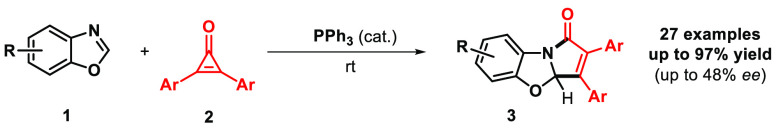
Abstract
The triphenylphosphine-catalyzed dearomative [3 + 2] cycloaddition of benzoxazoles with 1,2-diphenylcyclopropenone is herein described. The reaction scope, mechanism, and possible future applications of this rare organocatalyzed cycloaddition are herein discussed.
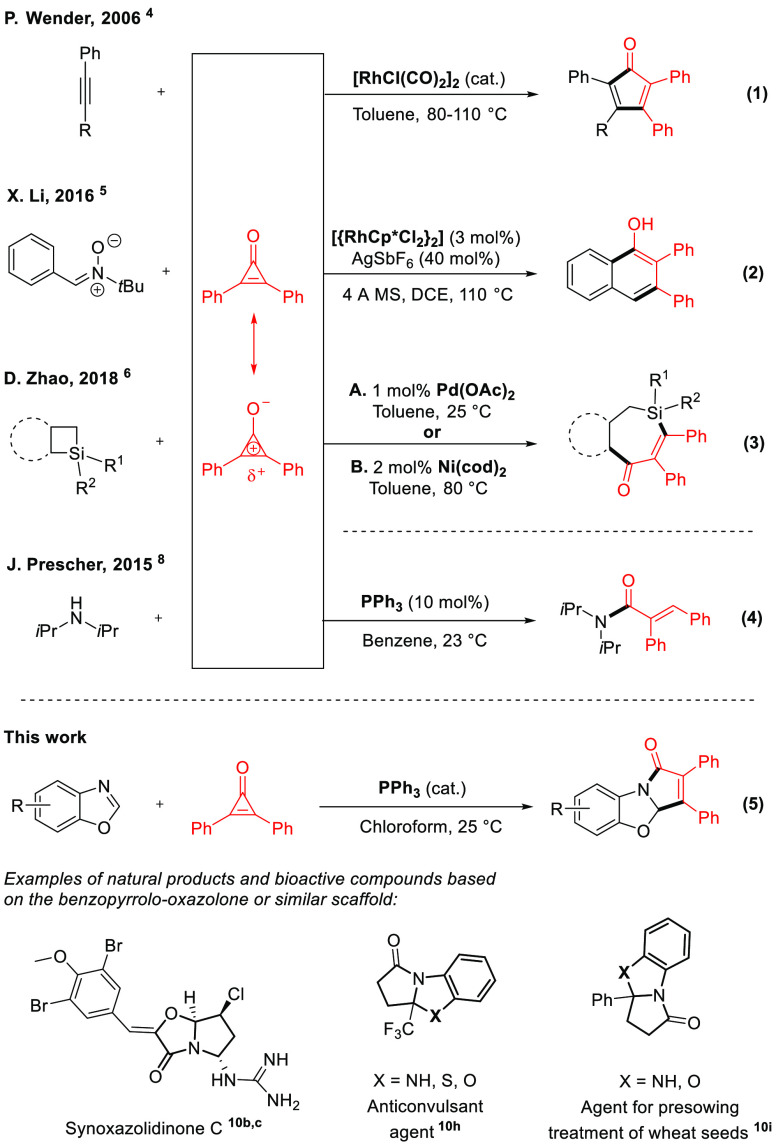
“[···] The cyclopropenone system must have strong resonance stabilization indeed to compensate for its high angle strain.” So did Breslow and his team express their surprise at the unexpected relative stability of 1,2-diphenylcyclopropenone (1959).1
The activation of C–C bonds is a powerful concept for the reorganization or coupling of organic scaffolds, yet it is a relatively challenging process to achieve in the context of synthetic methodology because of their inherent stability.2 In order to enable such methods, one can use C–C-strained, often cyclic, building blocks that are consequently spring loaded for C–C bond activation.3 In this context, 1,2-diphenylcyclopropenone, a particularly strained cyclic substance known since the late 1950s,1 is currently witnessing a spectacular rebirth in the context of synthetic method developments that rely on C–C bond activation. Even though its highly strained structure makes it an ideal building block for C–C bond activation, it usually still requires a precious metal salt as catalyst.4−7 Because 1,2-diphenylcyclopropenone is a particularly versatile building block for organic coupling reactions, yielding both open and (poly)cyclic complex skeletons (Scheme 1, eqs 1–4), its activation with more trivial and less onerous (organo)catalysts would constitute an important objective for rendering such methods sustainable and practical.8 We propose herein such a method with the simple triphenylphosphine-catalyzed9 dearomative [3 + 2] cycloaddition of benzoxazoles with 1,2-diphenylcyclopropenone.
Scheme 1. Selected Couplings with Cyclopropenones.
Prescher and co-workers recently utilized a triphenylphosphine organocatalyst in order to elegantly ring open 1,2-diphenylcyclopropenone with amines (Scheme 1, eq 4).8 We therefore reasoned that other highly important coupling partners, such as benzoxazoles, might intercept the cyclopropenone ring opening under simple phosphine catalysis, leading to unprecedented fused poly-heterocyclic rings.
