Abstract
Quinoa (Chenopodium quinoa Willd.) is a nutrient-rich grain native to South America and eaten worldwide as a healthy food, sometimes even referred to as a ”superfood”. Like quinoa grains, quinoa greens (green leaves, sprouts, and microgreens) are also rich in nutrients and have health promoting properties such as being antimicrobial, anticancer, antidiabetic, antioxidant, antiobesity, and cardio-beneficial. Quinoa greens are gluten-free and provide an excellent source of protein, amino acids, essential minerals, and omega-3 fatty acids. Quinoa greens represent a promising value-added vegetable that could resolve malnutrition problems and contribute to food and nutritional security. The greens can be grown year-round (in the field, high tunnel, and greenhouse) and have short growth durations. In addition, quinoa is salt-, drought-, and cold-tolerant and requires little fertilizer and water to grow. Nevertheless, consumption of quinoa greens as leafy vegetables is uncommon. To date, only a few researchers have investigated the nutritional properties, phytochemical composition, and human health benefits of quinoa greens. We undertook a comprehensive review of the literature on quinoa greens to explore their nutritional and functional significance to human health and to bring awareness to their use in human diets.
Keywords: quinoa, nutrients, bioactive components, greens, microgreens, sprouts, leafy vegetable, health benefits
1. Introduction
Quinoa (Chenopodium quinoa Willd.) is a summer annual dicotyledonous herbaceous crop of the Amaranthaceae family. It was domesticated first in the Andean countries of South America about 7000 years ago. The Andean people began the cultivation of quinoa, possibly for its nutritional values, drought tolerance, and ability to grow in high salt conditions. The Incas considered quinoa a sacred crop and called it the ”mother grain”. Although overlooked for thousands of years, the agronomic and nutritional importance of this crop was rediscovered during the last 50 years, leading to a resurgence in its production. The number of countries growing quinoa has increased rapidly from 8 in 1980 to 40 in 2010 and to more than 100 countries in 2021. South American countries, namely Bolivia, Ecuador, and Peru, lead production, and these together account for more than 80% of the world’s production [1,2,3]. There are about 250 species of Chenopodium worldwide [4]. Quinoa grains are the main edible part, and gluten-free grains contain high quantities of protein, essential amino acids, and essential minerals and vitamins. Because of these nutritional properties and health benefits, quinoa is considered a novel healthy food, occasionally referred to as a ”superfood”. Considering its importance, the United Nations General Assembly declared 2013 as the ”International Year of Quinoa”.
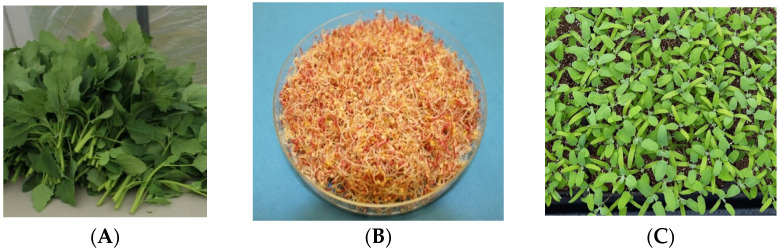
Like quinoa grains, quinoa greens (quinoa green leaves, sprouts, and microgreens; Figure 1) are rich in nutritional values and health promoting properties such as antimicrobial, anticancer, antidiabetic, antiobesity, antioxidant, and cardio-beneficial. The Incas consumed quinoa grains and leaves in their diet to balance the lack of animal proteins [5]. The people of the Andean regions have traditionally consumed quinoa leaves. The fresh leaves and tender shoots can be eaten as cooked vegetables and as a salad where quinoa sprouts can be added [6,7,8,9]. Researchers have recently reported that quinoa leaves contain high quantities of protein and all amino acids necessary for humans and low quantities of carbohydrates. Quinoa leaves also contain high levels of potassium, manganese, and copper and moderate levels of calcium, phosphorus, sodium, and zinc [10,11,12]. One of the species of genus Chenopodium, Chenopodium album, a close relative of quinoa, is a common weed known as ”lamb’s quarters”. Chenopodium album (C. album) originated in India and has been cultivated as a non-traditional vegetable in India and Bangladesh (Known as ”Bathua” in Hindi and Bengali). In the Indian subcontinent, young leaves and shoots of lamb’s quarters are eaten as vegetables. Although it is a weed, the young green leaves and tender stems are rich in different nutrients such as protein, fat, fiber, essential amino acids, minerals, and vitamins. They are rich in various bioactive compounds and have many medicinal properties for humans, such as antimicrobial, antihelmintic, antioxidant, antidiarrheal, and hepato-protective [13,14,15,16,17]. Phenotypically both Chenopodium quinoa and C. album appear very similar and hard to differentiate. Both are rich in nutritional and functional properties.
Figure 1.
Representative pictures of quinoa greens, (A) green leaves, (B) sprouts, and (C) microgreens.
Many research and review articles have been published on the nutritional and bioactive components of quinoa grains [18,19,20,21]. However, only a few research articles on quinoa greens are available regarding their nutritional and phytochemical composition and human health benefits (Table 1). Furthermore, most research has been conducted in Europe and Asia, whereas only a few investigations have been carried out in the USA. Until now, no review article has been available covering this topic. To the best of our knowledge, this is the first review article covering the potential benefits of quinoa greens.
Table 1.
Recent studies on nutritional composition and bioactive components in quinoa (Chenopodium quinoa) plant parts. Numbers in parenthesis refer to citations of articles of recent studies on quinoa plant parts.
| Plant Parts | Study Description | Study Location | References |
|---|---|---|---|
| Leaf | Antioxidant and anticancer activities | Poland | [7] |
| Leaf | Nutritional and chemical composition | Egypt | [10] |
| Leaf | Nutritional parameters | Poland | [11] |
| Leaf | Nutritional contents | USA | [12] |
| Leaf | Proximate and chemical composition | Peru | [22] |
| Leaf | Antioxidant activity and nitric oxide production | Taiwan | [23] |
| Leaf | Nutritional composition and antioxidant capacity | Poland | [24] |
| Leaf | Nutritional and chemical composition | Egypt | [25] |
| Leaf | Nutritional composition, polyphenols, flavonoids, and antioxidant capacity | Mexico | [26] |
| Leaf and sprout | Phenolic composition and antioxidant capacity in colored seeds | Poland | [27] |
| Seed and plant | Fatty acid and nutritive value of quinoa grains and plants at different growth stages | Italy | [28] |
| Sprout | Nutritional and functional contents in sprouts | China | [29] |
| Sprout | Effect of processing on nutritional composition | India | [30] |
| Sprout | Phenolic contents in colored grains | Peru | [31] |
| Sprout | Phenolic composition and antioxidant capacity | Argentina | [32] |
| Sprout | Polyphenolic contents and antioxidant capacity | Ireland | [33] |
| Sprout | Phenolic profiles and antioxidant capacity | Saudi Arabia | [34] |
| Sprout | Polyphenols and antioxidant capacity | Poland | [35,36] |
| Sprout | Polyphenols and antioxidant capacity | China | [37] |
| Infructescence | Nutritional composition and antioxidant capacity | Poland | [24] |
| Inflorescence | Anticancer, antimicrobial, and antioxidant compounds | Pakistan | [38] |
2. Nutritional Composition
A comparison of nutritional composition (proximate composition: protein, fiber, ash, and moisture; essential amino acids; and essential minerals) of quinoa (Chenopodium quinoa) greens (quinoa green leaves, sprouts), quinoa grains, and C. album leaves is presented in Table 2. The table was prepared based on available published articles. We noticed that data are variable. This may be due to the different locations and conditions under which the studies were conducted, the experimental variants used (different varieties, soil types, mineral composition, fertilizer application, and sampling time), and the analytical methods used.
