Abstract
Background
Hip fractures are a major healthcare problem, presenting a huge challenge and burden to individuals and healthcare systems. The number of hip fractures globally is rising rapidly. The majority of hip fractures are treated surgically. This review evaluates evidence for types of arthroplasty: hemiarthroplasties (HAs), which replace part of the hip joint; and total hip arthroplasties (THAs), which replace all of it.
Objectives
To determine the effects of different designs, articulations, and fixation techniques of arthroplasties for treating hip fractures in adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, seven other databases and one trials register in July 2020.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing different arthroplasties for treating fragility intracapsular hip fractures in older adults. We included THAs and HAs inserted with or without cement, and comparisons between different articulations, sizes, and types of prostheses. We excluded studies of people with specific pathologies other than osteoporosis and with hip fractures resulting from high‐energy trauma.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We collected data for seven outcomes: activities of daily living, functional status, health‐related quality of life, mobility (all early: within four months of surgery), early mortality and at 12 months after surgery, delirium, and unplanned return to theatre at the end of follow‐up.
Main results
We included 58 studies (50 RCTs, 8 quasi‐RCTs) with 10,654 participants with 10,662 fractures. All studies reported intracapsular fractures, except one study of extracapsular fractures. The mean age of participants in the studies ranged from 63 years to 87 years, and 71% were women.
We report here the findings of three comparisons that represent the most substantial body of evidence in the review. Other comparisons were also reported, but with many fewer participants.
All studies had unclear risks of bias in at least one domain and were at high risk of detection bias. We downgraded the certainty of many outcomes for imprecision, and for risks of bias where sensitivity analysis indicated that bias sometimes influenced the size or direction of the effect estimate.
HA: cemented versus uncemented (17 studies, 3644 participants)
There was moderate‐certainty evidence of a benefit with cemented HA consistent with clinically small to large differences in health‐related quality of life (HRQoL) (standardised mean difference (SMD) 0.20, 95% CI 0.07 to 0.34; 3 studies, 1122 participants), and reduction in the risk of mortality at 12 months (RR 0.86, 95% CI 0.78 to 0.96; 15 studies, 3727 participants). We found moderate‐certainty evidence of little or no difference in performance of activities of daily living (ADL) (SMD ‐0.03, 95% CI ‐0.21 to 0.16; 4 studies, 1275 participants), and independent mobility (RR 1.04, 95% CI 0.95 to 1.14; 3 studies, 980 participants). We found low‐certainty evidence of little or no difference in delirium (RR 1.06, 95% CI 0.55 to 2.06; 2 studies, 800 participants), early mortality (RR 0.95, 95% CI 0.80 to 1.13; 12 studies, 3136 participants) or unplanned return to theatre (RR 0.70, 95% CI 0.45 to 1.10; 6 studies, 2336 participants). For functional status, there was very low‐certainty evidence showing no clinically important differences.
The risks of most adverse events were similar. However, cemented HAs led to less periprosthetic fractures intraoperatively (RR 0.20, 95% CI 0.08 to 0.46; 7 studies, 1669 participants) and postoperatively (RR 0.29, 95% CI 0.14 to 0.57; 6 studies, 2819 participants), but had a higher risk of pulmonary embolus (RR 3.56, 95% CI 1.26 to 10.11, 6 studies, 2499 participants).
Bipolar HA versus unipolar HA (13 studies, 1499 participants)
We found low‐certainty evidence of little or no difference between bipolar and unipolar HAs in early mortality (RR 0.94, 95% CI 0.54 to 1.64; 4 studies, 573 participants) and 12‐month mortality (RR 1.17, 95% CI 0.89 to 1.53; 8 studies, 839 participants). We are unsure of the effect for delirium, HRQoL, and unplanned return to theatre, which all indicated little or no difference between articulation, because the certainty of the evidence was very low. No studies reported on early ADL, functional status and mobility.
The overall risk of adverse events was similar. The absolute risk of dislocation was low (approximately 1.6%) and there was no evidence of any difference between treatments.
THA versus HA (17 studies, 3232 participants)
The difference in the risk of mortality at 12 months was consistent with clinically relevant benefits and harms (RR 1.00, 95% CI 0.83 to 1.22; 11 studies, 2667 participants; moderate‐certainty evidence). There was no evidence of a difference in unplanned return to theatre, but this effect estimate includes clinically relevant benefits of THA (RR 0.63, 95% CI 0.37 to 1.07, favours THA; 10 studies, 2594 participants; low‐certainty evidence). We found low‐certainty evidence of little or no difference between THA and HA in delirium (RR 1.41, 95% CI 0.60 to 3.33; 2 studies, 357 participants), and mobility (MD ‐0.40, 95% CI ‐0.96 to 0.16, favours THA; 1 study, 83 participants). We are unsure of the effect for early functional status, ADL, HRQoL, and mortality, which indicated little or no difference between interventions, because the certainty of the evidence was very low.
The overall risks of adverse events were similar. There was an increased risk of dislocation with THA (RR 1.96, 95% CI 1.17 to 3.27; 12 studies, 2719 participants) and no evidence of a difference in deep infection.
Authors' conclusions
For people undergoing HA for intracapsular hip fracture, it is likely that a cemented prosthesis will yield an improved global outcome, particularly in terms of HRQoL and mortality. There is no evidence to suggest a bipolar HA is superior to a unipolar prosthesis. Any benefit of THA compared with hemiarthroplasty is likely to be small and not clinically appreciable. We encourage researchers to focus on alternative implants in current clinical practice, such as dual‐mobility bearings, for which there is limited available evidence.
Plain language summary
Hip replacement surgery in adults
This review assessed evidence from randomised controlled trials (RCTs) and quasi‐RCTs, on the benefits and harms of different types of hip replacement used to treat hip fracture in adults.
Background
A hip fracture is a break at the top of the leg bone. These types of breaks are common in older adults whose bones may be fragile because of a condition called osteoporosis. One method of treatment is to replace the broken hip with an artifical one. This can be done using a hemiarthroplasty (HA), which replaces part of the hip joint (the ball part of the joint). These replacements can be unipolar (a single artificial joint), or bipolar (with an additional joint within the HA). Alternatively, surgery may replace the whole hip joint, which also includes the socket in which the ball of the hip joint sits ‐ this a total hip arthroplasty (THA). Both of these artificial joints can be fixed in place with or without bone cement.
Search date
We searched for RCTs (clinical studies where people are randomly assigned to treatment groups), and quasi‐RCTs (in which people are put into groups by a method which is not randomised, such as date of birth or hospital record number) up to 6 July 2020.
Study characteristics
We included 58 studies, involving 10,654 adults with 10,662 hip fractures. Study participants ranged from 63 to 87 years of age, and 71% were women, which is usual for people who have this type of hip fracture.
Key results
Cemented HAs compared to uncemented HAs (17 studies, 3644 participants)
We found that cemented HAs improve health‐related quality of life (HRQoL) and reduce the risk of death at 12 months after surgery. The sizes of these benefits ranged from a small to a large effect. There may be little or no difference between treatments in the ability to use the hip (functional status), but this evidence was very uncertain. Whether or not the HA is cemented probably makes little or no difference to performance in activities of daily living (ADL) or the ability to walk independently, how many people experience confusion after surgery (delirium), die within four months of surgery, or need additional surgery. Most complication risks were similar, but we noted that some risks related directly to hip replacement surgery (such as causing a break during surgery) were increased with uncemented HAs.
Bipolar HAs compared to unipolar HAs (13 studies, 1499 participants)
The type of HA probably makes little or no difference to how many people die within four months or up to 12 months after surgery, and may make little or no difference to the need for additional surgery. No studies reported four‐month ADL and functional status. The evidence was very uncertain whether using a bipolar or unipolar HA makes any difference to delirium or HRQoL within four months of surgery. Again, complication risks were similar, and we found no evidence of a difference in the risk of hip dislocation.
THAs compared to HAs (17 studies, 3232 participants)
We are uncertain whether ADL, functional status, delirium, mobility, or deaths within four months or up to 12 months after surgery are different between these treatments. The evidence did not show a difference in the risk of additional surgery but we could not exclude the possibility of an important benefit of THA. Although the risk of most complications was similar, hip dislocation is increased with THA.
Certainty of the evidence
The evidence for many of the comparisons is based on only a few participants, and many studies used methods which may not be reliable. Most of the evidence for ADL, functional status, HRQoL, and independent walking was of low and very low certainty, meaning that we are not confident in the findings. We had limited confidence or were moderately confident in our other findings.
Conclusions
For people having a HA, it is likely that a cemented replacement produces a better outcome overall than an uncemented replacement. There is no evidence to suggest that a bipolar HA leads to different outcomes from a unipolar HA. The differences between a total hip replacement and partial hip replacement are small and may not be clinically important.
Summary of findings
Background
Description of the condition
Epidemiology
A hip fracture, or proximal femoral fracture, is a break in the upper region of the femur (thigh bone) between the subcapital region (the area just under the femoral head) and 5 cm below the lesser trochanter (a bony projection of the upper femur). The incidence of hip fractures increases with age, and are most common in the older adult population (Court‐Brown 2017; Kanis 2001). Hip fractures in younger adults are usually associated with poor bone health (Karantana 2011; Rogmark 2018). A small proportion of fractures occurring in younger people are a result of high‐energy trauma, such as road traffic collisions and sports injuries. Most hip fractures are fragility fractures associated with osteoporosis, and resulting from mechanical forces that would not ordinarily result in fracture. The World Health Organization (WHO) has defined fragility fracture as those sustained from injuries equivalent to a fall from a standing height or less (Kanis 2001). In the UK, the mean age of a person with hip fracture is 83 years and approximately two‐thirds occur in women (NHFD 2017).
Hip fractures are a major healthcare problem at the individual and population level, and present a huge challenge and burden to individuals, healthcare systems, and societies. The increased proportion of older adults in the world population means that the absolute number of hip fractures is rising rapidly worldwide. For example, in 2016 there were 65,645 new presentations of hip fracture to 177 trauma units in England, Wales, and Northern Ireland (NHFD 2017). Based on mid‐2016 population estimates for these regions, this equates to an incidence rate of 108 cases per 100,000 population (ONS 2018). By 2050, the annual worldwide incidence is estimated to be 6 million hip fractures (Cooper 2011; Johnell 2004). Incident hip fracture rates are higher in high‐income countries compared to low‐ or middle‐income countries. The highest hip fracture rates are seen across northern Europe and the USA, and the lowest in Latin America and Africa (Dhanwal 2011). There is also a north‐south gradient seen in European studies, and similarly, more fractures are seen in the north of the USA than in the south (Dhanwal 2011). The factors responsible for the variation in the incidence of hip fractures and osteoporosis are thought to be population demographics (with more elderly populations in countries with higher incidence rates), and the influence of ethnicity, latitude, and environmental factors such as socioeconomic deprivation (Bardsley 2013; Cooper 2011; Dhanwal 2011; Kanis 2012).
Burden of disease
Hip fractures are also associated with a high risk of death. For example, in England, Wales, and Northern Ireland, the 30‐day mortality rate in 2016 remained high at 6.7%, despite a decline from 8.5% in 2011 and 7.1% in 2015 (NHFD 2017). Mortality at one year following a hip fracture is approximately 30%. However, fewer than half of deaths are attributable to the fracture itself, reflecting the frailty of the individuals and associated high prevalence of comorbidities and complications (Parker 1991; SIGN 2009). Morbidity associated with hip fractures is similar to stroke in terms of impact, with a substantial loss of healthy life‐years in older people (Griffin 2015). As such, hip fractures commonly result in reduced mobility and greater dependency, with many people failing to return to their pre‐injury residence. In addition, the public health impact of hip fractures is significant: data from large prospective cohorts show the burden of disease due to hip fracture is 27 disability‐adjusted life years (DALYs) per 1000 individuals, which equates to an average loss of 2.7% of the healthy life expectancy in this population at risk of fragility hip fracture (Papadimitriou 2017).
The direct economic burden of hip fractures is also substantial. Hip fractures are among the most expensive conditions seen in hospitals, with an aggregated cost of nearly 4900 million US dollars (USD) for 316,000 inpatient episodes in the USA in 2011 (Torio 2013). In England, Wales, and Northern Ireland, people with hip fracture occupy 1.5 million hospital bed days each year, and cost the National Health Service (NHS) and social care 1000 million pounds sterling (GBP) (NHFD 2017). Combined health and social care costs incurred during the first year following a hip fracture have been estimated at USD 43,669, which is greater than the cost for non‐communicable diseases, such as acute coronary syndrome (USD 32,345) and ischaemic stroke (USD 34,772) (Williamson 2017). In established market economies, hip fractures represent 1.4% of the total healthcare burden (Johnell 2004).
Types of hip fracture
Hip fractures either involve the region of the femur that is enveloped by the ligamentous hip joint capsule (intracapsular), or that is outside the capsule (extracapsular).
Intracapsular fractures include subcapital (immediately below the femoral head), transcervical (across the mid‐femoral neck), or basicervical (across the base of the femoral neck). These injuries are also commonly termed fractures of the ‘neck of femur' (Lloyd‐Jones 2015). Intracapsular fractures can be further subdivided by fracture morphology using several different classification systems, such as the Garden (Garden 1961) or Pauwels classifications (Pauwels 1935). The reliability of these various classifications is poor (Parker 1993a; Parker 1998). A more appropriate grouping distinguishes only those fractures that are displaced, where the anatomy of the bone has been disrupted at the fracture site, and those that are undisplaced (Blundell 1998; Parker 1999). This system broadly corresponds with prognosis: the more displaced, the more likely the blood supply to the femoral head is compromised, which can lead to complications such as avascular necrosis and collapse of the femoral head. More recently, this classification has been refined with additional consideration of posterior tilt ‐ this is not a component of earlier classification, but may be useful in predicting poor outcomes from osteosynthesis (Palm 2009). Furthermore, displaced fractures are less stable, so that treatments involving fixation have a higher risk of failure compared with undisplaced fractures. Approximately 60% of hip fractures are intracapsular; of these, approximately 70% to 90% are displaced (Keating 2010; NHFD 2017).
Extracapsular fractures traverse the femur within the area of bone bounded by the intertrochanteric line proximally up to a distance of 5 cm from the distal part of the lesser trochanter. Several classification methods have been proposed to define different types of extracapsular fractures (AO Foundation 2018; Evans 1949; Jensen 1980). They are generally subdivided depending on their relationship to the greater and lesser trochanters, the two bony projections present at the upper end of the femur, and the complexity of the fracture configuration. It is increasingly clear that each of these classifications is limited in its generalisability since inter‐ and intra‐observer agreement is poor. Table 4 provides a description of the most recent classification of trochanteric fractures (AO Foundation 2018). For this Cochrane Review, we plan to use a pragmatic simplification of these classifications as follows.
1. Trochanteric region fractures: type and surgical management (revised AO/OTA classification, January 2018).
| Type | Features | Stability | Description |
| Simple, pertrochanteric fractures (A1) |
|
Stable | The fracture line can begin anywhere on the greater trochanter and end either above or below the lesser trochanter. The medial cortex is interrupted in only 1 place. |
| Multifragmentary pertrochanteric fractures (A2) |
|
Unstable | The fracture line can start laterally anywhere on the greater trochanter and runs towards the medial cortex which is typically broken in 2 places. This can result in the detachment of a third fragment which may include the lesser trochanter. |
| Intertrochanteric fractures (A3) |
|
Unstable | The fracture line passes between the 2 trochanters, above the lesser trochanter medially and below the crest of the vastus lateralis laterally. |
AO/OTA: Arbeitsgemeinschaft für Osteosynthesefragen (German for "Association for the Study of Internal Fixation") / Orthopaedic Trauma Association
Trochanteric fractures: those that lie mostly between the intertrochanteric line and a transverse line at the level of the lesser trochanter. These can be further divided into simple two‐part stable fractures and comminuted or reverse obliquity unstable fractures.
Subtrochanteric fractures: those that mostly lie in the region bordered by the lesser trochanter and 5 cm distal to the lesser trochanter.
Approximately 40% of hip fractures are extracapsular, of which 90% are trochanteric and 10% are subtrochanteric (NHFD 2017).
Description of the intervention
Internationally, many guidelines exist concerning hip fracture management (e.g. AAOS 2014; NICE 2011; SIGN 2009). Each recommends that early surgical management, generally within 24 to 48 hours, is the mainstay of care for most hip fractures. The overall goal of surgery in the older population is to facilitate early rehabilitation, enabling early mobilisation and the return to premorbid function while minimising the complication risk. This approach has been associated with reductions in mortality in many worldwide registries (Neufeld 2016; Sayers 2017). A proposed grouping of arthroplasty interventions is given in Table 5.
2. Proposed grouping of different types of arthroplasty for hip fracture in adults.
| Implant category | Variable (articulation/fixation technique) | Implant subcategory | Examplesa | Description |
| Total hip arthroplasty | Articulation | Femoral head and acetabular bearing surface materials |
|
Bearing surfaces may be grouped into hard (ceramic and metal) and soft (polyethylene variants). Arthroplasties exist with many of the possible combinations of these bearing surfaces. |
| Femoral head size |
|
Over the development of hip arthroplasty, different sizes of femoral head have been used, from 22 mm to very large diameters approximating that of the native femoral head. The size of the head represents a compromise between stability and linear and volumetric wear at the articulation. The optimum size varies by indication and bearing materials. 36 mm is considered as a cut‐off between standard and large sizes. | ||
| Acetabular cup mobility |
|
A standard THA has a single articulating surface between the femoral head and acetabulum bearing surface. Alternative designs incorporate a further articulation within the structure of the femoral head. | ||
| Fixation technique | Cemented |
|
Both components are cemented with polymethylmethacrylate bone cement that is inserted at the time of surgery. It sets hard and acts a grout between the prosthesis and the bone. | |
| Modern uncemented |
|
Neither component is cemented but rely on osseous integration forming a direct mechanical linkage between the bone and the implant. The femoral prosthesis may be coated with a substance such as hydroxyapatite which promotes bone growth into the prosthesis. Alternatively, the surface of the prosthesis may be macroscopically and microscopically roughened so that bone grows onto the surface of the implant. The acetabular component may be prepared similarly and may or may not be augmented with screws fixed into the pelvis. | ||
| Hybrid | Combinations | The femoral stem is cemented and the acetabular cup is uncemented. | ||
| Reverse hybrid | Combinations | The acetabular cup is cemented and the femoral stem is uncemented. | ||
| Hemiarthroplasty | Articulation | Unipolar |
|
A single articulation between the femoral head and the native acetabulum. The femoral component can be a single ‘monoblock’ of alloy or be modular, assembled from component parts during surgery. |
| Bipolar |
|
The object of the second joint is to reduce acetabular wear. This type of prosthesis has a spherical inner metal head with a size between 22 to 36 mm in diameter. This fits into a polyethylene shell, which in turn is enclosed by a metal cap. There are a number of different types of prostheses with different stem designs. | ||
| Fixation technique | First‐generation uncemented |
|
These prostheses were designed before the development of polymethylmethacrylate bone cement and were therefore originally inserted as a ‘press fit’. Long‐term stability through osseus integration was not part of the design concept. | |
| Cemented |
|
The femoral stem is cemented with polymethylmethacrylate bone cement that is inserted at the time of surgery. It sets hard and acts a grout between the prosthesis and the bone. | ||
| Modern uncemented |
|
The femoral stem relies on osseous integration forming a direct mechanical linkage between the bone and the implant. A prosthesis may be coated with a substance such as hydroxyapatite, which promotes bone growth into the prosthesis. Alternatively, the surface of the prosthesis may be macroscopically and microscopically roughened so that bone grows onto the surface of the implant. |
aThis list is not exhaustive.
Abbreviations: CoC: Ceramic‐on‐ceramic CoP: Ceramic‐on‐polyethylene CPT: collarless polished tapered HCL: Highly cross‐linked MoM: Metal‐on‐metal MoP: Metal‐on‐polyethylene THA: total hip arthroplasty
Arthroplasty
Arthroplasty entails replacing part or all of the hip joint with an endoprosthesis: an implant constructed of non‐biological materials such as metal, ceramic, or polyethylene. Arthroplasties can be grouped into two main categories: hemiarthroplasty (HA) where only the femoral head and neck are replaced, and total hip arthroplasty (THA) where both the femoral head and the acetabulum or socket are replaced.
Hemiarthroplasty
Hemiarthroplasty involves replacing the femoral head with a prosthesis whilst retaining the natural acetabulum and acetabular cartilage. The type of HA can be broadly divided into two groups: unipolar and bipolar. In unipolar HAs, the femoral head is a solid block of metal. Bipolar femoral heads include a single articulation that allows movement to occur, not only between the acetabulum and the prosthesis, but also at this joint within the prosthesis itself.
The best known of the early HA designs are the Moore prosthesis (1952) and the FR Thompson Hip Prosthesis (1954). These are both monoblock implants and were designed before the development of polymethylmethacrylate bone cement. They were therefore originally inserted as a ‘press fit’. The Moore prosthesis has a square femoral stem, which is fenestrated and has a shoulder to enable stabilisation within the femur; this resists rotation within the femoral canal. It is generally used without cement and, in the long term, bone in‐growth into the fenestrations can occur. The Thompson prosthesis has a smaller stem without fenestrations and is now often used in conjunction with cement. Numerous other designs of unipolar HAs exist, based on stems that have been used for THAs.
In bipolar prostheses, there is an articulation within the femoral head component itself. In this type of prosthesis, there is a spherical inner metal head between 22 mm and 36 mm in diameter. This fits into a polyethylene shell, which in turn is enclosed by a metal cap. The objective of the second joint is to reduce acetabular wear by promoting movement at the intraprosthetic articulation rather than with the native acetabulum. There are a number of different types of prostheses with different stem designs. Examples of bipolar prostheses are the Charnley‐Hastings, Bateman, Giliberty, and the Monk prostheses, but many other types with different stem designs exist.
Total hip arthroplasty
Total hip arthroplasty (also known as total hip replacement) involves the replacement of the acetabulum in addition to the femoral head. The first successful THA was developed by John Charnley, using metal alloy femoral heads articulating with polyethylene acetabular components. Subsequently, the articulating materials have diversified, and designs using metal alloys, ceramics, and various polyethylenes in various combinations have all been used.
Component fixation
Irrespective of the nature of the articulating surfaces, the components must be fixed to the bone to ensure longevity of the arthroplasty. The two approaches used to achieve this fixation are cemented and uncemented designs.
Cemented systems
Polymethylmethacrylate bone cement may be inserted at the time of surgery. It sets hard and acts as a grout between the prosthesis and the bone at the time of surgery. Potential advantages of cement are a reduced risk of intraoperative fracture, later periprosthetic fracture, and not relying on integration of the prosthesis with osteoporotic bone. Major side effects of cement are cardiac arrhythmias and cardiorespiratory collapse, which occasionally occur following its insertion. These complications may be fatal, leading either to embolism from marrow contents forced into the circulation (Christie 1994), or a direct toxic effect of the cement.
Uncemented systems
Uncemented systems rely on osseous integration forming a direct mechanical linkage between the bone and the implant. A prosthesis may be coated with a substance, such as hydroxyapatite, which promotes bone growth into the prosthesis. Alternatively, the surface of the prosthesis may be macroscopically and microscopically roughened so that bone grows onto the surface of the implant.
The general complications of both types of arthroplasty are those general to surgical management of hip fracture ‐ namely, pneumonia, venous thromboembolism, infection, acute coronary syndrome, and cerebrovascular accident ‐ and those specific to arthroplasty, including dislocation of the prosthesis, loosening of the components, acetabular wear, and periprosthetic fracture.
Why it is important to do this review
This review replaces the Cochrane Review, Parker 2010a, on the same topic. We used up‐to‐date review methods and have optimised current relevance in terms of patient population, implants used, and outcomes for policymaking bodies, such as the National Institute for Health and Care Excellence (NICE) in the UK, as well as international audiences. Since Parker 2010a, clinical uncertainty remains as to the optimum implant for older adults. Moreover, further studies have been reported since the last literature search in September 2009.
Appraisal and synthesis of contemporary evidence may enable more robust conclusions to be made to better inform practice. Furthermore, for displaced intracapsular fractures, the recommended treatment is either HA or THA (Parker 2010a; Hopley 2010; NICE 2011). However, there is a lack of evidence regarding whether older adults experience better outcomes with THA or HA. Recent research has also found interhospital variation and systematic inequalities in the provision of THA (Perry 2016). Further evidence is necessary to verify which individuals gain the most from THA. For treatment of undisplaced intracapsular fractures, there is also a gap in the evidence that resulted in the recently updated NICE guideline being unable to make an evidence‐based recommendation on the best surgical management strategy (NICE 2011). Other reviews that will address other types of interventions are in preparation; we focus on arthroplasty in this review.
Objectives
To determine the effects of different designs, articulations, and fixation techniques of arthroplasties for treating hip fractures in older adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs that assessed surgical interventions for the management of people with hip fracture. Quasi‐RCTs are trials in which the methods of allocating people to a trial are not properly random, but are intended to produce similar groups (Cochrane 2018). We included trials published as conference abstracts, provided the trial authors reported sufficient data relating to the methods and outcomes of interest. We aimed to include unpublished data if identified in the searches.
Types of participants
We included adults undergoing surgery in a hospital setting for fragility (low‐energy trauma) hip fractures. We included displaced and undisplaced intracapsular or extracapsular fractures which we expected to be caused by low‐energy trauma.
We expected trial populations to have a mean age of between 80 to 85 years, and include 70% women, 30% with chronic cognitive impairment, and 50% with an American Society of Anesthesiologists (ASA) score greater than II, indicating that people may have a disease or condition affecting their fitness before surgery (NHFD 2017; NICE 2011). These characteristics would be representative of the general hip fracture population.
We excluded studies that focused exclusively on the treatment of participants: younger than 16 year of age; with fractures caused by specific pathologies other than osteoporosis; and with high‐energy traumas. However, we took a pragmatic approach to study inclusion criteria, and included studies with mixed populations (fragility and other mechanisms, ages, or pathologies). We expected that participants with standard fragility fractures were most likely to outnumber those with high‐energy trauma or local pathological fractures; therefore, the results will be generalisable to the fragility fracture population. If the data were reported separately for standard fragility fractures, we planned to use this subgroup data in our main analysis.
We did not pool studies in which the fracture type is mixed (intracapsular and extracapsular).
Types of interventions
We included all hip prostheses: unipolar HA, bipolar HA, or THA (small and large head), applied with or without cement. We included the following comparisons in the review.
Prostheses inserted with cement versus without cement (stratified by THA versus HA; HA group subgrouped by modern versus first‐generation uncemented stems).
Bipolar HA versus unipolar HA (subgrouped by cemented versus uncemented).
HAs versus other HAs (subgrouped by modern stem design (‘ODEP 3A rating') and first‐generation stem design (e.g. Austin‐Moore or Thompson).
THA versus HA (cemented or uncemented, subgrouped by old versus new, as described above);
Single versus multiple (dual/triple) articulations of THA.
Large‐head THA (36 mm diameter or larger) versus other arthroplasty (stratified by THA versus HA).
We created a detailed table of interventions, grouping them by characteristics, and indicating which are in worldwide use. We prepared this table for the protocol with clinical authors and with the International Fragility Fracture Network (www.fragilityfracturenetwork.org/), and we updated it during review preparation to include all implants used in the included studies (Table 5).
Types of outcome measures
Depending on the length of follow‐up reported, we categorised the endpoints for outcomes into 'early' (up to and including 4 months), 12 months (prioritising 12‐month data, but in its absence including data after 4 months and up to 24 months) and 'late' (after 24 months, up to the end of study follow‐up). We selected four months as the definition of 'early' because most of early recovery has been achieved at this time point (Griffin 2015). This decision is also in accordance with the core outcome set for hip fracture, which prioritises early outcome over late recovery (Haywood 2014). Although priority was given to early outcomes in the presentation of our data, we also included outcome data at the '12 months' and 'late' times points.
Critical outcomes
We extracted information on the following seven 'critical' outcomes.
Activities of daily living (e.g. Barthel Index (BI), Functional Independence Measure (FIM)).
Delirium using recognised assessment scores, such as Mini‐Mental State Examination (MMSE) mental test score and the four 'A's test (4AT).
Functional status (region‐specific) (e.g. hip rating questionnaire, Harris Hip Score, Oxford Hip Score).
Health‐related Quality of Life (HRQoL) (e.g. Short Form Health Survey (SF‐36), EuroQol‐5 Dimensions (EQ‐5D)).
Mobility (e.g. indoor/outdoor walking status, Cumulated Ambulation Score, Elderly Mobility Scale Score, Timed Up and Go (TUG) test, Short Physical Performance Battery, self‐reported walking scores (e.g. Mobility Assessment Tool ‐ short form)).
Mortality.
Unplanned return to theatre: secondary procedure required for a complication resulting directly or indirectly from the index operation or primary procedure.
Other important outcomes
We also reported the following 'important' outcomes.
Pain (verbal rating or visual analogue scale (VAS)).
Length of in‐hospital stay.
Discharge destination. We used study authors’ definitions, which were variably defined in the included studies.
Adverse events.
We grouped adverse events by relatedness to the implant or fracture, or both. We reported each adverse event type separately for maximum clarity, and included the following.
Related
Damage to a nerve, tendon, or blood vessel.
Intraoperative periprosthetic fracture.
Postoperative periprosthetic fracture.
Loosening of prosthesis.
Wound infection. We used study authors' definitions, which often distinguished deep infection and superficial infection.
Dislocation.
Unrelated
Acute kidney injury.
Blood transfusion.
Cerebrovascular accident.
Chest infection/pneumonia.
Decreased cognitive ability.
Myocardial infarction/acute coronary syndrome.
Sepsis.
Urinary tract infection.
Venous thromboembolic phenomena.
Search methods for identification of studies
As well as developing a strategy for this review, we developed general search strategies for the large bibliographic databases to find records to feed into a number of Cochrane Reviews and review updates on hip fracture surgery (Lewis 2021; Lewis 2022a; Lewis 2022b; Lewis 2022c). We searched the main databases up to July 2020.
Electronic searches
We identified RCTs and quasi‐RCTs through literature searching with systematic and sensitive search strategies, as outlined in Chapter 4 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2019, hereafter referred to as the Cochrane Handbook). We applied no restrictions on language, date, or publication status. We searched these databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL; CRS Web; 8 July 2020);
MEDLINE (Ovid; 1946 to 6 July 2020);
Embase (Ovid; 1980 to 7 July 2020);
Web of Science (SCI EXPANDED; 1900 to 8 July 2020);
Cochrane Database of Systematic Reviews (CDSR; Cochrane Library; 7 July 2020);
Database of Abstracts of Reviews of Effects (DARE; www.crd.york.ac.uk/CRDWeb/; 17 December 2018);
Health Technology Assessment (HTA) database (www.crd.york.ac.uk/CRDWeb/; 17 December 2018);
Epistemonikos (www.epistemonikos.org/; 9 July 2020);
Proquest Dissertations and Theses (Proquest; 1743 to 8 July 2020);
National Technical Information Service (NTIS, for technical reports; www.ntis.gov/; 10 July 2020).
We developed a subject‐specific search strategy in MEDLINE and other listed databases. We adapted strategies with consideration of database interface differences as well as different indexing languages. In MEDLINE, we used the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2019). In Embase, we used the Cochrane Embase filter (www.cochranelibrary.com/central/central-creation) to focus on RCTs. We ran the initial searches in November and December 2018, and a top‐up search in July 2020 in all databases except for DARE and HTA, in which no new records had been added since the initial search. At the time of the search, CENTRAL was fully up to date with all records from the Cochrane Bone, Joint, and Muscle Trauma (BJMT) Group's Specialised Register, and so it was not necessary to search this separately. We developed the search strategy in consultation with Information Specialists (see Acknowledgements) and the Information Specialist for the BJMT Group. Search strategies can be found in Appendix 1.
We scanned ClinicalTrials.gov (www.clinicaltrials.gov/) for ongoing and unpublished trials on 10 July 2020.
Searching other resources
We handsearched these conference abstracts from 2016 to November 2018:
Fragility Fractures Network Congress;
British Orthopaedic Association Congress;
Orthopaedic World Congress (SICOT);
Orthopaedic Trauma Association Annual Meeting;
Bone and Joint Journal Orthopaedic Proceedings;
American Academy of Orthopaedic Surgeons Annual Meeting.
To identify further studies, we screened the reference lists of studies included in Parker 2010a as well as the reference lists of eligible studies and systematic reviews published within the last five years that were retrieved by the searches.
Data collection and analysis
In order to reduce bias, we ensured that any review author who is a co‐applicant, study author, or has or has had an advisory role on any potentially relevant study, remained independent of study selection decisions, risk of bias assessment, and data extraction for their study.
Selection of studies
Two review authors independently screened titles and abstracts of all the retrieved bibliographic records in a web‐based systematic reviewing platform, Rayyan (Ouzzani 2016), and in the top‐up search using Covidence. Full texts of all potentially eligible records passing the title‐ and abstract‐screening level were retrieved and examined independently by two review authors against the eligibility criteria described in Criteria for considering studies for this review. We conducted full‐text screening using Covidence. We resolved disagreements through discussion or by adjudication of a third review author. We excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We prepared a PRISMA flow diagram to outline the study selection process, numbers of records at each stage of selection, and reasons for exclusions of full‐text articles (Moher 2009). We reported in the review details of key excluded studies, rather than all studies that were excluded from consideration of full‐text articles.
Data extraction and management
All review authors conferred on the essential data for extraction. We designed a data extraction form that aligns with the default headings in the Characteristics of included studies (see Appendix 2). Two review authors independently piloted the form on five studies and compared results. We then made changes to the template following additional discussion with the review author team. For the remaining data extraction, one review author independently extracted data and a second review author checked all the data for accuracy. We extracted the following data.
Study methodology: publication type; sponsorship/funding/notable conflicts of interest of trial authors; study design; numbers of centres and locations; size and type of setting; study inclusion and exclusion criteria; randomisation method; number of randomised participants, losses (and reasons for losses), and number analysed for each outcome. (Collecting information relating to the participant flow helped the assessment of risk of attrition bias.)
Population: baseline characteristics of the participants by group and overall (age, gender, smoking history, medication, body mass index (BMI), comorbidities, functional status such as previous mobility, place of residence before fracture, cognitive status, American Society of Anesthesiologists (ASA) status, fracture type and displacement).
Interventions: details of each intervention (number and type, manufacturer details); general surgical details (number of clinicians and their skills and experience, perioperative care such as use of prophylactic antibiotics or antithromboembolics, mobilisation or weight‐bearing protocols).
Outcomes: all outcomes measured or reported by study authors; outcomes relevant to the review (including measurement tools and time points of measure); extraction of outcome data into data and analysis tables or additional tables in Review Manager 2014.
Assessment of risk of bias in included studies
We assessed risk of bias in the included studies using the Cochrane risk of bias tool (Higgins 2011a). We assessed the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants, personnel (performance bias).
Blinding of outcome assessors (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other risks of bias.
We considered risk of detection bias separately for: subjective outcomes measured by clinicians, objective outcomes measured by clinicians, and participant‐reported outcomes (e.g. pain and HRQoL). For each domain, two review authors judged whether study authors made sufficient attempts to minimise bias in their design. For each domain, we made judgements using three measures ‐ high, low, or unclear risk of bias ‐ and we recorded these judgements in risk of bias tables.
Measures of treatment effect
We calculated risk ratios (RRs) for dichotomous data outcomes with 95% confidence intervals (CIs); it was not appropriate to use Peto odds ratio (OR) to calculate effects because no outcomes had very low numbers of observed events. We expressed treatment effects for continuous data outcomes evaluated using the same measurement scales as mean differences (MD) with 95% CI. For outcomes measured using different scales, we used standardised mean differences (SMD) with 95% CI.
In the event that studies reported dichotomous data using more than one category, we selected these cut‐off points in the distribution of categories:
for functional status: we reported data for those with a score of excellent or good (using Harris Hip Score (HHS)) versus those with a score of moderate or poor;
for mobility: we reported data for those who were able to walk independently out of doors with no more than the use of one stick (NICE 2011), versus those who were more dependent;
for pain: we reported data for participants who reported no pain versus those who reported any category of pain;
for discharge destination: we reported data for participants who were discharged home versus those who were discharged to a care environment.
Unit of analysis issues
In preparation of the review, we encountered potential unit of analysis issues. We found that some studies reported number of hip fractures (or cases) as well as the number of participants, with a very small number of participants having two fractured hips. Often, differentiating the denominators within a report was challenging. In such studies, depending on the outcome, the unit of analysis was either the participant (for example, for outcomes such as mortality, discharge destination, or some adverse events), or the case (for example, for outcomes such as unplanned return to theatre). We noted this differentiation where applicable and used the unit of analysis (participants or case) that was appropriate for the outcome within these studies. One study included three intervention groups (Dorr 1986). We created a pairwise comparison by combining the data for the two HA groups (cemented and uncemented) and comparing these data with the THA group. Although the review included a comparison of cemented HA versus uncemented HA, we did not use data from these two study arms in this comparison because recruitment to these two groups was completed at different time points within the study period and thus it was not appropriate to compare these against one another.
Dealing with missing data
For each included study, we recorded the number of participant losses for each outcome. Unless reported otherwise, we assumed complete case data for mortality, unplanned return to theatre, and adverse events. For outcomes that required participant assessment at end of follow‐up (such as HRQoL), we prioritised intention‐to‐treat (ITT) data where these data were available. If ITT data were unavailable for these outcomes, and if study authors did not clearly report denominator figures for each group for the outcome, we reduced the denominator figure in each group to account for reported mortality. We did not impute missing data. We used the risk of bias tool to judge attrition bias. We judged studies to be at high risk of attrition bias if we noted large amounts of unexplained missing data, loss that could not be easily justified in the study population, or losses were not sufficiently balanced between intervention groups. If we included a study with high attrition bias, we explored the effect during sensitivity analysis. We completed sensitivity analysis only for critical review outcomes and only considered attrition for outcomes that may be affected by these losses.
We attempted to contact study authors of more recently published trials when we noted that data for critical outcomes appeared to be measured but not reported. Where standard deviations were not reported, we attempted to determine these from other reported data (such as standard errors, confidence intervals, or exact P values). We noted in the Characteristics of included studies when we could not use outcome data because they were insufficiently reported or because numbers of losses in each group were not clearly specified.
Assessment of heterogeneity
We used the I2 statistic, automatically calculated in Review Manager 2014 software, to quantify the possible degree of heterogeneity of treatment effects between trials. We assumed moderate heterogeneity when the I2 was between 30% and 60%; substantial heterogeneity when it was between 50% and 90%; and considerable heterogeneity when it was between 75% and 100%. We noted the importance of I2 depending on: 1) magnitude and direction of effects; and 2) strength of evidence for heterogeneity. We did not have sufficient studies to investigate statistical heterogeneity (Deeks 2017).
We assessed clinical and methodological diversity in terms of participants, interventions, outcomes, effect modifiers, and study characteristics for the included studies to determine whether a meta‐analysis was appropriate; we used the information collected during data extraction (Data extraction and management).
We visually inspected forest plots to look at the consistency of intervention effects across included studies. If the studies were estimating the same intervention effect, there should be overlap between the CIs for each effect estimate on the forest plot, but if overlap is poor, or there are outliers, then statistical heterogeneity may be likely.
Assessment of reporting biases
We planned to investigate the potential for publication bias and explore possible small‐study biases using funnel plots. However, we had insufficient studies (fewer than 10 studies) for most outcomes (Sterne 2017). For outcomes with 10 or more studies, we constructed a funnel plot and interpreted the plot using a visual inspection and the Harbord modified test in Stata; for the critical review outcomes, we reported P values for the Harbord modified test. We incorporated this judgement into the assessment of publication bias within the GRADE assessment.
To assess outcome reporting bias, we screened clinical trials registers for protocols and registration documents of included studies that were prospectively published, and we sourced all clinical trials register documents that were reported in the study reports of included studies. We used evidence of prospective registration to judge whether studies were at risk of selective reporting bias.
Data synthesis
We conducted meta‐analyses only when meaningful; that is, when the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. We pooled results of comparable groups of trials using random‐effects models. We chose this model after careful consideration of the extent to which any underlying effect could truly be thought to be fixed, given the complexity of the interventions included in this review. We presented 95% CIs throughout. We found that some studies reported outcome data at more than one time point and we reported the data within three time point windows for these studies. Early data included data up to four months, with priority given to data closest to four months; 12‐month data included a window from later than four months up to 24 months, but with priority given to data at 12 months; and late data, which included data reported after 24 months at the latest time point reported by study authors. For studies that reported outcome data using more than one measurement tool, we selected the tool that was used most commonly by other studies in the comparison group, or which reported data for the largest number of participants.
We considered the appropriateness or otherwise of pooling data where there was considerable heterogeneity (I2 statistic value of greater than 75%) that could not be explained by the diversity of methodological or clinical features amongst trials. We presented data from these studies in the analyses and clearly reported these observations in the text for the critical outcomes in the review.
If effect sizes were statistically significant, we considered whether the effect was clinically important. We based these decisions on established minimal clinically important differences (MCIDs) for the measurement tool, or used Cohen's effect sizes as a guide if MCIDs were unavailable (Schünemann 2019a).
Subgroup analysis and investigation of heterogeneity
Few outcomes provided evidence from at least 10 studies to justify subgroup analysis. Although we aimed to explore possible sources of heterogeneity between studies (key effect modifiers such as age, gender, cognitive impairment, and fracture displacement and location), these possible effect modifiers were insufficiently reported to allow for meaningful subgroup analysis.
We planned to subgroup prostheses according to whether a modern or first‐generation uncemented stem was used (see Types of interventions), and we reported the test for subgroup differences in outcomes that had at least 10 studies.
There is no explicit means of accounting for step changes in co‐interventions, certainly not one that would be applicable to the worldwide totality of the evidence. Therefore, we could not try to explain any heterogeneity by statistical test of subgroups defined by co‐intervention. However, we ordered forest plots by date of recruitment so that any temporal trend could be inspected visually and commented on.
Sensitivity analysis
We used sensitivity analysis to explore the effects of risks of bias on the review's critical outcomes. If pooled analyses had at least two studies, we excluded studies that were:
at high or unclear risk of selection bias for sequence generation (this included studies described as quasi‐randomised, or those that did not adequately describe methods used to randomise participants to intervention groups); or
at high risk of attrition bias (because studies reported a large number of losses that were unexplained or not justified for this population, or losses that were unbalanced between groups, and that we expected could influence outcome data).
We compared the effect estimates in the sensitivity analysis with the effect estimates in the primary analysis, and we reported the effect estimates from sensitivity analyses only if we noted a difference in our interpretation of the effect. We planned to conduct sensitivity analysis by excluding studies that had mixed populations, but these data were inadequately reported by study authors and did not allow for meaningful analysis. We also planned, but did not conduct, sensitivity analysis by excluding studies of interventions that are not currently in clinical use. We obtained the general view that all interventions at the major‐grouping level (implant sub‐category level in Table 5) remain in current use. Although some types of implant may no longer be manufactured, we believe the distinction between implants within the same category is marginal and that sensitivity analysis would not be meaningful.
Summary of findings and assessment of the certainty of the evidence
Two review authors used the GRADE system to assess the certainty of the body of evidence associated with the seven critical outcomes in the review (Schünemann 2019b):
activities of daily living (ADL);
delirium;
functional status;
health‐related quality of life (HRQoL);
mobility;
mortality (measured within four months of surgery, and at 12 months);
unplanned return to theatre.
For outcomes that were reported using more than one measurement tool, and that could not be combined in analysis, we assessed the certainty of the evidence for the outcome that used a measurement tool with the most participants.
The GRADE approach assesses the certainty of a body of evidence based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias (study limitations), directness of the evidence (indirectness), heterogeneity of the data (inconsistency), precision of the effect estimates (imprecision), and risk of publication bias. The certainty of the evidence could be high, moderate, low or very low, being downgraded by one or two levels depending on the presence and extent of concerns in each of the five GRADE domains. We used footnotes to describe reasons for downgrading the certainty of the evidence for each outcome, and we used these judgements when drawing conclusions in the review.
We did not construct summary of findings tables for all comparisons in this review. Instead, we selected three comparisons that provided the most substantial body of evidence. These provided evidence for each of our comparison types in our review objectives (different fixation techniques, different articulations, and different designs). We therefore constructed summary of findings tables for the following comparisons in this review, using the GRADE profiler software (GRADEpro GDT).
Cemented HA versus uncemented HA.
Bipolar HA versus unipolar HA.
THA versus HA.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
Results of the search
After the removal of duplicates from the search results, we screened 28,509 titles and abstracts, which included backward citation searches and searches of clinical trials registers. We reviewed the full texts of 1135 records and selected 58 studies (with 101 records) for inclusion in this review. We linked any references pertaining to the same study under a single study ID. We excluded 1023 records, and report the details of eight key studies from these excluded records. Four studies are awaiting classification, and we identified seven ongoing studies. See Figure 1.
1.
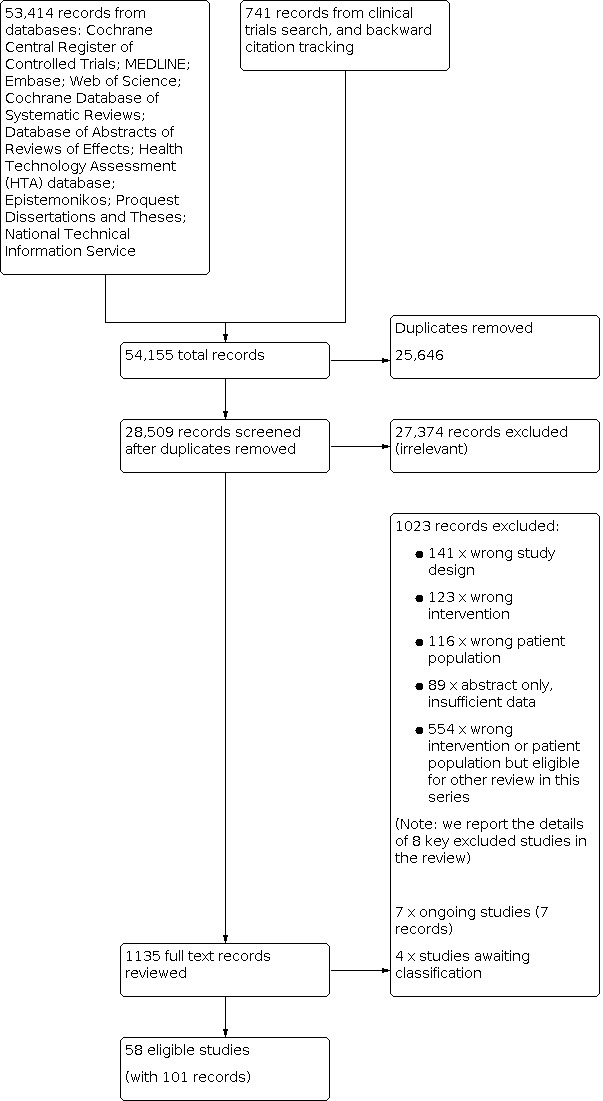
PRISMA flow diagram
Included studies
See Characteristics of included studies. Two studies were reported only as abstracts with limited study characteristics (Moroni 2002; Patel 2008).
Types of studies and setting
Whilst most studies were RCTs, eight studies used methods to allocate participants to interventions which we assessed as quasi‐randomised (Abdelkhalek 2011; Dorr 1986; Iorio 2019; Livesley 1993; Ravikumar 2000; Santini 2005; Sonaje 2017; Stoffel 2013).
Eleven were multicentre studies, and the remainder were single centre studies. Eighteen studies were completed in the UK (Baker 2006; Brandfoot 2000; Calder 1995; Calder 1996; Davison 2001; Emery 1991; Fernandez 2022; Griffin 2016; Harper 1994; Keating 2006; Livesley 1993; Parker 2010c; Parker 2012; Parker 2019; Parker 2020; Ravikumar 2000; Sadr 1977; Sims 2018), six in Sweden (Blomfeldt 2007; Chammout 2017; Chammout 2019; Cornell 1998; Hedbeck 2011; Inngul 2015), four in South Asia (Malhotra 1995; Rehman 2014; Sharma 2016; Sonaje 2017), four in Italy (Cadossi 2013; Iorio 2019; Moroni 2002; Santini 2005), four in the USA (DeAngelis 2012; Dorr 1986; Macaulay 2008; Raia 2003), three in Norway (Figved 2009; Figved 2018; Talsnes 2013), three in China (Cao 2017; Ren 2017; Xu 2017), three in Australasia (Jeffcote 2010; Stoffel 2013; Taylor 2012), two in the Netherlands (Moerman 2017; Van den Bekerom 2010), two in Egypt (Abdelkhalek 2011; Rashed 2020), and two in South Korea (Kim 2012; Lim 2020). HEALTH 2019 was an international study conducted in Canada, Finland, the Netherlands, New Zealand, Norway, South Africa, Spain, the UK, and the USA. The remainder were conducted in other European countries (Kanto 2014; Mouzopoulos 2008; Movrin 2020; Sonne‐Holm 1982; Vidovic 2013), and one study did not report where the study was conducted (Patel 2008).
Studies were published between 1977 and 2020, and we include one study that we expect to be published in 2021. Approximately two‐thirds of the studies were published since 2010.
Types of participants
In total, 10,654 participants, with 10,662 hip fractures, were recruited across the 58 studies. All studies included only participants with intracapsular fractures, except for Cao 2017 (85 participants), which included only participants with extracapsular fractures. Blomfeldt 2007 is the only study to report the inclusion of undisplaced fractures, which was only 2% of the reported study population. Nine studies did not report whether the fracture was displaced (Cadossi 2013; Cao 2017; Dorr 1986; Malhotra 1995; Moroni 2002; Patel 2008; Santini 2005; Sonne‐Holm 1982; Xu 2017), and the remainder included displaced fractures only. One study recruited participants that had neglected fractures, more than 30 days old (Xu 2017).
Most studies specified a lower age limit for recruited participants of at least 50 years (HEALTH 2019; Macaulay 2008), 55 years (DeAngelis 2012; Dorr 1986; Rashed 2020), 60 years (Baker 2006; Fernandez 2022; Griffin 2016; Iorio 2019; Jeffcote 2010; Parker 2010c; Parker 2019; Rehman 2014; Sharma 2016; Sims 2018; Sonaje 2017; Xu 2017), 65 years (Cao 2017; Chammout 2017; Cornell 1998; Davison 2001; Kanto 2014; Lim 2020; Raia 2003; Ravikumar 2000; Santini 2005), 70 years (Blomfeldt 2007; Cadossi 2013; Figved 2009; Figved 2018; Moerman 2017; Patel 2008; Sonne‐Holm 1982; Taylor 2012; Van den Bekerom 2010; Vidovic 2013), 75 years (Moroni 2002; Movrin 2020; Talsnes 2013), and 80 years (Calder 1996; Chammout 2019; Hedbeck 2011; Inngul 2015). Only five studies applied an upper age limit, which was 79 years (Calder 1995; Chammout 2017; Davison 2001), 80 years (Rashed 2020), and 90 years (Blomfeldt 2007). Where reported, the mean ages of participants ranged from 63 years to 87 years.
Seven studies did not report the baseline sex of the participants (Griffin 2016; Livesley 1993; Patel 2008; Ravikumar 2000; Sonaje 2017; Sonne‐Holm 1982; Stoffel 2013). In studies that reported sex distribution, there were 6835 female participants, which represented 71% of the sample in these studies. Approximately one third of the studies specified the ability to walk prior to surgery as an inclusion criteria or required participants to be free of any cognitive impairment. Almost half of the studies stated that pathological fractures would not be included.
The mean follow‐up time period was 24.3 (SD ± 109) months, with a range from 1 week (Malhotra 1995), to 13 years (Ravikumar 2000).
Types of interventions
We included 21 studies with 4282 participants that compared prostheses that were cemented or uncemented; as part of treatment with a THA (Chammout 2017), a HA (Brandfoot 2000; Cao 2017; DeAngelis 2012; Emery 1991; Fernandez 2022; Figved 2009; Harper 1994; Moerman 2017; Movrin 2020; Parker 2010c; Parker 2020; Rehman 2014; Sadr 1977; Santini 2005; Sonne‐Holm 1982; Talsnes 2013; Taylor 2012; Vidovic 2013), or a mixture of either a THA or HA (Inngul 2015; Moroni 2002). We briefly summarise the characteristics of these studies and the critical review outcomes they report that are relevant to this review in Table 6.
3. Implant and study characteristics. Prostheses implanted with cement versus without cement.
| Study ID |
Type of cemented implant Type of uncemented implant |
Study design (N) | Displaced fractures, % | Critical review outcomes (time point, n) |
| Brandfoot 2000 | 1. Cemented, Thompson, unipolar 2. Uncemented Thompson, unipolar |
RCT (91) | 98 | Mortality (16 months, 91) |
| Cao 2017 | 1. Cemented, stem type and uni/bipolar NR 2. Uncemented, stem type and uni/bipolar NR |
RCT (85) | NR | Function (3 and 6 months, 85) |
| Chammout 2017 | 1. Cemented, modular CPT, 32 mm head, cemented cup 2. Uncemented, Bi‐Metric stem, 32 mm head, cemented cup |
RCT (69) | 100 | ADL (3 months, 65; 24 months, 59) Function (24 months, 65) HRQoL (3 months, 64; 12 months, 62) Mortality (12 months, 69) Unplanned return to theatre (24 months, 69) |
| DeAngelis 2012 | 1. Cemented, VerSys stem, unipolar 2. Uncemented, beaded stem, unipolar |
RCT (130) | 100 | Unplanned return to theatre (12 months, 130) |
| Emery 1991 | 1. Cemented, Thompson, bipolar 2. Uncemented, Moore, bipolar |
RCT (53) | 100 | Mobility (3 months, 39) Mortality (3 and 17/18 months, 53) |
| Figved 2009 | 1. Cemented, Spectron, bipolar 2. Uncemented, Corail, bipolar |
RCT (230 fractures, 223 participants) | 100 | ADL (3 months, 190; 12 months, 168) Function (3 months, 189; 12 months, 167) HRQoL (3 months, 143; 12 months, 113) Mobility (3 months, 190; 12 months, 168) Mortality (3 and 12 months, 213) Unplanned return to theatre (12 months, 217) |
| Harper 1994 | 1. Cemented, Thompson, unipolar 2. Uncemented, Thompson, unipolar |
RCT (137) | 100 | Mortality (3 and 12 months, 137) |
| Inngul 2015 | 1. Cemented, Exeter stem, unipolar or 32mm, cemented cross‐linked polyethylene cup 2. Uncemented, HAC Bimetric stem, unipolar or 32 mm, cemented cross‐linked polyethylene cup |
RCT (141) | 100 | Mortality (4 and 12 months, 141) Unplanned return to theatre (12 months, 141) |
| Moerman 2017 | 1. Cemented, Muller, bi/unipolar NR 2. Uncemented, DB10, bi/unipolar NR |
RCT (201) | 100 | ADL (3 months, 114; 12 months, 96) HRQoL (3 months, 102; 12 months, 90) Mobility (3 months, 88; 12 months, 74) Mortality (12 months, 201) Unplanned return to theatre (12 months, 201) |
| Moroni 2002 | 1. Cemented, AHS prosthesis, unipolar or THA 2. Uncemented (HAC), Furlong, unipolar or THA |
RCT (28) | NR | Function (24 months, 28) HRQoL (24 months, 28) Mortality (24 months, 28) |
| Movrin 2020 | 1. Cemented, Muller, bi/unipolar NR 2. Uncemented, DB10, bi/unipolar NR |
RCT (158) | 100 | Function (3 month, 148; 24 months, 94) Mortality (7 days and 24 months, 158) |
| Parker 2010c | 1. Cemented, Thompson, unipolar 2. Uncemented, Moore, unipolar |
RCT (400) | 100 | Delirium (60 months, 400) Mobility (3 months, 327; 60 months, 64) Mortality (12 and 60 months, 400) Unplanned return to theatre (60 months, 400) |
| Parker 2020 | 1. Cemented, Exeter Trauma or CPT, unipolar 2. Uncemented, Furlong, unipolar |
RCT (400) | 100 | ADL (4 months, 329; 12 months 283) Delirium (12 months, 400) Mobility (3 months, 329; 12 months, 282) Mortality (3 and 12 months, 400) |
| Rehman 2014 | 1. Cemented, Thompson, unipolar 2. Uncemented, Moore, unipolar |
RCT (110) | 100 | Mobility (3 months, 110) |
| Sadr 1977 | 1. Cemented, Thompson, unipolar 2. Uncemented, Thompson, unipolar |
RCT (40) | 100 | Function (17 months, 25) Mortality (6 weeks and 12 months, 40) |
| Santini 2005 | 1. Cemented, stem type NR, unipolar 2. Uncemented, stem type NR, unipolar |
RCT (106) | NR | ADL (12 months, 106) Function (12 months, 106) Mobility (unknown time point, 106) Mortality (at hospital discharge and 12 months, 106) |
| Sonne‐Holm 1982 | 1. Cemented, Moore, unipolar 2. Uncemented, Moore, unipolar |
RCT (112) | NR | Function (3 and 12 months, 75) Mobility (3 and 12 months, 75) Mortality (6 weeks, 112) |
| Talsnes 2013 | 1. Cemented, Landos Titan, bipolar 2. Uncemented, Landos Corail, bipolar |
RCT (334) | 100 | Mortality (12 months, 334) |
| Taylor 2012 | 1. Cemented, Exeter, unipolar 2. Uncemented, Zweymuller Alloclassic, unipolar |
RCT (160) | 100 | Mortality (6 weeks and 12 months, 160) Unplanned return to theatre (24 months, 160) |
| Vidovic 2013 | 1. Cemented, modular, unipolar 2. Uncemented, Moore, unipolar |
RCT (79) | 100 | Function (3 months, 79; 12 months, 60) Mortality (12 months, 79) |
| Fernandez 2022 | 1.Cemented HA, stem and head at surgeon's preference 2.Uncemented HA, stem and head at surgeon's preference |
RCT (1225) | 99 | ADL (4 months, 715; 12 months, 580) HRQoL (4 months, 877; 12 months, 876) Mobility (4 months, 715; 12 months, 583) HRQoL (4 months, 877; 12 months, 876) Unplanned return to theatre (12 months, 1225) Mortality (12 months, 1225) |
ADL: activities of daily living AHS: manufacturer's name for implant CPT: collarless, polished, double‐taper design concept DB: manufacturer's name for implant HAC: hydroxyapatite‐coated HRQoL: health‐related quality of life N: total number randomised n: number analysed NR: not reported RCT: randomised controlled trial
We included 13 studies with 1499 participants that compared a bipolar HA with a unipolar HA (Abdelkhalek 2011; Calder 1995; Calder 1996; Cornell 1998; Davison 2001; Figved 2018; Hedbeck 2011; Jeffcote 2010; Kanto 2014; Malhotra 1995; Patel 2008; Raia 2003; Stoffel 2013). We briefly summarise the characteristics of these studies and the outcomes they report that are relevant to this review in Table 7.
4. Implant and study characteristics. Bipolar HA versus unipolar HA.
| Study ID |
Type of HA bipolar Type of HA unipolar |
Study design (N) | Displaced fractures, % | Critical review outcomes (time point, n) |
| Abdelkhalek 2011 | 1. Mixed cemented/uncemented, bipolar; 2. Mixed cemented/uncemented, unipolar |
Quasi RCT (50) | 100 | Function (4.4 years, 50) Unplanned return to theatre (24 months, 50) |
| Calder 1995 | 1. Monk, cemented, bipolar 2. Thompson, cemented, unipolar | RCT (73) | 100 | Pain (6 months, 73) Mobility (6 months, 73) |
| Calder 1996 | 1. Monk, cemented, bipolar 2. Thompson, cemented, unipolar |
RCT (250) | 100 | Mortality (4 and 12 months, 250) |
| Cornell 1998 | 1. Cemented modular, bipolar 2. Cemented modular, unipolar |
RCT (48) | 100 | Function (6 months, 48) Mobility (6 months, 48) Mortality (6 months, 48) |
| Davison 2001 | 1. Cemented, Monk, bipolar 2. Cemented, Thompson, unipolar |
RCT (187) | 100 | Mortality (12 and 36 months, 187) Unplanned return to theatre (36 months, 187) |
| Figved 2018 | 1. Cemented, 28 mm cobalt chromium head and a SelfCentering Bipolar (DePuy) 2. Cemented, Modular Cathcart Unipolar (DePuy) |
RCT (28) | 100 | Function (48 months, 19) HRQoL (12 months, 25; 48 months, 19) Mortality (3 and 12 months, 28) |
| Hedbeck 2011 | 1. Cemented, UHR (Stryker), from 42 to 72 mm, bipolar 2. Cemented, Exeter modular, unipolar |
RCT (120) | 100 | ADL (12 months, 99) HRQoL (4 months, 115; 12 months, 99) Mortality (4 and 12 months, 120) Unplanned return to theatre (12 months, 120) |
| Jeffcote 2010 | 1. Cemented, Centrax, bipolar 2. Cemented, Unitrax, unipolar |
RCT (51) | 100 | Mortality (24 months, 51) |
| Kanto 2014 | 1. Cemented, Vario cup, bipolar 2. Cemented, Lubinus, unipolar |
RCT (175) | 100 | Mortality (during hospital stay and 5 years, 175) Unplanned return to theatre (5 years, 175) |
| Malhotra 1995 | 1. Uncemented, Bateman type, bipolar 2. Uncemented; Austin‐Moore; unipolar |
RCT (68) | NR | Function (NR, 66) |
| Patel 2008 | 1. Uncemented, medical internation stem, bipolar 2: Uncemented, Thompson; unipolar |
RCT (40) | 100 | Mortality (13 months, 40) |
| Raia 2003 | 1. Centrax, appropriate‐sized cemented Premise stem, bipolar 2. Unitrax; appropriate‐sized cemented Premise stem, unipolar |
RCT (115) | 100 | Mortality (12 months, 115) |
| Stoffel 2013 | 1. Cemented, collarless polished stem, bipolar 2. Cemented, collarless polished stem, unipolar |
RCT (294) | 100 | Delirium (12 months, 261) Function (12 months, 251) Mobility (12 months, 186) |
ADL: activities of daily living HA: hemiarthroplasty HRQoL: health‐related quality life N: total number randomised n: number analysed NR: not reported RCT: randomised controlled trial UHR: universal head system (manufacturer's name)
We included four studies with 1397 participants that compared different types of HAs. These comparisons were between a short stem and standard stem (Lim 2020), a Thompson and a Exeter Trauma Stem (Parker 2012; Sims 2018), and an Austin‐Moore and a Furlong (Livesley 1993). We briefly summarise the characteristics of these studies and the outcomes they report that are relevant to this review in Table 8.
5. Implant and study characteristics. HAs versus other HAs.
| Study ID | Type of HA in each intervention group | Study design (N) | Displaced fractures, % | Critical review outcomes (time point, n) |
| Lim 2020 | 1. Short stem, Bencox M stem, proximal Ti‐plasma spray microporous coating, uncemented, bipolar 2. Standard stem, Bencox ID stem, proximal Ti‐plasma spray microporous coating, uncemented, standard stem, bipolar |
RCT (151) | 100 | ADL (24 months, 75) Mortality (24 months, 151) |
| Livesley 1993 | 1. HAC bipolar 2. Uncemented; press‐fit Moore‐bipolar |
Quasi‐RCT (82) | 100 | Mortality (1 and 12 months, 82) Unplanned return to theatre (12 months, 82) |
| Parker 2012 | 1. Uncemented, Exeter, unipolar 2. Cemented, Thompson, unipolar | RCT (200) | 100 | Delirium (12 months, 200) Mortality (3 and 12 months, 200) Unplanned return to theatre (12 months, 200) |
| Sims 2018 | 1. Uncemented, Exeter, unipolar 2. Cemented, Thompson, unipolar |
RCT (964) | 100 | HRQoL (4 months, 618) Mobility (4 months, 494) Mortality (4 months, 964) Unplanned return to theatre (12 months, 964) |
ADL: activities of daily living HA: hemiarthroplasty HAC: hydroxyapatite‐coated HRQoL: health‐related quality of life N: total number randomised n: number analysed RCT: randomised controlled trial
We included 17 studies with 3232 participants that compared a THA with a HA (Baker 2006; Blomfeldt 2007; Cadossi 2013; Chammout 2019; Dorr 1986; HEALTH 2019; Iorio 2019; Keating 2006; Macaulay 2008; Mouzopoulos 2008; Parker 2019; Ravikumar 2000; Ren 2017; Sharma 2016; Sonaje 2017; Van den Bekerom 2010; Xu 2017). We briefly summarise the characteristics of these studies and the outcomes they report that are relevant to this review in Table 9.
6. Implant and study characteristics. THA versus HA.
| Study ID |
Type of THA Type of HA |
Study design (N) | Displaced fractures, % | Critical review outcomes (time point, n) |
| Baker 2006 | 1. 28 mm femoral head articulating with an all‐polyethylene Zimmer cemented acetabular cup 2. Endo Femoral Head (Zimmer); cemented; unipolar |
RCT (81) | 100 | Mortality (39 months, 81) |
| Blomfeldt 2007 | 1. Modular Exeter femoral component; 28 mm head; OGEE cemented acetabular component 2. Bipolar; modular Exeter, 28 mm head |
RCT (120) | 100 | ADL (4 months, 114; 12 months, 111) Delirium (4 months, 116) Function (48 months, 83) Mortality (4, 12 and 48 months, 120) |
| Cadossi 2013 | 1. Uncemented Conus stem and a large‐diameter femoral head 2. Uncemented, bipolar |
RCT (96) | 100 | Mortality (12 and 36 months, 96) |
| Chammout 2019 | 1. Cemented 32 mm cobalt‐chromium head; cemented highly cross‐linked polyethylene acetabular component 2. Cemented, unipolar |
RCT (120) | 100 | ADL (3 months, 111; 24 months, 99) Delirium (3 months, 111) Function (24 months, 103) HRQoL (3 months, 111; 12 months, 106) Mortality (24 months, 120) Unplanned return to theatre (24 months, 120) |
| Dorr 1986 | 1. 28 mm head size was used 2. Cemented (n = 37) or uncemented (n = 13), bipolar |
RCT (89) | 100 | Unplanned return to theatre (48 months, 89) |
| HEALTH 2019 | 1. Surgeon's preference 2. Surgeon's preference |
RCT (1495) | 100 | Function (24 months, 669) HRQoL (24 months, 844) Mobility (24 months, 535) Mortality (24 months, 1441) Unplanned return to theatre (24 months, 1441) |
| Iorio 2019 | 1. Dual mobility cup with cementless femoral stem 2. Cementless femoral stem with bipolar head |
RCT (60) | 100 | Mortality (1 and 12 months, 60) Unplanned return to theatre (12 months, 60) |
| Keating 2006 | 1. NR 2. Bipolar, cemented |
RCT (180) | 100 | Delirium (24 months, 168) Function (24 months, 168) HRQoL (4 and 12 months, 168) Mortality (24 months, 180) Unplanned return to theatre (12 months, 180) |
| Macaulay 2008 | 1. Surgeon's preference 2. Surgeon's preference |
RCT (41) | 100 | Function (24 months, 40) HRQoL (12 months, 40) Mobility (12 and 24 months, 40) Mortality (24 months, 40) |
| Mouzopoulos 2008 | 1. Plus (DePuy) 2. Merete |
RCT (86) | 100 | ADL (48 months, 43) Function (48 months, 43) Mortality (12 and 48 months, 86) Unplanned return to theatre (48 months, 49) |
| Parker 2019 | 1. CPCS stem (n=29), CPT Zimmer (n=23) 2. Monoblock Exeter Trauma Stem (n=22), CPT bipolar (n=4), CPT modular (n=27) |
RCT (105) | 100 | ADL (12 months, 78) Delirium (12 months, 105) Mobility (12 months, 78) Mortality (4 and 12 months, 105) Unplanned return to theatre (12 months, 105) |
| Ravikumar 2000 | 1. Cemented with Howse II 2. Uncemented Austin‐Moore |
RCT (180) | 100 | Mobility (13 years, 32) Mortality (4 and 12 months and 13 years, 180) Unplanned return to theatre (12 months, 180) |
| Ren 2017 | 1. Surgeon's preference 2. Cemented |
RCT (100) | NR | Function (NR, 100) |
| Sharma 2016 | 1. NR 2. NR |
RCT (80) | 100 | Mortality (1 week, 80) |
| Sonaje 2017 | 1. NR 2. NR |
Quasi‐RCT (42) | 100 | Function (24 months, 40) |
| Van den Bekerom 2010 | 1. Cemented; 32 mm diameter modular head 2. Cemented, bipolar |
RCT (281) | 100 | Mortality (12 and 60 months, 252) Unplanned return to theatre (60 months, 252) |
| Xu 2017 | 1. Uncemented prosthesis 2. Bipolar; uncemented |
RCT (76) | NR | Function (60 months, 76) Mortality (60 months, 76) |
ADL: activity of daily living CPCS: collarless, polished, cemented stem CPT: collarless, polished, double‐taper design concept HA: hemiarthroplasty N: total number randomised n: number analysed NR: not reported OGEE: manufacturer's name for implant RCT: randomised controlled trial THA: total hemiarthroplasty
We included three studies with 244 participants that compared different types of THAs. These comparisons were between a single articulation and a dual‐mobility articulation (Griffin 2016; Rashed 2020), and a short stem and standard stem (Kim 2012). We briefly summarise the characteristics of these studies and the outcomes they report that are relevant to this review in Table 10.
7. Implant and study characteristics. THAs versus other THAs.
| Study ID | Type of THA | Study design (N) | Displaced fractures % | Critical review outcomes (time point, n) |
| Griffin 2016 | 1. Single articulation: surgeon's preference 2. Dual mobility: surgeon's preference for prosthesis, Novae DM acetabular component; uncemented |
RCT (21) | 100 | Function (12 months, 19) HRQoL (12 months, 19) Mortality (12 months, 21) |
| Rashed 2020 | 1. Single articulation: cemented 32 mm head 2. Dual mobility: cemented dual‐mobility cup (Ecofit 2M) |
RCT (108) | 100 | Function (12 months, 60) Mortality (12 months, 62) |
| Kim 2012 | 1. Short stem: short, anatomical metaphyseal‐fitting cementless femoral component, 36 mm modular head, cementless acetabular component 2. Standard stem: anatomical medullary locking fully porous coated cementless femoral component, 36 mm Biolox delta ceramic modular head |
RCT (161) | 100 | Function (24 months, 140) Mobility (24 months, 142) Mortality (12 months, 162) |
DM: dual‐mobility HRQoL: health‐related quality of life N: total number randomised n: number analysed RCT: randomised controlled trial THA: total hip arthroplasty
We found no studies of large‐head THAs compared with other arthroplasties.
Types of outcome measures
All studies in our main comparison groups reported data for at least one of our critical review outcomes.
Sources of funding and declarations of interest
Fourteen studies reported that they received no commercial or external funding (Baker 2006; Cadossi 2013; Calder 1996; Davison 2001; Emery 1991; Inngul 2015; Livesley 1993; Parker 2010c; Parker 2012; Parker 2019; Parker 2020; Santini 2005; Sonaje 2017; Van den Bekerom 2010), and six studies reported funding from independent sources such as research foundations (Chammout 2019; Fernandez 2022; Griffin 2016; HEALTH 2019; Keating 2006; Macaulay 2008). Eleven studies received support from manufacturers or insurance companies (Blomfeldt 2007; DeAngelis 2012; Dorr 1986; Figved 2009; Figved 2018; Hedbeck 2011; Raia 2003; Ravikumar 2000; Sims 2018; Talsnes 2013; Taylor 2012). Eight studies declared that the study investigators had no conflicts of interest (Cao 2017; Chammout 2017; Iorio 2019; Lim 2020; Movrin 2020; Rashed 2020; Vidovic 2013; Xu 2017). The remaining studies reported no information about their funding sources nor provided declarations about conflicts of interest.
Excluded studies
Because the searches in this review were designed to feed into a series of related Cochrane Reviews about the surgical management of hip fracture, we have not included a bibliographic list of all excluded studies. We excluded most studies because they were study designs that were not eligible for inclusion in this review, or were not treating participants with the type of fractures or with the types of interventions that were eligible for this review. Some of the excluded studies were eligible for inclusion in the related Cochrane Reviews.
Here, we report the details of eight key excluded studies (see Characteristics of excluded studies). We excluded five studies because they were abstracts with insufficient detail on the numbers of participants in each group, meaning extraction of outcome data was not feasible (Karpman 1992; Kavcic 2006; Rosen 1992; Stock 1997; Van Thiel 1988). We excluded one study that appeared to be randomised, but on closer inspection, we believed was not randomised (Somashekar 2013). We excluded one study that investigated the surgical approach rather than the type of intervention (Aydin 2009). We excluded one study from our clinical trials register search which was abandoned because of lack of funding, and its results are not reported (ISRCTN42349821).
Studies awaiting classification
We found four studies from the search of clinical trials registries that were registered as completed but do not have a published study report in the literature (NCT00800124; NCT00859378; NCT01432691; NTR1782). These studies potentially recruited 1204 participants and investigated the following comparison groups: cemented HA versus uncemented HA (NCT00800124; NCT00859378; NTR1782), and THA versus HA (NCT01432691). See Characteristics of studies awaiting classification.
Ongoing studies
We found seven ongoing studies (ChiCTR1800019531; ISRCTN15606075; NCT01109862; NCT01578408; NCT01787929; UMIN000011303; Wolf 2020). These studies have an estimated enrolment of 7199 participants, and evaluate the following comparison groups: THA versus HA (ChiCTR1800019531; NCT01109862; UMIN000011303), cemented HA versus uncemented HA (NCT01787929), cemented THA versus uncemented THA (NCT01578408), dual mobility THA versus standard THA (Wolf 2020), and single versus dual antibiotic cement HA (ISRCTN15606075). See Characteristics of ongoing studies.
Risk of bias in included studies
We conducted risk of bias according to outcomes relevant to this review. Blank spaces in the risk of bias figure for some detection bias domains indicate that risk of bias assessment was not applicable as the outcome category was not reported. See Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Blank spaces indicate that risk of bias was not conducted because study authors did not report outcomes relevant to these domains.
Allocation
Twenty studies reported an adequate method to randomise participants to groups, and we judged these studies be at low risk of selection bias for random sequence generation (Calder 1996; Cao 2017; Chammout 2017; Chammout 2019; Davison 2001; Fernandez 2022; Figved 2009; Figved 2018; Griffin 2016; HEALTH 2019; Kim 2012; Lim 2020; Macaulay 2008; Parker 2020; Raia 2003; Rashed 2020; Sims 2018; Taylor 2012; Van den Bekerom 2010; Xu 2017). We judged 11 studies to be at high risk of selection bias for random sequence generation because study authors used quasi‐randomised methods to randomise participants to intervention groups or because other information within the study report indicated that selection bias may have been present (Abdelkhalek 2011; Cornell 1998; Dorr 1986; Iorio 2019; Keating 2006; Livesley 1993; Mouzopoulos 2008; Ravikumar 2000; Santini 2005; Sonaje 2017; Stoffel 2013). The remaining studies reported insufficient information and we judged risk of selection bias for random sequence generation to be unclear.
We judged 11 studies to be at high risk of bias for allocation concealment (Abdelkhalek 2011; Dorr 1986; Iorio 2019; Keating 2006; Livesley 1993; Mouzopoulos 2008; Ravikumar 2000; Santini 2005; Sharma 2016; Sonaje 2017; Stoffel 2013). Twenty‐four studies reported insufficient information and we judged risk of selection bias for allocation to be unclear (Baker 2006; Blomfeldt 2007; Brandfoot 2000; Cadossi 2013; Calder 1995; Calder 1996; Cao 2017; Chammout 2017; Chammout 2019; Cornell 1998; Davison 2001; DeAngelis 2012; Emery 1991; Harper 1994; Jeffcote 2010; Malhotra 1995; Moroni 2002; Patel 2008; Raia 2003; Ren 2017; Sadr 1977; Sonne‐Holm 1982; Talsnes 2013; Vidovic 2013). The remaining studies reported sufficient information, and we judged these to be at low risk of selection bias.
Blinding
It is not possible to blind the operating surgeon to the type of arthroplasty or the implant fixation methods used in these studies. In making judgements about performance bias, we considered whether surgeons were equally experienced with the types of implants used in their study. We judged only 19 studies to report this sufficiently well and we assessed these studies to be at low risk of performance bias (Baker 2006; Blomfeldt 2007; Chammout 2017; Hedbeck 2011; Inngul 2015; Jeffcote 2010; Keating 2006; Kim 2012; Livesley 1993; Moerman 2017; Movrin 2020; Parker 2010c; Parker 2012; Parker 2019; Parker 2020; Ren 2017; Santini 2005; Sharma 2016; Taylor 2012). We expected that all surgeons would aim for the same standard of performance when carrying out all surgical procedures. However, unless otherwise stated, we could not be certain in the remaining studies that surgeons were equally experienced with the prostheses and we judged risk of performance bias in these studies to be unclear.
For detection bias, we considered the type of outcome and who was measuring it. We found that most studies did not report who measured clinically‐assessed outcomes that may be influenced by subjective decisions (such as performance in ADL, hip function or unplanned return to theatre). In these cases, we assumed that these judgements were made by surgeons who were aware of the intervention, which could influence their decision‐making. Thus, we judged detection bias for these clinically‐assessed subjective outcomes to be at high risk of bias. Only six studies reported that assessment of these outcomes was made by personnel who were unaware of treatment, and we judged these six studies to be at low risk of bias for these outcomes (Cornell 1998; Kim 2012; Parker 2012; Sims 2018; Sonne‐Holm 1982; Taylor 2012). Although some participants may have been aware of the type of intervention used during their surgery, we did not expect that knowledge of this would influence how they reported information that contributed towards outcome data such as mobility, pain, and HRQoL. We therefore judged risk of detection bias for all participant‐reported outcomes to be at low risk. We also judged detection bias to be at low risk for objective outcomes (such as mortality and length of stay), and we therefore judged all studies reporting these outcomes to be at low risk of detection bias.
Incomplete outcome data
Because of the high mortality in study population, we expected a large and unavoidable loss of outcome data from participants for outcomes measured with a longer follow‐up. We judged most studies to be at low risk of attrition bias because other losses were few, were well‐explained by study authors, and were balanced between groups. We judged only seven studies to be at high risk of attrition bias (Cadossi 2013; Calder 1995; Fernandez 2022; HEALTH 2019; Inngul 2015; Lim 2020; Moerman 2017). In these seven studies, we noted a large number of losses for outcomes reported at the end of follow‐up (such as HRQoL or functional status) that could not be explained by death. In four studies, we could not be certain if data were complete, particularly for outcomes reported at the end of study follow‐up (DeAngelis 2012; Dorr 1986; Malhotra 1995; Moroni 2002), and in two studies we could not be certain if the number of losses were balanced between intervention groups because of limited information in the study report (Stoffel 2013; Van den Bekerom 2010). We judged risk of attrition bias in these six studies to be unclear.
Selective reporting
Most studies did not report whether they were registered with a clinical trials register and did not provide details of protocols published prior to the study. Nine studies were registered retrospectively with a clinical trials register (Chammout 2017; Chammout 2019; DeAngelis 2012; Figved 2009; Figved 2018; Inngul 2015; Kanto 2014; Parker 2019; Parker 2020). Two reported registration with a clinical trials register, but because they did not report registration numbers, we were unable to source the trials register documents (Talsnes 2013; Taylor 2012). It is not feasible to effectively assess risk of selective reporting bias without these documents, and we judged risk of bias in all of these studies to be unclear.
Only five studies reported prospective clinical trials registration, with outcomes listed in these documents which were consistent with those measured and reported in the study report (Fernandez 2022; Griffin 2016; HEALTH 2019; Moerman 2017; Sims 2018). We judged these four studies to be at low risk of selective reporting bias.
Other potential sources of bias
Two studies were reported as abstracts, with limited study details, and we judged these to be at high risk of other bias because the reports were not peer‐reviewed (Moroni 2002; Patel 2008). We judged other bias to be unclear in another study in which the study methods had limited detail and we could not be certain of bias (Ren 2017). We identified no other sources of bias in the remaining studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Cemented versus uncemented hemiarthroplasty for hip fracture in adults.
| Cemented versus uncemented hemiarthroplasty for hip fracture in adults | ||||||
| Patient or population: adults with displaced and undisplaced hip fractures; included studies were for intracapsular fractures, except for one study of extracapsular fractures Setting: hospitals; included studies were conducted in China, Croatia, Denmark, Italy, New Zealand, Norway, Pakistan, Slovenia, Sweden, the UK and USA Intervention: HA fixed with cement (included studies which used unipolar or bipolar articulations) Comparison: HA fixed without cement (included studies which used unipolar or bipolar articulations. Designs of HA in 6 studies were first‐generation, and in 2 studies were unknown. We categorised them as first‐generation.) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with uncemented HA | Risk with cemented HA | |||||
|
Activities in daily living, early (within 4 months): using GARS (range from 18 to 72), a social dependency scale (range of scores 1 to 9); lower values in these scales indicate more independence. Also using OARS‐IADL (range from 0 to 14) and a 5‐point Likert scale derived from EQ‐5D; higher values in these scales indicate more independence Follow‐up: time points in the included studies were at 3 months and 4 months |
The mean GARS score in the uncemented group was 45.7. The mean social mobility scale score in the uncemented group was 4.6. The mean OARS‐IADL score in the uncemented group was 3.7. The mean Likert score in the uncemented group was 3.15. |
SMD 0.03 lower (0.21 lower to 0.16 higher) |
‐ | 1275 (4 studies) | ⊕⊕⊕⊝ moderatea | This effect did not indicate a clinically important difference, based on a 'rule of thumb' of: 0.2 for a small difference, 0.5 for a medium difference, and 0.8 for a large difference. |
|
Delirium (end of follow‐up) Follow‐up: time points in the included studies were at 12 months and 5 years |
Study population | RR 1.06 (0.55 to 2.06) | 800 (2 studies) | ⊕⊕⊝⊝ lowc | ||
| 40 per 1000b | 42 per 1000 (22 to 82) | |||||
|
Functional status, early (within 4 months): using HHS (range from 0 to 100); higher values indicate better function Follow‐up: time points in the included studies were at 6 weeks and 3 months |
The mean HHS scores in the uncemented groups ranged from 62.53 to 72.1. |
MD 3.38 higher (0.05 higher to 6.70 higher) |
‐ | 416 (3 studies) | ⊕⊝⊝⊝ very lowd | This effect did not indicate a clinically important improvement (based on a MCID of 15.9 to 18 points). In addition, data were available in 1 study with extracapsular fractures which showed improvement with cemented HAs (MD 14.70, 95% CI 11.78 to 17.62; 85 participants). We noted that the CI in this effect may indicate a clinically important improvement with cemented HAs in extracapsular fractures (based on a MCID of 15.9 to 18 points). |
|
HRQoL, early (within 4 months): using EQ‐5D (range 0 to 1), and SF‐12 (range 0 to 100); higher values indicate better quality of life. Follow‐up: time points in the included studies were at 3 months and 4 months |
The mean EQ‐5D score in the uncemented group ranged from 0.31 to0.58. The mean SF‐12 score in the uncemented group was 33.8. | SMD 0.20 higher (0.02 higher to 0.10 higher) | ‐ | 1122 (3 studies) | ⊕⊕⊕⊝ moderatea | The difference between fixation techniques was compatible with no effect or a clinically important benefit of cemented HAs based on a MCID for EQ‐5D of 0.07. |
|
Mobility, early (within 4 months): able to walk outdoors using no more than 1 walking aid. Follow‐up: time points in the included studies were at 3 months and 4 months |
Study population |
RR 1.04 (0.95 to 1.14) |
980 (3 studies) |
⊕⊕⊕⊝ moderatea | ||
| 354 per 1000b |
369 per 1000 (227 to 404) |
|||||
|
Mortality, early (within 4 months) Follow‐up: time points in the included studies were at hospital discharge, 7 days, 6 weeks, 3 months and 4 months |
Study population | RR 95 (0.80 to 1.13) | 3136 (12 studies) | ⊕⊕⊝⊝ lowe | ||
| 143 per 1000b | 136 per 1000 (114 to 162) | |||||
|
Mortality at 12 months Follow‐up: time points in the included studies were at 12 months, 16 months, 18 months, and 24 months |
Study population | RR 0.86 (0.78 to 0.96) | 3727 (15 studies) | ⊕⊕⊕⊝ moderatea | ||
| 283 per 1000b | 243 per 1000 (221 to 272) | |||||
|
Unplanned return to theatre (end of follow‐up)f Follow‐up: time points in the included studies were at 12 months, 2 years and 5 years |
Study population | RR 0.70 (0.45 to 1.10) | 2336 (6 studies) | ⊕⊕⊝⊝ lowg | ||
| 39 per 1000b | 27 per 1000 (17 to 43) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EQ‐5D: EuroQoL 5 Dimensions instrument; GARS: Groningen Activity Restriction Scale;HA: hemiarthroplasty; HRQoL: health‐related quality of life; HHS: Harris Hip Score; MCID: minimal clinically important difference; MD: mean difference; OARS‐IADL: Older Americans Resources Scale of Instrumental Activities of Daily Living; RR: risk ratio; SF‐12: Short‐form 12; SMD: standardised mean difference; THA: total hip arthroplasty | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for study limitations because included studies had some high/unclear risks of bias. bDerived from the pooled estimate of the uncemented HA group cWe downgraded by two levels: one level for imprecision because we noted a wide CI in the estimate, and one level for study limitations because the studies had unclear risks of bias. dWe downgraded by three levels: one level for imprecision because we noted a wide CI in the estimate, and two levels for study limitations because some studies had unclear risks of bias, and we found during sensitivity analyses that the estimate was influenced by these studies. eDowngraded by two levels: one level for imprecision because the CI included possible benefits and possible harms, and one level for study limitations because the studies had unclear risks of bias. fSome re‐operations were because of infection, acetabular wear, dislocation, periprosthetic fracture or loosening. We noted that types of re‐operation included replacement with THA, Girdlestone and drainage of infection. gWe downgraded by two levels for study limitations because some studies had unclear risks of bias and all studies were at high risk of detection bias.
Summary of findings 2. Bipolar hemiarthroplasty compared with unipolar hemiarthroplasty for hip fracture in adults.
| Bipolar hemiarthroplasty compared with unipolar hemiarthroplastyfor hip fracture in adults | ||||||
|
Patient or population: adults with displaced and undisplaced hip fractures Setting: hospitals; included studies were conducted in Australia, Egypt, Finland, India, Norway, Sweden, the UK and USA Intervention: bipolar HA. These were fixed with cement in 9 studies, without cement in 3 studies, and at the discretion of the surgeon in 1 study. Comparison: unipolar HA. These were fixed with cement in 9 studies, without cement in 3 studies, and at the discretion of the surgeon in 1 study. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with unipolar HA | Risk with Bipolar HA | |||||
| Activities of daily living, early (within 4 months) | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome |
|
Early delirium Follow‐up: postoperative period |
Study population | RR 0.48 (0.09 to 2.58) | 261 (1 study) | ⊕⊝⊝⊝ very lowb | ||
| 31 per 1000a | 15 per 1000 (3 to 81) | |||||
| Functional status, early (within 4 months) | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome |
|
HRQoL, early (within 4 months): using EQ‐5D (range 0 to 1); higher values indicate better quality of life Follow‐up: 4 months |
The mean EQ‐5D score in the unipolar group was 0.54 | MD 0.08 higher (0.03 lower to 0.19 higher) | ‐ | 115 (1 study) | ⊕⊝⊝⊝ very lowb | |
| Mobility, early (within 4 months) | ‐ | ‐ | No studies reported this outcome | |||
|
Mortality, early (within 4 months) Follow‐up: time points in the included studies were during hospital stay, at 3 months and at 4 months |
Study population | RR 0.94 (0.54 to 1.64) | 573 (4 studies) | ⊕⊕⊝⊝ lowc | ||
| 105 per 1000a | 99 per 1000 (57 to 173) | |||||
|
Mortality at 12 months Follow‐up: time points in the included studies were at 6 months, 12 months, 13 months, and 24 months |
Study population | RR 1.17 (0.89 to 1.53) | 839 (8 studies) | ⊕⊕⊝⊝ lowc | ||
| 184 per 1000a | 216 per 1000 (164 to 282) | |||||
|
Unplanned return to theatre (end of follow‐up)d Follow‐up: time points in the included studies were at 12 months, 24 months, 48 months, and 60 months |
Study population | RR 1.08 (0.44 to 2.64) | 532 (4 studies) | ⊕⊝⊝⊝ very lowe | ||
| 57 per 1000a | 62 per 1000 (25 to 151) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EQ‐5D: EuroQoL 5 Dimensions instrument; HA: hemiarthroplasty; HRQoL: health‐related quality of life; MD: mean difference; RR: risk ratio; THA: total hip arthroplasty | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDerived from the unipolar HA group if results from a single study, or otherwise, from the pooled estimate of the unipolar group bWe downgraded by three levels: two levels for imprecision because the evidence included very few participants, and one level for study limitations because the included study had high and/or unclear risks of bias. cWe downgraded by two levels: one level for imprecision because we noted a wide CI in the effect estimate, and one level for study limitations because some of the included studies had unclear risks of bias. dSome re‐operations were because of dislocation, acetabular wear, pain, periprosthetic fracture or infection. We noted that types of re‐operation included replacement with THA, revised HA, open reduction and drainage of infection. eWe downgraded by three levels: one level for imprecision, and two levels for study limitations because studies had high and unclear risks of bias, which included high risks of detection bias.
Summary of findings 3. Total hip arthroplasty compared with hemiarthroplasty for hip fracture in adults.
| Total hip arthroplasty compared with hemiarthroplasty for hip fracture in adults | ||||||
| Patient or population: adults with displaced and undisplaced hip fractures Setting: hospitals; included studies were conducted in Canada, China, Greece, Finland, India, Italy, the Netherlands, New Zealand, Norway, South Africa, Spain, Sweden, the UK and USA Intervention: THA Comparison: HA (in 1 of the included studies, this was a first‐generation design of HA) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with HA | Risk with THA | |||||
|
Activities of daily living, early (within 4 months): using Katz Index and an undefined measurement tool to identify people who were independent Follow‐up: time points in the included studies were at 3 months and 4 months |
Study population | RR 1.03 (0.91 to 1.18) | 225 (2 studies) | ⊕⊝⊝⊝ very lowb | ||
| 764 per 1000a | 787 per 1000 (695 to 901) | |||||
|
Delirium (end of follow‐up) Follow‐up: time point in the included studies was 12 months |
Study population |
RR 1.41 (0.60 to 3.33) |
357 (2 studies) | ⊕⊕⊝⊝ lowc | ||
| 47 per 1000a | 67 per 1000 (28 to 158) | |||||
|
Functional status, early (within 4 months): using HHS (range from 0 to 100) and Johansen hip score (range from 0 to 100); higher scores indicate better function Follow‐up: time points in the included studies were at 3 months and 4 months |
The mean HHS scores in HA groups ranged from 69 to 77.5. The mean Johansen hip score in the HA group was 71.4. |
SMD 0.27 higher (0.07 higher to 0.47 higher) |
‐ | 395 (3 studies) | ⊕⊝⊝⊝ very lowd | There appeared to be no clinically important difference in this effect, based on a MCID for HHS of 16 to 18 |
|
HRQoL, early (within 4 months): using EQ‐5D (range from 0 to 1); higher scores indicate better quality of life Follow‐up: time points in the included studies were at 3 months and 4 months |
The mean EQ‐5D scores in the HA groups ranged from 0.61 to 0.67. | MD 0.03 higher (0.06 lower to 0.12 higher) | ‐ | 279 (2 studies) | ⊕⊝⊝⊝ very lowe | Compatible with no effect or a clinically important benefit of THA, based on a MCID for EQ‐5D of 0.07 |
|
Mobility, early (within 4 months): using a 9‐point mobility scale; lower scores indicate better mobility Follow‐up: time point in the included study was 3 months |
The mean mobility score in the HA group was 3.8 |
MD 0.40 lower (0.96 lower to 0.16 higher) |
‐ | 83 (1 study) |
⊕⊕⊝⊝ lowf | |
|
Mortality, early (within 4 months) Follow‐up: time points in the included studies were at 1 week, 1 month, 2 months, and 4 months |
Study population | RR 0.77 (0.42 to 1.42) | 725 (6 studies) | ⊕⊝⊝⊝ very lowg | ||
| 62 per 1000a | 48 per 1000 (26 to 89) |
|||||
|
Mortality at 12 months Follow‐up: time points in the included studies were at 12 months and 24 months |
Study population | RR 1.00 (0.82 to 1.34) | 2667 (11 studies) | ⊕⊕⊕⊝ moderateh | ||
| 135 per 1000a | 135 per 1000 (112 to 165) |
|||||
|
Unplanned return to theatre (end of follow‐up)i Follow‐up: time points in the included studies were at 12 months, 24 months, 48 months, 60 months, and 13 years |
Study population | RR 0.68 (0.41 to 1.15) | 2476 (9 studies) | ⊕⊕⊝⊝ lowj | ||
| 84 per 1000a | 57 per 1000 (35 to 97) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EQ‐5D: EuroQoL 5 Dimensions instrument; HA: hemiarthroplasty; HHS: Harris Hip Score; MCID: minimal clinically important difference; RR: risk ratio; THA: total hip arthroplasty | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDerived from the pooled estimate of the HA group bWe downgraded by three levels: one level for imprecision because the evidence included very few participants, and two levels for study limitations because one of the studies had unclear risk of selection bias and we found during sensitivity analyses that this may influence the estimate. cWe downgraded by two levels: one level for imprecision because we noted a wide CI in the effect, and one level for study limitations because of unclear risks of bias. dWe downgraded by three levels: one level for imprecision because the evidence included few participants, and two levels for study limitations because some studies had high and unclear risks of bias and we found during sensitivity analysis that the direction of effect was influenced by these studies. eWe downgraded by three levels: two levels for imprecision because the evidence was compatible with no difference and a clinically meaningful difference (based on a MCID for EQ‐5D of 0.07), and one level for study limitations because studies had high and unclear risks of bias. fWe downgraded by two levels: one level for imprecision because the evidence included few participants, and one level for study limitations because the study included unclear risks of bias. gWe downgraded by three levels: two levels for imprecision because the evidence was consistent with both benefits and harms, and one level for study limitations because some included studies had high and unclear risks of bias. hWe downgraded by one level for study limitations because included studies were at high or unclear risks of bias. iSome re‐operations were because of dislocation, acetabular wear, pain, periprosthetic fracture or infection. We noted that types of re‐operation included replacement with THA, open reduction, and internal fixation. jWe downgraded by two levels: one level for imprecision because the evidence was consistent with both benefits and harms, and one level for study limitations because included studies had high and unclear risks of bias which included high risks of detection bias.
We report data available at three possible time points: early (within four months of surgery), 12 months (after four months and up to 24 months after surgery, prioritising data at 12 months if possible), and late (more than 24 months after surgery, at the latest time point reported by study authors). In the following, we report subgroup and sensitivity analyses only for comparisons for which they were appropriate and possible.
1. Prostheses implanted with cement versus without cement
Here we present three comparisons of cemented and uncemented prostheses ‐ stratified by the categories THA, HA, and a mixed intervention of THA and HA (participants were treated with either a THA or HA which is cemented, or a THA or HA which is uncemented). A summary of the implant and study characteristics is presented in Table 6. For outcomes measured with scales, we present range of scores and direction of effect for each scale in Appendix 3.
THA: cemented versus uncemented
This comparison includes data from only one study with 69 participants (Chammout 2017).
Here we report the effects for critical outcomes, and we summarise the effects of other important outcomes in Table 11. All outcomes in this comparison are reported without GRADE assessments.
8. THA (cemented vs uncemented): effects of other important outcomes and adverse events.
| Number of studies | Studies | Participants | Effect estimate | |
| Other important outcomes | ||||
| Pain at ≤ 4 months Using Pain Numerical Rating Score (range of scores from 0 to 11; lower scores indicate less pain) |
1 | Chammout 2017 | 64 | MD ‐0.90, 95% CI ‐1.82 to 0.02 (favours cemented); Analysis 1.6 |
| Pain at 12 months Using Pain Numerical Rating Score (range of scores from 0 to 11; lower scores indicate less pain) |
1 | Chammout 2017 | 63 | MD 1.00, 95% CI 0.03 to 1.97 (favours uncemented); Analysis 1.6 |
| Adverse events related to implant or fracture, or both | ||||
| Intraoperative periprosthetic fracture | 1 | Chammout 2017 | 69 | RR 0.14, 95% CI 0.01 to 2.59 (favours cemented); Analysis 1.7 |
| Postoperative periprosthetic fracture | 1 | Chammout 2017 | 69 | RR 0.97, 95% CI 0.06 to 14.91 (favours cemented); Analysis 1.7 |
| Loosening | 1 | Chammout 2017 | 69 | RR 0.32, 95% CI 0.01 to 7.69 (favours cemented); Analysis 1.7 |
| Superficial infection | 1 | Chammout 2017 | 69 | RR 0.32, 95% CI 0.01 to 7.69 (favours cemented); Analysis 1.7 |
| Dislocation | 1 | Chammout 2017 | 69 | RR 0.32, 95% CI 0.04 to 2.96 (favours cemented); Analysis 1.7 |
CI: confidence interval MD: mean difference RR: risk ratio
Critical outcomes
Activities of daily living (ADL)
Chammout 2017 provided scores for the ability to perform ADL but did not report the measurement tool used to score this data. We found no evidence of a difference in performing ADL at 3 months (mean difference (MD) 0.00, 95% confidence interval (CI) ‐0.17 to 0.17; 1 study, 65 participants; Analysis 1.1) and at 12 months after surgery (MD 0.10, 95% CI ‐0.22 to 0.42, favours uncemented; 1 study, 63 participants; Analysis 1.1).
1.1. Analysis.

