Abstract
Complements and neutrophils are two key players of the innate immune system that are widely implicated as drivers of severe COVID-19 pathogenesis, as evident by the direct correlation of respiratory failure and mortality with elevated levels of terminal complement complex C5b-9 and neutrophils. In this study, we identified a feed-forward loop between complements and neutrophils that could amplify and perpetuate the cytokine storm seen in severe SARS-CoV-2–infected patients. We observed for the first time that the terminal complement activation complex C5b-9 directly triggered neutrophil extracellular trap (NET) release and interleukin (IL)-17 production by neutrophils. This is also the first report that the production of NETs and IL-17 induced by C5b-9 assembly on neutrophils could be abrogated by mesenchymal stem cell (MSC) exosomes. Neutralizing anti-CD59 antibodies abolished this abrogation. Based on our findings, we hypothesize that MSC exosomes could alleviate the immune dysregulation in acute respiratory failure, such as that observed in severe COVID-19 patients, by inhibiting complement activation through exosomal CD59, thereby disrupting the feed-forward loop between complements and neutrophils to inhibit the amplification and perpetuation of inflammation during SARS-CoV-2 infection.
Keywords: complements, exosomes, IL-17, netosis, neutrophils
Introduction
The COVID-19 pandemic is a global public health emergency caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), affecting >200 million individuals to date, with a significant mortality rate of ∼2% (WHO, covid19.who.int). The unprecedented rapid development and rollout of vaccines have significantly reduced mortality. However, a significant number of people remain unvaccinated and are at risk of developing severe disease. Severe COVID-19 disease is essentially a manifestation of dysregulated immune responses in the lungs that could lead to multi-organ pathologies. Current COVID-19 interventions are generally symptomatic and palliative treatments, and there is an urgent unmet need for effective drugs to alleviate immune dysfunctions in the lungs of severely ill COVID-19 patients to reduce mortality and improve recovery.
Mesenchymal stromal cell (MSC)-derived exosomes, which have documented immunomodulatory and regenerative properties, have been proposed as a promising therapeutic to alleviate immune dysfunction and reduce disease severity in fatally ill COVID-19 patients [1]. Although several preclinical and clinical studies have demonstrated their safety and efficacy in mitigating the symptoms associated with severe COVID-19 [2,3], the precise mechanism by which MSC exosomes exert their effects to benefit COVID-19 patients remains elusive.
Our innate immune system serves as the first line of defense against viral invasion. However, an augmented or dysregulated innate immune response could exacerbate the pathogenesis of a viral infection such as the development of acute respiratory distress syndrome (ARDS) and cytokine storm syndrome in severe COVID-19 [4], [5], [6], [7]. Complement is an integral component of the innate immune system that eliminates viral pathogens directly and indirectly through opsonization, formation of the membrane attack complex (MAC) C5b-9 and recruitment of other leukocytes to promote an anti-viral inflammatory response [8,9]. However, clinical data have revealed that increased activation of the complement system is a distinct immunologic feature correlating with worse COVID-19 outcomes [10], [11], [12], [13], [14], [15], [16], [17]. Elevated levels of circulating complement markers were present in patients with COVID-19 compared with other etiologies of acute respiratory failure, and these markers can distinguish those with worse outcomes in the setting of SARS-CoV-2 infection [10]. Indeed, complement activation has been known to play a central role in endothelial injury and hypercoagulability, leading to multi-organ failure and pathogenesis of COVID-19. As such, complement inhibitors to combat SARS-CoV-2 infection are currently being explored in multiple phase II and III clinical trials [18], [19], [20], [21].
Another immunologic indicator of poor disease outcome in COVID-19 is the increased number of circulating neutrophils. In critically ill patients, extensive neutrophil extracellular traps (NETs), a prominent signature of neutrophil activation, are detected in sera and lungs, thereby predisposing the patients to thrombosis, organ damage and, eventually, death [22], [23], [24], [25], [26], [27], [28]. This is further exacerbated by the hyperactivation of complements in the lungs of severely ill COVID-19 patients [2], as activated complements are potent chemoattractants for neutrophils [29,30]. Together, the clinical data suggest that the cardinal roles of complements and neutrophils in the pathogenesis of severe COVID-19 intersect. Therefore, elucidating and modulating this intersection represents a potential strategy to prevent the devastating complications in SARS-CoV-2–infected patients [31,32].
In this study, we show that the terminal complement activation complex C5b-9 can directly drive the release of NETs and interleukin (IL)-17 by neutrophils. MSC exosomes can suppress this complement-mediated neutrophil activation via a CD59-dependent mechanism. Together, our data revealed a novel cross-talk between two innate immune components, complement and neutrophil, and further highlighted the potency of MSC exosomes in suppressing the activation of this innate immune axis. Our data also provide a compelling rationale for the use of MSC exosome to treat severe COVID-19.
