Abstract
It has been suggested that isoprene synthesis by isoprene synthase (IspS) proceeds via a substrate-assisted mechanism. The authors observed a non-enzymatic isoprene formation by Mn2+, which represents the basis of IspS enzyme reaction. Because IspS and many other terpene synthases require Mn2+ metal ions as cofactor, this study characterized the formation reaction for the first time. Metal ions including Mn2+ non-enzymatically produced both isoprene and 2-methyl-3-buten-2-ol (2-MBO) from dimethylallyl pyrophosphate (DMADP). Isoprene formation was most enhanced by Fe2+ and, to a lesser extent, by Mn2+ or Cu2+. Ni2+, Co2+, Mg2+, and Ba2+ exhibited a low activity to generate both isoprene and 2-MBO. The proportion of isoprene and 2-MBO varied with the Mn2+ concentration: isoprene predominated over 2-MBO at a higher Mn2+ concentration. Similarly, isoprene formation by Mn2+ increased exponentially as temperature increased with predominance of isoprene over 2-MBO at higher temperature. Both isoprene and 2-MBO formation was enhanced by acidic and neutral pH compared to alkaline conditions. Molecular dynamic simulation of DMADP suggested that the formation reaction is initiated by deprotonation of hydrogen on allyl terminal carbon by phosphate oxygen and generates carbocation and allyl anion intermediates. This is followed by quenching to produce isoprene or by hydroxyl addition to form 2-MBO. Thus, this study provided an insight into reaction mechanism of isoprene and 2-MBO biosynthesis and highlighted some parts of isoprene emission from terrestrial plants, which could be formed by non-enzymatic mechanism.
Subject terms: Biochemistry, Plant sciences, Environmental sciences
Introduction
Isoprene (2-methyl-1,3-butadiene) emitted from a terrestrial plants is an essential and the most abundant component in tropospheric chemistry1–3. Isoprene has a great impact on the chemistry of atmosphere through reaction with hydroxy radicals and nitrogen oxide leading to production of ozone or to increased life span of the greenhouse gas methane, while isoprene is considered to confer some advantages to plants including better stabilization of photosynthetic apparatus under stressed conditions4.
In addition, it is a precursor in the synthetic chemistry industry for the production of rubber, pharmaceuticals, and potential biofuel5–7. Isoprene for industrial chemistry has been mainly obtained from petrochemical sources and its supply is dependent on the petroleum industry wherein the production is energy consuming and environment-unfriendly. Bio-generation of isoprene using isoprene synthase (IspS) therefore has been attracting attention to overcome above mentioned disadvantages in chemical process7,8.
Furthermore, volatile organic compounds in breath can be a biochemical probe providing both non-invasive and continuous information on the metabolic and physiological state of an individuals. In terms of human exhalation, isoprene accounts for up to 70% of total hydrocarbon removal via exhalation and may act as a noninvasive indicator of several metabolic effects in the human body9.
In plants and bacteria, isoprene is biosynthesized by isoprene synthase10–12 whilst its formation mechanism in the human body remains unclear9. IspS and many other terpene synthases require Mg2+ or Mn2+ metal ions as a cofactor13–17. The enzyme rection of terpenoid biosynthesis depends for activation on a trinuclear cluster, usually containing Mg2+ or Mn2+. This cluster not only activate the reaction, but also control product specificity of the enzymes18. The authors have studied the metal requirements of IspSs in a series of gene cloning and characterization of IspS from tropical trees and found that these IspSs were most activated by Mn2+ or Mg2+ similarly to other terpenoid synthases19. During the study, the authors observed non-enzymatic formation of isoprene by divalent cations, especially to a greater extent by manganese ions (Mn2+). It has been implicated that isoprene synthesis by IspS proceeds via a substrate-assisted mechanism mediated by diphosphate oxygen as a base20. Thus, the substrate-assisted catalysis of isoprene formation constitutes the basis of the IspS enzyme reaction. The authors of this study characterized the non-enzymatic formation of isoprene by Mn2+ and investigated the reaction mechanism via molecular dynamic simulation for the first time.
Materials and method
Reagents
Dimethylallyl pyrophosphate (DMADP) was purchased from Merk Japan (Tokyo, Japan). The metal salts used include MnCl2∙4H2O, MgCl2∙6H2O, FeSO4∙7H2O, CuCl2∙2H2O, NiCl2∙6H2O, CoCl2∙6H2O, BaCl2∙2H2O, CaCl2, KCl, and NaCl. Guaranteed reagents were used, which were purchased from Nacalai Tesque (Tokyo, Japan) or Fujifilm Wako Pure Chemical Corporation (Tokyo, Japan).
