31.7 (10.3) | SCID‐IV | No (lifetime) | No (lifetime, except of past depression) | CAPS [PTSD = 69.1 (17.6)] | Reinterpretation/downregulation and upregulation | IAPS (negative images) | NA | FWE (REST, AlphaSim) | SPM 2 | 3 T |
| Bipolar disorder (BD) | ||||||||||||||
| Zhang et al. (2020) | 15 (6:9) | 15 (10:5) | 39.87 (12.5) | 33.60 (11.1) | DSM‐IV and ICD‐10 | NA | No (current) | YMRS [BD = 1.4 (1.5)] and QIDS [BD = 5.27 (5.4)] | Reinterpretation/downregulation | IAPS (negative pictures) | NA | FWE (AFNI 2018, 3dClustSim) | SPM 12 | 3 T |
| Townsend et al. (2013) | 30 (19:11) | 26 (15:11) | 37.9 (12.6) | 35.5 (12.4) | SCID‐IV | 21 | No (current psychiatric and lifetime substance use/abuse) | YMRS [BD = 1.7 (2.2)] and HAMD [BD = 3.8 (1.9)] | Reinterpretation/downregulation | IAPS (negative images) | Normative mean valence negative = 2.8; mean arousal negative = 6.5 | NA | FSL | 3 T |
| Morris et al. (2012) | 13 (8:5) | 15 (6:9) | 41 (3) | 35 (2) | DSM‐IV | 13 | NA | NA | Distancing/downregulation | IAPS (negative threat and suffering images) | NA | FWE | SPM 8 | 3 T |
| Borderline personality disorder (BPD) | ||||||||||||||
| van Zutphen et al. (2018) | 55 (0:55) | 42 (0:42) | 30.88 (8.78) | 28.33 (10.50) | SCID‐IV | 55 | 49 | BPD checklist [BPD = 120.6 (26.92); controls = 50.73 (5.03)] | Reinterpretation/downregulation and upregulation | IAPS (negative and positive), additional erotic pictures | NA | FWE | BrainVoyager | 3 T |
| Schulze et al. (2011) | 15 (0:15) | 15 (0:15) | 27.60 (7.85) | 24.53 (2.85) | SCID‐IV | NA | 7 | BSL [BPD = 183.87 (53.64); controls = 49.60 (16.04)] | Distancing/downregulation and upregulation | IAPS (negative threat and suffering images) | NA | FWE | SPM 5 | 1.5 T |
| Koenigsberg et al. (2009) | 18 (8:10) | 16 (9:9) | 32.6 (10.4) | 31.8 (7.7) | SCID‐IV | No (within last 4 weeks) | No (lifetime) | ALS [BPD = 94.9 (23.7); controls = 20.3 (16.0)] | Distancing/downregulation | IAPS (interpersonal situations) | Normative mean valence negative = 2.35; mean arousal negative = 5.9; mean valence neutral = 5.2; mean arousal neutral = 3.65 | FWE (REST, AlphaSim) | SPM 2 | 3 T |
| Miscellaneous anxiety disorders | ||||||||||||||
| Blair et al. (2012) | 53 (17:36) | 18 (8:10) | 33.73 (9.99) | 33.4 (9.65) | SCID‐IV | No (within last 6 months) | No (current) | BAI [miscellaneous = 10.93 (7.17); controls = 2.3 (2.02)] | Reinterpretation/downregulation | IAPS (positive and negative images) | Normative mean valence negative = 3.08; mean arousal negative = 5.43; mean valence positive = 7.21; mean arousal positive = 5.15 | FWE (AlphaSim) | AFNI | 1.5 T |
| Ball, Ramsawh, Campbell‐Sills, Paulus, and Stein (2013) | 41 (9:32) | 22 (11:11) | 32 (9) | 27 (9) | DSM‐IV | No (within last 2 weeks) | 17 | QASIS [miscellaneous = 8.6 (3.3); controls = .8 (1.2)] | Not‐specified/downregulation | IAPS (negative images) | NA | FWE (3dClustSim) | AFNI | 3 T |
| Campbell‐Sills et al. (2011) | 13 (2:11) | 13 (2:11) | NA | NA | SCID‐IV | No (lifetime) | 12 | NA | Reinterpretation/downregulation | IAPS (negative images) | NA | FWE (AlphaSim) | AFNI | 3 T |
| Panic disorder (PD) | ||||||||||||||
| Reinecke et al. (2015) | 18 (4:14) | 18 (4:14) | 36.5 (13.8) | 32.3 (12.1) | SCID‐IV | 3 | 13 | HADS‐anxiety [PD = 14.6 (4.1); controls = 2.0 (1.6)] | Reinterpretation/downregulation | IAPS (accidents or funerals) | Normative mean valence negative = 2.8 (1.7); mean arousal negative = 6.0 (2.2) | NA | FSL | 3 T |
| Pre‐menstrual dysphoric disorder (PMDD) | ||||||||||||||
| Petersen et al. (2018) | 18 (0:18) | 18 (0:18) | 29.2 (7.24) | 25.4 (6.99) | SCID‐IV | No (current) | No (lifetime except of unipolar mood disorders) | DRSP [PMDD = 3.53 (.63); controls = 1.01 (.05)] | Distancing/downregulation | IAPS (negative images) and other ones | NA | NA | FSL | 3 T |
| Obsession‐compulsion disorder (OCD) | ||||||||||||||
| Thorsen et al. (2019) | 43 (21:22) | 38 (18:20) | 37.58 (10) | 39.05 (11.27) | SCID‐IV | No (within last 4 weeks) | 29 | OCI‐R [OCD = 24.67 (11.79); controls = 3.37 (4.71)] | Not‐specified/downregulation | Fearful and OCD‐related pictures | NA | NA | SPM 8 | 3 T |
| Substance use disorder (SUD) | ||||||||||||||
| Albein‐Urios et al. (2014) | 17 (16:1) | 18 (17:1) | 36.41 (5.99) | 30.50 (4.64) | SCID‐IV | NA | NO (current psychiatric and lifetime neurologic) | UPPS‐negative urgency [SUD = 33.17 (6.51); controls = 22.22 (5.1)] | Not‐specified/downregulation | IAPS (negative images) | Normative mean valence negative = 3.51 (.86); mean arousal negative = 5.70 (0.6) | FDR | SPM 8 | 3 T |
| Gambling disorder (GD) | ||||||||||||||
| Navas et al. (2017) | 17 (16:1) | 21 (20:1) | 32.94 (7.77) | 31 (4.6) | SCID‐IV | NA | No (current psychiatric and lifetime neurologic) | UPPS‐negative urgency [GD = 29.18 (4.7); controls = 23.19 (5.46)] | Reinterpretation/downregulation | IAPS (negative mutilation pictures) | Normative mean valence negative = 3.51 (.86); mean arousal negative = 5.70 (0.6) | FWE (REST, AlphaSim) | SPM 8 | 3 T |
Abbreviations: ALS, Affect Lability Scale; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BSL, Borderline Symptom List; CAPS, Clinician‐Administered PTSD Scale; DRSP, Daily Record of Severity of Problems; HADS, Hospital Anxiety and Depression Scale; HAMD, Hamilton Depression Rating Scale; LSAS‐SR, Liebowitz Social Anxiety Scale—Self‐Report; MADRS, Montgomery–Asberg Depression Rating Scale; OCI‐R, Obsessive Compulsive Inventory‐Revised; PANASS, Positive and Negative Syndrome Scale; PDS, Posttraumatic Diagnostic Scale; QASIS, Overall Anxiety Severity and Impairment Scale; QIDS, Quick Inventory of Depressive Symptomatology; UPPS, urgency, premeditation, perseverance, and sensation seeking scale; YMRS, Young Mania Rating Scale.
2.3. Activation likelihood estimation
A revised version of ALE (Eickhoff et al., 2012) was used to assess whether the activation foci reported in peak coordinates significantly clustered into specific spatial locations, rather than randomly distributed across the whole brain. The ALE analyses were performed in three steps: First, spatial 3D Gaussian probability distributions were modeled around the peak coordinates of activated foci from experiments of interest. The width of the aforementioned probability determines the spatial uncertainty associated with variations in sampling effects, data processing, and data analysis. Since the foci of contrasts with smaller sample size have a smaller effect on the modeled uncertainty, it was adjusted for the number of subjects in the smaller group. Then, “modeled activation” maps of all foci from each experiment were pooled into an ALE activation map by computing their overlap across the experiments (Eickhoff et al., 2012; Turkeltaub et al., 2012). Finally, the ALE maps were assessed against a null distribution map to enable the random‐effects inference by using nonlinear histogram integration (Eickhoff et al., 2012; Turkeltaub et al., 2012). As suggested previously, the above‐chance clustering activation foci were assessed by setting the threshold at p < .05 family‐wise error at the cluster level (cFWE) to correct for multiple comparisons and avoid spurious findings.
