Abstract
Overdiagnosis refers to detection of disease that would not otherwise become clinically apparent during a patient’s lifetime. Overdiagnosis is common and has been reported for several cancer types, although there are few studies describing its prevalence in hepatocellular carcinoma (HCC) surveillance programs. Overdiagnosis can have serious negative consequences including overtreatment and associated complications, financial toxicity, and psychological harms related to being labeled with a cancer diagnosis. Overdiagnosis can occur for several different reasons including inaccurate diagnostic criteria, detection of premalignant or very early malignant lesions, detection of indolent tumors, and competing risks of mortality. The risk of overdiagnosis is partly mitigated, albeit not eliminated by several guideline recommendations, including definitions for the at-risk population in whom surveillance should be performed, surveillance modalities, surveillance interval, recall procedures, and HCC diagnostic criteria. Continued research is needed to further characterize the burden and trends of overdiagnosis as well as identify strategies to reduce overdiagnosis in the future.
Hepatocellular carcinoma (HCC) is the 3rd leading cause of cancer-related death worldwide with an increasing mortality rate in the United States.1, 2 HCC prognosis highly depends on tumor stage at diagnosis; patients diagnosed with early-stage HCC are eligible for curative treatments and can achieve 5-year survival exceeding 60%, whereas those with more advanced tumor burden have a median survival of 1–2 years.3, 4 This contrast in survival underlies guideline recommendations from the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) for semi-annual surveillance using ultrasound with or without AFP in at-risk patients, including those with chronic hepatitis B virus (HBV) infection or those with cirrhosis from any etiology.5, 6
The goal of any cancer screening program is to improve overall survival by detecting cancer at an early, asymptomatic stage and facilitating curative treatments. The overall value of cancer screening programs is determined by the balance of screening benefits versus potential physical, financial, and psychological harms.7, 8 The benefits of HCC surveillance have been best demonstrated in a large randomized clinical trial (RCT) among >18,000 patients with HBV infection.9 Among patients with cirrhosis, HCC surveillance is supported by several cohort studies showing a consistent association with improved outcomes including early detection, curative treatment receipt, and overall survival.10 Notably, available cohort studies have recognized limitations including lead time bias, length time bias, and risk of residual confounding, which may result in overestimation of surveillance benefits.11–13
Cancer screening can result in physical, financial, or psychological harms – which has been defined using a standardized nomenclature across cancer types.14–16 Physical harm is defined as any diagnostic testing related to false positive or indeterminate surveillance results. Financial harms include direct costs of screening and diagnostic evaluations plus any indirect costs, such as missed work. Psychological harms can occur at multiple steps of the screening process and include fear of abnormal results, cancer-specific worry after positive screening results, and depression after cancer diagnosis. Studies have reported varying degrees of physical harms associated with HCC surveillance8, 17–19, although most harms appear to be mild in severity.
Most patients report placing increased importance on screening benefits over screening-related harms. This may be in part due to public health messaging over the past several decades that has equated cancer diagnosis with death and cancer screening programs with hope, resulting in a firm commitment of many people to desire cancer screening.20 This bias toward valuing screening benefits over harms also applies to patients with cirrhosis when making decisions regarding HCC surveillance.21 However, this assessment often fails to account for the risk of overdiagnosis – a common issue for several cancer types that can negatively impact the risk-benefit ratio of screening but is more difficult for patients and providers to understand.22 Herein, we detail the concept of overdiagnosis, why this may occur in HCC surveillance, and its importance when assessing the value of an HCC surveillance program.
Clinical significance of overdiagnosis
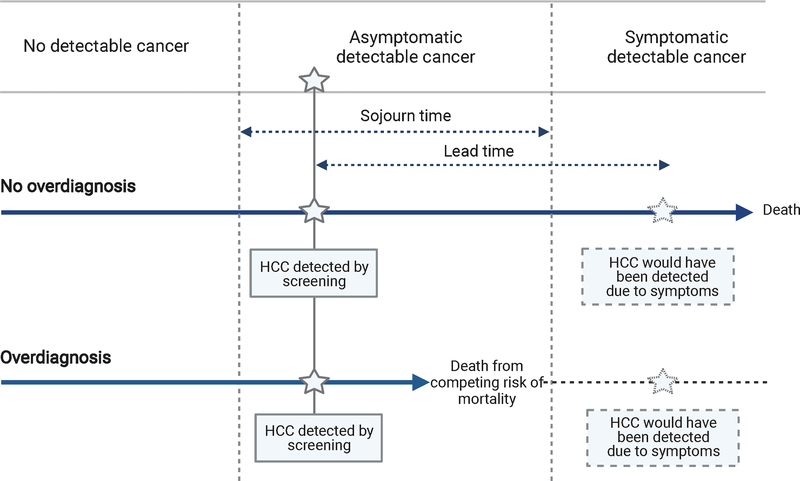
Overdiagnosis refers to any diagnosis that would not otherwise become symptomatic or clinically apparent during a patient’s lifetime – a form of “pseudodisease”.23–25 Notably, the concept of overdiagnosis is distinct from low specificity and false positive findings; rather, it refers to “true positives” that are not clinically significant and would not have harmed the patient. In these cases, a cancer screening program would appear to be successful by detecting the tumor at an early stage but not truly provide a net benefit since the patient’s survival would be unchanged (Figure 1). It is broadly related to (but not synonymous with) over-testing and often leads to over-treatment.26, 27
Figure 1.
Schema of overdiagnosis and lead time in patients with hepatocellular carcinoma
Overdiagnosis is common and has been reported as an inevitable consequence of screening in many cancer types, including breast, prostate, lung, and colorectal cancers.28–31 Most commonly, it is first observed (inferred from) population-based studies when the incidence and mortality rates of a cancer begin to diverge over time. The magnitude and extent of overdiagnosis has been measured using various methods, including autopsy studies, studies with imaging and pathological correlation, modelling studies, ecological and cohort studies and extended follow-up of randomized controlled trials.32 The magnitude of estimated overdiagnosis for a given cancer can be highly variable33, which leads to difficulty translating these data into guidelines and informed decisions with patients. For example, the published estimates of the magnitude of overdiagnosis in breast cancer vary widely from 0% to 57%.34, 35 Welch and colleagues, using Surveillance, Epidemiology, and End Results (SEER) data, noted increasing use of screening mammography over time resulted in significant increase in small tumor detection (162 more cases per 100,000 women); however, there was not a proportional decrease in large tumor detection (only 30 fewer per 100,000 women), suggesting a significant number of tumors were likely overdiagnosed.28 Similarly, a multicenter, randomized screening trial in Canada found nearly one-fourth (22%) of tumors detected by annual mammograms in women aged 40–59 were classified as overdiagnosis.36
Though seemingly counterintuitive, overdiagnosis can have serious negative consequences from both an individual and public health perspective. From an individual perspective, overdiagnosis can lead to overtreatment and its associated risk of complications, related financial harms, and the stigma and impaired psychological well-being related to the label of a cancer diagnosis.27, 37 The issue of overdiagnosis resulting in overtreatment has been described with prostate cancer, in which PSA screening has been reported to result in over 1 million additional men receiving aggressive prostate cancer treatment in the U.S. despite its traditionally indolent nature.38 Several studies have reported the financial toxicity of cancer treatment, including high out-of-pocket costs for palliative therapies with limited survival benefit.39, 40 The total expenditures for cancer care have increased by over 33% over the past decade, and out-of-pocket costs have increased from $1800 to $2900 in the month of diagnosis alone.41, 42 From a psychological harms perspective, a cancer diagnosis may result in negative self-perception, altered life goals and significant worry about future health outcomes.15 Family and friends may also experience undue stress and burden as a consequence of the diagnosis.43, 44 Finally, overdiagnosis may distract from other health issues, as patients and providers may assume other health conditions as well as general preventive care have a lower priority compared to the new cancer diagnosis. For HCC, most data have focused on potential physical harms of treatment, which may be a consequence of overdiagnosis, with fewer data reporting financial or psychological harms of HCC treatment.8, 18 For example, local ablation and surgical resection – traditionally curative procedures for HCC - can be associated with risks of bleeding, infection, hepatic decompensation, and injury to nearby organs.45–47 In the setting of overdiagnosis, the patient would be subjected to these risks in the absence of any potential benefit.
