Abstract
Objective
Old or frail acutely hospitalised patients can benefit from geriatric rehabilitation but criteria concerning referral decisions are unclear. This review presents an overview of clinical factors associated with referral to geriatric rehabilitation that may further consensus between hospital and rehabilitation professionals on triage.
Design
Scoping review.
Methods
A review was conducted following Arksey and O’Malley’s framework. The search included literature concerning a broad spectrum of acutely hospitalised patients and factors associated with their referral to geriatric rehabilitation.
Results
Selected abstracts were categorised into distinct geriatric rehabilitation care pathways such as stroke, hip fracture, amputation of lower limb, cardiac and oncologic rehabilitation. Abstracts on internal medical patients were further reviewed and 29 studies were included. A total of 13 studies focused on factors identifying rehabilitation needs and 16 on factors associated with outcome of geriatric rehabilitation. Triage factors were diverse and included frailty status, functional decline, cognitive symptoms and multimorbidity. Mood symptoms and living situation further specified post-acute care needs. In overview, triage factors could be characterised as demographic (n = 4), diagnosis-related (n = 8), mental (n = 6), functional (n = 10) or multi-domain (n = 12) and mapped in a transitional care pathway.
Conclusions and implications
Frailty and functional decline are characteristics frequently associated with referral to geriatric rehabilitation of acutely hospitalised internal medical patients. A comprehensive geriatric assessment or a simpler multi-domain set of tests reveals rehabilitation needs and approximates a functional prognosis. Professional consensus on factors and timing of triage in hospital is within reach.
Keywords: geriatric rehabilitation, triage, post-acute care, rehabilitation needs, frailty, older people
Key Points
Criteria for referral to geriatric rehabilitation are unclear.
Rehabilitation potential correlates with frailty status and psychosocial needs.
Comprehensive Geriatric Assessment and multi-domain tools support triage decisions.
Professional consensus on triage is in reach.
Introduction
Geriatric rehabilitation is post-acute restorative care that is adapted to older or frail hospitalised patients, especially those with pre-existing functional decline or specific care needs [1–3]. Its central goal is to optimise functional capacities and support societal participation despite impairments [4]. Old or frail patients with stroke, fractures, amputation, or undergoing orthopaedic surgery can profit from this kind of post-acute care. Patients who are hospitalised with acute internal illnesses such as infections, organ failure or exacerbations of chronic diseases can benefit from rehabilitation as well [4–7]. Since acute hospitalisation of older patients is often associated with functional decline, geriatric rehabilitative care has become an important post-acute care pathway enabling patients to continue living at home [8, 9]. It is either a home-based service offered by community care organisations or an inpatient care trajectory in geriatric hospitals, geriatric wards, rehabilitation hospitals, skilled nursing facilities or nursing homes with rehabilitation units [4].
Accurate identification of patients for rehabilitative care is pivotal to optimise targeting of care and prevent unnecessary transitions. In the triage process a patient’s care needs, his functional prognosis and personal wishes should serve as building blocks for the decision-making [10–12]. Triage for rehabilitative care assumes a multifaceted, patient-oriented examination and evaluation of all relevant factors to establish the rehabilitation potential [10, 13, 14]. The assessment of a patient’s rehabilitation prognosis, however, is predominantly based on clinical intuition. A strong evidence base for the clinical factors that contribute to post-acute care decision-making is absent [15, 16].
Apart from clinical factors, organisational aspects play an important role in referral practice [17, 18]. Pressure to discharge early is a key driver for hospital referral practice [19]. Other non-clinical factors in referral procedures are the capacity of local facilities and their distance from the patient’s home [20]. Healthcare regulations and insurance policy also represent limitations for rehabilitation facility placement [6, 21].
In the absence of consensus on clinical criteria for rehabilitation needs and potential of old or frail acutely hospitalised patients we undertook a scoping review of the literature on geriatric rehabilitation triage decisions. Scoping reviews are a form of knowledge synthesis that addresses broad or fragmented areas of research, aiming to map the literature on a practice that is less studied or understood in literature [22]. The purpose of this review is to present an overview of factors considered relevant to assess the eligibility of hospital patients for geriatric rehabilitation in order to advance professional consensus concerning triage.
Methods
We followed the framework for scoping reviews by Arksey and O’Malley and refined by Levac, starting with a broad definition of the study population [22–24].
The research team consisted of care of older people physicians, an internal medical resident, geriatric rehabilitation specialists and researchers. The core elements of the search string (Appendix B) were key words associated with ‘geriatric patients’, ‘rehabilitation’, ‘referral/triage’ and ‘in-hospital’. Growing numbers of patients have received geriatric rehabilitation care since 2000; we therefore limited our search to articles published between January 2000 and July 2020. We included English, French or German articles extracted from PubMed, Embase, CINAHL, PsycINFO and the Cochrane Library. Our protocol is in Appendix A.
Selection of abstracts
A priori inclusion and exclusion criteria were set. Two reviewers (X, Y) independently screened the abstracts. A third member of the scoping team (Z) was consulted when consensus about selection was not reached.
We included studies
on referral to rehabilitative post-acute care of vulnerable, community dwelling, acutely hospitalised older patients.
on prognostic factors influencing functional recovery in acutely hospitalised old or vulnerable community dwelling persons.
targeting rehabilitative post-acute care referral and involving family caregivers or professionals.
on interventions concerning selection for geriatric rehabilitation.
We excluded studies
reporting exclusively on prevention of adverse outcomes in frail older hospital patients.
involving hospitalised long-term care patients.
on efficacy of a specific geriatric rehabilitation intervention.
focusing only on burden of family caregivers.
