Abstract
This review addresses the role of the essential trace element, selenium, in type-2 diabetes mellitus (T2DM) and its metabolic co-morbidities, i.e., metabolic syndrome, obesity and non-alcoholic fatty liver disease. We refer to the dietary requirements of selenium and the key physiological roles of selenoproteins. We explore the dysregulated fuel metabolism in T2DM and its co-morbidities, emphasizing the relevance of inflammation and oxidative stress. We describe the epidemiology of observational and experimental studies of selenium in diabetes and related conditions, explaining that the interaction between selenium status and glucose control is not limited to hyperglycemia but extends to hypoglycemia. We propose that the association between high plasma/serum selenium and T2DM/fasting plasma glucose observed in many cross-sectional studies may rely on the upregulation of hepatic selenoprotein-P biosynthesis in conditions of hyperglycemia and insulin resistance. While animal studies have revealed potential molecular mechanisms underlying adverse effects of severe selenium/selenoprotein excess and deficiency in the pathogenesis of insulin resistance and β-cell dysfunction, their translational significance is rather limited. Importantly, dietary selenium supplementation does not appear to be a major causal factor for the development of T2DM in humans though we cannot currently exclude a small contribution of selenium on top of other risk factors, in particular if it is ingested at high (supranutritional) doses. Elevated selenium biomarkers that are often measured in T2DM patients are more likely to be a consequence, rather than a cause, of diabetes.
Keywords: Selenium, Diabetes, Obesity, Metabolic syndrome, NAFLD, Hyperglycemia, Hypoglycemia, Glucose, β-cell
1. Introduction
Known as a toxic element since the 1930s, selenium (Se) was first recognized as essential to mammals in 1957, when it was shown to prevent necrotic liver degeneration [1]. Now, many decades later, as the only trace element to be specified in the human genome by a codon for the Se-containing amino acid, selenocysteine [2], to give selenoproteins, the uniqueness of Se, has become apparent [3].
Due to antioxidant and anti-inflammatory effects of selenoproteins [3], it was at first expected that Se would be beneficial for diabetes patients, given that type-2 diabetes mellitus (T2DM) is associated with oxidative stress [4]. Indeed, Se (as selenate) has anti-diabetic, and insulin-mimetic effects, at high supranutritional doses [4]. It was thus unexpected that observational epidemiological studies showed that higher plasma Se was associated with a greater occurrence of T2DM [5].
The role of Se in diabetes is still a matter of discussion with respect both to Se deficiency and Se supply above dietary requirements [6]. We are aware of the frequent appearance of associated metabolic disorders such as obesity, metabolic syndrome (MetS), and non-alcoholic fatty liver disease (NAFLD) in preparing the ground for T2DM and vice versa [7,8]. We have enlarged our topic from a review of Se and T2DM which has been covered before [5,9] to include those diabetic comorbidities. In this review of T2DM and associated disorders, we discuss dysregulation of fuel metabolism together with epidemiological findings and studies on potential molecular mechanisms that may help to illuminate the inconsistent results in this area of research [5,9].
2. The essential trace element Se and its importance for human health
2.1. How much Se do we need and how much do we get?
Dietary Se is essential at a very low level of intake, from 55 to 75 μg/d [[10], [11]]. It is defined as being toxic above 800 μg/d, with the Safe Upper Limit being set at 400 μg/d [10]. Se gets into the food chain through plants which take it up from the soil [12]. The amount taken up is dependent not only on the Se content of the soil which relates to the underlying geology, but to soil pH, the presence of organic matter and climatic conditions [12]. Animals that eat forage acquire Se in that way and provide a good supply of Se to those that consume them. Se is also supplied by fish and shellfish which are a particularly important source in some communities [13]. Though Se is present in many environmental sources, those from plants usually contain selenomethionine (SeMet), from animals SeMet and selenocysteine, and from fish SeMet and selenoneine [3,13]. Supplements usually contain SeMet, Se from yeast (largely SeMet) and sodium selenite or selenate [3].
2.2. The variation in intake and status of Se across the globe
There is an extremely wide range of intake of Se seen across the world [14]. Both the lowest and highest intakes are seen in China where plasma concentrations of Se range from 22 to 550 μg/L [[15], [16]]. Levels of intake are quite high in N America [12]: mean (SD) serum Se concentration in US residents measured in the 2003–2004 National Health and Nutrition Examination Survey (NHANES) was 137 (20) μg/L, in agreement with other later NHANES studies [3,17,18]. By contrast, Se intake in ten European countries has recently been measured in the EPIC study where a much lower mean serum Se concentration of 85.6 μg/L was found [19]. These deviations in intake and status mean that the location where studies have been carried out should always be stated.
2.3. Human selenoproteins
The biological actions of Se are mainly mediated by selenoproteins. The human genome contains 25 genes that encode selenoproteins [20]. Selenoproteins have a wide range of essential functions including antioxidant, anti-inflammatory, immune, and antiviral effects, and are needed by the endoplasmic reticulum (ER), for thyroid-hormone production, brain activity, and the reproductive system [3,20]. A table of selenoproteins with their updated nomenclature can be found in Pitts and Hoffman, 2018 [21].
Selenoproteins are synthesized in a unique manner; in the presence of a selenocysteine insertion sequence (SECIS) in the 3′-untranslated region of mRNA, the UGA codon, which normally acts as a stop codon, is recoded to specify the insertion of selenocysteine [20]. A SECIS-binding protein (SECISBP2) recognizes the SECIS element and recruits the selenocysteine specific elongation factor (EFSec) and the unique transfer RNA, tRNA[Ser]Sec, for ribosomal translation [20]. Some individuals with compound heterozygous defects in the SECISBP2 gene show lowered synthesis of most selenoproteins resulting in a complex phenotype [22]. Interestingly, oxidative stress in individuals with that phenotype was associated with enhanced insulin sensitivity, similar to findings in mice that lack the antioxidant selenoenzyme GPX1 [22].
The selenoproteins that will mainly feature in this review are the glutathione peroxidases (e.g. cytosolic GPX1, plasma GPX3) which remove hydroperoxides including H2O2, the thioredoxin reductases (e.g. cytosolic TXNRD1) which control redox function, selenoprotein P (SELENOP), which is made in the liver and transports Se to other tissues, and selenoprotein S (SELENOS), which is involved in ER function [21]. Moreover, polymorphisms in at least four selenoprotein genes affect the risk of T2DM or its associated metabolic comorbidities (see Supplemental Data, Supplemental Table 1) [[23], [24], [25], [26], [27], [28], [29], [30]].
3. T2DM and associated metabolic comorbidities
3.1. Dysregulated fuel metabolism in T2DM, obesity, MetS and NAFLD
In many modern-day societies where lifestyle is largely sedentary, access to an abundance of high-energy food has given rise to a global pandemic of disorders that are attributed to dysregulated fuel metabolism. The metabolic derangement may become apparent in epidemiologically- and etiologically related widespread diseases such as MetS, obesity, T2DM, and NAFLD. Central obesity, hypertension, and disturbances of the insulin-regulated lipid and carbohydrate metabolism resulting in dyslipidaemia and impaired glucose tolerance are hallmarks of MetS [7]. Both obesity and MetS increase the risk for T2DM and NAFLD, even though the subgroup of metabolically-healthy obese individuals present with few or no metabolic abnormalities [31]. In this regard, a recent study reported increased insulin secretion in insulin-sensitive obese individuals that was associated with excess adiposity [32]. This is an interesting finding, as hyperinsulinemia caused by overconsumption of insulinogenic nutrients or by exposure to some environmental toxins, is increasingly being recognized as related to the development of insulin resistance [33].
By contrast, the current majority view considers that insulin resistance, particularly of skeletal muscle and visceral white adipose tissue (WAT), is a primary factor in the pathogenesis of T2DM. When insulin sensitivity is progressively lost, the pancreatic islets undergo an adaptive response with increased β-cell proliferation and differentiation; this results in initially preserved normoglycemia at the expense of hyperinsulinemia. However, permanently elevated blood-insulin levels may provoke adverse metabolic effects such as WAT expansion as well as hepatic de novo lipogenesis and lipid accumulation thus establishing a vicious cycle of expanding insulin resistance. Later on, sometimes after decades, this is thought to cause β-cell exhaustion and decline. Eventually, when insulin secretion is insufficient to maintain fasting and postprandial normoglycemia, overt T2DM arises [8]. T2DM, just as in the case of obesity and MetS, is often associated with NAFLD. Patients with T2DM already have an increased risk for NAFLD at the time of diagnosis and hepatic lipid accumulation has been shown to further increase rapidly thereafter, even when blood glucose levels were well controlled by medication [34].
The two models that explain the pathogenesis of T2DM – based either on primary insulin resistance or primary hyperinsulinemia – do not necessarily exclude one another but might rather reflect the heterogeneous nature of the disease and the different ways in which metabolic dysfunction is developing. In this regard, a “palette model” assumes that each diabetes patient exhibits a mixture of defects in different metabolic traits (e.g., β-cell function, insulin and incretin action, fat distribution) at different degrees [35]. Moreover, a novel bioinformatics approach defined clusters of diabetes patients showing differences in pathogenesis, disease progression, and risk of complications. While one cluster represented individuals with type-1 diabetes mellitus, the T2DM patients were subdivided into four clusters, which might allow for an improved treatment based on the underlying metabolic defect(s) in each case [36].
3.2. Pathomechanisms underlying the development of insulin resistance and β-cell dysfunction
A large number of potentially interactive pathological mechanisms may affect the insulin target tissues as well as the pancreatic islets and the intestinal tract, thus contributing to the development of metabolic dysfunction. These include chronic low-grade inflammation, redox imbalance, mitochondrial dysfunction, ER stress, alterations in plasma levels of factors involved in inter-organ communication, leakage of the intestinal barrier, and alterations in the intestinal microbiota [4,8,[37], [38], [39], [40], [41], [42]].
Inflammation of the visceral WAT, the liver, and the pancreatic islets is considered as a major cause of insulin resistance and β-cell dysfunction. During the development of obesity and T2DM, M1-like macrophages become the most abundant immune cells in these tissues; they are then the primary source of pro-inflammatory cytokines. An inflamed WAT exhibits increased lipolysis, resulting in lipid overflow and ectopic lipid accumulation in liver, skeletal muscle, and β-cells. Chronic inflammation is associated with lowered glucose uptake in WAT and skeletal muscle, elevated hepatic lipogenesis and gluconeogenesis, dysregulated secretion and/or activity of adipokines, hepatokines and incretins, decreased glucose-induced insulin secretion (GSIS), increased β-cell proliferation, and dysfunction of the intestinal barrier [8,39,42].
In addition, the insulin-regulated fuel metabolism is closely intertwined with redox homeostasis. Reactive oxygen species (ROS), in particular H2O2, are required for proper insulin biosynthesis, secretion, and signaling [4,37,40]. ROS at low levels do not injure cells. However, an imbalance between the generation and degradation of ROS may cause oxidative stress, resulting in disruption of signaling pathways, mitochondrial dysfunction, and ultimately, cell death [43]. Nutrient oversupply, hyperglycemia, dyslipidemia and chronic inflammation have all been reported to be associated with oxidative stress. Disturbance of redox homeostasis may promote mitochondrial dysfunction and insulin resistance as well as ER stress, impaired insulin biosynthesis and β-cell de-differentiation [4,37,40]. While diabetes has been found to be associated with decreased levels of intracellular antioxidants such as glutathione or vitamins C and E, it should be noted, however, that an “antioxidant therapy” by simply supplementing antioxidants was generally not effective in diabetic patients [44].