Comparison 1: THA: cemented vs uncemented, Outcome 1: ADL (measurement tool not defined)
Delirium
Chammout 2017 did not report this outcome.
Functional status
Chammout 2017 reported functional status measured with the Harris Hip Score (HHS), with higher scores indicating better function. We found no evidence of a difference in function at 3 months after surgery (MD 1.00, 95% CI ‐5.37 to 7.37, favours cemented; 1 study, 65 participants; Analysis 1.2) and at 12 months after surgery (MD ‐3.00, 95% CI ‐11.29 to 5.29, favours uncemented; 1 study, 65 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: THA: cemented vs uncemented, Outcome 2: Functional status (using HHS, range for scores from 0 to 100; higher scores indicate better function)
Health‐related quality of life (HRQoL)
Chammout 2017 also reported HRQoL, measured using the EQ‐5D (range of scores from 0 to 1; higher scores indicate better quality of life). We found no evidence of a difference in HRQoL at 3 months after surgery (MD 0.00, 95% CI ‐0.12 to 0.12; 1 study, 64 participants; Analysis 1.3) and at 12 months after surgery (MD 0.00, 95% CI ‐0.13 to 0.13; 1 study, 62 participants; Analysis 1.3).
1.3. Analysis.

Comparison 1: THA: cemented vs uncemented, Outcome 3: HRQoL (using EQ‐5D, range of scores from o to 1; higher scores indicate better quality of life)
Mobility
Chammout 2017 did not report this outcome.
Mortality
We found no evidence of a difference in mortality at 12 months (risk ratio (RR) 0.49, 95% CI 0.05 to 5.11, favours cemented; 1 study, 69 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1: THA: cemented vs uncemented, Outcome 4: Mortality (12 months)
Unplanned return to theatre
We found no evidence of a difference in return to theatre at 24 months (RR 0.11, 95% CI 0.01 to 1.93, favours cemented; 1 study, 69 participants; Analysis 1.5). Some re‐operations were because of dislocation, subsidence or pain. We noted that types of re‐operation included revision to HA and to change the liner to an elevated rim.
1.5. Analysis.

Comparison 1: THA: cemented vs uncemented, Outcome 5: Unplanned return to theatre (end of follow‐up)
Other important outcomes
Effect estimates were imprecise; we found no evidence of a difference in pain or adverse events (intra‐ or postoperative periprosthetic fracture, loosening, superficial infection, and dislocation). We report the summary effects of important outcomes and adverse effects in Table 11.
HA: cemented versus uncemented
This comparison includes data from 17 studies with 3644 participants (Brandfoot 2000; Cao 2017; DeAngelis 2012; Emery 1991; Fernandez 2022; Figved 2009; Harper 1994; Moerman 2017; Movrin 2020; Parker 2010c; Rehman 2014; Sadr 1977; Santini 2005; Sonne‐Holm 1982; Talsnes 2013; Taylor 2012; Vidovic 2013). We analysed the data from Cao 2017 separately because this study includes only participants with extracapsular fractures.
Here we report effects for critical outcomes. Where pooled analyses included at least one study in each category, we subgrouped the analysis according to whether studies used a modern or a first‐generation, uncemented stem design in one of the intervention groups. Of the 16 studies including participants with intracapsular fractures, eight reported a comparison between cemented and modern uncemented HAs.
We used GRADE to assess the certainty of the evidence for the critical outcomes measured within four months of surgery (ADL, functional status, HRQoL, and mobility), within four months and at 12 months for mortality, and at the end of follow‐up for delirium and unplanned return to theatre. See Table 1.
We summarise the effects of other important review outcomes in a table, which are not subgrouped according to the stem design; these outcomes are reported without GRADE assessments.
Critical outcomes
ADL
Seven studies reported performance of ADL. The uncemented stem designs in these studies were modern (DeAngelis 2012; Fernandez 2022; Figved 2009; Moerman 2017; Parker 2020), first generation (Brandfoot 2000), or the type of stem was unknown (Santini 2005).
Early:
Moerman 2017 used the Groningen Activity Restriction Scale (GARS) at four months, and Parker 2020 used a social dependency scale at four months. For both instruments, lower scores indicate more independence. DeAngelis 2012 used the Older Americans Resources Scale of Instrumental Activities of Daily Living (OARS‐IADL), and Fernandez 2022 used a five‐point Likert scale for 'usual activities' derived from the EQ‐5D utility index; we inverted the data in these studies to account for these instruments in which higher scores indicate more independence. We found no evidence of a difference in performance of ADL (SMD ‐0.03, 95% CI ‐0.21 to 0.16, favours cemented; 4 studies, 1275 participants; I2 = 53%; moderate‐certainty evidence; Analysis 2.1). We downgraded the certainty of the evidence by one level for study limitations because studies had unclear risks of bias.
In addition, Figved 2009 reported the number of people who were independent at three months, defined as those scoring 19 or 20 on the Barthel Index Score. From these data, we found no evidence of a difference in performance of ADL (RR 0.88, 95% CI 0.65 to 1.19, favours uncemented; 1 study, 190 participants; Analysis 2.2).
2.1. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 1: Early ADL (≤ 4 months, continuous data)
2.2. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 2: Early ADL (≤ 4 months, categorical data)
At 12 months:
Moerman 2017 used the GARS and Parker 2020 used a social dependency scale. Santini 2005 used the Verona Elderly Care (VELCA) scoring system, DeAngelis 2012 used the OARS‐IADL, and Fernandez 2022 used a five‐point Likert scale; we inverted the data for these studies to account for those instruments in which higher scores indicate more independence. We found no evidence of a difference in performance of ADL (SMD ‐0.09, 95% CI ‐0.21 to 0.02, favours cemented; 5 studies, 1173 participants; I2 = 0%; Analysis 2.3). Data in this analysis were reported at 12 months in all studies.
Figved 2009 reported the number of people who were independent at 12 months, defined as those scoring 19 or 20 on the Barthel Score. From these data, we found no evidence of a difference in performance of ADL (RR 0.79, 95% CI 0.61 to 1.04, favours uncemented; 1 study, 168 participants; Analysis 2.4).
In addition, Brandfoot 2000 reported ADL outcome at 16 months using a modification of the HHS, extracting responses from the instrument related to using stairs, socks, shoes, and bathing. We did not calculate an effect estimate because data were reported as a measure of the variance. See Appendix 4 for mean scores as reported by study authors.
2.3. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 3: ADL (12 months, continuous data)
2.4. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 4: ADL (12 months, categorical data)
Late:
Figved 2009 reported the number of people who were independent at five years, defined as those scoring 19 or 20 on the Barthel Score. From these data, we found no evidence of a difference in performance of ADL (RR 0.87, 95% CI 0.63 to 1.21, favours uncemented; 1 study, 80 participants; Analysis 2.5).
2.5. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 5: Late ADL (> 24 months; categorical data)
Delirium
Two studies reported data for delirium. The uncemented stem designs in these studies were modern (Parker 2020), and first generation (Parker 2010c). We found no evidence of a difference for this outcome (RR 1.06, 95% CI 0.55 to 2.06, favours uncemented; 2 studies, 800 participants; I2 = 0%; low‐certainty evidence; Analysis 2.6). We downgraded the certainty of the evidence by two levels ‐ one level for imprecision because we noted a wide CI in the estimate, and one level for study limitations because the studies had unclear risks of bias.
2.6. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 6: Delirium (end of follow‐up)
Functional status
Eight studies reported data for functional status. The uncemented stem designs in these studies were modern (Cao 2017; Figved 2009; Movrin 2020), first generation (Brandfoot 2000; Sadr 1977; Sonne‐Holm 1982; Vidovic 2013), or unknown (Santini 2005).
Early:
Three studies reported function using the HHS (Figved 2009; Movrin 2020; Vidovic 2013). For this instrument, higher scores indicate better function. We found improved function with cemented HAs (MD 3.38, 95% CI 0.05 to 6.70, favours cemented; 3 studies, 416 participants; very low‐certainty; Analysis 2.7). We noted that this estimate did not indicate a clinically important improvement (based on a minimal clinically important difference (MCID)) of 16 to 18 points (Singh 2016). We downgraded the certainty of the evidence by one level for imprecision because the evidence included few studies, and two levels for study limitations because the studies had unclear risks of bias, and we found during sensitivity analysis that the effect estimate was influenced by these studies (see below).
In addition, Sonne‐Holm 1982 reported the number of participants with a maximum score on the D'Aubigne scale indicating good functional status (D'Aubigne 1954. From these data, we found no evidence of a difference in functional status (RR 1.15, 95% CI 0.84 to 1.59, favours cemented; 1 study, 75 participants; Analysis 2.8).
Cao 2017 reported function using the HHS at three months, and we found improved function with cemented HAs for extracapsular fractures (MD 14.70, 95% CI 11.78 to 17.62, favours cemented; 1 study, 85 participants; Analysis 2.9). We noted that the CI in this estimate may indicate a clinically important improvement with cemented HAs based on a MCID of 16 to 18 points (Singh 2016).
2.7. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 7: Early functional status (≤ 4 months, continuous data)
2.8. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 8: Early functional status (≤ 4 months; categorical data)
2.9. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 9: Early functional status: extracapsular fractures (≤ 4 months. HHS; higher scores indicate better function)
At 12 months:
Three studies reported this outcome using the HHS (Figved 2009; Movrin 2020; Vidovic 2013), Taylor 2012 used the Oxford Hip Score (OHS), and Santini 2005 used the VELCA scoring system. For all instruments, higher values indicate better function. The estimate was imprecise, including clinically relevant benefits in favour of cemented implants but also no difference between interventions (SMD 0.13, 95% CI ‐0.09 to 0.35, favours cemented; 5 studies, 494 participants; I2 = 31%; Analysis 2.10). We pooled data reported at 12 months (Figved 2009; Santini 2005; Taylor 2012; Vidovic 2013), and at 24 months (Movrin 2020).
Sadr 1977 reported data using the HHS measured at 17 months; scores were categorically reported as excellent, good, medium or poor. We combined the good and excellent scores with maximum scores from Sonne‐Holm 1982, reported at 12 months. We found no evidence of a difference in functional status (RR 1.15, 95% CI 0.91 to 1.45, favours cemented; 2 studies, 100 participants; I2 = 0%; Analysis 2.11).
Cao 2017 reported function using the HHS at 6 months, and we found improved function with cemented HAs for extracapsular fractures (MD 11.09, 95% CI 7.70 to 14.48, favours cemented; 1 study, 85 participants; Analysis 2.12). We noted that the CI is unlikely to be compatible with a clinically important improvement (Singh 2016).
In addition, Brandfoot 2000 reported this outcome at 16 months using the HHS. We did not pool data from this study in the analysis because the data were reported without any measures of variance. See Appendix 4 for mean scores as reported by study authors.
2.10. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 10: Functional status (12 months; continuous data using HHS, OHS and VELCA; higher scores indicate better function)
2.11. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 11: Functional status (12 months, categorical data using HHS)
2.12. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 12: Functional status: extracapsular fractures (12 months. HHS; higher scores indicate improved function)
Late:
Figved 2009 also reported functional status outcome at five years, but the estimate was imprecise (MD ‐9.90, 95% CI ‐17.75 to ‐2.05, favours uncemented; 1 study, 78 participants; Analysis 2.13).
2.13. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 13: Late functional status (> 24 months using HHS; higher scores indicate better function)
HRQoL
Three studies reported HRQoL (Fernandez 2022; Figved 2009; Moerman 2017). These studies used modern, uncemented stem designs. We extracted data for the physical component of the Short‐Form 12 (SF‐12) for Moerman 2017, and EQ‐5D for Fernandez 2022 and Figved 2009. In both scales, higher scores indicate better quality of life.
Early:
We found improved HRQoL for cemented HAs (SMD 0.20, 95% CI 0.07 to 0.34, favours cemented; 3 studies, 1122 participants; I2 = 9%; moderate‐certainty evidence; Analysis 2.14). The outcome was measured at four months in Fernandez 2022 and at three months in the other studies. After converting this effect estimate onto the EQ‐5D utility scale, the difference between fixation techniques was compatible with clinically small and large benefits of cemented HAs (0.06, 95% CI 0.02 to 0.10); this was based on a MCID for EQ‐5D of 0.07 (Walters 2005). We downgraded the certainty of the evidence by one level for study limitations because the studies had high and unclear risks of bias.
2.14. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 14: Early HRQoL (≤ 4 months)
At 12 months:
We found improved HRQoL for cemented HAs (SMD 0.12, 95% CI 0.00 to 0.24, favours cemented; 3 studies, 1079 participants; I2 = 0%; Analysis 2.15). After converting this effect estimate onto the EQ‐5D utility scale, the difference was compatible with no effect or a clinically important benefit of cemented HAs (0.03, 95% CI 0.00 to 0.07); this was based on a MCID for EQ‐5D of 0.07 (Walters 2005).
2.15. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 15: HRQoL (12 months)
Late:
Figved 2009 also reported HRQoL at five years, with no evidence of a difference between fixation techniques (MD ‐0.09, 95% CI ‐0.23 to 0.05, favours uncemented; 1 study, 71 participants; I2 = 0%; Analysis 2.16).
2.16. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 16: Late HRQoL (> 24 months)
Mobility
Eleven studies reported data for mobility. The uncemented stem designs in these studies were modern (Fernandez 2022; Figved 2009; Moerman 2017; Parker 2020; Taylor 2012), first generation (Brandfoot 2000; Emery 1991; Parker 2010c; Rehman 2014; Sonne‐Holm 1982), and unknown (Santini 2005).
Early:
Three studies reported the proportion of people who were able to walk independently at three months (Figved 2009; Sonne‐Holm 1982), and four months (Fernandez 2022). We found no evidence of a difference in mobility (RR 1.04, 95% CI 0.95 to 1.14, favours cemented; 3 studies, 980 participants; I2 = 5%; moderate‐certainty evidence; Analysis 2.17). We downgraded the certainty of the evidence by one level for study limitations because included studies had unclear risks of bias.
Parker 2010c and Parker 2020 used a nine‐point mobility scale in which lower scores indicate better mobility, and Moerman 2017 used a nine‐point mobility scale in which higher scores indicate better mobility. We inverted the data in Moerman 2017 before pooling. We found that mobility was improved with cemented prostheses (SMD ‐0.26, 95% CI ‐0.40 to ‐0.12, favours cemented; 3 studies, 766 participants; I2 = 0%; Analysis 2.18). This effect size is likely to be small to medium (Cohen 1988).
Rehman 2014 used a nine‐point mobility rating scale, in which higher scores indicated better mobility; these data were reported as mean reduction values. We found that mobility was improved with uncemented HA (MD ‐0.40, 95% CI ‐0.68 to ‐0.12, favours uncemented; 1 study, 110 participants; Analysis 2.19). This effect estimate was imprecise, including clinically relevant benefits and harms (Cohen 1988).
2.17. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 17: Early mobility (≤ 4 months, independent mobility)
2.18. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 18: Early mobility (≤ 4 months, continuous data)
2.19. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 19: Early mobility (mean reduction values at ≤ 4 months; higher scores indicate better mobility)
At 12 months:
Parker 2010c and Parker 2020 used a nine‐point scale in which lower scores indicate better mobility. Moerman 2017 used a nine‐point scale and Santini 2005 reported a six‐point subscale for walking abilities from the VELCA scoring system; for both scales, higher scores indicate better mobility. We inverted the data in Moerman 2017 and Santini 2005 before pooling. We found that mobility was improved with a cemented HA (SMD ‐0.24, 95% CI ‐0.42 to ‐0.06, favours cemented; 4 studies, 762 participants; I2 = 32%; Analysis 2.20). This effect size is likely to be small to medium (Cohen 1988).
Three studies reported the proportion of people who were able to walk independently at 12 months (Fernandez 2022; Figved 2009; Sonne‐Holm 1982). We found no evidence of a difference in mobility (RR 0.98, 95% CI 0.70 to 1.37, favours uncemented; 3 studies, 826 participants; I2 = 84%; Analysis 2.21).
Emery 1991 reported the number of people who were more dependent on walking aids at 17 months after surgery than before their injury. We found that mobility was better using a cemented HA (RR 0.53, 95% CI 0.30 to 0.93, favours cemented; 1 study, 39 participants; Analysis 2.22).
In addition, Brandfoot 2000 reported this outcome at 16 months, using responses extracted from the HHS, and Taylor 2012 reported this using the Timed Up and Go (TUG) test at 24 months. We did not pool data from these studies in the analyses because the data were reported without variances. See Appendix 4 for mean scores, as reported by study authors.
2.20. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 20: Mobility (12 months, continuous data using different mobility scales; lower scores indicate better mobility)
2.21. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 21: Mobility (12 months, independent mobility)
2.22. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 22: Mobility (12 months, dependent on walking aid)
Late:
Parker 2010c reported data at five years, using a nine‐point mobility scale in which lower scores indicate better mobility. We found no evidence of a difference in mobility (MD ‐0.60, 95% CI ‐1.79 to 0.59, favours cemented; 1 study, 64 participants; Analysis 2.23).
Figved 2009 reported the number of people who were able to walk independently at five years. We found no evidence of a difference in mobility (RR 0.88, 95% CI 0.75 to 1.02, favours uncemented; 1 study, 79 participants; Analysis 2.24).
2.23. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 23: Late mobility (> 24 months)
2.24. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 24: Late mobility (> 24 months; independent mobility)
Mortality
Fifteen studies reported mortality. The uncemented stem designs in these studies were modern (Fernandez 2022; Figved 2009; Moerman 2017; Movrin 2020; Parker 2020; Talsnes 2013; Taylor 2012), first generation (Brandfoot 2000; Emery 1991; Harper 1994; Parker 2010c; Sadr 1977; Sonne‐Holm 1982; Vidovic 2013), or unknown (Santini 2005).
Early:
The estimate for mortality within four months of surgery includes clinically relevant harms and benefits (RR 0.95, 95% CI 0.80 to 1.13, favours cemented; 12 studies, 3136 participants; I2 = 0%; low‐certainty evidence; Analysis 2.25). We downgraded the certainty of the evidence by one level for imprecision because the CI included both possible harms and benefits, and one level for study limitations because most studies in this analysis had unclear or high risks of bias. We generated a funnel plot (Figure 3), and we found evidence of small study effects which tend to favour cemented HAs (Harbord modified test, P value = 0.003).
2.25. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 25: Early mortality (≤ 4 months)
3.