Narrowing down and re-evaluation
Categories of abstracts were formed according to the main hospital diagnosis of the study population and the associated rehabilitative care pathway. For the remaining abstracts three overarching categories were formed: triage education of hospital staff, organisation of the referral process and health economic aspects of access to geriatric rehabilitation. Confronted with an overwhelming amount of data after this first phase of the selection procedure, the second phase of selection exclusively focused on internal medical patients. This inclusion criterion was added. The research team assumed that literature concerning this heterogeneous group of rehabilitation candidates would present rich data on triage factors. Referral decisions concerning patients with classic rehabilitation diagnoses such as stroke or hip-fracture might be more routine.
Two researchers (X, Q) re-evaluated the selection of abstracts in the internal medical category to assess their fit with the purpose of our research: an inventory of patient-related factors concerning referral to geriatric rehabilitation. They continued with the selection for full text evaluation. All through the selection phases arguments to amend inclusion and exclusion criteria were discussed.
Charting of data
Included studies were scrutinised to extract data about aims, design and findings and papers were categorised according to their focus, whether on rehabilitation needs or on potential to recover. Triage factors were extracted, categorised and presented.
Results
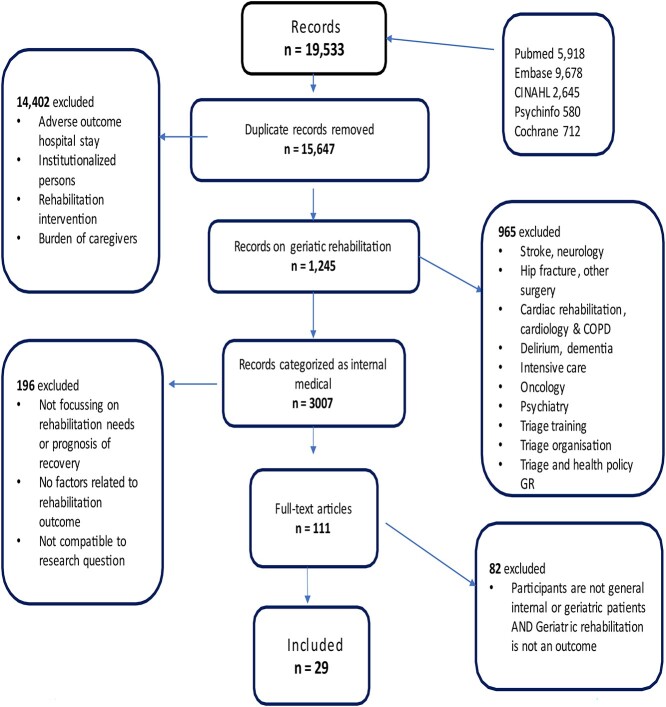
The literature search resulted in a total of 1,245 abstracts, which were assigned to diagnostic categories associated with rehabilitation care pathways. Reports on stroke patients represented the largest group followed by patients with internal medical diagnoses such as infections, organ failure, malnutrition, deconditioning, ulcers or a deterioration of chronic illness. This category of abstracts was further reviewed. Other categories concerned cardiac rehabilitation patients, patients with hip-fracture, other trauma, amputation, elective orthopaedic surgery and patients with delirium, dementia or psychiatric diagnoses. Figure 1 shows the flow diagram of the selection process.
Figure 1.
Flow chart of the scoping review process.
Included studies
Design
We found 29 studies on factors related to recovery of internal medical patients; 19 of these were prospective cohort studies [27, 29–37, 39, 40, 42, 45, 48–53], eight retrospective [28, 38, 41, 43–47, 50] and two used mixed methods, combining a cohort study with interviews or a survey [25, 26]. The sample size varied from 100 [27] to over 60,000 [28]. Duration of follow-up was 3 months [29, 30], 6 months [31, 32] or 1 year after discharge [33, 34].
Settings and participants
A total of 11 studies were situated in acute hospital wards and included only internal medical or acute geriatric hospital patients [30, 33, 35–38, 40–44], another four also included other acute hospital patients [25, 31, 35, 36]. A total of 14 studies were situated in rehabilitation settings: one outpatient rehabilitation setting [31], the other 13 situated in intermediate care units in skilled nursing facilities, rehabilitation hospitals or geriatric rehabilitation hospital wards [27–29, 32, 45–52, 55].
Outcome
Primary outcome of the hospital studies was discharge disposition: discharge home versus non-home or transition to geriatric rehabilitation. In geriatric rehabilitation settings the primary outcome was discharge to independent living versus long-term care. In our selection two studies fitted best to our research purpose, focusing exclusively on referral of internal medical patients to geriatric rehabilitation [37, 38]. An overview of participants, settings and primary outcome is given in Table 1.
Table 1.
Participants and outcome in selected studies
| Transition | |||
|---|---|---|---|
| Participants | Hospital to geriatric rehabilitation | Hospital to post-acute care | Rehabilitation or post-acute care to home |
| Internal medical patients | Luthy [37], Meyer [42] | Boyd [33], Cullum [35], D’Souza [40], Koch [36], Liu [41] | |
| Acute geriatric patients | Hartley [43] | Gijzel [30], Hartley [38], Lyons [44] | |
| Acute hospital patients | Buurman [34], Koné [26] | Bowles [25], Jackson [39] | |
| Medical geriatric rehabilitation patients | Hubbard [49], Kortebein [28], Luk [50], Singh [51], Wakabayashi [48] | ||
| Geriatric rehabilitation patients | Abrahamsen [29], Abrahamsen [32], Arjunan [52], Gill [45], Jupp [55], Leung [27], Ling [46], Simning [47], Peel [31] | ||
Acute geriatric patients: admitted to Department of Medicine for the elderly wards or to geriatric wards. Medical geriatric rehabilitation patients: patients with neurological or internal, non-surgical, rehabilitation diagnoses.