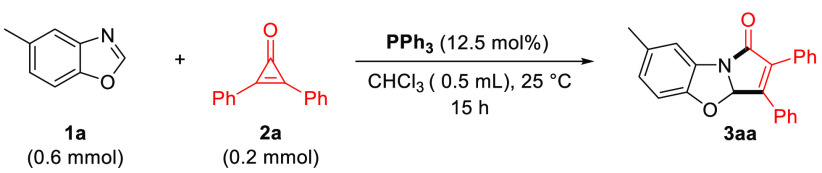
We commenced our study by engaging 1,2-diphenylcyclopropenone 2a in the presence of an excess of test substrate benzoxazole 1a and triphenylphosphine (PPh3, 12.5 mol %, 1:8 ratio) in chloroform at 25 °C for 15 h. This afforded a new dearomatized polycyclic substance 3aa in impressive 96% isolated yield (Table 1, entry 1). This particular scaffold, a benzopyrrolo-oxazolone, is relevant, as similar structures are found at the core of several bioactive substances of interest (Scheme 1).10 Its direct synthesis from trivial building blocks such as presented here would therefore represent a significant advancement for the field. No conversion was observed in the absence of the PPh3 catalyst (Table 1, entry 2) nor with bulkier phosphines such as BINAP (Table 1, entry 3). This is an important result because PPh3 is by far the cheapest triarylphosphine available. No other solvents performed any better than chloroform (entries 4–7), nor any other relative ratio between the coupling partners (entries 8–10).
Table 1. Optimization Tablea.
| entry | variations from standard conditionsa | yield (%)b of 3aa |
|---|---|---|
| 1 | none | 97 (96)c |
| 2 | without PPh3 | NR |
| 3 | BINAP instead of PPh3 | NR |
| 4 | toluene instead of CHCl3 | 60 |
| 5 | DCM instead of CHCl3 | 78 |
| 6 | EtOAc instead of CHCl3 | 64 |
| 7 | CDCl3 instead of CHCl3 | 93 |
| 8 | 1 equiv of 1a | 46 |
| 9 | 2 equiv of 1a | 82 |
| 10 | 0.2 mmol 1a and 2 equiv of 2a | 20 |
Unless otherwise noted, the standard reaction conditions were as follows: 1a (0.6 mmol), 2a (0.2 mmol), solvent (0.5 mL).
The yield was determined by 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethoxybenzene as an internal standard.
Isolated yield.
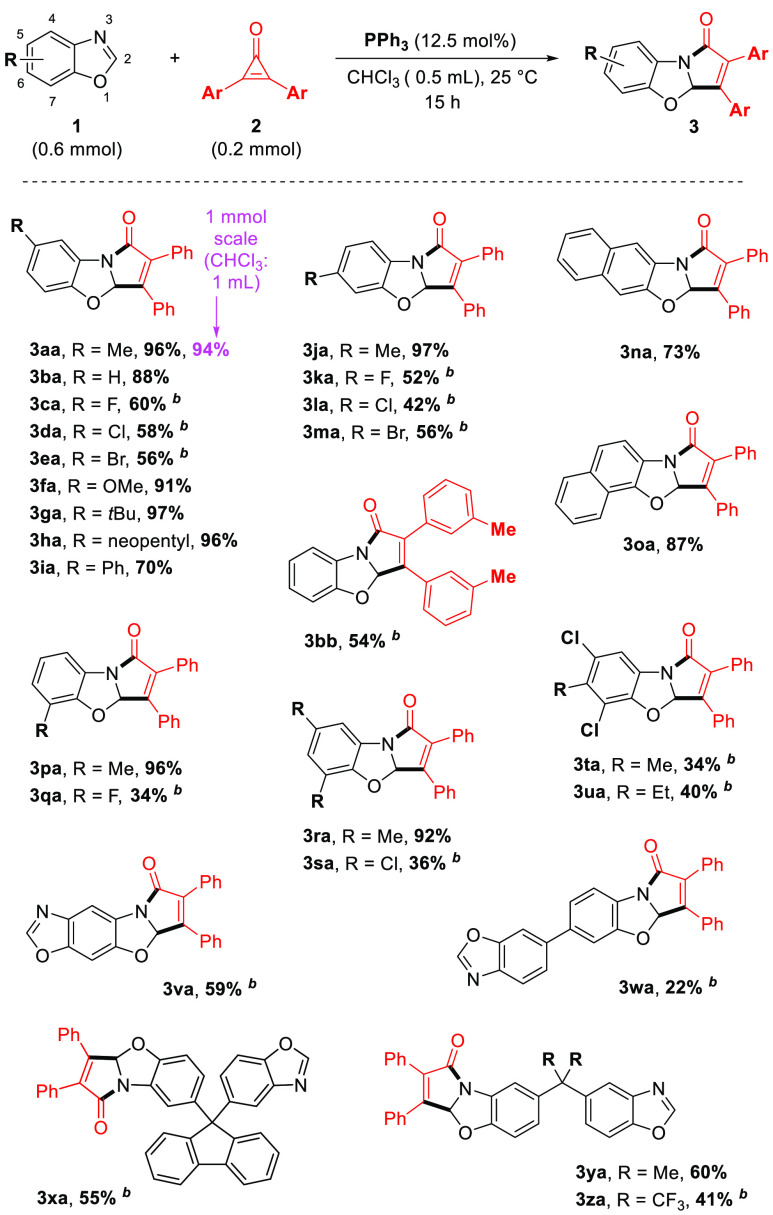
With these simple reaction conditions in hand, we then investigated the reaction scope with various benzoxazoles (Scheme 2). First, we tested C5-substituted benzoxazole substrates. Electron-neutral (3ba) and electron-donating (3aa, 3fa, 3ga, 3ha) functional groups afforded the corresponding benzopyrrolo-oxazolone coupling products in excellent yields (88–97%). Although electron-withdrawing substituents performed somewhat less well at 25 °C (3ca–3ea), increasing the reaction temperature to 70 °C afforded promising yields (56–60%). Next, C6-substitution was also explored (3ja–3ma), as well as C7 (3pa, 3qa) with promising to excellent yields. Di- and trisubstituted benzoxazole structures (3na, 3oa, 3ra–3ua) as well as bulky C4-substitutents were likewise well tolerated (3ga, 3ha), with 97 and 96% yields, respectively. Interestingly, even fused or alternatively tethered dibenzoxazole substrates were found applicable, yielding the corresponding single coupling cycloaddition products (3va–3za) in 22–60% yields. Moreover, the 1,2-diphenylcyclopropen-3-one 2a could be replaced with a different cyclopropenone 2b (product 3bb).