Results
Terminal complement complex triggers NET formation and IL-17 release by neutrophils
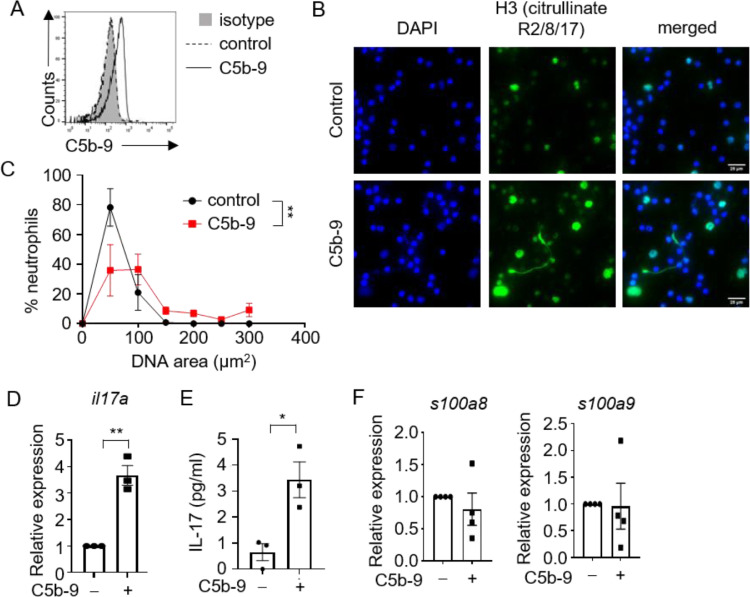
To characterize the effects of complement activation on neutrophil responses, we purified neutrophils from the blood of healthy human donors and initiated complement activation using a standard protocol of adding complements C5b-6 and C7 followed by C8 and C9. The assembly of terminal complement complex C5b-9 on neutrophil surfaces was confirmed by flow cytometry (Figure 1 A). Complement activation triggered the release of NETs in vitro, as visualized microscopically via the colocalization of DAPI-positive extracellular DNA fibers with citrullinated histone H3. In particular, >60% of neutrophils underwent NETosis upon activation of the complement cascade, as determined by DNA size, and the area of released NETs was significantly larger than that from the control group (Figure 1B, C). As the severity of COVID-19 also correlates positively with the level of neutrophil-associated inflammatory mediators such as IL-17 and calprotectin S100A8 and S100A9 [33], [34], [35], [36], [37], [38], we determined whether these inflammatory mediators were induced in the neutrophil response to complement activation. Quantitative reverse-transcription real-time polymerase chain reaction (PCR) and ELISA analysis revealed a significant induction of IL-17 transcription and secretion upon complement activation (Figure 1D, E), while levels of s100a8 and s100a9 transcripts remain unchanged (Figure 1F). Together, these data showed that complement activation can specifically induce NETs and IL-17 production by neutrophils. These data also provided a possible mechanistic link between complements and neutrophils to induce hyperinflammatory immune responses in severely ill COVID-19 patients.
Figure 1.
Complement C5b-9 complex induces NET formation and IL-17 release by neutrophils. (A) Flow cytometric analysis of C5b-9 assembly on neutrophils. (B) Representative images of NET formation by neutrophils upon assembly of complements C5b-9. Cells were stained for nuclei (DAPI, blue) and H3 Citrullinate R2/8/17 (green). Scale bar, 25 µm. (C) Quantification of NET release by neutrophils pooled from three independent experiments performed using neutrophils from four unique donors. At least 100 cells were quantified from each experiment. Data shown as mean ± SEM (n = 4). **P = 0.006, two-way ANOVA. (D) mRNA expression levels of il17a relative to hprt1 in neutrophils upon assembly of C5b-9 (n = 3). Three independent experiments were performed using neutrophils from three unique donors. **P = 0.002, unpaired two-tailed Student's t test. (E) C5b-9 were assembled on neutrophils, and culture supernatants were assayed for IL-17 by ELISA (n = 3). Three independent experiments were performed using neutrophils from three unique donors. *P = 0.02, unpaired two-tailed Student's t test. (F) mRNA expression levels of s100a8 and s100a9 relative to hprt1 in neutrophils upon assembly of C5b-9 (n = 4). Four independent experiments were performed using neutrophils from four unique donors. (Color version of figure is available online.)
Exosomes inhibit complement-induced neutrophil activation
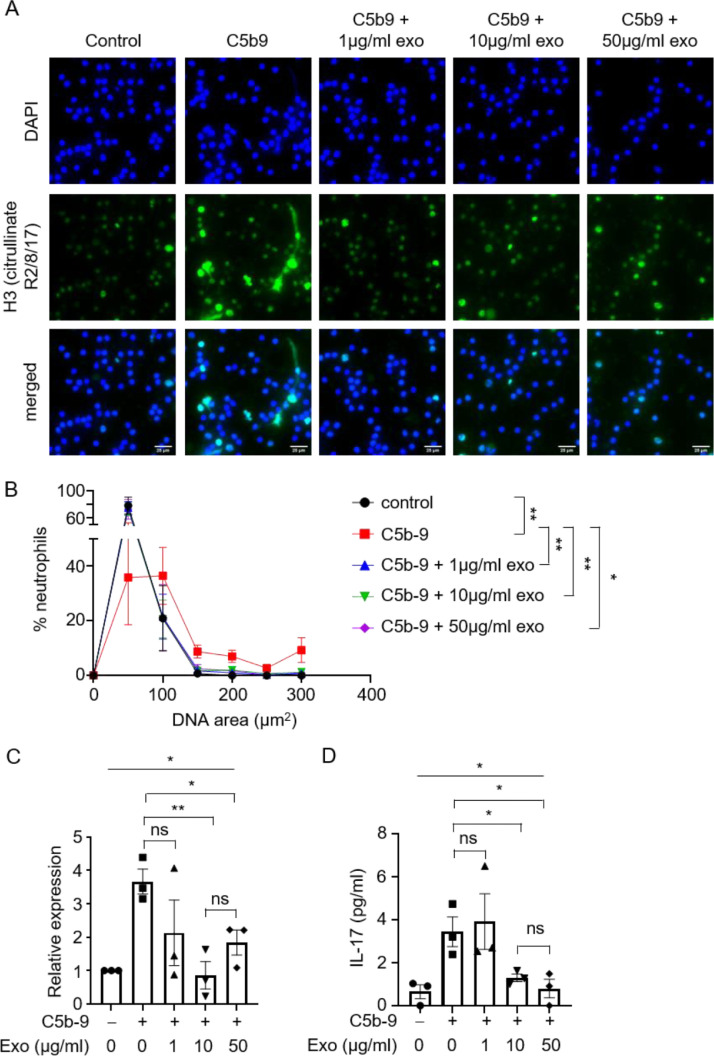
MSC exosomes, which possess immunomodulatory properties, have been proposed as a therapeutic option for COVID-19 to alleviate complications such as acute respiratory distress syndrome [2,3,39]. However, the mechanism by which exosomes exert their functions remains unclear. To test whether MSC exosomes can intervene in complement-induced neutrophilic responses, we incubated neutrophils with exosomes and complements C5b-6 and C7 followed by C8 and C9. Activation of neutrophils was attenuated upon addition of exosomes. Specifically, exosomes abrogated the release of NETs by neutrophils at concentration as low as 1 µg/mL (Figure 2 A, B). Exosomes also inhibited IL-17 production at the transcript and protein levels by C5b-9–assembled neutrophils at 10 and 50 µg/mL (Figure 2C, D). The inhibition was statistically significant at 10 but not 1 µg/mL exosome, indicating a dose-dependent response. However, the level of inhibition at 10 and 50 µg/mL was not significantly different, suggesting that maximum inhibition was reached at 10 µg/mL. Therefore, MSC exosomes can attenuate the complement-mediated NET formation and neutrophil IL-17 production.
