Abstract
Chimeric antigen receptor (CAR) T-cell therapy is a dynamic therapy of engineered T-cells targeting neoplastic cells, which offers impressive long-term remissions for aggressive relapsed/refractory hematologic malignancies. However, side effects including severe infections can be life-threatening. Multiple factors, including cytokine release syndrome, B-cell aplasia, and hypogammaglobulinemia, contribute to infection risk. B-cell aplasia is an expected on-target, off-tumor effect of CD19+-targeted CAR T-cells and leads to hypogammaglobulinemia. We review hypogammaglobulinemia observed in the five currently FDA-approved CAR T-cell therapies and other CAR T-cell products evaluated in clinical trials, and discuss hypogammaglobulinemia onset, duration, and immune recovery. We review associations between hypogammaglobulinemia and infections, with a discussion informed by other known B-cell depleting contexts. Differences in hypogammaglobinemia between children and adults are identified. We integrate management strategies for evaluation and immunoglobulin replacement from clinical studies, expert recommendations, and organizational guidelines. Notably, our review also highlights newer CAR T-cell products targeting different B-cell antigens – including BCMA, SLAMF7, and κ light chains. Finally, we identify key areas for future study to mitigate and treat hypogammaglobulinemia resulting from this transformative therapy.
Keywords: Chimeric antigen receptor T-cell therapy, CAR T-cell, hypogammaglobulinemia, B-cell aplasia, infections, IgG, IVIG, immunoglobulin replacement, review
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has dramatically improved the outcomes for patients with relapsed or refractory hematopoietic malignancies that previously had poor prognoses. CAR T-cells utilize an engineered receptor combining an antigen recognition domain with T-cell activation domains to stimulate T cell elimination of neoplastic cells (1,2). Serious adverse side effects including immune related adverse events and severe infections have been associated with CAR T-cell therapy and can be fatal. While infection risk is due to multiple factors, two of the direct side effects of CAR T-cell therapy, B-cell aplasia and hypogammaglobulinemia, can predispose to infections both in the immediate post-infusion period and even years later.
Although hypogammaglobulinemia can be a long-lasting side effect contributing to infection, standardized guidelines for managing hypogammaglobulinemia after CAR T-cell therapy have not been well established. We describe immunoglobulin replacement across studies and summarize an integrated management approach from expert recommendations, experiences from other immunodeficiencies, and organizational guidelines. We propose future directions to further our understanding of hypogammaglobulinemia and strategies to predict and reduce hypogammaglobulinemia utilizing new CAR T-cell approaches and technologies.
Mechanism of B-cell aplasia in CAR T-cell therapy and correlation with tumor responses
B-cell aplasia and hypogammaglobulinemia after CAR T-cell therapy are expected consequences – “on target, off tumor” effects – of CAR T-cell therapy targeting CD19, a transmembrane glycoprotein antigen on malignant B-cells in B-cell lymphomas and leukemias and also on normal B cells (3). B-cell aplasia results from the CAR T-cell attack on B cells, which affects both normal and malignant B-cells expressing CD19, and can be profound and persistent (4–9). The severity and duration of B-cell aplasia can serve as a pharmacodynamic measure of the persistence and functionality of infused CAR T-cells (8).
Temporally, B-cell aplasia occurs around the time of CAR T-cell expansion, within two weeks to one month of infusion (4,10). B-cell aplasia often persists for over 6 months, extending to years (2,6,8,11). However, B-cell recovery is variable across different studies (10,12,13).
Hypogammaglobulinemia: Timing, Variability, and Recovery
Hypogammaglobulinemia has been described following CAR T-cell therapy, with variable incidence. Within the first 90 days post-CAR T-cell infusion (CTI), hypogammaglobulinemia (defined as IgG < 400 mg/dL) was present in 35%, 27%, and 46% of adult patients between days 15–30, 31–60, and 61–90 post-CTI, respectively (10). At 90 days or later after CAR T-cell infusion, 67% of adults had hypogammaglobulinemia at some point, either with IgG < 400 mg/dL or requiring at least one intravenous immunoglobulin (IVIG) infusion (14). Hypogammaglobulinemia has been reported to last up to four years (6,9). Similarly, 40% of adults treated with axicabtagene ciloleucel experienced hypogammaglobulinemia overall (13), whereas hypogammaglobulinemia occurred in only 14% for lisocabtagene maraleucel (15). Table 1 presents the hypogammaglobulinemia and infection incidence and management of hypogammaglobulinemia associated with the five currently U.S. Food and Drug Administration (FDA)-approved CAR T-cell treatments.
