Abstract
There is growing interest in utilizing pharmacogenetic (PGx) testing to guide antidepressant use, but there is lack of clarity on how to implement testing into clinical practice. We administered two surveys at 17 sites that had implemented or were in the process of implementing PGx testing for antidepressants. Survey 1 collected data on the process and logistics of testing. Survey 2 asked sites to rank the importance of Consolidated Framework for Implementation Research (CFIR) constructs using best‐worst scaling choice experiments. Of the 17 sites, 13 had implemented testing and four were in the planning stage. Thirteen offered testing in the outpatient setting, and nine in both outpatient/inpatient settings. PGx tests were mainly ordered by psychiatry (92%) and primary care (69%) providers. CYP2C19 and CYP2D6 were the most commonly tested genes. The justification for antidepressants selected for PGx guidance was based on Clinical Pharmacogenetics Implementation Consortium guidelines (94%) and US Food and Drug Administration (FDA; 75.6%) guidance. Both institutional (53%) and commercial laboratories (53%) were used for testing. Sites varied on the methods for returning results to providers and patients. Sites were consistent in ranking CFIR constructs and identified patient needs/resources, leadership engagement, intervention knowledge/beliefs, evidence strength and quality, and the identification of champions as most important for implementation. Sites deployed similar implementation strategies and measured similar outcomes. The process of implementing PGx testing to guide antidepressant therapy varied across sites, but key drivers for successful implementation were similar and may help guide other institutions interested in providing PGx‐guided pharmacotherapy for antidepressant management.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Many centers are beginning to utilize pharmacogenetic (PGx) testing to guide antidepressant therapy but this is not yet part of routine clinical practice. Implementation science, with its focus on systematically assessing implementation barriers and strategies, can enhance the integration of PGx into clinical care.
WHAT QUESTION DID THIS STUDY ADDRESS?
We evaluated the approaches taken by early adopters to operationalize clinical PGx testing for antidepressant management and assessed what factors were perceived to be important to the implementation.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The process of providing PGx testing, such as where the testing was performed (i.e., in‐house vs. commercial laboratory) and how results were returned to patients and providers, varied across sites. However, there were several common key factors that determined successful PGx implementation, such as the use of Clinical Pharmacogenetic Implementation Consortium guidelines, leadership engagement, identification of clinical champions, and deployment of educational strategies for clinical providers.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Experiences gained by early adopters of PGx implementation may help guide other institutions interested in providing PGx‐guided pharmacotherapy for antidepressant medications.
INTRODUCTION
Antidepressants are commonly prescribed medications used by ~ 13% of the population. 1 Although originally developed and approved for the treatment of major depressive disorder (MDD), many antidepressants are also used to treat other conditions, such as anxiety disorders, obsessive‐compulsive disorder, and post‐traumatic stress disorder. Approximately one in five people in the United States meets diagnostic criteria for a depressive or anxiety disorder at some point in their lifetime, both of which are commonly treated with antidepressants. 2 Identifying effective treatment(s) for a given patient can be difficult as clinicians are challenged to select antidepressants based on a number of factors. 3 Less than half of patients with depression achieve some response to a first antidepressant, only a third experience remission, and a third do not have adequate improvement or tolerability to two or more trials of an antidepressant. 4 Each trial involving a new antidepressant medication or dose in a given patient requires 4–8 weeks to evaluate effectiveness. This can result in extended trial‐and‐error odysseys and adverse effect experiences across a period of months to years, while a patient’s depression remains inadequately treated.
Common genetic variation may explain 42% of individual differences in antidepressant response, 5 highlighting the potential opportunities for using pharmacogenetic (PGx) information as part of clinical care for some psychiatric conditions. Genetic variability in the CYP2D6 and CYP2C19 drug metabolizing enzymes impacts dose‐adjusted exposure to a number of commonly used antidepressants. 6 Minimal doses and exposures of antidepressant medications are required for clinical efficacy and dose relationships with side effects and tolerability are well‐established. 7 Large cohort studies suggest that poor and ultrarapid metabolizer status is associated with treatment discontinuation, side effects, or lack of efficacy to some antidepressants. 8 Prospective PGx test guidance may improve symptom remission in patients with MDD requiring antidepressant therapies. 9
Although many psychiatrists and primary care providers agree that PGx testing will become part of standard care when prescribing psychotropics, 10 established processes of how best to incorporate this technology into clinical workflows are lacking. To aid the translation of PGx results for implementation into clinical practice, the Clinical Pharmacogenetics Implementation Consortium (CPIC) was established to create peer‐reviewed, evidence‐based guidelines. 11 The CPIC has published guidelines for how to use existing PGx information for greater than 50 medications, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs). 12 , 13 Furthermore, the US Food and Drug Administration (FDA) recognizes PGx associations with antidepressants that may be clinically relevant. 14 However, there are cautionary recommendations or position statements on clinical use of PGx testing from society guidelines focused on mental health. 15 , 16
Evidence‐based practices can take 15–20 years to be incorporated into routine clinical care. 17 Assessing and quantifying barriers to implementation within and external to a healthcare organization are essential for promoting the efficient adoption of novel interventions. Implementation science is an emerging field that can evaluate strategies to enhance the integration of genomic medicine interventions as applied to mental health clinical practice. 18
The Implementing Genomics in Practice (IGNITE) network is a multidisciplinary collaboration focused on the development, implementation, and dissemination of methods that incorporate genomic medicine into clinical care. 19 Previous work by the network identified factors based on the Consolidated Framework for Implementation Research (CFIR) that were vital to the adoption of genomic medicine interventions across six clinical sites, 20 , 21 although these were not necessarily specific to PGx. The growing availability of PGx tests to guide prescribers of mental health medications along with previously described challenges with clinical application presents a need to identify successful approaches to implementation for antidepressants. To understand factors important for the implementation of PGx testing to guide antidepressant prescribing, we surveyed 17 institutions of the IGNITE PGx Working Group. We conducted two surveys to understand (1) how sites were operationalizing PGx testing within their center and (2) the relative importance of implementation characteristics at institutions with planned or established programs to guide antidepressant use with PGx.
