Summary
Background
Little is known about the epidemiology and patterns of care of intrahepatic cholangiocarcinoma (iCCA) in daily clinical practice. The aims of this study were to estimate the number of declared cases during the study period 2014–2015 in France from a hospitalization database and to describe the healthcare trajectories of these patients.
Methods
A retrospective analysis was carried out using the French nationwide prospective hospitalization database. All pts with a new diagnosis of "carcinoma of the intrahepatic bile duct" who had a first hospital stay in the Medicine, Surgery and Obstetrics departments (MSO) between 2014 and 2015 with a 2-year follow-up were included. Data related to the first identified stay (S1) in the MSO and on all subsequent stays in the MSO, aftercare and rehabilitation or home hospitalization were analysed.
Findings
A total of 3650 new iCCA cases were identified. At S1 (admission via emergency room (ER) in 28%), the median age of the patients was 73 years, 57% were male and 35% had metastases. Jaundice/anaemia/ascites/cholangitis were reported in 17%/16%/12%/7% of patients, respectively. The care of patients at S1 was mainly provided in general hospitals (CHG, 60%). A total of 896 (24%) patients died during S1. They were more frequently hospitalized via the ER (48% vs 23%), metastatic (52% vs 35%) and symptomatic. Subsequent stays were identified for 2507 (69%) patients. Three healthcare pathways were defined: surgery (n = 519; 14%), chemotherapy (CT) without surgery (n = 812; 22%) and best supportive care (BSC) (n = 2319; 63%). CT, surgery and BSC were most frequently performed in the cancer centres, university hospitals and CHG, respectively.
Interpretation
This medico administrative study reveals a higher number of iCCA cases than that previously reported by registries and highlights the severity of this disease.
Funding
This study was sponsored by Incyte Biosciences International Sàrl., Geneva, Switzerland. INCYTE validated the design of the study, the analysis, the interpretation of data and the writing of the manuscript which was first written by the 2 experts and CEMKA.
Keywords: Epidemiology, Patterns of care, Intrahepatic cholangiocarcinoma, Real life, French PMSI database
Research in context panel.
Evidence before this study
The incidence of biliary tract cancers was reported as low, with approximately 2000 per year in France, all primary sites taken together, according to registries. Data specific to intrahepatic cholangiocarcinoma were scarce. Findings from other industrialized countries (SEER database in the US) suggested increased incidence of intrahepatic cholangiocarcinoma but no real-life data were available about the oncological care pathways of these patients.
Added value of this study
We analyzed national data from the exhaustive PMSI system, recording all day hospital and conventional hospital stays in France. We described a higher incidence for intrahepatic cholangiocarcinoma than previously estimated in registries, highlighting the severity of the disease with 25% of patients dying at first hospital stay and 60% of patients receiving no active treatment. We also highlighted the central role of small centres and community hospitals in the management of these patients.
Implications of all the available evidence
Our results have implications for the discussions about the optimization of the oncological care pathways for patients with intrahepatic cholangiocarcinoma. Treatments for biliary tract cancers are evolving fast, with notably the approval of targeted therapies requiring access to molecular screening for these patients. Particularly, the decentralized organisation for treatment of these patients is challenging and there is a need to increase the awareness about treatment options for biliary tract cancers. In addition, regional discussions should be launched to implement networks between centres to ensure access to active treatments and molecular screening.
