Abstract
Mice deficient in the antioxidant enzyme Cu/Zn-superoxide dismutase (Sod1−/− or Sod1KO mice) have increased oxidative stress, show accelerated aging and develop spontaneous hepatocellular carcinoma with age (HCC). Similar to humans, HCC development in Sod1KO mice progresses from non-alcoholic fatty liver disease (NAFLD) to non-alcoholic steatohepatitis (NASH) with fibrosis, which eventually progresses to HCC. Oxidative stress plays a role in NAFLD to NASH progression, and liver inflammation is the main mechanism that drives the disease progression from NASH to fibrosis. Because necroptosis is a major source of inflammation, we tested the hypothesis that increased necroptosis in the liver plays a role in increased inflammation and fibrosis in Sod1KO mice. Phosphorylation of MLKL (P- MLKL), a well-accepted marker of necroptosis, and expression of MLKL protein were significantly increased in the livers of Sod1KO mice compared to wild type (WT) mice indicating increased necroptosis. Similarly, phosphorylation of RIPK3 and RIPK3 protein levels were also significantly increased. Markers of pro-inflammatory M1 macrophages, NLRP3 inflammasome, and transcript levels of pro-inflammatory cytokines and chemokines, e.g., TNFα, IL-6, IL-1β, and Ccl2 that are associated with human NASH, were significantly increased. Expression of antioxidant enzymes and heat shock proteins, and markers of fibrosis and oncogenic transcription factor STAT3 were also upregulated and autophagy was downregulated in the livers of Sod1KO mice. Short term treatment of Sod1KO mice with necrostatin-1s (Nec-1s), a necroptosis inhibitor, reversed these conditions. Our data show for the first time that necroptosis-mediated inflammation contributes to fibrosis in a mouse model of increased oxidative stress and accelerated aging, that also exhibits progressive HCC development.
Keywords: Necroptosis, Inflammation, Hepatocellular Carcinoma, Fibrosis, Cu/Zn Superoxide Dismutase, Oxidative Stress, Necrostatin-1s, Non-alcoholic hepatosteatosis, Non-alcoholic fatty liver disease, Autophagy
Graphical Abstract
Introduction
Chronic liver disease (CLD) is a major health problem affecting over 800 million people worldwide with an estimated mortality rate of 2 million deaths per year [1,2]. CLD involves a spectrum of diseases ranging from viral infection (hepatitis B and C) to alcohol induced CLD and metabolic CLD such as non- alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and fibrosis. Due to the obesity epidemic in recent years, the increased incidence of NAFLD affecting nearly 25% of the world population has become one of the leading risk factors for CLD-associated mortality [3]. NAFLD arising from obesity can eventually progress to non-alcoholic steatohepatitis (NASH), a condition characterized by increased steatosis, liver inflammation and hepatocellular damage. Nearly one-third of patients with NASH develop advanced fibrosis or cirrhosis that can progress to hepatocellular carcinoma (HCC) [4,5], which is the fastest-growing cause of cancer-related deaths in the United States and world [6]. ‘Two-hit hypothesis’ was proposed to describe the evolution of inflammation in NAFLD: the first hit is hepatic steatosis followed by the second hit, which could be one or more of the following: oxidative stress, endoplasmic reticulum stress, lipotoxicity, extrinsic factors derived from gut or adipose tissue and intrinsic factors in liver, e.g. innate immune response etc. [7]. Recently, ‘multiple-hits hypothesis’ was proposed, which states that many of the events may take place in parallel, rather than consecutively [8].
Liver inflammation has been identified as one of the major mechanisms driving the progression of NASH to HCC [9]. Cell death has been identified as one of the triggers for liver inflammation in CLD, in addition to many other factors mentioned above [10]. Of these, hepatocyte cell death is one of the key factors that can cause liver inflammation in CLD [11]. Several forms of hepatocyte cell death have been reported in CLD, e.g., necrosis, apoptosis, pyroptosis and necroptosis (programmed necrosis). Whereas apoptosis is considered as a non-inflammatory mode of cell death, several studies have demonstrated that necroptosis plays a major role in inflammation [12–14]. Necroptotic stimuli (e.g., TNF-α, oxidative stress, and mTOR/Akt signaling) sequentially activate the three key kinases in the necroptosis pathway through phosphorylation: receptor-interacting serine/threonine-protein kinase 1 (RIPK1), RIPK3, and mixed lineage kinase domain like pseudokinase (MLKL). Phosphorylation of MLKL leads to its oligomerization and binding to the plasma membrane leading to the disruption of the membrane and release of cellular components including damage associated molecular patterns (DAMPs) (e.g., mitochondrial DNA, S100A9, HMGB-1, ATP, IL-33 etc.). DAMPs initiate and exacerbate the inflammatory process by binding to cell surface receptors of innate immune cells [15,16]. Studies have shown that necroptosis is increased in NAFLD/NASH in mice, and inflammation associated with NAFLD/NASH with high fat feeding in mice is reduced by inhibiting necroptosis [17–23]. In addition, studies with human subjects show that increased necroptosis is associated with NASH [18,19].
To study the role of necroptosis in CLD, we used a novel mouse model that develops spontaneous NAFLD/NASH and progresses to fibrosis and HCC with age: mice deficient in the antioxidant enzyme, Cu/Zn superoxide dismutase (Sod1−/− or Sod1KO mice). Sod1KO mice are characterized by high levels of oxidative stress in various tissues, including liver [24,25] and show increased accumulation of triglycerides in the liver, a characteristic feature of NAFLD, as early as 3 weeks of age [26]. The Sod1KO mice also show increased accumulation of collagen in liver indicating increased fibrosis [27] and develop spontaneous HCC around 18 to 20 months of age [28]. The Sod1KO mice have several features that make them particularly relevant to the development of CLD in humans. First, although they do not become obese, the Sod1KO mice develop fatty liver from an imbalance in triglyceride deposition and removal arising from impaired very low density lipoprotein (VLDL) secretion because apolipoprotein B (ApoB) levels are reduced in the livers of Sod1KO mice [26], and APOB mutations are associated with HCC in humans [29]. Second, the increased oxidative stress associated with knocking out Sod1 is a key factor in the development of NASH and HCC in the Sod1KO mice [25,28], and oxidative stress is one of the factors that has been proposed to play a role in HCC in humans [30].
Previously we reported that necroptosis and expression of proinflammatory cytokines are increased in the adipose tissue of Sod1KO mice [31]. Therefore, we hypothesized that increased necroptosis in the liver plays a role in increased inflammation and progression to fibrosis and possibly HCC reported in Sod1KO mice. To test this hypothesis, we treated Sod1KO mice with a necroptosis inhibitor, necrostatin-1s (Nec-1s), which targets RIPK1 [32] and has been shown to block necroptosis and reduce expression of proinflammatory cytokines in mouse models of multiple sclerosis and amyotrophic lateral sclerosis [33,34]. Our findings show that markers of necroptosis, inflammation, fibrosis, and expression of heat shock proteins are increased and autophagy is reduced, and oncogenic transcription factor STAT3 is activated in the livers of Sod1KO mice and Nec-1s treatment reversed these effects.
Experimental Procedures
Animals.
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Oklahoma Health Sciences Center (OUHSC). The Sod1KO mice were generated as described previously [24,28]. Experimental cohorts were raised in the OUHSC rodent facility. Ten to 13-month-old male Sod1KO mice (C57Bl/6 background) and WT litter male controls were used for the study. The mice were group housed in ventilated cages 20 ± 2 °C, 12-h/12-h dark/light cycle and were fed rodent chow (5053 Pico Lab, Purina Mills, Richmond, IN) ad libitum.
Administration of Nec-1s.
Three groups of mice (10 to 13-month-old) were used for the study: WT mice (littermates) treated with vehicle (n=6), Sod1KO mice treated with vehicle (n=7) and Sod1KO mice treated with Nec-1s (n=5). On the first day of treatment, mice were given a single intraperitoneal injection of 10mg/kg Nec-1s (7-Cl-O-Nec-1; Focus Biomolecules, Plymouth Meeting, PA) or vehicle, followed by administration of Nec-1s in drinking water for 25 days. For supplementation in drinking water, Nec-1s was first dissolved in dimethylsufoxide (DMSO, 50% w/v) and was transferred into 35% polyethylene glycol (PEG) solution and was suspended in water containing 2% sucrose (final concentration: 0.5mg/mL of Nec-1s). Daily consumption of Nec-1s based on this protocol is reported to be 2.5–5 mg/day [34].
Quantitative real-time PCR.
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA, USA) from 20mg frozen liver tissues. First-strand cDNA was synthesized using a high capacity cDNA reverse transcription kit [ThermoFisher Scientific (Applied Biosystems), Waltham, MA] and quantitative real-time PCR was performed with ABI Prism using Power SYBR Green PCR Master Mix [ThermoFisher Scientific (Applied Biosystems), Waltham, MA]. PCR arrays were performed using RT2 Profiler PCR Arrays from Qiagen: RT² Profiler™ PCR Array Mouse Cytokines & Chemokines (PAMM-150Z), RT² Profiler™ PCR Array Mouse Fatty Liver (PAMM-157Z), RT² Profiler™ PCR Array Mouse Fibrosis (PAMM-120Z). Calculations were performed by a comparative method (2−ΔΔCt) using β-microglobulin, β-actin, and hypoxanthine phosphoribosyltransferase 1 (HPRT) as controls, as described previously [35].
Western Blotting.
Liver tissues collected during sacrifice were immediately frozen in liquid nitrogen and stored at −80 °C until use. For western blotting, 50 mg of liver tissues were homogenized in extraction buffer [50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.6; 150 mM sodium chloride; 20 mM sodium pyrophosphate; 20 mM β-glycerophosphate; 2 mM ethylenediaminetetraacetic acid (EDTA); 1% Nonidet P-40; 10% glycerol; 2 mM phenylmethylsulfonyl fluoride; and protease inhibitor cocktail (GoldBio, St Louis, MO)] or RIPA lysis buffer (ThermoFisher Scientific, Waltham, MA), and western blotting was performed using 40 μg protein/well as previously described [36]. Images were taken using a chemidoc imager (Bio-Rad) and quantified using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). Primary antibodies against the following proteins were used: MLKL (phospho S345) and RIP3 (phospho T231+S232) from Abcam (Cambridge, MA); phospho-NF-κB p65 (Ser536), NF-κB p65, TGF-β, STAT-3 (phospho Tyr705), STAT-3, phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), p44/42 MAPK (Erk1/2), phospho-p38 MAPK (Thr180/Tyr182), p38 MAPK, ACC, phospho-ACC (Ser79), LC3B, AMPKα, phospho-AMPKα (Thr172), caspase-3 and cleaved caspase-3 antibody from Cell Signaling Technology (Danvers, MA); RIPK3 antibody from Novus Biologicals (Centennial, CO); MLKL from Millipore Sigma (Burlington, MA); NLRP3 from Adipogen (San Diego, CA); Desmin from ThermoFisher Scientific (Invitrogen) (Waltham, MA); p62 from R&D systems (Minneapolis, MN), SREBP1 and SREBP2 from Santa Cruz Biotechnology (Dallas, TX), β- tubulin and β- actin were from Sigma-Aldrich (St. Louis, MO). HRP-linked anti-rabbit IgG, HRP-linked anti-mouse IgG and HRP-linked anti-rat IgG from Cell Signaling Technology (Danvers, MA) were used as secondary antibodies.
Targeted quantitative proteomics.