Table 2.
Nutritional composition of quinoa plant parts and C. album leaves. Numbers in parenthesis refer to citations from which the nutrients data for quinoa plant parts and C. album were obtained.
| Nutrients | Quinoa Plant Parts | C. album Leaves | ||
|---|---|---|---|---|
| Leaves | Sprouts | Grains | ||
| Proximate composition | ||||
| Crude protein% | 28.2–37.0 [10,12] | 6.1–12.3 [30,39] | 9.1–15.7 [40] | 28.7 [13] |
| Crude fat% | 2.4–4.5 [10,12] | 0.1–3.8 [30,39] | 4.0–7.6 [40] | 4.4 [13] |
| Crude fiber% | 6.9–7.8 [10,12] | 4.6–23.5 [30,39] | 7.0–14.1 [40,41] | 0.1 [13] |
| Carbohydrate% | 34.0 [12] | 9.6–73.0 [30,39] | 48.5–69.8 [40] | 40.8 [13] |
| Ash% | 2.1–20.0 [10,12] | 0.9–3.4 [30,39] | 2.0–7.7 [40] | 21.0 [13] |
| Energy (kcal) | 325 [12] | 69 [39] | 331–381 [40] | 317.8 [13] |
| Essential amino acids (g 100 g−1 DW) | ||||
| Histidine (His) | 0.7 [12] | 0.7 [29] | 1.4–5.4 [40] | 0.4 [42] |
| Isoleucine (Ile) | 1.6 [12] | 1.1 [29] | 0.8–7.4 [40] | 0.5 [42] |
| Leucine (Leu) | 2.7 [12] | 2.0 [29] | 2.3–9.4 [40] | 1.3 [42] |
| Lysine (Lys) | 1.9 [12] | 1.3 [29] | 2.4–7.5 [40] | 1.8 [42] |
| Methionine (Met) | 0.6 [12] | 0.2 [29] | 0.3–9.1 [40] | 0.2 [42] |
| Phenylalanine (Phe) | 1.8 [12] | 1.2 [29] | 0.1–2.7 [40] | 0.9 [42] |
| Threonine (Thr) | 1.5 [12] | 1.0 [29] | 2.1–8.9 [40] | 0.8 [42] |
| Tryptophan (Trp) | 1.2 [12] | NA | 0.6–1.9 [40] | NA |
| Valine (Val) | 1.8 [12] | 1.3 [29] | 0.8–6.1 [40] | 0.7 [42] |
| Minerals (mg 100 g−1 DW) | ||||
| Calcium (Ca) | 147.0–1535.0 [10,12] | 21.7 [29] | 27.5–148.7 [40] | 1438.9 [13] |
| Copper (Cu) | 1.0–1.1 [10,12] | 0.2 [29] | 1.0–9.5 [40] | 1.1 [13] |
| Iron (Fe) | 11.6–148.0 [10,12] | NA | 1.4–16.7 [40] | 15.2 [13] |
| Magnesium (Mg) | 14.0–902.0 [12,43] | 219.3 [29] | 26.0–502.0 [40] | 1301.1 [13] |
| Phosphorus (P) | 39.0–405.6 [12,43] | NA | 140.0–530.0 [40] | 419.7 [13] |
| Potassium (K) | 474.0–8769.0 [12,43] | 525.2 [29] | 696.7–1475.0 [40] | 8125.2 [13] |
| Sodium (Na) | 3.0–15.1 [10,12] | NA | 11.0–31.0 [40] | 573.9 [13] |
| Zinc (Zn) | 3.3–6.8 [10,12] | NA | 2.8–4.8 [40] | 4.8 [13] |
DW: dry weight; NA, not available.
2.1. Protein
The protein content of young (30–45 days old) dry leaves of quinoa ranged from 28.2 to 37.0 g 100 g−1 on a dry weight (DW) basis, and protein content of quinoa grains ranged from 9.1 to 15.7 g 100 g−1 (Table 2), indicating that dried leaves of quinoa contain a higher amount of protein than grains. Alternatively, Chacaliaza-Rodríguez et al. (2016) [22] used fresh leaf quinoa and reported that protein was low, about 4.6% at 86.4% moisture content. Quinoa sprouts contain a low amount of protein ranging from 6.1 to 12.3% [30,39]. Protein content in C. album dry and fresh leaf was about 28.7% and 5.0%, respectively [13,44]. Protein contents in both dry and fresh leaves of quinoa and C. album are comparable. Quinoa has a high biological value (the proportion of absorbed protein from a food which becomes incorporated into the proteins of the organism’s body) (73%), similar to that of beef (74%) and higher than wheat (49%) and corn (36%) [4,42]. The main proteins in quinoa are albumins (35%) and globulins (37%), whereas the prolamins are present in very low quantities [45].
Proteins of green leaves of quinoa are high in lysine (1.9 g), methionine (0.6 g), and threonine (1.5 g 100 g−1 protein) [12]; these are the limiting amino acids in conventional cereals, for example, wheat and maize [46].
2.2. Fat
The fat content of green leaves of quinoa ranged from 2.4 to 4.5%, lower than in quinoa grains (4.0 to 7.6%; Table 2). The fatty acid (FA) profile of quinoa plant parts at different growth stages compared with spinach, kale, and quinoa grains is presented in Table 3. Peiretti et al. (2013) [28] analyzed fatty acids and the nutritive value of quinoa grains and plant parts at different growth stages. At the early vegetative stage of quinoa, alpha-linolenic acid (ALA) was most abundant at about 47%, and linoleic acid (LA) was 16% of total fatty acid (TFA). In contrast, quinoa grain was characterized by a high amount of LA ranging from 46.69 to 58.10% and a low amount of ALA ranging from 6.10 to 8.44% of TFA [9,47]. It is clear that early and mid-vegetative growth stages of quinoa plants contain more ALA than LA. Both spinach and kale contain more ALA than LA [48,49], and results are comparable with the results of the quinoa early vegetative stage. The balance between omega-6 and omega-3 is critical in health risk reduction [50]. The above reports summarized that quinoa greens and early to mid-vegetative plants contain a significantly high amount of omega-3 (ALA); however, quinoa grains contain a high amount of omega-6 (LA). It is suggested that higher dietary intake of omega-3 fatty acids is associated with risk reduction in cardiovascular disease that may suppress inflammatory responses and benefit individuals with other chronic diseases.
