Abstract
A previously uninvestigated essential oil (EO) was distilled from Gynoxys miniphylla Cuatrec. (Asteraceae) and submitted to chemical and enantioselective analyses. The qualitative and quantitative analyses were conducted by GC-MS and GC-FID, over two orthogonal columns (5%-phenyl-methylpolysiloxane and polyethylene glycol stationary phases). Major constituents (≥2%) were, on both columns, respectively, as follows: α-phellandrene (16.1–17.2%), α-pinene (14.0–15.0%), germacrene D (13.3–14.8%), trans-myrtanol acetate (8.80%), δ-cadinene (4.2–4.6%), β-phellandrene (3.3–2.8%), (E)-β-caryophyllene (3.1–2.0%), o-cymene (2.4%), α-cadinol (2.3–2.6%), and α-humulene (1.7–2.0%). All the quantified compounds corresponded to 93.5–97.3% by weight of the whole essential oil, with monoterpenes counting for 53.8–55.6% of the total, and sesquiterpenes for 38.5–41.4%. For what concerns the enantioselective analyses, the chiral components were investigated through a β-cyclodextrin-based enantioselective column (2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin). A total of six chiral metabolites were analysed and the respective enantiomeric excess calculated as follows: (1S,5S)-(−)-α-pinene (98.2%), (1S,5S)-(−)-β-pinene (11.9%), (1R,5R)-(+)-sabinene (14.0%), (R)-(−)-α-phellandrene (100.0%), (R)-(−)-β-phellandrene (100.0%), and (S)-(−)-germacrene D (95.5%). According to the chemical composition and enantiomeric distribution of major compounds, this EO can be considered promising as a cholinergic, antiviral and, probably, analgesic product.
Keywords: Gynoxys miniphylla, Asteraceae, essential oil, enantioselective analysis, Ecuador
1. Introduction
Since the beginning of 19th century, plants have been thoroughly investigated as sources of new active principles. Nowadays, most of the European vegetal species have been deeply studied and their phytochemical profiles are well known. Therefore, the search for new natural products moved from temperate to tropical countries, where an incredible biodiversity, together with historical and logistical reasons, make the phytochemical investigation more difficult but also more suitable of interesting findings. Seventeen countries have been notably defined as “megadiverse” by the UN Environment Programme, for possessing two thirds of all non-fish vertebrate and three quarters of all higher plant species in the world [1]. Among these countries we can mention Ecuador, whose flora has been very little investigated so far from the chemical point of view [2,3]. For this reason, some of the authors (O.M. and G.G.) have been working for more than 20 years in the purification and structure elucidation of secondary metabolites, isolated from species of the Ecuadorian biodiversity [4,5,6,7,8,9,10]. During the last few years, our attention has been especially drawn by the chemistry of the essential oils (EOs), including the enantiomeric distribution of their chiral components [11,12,13,14,15,16]. Since volatile fractions are not only biologically interesting mixtures but also economically attractive products, we have recently decided to systematically investigate the genus Gynoxys, belonging to the family Asteraceae, with a special focus on the EOs. In fact, they find nowadays many commercial applications as flavours and flagrances, for example in foods, pharmaceuticals, cosmetics, perfumery, aromatherapy and household detergents.
The genus Gynoxys counts for 162 accepted species and is spread through the Andean region, from Colombia to Bolivia, with a few species also observed in Venezuela [17]. Of all these taxa, 33 have been described in Ecuador, most of them (23) being endemic [18]. According to literature, only a few species have been phytochemically studied so far. On one hand, G. acostae, G. buxifolia, G. nitida, G. verrucosa, G. oleifolia, G. sancto antonii, G. dielsiana, and G. psilophylla have been studied for their non-volatile constituents, with furanoeremophilanes and sesquiterpene lactones as typical secondary metabolites [19,20,21,22,23,24,25]. On the other hand, only from G. meridana and G. verrucosa, two EOs, dominated by sesquiterpenes, have been described [26,27].
For what concerns Gynoxys miniphylla Cuatrec., it is an Andean endemic shrub, apparently only present in Ecuador, where it grows between 3500 and 4000 m above the sea level. Azuay, Chimborazo, Loja and Morona-Santiago are the provinces where this species has been observed [17,18]. However, no ethnobotanical use is known for this plant. To the best of the authors’ knowledge, this is the first chemical and enantioselective description of an EO distilled from G. miniphylla.
2. Results
2.1. Distillation of the Essential Oil
The fresh leaves of G. miniphylla produced, after a 4 h distillation, 2.05 g of a yellow spicy essential oil, that spontaneously separated from the aqueous phase. The yield corresponded to 0.02% by weight, with a density of 0.819 g/cm3.
2.2. Qualitative and Quantitative Analyses
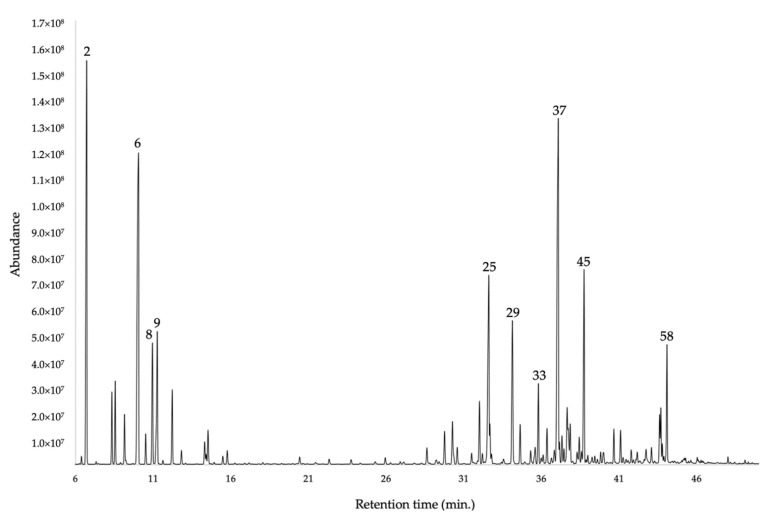
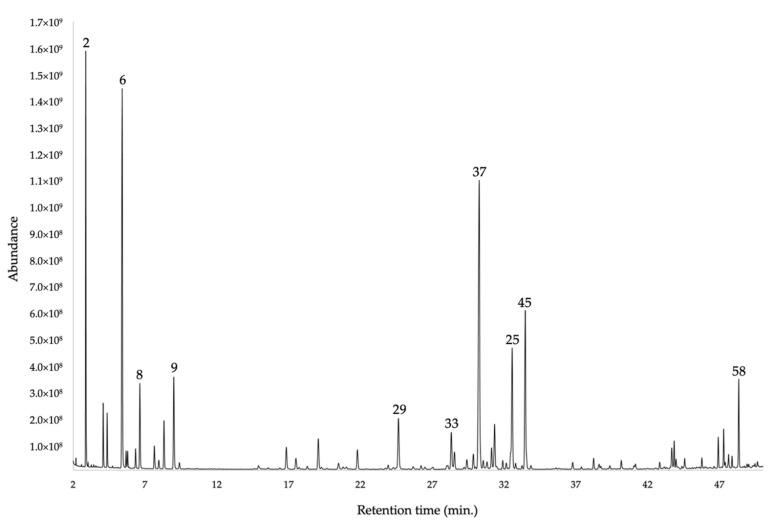
The qualitative chemical analysis of the EO resulted in the identification of 59 compounds, all quantified with at least one column. The total quantification of the components corresponded to 93.5–97.3% by weight of the whole EO, through a non-polar (5%-phenyl-methylpolysiloxane) and a polar (polyethylene glycol) stationary phase, respectively. The main constituents (≥2%) were α-phellandrene (16.1–17.2%), α-pinene (14.0–15.0%), germacrene D (13.3–14.8%), trans-myrtanol acetate (8.80%), δ-cadinene (4.2–4.6%), β-phellandrene (3.3–2.8%), (E)-β-caryophyllene (3.1–2.0%), o-cymene (2.4% by both columns), α-cadinol (2.3–2.6%), and α-humulene (1.7–2.0%). Quantitatively, the monoterpene and the sesquiterpene fractions (53.8–55.6% and 38.5–41.4%, respectively) were almost comparable, with a slight excess (about 10%) of monoterpenes. However, the number of sesquiterpenes is quite greater than the one of monoterpenes, as it appears in Figure 1 and Figure 2. The quantitative analysis was carried out in four repetitions for each column, and the results were expressed as mean values and standard deviations. All the qualitative and quantitative results are reported in Table 1. The gas-chromatography (GC) profiles are represented in Figure 1 and Figure 2.
