Abstract
Study Objectives
Chronic kidney disease (CKD) is a global health concern and a major risk factor for cardiovascular morbidity and mortality. Obstructive sleep apnea (OSA) may exacerbate this risk by contributing to the development of CKD. This study investigated the prevalence and patient awareness of the risk of CKD progression in individuals with OSA.
Methods
Adults referred to five Canadian academic sleep centers for suspected OSA completed a questionnaire, a home sleep apnea test or in-lab polysomnography and provided blood and urine samples for measurement of estimated glomerular filtration rate (eGFR) and the albumin:creatinine ratio (ACR), respectively. The risk of CKD progression was estimated from a heat map incorporating both eGFR and ACR.
Results
1295 adults (42% female, 54 ± 13 years) were categorized based on the oxygen desaturation index (4% desaturation): <15 (no/mild OSA, n = 552), 15−30 (moderate OSA, n = 322), and >30 (severe OSA, n = 421). After stratification, 13.6% of the no/mild OSA group, 28.9% of the moderate OSA group, and 30.9% of the severe OSA group had a moderate-to-very high risk of CKD progression (p < .001), which was defined as an eGFR <60 mL/min/1.73 m2, an ACR ≥3 mg/mmol, or both. Compared to those with no/mild OSA, the odds ratio for moderate-to-very high risk of CKD progression was 2.63 (95% CI: 1.79−3.85) for moderate OSA and 2.96 (2.04–4.30) for severe OSA after adjustment for CKD risk factors. Among patients at increased risk of CKD progression, 73% were unaware they had abnormal kidney function.
Conclusion
Patients with moderate and severe OSA have an increased risk of CKD progression independent of other CKD risk factors; most patients are unaware of this increased risk.
Keywords: obstructive sleep apnea, chronic kidney disease, glomerular filtration rate, albumin:creatinine ratio
Statement of Significance.
Chronic kidney disease (CKD) and obstructive sleep apnea (OSA) are common global health concerns associated with increased cardiovascular morbidity and mortality. OSA may contribute to the pathogenesis of CKD thereby promoting their co-existence and further increasing cardiovascular complications. We estimated the risk of CKD progression, based on conventional measurements of kidney function, in a cohort of 1295 patients referred for assessment of OSA. We found that ~30% of patients with severe OSA had a 3 times greater risk of CKD progression compared to those with mild/no OSA. Furthermore, most patients were unaware of this risk. These results highlight a vulnerable patient population and the opportunity to reduce the risk of CKD progression and cardiovascular disease in patients with newly diagnosed OSA.
Introduction
Chronic kidney disease (CKD) is a global epidemic that is found in more than 10% of the adult population; this prevalence is expected to increase as the population ages [1–4]. In addition to being a direct cause of morbidity and mortality, CKD is a major risk factor for cardiovascular disease [5–7]. The key diagnostic criteria for CKD are reduced glomerular filtration rate and/or proteinuria [8]. The stages of CKD range from 1 to 5 (most severe) depending on the degree of reduced glomerular filtration rate and proteinuria [8]. If CKD is not treated, it may progress to end-stage kidney disease (ESKD), requiring renal function replacement with chronic dialysis or kidney transplantation. This has enormous implications for individual patients and healthcare systems [9–11]. Consequently, identification of treatable risk factors for the development and progression of CKD are urgently needed.
Obstructive sleep apnea (OSA) is also very common; it is estimated that 38% of the world’s adult population has moderate-to-severe OSA [12]. Furthermore, OSA is common in patients with CKD [13], and has been reported to contribute to the pathogenesis of CKD through exposure to intermittent hypoxia in both experimental animal models [14, 15] and in human studies [16–18]. The interaction of these two common and increasingly prevalent conditions present both an urgent global health concern and an opportunity for mitigation since OSA is a treatable CKD risk factor.
Substantial medical literature support a bi-directional relationship between OSA and CKD [19, 20]. The prevalence of CKD in patients presenting with OSA has been evaluated in cross-sectional and longitudinal studies, using both biochemical and administrative data, with virtually all reporting a significant association between OSA and CKD [21]. Cross-sectional studies have used estimated glomerular filtration rate (eGFR) based on serum creatinine, with or without a measurement of proteinuria (reflected by the albumin:creatinine ratio (ACR)) to determine the prevalence of CKD and its relationship to an index of OSA severity such as the apnea-hypopnea index [22–25]. Longitudinal studies have used biochemical and administrative data to demonstrate an association between OSA and incident CKD [26–29], notwithstanding the high prevalence of undiagnosed OSA in the community limiting the accuracy of administrative data. Importantly, most of these results were independent of common comorbidities that can cause CKD including hypertension, diabetes [4], and obesity [30].
Notwithstanding this literature, significant gaps remain in our understanding of the relationship between OSA and CKD. Firstly, rather than simply using eGFR and ACR independently to determine the prevalence of CKD, as has been done by Adams et al. in a community cohort [25], these measurements can be combined to provide a risk estimate of CKD progression [8]; this has not been done in patients with OSA either in a community or sleep clinic population. Secondly, none of the previous literature has evaluated the awareness among patients with OSA and their healthcare providers of the association between OSA and the potential risk of CKD. This is particularly relevant since the early stage of CKD is clinically silent [31–33] and may go undetected without specific screening driven by greater awareness of this association. The objectives of this study were to address these knowledge gaps in an observational cohort of patients referred for evaluation of OSA to multiple academic sleep centers.
Methods
This study included individuals ≥18 years of age enrolled in the multi-center Canadian Sleep and Circadian Network’s (CSCN) adult OSA observational cohort database between July 2016 and March 2021. Participants were referred to one of five participating sleep centers for suspected OSA, which was diagnosed by unattended home sleep apnea testing (HSAT) or in-laboratory polysomnography (PSG). The current study included all participants who provided a venous blood sample and a urine sample upon enrollment, and answered all questions related to medical history of renal function included in a comprehensive sleep questionnaire. Exclusion criteria were current dialysis and/or a prior kidney transplant.
The study was approved by the Conjoint Health Research Ethics Board of the University of Calgary (UC; REB16-0211), the Biomedical Research Ethics Board of the University of Saskatchewan (US; BIO-REB16-106), the University of British Columbia Clinical Research Ethics Board (UBC; H16-00422), the McGill University Health Centre (MEO-10-2019-4718), and the Institut Universataire de Cardiologie et de Pneumologie de Quebec at Université Laval (MP-10-2018-2938). All participants were informed of study requirements prior to providing written informed consent.