Alt-text: Unlabelled box
Introduction
Biliary tract cancers (BTCs) are a heterogeneous group of epithelial neoplasms (adenocarcinoma in 90% of cases).1 They are considered rare tumours, with less than six new cases per 100,000 people each year (10,000 and 12,000 new cases/year in Europe and the United States, respectively).2,3 They are classified into three main subtypes based on their anatomical origin, with specific epidemiological, clinical, molecular, and therapeutic features: intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA, including hilar cholangiocarcinoma), and gallbladder carcinoma.1 Overall, BTCs display a poor prognosis, with a five-year overall survival (OS) rate for all stages taken together of less than 20%.2,3 iCCAs develop within the liver parenchyma and represent the second most frequent primary liver malignancy after hepatocellular carcinoma (HCC).4 The incidence of iCCA is increasing worldwide, particularly in Europe and North America.5 Risk factors include cirrhosis, nonalcoholic steatohepatitis (NASH), obesity and diabetes mellitus, chronic hepatitis B or C infection, Opisthorchis and Clonorchis infections in Asia, Lynch syndrome, primary sclerosing cholangitis, and intrahepatic lithiasis.4 The diagnosis of iCCA can be challenging, and the main differential diagnoses include liver metastases from other carcinomas or HCC, especially in cirrhotic patients.4
The reference therapy for localized iCCA (20–30%) is surgical resection, which should be considered whenever possible, within a multidisciplinary team experienced in hepatobiliary surgery.6 Surgery is followed by six months of adjuvant chemotherapy (CT) with capecitabine, based on the results of the BILCAP phase III study, with a 15-month OS benefit over observation, as per the results of the intention-to-treat analysis (non-significant but clinically relevant) and the adjusted and per-protocol pre-specified sensitivity analyses (statistically significant).7
The vast majority (70–80%) of iCCAs are diagnosed at an advanced, nonresectable stage, and their management relies on best supportive care (BSC, including biliary drainage in case of jaundice) and CT if the patient's general condition allows.6 Standard first-line (L1) CT is gemcitabine plus cisplatin (CISGEM), which shows superiority over gemcitabine alone with regard to OS in the ABC-02 randomized phase III study.8 In the second-line (L2) setting, the 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX) doublet recently demonstrated superiority over BSC alone in the ABC-06 phase III study,9 but the question of the best L2 regimen is still open.10 Finally, patients with iCCA with liver-predominant disease (50% of cases) can also be treated with locoregional therapies, including liver intra-arterial CT, selective internal radiation therapy, chemoembolization, and radiotherapy.6
In recent years, knowledge about the molecular heterogeneity of iCCA has considerably increased with the advent of large-scale genomic and transcriptomic analyses, opening new perspectives for so-called personalized targeted therapies.11,12 Among the ongoing therapeutic developments, targeting fibroblast growth factor receptor (FGFR) and isocitrate dehydrogenase (IDH) alterations, as well as “agnostic” neurotrophic tyrosine receptor kinase (NTRK) gene fusion and microsatellite instability (MSI), are the most advanced candidates and hold much promise for the future.13
Little is known about the epidemiology of iCCA and the care pathways of patients with iCCA in daily clinical practice. The aims of this study were to estimate the number of declared cases during the study period 2014–2015 in France from a hospitalization database and to describe the healthcare trajectories of these patients.
Methods
Data source
A retrospective analysis was carried out using the nationwide hospitalization database (Programme de Médicalisation des Systèmes d'Information (PMSI)).
The PMSI is a comprehensive and exhaustive database recording all hospitalizations in France (approximately 25 million in 2012) in both public and private care facilities.14 All stays are chainable for a given patient with a unique identification number of anonymizations since 1 January 2006. This database provides detailed clinical information, such as the main diagnosis, comorbidities and main procedures performed (e.g., surgery and imaging): (i) patient characteristics (age and gender); (ii) diagnoses (main and secondary), coded according to the World Health Organization's International Classification of Diseases, 10th revision (WHO-ICD-10); (iii) the disease-related group (DRG), a classification system summarizing medical and surgical interventions; (iv) duration of hospital stay in days; (v) medical procedures performed during the stay; (vi) origin of the patient before admission (home, same hospital or other hospital); (vii) discharge destination (home or another hospital); (vii) vital status of patient (alive or dead); and (viii) type of care facility (public hospitals, including university hospitals, or private clinics).
Three subdatabases of the PMSI were used for this study:
-
•
The Medicine, Surgery and Obstetrics (MSO) database records acute hospitalization stays.
-
•
The Soins de Suite et de Réhabilitation (SSR) database records post-acute care and rehabilitation hospitalization stays.
-
•
The Hospitalisation à domicile (HAD) database records at-home hospitalizations.
Study design
The diagnosis of iCCA was based on the WHO-ICD-10 code C221 (intrahepatic bile duct carcinoma). All patients who had a first hospital stay (S1) in the MSO between 2014 and 2015 with this diagnosis as a main diagnosis were included. The date of S1 was referred to as the “index date”. Data related to the S1 in the MSO and on all subsequent stays in the MSO, SSR or HAD were included.