4. What we can learn from epidemiology on the relationship between Se and diabetes
4.1. Results from observational studies on Se and T2DM or its correlates
Many observational studies, both cross-sectional and longitudinal, have investigated the association between Se status and diabetes, fasting plasma glucose or insulin resistance. It is hoped that such studies would have adjusted for BMI which will also affect the risk [45].
Though by far the majority found significant positive associations between serum/plasma Se and diabetes/T2DM [9,[46], [47], [48]], a number found a rather complex non-linear association with increasing Se status, e.g., in NHANES, and in a five-study meta-analysis [47,49,50]. Some studies even found a significant inverse association, most often where markers of diabetes [51,52], rather than diabetes itself, or toenail Se [53,54] were measured.
Dealing first with the toenail analysis, in a cross-sectional analysis of men from the Health Professionals’ Follow-up Study (HPFS), toenail Se was compared between 361 healthy control men and 688 men with diabetes [53]. After controlling for potential confounders, toenail Se status was lower among men with diabetes than among healthy controls [53]. A later cohort study followed two separate cohorts of 3,630 women from the Nurses’ Health Study and 3,535 HPFS men, who were free of prevalent T2DM and heart disease at baseline in 1982–1983 and 1986–1987, respectively. Toenail Se concentration was measured at baseline and over the subsequent 21–26 years when 780 cases of incident T2D occurred [54]. After multivariable adjustment, the risk of T2D was lower across increasing quintiles of Se. However, an Italian cohort study that measured baseline toenail Se in women found a U-shaped relationship with T2DM 16 years later [46,55]. One study used a cross-sectional design to measure the association between fingernail Se and the prevalence of diabetes in 163 of 1856 elderly subjects from four Chinese rural counties. The mean nail Se was 0.461 ± 0.190 μg/g, denoting a low concentration of Se. The adjusted odds ratios for diabetes rose from quartile 1 to the other quartiles by a factor of around 2.8 with no significant difference between the top three quartiles.
It is difficult to rationalize these toenail/fingernail studies. Se status rose from 0.46 μg/g in China to approximately 0.63 (0.33–0.94) μg/g in Italy to 0.77/0.84 (women/men) μg/g in the USA. Diabetes rose in China with increasing nail Se, showed a U-shaped relationship in Italy as toenail Se rose, and fell in the USA as toenail Se increased, implying a non-linear association with increasing Se status.
Toenail Se has been shown to correlate with Se intake [56] and with whole blood, plasma and serum Se, even after years [57]. However, homeostasis of an essential trace element is tightly and specifically regulated because of its importance to the organism; this regulation may modify the relationship between exposure and the biomarker, making it more difficult to interpret [57]. In this context, an Italian environmental study saw an inverse correlation with Se status for fish and seafood intake though these are normally a good source of Se [58].
A number of meta-analyses have been carried out (see Table 1); in the 2016 five-study meta-analysis described above, there was a non-linear dose response relationship between T2DM in both the highest (>132.5 μg/L) and the lowest category of serum Se (<97.5 μg/L) [47]. A 2018 meta-analysis included 13 studies where Se was measured in blood/serum/plasma or Se intake was assessed (studies that measured Se in toenails were excluded) [9]. The summary odds ratio of T2DM was around 2 (see Table 1) [9]. In a 2019 meta-analysis of 20 observational studies evaluating 47,930 subjects, high levels of Se were significantly associated with the presence of DM though significant heterogeneity was found [46]. That study used different Se subgroups based on blood, diet, urine, and nails and found a significant association between high Se and the presence of DM in the studies using blood, diet, urine, but not nails/toenails [46].
Table 1.
Summary of meta-analyses of observational studies on the association of Se with DM/T2DM.
| Study Citation | Participants | Observation | Result |
|---|---|---|---|
| Wang XL et al., 2016 [47] | 13,460 adults from five studies of Se and T2DM met the inclusion criteria | MEDLINE and EMBASE databases were used for a literature search. | The pooled OR indicated that there was a significantly higher prevalence of T2DM in the highest category of serum Se vs. the lowest; OR = 1.63, 95% CI: 1.04, 2.56, P = 0.033. Serum Se levels were positively associated with T2DM in populations with relatively low serum Se (<97.5 μg/L) & those with high serum Se (>132.5 μg/L). A U-/S-shaped, non-linear dose-response relationship between serum Se and T2DM appears probable. |
| Kohler et al., 2018 [9] | 13 studies on Se and T2DM met the inclusion criteria | Se was measured in blood/serum/plasma but not in toenails. Se intake assessed from an FFQ. | Of the 13 studies included, eight demonstrated a statistically significant positive association between concentrations of Se and odds for T2DM, with OR (95% CI) ranging from 1.52 (1.01–2.28) to 7.64 (3.34–17.46), and a summary OR (95% CI) of 2.03 (1.51–2.72). |
| Kim et al., 2019 [46] | 20 articles evaluating 47,930 participants were included | Subgroup analyses were performed based on the Se measurement methods used in each study. | High levels of Se were significantly associated with the presence of diabetes: OR (95% CI), 1.88 (1.44, 2.45), though with significant heterogeneity (I2 = 82%). High Se was significantly associated with the presence of diabetes in studies that measured Se in: blood [2.17 (1.60, 2.93) I2 = 77%]; diet [1.61 (1.10, 2.36) I2 = 0%]; urine [1.49 (1.02, 2.17) I2 = 0%] but not in studies that used nails [1.24 (0.52, 2.98) I2 = 91%]. |
| Vinceti et al., 2021 [48] | 34 non-experimental studies (update of a previous m-analysis of 13 studies [59]) | Direct relationship between blood/serum/plasma, dietary and urinary levels of Se and risk of T2DM, but not with nail Se. | The association was nonlinear, with risk increasing above 80 μg/day of dietary Se. Whole blood/plasma/serum Se concentrations of 160 μg/L corresponded to a risk ratio of 1.96 (95% CI 1.27, 3.03) compared with a concentration of 90 μg/L. Cohort studies indicated increased risk for both blood/urine concentrations and dietary selenium intake. |
DM = diabetes mellitus; FFQ = food frequency questionnaire.
The largest meta-analysis of observational studies to date was published by Vinceti and collaborators [48]. It evaluated 34 non-experimental studies updating a previous meta-analysis of 13 studies and looked at the dose-response [59]. Studies that appeared in that meta-analysis are not listed separately in this review. A direct relationship was found with blood/serum/plasma, dietary and urinary levels of Se and risk of T2DM, but there was no relationship with nail Se. The association was nonlinear with risk increasing above 80 μg/day of dietary Se. Whole blood/plasma/serum Se concentrations of 160 μg/L vs. 90 μg/L gave a risk ratio of almost 2 (see Table 1). Cohort studies, a more reliable source of data, indicated increased risk for both blood and urine concentrations and dietary Se intake. However, a cohort study that was published subsequent to that meta-analysis looked at dietary Se intake and T2DM in 4,106 Brazilians from the CUME (Cohort of Universities of Minas Gerais) population and found that dietary Se intake was not associated with the prevalence of T2DM, despite the high mean intake of Se, i.e., 157.4 μg/d, in the sample (Supplemental Table 2) [60].
Other studies not included in a meta-analyses [46,48] were a number from the USA NHANES, e.g., in NHANES 2011–2014, an increase of 10 μg/L in Se increased the prevalence of diabetes by 12% (OR: 1.12; 95% CI: 1.06, 1.18) [61]. In NHANES 2003–2014, published in 2021, which included six survey cycles, almost 19,000 people were followed up for diabetes and mortality for a mean of 6.6 years. Cross-sectionally, comparing the top with the bottom quartile of Se intake, the OR for diabetes was 1.44 (95% CI 1.09, 1.89) [62]. However, for all-cause mortality, comparing the highest with the lowest quartiles of Se intake gave a Hazard Ratio (HR) for all-cause mortality of 0.77 (95% CI 0.59, 1.01), suggesting that a higher Se status was a bonus overall [62].
The most recently published NHANES study (2013–2018) is recorded in Supplemental Table 2 which shows the relationship of Se with markers of diabetes. That study measured whole blood Se concentration, fasting plasma insulin and glucose, HbA1c and HOMA-IR in 4,339 participants [63]. When models were adjusted for age, sex, smoking status, physical activity, metabolic syndrome and BMI, Se status was associated with insulin and HOMA-IR but the association with glucose was no longer significant showing that there was “no evidence of an association between selenium and diabetes prevalence” [63].
In a further example showing the relationship of Se with markers of diabetes, 2,420 subjects without diabetes from the CODING (Complex Diseases in the Newfoundland Population: Environment and Genetics) study were assessed for Se intake and fasting glucose and insulin were measured in blood samples; insulin resistance was determined with the HOMA-IR (Supplemental Table 2) [51]. Se intake was negatively correlated with HOMA-IR after adjusting for Se confounding factors in those whose dietary Se intake was <1.6 μg/kg/day, i.e., 112 μg/day in someone of 70 kg weight. Above this cut-off, the beneficial effect disappeared [51]. Similarly, a cross-sectional Brazilian study of 270 individuals with adequate body weight but excess body fat found that Se intake lower than the Estimated Average Requirement (i.e. 45 μg/d) was associated with higher values of HbA1c (Supplemental Table 2) [64].
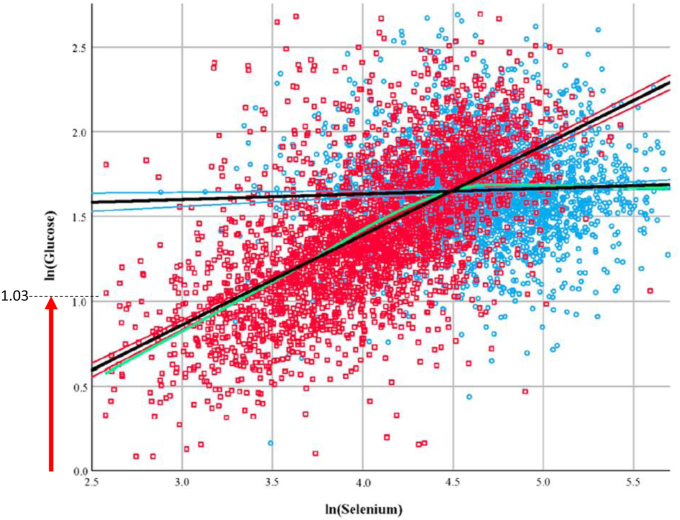
Supplemental Table 2 also shows an interesting study which is based on two regions of the Shaanxi province in China, one of which has moderate Se status (Ziyang, plasma Se 103 μg/d) and the other of which has low Se status (Ningshan, 58 μg/d) [52]. Overt hypoglycemia was observed in 19.2% of the random sample of 2889 Ningshan subjects and in 1.4% of the 2797 Ziyang subjects (see Fig. 1). The lines intersect at 88.9 μg/L which is a concentration likely to optimize plasma GPX3 but certainly not sufficient to optimize SELENOP. This study shows that the interaction between Se status and glucose control is not limited only to hyperglycemia, but apparently extends to hypoglycemia risk in Se deficiency.
Fig. 1.