Change in mitochondrial enzymes in liver was analyzed by quantitative proteomics as previously described [37,38]. Briefly, 20 μg total liver homogenate/sample was run 1.5 cm into a 12.5% SDS–PAGE gel (Criterion, Bio-Rad) followed by fixation and staining with GelCode Blue (Pierce). The entire lane was cut into ~1 mm3 pieces, washed, reduced with DTT, alkylated with iodoacetamide, and digested with trypsin. The peptides generated were extracted with 50% methanol/10% formic acid in water, dried, reconstituted in 1% acetic acid, and analyzed using selected reaction monitoring (SRM) with a triple quadrupole mass spectrometer (ThermoScientific TSQ Vantage) configured with a splitless capillary column HPLC system (Eksigent). Data processing was done using the program Pinpoint (ThermoScientific), which aligned the various collision-induced dissociation reactions monitored for each peptide and determines the chromatographic peak areas. The response for each protein was taken as the total response for all peptides monitored. Changes in the relative abundance of the proteins were determined by normalization to the BSA internal standard, with confirmation by normalization to the housekeeping proteins.
Detection of 4-Hydroxynonenal (4-HNE) adducts.
For the detection of 4-HNE modified proteins, liver tissue homogenates containing equal amounts of protein (40 μg/well) were separated by SDS–PAGE, transferred to polyvinylidene difluoride membranes and treated with 250 mM sodium borohydride in 100mM (3-(N-morpholino)propanesulfonic acid, MOPS), pH 8.0 for 15 min. The membrane was washed with water, followed by tris buffered saline with tween-20 (TBS-T), and blocked with 5% non-fat milk/TBS-T. The membrane was incubated with a 1:2000 dilution of polyclonal antibody against 4-HNE, as described [35,36]. The antibody recognizes cysteine, lysine, and histidine 4-HNE protein adducts and is highly specific to 4-HNE derived protein adducts (gift from Dr. Luke Szweda, Oklahoma Medical Research Foundation) [39]. This was followed by incubation with anti-rabbit IgG HRP conjugated antibody and the blot was developed using ECL Western Blotting Substrate. Images were taken using a Chemidoc imager (Bio-Rad) and quantified using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Serum alanine aminotransferase (ALT) measurement.
Serum levels of ALT was measured using alanine transaminase colorimetric activity assay kit from Cayman Chemical Company (Ann Arbor, MI) following manufacturer’s instructions.
Statistical analyses.
Ordinary one-way ANOVA with Tukey’s post hoc test was used to analyze data.
Results
In these experiments, we tested whether necroptosis and proinflammatory cytokines are increased in the livers of Sod1KO mice and whether blocking necroptosis has any effect on the expression of proinflammatory cytokines. To block necroptosis, we treated the mice with Nec-1s for 25 days, which inhibits RIPK1 and has been shown to effectively block necroptosis and reduce expression of proinflammatory cytokines in multiple sclerosis [33] and amyotrophic lateral sclerosis [34]. Three groups of 10 to 13-month-old male mice were used for the study: (1) WT mice (vehicle treated), (2) Sod1KO mice (vehicle treated), and (3) Sod1KO mice (Nec-1s treated). Because Sod1KO mice are reported to have reduced body weight and increased liver weight compared to control mice [25], we assessed the effect of Nec-1s treatment of these parameters. We found that Nec-1s treatment had no effect on body weight or liver weight of Sod1KO mice (Supplementary Figures 1A and 1B). Thus Nec-1s treatment had no obvious negative effect on Sod1KO mice.
Effect of Sod1 deficiency on markers of necroptosis.
Phosphorylation of MLKL (P-MLKL) is a well-accepted marker of necroptosis because the membrane binding of P-MLKL is the key step in disrupting the cell membrane [40]. Therefore, we first tested whether necroptosis is activated in the livers of Sod1KO mice by measuring the levels of P-MLKL and MLKL. As shown in Figure 1A, the levels of both P-MLKL and MLKL were significantly increased (1.7- and 5.8-fold, respectively) in the livers of Sod1KO mice compared to WT mice indicating increased necroptosis. Nec-1s treatment resulted in a significant reduction in the expression of P-MLKL and MLKL in the livers of Sod1KO mice, such that P- MLKL levels were not significantly different from the WT control mice (Figure 1A). Next, we assessed changes in the expression and phosphorylation of RIPK3, the kinase that phosphorylates MLKL. Phosphorylation of RIPK3 (P-RIPK3) and RIPK3 protein levels were significantly increased (2.5-fold and 2-fold, respectively) in the livers of Sod1KO mice, and Nec-1s treatment significantly reduced the levels of P-RIPK3 and RIPK3 (Figure 1B). Figure 1C shows the effect of Nec-1s on the transcript levels of RIPK3 and MLKL. Transcript levels of RIPK3 (1.8-fold) and MLKL (4-fold) were significantly increased in the livers of Sod1KO mice compared to WT mice, and Nec-1s resulted in a significant reduction in the expression of RIPK3 and MLKL in the livers of Sod1KO mice. Thus, RIPK3 and MLKL components of the necroptosis pathway are increased in the livers of Sod1KO mice largely through an increase in transcription, and Nec-1s effectively blocked the increase in the expression of these components of necroptosis.
Figure 1. Effect of Sod1 deficiency on necroptosis.
(A) Left panel: Immunoblots of liver extracts prepared from WT (blue bars), Sod1KO untreated (red bars) and Sod1KO mice treated with Nec-1s (green bars) for P-MLKL, MLKL and β-tubulin. Right panel: Graphical representation of quantified blots normalized to β-tubulin. (B) Left panel: Immunoblots of liver extracts for P-RIPK3, RIPK3, and β-tubulin. Right panel: Graphical representation of quantified blots normalized to β-tubulin. (C) Transcript levels for MLKL and RIPK3. (D) Left panel: Immunoblots of liver extracts for cleaved caspase-3 and total caspase-3. Right panel: Graphical representation of quantified blots normalized to total caspase-3. (E) Left panel: Immunoblots of liver extracts for LC3-I, LC3-II, p62 and β-actin. Right panel: Graphical representation of quantified blots normalized to β-actin. Data were obtained from 5 to 7 mice per group and are expressed as the mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Because RIPK3 protein is involved in the activation of both apoptosis and necroptosis, we tested whether apoptosis was activated in the livers of Sod1KO mice and blocking necroptosis had any effect on apoptosis. Apoptosis was assessed by measuring the level of cleaved caspase-3, the active form of caspase-3. Levels of cleaved caspase-3 normalized to caspase-3 was significantly increased in the livers of Sod1KO mice compared to WT mice, and Nec-1s had no effect on the levels of cleaved caspase-3 in Sod1KO mice liver (Figure 1D). Thus, both apoptosis and necroptosis are activated in Sod1KO mice liver. However, Nec-1s treatment attenuated only necroptosis; it had no effect on apoptosis in the livers of Sod1KO mice.
Autophagy is a conserved cell survival pathway that catabolizes damaged proteins and organelles to maintain cellular homeostasis. A recent study by Wu et al. (2020) showed an association between autophagy and necroptosis in the development of NAFLD and NASH [41]. They reported that necroptosis is activated and autophagy is reduced when mice were fed a western diet. Western diet increased levels of the adaptor protein p62/SQSTM-1 (that interacts with autophagic substrates and delivers them to autophagosomes for degradation) and autophagy marker microtubule-associated protein 1 light chain 3 II (LC3-II), indicating reduced autophagic flux, and deficiency of Mlkl blocked this effect of western diet. Therefore, we tested whether autophagy is altered in the livers of Sod1KO mice by measuring the levels of LC3-II and p62 by western blotting. Protein expression of LC3-II (2.5-fold) and p62 (2.8-fold) were upregulated in the livers of Sod1KO mice compared to WT mice, and Nec-1s treatment significantly reduced their expression in the livers of Sod1KO mice (Figure 1E). Thus, autophagic flux is reduced in the livers of Sod1KO mice and blocking necroptosis improved autophagic flux in Sod1KO mice.
Effect of Sod1 deficiency and necroptosis on inflammation.
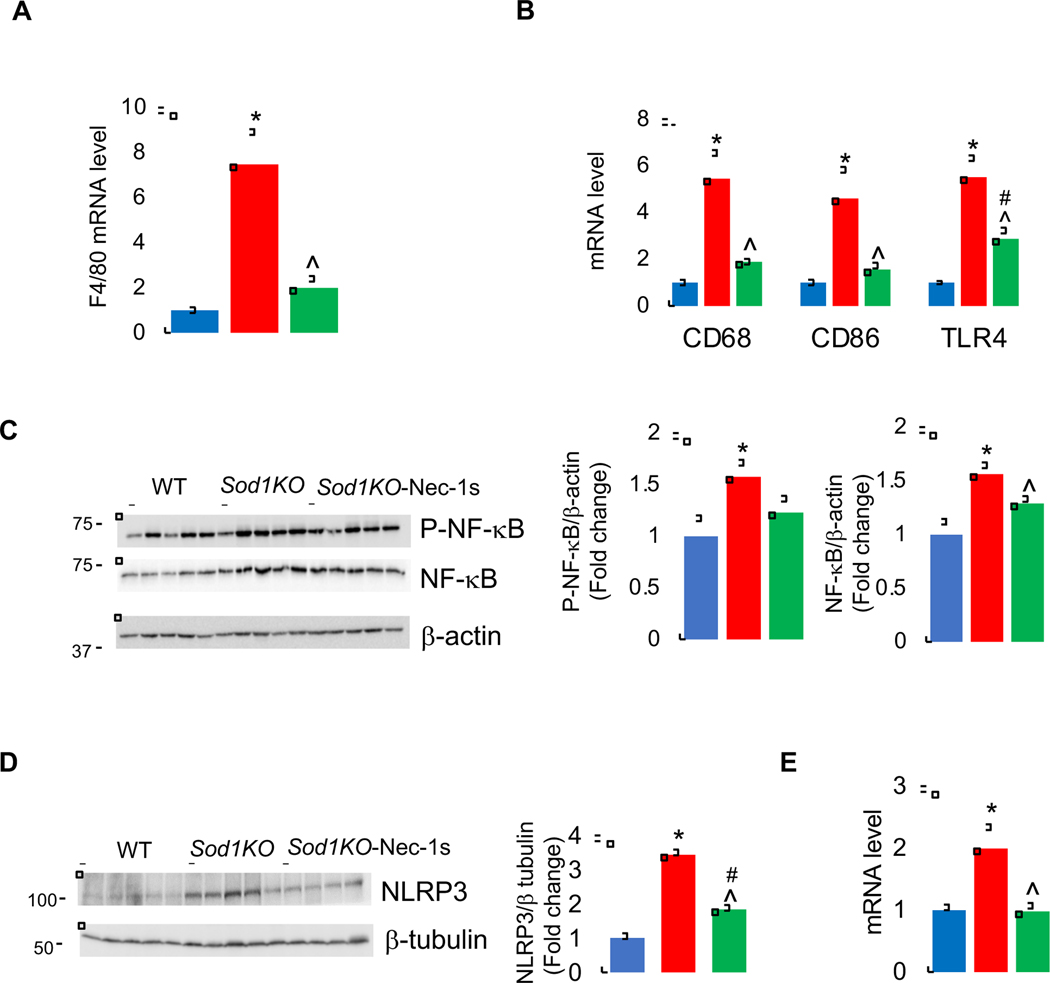
DAMPs released by necroptosis are key in initiating an inflammatory response by activating immune cells such as macrophages. Therefore, we measured the expression of F4/80, a marker of mice liver macrophages. A significant increase in the transcript levels of macrophage marker F4/80 (7.4-fold) was observed in the livers of Sod1KO mice, and Nec-1s treatment significantly reduced its expression (Figure 2A). Macrophages are categorized into M1 or M2 phenotypes. In general, M1 macrophages play a more proinflammatory role in liver injury and M2 macrophages exert an anti-inflammatory effect. In the livers of Sod1KO mice, markers of M1 macrophages CD68 (5.4-fold), CD86 (4.6-fold), and TLR4 (5.5-fold) were significantly elevated compared to WT mice, and Nec-1s treatment significantly reduced these markers of M1 macrophages (Figure 2B). Markers of M2 macrophages were either down regulated (Arg1) or unaltered (CD206) in the livers of Sod1KO mice. Nec-1s treatment had no effect on the expression of Arg1 or CD206 (Supplementary Figure 2A).
