Abstract
Mesenchymal stem cells (MSCs), multipotent stromal cells, have attracted extensive attention in the field of regenerative medicine and cell therapy due to the capacity of self-renewal, multilineage differentiation, and immune regulation. MSCs have different cellular effects in different diseases, and even have markedly different curative effects with different tissue sources, indicating the plasticity of MSCs. The phenotypes, secreted factors, and proliferative, migratory, differentiating, and immunomodulatory effects of MSCs depend on certain mediators present in their microenvironment. Understanding microenvironmental factors and their internal mechanisms in MSC responses may help in subsequent prediction and improvement of clinical benefits. This review highlighted the recent advances in MSC plasticity in the physiological and pathological microenvironment and multiple microenvironmental factors regulating MSC plasticity. It also highlighted some progress in the underlying molecular mechanisms of MSC remodeling in the microenvironment. It might provide references for the improvement in vitro culture of MSCs, clinical application, and in vivo induction.
Keywords: Immune regulation, Mesenchymal stem cells, Microenvironment factors, Molecular mechanism, Multidirectional differentiation, Plasticity
Introduction
Mesenchymal stem cells (MSCs) have been widely studied since their discovery in the late 1960s.1 As adult stem cells, they have high potential in the field of regenerative medicine and disease treatment due to their multiple differentiation potential, immunomodulatory function, and characteristics of promoting tissue and organ repair. Boada et al found that the volume of MSCs increases and the number of branches decreases in myelodysplastic syndrome (MDS), while producing higher levels of interleukin (IL)-6, IL-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α.2 A study on colitis also reported anti-inflammatory responses mediated by chemokine (C–C motif) ligand (CCL)-2 and chemokine (C-X-C motif) ligand (CXCL)-12 derived from adoptively transferred MSCs.3 Numerous studies have shown the changes and differences in the phenotype and functions of MSCs under pathological or physiological conditions, suggesting the plasticity of MSCs, namely the ability to change. The plasticity of MSCs is the foundation of clinical applications, making it play different therapeutic roles in different diseases.
The factors regulating the plasticity of MSCs and their mechanism of action have triggered further explorations. In view of the physiological structure of MSCs as stromal cells, the complexity of the microenvironment may be one of the important reasons for MSCs to have a variety of stem cell characteristics, so that they can adapt to the microenvironment and change their phenotype and functions. Based on this concept, various studies were performed on the influencing factors in the microenvironment acting on MSCs. A study on the osteogenic differentiation of MSCs by Wan showed that higher matrix rigidity induced the osteogenic bias of MSCs, while IL-β inhibited osteogenic differentiation.4 Liu found that the production of miR-126 in MSC exosomes increased during hypoxia treatment.5 These results confirmed the significance of the influence of the microenvironment on the plasticity of MSCs. Different intrinsic factors comprehensively induce changes in MSCs through different mechanisms. Therefore, the plasticity manifestations of MSCs (phenotype, proliferation, apoptosis, migration, differentiation, paracrine, etc.), microenvironmental factors (physical, chemical, and biological factors) that affect plasticity, and molecular mechanisms related to MSC plasticity were reviewed. The review might provide an important reference for the selection of MSC tissue sources, clinical function prediction, optimization of characteristics and functions in vitro, in vivo effect induction, and other clinical practical applications.
Plasticity of MSCs
Many recent studies explored and demonstrated the changes in various characteristics and functions of MSCs in different contexts, including cell phenotype, proliferation, differentiation, migration, apoptosis, factor secretion, and so forth. The ability of MSCs to change is called plasticity. The findings on MSC plasticity in recent years are summarized in the following sections.
Variations in morphology and phenotype
Changes in MSC morphology and surface marker expression are the most intuitive plasticity phenomena. Studies related to aging showed an increase in the volume of MSCs,6 reduction in the expression of TNF receptor (TNFR), IFN gamma receptor (IFNGR), and chemokine receptor (CCR)-7.7 MSCs are biased toward senescence-like phenotypes in various diseases. In systemic lupus erythematosus (SLE), the expansion, distortion, and swelling of MSCs, cytoskeletal tissue disorder, and abnormal F-actin skeleton have been observed.8 In obesity, the bone marrow-derived MSC (BM-MSC) molecular phenotype shifts to specific adipocyte progenitor cells, the abundance of insulin receptor–positive (IR+) and leptin receptor–positive (LEPR+) cells increases, and the overactivated metabolic state is accompanied by accelerated aging.9 In multiple myeloma (MM), a transition from MSCs to an aging-like phenotype is also observed.10
The supporting or inhibitory role of MSCs in tumor therapy is closely related to the type of tumor.11 El-Badawy found that soluble factors of cancer cells induced a series of phenotypic changes in BM-MSCs, and their cytoskeleton shows greater variability. The surface of the cell produces many protrusions in the form of microbuds as well as obvious microvilli, which is similar to the surface of HeLa cancer cells. It is possible that CD44+CD24+ are transformed into the CD44+CD24low phenotype.12 However, a study on acute lymphoblastic leukemia (ALL) demonstrated that the upregulation of the specific T cell receptor (TCR) expression of MSCs promoted the anti-cancer effect of the body.13 In addition, in acute lung injury, MSCs and alveolar epithelium formed a gap junction channel of LX43.14 In a long-term in vitro culture, the expression of MSC markers reduced, and the morphology changed to fibroblasts with reduced proliferation.15
Physiological functions of MSC from different sources
MSCs from different tissues in the normal body have obvious differences in their physiological functions (proliferation, growth, migration, differentiation, paracrine, etc.). In terms of proliferation and growth characteristics, Calle compared MSCs derived from skin, abdomen, and subcutaneous adipose tissue, and found that the population doubling time and growth rate were different even if all MSCs originated from adipose tissue.16 The comparative studies on BM-MSCs and adipose tissue-derived MSCs (AD-MSCs) showed that the proliferation rate of AD-MSCs was 40 times that of BM-MSCs.17 Compared with adult BM-MSCs and AD-MSCs, MSCs derived from Wharton's jelly of the umbilical cord (WJ-MSCs) had higher proliferation potential and faster growth rate, with more passaging potential in vitro.18
In terms of differentiation, MSCs preferentially differentiate into the cell lineage of the source tissue.19 The comparative studies on BM-MSCs and AD-MSCs revealed that BM-MSCs had a higher ability to differentiate into chondrocytes, while AD-MSC differentiated into adipocytes.20 The studies also found that MSCs derived from the human umbilical cord (UC-MSCs) and the human amniotic fluid (hAF-MSCs) had a wider differentiation potential,17 while MSCs derived from the placenta had a lower potential for fat formation.7 Nguyen compared BM-MSCs with MSCs derived from the acetabulum and femur, and found that the bone marrow and femur had more calcium deposits under osteogenic differentiation conditions, while BM-MSCs had the main differentiation ability under cartilaginous and adipogenic differentiation conditions.21 Moreover, MSCs derived from the ilium and vertebral bone marrow had a stronger response to cartilage induction than those isolated from femoral heads.22
In addition, AD-MSCs had a stronger immunosuppressive effect and lower immunogenicity compared with BM-MSCs,20 while BM-MSCs had higher expression levels of transforming growth factor (TGF)-β1, prostaglandin E (PGE)-2, and IL-6.23 The mRNA expression in their secreted extracellular vesicles was less than 3%,7 while more than 1400 genes were differentially expressed.24 Compared with AD-MSCs and BM-MSCs, WJ-MSCs inhibited the mitogen-induced T-cell response to a greater extent,25 and MSCs derived from the umbilical cord matrix had the highest angiogenic capacity in vitro.26 UC-MSCs and MSCs derived from embryos had lower immunogenicity.17 In addition, the differential secretion of milk fat globule epidermal-growth factor (MFGE)-8 was found in BM-MSCs, UC-MSCs, and MSCs derived from embryos and teeth.27 Table 1 shows the functional differences in MSCs from different tissue sources.
Table 1.
Comparison of MSC functions from different tissue sources.
| English abbreviations | MSC source | Functional characteristics | References |
|---|---|---|---|
| BM-MSC | The bone marrow | Higher ability to differentiate into chondrocytes. The secretion group is rich in MFGE8; higher expression of TGF-β1, PGE2, and IL-6 than AD-MSC. |
20,23,27 |
| AD-MSC | The adipose tissue | The proliferation rate is higher than BM-MSC. Higher ability to differentiate into adipocytes. Immunosuppression is stronger than that of BM-MSC. The immunogenicity is lower than that of BM-MSC, and the expressions of VEGF, HGF, Nestin and neurotrophic factor are higher. |
17,20,23 |
| WJ-MSC | Wharton's jelly of umbilical cord | Compared with BM-MSC and AD-MSC, the proliferation potential and growth rate are higher, more passage potential in vitro, and the mitogen-induced T cell response is inhibited to a greater extent. | 18,25 |
| UC-MSC | Human umbilical cord | Wider differentiation potential. Lower immunogenicity. The secreted group is rich in MFGE8, and does not express the tumor-related fibroblast phenotype. It has stronger angiogenic capacity than BM-MSC and AD-MSC, and higher expression of PGE2 and IL-6 than AD-MSC. |
7,17,23,26,27 |
| Human embryo | Low immunogenicity. Not involved in proliferation. The secreted group do not contain MFGE8. |
17,27 | |
| hAF-MSC | Human Amniotic fluid | Higher proliferation rate than MSC from adult source. The ability to differentiate more widely than that of an adult-derived MSC. Compared with MSC separated from dermal, it shares stem related miRNAs, but is significantly different from fat-forming miRNAs. |
7,17 |
| The placenta | Less fat-forming potential. Good migration ability. |
7 | |
| SHED | Human exfoliated deciduous teeth | Higher proliferation capacity than BM-MSC. Secretion group is rich in MFGE8. |
17,27 |
Furthermore, extracellular matrix (ECM) is a complex noncellular network in all tissues and organs in which cells reside. Cell surface receptors transduce signals into cells from the ECM, which control diverse cellular functions and differentiation. BM-MSC- and AD-MSC-derived ECM can induce changes in the quantity, morphology, and function of MSCs.28 The proliferation is enhanced when the tissue origin of MSC matches the tissue origin of cultured ECM.29 Compared with the three-dimensional scaffold cultured MSCs, the combination of ECM and three-dimensional scaffold promoted the proliferation and growth of MSCs and better maintained the stemness.30
In addition to tissue origin, based on the practical limitations such as isolation and culture of natural MSCs, it has been extensively studied to induce differentiation of other types of stem cells to produce MSCs with sufficient quantity and better quality. At present, MSCs have been successfully obtained from induced pluripotent stem cells (iPSCs)31 and human embryonic stem cells (hESCs).32 It overcomes the limitations of adult MSC in practical applications, including MSC aging,33 batch differences,34 etc., and because of the difference in gene expression regulation between it and natural MSC, it may mediate a more effective therapeutic effect in the treatment of diseases.35 Recently, there have been reports of clinical application of iPSC-MSCs.36
Changes in secreted factors and differentiating, proliferative, migratory, and apoptotic abilities of MSCs
The effects of MSC-mediated immunosuppression or immunostimulation are closely related to the type and amount of inflammatory factors secreted. These factors control the migration, differentiation, and functions of immune cells by mediating the formation of anti-inflammatory or pro-inflammatory microenvironment. Some studies detected an induced increase in the levels of MSC anti-inflammatory factors. For example, an increase in the levels of anti-inflammatory cytokines was found in diabetes mellitus (DM)37 and experimental autoimmune encephalomyelitis (EAE).38 MSCs secreted indoleamine 2,3-dioxygenase (IDO), inhaled nitric oxide (iNO), PGE2, and IL-10 in lung injury.39 The immunosuppressive effects of MSCs have been reported in a variety of diseases such as neurodegenerative diseases,40 traumatic brain injury (TBI),41 DM,42 liver43/lung39/kidney44 injury, colitis,45 acute pancreatitis,46 MDS47 and other tissue injuries,48 primary Sjögren's syndrome,49 SLE50 and other autoimmune diseases, and allogeneic transplantation.51 In contrast, the upregulation of MSC pro-inflammatory factors has also been detected. For example, MSCs are induced to increase the expression of IL-1β, IL-6, and IL-32 in acute myeloid leukemia (AML).52 MSCs produce high levels of IL-6, IL-17, IFN-γ, and TNF-α in MDS,2 and the expression of IL-6 and IL-8 is also upregulated in MM.53 Pro-inflammatory phenotypic changes in MSCs were detected in the head and neck squamous cell carcinoma,54 MM,10 and other diseases. Kim concluded that acute inflammation induced the immunosuppressive effect of MSCs, while chronic inflammation caused inflammatory exacerbation.55 Studies on damaged MSCs, such as SLE8 and atherosclerosis,56 also demonstrated the induction of pro-inflammatory effects.