Table 1. FDA-approved CAR T-cell therapies and hypogammaglobulinemia and infections.
Data for each of the FDA-approved CAR T-cell products, including hypogammaglobulinemia (all grades and grades ≥ 3), infections (all grades and grades ≥ 3), infection types, treatments, population and malignancy demographics, and additional notes on each product. Data gathered from applications submitted to the FDA.
| Hypogammaglobulinemia | Infections | Infection Types | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Target/Agent/Manufacturer* | Population / Malignancy | All Grades, n (%) | Grade ≥ 3, n (%) | All Grades N (%) | Grade ≥ 3, N (%) | Infection Types | All Grades N (%) | Grade ≥ 3, N (%) | Hypogammaglobulinemia treatment | Notes |
| CD19 CAR-T, Tisagenlecleucel (Kymriah, Novartis) 45 | Ages 3–23 years (safety set) relapsed/refractory B-cell precursor ALL | 20 (29%) (Post-CAR-T time period) | 3 (4%) (Post-CAR-T time period) | 40 (59%) | 19 (27%) | Not specified | % not specified | % not specified | Patients maintained on supplemental treatment with intravenous gamma globulin (IVIG) post-tisagenlecleucel. Prolonged hypogammaglobulinemia necessitated the use of routine infusions of IVIG. | Hypogammaglobulinemia reported in 24 of 68 patients in the safety set pre- and post- infusion of tisagenlecleucel |
| CD19 CAR-T, Axicabtagene ciloleucel (Yescarta, Kite Pharma) 46 | 108 adult patients with relapsed or refractory aggressive B-cell non-Hodgkin (primary safety population for ZUMA-1) | 16 (15%) (unclear if post-CAR-T rate) | 0% | 41 (38%), serious infections | 25 (23%) | Unspecified: Viral Bacterial Lung Fungal |
28 (26%) 17 (16%) 14 (13%) 13 (12%) 5 (5%) |
17 (16%) 4 (4%) 10 (9%) 11 (10%) 0 (0%) |
Patients were maintained on supplemental treatment with intravenous gamma globulin. (6% of patients were started on immunoglobulins following the first dose of KTE-C19 & prior to hospital discharge) | Hypogammaglobulinemia following YESCARTA occurred in 15% of patients and required monitoring and intervention |
| CD19 CAR-T, Lisocabtagene maraleucel (Breyanzi, Juno Therapeutics) 47 | Age > 18 y/o; Adult patients with relapsed or refractory (R/R) large B-cell lymphoma after at least 2 prior therapies | 32% (defined as IgG <500 mg/dl) | 0% | 45% (121/268) | 19% (52/268) | Unspecified Bacterial URTI Viral |
29% 13% 13% 10% |
16% 5% 0.7% 1.5% |
Intravenous immune globulin (IVIG) therapy was not mandated in the protocol for a defined IgG cutoff level and was left to clinician discretion. As the vast majority of subjects with hypogammaglobulinemia did not receive IVIG, IVIG replacement can be left to institutional clinical practice and judgment | The analysis of IVIG replacement therapy by the company included all events of hypogammaglobulinemia based on laboratory analyses during the study and was not restricted to the treatment emergent period. |
| CD19 CAR-T, Brexucabtagene autoleucel (Tecartus, Kite Pharma) 48 | Adult patients with relapsed/refractory mantle cell lymphoma. 82 pts received treatment in the safety population (ZUMA-2) | 12 (15%) | 1 (1%) (Grade 3 or higher) | 47 (57%) | 26 (32%) | Unspecified Viral Bacterial Pneumonia Fungal |
35 (43%) 14 (17%) 13 (16%) 15 (17%) 8 (10%) |
23 (28%) 4 (5%) 6 (7%) 10 (12%) 0 (0%) |
Hypogammaglobulinemia can persist for months and requires monitoring and intervention | Hypogammaglobulinemia risks managed with appropriate risk mitigation strategies. |
| BCMA CAR-T, Idecabtagene vicleucel (Abecma, Celgene Corporation) 49 | Adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy | 41% (52/127) of subjects | 0.80% | 89 (70%) | 29 (23%) |
Unspecified Bacterial Viral Pneumonia URTI |
(%) 51 15 27 17 34 |
(%) 15 3.9 9.4 9.4 1.6 |
Overall, 77/127 (61%) of ABECMA treated subjects received IVIG (intravenous immunoglobulin) therapy for serum IgG level less than 400 mg/dl as needed to maintain an IgG level above 400 mg/dl. | Newly diagnosed hypogammaglobulinemia, based either on laboratory value defined as IgG <500 mg/dl post-ABECMA infusion or an adverse event was reported in 41% (52/127) of subjects |
The commercial trade name is provided in parentheses with manufacturer
Rates of hypogammaglobulinemia may vary across studies due to differences in hypogammaglobulinemia definitions, timing of immunoglobulin measurements, and study protocols. Pre-treatment with chemotherapy including rituximab-based regimens, resulting in undetectable circulating B-cells and decreased immunoglobulin levels even prior to CAR T-cell infusion, serve as another confounder (16). However, a CAR T-cell specific effect on hypogammaglobulinemia was demonstrated in a study examining 46 patients with immunoglobulin levels both prior to and after CAR T-cell-infusion, which found significantly lower post therapy immunoglobulin levels compared to pre-therapy (692 mg/dL pre- vs. 392 mg/dL post-; p <0.0001) (17). In the trials, IVIG was given at clinician discretion or by institutional protocol. In four adult trials, IVIG supplementation ranged from 11% to 32% (13,16,18,19).