Figure 2. Effect of Sod1 deficiency and necroptosis on inflammation.
Transcript levels of F4/80 (A), and proinflammatory M1 macrophage markers CD68,CD86, and TLR4 (B) in the livers of WT (blue bars), Sod1KO untreated (red bars) and Sod1KO mice treated with Nec-1s (green bars). (C) Left panel: Immunoblots of liver extracts for phospho-NF-κB (p65), NF-κB and β-actin. Right panel: Graphical representation of quantified blots normalized to β-actin. (D) Left panel: Immunoblots of liver extracts for NLRP3 and β-tubulin. Right panel: Graphical representation of quantified blots normalized to β-tubulin. (E) Transcript levels of NLRP3. Data were obtained from 5 to 7 mice per group and are expressed as the mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Activation of the NF-κB pathway in macrophages by DAMPs is one of mechanisms proposed for increased production of pro-inflammatory cytokines [42]. Therefore, we determined whether NF-κB pathway is activated in the livers of Sod1KO mice by assessing the expression of active form of NF-κB, phospho-NF-κB (p65). The levels of phospho-NF-κB (p65) were significantly increased in the livers of Sod1KO mice (1.5-fold) compared to WT mice, and Nec-1s treatment showed a tendency to reduce the levels of phospho-NF-κB p65; however, this decrease was not statistically significant. The levels of NF-κB protein were also significantly increased in the livers of Sod1KO mice (1.5-fold) and was significantly reduced by Nec-1s treatment (Figure 2C).
DAMPs activate the NLRP3 inflammasome complex in macrophages, which leads to the secretion of proinflammatory cytokines such as IL-1β, IL-18 [43]. Therefore, we measured the levels of NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3), which is a component of the inflammasome. NLRP3 protein and transcript levels were significantly increased (3.4-fold) in the livers of Sod1KO mice compared to WT mice, and Nec-1s treatment significantly reduced NLRP3 protein and transcript levels in the livers of Sod1KO mice to levels similar to the WT control mice (Figures 2D and 2E).
We next measured the transcript levels of 84 proinflammatory cytokines and chemokines in the livers of WT, Sod1KO and Sod1KO mice treated with Nec-1s. The data are shown as heat map in Supplementary Figure 3A, and levels of the transcripts of the proinflammatory cytokines and chemokines are presented in Supplementary Table 1. The expression of 16 of the 26 chemokines measured were significantly upregulated (Ccl1, Ccl12, Ccl17, Ccl2, Ccl20, Ccl22, Ccl3, Ccl4, Ccl5, Ccl7, Cx3cl1, Cxcl10, Cxcl16, Cxcl3, Pf4, Xcl1) in the livers of Sod1KO mice compared to WT mice (Figure 3A), and Nec-1s treatment resulted in a significant reduction in the expression of 7 of the upregulated cytokines (Ccl1, Ccl12, Ccl17, Ccl2, Ccl20, Ccl3, and Cxcl3). Of the 25 interleukins analyzed, 9 of them (IL10, IL11, IL12α, IL12β, IL1β, IL1α, IL1rn, IL6, and IL7) showed a significant increase in the livers of Sod1KO mice compared to WT mice (Figure 3B), and Nec-1s treatment significantly reduced the expression of all of these interleukins, except IL11 and IL12α. Of the 10 TNF superfamily members, 5 showed a significant increase in the livers of Sod1KO mice compared to WT mice: Cd40lg, Ltb, TNFα, Tnfrsf11β, and Tnfsf13β (Figure 3C). Nec-1s treatment significantly reduced the expression of TNFα and Tnfsf13b. Thus, absence of Sod1 is associated with increased expression of proinflammatory cytokines and chemokines in the liver and blocking necroptosis reduced the expression of the majority of the cytokines and chemokines, suggesting that increased necroptosis is a major contributor to increased expression of proinflammatory cytokines in livers of Sod1KO mice.
Figure 3. Effect of Sod1 deficiency and necroptosis on proinflammatory cytokines and chemokines.
Transcript levels of proinflammatory cytokines and chemokines (A), interleukins (B), and members of TNFα family (C) in the livers of WT (blue bars), Sod1KO untreated (red bars) and Sod1KO mice treated with Nec-1s (green bars). Data were obtained from 5 to 7 mice per group and are expressed as the mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh;#WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Effect of Sod1 deficiency and necroptosis on metabolic pathways.
Deficiency of Sod1 is reported to alter glucose and lipid metabolism in the livers of Sod1KO mice [44]. Therefore, we tested the effect of blocking necroptosis on glycolysis and lipid metabolism in the livers of Sod1KO mice using a targeted quantitative proteomic approach. Several of the enzymes in the glycolytic pathway were significantly upregulated in the livers of Sod1KO mice compared to WT mice: hexokinase (HK), glucose phosphate isomerase (GPI), phosphofructokinase (PFK), aldolase (ALDO), triosephosphate isomerase (TPI), glyceraldehyde phosphate dehydrogenase (GAPDH), and lactate dehydrogenase (LDH). Nec-1s significantly reduced the protein levels of HK, GAPDH, LDH and pyruvate carboxylase (PC) in the livers of Sod1KO mice (Figure 4). Other proteins in the glycolysis pathway also showed a tendency to decrease with Nec-1s treatment, however, they did not reach statistical significance. Thus, carbohydrate utilization through glycolysis pathway is upregulated in the livers of Sod1KO mice and blocking necroptosis reduced this effect. Sod1KO mice is reported to have reduced gluconeogenesis due to a 50% increase in the levels of AMPK and P-AMPK [44]. Therefore, we assessed levels of AMPK and P-AMPK. As shown in Supplementary Figure 4A, AMPK levels were upregulated (1.3-fold) in the livers of Sod1KO mice and Nec-1s treatment significantly reduced AMPK levels, however, P-AMPK levels were similar in the livers of WT and Sod1KO mice and Nec-1s treatment had no effect on P-AMPK level, suggesting that gluconeogenesis is unaltered in Sod1KO mice.
Figure 4. Effect of Sod1 deficiency and necroptosis on glycolytic pathway.
The relative expression of glycolytic enzymes in the livers Sod1KO mice (red bars) and Sod1KO mice treated with Nec-1s (green bars), compared to WT mice, as assessed by targeted quantitative proteomics. Expression of proteins in WT mice is taken as 100%. The values are normalized to internal standards and housekeeping proteins. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
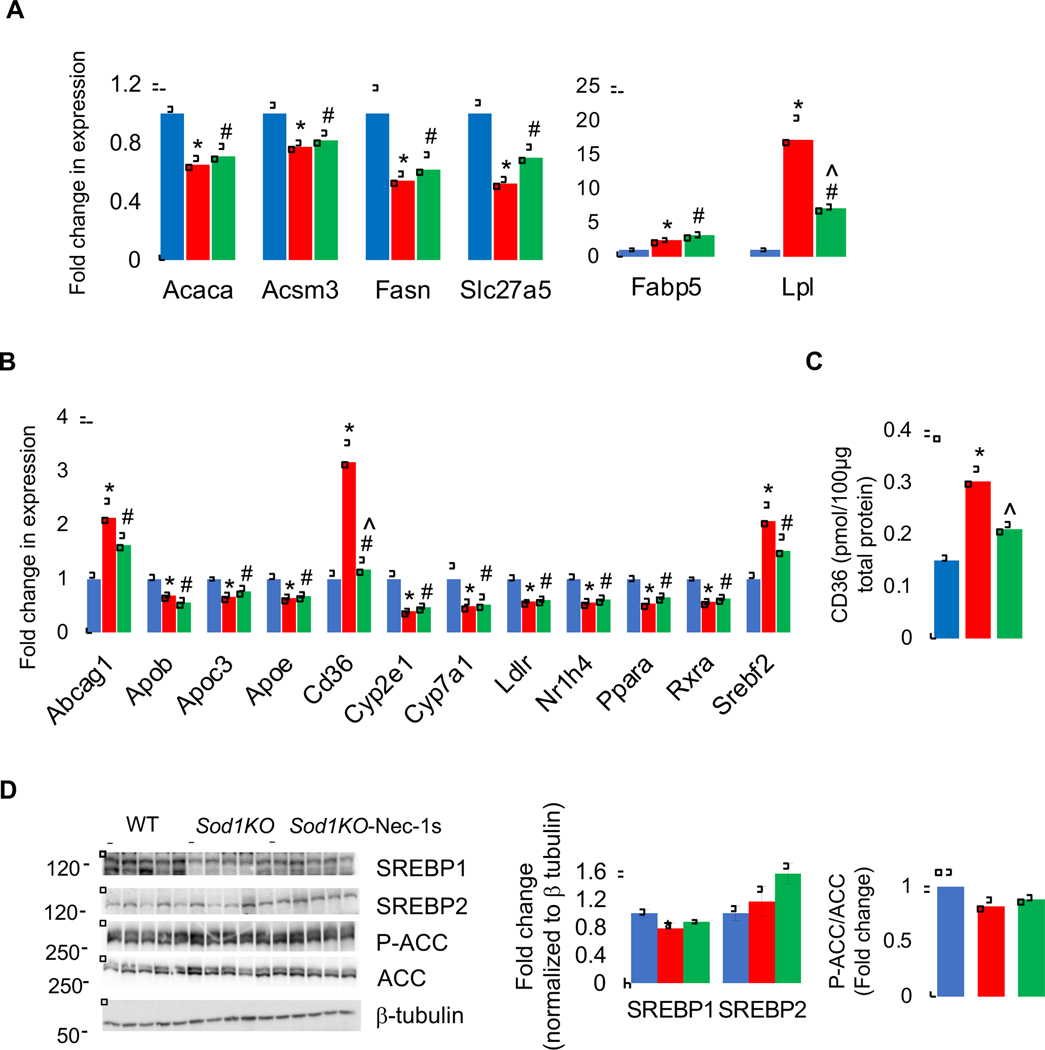
To study the effect of Sod1 deficiency on lipid metabolism, first we analyzed changes in the expression of genes involved in lipid and cholesterol metabolism. Of the 13 genes analyzed in lipid metabolism and transport, 6 genes showed a significant change in the livers of Sod1KO mice compared to WT mice: 2 were upregulated (fatty acid binding protein 5, Fabp5 and lipoprotein lipase, Lpl) and 4 were downregulated [acetyl CoA carboxylase (Acaca), acyl-CoA synthetase medium chain family member 3 (Acsm3), fatty acid synthase (Fasn), solute carrier family 27a5 (Slc27a5)]. Nec-1s treatment downregulated the expression of Lpl and had no effect on the expression of other genes (Figure 5A). In the cholesterol metabolism and transport pathway, 23 genes were analyzed, of which 13 showed a significant change in the livers of Sod1KO mice compared to WT mice: 9 genes were significantly downregulated [apolipoprotein B (Apob), Apoc3, Apoe, cytochrome P450 family 2 subfamily E member 1 (Cyp2e1), Cyp7a1, low density lipoprotein receptor (Ldlr), nuclear receptor subfamily 1 group H member 4 (Nr1h4), peroxisome proliferator activated receptor alpha (Pparα), and retinoid X receptor alpha (Rxrα)] 3 genes were significantly upregulated [ATP binding cassette subfamily G member 1 (Abcg1), CD36 Molecule (CD36), and sterol regulatory element binding transcription factor 2 (Srebp2)]. Blocking necroptosis did not affect the expression of any of the downregulated genes in the livers of Sod1KO mice, however, Nec-1s significantly reduced the expression of CD36 in Sod1KO mice (Figure 5B). Analysis of the protein expression also showed a significant increase (2-fold) in CD36 levels in Sod1KO mice liver compared to WT mice, and Nec-1s treatment significantly reduced its expression (Figure 5C). We also assessed changes in lipid metabolism by assessing protein levels of sterol regulatory element binding transcription factor 1 (SREBP1) and SREBP2, key transcription factors involved in lipid synthesis, that were reported to be upregulated in Sod1KO mice [44]. In our study, levels of SREBP1 was reduced in the livers of Sod1KO mice, whereas SREBP2 expression was similar in the livers of WT and Sod1KO mice suggesting that lipid synthesis is reduced or unaltered, and Nec-1s treatment did not alter the expression of SREBP1 or SREBP2. This finding was further confirmed by assessing phosphorylation of acetyl CoA carboxylase (ACC): phosphorylation of ACC inhibits its enzymatic activity and shifts fat synthesis to fatty acid oxidation [45]. The ratio of P-ACC to ACC was similar in the livers of Sod1KO mice, and Nec-1s had no effect on the phosphorylation of ACC (Figure 5D). Thus, in 10- to 13-month-old Sod1KO mice, we found changes the expression of proteins involved in fatty acid transport (CD36) and VLDL secretion (Apob) with no change in enzymes involved in fat synthesis.