Patients who had the WHO-ICD-10 code C221 as a main or secondary diagnosis during a hospital stay over the 12 previous months were excluded. Patients who were hospitalized (MSO, SSR or HAD) between 2014 and 2017 with the following codes were also excluded: C18 (malignant neoplasm of the colon), C21 (malignant neoplasms of the anus and anal canal), C220 (malignant neoplasm of the rectum), C227 (other specified carcinomas of the liver), C23 (malignant neoplasm of the gallbladder), C24 (malignant neoplasm of other and unspecified parts of the biliary tract, except C249), and C25 (malignant neoplasm of the pancreas). In addition, if a patient had a cholecystectomy procedure during S1 coded C221, he/she was excluded from the analysis. The selection of these codes and exclusion criteria were discussed and validated with experts (CN and AL).
The population was analysed as a historical cohort followed-up for 24 months from the index date. The database was censored on 31 December 2017.
Statistical analyses
The statistical management and analysis of the data were carried out using SAS® V9.4 software (North Carolina, USA).
Continuous variables were described for the overall population using the following descriptive statistics: number of patients, number of missing values, mean, standard deviation, median, minimum, and maximum. Categorical variables were described as follows: number of patients, number of missing values and percentage of each modality, which were calculated on the answers expressed.
Bivariate statistical analyses were performed with different statistical tests based on the nature of the variables. For categorical variables, the chi-squared test was applied, except for theoretical sample sizes less than 5; in this case, the Yates continuity correction or Fisher exact test was used. For quantitative variables, when the distribution was close to normal (nonsignificant Shapiro-Wilk test), Student's t-test or analysis of variance was performed. Otherwise, nonparametric tests were used (Wilcoxon and Kruskal-Wallis).
As the PMSI database covered the whole country of France, the number of patients identified with a diagnosis of ICCA was exhaustive and estimating a sample size was therefore not relevant in this type of analysis.
Regulatory declaration
This study complied with MR006 (Méthodologie de Référence) and was declared on the National Institute of Health Data (INDS: Institut National des données de santé) site.
Role of the funding source
INCYTE validated the design of the study, the analysis, the interpretation of data and the writing of the manuscript which was first written by the 2 experts and CEMKA.
Results
Estimated number of new iCCA declared cases in the database and patient characteristics at S1
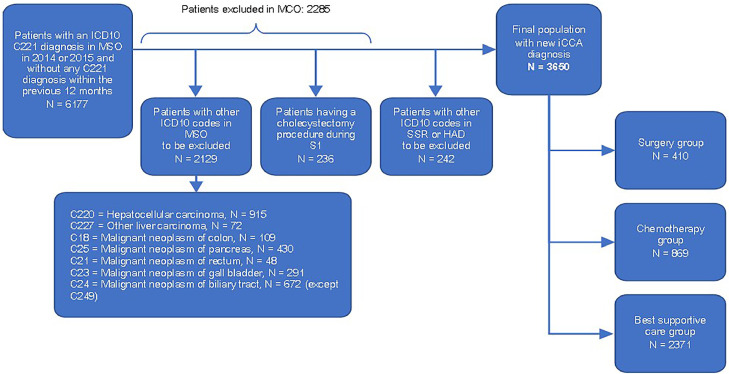
A total of 3650 new iCCA cases declared in the database were identified in 2014–2015 (1825 patients/year) (Figure 1). Patient characteristics at S1 are displayed in Table 1. The median age of the patients was 73 years (range: 23–101 years), with 60·2% of patients aged ≥ 70 years and 57·0% male. More than one-third (35·3%) of patients had metastases at diagnosis. The main metastatic sites were the liver (18·8%), followed by the peritoneum (10·7%) and lungs (8·9%). Jaundice, cholangitis, anaemia, and ascites were reported in 17·5%, 7·5%, 16·3%, and 12·2% of patients, respectively.
Figure. 1.
Flowchart of patients.
Table 1.
