Abstract
Obesity-induced inflammation, characterized by augmented infiltration and altered balance of macrophages, is a critical component of systemic insulin resistance. Chemokine-chemokine receptor system plays a vital role in the macrophages accumulation. CC-Chemokine Receptor-like 2 (Ccrl2) is one of the receptors of Chemerin, which is a member of atypical chemokine receptors (ACKR) family, reported taking part in host immune responses and inflammation-related conditions. In our study, we found ccrl2 expression significantly elevated in visceral adipose tissue (VAT) of high fat diet (HFD) induced obese mice and ob/ob mice. Systemic deletion of Ccrl2 gene aggravated HFD induced obesity and insulin resistance and ccrl2−/− mice showed aggravated VAT inflammation and increased M1/M2 macrophages ratio, which is due to the increase of macrophages chemotaxis in Ccrl2 deficiency mice. Cumulatively, these results indicate that Ccrl2 has a critical function in obesity and obesity-induced insulin resistance via mediating macrophages chemotaxis.
Keywords: Ccrl2, Inflammation, Insulin resistance, Macrophages, Obesity
Introduction
Obesity is becoming prevalent worldwide in not only western societies but also in Asian countries because of a sedentary lifestyle and overnutrition.1 It is well known that obesity is related to chronic, low-grade tissue inflammation that can reduce insulin sensitivity and eventually contributes to insulin resistance and type 2 diabetes.2, 3, 4 The inflammatory response triggers immune cells into adipose tissue and ultimately impairs adipocyte function.5 Among these immune cells, macrophages account for the highest proportion up to about 40%–60%, which are named as adipose tissue macrophages(ATMs).6 The ATMs can release proinflammatory cytokines, such as TNFα, IL1β, and IL6. These cytokines have paracrine effects in the adipose tissue and may also be transported via the systemic circulation, impairing insulin sensitivity in other tissues.7,8
It is reported that the chemokine-chemokine receptors system plays a crucial role in the differentiation and activation of mononuclear phagocytes.9 For example, monocyte chemoattractant protein–1 (MCP1), reported increasing in obesity, causes bone marrow-derived macrophages (BMDMs) infiltrating into obesity adipose tissue via binding to the CCR2 receptor and is involved in the development of insulin resistance.10,11 Chemokine receptors, with seven transmembrane domains, belong to the G-protein-coupled receptors (GPCRs) superfamily. Chemokine receptors are differentially expressed by all leukocytes and can be divided into the following two groups: a more significant subgroup of G protein-coupled leukocyte chemotactic receptors and a smaller subgroup of atypical chemokine receptors (ACKRs).12, 13, 14 Compared to G protein-coupled chemokine receptors, ACKRs do not signal through G proteins, but instead, they promote ligand internalization and degradation.15 ACKR family comprises five receptors: ACKR1, ACKR2, ACKR3, ACKR4, and CC-Chemokine Receptor-like 2.16ACKRs are regulators of leukocyte traffic, inflammation, and immunity.17
ACKR5-Ccrl2 is expressed by a variety of leukocytes, including macrophages, dendritic cells, neutrophils, microglia, and is rapidly upregulated following stimulation with pro-inflammatory stimuli, such as LPS and TNF-α.18, 19, 20, 21 Ccrl2 is thought to bind several chemokines, such as Chemerin, CCL2, CCL5, CCL7, and CCL8.22,23 Among these ligands, Ccrl2 is reported to connect tightly with Chemerin, an adipokine produced by visceral adipose tissue (VAT), placenta, and liver.24 Ccrl2 was shown to bind and present the adipokine Chemerin to the functional receptor ChemR23, a function that may be relevant for leukocyte extravasation.18 The Chemerin system is tightly associated with obesity, diabetes, and adipogenesis. As a receptor of Chemerin, functional studies have shown that Ccrl2 may play an essential role in the NAFLD, dendritic cells transport to secondary immune organs, tumor metastasis, and invasion.25, 26, 27, 28 However, there was no report about whether Ccrl2 takes a role in obesity and related inflammation by affecting macrophages in adipose tissue.
We report, for the first time, that mice lacking chemokine receptor Ccrl2 displayed aggravated insulin resistance and inflammation, which is due to the exaggerated inflammatory monocyte recruitment to VAT.
Materials and methods
Animals
C57BL/6J mice were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). Male ob/ob and db/db mice used in the research were purchased from the Model Animal Research Center of Nanjing University at the age of four-five weeks. Cyagen Biosciences Inc created Ccrl2 deficiency mice in C57BL/6 mice by microinjection of TALENs in fertilized eggs. Littermate mice (ccrl2+/+)/heterozygous (ccrl2+/−)/Homozygous (ccrl2−/−) mice were obtained by mating between heterozygotes. Mice were housed in colony cages under 12h light-dark cycles with free access to food and water at 22–24 °C. Male C57BL/6J ccrl2+/+/ccrl2+/−/ccrl2−/− mice were fed a high-fat diet (60% fat; Research Diet)for 13–15 weeks since the eighth week or fed with a normal diet since weaning. Male ob/ob and db/db mice were fed a normal diet. All the experiments were performed under the approved guidelines of the institutional Animal Care and Use Committee of Chongqing medical university and follow the national institute of health guidelines on the care and use of animals.
Glucose tolerance tests and insulin tolerance tests
For glucose tolerance test (GTT), mice were fasted for 14 h (from 19:00 to 9:00). After that, the mice were intraperitoneally injected with glucose (2 mg/g glucose, 50% glucose solution). The glucose level was monitored at the indicated time points(30min,60min,90min,120min). For insulin tolerance test (ITT), mice were fasted for four hours (from 9:00 to 13:00). After that, mice were intraperitoneally injected with insulin (0.75 mU/g). The glucose level was monitored at the indicated time points(15min,30min,45min,60min).
Western blot analyses and antibodies
Cells were scraped into lysis buffer and tissue samples need to be ground in lysis buffer (70HZ, 90s). Then the lysates were degenerated under 100 °C and centrifugated and then quantitated, equal amounts of protein were electrophoresed, blotted, and then incubated with corresponding antibodies and appropriate secondary HRP-conjugated antibodies. Antibodies used for our experiments were shown in Table 1.
Table 1.
Antibodies used in this study.
| Antibody Name | Catalog No. | Host Species | MW (KDa) | Working Dilition | Company |
|---|---|---|---|---|---|
| Β-Actin | 4967S | Rabbit | 45 | 1:1000 | Cell Signaling Technology |
| P-Akt | 4058S | Rabbit | 60 | 1:1000 | Cell Signaling Technology |
| Akt | 9272S | Rabbit | 60 | 1:1000 | Cell Signaling Technology |
| SAPK/JNK | 9252S | Rabbit | 46,54 | 1:1000 | Cell Signaling Technology |
| P44/22MAPK ERK | 9102S | Rabbit | 42,44 | 1:1000 | Cell Signaling Technology |
| p-P44/42MAPK ERK | 9101S | Rabbit | 42,44 | 1:1000 | Cell Signaling Technology |
| p-STAT3 | 9145S | Rabbit | 79,86 | 1:1000 | Cell Signaling Technology |
| STAT3 | 9139S | mouse | 79,86 | 1:1000 | Cell Signaling Technology |
| p-NF-Κb P65 | 3033S | Rabbit | 65 | 1:1000 | Cell Signaling Technology |
| NF-Κb P65 | 8242S | Rabbit | 65 | 1:1000 | Cell Signaling Technology |
| p-P38 MAPK | 9216S | mouse | 43 | 1:1000 | Cell Signaling Technology |
| P38 MAPK | 9212S | Rabbit | 40 | 1:1000 | Cell Signaling Technology |
RNA isolation, a quantitative real-time polymerase chain reaction
Total RNA was isolated by using TRIzol™ Reagent (Thermo Scientific, 15596026). For quantitative real time polymerase chain reaction (qRT-PCR), 1 μg total RNAs were reverse transcribed by using a Revert Aid first-strand cDNA synthesis kit (Thermo Scientific, 00698284). The samples were analyzed by using the Power SYBR Green PCR Master Mix (Thermo Scientific, 00736756) with the Quantstudio3/5 (Thermo Scientific). Target gene expression levels were calculated after normalization to the standard housekeeping gene 18s using the ΔΔCT method and expressed as relative mRNA levels compared with internal control. The primers used for qRT-PCR in this study were synthesized by sangon biotech and were shown in Table 2.
Table 2.