Figure 2.
Exosomes suppress C5b-9–induced NETs and IL-17 release by neutrophils. (A) Representative images of NET formation by neutrophils upon assembly of C5b-9, in the presence or absence of different concentrations of exosomes. Cells were stained for nuclei (DAPI, blue) and H3 Citrullinate R2/8/17 (green). Scale bar, 25 µm. (B) Quantification of NET release by neutrophils pooled from three independent experiments performed using neutrophils from four unique donors. At least 100 cells were quantified from each experiment. The neutrophils used in these experiments were from the same donors as those in Figures 1 and 4. Data shown as mean ± SEM (n = 4). *P = 0.03, **P = 0.006, 0.004, 0.005 (from left to right), two-way ANOVA. (C) mRNA expression levels of il17a relative to hprt1 in neutrophils upon assembly of C5b-9, in the presence or absence of different concentrations of exosomes (n = 3). Three independent experiments were performed using neutrophils from three unique donors. The neutrophils used in these experiments were from the same donors as those in Figures 1 and 4. *P = 0.02, one-way ANOVA. *P = 0.02, **P = 0.007, unpaired two-tailed Student's t test. (D) C5b-9 were assembled on neutrophils in the presence or absence of different concentrations of exosomes, and culture supernatants were assayed for IL-17 by ELISA (n = 3). Three independent experiments were performed using neutrophils from three unique donors. The neutrophils used in these experiments were from the same donors as those in Figures 1 and 4. *P = 0.02, one-way ANOVA. *P = 0.03, unpaired two-tailed Student's t test. (Color version of figure is available online.)
Exosomes do not affect PMA-induced NETosis and IL-17 release
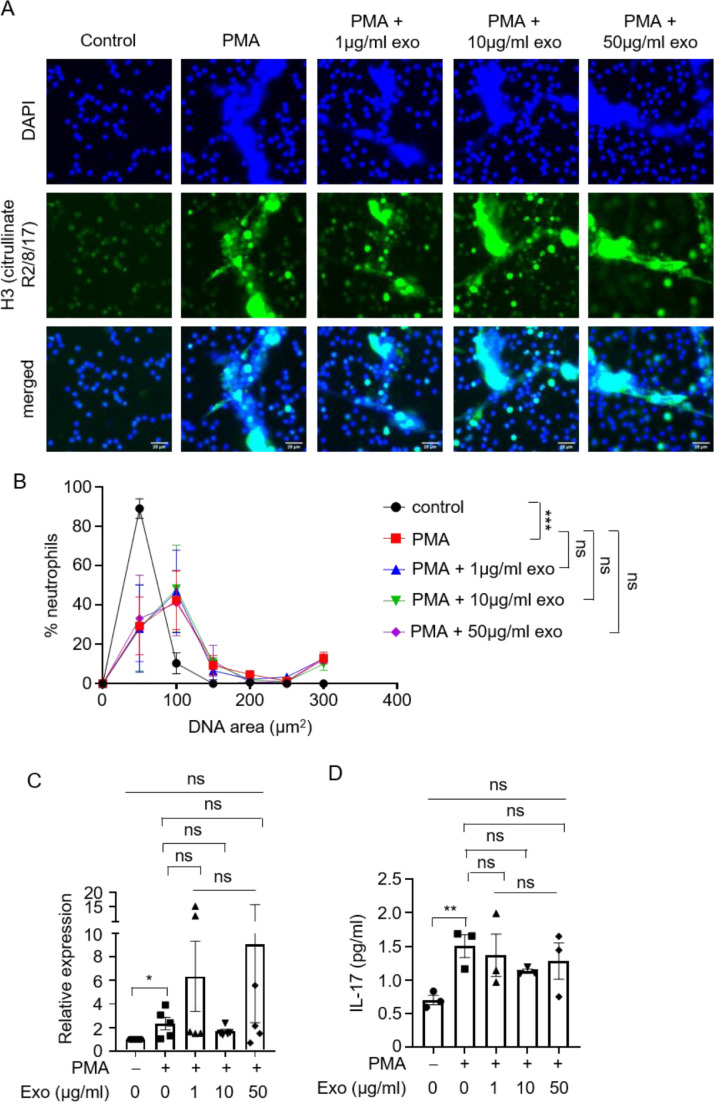
Several stimuli are known to trigger NET formation and IL-17 production by neutrophils. To determine whether inhibition of NETosis and IL-17 release by exosomes is specific to complement-mediated activation of neutrophils, we treated neutrophils with another known neutrophil activator, phorbol myristate acetate (PMA), which activates neutrophils through the signal transduction enzyme protein kinase C [40]. PMA induced NETosis and IL-17 production at the transcript and protein levels in neutrophils, but MSC exosomes did not have any effect on these processes (Figure 3 A–D). Therefore, exosomes suppressed complement but not PMA-mediated activation of neutrophils. This suggested that exosomes exert their inhibition of complement-mediated neutrophil activation through the complement cascade and not through the neutrophil per se.