To identify potential transdiagnostic patterns of disturbed recruitments of neural mechanisms responsible for reappraisal, we conducted a set of complementary ALE analyses at three hierarchical levels based on the homogeneity of experiments: global level, experimental‐contrast level, and disorder‐group level (see Table 2). At the global level, we assessed convergence across all reported effects by pooling all experiments, and further, analyzed increased/decreased activations separately. At the experimental‐contrast level, we first performed three independent analyses on the regulation goal (downregulation/upregulation), stimulus valence (negative/positive), and reappraisal instruction (reinterpretation/distancing/not‐specified). Notably, a valid analysis was only possible for “downregulation” and “negative” as there were not enough experiments in the other groups of data (<17) (Eickhoff et al., 2016). Then, we restricted the analysis to the experiments that reported peak coordinates for “downregulation” and “negative valence” simultaneously (i.e., negative downregulation). At the disorder‐group level, we performed the analyses on the negative downregulation experiments to explore the effects of increasing disorder homogeneity on the nature of disturbances, while patients were applying reappraisal to downregulate their negative emotions. In this regard, we used a stepwise narrowing down approach to group the experiments. For the first step, we restricted the experiments to nonpsychotic disorders by excluding the ones belonging to schizophrenia. For the second step, we restricted the experiments of nonpsychotic disorders to those belonging to emotional disorders which are identified with emotional disturbances as their hallmark (i.e., borderline personality as well as mood and anxiety disorders; Bullis, Boettcher, Sauer‐Zavala, Farchione, & Barlow, 2019). Finally, for the third step, we restricted the experiments of emotional disorders to those belonging to the disorders with shared neural phenotypes (i.e., mood and anxiety disorders; Janiri et al., 2020). No other sets of experiments (i.e., specific category or type of disorders) had enough data for a valid ALE analysis.
TABLE 2.
Findings of conducted meta‐analyses in patients compared with healthy subjects
| ALE analysis | Experiments | Contrast | p‐value | Number of experiments | |
|---|---|---|---|---|---|
| TFCE | cFWE | ||||
| Global level | Pooled | All | .406 | .418 | 28 |
| Decreased | Patients < controls | .436 | .570 | 21 | |
| Increased | Patients > controls | .675 | .832 | 20 | |
| Experimental‐contrast level | Downregulation | All | .662 | .859 | 27 |
| Negative | All | .615 | .930 | 28 | |
| Negative downregulation | All | .759 | .850 | 27 | |
| Disorder‐group level | 1. Across nonpsychotic disorders | All | .632 | .751 | 25 |
| 2. Across emotional disorders | All | .889 | .859 | 24 | |
| 3. Across mood and anxiety disorders | All | .658 | .655 | 21 | |
Abbreviations: ALE, activation likelihood estimation; cFWE, cluster‐level family‐wise error; TFCE, threshold‐free cluster enhancement.
3. RESULTS
A pool of 1,608 records was screened and 107 full‐text publications were assessed for eligibility. Subsequently, 75 studies were excluded for the following reasons: lacking either healthy controls or group comparison analyses, restricting samples to adolescents or older adults, not performing whole‐brain analysis, not reporting significant group comparison results, and including individuals without DSM/ICD‐based diagnosis (e.g., at a high risk of mental illnesses or with subclinical conditions) (Table S1). Finally, 32 publications were included in our meta‐analysis (Figure 1), with three overlapping samples reported in multiple publications, one sample used in three articles (Larabi et al., 2018; van der Meer et al., 2014; Zhang et al., 2020), and two samples each used in two articles (Goldin, Manber‐Ball, et al., 2009; Heller et al., 2009; Johnstone et al., 2007; Ziv et al., 2013). As mentioned earlier, we rigorously avoided including the same/overlapping samples within and across articles. Accordingly, we merged all studies with overlapping samples that resulted in 28 independent samples. Demographic, clinical, and experimental characteristics of the included articles are shown in Table 1. Overall, we conducted several ALE meta‐analyses (Table 2).
FIGURE 1.
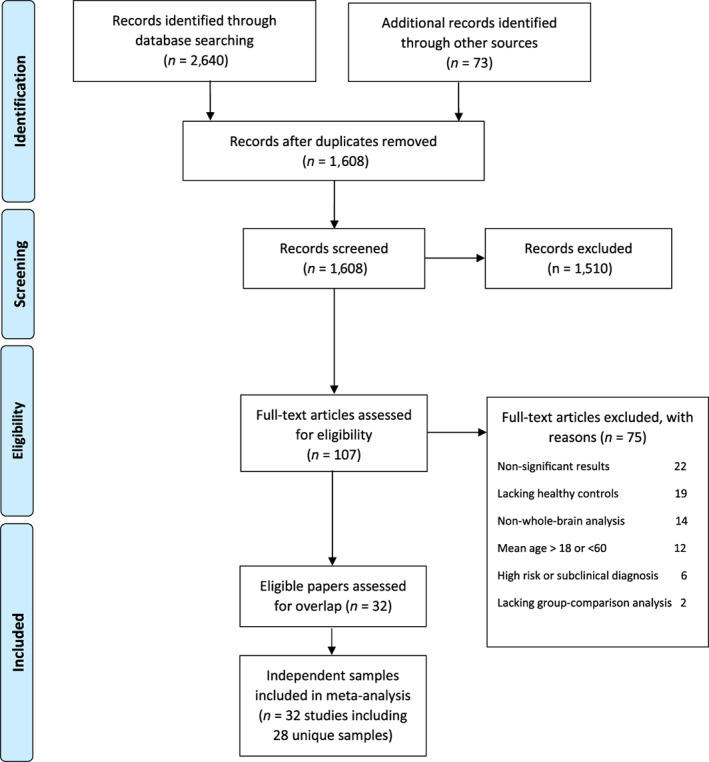
Study selection strategy flow chart
Surprisingly, none of our global level, experimental‐contrast level or disorder‐group level analyses yielded significant results:
global level analyses included [“pooled”: 28 experiments (p = .418), “decreased”: 21 experiments (p = .570) and “increased”: 20 experiments (p = .832)],
experimental‐contrast level analyses included [“downregulation”: 27 experiments (p = .859), “negative”: 28 experiments (p = .930), “negative downregulation”: 27 experiments (p = .850)],
-
disorder‐group level analyses included [“nonpsychotic disorders”: 25 experiments (p = .751), “emotional disorders”: 24 experiments (p = .859), “mood and anxiety disorders”: 21 experiments (p = .655)].
Of note, repeating all analyses with a more liberal statistical threshold (i.e., threshold‐free cluster enhancement, TFCE) demonstrated no significant convergence either (Table 2). Figure 2 displays the sporadic distribution of foci across the included experiments.
FIGURE 2.
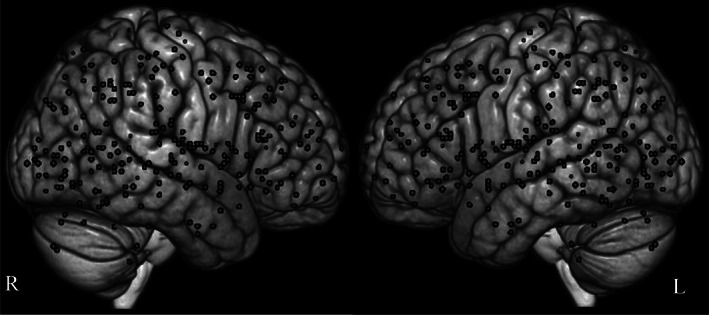
Distribution of the included peak coordinates in the current study. The represented foci reflect functional alterations related to the reappraisal task in patients with various psychiatric disorders compared with healthy subjects
4. DISCUSSION
In the current meta‐analysis, we explored whether there is a convergent regional brain abnormality related to dysfunctional reappraisal across various psychiatric disorders. Considering the homogeneity of included tasks (i.e., reappraisal) and using a strictly statistical approach for multiple comparison correction (i.e., cFWE), our ALE analyses did not yield any significant results, indicating the divergence of reappraisal impairments across different forms of psychopathology. Our results are in line with some previous meta‐analytic findings in patient populations that did not reveal spatial convergence of brain abnormalities (Degasperi, Cristea, Di Rosa, Costa, & Gentili, 2021; Giehl, Tahmasian, Eickhoff, & van Eimeren, 2019; Huang, Rootes‐Murdy, Bastidas, Nee, & Franklin, 2020; Müller et al., 2017; Nickl‐Jockschat, Janouschek, Eickhoff, & Eickhoff, 2015; Saberi, Mohammadi, Zarei, Eickhoff, & Tahmasian, 2021; Samea et al., 2019; Sheng et al., 2020; Tahmasian et al., 2018). This variance could be attributable to the complex physiological and pathophysiological mechanisms of reappraisal, heterogeneity in clinical populations, and/or experimental or methodological divergence (Tahmasian et al., 2018). We further discussed this heterogeneity as follows.