Primers used in this study.
| Primer Name | sequence(5′ to 3′) |
|---|---|
| 18S-F | CGCCGCTAGAGGTGAAATTCT |
| 18S-R | CATTCTTGGCAAATGCTTTCG |
| F4/80-F | CTTTGGCTATGGGCTTCCAGTC |
| F4/80-R | GCAAGGAGGACAGAGTTTATCGTG |
| CD11C-F | GGAAGGGATAAGAGCCAGTTTG |
| CD11C-R | CCCGGAATCTCTCACTTGGA |
| IL-1b-F | GCTGCTTCCAAACCTTTGACC |
| IL-1b-R | GAGTGATACTGCCTGCCTGAA |
| IL-6-F | GACAACCACGGCCTTCCCTAC |
| IL-6-R | TCATTTCCACGATTTCCCAGA |
| MCP-1-F | CTGGATCGGAACCAAATGAG |
| MCP-1-R | CGGGTCAACTTCACATTCAA |
| Mrc1-F | CTCTGTTCAGCTATTGGACGC |
| Mrc1-F | TGGCACTCCCAAACATAATTTGA |
| TNFα-F | CGTCGTAGCAAACCACCAA |
| TNFα-R | GGGCAGCCTTGTCCCTTGA |
| Ccrl2-F | GCCCCGGACGATGAATATGAT |
| Ccrl2-R | CACCAAGATAAACACCGCCAG |
H&E staining and immunohistochemistry
For H&E staining or immunohistochemistry, adipose tissue tissues were fixed by using 4%(vol./vol.) formaldehyde, embedded in paraffin and cut. H&E staining was performed on the slices after rehydration staining with hematoxylin and eosin. Immunohistochemistry was performed to evaluate the macrophages recruitment to adipose tissues by immunostaining against antibody F4/80 and DAPI. Images were obtained with an optical microscope.
SVF isolation and flow cytometry analysis
Adipose tissue tissues were excised and minced in PBS, containing 0.075% collagenase (Sigma, C2139). After incubated at 37 °C for 30min and filtrated with 100 μm filter, cell suspensions were centrifuged at 1500 rpm for 5 min to remove adipocyte. Isolated stromal vascular fraction (SVF) pellet was collected from the bottom. The SVF pellet was resuspended in PBS containing 3% BSA, then red blood cell lysis buffer was added and incubated for 3 min. After washing in 3% FBS, bottom cells were incubated with Fc Block (CD16/32,12-0161-85,ebioscience) for 20 min at 4 °C. Antibodies against CD45-FITC (11-0451-82, ebioscience), F4/80-PE (123110,Biolegend), CD11b-PerCP/Cy5.5 (101227, Biolegend), CD206-APC (141707, Biolegend) and CD11c-APC (117310, Biolegend) were added, and incubated for 20 min followed by washing in PBS containing 3% FBS. The analysis was determined by flow cytometry (BD Biosciences).
Peritoneal macrophages isolation, culture, and polarization
Peritoneal macrophages were isolated from 1640 that containing 50% FBS injected mice. Mice were injected 5 ml 1640 containing 50% FBS, 30 min later, the injection was obtained then centrifuged at 1200 rpm for 5 min. Pellets were pooled from 4 or 5 mice per group, resuspended in red blood cell lysis buffer, and then the cells seeded in the same medium as for 1640. After 1–2h, unattached cells were removed, and the adherent cells were washed twice with medium alone before the addition of fresh culture medium. The next day, Macrophages were treated with 10 ng/ml LPS for polarization.
BMDMs isolation, culture and polarization
To isolate BMDMs, femur tibias of four-six weeks old male C57BL/6J mice were collected. Bone marrow was cultured and differentiated for seven days, in DMEM medium with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S), and M-SCF (10 ng/ml). On the last day, macrophages were treated with 10 ng/ml LPS for M1 polarization.
Monocyte preparation and vivo migration
After red blood cell lysis, Leukocyte pools from 12 weeks male C57BL/6 WT, ccrl2−/−mice were obtained. Then monocyte subsets were enriched with the EasySep mouse monocyte enrichment kit (STEMCELL Technologies), following the manufacturer's instructions.
Isolated monocytes (5 × 106 to 10 × 106) were washed once in serum-free medium (RPMI-1640) at 1200 rpm for 5 min and then suspended in 2 mL diluent solution C (PKH26 labeling kit, PKH67 labeling kit, Sigma). A total of 2 mL PKH26/PKH67 (Sigma) at 2 × 10−3 mol/L in diluent C was added and mixed, incubated for 10 min at room temperature in the dark. The staining reaction was stopped by the addition of an equal volume (2 mL) of 1% BSA in PBS. After 1 min, the mixture was centrifuged at 1200 rpm for 10 min, and the cells were washed once and resuspended in a serum-containing medium. After labeled with PKH26/PKH67, the monocytes were counted, equal monocytes about 1 × 106 were mixed (WT-PKH26/ccrl2−/−-PKH67) and suspended in 0.2 mL PBS and then injected into HFD C57/BL6 WT mice via the femoral vein. Seven days after the injection, the ATMs were immediately isolated from VAT and analyzed by using fluorescence microscope.
In vitro chemotaxis assay
For the migration assay, 100,000 Peritoneal Macrophages were taken out, as previously mentioned, obtained from WT or ccrl2−/− mice. The Peritoneal Macrophages were placed in the upper chamber of an 8 μm polycarbonate filter (24-transwell format; Corning), whereas the MCP1 conditioned medium was placed in the lower chamber. After three hours of migration, cells were fixed in formalin and stained with crystal violet and observed.
Immunofluorescence staining
Adipose tissue was cut and snap-frozen in optimum cutting temperature (O.C.T., Fisher Healthcare). 15 mm cryo-sections of tissue sections were cut and fixed with pre-cold acetone for 20 min. Before adding primary mAbs, sections were blocked with 5% normal donkey serum for 2h. Shake off the blocking solution and add F4/80 antibody (PBST dilution: 0.1% Tween-20 and 0.5% BSA, 1: 100 dilution) dropwise, and incubate the slides in a wet box at 4 °C overnight (>8h). Take out and rewarming for more than 30min the next day, soak and shake the sections in PBS buffer for 3min three times, dry the remaining PBS buffer, and add Ki67 fluorescent dye (PBST dilution: 0.1% Tween-20 and 0.5% BSA, 1: 100 dilution) and Incubate for 1h at room temperature. Soak and shake the sections in PBS buffer for 3min three times, wipe off the remaining PBS buffer, and stain the nuclei with DAPI (4′,6-Diamidino-2-28 phenylindole dihydrochloride) for 10 min at room temperature. Soak and shake the sections in PBS buffer solution for 3min three times, dry the remaining PBS buffer solution, cover with 50% glycerol (diluted with PBS), and use nail polish on the surrounding map to block the air. Observe immediately by a fluorescence microscope.
ATMs isolated from SVF
SVF isolation was according to the previous method and resuspended in 2 ml of medium (1640 + 10% FBS + P/S), Then gently add 2 ml of 50% percoll, 2 ml of 25% percoll in a new test tube. Cell suspension (percoll preparation according to the instructions, Sigma–Aldrich, P1644-500ML), centrifugation at 4 °C, 1800g for 15min, suck the middle cloud-like suspension, then centrifuge at 650g for 5min, wash the pellet with 3 ml culture medium twice, discard the supernatant, add TRIzol™ Reagent (Invitrogen) for RNA isolation.
ELISA
Serum insulin levels and IL-6 levels were analyzed by ELISA kit buying from MULTI SCIENCES and the specific operation steps were according to the manufacturer's protocol.
Statistical analysis
All data are presented as mean ± SEM. Mean value differences between two groups were assessed by two-tailed Student t-test. P values of 0.05 or less were considered to be statistically significant. Statistical analyses were performed with Graph Pad Prism 5.