Scatterplot showing the relationship between ln (Glucose) and ln (Se) in subjects living in Ziyang (moderate Se status, 103 μg/L, blue circles) and Ningshan (low Se status, 58 μg/L, red squares). The lines intersect at a serum Se concentration of 88.9 μg/L. The relationships are: ln(Glucose) = 1.501 + 0.033ln(Se) for Ziyang; ln(Glucose) = -0.7307 + 0.53ln(Se) for Ningshan. The red arrow on the Y axis shows hypoglycemia, i.e. ln (Glucose < 2.8 mM) = 1.03, in this random sample of subjects in the Se-deficient region. The green line represents the locally estimated scatterplot smoothing curve (α = 50%) of the combined regions (amended figure published with permission from Redox Biology) [52]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4.2. Results from observational studies on Se and associated metabolic comorbidities
Se intake was measured in a cross-sectional study of 2,069 Chinese people where the prevalence of MetS was 17.0% [65]. According to the multi-variable adjusted analysis, dietary Se intake had a moderate negative association with MetS [65]. A median dietary Se intake of 98.3 μg/d was found in a Brazilian study of 39,568 adolescents between 12 and 17 years old (ERICA study) [66]. The prevalence of MetS was only 2.6% but there was no association with dietary Se intake [66].
Some studies have investigated the effect of serum/plasma Se with obesity, prediabetes, MetS, and NAFLD. The relationship between Se status with T2DM and obesity was investigated in a Thai study of 655 men of whom 165 were overweight, and 290 were obese [67]. When the prevalence of prediabetes (HbA1c 5.7–6.4%) or T2DM (HbA1c ≥ 6.5%) was determined, the adjusted odds ratios (ORs) (95% CI) were 1.991 (1.318, 3.009) and 3.786 (2.087, 6.896) when the third tertile of serum Se concentration was compared with the first, in the entire sample and obese participants, respectively [67].
Other studies have shown an inverse association of serum Se with metabolic co-morbidities. In an analysis of 33,944 patients recruited from the Third NHANES, NAFLD was diagnosed by sonography after the exclusion of other liver diseases [68]. The odds ratio of advanced liver fibrosis (fibrosis score of >0.676) was significantly reduced with increasing serum Se concentration; OR (95% CI) 0.55 (0.32, 0.94) in the highest vs the lowest Se quartile. The relationship was significant regardless of BMI [68].
Two studies showed non-linear relationships with serum/plasma Se. One included 3,827 adults aged ≥20 years without any hepatic disease who participated in the NHANES 2011–2016. The median serum Se concentration was 127.9 μg/L [69]. Non-linear associations of serum Se with NAFLD prevalence and serum alanine aminotransferase (ALT) were found. However, following adjustment, positive associations were observed with NAFLD and ALT at serum Se above 130 μg/L whereas no association was observed below this value [69]. The second was a matched case-control study in 1,279 cases of MetS and 1,279 sex- and age-matched controls in a Chinese population [70]. Plasma Se showed a U-shaped association with MetS which was lowest when plasma Se was 93.7 μg/L; however, below and above this value, risk was increased. U-shaped associations were also seen between plasma Se with central obesity and high blood pressure [70]. Higher plasma Se was positively associated with odds of hypertriglyceridemia and hyperglycemia and negatively associated with odds of low HDL-C. This study helps to explain the discrepancies in results from previous studies which assumed linear relationships rather than the nonlinearity of the Se-MetS relationship [70].
It is also worthwhile looking at studies that investigated the relationship between SELENOP concentration and T2DM metabolic abnormalities because the A allele of SELENOP rs7579 polymorphism has been associated with higher odds of MetS [70]. However, while we know that SELENOP is an excellent biomarker of Se status, the different ELISA kits currently used for its quantitation in serum/plasma give different results and are not standardised [71,72]. A number of studies have found differences between SELENOP in healthy and diabetic persons but these can be criticized as being too small, using unreliable ELISA kits, and providing values of SELENOP that are either too high or too low [[73], [74], [75]]. Studies should present some quality control of their quantification, e.g., a second Se biomarker measured in parallel, they should find a reasonable concentration range for SELENOP (more than 2 and less than 8 mg/L) and those with fewer than 100 samples are strongly under-powered to provide meaningful results on such a complex disease as diabetes mellitus (Prof L Schomburg, personal communication September 29, 2021). Though small, a case-control pilot study of patients with biopsy-proven NAFLD indicated a declining trend in SELENOP levels due to deterioration of NAFLD [76].
4.3. Results from randomized trials of Se and T2DM
Randomized trials were largely based in the USA where Se status is high [3]. The first trial to investigate whether higher supplemental Se increased diabetes was the NPC (Nutritional Prevention of Cancer) trial, a randomized, double-blind, placebo-controlled study (RCT) in areas of low Se intake (plasma Se 114 μg/L) in eastern regions of the USA which was designed primarily to look at skin cancer risk (see Table 2). The trial included 1,202 patients with non-melanoma skin cancer who attended dermatology clinics and were randomized to 200 μg Se daily as Se-yeast tablets or a yeast-placebo [77]. During the 7.7- year follow-up, diabetes was diagnosed in 58 people who received Se and in 39 who received placebo, respectively (HR 1.55, 95% CI 1.03, 2.33). Diabetes was self-reported, was the secondary outcome and the excess number of cases was higher in men. Nevertheless, a noticeably increased risk for diabetes was detected in the highest tertile of baseline plasma Se level (HR 2.70, 95% CI 1.30, 5.61) [77].
Table 2.
Randomized trials and meta-analyses of randomized trials on the effects of Se supplementation on the incidence or markers of DM/T2DM.
| Study, Country (where applicable), Reference | Trial, Sample Size Intervention/placebo |
Duration | Outcome: Incidence of T2DM/DM, serum glucose, β-cell function, insulin sensitivity |
|---|---|---|---|
| Stranges S et al., 2007 [77] USA |
NPC trial: 1,202 people with previous history of non-melanoma skin cancer randomly assigned to 200 μg Se (as Se-yeast) or placebo. | 7.7 years | Cases: 58 (Se)/39 (placebo). HR (95% CI) overall 1.55 (1.03, 2.33); for men 1.62 (1.04, 2.55) and for women 1.38 (0.52, 3.64), suggesting a more important effect in men than in women. Significantly increased risk for T2DM in the highest tertile of baseline plasma Se concentration, i.e. 121.6 μg/L; HR (95% CI) 2.70 (1.30, 5.61). |
| Lippman S et al., 2009 [78] Klein E et al., 2011 [79] USA |
SELECT: 32,400 men randomly assigned to Se 200 μg (as SeMet), vitamin E or placebo alone or combined. | 7–12 years | Results were most compatible with no important effect of Se on risk of T2DM and the effect size reduced after 2008 as the number of cases rose (treatment stopped in 2008). Relative risk (RR), 1.07; 99% CI 0.94, 1.22; P = 0.16 (Oct 2008) RR, 1.04; 99% CI 0.91, 1.18; P = 0.34 (July 2011) |
| Algotar AM et al., 2013 [80] USA and New Zealand |
699 men at high risk for prostate cancer: PSA >4 ng/ml, and/or suspicious digital rectal examination and/or PSA velocity >0.75 ng/ml/year, but with a negative prostate biopsy. They were treated with placebo, 200 or 400 μg Se-yeast/day. | Men were followed every 6 mth for ≤ 5yr | At the end of the study, Supplemental Table 1 showed: - 7 new cases of diabetes in the 209 placebo men; - 12 new cases of diabetes in the 212 men treated with 200 μg; - 12 new cases of diabetes in the 210 men treated with 400 μg. Risk of diabetes not reported. |
| Karp DD et al., 2013 [82] USA |
1561 patients with resected Stage I non–small-cell lung cancer randomly assigned to 200 μg Se (Se-yeast) or placebo. | 48 months | There was no benefit in the prevention of second primary tumors in patients receiving Se. 26 patients of 865 (39.7%) in the Se arm and 12 patients of 477 (33.2%) in the placebo arm had a diagnosis of diabetes during the long-term follow-up period; this was reported as “no increase in diabetes”. |
| Thompson PA et al., 2016 [83] USA |
Following removal of colorectal adenomas, 1374 people aged 40–80 years were randomly assigned to Se (Se-yeast) 200 μg or placebo/day. | 33 months | In participants receiving Se, the HR (95% CI) for new-onset T2DM was 1.25 (0.74, 2.11) showing no important effect. There was a noticeably increased risk of Se-associated T2DM among older participants RR (95% CI) 2.21 (1.04, 4.67). |
| Algotar AM et al., 2013 [81] USA and New Zealand |
699 elderly men at high risk for prostate cancer, randomly assigned to Se 200 or 400 μg (as Se-yeast) or placebo. | Men were followed every 6 mth for ≤ 5yr | The changes in serum glucose levels during the course of the trial were not appreciably different from placebo for the Se 200 μg/day (p = 0.98) or Se 400 μg/day (p = 0.81) treatment groups. |
| Jacobs ET et al., 2019 [85] USA |
In 400 people in trial of Se (200 μg/day as Se-yeast) vs placebo for colorectal adenomatous polyps, fasting plasma glucose and insulin were measured before randomization and after completing the intervention. | A mean of 2.9 years. | When change in HOMA2-%β and insulin sensitivity (HOMA2-%S) were compared, there were no statistically significant differences between Se and placebo groups. 175 participants underwent a modified oral glucose tolerance test at the end of intervention, mean baseline fasting blood glucose concentration was significantly higher (p = 0.04) in those in the placebo group (96.6 ± 14.6) than in those in the Se group (92.3 ± 12.0). There was no adverse effect of 200 μg/day Se supplementation. |
| Rayman MP et al., 2012 [87] UK |
PRECISE: 501 elderly volunteers randomly assigned to 100, 200 or 300 μg Se (as Se-yeast) or placebo. | 6 months | In baseline analyses, the fully adjusted geometric mean of plasma adiponectin was 14% lower (95% CI 0, 27) in the highest than in the lowest quartile of plasma Se (Ptrend = 0.04). However, there was no effect on adiponectin, a predictor of T2DM risk [95]. |
| Meta-analyses and Systematic Reviews of Randomized Trials Showing the Effect of Se on T2DM or Glycemic Indices | |||
| Mao S et al., 2014 [84] | Four RCTs involving 20,294 participants were included in this meta-analysis. | RCTs published from 2007 to 2013 | RR (95% CI) for those given Se vs placebo was 1.09 (0.99, 1.20) for T2DM but this estimate is imprecise. |
| Vinceti M et al., 2018 [59] | Five RCTs involving 23,656 participants were included in this meta-analysis. | RCTs published from 2007 to 2016 | RRs (95% CI) for T2DM for those given Se vs placebo showed an 11% increase: 1.11 (1.01, 1.22). RR (95% CI) of 1.10 (1.00, 1.21) in men (4 studies) and a much less precise estimate of 1.43 (0.74, 2.77) in women (2 studies). |
| Kohler LN et al., 2018 [9] | Three RCTs involving 20,290 participants were included in this meta-analysis using PRISMA. | RCTs published from 2007 to 2016 | OR (95% CI) for those given Se vs placebo had a 1.18 (0.95, 1.47) increased rate for T2DM but this estimate is imprecise. |
| Mahdavi Gorabi A et al., 2019 [86] | 12 RCTs and cross-over trials involving 1,441 participants were included to show the effect of Se supplementation on glycemic indices. | Trials published between 2004 and 2016. | Se supplementation significantly decreased β-cell function (HOMA-B) and increased insulin sensitivity (QUICKI score): - Decrease in HOMA-B: SMD: −0.63; 95% CI: −0.89 to −0.38; - Increase in QUICKI: SMD: by 0.74; 95% CI: 0.49 to 1.0. No improvement in fasting plasma glucose, HOMA-IR, HbA1c, or adiponectin levels. |
In an RCT to look at the effect on prostate cancer, the Selenium and Vitamin E Cancer Prevention Trial (SELECT), 35,533 men from the USA, Canada and Puerto Rico were randomly assigned to four groups (Se, vitamin E, Se + vitamin E, and placebo) [78]. Se (200 μg Se/d as selenomethionine) or vitamin E, alone or in combination, did not prevent prostate cancer but when the men were investigated in a secondary end-point to see whether either Se or vitamin E or the two increased the risk of T2DM, results were most compatible with no important effect on risk (Table 2) [78,79]. In SELECT, Se status was already high at baseline in these men (serum Se 136 μg/L) [78]; it appeared that an additional 200 μg Se/d, as selenomethionine (as opposed to Se-yeast as in the NPC trial), had little or no effect on increasing the risk of diabetes.