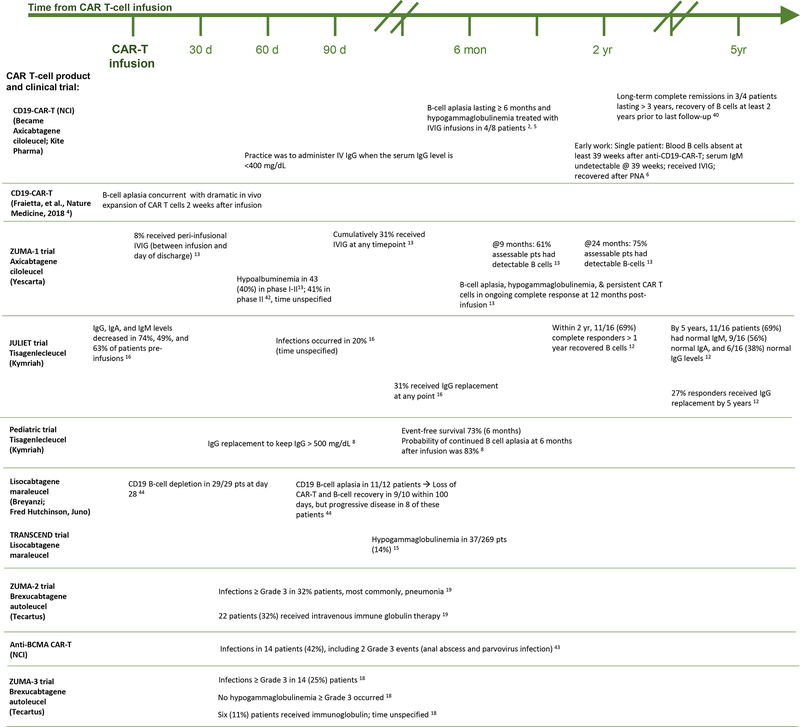
Long-term immunoglobulin recovery has been reported, even in complete responders. At five-year follow-up of 16 adult patients in complete remission after tisagenlecleucel, 11 had normal Immunoglobulin (Ig) M, 9 had normal IgA, and 6 had normal IgG levels (12). Figure 1 displays temporally the B-cell aplasia, hypogammaglobulinemia, recovery, and immunoglobulin supplementation practices for CAR T-cell products.
Figure 1. Temporal Events of Hypogammaglobulinemia, Treatments, and Infections from CAR-T therapy.
Rates of hypogammaglobulinemia, supplementation, and infection associated with each CAR-T product, as reported in clinical trials, are arranged temporally from pre-CAR-T infusion onward. Product names are listed to the left, with corresponding events for each product depicted to the right along the timeline. Each event is listed with its corresponding citation. (Events with an unspecified time frame are also noted.)
(Hypogammaglobulinemia after CAR-T cell therapy: characteristics, management, and future directions; Wat and Barmettler)
Underlying Differences in Children and Adults
Hypogammaglobulinemia is more frequent in children compared to adults. In one study, 100% of children and young adults receiving CAR T-cell therapy for B-cell Acute Lymphocytic Leukemia (ALL) experienced hypogammaglobulinemia (7). In another study, 14% had hypogammaglobulinemia (IgG < 400 mg/dL) at day 21, and 29% were hypogammaglobulinemic at day 63 (with median IgG of 455 mg/dL regardless of IgG supplementation) (20). In children, IVIG supplementation was more frequent, with all 25 pediatric and young adult patients in one trial receiving supplementation, which was given for IgG < 500 mg/dL (8).