Figure 3.
Exosomes do not affect PMA-induced NETs and IL-17 release by neutrophils. (A) Representative images of NET formation by neutrophils PMA stimulation, in the presence or absence of different concentrations of exosomes. Cells were stained for nuclei (DAPI, blue) and H3 Citrullinate R2/8/17 (green). Scale bar, 25 µm. (B) Quantification of NET release by neutrophils pooled from three independent experiments performed using neutrophils from three unique donors. At least 100 cells were quantified from each experiment. The neutrophils used in these experiments were from different donors than those in Figures 1, 2 and 4. Data shown as mean ± SEM (n = 3). ***P < 0.0001, two-way ANOVA. (C) mRNA expression levels of il17a relative to hprt1 in neutrophils upon PMA stimulation, in the presence or absence of different concentrations of exosomes (n = 5). Five independent experiments were performed using neutrophils from five unique donors. The neutrophils used in these experiments were from different donors than those in Figures 1, 2 and 4. *P = 0.03, unpaired two-tailed Student's t test. (D) Neutrophils were stimulated with PMA in the presence or absence of different concentrations of exosomes, and culture supernatants were assayed for IL-17 by ELISA (n = 3). Three independent experiments were performed using neutrophils from three unique donors. **P = 0.01, unpaired two-tailed Student's t test. (Color version of figure is available online.)
MSC exosomes inhibit complement-induced neutrophil activation through a CD59-dependent mechanism
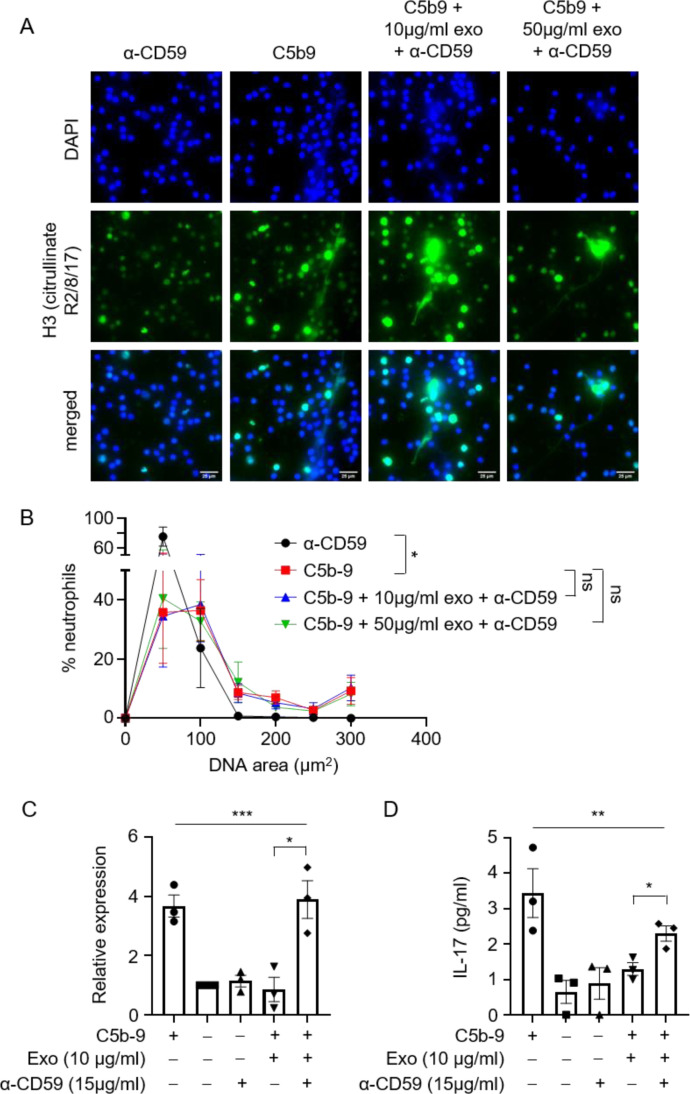
MSC exosomes have been reported to inhibit the formation of the terminal complement complex, C5b-9, through their expression of CD59 [41]. To investigate whether exosomal CD59 is required for exosome-mediated suppression of complement-induced neutrophil activation, a neutralizing antibody against CD59 was added to the mixture of activated complements and neutrophils. The anti-CD59 antibody abrogated the inhibitory effects of exosomes on complement-induced NETosis. Notably, the NETs released by neutrophils in the presence of exosomes and the anti-CD59 antibody were of comparable size to those found in the control group without exosome treatment (Figure 4 A, B). Consistent with this, the inhibition of IL-17 release by exosomes was also abolished by anti-CD59 antibody treatment (Figure 4C, D). Of note, anti-CD59 antibody alone does not trigger the release of NETs or IL-17, ruling out nonspecific effects of anti-CD59 antibody on neutrophil activation (Figure 4A–D). Together, these results indicate that inhibition of C5b-9 formation by CD59 on the MSC exosome abolished complement-induced neutrophil NETosis and IL-17.
Figure 4.