Patient characteristics at first hospital stay (N = 3650 patients).
| OverallN (%) | Surgery group N (%) | Chemotherapy group N (%) | Best supportive care group N (%) | |
|---|---|---|---|---|
| Global population with iCCA | 3 650 (100) | 410 (11·2%) | 869 (23·8) | 2 371 (65·0%) |
| Gender | ||||
| Male | 2 082 (57·0) | 257 (62·7%) | 513 (59·0) | 1 312 (55·3) |
| Female | 1 568 (43·0) | 153 (37·3%) | 356 (41·0) | 1 059 (44·7) |
| Age (years) | ||||
| Mean | 72·0 | 66·0 | 66·8 | 74·9 |
| Median [Min / Max] | 73·0 / 23·0 / 101·0 | 67·0 / 23·0 / 95·0 | 68·0 / 27·0 / 92·0 | 77·0 / 23·0 / 101·0 |
| Age in classes | ||||
| < 50 years | 35 (6·7) | 50 (6·2) | 73 (3·1) | 158 (4·3) |
| 50–69 years | 265 (51·1) | 414 (51·0) | 614 (26·5) | 1293 (35·4) |
| 70–79 years | 168 (32·4) | 259 (31·9) | 642 (27·7) | 1069 (29·3) |
| ≥ 80 years | 51 (9·8) | 89 (11·0) | 990 (42·7) | 1130 (31·0) |
| Symptoms at inclusion | ||||
| Jaundice (1) | 639 (17·5) | 36 (8·8) | 70 (8·1) | 533 (22·5) |
| Angiocholitis (2) | 273 (7·5) | 27 (6·6) | 21 (2·4) | 225 (9·5) |
| Ascites (3) | 444 (12·2) | 31 (7·6) | 34 (3·9) | 379 (16·0) |
| Anaemia (4) | 596 (16·3) | 69 (16·8) | 70 (8·1) | 457 (19·3) |
| Distant metastasis Liver metastasis |
1 290 (35·3) 686 (18·8) |
70 (17·1) 21 (5·1) |
331 (38·1) 169 (19·4) |
889 (37·5) 496 (20·9) |
| Peritoneal metastasis Lung metastasis Lymph node metastasis |
389 (10·7) 325 (8·9) 264 (7·2) |
14 (3·4) 11 (2·7) 27 (6·6) |
81 (9·3) 71 (8·2) 99 (11·4) |
294 (12·4) 243 (10·2) 138 (5·8) |
| Bone metastasis | 245 (6·7) | 12 (2·9) | 61 (7·0) | 172 (7·3) |
| Patients hospitalized in their region of residence (at S1) | 3 428 (93·9) | 367 (89·5) | 819 (94·2) | 2 242 (94·6) |
iCCA : intra-hepatic cholangiocarcinoma; (1) R17 (2) K803, K830 (3) R18 (4) D50, D510, D519, D52, D539, D598, D599, D62, D630, D638, D648, D649
All statistical tests between columns are statistically significant at 0·05%.
Characteristics of centres at S1
A total of 655 centres were involved in the patient's first hospital stay. Patient care was mainly provided by general hospitals (CHGs, 59·8%) rather than university hospitals (CHUs, 15·0%) and private (19·2%) or cancer centres (CLCCs, 6·0%) (Table 2). In all, 28% of patients were admitted via the emergency room (ER). Patients were mainly admitted for S1 in a hospital from their region of residence (Table 1).
Table 2.
Centers characteristics at first hospital stay (N = 3650 patients).
| OverallN (%) | Surgery group N (%) | Chemotherapy group N (%) | Best supportive care group N (%) | |
|---|---|---|---|---|
| Global population with iCCA+ | 3650 (100) | 410 (11,2) | 869 (23,8) | 2 371 (65,0) |
| Entry mode by emergency room | 994 (28·4) | 28 (6·9%) | 104 (12·2) | 862 (38·4) |
| Type of establishment | ||||
| Cancer center (CLCC) | 218 (6·0) | 27 (6·6) | 108 (12·4) | 83 (3·5) |
| University Hospital (CHU) | 549 (15·0) | 94 (22·9) | 138 (15·9) | 317 (13·4) |
| General Hospital (CHG) | 2 184 (59·8) | 213 (52·0) | 462 (53·2) | 1 509 (63·6) |
| Private centers | 699 (19·2) | 76 (18·5) | 161 (18·5) | 462 (19·5) |
| Volume of centers * | ||||
| Low (<5 patients) | 1052 (28·8) | 63 (15·4) | 220 (25·3) | 769 (32·4) |
| Intermediate (5–20 patients) | 1551 (42·5) | 123 (30·0) | 391 (45·0) | 1037 (43·7) |
| High (>20 patients) | 1047 (28·7) | 224 (54·6) | 258 (29·7) | 565 (23·8) |
+iCCA : intra-hepatic cholangiocarcinoma; *Number of patients identified during the 2014–2015 period.
