Summary:
Readthrough transcription caused by inefficient 3´-end cleavage of nascent mRNAs has emerged as a hallmark of the mammalian cellular stress response and results in the production of long noncoding RNAs known as downstream-of-gene (DoG) containing transcripts. DoGs arise from around ten percent of human protein-coding genes and are retained in the nucleus. They are produced minutes after cell exposure to stress and can be detected hours after stress removal. However, their biogenesis and the role(s) that DoGs or their production play in the cellular stress response are incompletely understood. Here, we discuss findings that implicate host and viral proteins in the mechanisms underlying DoG production, as well as the transcriptional landscapes that accompany DoG induction under different stress conditions.
Keywords: cellular stress, long noncoding RNAs, readthrough transcription, 3´-end cleavage, transcription termination
Cellular stress induces readthrough transcription
Cellular stress causes a variety of changes in the molecular organization of cells, which affects cellular structure and molecular interactions. Examples include the aggregation of filaments and other proteins upon heat shock [1, 2] (see Glossary), as well as nuclear shrinkage and chromatin collapse upon hyperosmotic stress [3-5]. Additionally, perturbations in the transcriptional profiles of cells due to stress have been documented and are fundamental to cell survival during heat shock [6-12]. Recently, several groups have found that cellular stress, including hyperosmotic stress [13], heat shock, oxidative stress [14, 15], renal cancer [16], hypoxia [17, 18], and certain viral infections [19, 20], inhibit the 3´-end cleavage of nascent transcripts from a subset of genes, leading to readthrough transcription [21]. This stress-induced readthrough transcription results in the production of downstream-of-gene (DoG) containing RNAs [18] (Box 1). In this review, we discuss what is currently known about the mechanisms of biogenesis of these stress-induced readthrough transcripts, including the chromatin marks and DNA sequences that characterize DoG regions, the transcriptional processes and protein factors involved in their production, as well as the different stressors and diseases that lead to their induction.
Box 1: The serendipitous discovery of DoGs.
Downstream-of-gene (DoGs) containing RNAs were originally characterized in 2015 when three groups independently published observations of widespread readthrough transcription in different contexts: hyperosmotic stress [13], Herpes simplex virus 1 (HSV-1) infection [19], and clear cell renal cell carcinoma (ccRCC) [16]. The serendipitous observations of these labs created a new field of research that promises to uncover additional layers of complexity in gene regulation, transcription termination, and the interconnections between different steps of transcription.
Our lab aimed to characterize a long noncoding RNA (lncRNA) whose expression had been shown to correlate with unfavorable outcomes in neuroblastoma patients [13]. To study the transcriptional regulation of this putative lncRNA, human neuroblastoma cells were exposed to hyperosmotic stress, considered at the time to be a transcriptional activator of neuronal cells [68, 69]. Assessment of nuclear RNA-sequencing (RNA-seq) data [13] led to the realization that the lncRNA of interest was in fact continuous with the transcript arising from the upstream gene, CXXC4, and was, therefore, a product of readthrough transcription. Hence, downstream of CXXC4 (doCXXC4) was the first transcript to be baptized a DoG. Additional analyses of nuclear RNA-seq data revealed the induction of DoGs from thousands of protein-coding genes upon KCl treatment.
Concurrently, the Dölken lab set out to understand the impact of HSV-1 infection on the transcription, processing, and translation of host mRNAs by combining ribosome profiling with the sequencing of recently synthesized RNAs [19]. They sequenced RNAs labeled with the uridine analogue 4-thiouridine (s4U) during a one-hour incubation period at various timepoints after infection. This approach revealed defects in the transcription termination of many host genes, resulting in the production of DoGs [19].
Meanwhile, Grosso et al. (2015) surveyed RNA populations in samples obtained from ccRCC tumors. By comparing RNA-seq data from tumors to normal matched tissues, they were able to detect accumulation of readthrough transcripts in samples obtained from renal cancer patients. Furthermore, the authors correlated mutations in the SETD2 gene with increased readthrough transcription in tumor samples by doing experiments that showed decreased readthrough transcription from a subset of genes upon expression of the wild-type SETD2 protein [16].
Rutkowski et al. and Grosso et al. (2015) both noted that the readthrough transcripts arising from viral infection and renal cancer, respectively, led to the invasive transcription of neighboring genes. Therefore, these groups provided the first glimpse of the impact that the production of DoG RNAs could have on transcription [16, 19].
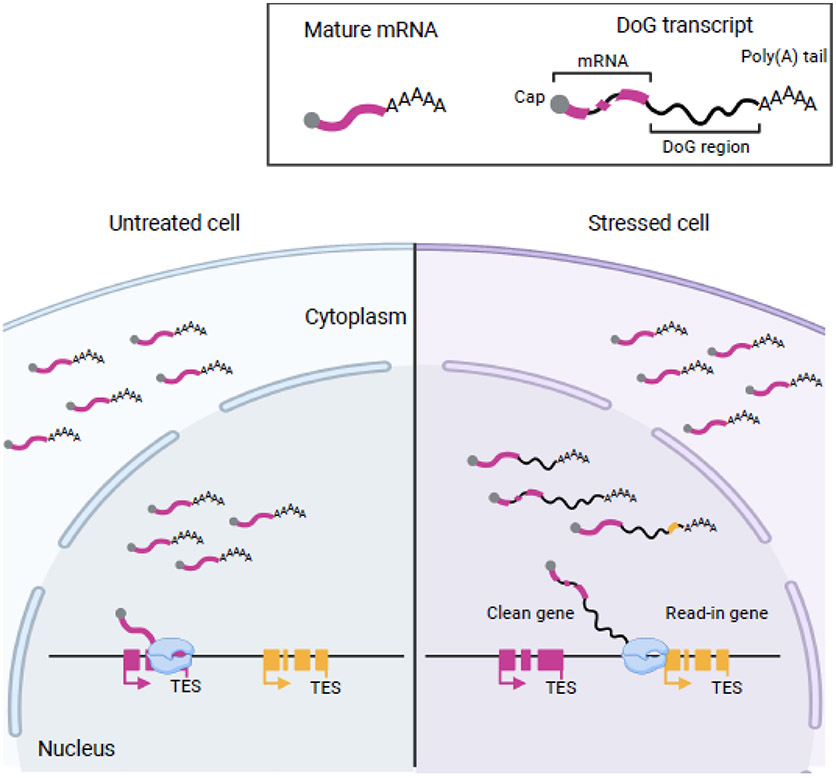
DoGs are long noncoding RNAs (lncRNAs) [22] that arise from around ten percent of human protein-coding genes and are continuous with their upstream messenger RNAs (mRNAs) [13]. Therefore, the production of these RNAs does not involve de novo transcription initiation from an independent transcription start site (TSS). DoGs are defined as having a minimal length of five kilobases beyond the annotated polyadenylation signal (PAS; also referred to here as TES for transcription end site) of their parent gene but can reach lengths of up to two hundred kilobases in mammalian cells [13]. Transcription units with other genes within 50 kb of their 3´-ends are more likely to experience readthrough transcription than those in gene-poor chromosomal regions [15]. Due to their lengths, DoGs often overlap downstream transcription units, which are referred to as “read-in genes” (Figure 1) [16, 19, 23]. The impact of DoG transcription on the expression of read-in genes is currently unclear (further discussed below).