Study protocol
OSA was diagnosed by HSAT at the University of Calgary and Université Laval, and by PSG at the University of Saskatchewan, University of British Columbia, and McGill University. All participants completed a sleep and medical history questionnaire and provided a venous blood sample and mid-stream urine sample prior to any treatment for OSA.
Home sleep apnea testing. Home sleep apnea testing was performed using monitors validated against PSG [34–36]. These included the Remmers Sleep Recorder (Sagatech, Calgary, AB, Canada), ApneaLink Air (Resmed, San Diego, CA, USA), Apnea Risk Evaluation System (ARES, SleepMed, Kennesaw, GA, USA), Embletta MPR Sleep System (Natus, Middleton, WI, USA) and the Alice PDx (Philips Healthcare, Markham, ON, Canada). As previously described [37] HSAT monitors record arterial oxyhemoglobin saturation using pulse oximetry (Spo2), respiratory airflow via nasal cannula connected to a pressure transducer, snoring via a microphone and sleep position (supine/not supine) from an accelerometer. The Spo2 signal is recorded at a minimum of 1 Hz and analyzed using proprietary scoring algorithms. For all HSAT monitors, the oxygen desaturation index (ODI) was calculated as the number of times Spo2 decreased by ≥4% divided by the total time of oximetry recording. In addition, mean Spo2 during the HSAT and the duration of Spo2 <90% (T90) were indexed to the total recording time.
Polysomnography. Polysomnography was performed according to American Academy of Sleep Medicine (AASM) guidelines [38]. Polysomnographic recordings included electroencephalography (EEG) channels (C3, C4, M1, M2, O1, O2), electro-oculograms (left and right), submental electromyograms (EMG), and bilateral tibialis anterior EMG using surface electrodes, airflow using nasal pressure and oral thermistor, respiratory efforts using inductance plethysmography with transducers placed around the chest and abdomen and Spo2 with finger pulse oximetry. All channels were continuously recorded at the minimum (or higher) frequencies recommended by the AASM [38] and stored electronically for later scoring (Sandman, Tyco Healthcare, Kanata, ON, Canada; Sleepware G3, Philips Healthcare, Amsterdam, Netherlands; or Polysmith, Nihon Kohden, Irvine, CA, USA). PSGs were manually scored by experienced registered polysomnographic technologists according to AASM criteria [38].
All HSAT and PSG studies were interpreted by a sleep physician to confirm that episodes of oxygen desaturation reflected a corresponding change in airflow; if oxygen desaturation was not accompanied by changes in airflow consistent with apnea, these patients were not recruited.
Sleep questionnaire
Details of the sleep questionnaire have been previously published [37]. Briefly, it included questions regarding demographics (age, height, weight, gender), medical history, comorbidities, medications, sleep schedule, symptoms of restless legs syndrome, and insomnia. The questionnaire included the following two specific questions regarding a previous physician diagnosis of abnormal kidney function and proteinuria: (1) “Have you ever been told by a physician that your kidney function is not normal? Yes/No”; and (2) “Have you ever been told by a physician that you have protein in your urine? Yes/No”. A positive answer to question (1) and/or (2) was used to indicate that the patient was aware that their kidney function was not normal. The questionnaire also included the Epworth Sleepiness Scale (ESS) [39] and the Pittsburgh Sleep Quality Index (PSQI) [40] to assess daytime sleepiness and sleep quality, respectively. Additional detail is provided in the online supplement.
Measurement of eGFR and urine ACR
Venous blood samples were collected into serum separator tubes by trained phlebotomists and analyzed by local laboratories for serum creatinine using enzymatic colorimetric assays. Estimated GFR was derived from serum creatinine values using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [41] as recommended by international guidelines [8].
Urine samples were collected into sterile containers and analyzed by local laboratories for albumin and creatinine levels. Albumin was quantified by immunoturbidimetric (UC, US, McGill), bromocresol blue dye-binding method (UBC) or immunonephelometric assays (Laval) while creatinine was measured using enzymatic colorimetric assays. Urine albumin values were indexed to urine creatinine values to calculate ACR. For urine albumin values below detectable limits (n = 275) a value 0.01 mg/L lower than the detectable limit was used [42]. The lower limits of detection were 3 (UC and US), 5 (UBC and Laval) and 7 mg/L (McGill). For albumin values above the detectable limit (n = 2), a value 0.01 mg/L higher than the detectable limit was used.
Details of techniques employed by local laboratories to quantify serum creatinine, urine creatinine, and albumin are provided in the online supplement.
Data analyses
The primary outcome was the prevalence of moderate-to-very high risk of CKD progression based upon the eGFR and ACR values at the time of enrollment in patients with untreated OSA. Each participant was categorized as being at low, moderate, high, and very high risk of kidney disease progression according to a heat map populated by their eGFR and ACR values as outlined in the Kidney Disease: Improving Global Outcome guidelines.[8] Low risk is defined as an eGFR ≥60 mL/min/1.73 m2 and an ACR <3 mg/mmol while moderate-to-very high risk was defined as an eGFR <60 mL/min/1.73 m2, an ACR ≥3 mg/mmol, or both.
Details for harmonizing HSAT measurements of OSA and nocturnal hypoxemia with those from PSG have been previously outlined [37]. Briefly, for PSG studies the ODI (based upon ≥4% desaturations), mean SpO2 and T90 were indexed to the total recording time (TRT; time between “lights off” and “lights on”), which is the denominator used in HSAT. Based upon the ODI, participants were categorized as having no/mild OSA (0 ≤ ODI < 15), moderate OSA (15 ≤ ODI ≤ 30) and severe OSA (ODI > 30). For participants who underwent PSG, OSA severity was additionally categorized based on the apnea-hypopnea index (AHI) as no/mild OSA (0 ≤ AHI < 15), moderate OSA (15 ≤ AHI ≤ 30) and severe OSA (AHI > 30).
An ESS >10 indicated excessive daytime sleepiness [39]; a PSQI >5 indicated poor sleep quality [40] and an ISI >7 indicated the presence of insomnia [43]. Sleep duration was quantified from the PSQI question 4 (“How many hours of actual sleep do you get at night?”). Participants were categorized as having short sleep duration if they reported ≤6 h of sleep per night [44].