Increased severity and duration of hypogammaglobulinemia in children may result from fundamental differences in immunoglobulin and plasma cell development between children and adults. For example, some immunoglobulin subclasses complete maturation only in late childhood or adulthood. Children also may have fewer plasma cells protective against pathogens. A subset of long-lived bone marrow plasma cells (CD38+CD19−), surviving after CAR T-cell infusion and protective against specific pathogens, such as tetanus, measles, and mumps, was detected only in subjects aged 17 and older, suggesting that this subset developed throughout childhood and adolescence and conferred protection starting only in late adolescence (11). Therefore, adults may possess a more robust plasma cell subset resistant to CD19+ CAR T-cell therapy compared to children, rendering children more susceptible to hypogammaglobulinemia and infections (21).
Infection risk from CAR T-cell therapy and hypogammaglobulinemia
Infections remain a major risk factor for mortality after CAR T-cell infusion (6,9). Figure 1 indicates infections after CAR T-cell therapy in clinical trials.
Generally, infections were greatest within the first month after CAR T-cell infusion and include bacterial, viral, and fungal infections, although bacterial were the most common type (10,20,22). Infections occurred in 23–42% of adult patients in the first month (10,22), with 80% of infections occurring within the first ten days in one study (10). At later time points, infections occurred in 21% of adults between days 29–90 (10) and 30% of adults at days 31–180 (22), consisting mainly of respiratory viral infections (10,22). In children, respiratory viral infections also predominated later but occurred in higher frequency than adults (20). Children were at higher risk for more severe infections than adults within the first 90 days, with 57% of infections being severe or life-threatening (20).
Between days 0 and 90, 50% of adult infections were mild or moderate and 41% were severe (10). Beyond 90 days post-CTI, infection density was 0.55 per 100 days-at-risk (14), and most infections were not severe, with 80% treated in the outpatient setting (14). Table 1 presents infection rates and infection types for the currently FDA-approved CAR T-cell therapies.
Hypogammaglobulinemia has been directly linked to both early and late infections in children. Hypogammaglobulinemia post-CAR T-cell infusion has been significantly associated with infection risk within the first 28 days (20). In another study of 28 children receiving subcutaneous immunoglobulin replacement (scIg) after CAR T-cell therapy, increasing IgG levels were significantly associated with a lower rate of sinopulmonary infection (p = 0.0072) (23).
In adults, few studies have examined infection risk factors specifically related to CAR T-cell therapy. While two studies found a significant association between infection and cytokine release syndrome severity, a statistical association with hypogammaglobulinemia was not found (10,24). These studies may have been underpowered to detect a difference, and variability in timing, degree, and duration of B-cell aplasia and hypogammaglobulinemia may have prevented detecting a direct causal relationship. An analysis of 101 patients revealed that infectious complications significantly increased in number after CAR T-cell therapy (p=0.0001), and that the number of infectious complications after CAR T-cell therapy were significantly greater in patients with moderate-severe compared to mild hypogammaglobulinemia (p=0.03) (17). Additional studies may lead to greater insight into the infectious consequences of post-CAR T-cell hypogammaglobulinemia and represent an area for future investigations.
Evidence for hypogammaglobulinemia and infections in other B-cell depleting contexts
Evidence from B-cell specific depletion in other contexts support hypogammaglobulinemia predisposing to infection. In chronic lymphocytic leukemia, a malignancy of dysfunctional B-cells that can suppress normal IgG production, regular immunoglobulin supplementation led to decreased bacterial infections (25). Specific agents targeting B-cells are also associated with infections and hypogammaglobulinemia. In a study of 4479 patients receiving rituximab, worsening hypogammaglobulinemia as well as a significant increase in severe infections was identified in patients following rituximab (26). In another study, 23.7% of 114 patients receiving rituximab for any reason developed hypogammaglobulinemia with IgG < 580 mg/dL (27).