Table 3.
Fatty acid profile of quinoa plant parts at different growth stages, spinach, and kale. Numbers in parenthesis refer to citations from which the fatty acid profile data were obtained.
| Plant Parts Studied | Saturated Fatty Acid (SFA) | Unsaturated Fatty Acid (UFA) | References | |||
|---|---|---|---|---|---|---|
| Palmitic (16:0) | Stearic (18:0) | Oleic (18:1) | Linoleic Acid (LA, 18:2) | Linolenic Acid (ALA, 18:3) | ||
| Quinoa early veg | 12.07 | 1.51 | 7.49 | 15.97 | 47.4 | [28] |
| Quinoa bud | 11.64 | 1.68 | 7.64 | 16.14 | 39.9 | [28] |
| Quinoa grain | 9.60–10.00 [9] | 0.84–0.94 [9] | 23.10–29.18 [9,47] | 46.69–58.10 [9,47] | 6.10–8.44 [9,47] | [9,47] |
| Spinach | 20.65 | 1.71 | 9.48 | 18.63 | 37.37 | [48] |
| Kale | 11.84 | 3.95 | 2.14 | 11.8 | 54 | [49] |
2.3. Fiber
Based on solubility, there are two types of fiber in plants, soluble and insoluble. Pectin and gums are soluble in water (soluble fiber), whereas polysaccharides, cellulose, and hemicellulose are insoluble in water (insoluble fiber). Based on digestibility, fibers are divided into neutral detergent fiber (NDF) fractions containing hemicelluloses, cellulose, lignin, and acid detergent fiber (ADF) fractions containing mainly cellulose and some lignin. The amount of fiber present in quinoa leaves, sprouts, and grains ranged from 6.9 to 7.8, 4.6 to 23.5, and 7.0 to 14.1%, respectively (Table 2). In fiber fraction analysis of sprouted quinoa, Bhathal et al. (2017) [30] found a significantly higher amount of neutral detergent fiber (NDF), about 77.73%, compared to acid detergent fiber (ADF), only 11.63%. Peiretti et al. (2013) [28] reported 44.63 and 21.85% NDF and ADF, respectively, in the early vegetative stage of the quinoa plant. Quinoa grains are also an excellent source of dietary fiber with 78% insoluble and 22% soluble fiber [8,51,52]. A moderate level of fiber in quinoa leaves, which is close to spinach, contributes to the overall nutritional value [12].
2.4. Carbohydrate
The carbohydrate content was typically estimated using the following equation: carbohydrate (%) = 100% (protein + ash + fat + moisture). Total carbohydrate content in quinoa leaves (34.0%) was significantly lower than that of quinoa grain (48.5–69.8%), whereas 40.8% carbohydrate was reported in C. album leaves (lamb’s quarters) (Table 2). Starch is the major component of carbohydrates and comprises about 60% of dry weight [3]. Quinoa starch comprises 58.1% to 64.2% of dry seed weight with a low glycemic index [4]. The starch mainly constitutes D-xylose (120 mg 100 g−1) and maltose (101 mg 100 g−1) with low glucose (19 mg 100 g−1) and fructose (19.6 mg 100 g−1) content [6]. Foods containing high protein and low carbohydrates are beneficial to human health, as they do not contribute to raised plasma glucose.
2.5. Essential Amino Acids
Protein and amino acids (AA) are macromolecular compounds that have an essential role in the body as catalysts for structural components, enzymatic reactions, energy sources, and protein synthesis [53]. The nutritional quality of protein is determined by the concentration of essential amino acids that humans cannot synthesize in their bodies. Interestingly, all essential amino acids necessary for human growth and function are present in quinoa greens. The composition of essential AA of quinoa leaves, sprouts, grains, and leaves of C. album is presented in Table 2. The most abundant essential AA in quinoa leaves are leucine (2.7), lysine (1.9), phenylalanine (1.8), and valine (1.8 g 100 g−1 DW). In quinoa sprouts, the abundant AA are leucine (2.0), lysine (1.3), valine (1.3), and phenylalanine (1.2 g 100 g−1 DW). Lysine is deficient in many grains [42,54]. Methionine is generally deficient in green leaves but is found in higher levels in quinoa leaves than amaranth, spinach, moringa, and C. album leaves [12]. Methionine and cysteine are potent antioxidants that help in the detoxification of harmful components and protection from radiation [55].
2.6. Minerals
The composition of essential minerals of quinoa leaves, sprouts, grains, and leaves of C. album are presented in Table 2. The ash content is a measure of the total quantity of minerals present in a food. Ash contents in quinoa leaves ranged from 2.1 to 20.0%. Quinoa greens, especially quinoa leaves are rich in essential minerals including calcium (Ca), magnesium (Mg), phosphorus (P), potassium (K), iron (Fe), and zinc (Zn), and their richness compared with quinoa grains and C. album leaves is presented in Table 2. On a dry weight basis (mg 100 g−1), quinoa leaves range for Ca from 147.0 to 1535.0, Mg 14.0 to 902.0, P 39.0 to 405.6, K 474.0 to 8769.0, Fe 11.6 to 148.0, and Zn 3.3 to 6.8 mg 100 g−1. These values are comparable with the values obtained for the leaves of amaranth and spinach [12] and C. album [13,42]. Only two studies reported that quinoa sprouts are also rich in K and Mg, and the values are 525.0 and 219.0 mg 100 g−1, respectively [29,39].
2.7. Vitamins
There are few reports of vitamins quantification in quinoa greens. Among different vitamins, vitamin C (ascorbic acid) is an essential nutrient with a robust antioxidant capacity that the human body cannot synthesize and must be acquired from fruits and vegetables. The amount of vitamin C per 100 g of quinoa leaves ranged from 70 to 230 mg [6,11], 4-day-old sprouts ranged from 40 to 52 mg [56], and 20-day-old seedlings ranged from 37 to 70 mg 100 g−1 [29]. Quinoa leaves contain vitamin A (2085 mg) and a small amount of vitamin E (2.9 mg 100 g−1) [57]. Common leafy green spinach contains vitamin C ranging from 30 to 130 mg 100 g−1 [58,59]. However, quinoa grains contain 16 mg 100 g−1, whereas wheat, corn, and rice contain non-traceable amounts of vitamin C [60,61]. The above data suggest that quinoa greens are a good source of vitamin C.
3. Bioactive Compounds/Functional Compounds
Bioactive compounds include a variety of compounds present in small quantities in plants and plant-derived foods and provide health benefits beyond the nutritional value. It is suggested that intake of bioactive compounds rich food help in reducing oxidative stress and in prevention of cancer, heart diseases, and other diseases. Important bioactive compounds include polyphenols, carotenoids, tocols, phytoecdysteroids, phytosterols, bioactive proteins, and peptides as well as saponins, the most studied anti-nutrients, and other anti-nutrients including tannins and phytic acid.