METHODS
Funded and affiliate members of the IGNITE Network that had either implemented or were planning to implement clinical laboratory testing (e.g., performed in a College of American Pathologists [CAP] accredited/Clinical Laboratory Improvement Amendment [CLIA] certified laboratory) to guide antidepressant prescribing were invited to participate in the surveys. Two electronic surveys (Supplementary Material) were developed to assess and describe institutional and practice environment characteristics and workflows for delivering PGx results and factors important for the implementation of PGx testing to guide antidepressant therapy. Survey 1 was developed by the IGNITE PGx Working Group to capture 34 measures regarding the process of implementing PGx for guiding antidepressant therapies. Data collection was completed at each site by IGNITE institutional representatives using a Research Electronic Data Capture (REDCap) database hosted at the University of Florida. 22 The data collection tool was piloted for feasibility and clarity prior to dissemination to participating sites. To reconcile completed survey irregularities, study investigators followed up with individual communications. Some questions allowed a free‐text response, which were subsequently recoded as an additional response or grouped into similar survey choices. Survey 1 was administered and completed between June 1, 2020, and October 1, 2020.
Survey 2 was distributed to those sites that completed survey 1 with the goal of identifying: (1) which factors were most important when implementing PGx for antidepressants, (2) which implementation outcomes, as defined by Proctor et al., 18 were observed or planned to be evaluated, and (3) the implementation strategies that were perceived to be most effective during active or planned implementation. Survey 2 was informed by the CFIR because (1) it is the framework that was used in prior IGNITE work 20 , 21 ; and (2) it is broadly applied in clinical implementation research and provides a stakeholder engaged framework. 23 The CFIR is composed of 37 constructs organized into five major domains that may influence implementation of an intervention. The domains are (1) Outer Setting (e.g., economic or political context), (2) Inner Setting (e.g., institutional climate or readiness for change), (3) Characteristics of Individuals (e.g., knowledge or beliefs about the intervention, individual stage of change), (4) Intervention Characteristics (e.g., evidence strength, quality, and cost), and (5) Process of Implementation (e.g., planning the interventions and engaging opinion leaders). To evaluate the importance of implementation factors, participants were asked to rank all constructs of the CFIR 24 across its five domains using best‐worst scaling (BWS) choice experiments: Outer Setting (4 constructs evaluated in 3 choice tasks), Inner Setting (14 constructs evaluated in 9 tasks), Intervention Characteristics (9 constructs evaluated in 6 tasks), Characteristics of Individuals (5 constructs evaluated in 3 tasks), and Process (8 constructs evaluated in 5 tasks). Choices included additional constructs from the Genomic Medicine Integrative Research Framework 25 not specified in CFIR but may be pertinent to PGx testing. The BWS method has been used to determine preferences for a wide range of health care applications. 26 BWS is a low‐burden method for quantitatively prioritizing a large number of observed factors that offers advantages to traditional rating or ranking techniques. Rather than only choosing the best alternative, respondents in BWS select the best (highest ranking) and worst (lowest ranking) items in a series of tasks, which provides ratio scales of importance. BWS circumvents common limitations with techniques that require individuals to rank alternatives, such as Likert‐style rating scales. Specifically, this method addresses concerns that respondents do not use the ratings the same way across responses as well as the challenge of evaluating multiple items that have similar importance. Survey 2 was administered and completed between September 15, 2020, and December 15, 2020.
This research was approved as exempt by the University of Florida Institutional Review Board.
Analysis
Descriptive statistics were characterized for all sites and then stratified by sites with established implementation programs and those in the planning stages. Exploratory comparisons between implemented and planning sites were conducted using the χ2 or Fisher’s exact tests for categorical data and independent t‐tests for continuous data. Both the BWS data collection and the quantitative analysis to estimate individual preferences for each construct were conducted using Lighthouse Studio (version 9.9.2; Sawtooth Software). We used multinomial logistic regression to estimate the probabilities of respondents choosing particular alternatives. Probability scores were then transformed into probability scaled scores (i.e., relative importance scores), which allowed for comparisons across attributes. The importance score summarizes how much impact the attribute had upon choice, given the range of constructs under evaluation. Importance scores are calculated as percentages of the range of constructs (that sum to 100% for each domain). Additionally, we applied a Bayesian approach using a Monte Carlo Markov chain to compare and update respondents’ estimates on the basis of the distribution of preferences from other respondents. Utility estimates of each construct were averaged after 10,000 random draws.
RESULTS
Survey 1
Institutional characteristics and programmatic drivers
Representatives of 17 sites responded and indicated that they had implemented (n = 13) or were in the planning phases (n = 4) of implementing PGx to guide antidepressant utilization. Detailed institutional characteristics are included in Table S1. All respondents completed both surveys. These were largely academic (n = 12) institutions implementing or planning implementation in the context of clinical care. Among sites that had already implemented, 13 (100%) were testing in the outpatient setting and nine (69%) were also testing in the inpatient setting.