Figure 1.
GC-MS chromatogram of G. miniphylla EO obtained with a 5%-phenyl-methylpolysiloxane capillary column. The numbers correspond to the main components (≥2% with at least one column).
Figure 2.
GC-MS chromatogram of G. miniphylla EO, obtained with a polyethylene glycol capillary column. The numbers correspond to the main components (≥2% with at least one column).
Table 1.
Chemical analyses of G. miniphylla EO obtained with non-polar (5%-phenyl-methylpolysiloxane) and polar (polyethylene glycol) capillary columns.
| N. | Compounds | 5%-phenyl-methylpolysiloxane | polyethylene glycol | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IRL a | IRL b | % | σ | IRL a | IRL c | Ref. | % | σ | ||
| 1 | tricyclene | 923 | 921 | 0.1 | 0.01 | 1013 | 1012 | [28] | 0.1 | 0.01 |
| 2 | α-pinene | 931 | 932 | 14.0 | 0.45 | 1025 | 1025 | [28] | 15.0 | 0.68 |
| 3 | sabinene | 969 | 969 | 1.3 | 0.04 | 1123 | 1122 | [28] | 1.4 | 0.06 |
| 4 | β-pinene | 974 | 974 | 1.7 | 0.05 | 1112 | 1110 | [28] | 1.8 | 0.09 |
| 5 | myrcene | 988 | 988 | 1.1 | 0.09 | 1203 | 1187 | [29] | 0.7 | 0.04 |
| 6 | α-phellandrene | 1007 | 1002 | 16.1 | 0.45 | 1166 | 1168 | [28] | 17.2 | 0.92 |
| 7 | α-terpinene | 1015 | 1014 | 0.5 | 0.01 | 1182 | 1178 | [28] | 0.5 | 0.03 |
| 8 | o-cymene | 1023 | 1022 | 2.4 | 0.07 | 1272 | 1276 | [30] | 2.4 | 0.12 |
| 9 | β-phellandrene | 1028 | 1025 | 3.3 | 0.09 | 1210 | 1209 | [28] | 2.8 | 0.15 |
| 10 | (E)-β-ocimene | 1044 | 1044 | 1.8 | 0.07 | 1254 | 1250 | [28] | 1.8 | 0.10 |
| 11 | ɣ-terpinene | 1055 | 1054 | 0.3 | 0.01 | 1245 | 1245 | [28] | 0.3 | 0.01 |
| 12 | camphenilone | 1081 | 1078 | 0.7 | 0.02 | 1177 | 1456 | [31] | 0.8 | 0.04 |
| 13 | terpinolene | 1082 | 1086 | 0.2 | 0.02 | 1282 | 1282 | [32] | 0.3 | 0.01 |
| 14 | linalool | 1101 | 1095 | 0.3 | 0.01 | 1565 | 1556 | [33] | 0.5 | 0.46 |
| 15 | n-nonanal | 1105 | 1100 | 0.5 | 0.01 | 1399 | 1387 | [34] | 0.3 | 0.04 |
| 16 | terpinen-4-ol | 1177 | 1174 | 0.2 | 0.01 | 1607 | 1601 | [28] | 0.2 | 0.02 |
| 17 | n-decanal | 1206 | 1201 | 0.2 | 0.01 | - | - | - | - | - |
| 18 | thymol methyl ether | 1228 | 1232 | 0.2 | 0.01 | 1596 | 1587 | [28] | 0.2 | 0.03 |
| 19 | 2-(E)-decenal | 1263 | 1260 | 0.5 | 0.04 | 1645 | 1640 | [28] | trace | - |
| 20 | carvacrol | 1306 | 1298 | 0.5 | 0.05 | 2213 | 2210 | [28] | 0.4 | 0.31 |
| 21 | α-cubebene | 1343 | 1348 | 0.3 | 0.01 | 1447 | 1460 | [28] | 0.5 | 0.05 |
| 22 | neryl acetate | 1361 | 1359 | 0.3 | 0.01 | 1734 | 1718 | [28] | 0.4 | 0.05 |
| 23 | α-copaene | 1370 | 1374 | 1.2 | 0.03 | 1476 | 1491 | [28] | 1.2 | 0.13 |
| 24 | modheph-2-ene | 1374 | 1382 | 0.2 | 0.01 | 1502 | 1496 | [35] | 0.3 | 0.03 |
| 25 | trans-myrtanol acetate | 1382 | 1385 | 8.8 | 0.24 | 1765 | 1746 | [36] | 8.8 | 1.44 |
| 26 | β-cubebene | 1383 | 1387 | 0.8 | 0.02 | 1526 | 1542 | [28] | 0.9 | 0.11 |
| 27 | β-elemene | 1385 | 1389 | 0.2 | 0.01 | - | - | - | - | - |
| 28 | α-gurjunene | 1399 | 1409 | 0.1 | 0.04 | 1512 | 1529 | [28] | 0.1 | 0.01 |
| 29 | (E)-β-caryophyllene | 1412 | 1417 | 3.1 | 0.08 | 1577 | 1578 | [37] | 2.0 | 0.39 |
| 30 | β-copaene | 1423 | 1430 | 0.4 | 0.04 | 1613 | 1631 | [38] | 0.2 | 0.04 |
| 31 | β-gurjunene | 1438 | 1431 | 0.2 | 0.02 | - | - | - | - | - |
| 32 | aromadendrene | 1444 | 1439 | 0.3 | 0.05 | 1622 | 1620 | [28] | 0.1 | 0.03 |