The pharmacokinetics of B-cell depletion due to CAR T-cell therapy differ greatly compared to passively transferred B-cell-depleting antibodies, resulting in a more robust B-cell aplasia (11). Thus, data and experience in other B-cell targeted therapies may help to inform the establishment of guidelines to manage long-term hypogammaglobulinemia post CAR T-cell infusion given the lack of data for CAR T-cell therapy specifically.
Management Recommendations
Due to lack of randomized, controlled clinical trials on treatment of hypogammaglobulinemia and infection risk, recommendations are based on expert opinion, center specific experience, and infection-prevention approaches and strategies from other contexts, such as the use of high-dose corticosteroids, B-cell targeting therapies including rituximab, primary immunodeficiencies, and post-hematopoietic cell transplantation (HSCT) (21).
Recently, guideline recommendations on infections and CAR T-cell treatment have been proposed. A multidisciplinary team designated by the Spanish Ministry of Health recommends a baseline assessment of lymphocyte subsets and immunoglobulin levels in all adult patients prior to lymphodepleting chemotherapy and subsequent monthly monitoring until the sixth month after infusion (28). The Society for Immunotherapy for Cancer (SITC) provides similar guidance, but without a specified end date (29). After the first six months, clinical criteria should dictate monitoring; patients without hypogammaglobulinemia at that time may undergo twice yearly measurements of immunoglobulin and annual measurements of immunoglobulin subsets (28).
A specific immunoglobulin level at which to begin replacement was not proposed in these guidelines; rather, the ideal immunoglobulin level to prevent infections is individualized and determined over time, and replacement should be considered for those with high infection risk or experiencing recurrent infections (28–30). If substitution is required, a starting dose of 400–600 mg/kg every 3–4 weeks (28,29) with a goal IgG trough > 400 mg/dL is recommended in adults (28). If a patient continues to have recurrent infections despite monthly supplementation, the dose or frequency can be increased or subcutaneous administration can considered (28). ScIg (at 100–200 mg/kg/week) may also be recommended for patients with long-term B-cell aplasia (29). Replacement in specific situations – such as absence of seroconversion after vaccination or subclass deficiency – may also be warranted (28).
Hill et al. (31) and Hill and Seo (21) propose an algorithm based on expert opinion, other contexts, and primary immunodeficiency. This includes monthly monitoring of serum total IgG for at least the first three months after CAR T-cell infusion. Patients with IgG ≤ 400 mg/dl within the first three months should be considered for supplementation, especially if they experience severe or recurrent bacterial infections (31). After the first 3 months, patients with IgG ≤ 400 mg/dl who are not experiencing infections may be trialed off immunoglobulin replacement with close monitoring (31). Supplementation can be considered for patients with IgG between 400 and 600 mg/dL who are experiencing recurrent or severe bacterial infections (31). Patients with total IgG >600 mg/dL, but with recurrent or serious infections, may undergo additional immunologic evaluation including CD19+ or CD20+ B cell flow cytometry, antibody titers for certain pathogens and immunoglobulin subclasses (21). If specific antibody levels are low or nonprotective, IgG replacement or determination of responses to vaccine challenge can be considered (21).
Management in Children
Generally, most pediatric patients in clinical trials have been supplemented monthly with IVIG (according to institutional protocol or physician discretion). One such protocol begins replacement at 400 mg/kg monthly within 30 days of CAR T-cell infusion (23).
For all children, Los-Arcos et al. (28) recommends a monthly IVIG replacement dose of 0.5 g/kg, aiming for an IgG level within the normal range for the child’s age, starting one month after CAR T-cell infusion. The SITC recommends supplementation for children with serum IgG <400 mg/dL (29), and Doan and Pulsipher (32) recommend beginning IVIG supplementation when IgG levels fall between 400–600 mg/dL (typically 1–3 months after CAR T-cell infusion) and replacement every 3–4 weeks to maintain appropriate trough levels.
For children, replacement therapy should begin even at a trough IgG levels higher than 400 mg/dL in order to minimize infection (32). Los-Arcos et al. (28) recommends a higher IgG trough level (800 mg/dL) in some cases, especially for children younger than ten years or with baseline pulmonary pathology or added immunosuppression, such as in graft-vs-host disease. Arnold et al. (23) suggest immunoglobulin replacement to a higher trough level (IgG > 1000 mg/dL) for all children, given that fewer infections occurred at this higher level in their cohort receiving scIg replacement post-CAR T-cell infusion.