MSCs secrete several factors including growth factors, chemokines, angiogenesis factors, and other cytokines, which are also closely related to tissue repair and disease treatment. A general upregulation of vascular endothelial growth factor (VEGF) secreted by MSCs was observed in diseases with tissue and organ damage, such as muscle necrosis,57 superficial wounds,58 liver fibrosis,59 multiple-system atrophy (MSA),60 and nervous system diseases,61 while VEGF was significantly downregulated in tumors.62 MSCs also secreted many important neuromodulators such as neurotrophic factors in a variety of nerve-damaging diseases, such as amyotrophic lateral sclerosis, TBI,41 EAE,38 and MSA.60 They secreted high levels of anti-apoptotic factors in cardiovascular diseases, liver/lung injury,63 liver fibrosis,59 primary ovarian insufficiencies,64 and other diseases. They had high expression of different chemokines in AML,65 MM,53 and other diseases. Recent studies have reported on the use of engineered MSCs to produce therapeutic factors to mediate more effective therapeutic effects.66,67 In addition, the levels of miR-10a, miR-15a, and other miRNAs increased in MSC-secreted exosomes in MDS68; however, the levels of miR-15a in MSC exosomes were lower in MM, but the levels of oncogene proteins, cytokines, and adhesion molecules were higher.69 Mitochondrial metastasis in MSCs was observed in acute respiratory distress syndrome, cardiomyopathy, corneal epithelial cell damage, and infectious diseases.70
MSCs can perform the function of tissue repair by differentiating into cells of different lineages, although the percentage of MSCs differentiated into tissue cells during treatment is very limited and the therapeutic effect of MSCs is mainly realized through paracrine rather than cell differentiation.71, 72, 73 The differentiation of MSCs into osteoblasts14 or cartilage74 is observed in bone damage. In neurological diseases, MSC can differentiate into neuron-like cells and glial cells.61 In retinal degenerative diseases, MSC can differentiate into retinal pigment epithelial cells and photoreceptor cells.75 However, abnormal or even impaired regulation of MSC differentiation has been detected in abnormal conditions such as diseases. MSCs are induced to differentiate into cancer-associated fibroblasts in cancer.76 The differentiation ability of MSCs is impaired to varying degrees in aging,77 obesity,20 SLE,8 and liver injury.59 An increase in adipogenesis and the inhibition of osteogenesis in MSCs have been found in MM and osteoporosis,10 but some studies showed an overall decline in the differentiation potential of MSCs in MM.78 Short-term inflammation can induce MSCs to increase bone formation, while the effect of long-term inflammation is opposite.79
Different pathological environments have different effects on the physiological functions of MSCs, such as growth, proliferation, migration, and even apoptosis, which also depends on the type and severity of the disease. The proliferation of MSCs is significantly downregulated in MM,75 AML,80 SLE,8 and senescence,74 while the proliferation of MSCs increases in obesity.81 The expression of MSC surface chemokine receptor is upregulated and the migration ability increases in tumors,82 DM,42 lung39/liver83 damage, and other pathological conditions, but the migration ability of MSCs is impaired in acute kidney injury (AKI), chronic kidney disease,84 and aging.20 An increase in the proportion of apoptosis of MSCs is found in patients with SLE.8 Colmegna found an increase in apoptosis and a decreased ability of MSCs to cope with stress in their study on atherosclerosis.56 In addition, MSC metabolic pathways and endocytosis are dysregulated in AML,85 and MSC chemoresistance is achieved in experiments related to cancer cell soluble factors.12 MSCs have plasticity under different pathological conditions. Thus, the possible functional changes in MSCs under different disease backgrounds should be explored and clarified before the clinical application of MSCs.
Mechanisms of the effect of microenvironment on MSC plasticity
The complexity of the microenvironment in which MSC is located may be one of the important reasons for its plasticity. The changes in the characteristics and functions of MSCs cannot be separated from the induction of the microenvironment. Hence, further analysis of the reasons affecting the plasticity of MSCs from the perspective of the microenvironment, understanding of specific factors acting on them in the microenvironment, and investigation of their effects and internal mechanisms can facilitate more accurate regulation of MSCs and help predict the effects of complex microenvironment on them.
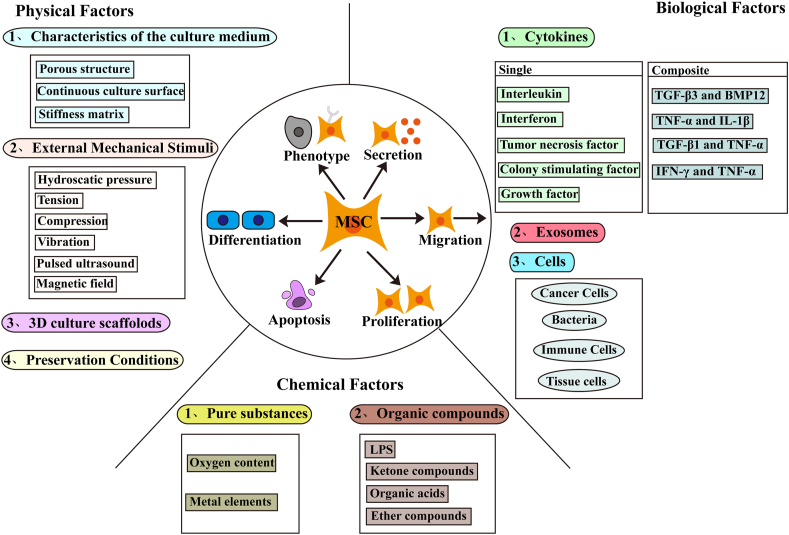
Physical factors
In terms of the characteristics of the culture medium, the three-dimensional foam with a porous and interconnected structure (>90%) and a low elastic modulus can support the survival, quiescence, and basal secretory activity of MSCs.86 Compared with the flat substrate, the porous structure can not only promote MSC osteogenesis but also upregulate the expression of stem-like markers.87 A continuous culture surface can promote the expression of MSC osteogenic differentiation markers, while a discontinuous culture surface is associated with low expression of osteogenic or adipogenic differentiation markers.88 The high stiffness matrix can support MSC bone formation, the soft substrate promotes neurogenesis, and the medium stiffness helps in myogenesis.4
External mechanical stimulation, such as hydrostatic pressure, tension, compression, vibration, pulsed ultrasound,89 magnetic field,90 and so forth, can promote osteogenesis and/or cartilage differentiation; tension can also promote the myogenic differentiation of MSCs. The low-frequency magnetic field (7.5 Hz, 0.4 T) can inhibit the adipogenic differentiation of MSCs. Besides differentiation, other functional changes in MSCs are also involved. For example, pulsed focused ultrasound,17 extracorporeal shock wave, and ultrasound-targeted microbubble destruction91 can all improve the homing of MSCs, while heat shock can enhance the survival of MSCs.44
Many recent studies tried to construct MSC 3D culture scaffolds with various materials, which not only supported the survival of MSCs,92,93 but also had the ability to improve cell adhesion91 and maintain cell stemness.94 Some scaffolds were involved in the regulation of MSC differentiation outcome, including the induction of musculoskeletal lineage.95
Preservation conditions also affect the quality of MSCs. The cryopreservation of umbilical cord tissue can maintain the diversity of MSCs. Repeated cryopreservation, with population doubling time similar to that of initial freeze-thaw, has no effect.96 However, freeze-thaw had a certain effect on the whole gene expression profile of MSCs.97
Chemical factors
Studies on the influence of chemical factors in the microenvironment on MSC plasticity focused on pure substances. As far as simple substances are concerned, the research on the oxygen content is the most extensive. As a common feature in the microenvironment of a variety of diseases or normal tissues,98 appropriate hypoxic pretreatment (2% and 5% hypoxia treatments are more common) can contribute to the survival and function of MSCs, which is manifested in the improvement in proliferation, differentiation, homing, and paracrine function.99 However, it should not be ignored that hypoxia-treated MSCs promote the proliferation and growth of liver cancer cells in the xenotransplantation model.100 The protein expression in MSCs under hypoxia is significantly different from that under normoxia,7 and hypoxia increases the expression of pluripotent markers of WJ-MSCs.99 Few studies have been performed on hyperoxic treatment,69 and currently more studies are available on the synergistic regulation of oxygen environmental treatment combined with other factors.91 In addition, reactive oxygen species (ROS) cause damage to MSCs, showing increased apoptosis, osteogenic inhibition, and adipogenic accumulation.101 Studies also explored elemental metal elements. For example, magnesium can improve osteogenesis and tissue mineralization in a dose-dependent manner,91 while the lithium treatment of senescent MSCs can induce an increase in the ectopic expression of β-catenin and promote myogenic differentiation.102
Organic compounds are more extensively studied than inorganic compounds. Among organic compounds, lipopolysaccharide (LPS) is the most commonly studied. It can reduce hypoxia- and nutrient-induced apoptosis and increase the expression of secreted proteins, including VEGF, hepatocyte growth factor (HGF), fibroblast growth factor, insulin-like growth factor (IGF), and so forth.103 Previous studies showed that the immunosuppressive effect of LPS-induced MSCs was up-regulated, and miRNA let-7b rich in exosomes could regulate the polarization of macrophages to M2.104 However, some studies also demonstrated that LPS led to immune enhancement by activating MSC Toll-like receptor (TLR)-4 and produced low levels of NO and IDO.55 In addition, studies related to ketone compounds demonstrated that ginsenoside Rg1 positively regulated the proliferation and multiple differentiation of MSCs,105 and the treatment using rapamycin,91 icariside II, and genistein induced the osteogenic differentiation of MSCs. Isorhamnetin (IsR) can promote the formation of fat, and the treatment using atractyloides promotes the formation of cartilage.106 As far as immune regulation is concerned, chlorzoxazones (CZ) can promote the immunosuppressive function of MSCs by regulating the phosphorylation of forkhead box O3 (FOXO3),107 and progesterone can improve the ability of immune regulation by upregulating the expression of PGE2 and IL-6.108 A study on organic acids found that poly-l-lysine increased the proliferation, growth, differentiation, and adhesion of MSCs,109 valproic acid significantly increased the migration ability of MSCs,110 and fatty acid (FFA) promoted adipogenic differentiation of MSCs.101 Atorvastatin treatment upregulated the expression of lncRNA H19 in MSC exosomes to promote their repair function.111 As far as the studies on ether compounds are concerned, sevoflurane can minimize MSC apoptosis during hypoxia and significantly enhance their migration ability.91 Medicarpin treatment promotes the osteogenesis of MSCs.106 In addition, compounds such as alcohols, phenols, alkanes, esters, and so forth, are involved in this process. For example, sphingosine-1-phosphate can upregulate the migration ability of MSCs and increase homing,110 while the treatment of 2,4-dinitrophenol in vivo renders MSC higher adhesion and viability.63
Cytokines