| 33 | α-humulene | 1448 | 1452 | 1.7 | 0.03 | 1650 | 1667 | [28] | 2.0 | 0.30 |
| 34 | allo-aromadendrene | 1452 | 1458 | 0.1 | 0.03 | 1624 | 1637 | [28] | 0.2 | 0.04 |
| 35 | (E)-β-farnesene | 1461 | 1454 | 0.8 | 0.01 | 1655 | 1664 | [28] | 1.0 | 0.14 |
| 36 | dauca-5,8-diene | 1467 | 1471 | 0.1 | 0.01 | 1644 | 1654 | [39] | 0.4 | 0.16 |
| 37 | germacrene D | 1476 | 1480 | 13.3 | 0.38 | 1690 | 1708 | [28] | 14.8 | 2.36 |
| 38 | ar-curcumene | 1478 | 1479 | 0.5 | 0.01 | 1767 | 1770 | [28] | 0.8 | 0.14 |
| 39 | cis-β-guaiene | 1482 | 1492 | 0.2 | 0.01 | 1677 | 1664 | [28] | trace | - |
| 40 | trans-muurola-4(14),5-diene | 1484 | 1493 | 0.3 | 0.01 | - | - | - | - | - |
| 41 | bicyclogermacrene | 1489 | 1500 | 1.9 | 0.06 | 1714 | 1730 | [28] | 1.8 | 0.26 |
| 42 | α-muurolene | 1493 | 1500 | 0.9 | 0.03 | 1709 | 1723 | [28] | 1.1 | 0.22 |
| 43 | (E,E)-α-farnesene | 1503 | 1505 | 0.2 | 0.01 | 1749 | 1744 | [28] | trace | - |
| 44 | δ-amorphene | 1510 | 1511 | 0.3 | 0.07 | 1702 | 1710 | [40] | 0.4 | 0.07 |
| 45 | δ-cadinene | 1514 | 1522 | 4.2 | 0.81 | 1743 | 1756 | [28] | 4.6 | 1.23 |
| 46 | β-sesquiphellandrene | 1520 | 1521 | 0.3 | 0.01 | 1759 | 1771 | [28] | trace | - |
| 47 | trans-cadina-1,4-diene | 1527 | 1533 | 0.1 | 0.01 | - | - | - | - | - |
| 48 | α-cadinene | 1531 | 1537 | 0.1 | 0.01 | 1774 | 1769 | [28] | 0.2 | 0.04 |
| 49 | (E)-nerolidol | 1561 | 1561 | 0.8 | 0.01 | 2057 | 2053 | [34] | 1.4 | 0.62 |
| 50 | trans-sesquisabinene hydrate | 1575 | 1577 | 0.2 | 0.02 | 2128 | 2092 | [28] | 0.5 | 0.16 |
| 51 | globulol | 1588 | 1590 | 0.3 | 0.02 | 2082 | 2082 | [28] | 0.6 | 0.15 |
| 52 | viridiflorol | 1597 | 1592 | 0.2 | 0.01 | 2023 | 2054 | [28] | 0.5 | 0.13 |
| 53 | junenol | 1613 | 1618 | 0.3 | 0.01 | 2052 | 2052 | [41] | trace | - |
| 54 | 1-epi-cubenol | 1624 | 1627 | 0.3 | 0.02 | 2062 | 2088 | [28] | 0.4 | 0.12 |
| 55 | epi-α-cadinol | 1640 | 1638 | 0.9 | 0.02 | 2176 | 2170 | [28] | 1.1 | 0.36 |
| 56 | epi-α-muurolol | 1642 | 1640 | 1.0 | 0.02 | 2192 | 2186 | [28] | 1.7 | 0.52 |
| 57 | α-muurolol | 1645 | 1644 | 0.3 | 0.01 | - | - | - | - | - |
| 58 | α-cadinol | 1654 | 1652 | 2.3 | 0.04 | 2230 | 2227 | [28] | 2.6 | 1.16 |
| 59 | cyperotundone | 1690 | 1695 | 0.1 | 0.01 | - | - | - | - | - |
| Monoterpene hydrocarbons | 42.8 | 44.3 | ||||||||
| Oxygenated monoterpenes | 11.0 | 11.3 | ||||||||
| Sesquiterpene hydrocarbons | 31.8 | 32.6 | ||||||||
| Oxygenated sesquiterpenes | 6.7 | 8.8 | ||||||||
| Other compounds | 1.2 | 0.3 | ||||||||
| Total identified | 93.5 | 97.3 | ||||||||
a Calculated linear retention index; b Linear retention index according to [42]; c Linear retention index according to reference (Ref.).
2.3. Enantioselective Analysis
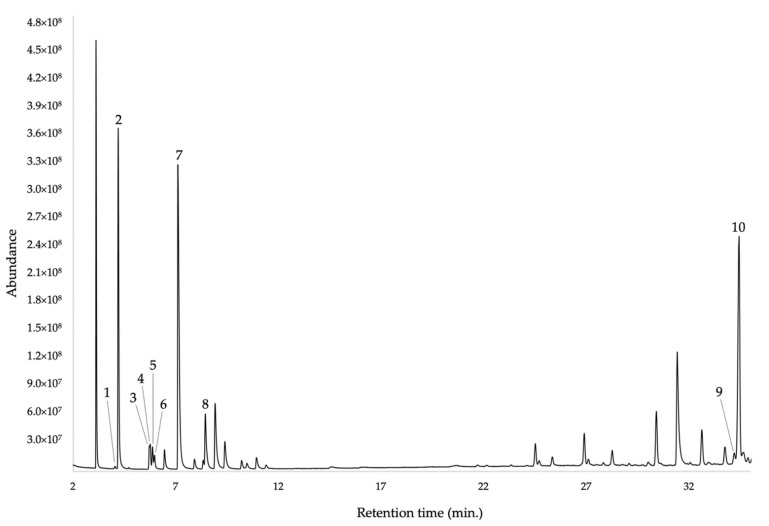
The enantioselective analysis of the EO was carried out on a 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin based capillary column. A total of 6 chiral terpenes were analysed, most of them being present as enantiomeric pairs. On the other hand, (R)-(−)-α-phellandrene and (R)-(−)-β-phellandrene resulted enantiomerically pure, whereas (1S,5S)-(−)-α-pinene and (S)-(−)-germacrene D presented an enantiomeric excess >95%. All the results from the enantioselective analysis are reported in Table 2, and the GC profile in Figure 3.