α-CD59 abolishes inhibitory effects of exosomes on C5b-9–induced NETosis and IL-17 production. (A) Representative images of NETs formation by neutrophils upon assembly of C5b-9, in the presence or absence of exosomes and α-CD59. Cells were stained for nuclei (DAPI, blue) and H3 Citrullinate R2/8/17 (green). Scale bar, 25 µm. (B) Quantification of NET release by neutrophils pooled from three independent experiments performed using neutrophils from four unique donors. At least 100 cells were quantified from each experiment. The neutrophils used in these experiments were from the same donors as those in Figures 1 and 2. Data shown as mean ± SEM (n = 4). *P = 0.02, two-way ANOVA. (C) mRNA expression levels of il17a relative to hprt1 in neutrophils upon assembly of C5b-9, in the presence or absence of exosomes and α-CD59 (n = 3). Three independent experiments were performed using neutrophils from three unique donors. The neutrophils used in these experiments were from the same donors as those in Figures 1 and 2. *P = 0.02, unpaired two-tailed Student's t test. ***P = 0.0003, one-way ANOVA. (D) C5b-9 were assembled on neutrophils in the presence or absence of exosomes and α-CD59, and culture supernatants were assayed for IL-17 by ELISA (n = 3). Three independent experiments were performed using neutrophils from three unique donors. The neutrophils used in these experiments were from the same donors as those in Figures 1 and 2. *P = 0.02, unpaired two-tailed Student's t test. **P = 0.004, one-way ANOVA. (Color version of figure is available online.)
Discussion
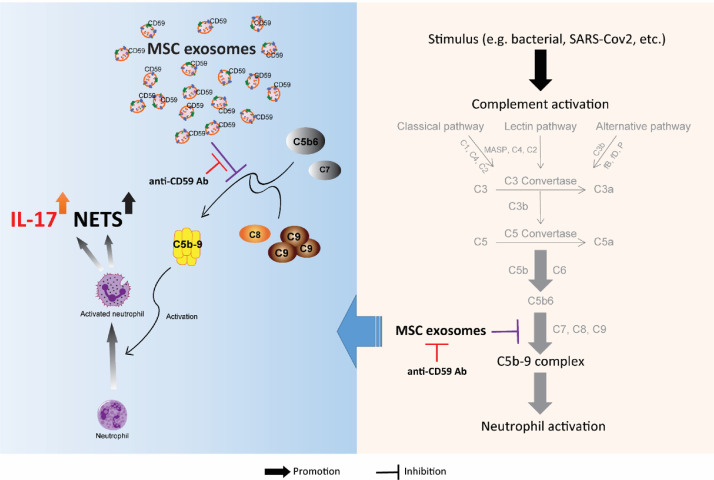
Hyperactivation of the complement system and neutrophilic immune responses have been associated with worse outcomes in COVID-19 patients [[10], [11], [12],16,23,28,35,42]. It remains unclear, however, whether these are two independent events or are interrelated components of a broader immune cascade. In this study, we demonstrated that initiation of the complement activation cascade induced NET release and IL-17 production by neutrophils. These neutrophilic responses were suppressed by MSC exosomes via a CD59-dependent mechanism (Figure 5 ). Because CD59 inhibits only the terminal step of the complement activation cascade, i.e., C5b-9 complex, our data also demonstrated that C5b-9, and not the other complement complexes, was the main effector of neutrophil activation. Collectively, our results provide a mechanistic basis for how exosomes can inhibit complement-induced neutrophil activation, potentially mitigating the vicious cycle that perpetuates inflammation in the lungs of severely ill COVID-19 patients.
Figure 5.
Schematic diagram showing MSC exosome inhibition of complement/neutrophil axis through the expression of CD59. (Color version of figure is available online.)
Clinical observations strongly indicate that patients with severe COVID-19 are predisposed to complement activation and dysregulated neutrophilic responses [24,25,43]. Interestingly, it has been reported that the sera from COVID-19 patients can trigger the release of NETs by healthy control neutrophils [24,44], consistent with a possibility that complements present in the sera can activate neutrophils[45]. In this study, we present novel findings on the cross-talk between the terminal complement complex and neutrophils. We showed that the assembly of complement complexes can directly trigger NET formation and IL-17 release by neutrophils. NETs are known to fuel pro-inflammatory immune responses, and their aggregation culminates in vascular occlusion and organ damage, whereas IL-17 has been reported to exacerbate inflammation and destruction of the lung parenchyma [37,46,47]. Hence, it is likely that the complement system and neutrophils form a feed-forward loop to escalate inflammation, tissue injury and thrombosis that, if left unchecked, would lead to coagulopathy, respiratory failure and other devasting complications observed in COVID-19.
MSC exosomes are an attractive cell-free therapeutic option to treat and manage symptoms associated with COVID-19, and they have demonstrated promising results toward alleviation of major COVID-19 complications such as cytokine storm and ARDS in a small but growing number of clinical trials [2,3]. The rationale for such a treatment approach was predicated on the superior regenerative and immunomodulatory capacity of MSC exosomes [3]. Exosomes have been shown to secrete anti-inflammatory cytokines that interact with immune cells such as T cells, B cells and macrophages, thereby preventing overactivation of the immune system. Furthermore, they can deliver various growth factors that help initiate tissue repair and regeneration during lung injury [48]. Together, these studies support the potential utility of exosomes as a treatment option for COVID-19 patients with severe complications. However, the precise therapeutic effect of exosomes on COVID-19 patients, and the exosome attribute responsible for mediating such an effect, have not been elucidated.
Here, we demonstrate for the first time that exosomes suppress complement-mediated neutrophil activation and inhibit the release of NETs and IL-17 by neutrophils (Figure 5). We identify CD59 as the MSC exosome attribute that mediates this suppression. We previously showed that MSC exosomes inhibit the assembly of terminal C5b-9 complement complex through CD59, a known inhibitor of the final stage in the complement activation cascade and an abundant MSC exosomal protein [41]. Consistent with the role of CD59 in mediating the inhibitory effect of MSC exosomes on neutrophil activation, this inhibitory effect was attenuated by a CD59-neutralizing antibody [41]. By inhibiting the formation of the terminal complement complex on neutrophils through CD59, MSC exosomes attenuated the activation of neutrophils and the manifestations of neutrophil activation, namely NETosis and IL-17 production. Significantly, we did not observe any inhibitory effect of exosomes on PMA-induced NETs and IL-17 release, indicating that MSC exosomes act specifically on complement-mediated activation of neutrophils. Together, these results also revealed for the first time that C5b-9, and not the other complement complex upstream of C5b-9, is the major complement complex that activates neutrophils.