Cemented hemiarthroplasty versus uncemented hemiarthroplasty. Funnel plot for early mortality (≤ 4 months), subgrouped by stem design
At 12 months:
We found that the risk of death at 12 months was reduced using cemented HA (RR 0.86, 95% CI 0.78 to 0.96, favours cemented; 15 studies, 3727 participants; I2 = 0%; moderate‐certainty evidence; Analysis 2.26). This analysis included data reported at 16 months (Brandfoot 2000), 18 months (Emery 1991), and 24 months (Movrin 2020). We downgraded the certainty of the evidence by one level for study limitations because most studies in this analyses had unclear or high risks of bias. We generated a funnel plot (Figure 4), and we found no statistical evidence of small study size effects (Harbord modified test, P value = 0.169).
2.26. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 26: Mortality (12 months)
4.

Cemented hemiarthroplasty versus uncemented hemiarthroplasty. Funnel plot for mortality at 12 months, subgrouped by stem design
Late:
Two studies reported data at a time point of five years (Figved 2009; Parker 2010c). We found no evidence of a difference in mortality according to the fixation technique (RR 1.01, 95% CI 0.89 to 1.25, favours uncemented; 2 studies, 620 participants; Analysis 2.27).
2.27. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 27: Late mortality (> 24 months)
Unplanned return to theatre
Six studies reported unplanned return to theatre at the end of study follow‐up, which was at 12 months (DeAngelis 2012; Fernandez 2022; Figved 2009; Moerman 2017), 24 months (Taylor 2012), and 60 months (Parker 2010c). The effect estimate was imprecise, including benefits and harms (RR 0.70, 95% CI 0.45 to 1.10, favours cemented; 6 studies, 2336 participants; I2 = 0%; low‐certainty evidence; Analysis 2.28). Some re‐operations were because of dislocation, loosening, acetabular wear, periprosthetic fracture or infection. We noted that types of re‐operation included replacement with THA, Girdlestone and open reduction and drainage of infection. We downgraded the certainty of the evidence by two levels for study limitations because most studies in the analysis had unclear risks of bias and all studies were at high risk of detection bias.
2.28. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 28: Unplanned return to theatre (end of follow‐up)
Other important outcomes
It was difficult to interpret pain outcomes because studies reported this outcome using different instruments and scales. We found no evidence of a difference in the number of people who were discharged to their own home according to the fixation technique. Similarly, we found no evidence of a difference in most adverse events unrelated to the implant or fracture, or both, according to whether or not cement was used to fix the HA (acute kidney injury, cerebrovascular accident, chest infection/pneumonia, myocardial infarction, urinary tract infection, deep vein thrombosis, and pulmonary infection). However, we noted that fewer people had a pulmonary embolism when the HA was fixed without cement. Cao 2017 reported no adverse events in either group of participants with extracapsular fractures (intraoperative fractures, loosening, deep infection, superficial infection, and dislocation). We report the summary effects of all these important outcomes and adverse effects in Table 12.
9. HA (cemented vs uncemented): effects of other important outcomes and adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Other important outcomes | ||||
| Paina Experiencing no pain at ≤ 4 months (We inverted data in 2 studies in which data were reported as complaining of pain or experiencing mid‐thigh pain) |
4 | Harper 1994; Figved 2009; Moerman 2017; Sonne‐Holm 1982 | 500 | RR 1.11, 95% CI 1.00 to 1.22 (favours uncemented); Analysis 2.29 |
| Pain at ≤ 4 months Using VAS, and a 9‐point pain scale (lower values indicate less pain) |
3 | Movrin 2020; Parker 2010c; Parker 2020 | 802 | Data not combined because of substantial statistical heterogeneity I2 = 91%; Analysis 2.30 |
| Paina Experiencing no pain at 12 months (We inverted data in 1 study in which data were reported as complaining of pain or experiencing mid‐thigh pain) |
4 | Emery 1991; Figved 2009; Moerman 2017; Sonne‐Holm 1982 | 376 | RR 1.17, 95% CI 0.85 to 1.63 (favours uncemented); I2 = 77%; Analysis 2.31 |
| Pain at 12 months Using VAS, and a 9‐point pain scale (lower values indicate less pain) |
4 | Figved 2009; Movrin 2020; Parker 2010c; Parker 2020 | 726 | SMD ‐0.06, 95% CI ‐0.33 to 0.21 (favours cemented); I2 = 66%; Analysis 2.32 |
| Pain at 12 months Mean reduction values (lower values indicate less pain) |
1 | Rehman 2014 | 110 | MD ‐0.27, 95% CI ‐0.48 to ‐0.06 (favours cemented); Analysis 2.33 |
| Pain at > 24 months. Reported by study authors at 5 years Using VAS (lower values indicate less pain) |
1 | Parker 2010c | 58 | MD ‐0.30, 95% CI ‐0.92 to 0.32 (favours cemented); Analysis 2.34 |
| Pain Experiencing no pain at 5 years |
1 | Figved 2009 | 80 | RR 1.00, 95% CI 0.77 to 1.30; Analysis 2.35 |
| Length of hospital stay | 9 | Emery 1991; Figved 2009; Harper 1994; Moerman 2017; Parker 2010c; Parker 2020; Santini 2005; Taylor 2012; Vidovic 2013 | 1801 | MD ‐0.40 days, 95% CI ‐1.03 to 0.23 (favours cemented); Analysis 2.36 |
| Discharge destination Living in own homea |
6 | DeAngelis 2012; Figved 2009; Parker 2010c; Santini 2005; Taylor 2012; Fernandez 2022 | 2331 | RR 1.05, 95% CI 0.98 to 1.13 (favours uncemented); Analysis 2.37 |
| Adverse events related to surgery | ||||
| Intraoperative periprosthetic fracture | 7 | DeAngelis 2012; Figved 2009; Moerman 2017; Movrin 2020; Parker 2010c; Parker 2020; Taylor 2012 | 1669 | RR 0.20, 95% CI 0.08 to 0.46 (favours cemented); Analysis 2.38 |
| Postoperative periprosthetic fracture | 6 | Figved 2009; Moerman 2017; Movrin 2020; Santini 2005; Taylor 2012; Fernandez 2022 | 2819 | RR 0.29, 95% CI 0.14 to 0.57 (favours cemented); Analysis 2.38 |
| Loosening | 4 | Brandfoot 2000; Figved 2009; Moerman 2017; Sadr 1977 | 537 | RR 0.52, 95% CI 0.14 to 1.89 (favours cemented); I2 = 45%; Analysis 2.38 |
| Deep infection | 7 | Figved 2009; Harper 1994; Moerman 2017; Movrin 2020; Parker 2010c; Santini 2005; Taylor 2012 | 1382 | RR 1.56, 95% CI 0.72 to 3.38 (favours uncemented); Analysis 2.38 |
| Superficial infection | 7 | DeAngelis 2012; Emery 1991; Figved 2009; Harper 1994; Moerman 2017; Parker 2010c; Parker 2020; Sonne‐Holm 1982; Taylor 2012; Fernandez 2022 | 1210 | RR 1.23, 95% CI 0.73 to 2.06 (favours uncemented); Analysis 2.38 |
| Dislocation | 8 | Figved 2009; Harper 1994; Moerman 2017; Movrin 2020; Parker 2010c; Parker 2020; Sadr 1977; Santini 2005; Taylor 2012; Fernandez 2022 | 3032 | RR 1.08, 95% CI 0.61 to 1.91 (favours uncemented); Analysis 2.38 |
| Adverse events unrelated to surgery | ||||
| Acute kidney injury | 4 | Moerman 2017; Parker 2010c; Parker 2020; Fernandez 2022 | 2226 | RR 1.23, 95% CI 0.76 to 2.00 (favours uncemented); Analysis 2.39 |
| Blood transfusion | 7 | DeAngelis 2012; Figved 2009; Moerman 2017; Parker 2010c; Parker 2020; Talsnes 2013; Fernandez 2022 | 2907 | RR 1.00, 95% CI 0.83 to 1.20 (favours cemented); I2 = 36%; Analysis 2.39 |
| Cerebrovascular accident | 5 | DeAngelis 2012; Moerman 2017; Parker 2010c; Parker 2020; Fernandez 2022 | 2356 | RR 0.93, 95% CI 0.41 to 2.10 (favours cemented); Analysis 2.39 |
| Chest infection/pneumonia | 8 | DeAngelis 2012; Emery 1991; Figved 2009; Moerman 2017; Parker 2010c; Parker 2020; Taylor 2012; Fernandez 2022 | 2789 | RR 0.78, 95% CI 0.50 to 1.21 (favours cemented); Analysis 2.39 |
| Myocardial infarction | 7 | DeAngelis 2012; Figved 2009; Moerman 2017; Parker 2010c; Parker 2020; Santini 2005; Fernandez 2022 | 1457 | RR 0.91, 95% CI 0.44 to 1.89 (favours cemented); Analysis 2.39 |
| Urinary tract infection | 5 | Emery 1991; Moerman 2017; Santini 2005; Taylor 2012; Fernandez 2022 | 1745 | RR 0.89, 95% CI 0.65 to 1.20 (favours cemented); Analysis 2.39 |
| Venous thromboembolic phenomena (DVT) | 7 | Cao 2017; DeAngelis 2012; Figved 2009; Moerman 2017; Parker 2010c; Parker 2020; Fernandez 2022 | 2661 | RR 1.28, 95% CI 0.56 to 2.90 (favours uncemented); Analysis 2.39 |
| Venous thromboembolic phenomena (pulmonary embolism) | 6 | Emery 1991; Figved 2009; Moerman 2017; Parker 2010c; Parker 2020; Fernandez 2022 | 2499 | RR 3.56, 95% CI 1.26 to 10.11 (favours uncemented); Analysis 2.39 |
aOther data is reported in Appendix 5
CI: confidence interval DVT: deep vein thrombosis MD: mean difference RR: risk ratio VAS: visual analogue scale
Subgroup analysis
We did not conduct subgroup analysis to explore differences between studies according to our pre‐specified effect modifiers (age, gender, and fracture displacement) because these variables were insufficiently reported.
Visual inspection of the forest plots for each outcome where data were available in both first generation and modern uncemented HA subgroups showed that the overall trend for benefits associated with cemented HA were reduced in the modern uncemented subgroup. However, these subgroups were sparse and few studies were available across most of the comparisons. We conducted a formal subgroup analysis for mortality at 12 months because this analysis had sufficient studies. Santini 2005 did not report the exact type of uncemented HA, and we chose to include this study in the first generation subgroup. On visual inspection of the data, we noted that the reduction in mortality was increased amongst studies reporting a comparison with a modern uncemented design of HA rather than a first generation uncemented design (RR 0.80, 95% CI 0.68 to 0.95; 6 studies, 1466 participants, favours cemented modern HA; I2 = 0%; Analysis 2.26; Figure 4). However, this was not supported by formal tests of interaction (P = 0.24).
Sensitivity analysis
Here, we report the results of sensitivity analyses only when we noted a difference in interpretation of the effect.
High or unclear risk of selection bias (for sequence generation)
Early functional status (≤ four months; continuous data): we excluded two studies from this analysis (Movrin 2020; Vidovic 2013). Although the effect continued to show no evidence of a difference between groups, we noted that the estimate favoured the alternative intervention (MD ‐1.20, 95% CI ‐6.66 to 4.26, favours uncemented; 1 study, 189 participants).
Functional status (at 12 months; continuous data): we excluded three studies from this analysis (Movrin 2020; Santini 2005; Vidovic 2013). Although the effect continued to show no evidence of a difference between groups, we noted that the estimate favoured the alternative intervention (MD ‐0.02, 95% CI ‐0.28 to 0.24, favours uncemented; 2 studies, 234 participants).
Early mobility (reported at ≤ four months; continuous data): we excluded two studies from this analysis (Moerman 2017; Parker 2010c). We found that the analysis no longer showed an improvement in mobility when the prosthesis was cemented (MD ‐0.40, 95% CI ‐0.81 to 0.01; 1 study, 329 participants).
High risk of attrition bias
In this sensitivity analysis, we considered attrition bias at the outcome level. We therefore conducted sensitivity analysis only on outcomes that included a study with high risk of attrition bias owing to losses for that specific outcome. We found no difference in the interpretation of the effect for all outcomes in this comparison.
Mixed HA and THA: cemented versus uncemented
This comparison includes data from two studies with 169 participants (Inngul 2015; Moroni 2002). In both studies, participants were randomised to a cemented or uncemented prosthesis, but the selection of a THA or HA was left to the treating surgeon and participant to select.
Here we report the effects for critical outcomes, and we summarise the effects of other important review outcomes in a table. These outcomes are reported without GRADE assessments.
Critical outcomes
ADL, delirium, and mobility
Neither study reported data for these outcomes.
Functional status
Both studies reported functional status measured using the HHS, with higher scores indicating better function.
At 12 months:
We calculated an effect estimate for Moroni 2002 but this estimate was very imprecise. We found no evidence of a difference in functional status at 24 months from surgery (MD ‐16.00, 95% CI ‐41.57 to 9.57, favours uncemented; 1 study, 28 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 1: Functional status (12 months, using HHS, range of scores from 0 to 100; higher scores indicate better function)
Inngul 2015 also reported data for this outcome at 4 months, 12 months and 4 years using the HHS, but we could not calculate effect estimates because the study authors did not clearly report the number of participants available in each group. See Appendix 4 for mean scores as reported by study authors.
HRQoL
Only Moroni 2002 reported this outcome, measured using SF‐36 at 24 months. This estimate was very imprecise. We found no evidence of a difference in HRQoL (MD ‐19.00, 95% CI ‐42.77 to 4.77, favours uncemented; 1 study, 28 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 2: HRQoL (12 months, using SF‐36, range of scores from 0 to 100; higher scores indicate better quality of life)
Mortality
Both studies reported mortality (Inngul 2015; Moroni 2002).
Early:
The effect estimate for mortality at four months was very imprecise, including clinically relevant harms and benefits (RR 4.42, 95% CI 0.51 to 38.55, favours uncemented; 1 study, 141 participants; Analysis 3.3).
3.3. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 3: Early mortality (≤ 4 months)
At 12 months:
Similarly, the estimate for mortality at 12 months was imprecise (RR 2.02, 95% CI 0.81 to 5.07, favours uncemented; 2 studies, 169 participants; I2 = 0%; Analysis 3.4). Moroni 2002 did not report mortality at 12 months, and this analysis includes data at 24 months from this study.
3.4. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 4: Mortality (12 months)
Late:
Data were available at four years in Inngul 2015, and the estimate was also imprecise (RR 0.88, 95% CI 0.50 to 1.56, favours cemented; 1 study, 141 participants; Analysis 3.5).
3.5. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 5: Late mortality (> 24 months)
Unplanned return to theatre
Only Inngul 2015 reported unplanned return to theatre. The estimate was imprecise, showing no evidence of a difference at four years after surgery (RR 0.74, 95% CI 0.22 to 2.50, favours uncemented; 1 study, 141 participants; Analysis 3.8). Indications for re‐operation were dislocation and periprosthetic fracture, and revision included THA.
3.8. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 8: Unplanned return to theatre (end of follow‐up)
Other important outcomes
We found no evidence of a difference in some adverse events related to the implant or fracture, or both (superficial infection and dislocation). However, we noted fewer intraoperative periprosthetic fractures when cement was used in Inngul 2015 (0.06, 95% CI 0.00 to 0.98; 1 study, 141 participants; Analysis 3.9). We found no evidence of a difference in adverse events unrelated to the implant or fracture, or both (acute kidney injury, pneumonia, myocardial infarction, urinary tract infection). We report the summary effects for these adverse events in Table 13.
3.9. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 9: Adverse events related to the implant, fracture, or both
10. THA (mixed HA and THA): cemented vs uncemented: effects of other important outcomes and adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Adverse events related to the implant or fracture, or both | ||||
| Intraoperative periprosthetic fracture | 1 | Inngul 2015 | 141 | RR 0.06, 95% CI 0.00 to 0.98 (favours cemented); Analysis 3.9 |
| Superficial infection | 1 | Inngul 2015 | 141 | RR 0.49, 95% CI 0.16 to 1.52 (favours cemented); Analysis 3.9 |
| Dislocation | 1 | Moroni 2002 | 28 | RR 0.87, 95% CI 0.14 to 5.32 (favours cemented); Analysis 3.9 |
| Adverse events unrelated to implant or fracture, or both | ||||
| Acute kidney injury | 1 | Inngul 2015 | 141 | RR 0.37, 95% CI 0.02 to 8.87 (favours cemented); Analysis 3.10 |
| Chest infection/pneumonia | 1 | Inngul 2015 | 141 | RR 0.55, 95% CI 0.05 to 5.95 (favours cemented); Analysis 3.10 |
| Myocardial infarction | 1 | Inngul 2015 | 141 | RR 0.37, 95% CI 0.02 to 8.87 (favours cemented); Analysis 3.10 |
| Urinary tract infection | 1 | Inngul 2015 | 141 | RR 1.42, 95% CI 0.56 to 3.60 (favours uncemented); Analysis 3.10 |
CI: confidence interval RR: risk ratio
2. Bipolar HA versus unipolar HA
This comparison includes 13 studies with 1499 participants (Abdelkhalek 2011; Calder 1995; Calder 1996; Cornell 1998; Davison 2001; Figved 2018; Hedbeck 2011; Jeffcote 2010; Kanto 2014; Malhotra 1995; Patel 2008; Raia 2003; Stoffel 2013). A summary of the types of implants and study characteristics is presented in Table 7. Whilst cemented prostheses were reported in most studies in this comparison, three studies reported outcomes with uncemented prostheses (Figved 2018; Malhotra 1995; Patel 2008), and in one study, mixed cemented and uncemented prostheses were included in both groups (Abdelkhalek 2011).
Here we report effects for critical outcomes. Where analyses included at least one study in each category, we subgrouped the analysis according to whether studies reported interventions with cemented or uncemented prostheses.
We used GRADE to assess the certainty of the evidence for the critical outcomes measured within four months of surgery (ADL, functional status, HRQoL, and mobility), within 4 months and at 12 months for mortality, and at the end of follow‐up for delirium and unplanned return to theatre. See Table 2.
We summarise the effects of other important review outcomes in a table, which are not subgrouped by stem fixation. These outcomes are reported without GRADE assessments.
For outcomes measured with scales, we present range of scores and direction of effect for each scale in Appendix 3.
Critical outcomes
ADL
Two studies reported performance of ADL; in both studies, the prostheses were cemented (Hedbeck 2011; Raia 2003).
At 12 months:
Hedbeck 2011 used the Katz Index to identify participants who were independent (Katz 1963). We found no evidence of a difference in the number of people who were independent in ADL at 12 months (RR 1.06, 95% CI 0.85 to 1.33, favours bipolar; 1 study, 99 participants; Analysis 4.1).
In addition, Raia 2003 reported this outcome at 12 months using the ADL subset score of the Musculoskeletal Functional Assessment Instrument. We did not calculate an effect estimate because data were reported without distribution variables. See Appendix 5 for average scores as reported by study authors.
4.1. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 1: ADL (12 months)
Delirium
Stoffel 2013 reported delirium following cemented HAs. We found no evidence of a difference in postoperative delirium (RR 0.48, 95% CI 0.09 to 2.58, favours bipolar; 1 study, 261 participants; very low‐certainty evidence; Analysis 4.2). We downgraded the certainty of the evidence by three levels ‐ two levels for imprecision because the evidence included very few participants, and one level for study limitations because the included study had high and unclear risks of bias.
4.2. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 2: Delirium/confusion
Functional status
Eight studies reported functional status. Studies included cemented (Cornell 1998; Davison 2001; Hedbeck 2011; Raia 2003; Stoffel 2013), uncemented (Figved 2018; Malhotra 1995), and a mixture of cemented and uncemented HAs in both the bipolar and unipolar groups (Abdelkhalek 2011).
Early:
Hedbeck 2011 reported this outcome using the HHS at four months. We did not calculate an effect estimate for this study because data were reported without measures of variance. See Appendix 5 for mean scores as reported by study authors.
At 12 months:
Two studies reported this outcome, using the HHS (Stoffel 2013), and the Johansen hip score (Cornell 1998). In both scales, higher scores indicate better function. This analysis included data at 12 months (Stoffel 2013), and at 6 months (Cornell 1998). This estimate was imprecise, including clinically relevant benefits and harms; we found no evidence of a difference according to the articulation of the HA (SMD ‐0.04, 95% CI ‐0.27 to 0.19, favours unipolar; 2 studies, 299 participants; I2 = 0%; Analysis 4.3).
Malhotra 1995 reported categorical data using the Devas 1983 system. Ranges of scores were reported as excellent, good, medium, or poor, and we combined data for scores which were excellent and good. We found no evidence of a difference according to the articulation of the HA (RR 1.17, 95% CI 0.95 to 1.43, favours bipolar; 1 study, 68 participants; Analysis 4.4).
In addition, four studies reported this outcome using the HHS at 12 months (Davison 2001; Figved 2018; Hedbeck 2011), and physical function scores of SF‐36 (Raia 2003). We did not calculate effect estimates for these studies because data were reported without means or without an appropriate measure of variance. See Appendix 5 for data as reported by study authors.
4.3. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 3: Functional status (12 months; using different measurement tools; higher scores indicate better function)
4.4. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 4: Functional status (12 months. HHS; excellent and good)
Late:
One study reported categorical data using the HHS (Abdelkhalek 2011). Ranges of scores were reported as excellent, good, medium, or poor, and we combined data for scores which were excellent and good. We found no evidence of a difference according to the articulation of the HA (RR 1.28, 95% CI 0.98 to 1.67, favours bipolar; 1 study, 50 participants; Analysis 4.5).
In addition, one study reported this outcome using the HHS at five years (Davison 2001). We did not calculate an effect estimate for this study because data were reported without an appropriate measure of variance. See Appendix 5 for mean scores as reported by study authors.
4.5. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 5: Functional status (> 24 months. HHS; excellent or good)
HRQoL
Three studies reported HRQoL. These studies included cemented (Hedbeck 2011; Raia 2003), and uncemented HAs (Figved 2018).
Early:
Hedbeck 2011 reported data using EQ‐5D up to four months since surgery; in this scale, higher scores indicate better quality of life. We found no evidence of a difference in HRQoL according to the articulation of the HA (MD 0.08, 95% CI ‐0.03 to 0.19, favours bipolar; 1 study, 115 participants; very low‐certainty evidence; Analysis 4.6). We downgraded the certainty of the evidence by three levels ‐ two levels for imprecision because data were available from only one small study, and one level for study limitations because this study had unclear risks of bias.
4.6. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 6: Early HRQoL (≤ 4 months)
At 12 months:
One study reported data using EQ‐5D at 12 months (Hedbeck 2011). We found no evidence of a difference in quality of life at 12 months (MD 0.03, 95% CI ‐0.08 to 0.14, favours bipolar; 1 study, 99 participants; very low‐certainty evidence; Analysis 4.7).
In addition, Figved 2018 reported this outcome using EQ‐5D, and Raia 2003 reported this outcome at 12 months using SF‐36. We did not pool data from these studies because data were reported without an appropriate measure of variance. See Appendix 5 for average scores as reported by study authors.
4.7. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 7: HRQoL (12 months)
Mobility
Five studies reported data for mobility within 12 months, and the HAs in all these studies were fixed with cement (Calder 1995; Calder 1996; Cornell 1998; Raia 2003; Stoffel 2013).
Cornell 1998 used TUG at six months, and we found no evidence of a difference in mobility according to articulation of the HA (MD 5.80, 95% CI ‐6.83 to 18.43, favours unipolar; 1 study, 48 participants; Analysis 4.8).
Stoffel 2013 used a six‐minute walk test at 12 months. We found the mobility was better when a unipolar HA was used (MD ‐45.00 metres, 95% CI ‐80.64 to ‐9.36, favours unipolar; 1 study, 186 participants; Analysis 4.9). The CI in this effect may suggest a clinically important improvement in mobility when a unipolar HA was used (based on a MCID of 59.4 metres in Overgaard 2017).
In addition, we did not calculate an effect estimate for three studies because these data were reported without distribution variables (Calder 1995; Calder 1996; Raia 2003). In Calder 1995 and Calder 1996, study authors reported mobility scores using a subscale of the Nottingham Health Profile. Raia 2003 reported average mobility scores using the Musculoskeletal Functional Assessment Instrument. See Appendix 5 for average scores as reported by study authors.
4.8. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 8: Mobility (Get up and Go Test; in seconds)
4.9. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 9: Mobility (6 minute walk test; in metres)
Mortality
Nine studies reported mortality. Studies included cemented (Calder 1996; Cornell 1998; Davison 2001; Hedbeck 2011; Jeffcote 2010; Kanto 2014; Raia 2003), and uncemented HAs (Figved 2018; Patel 2008).
Early:
The estimate for mortality within four months of surgery was very imprecise, including clinically relevant benefits and harms (RR 0.94, 95% CI 0.54 to 1.64, favours bipolar; 4 studies, 573 participants; I2 = 3%; low‐certainty evidence; Analysis 4.10). We downgraded the certainty of the evidence by one level for imprecision because we noted a wide CI in the effect estimate, and one level because some of the included studies had unclear risks of bias.
4.10. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 10: Early mortality (≤ 4 months)
At 12 months:
Similarly, the estimate for mortality at 12 months from surgery was very imprecise, including clinically relevant benefits and harms (RR 1.17, 95% CI 0.89 to 1.53, favours unipolar; 8 studies, 839 participants; I2 = 0%; low‐certainty evidence; Analysis 4.11). This analysis included data reported at 6 months (Cornell 1998), 13 months (Patel 2008), and 24 months (Jeffcote 2010). We downgraded the certainty of the evidence by one level for imprecision because we noted a wide CI in the effect estimate, and one level because some of the included studies had unclear risks of bias.
4.11. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 11: Mortality (12 months)
Late:
Two studies also reported mortality after 24 months from surgery, at 36 months (Davison 2001), and 60 months (Kanto 2014). We found no evidence of a difference in mortality at this late time point according to the articulation of the HA (RR 0.94, 95% CI 0.72 to 1.23, favours bipolar; 2 studies, 362 participants; I2 = 0%; Analysis 4.12).
4.12. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 12: Late mortality (> 24 months)
Unplanned return to theatre
Four studies reported unplanned return to theatre. Studies included cemented (Davison 2001; Hedbeck 2011; Kanto 2014), and a mixture of cemented and uncemented stems in both the bipolar and unipolar groups (Abdelkhalek 2011).
We found no evidence of a difference in unplanned return to theatre (RR 1.08, 95% CI 0.44 to 2.64, favours unipolar; 4 studies, 532 participants; I2 = 31%; very low‐certainty evidence; Analysis 4.13). Data were reported at the end of study follow‐up which was at 12 months (Hedbeck 2011), 24 months (Abdelkhalek 2011), 48 months (Davison 2001), and 60 months (Kanto 2014). Some Indications for re‐operations were dislocation, acetabular wear, pain, periprosthetic fracture or infection. We noted that types of re‐operation included replacement with THA, revised HA, open reduction and drainage of infection. We downgraded the certainty of the evidence by one level for imprecision because we noted a wide CI in the effect estimate, and two levels for study limitations because the studies were at high risk of detection bias and had high and unclear risks of bias in other domains.
4.13. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 13: Unplanned return to theatre (end of follow‐up)
Other important outcomes
We found no evidence of a difference in pain when reported using categorical data, although one small study that used a numerical rating score to measure this outcome found that pain was reduced when a bipolar articulation was used. We found no evidence of a difference according to the articulation of the HA for discharge destination. We also found no evidence of a difference in adverse events related to the implant or fracture, or both (periprosthetic fracture, deep infection, superficial infection, dislocation) (Figure 5), or in adverse events unrelated to the implant or fracture, or both (acute kidney injury, blood transfusion, cerebrovascular accident, chest infection/pneumonia, myocardial infarction, urinary tract infection, deep vein thrombosis, and pulmonary embolism). We report the summary effects of all these important outcomes and adverse effects in Table 14.
5.

Bipolar hemiarthroplasty versus unipolar hemiarthroplasty. Forest plot for adverse events related to the implant, fracture, or both
11. HA (bipolar vs unipolar): effects of other important outcomes and adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Other important outcomes | ||||
| Pain (categorical data; no pain, or mild pain) | 2 | Abdelkhalek 2011; Calder 1996 | 300 | RR 1.22, 95% CI 0.82 to 1.82; I2 = 61% (favours bipolar); Analysis 4.14 |
| Paina Using Numerical Rating Scale (lower scores indicate less pain) |
1 | Stoffel 2013 | 233 | MD ‐0.60, 95% CI ‐1.07 to ‐0.13 (favours bipolar); Analysis 4.15 |
| Length of hospital staya | 1 | Stoffel 2013 | 261 | MD 0.20 days, 95% CI ‐0.95 to 1.35 (favours unipolar); Analysis 4.16 |
| Discharge destination | 2 | Calder 1996; Kanto 2014 | 381 | RR 0.95, 95% CI 0.84 to 1.08 (favours bipolar); Analysis 4.17 |
| Adverse events related to surgery | ||||
| Periprosthetic fracture | 1 | Hedbeck 2011 | 120 | RR 7.00, 95% CI 0.37 to 132.66 (favours unipolar); Analysis 4.18 |
| Deep infection | 7 | Calder 1996; Davison 2001; Hedbeck 2011; Jeffcote 2010; Kanto 2014; Malhotra 1995; Stoffel 2013 | 1122 | RR 1.10, 95% CI 0.44 to 2.71 (favours unipolar); Analysis 4.18 |
| Superficial infection | 1 | Stoffel 2013 | 261 | RR 2.41, 95% CI 0.48 to 12.18 (favours unipolar); Analysis 4.18 |
| Dislocation | 9 | Abdelkhalek 2011; Calder 1996; Cornell 1998; Davison 2001; Hedbeck 2011; Kanto 2014; Malhotra 1995; Raia 2003; Stoffel 2013 | 1274 | RR 0.62, 95% CI 0.28 to 1.38 (favours bipolar); Analysis 4.18 |
| Adverse events unrelated to surgery | ||||
| Acute kidney injury | 1 | Stoffel 2013 | 261 | RR 2.89, 95% CI 0.12 to 70.25 (favours unipolar); Analysis 4.19 |
| Blood transfusion | 1 | Raia 2003 | 115 | RR 0.91, 95% CI 0.51 to 1.62 (favours bipolar); Analysis 4.19 |
| Cerebrovascular accident | 2 | Kanto 2014; Stoffel 2013 | 436 | RR 1.57, 95% CI 0.20 to 12.69 (favours unipolar); Analysis 4.19 |
| Chest infection/pneumonia | 3 | Hedbeck 2011; Kanto 2014; Stoffel 2013 | 556 | RR 0.61, 95% CI 0.10 to 3.86 (favours bipolar); Analysis 4.19 |
| Myocardial infarction | 3 | Hedbeck 2011; Kanto 2014; Stoffel 2013 | 556 | RR 0.69, 95% CI 0.11 to 4.32 (favours bipolar); Analysis 4.19 |
| Urinary tract infection | 1 | Stoffel 2013 | 261 | RR 0.96, 95% CI 0.29 to 3.25 (favours bipolar); Analysis 4.19 |
| Venous thromboembolic phenomena (DVT) | 2 | Hedbeck 2011; Stoffel 2013 | 381 | RR 3.84, 95% CI 0.43 to 34.45 (favours unipolar); Analysis 4.19 |
| Venous thromboembolic phenomena (pulmonary embolism) | 1 | Hedbeck 2011 | 120 | RR 3.00, 95% CI 0.12 to 72.20 (favours bipolar); Analysis 4.19 |
| aAdditional data are reported in Appendix 4. We did not calculate effect estimates for the data in Appendix 4 because study authors did not report distribution variables that we required for analysis. | ||||
CI: confidence interval DVT: deep vein thrombosis MD: mean difference RR: risk ratio
Sensitivity analysis
High or unclear risk of selection bias (for sequence generation)
We excluded studies with high or unclear risk of selection bias from the primary analyses. This did not alter our interpretation of the effect for any outcomes.
High risk of attrition bias
No studies in this comparison group were at high risk of attrition bias.
3. HAs versus other HAs
Here we present three comparisons that evaluate one design of HA with another design: short stem versus long stem; Thompson versus Exeter Trauma Stem; Moore versus Furlong. A summary of the implant and study characteristics is presented in Table 8.
For each of these comparisons, we report here the effects for critical outcomes and we summarise the effects of other important outcomes in a table. All outcomes in these three comparisons are reported without GRADE assessment. For outcomes measured with scales, we present range of scores and direction of effect for each scale in Appendix 3.
HA: short stem versus standard stem
This comparison includes data from only one study with 151 participants (Lim 2020).
Critical outcomes
ADL, delirium, functional status, HRQoL, and unplanned return to theatre
Lim 2020 did not report data for these outcomes.
Mobility
Lim 2020 measured this outcome using dichotomised Koval's categories (see Appendix 3). We found no evidence of a difference in mobility at two years according to whether a short or standard stem was used (RR 0.98, 95% CI 0.72 to 1.34, favours standard stem; 1 study, 75 participants; Analysis 5.1).
5.1. Analysis.

Comparison 5: HA: short stem vs standard stem, Outcome 1: Mobility (24 months)
Mortality
Lim 2020 reported data for mortality at 24 months. The estimate was very imprecise; we found no evidence of a difference in mortality two years after surgery (RR 0.77, 95% CI 0.43 to 1.37, favours short stem; 1 study, 151 participants; Analysis 5.2).
5.2. Analysis.

Comparison 5: HA: short stem vs standard stem, Outcome 2: Mortality (24 months)
Other important outcomes
We found no evidence of a difference in pain according to whether a short stem or standard stem was used. We found no evidence of a difference in adverse events related to the implant or fracture, or both, according to whether a short stem or a standard stem was used (postoperative periprosthetic fracture, loosening, superficial infection, and dislocation). We report the summary effects of all these important outcomes and adverse effects in Table 15.
12. HA (short stem vs standard stem): effects of other important outcomes and adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Other important outcomes | ||||
| Pain (experiencing thigh pain; at 2 years) |
1 | Lim 2020 | 71 | RR 0.87, 95% CI 0.13 to 5.83 (favours short stem) Analysis 5.3 |
| Adverse events related to the implant or fracture, or both | ||||
| Postoperative periprosthetic fracture | 1 | Lim 2020 | 151 | RR 0.96, 95% CI 0.14 to 6.65 (favours short stem); Analysis 5.3 |
| Loosening | 1 | Lim 2020 | 151 | Not estimable. No events in either group |
| Superficial infection | 1 | Lim 2020 | 151 | Not estimable. No events in either group |
| Dislocation | 1 | Lim 2020 | 112 | RR 0.93, 95% CI 0.06 to 14.52 (favours short stem); Analysis 5.3 |
CI: confidence interval RR: risk ratio
HA: Exeter Trauma Stem (ETS) versus Thompson
This comparison includes two studies with 1164 participants (Parker 2012; Sims 2018).
Critical outcomes
ADL and functional status
Neither study reported data for these outcomes.
Delirium
Parker 2012 reported delirium; the estimate was very imprecise such that no meaningful inference was possible (RR 5.00, 95% CI 0.24 to 102.85, favours Thompson; 1 study, 200 participants; Analysis 6.1).
6.1. Analysis.

Comparison 6: HA: ETS vs Thompson, Outcome 1: Delirium
HRQoL
Sims 2018 reported HRQoL at four months, measured using EQ‐5D, in which higher scores indicate better HRQoL. We found that HRQoL was slightly improved when an ETS was used (MD 0.06, 95% CI 0.00 to 0.11, favours ETS; 1 study, 618 participants; Analysis 6.2). We noted that the CI is compatible with no difference or a small clinically important benefit with an ETS, based on a MCID of 0.07 (Walters 2005).
6.2. Analysis.

Comparison 6: HA: ETS vs Thompson, Outcome 2: Early HRQoL (≤ 4 months)
Mobility
Sims 2018 reported mobility, using categorical data according to whether participants could walk outdoors with or without a walking stick. We combined data for those that were freely mobile or able to walk outdoors with one walking stick, and found no evidence of a difference according to whether an ETS or a Thompson HA was used (RR 1.14, 95% CI 0.83 to 1.57, favours ETS; 1 study, 494 participants; Analysis 6.3). We report data for other categories in Appendix 6.
6.3. Analysis.

Comparison 6: HA: ETS vs Thompson, Outcome 3: Early mobility (freely mobile without aids, or able to walk outdoors with one aid)
In addition, Parker 2012 reported mean change in mobility at 3 months and 12 months after surgery. We did not calculate effect estimates for this study because the data were reported without an appropriate measure of variance. See Appendix 7 for mean scores as reported by study authors.
Mortality
Both studies reported mortality.
Early:
The estimate of this effect was imprecise, including clinically relevant benefits and harms. We found no evidence of a difference in mortality at up to four months from surgery (RR 1.20, 95% CI 0.76 to 1.88, favours Thompson; 2 studies, 1164 participants; I2 = 45%; Analysis 6.4).
6.4. Analysis.

Comparison 6: HA: ETS vs Thompson, Outcome 4: Early mortality (≤ 4 months)
At 12 months:
We also found no evidence of a difference in mortality at 12 months after surgery (RR 1.44, 95% CI 0.94 to 2.21, favours Thompson; 1 study, 200 participants; Analysis 6.5).
6.5. Analysis.

Comparison 6: HA: ETS vs Thompson, Outcome 5: Mortality (12 months)
Unplanned return to theatre
Both studies reported unplanned return to theatre. We found no evidence of a difference in unplanned return to theatre (RR 0.46, 95% CI 0.05 to 3.89, favours ETS; 2 studies, 1164 participants; I2 = 45%; Analysis 6.6). Re‐operations were due to dislocation and acetabular wear, and resolved with revision of the HA.
6.6. Analysis.

Comparison 6: HA: ETS vs Thompson, Outcome 6: Unplanned return to theatre (end of follow‐up)
Other important outcomes
We found no evidence of a difference in adverse events related to the implant or fracture, or both, according to whether an ETS or a Thompson HA was used (intraoperative periprosthetic fracture, deep or superficial infection, dislocation). We also found no evidence of a difference in adverse events unrelated to the implant or fracture, or both, according to whether a Thompson HA or ETS was used (acute kidney injury, blood transfusion, cerebrovascular accident, chest infection/pneumonia, myocardial infarction, DVT, or pulmonary embolism). We report the summary effects for these adverse events in Table 16. Additional outcome data for pain and length of stay is included in Appendix 7, since these data were reported without appropriate measures of variance such that we could not calculate effect estimates.
13. HA: ETS vs Thompson: effects of adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Adverse events related to the implant or fracture, or both | ||||
| Intraoperative periprosthetic fracture | 1 | Parker 2012 | 200 | RR 1.00, 95% CI 0.21 to 4.84 (favours neither); Analysis 6.7 |
| Deep infection | 1 | Parker 2012 | 200 | Not estimable; zero events in both groups |
| Superficial infection | 1 | Parker 2012 | 200 | RR 3.00, 95% CI 0.32 to 28.35 (favours Thompson); Analysis 6.7 |
| Dislocation | 1 | Parker 2012 | 200 | RR 0.20, 95% CI 0.01 to 4.11 (favours ETS); Analysis 6.7 |
| Adverse events unrelated to implant or fracture, or both | ||||
| Acute kidney injury | 1 | Parker 2012 | 200 | RR 1.00, 95% CI 0.06 to 15.77 (favours neither); Analysis 6.8 |
| Blood transfusion | 1 | Parker 2012 | 200 | RR 1.00, 95% CI 0.54 to 1.84 (favours neither); Analysis 6.8 |
| Cerebrovascular accident | 1 | Parker 2012 | 200 | RR 2.00, 95% CI 0.18 to 21.71 (favours Thompson); Analysis 6.8 |
| Chest infection/pneumonia | 1 | Parker 2012 | 200 | RR 1.67, 95% CI 0.41 to 6.79 (favours Thompson); Analysis 6.8 |
| Myocardial infarction | 1 | Parker 2012 | 200 | RR 5.00, 95% CI 0.24 to 102.85 (favours Thompson); Analysis 6.8 |
| Venous thromboembolic phenomena (DVT) | 1 | Parker 2012 | 200 | RR 1.00, 95% CI 0.21 to 4.84 (favours neither); Analysis 6.8 |
| Venous thromboembolic phenomena (pulmonary embolism) | 1 | Parker 2012 | 200 | Not estimable; zero events in both groups |
CI: confidence interval DVT: deep vein thrombosis ETS: Exeter trauma stem HA: hemiarthroplasty RR: risk ratio
Sensitivity analysis
We excluded Parker 2012 from the primary analysis of early mortality (at ≤ four months) and unplanned return to theatre because the study was at unclear risk of selection bias (for sequence generation). This did not alter our interpretation of the effect for these outcomes. Neither study in this comparison was at high risk of attrition bias.
HA: hydroxyapatite (HAC)‐coated Furlong versus Moore
This comparison includes one study with 82 participants and compares a first generation with a modern uncemented HA (Livesley 1993).
Critical outcomes
ADL, delirium, HRQoL, and mobility
Livesley 1993 did not report data for these outcomes.
Functional status
Livesley 1993 used a five‐point hip function assessment according to Benjamin 1990 to evaluate functional status at 12 months (higher scores indicate better function). We did not calculate effect estimates for this study because data were reported without an appropriate measure of variance. The study authors reported a mean of 33.0 for participants who had a Furlong prosthesis, and a mean of 27.3 for participants who had a Moore prosthesis.
Mortality
Early:
This effect estimate was imprecise. We found no evidence of a difference in mortality according to whether a Furlong or Moore HA was used (RR 0.35, 95% CI 0.07 to 1.82, favours Furlong; 1 study, 82 participants; Analysis 7.1).
7.1. Analysis.

Comparison 7: HA: Furlong vs Moore, Outcome 1: Early mortality (≤ 4 months)
At 12 months:
Similarly, we found an imprecise estimate at 12 months. There was no evidence of a difference in mortality according to the type of prosthesis (RR 0.81, 95% CI 0.46 to 1.43, favours Furlong; 1 study, 82 participants; Analysis 7.2).
7.2. Analysis.

Comparison 7: HA: Furlong vs Moore, Outcome 2: Mortality (12 months)
Unplanned return to theatre
Livesley 1993 reported unplanned return to theatre. The estimate was very imprecise, precluding meaningful interpretation. We found no evidence of a difference in mortality according to the type of prosthesis (RR 1.42, 95% CI 0.13 to 15.00, favours Moore; 1 study, 82 participants; Analysis 7.3). Re‐operations were because of pain, periprosthetic fracture, or infection. The types of re‐operation were not reported.
7.3. Analysis.

Comparison 7: HA: Furlong vs Moore, Outcome 3: Unplanned return to theatre (at end of follow‐up)
Other important outcomes
We found no evidence of a difference in pain at rest, or in adverse events related to the implant or fracture according to the type of prosthesis (periprosthetic fracture, superficial infection, or dislocation). We report the summary effects for these adverse events in Table 17.
14. HA: Furlong vs Austin‐Moore: effects of other important outcomes and adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Other important outcomes | ||||
| Pain at rest (at 12 months) | 1 | Livesley 1993 | 82 | RR 0.71, 95% CI 0.22 to 2.26 (favours Furlong); Analysis 7.4 |
| Adverse events related to surgery | ||||
| Periprosthetic fracture | 1 | Livesley 1993 | 82 | RR 10.71, 95% CI 0.63 to 181.50 (favours Moore); Analysis 7.5 |
| Superficial infection | 1 | Livesley 1993 | 82 | RR 0.71, 95% CI 0.05 10.93 (favours Furlong); Analysis 7.5 |
| Dislocation | 1 | Livesley 1993 | 82 | RR 2.14, 95% CI 0.09 to 51.07 (favours Moore); Analysis 7.5 |
CI: confidence interval RR: risk ratio
4. THA versus HA
This comparison includes 17 studies with 3232 participants (Baker 2006; Blomfeldt 2007; Cadossi 2013; Chammout 2019; Dorr 1986; HEALTH 2019; Iorio 2019; Keating 2006; Macaulay 2008; Mouzopoulos 2008; Parker 2019; Ravikumar 2000; Ren 2017; Sharma 2016; Sonaje 2017; Van den Bekerom 2010; Xu 2017). A summary of the implant and study characteristics is presented in Table 9. Whilst most designs of HA used in this comparison were modern, one study included a first generation uncemented HA (Ravikumar 2000), and Sharma 2016 did not specify whether a first generation or modern design was used.
Here we report effects for critical outcomes. Where analyses included at least one study in each category, we subgrouped the analysis according to whether studies used a first generation or modern HA stem design in one of the intervention groups.
We used GRADE to assess the certainty of the evidence for the critical outcomes measured within four months of surgery (ADL, functional status, HRQoL, and mobility), within four months and at 12 months for mortality, and at the end of follow‐up for delirium and unplanned return to theatre. See Table 3.
We summarise the effects of other important review outcomes in a table, which are not subgrouped by stem design, and these outcomes are reported without GRADE assessments. For outcomes measured with scales, we present range of scores and direction of effect for each scale in Appendix 3.
Critical outcomes
ADL
Four studies reported performance of ADL (Blomfeldt 2007; Chammout 2019; Mouzopoulos 2008; Parker 2019).
Early:
We could only combine data from two of the studies. Blomfeldt 2007 used the Katz Index to identify participants that were independent (Katz 1963), and Chammout 2019 did not describe a measurement tool for this outcome. We found evidence that any difference in the number of people who were independent in ADL within four months of surgery is likely to be small (RR 1.03, 95% CI 0.91 to 1.18, favours THA; 2 studies, 225 participants; I2 = 0%; very low‐certainty evidence; Analysis 8.1). We downgraded the certainty of the evidence by one level for imprecision because the evidence included few participants, and two levels for study limitations because one of the studies had unclear risks of selection bias, and we found during sensitivity analyses that this may influence the direction of the estimate.
Parker 2019 used a social mobility scale, in which lower scores indicate more independence. We found no evidence of a difference in mobility at 3 months (MD ‐0.10, 95% CI ‐0.46 to 0.26, favours THA; 1 study, 83 participants; Analysis 8.2).
8.1. Analysis.

Comparison 8: THA vs HA, Outcome 1: Early ADL (≤ 4 months, using categorical data)
8.2. Analysis.

Comparison 8: THA vs HA, Outcome 2: Early ADL (≤ 4 months; using social mobility scale (lower scores indicate better mobility)
At 12 months:
We also found no evidence of a difference in the number of people who were independent in ADL at 12 months in Blomfeldt 2007 and Chammout 2019 (RR 0.96, 95% CI 0.86 to 1.07, favours HA; 2 studies, 217 participants; I2 = 0%; Analysis 8.3).
We also considered data from two studies that used continuous data, measured at 12 months (Mouzopoulos 2008; Parker 2019). Parker 2019 used a social mobility scale, in which lower scores indicate more independence. Mouzopoulos 2008 used the Barthel Index, in which higher scores indicate more independence; accordingly, we inverted the data from Mouzopoulos 2008 in this analysis. However, we did not pool these data owing to substantial statistical heterogeneity (I2= 80%). Data from individual studies are reported in Analysis 8.4.
8.3. Analysis.

Comparison 8: THA vs HA, Outcome 3: ADL (12 months, using categorical data)
8.4. Analysis.

Comparison 8: THA vs HA, Outcome 4: ADL (12 months; using different measurement tools; lower scores indicate more independence))
Late:
Mouzopoulos 2008 also reported data at four years from surgery, and we found no evidence of a difference in performance of ADL at this later time point (MD 5.70, 95% CI 0.21 to 11.19, favours THA; 1 study, 43 participants; very low‐certainty evidence; Analysis 8.5).
8.5. Analysis.

Comparison 8: THA vs HA, Outcome 5: Late ADL (> 24 months; using Barthel Index, range of scores from 0 to 100; higher scores indicate more independence)
Delirium
Two studies measured delirium at 12 months (Parker 2019; Van den Bekerom 2010). We found no evidence of a difference in delirium according to the type of arthroplasty (RR 1.41, 95% CI 0.60 to 3.33, favours HA; 2 studies, 357 participants; low‐certainty evidence; Analysis 8.6). We downgraded the certainty of the evidence by two levels ‐ one level for imprecision because we noted a wide CI in the effect, and one level for study limitations because of unclear risks of bias.
8.6. Analysis.

Comparison 8: THA vs HA, Outcome 6: Delirium
Functional status
Thirteen studies reported functional status, and all studies used modern stem designs in both intervention groups (Baker 2006; Blomfeldt 2007; Cadossi 2013; Chammout 2019; HEALTH 2019; Keating 2006; Macaulay 2008; Mouzopoulos 2008; Ren 2017; Sharma 2016; Sonaje 2017; Van den Bekerom 2010; Xu 2017).
Early:
Blomfeldt 2007 and Chammout 2019 reported mean data within four months of surgery using the HHS, and Keating 2006 reported mean scores using the Johansen hip score; in both scales, higher scores indicate better function. We found improved function within four months of surgery in people who received a THA (SMD 0.27, 95% CI 0.07 to 0.47, favours THA; 3 studies, 395 participants; very low‐certainty evidence; I2 = 0%; Analysis 8.7). After converting this effect estimate to the HHS, there appeared to be no clinically important difference in functional status between THAs and HAs (MD 3.44, 95% CI 0.89 to 5.98); this was based on a MCID for HHS of 16 to 18 (Singh 2016).
In addition, Cadossi 2013 reported function using the HHS. We could not calculate effect estimates for this study because data were reported without an appropriate measure of variance. See Appendix 8 for mean scores as reported by study authors.
We downgraded the evidence by three levels to very low certainty ‐ one level for imprecision because the evidence included few participants, and two levels for study limitations because some studies had high and unclear risks of bias, and we found during sensitivity analysis that the direction of effect estimate was influenced by these studies.
8.7. Analysis.

Comparison 8: THA vs HA, Outcome 7: Early functional status (≤ 4 months)
At 12 months:
Six studies reported functional status using the HHS, in which higher scores indicate better function (Blomfeldt 2007; Chammout 2019; Macaulay 2008; Mouzopoulos 2008; Sonaje 2017; Xu 2017); one study reported this outcome using the Johansen hip score in which higher scores indicate better function (Keating 2006); and one study reported this outcome using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (HEALTH 2019). Because the WOMAC score has an opposite direction of effect (i.e. lower scores indicate better function), we inverted the data in HEALTH 2019. We found improved function at 12 months in people who had a THA (SMD 0.23, 95% CI 0.14 to 0.44, favours THA; 8 studies, 1273 participants; I2 = 0%; Analysis 8.8; Figure 6). After converting this effect estimate to the HHS, there appeared to be no clinically important difference in functional status between THAs and HAs (MD 2.23, 95% CI 1.35 to 4.26); this was based on a MCID for HHS of 16 to 18 (Singh 2016).
Ren 2017 and Sonaje 2017 reported categorical data using the HHS; ranges of scores were reported as excellent, good, medium, or poor, and we combined data for scores which were excellent and good. The time point of measurement was not reported in Ren 2017, and was at 24 months in Sonaje 2017. We found evidence that any difference in excellent and good scores is likely to be small (RR 1.07, 95% CI 0.98 to 1.17, favours THA; 2 studies, 140 participants; I2 = 0%; Analysis 8.9). We report data for other categories in Appendix 6.
In addition, three studies reported data at 12 months using the HHS (Cadossi 2013; Sharma 2016; Van den Bekerom 2010). We did not calculate effect estimates for these studies because data were reported without an appropriate measure of variance. See Appendix 8 for mean scores as reported by study authors.
8.8. Analysis.

Comparison 8: THA vs HA, Outcome 8: Functional status (12 months)
6.

Total hip arthroplasty versus hemiarthroplasty. Forest plot of functional status at 12 months
8.9. Analysis.

Comparison 8: THA vs HA, Outcome 9: Functional status (HHS; excellent or good)
Late:
Three studies reported mean scores using the HHS at more than 24 months since surgery (Blomfeldt 2007; Mouzopoulos 2008; Xu 2017), and one study used the Oxford Hip Score (Baker 2006). Time points of measurement were at four years (Blomfeldt 2007; Mouzopoulos 2008), five years (Xu 2017), and nine years (Baker 2006). We found that hip function was improved when a THA was used (SMD 0.65, 95% CI 0.23 to 1.08, favours THA; 4 studies, 224 participants; I2 = 56%; Analysis 8.10). We noted that this effect did not suggest a clinically important improvement in hip function (based on a MCID of 16 to 18 points; Singh 2016).
In addition, two studies reported late data at three years using the HHS (Cadossi 2013), and at five years using the HHS (Van den Bekerom 2010). We did not calculate effect estimates for these studies because data were reported without an appropriate measure of variance. See Appendix 8 for mean scores as reported by study authors.
8.10. Analysis.

Comparison 8: THA vs HA, Outcome 10: Late functional status (> 24 months; using OHS and HHS; higher scores indicate better function)
HRQoL
Five studies reported HRQoL (Baker 2006; Chammout 2019; HEALTH 2019; Keating 2006; Macaulay 2008).
Early:
Two studies reported EQ‐5D at four months after surgery (Chammout 2019; Keating 2006); in this scale, higher scores indicate improved quality of life. We found no evidence of a difference in HRQoL at four months after surgery according to the type of arthroplasty (MD 0.03, 95% CI ‐0.06 to 0.12, favours THA; 2 studies, 279 participants; I2 = 51%; very low‐certainty evidence; Analysis 8.11). We downgraded the certainty of the evidence by three levels ‐ two levels for imprecision because the evidence was compatible with no difference and a clinically meaningful difference (based on a MCID for EQ‐5D of 0.07; Walters 2005), and one level for study limitations because studies had high and unclear risks of bias.
8.11. Analysis.

Comparison 8: THA vs HA, Outcome 11: Early HRQoL (≤ 4 months)
At 12 months:
Four studies reported this outcome at 12 months using EQ‐5D (Chammout 2019; HEALTH 2019; Keating 2006), or SF‐36 (Macaulay 2008); in both scales, higher scores indicate improved quality of life. We found that HRQoL at 12 months was improved when a THA was used (SMD 0.19, 95% CI 0.07 to 0.31, favours THA; 4 studies, 1158 participants; I2 = 0%; moderate‐certainty evidence; Analysis 8.12). After converting this effect estimate to the EQ‐5D scale, it is likely that the evidence is most compatible with no clinically important difference in HRQoL between THAs and HAs (0.05, 95% CI 0.02 to 0.08): this was based on a MCID for EQ‐5D of 0.07 (Walters 2005).
8.12. Analysis.

Comparison 8: THA vs HA, Outcome 12: HRQoL (12 months)
Late:
In addition, one study also reported HRQoL using SF‐36 at nine years (Baker 2006). This effect was imprecise; we found no evidence of a difference in health‐related quality of life (MD 5.90, 95% CI ‐1.99 to 13.79, favours THA; 1 study, 34 participants; Analysis 8.13).
8.13. Analysis.

Comparison 8: THA vs HA, Outcome 13: HRQoL (> 24 months. Using SF‐36; higher scores indicate better quality of life)
Mobility
Five studies reported mobility (Baker 2006; HEALTH 2019; Macaulay 2008; Parker 2019; Ravikumar 2000).
Early:
Parker 2019 used a nine‐point mobility scale, with lower scores indicating better mobility. We found no evidence of a difference in mobility at three months after surgery (MD ‐0.40, 95% CI ‐0.96 to 0.16, favours THA; 1 study, 83 participants; low‐certainty evidence; Analysis 8.14). We downgraded the evidence by one level for imprecision because the evidence included few participants, and one level for study limitations because the study included unclear risks of bias.
Dorr 1986 reported mobility using a six‐point scale to describe ambulation. We did not calculate effect estimates for this study because data were reported without an appropriate measure of variance. See Appendix 8 for mean scores as reported by study authors.
8.14. Analysis.

Comparison 8: THA vs HA, Outcome 14: Early mobility (≤ 4 months; lower scores indicate better mobility
At 12 months:
We combined data from two studies which used the Timed Up and Go (TUG) test to measure mobility (Macaulay 2008; HEALTH 2019); lower values (a shorter length of time) indicate better mobility. We found no evidence of a difference in mobility (MD ‐2.74, 95% CI ‐6.82 to 1.35, favours THA; 2 studies, 575 participants; I2 = 9%; Analysis 8.15).
Parker 2019 used a nine‐point mobility scale, with lower scores indicating better mobility. We found no evidence of a difference in mobility according to the type of arthroplasty (MD 0.40, 95% CI ‐0.32 to 1.12, favours HA; 1 study, 78 participants; Analysis 8.16).
Macaulay 2008 and Ravikumar 2000 reported the number of people who were able to ambulate independently at 12 months. We also found no evidence of a difference according to the type of arthroplasty with this mobility measure (RR 0.96, 95% CI 0.71 to 1.31, favours HA; 2 studies, 175 participants; I2 = 49%; Analysis 8.17).
In addition, one study reported mobility using a six‐point scale to describe ambulation (Dorr 1986). We did not calculate effect estimates for this study because data were reported without an appropriate measure of variance. See Appendix 8 for mean scores as reported by study authors.
8.15. Analysis.

Comparison 8: THA vs HA, Outcome 15: Mobility (12 months, using TUG; lower values indicate better mobility)
8.16. Analysis.