Table 2.
Enantioselective analysis of G. miniphylla EO, obtained with a β-cyclodextrin-based capillary column.
| N. | Enantiomers | 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin | ||
|---|---|---|---|---|
| LRI 1 | ED 2 (%) | ee3 (%) | ||
| 1 | (1R,5R)-(+)-α-pinene | 932 | 0.9 | 98.2 |
| 2 | (1S,5S)-(–)-α-pinene | 938 | 99.1 | |
| 3 | (1R,5R)-(+)-β-pinene | 993 | 44.1 | 11.9 |
| 4 | (1S,5S)-(–)-β-pinene | 995 | 55.9 | |
| 5 | (1R,5R)-(+)-sabinene | 999 | 57.0 | 14.0 |
| 6 | (1S,5S)-(–)-sabinene | 1001 | 43.0 | |
| 7 | (R)-(–)-α-phellandrene | 1027 | 100.0 | 100.0 |
| 8 | (R)-(–)-β-phellandrene | 1056 | 100.0 | 100.0 |
| 9 | (R)-(+)-germacrene D | 1499 | 4.5 | 91.0 |
| 10 | (S)-(–)-germacrene D | 1504 | 95.5 | |
1 Linear retention index; 2 Enantiomeric distribution; 3 Enantiomeric excess.
Figure 3.
GC-MS chromatogram of G. miniphylla EO obtained with a 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin capillary column. 1: (1R,5R)-(+)-α-pinene; 2: (1S,5S)-(−)-α-pinene; 3: (1R,5R)-(+)-β-pinene; 4: (1S,5S)-(−)-β-pinene; 5: (1R,5R)-(+)-sabinene; 6: (1S,5S)-(−)-sabinene; 7: (R)-(−)-α-phellandrene; 8: (R)-(−)-β-phellandrene; 9: (R)-(+)-germacrene D; 10: (S)-(−)-germacrene D.
3. Discussion
3.1. The EOs of Genus Gynoxys
Despite very few studies having been published on genus Gynoxys, especially for what concerns EOs, the following two species have been described for their volatile fractions: G. meridana and G. verrucosa [26,27]. On one hand, the main components of G. meridana EO (>2%) were: γ-curcumene (31.9%), β-pinene (9.5%), α-phellandrene (7.1%), α-pinene (5.7%), valencene (3.8%), and ar-curcumene (2.7%). On the other hand, the major constituents of G. verrucosa EO were: α-zingiberene (45.6%), α-amorphene (11.1%), p-cymene (15.2%), α-phellandrene (11.7%), thymol methyl ether (3.4%), and (E)-β-cariophyllene (3.2%). The comparison of these two EOs with the one from G. miniphylla demonstrated that they are all characterized by both an important monoterpene and sesquiterpene fractions, where the number of sesquiterpenes is clearly prevalent, even when the monoterpenes are quantitatively majoritarian (e.g., G. miniphylla). The preliminary and still unpublished data so far available effectively demonstrate that the EOs from this genus are tendentially dominated by sesquiterpenes.
3.2. Biological Activities of the Main Components
In the EO from the leaves of G. miniphylla, the sum of three common terpenes counts for about 50% of the whole amount. According to the elution order, they are α-pinene, α-phellandrene, and germacrene D, each corresponding to more than 15% of the total EO mass. Since the biological properties of the EOs can be partially deduced by the activities of their major compounds, we conducted a short review of the three main terpenes. For what concerns α-pinene, this very common monoterpene is known to possess a wide range of biological activities. It is anti-inflammatory, a human bronchodilator, antibacterial against methicillin-resistant Staphylococcus aureus (MRSA), and antifungal against Cryptococcus neoformans and Candida albicans [43,44,45,46]. Furthermore, α-pinene manifested an interesting activity against the promastigotes of Leishmania amazonensis and the larvae of Anopholes subpictus (a vector of malaria), Aedes albopictus (a vector of dengue), and Culex tritaeniorhynchus (vector of Japanese encephalitis) [47,48]. Nevertheless, we agree with some authors that consider the inhibition activity of acetylcholinesterase (AChE) to be the most important property of α-pinene. This activity probably explains, to a great extent, the strong AChE inhibition capacity observed for many monoterpene-based EOs [49,50]. In some previous studies, we could personally observe that this activity is often selectively stronger versus butyrylcholinesterase (BChE) than AChE [51,52]. Many other biological activities have been described for α-pinene, including a very peculiar in vivo anxiolytic effect by inhalation [53,54,55]. Another important component is α-phellandrene. Like α-pinene, also α-phellandrene is one of the most common monoterpenes in EOs, despite its biological properties are less studied than those of the previous metabolite. The most interesting property of α-phellandrene is probably the in vivo antinociceptive activity in rodents [56]. A subsequent study confirmed this activity, by observing an antihyperalgesic action in a neuropathic pain model [57]. Furthermore, α-phellandrene, that apparently does not exert any interesting in vitro anti-microbial action, enhanced the macrophage phagocytosis and the activity of killer cells. This property could result in an in vivo increased immune reaction to pathogenic agents [58]. Additionally, α-phellandrene induced a DNA damage in murine leukaemia cells, also affecting their DNA-repairing capacity in an in vitro study [59,60]. Finally, some literature about germacrene D will be analysed. The main biological property of this sesquiterpene is to interact with specific antennal receptors, located in the moths’ olfactory neurons of some species from genera Heliothis and Helicoverpa. The effect of this pheromone-like interaction is to increase the attraction and oviposition in these insects [61,62,63]. Anyway, the property of some EO components to act as insect pheromones is well described in literature and quite common in nature [64].
3.3. Biological Properties of the Main Enantiomers
The three major constituents α-pinene, α-phellandrene, and germacrene D were among the chiral metabolites, that could be enantioselectively analysed in the present study. Since different enantiomers are notoriously characterised by different biological activities, the enantiomeric composition of an EO must be investigated, in order to get a comprehensive information about its potential properties. In fact, as described in the previous section, bicyclic monoterpenes are important inhibitors of AChE, but the two enantiomers are sometimes characterized by different activities. However, in the case of α-pinene, both enantiomeric forms practically present the same inhibition capacity [50]. Nevertheless, other biological activities of α-pinene are influenced by stereochemistry. Whereas the laevorotatory isomer is active against the infectious bronchitis virus (IBV), the dextrorotatory form (minority in G. minyphilla EO) is the most effective antifungal enantiomer against C. albicans, C. neoformans, and Rhizopus oryzae and the strongest antibacterial isomer versus MRSA. Similarly, in the cytotoxicity of α-pinene versus mouse peritoneal macrophages, the dextrorotatory isomer is the most active form [46]. On the other hand, no information has been found in literature about the enantiomerically based properties of α-phellandrene. For what concerns germacrene D, the laevorotatory isomer, dominant in our EO, appeared to be ten times more active than the dextrorotatory form in the previously described pheromone-like activity [62,63]. According to the facts discussed in this section, the frequent lack of enantioselective analysis in the EO studies can explain the discrepancies, often observed in the literature, with respect to the bioactivity data.