Formation of isoprene
The reaction mixture consisted of 45-mM Tris–HCl buffer (pH 8.5) containing 5% glycerol, 2-mM DTT, 10-mM metal ions, and 10-mM DMADP in 100 μL, unless otherwise specified. Isoprene formation was measured in 2-mL glass vials with a silicon septum cap. DMADP and metal ions were mixed on ice, and the formation reactions were induced by incubating the reaction mixture at 40 °C and then stopped by removing the reaction mixture from the vials on ice. The headspace gas of the reaction mixture (100 µL) was analyzed using a gas chromatograph (Agilent GC6890N or Shimadzu GC 14A) equipped with a flame ionization detector. The samples spit by the ratio of 5:1 were analyzed on DB-VRX columns (0.25 mm × 30 m, Agilent Technologies, CA, USA) at an isocratic constant temperature of 60 °C with helium as the carrier gas and flow rate of 30 cm/s. The chemical structure of the reaction product was identified by comparing the retention time with authentic standard or via gas chromatography–mass spectrometry (GC–MS) analysis using Shimadzu QP-2010. The columns and separation conditions of the GC–MS analysis were the same as those of gas chromatography. Ionization for GC–MS was by electron impact at 70 eV. The chemical structures of the compounds were identified by comparing the spectrum with the NIST Mass Spectral Library.
Molecular dynamic simulation of DMADP
The DMADP structure was simulated at constant normal pressure and temperature (NPT ensemble). The models of DMADP and Mn2+ or Mg2+in the water was fabricated using “tleap” program of Amber16 (AMBER 2016, Case et al. 201621, https://ambermd.org/index.php) with a force field of ions 234lm_1264_tip3p for metal ions and with gaff2 for DMADP. One DMDP molecule and three metal salts were placed in 15 Å tip3p solvate box (43.9 × 42.1 × 42.9 Å). The system was energy-minimized by 3500 steps and solvent-relaxed for 40 ps under constant volume (ntb = 1), followed by all-relaxation under constant pressure (ntb = 2, NPT ensemble) for 100 ps. The system was further equilibrated by NPT ensemble at 313 K for 100 ps, and the simulation was continued for another 10 ns. The simulation parameters were as follows: nstlim = 10,000,000, dt = 0.001, imin = 0, irest = 1, ntx = 5, ntb = 2, pres0 = 1.0, ntp = 1, taup = 2.0, cut = 12, ntr = 0, ntc = 2, ntf = 2, tempi = 313.0, temp0 = 313.0, ntt = 1, tautp = 1.0, ntpr = 100, ntwx = 100, ntwr = 100, iwrap = 1. Simulation of 10 ns (100,000 trajectories) was used for analysis. The atom distance and dihedral angle were measured using the “CPPTRAJ” program of Amber Tools.
Results
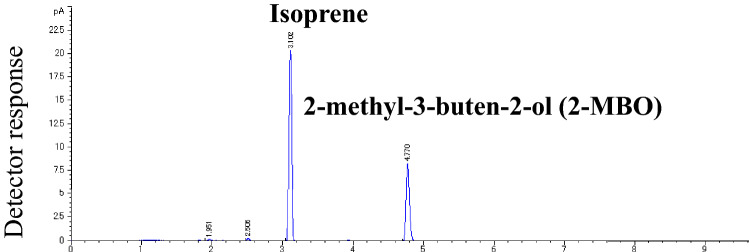
Figure 1 presents the gas chromatogram of the headspace gas obtained from the reaction of DMADP with Mn2+. DMADP (4 mM) was reacted with Mn2+ (20 mM) at 55 °C for 1 h. There are two prominent peaks: one eluted at 3.10 min and another eluted at 4.77 min. The first and second peaks were respectively identified as isoprene and 2-methyl-4-buten-2-ol (2-MBO) by comparing the retention time with authentic standard and the MS spectrum with NIST MS library. (Supplementary Fig. S1). The ratio of isoprene to 2-MBO under this condition was almost 2:1. To scan the side-product of the reaction, ESI–MS analysis of the liquid phase was conducted after 90 min reaction of DMADP with Mn2+ at 40 °C. No accumulation of specific molecule was observed in the reaction mixture suggesting occurrence of no other side-products (data not shown).
Figure 1.
GC chromatogram of head space gas of the reaction mixture. Four mM DMADP was reacted with 20 mM Mn2+ at 55 °C with pH 8.5 for 1 h and the head space gas was analyzed by gas-chromatography. The first and second peaks were respectively identified as isoprene and 2-methyl-4-buten-2-ol (2-MBO) by comparing the retention time with authentic standard and the MS spectrum with NIST MS library.
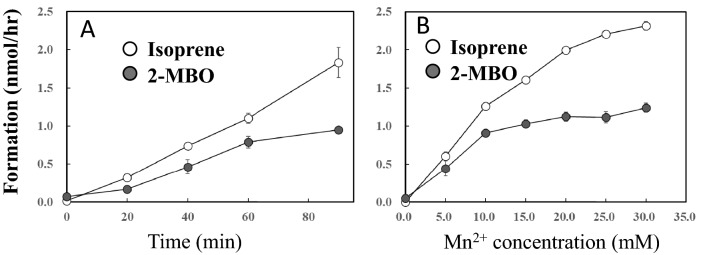
Formation of both isoprene and 2-MBO increased linearly up to 90 min incubation time (Fig. 2A). Isoprene formation increased linearly with Mn2+ concentration up to 20 mM and almost reached plateau level beyond this concentration whilst 2-MBO formation reached the plateau level at 10 mM (Fig. 2B). Thus, the proportion of isoprene versus 2-MBO becomes prominent at the concentration greater than 20 mM of Mn2+.