Figure 5. Effect of Sod1 deficiency and necroptosis on lipid metabolism.
Transcript levels of proteins involved lipid metabolism and transport (A) and cholesterol metabolism and transport (B) in the livers Sod1KO mice (red bars) and Sod1KO mice treated with Nec-1s (green bars), compared to WT mice. (C) Protein expression of as assessed by targeted quantitative proteomics. (D) Left panel: Immunoblots of liver extracts for SREBP1, SREBP2, P-ACC, ACC, and β-tubulin. Right panel: Graphical representation of SREBP1 and SREBP2 blots normalized to β-tubulin and P-ACC normalized to ACC. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Effect of Sod1 deficiency and necroptosis on mitochondrial enzymes/proteins, stress response, and oxidative stress.
Mitochondrial maladaptation contributes to detrimental effects on hepatocyte bioenergetics, reactive oxygen species homeostasis, inflammation, and cell death leading to NASH and fibrosis [46,47]. A targeted quantitative proteomic approach was employed for a detailed analysis of the changes in levels of mitochondrial metabolic enzymes, proteins, antioxidants and stress proteins in the livers of WT, Sod1KO mice and Sod1KO mice treated with Nec-1s.
Expression of most of the proteins involved in the beta oxidation pathway were similar in WT and Sod1KO mice, except for acyl-CoA thioesterase 13 (Acot13), acyl-coa synthetase long chain family member 1 (Acsl1), carnitine O-acetyltransferase (Crat), enoyl-CoA hydratase and 3-hydroxyacyl CoA dehydrogenase (Ehhadh), 3-hydroxybutyrate dehydrogenase 1 (Bdh1), fatty acid binding protein 4 (Fabp4), and 3-hydroxy-3-methylglutaryl-coA synthase 1 (Hmgcs1) that were upregulated in the livers of Sod1KO mice (Supplementary Figure 5). In the tricarboxylic acid (TCA) cycle, the following proteins were significantly upregulated in the livers of Sod1KO mice compared to WT mice: aconitase 2 (Aco2), citrate synthase (Cs), dihydrolipoamide S-succinyltransferase (Dlst), isocitrate dehydrogenase 3a (Idh3a), Idh3b, Idh3g, and malate dehydrogenase 2 (Mdh2). Nec-1s reduced the expression of Mdh2 with no effect on the expression of other proteins (Supplementary Figure 6A). Expression of proteins in the electron transport chain (ETC) were similar in the livers of WT and Sod1KO mice, except for NADH dehydrogenase flavoprotein (Ndufv1) that was increased, and Nec-1s had no effect on the expression of these proteins (Supplementary Figure 6B).
Analysis of protein levels of antioxidant enzymes showed that absence of Sod1 resulted in a significant increase in the level of the antioxidant enzymes glutathione peroxidase 4 (Gpx4), glutathione-disulfide reductase (Gsr), glutathione s-transferase mu 1 (Gstm1), peroxiredoxin 1 (Prdx1), Prdx3, thioredoxin (Txn1), and thioredoxin reductase 1 (Txnrd1) and a reduction in the levels of glutathione peroxidase 1 (Gpx1). Even though Nec-1s treatment downregulated the expression of these antioxidant enzymes in the livers of Sod1KO mice, it did not reach statistical significance (Figure 6A). Protein levels of Lon protease 1 (LonP1), a mitochondrial matrix protease involved in the degradation of unfolded proteins, was also upregulated in the livers of Sod1KO mice compared to WT control, and Nec-1s treatment reduced its expression (Figure 6B). Among the stress-related proteins, Hspa1a (Hsp70), Hspa9 (mitochondrial Hsp70), and Hspd1 (Hsp60) were significantly upregulated in the livers of Sod1KO mice and Nec-1s treatment significantly reduced their expression (Figure 6C). Thus, absence of Sod1 in the liver did not result in mitochondrial dysfunction as indicated by the changes in the expression of proteins involved in fatty acid oxidation pathway or TCA or mitochondrial ETC chain, rather it resulted in an increase in heat shock proteins and antioxidant enzymes, and Nec-1s reduced their expression.
Figure 6. Effect of Sod1 deficiency and necroptosis on antioxidant proteins and stress response.
The relative expression of antioxidant enzymes (A), Lon protease 1 (B), and stress-related proteins (C) in the livers of Sod1KO mice (red bars) and Sod1KO mice treated with Nec-1s (green bars), compared to WT mice, as assessed by targeted quantitative proteomics. Expression of these proteins in WT mice is taken as 100%. The values are normalized to internal standards and housekeeping proteins. (D) Left panel: Immunoblots of liver extracts for 4-HNE. Right panel: Graphical representation of quantified blots normalized to total protein in Coomassie stained gel. Data were obtained from 5 to 6 mice per group and are expressed as the mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Veh; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Sod1KO mice is reported to have high levels of oxidative stress in various organs, including liver [24,25]. Therefore, we tested whether Nec-1s treatment has an effect on oxidative stress in the livers of Sod1KO mice. To assess changes in oxidative stress, levels of 4-HNE were measured, which is a product of lipid peroxidation and a routinely used biomarker of oxidative stress [48,49]. Western blotting of liver extracts showed a significant increase in 4-HNE levels in the livers of Sod1KO mice (1.4-fold) compared to WT mice, and Nec-1s treatment did not show any significant effect on 4-HNE levels (Figure 6D).
Effect of Sod1 deficiency and necroptosis on markers of fibrosis.
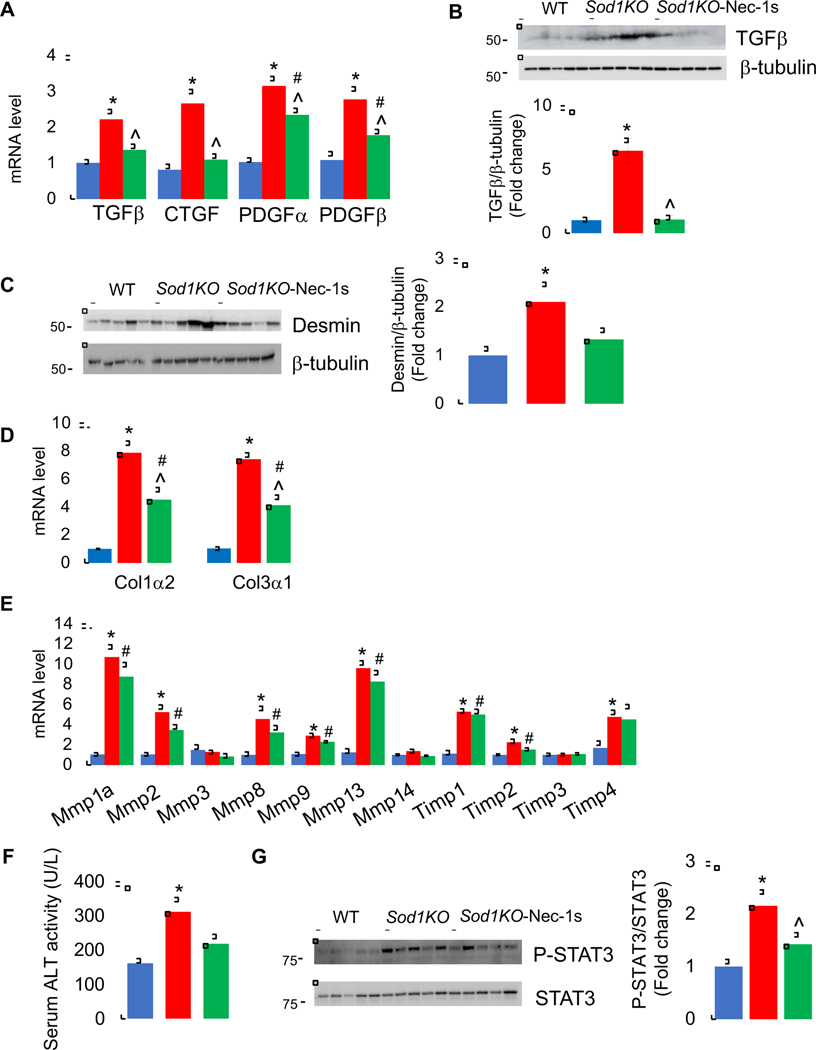
The Sod1KO mice are characterized by the development of liver fibrosis, a condition that proceeds NASH [27]. Because inflammation is a key driver of fibrosis in the liver [50,51], we determined if short-term Nec-1s treatment had any effect on markers of fibrosis in the Sod1KO mice. We first measured the expression of genes associated with fibrosis in the livers of WT and Sod1KO mice treated without or with Nec-1s. The pro-fibrogenic cytokine transforming growth factor beta (TGFβ) is produced by activated macrophages and plays a critical role in fibrosis by transforming quiescent hepatic stellate cells (HSCs) to trans differentiated myoblasts, which produce extracellular matrix components (ECM) [52]. In addition to TGFβ, pro-fibrotic cytokines connective tissue growth factor (CTGF) and platelet-derived growth factor (PDGF) are also involved in fibrosis [53]. Therefore, we first tested whether expression of these pro-fibrogenic cytokines are increased in Sod1KO mice. As shown in Figure 7A, levels of TGFβ (2.2-fold), CTGF (2.6-fold), PDGFα (3.2-fold) and PDGFβ (2.7-fold) transcripts were significantly up-regulated in the livers of Sod1KO mice compared to WT mice, and Nec-1s treatment significantly reduced the expression of these pro-fibrogenic cytokines. Consistent with an increase in the transcripts of TGFβ, levels of TGFβ protein were also up-regulated in the livers of Sod1KO mice, and Nec-1s treatment reduced TGFβ protein expression in Sod1KO mice (Figure 7B). Next, we measured the expression of desmin, a protein that is strongly upregulated in liver fibrosis and is produced by activated HSCs [54]. Levels of desmin protein expression were significantly upregulated in the livers of Sod1KO mice (2.1-fold) compared to WT mice (Figure 7C). Nec-1s treatment reduced levels of desmin protein by 38%, however, this decrease did not reach statistical significance. Consistent with the activation of HSCs, expression of ECM components Col1α2 (7.8-fold) and Col3α1 (7.4-fold) were significantly elevated in the livers of Sod1KO mice, and Nec-1s significantly reduced their expression (Figure 7D). In addition to ECM components, activated HSCs also express matrix metalloproteinases (MMPs) that degrade ECM, and inhibitors of metalloproteinases (TIMPs) that stabilize the ECM against MMP degradation [55]. Levels of MMPs and TIMPs transcripts were significantly upregulated in Sod1KO mice liver; however, Nec-1s had no effect on the expression of these MMPs and TIMPs (Figure 7E and Supplementary Table 2). Thus, fibrosis markers are upregulated in the livers of Sod1KO mice, and short-term Nec-1s treatment attenuated expression of most of the fibrosis markers associated with HSC activation. We also tested whether Nec-1s treatment had any effect on liver function by assessing the levels of ALT in serum. ALT levels were 1.9-fold higher in Sod1KO mice compared to WT mice and Nec-1s treatment reduced its level, however, this decrease did not reach statistical significance (Figure 7F).