Some subsequent RCTs were run in north American participants who were primarily being treated for other conditions where diabetes or high serum glucose was a secondary end-point, i.e., high-risk of prostate cancer [80,81], previous lung cancer [82], or previous colorectal adenoma (see Table 2) [83]. These RCTs were included in a number of meta-analyses of Se and T2DM. In a 2014 meta-analysis, four RCTs including 20,294 participants were analyzed [84]. The combined relative risk (RR) for subjects administered Se compared with control groups did not show an important effect on T2DM. In 2018, a systematic literature search found five RCTs and meta-analyzed a direct relationship between Se exposure and risk of diabetes with a linear trend in subjects with higher plasma or serum Se levels, RR (95% CI): 1.11 (1.01, 1.22) [59]. In a later systematic review and meta-analysis which used the PRISMA method [9], three studies were included. A higher risk of T2DM was not observed for those who received Se as opposed to placebo (Table 2) [9].
It is interesting that the last two meta-analyses used the SELECT data from Lippman et al. (2009) rather than the data from the already published follow-up data on the same study by Klein et al. (2011). Though treatment stopped in 2008, the Klein study had 28% additional data on T2DM from Se and controls after an additional 33 months. The Klein/Lippman studies are heavily weighted in the data analysis owing to their size so any changes to them will have a large impact on the overall result. Re-analysis of the data from Vinceti et al. [59] using the later data [79] gave a change in risk ratio (Fixed Effect Model) of 1.09 (0.98, 1.21), showing a reduced effect size (Dr A Darling, Personal communication, July 15, 2021, endorsed by Dr Eric Klein).
In 2019, the hypothesis that Se supplementation might adversely affect pancreatic β-cell function, insulin sensitivity, and glycemic indices was tested (see Table 2) [85,86]. In an RCT of Se at 200 μg/day for colorectal adenomatous polyps conducted in a subset of 400 individuals, fasting plasma glucose and insulin were measured before randomization and within 6 months after the end of the intervention. Following the intervention, a subgroup of 175 (79 Se and 96 placebo) participants were submitted to a modified oral glucose tolerance test evaluating change in glucose values. Mean baseline, fasting blood glucose concentration was significantly higher in those in the placebo group than in those in the Se group (Table 2) [85]. After a follow-up of 2.9 years, no statistically significant differences were observed for changes in HOMA2-%β or HOMA2-%S between Se and placebo groups. These findings do not support a significant adverse effect of 200 μg Se/day (as Se-yeast) on β-cell function or insulin sensitivity as an explanation for previously reported associations between Se and T2DM [85].
In an RCT which was part of the UK PRECISE Pilot Trial, the effects of Se on T2DM risk were studied in 501 elderly volunteers with relatively low Se status (plasma Se 90.8 μg/L) [87]. They were given placebo, 100, 200, or 300 μg Se/d of Se or placebo yeast and the biomarker was adiponectin, a reliable predictor of T2DM risk [87]. In analyses across randomized groups, Se supplementation had no effect on adiponectin levels after six months of treatment.
In a meta-analysis of RCTs that looked at glycemic indices and adiponectin, Se supplementation resulted in a decrease in homeostasis model of assessment-estimated β-cell function (HOMA-B) and an increase in quantitative insulin sensitivity check index (QUICKI) [86]. There was no improvement in glycemic indices such as fasting plasma glucose, insulin, homeostasis model of assessment-estimated insulin resistance (HOMA-IR), HbA1c, or adiponectin (Table 2) [86].
Inconsistent results of the studies discussed above may be due to the heterogeneity of the studies performed, the relatively small number of RCTs, the different study populations, the dosage and duration of Se supplementation, as well as the variable duration of T2DM [85].
4.4. Summary of the evidence from epidemiological studies
In conclusion, most of the observational studies found significant positive associations between serum/plasma Se and T2DM or fasting plasma glucose though the association was often non-linear. However, a number found no association, a complex non-linear association or an inverse association with increasing Se status, particularly when Se status was low or Se was measured in toenails [[52], [53], [54]]. Effects of Se on T2DM comorbidities were variable, but no effect, a negative effect, a non-linear or U-shaped effects with NAFLD and MetS were found [66,69,70]. Results with SELENOP on T2DM comorbidities were difficult to categorize though SELENOP did appear to be lower in patients with NASH [75,76,88]. Circulating SELENOP derives primarily from hepatocytes, thus, severe liver damage will eventually result in a decline of plasma SELENOP levels, as also observed in patients with liver cirrhosis [88].
Looking at the five RCTs, only one (SELECT) was carried out in healthy people, the others were carried out in people who had had cancer or were at high risk of cancer. Of these five RCTs, only the NPC trial (subjects with previous non-melanoma skin cancer) showed an importantly increased risk of Se on T2DM with an overall HR (95% CI) of 1.55 (1.03, 2.33) and a HR for men of 1.62 (1.04, 2.55) [77]; the others showed no important effect of Se on risk of T2DM (Table 2). Comparing the NPC trial with SELECT, its results derive from a post-hoc analysis of a small trial – only 58 subjects on Se and 39 on placebo developed T2DM [77]; SELECT was a much larger study – 913 on Se and 869 on placebo, and its findings are therefore more robust [79]. The baseline Se status of the men in SELECT was higher than in the NPC trial [78,89]; was it already above a threshold of risk so that further treatment with Se made little or no difference? Was the form of Se used in SELECT (selenomethionine) less effective in promoting T2DM than that used in the NPC and other trials (Se-yeast)? As mentioned by Duffield-Lillico et al. (2003) [90], 60% of the NPC participants worked on farms with great potential for arsenic pesticide exposure and had at least one punctate keratosis of the palm, believed to result from arsenic contact [90,91]. Arsenic exposure increases the risk of T2DM as shown by data from 788 adults in NHANES 2003–2004 who had urine arsenic determinations; the OR for T2DM in participants at the 80th vs the 20th percentile of total arsenic was 3.58 (95% CI, 1.18, 10.83) [92]. (Several authors have shown that arsenic interferes with the metabolism of Se and Se incorporation into proteins [93,94].) These are all points that were made in a 2013 review by Rayman and Stranges who discussed the inconsistent results of RCTs of Se and T2DM [5]. Furthermore, treatment with Se did not appear to support an increased risk of impaired pancreatic β-cell function and insulin sensitivity or glycemic indices [81,85,86]. Importantly, dietary Se supplementation does not appear to be a major causal factor for the development of T2DM in humans, though we cannot currently exclude a small contribution of Se on top of other risk factors, and in particular if it is ingested at high (supranutritional) doses [78,79].
5. What can we learn from animal studies about a link between Se homeostasis and the insulin-regulated fuel metabolism?
5.1. Both supranutritional Se intake and selenoprotein deficiency may promote metabolic derangement but do not necessarily cause overt diabetes
Succeeding the first worrying report on high Se intake as a potential risk factor for T2DM [77], many animal studies investigated how dietary Se supply and the manipulation of selenoprotein levels may affect fuel metabolism. The best characterized selenoproteins in this context are GPX, TXNRD, and SELENOP [4,96]. In mice, global GPX1 overexpression or injection of SELENOP at supraphysiological doses resulted in development of T2DM/MetS-like phenotypes, with hyperinsulinemia and/or impaired insulin action [97,98]. Similar metabolic derangement was observed in mice exhibiting suppressed selenoprotein biosynthesis due to genetic manipulation [99,100]. This fits with the observation that both maximal expression of selenoproteins and selenoprotein deficiency promoted development of a T2DM-like phenotype in mice [99]. Though dietary supplementation with Se at supranutritional doses can also affect functioning and secretion of insulin, the corresponding alterations in signaling and metabolic pathways are often more subtle and do not necessarily induce diabetes [99,[101], [102], [103], [104], [105], [106]]. Interestingly, maternal Se status may predispose the offspring to metabolic disorders: diabetes-like phenotypes associated with either insulin deficiency or resistance were observed in rat pups, depending on whether the dams received Se-deficient or high-Se diets during gestation and lactation [107]. Taken together, both very low and high Se intake in addition to deficient or supraphysiological selenoprotein biosynthesis/activity can promote or exacerbate metabolic derangement. Nevertheless, marginal Se deficiency or oversupply (as also occurs in humans) does not generate overt diabetes/MetS [4,[97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109]].
5.2. Se status and selenoproteins affect tissue homeostasis and metabolic pathways in pancreatic islets and in the major insulin target tissues
Impaired Se homeostasis was frequently associated with either hyper- or hypoinsulinemia in the animal models discussed above. A likely explanation for these observations stems from the fragile redox homeostasis in pancreatic β-cells and the role of selenoenzymes therein [4,37]. Due to the low activity of the common antioxidant enzymes, superoxide dismutase, catalase, and GPX [110], β-cells rely for protection against oxidative stress mainly on an antioxidant system composed of peroxiredoxins, thioredoxin, and TXNRDs [111]. As cellular GPX and TXNRD activities depend on sufficient Se availability, severe Se deficiency may result in oxidative damage of β-cells and lowered insulin secretion. The cytoprotective role of these selenoenzymes for β-cells becomes obvious under conditions of metabolic or oxidative stress: TXNRD inhibition made β-cells more prone to H2O2-induced cell death [111] while GPX1 knock-out mice developed more severe defects in insulin production/secretion than their wild-type littermates when the animals were fed an obesogenic diet [112]. Conversely, GPX1 overexpression protected mice from β-cell loss induced by treatment with streptozotocin and reversed both hypoinsulinemia and hyperglycemia in db/db mice, a model that spontaneously develops a T2DM-like phenotype [113]. The rescue of β-cell function by GPX1 overexpression has been attributed to transcription factors (e.g. MafA, Pdx1, Nkx-6.1) controlling β-cell proliferation and differentiation as well as insulin production. GPX1 overexpression resulted in elevated expression and/or nuclear localization of these factors, associated with increases in β-cell mass and insulin content [[113], [114], [115]]. However, such changes are not necessarily beneficial, as global GPX1 overexpression eventually triggered the development of a T2DM-like phenotype, through dysregulation of basal insulin secretion resulting in hyperinsulinaemia [97,115]. This seemingly paradoxical outcome of supraphysiological GPX1 expression is reminiscent of the hyperinsulinemia occurring in animals fed a high-Se diet, and illustrates that expression/activity of selenoenzymes in β-cells should rather not be maximized or even pushed above physiological levels in order to avoid any severe disturbance of redox homeostasis. This is further highlighted by a recent study that reported a decreased insulin secretory pathway of islet β-cells in response to treatment with SELENOP [116]; however, these findings await independent confirmation as they are in contrast to the previously reported stimulation of insulin secretion by high Se in vivo and in vitro [4,117]. Generally, redox signaling is implicated in differentiation and maturation of β-cells as well as in insulin secretion, and thus, antioxidant enzymes (including selenoenzymes) may interfere with those processes [4,37].