3.1. Total Phenolic Content (TPC)
The TPC was measured in quinoa leaves and sprouts, mostly using the Folin–Ciocalteu assay, and data are expressed as mg gallic acid equivalent (GAE) per 100 g of sample dry weight basis (Table 4). The values ranged in the leaf from 131.80 to 544.00 and in the sprout from 49.02 to 417.75 mg GAE 100 g−1 DW, excluding some very high values [23,27,29]. The highest value of 544.00 was found in quinoa leaf [10], and the lowest of 79.04 mg GAE g−1 DW was found in quinoa sprout [32]. The TPC in quinoa grains ranged from 39.29 to 198.23 mg GAE 100 g−1 DW [31,32] (Table 4). During germination, biochemical, physical, and enzymatic activities lead to an increase in TPC in sprouts [62]. Different authors [33,63,64] have reported an increase in TPC during the seed germination process of quinoa. Hence, germination is used as a strategy to increase the quantity of bioactive compounds such as phenols, anthocyanins, and flavonoids [65,66].
Table 4.
Total phenolic content (TPC), total flavonoid content (TFC), and antioxidant capacity (AC, measured using 2,2-diphenyl-1-picrylhydrazyl-DPPH assay) in quinoa plant parts. Numbers in parenthesis refer to citations from which the TPC, TFC, and AC data were obtained.
| Plant Parts Studied | TPC | TFC | AC_DPPH | References |
|---|---|---|---|---|
| (mg GAE 100 g−1 DW) | (mg QE 100 g−1 DW) | (mg TE 100 g−1 DW) | ||
| Leaf | 418.00–544.00 | 14.00–23.00 | 29.90–55.40 | [10] |
| Leaf | 10.55–10.75 mg/g | 8.69–9.14 mg/g | 46.00–62.65 | [22] |
| Leaf | 569.50 mg/g | NA | 50.70–65.30 mg/g | [23] |
| Leaf | 188 | NA | 34 | [24] |
| Leaf | 131.8 | 62.07 | NA | [26] |
| 1 Leaf | 16.03–16.10 mg/g | 2.02–2.54 mg/g | NA | [27] |
| Sprout | 58.91–71.01 mg/g | 3.29–9.05 mg/g | NA | [29] |
| Sprout | 101.2 | 18.02 | 61.41 | [61] |
| 1 Sprout | 308.82–417.75 | NA | NA | [31] |
| Sprout | 79.04 | NA | 27.39 | [32] |
| Sprout | 147 | 122.6 | 50.4 | [33] |
| 1 Sprout | 259.02–293.35 | 10.38–24.00 | 5.26–7.39 | [34] |
| Sprout | 49.02–51.63 | 290.00–304.10 | NA | [35,36] |
| 1 Sprout | 15.15–28.79 mg/g | 0.63–3.34 mg/g | NA | [27] |
| Infructescence | 172 | NA | 95 | [24] |
| Quinoa grain, raw | 39.29–198.23 [31,32] | 11.40–223.80 [36,61] | 13.61–59.61 [33,61] |
NA, not available; 1 Colored grain.
The differences in the TPC as reported by different authors are due to growing conditions, soil type, as well as the variety, since the colored varieties have a higher TPC [9,31,34,67,68,69].
3.2. Total Flavonoid Content (TFC)
The TFC was measured in quinoa leaves and sprouts and expressed as mg quercetin equivalent (QE) per 100 g of sample dry weight basis (Table 4). The values ranged from 10.38 to 304.10 mg QE 100 g−1 DW, excluding some very high values [22,27,29]. The highest value of 304.10 was found in quinoa sprouts [35] and the lowest value in quinoa sprouts (10.38 mg QE 100 g−1 DW) [34]. The TFC in quinoa grains was about 11.40 to 223.80 mg QE 100 g−1 DW [36,61]. Flavonoids, such as rutin, quercetin, and kemferol, gradually increased with the progression of germination (9 days) and values were higher in yellow quinoa sprouts than red quinoa sprouts [34]. Exceptionally high TPC and TFC values were reported [22,23,27,29] for leaves and sprouts.
3.3. Antioxidants
Antioxidants are molecules that can prevent or reduce damage to cells caused by free radicals (known as reactive oxygen species, ROS), and plant-based foods are rich in antioxidants [70]. Different assays have been used to determine antioxidant capacity (AC) of quinoa greens, including chemical assays such as the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method (Table 4). A comparison of results on quinoa greens obtained by different researchers is not always possible because of the use of different extraction methods, different assays for quantification, different varieties with different seed colors, samples from different plant parts, and growth stages used.
In general, radical scavenging activity measured in quinoa greens using DPPH was higher in infructescence (the fruiting stage of an inflorescence) followed by leaves and sprouts. Debski et al. (2018) [24] reported antioxidant activity in quinoa leaves, stems, and infructescence and compared with C. album and found similar trends in both the species. Alvarez-Jubete et al. (2010) [33] reported that the capacity of DPPH was comparable in quinoa sprouts and quinoa grains. Quinoa sprouts contain more antioxidant than quinoa grains and increase in antioxidant activity depends on the length of germination (up to 6–9 days) [34,36]. Red quinoa sprouts have a higher antioxidant capacity than yellow quinoa. Germinated quinoa grains (quinoa sprouts) revealed a considerable increase in antioxidant capacity due to physiological and biological changes during seed germination [63].
3.4. Carotenoids
Carotenoids are a group of pigments that are largely produced by plants and algae. Carotenoids play an important role in human health nutrition as precursors for vitamin A and as antioxidants. The most important and most studied carotenoids are beta-carotene, lycopene, and lutein. Bhargava et al. (2006) [6] evaluated 27 Chenopodium quinoa germplasm lines, and the average leaf carotenoid content was 48.41 mg, with a range from 23.02 to 66.96 mg 100 g−1 fresh weight (FW). Prakash et al. (1993) [71] reported a much lower quantity of total carotenoids in quinoa leaves in 10 germplasm lines, ranging from 8.20 to 19.00 mg 100 g−1. On the contrary, a higher quantity of carotenoids detected in leaves of two quinoa lines ranged from 64.24 to 90.50 mg 100 g−1 DW [22]. Total carotenoid content determined in sprouts of 6 days after germination in red and yellow quinoa grains was 15.58 and 8.11 mg 100 g−1, respectively [34]. Le at al. (2021) [29] reported that the amount of beta-carotene in 25-day old sprouts of 10 quinoa lines ranged from 12.42 to 32.71 mg 100 g−1 FW.
4. Antinutritional Factors
Molecules that react with nutrients, thus interfering with their absorption, are referred to as antinutrients [63]. Saponins, phytic acids, oxalates, tannins, and trypsin inhibitors are the main antinutritional elements found in quinoa.