Comparison 8: THA vs HA, Outcome 16: Mobility (12 months, using 9‐point mobility scale; lower scores indicate better mobility)
8.17. Analysis.

Comparison 8: THA vs HA, Outcome 17: Mobility (12 months; able to ambulate independently)
Late:
Ravikumar 2000 also reported the number of people able to ambulate independently at the end of the study follow‐up, which was at 13 years. We found no evidence of a difference in mobility according to the type of arthroplasty (RR 1.27, 95% CI 0.71 to 2.29, favours THA; 1 study, 32 participants; Analysis 8.18).
8.18. Analysis.

Comparison 8: THA vs HA, Outcome 18: Late mobility (> 24 months; able to ambulate independently)
Mortality
Fourteen studies reported mortality (Baker 2006; Blomfeldt 2007; Cadossi 2013; Chammout 2019; HEALTH 2019; Iorio 2019; Keating 2006; Macaulay 2008; Mouzopoulos 2008; Parker 2019; Ravikumar 2000; Sharma 2016; Van den Bekerom 2010; Xu 2017).
Early:
The effect estimate for mortality within four months of surgery was very imprecise, including clinically relevant benefits and harms. We found no evidence of a difference (RR 0.77, 95% CI 0.42 to 1.42, favours THA; 6 studies, 725 participants; I2 = 0%; very low‐certainty evidence; Analysis 8.19). We downgraded the certainty of the evidence by two levels for imprecision because the wide CI included relevant benefits and harms, and one level for study limitations because included studies had high and unclear risks of bias.
8.19. Analysis.

Comparison 8: THA vs HA, Outcome 19: Early mortality (≤ 4 months)
At 12 months:
We found no evidence of a difference in mortality at 12 months from surgery according to the type of arthroplasty (RR 1.00, 95% CI 0.83 to 1.22, favours THA; 11 studies, 2667 participants; I2 = 0%; moderate‐certainty evidence; Analysis 8.20). We downgraded the certainty of the evidence by one level because included studies had high and unclear risks of bias. We generated a funnel plot which showed no evidence of publication bias from visual inspection; we also found no statistical evidence of small study size effects (Harbord modified test, P value = 0.966).
8.20. Analysis.

Comparison 8: THA vs HA, Outcome 20: Mortality (12 months)
Late:
Seven studies reported a late follow‐up after surgery. These data were reported at 36 months (Cadossi 2013), 39 months (Baker 2006), 48 months (Blomfeldt 2007; Mouzopoulos 2008), 60 months (Van den Bekerom 2010; Xu 2017), and 13 years (Ravikumar 2000). We found no evidence of a difference at this late follow‐up according to the type of arthroplasty (RR 1.00, 95% CI 0.81 to 1.23, favours HA; 7 studies, 891 participants; I2 = 46%; Analysis 8.21).
8.21. Analysis.

Comparison 8: THA vs HA, Outcome 21: Late mortality (> 24 months)
Unplanned return to theatre
Ten studies reported unplanned return to theatre, which was reported at 12 months (Iorio 2019, Parker 2019), 24 months (Chammout 2019; HEALTH 2019, Keating 2006), 39 months (Baker 2006), 48 months (Dorr 1986; Mouzopoulos 2008), 60 months (Van den Bekerom 2010), and 13 years (Ravikumar 2000). We found no evidence of a difference in unplanned return to theatre according to the type of arthroplasty (RR 0.63, 95% CI 0.37 to 1.07, favours THA; 10 studies, 2594 participants; I2 = 40%; low‐certainty evidence; Analysis 8.22). Some re‐operations were because of dislocation, acetabular wear, pain, periprosthetic fracture or infection. We noted that types of re‐operation included replacement with THA, revised HA, open reduction, and drainage of infection. We downgraded the certainty of the evidence by one level for imprecision because the wide CI was consistent with both benefit and harms, and one level for study limitations because the evidence included studies with high and unclear risks of bias which included high risks of detection bias.
8.22. Analysis.

Comparison 8: THA vs HA, Outcome 22: Unplanned return to theatre (end of follow‐up)
Other important outcomes
We found substantial levels of statistical heterogeneity in data for some pain outcomes, and did pool data in these instances. We found no evidence of a difference in discharge destination according to the type of arthroplasty. We also found no evidence of a difference in adverse events related to the implant or fracture, or both (periprosthetic fracture, loosening, deep infection, superficial infection, dislocation) or in adverse events unrelated to the implant or fracture, or both (acute kidney injury, cerebrovascular accident, chest infection/pneumonia, myocardial infarction, urinary tract infection, deep vein thrombosis, and pulmonary embolism). We found that fewer participants had a blood transfusion when a HA was used; however, this analysis was from only two small studies. We report the summary effects of all these important outcomes and adverse effects in Table 18.
15. THA vs HA: effects of other important outcomes and adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Other important outcomes | ||||
| Paina (reported at ≤ 4 months) Using Hip Rating Questionnaire or HHS (higher scores indicate less pain), and VAS and 8‐point pain scale (lower scores indicate less pain; data inverted in meta‐analysis) |
5 | Blomfeldt 2007; Cadossi 2013; Chammout 2019; Keating 2006; Parker 2019 | 572 | SMD 0.10, 95% CI ‐0.10 to 0.30 (favours THA); Analysis 8.27 |
| Paina (at 12 months) Using VAS, 8‐point pain scale or WOMAC (lower scores indicate less pain); and Hip Rating Questionnaire, WOMACb or HHS (higher scores indicate less pain; data inverted in meta‐analysis) Follow‐up: 12 months and 24 months |
7 | Blomfeldt 2007; Cadossi 2013; Chammout 2019; HEALTH 2019; Keating 2006; Macaulay 2008; Parker 2019; Sonaje 2017 | 1359 | SMD ‐0.19, 95% CI ‐0.44 to 0.06 (favours THA); I2 = 73%; Analysis 8.24 |
| Pain (> 24 months) Using HHS (higher scores indicate less pain) Follow‐up: 48 months |
2 | Blomfeldt 2007; Cadossi 2013 | 83 | We did not combine data because of substantial statistical heterogeneity (I2 = 96%); Analysis 8.25 |
| Pain (> 24 months) Using categorical data; we report data for those experiencing no painc Follow‐up: 13 years |
1 | Ravikumar 2000 | 135 | RR 1.47, 95% CI 1.07 to 2.00 (favours THA); Analysis 8.26 |
| Length of hospital staya | 4 | Keating 2006; Macaulay 2008; Mouzopoulos 2008; Xu 2017 | 382 | MD 0.72 days, 95% CI ‐0.21 to 1.64 (favours HA); Analysis 8.23 |
| Discharge destination (own home) | 2 | HEALTH 2019; Keating 2006 | 1612 | RR 0.97, 95% CI 0.87 to 1.08 (favours HA); Analysis 8.28 |
| Discharge destination (geriatric ward) | 1 | Chammout 2019 | 120 | RR 0.88, 95% CI 0.34 to 2.26 (favours HA); Analysis 8.29 |
| Adverse events related to the implant or fracture, or both | ||||
| Postoperative periprosthetic fracture | 3 | HEALTH 2019; Sonaje 2017; Xu 2017 | 1557 | RR 1.08, 95% CI 0.70 to 1.66 (favours HA); Analysis 8.30 |
| Prosthetic loosening | 4 | Blomfeldt 2007; HEALTH 2019; Van den Bekerom 2010; Xu 2017 | 1889 | RR 0.64, 95% CI 0.17 to 2.41 (favours THA); Analysis 8.30 |
| Deep infection | 8 | Chammout 2019; Dorr 1986; HEALTH 2019; Parker 2019; Ravikumar 2000; Sharma 2016; Xu 2017; Van den Bekerom 2010 | 2343 | RR 0.87, 95% CI 0.50 to 1.54 (favours THA); Analysis 8.30 |
| Superficial infection | 10 | Baker 2006; Blomfeldt 2007; Chammout 2019; Dorr 1986; HEALTH 2019; Keating 2006; Macaulay 2008; Parker 2019; Sharma 2016; Van den Bekerom 2010 | 2495 | RR 1.25, 95% CI 0.67 to 2.30 (favours HA); Analysis 8.30 |
| Dislocation | 12 | Baker 2006; Blomfeldt 2007; Chammout 2019;Dorr 1986; HEALTH 2019; Iorio 2019; Keating 2006; Macaulay 2008; Ravikumar 2000; Sharma 2016; Van den Bekerom 2010; Xu 2017 | 2719 | RR 1.96, 95% CI 1.17 to 3.27 (favours HA); Analysis 8.30 |
| Adverse events unrelated to the implant or fracture, or both | ||||
| Acute kidney injury | 2 | Chammout 2019; HEALTH 2019 | 1561 | RR 1.09, 95% CI 0.62 to 1.92 (favours HA); Analysis 8.31 |
| Blood transfusion | 2 | Keating 2006; Parker 2019 | 285 | RR 2.14, 95% CI 1.27 to 3.61 (favours HA); Analysis 8.31 |
| Cerebrovascular accident | 4 | Chammout 2019; Keating 2006; Parker 2019; Van den Bekerom 2010 | 657 | RR 1.63, 95% CI 0.63 to 4.21 (favours HA); Analysis 8.31 |
| Chest infection/pneumonia (reported at > 4 months) | 5 | Baker 2006; Blomfeldt 2007; Chammout 2019; Macaulay 2008; Van den Bekerom 2010 | 613 | RR 0.87, 95% CI 0.38 to 2.00 (favours THA); Analysis 8.31 |
| Myocardial infarction | 4 | Blomfeldt 2007; Chammout 2019; Keating 2006; Macaulay 2008 | 460 | RR 1.48, 95% CI 0.48, 4.58 (favours HA); Analysis 8.31 |
| Urinary tract infection | 1 | Macaulay 2008 | 40 | RR 0.19, 95% CI 0.01 to 3.46 (favours THA); Analysis 8.31 |
| Venous thromboembolic phenomena (DVT) | 4 | Baker 2006; Blomfeldt 2007; Keating 2006; Parker 2019 | 486 | RR 4.25, 95% CI 0.86 to 21.06 (favours HA); Analysis 8.31 |
| Venous thromboembolic phenomena (pulmonary embolism) | 5 | Baker 2006; Chammout 2019; Keating 2006; Macaulay 2008; Van den Bekerom 2010 | 673 | RR 0.49, 95% CI 0.14 to 1.63 (favours THA); Analysis 8.31 |
|
aAdditional data are reported in Appendix 8. We did not calculate effect estimates for the data in Appendix 8 because study authors did not report distribution variables that we required for analysis. bTwo studies reported data from different versions of the WOMAC scale, with opposite directions of effect. We inverted the data from one of these studies so that the direction was consistent across the analysis. cData for additional categories are reported in Appendix 5. | ||||
CI: confidence interval DVT: deep vein thrombosis HA: hemiarthroplasty MD: mean difference RR: risk ratio SMD: standardised mean difference THA: total hip arthroplasty VAS: visual analogue scale WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Sensitivity analysis
We performed sensitivity analysis on critical outcomes in which data were available from more than one study for risk of bias judgements (sequence generation and attrition bias). We did not perform sensitivity analysis on mixed populations because most studies reported insufficient information for us to judge whether participants' characteristics in the included studies were mixed. We did not perform sensitivity analysis according to whether interventions are no longer in current use since this was not relevant.
Here, we report the findings of sensitivity analyses only for those outcomes in which we noted an effect which differed in interpretation to the primary analysis.
High or unclear risk of selection bias (for sequence generation)
Early ADL (≤ 4 months; categorical data): we excluded Blomfeldt 2007 from the primary analysis. Only one study remained in analysis. Although the estimate continued to show no evidence of a difference in performance of ADL, we noted the direction favoured the alternative intervention (RR 1.00, 95% CI 0.78 to 1.29, favours HA; 1 study, 111 participants).
Early functional status (≤ 4 months; continuous data): we excluded Blomfeldt 2007 and Keating 2006 from the primary analysis. Only one study remained in analysis; this estimate no longer indicated a benefit in favour of HA (MD 1.00, 95% CI ‐4.03 to 6.03; 1 study, 111 participants).
Late functional status (at > 24 months): we excluded two studies from the primary analysis (Blomfeldt 2007; Mouzopoulos 2008), and found that the effect estimate no longer demonstrated an improvement in hip function when THA was used (MD 4.83, 95% CI 0.48 to 9.18; 1 study, 64 participants).
Early HRQoL (≤ 4 months): we excluded Keating 2006 from the primary analysis. Only one study remained in analysis. Although the estimate continued to show no evidence of a difference in early HRQoL, we noted the direction favoured the alternative intervention (MD ‐0.02, 95% CI ‐0.11 to 0.07, favours HA; 1 study, 111 participants).
High risk of attrition bias
HRQoL (at 12 months): we excluded HEALTH 2019 from the primary analysis, including only studies at low risk of attrition bias for this outcome. We found that the effect estimate no longer showed evidence of a difference between interventions (SMD 0.17, 95% CI ‐0.05 to 0.40; 4 studies, 314 participants; I2 = 0%).
5. Single versus multiple articulations of THA
This comparison included two studies with 83 participants in which a standard cup (single articulation) was compared to a dual‐mobility cup (Griffin 2016; Rashed 2020). A summary of the implant and study characteristics is presented in Table 10. For outcomes measured with scales, we present range of scores and direction of effect for each scale in Appendix 3.
Critical outcomes
ADL, delirium, mobility, and unplanned return to theatre
Neither study reported data for these outcomes.
Functional status
This outcome was measured using the Oxford Hip Score in Griffin 2016, and the HHS in Rashed 2020. In both scales, higher scores indicate better function.
Early:
We found no evidence of a difference in functional status within four months of surgery according to the articulation type (SMD ‐0.33, 95% CI ‐0.78 to 0.12, favours dual‐mobility; 2 studies, 78 participants; I2 = 0%; Analysis 9.1).
9.1. Analysis.

Comparison 9: THA: single articulation vs dual‐mobility, Outcome 1: Early functional status (≤ 4 months, using different scales; higher scores indicate better function)
At 12 months:
When measured at 12 months, we found that functional status was improved when a dual‐mobility cup was used (SMD ‐0.60, 95% CI ‐1.05 to ‐0.15, favours dual‐mobility; 2 studies, 79 participants; I2 = 0%; Analysis 9.2).
9.2. Analysis.

Comparison 9: THA: single articulation vs dual‐mobility, Outcome 2: Functional status (12 months, using OHS and HHS; higher scores indicate better function)
HRQoL
Only one study reported HRQoL (Griffin 2016). This was measured using EQ‐5D, with a range of scores from 0 to 1 (higher scores indicate better quality of life).
Early:
We found no evidence of a difference in HRQoL at four months after surgery according to the articulation (MD 0.24, 95% CI ‐0.21 to 0.69, favours single articulation; 1 study, 16 participants; Analysis 9.3).
9.3. Analysis.

Comparison 9: THA: single articulation vs dual‐mobility, Outcome 3: HRQoL (using EQ‐5D, range of scores from 0 to 1; higher scores indicate better quality of life)
At 12 months:
We found improved HRQoL at 12 months after surgery when a standard cup was used (MD 0.30, 95% CI 0.08 to 0.52, favours single articulation; 1 study, 19 participants; Analysis 9.3).
Mortality
Both studies reported mortality (Griffin 2016; Rashed 2020). We found no evidence of a difference in mortality at 12 months after surgery according to whether a dual‐mobility cup or a standard cup was used with the THA (RR 0.62, 95% CI 0.08 to 4.77, favours single articulation; 2 studies, 82 participants; I2 = 0%; Analysis 9.4).
9.4. Analysis.

Comparison 9: THA: single articulation vs dual‐mobility, Outcome 4: Mortality (12 months)
Other important outcomes
We found no evidence of a difference in adverse events related to the implant or fracture, or both (deep infection, superficial infection, and dislocation); we noted zero events for dislocation from two small studies. We also found no evidence of a difference in adverse events unrelated to the implant or fracture, or both (DVT). We report the summary statistics for these adverse events in Table 19.
16. THA (dual‐mobility cup vs standard cup): effects of other important outcomes and adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Adverse events related to implant or fracture, or both | ||||
| Deep infection | 1 | Rashed 2020 | 62 | RR 1.00, 95% CI 0.07 to 15.28 (favours neither); Analysis 9.5 |
| Superficial infection | 1 | Rashed 2020 | 62 | RR 3.00, 95% CI 0.33 to 27.29 (favours DM); Analysis 9.5 |
| Dislocation | 2 | Griffin 2016; Rashed 2020 | 82 | Not estimable; zero events in both groups |
| Adverse events unrelated to implant or fracture, or both | ||||
| Venous thromboembolic phenomena | 1 | Rashed 2020 | 62 | RR 0.33, 95% CI 0.01 to 7.88 (favours single); Analysis 9.6 |
CI: confidence interval DM: dual‐mobility RR: risk ratio
6. Short stem versus standard stem of THA
This comparison includes only one study with 161 participants, comparing a short stem THA with a standard stem (Kim 2012). A summary of the implant and study characteristics is presented in Table 10. For outcomes measured with scales, we present range of scores and direction of effect for each scale in Appendix 3.
Critical outcomes
ADL, delirium, HRQoL, and unplanned return to theatre
Kim 2012 did not report data for these outcomes.
Functional status
Kim 2012 reported functional status measured using the HHS. We found no evidence of a difference in functional status at 24 months after surgery according to whether a short or standard stem was used in the THA, when measured with the HHS (MD ‐0.40, 95% CI ‐3.19 to 2.39, favours standard stem; 1 study, 140 participants; Analysis 10.1).
10.1. Analysis.

Comparison 10: THA: short stem vs standard stem, Outcome 1: Functional status (at 24 months; using HHS, range of scores from 0 to 100; higher scores indicate better function)
Mobility
Kim 2012 reported mobility using categorical data according to distance walked (walks > six blocks with or without aids, walks < six blocks, walks indoors only). We found no evidence of a difference in being able to walk more than six blocks with or without aids at 24 months according to whether a short or standard stem was used in the THA (RR 1.10, 95% CI 0.84 to 1.44, favours short stem; 1 study, 424 participants; Analysis 10.2). We report data for other categories in Appendix 6.
10.2. Analysis.

Comparison 10: THA: short stem vs standard stem, Outcome 2: Mobility
Mortality
We found no evidence of a difference in mortality at 12 months from surgery according to whether a short or standard stem was used in the THA (RR 1.20, 95% CI 0.38 to 3.78, favours standard stem; 1 study, 161 participants; Analysis 10.3).
10.3. Analysis.

Comparison 10: THA: short stem vs standard stem, Outcome 3: Mortality (12 months)
Other important outcomes
We found no evidence of a difference in pain according to whether a short or standard stem was used. We found no evidence of a difference in some adverse events related to the implant or fracture, or both (superficial infection and dislocation), and for adverse events unrelated to the implant or fracture, or both (acute kidney injury, pneumonia, urinary tract infection). We noted fewer intraoperative periprosthetic fractures when a short stem was used, but, as for all adverse events, data were available from only one small study (Kim 2012). We report the summary effects of important outcomes and adverse events in Table 20.
17. THA (short stem vs standard stem): effects of other important outcomes and adverse events.
| Outcome | Number of studies | Studies | Participants | Effect estimate |
| Other important outcomes | ||||
| Pain Number of people experiencing thigh pain at 24 months |
1 | Kim 2012 | 140 | RR 0.04, 95% CI 0.00 to 0.72 (favours short stem); Analysis 10.4 |
| Adverse events related to implant or fracture, or both | ||||
| Intraoperative periprosthetic fracture | 1 | Kim 2012 | 140 | RR 0.13, 95% CI 0.02 to 0.97 (favours short stem); Analysis 10.5 |
| Superficial infection | 1 | Kim 2012 | 140 | RR 1.00, 95% CI 0.06 to 15.67 (favours neither); Analysis 10.5 |
| Dislocation | 1 | Kim 2012 | 140 | RR 0.25, 95% CI 0.03 to 2.18 (favours short stem); Analysis 10.5 |
| Adverse events unrelated to implant or fracture, or both | ||||
| Acute kidney injury | 1 | Kim 2012 | 140 | RR 0.50, 95% CI 0.05 to 5.39 (favours short stem); Analysis 10.6 |
| Chest infection/pneumonia | 1 | Kim 2012 | 140 | RR 0.67, 95% CI 0.11 to 3.87 (favours short stem); Analysis 10.6 |
| Urinary tract infection | 1 | Kim 2012 | 140 | RR 0.47, 95% CI 0.20 to 1.07 (favours short stem); Analysis 10.6 |
CI: confidence interval RR: risk ratio
Discussion
Summary of main results
We included 58 studies (50 RCTs, eight quasi‐RCTs) with 10,654 participants with 10,662 hip fractures. All hip fractures were intracapsular, except in one study that included only extracapsular fractures. We also identified seven ongoing studies with an estimated recruitment of 7199 participants.
We found evidence for 10 different comparisons of types of arthroplasties. We report below the main findings of three of these comparisons, representing the most substantial bodies of evidence in the review.
Cemented versus uncemented HA (17 studies, 3644 participants)
Eight studies compared cemented prostheses with first‐generation uncemented prostheses, and nine studies with modern uncemented prostheses. Moderate‐certainty evidence indicated no clinically important difference between interventions in performance of ADL and independent mobility at four months. The estimates for treatment effects in delirium, the risk of mortality within four months of surgery, and unplanned return to theatre were imprecise, of low certainty, and compatible with clinically relevant benefits and harms. There were, however, statistically significant benefits with cemented prostheses in HRQoL at 4 months, and mortality at 12 months, with moderate‐certainty evidence. The magnitude of these effects were compatible with small to large benefits. The evidence for function was of very low certainty, and although the estimate included benefits and harms, these were not clinically important. Subgroup analysis by uncemented prosthesis design suggested that the mortality benefit from cemented prostheses cannot be explained by higher mortality reported in the uncemented group from studies including first‐generation prostheses.
There was no difference in the overall risk of adverse events. However, within this overall risk profile, we found evidence that the risk of intra‐ and postoperative periprosthetic fracture was lower with cemented HA, but the risk of pulmonary embolic events was greater.
We analysed the data for extracapsular fractures separately, and found very low‐certainty evidence of an improvement in functional status within four months of surgery. This difference may be clinically important.
Bipolar HA versus unipolar HA (13 studies, 1499 participants)
Prostheses were fixed with cement in nine studies, and without cement in three studies. No studies reported performance of ADL or functional status within four months of surgery. For the outcomes of delirium, HRQoL within four months of surgery, and unplanned return to theatre, the evidence was of very low certainty, and plausibly included clinically relevant benefits and harms. For mortality at both 4 and 12 months from surgery, the evidence was of low certainty, and plausibly included clinically relevant benefits and harms.
We found no difference in the overall risk of adverse events.
THA versus HA (17 studies, 3232 participants)
We found very low‐certainty evidence in performance of ADL and HRQoL; the findings were compatible both with no effect and a clinically relevant improvement. Similarly, the moderate‐certainty evidence for mortality at 12 months plausibly included clinically relevant benefits and harms. These findings were the same for delirium, mortality at four months, and unplanned return to theatre, but were supported by low‐certainty evidence. For functional status, we noted that an improvement which favoured THA was not clinically important, and that this evidence was of very low certainty.
We found no difference in the overall risk of adverse events.
Other comparisons, which had fewer participants contributing to the available evidence and for which the estimates were generally too imprecise to yield meaningful inferences, were between: cemented and uncemented THAs; a combination of THAs and HAs which were cemented or uncemented; short or standard stem HAs; Exeter Trauma Stem or Thompson HAs; Furlong or Austin‐Moore HAs; single‐ or dual‐mobility articulations of THA; and short or standard stem THAs.
Overall completeness and applicability of evidence
We included 58 studies with 10,654 participants with a hip fracture. Most of our evidence is applicable only to people with intracapsular fractures as only one of the studies included participants with extracapsular fractures. Most extracapsular fractures are treated primarily with fixation rather than arthroplasty. Where reported, we noted a range of mean ages from 63 years to 87 years, and 73% of participants were female. We expected that most studies would include some participants with cognitive impairment, although approximately one‐third of studies excluded participants with cognitive impairment. Studies did not consistently report American Society of Anesthesiologists (ASA) status scores to indicate participants' fitness for surgery. In general, we assess that the review includes participants who are largely representative of the general hip fracture population undergoing arthroplasty surgery.
The studies reported outcomes following interventions that are all still in use worldwide. We recognise that there is variation in practice in different countries. The provision of cemented or modern uncemented HA and THA treatments may be particularly variable across different resource settings. We assess that the findings of this review are therefore applicable only to the countries in which studies were conducted, of which two‐thirds were in European and western countries.
The included studies were conducted between 1977 and 2020. There have been very substantial changes in co‐interventions in hip fracture care over this period of time. This may mean that, in older studies, the absolute effects are not directly applicable to contemporary care, but we found no evidence that the relative effects varied across time. Therefore, we assess that the historical literature is relevant and appropriate for pooling with more contemporary studies.
We identified studies that evaluated most of our clinically relevant, prespecified comparisons. The majority of studies provided evidence for one of three major groups of comparisons: cemented versus uncemented HAs; unipolar versus bipolar HAs; and THA versus HA. Even within these comparisons, with relatively more included studies, we found that many did not report fully outcomes such as performance of ADL or HRQoL. These are key components of the core outcome set for hip fracture, and yet our ability to draw inferences on the effect of interventions on these outcomes was limited. However, mortality was generally well‐reported, an outcome that is valued by individuals and clinicians in assessing intervention effects.
We were unable to fully perform our prespecified subgroup analyses to explore the impact of specific participant characteristics on the outcomes, such as the effect of age or cognitive impairment, since study characteristics were inconsistently reported within and between studies.
We prioritised short‐term outcomes in this review. We attempted to explore the longer‐term outcomes of the interventions, adding a long‐term measure of outcomes after 24 months from surgery. Longer‐term outcome could help to determine cost‐benefit decisions around intervention choices. Although some studies did present longer‐term data, these findings were often less precise due to attrition from death in this older, frail population.
Quality of the evidence
We used GRADE to formally assess the certainty of the evidence for the critical outcomes for the three main comparisons. The certainty of the evidence ranged from moderate to very low certainty. This was often due to imprecision in the estimate and the risk of bias in the included studies.
We judged several studies to have unclear risk of selection bias because they did not provide information about the allocation methods, or high risk of selection bias because they used quasi‐randomised methods to allocate participants to groups. We used sensitivity analysis to explore this, and found that re‐analysing the data without these studies sometimes influenced the effect: either importantly changing the size of the effect by including or excluding clinically relevant effects, or even changing the direction of the effect. All outcomes in the analyses of our main comparisons included studies with unclear or high risks of selection bias, and we therefore downgraded the certainty of the evidence for all outcomes in our main comparison groups owing to study limitations. We also downgraded the evidence for unplanned return to theatre because studies were at a high risk of detection bias for this outcome.
As well as the risks of bias, the majority of the studies had few participants, reported imprecise estimates, and were likely to be at high risk of a type II error (when a researcher may conclude that there is not a signficant effect when actually there is). The potential benefit of meta‐analysis to overcome this limitation was confounded by the reporting of widely different sets of outcomes across the included studies. Approximately two‐thirds of the studies predated the publication of the hip core outcome set which guided the selection of the critical outcomes in this review (Haywood 2014).
We did not downgrade for indirectness as the study populations and types of interventions were consistent with our intended criteria. We did not downgrade for inconsistency. We evaluated the risk of publication bias in only two analyses (in which we had more than 10 studies), and found no reason to downgrade for this potential limitation.
Potential biases in the review process
The review authors conducted a thorough search and independently assessed study eligibility, extracted data, and assessed risk of bias in the included studies before reaching consensus together or with one other review author.
During the review process, we made changes to the methods, which we describe in Differences between protocol and review. The most significant change was to collect data at three time points rather than two time points. This reflected the wider than expected variation in the outcome time points in the included studies. We aggregated outcome data for the 12‐months time point across a window between 4 and 24 months. Due to the high rates of attrition, we recognise that estimates based on later time points may systematically tend towards no effect. However, we believed it was important to report available data, but recognise that the decision may have influenced the pooled effect estimates.
Although data were sometimes more frequently reported after four months from surgery (typically at 12 months), we prioritised early outcomes in the summary of findings tables. The consequence of this decision is that some critical outcomes for the bipolar versus unipolar comparison have no data in the relevant table. However, this is consistent with our protocol, based on a core outcome set for hip fracture, which prioritises early outcomes over late recovery (Haywood 2014). We reached the decision to report mortality at two time points ‐ within four months of surgery and at 12 months after surgery ‐ following discussion with the Cochrane Bone, Joint, and Muscle Trauma Group. Mortality at 12 months still remains a more common time point reported by study authors, reflecting the expectations of organisations that fund research and journal editors.
Each of the interventions included in the review is complex: they are a combination of different design components which are not mutually exclusive. They are described more fully in Table 5. We made prespecified decisions in our protocol in stratifying our comparisons. This had most effect in the comparison of cemented and uncemented prostheses, where we had to divide the pooled analyses into those including studies of HA, THA, and a mixed intervention. This may have reduced the precision of some of the effect estimates by reducing the available studies in any one pooled analysis. However, the effects were largely concordant across the comparison, and we assess this to be unlikely to have substantially changed the inferences from the available data.
We did not explore adverse events related to implants beyond those described in the protocol for this review. We listed all outcomes reported by each study in the Characteristics of included studies, and these lists include additional adverse events for which we did not report data. Data for these additional adverse events for studies previously included are available in a previous version of this review (Parker 2010c). We attempted to collect information about the reasons for unplanned return to theatre, or the types of re‐operation, but found that this information was not clearly reported in many of the studies. This limited our ability to comment further on these events.
Newer studies were typically reported more completely. However, the majority of the available data in this review are derived from the historical literature. Where possible, we have presented the data in chronological order to try to indicate visually if effect estimates have varied systematically with time. We recognise that there may be an interaction, too, with the changes in co‐interventions with time.
We used GRADE only to assess the certainty of the evidence for the critical outcomes in this review that are included in our summary of findings tables. Therefore, we did not report any judgements of certainty for the remaining review outcomes. We highlighted this distinction when introducing the results for each comparison group. Given the risks of bias in all studies, as well as the imprecision in many of the findings, we anticipate that the certainty of most of these remaining review outcomes is likely to range from low to very low.
Agreements and disagreements with other studies or reviews
Although we found no recent comprehensive systematic reviews that evaluate all types of HA and THA within a single review, we found reviews comparing fixation techniques, articulations, and stem designs similar to the comparisons included in this Cochrane Review: cemented versus uncemented HAs (Azegami 2011; Imam 2019a), unipolar with bipolar HAs (Imam 2019b), and HA with THA (Hopley 2010; Lewis 2019; Liu 2020; Metcalfe 2019).
Azegami 2011 included eight RCTs, and reported the potential for reduced pain and improved mobility in cemented HAs compared to uncemented HAs. A more recent review of nine RCTS found no significant differences in pain or other complications, although the review authors observed that cemented HAs may lead to fewer intraoperative fractures (Imam 2019a). The improved mobility and reduction in intraoperative fractures is compatible with our findings. The reporting of pain in our included studies was highly variable, precluding effective pooling of studies, so that in this Cochrane Review, the reduction in pain in the cemented group was not evident. Whilst our data are compatible with this finding, they are also compatible with an alternative hypothesis that modern uncemented prostheses may yield reduced pain.
Imam 2019b included 13 RCTs and 17 observational studies, and found no significant difference in function and mortality between bipolar and unipolar HAs. Although review authors concluded that bipolar HAs lead to lower rates of re‐operation, their analysis included observational studies. An analysis with only RCTs was consistent with our findings that the re‐operation risk is similar with both interventions.
A larger number of reviews comparing HA to THA have been completed in recent years (Hopley 2010; Lewis 2019; Liu 2020; Metcalfe 2019). Results vary across the reviews, with reduced risk of re‐operation and improved function being reported for THA in three reviews (Hopley 2010; Lewis 2019; Liu 2020). Metcalfe 2019 combined a meta‐analysis of five RCTs with data from a comprehensive national cohort of hip fractures of 143,000 individuals, and reported no difference in re‐operation rates or function, which reflects the findings in this review.
This review included two large multicentre studies. The findings of Fernandez 2022 for cemented compared to uncemented HAs, and of HEALTH 2019 for THA compared to HA, provide substantial data which are consistent with the findings in this review.
Authors' conclusions
Implications for practice.
For people undergoing hemiarthroplasty for intracapsular hip fracture, it is likely that a cemented prosthesis will yield an improved global outcome, particularly in terms of clinically appreciable improvements in HRQoL and mortality. For every 26 people treated with a cemented hemiarthroplasty, one more person will be alive at 12 months following surgery.
Currently, there is insufficient evidence to determine whether a bipolar hemiarthroplasty yields different outcomes compared to a unipolar prosthesis. Both are appropriate treatments for people with intracapsular hip fracture.
Any benefit of THA compared with hemiarthroplasty is likely to be small and not clinically appreciable.
Implications for research.
Considerable research resources have been and are being committed to this field; we identified seven ongoing studies that may contribute data in future review updates. It is unlikely that future research will importantly alter our inferences about the relative clinical effectiveness of cemented and uncemented HAs, which now include data from a large multicentre study (Fernandez 2022). There is a relative paucity of evidence available from generally small studies for the comparison of bipolar and unipolar hemiarthroplasty. The estimates of any difference between total hip arthroplasty and hemiarthroplasty for some of the critical outcomes are imprecise. However, available data provide little to suggest that any effect is likely to be clinically meaningful. This is consistent with the findings of the large, international HEALTH 2019 study, and suggests that repeating such a study may not yield high‐value information.
We therefore encourage investigators interested in these comparisons to focus on conducting studies of alternative implant designs ‐ such as dual mobility bearings ‐ that are being incorporated widely into clinical practice, with scant evidence to support their use. We encourage investigators to address the limitations in the quality of the evidence in the field through better study design and clear reporting about methods of randomisation and allocation concealment, as well as attempting to minimise attrition for participant‐reported outcomes. We raise the awareness amongst investigators of the core outcome set for hip fracture that should be included in every RCT in hip fracture (Haywood 2014). To date, few studies have considered patient‐relevant outcomes, such as performance of activities of daily living, health‐related quality of life, mobility, or delirium.
Given the recommendations in Haywood 2014, we recommend that future studies are large enough to detect differences in HRQoL. Having reviewed the included studies, we estimate that the standard deviation for EQ‐5D at four months' post‐diagnosis is approximately 0.3. Assuming a minimal clinically important difference of 0.07 (Walters 2005), and an observed attrition in the included studies approaching 40%, we recommend future samples of not less than 1000 participants in order to yield sufficiently precise estimates.
History
Protocol first published: Issue 8, 2019
Acknowledgements
We thank Joanne Elliott, Helen Handoll, and Maria Clarke for editorial base support and helpful feedback on the review. We thank Liz Bickerdike for her comments and help at editorial review. We thank Cecilia Rogmark and Olof Wolf (external referees) for their helpful comments on the review. For their helpful comments on the protocol, we thank Cecilia Rogmark and Emil Schemitsch (external referees) and MingHui Yang (content expert, Trauma and Orthopaedics). We thank Ashwini Sreekanta, Alex Jubb, Julie Glanville, and Hannah Wood for their contributions to the development and publication of the protocol, and for developing and running the searches in December 2018.
This project was funded and supported by the National Institute for Health Research (NIHR). The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS, or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (CRS‐Web)
#1 MESH DESCRIPTOR Femoral Fractures EXPLODE ALL AND CENTRAL:TARGET #2 ((hip or hips or cervical) NEAR5 (fracture* or break* or broke*)) AND CENTRAL:TARGET #3 ((femoral* or femur* or acetabul*) NEAR5 (fracture* or break* or broke*)) AND CENTRAL:TARGET #4 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) NEAR5 (fracture* or break* or broke*)) AND CENTRAL:TARGET #5 ((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) NEAR5 (fracture* or break* or broke*)) AND CENTRAL:TARGET #6 ((head or neck or proximal) NEAR5 (fracture* or break* or broke*)) and (femoral* or femur*) AND CENTRAL:TARGET #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 AND CENTRAL:TARGET #8 MESH DESCRIPTOR Arthroplasty, Replacement, Hip AND CENTRAL:TARGET #9 MESH DESCRIPTOR Hip Prosthesis AND CENTRAL:TARGET #10 MESH DESCRIPTOR Arthroplasty, Replacement AND CENTRAL:TARGET #11 MESH DESCRIPTOR Hemiarthroplasty AND CENTRAL:TARGET #12 MESH DESCRIPTOR Joint Prosthesis AND CENTRAL:TARGET #13 ((arthroplast* or hemiarthroplast*) NEAR5 (hip or hips or femur* or femoral* or acetabul*)) AND CENTRAL:TARGET #14 ((hip or hips) NEAR5 (replac* or prosthes* or implant*)) AND CENTRAL:TARGET #15 ((joint* NEAR5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*)) AND CENTRAL:TARGET #16 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 AND CENTRAL:TARGET #17 MESH DESCRIPTOR Fractures, Bone AND CENTRAL:TARGET #18 MESH DESCRIPTOR Fracture Dislocation EXPLODE ALL AND CENTRAL:TARGET #19 MESH DESCRIPTOR Fractures, Closed AND CENTRAL:TARGET #20 MESH DESCRIPTOR Fractures, Comminuted AND CENTRAL:TARGET #21 MESH DESCRIPTOR Fractures, Compression AND CENTRAL:TARGET #22 MESH DESCRIPTOR Fractures, Malunited AND CENTRAL:TARGET #23 MESH DESCRIPTOR Fractures, Multiple AND CENTRAL:TARGET #24 MESH DESCRIPTOR Fractures, Open AND CENTRAL:TARGET #25 MESH DESCRIPTOR Fractures, Spontaneous AND CENTRAL:TARGET #26 MESH DESCRIPTOR Fractures, Stress AND CENTRAL:TARGET #27 MESH DESCRIPTOR Fractures, Ununited AND CENTRAL:TARGET #28 MESH DESCRIPTOR Intra‐Articular Fractures AND CENTRAL:TARGET #29 MESH DESCRIPTOR Osteoporotic Fractures AND CENTRAL:TARGET #30 MESH DESCRIPTOR Periprosthetic Fractures AND CENTRAL:TARGET #31 fracture* AND CENTRAL:TARGET #32 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 AND CENTRAL:TARGET #33 #32 AND #16 AND CENTRAL:TARGET #34 (pin or pins or nail or nails or screw or screws or plate or plates) AND CENTRAL:TARGET #35 MESH DESCRIPTOR Internal Fixators AND CENTRAL:TARGET #36 MESH DESCRIPTOR Bone Nails AND CENTRAL:TARGET #37 MESH DESCRIPTOR Bone Plates AND CENTRAL:TARGET #38 MESH DESCRIPTOR Bone Screws EXPLODE ALL AND CENTRAL:TARGET #39 (static NEXT (device* or implant*)) AND CENTRAL:TARGET #40 (dynamic NEXT (device* or implant*)) AND CENTRAL:TARGET #41 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 AND CENTRAL:TARGET #42 ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) AND CENTRAL:TARGET #43 (hip or hips or femur* or femoral* or acetabul*) AND CENTRAL:TARGET #44 #43 AND (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) AND CENTRAL:TARGET #45 #42 OR #44 AND CENTRAL:TARGET #46 #41 AND #45 AND CENTRAL:TARGET #47 #7 OR #33 OR #46 AND CENTRAL:TARGET #48 14/11/2018_TO_08/07/2020:CRSCREATED AND CENTRAL:TARGET #49 #47 AND #48
MEDLINE (Ovid)
1 exp Femoral Fractures/ 2 ((hip or hips or cervical) adj5 (fracture$ or break$ or broke$)).ti,ab,kf. 3 ((femoral$ or femur$ or acetabul$) adj5 (fracture$ or break$ or broke$)).ti,ab,kf. 4 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) adj5 (fracture$ or break$ or broke$)).ti,ab,kf. 5 ((extracapsular or extra‐capsular or trochant$ or subtrochant$ or pertrochant$ or intertrochant$) adj5 (fracture$ or break$ or broke$)).ti,ab,kf. 6 (((head or neck or proximal) adj5 (fracture$ or break$ or broke$)) and (femoral$ or femur$)).ti,ab,kf. 7 or/1‐6 8 randomized controlled trial.pt. 9 controlled clinical trial.pt. 10 randomized.ab. 11 placebo.ab. 12 clinical trials as topic.sh. 13 randomly.ab. 14 trial.ti. 15 or/8‐14 16 7 and 15 17 Arthroplasty, Replacement, Hip/ or Hip Prosthesis/ 18 Arthroplasty, Replacement/ or Hemiarthroplasty/ or Joint Prosthesis/ 19 ((arthroplast$ or hemiarthroplast$) adj5 (hip or hips or femur$ or femoral$ or acetabul$)).ti,ab,kf. 20 ((hip or hips) adj5 (replac$ or prosthes$ or implant$)).ti,ab,kf. 21 ((joint$1 adj5 (replac$ or prosthes$ or implant$)) and (hip or hips or femur$ or femoral$ or acetabul$)).ti,ab,kf. 22 or/17‐21 23 fractures, bone/ or exp fracture dislocation/ or fractures, closed/ or fractures, comminuted/ or fractures, compression/ or fractures, malunited/ or fractures, multiple/ or fractures, open/ or fractures, spontaneous/ or exp fractures, stress/ or fractures, ununited/ or intra‐articular fractures/ or osteoporotic fractures/ or periprosthetic fractures/ 24 fracture$.ti,ab,kf. 25 23 or 24 26 22 and 25 and 15 27 (pin or pins or nail or nails or screw or screws or plate or plates).ti,ab,kf. 28 internal fixators/ or bone nails/ or bone plates/ or exp bone screws/ 29 (static adj (device$1 or implant$1)).ti,ab,kf. 30 (dynamic adj (device$1 or implant$1)).ti,ab,kf. 31 or/27‐30 32 ((hip or hips or femur$ or femoral$ or acetabul$) and (fracture$ or break$ or broke$)).ti,ab,kf. 33 (hip or hips or femur$ or femoral$ or acetabul$).ti,ab,kf. and (fractures, bone/ or exp fracture dislocation/ or fractures, closed/ or fractures, comminuted/ or fractures, compression/ or fractures, malunited/ or fractures, multiple/ or fractures, open/ or fractures, spontaneous/ or exp fractures, stress/ or fractures, ununited/ or intra‐articular fractures/ or osteoporotic fractures/ or periprosthetic fractures/) 34 or/32‐33 35 31 and 34 and 15 36 16 or 26 or 35 37 exp animals/ not humans/ 38 36 not 37
Embase (Ovid)
1 exp Femur Fractures/ or exp hip fracture/ 2 ((hip or hips or cervical) adj5 (fracture$ or break$ or broke$)).ti,ab,kw. 3 ((femoral$ or femur$ or acetabul$) adj5 (fracture$ or break$ or broke$)).ti,ab,kw. 4 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) adj5 (fracture$ or break$ or broke$)).ti,ab,kw. 5 ((extracapsular or extra‐capsular or trochant$ or subtrochant$ or pertrochant$ or intertrochant$) adj5 (fracture$ or break$ or broke$)).ti,ab,kw. 6 (((head or neck or proximal) adj5 (fracture$ or break$ or broke$)) and (femoral$ or femur$)).ti,ab,kw. 7 or/1‐6 8 exp hip surgery/ or (joint surgery/ and exp hip/) 9 exp Hip Prosthesis/ 10 joint prosthesis/ and exp hip/ 11 Replacement Arthroplasty/ and exp hip/ 12 exp Hip arthroplasty/ 13 Arthroplasty/ and exp hip/ 14 Hemiarthroplasty/ and exp hip/ 15 Hip hemiarthroplasty/ 16 ((arthroplast$ or hemiarthroplast$) adj5 (hip or hips or femur$ or femoral$ or acetabul$)).ti,ab,kw. 17 ((hip or hips) adj5 (replac$ or prosthes$ or implant$)).ti,ab,kw. 18 ((joint$1 adj5 (replac$ or prosthes$ or implant$)) and (hip or hips or femur$ or femoral$ or acetabul$)).ti,ab,kw. 19 or/8‐18 20 fracture/ 21 Fracture dislocation/ 22 Comminuted fracture/ 23 Multiple fracture/ 24 Open fracture/ 25 Fragility fracture/ 26 exp Fracture healing/ 27 Stress fracture/ 28 intraarticular fracture/ 29 periprosthetic fracture/ 30 fracture$.ti,ab,kw. 31 or/20‐30 32 19 and 31 33 (pin or pins or nail or nails or screw or screws or plate or plates).ti,ab,kw. 34 internal fixator/ or exp bone nail/ or exp bone plate/ or exp bone pin/ or exp bone screw/ or exp femoral fixation device/ 35 (static adj (device$1 or implant$1)).ti,ab,kw. 36 (dynamic adj (device$1 or implant$1)).ti,ab,kw. 37or/33‐36 38 ((hip or hips or femur$ or femoral$ or acetabul$) and (fracture$ or break$ or broke$)).ti,ab,kw. 39 (hip or hips or femur$ or femoral$ or acetabul$).ti,ab,kw. 40 39 and 31 41 37 and (38 or 40) 42 7 or 32 or 41 43 Randomized controlled trial/ 44 Controlled clinical study/ 45 Random$.ti,ab. 46 randomization/ 47 intermethod comparison/ 48 placebo.ti,ab. 49 (compare or compared or comparison).ti. 50 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 51 (open adj label).ti,ab. 52 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 53 double blind procedure/ 54 parallel group$1.ti,ab. 55 (crossover or cross over).ti,ab. 56 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 57 (assigned or allocated).ti,ab. 58 (controlled adj7 (study or design or trial)).ti,ab. 59 (volunteer or volunteers).ti,ab. 60 human experiment/ 61 trial.ti. 62 or/43‐61 63 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 64 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 65 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 66 (Systematic review not (trial or study)).ti. 67 (nonrandom$ not random$).ti,ab. 68 "Random field$".ti,ab. 69 (random cluster adj3 sampl$).ti,ab. 70 (review.ab. and review.pt.) not trial.ti. 71 "we searched".ab. and (review.ti. or review.pt.) 72 "update review".ab. 73 (databases adj4 searched).ab. 74 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ 75 Animal experiment/ not (human experiment/ or human/) 76 or/63‐75 77 62 not 76 78 42 and 77
Web of Science
# 1 TOPIC: (((hip or hips or cervical) NEAR/5 (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 2 TOPIC: (((femoral* or femur* or acetabul*) NEAR/5 (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 3 TOPIC: (((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) NEAR/5 (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 4 TOPIC: (((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) NEAR/5 (fracture* or break* or broke*))) Indexes=SCIEXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 5 TOPIC: (((head or neck or proximal) NEAR/5 (fracture* or break* or broke*)) and (femoral* or femur*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 6 #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 7 TS=(((arthroplast* or hemiarthroplast*) NEAR/5 (hip or hips or femur* or femoral* or acetabul*)) and fracture*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 8 TS=( ((hip or hips) NEAR/5 (replac* or prosthes* or implant*)) and fracture*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 9 TS=(((joint* NEAR/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*)) and fracture*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 10 TS=( (pin or pins or nail or nails or screw or screws or plate or plates or fixator*) and ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 11 TS=((“static device*” OR “static implant*”) and ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 12 TS=((“dynamic device*” or “dynamic implant*”) and ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 13 #12 OR #11 OR #10 OR #9 OR #8 OR #7 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 14 #13 OR #6 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 15 TS=( random* or factorial* or crossover* or "cross‐over*" or placebo* or "doubl* blind*" or "singl* blind*" or assign* or allocat* or volunteer* or "trial" or "groups" or "controlled") Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 16 #15 AND #14 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years # 17 #16 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2018 # 18 TI=(RAT OR RATS OR MOUSE OR MOUSE OR DOG OR DOGS OR RABBIT OR RABBITS OR PIG OR PIGS OR SWINE OR PORCINE) Indexes=SCIEXPANDED, CPCI‐S Timespan=1900‐2020 # 19 #17 NOT #18 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=1900‐2020
Cochrane Database of Systematic Reviews (CDSR)
#1 MeSH descriptor: [Femoral Fractures] explode all trees #2 ((hip or hips or cervical) NEAR/5 (fracture* or break* or broke*)) #3 ((femoral* or femur* or acetabul*) NEAR/5 (fracture* or break* or broke*)) #4 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) NEAR/5 (fracture* or break* or broke*)) #5 ((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) NEAR/5 (fracture* or break* or broke*)) #6 ((head or neck or proximal) NEAR/5 (fracture* or break* or broke*)) and (femoral* or femur*) #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 #8 MeSH descriptor: [Arthroplasty, Replacement, Hip] this term only #9 MeSH descriptor: [Hip Prosthesis] this term only #10 MeSH descriptor: [Arthroplasty, Replacement] this term only #11 MeSH descriptor: [Hemiarthroplasty] this term only #12 MeSH descriptor: [Joint Prosthesis] this term only #13 ((arthroplast* or hemiarthroplast*) NEAR/5 (hip or hips or femur* or femoral* or acetabul*)) #14 ((hip or hips) NEAR/5 (replac* or prosthes* or implant*)) #15 ((joint* NEAR/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*)) #16 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 #17 MeSH descriptor: [Fractures, Bone] this term only #18 MeSH descriptor: [Fracture Dislocation] explode all trees #19 MeSH descriptor: [Fractures, Closed] this term only #20 MeSH descriptor: [Fractures, Comminuted] this term only #21 MeSH descriptor: [Fractures, Compression] this term only #22 MeSH descriptor: [Fractures, Malunited] this term only #23 MeSH descriptor: [Fractures, Multiple] this term only #24 MeSH descriptor: [Fractures, Open] this term only #25 MeSH descriptor: [Fractures, Spontaneous] this term only #26 MeSH descriptor: [Fractures, Stress] explode all trees #27 MeSH descriptor: [Fractures, Ununited] this term only #28 MeSH descriptor: [Intra‐Articular Fractures] this term only #29 MeSH descriptor: [Osteoporotic Fractures] this term only #30 MeSH descriptor: [Periprosthetic Fractures] this term only #31 fracture* #32 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 #33 #16 AND #32 #34 (pin or pins or nail or nails or screw or screws or plate or plates) #35 MeSH descriptor: [Internal Fixators] this term only #36 MeSH descriptor: [Bone Nails] this term only #37 MeSH descriptor: [Bone Plates] this term only #38 MeSH descriptor: [Bone Screws] explode all trees #39 (static NEXT (device* or implant*)) #40 (dynamic NEXT (device* or implant*)) #41 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 #42 ((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) #43 (hip or hips or femur* or femoral* or acetabul*) #44 #43 AND (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) #45 #42 OR #44 #46 #41 AND #45 #47 #7 OR #33 OR #46 in Cochrane Reviews
Database of Abstracts of Reviews of Effects (DARE)
1 (MeSH DESCRIPTOR Femoral Fractures EXPLODE ALL TREES) 2 ((hip or hips or cervical) near5 (fracture* or break* or broke*)) 3 ((fracture* or break* or broke*) near5 (hip or hips or cervical) ) 4 ((femoral* or femur* or acetabul*) near5 (fracture* or break* or broke*)) 5 ((fracture* or break* or broke*) near5 (femoral* or femur* or acetabul*)) 6 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) near5 (fracture* or break* or broke*)) 7 ((fracture* or break* or broke*) near5 (intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical)) 8 ((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near5 (fracture* or break* or broke*)) 9 ((fracture* or break* or broke*) near5 (extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*)) 10 ((head or neck or proximal) near5 (fracture* or break* or broke*) ) AND (femoral* or femur*) 11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 12 (MeSH DESCRIPTOR Arthroplasty, Replacement, Hip) OR (MeSH DESCRIPTOR Hip Prosthesis) 13 (MeSH DESCRIPTOR Arthroplasty, Replacement ) OR (MeSH DESCRIPTOR Hemiarthroplasty) OR (MeSH DESCRIPTOR Joint Prosthesis) 14 ((arthroplast* or hemiarthroplast*) near5 (hip or hips or femur* or femoral* or acetabul*)) 15 ((hip or hips or femur* or femoral* or acetabul*) near5 (arthroplast* or hemiarthroplast*)) 16 ((hip or hips) near5 (replac* or prosthes* or implant*)) 17 ((replac* or prosthes* or implant*) near5 (hip or hips)) 18 (joint* near5 (replac* or prosthes* or implant*) ) AND (hip or hips or femur* or femoral* or acetabul*) 19 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 20 (MeSH DESCRIPTOR fractures, bone) 21 (MeSH DESCRIPTOR fracture dislocation EXPLODE ALL TREES ) 22 (MeSH DESCRIPTOR fractures, closed ) 23 (MeSH DESCRIPTOR fractures, comminuted ) 24 (MeSH DESCRIPTOR fractures, compression ) 25 (MeSH DESCRIPTOR fractures, malunited ) 26 (MeSH DESCRIPTOR fractures, open ) 27 (MeSH DESCRIPTOR fractures, spontaneous) 28 (MeSH DESCRIPTOR fractures, stress EXPLODE ALL TREES ) 29 (MeSH DESCRIPTOR fractures, ununited ) 30 (MeSH DESCRIPTOR intra‐articular fractures ) 31 (MeSH DESCRIPTOR osteoporotic fractures ) 32 (MeSH DESCRIPTOR periprosthetic fractures ) 33 (MeSH DESCRIPTOR fractures, multiple ) 34 (fracture*) 35 #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 36 #19 AND #35 37 (pin or pins or nail or nails or screw or screws or plate or plates) 38 (MeSH DESCRIPTOR internal fixators ) 39 (MeSH DESCRIPTOR bone nails) 40 (MeSH DESCRIPTOR bone plates ) 41 (MeSH DESCRIPTOR bone screws EXPLODE ALL TREES ) 42 (static near (device* or implant*)) 43 ((device* or implant*) near static) 44 (dynamic near (device* or implant*)) 45 ((device* or implant*) near dynamic) 46 #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 47 ( (hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) 48 (hip or hips or femur* or femoral* or acetabul* ) 49 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33) 50 #48 AND #49 51 #47 OR #50 52 #46 AND #51 53 #11 OR #36 OR #52 54 * IN DARE 55 #53 AND #54
Health Technology Assessment (HTA)
1 (MeSH DESCRIPTOR Femoral Fractures EXPLODE ALL TREES) 2 ((hip or hips or cervical) near5 (fracture* or break* or broke*)) 3 ((fracture* or break* or broke*) near5 (hip or hips or cervical) ) 4 ((femoral* or femur* or acetabul*) near5 (fracture* or break* or broke*)) 5 ((fracture* or break* or broke*) near5 (femoral* or femur* or acetabul*)) 6 ((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or transcervical or basicervical or basi‐cervical) near5 (fracture* or break* or broke*)) 7 ((fracture* or break* or broke*) near5 (intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical)) 8 ((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near5 (fracture* or break* or broke*)) 9 ((fracture* or break* or broke*) near5 (extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*)) 10 ((head or neck or proximal) near5 (fracture* or break* or broke*) ) AND (femoral* or femur*) 11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 12 (MeSH DESCRIPTOR Arthroplasty, Replacement, Hip) OR (MeSH DESCRIPTOR Hip Prosthesis) 13 (MeSH DESCRIPTOR Arthroplasty, Replacement ) OR (MeSH DESCRIPTOR Hemiarthroplasty) OR (MeSH DESCRIPTOR Joint Prosthesis) 14 ((arthroplast* or hemiarthroplast*) near5 (hip or hips or femur* or femoral* or acetabul*)) 15 ((hip or hips or femur* or femoral* or acetabul*) near5 (arthroplast* or hemiarthroplast*)) 16 ((hip or hips) near5 (replac* or prosthes* or implant*)) 17 ((replac* or prosthes* or implant*) near5 (hip or hips)) 18 (joint* near5 (replac* or prosthes* or implant*) ) AND (hip or hips or femur* or femoral* or acetabul*) 19 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 20 (MeSH DESCRIPTOR fractures, bone) 21 (MeSH DESCRIPTOR fracture dislocation EXPLODE ALL TREES ) 22 (MeSH DESCRIPTOR fractures, closed ) 23 (MeSH DESCRIPTOR fractures, comminuted ) 24 (MeSH DESCRIPTOR fractures, compression ) 25 (MeSH DESCRIPTOR fractures, malunited ) 26 (MeSH DESCRIPTOR fractures, open ) 27 (MeSH DESCRIPTOR fractures, spontaneous) 28 (MeSH DESCRIPTOR fractures, stress EXPLODE ALL TREES ) 29 (MeSH DESCRIPTOR fractures, ununited ) 30 (MeSH DESCRIPTOR intra‐articular fractures ) 31 (MeSH DESCRIPTOR osteoporotic fractures ) 32 (MeSH DESCRIPTOR periprosthetic fractures ) 33 (MeSH DESCRIPTOR fractures, multiple ) 34 (fracture*) 35 #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 36 #19 AND #35 37 (pin or pins or nail or nails or screw or screws or plate or plates) 38 (MeSH DESCRIPTOR internal fixators ) 39 (MeSH DESCRIPTOR bone nails) 40 (MeSH DESCRIPTOR bone plates ) 41 (MeSH DESCRIPTOR bone screws EXPLODE ALL TREES ) 42 (static near (device* or implant*)) 43 ((device* or implant*) near static) 44 (dynamic near (device* or implant*)) 45 ((device* or implant*) near dynamic) 46 #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 47 ( (hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) 48 (hip or hips or femur* or femoral* or acetabul* ) 49 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33) 50 #48 AND #49 51 #47 OR #50 52 #46 AND #51 53 #11 OR #36 OR #52 54 * IN HTA 55 #53 AND #54
Epistemonikos
Search 1: Title/abstract (fracture* or break* or broke) AND Title/abstract (hip or hips or cervical or femoral* or femur* or acetabul* or intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical or extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*)
Search 2: Title/abstract (hip or hips or femur* or femoral* or acetabul*) and (replac* or prosthes* or implant*) and fracture* OR Title/abstract (arthroplast* or hemiarthroplast*) and (hip or hips or femur* or femoral* or acetabul*) and fracture*
Search 3: Title/abstract (pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators) AND Title/abstract (hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke)
Proquest DISSERTATIONS AND THESES
S1 ti(((hip or hips or cervical) near/5 (fracture* or break* or broke*))) OR ab(((hip or hips or cervical) near/5 (fracture* or break* or broke*))) S2 ti(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*))) OR ab(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*))) S3 ti(((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) near/5 (fracture* or break* or broke*))) OR ab(((intracapsular or intracapsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) near/5 (fracture* or break* or broke*))) S4 ti(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*))) OR ab(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*))) S5 ti((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))) OR ab((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))) S6 (ti(((hip or hips or cervical) near/5 (fracture* or break* or broke*))) OR ab(((hip or hips or cervical) near/5 (fracture* or break* or broke*)))) OR (ti(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*))) OR ab(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*)))) OR (ti(((intracapsular or intracapsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) near/5 (fracture* or break* or broke*))) OR ab(((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basicervical) near/5 (fracture* or break* or broke*)))) OR (ti(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*))) OR ab(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*)))) OR (ti((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))) OR ab((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*)))) S7 ti((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) OR ab((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) S8 ti( (hip or hips) near/5 (replac* or prosthes* or implant*)) OR ab( (hip or hips) near/5 (replac* or prosthes* or implant*)) S9 ti(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) OR ab(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) S10 (ti((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) OR ab((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*))) OR (ti( (hip or hips) near/5 (replac* or prosthes* or implant*)) OR ab( (hip or hips) near/5 (replac* or prosthes* or implant*))) OR (ti(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) OR ab(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*)))) S11 ti(fracture*) OR ab(fracture*) S12 ((ti((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) OR ab((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*))) OR (ti( (hip or hips) near/5 (replac* or prosthes* or implant*)) OR ab( (hip or hips) 183 near/5 (replac* or prosthes* or implant*))) OR (ti(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) OR ab(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))))) AND (ti(fracture*) OR ab(fracture*)) S13 ti((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) OR ab((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) S14 ti(static near (device* or implant*)) OR ab(static near (device* or implant*)) S15 ti(dynamic near (device* or implant*)) OR ab(dynamic near (device* or implant*)) S16 (ti((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) OR ab((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators))) OR (ti(static near (device* or implant*)) OR ab(static near (device* or implant*))) OR (ti(dynamic near (device* or implant*)) OR ab(dynamic near (device* or implant*))) S17 ti((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) OR ab((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) S18 ((ti((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) OR ab((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators))) OR (ti(static near (device* or implant*)) OR ab(static near (device* or implant*))) OR (ti(dynamic near (device* or implant*)) OR ab(dynamic near (device* or implant*)))) AND (ti((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) OR ab((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))) S19 ((ti(((hip or hips or cervical) near/5 (fracture* or break* or broke*))) OR ab(((hip or hips or cervical) near/5 (fracture* or break* or broke*)))) OR (ti(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*))) OR ab(((femoral* or femur* or acetabul*) near/5 (fracture* or break* or broke*)))) OR (ti(((intracapsular or intracapsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basi‐cervical) near/5 (fracture* or break* or broke*))) OR ab(((intracapsular or intra‐capsular or subcapital or sub‐capital or transcervical or trans‐cervical or basicervical or basicervical) near/5 (fracture* or break* or broke*)))) OR (ti(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*))) OR ab(((extracapsular or extra‐capsular or trochant* or subtrochant* or pertrochant* or intertrochant*) near/5 (fracture* or break* or broke*)))) OR (ti((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))) OR ab((((head or neck or proximal) near/5 (fracture* or break* or broke*)) and (femoral* or femur*))))) OR (((ti((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*)) OR ab((arthroplast* or hemiarthroplast*) near/5 (hip or hips or femur* or femoral* or acetabul*))) OR (ti( (hip or hips) near/5 (replac* or prosthes* or implant*)) OR ab( (hip or hips) near/5 (replac* or prosthes* or implant*))) OR (ti(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))) OR ab(( (joint* near/5 (replac* or prosthes* or implant*)) and (hip or hips or femur* or femoral* or acetabul*))))) AND (ti(fracture*) OR ab(fracture*))) OR (((ti((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators)) OR ab((pin or pins or nail or nails or screw or screws or plate or plates or fixator or fixators))) OR (ti(static near (device* or implant*)) OR ab(static near (device* or implant*))) OR (ti(dynamic near (device* or implant*)) OR ab(dynamic near (device* or implant*)))) AND (ti((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*)) OR ab((hip or hips or femur* or femoral* or acetabul*) and (fracture* or break* or broke*))))
National Technical Information Service (NTIS)
Title: hip fractures OR Keyword: hip fractures
Keyword: Hip AND Keyword: Bone fractures
ClinicalTrials.gov
Advanced search limited to intervention studies in Condition or disease
Interventional Studies | (fracture OR fractures OR break OR broke OR broken ) AND ( hip OR hips OR femoral OR femur OR acetabular OR intracapsular OR intra‐capsular OR subcapital OR sub‐capital OR transcervical OR trans‐cervical OR basicervical OR basi‐cervical)
Interventional Studies | (fracture OR fractures OR break OR broke OR broken ) AND ( extracapsular OR extracapsular OR trochanter OR trochanteric OR subtrochanter OR subtrochanteric OR pertrochanter OR pertochanteric OR intertrochanter OR intertochanteric )
Interventional Studies | (hip OR hips OR femur OR femoral OR acetabular) AND (replace OR replacement OR prosthesis OR prostheses OR implant OR implants) AND (fracture OR fractures OR break OR broke OR broken)
Interventional Studies | (arthroplasty OR hemiarthroplasty) AND (hip OR hips OR femur OR femoral OR acetabular) AND (fracture OR fractures OR break OR broke OR broken)
Appendix 2. Template data extraction form
| Methods |
RCT or quasi‐randomised; parallel design Review comparison group: |
| Participants |
Total number of randomised participants: Total number of participants that completed the study: Inclusion criteria: Exclusion criteria: Setting: type of setting, how many sites & country Baseline characteristics Intervention group 1 (specify by name)
Intervention group 2 (specify by name)
Overall:
Note:
|
| Interventions |
General details: to include number of clinicians (and their skills and experience), type of anaesthesia, pre‐ and postoperative care (e.g. use of prophylactic antibiotics or anti‐thromboembolics), rehabilitation (e.g. time to mobilisation or weight‐bearing) Intervention group 1: type of implant (with manufacturer details), description of use; number randomised to group, number of losses (for relevant outcomes, and with reasons for losses), number analysed by review authors for each review outcome Intervention group 2: type of implant (with manufacturer details), description of use; number randomised to group, number of losses (for relevant outcomes, and with reasons for losses), number analysed by review authors for each review outcome Note:
|
| Outcomes | Outcomes measured/reported by study authors: Outcomes relevant to the review: include measurement tools and time point of measure used in review analysis Note:
|
| Notes |
Funding/sponsor/declarations of interest: Study dates: |
Appendix 3. Scales used in 'critical outcomes'
| Outcome | Scale | Range | Direction of effect |
| ADL | Katz ADL (Katz 1963) |
0 to 6 | 6 indicating full function; 2 or less indicates severe functional impairment |
| Katz ADL (Katz 1963) |
A to G | A: independence in all six functions B: independence in all but one of the six functions. C–G: dependence in bathing and at least one more function. |
|
| Groningen Activity Restriction Scale (Suurmeijer 1994) |
18 to 72 | Lower scores indicate greater independence |
|
| OARS‐IADL (Fillenbaum 1981) |
0 to 14 | Higher scores indicate greater independence |
|
| Parker social dependency (Parker 2020) |
1 to 8 | Lower scores indicate greater independence |
|
| Barthel Index ‐ ADL (Mahoney 1965) |
0 to 20 | Higher scores indicate greater independence |
|
| Barthel Index – ADL (Wade 1988) |
0 to 100 | Higher scores indicate greater independence | |
| VELCA (Spolaore 2001) | 1 to 18 | Higher scores indicate greater independence | |
| WOMAC (Roos 1999) | 0 to 96 | Lower scores indicate better function | |
| Functional status | HHS (Singh 2016) | 0 to 100 | Higher scores indicate better function |
| D’Aubigne (D'Aubigne 1954) | 0 to 6 | Higher scores indicate better function | |
| Hip Rating Questionnaire (Johanson 1992) | 0 to 100 | Higher score indicates better function | |
| Devas and Hinves (Devas 1983) | Categorical | Good, medium, poor | |
| Assessment of Hip and Knee surgery (Benjamin 1990) | 0 to 5; over 9 domains; overall score up to 45 | Higher score indicates better function | |
| Oxford Hip Score (Dawson 1996) |
0 to 48 | Higher score indicates better function | |
| HRQoL | EQ‐5D (EuroQol 1990) | ‐0.654 (worst quality of life) 0 (dead) 1 (best quality of life) |
Higher scores indicate better quality of life |
| SF‐12 (Mols 2009) |
0 to 100 | Higher scores indicate better quality of life |
|
| SF‐36 (SF‐36) | 0 to 100 | Higher scores indicate better quality of life | |
|
Mobility |
Parker scale (Parker 1993b) | 0 to 9 | Higher scores indicate better mobility |
| Timed Up and Go (TUG) (Podsiadlo 1991) | To stand from a seated position and walk 6 steps | Lower time indicates better mobility | |
| 6 minute walk test (Overgaard 2017) | Distance walked in 6 mins | Higher distance indicates better mobility | |
| Parker scale (Parker 2019) |
1 to 9 | Lower scores indicate better mobility | |
| Nottingham Health Profile: mobility sub‐scale (Wiklund 1990) | 0 to 100 | Lower scores indicate better mobility | |
| VELCA ‐ walking (Spolaore 2001) | 0 to 6 | Higher scores indicate better mobility | |
| Koval (Koval 1995 ) |
Level I to VII | Dichotomised to either ambulatory indoors or outdoors; lower scores indicate greater independent mobility | |
| Footnotes: ADL: activities of daily living; EQ‐5D: EuroQoL 5 Dimensions instrument; HHS: Harris Hip Score; OARS‐IADL: Older Americans Resources Scale of Instrumental Activities of Daily Living; SF‐36 or SF‐12: short‐form 36 or short‐form 12; WOMAC: Western Ontario and McMAster Osteoarthritis index; VELCA: Verona Elderly Care | |||
Appendix 4. Prostheses implanted with cement versus without cement: data incomplete and not included in analysis
| Measurement tool | Interventions | Study ID | Data for Intervention 1 | Data for Intervention 2 | Additional information P value reported by study authors | |
| HA: cemented versus uncemented | ||||||
|
ADL (12 months) |
Modified HHS activities domain Follow‐up: 16 months |
1. Cemented; unipolar (Thompson) 2. Uncemented; unipolar (Thompson) |
Brandfoot 2000 | Mean: 1.64 n = 31 |
Mean: 1.61 n = 39 |
No SD No P value |
|
Functional status (12 months) |
HHS total Follow‐up: 16 months |
1. Cemented; unipolar (Thompson) 2. Uncemented; unipolar (Thompson) |
Brandfoot 2000 | Mean: 6.15 n = 31 |
Mean: 5.97 n = 39 |
No SD No P value |
|
Mobility (12 months) |
HHS mobility domain Follow‐up: 16 months |
1. Cemented; unipolar (Thompson) 2. Uncemented; unipolar (Thompson) |
Brandfoot 2000 | Mean: 1.38 n = 31 |
Mean: 1.37 n = 39 |
No SD No P value |
| TUG Follow‐up: 24 months |
1. Cemented 2. Uncemented |
Taylor 2012 | Mean: 24.7 n = 21 |
Mean: 26.9 n = 27 |
No SD No P value |
|
|
Pain (12 months) |
HHS pain domain Follow‐up: 16 months |
1. Cemented; unipolar (Thompson) 2. Uncemented; unipolar (Thompson) |
Brandfoot 2000 | Mean: 0.42 n = 31 |
Mean: 0.24 n = 39 |
No SD No P value |
| VAS Follow‐up: 24 months |
1. Cemented 2. Uncemented |
Taylor 2012 | Mean: 2.24 n = 21 |
Mean: 2.77 n = 27 |
No SD No P value |
|
| Mixed HA/THA: cemented versus uncemented | ||||||
|
Functional status (≤ 4 months) |
HHS total Follow‐up: 4 months |
1. Cemented; unipolar or THA 2. Uncemented; unipolar or THA |
Inngul 2015 | Mean (SD): 78 (± 14) |
Mean (SD): 70.7 (± 14.6) |
Number of participants not reported for each group P = 0.004; N = 127 |
|
Functional status (12 months) |
HHS total Follow‐up: 12 months |
1. Cemented; unipolar or THA 2. Uncemented; unipolar or THA | Inngul 2015 | Mean (SD): 82.3 (± 13.1) |
Mean (SD): 78.6 (± 17.1) |
Number of participants not reported for each group P = 0.93; N = 123 |
|
Pain (≤ 4 months) |
HHS pain score Follow‐up: 4 months |
1. Cemented; unipolar or THA 2. Uncemented; unipolar or THA | Inngul 2015 | Mean (SD): 39.6 (± 8.2) |
Mean (SD): 37.2 (± 9.1) |
Number of participants not reported for each group P = 0.065; N = 127 |
|
Pain (12 months) |
HHS pain score Follow‐up: 12 months |
1. Cemented; unipolar or THA 2. Uncemented; unipolar or THA | Inngul 2015 | Mean (SD): 40.7 (± 8.8) |
Mean (SD): 38.9 (± 9) |
Number of participants not reported for each group P = 0.101; N = 123 |
ADL: activities of daily living HA: hemiarthroplasty HHS: Harris hip score OHS: Oxford hip score n: number analysed per group N: number randomised to both groups SD: standard deviation SMFA: Short Musculoskeletal Function Assessment THA: total hip arthroplasty TUG: Timed Up and Go VAS: visual analogue scale
Appendix 5. HA bipolar versus unipolar: data incomplete and not included in analysis
| Measurement tool | Interventions | Study ID | Data for Intervention 1 | Data for Intervention 2 | Additional information P value reported by study authors | |
| Bipolar versus unipolar | ||||||
|
ADL (12 months) |
Musculoskeletal Functional Assessment Instrument; self care ADL Follow‐up: 12 months |
1, Bipolar 2. Unipolar |
Raia 2003 | Average: 37.0 n = 55 |
Average: 32.9 n = 60 |
Not specified whether mean or median; no SD or IQR P = 0.65 |
|
Functional status (≤ 4 months) |
HHS Follow‐up: 4 months |
1. Bipolar; cemented 2. Unipolar; cemented |
Hedbeck 2011 | Mean (range): 75.5 (24‐95) n = 56 |
Mean (range): 73.8 (44‐98) n = 59 |
No SD P = 0.17 |
|
Functional status (12 months) |
HHS Follow‐up: 12 months |
1. Bipolar; cemented (Monk) 2. Unipolar; cemented (Thompson) |
Davison 2001 | Mean: 73.2 n = 85 |
Mean: 71.1 n = 80 |
No SD No P value |
| HHS Follow‐up: 12 months |
1. Bipolar, uncemented 2. Unipolar, uncemented |
Figved 2018 | Median (IQR): 100 (95 to 100) n = 10 |
Median (IQR): 75 (70 to 85) n = 12 |
We reported median values owing to small sample size P = 0.001 |
|
| HHS Follow‐up: 12 months |
1. Bipolar; cemented 2. Unipolar; cemented |
Hedbeck 2011 | Mean (range): 77.7 (33‐100) n = 46 |
Mean (range): 78.2 (34‐100); n = 53 |
No SD P = 1.0 |
|
| Physical function from SF‐36 Follow‐up:12 months |
1. Bipolar 2. Unipolar |
Raia 2003 | Average: 54.2 n = 55 |
Average: 51.6 n = 60 |
Not specified whether mean or median; no SD or IQR P > 0.05 |
|
|
Functional status (> 24 months) |
HHS Follow‐up: 60 months |
1. Bipolar; cemented (Monk) 2. Unipolar; cemented (Thompson) |
Davison 2001 | Mean: 73.6 n = unknown |
Mean: 71.8 n = unknown |
No SD No P value |
|
HRQoL (12 months) |
EQ‐5D Follow‐up: 12 months |
1. Bipolar, uncemented 2. Unipolar, uncemented |
Figved 2018 | Median (IQR): 1.0 (0.84 to 1.0) n = 12 |
Median (IQR): 0.68 (0.52 to 0.82) n = 12 |
We reported median values owing to small sample size P = 0.003 |
| SF‐36 general health Follow‐up: 12 months |
1. Bipolar 2. Unipolar |
Raia 2003 | Average: 74.3 n = 55 |
Average 72.7 n = 60 |
Not specified whether mean or median; no SD or IQR P > 0.05 |
|
|
Mobility (12 months) |
Nottingham Health Profilea physical mobility domain Follow‐up: 6 months |
1. Bipolar; cemented (Monk) 2. Unipolar; cemented (Thompson) |
Calder 1995 | Median: Male, 23.4 Female 12.7 n = 39 |
Median: Male, 44.3 Female 67.0 n = 34 |
No SD No P value |
| Nottingham Health Profile physical mobility domain Follow‐up: 6 months |
1. Bipolar; cemented (Monk) 2. Unipolar; cemented (Thompson) |
Calder 1996 | Median: Male, 46.1 Female, 66.6 n = 56 |
Median: Male, 46.2 Female, 61.5 n = 72 |
No SD No P value |
|
| Mobility domain of Musculoskeletal Functional Assessment Instrumentb Follow‐up: 12 months |
1. Bipolar 2. Unipolar |
Raia 2003 | Average: 46.9 | Average: 47.4 | Not specified whether mean or median; no SD or IQR P = 0.94 |
|
|
Pain (≤ 4 months) |
HHS pain score Follow‐up: 4 months |
1. Bipolar; cemented 2. Unipolar; cemented |
Hedbeck 2011 | Mean (range): 40.3 (10 to 44) n = 56 |
Mean (range): 39.5 (20 to 44) n = 59 |
No SD P = 0.22 |
|
Pain (12 months) |
Nottingham Health Profile pain domain Follow‐up: 6 months |
1. Bipolar; cemented (Monk) 2. Unipolar; cemented (Thompson) |
Calder 1995 | Median: Male, 10.6 Female, 0.0 n = 39 |
Median: Male, 5.8 Female, 26.0 n = 34 |
No SD No P value |
| Nottingham Health Profile pain domain Follow‐up: 6 months |
1. Bipolar; cemented (Monk) 2. Unipolar; cemented (Thompson) |
Calder 1996 | Median: Male, 0.0 Female, 38.8 n = 56 |
Median: Male, 10.0 Female, 11.4 n = 72 |
No SD No P value |
|
| HHS pain score Follow‐up: 12 months |
1. Bipolar; cemented 2. Unipolar; cemented |
Hedbeck 2011 | Mean (range): 40.5 (20 to 44) n = 46 |
Mean (range): 41.3 (20 t0 44) n = 53 |
No SD P = 0.92 |
|
| SF‐36; bodily pain function Follow‐up: 12 months |
1. Bipolar 2. Unipolar |
Raia 2003 | Average: 77.8 | Average: 80 | Not specified whether mean or median; no SD or IQR P > 0.05 |
|
| Length of stay (LOS) in hospital | LOS (days) | 1. Bipolar; cemented (Monk) 2. Unipolar; cemented (Thompson) |
Calder 1996 | Median (IQR): 17 (13‐22) n = 118 |
Median (IQR): 18 (13‐23) n = 132 |
No mean or SD No P value |
| LOS (days) | 1. Bipolar; cemented 2. Unipolar; cemented |
Cornell 1998 | Mean (range): 13.4 (4 to 30) n = 33 |
Mean (range): 10.3 (5 to 23) n = 15 |
No SD No P value |
|
| LOS (days) | 1. Bipolar; cemented (Monk) 2. Unipolar; cemented (Thompson) |
Davison 2001 | Median (IQR): 15 (13‐21) n = 97 |
Median (IQR): 15 (1‐2) n = 90 |
No mean or SD No P value |
|
| LOS (days) | 1. Bipolar; Bateman type; uncemented 2. Unipolar; Austin‐Moore; uncemented |
Malhotra 1995 | Average: 17.24 n = 32 |
Average: 18.10 n = 36 |
No SD; mean/median not clarified No P value |
|
| LOS (days) | 1. Bipolar 2. Unipolar |
Raia 2003 | Mean: 5.2 n = 55 |
Mean: 5.5 n = 60 |
No SD No P value |
|
| LOS (days) | 1. Bipolar; cemented 2. Unipolar; cemented (Thompson) |
Patel 2008 | Average: 7 n = 20 |
Average: 13 n = 19 |
No SD No P value |
|
|
aNottingham Health profile; scores out of 100; lower score indicates better performance bMusculoskeletal Functional Assessment Instrument; lower score indicates better function | ||||||
ADL: activities of daily living HA: hemiarthroplasty HHS: Harris hip score HRQoL: health‐related quality of life IQR: interquartile range LOS: length of stay in hospital n: number analysed SD: standard deviation SF‐36: Short‐Form 36
Appendix 6. Categorical outcome data: complete data for all categories
| HA vs THA | ||||
| Outcome | Study ID | Short stem: n/N | Standard stem: n/N | Effect estimate as reported by study authors |
| Functional status | Ren 2017; Sonaje 2017 | Excellent: 33/70 Good: 29/70 Medium: 7/70 Poor: 1/70 |
Excellent: 40/70 Good: 27/70 Medium: 3/70 Poor: 0/70 |
Effect estimate not reported |
| Pain | Ravikumar 2000 | No pain: 30/66 Occasional pain: 18/66 Occasional analgesia: 3/66 Regular analgesia: 15/66 |
No pain: 46/69 Occasional pain: 23/69 Occasional analgesia: 0/69 Regular analgesia: 0/69 |
Effect estimate not reported |
| HA: bipolar vs unipolar | ||||
| Functional status | Abdelkhalek 2011; Malhotra 1995 | Excellent: 46/57 Good: 6/57 Fair: 4/57 Poor: 1/57 |
Excellent: 27/61 Good: 19/61 Fair: 9/61 Poor: 6/61 |
Effect estimate not reported |
| Pain (studies did not report outcome for all categories included in this table) |
Abdelkhalek 2011; Calder 1996; Livesley 1993 | No pain: 17/41 Mild pain: 10/41; No pain, or mild pain: 65/118; Pain at rest: 5/34 Pain on rising from a chair: 5/34 Activity pain: 5/34 |
No pain: 7/37 Mild pain: 11/37; No pain, or mild pain: 70/132; Pain at rest: 5/38 Pain on rising from a chair: 5/38 Activity pain: 2/38 |
Effect estimate not reported |
| HA: cemented vs uncemented | ||||
| Functional status (Studies did not report data for all categories included in this table) |
Sadr 1977; Sonne‐Holm 1982 | Excellent: 1/11 Good: 6/11 Fair: 3/11 Poor: 1/11; Maximal score (3 months): 29/40 Maximal score (12 months): 33/40 |
Excellent: 0/14 Good: 8/14 Fair: 5/14 Poor: 1/11; Maximal score (3 months): 22/35 Maximal score (12 months): 25/35 |
Effect estimate not reported |
| Mobility | Fernandez 2022 (4, 12 months) | Freely mobile (no aids): 18, 18 Mobile outdoors (one aid): 64, 57 Mobile outdoors (two aids/frame): 102, 78 Indoor only: 134, 113 No mobility: 48, 36 Missing: 244, 308 |
Freely mobile (no aids): 15, 21 Mobile outdoors (one aid): 61, 70 Mobile outdoors (two aids/frame): 100, 57 Indoor only: 119, 90 No mobility: 54, 43 Missing: 266, 334 |
Odds ratios: 4 months 0.93 (95% CI; 0.72‐1.22), P value 0.610 12 months 1.09 (95% CI; 0.81‐1.46), P value 0.556 |
| Discharge destination (Studies did not report data for all categories included in this table) |
DeAngelis 2012; Figved 2009; Santini 2005; Taylor 2012 | Own home: 43/305 Other medical department: 8/50 Rehabilitation facility: 3/66 Assisted living: 62/66 Geriatric institution: 29/50 Unknown: 1/66 |
Own home: 50/301 Other medical department: 5/51 Rehabilitation facility: 3/64 Assisted living: 60/64 Geriatric institution: 28/51 Unknown: 1/64 |
Effect estimate not reported |
| THA: short stem vs standard stem | ||||
| Outcome | Study ID | Short stem: n/N | Standard stem: n/N | Effect estimate as reported by study authors |
| Mobility | Kim 2012 | Walks > 6 blocks with or without aid: 44/72 Walks < 6 blocks: 22/72 Walks indoors only: 6/72 |
Walks > 6 blocks with or without aid: 40/70 Walks < 6 blocks: 25/70 Walks indoors only: 5/70 |
Effect estimate not reported |
| HA: Thompson vs Exeter Trauma Stem | ||||
| Mobility | Sims 2018 | Freely mobile without aids: 15/242 Mobile outdoors with 1 aid: 38/242 Mobile outdoors with 2 aids/frame: 19/242 Some indoor mobility but never goes out without help: 135/242 No functional mobility: 35/242 |
Freely mobile without aids: 16/252 Mobile outdoors with 1 aid: 47/252 Mobile outdoors with 2 aids/frame: 34/252 Some indoor mobility but never goes out without help: 123/242 No functional mobility: 32/252 |
Effect estimate not reported |
| Footnotes: CI: confidence interval; n: number of participants with an event; N: total number of participants in group;RR: risk ratio | ||||
Appendix 7. HAs versus other HAs: data incomplete and not included in analysis
| Measurement tool | Interventions | Study ID | Data for Intervention 1 | Data for Intervention 2 | Comment | |
| Exeter vs Thompson | ||||||
|
Mobility (≤ 4 months) |
Parker mobility scale (lower scores indicate better mobility) Follow‐up: 3 months |
1. Exeter; modern stem; cemented 2. Thompson; traditional stem; cemented |
Parker 2012 | Mean (change from baseline): 2.2 n = 64 |
Mean (change from baseline): 1.3 n= 75 |
No SD P = 0.05 |
|
Mobility (12 months) |
Parker mobility scale (lower scores indicate better mobility) Follow‐up: 12 months |
1. Exeter; modern stem; cemented 2. Thompson; traditional stem; cemented |
Parker 2012 | Mean (change from baseline): 1.7 n = 64 |
Mean (change from baseline): 1.1 n = 75 |
No SD P = 0.05 |
|
Pain (≤ 4 months) |
Degree of residual pain Follow‐up: 3 months |
1. Exeter; modern stem; cemented 2. Thompson; traditional stem; cemented |
Parker 2012 | Mean: 1.6 n = 64 |
Mean: 1.8 n = 75 |
No SD P = 0.6 |
|
Pain (12 months) |
Follow‐up: 12 months | 1. Exeter; modern stem; cemented 2. Thompson; traditional stem; cemented |
Parker 2012 | Mean: 1.5 n = 64 |
Mean: 1.6 n = 75 |
No SD P = 0.8 |
| Length of stay in hospital | LOS (days) | 1. Exeter; modern stem; cemented 2. Thompson; traditional stem; cemented |
Parker 2012 | Mean: 17.6 n = 100 |
Mean: 17.6 n = 100 |
No SD P = 1.0 |
| LOS (days) | 1. Exeter; modern stem; cemented 2. Thompson; traditional stem; cemented |
Sims 2018 | Mean: 9.67 n = 303 |
Mean: 9.0 n = 315 |
No SD No P value |
|
HA: hemiarthroplasty LOS: length of stay in hospital n: number of analysed participants SD: standard deviation
Appendix 8. THA versus HA: data incomplete and not included in analysis
| Measurement tool | Interventions | Study ID | Data for Intervention 1 | Data for Intervention 1 | Comment | |
|
Functional status (≤ 4 months) |
HHS Follow‐up: 3 months |
1. THA 2. HA |
Cadossi 2013 | Mean (range): 24.6 (5 to 40) n = 37 |
Mean (range): 20.8 (5 to 45) n = 37 |
No SD P = 0.471 |
|
Functional status (12 months) |
HHS Follow‐up: 12 months |
1. THA 2. HA |
Cadossi 2013 | Mean (range): 26.4 (5 to 45) n = 36 |
Mean (range): 23.9 (5 to 45) n = 33 |
No SD P = 0.466 |
| HHS Follow‐up: 12 months |
1. THA 2. HA |
Sharma 2016 | Mean (range): 90 (97 to 95) n = 39 |
Mean (range): 80 (67 to 85) n = 39 |
No SD No P value |
|
| HHS (modified) Follow‐up: 12 months |
1. THA 2. HA |
Van den Bekerom 2010 | Mean (range): 76 (44 to 100) n = 115 |
Mean (range): 73.9 (23 to 100) n = 137 |
No SD P = 0.40 |
|
|
Functional status (> 24 months) |
HHS Follow‐up: 36 months |
1. THA 2. HA |
Cadossi 2013 | Mean (range): 24.7 (5 to 40) n = 16 |
Mean (range): 28.2 (5 to 45) n = 16 |
No SD P = 0.417 |
| HHS (modified) Follow‐up: 60 months |
1. THA 2. HA |
Van den Bekerom 2010 | Mean (range): 75.2 (45 to 96) n = 115 |
Mean (range): 71.9 (33 to 99) n = 137 |
No SD P = 0.22 |
|
|
Mobility (≤ 4 months) |
Ambulationa Follow‐up: 3 months |
1. THA 2. HA |
Dorr 1986 | Mean: 4.1 n = 39 |
Mean: Cemented HA: 4.0; n = 37 Uncemented HA: 3.7; n = 13 |
No SD No P value |
|
Mobility (12 months) |
Ambulationa Follow‐up: 12 months |
1. THA 2. HA |
Dorr 1986 | Mean: 4.1 n = 39 |
Mean: Cemented HA: 4.2; n = 37 Uncemented HA: 3.0; n = 13 |
No SD No P value |
|
Mobility (> 24 months) |
Distance walkedb (km) Follow‐up: 36 month |
1. THA 2. HA |
Baker 2006 | Mean (range): 3.6 (0 to 40.2) n = 36 |
Mean (range): 1.9 (0 to 6.4) n = 33 |
No SD No P value |
|
Pain (≤ 4 months) |
HHS ‐ pain Follow‐up: 3 months |
1. THA 2. HA |
Cadossi 2013 | Mean (range): 39.5 (20 to 44) n = 37 |
Mean (range): 43.7 (30 to 44) n = 37 |
No SD P = 0.158 |
| Painc Follow‐up: 3 months |
1. THA 2. HA |
Dorr 1986 | Mean: 4.9 n = 39 |
Mean: Cemented HA: 5.4; n = 37 Uncemented HA: 3.7; n = 13 |
No SD No P value |
|
|
Pain (12 months) |
HHS ‐ pain Follow‐up: 12 months |
1. THA 2. HA |
Cadossi 2013 | Mean (range): 39.5 (20 to 44) n = 36 |
Mean (range): 43.3 (30 to 44) n = 33 |
No SD P = 0.006 |
| Painc Follow‐up: 3 months |
1. THA 2. HA |
Dorr 1986 | Mean: 5.5 n = 39 |
Mean: Cemented HA: 5.2; n = 37 Uncemented HA: 3.6; n = 13 |
No SD No P value |
|
|
Pain (> 24 months) |
HHS ‐ pain Follow‐up: 36 months |
1. THA 2. HA |
Cadossi 2013 | Mean (range): 40.5 (20 to 44) n = 16 |
Mean (range): 44 (44 to 44) n = 16 |
No SD P = 0.073 |
| HHS ‐ pain Follow‐up: 36 months |
1. THA 2. HA |
Van den Bekerom 2010 | Mean (range): 40.1 (20 to 44) n = 115 |
Mean (range): 38.6 (10 to 44) n = 137 |
No SD No P value |
|
| Length of stay in hospital | LOS (days) | 1. THA 2. HA |
Cadossi 2013 | Mean (range): 8.7 (4 to 21) n = 41 |
Mean (range): 9.9 (5 to 21) n = 42 |
No SD No P value |
| LOS (days) | 1. THA 2. HA |
Parker 2019 | Mean: 14.5 n = 52 |
Mean: 9.2 n = 53 |
No SD P = 0.055 |
|
| LOS (days) | 1. THA 2. HA |
Van den Bekerom 2010 | Mean (range): 17.1 (2 to 89) n = 137 |
Mean (range): 18.4 (4 to 86) n = 115 |
No SD No P value |
|
| LOS (days) | 1. THA 2. HA |
Iorio 2019 | Mean (range): 6.1 (5 to 8) n = 30 |
Mean (range): 5.5 (5 to 7) n = 30 |
No SD P > 0.05 |
|
|
aAmbulation (6 point scale; higher scores indicate better mobility) bParticipant‐reported but not specified how this was measured c6‐point scale; higher scores indicate less pain | ||||||
HA: hemiarthroplasty HHS: Harris hip score HRQoL: health‐related quality of life LOS: length of stay in hospital SD: standard deviation SF‐36: Short‐Form 36 THA: total hip arthroplasty
Data and analyses
Comparison 1. THA: cemented vs uncemented.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 ADL (measurement tool not defined) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Early (≤ 4 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Functional status (using HHS, range for scores from 0 to 100; higher scores indicate better function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Early (reported at ≤ 4 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 HRQoL (using EQ‐5D, range of scores from o to 1; higher scores indicate better quality of life) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Early (≤ 4 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Mortality (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Unplanned return to theatre (end of follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 Pain (using PNRS, range of scores from 0 to 11: lower values indicate less pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 Early (reported at ≤ 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.2 Reported at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7 Adverse events related to the implant, fracture, or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 Intraoperative periprosthetic fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.2 Postoperative periprosthetic fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.3 Loosening | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.4 Superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.5 Dislocation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.6. Analysis.