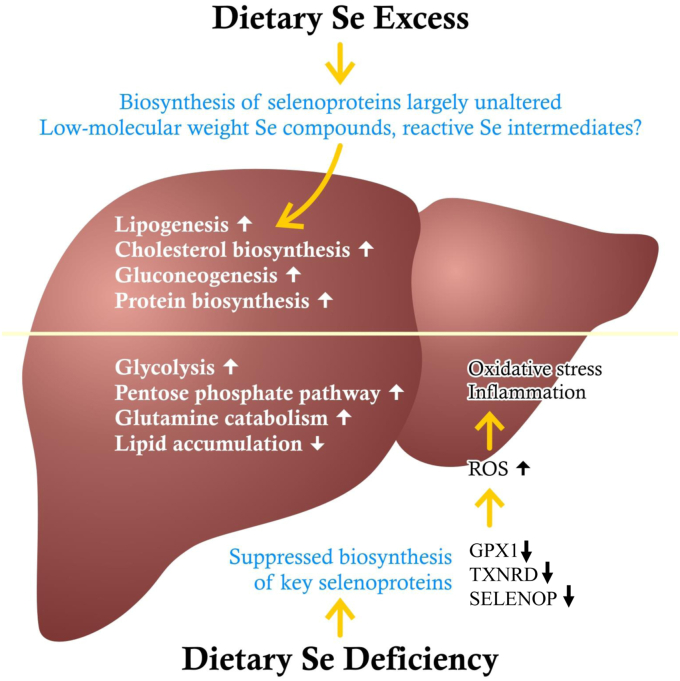
Besides elevated serum insulin concentrations, impaired insulin sensitivity has frequently been observed when rodents were exposed to high-Se diets or exhibited supraphysiological selenoprotein expression/activity [[97], [98], [99], [100],103,105,107,109]. A mechanistic rationale for insulin-antagonistic actions of selenoproteins derives from transient inactivation of counter-regulatory phosphatases (e.g., PTP-1B, PTEN) in the insulin signaling cascade by small amounts of H2O2. Excessive activity of antioxidant enzymes may thus interfere with and attenuate insulin signaling, whereas low antioxidant levels can support insulin sensitivity as long as an unbalanced ROS generation does not provoke oxidative stress resulting in disruption of insulin signalling [4,40]. In this regard, GPX1-deficient mice were protected from developing insulin resistance in liver and skeletal muscle when fed an obesogenic diet [112,118]. Insulin sensitivity and parameters of carbohydrate metabolism were improved in the livers of mice with hepatocyte-specific GPX1 knock-down; these mice developed less hepatic inflammation when they were fed obesity- or steatohepatitis-promoting diets [119]. Nevertheless, peroxiredoxins are quantitatively more important than GPX1 for the degradation of intracellular H2O2 [120] hence TXNRDs, by recycling the peroxiredoxin co-substrate, thioredoxin, also indirectly contribute to H2O2 removal. Supporting the importance of further selenoproteins for liver homeostasis, a recent study in pigs reported more serious consequences of a Se-deficient diet than a GPX1 knock-out: redox imbalance in the liver, induced by severe Se deficiency, was associated with enhanced glucose and glutamine catabolism, suppressed lipid synthesis, and inflammation (Fig. 2) [108]. GPX1 and GPX3 levels were lowered in the WAT of db/db mice and of obese and T2DM patients, and this was associated with insulin resistance, oxidative stress, and inflammation [121]. Down-regulation of both GPX1 and GPX3 impaired the sensitivity of adipocytes for insulin [[122], [123], [124]]. Thus, GPX selenoenzymes support the function of WAT by counteracting oxidative stress and inflammation. In contrast to the insulin-sensitivity-promoting impact of GPX1 and GPX3 in WAT, however, lack of the selenoenzyme TXNRD1 resulted in augmented adipogenesis as well as increased insulin responsiveness and lipogenesis in adipocytes thus suggesting an inhibitory effect of TXNRD1 on insulin signaling [125].
Fig. 2.
Both severe Se excess and severe Se deficiency may alter hepatic metabolism in animals. Dietary Se supply above adequate levels does not enhance the biosynthesis of hepatic selenoproteins or the activity of key selenoenzymes but at very high doses, it may generate reactive Se metabolites that interfere with signaling or metabolic pathways. As a result, several anabolic pathways and lipid accumulation may become augmented. In contrast, biosynthesis/activity of key antioxidant selenoproteins is suppressed at dietary Se deficiency, resulting in oxidative stress and inflammation that is associated with elevated glucose and glutamine catabolism as well as decreased lipid accumulation [101,106,108]. Note that the fluctuations in the Se supply of humans are usually less pronounced and thus will result in less, if any, metabolic derangement, when compared to the experimental conditions in many animal studies.
In addition to effects on insulin sensitivity, supranutritional Se has been reported to modify the expression and/or activity of transcription factors and enzymes implicated in carbohydrate, lipid and protein metabolism in a tissue-selective manner: key factors related to gluconeogenesis, lipogenesis, cholesterol, and protein biosynthesis were up-regulated in the liver of mice and pigs fed high doses of selenite, whereas genes encoding enzymes for glycolysis and cholesterol hydrolysis became down-regulated (Fig. 2) [101,106,109]. In skeletal muscle, the pattern of molecular alterations induced by supranutritional Se indicated a switch in fuel usage from glucose to fatty acids as well as lowered lipogenesis and elevated protein biosynthesis [102,106]. In WAT, high Se-supply resulted in molecular alterations pointing to an increased lipid turnover, attenuated inflammation and insulin resistance induced by an obesogenic diet [102,122]. Again, the metabolic alterations were much more pronounced if high Se doses were fed, rather than marginal Se oversupply.
5.3. The hepatokine, SELENOP, is regulated like a gluconeogenic enzyme and may induce insulin resistance at supraphysiological doses
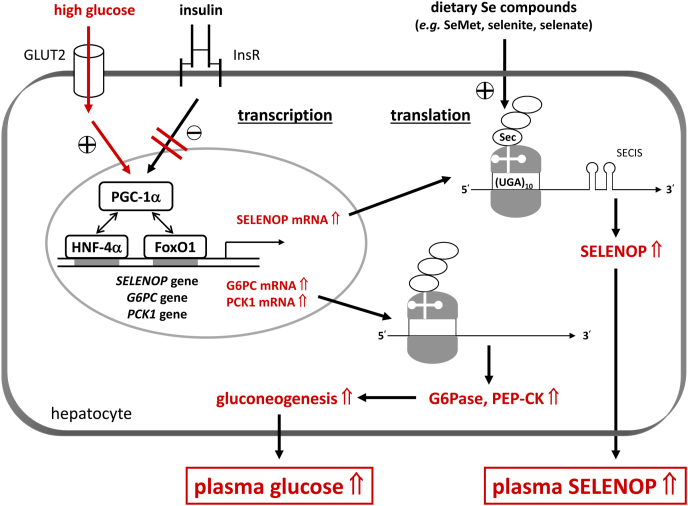
Liver-derived SELENOP is essential for systemic Se homeostasis as it supplies extrahepatic tissues with Se required for the biosynthesis of selenoproteins [4]. Elevated plasma SELENOP levels are associated with hyperglycemia in patients with T2DM [4] and more recently with hepatic steatosis and fibrosis in NAFLD patients [75]. Insulin sensitivity in liver and skeletal muscle was improved in SELENOP-deficient mice, and conversely, intraperitoneal SELENOP injection impaired insulin signaling, hence, SELENOP has been designated as a hepatokine that is capable of inducing insulin resistance [98,126]. Beside SELENOP, other hepatokines associated with insulin resistance have been found in the 69 proteins upregulated in the plasma proteome of T2DM patients when compared to normal subjects [127,128]. A SELENOP-neutralizing antibody has recently been shown to improve glucose metabolism in mice treated with excess SELENOP and the authors of this study proposed that snatching away SELENOP from the blood may provide a novel therapeutic option for T2DM [116]. However, such a therapy might result in other health problems due to an inadequate Se supply of extrahepatic tissues. Moreover, the hypothesis that SELENOP induces insulin resistance is unlikely to explain the above-mentioned cross-sectional associations, as high (supraphysiological) doses of SELENOP were required to provoke metabolic derangement. Also, Se-supplementation at high dietary levels consistently failed to increase hepatic SELENOP gene expression in animal models, even though elevated hepatic protein levels of SELENOP were measured in one study [101,102,106]. More importantly, intervention studies in humans have shown that plasma SELENOP levels were saturated at a daily intake of ∼50–100 μg Se and did not further increase by ingesting Se supplements in larger doses [[129], [130], [131]]. Elevated plasma SELENOP levels may be considered as an accessory phenomenon of insulin resistance and hyperglycemia, as hepatic SELENOP biosynthesis has been shown to be suppressed by insulin and increased by high glucose concentrations [98,132,133]. Thus, hepatic SELENOP transcription is regulated like that of a gluconeogenic enzyme through the transcription factors FoxO1 and HNF-4α together with the co-activator PGC-1α and may also become dysregulated under conditions of hyperglycemia and insulin resistance (Fig. 3) [98,133,134].
Fig. 3.
Hyperglycaemia and insulin resistance, the characteristic features of T2DM, increase hepatic biosynthesis of both SELENOP and gluconeogenic enzymes. SELENOP as well as G6PC (glucose-6-phospatase, G6Pase, catalytic subunit) and PCK1 (phosphoenolpyruvate carboxykinase, PEP-CK) transcription is governed through FoxO1 and HNF-4α that are co-activated by PGC-1α. In metabolically healthy persons, insulin switches off transcription of the three genes. Under conditions of high extracellular glucose concentrations and insulin resistance, the dysregulated transcriptional activity of FoxO1 causes an increase in biosynthesis of SELENOP and the gluconeogenic enzymes, resulting in elevated plasma Se and SELENOP levels as well as in a vicious cycle with further elevated plasma glucose levels (marked in red). Note that ribosomal translation of SELENOP depends on sufficient Se availability [98,[132], [133], [134]]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
5.4. Se compounds may exert specific actions on signaling and metabolic pathways independent of selenoproteins
Given that expression and activity of most selenoproteins are already saturated at adequate or slightly supranutritional Se intake, high Se-induced alterations in fuel metabolism cannot be mediated solely through selenoproteins [4,101,102]. It has been hypothesized that low-molecular-weight Se compounds and reactive Se intermediates may act through modulation of redox-sensitive cysteine residues in proteins or by interference with the thioredoxin-dependent antioxidant system [101]. Moreover, high doses of dietary Se compounds can have opposite effects: while selenite has been shown to counteract insulin signaling and exacerbate insulin resistance, selenate may act as an insulin mimic and ameliorate hyperglycemia in diabetic mice [4,104,109,135]. In particular, selenite is known to induce oxidative stress through redox cycling at high concentrations [136]; this may also explain its adverse effects on insulin signaling. Selenoneine, a novel organic Se compound occurring in fish, ameliorated hepatic steatosis and hepatocellular injury in a mouse model of NAFLD, despite decreased hepatic selenoprotein mRNA levels [13,137]. A novel non-canonical form of Se incorporation into proteins, named facultative protein selenation, has recently been discovered to occur at high Se-supplementation with SeMet; key metabolic proteins in the brown adipose tissue of mice were modified, and the animals were protected from obesity through enhanced thermogenesis [138]. Taken together, effects of Se supplementation depend not only on the dose but also on the Se compound ingested. It should be noted, however, that SeMet is the major Se compound in the normal human diet as well as in many Se-containing dietary supplements.
