Abstract
Background: Evaluating muscle mass and function among stroke patients is important. However, evaluating muscle volume and function is not easy due to the disturbances of consciousness and paresis. Temporal muscle thickness (TMT) has been introduced as a novel surrogate marker for muscle mass, function, and nutritional status. We herein performed a narrative literature review on temporal muscle and stroke to understand the current meaning of TMT in clinical stroke practice. Methods: The search was performed in PubMed, last updated in October 2021. Reports on temporal muscle morphomics and stroke-related diseases or clinical entities were collected. Results: Four studies reported on TMT and subarachnoid hemorrhage, two studies on intracerebral hemorrhage, two studies on ischemic stroke, two studies on standard TMT values, and two studies on nutritional status. TMT was reported as a prognostic factor for several diseases, a surrogate marker for skeletal muscle mass, and an indicator of nutritional status. Computed tomography, magnetic resonance imaging, and ultrasonography were used to measure TMT. Conclusions: TMT is gradually being used as a prognostic factor for stroke or a surrogate marker for skeletal muscle mass and nutritional status. The establishment of standard methods to measure TMT and large prospective studies to further investigate the relationship between TMT and diseases are needed.
Keywords: frailty, muscle volume, nutritional status, prognostic factor, sarcopenia, skeletal muscle mass, stroke, temporal muscle thickness
1. Introduction
Stroke is a widely known cause of disability [1]. Stroke also increases the risk of skeletal muscle loss [2]—sarcopenia—which contributes to further disability related to stroke [3]. Furthermore, pre-stroke sarcopenia is also associated with poor functional outcomes [4,5]. Therefore, evaluating muscle mass and function among stroke patients is important [6,7], and aggressive nutrition therapy [8,9,10], deprescribing [11], and rehabilitation [12,13] re applicable for those with stroke, as well as those at high risk for muscle loss.
Measuring skeletal muscle mass and function is an evolving parameter for the clinical evaluation of physiological conditions [14]. The gold standard to evaluate sarcopenia are muscle function tests such as the gait speed test and the grip strength test, according to the European Working Group on Sarcopenia in Older People (EWGSOP), EWGSOP2, and the Asian Working Group for Sarcopenia (AGWS) [15,16]. However, measuring muscle function such as grip strength and gait speed sometimes cannot be performed because stroke patients often have disturbances of consciousness, are sedated, are resting due to surgical treatment, or experience paresis. Therefore, an alternative method to evaluate muscle mass and function is needed.
Recently, temporal muscle thickness (TMT) on computed tomography (CT) images or magnetic resonance images (MRI) has been introduced as a novel surrogate marker with which to measure muscle mass [17], function [18], and nutritional status [19,20]. CT and MRI are routinely performed for stroke patients, and TMT measurement is easier than other methods such as quantitative measurements of the cross-sectional skeletal muscle area at the third lumbar vertebra using CT imaging, which is known to be significantly correlated with whole-body muscle [16]. Therefore, TMT is attractive as an alternative method to evaluate muscle mass and function for stroke patients. The first purpose of the narrative literature review that we present herein is to investigate reports on TMT and stroke. The second purpose is to understand the current meaning of TMT in clinical stroke practice. In addition to TMT [21,22], we also examined temporal muscle area (TMA) [21,23,24] and temporal muscle volume (TMV) [9] as novel TM-related surrogate markers for skeletal muscle mass.
2. Materials and Methods
Studies regarding TMT and stroke were examined. The search was performed in PubMed, last updated in October 2021, using the terms “stroke” OR “intracerebral hemorrhage (ICH)” OR “subarachnoid hemorrhage (SAH)” OR “cerebral infarction” OR “rehabilitation” OR “sarcopenia” OR “frailty” OR “nutrition” AND “temporal muscle thickness”. The PubMed search resulted in a total of 73 articles. We systematically read through the abstracts of all original articles available in English. We included studies on the association between stroke and TMT, with a sample size of around 50 cases and appropriate statistical analyses. We also checked through the lists of references to complete our collection of studies. All the authors verified the correct transcription of the data to our manuscript. Finally, we included eight studies related to stroke in our review.