Cytokines are roughly divided into six categories according to their functions. Among them, interleukin-related studies found that IL-1α,55 IL-1β,112 and IL-2113 induced the immunosuppressive phenotype of MSCs. Previous studies showed that IL-1β increased the expression of MSC cyclooxygenase (Cox)-2, IL-6, and IL-8, which in turn affected the polarization of macrophages.112 However, the results of studies on the effects of migration and osteogenic differentiation were not uniform.4,16,91 The related studies on IL-363 and IL-17114 showed that both promoted the osteoinduction of MSCs. Interferon research found that IFN-γ increased the immunosuppressive activity,115 increased migration, and inhibited proliferation and differentiation of MSCs.7 TNF-α induced MSC immunosuppression and promoted the survival, proliferation,63 migration, and osteogenic differentiation of MSCs,7 but a few studies suggested that TNF-α induced a decline in MSC immunomodulatory properties.116 A study on colony-stimulating factor (CSF) found that G-CSF significantly downregulated the secretion of PGE2 by MSCs and promoted their migration.117 VEGF induced MSC neural differentiation14 and promoted migration,63 while stromal cell–derived factor 1 upregulated MSC migration, survival, proliferation, and paracrine function.91 In addition, TGF-β, bone morphogenetic protein (BMP),91 and epidermal growth factor (EGF)77 have been shown to effectively enhance the osteogenic induction of MSCs. Insulin growth factor-1 (IGF-1) and hypoxia-inducible factor-1α (HIF-1α) enhanced the migration ability of MSCs by promoting the expression of chemokine receptors.110 Previous studies found that TGF-β3 in the decidua of patients with preeclampsia (PE) induced decidual-derived MSCs (dMSCs) to reduce the secretion of PGE2 and cause immune imbalance.118
The comprehensive effect of multiple cytokines in the real microenvironment is the trend of research at present. For example, the combined action of TGF-β3 and BMP12 can induce the generation of the tendon phenotype of MSCs.119 Pretreatment of TNF-α and IL-1β can enhance the repairing effect of MSCs on lung epithelium.103 TGF-β1 and TNF-α can synergistically induce the expression of CCL2, CXCL8, and Cox-2.120 In addition, the concentration of cytokines is also one of the important factors for exploring the plasticity of MSCs. For example, the survival and differentiation of MSCs are severely impaired under the conditions of CK50/20 (50/20 ng/mL for IFN-γ and TNF-α).121 However, under the CK5 condition (5 ng/mL for IFN-γ and TNF-α), MSCs not only maintain their vitality and differentiation but also show enhanced immune-regulatory characteristics.122
Exosomes and cells
Exosomes are another way of interaction between cells. Studies have found that exosomes produced by tumor cells can deliver proteins and genetic material to MSCs and induce MSCs to transform into tumorigenic MSCs, which become a producer of factors necessary for tumor growth, thus directly or indirectly enhancing their tumor-promoting function.123 For example, exosomes secreted by human hepatoma HepG2 cells,124 ovarian cancer cells,125 chronic myeloid leukemia K562 cells,126 and MM cells53 can induce MSCs to support the proliferative, growth, or migratory and invasive abilities of tumors in different ways. However, a previous study also showed that gastric cancer cell line AGS-derived exosomes enhanced the abilities of MSCs to activate immune cells and maintain an inflammatory environment.127
Compared with cytokines and exosomes, the microenvironmental factors involved in cell co-cultivation are more complex but real. The co-cultivation of MSC and cancer cells is still a research hotspot. In this environment, changes in the morphology, growth, differentiation, and other characteristics and functions of MSCs have been found,128 besides MSC mitochondrial transfer to cancer cells129 and the fusion of cancer cells and MSCs to form cancer hybrid cells,130 which are closely related to the type of cancer cells. The co-culture of osteosarcoma (OS) cells,131 breast cancer cells,132 leukemia stem cells (LSC)65 with MSCs revealed that MSCs promoted the occurrence and development of tumors in different ways. Further, bacteria were shown to increase the absolute number of MSCs and maintain the plasticity of MSCs. Even the inactivated bacteria could increase the proliferation of MSCs, improve the immune regulation properties, and increase the expression of anti-inflammatory factors.133
The research on MSCs and autologous normal cells mainly focuses on immune cells and tissue cells. The co-cultivation of immune cells has revealed that monocytes/macrophages can inhibit the osteogenic differentiation of MSCs,134 and monocytes,135 macrophages M1, Tregs,14 and activated CD4+ cells136 play an immunosuppressive role by regulating the secretion of MSC proteins. In vitro co-cultivation experiments also found a dose-dependent transfer of MSC mitochondria to peripheral blood mononuclear cells (PBMC) (especially CD4+T cells), which seems to be able to induce CD4+T cells to reprogram into a highly inhibitory population.137 Co-culture with endothelial cells,14 stromal vascular fraction cells,138 and human gingival fibroblasts can induce multiple differentiation of MSCs.139 Amniotic epithelial cells110 and freeze-dried platelets140 can promote the proliferation of MSCs. At present, it has been found that some pro-inflammatory factors seem to be able to induce the occurrence of MSC mitochondrial transfer.141 In diabetic nephropathy,142 asthma143 and other disease models, mitochondrial transfer in MSCs was found in co-culture studies with damaged epithelial cells, and the phenomenon that retinal ganglion cells obtained mitochondria from MSCs was also found in studies related to nervous system diseases.144 Fig. 1 summarizes the specific physical, chemical, and biological factors that regulate MSC plasticity in the microenvironment.
Figure 1.
Specific factors involved in the regulation of MSC plasticity in microenvironment.
Signaling pathways
Studies on the signaling pathways of the microenvironment regulating MSC plasticity are relatively extensive and complex. Several common and widely studied signaling pathways are introduced in the following text.
The classical Wnt signaling pathway is involved in the osteogenic and chondrogenic differentiation and adipogenic inhibition of MSCs, while the nonclassical Wnt pathways have a wide range of roles in bone development and bone metabolism, such as the activation of histone methyltransferase that inhibits peroxisome proliferator-activated receptors (PPAR)-γ transactivation through the H3K9 methylation of their target genes.145 Benzyl butyl ester (BBP) in endocrine-disrupting chemicals (EDC) can reduce H3K9 methylation in the nonclassical Wnt pathway, inducing MSC adipogenic differentiation and inhibition of bone formation.79 The accumulation of ROS activates the redox-sensitive factor FOXO. The transfer of β-catenin to FOXO-mediated transcription leads to reduced signal transduction of Wnt/β-catenin.101 SKL2001 increases the expression level of β-catenin in the cytoplasm and nucleus by inhibiting β-catenin phosphorylation and hindering its interaction with the degradation medium β-transducin repeat-containing protein. The addition of Ginkgo biloba extract significantly enhances calcium deposition and alkaline phosphatase (ALP) activity, and can reduce lipid accumulation and inhibit the expression of fat-forming genes.106 Previous studies demonstrated that the extremely low-frequency magnetic field inhibited the adipogenic differentiation of MSCs through the Jun N-terminal kinase (JNK)-dependent Wnt signaling pathway, and the activation of the nonclassical Wnt pathway caused the inhibition of PPARγ2 expression.90 They also found that CXCL16 induced by Wnt5a-Ror2 signaling in MSC promoted the migration and proliferation of MKN45 gastric cancer cells,146 and the Wnt/β-catenin pathway was also involved in the development of skeletal abnormalities, including endogenous chondroma and OS.147
The mitogen-activated protein kinases (MAPK) signaling pathway is involved in regulating various cellular behaviors of MSCs. It also can regulate the osteogenic and chondrogenic differentiation of MSCs. Two-dimensional nanosilicates can activate the MAPK cascade in MSCs, especially the ERK and p38 pathways. Further, the levels of p-MAPK and ERK kinase (MEK)-1/2 increase more than sixfold after nanosilicate treatment, and various genes are expressed differently, including thousand-and-one amino acid kinases 1 and MEK1/2 upstream and downstream genes and a large number of genes related to osteogenic and chondrogenic differentiation.148 Matrix stiffness–induced MSC osteogenesis is attributed to the high phosphorylation level of ERK1/2. IL-1β can activate ERK1/2 and p38 signals in hMSCs, but the inhibition of p38 signal is more significant than the promotion of ERK1/2 signal during osteogenic differentiation; even under the condition of high matrix stiffness, the osteogenic effect is almost negligible.4 After icariin (ICA) treatment, MAPK signaling molecules, including ERK and JNK, are phosphorylated, and the expression of genes related to osteogenesis increases in a dose-dependent manner.149
The TGF-β/Smad signaling pathway is also involved in the induction of MSC osteogenesis and chondrogenesis, of which BMP is the most studied. It is also involved in the regulation of the expression of some cellular molecules of MSCs. Except for BMP8, a series of BMP (1–7) transcripts are upregulated after genistein treatment. Also, the expression of Smad 1 and Smad 5 increases, but the expression of BMP signaling pathway inhibitors Smad 6 and Smad 7 decreases. BMP2-dependent Smad5/Runx 2 signaling has been shown to mediate genistein-induced osteogenic differentiation and maturation of BM-MSCs. T63 treatment can upregulate the gene expression levels of BMP2, BMP4, and BMP7 in the BMP signaling pathway and increase the phosphorylation of BMP downstream mediator Smad1/5/8 in a dose-dependent manner. The addition of T36 in the osteogenic induction medium significantly increases the expression of genes.106 Hypoxia-induced chondrogenesis is related to the activation of the TGF-β pathway (upregulation of TGF-β1 expression) and the upregulation of the BMP signal inhibitor Gremlin-1. VEGF blockade inhibits angiogenesis and also indirectly induces the differentiation of MSCs into cartilage by inducing the formation of a hypoxic environment.100 TGF-β1 induces increased expression of Cox-2 and CCL2 by activating Smad 3. Cox-2 induction still mainly depends on Smad3 even under the synergistic effect of TGF-β1 and TNF-α.120
The phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway participates in the regulation of a variety of MSC cell functions, including the regulation of osteogenesis and adipogenesis, and as an inhibitor of apoptosis in regulating cell survival. The treatment with Plastrum testudinis (PTE)150 or psoralidin151 increases the protein expression of p-PI3K, p-Akt, p-GSK3β, and various osteogenic genes in MSCs. Icariside II treatment increases the phosphorylation level of Akt and S6K1 in BM-MSCs and promotes the osteogenic differentiation of MSC through PI3K/Akt/mTOR/S6K1 signaling pathway.152 Risedronate can inhibit the phosphorylation of downstream effectors of mTOR and impair the adipogenic differentiation of MSCs.106 Under hypoxic preconditioning, HIF-1α-mediated 78 kDa glucose regulatory protein (GRP78) regulates the activation of Akt, mTOR, and p70S6k, and the expression of cell cycle–related proteins increases through the GRP78–Akt axis. It is vital in promoting the survival, proliferation, migration, and secretion of angiogenic cytokines in transplanted MSCs.153