Figure 7. Effect of Sod1 deficiency and necroptosis on markers of fibrosis.
(A) Transcript levels of pro-fibrotic cytokines TGFβ, CTGF, PDGFα, and PDGFβ in the livers of WT (blue bars), Sod1KO untreated (red bars) and Sod1KO mice treated with Nec-1s (green bars). (B) Top panel: Immunoblots of liver extracts for TGFβ and β-tubulin. Bottom panel: Graphical representation of quantified blots normalized to β-tubulin. (C) Left panel: Immunoblots of liver extracts for desmin and β-tubulin. Right panel: Graphical representation of quantified blots normalized to β-tubulin. (D) Transcript levels of Col1α2 and Col3α1. (E) Transcript levels of MMPs and TIMPs. (F) ALT activity as measured in the serum of WT (blue bars), Sod1KO (red bars) and Sod1KO mice treated with Nec-1s (green bars). (G) Left panel: Immunoblots of liver extracts prepared from WT (blue bars), Sod1KO untreated (red bars) and Sod1KO mice treated with Nec-1s (green bars) for P-STAT3 and STAT3. Right panel: Graphical representation of quantified phospho-blots normalized to total protein. Data were obtained from 5 to 7 mice per group and are expressed as the mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Because fibrosis can lead to HCC, we tested whether signaling pathways that are known to modulate HCC progression and to be affected by inflammation are altered in the livers of Sod1KO mice: STAT3, JNK, ERK and p38 pathways [56–59]. Activation of the oncogenic transcription factor STAT3 (P-STAT3/STAT3) was significantly upregulated in the livers of Sod1KO mice (2-fold) compared to WT mice, and short-term Nec-1s treatment significantly reduced STAT3 activation (Figure 7G). JNK activity (P-JNK/JNK) was significantly increased in the livers of Sod1KO mice(1.7-fold). Nec-1s reduced its activity; however, this reduction did not reach statistical significance (Supplementary Figure 7A). ERK activity (phospho-ERK/ERK) was similar in the livers of Sod1KO and WT mice, and Nec-1s treatment had no effect on ERK activity in Sod1KO mice (Supplementary Figure 7B). The activity of p38 (P-p38/p38) was significantly lower in Sod1KO mice liver compared to WT mice, and Nec-1s treatment had no effect on p38 activity (Supplementary Figure 7C).
Discussion
Several studies have identified non-resolving chronic inflammation as a key driver of chronic liver disease [60,61]. Therefore, we were interested in determining if inflammation plays a role in chronic liver disease that is observed in Sod1KO mice. Median lifespan of Sod1KO mice is ~22 months [62] and in our study, we used 10 to 13-month-old mice WT and Sod1KO mice to assess the effect of blocking necroptosis at an advanced stage of CLD, i.e. fibrosis. Previously, we showed that circulating proinflammatory cytokines levels were increased in Sod1KO mice [63], and in this study, we demonstrated that the expression of proinflammatory cytokines and chemokines, and markers of proinflammatory macrophages are dramatically increased in the livers of Sod1KO mice. For example, the expression of TNFα, IL-6, IL-1β, and Ccl2, which have been shown to increase in humans with NASH and HCC [64,65], were increased in the livers of Sod1KO mice (TNFα, 4.3-fold; IL6, 2.3-fold; IL-1β, 5-fold; and Ccl2, 13.7-fold). In addition, expression of proinflammatory M1 macrophages and levels of NLRP3 were also increased. These data are consistent with the human data showing that these measures of inflammation in the liver are associated with CLD.
Studies have shown that necroptosis is a major source of inflammation in a variety of tissues, and genetic or pharmacological inhibition of necroptosis can block/reduce inflammation in various mouse models [31]. In addition, necroptosis has been reported to be elevated in the livers of patients with NASH [18,19]. Therefore, we measured necroptosis in the livers of Sod1KO mice and found that necroptosis was dramatically elevated in the livers of Sod1KO mice. For example, the level of P-MLKL, the biomarker of necroptosis was increased 1.7-fold, as well as levels of MLKL (5.8-fold), RIPK3 (2.1-fold) and P-RIPK3 (2.4-fold). Several factors have been proposed to induce necroptosis, and oxidative stress is one of them [31], e.g., deficiency of the antioxidant enzyme Gpx4 in hematopoietic cells resulted in increased ROS generation and necroptosis in erythroid precursor cells [66]; excessive acetaminophen treatment resulted in increased ROS production and necroptosis in the livers of WT mice [67], and exposure to hypoxia increased oxidative stress and necroptotic cell death in the lung tissue of rats [68]. Due to the lack of the antioxidant enzyme Cu/Zn-SOD, the Sod1KO mice show an increase in oxidative stress as shown by elevated levels of 4-HNE in liver tissues and previous reports support our finding [24,25]. Thus, our data are consistent with oxidative stress playing an important role in inducing necroptosis. We did not see an effect of Nec-1s on 4-HNE levels suggesting that effect of Nec-1s on inflammation arises from its inhibition on necroptosis and is independent of oxidative stress. One possible mechanism for oxidative stress mediated necroptosis in Sod1KO mice could be the upregulation of heat shock protein, Hsp70 that has been shown to interact with the N-terminal domain of MLKL to promote polymerization of MLKL leading to cell membrane rapture [69]. The necroptosis-blocking compound 1 (NBC1), a known Hsp70 substrate-binding inhibitor, is shown to blocks necroptosis by inhibiting MLKL polymerization. In addition, knockdown of Hsp70 in cells is shown to destabilize MLKL suggesting that Hsp70 stabilizes MLKL protein under normal condition and promotes MLKL polymerization through its substrate-binding domain during necroptosis [69]. While we found that Hsp70 is upregulated in the livers of Sod1KO mice, the reason(s) for its downregulation with Nec-1s treatment is not clear. Hsp70 is a chaperone that binds to unfolded/misfolded proteins under conditions of stress and is also known to be involved in chaperone-mediated autophagy [70]. It is possible that when autophagy is blocked in the livers of Sod1KO mice, Hsp70 is upregulated to stabilize unfolded/misfolded proteins and activation of autophagy by Nec-1s might reduce Hsp70 levels through chaperone-mediated autophagy.
Although increased apoptosis in the livers of Sod1KO mice were unaffected by Nec-1s treatment, autophagic flux that was reduced in Sod1KO mice liver was up-regulated when necroptosis was inhibited. Kurahashi et al, (2015) [71] also reported impaired autophagy in Sod1KO mice liver. Our finding that blocking necroptosis increased autophagy is in line with a recent report that reduced autophagic flux in the livers of western diet fed mice is upregulated in the absence of Mlkl [41]. This demonstrates the existence of a cross talk between autophagy and necroptosis and suggest that blocking necroptosis activates autophagy to maintain cellular homoeostasis.
Because our data showed that the increase in inflammation in the livers of Sod1KO mice was associated with increased necroptosis, we directly tested whether necroptosis was responsible for the increased inflammation using a RIPK1 inhibitor, Nec-1s to block necroptosis [32]. Nec-1s reduced markers of necroptosis to the levels seen in wild type mice and dramatically reduced inflammation in the livers of Sod1KO mice. In particular, levels of TNFα, IL6, IL-1β and Ccl2 expression that are associated with human NASH [64], are significantly downregulated by Nec-1s treatment in the livers of Sod1KO mice. This reduction in inflammation in response to Nec-1s appears to arise from a decrease in M1 macrophages and reduced NLRP3 expression. Even though apoptosis was increased in Sod1KO mice liver, Nec-1s treatment had no effect on apoptosis. Thus, our data conclusively demonstrates that necroptosis contributes to increased inflammation observed in livers of Sod1KO mice. These findings are consistent with previous reports showing that blocking necroptosis in mouse models of diet-induced NAFLD and NASH reduced hepatic inflammation [17–20].
Absence of Sod1 is reported to cause alterations in glucose and lipid metabolism [44]. Even though we found increased glycolysis in the livers of Sod1KO mice, we did not find any change in lipid metabolism in our study. The reason for this discrepancy in lipid metabolism could be the age of mice used for the study: we used 10 to 13-month-old mice, whereas Wang et al. (2012) used 6-month-old mice [44] and increased lipid accumulation is reported in the livers of young (2 to 3-month-old) Sod1KO mice [26]. Therefore, it is possible that in 10 to 13-month-old Sod1KO mice NAFLD has progressed to more severe form of CLD, viz. fibrosis. In support of this, expression of SREBP1 the key transcription factor regulating lipid synthesis is reduced in 10 to 13-month-old Sod1KO mice, whereas it is increased in 6-month-old mice [44]. Mitochondrial dysfunction is one of the contributing factors to the development of diet-induced NAFLD [46,47]. Interestingly, our data shows that proteins in the mitochondrial ETC, TCA cycle or fatty acid oxidation pathway are unaltered in the livers of Sod1KO mice, suggesting that alteration in these pathways does not contribute to NAFLD development in Sod1KO mice. It is important to note that Sod1 KO mice develop fatty liver from an imbalance in triglyceride deposition and removal arising from impaired very low density lipoprotein (VLDL) secretion because apolipoprotein B (ApoB) levels are reduced in the livers of Sod1KO mice [26]. Whether mitochondrial functions are altered in young Sod1KO mice and whether blocking necroptosis can improve mitochondrial respiration and reduce steatosis in young Sod1KO mice, similar to high fat diet-mediated NAFLD need to be tested [23].
Since chronic inflammation is believed to be a major driver of fibrosis [9,72], we were interested in determining whether blocking necroptosis and inflammation for a month had any impact on markers of fibrosis in the livers of the 10- to 13-month-old Sod1KO mice. Fibrosis markers were strongly up-regulated in the livers of Sod1KO mice, and short-term Nec-1s treatment reduced levels of fibrosis markers involved in ECM synthesis. A tendency for reduction in the levels of ALT, a marker of liver injury, with Nec-1s treatment suggest improvement in liver function. Similarly, the increase in activated STAT3, which mediates tumor promotion, is significantly reduced by Nec-1s treatment. Obesity-promoted HCC development has been shown to be dependent on enhanced production of the tumor promoting cytokines IL-6 and TNF, which cause hepatic inflammation and activation of the oncogenic transcription factor STAT3 [73,74]. Thus, our preliminary data show that short-term treatment with necroptosis inhibitor, which reduces inflammation, has the potential to reduce fibrosis, liver injury and possibly tumor promotion in Sod1KO mice.