Scheme 2. Scope, Isolated Yields.
All reactions were carried out on a 0.2 mmol scale for 15 h under the standard conditions.
The reaction was carried out at 70 °C.
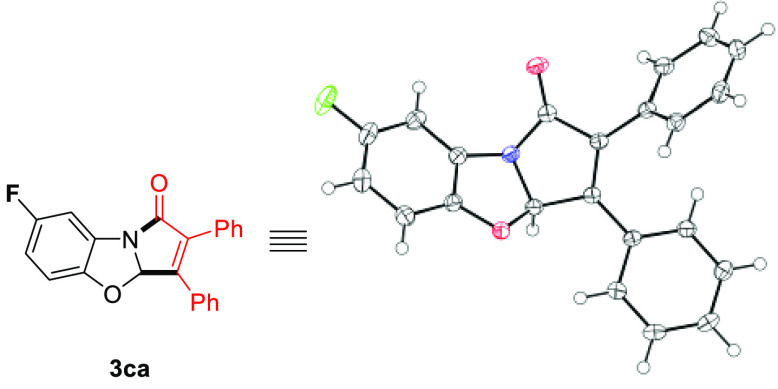
In order to demonstrate the practicality of our reaction, a 1 mmol scale batch was conducted for product 3aa. This product was thus obtained in remarkably preserved 94% isolated yield (320 mg) in moreover only 1 mL of chloroform. In addition, the X-ray diffraction analysis of product 3ca confirmed the structural interpretation, in particular its fused cyclic nature (Figure 1).
Figure 1.
X-ray structure of product 3ca (CCDC: 2093753), ORTEP view,11 50% probability level.
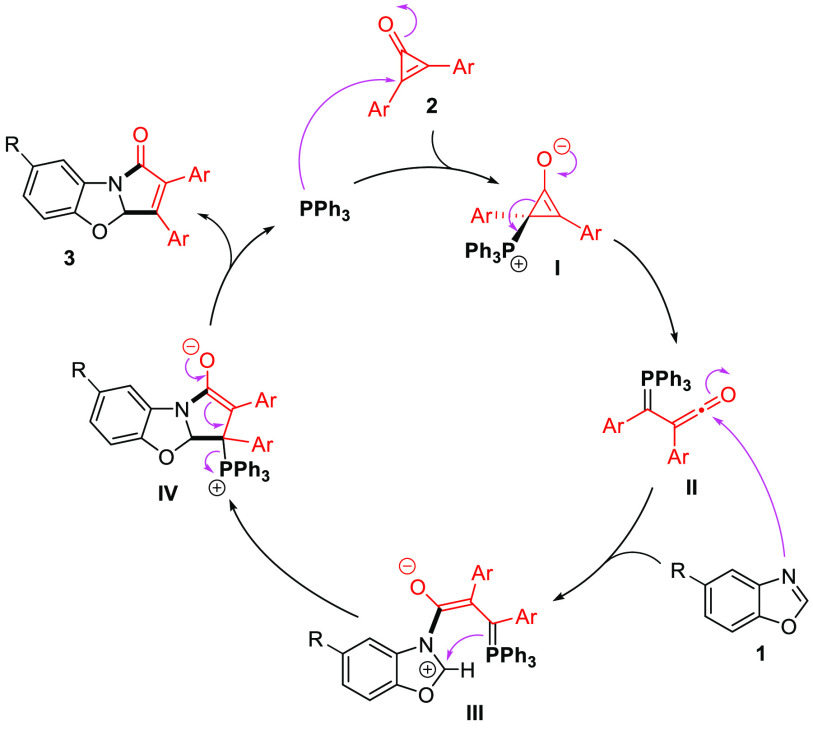
Based on some literature precedents,12 we assume that the phosphine organocatalyst activates the strained and electrophilic cyclopropenone to form zwitterionic intermediate I, which would then progress to ketene ylide intermediate II (Scheme 3). The latter species would then undergo a nucleophilic dearomative attack from the benzoxazole coupling partner to generate intermediate III. This would rapidly cyclize to form the second C–C bond toward intermediate IV. Phosphine elimination would then regenerate the organocatalyst, releasing coupling product 3.
Scheme 3. Proposed Mechanism.