The Notch signaling pathway participates in the regulation of MSC osteogenic and chondrogenic differentiation, regulates the secretion of cytokines, and is involved in exerting immunomodulatory effects. The expression of hairy/enhancer-of-split related with YRPW motif (HEY)-1 is upregulated and the expression of cartilage-specific markers SRY (sex determining region Y)-box (Sox)-9, cartilage oligomeric matrix protein (COMP), and collagen type II alpha 1 chain (Col2a1) is inhibited in MSCs treated with dihydroartemisinin (DHA).154 The addition of magnesium chloride promotes the nuclear ectopic expression of Notch intracellular domain (NICD) and HEY2 in MSCs, activating the Notch 1 signal to enhance MSC proliferation and induce osteogenic differentiation.155 The transfer of miR-146a in MM exosomes activates the endogenous Notch pathway by targeting the 3′-UTR of the Notch signal inhibitor Numb, which helps induce the secretion of various cytokines in MSCs and promotes the cell viability and migration in MM.53 A contact with chronic lymphocytic leukemia (CLL) cells induces the activation of Notch 2 in MSCs, which is necessary for Wnt signal activation in malignant B cells.156 The expression of TLR4 and Jagged 1 in MSCs derived from ALL significantly increases, and Jagged1 is at least partially involved in the ability of ALL-MSCs to stimulate the anti-tumor activity of natural killer (NK) cells during diagnosis.13
As an important nuclear transcription factor in cells, NF-κB participates in the body's inflammatory and immune responses, and can regulate cell apoptosis and stress response. Studies have found that the paracrine function of MSC is closely regulated by NF-κB, and down-regulation of this signaling pathway can reduce the sensitivity of MSC to stress-induced pro-inflammatory factors and reduce apoptosis.157,158 ROS stimulation promotes the JNK/Src-mediated phosphorylation of IκB and enhances the translocation of NF-κB from the cytoplasm to the nucleus, finally contributing to the inflammatory microenvironment. The secretion of CCL2 and CXCL8 in the synergistic induction of TNF-α and TGF-β1 is determined by the activation of p65. Further, p65 can directly bind to the promoter/enhancer region of these genes to regulate gene expression.120 TNF-α also mediates the formation of tunneling nanotubes (TNTs) in MSCs through TNF-α/NF-κB/TNFαIP2, and promotes the transfer of mitochondria to cardiomyocytes.14 A recent study demonstrated the involvement of TNF-α-mediated downstream NF-κB activation in the fusion of cancer cells and MSCs.132
The AMP-activated protein kinase (AMPK) signaling pathway is also involved in the regulation of MSC osteogenic and adipogenic differentiation. During the osteogenic differentiation, the expression and phosphorylation of AMPK increase and the inhibition of the AMPK signaling pathway can promote adipogenesis. Arctigenin (ARC)-159 and platycodi D (PD)-160 induced AMPK phosphorylation significantly increases in the inhibition of AD-MSC lipogenesis. The JAK/STAT signaling pathway is involved in the regulation of MSC immune function. IL-6 deficiency leads to the disorder of the IL-6/STAT3 signaling pathway, and MSC presents a pro-inflammatory phenotype.161
Cellular reprogramming
Common epigenetic modification methods include DNA methylation, histone modification, non-coding RNA expression changes, and so forth. Existing studies have also shown that epigenetic mechanisms regulate the changes in MSC fate and maintain the homeostasis of MSCs in vivo, and epigenetic disorders may lead to abnormal MSC function.162
Regarding the well-known DNA methylation modification methods, the downregulation of MSC DNA methyltransferase 1 is found in lupus bone loss. Also, the coordinated disorder of epigenetic markers and corresponding enzymes in BM-MSCs is found in osteoporosis caused by estrogen deficiency, including reduced 5-hydroxymethylcytosine (5-hmC) levels and downregulation of DNA hydroxylases and demethylases Tet 1 and Tet 2. In addition, epigenetic regulators have become the regulators of MSC self-renewal, characteristics, and functional homeostasis in vivo,162 confirming the regulation of MSC plasticity using epigenetic mechanisms. In the case of DNA methyltransferase inhibitor 5-azacytidine, a significant decrease in the methylation levels in the chromatin region of Wnt10α leads to the activation of Wnt10α, thereby downregulating the expression of fat-forming markers and inhibiting the adipogenic differentiation of MSCs.146 The effect of 5-azacytidine treatment on the differentiation of MSCs from different tissue sources is significantly different. For example, MSCs from dense bone source show enhanced osteogenic and adipogenic differentiation, while the differentiation ability of AD-MSCs is poor.40
The histone modification methods are complex and diverse, and common forms include acetylation and methylation. The increased expression of Gfi1 in MM MSCs recruits histone deacetylase 1 (HDAC1), enhancer of zeste homolog 2 (EZH2) and other targeted gene promoters. Of these, EZH2 partly inhibits several types of osteogenic gene promoters by generating H3K27me3, can also directly target Wnt genes to suppress osteogenic signaling, and is dedicated to adipocyte differentiation.10 EDC can induce a variety of epigenetic changes in MSCs, including histone acetylation through changes in oxidative stress ligand signal transduction, thereby inhibiting osteogenic differentiation and inducing adipogenic differentiation. For example, BBP can enhance H3K9 acetylation, increase the expression of histone acetyltransferases (such as p300 and GCN5), reduce the expression of histone deacetylase, and decrease the methylation of H3K9.79 In osteoporosis caused by estrogen deficiency, H3K4me3 decreases and H3K27me3 increases in BM-MSCs. The downregulation of lysine acetyltransferase 2 A (KAT2A) and upregulation of histone deacetylase 9 (HDAC9) are observed in periodontitis MSCs.
Abnormal expression of noncoding RNA has been found under various pathological conditions, such as osteoporosis caused by estrogen deficiency, lupus bone loss, periodontitis, and so forth. In elderly people and mice, miR-188 is downregulated and lncRNA-Bmncr is upregulated in BM-MSCs, which helps offset the bone mass loss and bone marrow fat accumulation. The high level of bone marrow TNF-α in the ovariectomy mouse model can regulate the expression levels of miR-21, miR-3077–5p, and miR-705. The inhibition of miR-21 can inhibit osteogenic differentiation through the negative regulator Spry 1 of osteoblasts, while miR-3077–5p and miR-705 target Runx 2 and HOXA10, respectively, to inhibit osteogenic differentiation.162 Previous studies found that the changes in the expression of multiple noncoding RNAs in UC-MSC and dMSC in patients with PE were involved in the regulation of cell proliferation, migration, angiogenesis, and immune function–related pathways.163 For example, miR-495,164 miR-30a,165 and miR-181a166 are highly expressed in UC-MSCs, while lncRNA-metastasis-associated lung adenocarcinoma transcript (MALAT)-1 is downregulated.167 High expression of miR-16168 and miR-494118,169 is detected in dMSCs. IL-1β pretreatment of UC-MSCs can strongly upregulate anti-inflammatory miR-146a, while exosome-mediated miR-146a transfer effectively enhances the immunomodulatory properties of UC-MSCs.170 TLR3 activation can enhance the immunosuppressive and anti-inflammatory effects of MSC through the downregulation of miR-143 in vivo and in vitro.171 LncRNA-highly up-regulated in liver cancer (HULC) has a positive regulatory effect on MSC proliferation, migration, and invasion; its expression may be affected by various immune factors.172 The silencing of CircFOXP1 greatly impairs the differentiation potential of MSCs. A direct interaction exists between circFOXP1 and miR-17–3p/miR-127–5p, leading to the regulation of nonclassical Wnt and other signaling pathways.173
In addition, ginkgo acid can act as an inhibitor of SUMOylation to regulate the adipogenic differentiation of MSCs, which can prevent the formation of the E1-SUMO thioester complex and act as a sulfonylation inhibitor to damage SUMOylation.106 SWI/SNF-(BAF) is known to be an ATP-dependent chromatin remodeling agent involved in multicellular development. BMP and long-term osteogenic signals can selectively induce the expression of polybromide BAF (PBAF) components Pbrm1, Arid 2, and Brd 7. Pbrm1/PBAF deficiency affects the expression of BMP early response genes by locus-specific epigenome remodeling (involving Pbrm1 bromodomains) and transcriptional downregulation of BMPR/TGF-βRII, which impairs Smad 1/5/8 activation and negatively affects osteogenesis.174
Extensive studies have been conducted on the aforementioned signaling pathways and cell reprogramming in the exploration of the plasticity mechanism of MSCs, and also the fundamental way to change gene expression. However, studies on gene mutations are relatively few. A study on AML showed the possibility of PLEC gene mutation in MSCs.85 A recent study showed that hMSCs lacking the glutathione S-transferase theta 1 (GSTT1) gene showed increased proliferation rate, clone formation, and longer telomeres.175
Conclusions
Numerous studies investigated the changes in MSC phenotype and functions. Different physiological or pathological conditions can induce different plasticity phenomena in MSCs, including changes in morphology, surface markers, and paracrine, differentiating, proliferative, migratory, and apoptotic abilities, which may be closely related to the complex microenvironments of MSCs. Various influencing factors (physical, chemical, and biological factors) in the microenvironment can induce phenotypic and functional changes in MSCs through different mechanisms of action (including signaling pathways, cell reprogramming, etc.) to affect or determine the functions of MSCs. Therefore, the role and mechanism of different microenvironmental factors inducing MSC plasticity are the focus of MSC clinical application, as they are the important factors influencing the treatment effect and the important parts of exploring the treatment mechanism. For example, a study on AKI found that MSCs induced by CZ changed to anti-inflammatory MSCs, and the significant enhancement of immunosuppressive ability effectively reduced the inflammatory infiltration and tissue damage in the rat model of AKI.107 Hypoxia-induced miR-126 production in MSC exosomes has been demonstrated to promote effective fracture healing in an in vivo fracture model.5 In addition, understanding and mastering the specific induction and internal mechanisms of different microenvironments on MSCs may help explain or resolve the potential treatment limitations or safety issues of MSC treatment. For example, some clinical trials have shown no therapeutic effects of MSCs; the transplanted MSCs have poor differentiation. The findings of this study have important reference value for predicting the therapeutic effects of MSCs and artificially regulating the characteristics and functions of MSCs. Also, they have an important guiding role for subsequent improvement in their clinical benefits. More studies on MSC plasticity are still needed.