4.1. Saponins
Saponins are the secondary metabolites, mostly found on the outer layer of grains, which provide bitter taste. Quinoa grains contain about 0.00–4.4% saponins [72,73]. In addition to grains, saponin contents have been also investigated in quinoa leaves, sprouts, stems, and bran [74,75,76]. Lim et al. (2017) [75] reported saponin contents in different parts of quinoa plants, such as leaves (0.97), grains (1.26), sprouts (1.29), stems (3.67), and bran (8.34%). Different factors such as locations, abiotic stresses, and varieties influence saponin contents. Environmental factors, such as drought and salinity, decrease the quantity of saponins, and the sweet quinoa variety has lower saponin contents than the bitter variety. In contrast to the cytotoxic effects of saponins, some health promoting properties of quinoa saponins have been reported such as anticancer, antiobesity, neutralizing free radicals, and reducing heart diseases [19,77].
4.2. Phytic Acid
An excessive amount of dietary phytate capable of chelating bivalent minerals, such as iron, calcium, magnesium and zinc reduces the absorption of these elements. The presence of a small amount of phytic acid in food can help in phosphorus storage. To the best of our knowledge, no report available on phytic acid amount in quinoa greens. Phytic acid contents in quinoa grains ranging from 200 to 880 mg 100 g−1 [78,79]. Phytic acid content in the leaves of several C. album varieties ranged from 238 to 268 mg 100 g−1 [80].
4.3. Oxalate
Oxalates are antinutrient components that can bind with Ca, Fe, and Mg ions and hinder availability of nutrients. High intake of soluble oxalate in diets decrease bioavailability of minerals and trace elements and can form calcium oxalate stones in the kidneys. Total oxalate content in quinoa ranged from 143 to 232 mg 100 g−1 in roots and grains, and from 874 to 1959 mg/100 g−1 in leaves and stems of quinoa [81]. Leaves of C. album contained oxalic acid ranged from 360 to 2000 mg 100 g−1 DW [82,83].
4.4. Trypsin Inhibitor (TI)
Trypsin inhibitor interacts with enzymes and makes them unavailable for protein digestion. TI is a thermolabile compound and is inactivated by heat treatment. To date, no reports are available on the detection of TI in quinoa leaves. However, Sood et al. (2012) [80] detected a very small amount of TI in C. album leaves, ranging from 0.11 to 0.17 TIU mg−1. TI contents in quinoa grains range from 1.36 to 5.04 TIU/mg which is much lower than in soybean (24.5 TIU mg−1) [84].
4.5. Tannins
Tannins are polyphenolic and antinutritional components and are able to interact with proteins and macromolecules, thus decreasing the nutritional value of food [63]. They are present in quinoa grains in small amounts, with values ranging from 23.00 to 31.00 mg 100 g−1 [47]. So far, there are no data available on the presence of tannins in quinoa greens.
5. Conclusions
Quinoa greens (green leaves, sprouts, and microgreens) are excellent sources of nutrients and health promoting compounds with antimicrobial, anticancer, antidiabetic, antioxidant, antiobesity, and cardio-beneficial properties. Greens are gluten-free and provide an outstanding source of protein, amino acids, essential minerals, and omega-3 fatty acids. Quinoa greens represent a promising value-added new vegetable that could resolve malnutrition problems and contribute to food and nutritional security. However, consumption of quinoa greens as vegetables is not common today. Greens can be eaten as both cooked vegetables and salads. In addition, quinoa greens may be combined as functional food ingredients in other gluten-free food products. The dried leaf powder may be added to soups, breads, and processed foods. Higher levels of total phenolic content and antioxidant capacity are found in germinated quinoa (quinoa sprouts) when compared to grains. After germination (sprouting), antioxidant capacity of quinoa sprouts increases for up to 6–9 days, and the amount of antioxidant is higher in red quinoa than yellow quinoa. Just like the sprouts and microgreens found in the stores today, quinoa sprouts and microgreens present a promising food for nutrition and human health. Further investigation is needed to draw a complete picture of the nutritional and functional significance to human health and to bring awareness to the use of quinoa greens in human diets as new ”super vegetables”.
Acknowledgments
The senior author (S.P.) would like to thank Lincoln University of Missouri for supporting this research and USDA-NIFA for providing funding for quinoa production research (award #2020-38821-31090). The authors also thank Grato Ndunguru of Lincoln University of Missouri for the photographs used in the article.
Author Contributions
S.P. and R.A.S. contributed equally to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bazile D., Jacobsen S.-E., Verniau A. The global expansion of quinoa: Trends and limits. Front. Plant Sci. 2016;7:622. doi: 10.3389/fpls.2016.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazile D., Baudron F. The dynamics of the global expansion of quinoa growing in view of its high biodiversity. In: Bazile D., Bertero H.D., Nieto C., editors. State of the Art Report of Quinoa in the World in 2013. FAO & CIRAD; Rome, Italy: 2015. [(accessed on 25 October 2021)]. pp. 42–55. Available online: http://www.fao.org/3/a-i4042e.pdf. [Google Scholar]
- 3.Repo-Carrasco R., Espinoza C., Jacobsen S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule) Food Rev. Int. 2003;19:179–189. doi: 10.1081/FRI-120018884. [DOI] [Google Scholar]
- 4.Vega-Galvez A., Miranda M., Vergara J., Uribe E., Puente L., Martinez E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010;90:2541–2547. doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]
- 5.Camaggio G., Amicarelli V. The ancient crop of quinoa for world food security. In: Miśniakiewicz M., Popek S., editors. Future Trends and Challenges in the Food Sector. Polish Society of Commodity Science; Cracow, Poland: 2014. [Google Scholar]
- 6.Bhargava A., Shukla S., Deepak O. Chenopodium quinoa: An Indian perspective. Ind. Crops Prod. 2006;23:73–87. doi: 10.1016/j.indcrop.2005.04.002. [DOI] [Google Scholar]