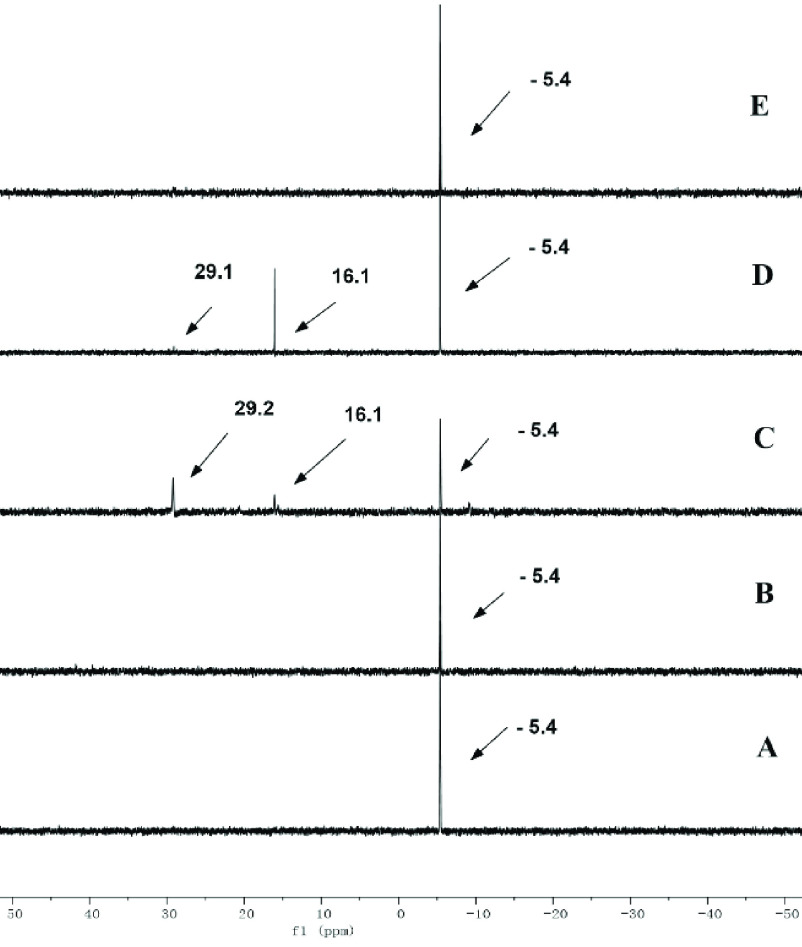
In order to further investigate this mechanism, we then performed some key 31P NMR experiments (Figure 2). Experiment A shows that the 31P NMR signal of PPh3 shifts at −5.4 ppm in CDCl3, a solvent which we know accommodates the reaction well (Table 1, entry 7). The addition of benzoxazole 1a does not alter this signal, even in large excess (24 equiv, experiment B). However, the addition of strained electrophilic cyclopropenone 2a (8 equiv) leads to the appearance of two new signals at +16.1 and +29.2 ppm, presumably corresponding to two new species (experiment C). One or both might correspond to intermediates I and/or II, as the observed chemical shifts are compatible. If one adds to this 24 equiv of benzoxazole 1a, the signal at +29.1 ppm disappears (experiment D), demonstrating that this particular species is probably a productive intermediate of the reaction. If one stirs this mixture for another 15 h, only the PPh3 signal remains (−5.4 ppm, experiment E), thus demonstrating the intermediacy of the noted signals in experiments C and D as well as the catalytic role of the phosphine.
Figure 2.
Comparison of the 31P NMR spectra of (A) only PPh3 in CDCl3; (B) PPh3 and 1a (1:24); (C) PPh3 and 2a (1:8); (D) PPh3, 1a, and 2a (1:24:8); (E) PPh3,1a and 2a (1:24:8) after the mixture was stirred for 15 h.
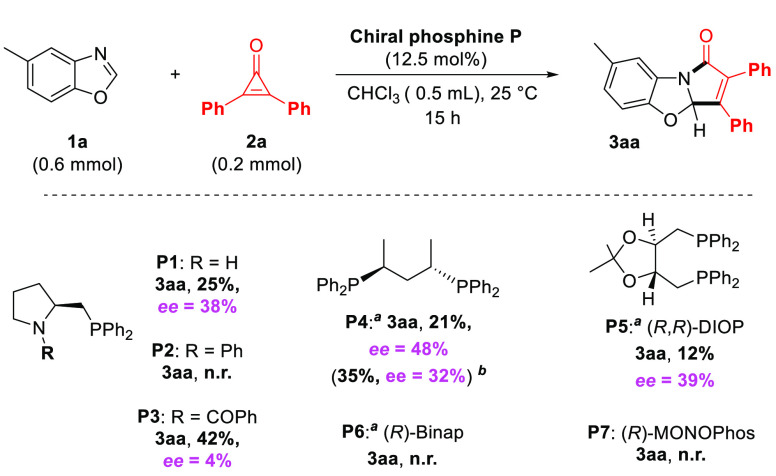
Finally, because of the envisaged mechanism involving a very rigid and covalent proximity of the catalyst to the reaction sites in intermediates II and III (Scheme 3), it occurred to us that an optically active phosphine might render the reaction enantioselective.13 In order to explore this possibility, we screened a series of commercially available chiral and optically active phosphines (phosphines P1–P7, Scheme 4). Unfortunately, none performed with an enantiomeric excess above 48% for product 3aa (chiral phosphine P4) in moreover moderate yields. While we could not improve these results so far, these at least demonstrate the feasibility of an enantioselective version of this organocatalyzed synthetic method. We are currently designing and synthetizing new chiral phosphines in order to achieve this objective.
Scheme 4. Action of Optically Active Phosphine Catalysts.
For the diphosphines, a catalytic loading of 6.25 mol % was utilized, thus giving a 1:16 ratio versus substrate 2a.
Control run under strict argon atmosphere.
In conclusion, we have developed a triphenylphosphine organocatalyzed dearomative [3 + 2] cycloaddition of benzoxazoles with 1,2-diphenylcyclopropenone. The cyclic and fused nature of the coupling product was confirmed by X-ray crystallography. Moreover, a mechanistic investigation was conducted with 31P NMR, leading to important insights regarding the existence of phosphorus based catalytic intermediates. This contribution should encourage the further development of organocatalyzed C–C bond activation coupling methods.
Acknowledgments
ERC project 716136:2O2ACTIVATION is acknowledged for generous financial support. We are also thankful to the Chinese Scholarship Council (CSC) for financial support to Xingben Wang (No. 202008330337).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c04045.