Author contributions
Y.Y.H. and H.D. provided conceptualization, supervision, reviewing and funding acquisition; L.P.T. wrote original draft; X.L. carried out the schematic diagram. All authors reviewed and approved the final manuscript.
Conflict of interests
Authors declare no conflict of interests.
Funding
This work was supported by grant from Provincial Key Research and Development of Jiangsu Province (No. BE2019706).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Huan Dou, Email: douhuan@nju.edu.cn.
Yayi Hou, Email: yayihou@nju.edu.cn.
References
- 1.Spees J.L., Lee R.H., Gregory C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boada M., Echarte L., Guillermo C., et al. 5-Azacytidine restores interleukin 6-increased production in mesenchymal stromal cells from myelodysplastic patients. Hematol Transfus Cell Ther. 2021;43(1):35–42. doi: 10.1016/j.htct.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giri J., Das R., Nylen E., Chinnadurai R., Galipeau J. CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury. Cell Rep. 2020;30(6):1923–1934.e4. doi: 10.1016/j.celrep.2020.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan W., Cheng B., Zhang C., et al. Synergistic effect of matrix stiffness and inflammatory factors on osteogenic differentiation of MSC. Biophys J. 2019;117(1):129–142. doi: 10.1016/j.bpj.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W., Li L., Rong Y., et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi: 10.1016/j.actbio.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Block T.J., Marinkovic M., Tran O.N., et al. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res Ther. 2017;8(1):239. doi: 10.1186/s13287-017-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji K., Kitamura S., Wada J. Secretomes from mesenchymal stem cells against acute kidney injury: possible heterogeneity. Stem Cell Int. 2018;2018:8693137. doi: 10.1155/2018/8693137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng R.-J., Xiong A.-J., Li Y.-H., et al. Mesenchymal stem cells: allogeneic MSC may Be immunosuppressive but autologous MSC are dysfunctional in lupus patients. Frontiers in cell and developmental biology. 2019;7:285. doi: 10.3389/fcell.2019.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tencerova M., Frost M., Figeac F., et al. Obesity-associated hypermetabolism and accelerated senescence of bone marrow stromal stem cells suggest a potential mechanism for bone fragility. Cell Rep. 2019;27(7):2050–2062. doi: 10.1016/j.celrep.2019.04.066. e2056. [DOI] [PubMed] [Google Scholar]
- 10.Adamik J., Roodman G.D., Galson D.L. Epigenetic-based mechanisms of osteoblast suppression in multiple myeloma bone disease. JBMR plus. 2019;3(3):e10183. doi: 10.1002/jbm4.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atiya H., Frisbie L., Pressimone C., et al. Mesenchymal stem cells in the tumor microenvironment. Adv Exp Med Biol. 2020;1234:31–42. doi: 10.1007/978-3-030-37184-5_3. [DOI] [PubMed] [Google Scholar]
- 12.El-Badawy A., Ghoneim M.A., Gabr M.M., et al. Cancer cell-soluble factors reprogram mesenchymal stromal cells to slow cycling, chemoresistant cells with a more stem-like state. Stem Cell Res Ther. 2017;8(1):254. doi: 10.1186/s13287-017-0709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Entrena A., Varas A., Vázquez M., et al. Mesenchymal stem cells derived from low risk acute lymphoblastic leukemia patients promote NK cell antitumor activity. Canc Lett. 2015;363(2):156–165. doi: 10.1016/j.canlet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Fan X.L., Zhang Y., Li X., Fu Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771–2794. doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng T.T., Mak K.H., Popp C., Ng R.K. Murine mesenchymal stromal cells retain biased differentiation plasticity towards their tissue of origin. Cells. 2020;9(3):756. doi: 10.3390/cells9030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calle A., Barrajón-Masa C., Gómez-Fidalgo E., et al. Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: in vitro characterization and migratory properties in inflammation. Stem Cell Res Ther. 2018;9(1):178. doi: 10.1186/s13287-018-0933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Missoum A. Recent updates on mesenchymal stem cell based therapy for acute renal failure. Current urology. 2020;13(4):189–199. doi: 10.1159/000499272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liau L.L., Ruszymah B.H.I., Ng M.H., et al. Characteristics and clinical applications of Wharton's jelly-derived mesenchymal stromal cells. Current research in translational medicine. 2020;68(1):5–16. doi: 10.1016/j.retram.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Klimczak A., Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cell Int. 2016;2016:4285215. doi: 10.1155/2016/4285215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ménard C., Dulong J., Roulois D., et al. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem cells (Dayton, Ohio) 2020;38(1):146–159. doi: 10.1002/stem.3077. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen V.T., Tessaro I., Marmotti A., et al. Does the harvesting site influence the osteogenic potential of mesenchymal stem cells? Stem Cell Int. 2019;2019:9178436. doi: 10.1155/2019/9178436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann M., Hildebrand M., Menzel U., et al. Phenotypic characterization of bone marrow mononuclear cells and derived stromal cell populations from human iliac crest, vertebral body and femoral head. Int J Mol Sci. 2019;20(14) doi: 10.3390/ijms20143454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakao M., Inanaga D., Nagase K., et al. Characteristic differences of cell sheets composed of mesenchymal stem cells with different tissue origins. Regenerative therapy. 2019;11:34–40. doi: 10.1016/j.reth.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X., Rui T., Zhang S., et al. Heterogeneity of MSC: origin, molecular identities, and functionality. Stem Cell Int. 2019;2019:9281520. doi: 10.1155/2019/9281520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieira Paladino F., de Moraes Rodrigues J., da Silva A., et al. The immunomodulatory potential of Wharton's jelly mesenchymal stem/stromal cells. Stem Cell Int. 2019;2019:3548917. doi: 10.1155/2019/3548917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto D.S., Ahsan T., Serra J., Fernandes-Platzgummer A., Cabral J.M.S., Cabral J.M.S. Modulation of the in vitro angiogenic potential of human mesenchymal stromal cells from different tissue sources. J Cell Physiol. 2020;235(10):7224–7238. doi: 10.1002/jcp.29622. [DOI] [PubMed] [Google Scholar]
- 27.Driscoll J., Patel T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J Gastroenterol. 2019;54(9):763–773. doi: 10.1007/s00535-019-01599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragelle H., Naba A., Larson B.L., et al. Comprehensive proteomic characterization of stem cell-derived extracellular matrices. Biomaterials. 2017;128:147–159. doi: 10.1016/j.biomaterials.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinkovic M., Block T.J., Rakian R., et al. One size does not fit all: developing a cell-specific niche for in vitro study of cell behavior. Matrix Biol : journal of the International Society for Matrix Biology. 2016;52–54:426–441. doi: 10.1016/j.matbio.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antebi B., Zhang Z., Wang Y., et al. Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds. Tissue Eng C Methods. 2015;21(2):171–181. doi: 10.1089/ten.tec.2014.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lian Q., Zhang Y., Liang X., et al. Directed differentiation of human-induced pluripotent stem cells to mesenchymal stem cells. Methods Mol Biol. 2016;1416:289–298. doi: 10.1007/978-1-4939-3584-0_17. [DOI] [PubMed] [Google Scholar]
- 32.Sze S.K., de Kleijn D.P.V., Lai R.C., et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol Cell Proteomics. 2007;6(10):1680–1689. doi: 10.1074/mcp.M600393-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Lian Q., Zhang Y., Zhang J., et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 34.Lian Q., Lye E., Suan Yeo K., et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cell. 2007;25(2):425–436. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Liang X., Liao S., et al. Potent paracrine effects of human induced pluripotent stem cell-derived mesenchymal stem cells attenuate doxorubicin-induced cardiomyopathy. Sci Rep. 2015;5(1):11235. doi: 10.1038/srep11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloor A.J.C., Patel A., Griffin J.E., et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: a phase I, multicenter, open-label, dose-escalation study. Nat Med. 2020;26(11):1720–1725. doi: 10.1038/s41591-020-1050-x. [DOI] [PubMed] [Google Scholar]
- 37.Päth G., Perakakis N., Mantzoros C.S., et al. Stem cells in the treatment of diabetes mellitus - focus on mesenchymal stem cells. Metabolism. 2019;90:1–15. doi: 10.1016/j.metabol.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Cuascut F.X., Hutton G.J. Stem cell-based therapies for multiple sclerosis: current perspectives. Biomedicines. 2019;7(2):26. doi: 10.3390/biomedicines7020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu T., Zhang Y., Chang P., Gong S., Shao L., Dong L. Mesenchymal stem cell-based therapy for radiation-induced lung injury. Stem Cell Res Ther. 2018;9(1):18. doi: 10.1186/s13287-018-0776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho J., D'Antuono M., Glicksman M., et al. A review of clinical trials: mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am J Stem Cells. 2018;7(4):82–93. [PMC free article] [PubMed] [Google Scholar]
- 41.Hernández R., Jiménez-Luna C., Perales-Adán J., et al. Differentiation of human mesenchymal stem cells towards neuronal lineage: clinical trials in nervous system disorders. Biomol Ther (Seoul) 2020;28(1):34–44. doi: 10.4062/biomolther.2019.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamal M.M., Kassem D.H. Therapeutic potential of Wharton's jelly mesenchymal stem cells for diabetes: achievements and challenges. Front Cell Dev Biol. 2020;8:16. doi: 10.3389/fcell.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong R., Liu Y., Yang Y., et al. MSC-derived exosomes-based therapy for peripheral nerve injury: a novel therapeutic strategy. BioMed Res Int. 2019;2019:6458237. doi: 10.1155/2019/6458237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia H., Yan Y., Liang Z., et al. Autophagy: a new treatment strategy for MSC-based therapy in acute kidney injury (Review) Mol Med Rep. 2018;17(3):3439–3447. doi: 10.3892/mmr.2017.8311. [DOI] [PubMed] [Google Scholar]