Figure 1: Cellular stress induces the production of nuclear-retained downstream-of-gene (DoG) RNAs.
After synthesis, many DoGs remain close to their sites of transcription, while others are released into the nucleoplasm [13, 25]. DoG production can lead to the invasive transcription of neighboring genes (read-in genes) [16, 19, 23]. Genes that are free of read-in transcription are referred to as clean genes. DoG RNAs have been detected at similar levels in both polyadenylated and unpolyadenylated forms [12].
DoG transcripts are retained in the nucleus and many remain close to their site of transcription, while others are detected as nucleoplasmic puncta [13, 15, 25] (Figure 1). It has been suggested that DoGs or their production are important for supporting chromatin structure throughout the cellular stress response [13, 21]. However, the exact function of these readthrough transcripts remains unknown. Experiments that specifically inhibit the production of DoG RNAs will be necessary to solidify insights into the potential effects of these RNAs on chromatin architecture and marks.
The timeline of DoG induction has been characterized by measuring their levels among steady-state RNAs obtained from cells exposed to environmental stressors, such as heat shock or hyperosmotic stress, for different lengths of time. Results reveal that these transcripts are induced within minutes of stress exposure [15]. However, the time required for DoGs to return to basal levels after stress removal ranges from several hours to a day, depending on the type of stress [15]. Previous work has demonstrated that the half-life of salt-induced DoGs is about an hour [13], similar to the median half-life of mRNAs [24]. Thus, DoG transcripts are neither quickly degraded nor particularly long-lived. The prolonged presence of DoGs in recovering cells suggests that these transcripts could have a role during stress recovery.
The identities of DoG-producing genes [13] show significant overlap within a given mammalian cell line exposed to different stress conditions [15, 25], but it is unclear whether the same set of genes produces DoGs across different cell types. However, the quick induction of these transcripts upon stress suggests that they are produced from genes that are being actively transcribed in a given cell line. Therefore, it is likely that some DoG-producing genes are induced in a cell type-specific manner even when cells are exposed to the same stress conditions. Moreover, a subset of pathways involved in DoG production could be specific to certain stress conditions regardless of the cell type. This idea is supported by the potential role of the temperature-responsive transcription factor, HSF1, in regulating DoG biogenesis upon heat shock. Analyses of heat-shocked cells demonstrated that the level of readthrough transcription downstream of a subset of genes is positively correlated with HSF1 binding to their promoters [15]. Whereas initial studies suggested that DoG induction was mediated by calcium signaling through the inositol 1,4,5-triphosphate (IP3) receptor upon hyperosmotic stress, but not upon heat shock [13, 15, 21], it was later discovered that the decrease in DoG levels observed after inhibiting calcium signaling was caused by a general decrease in transcription [25]. Therefore, the involvement of calcium signaling in DoG production is currently unclear. Additional studies addressing the stress-specific pathways that regulate termination factors involved in DoG biogenesis are necessary to understand the signaling strategies used to initiate this rapid cellular response to stress.
Genomic and chromatin features of DoGs and their parent genes
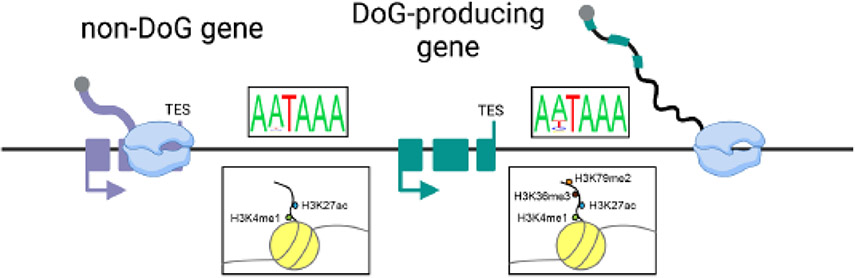
To understand why DoGs arise from only a subset of protein-coding genes upon stress, several groups have investigated whether DoG-producing genes have signature chromatin features or DNA sequences that distinguish them from non-DoG genes. Interestingly, towards the latter point, genes that produce readthrough transcripts in the context of Herpes simplex virus 1 (HSV-1) infection tend to have weaker polyadenylation signals than non-DoG genes [19]. Moreover, DoG regions themselves are also slightly depleted of the canonical poly(A) signal, AATAAA (Figure 2), and of GU repeats, which are important for 3´-end processing of pre-mRNAs [13, 15]. The relative depletion of DNA sequences important for transcription termination observed in pan-stress DoG regions compared to regions downstream of genes that do not experience readthrough transcription (non-DoG genes) suggests that DoG-producing genes are predisposed to transcription beyond their annotated transcription end sites.
Figure 2: Characteristics of downstream-of-gene (DoG)-producing genes.
A canonical poly(A) signal is found within 50 base pairs of the 3´-end of 79 percent of non-DoG genes and 61 percent of genes that produce DoGs upon HSV-1 infection [19]. Similarly, comparing the frequency of appearance of the canonical poly(A) signal in the sense versus the antisense strand across the region 5 kb downstream of the annotated transcription end sites (TES) of DoG-producing genes induced by hyperosmotic stress revealed that this sequence is less prevalent at DoG producing genes compared to non-DoG genes (ratio of 0.8 at DoG-producing genes versus 1.1 in non-DoG genes) [13]. The estimated frequency of the canonical poly(A) signal, AATAAA, and of the weaker ATTAAA are depicted in the figure. Additionally, DoG regions are slightly enriched for histone marks that favor transcription elongation throughout the first 5 kb after their annotated TES (H3K36me3 and H3K79me2). DoG regions are also enriched for histone marks typically found in intergenic regions, such as H3K4me1 and H3K27ac [15].