4.1. Distinct pathophysiology of impaired reappraisal across psychiatric disorders
4.1.1. Cognitive view
Theoretically, emotion dysregulation may take place in different stages including the identification of regulation necessity, selection of regulatory strategy, implementation of selected strategy, and stopping/switching of the implemented process (Fernandez et al., 2016; Gross, Uusberg, & Uusberg, 2019). Indeed, clinical conditions can be characterized by cognitive impairments in different regulatory stages and may not involve disruptions in common brain regions. For example, major depressive disorder is involved with overestimation of mood‐congruent stimuli, (Zilverstand et al., 2017) and conversely, helplessness to ignite a regulatory action (Sheppes, Suri, & Gross, 2015). Patients with bipolar disorder overvalue the hedonic benefits of manic states and are not usually convinced to downregulate the positive affect (Fernandez et al., 2016). Anxiety is associated with attentional biases toward threat stimuli, (Zilverstand et al., 2017) and consequently an amplified representation of regulation urgency (Gross & Jazaieri, 2014). An exaggerated sense of regulation necessity and decreased flexibility in strategy selection are mainly observed in posttraumatic stress disorder and obsessive–compulsive disorder (Taylor & Liberzon, 2007). Patients with social anxiety disorder may be uncertain about the effectiveness of reappraisal implementation due to insufficient self‐efficacy, which probably results in premature stopping of regulatory effort (Sheppes et al., 2015). Borderline personality disorder is associated with monitoring deficits related to impulsive strategy switching (Gross et al., 2019). And finally, failing to stop maladaptive strategies (e.g., rumination) in depressive and anxiety disorders may affect the implementation of adaptive strategies including reappraisal (Dryman & Heimberg, 2018). All these examples show that differences in clinical populations regarding the awareness of emotions, beliefs about the controllability of emotions, tendency to regulate emotions, and availability of regulatory resources (Kim, Bigman, & Tamir, 2015) are critical factors influencing the successful reappraisal performance.
4.1.2. Neurobiological view
Abnormal reappraisal in individuals with psychopathology generally occurs in the form of disrupted top‐down modulation of emotion processing (Zilverstand et al., 2017). However, the exact neurophysiological patterns may vary across specific disorders. In fact, the intimate connection between emotion generation and emotion regulation systems (Ochsner et al., 2004) can make it difficult to determine the extent to which the cross‐disorder regulation impairments can be explained in terms of common pathways. In other words, cognitive regulatory mechanisms are thought to rely on a set of cortical–cortical and cortical–subcortical networks (Morawetz et al., 2020; Sripada et al., 2014) that facilitate top‐down modulation of emotions across hierarchical levels of emotion processing (Smith & Lane, 2015). Thus, it is plausible that distinct disturbances in different hierarchical networks may lead to emotion dysregulation in the form of reappraisal impairments. For example, in anxiety disorders, excessive activation in the amygdala during the appraisal of aversive stimuli may result in the generation of intensive emotions, which can challenge the regulation system (Brehl, Kohn, Schene, & Fernández, 2020; Silvers et al., 2014). On the other hand, atypical recruitment of prefrontal regulatory networks may be the underlying cause of dysfunctional reappraisal among patients with schizophrenia or bipolar disorder (Silvers et al., 2014; Tully & Niendam, 2014). Abnormal network interactions between the prefrontal and subcortical structures may be another source of regulation disruption (Berboth & Morawetz, 2021). For instance, poor top‐down regulation in major depressive disorder can be recognized with a diminished negative correlation between the amygdala and prefrontal cortices (Park et al., 2019). Collectively, these examples indicate that ineffective reappraisal in psychopathology might result from disturbances at different levels of emotional processing rather than just a particular regional abnormality.
4.2. Experimental task design issues
The absence of convergent regional abnormality due to the reappraisal impairment can be further explained by the taxonomy of experimental designs in neuroimaging studies of reappraisal. Although we only included studies that used the prototypical reappraisal paradigm, some experimental factors and underlying cognitive functions could well affect the neural basis of cognitive reappraisal. Attentional engagement is a relevant example that can be modulated with interrelated exogenous factors such as arousal and valence (Sussman, Heller, Miller, & Mohanty, 2013) and endogenous factors like needs, goals, and motivations (Ochsner & Gross, 2005). Remarkably, reappraising high arousal stimuli involves greater cognitive demands (Ortner, Ste Marie, & Corno, 2016), as appeared in differential prefrontal recruitment (Silvers, Weber, Wager, & Ochsner, 2015). Relatedly, various negative stimuli (e.g., sad, disgusting, and fearful) are reported to reflect similar arousals, but involve different emotion processing networks (Fusar‐Poli et al., 2009; Vytal & Hamann, 2010), suggesting their potentially distinct regulatory circuitries. In particular, disorder‐relevant stimuli are expected to be more salient than irrelevant ones (Hagemann, Straube, & Schulz, 2016). Thus, at least a part of nonreplicable results may stem from uncontrolled moderating factors related to external heterogeneous stimuli and/or internal diverse representations.
Another important factor that may not be well indexed by prototypical reappraisal tasks is the temporal dynamics of reappraisal, which is thought to engage different neural substrates for implementing and maintaining new appraisals (Kalisch, 2009). In a standard reappraisal task, individuals are given instructions on how and when to regulate their emotions. This paradigm usually measures the capability of individuals to implement reappraisal‐related cognitive processes to regulate their emotions (Silvers & Moreira, 2019). However, their tendency to engage regulatory mechanisms without being instructed (Doré, Weber, & Ochsner, 2017) or their ability to keep and monitor reappraised images or thoughts (Kalisch, 2009) are not generally evaluated by these experiments. Therefore, by using a standard reappraisal paradigm, it is not possible to capture the difficulties psychiatric patients may encounter while self‐initiating the regulation process or maintaining the reframed emotional states after applying the reappraisal successfully.
4.3. Heterogeneity of demographic and clinical characteristics of psychiatric patients
In this meta‐analysis, we included adult patients aged between 18 and 60 years in order to exclude the potential effect of adolescent and aged brains on the neural correlates of reappraisal (Ahmed, Bittencourt‐Hewitt, & Sebastian, 2015; Lantrip & Huang, 2017; Nashiro, Sakaki, & Mather, 2012). However, emotion regulation is a dynamic process and may change across the lifespan (Consedine & Mauss, 2014; Livingstone & Isaacowitz, 2021). Thus, a part of the neural heterogeneity can be explained by the fact that inevitable brain changes may occur across different ages even in our restricted age range (Allard & Kensinger, 2014). Other characteristics of patients such as gender (McRae, Ochsner, Mauss, Gabrieli, & Gross, 2008; Whittle, Yücel, Yap, & Allen, 2011), medication status (Roiser & Sahakian, 2013), and severity of their illness (Dixon et al., 2020; Stephanou et al., 2017) can also be confounders for our divergent findings (Table 1). Additionally, heterogeneity of psychiatric disorders (Feczko et al., 2019), which are expressed both across diagnostic criteria and underlying neural substrates can be another source of inconsistency. For example, major depressive disorder is a highly heterogeneous syndrome (Lynch, Gunning, & Liston, 2020) that presents itself in a number of variants with different somatic/emotional/cognitive and clinical states (Drysdale et al., 2017; Tokuda et al., 2018). Specifically, in the case of emotion dysregulation, various trends of neural disturbances have emerged across patients with depression (Rive et al., 2013; Silvers et al., 2014). Similarly, in other psychiatric disorders, such as generalized anxiety disorder and borderline personality disorder several patterns of atypical brain activation during reappraisal performance have been recognized (Silvers et al., 2014). Taken together, these findings suggest that heterogeneity in clinical or demographic characteristics of patients may importantly contribute to the inconsistent findings regarding the neural correlates of impaired reappraisal.
4.4. Flexible methodology and publication bias
Methodological flexibility in neuroimaging studies (e.g., image acquisition, preprocessing, and analysis pipeline; Bowring, Maumet, & Nichols, 2019; Masouleh, Eickhoff, Hoffstaedter, Genon, & Initiative, 2019) could also explain our null findings. The noticeable effects of analytical variability on the results of neuroimaging studies and their interpretation have been indicated in a recent neuroimaging study in which 70 independent teams analyzed the same dataset and even no two teams followed identical analysis workflows (Botvinik‐Nezer et al., 2020). As a relevant example in our meta‐analysis, the different approaches that are employed for multiple testing adjustment might be a potential reason for our nonreplicable findings (Table 1).