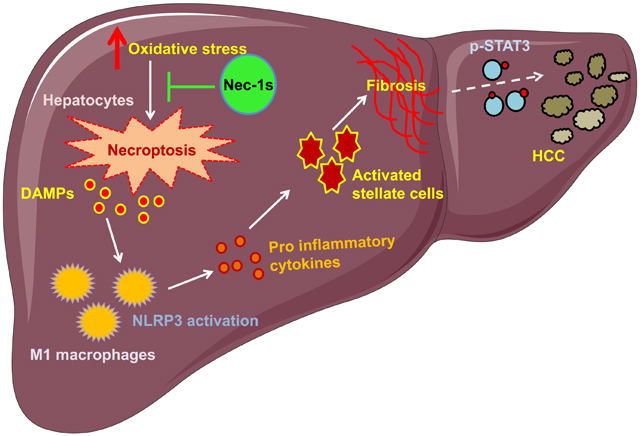
In summary, our data demonstrate that increased oxidative stress in Sod1KO mice leads to increased necroptosis and necroptosis-mediated inflammation, which appears to contribute to CLD, and possibly HCC seen in the Sod1KO mice. Because inflammation is a major factor in CLD in humans and markers of necroptosis are increased in CLD, our data lead us to propose that necroptosis plays an important role in CLD. We propose that excess fat accumulation in the livers of Sod1KO mice due to impaired VLDL secretion leads to NAFLD and the high oxidative stress triggers necroptosis in the liver leading to the generation of DAMPs. The DAMPs, in turn activate macrophages and the inflammasome leading to the production of pro-inflammatory cytokines resulting in non-resolving chronic inflammation (NASH). The increased inflammation activates HSC causing fibrosis through increased production of ECM. Based on our model, we predict that blocking/reducing necroptosis would be an effective strategy for treating/preventing the development of NASH and fibrosis and possibly HCC because we demonstrated that short-term (~1 month) administration of Nec-1s completely blocked necroptosis and dramatically reduced inflammation in the livers of Sod1KO mice. Currently, we are testing the ability of genetic and pharmaceutical strategies that block necroptosis to prevent the progression of NAFLD to HCC in various mouse models, including Sod1KO mice. Several drugs are currently available that target necroptosis in addition to Nec-1s, e.g., RIPK1 inhibitor RIPA-56 [75], RIPK3 inhibitors dabrafenib [76] and GSK872 [77], and MLKL inhibitor necrosulfonamide [78]. Because some of these drugs are approved for use in humans, targeting necroptosis is a potential translatable treatment to prevent NAFLD-HCC progression in humans.
Supplementary Material
Supplementary Figure 1. Effect of Sod1 deficiency and necroptosis on body weight and liver weight.
The body weight (A) and liver weight (B) of WT (blue circles), Sod1KO (red circles) and Sod1KO mice treated with Nec-1s (green circles) are compared. Data were obtained from 5 to 7 mice per group and are expressed as mean + SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Supplementary Figure 2. Effect of Sod1 deficiency and necroptosis on expression of M2 macrophage markers.
(A) Transcript levels of M2 macrophage markers in the livers of WT (blue bars), Sod1KO (red bars) and Sod1KO mice treated with Nec-1s (green bars). Data were obtained from 5 to 7 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs 0.05).
Supplementary Figure 3. Effect of Sod1 deficiency and necroptosis on expression of proinflammatory cytokines and chemokines.
(A) Transcript levels of proinflammatory cytokines and chemokines in the livers of WT, Sod1KO and Sod1KO mice treated with Nec-1s as measured by RT2 Profiler PCR array. Data were obtained from 5 to 6 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO- Nec-1s; */#/^ P ≤ 0.05).
Supplementary Figure 4. Left panel: Immunoblots of liver extracts prepared from WT (blue bars), Sod1KO (red bars) and Sod1KO mice treated with Nec-1s (green bars) for P-AMPK, AMPK and β-tubulin. Right panel: Graphical representation of quantified blots normalized to β-tubulin. Data were obtained from 5 to 7 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Supplementary Figure 5. The relative expression of proteins involved in β-oxidation pathway and fatty acid metabolism in the livers of Sod1KO mice (red bars) and Sod1KO mice treated with Nec-1s (green bars), compared to WT mice, as assessed by targeted quantitative proteomics. Expression of these proteins in WT mice is taken as 100%. The values are normalized to internal standards and housekeeping proteins. Data were obtained from 5 to 6 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05). ABCD3-ATP- binding cassette, sub-family, member 3; ACAA1A/B-Acetyl Coenzyme A acyltransferase 1A/B; ACAA2-Acetyl Coenzyme A acyltransferase2; ACAD11-Acyl coenzyme A dehydrogenase family, member 11; ACADL-Acyl coenzyme A dehydrogenase, long chain; ACADM-Acyl coenzyme A dehydrogenase, medium chain; ACADS-Acyl coenzyme A dehydrogenase, short chain; ACADVL-Acyl coenzyme A dehydrogenase, very long chain; ACOT13-Acyl CoA thioesterase 13; ACSL1-Acyl CoA synthetase long chain family member 1; BDH1–3- hydroxybutyrate dehydrogenase, type 1; CPT1A-Carnitine palmitoyl transferase 1a; CPT2-Carnitine palmitoyl transferase 2; CRAT-Carnitine acetyltransferase; CROT-Carnitine O-octanoyltransferase; DECR1–2,4- dienoyl CoA reductase; ECH1-Enoyl Coenzyme A hydratase 1, ECHS1-Enoyl Coenzyme A hydratase, short chain; ECI1-Enoyl Coenzyme A delta isomerase 1; ECI2-Enoyl Coenzyme A delta isomerase 2; EHHADH-Enoyl Coenzyme A, hydratase/3-hydroxyacyl coenzyme A dehydrogenase; FABP4-Fatty acid binding protein 4; HADH-Hydroxy acyl Coenzyme A dehydrogenase; HADHA-Hydroxy acyl Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase, alpha subunit; HADHB-Hydroxy acyl Coenzyme A dehydrogenase/3-ketoacyl Coenzyme A thiolase/enoyl Coenzyme A hydratase, beta subunit; HMGC1–3-hydroxy-3-methyl glutaryl-Coenzyme A lyase; HMGCS1/2– 3- hydroxy- 3- methyl glutaryl –Coenzyme A synthase1/2; HSD17B4-Hydroxy steroid (17-beta) dehydrogenase 4; SLC25A20-Solute carrier family 25, member 20.
Supplementary Figure 6. The relative expression of proteins involved in TCA cycle and electron transport chain in the livers of Sod1KO mice (red bars) and Sod1KO mice treated with Nec-1s (green bars), compared to WT mice, as assessed by targeted quantitative proteomics. Expression of these proteins in WT mice is taken as 100%. The values are normalized to internal standards and housekeeping proteins. Data were obtained from 5 to 6 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05). ACO2 – Aconitase 2; ATP2A2-ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2; ATP5A1-ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit 1; ATP5B-ATP synthase, H+ transporting, mitochondrial F1 complex, beta subunit; CS-Citrate synthase; DLAT-Dihydrolipoamide S-acetyl transferase; DLD-dihydrolipoamide dehydrogenase; DLST-dihydrolipoamide S-succinyl transferase; ETFA-Electron transferring flavoprotein, alpha polypeptide; ETFB-Electron transferring flavoprotein, beta polypeptide; ETFDH-Electron transferring flavoprotein, dehydrogenase; FH1-Fumarate hydratase 1; IDH1, 2, 3A, 3B, 3G-Isocitrate dehydrogenase 1, 2, 3 alpha,3 beta, 3 gamma; MDH 1, 2-Malate dehydrogenase 1, 2; NDUFS1-NADH dehydrogenase Fe-S protein; NDUFV1-NADH dehydrogenase flavoprotein; OGDH-Oxoglutarate (alpha-ketoglutarate) dehydrogenase; PDHA1-Pyruvate dehydrogenase E1 alpha 1; PDHB-Pyruvate dehydrogenase beta; SDHA, B, C-Succinate dehydrogenase complex, subunit A, B, C; SUCLA2-Succinate-Coenzyme A ligase, ADP-forming, beta subunit; SUCLG1-Succinate-CoA ligase, GDP-forming, alpha subunit; UQCRC1-Ubiquinol-cytochrome C reductase core protein 1.
Supplementary Figure 7. Effect of Sod1 deficiency and necroptosis on signaling pathways associated with HCC.
Left panel: Immunoblots of liver extracts prepared from WT (blue bars), Sod1KO (red bars) and Sod1KO mice treated with Nec-1s (green bars) for P-JNK and JNK (A), P-ERK and ERK, (B) and P-p38 and p38 (C). Right panel: Graphical representation of quantified phospho-blots normalized to total protein. Data were obtained from 5 to 7 mice per group and are expressed as the mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Supplementary Table 1. Effect of Sod1 deficiency and necroptosis on the expression of proinflammatory cytokines and chemokines.
Transcript levels of proinflammatory cytokines and chemokines in the livers of WT, Sod1KO and Sod1KO mice treated with Nec-1s as measured by RT2 Profiler PCR array, normalized to HPRT. Average value of WT mice was used to normalize values of WT, Sod1KO and Sod1KO mice treated with Nec-1s mice. The darker the red indicates the greater the increase in expression, and the darker the blue indicates the greater the decrease in expression.
Supplementary Table 2. Effect of Sod1 deficiency and necroptosis on the expression of MMPs and TIMPs.
Transcript levels of MMPs and TIMPs in the livers of WT, Sod1KO and Sod1KO mice treated with Nec-1s as measured by RT2 Profiler PCR array. Data were obtained from 5 to 6 mice per group and are expressed as the mean ± SEM. P value ≤ 0.05 is considered significant.
Highlights.
Inflammation and necroptosis are increased in the livers of Sod1KO mice, a mouse model of increased oxidative stress and accelerated aging.
Blocking necroptosis reduced expression of proinflammatory cytokines in livers of Sod1KO mice.
Blocking necroptosis reduced fibrosis and activation of oncogenic transcription factor STAT3 in the livers of Sod1KO mice.
Necroptosis-mediated inflammation play a role in chronic liver disease in Sod1KO mice.