3. Results
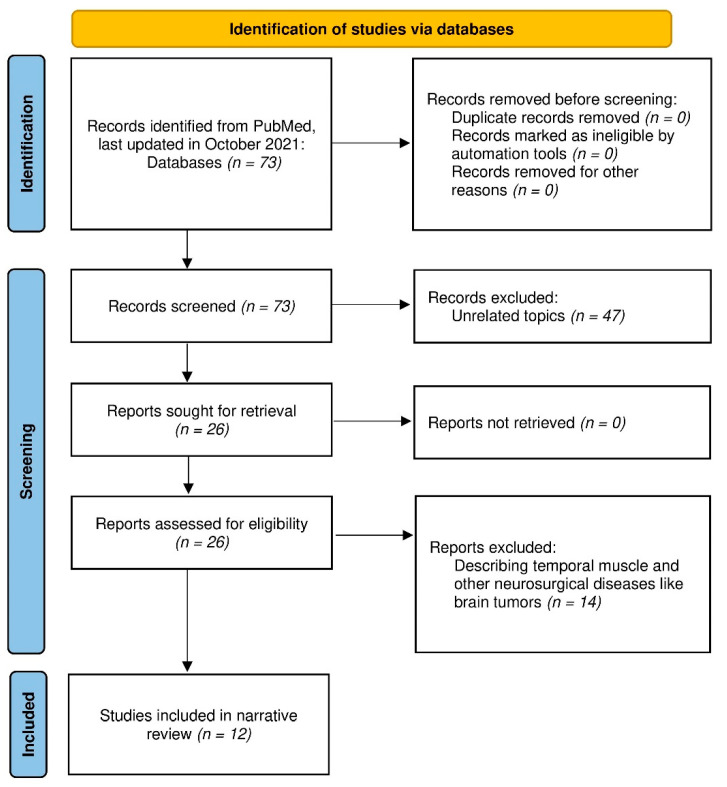
Four studies reported the association between TMT and SAH [9,21,23,24], two studies between TMT and ICH [25,26], and two studies between TMT and ischemic stroke [27,28]. The other four studies described the standard TMT values [18,29] and the relationship between TMT and nutritional status [19,20]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram [30] for systematic review is shown as Figure 1.
Figure 1.
PRISMA 2020 flow diagram for this review.
3.1. Temporal Muscle and SAH
Katsuki et al. first reported TMT as a prognostic factor for SAH outcomes in 2019, investigating 49 SAH patients over 75 years of age who were treated by clipping with craniotomy [21]. TMT was measured on CT images on admission, using Aquilion ONE (Canon Medical Systems Corporation, Tochigi, Japan) with 0.5 × 0.5 x 1.0 mm voxels. The slice thickness was reconstructed to 5 mm. The window width was adjusted to 300 Hounsfield units and the window level was adjusted to 20 Hounsfield units. TMT was measured bilaterally perpendicular to the long axis of the temporal muscle at a slice 5 mm above the orbital roof using SYNAPSE V 4.1.5 imaging software (Fujifilm Medical, Tokyo, Japan). Then, the averages of the left and right of the TMTs were used. The method to measure TMT on CT was thereby defined. Katsuki et al. then performed univariate analysis regarding TMT and functional outcome at six months. The study was preliminary, but the study suggested that greater TMT was related to favorable outcomes among elderly SAH.
Katsuki et al. next investigated the relationship between temporal muscle and Hunt and Kosnik grade on admission and functional outcome at six months [23]. They examined 298 all age-group patients, and all patients were treated by endovascular coiling. They revealed that the Hunt and Kosnik grade on admission and functional outcome were related to TMT and TMA. TMA was measured manually by tracing the outline of the temporal muscle on the same CT slice as that used for measuring TMT. Notably, this study suggests that TMT and TMA are related to both the severity of SAH and functional outcome regardless of age, not only for the elderly.
They then investigated 127 SAH patients under 75 years of age who were treated by clipping [24]. They examined the cut-off values for the functional outcomes. Receiver operating characteristic analysis found that the threshold of TMT was 4.9 mm in women and 6.7 mm in men, and that of TMA was 193 mm2 in women and 333 mm2 in men, which were the cut-off values for the functional outcomes among SAH patients under 75 years of age.
Onodera et al. [9] examined TMV using volume rendering software (Ziostation 2 version 2.9.5.1, Ziosoft, Tokyo), because TMT may be less reproducible. They investigated 60 SAH patients and measured TMV on the CT images at admission and two weeks after aneurysm treatment. Patients whose TMV had decreased by ≥20% were classified into the “atrophy group,” whereas those whose TMV had decreased by <20% were classified into the “maintenance group.” Their study showed that the food intake score and the functional outcome were significantly more positive in the TMV maintenance group than the TMV atrophy group. Therefore, this study suggests the importance of early high-protein administration to maintain TMV in the acute term (Table 1).
Table 1.