6. Conclusions
The issue of Se as a potential risk factor for diabetes and its co-morbidities has been discussed for several years in the scientific community, with differing opinions. Indeed, Se homeostasis, selenoproteins, insulin signaling/secretion and carbohydrate/lipid metabolism are inextricably linked in numerous and complex ways so that a change in one may affect others, sometimes even in a paradoxical manner. A subtle understanding of the molecular mechanisms that link Se to derangements in fuel metabolism is required to fully comprehend what is happening. Based on our review of the current available literature, we conclude:
-
⁃
In humans, fluctuations in Se supply and in selenoprotein expression or activity are usually less pronounced than those applied in many experimental animal studies. Thus, the translational value of many animal studies is rather limited, even though they provide important mechanistic insights into the role of Se in fuel metabolism.
-
⁃
The interaction between Se status and glucose control is not limited to hyperglycemia but extends to hypoglycemia risk in Se deficiency (Fig. 1) [52].
-
⁃
While SELENOP is an excellent biomarker of Se status, the different ELISA kits currently used for its quantitation in serum/plasma urgently need to be standardized to allow better comparison between the published results and assessment of their reliability [71,72].
-
⁃Many cross-sectional studies show an association between high plasma/serum Se and T2DM/fasting plasma glucose. These findings may be explained by the fact that:
-
⁃
Results from RCTs must carry more weight than those of observational studies when evaluating whether there is a truly causal relationship between Se, T2DM and insulin resistance [85].
-
⁃
Importantly, dietary Se supplementation does not appear to be a major causal factor for the development of insulin resistance/T2DM in humans, though we cannot currently exclude a small contribution of Se on top of other risk factors, and in particular if it is ingested at high (supranutritional) doses [78,79].
-
⁃
As biosynthesis of selenoproteins is saturated at adequate or slightly supranutritional Se supply, additional Se species beyond selenoproteins may be involved in the adverse effects of a high Se supply. In this context, further research is required on potential differences in the effect of dietary Se compounds on fuel metabolism.
7. Implications for clinical practice
An adequate Se supply constitutes an important factor for glucose/lipid homeostasis in healthy persons as well as in patients with T2DM or one of its metabolic co-morbidities.
-
⁃It is important to take into account the likely Se status of the patient:
-
⁃
If you cannot determine the Se status of the patient, have it measured in a laboratory that is part of a trace-element External Quality Assessment Scheme to ensure that the result is accurate.
-
⁃
Our data suggest that only where Se status is low (e.g., below 89 μg/L [52]), it may be appropriate to increase Se intake through diet or via a modest Se supplementation (e.g. 50–100 μg/d). Ideally keep plasma Se < 122 μg/L [3,77].
-
⁃High Se can be toxic:
-
−there was increased mortality in Danish volunteers ten years after they had received a supplement of 300 μg Se/d for five years [141];
-
−the SELECT study shows alopecia and dermatitis in N American men given a supplement of 200 μg/d Se for five years [78];
-
−there was an increased risk of non-melanoma skin cancer in volunteers from the NPC Trial that had previously had non-melanoma skin cancer [90].
-
−
-
⁃
Though a Se-adequate diet is important for human health, Se supplementation beyond the adequate level [3] is not recommended as an adjuvant “antioxidant” treatment for T2DM or its metabolic comorbidities [44]. Selenite-containing dietary supplements should be taken with particular care, as selenite can induce oxidative stress at high doses, which might also contribute to derangements in fuel metabolism [136].
8. Search strategy and selection criteria
References for this review were identified through searches of PubMed for articles published to September 2021, by use of the terms, “selenium” or “selenoprotein P” in combination with the term “diabetes”, “obesity”, “metabolic syndrome”, “non-alcoholic fatty liver disease”, “NAFLD”, “hyperglycemia”, “hypoglycemia”, glucose” and “β-cell”. Articles resulting from these searches, from authors’ personal files and relevant references cited in those articles were reviewed. Articles published in English and German were included.
Authors’ contributions
HS wrote the sections on T2DM, its metabolic comorbidities, the molecular mechanisms that underlie the roles of Se in the pathogenesis of T2DM and its associated disorders. LHD wrote the sections on the epidemiology of randomized trials with Se on the incidence of DM/T2DM and on the effect of Se treatment on pancreatic β-cell function, insulin sensitivity, and glycemic indices. MPR wrote the Introduction, the health effects of Se, selenoproteins and their polymorphisms that affect T2DM or its co-morbidities, the epidemiology of observational studies of Se on T2DM, the Supplemental Material, prepared the tables and revised all the text. The figures were prepared jointly.
Declaration of competing interest
None of the authors has a conflict of interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102236.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Schwarz K., Foltz C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. 1951. Nutrition. 1999;15(3):255. [PubMed] [Google Scholar]
- 2.Hatfield D.L., Gladyshev V.N. How selenium has altered our understanding of the genetic code. Mol. Cell Biol. 2002;22(11):3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rayman M.P. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 4.Steinbrenner H. Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic. Biol. Med. 2013;65:1538–1547. doi: 10.1016/j.freeradbiomed.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Rayman M.P., Stranges S. Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radic. Biol. Med. 2013;65:1557–1564. doi: 10.1016/j.freeradbiomed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Schomburg L. Selenium deficiency due to diet, pregnancy, severe illness, or COVID-19-A preventable trigger for autoimmune disease. Int. J. Mol. Sci. 2021;22(16) doi: 10.3390/ijms22168532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudish L.I., Reusch J.E., Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J. Clin. Invest. 2019;129(10):4001–4008. doi: 10.1172/JCI129188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roden M., Shulman G.I. The integrative biology of type 2 diabetes. Nature. 2019;576(7785):51–60. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 9.Kohler L.N., Foote J., Kelley C.P., et al. Selenium and type 2 diabetes: systematic review. Nutrients. 2018;10(12) doi: 10.3390/nu10121924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine (US) National Academies Press (US); Washington (DC): 2000. Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. [PubMed] [Google Scholar]
- 11.Department of Health . Dietary Reference Values for Food, Energy and Nutrients for the United Kingdom.: the Stationery Office: London. 1991. Dietary reference values of the committee on medical aspects of food policy (COMA) [PubMed] [Google Scholar]
- 12.Johnson C.C., Fordyce F.M., Rayman M.P. Symposium on 'Geographical and geological influences on nutrition': factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc. Nutr. Soc. 2010;69(1):119–132. doi: 10.1017/S0029665109991807. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita M., Yamashita Y., Ando T., Wakamiya J., Akiba S. Identification and determination of selenoneine, 2-selenyl-N α, N α, N α -trimethyl-L-histidine, as the major organic selenium in blood cells in a fish-eating population on remote Japanese Islands. Biol. Trace Elem. Res. 2013;156(1–3):36–44. doi: 10.1007/s12011-013-9846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winther K.H., Rayman M.P., Bonnema S.J., Hegedus L. Selenium in thyroid disorders - essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020;16(3):165–176. doi: 10.1038/s41574-019-0311-6. [DOI] [PubMed] [Google Scholar]
- 15.Xia Y., Hill K.E., Byrne D.W., Xu J., Burk R.F. Effectiveness of selenium supplements in a low-selenium area of China. Am. J. Clin. Nutr. 2005;81(4):829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 16.Yang G.Q., Xia Y.M. Studies on human dietary requirements and safe range of dietary intakes of selenium in China and their application in the prevention of related endemic diseases. Biomed. Environ. Sci. 1995;8(3):187–201. [PubMed] [Google Scholar]
- 17.Laclaustra M., Navas-Acien A., Stranges S., Ordovas J.M., Guallar E. Serum selenium concentrations and diabetes in U.S. Adults: national health and nutrition examination survey (NHANES) 2003-2004. Environ. Health Perspect. 2009;117(9):1409–1413. doi: 10.1289/ehp.0900704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y.Q., Shen G., Lo K., et al. Association of circulating selenium concentration with dyslipidemia: results from the NHANES. J. Trace Elem. Med. Biol. 2020;58:126438. doi: 10.1016/j.jtemb.2019.126438. [DOI] [PubMed] [Google Scholar]
- 19.Hughes D.J., Fedirko V., Jenab M., et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int. J. Cancer. 2015;136(5):1149–1161. doi: 10.1002/ijc.29071. [DOI] [PubMed] [Google Scholar]
- 20.Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 2014;94(3):739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitts M.W., Hoffmann P.R. Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium. 2018;70:76–86. doi: 10.1016/j.ceca.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenmakers E., Agostini M., Mitchell C., et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J. Clin. Invest. 2010;120(12):4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellwege J.N., Palmer N.D., Ziegler J.T., et al. Genetic variants in selenoprotein P plasma 1 gene (SEPP1) are associated with fasting insulin and first phase insulin response in Hispanics. Gene. 2014;534(1):33–39. doi: 10.1016/j.gene.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu S.S., Du J.L. Selenoprotein S: a therapeutic target for diabetes and macroangiopathy? Cardiovasc. Diabetol. 2017;16(1):101. doi: 10.1186/s12933-017-0585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L., Zheng Y.Y., Chen Y., et al. Association of genetic polymorphisms of SelS with Type 2 diabetes in a Chinese population. Biosci. Rep. 2018;38(6) doi: 10.1042/BSR20181696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsson M., Olsson B., Jacobson P., et al. Expression of the selenoprotein S (SELS) gene in subcutaneous adipose tissue and SELS genotype are associated with metabolic risk factors. Metabolism. 2011;60(1):114–120. doi: 10.1016/j.metabol.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dora J.M., Machado W.E., Rheinheimer J., Crispim D., Maia A.L. Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: case-control study and meta-analysis. Eur. J. Endocrinol. 2010;163(3):427–434. doi: 10.1530/EJE-10-0419. [DOI] [PubMed] [Google Scholar]
- 28.Canani L.H., Capp C., Dora J.M., et al. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2005;90(6):3472–3478. doi: 10.1210/jc.2004-1977. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X., Sun J., Han W., et al. The type 2 deiodinase Thr92Ala polymorphism is associated with worse glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J. Diabetes Res. 2016:5928726. doi: 10.1155/2016/5928726. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzuya M., Ando F., Iguchi A., Shimokata H. Glutathione peroxidase 1 Pro198Leu variant contributes to the metabolic syndrome in men in a large Japanese cohort. Am. J. Clin. Nutr. 2008;87(6) doi: 10.1093/ajcn/87.6.1939. 1939-44. [DOI] [PubMed] [Google Scholar]