All statistical tests between columns are statistically significant at 0·05%.
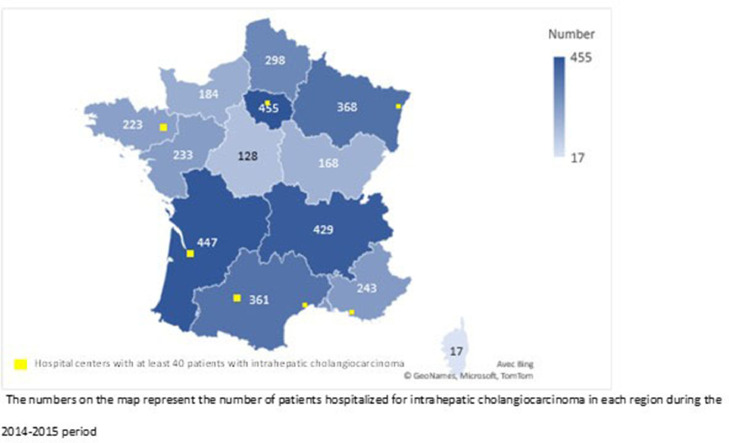
Based on the number of patients treated at S1, the centres were classified as low-volume (< 5 patients, representing 68% of centres, N = 446), intermediate-volume (5–20 patients, 26%, N = 170) and high-volume (> 20 patients, 6%, N = 39); 47% of the high-volume centres were CHU or CLCC. The geographical distribution of patients and the seven centres with the highest patient volume (> 40) are displayed in Figure 2.
Figure. 2.
Geographical distribution of patents and centers with the highest patent volume involved at first hospital stay.
Healthcare trajectories
In all, 896 (24%) patients died during S1; they were older (mean age: 75 years vs 71 years) and more frequently hospitalized via the ER (48% vs 23%), metastatic (52% vs 35%) and symptomatic (ascites 25% vs 8%/cholangitis 11% vs 7%) than those who did not die during S1.
Of the 2754 patients alive at S1, 2397 (87%) had subsequent stays in the MSO, 308 (11%) had subsequent stays in the SSR and 303 (11%) had subsequent stays in the HAD.
Three healthcare trajectories were defined: surgery (N = 410; 11·2%), CT without surgery (N = 869; 23·8%) and best supportive care (BSC) (N = 2371; 65·0%) (Table 1).
In the surgery group, patients had less frequent metastatic disease (17·1%) than in the other groups (Table 1). Surgical intervention was most often carried out in CHG (51·2%) or CHU (27·6%). The mean duration of hospital stay for surgery was 19·98 (± 17·72) days, and the postoperative mortality rate was 4·6%.
Patients who received CT were younger, less frequently hospitalized via the ER and less symptomatic at S1 than patients from the BSC group (Tables 1 and 2). The mean time between S1 and the start of CT was 1·9 (± 4·7) months. The median number of CT sessions was 10 (range: 1–122). CT infusions were mainly administered in CHG (54·6%), in private centres (16·5%) or cancer centres (CLCC 15·5%).
A palliative care code was associated with S1 in 25% of patients and with a subsequent MSO, SSR, or HAD stay in 60% of patients.