Self-reported medications were categorized according to their drug classification [45]. We specifically identified the use of non-steroidal anti-inflammatory drugs (NSAIDs) and proton pump inhibitors (PPIs), since chronic use of these medications have been associated with CKD progression [46, 47].
Statistical analyses
Participant characteristics were compared across OSA groups using one-way ANOVAs for continuous, normally distributed variables and Kruskal−Wallis H tests for continuous, non-normally distributed variables. Post hoc group comparisons were adjusted using a Tukey−Kramer correction or the Dwass, Steel, Critchlow-Fligner (DSCF) analyses, respectively. A chi-square goodness-of-fit was used to test for group differences in categorical variables. Binary logistic regression was used to assess if there was a linear trend across OSA groups for the prevalence of participants at moderate-to-very high risk of kidney disease progression controlling for age, sex, and body mass index (BMI) and to estimate the odds ratios (ORs) for participants with moderate and severe OSA being at moderate-to-very high risk of kidney disease progression (participants with no/mild OSA formed the reference group since their OSA and nocturnal hypoxemia profile was similar to that of the control group of obese, non-apneic subjects in a previous publication [17]. Primary analyses were performed using all participants. Secondary analyses excluded participants at moderate-to-very high risk of CKD progression with a history of reduced kidney function and/or proteinuria. Tertiary analyses additionally excluded participants with a history of common CKD risk factors (diabetes, hypertension, NSAID or PPI use). For our analyses, ORs were first estimated adjusting for age, self-identified gender, and BMI (model 1); model 2 added adjustments for excessive daytime sleepiness (ESS > 10) and poor sleep quality (PSQI > 5); model 3 added adjustments for comorbidities; and model 4 added adjustment for use of NSAID and PPI medications. For OR analyses, missing data were handled using multiple imputation (procedures outlined in the online supplement). Complete case analyses were also performed as sensitivity analyses. Additionally, the associations between measures of renal function (eGFR and ACR) and measures of nocturnal hypoxemia [ODI, mean SpO2 (%; TRT) and T90 (% TRT)] were assessed using multivariable linear regression incorporating all variables included in model 4 of the binary logistic regression analyses. ACR values were log transformed to satisfy the assumption of normality of residual. Finally, binary logistic regression analyses were repeated in a cohort restricted to participants who had a PSG in order to substitute AHI for ODI.
All analyses were performed with Statistical Analysis Software (v9.4, Cary, North Carolina, USA) and alpha ≤0.05 was considered significant.
Results
Between July 2016 and March 2021, 2083 participants were enrolled in the CSCN’s adult OSA database. Our primary analyses were performed on 1295 participants after removing those whose data did not include (1) eGFR and/or ACR results (n = 732), (2) ODI based upon 4% desaturations (n = 18), and (3) an answer to the two specific questions regarding a history or abnormal kidney function and proteinuria (n = 33). We also removed two participants who were on dialysis and three participants who had a prior kidney transplant. Of the 1295 participants enrolled, 824 had a PSG.
Participants were predominantly white; male gender and BMI increased with OSA severity (Table 1). Participants with severe OSA reported greater daytime sleepiness, with a higher proportion of short sleepers (≤6 h sleep/night) and a higher ISI score. Compared to the no/mild OSA group, participants with moderate and severe OSA had lower eGFR and higher ACR. A history of hypertension, high cholesterol, diabetes, and coronary artery disease were more prevalent in participants with moderate and severe OSA. There was no difference between the groups in the use of NSAIDs and PPI medications.
Table 1.
Participant characteristics for the entire cohort and categorized by OSA severity
| All participants | No/mild OSA | Moderate OSA | Severe OSA | P-value | |
|---|---|---|---|---|---|
| N | 1295 | 552 | 322 | 421 | |
| Female (%) | 538 (41.5) | 282 (51.1) | 117 (36.3)* | 139 (33.0)* | <.001 |
| Age (years) | 54.0 ± 12.7 | 53.3 ± 13.4 | 55.4 ± 12.0 | 53.9 ± 12.3 | .060 |
| BMI (kg/m²) | 34.2 ± 8.3 | 30.7 ± 6.6 | 34.7 ± 8.1* | 38.2 ± 8.6* † | <.001 |
| White (%) | 1060 (81.9) | 464 (84.1) | 257 (79.8) | 339 (80.5) | .201 |
| OSA severity | |||||
| ODI (4%, events/h; TRT) | 17.7 (5.2−37.8) | 4.0 (1.5−9.6) | 20.8 (17.2−24.9)* | 50.3 (38.6−66.8)* † | <.001 |