Studies assessing the chromatin environment of stress-induced DoG-producing genes prior to stress (homeostasis) have uncovered additional landmarks of transcription elongation beyond the polyadenylation signal of these transcription units. Histone marks contribute to the progression of transcription by recruiting protein factors that mediate various steps of the process or by favoring certain chromatin conformations [26, 27]. In untreated cells, the 3´-ends and downstream regions of pan-stress DoG-producing genes are slightly enriched for H3K79me2 and H3K36me3 compared to the downstream regions of non-DoG genes. These histone marks are known to favor transcription elongation [15, 28]. Enrichment of H3K4me1 and H3K27ac was also observed in pan-stress DoG regions in unstressed cells. Yet, these two histone marks are also enriched across all intergenic regions, consistent with their presence in enhancers [29, 30, 31], which suggests that they are not indicative of DoG-producing regions (Figure 2). The enrichment of H3K36me3 in regions downstream of genes in unstressed cells was confirmed by the Friedel and Dölken groups for genes that read through upon early HSV-1 infection [25]. Furthermore, readthrough transcription induced upon prolonged HSV-1 infection negatively correlates with general (H3K4me3) and repressive (H3K27me3) promoter marks in unstressed cells [25]. The negative correlation between readthrough and repressive histone marks could further support active transcription through these downstream regions upon infection. However, how chromatin marks change in DoG-producing genes and their downstream regions in response to different stressors, and whether or not changes to these marks are the cause or a consequence of DoG production, is yet to be assessed [15, 25].
Chromatin organization is characterized in part by transcriptional activity [32, 33, 34] (Box 2). Moreover, regions of active transcription generally exhibit hallmarks of open chromatin conformation, whereas regions that are transcriptionally silent have closed chromatin features [35]. In untreated cells, regions of accessible chromatin are prevalent near promoters and in regions downstream of pan-stress DoG-producing genes as measured by DNase 1 hypersensitive sites sequencing (DNase-seq) or by the Assay for Transposase-Accessible Chromatin sequencing (ATAC-seq) [15]. However, open chromatin was not prevalent when DoG-regions that are selectively induced only upon hyperosmotic stress or heat shock were included in analyses of unstressed cells [25]. Consistently, ATAC-seq experiments performed on cells exposed to hyperosmotic stress, heat shock, or HSV-1 infection for one or two hours did not show increased chromatin accessibility in DoG regions upon stress [25]. Conversely, an increase in chromatin accessibility was observed across readthrough regions at least four hours after HSV-1 infection, suggesting that, in DoG regions, chromatin architecture continues to change upon prolonged stress exposure. Furthermore, these results imply that the impact of DoG production on chromatin architecture is not immediately evident, but depends on persistent transcription through these regions [25]. Nevertheless, such discrepancies further emphasize the need for comprehensive mechanistic investigations of DoG induction by specific stress conditions versus pan-stress DoG induction.
Box 2: Chromatin topology and DoG production.
Chromatin organization is divided into several layers underlying important interactions between enhancers, promoters, and their target genes. These layers include topologically associated domains (TADs), which span tens to hundreds of kilobases and have defined boundaries established by the binding of architectural proteins such as CTCF and cohesin [70]. A/B compartments provide an additional layer of chromatin organization, based on transcriptional activity and the presence of activating or repressive histone marks [33]. The establishment and maintenance of chromatin architecture are critical for programs of gene expression and are altered in different disease states.
Several groups have published genome-wide assessments of chromatin reorganization upon stress. A recent study demonstrated that cells preserve A/B compartments and TAD boundaries upon heat shock [71]. In contrast, upon hyperosmotic stress or influenza A infection, the reorganization of chromatin compartments is accompanied by a loss of CTCF and cohesin [37, 72]. Specifically, after influenza A infection, the CTCF and cohesin molecules that bind DoG regions are displaced by elongating Pol II molecules [72]. In agreement with these observations, experiments in cells exposed to hyperosmotic stress show that the redistribution of Pol II molecules into DoG regions weakens TAD boundaries, supporting a role for DoGs in the observed disruption of local chromatin organization [37]. Stress-recovery experiments demonstrate that chromatin organization is restored one hour after removing cells from hypertonic media. Yet, exposing cells to hyperosmotic stress as well as an inhibitor of transcription initiation suggests that some aspects of the observed chromatin reorganization are independent of new transcription [37], contradicting observations in influenza A-infected cells [72].
Transcriptional landscapes accompanying DoG induction
Recent studies have documented that cellular stress conditions leading to the production of DoGs can also induce other changes in the transcriptional landscape of cells [11, 12]. Recently, Transient Transcriptome TimeLapse sequencing (TT-TL seq) [36] was combined with the use of spike-in RNA controls to investigate the nascent (rather than steady-state) transcription profiles that accompany DoG production after hyperosmotic stress. Analysis of a subset of genes that are free from invasive transcription originating on either strand from neighboring DoG-producing genes (clean genes) revealed that hyperosmotic stress induces widespread transcriptional repression [12]. Accordingly, recent experiments using anti-Polymerase II (Pol II) chromatin immunoprecipitation combined with high throughput sequencing (ChIP-seq) also demonstrated a decrease in Pol II occupancy across gene bodies after hyperosmotic stress, which is accompanied by a redistribution of Pol II molecules into DoG regions [12, 37]. The decreased occupancy of Pol II molecules across gene bodies and the consequent transcriptional repression induced by hyperosmotic stress can be explained in part by an increase in termination occurring shortly downstream of transcription initiation sites, which prevents Pol II from progressing into the elongation phase [38]. Other reports demonstrated transcriptional repression in the context of viral infection or heat shock similarly accompanied by a shift of Pol II molecules into downstream regions [11, 20, 39, 40]. Upon heat shock, inhibition of Pol II pause release was shown to contribute to transcriptional repression [11]. These results highlight common features of the chromatin landscape accompanying DoG transcription. Furthermore, these studies demonstrate that DoG production displaces Pol II molecules into downstream regions, thereby, impacting Pol II distribution genome wide during a variety of stress conditions.