Results
The ccrl2 expression is upregulated in adipose tissues of obesity and diabetic mice
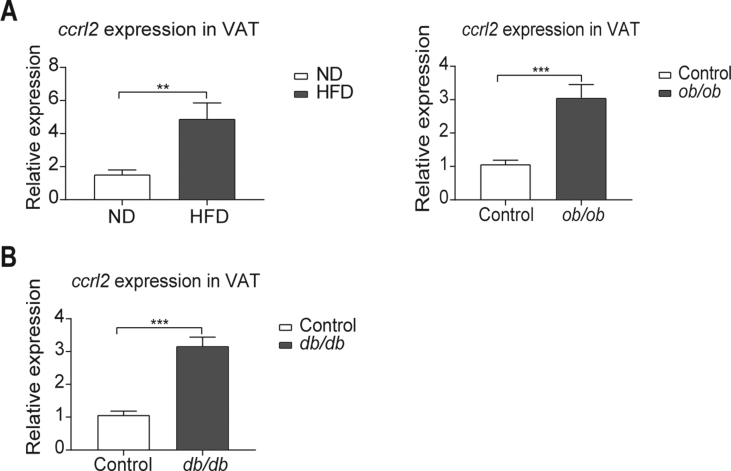
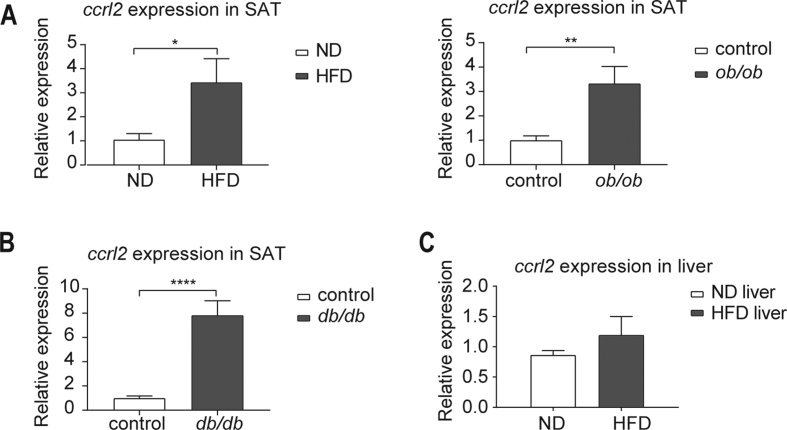
In obesity, the expansion of VAT (visceral adipose tissue) is closely linked to metabolic diseases, including insulin resistance.29,30 To address the potential role of Ccrl2 in obesity and diabetes in mice, the ccrl2 expression was detected in VAT of obesity mice. Data showed that ccrl2 expression was up-regulated in VAT of high-fat diet (HFD)-induced obese (DIO) mice and ob/ob mice comparing with control mice (Fig. 1A). In diabetic db/db mice, ccrl2 was also significantly up-regulated (Fig. 1B). Besides VAT, the ccrl2 expression was also up-regulated in the subcutaneous adipose tissue (SAT) in both obesity and diabetic mice (Fig. S1A, B), while no difference in the liver of HFD mice (Fig. S1C). These results indicate that Ccrl2 might play roles in obesity and diabetes especially by affecting adipose tissue.
Figure 1.
ccrl2 gene expression is positively correlated with obesity and type 2 diabetes. (A) qPCR analysis of ccrl2 mRNA expression in viscera adipose tissue of C57BL/6J mice fed with ND or HFD for 12 weeks (n = 9), and ob/ob mice (n = 8) and their wild-type (WT) littermates (n = 8). (B) qPCR analysis of ccrl2 mRNA expression in viscera adipose tissue of db/db mice(n = 8) and their wild type(WT) littermates(n = 8). Data are expressed as Mean ± SEM, ∗∗P < 0.01, ∗∗∗P < 0.001.
Ccrl2 deficiency mice showed deteriorated obesity and insulin sensitivity on normal chow diet
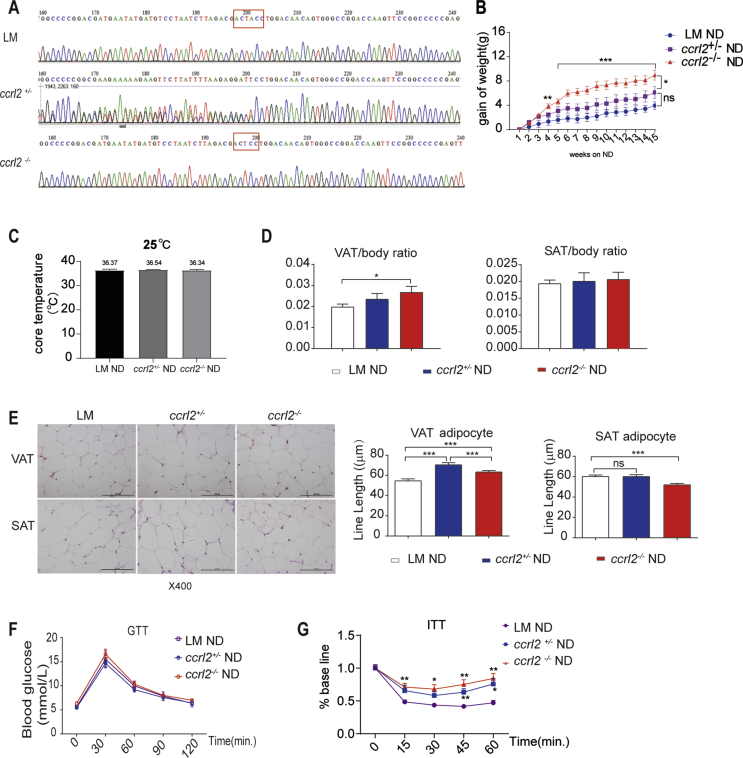
To elucidate the roles of Ccrl2 in metabolism, we created ccrl2−/− mice in C57BL/6 mice by microinjection of TALENs, which is the target of Exon 2 in fertilized eggs. The ccrl2−/− mice were fertile, with no apparent developmental defects. The gene deletion of ccrl2 was identified by sequencing analysis (Single base knockout) in the present study (Fig. 2A). From the 8th week, ccrl2−/−mice, ccrl2+/-mice and WT littermates were fed with normal diet (ND) for 15 weeks, the bodyweight of mice was measured weekly, statistics showed that the gain of the bodyweight of ccrl2−/−mice was significantly higher than that of WT littermates since the 4th week on ND, while no difference showed between ccrl2+/− mice and WT littermates (Fig. 2B). Systemic ccrl2 deletion had no significant effect on rectal temperature (Fig. 2C). To further assess body composition changes accompanying this increase in obesity, the weight of organs that are prone to lipid accumulation was weighed, ccrl2−/− mice exhibited an increased mass in VAT rather than SAT or liver (Fig. 2D, Fig. S2A). H&E staining revealed that ccrl2−/− mice exacerbated the increase in adipocyte size of VAT rather than the SAT or the liver after 15-weeks ND-fed (Fig. 2E, Fig. S2B). These data showed that Ccrl2 deficiency had a more significant effect on VAT. Strong epidemiologic data demonstrate that excess VAT, comparing with SAT, is a significant contributor to metabolic risk.31,32 Fig. 2F, G showed that although ccrl2−/− mice displayed no significance in glucose tolerance test (GTT) on ND, insulin tolerance test (ITT) indicated ccrl2−/−mice showed a deteriorated insulin sensitivity. This founding suggested that Ccrl2 deficiency leads to a deterioration of obesity, increased VAT adipocyte size and impaired insulin sensitivity.
Figure 2.
Ccrl2 deficiency mice showed aggravated obesity and insulin resistance on ND. (A) Gene sequencing results of knock out (one base knockout). (B) Body weight gain of Littermates ccrl2+/−ccrl2−/− mice fed with normal diet (n = 7, 10, 10). (C) core temperature of ND fed WT ccrl2+/−ccrl2−/− mice. (D) ratio of VAT/SAT to body weight in Littermates ccrl2+/−ccrl2−/− mice (n = 7, 10, 10). (E) HE and line length statistics of VAT and SAT, scale bar = 100 mm. (F) GTT (n = 7, 10, 10). (G) ITT (n = 7, 10, 10). Data are expressed as Mean ± SEM, ns = not significant, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Ccrl2 deficiency exacerbated HFD-induced obesity and insulin resistance
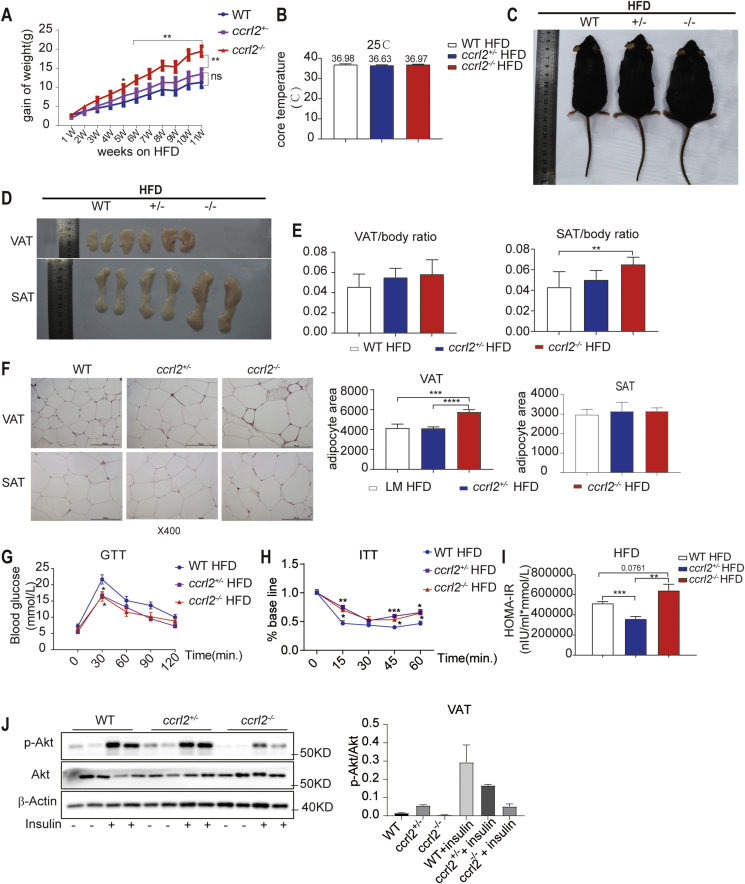
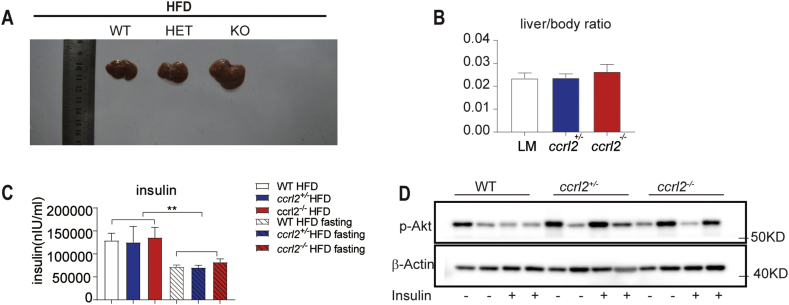
Then we assessed the susceptibility of ccrl2−/− mice to high-fat diet (HFD)-induced obesity. The mice were fed with HFD since the eighth week, the bodyweight of the mice was measured weekly, the body-weight gain was significantly increased on HFD ccrl2−/− mice relative to HFD WT mice with no significant effect on rectal temperature (Fig. 3A, B). Fig. 3C showed that HFD exacerbated the obesity of ccrl2−/− mice. The ccrl2−/− mice exhibited an increased mass in SAT, VAT, and liver, the weight ratio of SAT of ccrl2−/−mice was significantly increased but no difference in the weight ratio of VAT or the liver (Fig. 3D, E, and Figs. S3A, B). H&E staining results showed that ccrl2−/− mice exacerbated the increase in adipocyte size of VAT and showed conspicuous crown-like structure (CLS), which stands for an accumulation of macrophages rather than in the SAT(Fig. 3F). GTT and ITT showed that Ccrl2 deficiency aggravated the HFD-induced insulin sensitivity impairment rather than glucose tolerance (Fig. 3G, H). HOMA-IR value and Akt pathway also showed that ccrl2−/−mice had worse insulin sensitivity than WT mice(Fig. 3I, J). These data indicated that Ccrl2 deficiency aggravated diet-induced obesity and insulin resistance.