| Mean Spo2 (%; TRT) | 91.1 (87.8−93.4) | 93.3 (91.6−94.7) | 90.8 (88.4−92.6)* | 87.2 (84.0−90.2)* † | <.001 |
| T90 (% TRT) | 19.6 (2.2−70.0) | 1.7 (0.2−14.8) | 20.9 (7.2−68.1)* | 68.3 (35.6−91.0)* † | <.001 |
| Daytime sleepiness and sleep quality | |||||
| ESS Score | 9.7 ± 5.2 (n=1276) | 9.1 ± 5.0 (n = 537) | 9.1 ± 5.2 (n = 320) | 10.8 ± 5.2* † (n = 419) | <.001 |
| ESS > 10 (%) | 629 (49.3) | 245 (45.6) | 145 (45.3) | 239 (57.0)* † | <.001 |
| PSQI Global Score | 8.8 ± 4.0 (n = 1242) | 8.6 ± 4.0 (n = 515) | 8.7 ± 4.1 (n = 311) | 9.0 ± 4.0 (n = 416) | .275 |
| PSQ I > 5 (%) | 1053 (84.8) | 430 (83.5) | 261 (83.9) | 362 (87.0) | .293 |
| Sleep duration (h) | 6.5 ± 1.5 (n = 1293) | 6.5 ± 1.4 (n = 551) | 6.5 ± 1.5 (n = 321) | 6.4 ± 1.7 (n = 421) | .193 |
| Sleep ≤ 6 h (%) | 609 (47.1) | 232 (42.1) | 150 (46.7) | 227 (53.9)* | .001 |
| ISI (total score) | 12.6 ± 5.8 (n = 1274) | 12.1 ± 5.7 (n = 536) | 12.6 ± 6.0 (n = 319) | 13.3 ± 5.8* (n = 419) | .006 |
| Insomnia (%) | 1006 (79.0) | 420 (78.4) | 249 (78.1) | 337 (80.4) | .664 |
| RLS (%) | 291 (22.7) | 128 (23.5) | 78 (24.5) | 85 (20.2) | .312 |
| RLS severity | 4.7 ± 1.6 | 4.6 ± 1.7 | 4.7 ± 1.8 | 5.0 ± 1.4 | .142 |
| Kidney function | |||||
| Serum creatinine (µmol/L) | 80.0 (69.0−91.0) | 76.0 (66.0−87.0) | 82.5 (71.0−92.3)* | 83.0 (71.0−96.0)* | <.001 |
| eGFR (mL/min/1.73 m²) | 87.0 (74.0−98.0) | 90.0 (77.0−100.0) | 87.0 (71.8−98.0)* | 84.0 (71.5−96.5)* | <.001 |
| Urine albumin (mg/L)# | 8.6 (5.0−21.4) | 8.0 (5.0−14.0) | 8.3 (5.0−26.0)* | 11.0 (5.0−31.6)* | <.001 |
| Urine creatinine (mmol/L) | 11.5 (6.7−16.6) | 11.4 (6.5−16.5) | 11.5 (7.0−16.4) | 11.6 (6.7−17.0) | .625 |
| ACR (mg/mmol) | 0.9 (0.5−2.0) | 0.8 (0.5−1.5) | 0.9 (0.5−2.3)* | 1.0 (0.5−2.9)* | <.001 |
| Comorbidities | |||||
| Kidney disease (%) | 102 (7.9) | 38 (6.9) | 24 (7.5) | 40 (9.5) | .307 |
| Proteinuria | 94 (7.3) | 33 (6.0) | 23 (7.1) | 38 (9.0) | .192 |
| Smoking | |||||
| Never smoker (%) | 636 (49.1) | 290 (52.5) | 153 (47.5) | 193 (45.8) | .153 |
| Past smoker (%) | 510 (39.4) | 210 (38.0) | 129 (40.1) | 171 (40.6) | |
| Current smoker (%) | 149 (11.5) | 52 (9.4) | 40 (12.4) | 57 (13.5) | |
| Hypertension (n, %) | 592 (45.7) | 200 (36.3) | 176 (54.7)* | 216 (51.3)* | <.001 |
| High cholesterol (%) | 510 (39.5) | 176 (32.1) | 156 (48.4)* | 178 (42.3)* | <.001 |
| Diabetes (%) | 239 (18.5) | 64 (11.7) | 60 (18.6)* | 115 (27.3)* | <.001 |
| Coronary artery disease (%) | 105 (8.1) | 32 (5.8) | 36 (11.2)* | 37 (8.8) | .016 |
| Heart failure (%) | 43 (3.3) | 17 (3.1) | 15 (4.7) | 11 (2.6) | .284 |
| Atrial fibrillation (%) | 88 (6.8) | 45 (8.2) | 18 (5.6) | 25 (5.9) | .232 |
| Past stroke (%) | 37 (2.9) | 13 (2.4) | 14 (4.4) | 10 (2.4) | .180 |
| COPD (%) | 71 (5.5) | 28 (5.1) | 16 (5.0) | 27 (6.4) | .595 |
| Asthma (%) | 275 (21.3) | 116 (21.1) | 62 (19.3) | 97 (23.1) | .444 |
| Medications | |||||
| NSAIDs (%) | 92 (7.1) | 37 (6.7) | 25 (7.8) | 30 (7.1) | .848 |
| Proton pump inhibitors (%) | 288 (22.3) | 120 (21.8) | 73 (22.7) | 95 (22.6) | .940 |
Number (%), categorical variables (p-value = χ2); mean ± SD or median (interquartile range), continuous variables (p-value = ANOVA or Kruskal−Wallis H).
#Urine albumin was below detectable limits in 275 participants and above detectable limits in two participants.
* p < .05 versus no/mild OSA.
† p < .05 versus moderate OSA.
Abbreviations: BMI, body mass index; ODI, oxygen desaturation index based upon 4% desaturations; mean SpO2, mean arterial oxyhemoglobin saturation; T90, percentage of total recording time (TRT) with SpO2 <90%; ESS, Epworth Sleepiness Scale; PSQI, Pittsburgh Sleep Quality Index; ISI, insomnia severity index; RLS, restless legs syndrome; eGFR, estimated glomerular filtration rate; ACR, urine albumin:creatinine ratio; COPD, chronic obstructive pulmonary disease; NSAID, non-steroidal anti-inflammatory drugs.
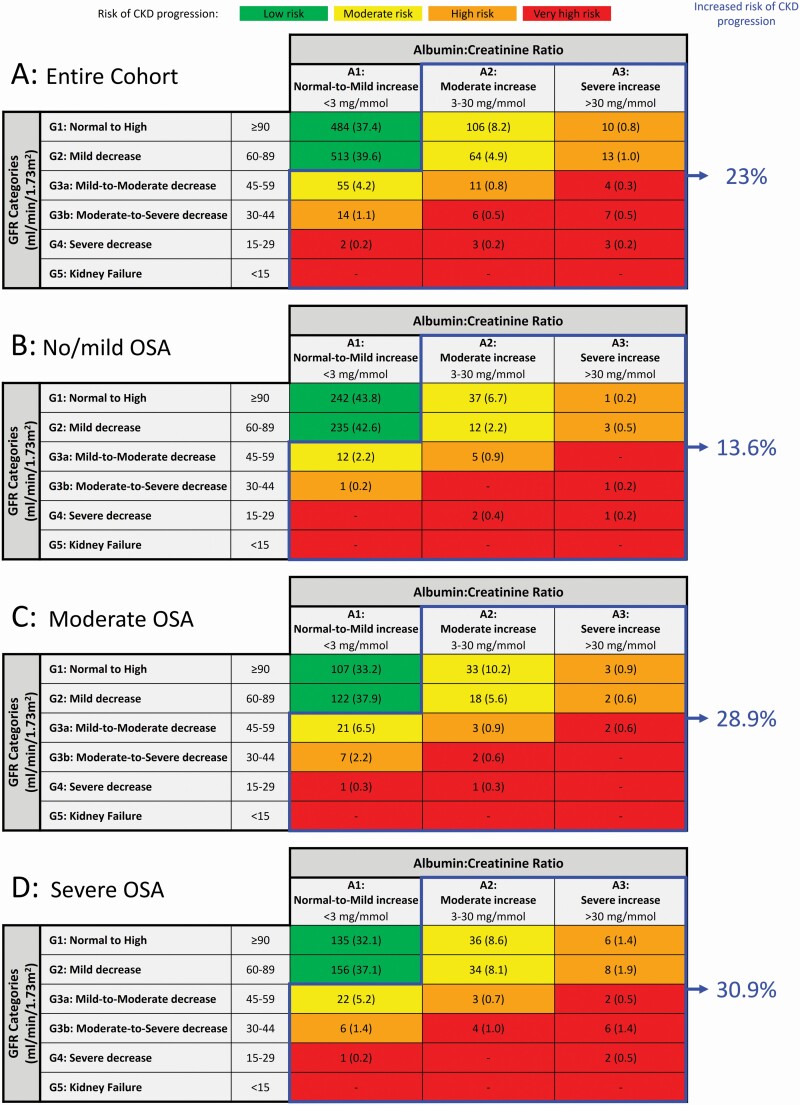
Prevalence of increased risk of CKD progression
Within our entire cohort, 23% were at moderate-to-very high risk of kidney disease progression (Figure 1, A; blue outline) and this risk increased from 13.6% of those with no/mild OSA to 28.9% and 30.9% of those with moderate and severe OSA, respectively, independent of age, sex, and BMI (p<0.001, Figure 1, B, C and D; blue outlines). Results were similar after removing 81 participants at moderate-to-very high risk of CKD progression who reported a history of reduced kidney function and/or proteinuria; specifically, 17.9% of all participants remained at moderate-to-very high risk of kidney disease progression with the prevalence increasing from 10.8% of those with no/mild OSA to 23.4% and 23.4% of those with moderate and severe OSA, respectively (p < .001, Figure S1, online supplement). Importantly, results were unchanged when analyses were restricted to participants who had a measurement of AHI from their PSG in both the entire cohort and following exclusion of those with a history of reduced kidney function and/or proteinuria (i.e. the proportion of participants with moderate-to-very high risk of CKD progression increased with OSA severity, based upon AHI, independent of age, sex and BMI (p < .001); Figures S2 and S3, online supplement).