Measurements of Pol II occupancy and nuclear RNA levels have shown that DoGs arise from genes that fall into different categories of transcriptional regulation (activated, repressed, or unchanged) upon stress [15, 40]. Consistent with these observations, recent TT-TL-seq experiments confirm that DoGs can arise independent of the transcriptional response of their parent gene upon hyperosmotic stress (Figure 3). Namely, similar fractions of genes that are activated, unchanged, or repressed upon stress are able to produce DoGs [12].
Figure 3: Termination factors involved in downstream-of-gene (DoG) production.
(Top) Under homeostasis, components of the cleavage and polyadenylation (CPA) machinery mediate the 3´-end formation of protein-coding gene transcripts [74]. The Integrator complex regulates RNA output near transcription start sites [59, 60, 61] and transcripts that continue to be elongated are normally cleaved and polyadenylated at the 3´-end. (Middle) Upon hyperosmotic stress, the interaction between Pol II and Integrator is disrupted and DoG-production is observed [12]. Dysregulated occupancy of CPA components upon hyperosmotic stress has been reported [40, 51]. However, whether this deficit directly affects DoG-producing genes remains unclear. The uncertain (yet likely) loss of CPA factors during DoG production is indicated in the figure with a question mark. Differential gene expression observed upon salt stress does not dictate a gene’s ability to produce DoGs. (Bottom) After Influenza A or HSV-1 infection, viral proteins NS1 and ICP27, respectively, sequester subunits of the CPA machinery leading to DoG biogenesis [63, 64]. Upon cellular stress, three types of transcripts have been detected and are, therefore, represented throughout this figure: mature mRNAs, polyadenylated DoGs, and non-polyadenylated DoGs.
DoG production can also lead to read-in transcription of neighboring genes, potentially altering their expression through transcriptional interference [12, 19, 41]. From a technical perspective, such read-in transcription complicates differential expression analysis, making it appear that many genes are transcriptionally activated by stress [23, 40]. Furthermore, production of DoGs as antisense transcripts may also negatively affect the transcription of read-in genes on the opposite strand [15, 42], perhaps contributing to the maintenance of transcriptional repression upon stress [43].
Previous gene ontology (GO) analyses of DoG-producing genes have been complicated by the fact that read-in transcription often causes DoGs to be assigned to incorrect parent genes [12]. Our recent assessments of only clean DoG-producing genes induced by hyperosmotic stress revealed enrichment for functions related to transcriptional repression [12]. In contrast, terms related to transcriptional repression were not among the ten most enriched functions for non-DoG genes. This finding supports the idea that DoGs might serve as a reservoir of unprocessed transcripts that could later be cleaved and polyadenylated, turning these into mature mRNAs that can be exported to the cytoplasm in order to perpetuate the transcriptional repression of certain genes upon prolonged exposure to mild stress conditions or to facilitate cellular recovery from stress [12]. However, experimental evidence is necessary to test the veracity of this hypothesis. Other groups have investigated functional aspects of DoG production by taking a gene-specific approach [25]. For example, Hennig et al. (2018) observed that the IRF1 gene, which encodes a transcription factor involved in regulating the host immune response [44], produces DoGs upon viral infection, heat shock, or hyperosmotic stress [25]. By taking advantage of their previously-published ribosome profiling experiments, which assessed the impact of HSV-1 infection on translation [19], the authors demonstrated that translation of the IRF1 mRNA decreases after infection. One possible explanation is that DoGs dampen the host immune response in the context of viral infection by retaining unprocessed IRF1 transcripts in the nucleus [25]. Whether DoGs represent a strategy that cells employ to survive extended periods of stress or a response orchestrated by viruses to promote their own replication is yet to be determined.
Splicing and transcription termination as interconnected processes
Co-transcriptional processing of pre-mRNAs requires interconnected steps. Although the splicing status of DoG-containing transcripts remains unclear, studies in yeast and mammalian cells have revealed that unspliced pre-mRNAs tend not to be cleaved at their 3´-ends and, therefore, exist as readthrough transcripts [45-48]. In budding yeast, readthrough transcription is induced upon depletion of Nab2 [47], which binds polyadenylated RNAs and had been thought to be involved in splicing [49]. Long-read sequencing experiments then revealed that splicing is disrupted only for transcripts arising from read-in transcription events, implying that the chimeric transcripts produced by intrusive transcription are not optimal splicing substrates [48]. Furthermore, experiments performed in differentiating mouse erythroblasts demonstrated that readthrough transcripts arise from genes whose pre-mRNAs experience inefficient co-transcriptional splicing [48]. Targeted long-read sequencing of beta-globin transcripts in these murine erythroleukemia cells further revealed that introducing a cryptic splice site that increases splicing efficiency also increases 3´-end cleavage, indicating a functional link between these processes [48]. Moreover, increased readthrough transcription of stress-responsive genes was also observed in the presence of the splicing inhibitor, PladB [50]; genes producing transcripts with retained terminal introns after PladB treatment are more likely to read through than other genes, further suggesting that splicing disruptions interfere with 3´-end cleavage and transcription termination for at least a subset of genes.
Disrupted splicing patterns have also been reported in several stress conditions that induce DoG production, including heat shock [10] and HSV-1 infection [19]. Upon HSV-1 infection, splicing disruptions were predominantly observed in read-in genes [19]. Further examination of the impact of cellular stress on splicing revealed that read-in transcription results in intergenic splicing, leading to the formation of chimeric exon-exon junctions [25]. This phenomenon was more evident after HSV-1 infection than after heat shock or hyperosmotic stress. More experiments that investigate the splicing status of DoG RNAs will be necessary to fully understand the extent of perturbation of RNA processing observed in cells exposed to stress.
Transcription termination factors contribute to DoG biogenesis upon environmental stress
DoGs arise as a consequence of decreased transcription termination due to impaired 3´-end cleavage of the nascent pre-mRNA [13] (Box 3). Recent studies have shed light on how cellular stress affects the occupancy of different termination factors on chromatin. For example, ChIP analyses of several components of the cleavage and polyadenylation (CPA) machinery, including CPSF73, CPSF6, and CPSF1, show that their occupancy at the TESs of certain genes decreases upon hyperosmotic stress or heat shock [40, 51]. Moreover, CPSF6 was shown to be sequestered into phase-separated droplets after hyperosmotic stress [51]. Consistent with the known roles of CPA factors in catalyzing the 3´-end formation of protein-coding gene transcripts, depletion of CPSF73 leads to readthrough transcription [13, 52, 53]. Cardiello et al. (2018) and Jalihal et al. (2020) suggest the direct involvement of CPA factors in DoG biogenesis upon stress. Yet, a thorough analysis assessing the differential binding of these factors relative to Pol II occupancy to DoG-producing versus non-DoG genes in stressed cells is needed to confirm this notion (Figure 3).