Previous reports on the association between temporal muscle and SAH.
| Author | Year | Number of Cases | Abstract |
|---|---|---|---|
| Katsuki [21] | 2019 | 49 | High TMT was related to favorable outcomes among elderly SAH. |
| Katsuki [23] | 2020 | 298 | TMT and TMA were related to Hunt and Kosnik grade and functional outcome at six months after endovascular coiling, regardless of age. |
| Katsuki [24] | 2021 | 127 | The threshold of TMT was 4.9 mm in women and 6.7 mm in men, and that of TMA was 193 mm2 in women and 333 mm2 in men, which were the cut-off values for the functional outcomes at six months among SAH patients under 75 years of age. |
| Onodera [9] | 2021 | 60 | The food intake score and the functional outcome at discharge were significantly more positive in the TMV maintenance group than the TMV atrophy group after SAH. |
Abbreviations: SAH: subarachnoid hemorrhage; TMA: temporal muscle area; TMT: temporal muscle thickness; TMV: temporal muscle volume.
3.2. Temporal Muscle and ICH
Katsuki et al. examined 75 ICH patients treated by endoscopic hematoma removal and investigated the factors related to the functional outcome [25]. They revealed that lower total protein level was related to poor outcomes at six months. In addition, they mentioned TMA as an indicator of nutrition, but TMA itself was not significantly related to the outcome (p = 0.08). However, they suggested that low nutritional status, indicated by lower total protein level and low TMA altogether, seemed to be associated with poor outcomes.
Gomes et al. examined 24 post-hemorrhagic stroke patients in the chronic stage and tested bite force and TMT [26]. Maximum molar bite force was verified using a digital dynamometer. TMT was measured using ultrasound images obtained at rest and during maximal voluntary contraction of the masseter and temporalis muscles. The TMT on the unaffected side was larger than on the affected side. This study first focused on the functional and morphological changes in the stomatognathic system after a hemorrhagic stroke. The clinical meaning of these changes was investigated (Table 2).
Table 2.
Previous reports on the association between temporal muscle and ICH.
| Author | Year | Number of Cases | Abstract |
|---|---|---|---|
| Katsuki [25] | 2019 | 75 | Low nutritional status, indicated by low total protein level and low TMA altogether, seemed to be associated with the poor functional outcomes at six months after endoscopic hematoma removal. |
| Gomes [26] | 2021 | 24 | TMT on the unaffected side was greater than on the affected side after a hemorrhagic stroke. |
Abbreviations: ICH: intracerebral hemorrhage; TMA: temporal muscle area; TMT: temporal muscle thickness.
3.3. Temporal Muscle and Stroke
Sakai et al. [27] investigated 70 acute cerebral infarction patients’ TMT on the T2-weight MR image and functional oral intake scales. They revealed that TMT was a significant explanator of dysphagia severity following acute ischemic stroke, along with age and the National Institute of Health Stroke Scale score. The measuring method of TMT using T2-weighted images was similar to the previous report from Furtner et al. using T1-weighted images [31]. They first reported the association between TMT and ischemic stroke-related dysphagia in the acute term.
Nozoe et al. [28] examined 289 acute elderly stroke patients and investigated TMT on CT images as an indicator of sarcopenia risk and its relationship with the functional outcome at three months. They found that sarcopenia risk was independently associated with TMT in older patients with acute stroke. However, TMT was not independently related to the functional outcome (Table 3).
Table 3.
Previous reports on the association between temporal muscle and stroke.
| Author | Year | Number of Cases | Abstract |
|---|---|---|---|
| Sakai [27] | 2021 | 70 | TMT was a significant explanator of dysphagia severity following acute ischemic stroke. |
| Nozoe [28] | 2021 | 289 | Sarcopenia risk was independently associated with TMT in older patients with acute stroke, but TMT was not independently related to the functional outcome. |
Abbreviations: TMT: temporal muscle thickness.
3.4. Standard Values of TMT
Steindl et al. [18] investigated a 624-individual MRI dataset to establish standard reference values of TMT on T1-weighted images. The cohort consisted of two MRI repositories: The Enhanced Nathan Kline Institute-Rockland Sample [32]; and the Designed Database of MR Brain Images of Healthy Volunteers [33]. TMT was measured on isovoxel (1 × 1 × 1 mm3) T1-weighted MR images perpendicular to the long axis of the temporal muscle on an axial plane, which was oriented parallel to the anterior commissure-posterior commissure line. They also examined 422 healthy volunteers and 130 cases as a prospective validation cohort and found that TMT and grip strength were correlated. This was the first report to validate the relationship between the TMT and grip strength, namely muscle function, prospectively.