Figure 1.
Risk of CKD progression for the entire cohort (A; n = 1295) and participants with no/mild OSA (B; n = 552), moderate OSA (C; n = 322) and severe OSA (D; n = 421). Colored boxes indicate the number (% of group) with low (green), moderate (yellow), high (orange), and very high (red) risk of CKD progression. Blue line surrounds the 23.0% of participants within the entire cohort, 13.6% of participants with no/mild OSA, 28.9% of participants with moderate OSA, and 30.9% of participants with severe OSA who are at moderate-to-very high risk of kidney disease progression.
Odds of moderate-to-very high risk of CKD progression
Correspondingly, the ORs for participants with moderate and severe OSA to be at moderate-to-very high risk of kidney disease progression were 2.67 and 3.11, respectively, compared to participants with no/mild OSA after adjusting for age, sex, and BMI ≥30 (model 1, Table 2 – primary analyses). Following adjustments for excessive daytime sleepiness and poor sleep quality (model 2), comorbidities (model 3), and medications (model 4) the ORs remained ≥2.63 for participants with both moderate and severe OSA in all models (p < .001 for all ORs). Results were similar following removal of participants at moderate-to-very high risk of CKD progression with a previous history of kidney disease and/or proteinuria (n = 81; Table 2 – secondary analyses, model 4). Finally, ORs remained ≥2.1 for participants with moderate OSA after further exclusion of participants with a history of risk factors for CKD (diabetes, hypertension, NSAID and PPI medications), and ≥3.21 for participants with severe OSA (model 3, Table 2 – tertiary analyses). For primary, secondary and tertiary analyses, values were imputed for ≤4.3% of participants across all variables included in each model. As a result, similar results were obtained in complete case analysis of 1208 participants (out of 1295) for our primary analyses, 1131 participants (out of 1214) for our secondary analyses, and 451 (out of 488) for our tertiary analyses (Table S1, online supplement). Similar results were observed for both imputed data and for complete case analyses following restriction of our cohort to participants who had a PSG, although the ORs associated with moderate OSA did not reach statistical significance for our secondary and tertiary analyses (Tables S2 and S3; online supplement).
Table 2.
Odds ratios (OR) for moderate-to-very high risk of kidney disease progression in all participants*
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OSA severity | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Primary analyses: all participants (n = 1295) | ||||||||
| No/mild OSA | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Moderate OSA | 2.67 (1.86−3.81) | <.001 | 2.69 (1.88−3.85) | <.001 | 2.63 (1.79−3.85) | <.001 | 2.63 (1.79−3.85) | <.001 |
| Severe OSA | 3.11 (2.19−4.40) | <.001 | 3.19 (2.24−4.53) | <.001 | 2.96 (2.04−4.30) | <.001 | 2.96 (2.04−4.30) | <.001 |
| Secondary analyses: participants without a history of kidney disease and/or proteinuria (n = 1214) | ||||||||
| No/mild OSA | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Moderate OSA | 2.68 (1.80−3.98) | <.001 | 2.71 (1.82−4.04) | <.001 | 2.53 (1.69−3.80) | <.001 | 2.53 (1.68−3.80) | <.001 |
| Severe OSA | 2.86 (1.93−4.23) | <.001 | 2.94 (1.98−4.36) | <.001 | 2.71 (1.81−4.04) | <.001 | 2.71 (1.81−4.04) | <.001 |
| Tertiary analyses: participants without CKD risk factors (diabetes, hypertension, NSAID or PPI use; n = 488) | ||||||||
| No/mild OSA | 1.0 | 1.0 | 1.0 | |||||
| Moderate OSA | 2.17 (1.01−4.69) | .049 | 2.21 (1.02−4.77) | .045 | 2.10 (0.93−4.75) | .076 | − | |
| Severe OSA | 3.66 (1.77−7.59) | <.001 | 3.80 (1.83−7.90) | <.001 | 3.21 (1.51−6.85) | .003 |
*Missing data from 87 patients with missing data handled using multiple imputation.
Model 1: Adjusted for age, sex and body mass index (BMI) > 30 kg/m2.
Model 2: Model 1 + adjustments for excessive daytime sleepiness (ESS > 10) and poor sleep quality (PSQI > 5).
Model 3: Model 2 + adjustments for smoking and medical history of kidney disease (not in secondary analyses), proteinuria (not in secondary analyses), hypertension, high cholesterol, diabetes, coronary artery disease, heart failure, atrial fibrillation, and stroke.