Figure 2.
Time course (A) and Mn2+ dose dependency (B) of isoprene and 2-methyl-3-buten-2-ol (2MBO) formation. Data are mean ± SE (n = 3). Two mM DMADP was reacted with shown incubation time (A), and concentration of Mn2+ at 40 °C and pH 8.5 for 1 h (B). Head space gas was analyzed by GC. Formation of isoprene and 2MBO was calculated by relative proportion of the peak area against that of standard isoprene gas. Yield of isoprene after 60 min reaction was roughly 1% of the total amount of DMADP in the reaction mixture.
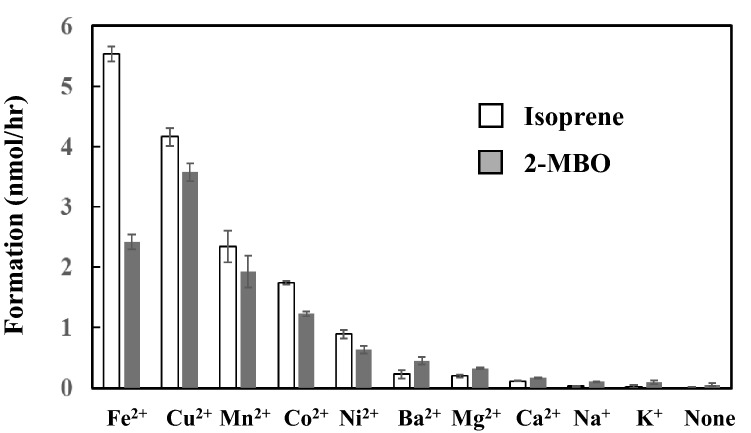
Figure 3 demonstrates the effect of divalent and monovalent cations on the formation of isoprene from DMADP. The authors attempted to test the effect of trivalent cations by using AlCl3. However, the AlCl3 solution was acidic, and the pH of the reaction mixture was estimated to be less than 4.5. For this reason, its activity to generate isoprene was not investigated.
Figure 3.
Effect of metal ions on the formation of isoprene and 2-methyl-3-buten-2-ol (2-MBO). Data are mean ± SE (n = 3). Two mM DMADP and 20 mM metal ions were reacted at 40 °C for 1 h at pH 8.5 and the head space gas was analyzed by GC. Formation rate of isoprene and 2-MBO was calculated by relative proportion against the peak area of standard isoprene gas.
Isoprene formation was most enhanced by Fe2+ and, to a lesser extent, by Mn2+ or Cu2+. Ni2+, Co2+, Mg2+, and Ba2+ exhibited a relatively low activity to generate both isoprene and 2-MBO. Isoprene formation by Mn2+ was almost 10 times that by Mg2+. Monovalent cations, K+ and Na+, exhibited much less activity to generate isoprene. The proportion of isoprene vs. 2-MBO was higher in high-isoprene-forming metal ions, such as Fe2+, Cu2 + and Mn2+. This relationship was reversed in low-isoprene-forming metal ions, such as Mg2+ and Ba2+. Low isoprene-emitting metal ions prefer 2-MBO formation rather than isoprene formation.
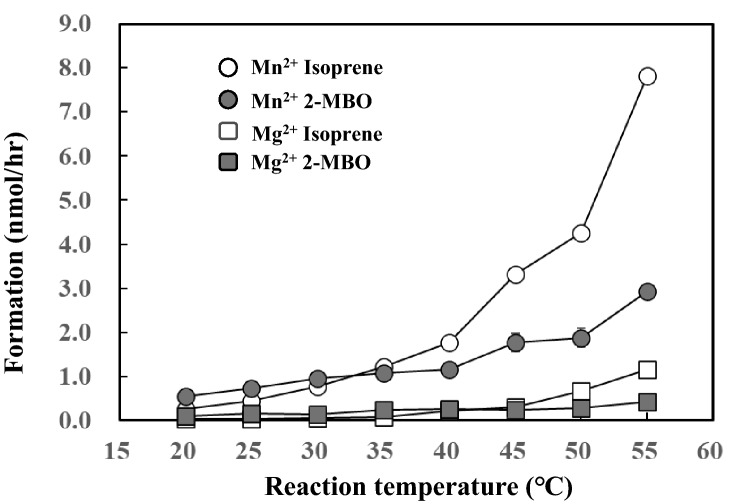
Isoprene formation by Mn2+ increased exponentially from 25 °C with temperature, as presented in Fig. 4. Furthermore, 2-MBO predominated over isoprene at a temperature less than 30 °C, whereas isoprene predominated over 2-MBO at a temperature greater than 40 °C. The Q10 (40 °C/30 °C) of isoprene formation was 2.3 for Mn2+ and 3.5 for Mg2+.