Acknowledgments
The efforts of authors were supported by Oklahoma Center for the Advancement of Science and Technology research grant (HR18-053) (SD), Presbyterian Health Foundation (OUHSC) Seed grant (SD), NIH grants R01AG059718 (SD),R01AG057424 (AR), and the Oklahoma Nathan Shock Aging Center [P30AG050911 (AR, MS)] and a Senior Career Research Award (AR) and a Merit grant I01BX004538 (AR) from the Department of Veterans Affairs.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- [1].Marcellin P, Kutala BK, Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening., Liver Int. Off. J. Int. Assoc. Study Liver. 38 Suppl 1 (2018) 2–6. 10.1111/liv.13682. [DOI] [PubMed] [Google Scholar]
- [2].Byass P, The global burden of liver disease: a challenge for methods and for public health., BMC Med. 12 (2014) 159. 10.1186/s12916-014-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M, Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes., Hepatology. 64 (2016) 73–84. 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- [4].Marengo A, Rosso C, Bugianesi E, Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis., Annu. Rev. Med. 67 (2016) 103–117. 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- [5].Goh GB-B, McCullough AJ, Natural History of Nonalcoholic Fatty Liver Disease., Dig. Dis. Sci. 61 (2016) 1226–1233. 10.1007/s10620-016-4095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rawla P, Sunkara T, Muralidharan P, Raj JP, Update in global trends and aetiology of hepatocellular carcinoma., Contemp. Oncol. (Poznan, Poland). 22 (2018) 141–150. 10.5114/wo.2018.78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Day CP, James OF, Steatohepatitis: a tale of two “hits”?, Gastroenterology. 114 (1998) 842–845. 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- [8].Tilg H, Moschen AR, Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis., Hepatology. 52 (2010) 1836–1846. 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- [9].Koyama Y, Brenner DA, Liver inflammation and fibrosis., J. Clin. Invest. 127 (2017) 55–64. 10.1172/JCI88881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schuster S, Cabrera D, Arrese M, Feldstein AE, Triggering and resolution of inflammation in NASH., Nat. Rev. Gastroenterol. Hepatol. 15 (2018) 349–364. 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- [11].Luedde T, Kaplowitz N, Schwabe RF, Cell death and cell death responses in liver disease: mechanisms and clinical relevance., Gastroenterology. 147 (2014) 765–783.e4. 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.[] Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J, Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury., Nat. Chem. Biol. 1 (2005) 112–119. 10.1038/nchembio711.. [DOI] [PubMed] [Google Scholar]
- [13].Newton K, Manning G, Necroptosis and Inflammation., Annu. Rev. Biochem. 85 (2016) 743–763. 10.1146/annurev-biochem-060815-014830. [DOI] [PubMed] [Google Scholar]
- [14].Weinlich R, Oberst A, Beere HM, Green DR, Necroptosis in development, inflammation and disease., Nat. Rev. Mol. Cell Biol. 18 (2017) 127–136. 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- [15].Pasparakis M, Vandenabeele P, Necroptosis and its role in inflammation., Nature. 517 (2015) 311–320. 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- [16].Yuan J, Amin P, Ofengeim D, Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases., Nat. Rev. Neurosci. 20 (2019) 19–33. 10.1038/s41583-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roychowdhury S, McCullough RL, Sanz-Garcia C, Saikia P, Alkhouri N, Matloob A, Pollard KA, McMullen MR, Croniger CM, Nagy LE, Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury., Hepatology. 64 (2016) 1518–1533. 10.1002/hep.28676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT, Bartneck M, Neumann UP, Canbay A, Reeves HL, Luedde M, Tacke F, Trautwein C, Heikenwalder M, Luedde T, A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis., EMBO Mol. Med. 6 (2014) 1062–1074. 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gautheron J, Vucur M, Schneider AT, Severi I, Roderburg C, Roy S, Bartneck M, Schrammen P, Diaz MB, Ehling J, Gremse F, Heymann F, Koppe C, Lammers T, Kiessling F, Van Best N, Pabst O, Courtois G, Linkermann A, Krautwald S, Neumann UP, Tacke F, Trautwein C, Green DR, Longerich T, Frey N, Luedde M, Bluher M, Herzig S, Heikenwalder M, Luedde T, The necroptosis-inducing kinase RIPK3 dampens adipose tissue inflammation and glucose intolerance., Nat. Commun. 7 (2016) 11869. 10.1038/ncomms11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Afonso MB, Rodrigues PM, Carvalho T, Caridade M, Borralho P, Cortez-Pinto H, Castro RE, Rodrigues CMP, Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis., Clin. Sci. (Lond). 129 (2015) 721–739. 10.1042/CS20140732. [DOI] [PubMed] [Google Scholar]
- [21].Xu H, Du X, Liu G, Huang S, Du W, Zou S, Tang D, Fan C, Xie Y, Wei Y, Tian Y, Fu X, The pseudokinase MLKL regulates hepatic insulin sensitivity independently of inflammation., Mol. Metab. 23 (2019) 14–23. 10.1016/j.molmet.2019.02.003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saeed WK, Jun DW, Jang K, Koh DH, Necroptosis signaling in liver diseases: An update, Pharmacol. Res. 148 (2019). 10.1016/j.phrs.2019.104439. [DOI] [PubMed] [Google Scholar]
- [23].Majdi A, Aoudjehane L, Ratziu V, Islam T, Afonso MB, Conti F, Mestiri T, Lagouge M, Foufelle F, Ballenghien F, Ledent T, Moldes M, Cadoret A, Fouassier L, Delaunay J-L, Aït-Slimane T, Courtois G, Fève B, Scatton O, Prip-Buus C, Rodrigues CMP, Housset C, Gautheron J, Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease., J. Hepatol. 72 (2020) 627–635. 10.1016/j.jhep.2019.11.008. [DOI] [PubMed] [Google Scholar]
- [24].Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang T-T, Epstein CJ, L.J. 2nd Roberts, M. Csete, J.A. Faulkner, H. Van Remmen, Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy., Free Radic. Biol. Med. 40 (2006) 1993–2004. 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- [25].Zhang Y, Liu Y, Walsh M, Bokov A, Ikeno Y, Jang YC, Perez VI, Van Remmen H, Richardson A, Liver specific expression of Cu/ZnSOD extends the lifespan of Sod1 null mice., Mech. Ageing Dev. 154 (2016) 1–8. 10.1016/j.mad.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uchiyama S, Shimizu T, Shirasawa T, CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice., J. Biol. Chem. 281 (2006) 31713–31719. 10.1074/jbc.M603422200. [DOI] [PubMed] [Google Scholar]
- [27].Sakiyama H, Fujiwara N, Yoneoka Y, Yoshihara D, Eguchi H, Suzuki K, Cu,Zn-SOD deficiency induces the accumulation of hepatic collagen., Free Radic. Res. 50 (2016) 666–677. 10.3109/10715762.2016.1164856. [DOI] [PubMed] [Google Scholar]
- [28].Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang T-T, CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life., Oncogene. 24 (2005) 367–380. 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- [29].Lee G, Jeong YS, Kim DW, Kwak MJ, Koh J, Joo EW, Lee J-S, Kah S, Sim Y-E, Yim SY, Clinical significance of APOB inactivation in hepatocellular carcinoma., Exp. Mol. Med. 50 (2018) 1–12. 10.1038/s12276-018-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Febbraio MA, Reibe S, Shalapour S, Ooi GJ, Watt MJ, Karin M, Preclinical Models for Studying NASH-Driven HCC: How Useful Are They?, Cell Metab. 29 (2019) 18–26. 10.1016/j.cmet.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Royce GH, Brown-Borg HM, Deepa SS, The potential role of necroptosis in inflammaging and aging., GeroScience. 41 (2019) 795–811. 10.1007/s11357-019-00131-w.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, Goossens V, Roelandt R, Van Hauwermeiren F, Libert C, Declercq W, Callewaert N, Prendergast GC, Degterev A, Yuan J, Vandenabeele P, Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models., Cell Death Dis. 3 (2012) e437. 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, Geng J, Py B, Zhou W, Amin P, Berlink Lima J, Qi C, Yu Q, Trapp B, Yuan J, Activation of necroptosis in multiple sclerosis., Cell Rep. 10 (2015) 1836–1849. 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L, Geng J, Amin P, DeWitt JP, Mookhtiar AK, Florez M, Ouchida AT, Fan J, Pasparakis M, Kelliher MA, Ravits J, Yuan J, RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS., Science. 353 (2016) 603–608. 10.1126/science.aaf6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Logan S, Royce GH, Owen D, Farley J, Ranjo-Bishop M, Sonntag WE, Deepa SS, Accelerated decline in cognition in a mouse model of increased oxidative stress., GeroScience. 41 (2019) 591–607. 10.1007/s11357-019-00105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bhaskaran S, Pharaoh G, Ranjit R, Murphy A, Matsuzaki S, Nair BC, Forbes B, Gispert S, Auburger G, Humphries KM, Kinter M, Griffin TM, Deepa SS, Loss of mitochondrial protease ClpP protects mice from diet-induced obesity and insulin resistance., EMBO Rep. 19 (2018). 10.15252/embr.201745009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fernandes J, Weddle A, Kinter CS, Humphries KM, Mather T, Szweda LI, Kinter M, Lysine Acetylation Activates Mitochondrial Aconitase in the Heart., Biochemistry. 54 (2015) 4008–4018. 10.1021/acs.biochem.5b00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kinter CS, Lundie JM, Patel H, Rindler PM, Szweda LI, Kinter M, A quantitative proteomic profile of the Nrf2-mediated antioxidant response of macrophages to oxidized LDL determined by multiplexed selected reaction monitoring., PLoS One. 7 (2012) e50016. 10.1371/journal.pone.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Uchida K, Itakura K, Kawakishi S, Hiai H, Toyokuni S, Stadtman ER, Characterization of epitopes recognized by 4-hydroxy-2-nonenal specific antibodies., Arch. Biochem. Biophys. 324 (1995) 241–248. 10.1006/abbi.1995.0036. [DOI] [PubMed] [Google Scholar]
- [40].He S, Huang S, Shen Z, Biomarkers for the detection of necroptosis., Cell. Mol. Life Sci. 73 (2016) 2177–2181. 10.1007/s00018-016-2192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wu X, Nagy LE, MLKL contributes to Western diet-induced liver injury through inhibiting autophagy, Autophagy. 16 (2020) 1351–1352. 10.1080/15548627.2020.1760624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Land WG, The Role of Damage-Associated Molecular Patterns in Human Diseases: Part I - Promoting inflammation and immunity., Sultan Qaboos Univ. Med. J. 15 (2015) e9–e21. [PMC free article] [PubMed] [Google Scholar]
- [43].Youm Y-H, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Münzberg H, Rosen CJ, Ingram DK, Salbaum JM, Dixit VD, Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging., Cell Metab. 18 (2013) 519–532. 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang L, Jiang Z, Lei XG, Knockout of SOD1 alters murine hepatic glycolysis, gluconeogenesis, and lipogenesis., Free Radic. Biol. Med. 53 (2012) 1689–1696. 10.1016/j.freeradbiomed.2012.08.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW, Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle., J. Appl. Physiol. 92 (2002) 2475–2482. 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- [46].Begriche K, Igoudjil A, Pessayre D, Fromenty B, Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it., Mitochondrion. 6 (2006) 1–28. 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- [47].Léveillé M, Estall JL, Mitochondrial Dysfunction in the Transition from NASH to HCC., Metabolites. 9 (2019). 10.3390/metabo9100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dalleau S, Baradat M, Guéraud F, Huc L, Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance., Cell Death Differ. 20 (2013) 1615–1630. 10.1038/cdd.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Breitzig M, Bhimineni C, Lockey R, Kolliputi N, 4-Hydroxy-2-nonenal: a critical target in oxidative stress?, Am. J. Physiol. Cell Physiol. 311 (2016) C537–C543. 10.1152/ajpcell.00101.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nikolic-Paterson DJ AR, Main IW, Tesch GH, H Y, Interleukin-1 in renal fibrosis., Kidney Int Suppl. 54: (1996) S88–90. [PubMed] [Google Scholar]
- [51].O’Reilly S, Ciechomska M, Cant R, Hügle T, van Laar JM, Interleukin-6, its role in fibrosing conditions., Cytokine Growth Factor Rev. 23 (2012) 99–107. 10.1016/j.cytogfr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- [52].Cong M, Iwaisako K, Jiang C, Kisseleva T, Cell signals influencing hepatic fibrosis., Int. J. Hepatol. 2012 (2012) 158547. 10.1155/2012/158547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Alegre F, Pelegrin P, Feldstein AE, Inflammasomes in Liver Fibrosis., Semin. Liver Dis. 37 (2017) 119–127. 10.1055/s-0037-1601350. [DOI] [PubMed] [Google Scholar]