Model 4: Model 3 + adjustments for use of non-steroidal anti-inflammatory (NSAID) and proton pump inhibitor (PPI) medications (not applied to tertiary analyses).
Association between renal function and nocturnal hypoxemia
Consistent with moderate and severe OSA increasing the odds of moderate-to-very high risk of kidney disease progression, eGFR was negatively associated with ODI (p = .010) while ACR was positively associated with ODI (p = .001) after adjusting for the same variables used in model 4 of the binary logistic regression analyses (Table 3). Furthermore, higher ACR was associated with lower mean SpO2 (p = .002). However, neither eGFR nor ACR were associated with T90, although there was a tendency for eGFR to be lower with greater T90 (p = .057).
Table 3.
Standardized beta coefficients (95% confidence interval) for associations between measures of renal function and nocturnal hypoxemia
| Measures of nocturnal hypoxemia | ||||||
|---|---|---|---|---|---|---|
| ODI (4%, events/h; TRT) |
P-value | Mean SpO2 (TRT) |
P-value | T90 (% TRT) |
P-value | |
| eGFR (mL/min/1.73 m²) | −0.06 (−0.11 to −0.02) |
.010 | 0.04 (−0.01 to 0.08) |
.160 | −0.05 (−0.10 to 0.002) |
.057 |
| Log ACR (mg/mmol) | 0.10 (0.04 to 0.15) |
.001 | −0.09 (−0.15 to −0.03) |
.002 | 0.03 (−0.03 to 0.09) |
.331 |
Beta coefficients adjusted for age, sex, body mass index (BMI) >30 kg/m2, excessive daytime sleepiness (ESS > 10), poor sleep quality (PSQI > 5), smoking, history of kidney disease, proteinuria, hypertension, high cholesterol, diabetes, coronary artery disease, heart failure, atrial fibrillation, stroke, and use of non-steroidal anti-inflammatory (NSAID) and proton pump inhibitor (PPI) medications.
Participant awareness of CKD
Although 23% of the entire study cohort (298 participants) met our combined eGFR and ACR criteria for CKD (Figure 1, A), only 27% (81 of 298) reported a previous physician diagnosis of abnormal kidney function and/or proteinuria (Table 4). This implies that the remaining 73% (217 out of 298) of participants with CKD were unaware that they had abnormal kidney function. This proportion remained the same within each category of sleep apnea severity. Furthermore, among the 217 participants who were unaware they had abnormal kidney function, 34.2% of those with moderate OSA and 24.7% of those with severe OSA had stage 3 CKD (Table 3).
Table 4.
Awareness and severity of CKD in participants with increased risk of CKD progression
| All participants | No/mild OSA | Moderate OSA | Severe OSA | |
|---|---|---|---|---|
| N | 1295 | 552 | 322 | 421 |
| Awareness of increased risk of CKD progression | ||||
| Increased risk CKD progression, n (%) | 298 (23.0) | 75 (13.6) | 93 (28.9) | 130 (30.9) |
| Participants unaware of their increased risk CKD progression, n (%)* | 217 (72.8) | 58 (77.3) | 70 (75.3) | 89 (68.5) |
| CKD severity in participants unaware of their increased risk of CKD* | ||||
| N | 217 | 58 | 70 | 89 |
| CKD Stage 1, n (%) | 96 (44.2) | 32 (55.2) | 31 (44.3) | 33 (37.1) |
| CKD Stage 2, n (%) | 61 (28.1) | 12 (20.7) | 15 (21.4) | 34 (38.2) |
| CKD Stage 3a, n (%) | 51 (23.5) | 14 (24.1) | 19 (27.1) | 18 (20.2) |
| CKD Stage 3b, n (%) | 9 (4.2) | 0 (0.0) | 5 (7.1) | 4 (4.5) |
| CKD Stage 4, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CKD Stage 5, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
* Lack of awareness determined by participant reporting they have no history of abnormal kidney function and/or proteinuria.
Discussion
We assessed the risk of CKD progression and awareness of kidney disease in a large cohort of patients referred to a sleep clinic for suspected OSA. The main findings were that (1) there was a linear increase in the proportion of participants at moderate-to-very high risk of CKD progression, ranging from ~14% in patients with no/mild OSA to 31% in participants with severe OSA; (2) moderate and severe OSA were associated with ~2.6 and 3 times greater risk of moderate-to-very high risk of CKD progression; (3) the risk of CKD progression was associated with the severity of intermittent nocturnal hypoxemia; and (4) most participants were unaware they had an elevated risk of CKD.
In our cohort, all participants were referred for evaluation of OSA without screening for the presence of kidney disease. Although our questionnaire included specific questions about a history of abnormal kidney function for our subsequent analysis, the answers to these questions did not alter patients’ participation in the study. The range and prevalence of co-existing sleep and medical disorders were consistent with a sleep clinic cohort. The prevalence of many medical disorders, such as hypertension and diabetes, increased in association with the severity of OSA, whereas the prevalence of RLS was similar across all categories of OSA severity.
By combining our measurements of eGFR and ACR in a heat map, we were able to estimate the risk of CKD progression using the method recommended for patients with CKD [8]. Although previous studies have reported these indices of kidney function in patients with OSA [21], this is the first study to combine eGFR and ACR to estimate the risk of CKD progression in a sleep clinic population. We found that approximately 30% of participants with moderate or severe OSA had an increased risk of CKD progression independent of age, sex, and BMI (Figure 1) with an odds ratio of 2.63 and 2.96, respectively, compared to participants with no/mild OSA (Table 2, primary analysis, model 4). Notably, even after exclusion of participants with an increased risk of CKD progression who reported a previous history of abnormal kidney function, the prevalence of increased risk of CKD progression remained elevated at 25% (Figure S1), with an odds ratio of 2.53 and 2.71 for moderate and severe OSA, respectively, compared to participants with no/mild OSA (Table 2, secondary analysis, model 4). Furthermore, the OR for moderate-to-very high risk of CKD progression remained significantly elevated after the additional exclusion of participants with risk factors for the development of CKD (diabetes, hypertension, NSAID and PPI medications) at 2.10 (moderate OSA) and 3.21 (severe OSA) (Table 2, tertiary analysis, model 3). Finally, the associations between OSA and risk of CKD progression were similar using both imputed data (Table 2) and complete case analyses (Table S1). Consequently, we believe that the risk of CKD progression is elevated in participants with moderate and severe OSA, independent of other risk factors.