Comparison 1: THA: cemented vs uncemented, Outcome 6: Pain (using PNRS, range of scores from 0 to 11: lower values indicate less pain)
1.7. Analysis.

Comparison 1: THA: cemented vs uncemented, Outcome 7: Adverse events related to the implant, fracture, or both
Comparison 2. HA: cemented vs uncemented.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Early ADL (≤ 4 months, continuous data) | 4 | 1275 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.21, 0.16] |
| 2.2 Early ADL (≤ 4 months, categorical data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 ADL (12 months, continuous data) | 5 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.21, 0.02] |
| 2.3.1 First generation uncemented stem | 1 | 106 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.56, 0.20] |
| 2.3.2 Modern stem | 4 | 1067 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.20, 0.04] |
| 2.4 ADL (12 months, categorical data) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Late ADL (> 24 months; categorical data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6 Delirium (end of follow‐up) | 2 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.55, 2.06] |
| 2.6.1 First generation uncemented stem | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.14, 7.03] |
| 2.6.2 Modern stem | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.53, 2.16] |
| 2.7 Early functional status (≤ 4 months, continuous data) | 3 | 416 | Mean Difference (IV, Random, 95% CI) | 3.38 [0.05, 6.70] |
| 2.7.1 First generation uncemented stem | 1 | 79 | Mean Difference (IV, Random, 95% CI) | 4.21 [1.77, 6.65] |
| 2.7.2 Modern stem | 2 | 337 | Mean Difference (IV, Random, 95% CI) | 2.43 [‐4.42, 9.29] |
| 2.8 Early functional status (≤ 4 months; categorical data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9 Early functional status: extracapsular fractures (≤ 4 months. HHS; higher scores indicate better function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.10 Functional status (12 months; continuous data using HHS, OHS and VELCA; higher scores indicate better function) | 5 | 494 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.09, 0.35] |
| 2.10.1 First generation uncemented stem | 2 | 166 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.30, 0.94] |
| 2.10.2 Modern stem | 3 | 328 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.18, 0.25] |
| 2.11 Functional status (12 months, categorical data using HHS) | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.45] |
| 2.12 Functional status: extracapsular fractures (12 months. HHS; higher scores indicate improved function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.13 Late functional status (> 24 months using HHS; higher scores indicate better function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.14 Early HRQoL (≤ 4 months) | 3 | 1122 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.07, 0.34] |
| 2.15 HRQoL (12 months) | 3 | 1079 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.00, 0.24] |
| 2.16 Late HRQoL (> 24 months) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| 2.17 Early mobility (≤ 4 months, independent mobility) | 3 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.14] |
| 2.17.1 First generation uncemented stem | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.84, 2.95] |
| 2.17.2 Modern stem | 2 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.12] |
| 2.18 Early mobility (≤ 4 months, continuous data) | 3 | 766 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.40, ‐0.12] |
| 2.18.1 First generation uncemented stem | 1 | 327 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.58, ‐0.14] |
| 2.18.2 Modern stem | 2 | 439 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.00] |
| 2.19 Early mobility (mean reduction values at ≤ 4 months; higher scores indicate better mobility) | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.68, ‐0.12] |
| 2.20 Mobility (12 months, continuous data using different mobility scales; lower scores indicate better mobility) | 4 | 762 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.42, ‐0.06] |
| 2.20.1 First generation uncemented stem | 2 | 386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.54, 0.30] |
| 2.20.2 Modern stem | 2 | 376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.53, ‐0.12] |
| 2.21 Mobility (12 months, independent mobility) | 3 | 826 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.37] |
| 2.21.1 First generation uncemented stem | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.81, 1.82] |
| 2.21.2 Modern | 2 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.52, 1.55] |
| 2.22 Mobility (12 months, dependent on walking aid) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.23 Late mobility (> 24 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.24 Late mobility (> 24 months; independent mobility) | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.02] |
| 2.25 Early mortality (≤ 4 months) | 12 | 3136 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.13] |
| 2.25.1 First generation uncemented stem | 7 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.79, 1.54] |
| 2.25.2 Modern stem | 5 | 2156 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.10] |
| 2.26 Mortality (12 months) | 15 | 3727 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.96] |
| 2.26.1 First generation uncemented stem | 8 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.78, 1.18] |
| 2.26.2 Modern stem | 7 | 2691 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.73, 0.94] |
| 2.27 Late mortality (> 24 months) | 2 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.15] |
| 2.28 Unplanned return to theatre (end of follow‐up) | 6 | 2336 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.45, 1.10] |
| 2.28.1 First generation uncemented stem | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.30, 1.26] |
| 2.28.2 Modern stem | 5 | 1936 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.44, 1.35] |
| 2.29 Early pain (≤ 4 months, experiencing no pain) | 4 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.22] |
| 2.29.1 First generation uncemented stem | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.81, 1.97] |
| 2.29.2 Modern stem | 2 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.24] |
| 2.30 Early pain (≤ 4 months; mean scores, lower scores indicate less pain) | 4 | 1507 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |
| 2.30.1 First generation uncemented stem | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.87, ‐0.33] |
| 2.30.2 Modern stem | 3 | 1187 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.01, 0.20] |
| 2.31 Pain (12 months, experiencing no pain) | 4 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.85, 1.63] |
| 2.31.1 First generation uncemented stem | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.97, 4.48] |
| 2.31.2 Modern stem | 2 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.82, 1.06] |
| 2.32 Pain (12 months, using continuous data; lower values indicate less pain) | 5 | 1305 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.21, 0.18] |
| 2.32.1 First generation uncemented stem | 1 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.56, ‐0.08] |
| 2.32.2 Modern stem | 4 | 1033 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.21] |
| 2.33 Pain (12 months; mean reduction values: lower scores indicate less pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.34 Late pain (> 24 months, using mean scores; lower scores indicate less pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.35 Late pain (> 24 months; experiencing no pain) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 2.36 Length of hospital stay (days) | 9 | 1741 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.03, 0.23] |
| 2.36.1 First generation uncemented stem | 5 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.84, 0.55] |
| 2.36.2 Modern stem | 4 | 976 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.73, 0.88] |
| 2.37 Discharge destination (own home) | 6 | 2231 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 2.37.1 First generation uncemented stem | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.34] |
| 2.37.2 Modern stem | 4 | 1730 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.95, 1.20] |
| 2.38 Adverse events related to the implant, fracture, or both | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.38.1 Intraoperative periprosthetic fracture | 7 | 1669 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.08, 0.46] |
| 2.38.2 Postoperative periprosthetic fracture | 8 | 2819 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.14, 0.57] |
| 2.38.3 Loosening | 4 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.14, 1.89] |
| 2.38.4 Deep infection | 7 | 1382 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.72, 3.38] |
| 2.38.5 Superficial infection | 10 | 3038 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.73, 2.06] |
| 2.38.6 Dislocation | 10 | 3032 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.61, 1.91] |
| 2.39 Adverse events unrelated to the implant, fracture, or both | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.39.1 Acute kidney injury | 4 | 2226 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.76, 2.00] |
| 2.39.2 Blood transfusion | 7 | 2907 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.20] |
| 2.39.3 Cerebrovascular accident | 5 | 2356 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.41, 2.10] |
| 2.39.4 Pneumonia/chest infection | 8 | 2789 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.50, 1.21] |
| 2.39.5 Myocardial infarction | 7 | 2682 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.44, 1.89] |
| 2.39.6 Urinary tract infection | 5 | 1745 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.65, 1.20] |
| 2.39.7 Venous thromboembolic phenomena (DVT) | 7 | 2661 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.56, 2.90] |
| 2.39.8 Venous thromboembolic phenomena (pulmonary embolism) | 6 | 2499 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [1.26, 10.11] |
2.29. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 29: Early pain (≤ 4 months, experiencing no pain)
2.30. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 30: Early pain (≤ 4 months; mean scores, lower scores indicate less pain)
2.31. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 31: Pain (12 months, experiencing no pain)
2.32. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 32: Pain (12 months, using continuous data; lower values indicate less pain)
2.33. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 33: Pain (12 months; mean reduction values: lower scores indicate less pain)
2.34. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 34: Late pain (> 24 months, using mean scores; lower scores indicate less pain)
2.35. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 35: Late pain (> 24 months; experiencing no pain)
2.36. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 36: Length of hospital stay (days)
2.37. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 37: Discharge destination (own home)
2.38. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 38: Adverse events related to the implant, fracture, or both
2.39. Analysis.

Comparison 2: HA: cemented vs uncemented, Outcome 39: Adverse events unrelated to the implant, fracture, or both
Comparison 3. Mixed HA and THA: cemented vs uncemented.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Functional status (12 months, using HHS, range of scores from 0 to 100; higher scores indicate better function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 HRQoL (12 months, using SF‐36, range of scores from 0 to 100; higher scores indicate better quality of life) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Early mortality (≤ 4 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 Mortality (12 months) | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.81, 5.07] |
| 3.5 Late mortality (> 24 months) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.50, 1.56] |
| 3.6 Pain (12 months, using HHS pain scales; higher values indicate less pain) | 1 | 106 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐0.87, 6.07] |
| 3.7 Pain (> 24 months, using HHS pain scales; higher values indicate less pain) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐0.01, 7.21] |
| 3.8 Unplanned return to theatre (end of follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9 Adverse events related to the implant, fracture, or both | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9.1 Intraoperative periprosthetic fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9.2 Superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9.3 Dislocation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.10 Adverse events unrelated to implant, fracture, or both | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.10.1 Acute kidney infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.10.2 Chest infection/pneumonia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.10.3 Myocardial infarction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.10.4 Urinary tract infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
3.6. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 6: Pain (12 months, using HHS pain scales; higher values indicate less pain)
3.7. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 7: Pain (> 24 months, using HHS pain scales; higher values indicate less pain)
3.10. Analysis.

Comparison 3: Mixed HA and THA: cemented vs uncemented, Outcome 10: Adverse events unrelated to implant, fracture, or both
Comparison 4. Bipolar HA vs unipolar HA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 ADL (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.2 Delirium/confusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3 Functional status (12 months; using different measurement tools; higher scores indicate better function) | 2 | 299 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.27, 0.19] |
| 4.4 Functional status (12 months. HHS; excellent and good) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.5 Functional status (> 24 months. HHS; excellent or good) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.6 Early HRQoL (≤ 4 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7 HRQoL (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.8 Mobility (Get up and Go Test; in seconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9 Mobility (6 minute walk test; in metres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10 Early mortality (≤ 4 months) | 4 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.54, 1.64] |
| 4.11 Mortality (12 months) | 8 | 839 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.53] |
| 4.11.1 Cemented | 7 | 799 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 4.11.2 Uncemented | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 4.12 Late mortality (> 24 months) | 2 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.23] |
| 4.13 Unplanned return to theatre (end of follow‐up) | 4 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.44, 2.64] |
| 4.13.1 Cemented | 3 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.73, 3.99] |
| 4.13.2 Cemented and uncemented | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.07, 1.24] |
| 4.14 Pain (categorical data; no pain, or mild pain) | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.82, 1.82] |
| 4.15 Pain (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.16 Length of hospital stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.17 Discharge destination: return to preoperative residence | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 4.18 Adverse events related to implant, fracture, or both | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.18.1 Periprosthetic fracture | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.37, 132.66] |
| 4.18.2 Superficial infection | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [0.48, 12.18] |
| 4.18.3 Deep infection | 7 | 1122 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.44, 2.71] |
| 4.18.4 Dislocation | 9 | 1274 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.28, 1.38] |
| 4.19 Adverse event unrelated to implant, fracture, or both | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.19.1 Acute kidney injury | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.12, 70.25] |
| 4.19.2 Blood transfusion | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.51, 1.62] |
| 4.19.3 Cerebrovascular accident | 2 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.20, 12.69] |
| 4.19.4 Pneumonia/chest infection | 3 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.10, 3.86] |
| 4.19.5 Myocardial infarction | 3 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.11, 4.32] |
| 4.19.6 Urinary tract infection | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.29, 3.25] |
| 4.19.7 Venous thromboembolic phenomena (DVT) | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 3.84 [0.43, 34.45] |
| 4.19.8 Venous thromboembolic phenomena (pulmonary embolism) | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 72.20] |
4.14. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 14: Pain (categorical data; no pain, or mild pain)
4.15. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 15: Pain (12 months)
4.16. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 16: Length of hospital stay (days)
4.17. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 17: Discharge destination: return to preoperative residence
4.18. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 18: Adverse events related to implant, fracture, or both
4.19. Analysis.

Comparison 4: Bipolar HA vs unipolar HA, Outcome 19: Adverse event unrelated to implant, fracture, or both
Comparison 5. HA: short stem vs standard stem.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mobility (24 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Mortality (24 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.3 Pain (24 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.4 Adverse events related to implant, fracture, or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.4.1 Postoperative periprosthetic fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.4.2 Loosening | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.4.3 Superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.4.4 Dislocation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.3. Analysis.

Comparison 5: HA: short stem vs standard stem, Outcome 3: Pain (24 months)
5.4. Analysis.

Comparison 5: HA: short stem vs standard stem, Outcome 4: Adverse events related to implant, fracture, or both
Comparison 6. HA: ETS vs Thompson.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Delirium | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2 Early HRQoL (≤ 4 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3 Early mobility (freely mobile without aids, or able to walk outdoors with one aid) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.4 Early mortality (≤ 4 months) | 2 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.76, 1.88] |
| 6.5 Mortality (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.6 Unplanned return to theatre (end of follow‐up) | 2 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.05, 3.89] |
| 6.7 Adverse events related to implant, fracture, or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.7.1 Intraoperative periprosthetic fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.7.2 Deep infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.7.3 Superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.7.4 Dislocation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.8 Adverse events unrelated to implant, fracture, or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.8.1 Acute kidney injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.8.2 Blood transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.8.3 Cerebrovascular accident | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.8.4 Chest infection/pneumonia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.8.5 Myocardial infarction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.8.6 Venous thromboembolic phenomena (DVT) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.8.7 Venous thromboembolic phenomena (pulmonary embolism) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
6.7. Analysis.

Comparison 6: HA: ETS vs Thompson, Outcome 7: Adverse events related to implant, fracture, or both
6.8. Analysis.

Comparison 6: HA: ETS vs Thompson, Outcome 8: Adverse events unrelated to implant, fracture, or both
Comparison 7. HA: Furlong vs Moore.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Early mortality (≤ 4 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.2 Mortality (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.3 Unplanned return to theatre (at end of follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4 Pain at rest | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5 Adverse events related to the implant, fracture, or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5.1 Periprosthetic fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5.2 Superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5.3 Dislocation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
7.4. Analysis.

Comparison 7: HA: Furlong vs Moore, Outcome 4: Pain at rest
7.5. Analysis.

Comparison 7: HA: Furlong vs Moore, Outcome 5: Adverse events related to the implant, fracture, or both
Comparison 8. THA vs HA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Early ADL (≤ 4 months, using categorical data) | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.18] |
| 8.2 Early ADL (≤ 4 months; using social mobility scale (lower scores indicate better mobility) | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 8.3 ADL (12 months, using categorical data) | 2 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.07] |
| 8.4 ADL (12 months; using different measurement tools; lower scores indicate more independence)) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.5 Late ADL (> 24 months; using Barthel Index, range of scores from 0 to 100; higher scores indicate more independence) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.6 Delirium | 2 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.60, 3.33] |
| 8.7 Early functional status (≤ 4 months) | 3 | 395 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.07, 0.47] |
| 8.8 Functional status (12 months) | 8 | 1273 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.14, 0.44] |
| 8.9 Functional status (HHS; excellent or good) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.98, 1.17] |
| 8.10 Late functional status (> 24 months; using OHS and HHS; higher scores indicate better function) | 4 | 224 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [0.23, 1.08] |
| 8.11 Early HRQoL (≤ 4 months) | 2 | 279 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
| 8.12 HRQoL (12 months) | 4 | 1158 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.07, 0.31] |
| 8.13 HRQoL (> 24 months. Using SF‐36; higher scores indicate better quality of life) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 5.90 [‐1.99, 13.79] |
| 8.14 Early mobility (≤ 4 months; lower scores indicate better mobility | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.96, 0.16] |
| 8.15 Mobility (12 months, using TUG; lower values indicate better mobility) | 2 | 575 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐6.82, 1.35] |
| 8.16 Mobility (12 months, using 9‐point mobility scale; lower scores indicate better mobility) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.17 Mobility (12 months; able to ambulate independently) | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.71, 1.31] |
| 8.17.1 Modern design | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.53, 1.17] |
| 8.17.2 First generation uncemented stem | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.87, 1.36] |
| 8.18 Late mobility (> 24 months; able to ambulate independently) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.19 Early mortality (≤ 4 months) | 6 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.42, 1.42] |
| 8.19.1 Modern design | 4 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.32, 2.41] |
| 8.19.2 First generation uncemented stem design | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.30, 1.44] |
| 8.19.3 Age of design is unknown | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.51] |
| 8.20 Mortality (12 months) | 11 | 2667 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.22] |
| 8.20.1 Modern design | 10 | 2487 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.82, 1.28] |
| 8.20.2 First generation uncemented stem design | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.52, 1.42] |
| 8.21 Late mortality (> 24 months) | 8 | 931 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.23] |
| 8.21.1 Modern design | 7 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.32] |
| 8.21.2 First generation uncemented stem design | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 8.22 Unplanned return to theatre (end of follow‐up) | 10 | 2594 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.37, 1.07] |
| 8.22.1 Modern design | 9 | 2414 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.59, 1.25] |
| 8.22.2 First generation uncemented stem design | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.12, 0.66] |
| 8.23 Length of hospital stay (days) | 3 | 306 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐1.12, 2.73] |
| 8.24 Pain (12 months: data not combined; lower scores indicate less pain) | 9 | 1435 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.38, 0.12] |
| 8.25 Late pain (> 24 months) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.26 Pain (> 24 months: categorical data: no pain) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.07, 2.00] |
| 8.27 Early pain (≤ 4 months: higher scores indicate less pain) | 5 | 572 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.10, 0.30] |
| 8.28 Discharge destination (own home) | 2 | 1612 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.08] |
| 8.29 Discharge destination (geriatric ward) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.30 Adverse events related to implant, fracture, or both | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.30.1 Postoperative perioprosthetic fracture | 3 | 1557 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.66] |
| 8.30.2 Prosthetic loosening | 4 | 1889 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.17, 2.41] |
| 8.30.3 Deep infection | 8 | 2343 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.50, 1.54] |
| 8.30.4 Superficial infection | 10 | 2495 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.67, 2.30] |
| 8.30.5 Dislocation | 12 | 2719 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.17, 3.27] |
| 8.31 Adverse events unrelated to implant, fracture, or both | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.31.1 Acute kidney injury | 2 | 1561 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.62, 1.92] |
| 8.31.2 Blood transfusion | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.27, 3.61] |
| 8.31.3 Cerebrovascular accident | 4 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.63, 4.21] |
| 8.31.4 Pneumonia/chest infection (reported at > 4 months) | 5 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.38, 2.00] |
| 8.31.5 Myocardial infarction | 4 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.48, 4.58] |
| 8.31.6 Urinary tract infection | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.46] |
| 8.31.7 Venous thromboembolic phenomena (DVT) | 4 | 486 | Risk Ratio (M‐H, Random, 95% CI) | 4.25 [0.86, 21.06] |
| 8.31.8 Venous thromboembolic phenomena (pulmonary embolism) | 5 | 673 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.14, 1.63] |
8.23. Analysis.

Comparison 8: THA vs HA, Outcome 23: Length of hospital stay (days)
8.24. Analysis.

Comparison 8: THA vs HA, Outcome 24: Pain (12 months: data not combined; lower scores indicate less pain)
8.25. Analysis.

Comparison 8: THA vs HA, Outcome 25: Late pain (> 24 months)
8.26. Analysis.

Comparison 8: THA vs HA, Outcome 26: Pain (> 24 months: categorical data: no pain)
8.27. Analysis.

Comparison 8: THA vs HA, Outcome 27: Early pain (≤ 4 months: higher scores indicate less pain)
8.28. Analysis.

Comparison 8: THA vs HA, Outcome 28: Discharge destination (own home)
8.29. Analysis.

Comparison 8: THA vs HA, Outcome 29: Discharge destination (geriatric ward)
8.30. Analysis.

Comparison 8: THA vs HA, Outcome 30: Adverse events related to implant, fracture, or both
8.31. Analysis.

Comparison 8: THA vs HA, Outcome 31: Adverse events unrelated to implant, fracture, or both
Comparison 9. THA: single articulation vs dual‐mobility.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Early functional status (≤ 4 months, using different scales; higher scores indicate better function) | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.78, 0.12] |
| 9.2 Functional status (12 months, using OHS and HHS; higher scores indicate better function) | 2 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.05, ‐0.15] |
| 9.3 HRQoL (using EQ‐5D, range of scores from 0 to 1; higher scores indicate better quality of life) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.3.1 Early (≤ 4 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.3.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.4 Mortality (12 months) | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.08, 4.77] |
| 9.5 Adverse events related to the implant, fracture, or both | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.5.1 Deep infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.5.2 Superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.5.3 Dislocation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.6 Adverse events unrelated to the implant, fracture, or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.6.1 Venous thromboembolic phenomena | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
9.5. Analysis.

Comparison 9: THA: single articulation vs dual‐mobility, Outcome 5: Adverse events related to the implant, fracture, or both
9.6. Analysis.

Comparison 9: THA: single articulation vs dual‐mobility, Outcome 6: Adverse events unrelated to the implant, fracture, or both
Comparison 10. THA: short stem vs standard stem.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Functional status (at 24 months; using HHS, range of scores from 0 to 100; higher scores indicate better function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.2 Mobility | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3 Mortality (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.4 Pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.5 Adverse events related to the implant, fracture, or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.5.1 Intraoperative periprosthetic fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.5.2 Superficial infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.5.3 Dislocation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.6 Adverse events unrelated to the implant, fracture, or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.6.1 Acute kidney injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.6.2 Chest infection/pneumonia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.6.3 Urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
10.4. Analysis.

Comparison 10: THA: short stem vs standard stem, Outcome 4: Pain
10.5. Analysis.

Comparison 10: THA: short stem vs standard stem, Outcome 5: Adverse events related to the implant, fracture, or both
10.6. Analysis.