From a public health perspective, overdiagnosis can inflate epidemiologic estimates for both HCC incidence and mortality.25 Increased implementation of surveillance programs and overdiagnosis can result in perceived increases in HCC incidence over time despite the true incidence remaining stable. It can also lead to biased comparisons for screening test performance and benefits, particularly if one modality is more prone to overdiagnosis. For example, overdiagnosis may be more likely with MRI than ultrasound- or biomarker-based HCC surveillance given increased detection of subcentimeter observations with imaging features that are suspicious for HCC and lesions between 1–2 cm that are classified as definite HCC – despite some of these lesions being low risk of becoming clinically significant.48, 49
Drivers of Overdiagnosis and Guideline Efforts to Mitigate Overdiagnosis
There are several potential drivers of overdiagnosis, most related to either patient- or tumor-related factors. Overdiagnosis can be related to inaccurate diagnostic criteria, detection of premalignant or small malignant lesions that would not otherwise become symptomatic, detection of indolent tumors, and competing risks of mortality.
Inaccurate diagnostic criteria
Overdiagnosis can occur when disease thresholds are lowered without evidence that this improves downstream outcomes and when patients with ambiguous are included as cases. Although often well-intended with the goal of intervening early to prevent worse disease, expanding diagnostic criteria often simply results in overdiagnosis. One of the best examples may be hypertension, for which the American Heart Association/American College of Cardiology lowered the threshold for systolic blood pressure from 140 mmHg to 130 mmHg in the 2017 hypertension guidelines.50 This expansion was controversial and not endorsed or accepted by many other societies including the American Academy of Family Medicine for fear of overdiagnosis and overtreatment.51 In oncology, this issue is observed with carcinoma in situ, which is reported in several organ systems including breast, bladder, and cervix. While the term “carcinoma” implies cancer and this is even sometimes referred to as stage 0 cancer, the cells are noninvasive and non-malignant in nature.52 Across organ systems, less than one-fourth of carcinoma in situ patients develop invasive cancer, although a higher proportion undergo intensive monitoring or even early treatment.53
HCC is unique compared to other tumors given its diagnosis can often be made solely on radiographic criteria using dynamic contrast-enhanced CT or MRI.54, 55 Although biopsy can be used in some cases with atypical imaging features, histologic confirmation is not required in most cases – which some argue increases the likelihood of misdiagnoses.56, 57 The LI-RADS (Liver Imaging Reporting and Data) system, proposed by the American College of Radiology (ACR) to standardize classification of liver nodules on diagnostic CT or MRI in at-risk patients,58 categorizes liver nodules on an ordinal scale from LR-1 (definitely benign) to LR-5 (definitely HCC) based on imaging features including size, arterial phase hyperenhancement, delayed phase washout, and capsule appearance. The presence of arterial phase hyperenhancement and delayed washout in lesions ≥1 cm is reported to have a ~95% positive predictive value for the presence of HCC, whereas subcentimeter lesions with these features have a substantially lower risk of HCC.59–61 However, the positive predictive value and accuracy of these imaging findings is directly proportional to tumor size, with substantially lower accuracy in lesions closer to the 1 cm size threshold.6, 62, 63 A meta-analysis evaluating the performance of HCC diagnostic imaging found MRI had positive likelihood ratios and diagnostic odds ratios of only 1.3 and 2.3, respectively, for lesions < 1cm; 5.5 and 17, respectively, for lesions 1–2 cm; and 6.5 and 64.7, respectively, for lesions > 2cm.63 Observations with features such as progressive concentric enhancement, rim arterial phase hyperenhancement, liver surface retraction, and target appearance on diffusion-weighted images have a >90% risk of malignancy but only 36% are HCC. 64 It is recommended that any cases with questionable or overlapping features be classified as LR-M instead of LR-5 and undergo biopsy to make a definitive diagnosis. Accurate distinction of LR-5 and LR-M observations can be difficult, particularly among non-expert readers, and thereby potentially lead to HCC overdiagnosis.65, 66 Similarly, non-invasive diagnostic criteria using LI-RADS have only been validated in at-risk populations including patients with cirrhosis or those with chronic HBV infection. Patients with liver masses outside of this setting, including patients with advanced fibrosis but not cirrhosis (as well as those with congenital hepatic fibrosis, Budd-Chiari syndrome and cardiac cirrhosis) require biopsy for HCC diagnosis, even if features of arterial phase hyperenhancement and delayed phase washout are present.6, 58 The positive predictive value for HCC diagnosis in these other settings is markedly lower and can lead to overdiagnosis if incorrectly applied.
Gadoxetate-enhanced MRI has been proposed as an alternative diagnostic strategy in patients with positive surveillance tests to increase sensitivity for early-stage HCC67–69; however, this may further compound the issue of overdiagnosis and has not been included in guideline recommendations from the AASLD or EASL. Prior studies suggest gadoxetate-enhanced MRI lacks perfect specificity and a proportion of high-grade dysplastic nodules will have overlapping features with early-stage HCC on imaging.5
Detection of Premalignant lesions
Overdiagnosis of premalignant lesions has been described for other cancer types including pancreatic cancer, esophageal cancer, and colorectal cancer.33, 70 Although there is a lack of robust data to support routine pancreatic or esophageal cancer screening programs, the issue of overdiagnosis (and resultant over-treatment) related to detection of premalignant lesions is common in clinical practice.71 72 There is similarly overdiagnosis of polyps in colorectal cancer screening, with studies reporting a prevalence of 25–35% for colon polyps compared to a substantially lower lifetime risk of CRC.73 These data suggest that many polypectomies are performed with no clinical gain but still result in increased surveillance intensity.
During HCC surveillance, indeterminate liver nodules on diagnostic CT or MRI (i.e., LR-3 or LR-4) are observed in over one-fourth of cirrhosis patients undergoing surveillance.74 Patients with LR-3 or LR-4 lesions have an elevated, yet variable, annual risk of HCC, with approximately one-third of LR-3 lesions and two-thirds of LR-4 lesions becoming HCC over time.75, 76 Although features such as size and AFP level are associated with risk of incident HCC, the predictive value of these features is imperfect so guidelines recommend monitoring all patients with LR-3 or LR-4 lesions with cross-sectional imaging every 3–6 months.5, 6 Intensive surveillance and follow-up of these lesions can result in negative financial and psychological consequences (e.g., anxiety and cancer-specific worry). Further, some centers empirically treat LR-4 lesions, without histologic confirmation, with locoregional therapies including ablation given high perceived risk of HCC, leading to additional harms of overtreatment. In those cases, it is difficult to determine if treatment was appropriate or overtreatment as the natural history in the lesion in the absence of any therapy is unknown. Although guidelines attempt to minimize the risk of overdiagnosis by recommending multidisciplinary discussion to consider close surveillance versus biopsy versus other management, there is a clear need for risk stratification and diagnostic biomarkers to aid in the clinical management of these patients.