Los-Arcos et al. (28) recommends continuing replacement as long as B-cell aplasia or low IgA or IgM levels persist, with the decision to stop made on a case-by-case basis once B-cells recover. However, B-cells can rise in number while remaining dysfunctional, and IgG levels can be absent even with detectable B-cells, so B-cell number should not serve as a sole surrogate for immunoglobulin levels (23,32).
Future directions
Further studies on the clinical consequences of prolonged B-cell aplasia, such as the relationship to infections and mortality, and optimal monitoring and management are greatly needed as the number of CAR T-cell products and their uses expand. Standardization of definitions of hypogammaglobulinemia, and classification into mild, moderate, and severe categories, can facilitate easier comparison across clinical trials and studies. Moreover, standardizing the timepoints for B-cell assessment and immunoglobulin level measurements would enable a better understanding of the temporality of B-cell aplasia and hypogammaglobulinemia and subsequent recovery. Additional studies that systematically examine the effects of hypogammaglobulinemia, while controlling for other factors, or prospective studies on hypogammaglobulinemia – measuring pre- and post-CAR T-cell infusion immunoglobulin levels and implementing consistent classifications for hypogammaglobulinemia severity– can provide more definitive insights into the nature of hypogammaglobulinemia and its consequences. Given the increasing use of CAR T-cell therapy for relapsed/refractory malignancy, consultation and input from Allergy/Immunology specialists with experience and training in immune evaluation and the use of immunoglobulin replacement may be helpful in managing these complicated patients.
In addition, factors predisposing to persistent B-cell aplasia and hypogammaglobulinemia require further exploration. Construct type, such as use of CD28 or 41-BB co-stimulation domains, distribution of persistent CAR T-cells, and ratio of CD4:CD8 T cells re-infused into the patient could all affect the persistence of CAR T-cells and resulting B-cell aplasia. Ideally, biomarkers to allow for a risk prediction model could be identified to allow for maintaining the cancer in remission while also allowing B-cell and immunoglobulin recovery.
With newer CAR T-cell constructs that target additional antigens – including the κ immunoglobulin light chain, B cell maturation antigen (BCMA), and signaling lymphocytic activation molecule (SLAMF7) – and other diseases such as multiple myeloma, the risks for hypogammaglobulinemia and infection remain to be elucidated and compared with those of CD19+ CAR T-cells. It is plausible that specifically targeting plasma cells may pose a higher infection and hypogammaglobulinemia risk.
To minimize the risk of B-cell aplasia, research on CAR T-cells targeting antigens that are less ubiquitously expressed in B-cell lineages is ongoing. One such approach includes CAR T-cells that target the κ light chain, with the purpose of sparing normal B cells that express the λ light chain and therefore limiting B-cell aplasia and hypogammaglobulinemia (33). Another strategy is to target BCMA, an antigen expressed by malignant cells from almost all multiple myeloma patients, but unlike CD19, expressed in normal B cells only late in differentiation (34). BCMA−/− mice retained intact early humoral responses, short-term immunoglobulin production, and germinal center responses (34), which may make targeting BCMA less likely to cause hypogammaglobulinemia than targeting CD19+. A third molecule includes the SLAMF7 antigen, which may be a promising target for multiple myeloma as it is uniformly and highly expressed in multiple myeloma and present on a subset of normal leukocytes (plasma cells, natural killer (NK), T cell subsets, NK-T, and dendritic cells) (35,36).
In addition to targeting more restricted antigens along the B-cell differentiation course, other strategies to limit CAR T-cell toxicity include incorporation of a ‘suicide gene’ into the construct or utilizing a switchable system. In developing a CAR T-cell construct targeting SLAMF7, Amatya et al. (37) incorporated a dimerization domain fused to a caspase-9 domain, such that administration of a dimerization agent activates caspase-9 and induces elimination of the CAR T-cells, thus limiting possible toxicities of persistent CAR T-cells on leukocyte subsets. Viaud et al. (38) described a murine switchable CAR T-cell system to limit persistence of CAR T-cell action during tumor relapse. Both of these recent technological developments will need to be further evaluated in clinical trials but may reduce extent and duration of B-cell aplasia.
Furthermore, alternative means of immunoglobulin replacement may mitigate the impact of hypogammaglobulinemia resulting from CAR T-cell administration. Dosing on a regular schedule (every 3–4 weeks for IVIG, and weekly for scIg) may be more beneficial than an as needed approach. Additionally, scIg replacement has been used in patients requiring long-term immunoglobulin replacement, with the advantages of home administration, more stable IgG levels, decreased systemic side effects, and cost-effectiveness compared to IVIG (23).