Focus of studies
Rehabilitation needs
Triage factors associated with rehabilitation needs were evaluated in 13 studies [25–27, 35–44]. These papers described patient characteristics and symptoms indicative of the necessity of post-acute care. Activities of Daily Living (ADL) dependency and cognitive decline were factors frequently associated with referral to rehabilitation [27, 36, 38–44]. Other examples of this type of triage factors were living without help at their own home, having a less than excellent self-rated health, symptoms of depression, multimorbidity, case complexity and length of hospital stay. Multi-domain triage tools assessing rehabilitation needs were the Hospital Admission Risk Profile, the INTERMED score and the Post-Acute Care Discharge score [36, 37, 41].
Rehabilitation outcome
The focus of the other 16 studies was to examine patient factors predicting rehabilitation outcome [28–34, 45–53]. Outcome was measured as duration of rehabilitation, functional gain or discharge destination. Pre-existing loss of instrumental ADL adversely affected functional gain during rehabilitation [28, 29, 35, 39, 45, 48–50]. Rehabilitation outcome was negatively associated with duration of the trajectory of functional loss before the acute illness and with the presence of mobility problems at admission [33, 34, 45]. The relation between severity of frailty and low functional gain during rehabilitation was reported in four studies [38, 45, 51, 52]. In a severely frail cohort, daily use of a measurement instrument for mobility and balance improved the prediction on discharge destination [49]. In-hospital deconditioning was associated with poor rehabilitation outcome when the patient was also malnourished [46]. Furthermore, oncologic or cardiovascular comorbidity reduced the outcome of geriatric rehabilitation [25, 29, 33].
Table 2 presents an overview of the triage factors in our selection.
Table 2.
Characteristics, symptoms and measures associated with referral to geriatric rehabilitation
| Demographic | Diagnoses, syndromes | Cognitive and mental status | Mobility and Functional status | Multi-domain tools and measures |
|---|---|---|---|---|
| Mobility | Frailty | |||
| Age [33, 36, 41, 51] | Admission diagnosis [29] | Clock in the Box [39] | Gait speed [31, 52] | Frailty Index. [51, 52] |
| Sex [26, 50, 51] | Non-surgical rehabilitation diagnosis [47] | MMSE [41] | Qualitative gait [52] | Frailty Index-CGA [49] |
| Multimorbidity [25, 37] | Cognitive impairment [43, 44] | Physical activity [30]Balance [25, 49] | Clinical Frailty Scale [38, 43, 44] | |
| Living without or with intermittent help [25, 37] | Metastatic cancer or cardiovascular disease as comorbidity [33] | Depressive symptoms [25, 35] | Hierarchal Assessment of Balance and Mobility (HABAM) [70] | Comprehensive Geriatric Assessment |
| Dementia [33] | Use of sedative medicine [55] | Comprehensive Geriatric Assessment (CGA) [29, 45] | ||
| Education [39, 45] | Vision impairment [55] | Momentary well-being [30] | Elderly Mobility Scale [44] | CGA Multidimensional Prognostic Index [42] |
| Low albumin [33] | De Morton Mobility Index, toilet transfer [40] | Multi-domain tools | ||
| Malnutrition, sarcopenia, in hospital deconditioning [26, 28, 34, 37, 48] | Functional status | Case complexity, amount of nursing care (INTERMED) [37] | ||
| Functional decline (ADL or i-ADL) [33, 38, 41, 47, 48] | Active medical problems, living with help at home, number of disabilities, age (Post-acute care discharge score, PACD) [36] | |||
| BI-decline 2 weeks before hospital admission, decline of Basic ADL [34, 71] | Gait, Eyesight, Mental state, Sedation (GEMS) [55] | |||
| Premorbid activity limitation [40, 46] | Age, i-ADL, MMSE, Hospital Admission Risk Profile score (HARP) [41] | |||
| Functional Independence Measure [27, 40] | Multi-domain measures | |||
| Less than excellent self-rated health [25] | ||||
| Resilience [30] | ||||
| Length of Hospital Stay [25, 26, 37] |
In italics: factors identifying rehabilitation needs. ADL = Activities of Daily Living. I-ADL = instrumental Activities of Daily Living. BI = Barthel Index. CGA = Comprehensive geriatric Assessment. MMSE = Mini Mental State Examination.
INTERMED is a system for classifying case complexity.
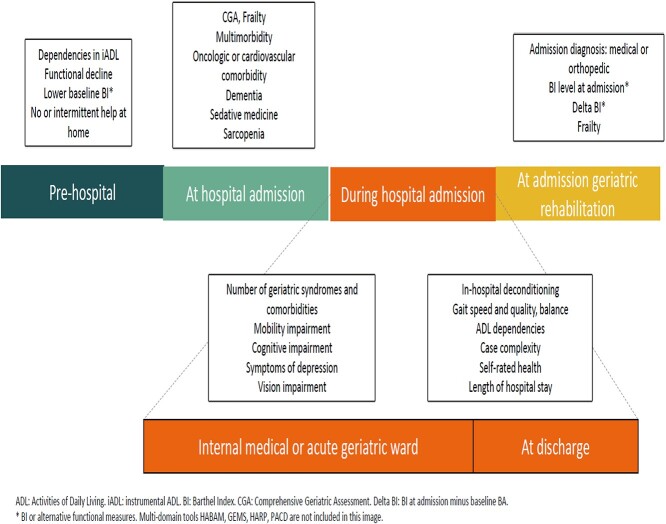
Figure 2 presents an overview of triage factors and the care pathway prior to geriatric rehabilitation admission. It visualises when triage information was assessed in the included studies.
Figure 2.
Triage factors visualised in a care trajectory.