Box 3: Canonical roles of transcription termination factors important for DoG production.
Transcription termination of protein-coding genes requires cleavage of the nascent mRNA after RNA Polymerase (Pol) II transcribes the polyadenylation signal (PAS) [73]. The cleavage and polyadenylation (CPA) machinery [74] cuts the nascent transcript and Pol II continues to transcribe, producing an uncapped RNA. Removal of Pol II from the DNA template is induced when the 5´ to 3´ exonuclease Xrn2 crashes into Pol II as it progressively degrades the attached uncapped transcript downstream of the polyadenylation signal [54]. This model of termination is known as the torpedo model. Another model for transcription termination, known as the allosteric model, posits that Pol II undergoes a conformational change when it transcribes through the polyadenylation signal of a gene, resulting in its dissociation from the DNA template [75]. These models may work in combination to achieve efficient transcription termination at the ends of mammalian protein-coding genes [53]. Therefore, it is not surprising that depletion of either CPSF73 or Xrn2 leads to genome-wide readthrough transcription [53, 54].
The Integrator (Int) complex plays important roles in the 3´-end formation of different types of noncoding RNAs: subunits of its cleavage module, composed of Int 4, 9, and 11, produce the 3´-ends of small nuclear RNAs (snRNAs) [55, 56, 76] and of certain viral microRNAs [77]. Depletion of Integrator subunits has also been found to impair transcription termination of replication-dependent histone genes, enhancer RNA genes, and lncRNA genes [78, 79, 80]. Furthermore, the Integrator complex plays a role in promoter-proximal Pol II pausing on protein-coding genes [57, 59, 60, 61] and has been proposed to police polymerases that are unfit to properly proceed into transcription elongation [81].
To identify additional termination factors that contribute to DoG induction upon stress, mass spectrometry analyses of anti-Pol II immunoprecipitates from chromatin fractions of untreated and KCl-treated SK-N-BE(2)C cells were performed. The results demonstrated that hyperosmotic stress alters the Pol II interactome [12]. Surprisingly, certain CPA factors and Xrn2, the nuclease that mediates the final step of transcription termination [54], were associated with Pol II in both untreated and stressed samples. Conversely, interactions between Pol II and subunits of the Integrator complex [55-61] decreased upon stress [12]. Furthermore, ChIP-seq experiments using antibodies against two different subunits of the Integrator complex demonstrated a genome-wide loss of occupancy at both DoG-producing genes and non-DoG genes. Accordingly, knockdown of the catalytical subunit of Integrator, Int11, led to the induction of hundreds of readthrough transcripts. Comparison of genes that produce readthrough transcripts after Int11 knockdown to clean genes that produce DoGs upon hyperosmotic stress showed an overlap of up to 25 percent, suggesting that the Integrator complex partially contributes to DoG biogenesis [12]. However, it remains unclear whether the Integrator complex plays a direct role in DoG induction. Further delineation of the transcriptional machinery present near the TESs of DoG-producing genes or the 3´-end of DoG transcripts using cross-linking and immunoprecipitation (CLIP) and ChIP should reveal additional features that make these genes prone to readthrough transcription upon stress.
Factors contributing to DoG induction in disease
DoG production has been observed in several disease contexts including viral infections and renal cancer, as well as in senescent cells. Viruses enter cells equipped to hijack host processes to prioritize their own replication [62]. Nuclear retention of readthrough transcripts was suggested to facilitate viral replication by increasing the source of capped RNA primers for influenza virus mRNA production [63]. It is possible that upon prolonged viral infection, DoG production also contributes to viral replication by simply retaining coding transcripts in the nucleus and preventing mature mRNAs from being exported to the cytoplasm where they can be translated. However, despite extensive characterization of DoG induction upon multiple stress conditions, the exact fraction of Pol II molecules that read through at the end of a DoG-producing gene has not yet been quantified using nascent RNA production as a metric. Therefore, the potential impact of the nuclear retention of DoG transcripts on mRNA export to the cytoplasm remains unknown.
Upon HSV-1 infection, the viral protein, ICP27, disrupts host transcription termination by interfering with the assembly of host CPA complexes, resulting in DoG production [64] (Figure 3). Similarly, the influenza A protein NS1 was observed to sequester CPSF30, leading to the production of readthrough transcripts from host genes upon infection [63] (Figure 3). However, later studies found that the induction of DoGs during influenza A infection is independent of interactions between NS1 and CPSF30 [20]. Perhaps the influenza A virus employs additional strategies to generate DoGs from host genes in the absence of NS1. Alternatively, infection by these viruses could also elicit a cellular stress response leading to DoG production through host mechanisms described above. Nonetheless, these findings showcase the apparently purposeful induction of readthrough transcripts upon viral infection.
Readthrough transcription has also been observed in tumor samples obtained from patients suffering from clear cell renal cell carcinoma (ccRCC). Here, high levels of readthrough transcription inversely correlate with patient survival [16]. One of the most frequently mutated genes in patients with this malignancy is SETD2, which encodes a histone modifying enzyme that mediates the trimethylation of H3K36 [65]. Interestingly, H3K36me3 has been found to be enriched in DoG regions (Figure 2). Importantly, expression of wild-type SETD2 in a ccRCC cell line expressing mutated SETD2 was sufficient to revert the defect in transcription termination at some DoG-producing genes [16], suggesting that changes in H3K36me3 explain the induction of certain DoGs in ccRCC.
DoGs have also been detected in senescence, where cells are not proliferative, in contrast to cancerous cells. Although beneficial in certain contexts, senescence is also associated with endocrine disease and ageing [66]. In senescent cells, DoGs are thought to play a key role in controlling gene expression by acting as antisense transcripts [42]. Compared to control cells, Muniz et al. (2017) observed that senescent cells exhibited decreased occupancy of H2A.Z, an alternative histone associated with DNA repair that is also dysregulated in cancer [67]. It has been proposed that the presence of the histone variant, H2A.Z, represses readthrough transcription under normal cellular conditions [42]. However, additional factors are likely involved in the induction of readthrough transcripts upon senescence because knockdown of H2A.Z leads only to partial DoG induction. These studies highlight the need for a better understanding of how important chromatin modifiers and histone marks respond to disease or to other stress conditions.