In conclusion, our study elucidates a feed-forward loop between two key immunologic indicators of severe COVID-19, complements and neutrophils, and provides an overarching mechanistic context for the association of elevated complement activation, neutrophils, NETosis and IL-17 with adverse COVID-19 outcomes. This study also determines that MSC exosomes can specifically inhibit this complement/neutrophil axis through their expression of CD59 and provides a scientific rationale for use of MSC exosomes in alleviating the severe manifestations of critically ill COVID-19 patients.
Materials and Methods
Preparation of MSC exosomes
The exosome preparation used in this study was batch AC109 from an ongoing production process that has been in operation for >10 years and has manufactured >100 batches to date. This process was first described in 2010 [1]. The cell source was replaced with the immortalized cell line in 2013 [5], and the process was simplified as described below. Immortalized E1-MYC 16.3 human ESC-derived mesenchymal stem cells were cultured in DMEM with 10% fetal calf serum as previously described [49]. For MSC exosome preparation, the conditioned medium was prepared by growing 80% confluent cells in a chemically defined medium for 3 days as previously described [50], [51], [52]. The defined medium was prepared as follows: 480 mL DMEM (31053; Thermo Fisher), 5 mL NEAA (11140-050; Thermo Fisher), 5 mL l-glutamine (25030-081; Thermo Fisher), 5 mL sodium pyruvate (11360; Thermo Fisher), 5 mL ITS-X (51500-056; Thermo Fisher) and 0.5 mL 2-mercaptoethanol (21985-02; Thermo Fisher). This was supplemented with 0.1 mL basic fibroblast growth factor (bFGF; 0.5 ng/μL 0.2% BSA in PBS(+)) and 0.005 mL platelet-derived growth factor (PDGF; 100 ng/μL PBS(+)). The latter components were obtained as follows: bovine serum albumin (BSA; A9647; Sigma-Aldrich), PDGF (100-00; CYTOLAB), bFGF (13256-029; Thermo Fisher) and PBS(+) (14040-133; Thermo Fisher). The conditioned medium (CM) was size-fractionated by tangential flow filtration and then concentrated 50× using a membrane with a molecular weight cutoff (MWCO) of 100 kDa (Sartorius, Gottingen, Germany). The MSC exosome preparation was assayed for protein concentration using a Coomassie Plus (Bradford) Assay Kit (Thermo Fisher). The properties of these exosome preparations have been extensively assayed in accordance with Minimum Information for the Study of Extracellular Vesicles 2018 [6] and reported in our earlier papers. These preparations carry exosome-associated markers CD81, ALIX and TSG101 [[50], [53]], and the presence of CD81 on bi-lipid membrane vesicles was confirmed using transmission electron microscopy [54]. For this study, AC109 was assayed for the key identity and potency parameters for MSC-sEV preparations as recommended [7,8]. Specifically, the preparation had a protein concentration of 1.419 ± 0.034 mg/mL, particle concentration of 2.13 × 1011 ± 5 × 109 particles/mL, particles with a modal size of 125.1 ± 6.0 nm, cholesterol of 8.16 ± 0.32 ng cholesterol/µg protein, CD73/ecto-5′-nucleotidase activity of 27.04 ± 0.79 mU/µg protein and CD59 concentration of 3.15 ± 0.43 ng CD59/mg protein. The exosome preparation was filtered with a 0.22-μm filter (Merck Millipore, Billerica, MA) and stored in a −80°C freezer until use or lyophilized.
Isolation of neutrophils
Venous blood of healthy human donors was collected and diluted 1:1 in PBS before overlaying on Ficoll-Paque (GE Healthcare). After density gradient centrifugation, the polymorphonuclear and erythrocyte-rich pellet was collected, and cells were treated with red blood cell lysis buffer for 15 min at room temperature. Cells were subsequently washed with PBS, and neutrophils obtained were resuspended in PBS and rested at 37°C for ≥1 h.
Assembly of C5b-9
Neutrophils were washed and then resuspended in PBS with C5b-6 (0.125 µg/mL) and C7 (0.5 µg/mL) in the presence or absence of exosomes. The mixture was incubated at 37°C for 15 min before C8 (0.5 µg/mL) and C9 (0.5 µg/mL) were added with or without a CD59 blocking antibody (YTH53.1; Invitrogen) at 37°C for an additional 30-min incubation. As a negative control, C9 was omitted.
Flow cytometry staining for C5b-9
Neutrophils were stained with anti-C5b-9 (aE11; Novus Biologicals), followed by anti-mouse FITC (Sigma-Aldrich), in staining buffer (PBS containing 1% BSA) at 4°C for 1 h. Data were acquired using LSRII (BD Biosciences) and analyzed using FlowJo software (TreeStar).
PMA stimulation
Neutrophils were stimulated with 10 ng/mL PMA, in the presence or absence of exosomes, for 30 min at 37°C.
NETosis assay
Neutrophils were seeded onto poly-l-lysine–coated 48-well plates and allowed to adhere for 30 min at 37°C. The cells were then stimulated with complement components or PMA as described above. Cells were then fixed in 2% paraformaldehyde at room temperature for 15 min and stained with anti-histone H3 (citrulline R2+R8+R17) antibody (ab5103; Abcam) and DAPI before visualization with a microscope (EVOS). DNA area was measured using DAPI staining in ImageJ software. Image acquisition and analysis were performed by a blinded investigator. At least 100 cells in each experimental group were analyzed.