- 7.Gawlik-Dziki U., Swieca M., Sulkowski M., Dziki D., Baraniak B., Czyz J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts—In vitro study. Food Chem. Toxicol. 2013;57:154–160. doi: 10.1016/j.fct.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Jancurová M., Minarovičová L., Dandár A. Quinoa—A review. Czech J. Food Sci. 2009;27:71–79. doi: 10.17221/32/2008-CJFS. [DOI] [Google Scholar]
- 9.Tang Y., Li X., Chen P.X., Zhang B., Hernandez M., Zhang H., Marcone M.F., Liu R., Tsao R. Characterization of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015;174:502–508. doi: 10.1016/j.foodchem.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Abd El-Samad E., Hussin S., El-Naggar A., El-Bordeny N., Eisa S. The potential use of quinoa as a new non-traditional leafy vegetable crop. Biosci. Res. 2018;15:3387–3403. [Google Scholar]
- 11.Adamczewska-Sowińska K., Sowiński J., Jama-Rodzeńska A. The effect of sowing date and harvest time on leafy greens of quinoa (Chenopodium quinoa willd.) yield and selected nutritional parameters. Agriculture. 2021;11:405. doi: 10.3390/agriculture11050405. [DOI] [Google Scholar]
- 12.Pathan S., Eivazi F., Valliyodan B., Paul K., Ndunguru G., Clark K. Nutritional composition of the green leaves of quinoa (Chenopodium quinoa Willd.) J. Food Res. 2019;8:55–65. doi: 10.5539/jfr.v8n6p55. [DOI] [Google Scholar]
- 13.Pandey S., Gupta R.K. Screening of nutritional, phytochemical, antioxidant and antibacterial activity of Chenopodium album (Bathua) J. Phar. Phytochem. 2014;3:1–9. [Google Scholar]
- 14.Poonia A., Upadhayay A. Chenopodium album Linn: Review of nutritive value and biological properties. J. Food Sci. Technol. 2015;52:3977–3985. doi: 10.1007/s13197-014-1553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan S., Tamang J.P. Ethnobiology of wild leafy vegetables of Sikkim. Indian J. Trad. Knowl. 2015;14:290–297. [Google Scholar]
- 16.Saini S., Saini K. Chenopodium album Linn: An outlook on weed cum nutritional vegetable along with medicinal properties. Emergent Life Sci. Res. 2020;6:28–33. doi: 10.31783/elsr.2020.612833. [DOI] [Google Scholar]
- 17.Yadav R.K., Tomar B.S., Pachauri N., Jain V. Studies of nutritional properties and antioxidant potential in green leafy vegetables. J. Sci. Food Agric. 2018;2:7–13. [Google Scholar]
- 18.Angeli V., Silva P., Massuela D., Khan M., Hamar A., Khajehei F., Graeff-Hönninger S., Piatti C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “golden grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods. 2020;9:216. doi: 10.3390/foods9020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Hazzam K., Hafsa J., Sobeh M., Mhada M., Taourirte M., Kacimi K.E.L., Yasri A. An insight into saponins from Quinoa (Chenopodium quinoa Willd): A review. Molecules. 2020;25:1059. doi: 10.3390/molecules25051059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melini V., Melini F. Functional components and anti-nutritional factors in gluten-free grains: A focus on quinoa seeds. Foods. 2021;10:351. doi: 10.3390/foods10020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilcacundo R., Hernández-Ledesma B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd.) Curr. Opin. Food Sci. 2017;14:1–6. doi: 10.1016/j.cofs.2016.11.007. [DOI] [Google Scholar]
- 22.Chacaliaza-Rodríguez L., Espinoza-Begazo G., Ramos-Escudero F., Servan K. Proximate chemical composition and content of biologically active components in leaves of two quinoa cultivars (Salcedo and Altiplano) produced in Peru. Res. J. Med. Plant. 2016;10:450–456. doi: 10.3923/rjmp.2016.450.456. [DOI] [Google Scholar]
- 23.Chen H.-L., Lan X.-Z., Wu Y.-Y., Ou Y.-W., Chen T.C., Wu W.-T. The antioxidant activity and nitric oxide production of extracts obtained from the leaves of Chenopodium quinoa Willd. Biomedicine. 2017;7:24. doi: 10.1051/bmdcn/2017070424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debski B., Gralak M.A., Bertrandt J., Klos A. Comparison of antioxidant potential and mineral composition of quinoa and lamb’s quarters weed (Chenopodium album) Probl. Hig. Epidemiol. 2018;99:88–93. [Google Scholar]
- 25.El-Naggar A., Hussin S., Abd El-Samad E., Eisa S. Quinoa as a new leafy vegetable crop in Egypt. Arab. Univ. J. Agric. Sci. 2018;26:745–753. doi: 10.21608/ajs.2018.16007. [DOI] [Google Scholar]
- 26.Vazquez-Luna A., Cortés V.P., Carmona F.F., Díaz-Sobac R. Quinoa leaf as a nutritional alternative. Cienc. Investig. Agrar. 2019;46:137–143. doi: 10.7764/rcia.v46i2.2098. [DOI] [Google Scholar]
- 27.Złotek U., Gawlik-Dziki U., Dziki D., Swieca M., Nowak R., Martinez E. Influence of drying temperature on phenolic acids composition and antioxidant activity of sprouts and leaves of white and red quinoa. J. Chem. 2019;2019:7125169. doi: 10.1155/2019/7125169. [DOI] [Google Scholar]
- 28.Peiretti P.G., Gai F., Tassone S. Fatty acid profile and nutritive value quinoa (Chenopodium quinoa Willd.) seeds and plants at different growth stages. Anim. Feed Sci. Technol. 2013;183:56–61. doi: 10.1016/j.anifeedsci.2013.04.012. [DOI] [Google Scholar]
- 29.Le L., Gong X., An Q., Xiang D., Zou L., Peng L., Wu X., Tan M., Nie Z., Wu Q., et al. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021;357:129752. doi: 10.1016/j.foodchem.2021.129752. [DOI] [PubMed] [Google Scholar]
- 30.Bhathal S., Kaur N., Gill J. Effect of processing on the nutritional composition of quinoa (Chenopodium quinoa Willd) Agric. Res. J. 2017;54:90–93. doi: 10.5958/2395-146X.2017.00015.1. [DOI] [Google Scholar]
- 31.Choque-Quispe D., LigardaSamanez C.A., RamosPacheco B.S., Legu’ıaDamiano S., CallaFlórez M., ZamalloaPuma L.M., ColqueCondeña L. Phenolic compounds, antioxidant capacity, and protein content of three varieties of germinated quinoa (Chenopodium quinoa Willd) Ing. Investig. 2021;41:e89831. doi: 10.15446/ing.investig.v41n2.89831. [DOI] [Google Scholar]
- 32.Carciochi R., Galván-D’Alessandro L., Vandendriessche P., Chollet S. Effect of germination and fermentation process on the antioxidant compounds of quinoa seeds. Plant Foods Hum. Nutr. 2016;71:361–367. doi: 10.1007/s11130-016-0567-0. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez-Jubete L., Wijngaard H., Arendt E., Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. doi: 10.1016/j.foodchem.2009.07.032. [DOI] [Google Scholar]