Finally, identifying biomarkers for hypogammaglobulinemia and predisposing genetic or environmental factors may permit prospective management of hypogammaglobulinemia and its complications. Studies have demonstrated that the expression profile of higher fitness CAR T-cells differs than that of CAR T-cells that did not expand well (4). Certain cytokine profiles after lymphodepletion (in particular, elevated MCP-1 and IL-7) were associated with superior progression-free survival (39), and further studies can elucidate whether certain cytokines correlate with CAR T-cell persistence or profound hypogammaglobulinemia. As such, cytokine profiling may lead to a better understanding of the causes of variation in hypogammaglobulinemia across individuals.
The future remains bright for understanding, evaluating, and managing the toxicities and risks of hypogammaglobulinemia from CAR T-cell therapy, which is a potentially life-saving option for patients with refractory/relapsed hematopoietic malignancies who have exhausted previous treatments. Increased understanding of the factors predisposing to persistent hypogammaglobulinemia and infectious complications will allow for better management and risk reduction. In the meantime, adoption of new management approaches, such as the use of scIg, may lead to better control of immunoglobulin levels in a cost-effective manner, improve quality of life, and minimize infection risk with more stable immunoglobulin levels. Much exciting work remains to be done in this area to optimize the outcomes for current and future patients on CAR T-cell therapy.
Funding/Support:
Sara Barmettler is supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number K23AI163350. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- CAR
Chimeric antigen receptor
- CTI
CAR T-cell infusion
- IVIG
intravenous immunoglobulin
- Ig
Immunoglobulin
- ALL
Acute Lymphocytic Leukemia
- FDA
U.S. Food and Drug Administration
- scIg
subcutaneous immunoglobulin replacement
- HSCT
hematopoietic cell transplantation
- BCMA
B cell maturation antigen
- SLAMF7
signaling lymphocytic activation molecule
Footnotes
Conflict of Interest: The authors declare no relevant conflicts of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–86. [DOI] [PubMed] [Google Scholar]
- 2.Kochenderfer J, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikulska M, Lanini S, Gudiol C, Drgona L, Ippolito G, Fernández-Ruiz M, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect. 2018/02/16. 2018;24 Suppl 2:S71–s82. [DOI] [PubMed] [Google Scholar]
- 4.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018/05/02. 2018;24(5):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer J, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010/07/16. 2010;116(19):3875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochenderfer J, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010/07/30. 2010;116(20):4099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018/02/01. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med [Internet]. 2014/10/16. 2014;371(16):1507–17. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25317870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015/09/04. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood [Internet]. 2017/10/19. 2018;131(1):121–30. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29038338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood [Internet]. 2016/05/12. 2016;128(3):360–70. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27166358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong EA, Ruella M, Schuster SJ. Five-Year Outcomes for Refractory B-Cell Lymphomas with CAR T-Cell Therapy. N Engl J Med. 2021/02/18. 2021;384(7):673–4. [DOI] [PubMed] [Google Scholar]
- 13.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2018/12/07. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, et al. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transpl. 2019/08/17. 2020;26(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet (London, England). 2020. Sep;396(10254):839–52. [DOI] [PubMed] [Google Scholar]
- 16.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med [Internet]. 2018/12/07. 2019;380(1):45–56. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30501490 [DOI] [PubMed] [Google Scholar]
- 17.Barmettler S, Yang N, Farmer J, Long A, Maus M, Camargo C. Significant hypogammaglobulinemia in patients receiving CAR T-cell therapy. In: Journal of Allergy and Clinical Immunology. 2021. p. AB1. [Google Scholar]
- 18.Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet (London, England). 2021. Jun; [DOI] [PubMed] [Google Scholar]