Most respondents indicated that programmatic initiatives for PGx‐guided antidepressant implementation were initially championed by a dedicated precision medicine or PGx service (71%) and that this group was also leading current activities at most institutions (88%; Figure 1). Although fewer respondents indicated PGx leadership roles from psychiatry service lines, they were noted as essential collaborators by most (94%). Collaborating clinical or academic units spanned a range of groups within institutions (e.g., informatics, laboratory medicine, nursing, pharmacy, primary care, and psychiatry). PGx tests were predominantly ordered by psychiatry (92% of respondents) and primary care (69%; Figure 1).
FIGURE 1.
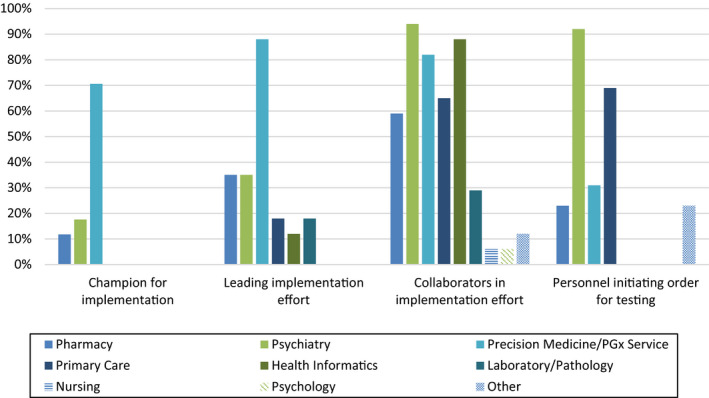
Personnel involved in antidepressant pharmacogenetic testing
Testing and operational workflow
Figure 2 provides an overview of PGx testing workflow. Most institutions (82%) indicated that prescribers were responsible for identifying patients for testing, whereas 53% noted options for patient self‐referral, and 35% indicated that pharmacy referrals were also available. Both institutional (in‐house) and commercial laboratories were used for testing.
FIGURE 2.
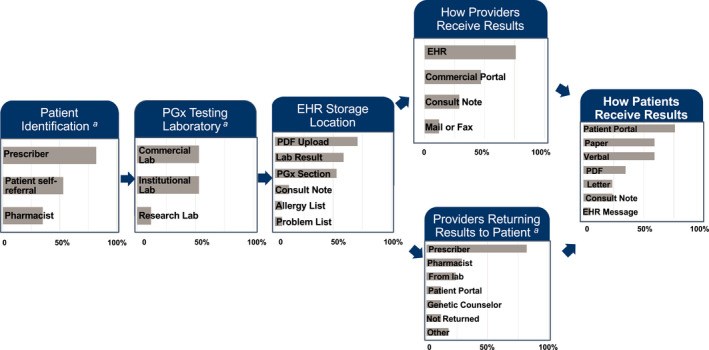
Pharmacogenetic testing and return of results workflow. The most common methods for PGx testing and return of results from 17 sites implementing or planning to implement PGx testing for tailoring antidepressant therapy are provided. EHR, electronic health record; PDF, portable document format; PGx, pharmacogenetic. aThe most common methods are displayed in the figure; additional options can be found in Table S2
Tables 1 and S2 provide additional details about testing and workflow considerations. Most (88%) respondents indicated that they did not require specific diagnoses or utilization of prior antidepressants for a test to be ordered. All institutions with established implementation programs utilized multigene PGx testing approaches, with five sites also offering single gene test orders. Most institutions included CYP2C19 and CYP2D6 genotypes as a core genetic basis for antidepressant guidance with five of the 13 implementing sites also offering testing for other drug metabolism or pharmacodynamic genes. Most respondents indicated PGx guidance was used for tailoring SSRIs and TCAs (Figure 3). The justification for antidepressants selected for PGx guidance was largely based on CPIC (94%) and FDA (75.6%) guidance with other groups referencing the Dutch Pharmacogenetics Working Group (DPWG; 23.5%) or internal evidence review (17.6%; Figure S1).
TABLE 1.
Pharmacogenetic testing and operational workflow for guiding antidepressant therapy
| Process |
Total (n = 17) N (%) |
Stage of implementation | |
|---|---|---|---|
|
Implemented total (n = 13) N (%) |
Planning Total (n = 4) N (%) |
||
| Prior antidepressant treatment required | |||
| No | 15 (88) | 12 (92) | 3 (75) |
| Age of patients eligible for PGx testing | |||
| <18 years | 1 (6) | 0 (0) | 1 (25) |
| ≥18 years | 4 (24) | 4 (31) | 0 (0) |
| No age restriction | 12 (71) | 9 (69) | 3 (75) |
| Type of PGx test a | |||
| Single gene | 7 (41) | 5 (39) | 2 (50) |
| Multigene | 16 (94) | 13 (100) | 3 (75) |
| Method used for genotyping | |||
| Genotyping | 17 (100) | 13 (100) | 4 (100) |
| Sequencing | 3 (18) | 3 (23) | 0 (0) |
| Testing payment method | |||
| Patient/self‐pay | 12 (71) | 8 (62) | 4 (100) |
| Insurance/third party billed | 11 (65) | 10 (77) | 1 (25) |
| Research funded | 7 (41) | 5 (39) | 2 (50) |
| Other | 3 (18) | 3 (23) | 0 (0) |
| Genes used to guide antidepressant therapy | |||
| CYP2C19 | 16 (100) b | 13 (100) | 3 (100) c |
| CYP2D6 | 15 (94) b | 12 (92) | 3 (100) c |
| Other | 5 (39) b | 5 (39) | 0 (0) |
| Established institutional workflow for ordering and return of results | |||
| Yes | 12 (71) | 10 (77) | 2 (50) |
| Results reported as discrete data | |||
| Yes | 11 (65) | 9 (69) | 2 (50) |
| Clinical decision support available for prescribing decisions | |||
| Consultation | 12 (71) | 10 (77) | 2 (50) |
| PDF report | 11 (65) | 9 (69) | 2 (50) |
| Electronic CDS | 9 (53) | 8 (62) | 1 (25) |
| None | 1 (6) | 0 (0) | 1 (25) |
| Other | 1 (6) | 1 (8) | 0 (0) |
| Results used to guide other therapies in addition to antidepressants | |||
| Yes | 10 (59) | 9 (69) | 1 (25) |
Abbreviations: CDS, clinical decision support; PGx, pharmacogenetic; PDF, portable document format.