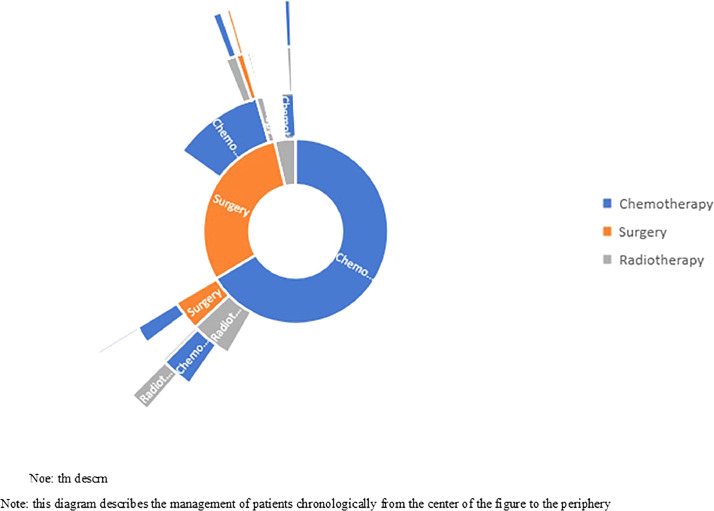
The sequence of specific therapies (surgery, CT and radiotherapy) is summarized in Figure 3. For two thirds of the patients, the first treatment modality was chemotherapy. Among them, very few patients had surgery after their chemotherapy. One third of the patients got first surgery and 40% of them had chemotherapy after their surgery during the study follow-up period (study period prior to BILCAP trial results).
Figure. 3.
Healthcare trajectories of patiens who received specific therapies: Sunburst diagram.
Patient referral and national organization of care
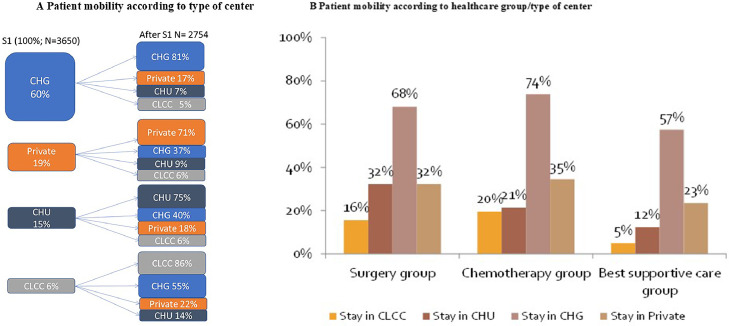
Patients were mainly managed in CHG (60%) at S1, of which 17%, 7%, and 5% were referred to other private centres, CHU, or CLCC after S1 (Figure 4A). Overall, patients initially managed in CHG, private centres or CHU mostly remained managed in the same type of care facility after S1.
Figure. 4.
(A) Patient mobility according to type of center. (B) Patient mobility according to healthcare group/type of center.
CHG was the main type of facility in charge of patient management, regardless of the healthcare group (surgery: 66·8% of patients; CT: 74·0% of patients; BSC: 57·6% of patients) (Figure 4B). Patients in the surgery group were referred to high-volume centres (79·0% of patients), while intermediate-volume centres were involved in CT (61·8%) and BSC (55%) (Figure 4C). Patients remained in their region of residence for follow-up in 91–99% of cases.
Discussion
In this study, we analysed the estimated number of cases of iCCA declared in the database during the study period 2014–2015 in France and described the characteristics and healthcare trajectories of these patients using the French nationwide hospitalization database.
These real-world data from the recent exhaustive PMSI database revealed a higher estimated number of new iCCA cases (1825 cases/year) than those previously reported and extrapolated from local cancer registers (0·2/100,000 inhabitants).15,16 Although the PMSI is not intended to be an epidemiological register (it aims to recover clinical activity for budgetary purposes), it provides complementary information to local registers in France. As the PMSI data are declarative and not checked from patient records, coding errors can occur, leading to underestimation as well as overestimation of the estimated number of new iCCA cases (e.g., in case of presumptive diagnosis based on imaging without full diagnostic work-up/histological confirmation). However, this increased number of iCCA cases is consistent with the current epidemiological trends observed in other developed countries.6,19,20 This can be explained by an increase in the number of individuals exposed to risk factors associated with iCCA, including chronic liver disease, particularly NASH related to obesity and metabolic syndrome.21,22 In addition, a greater awareness of the diagnosis of iCCA among oncologists may have led to a better identification of these tumours and reclassification of misdiagnosed HCC, metastasis from extrahepatic primary tumours or carcinoma of unknown primary.