- 45.Hidalgo-Garcia L., Galvez J., Rodriguez-Cabezas M.E., et al. Can a conversation between mesenchymal stromal cells and macrophages solve the crisis in the inflamed intestine? Front Pharmacol. 2018;9:179. doi: 10.3389/fphar.2018.00179. -179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawakubo K., Ohnishi S., Kuwatani M., et al. Mesenchymal stem cell therapy for acute and chronic pancreatitis. J Gastroenterol. 2018;53(1):1–5. doi: 10.1007/s00535-017-1363-9. [DOI] [PubMed] [Google Scholar]
- 47.Sarhan D., Wang J., Sunil Arvindam U., et al. Mesenchymal stromal cells shape the MDS microenvironment by inducing suppressive monocytes that dampen NK cell function. JCI insight. 2020:130155. doi: 10.1172/jci.insight.130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi K., Li N., Zhang Z., et al. Tissue regeneration: the crosstalk between mesenchymal stem cells and immune response. Cell Immunol. 2018;326:86–93. doi: 10.1016/j.cellimm.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Yao G., Qi J., Liang J., et al. Mesenchymal stem cell transplantation alleviates experimental Sjögren's syndrome through IFN-β/IL-27 signaling axis. Theranostics. 2019;9(26):8253–8265. doi: 10.7150/thno.37351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pistoia V., Raffaghello L. Mesenchymal stromal cells and autoimmunity. Int Immunol. 2017;29(2):49–58. doi: 10.1093/intimm/dxx008. [DOI] [PubMed] [Google Scholar]
- 51.Weiss A., Lee O., Eggenhofer E., et al. Differential effects of heat-inactivated, secretome-deficient MSC and metabolically active MSC in sepsis and allogenic heart transplantation. Stem Cells. 2020;38(6):797–807. doi: 10.1002/stem.3165. [DOI] [PubMed] [Google Scholar]
- 52.Lopes M.R., Pereira J.K.N., de Melo Campos P., et al. De novo AML exhibits greater microenvironment dysregulation compared to AML with myelodysplasia-related changes. Sci Rep. 2017;7:40707. doi: 10.1038/srep40707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Veirman K., Wang J., Xu S., et al. Induction of miR-146a by multiple myeloma cells in mesenchymal stromal cells stimulates their pro-tumoral activity. Canc Lett. 2016;377(1):17–24. doi: 10.1016/j.canlet.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 54.Schuler P.J., Westerkamp A.-M., Kansy B.A., et al. Adenosine metabolism of human mesenchymal stromal cells isolated from patients with head and neck squamous cell carcinoma. Immunobiology. 2017;222(1):66–74. doi: 10.1016/j.imbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Kim N., Cho S.-G. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol. 2016;103(2):129–137. doi: 10.1007/s12185-015-1918-6. [DOI] [PubMed] [Google Scholar]
- 56.Colmegna I., Stochaj U. Msc - targets for atherosclerosis therapy. Aging. 2018;11(2):285–286. doi: 10.18632/aging.101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linard C., Brachet M., L'Homme B., et al. Long-term effectiveness of local BM-MSCs for skeletal muscle regeneration: a proof of concept obtained on a pig model of severe radiation burn. Stem Cell Res Ther. 2018;9(1):299. doi: 10.1186/s13287-018-1051-6. -299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isakson M., de Blacam C., Whelan D., et al. Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem Cell Int. 2015;2015:831095. doi: 10.1155/2015/831095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiore E.J., Domínguez L.M., Bayo J., et al. Taking advantage of the potential of mesenchymal stromal cells in liver regeneration: cells and extracellular vesicles as therapeutic strategies. World J Gastroenterol. 2018;24(23):2427–2440. doi: 10.3748/wjg.v24.i23.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu H., Yuan X., Liu L., Wang T., Gong D. Treatment of multiple system atrophy - the past, present and future. Am J Clin Exp Immunol. 2018;7(5):88–94. [PMC free article] [PubMed] [Google Scholar]
- 61.Wang F., Zhang C., Hou S., Geng X. Synergistic effects of mesenchymal stem cell transplantation and repetitive transcranial magnetic stimulation on promoting autophagy and synaptic plasticity in vascular dementia. J Gerontol A Biol Sci Med Sci. 2019;74(9):1341–1350. doi: 10.1093/gerona/gly221. [DOI] [PubMed] [Google Scholar]
- 62.Hu P., Yang Q., Wang Q., et al. Mesenchymal stromal cells-exosomes: a promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burns Trauma. 2019;7:38. doi: 10.1186/s41038-019-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ocansey D.K.W., Pei B., Yan Y., et al. Improved therapeutics of modified mesenchymal stem cells: an update. J Transl Med. 2020;18(1):42. doi: 10.1186/s12967-020-02234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng X., Ling L., Zhang W., et al. Effects of human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation in situ on primary ovarian insufficiency in SD rats. Reprod Sci. 2020;27(7):1502–1512. doi: 10.1007/s43032-020-00147-0. [DOI] [PubMed] [Google Scholar]
- 65.Kim J.A., Shim J.S., Lee G.Y., et al. Microenvironmental remodeling as a parameter and prognostic factor of heterogeneous leukemogenesis in acute myelogenous leukemia. Canc Res. 2015;75(11):2222–2231. doi: 10.1158/0008-5472.CAN-14-3379. [DOI] [PubMed] [Google Scholar]
- 66.Liang X., Ding Y., Zhang Y., et al. Activation of NRG1-ERBB4 signaling potentiates mesenchymal stem cell-mediated myocardial repairs following myocardial infarction. Cell Death Dis. 2015;6(5):e1765. doi: 10.1038/cddis.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi X., Ng K.T.-P., Lian Q., et al. Glutathione peroxidase 3 delivered by hiPSC-MSCs ameliorated hepatic IR injury via inhibition of hepatic senescence. Theranostics. 2018;8(1):212–222. doi: 10.7150/thno.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muntión S., Ramos T.L., Diez-Campelo M., et al. Microvesicles from mesenchymal stromal cells are involved in HPC-microenvironment crosstalk in myelodysplastic patients. PloS One. 2016;11(2):e0146722. doi: 10.1371/journal.pone.0146722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng H., Sun C., Sun Y., et al. Lipid, protein, and MicroRNA composition within mesenchymal stem cell-derived exosomes. Cell Reprogr. 2018;20(3):178–186. doi: 10.1089/cell.2017.0047. [DOI] [PubMed] [Google Scholar]
- 70.Zhao L., Hu C., Zhang P., et al. Mesenchymal stem cell therapy targeting mitochondrial dysfunction in acute kidney injury. J Transl Med. 2019;17(1):142. doi: 10.1186/s12967-019-1893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mancuso P., Raman S., Glynn A., et al. Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Front Bioeng Biotechnol. 2019;7:9. doi: 10.3389/fbioe.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y., Liang X., Lian Q., et al. Perspective and challenges of mesenchymal stem cells for cardiovascular regeneration. Expert Rev Cardiovasc Ther. 2013;11(4):505–517. doi: 10.1586/erc.13.5. [DOI] [PubMed] [Google Scholar]
- 73.Siu D., Liao S.Y., Liu Y., Lian Q., Tse H.F. Stem cells for myocardial repair. Thromb Haemost. 2010;104:6–12. doi: 10.1160/TH09-05-0336. [DOI] [PubMed] [Google Scholar]
- 74.Armiento A.R., Alini M., Stoddart M.J. Articular fibrocartilage - why does hyaline cartilage fail to repair? Adv Drug Deliv Rev. 2019;146:289–305. doi: 10.1016/j.addr.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 75.Nuzzi R., Tridico F. Perspectives of autologous mesenchymal stem-cell transplantation in macular hole surgery: a review of current findings. Journal of ophthalmology. 2019;2019:3162478. doi: 10.1155/2019/3162478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin S., Zhu B., Huang G., et al. Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: the role of PI3K/AKT and HIF-1α. Hum Cell. 2019;32(1):64–74. doi: 10.1007/s13577-018-0224-z. [DOI] [PubMed] [Google Scholar]
- 77.Drela K., Stanaszek L., Nowakowski A., et al. Experimental strategies of mesenchymal stem cell propagation: adverse events and potential risk of functional changes. Stem Cell Int. 2019;2019:7012692. doi: 10.1155/2019/7012692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehdi S.J., Johnson S.K., Epstein J., et al. Mesenchymal stem cells gene signature in high-risk myeloma bone marrow linked to suppression of distinct IGFBP2-expressing small adipocytes. Br J Haematol. 2019;184(4):578–593. doi: 10.1111/bjh.15669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bateman M.E., Strong A.L., McLachlan J.A., et al. The effects of endocrine disruptors on adipogenesis and osteogenesis in mesenchymal stem cells: a review. Front Endocrinol. 2017;7:171. doi: 10.3389/fendo.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desbourdes L., Javary J., Charbonnier T., et al. Alteration analysis of bone marrow mesenchymal stromal cells from de novo acute myeloid leukemia patients at diagnosis. Stem Cell Dev. 2017;26(10):709–722. doi: 10.1089/scd.2016.0295. [DOI] [PubMed] [Google Scholar]
- 81.da Silva S.V., Renovato-Martins M., Ribeiro-Pereira C., et al. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity. 2016;24(12):2522–2532. doi: 10.1002/oby.21660. [DOI] [PubMed] [Google Scholar]
- 82.Timaner M., Tsai K.K., Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Canc Biol. 2020;60:225–237. doi: 10.1016/j.semcancer.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Hu C., Wu Z., Li L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int J Biol Sci. 2020;16(5):893–903. doi: 10.7150/ijbs.39725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao L., Hu C., Zhang P., et al. Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J Cell Mol Med. 2020;24(1):25–33. doi: 10.1111/jcmm.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.von der Heide E.K., Neumann M., Vosberg S., et al. Molecular alterations in bone marrow mesenchymal stromal cells derived from acute myeloid leukemia patients. Leukemia. 2017;31(5):1069–1078. doi: 10.1038/leu.2016.324. [DOI] [PubMed] [Google Scholar]
- 86.Aubert L., Dubus M., Rammal H., et al. Collagen-based medical device as a stem cell carrier for regenerative medicine. Int J Mol Sci. 2017;18(10):2210. doi: 10.3390/ijms18102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hsieh W.-T., Liu Y.-S., Lee Y.-H., et al. Matrix dimensionality and stiffness cooperatively regulate osteogenesis of mesenchymal stromal cells. Acta Biomater. 2016;32:210–222. doi: 10.1016/j.actbio.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 88.Vega S.L., Arvind V., Mishra P., et al. Substrate micropatterns produced by polymer demixing regulate focal adhesions, actin anisotropy, and lineage differentiation of stem cells. Acta Biomater. 2018;76:21–28. doi: 10.1016/j.actbio.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boeri L., Albani D., Raimondi M.T., et al. Mechanical regulation of nucleocytoplasmic translocation in mesenchymal stem cells: characterization and methods for investigation. Biophysical reviews. 2019;11(5):817–831. doi: 10.1007/s12551-019-00594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du L., Fan H., Miao H., et al. Extremely low frequency magnetic fields inhibit adipogenesis of human mesenchymal stem cells. Bioelectromagnetics. 2014;35(7):519–530. doi: 10.1002/bem.21873. [DOI] [PubMed] [Google Scholar]