Sites could select more than one option.
Out of 16 sites.
Out of 3 sites.
FIGURE 3.
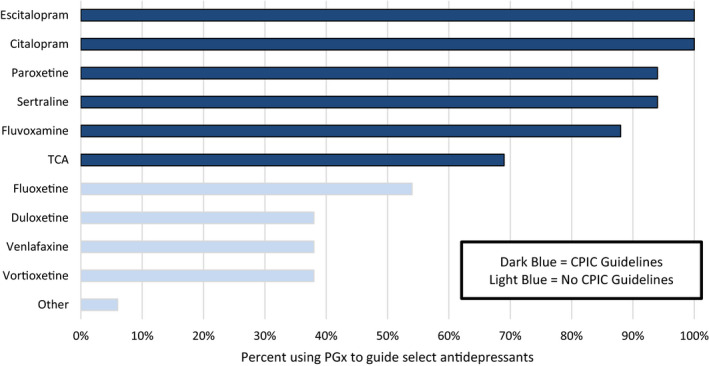
Antidepressants considered for pharmacogenetic guidance. More than one response was allowed. Only 16 of 17 sites responded. Providers may have access to the PGx report and use it to tailor additional psychotropic medications. CPIC, Clinical Pharmacogenetics Implementation Consortium; PGx, pharmacogenetic, TCA, tricyclic antidepressants
Return of results
The majority (76%) of respondents indicated that results were (or will be) returned to providers via the electronic health record (EHR), whereas 47% utilized prescriber‐specific portals associated with commercial tests (Figure 2, Table S2). Most (82%) respondents indicated that prescribers communicated results to patients, whereas less than 30% of institutions reported that results were communicated by pharmacists, genetic counselors, or through laboratory reports.
Of the sites that had already implemented, prescribing decisions were further supported using expert consultation (77%), portable document format (PDF) reports (69%), and electronic clinical decision support (CDS; 62%; Table 1). Most institutions indicated that they uploaded the genotype results as discrete variables (e.g., with star [*] allele nomenclature) into the EHR (65%).
Survey 2
Implementation characteristics
Using BWS, respondents from each institution ranked CFIR constructs important to the uptake of PGx testing to guide antidepressant prescribing. Figure 4 shows the ranking of the top three constructs within each domain across all sites. The top constructs for each domain identified as most important for PGx implementation were: patient needs/resources (domain: Outer Setting), leadership engagement (domain: Inner Setting), clinician knowledge and beliefs about the intervention (domain: Characteristics of Individuals), strength and quality of the evidence supporting PGx testing (domain: Intervention Characteristics) and the identification of champions to support PGx testing (domain: Process). A complete ranking of all constructs within each domain can be found in Table S3 with further stratification by sites that had already implemented PGx testing for antidepressants and those that were in the planning phases. The top ranked constructs between sites that had already implemented PGx testing and those in the planning phase were largely consistent with a few exceptions. Compatibility (domain: Inner Setting) or fit of antidepressant PGx testing with individual values or institutional workflow, was ranked second among those in the planning phase, whereas it ranked sixth among those that had implemented. Formally appointed internal implementation leaders (domain: Process) was ranked as the third most important construct among sites that had implemented, whereas it ranked fifth among those in the planning phase. Sites were also consistent in those constructs that were least important for implementing PGx testing, which included cosmopolitanism (domain: Outer Setting) or the degree to which the organization is connected to other institutions; organization incentives and awards (domain: Inner Setting); individual identification with the organization (domain: Characteristics of Individuals); design quality and packaging of the PGx intervention (domain: Intervention Characteristics); and identifying external change agents (domain: Process).
FIGURE 4.