Figure 3.
Ccrl2 deficiency exacerbates diet-induced obesity and insulin resistance. (A) weight gain of WT ccrl2+/−ccrl2−/−mice fed with HFD (n = 7, 6, 7). (B) core temperature of HFD WT ccrl2+/−ccrl2−/−mice (n = 7, 6, 7). (C) The general picture of the mice's body. (D) The general picture of the SAT and VAT. (E) ratio of VAT/SAT to body weight in WT ccrl2+/−ccrl2−/−mice (n = 7, 6 ,7). (F) HE and adipocyte area statistics of VAT and SAT, scale bar = 100 mm. (G) GTT (n = 7, 6, 7). (H) ITT (n = 7, 6, 7). (I) HOMO-IR (n = 7, 6, 7). (J) The protein levels of p-Akt, Akt in VAT of different groups on HFD were evaluated by western blotting. Data are expressed as Mean ± SEM, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Ccrl2 deficiency aggravated VAT macrophages accumulation and increased the ratio of M1/M2
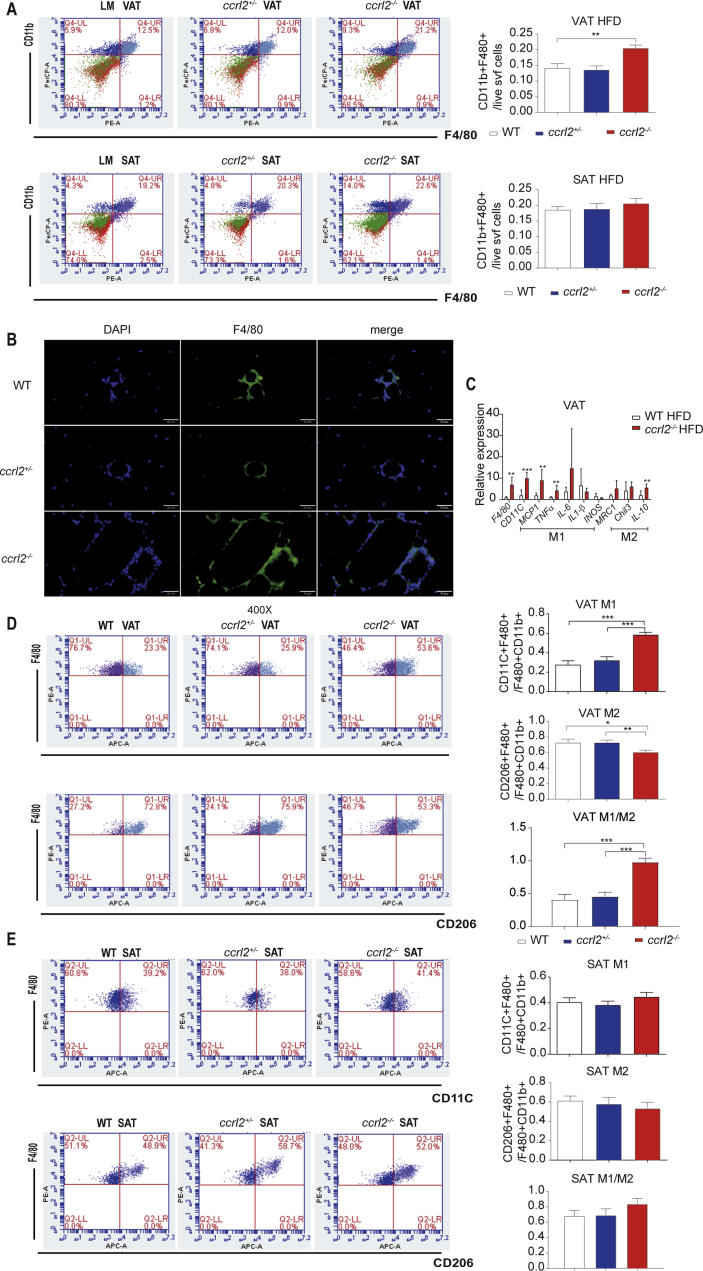
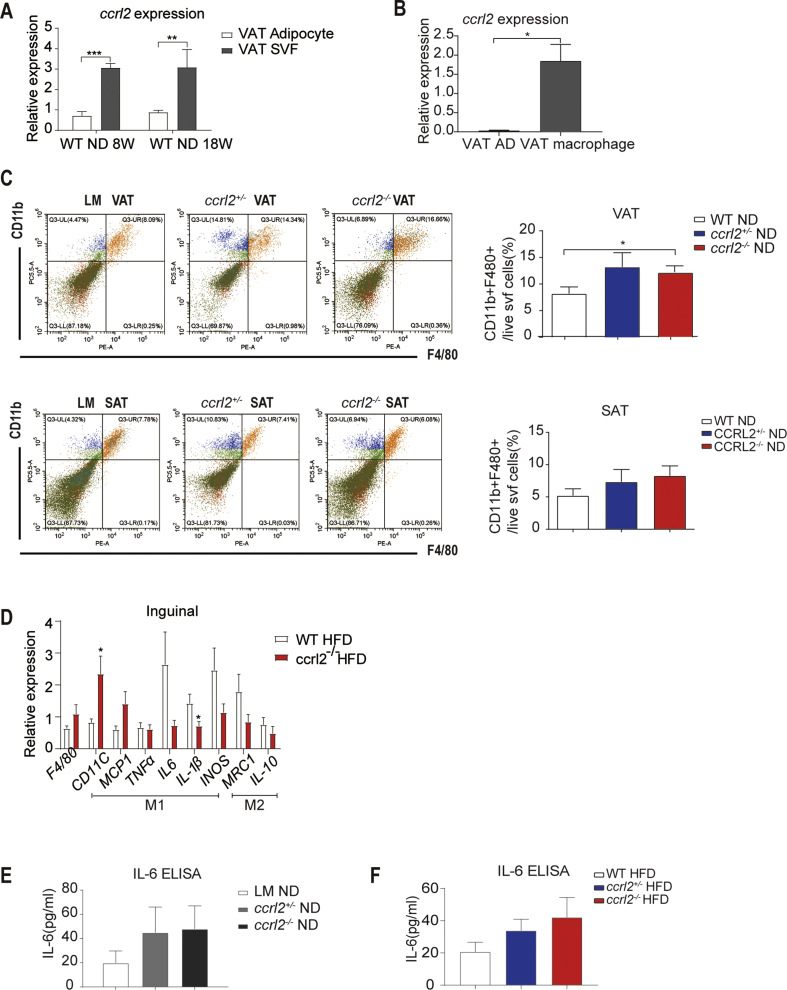
Response to obesity, VAT exhibits increased expression of inflammatory markers and increased ATM infiltration compared to SAT.5,33,34 Macrophage-induced inflammation is an important turning point in the development of obesity-related insulin resistance.35 To better understand the roles of Ccrl2 played in the inflammation, firstly we confirmed that ccrl2 was highly expressed in stromal vascular fraction (SVF) isolated from VAT rather than mature adipocytes (Fig. S4A). Comparing with mature adipocytes, ccrl2 was significantly increased in macrophages isolated from SVF (Fig. S4B). Flow cytometry analysis showed accumulation of macrophages in VAT of ccrl2−/− mice was increased comparing with WT by counting the number of CD45+F4/80+CD11b+ macrophages while there was no difference in SAT in both ND and HFD (Fig. 4A, Fig. S4C). Furthermore, F4/80 immunofluorescent staining showed more accumulation of macrophages in VAT from ccrl2−/− mice (Fig. 4B). These data showed that Ccrl2 deficiency aggravates inflammation in adipose tissue, especially by influencing adipose tissue macrophages (ATMs).