Moreover, our results underlined the aggressiveness of this disease, with one-quarter of patients dying at S1 and two-thirds of patients not having access to active treatment. This may be related to the late diagnosis of iCCA at an advanced stage in the majority of patients.6 In our study, 35% of patients were diagnosed with distant metastases, and only 11% had access to surgery, which is inferior to the 22–25% rate of curative resection previously reported for iCCA by French registries.15,16 This may be due to coding errors, with perihilar CCA (which has a lower resection rate) coded as iCCA, for example. Unfortunately, no screening is available for iCCA given the rarity of this disease, except for the case of cirrhotic patients (ultrasound screening intended to diagnose HCC but that may detect early iCCA as well). Disease with a high tumour burden can result in altered general condition and nutritional status, which can compromise the possibility of administering specific treatments, particularly in frail, older patients or in the context of biliary complications (jaundice and angiocholitis). This finding highlights the importance of supportive care in these patients, a topic on which data are critically missing in iCCA (no prospective study available on the benefit of nutritional management or physical activity or even of the management of biliary stenosis and associated infectious complications in patients with advanced iCCA) and that would warrant more attention and investigation. Furthermore, the lack of access to specific treatment may also be related to insufficient knowledge of clinicians about the therapeutic options for iCCA, as this disease has been labelled with a poor prognosis and chemoresistant cancer for a long time. This is underlined in our study by the small proportion of patients referred to high-volume centres, except in the surgery group. iCCA management has been a rapidly moving field with many evolutions over the last years (particularly, with its molecular dismantlement into molecularly actionable subgroups, with recent drug approvals for ivosidenib17 and pemigatinib,18 yet posterior to our study), but oncologists might not be aware of these new opportunities for this rare condition, especially when they take care of only one or two patients per year, as was the case for the majority of centres in France in our study. This could be approached by education programmes and information directed towards oncologists and gastroenterologists; this is the aim of national guidelines published by the Societé Nationale Française de Gastro-entérologie (SNFGE)23 and soon by the Association Française pour l’étude du Foie (AFEF).
We also identified the central role of a high number of low-volume and CHG centres in the management of the majority of patients. This raises the question of the territorial organization and potential centralization of iCCA patient care in France, with the issues of referral of patients to high-volume centres for surgery by expert teams given the complexity and high morbidity associated with these procedures and sending of samples to genetic platforms for molecular testing as recently recommended by the ESMO guidelines.24
The main strength of the PMSI is that it is an exhaustive database for all French hospitalized patients, providing real world, recent data. It is a particularly relevant to approach iCCA care, as the hospital plays an important role in the management of these patients. In addition, it is easy to access and operate. In contrast, as mentioned above, data entered in the PMSI are declarative and limited by the existing codes, and coding errors may occur. Finally, events outside the hospital are not captured (including deaths).
Overall, our study provides new data about iCCA in the real-world setting, revealing its increasing number of cases and aggressiveness, and shows that the patient population from clinical trials and observational cohorts of specific treatments only represents the visible part of the iceberg in this disease. This study also illustrates that the PMSI is a valuable source of information about patient care, which is complementary to other systems, including cancer and death registries, representing a model potentially applicable to other poorly described pathologies.
Contributors
Cindy Neuzillet participated in the writing of the manuscript and the interpretation of the study results.
Astrid Lièvre participated in the writing of the manuscript and the interpretation of the study results.
Corinne Emery participated in the study design, performed the statistical analysis, and participated in the writing of the manuscript and the interpretation of the study results. Clément Teissier performed the statistical analysis. Stéphane Bouée participated in the study design, the writing of the manuscript and the interpretation of the study results. All authors have read and approved the manuscript.
Funding source
This study was sponsored by Incyte Biosciences International Sàrl., Geneva, Switzerland. INCYTE validated the design of the study, the analysis, the interpretation of data and the writing of the manuscript which was first written by the 2 experts and CEMKA.
Ethics approval and consent to participate
The study was approved by the Committee of Expertise for Research, Studies and Evaluations in the field of Healthcare (Comité d'Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé) and by the National Commission on Informatics and Liberty (Commission nationale de l'informatique et des libertés).
Declaration of interests
Honoraria from INCYTE for being experts of scientific committee.