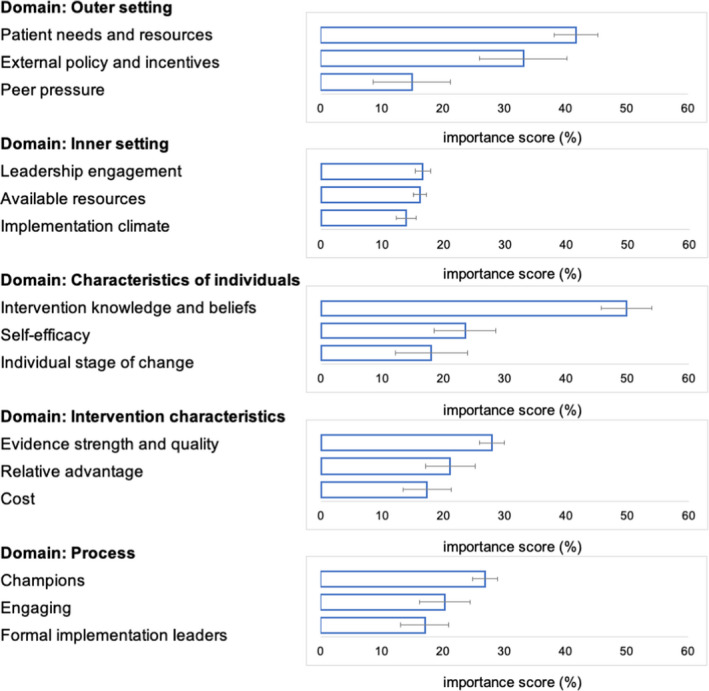
The top three constructs within each domain from the Consolidated Framework for Implementation Research (CFIR) rated as most important for implementation of pharmacogenetic testing to guide antidepressant treatment with importance scores and 95% confidence intervals
Sites were also asked about what outcomes they were collecting to assess implementation success. Table 2 reports the most common outcomes measured. Most sites were measuring implementation outcomes, including acceptability, adoption, and costs of the PGx intervention. Sites that had already implemented testing were more likely to measure patient and service outcomes compared with sites in the planning phase. In addition, sites indicated that they deployed a multifaceted implementation strategy to enhance uptake of PGx testing, with the most common strategies being identifying barriers for implementation, developing educational materials for providers, and facilitating the relay of PGx test results to providers within the EHR (Table S4). All but one site (94%) indicated that they received internal institutional‐level funding to support implementation (Table S5). Sites that had already implemented were more likely to have additional sources of funding, including external funding, such as the National Institutes of Health (NIH) grants and philanthropic sources (Table S5). Four sites (n = 3 implemented and n = 1 planning) indicated that clinical revenue was also a current or planned source of support.
TABLE 2.
Outcomes assessed or planned to be assessed during implementation of pharmacogenetic testing for antidepressants
| Outcomes |
All (n = 17) N (%) |
Stage of implementation | |
|---|---|---|---|
|
Implemented (n = 13) N (%) |
Planning (n = 4) N (%) |
||
| Implementation outcomes | |||
| Acceptability | 14 (82) | 11 (85) | 3 (75) |
| Adoption | 13 (76) | 10 (77) | 3 (75) |
| Costs | 13 (76) | 10 (77) | 3 (75) |
| Feasibility | 13 (76) | 11 (85) | 2 (50) |
| Penetration | 12 (71) | 11 (95) | 1 (25) |
| Appropriateness | 10 (59) | 8 (62) | 2 (50) |
| Fidelity | 10 (59) | 8 (62) | 2 (50) |
| Sustainability | 9 (53) | 7 (54) | 2 (50) |
| Service outcomes | |||
| Effectiveness | 14 (82) | 13 (100) | 1 (25) |
| Safety | 13 (76) | 12 (92) | 1 (25) |
| Timeliness | 12 (71) | 10 (77) | 2 (50) |
| Patient‐centeredness | 10 (59) | 9 (69) | 1 (25) |
| Efficiency | 6 (35) | 4 (31) | 2 (50) |
| Equity | 6 (35) | 6 (46) | 0 (0) |
| Patient outcomes | |||
| Symptomatology | 12 (71) | 11 (85) | 1 (25) |
| Satisfaction | 9 (53) | 8 (62) | 1 (25) |
| Function (QOL) | 6 (35) | 5 (38) | 1 (25) |
| Impact on health and social policy | 3 (18) | 3 (23) | 0 (0) |
Outcomes from Proctor et al. 20
Abbreviation: QOL, quality of life.
DISCUSSION
Our study assessed testing processes and operational workflow considerations from institutions that are part of the IGNITE Network PGx Working Group and are implementing or planning to implement PGx guidance for antidepressant therapy. We also asked sites to rank the relative importance of constructs from the CFIR. We found notable similarities across sites in testing process and CFIR rankings. Most sites reported their implementation being led by a multidisciplinary PGx service, providing recommendations according to CPIC guidelines, and communicating recommendations through electronic CDS. The primary constructs within the CFIR that were identified as most important included patient needs and resources (domain: Outer Setting), leadership engagement (domain: Inner Setting), intervention knowledge and beliefs (domain: Characteristics of Individuals), evidence strength and quality (domain: Intervention Characteristics), and the identification of champions (domain: Process). The results from our study provide important information for institutions seeking to advance precision medicine approaches for mental health.
Other centers implementing PGx testing for the management of antidepressants have described similar processes. Common themes highlighted across those institutions as well as those examined herein include leadership by a multidisciplinary team, extensive efforts to integrate results into the EHR, and use of electronic CDS. 27 , 28 , 29 In developing CDS for prescribers, PGx management recommendations in these centers are largely based on CPIC or DPWG guidelines, which is similar to our findings.
Prior studies that have evaluated the clinical or economic impact of PGx testing in psychiatry clinics have primarily examined commercial tests that provide results to prescribers through provider‐specific portals established by the testing lab outside the EHR. 30 , 31 Although this approach informs mental health care with PGx data at a specific point in therapy, it presents challenges for how best to store results, make them available to other providers, determine relevance to medications beyond those used for mental health indications, and assure accessibility for future treatment decisions. Many commercially available PGx tests provide information for multiple genes beyond those included in guidelines and FDA labeling and results that are formatted differently across laboratories. Some of these include combinatorial tests that use proprietary algorithms to provide pharmacotherapy recommendations and may include genes with weaker levels of evidence. Despite some of the positive outcomes reported with the use of commercial testing that report results directly to providers, 30 , 31 , 32 there are no gold standard approaches for how best to implement or organize this process. Due to this situation and concerns about differences across commercial tests, mental health professional organizations have issued cautionary statements suggesting that PGx testing is either not recommended or not ready for widespread use. 15 Institution and implementation characteristics identified herein bridge this gap and identify considerations and strategies for other organizations considering application of evidence‐based PGx information to guide antidepressant use. Although this may provide some optimism, a reality is that the institutions responding to our surveys have developed programmatic efforts with defined leadership and interprofessional collaboration that took time and capital to create.