Indolent Tumors
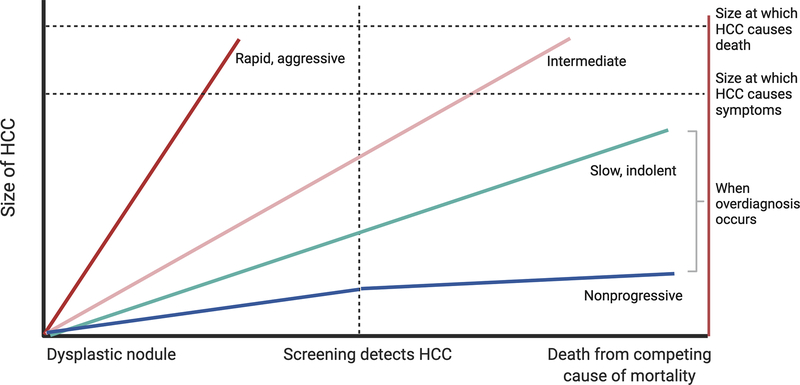
Cancer screening programs are built on the premise that most tumors have a consistent, predictable, and gradual growth pattern, as they progress from very early and early stages to intermediate or advanced stage disease (Figure 2). Based on this assumption, anticipated tumor doubling times can then inform optimal surveillance intervals to detect cancer at an early stage. Aggressive tumors, with short tumor doubling times and rapid growth patterns, are less likely to be detected at an early stage by screening. These tumors may also be less likely to respond to therapies or be associated with higher risk of recurrence even if treated surgically. For example, pancreatic cancer’s aggressive tumor biology is one reason why cancer screening programs have been difficult to implement, even in higher risk populations.77 In contrast, indolent tumors, with longer tumor doubling times and slow growth patterns, are very likely to be detected at an early stage by screening and may have excellent prognosis with cancer-directed therapy; however, the natural history of these patients in the absence of screening and treatment may have been similar without exposing the patient to the risks and financial toxicity of the therapies.
Figure 2.
Overdiagnosis due to indolent tumor growth patterns and competing risk of mortality
Some cancers are so slow growing that they would not have any clinical significance if left undetected and untreated. This issue is perhaps best epitomized by neuroblastoma in infants, which can often spontaneously regress, without any treatment.78 Prostate cancer is another traditionally indolent cancer where overdiagnosis is common.79 This is evidenced by results of the PIVOT Trial, in which only 31 (8%) of 367 men randomly assigned to observation died of prostate cancer after a median follow-up of 10 years.80 Prostate specific antigen (PSA) testing is unable to distinguish aggressive cancers from slow-growing, indolent cancers, and treatment side effects can result in harms that significantly impact quality of life, leading the USPSTF to recommends against routine PSA screening (without discussion of goals and preferences).81
With an incidence-to-mortality ratio near 1, HCC has historically not been considered an indolent (or overdiagnosed) tumor. Early studies found the tumor doubling time (TDT) for HCC was approximately 90 days, informing a suggested surveillance interval of 6 months.82, 83 However, increasing data highlight heterogeneity in tumor growth patterns. A retrospective multi-center cohort study from the US and Europe revealed a median tumor doubling time of 7.5 months, with 38% of HCC having indolent growth patterns (TDT > 365 days), 37% having intermediate growth patterns (TDT 90–365 days), and 25% having rapid growth patterns (TDT <90 days).84 A subsequent systematic review and meta-analysis with 20 studies reported a pooled tumor doubling time of 4.6 months, with 38% of HCC being classified as indolent with TDT exceeding 9 months.85 There were few consistent correlates of tumor growth patterns, although some studies reported associations between indolent growth and non-viral liver disease, larger tumor size, lower AFP levels, and well differentiated histology.85 In the absence of clinical risk models, blood-based biomarkers, or radiomics features to distinguish tumor biology and growth patterns, guidelines have not remarked on how to best minimize overdiagnosis of indolent HCC tumors or how this should be incorporated into clinical decision making to prevent overtreatment.
Overdiagnosis due to a tumor’s indolent nature is encompassed by the concept of length time bias, a recognized limitation of cohort studies assessing screening benefits. Length time bias is an overestimation of survival benefit due to excess detection of indolent tumors by screening, whereas aggressive, rapidly growing tumors are more likely to present symptomatically. Although several cohort studies assessing the association between HCC surveillance and survival have statistically adjusted for lead time bias12, 13, future studies should also aim to address concerns of length time bias.11
Competing risks of mortality
Overdiagnosis due to competing risk of mortality can be difficult to detect as it typically must be identified in asymptomatic individuals who died of other causes. Autopsy studies have confirmed a large reservoir of pseudodisease for other cancers, particularly prostate, lung and papillary thyroid cancers86–89; however, few postmortem studies are available in patients with cirrhosis and HCC.90, 91 A proportion of tumors detected by any screening program will be inconsequential due to competing mortality risk from other causes. The proportion of overdiagnosis related to competing risks of mortality vary by cancer type. For example, lung cancer screening may be more prone to this cause of overdiagnosis given competing mortality from chronic obstructive pulmonary disease and other smoking-related complications.92 93 “Excess” screening and risk of overdiagnosis in patient populations with competing risks of mortality may be driven by patients’ anxiety regarding cancer and physicians’ fear of litigation94, 95, as patients and providers may not find it acceptable to stop screening.
For HCC, there can be competing risks of mortality from liver-related and non-liver-related causes given that 80–90% of HCC occur in the setting of cirrhosis.96, 97 Liver function is an important determinant of eligibility for HCC-directed therapy and cirrhosis-related mortality and is therefore reflected in the Barcelona Clinic Liver Cancer (BCLC) staging system which includes not only tumor burden, but also liver disease severity and ECOG performance status.98 Independent of tumor burden, patients with significant liver dysfunction, i.e., Child Pugh class C cirrhosis, are classified as BCLC stage D (terminal stage); these patients are not eligible for HCC-directed therapy, outside of liver transplantation, given lack of improvement in overall survival.
The assessment of competing risks in various states of cirrhosis is inherently complex, and the use of multistate models may improve risk estimates and guide decision making in patients with cirrhosis and HCC.99 Further, the trajectory of cirrhosis is no longer considered to be unidirectional, particularly with lifestyle changes (e.g., alcohol abstinence) and effective treatments for the underlying insult (e.g., antiviral therapies for hepatitis B or C infection) that can improve fibrosis and result in “recompensation”.100 Antiviral treatment for HBV and HCV in patients with HCC may also preserve liver function and reduce the risk of and/or improve decompensation and therefore make subsequent HCC treatments feasible.101–103 In the future, the availability of antifibrotic drugs may further affect competing risk of liver-related mortality and shift the risk-benefit ratio of HCC surveillance in patients with Child Pugh B or C cirrhosis.
There are limited data available on competing risk of non-liver mortality in patients with HCC, which is in part due to the inherent difficulty ascertaining cause of death in patients with cirrhosis and HCC. Although the competing risk of non-liver mortality may be low in patients with advanced stage HCC, this needs to be considered for patients with pre-malignant lesions or early-stage HCC, who can have multi-year survival even in the absence of HCC-directed therapies.4 This concept may become increasingly important as the epidemiology of HCC shifts from a viral hepatitis-related condition to one driven by non-viral etiologies, such as alcohol-associated cirrhosis and nonalcoholic steatohepatitis (NASH).104, 105 A population-based Danish study reported patients with alcohol-associated cirrhosis had a high 5-year cumulative mortality of 43.7% (95%CI 42.6–44.7%); however, only 1.8% of deaths were related to HCC, suggesting a high competing mortality risk and possibility of HCC overdiagnosis106; however, further studies are needed. Compared to patients with HCC of other etiologies, patients with NASH-HCC are often older with more non-liver comorbidities, particularly obesity, cardiovascular disease, and renal insufficiency.107, 108 These comorbidities may preclude curative surgical treatments (i.e., resection and transplant) as well as systemic therapy regimens (e.g., anti-VEGF agents109), mitigating benefits of surveillance and increasing the possibility of overdiagnosis.