Figure 4.
Ccrl2 deficiency mice showed aggravated VAT inflammation manifested in accumulation of macrophages and increased M1/M2 ratio. (A) FCM analysis of proportion of macrophages in VAT and SAT of mice of different genotypes (n = 6, 8, 8). (B) F4/80 immunofluorescence staining of VAT. (C) ATMs associated inflammation markers in VAT (n = 6 in per group). (D) FCM results of the M1 M2 macrophage ratio of VAT (n = 7, 6, 7). (E) FCM results of the M1 M2 macrophage ratio of SAT (n = 7, 6, 7). Data are expressed as Mean ± SEM,∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Obesity is a state of chronic inflammation with infiltration of macrophages, characterized by an increased M1/M2 macrophages ratio, contributing to insulin resistance.36 Macrophages associated inflammation markers were detected in ccrl2−/− mice and WT mice and qPCR showed that proinflammation markers (CD11C, MCP1, TNFα) were significantly increased in VAT While no significant difference in SAT (Fig. 4C, Fig. S4D). Flow cytometry analysis showed the increased percentage of proinflammation M1 macrophages in ccrl2−/− mice than WT mice by counting the ratio of CD45+F4/80+CD11b+CD11C+ pro-inflammation M1 macrophages to total CD45+F4/80+CD11b+ macrophages. Meanwhile, the ratio of CD45+F480+CD11b+CD206+ anti-inflammation M2 macrophages to total CD45+F4/80+CD11b+ macrophages was downregulated in the VAT rather than the SAT on HFD (Fig. 5D, E).
Figure 5.
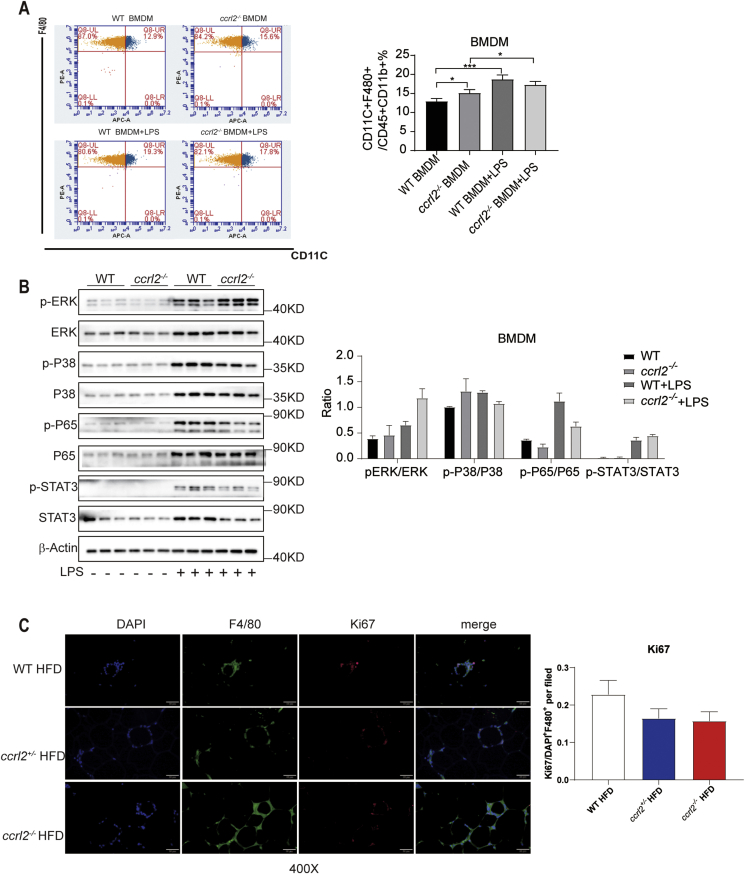
Accumulation of macrophage and the ratio of M1/M2 had nothing to do with polarization and proliferation. (A) FCM results of LPS Statistical stimulated bone marrow-derived macrophage from WT and ccrl2−/− mice. (B) Western Blot of inflammation associated protein of LPS stimulated bone marrow-derived macrophage from WT and ccrl2−/− mice. (C) results of VAT of WT/ccrl2+/−/ccrl2−/− mice containing CLS stained with antibodies against Ki67 (red) and F4/80 (green), scale bar = 50 mm. Data are expressed as Mean ± SEM,∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Obesity contributes to the development of insulin resistance through the so-called obesity-associated low-grade inflammation or metaflammation. Clamp experiments indicating that IL-6 infusion causes insulin resistance and obesity have a positive correlation with and the circulation IL6, IL-6 takes part in the connection between insulin resistance and inflammation.37, 38, 39, 40 Other studies showed IL-6 took park in the infiltration of macrophages.41 IL-6 level was detected both in the serum of WT and ccrl2−/− mice on ND and HFD and found that IL-6 of ccrl2 deficiency mice were more than that of WT mice, which could explain the impaired insulin sensitivity in ccrl2−/− mice (Fig. S4E, F). Taken together, these results suggested that ccrl2−/− mice showed impaired insulin sensitivity accompanying increased serum IL6 level and M1/M2 ratio in VAT in HFD induced obesity.
No difference on polarization or proliferation between ccrl2−/− and WT mice derived macrophage
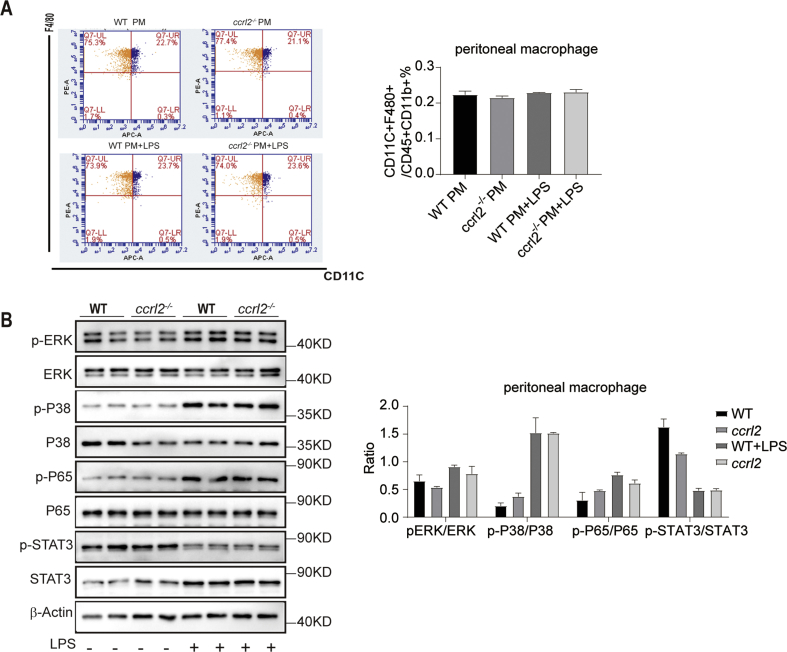
Considering the VAT of ccrl2−/− mice had increased M1/M2 ratio, to confirm the direct effect of Ccrl2 on the macrophages polarization, the bone marrow derived macrophages (BMDMs) were isolated from WT and ccrl2−/− mice. Lipopolysaccharide (LPS) (100 ng/ml) was used to drive macrophages polarizing to M1 macrophages. Western blot showed no difference between ccrl2−/− mice derived macrophages and WT derived macrophages (Fig. 5B). Flow cytometry analysis was consistent with Western blot results in the case of LPS stimulation, while M1 macrophages showed a significant increase without LPS stimulation (Fig. 5A). Besides BMDMs, peritoneal macrophages were taken from WT or ccrl2−/− mice, FCM and Western blot showed no difference between WT and ccrl2−/− mice in polarization (Fig. S5A, B).