Katsuki et al. [29] investigated a database of 360 Japanese individuals’ brain check-ups obtained by MRI. They measured TMT in the same way previously reported in [18] to obtain standard values of TMT among Japanese individuals. They compared their result to Steindl’s results to obtain the racial difference, but the background of the participants differed. They did not perform any muscle function test, so further investigation is needed (Table 4).
Table 4.
TMT and nutritional status.
| Author | Year | Number of Cases | Abstract |
|---|---|---|---|
| Steindl [18] | 2020 | 1175 | Standard values of TMT were investigated, and TMT and grip strength were correlated. |
| Katsuki [29] | 2021 | 360 | Standard values of TMT were investigated among Japanese individuals who underwent brain check-ups. |
Abbreviations: TMT: temporal muscle thickness.
3.5. TMT and Nutritional Status
Hasegawa et al. [20] investigated 73 elderly individuals to measure their TMT using ultrasonography and nutritional status assessed with anthropometric measurements and laboratory tests. Arm circumference (AC) was measured in the middle of the non-dominant upper arm using a measuring tape. Arm muscle circumference (AMC) was calculated based on the standard procedure using the following formula: AMC (cm) = AC (cm)−π x the triceps skinfold thickness (cm) [34]. Calf circumference (CC) was measured with a tape at the maximum girth of the right calf with the leg in a lying position. TMT was strongly correlated with CC and AMC. However, there were no strong correlations with serum protein levels, nor was fat mass evaluated in the triceps skinfold thickness. They also examined the reliability to measure TMT using ultrasonography; the inter-rater reliability was 0.99.
Hasegawa et al. also performed a prospective study [19]. The study aimed to examine whether a change in TMT evaluated by the ultrasonography was directly correlated with energy adequacy, and to determine the cut-off value of a change in TMT to detect energy inadequacy. They investigated 48 bedridden elderly patients and revealed that percentage change in TMT was significantly correlated with energy adequacy. They suggested that the assessment of TMT changes could be helpful for performing better nutritional therapy (Table 5).
Table 5.
TMT and nutritional status.
| Author | Year | Number of Cases | Abstract |
|---|---|---|---|
| Hasegawa [20] | 2019 | 73 | TMT was strongly correlated with CC and ACM. However, there were no strong correlations with serum protein levels, nor was fat mass evaluated in the triceps skinfold thickness. |
| Hasegawa [19] | 2021 | 48 | TMT changes were directly correlated with energy adequacy in bedridden older adults. |
Abbreviations: ACM: arm muscle circumference; CC: Calf circumference; TMT: temporal muscle thickness.
4. Discussion
We herein reviewed reports on TMT and stroke. TMT is useful as a prognostic marker for SAH, ICH, and dysphagia after stroke. It also indicates nutritional status and risk of sarcopenia. As the number of reports on TMT and stroke has been increasing rapidly in recent years, we believe that TMT is one of the important factors in clinical practice. In addition to this review, we discussed the TMT measurement method and TMT use in other neurosurgical practices.
4.1. TMT Measurement Method
A standard TMT measurement method has not been established. Old reports used volume rendering software [35,36], and Onodera et al. also used a similar approach [9] to measure TMV, but not TMT. Then, Furtner et al. established TMT measurement using T1-weighted MR images. They measured TMT perpendicular to the long axis of the temporal muscle at the level of the orbital roof [14,17,18,37,38]. This method is widely used, but low accessibility to MRI in routine work is a problem. Sakai et al. [27] used T2-weighted MR images, rather than T1-weighted images. The difference between the T1- and T2-weighted images should be discussed. Katsuki et al.first defined TMT and TMA on CT images [21]. CT is more accessible than MRI, so TMT measurement on CT seems better for routine clinical work. Hasegawa et al. used ultrasonography (M-Turbo; SonoSite, Bothell, WA, USA) to measure TMT at 4 cm from the eyelid and 2 cm above the reference line, which was the orbitomeatal line [20]. Ultrasonography is not so reproducible, but their study reported that TMT measurement by ultrasonography is reliable.
As described above, there are some ways to measure temporal muscle morphomics, including TMT, TMA, and TMV. Easiness and high reproducibility are needed to establish a standard method. Further study on the measurement method is desirable.