In addition to the lack of a validated analytical workflow, positive‐results bias or tendency to publish significant findings is another potential explanation for our nonreplicable results (Jennings & van Horn, 2012). Moreover, when nonsignificant results are published, they are not entered in ALE meta‐analyses (Müller et al., 2018). So, the insensitivity of ALE to nonsignificant results increases the publication bias. Accordingly, we had to exclude 22 eligible studies because of their null findings (Table S1), despite knowing the importance of their valuable results (Mervis, 2014). Thus, at least in some cases, the identified reappraisal disturbances in clinical populations could have resulted from a biased overestimation. For example, regarding depression, there is evidence for the intact neural underpinning of reappraisal (Davis, Foland‐Ross, & Gotlib, 2018; Doré et al., 2018; Loeffler et al., 2019; Rubin‐Falcone et al., 2018) or at least effective implementation of reappraisal when patients are explicitly trained to do so (Ebneabbasi et al., 2021; Liu & Thompson, 2017). Hence, some of the observed heterogeneity in reappraisal literature might be due to methodological diversity or publication bias.
4.5. Collation with previous meta‐analyses
Following the best‐practice guideline for neuroimaging meta‐analysis (Müller et al., 2018; Tahmasian et al., 2019) and the rigorous methodological approach, our null result is expected to reflect a representation of the existing reappraisal studies across psychiatric disorders and should not be attributable to a lack of statistical power or methodological issues. Up to now, three meta‐analyses have been performed on the functional organization of reappraisal in psychiatrically ill populations (McTeague et al., 2020; Picó‐Pérez et al., 2017; Wang et al., 2018). Two of these meta‐analyses are conducted on specific categories of disorders including a combination of mood and anxiety disorders (Picó‐Pérez et al., 2017) with a higher proportion of mood disorders (9/4), and a range of anxiety disorders (Wang et al., 2018). Although both of these meta‐analyses have yielded convergence of brain abnormalities across their included studies, they do not indicate consistent findings compared with each other. The heterogeneity of their findings supports our null result, especially, when we restricted the analysis to only those experiments representing the merged category of mood and anxiety disorders (Table 2). However, our study has some differences compared with these meta‐analyses that make the comparison between the results difficult. Firstly, these meta‐analyses used the effect size signed differential mapping (ES‐SDM) method, which is statistically more lenient than ALE (Müller et al., 2018). Secondly, these studies performed the analyses on a low number of studies (13 and 11, respectively), and each of them included two nonsignificant studies. Thus, their findings of significant convergence might be driven by only a few experiments. Thirdly, due to the nature of ALE method, we did not have enough experiments to perform analysis on each category of mood and anxiety disorders (Table 2) to see if we could replicate their disorder‐specific findings. Lastly, our search was not restricted to mood and anxiety disorders, and thereby, additional relevant disorders were covered. The third transdiagnostic meta‐analysis (McTeague et al., 2020) was performed on a pool of 18 studies including patients with various psychiatric disorders. This study found a consistent brain abnormality located in the right ventrolateral prefrontal cortex. Despite adhering to the same analytic method (i.e., ALE), we did not replicate their result, indicating that by increasing the number of studies, as well as covering additional relevant disorders (i.e., borderline personality disorder, premenstrual depressive disorder, and gambling disorder), the obtained consistency was not observed anymore. Of note, having included two regulation studies other than reappraisal may have also influenced their study results. However, similar to our study they did not find significant results for the increased/decreased analyses, which may indicate the fragility of their finding for the pooled analysis. We additionally explored the role of regulation goal and stimulus valence both separately and in combination, as well as the effect of homogeneity of disorders by narrowing down the spectrum of included disorders in three subsequent steps to see where we can get transdiagnostic patterns of disturbances. Collectively, none of our complementary analyses yielded significant findings.
4.6. Limitations and recommendations for future studies
Our study has some limitations as well. First, the number of patients in the included studies differed substantially across the included psychiatric groups. The disproportionate share of coordinates, therefore, may affect the sensitivity of results and may lead to overemphasizing the larger diagnostic groups (e.g., major depressive disorder). Second, none of the particular diagnostic groups reached the minimum number of experiments to obtain sufficient power for ALE analysis, which forecloses further representation of their pathologically related differences in brain activation. And third, 22 eligible studies with nonsignificant group comparison results were excluded due to the insensitivity of ALE to nonsignificant results, which may have affected the robustness of our findings. These substantial limitations restrict the generalizability of our null findings and emphasize the need for further original studies on various psychiatric patients using larger sample sizes and standard unbiased methodologies, ideally through collaborations to ameliorate site idiosyncrasies, as well as sharing data openly to allow replication and future integration.
Furthermore, to differentiate a true lack of localized consistency from clinical/methodological divergence, we propose the following recommendations for future clinical neuroimaging studies: (a) investigate the neural correlates of model‐driven/stage‐based regulatory dysfunctions in clinical populations; (b) design the experiments considering the temporal dynamics of the reappraisal process (i.e., initiation, implementation, and maintenance), and experimental moderating factors such as stimulus features (e.g., valence, arousal, relevancy to disorder); (c) report clinical (e.g., comorbidity, medication, age/gender, symptom severity) as well as methodological (e.g., preprocessing, software and analysis pipeline) characteristics for replication feasibility; and, (d) utilize stringent statistical thresholds to minimize the potential nonreplicable spurious results. Moreover, we recommend future transdiagnostic meta‐analyses to use other techniques such as hierarchical clustering (Morawetz et al., 2020) or psychophysiological interaction (Berboth & Morawetz, 2021) analyses to investigate the regulation disruptions beyond regional disturbances, if enough experiments are available.
5. CONCLUSION
The present meta‐analysis demonstrated that the existing literature on emotion dysregulation has not yielded consistent, localized, and cross‐cutting neural abnormality during reappraisal performance. We highlighted the distinct psychopathology of impaired reappraisal across different clinical populations as well as divergent experimental, clinical, and methodological factors that could explain our null results. Our transdiagnostic neuroimaging meta‐analysis highlights the importance of simultaneous evaluation of psychiatric disorders in order to construct a multilevel understanding of neuropsychopathology (Barch, 2020; Fusar‐Poli et al., 2019). Even though a transdiagnostic approach generally helps to map the commonalities of psychiatric disorders (Goodkind et al., 2015; McTeague et al., 2017; McTeague et al., 2020; Sha, Wager, Mechelli, & He, 2019; Yaple, Tolomeo, & Rongjun, 2021; Zhang et al., 2016), our study underscores the complexity of studying the neural abnormalities related to higher‐order cognitive processes including reappraisal.
CONFLICT OF INTERESTS
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGMENTS
Author SBE was supported by the Deutsche Forschungsgemeinschaft (DFG) (EI 816/21‐1), the National Institute of Mental Health (R01‐MH074457), and the European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement No. 945539 (HBP SGA3).
Khodadadifar, T. , Soltaninejad, Z. , Ebneabbasi, A. , Eickhoff, C. R. , Sorg, C. , Van Eimeren, T. , Vogeley, K. , Zarei, M. , Eickhoff, S. B. , & Tahmasian, M. (2022). In search of convergent regional brain abnormality in cognitive emotion regulation: A transdiagnostic neuroimaging meta‐analysis. Human Brain Mapping, 43(4), 1309–1325. 10.1002/hbm.25722
Tina Khodadadifar and Zahra Soltaninejad contributed equally to this study.