Experimental procedures, characterization, and NMR spectra of new compounds (PDF)
Accession Codes
CCDC 2093753 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- a Breslow R.; Haynie R.; Mirra J. The synthesis of diphenyl-cyclopropenone. J. Am. Chem. Soc. 1959, 81, 247. 10.1021/ja01510a060. [DOI] [Google Scholar]; b Breslow R.; Eicher T.; Krebs A.; Peterson R. A.; Posner J. Diphenylcyclopropenone. J. Am. Chem. Soc. 1965, 87, 1320. 10.1021/ja01084a029. [DOI] [Google Scholar]
- C–C Bond Activation; Dong G., Ed.; Springer: Berlin, 2014; Vol. 346, pp 1 – 258. [Google Scholar]
- a Jones W. D. The fall of the C-C bond. Nature 1993, 364, 676. 10.1038/364676a0. [DOI] [Google Scholar]; b Rybtchinski B.; Milstein D. Metal Insertion into C-C Bonds in Solution. Angew. Chem., Int. Ed. 1999, 38, 870.. [DOI] [PubMed] [Google Scholar]; c Jun C.-H. Transition metal-catalyzed carbon–carbon bond activation. Chem. Soc. Rev. 2004, 33, 610. 10.1039/B308864M. [DOI] [PubMed] [Google Scholar]; d Seiser T.; Cramer N. Enantioselective metal-catalyzed activation of strained rings. Org. Biomol. Chem. 2009, 7, 2835. 10.1039/b904405a. [DOI] [PubMed] [Google Scholar]; e Aissa C. Transition-Metal-Catalyzed Rearrangements of Small Cycloalkanes: Regioselectivity Trends in β-Carbon Elimination Reactions. Synthesis 2011, 2011, 3389. 10.1055/s-0030-1260233. [DOI] [Google Scholar]; f Cramer N.; Seiser T. β-Carbon Elimination from Cyclobutanols: A Clean Access to Alkylrhodium Intermediates Bearing a Quaternary Stereogenic Center. Synlett 2011, 2011, 449. 10.1055/s-0030-1259536. [DOI] [Google Scholar]; g Murakami M.; Matsuda T. Metal-catalysed cleavage of carbon–carbon bonds. Chem. Commun. 2011, 47, 1100. 10.1039/C0CC02566F. [DOI] [PubMed] [Google Scholar]; h Korotvicka A.; Necas D.; Kotora M. Rhodium-catalyzed C-C Bond Cleavage Reactions - An Update. Curr. Org. Chem. 2012, 16, 1170. 10.2174/138527212800564213. [DOI] [Google Scholar]; i Dermenci A.; Coe J. W.; Dong G. Direct activation of relatively unstrained carbon–carbon bonds in homogeneous systems. Org. Chem. Front. 2014, 1, 567. 10.1039/C4QO00053F. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Marek I.; Masarwa A.; Delaye P. O.; Leibeling M. Selective Carbon–Carbon Bond Cleavage for the Stereoselective Synthesis of Acyclic Systems. Angew. Chem., Int. Ed. 2015, 54, 414. 10.1002/anie.201405067. [DOI] [PubMed] [Google Scholar]; k Souillart L.; Cramer N. Catalytic C–C Bond Activations via Oxidative Addition to Transition Metals. Chem. Rev. 2015, 115, 9410. 10.1021/acs.chemrev.5b00138. [DOI] [PubMed] [Google Scholar]; l Murakami M.; Ishida N. Potential of Metal-Catalyzed C–C Single Bond Cleavage for Organic Synthesis. J. Am. Chem. Soc. 2016, 138, 13759. 10.1021/jacs.6b01656. [DOI] [PubMed] [Google Scholar]; m Kim D.-S.; Park W.-J.; Jun C.-H. Metal–Organic Cooperative Catalysis in C–H and C–C Bond Activation. Chem. Rev. 2017, 117, 8977. 10.1021/acs.chemrev.6b00554. [DOI] [PubMed] [Google Scholar]; n Chen P.-h.; Billett B. A.; Tsukamoto T.; Dong G. “Cut and Sew” Transformations via Transition-Metal-Catalyzed Carbon–Carbon Bond Activation. ACS Catal. 2017, 7, 1340. 10.1021/acscatal.6b03210. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Zheng Q.-Z.; Jiao N. Ag-catalyzed C–H/C–C bond functionalization. Chem. Soc. Rev. 2016, 45, 4590. 10.1039/C6CS00107F. [DOI] [PubMed] [Google Scholar]; p Zhao B.; Rogge T.; Ackermann L.; Shi Z. Metal-catalysed C–Het (F, O, S, N) and C–C bond arylation. Chem. Soc. Rev. 2021, 50, 8903. 10.1039/C9CS00571D. [DOI] [PubMed] [Google Scholar]; q Vicente R. C–C Bond Cleavages of Cyclopropenes: Operating for Selective Ring-Opening Reactions. Chem. Rev. 2021, 121, 162. 10.1021/acs.chemrev.0c00151. [DOI] [PubMed] [Google Scholar]; r Wang J.; Blaszczyk S. A.; Li X.; Tang W. Transition Metal-Catalyzed Selective Carbon–Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions. Chem. Rev. 2021, 121, 110. 10.1021/acs.chemrev.0c00160. [DOI] [PMC free article] [PubMed] [Google Scholar]; s Murakami M.; Ishida N. Cleavage of Carbon–Carbon σ-Bonds of Four-Membered Rings. Chem. Rev. 2021, 121, 264. 10.1021/acs.chemrev.0c00569. [DOI] [PubMed] [Google Scholar]