Comparison 10: THA: short stem vs standard stem, Outcome 6: Adverse events unrelated to the implant, fracture, or both
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelkhalek 2011.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: HA: bipolar vs unipolar |
|
| Participants |
Total number of randomised participants: 50 Inclusion criteria: elderly people with displaced femoral neck fractures Exclusion criteria: not reported Setting: single centre; hospital; Egypt Baseline characteristics (overall)
Note:
|
|
| Interventions |
General details: posterior surgical approach; the decision to use cement was applied on an individual basis; prophylactic low‐molecular‐weight heparin 12 hours preoperatively, and daily postoperatively for 5 days; ambulation with weight‐bearing as tolerated was started on POD2 or POD3. All participants were followed up and clinically evaluated at 6 weeks, 3 months, 6 months, 12 months and then annually. Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS (> 90 excellent, 80 to 90 good, 70 to 80 fair, < 70 poor); migration; acetabular erosion; subsidence; femoral loosening; pain (none, slight, mild, severe); dislocation; infection; DVT; range of motion; limping Outcomes relevant to the review: HHS (categorical data: excellent, good, fair, poor); pain (categorical data: none, slight, mild, severe); dislocation; infection; DVT; unplanned return to theatre Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: 2002 to 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternately allocated to groups |
| Allocation concealment (selection bias) | High risk | Not possible to conceal alternate allocation |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report prepublished protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Baker 2006.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 81 Inclusion criteria: diagnosis of a displaced fracture; > 60 years of age, a normal Abbreviated Mini Mental Test score, ability to walk ≥ 0.5 miles (≥ 0.8 km), ability to live independently (without reliance on a caregiver), a nonpathological fracture, a hip with no or minimal osteoarthritic changes Exclusion criteria: age of < 60 years, medical or physical comorbidities that limited the walking distance to < 0.5 miles (0.8 km), a pre‐existing hip abnormality requiring total hip arthroplasty, a pathological fracture secondary to malignant disease Setting: 3 centres; hospital; UK Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: surgeons of similar levels of training; HA: 31 by residents, 7 by consultants, 2 by senior house officers, 1 not documented; THA: 31 by residents, 9 by consultants; all received the same cemented femoral component (collarless polished tapered stem (Zimmer, Warsaw, Indiana)); transgluteal lateral approach. Followed up at 3 months, 1 year and 3 years after surgery Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (at 3 years and 9 years); OHS (3 years and 9 years); HRQoL (SF‐36, PCS and MCS; at 3 years and 9 years); walking distance (participant reported); postoperative complications within 30 days after surgery using anteroposterior and lateral radiographs: acetabular erosion, polyethylene wear, femoral stem subsidence, and component migration, dislocation, infection, thromboembolic events, pneumonia, atrial fibrillation, haematemesis, pressure sore, hypnotremia Outcomes relevant to the review: mortality (at 9 years); dislocation; infection; venous thromboembolic phenomena (pulmonary emboli, and DVT); pneumonia; functional status (OHS); mobility (walking distance, participant reported); HRQoL (SF‐36, PCS; at 9 years); unplanned return to theatre Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: no grants or external funding Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | "Randomization was performed with use of sealed envelopes that were opened before surgery"; insufficient information because study authors do not report if envelopes were sealed or opaque |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were all performed by surgeons of similar training but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report prepublished protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Blomfeldt 2007.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 120 Inclusion criteria: 70 to 90 years of age; absence of severe cognitive dysfunction demonstrated by ≥ 3 correct answers on the 10‐item SPMSQ; non‐institutionalised independent living status; pre‐injury independent walking capability with or without aids. Exclusion criteria: pathological fractures; displaced fractures present for > 48 hours before presentation; rheumatoid arthritis; osteoarthritis Setting: single centre; hospital; Sweden Intervention group 1 (THA)
Intervention group 2 (HA)
Notes:
|
|
| Interventions |
General details: 1 of 9 consultants experienced in both procedures; same cementing technique was used in both groups; low‐molecular‐weight heparin preoperatively and for ≥ 10 days postoperatively; cefuroxime 1.5 g was given preoperatively followed by 2 additional doses during the first 24 hours; mobilised bearing full weight with the aid of 2 crutches as tolerated Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: ADL (Katz; available at 4 and 12 months); HRQoL (EQ‐5D); living conditions (independent or institutional); intra‐operative blood loss, need for blood transfusion and duration of surgery; HHS and pain (available at 4, 12, 24, and 48 months); complications (dislocation, periprosthetic fracture, radiological signs of loosening of the femoral component, radiological signs of erosion in the acetabulum with a hemiarthroplasty, or loosening of the acetabular component in a THA, deep wound infection, superficial wound infection, pressure sores, cardiac, pulmonary, thromboembolic or cerebrovascular complications, any new fracture of the lower limb): mortality (at 12 months, 24 months, 48 months) Outcomes relevant to the review: mortality (at 12 months and 48 months); ADL (Katz) number categorised as A or B (at 4 months and 12 months); functional status (using HHS; at 4 months, 12 months and 48 months); pain (using HHS; at 4 months, 12 months, and 48 months); loosening (12 months); complications (DVT; MI; pneumonia; at 4 months); dislocation (12 months); perioperative complications (superficial infection) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: supported in part by a grant from the Trygg‐Hansa Insurance Company and the Stockholm County Council Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised by a sealed‐envelope technique" Comment: no additional details |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes; no additional details |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeons in the study were experienced in both techniques, and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most participant loss was because of death, which is expected in this population. Few lost to follow‐up, and these losses were relatively balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report prepublished protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Brandfoot 2000.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 91 Inclusion criteria: all participants to be treated with HA Exclusion criteria: pathological fractures; selected for internal fixation or THA Setting: single centre; hospital; UK Baseline characteristics (overall)
Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: Thompson HA for both groups; performed by a consultant 9 times, specialist registrar 70, senior house officer 12; all received the same postoperative care. Routine follow‐up at approximately 6 weeks and 6 to 9 months (and later, if problems identified) Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality; radiographs (dislocation and failures) and telephone interview; modified HHS; mean follow‐up 16 months (range 8 to 20) for functional assessment Outcomes relevant to the review: mortality, loosening; total function scores; mobility; ADL (using modified HHS for activities: stairs, shoes, socks, bath; higher scores indicate more independence); pain; all at 16 months |
|
| Notes |
Declarations/sponsorship/declarations of interest: not reported Study dates: 1 January 1998 to 31 December 1998 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used, but no further details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report seniority of staff involved in the surgery but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Cadossi 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 96 Inclusion criteria: displaced intracapsular femoral neck fracture, Garden type III or IV; ≥ 70 years of age; pre‐injury independent walking capability without aids Exclusion criteria: advanced radiological osteoarthritis or rheumatoid arthritis in the fractured hip; suspected pathological fracture; senile dementia Setting: single centre; hospital; Italy Baseline characteristics Intervention group 1 (THA; data reported only for 42 participants)
Intervention group 2 (HA; data reported only for 41 participants)
|
|
| Interventions |
General details: performed by 2 experienced surgeons; mobilised bearing full‐weight with the aid of 2 crutches as tolerated Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (data available at 1 year, 2 years, 3 years); HHS (data available at: 3 months,1 year, 2 years, 3 years); dislocation; revision operations and implant‐related complications: stem subsidence, osteoarthritis of the acetabulum, protrusio acetabuli, fractures and fissures, and heterotopic ossification according to the classification of Brooker Outcomes relevant to the review: mortality (at 12 months and 3 years), functional status (using HHS; at 3 months, 12 months, and 3 years), pain (using HHS at 3 months, 12 months, 3 years), length of stay in hospital Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: no external funding Study dates: March 2008 to April 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | Described as sealed envelopes, no further details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were all performed by experienced staff, but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For functional status and pain, we noted a large number of losses in each group; some losses could be explained by death but other losses are not explained. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Calder 1995.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar vs unipolar Note:
|
|
| Participants |
Total number of analysed participants: 73 (total randomised participants not reported) Inclusion criteria: 65 to 79 years of age; displaced intracapsular fracture (Garden stage III to IV) Exclusion criteria: mental test score < 5; uncontrolled Parkinson’s disease; disseminated malignancy or pathological fracture; rheumatoid arthritis; long‐term steroid therapy Setting: single centre; hospital; UK Intervention group 1 (bipolar; data available only for analysed participants)
Intervention group 2 (unipolar; data available only for analysed participants)
Note:
|
|
| Interventions |
General details: no details Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: Nottingham Health Profile (pain, physical mobility, sleep, energy, social, emotion) Outcomes relevant to the review: pain and mobility (using Nottingham Health Profile; at 6 months) |
|
| Notes |
Funding/sponsor/declarations of interest: no commercial funding Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. We could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | It is not reported whether participants were blinded to the intervention group; however, we did not expect this would influence outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of randomised participants is not reported for the intervention groups in this study, but it is understood that only a few participants responded to the questionnaire and provided outcome data for this study. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Calder 1996.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 250 Inclusion criteria: > 80 years of age; displaced intracapsular fracture (Garden stage III to IV) Exclusion criteria: mental test score < 5; uncontrolled Parkinson’s disease; disseminated malignancy or pathological fracture; Paget’s disease involving the proximal femur on the side of the fracture; rheumatoid arthritis; long‐term steroid therapy Setting: single centre; hospital; UK Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: all carried out by one surgeon; "a Hardinge direct lateral approach was used in the same conventional operating theatre which did not have laminar flow air supply. The prostheses were cemented into the femur with normal viscosity cement in an orthograde manner using a syringe and a vent but no cement restriction"; mobilised fully weight‐bearing after 24 to 48 hours with assistance from physiotherapists. Outpatient assessment carried out at 6 to 8 weeks, followed by annual reviews Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (in hospital; at 2 monthly intervals up to 12 months); return to preoperative place of residence; return to pre‐injury state; no limp; no or mild pain; satisfied with operation; HHS; length of hospital stay Outcomes relevant to the review: mortality (at 4 and 12 months); return to preoperative place of residence; no pain or mild pain; dislocation; deep infection; length of hospital stay; mobility and pain scores (Nottingham Health Profile) Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: no commercial funding Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerised random‐number generation" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by one surgeon but we could not be certain whether they were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Not reported whether participants were blinded to the intervention; although unlikely that this would influence participants' decisions. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We noted a large number of deaths, but these were balanced between groups, and we assumed that data were complete for other outcomes. We included data from an interim report for mobility and pain, which included fewer participants, and we could not be certain whether this data included attrition. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Cao 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 85 Inclusion criteria: intertrochanteric fracture; ≥ 66 years old; normal walking without or with single stick before fracture; Evans‐Jensen type III to V fracture complicated with many underlying diseases Exclusion criteria: new or old cerebral thrombosis or Evans‐Jensen type I fracture; cardiac insufficiency; pathological fracture; complicated with coagulation disorders; mental diseases Setting: single centre; hospital; China Baseline characteristics (overall)
Note:
|
|
| Interventions |
General details: subarachnoid block combined epidural anaesthesia; post‐lateral approach; prophylactic anticoagulation and antibiotics; DVT prevention; mobilisation within 3 days to 1 week postoperatively; partial weight‐bearing after 3 weeks post‐surgery; full weight‐bearing after 3 months. Follow‐up at 1, 3, 6 months and 1 year after the surgery Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; total blood loss; ambulation time; HHS (available at 1, 3, and 6 months); loosening; neurovascular injury; infection; fracture; dislocation; pain in non‐femoral region; mortality Outcomes relevant to the review: HHS (3 months, 6 months); superficial and deep infection; fracture; loosening; dislocation; venous thromboembolic phenomena (neurovascular injury); intraoperative fracture Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: January 2012 to January 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number table method" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We assumed no losses. We noted a minor discrepancy in the number of reported participants in the study report between text and tables; we did not expect this to influence outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Chammout 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 69 Inclusion criteria: displaced femoral neck fracture (Garden III–IV); surgery within 48 hours; age 65–79 years; no concurrent joint disease or previous fracture in the lower extremities; intact cognitive function (no diagnosis of dementia and at least 7 correct answers on a 10‐item SPMSQ); ability to ambulate independently with or without walking aids Exclusion criteria: pathological fractures; rheumatoid arthritis; symptomatic osteoarthritis; severe comorbidities; deemed unsuitable for a THA by the anaesthesiologist Setting: single centre; hospital; Sweden Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Notes:
|
|
| Interventions |
General details: 22 surgeons (at consultant or specialist level) who were experienced in both procedures; direct lateral approach; preoperative surgical planning was performed; 32 mm cobalt‐chromium head was used in all participants; low‐molecular‐weight heparin postoperatively for at least 10 days; preoperative antibiotic prophylaxis with cloxacillin (2 g); 3 additional doses during the first 24 hours; participants were mobilised without any restrictions Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: hip‐related complications and re‐operations, HRQoL (assessed with EQ‐5D index; at 3 months, 12 months, and 24 months); complications: intraoperative and postoperative periprosthetic fracture, dislocations, wound infection (both superficial and deep), early and late loosening, and re‐operation of the hip for any reason; at 24 months; mortality and hip function at 3, 12, and 24 months (using HHS; at 3 months, 12 months, and 24 months); pain (using Pain Numerical Rating Score; at 3 months, 12 months, and 24 months); ADL (at 3 months, 12 months, and 24 months); intraoperative bleeding, duration of surgery, and intraoperative vital signs; serological markers of inflammation and thrombosis preoperatively and postoperatively; cardiovascular events; acute heart infarction; cerebral vascular lesions; pulmonary embolism; DVT; heterotopic ossification at 24 months Outcomes relevant to the review: unplanned return to theatre, dislocation, intraoperative periprosthetic fracture, postoperative periprosthetic fracture, superficial infection, loosening of prosthesis (defined by study authors as unstable stem) (all reported at 24 months); mortality (at 12 months); HRQoL (using EQ‐5D), functional status (using HHS), ADL, pain (using Pain Numerical Rating Score), (all functional outcomes reported at 3 and 12 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: September 2009 to 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes, but does not state whether envelopes were opaque or numbered |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Participants were blind to allocation. It is not possible to blind surgeons to treatment groups. The surgeons in the study were experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Participants were blind to allocation. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was mostly clearly reported, with few losses which were reasonably balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors reported a pre‐published protocol and clinical trials registration (NCT02247791). The study commenced in 2009 but the clinical trial registration did not occur until 2013 and the protocol was published in 2016. We cannot feasibly assess the risk of reporting bias using these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Chammout 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 120 Inclusion criteria: acute displaced femoral neck fracture (Garden III or IV); occurred < 36 hours previously; ≥ 80 years of age; ability to walk independently with or without walking aids; intact cognitive function with a SPMSQ score of 8 to 10 points Exclusion criteria: pathological fracture; osteoarthritis; people with rheumatoid arthritis in the fractured hip; people who were non‐walkers; deemed unsuitable for participation Setting: single centre; hospital; Sweden Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: performed either by a consultant orthopaedic surgeon or by a registrar with assistance from a consultant; direct lateral approach with the patient in the lateral decubitus position; modular, collarless, polished, tapered cemented femoral component (CPT; Zimmer) was used until 2014 ‐ changed to an anatomically shaped cemented stem (Lubinus SP2; Waldemar Link); vacuum‐mixed low‐viscosity cement with gentamicin (Palacos with gentamicin; Schering‐Plough) was used in all patients; antibiotic and anticoagulant prophylaxis, weight bearing the day after surgery Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS, HRQoL (EQ‐5D), Pain Numerical Rating score, ADL (available at 3, 12, and 24 months), mortality (at 24 months); surgical time, intraoperative bleeding, ability to regain previous walking function (at 2 years); adverse events, including cardiovascular events (at 2 years): dislocation, superficial infection, deep periprosthetic infection, non‐healing fracture, total number of hip complications, number of participants with re‐operation, closed reduction, surgical debridement, another major re‐operation, pneumonia, pulmonary embolism, myocardial infarct, cerebrovascular lesion, acute kidney failure Outcomes relevant to the review: ADL (number of people who were fully independent in ADL; at 3 months and 12 months), functional status (using HHS; at 3 months and 12 months), HRQoL (using EQ‐5D utility index ‐ VAS not reported; at 3 months and 12 months), pain (using VAS; at 3 months and 12 months); adverse events (at 2 years): MI, pulmonary embolism, cerebrovascular accident, infection, acute kidney injury, dislocation, pneumonia; mortality (at 24 months); unplanned returned to theatre (2 years); discharged to geriatric ward Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: funded by grants from the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet. Study dates: September 2009 to 2018; recruitment September 2009 to April 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes; however, study authors do not report if envelopes are opaque and sequentially numbered and we have therefore judged that there is insufficient information. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | Particpants blinded but It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed or supervised by consultants but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Participants blinded to intervention |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most participant loss was because of death, which is expected in this population. Other losses (owing to participants that withdrew from the study) were relatively balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Protocol published in 2015, and retrospective clinical trials registration in 2014 (NCT02246335; first received September 2014). Because the study started in 2009, it is not feasible to effectively assess risk of selective reporting bias with these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Cornell 1998.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 48 Inclusion criteria: displaced femoral neck fracture Exclusion criteria: < 65 years; previous surgery involving the fractured hip; pathological fracture; life expectancy < 1 year; inability to make competent decisions regarding healthcare Setting: single centre; hospital; Sweden Baseline characteristics Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: all performed through posterior approach with a cemented modular femoral component; preoperative antibiotics; spinal or general anaesthetic; postoperative thromboembolic prophylaxis; weight‐bearing where tolerated; postoperative clinical follow‐up at 6 weeks, 3 months and 6 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: postoperative complications: dislocation; range of motion; length of hospitalisation; cost of prosthesis; operative time; estimated blood loss; functional (Johansen hip score); 6MWT; Get Up and Go test Outcomes relevant to the review: mortality (at 6 months); functional status (Johansen hip rating questionnaire); mobility (Get Up and Go, in seconds; at 6 months); dislocation; length of stay Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: study started in July 1996; finish date not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random generated order with sealed envelopes, opened prior to anaesthesia; method of randomisation not clearly explained. We noted an uneven number of participants in each group which could indicate that the method of randomisation was not adequate. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes; study authors did not state whether envelopes were opaque and sequentially numbered and we have therefore judged that there is insufficient information |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | Participants blinded. It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | Low risk | Assessments for functional outcomes/mobility completed by a physical therapist blinded to allocation |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect that lack of blinding would influence participant‐reported outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Davison 2001.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar (versus internal fixation) Note:
|
|
| Participants |
Total number of randomised participants: 187 Inclusion criteria: displaced intracapsular fracture of the proximal femur; aged 65 to 79 years Exclusion criteria: AMTS < 5/13; uncontrolled Parkinson’s disease; pathological fracture; disseminated malignancy; Paget’s disease; rheumatoid arthritis; long‐term steroid therapy Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: lateral (Hardinge) approach; identical collar‐and‐stem profiles; methylmethacrylate cement; immediate weight‐bearing; clinical follow‐up at 6 weeks, then annually for 5 years ‐ a home assessment was carried out annually by a research occupational therapist who was blind to the participant's operative treatment Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: verbally‐conducted functional assessment questionnaire, in addition to HHS (HHS; data available at 1, 2, 3, 4, and 5 years); loosening and subsidence; mortality (data available at 6, 12, 18, 24, 30, and 36 months); revision (data available at 6, 12, 18, 24, 30, and 36 months); Barthel Index; return to pre‐injury state, patient satisfaction Outcomes relevant to the review: mortality (at 12 months and 36 months); complications (infection and dislocation ‐ up to 5 years); unplanned return to theatre; HHS (12 months and 5 years), Barthel Index Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: no funding from commercial funding; study report states that "benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non‐profit organisation with which one or more of the authors are associated" Study dates: January 1991 to January 1996 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generation of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
DeAngelis 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 130 Inclusion criteria: > 55 years; nonpathologic displaced femoral neck fracture; scheduled for HA by the attending orthopaedic surgeon; able to ambulate 10 feet before presentation Exclusion criteria: multiple extremity trauma; clinically recognised acute MI within 30 days before enrolment; anaemia; pre‐existing metabolic bone disease Setting: single centre; hospital; USA Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Overall
Note:
|
|
| Interventions |
General details: performed by the attending orthopaedic surgeon; spinal or general anaesthetic; placed in the lateral decubitus position, and a standard anterolateral or posterolateral approach was used; all participants received a unipolar head; all participants were allowed to weight bear to tolerance Intervention group 1
Intervention group 2
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional outcome at 1 year; IADL and PADL scales were obtained using a modified version of the Older Americans Resources and Services Instrument; mortality (in hospital and at 30 days, 60 days, and 1 year); postoperative unstable angina, and MI; unstable angina; pneumonia, wound infection, thromboembolism, and stroke; ability to walk independently; discharge destination; functional outcome questionnaire was completed by telephone at 30 days, 60 days, and 1 year. Outcomes relevant to the review: mortality (at 60 days, and 1 year); functional: IADL (at 60 days and 1 year); acute postoperative complications (pneumonia, MI, wound infection, CVA, thromboembolic event, re‐operation); intraoperative fracture; blood transfusion; discharge destination (assisted living, rehabilitation facility, home) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: supported by a restricted research grant from Zimmer, Inc (Warsaw, IN). Funds allocated to hospital costs associated with randomisation (implants and surgical supplies), and not for salary costs Study dates March 2005 and May 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were all performed by orthopaedic surgeons but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss of participants is unknown. We attempted to contact study authors to clarify numbers of participants who died, and numbers of participants available for ADL data. For complications data and discharge destination, data appear to be complete |
| Selective reporting (reporting bias) | Unclear risk | Study is retrospectively registered with a clinical trials register (NCT01114646; first posted in May 2010). It was not feasible to use these retrospective documents to assess risk of selective reporting bias. |
| Other bias | Low risk | We identified no other sources of bias. |
Dorr 1986.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA: cemented versus uncemented Note:
|
|
| Participants |
Total number of randomised participants: 89 Inclusion criteria: oriented and ambulatory patients (classes 1 and 2); Garden type III or IV Exclusion criteria: < 55 years of age (apart from 5 included younger patients); "totally confused and nonambulatory patients" Setting: single centre; hospital; USA Baseline characteristics Intervention group 1 (THA)
Intervention group 2 (HA cemented)
Intervention group 3 (HA uncemented)
Overall
Note:
|
|
| Interventions |
General details: performed through a posterior approach; capsule and external rotators were re‐attached; antibiotics for 72 hours, aspirin for thromboembolism prophylaxis, and progressive ambulation beginning on the second day after operation Intervention group 1
Intervention group 2
Intervention group 3
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality; infections; reoperation; dislocation; modified d'Aubigne and Postel hip score (D'Aubigne 1954); heterotopic ossification; progressive femoral and acetabular cement‐bone demarcatlon; subsidence; calcar resorption; calcar sclerosis; gait analysis; not walking at final follow‐up; pain and ambulation (available at 3, 12, and 24 months) Outcomes relevant to the review: mortality; not walking at final follow‐up (between 2 and 4 years); infections (between 2 and 4 years); re‐operation and dislocations (between 2 and 4 years); pain and ambulation (using 6 point scale in which high scores indicate less pain/better mobility; at 3 and 24 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: supported by grants from the Canadian Institutes of Health Research, the National Institutes of Health, ZorgOnderzoek Nederland‐Medische Wetenschappen, Sphies Minde Foundation for Orthopaedic Research, McMaster Surgical Associates, and Stryker Orthpaedics Study dates March 1980 and July 1992 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation based on odd or even hospital numbers |
| Allocation concealment (selection bias) | High risk | It is not feasible to conceal allocation because of the quasi‐randomised methods used to allocate participants to groups. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect that lack of blinding of participant‐reported outcomes would influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We could not be certain whether data were complete because numbers of losses were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Emery 1991.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 53 Inclusion criteria: displaced subcapital fracture of the femoral neck Exclusion criteria: admitted from nursing homes or from other hospitals; use > 1 stick to walk Setting: single centre; hospital; UK Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: operations performed by same group of junior staff; Monk duoplet design; participants were mobilised, partial weight‐bearing using crutches or a frame; full weight‐bearing allowed when comfortable (2 or 3 months) Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: complications: pulmonary embolus, wound infection, chest infection, bedsore, renal failure secondary to a gastro‐intestinal bleed, urinary tract infection, aortic aneurysm; mortality (at 2 weeks, 3 months, 17 months); pain (measured as presence of any pain); increased dependency on walking aids; change in residential setting (moved to more supportive accommodation) Outcomes relevant to the review: mortality (3 months and 17 months); pain (measured as presence of any pain; at 17 months); increased dependency on walking aids; wound infection; chest infection; pulmonary embolism; length of stay (excluding those who died before hospital discharge) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: no funding from commercial funding; study report states that "benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non‐profit organisation with which one or more of the authors are associated" Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the time of the operation a randomised card was drawn from a sealed envelope ; this decided whether each patient had an uncemented bipolar hemiarthroplasty with a Moore stem, or a cemented prosthesis with a Thompson stem" Comment: study authors do not report method used to ensure that cards are randomised |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. Study authors do not report whether envelopes are numbered or opaque and we have therefore judged that there is insufficient information. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by all junior staff, we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding of participant‐reported outcomes to influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Fernandez 2022.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 1225 Inclusion criteria: all people, with and without capacity, presenting with a displaced intracapsular fracture of the hip suitable for HA Exclusion criteria: < 60 years old; managed non‐operatively; treated with a THA Setting: multicentre; 14 hospitals; UK Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: appropriate preparation, positioning and surgical technique will be left to the discretion of the operating surgeon, according to their normal clinical practice; range of surgeon experience including consultant, specialty and associate specialist; speciality trainee surgeons and staff grade Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality; HRQoL; discharge destination; mobility; adverse events: dislocation; DVT; cerebrovascular injury; wound infection; venous thromboembolism; pneumonia; UTI; MI; blood transfusion; acute kidney injury; per‐prosthetic fracture; neurological injury; vascular injury; tendon injury; erythema; dehiscence; chest infection; failure of fixation; unplanned return to theatre Outcomes relevant to the review: mortality (4 and 12 months); HRQoL (EQ‐5D, 4 and 12 months); ADL ('usual activities'; using 5‐point Likert scale from EQ‐5D; at 4 and 12 months); pain (using 5‐point Likert scale from EQ‐5D; at 4 and 12 months); discharge destination; mobility (mobile/no aids/one aid/two aids/indoor only/none; 4 and 12 months); adverse events: dislocation; DVT; cerebrovascular injury; wound infection; pneumonia; UTI; MI; blood transfusion; acute kidney injury; pulmonary embolism; periprosthetic fracture; unplanned return to theatre Note:
|
|
| Notes |
Funding/sponsor/declarations of interest: National Institute for Health Research, Research for Patient Benefit Study dates: March 2017 to December 2019 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated by CTU |
| Allocation concealment (selection bias) | Low risk | Allocation concealed due to randomisation being performed by the statistician from the CTU |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. We could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Participants blind to intervention |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | We noted a large number of losses for participant‐reported outcomes at 12 months. These losses were mostly owing to death, but some were because of missing data and owing to withdrawn participants. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered with clinical trials register (ISRCTN18393176; first received March 2017). Outcome data supplied by study authors are consistent with outcomes in the clinical trials register. |
| Other bias | Low risk | We identified no other sources of bias. |
Figved 2009.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 230 fractures (223 participants; 7 participants with both hips were included); 3 protocol violations results in 220 patients Inclusion criteria: ≥ 70 years of age; displaced intracapsular fracture of femoral neck Exclusion criteria: unfit for arthroplasty according to the anaesthesiologist on call; osteoarthritis; fracture caused by malignant disease; ongoing infectious disease; unable to walk before the fracture Setting: 2 centres; hospitals; Norway Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: 36 surgeons; 11 patients received a 28 mm cobalt‐chromium head and the same bipolar head (Mobile Cup; DePuy); posterior approach with the patient in a lateral decubitus position; spinal anaesthesia; 2 g preoperative intravenous cefalotin and an additional three doses the first 16 hours after the operation; 5000 IU low‐molecular‐weight heparin subcutaneously daily for at least 7 days; early mobilisation was encouraged in all participants with weight bearing as tolerated. Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: duration of surgery; blood loss; blood transfusions; length of stay in hospital; mortality (at 7, 30, 90 days; and at 12, 24 months, 5 years); HHS, Barthel Index and EQ‐5D (available at 3 months, 12 months, 5 years); living in own home (discharge, 3 and 12 months); no pain medication (discharge, 3, 12 months, 5 years); walking independently (at discharge, 3 and 12 months); pneumonia; dislocation; DVT; superficial (wound) infection; pulmonary embolism; fracture of the contralateral hip; deep infection; intraoperative periprosthetic fracture; postoperative periprosthetic fracture; postoperative MI not leading to death; perioperative death; intraoperative severe decrease in blood pressure during preparation of the femoral canal; perioperative MI leading to death; intraoperative cardiac arrest Outcomes relevant to the review: blood transfusions; length of stay in hospital; mortality (3, 12 months, 5 years); HHS (3, 12 months, 5 years); ADL (participants with Barthel Index of 19 or 20; at 3, 12 months, 5 years); EQ‐5D (we have used data from the VAS in analysis; index score also available; at 3 months, 12 months, 5 years); living in own home (at discharge); no pain medication (3 months, 12 months, 5 years); walking independently (3 and 12 months); unplanned return to theatre (12 months); intraoperative fracture; loosening of prosthesis; MI; pneumonia; dislocation; DVT; superficial infection; pulmonary embolism; deep infection; intraoperative periprosthetic fracture; postoperative periprosthetic fracture; postoperative MI not leading to death and perioperative MI leading to death Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: funding from Eastern Norway Regional Health Authority (nonprofit, governmental). At least 1 study author received funding from Smith & Nephew, Inc, and from OrtoMedic AS Study dates: September 2004 to August 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed separately for the two hospitals using a computer random number generator with permuted blocks of five" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was done by the surgeon on call using sealed, numbered, opaque envelopes" |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed according to usual hospital procedures but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Collected with assistance of research nurses who were blind to intervention |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death or otherwise clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered with a clinical trials register (NCT00491673; first received June 2007). Study commenced in 2004 and it was not feasible to effectively assess risk of selective reporting bias with these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Figved 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 28 Inclusion criteria: ≥ 70 years of age; displaced intracapsular fracture femoral neck; living independently; walking without aids Exclusion criteria: cognitive impairment; osteoarthritis; a fracture caused by malignant disease; ongoing infectious disease Setting: single centre; hospital; Norway Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: uncemented pressfit hydroxyapatite‐coated femoral stem (Corail, DePuy Orthopaedics Inc, Warzaw, IN, USA); posterior approach with the patient in the lateral decubitus position; spinal anaesthesia; 6 experienced surgeons; preoperative IV cefalotin 2 g and a further 3 doses in the first 12 hours after the operation; 5000 IU low‐molecular‐weight heparin subcutaneously daily for at least 10 days; early mobilisation was encouraged, with weight bearing as tolerated Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: migration of femoral head, cartilage wear; HHS, EQ‐5D index and VAS (at 3, 12, and 24 months); mortality (data available at 12 months and 24 months) Outcomes relevant to the review: functional status (HHS; at 12 months) HRQoL (EQ‐5D index; at 12 months); mortality (at 12 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: research grant from Smith & Nephew, Norway. Study authors declare no other conflicts of interest Study dates: Sept 2004 to August 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computer random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was done by the surgeon on call using sealed envelopes" Comment: study authors do not report if envelopes are opaque and sequentially numbered. However, because the same study authors report using opaque, numbered envelopes in Figved 2009, we have assumed this to also be the case in this study. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by all experienced surgeons but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Uncertain whether participants were blind of the intervention, but unlikely to effect their decisions on HHS or EQ‐5D |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for attrition are clearly reported in CONSORT diagram; losses are few and are balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered with clinicaltrials.gov (NCT00746876, first received September 2008). It is not feasible to use these documents to effectively assess risk of selective reporting bias. |
| Other bias | Low risk | We identified no other sources of bias. |
Griffin 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA with single articulation vs THA with dual mobility (DM) |
|
| Participants |
Total number of randomised participants: 21 Inclusion criteria: aged > 60 years; displaced intracapsular fracture Exclusion criteria: chronic cognitive impairment; in the opinion of the consultant trauma surgeon the patient would not benefit from a THA; treated non‐operatively Setting: single centre; hospital; UK Intervention group 1 (THA)
Intervention group 2 (THA‐DM)
Note:
|
|
| Interventions |
General details: antibiotic and venous thromboembolic prophylaxis, procedure undertaken in lateral position; routine follow‐up at 1, 4 and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: dislocation; OHS, EQ‐5D, ICECAP‐O ‐ available at 1 month. 4 months, and 12 months; mortality (12 months); re‐operation. Outcomes relevant to the review: dislocation; EQ‐5D and OHS (4 months and 12 months); mortality (12 months); re‐operation Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: funded by National Institute for Health Research Portfolio Study dates: June 2013 to May 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to treatment groups. |
| Allocation concealment (selection bias) | Low risk | Randomisation was administered via an online service administered by an independent Clinical Trials Unit. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | Participants and research associates, but not the operating surgeon, were blinded to the allocation of treatment. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Participants were blind to the intervention. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up is clearly reported. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered with a clinical trials register (ISRCTN90544391, first received April 2013). Outcomes in the published report are consistent with those in clinical trial registration and protocol. |
| Other bias | Low risk | We identified no other sources of bias. |
Harper 1994.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 137 Inclusion criteria:
Exclusion criteria: none reported Setting: single centre; hospital; UK Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: a direct lateral approach was used; patient supine; femoral head diameter was measured and a prosthesis of appropriate size used; Thompson prostheses; weight‐bearing after 48 hours Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: dislocation; mortality; superficial and deep infection; length of stay in hospital; pain Outcomes relevant to the review: dislocation (at 2 months); mortality (3 and 12 months); dislocations (2 month), superficial and deep infection (2 months); length of stay in hospital; pain (3 months) |
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: January 1989 to January 1990 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not clearly described |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
HEALTH 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 1495 Inclusion criteria: adult men or women ≥ 50 years of age (with no upper age limit); fracture of the femoral neck confirmed with anteroposterior and lateral radiographs, or CT or MRI; displaced fracture that is not, in the judgment of the attending surgeon, optimally managed by reduction and internal fixation; operative treatment within 72 hours of the patient being medically cleared for surgery; patient was ambulatory prior to fracture, though they may have used an aid such as a cane or a walker; anticipated medical optimisation for arthroplasty of the hip; provision of informed consent by patient or proxy; low‐energy fracture (defined as a fall from standing height); no other major trauma (defined as an ISS < 17); assurance that surgeons with expertise in both THA and HA are available to perform surgery Exclusion criteria: not suitable for HA (e.g. inflammatory arthritis, rheumatoid arthritis, pathological fracture (secondary to cancer) or severe osteoarthritis of the hip); associated major injuries of the lower extremity (e.g. ipsilateral or contralateral fractures of the foot, ankle, tibia, fibula, knee or femur; dislocations of the ankle, knee or hip; or femoral head defects or fracture); retained hardware around the affected hip that will interfere with arthroplasty; infection around the hip (soft tissue or bone); disorder of bone metabolism other than osteoporosis (e.g. Paget’s disease, renal osteodystrophy, osteomalacia); previous history of frank dementia that would interfere with assessment of the primary outcome (i.e. secondary procedures at 2 years); likely problems, in the judgement of the investigators, with maintaining follow‐up (e.g. people with no fixed address, report a plan to move out of town, alcohol abuse issues or intellectually‐challenged people without adequate family support); fracture occurred as a result of an act of violence Setting: multicentre; hospital; Canada, USA, Spain, UK, the Netherlands, Norway, Finland, New Zealand, South Africa Intervention group 1 (THA; data missing for small number of participants for some outcomes)
Intervention group 2 (HA; data missing for small number of participants for some outcomes)
Note:
|
|
| Interventions |
General details: each surgical team used their preferred implant, surgical technique, type of anaesthesia, postoperative mobility/weight‐bearing regimen approach. All are reported in study appendices along with clinicians' skills and experience. Preoperative antibiotic prophylaxis; thromboprophylaxis; medical consultation to optimise condition prior to surgery; postoperative antibiotic prophylaxis for 24 hours; thromboprophylaxis; weight‐bearing as tolerated; 600 mg calcium by mouth daily; 1000 IU vitamin D per day Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: unplanned secondary hip procedure within 24 months; death; serious adverse events; hip related complications; HRQoL (SF‐12 and EQ‐5D); function (WOMAC and TUG scores) Outcomes relevant to the review: unplanned return to theatre; mortality (at 2 years); periprosthetic fracture; dislocation; deep and superficial infection; loosening; discharge destination; functional status (WOMAC); pain (WOMAC); mobility (TUG); HRQoL (EQ‐5D; at 24 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: supported by grants from the Canadian Institutes of Health Research, the National Institutes of Health, ZorgOnderzoek Nederland‐Medische Wetenschappen (ZonMw), Sophies Minde Foundation for Orthopaedic Research, McMaster Surgical Associates, and Stryker Orthopaedics Study dates: January 2009 to May 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation with minimisation |
| Allocation concealment (selection bias) | Low risk | Quote: "centralised 24 h computerised randomisation system that will allow internet‐based randomisation" |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors describe the experience level of surgeons in each group, and we noted these were evenly balanced. However, it is unclear if each surgeon was equally experienced with both types of implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect that lack of blinding for participant‐reported outcomes would influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial numbers of participants lost to follow‐up and reported as "unable to locate" and "withdrew consent" |
| Selective reporting (reporting bias) | Low risk | Protocol published and prospectively registered with a clinical trials register (NCT00556842; first received November 2007). Outcomes reported in the published report are consistent with those in the prospectively published documents. |
| Other bias | Low risk | We identified no other sources of bias |
Hedbeck 2011.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 120 Inclusion criteria: acute displaced femoral neck fracture (Garden III and IV); > 80 years of age; absence of severe cognitive dysfunction; independent living status; independent walking capability Exclusion criteria: pathological fractures; displaced fractures older than 48 hours; people with rheumatoid arthritis or osteoarthritis Setting: single centre; hospital; Sweden Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: 1 of 16 surgeons, all specialists in orthopaedic surgery experienced in both procedures; anterolateral approach; Exeter stem (modular); low‐molecular‐weight heparin given preoperatively and for at least 10 days postoperatively; cloxacillin 2 g was given preoperatively, followed by 2 additional doses during the first 24 hours; mobilised with full weight‐bearing as tolerated; clinical follow‐up at 4 months and 12 months Intervention group 1
Intervention group 2
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality; hip complications; general complications; ADL status (at 12 months); hip function (HHS; available at 4 months and 12 months); EQ‐5D (available at 4 months and 12 months); independent living; perioperative parameters (blood loss, duration of surgery) ; dislocations, infection; Outcomes relevant to the review: mortality (at 4 and 12 months); EQ‐5D index (VAS not available; at 4 months and 12 months), ADL Katz index A or B (at 12 months); unplanned return to theatre (at 12 months); adverse events: dislocation, deep infection, periprosthetic fracture; pneumonia; cardiac complication, DVT, pulmonary embolism (all at 12 months); function and pain (HHS; at 4 months and 12 months) |
|
| Notes |
Funding/sponsor/declarations of interest: grants from the Trygg‐Hansa Insurance Company and through the Regional Agreement on Medical Training and Clinical Research (ALF) between the Stockholm County Council and Karolinska Institutet Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on method of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed‐envelope technique, independently prepared" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeons in the study were experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | Outcomes assessed by a nurse independent to the surgical team; however, the "research nurse was not blinded to the type of surgical intervention" |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Not reported whether participants were blind to intervention, although unlikely to effect outcomes |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Inngul 2015.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA & HA: cemented versus uncemented
|
|
| Participants |
Total number of randomised participants: 141 Inclusion criteria: acute, displaced (Garden's III or IV) fracture of the femoral neck following low‐energy trauma Exclusion criteria: people who sustained a fracture > 48 hours before admission and those with rheumatoid arthritis and symptomatic osteoarthritis Setting: single centre; hospital; Sweden Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: performed by consultant orthopaedic surgeons experienced in the use of cemented and uncemented stems; lateral decubitus position via a direct lateral approach; spinal anaesthesia; prophylactic antibiotics 30 to 60 minutes preoperatively, and 3 and 6 hours later; low‐molecular‐weight heparin, postoperatively and continued for 30 days; weight‐bearing as tolerated Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: HRQoL questionnaire (EQ‐5D); SMFA; HHS; bleeding and operating time; adverse events; post‐operative heterotopic ossification; acetabular erosion; mortality (4 months and 12 months); intra‐operative femoral fracture; intra‐operative fracture of the tip of the greater trochanter
Outcomes relevant to the review: adverse events (at 12 months): intraoperative periprosthetic fracture (intra‐operative femoral fracture); unplanned return to theatre (for dislocation, periprosthetic fracture and for deep infection); superficial wound infection; UTI; pneumonia; acute MI; acute renal failure; mortality (4 and 12 months); HRQoL (EQ‐5D), functional status (HHS); pain using HHS (24 and 48 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: no commercial funding Study dates: October 2009 to April 2013 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomised using sealed, numbered, opaque envelopes" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeons in the study were experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some losses owing to death, which is expected in this population. However, we noted some participant loss which was not explained. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered with a clinical trials register (NCT01798472; first received February 2013). It is not feasible to assess risk of selective reporting bias because study was registered at the end of the study period. |
| Other bias | Low risk | We identified no other sources of bias. |
Iorio 2019.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group:THA (with dual‐mobility cup) versus HA |
|
| Participants |
Total number of randomised participants: 60 Inclusion criteria: displaced intracapsular fracture (Garden III or IV); dementia diagnosis made by a professional Geriatric Assessment Team (DSM‐5 criteria); Mini‐Mental Test score < 18; people > 60 years of age; able to walk unaided before fracture Exclusion criteria: pathological fracture secondary to malignant disease; concomitant fracture requiring surgery Setting: single centre; hospital; Italy Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: antibiotic and venous thromboembolic prophylaxis; direct lateral approach; weight‐bearing was allowed (POD2); guided rehabilitation protocol Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: dislocation; re‐operation rate; time to surgery; surgical time; length of hospital stay (available at 30 days and 1 year) Outcomes relevant to the review: mortality (at 30 days and 1 year); dislocation, re‐operation, length of stay (all at 12 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: October 2015 to September 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocated "with an alternate assignment on the basis of their order of admission" |
| Allocation concealment (selection bias) | High risk | Not possible to conceal an alternate allocation method |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Jeffcote 2010.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 51 participants (52 hip fractures) Inclusion criteria: displaced (Garden's III and IV) subcapital fracture Exclusion criteria: < 60 years of age; significant arthritic change; pathological fracture; living outside the metropolitan area Setting: single centre; hospital; Australia Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: cemented Exeter femoral stem (Stryker, Kalamazoo, MI, USA); performed by consultants or registrars; postoperative 24 hour IV antibiotics, thromboprophylaxis, early mobilisation; follow‐up with radiographs at first week postoperatively and at 3, 12 and 24 months Intervention group 1
Intervention group 2
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS; WOMAC; migration of the HA head; 6MWT (available at 3, 12, and 24 months); mortality (3 months and 2 years)
Outcomes relevant to the review: mortality (at 2 years); functional status (using HHS and WOMAC) and 6MWT; deep infection Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: April 2001 and August 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated to either the bipolar or unipolar group using a list with random numbers" Comment: it is unclear how the random numbers were generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Although participants may have been aware of the type of intervention used, we did not expect that this knowledge would influence their assessments of hip function. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We noted a large loss to follow‐up at 12 and 24 months, but we did not extract data for these outcomes because the data were unclearly reported. We included only data for mortality which were complete. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Kanto 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 175 Inclusion criteria: > 65 years; displaced (Garden III to IV) femoral neck fracture; enrolled in the study within 24 hours of hospital admission Exclusion criteria: < 65 years; fracture of pathological origin; non‐displaced (Garden I to II) fracture; alcohol or drug abuse; cognitively not intact; known bone diseases or known malignancy; high‐energy trauma; rheumatoid arthritis; osteoarthritis Setting: 2 trauma centres, 1 secondary trauma centre and 1 tertiary trauma centre; Finland Intervention group 1 (bipolar; data are incomplete for gender which is unexplained by study authors)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: cemented Lubinus SP II stem (Waldemar Link GmbH & Co, Hamburg, Germany); posterior decubitus approach; lateral position; cemented with Palacos cum gentamycin antibiotic cement (Heraeus Holding GmbH, Hanau, Germany); multiple surgeons performing the operations, senior consultants 27%, 73% orthopaedic residents; spinal anaesthesia; preoperative prophylactic cefuroxime, or clindamycin in case of cefuroxime allergy, was infused 30 min prior to surgery; low‐molecular‐weight mini‐heparin starting at 6 hours preoperatively and continuing for 4 weeks postoperatively except those with permanent preoperative warfarin treatment when mini‐heparin was given until the international normalisation ratio (INR) had been between 2 and 3 for 2 days; participants were mobilised to full weight‐bearing as tolerated Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: implant survival, with revision; mortality (reported in hospital, and at 1, 3, 12 months, and 3 and 5 years); categories of ambulatory ability; general complications; radiographic analysis; operating time; estimated blood loss; dislocations; protrusion; revisions
Outcomes relevant to the review: mortality (in hospital, and at 5 years); unplanned return to theatre (revision); dislocation Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: March 2003 and November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively numbered and sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by all senior consultants or orthopaedic residents but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registration with a clinical trials register (ACTRN12613000092796, first received in 2013). It is not feasible to use these documents to effectively assess risk of selective reporting bias. |
| Other bias | Low risk | We identified no other sources of bias. |
Keating 2006.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA Note:
|
|
| Participants |
Total number of randomised participants: 180 Inclusion criteria: displaced intracapsular hip fracture; normal cognitive function (a mini‐mental test score of > 6), an ability to be mobile independent of another person prior to the fracture, and no serious concomitant disease (or other clinical reason for exclusion) Exclusion criteria: undisplaced or valgus impacted intracapsular fracture; "if a surgeon believed that a particular procedure was clearly indicated or clearly contraindicated, then that patient was not eligible for the trial" Setting: 11 orthopaedic units; 5 university‐affiliated teaching hospitals, 6 district general hospitals; UK Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: 46 surgeons; surgical approach (lateral or posterior) for the arthroplasty, the type of cemented implant, and the use of antibiotics or thromboprophylaxis, were made by the treating surgeon Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: hip‐rating questionnaire (100‐point scale across 4 domains: global, pain, walking, function; available at 4, 12, and 24 months); HRQoL (using EQ‐5D; available at 4, 12, 24 months); mortality (at 4 months and 24 months); re‐admission; re‐operation; fixation failure; non‐union; osteonecrosis; prosthetic dislocation; postoperative complications: wound infection, septicaemia, deep venous thrombosis, pulmonary embolism, stroke, and MI; blood transfusion; discharge destination; length of stay Outcomes relevant to the review: hip‐rating questionnaire: pain and function at 4 and 12 months reported; HRQoL using EQ‐5D (utility index score, no VAS reported) at 4 and 12 months; mortality (at 4 months and 24 months), re‐operation, dislocation, infection, DVT, pulmonary embolism, MI, blood transfusion all at 24 months; discharged to own home; length of stay Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: National Health Service R&D Health Technology Assessment Programme Study dates: June 1996 May 2000 (recruitment period) Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | We noted 3 levels to the randomisation process, with high risk of bias in the initial decision to allocate participants to a 3‐arm comparison (to include internal fixation) or to a 2‐arm comparison using the surgeon's decision on selection. Once selected to a comparison group, allocation was completed using a centralised, computer‐based system. |
| Allocation concealment (selection bias) | High risk | Because of the initial selection process, we have judged this to be high risk of selection bias. However, we acknowledge that the second process of randomisation to treatment groups (using a centralised system) indicated low risk of bias. |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeons in the study were competent to undertake the allocated procedure and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Not certain whether participants were blind to intervention, but low risk of bias as it is unlikely to effect outcomes |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was not explained, but ITT analysis was used, and we noted few losses in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Kim 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA: short stem versus conventional stem |
|
| Participants |
Total number of randomised participants: 161 participants/hips Inclusion criteria: acute Garden III or IV fracture of the femoral neck Exclusion criteria: none reported Setting: single centre; hospital; South Korea Intervention group 1 (THA ‐ short; reported for analysed participants)
Intervention group 2 (THA ‐ conventional; reported for analysed participants)
Note:
|
|
| Interventions |
General details: both groups received a cementless Pinnacle acetabular component (DePuy) with a 36 mm inner diameter Biolox delta ceramic liner (CeramTec); 2 surgeons had experience with each of the 2 stems in more than 200 implantations with each of the stems under investigation; posterolateral approach; mobilised on the second post‐operative day; follow‐up at 3 months, 1 year and yearly thereafter Intervention group 1
Intervention group 2
Notes: 161 recruited, 11 died, 10 lost to follow‐up at 24 months |
|
| Outcomes |
Outcomes measured/reported by study authors: HHS; WOMAC; thigh pain (10‐point visual analogue scale, where 0 represents no pain and 10 severe pain); activity level using UCLA score; adverse events; acute kidney injury; pneumonia; transfusion reaction; mental status change; fracture; dislocation; superficial infection; pain; walking ability
Outcomes relevant to the review: functional status (HHS); thigh pain (number of people experiencing thigh pain); UTI; acute kidney injury; pneumonia; mortality; fracture; dislocation; superficial infection (at 24 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: November 2006 and November 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly assigned, by means of a computer‐generated random number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation table was stored at the co‐ordinating centre" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeons in the study were experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | Low risk | Assessed by nurse separate from surgical team but we judged that this nurse was unaware of the types of interventions |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Unclear whether participants were blind to intervention, but unlikely that this would bias participant reported outcomes |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We noted that participant loss was because of death and because of loss to follow‐up. These losses were balanced between groups and therefore we did not expect losses to introduce attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Lim 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: short stem versus standard stem |
|
| Participants |
Total number of randomised participants: 151 (study authors report numbers of participants and numbers of hips inconsistently throughout the paper. Because the baseline data is reported for 151 participants, we have used this number as the total number randomised.) Inclusion criteria: people ≥ 65 years of age; femoral neck fractures (Garden type III or IV) Exclusion criteria: history of hip surgery; pathologic fracture; immunologic disorders such as rheumatoid arthritis, avascular necrosis of the femur head; Legg–Calvé–Perthes disease Setting: single site; orthopaedics department; South Korea Intervention group 1 (short stem)
Intervention group 2 (standard)
Note:
|
|
| Interventions |
General details: all cementless; 5 mg of zoledronate intravenously annually and calcium and vitamin D supplements orally; posterolateral approach ‐ single experienced hip surgeon; immediate weight‐bearing; both bipolar; clinical follow‐up at 6 weeks, 3, 6, 9, and 12 months, and every year thereafter Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: activity level (Koval’s categories); thigh pain; stability of the femoral stem; fixation status; stress shielding grade; leg‐length discrepancy; heterotopic ossification; BMD Outcomes relevant to the review: mortality; superficial Infection at 12 months; pain (without/with); mobility (outdoors/housebound) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: study authors received no funding and declared no conflicts of interest Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used software to generate random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation completed by independent statistician |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding for participant‐reported outcomes to influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High proportion of loss to follow‐up; described only as lost or refused |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Livesley 1993.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: HA: uncemented (Furlong HAC) versus uncemented |
|
| Participants |
Total number of randomised participants: 82 Inclusion criteria: displaced subcapital fracture of the femur; walking normally before surgery Exclusion criteria: none reported Setting: single site; general hospital; UK Intervention group 1 (HAC)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: "several surgeons", postoperative management the same in both groups (details not specified) Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: hip function assessment; mortality; discharge destination; adverse events: perioperative fractures, dislocation, wound infection, revision (for infection, anterior thigh pain, or fracture blow prosthesis); foot drop; pressure sores; perioperative complications (calcar splits, shaft fracture, greater trochanteric detachment, lesser trochanter detachment, prosthesis placed in internal rotation) Outcomes relevant to the review: mortality (at 30 days, and 1 year); functional assessment (using a 5‐point scale across 9 domains by Benjamin 1990; higher scores indicate better function); discharge destination; adverse events: perioperative fractures, dislocation, infection, revision Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: no commercial funding Study dates: October 1989 to September 1990 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocated by week of admission |