Figure 4.
Effect of reaction temperatures on the formation of isoprene and 2-methyl-3-butene-2-ol (2-MBO). Data are mean ± SE (n = 3). Two mM DMADP and 20 mM metal ions were reacted at shown reaction temperature for 1 h with pH 8.5 and the head space gas was analyzed by GC. Formation rate of isoprene and 2-MBO was calculated by relative proportion of peak area against that of standard isoprene gas.
Formation of isoprene and 2-MBO was significantly enhanced by Mn2+ at acidic and neutral pH (Fig. 5), whereas no significant change was observed with Mg2+ or without addition of metal ions. The isoprene formation was prominent for Mn2+ throughout the pH variation whereas MBO was largely dominant at entire range of pH for Mg2+.
Figure 5.
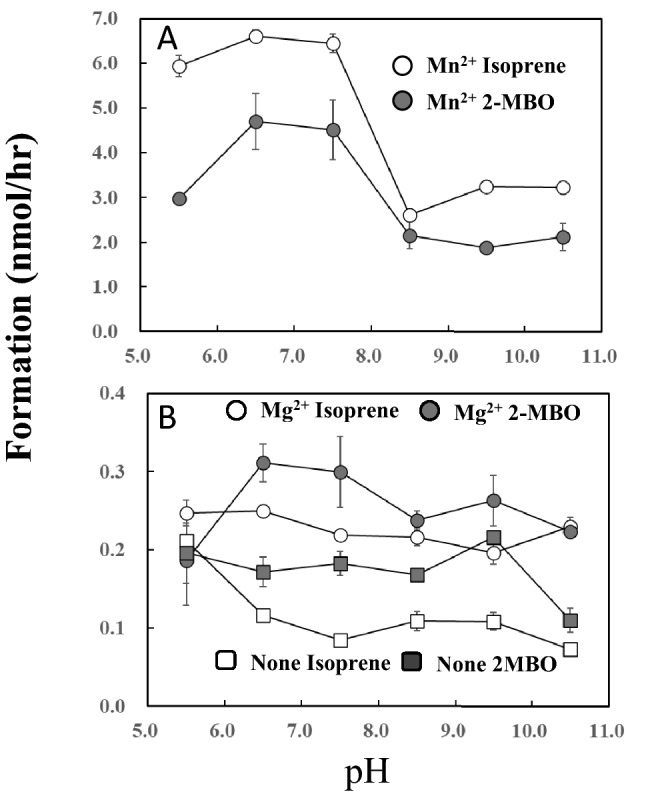
Effect of reaction pH on the formation of isoprene and 2-methyl-3-butene-2-ol (2-MBO). Data are mean ± SE (n = 3). Two mM DMADP and 20 mM metal ions were reacted at 40 ℃ for 1 h at shown pH and the head space gas was analyzed by GC. For pH 5.5 and 6.5, used buffer was 45 mM 2-(N-morpholino) ethanesulfonic acid (MES) and for pH 7.5–10.5, 45 mM Tris–HCl was used. Formation rate of isoprene and 2-MBO was calculated by relative proportion of the peak area against that of standard isoprene gas.
To gain more insight into the mechanism of non-enzymatic isoprene formation, the DMADP structure in the water at 313 K (40 °C) was simulated for 10 ns using the Amber software. Figure 6 presents the structure of DMADP bound with two Mn2+. One of the models showing attractive interaction of H7 and O3 with atom distance 2.2 Å was selected from 10 ns ensemble trajectory. Three negative charges of DMADP were stoichiometrically neutralized by two Mn2+, and one excess positive charge of Mn2+ was antagonized by one negative chloride ion (Fig. 6). The authors included three Mn2+ molecules in the system. One of these three Mn2+ was not involved in the binding to the DMADP molecule.
Figure 6.
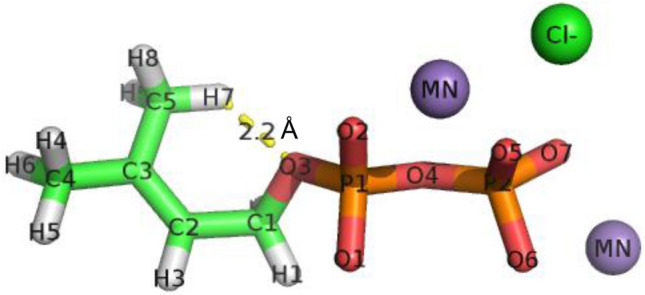
Chemical structure of DMDP bound to MgCl2. The DMADP structure in the water at 313 K (40 °C) was simulated for 10 ns using the Amber software. One of the models showing attractive interaction of H7 and O3 with atom distance 2.2 Å was selected. Two Mn2+ bound to one DMADP molecule. Three negative charges of DMADP were stoichiometrically neutralized by 2 Mn2+, and one excess positive charge of Mn2+ was antagonized by one negative chloride ion. The atom labels were by the Amber program (AMBER 2016, Case et al. 201621, https://ambermd.org/index.php).