IL-17 ELISA
2 × 106 neutrophils were stimulated with C5b-9 or PMA as described above. Culture supernatants were collected after 4 h, and IL-17 was detected by human IL-17A ELISA kit (ab216167; Abcam) according to the manufacturer's protocol.
Quantitative PCR
Purified neutrophils were lysed with TRIzol (Gibco, Thermo Fisher), and RNA was purified using phenol/chloroform extraction. Complementary DNA was reversed transcribed using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher). The following primers were used for real-time PCR using SYBR Green PCR Master Mix (Applied Biosystems):
Il17a (forward): TCCCACGAAATCCAGGATGC
Il17a (reverse): GGATGTTCAGGTTGACCATCAC
s100a8 (forward): GGGCATCATGTTGACCGAGC
s100a8 (reverse): GTAACTCAGCTACTCTTTGTGGCTT
s100a9 (forward): CGATGACTTGCAAAATGTCGCAG
s100a9 (reverse): GCCACTGTGGTCTTAGGGGGT
hprt1 (forward): GAAAAGGACCCCACGAAGTGT
hprt1 (reverse): AGTCAAGGGCATATCCTACAACA
The mRNA expression of il17a, s100a8 and s100a9 were normalized to hprt1 using the 2−ΔΔCt method.
Statistics
Figures and statistical analyses were generated using GraphPad Prism software. For NETosis analyses, two-way ANOVA was performed. For other analyses, 1-way ANOVA or unpaired two-tailed Student's t test was performed. A P value of <0.05 was considered significant.
Study approval
Human blood was obtained for research with approval from the Centralised Institutional Research Board of the Singapore Health Services in Singapore.
Funding
This work was funded by institutional core funds from A*STAR and IAF-PP (H19H6a0026, TEx2Pharm) to SKL and KPL.
Author Contributions
JTL, BZ, KPL and SKL were involved in the conceptualization and design of study. JTL and JKHT performed the experiments. ABHC produced the MSC exosomes, which were characterized by RCL. JTL, BZ, KPL and SKL analyzed and interpreted the data. The manuscript was drafted by JTL and revised by BZ, KPL and SKL. All authors have approved the final article.
Declaration of Competing Interests
SKL holds founding shares in Paracrine Therapeutics.
Acknowledgments
Human PBMCs, from which neutrophils were isolated, are provided by Singapore Health Science Authority (HSA) under project no: 201306-05 to KPL (SIgN).
References
- 1.Börger V, Weiss DJ, Anderson JD, Borràs FE, Bussolati B, Carter DRF, et al. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy. 2020;22:482–485. doi: 10.1016/j.jcyt.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Kashte S, Gupta M, Rodriguez HC, Gautam SS, Kadam S. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Human Cell. 2020;33:907–918. doi: 10.1007/s13577-020-00407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazini L, Rochette L, Malka G. Exosomes contribution in COVID-19 patients’ treatment. Journal of Translational Medicine. 2021;19:234. doi: 10.1186/s12967-021-02884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. The Lancet Respiratory Medicine. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb BJ, Peltan ID, Jensen P, Hoda D, Hunter B, Silver A, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. The Lancet Rheumatology. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LYC, Quach TTT. COVID-19 cytokine storm syndrome: a threshold concept. The Lancet Microbe. 2021;2:e49–e50. doi: 10.1016/S2666-5247(20)30223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson JG, Simpson LJ, Ferreira A-M, Rustagi A, Roque J, Asuni A, et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5 doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reis ES, Mastellos DC, Hajishengallis G, Lambris JD. New insights into the immune functions of complement. Nature Reviews Immunology. 2019;19:503–516. doi: 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Research. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Sahu SK, Cano M, Kuppuswamy V, Bajwa J, McPhatter J, et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Science Immunology. 2021;6:eabh2259. doi: 10.1126/sciimmunol.abh2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Java A, Apicelli AJ, Liszewski MK, Coler-Reilly A, Atkinson JP, Kim AH, et al. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5 doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risitano AM, Mastellos DC, Huber-Lang M, Yancopoulou D, Garlanda C, Ciceri F, et al. Complement as a target in COVID-19? Nature Reviews Immunology. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Translational Research : The Journal of Laboratory and Clinical Medicine. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holter JC, Pischke SE, de Boer E, Lind A, Jenum S, Holten AR, et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proceedings of the National Academy of Sciences. 2020;117:25018. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Chen Z. An update: the emerging evidence of complement involvement in COVID-19. Medical Microbiology and Immunology. 2021;210:101–109. doi: 10.1007/s00430-021-00704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvelli J, Demaria O, Vély F, Batista L, Chouaki Benmansour N, Fares J, et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588:146–150. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelek WM, Cole J, Ponsford MJ, Harrison RA, Schroeder BE, Webb N, et al. Complement Inhibition with the C5 Blocker LFG316 in Severe COVID-19. American Journal of Respiratory and Critical Care Medicine. 2020;202:1304–1308. doi: 10.1164/rccm.202007-2778LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annane D, Heming N, Grimaldi-Bensouda L, Frémeaux-Bacchi V, Vigan M, Roux A-L, et al. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlaar APJ, de Bruin S, Busch M, Timmermans SAMEG, van Zeggeren IE, Koning R, et al. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. The Lancet Rheumatology. 2020;2:e764–e773. doi: 10.1016/S2665-9913(20)30341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastaglio S, Ruggeri A, Risitano AM, Angelillo P, Yancopoulou D, Mastellos DC, et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clinical Immunology (Orlando, Fla) 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackermann M, Anders H-J, Bilyy R, Bowlin GL, Daniel C, de Lorenzo R, et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death & Differentiation. 