- [54].Puche JE, Saiman Y, Friedman SL, Hepatic stellate cells and liver fibrosis., Compr. Physiol. 3 (2013) 1473–1492. 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- [55].Roeb E, Matrix metalloproteinases and liver fibrosis (translational aspects)., Matrix Biol. 68–69 (2018) 463–473. 10.1016/j.matbio.2017.12.012. [DOI] [PubMed] [Google Scholar]
- [56].Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF, p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway., Nat. Genet. 39 (2007) 741–749. 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- [57].Maeda S, Kamata H, Luo J-L, Leffert H, Karin M, IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis., Cell. 121 (2005) 977–990. 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- [58].Sakurai T, Maeda S, Chang L, Karin M, Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation., Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 10544–10551. 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sakurai T, He G, Matsuzawa A, Yu G-Y, Maeda S, Hardiman G, Karin M, Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis., Cancer Cell. 14 (2008) 156–165. 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yu R, Shi Q, Liu L, Chen L, Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non-alcoholic fatty liver disease: a meta-analysis., BMC Gastroenterol. 18 (2018) 51. 10.1186/s12876-018-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Villanueva A, Luedde T, The transition from inflammation to cancer in the liver., Clin. Liver Dis. 8 (2016) 89–93. 10.1002/cld.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang Y, Ikeno Y, Bokov A, Gelfond J, Jaramillo C, Zhang H-M, Liu Y, Qi W, Hubbard G, Richardson A, Van Remmen H, Dietary restriction attenuates the accelerated aging phenotype of Sod1(−/−) mice., Free Radic. Biol. Med. 60 (2013) 300–306. 10.1016/j.freeradbiomed.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, Sosnowska D, Van Remmen H, Richardson A, A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1(-/)(−) mice is correlated to increased cellular senescence., Redox Biol. 11 (2017) 30–37. 10.1016/j.redox.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Braunersreuther V, Viviani GL, Mach F, Montecucco F, Role of cytokines and chemokines in non-alcoholic fatty liver disease., World J. Gastroenterol. 18 (2012) 727–735. 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E, The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages., Biomed Res. Int. 2013 (2013) 187204. 10.1155/2013/187204.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Canli Ö, Alankuş YB, Grootjans S, Vegi N, Hültner L, Hoppe PS, Schroeder T, Vandenabeele P, Bornkamm GW, Greten FR, Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors., Blood. 127 (2016) 139–148. 10.1182/blood-2015-06-654194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Takemoto K, Hatano E, Iwaisako K, Takeiri M, Noma N, Ohmae S, Toriguchi K, Tanabe K, Tanaka H, Seo S, Taura K, Machida K, Takeda N, Saji S, Uemoto S, Asagiri M, Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure., FEBS Open Bio. 4 (2014) 777–787. 10.1016/j.fob.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Han CH, Guan ZB, Zhang PX, Fang HL, Li L, Zhang HM, Zhou FJ, Mao YF, Liu WW, Oxidative stress induced necroptosis activation is involved in the pathogenesis of hyperoxic acute lung injury., Biochem. Biophys. Res. Commun. 495 (2018) 2178–2183. 10.1016/j.bbrc.2017.12.100. [DOI] [PubMed] [Google Scholar]
- [69].Johnston AN, Ma Y, Liu H, Liu S, Hanna-Addams S, Chen S, Chen C, Wang Z, Necroptosis-blocking compound NBC1 targets heat shock protein 70 to inhibit MLKL polymerization and necroptosis., Proc. Natl. Acad. Sci. U. S. A. 117 (2020) 6521–6530. 10.1073/pnas.1916503117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kaushik S, Cuervo AM, Chaperone-mediated autophagy: a unique way to enter the lysosome world., Trends Cell Biol. 22 (2012) 407–417. 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kurahashi T, Hamashima S, Shirato T, Lee J, Homma T, Kang ES, Fujii J, An SOD1 deficiency enhances lipid droplet accumulation in the fasted mouse liver by aborting lipophagy., Biochem. Biophys. Res. Commun. 467 (2015) 866–871. 10.1016/j.bbrc.2015.10.052. [DOI] [PubMed] [Google Scholar]
- [72].Friedman SL, Mechanisms of hepatic fibrogenesis., Gastroenterology. 134 (2008) 1655–1669. 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Park EJ, Lee JH, Yu G-Y, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M, Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression., Cell. 140 (2010) 197–208. 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].He G, Yu G-Y, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck- Radosavljevic M, Leffert HL, Karin M, Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation., Cancer Cell. 17 (2010) 286–297. 10.1016/j.ccr.2009.12.048.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ren Y, Su Y, Sun L, He S, Meng L, Liao D, Liu X, Ma Y, Liu C, Li S, Ruan H, Lei X, Wang X, Zhang Z, Discovery of a Highly Potent, Selective, and Metabolically Stable Inhibitor of Receptor-Interacting Protein 1 (RIP1) for the Treatment of Systemic Inflammatory Response Syndrome., J. Med. Chem. 60 (2017) 972–986. 10.1021/acs.jmedchem.6b01196. [DOI] [PubMed] [Google Scholar]
- [76].Li J-X, Feng J-M, Wang Y, Li X-H, Chen X-X, Su Y, Shen Y-Y, Chen Y, Xiong B, Yang C-H, Ding J, Miao Z-H, The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury., Cell Death Dis. 5 (2014) e1278. 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK-M, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ, RIP3 induces apoptosis independent of pronecrotic kinase activity., Mol. Cell. 56 (2014) 481–495. 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liao D, Sun L, Liu W, He S, Wang X, Lei X, Necrosulfonamide inhibits necroptosis by selectively targeting the mixed lineage kinase domain-like protein, Medchemcomm. 5 (2014) 333–337. 10.1039/C3MD00278K. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Effect of Sod1 deficiency and necroptosis on body weight and liver weight.
The body weight (A) and liver weight (B) of WT (blue circles), Sod1KO (red circles) and Sod1KO mice treated with Nec-1s (green circles) are compared. Data were obtained from 5 to 7 mice per group and are expressed as mean + SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Supplementary Figure 2. Effect of Sod1 deficiency and necroptosis on expression of M2 macrophage markers.
(A) Transcript levels of M2 macrophage markers in the livers of WT (blue bars), Sod1KO (red bars) and Sod1KO mice treated with Nec-1s (green bars). Data were obtained from 5 to 7 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs 0.05).
Supplementary Figure 3. Effect of Sod1 deficiency and necroptosis on expression of proinflammatory cytokines and chemokines.
(A) Transcript levels of proinflammatory cytokines and chemokines in the livers of WT, Sod1KO and Sod1KO mice treated with Nec-1s as measured by RT2 Profiler PCR array. Data were obtained from 5 to 6 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO- Nec-1s; */#/^ P ≤ 0.05).
Supplementary Figure 4. Left panel: Immunoblots of liver extracts prepared from WT (blue bars), Sod1KO (red bars) and Sod1KO mice treated with Nec-1s (green bars) for P-AMPK, AMPK and β-tubulin. Right panel: Graphical representation of quantified blots normalized to β-tubulin. Data were obtained from 5 to 7 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Supplementary Figure 5. The relative expression of proteins involved in β-oxidation pathway and fatty acid metabolism in the livers of Sod1KO mice (red bars) and Sod1KO mice treated with Nec-1s (green bars), compared to WT mice, as assessed by targeted quantitative proteomics. Expression of these proteins in WT mice is taken as 100%. The values are normalized to internal standards and housekeeping proteins. Data were obtained from 5 to 6 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05). ABCD3-ATP- binding cassette, sub-family, member 3; ACAA1A/B-Acetyl Coenzyme A acyltransferase 1A/B; ACAA2-Acetyl Coenzyme A acyltransferase2; ACAD11-Acyl coenzyme A dehydrogenase family, member 11; ACADL-Acyl coenzyme A dehydrogenase, long chain; ACADM-Acyl coenzyme A dehydrogenase, medium chain; ACADS-Acyl coenzyme A dehydrogenase, short chain; ACADVL-Acyl coenzyme A dehydrogenase, very long chain; ACOT13-Acyl CoA thioesterase 13; ACSL1-Acyl CoA synthetase long chain family member 1; BDH1–3- hydroxybutyrate dehydrogenase, type 1; CPT1A-Carnitine palmitoyl transferase 1a; CPT2-Carnitine palmitoyl transferase 2; CRAT-Carnitine acetyltransferase; CROT-Carnitine O-octanoyltransferase; DECR1–2,4- dienoyl CoA reductase; ECH1-Enoyl Coenzyme A hydratase 1, ECHS1-Enoyl Coenzyme A hydratase, short chain; ECI1-Enoyl Coenzyme A delta isomerase 1; ECI2-Enoyl Coenzyme A delta isomerase 2; EHHADH-Enoyl Coenzyme A, hydratase/3-hydroxyacyl coenzyme A dehydrogenase; FABP4-Fatty acid binding protein 4; HADH-Hydroxy acyl Coenzyme A dehydrogenase; HADHA-Hydroxy acyl Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase, alpha subunit; HADHB-Hydroxy acyl Coenzyme A dehydrogenase/3-ketoacyl Coenzyme A thiolase/enoyl Coenzyme A hydratase, beta subunit; HMGC1–3-hydroxy-3-methyl glutaryl-Coenzyme A lyase; HMGCS1/2– 3- hydroxy- 3- methyl glutaryl –Coenzyme A synthase1/2; HSD17B4-Hydroxy steroid (17-beta) dehydrogenase 4; SLC25A20-Solute carrier family 25, member 20.
Supplementary Figure 6. The relative expression of proteins involved in TCA cycle and electron transport chain in the livers of Sod1KO mice (red bars) and Sod1KO mice treated with Nec-1s (green bars), compared to WT mice, as assessed by targeted quantitative proteomics. Expression of these proteins in WT mice is taken as 100%. The values are normalized to internal standards and housekeeping proteins. Data were obtained from 5 to 6 mice per group and are expressed as mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO- Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05). ACO2 – Aconitase 2; ATP2A2-ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2; ATP5A1-ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit 1; ATP5B-ATP synthase, H+ transporting, mitochondrial F1 complex, beta subunit; CS-Citrate synthase; DLAT-Dihydrolipoamide S-acetyl transferase; DLD-dihydrolipoamide dehydrogenase; DLST-dihydrolipoamide S-succinyl transferase; ETFA-Electron transferring flavoprotein, alpha polypeptide; ETFB-Electron transferring flavoprotein, beta polypeptide; ETFDH-Electron transferring flavoprotein, dehydrogenase; FH1-Fumarate hydratase 1; IDH1, 2, 3A, 3B, 3G-Isocitrate dehydrogenase 1, 2, 3 alpha,3 beta, 3 gamma; MDH 1, 2-Malate dehydrogenase 1, 2; NDUFS1-NADH dehydrogenase Fe-S protein; NDUFV1-NADH dehydrogenase flavoprotein; OGDH-Oxoglutarate (alpha-ketoglutarate) dehydrogenase; PDHA1-Pyruvate dehydrogenase E1 alpha 1; PDHB-Pyruvate dehydrogenase beta; SDHA, B, C-Succinate dehydrogenase complex, subunit A, B, C; SUCLA2-Succinate-Coenzyme A ligase, ADP-forming, beta subunit; SUCLG1-Succinate-CoA ligase, GDP-forming, alpha subunit; UQCRC1-Ubiquinol-cytochrome C reductase core protein 1.
Supplementary Figure 7. Effect of Sod1 deficiency and necroptosis on signaling pathways associated with HCC.
Left panel: Immunoblots of liver extracts prepared from WT (blue bars), Sod1KO (red bars) and Sod1KO mice treated with Nec-1s (green bars) for P-JNK and JNK (A), P-ERK and ERK, (B) and P-p38 and p38 (C). Right panel: Graphical representation of quantified phospho-blots normalized to total protein. Data were obtained from 5 to 7 mice per group and are expressed as the mean ± SEM. (ANOVA, *WT-Veh vs Sod1KO-Veh; #WT-Veh vs Sod1KO-Nec-1s; ^ Sod1KO-Veh vs Sod1KO-Nec-1s; */#/^ P ≤ 0.05).
Supplementary Table 1. Effect of Sod1 deficiency and necroptosis on the expression of proinflammatory cytokines and chemokines.
Transcript levels of proinflammatory cytokines and chemokines in the livers of WT, Sod1KO and Sod1KO mice treated with Nec-1s as measured by RT2 Profiler PCR array, normalized to HPRT. Average value of WT mice was used to normalize values of WT, Sod1KO and Sod1KO mice treated with Nec-1s mice. The darker the red indicates the greater the increase in expression, and the darker the blue indicates the greater the decrease in expression.
Supplementary Table 2. Effect of Sod1 deficiency and necroptosis on the expression of MMPs and TIMPs.
Transcript levels of MMPs and TIMPs in the livers of WT, Sod1KO and Sod1KO mice treated with Nec-1s as measured by RT2 Profiler PCR array. Data were obtained from 5 to 6 mice per group and are expressed as the mean ± SEM. P value ≤ 0.05 is considered significant.