Guidelines for other cancer types have incorporated competing risk of mortality when defining at-risk populations in whom screening is recommended. For example, US Preventive Services Task Force recommendations for CRC screening recommend screening until age 75 and the net benefit of screening is small in those aged 76–85 years.110 They recommend providers selectively offer screening in this age group based on prior screening and overall health. Recent data show lower benefits of CRC screening in younger patients with significant comorbidity111, although this has not been officially incorporated into guideline recommendations. AASLD and EASL guidelines recommend against HCC surveillance in patients with Child Pugh class C cirrhosis who are ineligible for transplant given the lack of available HCC-directed therapies, although do not include limitations based on age or comorbidity.5, 6 This recommendation was supported in a multicenter Italian study by Trevisani et al, in which HCC surveillance was associated with a survival benefit in patients with Child Pugh class A cirrhosis, marginal benefit in Child Pugh B cirrhosis and no benefit in those with Child Pugh C cirrhosis.112 However, in contrast to other cancer types, currently available data have failed to show a consistent difference in treatment eligibility and prognosis between younger and older patients with HCC.113–116
Further research (both in prospective cohorts and modeling studies) is needed to provide more accurate estimates of competing risks of mortality in HCC to determine who to enroll in and when to stop surveillance programs. Novel multistate competing risk models that adjust for the risk of mortality in various states of cirrhosis (e.g., compensated with clinically significant portal hypertension vs late decompensated cirrhosis) and the fluidity of this risk over time may improve prognostication in patients with HCC.99, 117
Choice of Surveillance Test and Interval
Current societal guidelines recommend semi-annual ultrasound with or without serum AFP for surveillance.5, 6 Although ultrasound is widely available and relatively inexpensive with acceptable sensitivity for any-stage HCC (84%; 95%CI 76 – 92%), it has imperfect sensitivity for detection of early HCC (47%; 95%CI 33 – 61%).118 Ultrasound is also highly operator dependent, resulting in variable sensitivity in clinical practice.118 Moreover, up to 1 in 4 ultrasound exams are inadequate due to impaired visualization due to actors such as body habitus, liver nodularity and/or presence of liver steatosis, which may result in further decreases in sensitivity.119
Given the limited effectiveness of ultrasound, other modalities (e.g., CT and MRI) have been investigated for use in surveillance programs.48, 120 However, the higher sensitivity of CT and MRI for early-stage HCC may result in increased overdiagnosis – both by detecting small LR-5 observations that may not truly be HCC as well as increased detection of premalignant lesions. MRI-based surveillance has been best evaluated to date in a cohort study from South Korea with 407 patients, of whom 43 developed HCC – 32 of whom were detected at a very early stage (unifocal lesion ≤2 cm in maximum diameter) and 20 of whom with pathologic confirmation.49 In this study, Kim and colleagues found MRI had a sensitivity of 54.5% for early-stage HCC when considering LR-5 lesions and 84.8% when considering LR-4 or LR-5 lesions, compared to only 27.3% for ultrasound. Although the sensitivity of MRI was higher than that of ultrasound, particularly for very early-stage HCC detection, it is unclear if this difference was partly related to overdiagnosis. Further head-to-head trials of MRI- and ultrasound-based surveillance, including in patients with non-viral related cirrhosis are still needed. At the present time, AASLD guidelines recommend against the routine use of CT or MRI as the primary modality for HCC surveillance in patients with cirrhosis.6
In parallel with advances in radiologic imaging, there has been increased interest in novel blood-based biomarker panels.121 AFP is the best studied biomarker for HCC early detection, having been validated in a phase V study and a meta-analysis showing benefit when added to surveillance ultrasound.118, 122–124 However, AFP has suboptimal sensitivity and specificity when used alone125, creating a need for novel biomarkers and biomarker panels. GALAD (a panel combining AFP, AFP-L3% and DCP with patient gender and age) and a methylated DNA marker panel have both recently demonstrated promising sensitivity in phase II case-control datasets126–128 and small preliminary cohort studies.129, 130 Interestingly, several biomarkers also have prognostic value131, 132, suggesting they are less likely to detect pre-malignant or indolent lesions so may be less prone to overdiagnosis. AFP, AFP-L3, and DCP have each been demonstrated to predict risk of recurrence after liver transplantation, independent of tumor burden, with AFP levels now incorporated into patient selection criteria.133, 134 Similarly, a study from the Circulating Cell-free Genome Atlas (CCGA) across cancer types found that circulating tumor fractions were higher for faster growing, more aggressive tumors that were more likely to be clinically significant.135 Although further validation of these emerging biomarker panels still require validation in large phase III and IV cohort studies124, it is possible that a shift to a biomarker-predominant surveillance strategy may also mitigate issues with overdiagnosis.
The recommended six-month interval for surveillance was initially based on tumor doubling times but subsequent studies have shown this interval to be superior to both longer and shorter intervals. Cohort studies have demonstrated that semi-annual surveillance is superior as it is associated with increased early HCC detection, curative treatment receipt and overall survival benefit compared to annual surveillance.136 Conversely, a RCT of 1278 patients in France and Belgium found that quarterly surveillance resulted in detection of an increased number of indeterminate liver nodules but did not improve early-stage HCC detection versus semi-annual surveillance. Only 4% of sub-centimeter indeterminate liver nodules in the study were subsequently determined to be HCC, with most remaining stable or regressing during follow-up, and there was no difference in 5-year survival between the two groups (84.9% vs 85.8%, p=0.38).137Based on these data, AASLD and EASL guidelines both recommend semi-annual surveillance.5, 6
Methods to Detect and Quantify Overdiagnosis
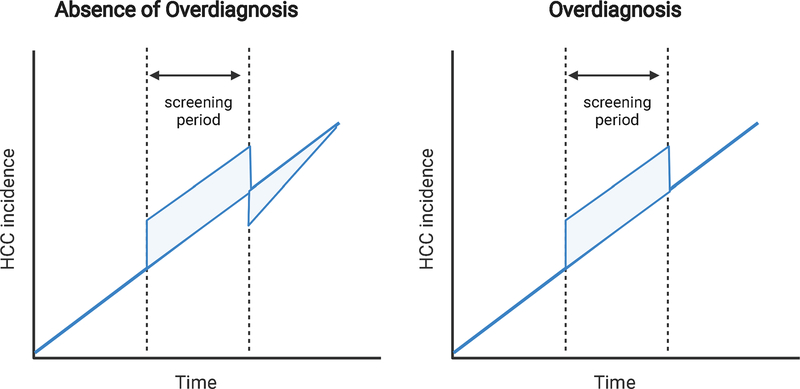
Figure 3 illustrates the expected changes in cancer incidence after implementation of a cancer screening program, with and without overdiagnosis.138 In the absence of overdiagnosis, screening should result in perceived increases in cancer incidence, related to detection of both incident and prevalent cases. After cessation of the screening program, whether related to completion of a RCT or stopping rules based on age and comorbidity, the incidence should decrease given time required for new incident cases to present symptomatically. After that lead-time period has passed, the incidence then converges back to baseline levels prior to implementation of the screening program. In the setting of overdiagnosis, one observes a similar increased cancer incidence with implementation of the screening program but fail to observe a compensatory drop in incidence after cessation of the program.
Figure 3.
Effect of overdiagnosis on cancer incidence
In the absence of overdiagnosis (left panel), there is an increase in cancer incidence during the screening period, which is then compensated with decreased incidence after screening is stopped. In the setting of overdiagnosis (right panel), there is an increased incidence but no associated decreased incidence after screening cessation.