- 91.García-Sánchez D., Fernández D., Rodríguez-Rey J.C., et al. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J Stem Cell. 2019;11(10):748–763. doi: 10.4252/wjsc.v11.i10.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Narayan R., Agarwal T., Mishra D., et al. Ectopic vascularized bone formation by human mesenchymal stem cell microtissues in a biocomposite scaffold. Colloids Surf B Biointerfaces. 2017;160:661–670. doi: 10.1016/j.colsurfb.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Ye J., Wang J., Zhu Y., et al. A thermoresponsive polydiolcitrate-gelatin scaffold and delivery system mediates effective bone formation from BMP9-transduced mesenchymal stem cells. Biomed Mater. 2016;11(2) doi: 10.1088/1748-6041/11/2/025021. 025021. [DOI] [PubMed] [Google Scholar]
- 94.Heo S.-J., Szczesny S.E., Kim D.H., et al. Expansion of mesenchymal stem cells on electrospun scaffolds maintains stemness, mechano-responsivity, and differentiation potential. J Orthop Res : official publication of the Orthopaedic Research Society. 2018;36(2):808–815. doi: 10.1002/jor.23772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ozdemir T., Higgins A.M., Brown J.L. Molecular mechanisms orchestrating the stem cell response to translational scaffolds. Annu Int Conf Eng Med Biol Soc. 2015:1749–1752. doi: 10.1109/EMBC.2015.7318716. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y., Melzer C., Bucan V., et al. Conditioned umbilical cord tissue provides a natural three-dimensional storage compartment as in vitro stem cell niche for human mesenchymal stroma/stem cells. Stem Cell Res Ther. 2016;7:28. doi: 10.1186/s13287-016-0289-0. -28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoogduijn M.J., de Witte S.F.H., Luk F., et al. Effects of freeze-thawing and intravenous infusion on mesenchymal stromal cell gene expression. Stem Cell Dev. 2016;25(8):586–597. doi: 10.1089/scd.2015.0329. [DOI] [PubMed] [Google Scholar]
- 98.Marsano A., Medeiros da Cunha C.M., Ghanaati S., et al. Spontaneous in vivo chondrogenesis of bone marrow-derived mesenchymal progenitor cells by blocking vascular endothelial growth factor signaling. Stem cells translational medicine. 2016;5(12):1730–1738. doi: 10.5966/sctm.2015-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu C., Wu Z., Li L. Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med. 2020;24(1):40–49. doi: 10.1111/jcmm.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y., Ren H., Zhou Y., et al. The hypoxia conditioned mesenchymal stem cells promote hepatocellular carcinoma progression through YAP mediated lipogenesis reprogramming. J Exp Clin Canc Res : CR (Clim Res) 2019;38(1):228. doi: 10.1186/s13046-019-1219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J., Liu X., Zuo B., et al. The role of bone marrow microenvironment in governing the balance between osteoblastogenesis and adipogenesis. Aging and disease. 2015;7(4):514–525. doi: 10.14336/AD.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yin S., Ji C., Wu P., et al. Human umbilical cord mesenchymal stem cells and exosomes: bioactive ways of tissue injury repair. Am J Tourism Res. 2019;11(3):1230–1240. [PMC free article] [PubMed] [Google Scholar]
- 103.Broekman W., Khedoe P.P.S.J., Schepers K., et al. Mesenchymal stromal cells: a novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax. 2018;73(6):565–574. doi: 10.1136/thoraxjnl-2017-210672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ti D., Hao H., Tong C., et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He F., Yu C., Liu T., et al. Ginsenoside Rg1 as an effective regulator of mesenchymal stem cells. Front Pharmacol. 2020;10:1565. doi: 10.3389/fphar.2019.01565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng Y.H., Dong J.C., Bian Q. Small molecules for mesenchymal stem cell fate determination. World J Stem Cell. 2019;11(12):1084–1103. doi: 10.4252/wjsc.v11.i12.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deng L., Li H., Su X., et al. Chlorzoxazones, a small molecule drug, augments immunosuppressive capacity of mesenchymal stem cells via modulation of FOXO3 phosphorylation. Cell Death Dis. 2020;11(3):158. doi: 10.1038/s41419-020-2357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao X., Liu L., Liu D., et al. Progesterone enhances immunoregulatory activity of human mesenchymal stem cells via PGE2 and IL-6. Am J Reprod Immunol. 2012;68(4):290–300. doi: 10.1111/j.1600-0897.2012.01163.x. [DOI] [PubMed] [Google Scholar]
- 109.Heo J.S., Kim H.O., Song S.Y., et al. Poly-L-lysine prevents senescence and augments growth in culturing mesenchymal stem cells ex vivo. BioMed Res Int. 2016;2016:8196078. doi: 10.1155/2016/8196078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ullah M., Liu D.D., Thakor A.S. Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience. 2019;15:421–438. doi: 10.1016/j.isci.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang P., Wang L., Li Q., et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116(2):353–367. doi: 10.1093/cvr/cvz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan H., Zhao G., Liu L., et al. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9(6):473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niu P., Smagul A., Wang L., et al. Transcriptional profiling of interleukin-2-primed human adipose derived mesenchymal stem cells revealed dramatic changes in stem cells response imposed by replicative senescence. Oncotarget. 2015;6(20):17938–17957. doi: 10.18632/oncotarget.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liao C., Zhang C., Jin L., et al. IL-17 alters the mesenchymal stem cell niche towards osteogenesis in cooperation with osteocytes. J Cell Physiol. 2020;235(5):4466–4480. doi: 10.1002/jcp.29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marklein R.A., Klinker M.W., Drake K.A., et al. Morphological profiling using machine learning reveals emergent subpopulations of interferon-γ-stimulated mesenchymal stromal cells that predict immunosuppression. Cytotherapy. 2019;21(1):17–31. doi: 10.1016/j.jcyt.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 116.Mohammadpour H., Pourfathollah A.A., Zarif M.N., et al. TNF-α modulates the immunosuppressive effects of MSCs on dendritic cells and T cells. Int Immunopharm. 2015;28(2):1009–1017. doi: 10.1016/j.intimp.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 117.Ok Bozkaya I., Azik F., Tavil B., et al. The effect of granulocyte colony-stimulating factor on immune-modulatory cytokines in the bone marrow microenvironment and mesenchymal stem cells of healthy donors. Biol Blood Marrow Transplant : journal of the American Society for Blood and Marrow Transplantation. 2015;21(11):1888–1894. doi: 10.1016/j.bbmt.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 118.Zhao G., Miao H., Li X., et al. TGF-β3-induced miR-494 inhibits macrophage polarization via suppressing PGE2 secretion in mesenchymal stem cells. FEBS Lett. 2016;590(11):1602–1613. doi: 10.1002/1873-3468.12200. [DOI] [PubMed] [Google Scholar]
- 119.Roth S.P., Schubert S., Scheibe P., et al. Growth factor-mediated tenogenic induction of multipotent mesenchymal stromal cells is altered by the microenvironment of tendon matrix. Cell Transplant. 2018;27(10):1434–1450. doi: 10.1177/0963689718792203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lerrer S., Liubomirski Y., Bott A., et al. Co-inflammatory roles of TGFβ1 in the presence of TNFα drive a pro-inflammatory fate in mesenchymal stem cells. Front Immunol. 2017;8:479. doi: 10.3389/fimmu.2017.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barrachina L., Remacha A.R., Romero A., et al. Inflammation affects the viability and plasticity of equine mesenchymal stem cells: possible implications in intra-articular treatments. J Vet Sci. 2017;18(1):39–49. doi: 10.4142/jvs.2017.18.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barrachina L., Remacha A.R., Romero A., et al. Priming equine bone marrow-derived mesenchymal stem cells with proinflammatory cytokines: implications in immunomodulation-immunogenicity balance, cell viability, and differentiation potential. Stem Cell Dev. 2017;26(1):15–24. doi: 10.1089/scd.2016.0209. [DOI] [PubMed] [Google Scholar]
- 123.Whiteside T.L. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol. 2018;35:69–79. doi: 10.1016/j.smim.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo F., Sun Z., Han Q., et al. Effect of human hepatocellular carcinoma HepG2 cell-derived exosome on the differentiation of mesenchymal stem cells and their interaction. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39(3):312–317. doi: 10.3881/j.issn.1000-503X.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 125.Sharma S., Alharbi M., Kobayashi M., et al. Proteomic analysis of exosomes reveals an association between cell invasiveness and exosomal bioactivity on endothelial and mesenchymal cell migration in vitro. Clinical science (London, England : 1979) 2018;132(18):2029–2044. doi: 10.1042/CS20180425. [DOI] [PubMed] [Google Scholar]
- 126.Jafarzadeh N., Safari Z., Pornour M., et al. Alteration of cellular and immune-related properties of bone marrow mesenchymal stem cells and macrophages by K562 chronic myeloid leukemia cell derived exosomes. J Cell Physiol. 2019;234(4):3697–3710. doi: 10.1002/jcp.27142. [DOI] [PubMed] [Google Scholar]
- 127.Shen Y., Xue C., Li X., et al. Effects of gastric cancer cell-derived exosomes on the immune regulation of mesenchymal stem cells by the NF-kB signaling pathway. Stem Cell Dev. 2019;28(7):464–476. doi: 10.1089/scd.2018.0125. [DOI] [PubMed] [Google Scholar]
- 128.Al-toub M., Vishnubalaji R., Hamam R., et al. CDH1 and IL1-beta expression dictates FAK and MAPKK-dependent cross-talk between cancer cells and human mesenchymal stem cells. Stem Cell Res Ther. 2015;6(1):135. doi: 10.1186/s13287-015-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li C., Cheung M.K.H., Han S., et al. Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Biosci Rep. 2019;39(5) doi: 10.1042/BSR20182417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Melzer C., von der Ohe J., Hass R. Concise review: crosstalk of mesenchymal stroma/stem-like cells with cancer cells provides therapeutic potential. Stem cells (Dayton, Ohio) 2018;36(7):951–968. doi: 10.1002/stem.2829. [DOI] [PubMed] [Google Scholar]