- Wender P. A.; Paxton T. J.; Williams T. J. Cyclopentadienone Synthesis by Rhodium(I)-Catalyzed [3 + 2] Cycloaddition Reactions of Cyclopropenones and Alkynes. J. Am. Chem. Soc. 2006, 128, 14814. 10.1021/ja065868p. [DOI] [PubMed] [Google Scholar]
- Xie F.; Yu S.; Qi Z.; Li X. Nitrone Directing Groups in Rhodium(III)-Catalyzed C–H Activation of Arenes: 1,3-Dipoles versus Traceless Directing Groups. Angew. Chem., Int. Ed. 2016, 55, 15351. 10.1002/anie.201609658. [DOI] [PubMed] [Google Scholar]
- Zhao W.-T.; Gao F.; Zhao D. Intermolecular σ-Bond Cross-Exchange Reaction between Cyclopropenones and (Benzo)silacyclobutanes: Straightforward Access towards Sila(benzo)cycloheptenones. Angew. Chem., Int. Ed. 2018, 57, 6329. 10.1002/anie.201803156. [DOI] [PubMed] [Google Scholar]
- For some related references, see:; a Yu S.; Li X. Mild Synthesis of Chalcones via Rhodium(III)-Catalyzed C–C Coupling of Arenes and Cyclopropenones. Org. Lett. 2014, 16, 1220. 10.1021/ol500140e. [DOI] [PubMed] [Google Scholar]; b Augustin A. U.; Sensse M.; Jones P. G.; Werz D. B. Stereospecific Reactions of Donor-Acceptor Cyclopropanes with Thioketones: Access to Highly Substituted Tetrahydrothiophenes. Angew. Chem., Int. Ed. 2017, 56, 14293. 10.1002/anie.201708346. [DOI] [PubMed] [Google Scholar]; c Kong L.; Zhou X.; Xu Y.; Li X. Rhodium(III)-Catalyzed Acylation of C(sp3)–H Bonds with Cyclopropenones. Org. Lett. 2017, 19, 3644. 10.1021/acs.orglett.7b01650. [DOI] [PubMed] [Google Scholar]; d Xu J.; Cao J.; Fang C.; Lu T.; Du D. Organocatalytic C–C bond activation of cyclopropenones for ring-opening formal [3 + 2] cycloaddition with isatins. Org. Chem. Front. 2017, 4, 560. 10.1039/C6QO00734A. [DOI] [Google Scholar]; e Row R. D.; Prescher J. A. A Cyclopropenethione-Phosphine Ligation for Rapid Biomolecule Labeling. Org. Lett. 2018, 20, 5614. 10.1021/acs.orglett.8b02296. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Ren J.-T.; Wang J.-X.; Tian H.; Xu J.-L.; Hu H.; Aslam M.; Sun M. Ag(I)-Catalyzed [3 + 2]-Annulation of Cyclopropenones and Formamides via C–C Bond Cleavage. Org. Lett. 2018, 20, 6636. 10.1021/acs.orglett.8b02612. [DOI] [PubMed] [Google Scholar]; g Niu B.; Jiang B.; Yua L.-Z.; Shi M. Base-promoted [3 + 3] cyclization of cyclopropenones and cyclopropenethiones with amides for the synthesis of 6H-1,3-oxazin-6-ones and 6H-1,3-thiazin-6-ones. Org. Chem. Front. 2018, 5, 1267. 10.1039/C8QO00091C. [DOI] [Google Scholar]; h Cao J.; Fang R.; Liu J.-Y.; Lu H.; Luo Y.-C.; Xu P.-F. Organocatalytic Regiodivergent C–C Bond Cleavage of Cyclopropenones: A Highly Efficient Cascade Approach to Enantiopure Heterocyclic Frameworks. Chem.—Eur. J. 2018, 24, 18863. 10.1002/chem.201803861. [DOI] [PubMed] [Google Scholar]; i Shan L.; Wu G.; Liu M.; Gao W.; Ding J.; Huang X.; Wu H. α,β-Diaryl unsaturated ketones via palladium-catalyzed ring-opening of cyclopropenones with organoboronic acids. Org. Chem. Front. 2018, 5, 1651. 10.1039/C8QO00241J. [DOI] [Google Scholar]; j Heiss T. K.; Prescher J. A. Cyclopropeniminium Ions Exhibit Unique Reactivity Profiles with Bioorthogonal Phosphines. J. Org. Chem. 2019, 84, 7443. 10.1021/acs.joc.9b00518. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Wang X.; Seidel F. W.; Nozaki K. Synthesis of Polyethylene with In-Chain α,β-Unsaturated Ketone and Isolated Ketone Units: Pd-Catalyzed Ring-Opening Copolymerization of Cyclopropenone with Ethylene. Angew. Chem., Int. Ed. 2019, 58, 12955. 10.1002/anie.201906990. [DOI] [PubMed] [Google Scholar]; l Liu Y.; Tian Y.; Su K.; Wang P.; Guo X.; Chen B. Rhodium(iii)-catalyzed [3 + 3] annulation reactions of N-nitrosoanilines and cyclopropenones: an approach to functionalized 4-quinolones. Org. Chem. Front. 2019, 6, 3973. 10.1039/C9QO01250H. [DOI] [Google Scholar]; m Pleschka D.; Uebing M.; Lange M.; Hepp A.; Webker A.-L.; Hansen M. R.; Werthwein E.-U.; Uhl W. Al/P- and Ga/P-Based Frustrated Lewis Pairs and Electronically Unsaturated Substrates: Ring Cleavage and Ring Closure, C–C and C–N Bond Formation. Chem.—Eur. J. 2019, 25, 9315. 10.1002/chem.201901659. [DOI] [PubMed] [Google Scholar]; n Wu J.; Gao W.-X.; Huang X.-B.; Zhou Y.-B.; Liu M.-C.; Wu H.-Y. Selective [3 + 2] Cycloaddition of Cyclopropenone Derivatives and Elemental Chalcogens. Org. Lett. 2020, 22, 5555. 10.1021/acs.orglett.0c01914. [DOI] [PubMed] [Google Scholar]; o Xu J.