There has been significant discussion over the past two decades on the optimal method to detect and quantify overdiagnosis.139, 140 Using the principle above, follow-up studies of RCTs can be an ideal way to quantify overdiagnosis by comparing cancer incidence in the screening and control arms. Overdiagnosis is quantified by comparing the number of cancers in the screening group to those in the no-screening group (including cases diagnosed after screening completion). Although this methodology has low risk of selection bias and confounding, it is often difficult to perform as it requires resources for the RCT and prolonged follow-up after the trial has been completed. This time requirement can also limit generalizability if there are changes in disease epidemiology, screening tools, or diagnostic standards over time. For example, it is unknown if studies in breast cancer that used this methodology from RCTs conducted >30 years ago still apply to contemporary practice. Modeling studies have also been proposed, using inputs for tumor growth patterns and doubling times, but these models are dependent on the quality of data for their inputs and often oversimplify the concept of overdiagnosis.138, 141
Given limitations of other methods, the most accurate approach to estimate overdiagnosis may be robust ecological and cohort studies, particularly if conducted in multiple settings (i.e., different time periods, geographic areas, various demographic populations), such as has been done in breast142–144 and prostate cancer.145 Using cohort studies, overdiagnosis is quantified as the difference in cumulative incidence between the screened group and the non-screened group. For example, this approach can either compare attendees vs. non-attendees in the setting of an existing screening program or find an external non-screening group (e.g., historical control prior to implementation of screening program or external geographic area where screening program does not exist). Limitations of this approach include self-selection bias, unmeasured confounding, lead-time bias, and misclassification bias.32 Self-selection bias is a concern as the patient choice to complete or not complete screening may be correlated with cancer risk, so this may result in either an over- or under-estimation of overdiagnosis. Lead-time bias artificially increases survival time of cases detected by screening and can be difficult to adjust, as it is heterogenous among individuals and differing screening strategies. Thus, no single lead-time estimate can capture this time period for an entire population and adjusting for the “mean” lead time among individuals presents its own risk of bias. Although classification of regular screening attendees and never attendees can be uncomplicated, irregular attendees can be misclassified and result in biased estimates for the magnitude of overdiagnosis. However, well designed ecological and cohort studies can still provide credible estimates of overdiagnosis as well as monitor effects over time. There is a paucity of such data in HCC and thus there is a need for international, well-conducted cohort studies to better understand the impact and temporal trends of different screening programs on overdiagnosis.
Steps to Mitigate Overdiagnosis
Given the negative consequences of overdiagnosis, standards for studying and reporting overdiagnosis in HCC are needed and should be incorporated into future studies when possible. We have outlined some steps to mitigate overdiagnosis in Table 1. Several guideline recommendations can reduce overdiagnosis including: targeting surveillance to high-risk populations, using ultrasound-based surveillance, performing surveillance on a semi-annual basis, and using strict diagnostic criteria for HCC cases. Patients with HBV or cirrhosis from any etiology are target populations in whom surveillance has been shown to be beneficial, and expansion of surveillance to lower risk populations such as those with advanced fibrosis may increase the risk of overdiagnosis. Similarly, guidelines recommend against HCC surveillance in patients with high risk competing risk of liver-related or non-liver related mortality, as it is unlikely to provide a survival benefit. Patient eligibility and stopping rules must be considered when implementing inreach and outreach interventions to address underuse of HCC surveillance.146, 147 If not done, benefits of these programs may be overestimated given unintended increases in overdiagnosis. We have discussed above how increasing surveillance intensity – whether by use of cross-sectional imaging such as MRI or by shortening surveillance intervals – also may increase overdiagnosis. It is also critical to adhere to guideline recommendations for HCC diagnosis, including appropriate use of standardized diagnostic criteria (i.e., LI-RADS), use of biopsy in patients with uncertain imaging features (e.g., LR-M) and not empirically treating patients with premalignant disease (e.g., LR-4). Patients in whom imaging features have not been validated, e.g., patients without cirrhosis or those with cardiac cirrhosis, also require biopsy for definitive HCC diagnosis.
Table 1.
Potential steps to mitigate overdiagnosis in HCC surveillance
| Method | Example |
|---|---|
| Target screening to the at-risk population | AASLD and EASL guidelines recommend HCC surveillance in at risk populations (i.e., cirrhosis and select individuals with hepatitis B infection) and against routine screening in non-cirrhotic NAFLD or hepatitis C infection Risk stratification biomarkers may facilitate precision/tailored surveillance according to individual patient risk in the future |
| Avoid screening in patients with high competing risks of mortality | AASLD and EASL guidelines recommend avoiding HCC surveillance in patients with significant liver dysfunction (e.g., Child Pugh C cirrhosis) and those with high comorbidity |
| Appropriate frequency of screening | AASLD and EASL guidelines recommend HCC surveillance with ultrasound +/- AFP every 6 months (shorter intervals result in increased overdiagnosis) |
| Avoid screening and diagnostic tests that have high potential for overdiagnosis | AASLD and EASL guidelines recommend ultrasound instead of routine use of CT or MRI for surveillance Emerging early detection biomarkers may preserve high sensitivity for early HCC while reducing potential for overdiagnosis |
| Adhere to guideline recommendations for recall procedures | AASLD and EASL guidelines recommend repeat short-interval ultrasound in patients with sub-centimeter lesions and perform diagnostic imaging for those with lesions ≥1 cm and those with elevated AFP levels. |
| Strictly apply diagnostic criteria | AASLD and EASL guidelines recommend strict radiologic criteria (e.g., LI-RADS) for HCC diagnosis and using biopsy in cases where diagnosis is uncertain and LI-RADS has not been validated (e.g., non-cirrhosis, cardiac cirrhosis) AASLD and EASL guidelines recommend multidisciplinary discussion for patients with LR-4 observations and recommend against routine treatment |
| Assess tumor biology and identify indolent tumors that may not require treatment | Novel prognostic biomarkers and radiomics may help distinguish indolent versus aggressive HCC |
| Inform patients of the possibility of overdiagnosis, the balance of benefits and harms and enable shared decision making | Discuss benefits and harms of various HCC surveillance modalities according to the patient’s individual risk Counsel patients on the potential outcomes and limitations of surveillance tests |
Development and validation of biomarkers could help improve processes at several steps to further reduce risk of overdiagnosis. For example, risk stratification biomarkers may help exclude low risk populations in whom surveillance is unlikely to be beneficial and identify patients, including those with indeterminate liver nodules, who may have the highest risk of developing HCC and to whom surveillance should be targeted. Compared to imaging, early detection biomarkers may be less prone to detecting premalignant and indolent tumors and could possibly reduce overdiagnosis. Finally, prognostic biomarkers would help discriminate between tumors that will have indolent or aggressive behavior and identify those that do not require immediate treatment Continued progress in this area will help improve the overall risk-benefit ratio of HCC surveillance and reduce the burden and harms of overdiagnosis.
Conclusion
In summary, overdiagnosis is a clinically important but understudied concept in HCC. It can result in various harms, including overestimation of screening benefits and overtreatment. Overdiagnosis is driven by several patient and tumor factors and has important implications from both patient and public health perspectives. This is a high priority area for future research, with studies needed to evaluate the burden and trends of overdiagnosis, considering shifting surveillance strategies, as well as the impact of potential interventions to reduce overdiagnosis.
Financial Source:
Dr. Singal’s effort is supported by NIH U01 CA230694, R01 CA222900, and R01 CA212008. Dr. Rich is supported by the American College of Gastroenterology Junior Faculty Development Award and the Texas Health Resources Clinical Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest:
Amit Singal has served as a consultant or on advisory boards for Genentech, AstraZeneca Bayer, Eisai, Exelixis, BMS, FujiFilm Medical Sciences, Exact Sciences, Glycotest, Roche, and GRAIL.