Grants or contracts from any entity: Roche
Consulting fees: Pierre Fabre, Servier, Roche, AstraZeneca, Bristol-Myers Squibb, Amgen, Merck, MSD, Novartis, Incyte Biosciences, Mylan, Baxter, Nutricia, Fresenius Kabi, Sanofi
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Squibb, Amgen, Merck, MSD, Novartis, Incyte Biosciences, Mylan, Baxter, Nutricia, Fresenius Kabi, Sanofi
Support for attending meetings and/or travel: MSD, Mylan, Merck, OSE Immunotherapeutics
Participation on a Data Safety Monitoring Board or Advisory Board: AstraZeneca, Mylan
Other financial or non-financial interests: Clinical trials: OSE Immunotherapeutics, AstraZeneca, Servier
Astrid Lièvre
Honoraria from INCYTE for being experts of scientific committee.
Grant for laboratory research and support for clinical trials: Bayer, Lilly, Novartis
Consulting fees: AAA, Amgen, Bayer, Incyte, Ipsen, Merck, Novartis Pierre Fabre, Sandoz and Servier
Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: AAA, Amgen, Bayer, BMS, HalioDx, Incyte, Ipsen, Merck, Novartis, Pierre Fabre, Roche, Sandoz, Sanofi and Servier.
Support for attending meetings and/or travel: AAA, Bayer, Ipsen, Merck, Mylan, Novartis, Pfizer, Roche and Servier
Corinne Emery, Stéphane Bouée and Clément Tessier are employed at CEMKA, a consultancy which received grants from INCYTE to perform the project.
Acknowledgments
Acknowledgments
This study was sponsored by Incyte Biosciences International Sàrl., Geneva, Switzerland. INCYTE validated the design of the study, the analysis, the interpretation of data and the writing of the manuscript which was first written by the 2 experts and CEMKA.
Data sharing
The data that support the findings of this study are available from the French PMSI database, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Editors’ note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
References
- 1.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lepage C., Capocaccia R., Hackl M., et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: results of EUROCARE-5. Eur J Cancer. 2015;51:2169–2178. doi: 10.1016/j.ejca.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Bridgewater J., Galle P.R., Khan S.A., et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Patel N., Benipal B. Incidence of cholangiocarcinoma in the USA from 2001 to 2015: a US cancer statistics analysis of 50 states. Cureus. 2019;11:e3962. doi: 10.7759/cureus.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valle J.W., Borbath I., Khan S.A., et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 7.Primrose J.N., Fox R.P., Palmer D.H., et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 8.Valle J., Wasan H., Palmer D.H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 9.Lamarca A., Palmer D.H., Wasan H.S., et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuzillet C., Casadei-Gardini A., Brieau B., et al. Fluropyrimidine single agent or doublet chemotherapy as second line treatment in advanced biliary tract cancer. Int J Cancer. 2020 doi: 10.1002/ijc.33146. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H., Arai Y., Totoki Y., et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 12.Valle J.W., Lamarca A., Goyal L., Barriuso J., Zhu A.X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7:943–962. doi: 10.1158/2159-8290.CD-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vienot A., Neuzillet C. Continuum of care for advanced biliary tract cancers. Clin Res Hepatol Gastroenterol. 2020 doi: 10.1016/j.clinre.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 14.http://www.atih.sante.fr.
- 15.Lepage C., Cottet V., Chauvenet M., et al. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol. 2011;54:306–310. doi: 10.1016/j.jhep.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Al Mahjoub A., Bouvier V., Menahem B., et al. Epidemiology of intrahepatic, perihilar, and distal cholangiocarcinoma in the French population. Eur J Gastroenterol Hepatol. 2019;31:678–684. doi: 10.1097/MEG.0000000000001337. [DOI] [PubMed] [Google Scholar]
- 17.https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3181994
- 18.https://www.ema.europa.eu/en/medicines/human/EPAR/pemazyre#authorisation-details-section
- 19.Banales J.M., Cardinale V., Carpino G., et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 20.DeOliveira M.L., Cunningham S.C., Cameron J.L., et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clements O., Eliahoo J., Kim J.U., Taylor-Robinson S.D., Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Hepatol. 2020;72:95–103. doi: 10.1016/j.jhep.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 22.https://www.snfge.org/content/8-cancer-des-voies-biliaires
- 23.De Lorenzo S., Tovoli F., Mazzotta A., et al. Non-alcoholic steatohepatitis as a risk factor for intrahepatic cholangiocarcinoma and its prognostic role. Cancers (Basel) 2020;12(11):3182. doi: 10.3390/cancers12113182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosele F., Remon J., Mateo J., et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. 2020;31:1491–1505. doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]