- 31.Smith G.I., Mittendorfer B., Klein S. Metabolically healthy obesity: facts and fantasies. J. Clin. Invest. 2019;129(10):3978–3989. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Vliet S., Koh H.E., Patterson B.W., et al. Obesity is associated with increased basal and postprandial beta-cell insulin secretion even in the absence of insulin resistance. Diabetes. 2020;69(10):2112–2119. doi: 10.2337/db20-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erion K., Corkey B.E. beta-Cell Failure or beta-Cell Abuse? Front. Endocrinol. 2018;9:532. doi: 10.3389/fendo.2018.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kupriyanova Y., Zaharia O.P., Bobrov P., et al. Early changes in hepatic energy metabolism and lipid content in recent-onset type 1 and 2 diabetes mellitus. J. Hepatol. 2021;74(5):1028–1037. doi: 10.1016/j.jhep.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy M.I. Painting a new picture of personalised medicine for diabetes. Diabetologia. 2017;60(5):793–799. doi: 10.1007/s00125-017-4210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahlqvist E., Storm P., Karajamaki A., et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 37.Benakova S., Holendova B., Plecita-Hlavata L. Redox homeostasis in pancreatic beta-cells: from development to failure. Antioxidants. 2021;10(4) doi: 10.3390/antiox10040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 39.Lee Y.S., Olefsky J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021;35(5–6):307–328. doi: 10.1101/gad.346312.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lennicke C., Cocheme H.M. Redox regulation of the insulin signalling pathway. Redox Biol. 2021;42:101964. doi: 10.1016/j.redox.2021.101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangwung P., Petersen K.F., Shulman G.I., Knowles J.W. Mitochondrial dysfunction, insulin resistance, and potential genetic implications. Endocrinology. 2020;161(4) doi: 10.1210/endocr/bqaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilg H., Zmora N., Adolph T.E., Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020;20(1):40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 43.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 44.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50(5):567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocourt C.R., Cheng W.H. Selenium supernutrition: are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients. 2013;5(4):1349–1365. doi: 10.3390/nu5041349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J., Chung H.S., Choi M.K., et al. Association between serum selenium level and the presence of diabetes mellitus: a meta-analysis of observational studies. Diabetes Metab. J. 2019;43(4):447–460. doi: 10.4093/dmj.2018.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X.L., Yang T.B., Wei J., Lei G.H., Zeng C. Association between serum selenium level and type 2 diabetes mellitus: a non-linear dose-response meta-analysis of observational studies. Nutr. J. 2016;15(1):48. doi: 10.1186/s12937-016-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinceti M., Filippini T., Wise L.A., Rothman K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021;197:111210. doi: 10.1016/j.envres.2021.111210. [DOI] [PubMed] [Google Scholar]
- 49.Bleys J., Navas-Acien A., Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30(4):829–834. doi: 10.2337/dc06-1726. [DOI] [PubMed] [Google Scholar]
- 50.Lin J., Shen T. Association of dietary and serum selenium concentrations with glucose level and risk of diabetes mellitus: a cross sectional study of national health and nutrition examination survey, 1999-2006. J. Trace Elem. Med. Biol. 2021;63:126660. doi: 10.1016/j.jtemb.2020.126660. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Lin M., Gao X., et al. High dietary selenium intake is associated with less insulin resistance in the Newfoundland population. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., Rijntjes E., Wu Q., et al. Selenium deficiency is linearly associated with hypoglycemia in healthy adults. Redox Biol. 2020;37:101709. doi: 10.1016/j.redox.2020.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajpathak S., Rimm E., Morris J.S., Hu F. Toenail selenium and cardiovascular disease in men with diabetes. J. Am. Coll. Nutr. 2005;24(4):250–256. doi: 10.1080/07315724.2005.10719472. [DOI] [PubMed] [Google Scholar]
- 54.Park K., Rimm E.B., Siscovick D.S., et al. Toenail selenium and incidence of type 2 diabetes in U.S. men and women. Diabetes Care. 2012;35(7):1544–1551. doi: 10.2337/dc11-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinceti M., Grioni S., Alber D., et al. Toenail selenium and risk of type 2 diabetes: the ORDET cohort study. J. Trace Elem. Med. Biol. 2015;29:145–150. doi: 10.1016/j.jtemb.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Longnecker M.P., Stampfer M.J., Morris J.S., et al. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am. J. Clin. Nutr. 1993;57(3):408–413. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]
- 57.Gutiérrez-González E., García-Esquinas E., de Larrea-Baz N.F., et al. Toenails as biomarker of exposure to essential trace metals: a review. Environ. Res. 2019;179(Pt A):108787. doi: 10.1016/j.envres.2019.108787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filippini T., Ferrari A., Michalke B., et al. Toenail selenium as an indicator of environmental exposure: a cross-sectional study. Mol. Med. Rep. 2017;15(5):3405–3412. doi: 10.3892/mmr.2017.6388. [DOI] [PubMed] [Google Scholar]
- 59.Vinceti M., Filippini T., Rothman K.J. Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Eur. J. Epidemiol. 2018;33(9):789–810. doi: 10.1007/s10654-018-0422-8. [DOI] [PubMed] [Google Scholar]
- 60.Dias J.P.V., Costa Sobrinho P.S., Pimenta A.M., Hermsdorff H.H.M., Bressan J., Nobre L.N. Dietary selenium intake and type-2 diabetes: a cross-sectional population-based study on CUME project. Front. Nutr. 2021;8:678648. doi: 10.3389/fnut.2021.678648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moon S., Chung H.S., Yu J.M., et al. Association between serum selenium level and the prevalence of diabetes mellitus in U.S. population. J. Trace Elem. Med. Biol. 2019;52:83–88. doi: 10.1016/j.jtemb.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Hoque B., Shi Z. Association between selenium intake, diabetes and mortality in adults: findings from National Health and Nutrition Examination Survey (NHANES) 2003-2014. Br. J. Nutr. 2021:1–8. doi: 10.1017/S000711452100177X. [DOI] [PubMed] [Google Scholar]
- 63.Cardoso B.R., Braat S., Graham R.M. Selenium status is associated with insulin resistance markers in adults: findings from the 2013 to 2018 national health and nutrition examination survey (NHANES) Front. Nutr. 2021;8:696024. doi: 10.3389/fnut.2021.696024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos A.C., Passos A.F.F., Holzbach L.C., Cominetti C. Selenium intake and glycemic control in young adults with normal-weight obesity syndrome. Front. Nutr. 2021;8:696325. doi: 10.3389/fnut.2021.696325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei J., Zeng C., Gong Q.Y., Li X.X., Lei G.H., Yang T.B. Associations between dietary antioxidant intake and metabolic syndrome. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Retondario A., Souza A.M., Bricarello L.P., et al. Selenium intake is not associated with the metabolic syndrome in Brazilian adolescents: an analysis of the Study of Cardiovascular Risk Factors in Adolescents. Br. J. Nutr. 2021:1–11. doi: 10.1017/S0007114521002385. [DOI] [PubMed] [Google Scholar]
- 67.Wongdokmai R., Shantavasinkul P.C., Chanprasertyothin S., et al. The involvement of selenium in type 2 diabetes development related to obesity and low grade inflammation. Diabetes Metab. Syndr. Obes. 2021;14:1669–1680. doi: 10.2147/DMSO.S303146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reja M., Makar M., Visaria A., Marino D., Rustgi V. Increased serum selenium levels are associated with reduced risk of advanced liver fibrosis and all-cause mortality in NAFLD patients: national Health and Nutrition Examination Survey (NHANES) III. Ann. Hepatol. 2020;19(6):635–640. doi: 10.1016/j.aohep.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Wang X., Seo Y.A., Park S.K. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: national Health and Nutrition Examination Survey (NHANES) 2011-2016. Environ. Res. 2021;197:111190. doi: 10.1016/j.envres.2021.111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou L., Luo C., Yin J., et al. Diverse associations of plasma selenium concentrations and SELENOP gene polymorphism with metabolic syndrome and its components. Oxid. Med. Cell. Longev. 2020:5343014. doi: 10.1155/2020/5343014. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schomburg L., Melander O. Letter by schomburg and melander regarding article, "selenoprotein P promotes the development of pulmonary arterial hypertension: a possible novel therapeutic target". Circulation. 2019;139(5):722–723. doi: 10.1161/CIRCULATIONAHA.118.035782. [DOI] [PubMed] [Google Scholar]
- 72.Saito Y., Misu H., Takayama H., et al. Comparison of human selenoprotein P determinants in serum between our original methods and commercially available kits. Biol. Pharm. Bull. 2018;41(5):828–832. doi: 10.1248/bpb.b18-00046. [DOI] [PubMed] [Google Scholar]
- 73.Chen M., Liu B., Wilkinson D., et al. Selenoprotein P is elevated in individuals with obesity, but is not independently associated with insulin resistance. Obes. Res. Clin. Pract. 2017;11(2):227–232. doi: 10.1016/j.orcp.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y., He X., Chen X., Li Y., Ke Y. SeP is elevated in NAFLD and participates in NAFLD pathogenesis through AMPK/ACC pathway. J. Cell. Physiol. 2021;236(5):3800–3807. doi: 10.1002/jcp.30121. [DOI] [PubMed] [Google Scholar]
- 75.Caviglia G.P., Rosso C., Armandi A., et al. Interplay between oxidative stress and metabolic derangements in non-alcoholic fatty liver disease: the role of selenoprotein P. Int. J. Mol. Sci. 2020;21(22) doi: 10.3390/ijms21228838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polyzos S.A., Kountouras J., Mavrouli M., et al. Selenoprotein P in patients with nonalcoholic fatty liver disease. Exp. Clin. Endocrinol. Diabetes. 2019;127(9):598–602. doi: 10.1055/a-0811-9136. [DOI] [PubMed] [Google Scholar]
- 77.Stranges S., Marshall J.R., Natarajan R., et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann. Intern. Med. 2007;147(4):217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 78.Lippman S.M., Klein E.A., Goodman P.J., et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein E.A., Thompson I.M., Jr., Tangen C.M., et al. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Algotar A.M., Stratton M.S., Ahmann F.R., et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73(3):328–335. doi: 10.1002/pros.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Algotar A.M., Hsu C.H., Singh P., Stratton S.P. Selenium supplementation has no effect on serum glucose levels in men at high risk of prostate cancer. J. Diabetes. 2013;5(4):465–470. doi: 10.1111/1753-0407.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karp D.D., Lee S.J., Keller S.M., et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J. Clin. Oncol. 2013;31(33):4179–4187. doi: 10.1200/JCO.2013.49.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thompson P.A., Ashbeck E.L., Roe D.J., et al. Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J. Natl. Cancer Inst. 2016;108(12) doi: 10.1093/jnci/djw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao S., Zhang A., Huang S. Selenium supplementation and the risk of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Endocrine. 2014;47(3):758–763. doi: 10.1007/s12020-014-0298-7. [DOI] [PubMed] [Google Scholar]
- 85.Jacobs E.T., Lance P., Mandarino L.J., et al. Selenium supplementation and insulin resistance in a randomized, clinical trial. BMJ Open Diabetes Res. Care. 2019;7(1) doi: 10.1136/bmjdrc-2018-000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahdavi Gorabi A., Hasani M., Djalalinia S., et al. Effect of selenium supplementation on glycemic indices: a meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2019;18(2):349–362. doi: 10.1007/s40200-019-00419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rayman M.P., Blundell-Pound G., Pastor-Barriuso R., Guallar E., Steinbrenner H., Stranges S. A randomized trial of selenium supplementation and risk of type-2 diabetes, as assessed by plasma adiponectin. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0045269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burk R.F., Hill K.E., Motley A.K., Byrne D.W., Norsworthy B.K. Selenium deficiency occurs in some patients with moderate-to-severe cirrhosis and can be corrected by administration of selenate but not selenomethionine: a randomized controlled trial. Am. J. Clin. Nutr. 2015;102(5):1126–1133. doi: 10.3945/ajcn.115.110932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clark L.C., Combs G.F., Jr., Turnbull B.W., et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276(24) 1957-63. [PubMed] [Google Scholar]