- 34.Al-Qabba M., El-Mowafy M., Althwab S., Alfheeaid H., Aljutaily T., Barakat H. Phenolic profile, antioxidant activity, and ameliorating efficacy of Chenopodium quinoa sprouts against ccl4-induced oxidative stress in rats. Nutrients. 2020;12:2904. doi: 10.3390/nu12102904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paśko P., Sajewicz M., Gorinstein S., Zachwieja Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chrom. 2008;20:661–672. doi: 10.1556/AChrom.20.2008.4.11. [DOI] [Google Scholar]
- 36.Paśko P., Bartoń H., Zagrodzki P., Gorinstein S., Fołta M., Zachwieja Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009;115:994–998. doi: 10.1016/j.foodchem.2009.01.037. [DOI] [Google Scholar]
- 37.Zhang Q., Xing B., Sun M., Zhou B., Ren G., Qin P. Changes in bio-accessibility, polyphenol profile and antioxidants of quinoa and djulis sprouts during in vitro simulated gastrointestinal digestion. Food Sci. Nutr. 2020;8:4232–4241. doi: 10.1002/fsn3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan I.H., Javaid A. Anticancer, antimicrobial and antioxidant compounds of quinoa inflorescence. Adv. Life Sci. 2020;8:68–72. [Google Scholar]
- 39.Mezzatesta P., Farah S., di Fabio A., Emilia R. Variation of the nutritional composition of quinoa according to the processing used. Proceedings. 2020;53:4. doi: 10.3390/proceedings2020053004. [DOI] [Google Scholar]
- 40.Nowak V., Du J., Charrondière U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.) Food Chem. 2016;193:47–54. doi: 10.1016/j.foodchem.2015.02.111. [DOI] [PubMed] [Google Scholar]
- 41.Navruz-Varli S., Sanlier N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.) J. Cereal Sci. 2016;69:371–376. doi: 10.1016/j.jcs.2016.05.004. [DOI] [Google Scholar]
- 42.Gesinski K., Nowak K. Comparative analysis of the biological value of protein of Chenopodium quinoa willd and Chenopodium album L. Part I. amino acid composition of the seed protein. Acta Sci. Pol.-Agric. 2011;10:47–56. [Google Scholar]
- 43.Eryilmaz-Acikgoz F., Adiloglu S., Solmaz Y., Adiloglu A. Determination of some mineral material content in quinoa greens (Chenopodium quinoa) as a vegetable. Fresenius Environ. Bull. Adv. Food Sci. 2018;27:7108–7111. [Google Scholar]
- 44.Odhav B., Beekrum S., Akula U., Baijnath H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J. Food Comp. Anal. 2007;20:430–435. doi: 10.1016/j.jfca.2006.04.015. [DOI] [Google Scholar]
- 45.Abugoch L. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009;58:1–31. doi: 10.1016/S1043-4526(09)58001-1. [DOI] [PubMed] [Google Scholar]
- 46.Craine E.B., Murphy K.M. Seed Composition and amino acid profiles for quinoa grown in Washington State. Front. Nutr. 2020;7:126. doi: 10.3389/fnut.2020.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saad-Allah K., Youssef M. Phytochemical and genetic characterization of five quinoa (Chenopodium quinoa Willd.) genotypes introduced to Egypt. Physiol. Mol. Biol. Plants. 2018;24:617–629. doi: 10.1007/s12298-018-0541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barzegar M., Erfani F., Jabbari A., Hassandokht M.R. Chemical composition of 15 spinach (Spinacea oleracea L.) cultivars grown in Iran. Ital. J. Food Sci. 2007;19:309–318. [Google Scholar]
- 49.Ayaz F.A., Glew R.H., Millson M., Huang H.S., Chuang L.T., Sanz C., Hayirlioglu-Ayaz S. Nutrient contents of kale (Brassica oleraceae L. var. acephala DC.) Food Chem. 2006;96:572–579. doi: 10.1016/j.foodchem.2005.03.011. [DOI] [Google Scholar]
- 50.Simopoulos A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.González Martín M.I., Wells Moncada G., Fischer S., Escuredo O. Chemical characteristics and mineral composition of quinoa by near-infrared spectroscopy. J. Sci. Food Agric. 2014;94:876–881. doi: 10.1002/jsfa.6325. [DOI] [PubMed] [Google Scholar]
- 52.Lamothe L.M., Srichuwong S., Reuhs B.L., Hamaker B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015;167:490–496. doi: 10.1016/j.foodchem.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Lee C., Giallongo F., Hristov A.N., Lapierre H., Cassidy T.W., Heyler K.S., Varga G.A., Parys C. Effect of dietary protein level and rumen-protected amino acid supplementation on amino acid utilization for milk protein in lactating dairy cows. J. Dairy Sci. 2015;98:1885–1902. doi: 10.3168/jds.2014-8496. [DOI] [PubMed] [Google Scholar]
- 54.Yang H., Ludewig U. Lysine catabolism, amino acid transport, and systemic acquired resistance: What is the link? Plant Signal. Behav. 2014;9:e28933-1. doi: 10.4161/psb.28933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brisibe E., Umoren U., Brisibe F., Magalhäes P., Ferreira J., Luthria D., Wu X., Prior R. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009;115:1240–1246. doi: 10.1016/j.foodchem.2009.01.033. [DOI] [Google Scholar]
- 56.Laus M.N., Cataldi M.P., Robbe C., D’Ambrosio T., Luisa Amodio M., Colelli G., de Santis G., Flagella Z., Pastore D. Antioxidant capacity, phenolic and vitamin C contents of quinoa (Chenopodium quinoa willd.) as affected by sprouting and storage conditions. Ital. J. Agron. 2017;12:63–68. doi: 10.4081/ija.2017.816. [DOI] [Google Scholar]
- 57.Koziol M.J. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.) J. Food Compos. Anal. 1992;5:35–68. doi: 10.1016/0889-1575(92)90006-6. [DOI] [Google Scholar]
- 58.Phillips K.M., Tarrago-Trani M.T., McGinty R.C., Rasor A.S., Haytowitz D.B., Pehrsson P.R. Seasonal variability of the vitamin C content of fresh fruits and vegetables in a local retail market. J. Sci. Food Agric. 2018;98:4191–4204. doi: 10.1002/jsfa.8941. [DOI] [PubMed] [Google Scholar]
- 59.Wang X., Cai X., Xu C., Zhao Q., Ge C., Dai S., Wang Q. Diversity of nitrate, oxalate, vitamin C and carotenoid contents in different spinach accessions and their correlation with various morphological traits. J. Hort. Sci. Biotechnol. 2018;93:409–415. doi: 10.1080/14620316.2017.1404438. [DOI] [Google Scholar]
- 60.Ahamed N., Singhai R., Kulkarni P., Pal M. A lesser-known grain, Chenopodium Quinoa: Review of the chemical composition of its edible parts. Food Nutr. Bull. 1998;19:61–70. doi: 10.1177/156482659801900110. [DOI] [Google Scholar]
- 61.Kaur I., Tanwar B., Reddy M., Chauhan A. Vitamin C, total polyphenols and antioxidant activity in raw, domestically processed and industrially processed Indian Chenopodium quinoa seeds. J. Appl. Pharm. Sci. 2016;6:139–145. doi: 10.7324/JAPS.2016.60419. [DOI] [Google Scholar]