It has been suggested that isoprene formation could proceed via allylic carbocation, followed by quenching via base-assisted proton abstraction by analogy with the initiation step in the most related plant monoterpene synthase22. It has also been suggested that the oxygen (O1 or O2 in Fig. 6) of the pyrophosphate group acts as a general base, and synchronous or asynchronous allylic carbocation and deprotonation could occur in a substrate-assisted manner to form isoprene20. In this mechanism, phosphate oxygen (O1, O2, or O3) acts as a base to abstract the hydrogen (H4, H5, H6, H7, H8, and H9) on allyl terminal carbon (C4 or C5, referred to as ally carbon in this paper). To explore the possibility of interactions between these atoms, the NPT ensemble of the DMADP simulation comprising of 100,000 trajectories was analyzed using the CPPTRAJ program of Amber16. The authors measured the frequencies of the interaction between phosphate oxygen and allyl carbon–hydrogen with an atom distance less than 3.5 Å (Table 1). The value of 3.5 Å is the arbitral cut-off in this study and is considered to be the longest atom distance to provide attractive interatomic potential. The highest attractive interaction frequency occurred between O3 and allyl carbon–hydrogen but not between O1 or O2 and allyl carbon–hydrogen throughout all ensembles. The attractive interaction frequencies within 3.5 Å between O3 and allyl carbon–hydrogen were17 times those of the interaction between O1 or O2 and allyl carbon–hydrogen. The addition of neither Mn2+ nor Mg2+ did not induce a significant change in the interaction profile.
Table 1.
Frequencies of interaction between phosphate oxygen and allyl carbon hydrogen with atom distance less than 3.5 Å.
| Divalent cations | O3 vs allyl carbon hydrogens | O1 vs allyl carbon hydrogens | O2 vs allyl carbon hydrogens | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H4 | H5 | H6 | H7 | H8 | H9 | H4 | H5 | H6 | H7 | H8 | H9 | H4 | H5 | H6 | H7 | H8 | H9 | |
| None | 0 | 0 | 0 | 17,627 | 15,789 | 17,280 | 0 | 0 | 0 | 803 | 674 | 948 | 0 | 0 | 1 | 1140 | 1138 | 1291 |
| Mn2+ | 0 | 0 | 0 | 16,648 | 16,509 | 16,745 | 0 | 0 | 0 | 906 | 812 | 902 | 0 | 1 | 0 | 988 | 1003 | 946 |
| Mg2+ | 0 | 0 | 0 | 16,864 | 18,900 | 18,415 | 0 | 0 | 0 | 1000 | 1152 | 1233 | 1 | 0 | 0 | 970 | 1050 | 1129 |
| Fe2+ | 0 | 0 | 0 | 17,062 | 16,285 | 16,258 | 0 | 0 | 0 | 928 | 966 | 1098 | 0 | 0 | 0 | 1078 | 912 | 1083 |
| Cu2+ | 0 | 0 | 0 | 17,101 | 16,417 | 16,836 | 6 | 1 | 1 | 1146 | 1111 | 1299 | 0 | 1 | 2 | 607 | 761 | 690 |
NPT ensemble for 10 ns comprised of 100,000 trajectories were analyzed by CPPTRAJ program.
The atom labels were via AMBER program (AMBER 2016, Case et al. 2016, https://ambermd.org/index.php) as shown in Fig. 6.
The average, minimum, and maximum atom distances between phosphate oxygen and allyl carbon–hydrogen has given as Supplementary Table S1. The average interaction distance between O3 and allyl carbon–hydrogen was 1.7 Å shorter than that between O1 or O2 and allyl carbon–hydrogen throughout the ensembles of control (None), Mn2+, Mg2+, Fe2+ and Cu2+. As was the case of the interaction frequencies, the atom distance profile did not exhibit any considerable difference between the control (none), Mn2+, and Mg2+ ensembles.