4. Materials and Methods
4.1. GC and GC-MS Analyses
The chemical and enantioselective analyses of G. miniphylla EO were carried out with a gas chromatography-mass spectrometry (GC-MS) equipment, consisting of a Trace 1310 gas chromatograph, coupled to a simple quadrupole mass spectrometry detector, model ISQ 7000 (Thermo Fisher Scientific, Walthan, MA, USA). Additionally, a common flame ionization detector (FID) complemented the same instrument. The mass spectrometer was operated in SCAN mode (scan range 35–350 m/z), with the electron ionization (EI) source set at 70 eV. A non-polar column, based on 5%-phenyl-methylpolysiloxane, and a polar one, based on a polyethylene glycol stationary phase, were applied to both the qualitative and quantitative analyses. The non-polar column was DB-5ms (30 m long, 0.25 mm internal diameter, and 0.25 μm film thickness), whereas the polar one was HP-INNOWax (30 m × 0.25 mm × 0.25 μm), both purchased from Agilent Technology (Santa Clara, CA, USA). The enantioselective analysis was carried out through an enantioselective capillary column, based on 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin as a chiral selector (25 m × 250 μm internal diameter × 0.25 μm phase thickness), and purchased from Mega, MI, Italy. GC purity grade helium, from Indura, Guayaquil, Ecuador, was used as carrier gas, set at the constant flow of 1 mL/min. For all the GC analysis, the analytical purity grade solvents, the mixture of n-alkanes (C9–C25), and the internal standard (n-nonane), were purchased from Sigma-Aldrich (St. Louis, MO, USA). The calibration standard was isopropyl caproate, obtained by synthesis in the authors’ laboratory and purified to 98.8% (GC-FID).
4.2. Plant Material
The leaves of G. miniphylla were collected on 11 March 2020, on the way to mount Fierro Urku, in the San Lucas parish, Province of Loja, Ecuador. The collection point corresponded to coordinates 3°42′59.6” S and 79°18′51.0” W, at 3388 m above the sea level. The species was identified and classified by one of the authors (N.C.), whereas a botanical specimen was deposited at the herbarium of the Universidad Técnica Particular de Loja, with voucher code HUTPL14301. This investigation was carried out under permission of the Ministry of Environment, Water and Ecological Transition of Ecuador, with MAATE registry number MAE-DNB-CM-2016-0048.
4.3. Sample Preparation and EO Distillation
The day after collection, 9.13 Kg of fresh leaves were steam distilled, for 4 h, in a stainless-steel Clevenger-type apparatus, where steam is produced in a separated compartment. The process afforded 2.05 g a yellow essential oil, that spontaneously separated from the aqueous phase. The EO was then dried over anhydrous sodium sulphate and stored in the darkness, at −15 °C, until use. All the GC analyses were conducted by injecting diluted samples, prepared as previously described in the literature [15].
4.4. Qualitative Chemical Analysis
The components of the EO were identified by comparing their mass spectrum and linear retention index (LRI) with data present in literature (see Table 1). The LRI was calculated for each constituent according to Van den Dool and Kratz, using the homologous series of n-alkanes, from C9 to C25 [65]. The qualitative analysis was repeated with two orthogonal columns (polar and non-polar), injecting in both 1 μL of the previously described sample in split mode (split ratio 40:1). With the 5%-phenyl-methylpolysiloxane column, the thermal program was as follows: initial temperature 60 °C for 5 min, followed by a first thermal gradient of 2 °C/min until 100 °C, then a second gradient of 3 °C/min until 150 °C, and a third one of 5 °C/min until 200 °C. Finally, a new gradient of 15 °C/min until 250 °C was applied. The final temperature was maintained for 5 min. With the polyethylene glycol column, the thermal program was the same, except for the final temperature that did not exceed 230 °C.
4.5. Quantitative Chemical Analysis
The metabolites, previously identified, were subsequently quantified through the same two columns, by means of a flame ionization detector (FID). The quantification was carried out calculating the relative response factor (RRF) of each analyte versus isopropyl caproate, used as a quantification standard. The RRFs were determined according to the combustion enthalpy of each compound, as described in the literature [66]. However, instead of using the isopropyl caproate as internal standard, it was applied for external calibration, whereas n-nonane was used for internal normalization [16]. For both columns, the calibration curve afforded a R2 > 0.995. The GC methods and conditions were the same as the qualitative analyses. The quantitative results were expressed as mean values and standard deviation, over four repetitions, with each column. The percentage values referred to the weight of each analyte with respect to the mass of the whole essential oil.
4.6. Enantioselective Analyses
The enantioselective analyses were conducted by injecting the same previously described samples into the same GC-MS system used for the qualitative analyses. The employed GC method was as follows: the initial temperature was 60 °C for 2 min, followed by a thermal gradient of 2 °C/min until 220 °C, that was maintained for 2 min. The homologous series of n-alkanes (C9–C25) was also injected, in order to calculate the linear retention indices of the stereoisomers. The enantiomers were identified for their MS spectrum and elution order, determined by injection of enantiomerically pure standards.
5. Conclusions
The leaves of G. miniphylla Cuatrec. produced an EO of monoterpene ad sesquiterpene composition, with the relatively low yield of 0.02% by weight over the fresh plant material. Despite the monoterpene fraction appeared quantitatively dominant, the sesquiterpenes numerically prevailed. According to the chemical composition and enantiomeric distribution of the major compounds, this volatile fraction can be considered promising as a cholinergic, antiviral and, probably, analgesic EO. This hypothesis should be experimentally verified in future studies.
Acknowledgments
We are grateful to the Universidad Técnica Particular de Loja (UTPL) for sup- porting this investigation (2nd call funding TFT, April–August 2020) and open access publication. We are also grateful to Carlo Bicchi (University of Turin, Italy) for providing enantiomerically pure standards.
Author Contributions
Conceptualization, G.G.; investigation, P.C., A.M. and N.C.; data curation, O.M. and G.G.; writing—original draft preparation, G.G.; writing—review and editing, O.M. and G.G.; supervision, O.M. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data are available from the authors (P.C.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Megadiverse Countries, UNEP-WCMC. [(accessed on 5 December 2021)]. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries.