-L.; Tian H.; Kang J.-H.; Kang W.-X.; Sun W.; Sun R.; Li Y.-M.; Sun M. Ag(I)-Catalyzed Addition of Cyclopropenones and Nitrones to Access Imides. Org. Lett. 2020, 22, 6739. 10.1021/acs.orglett.0c02099. [DOI] [PubMed] [Google Scholar]; p Nanda T.; Ravikumar P. C. A Palladium-Catalyzed Cascade C–C Activation of Cyclopropenone and Carbonylative Amination: Easy Access to Highly Functionalized Maleimide Derivatives. Org. Lett. 2020, 22, 1368. 10.1021/acs.orglett.9b04656. [DOI] [PubMed] [Google Scholar]; q Bai D.; Yu Y.; Guo H.; Chang J.; Li X. Nickel(0)-Catalyzed Enantioselective [3 + 2] Annulation of Cyclopropenones and α,β-Unsaturated Ketones/Imines. Angew. Chem., Int. Ed. 2020, 59, 2740. 10.1002/anie.201913130. [DOI] [PubMed] [Google Scholar]; r Xing H.; Chen J.; Shi Y.; Huang T.; Hai L.; Wu Y. Synthesis of 4-ethenyl quinazolines via rhodium(iii)-catalyzed [5 + 1] annulation reaction of N-arylamidines with cyclopropenones. Org. Chem. Front. 2020, 7, 672. 10.1039/C9QO01372E. [DOI] [Google Scholar]; s Chen J.; Tang B.; Liu X.; Lv G.; Shi Y.; Huang T.; Xing H.; Guo X.; Hai L.; Wu Y. Ruthenium(ii)-catalyzed [5 + 1] annulation reaction: a facile and efficient approach to construct 6-ethenyl phenanthridines utilizing a primary amine as a directing group. Org. Chem. Front. 2020, 7, 2944. 10.1039/D0QO00769B. [DOI] [Google Scholar]; t Yao L.; Hu Q.; Lei Y.; Bao L.; Hu Y. C–O/C–S difunctionalized benzene derivatives via multicomponent coupling of tetraynes. Org. Chem. Front. 2020, 7, 3633. 10.1039/D0QO00967A. [DOI] [Google Scholar]; u Yao L.; Hu Q.; Bao L.; Zhu W.; Hu Y. Fully Substituted Conjugate Benzofuran Core: Multiyne Cascade Coupling and Oxidation of Cyclopropenone. Org. Lett. 2021, 23, 4971. 10.1021/acs.orglett.1c01304. [DOI] [PubMed] [Google Scholar]; v Chen Q.; Teng Y.; Xu F. Lanthanide Silylamide-Catalyzed Synthesis of Pyrano[2,3-b]indol-2-ones. Org. Lett. 2021, 23, 4785. 10.1021/acs.orglett.1c01506. [DOI] [PubMed] [Google Scholar]; w Liu B.; Yang L.; Dong Z.; Chang J.; Li X. Rh(III)-Catalyzed Annulation of 2-Biphenylboronic Acid with Diverse Activated Alkenes. Org. Lett. 2021, 23, 7199. 10.1021/acs.orglett.1c02597. [DOI] [PubMed] [Google Scholar]; x Jiang Z.; Niu S.-L.; Zeng Q.; Ouyang Q.; Chen Y.-C.; Xiao Q. Selective Alkynylallylation of the C–C σ Bond of Cyclopropenes. Angew. Chem., Int. Ed. 2021, 60, 297. 10.1002/anie.202008886. [DOI] [PubMed] [Google Scholar]; y Zhu W.-Q.; Fang Y.-C.; Han W.-Y.; Li F.; Yang M.-G.; Chen Y.-Z. Palladium-catalyzed [2 + 2 + 1] annulation: access to chromone fused cyclopentanones with cyclopropenone as the CO source. Org. Chem. Front. 2021, 8, 3082. 10.1039/D1QO00222H. [DOI] [Google Scholar]; z Bai D.; Liu S.; Chen J.; Yu Y.; Wang M.; Chang J.; Lan Y.; Li X. Mechanistic studies on nickel-catalyzed enantioselective [3 + 2] annulation for γ-butenolide synthesis via C–C activation of diarylcyclopropenones. Org. Chem. Front. 2021, 8, 3023. 10.1039/D1QO00322D. [DOI] [Google Scholar]
- Shih H.-W.; Prescher J. A. A Bioorthogonal Ligation of Cyclopropenones Mediated by Triarylphosphines. J. Am. Chem. Soc. 2015, 137, 10036. 10.1021/jacs.5b06969. [DOI] [PubMed] [Google Scholar]
- Phosphine organocatalysis, selected references:; a Denmark S. E.; Beutner G. L. Lewis Base Catalysis in Organic Synthesis. Angew. Chem., Int. Ed. 2008, 47, 1560. 10.1002/anie.200604943. [DOI] [PubMed] [Google Scholar]; b Ye L.-W.; Zhou J.; Tang Y. Phosphine-triggered synthesis of functionalized cyclic compounds. Chem. Soc. Rev. 2008, 37, 1140. 10.1039/b717758e. [DOI] [PubMed] [Google Scholar]; c Guo H.; Fan Y. C.; Sun Z.; Wu Y.; Kwon O. Phosphine Organocatalysis. Chem. Rev. 2018, 118, 10049. 10.1021/acs.chemrev.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Huang Y.; Liao J.; Wang W.; Liu H.; Guo H. Synthesis of heterocyclic compounds through nucleophilic phosphine catalysis. Chem. Commun. 2020, 56, 15235. 10.1039/D0CC05699E. [DOI] [PubMed] [Google Scholar]; e Xie C.; Smaligo A. J.; Song X.-R.; Kwon O. Phosphorus-Based Catalysis. ACS Cent. Sci. 2021, 7, 536. 10.1021/acscentsci.0c01493. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Khong S.; Venkatesh T.; Kwon O. Nucleophilic Phosphine Catalysis: The Untold Story. Asian J. Org. Chem. 2021, 10, 2699. 