Multi-domain measurements
Instruments measuring multiple domains of functioning supported triage and discharge planning in five of the included studies. The Hospital Admission Risk Profile score, consisting of age, cognitive status and i-ADL 2 weeks before admission identified individuals at risk of hospital-related functional decline and predicted risk of facility placement [41]. In Luthy’s study case complexity and nursing workload was taken into account, next to biomedical and psychosocial case-complexity [37]. More recently the Post-acute Care Discharge score and the Selfcare Index (SPI) were developed, two complementary and more elaborate triage instruments [36, 42]. A study on resilience concluded that frequent assessments of both physical and psychological indicators supported prediction of recovery (of geriatric patients by clinicians) [30].
Table 3 shows additional information on the selected studies.
Table 3.
Characteristics of selected studies
| Author and year | Subject and hypothesis | Population and setting | Exclusion | Triage factors |
|---|---|---|---|---|
| Bowles [25] 2009. |
Expert knowledge of important factors in post-acute care (PAC) referral, identification of characteristics hospitalised patients needing PAC | ≥65 years Six hospitals, urban, suburban and rural |
Not cognitively intact | Living without or with intermittent help, multimorbidity, depressive symptoms, balance, less than excellent self-rated health |
| Cullum [35] 2008. |
Relationship between depressive symptoms and hospital outcomes | ≥65 years General hospital |
Severe dysphasia, severe deafness, moderately impaired cognitive function. | Depressive symptoms. |
| D’Souza [40] 2020 |
Association between patient factors and patients’ discharge destination from acute medical wards. | Acute general medical patients admitted to physical therapy. Tertiary Hospital. |
Palliative care patients or transferred from other units | Premorbid physical function, current functional status, mobility, toilet transfer. |
| Hartley, Adamson [38] 2017 |
Association between Clinical Frailty Scale and functional trajectories. | ≥75 years Acute patients first admitted to Department of Medicine for the Elderly. Tertiary Hospital. |
Patients outside hospital region. | Functional decline, frailty. |
| Hartley Alexander [43] 2017 |
Compare functional trajectories of patients with and without cognitive impairment | ≥75 years Acute patients first admitted to Department of Medicine for the Elderly. Tertiary Hospital. |
Patients outside hospital region. Palliative or terminally ill patients. | Cognitive impairment, frailty. |
| Jackson [39] 2016. |
Predictive validity for discharge location of the Clock in the Box at admission. | ≥55 years Tertiary VA medical centre |
Detoxification or palliative admission, cognitive or sensory impairment, delirium | Cognitive screening. |
| Koch [36] 2019 |
Predict post-acute care needs early after admission by combining a self-care index with PAC-Discharge score | ≥16 years Acute medical or neurological patients. Tertiary hospital |
Patients transferred from other hospital, from NH, terminally ill patients. | Self-care abilities, amount of nursing care, active medical diagnoses at admission, living with help at home, disabilities, age. |
| Koné [26] 2018 |
Factors associated with transfer to transitional care or to geriatric rehabilitation | ≥18 years Patients with care needs after hospital stay Municipal hospital |
Sex, length of hospital stay. | |
| Leung [27] 2016 |
Characteristics and outcomes of elderly patients admitted to a slow stream, low-intensity and long-duration inpatient rehabilitation program | ≥60 years Patients admitted to a 30-bed Slow Stream Rehabilitation Unit. |
Medically unstable, palliative, undergoing chemotherapy or dialysis, wandering behaviour. | Functional decline |
| Liu [41] 2016. |
Association of the Hospital admission risk profile (HARP) score with discharge to SNF or Acute Rehab Unit. | ≥70 years Internal medicine inpatient unit Rural medical center |
Age, cognitive status, instrumental ADL. | |
| Luthy [37] 2007. |
Biomedical and psychosocial characteristics associated with PAC utilisation. | ≥18 years Internal medicine ward tertiary hospital; facility for rehabilitation and psycho-social care |
Other diagnose than congestive heart failure, community acquired pneumonia, malaise or fall. | Psychosocial complexity, comorbidity, medical diagnoses. |
| Lyons [44] 2019 |
Mobility trajectories and the associated patient characteristics (frailty and cognitive impairment) | Department of Medicine for the Elderly, first admittances Tertiary hospital |
Cognitive impairment, mobility, frailty. | |
| Meyer [42] 2019 |
Predictive value of the Multidimensional Prognostic Index concerning nursing needs and discharge allocation. | >70 years Renal, rheumatoid, diabetic or internal medical patients with comorbidity Tertiary hospital |
Inability to consent or to speak, terminal situation. | CGA, Multimorbidity, medication, pressure ulcer risk, nutrition, ADL and instrumental ADL, cognitive status, living situation. |
| Abrahamsen Haugland, Nilsen [32] 2016. |
Better post-acute care decision-making. Potential predictors for not returning to own home after rehabilitation. |
≥70 years Intermediate Care Unit with short-term rehabilitation |
Major cognitive impairment, delirium. NH decides if suitable for Intermediate Care. |
Functional decline before admission. |
| Abrahamsen Haugland, Ranhoff [29] 2016. |
Predictive value of admission diagnoses, degree of functional loss; simple versus comprehensive assessment. | ≥70 years Intermediate Care Unit with short-term rehabilitation |
Major cognitive impairment, delirium. NH decides if suitable for Intermediate Care. |
CGA. |
| Arjunan [52] 2019 |
Compare predictive value of Frailty Index and gait speed concerning geriatric rehabilitation outcome. | >65 years Inpatient rehabilitation ward Tertiary hospital. |