2021 doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathologySARS-CoV-2 directly triggers ACE-dependent NETs. Journal of Experimental Medicine. 2020;217 doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reusch N, de Domenico E, Bonaguro L, Schulte-Schrepping J, Baßler K, Schultze JL, et al. Neutrophils in COVID-19. Frontiers in Immunology. 2021;12 doi: 10.3389/fimmu.2021.652470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazeldine J, Lord JM. Neutrophils and COVID-19: Active Participants and Rational Therapeutic Targets. Frontiers in Immunology. 2021;12:2097. doi: 10.3389/fimmu.2021.680134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meizlish ML, Pine AB, Bishai JD, Goshua G, Nadelmann ER, Simonov M, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Advances. 2021;5:1164–1177. doi: 10.1182/bloodadvances.2020003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lévy Y, Wiedemann A, Hejblum BP, Durand M, Lefebvre C, Surénaud M, et al. CD177, a specific marker of neutrophil activation, is associated with coronavirus disease 2019 severity and death. IScience. 2021;24 doi: 10.1016/j.isci.2021.102711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price PJR, Bánki Z, Scheideler A, Stoiber H, Verschoor A, Sutter G, et al. Complement Component C5 Recruits Neutrophils in the Absence of C3 during Respiratory Infection with Modified Vaccinia Virus Ankara. The Journal of Immunology. 2015;194:1164. doi: 10.4049/jimmunol.1301410. [DOI] [PubMed] [Google Scholar]
- 30.Vandendriessche S, Cambier S, Proost P, Marques PE. Complement Receptors and Their Role in Leukocyte Recruitment and Phagocytosis. Frontiers in Cell and Developmental Biology. 2021;9:144. doi: 10.3389/fcell.2021.624025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strich JR, Ramos-Benitez MJ, Randazzo D, Stein SR, Babyak A, Davey RT, et al. Fostamatinib Inhibits Neutrophils Extracellular Traps Induced by COVID-19 Patient Plasma: A Potential Therapeutic. The Journal of Infectious Diseases. 2021;223:981–984. doi: 10.1093/infdis/jiaa789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delaveris CS, Wilk AJ, Riley NM, Stark JC, Yang SS, Rogers AJ, et al. Synthetic Siglec-9 Agonists Inhibit Neutrophil Activation Associated with COVID-19. ACS Central Science. 2021;7:650–657. doi: 10.1021/acscentsci.0c01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvin A, Chapuis N, Dunsmore G, Goubet A-G, Dubuisson A, Derosa L, et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell. 2020;182:1401–1418. doi: 10.1016/j.cell.2020.08.002. .e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahler M, Meroni P-L, Infantino M, Buhler KA, Fritzler MJ. Circulating Calprotectin as a Biomarker of COVID-19 Severity. Expert Review of Clinical Immunology. 2021;17:431–443. doi: 10.1080/1744666X.2021.1905526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Q, Zhao Y, Li J, Liu J, Yang X, Guo X, et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host & Microbe. 2021;29:222–235. doi: 10.1016/j.chom.2020.12.016. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacha O, Sallman MA, Evans SE. COVID-19: a case for inhibiting IL-17? Nature Reviews Immunology. 2020;20:345–346. doi: 10.1038/s41577-020-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibabaw T. Inflammatory Cytokine: IL-17A Signaling Pathway in Patients Present with COVID-19 and Current Treatment Strategy. Journal of Inflammation Research. 2020;13:673–680. doi: 10.2147/JIR.S278335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. Journal of Microbiology, Immunology and Infection. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal Stem Cells Secrete Immunologically Active Exosomes. Stem Cells and Development. 2013;23:1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 40.Robinson PJ. Differential stimulation of protein kinase C activity by phorbol ester or calcium/phosphatidylserine in vitro and in intact synaptosomes. Journal of Biological Chemistry. 1992;267:21637–21644. doi: 10.1016/S0021-9258(19)36659-1. [DOI] [PubMed] [Google Scholar]
- 41.Lai RC, Yeo RWY, Tan SS, Zhang B, Yin Y, Sze NSK, et al. Mesenchymal stem cell exosomes: the future MSC-based therapy? Mesenchymal stem cell therapy. 2013:39–61. Springer. [Google Scholar]
- 42.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney International. 2020;98:314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye W, Chen G, Li X, Lan X, Ji C, Hou M, et al. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respiratory Research. 2020;21:169. doi: 10.1186/s12931-020-01428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Middleton EA, He X-Y, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. The Journal of Clinical Investigation. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mutua V, Gershwin LJ. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clinical Reviews in Allergy & Immunology. 2021;61:194–211. doi: 10.1007/s12016-020-08804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. Journal of Immunology (Baltimore, Md : 1950) 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochemical Society Transactions. 2018;46:843–853. doi: 10.1042/BST20180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo ABH, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. Journal of Translational Medicine. 2011;9:47. doi: 10.1186/1479-5876-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Lai RC, Arslan F, Tan SS, Tan B, Choo A, Lee MM, et al. Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles. Journal of Molecular and Cellular Cardiology. 2010;48:1215–1224. doi: 10.1016/j.yjmcc.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 52.Sze SK, de Kleijn DP v, Lai RC, Tan EKW, Zhao H, Yeo KS, et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Molecular & Cellular Proteomics. 2007;6:1680–1689. doi: 10.1074/mcp.M600393-MCP200. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis and Cartilage. 2016;24(12):2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 54.Accarie A, I’Homme B, Benadjaoud MA, Lim SK, Guha C, Benderitter M, et al. Extracellular vesicles derived from mesenchymal stromal cells mitigate intestinal toxicity in a mouse model of acute radiation syndrome. Stem Cell Research & Therapy. 2020;371 doi: 10.1186/s13287-020-01887-1. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]