4.2. Temporal Muscle in Other Neurosurgical Practice
The first report on the temporal muscle as a prognostic factor in neurosurgical practice evaluated the operative risk in non-syndromic craniosynostosis in 2013 [36]. The authors used volume rendering software to assess the temporal fat pad. Since this report, there have been several papers on the temporalis muscle and prognosis, especially in brain tumors. There are several reports on overall survival and temporal muscle in glioblastoma [38,39,40,41,42,43,44,45], metastatic brain tumor [31,46,47], and primary central nervous system lymphoma [37,48]. As in reports on TMT and stroke, all of these reports used temporal muscle to indicate nutritional status and skeletal muscle mass volume. The greater the temporal muscle, the better the outcome, probably due to better nutritional status and more skeletal muscle mass. Furthermore, deep learning-based quantification of TMA has been reported [49], so it is expected that TMA measuring will be widely performed.
4.3. Limitations
As described above, TMT is now attractive, and many studies have been performed, but some issues should be addressed. First, most of the studies were retrospective, so further prospective study is needed. Second, the sample sizes were small, so studies with large sample sizes are desirable. Third, a standard TMT measurement method has not been established, and several methods can be used, such as MRI, CT, and ultrasonography. A standard approach to measuring TMT is needed. Fourth, the direct mechanism of why large temporal muscle relates to favorable prognosis has not been clarified. The true mechanism between TMT and outcomes should be discussed from several perspectives, such as rehabilitation, nutrition, frailty, deglutition, or basic medicine. Some of the problems may be resolved as TMT measurements are routinely taken, thereby tracking time-course changes.
5. Conclusions
TMT seems to be useful surrogate marker for skeletal muscle volume and function, and is a potential prognostic factor. Research on the association between stroke and TMT is increasing. Further research is needed to establish the usefulness of TMT.
Acknowledgments
We thank the hospital staff.
Funding
None.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This is a review of literature, so informed consent statement is not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Toyoda K., Sonoda K., Sato S., Yoshimura S. The Japan Stroke Databank -Current stroke care in Japan and ideal stroke registries. Neurol. Ther. 2018;35:188–192. doi: 10.15082/jsnt.35.3_188. (In Japanese) [DOI] [Google Scholar]
- 2.Ryan A.S., Ivey F.M., Serra M.C., Hartstein J., Hafer-Macko C.E. Sarcopenia and Physical Function in Middle-Aged and Older Stroke Survivors. Arch. Phys. Med. Rehabilitation. 2016;98:495–499. doi: 10.1016/j.apmr.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherbakov N., von Haehling S., Anker S.D., Dirnagl U., Doehner W. Stroke induced Sarcopenia: Muscle wasting and disability after stroke. Int. J. Cardiol. 2013;170:89–94. doi: 10.1016/j.ijcard.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura Y., Wakabayashi H., Bise T., Nagano F., Shimazu S., Shiraishi A., Yamaga M., Koga H. Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition. 2019;61:111–118. doi: 10.1016/j.nut.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Nozoe M., Kanai M., Kubo H., Yamamoto M., Shimada S., Mase K. Prestroke sarcopenia and functional outcomes in elderly patients who have had an acute stroke: A prospective cohort study. Nutrition. 2019;66:44–47. doi: 10.1016/j.nut.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Nozoe M., Kubo H., Kanai M., Yamamoto M. Relationships between Pre-Stroke SARC-F Scores, Disability, and Risk of Malnutrition and Functional Outcomes after Stroke—A Prospective Cohort Study. Nutrients. 2021;13:3586. doi: 10.3390/nu13103586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakanishi N., Okura K., Okamura M., Nawata K., Shinohara A., Tanaka K., Katayama S. Measuring and Monitoring Skeletal Muscle Mass after Stroke: A Review of Current Methods and Clinical Applications. J. Stroke Cerebrovasc. Dis. 2021;30:105736. doi: 10.1016/j.jstrokecerebrovasdis.2021.105736. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura Y., Wakabayashi H., Momosaki R., Nagano F., Bise T., Shimazu S., Shiraishi A. Stored Energy Increases Body Weight and Skeletal Muscle Mass in Older, Underweight Patients after Stroke. Nutrients. 2021;13:3274. doi: 10.3390/nu13093274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onodera H., Mogamiya T., Matsushima S., Sase T., Kawaguchi K., Nakamura H., Sakakibara Y. High protein intake after subarachnoid hemorrhage improves oral intake and temporal muscle volume. Clin. Nutr. 2021;40:4187–4191. doi: 10.1016/j.clnu.2021.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Onodera H., Mogamiya T., Mori M., Matsushima S., Sase T., Nakamura H., Sakakibara Y. High protein intake after subarachnoid hemorrhage improves ingestion function and temporal muscle volume. Clin. Nutr. ESPEN. 2020;40:595. doi: 10.1016/j.clnesp.2020.09.566. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto A., Yoshimura Y., Wakabayashi H., Kose E., Nagano F., Bise T., Kido Y., Shimazu S., Shiraishi A. Deprescribing Leads to Improved Energy Intake among Hospitalized Older Sarcopenic Adults with Polypharmacy after Stroke. Nutrients. 2022;14:443. doi: 10.3390/nu14030443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura Y., Wakabayashi H., Nagano F., Bise T., Shimazu S., Shiraishi A., Kido Y., Matsumoto A. Chair-Stand Exercise Improves Sarcopenia in Rehabilitation Patients after Stroke. Nutrients. 2022;14:461. doi: 10.3390/nu14030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimura Y. Treating sarcopenia with a hybrid of aggressive exercise therapy and nutritional therapy: Approaches at Kumamoto Rehabilitation Hospital. Mon. B Med. Rehabil. 2018;224:16–24. (In Japanese) [Google Scholar]
- 14.Furtner J., Weller M., Weber M., Gorlia T., Nabors B., Reardon D.A., Tonn J.-C., Stupp R., Preusser M. Temporal Muscle Thickness as a Prognostic Marker in Patients with Newly Diagnosed Glioblastoma: Translational Imaging Analysis of the CENTRIC EORTC 26071–22072 and CORE Trials. Clin. Cancer Res. 2021;28:129–136. doi: 10.1158/1078-0432.CCR-21-1987. [DOI] [PubMed] [Google Scholar]
- 15.Chen L.-K., Liu L.-K., Woo J., Assantachai P., Auyeung T.-W., Bahyah K.S., Chou M.-Y., Chen L.-Y., Hsu P.-S., Krairit O., et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitner J., Pelster S., Schöpf V., Berghoff A.S., Woitek R., Asenbaum U., Nenning K.-H., Widhalm G., Kiesel B., Gatterbauer B., et al. High correlation of temporal muscle thickness with lumbar skeletal muscle cross-sectional area in patients with brain metastases. PLoS ONE. 2018;13:e0207849. doi: 10.1371/journal.pone.0207849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steindl A., Leitner J., Schwarz M., Nenning K.-H., Asenbaum U., Mayer S., Woitek R., Weber M., Schöpf V., Berghoff A.S., et al. Sarcopenia in Neurological Patients: Standard Values for Temporal Muscle Thickness and Muscle Strength Evaluation. J. Clin. Med. 2020;9:1272. doi: 10.3390/jcm9051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa Y., Yoshida M., Sato A., Fujimoto Y., Minematsu T., Sugama J., Sanada H. A change in temporal muscle thickness is correlated with past energy adequacy in bedridden older adults: A prospective cohort study. BMC Geriatr. 2021;21:1–9. doi: 10.1186/s12877-021-02086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa Y., Yoshida M., Sato A., Fujimoto Y., Minematsu T., Sugama J., Sanada H. Temporal muscle thickness as a new indicator of nutritional status in older individuals. Geriatr. Gerontol. Int. 2019;19:135–140. doi: 10.1111/ggi.13570. [DOI] [PubMed] [Google Scholar]
- 21.Katsuki M., Yamamoto Y., Uchiyama T., Wada N., Kakizawa Y. Clinical characteristics of aneurysmal subarachnoid hemorrhage in the elderly over 75; would temporal muscle be a potential prognostic factor as an indicator of sarcopenia? Clin. Neurol. Neurosurg. 2019;186:105535. doi: 10.1016/j.clineuro.2019.105535. [DOI] [PubMed] [Google Scholar]