The awareness of participants with OSA that they have abnormal kidney function has not been addressed in previous studies. We found that 73% of participants with OSA who had an increased risk of CKD progression did not report a previous physician diagnosis of abnormal kidney function or proteinuria (Table 4). This is not surprising in that early stages of CKD are usually clinically silent and require objective testing to diagnose [31] and similar findings have been reported in CKD cohorts [48]. Nevertheless, it does suggest that both patients with OSA and their healthcare providers need to be more aware of the association between OSA and CKD. This is clinically relevant for several reasons. Firstly, approximately 30% of participants who were not aware they had abnormal kidney function, had stage 3 CKD (Table 4) which is the level of CKD at which cardiovascular morbidity and mortality increase [6, 7]. Secondly, the potential for treatment of OSA with continuous positive airway pressure to improve kidney function may be greatest in those with early CKD, as suggested by a recent clinical trial at our center [49].
This study has limitations. First, cross-sectional data for eGFR and ACR were used to estimate the longitudinal risk of CKD progression. The combined assessment of eGFR and ACR to estimate the risk of CKD progression is based on a clinical practice guideline authored by experts in nephrology and is widely used in the management of patients with CKD [8]. Secondly, our estimation of patients’ awareness of CKD relied on self-report which can be confounded by other factors such as poor memory, which is commonly found in patients with OSA [37]. We were careful to frame the questions about possible CKD in lay terms that included a “physician-diagnosis” and to ask about both “abnormal kidney function” and “protein in the urine,” which are key diagnostic criteria for CKD. Although a large proportion of patients appeared unaware that they had CKD, this high prevalence has also been reported in the general population [48]. The strengths of the study are that we recruited a large sample size from multiple sleep centers across Canada which was representative of the full spectrum of OSA severity. Furthermore, objective testing was used to diagnose both OSA and CKD. Finally, our multivariate analysis was comprehensive in that we controlled for all known causes of CKD that we could measure.
The findings of this study have important clinical implications. First, the development and progression of CKD has significant implications for patients with OSA. A fall in eGFR below 60 mL/min/1.73 m2 and a rise in ACR >3 mg/mmol have both been shown to independently increase cardiovascular mortality [6, 7]. Since OSA is also a recognized risk factor for cardiovascular disease [50], the addition of CKD is likely to increase this risk further. This is supported by a recent study of patients with CKD who also had OSA in whom the hypoxic burden was associated with increased mortality which was predominantly due to cardiovascular events [51]. Second, progression of CKD to ESKD, requiring renal function replacement with chronic dialysis or renal transplantation, has major implications for patient well-being and the associated costs to the healthcare system [9, 11]. Identification and treatment of OSA patients who have an increased risk of CKD progression provides an opportunity to reduce these complications in the era of precision-based medicine.
In summary, we found that a large proportion of patients who present with OSA have an increased risk of CKD progression which is independent of other CKD risk factors. Both patients, and likely their healthcare providers, are unaware of this association. This results in a potential missed opportunity to detect CKD in its early stages and to initiate interventions, including treatment of OSA, that may reduce the risk of CKD progressing to more severe kidney disease with its associated risk of increased morbidity and mortality. Accordingly, it may be worthwhile to assess renal function in patients who present with moderate-to-severe OSA, particularly when accompanied by significant nocturnal hypoxemia. Further studies are required to evaluate the impact of such interventions on these important clinical outcomes.
Supplementary Material
Acknowledgments
The authors would like to thank our participants for the donation of their time and interest in this study. We would like to thank the staff at the Sleep Centre, Foothills Medical Centre, University of Calgary, the Sleep Disorders Center at Saskatoon City Hospital, the Leon Judah Blackmore Centre for Sleep Disorders, University of British-Columbia Hospital, the Sleep Centre, McGill University Health Centre, and the Sleep Centre, Hôpital Hôtel-Dieu de Lévis, Université Laval for their assistance. In addition, we thank Healthy Heart Sleep Company, Dream Sleep Respiratory Services and Rimer Alco North America (RANA) in Calgary for their help with patient recruitment and other community CPAP providers in Calgary for their support with data collection.
Data Availability
Anonymized data will be made available to other qualified researchers on reasonable request to the corresponding author.
Authors’ Contributions
A.E.B., S.B.A., F.S., J.K., R.P.S., N.T.A., and P.J.H. conceived the experimental design; J.K.R., A.J.M.H.A., A.N., T.G., and S.G. helped with participant recruitment and data acquisition; A.E.B., A.J.M.H.A., and P.J.H. performed the statistical analyses. All authors contributed to the interpretation of the data. A.E.B. and P.J.H., wrote the first draft of the manuscript. A.E.B., J.K.R., S.B.A., A.J.M.H.A., A.N., T.G., S.G., F.S., J.K., R.R.S., N.T.A., and P.J.H. critically reviewed the manuscript for important intellectual property. A.E.B., J.K.R., S.B.A., A.J.M.H.A., A.N., T.G., S.G., F.S., J.K., R.R.S., N.T.A., and P.J.H. approved of the final manuscript.
Funding
A.E.B. was supported by postdoctoral fellowships from the Canadian Institutes of Health Research (CIHR). J.K.R. was supported by funds from the Cumming School of Medicine, Sleep Research Program (University of Calgary) and the Canadian Sleep and Circadian Network (CSCN). A.J.M.H.A., A.N., T.G., and S.G. were supported by funds provided by the CSCN. Funding for this study was provided by the respiratory network of the Fonds de Recherche du Québec- Santé (FRQS), Fonds de support aux activités d’enseignement et de recherche sur les troubles respiratoires di sommeil de la Fondation IUCPQ and the CSCN, which is funded through a CIHR Community Development Program grant.
Disclosure Statement
Financial Disclosure: R.P.S reports having received consulting fees from Resmed, GSK and AstraZeneca outside the submitted work; P.J.H reports having received financial support and equipment (CPAP units) from Philips Respironics for clinical research. All remaining authors report no financial conflicts of interests.
Non-financial Disclosure: None.