Funding information European Union's Horizon 2020 Research and Innovation Programme, Grant/Award Number: 945539; National Institute of Mental Health, Grant/Award Number: R01‐MH074457; Deutsche Forschungsgemeinschaft (DFG), Grant/Award Number: EI 816/21‐1
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ahmed, S. P. , Bittencourt‐Hewitt, A. , & Sebastian, C. L. (2015). Neurocognitive bases of emotion regulation development in adolescence. Developmental Cognitive Neuroscience, 15, 11–25. 10.1016/j.dcn.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albein‐Urios, N. , Verdejo‐Román, J. , Asensio, S. , Soriano‐Mas, C. , Martínez‐González, J. M. , & Verdejo‐García, A. (2014). Re‐appraisal of negative emotions in cocaine dependence: Dysfunctional corticolimbic activation and connectivity. Addiction Biology, 19, 415–426. 10.1111/j.1369-1600.2012.00497.x [DOI] [PubMed] [Google Scholar]
- Aldao, A. (2016). Introduction to the special issue: emotion regulation as a transdiagnostic process. Cognitive Therapy and Research, 40(3), 257–261. 10.1007/s10608-016-9764-2 [DOI] [Google Scholar]
- Aldao, A. , Nolen‐Hoeksema, S. , & Schweizer, S. (2010). Emotion‐regulation strategies across psychopathology: A meta‐analytic review. Clinical Psychology Review, 30, 217–237. 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Allard, E. S. , & Kensinger, E. A. (2014). Age‐related differences in functional connectivity during cognitive emotion regulation. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 69(6), 852–860. 10.1093/geronb/gbu108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, T. M. , Ramsawh, H. J. , Campbell‐Sills, L. , Paulus, M. P. , & Stein, M. B. (2013). Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychological Medicine, 43, 1475–1486. 10.1017/s0033291712002383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch, D. M. (2020). What does it mean to be transdiagnostic and how would we know?. American Journal of Psychiatry, 177(5), 370–372. 10.1176/appi.ajp.2020.20030243 [DOI] [PubMed] [Google Scholar]
- Beauchaine, T. P. , & Cicchetti, D. (2019). Emotion dysregulation and emerging psychopathology: A transdiagnostic, transdisciplinary perspective. Development and Psychopathology, 31, 799–804. 10.1017/s0954579419000671 [DOI] [PubMed] [Google Scholar]
- Berboth, S. , & Morawetz, C. (2021). Amygdala‐prefrontal connectivity during emotion regulation: A meta‐analysis of psychophysiological interactions. Neuropsychologia, 153, 107767. 10.1016/j.neuropsychologia.2021.107767 [DOI] [PubMed] [Google Scholar]
- Blair, K. S. , Geraci, M. , Smith, B. W. , Hollon, N. , DeVido, J. , Otero, M. , … Pine, D. S. (2012). Reduced dorsal anterior cingulate cortical activity during emotional regulation and top‐down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biological Psychiatry, 72, 476–482. 10.1016/j.biopsych.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinik‐Nezer, R. , Holzmeister, F. , Camerer, C. F. , Dreber, A. , Huber, J. , Johannesson, M. , … Schonberg, T. (2020). Variability in the analysis of a single neuroimaging dataset by many teams. Nature, 582, 84–88. 10.1038/s41586-020-2314-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowring, A. , Maumet, C. , & Nichols, T. E. (2019). Exploring the impact of analysis software on task fMRI results. Human Brain Mapping, 40, 3362–3384. 10.1002/hbm.24603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehl, A.‐K. , Kohn, N. , Schene, A. H. , & Fernández, G. (2020). A mechanistic model for individualised treatment of anxiety disorders based on predictive neural biomarkers. Psychological Medicine, 50, 727–736. 10.1017/s0033291720000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullis, J. R. , Boettcher, H. , Sauer‐Zavala, S. , Farchione, T. J. , & Barlow, D. H. (2019). What is an emotional disorder? A transdiagnostic mechanistic definition with implications for assessment, treatment, and prevention. Clinical Psychology: Science and Practice, 26, e12278. 10.1111/cpsp.12278 [DOI] [Google Scholar]
- Butler, O. , Willmund, G. , Gleich, T. , Zimmermann, P. , Lindenberger, U. , Gallinat, J. , & Kühn, S. (2018). Cognitive reappraisal and expressive suppression of negative emotion in combat‐related posttraumatic stress disorder: A functional MRI study. Cognitive Therapy and Research, 43, 236–246. 10.1111/cpsp.12278 [DOI] [Google Scholar]
- Campbell‐Sills, L. , Simmons, A. N. , Lovero, K. L. , Rochlin, A. A. , Paulus, M. P. , & Stein, M. B. (2011). Functioning of neural systems supporting emotion regulation in anxiety‐prone individuals. NeuroImage, 54, 689–696. 10.1016/j.neuroimage.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cludius, B. , Mennin, D. , & Ehring, T. (2020). Emotion regulation as a transdiagnostic process. Emotion, 20(1), 37. 10.1037/emo0000646 [DOI] [PubMed] [Google Scholar]
- Consedine, S. , & Mauss, I. (2014). Tasks, capacities, and tactics: A skill‐based conceptualization of emotion regulation across the lifespan. The Oxford Handbook of Emotion, Social Cognition, and Problem Solving in Adulthood. 1 (pp. 142–154). Oxford, England: Oxford University Press. [Google Scholar]
- D'Agostino, A. , Covanti, S. , Monti, M. R. , & Starcevic, V. (2017). Reconsidering emotion dysregulation. Psychiatric Quarterly, 88(4), 807–825. 10.1007/s11126-017-9499-6 [DOI] [PubMed] [Google Scholar]
- Davis, E. G. , Foland‐Ross, L. C. , & Gotlib, I. H. (2018). Neural correlates of top‐down regulation and generation of negative affect in major depressive disorder. Psychiatry Research: Neuroimaging, 276, 1–8. 10.1016/j.pscychresns.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degasperi, G. , Cristea, I. A. , Di Rosa, E. , Costa, C. , & Gentili, C. (2021). Parsing variability in borderline personality disorder: A meta‐analysis of neuroimaging studies. Translational Psychiatry, 11(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, M. L. , Moodie, C. A. , Goldin, P. R. , Farb, N. , Heimberg, R. G. , & Gross, J. J. (2020). Emotion regulation in social anxiety disorder: Reappraisal and acceptance of negative self‐beliefs. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(1), 119–129. 10.1016/j.bpsc.2019.07.009 [DOI] [PubMed] [Google Scholar]
- Doré, B. P. , Rodrik, O. , Boccagno, C. , Hubbard, A. , Weber, J. , Stanley, B. , … Ochsner, K. N. (2018). Negative autobiographical memory in depression reflects elevated amygdala‐hippocampal reactivity and hippocampally associated emotion regulation. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(4), 358–366. [DOI] [PubMed] [Google Scholar]
- Doré, B. P. , Weber, J. , & Ochsner, K. N. (2017). Neural predictors of decisions to cognitively control emotion. Journal of Neuroscience, 37(10), 2580–2588. 10.1523/jneurosci.2526-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryman, M. T. , & Heimberg, R. G. (2018). Emotion regulation in social anxiety and depression: A systematic review of expressive suppression and cognitive reappraisal. Clinical Psychology Review, 65, 17–42. [DOI] [PubMed] [Google Scholar]
- Drysdale, A. T. , Grosenick, L. , Downar, J. , Dunlop, K. , Mansouri, F. , Meng, Y. , … Etkin, A. (2017). Resting‐state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine, 23(1), 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneabbasi, A. , Mahdipour, M. , Nejati, V. , Li, M. , Liebe, T. , Colic, L. , … Walter, M. (2021). Emotion processing and regulation in major depressive disorder: A 7T resting‐state fMRI study. Human Brain Mapping, 42(3), 797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood estimation meta‐analysis revisited. NeuroImage, 59(3), 2349–2361. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Nichols, T. E. , Laird, A. R. , Hoffstaedter, F. , Amunts, K. , Fox, P. T. , Bzdok, D. , Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70–85. 10.1016/j.neuroimage.2016.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , Büchel, C. , & Gross, J. J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693–700. 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- Feczko, E. , Miranda‐Dominguez, O. , Marr, M. , Graham, A. M. , Nigg, J. T. , & Fair, D. A. (2019). The heterogeneity problem: approaches to identify psychiatric subtypes. Trends in Cognitive Sciences, 23(7), 584–601. 10.1016/j.tics.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez, K. C. , Jazaieri, H. , & Gross, J. J. (2016). Emotion regulation: a transdiagnostic perspective on a new RDoC domain. Cognitive Therapy and Research, 40(3), 426–440. 10.1007/s10608-016-9772-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, B. Q. , Karnilowicz, H. R. , & Mauss, I. B. (2017). Understanding reappraisal as a multicomponent process: The psychological health benefits of attempting to use reappraisal depend on reappraisal success. Emotion, 17(6), 905–911. 10.1037/emo0000310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar‐Poli, P. , Placentino, A. , Carletti, F. , Landi, P. , Allen, P. , Surguladze, S. , Benedetti, F. , Abbamonte, M. , Gasparotti, R. , Barale, F. , Perez, J. , McGuire, P. , & Politi, P. (2009). Functional atlas of emotional faces processing: A voxel‐based meta‐analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry Neuroscience, 34(6), 418–432. [PMC free article] [PubMed] [Google Scholar]