- 2.Malagón O., Ramírez J., Andrade J., Morocho V., Armijos C., Gilardoni G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016;11:297. doi: 10.1177/1934578X1601100307. [DOI] [PubMed] [Google Scholar]
- 3.Armijos C., Ramírez J., Salinas M., Vidari G., Suárez A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals. 2021;14:1145. doi: 10.3390/ph14111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiriboga X., Gilardoni G., Magnaghi I., Vita Finzi P., Zanoni G., Vidari G. New Anthracene Derivatives from Coussarea macrophylla. J. Nat. Prod. 2003;66:905. doi: 10.1021/np030066i. [DOI] [PubMed] [Google Scholar]
- 5.Quílez A., Berenguer B., Gilardoni G., Souccar C., De Mendonça S., Oliveira L.F.S., Martin-Calero M.J., Vidari G. Anti- secretory, Anti-inflammatory, and Anti-Helicobacter pylori Activities of Several Fractions Isolated from Piper carpunya Ruiz & Pav. J. Ethnopharmacol. 2010;128:583. doi: 10.1016/j.jep.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 6.Gilardoni G., Tosi S., Mellerio G., Maldonado M.E., Chiriboga X., Vidari G. Lipophilic Components from the Ecuadorian Plant Schistocarpha eupatorioides. Nat. Prod. Commun. 2011;6:767. doi: 10.1177/1934578X1100600606. [DOI] [PubMed] [Google Scholar]
- 7.Gilardoni G., Malagon O., Morocho V., Negri R., Tosi S., Guglielminetti M., Vidari G., Vita Finzi P. Phytochemical Research and Antimicrobial Activity of Clinopodium nubigenum Kunth (Kuntze) Raw Extracts. Rev. Bras. Farmacogn. 2011;21:850. doi: 10.1590/S0102-695X2011005000139. [DOI] [Google Scholar]
- 8.Gilardoni G., Chiriboga X., Finzi P.V., Vidari G. New 3,4-Secocycloartane and 3,4-Secodammarane Triterpenes from the Ecuadorian Plant Coussarea macrophylla. Chem. Biodivers. 2015;12:946. doi: 10.1002/cbdv.201400182. [DOI] [PubMed] [Google Scholar]
- 9.Herrera C., Pérez Y., Morocho V., Armijos C., Malagón O., Brito B., Tacán M., Cartuche L., Gilardoni G. Preliminary Phytochemical Study of the Ecuadorian Plant Croton elegans Kunth. (Euphorbiaceae) J. Chil. Chem. Soc. 2018;63:3788. doi: 10.4067/s0717-97072018000103875. [DOI] [Google Scholar]
- 10.Morocho V., Valarezo L.P., Tapia D.A., Cartuche L., Cumbicus N., Gilardoni G. A Rare Dirhamnosyl Flavonoid and Other Radical-scavenging Metabolites from Cynophalla mollis (Kunth) J. Presl and Colicodendron scabridum (Kunt) Seem. (Capparaceae) of Ecuador. Chem. Biodivers. 2021;16:e2100260. doi: 10.1002/cbdv.202100260. [DOI] [PubMed] [Google Scholar]
- 11.Gilardoni G., Montalván M., Vélez M., Malagón O. Chemical and Enantioselective Analysis of the Essential Oils from Different Morphological Structures of Ocotea quixos (Lam.) Kosterm. Plants. 2021;10:2171. doi: 10.3390/plants10102171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvopiña K., Malagón O., Capetti F., Sgorbini B., Verdugo V., Gilardoni G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants. 2021;10:2102. doi: 10.3390/plants10102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramírez J., Andrade M.D., Vidari G., Gilardoni G. Essential Oil and Major Non-Volatile Secondary Metabolites from the Leaves of Amazonian Piper subscutatum. Plants. 2021;10:1168. doi: 10.3390/plants10061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa S., Bec N., Larroque C., Ramírez J., Sgorbini B., Bicchi C., Cumbicus N., Gilardoni G. A Novel Chemical Profile of a Selective In Vitro Cholinergic Essential Oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a Native Andean Species of Ecuador. Molecules. 2021;26:45. doi: 10.3390/molecules26010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilardoni G., Montalván M., Ortiz M., Vinueza D., Montesinos J.V. The Flower Essential Oil of Dalea mutisii Kunth (Fabaceae) from Ecuador: Chemical, Enantioselective, and Olfactometric Analyses. Plants. 2020;9:1403. doi: 10.3390/plants9101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilardoni G., Matute Y., Ramírez J. Chemical and Enantioselective Analysis of the Leaf Essential Oil from Piper coruscans Kunth (Piperaceae), a Costal and Amazonian Native Species of Ecuador. Plants. 2020;9:791. doi: 10.3390/plants9060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tropicos.org. Missouri Botanical Garden. [(accessed on 5 December 2021)]. Available online: https://www.tropicos.org.