Then, we tried to explore why the accumulation of ATM increased in VAT of ccrl2−/− mice. ATM accumulation could be determined by proliferation.5 The ATM proliferation was detected through Ki67, a protein expressed during all active phases of the cell cycle. The data showed no significant difference between ccrl2−/− mice and WT mice in the macrophages proliferation (Fig. 5C). These data indicated that Ccrl2 deficiency aggravated macrophages accumulation was not through proliferation and the M1/M2 ratio increase was not due to the polarization of macrophages.
The absence of Ccrl2 contributed to adipose tissue inflammation was depending on the chemotaxis of macrophages
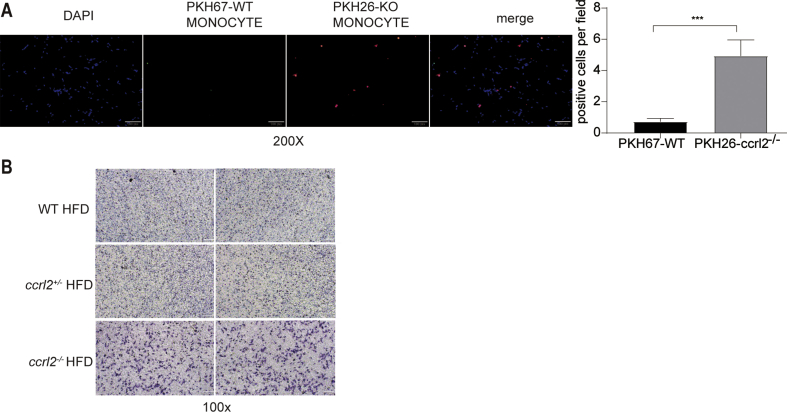
Besides proliferation, infiltration is another important reason for macrophages accumulation.42,43 Monocytes were isolated from the peripheral blood, monocytes derived from WT mice were dyed with PKH67 (green fluorescence) while monocytes from ccrl2−/− mice were dyed with PKH26 (red fluorescence). Dyed monocytes were mixed with equal cells and injected into WT mice through the tail vein, which was fed on HFD for two weeks. VAT was prepared after injection for seven days. Using fluorescence microscope, Fig. 6A showed that PKH26 positive monocytes derived from ccrl2−/− mice were more than WT derived monocytes stained with PKH67. To further confirm the chemotaxis of macrophages, in vitro trans-well chemotaxis assay was employed to detect the migratory capacity of peritoneal macrophages from ccrl2−/− mice and WT littermates. As shown in Fig. 6B, macrophages from ccrl2−/− mice migrated rapidly than WT mice. These data suggested that more macrophages accumulation in VAT of ccrl2−/− mice was due to the increased chemotaxis of macrophages.
Figure 6.
The absence of Ccrl2 increases macrophage chemotaxis. (A) fluorescence microscope results of vivo migration results of monocytes derived from WT and ccrl2−/− mice, scale bar = 100 mm. (B) transwell results of peritoneal macrophage, scale bar = 100 mm. Data are expressed as Mean ± SEM, ∗∗∗P < 0.001.
Discussion
It is well known that obesity contributes to the development of insulin resistance through the obesity-associated low-grade inflammation and increased accumulation of proinflammatory macrophages in adipose tissue and liver.44, 45, 46 Inflammation triggered by macrophages constitutes a turning point in the development of obesity-related insulin resistance.35 Chronic inflammation is mainly supported by chemokine-chemokine receptor systems like CCL2-CCR2 and some other essential combinations.47 In the present study, we found that deficiency of Ccrl2, which is a member of ACKRs, led to a robust deterioration in systemic insulin sensitivity as well as obesity. The deterioration in insulin sensitivity was traced to a marked increase in VAT inflammation, with more ATMs infiltration and increased M1/M2 ratio in adipose tissue of HFD-fed ccrl2−/− mice. Our data shed light on the impact of Ccrl2 in the inflammation and suggest that Ccrl2 may be a new target in the intervention of inflammation and insulin resistance in obesity.
Firstly, the ccrl2 expression was found significantly increased in both obesity and diabetic mice models. It is well known that obesity is associated with an increased risk of type 2 diabetes, and in the process of obesity to diabetes, insulin resistance played a very important role.2 Evidence showed with the progression of obesity, excess adiposity secretes some adipokines like MCP1 that could promote the infiltration of macrophages.48 These ATMs are a prominent source of proinflammatory cytokines like IL-6 and TNFα and further induce systemic insulin resistance.49,50 Ccrl2 is a receptor of many inflammation associated adipokines like chemerin, MCP1, and could adaptive change along with these adipokines. Thus, the increased expression of ccrl2 in obesity and diabetes may mainly associate with the development of obesity-related inflammation and insulin resistance. In our article, we showed that Ccrl2 deficiency deteriorates obesity and insulin resistance while it was interesting to find that the ccrl2 expression was significantly increased in the adipose tissue of obesity (HFD and ob/ob mice) and diabetes mice (db/db mice). The results could be explained by a compensatory mechanism. During obesity development process, the chemokine like MCP1 increased and promotes more macrophage accumulation into the adipose tissue, the infiltrated macrophages may in turn secrete a variety of chemokines and other cytokines that thus further promote adipose tissue inflammatory response, resulting in systemic insulin resistance.10 Our results showed that Ccrl2 deficiency increased adipose tissue macrophages infiltration, as a member of ACKRs, Ccrl2 lacks the structure of signal transmission and could not transduct the ligand signals into the cells, in the situation of obesity, to counteract the process of macrophages accumulation, the expression of ccrl2 may increase and reduce the macrophages infiltration through competitively binding to ligands, thereby weakening the role of functional receptors which similar to the process of combining with Chemerin.15,18 However, this compensation mechanism did not be strong enough to result in a retrograde change in the number of adipose tissue macrophages in obese and diabetic mice. This compensatory response is common in the body, for example, leptin, which is an important adipokine reported to inhibit feeding thereby to resistant obesity and also has a correlation with adipose tissue inflammation while a study showed the serum leptin levels were approximately four times higher in obese compared to normal subjects.51, 52, 53, 54
Chemerin is the most crucial ligand of Ccrl2, plays an essential role in adipogenesis and adipocyte metabolism, shows a great importance in obesity.55 Many reports show that Chemerin plays different roles in different tissues or experimental models.56, 57, 58 In obesity, Chemerin is elevated in the serum of obese patients.59 Chemerin deficiency mice fed with normal diets and high-fat diets have lower insulin sensitivity.60 Insulin sensitivity is also worse in CMKLR1 deficiency mice.61 In our study, ITT and AKT signaling pathways indicated that insulin sensitivity of ccrl2−/− mice was impaired, which was consistent with the results of Chemerin deficiency and CMKLR1 deficiency results, but it seemed that the specific mechanism was different. In the Chemerin knockout mice and chemR23 knockout mice, the infiltration of macrophages is reduced.60,61 Our results showed macrophages accumulation in VAT of ccrl2−/− mice was more than that in control mice in both ND and HFD and M1 proinflammatory macrophages were increased in ccrl2 −/− mice which was responsible for the worsening of insulin sensitivity. Besides, the weight of Chemerin deficiency mice was similar to the WT mice, and CMKLR1 deficiency mice were significantly reduced while our research showed the weight gain of ccrl2−/−mice was significantly higher than that of WT control mice. It is reported that Ccrl2 enriches Chemerin after binding, further presents it to the critical receptor-CMKLR1, and thus helps magnify the function of Chemerin. However, in our research, it seemed that Ccrl2 was not through magnifying the function of Chemerin-CMKLR1.CCL2 is also a ligand of Ccrl2 and has been shown to play an important role in obesity and insulin resistance.11,62 Lei-Ping Wang et al showed overexpression of Ccrl2 inhibits CCL2-induced chemotaxis and invasion in breast cancer which could indicate a relationship between Ccrl2 and CCL228. In the adipose tissue, it is reported that CCL2(MCP1) could regulate the migration and infiltration of monocytes, in this way, whether Ccrl2 could lead to a decrease in monocyte chemotaxis by competitive binding to CCL2 with CCR2 needs to be studied. However, another research showed CCL2 is released by adipocytes in crown-like structure and could stimulate the proliferation of surrounding ATMs, but in our study, ATMs proliferation in VAT of ccrl2−/− mice had no significant difference from WT mice which (Fig. 5C).5 As for another receptor-CCL5, although CCL5 takes part in diseases that are closely related to inflammation like systemic lupus erythematosus (SLE), autoimmune nephritis, atherosclerosis and liver fibrosis, and CCL5/CCR5 axis directs infiltration and interactions with monocytes/macrophages and mesenchymal stem cells.63,64 But there is no evidence that CCL5 takes part in ATMs infiltration, whether in adipose tissues Ccrl2 affects macrophage chemotaxis by interrupting CCL5/CCR5 needs further research. Another reported ligand of Ccrl2 is CCL19. The most crucial functional receptor of CCL19 is CCR7, and mice lack CCR7 are protected from diet-induced obesity and insulin resistance.65 Considering this, it was possible that Ccrl2 could internalize CCL19 binding, Ccrl2 knock out might led to more CCL19-CCR7 connection, which resulted in obesity and IR, but this needs to be further explored in the future.23
Ccrl2 is expressed by a variety of leukocytes, especially macrophages. In the process of obesity, adipose tissue macrophages played important roles, imbalance of proinflammatory M1 macrophages and anti-inflammatory M2 macrophages is well correlated with inflammation and insulin resistance in obesity.66 In our study, FCM and gene expression showed an increased M1/M2 macrophages ratio. Besides, we found that the pro-inflammatory cytokines IL-6 level of ccrl2−/−mice was more than that of WT mice in the serum of HFD mice, which could further explain the insulin sensitivity of ccrl2−/−mice was impaired. ITT and Akt signaling pathways indicated that insulin sensitivity of ccrl2−/− mice became worse. However, it was interesting that there was no difference in GTT between ccrl2−/− mice and WT mice. We know that GTT mainly shows the sensitivity of body insulin to glucose, while ITT mainly focuses on tissue sensitivity to insulin, which means that in ccrl2−/− mice, tissue sensitivity to insulin is impaired. On HFD, the primary affected tissue is VAT rather than SAT, which manifested in the size of adipocytes while the weight of SAT was more than VAT. Compared to SAT, VAT hypertrophy is positively related to insulin sensitivity impairment.31,32 We found the p-Akt protein did not change in SAT (Fig. S3D). Whether long-term HFD stimulated obesity will affect GTT is unclear.