- Fusar‐Poli, P. , Solmi, M. , Brondino, N. , Davies, C. , Chae, C. , Politi, P. , … McGuire, P. (2019). Transdiagnostic psychiatry: A systematic review. World Psychiatry, 18(2), 192–207. 10.1002/wps.20631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl, K. , Tahmasian, M. , Eickhoff, S. B. , & van Eimeren, T. (2019). Imaging executive functions in Parkinsons disease: An activation likelihood estimation meta‐analysis. Parkinsonism & Related Disorders, 63, 137–142. [DOI] [PubMed] [Google Scholar]
- Goldin, P. R. , Manber‐Ball, T. , Werner, K. , Heimberg, R. , & Gross, J. J. (2009). Neural mechanisms of cognitive reappraisal of negative self‐beliefs in social anxiety disorder. Biological Psychiatry, 66(12), 1091–1099. 10.1016/j.biopsych.2009.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin, P. R. , Manber, T. , Hakimi, S. , Canli, T. , & Gross, J. J. (2009). Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66, 170–180. 10.1001/archgenpsychiatry.2008.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind, M. , Eickhoff, S. B. , Oathes, D. J. , Jiang, Y. , Chang, A. , Jones‐Hagata, L. B. , … Korgaonkar, M. S. (2015). Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry, 72(4), 305–315. 10.1001/jamapsychiatry.2014.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. J. , & Malhi, G. S. (2006). Neural mechanisms of the cognitive control of emotion. Acta Neuropsychiatrica, 18(3‐4), 144–153. 10.1111/j.1601-5215.2006.00149.x [DOI] [PubMed] [Google Scholar]
- Greening, S. G. , Osuch, E. A. , Williamson, P. C. , & Mitchell, D. G. V. (2014). The neural correlates of regulating positive and negative emotions in medication‐free major depression. Social Cognitive and Affective Neuroscience, 9(5), 628–637. 10.1093/scan/nst027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. J. (1998). Antecedent‐and response‐focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74(1), 224. 10.1037/0022-3514.74.1.224 [DOI] [PubMed] [Google Scholar]
- Gross, J. J. , & Jazaieri, H. (2014). Emotion, emotion regulation, and psychopathology: An affective science perspective. Clinical Psychological Science, 2(4), 387–401. [Google Scholar]
- Gross, J. J. , Uusberg, H. , & Uusberg, A. (2019). Mental illness and well‐being: An affect regulation perspective. World Psychiatry, 18(2), 130–139. 10.1002/wps.20618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann, J. , Straube, T. , & Schulz, C. (2016). Too bad: Bias for angry faces in social anxiety interferes with identity processing. Neuropsychologia, 84, 136–149. 10.1016/j.neuropsychologia.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Heller, A. S. , Johnstone, T. , Shackman, A. J. , Light, S. N. , Peterson, M. J. , Kolden, G. G. , … Davidson, R. J. (2009). Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto‐striatal brain activation. Proceedings of the National Academy of Sciences, 106(52), 22445–22450. 10.1073/pnas.0910651106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, T. , Zhang, D. , Wang, J. , Mistry, R. , Ran, G. , & Wang, X. (2014). Relation between emotion regulation and mental health: A meta‐analysis review. Psychological Reports, 114(2), 341–362. 10.2466/03.20.pr0.114k22w4 [DOI] [PubMed] [Google Scholar]
- Huang, X. , Rootes‐Murdy, K. , Bastidas, D. M. , Nee, D. E. , & Franklin, J. C. (2020). Brain differences associated with self‐injurious thoughts and behaviors: A meta‐analysis of neuroimaging studies. Scientific Reports, 10(1), 1–13. 10.1038/s41598-020-59490-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri, D. , Moser, D. A. , Doucet, G. E. , Luber, M. J. , Rasgon, A. , Lee, W. H. , … Frangou, S. (2020). Shared neural phenotypes for mood and anxiety disorders: A meta‐analysis of 226 task‐related functional imaging studies. JAMA Psychiatry, 77(2), 172–179. 10.1001/jamapsychiatry.2019.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaieri, H. , Urry, H. L. , & Gross, J. J. (2013). Affective disturbance and psychopathology: An emotion regulation perspective. Journal of Experimental Psychopathology, 4, 584–599. 10.5127/jep.030312 [DOI] [Google Scholar]
- Jennings, R. G. , & van Horn, J. D. (2012). Publication bias in neuroimaging research: Implications for meta‐analyses. Neuroinformatics, 10(1), 67–80. 10.1007/s12021-011-9125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone, T. , van Reekum, C. M. , Urry, H. L. , Kalin, N. H. , & Davidson, R. J. (2007). Failure to regulate: counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. Journal of Neuroscience, 27(33), 8877–8884. 10.1523/jneurosci.2063-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch, R. (2009). The functional neuroanatomy of reappraisal: Time matters. Neuroscience & Biobehavioral Reviews, 33(8), 1215–1226. 10.1016/j.neubiorev.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Kebets, V. , Favre, P. , Houenou, J. , Polosan, M. , Perroud, N. , Aubry, J.‐M. , Van De Ville, D. , & Piguet, C. (2021). Fronto‐limbic neural variability as a transdiagnostic correlate of emotion dysregulation. Translational Psychiatry, 11(1), 1–8. 10.1038/s41398-021-01666-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. Y. , Bigman, Y. , & Tamir, M. (2015). Emotional regulation. International Encyclopedia of the Social & Behavioral Sciences. 2(452–456). Oxford, England: Elsevier. 10.1016/B978-0-08-097086-8.25055-1 [DOI] [Google Scholar]
- Koenigsberg, H. W. , Fan, J. , Ochsner, K. N. , Liu, X. , Guise, K. G. , Pizzarello, S. , … Goodman, M. (2009). Neural correlates of the use of psychological distancing to regulate responses to negative social cues: A study of patients with borderline personality disorder. Biological Psychiatry, 66(9), 854–863. 10.1016/j.biopsych.2009.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, J. L. , Tordesillas‐Gutiérrez, D. , Martinez, M. , Salinas, F. , Evans, A. , Zilles, K. , … Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Human Brain Mapping, 28(11), 1194–1205. 10.1002/hbm.20345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantrip, C. , & Huang, J. H. (2017). Cognitive control of emotion in older adults: a review. Clinical Psychiatry, 03(01), 9. 10.21767/2471-9854.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabi, D. I. , van der Meer, L. , Pijnenborg, G. H. M. , Ćurčić‐Blake, B. , & Aleman, A. (2018). Insight and emotion regulation in schizophrenia: A brain activation and functional connectivity study. NeuroImage: Clinical, 20, 762–771. 10.1016/j.nicl.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. Y. , & Thompson, R. J. (2017). Selection and implementation of emotion regulation strategies in major depressive disorder: An integrative review. Clinical Psychology Review, 57, 183–194. 10.1016/j.cpr.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Livingstone, K. M. , & Isaacowitz, D. M. (2021). Age and emotion regulation in daily life: Frequency, strategies, tactics, and effectiveness. Emotion, 21(1), 39–51. 10.1037/emo0000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler, L. A. , Kathrin, T. D. , Satterthwaite, U. H. , Schneider, F. , Radke, S. , & Derntl, B. (2019). Attention control and its emotion‐specific association with cognitive emotion regulation in depression. Brain Imaging and Behavior, 13(6), 1766–1779. 10.1007/s11682-019-00174-9 [DOI] [PubMed] [Google Scholar]
- Lynch, C. J. , Gunning, F.M. , & Liston, C. (2020). Causes and consequences of diagnostic heterogeneity in depression: Paths to discovering novel biological depression subtypes. Biological Psychiatry, 88(1), 83–94. 10.1016/j.biopsych.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Malloy‐Diniz, L.F. , Miranda, D.M. , & Grassi‐Oliveira, R. (2017). Editorial: executive functions in psychiatric disorders. Frontiers in Psychology, 8, 10.3389/fpsyg.2017.01461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharabian Masouleh, S. , Eickhoff, S. B. , Hoffstaedter, F. , Genon, S. S , (2019). Empirical examination of the replicability of associations between brain structure and psychological variables. eLife, 8, 10.7554/elife.43464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae, K. , Jacobs, S. E. , Ray, R. D. , John, O. P. , Gross, J. J. (2012). Individual differences in reappraisal ability: Links to reappraisal frequency, well‐being, and cognitive control. Journal of Research in Personality, 46(1), 2–7. 10.1016/j.jrp.2011.10.003 [DOI] [Google Scholar]