Discussion
This study demonstrated the non-enzymatic formation of isoprene by Mn2+ for the first time. It has been proposed that allylic carbocation and quenching by deprotonation proceed in a self-catalyzed manner with pyrophosphate oxygen as the general base20. In this proposal, the negative charge of phosphate oxygen (O1 or O2 in Fig. 6) plays a significant role in attracting the hydrogen on terminal ally carbon (H7, H8, and H9 in Fig. 6). DMADP bears three negative charges under reaction condition of pH 8.5. Given that allyl carbon hydrogen is deprotonated via the electrostatic interaction through the negative charge of phosphate oxygen, isoprene should be formed without addition of metal ions. However, no significant formation of isoprene was observed without addition of metal ions throughout the pH range from 5.5 to 10.5 (Fig. 5). Metal ions stoichiometrically neutralizes the negative charge of oxygen as illustrated in Fig. 6 and may inhibit the electrostatic interaction between phosphate oxygen (O1 or O2 in Fig. 6) and allyl carbon hydrogen (H7, H8 or H9) hence reduce the formation of isoprene and 2-MBO. Nevertheless, isoprene and 2-MBO formation requires metal ions suggesting that the negative charge and electrostatic interaction is not involved in the deprotonation reaction. Furthermore, the molecular dynamic simulation of DMADP indicated much less attractive interaction frequencies between phosphate oxygen O1 or O2 and allyl carbon–hydrogen (Table 1 and Supplementary Table S1).
Taking these observations together, the authors propose the isoprene and 2-MBO formation mechanism, which is illustrated in Fig. 7. The formation reaction is initiated by the deprotonation of hydrogen on allyl carbon (H7, H8, and H9 in Fig. 7) by phosphate oxygen (O3). It generates carbocation and allyl anion intermediate followed by quenching to produce isoprene or by hydroxyl addition (SN1 reaction) to form 2-MBO. The ratio of isoprene to 2-MBO showed variation depending on reaction conditions of time (Fig. 2A), Mn2+ concentration (Fig. 2B), temperature (Fig. 4) and pH (Fig. 5). Relative proportion of 2-MBO appeared to be decreased with increase in the isoprene formation in all cases. These observations therefore may be explained by trade-off of isoprene and 2-MBO formation due to competition for common precursor carbocation as illustrated in Fig. 7.
Figure 7.
Reaction mechanism of the formation of isoprene and 2-MBO from DMADP. The formation reaction is initiated by the deprotonation of hydrogen on allyl carbon (H7, H8, and H9 in Fig. 7) by phosphate oxygen (O3). It generates carbocation and allyl anion intermediate followed by quenching to produce isoprene or by hydroxyl addition (SN1 reaction) to form 2-MBO.
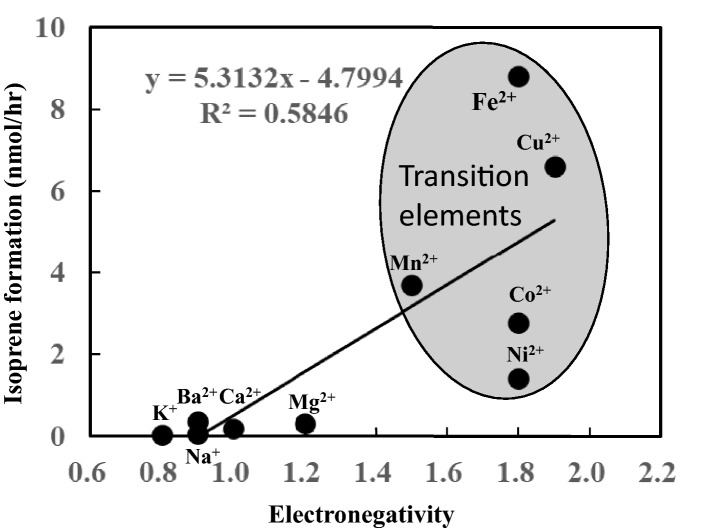
Mn2+ exhibited high activity to induce isoprene and 2-MBO formation (Fig. 3). IspS and many other terpene synthases require Mg2+ or Mn2+metal ions as a cofactor12,14–17. The formation of isoprene by Mn2+ was almost ten times that by Mg2+. The authors speculated that Mn2+ accelerates the attractive interaction frequencies by binding to pyrophosphate. However, the molecular dynamic simulation revealed no difference in the interaction frequencies between Mn2+ and Mg2+ (Table 1). The binding of Mn2+ could affect the electron localization or electrostatic potential of allyl terminal carbon–oxygen (O3) to subtract allyl hydrogen and facilitate the carbocation formation. Thus, the higher electronegativity of Mn2+ (electronegativity = 1.5) than that of Mg2+ (electronegativity = 1.2) may explain the difference. However, no strong linear correlation was observed between electronegativity and isoprene formation (Fig. 8). Mn2+, Fe2+, and Cu2+ are transition elements, and their electron configuration in the d orbital of the M electron shell may bear some relevance with the high formation rate of carbocation. This hypothesis needs further investigation.
Figure 8.
Correlation between electronegativity and isoprene formation.
Here, we demonstrated the non-enzymatic formation of isoprene and 2-MBO by Mn2+ for the first time. Isoprene has been considered to be synthesized by only IspS in the plant kingdom. However, this study highlighted some parts of isoprene emission from terrestrial plants, which could be formed by non-enzymatic mechanism. It has been suggested that isoprene protects the photosynthetic system from thermal stress23,24. Moreover, it stabilizes the photosynthetic protein complex in the thylakoid membrane25. Elevated temperature disrupts the manganese cluster and releases manganese from its binding site26,27. Therefore, the authors speculate that liberated manganese in chloroplast on high temperature binds to DMADP and instantly generates isoprene and may serve as an acute safety net to protect the photosystem. However, the physiological significance of non-enzymatic isoprene formation is yet to be demonstrated.