The process of ATM accumulation in the adipose tissue is multifactorial; among these factors, proliferation and infiltration are of the most importance. Proliferation results of Ki67 immunofluorescence staining Showed no significant difference between ccrl2−/− mice and WT mice. Depends on the data from in vivo migration and in vitro chemotaxis assay, we found that monocytes or macrophages isolated from ccrl2−/− mice showed more chemotaxis than WT mice, which might explain why in the situation of both ND and HFD, the number of macrophages was more in VAT of ccrl2−/− mice than that in WT mice. In addition to macrophages regionalization ability, adipose tissue secretes some adipokine to affect the chemotaxis of macrophages. Our experimental results showed that the cell size of visceral adipocytes was significantly enlarged rather than hyperplasia, both in ND or HFD. It is known that hypertrophic adipocytes, especially viscera adipose tissue, could secrete chemokines to attract various inflammatory cells and promote chronic adipose tissue inflammation.67 However, in our research, an essential macrophage-related chemokine-MCP1 expression was not significantly different between the two groups of mice at ND, so we excluded the possibility that increased secretion of chemokines led to macrophages accumulation. MCP1 increased in HFD might indeed help more macrophages infiltrate into VAT, but it is not the starting factor.
The recruitment of macrophages in adipose tissue is the first event and primary contributor to inflammation in obesity.68 On HFD, some proinflammation markers like CD11C, MCP1, TNFα were upregulated, but it was interesting that although the number of macrophages was significantly increased during ND, the qPCR results showed that the expression of pro-inflammatory chemokines related genes in VAT was not significantly increased. However, Like HFD, ND fed ccrl2−/− mice showed significant deterioration in ITT results. Therefore, we tested the peripheral blood IL-6 levels in ND mice and found that the peripheral blood IL-6 levels in ND mice were indeed upregulated (Fig. S4E). It is speculated that in the case of ND, there is compensatory regulation of mRNA levels.
Conclusion
The study firstly demonstrated that Ccrl2 played an essential role in the pathogenesis of obesity and obesity-associated insulin assistance and has shown that Ccrl2 takes part in the migration of macrophages into VAT.
Author contributions
M.X, Y.M.W and X.L, conceived and designed the experiments. M.X, Y.M.W, W.Q.L, C.L.H, J.L performed the experiments. M.X, Y.M.W and X.L analyzed the data. W.H.X, H.X.Z and L.F.T help completed relevant experiments. M.X, Y.M.W and X.L wrote the paper.
Conflict of interests
The author declares no conflict interest.
Funding
This work was supported by National Key R&D Program of China (No. 2018YFA0800401 to X. Li); National Natural Science Foundation of China (No. 81770861 and 31571401 to X. Li); Chongqing Science and Technology Foundation (No. cstc2018jcyjAX0232); Science and Technology Research Program of Chongqing Municipal Education Commission (No. KJZD-K201800402).
Acknowledgements
We are grateful to Dr. Rui He, from Department of Immunology, Fudan University Shanghai Medical College, for her kindly share of ccrl2−/− mice.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2020.08.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
supplementary figure 1.
ccrl2 gene expression is upregulated in obesity and type 2 diabetic mice model. (A) qPCR analysis of ccrl2 mRNA expression in subcutaneous adipose tissue of C57BL/6J mice fed with ND or HFD for 12 weeks (n = 6), and ob/ob mice (n = 8) and their wild-type(WT) littermates (n = 8). (B) qPCR analysis of ccrl2 mRNA expression in subcutaneous adipose tissue of db/db mice(n = 8) and their wild type(WT) littermates(n = 8). (C) qPCR analysis of ccrl2 mRNA expression in liver of C57BL/6J mice fed with ND or HFD for 12 weeks (n = 5).Data are expressed as Mean ± SEM,∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Supplementary figure 2.
Ccrl2 deficiency mice showed aggravated obesity and insulin resistance on ND. (A)ratio of Liver to body weight in Littermates ccrl2+/−ccrl2−/−mice(n = 7,10,10). (B) HE results of liver.
Supplementary figure 3.
Ccrl2 deficiency exacerbates diet-induced obesity and insulin resistance. (A) The general picture of the liver. (B) ratio of liver to body weight in WT ccrl2+/−ccrl2−/− mice(n = 7,6,7). (C) Serum insulin concentration(n = 7,6,7). (D) The protein levels of p-Akt in SAT of different groups on HFD were evaluated by western blotting. Data are expressed as Mean ± SEM,∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Supplementary figure 4.
Ccrl2 deficiency mice showed aggravated VAT inflammation manifested in accumulation of macrophages and increased M1/M2 ratio. (A) ccrl2 expression in VAT of ND 18w mice(n = 6) and ND 8W mice (AD n = 4 SVF n = 6). (B) ccrl2 expression in adipocyte and isolated macrophage from SVF of VAT(n = 3). (C) FCM analysis of proportion of macrophages in VAT or SAT of mice fed with ND of different genotypes(n = 6,8,8). (D) ATMs associated inflammation markers in SAT(n = 6 in per group). (E) serum IL-6 level in ND mice. (F) serum IL-6 level in HFD mice. Data are expressed as Mean ± SEM, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Supplementary figure 5.