Concluding remarks
Stress-induced readthrough transcription has been shown to be a marker of the cellular stress response after exposure to certain environmental stressors or disease. The transcripts that arise have been well characterized over the last few years. Yet, much work is still required to gain insights into their functions, as well as the mechanisms underlying their production (see Outstanding questions).
Outstanding questions.
Are downstream-of-gene (DoG) containing RNAs produced in response to specific signaling pathways activated upon stress?
Do cells employ multiple strategies to produce DoGs or do known factors converge into a single pathway?
Are there as-yet unrecognized signals and factors involved in DoG biogenesis?
What characteristic features make DoG-producing genes more likely to read through than non-DoG genes?
How does DoG transcription impact the levels of neighboring read-in genes?
What fraction of Pol II molecules reads through a DoG-producing gene whose transcription is either activated, unchanged, or repressed upon stress?
Can DoG RNAs be processed post-transcriptionally leading to the production of mature mRNAs corresponding to their parent genes?
What is the function of DoGs?
Recent findings have expanded our understanding of the biogenesis of stress-induced readthrough transcripts. The data presented in the articles reviewed here suggest the involvement of termination factors, including components of the CPA machinery [40, 51] and the Integrator complex [12], as well as histone modifying enzymes in the biogenesis of these stress-induced lncRNAs [16]. It is possible that cells undergoing long-lasting changes, such as cancer cells, rely on histone modifiers to regulate DoG production, whereas conditions that require a rapid response mediate DoG formation through the differential occupancy of termination factors. The interplay between these alternative mechanisms is yet to be investigated and promises to provide a more comprehensive picture of how termination is regulated across different genes.
The association of DoG induction with disease and disease-like contexts, such as heat shock and hyperosmotic stress, underscores the question of whether DoG RNAs or the act of their production play a protective role in cells or whether they instead promote disease progression. Whatever the case, mechanistic studies of DoG biogenesis reviewed here have laid the foundation for future functional studies and therapeutic applications.
Highlights:
Stress-induced readthrough transcription leads to the synthesis of long noncoding RNAs called DoGs (downstream-of-gene containing transcripts), which are retained in the nucleus.
DoG regions have characteristic features that may support transcription elongation, including enrichment of weak polyadenylation signals and of histone marks associated with active transcription.
Several conditions that induce DoGs also generate changes in transcription regulation, including disrupted splicing, and repression of many genes, and DoG production often leads to invasive transcription into neighboring genes that may impact the transcription of the downstream gene.
While the molecular mechanisms and signaling pathways underlying DoG production upon stress are still unclear, known factors involved in DoG induction include histone modifying enzymes, histone variants, and transcription termination factors.
Acknowledgments
We thank Annsea Park, Kazimierz Tycowski, Salehe Ghasempur, Shervin Takyar and Karla Neugebauer for critical discussion of the manuscript. We also thank other members of the Steitz lab for their input as well as Angela Miccinello for editorial assistance. This work was supported by the NIH grant R01GM140735. N.A.R.M. is a Ford Foundation Pre-doctoral fellow and J.A.S. is an investigator of the Howard Hughes Medical Institute.
Glossary
- Clean genes
expressed genes that do not experience invasive transcription from neighboring DoG-producing genes. Clean genes may or may not produce DoGs
- Cleavage and polyadenylation machinery (CPA)
composed of four different protein complexes (CPSF, CstF, CFIm, and CFIIm) that catalyze RNA cleavage, allowing subsequent polyadenylation of nascent pre-mRNAs in metazoans by recognizing the polyadenylation signal as well as auxiliary motifs
- DoG-producing genes
genes that produce readthrough transcripts of five kilobases or more beyond their annotated transcription end sites
- Downstream-of-gene (DoG) containing RNAs
DoGs are stress-induced long noncoding RNAs that are continuous with an upstream gene transcript for at least 5 kilobases (kb) beyond the gene’s annotated polyadenylation signal
- H2A.Z
histone variant predominantly found at enhancer and promoter regions. Activates or represses the transcription of certain genes depending on its post-translational modifications. The levels of this non-canonical histone are dysregulated in diseases such as cancer
- Heat shock
refers to the stress experienced by cells when they are exposed to increased temperatures
- Hyperosmotic stress
occurs when cells are exposed to increased osmolarity
- Hypoxia
stress that is induced when cells experience low levels of oxygen
- Integrator complex
protein complex composed of at least fourteen subunits that catalyzes the 3´-end formation of several noncoding RNAs. This complex has also been implicated in regulating promoter proximal Pol II pausing near the transcription start site of protein-coding genes, thereby affecting gene output
- Long noncoding RNA (lncRNA)
RNA molecules longer than 200 nucleotides that do not undergo translation. Many lncRNAs have roles in maintaining chromatin structure and in regulating gene expression
- Non-DoG genes
genes that undergo efficient transcription termination despite exposure to cellular stress
- Oxidative stress
induced when cells experience an accumulation of oxidants that exceeds the levels of antioxidant
- Pan-stress DoGs
DoGs that are induced by several different types of cellular stress in a given cell line
- Read-in genes
loci that are invaded by readthrough transcription arising from neighboring DoG-producing genes
- Readthrough transcription
transcription extending beyond the annotated transcription end site of a gene
- Transient Transcriptome TimeLapse sequencing (TT-TL-seq)
A technique that combines short periods of metabolic labeling using the uridine analogue, s4U followed by streptavidin-based enrichment strategies that allow specific isolation of nascent RNAs. In contrast to other techniques that rely on the isolation of s4U-labeled RNA, TT-TL-seq requires that the uridine analogue in the isolated RNAs be chemically converted to a cytosine analogue using TimeLapse chemistry. This strategy decreases the likelihood of contaminating reads being called as nascent RNAs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richter K et al. (2010) The heat shock response: life on the verge of death. Mol Cell 40 (2), 253–66. [DOI] [PubMed] [Google Scholar]