In survey 2, we asked sites to rank constructs within the CFIR that were most (and least) important for implementation of PGx testing for antidepressants. The sites were consistent in the selection of factors from the CFIR deemed most important for implementation. The constructs of evidence strength and quality (domain: Intervention Characteristics) and provider knowledge and beliefs (domain: Characteristics of Individuals) were among the top constructs identified as important for implementation. As shown by this study, rankings of these constructs reinforce the results of previous surveys of psychiatrists and primary care providers in which providers expressed concern about the evidence supporting PGx testing and its clinical utility as well as provider lack of comfort in interpreting PGx test results to guide drug therapy decisions. 33 , 34 Much of the evidence supporting genotype‐guided antidepressant therapy consists of pharmacokinetic data, with differences in serum drug concentrations observed across genotype groups, rather than evidence of improved remission rates with genotype‐guided therapy from prospective trials. 6 While we await the results of large randomized controlled trials showing the clinical utility of PGx guided approaches, 34 , 35 sites that have implemented PGx testing for antidepressants are concurrently collecting effectiveness and safety outcomes needed to grow the evidence base, which may be needed to support broader clinician and payer uptake and acceptance of PGx testing. All sites surveyed in the present study were focused on collecting data regarding implementation outcomes, such as acceptability, adoption, and costs of PGx testing.
Successful implementation is inherently collaborative and complex, involving multiple stakeholders across institutional hierarchies. 17 The CFIR constructs of leadership engagement, available resources, and implementation climate were also ranked highly among sites both that have implemented and were in the planning phases of implementation. PGx implementation can be resource intensive and involves buy‐in from multiple stakeholders, such as precision medicine leadership, laboratory medicine, provider groups, pharmacists, and information services. As such, 16 of the 17 sites surveyed indicated that they received some degree of internal funding to support this initiative. Genomic medicine and PGx face the additional burden of the costs or reimbursement for genetic tests. However, US payer coverage for PGx tests is improving and now includes Medicare patients through new Molecular Diagnostic Services (MolDx) local coverage determinations (LCDs). 36 The LCD includes gene‐drug pairs that are clinically actionable as defined by the CPIC or the FDA and includes CYP2C19/CYP2D6 for antidepressant prescribing.
These results expand findings from previous implementation science work conducted by the IGNITE network, which identified system‐level barriers for genomic medicine implementation more broadly. 20 , 37 Prior research from IGNITE identified three common challenges to genomic medicine implementation, including integration of genomics in the EHR, improving clinician knowledge and beliefs about genomic medicine, and engaging patients to become active participants in genomic medicine studies, for example, by giving feedback on specific implementation activities. 20 These themes are reinforced in our study where sites implementing PGx testing ranked constructs related to knowledge and beliefs about the intervention and patient needs and resources as critical to implementation success. Previous IGNITE research also ranked CFIR constructs important to genomic medicine implementation and created standardized measurement instruments for those identified as high priority. 21 There was some overlap in the rankings of these constructs in our study, including knowledge and beliefs about the intervention and self‐efficacy (domain: Characteristics of Individuals); implementation climate and readiness for implementation (domain: Inner Setting); relative advantage and cost (domain: Intervention Characteristics); and engaging (domain: Process). Additional constructs that were highly ranked in our study unique to PGx implementation were leadership engagement and available resources (domain: Inner Setting) and the identification of champions (domain: Process). The rankings of the outer and inner setting constructs are aligned with the notion that successful implementation (and sustainability) is predicated upon increasing the fit of the PGx testing program with its inner and outer settings (e.g., institutional support and available resources).
The IGNITE PGx working group has also previously published implementation barriers and offered strategies for implementation of CYP2C19 for selection of antiplatelet medications 38 and CYP2D6 for prescribing opioid medications. 39 These previous papers highlight the importance of establishing multidisciplinary teams, identifying a physician champion, educating providers, creating electronic CDS to facilitate PGx testing, and collection of clinical outcomes data to support the utility of PGx testing. In the current study, sites reiterated the importance of these themes and are using similar strategies to facilitate PGx testing for antidepressants, such as developing educational materials for physicians and pharmacists, identifying and training physician champions, relaying PGx results with CDS within the EHR, and creating a centralized PGx consult service.
The results of our study should be interpreted in the context of their limitations. Respondents of the surveys were sites participating in the IGNITE network; therefore, some findings from our study may not be representative to the broader community. For example, most sites indicated support by a dedicated precision medicine or PGx service to oversee their implementation, which may not be readily accessible at all centers. However, processes for implementing PGx testing and factors important for implementation from these early adopter sites may be beneficial to centers seeking to newly implement PGx testing. The unfunded affiliate sites that constituted most of the respondents herein, are expected to be representative of many institutions in the active or planning stages of implementation. Formalized PGx implementation processes to guide antidepressant use are relatively new, and the overall number of respondents, particularly those in the planning phase was small. We did not collect the specific genotypes that were used for phenotype translation because of the large number of genotypes included on many commonly used test panels. Our approach was not suitable to quantify organizational capacity for incorporating a PGx program. This is an important characteristic connected to sustainability to assess in future studies. Implementation is a constantly evolving process, and most sites are observing early‐ to mid‐stage implementation outcomes (e.g., acceptability), whereas only a minority of sites are observing later‐stage outcomes (e.g., sustainability). As implementation progresses across sites, these later‐stage outcomes will be important to evaluate. Similar to other stakeholder engagement approaches, the primary limitation of BWS is its focus on stated preferences, or respondents’ perceptions of what multilevel factors are most and least likely to influence implementation. These responses may not be reflective of actual practice, and there may be other important factors influencing implementation that were not observed in this study.