| Allocation concealment (selection bias) | High risk | It is not feasible to conceal allocation because selection was made according to week of admission. |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. Data for all outcomes were complete. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Macaulay 2008.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 41 Inclusion criteria: > 50 years of age; independent ambulation before fracture; displaced femoral neck fracture (Garden III or IV which the surgeon considered not amenable to treatment with internal fixation); ability to comprehend and read either English or Spanish Exclusion criteria: chronic severe dementia (defined as < 23 out of 30 on Folstein MMSE); pathologic fracture; other concomitant long bone fractures or fractures requiring surgical repair; pre‐existing arthritis of the hip Setting: five sites; medical centres; USA Intervention group 1 (THA; baseline data missing for 1 participant)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: surgeon choice: posterior (posterolateral) approach with enhanced soft tissue repair or direct lateral (Modified Hardinge) approach Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: Function (WOMAC and HHS; data available at 12 and 24 months); HRQoL (SF‐36; data available at 12 and 24 months); functional tasks; HHS (data available at 12 and 24 months); mobility (TUG; data available at 12 and 24 months); Complications: additional hospitalisations, care utilisation, re‐operations, ambulatory status; length of stay in hospital; mortality (6 months and 34 months) Outcomes relevant to the review: length of stay in hospital, mortality (at 6 months, and 34 months); dislocation, MI, pneumonia, UTI, wound infections (at 6 months); SF‐36 (physical components), WOMAC (pain), functional status (HHS), mobility (TUG) (all at 12 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: partial or total financial support from: American Assoication of Hip and Knee Surgeons and Orthopaedic Research and Education Foundation grants Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each site had an individual blocked randomization scheme, which was verified at the coordinating site for compliance. " |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed‐envelope technique" |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Although participants may have been aware of the type of intervention used, we did not expect that this would influence their assessments of relevant outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data complete for all outcomes |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Malhotra 1995.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 68 Inclusion criteria: elderly people with femoral neck fractures Exclusion criteria: none reported Setting: single site; general hospital; India Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: Moore's posterior approach for both groups; no cement fixation; antibiotic prophylaxis (10 days); prophylactic anti‐coagulation not routinely used; weight‐bearing after 3 days; clinical follow‐up at 6 weeks, 6 months, and then annually Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: "results of surgery"; loosening; angular shift; settling; deep infection; dislocation; acetabular erosion; subsidence; mobility; length of stay in hospital; functional status (using Devas 1983) Outcomes relevant to the review: dislocation (first week); deep infection (two year follow‐up); length of hospital stay; functional status (using Devas 1983; categorical data as excellent, good, fair, and unsatisfactory; at 12 months) Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: commenced January 1989; 4 year period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participant loss reported, and we could not be certain whether the study included participants who died during study follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Moerman 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 201 Inclusion criteria: ≥ 70 years of age; displaced femoral neck fracture (Garden type III or IV) Exclusion criteria: pathological fracture, a fracture > 7 days, or ASA IV or V Setting: 5 medical centres; USA Intervention group 1 (cemented; some characteristics not reported for all participants)
Intervention group 2 (uncemented; some characteristics not reported for all participants)
Note:
|
|
| Interventions |
General details: orthopaedic surgeon or registrar performed the operation; approach decided by surgeon; physiotherapy therapy; analgesia and thromboembolic prophylaxis; clinical follow‐up at 6 weeks, 12 weeks, and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: operation time; blood loss; length of stay, decrease in haemoglobin level; transfusion rate; TUG score, GARS, NMS, HRQoL (SF‐12 PCS and MCS), mid‐thigh pain (reported at 6 weeks, 12 weeks, and 1 year); mortality; complications (death, tachyarrhythmia, MI, pulmonary embolism, acute renal failure, stroke and/or TIA, bowel obstruction, anaemia, UTI, mental status change, gastric hypomotility, DVT, pneumonia, social complication, peripheral nerve injury, infection leading to revision, periprosthetic fracture (intra‐ and postoperatively), dislocation, haematoma, persistent wound drainage, superficial wound infection, skin blisters Outcomes relevant to the review: mortality (12 months); MI; venous thromboembolic phenomena (pulmonary embolus, DVT); acute renal failure; CVA (stroke/TIA); urinary tract infection; infection leading to revision; periprosthetic fracture (intra‐ and postoperatively); dislocation; superficial wound infection (all complications at 1 year); mobility (9‐point mobility scale; 12 weeks and at 1 year); ADL (GARS; at 12 weeks and 1 year); HRQoL: SF‐12 (physical component; at 12 weeks and 1 year); mid‐thigh pain; length of hospital stay; blood transfusion Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: August 2008 and June 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized following a simple randomization procedure in the operation theatre" Comment: insufficient information on methods of randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by orthopaedic surgeons or registrars but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Participants blind to intervention |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | We noted a large number of participants lost to follow‐up at 12 months, with more lost in the cemented group. We also noted some variation in the number of reported participants for each outcome at each time point which was not explained. |
| Selective reporting (reporting bias) | Low risk | Registered with a clinical trials register (NTR1508; first received October 2008). Registration soon after start of trial. All outcomes in the published report are consistent with those in the clinical trials register documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Moroni 2002.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: mixed HA and THA: uncemented versus cemented |
|
| Participants |
Total number of randomised participants: 28 Inclusion criteria: AO/OTA fracture type B2 and B3; female ≥75 years of age, fracture resulting from minor trauma, ability to communicate and BMD T‐score at the contralateral hip < ‐2.5 SD Exclusion criteria: none reported Setting: single centre; hospital; Italy Intervention group 1 (uncemented)
Intervention group 2 (cemented)
Note:
|
|
| Interventions |
General details: none reported Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS; SF‐36; mortality; revision (due to loosening) Outcomes relevant to the review: mortality; functional status (HHS); HRQoL (SF‐36); dislocation Notes:
|
|
| Notes |
Funding/sponsor/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect that lack of blinding for participant‐reported outcomes would influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study authors did not report whether there were any losses, and because of other limited details in the abstract, we could not be certain whether data were complete. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | High risk | Published only as an abstract with limited detail on study characteristics. In addition, we expected the abstract publication was not peer‐reviewed and we judged this to increase risks of other bias. |
Mouzopoulos 2008.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA Note:
|
|
| Participants |
Total number of randomised participants: 86 Inclusion criteria: displaced subcapital hip fracture (Garden III or IV) after falling down Exclusion criteria: previous hip fracture; history of cancer or Paget's disease; rheumatic arthritis Setting: hospital; single centre; Greece Baseline characteristics Intervention group 1 (THA; data only reported for 37 participants)
Intervention group 2 (HA; data only reported for 34 participants)
Note:
|
|
| Interventions |
General details: 2 orthopaedic surgeons; postoperative strengthening exercises and range‐of‐motion exercises for the hip and knee joint Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: BI (available at 12 months and 4 years); HHS (available at 12 months and 4 years); range of passive hip motion; gait speed; mortality (available at 12 months and 4 years); length of hospital stay; revision Outcomes relevant to the review: ADL (BI; scores 0 to 100; higher scores indicate more independence; at 12 months and 4 years); functional status (HHS, mean scores; at 12 months and 4 years); mortality (at 12 months and 4 years); length of hospital stay; unplanned return to theatre (revision; at 4 years) Notes:
|
|
| Notes |
Funding/sponsorship/declarations of interest: not reported Study dates: April 1999 to April 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Two levels of randomisation; every third participant is selected to be included in the study, and then participants are "randomly divided" into groups by two orthopaedic surgeons. We believed the first level of randomisation indicated the potential to manipulate the order of participants included in the study. |
| Allocation concealment (selection bias) | High risk | Not described. Because the initial methods selected participants according to order, we judged there to be no allocation concealment. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by all orthopaedic surgeons but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect that lack of blinding for participant‐reported outcomes would influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some losses are owing to death, which is expected in this population with a long study follow‐up. Other losses were explained and relatively balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Movrin 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 158 Inclusion criteria: ≥ 76 years of age; displaced femoral neck fracture (Garden's III to IV); no concurrent joint disease; no previous hip fractures; intact cognitive functions; ability to ambulate independently with or without walking aids Exclusion criteria: Garden's I to II fractures; pathological fractures; rheumatoid arthritis; symptomatic osteoarthritis; deemed unsuitable for surgical procedures by the anaesthesiologist Setting: hospital; single centre; Slovenia Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: 9 consultant or specialist orthopaedic‐trauma surgeons performed all operations and were experienced in the use of cemented and uncemented stems; standard anterolateral approach; both implants produced by Ecofit (Implantcast); closed‐suction drains were placed in all participants; 2 g tranexamic acid; perioperative antibiotic prophylaxis; low‐molecular‐weight heparin as a thromboembolic prophylaxis; mobilised immediately with weight‐bearing; initially reviewed after discharge at 6 weeks; subsequent assessments were made at 3, 6, and 12 months Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain (VAS; at 6 weeks and 6 months); intraoperative parameters; bleeding; fracture (intraoperative and postoperative); dislocation; deep infection; mortality (intraoperative, 7 days, 24 months); HHS (6 weeks and 24 months); re‐operations Outcomes relevant to the review: pain (6 months); fracture (intraoperative and postoperative); dislocation; deep infection; mortality (7 days and 24 months); functional status (HHS; 6 weeks and 24 months) Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: study received no funding and study authors declared no conflicts of interest Study dates: January 2013 and December 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote:"randomized using sealed, numbered, and opaque envelopes " |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeons in the study were experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Participants blinded to intervention |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. We noted loss of 3 participants for HHS data in the uncemented group which was not explained, but we did not expect these few losses to influence outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Parker 2010c.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 400 Inclusion criteria: displaced intracapsular fracture, > 60 years of age Exclusion criteria: undisplaced or minimally displaced intracapsular fracture; < 60 years of age; 60 to 75 years of age with no restriction in mobility at the time of injury; declined to participate; senile dementia for whom the assent of their next of kin was not obtained; pathological fracture from a tumour or Paget's disease; previous treatment of the same hip for a fracture; not considered to be fit for either of the surgical procedures; significant arthritis of the hip that necessitated treatment with THA; admitted when the lead trialist was not available to supervise the procedure Setting: hospital; single centre; UK Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: all operations were performed or supervised by 1 orthopaedic surgeon; all received perioperative prophylactic antibiotics and 14 days of low‐molecular‐weight heparin as thromboembolic prophylaxis; mobilisation as soon as able to, with no restrictions on hip movements or weight‐bearing; routine follow‐up at 6 weeks, then by telephone at 3, 6, 9 and 12 months, then annually up to 5 years Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain (VAS; scale of 1 to 10, lower numbers indicate less pain; data available at: 8 weeks: 3, 6, and 9 months; 1, 2, 3, 4, 5 years); mobility scale (Parker mobility score: 0 to 9; lower scores indicate better mobility; data available at: 8 weeks: 3, 6, and 9 months; 1, 2, 3, 4, 5 years); mortality; length of hospital stay; need for blood transfusion; complications (confusion, pneumonia, pressure sores, DVT, pulmonary embolism, CVA, GI bleed, cardiac failure, acute renal failure, MI, acute cardiac arrhythmia, acute confusion state, intestinal obstruction, clostridia diarrhoea, peritonitis); wound healing complications (wound haematoma, superficial infection, deep wound infection, dislocation, drainage of infection or haematoma, internal fixation revised to HA, revision arthroplasty for periprosthetic fracture, revision for pain to THA, revision for dislocation to THA, Girdlestone arthroplasty, Girdlestone arthroplasty and later THA, any re‐operation) Outcomes relevant to the review: operative fracture; length of stay in hospital; pneumonia; DVT; pulmonary embolism; CVA; acute renal failure; MI; superficial and deep infection; dislocation; revision; postoperative fracture requiring revision; blood transfusion; delirium (acute confusional state); pain (at 3 months, 12 months, and 5 years); mobility (at 12 months, and 5 years); mortality (at 2 to 3 months, 12 months and 5 years); return to original residence Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: support by a grant from the Peterborough Hospital Hip Fracture Fund Study dates: March 2001 to November 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not described |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised by the opening of a sealed opaque numbered envelope, prepared by a person independent of the study" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeon in the study was experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding for participant‐reported outcomes to influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. We noted data were not complete for pain and mobility at 5 years. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Parker 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented ETS versus cemented Thompson |
|
| Participants |
Total number of randomised participants: 200 Inclusion criteria: people with a displaced intracapsular fracture Exclusion criteria: pathological fractures from secondary tumour or local bone disease; fracture of the same hip that had previous surgical treatment; fractures being treated conservatively; declined to participate; senile dementia; significant arthritis of the hip to be treated with THA; fractures treated by internal fixation; people treated when lead trialist was not available to supervise the surgical procedure Setting: hospital; single centre; UK Baseline characteristics Intervention group 1 (Exeter Trauma Stem)
Intervention group 2 (Thompson)
Note:
|
|
| Interventions |
General details: performed or supervised by 1 orthopaedic surgeon (study author) with participant in the lateral position; all participants mobilised as soon as able with restrictions placed on hip movements or weight‐bearing; routine follow‐up at 6 weeks, then by telephone at 3, 6, 9 and 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: length of surgery, difficulty level of surgery, retained cement in the joint, laceration of the limb at surgery, operative fracture femur, required blood transfusion, volume of blood transfused, wound haematoma, superficial or deep wound infection, dislocation, acetabular wear, length of hospital stay, complications (cardiac arrest at surgery, pneumonia, pressure sores, DVT, pulmonary embolism, delirium, CVA, cardiac failure, cardiac arrhythmia, clostridia diarrhoea, GI bleed, urine retention, acute renal failure), mean pain scores and mean change in mobility scores (data available at 8 weeks, and at 3, 6, 9 and 12 months); mortality (30 days, 90 days, 120 days, 1 year); unplanned return to theatre Outcomes relevant to the review: mortality (120 days and 1 year); length of hospital stay; blood transfusion; superficial infection; deep infection; dislocation; periprosthetic fracture (operative fracture femur); complications (pneumonia, DVT, pulmonary embolism, CVA, cardiac failure, delirium; acute renal failure); pain (mean scores); mobility (change in mean scores; at 1 year); unplanned return to theatre Notes:
|
|
| Notes |
Funding/sponsorship/declarations of interest: no external sources of funding; internal funding from the Peterborough Hospital Hip Fracture fund Study dates: November 2006 to July 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised by the opening of a sealed opaque numbered envelope" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeon in the study was experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | Low risk | Quote: "all assessments were made by a nurse who was blinded to the treatment allocation" |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding for participant‐reported outcomes to influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses were owing to death, which is expected in this population. No participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Parker 2019.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 105 Inclusion criteria: displaced intracapsular fracture; able to walk independently out of doors with no more than the use of a stick; not cognitively impaired; medically fit Exclusion criteria: < 60 years of age; where internal fixation was felt to be the best treatment; degenerative arthritis of the hip; acetabular dysplasia; senile dementia Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: performed or supervised by 1 orthopaedic surgeon; both interventions were cemented; general anaesthesia was given to 26 participants in the HA group and 29 participants in the THA group; weight‐bearing as able; routine follow‐up at 8 weeks; clinical follow‐up phone calls at 3, 6, 9 and 12 months from injury and then annually. Mean follow‐up was approximately 3 years and all participants had a minimum follow‐up of 1 year Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain (scale: 1 (no pain) to 8 (constant and severe); available at 8 weeks, 3 months, 6 months, 9 months, 12 months); walking/mobility ability (scale: 1 (no walking aid) to 9 (wheelchair bound); available at 8 weeks, 3 months, 6 months, 9 months, 12 months); social dependence (scale: 1 (completely independent) to 8 (hospital inpatient); available at 8 weeks, 3 months, 6 months, 9 months, 12 months); length of stay in hospital; superficial wound infection; deep wound infection; haematoma; urinary retention; DVT; pressure sores; delirium; CVA; fat embolism/cement reaction; blood transfusion; mortality (data available at 30 days, 4 months and 1 year) Outcomes relevant to the review: mortality (4 months and 12 months); unplanned return to theatre; blood transfusion; superficial wound infection; deep wound infection; DVT; CVA; length of hospital stay; delirium; ADL (social dependency scale; 3 months and 12 months); mobility (3 months and 12 months); pain (3 months and 12 months) Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: study authors report no commercial funding Study dates: December 2012 to February 2018 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Quote: "numbered sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeon in the study was experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Unclear whether participants were blind to intervention but unlikely to effect results. Study authors reported that a research nurse who was blinded to the treatment allocation measured function and pain outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant loss was because of death, which is expected in this population. Study authors reported that no participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registration with a clinical trials register (NCT02998359; first received December 2016); only mobility stated as outcome a priori with more outcomes reported in paper. We could not feasibly use these retrospectively‐registered documents to assess risk of selective reporting bias. |
| Other bias | Low risk | We identified no other sources of bias. |
Parker 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 400 Inclusion criteria: displaced intracapsular fracture; able to walk independently out of doors with no more than the use of a stick; not cognitively impaired Exclusion criteria: "younger patients"; where internal fixation or total hip arthroplasty were felt to be the best treatment; mental impairment; considered unfit for a cemented arthroplasty; degenerative arthritis of the hip; pathological fractures; acetabular dysplasia Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (cemented)
Intervention group (uncemented)
Note:
|
|
| Interventions |
General details: Hardinge direct lateral approach to the hip; surgery was undertaken or directly supervised by the lead trialist (in all but 8 operations); general anaesthesia given to 91 participants in the cemented group and 101 participants in the uncemented group; fully weight‐bearing with no postoperative restrictions on weight‐bearing or hip movement Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: functional assessments; hip movements; limb shortening; pain (data available at 8 weeks; 3, 6, 9, and 12 months); walking/mobility (data available at 8 weeks; 3, 6, 9, and 12 months); social dependence (data available at 8 weeks; 3, 6, 9, and 12 months); pneumonia; congestive cardiac failure; MI; cardiac arrhythmia; urinary retention; DVT; pulmonary embolism; pressure sores; delirium; CVA; gastrointestinal bleed; acute renal failure; clostridia diarrhoea; fat embolism; mortality (data available at 30 days, 120 days, and 1 year); blood transfusion; length of hospital stay Outcomes relevant to the review: blood transfusion; length of hospital stay; mortality (4 and 12 months); complications (pneumonia, MI, DVT, pulmonary embolism, delirium, CVA, acute renal failure); pain (at 3 months and 12 months); mobility (at 3 months and 12 months); ADL (social dependency; at 3 months and 12 months) |
|
| Notes |
Funding/sponsorship/declarations of interest: no commercial funding. Funding for research nurse was provided by Peterborough Hip Fracture Project Research Fund Study dates: December 2012 to February 2018 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "envelopes were prepared, sealed, randomly mixed, and then numbered by an individual independent of the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed, identical, opaque envelopes " |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeon in the study was experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding to influence participant‐reported outcomes. Function and pain measured by a research nurse who was blinded to the treatment allocation |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most participant loss was because of death, which is expected in this population. Although study authors reported no other participant losses, we noted missing data for a very small number of participants for participant‐reported outcomes. We did not expect these losses to influence effect estimates for these outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registration with clinical trials register (NCT02998034: first received December 2016). It was not feasible to effectively assess risk of reporting bias using these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Patel 2008.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 40 Inclusion criteria: people > 70 years of age, presenting with intracapsular hip fractures (Gardens III or IV) Exclusion criteria: not reported Setting: single centre; hospital; location not reported Baseline characteristics not reported Note:
|
|
| Interventions |
General details: all operations performed through a Hardinge approach by the same surgical team. All prostheses were uncemented. Rehabilitation with same physiotherapist using same routine Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (in hospital); length of hospital stay; deep infections; periprosthetic fracture; return to pre‐injury state; pain; participant satisfaction with procedure Outcomes relevant to the review: mortality; length of hospital stay; deep infection; periprosthetic fracture; pain Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: not reported Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as a randomised study, but no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to types of interventions. Study authors report that all interventions were performed by the same team but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of only 1 participant |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | High risk | Study reported only as an abstract which we assumed was not peer‐reviewed. In addition, there is limited information in the study report and we could not be certain of other potential biases. |
Raia 2003.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: bipolar vs unipolar |
|
| Participants |
Total number of randomised participants: 115 Inclusion criteria: ≥ 65 years of age, with an acute displaced femoral neck fracture (Garden's III to IV) Exclusion criteria: people with dementia; nonambulatory; pathologic femoral neck fractures; additional acute lower extremity fracture in addition to the femoral neck fracture; living in nursing homes Setting: single centre; hospital; USA Baseline characteristics Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: surgery done within 24 to 48 hours of hospital admission. Preoperative heparin, prophylactic antibiotics started preoperatively, and warfarin for 6 weeks postoperatively. Anaesthesia type at the discretion of the anaesthetists (majority were regional anaesthesia). Mobilised to full‐weight bearing on POD 1 with supervision of physical therapists Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality; estimated blood loss, number of participants requiring blood transfusion; length of stay on orthopaedic ward; complications (urinary tract and haematoma; pulmonary embolism and re‐operation); dislocations; QoL (SF‐36; separately reports scores for physical function; bodily pain; role limitations physical; role limitations emotional; mental health; social functioning; vitality; general health); mobility and ADL (Musculoskeletal Functional Assessment Instrument Scores; lower score indicates better function; at 1 year) Outcomes relevant to the review: mortality (1 year); blood transfusion; length of stay; dislocations; HRQoL (SF‐36; physical function; at 1 year); pain (SF‐36; bodily pain; at 1 year); mobility and ADL (Musculoskeletal Functional Assessment Instrument Scores; lower score indicates better function; at 1 year) Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: 1 study author received funding as a consultant for Stryker Howmedica Osteonics Study dates: May 1997 to January 2000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We were not certain whether participants were blinded to the intervention. However, we did not expect lack of blinding to influence reporting of mobility, ADL, or HRQoL. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect lack of blinding of objective measures to be influence the outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses were owing to death, which is expected in this population. Loss to follow‐up at 12 months was clearly explained and balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Rashed 2020.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA: dual mobility cups versus conventional large head |
|
| Participants |
Total number of randomised participants: 62 Inclusion criteria: 55 to 80 years of age, and a displaced femoral neck fractures (Garden III and IV) Exclusion criteria: cognitive dysfunction (as evidenced by > 4 errors on the SPMSQ); dependency on daily living activities as proved by the Katz ADL index; previous hip surgery; old non‐united femoral neck fractures; neuromuscular disorders; previous prolonged nonambulation; preoperative ASA score > III; presence of other injuries or fractures; upper or lower limb amputation; inflammatory arthropathies; arthritic acetabulum; pathological femoral neck fractures Setting: single centre; hospital; Egypt Baseline characteristics Intervention group 1 (dual mobility cups)
Intervention group 2 (conventional)
Note:
|
|
| Interventions |
General details: 4 senior arthroplasty surgeons using the posterior approach; physiotherapy was initiated as per a modified protocol, participants routinely followed up at 12 weeks, 16 weeks, 6 months, and 1 year Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS (available at 3, 4, 6 and 12 months); range of motion; HRQoL (SF‐36); mortality; superficial wound infection; deep infection; dislocation; DVT; heterotopic ossification; neurovascular injury; limb‐length discrepancy Outcomes relevant to the review: HHS (categorical data: excellent, good, fair, and poor; at 12 months; and mean scores at 12 months); mortality; superficial wound infection; deep infection; dislocation; DVT Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: study authors received no funding and declared no conflicts of interest Study dates: April 2014 to May 2015 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomisation list that was created by a statistician prior to the commencement of the study" |
| Allocation concealment (selection bias) | Low risk | Managed by a statistician |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were all performed by senior surgeons but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. We noted that some outcomes were assessed by a physiotherapist who was blinded to the intervention. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | Participants were not blinded to intervention but unlikely to effect the HRQoL outcome |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported that no participants were lost to follow‐up. Only participant loss was because of death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Ravikumar 2000.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: THA versus HA Note:
|
|
| Participants |
Total number of randomised participants: 180 Inclusion criteria: > 65 years of age; displaced subcapital femoral neck fracture (Gardens III and IV) Exclusion criteria: old fractures; pathological fractures; rheumatoid arthritis Setting: single centre; UK Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: surgery by orthopaedic trainees and occasionally consultants; mobilised with full‐weight bearing Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain and mobility (Sikorski 1981; available at 1 year and 13 years); HHS (at 13 years); loss of mobility; infection (13 years); dislocation (13 years); revision (13 years); adverse events: pulmonary embolism; myocardial infarction; perioperative deaths; peroneal nerve palsy; iatrogenic femoral fracture; mortality (available at 2 months, 12 months, 13 years) Outcomes relevant to the review: pain (at 1 year and 13 years; categorical data: no pain, occasional pain; occasional analgesia; regular analgesia); mobility (at 1 year and 13 years; categorical data: independent (does shopping); independent with aids; housebound unless accompanied; uses aids indoors; chair or bedbound); mortality (2 months, 12 months and 13 years); infection (deep and superficial combined); dislocation; unplanned return to theatre (revision); functional status (HHS) Notes
|
|
| Notes |
Funding/sponsorship/declarations of interest: funding by Johnson & Johnson Study dates: December 1984 to December 1986 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised according to the day of the week on which participants were admitted |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation because of the randomisation methods. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We were not certain whether participants were blinded to the intervention. However, we did not expect lack of blinding to influence data for participant‐reported outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect lack of blinding of objective measures to be influence the outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were owing to death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration or pre‐published protocol. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Rehman 2014.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 110 Inclusion criteria: displaced intracapsular hip fracture (Gardens type III and IV); > 60 years of age; either gender Exclusion criteria: pathological hip fractures; previous treatment to same hip for a fracture; significant arthritis for the hip assessed radiologically Setting: multicentre; 2 hospitals and 1 research institute; Pakistan Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: operations performed or supervised by the same orthopaedic surgeon, and by a standard lateral approach. All participants received perioperative prophylactic antibiotics, and 14 days of low‐molecular‐weight heparin as thromboembolic prophylaxis. After surgery, all participants were mobilised as soon as possible, with no restriction on hip movement or weight‐bearing; participants reviewed at 4, 8 and 12 weeks Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain (assessed using a pain scale of 0 to 6); mobility (scale of 0 to 9); reported at 12 weeks Outcomes relevant to the review: pain (assessed using a pain scale of 0 to 6; higher numbers indicate more pain; at 12 weeks); mobility (scale of 0 to 9; higher scores indicate better mobility; at 12 weeks) |
|
| Notes |
Funding/sponsorship/declarations of interest: not reported Study dates: August 2010 to August 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was prepared by a person who was independent of the study; insufficient information |
| Allocation concealment (selection bias) | Low risk | Use of sealed, opaque, numbered envelopes |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that all interventions were performed by all same orthopaedic team but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We are uncertain whether participants were blinded to the intervention, but we did expect this influence reporting of data for mobility or pain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Ren 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 100 Inclusion criteria: people with femoral neck fractures Exclusion criteria: not reported Setting: single centre; hospital; China Baseline characteristics Intervention group 1 (THA)
Intervention group 2 (HA)
Notes:
|
|
| Interventions |
General details: no details of procedure are reported Intervention group 1
Intervention group 2
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operative variables (operation time, volume of blood loss); time until out of bed; complications (types not defined); functional status (with HHS; time point not specified) Outcomes relevant to the review: functional status (HHS; excellent ≥ 90; good = 80 to 90; medium = 70 to 90; poor ≤ 70); time point not specified |
|
| Notes |
Funding/sponsorship/declarations of interest: not reported Study dates: October 2015 to March 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Unclear risk | We could not be certain of other risks of bias because of limited detail in the methods section. |
Sadr 1977.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented vs uncemented |
|
| Participants |
Total number of randomised participants/cases: 40 participants/40 cases Inclusion criteria: emergency admissions with subcapital fractures of the femoral neck; displaced fractures (Gardens III or IV) Exclusion criteria: undisplaced (Gardens I); pathological fractures Setting: single centre; hospital; UK Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: surgery within first week of injury (usually within 72 hours); "a number of different surgeons"; using anterolateral and posterior approaches; early mobility with unrestricted weight‐bearing on POD 2; discharged from hospital when independently mobile with a walking aid, or transferred to a rehabilitation unit within 3 to 4 weeks of surgery Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: loosening of prosthesis; dislocation; ectopic calcification; mortality; functional status Outcomes relevant to the review: loosening of prosthesis; dislocation; mortality (6 weeks and 12 months); functional status (excellent = flexion > 90°, no pain, able to walk outdoors unaided; good = flexion 60° to 90°, slight pain, able to walk outdoors with walking aids; fair = flexion 30° to 60°, moderate pain, confined indoors; or poor: flexion under 30° or severe pain) Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated to one or other group by random selection". Comment: no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We are uncertain whether participants were blinded to the intervention, but we did not expect this to influence reporting of data which contributed to the functional status outcome. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were clearly reported with most owing to death. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Santini 2005.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: HA: cemented vs uncemented |
|
| Participants |
Total number of randomised participants: 106 Inclusion criteria: ≥ 65 years of age with femoral neck fractures; also included participants < 65 years old with fractures secondary to malignant tumours but with life expectancy > 3 months Exclusion criteria: pathological fractures, with life expectancy inferior to 3 months Setting: single centre; hospital; Italy Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: skin traction until surgery, spinal anaesthesia in all participants, surgical procedure using a lateral approach in supine position, full weight‐bearing on POD3; blood transfusion according to haemoglobin levels preoperatively and postoperatively; radiographic follow‐up at 6 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (in‐hospital; at 1 year); postoperative complications (MI, cardiac arrhythmia, pneumonia, pulmonary embolism, thrombophlebitis, UTI, gastric disease; deep wound infection, prosthesis dislocation, iatrogenic femoral fracture); length of hospital stay; functional recovery; discharge destination Outcomes relevant to the review: length of stay (days); ADL (using functional score with VELCA); mobility (using functional score with VELCA, at 12 months); functional status (using total functional score with VELCA, at 12 months); mortality (at hospital discharge and 12 months); postoperative complications (deep wound infections; prosthesis dislocations; intraoperative periprosthetic fracture (iatrotrogenic femoral fracture); arrhythmias/MI; UTI; pneumonia/pulmonary embolism); discharge destination (geriatric institutions, home, hospital), all adverse events at 12 months Notes:
|
|
| Notes |
Funding/sponsorship/declarations of interest: no external funding Study dates: September 2000 to December 2001 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised trial; participants allocated to each treatment on alternate days |
| Allocation concealment (selection bias) | High risk | It is unlikely that allocation could be effectively concealed because of the method of sequence generation. |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding for participant‐reported outcomes to influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality and length of stay) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses were owing to death, which is expected in this population. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Sharma 2016.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 80 Inclusion criteria: people with displaced femoral neck fractures, > 60 years of age Exclusion criteria: associated osteoarthritis, AVN, rheumatoid arthritis, pathological fractures due to any other cause; people with significant comorbidities Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: all surgeries performed by one of two senior arthroplasty surgeons using modified Gibson approach (Gibson 1950); weight‐bearing allowed as soon as pain threshold permitted Intervention group 1
Intervention group 2
Notes:
|
|
| Outcomes |
Outcomes measured/reported by study authors: operative variables (surgery time, volume of blood loss, mean units of transfused blood); wound infection; time to ambulation; time to achieve pre‐ambulation status; dislocation; abductor laxity; functional status; early mortality Outcomes relevant to the review: mortality (reported for 1 participant at 7 days); wound infection (superficial and deep infection; assumed time point to be during postoperative period up to 1 week); dislocation; functional status (HHS; at 12 months) |
|
| Notes |
Funding/sponsorship/declarations of interest: not reported Study dates: 2010 to 2014 Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "simple randomisation technique (cards in a box)" Comment: insufficient information to judge whether randomisation is likely to be adequate |
| Allocation concealment (selection bias) | High risk | Not described. By selecting cards from a box, it is possible that allocation could be manipulated. |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by consultant surgeons but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of one participant in each group, which was explained |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Sims 2018.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: ETS versus Thompson |
|
| Participants |
Total number of randomised participants: 964 Inclusion criteria: > 60 years of age; type B3 fracture (displaced) Exclusion criteria: pre‐existing symptomatic hip arthritis Setting: multicentre; 5 hospitals; UK Baseline characteristics Intervention group 1 (Exeter/ Unitrax)
Intervention group 2 (Thompson)
Note:
|
|
| Interventions |
General details: multiple surgeons; pre‐ and postoperative management was as per the standard of care in the unit, according to NICE guidance Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: EQ‐5D‐5L (4 months); mortality; walking ability; length of stay; complications; radiological neck length Outcomes relevant to the review: EQ‐5D‐5L (4 months); mobility; mortality (4 months); length of stay Notes:
|
|
| Notes |
Funding/sponsorship/declarations of interest: funded by Stryker Study dates: February 2015 and March 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random number sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "via an online randomization portal" |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | Low risk | Subjective outcomes were obtained by individuals distanced from the surgical team. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | "Participants were blinded to the treatment allocation" |
| Blinding of outcome assessment (detection bias) objective outcomes | Unclear risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Large number of participants lost after allocation, but most of these losses are either participants withdrawn before consent or are owing to death |
| Selective reporting (reporting bias) | Low risk | Prospectively registered with clinical trials register (ISRCTN39085558; first received October 2014). All reported outcomes are consistent with those in the clinical trials documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Sonaje 2017.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 42 Inclusion criteria: > 60 years of age with closed intracapsular displaced femoral neck fracture, giving informed consent Exclusion criteria: ipsilateral lower limb fractures, with psychiatric and neurological disorders, not giving informed consent Setting: single centre; hospital; India Baseline characteristics Intervention group 1 (THA; for analysed participants only)
Intervention group 2 (HA; for analysed participants only)
Note:
|
|
| Interventions |
General details: all surgeries performed on elective basis, using standard aseptic procedures, under spinal anaesthesia. In all cases, the stem was cemented in place using standard cement techniques. Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative variables (duration of surgery, volume of blood loss); pain scores; limp; use of walking support; walking distance; ability to put on shoes and socks; stair climbing; sitting; entering public transportation; deformity of the hip; range of movements; functional modified HHS; complications (death, periprosthetic fracture, bed sore, prosthetic dislocation, minor limb length discrepancy) Outcomes relevant to the review: pain (using modified HHS; mean score ‐ higher score indicated less pain); functional status (modified HHS; mean score, and distribution of scores for excellent, good, fair, and poor); periprosthetic fracture Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: no external funding. Study authors declare no conflicts of interest Study dates: September 2011 to November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "First simple random technique thereafter alternate systemic random sampling was used" Comment: we have interpreted this as a quasi‐randomised method of allocation |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation if an alternative sequence is used. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We are uncertain whether participants were blinded to the intervention, but we did not expect this to influence reporting of data for mobility or pain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although study authors do not report to which group the participants belonged who either died or were lost to follow‐up, these losses were only one per group. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Sonne‐Holm 1982.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 112 Inclusion criteria: admitted to hospital with a femoral neck fracture, > 70 years of age, with fracture sustained within the past week, with no orthopaedic or neurological disorders influencing gait function Exclusion criteria: not specified Setting: single centre; hospital; Denmark Baseline characteristics not reported Note:
|
|
| Interventions |
General details: performed as emergency procedures Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: hip function (includes total scores, and scores for pain, mobility and gait function at 6 weeks, 3 months, 6 months, and 12 months; mortality; superficial infection; periarticular calcification; osteolysis; settling of the prosthesis Outcomes relevant to the review: hip function (number of people achieving total score on a scale of 0 to 6, according to D'Aubigne 1954; high scores indicate better hip function (at 6 weeks; 3, 6 and 12 months); pain (number of people achieving total score on a scale of 0 to 6, according to D'Aubigne 1954; high scores indicate least pain; at 6 weeks; 3, 6 and 12 months); mobility (number of people achieving total score on a scale of 0 to 6, according to D'Aubigne 1954; high scores indicate better mobility; at 6 weeks; 3, 6 and 12 months); mortality (before first follow‐up; we assumed that this was at 6 weeks); infection (superficial) |
|
| Notes |
Funding/sponsorship/declarations of interest: not reported Study dates: all recruited in 1979 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to groups but no additional details. We also noted that baseline characteristics were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | Low risk | Quote: "The patients were evaluated by the authors without knowledge of the type of prosthesis inserted" |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We did not expect lack of blinding for participant‐reported outcomes to influence outcome data. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Large number of losses, but mostly caused by death which is expected in this population. All losses were well reported. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Stoffel 2013.
| Study characteristics | ||
| Methods | Quasi‐RCT; parallel design Review comparison group: HA: bipolar versus unipolar |
|
| Participants |
Total number of randomised participants: 294 Inclusion criteria: people with displaced intracapsular fracture of the femoral neck who met the criteria for treatment with cemented hemiarthroplasty Exclusion criteria: significant communication disorders, non‐ambulatory after surgery, previous symptomatic hip pathology, residence outside the hospital's service zone Setting: hospital; single centre; Australia Baseline characteristics (overall; only for those who were not excluded)
Intervention group 1 (bipolar)
Intervention group 2 (unipolar)
Note:
|
|
| Interventions |
General details: procedures done by 15 registrars and 8 consultants; standardised rehabilitation programme Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: OHS; HHS; verbal numerical rating score for pain; 6MWT; hip range of motion (all at 12 months after surgery); postoperative complications (dislocation, CVA, delirium/confusion, encephalopathy, DVT, MI, chest infection, pneumonia, heart failure/pulmonary oedema, renal failure/acidosis, UTI, wound infection (superficial; deep) Outcomes relevant to the review: postoperative complications (delirium; dislocation; wound infection ‐ superficial and deep; CVA, DVT; MI, pneumonia; UTI; renal failure/acidosis); length of hospital stay; functional status at 12 months (modified HHS ‐ more points indicate high functioning); pain at 12 months (verbal numerical rating score; 0 = no pain, 10 = worst imaginable pain); 6MWT at 12 months Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: not reported Study dates: June 2005 to June 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Study authors report 2 conflicting descriptions of selection: random allocation to groups by coin toss, but all participants in group 1 have an odd year of birth, and all in group 2 have an even year of birth. We have judged this to indicate that the study is quasi‐randomised because it appears that participants were allocated to groups according to odd/even year of birth. |
| Allocation concealment (selection bias) | High risk | It is not possible to conceal allocation because of the quasi‐randomised methods used to allocate participants to groups. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by registrars and consultants but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We are uncertain whether participants were blinded to the intervention, but we did not expect this to influence reporting of data for mobility or pain. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We could not be certain whether losses (which were approximately 11%), and reasons for these losses, were balanced between groups because the number randomised to each group was not reported. We also noted a higher number of losses for measurement of mobility which were not explained, and variable losses for participant‐reported outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Talsnes 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 334 Inclusion criteria: admitted for cervical hip fracture with displaced Gardens III to IV fractures; > 75 years of age Exclusion criteria: not residing locally (because of the difficulties with follow‐up) Setting: multicentre; 2 hospitals; Norway Baseline characteristics Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: no details on surgery were reported Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: all‐cause mortality (12 months); surgery time; volume of blood loss; need for blood transfusion; haemoglobin concentration Outcomes relevant to the review: mortality (12 months); need for blood transfusion (≥ 2 units PRBC before discharge) |
|
| Notes |
Funding/sponsorship/declarations of interest: Charnley Grant from Orthomedic, and financial support from Centre of Medical Science, Innlandet Hospital Trust, Elverum, Norway Study dates: 2005 to 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A nurse in the operating theatre conducted the randomisation by opening one of the block randomised envelopes stating whether the prosthesis should be cemented or non cemented" Comment: insufficient information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Use of envelopes, but study authors do not report if envelopes are opaque, sealed, and sequentially numbered |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors did not describe how many surgeons were involved in the study and whether they were equally experienced with the types of implants used in this study. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality and blood transfusion) would influence objective outcome data . |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study report states that the study is registered with a clinical trials register; no identification number is reported, and we were unable to verify this. It is not feasible to effectively assess risk of selective reporting bias without this information. |
| Other bias | Low risk | We identified no other sources of bias. |
Taylor 2012.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented versus uncemented |
|
| Participants |
Total number of randomised participants: 160 Inclusion criteria: ≥ 70 years of age; acutely displaced fracture deemed by the attending surgeon to be suitable for hemiarthroplasty Exclusion criteria: people with a previous fracture of the same hip; pathological fracture; suitability for receiving a cemented component was made by the attending anaesthetist ‐ participants were excluded if the risk of death was unacceptable (based on patient age, pre‐existing cardiovascular or respiratory disease, or history of bone cement implantation syndrome) Setting: single centre; hospital; New Zealand Baseline characteristics (overall)
Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: carried out using modified Hardinge surgical approach, performed under supervision of 12 consultant surgeons experienced with both procedures (majority of procedures performed by registrars); all participants received 1 g cephazolin intraoperatively and 2 additional doses at 8 and 16 hours postoperatively; all received routine observation, analgesia, and prophylaxis against DVT; allowed to mobilise with full weight‐bearing as tolerated; clinical examinations at 6 weeks, 6 months, 1 and 2 years Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: pain (VAS); functional status (OHS; and SMFA); cognitive function (MMSE); mobility (TUG, use of walking aids); ability to live independently; mortality (6 weeks, 6 months, 1 year, 2 years); unplanned return to theatre; complications (cardiovascular, respiratory infections, superficial or deep wound infection, UTI, postoperative fracture, intraoperative fracture, dislocation, re‐operation); length of stay Outcomes relevant to the review: length of hospital stay; mortality (6 weeks, 1 year); unplanned return to theatre (assumed to be within 2‐year follow‐up period); complications (respiratory infections, superficial or deep wound infections, UTI, postoperative and intraoperative fractures, dislocation); discharge destination (discharged to own home); pain (VAS); functional status (OHS); mobility (TUG and use of walking aids) Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: funded by the New Zealand Orthopaedic Association, the Wishbone Trust, and the Accident Compensation Corporation (Wellington, New Zealand) Study dates: May 2006 to November 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Use of sequentially numbered, sealed and opaque envelopes |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | It is not possible to blind surgeons to treatment groups. The surgeons in the study were experienced in both techniques and we did not expect that lack of blinding would influence outcome performance or outcome data. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | Low risk | Quote: "All clinical variables were assessed by an unbiased observer (a research nurse who was not involved in the surgery or clinical decisions and who was blinded to the treatment group" |
| Blinding of outcome assessment (detection bias) participant‐reported outcomes | Low risk | We are uncertain whether participants were blinded to the intervention, but we did not expect this to influence reporting of data for mobility, pain or ability to live independently. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality and length of stay) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant losses |
| Selective reporting (reporting bias) | Unclear risk | Registered with Australian New Zealand Clinical Trials Register. Study authors do not report identification number and we were unable to check whether the study was registered prospectively. It is not feasible to effectively assess selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Van den Bekerom 2010.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 281 Inclusion criteria: displaced intracapsular femoral neck fractures, capability to give informed consent, no known metastatic disease, no contraindication to anaesthesia, ≥ 70 years of age; ability to understand written Dutch Exclusion criteria: inability to fulfil the inclusion criteria including refusal to consent, advanced radiological osteoarthritis or rheumatoid arthritis in the fractured hip; suspected pathological fracture; bedridden or barely mobile bed to chair; significant senile dementia Setting: multicentre; 7 district hospitals and 1 university hospital; Netherlands Baseline characteristics Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: all operations performed by experienced surgeons or residents under direct supervision of an experienced surgeon; participating surgeons used their own judgement to manage care (such as antibiotic and thromboembolic prophylaxis and surgical approach to the hip); type of anaesthesia reported by group (HA ‐ spinal: 92; epidural: 5; general: 25; psoas block: 2; unknown: 13; THA ‐ spinal: 71; epidural: 11; general: 30; psoas block: 0; unknown: 3); mobilised and full weight‐bearing as tolerated; use of participant education and physiotherapy supervision in ADL; after 6 weeks, allowed to mobilise without further restriction Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: mortality (during hospital stay; at 12 months; at 5 years); length of hospital stay; functional status (modified HHS, pain using HHS, function using HHS; at 12 months, and at 5 years); revision surgery (at 5 years); dislocation (at 5 years); loosening of femoral component, loosening of acetabular; polythene wear; osteoarthritis at the acetabulum; protrusio acetabuli; fracture/fissure at the acetabulum; heterotopic ossification; complications (defined as general, and local) Outcomes relevant to the review: mortality (during hospital stay; at 12 months; at 5 years); length of hospital stay; functional status (modified HHS, pain using HHS, function using HHS; at 12 months, 5 years, and 12 years); revision surgery (at 12 months, 5 years, and 12 years); dislocation (at 5 years, and at 12 years); loosening of femoral component (at 5 years, and at 12 years); superficial infection; deep infection; pulmonary embolism; pneumonia; CVA; delirium Note:
|
|
| Notes |
Funding/sponsorship/declarations of interest: no funding Study dates: not reported Note:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted externally |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by experienced surgeons but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality and length of stay) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although the study authors report the total number randomised and overall number of losses, these numbers are not reported by group and we could not be certain whether losses were evenly balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Vidovic 2013.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: HA: cemented vs uncemented |
|
| Participants |
Total number of randomised participants: 79 Inclusion criteria: female; > 70 years of age; displaced femoral neck fracture (Garden's III or IV) Exclusion criteria: participants that could not comprehend the study protocol; participants with sustained pathological fracture; presence of local or systemic infection; hip osteoarthritis; complete pre‐injury immobility; previous fracture of lower limbs; immunosuppression or other disease that interfere with bone metabolism Setting: hospital; single centre; Croatia Baseline characteristics (overall)
Intervention group 1 (cemented)
Intervention group 2 (uncemented)
Note:
|
|
| Interventions |
General details: 5 surgeons skilled in hip replacement surgery with the assistance of surgical residents; carried out using direct lateral, Hardinge approach; protocols followed for anticoagulation, antibiotics, and anaesthesia for hip fracture (low‐molecular‐weight heparin‐deltaparin 5000 IU once a day starting on POD1; 3 doses of cefazolin perioperatively; bupivacaine 0.5% and fentanyl for spinal and epidural anaesthesia); standard protocols for rehabilitation during hospitalisation followed by 21 days at rehabilitation centre; routine follow‐up and scans were scheduled for 1, 6 and 12 months Intervention group 1
Intervention group 2
Note:
|
|
| Outcomes |
Outcomes measured/reported by study authors: HHS (available at 3, 6 and 12 months); BMD; duration of surgery; length of hospital stay; complication rates (overall); mortality Outcomes relevant to the review: length of in‐hospital stay; mortality (12 months); HHS (at 3 and 12 months) |
|
| Notes |
Funding/sponsorship/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: January 2007 to December 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized into group A and B by an envelope" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were all performed by experienced surgeons but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality and length of stay) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We noted a discrepancy in the number of reported losses and the number of deaths. However, because this discrepancy was for only one participant, we used data in the results section for mortality to infer the number of losses. Most losses were owing to death. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
Xu 2017.
| Study characteristics | ||
| Methods | RCT; parallel design Review comparison group: THA versus HA |
|
| Participants |
Total number of randomised participants: 76 Inclusion criteria: neglected femoral neck fracture (defined as > 30 days after injury); ≥ 60 years of age; able to walk without aids before injury; able to provide informed consent Exclusion criteria: refusal to undergo surgery; any contraindication to surgery or anaesthesia; chronic hip pain and imaging revealing osteoarthritis or atrophic arthritis; metastatic cancer; active inflammatory disease Setting: hospital; single centre; China Baseline characteristics Intervention group 1 (THA)
Intervention group 2 (HA)
Note:
|
|
| Interventions |
General details: 1 experienced chief orthopaedic surgeon specialising in hip joint surgery; performed with spinal anaesthesia (or spinal and epidural, for THA); prophylactic antibiotics and anti‐thromboembolics given; functional exercises started on day of surgery, plan for full weight‐bearing 6 weeks after surgery; routine follow‐up annually (1 to 5 years) Intervention group 1
Intervention group 2
|
|
| Outcomes |
Outcomes measured/reported by study authors: intraoperative blood loss, operation time, duration of hospital stay, postoperative length discrepancy in lower extremities, HHS (before surgery; 1 year and 5 year postoperatively), complications (deep infection, prosthetic loosening, dislocation, periprosthetic fracture, acetabular osteoarthritis, all‐cause mortality (5 years) Outcomes relevant to the review: length of hospital stay; mortality (5 years); HHS (1 and 5 years); complications (deep infection, prosthetic loosening, dislocation, periprosthetic fracture; all at 5 years) |
|
| Notes |
Funding/sponsorship/declarations of interest: funding not reported; study authors declare no conflicts of interest Study dates: June 2000 to November 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Independent statistician prepared sequential sealed envelopes |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | It is not possible to blind surgeons to treatment groups. Study authors report that the interventions were performed by an experienced surgeon but we could not be certain whether surgeons were equally experienced in using the study implants. |
| Blinding of outcome assessment (detection bias) clinically‐assessed subjective outcomes | High risk | It is not possible to blind surgeons to treatment groups. We judged that knowledge of the type/fixation method of arthroplasty could influence judgments made by surgeons when assessing subjective outcomes. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | We did not expect that lack of blinding of assessors of objective measures (mortality and length of stay) would influence objective outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report pre‐published protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias. |
ADL: activities of daily living; AHS: manufacturers name for implant; AMTS: Abbreviated Mental Test Score; AO: Arbeitsgemeinschaft für Osteosynthesefragen (system for classification of fractures); ASA: American Society of Anesthesiologists; AVN: avascular necrosis; BI: Barthel Index; BMD: bone mineral density; BMI: body mass index; CCI: Charlson Comorbidity Index; CPCS: Collarless Polished Cemented Stem; CPT: collarless, polished, double‐taper design concept; CT: chromatography; CTU: Clinical Trials Unit; CVA: cerebrovascular accident; DB: manufacturers name for implant; DM: dual mobility; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; DVT: deep vein thrombosis; EQ‐5D: European Quality of Life ‐ 5 dimensions; ETS: Exeter trauma stem; GARS: Groningen Activity Restriction Scale; GI: gastrointestinal; HA: hemiarthroplasty; HAC: hydroxyapatite‐coated; HHS: Harris Hip Score; HRQoL: health‐related quality of life; IADL: instrumental activities of daily living; ICECAP‐O: ICEpop CAPability measure for older people; IQR: interquartile range; ISS: Injury Severity Score; ITT: intention‐to‐treat; IU: international units; IV: intravenous(ly); LD/Fx: manufacturers name for implant; M/F: male/female; MI: myocardial infarction; MMSE: Mini‐Mental State Examination; MRI: magnetic resonance imaging; NICE: National Institute for Health and Care Excellence; NMS: New Mobility Score; OGEE: manufacturers name for implant; OHS: Oxford Hip Score; OTA: orthopaedic trauma association; PADL: physical activities of daily living; POD: postoperative day; PRBC: packed red blood cells; PCU: polycarbonate–urethane ; QoL: quality of life; RCT: randomised controlled trial; SD: standard deviation; SF‐36/12 (PCS or MCS): Short‐Form General Health Survey ‐36/12 (physical component score or mental component score): SMFA: Short Musculoskeletal Function Assessment; 6MWT: six‐minute walk test; SPMSQ: Short Portable Mental Status Questionnaire; THA: total hip arthroplasty; TIA: transient ischaemic attack; TUG: Timed Up and Go; UCLA: University of California, Los Angeles; UHR: universal head system (manufacturer name); UTI: urinary tract infection; VAS: visual analogue scale; VELCA: Verona Elderly Care Study; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aydin 2009 | RCT, comparing distal and proximal centralising devices for arthroplasty. We excluded this study because it investigated surgical approaches rather than implants and the interventions were therefore not eligible. |
| ISRCTN42349821 | Abandoned due to lack of funding |
| Karpman 1992 | RCT, comparing Austin‐Moore with cemented and uncemented bipolar hemiarthroplasty. We excluded this study because it was published only as an abstract with limited detail, and it did not report the number of participants randomised to each group. |
| Kavcic 2006 | RCT, comparing THA and HA. We excluded this study because it was published only as an abstract with limited detail, and it did not report the number of participants randomised to each group. |
| Rosen 1992 | RCT, comparing bipolar versus unipolar hemiarthroplasty in displaced subcapital fractures of the hip in an elderly population. We excluded this study because it was published only as an abstract with insufficient information on numbers of participants in each group and insufficient quantitative outcome data. |
| Somashekar 2013 | Study comparing unipolar with bipolar hemiarthroplasty in adults > 60 years of age. We judged that this study was not randomised because study authors described the use of purposive sampling to select participants. |
| Stock 1997 | RCT, comparing ceramic arthroplasty with Thomson's hemiarthroplasty. We excluded this study because it was published only as an abstract with limited detail, and it did not report the number of participants randomised to each group. |
| Van Thiel 1988 | RCT, comparing a Moore and Bateman bipolar prosthesis. We excluded this study because it was published only as an abstract with insufficient detail and no quantitative outcome data. |
HA: hemiarthroplasty RCT: randomised controlled trial THA: total hip arthroplasty
Characteristics of studies awaiting classification [ordered by study ID]
NCT00800124.
| Methods | RCT, parallel group Comparison: HA (cemented) versus HA (modern uncemented) |
| Participants |
Number of recruited participants: 334 Inclusion criteria: people aged > 70 years with a Garden III or IV acute hip fracture Exclusion criteria: person or relative refuse enrollment Settings: hospital, Norway |
| Interventions | HA: cemented Landos prosthesis HA: modern uncemented Landos prosthesis |
| Outcomes | Mortality (1 year) |
| Notes | Study completed June 2011 |
NCT00859378.
| Methods | RCT, parallel group Comparison: HA (modern uncemented) versus HA (cemented) |
| Participants |
Number of expected participants: 400 Inclusion criteria: proximal femoral fracture Exclusion criteria: rheumatoid arthritis, pathologic fracture, severe dementia (preventing the informed consent) Setting: Finland |
| Interventions | Cemented semi‐endoprosthesis (Basis, Smith & Nephew) Uncemented semi‐endoprosthesis (Biomet Taperloc, Biomet Inc.) |
| Outcomes | Mortality (3 months); prosthetic complications (1 year) |
| Notes | Active, not recruiting; last updated 7 April 2015 |
NCT01432691.
| Methods | RCT, parallel group Comparison: THA versus HA |
| Participants |
Number of participants: 70 Inclusion criteria: people aged > 70 years, admitted to hip fracture department with a III to IV Garden femoral neck fracture or a fracture Garden I to II with over 20‐degree posterior tilt, with a preoperative New Mobility Score ≥ 6, ASA score ≤ III, are able to give informed consent, be cognitively intact (Hindsøe score ≥ 6) and speak and understand Danish Exclusion criteria: none Settings: hospital, Denmark |
| Interventions | THA: BFX (Biomet CE‐number: 00520) HA: hemialloplastik |
| Outcomes | Migration/rotation (RSA); function (WOMAC); HRQoL (EQ‐5D) |
| Notes | Study completed in June 2015 |
NTR1782.
| Methods | RCT, parallel design Comparison group HA (cemented) vs HA (modern uncemented) |
| Participants |
Number of expected participants: 400 Inclusion criteria: people aged > 65 years of age with a proximal intracapsular femoral fracture who should be treated with a hemiarthroplasty Exclusion criteria: multiple trauma patient, pathological fracture, symptomatic, coxarthritis at the ipsilateral side, osteosynthesis revision Setting: Netherlands |
| Interventions | HA (cemented stem) vs HA (modern, hydroxyapatite‐coated uncemented stem) |
| Outcomes | Composite endpoint of serious adverse events; post‐surgery delirium; surgical time; radiological evaluation; pain; complications and mobilisation. Follow‐up: 0 to 30 days (serious adverse events), 6 weeks, 12 weeks and 1 year |
| Notes | Study completed 30 June 2012 but no trial report available |
ASA: American Society of Anesthesiologists HA: hemiarthroplasty HRQoL: health‐related quality of life RCT: randomised controlled trial RSA: radiostereometric analysis THA: total hip arthroplasty WOMAC: Western Ontario and McMaster Osteoarthritis index
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800019531.
| Study name | A randomised controlled trial for comparing the hemiarthroplasty with the total hip arthroplasty in the treatment of femoral neck fractures in patients older than 75 years |