- 62.Sani I.M., Iqbal S., Chan K.W., Ismail M. Effect of acid and base catalyzed hydrolysis on the yield of phenolics and antioxidant activity of extracts from germinated brown rice (GBR) Molecules. 2012;17:7584–7594. doi: 10.3390/molecules17067584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filho A.M.M., Pirozi M.R., Borges J.T.D.S., Sant’Ana H.M.P., Chaves J.B.P., Coimbra J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017;57:1618–1630. doi: 10.1080/10408398.2014.1001811. [DOI] [PubMed] [Google Scholar]
- 64.Piñuel L., Boeri P., Zubillaga F., Barrio D.A., Torreta J., Cruz A., Vásquez G., Pinto A., Carrillo W. Production of white, red and black quinoa (Chenopodium quinoa Willd Var. Real) protein isolates and its hydrolysates in germinated and nongerminated quinoa samples and antioxidant activity evaluation. Plants. 2019;8:257. doi: 10.3390/plants8080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oghbaei M., Prakash J. Nutritional properties of green gram germinated in mineral fortified soak water: I. Effect of dehulling on total and bioaccessible nutrients and bioactive components. J. Food Sci. Technol. 2017;54:880889. doi: 10.1007/s13197-016-2382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Televiciute D., Taraseviciene Z., Danilcenko H., Barcauskaite K., Kandaraite M., Paulauskiene A. Changes in chemical composition of germinated leguminous under abiotic stress conditions. Food Sci. Technol. 2020;40:415421. doi: 10.1590/fst.23019. [DOI] [Google Scholar]
- 67.Abderrahim F., Huanatico E., Segura R., Arribas S., Gonzalez M., Condezo-Hoyos L. Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015;183:83–90. doi: 10.1016/j.foodchem.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 68.Han Y., Chi J., Zhang M., Zhang R., Fan S., Huang F., Xue K., Liu L. Characterization of saponins and phenolic compounds: Antioxidant activity and inhibitory effects on glucosidase in different varieties of colored quinoa (Chenopodium quinoa Willd) Biosci. Biotechnol. Biochem. 2019;83:2128–2139. doi: 10.1080/09168451.2019.1638756. [DOI] [PubMed] [Google Scholar]
- 69.Huang J., Qin L., Shi Q., Wen A. Effect of quinoa saponins extraction and sprouting on saponins content. [(accessed on 27 October 2021)];J. Chin. Cereals Oils Assoc. 2017 32:3439. Available online: https://en.cnki.com.cn/Article_en/CJFDTotalZLYX201711008.htm. [Google Scholar]
- 70.Baiano A., del Nobile M.A. Antioxidant Compounds from Vegetable Matrices: Biosynthesis, Occurrence, and Extraction Systems. Crit. Rev. Food Sci. Nutr. 2016;56:2053–2068. doi: 10.1080/10408398.2013.812059. [DOI] [PubMed] [Google Scholar]
- 71.Prakash D., Nath P., Pal M. Composition, variation of nutritional content in leaves, seed protein, fat and fatty acid profile of Chenopodium species. J. Sci. Food. Agric. 1993;62:203–205. doi: 10.1002/jsfa.2740620214. [DOI] [Google Scholar]
- 72.Diaz-Valencia Y.K., Alca J.J., Calori-Domingues M.A., Zanabria-Galvez S.J., da Cruz S.H. Nutritional composition, total phenolic compounds and antioxidant activity of quinoa (Chenopodium quinoa Willd.) of different colours. Nova Biotechnol. Chim. 2018;17:74–85. doi: 10.2478/nbec-2018-0008. [DOI] [Google Scholar]
- 73.Navarro del Hierro J., Herrera T., García-Risco M.R., Fornari T., Reglero G., Martin D. Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: Quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 2018;109:440–447. doi: 10.1016/j.foodres.2018.04.058. [DOI] [PubMed] [Google Scholar]
- 74.Fiallos-Jurado J., Pollier J., Moses T., Arendt P., Barriga-Medina N., Morillo E., Arahana V., de Lourdes Torres M., Goossens A., Leon-Reyes A. Saponin determination, expression analysis and functional characterization of saponin biosynthetic genes in Chenopodium quinoa leaves. Plant Sci. 2016;250:188–197. doi: 10.1016/j.plantsci.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 75.Lim J.G., Park H.M., Yoon K.S. Analysis of saponin composition and comparison of the antioxidant activity of various parts of the quinoa plant (Chenopodium quinoa Willd.) Food Sci. Nutr. 2020;8:694–702. doi: 10.1002/fsn3.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mastebroek H.D., Limburg H., Gilles T., Marvin H.J.P. Occurrence of sapogenins in leaves and seeds of quinoa (Chenopodium quinoa Willd) J. Sci. Food Agric. 2000;80:152–156. doi: 10.1002/(SICI)1097-0010(20000101)80:1<152::AID-JSFA503>3.0.CO;2-P. [DOI] [Google Scholar]
- 77.Graf B.L., Rojas-Silva P., Rojo L.E., Delatorre-Herrera J., Baldeón M.E., Raskin I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.) Compr. Rev. Food Sci. Food Saf. 2015;14:431–445. doi: 10.1111/1541-4337.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castro-Alba V., Lazarte C.E., Perez-Rea D., Carlsson N.G., Almgren A., Bergenståhl B., Granfeldt Y. Fermentation of pseudocereals quinoa, canihua, and amaranth to improve mineral accessibility through degradation of phytate. J. Sci. Food Agric. 2019;99:5239–5248. doi: 10.1002/jsfa.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reguera M., Conesa C.M., Gil-Gómez A., Haros C.M., Pérez-Casas M.Á., Briones-Labarca V., Bolaños L., Bonilla I., Álvare B., Pinto K., et al. The impact of different agroecological conditions on the nutritional composition of quinoa seeds. PeerJ. 2018;2018:e4442. doi: 10.7717/peerj.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sood P., Modgil R., Sood M., Chuhan P.K. Anti-nutrient profile of different chenopodium cultivars leaves. Annals Food Sci. Technol. 2012;13:68–74. [Google Scholar]
- 81.Siener R., Hönow R., Seidler A., Voss S., Hesse A. Oxalate contents of species of the Polygonaceae, Amaranthaceae and Chenopodiaceae families. Food Chem. 2006;98:220–224. doi: 10.1016/j.foodchem.2005.05.059. [DOI] [Google Scholar]
- 82.Guil J.L., Torija M.E., Giménez J.J., Rodríguez-García I., Giménez A. Oxalic acid and calcium determination in wild edible plants. J. Agric. Food Chem. 1996;44:1821–1823. doi: 10.1021/jf950472a. [DOI] [Google Scholar]
- 83.Savage G., Vanhanen L. Oxalate contents of raw, boiled, wok-fried and pesto and juice made from fat hen (Chenopodium album) leaves. Foods. 2019;8:2. doi: 10.3390/foods8010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valencia-Chamorro S. Quinoa-Overview. In: Wrigley C., Corke H., Seetharaman K., Faubion J., editors. Encyclopedia of Food Grains. 2nd ed. Volume 1. Academic Press; Oxford, UK: 2016. pp. 341–348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.