Accumulation of macrophage and the ratio of M1/M2 had nothing to do with polarization and proliferation. (A) FCM results of LPS Statistical stimulated peritoneal macrophages from WT and ccrl2−/− mice. (B) Western Blot of inflammation associated protein of LPS stimulated peritoneal macrophages from WT and ccrl2−/− mice. Data are expressed as Mean ± SEM, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
References
- 1.Fan J.G., Kim S.U., Wong V.W. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67(4):862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 4.Han M.S., Jung D.Y., Morel C., et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amano S.U., Cohen J.L., Vangala P., et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metabol. 2014;19(1):162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 8.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Kanda H., Tateya S., Tamori Y., et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L., Kitade H., Ni Y., Ota T. Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules. 2015;5(3):1563–1579. doi: 10.3390/biom5031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonecchi R., Savino B., Borroni E.M., Mantovani A., Locati M. Chemokine decoy receptors: structure-function and biological properties. Curr Top Microbiol Immunol. 2010;341:15–36. doi: 10.1007/82_2010_19. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A., Bonecchi R., Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6(12):907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 14.Bachelerie F., Ben-Baruch A., Burkhardt A.M., et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66(1):1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzotti C., Gagliostro V., Bosisio D., et al. The atypical receptor CCRL2 (C-C chemokine receptor-like 2) does not act as a decoy receptor in endothelial cells. Front Immunol. 2017;8:1233. doi: 10.3389/fimmu.2017.01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvi V., Sozio F., Sozzani S., Del Prete A. Role of atypical chemokine receptors in microglial activation and polarization. Front Aging Neurosci. 2017;9:148. doi: 10.3389/fnagi.2017.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nibbs R.J., Graham G.J. Immune regulation by atypical chemokine receptors. Nat Rev Immunol. 2013;13(11):815–829. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- 18.Zabel B.A., Nakae S., Zuniga L., et al. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med. 2008;205(10):2207–2220. doi: 10.1084/jem.20080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monnier J., Lewen S., O'Hara E., et al. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J Immunol. 2012;189(2):956–967. doi: 10.4049/jimmunol.1102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel L., Charlton S.J., Chambers J.K., Macphee C.H. Expression and functional analysis of chemokine receptors in human peripheral blood leukocyte populations. Cytokine. 2001;14(1):27–36. doi: 10.1006/cyto.2000.0851. [DOI] [PubMed] [Google Scholar]
- 21.Galligan C.L., Matsuyama W., Matsukawa A., et al. Up-regulated expression and activation of the orphan chemokine receptor, CCRL2, in rheumatoid arthritis. Arthritis Rheum. 2004;50(6):1806–1814. doi: 10.1002/art.20275. [DOI] [PubMed] [Google Scholar]
- 22.Biber K., Zuurman M.W., Homan H., Boddeke H.W. Expression of L-CCR in HEK 293 cells reveals functional responses to CCL2, CCL5, CCL7, and CCL8. J Leukoc Biol. 2003;74(2):243–251. doi: 10.1189/jlb.0802415. [DOI] [PubMed] [Google Scholar]
- 23.Leick M., Catusse J., Follo M., et al. CCL19 is a specific ligand of the constitutively recycling atypical human chemokine receptor CRAM-B. Immunology. 2010;129(4):536–546. doi: 10.1111/j.1365-2567.2009.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inci S., Aksan G., Dogan P. Chemerin as an independent predictor of cardiovascular event risk. Therapeut Adv Endocrinol Metabol. 2016;7(2):57–68. doi: 10.1177/2042018816629894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimny S., Pohl R., Rein-Fischboeck L., et al. Chemokine (CC-motif) receptor-like 2 mRNA is expressed in hepatic stellate cells and is positively associated with characteristics of non-alcoholic steatohepatitis in mice and men. Exp Mol Pathol. 2017;103(1):1–8. doi: 10.1016/j.yexmp.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura T., Oppenheim J.J. Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp Cell Res. 2011;317(5):674–684. doi: 10.1016/j.yexcr.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin F., Xu Z., Wang Z., et al. Elevated chemokine CC-motif receptor-like 2 (CCRL2) promotes cell migration and invasion in glioblastoma. Biochem Biophys Res Commun. 2012;429(3–4):168–172. doi: 10.1016/j.bbrc.2012.10.120. [DOI] [PubMed] [Google Scholar]
- 28.Wang L.P., Cao J., Zhang J., et al. The human chemokine receptor CCRL2 suppresses chemotaxis and invasion by blocking CCL2-induced phosphorylation of p38 MAPK in human breast cancer cells. Med Oncol. 2015;32(11):254. doi: 10.1007/s12032-015-0696-6. [DOI] [PubMed] [Google Scholar]
- 29.Pouliot M.C., Despres J.P., Nadeau A., et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41(7):826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki Y., Glass L., Triplitt C., Wajcberg E., Mandarino L.J., DeFronzo R.A. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metabol. 2002;283(6):E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- 31.Banerji M.A., Faridi N., Atluri R., Chaiken R.L., Lebovitz H.E. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metabol. 1999;84(1):137–144. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 32.Carey V.J., Walters E.E., Colditz G.A., et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol. 1997;145(7):614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 33.Cancello R., Henegar C., Viguerie N., et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Ambrosi J., Catalan V., Diez-Caballero A., et al. Gene expression profile of omental adipose tissue in human obesity. FASEB J: Offic Publ Fed Am Soc Exp Biol. 2004;18(1):215–217. doi: 10.1096/fj.03-0591fje. [DOI] [PubMed] [Google Scholar]
- 35.Dong J., Xian Z., Lei Z., et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKα1/SIRT. J Lipid Res. 2014;55(3):363–374. doi: 10.1194/jlr.M038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauer J., Chaurasia B., Goldau J., et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15(5):423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 39.Weiss R., Dziura J., Burgert T.S., et al. Obesity and the metabolic syndrome in children and adolescents. 2004;350(23):2362. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 40.Kim H.-J., Takamasa Higashimori S.-Y.P., Choi Hyejeong, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 41.Wang X., Yang X., Tsai Y., et al. IL-6 mediates macrophage infiltration after irradiation via up-regulation of CCL2/CCL5 in non-small cell lung cancer. Radiat Res. 2017;187(1):50–59. doi: 10.1667/RR14503.1. [DOI] [PubMed] [Google Scholar]
- 42.Oh D.Y., Morinaga H., Talukdar S., Bae E.J., Olefsky J.M. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61(2):346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lumeng C.N., Deyoung S.M., Bodzin J.L., Saltiel A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 44.Belkina A.C., Denis G.V., Becc M. Obesity genes and insulin resistance. 2010;17(5):472. doi: 10.1097/MED.0b013e32833c5c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olefsky Jerrold M, Glass Christopher K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72(1):219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 46.Xu H., Barnes G.T., Yang Q., et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao L., Herlea-Pana O., Heuser-Baker J., Chen Y., Barlic-Dicen J. Roles of the chemokine system in development of obesity, insulin resistance, and cardiovascular disease. J Immunol Res. 2014;2014:181450. doi: 10.1155/2014/181450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amin M.N., Hussain M.S., Sarwar M.S., et al. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab Syndrome. 2019;13(2):1213–1224. doi: 10.1016/j.dsx.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 49.Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Gurmaches J., Tang Y., Jespersen N.Z., et al. Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metabol. 2018;27(1):195–209. doi: 10.1016/j.cmet.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farr O.M., Fiorenza C., Papageorgiou P., et al. Leptin therapy alters appetite and neural responses to food stimuli in brain areas of leptin-sensitive subjects without altering brain structure. J Clin Endocrinol Metabol. 2014;99(12):E2529–E2538. doi: 10.1210/jc.2014-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kissileff H.R., Thornton J.C., Torres M.I., et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr. 2012;95(2):309–317. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha M.K., Caro J.F. Clinical aspects of leptin. Vitam Horm. 1998;54:1–30. doi: 10.1016/s0083-6729(08)60919-x. [DOI] [PubMed] [Google Scholar]
- 54.Bulló M., García-Lorda P., Megias I., Salas-Salvadó J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11(4):525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 55.Goralski K.B., McCarthy T.C., Hanniman E.A., et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282(38):28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 56.Luangsay S., Wittamer V., Bondue B., et al. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J Immunol. 2009;183(10):6489–6499. doi: 10.4049/jimmunol.0901037. [DOI] [PubMed] [Google Scholar]
- 57.Skrzeczyńska-Moncznik J., Stefańska A., Zabel B.A., Kapińska-Mrowiecka M., Butcher E.C., Cichy J. Chemerin and the recruitment of NK cells to diseased skin. Acta Biochim Pol. 2009;56(2):355–360. [PMC free article] [PubMed] [Google Scholar]
- 58.Parolini S., Santoro A., Marcenaro E., et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109(9):3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 59.Chakaroun R., Raschpichler M., Klöting N., et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism. 2012;61(5):706–714. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi M., Okimura Y., Iguchi G., et al. Chemerin regulates β-cell function in mice. Sci Rep. 2011;1:123. doi: 10.1038/srep00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ernst M.C., Haidl I.D., Zúñiga L.A., et al. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology. 2012;153(2):672–682. doi: 10.1210/en.2011-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taku W., Kazuyuki T., Takashi K., et al. Insulin resistance in central nerve system. Nihon Rinsho Jpn J Clin Med. 2006;64(Suppl 9):158–162. [PubMed] [Google Scholar]
- 63.Marques R.E., Guabiraba R., Russo R.C., Teixeira M.M. Targeting CCL5 in inflammation. Expert Opin Ther Targets. 2013;17(12):1439–1460. doi: 10.1517/14728222.2013.837886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kranjc M.K., Novak M., Pestell R.G., Lah T.T. Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol Oncol. 2019;53(4):397–406. doi: 10.2478/raon-2019-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sano T., Iwashita M., Nagayasu S., et al. Protection from diet-induced obesity and insulin resistance in mice lacking CCL19-CCR7 signaling. Obesity. 2015;23(7):1460–1471. doi: 10.1002/oby.21127. [DOI] [PubMed] [Google Scholar]
- 66.Oliveira A.G., Araujo T.G., Carvalho B.M., et al. Acute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet-induced obese rats. Obesity. 2013;21(12):2545–2556. doi: 10.1002/oby.20402. [DOI] [PubMed] [Google Scholar]
- 67.Ouchi N., Parker J.L., Lugus J.J., Walsh K.J.N.R.I. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Osborn O., Olefsky J.M.J.N.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]