| Methods | RCT, parallel group Comparison: THA versus HA |
| Participants |
Expected number of participants:100 Inclusion criteria: people who are willing to participate this study with a displaced femoral neck fracture, diagnosed by CT or X‐ray, aged > 75 years with a history of injury Exclusion criteria: pathological fractures; fracture with tumour or immunodeficiency disease; fracture with spinal cord injury or nerve injury, refusal to sign informed consent Settings: hospital, China |
| Interventions | THA (unspecified) HA (unspecified) |
| Outcomes | Total blood loss; maximum haemoglobin decline; blood transfusion rate; pain score (VAS); range of hip flexion and abduction; length of stay; postoperative compliance; function (HHS & WOMAC); incidence of thrombosis |
| Starting date | 2 November 2018 |
| Contact information | Zha Guo‐chun, 41049015@qq.com, Affiliated Hospital of Xuzhou Medical University, China |
| Notes | Recruiting, expected completion date 2 October 2019 |
ISRCTN15606075.
| Study name | WHiTE 8 COPAL: a randomised controlled trial of low dose single antibiotic loaded cement versus high dose dual antibiotic loaded cement in patients receiving a hip hemiarthroplasty after fracture |
| Methods | RCT, parallel group Comparison: HA (modern, cemented) versus HA (modern, cemented) |
| Participants |
Expected number of participants: 4920 Inclusion criteria: people aged > 60 years with an intracapsular hip fracture, which in the opinion of the treating surgeon requires acute surgical treatment with a cemented hip hemiarthroplasty Exclusion criteria: people will be excluded if they are allergic to gentamicin or clindamycin Settings: hospital, multicentre, UK |
| Interventions | HA: cemented hemiarthroplasty with low dose single antibiotic cement with choice of femoral head and stem. Cement used will be Heraeus Palacos R+G cement (Hanau, Germany) – contains gentamicin 0.5 g per 40 g mix of cement. HA: cemented hemiarthroplasty with high dose dual antibiotic cement with choice of femoral head and stem. Cement used will be Heraeus Copal G+C cement (Hanau, Germany) – contains gentamicin 1 g and clindamycin 1 g per 40 g mix of cement. |
| Outcomes | Deep infection (CDC definition); mortality; HRQoL (EQ‐5D‐5L); complications; antibiotic use; resource use; mobility; residential status |
| Starting date | 15 December 2017 |
| Contact information | Stephanie Wallis, white8‐copal@ndorms.ox.ac.uk |
| Notes | Expected to complete 15 November 2021 |
NCT01109862.
| Study name | Prospective randomised comparison of bipolar hemiarthroplasty and total hip arthroplasty with large femoral heads for the treatment of displaced intracapsular femoral neck fractures in the elderly |
| Methods | RCT, parallel group Comparison: HA (bipolar, cemented) versus THA (large head, cemented) |
| Participants |
Expected number of participants: 80 Inclusion criteria: people aged from 70 to 90 years, with an acute femoral neck fracture, independent community ambulator (more than 0.5 km, without the aid of another person, use of a cane is permitted) and an abbreviated mental test score > 6 Exclusion criteria: pathological fracture (excluding osteoporosis), rheumatoid arthritis, symptomatic arthrosis of the involved hip, neurological disorder that may significantly influence walking ability and/or tendency to dislocate, chronic corticosteroid use, concomitant other fracture or very high surgical risk Settings: hospitals, multicentre, UK |
| Interventions | All cemented THA Cemented bipolar HA |
| Outcomes | Function (OHS); HRQoL (SF‐36); dislocation risk; mortality. Follow‐up: 2 years |
| Starting date | April 2010 |
| Contact information | Dror Lakstein, drorale@gmail.com |
| Notes | Recruiting |
NCT01578408.
| Study name | Corail‐SP study ‐ a prospective randomised comparison between cemented and uncemented hydroxyapatite coated prosthesis stems in total hip arthroplasty in patients with femoral neck fractures |
| Methods | RCT, parallel group Comparison THA (cemented) versus THA (modern uncemented) |
| Participants |
Expected number of participants: 109 Inclusion criteria: people approximately 60 to 85 years of age, who are acutely admitted to Mölndal's Hospital with a dislocated intracapsular femoral neck fracture, that in clinical practice is treated with a hip prosthesis operation and live independently. Exclusion criteria: people who have difficulties in understanding the intent of the study, have rheumatic disorders (RA, Bechterew, SLE), current cortisone treatment, stroke with remaining weakness or neurological disorders with affection of locomotion, dementia, grave obesity with BMI ≥ 30 to 35 kg/m2 or a delay between time of injury and time of surgery exceeding 72 hours Setting: Sweden |
| Interventions | Surgery with a reverse hybrid arthroplasty with an uncemented hydroxyapatite‐coated Corail stem and a cemented Marathon cup (DePuy) Surgery with a totally cemented option with a Lubinus SPII stem and a IP cup (Link) |
| Outcomes | Time to mobilisation (days); cognitive status (SPMSQ); intraoperative partial pressure oxygen with a pulmonary catheter; bone remodelling (hip DEXA); inflammatory response (blood samples); fixation / migration / loosening of the hip prosthesis components (RSA) and conventional pelvis and hip X‐ray exams; reoperation; HRQoL (EQ‐5D); activity level (UCLA); function (HHS). Follow‐up visits at 3, 6 months, 1, 2, 5, 7, 10 years. |
| Starting date | 11 May 2010 |
| Contact information | Johan Kärrholm, Orthopaedic Department, Sahlgrenska University Hospital, Gothenburg, Sweden |
| Notes | Outcome data collection completed 19 February 2020 |
NCT01787929.
| Study name | Cemented versus uncemented hemiarthroplasty for displaced femoral neck fracture in elderly patients: a randomised prospective trial |
| Methods | RCT, parallel group Comparison: HA (cemented) versus HA (uncemented) |
| Participants |
Expected number of participants: 150 Inclusion criteria: people aged > 70 years with displaced femoral neck fractures (Garden III and IV), ASA score ≤ III, Lee score ≤ 2 Exclusion criteria: Parker score < 4, pathological femoral neck fracture (Paget disease or tumour) Settings: hospital, France |
| Interventions | HA (cemented): hemiarthroplasty surgery with cement for displaced femoral neck fractures HA (uncemented): hemiarthroplasty surgery without cement is a surgery for displaced femoral neck fractures |
| Outcomes | Function (HHS) at 3 and 12 months |
| Starting date | 7 February 2016, expected primary outcome completion 7 February 2018 |
| Contact information | bernard‐de‐dompsure.r@chu‐nice.fr |
| Notes |
UMIN000011303.
| Study name | A randomised controlled trial comparing bipolar hemiarthroplasty with total hip replacement for displaced intracapsular fractures of the femoral neck in active patients |
| Methods | RCT, parallel group Comparison: THA versus HA (bipolar) |
| Participants |
Expected number of participants: 240 Inclusion criteria: 20 to 76 years of age, with displaced intracapsular fracture of femoral neck suitable for treatment with either THA or bipolar HA, femoral head size > 36 mm, walking independently without any orthosis, able to give informed consent and adhere to follow‐up Exclusion criteria: history of infectious disease, previous hip surgery, BMI > 40 kg/m2, pregnancy, history of neurological disease, history of Paget's disease, history of steroid therapy or immunosuppression therapy Settings: Japan |
| Interventions | THA Bipolar HA |
| Outcomes | Functional outcome (JOA score, walking ability); patient satisfaction (EQ‐5D, JHEQ); radiographic evaluation |
| Starting date | 1 October 2013 |
| Contact information | Yukiharu Hasegawa; taekgami‐toyomh@umin.ac.jp |
| Notes | Recruiting |
Wolf 2020.
| Study name | The DUALITY trial ‐ a register‐based, randomised controlled trial to investigate dual mobility cups in hip fracture patients |
| Methods | Multicentre, register‐nested, randomised controlled trial |
| Participants |
Expected number of participants: 1600 Inclusion criteria: > 65 years of age, with a displaced femoral neck fracture who are eligible for a THA ; Garden 3–4 fracture Exclusion criteria: cognitive impairment, previous inclusion of a contralateral THA in the ongoing trial, delayed fracture surgery (date of injury more than seven days prior to date of screening), pathological or stress fracture of the femoral neck, and fracture adjacent to a previous ipsilateral hip implant, such as a previously inserted screw or plate Settings: Sweden |
| Interventions | Dual mobility cup (Avantage (Zimmer Biomet, Warsaw, IN, USA), Polar (Smith & Nephew, London, UK), or Ades (Zimmer Biomet); surgeon preference Standard cup (Lubinus (Waldemar Link, Hamburg, Germany), Marathon (DePuy Synthes, Warsaw, IN, USA), Exeter RimFit (Stryker, Kalamazoom MI, USA), or Lubinus IP (Waldemar Link) cups); surgeon preference |
| Outcomes | Dislocation; reoperation; mortality; HRQoL (EQ‐5D) |
| Starting date | January 2020 |
| Contact information | olof.wolf@surgsci.uu.se |
| Notes |
ASA: American Society of Anesthesiologists BMI: body mass index CDC: Centre for Disease Control CT: computed tomography DEXA: dual energy x‐ray absorptiometry EQ‐5D: EuroQoL 5 Dimensions instrument EQ‐5D‐5L: EuroQoL 5 Dimensions, 5 levels instrument HA: hemiarthroplasty HHS: Harris hip score HRQoL: health‐related quality of life JHEQ: Japanese Orthopaedic Association hip disease evaluation questionnaire JOA: Japanese Orthopaedic Association OHS: Oxford hip score RA: rheumatoid arthritis RCT: randomised controlled trial RSA: radiostereometric analysis SF‐36: Short form‐36 SLE: systemic lupus erythematosis SPMSQ: short portable mental status questionnaire UCLA: University of California, Los Angeles THA: total hip arthroplasty VAS: visual analogue score WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Differences between protocol and review
Review information
Review authors: two new review authors joined the review team (SL, RM, JC), and four authors left the review author team (AS, AJ, HW, JMG).
Objectives
we edited the objectives in line with Cochrane guidance, using a single sentence.
Methods
Criteria for considering studies for this review
Types of interventions: we specified that the bipolar HA versus unipolar HA was subgrouped by type of cement. We did not organise the interventions groups according to a direction (intervention named first and the control second), because the comparative groups in all studies were for active interventions and did not include control group.
We did not organise the data by naming the intervention first and the control second. The studies in this review evaluated comparisons between established treatments which are still in active use. In order to keep the interventions distinct and provide relevant information to the reader, we specified the direction of effect in each effect estimate.
Types of outcome measures: we edited the time points in the review to reflect the wider variation in data in the included studies. In addition to the early data at 4 months or less, we added collection of data at 12 months (prioritising 12‐month data, but in its absence including data after 4 months and up to 24 months) and late (after 24 months).
Search methods for identification of studies
Electronic searches: we did not search the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/en/) because, at the time of searching, the platform was not available because of the COVID‐19 pandemic. We believed that clinical trials register searches remained comprehensive because CENTRAL also includes studies from international trials registers.
Data collection and analysis
Data extraction and management: we planned that data extraction would be completed independently by two reviewers. In practice, one author extracted data which was checked for accuracy by a second review author. We edited the data collected to describe the flow of study participants. Rather than collecting "study disposition (number randomised, number by protocol, number available for analysis)", we collected "number of randomised participants, losses (and reasons for losses), and number analysed for each outcome".
Measures of treatment effect: we found that some studies reported outcomes using categorical data. We added an explanation of our methods to report effect estimates for these data.
Unit of analysis issues: we edited this section of the review to describe how we managed potential unit of analysis issues with the included studies. We reported methods for managing multi‐arm studies and for managing outcome data in studies that reported participants as well as fracture cases.
Dealing with missing data: we attempted to contact study authors of recently published studies (since 2012) when we noted data were missing or not clearly reported for critical review outcomes. Most studies in the review were published more than 20 years ago, and we did not expect study authors of older studies to have access to study data. We specified that we used the Characteristics of included studies to note when study authors reported data that we were unable to use because of an unknown number of losses or because data were reported unclearly.
Assessment of reporting biases: we stated that we required 10 studies to explore publication bias with a funnel plot. We stated that this assessment was therefore only conducted for a few outcomes.
Data synthesis: we did not pool data using the generic inverse variance method in RevMan 5.4 (Review Manager 2014), because it was not necessary, as we found that study authors reported outcomes that could be pooled appropriately as dichotomous and continuous data. In this section, we added detail to describe how we decided which data to pool in analysis if data were reported at more than one point, and if data were reported using more than one measurement tool.
Subgroup analysis: we clarified that we conducted subgroup analysis only when we had at least 10 studies. We were unable to explore key effect modifiers because these were insufficiently reported in studies. In this section, we also specified the plan to test for subgroup differences for prostheses according to whether a modern or old uncemented stem was used.
Sensitivity analysis: we clarified that sensitivity analysis was conducted when pooled analyses included more than two studies. We reported additional detail, for clarity, to describe how we managed sensitivity analysis in the review. We did not perform sensitivity analysis on mixed populations because most studies reported insufficient information for us to judge whether participants' characteristics in the included studies were mixed. We also did not perform sensitivity analysis for studies of implants that are currently not in clinical use. We obtained the general view that all interventions at the major‐grouping level remain in current use, and although some examples of implants within these categories may no longer be manufactured, we believe the distinction between these implants within the same category is marginal and the sensitivity analysis would not be meaningful.
Summary of findings: we specified the comparison groups for which we constructed summary of findings tables. We also explained a choice to select one measurement tool for the summary of findings tables when outcomes were reported using different measurement tools or measurement values that could not all be combined in meta‐analysis.
Contributions of authors
SL (systematic reviewer): sifted and identified included studies, extracted study data, interpreted the findings, and drafted the review. RM (systematic reviewer): sifted and identified included studies, extracted study data, interpreted the findings, and drafted the review. MP (content expert, Trauma and Orthopaedics): interpreted the findings, reviewed and approved the final review. JC (statistician): reviewed and approved the final review. XG (guarantor and content expert, Trauma and Orthopaedics): interpreted the findings, reviewed and approved the final review, and is the guarantor of the content.
Editorial contributions
Faith Armitage (Copy Editor): copy‐edited the review. Liz Bickerdike (Acute and Emergency Care Network Associate Editor): advised on methodology and review content. Mike Brown (Acute and Emergency Care Network Senior Editor): approved the final version for publication. Maria Clarke (Information Specialist): ran literature searches and edited the search methods section. Joanne Elliott (Managing Editor): co‐ordinated the editorial process and edited the review.
Xavier Griffin and Sharon Lewis are members of the editorial base but were not involved in the editorial process or decision‐making for this review.
Sources of support
Internal sources
-
National Institute for Health Research (NIHR), UK
Oxford Biomedical Research Centre, Oxford
External sources
-
NIHR, UK
NIHR Systematic Review Programme Grant 16/114/15. A programme of high‐priority reviews for the management of patients with hip fracture: a collaboration which can inform future healthcare policy guidance. NIHR Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group
Declarations of interest
SL has no known conflicts of interest. RM has no known conflicts of interest. MP has received expenses and honoraria from a number of commercial companies and organisations for giving lectures on different aspects of hip fracture treatment. In addition, he has received royalties from BBrawn Ltd related to the design and development of an implant used for the internal fixation of intracapsular hip fractures. He remained independent of study selection decisions, risk of bias assessment, and any data extraction of any of the studies on which he is an author, co‐applicant, or has had an advisory role. JC remained independent of study selection decisions for ongoing studies. XG is funded by a National Institute for Health Research Clinician Scientist Grant. Further funding from industry and charitable grants are and have been made available to his institution. All decisions relating to the design, conduct, analysis, write‐up and publication of research are independent of these funding organisations. He has ongoing expert consultancy with several companies; none involve the development of any implant for use in hip fracture care. He remained independent of study selection decisions, risk of bias assessment, and any data extraction of any of the studies on which he is an author, co‐applicant, or has had an advisory role.
New
References
References to studies included in this review
Abdelkhalek 2011 {published data only}
- Abdelkhalek M, Abdelwahab M, Ali A M.Bipolar versus fixed-head hip arthroplasty for femoral neck fractures in elderly patients. Strategies in Trauma & Limb Reconstruction 2011;6(1):1-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2006 {published data only}
- Avery PP, Baker RP, Walton MJ, Rooker JC, Squires B, Gargan MF, et al.Total hip replacement and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck: a seven- to ten-year follow-up report of a prospective randomised controlled trial. Journal of Bone and Joint Surgery - British Volume 2011;93(8):1045-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Baker RP, Squires B, Gargan MF, Bannister GC.Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. Journal of Bone & Joint Surgery - American Volume 2006;88(12):2583-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Baker RP, Squires B, Gargan MF, Bannister GC.A randomised controlled comparison of total hip arthroplasty and hemiarthroplasty in mobile independent patients with displaced intracapsular femoral neck fracture [abstract]. Journal of Bone and Joint Surgery - British Volume 2006;88:431-2. [DOI] [PubMed] [Google Scholar]
- ISRCTN70736853.A randomised prospective trial comparing unipolar hemiarthroplasty, bipolar hemiarthroplasty and total hip replacement in the treatment of displaced intracapsular femoral neck fractures. www.isrctn.com/ISRCTN70736853 (first received 20 September 2004).
Blomfeldt 2007 {published data only}
- Blomfeldt R, Tornkvist H, Eriksson K, Soderqvist A, Ponzer S, Tidermark J.A randomised controlled trial comparing bipolar hemiarthroplasty with total hip replacement for displaced intracapsular fractures of the femoral neck in elderly patients. Journal of Bone & Joint Surgery - British Volume 2007;89(2):160-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Blomfeldt R, Tornkvist H, Eriksson K, Soderqvist A, Ponzer S, Tidermark J.Bipolar hemiarthroplasty compared with total hip replacement for displaced femoral neck fractures in the elderly. A randomised, controlled trial [abstract]. Journal of Bone & Joint Surgery - British Volume 2009;91:169. [Google Scholar]
- Hedbeck CJ, Enocson A, Lapidus G, Blomfeldt R, Tornkvist H, Ponzer S, et al.Comparison of bipolar hemiarthroplasty with total hip arthroplasty for displaced femoral neck fractures: a concise four-year follow-up of a randomized trial. Journal of Bone & Joint Surgery - American Volume 2011;93(5):445-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brandfoot 2000 {published data only}
- Brandfoot T, Faraj AA, Porter P.Cemented versus uncemented Thompson's prosthesis: a randomised prospective functional outcome study. Injury 2000;31:280-1. [Google Scholar]
Cadossi 2013 {published data only}
- Cadossi M, Chiarello E, Savarino L, Tedesco G, Baldini N, Faldini C, et al.A comparison of hemiarthroplasty with a novel polycarbonate-urethane acetabular component for displaced intracapsular fractures of the femoral neck: a randomised controlled trial in elderly patients. Bone & Joint Journal 2013;95(5):609-15. Erratum in: Bone & Joint Journal 2013; 95-B(11):1582. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cadossi M, Chiarello E, Savarino L, Tedesco G, Baldini N, Faldini C, et al.Erratum: A comparison of hemiarthroplasty with a novel polycarbonate- urethane acetabular component for displaced intracapsular fractures of the femoral neck: a randomised controlled trial in elderly patients. Bone & Joint Journal 2013;95(11):1582. Erratum for: Bone & Joint Journal 2013; 95B: 609-15. [DOI] [PubMed] [Google Scholar]
Calder 1995 {published data only}
- Calder SJ, Anderson GH, Harper WM, Jagger C, Gregg PJ.A subjective health indicator for follow-up. A randomised trial after treatment of displaced intracapsular hip fractures. Journal of Bone and Joint Surgery - British Volume 1995;77(3):494-6. [PMID: ] [PubMed] [Google Scholar]
Calder 1996 {published data only}
- Calder SJ, Anderson GH, Jagger C, Harper WM, Gregg PJ.Unipolar or bipolar prosthesis for displaced intracapsular hip fracture in octogenarians: a randomised prospective study. Journal of Bone and Joint Surgery - British Volume 1996;78(3):391-4. [PMID: ] [PubMed] [Google Scholar]
Cao 2017 {published data only}
- Cao X, Kong X, Li A.Auxiliary biological cemented femoral stem was effective in treating elderly patients with intertrochanteric fracture. Biomedical Research (India) 28;9:4071-5. [ISSN: 0970-938X] [Google Scholar]
Chammout 2017 {published data only}
- Chammout G, Muren O, Boden H, Salemyr M, Skoldenberg O.Cemented compared to uncemented femoral stems in total hip replacement for displaced femoral neck fractures in the elderly: study protocol for a single-blinded, randomized controlled trial (CHANCE-trial). BMC Musculoskeletal Disorders 2016;17(1):398. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chammout G, Muren O, Laurencikas E, Boden H, Kelly-Pettersson P, Sjoo H, et al.More complications with uncemented than cemented femoral stems in total hip replacement for displaced femoral neck fractures in the elderly. Acta Orthopaedica 2017;88(2):145-51. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02247791.Study of prosthesis choice in older patients with a dislocated femoral neck fracture of the hip. clinicaltrials.gov/ct2/show/NCT02247791 (first received 25 September 2014).
Chammout 2019 {published data only}
- Chammout G, Kelly-Pettersson P, Hedbeck C-J, Stark A, Mukka S, Skoldenberg O.HOPE-Trial: Hemiarthroplasty compared with total hip arthroplasty for displaced femoral neck fractures in octogenarians: a randomized controlled trial. Journal of Bone and Joint Surgery - American Volume 2019;4((2) e0059):1-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02246335.Hemiarthroplasty compared to total hip arthroplasty for displaced femoral neck fractures in the elderly. A randomised controlled trial. clinicaltrials.gov/ct2/show/NCT02246335 (first received 22 September 2014).
- Skoldenberg O, Chammout G, Mukka S, Muren O, Nasell H, Hedbeck CJ, et al.HOPE-trial: hemiarthroplasty compared to total hip arthroplasty for displaced femoral neck fractures in the elderly-elderly, a randomized controlled trial. BMC Musculoskeletal Disorders 2015;16:307. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornell 1998 {published data only}
- Cornell CN, Levine D, O'Doherty J, Lyden J.Unipolar versus bipolar hemiarthroplasty for the treatment of femoral neck fractures in the elderly. Clinical Orthopaedics & Related Research 1998;348:67-71. [PMID: ] [PubMed] [Google Scholar]
Davison 2001 {published data only}
- Calder SJ, Anderson GH, Gregg PJ.Bipolar hemiarthroplasty for displaced intracapsular hip fractures: a randomised prospective trial [abstract]. Journal of Bone and Joint Surgery - British Volume 1995;77:214. [PubMed] [Google Scholar]
- Calder SJ, Anderson GH, Harper WM, Gregg PJ.The use of bipolar hemi-arthroplasty in intracapsular hip fracture - a prospective randomised trial [abstract]. Journal of Bone and Joint Surgery - British Volume 1995;77(1):no pagination. [Google Scholar]
- Davison J, Harper WM, Gregg PJ.Which treatment for the displaced fractures of the femoral neck? A prospective randomised comparison of three surgical procedures [abstract]. Journal of Bone and Joint Surgery 1997;79(2):243-4. [Google Scholar]
- Davison JN, Calder SJ, Anderson GH, Ward G, Jagger C, Harper WM, et al.Treatment for displaced intracapsular fracture of the proximal femur. Journal of Bone and Joint Surgery 2001;83(2):206-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
DeAngelis 2012 {published data only}
- DeAngelis JP, Ademi A, Staff I, Lewis CG.Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. Journal of Orthopaedic Trauma 2012;26(3):135-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01114646.Functional status, morbidity and mortality in cemented versus uncemented hemiarthroplasty for subcapital hip fractures: a prospective randomised trial. clinicaltrials.gov/ct2/show/results/NCT01114646 (first received 3 May 2010).
Dorr 1986 {published data only}
- Dorr LD, Glousman R, Hoy AL, Vanis R, Chandler R.Treatment of femoral neck fractures with total hip replacement versus cemented and noncemented hemiarthroplasty. Journal of Arthroplasty 1986;1(1):21-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Emery 1991 {published data only}
- Emery RJ, Broughton NS, Desai K, Bulstrode CJ, Thomas TL.Bipolar hemiarthroplasty for subcapital fracture of the femoral neck. A prospective randomised trial of cemented Thompson and uncemented Moore stems. Journal of Bone and Joint Surgery - British Volume 1991;73(2):322-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fernandez 2022 {published data only}
- Fernandez MA, Achten J, Lerner RG, Mironov K, Parsons N, Dritsaki M, et al.Randomised controlled trial comparing hydroxyapatite coated uncemented hemiarthroplasty with cemented hemiarthroplasty for the treatment of displaced intracapsular hip fractures: a protocol for the WHITE 5 study. BMJ Open 2019;9(12):e033957. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MA, Achten J, Parsons N, Griffin XL, Png M-E, Gould J, et al.Cemented or uncemented hemiarthroplasty for intracapsular hip fracture. New England Journal of Medicine 2022;386:521-30. [DOI] [PubMed] [Google Scholar]
- ISRCTN18393176.A randomised controlled trial to compare contemporary un-cemented hemiarthroplasty with the standard-of-care cemented hemiarthroplasty for the treatment of displaced intracapsular hip fractures. www.isrctn.com/ISRCTN18393176 (first received 13 March 2017).
Figved 2009 {published data only}
- Figved W, Opland V, Frihagen F, Jervidalo T, Madsen JE, Nordsletten L.Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures. Clinical Orthopaedics & Related Research 2009;467(9):2426-35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langslet E, Frihagen F, Opland V, Madsen JE, Nordsletten L, Figved W.Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: 5-year followup of a randomized trial. Clinical Orthopaedics & Related Research 2014;472(4):1291-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00491673.A prospective randomised trial of uncemented versus cemented hemiarthroplasty for displaced femoral neck fractures. clinicaltrials.gov/ct2/show/record/NCT00491673 (first received 25 June 2007).
Figved 2018 {published data only}
- Figved W, Svenoy S, Rohrl SM, Dahl J, Nordsletten L, Frihagen F.Higher cartilage wear in unipolar than bipolar hemiarthroplasties of the hip at 2 years: a randomized controlled radiostereometric study in 19 fit elderly patients with femoral neck fractures. Acta Orthopaedica 2018;89(5):503-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00746876.Unipolar or bipolar hemiarthroplasty in the treatment of displaced femoral neck fractures. A randomised trial of RSA measurements of acetabular wear. clinicaltrials.gov/ct2/show/NCT00746876 (first received 4 September 2008).
Griffin 2016 {published data only}
- Griffin XL, McArthur J, Achten J, Parsons N, Costa ML.The Warwick Hip Trauma Evaluation Two -an abridged protocol for the WHiTE Two Study: an embedded randomised trial comparing the dual-mobility with polyethylene cups in hip arthroplasty for fracture. Bone & Joint Research 2013;2(10):210-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin XL, Parsons N, Achten J, Costa ML.A randomised feasibility study comparing total hip arthroplasty with and without dual mobility acetabular component in the treatment of displaced intracapsular fractures of the proximal femur : the Warwick Hip Trauma Evaluation Two: WHiTE Two. The Bone & Joint Journal 2016;98(11):1431-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN90544391.A randomised controlled trial comparing total hip replacement with and without a dual mobility cup in the treatment of displaced intracapsular fractures of the proximal femur. www.isrctn.com/ISRCTN90544391 (first received 9 April 2013).
Harper 1994 {published data only}
- Anderson GH, Dias JJ, Hoskinson J, Harper WM.A randomized study of the use of bone cement with Thompson's prosthesis in the treatment of intracapsular fractures of the femoral neck [abstract]. Journal of Bone and Joint Surgery - British Volume 1992;74(2):132-3. [Google Scholar]
- Harper WM.Treatment of intracapsular proximal femoral fractures. A prospective randomised trial comparing cemented and uncemented Thompson hemiarthroplasty in the treatment of displaced intracapsular proximal femoral fractures [dissertation]. Leicester, UK: University of Leicester, 1994. [Google Scholar]
HEALTH 2019 {published data only}
- Bhandari M, Devereaux PJ, Einhorn TA, Thabane L, Schemitsch EH, Koval KJ, et al, Investigators Health.Hip fracture evaluation with alternatives of total hip arthroplasty versus hemiarthroplasty (HEALTH): protocol for a multicentre randomised trial. BMJ Open 2015;5(2):e006263. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Investigators.Total hip arthroplasty or hemiarthroplasty for hip fracture. New England Journal of Medicine 2019;381(23):2199-2208. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NTR1623.Hip fracture evaluation with alternatives of total hip arthroplasty versus hemi-arthroplasty (HEALTH): a multi-centre randomised trial comparing total hip arthroplasty and hemi-arthroplasty on revision surgery and quality of life in patients with displaced femoral neck fractures. www.trialregister.nl/trial/685 (first received 12 January 2009).
Hedbeck 2011 {published data only}
- Hedbeck CJ, Blomfeldt R, Lapidus G, Tornkvist H, Ponzer S, Tidermark J.Unipolar hemiarthroplasty versus bipolar hemiarthroplasty in the most elderly patients with displaced femoral neck fractures: a randomised, controlled trial. International Orthopaedics 2011;35(11):1703-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inngul C, Hedbeck CJ, Blomfeldt R, Lapidus G, Ponzer S, Enocson A.Unipolar hemiarthroplasty versus bipolar hemiarthroplasty in patients with displaced femoral neck fractures: a four-year follow-up of a randomised controlled trial. International Orthopaedics 2013;37(12):2457-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inngul 2015 {published data only}
- Barenius B, Inngul C, Alagic Z, Enocson A.A randomized controlled trial of cemented versus cementless arthroplasty in patients with a displaced femoral neck fracture. Bone & Joint Journal 2018;100(8):1087-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Inngul C, Blomfeldt R, Ponzer S, Enocson A.Cemented versus uncemented arthroplasty in patients with a displaced fracture of the femoral neck: a randomised controlled trial. Bone & Joint Journal 2015;97(11):1475-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01798472.Cemented versus uncemented arthroplasty in elderly patients with displaced femoral neck fractures: a randomized controlled trial. clinicaltrials.gov/ct2/show/record/NCT01798472 (first received 25 February 2013).
Iorio 2019 {published data only}
- Iorio R, Iannotti F, Mazza D, Speranza A, Massafra C, Guzzini M, et al.Is dual cup mobility better than hemiarthroplasty in patients with dementia and femoral neck fracture? A randomized controlled trial. SICOT-J 5;38:1-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffcote 2010 {published data only}
- Jeffcote B, Li MG, Barnet-Moorcroft A, Wood D, Nivbrant B.Roentgen stereophotogrammetric analysis and clinical assessment of unipolar versus bipolar hemiarthroplasty for subcapital femur fracture: a randomized prospective study. ANZ Journal of Surgery 2010;80(4):242-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kanto 2014 {published data only}
- Kanto K, Sihvonen R, Eskelinen A, Laitinen M.Uni- and bipolar hemiarthroplasty with a modern cemented femoral component provides elderly patients with displaced femoral neck fractures with equal functional outcome and survivorship at medium-term follow-up. Archives of Orthopaedic & Trauma Surgery 2014;134(9):1251-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Keating 2006 {published data only}
- ISRCTN32884890.Scottish trial of arthroplasty or reduction for subcapital fractures (STARS). www.isrctn.com/ISRCTN32884890 (first received 25 April 2003).
- Keating JF, Grant A, Masson M, Scott NW, Forbes JF.Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty. Health Technology Assessment 2005;9(41):iii-iv, ix. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Keating JF, Grant A, Masson M, Scott NW, Forbes JF.Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. Journal of Bone and Joint Surgery - American Volume 2006;88(2):249-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim YH, Oh JH.A comparison of a conventional versus a short, anatomical metaphyseal-fitting cementless femoral stem in the treatment of patients with a fracture of the femoral neck. Journal of Bone and Joint Surgery - British Volume 2012;94(6):774-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lim 2020 {published data only}
- Lim YJ, Park HJ, Lee YK, Ha YC, Koo KH.Comparison of bone preservation in elderly patients with femoral neck fracture after bipolar hemiarthroplasty using shorter femoral stem and standard femoral stem. Indian Journal of Orthopaedics 2020;54(6):868-78. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Livesley 1993 {published data only}
- Livesley PJ, Srivastiva VM, Needoff M, Prince HG, Moulton AM.Use of a hydroxyapatite-coated hemiarthroplasty in the management of subcapital fractures of the femur. Injury 1993;24(4):236-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Macaulay 2008 {published data only}
- Macaulay W, Nellans K, Garvin K, Iorio R, Healy W, Teeny S, et al, Dfacto Consortiuim.Prospective randomized clinical trial comparing hemiarthroplasty to total hip arthroplasty: Functional outcomes in the treatment of displaced femoral neck fractures. Journal of Arthroplasty 2006;17:S238-9. [DOI] [PubMed] [Google Scholar]
- Macaulay W, Nellans KW, Garvin KL, Iorio R, Healy WL, Rosenwasser MP, et al, Dfacto Consortium.Prospective randomized clinical trial comparing hemiarthroplasty to total hip arthroplasty in the treatment of displaced femoral neck fractures: winner of the Dorr Award. Osteoporosis International 2008;23(6):2-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Macaulay W, Nellans KW, Iorio R, Garvin KL, Healy WL, Rosenwasser MP, Consortium Dfacto.Total hip arthroplasty is less painful at 12 months compared with hemiarthroplasty in treatment of displaced femoral neck fracture. HSS Journal 2008;4(1):48-54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellans KW, Parsley BS, Teeny SM, Healy WL, Yoon, RS, Greisberg JK.RCT comparing hemiarthroplasty to THA in treating displaced femoral neck fractures. In: American Academy of Orthopaedic Surgeons 75th Annual Meeting; 2008 Mar 5-9; San Francisco (CA). 2008.
Malhotra 1995 {published data only}
- Malhotra R, Arya R, Bhan S.Bipolar hemiarthroplasty in femoral neck fractures. Archives of Orthopaedic & Trauma Surgery 1995;114(2):79-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moerman 2017 {published data only}
- Moerman S, Mathijssen NM, Niesten DD, Riedijk R, Rijnberg WJ, Koeter S, et al.More complications in uncemented compared to cemented hemiarthroplasty for displaced femoral neck fractures: a randomized controlled trial of 201 patients, with one year follow-up. BMC Musculoskeletal Disorders 2017;18(1):169. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR1508.Cemented versus non-cemented hemiarthroplasty of the hip as a treatment for a displaced femoral neck fracture; a multi center randomised trial. www.trialregister.nl/trial/1447 (first received 27 October 2008).
Moroni 2002 {published data only}
- Moroni A, Pegreffi F, Romagnoli M, Giannini S.Cemented vs uncemented fixation of femoral neck fractures in osteoporotic patients. A prospective randomized study [abstract]. Hip international 2002;12(2):245-6. [Google Scholar]
- Moroni A, Pegreffi F, Romagnoli M, Hoang-Kim A, Tesei F, Giannini S.Result in osteoporotic femoral neck fractures treated with cemented versus uncemented hip arthroplasty (Abstract). Journal of Bone and Joint Surgery - British Volume 2009;91:167. [Google Scholar]
Mouzopoulos 2008 {published data only}
- Mouzopoulos G, Stamatakos M, Arabatzi H, Vasiliadis G, Batanis G, Tsembeli A, et al.The four-year function results after a displaced subcapital fracture treated with three different surgical options. International Orthopaedics 2008;32(3):367-73. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Movrin 2020 {published data only}
- Movrin I.Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: A randomized controlled trial with two years follow-up. Acta Orthopaedica et Traumatoligica Turcica 2020;54(1):83-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2010c {published data only}
- Cumming D, Parker M.Randomised trial of cemented versus uncemented hemiarthroplasty for displaced intracapsular fractures. Orthopaedic Proceedings 2012;94-B:Supp III. [Google Scholar]
- Haleem S, Pryor GA, Parker MJ.Randomised controlled trial of cemented versus uncemented hemiarthroplasty for displaced intracapsular fractures. Journal of Bone and Joint Surgery - British Volume 2008;90:535. [Google Scholar]
- Parker M, Cumming D.Randomised trial of cemented versus uncemented hemiarthroplasty for displaced intracapsular fractures. In: British Orthopaedic Association Annual Congress; 2006 Sep 26-29; Glasgow (UK). 2006.
- Parker MJ, Pryor G, Gurusamy K.Cemented versus uncemented hemiarthroplasty for intracapsular hip fractures: a randomised controlled trial in 400 patients. Journal of Bone & Joint Surgery - British Volume 2010;92(1):116-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 2012 {published data only}
- ISRCTN04635269.Randomised trial of hip fractures treated with two different types of hip replacements. www.isrctn.com/ISRCTN04635269 (first received 28/09/2007).
- Parker MJ.Cemented Thompson hemiarthroplasty versus cemented Exeter Trauma Stem (ETS) hemiarthroplasty for intracapsular hip fractures: a randomised trial of 200 patients. Injury 2012;43(6):807-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 2019 {published data only}
- NCT02998359.Randomised controlled trial of hemiarthroplasty versus total hip replacement for intracapsular hip fractures. clinicaltrials.gov/ct2/show/study/NCT02998359 (first received 20 December 2016).
- Parker M, Cawley S.Treatment of the displaced intracapsular fracture for the 'fitter' elderly patients: a randomised trial of total hip arthroplasty versus hemiarthroplasty for 105 patients. Injury 2019;50(11):2009-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 2020 {published data only}
- NCT02998034.Randomised controlled trial of cemented hemiarthroplasty versus uncemented Furlong hemiarthroplasty. clinicaltrials.gov/ct2/show/record/NCT02998034 (first received 16 December 2016).
- Parker M, Cawley S.Cemented or uncemented hemiarthroplasty for displaced intracapsular fractures of the hip: a randomized trial of 400 patients. The Bone and Joint Journal 2020;102(B1):11-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patel 2008 {published data only}
- Patel N, Brijlall S.Bipolar versus unipolar for displaced intracapsular hip fractures - a preliminary report [abstract]. Journal of Bone and Joint Surgery - British Volume 2008;90:473. [Google Scholar]
Raia 2003 {published data only}
- Raia FJ, Chapman CB, Herrera MF, Schweppe MW, Michelsen CB, Rosenwasser MP.Unipolar or bipolar hemiarthroplasty for femoral neck fractures in the elderly? Clinical Orthopaedics & Related Research 2003;414:259-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schweppe MW, Vitale MG, Yaron I, Herrera MF, Sollano JA, Michelsen CB, et al.Randomized evaluation of bipolar versus unipolar hemiarthroplasty for displaced femoral neck fractures in the elderly: preliminary results. Orthopaedic Trauma Association Annual Meeting 1998;25(11):ota.org/sites/files/legacy_abstracts/ota98/otapa/OTA98511.htm. [Google Scholar]
Rashed 2020 {published data only}
- Rashed RA, Sevenoaks H, Choudry QA, Kasem MS, Elkhadrawe TA, Eldakhakhny MM.Comparison of functional outcome of cemented total hip replacement versus cemented dual-mobility cup total hip replacement for the management of displaced femoral neck fractures in the active elderly patients. HIP International 2020;March(4):1120700020910414. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ravikumar 2000 {published data only}
- Ravikumar KJ, Marsh G.Internal fixation versus hemiarthroplasty versus total hip arthroplasty for displaced subcapital fractures of femur--13 year results of a prospective randomised study. Injury 2000;31(10):793-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B.Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury 1989;20(5):291-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rehman 2014 {published data only}
- Rehman ur M, Imran M, Kang TA.Functional outcome of cemented versus uncemented hemiarthroplasty for intracapsular hip fractures. Medical Forum Monthly 2014;25(1):44-8. [PMID: 30919045]30919045 [Google Scholar]
Ren 2017 {published data only}
- Ren CG, Gao Y.Comparison of total hip arthroplasty and hemiarthroplasty in elderly patients with femoral neck fracture. Biomedical Research (India) 2017;28(16):7127-30. [Google Scholar]
Sadr 1977 {published data only}
- Sadr B, Arden GP.A comparison of the stability of proplast-coated and cemented Thompson prostheses in the treatment of subcapital femoral fractures. Injury 1977;8(3):234-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Santini 2005 {published data only}
- Santini S, Rebeccato A, Bolgan I, Turi G.Hip fractures in elderly patients treated with bipolar hemiarthroplasty: comparison between cemented and cementless implants. Journal of Orthopaedics & Traumatology 2005;6(2):80-7. [DOI: 10.1007/s10195-005-0086-5] [DOI] [Google Scholar]
Sharma 2016 {published data only}
- Sharma V, Awasthi B, Kumar K, Kohli N, Katoch P.Outcome analysis of hemiarthroplasty vs. total hip replacement in displaced femoral neck fractures in the elderly. Journal of Clinical and Diagnostic Research 2016;10(5):RC11-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sims 2018 {published data only}
- ISRCTN39085558.The World Hip Trauma Evaluation Study 3: hemiarthroplasty evaluation multi-centre investigation. www.isrctn.com/ISRCTN39085558 (first received 23 September 2009).
- Sims AL, Parsons N, Achten J, Griffin XL, Costa ML, Reed MR, Cornet trainee collaborative.A randomized controlled trial comparing the Thompson hemiarthroplasty with the Exeter polished tapered stem and Unitrax modular head in the treatment of displaced intracapsular fractures of the hip. Bone & Joint Journal 2018;100(3):352-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims AL, Parsons N, Achten J, Griffin XL, Costa ML, Reed MR.The World Hip Trauma Evaluation Study 3. Hemiarthroplasty evaluation by multicentre investigation - WHiTE 3: HEMI - An abridged protocol. Bone and Joint Research 2016;5(1):18-25. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonaje 2017 {published data only}
- Sonaje JC, Meena PK, Bansiwal RC, Bobade SS.Comparison of functional outcome of bipolar hip arthroplasty and total hip replacement in displaced femoral neck fractures in elderly in a developing country: a 2-year prospective study. European Journal of Orthopaedic Surgery & Traumatologie 2017;13:13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sonne‐Holm 1982 {published data only}
- Sonne-Holm S, Walter S, Jensen JS.Moore hemi-arthroplasty with and without bone cement in femoral neck fractures. A clinical controlled trial. Acta Orthopaedica Scandinavica 1982;53(6):953-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoffel 2013 {published data only}
- Stoffel KK, Nivbrant B, Headford J, Nicholls RL, Yates PJ.Does a bipolar hemiprosthesis offer advantages for elderly patients with neck of femur fracture? A clinical trial with 261 patients. ANZ Journal of Surgery 2013;83(4):249-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Talsnes 2013 {published data only}
- Talsnes O, Hjelmstedt F, Pripp AH, Reikeras O, Dahl OE.No difference in mortality between cemented and uncemented hemiprosthesis for elderly patients with cervical hip fracture. A prospective randomized study on 334 patients over 75 years. Archives of Orthopaedic & Trauma Surgery 2013;133(6):805-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2012 {published data only}
- ACTRN12609000367246.A randomised clinical trial of pain and mobility folllowing cemented vs uncemented hemiarthroplasty in elderly patients with displaced subcapital neck of femur fracture. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000367246 (first received 10 February 2009).
- Taylor F, Wright M, Zhu M.Hemiarthroplasty of the hip with and without cement: a randomized clinical trial. Journal of Bone & Joint Surgery - American Volume 2012;94(7):577-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van den Bekerom 2010 {published data only}
- Tol MC, Van den Bekerom MP, Sierevelt IN, Hilverdink EF, Raaymakers EL, Goslings JC.Hemiarthroplasty or total hip arthroplasty for the treatment of a displaced intracapsular fracture in active elderly patients: 12-year follow-up of randomised trial. Bone & Joint Journal 2017;99(2):250-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van den Bekerom MP, Hilverdink EF, Sierevelt IN, Reuling EM, Schnater JM, et al.A comparison of hemiarthroplasty with total hip replacement for displaced intracapsular fracture of the femoral neck: a randomised controlled multicentre trial in patients aged 70 years and over. Bone & Joint Journal - British Version 2010;92(10):1422-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vidovic 2013 {published data only}
- Vidovic D, Matejcic A, Punda M, Ivica M, Tomljenovic M, Bekavac-Beslin M, et al.Periprosthetic bone loss following hemiarthroplasty: a comparison between cemented and cementless hip prosthesis. Injury 2013;44:S62-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Vidovic D, Punda M, Darabos N, Bekavac-Beslin M, Bakota B, Matejcic A.Regional bone loss following femoral neck fracture: A comparison between cemented and cementless hemiarthroplasty. Injury 2015;46:S52-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Xu 2017 {published data only}
- Xu F, Ke R, Gu Y, Qi W.Bipolar hemiarthroplasty vs. total hip replacement in elderly. International Journal of Clinical and Experimental Medicine 2017;10(5):7911-20. [CORPUS ID: 53049671] [Google Scholar]
References to studies excluded from this review
Aydin 2009 {published data only}
- Aydin N, Bezer M, Akgulle AH, Saygi B, Kocaoglu B, Guven O.Comparison of distal and proximal centralising devices in hip arthroplasty. International Orthopaedics 2009;33(4):945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN42349821 {published data only}
- ISRCTN42349821.Comparing outcomes of fractured neck of femur patients treated with Thompsons hemiarthroplasty versus Exeter Trauma Stem. www.isrctn.com/ISRCTN42349821 (first received 28 July 2014).
Karpman 1992 {published data only}
- Karpman RR, Lee TK, Moore BM.Austin-Moore versus Bipolar hemiarthroplasty for displaced femoral neck fractures: a randomized prospective study [abstract]. Orthopaedic Transactions 1992;16:no pagination. [Google Scholar]
Kavcic 2006 {published data only}
- Kavcic G, Hudoklin P, Mikek M, Hussein M.Hemiarthroplasty versus total arthroplasty for treatment of femoral neck fractures. European Journal of Trauma 2006;32:24. [Google Scholar]
Rosen 1992 {published data only}
- Rosen LL, Miller BJ, Dupuis PR, Jarzem P, Hadjipavlou A.A prospective randomized study comparing bipolar hip arthroplasty and hemiarthroplasty in elderly patients with subcapital fractures. Journal of Bone and Joint Surgery - British Volume 1992;74:282. [Google Scholar]
Somashekar 2013 {published data only}
- Somashekar, Krishna SV, Murthy JS.Treatment of femoral neck fractures: unipolar versus bipolar hemiarthroplasty. Malaysian Orthopaedic Journal 2013;7(2):6-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stock 1997 {published data only}
- Stock M, Halliday M, Wood D, Brennon J, Smith A.A randomised prospective trial comparing ceramic and Thompsons hemiarthroplasty in subcapital femoral fractures. Journal of Bone and Joint Surgery - British Volume 1997;79:386. [Google Scholar]
Van Thiel 1988 {published data only}
- Van Thiel PH, Snellen JP, Jansen WB, Van de Slikke W.Moore prosthesis versus bipolar Bateman prosthesis: a prospective randomised clinical study. Journal of Bone and Joint Surgery - British Volume 1988;70(4):677. [Google Scholar]
References to studies awaiting assessment
NCT00800124 {published data only}
- NCT00800124.A prospective randomised multicenter study on cemented and non-cemented hemiprosthesis in older patients with dislocated hip fracture. clinicaltrials.gov/ct2/show/record/NCT00800124 (first received 27 November 2008).
NCT00859378 {published data only}
- NCT00859378.A prospective, randomized study comparing cemented and non-cemented semiendoprostheses in the treatment of proximal femoral fractures in the elderly patients. clinicaltrials.gov/ct2/show/study/NCT00859378 (first received 10 March 2008).
NCT01432691 {published data only}
- NCT01432691.Clinical and RSA comparison of hemi versus total hip arthroplasty after displaced femoral neck fracture. A randomized double-blind study. clinicaltrials.gov/ct2/show/study/NCT01432691 (first received 9 September 2011).
NTR1782 {published data only}
- NTR1782.Randomised clinical trial comparing cemented and hydroxy-apatite coated uncemented hemi-arthroplasty in the elderly patient with a proximal intracapsular femoral fracture. trialregister.nl/trial/1681 (first received 28 April 2009).
References to ongoing studies
ChiCTR1800019531 {published data only}
- ChiCTR1800019531.A randomized controlled trial for comparing the hemiarthroplasty with the total hip arthroplasty in the treatment of femoral neck fractures in patients older than 75 years. www.chictr.org.cn/showproj.aspx?proj=31624 (first received 16 November 2018).
ISRCTN15606075 {published data only}
- ISRCTN15606075.A randomised controlled trial of single antibiotic cement versus dual antibiotic cement in patients receiving a partial hip joint replacement after fracture. www.isrctn.com/ISRCTN15606075 (first received 16 July 2018).
NCT01109862 {published data only}
- NCT01109862.Prospective randomized comparison of bipolar hemiarthroplasty and total hip arthroplasty with large femoral heads for the treatment of displaced intracapsular femoral neck fractures in the elderly. clinicaltrials.gov/ct2/show/record/NCT01109862 (first received 22 April 2010).
NCT01578408 {published data only}
- NCT01578408.Corail-SP study - a prospective randomized comparison between cemented and uncemented hydroxyapatite coated prosthesis stems in total hip arthroplasty in patients with femoral neck fractures. clinicaltrials.gov/ct2/show/record/NCT01578408 (first received 11 April 2012).
NCT01787929 {published data only}
- NCT01787929.Cemented versus uncemented hemiarthroplasty for displaced femoral neck fracture in elderly patient: a randomised prospective trial. clinicaltrials.gov/ct2/show/record/NCT01787929 (first received 11 February 2013).
UMIN000011303 {published data only}
- UMIN000011303.A randomised controlled trial comparing bipolar hemiarthroplasty with total hip replacement for displaced intracapsular fractures of the femoral neck in active patients. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000013209 (first received 1 October 2013).
Wolf 2020 {published data only}
- NCT03909815.Do dual mobility cups prevent dislocation after total hip arthroplasty performed due to femoral neck fracture? A registry-based, pragmatic, randomized controlled trial comparing dual mobility with standard cups. clinicaltrials.gov/ct2/show/nct03909815 (first received 10 April 2019).
- Wolf O, Mukka S, Notini M, Moller M, Hailer NP.Study protocol: The DUALITY trial-a register-based, randomized controlled trial to investigate dual mobility cups in hip fracture patients. Acta Orthopaedica 2020;June:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAOS 2014
- American Academy of Orthopaedic Surgeons (AAOS).Management of hip fractures in the elderly. Evidence-based clinical practice guideline. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors, 5 September 2014. www.aaos.org/research/guidelines/HipFxGuideline.pdf (accessed 29 July 2019).
AO Foundation 2018
- AO Foundation.Femur. Journal of Orthopaedic Trauma 2018;32(Suppl 1):S33-44. [DOI] [PubMed] [Google Scholar]
Azegami 2011
- Azegami S, Gurusamy KS, Parker MJ.Cemented versus uncemented hemiarthroplasty for hip fractures: a systematic review of randomised controlled trials. Hip International 2011;21(5):509-17. [DOI] [PubMed] [Google Scholar]
Bardsley 2013
- Smith P, Ariti C, Bardsley M.Nuffield Trust & Health Foundation: focus on hip fracture. Trends in emergency admissions for fractured neck of femur, 2001 to 2011. www.nuffieldtrust.org.uk/research/focus-on-hip-fracture (accessed 9 January 2019).
Benjamin 1990
- Benjamin A, Egan J.Assessment of hip and knee surgery. 3M Health Care Ltd 1990.
Blundell 1998
- Blundell CM, Parker MJ, Pryor GA, Hopkinson-Wooley J, Bonsall SS.An assessment of the AO classification of intracapsular fractures of the proximal femur. Journal of Bone and Joint Surgery - British Volume 1998;80(4):679-83. [DOI] [PubMed] [Google Scholar]
Ceder 1980
- Ceder L, Thorngren KG, Wallden B.Prognostic indicators and early home rehabilitation in elderly patients with hip fractures. Clinical Orthopaedics and Related Research 1980;152:173-84. [PMID: ] [PubMed] [Google Scholar]
Christie 1994
- Christie J, Burnett R, Potts HR, Pell AC.Echocardiography of transatrial embolism during cemented and uncemented hemiarthroplasty of the hip. Journal of Bone and Joint Surgery - British Volume 1994;76(3):409-12. [PubMed] [Google Scholar]
Cochrane 2018
- Cochrane.Glossary. community.cochrane.org/glossary (accessed 2 November 2018).
Cohen 1988
- Cohen, J.Statistical Power Analysis for the Behavioral Sciences. 2nd edition. New York: Routledge, 1988. [ ISBN 9781134742707] [Google Scholar]
Cooper 2011
- Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, et al.Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporosis International 2011;22(5):1277-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Court‐Brown 2017
- Court-Brown CM, Clement ND, Duckworth AD, Biant LC, McQueen MM.The changing epidemiology of fall-related fractures in adults. Injury 2017;48(4):819-24. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence.Version accessed 29 July 2019. Melbourne, Australia: Veritas Health Innovation, 2003. Available at covidence.org.
D'Aubigne 1954
- D'Aubigne RM, Postel M.Functional results of hip arthroplasty with acrylic prosthesis. Journal of Bone and Joint Surgery 1954;36(A (3)):451-75. [PMID: ] [PubMed] [Google Scholar]
Dawson 1996
- Dawson J, Fitzpatrick R, Carr A, Murray D.Questionnaire on the perceptions of patients about total hip replacement. Journal of Bone and Joint Surgery - British Volume 1996;78(2):185-9. [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, (editors), on behalf of the Cochrane Statistical Methods Group.Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Devas 1983
- Devas M, Hinves B.Prevention of acetabular erosion after hemiarthroplasty for fractured neck of femur. Journal of Bone and Joint Surgery - British Volume 1983;65(5):548-51. [DOI] [PubMed] [Google Scholar]
Dhanwal 2011
- Dhanwal DK, Dennison EM, Harvey NC, Cooper C.Epidemiology of hip fracture: worldwide geographic variation. Indian Journal of Orthopaedics 2011;45(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol 1990
- The EuroQol Group.EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. [DOI] [PubMed] [Google Scholar]
Evans 1949
- Evans EM.The treatment of trochanteric fractures of the femur. Journal of Bone and Joint Surgery - British Volume 1949;31(B(2)):190-203. [PubMed] [Google Scholar]
Fillenbaum 1981
- Fillenbaum GG, Smyer MA.The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. Journals of Gerontology 1981;36:428-34. [DOI] [PubMed] [Google Scholar]
Garden 1961
- Garden RS.Low-angle fixation in fractures of the femoral neck. Bone & Joint Journal 1961;43-B(4):647-63. [Google Scholar]
Gibson 1950
- Gibson A.Posterior exposure of the hip joint. Journal of Bone and Joint Surgery - British Volume 1950;32:183-6. [DOI] [PubMed] [Google Scholar]
Griffin 2015
- Griffin XL, Parsons N, Achten J, Fernandez M, Costa ML.Recovery of health-related quality of life in a United Kingdom hip fracture population: the Warwick Hip Trauma Evaluation--a prospective cohort study. Bone & Joint Journal 2015;97-B(3):372-82. [DOI] [PubMed] [Google Scholar]
Haywood 2014
- Haywood KL, Griffin XL, Achten J, Costa ML.Developing a core outcome set for hip fracture trials. Bone & Joint Journal 2014;96-B(8):1016-23. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hopley 2010
- Hopley C, Stengel D, Ekkernkamp A, Wich M.Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ (Clinical Research Ed.) 2010;340:c2332. [DOI] [PubMed] [Google Scholar]
Imam 2019a
- Imam MA, Shehata MSA, Elsehili A, Morsi M, Martin A, Shawq M, et al.Contemporary cemented versus uncemented hemiarthroplasty for the treatment of displaced intracapsular hip fractures: a meta-analysis of forty-two thousand forty-six hips. International Orthopaedics 2019;42(7):1715-23. [DOI] [PubMed] [Google Scholar]
Imam 2019b
- Imam MA, Shehata M, Abdallah AR, Ahmed H, Kader N, Ernstbrunner L, et al.Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a pooled analysis of 30,250 participants' data. Injury-International Journal of the Care of the Injured 2019;50(10):1694-708. [DOI] [PubMed] [Google Scholar]
Jensen 1980
- Jensen JS.Classification of trochanteric fractures. Acta Orthopaedica Scandinavica 1980;51(5):803-10. [DOI] [PubMed] [Google Scholar]
Johanson 1992
- Johanson NA, Charlson ME, Szatrowski TP, Ranawat CS.A self-administered hip-rating questionnaire for the assessment of outcome after total hip replacement. Journal of Bone and Joint Surgery - American Volume 1992;74:587-97. [PMID: ] [PubMed] [Google Scholar]
Johnell 2004
- Johnell O, Kanis JA.An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporosis International 2004;15(11):897-902. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kanis 2001
- Kanis JA, Oden A, Johnell O, Jonsson B, Laet C, Dawson A.The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporosis International 2001;12(5):417-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kanis 2012
- Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl DA, Cooper C, IOF Working Group on Epidemiology and Quality of Life.A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporosis International 2012;23(9):2239-56. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karantana 2011
- Karantana A, Boulton C, Bouliotis G, Shu KS, Scammell BE, Moran CG.Epidemiology and outcome of fracture of the hip in women aged 65 years and under: a cohort study. Journal of Bone and Joint Surgery - British Volume 2011;93(5):658-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Katz 1963
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185(12):914-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Keating 2010
- Keating J.Femoral neck fractures. In: Bucholz RW, Heckman JD, Court-Brown CM, Tornetta P 3rd, editors(s). Rockwood and Green's Fractures in Adults. 7th edition. Vol. 2. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins, 2010. [Google Scholar]
Koval 1995
- Koval KJ, Skovron ML, Aharonoff GB, Meadows SE, Zuckerman JD.Ambulatory ability after hip fracture. A prospective study in geriatric patients. Clinical Orthopaedics and Related Research 1995;310:150-9. [PMID: ] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Lewis 2019
- Lewis DP, Waever D, Thorninger R, Donnelly WJ.Hemiarthroplasty vs total hip arthroplasty for the management of displaced neck of femur fractures: a systematic review and meta-analysis. Journal of Arthroplasty 2019;34(8):1837-43 e2. [DOI] [PubMed] [Google Scholar]
Lewis 2021
- Lewis SR, Macey R, Eardley WG, Dixon JR, Cook J, Griffin XL.Internal fixation implants for intracapsular hip fractures in adults. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013409. [DOI: 10.1002/14651858.CD013409.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2022a
- Lewis SR, Macey R, Gill JR, Parker MJ, Griffin XL.Cephalomedullary nails versus extramedullary implants for extracapsular hip fractures in older adults. Cochrane Database of Systematic Reviews 2022, Issue 1. Art. No: CD000093. [DOI: 10.1002/14651858.CD000093.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2022b
- Lewis SR, Macey R, Lewis J, Stokes J, Gill JR, Cook JA, et al.Surgical interventions for treating extracapsular hip fractures in older adults: a network meta-analysis. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No: CD013405. [DOI: 10.1002/14651858.CD013405.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2022c
- Lewis SR, Macey R, Stokes J, Cook JA, Eardley WGP, Griffin XL.Surgical interventions for treating intracapsular hip fractures in older adults: a network meta-analysis. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No: CD013404. [DOI: 10.1002/14651858.CD013404.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2020
- Liu Y, Chen X, Zhang P, Jiang B.Comparing total hip arthroplasty and hemiarthroplasty for the treatment of displaced femoral neck fracture in the active elderly over 75 years old: a systematic review and meta-analysis of randomized control trials. Journal of Orthopaedic Surgery. 2020;15(1):215 2020;15(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd‐Jones 2015
- Lloyd-Jones G.Trauma x-ray - lower limb. Hip fracture. www.radiologymasterclass.co.uk/tutorials/musculoskeletal/x-ray_trauma_lower_limb/hip_fracture_x-ray (accessed 10 August 2018).
Mahoney 1965
- Mahoney FI, Barthel DW.Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61-5. [PMID: ] [PubMed] [Google Scholar]
Metcalfe 2019
- Metcalfe D, Judge A, Perry DC, Gabbe B, Zogg CK, Costa ML.Total hip arthroplasty versus hemiarthroplasty for independently mobile older adults with intracapsular hip fractures. BMC Musculoskeletal Disorders 2019;20(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mols 2009
- Mols F, Pelle AJ, Kupper N.Normative data of the SF-12 health survey with validation using postmyocardial infarction patients in the Dutch population. Quality of Life Research 2009;18:403-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Neufeld 2016
- Neufeld ME, O'Hara NN, Zhan M, Zhai Y, Broekhuyse HM, Lefaivre KA, et al.Timing of hip fracture surgery and 30-day outcomes. Orthopedics 2016;39(6):361-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
NHFD 2017
- National Hip Fracture Database (NHFD).The National Hip Fracture Database (NHFD) annual report 2017. www.nhfd.co.uk/ (accessed 30 October 2018).
NICE 2011
- National Clinical Guideline Centre (UK).The management of hip fracture in adults. NICE clinical guidelines, no. 124. London: Royal College of Physicians (UK); 2011. Available at www.nice.org.uk/guidance/cg124/evidence/full-guideline-pdf-183081997. [PubMed]
ONS 2018
- Office for National Statistics.Population estimates for the UK, England and Wales, Scotland and Northern Ireland: mid-2018. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2018 (accessed 1 August 2019).
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.Rayyan-a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overgaard 2017
- Overgaard JA, Larsen CM, Holtze S, Ockholm K, Kristensen MT.Interrater reliability of the 6-minute walk test in women with hip fracture. Journal of Geriatric Physical Therapy 2017;40(3):158-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Palm 2009
- Palm H, Gosvig K, Krasheninnikoff M, Jacobsen S, Gebuhr P.A new measurement of posterior tilt predicts reoperation in undisplaced femoral neck fractures: 113 consecutive patients treated by internal fixation and followed for 1 year. Acta Orthopaedica 2009;80(3):303-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papadimitriou 2017
- Papadimitriou N, Tsilidis KK, Orfanos P, Benetou V, Ntzani EE, Soerjomataram I, et al.Burden of hip fracture using disability-adjusted life-years: a pooled analysis of prospective cohorts in the CHANCES consortium. Lancet Public Health 2017;2(5):e239-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 1991
- Parker MJ, Anand JK.What is the true mortality of hip fractures? Public Health 1991;105(6):443-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 1993a
- Parker MJ.Garden grading of intracapsular fractures: meaningful or misleading? Injury 1993;24(4):241-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 1993b
- Parker MJ, Palmer CR.A new mobility score for predicting mortality after hip fracture. Journal of Bone and Joint Surgery - British Volume 1993;75:797. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 1998
- Parker MJ, Dynan Y.Is Pauwels classification still valid? Injury 1998;29(7):521-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 1999
- Parker MJ.Proximal femoral fractures. In: Pynsent PB, Fairbank JCT, Carr AJ, editors(s). Classification of Musculo-Skeletal Trauma. Oxford (UK): Butterworth-Heinemann Publications, 1999. [Google Scholar]
Parker 2010a
- Parker MJ, Gurusamy KS, Azegami S.Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No: CD001706. [DOI: 10.1002/14651858.CD001706.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pauwels 1935
- Pauwels F.Der Schenkelhalsbruch. Ein mechanisches Problem. In: Gesammelte Abhandlungen zur funktionellen Anatomie des Bewegungsapparates. Heidelberg, Germany: Springer, 1935. [Google Scholar]
Perry 2016
- Perry DC, Metcalfe D, Griffin XL, Costa ML.Inequalities in use of total hip arthroplasty for hip fracture: population based study. BMJ 2016;353:i2021. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Podsiadlo 1991
- Podsiadlo D, Richardson S.The timed "Up & Go": a test of basic functional mobility of frail elderly persons. Journal of the American Geriatrics Society 1991;39:142-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogmark 2018
- Rogmark C, Kristensen MT, Viberg B, Ronnquist SS, Overgaard S, Palm H.Hip fractures in the non-elderly - who, why and whither? Injury 2018;49(8):1445-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roos 1999
- Roos EM, Klässbo M, Lohmander LS.WOMAC osteoarthritis index: reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scandinavian Journal of Rheumatology 1999;28(4):210-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sayers 2017
- Sayers A, Whitehouse MR, Berstock JR, Harding KA, Kelly MB, Chesser TJ.The association between the day of the week of milestones in the care pathway of patients with hip fracture and 30-day mortality: findings from a prospective national registry – the National Hip Fracture Database of England and Wales. BMC Medicine 2017;15:62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019a
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al.Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:403-32. [Google Scholar]
Schünemann 2019b
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. Draft version (29 January 2019) for inclusion in: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1 (updated September 2020), Cochrane, 2020. Available from handbook.cochrane.org.
SF‐36
- RAND Corporation.36-Item Short Form Survey (SF-36). www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html.
SIGN 2009
- Scottish Intercollegiate Guidelines Network (SIGN).Management of hip fracture in older people. A national clinical guideline. www.sign.ac.uk/assets/sign111.pdf (accessed 30 October 2018).
Sikorski 1981
- Sikorski JM, Barrington R.Internal fixation versus hemiarthroplasty for the displaced subcapital fracture of the femur. A prospective randomised study. Journal of Bone and Joint Surgery - British Volume 1981;63(3):357-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Singh 2016
- Singh JA, Schleck C, Harmsen S, Lewallen D.Clinically important improvement thresholds for Harris Hip Score and its ability to predict revision risk after primary total hip arthroplasty. BMC Musculoskeletal Disorders 2016;10(17):256. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spolaore 2001
- Spolaore P, Maggi S, Trabucchi M.The elderly in the network of services [L’anziano nella rete dei servizi]. Il Poligrafo 2001;64:197-269. [Google Scholar]
Stata [Computer program]
- Stata.Version 15. College Station, TX, USA: StataCorp, 2017. Available at www.stata.com.
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors).Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Suurmeijer 1994
- Suurmeijer TP, Doeglas DM, Moum T, Briancon S, Krol B, Sanderman R, et al.The Groningen Activity Restriction Scale for measuring disability: its utility in international comparisons. American Journal of Public Health 1994;84:1270-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torio 2013
- Torio CM, Andrews RM.National inpatient hospital costs: the most expensive conditions by payer, 2011. Healthcare Cost and Utilization Project Statistical Brief #160; August 2013. Available at www.hcup-us.ahrq.gov/reports/statbriefs/sb160.pdf.
Wade 1988
- Wade T, Collin C.The Barthel ADL Index: a standard measure of physical disability? International Disability Studies 1988;10:64-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walters 2005
- Walters SJ, Brazier JE.Comparison of the minimally important difference for two health state utility measures: EQ-SD and SF-6D. Quality of Life Research 2005;14(6):1523-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wiklund 1990
- Wiklund I.The Nottingham Health Profile - a measure of health-related quality of life. Scandinavian Journal of Primary Health Care 1990;Supplement:15-8. [PubMed] [Google Scholar]
Williamson 2017
- Williamson S, Landeiro F, McConnell T, Fulford-Smith L, Javaid MK, Judge A, et al.Costs of fragility hip fractures globally: a systematic review and meta-regression analysis. Osteoporosis International 2017;28(10):2791-800. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sreekanta 2019
- Sreekanta A, Parker MJ, Wood H, Glanville JM, Cook J, Griffin XL.Arthroplasties for hip fracture in adults. Cochrane Database of Systematic Reviews 2079, Issue 8. Art. No: CD013410. [DOI: 10.1002/14651858.CD013410] [DOI] [PMC free article] [PubMed] [Google Scholar]