- McRae, K. , Ochsner, K. N. , Mauss, I. B. , Gabrieli, J. J. D. , Gross, J. J. (2008). Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations, 11(2), 143–162. 10.1177/1368430207088035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague, L. M. , Goodkind, M. S. , & Etkin, A. (2016). Transdiagnostic impairment of cognitive control in mental illness. Journal of Psychiatric Research, 83, 37–46. 10.1016/j.jpsychires.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague, L. M. , Huemer, J. , Carreon, D. M. , Jiang, Y. , Eickhoff, S. B. , & Etkin, A. (2017). Identification of common neural circuit disruptions in dognitive dontrol dcross psychiatric disorders. American Journal of Psychiatry, 174(7), 676–685. 10.1176/appi.ajp.2017.16040400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague, L. M. , Rosenberg, B. M. , Lopez, J. W. , Carreon, D. M. , Huemer, J. , Jiang, Y. , Chick, C. F. , Eickhoff, S. B. , & Etkin, A. (2020). Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. American Journal of Psychiatry, 177(5), 411–421. 10.1176/appi.ajp.2019.18111271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis, J. (2014). Why null results rarely see the light of day. Science, 345(6200), 992–992. 10.1126/science.345.6200.992 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. , (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz, C. , Riedel, M. C. , Salo, T. , Berboth, S. , Eickhoff, S. B. , Laird, A. R. , & Kohn, N. (2020). Multiple large‐scale neural networks underlying emotion regulation. Neuroscience & Biobehavioral Reviews, 116, 382–395. 10.1016/j.neubiorev.2020.07.001 [DOI] [PubMed] [Google Scholar]
- Morris, R. W. , Sparks, A. , Mitchell, P. B. , Weickert, C. S. , & Green, M. J. (2012). Lack of cortico‐limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Translational Psychiatry, 2(3), e90–e90. 10.1038/tp.2012.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, V. I. , Cieslik, E. C. , Laird, A. R. , Fox, P. T. , Radua, J. , Mataix‐Cols, D. , Tench, C. R. , Yarkoni, T. , Nichols, T. E. , Turkeltaub, P. E. , & Wager, T. D. , Eickhoff, S. B. (2018). Ten simple rules for neuroimaging meta‐analysis. Neuroscience & Biobehavioral Reviews, 84, 151–161. 10.1016/j.neubiorev.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, V. I. , Cieslik, E. C. , Serbanescu, I. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2017). Altered brain activity in unipolar depression revisited. JAMA Psychiatry, 74(1), 47. 10.1001/jamapsychiatry.2016.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro, K. , Sakaki, M. , & Mather, M. (2012). Age differences in brain activity during emotion processing: Reflections of age‐related decline or increased emotion regulation. Gerontology, 58(2), 156–163. 10.1159/000328465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas, J. F. , Contreras‐Rodríguez, O. , Verdejo‐Román, J. , Perandrés‐Gómez, A. , Albein‐Urios, N. , Verdejo‐García, A. , & Perales, J. C. (2017). Trait and neurobiological underpinnings of negative emotion regulation in gambling disorder. Addiction, 112(6), 1086–1094. [DOI] [PubMed] [Google Scholar]
- New, A. S. , Fan, J. , Murrough, J. W. , Liu, X. , Liebman, R. E. , Guise, K. G. , … Charney, D. S. (2009). A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biological Psychiatry, 66(7), 656–664. 10.1016/j.biopsych.2009.05.020 [DOI] [PubMed] [Google Scholar]
- Nezlek, J. B. , & Kuppens, P. (2008). Regulating positive and negative emotions in daily life. Journal of Personality, 76, 561–580. 10.1111/j.1467-6494.2008.00496.x [DOI] [PubMed] [Google Scholar]
- Nickl‐Jockschat, T. , Janouschek, H. , Eickhoff, S. B. , & Eickhoff, C. R. (2015). Lack of meta‐analytic evidence for an impact of COMT Val158Met genotype on brain activation during working memory tasks. Biological Psychiatry, 78(11), e43–e46. 10.1016/j.biopsych.2015.02.030 [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Ray, R. D. , Cooper, J. C. , Robertson, E. R. , Chopra, S. , Gabrieli, J. D. E. , & Gross, J. J. (2004). For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. NeuroImage, 23(2), 483–499. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1.(1), E1–E24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öner, S. (2018). Neural substrates of cognitive emotion regulation: a brief review. Psychiatry and Clinical Psychopharmacology, 28(1), 91–96. 10.1080/24750573.2017.1407563 [DOI] [Google Scholar]
- Ortner, C. N. , Ste Marie, M. , & Corno, D. (2016). Cognitive costs of reappraisal depend on both emotional stimulus intensity and individual differences in habitual reappraisal. PLoS One, 11, e0167253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C. , Rosenblat, J. D. , Lee, Y. , Pan, Z. , Cao, B. , Iacobucci, M. , & McIntyre, R. S. (2019). The neural systems of emotion regulation and abnormalities in major depressive disorder. Behavioural Brain Research, 367, 181–188. [DOI] [PubMed] [Google Scholar]
- Petersen, N. , Ghahremani, D. G. , Rapkin, A. J. , Berman, S. M. , Liang, L. , & London, E. D. (2018). Brain activation during emotion regulation in women with premenstrual dysphoric disorder. Psychological Medicine, 48(11), 1795–1802. 10.1017/s0033291717003270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó‐Pérez, M. , Radua, J. , Steward, T. , Menchón, J. M. , & Soriano‐Mas, C. (2017). Emotion regulation in mood and anxiety disorders: A meta‐analysis of fMRI cognitive reappraisal studies. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 79, 96–104. 10.1016/j.pnpbp.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Radke, S. , Hoffstaedter, F. , Löffler, L. , Kogler, L. , Schneider, F. , Blechert, J. , & Derntl, B. (2018). Imaging the up's and down's of emotion regulation in lifetime depression. Brain Imaging and Behavior, 12(1), 156–167. [DOI] [PubMed] [Google Scholar]
- Reinecke, A. , Nicola Filippini, C. , Berna, D. G. W. , Hanson, B. , Cooper, M. J. , Taggart, P. , & Harmer, C. J. (2015). Effective emotion regulation strategies improve fMRI and ECG markers of psychopathology in panic disorder: Implications for psychological treatment action. Translational Psychiatry, 5, e673. 10.1038/tp.2015.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive, M. M. , van Rooijen, G. , Veltman, D. J. , Phillips, M. L. , Schene, A. H. , & Ruhé, H. G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(10), 2529–2553. [DOI] [PubMed] [Google Scholar]
- Roiser, J. P. , & Sahakian, B. J. (2013). Hot and cold cognition in depression. CNS Spectrums, 18(3), 139–149. 10.1017/s1092852913000072 [DOI] [PubMed] [Google Scholar]
- Rubin‐Falcone, H. , Weber, J. , Kishon, R. , Ochsner, K. , Delaparte, L. , Doré, B. , … Miller, J. M. (2018). Longitudinal effects of cognitive behavioral therapy for depression on the neural correlates of emotion regulation. Psychiatry Research: Neuroimaging, 271, 82–90. 10.1016/j.pscychresns.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi, A. , Mohammadi, E. , Zarei, M. , Eickhoff, S. B. , & Tahmasian, M. (2021). Structural and functional neuroimaging of late‐life depression: A coordinate‐based meta‐analysis. Brain Imaging and Behavior, 1–14. 10.1007/s11682-021-00494-9 [DOI] [PubMed] [Google Scholar]
- Samea, F. , Soluki, S. , Nejati, V. , Zarei, M. , Cortese, S. , Eickhoff, S. B. , … Eickhoff, C. R. (2019). Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta‐analysis of 96 structural and functional studies. Neuroscience & Biobehavioral Reviews, 100, 1–8. 10.1016/j.neubiorev.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel, B. J. , & Tang, D. (2015). Individual differences in executive functioning and their relationship to emotional processes and responses. Current Directions in Psychological Science, 24(2), 93–98. 10.1177/0963721414555178 [DOI] [Google Scholar]
- Schulze, L. , Domes, G. , Krüger, A. , Berger, C. , Fleischer, M. , Prehn, K. , … Herpertz, S. C. (2011). Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biological Psychiatry, 69(6), 564–573. 10.1016/j.biopsych.2010.10.025 [DOI] [PubMed] [Google Scholar]
- Schulze, L. , Schulze, A. , Renneberg, B. , Schmahl, C. , & Niedtfeld, I. (2019). Neural correlates of affective disturbances: A comparative meta‐analysis of negative affect processing in borderline personality disorder, major depressive disorder, and posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(3), 220–232. 10.1016/j.bpsc.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Sha, Z. , Wager, T. D. , Mechelli, A. , & He, Y. (2019). Common dysfunction of large‐scale neurocognitive networks across psychiatric disorders. Biological Psychiatry, 85(5), 379–388. 10.1016/j.biopsych.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Sheline, Y. I. , Barch, D. M. , Price, J. L. , Rundle, M. M. , Neil Vaishnavi, S. , Snyder, A. Z. , … Raichle, M. E. (2009). The default mode network and self‐referential processes in depression. Proceedings of the National Academy of Sciences, 106(6), 1942–1947. 10.1073/pnas.0812686106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, L. Q. , Zhao, P. W. , Ma, H. R. , Yuan, C. H. , Zhong, J. G. , Dai, Z. Y. , & Pan, P. L. (2020). A lack of consistent brain grey matter alterations in migraine. Brain, 143, e45. 10.1093/brain/awaa123 [DOI] [PubMed] [Google Scholar]