10.1002/ajoc.202100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kumar D.; Jacob M. R.; Reynolds M. B.; Kerwin S. M. Synthesis and Evaluation of Anticancer Benzoxazoles and Benzimidazoles Related to UK-1. Bioorg. Med. Chem. 2002, 10, 3997. 10.1016/S0968-0896(02)00327-9. [DOI] [PubMed] [Google Scholar]; b Trepos R.; Cervin G.; Hellio C.; Pavia H.; Stensen W.; Stensvag K.; Svendsen J. S.; Haug T.; Svenson J. Antifouling Compounds from the Sub-Arctic Ascidian Synoicum pulmonaria: Synoxazolidinones A and C, Pulmonarins A and B, and Synthetic Analogues. J. Nat. Prod. 2014, 77, 2105. 10.1021/np5005032. [DOI] [PubMed] [Google Scholar]; c Tadesse M.; Svenson J.; Jaspars M.; Strøm M. B.; Abdelrahman M. H.; Andersen J. H.; Hansen E.; Kristiansen P. E.; Stensvåg K.; Haug T. Synoxazolidinone C; a bicyclic member of the synoxazolidinone family with antibacterial and anticancer activities. Tetrahedron Lett. 2011, 52, 1804. 10.1016/j.tetlet.2011.02.027. [DOI] [Google Scholar]; d Sheehan D. J.; Hitchcock C. A.; Sibley C. M. Current and Emerging Azole Antifungal Agents. Clin. Microbiol. Rev. 1999, 12, 40. 10.1128/CMR.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Noel S.; Cadet S.; Gras E.; Hureau C. The benzazole scaffold: a SWAT to combat Alzheimer’s disease. Chem. Soc. Rev. 2013, 42, 7747. 10.1039/c3cs60086f. [DOI] [PubMed] [Google Scholar]; f Cherblanc F. L.; Chapman K. L.; Reid J.; Borg A. J.; Sundriyal S.; Fuoli L. A.; Bignell E.; Demetriades M.; Schofield C. J.; DiMaggio P. A.; Brown R.; Fuchter M. J. On the Histone Lysine Methyltransferase Activity of Fungal Metabolite Chaetocin. J. Med. Chem. 2013, 56, 8616. 10.1021/jm401063r. [DOI] [PubMed] [Google Scholar]; g Mertens A.; Zilch H.; Kónig B.; Scháfer W.; Poll T.; Kampe W.; Seidel H.; Leser U.; Leinert H. Selective Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors. New 2,3-Dihydrothiazolo[2,3-a]isoindol-5(9bH)-ones and Related Compounds with Anti-HIV-1 Activity. J. Med. Chem. 1993, 36, 2526. 10.1021/jm00069a011. [DOI] [PubMed] [Google Scholar]; h Mao H.; Wan W.; Jiang H.; Hao J.. CN102766145 A, 2012.; i Egorova A. Y.; Grinev V. S.; Lyubun E. V.. RU2468580 C2, 2012.
- Farrugia L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849. 10.1107/S0021889812029111. [DOI] [Google Scholar]
- a Hamada A.; Takizawa T. Synthesis of phosphorane having ketene group at α-position. Tetrahedron Lett. 1972, 13, 1849. 10.1016/S0040-4039(01)85286-2. [DOI] [Google Scholar]; b Cunha S.; Kascheres A. On the Reactivity of α-(Triphenylphosphoranylidene)-benzylphenylketene with Nitrogen Compounds: Synthetic and Mechanistic Implications. J. Braz. Chem. Soc. 2002, 13, 687. 10.1590/S0103-50532002000500025. [DOI] [Google Scholar]; c Xie P.; Huang Y. Morita–Baylis–Hillman adduct derivatives (MBHADs): versatile reactivity in Lewis base-promoted annulation. Org. Biomol. Chem. 2015, 13, 8578. 10.1039/C5OB00865D. [DOI] [PubMed] [Google Scholar]; d Nguyen S. S.; Ferreira A. J.; Long Z. G.; Heiss T. K.; Dorn R. S.; Row R. D.; Prescher J. A. Butenolide Synthesis from Functionalized Cyclopropenones. Org. Lett. 2019, 21, 8695. 10.1021/acs.orglett.9b03298. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Li E.-Q.; Huang Y. Recent advances in phosphine catalysis involving γ-substituted allenoates. Chem. Commun. 2020, 56, 680. 10.1039/C9CC08241G. [DOI] [PubMed] [Google Scholar]
- Selected references:; a Chung Y. K.; Fu G. C. Phosphine-Catalyzed Enantioselective Synthesis of Oxygen Heterocycles. Angew. Chem., Int. Ed. 2009, 48, 2225. 10.1002/anie.200805377. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Li W.; Zhang J. Recent developments in the synthesis and utilization of chiral β-aminophosphine derivatives as catalysts or ligands. Chem. Soc. Rev. 2016, 45, 1657. 10.1039/C5CS00469A. [DOI] [PubMed] [Google Scholar]; c Dutartre M.; Bayardon J.; Jugé S. Applications and stereoselective syntheses of P-chirogenic phosphorus compounds. Chem. Soc. Rev. 2016, 45, 5771. 10.1039/C6CS00031B. [DOI] [PubMed] [Google Scholar]; d Han B.; He X.-H.; Liu Y.-Q.; He G.; Peng C.; Li J.-L. Asymmetric organocatalysis: an enabling technology for medicinal chemistry. Chem. Soc. Rev. 2021, 50, 1522. 10.1039/D0CS00196A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.