- 18.Jorgensen P., Leon-Yanez S. Catalogue of the Vascular Plants of Ecuador. Missouri Botanical Garden Press; St. Louis, MO, USA: 1999. pp. 286–288. [Google Scholar]
- 19.Jakupovic J., Zdero C., King R.M. Furanoeremophilanes from Gynoxys Species. Phytochemistry. 1995;40:1677. doi: 10.1016/0031-9422(95)00543-G. [DOI] [Google Scholar]
- 20.Ordóñez P.E., Quave C.L., Reynolds W.F., Varughesea K.I., Berry B., Breena P.J., Malagón O., Smeltzer M.S., Compadre C.M. Sesquiterpene Lactones from Gynoxys verrucosa and their Anti-MRSA Activity. J. Ethnopharmacol. 2011;137:1055. doi: 10.1016/j.jep.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ordóñez P.E., Sharma K.K., Bystrom L.M., Alas M.A., Enriquez R.G., Malagón O., Jones D.E., Guzman M.L., Compadre C.M. Dehydroleucodine, a Sesquiterpene Lactone from Gynoxys verrucosa, Demonstrates Cytotoxic Activity against Human Leukemia Cells. J. Nat. Prod. 2016;79:691. doi: 10.1021/acs.jnatprod.5b00383. [DOI] [PubMed] [Google Scholar]
- 22.Catalano S., Cioni P.L., Menichini A., Bilia A.B., Morelli L., De Feo V. Kauranoid Diterpenes in Gynoxys oleifolia. Planta Med. 1993;59:278. doi: 10.1055/s-2006-959671. [DOI] [PubMed] [Google Scholar]
- 23.Bohlmann F., Grenz M., Suwita A. Inhaltsstoffe aus Gynoxys- und Pseudogynoxys-arten. Phytochemistry. 1977;16:774. doi: 10.1016/S0031-9422(00)89257-2. [DOI] [Google Scholar]
- 24.Zdero C., Bohlmann F., Robinson H., King R.M. Neue Furanoeremophilane aus Gynoxys dielsiana. Phytochemistry. 1980;19:975. doi: 10.1016/0031-9422(80)85155-7. [DOI] [Google Scholar]
- 25.Bohlmann F., Zdero C. Ein Neues Furanoeremophilon-derivat aus Gynoxys psilophylla. Phytochemistry. 1979;18:339. doi: 10.1016/0031-9422(79)80094-1. [DOI] [Google Scholar]
- 26.Hernández J., Rojas-Fermina L.B., Amaro-Luis J., Pouységu L., Quideau S., Usubillaga A. Chemical Composition of the Essential Oil of Gynoxys meridana from Mérida, Venezuela. Nat. Prod. Commun. 2015;10:653. doi: 10.1177/1934578X1501000431. [DOI] [PubMed] [Google Scholar]
- 27.Valarezo E., Aguilera-Sarmiento R., Meneses M.A., Morocho V. Study of Essential Oils from Leaves of Asteraceae Family Species Ageratina dendroides and Gynoxys verrucose. J. Essent. Oil-Bear. Plants. 2021;24:400. doi: 10.1080/0972060X.2021.1948919. [DOI] [Google Scholar]
- 28.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 29.Chisholm M.G., Wilson M.A., Gaskey G.M. Characterization of Aroma Volatiles in Key Lime Essential Oils (Cirtrus aurantifolia Swingle) Flavour Fragr. J. 2003;18:106. doi: 10.1002/ffj.1172. [DOI] [Google Scholar]
- 30.Elmore J.S., Nisyrios I., Mottram D.S. Analysis of the Headspace Aroma Compounds of Walnuts (Juglans regia L.) Flavour Fragr. J. 2005;20:501. doi: 10.1002/ffj.1477. [DOI] [Google Scholar]
- 31.Cozzani S., Muselli A., Desjobert J.-M., Bernardini A.-F., Tomi F., Casanova J. Chemical Composition of Essential Oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr. J. 2005;20:436. doi: 10.1002/ffj.1463. [DOI] [Google Scholar]
- 32.Hachicha S.F., Skanji T., Barrek S., Ghrabi Z.G., Zarrouk H. Composition of the Essential Oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007;22:101. doi: 10.1002/ffj.1764. [DOI] [Google Scholar]
- 33.Kundakovic T., Fokialakis N., Kovacevic N., Chinou I. Essential Oil Composition of Achillea lingulata and A. umbellate. Flavour Fragr. J. 2007;22:184. doi: 10.1002/ffj.1778. [DOI] [Google Scholar]
- 34.Saroglou V., Marin P.D., Rancic A., Veljic M., Skaltsa H. Composition and Antimicrobial Activity of the Essential Oil of Six Hypericum Species from Serbia. Biochem. Syst. Ecol. 2007;35:146. doi: 10.1016/j.bse.2006.09.009. [DOI] [Google Scholar]
- 35.Muselli A., Rossi P.-G., Desjobert J.-M., Bernardini A.-F., Berti L., Costa J. Chemical Composition and Antibacterial Activity of Otanthus maritimus (L.) Hoffmanns. Link Essential Oils from Corsica. Flavour Fragr. J. 2007;22:217. doi: 10.1002/ffj.1787. [DOI] [Google Scholar]
- 36.Gonny M., Cavaleiro C., Salgueiro L., Casanova J. Analysis of Juniperus communis subsp. alpina Needle, Berry, Wood and Root Oils by Combination of GC, GC/MS and 13C-NMR. Flavour Fragr. J. 2006;21:99. [Google Scholar]
- 37.Skocibusic M., Bezic N., Dunkic V. Phytochemical Composition and Antimicrobial Activities of the Essential Oils from Satureja subspicata Vis. Growing in Croatia. Food Chem. 2006;96:20. doi: 10.1016/j.foodchem.2005.01.051. [DOI] [Google Scholar]
- 38.Stashenko E., Wiame H., Dassy S., Martinez J.R., Shibamoto T. Catalytic Transformation of Copaiba (Copaifera officinalis) Oil over Zeolite ZSM-5. J. High Res. Chromatogr. 1995;18:54. doi: 10.1002/jhrc.1240180112. [DOI] [Google Scholar]
- 39.Mazzoni V., Tomi F., Casanova J. A Daucane-type Sesquiterpene from Faucus carota Seed Oil. Flavour Fragr. J. 1999;14:268. doi: 10.1002/(SICI)1099-1026(199909/10)14:5<268::AID-FFJ823>3.0.CO;2-Z. [DOI] [Google Scholar]
- 40.Martinez J., Rosa P.T.V., Menut C., Leydet A., Brat P., Pallet D., Meireles M.A.A. Valorization of Brazilian Vetiver (Vetiveria zizanioides (L.) Nash ex Small) Oil. J. Agric. Food Chem. 2004;52:6578. doi: 10.1021/jf049182x. [DOI] [PubMed] [Google Scholar]
- 41.Wong K.C., Lim T.B., Ali D.M.H. Essential Oil of Homalomena sagittifolia Jungh. Flavour Fragr. J. 2006;21:786. doi: 10.1002/ffj.1714. [DOI] [Google Scholar]
- 42.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 43.Gil M.L., Jimenez J., Ocete M.A., Zarzuelo A., Cabo M.M. Comparative Study of Different Essential Oils of Bupleurum gibraltaricum Lamarck. Pharmazie. 1989;44:284. [PubMed] [Google Scholar]
- 44.Falk A.A., Hagberg M.T., Lof A.E., Wigaeus-Hjelm E.M., Wang Z.P. Uptake, Distribution, and Elimination of alpha-Pinene in Man after Exposure by Inhalation. Scand. J. Work Environ. Health. 1990;16:372. doi: 10.5271/sjweh.1771. [DOI] [PubMed] [Google Scholar]