- 131.Pietrovito L., Leo A., Gori V., et al. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Molecular oncology. 2018;12(5):659–676. doi: 10.1002/1878-0261.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hass R., von der Ohe J., Ungefroren H. Potential role of MSC/cancer cell fusion and EMT for breast cancer stem cell formation. Cancers. 2019;11(10):1432. doi: 10.3390/cancers11101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Silveira G.D.P., Ishimura M.E., Teixeira D., et al. Improvement of mesenchymal stem cell immunomodulatory properties by heat-killed propionibacterium acnes via TLR2. Front Mol Neurosci. 2018;11:489. doi: 10.3389/fnmol.2018.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang H., Husch J.F.A., Zhang Y., et al. Coculture with monocytes/macrophages modulates osteogenic differentiation of adipose-derived mesenchymal stromal cells on poly(lactic-co-glycolic) acid/polycaprolactone scaffolds. Journal of tissue engineering and regenerative medicine. 2019;13(5):785–798. doi: 10.1002/term.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Crippa S., Bernardo M.E. Mesenchymal stromal cells: role in the BM niche and in the support of hematopoietic stem cell transplantation. HemaSphere. 2018;2(6):e151. doi: 10.1097/HS9.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kadle R.L., Abdou S.A., Villarreal-Ponce A.P., et al. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PLoS One. 2018;13(3):e0193178. doi: 10.1371/journal.pone.0193178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Court A.C., Le-Gatt A., Luz-Crawford P., et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21(2):e48052. doi: 10.15252/embr.201948052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi J.W., Shin S., Lee C.Y., et al. Rapid induction of osteogenic markers in mesenchymal stem cells by adipose-derived stromal vascular fraction cells. Cell Physiol Biochem : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;44(1):53–65. doi: 10.1159/000484582. [DOI] [PubMed] [Google Scholar]
- 139.Kaneda-Ikeda E., Iwata T., Mizuno N., et al. Regulation of osteogenesis via miR-101-3p in mesenchymal stem cells by human gingival fibroblasts. J Bone Miner Metab. 2020;38(4):442–455. doi: 10.1007/s00774-019-01080-2. [DOI] [PubMed] [Google Scholar]
- 140.Xie Y., Chen M., Chen Y., et al. Effects of PRP and LyPRP on osteogenic differentiation of MSCs. J Biomed Mater Res. 2020;108(1):116–126. doi: 10.1002/jbm.a.36797. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Y., Yu Z., Jiang D., et al. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Reports. 2016;7(4):749–763. doi: 10.1016/j.stemcr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Konari N., Nagaishi K., Kikuchi S., et al. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci Rep. 2019;9(1):5184. doi: 10.1038/s41598-019-40163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yao Y., Fan X.-L., Jiang D., et al. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Reports. 2018;11(5):1120–1135. doi: 10.1016/j.stemcr.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang D., Xiong G., Feng H., et al. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics. 2019;9(8):2395–2410. doi: 10.7150/thno.29422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yuan Z., Li Q., Luo S., et al. PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2016;11(3):216–225. doi: 10.2174/1574888x10666150519093429. [DOI] [PubMed] [Google Scholar]
- 146.Ikeda T., Nishita M., Hoshi K., et al. Mesenchymal stem cell-derived CXCL16 promotes progression of gastric cancer cells by STAT3-mediated expression of Ror1. Canc Sci. 2020 doi: 10.1111/cas.14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deng Q., Li P., Che M., et al. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/β-Catenin. Elife. 2019;8 doi: 10.7554/eLife.50208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Carrow J.K., Cross L.M., Reese R.W., et al. Widespread changes in transcriptome profile of human mesenchymal stem cells induced by two-dimensional nanosilicates. Proc Natl Acad Sci U S A. 2018;115(17):E3905–E3913. doi: 10.1073/pnas.1716164115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu Y., Xia L., Zhou Y., et al. Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a MAPK-dependent manner. Cell Prolif. 2015;48(3):375–384. doi: 10.1111/cpr.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen G.Y., Ren H., Huang J.J., et al. Plastrum testudinis extracts promote BMSC proliferation and osteogenic differentiation by regulating let-7f-5p and the TNFR2/PI3K/AKT signaling pathway. Cell Physiol Biochem. 2018;47(6):2307–2318. doi: 10.1159/000491541. [DOI] [PubMed] [Google Scholar]
- 151.Zhai Y., Wang Q., Li Y., et al. The higher osteoprotective activity of psoralidin in vivo than coumestrol is attributed by its presence of an isopentenyl group and through activated PI3K/Akt axis. Biomed Pharmacother. 2018;102:1015–1024. doi: 10.1016/j.biopha.2018.03.166. [DOI] [PubMed] [Google Scholar]
- 152.Luo G., Xu B., Huang Y. Icariside II promotes the osteogenic differentiation of canine bone marrow mesenchymal stem cells via the PI3K/AKT/mTOR/S6K1 signaling pathways. Am J Transl Res. 2017;9(5):2077–2087. [PMC free article] [PubMed] [Google Scholar]
- 153.Lee J.H., Yoon Y.M., Lee S.H. Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF-1α-GRP78-Akt Axis. Int J Mol Sci. 2017;18(6) doi: 10.3390/ijms18061320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao Z., Liu C., Bai Y., et al. Inhibitory effect of dihydroartemisinin on chondrogenic and hypertrophic differentiation of mesenchymal stem cells. Am J Transl Res. 2017;9(6):2748–2759. [PMC free article] [PubMed] [Google Scholar]
- 155.Díaz-Tocados J.M., Herencia C., Martínez-Moreno J.M., et al. Magnesium chloride promotes osteogenesis through Notch signaling activation and expansion of mesenchymal stem cells. Sci Rep. 2017;7(1):7839. doi: 10.1038/s41598-017-08379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mangolini M., Götte F., Moore A., et al. Notch 2 controls non-autonomous Wnt-signalling in chronic lymphocytic leukaemia. Nat Commun. 2018;9(1):3839. doi: 10.1038/s41467-018-06069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Y., Zhang Z., Gao F., et al. Paracrine regulation in mesenchymal stem cells: the role of Rap 1. Cell Death Dis. 2015;6(10):e1932. doi: 10.1038/cddis.2015.285. -e1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Y., Chiu S., Liang X., et al. Rap 1-mediated nuclear factor-kappaB (NF-κB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discovery. 2015;1(1):15007. doi: 10.1038/cddiscovery.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han Y.H., Kee J.Y., Park J., et al. Arctidens inhibits adipogenesis by inducing AMPK activation and reduces weight gain in high-fat diet-induced obese mice. J Cell Biochem. 2016;117(9):2067–2077. doi: 10.1002/jcb.25509. [DOI] [PubMed] [Google Scholar]
- 160.Kim H.L., Park J., Jung Y., et al. Platycodin D, a novel activator of AMP-activated protein kinase, attenuates obesity in db/db mice via regulation of adipogenesis and thermogenesis. Phytomedicine. 2019;52:254–263. doi: 10.1016/j.phymed.2018.09.227. [DOI] [PubMed] [Google Scholar]
- 161.van de Vyver M., Niesler C., Myburgh K.H., et al. Delayed wound healing and dysregulation of IL6/STAT3 signalling in MSCs derived from pre-diabetic obese mice. Mol Cell Endocrinol. 2016;426:1–10. doi: 10.1016/j.mce.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 162.Sui B.D., Zheng C.X., Li M., et al. Epigenetic regulation of mesenchymal stem cell homeostasis. Trends Cell Biol. 2019;S0962–8924(919):30202–30208. doi: 10.1016/j.tcb.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 163.Zhao G., Zhou X., Chen S., et al. Differential expression of microRNAs in decidua-derived mesenchymal stem cells from patients with pre-eclampsia. J Biomed Sci. 2014;21(1):81. doi: 10.1186/s12929-014-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X., Song Y., Liu D., et al. MiR-495 promotes senescence of mesenchymal stem cells by targeting bmi-1. Cell Physiol Biochem. 2017;42(2):780–796. doi: 10.1159/000478069. [DOI] [PubMed] [Google Scholar]
- 165.Hu E., Ding L., Miao H., et al. MiR-30a attenuates immunosuppressive functions of IL-1β-elicited mesenchymal stem cells via targeting TAB3. FEBS Lett. 2015;589(24 Pt B):3899–3907. doi: 10.1016/j.febslet.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 166.Liu L., Wang Y., Fan H., et al. MicroRNA-181a regulates local immune balance by inhibiting proliferation and immunosuppressive properties of mesenchymal stem cells. Stem Cell. 2012;30(8):1756–1770. doi: 10.1002/stem.1156. [DOI] [PubMed] [Google Scholar]
- 167.Li X., Song Y., Liu F., et al. Long non-coding RNA MALAT1 promotes proliferation, angiogenesis, and immunosuppressive properties of mesenchymal stem cells by inducing VEGF and Ido. J Cell Biochem. 2017;118(9):2780–2791. doi: 10.1002/jcb.25927. [DOI] [PubMed] [Google Scholar]
- 168.Wang Y., Fan H., Zhao G., et al. miR-16 inhibits the proliferation and angiogenesis-regulating potential of mesenchymal stem cells in severe pre-eclampsia. FEBS J. 2012;279(24):4510–4524. doi: 10.1111/febs.12037. [DOI] [PubMed] [Google Scholar]
- 169.Chen S., Zhao G., Miao H., et al. MicroRNA-494 inhibits the growth and angiogenesis-regulating potential of mesenchymal stem cells. FEBS Lett. 2015;589(6):710–717. doi: 10.1016/j.febslet.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 170.Song Y., Dou H., Li X., et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cell. 2017;35(5):1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 171.Zhao X., Liu D., Gong W., et al. The toll-like receptor 3 ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR-143. Stem Cell. 2014;32(2):521–533. doi: 10.1002/stem.1543. [DOI] [PubMed] [Google Scholar]
- 172.Li X., Wang J., Pan Y., et al. Long non-coding RNA HULC affects the proliferation, apoptosis, migration, and invasion of mesenchymal stem cells. Exp Biol Med. 2018;243(13):1074–1082. doi: 10.1177/1535370218804781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cherubini A., Barilani M., Rossi R.L., et al. FOXP1 circular RNA sustains mesenchymal stem cell identity via microRNA inhibition. Nucleic Acids Res. 2019;47(10):5325–5340. doi: 10.1093/nar/gkz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sinha S., Biswas M., Chatterjee S.S., et al. Pbrm1 steers mesenchymal stromal cell osteolineage differentiation by integrating PBAF-dependent chromatin remodeling and BMP/TGF-β signaling. Cell Rep. 2020;31(4):107570. doi: 10.1016/j.celrep.2020.107570. [DOI] [PubMed] [Google Scholar]
- 175.Sathiyanathan P., Samsonraj R.M., Tan C.L.L., et al. A genomic biomarker that identifies human bone marrow-derived mesenchymal stem cells with high scalability. Stem Cell. 2020;38(9):1124–1136. doi: 10.1002/stem.3203. [DOI] [PubMed] [Google Scholar]