In summary, the process of implementing PGx testing to guide antidepressant use in sites from the IGNITE PGx working group is varied with respect to test ordering process and the process for returning results to the providers and patients. However, sites were consistent with respect to dedicated PGx program leadership of a multidisciplinary implementation team and efforts to integrate results into the EHR. Furthermore, the genes and medications used to guide antidepressant therapy were largely informed by CPIC guidelines. Additionally, sites identified similar CFIR constructs that were important to drive their implementation, used strategies to address implementation barriers, and collected similar implementation and effectiveness outcomes to measure the success of their implementations.
CONFLICT OF INTEREST
D.M.S. has institution‐associated research funding from Kailos Genetics. P.E.E. performs consulting at Cipherome. S.M.S. has a consulting/advisory or non‐promotional speaking role from Exact Sciences (Genomic Health), Genentech/Roche, Daiichi Sankyo, Athenex, Natura, and Silverback Therapeutics, IDMC from AstraZeneca, support for third party writing assistance from Genentech/Roche, and institution‐associated research funding from Genentech and Kailos Genetics. L.B.R. receives research funding from BTG, Intl. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
S.T., R.G.S., A.L.E., D.M.S., E.R., K.V.B., N.A.L., C.L.A., J.B., A.L.B., A.C., B.Q.D., P.E.E., C.M.F., J.K.H., P.M., D.W.O., A.L.P., N.P., C.B.R., A.S., S.M.S., K.M.W., K.W., T.C.S., S.L.V., L.H.C., and J.R.B. wrote the manuscript. S.T., R.G.S., L.H.C., and J.R.B. designed the research. S.T., R.G.S., A.L.E., D.M.S., E.R., K.V.B., N.A.L., C.L.A., J.B., A.L.B., A.C., B.Q.D., P.E.E., C.M.F., J.K.H., P.M., D.W.O., A.L.P., N.P., C.B.R., A.S., S.M.S., K.M.W., K.W., T.C.S., S.L.V., L.H.C., and J.R.B. performed the research. S.T., R.G.S., A.L.E., L.H.C., and J.R.B. analyzed the data.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge the following for assistance in completion of the surveys: Emily Cicali from the University of Florida; the Pharmacogenomics Implementation Committee Colorado (PICColo) Evaluation Working Group at the University of Colorado; Whitney Mason and Joy Thomas from Intermountain Precision Genomics; April Schultz from Sanford Health Imagenetics; Cindy Prows from the University of Cincinnati; and Michelle Liu and Bart Roland from Vanderbilt University Medical Center.
Tuteja S, Salloum RG, Elchynski AL, et al; for the IGNITE Pharmacogenetics Working . Multisite evaluation of institutional processes and implementation determinants for pharmacogenetic testing to guide antidepressant therapy. Clin Transl Sci. 2022;15:371–383. 10.1111/cts.13154
Funding information
This work was supported by grants from the National Institutes of Health (U01 HG007269, U01 HG010232, and U01 HG010245, and by the NIH IGNITE Network (https://gmkb.org/ignite/). Additional funding was provided by K23HL143161 and the Penn Center for Precision Medicine for ST; Indiana University Precision Health Initiative for E.R. and T.C.S.; Propeller Health, American Lung Association for K.B.; NIH for K.B., B.Q.D., and L.B.R.; K24HL133373 for N.A.L.; U01HG007775 for A.L.B.; U54TR001857 and the Pitt/UPMC Institute for Precision Medicine for P.E.E.; Agency for Healthcare Research and Quality (AHRQ) and Patient‐Centered Outcomes Research Institute (PCORI) K12HS026379, NIH/NCATS KL2TR002492 for P.M.; NIH/NCATS UL1TR001427 for L.H.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ, PCORI, Minnesota Learning Health System Mentored Career Development Program (MN‐LHS), or the National Institutes of Health.
REFERENCES
- 1. Brody DJ, Gu Q. Antidepressant use among adults: United States, 2015–2018. NCHS Data Brief. 2020;377:1–8. [PubMed] [Google Scholar]
- 2. Ornstein SM, Nietert PJ, Jenkins RG, Litvin CB. The prevalence of chronic diseases and multimorbidity in primary care practice: a PPRNet report. J Am Board Fam Med. 2013;26:518‐524. [DOI] [PubMed] [Google Scholar]
- 3. Gabriel FC, de Melo DO, Fráguas R, Leite‐Santos NC, Mantovani da Silva RA, Ribeiro E. Pharmacological treatment of depression: a systematic review comparing clinical practice guideline recommendations. PLoS One. 2020;15:e0231700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905‐1917. [DOI] [PubMed] [Google Scholar]
- 5. Tansey KE, Guipponi M, Hu X, et al. Contribution of common genetic variants to antidepressant response. Biol Psychiatry. 2013;73:679‐682. [DOI] [PubMed] [Google Scholar]
- 6. Milosavljevic F, Bukvic N, Pavlovic Z, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta‐analysis. JAMA Psychiatry. 2021;78:270‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jakubovski E, Varigonda AL, Freemantle N, Taylor MJ, Bloch MH. Systematic review and meta‐analysis: dose‐response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jukic MM, Haslemo T, Molden E, Ingelman‐Sundberg M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: a retrospective study based on 2,087 patients. Am J Psychiatry. 2018;175:463‐470. [DOI] [PubMed] [Google Scholar]
- 9. Bousman CA, Arandjelovic K, Mancuso SG, Eyre HA, Dunlop BW. Pharmacogenetic tests and depressive symptom remission: a meta‐analysis of randomized controlled trials. Pharmacogenomics. 2019;20:37‐47. [DOI] [PubMed] [Google Scholar]
- 10. Walden LM, Brandl EJ, Changasi A, et al. Physicians’ opinions following pharmacogenetic testing for psychotropic medication. Psychiatry Res. 2015;229:913‐918. [DOI] [PubMed] [Google Scholar]
- 11. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15:209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98:127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hicks JK, Sangkuhl K, Swen JJ, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. United States Food and Drug Administration . Table of pharmacogenetic associations. https://www.fda.gov/medical‐devices/precision‐medicine/table‐pharmacogenetic‐associations. Accessed April 6, 2016.