- 2.Velichko AK et al. (2013) Mechanisms of heat shock response in mammals. Cell Mol Life Sci 70 (22), 4229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finan JD and Guilak F (2010) The effects of osmotic stress on the structure and function of the cell nucleus. J Cell Biochem 109 (3), 460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finan JD et al. (2011) Osmotic stress alters chromatin condensation and nucleocytoplasmic transport. Biochem Biophys Res Commun 408 (2), 230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocker C et al. (2012) The role of hyperosmotic stress in inflammation and disease. Biomol Concepts 3 (4), 345–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Nadal E et al. (2011) Controlling gene expression in response to stress. Nat Rev Genet 12 (12), 833–45. [DOI] [PubMed] [Google Scholar]
- 7.Himanen SV and Sistonen L (2019) New insights into transcriptional reprogramming during cellular stress. J Cell Sci 132 (21). [DOI] [PubMed] [Google Scholar]
- 8.Åkerfelt M et al. (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nature Reviews Molecular Cell Biology 11 (8), 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vihervaara A et al. (2018) Molecular mechanisms driving transcriptional stress responses. Nat Rev Genet 19 (6), 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shalgi R et al. (2014) Widespread inhibition of posttranscriptional splicing shapes the cellular transcriptome following heat shock. Cell Rep 7 (5), 1362–1370. [DOI] [PubMed] [Google Scholar]
- 11.Mahat DB et al. (2016) Mammalian Heat Shock Response and Mechanisms Underlying Its Genome-wide Transcriptional Regulation. Mol Cell 62 (1), 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosa-Mercado NA et al. (2021) Hyperosmotic stress alters the RNA polymerase II interactome and induces readthrough transcription despite widespread transcriptional repression. Mol Cell 81 (3), 502–513.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vilborg A et al. (2015) Widespread inducible transcription downstream of human genes. Mol Cell 59 (3), 449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82 (2), 291–5. [DOI] [PubMed] [Google Scholar]
- 15.Vilborg A et al. (2017) Comparative analysis reveals genomic features of stress-induced transcriptional readthrough. Proc Natl Acad Sci U S A 114 (40), E8362–E8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosso AR et al. (2015) Pervasive transcription read-through promotes aberrant expression of oncogenes and RNA chimeras in renal carcinoma. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hockel M and Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93 (4), 266–76. [DOI] [PubMed] [Google Scholar]
- 18.Wiesel Y et al. (2018) DoGFinder: a software for the discovery and quantification of readthrough transcripts from RNA-seq. BMC Genomics 19 (1), 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutkowski AJ et al. (2015) Widespread disruption of host transcription termination in HSV-1 infection. Nat Commun 6, 7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer DLV et al. (2018) Influenza Virus Mounts a Two-Pronged Attack on Host RNA Polymerase II Transcription. Cell Rep 23 (7), 2119–2129 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilborg A and Steitz JA (2017) Readthrough transcription: How are DoGs made and what do they do? RNA Biol 14 (5), 632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao R-W et al. (2019) Cellular functions of long noncoding RNAs. Nature Cell Biology 21 (5), 542–551. [DOI] [PubMed] [Google Scholar]
- 23.Roth SJ et al. (2020) ARTDeco: automatic readthrough transcription detection. BMC Bioinformatics 21 (1), 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwalb B et al. (2016) TT-seq maps the human transient transcriptome. Science 352 (6290), 1225–8. [DOI] [PubMed] [Google Scholar]
- 25.Hennig T et al. (2018) HSV-1-induced disruption of transcription termination resembles a cellular stress response but selectively increases chromatin accessibility downstream of genes. PLoS Pathog 14 (3), e1006954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bannister AJ and Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Research 21 (3), 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gates LA et al. (2017) Histone Marks in the 'Driver's Seat': Functional Roles in Steering the Transcription Cycle. Trends Biochem Sci 42 (12), 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C and Zhu B (2018) Roles of H3K36-specific histone methyltransferases in transcription: antagonizing silencing and safeguarding transcription fidelity. Biophys Rep 4 (4), 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo YH and Li W-H (2012) Evolutionary Conservation of Histone Modifications in Mammals. Molecular Biology and Evolution 29 (7), 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heintzman ND et al. (2007) Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39 (3), 311–8. [DOI] [PubMed] [Google Scholar]
- 31.Creyghton MP et al. (2010) Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A 107 (50), 21931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman-Aiden E et al. (2009) Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326 (5950), 289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sexton T et al. (2012) Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148 (3), 458–72. [DOI] [PubMed] [Google Scholar]
- 34.Ulianov SV et al. (2016) Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res 26 (1), 70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y-J et al. (2004) Opening the chromatin for transcription. The International Journal of Biochemistry & Cell Biology 36 (8), 1411–1423. [DOI] [PubMed] [Google Scholar]
- 36.Schofield JA et al. (2018) TimeLapse-seq: adding a temporal dimension to RNA sequencing through nucleoside recoding. Nat Methods 15 (3), 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amat R et al. (2019) Rapid reversible changes in compartments and local chromatin organization revealed by hyperosmotic shock. Genome Res 29 (1), 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmer JT et al. (2021) STL-seq reveals distinct roles for release and termination from the promoter-proximal pause site. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birkenheuer CH et al. (2018) Herpes Simplex Virus 1 Dramatically Alters Loading and Positioning of RNA Polymerase II on Host Genes Early in Infection. Journal of Virology 92 (8), e02184–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardiello JF et al. (2018) Heat Shock Causes a Reversible Increase in RNA Polymerase II Occupancy Downstream of mRNA Genes, Consistent with a Global Loss in Transcriptional Termination. Mol Cell Biol 38 (18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazo A et al. (2007) Transcriptional interference: an unexpected layer of complexity in gene regulation. J Cell Sci 120 (Pt 16), 2755–61. [DOI] [PubMed] [Google Scholar]
- 42.Muniz L et al. (2017) Control of Gene Expression in Senescence through Transcriptional Read-Through of Convergent Protein-Coding Genes. Cell Rep 21 (9), 2433–2446. [DOI] [PubMed] [Google Scholar]
- 43.Pelechano V and Steinmetz LM (2013) Gene regulation by antisense transcription. Nat Rev Genet 14 (12), 880–93. [DOI] [PubMed] [Google Scholar]