Amputees | Gait, frailty. |
| Boyd [33] 2008. |
Functional outcomes in the year after discharge; identify predictors of failure to recovery to baseline function |
≥70 years Tertiary care hospital, community teaching hospital |
Hospital stay of less than two days, admission to Intensive Care Unit. | Age, co-morbidity, dementia, nutritional status |
| Buurman [34] 2015. |
Disability trajectories in the year before and after SNF admission, association with adverse outcome | ≥ 70 years Community dwelling |
Disabled in ADL at baseline. | Decline of basic ADL. |
| Gijzel [30] 2020 |
Develop dynamical indicators of resilience | ≥ 65 years Geriatric ward Tertiary hospital |
LoHS<3 days, inability to respond, contact isolation. | Resilience, wellbeing. |
| Gill [45] 2009. |
Factors associated with recovery of prehospital function | ≥70 years Community dwelling. |
Disabled in ADL at baseline. | Mobility, nutritional status, cognitive status. |
| Hubbard [49] 2011. |
Bedside assessment of balance and mobility. Association of mobility and balance impairments to adverse outcomes. | ≥65 years Tertiary care hospital |
Mobility, balance | |
| Jupp [55] 2011. |
Factors linked to discharge to residential placement after rehabilitation. Tool to guide rehabilitation requirements |
≥65 years Two non-acute rehabilitation hospitals |
Medication, vision, mental state, mobility. | |
| Kortebein [28] 2007. |
Inpatient rehabilitation outcomes of older adults diagnosed with debility. Hypothesis: functional improvement of patients with a primary diagnosis of debility is lower than in comorbid debility |
≥65 years 70% of rehabilitation facilities USA (IRF’s) |
Patients without a primary or comorbid deconditioning diagnosis. | Deconditioning. |
| Ling [46] 2019 |
Association of premorbid activity limitation stages with post-hospital discharge disposition | ≥65 years Medicare enrolees. All cause hospitalisation |
ADL and instrumental ADL. | |
| Luk [50] 2011. |
Relationship between gender and rehabilitation outcome. Efficiency and efficacy of motor and functional outcomes. Hypothesis: there are important gender differences in geriatric rehabilitation outcome. |
≥65 years Two Geriatric Units Geriatric medical care. |
Not admitted from acute geriatric unit. | Sex. |
| Peel [31] 2014. |
Meaningful improvement in gait speed. Predictive properties gait speed at follow-up. | Six sites of a community-based Transition Care Program (TCP). | Mobility. | |
| Singh [51] 2012 |
Comparison of chronological age, gender, co-morbidities and frailty as predictors of adverse outcomes. | Acute geriatric medicine rehabilitation unit Tertiary care teaching hospital |
Severe dementia, acute stroke, chronically bedbound. | Age, sex, frailty. |
| Simning [47] 2019 |
Patient characteristics associated with patient-reported lack of functional improvement. Hypothesis: demographic, socioeconomic, health status and rehabilitation characteristics are associated with patient reported outcome of rehabilitation. |
≥65 years National Health and Aging Trends Study of Medicare beneficiaries receiving rehabilitation services in 2015 and 2016 |
Functional decline. | |
| Wakaba-yashi [48] 2014 |
Association nutritional status and rehabilitation outcome in older inpatients with hospital-associated deconditioning. Hypothesis: hospital-associated deconditioning is a result of inactivity and malnutrition. |
≥65 years Tertiary-care acute general hospital department of rehabilitation medicine |
Not diagnosed with hospital-associated deconditioning. | Nutritional status. |
ADL: Activity of Daily Living. IC: Intermediate Care. CGA: Comprehensive Geriatric Assessment. IRF: Inpatient Rehabilitation Facility. LoHS: Length of Hospital Stay. MDCC: Multi- Disciplinary Case Conference. NH: Nursing Home. PAC: Post-Acute Care. NH: Nursing Home. SNF: Skilled Nursing Facility. TCP: Transition Care Program. VA: Veteran’s Affairs.
Discussion
This review presents an overview and categorisation of relevant triage factors. It shows that triage decisions are based on symptoms and measurements of frailty, functional decline, geriatric syndromes such as cognitive impairment or deconditioning and new or pre-existing care needs. Triage factors relate to rehabilitation needs and influence rehabilitation outcome. A minimal, multidisciplinary set of clinical data regarding the relevant domains, which is assembled as early as possible during hospital stay, can support identification of rehabilitation needs, as well as assessment of rehabilitation eligibility.
Triage support
Referral decision-making is part of clinical routine, but professionals receive little training for this task [53, 54]. To support triage decisions, use of multi-domain tools help to identify rehabilitation needs or predict rehabilitation outcome [36, 41, 42, 55]. These tools inform referral decision-making by adding up criteria that are deemed relevant for triage. Although a comprehensive geriatric assessment explores all relevant domains and facilitates a personalised care plan, a set of multi-domain tools assessing ADL function, frailty status, comorbidity and cognition may also give sufficient information regarding rehabilitation eligibility [46, 56–58]. A potentially promising and evidence-based approach to support decision-making is to use structured patient data for automated triage support [59, 60]. These alternative tools and methods would be less extensive than performing a comprehensive geriatric assessment and they are applicable in settings where geriatric medical care is not available. Interpretation of clinical data concerning referral decision-making, however, requires geriatric rehabilitation expertise. Evaluation of the patients’ situation and the dialogue with patients and their family on preferred treatment remains essential despite availability of triage support.