Nicole Rich has served as a consultant for AstraZeneca.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clinical Gastroenterology and Hepatology 2020;18:2650–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification, In Seminars in liver disease, © 1999 by Thieme Medical Publishers, Inc., 1999. [DOI] [PubMed] [Google Scholar]
- 4.Khalaf N, Ying J, Mittal S, et al. Natural history of untreated hepatocellular carcinoma in a US cohort and the role of cancer surveillance. Clinical Gastroenterology and Hepatology 2017;15:273–281. e1. [DOI] [PubMed] [Google Scholar]
- 5.Liver EAFTSOT. EASL clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 7.Woolf SH, Harris R. The Harms of Screening: New Attention to an Old Concern. JAMA 2012;307:565–566. [DOI] [PubMed] [Google Scholar]
- 8.Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–22. [DOI] [PubMed] [Google Scholar]
- 10.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cucchetti A, Garuti F, Pinna AD, et al. Length time bias in surveillance for hepatocellular carcinoma and how to avoid it. Hepatol Res 2016;46:1275–1280. [DOI] [PubMed] [Google Scholar]
- 12.Cucchetti A, Trevisani F, Pecorelli A, et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J Hepatol 2014;61:333–41. [DOI] [PubMed] [Google Scholar]
- 13.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:976–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heleno B, Thomsen MF, Rodrigues DS, et al. Quantification of harms in cancer screening trials: literature review. Bmj 2013;347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RP, Sheridan SL, Lewis CL, et al. The Harms of Screening: A Proposed Taxonomy and Application to Lung Cancer Screening. JAMA Internal Medicine 2014;174. [DOI] [PubMed] [Google Scholar]
- 16.Petrasek J, Singal AG, Rich NE. Harms of hepatocellular carcinoma surveillance. Curr Hepatol Rep 2019;18:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh ND, Singal AG, Hutton DW, et al. Cost-effectiveness of hepatocellular carcinoma surveillance: an assessment of benefits and harms. Official journal of the American College of Gastroenterology| ACG 2020;115:1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singal AG, Patibandla S, Obi J, et al. Benefits and harms of hepatocellular carcinoma surveillance in a prospective cohort of patients with cirrhosis. Clinical Gastroenterology and Hepatology 2021;19:1925–1932. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor EJ, Jones RL, Guthrie JA, et al. Modeling the benefits and harms of surveillance for hepatocellular carcinoma: information to support informed choices. Hepatology 2017;66:1546–1555. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz LM, Woloshin S, Fowler FJ Jr, et al. Enthusiasm for cancer screening in the United States. Jama 2004;291:71–78. [DOI] [PubMed] [Google Scholar]
- 21.Woolen SA, Singal AG, Davenport MS, et al. Patient Preferences for Hepatocellular Carcinoma Surveillance Parameters. Clinical Gastroenterology and Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodersen J, Schwartz LM, Heneghan C, et al. Overdiagnosis: what it is and what it isn’t. BMJ Evidence-Based Medicine 2018;23:1. [DOI] [PubMed] [Google Scholar]
- 23.Black WC. Overdiagnosis: an underrecognized cause of confusion and harm in cancer screening. Journal of the National Cancer Institute 2000;92:1280–1282. [DOI] [PubMed] [Google Scholar]
- 24.Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ : British Medical Journal 2012;344:e3502. [DOI] [PubMed] [Google Scholar]
- 25.Rich NE, Parikh ND, Singal AG. Overdiagnosis: An Understudied Issue in Hepatocellular Carcinoma Surveillance. Semin Liver Dis 2017;37:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kale MS, Korenstein D. Overdiagnosis in primary care: framing the problem and finding solutions. Bmj 2018;362:k2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. The lancet oncology 2014;15:e234–e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welch HG, Prorok PC, O’Malley AJ, et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. New England Journal of Medicine 2016;375:1438–1447. [DOI] [PubMed] [Google Scholar]
- 29.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from US prostate cancer incidence trends. Journal of the National Cancer Institute 2002;94:981–990. [DOI] [PubMed] [Google Scholar]
- 30.Patz EF, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA internal medicine 2014;174:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner H, Altenhofen L, Stock C, et al. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clinical gastroenterology and hepatology 2015;13:717–723. [DOI] [PubMed] [Google Scholar]
- 32.Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. Bmj 2015;350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava S, Koay EJ, Borowsky AD, et al. Cancer overdiagnosis: a biological challenge and clinical dilemma. Nature Reviews Cancer 2019;19:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etzioni R, Xia J, Hubbard R, et al. A reality check for overdiagnosis estimates associated with breast cancer screening. Journal of the National Cancer Institute 2014;106:dju315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med 2013;158:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller AB, Wall C, Baines CJ, et al. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. Bmj 2014;348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. European urology 2014;65:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. Journal of the National Cancer Institute 2009;101:1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA: a cancer journal for clinicians 2018;68:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lentz R, Benson AB III, Kircher S. Financial toxicity in cancer care: prevalence, causes, consequences, and reduction strategies. Journal of surgical oncology 2019;120:85–92. [DOI] [PubMed] [Google Scholar]
- 41.Desai A, Gyawali B. Financial toxicity of cancer treatment: Moving the discussion from acknowledgement of the problem to identifying solutions. EClinicalMedicine 2020;20:100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011;103:117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grunfeld E, Coyle D, Whelan T, et al. Family caregiver burden: results of a longitudinal study of breast cancer patients and their principal caregivers. Cmaj 2004;170:1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welch HG, Black WC. Overdiagnosis in cancer. Journal of the National Cancer Institute 2010;102:605–613. [DOI] [PubMed] [Google Scholar]
- 45.Xu X-L, Liu X-D, Liang M, et al. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology 2018;287:461–472. [DOI] [PubMed] [Google Scholar]
- 46.Akahane M, Koga H, Kato N, et al. Complications of Percutaneous Radiofrequency Ablation for Hepato-cellular Carcinoma: Imaging Spectrum and Management. RadioGraphics 2005;25:S57–S68. [DOI] [PubMed] [Google Scholar]
- 47.Chok K, Ng K, Poon R, et al. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Journal of British Surgery 2009;96:81–87. [DOI] [PubMed] [Google Scholar]
- 48.Yu NC, Chaudhari V, Raman SS, et al. CT and MRI Improve Detection of Hepatocellular Carcinoma, Compared With Ultrasound Alone, in Patients With Cirrhosis. Clinical Gastroenterology and Hepatology 2011;9:161–167. [DOI] [PubMed] [Google Scholar]
- 49.Kim SY, An J, Lim YS, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol 2017;3:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 51.Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Annals of internal medicine 2017;166:430–437. [DOI] [PubMed] [Google Scholar]
- 52.Yen M-F, Tabar L, Vitak B, et al. Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. European journal of cancer 2003;39:1746–1754. [DOI] [PubMed] [Google Scholar]