- 44.Forero A et al. (2019) Differential Activation of the Transcription Factor IRF1 Underlies the Distinct Immune Responses Elicited by Type I and Type III Interferons. Immunity 51 (3), 451–464.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dye MJ and Proudfoot NJ (1999) Terminal exon definition occurs cotranscriptionally and promotes termination of RNA polymerase II. Mol Cell 3 (3), 371–8. [DOI] [PubMed] [Google Scholar]
- 46.Herzel L et al. (2018) Long-read sequencing of nascent RNA reveals coupling among RNA processing events. Genome Res 28 (7), 1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alpert T et al. (2020) Widespread Transcriptional Readthrough Caused by Nab2 Depletion Leads to Chimeric Transcripts with Retained Introns. Cell Rep 33 (4), 108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reimer KA et al. (2021) Co-transcriptional splicing regulates 3' end cleavage during mammalian erythropoiesis. Mol Cell 81 (5), 998–1012.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soucek S et al. (2016) The Evolutionarily-conserved Polyadenosine RNA Binding Protein, Nab2, Cooperates with Splicing Machinery to Regulate the Fate of pre-mRNA. Mol Cell Biol 36 (21), 2697–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castillo-Guzman D et al. (2020). SF3B1-targeted splicing inhibition triggers global alterations in transcriptional dynamics and R-loop metabolism. bioRxiv. Doi: 10.1101/2020.06.08.130583 [DOI] [Google Scholar]
- 51.Jalihal AP et al. (2020) Multivalent Proteins Rapidly and Reversibly Phase-Separate upon Osmotic Cell Volume Change. Mol Cell 79 (6), 978–990.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandel CR et al. (2008) Protein factors in pre-mRNA 3'-end processing. Cell Mol Life Sci 65 (7-8), 1099–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eaton JD et al. (2020) A unified allosteric/torpedo mechanism for transcriptional termination on human protein-coding genes. Genes Dev 34 (1-2), 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fong N et al. (2015) Effects of Transcription Elongation Rate and Xrn2 Exonuclease Activity on RNA Polymerase II Termination Suggest Widespread Kinetic Competition. Mol Cell 60 (2), 256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baillat D et al. (2005) Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell 123 (2), 265–76. [DOI] [PubMed] [Google Scholar]
- 56.Albrecht TR and Wagner EJ (2012) snRNA 3' end formation requires heterodimeric association of integrator subunits. Mol Cell Biol 32 (6), 1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stadelmayer B et al. (2014) Integrator complex regulates NELF-mediated RNA polymerase II pause/release and processivity at coding genes. Nat Commun 5, 5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baillat D and Wagner EJ (2015) Integrator: surprisingly diverse functions in gene expression. Trends Biochem Sci 40 (5), 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elrod ND et al. (2019) The Integrator Complex Attenuates Promoter-Proximal Transcription at Protein-Coding Genes. Mol Cell 76 (5), 738–752.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tatomer DC, Elrod ND, Liang D, Xiao MS, Jiang JZ, Jonathan M, Huang KL, Wagner EJ, Cherry S, and Wilusz JE (2019). The Integrator complex cleaves nascent mRNAs to attenuate transcription. Genes Dev 33, 1525–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beckedorff F et al. (2020) The Human Integrator Complex Facilitates Transcriptional Elongation by Endonucleolytic Cleavage of Nascent Transcripts. Cell Rep 32 (3), 107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Withers JB et al. (2019) Idiosyncrasies of Viral Noncoding RNAs Provide Insights into Host Cell Biology. Annu Rev Virol 6 (1), 297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nemeroff ME et al. (1998) Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3'end formation of cellular pre-mRNAs. Mol Cell 1 (7), 991–1000. [DOI] [PubMed] [Google Scholar]
- 64.Wang X et al. (2020) Herpes simplex virus blocks host transcription termination via the bimodal activities of ICP27. Nat Commun 11 (1), 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duns G et al. (2010) Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer Res 70 (11), 4287–91. [DOI] [PubMed] [Google Scholar]
- 66.Khosla S et al. (2020) The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol 16 (5), 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giaimo BD et al. (2019) The histone variant H2A.Z in gene regulation. Epigenetics Chromatin 12 (1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim T-K et al. (2010) Widespread transcription at neuronal activity-regulated enhancers. Nature 465 (7295), 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greer PL and Greenberg ME (2008) From Synapse to Nucleus: Calcium-Dependent Gene Transcription in the Control of Synapse Development and Function. Neuron 59 (6), 846–860. [DOI] [PubMed] [Google Scholar]
- 70.Beagan JA and Phillips-Cremins JE (2020) On the existence and functionality of topologically associating domains. Nature Genetics 52 (1), 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ray J et al. (2019) Chromatin conformation remains stable upon extensive transcriptional changes driven by heat shock. Proceedings of the National Academy of Sciences 116 (39), 19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heinz S et al. (2018) Transcription Elongation Can Affect Genome 3D Structure. Cell 174 (6), 1522–1536.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Proudfoot NJ (2016) Transcriptional termination in mammals: Stopping the RNA polymerase II juggernaut. Science 352 (6291), aad9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neve J et al. (2017) Cleavage and polyadenylation: Ending the message expands gene regulation. RNA biology 14 (7), 865–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H. et al. (2015) Poly(A) Signal-Dependent Transcription Termination Occurs through a Conformational Change Mechanism that Does Not Require Cleavage at the Poly(A) Site. Mol Cell 59 (3), 437–48. [DOI] [PubMed] [Google Scholar]
- 76.Albrecht TR et al. (2018) Integrator subunit 4 is a ‘Symplekin-like’ scaffold that associates with INTS9/11 to form the Integrator cleavage module. Nucleic Acids Research 46 (8), 4241–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie M et al. (2015) The host Integrator complex acts in transcription-independent maturation of herpesvirus microRNA 3' ends. Genes Dev 29 (14), 1552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skaar JR et al. (2015) The Integrator complex controls the termination of transcription at diverse classes of gene targets. Cell Res 25 (3), 288–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai F et al. (2015) Integrator mediates the biogenesis of enhancer RNAs. Nature 525 (7569), 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nojima T et al. (2018) Deregulated Expression of Mammalian lncRNA through Loss of SPT6 Induces R-Loop Formation, Replication Stress, and Cellular Senescence. Mol Cell 72 (6), 970–984.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lykke-Andersen S et al. (2021) Integrator is a genome-wide attenuator of non-productive transcription. Mol Cell 81 (3), 514–529.e6. [DOI] [PubMed] [Google Scholar]