- 45.Kose E.O., Deniz I.G., Sarikurkcu C., Aktas O., Yavuz M. Chemical Composition, Antimicrobial and Antioxidant Activities of the Essential Oils of Sideritis erythrantha Boiss. and Heldr. (var. erythrantha and var. cedretorum P.H. Davis) Endemic in Turkey. Food Chem. Toxicol. 2010;48:2960. doi: 10.1016/j.fct.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 46.Rivas da Silva A.C., Lopes P.M., Barros de Azevedo M.M., Costa D.C., Alviano C.S., Alviano D.S. Biological Activities of alpha-Pinene and beta-Pinene Enantiomers. Molecules. 2012;17:6305. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodrigues K.A., Amorim L.V., Dias C.N., Moraes D.F., Carneiro S.M., Carvalho F.A. Syzygium cumini (L.) Skeels Essential Oil and Its Major Constituent alpha-Pinene Exhibit anti-Leishmania Activity through Immunomodulation in vitro. J. Ethnopharmacol. 2015;160:32. doi: 10.1016/j.jep.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 48.Govindarajan M., Rajeswary M., Hoti S.L., Bhattacharyya A., Benelli G. Eugenol, alpha-Pinene and beta-Caryophyllene from Plectranthus barbatus Essential Oil as Eco-friendly Larvicides against Malaria, Dengue and Japanese Encephalitis Mosquito Vectors. Parasitol. Res. 2016;115:807. doi: 10.1007/s00436-015-4809-0. [DOI] [PubMed] [Google Scholar]
- 49.Perry N.S., Houghton P.J., Theobald A., Jenner P., Perry E.K. In-vitro Inhibition of Human Erythrocyte Acetylcholinesterase by Salvia lavandulaefolia Essential Oil and Constituent Terpenes. J. Pharm. Pharmacol. 2000;52:895. doi: 10.1211/0022357001774598. [DOI] [PubMed] [Google Scholar]
- 50.Miyazawa M., Yamafuji C. Inhibition of Acetylcholinesterase Activity by Bicyclic Monoterpenoids. J. Agric. Food Chem. 2005;53:1765. doi: 10.1021/jf040019b. [DOI] [PubMed] [Google Scholar]
- 51.Montalván M., Peñafiel M.A., Ramírez J., Cumbicus N., Bec N., Larroque C., Bicchi C., Gilardoni G. Chemical Composition, Enantiomeric Distribution, and Sensory Evaluation of the Essential Oils Distilled from the Ecuadorian Species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) DC. (Myrtaceae) Plants. 2019;8:511. doi: 10.3390/plants8110511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espinosa S., Bec N., Larroque C., Ramírez J., Sgorbini B., Bicchi C., Gilardoni G. Chemical, Enantioselective, and Sensory Analysis of a Cholinesterase Inhibitor Essential Oil from Coreopsis triloba S.F. Blake (Asteraceae) Plants. 2019;8:448. doi: 10.3390/plants8110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salehi B., Upadhyay S., Erdogan Orhan I., Kumar Jugran A., Jayaweera S.L.D., A. Dias D., Sharopov F., Taheri Y., Martins N., Baghalpour N., et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules. 2019;9:738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasuya H., Okada N., Kubohara M., Satou T., Masuo Y., Koike K. Expression of BDNF and TH mRNA in the Brain Following Inhaled Administration of alpha-Pinene. Phytother. Res. 2015;29:43. doi: 10.1002/ptr.5224. [DOI] [PubMed] [Google Scholar]
- 55.Satou T., Kasuya H., Maeda K., Koike K. Daily Inhalation of alpha-Pinene in Mice: Effects on Behavior and Organ Accumulation. Phytother. Res. 2014;28:1284. doi: 10.1002/ptr.5105. [DOI] [PubMed] [Google Scholar]
- 56.Lima D.F., Brandão M.S., Moura J.B., Leitão J.M.R.S., Carvalho F.A.A., Miúra L.M.C.V., Leite J.R.S.A., Sousa D.P., Almeida F.R.C. Antinociceptive Activity of the Monoterpene α-Phellandrene in Rodents: Possible Mechanisms of Action. J. Pharm. Pharmacol. 2012;64:283. doi: 10.1111/j.2042-7158.2011.01401.x. [DOI] [PubMed] [Google Scholar]
- 57.Piccinelli A.C., Santos J.A., Konkiewitz E.C., Oesterreich S.A., Nazari-Formagio A.S., Croda J., Ziff E.B., Leite-Kassuya C.A. Antihyperalgesic and Antidepressive Actions of (R)-(+)-Limonene, α-Phellandrene, and Essential Oil from Schinus terebinthifolius Fruits in a Neuropathic Pain Model. Nutr. Neurosci. 2014;18:217. doi: 10.1179/1476830514Y.0000000119. [DOI] [PubMed] [Google Scholar]
- 58.Lin J.J., Lin J.H., Hsu S.C., Weng S.W., Huang Y.P., Tang N.Y., Lin J.G., Chung J.G. Alpha-phellandrene Promotes Immune Responses in Normal Mice Through Enhancing Macrophage Phagocytosis and Natural Killer Cell Activities. In Vivo. 2013;27:809. [PubMed] [Google Scholar]
- 59.Lin J.J., Wu C.C., Hsu S.C., Weng S.W., Ma Y.S., Huang Y.P., Lin J.G., Chung J.G. Alpha-Phellandrene-Induced DNA Damage and Affect DNA Repair Protein Expression in WEHI-3 Murine Leukemia Cells In Vitro. Environ. Toxicol. 2015;30:1322. doi: 10.1002/tox.22003. [DOI] [PubMed] [Google Scholar]
- 60.Hsieh L.C., Hsieh S.L., Chen C.T., Chung J.G., Wang J.J., Wu C.C. Induction of α-Phellandrene on Autophagy in Human Liver Tumor Cells. Am. J. Chin. Med. 2015;43:1. doi: 10.1142/S0192415X15500081. [DOI] [PubMed] [Google Scholar]
- 61.Røstelien T., Borg-Karlson A.K., Fäldt J., Jacobsson U., Mustaparta H. The Plant Sesquiterpene Germacrene D Specifically Activates a Major Type of Antennal Receptor Neuron of the Tobacco Budworm Moth Heliothis virescens. Chem. Senses. 2000;25:141. doi: 10.1093/chemse/25.2.141. [DOI] [PubMed] [Google Scholar]
- 62.Mozuraitis R., Stranden M., Ramirez M.I., Borg-Karlson A.K., Mustaparta H. (-)-Germacrene D Increases Attraction and Oviposition by the Tobacco Budworm Moth Heliothis virescens. Chem. Senses. 2002;27:505. doi: 10.1093/chemse/27.6.505. [DOI] [PubMed] [Google Scholar]
- 63.Stranden M., Liblikas I., Koenig W.A., Almaas T.J., Borg-Karlson A.K., Mustaparta H. (–)-Germacrene D Receptor Neurones in Three Species of Heliothine Moths: Structure-activity Relationships. J. Comp. Physiol. A. 2003;189:563. doi: 10.1007/s00359-003-0434-y. [DOI] [PubMed] [Google Scholar]
- 64.Müller M., Buchbauer G. Essential Oil Components as Pheromones. A Review. Flavour Fragr. J. 2011;26:357. doi: 10.1002/ffj.2055. [DOI] [Google Scholar]
- 65.Van Den Dool H., Kratz P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. 1963;11:463. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 66.Tissot E., Rochat S., Debonneville C., Chaintreau A. Rapid GC-FID Quantification Technique without Authentic Samples Using Predicted Response Factors. Flavour Fragr. J. 2012;27:290. doi: 10.1002/ffj.3098. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are available from the authors (P.C.).