- 22.Gonçalves R.C.G., Rabelo N.N., Figueiredo E.G., Welling L.C. Oral health and temporal muscle thickness. Surg. Neurol. Int. 2021;12:527. doi: 10.25259/SNI_905_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsuki M., Suzuki Y., Kunitoki K., Sato Y., Sasaki K., Mashiyama S., Matsuoka R., Allen E., Saimaru H., Sugawara R., et al. Temporal Muscle as an Indicator of Sarcopenia is Independently Associated with Hunt and Kosnik Grade on Admission and the Modified Rankin Scale Score at 6 Months of Patients with Subarachnoid Hemorrhage Treated by Endovascular Coiling. World Neurosurg. 2020;137:e526–e534. doi: 10.1016/j.wneu.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Katsuki M., Kakizawa Y., Nishikawa A., Yamamoto Y., Uchiyama T. Temporal muscle thickness and area are an independent prognostic factors in patients aged 75 or younger with aneurysmal subarachnoid hemorrhage treated by clipping. Surg. Neurol. Int. 2021;12:151. doi: 10.25259/SNI_814_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsuki M., Kakizawa Y., Nishikawa A., Yamamoto Y., Uchiyama T. Lower total protein and absence of neuronavigation are novel poor prognostic factors of endoscopic hematoma removal for intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2020;29:105050. doi: 10.1016/j.jstrokecerebrovasdis.2020.105050. [DOI] [PubMed] [Google Scholar]
- 26.Gomes G.G.C., Palinkas M., da Silva G.P., Gonçalves C.R., Lopes R.F.T., Verri E.D., Fabrin S.C.V., Fioco E.M., Siéssere S., Regalo S.C.H. Bite Force, Thickness, and Thermographic Patterns of Masticatory Muscles Post-Hemorrhagic Stroke. J. Stroke Cerebrovasc. Dis. 2021;31:106173. doi: 10.1016/j.jstrokecerebrovasdis.2021.106173. [DOI] [PubMed] [Google Scholar]
- 27.Sakai K., Katayama M., Nakajima J., Inoue S., Koizumi K., Okada S., Suga S., Nomura T., Matsuura N. Temporal muscle thickness is associated with the severity of dysphagia in patients with acute stroke. Arch. Gerontol. Geriatr. 2021;96:104439. doi: 10.1016/j.archger.2021.104439. [DOI] [PubMed] [Google Scholar]
- 28.Nozoe M., Kubo H., Kanai M., Yamamoto M., Okakita M., Suzuki H., Shimada S., Mase K. Reliability and validity of measuring temporal muscle thickness as the evaluation of sarcopenia risk and the relationship with functional outcome in older patients with acute stroke. Clin. Neurol. Neurosurg. 2021;201:106444. doi: 10.1016/j.clineuro.2020.106444. [DOI] [PubMed] [Google Scholar]
- 29.Katsuki M., Narita N., Sasaki K., Sato Y., Suzuki Y., Mashiyama S., Tominaga T. Standard values for temporal muscle thickness in the Japanese population who undergo brain check-up by magnetic resonance imaging. Surg. Neurol. Int. 2021;12:67. doi: 10.25259/SNI_3_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furtner J., Berghoff A.S., Albtoush O.M., Woitek R., Asenbaum U., Prayer D., Widhalm G., Gatterbauer B., Dieckmann K., Birner P., et al. Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur. Radiol. 2017;27:3167–3173. doi: 10.1007/s00330-016-4707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nooner K.B., Colcombe S.J., Tobe R.H., Mennes M., Benedict M.M., Moreno A.L., Panek L.J., Brown S., Zavitz S.T., Li Q., et al. The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Front. Behav. Neurosci. 2012;6:152. doi: 10.3389/fnins.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullitt E., Zeng D., Gerig G., Aylward S., Joshi S., Smith J.K., Lin W., Ewend M.G. Vessel Tortuosity and Brain Tumor Malignancy: A Blinded Study1. Acad. Radiol. 2005;12:1232–1240. doi: 10.1016/j.acra.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriwaki E.-I., Enomoto H., Saito M., Hara N., Nishikawa H., Nishimura T., Iwata Y., Iijima H., Nishiguchi S. The Anthropometric Assessment with the Bioimpedance Method Is Associated with the Prognosis of Cirrhotic Patients. Vivo. 2020;34:687–693. doi: 10.21873/invivo.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranganathan K., Terjimanian M., Lisiecki J., Rinkinen J., Mukkamala A., Brownley C., Buchman S.R., Wang S.C., Levi B. Temporalis muscle morphomics: The psoas of the craniofacial skeleton. J. Surg. Res. 2014;186:246–252. doi: 10.1016/j.jss.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 36.Rinkinen J., Zhang P., Wang L., Enchakalody B., Terjimanian M., Holcomb S., Wang S.C., Buchman S.R., Levi B. Novel Temporalis Muscle and Fat Pad Morphomic Analyses Aids Preoperative Risk Evaluation and Outcome Assessment in Nonsyndromic Craniosynostosis. J. Craniofacial Surg. 2013;24:250–255. doi: 10.1097/SCS.0b013e31827006f5. [DOI] [PubMed] [Google Scholar]