References
- 1. Bikbov MM, et al. Chronic kidney disease in Russia: the Ural eye and medical study. BMC Nephrol. 2020;21(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coresh J, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. [DOI] [PubMed] [Google Scholar]
- 3. Eckardt KU, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–169. [DOI] [PubMed] [Google Scholar]
- 4. Jha V, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. [DOI] [PubMed] [Google Scholar]
- 5. Go AS, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 6. Gansevoort RT, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. [DOI] [PubMed] [Google Scholar]
- 7. Matsushita K, et al. ; Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
- 9. James MT, et al. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375(9722):1296–1309. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from kidney disease improving global outcomes. Kidney Int. 2007;72(3):247–259. [DOI] [PubMed] [Google Scholar]
- 11. Kroeker A, et al. An operating cost comparison between conventional and home quotidian hemodialysis. Am J Kidney Dis. 2003;42(1 Suppl):49–55. [DOI] [PubMed] [Google Scholar]
- 12. Benjafield AV, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nicholl DDM, et al. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest. 2012;141(6):1422–1430. [DOI] [PubMed] [Google Scholar]
- 14. Evans RG, et al. Factors that render the kidney susceptible to tissue hypoxia in hypoxemia. Am J Physiol Regul Integr Comp Physiol. 2011;300(4):R931–R940. [DOI] [PubMed] [Google Scholar]
- 15. Abuyassin B, et al. Intermittent hypoxia causes histological kidney damage and increases growth factor expression in a mouse model of obstructive sleep apnea. PLoS One. 2018;13(2):e0192084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foster GE, et al. Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension. 2010;56(3):369–377. [DOI] [PubMed] [Google Scholar]
- 17. Zalucky AA, et al. Nocturnal hypoxemia severity and renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med. 2015;192(7):873–880. [DOI] [PubMed] [Google Scholar]
- 18. Nicholl DDM, et al. Nocturnal hypoxemia severity influences the effect of CPAP therapy on renal renin-angiotensin-aldosterone system activity in humans with obstructive sleep apnea. Sleep. 2021;44(5). [DOI] [PubMed] [Google Scholar]
- 19. Abuyassin B, et al. Obstructive sleep apnea and kidney disease: a potential bidirectional relationship? J Clin Sleep Med. 2015;11(8):915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanly PJ, et al. Sleep apnea and the kidney: is sleep apnea a risk factor for chronic kidney disease? Chest. 2014;146(4):1114–1122. [DOI] [PubMed] [Google Scholar]
- 21. Umbro I, et al. A systematic review on the association between obstructive sleep apnea and chronic kidney disease. Sleep Med Rev. 2020;53:101337. [DOI] [PubMed] [Google Scholar]
- 22. Iseki K, et al. High Prevalence of chronic kidney disease among patients with sleep related breathing disorder (SRBD). Hypertens Res. 2008;31(2):249–255. [DOI] [PubMed] [Google Scholar]
- 23. Chou YT, et al. Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant. 2011;26(7):2244–2250. [DOI] [PubMed] [Google Scholar]
- 24. Kanbay A, et al. Obstructive sleep apnea syndrome is related to the progression of chronic kidney disease. Int Urol Nephrol. 2012;44(2):535–539. [DOI] [PubMed] [Google Scholar]
- 25. Adams RJ, et al. Chronic Kidney Disease and Sleep Apnea Association of kidney disease with obstructive sleep apnea in a population study of Men. Sleep. 2017;40(1):zsw015. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed SB, et al. Nocturnal hypoxia and loss of kidney function. PLoS One. 2011;6(4):e19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee YC, et al. Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep. 2015;38(2):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Molnar MZ, et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax. 2015;70(9):888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin YS, et al. Simple obstructive sleep apnea patients without hypertension or diabetes accelerate kidney dysfunction: a population follow-up cohort study from Taiwan. Sleep Breath. 2017;21(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lakkis JI, et al. Obesity and kidney disease. Prog Cardiovasc Dis. 2018;61(2):157–167. [DOI] [PubMed] [Google Scholar]
- 31. Chen TK, et al. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ng SS, et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS). Respirology. 2010;15(2):336–342. [DOI] [PubMed] [Google Scholar]
- 33. Nilius G, et al. A randomized controlled trial to validate the Alice PDX ambulatory device. Nat Sci Sleep. 2017;9:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Issa FG, et al. Digital monitoring of sleep-disordered breathing using snoring sound and arterial oxygen saturation. Am Rev Respir Dis. 1993;148(4 Pt 1):1023–1029. [DOI] [PubMed] [Google Scholar]
- 35. Westbrook PR, et al. Description and validation of the apnea risk evaluation system: a novel method to diagnose sleep apnea-hypopnea in the home. Chest. 2005;128(4):2166–2175. [DOI] [PubMed] [Google Scholar]
- 36. Erman MK, et al. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3(4):387–392. [PMC free article] [PubMed] [Google Scholar]
- 37. Beaudin AE, et al. Cognitive function in a sleep clinic cohort of patients with obstructive sleep apnea. Ann Am Thorac Soc. 2021;18(5):865–875. [DOI] [PubMed] [Google Scholar]
- 38. Berry RB, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rule, Terminology and Technical Specifications, Version 2.2. Darien, Illinois: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 39. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 40. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 41. Levey AS, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dominguez JR, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group. Relationships between serum and urine phosphorus with all-cause and cardiovascular mortality: the Osteoporotic Fractures in Men (MrOS) Study. Am J Kidney Dis. 2013;61(4):555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bastien CH, et al. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 44. Grandner MA, et al. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14(4):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kane SP. Top 250 Drugs: Drug List by Therapeutic Category. ClinCalc LLC. https://clincalc.com/Downloads/Top250Drugs-DrugList.pdf. Accessed April 7, 2021.
- 46. Gooch K, et al. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120(3):280.e1–280.e7. [DOI] [PubMed] [Google Scholar]
- 47. Al-Aly Z, et al. Proton pump inhibitors and the kidney: implications of current evidence for clinical practice and when and how to deprescribe. Am J Kidney Dis. 2020;75(4):497–507. [DOI] [PubMed] [Google Scholar]
- 48. Tuot DS, et al. ; Centers for Disease Control Chronic Kidney Disease Surveillance Team. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6(8):1838–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rimke AN, et al. Effect of CPAP therapy on kidney function in patients with chronic kidney disease: a pilot randomized controlled trial. Chest. 2021;159(5):2008–2019. [DOI] [PubMed] [Google Scholar]
- 50. Marin JM, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. [DOI] [PubMed] [Google Scholar]
- 51. Jhamb M, et al. Association of sleep apnea with mortality in patients with advanced kidney disease. Clin J Am Soc Nephrol. 2020;15(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be made available to other qualified researchers on reasonable request to the corresponding author.