There is growing evidence that tropical trees emit trace gases, such as 2-MBO and dimethyl sulfide28,29. Both isoprene and 2-MBO are produced from the common precursor DMADP. It therefore could be possible that 2-MBO emitted from terrestrial plants is formed non-enzymatically from DMADP as our proposal in this study or enzymatically via 2-MBO synthase. It has been shown that 2-MBO synthase produces 2-MBO to isoprene at a ratio of 90:130. This observation suggests that isoprene synthase and 2-MBO shares the same formation mechanism. Given that the 2-MBO synthesis is also by substrate assisted mechanism as our proposal, the active site of 2-MBO synthase may provide a niche to allow entry of water. By analogy, the active site of isoprene synthase excludes water molecule to specifically enhance isoprene formation from carbocation as illustrated in Fig. 7. The hydrophilicity or hydrophobicity of active pocket could be crucial for product specificity of isoprene or 2-MBO biosynthesis.
Some of isoprene synthase from tropical tree preferred Mn2+ as cofactor rather than Mg2+31. Our previous19 and ongoing work found that 7 of 9 IspSs from tropical trees showed higher dependency on Mn2+ compared to Mg2+ (unpublished observation). The isoprene synthase kinetics and enzyme activation are an important controlling factor of isoprene emission. The dependency of IspS on Mn2+ therefore may relate with the isoprene emission behavior of tropical trees and this may need further investigation.
Supplementary Information
Acknowledgements
This study was supported by JSPS KAKENHI Grant Number 19H03089.
Author contributions
H.O. operated G.C. H.O. and I.M. prepared the manuscript. M.F. conducted M.D. simulation. M.I. assayed non-enzymatic and enzymatic isoprene formation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06520-0.
References
- 1.Claeys M, et al. Formation of secondary organic aerosols through photooxidation of isoprene. Science. 2004;303:1173–1176. doi: 10.1126/science.1092805. [DOI] [PubMed] [Google Scholar]
- 2.Poisson N, Kanakidou M, Crutzen PJ. Impact of non-methane hydrocarbons on tropospheric chemistry and the oxidizing power of the global troposphere: 3-dimensional modelling results. J. Atmos. Chem. 2000;36:157–230. doi: 10.1023/a:1006300616544. [DOI] [Google Scholar]
- 3.Trainer M, et al. Models and observations of the impact of natural hydrocarbons on rural ozone. Nature. 1987;329:705–708. doi: 10.1038/329705a0. [DOI] [Google Scholar]
- 4.Loreto F, Schnitzler JP. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Frank L, et al. Isoprene and beta-caryophyllene confer plant resistance via different plant internal signalling pathways. Plant Cell Environ. 2021;44:1151–1164. doi: 10.1111/pce.14010. [DOI] [PubMed] [Google Scholar]
- 6.Dani KGS, Fineschi S, Michelozzi M, Loreto F. Do cytokinins, volatile isoprenoids and carotenoids synergically delay leaf senescence? Plant Cell Environ. 2016;39:1103–1111. doi: 10.1111/pce.12705. [DOI] [PubMed] [Google Scholar]
- 7.Yeom, S. J. et al. Molecular and biochemical characterization of a novel isoprene synthase from Metrosideros polymorpha. BMC plant boil.18, Artn 118. 10.1186/S12870-018-1315-4 (2018). [DOI] [PMC free article] [PubMed]
- 8.Kim, J. H. et al. Isoprene production by Escherichia coli through the exogenous mevalonate pathway with reduced formation of fermentation byproducts. Microb Cell Fact15, Artn 214. 10.1186/S12934-016-0612-6 (2016). [DOI] [PMC free article] [PubMed]
- 9.Eom S, et al. Molecular determinants of alpha 3 beta 4 nicotinic acetylcholine receptors inhibition by triterpenoids. Biol. Pharm Bull. 2018;41:65–72. doi: 10.1248/bpb.b17-00576. [DOI] [PubMed] [Google Scholar]
- 10.Sivy TL, Shirk MC, Fall R. Isoprene synthase activity parallels fluctuations of isoprene release during growth of Bacillus subtilis. Biochem. Bioph. Res. Co. 2002;294:71–75. doi: 10.1016/S0006-291x(02)00435-7. [DOI] [PubMed] [Google Scholar]
- 11.Kuzma J, Nemecekmarshall M, Pollock WH, Fall R. Bacteria Produce the Volatile Hydrocarbon Isoprene. Curr. Microbiol. 1995;30:97–103. doi: 10.1007/Bf00294190. [DOI] [PubMed] [Google Scholar]
- 12.Miller B, Oschinski C, Zimmer W. First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta. 2001;213:483–487. doi: 10.1007/s004250100557. [DOI] [PubMed] [Google Scholar]