- 37.Furtner J., Nenning K.-H., Roetzer T., Gesperger J., Seebrecht L., Weber M., Grams A., Leber S., Marhold F., Sherif C., et al. Evaluation of the Temporal Muscle Thickness as an Independent Prognostic Biomarker in Patients with Primary Central Nervous System Lymphoma. Cancers. 2021;13:566. doi: 10.3390/cancers13030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furtner J., Genbrugge E., Gorlia T., Bendszus M., Nowosielski M., Golfinopoulos V., Weller M., Bent M.J.V.D., Wick W., Preusser M. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: Translational imaging analysis of the EORTC 26101 trial. Neuro-Oncol. 2019;21:1587–1594. doi: 10.1093/neuonc/noz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh K., Hwang M., Estevez-Inoa G., Saraf A., Spina C., Smith D., Wu C., Wang T. Temporalis Muscle Width as a Measure of Sarcopenia Independently Predicts Overall Survival in Patients with Newly Diagnosed Glioblastoma. Int. J. Radiat. Oncol. 2018;102:e225. doi: 10.1016/j.ijrobp.2018.07.771. [DOI] [Google Scholar]
- 40.An G., Ahn S., Park J.-S., Jeun S.S., Kil Hong Y. Association between temporal muscle thickness and clinical outcomes in patients with newly diagnosed glioblastoma. J. Cancer Res. Clin. Oncol. 2020;147:901–909. doi: 10.1007/s00432-020-03386-5. [DOI] [PubMed] [Google Scholar]
- 41.Cinkir H.Y., Er H.C. Is temporal muscle thickness a survival predictor in newly diagnosed glioblastoma multiforme? Asia-Pacific J. Clin. Oncol. 2020;16:e223–e227. doi: 10.1111/ajco.13369. [DOI] [PubMed] [Google Scholar]
- 42.Muglia R., Simonelli M., Pessina F., Morenghi E., Navarria P., Persico P., Lorenzi E., Dipasquale A., Grimaldi M., Scorsetti M., et al. Prognostic relevance of temporal muscle thickness as a marker of sarcopenia in patients with glioblastoma at diagnosis. Eur. Radiol. 2020;31:4079–4086. doi: 10.1007/s00330-020-07471-8. [DOI] [PubMed] [Google Scholar]
- 43.Liu F., Xing D., Zha Y., Wang L., Dong W., Li L., Gong W., Hu L. Predictive Value of Temporal Muscle Thickness Measurements on Cranial Magnetic Resonance Images in the Prognosis of Patients with Primary Glioblastoma. Front. Neurol. 2020;11:523292. doi: 10.3389/fneur.2020.523292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guven D.C., Aksun M.S., Cakir I.Y., Kilickap S., Kertmen N. The association of BMI and sarcopenia with survival in patients with glioblastoma multiforme. Futur. Oncol. 2021;17:4405–4413. doi: 10.2217/fon-2021-0681. [DOI] [PubMed] [Google Scholar]
- 45.Huq S., Khalafallah A.M., Ruiz-Cardozo M.A., Botros D., Oliveira L.A., Dux H., White T., Jimenez A.E., Gujar S.K., Sair H.I., et al. A Novel Radiographic Marker of Sarcopenia with Prognostic Value in Glioblastoma. Clin. Neurol. Neurosurg. 2021;207:106782. doi: 10.1016/j.clineuro.2021.106782. [DOI] [PubMed] [Google Scholar]
- 46.Furtner J., Berghoff A.S., Schöpf V., Reumann R., Pascher B., Woitek R., Asenbaum U., Pelster S., Leitner J., Widhalm G., et al. Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J. Neuro-Oncol. 2018;140:173–178. doi: 10.1007/s11060-018-2948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ilic I., Faron A., Heimann M., Potthoff A.-L., Schäfer N., Bode C., Borger V., Eichhorn L., Giordano F., Güresir E., et al. Combined Assessment of Preoperative Frailty and Sarcopenia Allows the Prediction of Overall Survival in Patients with Lung Cancer (NSCLC) and Surgically Treated Brain Metastasis. Cancers. 2021;13:3353. doi: 10.3390/cancers13133353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leone R., Sferruzza G., Calimeri T., Steffanoni S., Conte G., De Cobelli F., Falini A., Ferreri A., Anzalone N. Quantitative muscle mass biomarkers are independent prognosis factors in primary central nervous system lymphoma: The role of L3-skeletal muscle index and temporal muscle thickness. Eur. J. Radiol. 2021;143:109945. doi: 10.1016/j.ejrad.2021.109945. [DOI] [PubMed] [Google Scholar]
- 49.Mi E., Mauricaite R., Pakzad-Shahabi L., Chen J., Ho A., Williams M. Deep learning-based quantification of temporalis muscle has prognostic value in patients with glioblastoma. Br. J. Cancer. 2021;126:196–203. doi: 10.1038/s41416-021-01590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.