Geriatric syndromes and rehabilitation eligibility
Frailty, cognitive decline, new ADL dependencies and deconditioning are geriatric syndromes that can indicate rehabilitation needs in older internal medical patients [10, 61–63]. Especially when symptoms of depression or delirium coexist, these syndromes give rise to rehabilitation needs. On the other hand, these clinical characteristics and their associated care needs represent factors that may influence the rehabilitation prognosis unfavourably [35, 39, 43]. Both frailty and cognitive impairment are related to negative health outcomes and diminished responsiveness to therapy [64–66]. Since geriatric rehabilitation wards strive to make specific care adjustments for these patients, mild or moderate cognitive decline need not be a criterion to exclude patients for rehabilitation oriented care [27]. Establishing the potential of the individual to profit from rehabilitation is a complex clinical judgement that calls for geriatric assessment and careful multidisciplinary observation of frail or cognitively impaired patients [62, 67]. When individual patient characteristics such as mood, coping style, motivation and family support are taken into account, rehabilitation programs that address these personal resources can support patients despite frailty [68–70]. Programs with a lower intensity of treatment and longer duration, including outpatient treatment represent promising options for frail patients, despite the relation between severity of frailty and low functional gain [3, 62]. The assessment of rehabilitation eligibility, in the presence of geriatric syndromes, thus calls for a multifaceted evaluation of triage factors, preferably a comprehensive geriatric assessment.
Strengths
Our review had several strengths. Firstly, the methodology of exploring literature without appraisal of the evidence allowed us to present a comprehensive overview of triage factors. Secondly, we focused on complex triage decisions: those concerning patients with internal medical diagnoses. In this domain, compared with orthopaedic or neurological rehabilitation, evidence is scarce.
Our data synthesis led to a distinction between patient characteristics that indicate rehabilitation needs and those associated with outcome of rehabilitation. This distinction may be helpful in decision-making and in developing a core-set.
Limitations
We described only triage factors concerning patients with internal medical diagnoses and these may be less applicable to patients with other diagnoses. Triage factors for the latter may have been missed. The assembling of triage factors for internal medical patients, however, provides the field with a starting point to reach consensus on a triage core-set. Essential diagnosis-specific triage elements of other patient groups can be added later. Furthermore, we refrained from reviewing the abstracts concerning professional triage training, organisation of triage processes and health economic factors regarding triage. A thorough exploration of these ‘non-clinical’ triage aspects calls for a comprehensive literature search in other sources.
Finally, we discussed our findings only within the research team and decided to consult other professionals in a later stage as part of a broad consensus procedure.
Recommendations
Geriatric rehabilitation triage factors are routinely assembled clinical criteria, though measured with different instruments by different professionals. Use of a core-set triage will advance communication of relevant triage factors in patient handovers. It will also facilitate the reports on course and outcome of geriatric rehabilitation.
Therefore, hospital and geriatric rehabilitation experts should achieve (to reach) consensus on a feasible and well-defined subset of triage factors. This should include at least pre-existing and actual functional and cognitive status, severity of frailty and profile of psycho-social needs.
Both implementation of a triage core-set and feedback between settings on geriatric rehabilitation trajectories will enhance the transparency and the quality of triage decisions.
Conclusions and implications
Triage factors concerning geriatric rehabilitation patients with internal medical diagnoses were measures of frailty, functional status, cognitive impairments and new or pre-existing care needs. They also referred to geriatric syndromes like in-hospital deconditioning and multimorbidity. Triage factors were assembled at various moments during hospital stay. A comprehensive geriatric assessment or a less extensive set of multi-domain tests including functional, cognitive and frailty status informs triage decisions and may contribute to awareness of rehabilitation needs earlier during hospital stay. Future steps should include consensus between hospital professionals and rehabilitation teams on a core-set of triage criteria, in order to support decision-making.
Supplementary Material
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
Amsterdam University Medical Center.
References
- 1. Bachmann S, Finger C, Huss Aet al. . Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ 2010; 340: c1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoenig H, Nusbaum N, Brummel-Smith K. Geriatric rehabilitation: state of the art. J Am Geriatr Soc 1997; 45: 1371–81. [DOI] [PubMed] [Google Scholar]
- 3. Achterberg WP, Cameron ID, Bauer JM, Schols JM. Geriatric rehabilitation-state of the art and future priorities. J Am Med Dir Assoc 2019; 20: 396–8. [DOI] [PubMed] [Google Scholar]
- 4. Grund S, Gordon AL, Balen Ret al. . European consensus on core principles and future priorities for geriatric rehabilitation: consensus statement. Eur Geriatr Med 2020; 11: 233–8. [DOI] [PubMed] [Google Scholar]
- 5. Everink IH, Haastregt JC, Hoof SJet al. . Factors influencing home discharge after inpatient rehabilitation of older patients: a systematic review. BMC Geriatr 2016; 16: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouwstra H, Wattel EM, Groot AJet al. . The influence of activity-based funding on treatment intensity and length of stay of geriatric rehabilitation patients. J Am Med Dir Assoc 2017; 18: 549.e15–22. [DOI] [PubMed] [Google Scholar]
- 7. Boston working group on improving health care outcomes through geriatric rehabilitation. Med Care 1997; 35: 4–20. [DOI] [PubMed] [Google Scholar]