- 13.Tashiro M, et al. Tweezing the cofactor preference of gymnosperm pinene synthase. Biosci. Biotech. Bioch. 2018;82:1058–1061. doi: 10.1080/09168451.2018.1459465. [DOI] [PubMed] [Google Scholar]
- 14.Kollner TG, et al. Protonation of a neutral (S)-beta-bisabolene intermediate is involved in (S)-beta-macrocarpene formation by the maize sesquiterpene synthases TPS6 and TPS11. J Biol. Chem. 2008;283:20779–20788. doi: 10.1074/jbc.M802682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyatt DC, et al. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5360–5365. doi: 10.1073/pnas.0700915104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips MA, Wildung MR, Williams DC, Hyatt DC, Croteau R. cDNA isolation, functional expression, and characterization of (+)-alpha-pinene synthase and (-)-alpha-pinene synthase from loblolly pine (Pinus taeda): Stereocontrol in pinene biosynthesis. Arch. Biochem. Biophys. 2003;411:267–276. doi: 10.1016/S0003-9861(02)00746-4. [DOI] [PubMed] [Google Scholar]
- 17.Lucker J, et al. Monoterpene biosynthesis in lemon (Citrus limon) - cDNA isolation and functional analysis of four monoterpene synthases. Eur. J. Biochem. 2002;269:3160–3171. doi: 10.1046/j.1432-1033.2002.02985.x. [DOI] [PubMed] [Google Scholar]
- 18.Frick S, et al. Metal ions control product specificity of isoprenyl diphosphate synthases in the insect terpenoid pathway. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4194–4199. doi: 10.1073/pnas.1221489110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oku H, et al. Molecular cloning and biochemical characterization of isoprene synthases from the tropical trees Ficus virgata, Ficus septica, and Casuarina equisetifolia. J. Plant Res. 2015;128:849–861. doi: 10.1007/s10265-015-0740-9. [DOI] [PubMed] [Google Scholar]
- 20.Koksal M, Zimmer I, Schnitzler JP, Christianson DW. Structure of isoprene synthase illuminates the chemical mechanism of teragram atmospheric carbon emission. J. Mol. Biol. 2010;402:363–373. doi: 10.1016/j.jmb.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case DA, Betz RM, Cerutti DS, Cheatham TE, III, Darden TA, Duke RE, Giese TJ, Gohlke H, Goetz AW, Homeyer N, Izadi S, Janowski P, Kaus J, Kovalenko A, Lee TS, LeGrand S, Li P, Lin C, Luchko T, Luo R, Madej B, Mermelstein D, Merz KM, Monard G, Nguyen H, Nguyen HT, Omelyan I, Onufriev A, Roe DR, Roitberg A, Sagui C, Simmerling CL, Botello-Smith WM, Swails J, Walker RC, Wang J, Wolf RM, Wu X, Xiao L, Kollman PA. AMBER 2016. University of California; 2016. [Google Scholar]
- 22.Cheema J, Faraldos JA, O'Maille PE. REVIEW: epistasis and dominance in the emergence of catalytic function as exemplified by the evolution of plant terpene synthases. Plant Sci. 2017;255:29–38. doi: 10.1016/j.plantsci.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Singsaas EL, Lerdau M, Winter K, Sharkey TD. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharkey TD, Chen X, Yeh S. Isoprene Increases Thermotolerance of Fosmidomycin-Fed Leaves. Plant Physiol. 2001;125:2001. doi: 10.1104/pp.125.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siwko ME, et al. Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Bba-Biomembranes. 2007;1768:198–206. doi: 10.1016/j.bbamem.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant. Sci. 2008;13:178–182. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Hideg E, Kalai T, Hideg K, Vass I. Do oxidative stress conditions impairing photosynthesis in the light manifest as photoinhibition? Philos. T R Soc. B. 2000;355:1511–1516. doi: 10.1098/rstb.2000.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jardine KJ, et al. Emissions of putative isoprene oxidation products from mango branches under abiotic stress. J. Exp. Bot. 2013;64:3697–3709. doi: 10.1093/jxb/ert202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jardine K, et al. Dimethyl sulfide in the Amazon rain forest. Global Biogeochem. Cy. 2015;29:19–32. doi: 10.1002/2014GB004969. [DOI] [Google Scholar]
- 30.Gray DW, Breneman SR, Topper LA, Sharkey TD. Biochemical characterization and homology modeling of methylbutenol synthase and implications for understanding hemiterpene synthase evolution in plants. J. Biol. Chem. 2011;286:20582–20590. doi: 10.1074/jbc.M111.237438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko JH, Han KH, Park S, Yang JM. Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiol. 2004;135:1069–1083. doi: 10.1104/pp.104.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.