- 53.van Seijen M, Lips EH, Thompson AM, et al. Ductal carcinoma in situ: to treat or not to treat, that is the question. British Journal of Cancer 2019;121:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, S taging, and M anagement of H epatocellular C arcinoma: 2018 P ractice G uidance by the A merican A ssociation for the S tudy of L iver D iseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 55.Osho A, Rich NE, Singal AG. Role of imaging in management of hepatocellular carcinoma: surveillance, diagnosis, and treatment response. Hepatoma Res 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta N, Dodge JL, Roberts JP, et al. Misdiagnosis of hepatocellular carcinoma in patients receiving no local-regional therapy prior to liver transplant: An analysis of the Organ Procurement and Transplantation Network explant pathology form. Clin Transplant 2017;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Tommaso L, Spadaccini M, Donadon M, et al. Role of liver biopsy in hepatocellular carcinoma. World J Gastroenterol 2019;25:6041–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61:1056–1065. [DOI] [PubMed] [Google Scholar]
- 59.van der Pol CB, Lim CS, Sirlin CB, et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology 2019;156:976–986. [DOI] [PubMed] [Google Scholar]
- 60.Liu W, Qin J, Guo R, et al. Accuracy of the diagnostic evaluation of hepatocellular carcinoma with LI-RADS. Acta Radiologica 2018;59:140–146. [DOI] [PubMed] [Google Scholar]
- 61.Basha MAA, El Sammak DAEA, El Sammak A. Diagnostic efficacy of the Liver Imaging-Reporting and Data System (LI-RADS) with CT imaging in categorising small nodules (10–20 mm) detected in the cirrhotic liver at screening ultrasound. Clinical radiology 2017;72:901. e1–901. e11. [DOI] [PubMed] [Google Scholar]
- 62.Marrero JA, Hussain HK, Nghiem HV, et al. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transplantation 2005;11:281–289. [DOI] [PubMed] [Google Scholar]
- 63.Roberts LR, Sirlin CB, Zaiem F, et al. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018;67:401–421. [DOI] [PubMed] [Google Scholar]
- 64.Fowler KJ, Potretzke TA, Hope TA, et al. LI-RADS M (LR-M): definite or probable malignancy, not specific for hepatocellular carcinoma. Abdom Radiol (NY) 2018;43:149–157. [DOI] [PubMed] [Google Scholar]
- 65.Fowler KJ, Tang A, Santillan C, et al. Interreader Reliability of LI-RADS Version 2014 Algorithm and Imaging Features for Diagnosis of Hepatocellular Carcinoma: A Large International Multireader Study. Radiology 2018;286:173–185. [DOI] [PubMed] [Google Scholar]
- 66.Yokoo T, Singal AG, Diaz de Leon A, et al. Prevalence and clinical significance of discordant LI-RADS® observations on multiphase contrast-enhanced MRI in patients with cirrhosis. Abdominal Radiology 2020;45:177–187. [DOI] [PubMed] [Google Scholar]
- 67.Kim Y-Y, An C, Kim S, et al. Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. European radiology 2018;28:2038–2046. [DOI] [PubMed] [Google Scholar]
- 68.Rhee H, Kim MJ, Park MS, et al. Differentiation of early hepatocellular carcinoma from benign hepatocellular nodules on gadoxetic acid-enhanced MRI. Br J Radiol 2012;85:e837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahn SS, Kim M-J, Lim JS, et al. Added Value of Gadoxetic Acid–enhanced Hepatobiliary Phase MR Imaging in the Diagnosis of Hepatocellular Carcinoma. Radiology 2010;255:459–466. [DOI] [PubMed] [Google Scholar]
- 70.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. Journal of the National Cancer Institute 2005;97:142–146. [DOI] [PubMed] [Google Scholar]
- 71.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Official journal of the American College of Gastroenterology| ACG 2010;105:2079–2084. [DOI] [PubMed] [Google Scholar]
- 72.Sangle NA, Taylor SL, Emond MJ, et al. Overdiagnosis of high-grade dysplasia in Barrett’s esophagus: a multicenter, international study. Modern Pathology 2015;28:758–765. [DOI] [PubMed] [Google Scholar]
- 73.Kalager M, Wieszczy P, Lansdorp-Vogelaar I, et al. Overdiagnosis in Colorectal Cancer Screening: Time to Acknowledge a Blind Spot. Gastroenterology 2018;155:592–595. [DOI] [PubMed] [Google Scholar]
- 74.Konerman MA, Verma A, Zhao B, et al. Frequency and Outcomes of Abnormal Imaging in Patients With Cirrhosis Enrolled in a Hepatocellular Carcinoma Surveillance Program. Liver Transpl 2019;25:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanabe M, Kanki A, Wolfson T, et al. Imaging Outcomes of Liver Imaging Reporting and Data System Version 2014 Category 2, 3, and 4 Observations Detected at CT and MR Imaging. Radiology 2016;281:129–139. [DOI] [PubMed] [Google Scholar]
- 76.Arvind A, Zaki TA, Yokoo T, et al. 16 RISK OF PROGRESSION TO HEPATOCELLULAR CARCINOMA IN PATIENTS WITH INDETERMINATE (LI-RADS 3) OBSERVATIONS. Gastroenterology 2021;160:S-759–S-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson GC, Maithel SK, Bentrem D, et al. Are the Current Guidelines for the Surgical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas Adequate? A Multi-Institutional Study. J Am Coll Surg 2017;224:461–469. [DOI] [PubMed] [Google Scholar]
- 78.Sawada T Past and future of neuroblastoma screening in Japan. Am J Pediatr Hematol Oncol 1992;14:320–6. [PubMed] [Google Scholar]
- 79.Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 2013;105:1050–8. [DOI] [PubMed] [Google Scholar]
- 80.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Force UPST. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018;319:1901–1913. [DOI] [PubMed] [Google Scholar]
- 82.Kubota K, Ina H, Okada Y, et al. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci 2003;48:581–6. [DOI] [PubMed] [Google Scholar]
- 83.Okada S, Okazaki N, Nose H, et al. Follow-up examination schedule of postoperative HCC patients based on tumor volume doubling time. Hepatogastroenterology 1993;40:311–5. [PubMed] [Google Scholar]
- 84.Rich NE, John BV, Parikh ND, et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology 2020;72:1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nathani P, Gopal P, Rich N, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut 2021;70:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med 1993;328:1237–43. [DOI] [PubMed] [Google Scholar]
- 87.Chan CK, Wells CK, McFarlane MJ, et al. More lung cancer but better survival. Implications of secular trends in “necropsy surprise” rates. Chest 1989;96:291–6. [DOI] [PubMed] [Google Scholar]
- 88.Furuya-Kanamori L, Bell KJL, Clark J, et al. Prevalence of Differentiated Thyroid Cancer in Autopsy Studies Over Six Decades: A Meta-Analysis. Journal of Clinical Oncology 2016;34:3672–3679. [DOI] [PubMed] [Google Scholar]
- 89.Bell KJ, Del Mar C, Wright G, et al. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer 2015;137:1749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schlageter M, Quagliata L, Matter M, et al. Clinicopathological Features and Metastatic Pattern of Hepatocellular Carcinoma: An Autopsy Study of 398 Patients. Pathobiology 2016;83:301–307. [DOI] [PubMed] [Google Scholar]
- 91.Sundaram C, Reddy CR, Ramana GV, et al. Hepatitis B surface antigen, hepatocellular carcinoma and cirrhosis in south India--an autopsy study. Indian J Pathol Microbiol 1990;33:334–8. [PubMed] [Google Scholar]
- 92.Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. Bmj 2004;328:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. New England Journal of Medicine 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pathirana T, Clark J, Moynihan R. Mapping the drivers of overdiagnosis to potential solutions. BMJ 2017;358:j3879. [DOI] [PubMed] [Google Scholar]
- 95.Hoffman JR, Kanzaria HK. Intolerance of error and culture of blame drive medical excess. BMJ : British Medical Journal 2014;349:g5702. [DOI] [PubMed] [Google Scholar]
- 96.Colombo M, de Franchis R, Del Ninno E, et al. Hepatocellular Carcinoma in Italian Patients with Cirrhosis. New England Journal of Medicine 1991;325:675–680. [DOI] [PubMed] [Google Scholar]
- 97.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2016;14:124–31.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329–38. [DOI] [PubMed] [Google Scholar]
- 99.D’Amico G, Morabito A, D’Amico M, et al. Clinical states of cirrhosis and competing risks. J Hepatol 2018;68:563–576. [DOI] [PubMed] [Google Scholar]
- 100.Jalan R, D’Amico G, Trebicka J, et al. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis. Journal of Hepatology 2021;75:S14–S26. [DOI] [PubMed] [Google Scholar]