- 8. Covinsky KE, Palmer RM, Fortinsky RHet al. . Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc 2003; 51: 451–8. [DOI] [PubMed] [Google Scholar]
- 9. Buurman BM, Hoogerduijn JG, Haan RJet al. . Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One 2011; 6: e26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka M, Yamamoto H, Kita T, Yokode M. Early prediction of the need for non-routine discharge planning for the elderly. Arch Gerontol Geriatr 2008; 47: 1–7. [DOI] [PubMed] [Google Scholar]
- 11. Gonçalves-Bradley DC, Lannin NA, Clemson LMet al. . Discharge planning from hospital. Cochrane Database Syst Rev 2016; 1: CD000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burke RE, Lawrence E, Ladebue Aet al. . It's hard to do the right thing: how hospital-based clinicians select patients for post-acute care in a skilled nursing facility. J Am Geriatr Soc 2017; 65: S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jesus TS, Hoenig H. Postacute rehabilitation quality of care: toward a shared conceptual framework. Arch Phys Med Rehabil 2015; 96: 960–9. [DOI] [PubMed] [Google Scholar]
- 14. Kennedy GM, Brock KA, Lunt AW, Black SJ. Factors influencing selection for rehabilitation after stroke: a questionnaire using case scenarios to investigate physician perspectives and level of agreement. Arch Phys Med Rehabil 2012; 93: 1457–9. [DOI] [PubMed] [Google Scholar]
- 15. Hoenig H, Siebens H. Research agenda for geriatric rehabilitation. Am J Phys Med Rehabil 2004; 83: 858–66. [DOI] [PubMed] [Google Scholar]
- 16. Gijzel SMW, Whitson HE, Leemput IAet al. . Resilience in clinical care: getting a grip on the recovery potential of older adults. J Am Geriatr Soc 2019; 67: 2650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buntin MB. Access to postacute rehabilitation. Arch Phys Med Rehabil 2007; 88: 1488–93. [DOI] [PubMed] [Google Scholar]
- 18. Ayele R, Jones J, Ladebue Aet al. . Perceived costs of care influence post-acute care choices by clinicians, patients, and caregivers. J Am Geriatr Soc 2019; 67: 703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poulos CJ, Gazibarich BM, Eagar K. Supporting work practices, improving patient flow and monitoring performance using a clinical information management system. Aust Health Rev 2007; 31: S79–85. [DOI] [PubMed] [Google Scholar]
- 20. Buntin MB, Garten AD, Paddock Set al. . How much is postacute care use affected by its availability? Health Serv Res 2005; 40: 413–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buntin MB, Colla CH, Escarce JJ. Effects of payment changes on trends in post-acute care. Health Serv Res 2009; 44: 1188–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Meth 2005; 8: 19–32. [Google Scholar]
- 24. Daudt HM, Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team's experience with Arksey and O'Malley's framework. BMC Med Res Methodol 2013; 13: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bowles KH, Holmes JH, Ratcliffe SJet al. . Factors identified by experts to support decision making for post acute referral. Nurs Res 2009; 58: 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koné I, Zimmermann B, Wangmo Tet al. . Hospital discharge of patients with ongoing care needs: a cross-sectional study using data from a city hospital under SwissDRG. Swiss Med Wkly 2018; 148: w14575. [DOI] [PubMed] [Google Scholar]
- 27. Leung G, Katz PR, Karuza Jet al. . Slow stream rehabilitation: a new model of post-acute care. J Am Med Dir Assoc 2016; 17: 238–43. [DOI] [PubMed] [Google Scholar]
- 28. Kortebein P, Bopp MM, Granger CV, Sullivan DH. Outcomes of inpatient rehabilitation for older adults with debility. Am J Phys Med Rehabil 2008; 87: 118–25. [DOI] [PubMed] [Google Scholar]
- 29. Abrahamsen JF, Haugland CRanhoff AH. Assessment of recovery in older patients hospitalized with different diagnoses and functional levels, evaluated with and without geriatric assessment. Eur Rev Aging Phys Act 2016; 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gijzel SMW, Rector J, Meulen FBet al. . Measurement of dynamical resilience indicators improves the prediction of recovery following hospitalization in older adults. J Am Med Dir Assoc 2020; 21: 525–30. [DOI] [PubMed] [Google Scholar]
- 31. Peel NM, Navanathan S, Hubbard RE. Gait speed as a predictor of outcomes in post-acute transitional care for older people. Geriatr Gerontol Int 2014; 14: 906–10. [DOI] [PubMed] [Google Scholar]
- 32. Abrahamsen JF, Haugland C, Nilsen RM, Ranhoff AH. Three different outcomes in older community-dwelling patients receiving intermediate care in nursing home after acute hospitalization. J Nutr Health Aging 2016; 20: 446–52. [DOI] [PubMed] [Google Scholar]
- 33. Boyd CM, Landefeld CS, Counsell SRet al. . Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc 2008; 56: 2171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buurman BM, Han L, Murphy TEet al. . Trajectories of disability among older persons before and after a hospitalization leading to a skilled nursing facility admission. J Am Med Dir Assoc 2016; 17: 225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial. Age Ageing 2008; 37: 690–5. [DOI] [PubMed] [Google Scholar]
- 36. Koch D, Schuetz P, Haubitz Set al. . Improving the post-acute care discharge score (PACD) by adding patients' self-care abilities: a prospective cohort study. PLoS One 2019; 14: e0214194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luthy C, Cedraschi C, Rutschmann OTet al. . Managing postacute hospital care: a case for biopsychosocial needs. J Psychosom Res 2007; 62: 513–9. [DOI] [PubMed] [Google Scholar]
- 38. Hartley P, Adamson J, Cunningham Cet al. . Clinical frailty and functional trajectories in hospitalized older adults: a retrospective observational study. Geriatr Gerontol Int 2017; 17: 1063–8. [DOI] [PubMed] [Google Scholar]
- 39. Jackson CE, Grande LJ, Doherty Ket al. . The clock-in-the-box, a brief cognitive screen, is associated with failure to return home in an elderly hospitalized sample. Clin Interv Aging 2016; 11: 1715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. AN DS, Granger CL, Patrick CJet al. . Factors associated with discharge destination in community-dwelling adults admitted to acute general medical units. J Geriatr Phys Ther 2021; 44: 94–100. [DOI] [PubMed] [Google Scholar]