- 90.Duffield-Lillico A.J., Slate E.H., Reid M.E., et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J. Natl. Cancer Inst. 2003;95(19):1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 91.Tseng W.P., Chu H.M., How S.W., Fong J.M., Lin C.S., Yeh S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J. Natl. Cancer Inst. 1968;40(3):453–463. [PubMed] [Google Scholar]
- 92.Navas-Acien A., Silbergeld E.K., Pastor-Barriuso R., Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300(7):814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 93.Ip C., Ganther H.E. Activity of methylated forms of selenium in cancer prevention. Cancer Res. 1990;50(4):1206–1211. [PubMed] [Google Scholar]
- 94.Styblo M., Thomas D.J. Selenium modifies the metabolism and toxicity of arsenic in primary rat hepatocytes. Toxicol. Appl. Pharmacol. 2001;172(1):52–61. doi: 10.1006/taap.2001.9134. [DOI] [PubMed] [Google Scholar]
- 95.Misu H., Ishikura K., Kurita S., et al. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tinkov A.A., Bjørklund G., Skalny A.V., et al. The role of the thioredoxin/thioredoxin reductase system in the metabolic syndrome: towards a possible prognostic marker? Cell. Mol. Life Sci. 2018;75(9):1567–1586. doi: 10.1007/s00018-018-2745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McClung J.P., Roneker C.A., Mu W., et al. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. U. S. A. 2004;101(24):8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Misu H., Takamura T., Takayama H., et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metabol. 2010;12(5):483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 99.Labunskyy V.M., Lee B.C., Handy D.E., Loscalzo J., Hatfield D.L., Gladyshev V.N. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxidants Redox Signal. 2011;14(12):2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seale L.A., Hashimoto A.C., Kurokawa S., et al. Disruption of the selenocysteine lyase-mediated selenium recycling pathway leads to metabolic syndrome in mice. Mol. Cell Biol. 2012;32(20):4141–4154. doi: 10.1128/MCB.00293-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu X., Chandler J.D., Orr M.L., et al. Selenium supplementation alters hepatic energy and fatty acid metabolism in mice. J. Nutr. 2018;148(5):675–684. doi: 10.1093/jn/nxy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pinto A., Juniper D.T., Sanil M., et al. Supranutritional selenium induces alterations in molecular targets related to energy metabolism in skeletal muscle and visceral adipose tissue of pigs. J. Inorg. Biochem. 2012;114:47–54. doi: 10.1016/j.jinorgbio.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 103.Stahel P., Kim J.J., Cieslar S.R., Warrington J.M., Xiao C., Cant J.P. Supranutritional selenium intake from enriched milk casein impairs hepatic insulin sensitivity via attenuated IRS/PI3K/AKT signaling and decreased PGC-1alpha expression in male Sprague-Dawley rats. J. Nutr. Biochem. 2017;41:142–150. doi: 10.1016/j.jnutbio.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Wang C., Yang S., Zhang N., et al. Long-term supranutritional supplementation with selenate decreases hyperglycemia and promotes fatty liver degeneration by inducing hyperinsulinemia in diabetic db/db mice. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X., Zhang W., Chen H., et al. High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicol. Lett. 2014;224(1):16–23. doi: 10.1016/j.toxlet.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 106.Zhao Z., Barcus M., Kim J., Lum K.L., Mills C., Lei X.G. High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J. Nutr. 2016;146(9):1625–1633. doi: 10.3945/jn.116.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ojeda M.L., Nogales F., Membrilla A., Carreras O. Maternal selenium status is profoundly involved in metabolic fetal programming by modulating insulin resistance, oxidative balance and energy homeostasis. Eur. J. Nutr. 2019;58(8):3171–3181. doi: 10.1007/s00394-018-1861-4. [DOI] [PubMed] [Google Scholar]
- 108.Tang C., Li S., Zhang K., et al. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. 2020;36:101519. doi: 10.1016/j.redox.2020.101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou J., Xu G., Bai Z., et al. Selenite exacerbates hepatic insulin resistance in mouse model of type 2 diabetes through oxidative stress-mediated JNK pathway. Toxicol. Appl. Pharmacol. 2015;289(3):409–418. doi: 10.1016/j.taap.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 110.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46(11):1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 111.Stancill J.S., Broniowska K.A., Oleson B.J., Naatz A., Corbett J.A. Pancreatic beta-cells detoxify H2O2 through the peroxiredoxin/thioredoxin antioxidant system. J. Biol. Chem. 2019;294(13):4843–4853. doi: 10.1074/jbc.RA118.006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Merry T.L., Tran M., Stathopoulos M., et al. High-fat-fed obese glutathione peroxidase 1-deficient mice exhibit defective insulin secretion but protection from hepatic steatosis and liver damage. Antioxidants Redox Signal. 2014;20(14):2114–2129. doi: 10.1089/ars.2013.5428. [DOI] [PubMed] [Google Scholar]
- 113.Harmon J.S., Bogdani M., Parazzoli S.D., et al. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150(11):4855–4862. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo S., Dai C., Guo M., et al. Inactivation of specific beta cell transcription factors in type 2 diabetes. J. Clin. Invest. 2013;123(8):3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X.D., Vatamaniuk M.Z., Wang S.K., Roneker C.A., Simmons R.A., Lei X.G. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51(8):1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 116.Mita Y., Nakayama K., Inari S., et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat. Commun. 2017;8(1):1658. doi: 10.1038/s41467-017-01863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Campbell S.C., Aldibbiat A., Marriott C.E., et al. Selenium stimulates pancreatic beta-cell gene expression and enhances islet function. FEBS Lett. 2008;582(15):2333–2337. doi: 10.1016/j.febslet.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 118.Loh K., Deng H., Fukushima A., et al. Reactive oxygen species enhance insulin sensitivity. Cell Metabol. 2009;10(4):260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Merry T.L., Tran M., Dodd G.T., et al. Hepatocyte glutathione peroxidase-1 deficiency improves hepatic glucose metabolism and decreases steatohepatitis in mice. Diabetologia. 2016;59(12):2632–2644. doi: 10.1007/s00125-016-4084-3. [DOI] [PubMed] [Google Scholar]
- 120.Rhee S.G., Kil I.S. Multiple functions and regulation of mammalian peroxiredoxins. Annu. Rev. Biochem. 2017;86:749–775. doi: 10.1146/annurev-biochem-060815-014431. [DOI] [PubMed] [Google Scholar]
- 121.Chung S.S., Kim M., Youn B.S., et al. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor gamma in human skeletal muscle cells. Mol. Cell Biol. 2009;29(1):20–30. doi: 10.1128/MCB.00544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hauffe R., Stein V., Chudoba C., et al. GPx3 dysregulation impacts adipose tissue insulin receptor expression and sensitivity. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.136283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobayashi H., Matsuda M., Fukuhara A., Komuro R., Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Am. J. Physiol. Endocrinol. Metab. 2009;296(6):E1326–E1334. doi: 10.1152/ajpendo.90921.2008. [DOI] [PubMed] [Google Scholar]
- 124.Lee Y.S., Kim A.Y., Choi J.W., et al. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol. Endocrinol. 2008;22(9):2176–2189. doi: 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peng X., Giménez-Cassina A., Petrus P., Conrad M., Rydén M., Arnér E.S. Thioredoxin reductase 1 suppresses adipocyte differentiation and insulin responsiveness. Sci. Rep. 2016;6:28080. doi: 10.1038/srep28080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Misu H., Takayama H., Saito Y., et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 2017;23(4):508–516. doi: 10.1038/nm.4295. [DOI] [PubMed] [Google Scholar]
- 127.Solovyev N., Vanhaecke F., Michalke B. Selenium and iodine in diabetes mellitus with a focus on the interplay and speciation of the elements. J. Trace Elem. Med. Biol. 2019;56:69–80. doi: 10.1016/j.jtemb.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 128.Kaur P., Rizk N.M., Ibrahim S., et al. iTRAQ-based quantitative protein expression profiling and MRM verification of markers in type 2 diabetes. J. Proteome Res. 2012;11(11):5527–5539. doi: 10.1021/pr300798z. [DOI] [PubMed] [Google Scholar]
- 129.Xia Y., Hill K.E., Li P., et al. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010;92(3):525–531. doi: 10.3945/ajcn.2010.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hurst R., Armah C.N., Dainty J.R., et al. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010;91(4):923–931. doi: 10.3945/ajcn.2009.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burk R.F., Hill K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015;35:109–134. doi: 10.1146/annurev-nutr-071714-034250. [DOI] [PubMed] [Google Scholar]
- 132.Speckmann B., Sies H., Steinbrenner H. Attenuation of hepatic expression and secretion of selenoprotein P by metformin. Biochem. Biophys. Res. Commun. 2009;387(1):158–163. doi: 10.1016/j.bbrc.2009.06.143. [DOI] [PubMed] [Google Scholar]
- 133.Speckmann B., Walter P.L., Alili L., et al. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1alpha with FoxO1a and hepatocyte nuclear factor 4 alpha transcription factors. Hepatology. 2008;48(6):1998–2006. doi: 10.1002/hep.22526. [DOI] [PubMed] [Google Scholar]
- 134.Jackson M.I., Cao J., Zeng H., Uthus E., Combs G.F., Jr. S-adenosylmethionine-dependent protein methylation is required for expression of selenoprotein P and gluconeogenic enzymes in HepG2 human hepatocytes. J. Biol. Chem. 2012;287(43):36455–36464. doi: 10.1074/jbc.M112.412932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pinto A., Speckmann B., Heisler M., Sies H., Steinbrenner H. Delaying of insulin signal transduction in skeletal muscle cells by selenium compounds. J. Inorg. Biochem. 2011;105(6):812–820. doi: 10.1016/j.jinorgbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 136.Brigelius-Flohé R., Flohé L. Selenium and redox signaling. Arch. Biochem. Biophys. 2017;617:48–59. doi: 10.1016/j.abb.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 137.Miyata M., Matsushita K., Shindo R., Shimokawa Y., Sugiura Y., Yamashita M. Selenoneine ameliorates hepatocellular injury and hepatic steatosis in a mouse model of NAFLD. Nutrients. 2020;12(6) doi: 10.3390/nu12061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jedrychowski M.P., Lu G.Z., Szpyt J., et al. Facultative protein selenation regulates redox sensitivity, adipose tissue thermogenesis, and obesity. Proc. Natl. Acad. Sci. U. S. A. 2020;117(20):10789–10796. doi: 10.1073/pnas.2001387117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fallon N., Dillon S.A. Low intakes of iodine and selenium amongst vegan and vegetarian women highlight a potential nutritional vulnerability. Front. Nutr. 2020;7:72. doi: 10.3389/fnut.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kristensen N.B., Madsen M.L., Hansen T.H., et al. Intake of macro- and micronutrients in Danish vegans. Nutr. J. 2015;14:115. doi: 10.1186/s12937-015-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rayman M.P., Winther K.H., Pastor-Barriuso R., Cold F., Thvilum M., Stranges S., Guallar E., Cold S. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018 doi: 10.1016/j.freeradbiomed.2018.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.