- 101.Singal AG, Rich NE, Mehta N, et al. Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection Is Associated With Increased Survival in Patients With a History of Hepatocellular Carcinoma. Gastroenterology 2019;157:1253–1263.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rich NE, Singal AG. Direct-Acting Antiviral Therapy and Hepatocellular Carcinoma. Clin Liver Dis (Hoboken) 2021;17:414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papatheodoridis GV, Sypsa V, Dalekos G, et al. Eight-year survival in chronic hepatitis B patients under long-term entecavir or tenofovir therapy is similar to the general population. J Hepatol 2018;68:1129–1136. [DOI] [PubMed] [Google Scholar]
- 104.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology & Hepatology 2021;18:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Serag HB, Kanwal F, Feng Z, et al. Risk Factors for Cirrhosis in Contemporary Hepatology Practices-Findings From the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology 2020;159:376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jepsen P, Ott P, Andersen PK, et al. Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis: a Danish nationwide cohort study. Ann Intern Med 2012;156:841–7, w295. [DOI] [PubMed] [Google Scholar]
- 107.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723–1730. [DOI] [PubMed] [Google Scholar]
- 108.Hester CA, Rich NE, Singal AG, et al. Comparative Analysis of Nonalcoholic Steatohepatitis- Versus Viral Hepatitis- and Alcohol-Related Liver Disease-Related Hepatocellular Carcinoma. J Natl Compr Canc Netw 2019;17:322–329. [DOI] [PubMed] [Google Scholar]
- 109.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. British Journal of Cancer 2007;96:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Force UPST. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:1965–1977. [DOI] [PubMed] [Google Scholar]
- 111.Klabunde CN, Zheng Y, Quinn VP, et al. Influence of Age and Comorbidity on Colorectal Cancer Screening in the Elderly. American Journal of Preventive Medicine 2016;51:e67–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trevisani F, Santi V, Gramenzi A, et al. Surveillance for early diagnosis of hepatocellular carcinoma: is it effective in intermediate/advanced cirrhosis? Am J Gastroenterol 2007;102:2448–57; quiz 2458. [DOI] [PubMed] [Google Scholar]
- 113.Nozawa A, Kubo S, Takemura S, et al. Hepatic resection for hepatocellular carcinoma in super-elderly patients aged 80 years and older in the first decade of the 21st century. Surgery Today 2015;45:851–857. [DOI] [PubMed] [Google Scholar]
- 114.Kim J, Ko ME, Nelson RA, et al. Increasing Age and Survival after Orthotopic Liver Transplantation for Patients with Hepatocellular Cancer. Journal of the American College of Surgeons 2014;218:431–438. [DOI] [PubMed] [Google Scholar]
- 115.Jo M, Yasui K, Kirishima T, et al. Efficacy and safety of sorafenib in very elderly patients aged 80 years and older with advanced hepatocellular carcinoma. Hepatol Res 2014;44:1329–38. [DOI] [PubMed] [Google Scholar]
- 116.Nishikawa H, Kimura T, Kita R, et al. Treatment for Hepatocellular Carcinoma in Elderly Patients: A Literature Review. Journal of Cancer 2013;4:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: The importance of multistate models and competing risks analysis. Hepatology 2015;62:292–302. [DOI] [PubMed] [Google Scholar]
- 118.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706–1718.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pocha C, Dieperink E, McMaken KA, et al. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography – a randomised study. Alimentary Pharmacology & Therapeutics 2013;38:303–312. [DOI] [PubMed] [Google Scholar]
- 121.Nathani P, Singal AG. Imaging and Biomarker Approaches to HCC Surveillance. Clinical Liver Disease 2021;17:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parikh ND, Mehta AS, Singal AG, et al. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev 2020;29:2495–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tayob N, Christie I, Richardson P, et al. Validation of the Hepatocellular Carcinoma Early Detection Screening (HES) Algorithm in a Cohort of Veterans With Cirrhosis. Clinical Gastroenterology and Hepatology 2019;17:1886–1893.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singal AG, Hoshida Y, Pinato DJ, et al. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology 2021;160:2572–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marrero JA, Feng Z, Wang Y, et al. α-Fetoprotein, Des-γ Carboxyprothrombin, and Lectin-Bound α-Fetoprotein in Early Hepatocellular Carcinoma. Gastroenterology 2009;137:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chalasani NP, Porter K, Bhattacharya A, et al. Validation of a Novel Multitarget Blood Test Shows High Sensitivity to Detect Early Stage Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology. [DOI] [PubMed] [Google Scholar]
- 127.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clinical Gastroenterology and Hepatology 2016;14:875–886.e6. [DOI] [PubMed] [Google Scholar]
- 128.Singal AG, Tayob N, Mehta A, et al. Doylestown Plus and GALAD Demonstrate High Sensitivity for HCC Detection in Patients With Cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Best J, Bechmann LP, Sowa JP, et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728–735.e4. [DOI] [PubMed] [Google Scholar]
- 130.Singal AG, Nabihah T, Mehta A, et al. GALAD Demonstrates High Sensitivity for HCC Surveillance in a Cohort of Patients with Cirrhosis. Hepatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saito Y, Shimada M, Utsunomiya T, et al. Prediction of recurrence of hepatocellular carcinoma after curative hepatectomy using preoperative Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein. Hepatol Res 2012;42:887–94. [DOI] [PubMed] [Google Scholar]
- 132.Matsuda M, Asakawa M, Amemiya H, et al. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol 2011;26:731–8. [DOI] [PubMed] [Google Scholar]
- 133.Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. Journal of Hepatology 2017;66:552–559. [DOI] [PubMed] [Google Scholar]
- 134.Mehta N, Dodge JL, Roberts JP, et al. Alpha-Fetoprotein Decrease from > 1,000 to < 500 ng/mL in Patients with Hepatocellular Carcinoma Leads to Improved Posttransplant Outcomes. Hepatology 2019;69:1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bredno J, Lipson J, Venn O, et al. Clinical correlates of circulating cell-free DNA tumor fraction. PLoS One 2021;16:e0256436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010;53:291–7. [DOI] [PubMed] [Google Scholar]
- 137.Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011;54:1987–97. [DOI] [PubMed] [Google Scholar]
- 138.Bae J-M. Overdiagnosis: epidemiologic concepts and estimation. Epidemiol Health 2015;37:e2015004–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. Bmj 2015;350:g7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ripping TM, ten Haaf K, Verbeek ALM, et al. Quantifying Overdiagnosis in Cancer Screening: A Systematic Review to Evaluate the Methodology. JNCI: Journal of the National Cancer Institute 2017;109. [DOI] [PubMed] [Google Scholar]
- 141.de Koning HJ, Draisma G, Fracheboud J, et al. Overdiagnosis and overtreatment of breast cancer: microsimulation modelling estimates based on observed screen and clinical data. Breast Cancer Res 2006;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012;367:1998–2005. [DOI] [PubMed] [Google Scholar]
- 143.Falk RS, Hofvind S, Skaane P, et al. Overdiagnosis among women attending a population-based mammography screening program. Int J Cancer 2013;133:705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jørgensen KJ, Zahl PH, Gøtzsche PC. Overdiagnosis in organised mammography screening in Denmark. A comparative study. BMC Womens Health 2009;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ciatto S, Gervasi G, Bonardi R, et al. Determining overdiagnosis by screening with DRE/TRUS or PSA (Florence pilot studies, 1991–1994). Eur J Cancer 2005;41:411–5. [DOI] [PubMed] [Google Scholar]
- 146.Singal AG, Tiro JA, Murphy CC, et al. Mailed Outreach Invitations Significantly Improve HCC Surveillance Rates in Patients With Cirrhosis: A Randomized Clinical Trial. Hepatology 2019;69:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wolf E, Rich NE, Marrero JA, et al. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology 2021;73:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]