- 41. Liu SK, Montgomery J, Yan Yet al. . Association between hospital admission risk profile score and skilled nursing or acute rehabilitation facility discharges in hospitalized older adults. J Am Geriatr Soc 2016; 64: 2095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meyer AM, Becker I, Siri Get al. . New associations of the multidimensional prognostic index. Z Gerontol Geriatr 2019; 52: 460–7. [DOI] [PubMed] [Google Scholar]
- 43. Hartley P, Alexander K, Adamson Jet al. . Association of cognition with functional trajectories in patients admitted to geriatric wards: a retrospective observational study. Geriatr Gerontol Int 2017; 17: 1438–43. [DOI] [PubMed] [Google Scholar]
- 44. Lyons A, Romero-Ortuno R, Hartley P. Functional mobility trajectories of hospitalized older adults admitted to acute geriatric wards: a retrospective observational study in an English university hospital. Geriatr Gerontol Int 2019; 19: 305–10. [DOI] [PubMed] [Google Scholar]
- 45. Gill TM, Gahbauer EA, Han L, Allore HG. Factors associated with recovery of prehospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol A Biol Sci Med Sci 2009; 64: 1296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ling N, Dawei X, Qiang Pet al. . Premorbid activity limitation stages are associated with posthospitalization discharge disposition. Am J Phys Med Rehabil 2018; 97: 440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simning A, Caprio TV, Seplaki CLet al. . Patient-reported outcomes in functioning following nursing home or inpatient rehabilitation. J Am Med Dir Assoc 2018; 19: 864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wakabayashi H, Sashika H. Malnutrition is associated with poor rehabilitation outcome in elderly inpatients with hospital-associated deconditioning a prospective cohort study. J Rehabil Med 2014; 46: 277–82. [DOI] [PubMed] [Google Scholar]
- 49. Hubbard RE, Eeles EM, Rockwood MRet al. . Assessing balance and mobility to track illness and recovery in older inpatients. J Gen Intern Med 2011; 26: 1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luk JK, Chiu PK, Chu LW. Gender differences in rehabilitation outcomes among older Chinese patients. Arch Gerontol Geriatr 2011; 52: 28–32. [DOI] [PubMed] [Google Scholar]
- 51. Singh I, Gallacher J, Davis Ket al. . Predictors of adverse outcomes on an acute geriatric rehabilitation ward. Age Ageing 2012; 41: 242–6. [DOI] [PubMed] [Google Scholar]
- 52. Arjunan A, Peel NM, Hubbard RE. Gait speed and frailty status in relation to adverse outcomes in geriatric rehabilitation. Arch Phys Med Rehabil 2019; 100: 859–64. [DOI] [PubMed] [Google Scholar]
- 53. Garner KK, Mendiratta P, Wei JY. Incorporating team-based learning for transitions in care into interprofessional geriatric education program. J Am Geriatr Soc 2019; S229–30. [Google Scholar]
- 54. Muniak J, Nicholas J, Birkland A, McCormick K. A novel educational experience for internal medicine residents incorporating principles of post-acute rehabilitation, transitional medicine, and communication skills. J Am Geriatr Soc 2019; 67: S74. [Google Scholar]
- 55. Jupp BJ, Mallela SK, Kwan Jet al. . Development and evaluation of the GEMS (gait, eyesight, mental state, sedation) tool as an aid to predict outcome after hospitalization. Geriatr Gerontol Int 2011; 11: 8–15. [DOI] [PubMed] [Google Scholar]
- 56. Ellis G, Gardner M, Tsiachristas Aet al. . Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017; 9: CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pilotto A, Cella A, Pilotto Aet al. . Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J Am Med Dir Assoc 2017; 18: 192.e1–11. [DOI] [PubMed] [Google Scholar]
- 58. Parker SG, McCue P, Phelps Ket al. . What is comprehensive geriatric assessment (CGA)? An umbrella review. Age Ageing 2018; 47: 149–55. [DOI] [PubMed] [Google Scholar]
- 59. Bowles KH, Ratcliffe SJ, Naylor MDet al. . Nurse generated EHR data supports post-acute care referral decision making: development and validation of a two-step algorithm. AMIA Annu Symp Proc 2017; 2017: 465–74. [PMC free article] [PubMed] [Google Scholar]
- 60. Bowles KH, Ratcliffe SJ, Holmes JHet al. . Using a decision support algorithm for referrals to post-acute care. J Am Med Dir Assoc 2019; 20: 408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scharf AC, Gronewold J, Dahlmann Cet al. . Clinical and functional patient characteristics predict medical needs in older patients at risk of functional decline. BMC Geriatr 2020; 20: 752020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cowley A, Goldberg SE, Gordon ALet al. . Exploring rehabilitation potential in older people living with frailty: a qualitative focus group study. BMC Geriatr 2021; 21: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morandi A, Onder G, Fodri Let al. . The association between the probability of sarcopenia and functional outcomes in older patients undergoing in-hospital rehabilitation. J Am Med Dir Assoc 2015; 16: 951–6. [DOI] [PubMed] [Google Scholar]
- 64. Dent E, Martin FC, Bergman Het al. . Management of frailty: opportunities, challenges, and future directions. Lancet 2019; 394: 1376–86. [DOI] [PubMed] [Google Scholar]
- 65. Vermeiren S, Vella-Azzopardi R, Beckwée Det al. . Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 2016; 17: 1163.e1–17. [DOI] [PubMed] [Google Scholar]
- 66. Theou O, Squires E, Mallery Ket al. . What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr 2018; 18: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Longley V, Peters S, Swarbrick C, Bowen A. What influences decisions about ongoing stroke rehabilitation for patients with pre-existing dementia or cognitive impairment: a qualitative study. Clin Rehabil 2018; 32: 1133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. De Donder L, Smetcoren AS, Schols Jet al. . Critical reflections on the blind sides of frailty in later life. J Aging Stud 2019; 49: 66–73. [DOI] [PubMed] [Google Scholar]
- 69. Puts MTE, Toubasi S, Andrew MKet al. . Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing 2017; 46: 383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Arjunan A, Peel NM, Hubbard RE. Feasibility and validity of frailty measurement in geriatric rehabilitation. Australas J Ageing 2018; 37: 144–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.