- Sheppes, G. , Suri, G. , & Gross, J. J. (2015). Emotion regulation and psychopathology. Annual Review of Clinical Psychology, 11, 379–405. 10.1146/annurev-clinpsy-032814-112739 [DOI] [PubMed] [Google Scholar]
- Silvers, J. A. , Buhle, J. T. , & Ochsner, K. N. (2014). The neuroscience of emotion regulation: Basic mechanisms and their role in development, aging, and psychopathology. The Handbook of Cognitive Neuroscience. 1, New York, NY: Oxford University Press. [Google Scholar]
- Silvers, J. A. , & Moreira, J. F. G. (2019). Capacity and tendency: A neuroscientific framework for the study of emotion regulation. Neuroscience Letters, 693, 35–39. 10.1016/j.neulet.2017.09.017 [DOI] [PubMed] [Google Scholar]
- Silvers, J. A. , Weber, J. , Wager, T. D. , & Ochsner, K. N. (2015). Bad and worse: Neural systems underlying reappraisal of high‐and low‐intensity negative emotions. Social Cognitive and Affective Neuroscience, 10(2), 172–179. 10.1093/scan/nsu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, R. , & Lane, R. D. (2015). The neural basis of ones own conscious and unconscious emotional states. Neuroscience & Biobehavioral Reviews, 57, 1–29. 10.1016/j.neubiorev.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Smoski, M. J. , Keng, S.‐L. , Schiller, C. E. , Minkel, J. , & Dichter, G. S. (2013). Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. Journal of Affective Disorders, 151(1), 171–177. 10.1016/j.jad.2013.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada, C. , Angstadt, M. , Daniel Kessler, K. , Phan, L. , Liberzon, I. , Evans, G. W. , … Swain, J. E. (2014). Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. NeuroImage, 89, 110–121. 10.1016/j.neuroimage.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou, K. , Davey, C. G. , Kerestes, R. , Whittle, S. , & Harrison, B. J. (2017). Hard to look on the bright side: Neural correlates of impaired emotion regulation in depressed youth. Social Cognitive and Affective Neuroscience, 12(7), 1138–1148. 10.1093/scan/nsx039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman, T. J. , Heller, W. , Miller, G. A. , & Mohanty, A. (2013). Emotional distractors can enhance attention. Psychological Science, 24(11), 2322–2328. 10.1177/0956797613492774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian, M. , Noori, K. , Samea, F. , Zarei, M. , Spiegelhalder, K. , Eickhoff, S. B. , … Eickhoff, C. R. (2018). A lack of consistent brain alterations in insomnia disorder: An activation likelihood estimation meta‐analysis. Sleep Medicine Reviews, 42, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian, M. , Sepehry, A. A. , Samea, F. , Khodadadifar, T. , Soltaninejad, Z. , Javaheripour, N. , … Eickhoff, C. R. (2019). Practical recommendations to conduct a neuroimaging meta‐analysis for neuropsychiatric disorders. Human Brain Mapping, 40(17), 5142–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian, M. , Zarei, M. , Noori, K. , Khazaie, H. , Samea, F. , Spiegelhalder, K. , … Eickhoff, C. R. (2018). Reply to Hua Liu, HaiCun Shi and PingLei Pan: Coordinate based meta‐analyses in a medium sized literature: Considerations, limitations and road ahead. Sleep Medicine Reviews, 42, 236. 10.1016/j.smrv.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S. F. , & Liberzon, I. (2007). Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Sciences, 11(10), 413–418. [DOI] [PubMed] [Google Scholar]
- Thorsen, A. L. , de Wit, S. J. , de Vries, F. E. , Cath, D. C. , Veltman, D. J. , van der Werf, Y. D. , … van den Heuvel, O. A. (2019). Emotion regulation in obsessive‐compulsive disorder, unaffected siblings, and unrelated healthy control participants. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(4), 352–360. [DOI] [PubMed] [Google Scholar]
- Tokuda, T. , Junichiro Yoshimoto, Y. , Shimizu, G. O. , Takamura, M. , Okamoto, Y. , Yamawaki, S. , & Doya, K. (2018). Identification of depression subtypes and relevant brain regions using a data‐driven approach. Scientific Reports, 8(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, J. D. , Torrisi, S. J. , Lieberman, M. D. , Sugar, C. A. , Bookheimer, S. Y. , & Altshuler, L. L. (2013). Frontal‐amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biological Psychiatry, 73(2), 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully, L. M. , & Niendam, T. A. (2014). Beyond “cold” cognition: Exploring cognitive control of emotion as a risk factor for psychosis. Current Behavioral Neuroscience Reports, 1(3), 170–181. [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Human Brain Mapping, 33(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer, L. , Swart, M. , van der Velde, J. , Pijnenborg, G. , Wiersma, D. , Bruggeman, R. , & Aleman, A. (2014). Neural correlates of emotion regulation in patients with schizophrenia and non‐affected siblings. PLoS One, 9, e99667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zutphen, L. , Siep, N. , Jacob, G. A. , Domes, G. , Sprenger, A. , Willenborg, B. , … Arntz, A. (2018). Always on guard: Emotion regulation in women with borderline personality disorder compared to nonpatient controls and patients with cluster‐C personality disorder. Journal of Psychiatry & Neuroscience, 43(1), 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal, K. , & Hamann, S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: A voxel‐based meta‐analysis. Journal of Cognitive Neuroscience, 22(12), 2864–2885. [DOI] [PubMed] [Google Scholar]
- Wang, H.‐Y. , Zhang, X.‐X. , Si, C.‐P. , Yang, X. , Liu, Q. , Bian, H.‐T. , … Yan, Z.‐R. (2018). Prefrontoparietal dysfunction during emotion regulation in anxiety disorder: A meta‐analysis of functional magnetic resonance imaging studies. Neuropsychiatric Disease and Treatment, 14, 1183. 10.2147/ndt.s165677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Zhou, X. , Dai, Q. , Ji, B. , & Feng, Z. (2017). The role of motivation in cognitive reappraisal for depressed patients. Frontiers in Human Neuroscience, 11, 516. 10.3389/fnhum.2017.00516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, T. L. , Miles, E. , & Sheeran, P. (2012). Dealing with feeling: A meta‐analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138(4), 775. [DOI] [PubMed] [Google Scholar]
- Werner, K. , & Gross, J. J. (2010). Emotion regulation and psychopathology: A conceptual framework. In Kring A. M. & Sloan D. M. (Eds.), Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment (pp. 13–37). New York, NY: Guilford Press. [Google Scholar]
- Whittle, S. , Yücel, M. , Yap, M. B. H. , & Allen, N. B. (2011). Sex differences in the neural correlates of emotion: Evidence from neuroimaging. Biological Psychology, 87(3), 319–333. 10.1016/j.biopsycho.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Xiong, K. , Zhang, Y. , Qiu, M. , Zhang, J. , Sang, L. , Wang, L. , … Li, M. (2013). Negative emotion regulation in patients with posttraumatic stress disorder. PLoS One, 8, e81957. 10.1371/journal.pone.0081957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaple, Z. A. , Tolomeo, S. , & Rongjun, Y. (2021). Mapping working memory‐specific dysfunction using a transdiagnostic approach. NeuroImage: Clinical, 31, 102747. 10.1016/j.nicl.2021.102747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Lin, P. , Shi, H. , Öngür, D. , Auerbach, R. P. , Wang, X. , … Wang, X. (2016). Mapping anhedonia‐specific dysfunction in a transdiagnostic approach: An ALE meta‐analysis. Brain Imaging and Behavior, 10, 920–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Ai, H. , Opmeer, E. M. , Marsman, J.‐B. C. , van der Meer, L. , Ruhé, H. G. , … van Tol, M.‐J. (2020). Distinct temporal brain dynamics in bipolar disorder and schizophrenia during emotion regulation. Psychological Medicine, 50, 413–421. 10.1017/s0033291719000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand, A. , Parvaz, M. A. , & Goldstein, R. Z. (2017). Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage, 151, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv, M. , Goldin, P. R. , Jazaieri, H. , Hahn, K. S. , & Gross, J. J. (2013). Emotion regulation in social anxiety disorder: Behavioral and neural responses to three socio‐emotional tasks. Biology of Mood & Anxiety Disorders, 3, 1–17. 10.1186/2045-5380-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