- 15. Zeier Z, Carpenter LL, Kalin NH, et al. Clinical implementation of pharmacogenetic decision support tools for antidepressant drug prescribing. Am J Psychiatry. 2018;175:873‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Genetic Testing Statement . International Society of Psychiatric Genetics. 2019. https://ispg.net/genetic‐testing‐statement/. Accessed May 18, 2021.
- 17. Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA. 2016;315:1941‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Proctor EK, Landsverk J, Aarons G, Chambers D, Glisson C, Mittman B. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. 2009;36:24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weitzel KW, Alexander M, Bernhardt BA, et al. The IGNITE network: a model for genomic medicine implementation and research. BMC Med Genomics. 2016;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sperber NR, Carpenter JS, Cavallari LH, et al. Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med Genomics. 2017;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orlando LA, Sperber NR, Voils C, et al. Developing a common framework for evaluating the implementation of genomic medicine interventions in clinical care: the IGNITE Network’s Common Measures Working Group. Genet Med. 2018;20:655‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horowitz CR, Orlando LA, Slavotinek AM, et al. The genomic medicine integrative research framework: a conceptual framework for conducting genomic medicine research. Am J Hum Genet. 2019;104:1088‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flynn TN, Louviere JJ, Peters TJ, Coast J. Best–worst scaling: What it can do for health care research and how to do it. J Health Econ. 2007;26:171‐189. [DOI] [PubMed] [Google Scholar]
- 27. Caraballo PJ, Bielinski SJ, St. Sauver JL, Weinshilboum RM. Electronic medical record‐integrated pharmacogenomics and related clinical decision support concepts. Clin Pharmacol Ther. 2017;102:254‐264. [DOI] [PubMed] [Google Scholar]
- 28. Dunnenberger HM, Biszewski M, Bell GC, et al. Implementation of a multidisciplinary pharmacogenomics clinic in a community health system. Am J Health Syst Pharmacy. 2016;73:1956‐1966. [DOI] [PubMed] [Google Scholar]
- 29. van der Wouden CH, Cambon‐Thomsen A, Cecchin E, et al. Implementing pharmacogenomics in Europe: Design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharmacol Ther. 2017;101:341‐358. [DOI] [PubMed] [Google Scholar]
- 30. Perlis RH, Mehta R, Edwards AM, Tiwari A, Imbens GW. Pharmacogenetic testing among patients with mood and anxiety disorders is associated with decreased utilization and cost: A propensity‐score matched study. Depress Anxiety. 2018;35:946‐952. [DOI] [PubMed] [Google Scholar]
- 31. Espadaler J, Tuson M, Lopez‐Ibor JM, Lopez‐Ibor F, Lopez‐Ibor MI. Pharmacogenetic testing for the guidance of psychiatric treatment: a multicenter retrospective analysis. CNS Spectr. 2017;22:315‐324. [DOI] [PubMed] [Google Scholar]
- 32. Brennan FX, Gardner KR, Lombard J, et al. A naturalistic study of the effectiveness of pharmacogenetic testing to guide treatment in psychiatric patients with mood and anxiety disorders. Prim Care Companion CNS Disord. 2015;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vest BM, Wray LO, Brady LA, et al. Primary care and mental health providers’ perceptions of implementation of pharmacogenetics testing for depression prescribing. BMC Psychiatry. 2020;20:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johansen Taber KA, Dickinson BD. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmacogenomics Pers Med. 2014;7:145‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oslin DW, Chapman S, Duvall SL, et al. Study design and implementation of the PRecision Medicine In MEntal health Care (PRIME Care) Trial. Contemp Clin Trials. 2021;101:106247. [DOI] [PubMed] [Google Scholar]
- 36. Empey PE, Pratt VM, Hoffman JM, Caudle KE, Klein TE. Expanding evidence leads to new pharmacogenomics payer coverage. Genet Med. 2021;23:830‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zebrowski AM, Ellis DE, Barg FK, et al. Qualitative study of system‐level factors related to genomic implementation. Genet Med. 2019;21:1534‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Empey PE, Stevenson JM, Tuteja S, et al. Multisite investigation of strategies for the implementation of CYP2C19 genotype‐guided antiplatelet therapy. Clin Pharmacol Ther. 2018;104:664‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cavallari LH, Van Driest SL, Prows CA, et al. Multi‐site investigation of strategies for the clinical implementation of CYP2D6 genotyping to guide drug prescribing. Genet Med. 2019;21:2255‐2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