- 19.Wang E, Cesano A, Butterfield LH, Marincola F. Improving the therapeutic index in adoptive cell therapy: key factors that impact efficacy. J Immunother Cancer. 2020/10/08. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vora SB, Waghmare A, Englund JA, Qu P, Gardner RA, Hill JA. Infectious Complications Following CD19 Chimeric Antigen Receptor T-cell Therapy for Children, Adolescents, and Young Adults. Open Forum Infect Dis. 2020/05/21. 2020;7(5):ofaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020/06/26. 2020;136(8):925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018/02/01. 2018;378(5):449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold DE, Maude SL, Callahan CA, DiNofia AM, Grupp SA, Heimall JR. Subcutaneous immunoglobulin replacement following CD19-specific chimeric antigen receptor T-cell therapy for B-cell acute lymphoblastic leukemia in pediatric patients. Pediatr Blood Cancer. 2019/12/04. 2020;67(3):e28092. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clin Infect Dis. 2018. Aug;67(4):533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale RP, Chapel HM, Bunch C, Rai KR, Foon K, Courter SG, et al. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled clinical trial. N Engl J Med. 1988. Oct;319(14):902–7. [DOI] [PubMed] [Google Scholar]
- 26.Barmettler S, Ong MS, Farmer JR, Choi H, Walter J. Association of Immunoglobulin Levels, Infectious Risk, and Mortality with Rituximab and Hypogammaglobulinemia. JAMA Netw Open. 2018;1(7):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makatsori M, Kiani-Alikhan S, Manson AL, Verma N, Leandro M, Gurugama NP, et al. Hypogammaglobulinaemia after rituximab treatment-incidence and outcomes. Qjm. 2014/04/30. 2014;107(10):821–8. [DOI] [PubMed] [Google Scholar]
- 28.Los-Arcos I, Iacoboni G, Aguilar-Guisado M, Alsina-Manrique L, Díaz de Heredia C, Fortuny-Guasch C, et al. Recommendations for screening, monitoring, prevention, and prophylaxis of infections in adult and pediatric patients receiving CAR T-cell therapy: a position paper. Infection. 2020/09/27. 2021;49(2):215–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maus MV, Alexander S, Bishop MR, Brudno JN, Callahan C, Davila ML, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020/12/19. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008/07/08. 2008;122(1):210–2. [DOI] [PubMed] [Google Scholar]
- 31.Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T - and a side order of IgG, to go? - Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev [Internet]. 2019/08/17. 2019;38:100596. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31416717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doan A, Pulsipher MA. Hypogammaglobulinemia due to CAR T-cell therapy. Pediatr Blood Cancer. 2017/12/13. 2018;65(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest. 2016/06/09. 2016;126(7):2588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004. Jan;199(1):91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin cancer Res an Off J Am Assoc Cancer Res. 2008. May;14(9):2775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veillette A, Guo H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit Rev Oncol Hematol. 2013. Oct;88(1):168–77. [DOI] [PubMed] [Google Scholar]
- 37.Amatya C, Pegues MA, Lam N, Vanasse D, Geldres C, Choi S, et al. Development of CAR T Cells Expressing a Suicide Gene Plus a Chimeric Antigen Receptor Targeting Signaling Lymphocytic-Activation Molecule F7. Mol Ther. 2020/11/02. 2021;29(2):702–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viaud S, Ma JSY, Hardy IR, Hampton EN, Benish B, Sherwood L, et al. Switchable control over in vivo CAR T expansion, B cell depletion, and induction of memory. Proc Natl Acad Sci U S A. 2018. Nov;115(46):E10898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019. Apr;133(17):1876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochenderfer J, Somerville RPT, Lu T, Yang JC, Sherry RM, Feldman SA, et al. Long-Duration Complete Remissions of Diffuse Large B Cell Lymphoma after Anti-CD19 Chimeric Antigen Receptor T Cell Therapy. Mol Ther. 2017/08/15. 2017;25(10):2245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol Ther. 2017/01/28. 2017;25(1):285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017. Dec;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019. May;380(18):1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J Clin Oncol. 2017/07/18. 2017;35(26):3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Food and Drug Administration Center for Biologics Evaluation and Research. KYMRIAH (tisagenlecleucel) BLA Clinical Review Memorandum. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel. Accessed October 2, 2021.
- 46.Food and Drug Administration Center for Biologics Evaluation and Research. YESCARTA (axicabtagene ciloleucel) BLA Clinical Review Memorandum. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel. Accessed October 2, 2021.
- 47.Food and Drug Administration Center for Biologics Evaluation and Research. BREYANZI (lisocabtagene maraleucel) BLA Clinical Review Memorandum. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/breyanzi-lisocabtagene-maraleucel. Accessed October 2, 2021.
- 48.Food and Drug Administration Center for Biologics Evaluation and Research. TECARTUS (brexucabtagene autoleucel) BLA Clinical Review Memorandum. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus-brexucabtagene-autoleucel. Accessed October 2, 2021.
- 49.Food and Drug Administration Center for Biologics Evaluation and Research. ABECMA (idecabtagene vicleucel) BLA Clinical Review Memorandum. https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel. Accessed October 2, 2021.